Abstract
Complex genetic and physiological variations as well as environmental factors that drive emergence of chromosomal instability, development of unscheduled cell death, skewed differentiation, and altered metabolism are central to the pathogenesis of human diseases and disorders. Understanding the molecular bases for these processes is important for the development of new diagnostic biomarkers, and for identifying new therapeutic targets. In 1973, a group of non-histone nuclear proteins with high electrophoretic mobility was discovered and termed High-Mobility Group (HMG) proteins. The HMG proteins include three superfamilies termed HMGB, HMGN, and HMGA. High-mobility group box 1 (HMGB1), the most abundant and well-studied HMG protein, senses and coordinates the cellular stress response and plays a critical role not only inside of the cell as a DNA chaperone, chromosome guardian, autophagy sustainer, and protector from apoptotic cell death, but also outside the cell as the prototypic damage associated molecular pattern molecule (DAMP). This DAMP, in conjunction with other factors, thus has cytokine, chemokine, and growth factor activity, orchestrating the inflammatory and immune response. All of these characteristics make HMGB1 a critical molecular target in multiple human diseases including infectious diseases, ischemia, immune disorders, neurodegenerative diseases, metabolic disorders, and cancer. Indeed, a number of emergent strategies have been used to inhibit HMGB1 expression, release, and activity in vitro and in vivo. These include antibodies, peptide inhbitiors, RNAi, anti-coagulants, endogenous hormones, various chemical compounds, HMGB1-receptor and signaling pathway inhibition, artificial DNAs, physical strategies including vagus nerve stimulation and other surgical approaches. Future work further investigating the details of HMGB1 localizationtion, structure, post-translational modification, and identifccation of additional partners will undoubtedly uncover additional secrets regarding HMGB1’s multiple functions.
1 Introduction and Historical Background
In 1879, Walther Flemming, a trailblazing German cytologist, identified chromosomes using aniline dyes (Paweletz, 2001). We now know that chromosomes contain all genes that transfer well-defined characteristics from parents to offsprings. Chromosomes are actually packages of condensed chromatin, which is a nucleoprotein complex, residua of our archaeal past. The precise interaction between DNA and chromosomal protein regulates the structure, dynamics, and function of chromosomes, which in turn facilitates genomic stability and gene regulation (Andrews and Luger, 2011; Elgin and Weintraub, 1975; Stein et al., 1974). Histones, including H1, H2A, H2B, H3, and H4, are the predominant class of positively-charged chromosomal proteins (Campos and Reinberg, 2009). Histones tightly bind to negatively-charged DNA to condense it into a more compact structure, termed a nucleosome. A nucleosome is the basic repeating unit of chromatin with repetitive histone octamer units, which are typically wrapped with 147 base pairs of DNA. The second class of chromosomal proteins is composed of several relatively low-abundance, tissue-specific, high salt-urea insoluble proteins (Earnshaw and Mackay, 1994; Wakabayashi et al., 1974). These proteins include the nuclear matrix proteins, the chromosome scaffold protein, and the enzymes responsible for regulation of major DNA-associated events (e.g., replication, transcription, repair, and recombination). The third class of chromosomal proteins, and second most abundant, is the high mobility group (HMG) proteins including HMGB, HMGN, and HMGA. Compared with histones, HMG proteins have been highly conserved throughout evolution and loosely bind to chromatin without targeting individual DNA sequences but rather DNA structure (Bianchi and Beltrame, 1998; Einck and Bustin, 1985). Besides performing nuclear functions, several HMG proteins, including HMGB1 (Scaffidi et al., 2002; Wang et al., 1999), HMGB2 (Pusterla et al., 2009), and HMGN1 (Yang et al., 2012a) exert significant extracellular activity and function as damage-associated molecular pattern molecules (DAMPs). Recent genetic, biochemical, and cell biological analyses have greatly improved our understanding of the structure and function of HMG proteins in DNA-related processes, development, differentiation, aging, and cancer (Bianchi and Agresti, 2005; Bustin et al., 1990; Hock et al., 2007). In this review, we will briefly introduce the HMG protein members and focus on the physiological and pathological role of HMGB1 in health and disease. We believe that understanding of the HMG family is a link to our evolutionary past and a deeper understanding the bridge to our future.
1.1 Discovery of the HMG Protein
Ernest Johns from the Chester Beatty Research Institute, London is the pioneer of HMG research. In 1973, Ernest Johns and colleagues Graham Goodwin and Clive Sanders first isolated two groups of proteins from calf thymus chromatin by 0.35M NaCl extraction (Goodwin and Johns, 1973; Goodwin et al., 1973). One group of proteins was easily soluble in 10% trichloroacetic acid and migrated rapidly in polyacrylamide gel electrophoresis systems with no signs of aggregation. Based on its mobility, they called them “high-mobility group” proteins, namely HMG proteins (Goodwin et al., 1973). Partial fractionation of these HMG proteins by gel filtration revealed that they contained at least two proteins, namely protein 1 (HMG-1) and protein 2 (HMG-2). These two proteins are composed of over 55% acidic/basic amino acids with about 105 molecules of HMG-1 and -2 per cell nucleus (Goodwin and Johns, 1973; Goodwin et al., 1973). In contrast, the other group of proteins contained much fewer basic amino acids and migrated more slowly in polyacrylamide gel electrophoresis systems. They therefore called them “low-mobility group” proteins (Goodwin et al., 1973). Johns and his colleagues further demonstrated that HMG proteins can be extracted by 5% perchloric acid and have 40–50 % α-helix structures which are sensitive to pH around neutrality and the urea concentration (Baker et al., 1976; Cary et al., 1976). In addition to HMG1 and 2, Ernest Johns and colleagues separated other HMG proteins with perchloric acid extracts and divided HMG in two groups: the higher (e.g., HMG-1 and HMG-2) and lower (e.g., HMG-14, HMG-17, and HMG-Y) molecular weight proteins (Brown et al., 1980; Cockerill et al., 1983; Goodwin et al., 1980). Currently, a number of HMG proteins have been discovered in several species with the following properties (Bustin et al., 1990): (1) extractable from chromatin using 0.35 M NaCl; (2) soluble in 5% perchloric acid or tricloroacetic acid; (3) < 30 kDa in molecular weight with a high content of charged amino acids; (4) rapidly mobile in polyacrylamide gels; (5) sensitive to extensive post-translational modifications such as phosphorylation, acetylation, and poly-ADP-ribosylation; and (6) tissue- and development-dependent expression. In general, HMGs serve as architectural transcription factors that regulate not only special gene transcription but also global genomic stability by interacting with nucleotides, histones, transcription factors, and other chromosomal or nuclear proteins (Bianchi and Agresti, 2005; Grosschedl et al., 1994; Zlatanova et al., 1999).
1.2 Nomenclature Changes
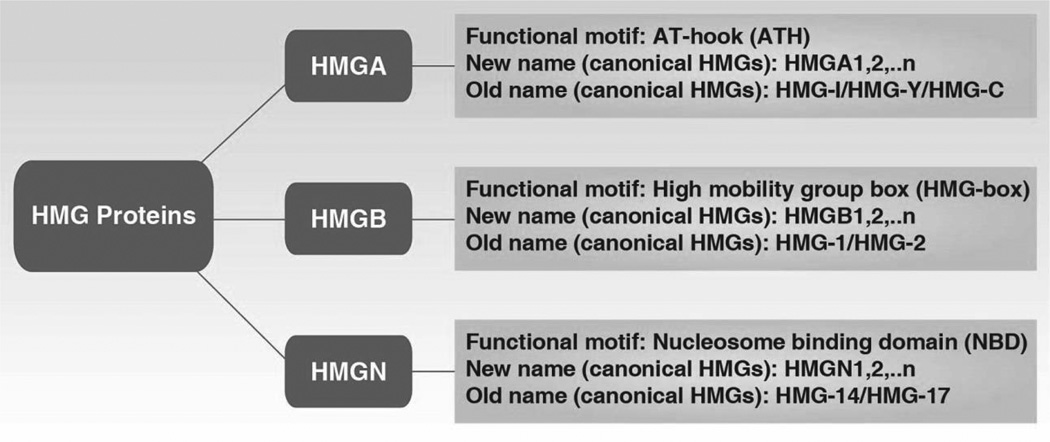
In 2001, Michael Bustin from the National Cancer Institute, USA organized the HMG Chromosomal Protein Nomenclature Committee and recategorized these proteins into three superfamilies, renaming them as HMGB (formerly known as HMG-1/2), HMGA (formerly known as HMG-14/17), and HMGN (formerly known as HMG-I/Y) (Figure 1) (Bustin, 2001). Members of a gene family are sequentially numbered (e.g., HMGB1, HMGB2, HMGA1, HMGA2, HMGN1, and HMGN2). Small letters indicate the splice variants of genes (e.g., HMGA1a, HMGA1b and HMGA1c). Each HMG superfamily contains a characteristic sequence motif with distinct cellular functions. HMG-box is the functional motif of the HMGB family; nucleosomal binding domain is the functional motif of the HMGN family; AT-hook is a DNA-binding motif with a preference for A/T rich regions and is the functional motif of the HMGA family. HMG proteins have the unique ability to recognize individual DNA structures from chromatin through functional motifs in a sequence-independent way. As architectural elements of chromosomes, HMG proteins bind or bend DNA structures, which contribute to sustaining DNA-dependent activity. Of note, the functional motifs of HMG proteins have also been discovered in other proteins, especially nuclear proteins, which are termed HMG-motif proteins such as the HMG-box family. There are two significant differences between canonical HMG proteins and HMG-motif proteins. HMG proteins are ubiquitous, abundant in almost all cells, and bind to DNA in a sequence-independent way, whereas HMG-motif proteins less abundant, are only present in specific cell types, and bind to DNA in a sequence-dependent manner (Bustin, 1999; Segall et al., 1994). The new nomenclature established rules to name genes and proteins belonging to the HMG family, which has contributed to our communication in research. For more detailed information about HMG nomenclature, a list of the HMG proteins in individual species can be found at the following link: http://www.informatics.jax.org/mgihome/nomen/hmg_family.shtml#G
Figure 1.
Revised nomenclature for the HMG chromosomal proteins (Bustin, 2001)
1.3 HMG Families
1.3.1 HMGAs
The HMGA family has two basic members (HMGA1 and HMGA2) and was firstisolated in HeLa cells by Søren Laland and colleagues in 1983 (Lund et al., 1983). HMGA1a (human, 107 amino acids, 11.6 kDa; previously, HMG-I; HMGI; HMG I; HMG-I/Y; a-protein), HMGA1b (human, 96 amino acids, 10.6 kDa; previously, HMG-Y; HMGY; HMG Y) and HMGA1c (human, 179 amino acids, 19.6 kDa; previously, HMG-I/R) are proteins produced from alternative splicing of HMGA1 genes (Friedmann et al., 1993; Johnson et al., 1989; Nagpal et al., 1999). HMGA1a and HMGA1b share a core sequence except for an internal deletion of 11 residues in HMGA1b. Chromosomal localization studies show that the HMGA1 gene is located at human chromosomal band 6p21 (Friedmann et al., 1993) and mouse chromosome 17 (Johnson et al., 1992), whereas HMGA2 (previously HMGI-C) is located at human chromosomal band 12q14–15 (Chau et al., 1995) and mouse chromosome 10 (Ashar et al., 1995). Each HMGA protein contains three small similar but independent functional AT-hook motifs and an acidic C terminal tail (Reeves, 2000, 2001). AT-hook motif is an auxiliary protein motif that cooperates with other DNA binding activities to regulate chromatin structure and transcription. The core AT-hook stretch sequence is positively-charged Pro-Arg-Gly-Arg-Pro (with RGRP being invariant), flanked on either side by other positively-charged lysine/arginine residues, first described in the HMGAs (Tkachuk et al., 1992). The multiple or single AT-hook motif is also found in many other non-HMGA proteins such as transcription factors and chromatin-remodeling components (Aravind and Landsman, 1998; Singh et al., 2006). As an architectural transcription factor, HMGA-mediated gene expression is completed mainly through AT-hook motif. Binding DNA by HMGA AT-hook can lead to DNA structural or conformational changes such as bending, straightening, unwinding, or inducing loop formation (Maher and Nathans, 1996). In addition to their DNA-binding characteristics, HMGAs have the ability to change the structure of bound protein substrates, including transcription factors, possibly by interacting through their acidic C terminal tail-mediated protein-protein (Yie et al., 1997). HMGAs not only have many partners, but also compete with the histone H1 for chromatin binding sites. A number of transcription factors can physically interact with HMGA proteins. HMGAs are not only key factors within enhanceosomes, which are multiple protein complexes including transcription factors and cofactors located upstream or downstream of the gene promoter (Reeves and Beckerbauer, 2001), but also mediate long-range enhancer-promoter interactions during gene transcription (e.g., β-globin and IL-2Rα) (Reeves, 2003). Thus, HMGAs regulate the expression of a large number of genes by DNA/protein binding and long/short-range mechanisms. In addition, post-translational modifications including phosphorylation, methylation, acetylation, sumoylation, and poly-ADP-ribosylation are essential to regulate HMGA substrate binding and their biological activities (Bianchi and Agresti, 2005; Reeves and Beckerbauer, 2001; Zhang and Wang, 2010). In particular, the HMGAs are the most heavily phosphorylated proteins in the nucleus (Lund et al., 1985), and HMGA phosphorylation by cyclin-dependent kinase 1 (CDK1/Cdc2) significantly decreases DNA binding affinity in the G2/M phase of the cell cycle (Nissen et al., 1991). In addition, casein kinase 2 (CK2) (Palvimo and Linnala-Kankkunen, 1989), protein kinase C (PKC) (Xiao et al., 2000), homeodomain-interacting protein kinase-2 (HIPK2) (Kim et al., 1999), and NIMA-related kinase 2 (Nek2) (Di Agostino et al., 2004) can phosphorylate HMGA1 proteins during cell death, the DNA damage response, and meiosis. The interaction between these upstream signal events remains unknown.
Both HMGA1 and HMGA2 are undetectable or very sparse in normal, fully-differentiated cells and adult tissues, but predominantly expressed during embryonic development in stem cells, undifferentiated cells, and neoplastic tissues (Cleynen and Van de Ven, 2008; Copley et al., 2013). In addition, HMGA1 is inducibly expressed following various environmental stimuli including the presence of growth factors, cytokines, endotoxin, retinoic acid, virus, or hypoxia (Liu et al., 2001). These expression properties indicate important roles of HMGAs in development, differentiation, cancer, and immunity. Indeed, HMGA1 and HMGA2 have different roles in development. HMGA1−/− mice have a cardiac hypertrophy phenotype (due to increased class II calcium/calmodulin-dependent protein kinase [CaMKII] expression), hematological malignancies, and type 2 diabetes (due to decreased insulin receptor expression) (Fedele et al., 2006; Foti et al., 2005), whereas HMGA2−/− mice have the dramatic phenotype called “pygmy,” which is characterized by treduced fat tissue, craniofacial defects, and slow growth due to a longer cell cycle of embryonic fibroblasts (Xiang et al., 1990; Zhou et al., 1995). In addition, both HMGA2−/− and HMGA1−/− male mice are infertile due to impaired spermatogenesis (Chieffi et al., 2002; Liu et al., 2003). HMGA1 is also important for lymphohematopoietic differentiation, because the loss of HMGA1 in embryonic stem cells causes impairment of T and myeloid cell development and leads to an increase in B-cells (Battista et al., 2003) by up regulation of recombination activating gene 2 (RAG2) expression (Battista et al., 2005).
Increasing evidence indicates that aberrantly-expressed HMGAs contribute to cancer initiation, promotion, and progression (Fedele and Fusco, 2010; Fusco and Fedele, 2007). HMGA overexpression and rearrangements are a hallmark of both malignant and benign neoplasia. Elevated HMGA1 expression correlates with metastatic potential in malignant epithelial tumors such as those of the lung, breast, prostate, colon, gastric, and pancreas, as well as renal and thyroid cancer. In contrast, elevated HMGA2 expression is observed in breast cancer, sarcomas, pancreatic cancer, head and neck/oral squamous cell carcinomas, melanoma, hepatoblastoma, and ovarian and non-small cell lung cancer. Rearrangements in HMGA2 genes are found in both benign (e.g., lipomas, uterine leiomyomas, and pulmonary chondroid hamartomas) and malignant (e.g., inflammatory myofibroblastic tumors and liposarcomas) mesenchymal tumors. HMGA1 is an independent prognostic factor in patients with pancreatic cancer. Patients with HMGA1-negative tumors have a better prognosis with longer median survival (Liau et al., 2008). Several transcription factors (e.g., Sp1, Sp3, AP-1, and E2F1), microRNAs (e.g., let-7, miR-196a-2, and microRNA-296) and signals (e.g., Wnt/β-catenin signaling) contribute aberrantly to HMGA expression in tumors (De Martino et al., 2009; Ferguson et al., 2003; Motoyama et al., 2008; Wend et al., 2013; Zhang et al., 2014b). In addition, oncogenic genes such as K-RAS, N-RAS, and c-Myc can induce HMGA1 overexpression during cell transformation (Li et al., 2013i). In vitro suppression of HMGA expression by RNAi decreases tumor cell proliferation and restores chemotherapy sensitivity (Liau et al., 2007; Watanabe et al., 2009), whereas overexpression of HMGAs by gene transfection promotes neoplastic transformation and increases chemotherapy resistance (Di Cello et al., 2008; Fedele et al., 1998). Moreover, transgenic mice overexpressing HMGA1 or HMGA2 produce a neoplastic phenotype (Arlotta et al., 2000; Baldassarre et al., 2001; Fedele et al., 2002; Fedele et al., 2005; Zaidi et al., 2006), whereas HMGB1−/− mice are resistant to chemically-induced skin carcinogenesis (Visone et al., 2008). Multiple molecular mechanisms contribute to the oncogenic activities of HMGAs. These mechanisms include uncontrolled cell cycling (Tessari et al., 2003), enhancement of transcription factor DNA-binding activity (Vallone et al., 1997), inhibition of apoptosis activity (Esposito et al., 2012), impairment of the DNA damage response (Pentimalli et al., 2008), promotion of inflammatory mediator production (Hillion et al., 2008; Perrella et al., 1999), regulation of cancer stem cells (Yanagisawa and Resar, 2013), downregulation of potential tumor-suppressor genes (Martinez Hoyos et al., 2009), upregulation of epithelial-mesenchymal transition (Morishita et al., 2013; Thuault et al., 2006), functioning as a competing endogenous RNA for microRNA (e.g., let-7 and MicroRNA-137) (Kumar et al., 2014; Liang et al., 2013a), and enhancement of autophagy-mediated aerobic glycolysis (Ha et al., 2012a). However, HMGAs also exerts anti-proliferative properties in some cells (Fedele et al., 2006), calling for further study of HMGA1 as potential therapeutic agent in cancer treatment.
1.3.2 HMGNs
The HMGN family has been found only in vertebrates and has five members: HMGN1 (human, 100 amino acids, 10.6 kDa), HMGN2 (human, 90 amino acids, 9.3 kDa), HMGN3 (human, 99 amino acids, 10.6 kDa), HMGN4 (human, 90 amino acids, 9.5 kDa), and HMGN5 (human, 282 amino acids, 31.5 kDa) (Furusawa and Cherukuri, 2010; Hock et al., 2007; Kugler et al., 2012). HMGN2 is the most conserved member of HMGNs. Chromosomal localization studies show that the HMGN1 gene is located at human chromosomal band 21p22 and mouse chromosome 16; the HMGN2 gene is located at human chromosomal band 1p36 and mouse chromosome 4; the HMGN3 gene is located at human chromosomal band 6p14 and mouse chromosome 9; the HMGN4 gene is located at human chromosomal band 6p21; and HMGA5 is located at human chromosomal band Xp13. HMGNs usually contain a bipartite nuclear localization signal (NLS), a highly-conserved nucleosome-binding domain (NBD), and a negatively charged regulatory domain (RD) within the C terminus. The major function of HMGNs is to bind nucleosomes and to regulate chromatin structure and function. The invariant sequence RRSARLSA in NBD is the core sequence of HMGNs that recognizes specifically generic structural features of the 147-bp nucleosome (Ueda et al., 2008). HMGNs have specific effects on gene transcription both locally and globally and sometimes acting in a cell-specific manner (Cuddapah et al., 2011; Kugler et al., 2012; Rochman et al., 2011). In addition, HMGNs are highly mobile and compete with the linker histone H1 for nucleosome access, which can cause chromosome relaxation and enhance gene transcription (Catez et al., 2002; Ding et al., 1997). Moreover, HMGNs facilitate epigenetic change by modulating the levels of posttranslational histone modifications (e.g., phosphorylation of H3, acetylation of H3K14, acetylation/methylation of H3K9, and phosphorylation of H2AS1) (Barkess et al., 2012; Lim et al., 2004; Lim et al., 2005). Although it binds to chromatin with very similar affinities, the expression and function of HMGNs in cellular differentiation and development are quite different.
HMGN1 (previously HMG-14; HMG14; HMG 14) and HMGN2 (previously HMG-17; HMG17; HMG 17) are ubiquitously expressed in embryonic tissues (Crippa et al., 1991; Lehtonen and Lehtonen, 2001), highly expressed in stem cells and undifferentiated cells, and downregulated in fully differentiated cells following organogenesis (Crippa et al., 1991; Furusawa et al., 2006; Lehtonen and Lehtonen, 2001; Pash et al., 1990). HMGN1−/− mice are subfertile and have slight defects in corneal epithelium maturation due to an absence of p63 expression (Birger et al., 2006). In particular, HMGN1−/− mice have identifiable phenotypes following stress. For example, loss of HMGN1 in mice renders them hypersensitive to both UV and ionizing radiation promoting increased tumorigenicity due to loss of the active G2-M checkpoint and impaired DNA repair (Birger et al., 2005; Birger et al., 2003). In addition, HMGN1−/− MEFs are hypersensitive to stressful stimuli and have an altered transcription profile and response (Birger et al., 2003; Lim et al., 2004; Lim et al., 2005; Rubinstein et al., 2005). Transient depletion of HMGN1 and HMGN2 proteins following injection of antisense oligonucleotides in one-cell or two-cell mouse embryos delayed cell cleavage during pre-implantation development (Mohamed et al., 2001). In contrast, overexpression of HMGN1 is observed in a mouse model of Down’s syndrome, suggesting a possible role for HMGN1 in post-natal development and mental retardation (Potier et al., 2006). In Xenopus, overexpression or disruption of HMGN1 or HMGN2 expression leads to significant developmental defects in post-blastula embryos (Korner et al., 2003). Thus, HMGN1 and 2 protein levels are tightly linked to cell differentiation and tumorigenesis. HMGN1 can also be released to the extracellular space and function as a DAMP (Yang et al., 2012a). Extracellular HMGN1 promotes antigen-specific immune responses by ligating TLR-4. Intracellular HMGN1 is required for OVA- and LPS-induced innate and adaptive immune responses (Yang et al., 2012a). The mechanism enabling HMGN1 release is unknown.
HMGN3 (previously, Trip7) with two splice variants (HMGN3a and HMGN3b) are expressed in a tissue-, variant-, and developmental stage-specific manner (Kugler et al., 2013; West et al., 2001). Although the tissue distributions and functions of HMGN3a and HMGN3b are similar, the structures of various splice variants are significantly different. HMGN3a contains classical HMGN domains (NLS, NBD, and RD), whereas HMGN3b only has NLS and NBD domains and lacks an RD domain. HMGN3 is highly expressed in the eye, brain, and pancreatic endocrine cells (β and α cells), suggesting a potential role for HMGN2 in eye development, brain activity, and glucose homeostasis (Ito and Bustin, 2002; Kurahashi et al., 2010; Ueda et al., 2009; West et al., 2001). Indeed, HMGN3−/− mice develop normally but have a mild diabetic phenotype due to partial downregulation of GLUT2 (a major transporter of glucose) as well as a glucagon-insulin production imbalance (Kurahashi et al., 2010; Ueda et al., 2009). In addition, HMGN3 is indirectly regulated by thyroid hormone and interacts with it in Xenopus laevis (Amano et al., 2002).
HMGN4 was discovered via a GenBank database search by Michael Bustin and colleagues in 2004 (Birger et al., 2001). However, at present, the biological function of HMGN4 is unknown, although its sequence is known to be closely related to that of HMGN2 (Birger et al., 2001). HMGN5 (previously BP1, NBD-45) was also discovered via a GenBank database search by Michael Bustin and colleagues in 2000 (Shirakawa et al., 2000). Compared to other HMGNs, HMGN5 has a unique molecular structure and size (Shirakawa et al., 2000). HMGN5 contains classical HMGN domains (NLS, NBD and RD), but the C-terminal domain is unusually long and contains a unique acidic amino acid repeat (Rochman et al., 2009). Although the structures are highly similar, the HMGN5 protein sequence has only a 59% amino acid conservation between human and mouse. The expression of HMGN5 protein varies significantly between cells and tissues during embryonic development (Shirakawa et al., 2000). In addition, HMGN5 expression is upregulated with the development of several cancers, including squamous cell carcinoma, breast and bladder cancer, renal cell carcinoma, and gliomas (Gerlitz, 2010; Ji et al., 2012b; Li et al., 2006a; Qu et al., 2011; Tang et al., 2008b; Wahafu et al., 2011). Knockout of HMGN5 by RNAi inhibits cell proliferation and increases apoptosis in cancer cells (Chen et al., 2012b; Ji et al., 2012b; Zhang et al., 2012c).
In summary, HMGNs bind directly to nucleosomes, modulate epigenetic modifications, and influence chromatin structure, which in turn have multiple roles in the regulation of cell differentiation, organ development, and tumorigenesis.
1.3.3 HMGBs
The high-mobility group box (HMGB) protein family is the most abundant protein family among HMGs (Goodwin and Johns, 1978). The HMGBs are highly conserved and have four members (HMGB1, HMGB2, HMGB3, and HMGB4). Interestingly, knockout of mouse HMGB1, HMGB2, and HMGB3 genes clearly results in identifiable phenotypes, although the encoded proteins share ~80% amino acid sequence identity. Each HMGB (HMGB1–4) contains two DNA binding domains (termed HMG boxes A and B). HMGB1–3 has an acidic C-terminal tail, whereas HMGB4 lacks this tail (Thomas and Travers, 2001). Each HMGB box binds to DNA without any significant sequence specificity and can induce DNA conformational and structural changes (Agresti and Bianchi, 2003; Ueda and Yoshida, 2010). The acidic C-tails of HMGBs can bind other nuclear proteins, even HMG boxes, to regulate their affinity for a variety of distorted DNA structures. Besides HMGBs, several proteins have been identified with the HMG box (termed the HMG-box family). The differences between the HMGBs and HMG-box family are described below.
1.3.3.1 The HMG-box Family
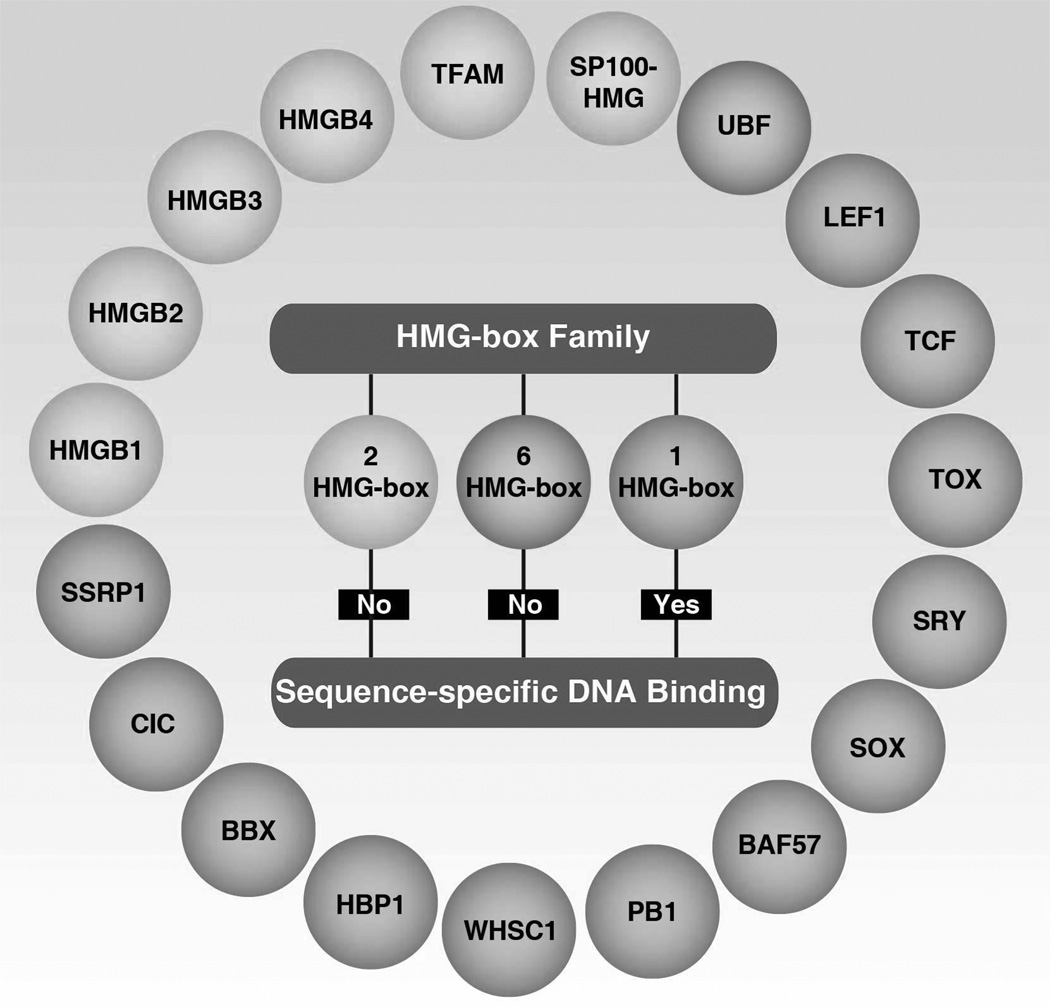
The HMG box is a novel type of protein motif that mediates DNA-binding (Landsman and Bustin, 1993). Each protein within the HMG-box family has at least one HMG-box (Figure 2). The mammalian HMG-box family can be divided into two main groups based on abundance, function, and DNA specificity (Griess et al., 1993; Laudet et al., 1993; van Houte et al., 1995). The first protein group contains multiple HMG-box domains with little or no sequence-specific DNA binding and an acidic C-tail. These proteins include HMGBs (HMGB1–4) with two HMG-boxes, mitochondrial transcription factor (TFAM) with two HMG-boxes, upstream binding factor (UBF) with six HMG-boxes, and SP100-HMG nuclear autoantigen with two HMG-boxes (Stros et al., 2007b). TFAM, a transcription factor for mitochondrial DNA, plays a critical role in maintaining the amount of mitochondrial DNA in a promoter-specific manner (Kanki et al., 2004) or a cAMP-dependent phosphorylation manner (Lu et al., 2013a). In addition, TFAM can be released into the extracellular space during infection and injury, and function as a DAMP to regulate immune and inflammatory responses (Chaung et al., 2012). The nuclear transcription factor UBF, including UBF1 and UBF2, is important for activation of ribosomal RNA transcription by associating with RNA polymerases (O'Mahony and Rothblum, 1991; Schnapp et al., 1994). In contrast, the tumor suppressor Rb can inhibit UBF expression, suggesting a potential role for UBF in tumorigenesis (Cavanaugh et al., 1995). SP100-HMG, a splicing variant of SP100, functions as a transcriptional activator or repressor (Seeler et al., 1998). The interrelationship between HMGBs and the various HMG-box families is unclear.
Figure 2.
The HMG-box proteins
The second group in the HMG-box family consists of highly diverse, much less abundant proteins and contains a single HMG-box domain with DNA binding sequence-specificity. These proteins include lymphoid enhancer-binding factor 1 (LEF1), T-cell factor 1 (TCF1), TCF3, thymocyte selection-associated HMG box protein (TOX), sex determining region Y (SRY), sex determining region Y-box (SOX), brahma-related gene -associated factor (BAF57), polybromo-1 (PB1), Wolf-Hirschhorn syndrome candidate 1 protein (WHSC1), HMG-box transcription factor 1 (HBP1), HMG box transcription factor (BBX), capicua transcriptional repressor (CIC), and structure-specific recognition protein 1 (SSRP1). The major functions of these proteins are described below.
LEF1, a key transcription factor of Wnt signaling, is expressed specifically in pre-B and T-cells and regulates cell differentiation (Giese et al., 1991; Metzeler et al., 2012; Milatovich et al., 1991). LEF1−/− mice die shortly after birth but have no apparent lymphoid defects (van Genderen et al., 1994). TCF1 is expressed specifically in cells of the T-cell lineage (Oosterwegel et al., 1993) and loss of TCF1 leads to deficient T-cell development (Verbeek et al., 1995). In contrast, TCF3 is expressed specifically in gastric epithelium, hair follicles, and keratinocytes of the skin (Korinek et al., 1998). TCF1 is an effector, whereas TCF3 is a repressor of Wnt signaling during embryonic development and gene expression (Yi et al., 2011). TOX is highly expressed in the thymus and has a critical role in immunity via three subfamily proteins: TOX2, TOX3, and TOX4 (Aliahmad et al., 2012; Wilkinson et al., 2002). Loss of TOX in mice leads to deficient development of T cells (Aliahmad and Kaye, 2008), natural killer cells, (Aliahmad et al., 2010) and lymphoid tissue inducer cells (Aliahmad et al., 2010). Mammalian SRY on the short arm of the Y chromosome encodes a nuclear factor-like protein harboring a DNA-binding domain known as the HMG box (Ferrari et al., 1992; Sinclair et al., 1990). SRY and its related SOX are sex-determining factors (Harley and Goodfellow, 1994; Werner et al., 1995). They have similar structures, but differing tissue-specific expression patterns. The SOX proteins comprise nearly half of all human HMG-box proteins. SRY and SOX are also important for organ development and cell type specification (Wegner, 1999). In humans, deletion or mutation of Sox proteins can cause developmental defects and congenital diseases. BAF57 and PB1 are chromatin-remodeling factors involved in gene regulation and cell cycle control by alteration of DNA-nucleosome topology (Domingos et al., 2002; Link et al., 2005). Mutation or aberrant expression of BAF57 has been observed in many tumor patients (Balasubramaniam et al., 2013; Hah et al., 2010; Kiskinis et al., 2006; Link et al., 2008). WHSC1 is expressed ubiquitously during early development and is involved in chromosomal translocations and histone-lysine N-methyltransferase activity (Hartlerode et al., 2012; Nimura et al., 2009; Pei et al., 2013; Sarai et al., 2013; Yang et al., 2012f). HBP1, as a tumor suppressor protein (Escamilla-Powers et al., 2010; Li et al., 2011b; Zhang et al., 2006), negatively regulates G and S1 phase progression and Wnt signaling (Berasi et al., 2004; Escamilla-Powers et al., 2010; Pan et al., 2013; Sampson et al., 2001; Tevosian et al., 1997; Xiu et al., 2003). BBX functions as a transcription factor and is necessary for cell cycle progression from the G1 to S phase (Stros et al., 2007b). CIC is a transcriptional repressor and plays a role in development of the central nervous system and lung alveolarization (Kim et al., 2013a; Lee et al., 2011b). SSRP1 is a component of the “facilitates chromatin transcription, FACT” complex, a general chromatin factor that acts to reorganize nucleosomes (Kasai et al., 2005). SSRP1 play multiple roles in mRNA elongation, DNA replication, and DNA damage response (Dyer et al., 1998; Keller et al., 2001; Orphanides et al., 1999; Spencer et al., 1999; Yarnell et al., 2001; Zeng et al., 2002).
Collectively, the HMG-box family has a unique role in DNA-dependent processes (transcription, replication, and repair) and their common mechanism of chromatin remodeling in biology and disease.
1.3.3.2 Mammalian HMGBs
1.3.3.2.1 HMGB1
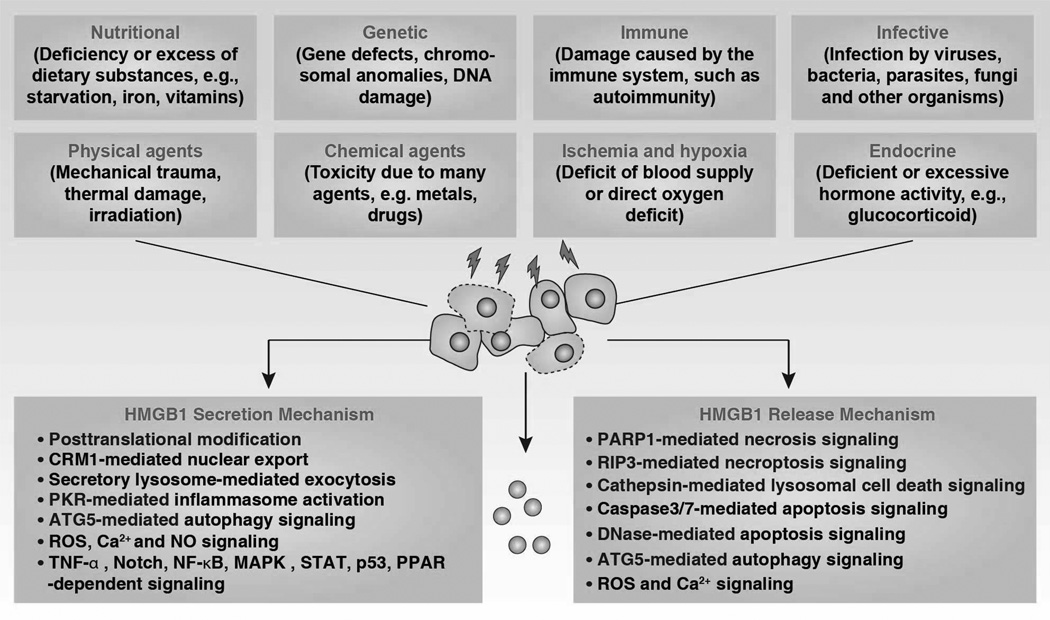
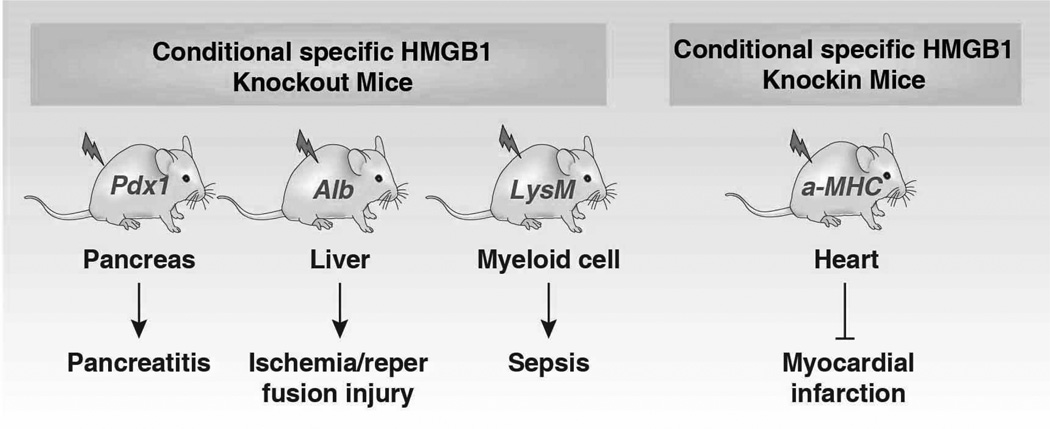
HMGB1 (previously HMG1; HMG-1; HMG 1; amphoterin; p30) expression is the most highly expressed of all the HMG family members. There are about 106 molecules of HMGB1 per cell, which is only an order of magnitude less than the core histones (Romani et al., 1979). HMGB1 has been extremely conserved during evolution and originated before the divergence of the protostomes and deuterostomes approximately 525 million years ago (Sharman et al., 1997). In contrast, HMGB1 pseudogenes, dysfunctional relatives of HMGB1 genes, arose relatively late in evolution, approximately one million years ago (Stros and Dixon, 1993). The homolog of mammalian HMGB1 has been identified in yeast (termed Nhp6A/B), drosophila (termed HMG-D and DSP1), chironomidae, echinoderms, bacteria, plants, fish, and C. elegans (Table 1) (Bustin, 2001; Giavara et al., 2005; Wu et al., 2003). The mRNA of HMGB1 is polyadenylated (Bustin et al., 1981), and the protein sequence of HMGB1 displays a 100% homology between mouse and rat and a 99% homology between rodent and human (Ferrari et al., 1994; Gariboldi et al., 1995; Wen et al., 1989). The C terminus contains two amino acids that differ between mice and humans. In all cells, HMGB1 can shuttle between the nucleus and cytoplasm, and normal HMGB1 accumulates in nuclei to bind chromatin (Isackson et al., 1980). HMGB1 is the most mobile protein in the nucleus, crossing this organelle into the cytosol within 1–2 seconds (Phair et al., 2004; Sapojnikova et al., 2005; Scaffidi et al., 2002). Given its mobility, HMGB1 has been found in the cytosol (e.g., mitochondria (Stumbo et al., 2008) and lysosome (Gardella et al., 2002)), in the cellular membrane, and extracellular space when its nuclear localization signal (NLS) is modified (Kuehl et al., 1985). The subcellular location of HMGB1 changes depending on cell type, tissue, and stress signals. HMGB1 is widely-expressed in various tissues and high HMGB1 levels are found particularly in the spleen and thymus (Prasad and Thakur, 1990a). The expression of HMGB1 in myeloid cells is higher than in lymphoid cells (Cabart et al., 1995) and correlates with the differentiation stage of these cells (Seyedin et al., 1981). Expression of HMGB1 is upregulated in cancer, but downregulated during aging (Muller et al., 2004; Prasad and Thakur, 1990a), suggesting a critical role in development and cancer. HMGB1 is an early maker of oligodendrocytes in the developing rat spinal cord (Daston and Ratner, 1994). HMGB1 is essential for life because HMGB1−/− mice die shortly after birth due to the downregulation of glucocorticoid receptor and the inability to use glycogen stored in the liver (Calogero et al., 1999). In contrast, glucose administration prolongs survival of HMGB1−/− mice, but these mice die before reaching sexual maturity (Calogero et al., 1999). Double knockout of HMGB1 and HMGB2 in mice or zebrafish embryos results in a significant deficiency in Wnt signaling and posterior digit development (Itou et al., 2011). Both endogenous and exogenous HMGB1 are required for pre-implantation embryo development in the mouse (Cui et al., 2008). Injection of HMGB1 siRNA into the zygote increases apoptosis (Cui et al., 2008). Overexpression of HMGB1 in cardiac tissue by transgenic methods significantly increases survival and protects mice against myocardial infarction by enhancing angiogenesis and cardiac function (Kitahara et al., 2008). We and others recently demonstrated that conditional knockout of HMGB1 in the pancreas (Kang et al., 2013b), liver (Huang et al., 2013a), or macrophages (Yanai et al., 2013) renders mice more sensitive to pancreatitis, liver ischemia/reperfusion injury, and sepsis, respectively. Of note, using various HMGB1 conditional knockout strategies may cause substantially different functional phenotypes in the liver and heart (Huebener et al., 2014). The threshold for the HMGB1 requirement to function in various biological processes may differ and may also depend on the cell type.
Table 1.
Multi-species Expression and Function of HMGB1
| Species | Function | References |
|---|---|---|
| Goldfish (Carassius auratus L.) | Inflammatory and immune response | (Xie et al., 2014) |
| Chlamys farreri | DNA-binding ability and pro-inflammatory activity | (Wang et al., 2014d) |
| Adult zebrafish | Regeneration after spinal cord injury and brain development | (Fang et al., 2014; Moleri et al., 2011; Zhao et al., 2011b) |
| Grass carp (Ctenopharyngodon idella) | Innate immune response | (Yang et al., 2013a) |
| Saccharomyces cerevisiae | DNA repair | (Thongsroy et al., 2013) |
| Pacific oyster (Crassostrea gigas) | Innate defense | (Li et al., 2013c) |
| Nematode Caenorhabditis elegans | Hypoxia response | (Lee, 2013) |
| Wuchereria bancrofti and Brugia malayi | Lymphatic filariasis | (Thirugnanam et al., 2012) |
| Aedes aegypti | DNA bending | (Ribeiro et al., 2012) |
| Lampetra japonica | Innate immunity | (Pang et al., 2012) |
| Arabidopsis thaliana | Chromatin dynamics and telomere maintenance | (Schrumpfova et al., 2011) |
| Yeast | Genome stability; DNA binding; Antiapoptosis | (Giavara et al., 2005; Guerin et al., 2008; Labazi et al., 2009) |
| Plasmodium falciparum | Inflammatory immune responses | (Kumar et al., 2008) |
| Saccharomyces cerevisiae | Chromosomal rearrangement Gene transcription | (Diffley and Stillman, 1991, 1992; Kim et al., 2007; Sikdar et al., 2008) |
| Human blood flukes Schistosoma mansoni and Schistosoma japonicum | Schistosomiasis | (de Oliveira et al., 2006; Gnanasekar et al., 2006) |
| Pelodiscus sinensis | Unknown | (Zheng et al., 2005) |
| Plodia interpunctella | Unknown | (Aleporou-Marinou et al., 2003) |
| Drosophila | DNA binding and bending | (Lehming et al., 1998; Lehming et al., 1994; Ner et al., 1993; Wagner et al., 1992) |
| Saccharomyces cerevisiae | Nucleosome | (Perez-Martin and Johnson, 1998a, b) |
| Lamprey lampetra fluviatilis | Unknown | (Sharman et al., 1997) |
| Sea-urchin | Unknown | (Niemeyer et al., 1995) |
| Trout | Unknown | (Stros et al., 1994a) |
| Chironomus thummi (Diptera) | DNA binding and bending | (Wisniewski and Schulze, 1992; Wisniewski et al., 1994) |
| Tetrahymena thermophila | Gene transcription | (Schulman et al., 1991) |
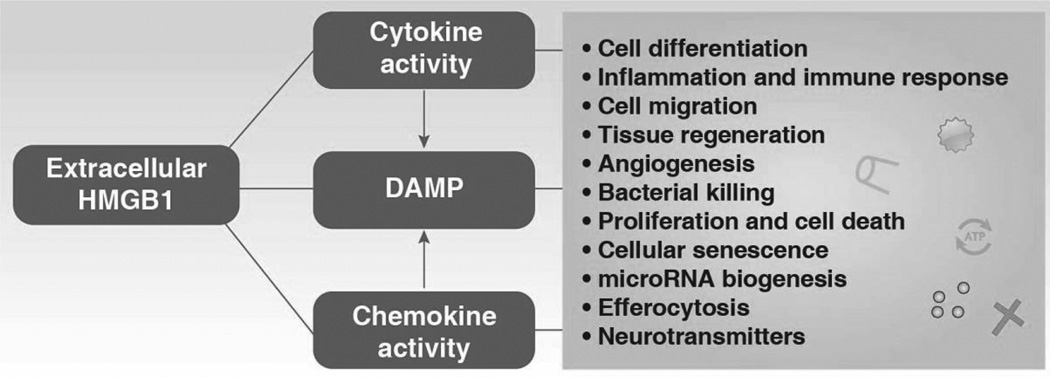
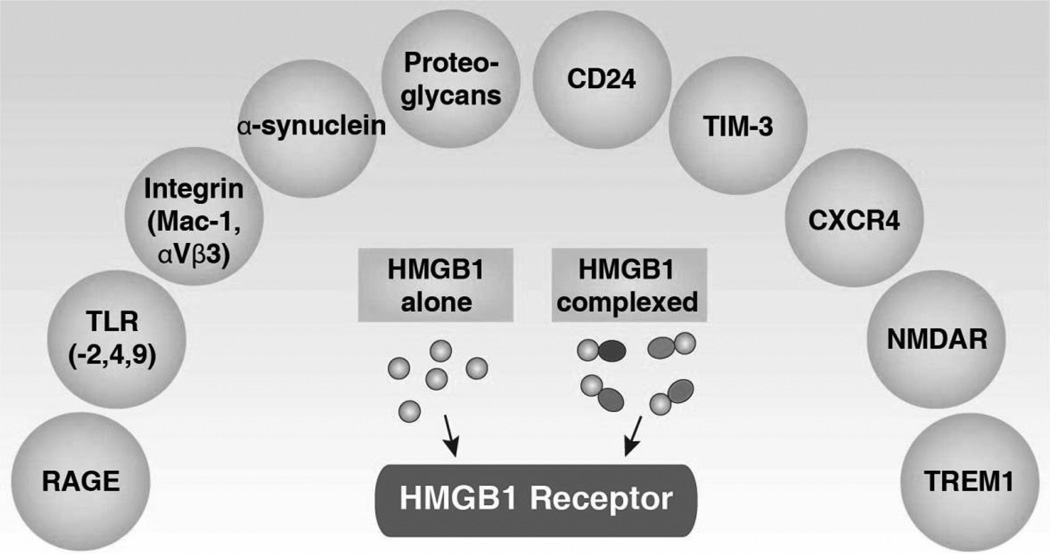
The HMGB1 protein is fully functional in cells of mammalian origin. Nuclear HMGB1 is engaged in many DNA activity-associated events (e.g., DNA replication, repair, recombination, transcription, and genomic stability). In addition to its nuclear function, HMGB1 plays a significant extracellular role in inflammation, immunity, cell growth, cell proliferation, and cell death. HMGB1 is massively released into the extracellular space by dead or dying cells. Extracellular HMGB1 functions as a DAMP to alert the innate immune system by recruiting inflammatory, smooth muscle cells, mesangioblasts, and stem cells. In addition, extracellular HMGB1 functions as an immune adjuvant to trigger a robust response to activation or suppression of T cells, dendritic cells, and endothelial cells. Activated immune cells (e.g., macrophages, monocytes, and dendritic cells) and endothelial cells also secrete HMGB1, which in turn forms a positive feedback loop that causes the release of additional cytokines and chemokines following engagement of multiple receptors. Thus, HMGB1 sustains a long-term inflammatory state under stress. Interestingly, extracellular HMGB1 has antibacterial, cell growth, and mitotic activity. These extracellular HMGB1 activities are not only mediated by receptors, but also by its Redox state and structure (Tang et al., 2012). Besides its nuclear and extracellular roles, cytoplasmic HMGB1 binds many proteins involved in autophagy (Tang et al., 2010c), cancer progression, and possibly the unconventional secretory pathway (Lee et al., 2010a). HMGB1 not only binds to DNA, but also interacts with many apparently unrelated proteins by recognizing short amino acid sequence motifs (Dintilhac and Bernues, 2002). For example, the motifs PXXPXP and WXXW (where X can be any amino acid) can interact with box A and box B of HMGB1, respectively (Dintilhac and Bernues, 2002). Thus, HMGB1 may be involved in many cell processes by promoting protein protein interactions (Table 2). These important structures and functions of mammalian HMGB1 both inside and outside the cell in health and disease will be discussed below.
Table 2.
Intracellular Binding Partners for HMGB1
| Name | Function | Site | Reference |
|---|---|---|---|
| H1, H2a, 2b, H3, H4 | Regulates nucleosome function | Nucleus | (Carballo et al., 1983; Kohlstaedt and Cole, 1994b; Smerdon and Isenberg, 1976; Stros and Kolibalova, 1987; Stros and Vorlickova, 1990; Totsingan and Bell, 2013; Watson et al., 1977) |
| Bric-a-brac | Inhibits HMGB1-induced oncogenesis | Nucleus | (Ko et al., 2014) |
| Sox9 | Inhibits E-selectin expression | Nucleus | (Zhang et al., 2013h) |
| RAG1 and RAG2 | Promotes V(D)J recombination | Nucleus | (Agrawal and Schatz, 1997; Ji et al., 2010; Little et al., 2013) |
| Gadd45a | Regulates DNA demethylation | Nucleus | (Li et al., 2013h) |
| p53 | Regulate gene transcription | Nucleus | (Banerjee and Kundu, 2003; Imamura et al., 2001; Jayaraman et al., 1998; Livesey et al., 2012c; McKinney and Prives, 2002; Rowell et al., 2012; Zhang et al., 2003) |
| Influenza virus nucleoprotein | Promotes viral replication | Nucleus | (Moisy et al., 2012) |
| Viral ribonucleoprotein | Promotes viral replication | Nucleus | (Matsumoto et al., 2012) |
| Estrogen receptor | Regulates gene transcription | Nucleus | (Joshi et al., 2012) |
| DFF40 | Regulates nuclease activity | Nucleus | (Kalinowska-Herok and Widlak, 2008; Ninios et al., 2010; Widlak and Garrard, 2005) |
| Oct1 | Regulates Rta expression | Nucleus | (Harrison and Whitehouse, 2008) |
| PU.1 | Regulates IL-1β expression | Nucleus | (Mouri et al., 2008) |
| Ets | Regulates transcription activity | Nucleus | (Shiota et al., 2008) |
| Mutant AT1 and Htt | Regulates genotoxic stress | Nucleus | (Qi et al., 2007) |
| Topoisomerase II alpha | Regulates catalytic activity | Nucleus | (Stros et al., 2007a) |
| Hsp72 | Regulates inflammatory response | Nucleus | (Tang et al., 2007a; Tang et al., 2007d) |
| GR | Regulates transcription activity | Nucleus | (Agresti et al., 2005) |
| Sterol regulatory element-binding proteins | Regulates DNA-binding activity | Nucleus | (Najima et al., 2005) |
| Replication protein A | Regulates DNA damage response | Nucleus | (Reddy et al., 2005) |
| Estrogen receptor | Regulates transcription activity | Nucleus | (Borrmann et al., 2001; Chau et al., 1998; Das et al., 2004; Melvin et al., 2004; Verrier et al., 1997) (Romine et al., 1998) |
| Replication and transcription activator | Regulates DNA-binding activity | Nucleus | (Song et al., 2004) |
| MutSalpha | Regulates DNA repair | Nucleus | (Yuan et al., 2004) |
| Rel family | Regulates transcription activity | Nucleus | (Agresti et al., 2003; Brickman et al., 1999) |
| HMGB1-HMGB2-HSC70-ERp60-glyceraldehyde 3-phosphate dehydrogenase complex | Regulates chemotherapy | Nucleus | (Krynetski et al., 2003) |
| Sleeping Beauty | Regulates transposition | Nucleus | (Zayed et al., 2003) |
| Groucho-related gene proteins 1 | Unknown | Unknown | (Dintilhac and Bernues, 2002) |
| Dof2 | Regulates transcription activity | Nucleus | (Krohn et al., 2002) |
| Steroid hormone subgroup of nuclear receptors (androgen, glucocorticoid, progesterone and mineralocorticoid receptors) | Regulates transcription activity | Nucleus | (Melvin et al., 2002; Onate et al., 1994; Verrijdt et al., 2002) |
| p73 | Regulates transcription activity | Nucleus | (Stros et al., 2002) |
| TATA-binding protein/TATA complex | Regulates DNA-binding activity | Nucleus | (Das and Scovell, 2001; Sutrias-Grau et al., 1999) |
| Up-stream stimulatory factor 1 | Regulates transcription activity | Nucleus | (Marmillot and Scovell, 1998) |
| SP100 nuclear bodies | Regulates transcription activity | Nucleus | (Seeler et al., 1998) |
| DNA-PKcs | Regulates DNA damage response | Nucleus | (Watanabe et al., 1994; Yumoto et al., 1998) |
| Transcription by RNA polymerase II | Regulates DNA binding activity | Nucleus | (Ge and Roeder, 1994) |
| Adeno-associated virus replication protein | Regulates virus replication | Nucleus | (Costello et al., 1997) |
| The conserved lymphokine elements-0 | Regulates DNA-binding activity | Nucleus | (Marrugo et al., 1996) |
| Hox | Regulates transcriptional activation | Nucleus | (Zappavigna et al., 1996) |
| SNCA/alpha-synuclein | Inhibits HMGB1-induced autophagy | Nucleus and cytosol | (Song et al., 2014) |
| Beclin-1 | Regulates autophagy | Cytosol | (Tang et al., 2010c) |
| Tubulin | Regulates cell skeleton | Cytosol | (Briolay et al., 1994) |
1.3.3.2.2 HMGB2
HMGB2 (previously HMG2; HMG-2; HMG 2) is very similar to HMGB1 (>80% identity) at the amino acid level. It is widely expressed in early embryos, especially in stem cells (Abraham et al., 2013a), and its expression is restricted mainly to the lymphoid organs and testis in adult mice (Ronfani et al., 2001). The mechanism of transcriptional regulation of HMGB2 expression is unclear. HMGB2−/− mice have increased susceptibility to apoptosis and have defects in spermatogenesis (Ronfani et al., 2001), chondrocyte development (Taniguchi et al., 2011; Taniguchi et al., 2009b), neurogenesis (Abraham et al., 2013b) and Wnt signaling (Taniguchi et al., 2009a). Thus, HMGB2 plays a critical role in the regulation of fertility, osteoarthritis, neuronal degeneration, and aging (Ly et al., 2000). Like HMGB1, HMGB2 participates in chromosomal processing and assembly by binding DNA with no sequence-specificity or specific proteins or post-translational modifications. HMGB2 can be phosphorylated by casein kinase 2 (CK2) (Stemmer et al., 2003; Stemmer et al., 2002) and acetylated by CREB-binding protein (CBP) (Pasheva et al., 2004). In vitro, HMGB2 can bind to multiple partner proteins, which in turn promotes or represses transcription and recombination activities of these partner proteins. These proteins include steroid hormone receptors (Boonyaratanakornkit et al., 1998), SSRP1 (Lichota and Grasser, 2001), p53 (Stros et al., 2002), p73 (Stros et al., 2002), chromatin transcription-enabling activity (CTEA) (Guermah et al., 2006), neurons expressing huntingtin (Htt) (Qi et al., 2007), endoplasmic reticulum-associated complex (SET) (Fan et al., 2002), Rag1 recombinase (Aidinis et al., 1999; Swanson, 2002a), EBV nuclear antigen 1 (EBNA-1) (Jourdan et al., 2012), ATP-binding cassette transporter 1 (ABCF1) (Lee et al., 2013c), pluripotency factor Oct4 (Campbell and Rudnicki, 2013), and LEF1 (Taniguchi et al., 2009a). In addition, increased HMGB2 levels h, like HHMGB1, facilitates efficient nonviral gene delivery (Sloots and Wels, 2005), which may be useful for gene therapy (Balani et al., 2009). Overexpression of HMGB2 increases topoisomerase II alpha expression (Stros et al., 2009) and correlates with the progression of several tumors such as skin, liver, and bladder cancer (Kwon et al., 2010a; Sharma et al., 2008; Wang et al., 2013j). Like HMGB1, HMGB2 is secreted by myeloid cells and has mitogenic and chemoattractant functions by binding to RAGE (Pusterla et al., 2009). However, the pro-inflammatory activity of extracellular HMGB2 is significantly lower than that of HMGB1 (Ueno et al., 2004). Extracellular HMGB2 is increased in experimental and clinical acute lung injury (Ueno et al., 2004), suggesting a possible role for HMGB2 in tissue injury. In addition, extracellular HMGB2 has antimicrobial activity in intestinal tissue, but the mechanism remains unknown (Kuchler et al., 2013). The presence of serum anti-HMGB2 antibodies may contribute to inflammatory bowel disease (Takaishi et al., 2012), suggesting a possible role for extracellular HMGB2 in the regulation of autoimmunity.
1.3.3.2.3 HMGB3
HMGB3 (previously HMG2a; HMG-2a; HMG 2a [HMG-4]) is an X-linked member of the HMGBs and was originally discovered in 1998 by Marco Bianchi and colleagues as an expressed sequence tag (EST) preferentially expressed in embryonic tissues (Vaccari et al., 1998). HMGB3 protein is highly expressed in the embryo and hardly detectable in adult tissues (Vaccari et al., 1998). HMGB3 expression is regulated by several miRNAs, including miR-206, miR-205, miR-10A, and miR-21 (Elgamal et al., 2013; Maciotta et al., 2012; Zhu et al., 2013c). HMGB3−/− mice are viable and HMGB3 is required for eye and brain development (Terada et al., 2006). Importantly, HMGB3 is expressed in most lymphoid and myeloid progenitors and HMGB3 levels are associated with myeloid and B-cell differentiation as well as hematopoietic stem cell self-renewal and proliferation (Nemeth et al., 2005; Nemeth et al., 2003; Nemeth et al., 2006; Somervaille et al., 2009; Tsuzuki and Seto, 2013). HMGB3 has a special role in leukemogenesis (Lilljebjorn et al., 2007). The formation of HMGB3-NPU98 fusion protein is a new oncogene identified in leukemia and significantly promotes malignant transformation in recipient mice (Petit et al., 2010). HMGB3 overexpression is associated with progression and poor prognosis of solid tumors such as breast, gastric, and non-small cell lung cancers (Elgamal et al., 2013; Gong et al., 2013; Song et al., 2013). However, the effect of HMGB3 in tumor therapy and the extracellular role of HMGB3 remain unknown.
1.3.3.2.4 HMGB4
HMGB4 was discovered as a new member of mammalian HMGBs in 2009 by Irwin Davidson and colleagues (Catena et al., 2009). HMGB4 is mainly expressed in germ cells of the testis and weakly in the brain, but not in other tissues. HMGB4 protein has a molecular mass of 21 kDa and lacks the acidic tail (Catena et al., 2009). Compared with HMGB1, HMGB4 is usually a transcriptional repressor and is encoded by an intronless gene (Catena et al., 2009). Similar to other HMGBs, HMGB4 has a potential role in tumor development. For example, overexpression of HMGB4 by gene transfection inhibits breast cancer cell proliferation through an LXCXE- or LXCXD-dependent mechanism, whereas it increases radiosensitivity through an LXCXE- or LXCXD-independent mechanism (Wang et al., 2012c). HMGB4 has high affinity to cisplatin-modified DNA, suggesting a potential role in the regulation of anticancer activity of cisplatin (Park and Lippard, 2012). Nothing is known about the phenotype of HMGB4-deficient mice. The biological function of HMGB4 remains largely unknown.
1.3.3.3 Plant HMGBs
Plant HMGs, first isolated from wheat germ, have a different structure than animal HMGs (Launholt et al., 2006; Spiker, 1984; Spiker et al., 1978). In the past few years, HMGAs and HMGBs, but not HMGNs, have been isolated and biochemically characterized from various plants (Grasser, 1995). HMGB proteins are expressed ubiquitously in the plant and usually in the nucleus (Grasser et al., 2007; Pedersen and Grasser, 2010). Compared with other eukaryotes, plant HMGBs have multiple members in the same species. For example, Arabidopsis thaliana has six HMGBs (HMGB1–HMGB6) with some common characteristic structural properties despite of the variable molecular size (Grasser et al., 2004). Each member contains a single, central HMG-box DNA-binding domain, a basic N-terminal domain, and an acidic C-terminal domain. This plant HMG-box domain has 75 amino acid residues and three α-helices to form an L-shaped fold with an 80° angle between the arms, which has a higher similarity to the B box domain of mammals. Plant HMGBs also are architectural chromosomal proteins and have a potential role in plant development and stress response by regulating transcription factor activity (Grasser et al., 2007; Pedersen and Grasser, 2010). Recent studies revealed that Arabidopsis HMGB2/3 and B4 proteins are predominantly nuclear but also exist in the cytoplasm, suggesting an as yet-unknown cytoplasmic function of these chromosomal HMG proteins (Merkle and Grasser, 2011).
The expressions of HMGB2, HMGB3, and HMGB4 are upregulated in response to cold stress, whereas the expression of HMGB2 and HMGB3 is downregulated in response to drought or salt stress (Kwak et al., 2007). Overexpressing HMGB2 and HMGB5, but not HMGB4, in Arabidopsis retarded germination and subsequent growth in response to salt and drought stress (Kwak et al., 2007). These findings suggest that different HMGBs are involved in response to several environmental stressors. Both the absence and overexpression of HMGB1 in Arabidopsis leads to shorter roots and affects their sensitivity to genotoxic agents (Lildballe et al., 2008). Further studies systematically analyzing plants lacking or overexpressing HMGB variants in varying environments will be essential to understand the role of these architectural chromosomal proteins in plant stress responses.
2. HMGB1 Structure
2.1 Primary Structure
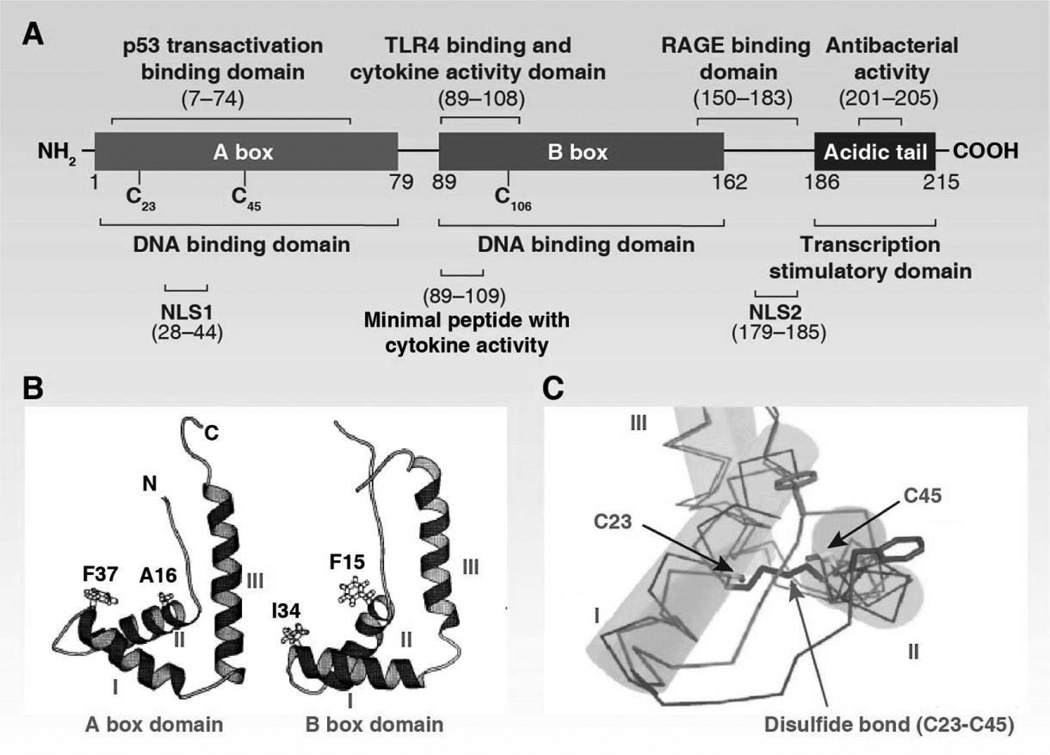
The primary structure of HMGB1 includes the linear sequence of its amino acid structural units. Human HMGB1 has 215 amino acid residues and forms two DNA binding domains (HMG A box [9–79aa], HMG B box [95–163aa]) and a C-terminal acidic tail (186–215aa) (Figure 3A) (Bianchi et al., 1992). The DNA binding domains are necessary for efficient DNA bending and flexure without sequence specificity. DNA binding domains contain nuclear-emigration signals (NES), which are mediated by nuclear exportin chromosome-region maintenance 1 (CRM1). In contrast, the steady state of HMGB1 is located in the nucleus due to two nuclear-localization signals: NLS1 (28–44aa) and NLS2 (179–185aa) (Bonaldi et al., 2003). The change of NES and NLS induce abnormal HMGB1 location. HMGB1 can bind a number of proteins and these interactions are important for HMGB1’s activity and function. Residues 150–183 are responsible for binding to RAGE for cell migration (Huttunen et al., 2002), whereas residues 89–108 and residues 7–74 are responsible for binding to TLR4 and p53 transactivating domains for inflammation and gene transcription, respectively. The extracellular B box has been reported to recapitulate pro-inflammatory activity, whereas the A box acts as an HMGB1 antagonist (Li et al., 2003). The anti-inflammatory activity of HMGB1 A box is enhanced when fused with the C-terminal acidic tail (Gong et al., 2010b). Residues 201–205 in the C-terminal acidic tail region are responsible for the antibacterial activity of HMGB1 (Gong et al., 2009). The C terminus is full of acidic amino acid residues (30 aspartate and glutamic acid) and this region was previously thought to protect the A-box and B-box during emigration from the nucleus. In addition, the C terminus i regulates DNA binding/bending by intramolecular interaction with the N-terminals of DNA-binding domains (especially cysteine residues) (Stros, 1998; Wang et al., 2007b) as well as intermolecular interaction with histones H1 and H3 (especially lysine residues) (Cato et al., 2008; Sheflin et al., 1993; Ueda et al., 2004) (Kawase et al., 2008). Removal of the C-terminal tail renders HMGB1 with low-affinity binding to DNA and protein in cell free systems (Stros et al., 1994c). In the cell, overexpression of HMGB1 lacking the C-terminal tail inhibits various reporter gene expression (Aizawa et al., 1994). HMGB1 mutations have been rarely identified in cancers from the stomach, endometrium, and bone according to the COSMIC cancer database (http://cancer.sanger.ac.uk/cosmic/gene/analysis?ln=HMGB1#muts). The function of HMGB1 mutations in cancer, if any, has not yet been determined (Xiang et al., 1997).
Figure 3.
Structure of the HMGB1 protein. (A) HMGB1 has two DNA-binding domains (A and B box) and an acidic C-terminal tail. (B) Helical secondary structure of A and B box domains (Thomas, 2001). (C) Intramolecular disulfide bond in the Adomain of HMGB1 (Wang et al., 2013e).
2.2 Secondary Structure
Secondary structure refers to highly regular local sub-structures motifs in proteins, including the alpha helix and the beta sheets, which was firstly described in 1951 by Linus Pauling and colleagues (Pauling et al., 1951). The two HMG boxes of HMGB1 are structurally similar to a characteristic DNA-binding domain consisting of 3 alpha helices (helix I, helix II, and helix III) and two loops (loop I and loop II), which then arranges in an “L” shape with an angle of 80° between the two arms (Figure 3B) (Hardman et al., 1995; Ohndorf et al., 1999; Stott et al., 2006; Thomas and Travers, 2001; Weir et al., 1993). Compared with the B box, the A box has a high alpha helix content and is more positively-charged and straight than that found in helices I/II (Abdul-Razzak et al., 1989; Webb and Thomas, 1999). The short arm contains helix I and helix II, whereas the long arm contains helix III and an N-terminal unstructured segment in parallel with the helix. The “L” shape structure from HMGB1 box B domain is defined by a number of conserved, predominantly aromatic residues (Phe14, Phe17, Trp45, Lys53, and Tyr56) that are located in the junction between the two helical arms (Weir et al., 1993). The rings of Phel7, Trp45, and TyrS6 pack at right angles to each other, while Phel4 lies between helix I and helix II. The conserved basic residues (Lys26, Lys 39, and Arg 22) are mainly distributed around the concave surface in between the two arms, indicating that they may be involved in DNA binding. The minor groove of the DNA molecule binds to the concave side of the boxes with no sequence specificity. The current model of HMGB1-mediated DNA binding/bending suggest that HMGB1 is involved in chromatin remodeling in a “hit and run” transient fashion (Gerlitz et al., 2009).
2.3 Tertiary Structure
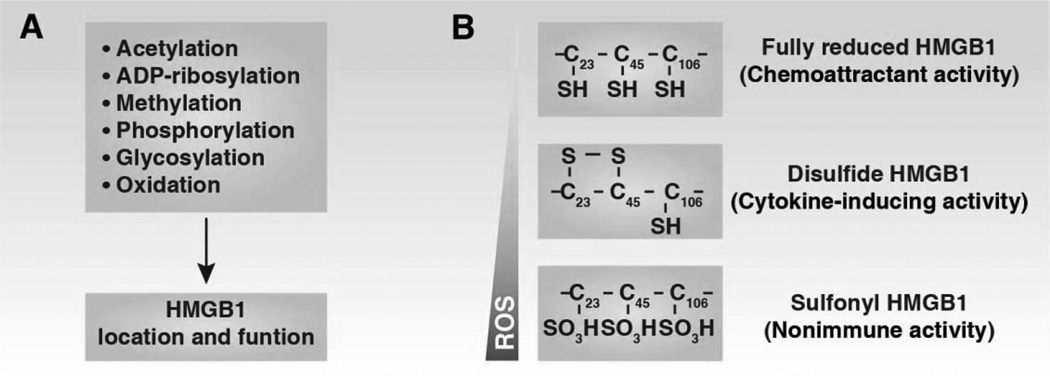
The tertiary structure of a protein is the final specific geometric shape that a protein assumes. The alpha helixes and beta sheets are folded into a compact tertiary structure by several molecular interactions including ionic bonds, hydrogen bonds, hydrophobic interaction, and disulfide bonds. The interaction between HMGB1 domains and the C terminus maintains the tertiary structure (Carballo et al., 1984; Cary et al., 1984). In addition, three cysteines are encoded at positions 23, 45, and 106 of HMGB1. The two vicinal Cys23–Cys45 residues can rapidly form an intramolecular disulfide bond via the process of oxidative folding (Figure 3C). Disulfide bonds are extremely rare in cytosolic proteins since cytosol is generally a reducing environment. A serine for cysteine substitution of C106 leads to HMGB1 translocation from the nucleus to the cytosol (Hoppe et al., 2006). In addition, reduced HMGB1 and oxidized HMGB1 interact with different receptors and have altered DNA binding activities. Thus, changes in the cellular redox environment can regulate the structure, location, and function of HMGB1 (Hoppe et al., 2006; Tang et al., 2011e).
2.4 Quaternary structure
The quaternary structure of a protein describes the interactions between different peptide chains that make up the protein. Complexes of two or more polypeptides (i.e. multiple subunits) are called multimers. Different methods used to extract HMGB1 may change HMGB1 structure. Indeed, native HMGB1 exists in homodimer and oligomer forms, whereas acid-extracted HMGB1 does not (Marekov et al., 1984). In addition, exogenous HMGB1 can bind to other proteins or chemicals in dimer, trimer, tetramer, and oligomer forms (Li et al., 2011e; Riuzzi et al., 2007). Thus, different purification steps and extraction methods may modify and impair the function of HMGB1.
3. HMGB1 Function
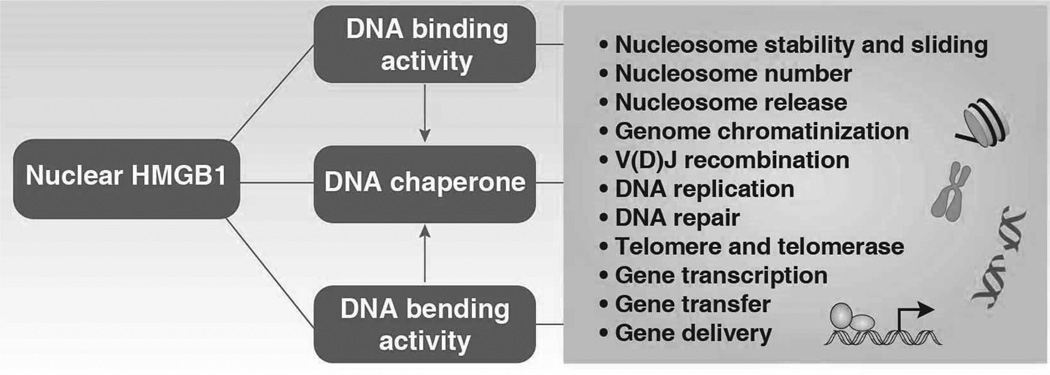
3.1 Nuclear HMGB1
Nuclear HMGB1 acts as a DNA chaperone with DNA binding and bending activities and regulates a number of key DNA events (Figure 4).
Figure 4.
Nuclear HMGB1 acts as a DNA chaperone with DNA binding and bending activities that regulate a number of nuclear events.
3.1.1 Nucleosome Stability and Sliding
Chromatin is a dynamic structure and the basic unit nucleosomes’ stability plays important roles in a number of DNA-related processes. HMGB1 is involved in nucleosome assembly and chromatin replication (Bonne-Andrea et al., 1984a, b; Mathew et al., 1979). It is clear that HMGB1 and linker histones (H1 and H5) are the major proteins that bind to linker DNA between successive nucleosomes in the chromatin fiber (Carballo et al., 1983; Nightingale et al., 1996; Smerdon and Isenberg, 1976; Thomas and Stott, 2012; Yamada et al., 2004; Yu and Spring, 1977). These proteins share many features of DNA-binding behavior, although they are structurally unrelated (Zlatanova and van Holde, 1998b). An early study indicated that HMGB1 protects linker DNA on the side opposite to that protected by linker histones (An et al., 1998). We now know that they have opposing effects on nucleosome assembly and stability (Paull et al., 1993). H1 stabilizes the nucleosome engendering less mobility, whereas HMGB1 relaxes the nucleosome and makes chromatin more accessible at the distorted site (Cato et al., 2008; Travers, 2003). The interaction between HMGB1 and linker histones (H1 and H5) occurs through their acidic and basic tails, respectively (Cato et al., 2008). This interaction between HMGB1 and H1 facilitates DNA-protein complex formation (Totsingan and Bell, 2013), DNA ligation reactions (Yamanaka et al., 2002) and enables stress-induced gene silencing (e.g., pro-inflammatory cytokine TNF-α) (El Gazzar et al., 2009). The interaction between HMGB1 and H1 is regulated by pH, local ionic concentration, and redox state (Kohlstaedt and Cole, 1994b; Kohlstaedt et al., 1987). HMGB1 suppresses nucleosome assembly at physiological ionic strength (Waga et al., 1989). Besides its cooperation with histones, HMGB1 can replace linker histones H1 and H5 in nucleosomes in vitro (Ner and Travers, 1994; Varga-Weisz et al., 1994) or in development (Ura et al., 1996) or directly compete with various DNA substrates (Zlatanova and van Holde, 1998b). Increased transient interaction between HMGB1 and nucleosomal linker DNA can activate ATP-utilizing chromatin assembly and remodeling factor/ chromatin accessibility complex (ACF/CHRAC) pathway, which in turn promotes nucleosome sliding (Bonaldi et al., 2002). The C terminus of HMGB1 is required for activation of the ACF/CHRAC pathway (Bonaldi et al., 2002). In addition, acetylated HMGB1 assists nucleosome mobilization induced by switch/sucrose nonfermentable (SWI/SNF), but does not affect its ATPase activity in in vitro assay (Ugrinova et al., 2009a). HMGB1 does not directly form a complex with either ACF/CHRAC or SWI/SNF, although some HMG-box proteins are direct components of chromatin remodeling complexes such as BAF57 in human SWI/SNF and SSRP1 in FACT. The interaction between HMGB1 and these HMG-box proteins in chromosome remolding is not yet clear. It is also important to determine if HMGB1 regulates specific histone modifications, which are also involved in nucleosome stability (Andrews and Luger, 2011). In addition to the the linker histones (H1 and H5), HMGB1 can interact with the core histones. HMGB1 binds H2A and H2B by it’s A and B boxes (Bernues et al., 1986; Bernues et al., 1983), whereas it binds H3 and H4 by its A box and acidic tail (Bernues et al., 1986; Bernues et al., 1983; Stros, 1987; Ueda et al., 2004). In general, the interaction between HMGB1 and nucleosomes is highly reversible during the dynamic process of chromatin remodeling (Falciola et al., 1997).
3.1.2 Nucleosome Number and Genome Chromatinization
The number of nucleosomes decrease and DNA damage increases during aging (Feser et al., 2010; O'Sullivan et al., 2010), suggesting that global alterations within nucleosomes reflect a programmed chromatin-level response to aging. Age-dependent reprogramming and epigenetic integrity may also serve as a target for cancer initiation. Early studies demonstrated that the expression of HMGB1 decreases, whereas the acetylation of HMGB1 increases with advancing age (Prasad and Thakur, 1988, 1990a; Thakur and Prasad, 1991). Age-dependent HMGB1 changes are associated with DNA double-strand break accumulation in the mouse brain (Enokido et al., 2008). Thus, HMGB1 levels and modifications may reflect the functional state of chromatin. Surprisingly, HMGB1 not only regulates nucleosome organization, but also biogenesis. Mammalian and yeast cells lacking HMGB1 contain 20–30% less histones and nucleosomes and more RNA transcripts (Celona et al., 2011), many of them promoting expression of inflammatory genes including chemokines. Exogenous HMGB1 promotes the assembly of chromatin in vitro by virtue of its DNA chaperone activity (Celona et al., 2011). These findings indicate that HMGB1 contributes to genome chromatinization by sustaining the number, and possibly location, of nucleosomes. Although total gene expression is increased in HMGB1−/− cells, some specific genes are significantly down regulated when HMGB1 is lost (Celona et al., 2011; Krynetskaia et al., 2008). In contrast, overexpression of HMGB1 in cells leads to gene transcription along with relaxation of chromatin structure (Ogawa et al., 1995). This finding raises some questions such as: how do HMGB1 levels balance global and local gene expression? What checkpoint is responsible for surveillance at the nucleosomal level? Interestingly both histones H3 and H4 exist in archaea, and although they are true ‘prokaryotes’, there may be some value in more closely examining them for ancient HMG motifs (Ammar et al., 2012).
3.1.3 Nuclear Catastrophe and Nucleosome Release
The nucleosome acts as a DAMP when it is released from the nuclei into the extracellular space during DNA damage (e.g. DNA strand scission), creation of neutrophil extracellular traps and cell death (Hamana and Kawada, 1989). Circulating nucleosomes, including histones and genomic DNA, are significantly elevated in patients with cancer, stroke, trauma, sepsis, and autoimmune diseases (Holdenrieder et al., 2008; Holdenrieder and Stieber, 2009). We recently demonstrated that deficiency of endogenous pancreatic HMGB1 led to exaggeration of L-arginine-induced pancreatitis, associated with nuclear catastrophe and nucleosome release. This in turn, recruited and activated inflammatory cells with subsequent HMGB1 release locally and into the circulation (Kang et al., 2013b). Serum levels of tissue enzymes (e.g., amylase, lactate dehydrogenase, and pancreatic myeloperoxidase) and pro-inflammatory cytokines were significantly higher in conditional pancreas-specific HMGB1 knockout mice when compared with their wild-type control littermates. Moreover, neutralizing extracellular histone and HMGB1 conferred protection against acute pancreatitis in these pancreas-specific HMGB1 knockout mice. Thus, intracellular HMGB1 may serve in a previously underappreciated negative regulator of inflammation, shedding light on the role of the innate immune response in infection and tissue damage. In addition, conditional knockout of HMGB1 in the liver also increases nucleosome release and accelerates liver reperfusion ischemic injury (Huang et al., 2013a). Thus, stress that induces HMGB1 translocation from the nucleus may enhance inflammation by allowing nucleosome release from terminally damaged cells. The nonspecific DNA-binding and -bending protein HMGB1 not only regulates nucleosome stability and biogenesis, but also nucleosome release, which provides a novel link between chromosomal instability and inflammation in disease. Intracellular HMGB1 and H1-mediated chromatin remodeling in leukocytes inhibits pro-inflammatory cytokine (TNF-α and IL-1β) transcription during endotoxin tolerance (El Gazzar et al., 2009). More recently, conditional knockout of HMGB1 in macrophages decreased autophagy, which in turn increased pro-inflammatory cytokine production in sepsis in an animal infection model (Yanai et al., 2013). These findings clearly suggest that HMGB1 is an intracellular, anti-inflammatory nuclear chromatin modulator.
3.1.4 DNA Binding
Besides acting as an architectural protein in chromosomes, HMGB1 acts as a DNA chaperone in the nucleus. HMGB1 binds to DNA with structure-specificity, but not sequence-specificity (Yu et al., 1977). Recognition and alteration of DNA structure plays a significant role in regulating DNA-related processes. The flanking sequences of HMG-boxes A and B as well as the acidic C terminal have the ability to regulate their DNA binding activities (Sheflin et al., 1993; Stros, 1998, 2001; Stros et al., 1994c; Teo et al., 1995b; Wisniewski and Schulze, 1994). Compared with the single HMGB-box, DNA binding is enhanced when the two domains are covalently connected (A+B) in vitro (Reddy et al., 2005; Stros, 1998, 2001; Yoshioka et al., 1999). Binding of HMGB1 to DNA is also regulated by post-translational modifications (e.g., phosphorylation, acetylation, and oxidization) (Assenberg et al., 2008), pH, ions (e.g., calcium and Mg2+) (Makiguchi et al., 1984; Stros et al., 1990) and the presence of another cationic factor, spermine (Van den Broeck et al., 1994). pH affects interactions between DNA and high-mobility group protein HMG1 (Kohlstaedt and Cole, 1994a). In many cases, HMG boxes bind to the minor groove of linear B-type DNA transiently and distort the double helix sharply to a larger bending angle of to 90° or greater (Zimmerman and Maher, 2008). These HMGB1-mediated architectural changes at the distorted site contribute to multiprotein complex assemblies that control DNA-related events. In addition, HMGB1 binds with relatively high affinity to distorted and damaged DNA. These DNA structures include H-DNA (Jain et al., 2005), hemicatenated DNA loops (hcDNA), (Jaouen et al., 2005), psoralen-DNA interstrand cross-link (ICL) (Reddy et al., 2005), hemicatenated DNA loops (Stros et al., 2004), duplex DNA[poly (dAdT). (dTdA) and poly (dGdC). (dCdG).] (Muller et al., 2001), four-way DNA junctions (Gaillard and Strauss, 1994; Grasser et al., 1998; Hill and Reeves, 1997; Stros and Muselikova, 2000; Teo et al., 1995a), supercoiled DNA and DNA modified with anticancer drug cisplatin (Stros, 2001), semicatenated DNA loops (Gaillard and Strauss, 2000), supercoiled plasmid DNA (Grasser et al., 1998), supercoiling of nicked-circular DNA (Sheflin et al., 1993), UV-damaged DNA (Pasheva et al., 1998), tandem repeats of dTG (Gibb et al., 1997), tandem repeats of (GCC)n (GGC)m DNA (Zhao et al., 1996), kinked DNA (Falciola et al., 1994), and supercoiled plasmids (Bustin and Soares, 1985). Of these structures, four-way DNA (Holliday) junctions and cisplatin-modified DNA are among the best-documented.
Four-way DNA junction is a mobile junction between four strands of DNA that is generated as an intermediate in genetic recombination and connected by mutual exchange of strands (Lilley and Clegg, 1993). Four-way DNA junction simulates the structure of the linker DNA strands near the entrance and exit points of nucleosomes. Except for genetic recombination, the four-way DNA junction is also involved in replication-related events. Proteins, including specific enzymes and HMG box proteins that bind and resolve four-way DNA junctions, can be regarded as a paradigm for the recognition of DNA structure (Zlatanova and van Holde, 1998a). The first reported HMGB1 binding to four-way DNA junction was published in 1989 by Marco E. Bianchi and colleagues (Bianchi et al., 1989). Reduced HMGB1, but not oxidized HMGB1, can effectively compete with H1 for binding to such four-way DNA junctions (Polanska et al., 2014; Varga-Weisz et al., 1994).
Cisplatin, or cis-diamminedichloroplatinum (II), is an effective anticancer drug in the treatment of several solid tumors including ovarian, genitourinary, lung, head, and neck cancers. Cisplatin and its second generation analogues carboplatin and oxaliplatin induce cell death by binding to DNA preferentially at the N7 position of guanine bases, inhibiting replication and transcription. The first report of HMGB1 binding to cisplatin-DNA by Stephen J. Lippard and colleagues was published in 1992 (Bruhn et al., 1992; Pil and Lippard, 1992). HMGB1 binds to platinated lesions on DNA with specificity for 1,2-d (GpG) and d (ApG) intrastrand cross-links (Pil and Lippard, 1992), which account for about 90% of the cisplatin-DNA adducts formed in vivo (Eastman, 1987; Fichtinger-Schepman et al., 1985). In addition, HMGB1 also binds to interstrand cross-linked versus undamaged DNA, but not 1,3-intrastrand cross-links (Kasparkova et al., 2003). Compared with box B, HMGB1 box A has a higher binding affinity for platinated DNA, although box B can enhance box A’s affinity (Jung and Lippard, 2003). This process is also regulated by the redox state of HMGB1 as well as the C-terminal domain (Park and Lippard, 2011; Yusein-Myashkova et al., 2013). For example, the reduced box A has a 10-fold greater platinated DNA binding affinity than the oxidized box A (Park and Lippard, 2011; Wang et al., 2013f). Although HMGB1 inhibits cisplatin-DNA damage repair (Huang et al., 1994; Ugrinova et al., 2009b), the final influence of HMGB1 expression in cisplatin sensitivity depends on cell types. For example, hormone-induced HMGB1 up-regulation in breast and ovarian cancer cells contributes to the anticancer activity of cisplatin (He et al., 2000). In contrast, knockout of HMGB1 in MEFs does not change cisplatin-mediated cell death (Wei et al., 2003). These findings suggest the importance of cell type in determining the ability of this and probably other cisplatin-DNA-binding proteins to influence the efficacy of the drug (Wei et al., 2003). The ability of H1 to bind cisplatin-DNA in an in vitro competition assay is much stronger than that of HMGB1 (Yaneva et al., 1997), suggesting that the dynamic change of H1 levels in the nucleus (Zlatanova and Van Holde, 1992) may regulate the HMGB1-mediated cisplatin-DNA damage response. In addition, HMGB1 levels regulate cisplatin sensitivity in different cells by directly affecting DNA replication (Hoffmann et al., 1997) as well as replication protein A’s DNA binding activity (Patrick and Turchi, 1998).
3.1.5 DNA Bending
After binding DNA, HMGB1 can bend and change DNA conformation by unwinding (Javaherian et al., 1978; Javaherian et al., 1979; Yoshida et al., 1984), looping (Paull et al., 1993; Paull and Johnson, 1995; Stros et al., 1994c), or compacting DNA (Javaherian et al., 1978). This HMGB1 DNA bending activity contributes to several DNA processes, especially enhancing DNA dynamic flexure. The DNA bending activity of HMGB1 was initially reported in 1993 by the research groups of Stephen J. Lippard and Reid C. Johnson (Paull et al., 1993; Pil et al., 1993). Stephen J. Lippard and colleagues demonstrated HMGB1’s DNA bending activity by ligase-mediated ring closure assays, and this activity is maintained by HMG boxes (Pil et al., 1993). Reid C. Johnson and colleagues found that HMGB proteins from HeLa or bovine nuclear extract have extraordinary DNA-bending activity, as demonstrated by their ability to promote circularization of very short DNA fragments. This DNA bending activity of HMGB1 promotes the assembly of the Hin invertasome, a topologically complex structure in Hin-mediated site-specific DNA inversion (Paull et al., 1993). Similar DNA bending activity has been confirmed in other HMGB1-related homologs, including yeast Nhp6A/B and drosophila HMG-D. The structural basis for the DNA bending activity of HMG box has been identified in a number of in vitro assays (Thomas and Travers, 2001). A basic DNA binding/bending model involves intercalation of bulky hydrophobic amino acid residues of the HMG-boxes between successive base-pairs within the DNA minor groove, accompanied by partial unwinding, widening of the minor groove, and bending towards the major grove (Stros, 2010). This process is tightly controlled by a number of factors, including intercalating residues of the HMG-box, N- and C-terminal flanking sequences of the HMG-box, post-translational modifications of HMGB1 (e.g., phosphorylation, acetylation, and oxidization) as well as structural features of bent DNA (Furuita et al., 2011; Stros, 1998, 2010; Thomas and Travers, 2001; Ugrinova et al., 2007). The HMGB1-mediated DNA recognition mechanism has been confirmed by NMR studies (Furuita et al., 2009). B box is known to be far more effective at bending linear DNA, whereas the A box binds preferentially to pre-bent DNA. This DNA binding and bending activity of HMGB1 can change the DNA helical structure by unwinding the double helix or inducing supercoiling of the DNA (Javaherian et al., 1978; Javaherian et al., 1979). The acidic tail is specifically involved in HMGB1-mediated Mg2+-, Ca2+-dependent unwinding of DNA double-helix (Yoshida, 1983, 1987). In addition, HMGB1 (either individual or arranged in tandems or multi-domain proteins) can enhance apparent DNA flexibility by looping, which is involved in the regulation of transcriptional initiation (Paull et al., 1993; Paull and Johnson, 1995; Stros et al., 1994c). The cysteine-sulfhydryl group of the HMG-box is specifically involved in HMGB1-mediated DNA looping, which is also regulated by the calcium level of the acidic C tail (Stros et al., 1994b). Microsatellites are repeating sequences of 2–6 base pairs of DNA. An in vitro study demonstrated that HMGB1 has varying DNA bending activities for individual microsatellites. Given that microsatellite instability is increased in human genetic diseases and cancer, HMGB1 may be involved in microsatellite instability-associated disease by regulation of DNA mismatch repair (Takayanagi et al., 1997).
3.1.6 V(D)J Recombination
V(D)J recombination, also known as somatic recombination, is the antigen receptor gene rearrangement process that generates diversity among T cell receptors (Sadofsky, 2001). Recombination is initiated by the lymphoid-specific recombination activating gene (RAG)-1 and RAG2 proteins, which recognize specific recognition sequences (termed recombination signal sequences or RSSs). Each RSS consists of a conserved heptamer and nonamer separated by a nonconserved spacer. RAG, which has nuclease activity, leads to double-strand breaks at RSSs. Following DNA cleavage by RAG, the nicked strand is converted to a hairpin and then the broken ends are joined by several proteins involved in DNA damage responses (Gellert, 2002). HMGB1 plays an essential role in V(D)J recombination by formation of RAG-RSS-HMG complexes to enhance RAG1/RAG2 activity (Agrawal and Schatz, 1997; Dai et al., 2005; Grundy et al., 2009; Little et al., 2013; Swanson, 2002a, b). The knowledge of their interconnection is essential for our understanding of immune cell development.
3.1.7 Gene Transcription
Transcription factors are proteins that bind to specific DNA sequences at promoters, thereby generally controlling gene transcription. HMGB1 is able to change transcription rates and gene expression via several different mechanisms (Singh and Dixon, 1990). These include: 1) sustaining nucleosome dynamics and number at a global level, 2) promoting interaction with the TBP (TATA binding protein)/TATA-box complex (Das and Scovell, 2001; Sutrias-Grau et al., 1999), the conserved lymphokine elements-0 (CLE0) (Marrugo et al., 1996) and long terminal repeat (LTR) (Naghavi et al., 2003) affecting recruitment of other general transcription factors, 3) enhancing interaction with RNA polymerase (transcription by RNA polymerase II), 4) promoting enhanceosome assembly (Ellwood et al., 2000; Mitsouras et al., 2002), 5) removing transcriptional blocks (Waga et al., 1988), or 6) acting as an activator, enhancer, repressor, or silencer locally by interfering with several sequence-specific transcription factors to their cognate DNA (Table 2). These transcription factors include steroid nuclear receptors (e.g., androgen, glucocorticoid, progesterone, and mineralocorticoid receptors) (Onate et al., 1994; Verrijdt et al., 2002), Hox, Sox (Zhang et al., 2013k), Oct (Harrison and Whitehouse, 2008), p53 (Banerjee and Kundu, 2003; Imamura et al., 2001; McKinney and Prives, 2002; Rowell et al., 2012; Zhang et al., 2003), p73 (Stros et al., 2002), the retinoblastoma protein (RB), nuclear factor-κB (NF-κB)/Rel (Agresti et al., 2003; Brickman et al., 1999), estrogen receptor (Chau et al., 1998; Romine et al., 1998), sterol regulatory element-binding proteins (SREBPs) (Najima et al., 2005), Up-stream stimulatory factor 1 (USF1) (Marmillot and Scovell, 1998), NF-Y (Stros et al., 2009), HNF1α (Yu et al., 2008), ETs (Shiota et al., 2008), PU.1 (Mouri et al., 2008), replication and transcription activator (RTA) (Song et al., 2004), and Dof2 (Krohn et al., 2002). Among them, the interaction between p53 and HMGB1 is the best documented.
p53 regulates a number of gene expressions and is the most frequently altered gene in human cancer. HMGB1 stimulated p53 DNA binding to linear DNA in vitro and increased p53 activity in vivo in a transfection assay (Jayaraman et al., 1998). That binding to pre-bent (e.g. minicircle) DNA is not facilitated by HMGB1 (McKinney and Prives, 2002) suggesting that HMGB1 promotes binding to linear DNA through its DNA-bending activity. Although the individual HMG boxes (A and B) and C-terminus can facilitate binding to p53 (McKinney and Prives, 2002), the A box has a strong p53 binding activity by using cross-linking chemical and biophysical measurements (Rowell et al., 2012). HMGB1 regulates p53 not only on the transcription activity, but also on its subcellular localization and phosphorylation (Krynetskaia et al., 2009). These findings suggest that HMGB1 regulates p53 at multiple levels.
HMGB1 is a critical member of the transcriptional regulatory network which regulates hematopoietic stem cell (HSC) multipotency and self-renewal. Many (18) genes confer a clear repopulation advantage to HSCs (Deneault et al., 2009). Among them, HMGB1, FOS, TCFEC, and SFPI1 regulate HCS activity via a non-cell-autonomous phenomenon (Deneault et al., 2009). In addition, HMGB1’s association with other transcription factors (OCT4/POU5F1, NANOG, and DPPA5) regulates embryonic stem cell differentiation (Adjaye et al., 2005). These findings suggest that HMGB1 regulates gene expression not only autonomously, but as part of a coordinated network.
3.1.8 DNA Replication
DNA contains two strands wrapped around each other in a helix, and its replication is the process of generating new DNA bands from one old single-stranded DNA. This single-stranded DNA serves as template for RNA priming and DNA synthesis. DNA polymerase is a critical enzyme in the process of DNA replication. In addition, helix-destabilizing protein is responsible for maintaining the single-stranded DNA structure by interaction with DNA polymerase during replication fork advances. Early studies indicated that HMGB1 isolated from rat liver acts as a DNA helix-destabilizing protein to stimulate activity of DNA polymerases α and β in vitro (Bonne et al., 1979; Bonne et al., 1982; Duguet et al., 1977). Thus, HMGB1 antibody inhibits the HMGB1-mediated polymerizing activity of DNA polymerase in vitro (Alexandrova et al., 1984). Interestingly, only isolated HMGB1 from regenerating liver, but not normal liver, has this activity. The role of HMGB1 in DNA replication is modulated by post-translational modification. The phosphorylated form of HMGB1 significantly decreases the HMGB1-mediated polymerizing activity of DNA polymerase, although it did not influence HMGB1 binding to single stranded DNA (Bonne et al., 1979; Duguet et al., 1977). In contrast, the acetylated form of HMGB1 proteins can bind and stimulate DNA polymerase activity in vitro (Alexandrova and Beltchev, 1988). A more recent study indicated that native, recombinant, and tailless HMGB1 proteins act significantly different in the regulation of DNA replication in an in vitro replication assay of closed circular DNA. In this study, the authors found that native HMGB1 isolated from tumor cells and phosphorylated recombinant HMGB1 by PKC inhibited DNA replication (Topalova et al., 2008). This inhibition effect can be further reversed by HMGB1 acetylation and removal of the acidic tail (Topalova et al., 2008). Taken together, these results show that HMGB1 participates in DNA replication from mammalian cells (Bonne-Andrea et al., 1986) and viruses (Cotmore and Tattersall, 1998) by positive or negative regulation of DNA polymerase activity. The role of HMGB1 in DNA replication remains unknown.
3.1.9 DNA Repair
DNA damage caused by various sources results in changes in molecular structure, such as a break in a DNA strand, a missing base from the DNA backbone, or a chemically changed base such as 8-OHdG. Recognition of DNA damage is a dynamic process, namely DNA damage response, including activation of cell cycle checkpoint, commencement of transcriptional programs, initiation of DNA repair, or induction of apoptosis if DNA repair fails. The DNA repair rate depends on many factors, including HMGB1 levels. HMGB1 has a dual role in DNA repair and cell death. In many cases, loss of HMGB1 or increased HMGB1 translocation from the nucleus to the cytoplasm could increase DNA damage, decrease DNA repair efficiency, and increase cell death in response to chemotherapy, irradiation, and oxidative stress. HMGB1 directly binds to a variety of bulky DNA lesions, allowing it to participate in DNA repair pathways.
3.1.9.1 DNA Mismatch Repair
DNA mismatch repair is an evolutionarily conserved genome maintenance pathway that corrects mismatches generated during DNA synthesis and homologous recombination. In 2004, HMGB1 was reported to be involved in DNA mismatch repair initiation and excision (Yuan et al., 2004). Recombinant human HMGB1 partially fractionated from HeLa cell extracts promotes mismatch excision through its interaction with MutSα, a critical component of DNA mismatch repair machinery (Yuan et al., 2004). In addition, recombinant human HMGB1 can replace replication protein A, a protein that binds single-stranded DNA, in a reconstituted human DNA mismatch repair system (Zhang et al., 2005). However, a recent study indicated that HMGB1 is not essential for 5-directed mismatch repair compared with the extracts derived from HMGB1+/+ and HMGB1−/− MEFs (Genschel and Modrich, 2009). One possible reason for this different finding is that mammalian cell extracts may possess a second activity, which provides a mismatch repair function that is redundant with respect to HMGB1 (Genschel and Modrich, 2009).
3.1.9.2 Base Excision Repair
Base excision repair is an evolutionarily-conserved pathway that corrects base lesions generated from oxidative, alkylation, deamination, and depurinatiation/depyrimidination damage (Robertson et al., 2009). There are two sub pathways, the short-patch and long-patch, involved in base excision repair. The short-patch pathway leads to insertion of a single nucleotide, whereas the long-patch pathway is involved in insertion of at least two nucleotides. These two sub pathways are initiated by DNA glycosylase that recognizes a damaged base or a base in a specific DNA sequence, and then removes the base by hydrolysis of the N-glycosylic bond. In 2007, HMGB1 was identified as a regulator of the base excision repair pathway by its DNA binding and protein interaction activity (Prasad et al., 2007). The major findings from this study include: HMGB1 accumulates in the sites of cellular oxidized DNA base damage and then binds to dRP lyase substrates, an intermediate in the base excision repair pathway; HMGB1 can physiologically interact with multiple key enzymes (e.g., APE, FEN-1, and pol β) to enhance their activity during base excision repair; HMGB1−/− MEFs exhibit more resistance to the methylating agent methyl methanesulfonate, suggesting that that the absence of HMGB1 does not significantly impact methyl methanesulfonate-induced damage repair. These findings suggest that HMGB1 inhibits the short-patch pathway and stimulates the long-patch pathway at different stages (Goula et al., 2009; Liu et al., 2010c).
3.1.9.3 Nucleotide Excision Repair
Nucleotide excision repair is an important general pathway that corrects many different types of DNA damage, including the UV component of sunlight, bulky chemical adducts, DNA intrastrand crosslinks, and some forms of oxidative damage. Given the affinity of HMGB1 for a number of nucleotide excision repair substrates (e.g. DNA damaged by cisplatin, UV, BPDE, etc.) (Malina et al., 2002), a number of studies have confirmed the critical role of HMGB1 in the regulation of the nucleotide excision repair pathway (Lange and Vasquez, 2009). HMGB1, molecularly “repair shielding” via DNA binding activity (Pil and Lippard, 1992; Takahara et al., 1995), inhibits nucleotide excision repair following cisplatin lesion (Huang et al., 1994; Lanuszewska and Widlak, 2000; Malina et al., 2002; Mitkova et al., 2005; Patrick et al., 1997; Ugrinova et al., 2009b; Yusein-Myashkova et al., 2013). The HMGB1-mediated inhibitory effect on the repair of cisplatin-damaged DNA is accomplished through the acidic domain (Mitkova et al., 2005). However, HMGB1’s protein interaction activity may enhance nucleotide excision repair in some cases. Indeed, recent studies indicate that HMGB1 can bind DNA damage recognition complex proteins such as XPC-RAD23B, XPA, and RPA to enhance interactions between nucleotide excision repair proteins on triplex-directed psoralen interstrand crosslinks (Lange et al., 2009; Lange and Vasquez, 2009; Reddy et al., 2005). Based on this finding, HMGB1−/− MEFs exhibit significantly decreased DNA repair ability, which in turn promotes cell death as well as gene mutation in response to UV irradiation and psoralen/UVA treatment (Lange et al., 2008). In contrast, some HMGB1−/− MEFs were more resistant to the nucleoside analogs 5-fluorouracil, araC, mercaptopurine, and thiopurine than the isogenic wild-type cell lines (Krynetskaia et al., 2008). Collectively, these findings from these studies suggest that HMGB1 has a dual role in the regulation of nucleotide excision repair depending on stimuli type and chromatin content.
3.1.9.4 Double Strand Break Repair
Double strand break repair is an important pathway during the cell cycle that corrects the DNA double-strand break generated by ionizing radiation, radio-mimetic chemicals, and other type of DNA lesion (Jackson, 2002). Two sub pathways, the nonhomologous end joining (NHEJ) and homologous recombination (HR) pathways, are responsible for double strand break repair (Rothkamm et al., 2003). NHEJ is initiated by the recognition and binding of the Ku70/Ku80 heterodimer proteins, which recruits and holds the catalytic subunit of DNA protein kinase (DNA-PKCS) to the ends of double-strand breaks (DSBs) to promote a repairing process. HR is mediated by using extensive homology to restore the sequence at the break site (Jackson, 2002). In vitro, recombinant mammalian HMGB1 can activate DNA-PKcs in the absence of Ku protein (Yumoto et al., 1998) and function as DNA-binding regulatory components for DNA-PKcs to enhance ligation reaction of DNA double strand breaks during V(D)J recombination (Nagaki et al., 1998; Yumoto et al., 1998). Given the role of HMGB1 in activating DNA-PKcs in vitro, it will be important to investigate whether HMGB1-mediated V(D)J recombination is completed partly by NHEJ (Downs, 2007).
3.1.10 Telomere and Telomerase
A telomere is a region of repetitive nucleotide sequences (TTAGGG) at the end of a chromosome. Telomeres not only protect chromosome ends against erosion and degradation, but also prevent activation of DNA damage checkpoints. Telomere length is maintained as a result of a dynamic equilibrium between lengthening and shortening. Telomere shortening is caused by incomplete DNA replication and nucleolytic degradation. In contrast, telomere lengthening primarily results from increased telomerase activity. Telomerase contains two main core components: a catalytic protein subunit (telomerase reverse transcriptase, TERT), and an RNA subunit (telomerase RNA, TR). In addition, shelterin, a six-subunit protein complex (TRF1, TRF2, TIN2, Rap1, TPP1, and POT1), protects human telomeres. An early study indicated that loss of HMGB1 in yeast and mammalian cells promotes chromosomal instability and telomere aberrant events (Giavara et al., 2005). A recent study demonstrated that HMGB1−/− MEFs exhibited mild telomere shortening, but significantly decreased telomerase activity and DNA damage. Possible reasons for this process include interaction between HMGB1 and TERT/TR and HMGB1-mediated transcription upregulation of TR (but not TERT). Interestingly, HMGB2 plays an opposing role in the regulation of telomerase activity, and HMGB2−/− MEFs exhibit increased telomerase activity. However, change HMGB1 level in Arabidopsis thaliana does not affect telomerase activity and chromatin architecture (Schrumpfova et al., 2011). The complex roles of HMGB1 in coordinating the DNA damage response and telomere dynamic and genomic stability remain to be further elucidated. It is important to determine whether HMGB1 directly binds or bends telomere’s repetitive nucleotide sequences.
3.1.11 Gene Transfer
Transposition of DNA is the process of a DNA sequence that can change its position within the genome, which will create or reverse mutations and alter genome size. Based on this theory, several DNA transposition systems, including Sleeping Beauty transposon system, have been developed to insert a gene or DNA sequence into chromosomes of vertebrate animals for gene therapy or offer a new model to study gene function and DNA recombination. The Sleeping Beauty transposon system contains a Sleeping Beauty (SB) transposase and a transposon designed in 1997 by Zsuzsanna Izsvák and colleagues (Ivics et al., 1997). This system-mediated transposition is a dynamic process including: (1) SB transposase binding to its cognate inverted repeat/direct repeat elements, (2) SB synaptic complex formation, (3) excision separating the transposon from the donor DNA, and (4) integration of the transposon into chromosomal DNA (Ivics et al., 1997; Ivics et al., 2004). HMGB1 acts as a cellular cofactor of SB transposase, and physically interacts with SB to enhance SB binding to the inner direct repeat element via its binding activity, which in turn stimulates synaptic complex formation and DNA recombination (Zayed et al., 2003). Thus, overexpression of HMGB1 by gene transfection has the ability to enhance SB-mediated transposition efficiency, which provides a novel DNA transposition system for gene transfer (Zayed et al., 2004). Besides the SB system, HMGB1 has the ability to enhance other DNA transposition systems such as herpes simplex virus/Sleeping Beauty (HSV/SB) amplicon vector platform (de Silva et al., 2010; Peterson et al., 2007). These findings make HMGB1 an excellent candidate for improving gene transfer in gene therapy.
3.1.12 Gene Delivery
Transfection is the process by which nucleic acids (DNA or RNA) are introduced into mammalian cells. It is a widely-used molecular and cellular technology to change gene expression by using various lipids, chemical, or physical methods. HMGB1 significantly enhances transfection efficiency in several systems by its nuclear localization signals and DNA binding ability (Bottger et al., 1988; Namiki et al., 1998; Shen et al., 2009b; Shen et al., 2010; Siu et al., 2012). The TAT-high mobility group box-1 A box peptide (TAT-HMGB1A) and its variants have the ability to deliver DNA into cells without cytotoxicity (Han et al., 2009; Yi et al., 2012). Thus, HMGB1 may be useful as a non-toxic gene delivery carrier in gene therapy (Kim et al., 2008b; Yi et al., 2012).
3.2 Cytosolic HMGB1
Early studies suggest that expression of HMGB1 is high in hepatic tissues and the brain, suggesting that HMGB1 functions in both the nucleus and cytoplasm (Bustin and Neihart, 1979; Mosevitsky et al., 1989). Subsequent studies investigated the levels and distribution of HMGB1 between the nucleus and cytoplasm in different cells and tissues. Localization of HMGB1 in the cytoplasm has been confirmed in living fibroblasts (Einck et al., 1984), thymocytes (Guillet et al., 1990) and several different tissues (e.g., liver, kidney, heart, and lung) (Kuehl et al., 1984). The normal ratio of nuclear to cytoplasmic HMGB1 is about 30:1 (Kuehl et al., 1984). Currently, we know that HMGB1 normally is located in the nucleus and translocates from the nucleus to the cytosol, including mitochondria and lysosome, following various stressors (e.g., cytokine, chemokine, heat, hypoxia, H2O2, and oncogene). Although the function of cytosolic HMGB1 still remains poorly studied, we demonstrated that the main function of HMGB1 in cytoplasm is to function as a positive regulator of autophagy, which we first reported in 2010 (Tang et al., 2010c). Autophagic stimuli promote the translocation of HMGB1 to the cytosol and cytosolic HMGB1 binds to Beclin-1 to induce autophagy to degrade damaged organelles and unused proteins (Tang et al., 2010c). We introduce details about the interaction between HMGB1 and autophagy in the “Autophagy” section.
Another potential function for cytosolic HMGB1 is involvement in the unconventional secretory pathway, found based on mass spectrometry-mediated binding partner analysis in 2010 (Lee et al., 2010a). In this study, the authors identified numerous HMGB1-binding partners in nuclear and cytosol fraction. Interestingly, cytoplasmic HMGB1 is overexpressed and colocalized with lysosomal protein in colon, liver, and gastric cancer cells. Among the cytoplasmic HMGB1-binding proteins, nine of the identified proteins are related to protein translocation and secretion. Of these, annexin A2, myosin IC isoform a, myosin-9, and Ras-related protein Rab10 are directly involved in the process of unconventional protein secretion, which has been confirmed by an immunopreciptation experiment (Lee et al., 2010a). These identified HMGB1-binding molecules provide new clues about the cytoplasmic functions of HMGB1 in cancer cells.
3.3 Membrane HMGB1
HMGB1 has been reported to be present on cell surface membranes involved in neurite outgrowth (Merenmies et al., 1991), platelet activation (Fuentes et al., 2014; Maugeri et al., 2012b), cell differentiation (Passalacqua et al., 1997), erythroid maturation (Hanspal and Hanspal, 1994), adhesion (Parkkinen and Rauvala, 1991) and innate immunity (Ciucci et al., 2011) through a different mechanism. In 1991, Heikki Rauvala and colleagues showed that HMGB1 is distributed to the filopodia of the advancing plasma membrane in process-growing neuroblastoma cells and is also deposited into the substrate-attached material, suggesting a role of HMGB1 in neurite outgrowth (Merenmies et al., 1991). Later, a study indicated that HMGB1 is distributed to spread laminin in N18 (mouse neuroblastoma) and HT1080 (human fibrosarcoma) cells and promote the generation of surface-bound plasmin, which mediates cell adhesion and invasion (Parkkinen et al., 1993; Parkkinen and Rauvala, 1991). During murine erythroleukemia (MEL) cell differentiation, HMGB1 is released and accumulates in cell membranes without extensive modification of the native molecular structure (Passalacqua et al., 1997). An unclassical secretory signal peptide, N-terminal 18 amino acids of HASPB, could efficiently deliver HMGB1 on the cell surface (Zhu et al., 2011a). HMGB1 is present in erythroblast-macrophage contact, which is involved in macrophage-mediated erythroid proliferation and maturation in a homophilic manner (Hanspal and Hanspal, 1994). Activated platelets induce the formation of neutrophil extracellular traps (NETs), an important innate immune mechanism to fight pathogenic bacteria. NETs are primarily composed of chromatin components bound to granular and selected cytoplasmic proteins (Brinkmann and Zychlinsky, 2012). During platelet activation, HMGB1 translocates to the membrane and is then released (Maugeri et al., 2012b), which mediates NET formation and function (Mitroulis et al., 2011; Tadie et al., 2013). HMGB1 is widely expressed on the cell-surfaces of human cord blood cells, especially myeloid dendritic cell precursors, which are involved in HMGB1 release and the immune response during inflammation (Ciucci et al., 2011).
3.4 Extracellular HMGB1
HMGB1 can be actively secreted by immune cells or passively released by dead, dying, or injured cells. Extracellular HMGB1 has multiple activities and is involved in several processes such as inflammation, immunity, migration, invasion, proliferation, differentiation, antimicrobial defense, and tissue regeneration (Figure 5). Native HMGB1 proteins from eukaryotic sources have the same (though less pronounced) biological activity in vitro compared to recombinant HMGB1 proteins from prokaryotic sources (Zimmermann et al., 2004).
Figure 5.
Extracellular HMGB1 acts as a DAMP with cytokine and chemokine activities that regulate a number of cellular processes.
3.4.1 Cell Differentiation
Cell differentiation is a process in which a less generic cell develops into a more specialized cell type. Many diseases are closely associated with problems from cell differentiation. Thus, it is important to investigate the structure and function of cell differentiation factors in development and disease. It is clear that the level of intracellular HMGB1 correlates with cell differentiation into several cells types such as T cells (Russanova and Ando, 1985), cancer cells (Seyedin et al., 1981), and stem cells (Adjaye et al., 2005; Deneault et al., 2009). Extracellular HMGB1 is also involved in cell differentiation. The first report about extracellular HMGB1 function from Edon Melloni, Bianca Sparatore, and colleagues was published in 1995 (Melloni et al., 1995a, b). They found that HMGB1 promotes MEL cell differentiation if present in cell culture medium. Indeed, the same group originally identified HMGB1 as differentiation-enhancing factor (DEF), which is intracellularly expressed in undifferentiated growing MEL cells (Sparatore et al., 1990). During chemical (e.g., hexamethylenebisacetamide)-induced cell differentiation, HMGB1 is released into the extracellular space in a calcium-dependent manner (Melloni et al., 1995a; Sparatore et al., 1996b; Sparatore et al., 1993a). Once released, HMGB1 binds to receptors on the membrane of MEL and then promotes erythroid differentiation by active protein kinase C, a critical protein in MEL cell differentiation (Melloni et al., 1995a; Passalacqua et al., 1997; Patrone et al., 1996; Sparatore et al., 1996b; Sparatore et al., 1993b). These receptors involved in HMGB1-mediated MEL cell differentiation include the ionotropic glutamate N-methyl-D-aspartate receptor (NMDAR) and unknown 65 kD protein, but not RAGE (Pedrazzi et al., 2012; Sparatore et al., 2002). Structurally, the N-terminal region of HMGB1 is responsible for promoting MEL cell differentiation (Sparatore et al., 1996a). A peptide from extracellular HMGB1 maintains the differentiation stimulatory activity of the whole protein by limited proteolysis (Sparatore et al., 2001). Besides MEL cells, extracellular HMGB1 promotes the differentiation of chronic lymphocytic leukemia (Jia et al., 2014a), stem cells (Pistoia and Raffaghello, 2011), dendritic cells, and T cells.
3.4.2 Inflammatory Response
In 1999, Haichao Wang and colleagues made breakthrough progress in uncovering the extracellular role of HMGB1 in inflammation and infection. They demonstrated that HMGB1 functions as a late mediator with cytokine activity in sepsis, a systemic inflammatory response syndrome resulting from microbial infection. Currently, a number of studies have demonstrated that HMGB1 can selectively bind multiple receptors (e.g., RAGE and TLRs) to active macrophages (He et al., 2012b), monocytes (Andersson et al., 2000), neutrophils (Park et al., 2003; Silva et al., 2007), eosinophils, astrocytes (Pedrazzi et al., 2007), fibroblasts (Guo et al., 2011; Hou et al., 2011b; Hreggvidsdottir et al., 2009), keratinocytes (Dejean et al., 2012), dendritic cells (Yang et al., 2007), natural killer cells, T and endothelial cells (e.g., vascular endothelial cells (Fiuza et al., 2003; Treutiger et al., 2003), airway epithelial cells (Kim et al., 2012a; Wolfson et al., 2011; Wu et al., 2013c), and intestinal epithelial cells (Huang et al., 2012c; Sappington et al., 2002)) to produce cytokines (e.g., TNF (Agnello et al., 2002; Andersson et al., 2000; Kim et al., 2010b; Park et al., 2003; Wu et al., 2013c), IL-1α (Andersson et al., 2000), IL-1β (Andersson et al., 2000; Park et al., 2003), IL-1RA (Andersson et al., 2000), IL-6 (Agnello et al., 2002; Andersson et al., 2000; Hou et al., 2011b; Kim et al., 2010b), IL-8 (Andersson et al., 2000; Dejean et al., 2012; Park et al., 2003; Treutiger et al., 2003; Wu et al., 2013c), IL-10 (Wu et al., 2013c), macrophage inflammatory protein (MIP)-1α (Andersson et al., 2000; Wu et al., 2013c), MIP-1β (Andersson et al., 2000; Wu et al., 2013c), IL-12 (Matsuoka et al., 2010), RANKL (Kim et al., 2010b), IL-11 (Kim et al., 2010b), IL-17 (Kim et al., 2010b)), chemokine (e.g., CCL5 (Pedrazzi et al., 2007), CXCL1 (Pedrazzi et al., 2007), CXCL2 (Pedrazzi et al., 2007), CCL2 (Pedrazzi et al., 2007), CCL20 (Pedrazzi et al., 2007) and CCL3 (Pedrazzi et al., 2007)), adhesion molecules (ICAM-1, VCAM-1 and E-selectin), growth factor (G-CSF (Treutiger et al., 2003)), antigen (CD40 (Matsuoka et al., 2010)) and other inflammatory associated proteins (e.g., tissue factor (TF) (Lv et al., 2009), inducible nitric oxide synthase (iNOS) (Ren et al., 2006; Sappington et al., 2002), mucin 8 (Kim et al., 2012a), inhibitor-signal transducer and activator of transcription-1 (SOCS-1) (Li et al., 2011c)). Molecular mechanisms underlying the potential pro-inflammatory activity of HMGB1 include active several signaling pathways (e.g., P38 (He et al., 2012b; Kim et al., 2010b; Park et al., 2003; Wolfson et al., 2011), ERK (Park et al., 2003), JNK (Kim et al., 2012a; Wu et al., 2013c), PI3K/Akt (Hou et al., 2011b; Kim et al., 2012a), JAK (Li et al., 2011c), Src (Hou et al., 2011b; Huang et al., 2012c), ELK-1 (Treutiger et al., 2003), SIRT1 (Kim et al., 2010b), HSP27 (Wolfson et al., 2011)) and transcriptional factors (NF-κB (Dejean et al., 2012; He et al., 2012b; Hou et al., 2011b; Kim et al., 2010b; Park et al., 2003; Silva et al., 2007; Treutiger et al., 2003; Wu et al., 2013c), STAT1 (Guo et al., 2011; Li et al., 2011c), and EGR1 (Lv et al., 2009)). In addition, high-purity HMGB1 has weak pro-inflammatory activity by itself (Rouhiainen et al., 2007), but can bind with DAMP or PAMP (e.g., IL-1β (Hreggvidsdottir et al., 2009), LPS (Hreggvidsdottir et al., 2009), CpG-ODN (Hreggvidsdottir et al., 2009), Pam3CSK4 (Hreggvidsdottir et al., 2009), lipids (Rouhiainen et al., 2007), DNA or nucleosome) to amplify their pro-inflammatory activity in a synergistic manner (Table 3) (Pisetsky, 2011; Pisetsky et al., 2011). Thus, HMGB1 is an important mediator of acute lung injury (Abraham et al., 2000), brain injury (Agnello et al., 2002), islet loss (Matsuoka et al., 2010), cytoskeletal rearrangement (Wolfson et al., 2011), intestinal barrier disruption (Sappington et al., 2002), vascular barrier disruption (Wolfson et al., 2011), precancerous lesions (Dejean et al., 2012), fibrinolysis (Roussel et al., 2011), and thrombosis (Ito et al., 2007c). Inhibition of HMGB1 release and/or activity significantly decreases the inflammatory response, tissue injury, and death in animals. The importance of HMGB1 as a pro-inflammatory cytokine has been shown in many inflammation- and immunity-associated diseases.
Table 3.
Extracellular Binding Partners for HMGB1
| Name | Function | Receptor | Reference |
|---|---|---|---|
| Inorganic polyphosphate | Amplifies inflammatory signaling | RAGE, P2Y1 | (Dinarvand et al., 2014) |
| CXCL12 | Promoting inflammatory cell recruitment and activation | CXCR4 | (Kew et al., 2012; Schiraldi et al., 2012) |
| DNA | Immunity regulation | TLR9, RAGE | (Jinushi, 2012; Pisetsky, 2012b; Tian et al., 2007) |
| Hemoglobin | Amplifies inflammatory signaling | TLR2, TLR4 | (Lin et al., 2012c) |
| LPS | Amplifies inflammatory signaling | CD14, TLR4 | (Hreggvidsdottir et al., 2012; Qin et al., 2009; Youn et al., 2008) |
| Pam(3)CSK(4) | Amplifies inflammatory signaling | TLR2 | (Hreggvidsdottir et al., 2012) |
| Heparin | Diminishes inflammatory signaling | RAGE | (Ling et al., 2011) |
| Kinked oligonucleotide duplexes | Diminishes inflammatory signaling | Unknown | (Musumeci et al., 2011) |
| IL-1α | Amplifies inflammatory signaling | IL-1R | (Harris et al., 2012; Wahamaa et al., 2011) |
| IL-1β | Amplifies inflammatory signaling | IL-1R | (Garcia-Arnandis et al., 2010b; Harris et al., 2012; Leclerc et al., 2013; Sha et al., 2008; Wahamaa et al., 2011) |
| RNA substrates | Regulates RNA processing as well as transcription | Unknown | (Bell et al., 2008) |
| Nucleosome (histone) | Amplifies inflammatory signaling | TLR2 TLR9 |
(Huang et al., 2011a; Urbonaviciute et al., 2008) |
| Lipids | Amplifies inflammatory signaling | Unknown | (Rouhiainen et al., 2007) |
| Thrombomodulin | Diminishes inflammatory signaling | Unknown | (Abeyama et al., 2005) |
3.4.3 Cell Migration
Cell migration, a central process in the development and maintenance of multicellular organisms, occurs in many physiological and pathological processes (Ilina and Friedl, 2009). Interactions of chemokines and their receptors mediate cell migration. A number of studies have indicated that HMGB1 acts as a potential host cell-derived, chemotactic factor to promote migration of several cell types (Degryse and de Virgilio, 2003). These cells include neurites (Fages et al., 2000), smooth muscle cells (Degryse et al., 2001), myoblast cells (Fages et al., 2000), tumor cells (Fages et al., 2000; Huttunen et al., 2002; Palumbo and Bianchi, 2004; Palumbo et al., 2004), hepatic stellate cells (Wang et al., 2013b), stem cells (Palumbo et al., 2009; Palumbo et al., 2007; Palumbo et al., 2004), endothelial cells (Furlani et al., 2012), keratinocytes (Ranzato et al., 2009), monocytes (Pedrazzi et al., 2007; Rouhiainen et al., 2004), dendritic cells (Dumitriu et al., 2007; Yang et al., 2007), and neutrophils (Orlova et al., 2007; Palumbo et al., 2009; van Zoelen et al., 2009). The potential mechanisms include HMGB1-mediated signaling transduction (e.g., ERK (Degryse et al., 2001; Ranzato et al., 2009), Cdc42 (Fages et al., 2000), Rac (Fages et al., 2000), JNK (Wang et al., 2013b), PI3K/AKT (Wang et al., 2013b) and Src (Palumbo et al., 2009)), transcriptional factor activation (NF-κB), and chemokine production. These findings suggest that extracellular HMGB1-mediated migration maybe contribute to the recruitment of innate immune cells (Degryse et al., 2001) and stem cells (Palumbo and Bianchi, 2004; Palumbo et al., 2004) to sites of infection and injury, wound healing and tissue regeneration (Degryse et al., 2001; Palumbo and Bianchi, 2004; Palumbo et al., 2004), atherosclerosis and restenosis after vascular damage (Degryse et al., 2001), microvascular rolling and adhesion (Furlani et al., 2012), and tumor invasion and metastasis (Huttunen et al., 2002) (Fages et al., 2000; Palumbo and Bianchi, 2004; Palumbo et al., 2004). In general, RAGE is required for HMGB1-mediated cell migration. Interestingly, HMGB1 not only stimulates, but also inhibits migration in some cells. For example, exogenous HMGB1 selectively inhibits VEGF-induced cell migration in pulmonary artery endothelial cells (HPAECs), but not human umbilical vein endothelial cells (HUVECs) (Bauer et al., 2013). Moreover, the IRF3-dependent TLR4 pathway is required for HMGB1-mediated migration inhibition in HPAECs (Bauer et al., 2013).
3.4.4 Tissue Regeneration
Tissue regeneration is a process of regeneration of a wide variety of complex structures, allowing host tissue to regrow after injury or damage. Several dynamic events contribute to tissue regeneration, including wound healing, cell death, dedifferentiation, and stem cell proliferation/recruitment. In addition, the polarity and position of structures in regrown tissues must integrate with preexisting body structures (King and Newmark, 2012). HMGB1 promotes scratch wound closure of keratinocytes via reorganization of the actin cytoskeleton and disruption of the key junctional protein (Ranzato et al., 2009).
Accumulating evidence indicates that HMGB1 can stimulate myocardial regeneration, which may facilitate cardiac repair (Abarbanell et al., 2011; Germani et al., 2007; Limana et al., 2013; Limana et al., 2005), cardiomyocyte hypertrophy (Su et al., 2012), or cardiac fibrosis (Wang et al., 2014e). In a mouse model of myocardial infarction, HMGB1 administration promoted proliferation and differentiation of cardiac stem cells into cardiomyocytes (Limana et al., 2005), suggesting that HMGB1 is a potent inducer of myocardial regeneration following myocardial infarction. HMGB1 regulates electrical remodeling (Liu et al., 2010b), inotropic effect (Hagiwara et al., 2008f), and Notch signaling (Limana et al., 2013), which maybe contribute to heart repair. In addition, HMGB1 leads to cardiac hypertrophy and cardiac fibrosis through activation of calcineurin (a calcium-dependent serine-threonine phosphatase) or upregulation of collagens I/III and TGF-β1, respectively (Su et al., 2012; Wang et al., 2014e). These finding suggests that HMGB1 is a therapy target of cardiac disorder. Except for myocardial regeneration, HMGB1 promotes myogenesis in skeletal muscle by activation of mitogen-activated protein kinase (MAPK), upregulation of myogenin and myosin heavy chain expression, and induction of muscle creatine kinase (De Mori et al., 2007; Sorci et al., 2004). HMGB1 also promotes epithelial regeneration through recruitment of epithelial progenitors to injured tissue (Tamai et al., 2011). HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption (Wolfson et al., 2011). In addition, HMGB1 promotes periodontal remodeling and repair (Wolf et al., 2012; Wolf et al., 2013a; Wolf et al., 2013b; Wolf et al., 2013c).
3.4.5 Angiogenesis
Angiogenesis, or the formation of new blood vessels from pre-existing capillaries, is involved in various physiologic and pathologic processes such as inflammation, wound repair, and tumor growth. In an endothelial-sprouting assay, exogenous HMGB1 induces endothelial cell migration and sprouting in a dose-dependent manner, suggesting that HMGB1 may be involved in angiogenesis (Schlueter et al., 2005). In 2006, Marco Presta and colleagues first confirmed that HMGB1 is an angiogenic molecule (Mitola et al., 2006). They demonstrated that extracellular HMGB1 induces a proangiogenic phenotype in endothelial cells and triggers a potent angiogenic response in vivo in the chicken embryo chorioallantoic membrane (Mitola et al., 2006). They also found that extracellular HMGB1 exerts potent angiogenic activity by activating the MAPK ERK1/2 pathway (Mitola et al., 2006). Activation of HMGB1 and its receptor RAGE results in the activation of NF-κB, which upregulates leukocyte adhesion molecules and the production of pro-inflammatory cytokines and angiogenic factors, thereby promoting inflammation and angiogenesis (van Beijnum et al., 2008). In addition, TLR4 is selectively required for HMGB1-mediated neovascularization (Lin et al., 2011). HMGB1 in complex with heparin also induces angiogenesis (Wake et al., 2009b). HMGB1 stimulates integrin-dependent homing of endothelial progenitor cells in ischemic regions and improves neovascularization (Chavakis et al., 2007). Importantly, HMGB1 has been shown to be upregulated in the serum or tissue specimens of patients with angiogenesis-associated diseases, especially cancer (Yang et al., 2014d). Rapid tumor growth is accompanied by reduced microvessel density, resulting in chronic hypoxia that often leads to necrotic areas within the tumor. These hypoxic and necrotic regions exhibit increased expression of angiogenic growth factors, including vascular endothelial growth factor (VEGF), and attracts macrophages, which also produce a number of potent angiogenetic cytokines and growth factors. Treatment with HMGB1 increases the secretion of VEGF, but not VEGF-C, in human oral squamous cell carcinoma cells. The effect of HMGB1 is abrogated by HMGB1-neutralizing antibody (van Beijnum et al., 2006) and suppression of RAGE expression (Sasahira et al., 2007).
3.4.6 Bacterial Killing
In 2002, Cecilia K. Zetterström and colleagues first found that HMGB1 is produced by human adenoid tissue and exerts potent antibiotic activity. The kinetics of bacterial killing by HMGB1 was found to be rapid within a time frame of seconds or minutes (Zetterstrom et al., 2002). Later, the same group discovered that HMGB1 is highly expressed by Sertoli cells in human as well as rat testes. The purification of HMGB1 from human and rat testes by reversed-phase high-performance liquid chromatography also exerts significant antibiotic activity against several types of bacteria (Zetterstrom et al., 2006). These findings suggest that HMGB1 may facilitate the first line of defense against invading bacteria. NET is an important mechanism of bacterial killing. Interestingly, HMGB1 promotes NET formation through interactions with TLR4 (Tadie et al., 2013), suggesting that HMGB1 may promote NET-mediated bacterial killing. However, exogenous HMGB1 also has the ability to diminish neutrophil-mediated bacterial killing after HMGB1 binds to RAGE (Tadie et al., 2012a). These findings suggest that HMGB1-mediated bacterial killing occurs in a receptor-dependent way.
3.4.7 Proliferation
HMGB1 activates cell proliferation signals in several cells, including smooth muscle cells (Porto et al., 2006), T cells (Sundberg et al., 2009), mesoangioblasts (Palumbo et al., 2004), cardiac stem cells (Limana et al., 2005), and various cancer cells. HMGB1 is not only released by smooth muscle cells, but also promotes the proliferation and migration of smooth muscle cells, which facilities atherosclerotic plaque formation (Porto et al., 2006). In vitro, HMGB1 induces the migration and proliferation of both adult and embryonic mesoangioblasts and disrupts the barrier function of endothelial monolayers (Palumbo et al., 2004), although some reports suggest that HMGB1 is a proliferation inhibitor of human mesenchymal stem cells (Meng et al., 2008). HMGB1 promotes proliferation of cardiac stem cells and myocardiocytes, which facilitates tissue regeneration (Limana et al., 2005) and cardiomyocyte hypertrophy (Su et al., 2012; Wang et al., 2014e). HMGB1 regulates the proliferation of lymphocytes in a time- and dose-dependent manner (Wang et al., 2008b). However, HMGB1 only acts as a proliferation signal for T cells in the presence of suboptimal doses of anti-CD3 antibody and RAGE (Sundberg et al., 2009). In cancer cells, reduced HMGB1 promotes proliferation, whereas oxidized HMGB1 induces apoptosis. Thus, the redox state of HMGB1 regulates tumor cell survival and death.
3.4.8 Cell Death
Several reports have indicated that an excessive accumulation of extracellular HMGB1 is cytotoxic and leads to cell death (e.g. apoptosis and necrosis) and tissue injury (Kikuchi et al., 2009a). In addition, HMGB1 can induce a special form of cell death in glioblastoma cells, which lack the typical features of apoptosis, autophagy, or classic necrosis (Gdynia et al., 2010). Exogenous HMGB1 can enter host mitochondria by an endocytosis-independent mechanism and result in the formation of vacuolated giant mitochondria and a rapid depletion of mitochondrial DNA (Gdynia et al., 2010). Exogenous HMGB1 can rhHMGB1 localizes to the mitochondria and induces the formation of giant mitochondria, which is independent of TLR2, TLR4, or RAGE signaling (Gdynia et al., 2010). However, reactive oxygen species-mediated JNK activation is required for this process (Gdynia et al., 2010). Future studies will have to examine the molecular basis of HMGB1 uptake and subsequent giant mitochondria formation.
3.4.9 Cellular Senescence
Cellular senescence is a state of permanent cell-cycle arrest when proliferating cells respond to stress, including oncogenetic stress (Campisi and d'Adda di Fagagna, 2007). In addition to cell death, senescence is considered an important mechanism for mammalian cells to suppress tumorigenesis. During senescence, HMGB1 translocates from the nucleus to the cytoplasm and is then released to the extracellular space (Davalos et al., 2013). Once released, oxidized HMGB1 binds to TLR4 and induces IL-6 production and release, which promotes age-associated inflammation. Interaction between HMGB1 and p53 determine the onset of senescence. p53, but not ATM, is required for HMGB1 translocation as well as altered HMGB1 expression (downregulation and upregulation)-induced senescence (Davalos et al., 2013).
3.4.10 microRNA Biogenesis
microRNAs (miRNAs) are approximately 22-nucleotide small RNAs that act as negative or positive gene transcriptional regulators involved in human health and disease. Several reports have indicated a potential role of HMGB1 in the regulation of microRNA expression involved in inflammation, cardiac remodeling, and cancer. The microRNA expression profile is significant in human peripheral blood mononuclear cells (PBMCs) following DAMP and PAMP stimulation. In particular, miR-34c expression in human PBMCs depends on the presence of HMGB1 within cells serving as a source of lysates or conditioned media from stressed cells (Unlu et al., 2012). HMGB1 increases the expression of miR-206, which contributes to downregulation of tissue inhibitor of metalloproteinase 3 (TIMP3), a physiological regulator of cardiac regeneration (Limana et al., 2011). In addition, the binding of HMGB1 to RAGE increases the expression of miR-221 and miR-222 in papillary thyroid cancer cells, which facilitates tumor growth and migration (Mardente et al., 2012). These findings suggest that HMGB1 facilitates microRNA biogenesis, although the molecular mechanism remains unknown.
3.4.11 Efferocytosis
Efferocytosis is an uptake process of apoptotic cells by macrophages and other phagocytic cell populations. Efferocytosis dysfunction may lead to excessive accumulation of late apoptotic and/or secondary necrotic cells and subsequent inflammatory response. Compared with milk fat globule-EGF factor 8 (MFG-E8) (Miksa et al., 2009; Wang et al., 2013k), HMGB1 is a negative regulator of efferocytosis by its C-terminal acidic tail (Banerjee et al., 2010) and poly(ADP-ribosyl)ation (Davis et al., 2012b). Extracellular HMGB1 inhibits efferocytosis by binding phosphatidylserine or ανβ3 integrin in apoptotic neutrophils or phagocytic macrophages, respectively (Friggeri et al., 2010; Liu et al., 2008a). Compared with unmodified HMGB1, PARylated HMGB1 has a higher affinity for phosphatidylserine and RAGE (Davis et al., 2012b). Thus, extracellular HMGB1-mediated efferocytosis inhibition may enhance inflammatory responses during apoptosis. Interestingly, intracellular HMGB1 is also a negative regulator of efferocytosis by binding to Src (Banerjee et al., 2011), whereas RAGE enhances efferocytosis in macrophages by binding to PtdSer (Friggeri et al., 2011; He et al., 2011) and to free DNA. It is thus important to determine why HMGB1 and RAGE both interact with PtdSer, but show opposite outcomes to efferocytosis.
3.4.12 Neurotransmitters
Neurotransmitters are endogenous chemicals (e.g., acetylcholine, norepinephrine, dopamine, gamma aminobutyric acid, glutamate, serotonin, and endorphin) that transmit signals from one neuron to the next across synapses. The vagus nerve can modulate systemic inflammation and HMGB1 release through the alpha7 nicotinic acetylcholine receptor (alpha7nAchR), suggesting a potential role of neurotransmitters in the regulation of HMGB1 release. In contrast, extracellular HMGB1 also has the ability to stimulate the release of glutamate and its analogues from gliosomes by binding to RAGE and glial glutamate-aspartate transporter (GLAST), suggesting a critical role of HMGB1 in the regulation of glutamate homeostasis in the brain (Bonanno et al., 2007).
3.4.13 Immune Response
The immune system is divided into two parts, the innate and adaptive systems, which protect the body against pathogens, destroying cancer cells and foreign substances. The innate immune system, including several cell types (e.g., macrophages, neutrophils, mast cells, basophils, eosinophils, dendritic cells, natural killer cells, and γδ T cells) is the first line of defense immediately available to fight against invading microorganisms. The adaptive immune response depends on B and T lymphocytes, which are specific for particular antigens and typically takes four to seven days to respond. It is clear that HMGB1 plays a critical role in the regulation of innate and adaptive immune responses by direct effects or cell-cell interaction (Bianchi and Manfredi, 2007; Dumitriu et al., 2005b; Manfredi et al., 2009). HMGB1 acts as an adjuvant for several vaccines (Grover et al., 2013; Li et al., 2013b; Li et al., 2011g; Wang et al., 2013h).
3.4.13.1 Macrophages
Macrophages differentiate from circulating PBMCs, common progenitor cells for many cell types such as dendritic cells and mast cells. Macrophages are important effector cells of the immune system that contribute to host defense, wound healing, and immune regulation. Many pro-inflammatory stimuli can induce macrophage secretion of HMGB1 into the extracellular space. Extracellular HMGB1 can further prolong the inflammatory response by stimulating macrophages to produce cytokines/chemokine by TLR2, TLR4, or RAGE (He et al., 2012b). In addition, HMGB1 has the ability to inhibit macrophage-mediated efferocytosis, which prevents macrophages from clearing dead cells and DAMP release (Friggeri et al., 2010; Liu et al., 2008a). Interestingly, pretreatment with HMGB1 leads to endotoxin and lipoteichoic acid tolerance in bone marrow-derived macrophages and the acute monocytic leukemia cell line THP-1 by downregulation NF-KB activity (Aneja et al., 2008; Robert et al., 2010). However, RAGE is required for HMGB1-mediated endotoxin tolerance, whereas TLR2 and TLR4 are required for HMGB1-mediated lipoteichoic acid tolerance (Aneja et al., 2008; Robert et al., 2010). More recently, endogenous HMGB1 has been found to be required in endotoxin tolerance in macrophages (Li et al., 2013d), and conditional knockout of HMGB1 in macrophages was found to cause animal death in response to endotoxemia and bacterial infection (Yanai et al., 2013). Thus, HMGB1 can activate or inhibit macrophage function in the innate immune response, which maybe depend on receptors, location, and reaction phase.
3.4.13.2 Neutrophils
Neutrophils, eosinophils, and basophils are known as granulocytes due to the presence of multi-lobed nuclei and granular cytoplasm. Neutrophils, also called polymorphonuclear cells (PMNs), are the most abundant leukocyte and kill microorganisms by phagocytosis, a process which typically leads to cytoplasmic vacuolar degeneration by production of reactive oxygen species. HMGB1 is an effective stimulus of neutrophil activation to produce cytokines (Park et al., 2003; Silva et al., 2007). Like in macrophages, HMGB1 also inhibits phagocytosis of apoptosis by neutrophils (Liu et al., 2008a). In contrast, HMGB1 mediates NET formation in neutrophils and facilitates type I IFN production in a TLR9-dependent way in systemic lupus erythematosus (Garcia-Romo et al., 2011).
3.4.13.3 Mast Cells
Mast cells are derived from haematopoietic stem cells and reside particularly in connective tissue and in the mucous membranes. When activated, mast cells can release granule-associated mediators such as histamine and heparin, which induce immediate allergic inflammation with recruitment of basophils, neutrophils and macrophages (Metcalfe et al., 1997). There is no information about the direct effect of exogenous HMGB1 on mast cell activity. Mast cell- deficient mice are protected from trauma partly through decreased circulating HMGB1 levels, suggesting a possible role of mast cells in the regulation of HMGB1 release (Cai et al., 2011). Similarly, mast cells were able to regulate cell death-mediated HMGB1 release in an animal sepsis model (Ramos et al., 2010). However, the mast cell effector histamine cannot induce HMGB1 release in rheumatoid arthritis (Adlesic et al., 2007). In contrast, histamine directly inhibits HMGB1 activity (Takahashi et al., 2013a) and mast cell protease chymase directly degrades HMGB1 (Roy et al., 2014). These findings suggest that mast cells have different roles in the regulation of HMGB1 release, activity, and levels during the inflammatory response.
3.4.13.4 Basophils
Basophils are an extremely rare type of granulocyte containing cytoplasmic granules that stain with basophilic dyes. When activated, basophils can release histamine, which is important in the development of allergic reactions such as asthma and allergic rhinitis. In addition, basophils play roles in the defense against parasites. Although sputum or serum HMGB1 is increased in patients with asthma (Watanabe et al., 2011) and allergic rhinitis (Salpietro et al., 2013), respectively, the direct interaction between HMGB1 and basophils is currently unknown.
3.4.13.5 Eosinophils
Eosinophil, a type of granulocyte, contains cytoplasmic granules that are easily stained by eosin or other acid dyes. When activated, eosinophils can release eosinophil granule proteins such as major basic protein (MBP), eosinophil peroxidase (EPO), eosinophil-derived neurotoxin (EDN), and eosinophilic cationic protein (ECP), which are implicated in the pathogenesis of numerous inflammatory processes such as parasites infection, asthma, allergy, and tumor (Lotfi et al., 2007). Exogenous HMGB1 has the ability to induce eosinophil migration, adhesion, survival, and degranulation (Lotfi et al., 2009). RAGE may be required for HMGB1-mediated eosinophil activation (Curran and Bertics, 2011; Lotfi et al., 2009). Upregulation of HMGB1 contributes to the pathogenesis of eosinophilic chronic rhinosinusitis with nasal polyps (Chen et al., 2014b).
3.4.13.6 Dendritic Cells
Dendritic cells (DCs) are the most powerful antigen-presenting cells (APCs) which play critical roles not only in the defense of microbial pathogens in the innate immune system but also in the regulation of the adaptive immune response. Major DC functions include antigen presentation and subsequent activation of T cells, immune tolerance, and immune memory. DC-based immunotherapy is an important way to treat human diseases, especially cancer and autoimmune disease. Increasing evidence from in vitro and in vivo studies suggests that HMGB1 is a critical regulator of DC maturation and function. Activated DCs (Tsung et al., 2007b) and other inflammatory cells, as well as dying cells, can release HMGB1 into the extracellular milieu (Dong Xda et al., 2007; Semino et al., 2005; Zou et al., 2013). This released HMGB1 further promotes DC as well as human plasmacytoid DC (Dumitriu et al., 2005a) maturation and migration (Campana et al., 2009) (Dumitriu et al., 2007) via several receptors such as RAGE (Manfredi et al., 2008; Tian et al., 2007), TLR2 (Curtin et al., 2009), TLR4 (Fang et al., 2013a), and TLR9 (Dumitriu et al., 2005c; Messmer et al., 2004; Tian et al., 2007). In addition, binding of HMGB1 to other DAMPs (nucleosome) (Urbonaviciute et al., 2008) or PAMPs (CpG-DNA) (Ivanov et al., 2007) can promote DC maturation. Released HMGB1 mediates natural killer cell-DC crosstalk (Balsamo et al., 2009; Melki et al., 2010; Saidi et al., 2008; Semino et al., 2005). HMGB1 suppresses the human plasmacytoid DC response to TLR9 agonists (Popovic et al., 2006). The interaction between HMGB1 and TLR4 is required for the DC-mediated antitumor immune response in conventional anticancer therapies such as chemotherapy and irradiation (Aguilera et al., 2011; Apetoh et al., 2007; Apetoh et al., 2008; Fucikova et al., 2011). In some cases, HMGB1 directly inhibits DC function in anti-cancer immunity (Kusume et al., 2009). In contrast, TIM3 decreases this effect (Chiba et al., 2012b; Tang and Lotze, 2012).
3.4.13.7 NK Cells
Natural killer (NK) cells, a type of lymphocyte, play a major role in the early phase of host-rejection to both tumors and virally-infected cells. When activated by interferon or macrophage/monocyte-derived cytokines, NKs release cytotoxic molecules such as granzymes and perforin, which lead to the destruction and killing of binding non-self cells and viruses. In addition to cytotoxic molecules, NKs secrete several cytokines, including IFN-γ, TNF-α, IL-12 and HMGB1. IL-18 contributes to HMGB1 release from NK-stimulated immature DCs. Once released, HMGB1 promotes DC maturation and limits NK’s cytotoxicity during NK-immature DC crosstalk (Semino et al., 2005; Semino et al., 2007). HMGB1-mediated NK-DC crosstalk is important in the regulation of HIV infection and viral replication partly by inhibition of apoptosis (Gougeon and Bras, 2011; Gougeon et al., 2012; Melki et al., 2010; Saidi et al., 2008). In addition to secretion, NK cells can passively release HMGB1 after acute toxic liver damage, which is CXCR3-dependent (Zaldivar et al., 2012). In addition, HMGB1 binding to TLR4 increases expression of natural killer group 2D (NKG2D) ligands by renal tubular epithelial cells (Chen et al., 2011a). HMGB1 combined with other cytokines (IL-2, IL-1, or IL-12) contributes to IFN-γ release from macrophage-stimulated NK cells (DeMarco et al., 2005). These findings suggest that HMGB1 regulates NK cell function at multiple levels such as cytotoxicity, cytokine release, and ligand expression.
3.4.13.8 T Cells
T cells, a type of lymphocyte, play a central role in cell-mediated adaptive immune responses. A specific combination of cytokine signals leads to the differentiation of naïve T cells into different effectors (Th1, Th2 and Th17) and regulatory T cell subsets. T cells can release HMGB1 in response to several stimuli or cell-cell contact in co-cultures (Jiang et al., 2013; Kawahara et al., 2007). In addition, HMGB1 released from DCs regulates polarization of CD4+ T cells (Messmer et al., 2004) and mediates cell-cell interactions (Kohka Takahashi et al., 2013). HMGB1 at low doses has no effect on the proliferation activity of CD4+ T cells, but promotes Th1 cytokine production. In contrast, HMGB1 at high doses suppresses the proliferative response and induces Th2 polarization of CD4+ T lymphocytes. HMGB1 also induces differentiation of splenic DCs to IL-10-producing CD11clowCD45RBhigh DCs, which in turn suppresses T lymphocyte function with shifting of Th1 to Th2 in vitro (Liu et al., 2011d). HMGB1 mediates proliferation of T cells, including CD4+ and CD8+, in response to suboptimal anti-CD3 antibody stimulation. (Sundberg et al., 2009). In addition to effector T cells, HMGB1 regulates the proliferation, function, and balance of regulator T cells (e.g., Treg and Th17). For example, HMGB1 inhibits CTLA4 and Foxp3 expression, as well as IL-10 release in Treg cells in a RAGE-dependent way (Dumitriu et al., 2005c; Zhang et al., 2011f). HMGB1 is a chemoattractant for Treg and promotes its survival and suppressive function (Wild et al., 2012a), indicating a pro-tumor role of HMGB1 in the tumor microenvironment (Wild et al., 2012b; Zhang et al., 2011f). Indeed, HMGB1 suppresses CD8 T cell-dependent antitumor immunity via enhancing Treg-mediated immune suppression by production of IL-10 (Liu et al., 2011f). However, in some cases, HMGB1 treatment induces downregulation expressions of Treg cell phenotypes (Huang et al., 2012b). These findings suggest a dual role of HMGB1 in the regulation of Treg. In contrast, HMGB1 promotes Th17 cell proliferation, differentiation, and activation in the setting of several autoimmune and inflammatory diseases, such as rheumatoid arthritis (He et al., 2012d; Shi et al., 2012b), myocarditis (Su et al., 2011), acute allograft injection (Duan et al., 2011), chronic hepatitis B (Li et al., 2014a).
γδ T cells represent a small subset of T cells that possess a distinct T-cell receptor (TCR) on their surface, which is important in innate immune responses, especially in the mucosal immunity of gut, respiratory tract and urogenital system. The HMGB1-TLR4-IL-23 pathway in macrophages induces the generation of IL-17-producing γδ T cells, which mediate neutrophil infiltration and damage-induced liver inflammation (Wang et al., 2013l).
3.4.13.9 B Cells
B cells, a type of lymphocyte, play a major role in making antibodies against antigen, performing the role of antigen-presenting cells in activation of immune memory. Moreover, B cells also secrete cytokines, especially IL-10, to inhibit the inflammatory response. This special subset is termed regulatory B cells (Mauri and Bosma, 2012). Several studies indicate that HMGB1 has a role in the regulation of B cell activation. Single HMGB1 or HMGB1-DNA immune complex promotes the proliferation and activation of auto reactive B cells, which requires several receptors including RAGE, TLR9, or B cell receptor (Avalos et al., 2010; McDonnell et al., 2011; Tian et al., 2007). In addition, the serum level of HMGB1 significantly correlates with autoantibody production (e.g., anti-dsDNA antibody) in patients with autoimmune diseases such as systemic lupus erythematosus (Wen et al., 2013), suggesting a potential role of HMGB1 in B cell-mediated antibody production. The function of HMGB1 in regulatory B cells remains unknown.
3.4.13.10 MDSC Cells
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of myeloid origin cells including myeloid progenitors and immature macrophages, immature granulocytes, and immature DCs at different stages of differentiation (Gabrilovich and Nagaraj, 2009). MDSCs are known to have remarkable ability to suppress both the cytotoxic activities of NK and NKT cells and the adaptive immune response mediated by CD4+ and CD8+ T cells during cancer and inflammation (Gabrilovich and Nagaraj, 2009). HMGB1 promotes recruitment and migration of MDSCs in the tumor microenvironment, which contributes to colon cancer metastasis after curative surgery (Li et al., 2013f). In addition, IL-17 and IL-23 production from the activated HMGB1-RAGE pathway increases accumulation of MDSCs in melanoma tumor tissues, which contributes to tumor growth. In addition, loss of RAGE in mice markedly delays oncogentetic K-RAS-derived neoplasia formation (Kang et al., 2012b) and limits MDSC accumulation (Vernon et al., 2013). These findings suggest that the HMGB1-RAGE pathway promotes tumor growth and metastasis partly by increasing MDSC accumulation and limiting the antitumor immune response.
4 HMGB1 Release
4.1 Active Release
In response to exogenous microbial products (such as endotoxin (Wang et al., 1999), CpG-DNA (Ivanov et al., 2007) (Jiang et al., 2005) lysophosphatidylcholine (LPC) (Gardella et al., 2002), or mycobacterial infection (Grover et al., 2008)) or endogenous host stimuli (e.g., TNF-α (Wang et al., 1999), IFN-α (Jiang and Pisetsky, 2006), IFN-β (Lu et al., 2014), IFN-γ (Rendon-Mitchell et al., 2003), hydrogen peroxide (Tang et al., 2007e), nitric oxide (Tamura et al., 2011), peroxynitrite (Loukili et al., 2011), hyperlipidemia (Haraba et al., 2011a), hyperglycemia (Kim et al., 2011a), kynurenic acid (Tiszlavicz et al., 2011), neuropeptide Y (Zhou et al., 2013a), ATP (Eun et al., 2014)) or other stimuli (ethanol (Whitman et al., 2013), photodynamic therapy (Korbelik et al., 2011), natural DNA or synthetic oligonucleotides (Jiang and Pisetsky, 2008b), and ultraviolet B (Chakraborty et al., 2013)), immune cells (e.g., macrophages, monocytes, neutrophils, DCs, NKs), fibroblasts, or epithelial cells actively release HMGB1 into the extracellular space. HMGB1 cannot be actively secreted via the classical endoplasmic reticulum-Golgi secretory pathway due to lack of a leader signal sequence. Instead, several mechanisms have been reported to be involved in HMGB1 translocation from the nucleus to the cytoplasm and subsequent release (Figure 6).
Figure 6.
HMGB1 is released in two different ways: active secretion and passive release.
4.1.1 Post Transcriptional Modifications
In 2003, Alessandra Agresti, Marco E. Bianchi, and colleagues first demonstrated that HMGB1 is acetylated at two NLS by PCAF, CBP, and p300 which results in HMGB1 cytoplamsic translocation and secretion by activated monocytic cells (Bonaldi et al., 2003). Similarly, both deacetylase inhibitors (TSA) and mimics acetylated lysine by mutation of six lysine to glutamine, causing the relocalization of HMGB1 from the nucleus to the cytoplasm (Bonaldi et al., 2003). Recent studies suggest that JAK/STAT1 mediates HMGB1 acetylation (Lu et al., 2014). Sirtuin 6 (SIRT6) belongs to the sirtuin family of NAD (+)-dependent deacetylases and has been implicated in the regulation of oxygen/glucose deprivation -induced HMGB1 release (Lee et al., 2013d). In addition to acetylation, phosphorylation and ADP-ribosylation may be other requisite steps for HMGB1 nucleocytoplasmic translocation. In particular, classical protein kinase C mediates HMGB1 phosphorylation (Oh et al., 2009). These findings suggest that the interaction between different post transcriptional modifications finally determines HMGB1 location. However, we still do not know whether these post transcriptional modifications are competitively, cooperatively, or independently regulated.
4.1.2 CRM1-mediated Nuclear Export
CRM1, a member of the importin β superfamily of nuclear transport receptors, recognizes and exports proteins containing a leucine-rich NES (Hutten and Kehlenbach, 2007). In addition to protein nuclear export, CRM1 also mediates RNA transport. In addition, CRM1 is also involved in centrosome duplication and spindle assembly during mitosis. Several inflammatory stimuli can enhance the interaction between CRM1 and HMGB1, and CRM1 inhibitor significantly inhibits HMGB1 translocation from the nucleus to the cytoplasm. Thus, CRM1 is the nuclear transport of HMGB1. In response to oxidative stress, cytoplasmic Hsp72 translocates to the nucleus, where it interacts with nuclear proteins including HMGB1, and prevents oxidative stress-induced HMGB1 cytoplasmic translocation and release (Tang et al., 2007b). Moreover, overexpression of Hsp72 inhibits CRM1 translocation and interaction between HMGB1 and CRM1 in macrophages following LPS or TNF-α treatment (Tang et al., 2007c).The expression of CRM1 is unregulated in several cancers and is a potential anti-cancer drug target. It is unclear whether the change of CRM1 expression in normal and cancer cells will cause HMGB1 translocation and release in cancer.
4.1.3 ROS Signaling Pathway
Reactive oxygen species (ROS) are free radicals that contain the oxygen atom, which are generated during various metabolic and biochemical reactions. ROS include superoxide anion (O2•−), hydroxyl radical (•OH), hydrogen peroxide (H2O2), and singlet oxygen (1O2). ROS have multifarious effects in signal transduction, but in excess can result in oxidative damage, cell death, and various diseases. It is clear that ROS are the major signals responsible for both HMGB1 active and passive release in immune and non-immune cells. H2O2 actives the MAPK and NF-κB pathways, which in turn promotes HMGB1 release in macrophages and monocytes (Tang et al., 2007e). Antioxidants such as NAC (Tsung et al., 2007a), quercetin (Tang et al., 2009), edaravone (Kato et al., 2009), pyrrolidine dithiocarbamate (Zhang et al., 2010), and resveratrol (Delucchi et al., 2012) significantly inhibit HMGB1 release in several animal infection and injury models.
Heme oxygenase-1 (HO-1) is a cytoprotective enzyme that plays a critical role in defending the body against oxidant-induced injury during inflammatory processes. HO catalyzes the first and rate-limiting step in the oxidative degradation of heme to CO, biliverdin, and ferrous iron. Biliverdin and CO have anti-inflammatory properties. Increasing evidence suggests that loss of HO-1 increases HMGB1 release, whereas upregulation of HO-1 inhibits HMGB1 release in response to inflammatory stimulus (Chen et al., 2013b; Clerigues et al., 2012; Garcia-Arnandis et al., 2010a; Gong et al., 2008; Jang et al., 2012; Park et al., 2013; Sakai et al., 2012; Tsoyi et al., 2009; Wang et al., 2013g; Yun et al., 2010).
The nuclear factor erythroid 2-related factor (Nrf2) is a master transcription factor that regulates redox balance and stress response by controlling the basal and induced expression of an array of antioxidant response element-dependent genes. Loss of NRF2 decreased HO-1 expression and increased HMGB1 release in CLP-induced sepsis and ischemic/reperfusion injury (Ha et al., 2011b; Ha et al., 2012b; Hwa et al., 2012; Kim et al., 2013b; Park et al., 2013; Tsoyi et al., 2011a; Wang et al., 2014c).
4.1.4 Calcium Signaling Pathway
Calcium ions, one type of important intracellular messenger, regulate multiple cellular processes by exerting allosteric regulatory effects on many enzymes and proteins. Numerous proteins, channels, and pumps regulate calcium ion production and move between the cytosol and intracellular stores. Calcium-mediated signaling is involved in HMGB1 nucleocytoplasmic shuttling and release during infection (Zhang et al., 2008c) and sterile inflammation (Tsung et al., 2007a). This process is tightly regulated by calcium/calmodulin-dependent protein kinase (CaMK) I and IV, which promote serine phosphorylation of HMGB1 (Zhang et al., 2011d; Zhang et al., 2008c). In addition, calcium regulates PKC activity and the IFN-β signaling pathway, which is also involved in HMGB1 release (Ma et al., 2012b; Oh et al., 2009). Thus, inhibition of calcium signaling by inhibitors (e.g., STO609 and CV159) or knockdown/knockout of CaMK I and IV decrease HMGB1 release and protect animals against ischemia/reperfusion injury or sepsis (Hataji et al., 2010; Tsung et al., 2007a; Zhang et al., 2008c).
4.1.5 NO Signaling Pathway
Nitric oxide (NO) is an important cellular signaling molecule produced by NO synthase from L-Arginine, O2, and NADPH. It is a powerful vasodilator with a short half-life of a few seconds in the blood and plays a role in many physiological and pathological processes. In macrophages, an inducible NO synthase (iNOS or NOS2) is produced after activation by endotoxins or cytokines and generates copious amounts of NO presumably to help kill or inhibit the growth of invading microorganisms or neoplastic tissue. However, excessive iNOS will cause inflammation partly by promoting HMGB1 release (Tsoyi et al., 2010). In addition, HMGB1 also induces iNOS expression, facilitating the development of inflammatory injury (Ren et al., 2006).
4.1.6 TNF-α Dependent Mechanism
IFN-γ and LPS stimulate macrophages to release TNF and HMGB1 in a time-dependent manner. Interestingly, the release of HMGB1 by IFN-γ and LPS depends on TNF production. TNF inhibition by using TNF−/− macrophages or TNF-neutralizing antibodies partly inhibits IFN-γ- and LPS-induced HMGB1 release in macrophages, suggesting that a TNF-dependent mechanism is involved in HMGB1 release (Chen et al., 2004; Rendon-Mitchell et al., 2003). In addition, CD14 and JAK/STAT1 act as upstream signal to regulate TNF production and HMGB1 release in response to LPS and IFN-γ, respectively (Rendon-Mitchell et al., 2003). Thus, there is crosstalk between different signals to regulate HMGB1 release from activated macrophages.
4.1.7 Notch Dependent Mechanism
Notch signaling is a highly conserved pathway involved in diverse developmental and physiological processes. Dysregulation of Notch signaling is associated with several human disorders, especially cancer. In mammals, there are four Notch receptors (Notch-1 to -4) and five Notch ligands (Delta-like-1, -3, and -4 and Jagged-1 and -2). The expression of Jagged-1 is increased in LPS-induced macrophages, which is JNK-dependent. A potent Notch inhibitor, DAPT, inhibits LPS-induced HMGB1 release in macrophages. In addition, recombinant soluble Jagged-1, Delta-like-1, or -4 amplified LPS induced inflammatory responses. These findings suggest that LPS-induced Notch signaling activation regulates HMGB1 release (Tsao et al., 2011).
4.1.8 NF-κB-Dependent Mechanism
Activation of NF-κB plays a central role in inflammation and immunity through its ability to induce production and release of multiple pro-inflammatory cytokines and chemokines (Oeckinghaus et al., 2011). There are two major NF-κB pathways, namely the canonical and the noncanonical pathways, in mammals. In the canonical pathway, NF-κB/p65 is retained in the cytoplasm until it is activated in response to several stimuli such as cytokines, LPS, growth factors, and antigen receptors. After activation by phosphorylates, IκB proteins, and its subsequent proteasomal degradation, NF-κB/p65 translocates to the nucleus to induce targeted gene expression either alone or in combination with other transcription factor families. Inhibition of the canonical NF-κB pathway decreases HMGB1 release in activated immune cells (Wang et al., 2013a; Watanabe et al., 2013; Yang et al., 2014a; Zhang et al., 2010). However, the essential role of NF-κB in the regulation of HMGB1 release remains a subject of future investigation.
4.1.9 MAPK-Dependent Mechanism
MAPKs, a family of highly-conserved serine/threonine protein kinases, are involved in the regulation of a number of cellular processes, including inflammation, immunity, differentiation, and cell survival and death. There are three major classes of MAPKs in mammals: ERK, JNK, and p38, which divergently contribute to HMGB1 release in different inflammatory and injury models (Zhou et al., 2013a).
4.1.10 STAT-Dependent Mechanism
STATs (signal transducers and activators of transcription) are transcription factors that contain STAT1–6. STAT1 and STAT2 have a central role in the interferon system. Binding of a growth factor or cytokine to its cell surface receptor results in the activation of the Janus kinases (JAK1 and JAK2), which in turn, induce STAT phosphorylation, dimerization, nuclear translocation, and STAT-mediated gene transcription. Increasing evidence suggests that JAK-mediated STAT1 and STAT3 activation is required for HMGB1 expression, modification, and/or release under several stressors such as LPS, IFN, and mechanical stress (Hao et al., 2013; Hui et al., 2009; Kim et al., 2009; Liu et al., 2007a; Wolfson et al., 2013). Thus, inhibition of the JAK/STAT pathway prevents HMGB1 release and protects against sepsis and ischemic reperfusion injury (Hui et al., 2009; Pena et al., 2010). Exogenous HMGB1 has the ability to induce activation of the STAT1 and STAT3 pathways (Conti et al., 2013; Guo et al., 2011; Li et al., 2011c). These findings suggest that a loop exists between HMGB1 and STAT1/3 signaling in inflammation.
4.1.11 Inflammasome-Dependent Mechanism
Inflammasome, a multiprotein oligomer, is activated by DAMPs and PAMPs that link the sensing of microbial products and metabolic stress to the proteolytic activation of proinflammatory cytokines such as IL-1β and IL-18 (Schroder and Tschopp, 2010). Inflammasomes have recently been shown to play an important role in mediating HMGB1 release from activated immune cells (Barlan et al., 2011; Craven et al., 2009; Lamkanfi et al., 2010; Lippai et al., 2013; Willingham et al., 2009; Willingham et al., 2007) or cancer cells (Miller et al., 2014). Thus, inflammasome-deficient mice or inflammasome inhibitor can inhibit HMGB1 release and protects mice against sepsis or I/R injury (Kamo et al., 2013; Xiang et al., 2011; Zhu et al., 2011b). LPS can induce ATP release and P2Y2 receptor upregulation, which in turn triggers the activation of inflammasome and enhanced HMGB1 release (Eun et al., 2014). Importantly, this process is mediated by double-stranded RNA (dsRNA)-dependent protein kinase (PKR), which promotes inflammasome assembly by directly interacting with inflammasome components (e.g., NOD-like receptor (NLR) family pyrin domain-containing 3 (NLRP3), NLRP1, NLR family CARD domain containing protein 4 (NLRC4), and absent in melanoma 2 (AIM2)) (Lu et al., 2012a; Schroder and Tschopp, 2010). Thus, loss of PKR or using PKR inhibitor in immune cells (macrophages and DCs) significantly inhibits inflammasome activation and release of IL-1β, IL-18, and HMGB1 (Li et al., 2013e; Lu et al., 2012b). Of note, some inflammasome components such as NLRP3 regulate HMGB1 release through inflammasome-independent pathways (Willingham et al., 2009). It is unclear whether other inflammatory signaling pathways are involved in inflammasome-mediated HMGB1 release (Lamkanfi and Dixit, 2011).
4.1.12 p53-Dependent Mechanism
HMGB1 and p53 form a complex that regulates DNA repair and the balance between tumor cell death and survival (Livesey et al., 2012c). Loss of HMGB1 increases p53 cytoplasmic translocation, whereas loss of p53 increases HMGB1 cytoplasmic translocation in colon cancer cells, suggesting that the HMGB1/p53 complex affects the cytoplasmic localization of the reciprocal binding partner (Livesey et al., 2012c). However, cytoplasmic translocation in hepatocytes and circulating levels of HMGB1 are greater in wild type rats than in p53+/− rats following carcinogen administration, suggesting that p53 promotes inflammation-associated hepatocarcinogenesis by inducing HMGB1 release (Yan et al., 2013a).
4.1.13 PPAR-Dependent Mechanism
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear hormone receptor family and function as transcription factors regulating gene expression. There are three PPARs (PPARα, PPARβ/δ, and PPARγ), which play essential roles in the regulation of cellular differentiation, development, metabolism, inflammation, and tumorigenesis. Recent studies indicate that PPAR is a negative regulator of HMGB1 release in activated macrophages after treatment with LPS or Poly (I:C). The administration of PPARγ ligand rosiglitazone protects against sepsis and decreases HMGB1 release in vivo and in vitro (Hwang et al., 2012).
4.1.14 Cell-Mediated HMGB1 Release
Several reports indicate that HMGB1 release is mediated by cell-cell interaction. HMGB1 release during NK-DC crosstalk has been well-studied. Interestingly, interplay between dying cells and immune cells also induces HMGB1 release. For example, exposure of apoptotic cells to macrophages stimulates the release of HMGB1 in macrophages, which provides an explanation for the mechanism for apoptosis-mediated sepsis lethality (Qin et al., 2006). In addition, impaired clearance of apoptotic cells also leads to HMGB1 release, which in turn induces TLR4-mediated cytokine production (Velegraki et al., 2013).
4.1.15 Lysosome-Dependent Mechanism
Christian de Duve first identified lysosomes in 1949 via cell fractionation as the organelles responsible for protein degradation within cells. In addition to lysosome-mediated degradation, a subset of lysosomes, namely secretory lysosomes, are found in different cells of the immune system and are responsible for lysosome exocytosis in response to external stimuli. Secretory lysosomes are Ca2+-regulated secretory organelles displaying features of both lysosomes and secretory granules. The best candidate for a molecular marker of secretory lysosomes is Rab27a. Lysosome exocytosis has been proposed to mediate secretion of leaderless cytokines such as IL-1β and HMGB1 (Gardella et al., 2002). There is a significant colocalization between HMGB1 and lysosomal marker LAMP1, but not early endosome marker EEA1, in activated monocytes after LPS treatment. The release kinetics of IL-1β and HMGB1 is significantly different in activated monocytes. IL-1β is rapidly released in response to ATP and LPS, whereas HMGB1 release is a late event in response to lysophosphatidylcholine (LPC) and LPS (Gardella et al., 2002). Like HMGB1, the release of the enzyme phosphatidylcholine sPLA2, responsible for the generation of extracellular LPC in the site of inflammation, is also a late event (Gardella et al., 2002). Gamma-interferon inducible lysosomal thiol reductase (GILT) can reduce protein disulfide bonds, which facilitate the complete unfolding of proteins destined for lysosomal degradation. Several studies reveal that NF-κB and STAT1 play direct roles in the regulation of LPS and IFN-γ-inducible GILT expression, respectively (Lackman and Cresswell, 2006; O'Donnell et al., 2004). Loss of GILT in immune cells such as T cells and monocytes has diminished cytokine production following Ag exposure or LPS (Lackman and Cresswell, 2006; Rausch and Hastings, 2012). Interestingly, loss of GILT increases mitochondrial oxidative damage and cytosolic HMGB1 accumulation (Chiang and Maric, 2011), suggesting a potential role of GILT in the regulation of secretory lysosome-mediated HMGB1 release (Lackman et al., 2007).
4.1.16 Other
In addition, ATF3−/− (Lai et al., 2013), CCR7−/− (Kawakami et al., 2012), IL-17A−/− (Ogiku et al., 2012), SLPI−/− (Nakamura et al., 2003), C5l2−/− (Rittirsch et al., 2008), C3−/− (Cai et al., 2010), and ADAMTS13−/− (Fujioka et al., 2012) mice increase HMGB1 release in response to sterile and infectious threat.
4.2 Passive Release of HMGB1
In addition, HMGB1 can be passively released in cell death (e.g., necrosis, apoptosis, lysosomal cell death, and autophagic cell death) and injury following various stimuli such as chemotherapy, irradiation, hypoxia, hyperthermia, hyperpressure (Fucikova et al., 2014), glucose deprivation (Lee et al., 2011a), bacillus Calmette-Guerin (Zhang et al., 2013c), virus (Whilding et al., 2013), free fatty acids (FFA) (Rockenfeller et al., 2010), toxin (Kennedy et al., 2009; Radin et al., 2011), foreign matter (Yang et al., 2010b), ATP (Kawano et al., 2012), ubiquitin isopeptidase inhibitor (Fontanini et al., 2009), and cytolytic cells (Ito et al., 2007b). Multiple signaling pathways regulate passive HMGB1 release as discussed below (Figure 6).
4.2.1 PARP1 Dependent Mechanism
PAR polymerases (PARPs) are a family of enzymes responsible for poly (ADP)-ribosylation reactions. PARP catalyzes the transfer of ADP-ribose moieties from NAD+ onto acceptor proteins in response to several stress responses such as DNA damage, heat shock, and the unfolded protein response. Among them, PARP-1 is the best-characterized and master regulator for poly (ADP)-ribosylation reactions in the mammalian cell. Extensive DNA damage-mediated PARP-1 overactivation leads to excessive consumption of NAD+, ATP depletion, and subsequent necrotic cell death (Zong et al., 2004). In addition to inducing necrosis by energy deletion, nuclear PARP-1 also interacts with transcriptional factors such as p53 and NF-Κb to regulate expression of several genes (e.g., TNF-α) which are involved in the cell death and inflammatory response (Jog et al., 2009). HMGB1 has generally been considered a necrosis marker to stimulate inflammation. A recent study indicated that PARP1 is responsible for DNA-damaging agent-induced HMGB1 release during necrosis. Compared with wild type cells, loss of PARP1 in MEFs or using PARP inhibitor significantly inhibits alkylating DNA damage-induced HMGB1 translocation and release. These findings suggest that targeting PARP can inhibit HMGB1 release and the necrosis-associated inflammatory response. Interestingly, endogenous HMGB1 regulates the DNA damage response and PARP1 activity. Loss of intracellular HMGB1 leads to PARP-1 over activation (Huang et al., 2013a).
4.2.2 RIP3-Dependent Mechanism
Necroptosis, also termed programmed necrosis, is an alternative form of programmed cell death that occurs when cells lack the capacity to activate caspase-8 following ligation of death receptors (e.g., TNF-α), the same ligands that activate apoptosis. Two members of the receptor-interacting serine-threonine kinase (RIP) family, RIP1 and RIP3, have recently been implicated in necroptosis due to their interaction to generate necrosomes (Li et al., 2012b; Vandenabeele et al., 2010). RIP1 and RIP3 interact via their RIP homotypic interaction motif (RHIM) domains. Compared with RIP1, RIP3 is more specific to the regulation of necroptosis. Overexpression of RIP3 in vitro increases necroptosis, whereas knockout of RIP3 in vitro or in vivo inhibits necroptosis in response to various stressors, especially inflammatory stimuli. For example, RIP3−/− mice are protected against sepsis and donor kidney inflammatory injury partly by inhibition of release of DAMPs, including HMGB1 (Lau et al., 2013). In addition, RIP3-mediated necroptosis has also been proposed to be required for dsRNA/poly (I:C)-induced HMGB1 release, which is important for the inflammatory response during retinal degeneration (Murakami et al., 2014). In contrast, IPS-1, an adaptor molecule for RIG-I-like receptors (RLRs) may be critical for poly (I:C)-induced necroptosis and HMGB1 release in DCs.
4.2.3 Cathepsin-Dependent Mechanism
Lysosomal cell death is a form of cell death mediated by lysosomal cathepsin proteases. Lysosomal membrane permeabilization is increased in response to several stressors such as chemotherapy and pathogens, which result in the release of cathepsins and other hydrolases from the lysosome to the cytosol. Lysosomal cell death has necrotic, apoptotic, or apoptosis-like features depending on the extent of the leakage and the cellular context (Aits and Jaattela, 2013). A recent study indicated that cathepsin B, a lysosomal cysteine protease, promotes L. pneumophila-induced lysosomal cell death and HMGB1 release (Morinaga et al., 2010). An early study indicated that minute amounts of cathepsin B are transferred abruptly to the nuclear compartment, including histone and HMGB1, in a variety of activated cells (Seeler et al., 1988). Cathepsin B may interact with histones and HMGB1 in pre-treatment with a specific cathepsin B inhibitor, CA074Me, inhibited L. pneumophila-induced cell death as well as HMGB1 release. The pattern of cathepsin B activation was important for subsequent PARP cleavage and type of cell death (Morinaga et al., 2010). In addition, cathepsin B is also involved in inflammasome activation and HMGB1 release (Duncan et al., 2009; Willingham et al., 2007). Moreover, cathepsin D is required for necroptosis-mediated HMGB1 release in DCs. These findings suggest that cathepsin plays a critical role in the regulation of HMGB1 release while cells undergo mixed cell death.
4.2.4 Antioxidant Enzyme-Dependent Mechanism
ROS affect cellular physiology in multiple ways, whereas excessive ROS can trigger death. ROS not only induce HMGB1 secretion, but also promote HMGB1 release during cell death such as necrosis, apoptosis, and necroptosis. Although there are multiple sources of ROS in the cell, mitochondria have been considered a major source of ROS production. Mitochondrial ROS (mtROS) are produced by increasing mitochondrial damage. Superoxide dismutases (SOD) are a class of metalloenzymes that catalyze the dismutation of superoxide (O2−) into O2 and H2O2 and play a critical role in protecting cells against oxidative injury. In humans, SOD1 (CuZn-SOD) is located in the cytoplasm, whereas SOD2 (Mn-SOD) is located in the mitochondria. SOD3 (EC-SOD) exists as a copper and zinc-containing tetramer and is located in extracellular spaces. Dysfunction of SOD1 and SOD2 is involved in the regulation of HMGB1 release in cell death (Kang et al., 2011b; Lo Coco et al., 2007; Tang et al., 2011d; Vezzoli et al., 2011; Yao and Brownlee, 2010). In addition, other antioxidant enzymes such as glutathione reductase (Chiang and Maric, 2011; Hoppe et al., 2006), thioredoxin (Vezzoli et al., 2011; Xiang et al., 2011; Xu et al., 2013a), and peroxiredoxins (Shichita et al., 2012) can regulate HMGB1 release.
4.2.5 DNase-Dependent Mechanism
Deoxyribonuclease (DNAse) is an enzyme for degrading DNA to fragmentation by catalyzing the hydrolytic cleavage of phosphodiester linkages in the DNA backbone. DNA is degraded during cell death that accompanies a number of diseases. DNA in apoptotic cells is specifically degraded into nucleosomal units by DNA endonuclease (DNase-gamma), whereas DNA in necrotic cells is usually degraded randomly by extracellular DNAse I from the pancreas or by lysosomal acid DNAse II (Nagata et al., 2003). An early study indicated that HMGB1 is released only by necrotic cells, but not apoptotic cells. We now know that apoptotic cells also release HMGB1 and nucleosome to mediate inflammatory and immune responses. The release of HMGB1 in apoptosis is triggered by DNase-gamma-mediated nucleosomal DNA fragmentation (Yamada et al., 2011a, b). Thus, DNase gamma inhibitors such as DR396 can limit HMGB1 release from apoptotic cells (Yamada et al., 2011a, b).
4.2.6 Caspase-Dependent Mechanism
Caspases are a group of intracellular cysteine-aspartic proteases that mediate apoptosis and other types of cell death such as pyroptosis. Caspases exist as inactive proenzymes that are activated by proteolytic cleavage. Caspase 8 is implicated in the death receptor-mediated apoptosis pathway and cytokine processing. Caspase 9 has been linked to the mitochondrial death pathway by triggering the release of cytochrome c from mitochondria. Caspase 3 and caspase 7 share similar substrate specificities and function as effector caspases through amplified initiation signals from caspase 8 and caspase 9. Recent studies indicate that caspase3/7 induce mitochondrial complex 1 protein p75 NDUFS1 cleavage, which in turn increases mitochondrial ROS production and subsequent HMGB1 release during apoptosis. This finding provided an explanation for the apoptosis-mediated HMGB1 release mechanism. Pyroptosis is an inflammatory cell death and is typically triggered by caspase 1 after its activation by various inflammasomes. Inhibition of caspapse 1 activity can diminish HMGB1 release and the inflammatory response during pyroptosis (Kamo et al., 2013; Lu et al., 2012b). In addition, a recent study indicated that HMGB1 at the sites of aa67, aa158, and aa169 can be directly cleaved by caspase 1, but not other caspases (-2, -3, -5, -7, -9 or -11) (Leblanc et al., 2014). Different from full length HMGB1, A box with anti-inflammatory activity, the caspase-1 generated A-box fragment (especially residues 23–50) binding to RAGE can rescue apoptosis-induced immune tolerance in a sepsis model (Leblanc et al., 2014). These findings suggest that caspase 1 regulates both the release and processing of HMGB1 in inflammation and immunity.
4.2.7 ATG-Dependent Mechanism
Autophagy is a lysosome-mediated, dynamic process including induction, cargo recognition, phagophore formation, autophagosme formation, autolysosme formation, and substrate degradation. This process is tightly regulated by ATG proteins that were first identified in yeast. Several ATG homologs have subsequently been identified in mammalian cells, suggesting that autophagy is a highly-conserved process from the evolution of prokaryotes to eukaryotes. Although autophagy is generally considered a survival mechanism against harmful stress, excessive autophagy can lead to cell death, namely autophagic cell death. In response to epidermal growth factor receptor (EGFR)-targeted diphtheria toxin (DT-EGF), epithelial and glioblastoma tumor cells can release HMGB1 in an autophagy-dependent way (Thorburn et al., 2009). Knockdown of ATG5, ATG7, or ATG12 by RNAi decreases autophagy and HMGB1 release in response to DT-EGF (Thorburn et al., 2009). In addition, the ATG5-mediated autophagy pathway is also involved in HMGB1 secretion from fibroblasts and macrophages in response to starvation and lipopolysaccharide (LPS) (Dupont et al., 2011; Tang et al., 2010c). This process requires ROS signaling (Tang et al., 2010c).
5. HMGB1 Receptors (Figure 7)
Figure 7.
HMGB1 signals through receptors.
5.1 RAGE
RAGE, a member of the immunoglobulin gene superfamily, is a transmembrane receptor with an extracellular domain, a short transmembrane domain, and a 43-amino acid cytoplasmic tail. The gene is localized on chromosome 6p21.3 near the HLA locus in the vicinity of the class III region of the major histocompatibility complex in humans and mice. This locus is in close proximity to the homeobox gene HOX12 and the human counterpart of the mouse mammary tumor gene int-3. A recent study indicated that RAGE is evolved from a cell adhesion molecule family and acts as an adhesion molecule in mammalian cells (Sessa et al., 2014). Extracellular domain, containing one “V”- type domain and two “C”-type-domains, is responsible for ligand binding, whereas a cytoplasmic tail is required for intracellular signaling transduction such as NF-κB signaling. There are multiple spliced transcript variants of RAGE which encode different isoforms, as well as non-protein-coding variants. In addition, the soluble form of RAGE (sRAGE) can be generated by ADAM10 or matrix metalloproteinase- mediated proteolysis (Raucci et al., 2008; Zhang et al., 2008a). RAGE was recognized as a receptor for AGEs and is now known as a multi-ligand receptor. In addition to AGE, RAGE binds HMGB1, S100, amyloid-β peptide, DNA, RNA, and other molecules to regulate multiple physiological and pathological processes. A number of studies demonstrate that RAGE is required for HMGB1-induced cell migration (Degryse et al., 2001; Fages et al., 2000; Palumbo and Bianchi, 2004; Palumbo et al., 2004), proliferation (Kang et al., 2010), regeneration (Degryse et al., 2001; Sorci et al., 2004), inflammation (He et al., 2012b; Lv et al., 2009; Treutiger et al., 2003; Wu et al., 2013c), autophagy (Kang et al., 2012a; Weiner and Lotze, 2012), injury (Huang et al., 2012c; Wolfson et al., 2011), metabolism (Kang et al., 2014; Luo et al., 2014), and immunity (Leblanc et al., 2014). In addition, extracellular HMGB1 can stimuli RAGE expression in several cell types (Li et al., 1998). Knockout of RAGE in vitro or in vitro decreases tumor growth and metastasis (Gebhardt et al., 2008; Heijmans et al., 2012; Kang et al., 2010; Taguchi et al., 2000), and increases chemotherapy resistance (Tang et al., 2011d). Currently, the HMGB1-RAGE signaling axis represents an important potential target for diseases such as diabetes (Manigrasso et al., 2014), neurogeneration (Li et al., 1998), cancer (Sims et al., 2010), and inflammatory (Liliensiek et al., 2004) and autoimmune diseases (Ullah et al., 2014).
5.2 TLR
The Toll-like receptors (TLRs) are an evolutionarily-conserved type I transmembrane superfamily that contain extracellular leucine-rich repeat (LRR) domains and a cytoplasmic Toll/IL-1 receptor (TIR) domain. TLRs recognize several danger signals, including PAMPs and DAMPs, to activate the innate immunity response for defense against infection and injury (Akira and Takeda, 2004). There are two major signal transduction pathways involved in TLR activation. The MyD88-dependent pathway is required for the production of inflammatory cytokines, whereas the MyD88-independent pathway is required for the production of type I IFN and the maturation of DCs. HMGB1 can interact with TLRs (TLR2, TLR4, and TLR9) to activate the NF-κB and IRF pathways and then produce cytokines and chemokines for the inflammation and immunity response. Of these, the structural basis for activation of the HMGB1-TLR4 pathway is well studied, and both an intra-molecular C23–C45 and a C106 amino acid of HMGB1 is required for TLR4 binding and activation (Yang et al., 2010a; Yang et al., 2012d). In addition, a lipid-bound HMGB1 may bind to CD14 and/or MD2 on the cell surface and induce signaling through TLR4 (Kim et al., 2013d). In addition to activating inflammatory responses, the interaction between HMGB1 and TLR4 mediates anti-cancer immunity during radio- or chemotherapy (Apetoh et al., 2007). Knockout of TLR4 in vitro or in vivo decreases HMGB1-induced tissue injury (Laird et al., 2014; Yang et al., 2013e; Ye et al., 2013a), cell migration and adhesion (Bauer et al., 2013) (Furlani et al., 2012; Wang et al., 2013m), angiogenesis (Lin et al., 2011), and the inflammation (Lv et al., 2009; Tadie et al., 2012b; Zong et al., 2013) and immunity responses (Apetoh et al., 2007). In addition to TLR4, TLR2 is required for tissue injury (Herzog et al., 2014; Kim et al., 2012h; Kruger et al., 2010; Leemans et al., 2009; Li et al., 2009), cell migration and adhesion (Furlani et al., 2012), inflammation (Park et al., 2006; Yu et al., 2006b), and self-renewal of stem cells (Conti et al., 2013). TLR9 is generally responsible for HMGB1-DNA complex-induced nucleotide immunity and RAGE can enhance this process (Bamboat et al., 2010; Hirata et al., 2013; Ivanov et al., 2007; Tian et al., 2007; Yanai et al., 2009).
5.3 Integrin
Integrins are adhesion proteins that play critical roles in leukocyte recruitment, a well-defined cascade immunological process enabling leukocytes to leave the microvasculature and migrate into inflamed tissue (Herter and Zarbock, 2013). Mac-1, also called αMβ2, ITGB2 and CD11b/CD18, is a leukocyte integrin involved in inflammatory cell recruitment and pathogen recognition by neutrophil phagocytosis. Previous studies have demonstrated that endothelial RAGE and sRAGE interact with Mac-1 on leukocytes (Chavakis et al., 2003; Pullerits et al., 2006), suggesting a potential role of Mac-1 in the regulation of HMGB1 activity. Indeed, HMGB1 not only enhances Mac-1 expression by activation of p47 NADPH oxidase and the NF-κB pathway, but also promotes subsequent interaction between Mac-1 and RAGE, which is required for HMGB1-mediated neutrophil recruitment (Gao et al., 2011a; Orlova et al., 2007). These findings suggest that HMGB1-mediated inflammatory cell recruitment is mediated by crosstalk between RAGE and Mac-1. In addition, the expression of Mac-1 in neutrophils is regulated by Rac1, a small G-protein (Hwaiz et al., 2013). Inhibition of Rac1 signaling by blocking Mac-1 expression and HMGB1 release in an animal sepsis model (Hwaiz et al., 2013) suggests an important role for Rac1 in the regulation of neutrophil activation and recruitment. In addition to Mac-1, HMGB1 inhibits macrophage activity in efferocytosis through binding to αVβ3 integrin (Friggeri et al., 2010).
5.4 α-synuclein Filaments
Deposition of α-synuclein aggregates as filaments in Lewy bodies and Lewy neurites represent a hallmark of Parkinson's disease and dementia with Lewy bodies. HMGB1 is identified as α-synuclein filament-binding protein and its direct interaction can inhibit HMGB1-mediated autophagy, which contributes to the neurodegenerative process (Lindersson et al., 2004; Song et al., 2014).
5.5 Proteoglycans
Proteoglycans are glycosylated proteins with covalently attached, highly anionic glycosaminoglycans (Yanagishita, 1993). Different types of proteoglycans are found in the extracellular matrices of connective tissues. Heparan sulfate, a linear polysaccharide, binds multiple protein ligands (e.g., cyclophilin A, cyclophilin B, and hepatoma-derived growth factor) and regulates a wide variety of biological activities. Using heparin-sepharose chromatography, HMGB1 was originally isolated from rat liver (Bianchi, 1988), suggesting its ability to bind cell surface proteoglycans. Indeed, some proteoglycans, including heparan sulfate (Xu et al., 2011a), syndecan (Salmivirta et al., 1992), neurocan (Milev et al., 1998), and phosphacan/PPTP-ζ/β (Milev et al., 1998), are identified as HMGB1 receptors. In addition, RAGE may be required for HMGB1 binding to heparan sulfate (Xu et al., 2011a), suggesting an interaction between RAGE and proteoglycan to mediate HMGB1 activity. However the functional significance of such interaction remains largely unknown in vivo.
5.6 CD24
CD24, first identified as a B cell differentiation marker, is a cell surface GPI-anchored mucin-like glycoprotein expressed by a variety of hematopoietic, neuronal, and epithelial cell types (Fang et al., 2010). Accumulating evidence shows that CD24 is involved in several physiological and pathological processes such as lymphocyte maturation, tissue regeneration, neuronal development, tumor development, and tumor metastasis. A recent study demonstrated that CD24 functions as a positive PAMP regulator, but serves as an important negative DAMP regulator. Upon recognizing HMGB1, CD24 inhibits HMGB1-induced NF-κB activation and subsequent pro-inflammatory cytokine production (Chen et al., 2009). Thus, CD24−/− mice exhibit increased susceptibility to DAMPs but not PAMPs (Chen et al., 2009). These findings provide a receptor switch to distinguish the immune response from intracellular and extracellular danger signals. In addition, CD24 expressed in CD103+ DCs is required for generation of effector CD8+ T cells by presenting HMGB1 to RAGE+ T cells (Kim et al., 2014).
5.7 TIM-3
TIM-3 is a member of the TIM (T-cell immunoglobulin domain and mucin domain) family. It was first identified as an immune checkpoint receptor that suppresses the activation of TH1 cells (Monney et al., 2002) thorough binding ligand galectin-9 (Zhu et al., 2005). TIM-3 is also expressed on other immune cells such as DCs (Anderson et al., 2007), monocytes and macrophages (Nakayama et al., 2009), and NK cells (Ndhlovu et al., 2012) to regulate inflammation, efferocytosis, and cytotoxicity, respectively (Tang and Lotze, 2012). In addition, TIM3 suppresses the activation of tumor-associated DCs in response to DNA vaccines and chemotherapy by binding to HMGB1 (Chiba et al., 2012a). As a negative receptor, TIM-3 impairs HMGB1-mediated recruitment of nucleic acids into the endosome, a key step in nucleic acid-mediated antitumor immunity (Chiba et al., 2012a). Therefore, blockade of TIM-3 by neutralizing antibody could improve therapeutic responses in combination with DNA vaccines and chemotherapy.
5.8 CXCR4
CXCR4, a member of the G protein-coupled receptors, is widely expressed by hematopoietic cells. In addition, CXCR4 is expressed lower in normal tissues, but expressed significantly higher in tumor tissues, indicating a critical role in tumor biology, including tumor proliferation and metastasis. After binding its ligands (e.g. CXCL12/SDF-1), the major function of CXCR4 is promoting cell migration and invasion by activation of downstream signaling pathways such as RAS/MAPKs, AKT/PI3K and JAK/STAT. In addition, acting as a chemokine receptor, CXCR4 acts as an HIV co-receptor for entry into T cells, which facilities T cell-mediated HIV clearance. Recent evidence demonstrates that ubiquitin and HMGB1 are also ligands of CXCR4. HMGB1 forms a heterocomplex with CXCL12 and then binds to CXCR4, but not RAGE and TLR4, to induce the recruitment of inflammatory cells to damaged tissues (Schiraldi et al., 2012). The NF-κB noncanonical pathway is required for CXCL12 production for cells to migrate toward HMGB1 (Kew et al., 2012), whereas the NF-κB canonical pathway is required for RAGE expression for cells to migrate toward HMGB1 (Penzo et al., 2010). These findings indicate that HMGB1-mediated cell migration is regulated by the NF-κB pathway and binding partner.
5.9 NMDAR
N-Methyl-D-aspartate receptor (NMDAR), a member of post-synaptic ionotropic glutamate receptors (iGluRs), is a heteromeric ligand-gated calcium ion channel mainly in the central nervous system. NMDAR mediates glutamate-induced calcium influx and sustains synaptic plasticity and neural excitatory. In contrast, excessive opening of NMDARs results in calcium overload and ultimately neuron death. Interestingly, NMDAR activation also causes HMGB1 cytoplasmic translocation and release. Cytosolic HMGB1 can bind to Beclin-1 to limit death by upregulated autophagy (Perez-Carrion and Cena, 2013), whereas extracellular HMGB1 plays both negative and positive roles in the regulation of neuronal cell death (Kim et al., 2010a; Kim et al., 2012c; Kim et al., 2006; Kim et al., 2012d; Kim et al., 2011b). A recent coimmunoprecipitation study demonstrated that HMGB1 physiologically interacts with GluN1 and GluN2B subunits of NMDAR via a specific region of HMGB1 localized in the B box upstream from the RAGE and downstream from the TLR4 binding sites (Pedrazzi et al., 2012). This interaction between HMGB1 and NMDAR on synaptosomes and cells of neuronal and non-neuronal origin promotes calcium influx and NO synthesis, which results in neuroblastoma cell motility and neurite outgrowth (Pedrazzi et al., 2012). Other studies indicate that activation of TLR4, but not RAGE, is required for the functional interaction between HMGB1 and NMDAR in neuronal cells (Balosso et al., 2014; Maroso et al., 2010). HMGB1 only in its disulfide form binds TLR4 to enhance neuronal calcium influx, which facilitates neuronal hyper excitability in seizures (Balosso et al., 2014; Maroso et al., 2010).
5.10 TREM1
The triggering receptor expressed on myeloid cells-1 (TREM-1) is a member of the immunoglobulin superfamily including three domains: extracellular domain (194aa), a membrane-spanning domain (29aa), and a short cytoplasmic tail (5 aa). The extracellular Ig-like domain contains the motif DxGxYxC, which corresponds to the V-type Ig-domain. The cytosolic domain of TREM-1 associates with the adaptor DAP12 that is required for intracellular signaling transduction. Soluble TREM-1 (sTREM-1) is created by cleavage of extracellular domain, namely TREM-1 shedding. TREM-1 mediates the inflammatory response initiated by TLRs in neutrophils, monocytes, and macrophages (Bouchon et al., 2001). Recent studies indicate that HMGB1 is a ligand of TREM1. Upon binding to TREM1, HMGB1 promotes cytokine production via the NF-κB pathway (El Mezayen et al., 2007), which plays a critical role in sepsis (Bouchon et al., 2001; El Mezayen et al., 2007) and tumor growth (Wu et al., 2012b).
6. HMGB1 Transcriptional Regulation (Figure 8)
Figure 8.
HMGB1 transcriptional regulation.
6.1 CTF2
The CCAAT-binding transcription factor (CTF)/nuclear factor I (NF-I) group of site-specific DNA-binding proteins that recognize the sequence TTGGC (N5)-GCCAA and are required for both viral DNA replication and various cellular gene expressions (Gronostajski, 2000). Human HMGB1 promoter is regulated by a silencer and an enhancer-containing intron (Lum and Lee, 2001). The sequence of HMGB1 promoter is TATA-less but contains three different CCAAT boxes (Nagatani et al., 2001). HMGB1 levels correlate with the anticancer activity of cisplatin. CTF2, one of the splice variants of CTF/NF-I, is overexpressed in cisplatin-resistant cells (KB-CP20) and is responsible for HMGB1 transactivation and DNA replication following cisplatin treatment (Nagatani et al., 2001).
6.2 p53/p73
The tumor suppressor p53 and its homologue p73 play opposite roles in the regulation of HMGB1 promoter activities by their physical interaction with CTF/NF-I. p53 binding to CTF2 downregulates the activity of the HMGB1 gene promoter, whereas p73a binding to CTF2 upregulates the HMGB1 gene promoter (Uramoto et al., 2003). In contrast, CTF2 has an opposite role in the regulation of p53/p73-dependent activation of the p21 promoter. CTF2 binding to p53 upregulates the p21 gene promoter, whereas CTF2 binding to p73 downregulates the p21 gene promoter (Uramoto et al., 2003). These findings suggest that interaction between same transcriptional factors play different roles in the regulation of different genes.
6.3 KLF4
KLF4, a member of the erythroid Kruppel-like factor (EKLF) multigene family, is widely expressed in a number of cells and tissues. KLF4 plays a critical role in proliferation, differentiation, development, inflammation, and cell death. KLF4 clearly acts as both a repressor and activator of gene transcription depending on target genes and cofactors. KLF4 can induce HMGB1 expression in activated macrophages by binding to the KLF4-binding element in the promoter of HMGB1 (Liu et al., 2008b). Overexpression of KLF4 increases HMGB1 expression and release, whereas suppression of KLF decreases HMGB1 expression and release in macrophages following treatment with LPS (Liu et al., 2008b).
6.4 C-myc
c-Myc, a member of the transforming Myc oncoprotein family, is overexpressed in many human cancers. c-Myc regulates a number of genes that are involved in the regulation of cell cycle, protein synthesis, cell adhesion, metabolism, cell death, and genomic integrity. Although c-Myc is a proto-oncogene and stimulates proliferation, downregulation of c-Myc promotes cancer cell survival under oxygen- and glucose-deprived conditions (Okuyama et al., 2010). This process occurs partly through deregulated intracellular levels of ATP and HMGB1 (Okuyama et al., 2010), suggesting c-myc acts as a positive regulator for HMGB1 expression.
6.5 C/EBP
CCAAT-enhancer-binding proteins (C/EBPs) are a family of transcription factors that are characterized by a leucine zipper motif that mediates dimerization and a basic DNA binding domain. The viral Tax protein encoded from human T-cell leukemia virus type-I (HTLV-1) is considered to play a central role in the process leading to adult T-cell leukemia. Tax acts as a transcriptional activator by associating with specific DNA-bound protein and transcript factors. A recent study indicated that Tax can interact with C/EBPs in HMGB1 promoter and promote subsequent HMGB1 expression in CD4+ T-cells (Zhang et al., 2013b). These findings suggest that HMGB1 may be involved in HTLV-1-mediated adult T-cell leukemia.
6.6 STAT3
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor which was first described as a DNA-binding activity from IL-6-stimulated hepatocytes. In addition to IL-6, Stat3 is also activated by multiple stimuli such as growth factors, cytokines, oncogenes, hypoxia, and oxidative stress. Human HMGB1 promoter has a STAT3 binding site that has located 872 base pairs from the transcription start site. Importantly, inhibition of STAT3 expression decreases HMGB1 upregulation during excessive mechanical stress in lung microvascular endothelial cells (Wolfson et al., 2013).
6.7 ErbB3
ErbB3, also called Her3, is a member of the ErbB receptor protein tyrosine kinase family that includes ErbB1 (EGFR), ErbB2 (Her2), ErbB3, and ErbB4. ErbB3 lacks tyrosine kinase activity, whereas ErbB2 is a ligand-less receptor. However, some ligands (e.g., neuregulin-1) can activate the ErbB3 pathway by inducing ErbB2/ErbB3 heterodimer formation. A recent study indicated that nuclear ErbB3 in Schwann cells significantly inhibits the transcriptional activity of HMGB1 promoters in response to neuregulin-1 (Adilakshmi et al., 2011).
6.8 HSF1
HSF1, a member of the heat shock factors (HSF), is activated under various stressors (e.g. hypoxia and high temperature) and subsequently induces up-regulation of heat shock genes (e.g., Hsp72) through binding to heat shock sequence elements (HSE) in promoters. Previous studies have demonstrated that HSP72 inhibits HMGB1 release in activated macrophages by direct protein-protein interaction in the nucleus. A recent study indicated that HSF1 overexpression inhibits the expression of HMGB1 and H2O2-induced cardiomyocyte death (Yu et al., 2012b). These findings suggest that HSF1 acts as a negative regulator of HMGB1 expression.
6.9 NAC1
Nucleus accumbens-1 (NAC1) is a transcription repressor that belongs to the BTB/POZ gene family. Knockdown of NAC1 inhibits cisplatin-induced HMGB1 expression, translocation, and release in ovarian cancer cells, which will limit autophagy and increase apoptosis (Zhang et al., 2012d; Zhang et al., 2011e).
6.10 microRNA
Several microRNAs (miR34A, miR218, miR181, and miR1192) are identified to function as negative regulators of HMGB1 gene expression by directly targeting its 3'-untranslated region. miR34A inhibits HMGB1 expression in retinoblastoma cells and leads to a decrease in autophagy under starvation conditions or chemotherapy treatment (Liu et al., 2014a). miR218 inhibits HMGB1 expression in lung cancer cells and leads to a decrease in cell migration and invasion (Zhang et al., 2013a). miR181b/c inhibits HMGB1 expression in astrocytes and leads to a decrease in the inflammatory response (Hutchison et al., 2013). miR181a inhibits HMGB1 expression in T- and B-Acute Lymphoblastic Leukemia (ALL) cells and leads to a decrease in cell proliferation and metabolic activity (Dahlhaus et al., 2013). miR-1192 inhibits HMGB1 expression in muscle cells and leads to a decrease in myogenesis, which is regulated by RNA-binding protein HuR (Dormoy-Raclet et al., 2013).
6.11 Other
CCR2−/− mice are protected against sepsis partly through downregulation of ERK-dependent HMGB1 expression (Alves et al., 2013). CXCR3−/−, but not CCR1−/−, CCR5−/− mice and NK cell-depleted mice display severe liver damage after CCl4 injection partly through increased HMGB1 expression in the liver (Zaldivar et al., 2012); HO1−/− mice show increased mortality in sepsis partly through increased HMGB1 expression in response to LPS or IL-1β (Garcia-Arnandis et al., 2010a; Takamiya et al., 2009); PI3Kγ−/− mice ameliorated the LPS-induced decrease in myocardial contractility and HMGB1 mycocardial expression (Xu et al., 2010); Single-Ig-interleukin-1 related receptor (SIGIRR)−/− mice show cognitive deficiencies and hippocampal dysfunction with enhanced expression of HMGB1 (Costello et al., 2011); Metalloproteinase Zmpste24−/− mice exhibit lipodystrophy with upregulated HMGB1 expression (Peinado et al., 2011).
7 HMGB1 Post-translational Modification
HMGB1 can undergo several post-translational modifications including acetylation ADP-ribosylation, methylation, phosphorylation, glycosylation, and oxidation as shown by studies with HPLC, gel electrophoresis, ion-exchange chromatography, and isoelectric focusing. These modifications and regulations are critical for HMGB1 localizations and functions (Figure 9A).
Figure 9.
HMGB1 post-translational modification. (A) These modifications and regulations are critical for HMGB1 location and function. (B) The redox status of HMGB1 regulates its cytokine-inducing and chemokine activities.
7.1 Acetylation
HMGB1 acetylation at Lys2 and Lys11 has been known for about 35 years (Sterner et al., 1979; Sterner et al., 1978), studies on the properties of HMGB1 acetylation started much later (Wong et al., 1991). In vivo acetylation of HMGB-1 at Lys 2 by histone acetyltransferases CBP (CREB-binding protein), but not PCAF, and Tip60 significantly enhanced its affinity to distort DNA structures (e.g., UV damaged- and cisplatinated DNA and with synthetic four-way junctions) (Pasheva et al., 2004; Ugrinova et al., 2001). In addition, removal of the highly charged C-terminal tail creates an additional acetylation site of HMGB1 at Lys81 in vitro by CBP, which has no effect on DNA binding affinity but increases DNA bending activity (Pasheva et al., 2004), suggesting that Lys-81 is critical for the DNA bending ability of truncated HMGB1 (Elenkov et al., 2011). There is a direct interaction between the acetylation and phosphorylation of HMGB1 lacking the C terminus in vitro. Phosphorylation by PKC prior to acetylation inhibits CBP activity, whereas acetylation by CBP has a stronger effect on the subsequent phosphorylation than the PKC modification (Pelovsky et al., 2009). Acetylated HMGB1 in cancer cells is tetrameric and interacts with homologous DNA polymerase alpha, which is involved in DNA replication (Alexandrova and Beltchev, 1987, 1988; Dimov et al., 1990). In addition to serving as a fine modulator of its “architectural” abilities, HMGB1 acetylation limits nuclear import of HMGB1 and mediates HMGB1 release in response to several stimuli such as LPS (El Gazzar, 2007), IFN (Lu et al., 2014), sodium butyrate (Carneiro et al., 2009) and I/R injury (Dhupar et al., 2011). Upregulation of pyruvate kinase M2 (PKM2) expression enables a metabolic switch to aerobic glycolysis, leading to excessive production of lactate. By inhibiting histone deacetylases activity, lactate in turn increases HMGB1 hyperacetylation, cytoplasmic translocation and release in systemic inflammatory response syndrome (Yang et al., 2014b).
7.2 ADP-ribosylation
ADP-ribosylation is the process of adding one or more ADP-ribose moieties to a protein by ADP-ribosyl transferases. ADP-ribosylation reactions can be divided into four groups: mono-ADP-ribosylation, poly-ADP-ribosylation, ADP-ribose cyclization, and formation of O-acetyl-ADP-ribose (Hassa et al., 2006). HMGB1 is a target of mono-ADP-ribosylation in intact cultured cells with or without DNA damage (Tanuma and Johnson, 1983; Tanuma et al., 1985a, 1986; Tanuma et al., 1985b). ADP-ribosylated HMGB1 does not change during aging, but increases in cancer cells (Alexandrova and Beltchev, 1987; Thakur and Prasad, 1990). Since ADP-ribosylation is generally inversely related to transcription, hyper ADP-ribosylation of HMGB1 downregulates gene transcription. In addition to mono-ADP-ribosylation, PARP1-mediated poly-ADP-ribosylation reactions are required for the nuclear export and release of HMGB1 during cell death, especially necrosis (Ditsworth et al., 2007; Zong et al., 2004). After release, hyper poly (ADP-ribosyl)ated HMGB1 enhances inhibition of efferocytosis by binding to PS and RAGE (Davis et al., 2012a). Lack of intracellular HMGB1 leads to excessive PARP1 activation and injury (Huang et al., 2013a). These findings suggest cross talk between HMGB1 and PARP1 in ADP-ribosylation reaction to regulate cell death.
7.3 Methylation
HMGB1 in neutrophils is mono-methylated at Lys42 during end differentiation from myelocytic cells. The DNA binding activity of methylated HMGB1 is significantly decreased by altering the conformation of box A. This change will cause HMGB1 to translocate from the nucleus to the cytoplasm in neutrophils (Ito et al., 2007a). In clear cell renal cell carcinoma, HMGB1 is mono-methylated at Lys112, which also contributes to HMGB1 relocation to the cytoplasm (Wu et al., 2013a). Thus, HMGB1 is a target for methylating agents in chromatin (Boffa and Bolognesi, 1985).
7.4 Phosphorylation
Phosphorylation of HMGB1 is mediated by several kinases such as PKC, casein kinase I (CK-I), CKII, and cyclin-dependent kinase 5 (Cdk5) in insect, chironomus, and vertebrates (Alami-Ouahabi et al., 1996; de Abreu da Silva et al., 2011; Kang et al., 2009; Kimura et al., 1985; Oh et al., 2009; Okano et al., 2001; Palvimo et al., 1987; Ugrinova et al., 2011; Ugrinova et al., 2012; Wisniewski et al., 1994; Wisniewski et al., 1999). In vitro, HMGB1 extracted from livers of young, but not old rats, is more sensitive to spermine and sodium butyrate-induced phosoporation (Prasad and Thakur, 1990b). Phosphorylated HMGB1 affects both its DNA binding/bending affinity and nucleocytoplasmic distribution and release (Alami-Ouahabi et al., 1996; Kang et al., 2009; Oh et al., 2009; Youn and Shin, 2006). PKC-phosphorylated HMGB1 increases DNA binding activity to cis-platinated DNA (Ugrinova et al., 2012) as well as HMGB1 release in cancer and by immune cells (Lee et al., 2012a). Cdk5-mediated HMGB1 phosphorylation regulates DNA end-joining activity, but not its ability to recognize distorted DNA structures (Ugrinova et al., 2011). Multiple HMGB1 serine residues (35, 39, 42, 46, 53, and 181) are phosphorylated within both NLSs in macrophages following TNF-α and OA treatments (Youn and Shin, 2006). Phosphorylated HMGB1 decreases its binding to nuclear import protein KAP-α1, which in turn promotes HMGB1 cytoplasmic relocation and its eventual secretion (Youn and Shin, 2006). This process is Ca2+ and PDK1- dependent (Oh et al., 2009). PDK1 is the downstream target of PI3K and regulates many PKC isoenzyme activities.
7.5 Glycosylation
HMGB1 is also able to undergo glycosylation, although the amount is insignificant and the function is unclear (Chao et al., 1994).
7.6 Oxidation
Increasing evidence indicates that changes in HMGB1 location and function largely depends on redox states (Tang et al., 2011e). Human HMGB1 contains three cysteines (C23, C45, and C106). Cys23/Cys45 in Box A can rapidly form an intramolecular disulfide bond; the cellular glutathione system alone is not enough to keep HMGB1 completely reduced within the cell (Sahu et al., 2008). C106 in Box B is required for HMGB1 nuclear location because C106 mutation, but not C23 and C45 mutations, promotes HMGB1 translocation from the nucleus to the cytosol. The native state of HMGB1 is rapidly lost via oxidation of sulfhydryl groups during storage (Kohlstaedt et al., 1986). Compared with oxidized HMGB1, reduced HMGB1 exhibits a stronger affinity for distorted DNA structures (Park and Lippard, 2011). Indeed, ROS significantly promote HMGB1 translocation and release in activated immune cells or injured cells (Tang et al., 2011e; Tang et al., 2007e). These findings suggest that ROS is a major signal that decreases nuclear HMGB1 DNA binding activation, which in turn promotes cytoplasmic translocation and release. In addition to location and release, the redox status of HMGB1 directly influences its extracellular activity, including immunity and autophagy (Venereau et al., 2012). The first research finding on HMGB1 redox and immunity was that oxidized HMGB1 released from apoptotic cell leads to DC immune tolerance (Kazama et al., 2008). However, this process can be reversed if HMGB1 is cleaved by caspase 1 and then binds to RAGE (Leblanc et al., 2014). Later, reduced HMGB1, but not oxidized HMGB1, promotes autophagy and cell proliferation by binding to RAGE in cancer cells (Tang et al., 2010a). Recent reports from several laboratories indicate that fully-reduced HMGB1 (also called all-cysteine-reduced HMGB1) acts as a chemokine by forming a heterocomplex with CXCL12 to bind CXCR4; disulfide HMGB1 with a disulfide bond connecting C23 and C45 acts as a proinflammatory cytokine by binding to TLR4 or NDMAR; sulfonyl HMGB1 (also called oxidized HMGB1) has no activity in cell migration or cytokine induction (Figure 9B) (Balosso et al., 2014; Liu et al., 2012a; Schiraldi et al., 2012; Venereau et al., 2012; Yang et al., 2010a). However, other reports indicate that oxidized HMGB1 still has the ability to activate neutrophils, vascular inflammation, and age-associated inflammation (Davalos et al., 2013; Maugeri et al., 2014). The different HMGB1 redox forms can be identified in serum, saliva, and cell culture medium by mass spectrometry (Balosso et al., 2014; Liu et al., 2012a; Schiraldi et al., 2012; Venereau et al., 2012; Yang et al., 2010a) and nuclear magnetic resonance (NMR) spectroscopy (Zandarashvili et al., 2013), although currently there is no convenient method. A systematic nomenclature for the redox states of HMGB1 and other HMGB proteins has been proposed by Marco E. Bianchi and colleagues (Antoine et al., 2014).
8 HMGB1 Cleavage and Degradation
Thrombin-thrombomodulin complexes, chlamydial-protease-like activity factor (CPAF), dipeptidyl peptidase IV, autophagic pathway, and caspase 1 have been identified as being involved in HMGB1 proteolytic cleavage and/or degradation under some conditions such as DIC (Ito et al., 2008) and infection with C. trachomatis (Yu et al., 2010) (Figure 10).
Figure 10.
HMGB1 cleavage and degradation.
8.1 Thrombomodulin
Thrombomodulin (TM) (also called CD141 or BDCA-3) is an integral membrane protein expressed on the surface of endothelial cells and composed of three domains: an N-terminal lectin-like domain, followed by an EGF-like domain consisting of six EGF-like repeats, and an O-glycosylation–rich domain. The major function of TM is as a cofactor for thrombin. An early study demonstrated that TM can bind HMGB1 thorough N-terminal lectin-like domain, which in turn prevents interaction between HMGB1 and RAGE during the inflammatory response (Abeyama et al., 2005). A recent study indicated that thrombin can directly cleave HMGB1 at the Arg10-Gly11 bond, and TM enhanced thrombin-mediated cleavage of HMGB1 to a less pro-inflammatory form (Ito et al., 2008). This cleaved HMGB1 form can be detected in serum from septic patients and animal models (Ito et al., 2008), suggesting that serum HMGB1 levels in patients in fact reflect active and inactive forms (Urbonaviciute et al., 2007). In addition, other thrombosis regulators such as activated protein C (Bae and Rezaie, 2011) and heparin (Wake et al., 2009a) have different roles in the regulation of HMGB1 degradation. Activated protein C promotes degradation of HMGB1 and other nDAMPs such as histone (Bae and Rezaie, 2011; Xu et al., 2009), whereas heparin inhibits the degradation of HMGB1 by plasmin (Wake et al., 2009a).
8.2 CPAF
During infection of epithelial cells, the obligate intracellular pathogen Chlamydia trachomatis secretes the serine protease Chlamydia protease-like activity factor (CPAF) into the host cytosol to regulate a range of host cellular processes through targeted proteolysis. A recent study indicated that CPAF mediated the cleavage and degradation of both HMGB1 and PARP-1 in C. trachomatis-infected HeLa cells (Yu et al., 2010). In contrast, lactacystin, an inhibitor of the 26S proteasome, can decrease CPAF activity and prevent HMGB1 degradation (Yu et al., 2010). These findings suggest that HMGB1 degradation contributes to immune tolerance during pathogen infection.
8.3 Autophagy
Autophagy plays a central role in the regulation of the cellular traffic, secretion, and degradation of HMGB1 in response to various stressors. Use of natural products including Chinese herbs is a novel way to inhibit HMGB1 release during sepsis; potential mechanisms of action are involved in promoting autophagy-mediated HMGB1 degradation. For example, Tanshinone IIA sodium sulfonate, a famous Chinese medicine which has been used in the treatment of cardiovascular disorders, induces clathrin- or caveolin-dependent endocytosis of exogenous HMGB1 and subsequently, autophagy-dependent degradation in macrophages (Zhang et al., 2012e). Green tea and its major constituent epigallocatechin gallate inhibit HMGB1 release in macrophages by stimulating its autophagic degradation (Li et al., 2011e).
8.4 DPP4
Dipeptidyl peptidase-4 (DPP4) (also called ADCP2 and CD26), a type II transmembrane glycoprotein, is a serine exopeptidase belonging to the S9B protein family that cleaves X-proline dipeptides from the N-terminus of polypeptides, such as chemokines, neuropeptides, and peptide hormones (Matteucci and Giampietro, 2009). A recent study indicated that HMGB1 is cleaved at its N-terminal region by DPP4, which in turn diminishes HMGB1’s angiogenic activity in vitro and in vivo (Marchetti et al., 2012). In contrast, diprotin A, a DPP-IV inhibitor, prevented HMGB1 degradation. The N-terminal truncated form of HMGB1 is detected in the serum of type 2 diabetic patients, which may affect diabetes-associated vascular complications.
8.5 Caspase-1
HMGB1 released from necrotic cells is immunogenic, whereas that released from apoptotic cells is tolerogenic. A recent study indicated that caspaspe1-mediated HMGB1 cleavage reversed apoptosis-induced tolerance through binding to RAGE in DCs (Leblanc et al., 2014). HMGB1 can be specifically processed to create an active A-box peptide by caspase-1, but not other caspases (-2, -3, -5, -7, -9 or -11) (Leblanc et al., 2014). These findings provide a new mechanism by which to regulate immune tolerance by HMGB1 cleavage and receptor recognition.
9 Regulation of Extracellular HMGB1 Activity
Extracellular HMGB1 plays multiple roles in the pathogenesis of human disease. The extracellular actions of HMGB1 depend on its forms (e.g., reduced or oxidized, dimer or multimer, full length or cleaved, single or partner), concentrations (e.g., high dose or low dose), receptor types (e.g., positive receptor or negative receptor) and downstream signaling (e.g., NK-κB, IRF, and STAT). Of note, ultra-pure HMGB1 (free from contaminating bacterial proteins and nucleic acids) at a low dose fails to trigger the inflammatory response. In contrast, HMGB1 is very “sticky” and can bind to various PAMPs (e.g., LPS), DAMPs (e.g., DNA), and other molecules (e.g., cytokines, chemokine and IgG) to regulate inflammatory and immune responses (Table 3) (Urbonaviciute et al., 2007). In many disease conditions, the oxidizing nature of the extracellular environment differs from the intracellular compartment’s highly reducing nature. In addition, normal and disease tissue (e.g., cancer) microenvironments also have a number of significantly different, even opposite properties regarding level of pH, O2, electrolytes, and glucose. These factors will determine HMGB1 activity and make HMGB1 biology extremely complex.
10 Anti-inflammatory and Anti-injury Activity of Intracellular HMGB1
It is clear that extracellular HMGB1 is generally a mediator of sterile inflammation and infection. Inhibition of HMGB1 release and activity has been demonstrated to prevent several inflammatory diseases such as sepsis and reperfusion injury. However, intracellular HMGB1 may function as an anti-inflammatory and anti-injury protein in these diseases, based on recent studies with HMGB1 conditional knockout mice. For example, conditional knockout of HMGB1 in the pancreas, liver, and myeloid cells decreases protection against experimental pancreatitis, liver ischemic reperfusion, and sepsis, respectively (Figure 11) (Huang et al., 2013a; Kang et al., 2013b; Yanai et al., 2013). The mechanism is involved in intracellular HMGB1-mediated nuclear hemostasis and autophagy. Loss of HMGB1 increases nuclear injury-mediated nucleosome release (Huang et al., 2013a; Kang et al., 2013b). In addition, loss of HMGB1 causes autophagy deficiency, which will increase oxidative stress and subsequent inflammasome signaling pathway activity, including proinflammatory IL-1β rerelease (Yanai et al., 2013). Nucleosome release from local tissue with HMGB1 deficiency will activate and recruit immune cells (e.g., macrophages and neutrophils) to increase systemic serum HMGB1 levels (Kang et al., 2013b). These findings provide a novel mechanism to explain why local injury causes systemic inflammation. Overexpression of HMGB1 in cardiac tissue by transgenic knockin methods significantly increases animal survival and protects mice against myocardial infarction by enhancing angiogenesis and cardiac function (Kitahara et al., 2008).
Figure 11.
Phenotype of the HMGB1 conditional knockout or knockin mouse in response to stress.
11 HMGB1 and Cell Death (Figure 12)
Figure 12.
HMGB1 release in cell death
11.1 Necrosis
The term “necrosis” was first used by morphologists to describe irreversible tissue damage in pathological circumstances lacking the morphological characteristics of apoptosis or autophagy (Zong and Thompson, 2006). Necrosis is not only accidental, but is also a specific form of programmed cell death, namely necroptosis (Galluzzi et al., 2012). Necroptosis refers to death-receptor-initiated cell death under conditions where cells lack the capacity to activate caspase-8. In response to TNFa, kinase receptor-interacting protein 1 (RIP1)/RIP3 form “necrosomes” to initiate necroptosis, which can be inhibited by necrostatin 1 (Degterev et al., 2008; Degterev et al., 2005; Li et al., 2012b; Vandenabeele et al., 2010). Morphologically, necrosis is characterized by cell swelling, cell rupture, and breakdown of cell organelles. The fundamental causes of necrosis include calcium overload, ROS generation, cellular energy depletion, membrane lipid injury, lysosomal destabilization, and release of lysosomal enzymes to digest liberated cellular components (Zong and Thompson, 2006). These factors can often cause HMGB1 release; therefore, HMGB1 is widely-used as a necrosis marker (Jeon et al., 2013). In addition to release from RIP3, PAPR1, and cathepsin-mediated necrotic cells (Zou et al., 2013), loss of intracellular HMGB1 also prompts necrosis in response to cytotoxic agents and inflammatory stimulus. Our unpublished data suggest that loss of HMGB1 causes necroptosis to switch to apoptosis, suggesting a potential role of HMGB1 in balancing different cell deaths.
11.2 Apoptosis
Apoptosis is activated through specific signaling pathways that result in a series of well-defined biochemical (e.g., activation of pro-apoptotic Bcl-2 family members, caspase activation, and substrate cleavages) and morphological (e.g., apoptotic bodies formation) changes. The two central apoptosis pathways include the extrinsic pathway triggered by cell death receptors (e.g., FasR, TNFR1, lymphotoxin receptor, DR3, and DR4/DR5) and the intrinsic pathway mediated by mitochondria and ER (Igney and Krammer, 2002). Although caspase activation is critical for apoptotic cell death, the caspase-independent pathway has been discovered by translocation of apoptosis-inducing factor (AIF) (Daugas et al., 2000; Susin et al., 1999) and endonuclease G (ENDOG) (Li et al., 2001) from the mitochondria to the nucleus, thereby mediating large-scale DNA fragmentation. HMGB1 not only regulates endonuclease activity, but is also a component of apoptotic body (Arends et al., 1990; Cockerill and Goodwin, 1983). Crosstalk between HMGB1 and apoptosis has been studied in many cancer cells. On one hand, HMGB1 can be released by apoptotic cancer cells at the late stage; on the other hand, extracellular oxidized HMGB1 can induce caspase-dependent apoptosis in cancer cells. Moreover, intracellular HMGB1 is generally an anti-apoptosis protein in response to several apoptotic stimuli such as ultraviolet radiation, CD95, TRAIL, Casp-8, and Bax (Brezniceanu et al., 2003). HMGB1 plays transcriptional-dependent (e.g., regulation of Bcl-2 family protein expression) and -independent roles (e.g., regulation of autophagy and p53 location) in the regulation of apoptosis. In some cases, overexpression of HMGB1 renders cells sensitive to apoptosis, suggesting that HMGB1 plays dual roles in the regulation of apoptosis (Guerin et al., 2008). The precise molecular mechanisms for this effect remain largely unknown.
11.3 Autophagy
Autophagy is a highly-conserved process in many species. It is a lysosomal-mediated degradation pathway that includes multiple steps such as phagophore, autophagosome, and autolysosome formation and progression. This dynamic process is primarily controlled by members of the autophagy related gene (Atg) family and share regulators derived from other cell processes such as trafficking, proteasome, and cell death pathways (Yang and Klionsky, 2010b). In contrast to bulk autophagy, selective autophagy involves targeted removal of damaged organelles, cellular debris, microorganisms, and pathogens (Reggiori et al., 2012). These special targets include mitochondria (mitophagy) (Youle and Narendra, 2011), peroxisomes (pexophagy) (Dunn et al., 2005), lysosomes (lysophagy) (Hung et al., 2013), lipid droplets (lipophagy) (Singh et al., 2009), secretory granules (zymophagy) (Grasso et al., 2011), the ER (reticulophagy) (Bernales et al., 2007), nucleus (nucleophagy) (Park et al., 2009), RNA (RNautophagy) (Fujiwara et al., 2013), pathogens (xenophagy) (Levine, 2005), ribosomes (ribophagy) (Kraft et al., 2008), and aggregate-prone proteins (aggrephagy) (Overbye et al., 2007).
Autophagy plays dual roles in cell death depending on the response context. In many cases, upregulated autophagy increases the ability to resist cell death, whereas autophagy deficiency contributes to cell death (Kroemer and Levine, 2008). In contrast, autophagy mediates cell death under specific circumstances such as apoptosis deficiency (Klionsky and Emr, 2000; Yang and Klionsky, 2010a). Autophagy dysfunction plays a critical role in human health and disease. Currently, autophagy is a hot research field and is thought to be involved in multiple physiological and pathological processes including HMGB1 release, secretion, and degradation. In many cases, autophagy inhibition prevents HMGB1 release, secretion, and degradation (Dupont et al., 2011; Li et al., 2011e; Liu et al., 2011b; Tang et al., 2010a; Tang et al., 2010c; Thorburn et al., 2009; Zhan et al., 2012; Zhang et al., 2012e). Autophagy is also regulated by HMGB1 at multiple levels. For example, Nuclear HMGB1 as a transcriptional cofactor regulates the expression of heat shock protein β-1 (HSPB1) (Tang et al., 2011c), which in turn sustains dynamic intracellular trafficking during autophagy and mitophagy (Tang et al., 2011c). Cytosolic HMGB1 competes with Bcl-2 for interaction with Beclin-1 by intra-molecular disulfide bridge (C23/45) of HMGB1, which in turn promotes Beclin-1-mediated autophagosomes (Tang et al., 2010b). The interaction between HMGB1 and Beclin-1 is positively regulated by ULK1 (Huang et al., 2012a), MAPK (Tang et al., 2010b), and NAC (Cheng et al., 2013), but negatively regulated by p53 (Livesey et al., 2012a) and synuclein (Song et al., 2014). Extracellular HMGB1 in its reduced form promotes autophagy through binding to RAGE (Tang et al., 2010a), which may contribute to lactate production and glutamine metabolism for tumor growth (Luo et al., 2014). Indeed, RAGE is a positive regulator of autophagy and a negative regulator of apoptosis during chemotherapy, oxidative stress, DNA damage, and hypoxia (Kang et al., 2011d; Kang et al., 2010). HMGB1-mediated autophagy increases chemoresistance in cancer cells, including colon cancer, pancreatic cancer, osteosarcoma, leukemia, gastric cancer, and ovarian cancer (Huang et al., 2012a; Kang et al., 2010; Liu et al., 2011c; Livesey et al., 2012b; Yang et al., 2012e; Zhan et al., 2012; Zhang et al., 2012d; Zhao et al., 2011a). In addition, HMGB1-mediated autophagy in vitro and/or in vivo prevents polyglutamine aggregates in Huntington’s disease (Min et al., 2013), systemic inflammation during sepsis (Hagiwara et al., 2012; Yanai et al., 2013), N-methyl-D-aspartate-induced excitotoxicity (Perez-Carrion and Cena, 2013), hepatic ischemia-reperfusion injury (Fang et al., 2013b; Shen et al., 2013), and sustains T cell survival in myositis (Zong et al., 2014). Conditional knockdown of HMGB1 in the liver or heart cannot change baseline autophagy level and mitochondrial quality (Huebener et al., 2014).
11.4 Pyroptosis
Pyroptosis is an inflammation-associated cell death characterized by rapid plasma-membrane rupture and release of pro-inflammatory intracellular molecules that occurs primarily with macrophages. Caspase 1 is not involved in apoptosis, but plays a central role in pyroptosis. In contrast, caspases 3, 6, and 8 are important for apoptosis, but not involved in pyroptosis. Unlike apoptosis, loss of mitochondrial integrity and release of cytochrome c and the cleaved PARP and ICAD are not observed in pyroptosis. Caspase-1 is activated during pyroptosis by inflammasome, a large supramolecular complex largely composed of dimers of the adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain). Increasing evidence indicates that HMGB1 released during pyroptosis is regulated by classical components of inflammasome as well as the recently-identified PKR (Van Opdenbosch et al., 2014). Thus, inhibition of inflammasome activation decreases serum HMGB1 level and protects against liver ischemic injury (Kamo et al., 2013).
11.5 NETosis
NETosis is a regulated, antimicrobial cell death that occurs primarily in neutrophils, the first cells recruited to the site of infection (Remijsen et al., 2011). In 2004, Volker Brinkmann, Arturo Zychlinsky, and colleagues first reported that neutrophils release DNA-protein structures called neutrophil extracellular traps (NETs) to kill pathogens (Brinkmann et al., 2004). Indeed, pro-inflammatory stimuli (e.g., LPS, IL-8, and TNF) and infections (e.g., microorganisms and pathogens) induce NET formation, which is regulated by ROS production, the activation of NADPH oxidase, and peptidylarginine deiminase 4 (PD4)-mediated histone citrullination. Increasing evidence indicates that NETs are released in the context of cell death, called NETosis (Steinberg and Grinstein, 2007). NETs are composed of DNA, histones, granule components, and some cytoplasmic proteins, as well as HMGB1. The release of HMGB1 during NETosis is regulated by autophagy (Kambas et al., 2012; Mitroulis et al., 2011), which may be implicated in inflammatory and autoimmune diseases (Branzk and Papayannopoulos, 2013).
12 HMGB1 and Disease
12.1 Sepsis
Severe sepsis and septic shock represent major clinical problems and are the leading causes of death in patients in intensive care units worldwide (Angus and van der Poll, 2013), with an overall mortality rate of 30% in the United States (Dombrovskiy et al., 2007). This medical problem results from an exuberant and excessive host response associated with a deleterious and non-resolving systemic inflammatory response syndrome, often caused by Gram-negative bacterial infection (Vincent et al., 2009). As the major component of Gram-negative bacteria, LPS can induce the secretion and release of multiple pro-inflammatory cytokines such as TNF-α (Tracey et al., 1987), IL-1β (Dinarello and Thompson, 1991), and HMGB1 (Wang et al., 1999). Early cytokines (e.g., TNF-α and IL-1β) peak within the first hours after infection, then circulatory levels revert to near baseline in three to four hours (Tracey and Cerami, 1993). This kinetic characteristic of “early” cytokines provides a narrow therapeutic time window for clinical intervention. In resting cells, most of the HMGB1 resides in the nucleus and functions as a DNA chaperone to regulate chromosomal structure and DNA biology (Malarkey and Churchill, 2012). When actively secreted by immune cells or passively leaked from necrotic or injured cells (Scaffidi et al., 2002; Tsung et al., 2005; Wang et al., 1999), HMGB1 functions as a DAMP to mediate innate immune responses (Lotze and Tracey, 2005). HMGB1 is one of the delayed cytokines secreted by macrophages 20 hours after activation with LPS (Wang et al., 1999). In vivo, HMGB1 is first detectable in the circulation eight hours after the onset of lethal endotoxemia and sepsis, subsequently increasing to plateau levels from 16 to 32 hours (Wang et al., 1999). Meanwhile, tissue HMGB1 mRNA levels are increased in various tissues (e.g., muscle, liver, and lung) during endotoxemia (Lang et al., 2003) or thermal injury-induced sepsis (Fang et al., 2002). Administration of recombinant HMGB1 to mice recapitulates the characteristic organ dysfunction of severe sepsis, including derangement of intestinal barrier function, acute lung injury, and lethal multiple organ failure (Andersson and Tracey, 2011; Wang et al., 2001). In addition, we and others have demonstrated that administration of anti-HMGB1 antibodies (Qin et al., 2006; Valdes-Ferrer et al., 2013; Wang et al., 1999; Yang et al., 2004a), inhibitors (e.g. ethyl pyruvate (Ulloa et al., 2002), nicotine (Wang et al., 2004a), stearoyl lysophosphatidylcholine (Chen et al., 2005), quercetin (Tang et al., 2009), chloroquine (Yang et al., 2013d), spermine (Zhu et al., 2009) and Chinese herbal extracts such as Angelica sinensis (Wang et al., 2006) and Salvia miltiorrhiza (Li et al., 2007b)) or endogenous hormones (e.g., insulin (Hagiwara et al., 2008b), vasoactive intestinal peptide (Chorny and Delgado, 2008), and ghrelin (Chorny et al., 2008; Wu et al., 2009)) protect mice against lethal endotoxemia and rescue mice from cecal ligation and puncture-induced lethal experimental sepsis even when the first doses are given 24 hours after the onset of sepsis. Taken together, these results suggest that HMGB1 is released in a delayed manner and functions as a late mediator of lethal sepsis and thus as a therapeutic target with a wider time window for clinical intervention (Andersson and Tracey, 2003, 2011; Huang et al., 2010; Wang et al., 2009a; Wang et al., 2004b; Wang et al., 2008a; Waterer, 2007). HMGB1 also causes endotoxin tolerance, suggesting another face for HMGB1 in immunity (Li et al., 2013d).
12.2 Ischemia Reperfusion Injury
Ischemia reperfusion (I/R) injury is a pathophysiologic process whereby hypoxic organ damage is accentuated following return of the blood supply, triggering a sterile inflammatory response. The ischemia is due to either arterial or venous occlusion. Such pathology occurs during solid organ transplantation, trauma, stroke, hypovolemic shock, myocardial infarction, and elective liver resection. I/R injury results in leukocyte recruitment and oxidative stress at the site of injury, which in turn promote inflammatory mediator release and complement activation. These events are thought to contribute to the local tissue damage and end-organ injury during I/R in various organs. Increasing evidence suggests that HMGB1 is an important mediator of I/R injury in the liver (Kamo et al., 2013; Liu et al., 2011a; Liu et al., 2014b; Tsung et al., 2007a; Tsung et al., 2005; Watanabe et al., 2005; Yang et al., 2013e), heart (Andrassy et al., 2008; Huang et al., 2007; Kaczorowski et al., 2009a; Oozawa et al., 2008; Zhai et al., 2012), kidney (Chen et al., 2011b; Chung et al., 2008; Rabadi et al., 2012; Wu et al., 2007; Wu et al., 2010), spinal cord (Esposito et al., 2009; Gong et al., 2012; Huang et al., 2011c; Wang et al., 2009c; Zhai et al., 2012; Zhou et al., 2013b), brain (Hayakawa et al., 2008a; Huang et al., 2013b; Kim et al., 2008a; Qiu et al., 2008; Schulze et al., 2013; Tang et al., 2013a), and intestine (Hagiwara et al., 2010a; He et al., 2012a; Kojima et al., 2012b; Tetteh, 2013) and triggers a potentially injurious innate immune response (Kaczorowski et al., 2009b). HMGB1 is also a biomarker of injury in human liver and kidney transplantation (Ilmakunnas et al., 2008; Kruger et al., 2009). HMGB1 levels were increased during mice liver I/R as early as one hour after reperfusion and then increased in a time-dependent manner for up to 24 hours (Tsung et al., 2005). Inhibition of HMGB1 activity with neutralizing antibody significantly decreased liver damage following I/R (Pardo et al., 2008), whereas administration of recombinant HMGB1 worsened I/R injury (Tsung et al., 2005). The release of HMGB1 during I/R is involved in ROS and Ca2+ signaling (Tsung et al., 2007a), whereas the activity of HMGB1 is mediated by several receptors such as TLR4 (Chen et al., 2011b; Kruger et al., 2009; Tsung et al., 2005; Wu et al., 2007; Zhang et al., 2013i), TLR-9 (Bamboat et al., 2009), and RAGE (Zeng et al., 2009). Treatment with HMGB1-neutralizing antibody remarkably ameliorates hepatic, kidney, and brain infarction (Levy et al., 2007; Liu et al., 2007c; Muhammad et al., 2008; Tsung et al., 2005; Watanabe et al., 2005; Wu et al., 2007). Like ischemic preconditioning, pretreatment of mice with HMGB1 can decrease I/R injury (Du et al., 2014; Izuishi et al., 2006; Wu et al., 2013b) and promote tissue regeneration (Biscetti et al., 2011). The protection observed in mice pretreated with HMGB1 partly depends on expression of IL-1R-associated kinase-M, a negative regulator of TLR4 signaling (Izuishi et al., 2006).
12.3 Central Nervous System
12.3.1 Aging
HMGB1 was initially recognized as a heparin-binding protein abundantly expressed in rat brain neurons promoting neurite outgrowth (Rauvala and Pihlaskari, 1987). In the early phase (E14.5-E16), HMGB1 is widely expressed throughout the brain; in the late phase (E18), HMGB1 is expressed in the cortical plate and thalamic area; in the adult, HMGB1 has limited expression in the regions of neurogenesis (Guazzi et al., 2003). Total HMGB1 expression is the highest in the brains of young adults and gradually decreases during aging in the mouse brain, which is a cause for the accumulation of DNA double-strand breaks in the aged brain (Enokido et al., 2008). HMGB1 is downregulated in the neurons of the aged brain, whereas it is upregulated in astrocytes, suggesting that HMGB1 expression during aging is differentially regulated between neurons and astrocytes (Enokido et al., 2008). Once released from neuronal death, HMGB1 binds to several receptors such as RAGE, TLR-2, TLR-4, and Mac1 in microglia, which in turn accelerates neuroinflammation, injury, and further HMGB1 release (Gao et al., 2011a). In addition to nervous system development, ischemia (e.g., stroke), and injury we discussed above, HMGB1 dysfunction plays dual roles in several neurodegenerative diseases, which are primary caused by polyglutamine (polyQ) expansions in diverse proteins (Fang et al., 2012).
12.3.2 Huntington’s Disease
Huntington's disease is a progressive brain disorder caused by an expanded trinucleotide repeat (CAG)n, encoding glutamine, on chromosome 4p16.3. The aggregates formed by polyQ induce neuronal cell toxicity. Huntington's disease affects muscle coordination and leads to uncontrolled movements, psychiatric problems, and cognitive decline. The expression of HMGB1 is decreased when mutant polyQ proteins are expressed in Huntington’s disease (Qi et al., 2007). In addition, downregulation of HMGB1 in the nucleus is associated with the DNA double-strand break (DDSB)-mediated neuronal damage in Huntington’s disease (Qi et al., 2007). In addition to DDSB, HMGB1 is the cofactor of base excision repair by increasing activity of apurinic/apyrimidinic endonuclease (APE1) and 5´-flap endonuclease-1 (FEN1) (Goula et al., 2009; Liu et al., 2009; Prasad et al., 2007). APE1 and FEN1 can prevent the neuronal CAG repeat expansion associated with Huntington’s disease. In addition, HMGB1 can direct bind to polyQ aggregates and then promote degradation by autophagy or lysosomal pathways (Min et al., 2013). These findings suggest that HMGB1 regulates somatic CAG expansion via two different mechanisms.
12.3.3 Alzheimer’s Disease
Alzheimer's disease is the most common form of dementia in which the death of brain cells causes memory loss and cognitive decline. One of the pathological characteristics of Alzheimer’s disease is the formation of extracellular senile plaques with global neuronal loss, which is caused by the production and deposition of the amyloid-beta peptide (Aβ) and the presence of intracellular tau protein tangles. RAGE is a receptor for Aβ in Alzheimer's disease (Yan et al., 1996). The secreted HMGB1 impairs memory by RAGE and TLR4 (Mazarati et al., 2011). In addition, the secreted HMGB1 can aggregate to neurotic plaques and then bind Aβ, which in turn inhibits the phagocytosis and degradation of Aβ by microglial cells (Takata et al., 2003; Takata et al., 2012).
12.3.4 Parkinson’s Disease
Abnormal accumulation of alpha-synuclein filaments in Lewy bodies is a neuropathological hallmark of Parkinson's disease and sequestration of cellular protein into these protein aggregates may contribute to the degenerative process. The alpha-synuclein can bind to HMGB1 in Lewy bodies, but the significance remains unknown (Lindersson et al., 2004). The interaction between HMGB1 and alpha-synuclein inhibits HMGB1 cytosolic translocation and subsequent HMGB1-Beclin-1 interaction, therefore limiting autophagy (Song et al., 2014). In contrast, corynoxine B inhibits the interaction between HMGB1 and alpha-synuclein and rescues the impaired autophagy (Song et al., 2014). These findings indicate that alpha-synuclein impairs the autophagy pathway by binding to HMGB1 in Parkinson’s disease.
12.3.5 Multiple Sclerosis
Multiple sclerosis, also known as disseminated sclerosis or encephalomyelitis disseminata, is a chronic inflammatory central nervous system disease involving the brain, spinal cord, and optic nerves. HMGB1 and its receptors are increased in the brains of patients with multiple sclerosis and mice with experimental autoimmune encephalomyelitis (Andersson et al., 2008). HMGB1-neutralizing antibody ameliorates experimental autoimmune encephalomyelitis (Robinson et al., 2013; Uzawa et al., 2013b). These findings suggest a direct role of HMGB1 in the regulation of innate immune and inflammatory responses in the central nervous system (Das, 2012; Hoarau et al., 2011).
12.3.6 Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis is a progressive neurodegenerative disorder caused by loss of motor neurons and extensive astrogliosis and microglial activation in the motor cortex and spinal cord. HMGB1 and its receptors such as TLR2, TLR4, and RAGE are increased in reactive glia, whereas they are decreased in degenerating motor neurons in patients with amyotrophic lateral sclerosis, suggesting a possible role in the progression of inflammation and motor neuron degeneration (Casula et al., 2011; Lo Coco et al., 2007). In addition, serum HMGB1 autoantibody is increased in patients with amyotrophic lateral sclerosis compared with patients with Alzheimer's disease and Parkinson's disease (Hwang et al., 2013). These findings suggest that HMGB1 autoantibody may be a biomarker for amyotrophic lateral sclerosis (Hwang et al., 2013).
12.3.7 Neuromyelitis Optica
Neuromyelitis optica, also known as Devic’s disease, is a rare autoimmune disorder involving repeated clinical symptoms of optic neuritis or myelitis. This disease can lead to loss of vision, muscle strength, and coordination, sensory impairment, and paraplegia or even tetraplegia. Serum and cerebrospinal fluid HMGB1 levels are significantly greater in patients with neuromyelitis optica, suggesting that HMGB1 might be a diagnostic marker for neuromyelitis optica in the early stage (Uzawa et al., 2013a; Wang et al., 2013c; Wang et al., 2012a).
12.3.8 Epilepsy
Epilepsy, also known as seizure disorder, is a sudden change in behavior including loss of consciousness caused by increased electrical activity in the brain. Serum HMGB1 levels are increased in child febrile seizure patients (Choi et al., 2011). Early evidence in experimental models of seizures and in temporal lobe epilepsy indicates that HMGB1 promotes seizures in a TLR-4-dependent pathway by triggering tissue damage and the inflammatory response (Kleen and Holmes, 2010; Maroso et al., 2010). Recent studies indicate that this process requires other receptors such as IL-1 receptor, TLR2, RAGE, and NMDAR (Balosso et al., 2014; Iori et al., 2013; Maroso et al., 2011; Vezzani et al., 2011; Zurolo et al., 2011). These findings suggest that a complex receptor interaction is required for HMGB1-induced seizure.
12.3.9 Neuropathic Pain
HMGB1 is involved in pathophysiological pain from cancer (Tong et al., 2010), acute appendicitis (Albayrak et al., 2011), type 2 diabetes (Ren et al., 2012), bladder pain (Tanaka et al., 2014), and neuropathic pain (Maeda et al., 2013). Neuropathic pain is caused by nervous system injury and persistent alterations in pain sensitivity. HMGB1 is released from neurons and satellite cells after nerve injury and can enhance pain hypersensitivity via RAGE or TLR4 (Feldman et al., 2012; Kuang et al., 2012; Maeda et al., 2013). In contrast, HMGB1-neutralizing antibody inhibited pain onset in a neuropathic pain model (Otoshi et al., 2011; Shibasaki et al., 2010). A recent study indicated that Panx1 channel-mediated HMGB1 released from neurons is a mediator of migraine from spreading depression (Karatas et al., 2013). Blockade of panx-1 channels by carbenoxolone inhibits HMGB1 release in neurons as well as macrophages, which may be involved in the PKR-signaling pathway (Karatas et al., 2013; Li et al., 2013e).
12.3.10 Meningitis
Meningitis is an acute inflammation of the protective membranes covering the brain and spinal cord. Meningitis may develop in response to a number of causes such as bacteria, viruses, physical injury, cancer, or drugs. HMGB1 in the cerebrospinal fluid sustains inflammation and brain damage in meningitis, including bacterial, aseptic, and tuberculous meningitis (Asano et al., 2011; Carrol et al., 2009; Eisenhut, 2008; Hohne et al., 2013; Tang et al., 2008a). Of them, HMGB1 levels are the highest in patients with bacterial meningitis (Tang et al., 2008a). These findings suggest that HMGB1 in the cerebrospinal fluid sustains biomarkers for neurological infection diseases.
12.4 Vascular Disease
The vascular system includes the arteries, veins, and capillaries that carry blood to and from the heart. Vascular disorders are diseases of the blood vessels. HMGB1 is implicated in vascular disorders, in particular systemic vasculitis and atherosclerosis (de Souza et al., 2012).
12.4.1 Systemic Vasculitis
Systemic vasculitis is thought to be an autoimmune disease characterized by inflammation of the blood vessel walls. Recent studies have found that serum HMGB1 levels are increased in patients with systemic vasculitis diseases such as Kawasaki syndrome (Eguchi et al., 2009; Hoshina et al., 2008), Churg-Strauss syndrome (Taira et al., 2007), Henoch-Schönlein purpura, and antineutrophil cytoplasmic antibody-associated vasculitis (Bruchfeld et al., 2011; de Souza et al., 2013a; Sato et al., 2008; Wibisono et al., 2010). Active immune cells as well as damaged cells are the sources of increased HMGB1 release in serum. These findings suggest that HMGB1 may be a biomarker for systemic vasculitis activity.
12.4.2 Atherosclerosis
Atherosclerosis is an inflammatory condition in which an artery wall thickens due to the accumulation of lipids. The pathogenesis of the atherosclerotic plaque in the vessel wall is a dynamic process that includes vascular injury, monocyte recruitment, macrophage activation, lipid deposition, platelet degranulation and aggregation, vascular smooth muscle cell migration, proliferation, and extracellular matrix synthesis (Caplice et al., 2003). In the normal human vessel wall, HMGB1 is expressed in endothelial cells, smooth muscle cells, and macrophages (Kalinina et al., 2004). In contrast, HMGB1 is overexpressed and released from several cell types in human atherosclerotic lesions including vascular smooth muscle cells, endothelial cells, foam cells, macrophages, and activated platelets (Inoue et al., 2007; Kalinina et al., 2004; Peng et al., 2006; Yao and Brownlee, 2009). Extracellular HMGB1 can stimulate vascular endothelial cells or smooth muscle cells to express and/or secrete adhesion molecules, cytokines, chemokines, plasminogen activator, vasoactive substances, lipid mediators, and matrix metalloproteinases, as well as the receptor RAGE (Fuentes et al., 2014; Jaulmes et al., 2006; Li et al., 2006b; Porto et al., 2006; Treutiger et al., 2003). In addition to unregulated HMGB1, RAGE was directly responsible for HMGB1 activity in atherosclerotic plaque formation in a transgenic mouse model of atherosclerosis (apolipoprotein E-deficient mice) (Basta, 2008; Harja et al., 2008; Inoue et al., 2007; Kanellakis et al., 2011; Lee et al., 2013b; Liu et al., 2013c; Soro-Paavonen et al., 2008). Oxidative stress and NF-κB activation is involved in HMGB1-RAGE mediated atherosclerosis (Soro-Paavonen et al., 2008). Of note, other RAGE ligands such as S100A8/A9 and AGE also contribute to the development of atherosclerosis (Basta, 2008; Harja et al., 2008).
12.4.3 Abdominal Aortic Aneurysm
An abdominal aortic aneurysm (AAA) refers to an aortic diameter at least one and one-half times the normal diameter at the level of the renal arteries, which is approximately 2.0 cm (Aggarwal et al., 2011). HMGB1 expression is increased in the aortic wall of AAA patients. HMGB1-neutralizing antibody prevented disease progression and inflammatory cell infiltrations in a mouse AAA model (Kohno et al., 2012), indicating a pathogenic role of HMGB1 in AAA.
12.5 Heart Diseases
12.5.1 Heart Failure
Heart failure is a chronic condition with considerable morbidity and mortality that occurs when the heart’s muscle becomes too damaged to adequately pump the blood through the body. Inflammation has emerged as a critical biological process contributing to nearly all aspects of cardiovascular diseases, including heart failure. HMGB1 and RAGE levels are increased in patients with heart failure (Volz et al., 2010b; Wang et al., 2011c) and serum HMGB1 is an independent predictor of death in heart failure from heart transplantation (Volz et al., 2012). The HMGB1-RAGE pathway sustains the inflammatory response in inflammatory cardiomyopathy, eventually leading to heart failure (Volz et al., 2010a). Thus, inhibition of HMGB1 activity and sustaining HMGB1 expression could prevent heart failure (Du et al., 2013; Funayama et al., 2013).
12.5.2 Myocardial Infarction
Myocardial infarction (MI), also known as heart attack, is the irreversible process of myocardial cell necrosis secondary to prolonged ischemia. HMGB1 plays dual roles in MI (Ding and Yang, 2010; Jiang and Liao, 2010; Li et al., 2006b; Volz et al., 2010b). On one hand, HMGB1 can enhance myocardial regeneration and repair by its angiogenic, vasculogenic, and stem cell self-renewal abilities (Germani et al., 2007; Limana et al., 2013; Limana et al., 2005; Rossini et al., 2008). Thus, administration of exogenous HMGB1 protein restores cardiac function and improves survival post-MI (Abarbanell et al., 2011; Kitahara et al., 2008; Takahashi et al., 2008). On the other hand, HMGB1 acts as a potent pro-inflammatory cytokine that accelerates MI and ischemic injury (Xu et al., 2011b; Zhai et al., 2012). Thus, elevated serum HMGB1 levels are associated with adverse clinical outcomes in patients with MI (Cirillo et al., 2009; Giallauria et al., 2010; Kohno et al., 2009; Sorensen et al., 2011b).
12.5.3 Acute Coronary Syndrome
Acute coronary syndrome refers to any condition brought on by sudden, reduced blood flow to the heart. Serum HMGB1 levels are higher in patients with acute coronary syndrome than in controls, suggesting that HMGB1 may be a potential and independent predictor of cardiovascular mortality in patients (Cirillo et al., 2009; Goldstein et al., 2006; Hashimoto et al., 2012; Peter and Bobik, 2012; Yamada et al., 2006).
12.5.4 Cardiac Hypertrophy
Cardiac hypertrophy is associated with many forms of heart disease, including ischemic disease, hypertensive heart disease, and valvular stenosis, and is a major risk factor for the development of heart failure and death (Funayama et al., 2013). Hypertrophic stimulation increases translocation of HMGB1 from the nucleus to the cytoplasm, which is associated with DNA damage and cardiomyocyte hypertrophy (Funayama et al., 2013). In contrast, activation of PPARα by fenofibrate inhibits HMGB1 cytoplasmic translocation and prevents cardiac hypertrophy development (Jia et al., 2014b). In addition, exogenous HMGB1 stimulates cardiac regeneration (Takahashi et al., 2008). These findings indicate that nuclear HMGB1 is a negative regulator of cardiomyocyte hypertrophy, whereas extracellular HMGB1 promotes cardiomyocyte hypertrophy.
12.6 Kidney Disease
12.6.1 Glomerulonephritis
Glomerulonephritis, also known as glomerular nephritis, is characterized by inflammation of the glomeruli, the basic filtration units of the kidney. HMGB1 is expressed in patients with glomerulonephritis (Sato et al., 2008). In vivo, the expression of HMGB1 and its receptors (RAGE and TLR4) are upregulated in granulomas in adenine-fed rat kidneys. Furthermore, HMGB1 increased the expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) expression, which in turn recruited more macrophages to sustain HMGB1 release during granulomatous inflammation and injury (Oyama et al., 2010). These findings suggest that the HMGB1-RAGE/TLR4-MCP-1 pathway is involved in glomerulonephritis.
12.6.2 Lupus Nephritis
Lupus nephritis is kidney inflammation caused by systemic lupus erythematosus. Serum HMGB1, serum anti-HMGB1 antibodies, and urinary HMGB1 levels are increased in patients with lupus nephritis (Abdulahad et al., 2012; Abdulahad et al., 2011; Ma et al., 2012a; Zickert et al., 2012). In addition, HMGB1 expression positively correlates with MCP-1 expression, suggesting interplay between HMGB1 and MCP-1 in the pathogenesis of lupus nephritis (Pisetsky, 2012a; Zhou et al., 2011a). HMGB1 acts as a pro-inflammatory mediator by RAGE or TLR2/4 in antibody-induced kidney damage in systemic lupus erythematosus (Qing et al., 2008). In lupus-prone MRL/lpr mice, HMGB1 released by DCs participates in the autoimmunity response (Iwata et al., 2009). Thus, inhibition of HMGB1 expression and/or release can alleviate lupus nephritis in MRL/lpr mice (Gu et al., 2010). These findings suggest that HMGB1 plays an important role in lupus nephritis (D'Agati and Schmidt, 2010; Zhu et al., 2013b).
12.6.3 Diabetic Nephropathy
Diabetic nephropathy, also known as Kimmelstiel-Wilson syndrome, is the leading cause of chronic kidney disease in the world. Hyperglycemia-mediated HMGB1 release induces renal injury and tubulointerstitial inflammation through the RAGE/TLR4/TLR2 receptor and downstream NF-κB activation (Bierhaus et al., 2005; Kim et al., 2011a; Li et al., 2010a; Lin et al., 2013; Lin et al., 2012b; Penfold et al., 2010). However, serum HMGB1 levels are decreased in patients with diabetic nephropathy (Penfold et al., 2010). Further study is needed to explain this paradox finding in the future.
12.6.4 Autosomal Dominant Polycystic Kidney Disease
Autosomal dominant polycystic kidney disease is a genetic condition that causes multiple cysts to develop on the kidneys. Serum HMGB1 level is increased and correlates with oxidative stress status in autosomal dominant polycystic kidney disease (Nakamura et al., 2011a; Nakamura et al., 2012a).
12.6.5 Chronic Allograft Dysfunction
Chronic allograft dysfunction is associated with a variety of fibrosing and sclerosing changes in the allograft that cause a gradual worsening of renal function. HMGB1 promotes inflammation through the TLR4-MyD88-TRIF pathway in chronic allograft dysfunction, which leads to renal transplant loss (Wang et al., 2010e).
12.6.6 Chronic Kidney Disease
Chronic kidney disease is associated with inflammation and malnutrition that cause a loss of kidney function over time. Serum HMGB1 level is increased in patients with chronic kidney disease (Bruchfeld et al., 2008; Nakamura et al., 2009b). Through binding to TLR2 and RAGE, HMGB1 enhances the inflammatory response, immune-mediated epithelial-mesenchymal transition, and renal fibrosis in the development of chronic kidney disease (D'Agati and Schmidt, 2010; Leelahavanichkul et al., 2011; Leemans et al., 2009; Zakiyanov et al., 2013a).
12.6.7 Clear Cell Renal Cell Carcinoma
Renal cell carcinoma is the most common kidney cancer in adults. HMGB1 expression is increased in clear cell renal cell carcinoma, in which HMGB1 is methylated and translocates to the cytoplasm (Wu et al., 2013a). The HMGB1-RAGE pathway is involved in the progression of clear cell renal cell carcinoma through activation of ERK1/2 signaling (Lin et al., 2012a).
12.7 Autoimmune Disease
Autoimmune diseases arise from an abnormal immune response against healthy body tissue. HMGB1 plays a critical role in regulating innate and adaptive immune responses by itself as well as in association with other endogenous and exogenous molecules (Bianchi and Manfredi, 2007; Harris et al., 2012; Magna and Pisetsky, 2014). Dysfunction of HMGB1 has been implicated in several autoimmune diseases that may affect one or more organ or tissue types such as blood vessels, connective tissues, endocrine glands (e.g., thyroid or pancreas), joints, muscles, red blood cells, and skin (Voll et al., 2008a; Voll et al., 2008b). Here, we focus on the role of HMGB1 in arthritis, systemic lupus erythematosus, and myositis.
12.7.1 Arthritis
Rheumatoid arthritis is a chronic, systemic, inflammatory disease that triggers an autoimmune reaction, resulting in synovial hypertrophy and joint inflammation. Rheumatoid arthritis is the first confirmed autoimmune disease linking HMGB1 to immune-mediated conditions (Andersson and Erlandsson-Harris, 2004; Pisetsky et al., 2008). The expression of HMGB1 is increased at the site of joint inflammation, including the synovial tissue and synovial fluid (Hamada et al., 2008; Kokkola et al., 2002; Taniguchi et al., 2003). Serum HMGB1 level is increased in patients with rheumatoid arthritis (Pullerits et al., 2011). Importantly, injection of HMGB1 into a normal joint caused NF-κB activation, IL-1β production, and the development of arthritis in 80% of animals in one study (Garcia-Arnandis et al., 2010b; Pullerits et al., 2003). HMGB1-triggered joint inflammation is not mediated through the TNF-α pathway (Goldstein, 2008; Pullerits et al., 2008; Sundberg et al., 2008). HMGB1 forms complexes with IL-1α, IL-1β, and LPS to enhance immune and inflammatory responses at the joint (Qin et al., 2014; Wahamaa et al., 2011). Moreover, therapeutic targeting of HMGB1 (e.g., HMGB1 neutralizing antibodies, recombinant A box, recombinant thrombomodulin, soluble RAGE, oxaliplatin, gold salts, and glucocorticoid) inhibits HMGB1 release and activity, which prevents the progression of arthritis in experimental animals (af Klint et al., 2005; Bossaller and Rothe, 2013; Goldstein et al., 2007; Hamada et al., 2008; Ostberg et al., 2010; Ostberg et al., 2008; Takahashi et al., 2013b; Yuan et al., 2008; Zetterstrom et al., 2008). These findings suggest that HMGB1 is an important target in the treatment of rheumatoid arthritis (Andersson and Harris, 2010).
12.7.2 Systemic Lupus Erythematosus
Systemic lupus erythematosus is a heterogeneous and multi-organ-involved autoimmune disease characterized by the production of a broad spectrum of autoantibodies directed to nuclear and cytoplasmic antigens. These autoantibodies can form immune complexes with other molecules, including nucleosome and HMGB1, to induce pro-inflammatory cytokine release in local tissue and whole body by RAGE and TLR9 receptors (Tian et al., 2007; Urbonaviciute et al., 2008). The expression of HMGB1 with translocation and release is increased in skin lesions in patients with systemic lupus erythematosus (Popovic et al., 2005). Ultraviolet radiation can enhance HMGB1 translocation and release, suggesting a mechanism for photosensitivity that flares disease (Barkauskaite et al., 2007). Serum HMGB1 is increased in patients with systemic lupus erythematosus and correlates with disease activity index and anti-dsDNA (Abdulahad et al., 2012; Abdulahad et al., 2011; Jiang and Pisetsky, 2008a; Ma et al., 2012a). HMGB1-nuclesome complex released from apoptotic cells can induce anti-DNA antibody production in mice, for which TLR2, MyD88, and microRNA-155 is required (Sanford et al., 2005; Urbonaviciute et al., 2008; Urbonaviciute et al., 2013; Wen et al., 2013). Interestingly, patients with systemic lupus erythematosus or other autoimmune diseases (e.g., arthritis) can create anti-HMGB1 autoantibodies (Abdulahad et al., 2011; Hayashi et al., 2009; Qing et al., 2008; Wittemann et al., 1990). Sadly, there is a clear lack of blocking the effects of HMGB1 in lupus animal models or in systemic lupus erythematosus patients (Pan et al., 2010; Pisetsky, 2010; Schaper et al., 2014).
12.7.3 Myositis
Myositis is a rare disease in which the immune system chronically inflames the body's own healthy muscle tissue. HMGB1 expression as well as HMGB1 cytoplasmic translocation and release are increased during myositis compared with control samples from healthy donors, suggesting a potential role of HMGB1 in mysitis (Ulfgren et al., 2004). Indeed, HMGB1 induced MHC-class I expression and muscle fatigue through binding to TLR4, but not RAGE (Grundtman et al., 2010; Zong et al., 2013). HMGB1-Beclin-1 complex-mediated autophagy may contribute to T cell survival in the muscles of patients with myositis (Zong et al., 2014). HMGB1 also has the ability to promote muscle fiber regeneration (De Mori et al., 2007; Vezzoli et al., 2011), suggesting a dual role of HMGB1 in the regulation of inflammatory myopathy.
12.8 AIDS
Acquired immunodeficiency syndrome (AIDS) is a disease of progressive human immune system failure caused by infection with human immunodeficiency virus (HIV) at advanced stage. Despite advances, HIV remains a major public health challenge since it was discovered in the early 1980s. Plasma HMGB1 levels are elevated and related to viral load and MD2/TLR4 in patients with HIV infection (Nowak et al., 2007; Troseid et al., 2013; Troseid et al., 2010). Intracellular HMGB1 inhibits long terminal repeat (LTR)-mediated HIV transcription (Naghavi et al., 2003), whereas extracellular HMGB1 has a dual role in the regulation of HIV transcription, depending on the stage of infection and type of cell (Cassetta et al., 2009; Nowak et al., 2006). HMGB1 can be passively released by virus-infected cells such as CD4 T (Barqasho et al., 2010), which is required for HIV-1-induced impairment of NK-DC crosstalk and subsequent viral persistence (Gougeon and Bras, 2011; Melki et al., 2010; Saidi et al., 2008). HMGB1 also forms a complex with PAMPs (e.g., LPS) to amplify inflammatory loops and increase viral replication in HIV disease (Troseid et al., 2010). These findings suggest that HMGB1 has an important role in HIV infection (Gougeon and Bras, 2011; Gougeon et al., 2012; Troseid et al., 2011).
12.9 Diabetes
Diabetes is a group of metabolic diseases associated with high blood sugar, either because of lack of insulin (Type 1), or insulin resistance (Type 2). Type 1 diabetes, also known as autoimmune or juvenile diabetes, is an autoimmune disease. Cytokine-mediated inflammatory damage is believed to contribute to the loss of β-cell mass and function during the development of autoimmune diabetes. IL-1β-induced β-cell necrosis and HMGB1 release in a nitric oxide-dependent manner may contribute to insulitis progression and diabetes onset (Han et al., 2008; Steer et al., 2006). Hyperglycemia-mediated oxidative stress promotes HMGB1 and RAGE expression (Yao and Brownlee, 2010). Loss of RAGE and TLR4 inhibits diabetes onset (Li et al., 2012c; Soro-Paavonen et al., 2008). During islet transplantation for the treatment of type 1 diabetes mellitus, the HMGB1-mediated inflammatory response can cause early islet loss through TLR2 and TLR4 (Itoh et al., 2012c; Kruger et al., 2010; Matsuoka et al., 2010; Tamura et al., 2011). In addition to type 1 diabetes, HMGB1-mediated inflammation and angiogenesis also facilitate the onset of type II diabetes (Biscetti et al., 2010; Skrha et al., 2012).
12.10 Lung Disease
12.10.1 Asthma
Asthma is a chronic obstructive lung disease characterized by chronic inflammation of the respiratory tract. Asthma is more commonly associated with Th2-mediated eosinophilic airway inflammation, although neutrophil inflammation also exists. Serum or sputum HMGB1 levels are increased in patients with asthma and are related to disease severity (Hou et al., 2011a; Shim et al., 2012; Watanabe et al., 2011; Zhou et al., 2012c). These findings suggest that HMGB1 plays a role in the pathogenesis of asthma and may be a useful marker for assessing the degree of airway obstruction.
12.10.2 Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD), a leading cause of morbidity and mortality worldwide, is associated with chronic inflammation predominantly in the small airways and lung parenchyma, which causes progressive airway obstruction (Barnes, 2013). Macrophages, neutrophils, and T lymphocytes can release cytokines, including IL-1β and HMGB1, which are responsible for sustaining inflammation and remodeling during COPD. Serum or sputum HMGB1 as well as other RAGE ligands are increased in COPD patients, suggesting that the RAGE pathway is critical in the pathophysiology of COPD (Ferhani et al., 2010; Hou et al., 2011a; Kanazawa et al., 2012; Ko et al., 2013; Pouwels et al., 2014; Sukkar et al., 2012).
12.10.3 Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome, a potentially devastating form of acute inflammatory lung injury, is a major cause of morbidity and mortality in critically ill patients. HMGB1 released by dying or dead cells is a mediator of lung inflammation and acute injury (Lutz and Stetkiewicz, 2004). Injection of HMGB1 in the lung, but not HMGB2, can cause acute respiratory distress syndrome in mice (Ueno et al., 2004). In addition, serum HMGB1 level is increased and related to death in patients with acute lung injury (Nakamura et al., 2011b; Ueno et al., 2004). In vivo, inhibition of HMGB1 release and activity prevents acute lung injury in animal studies (Gong et al., 2008; Hagiwara et al., 2008a; Hagiwara et al., 2008b; Hagiwara et al., 2008c; Hagiwara et al., 2008d; Hagiwara et al., 2007; Kim et al., 2005; Kudo et al., 2013; Lin et al., 2005). These findings suggest that HMGB1 is a therapeutic target for acute respiratory distress syndrome.
12.10.4 Cystic Fibrosis
Cystic fibrosis is a genetic disease that causes thick, sticky mucus to form in the lungs, digestive tract, and other organs. In the lung, mucus causes progressive obstruction of the airways, lung damage, neutrophil-predominant airway inflammatory response, as well as acute pulmonary exacerbations (Gaggar et al., 2010). HMGB1 expression in the sputum contributes to the inflammatory response and lung matrix degradation in cystic fibrosis airway disease (Rowe et al., 2008) and is a potential biomarker for predicting clinical outcome and treatment response (Liou et al., 2012). Moreover, inhibition of the HMGB1-TLR4 signaling pathway prevents development of cystic fibrosis airway disease partly through enhancing phagocytic activity and bacterial clearance (Enomoto et al., 2008; Entezari et al., 2012; Griffin et al., 2013).
12.10.5 Pneumonia
Pneumonia is an inflammatory condition of the lung caused by bacteria, a virus, or fungi. Community-acquired pneumonia is pneumonia acquired infectiously from normal social contact, as opposed to being acquired during hospitalization (termed hospital-acquired pneumonia). Increased serum HMGB1 level is caused by bacteria or virus in both community-acquired and hospital-acquired pneumonia (Achouiti et al., 2013; Angus et al., 2007; Entezari et al., 2012; Ito et al., 2011; Kosai et al., 2008; Sharma et al., 2013; Tasaka et al., 2010; van Zoelen et al., 2008; Zhou et al., 2011d). Both RAGE and TLR4 play roles in the regulation of HMGB1-mediated inflammatory response during pneumonia (Achouiti et al., 2013; Entezari et al., 2012; Ramsgaard et al., 2011).
12.10.6 Lung Cancer
Lung cancer, one of the most common cancers, is a leading cause of cancer death in the world. About 85–90% of lung cancer is non-small-cell lung cancer (NSCLC). Mutations in epidermal growth factor receptor (EGFR), K-RAS, and anaplastic lymphoma kinase (ALK) have been proposed as the initiating genetic lesions in NSCLC. Cigarette smoking is the number one risk factor for lung cancer. HMGB1 expression is increased in the lungs of patients with NSCLC and correlates with disease development, invasion, and metastasis (Liu et al., 2010a; Shen et al., 2009a; Zhang et al., 2013a; Zhang et al., 2013g). Moreover, serum HMGB1 may be a biomarker for NSCLC (Shang et al., 2009). miR-218 functions as a tumor suppressor in lung cancer partly thorough downregulation of HMGB1 expression and subsequently, metastasis (Zhang et al., 2013a). HMGB1 regulates MMP-9 expression and cellular metastatic ability in lung cancer cells thorough active PI3K-Akt and NF-κB pathways (Liu et al., 2010a).
12.11 Pancreas Disease
12.11.1 Pancreatitis
Acute pancreatitis (AP) is an inflammatory process of the pancreatic gland that exhibits a broad clinical spectrum; its severity may vary from mild and edematous to a serious, necrotizing disease with high morbidity and mortality (Whitcomb, 2006). AP is involved in a complex cascade of immunological events, including inflammatory mediator production, which affects not only the pathogenesis but also the course of the disease. Some of these inflammatory mediators are initially released by pancreatic acinar cells and result in the recruitment and activation of neutrophils, monocytes, and macrophages (Gea-Sorli and Closa, 2010; Satoh et al., 1998; Shrivastava and Bhatia, 2010; Sugita et al., 1997), which lead to further acinar cell injury. When released, these mediators gain access to the systemic circulation and play a central role in the progression of SIRS and multisystem organ failure (Hegyi et al., 2011). In patients and animals with AP, serum levels of HMGB1 are significantly elevated and positively correlate with the severity of this disease (Kocsis et al., 2009; Yasuda et al., 2007; Yasuda et al., 2006). Importantly, inhibiting the release or cytokine activity of HMGB1 (e.g., HMGB1 neutralizing antibody, ethyl pyruvate, danaparoid sodium, cisplatin, A box, antithrombin III, and pyrrolidine dithiocarbamate) confers protection against experimental AP (Cheng et al., 2007; Hagiwara et al., 2009g; Jo et al., 2013; Luan et al., 2013a; Luan et al., 2012; Luan et al., 2013b; Sawa et al., 2006; Weng et al., 2012; Yan et al., 2012a; Yang et al., 2009b; Yang et al., 2008; Yuan et al., 2009; Zhang et al., 2010). However, intracellular HMGB1 in the pancreas protects against acute pancreatitis (Kang et al., 2013b). Loss of endogenous HMGB1 within the injured pancreatic acinar cell worsens the severity of experimental AP with increased DNA damage, cell death, nDAMP release, and inflammatory response (Kang et al., 2013b). These findings suggest that HMGB1 plays dual roles in AP.
12.11.2 Pancreatic Cancer
Pancreatic cancer is a major, unsolved, public health problem in the world. It ranks as the fourth leading cause of cancer death in USA (Siegel et al., 2011), with advanced stage at diagnosis and poor response to current treatments (Hidalgo, 2010; Mazur and Siveke, 2011). Pancreatic ductal adenocarcinoma (PDAC), the most common type of pancreatic cancer, progresses from non-invasive pancreatic lesions termed pancreatic intraepithelial neoplasias (Hruban and Adsay, 2009). Mutations of the K-RAS gene occur in over 90% of pancreatic carcinomas and are proposed to be the initiating genetic lesion in PDAC (Bardeesy and DePinho, 2002; Hong et al., 2011; Maitra and Hruban, 2008; Morris et al., 2010b). In addition, PDAC commonly displays reactivation of embryonic signaling pathways in the early stage (Morris et al., 2010a; Thayer et al., 2003; Wang et al., 2009b). Serum HMGB1 is increased in pancreatic cancer patients with or without chemotherapy (Dong Xda et al., 2007; Wittwer et al., 2013a). HMGB1 release from necrotic or inflammatory cells promotes ATP production and pancreatic cancer growth through RAGE (Kang et al., 2014). Knockdown of HMGB1 or its receptor RAGE by RNAi or antisense nucleotide inhibits pancreatic cancer cell invasion and enhances chemotherapy sensitivity partly by downregulation of autophagy (Huang et al., 2004; Kang and Tang, 2012; Kang et al., 2011d; Kang et al., 2011e; Kang et al., 2010; Tang et al., 2010e). Moreover, loss of RAGE inhibits oncogenic K-RAS driven pancreatic tumorigenesis (Kang et al., 2012b). These findings suggest that the HMGB1-RAGE pathway is an important regulator of pancreatic cancer growth and therapy.
12.12 Stomach
12.12.1 Gastritis
Helicobacter pylori, discovered by Barry Marshall and Robin Warren, is a Gram-negative bacterium that infects over half of the world's population (1983). H. pylori infection causes gastric mucosal inflammation, which is an established risk factor for the development of peptic ulcer disease and gastric cancer. H. pylori and its active component VacA can induce HMGB1 release in gastric epithelial cells (Radin et al., 2011). This process is regulated by PARP-1-dependent necrosis (Radin et al., 2011).
12.12.2 Gastric Mucosal Injury
Gastric mucosal injury occurs when causative agents such as gastric acid, ethanol, and drugs (e.g., nonsteroidal anti-inflammatory drugs) overwhelm the mucosal defense. Recent studies indicate that HMGB1 is an important mediator of gastric mucosal injury by activation of RAGE and the TLR4-dependent inflammatory pathway (Nadatani et al., 2013; Ye et al., 2013a; Ye et al., 2013b). Inhibition of the HMGB1signaling pathway decreases gastric mucosal injury.
12.12.3 Gastric Cancer
Gastric cancer is the second most common cause of cancer-related death in the world and is most frequently discovered in advanced stages. The mRNA and protein expression levels of HMGB1 are increased and associated with inflammatory status in gastric cancer (Akaike et al., 2007; Xiang et al., 1997). Serum HMGB1 is a potential early diagnostic marker for gastric cancer (Chung et al., 2009). In addition to HMGB1, expression of RAGE is highly related to the invasive and metastatic activity of gastric cancer by enhancing NF-κB activity (Kuniyasu et al., 2002; Oue et al., 2005; Zhang et al., 2014a; Zhang et al., 2012a). During chemotherapy, HMGB1-mediated autophagy decreases vincristine-induced apoptosis in gastric cancer partly through upregulation of Mcl-1, a Bcl-2 family member (Zhan et al., 2012). However, overexpression of HMGB1 may mark good prognosis of gastric cancer after anticancer therapy (Bao et al., 2010). These findings suggest that HMGB1 plays different roles in the different gastric cancer stages.
12.13 Intestine
12.13.1 Intestinal Inflammation Disease
Inflammatory bowel disease refers to two chronic intestinal inflammation diseases: ulcerative colitis and Crohn's disease. In the gastrointestinal tract, increased levels of HMGB1 have positively correlated with intestinal barrier dysfunction and colonic inflammation in mice (Dave et al., 2009; Liu et al., 2006; Luan et al., 2010; Maeda et al., 2007; Sappington et al., 2002; Wu et al., 2009; Yang et al., 2009a). Moreover, fecal HMGB1 is a novel biomarker of intestinal mucosal inflammation in patients with inflammatory bowel disease (Vitali et al., 2011), whereas serum HMGB1 is diagnostic marker in patients with acute appendicitis (Albayrak et al., 2011; Soreide, 2011; Wu et al., 2012a). TLR4 and TLR2 are required for the HMGB1-mediated inflammatory response and injury in inflammatory bowel disease, as well as necrotizing enterocolitis (Dai et al., 2010; Downard et al., 2011; Lu et al., 2013b; McDonnell et al., 2011; Zamora et al., 2005).
12.13.2 Colorectal Cancer
Colorectal cancer is the second leading cause of cancer-related mortality in men and women in the United States. Gene mutations (e.g., APC, K-RAS, and p53), chromosomal instability, DNA-repair defects, and aberrant DNA methylation have been identified as molecular genetic bases of colorectal cancer. Nuclear HMGB1 may be an important regulatory factor for these molecular genetic bases (Balasubramani et al., 2006; Breikers et al., 2006; Yu et al., 2006a). HMGB1 expression is increased in patients with colorectal cancer and correlates with tumor progression and poor prognosis (Liu et al., 2008c; Wiwanitkit, 2010; Yao et al., 2010). Interestingly, anti-HMGB1 autoantibody is increased in serum from patients with colorectal cancer, although the significance of this change remains unclear (Kijanka et al., 2010). E-selectin promotes HMGB1 release in metastatic colorectal carcinoma cells, which in turn enhances E-selectin expression by endothelial cells, suggesting a novel mechanism regulating HMGB1 release and cancer metastasis. An early study indicated that extracellular HMGB1 induces macrophage apoptosis and inhibits macrophage infiltration into colon cancer, which might affect host immunity against cancer (Aychek et al., 2008; Kuniyasu et al., 2003a; Kuniyasu et al., 2004; Sasahira et al., 2005b). Recent studies indicate that phosphorylated HMGB1 released from colon cancer cells can promote tumor cell migration (Kang et al., 2009) by RAGE (Harada et al., 2007). Knockdown of HMGB1 increases chemotherapy sensitivity in clone cancer cells partly by regulating p53-mediated autophagy and apoptosis (Livesey et al., 2012c; Livesey et al., 2012d). HMGB1 released from chemotherapy (e.g., oxaliplatin)-induced cell death could increase antitumor immunity in colon cancer cells (Tesniere et al., 2010).
12.14 Liver Disease
In addition to liver I/R (Bamboat et al., 2010; Cai et al., 2013; Cardinal et al., 2009; Dhupar et al., 2011; Evankovich et al., 2010; Huang et al., 2013a; Izuishi et al., 2006; Kang et al., 2011a; Li et al., 2013a; Liu et al., 2013b; Nace et al., 2013; Ogiku et al., 2011; Oishi et al., 2012; Tsung et al., 2005; Watanabe et al., 2005; Zeng et al., 2009), transplantation (Ilmakunnas et al., 2008; Kao et al., 2008) and hepatocyte regeneration (Ogiku et al., 2011; Yang et al., 2012g; Zhou et al., 2011b) (discussed above), HMGB1 is implicated in several liver diseases (discussed below) (Chen et al., 2013c).
12.14.1 Viral Hepatitis
Viral hepatitis is inflammation of the liver caused by viruses, including hepatitis A-E. Viral hepatitis infection may eventually develop into liver cirrhosis and hepatocellular carcinoma (Albayrak et al., 2010). HMGB1 serum levels are increased in patients with chronic hepatitis B virus (HBV) and are related to the disease stage (Cheng et al., 2008). In response to HCV or HBV infection, HMGB1 translocates to the cytoplasm and is then released into the extracellular space. Extracellular HMGB1 is an important mediator for the inflammatory and immune responses through activating a TLR4-dependent antiviral response (Jung et al., 2011; Zhou et al., 2011b). HMGB1 also inhibits regulatory T-cell activity that contributes to liver failure in chronic HBV patients (Wang et al., 2010d). These findings shed light on the pathogenesis of viral hepatitis, which is mediated by HMGB1.
12.14.2 Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease (NAFLD), one of the most common causes of chronic liver disease, is an inflammation of the liver characterized by an accumulation of lipid deposits, which can result in liver damage, including steatosis, nonalcoholic steatohepatitis, fibrosis, and cirrhosis (Day and James, 1998). Extracellular HMGB1 enhances the inflammatory response and liver damage at the early stage of NAFLD. This process requires the TLR4-MyD88 signaling pathway; TLR4−/− and MyD88−/− mice showed impaired HMGB1-induced liver dysfunction after high fat treatment (Li et al., 2011d). Inhibition of HMGB1 activity by neutralizing antibody decreased production of pro-inflammatory cytokines (e.g., TNF-α and IL-6) and protected against experimental NAFLD (Li et al., 2011d). These findings indicate that HMGB1 functions as a novel mediator linking acute damage and the inflammatory response in NAFLD.
12.14.3 Liver Fibrosis
Liver fibrosis, the final common pathway of chronic liver diseases, is characterized by the accumulation of excess extracellular matrix, including collagen (Guo and Friedman, 2007). In vitro evidence indicates that HMGB1 is involved in liver fibrosis through single stimulating hepatic stellate cell proliferation (Kao et al., 2008) or combining with transforming growth factor β1 to induce fibrogenic protein expression (Zhang et al., 2012f). Moreover, the TLR4-MyD88 and MAPK-NF-κB pathways are required for HMGB1-mediated liver fibrosis (Wang et al., 2013b; Zhang et al., 2012f). An in vivo hepatic fibrosis animal study showed that HMGB1 expression closely correlates with collagen deposition. Knockdown of HMGB1 by siRNA significantly decreases expression of α-SMA and collagen (type I and III) in hepatic stellate cells (Ge et al., 2011). These studies have demonstrated that both intracellular and extracellular HMGB1 are positive regulators for liver fibrosis (Albayrak et al., 2010).
12.14.4 Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC), the fifth most common form of cancer in the world, is a typical inflammation-related carcinoma, characterized by extensive inflammation and fibrosis. Serum HMGB1 levels are increased in patients with HCC and expression of HMGB1 in the liver closely correlates with pathological grade, distant metastases, and drug resistance of liver cancer (Dong et al., 2013; Jiang et al., 2012a; Kawahara et al., 1996; Liu et al., 2012b; Xiao et al., 2014). In addition, serum HMGB1 levels were significantly higher in HCC patients with HCV or HBV infection (Cheng et al., 2008; Jiang et al., 2012a). HMGB1 released from the hypoxic tumor microenvironment can bind to TLR4 and RAGE, which in turn mediate HCC invasion and metastasis by activating inflammasome, NF-κB, and AKT pathways (Chen et al., 2014c; Cheng et al., 2014; Kostova et al., 2010; Yan et al., 2012b; Yaser et al., 2012). Interestingly, p53 may be a positive regulator for HMGB1 release in hepatocarcinogenesis (Yan et al., 2013b). Moreover, therapeutic targeting of HMGB1 (e.g., RNAi, ethyl pyruvate, and N-acetylcysteine) inhibits HMGB1 expression, release, and activity, which in turn suppresses tumor growth in liver metastasis models of colon cancer (Cheng et al., 2014; Cheng et al., 2013; Jiang et al., 2012b; Liang et al., 2009). These findings suggest that HMGB1 plays a critical role in the pathogenesis and treatment of HCC.
12.14.5 Drug-induced Liver Injury
Drug-induced liver injury (DILI), the first cause of acute liver failure, refers to liver injury caused by drugs or chemical agents. Acetaminophen overdose is a well-known cause of DILI (Lee, 2004). A number of animal and human studies have demonstrated that serum HMGB1 is a diagnosis and severity-assessment biomarker of acute liver injury induced by acetaminophen, as well as other drugs (Antoine et al., 2013; Antoine et al., 2012a; Antoine et al., 2010; Dragomir et al., 2011; Gong et al., 2010a; Yang et al., 2012g; Zhou et al., 2011c). HMGB1 release during acetaminophen-mediated liver injury impairs the innate immune response and accelerates the inflammatory response (Scaffidi et al., 2002; Wang et al., 2007a; Wang et al., 2013m). The activity of HMGB1 after release in DILI depends on post translational modification. For example, oxidized HMGB1 diminishes the inflammatory response in mice treated with acetaminophen (Antoine et al., 2010), whereas acetylated HMGB1 is related to DILI prognosis (Antoine et al., 2012b; Antoine et al., 2009). Therapeutic targeting of HMGB1 (e.g., neutralizing antibody and ethyl pyruvate) prevents acetaminophen-induced hepatotoxicity, promotes regeneration, and restores liver function (Dragomir et al., 2011; Yang et al., 2012g).
12.15 Cancer
In 2011, Hanahan and Weinberg (Hanahan and Weinberg, 2011) updated their cancer hallmark model and outlined ten fundamental properties that drive tumor development and growth. These cancer hallmarks include: sustainment of proliferative signaling; evasion of growth suppressors; avoidance of immune destruction; enablement of replicative immortality; tumor-promoting inflammation; activation of invasion and metastasis; induction of angiogenesis; genome instability and mutation; resistance to cell death; and deregulation of cellular energetics. Over the past two decades, HMGB1 has been demonstrated as one of the major players in a number of cancers including colon, breast, lung, prostate, cervical, skin, kidney, stomach, pancreatic, liver, bone, and blood cancer (Ellerman et al., 2007; Lotze and DeMarco, 2003; Sims et al., 2010; Tang et al., 2010d). HMGB1 acts as both a tumor suppressor and an oncogenic factor in tumorigenesis and cancer therapy depending on the context and the study conditions, as well as HMGB1 location and modification (Kang et al., 2013c).
12.15.1 Oncogenic Roles in Tumorigenesis
The tumor microenvironment consists of tumor cells and nontumor cells, including several immune cells. HMGB1 can be released, including by autocrine from the tumor cells and the surrounding cells under hypoxia or other environmental stimuli (Jube et al., 2012; Tafani et al., 2011; van Beijnum et al., 2013; Yan et al., 2012b). Extracellular HMGB1 mediates communication between cells in the tumor microenvironment by several receptors (e.g., RAGE and TLR4), which contributes to tumor growth and spreads by several mechanisms including sustenance of the inflammatory microenvironment (Bald et al., 2014; Gebhardt et al., 2008; Mittal et al., 2010), fulfillment of metabolic requirements (Kang et al., 2013a; Tang et al., 2011b), promotion of invasion and metastasis (Huttunen et al., 2002; Kuniyasu et al., 2002; Sasahira et al., 2005a; Taguchi et al., 2000), inhibition of antitumor immunity (He et al., 2012c; Kusume et al., 2009; Liu et al., 2011f), and promotion of angiogenesis (Sasahira et al., 2007; van Beijnum et al., 2012). Thus, inhibition of HMGB1 release and activity can block tumor growth and development.
12.15.2 Tumor Suppressor Roles in Tumorigenesis
Several studies indicate that intracellular HMGB1 may be a tumor suppressor. For example, nuclear HMGB1 binds to tumor suppressor RB, which leads to RB-dependent G1 arrest and apoptosis induction and prevents tumorigenicity in breast cancer cells in vitro and in vivo (Jiao et al., 2007). Nuclear HMGB1 is an important architectural factor with DNA chaperone activity. Loss of HMGB1 leads to genome instability with telomere shortening, which is major driving force in tumorigenesis (Celona et al., 2011; Giavara et al., 2005; Polanska et al., 2012). In addition, recent studies indicate that deficiencies of autophagy gene (e.g., Beclin-1, ATG5, UVRAG, Bif-1) increase tumorigenesis due to genome instability, inflammation, and organelle injury (Degenhardt et al., 2006; Mathew et al., 2007; Takahashi et al., 2013c; Zhao et al., 2012). Given that HMGB1 is a positive regulator of autophagy (Tang et al., 2010c; Tang et al., 2011b), HMGB1 deficiency leading to autophagy dysfunction may cause genome instability and inflammation, which promotes tumorigenesis. The translational potential of these findings still needs further investigation.
12.15.3 Sensitivity to Anticancer Therapy
Depending on the type of anticancer therapy, cancer cell death can be immunogenic or nonimmunogenic (Green et al., 2009; Hou et al., 2013; Krysko et al., 2012). HMGB1 can be released by dead or dying cells, which in turn mediate immunogenic cell death and subsequent anti-tumor immunity and tumor clearance by binding to TLR4 (Apetoh et al., 2007; Fucikova et al., 2011; Suzuki et al., 2012; Yamazaki et al., 2014). TLR2, but not TLR4 in DCs, mediates the T-cell-dependent antitumor immune response that induces brain tumor regression (Curtin et al., 2009). These findings suggest that HMGB1 release contributes to anticancer immunity. In contrast, HMGB1 released during cell death may mediate immunogenic tolerance if HMGB1 binds to TIM-3 or undergoes a redox change to oxidized form (Chiba et al., 2012b; Kazama et al., 2008). Furthermore, HMGB1 released during chemotherapy enhances the ability of remnant cancer cells to regrow and metastasize in a RAGE-dependent way (Luo et al., 2013). Inhibition of the HMGB1-RAGE pathway improves the effectiveness of chemotherapy (Kang et al., 2010). Collectively, many factors including receptor, death type, and redox state determine the activity of HMGB1 in the anticancer immune response.
12.15.4 Resistance to Anticancer Therapy
In addition to the important role of extracellular HMGB1 in anticancer therapy, intracellular HMGB1 is a general negative regular for the effectiveness of anticancer therapy. Several chemotherapy agents such as platinating agents can increase HMGB1 expression (Rabik and Dolan, 2007). HMGB1 is a novel therapeutic target for chemotherapy resistance. Downregulation of HMGB1 expression by RNAi increased the anticancer activity of cytotoxic agents, whereas upregulation of HMGB1 expression by gene transfection increased drug resistance (Huang et al., 2012a; Liu et al., 2011b; Livesey et al., 2012b). Increased HMGB1 expression in cancer cells facilitates chemotherapy resistance partly through inhibition of apoptosis and promotion of autophagy, which determine cell fate in anticancer therapy (Huang et al., 2012a; Kang et al., 2010; Liu et al., 2014a; Liu et al., 2011c; Livesey et al., 2012b; Ni et al., 2013; Yang et al., 2012e; Zhan et al., 2012; Zhang et al., 2012d; Zhao et al., 2011a). Interestingly, HMGB1 differs in the regulation of chemotherapeutic agent toxicity in cancer cells and normal cells (Krynetskaia et al., 2008). The potential mechanisms for these differences need further study.
13 HMGB1-targeting Therapeutic Strategies
Currently, several strategies have been proposed from cell, animal, and human studies to inhibit HMGB1 expression, release, and activity in a direct or indirect manner. These strategies include antibodies, peptide, RNAi, anti-coagulant agents, endogenous hormones, chemicals including natural product, HMGB1-receptor and signaling pathway inhibition, artificial DNAs, physical methods (e.g., medical hydrogen gas), vagus nerve stimulation, and surgery. The details of these strategies and associated experimental models are listed in Table 4.
Table 4.
HMGB1-targeting Therapeutic Strategies
| Therapeutic strategies | Inhibition of HMGB1 |
Model | References |
|---|---|---|---|
| Antibody | |||
| Mouse anti-HMGB1 DPH1.1 antibody | Activity | Migration | (Venereau et al., 2012) |
| Mouse monoclonal anti-HMGB1 2G7 antibody | Activity | Sepsis | (Yang et al., 2010a) |
| Anti-HMGB1 chicken IgY polyclonal antibody | Activity | UV-induced inflammation | (Abeyama et al., 2005) |
| IFNγ antibody | Release | Sepsis | (Yin et al., 2005) |
| TNF-α antibody | Release | Acute-on-chronic liver failure | (Yang et al., 2014a) |
| Peptide and protein | |||
| A box | Activity | Sepsis; thromboangiitis obliterans; postischemic brain; acute pancreatitis | (Jin et al., 2011; Kong et al., 2013; Yang et al., 2004b) |
| Fusion protein HMGB1 A box-TMD1 | Activity | Macrophage activation | (Li et al., 2010d) |
| LPS-binding peptide regions within HMGB1 | Activity | Sepsis | (Youn et al., 2011) |
| The fibrin-derived peptide Bbeta15–42 | Release and activity | Liver ischemia-reperfusion injury | (Liu et al., 2013a) |
| Recombinant bactericidal/permeability-increasing protein (rBPI21) | Expression and activity | Sepsis | (Zhang et al., 2008b) |
| Cationic antibacterial polypeptide of 11-kDa | Release | Sepsis, phagocytosis | (Murakami et al., 2009; Shibusawa et al., 2009) |
| HMGB1 binding heptamer peptide (HBHP; HMSKPVQ) | Activity | Ischemic brain injury | (Kim and Lee, 2013) |
| Fetuin-A | Release | Sepsis; cerebral ischemia injury | (Li et al., 2011f; Wang et al., 2010a) |
| Recombinant kallistatin | Release and expression | Sepsis | (Li et al., 2014b) |
| HMGB1 mutant protein | Activity | Monocyte activation | (Yuan et al., 2008) |
| RNAi | |||
| HMGB1 siRNA | Expression | Postischemic brain injury | (Kim et al., 2012b) |
| HMGB1 shRNA | Expression | Type 1 diabetes; cancer | (Livesey et al., 2012a; Wang et al., 2014f) |
| Physical method | |||
| Hemoperfusion with a HMGB1 adsorption column | Activity | Liver ischemia-reperfusion injury; sepsis | (Yamamoto et al., 2010; Yasuda et al., 2012) |
| Polymyxin B-immobilized fiber column | Activity | Patients with idiopathic pulmonary fibrosis with acute exacerbation; septic shock patient | (Abe et al., 2011; Nakamura et al., 2011c) (Sakamoto et al., 2006; Yamato et al., 2013) |
| Membranes for continuous hemofiltration (AN69ST) | Activity | Experimental hemofiltration in vitro | (Yumoto et al., 2011) |
| Calorie restriction | Release and expression | Sepsis | (Hasegawa et al., 2012) |
| Low temperature condition | Release | Hypoxia-induced islet cell damage | (Itoh et al., 2012b) |
| Xenon treatment | Release | Cold ischemia injury | (Zhao et al., 2013) |
| Controlled oxygen reperfusion | Release | Cardiopulmonary bypasses | (Rong et al., 2013) |
| Molecular hydrogen | Release | Sepsis; liver/spinal cord/ cerebral ischemia-reperfusion injury | (Huang et al., 2011c; Li et al., 2012a; Liu et al., 2014b; Xie et al., 2012; Xie et al., 2010; Zhou et al., 2013b) |
| Surgery | |||
| Splenectomy | Release | Sepsis | (Huston et al., 2008) |
| Chemicals | |||
| Carboxylated N-glycans | Activity | Neurite outgrowth | (Srikrishna et al., 2002) |
| Antileukinate (alpha-chemokine receptor inhibitor) | Release | Sepsis | (Lin et al., 2005) |
| Pyrrolidine dithiocarbamate (NF-κB inhibitor) | Release | Acute pancreatitis | (Zhang et al., 2010) |
| 20-5,14-HEDGE | Expression | Lung ischemia-reperfusion injury | (Ali et al., 2012) |
| Glutamine | Expression | Sepsis | (Kwon et al., 2010b) |
| Endothelin receptor antagonist | Release | Sepsis | (Goto et al., 2010) |
| 2-(2-N,N-dimethylaminoethylamine-1-carbonyl)-1H-quinolin-4-one hydrochloride (an analogue of Kynurenic acid) | Release | Monocyte activation | (Tiszlavicz et al., 2011) |
| Rosiglitazone (a PPAR-γ agonist) | Expression | Sepsis | (Wang et al., 2014a) |
| D-Ala2-D-Leu5-enkephalin (a delta-opioid receptor agonist) | Release | Sepsis | (Tang et al., 2011a) |
| Resolvin D1 | Release | Sepsis | (Murakami et al., 2011) |
| DR396 (an apoptotic DNase gamma inhibitor) | Release | Apoptosis | (Yamada et al., 2011a) |
| Farnesyltransferase inhibitor FTI-277 | Release | Sepsis | (Yang et al., 2011) |
| TNF-α inhibitor | Release | Arthritis; sepsis | (Fei et al., 2011) |
| tricarbonylchloro(glycin ate) ruthenium (II) (CORM-3) | Expression | Arthritis | (Maicas et al., 2010) |
| JAK2 inhibition with AG490 | Release | Sepsis | (Hui et al., 2009; Pena et al., 2010) |
| Glutamine | Expression | Sepsis | (Hu et al., 2012c) |
| Rosiglitazone (a specific ligand for PPAR-γ) | Release | Macrophage activation | (Hwang et al., 2012) |
| Alpha 2A-adreno receptor | Release and expression | Sepsis | (Ji et al., 2012a) |
| T-5224 (a selective inhibitor of c-Fos/activator protein-1) | Release | Sepsis | (Izuta et al., 2012) |
| Carbon monoxide from CORM-2 (Carbon monoxide-releasing molecule 2) | Release | Macrophage activation | (Tsoyi et al., 2010) |
| Galectin-9 | Release | Sepsis | (Kadowaki et al., 2013) |
| PNU-282987 (a selective alpha7 nicotinic acetylcholine receptor agonist) | Release | Liver ischemia-reperfusion injury | (Li et al., 2013a) |
| Sodium butyrate | Expression | Severe burn injury | (Liang et al., 2013b) |
| Tolerization with bacterial lipoprotein | Expression and release | Sepsis | (Coffey et al., 2007) |
| Tetrahydroisoquinoline alkaloid THI-28 | Release | Sepsis | (Kim et al., 2013b) |
| Exendin-4 (a glucagon-like peptide-1 receptor agonist) | Expression | Myocardial ischemia-reperfusion injury | (Hu et al., 2013) |
| 4,4'-diphenylmethane-bis (methyl) carbamate | Activity | Endothelial cell activation | (Feng et al., 2013) |
| Ethyl pyruvate | Release and expression | Sepsis; postischemic brain;hepatitis;hepatoce llular carcinoma; liver and myocardial ischemia-reperfusion injury; acute pancreatitis; murine colitis | (Cheng et al., 2014; Dave et al., 2009; Hu et al., 2012b; Luan et al., 2012; Shen et al., 2014; Shin et al., 2014; Ulloa et al., 2002) |
| Troglitazone (a PPAR-γ agonist) | Expression | EA.hy926 cell activation | (Gao et al., 2011b) |
| Glucan phosphate | Release | Sepsis | (Ha et al., 2011a) |
| Heme oxygenase-1 inducer | Release | Sepsis | (Ha et al., 2011b) |
| Clopidogrel sulfate (a adenosine diphosphate receptor antagonist) | Expression | Sepsis | (Hagiwara et al., 2011a) |
| Spermine | Release and activity | Sepsis | (Zhu et al., 2009) |
| Gold chloride | Release | Macrophage activation | (Zetterstrom et al., 2008) |
| Oroxylin-A | Release | Sepsis | (Tseng et al., 2012) |
| EPC-K1 (a vitamin E derivative) | Expression | Liver ischemia-reperfusion injury | (Oishi et al., 2012) |
| Geranylgeranylacetone | Expression | Myocardial ischemia-reperfusion injury | (Wang et al., 2012d) |
| Penehyclidine hydrochloride | Release | Sepsis | (Yang et al., 2014c) |
| Pyrrolidine dithiocarbamate (a NF-κB inhibitor) | Expression and release | Acute-on-chronic liver failure; chronic obstructive pulmonary disease | (Wang et al., 2013a; Yang et al., 2014a) |
| Dehydroxymethylepoxy quinomicin (a NF-κB inhibitor) | Activity | Islet transplantation | (Watanabe et al., 2013) |
| Probenecid (a pannexin 1 channel inhibitor) | Release | Middle cerebral artery occlusion | (Xiong et al., 2014) |
| AS605240 (a PI3Kgamma inhibitor) | Release | Sepsis | (Xu et al., 2010) |
| GW9662 (PPAR-γ antagonist) | Release | Cerebral ischemia injury | (Haraguchi et al., 2009) |
| Melatonin | Expression and release | Hemorrhage; hepatitis; liver ischemia-reperfusion injury | (Kang et al., 2011a; Laliena et al., 2012; Wang et al., 2013o) |
| Hemopexin | Activity | Macrophage activation | (Lin et al., 2012c) |
| Adiponectin | Release | Sepsis | (Li et al., 2012d) |
| Chymase | Degradation | Danger-induced inflammation | (Roy et al., 2014) |
| Glyceollins | Release | Sepsis | (Lee et al., 2014) |
| Prorenin receptor blocker | Release and expression | Sepsis | (Hirano et al., 2014) |
| Sodium hydrosulfide | Release | Hemorrhagic shock | (Xu et al., 2013a) |
| 3-(3-chloro-phenyl)-5-(4-pyridyl)-4,5-dihydroisoxazole | Release | Sepsis | (Vicentino et al., 2012) |
| Natural chemicals | |||
| Quercetin (a flavonoid) | Release and activity | Sepsis | (Tang et al., 2009) |
| An extract from Machilus zuihoensis | Release | Macrophage activity | (Mao et al., 2011) |
| Osthole (an active compound from Cnidium monnieri and Angelica pubescens) | Expression | Myocardial ischemia-reperfusion injury | (Wang et al., 2013n) |
| Tanshinone II A | Release and expression | Sepsis; cerebral ischemia-reperfusion injury | (Li et al., 2007b; Wang et al., 2010b; Zhan et al., 2014) |
| An extract from Helleborus purpurascens | Release | Sepsis | (Apetrei et al., 2011) |
| Chloroquine | Release and activity | Sepsis | (Yang et al., 2013d) |
| Lycopene | Release and activity | Vascular inflammation | (Lee et al., 2012d) |
| Withaferin A (an active compound from Withania somnifera) | Release and activity | Leukocyte migration | (Lee et al., 2012c) |
| Pellitorine (an active amide compound from Asarum sieboldii) | Release | Sepsis | (Ku et al., 2013) |
| Persicarin (an active compound from Oenanthe javanica) | Release and activity | Sepsis | (Kim et al., 2013f) |
| Isorhamnetin-3-O-galactoside (I3G) (an active compound from O. javanica) | Release | Sepsis | (Kim et al., 2013e) |
| Omega-3 polyunsaturated fatty acids | Release | Liver ischemia-reperfusion injury | (Kim et al., 2013c) |
| Curcumin | Expression | Hepatitis; Cardiac ischemia-reperfusion injury | (Tu et al., 2013) (Kim et al., 2012h) |
| 18alpha-glycyrrhetinic acid | Expression | Cancer | (Shetty et al., 2011) |
| Glycyrrhizin | Activity and release | Traumatic brain injury; Sepsis; hemorrhage-induced injury | (Gu et al., 2014; Mollica et al., 2007; Ohnishi et al., 2011; Vitali et al., 2013) |
| An extract of Prunus mume Sieb. et Zucc | Release | Macrophage activation | (Kawahara et al., 2009) |
| 2-methoxycinnamaldehyde (one of active ingredients of Cinnamomum cassia) | Expression | Myocardial ischemia-reperfusion injury | (Hwa et al., 2012) |
| Astragaloside IV (an important component of astragalus mongholicus) | Activity | T cell activation | (Hwa et al., 2012) |
| Danggui (Angelica sinensis) | Release | Sepsis | (Wang et al., 2006) |
| Oleanolic acid | Release | Endothelial cell activation | (Yang et al., 2012b) |
| Protocatechuic aldehyde | Release | Sepsis | (Xu et al., 2012) |
| n-3 polyunsaturated fatty acids | Expression | Chronic vasculopathy of small bowel allografts | (Wei et al., 2013) |
| Mung bean (Vigna Radiata) | Degradation | Sepsis | (Wei et al., 2013; Zhu et al., 2012a) |
| Danshen (Salvia miltiorrhiza) | Release | Sepsis | (Li et al., 2007b; Wang et al., 2006) |
| Green tea (Camellia sinensis) | Release and activity | Sepsis | (Li et al., 2007a; Li et al., 2011e) |
| Epi-sesamin (an active compound from Asarum sieboldii roots) | Release | Sepsis | (Lee et al., 2013e) |
| Berberine | Release | Macrophage activation | (Lee et al., 2013a) |
| 18 beta-glycyrrhetic acid | Activity | Macrophage activation | (Cavone et al., 2011b) |
| Paeoniflorin | Expression and release | Endothelial cell activation | (Wan et al., 2013) |
| Saponins (an active compound from Dioscorea nipponica Makino) | Expression | Liver injury | (Yu et al., 2014) |
| Shikonin | Release | Sepsis | (Yang et al., 2014e) |
| Higenamine | Expression | Brain injury | (Ha et al., 2012b) |
| Asperosaponin X (an active compound from the roots of Dipsacus asper) | Release | Myocardial ischemia injury | (Jiang et al., 2012e) |
| Forsythoside B | Release | Sepsis | (Jiang et al., 2012d) |
| Tricin 7-glucoside | Expression | Cerebral ischemia | (Jiang et al., 2012c) |
| Vitamin E derivative, E-Ant-S-GS | Expression and release | Sepsis; liver ischemia-reperfusion injury | (Kono et al., 2012) (Koga et al., 2012) |
| EPCK1 (a vitamin C and E analogue) | Release | Sepsis | (Shingu et al., 2011) |
| Genipin (an aglycon of geniposide) | Release | Sepsis | (Kim et al., 2012g) |
| Phlorotannins (active compounds of Eisenia bicyclis) | Release | Leukocyte migration | (Kim et al., 2012f) |
| Kaempferol-3-O-sophoroside (active compounds from leaves of cultivated mountain ginseng) | Release | Endothelial cell activation | (Kim et al., 2012e) |
| Gelam honey | Release | Sepsis | (Kassim et al., 2012) |
| Matrine | Release | Sepsis | (Zhang et al., 2011a) |
| Acanthopanax gracilistylus-extracted Acankoreanogenin A | Release | Hepatitis | (Zhang et al., 2011b) |
| 8-O-acetyl shanzhiside methylester (active compounds from leaves of Lamiophlomis rotata (Benth.) Kudo) | Expression | Myocardial ischemia injury | (Kang et al., 2012c) |
| Ethanol extract of Prunella vulgaris var. lilacina | Release and expression | Sepsis | (Jun et al., 2012) |
| Cannabidiol | Release and expression | Cerebral ischemia injury | (Hayakawa et al., 2008a) |
| Baicalin (the main active ingredient of the root from Scutellaria) | Release | Sepsis | (Wang and Liu, 2014) |
| Piperlonguminine (an active compound from Piper longum fruit) | Release and expression | Sepsis | (Ku et al., 2014) |
| Astilbin (an active compound from the rhizome of Smilax china L.) | Expression | Cardiac ischemia-reperfusion injury | (Diao et al., 2014) |
| Luteolin | Release and activity | Macrophage activation | (Chen et al., 2014a) |
| Qinggan Huoxue Recipe (a traditional Chinese medicine) | Expression | Acute liver failure | (Zhu et al., 2013a) |
| Betaine | Expression | Liver injury | (Zhang et al., 2013f) |
| Fucoidan (a sulfated polysaccharide from brown algae) | Expression | Myocardial ischemia-reperfusion injury | (Li et al., 2011a) |
| Curculigoside A | Expression | Cerebral ischemia injury | (Jiang et al., 2011) |
| Emodin-6-O-beta-D-glucoside (an active compound from Reynoutria japonica) | Release and activity | Sepsis | (Lee et al., 2013f) |
| Rutin (an active flavonoid compound) | Release and activity | Sepsis | (Yoo et al., 2014) |
| Rosmarinic acid (an active compound from leaves of Perilla frutescens) | Expression and activity | Sepsis | (Yang et al., 2013b) |
| Fish oil | Expression | Chronic allograft vasculopathy | (Wei et al., 2013) |
| Anethole (a major component of Foeniculum vulgare) | Release | Liver ischemia-reperfusion injury | (Cho et al., 2013) |
| Ursolic acid | Expression and release | Sepsis | (Chen et al., 2013e) |
| Scolopendra subspinipes mutilans | Release | Acute pancreatitis | (Jo et al., 2013) |
| Clinical drugs | |||
| Atorvastatin | Expression | Brains focal ischemia; patients with hyperlipidemia | (Jin et al., 2012; Wang et al., 2010c) |
| Bicyclol | Release | Sepsis | (Luo et al., 2011) |
| Carbenoxolone | Release | Sepsis | (Li et al., 2013e) |
| CKD712, (S)-1-(alpha-naphthylmethyl)-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline | Release | Macrophage activation | (Oh et al., 2011) |
| CuZnSOD and MnSOD | Release | Tumor growth | (Lee et al., 2010b) |
| Cholinergic agonist physostigmine | Expression | Forebrain ischemia-reperfusion injury | (Kutsuna et al., 2010) |
| Cisplatin | Release | Liver ischemia-reperfusion injury; acute liver failure | (Cardinal et al., 2009; Li et al., 2013g) |
| Candesartan | Activity | Stroke | (Kikuchi et al., 2013) |
| Chloroquine | Release | Sepsis; monocyte activation; cancer | (Schierbeck et al., 2010) |
| Dobutamine | Release | Myocardial ischemia-reperfusion injury | (Wang et al., 2013g) |
| Dihydropyridine-type calcium-channel blocker--CV159 | Release | Liver ischemia-reperfusion injury | (Hataji et al., 2010) |
| Dexamethasone | Release | Monocyte activation | (Schierbeck et al., 2010) |
| DL-sulforaphane | Release | Macrophage activation | (Killeen et al., 2006) |
| Dexmedetomidine | Release | Macrophage activation; sepsis | (Chang et al., 2013; Xu et al., 2013b) |
| Daunomycin | Release | Cancer | (Lotfi et al., 2013) |
| Ethacrynic acid | Release | Macrophage activation | (Killeen et al., 2006) |
| Fluvastatin | Expression | Hyperlipidemia | (Haraba et al., 2011b) |
| Fluorocitrate | Release | Focal cerebral ischemia | (Hayakawa et al., 2010) |
| Edaravone | Release | Sepsis; cerebral infarction | (Kato et al., 2009; Kikuchi et al., 2009b) |
| Forsythoside B | Expression | Myocardial ischemia-reperfusion injury | (Jiang et al., 2010) |
| Enalapril (an angiotensin-converting enzyme inhibitor) | Release and expression | Sepsis | (Hagiwara et al., 2009e) |
| Etanercept | Expression | Chronic constriction injury | (Wang et al., 2013i) |
| Gold sodium thiomalate | Release | Monocyte activation | (Schierbeck et al., 2010) |
| Gabexate mesilate | Expression | Sepsis | (Hidaka et al., 2011) |
| Glucocorticoid | Expression | Obstructive jaundice model | (Huang et al., 2011d) |
| Intravenous immunoglobulin G | Release | Sepsis | (Hagiwara et al., 2008a; Yoshikawa et al., 2012) |
| Intravenous parecoxib | Release | Cerebral ischemia-reperfusion injury | (Wang et al., 2011d) |
| Irbesartan | Activity | Stroke | (Kikuchi et al., 2013) |
| Lidocaine | Release | Macrophage activation | (Wang et al., 2013d; Wang et al., 2011b) |
| Losartan (a type 1 angiotensin II receptor antagonist) | Release | Sepsis | (Hagiwara et al., 2009b) |
| Landiolol (an ultrashort-acting beta1-adrenoceptor antagonist) | Release and expression | Sepsis | (Hagiwara et al., 2009d) |
| Lercanidipine | Release | Vascular smooth muscle cell activation | (Yeh et al., 2013) |
| Labedipinedilol-A | Release | Vascular smooth muscle cell activation | (Yeh et al., 2013) |
| Minocycline | Expression | Myocardial ischemia-reperfusion injury; microglia | (Hayakawa et al., 2008b; Hu et al., 2010) |
| Methotrexate | Release | Macrophage activation; cancer | (Kuroiwa et al., 2013) |
| Metformin | Release | Sepsis; Hyperglycemia-treated neonatal rat ventricular myocytes | (Tsoyi et al., 2011b; Zhang et al., 2014c) |
| Magnesium sulfate | Expression and release | Macrophage activation | (Liu et al., 2013d) |
| Niaspan | Release | Type-1 diabetes | (Ye et al., 2011) |
| Nafamostat mesilate | Expression and release | Sepsis | (Hagiwara et al., 2007) |
| Oxaliplatin | Release | Arthritis | (Ostberg et al., 2008) |
| Oltipraz | Release | Macrophage activation | (Killeen et al., 2006) |
| Polymyxin B | Release | Sepsis | (Morita et al., 2004) |
| Propofol | Expression | Gastric mucosal injury | (Ye et al., 2013a) |
| Rosuvastatin | Expression | Myocardial ischemia-reperfusion injury | (Du et al., 2014) |
| Sivelestat | Release | Liver resection injury; macrophage activation; sepsis | (Hagiwara et al., 2009c; Hagiwara et al., 2008e; Tsujii et al., 2012) |
| Statins | Activity | Endothelial cell activation | (Yang et al., 2010c) |
| Sodium butyrate | Release | Sepsis | (Zhang et al., 2007) |
| Stearoyl lysophosphatidylcholine | Release | Sepsis | (Chen et al., 2005) |
| Simvastatin | Release and expression | Sepsis; vascular inflammation and atherosclerosis | (Liu et al., 2013c; Zhang et al., 2012b) |
| Surfactant protein A | Release and activity | Dendritic cell maturation | (Ledford et al., 2010) |
| Sodium salicylate | Release | Cancer and necrosis | (Lim et al., 2008) |
| Tetrandrine (a bisbenzylisoquinoline alkaloid) | Release | Sepsis | (Lin et al., 2009) |
| Telmisartan | Activity | Stroke | (Kikuchi et al., 2013) |
| Ulinastatin (a human urinary trypsin inhibitor) | Release and expression | Sepsis; Forebrain ischemia-reperfusion injury | (Koga et al., 2010; Tanaka et al., 2010) |
| HMGB1-receptor and signaling pathway inhibition | |||
| sRAGE | Inhibition of HMGB1-RAGE pathway | Sepsis, cancer | (Liliensiek et al., 2004) |
| Anticoagulant agents | |||
| Thrombomodulin | Activity and release | Sepsis | (Abeyama et al., 2005; Nagato et al., 2009) |
| Danaparoid sodium | Expression | Heat stroke; sepsis; acute pancreatitis | (Hagiwara et al., 2008c; Hagiwara et al., 2011b; Hagiwara et al., 2009g) |
| Activated protein C | Release and activity | Endothelial cell activation | (Bae and Rezaie, 2011) |
| Antithrombin III | Activity, expression and release | Innate immune rejection; acute pancreatitis; sepsis | (Hagiwara et al., 2008d; Hagiwara et al., 2009f, 2010b; Kojima et al., 2012a) |
| Low molecular weight heparin | Expression and activity | Sepsis | (Luan et al., 2014) |
| 2-O, 3-O-desulfated heparin | Release | Airway inflammation | (Griffin et al., 2013) |
| Lower concentration thrombin | Release and activity | Endothelial cell activation | (Bae, 2012) |
| Endogenous hormones | |||
| Insulin | Release | Sepsis; cardiopulmonary bypass | (Hagiwara et al., 2009a; Hagiwara et al., 2008b; Hasegawa et al., 2011; Liu et al., 2012c) |
| Neuropeptides | Release and activity | Sepsis | (Chorny and Delgado, 2008; Tang et al., 2008c) |
| Insulin-like growth factor 1 | Release | ox-LDL-induced inflammation | (Yu et al., 2012a) |
| Ghrelin | Release | Sepsis | (Chorny et al., 2008; Wang et al., 2009d) |
| Glucagon-like peptide-1 (a gut incretin hormone secreted from L cells) | Expression | Cardiocyte injury | (Cai et al., 2012) |
| Vagus nerve stimulation | |||
| Chemical (nicotine, GTS-21, choline) ; Electrical; Mechanical | Release | Arthritis; sepsis | (Huston et al., 2007; Li et al., 2010c; Ni et al., 2011; Parrish et al., 2008; Pavlov et al., 2007; Rosas-Ballina et al., 2009; Wang et al., 2004a) |
| Artificial DNAs | |||
| Kinked oligonucleotide duplexes | Activity | Sepsis | (Musumeci et al., 2011; Yanai et al., 2011) |
| Bent oligonucleotide duplexes | Activity | Cell free system | (Musumeci et al., 2007; Yanai et al., 2011) |
14 HMGB1 Measurements and Significance in Clinical Practice
Biomarkers are characteristics that are objectively measured and evaluated as indicators of normal biological processes, pathogenic processes, or pharmacologic responses to therapeutic intervention (Atkinson et al., 2001). A number of studies have indicated that the level of HMGB1 in samples (e.g., serum, plasma, cerebrospinal fluid, sputum, urine, fecal, and tissue) may be a biomarker of human disease which can be used for detection and diagnosis of disease, prediction of response to therapeutic interventions, and prognosis of outcome. Interestingly, circulating HMGB1 levels have been positively or inversely associated with sRAGE levels, suggesting that sRAGE not only regulates HMGB1 activity, but also eliminates circulating HMGB1 in human disease (Fukami et al., 2009). Currently, ELISA and Western blot have been used to detect HMGB1 in serum, plasma, and body fluid. Of note, HMGB1 levels in serum or plasma may be five times higher when analyzed by Western blot compared to ELISA because serum and plasma components (e.g., immunoglobulins, phospholipids, thrombomodulin, and proteoglycans) can interfere with the detection of HMGB1 by ELISA (Urbonaviciute et al., 2007). In contrast, perchloric acid-modified ELISA can detect masked forms of HMGB1 (Barnay-Verdier et al., 2011). However, currently, the only commercially available HMGB1 ELISA kit is from Shino-Test Corporation in Japan, first reported in 2006 (Yamada et al., 2006). In addition to ELISA (Lepp and Martinez, 1989; Urbonaviciute et al., 2007; Wahamaa et al., 2007; Yamada et al., 2003), several new techniques (e.g., DNA nanostructure-based assay) have been proposed to test HMGB1 concentration in serum or supernatants (Gaillard et al., 2008). Immunohistochemical staining is widely used in the assessment of HMGB1 expression and localization in tissues. RT-PCR and q-PCR are widely used to test HMGB1 mRNA expression in tissues. HMGB1 gene polymorphisms (Kornblit et al., 2007) are involved in several human disease such as chronic HBV infection (Deng et al., 2013), trauma (Zeng et al., 2012), allogeneic hematopoietic cell transplantation (Kornblit et al., 2010), and systemic inflammatory response syndrome (Kornblit et al., 2008). Serum anti-HMGB1 autoantibody is increased in several autoimmune diseases (Urbonaviciute and Voll, 2011). The details of HMGB1 measurement and significance in clinical practice are listed in Table 5.
Table 5.
HMGB1 Measurements and Significance in Clinical Practice
| Disease | Sample | Significance | References |
|---|---|---|---|
| Respiratory system | |||
| Chronic rhinosinusitis with/without nasal polyposis | Nasal mucosa epithelial cells and inflammatory cells of patients | Expression and secretion markers of upper airway inflammatory diseases | (Bellussi et al., 2013) |
| Asthma | Sputum | HMGB1 plays a key role in the pathogenesis of clinical and experimental asthma characterized by eosinophilic airway inflammation | (Shim et al., 2012) |
| Acute respiratory distress syndrome (ARDS) | Plasma | Active involvement of HMGB1-RAGE axis in poor prognosis of ARDS | (Splichalov a et al., 2011) |
| Non-small cell lung cancer (NSCLC) | Serum | No clinical significance in the prognosis of the survival time in lung cancer | (Naumnik et al., 2009) |
| Community-acquired pneumonia (CAP) | Serum | HMGB1 is elevated in almost all CAP subjects, and higher circulating HMGB1 is associated with mortality of CAP | (Angus et al., 2007) |
| Chronic obstructive pulmonary disease (COPD) | Broncho-alveolar lavage | Elevated HMGB1 expression in COPD airways may sustain inflammation and remodeling through its interaction with IL-1β and RAGE | (Ferhani et al., 2010) |
| Non-small cell lung cancer (NSCLC) | Tissue | Diagnosis and prognosis of outcome | (Liu et al., 2010a) |
| Non-small cell lung cancer (NSCLC) | Serum | Useful clinical marker for evaluating NSCLC progression and potential prognostic value | (Shang et al., 2009) |
| Acute lung injury (ALI) | Plasma and lung epithelial lining fluid | Extracellular HMGB1 may play a key role in the pathogenesis of clinical and experimental ALI | (Ueno et al., 2004) |
| Cystic fibrosis (CF) with acute pulmonary exacerbations | Sputum | HMGB1 is potential CF reporting tools and treatment targets | (Liou et al., 2012) |
| Lung injury | Blood and Bronchoalveolar lavage (BALF), BALF macrophages | Diagnosis and prognosis of outcome | (Bitto et al., 2010) |
| Thoracic esophagectomy | Serum | A predictive marker for complications in this setting | (Suda et al., 2006) |
| Chronic obstructive pulmonary disease (COPD) | Plasma | In smokers, high expression of HMGB1 in the blood and lungs is related to lung function impairment and appears to be associated with the development of COPD | (Ko et al., 2013) |
| Asthma and chronic obstructive pulmonary disease (COPD) | Sputum | HMGB1 may contribute to airway inflammation through its higher expression in bronchial asthma and COPD patients | (Cheng et al., 2011b) |
| Chronic obstructive pulmonary disease (COPD) | Peripheral airways | A potentially interesting target for treatment | (Kanazawa et al., 2012) |
| Bronchial asthma | Sputum | Predictors of therapeutic effects | (Cheng et al., 2011a) |
| Asthma and chronic obstructive pulmonary disease (COPD) | Bronchial lavage fluid | Neutrophilic airway inflammation in asthma and COPD is associated with reduced sRAGE | (Sukkar et al., 2012) |
| Chronic obstructive pulmonary disease (COPD) | Sputum and plasma | A potential role for HMGB1 as a biomarker and diagnostic tool for the differential diagnosis of asthma and COPD | (Hou et al., 2011a) |
| Pneumonia | Serum | Differential diagnosis | (Zhou et al., 2011d) |
| Acute respiratory distress syndrome (ARDS) | Blood | HMGB1 and oxidative stress play a role in the pathogenesis of ARDS | (Nakamura et al., 2009a) |
| Cystic fibrosis airway disease | Sputum | HMGB1 expression contributes to pulmonary inflammation and lung matrix degradation in CF airway disease | (Rowe et al., 2008) |
| Urogenital system | |||
| Bladder cancer (BC) | Tumor tissue | A new molecular marker to predict the prognosis of patients with BC | (Yang et al., 2012c) |
| Clear cell renal cell carcinoma (CCRCC) | Tissue | HMGB1 promotes the development and progression of CCRCC | (Lin et al., 2012a) |
| Recurrent squamous cell carcinoma of uterine cervix | Tissue and serum | A useful and specific marker for evaluating the disease recurrence and predicting prognosis | (Sheng et al., 2009) |
| Acute kidney injury | Serum | HMGB1 is related to inflammatory parameters | (Zakiyanov et al., 2013b) |
| Bladder cancer | Tissue | HMGB1 serves as a potential diagnostic and therapeutic target | (Wang et al., 2013j) |
| Prostate cancer | Tissue | HMGB1 presents as a novel prognostic factor | (Li et al., 2012e) |
| Kidney transplantation after donor brain death | Tissue | Prognosis of outcome | (Kaminska et al., 2011) |
| Clear cell renal cell carcinoma | Tissue | Relocation of HMGB1 to cytoplasm was correlated with tumor grades | (Wu et al., 2013a) |
| Renal clear cell cancer | Tissue | HMGB1 expressed in the cytoplasm may be an effective marker of tumor grade | (Takeuchi et al., 2013) |
| Renal diseases | Serum | Predictor of disease progression | (Sato et al., 2008) |
| Immune system | |||
| Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV) | Serum | In contrast to systemic lupus erythematosus, HMGB1 is not a useful biomarker in AAV | (de Souza et al., 2013b) |
| Juvenile idiopathic arthritis (JIA) | Blood and synovial fluid | A mediator of JIA pathogenesis as well as a biomarker for inflammatory activity and as a target for therapy | (Schierbeck et al., 2013) |
| systemic lupus erythematosus (SLE) | Serum | HMGB1 contributes to the development of inflammatory lesions in the skin of SLE patients upon UVB exposure | (Abdulahad et al., 2013) |
| Systemic lupus erythematosus | Serum and urinary | Urinary HMGB1 might reflect both local renal inflammation as well as systemic inflammation | (Abdulahad et al., 2012) |
| Rheumatoid Arthritis (RA) | Peripheral blood mononuclear cells | Correlates with RA immunotherapy and pathogenesis | (Shi et al., 2012a) |
| Rheumatoid Arthritis (RA) | Serum | serum HMGB1 levels in postmenopausal women with RA could serve as a useful marker of inflammatory activity in these patients | (Pullerits et al., 2011) |
| Systemic lupus erythematosus (SLE) | Serum | HMGB1, HMGB1-anti-HMGB1 immune complexes play a role in the pathogenesis of SLE | (Abdulahad et al., 2011) |
| Rheumatoid Arthritis (RA) | Synovial biopsy specimen | Therapeutic target in RA | (Sundberg et al., 2008) |
| Systemic lupus erythematosus (SLE) | Tissue | HMGB1 is an important factor in the inflammatory autoimmune process of CLE | (Barkauskaite et al., 2007) |
| Alopecia areata (AA) | Serum | A promising predictor of prognosis and treatment response, a new potential therapeutic target for the treatment of AA | (Lee et al., 2013g) |
| Lupus nephritis (LN) | Tissue | This study clearly indicates a role for HMGB1 in LN | (Zickert et al., 2012) |
| Behçet's disease (BD) | Serum | extracellular HMGB1 may play an important role in the pathogenesis of BD | (Ahn et al., 2011) |
| Rheumatoid arthritis (RA) | PBMCs | HMGB1 plays a pivotal role in the pathogenesis of RA and may be a target of therapy as a novel cytokine | (Zuo et al., 2007) |
| Systemic sclerosis (SSc) | Serum | Associated with the disease severity and immunological abnormalities in SSc | (Yoshizaki et al., 2009) |
| Polymyositis and dermatomyositis | Endothelial cells and muscle fibers | Correlated with pathogenesis of polymyositis and dermatomyositis | (Ulfgren et al., 2004) |
| Juvenile SLE | Serum | A potential marker of disease activity | (Kanakoudi-Tsakalidou et al., 2014) |
| Fibromyalgia (FM) | Serum | HMGB1 protein might be a good laboratory-sourced candidate for the assessment of functional status and disease severity in patients with FM | (Li et al., 2014a) |
| Ankylosing spondylitis (AS) | Serum | HMGB1 might play an important role in the pathogenesis of AS as well as a new therapy option in AS | (Oktayoglu et al., 2013) |
| Neuromyelitis optica (NMO) and multiple sclerosis (MS) | Cerebrospinal fluid | Reflect the neuroinflammatory process in disease | (Wang et al., 2013c) |
| Neuromyelitis optical | Plasma | Serve as a surrogate marker for NMO disease activity and aid in differential diagnosis. | (Wang et al., 2012a) |
| Allergic rhinitis (AR) | Nasal lavage fluid | Nasal HMGB1 has significantly increased in children with AR and is significantly related to symptom severity | (Salpietro et al., 2013) |
| Systemic sclerosis | Serum | Contribute to disease progression | (Maugeri et al., 2012a) |
| Myasthenia gravis (MG) | Serum | Correlate with the pathophysiology of MG. Further studies are warranted to elucidate more about this immunological axis in patients with MG | (Moser et al., 2012) |
| Granulomatosis with polyangiitis | Serum | HMGB1 may be used as a marker of the burden of granulomatous inflammation in GPA | (Henes et al., 2011) |
| Systemic lupus erythematosus (SLE) | Plasma | HMGB1 associated with disease activity in SLE | (Ma et al., 2012a) |
| Wegener's granulomatosis (WG) and ANCA-associated vasculitis (AAV) | Serum | HMGB1 may be useful as a marker of disease activity in WG and as a discriminating marker between different forms of AAV | (Wibisono et al., 2010) |
| Systemic lupus erythematosus (SLE) | Serum | HMGB1 in SLE may be associated with lupus disease activity | (Li et al., 2010b) |
| (ANCA)-associated vasculitis (AAV) | Serum | HMGB1 is useful as a marker of disease activity and a predictor of outcome in AAV | (Bruchfeld et al., 2011) |
| Kawasaki syndrome | Serum | In conclusion, an elevated HMGB1 value could be a potential marker for poor-responders | (Eguchi et al., 2009) |
| Kawasaki disease (KD) | Serum | HMGB1 plays an important role in immune responses in KD patients | (Hoshina et al., 2008) |
| Rheumatoid arthritis (RA) | Serum | Correlated with disease progression | (Goldstein et al., 2007) |
| Churg-Strauss syndrome | Serum | HMGB1 might contribute to the pathogenesis of CSS | (Taira et al., 2007) |
| Circulatory system | |||
| Cardiopulmonary bypass (CPB) | Serum | HMGB1 levels may be used as an indicator of inflammation and may be a novel target during CPB | (Zhang et al., 2013j) |
| Non-ST-segment elevation myocardial infarction (NSTEMI) | Serum | A potential and independent predictor of outcome | (Hashimoto et al., 2012) |
| ST-segment elevation myocardial infarction (STEMI) | Plasma | Plasma HMGB1 may be used as a new prognostic biomarker in STEMI patients | (Sorensen et al., 2011a) |
| Effects of exercise training on acute myocardial infarction | Serum | Prognosis of outcome | (Giallauria et al., 2011) |
| Acute myocardial infarction | Serum | Prognosis of outcome | (Giallauria et al., 2010) |
| ST-elevation MI | Serum | Predictor of adverse clinical outcomes | (Kohno et al., 2009) |
| Atrial fibrillation (AF) | Serum | Significant independent predictors of AF | (Wu et al., 2013d) |
| Coronary artery disease | Serum | The levels of HMGB1 were correlated with the levels of high-sensitivity C-reactive protein (hs-CRP) and cardiac troponin | (Yao et al., 2013) |
| Cardiomyopathy | Tissue | Correlated with disease outcome | (Funayama et al., 2013) |
| Abdominal aortic aneurysm (AAA) | Human AAA samples | These findings suggest a significant role for HMGB1 in the pathogenesis of AAA | (Kohno et al., 2012) |
| Cardiac arrest | Serum | Associated with outcome | (Oda et al., 2012) |
| Exertional Heatstroke | Plasma | An indicator of the severity of illness and a useful mortality predictor in exertional heatstroke | (Tong et al., 2011) |
| Cardiac surgery patient | Plasma | Contribute to disease progression | (Haque et al., 2011) |
| Heart failure | Serum | Related with disease progression | (Wang et al., 2011c) |
| Myocardial infarction (MI) | Serum | A highly valuable surrogate marker for infarct transmurality and for the prediction of residual left ventricular function after MI | (Andrassy et al., 2011) |
| Thoracic aortic aneurysm (TAA) repair | Serum | HMGB1 might play a key role in the pathogenesis of SIRS after surgical TAA repair | (Kohno et al., 2011) |
| Coronary artery stenosis | Serum | Correlated with disease severity | (Hu et al., 2009) |
| Coronary artery disease in nondiabetic and type 2 diabetic patients | Serum | Correlated with disease severity | (Yan et al., 2009) |
| Digestive system | |||
| Pancreatic cancer (PC) | Serum | Courses of HMGB1 show prognostic relevance in PC patients undergoing chemotherapy | (Wittwer et al., 2013b) |
| Colorectal cancer after radioembolization | Serum | A valuable serum biomarker for early estimation of therapy response and prognosis in colorectal cancer patients | (Fahmueller et al., 2013) |
| Human primary liver cancer | Tissue | Represent a potential therapeutic target for this aggressive malignancy | (Dong et al., 2013) |
| Liver cancer patients receiving transarterial chemoembolization therapy | Serum | There was no difference with respect to treatment response for DNase and HMGB1 | (Kohles et al., 2012) |
| Acetaminophen hepatotoxicity | Serum | HMGB1 represent blood-based tools to investigate the cell death balance clinical APAP hepatotoxicity. Acetylated HMGB1 was associated with worse outcome | (Antoine et al., 2012b) |
| Gastric cancer | Serum | HMGB1 appears to be a useful serological biomarker for early diagnosis as well as evaluating the tumorigenesis, stage, and prognosis of gastric cancer | (Chung et al., 2009) |
| Esophageal squamous cell carcinoma (ESCC) | Serum | HMGB1 may be used as a marker in diagnosis, prediction of prognosis and monitor of postoperative recurrence of ESCC | (Chen et al., 2013a) |
| Autologous islet transplantation | Serum | Therapeutic target | (Itoh et al., 2012a) |
| Esophageal squamous cell carcinoma (ESCC) | Serum | Related to clinical outcome after chemoradiation | (Suzuki et al., 2012) |
| Liver resection | Serum | Correlated with therapy effect | (Tsujii et al., 2012) |
| Acute appendicitis (AA) | Serum | HMGB-1 might be useful in the diagnosis of AA | (Albayrak et al., 2011) |
| Inflammatory bowel disease | Serum | Correlated with disease progression | (McDonnell et al., 2011) |
| Carcinoma of the thoracic esophagus who underwent transthoracic esophagectomy | Serum | Predictor of therapy effect | (Suda et al., 2007) |
| Severe acute pancreatitis (SAP) | Serum | These results suggest that HMGB1 may act as a key mediator for inflammation and organ failure in SAP | (Yasuda et al., 2006) |
| Hepatocellular carcinoma (HCC) | Tissue | An important pathogenetic factor in HCC | (Jiang et al., 2012a) |
| Gastrointestinal surgery | Serum | Correlated with disease progression | (Takahata et al., 2011) |
| Colorectal carcinoma | Tissue | Co-expression of RAGE and amphoterin is closely associated with invasion and metastasis of colorectal cancer | (Kuniyasu et al., 2003b) |
| Hepatocellular carcinoma (HCC) | Serum | A useful marker for evaluating the tumor stage and predicting prognosis in HCC, as well as therapeutic target. | (Cheng et al., 2008) |
| Acetaminophe n-induced acute liver injury | Plasma | The application of such a biomarker panel could improve the speed of clinical decision-making. | (Antoine et al., 2013) |
| Acute liver failure | Tissue | Cytoplasmic HMGB1 translocation contributes to the pathogenesis of liver inflammatory diseases | (Zhou et al., 2011b) |
| Malignant peritoneal mesothelioma | Serum | A useful serum marker for diagnosis | (Tabata et al., 2013a) |
| Esophageal squamous cell carcinoma | Carcinoma cells | HMGB1 might serve as the marker of progression and potential target for anti-lymphangiogenesis therapy | (Chuangui et al., 2012) |
| Acute-on-chronic liver failure (ACLF) | PBMCs and serum | HMGB1 plays a critical role in the systemic inflammation of ACLF and could be a potential therapeutic target in the treatment of ACLF | (Zhou et al., 2012b) |
| Gastric cancer | Tissue | Combined evaluation of HMGB1 and VEGF-C may serve as a valuable independent prognostic factor for GC patients | (He et al., 2013) |
| Malignant pleural mesothelioma (MPM) | Serum | A useful prognostic factor for MPM | (Tabata et al., 2013b) |
| Hepatocellular carcinoma (HCC) | Tissue | Contribute to disease progression and become useful prognosis factor | (Liu et al., 2012b) |
| Pancreatic ductal adenocarcinoma | Serum | A desirable diagnostic and prognostic biomarker for PDAC | (Chung et al., 2012) |
| Acute liver failure (ALF) | Plasma | HMGB1 levels were increased in patients with ALF | (Oshima et al., 2012) |
| Colorectal carcinoma | Serum | A supportive diagnostic marker for colorectal carcinomas | (Lee et al., 2012b) |
| Colon cancer | Tissue | Diagnosis value | (Soldevilla et al., 2011) |
| Pediatric inflammatory bowel disease | Fecal | A novel marker for intestinal inflammation | (Vitali et al., 2011) |
| Esophageal squamous cell carcinoma | Tissue | could be used as tumor-associated antigen (TAA) biomarkers in cancer diagnosis | (Zhang et al., 2011c) |
| Acute pancreatitis | Serum | Predictor for prognosis | (Lindstrom et al., 2009) |
| Colorectal cancer | Tissue | Contribute to disease progression | (Kijanka et al., 2010) |
| Acute pancreatitis | Serum | A complex study of the plasma levels of HMGB1, sRAGE and circulating DNA can be informative in evaluations of acute pancreatitis with different levels of severity | (Kocsis et al., 2009) |
| Human liver transplantation | Serum | A marker of hepatocellular injury in human liver transplantation | (Ilmakunnas et al., 2008) |
| Colon cancer | Tissue | HMGB1 might contribute to the progression of colon cancer via modulation of the local immune response | (Peng et al., 2010) |
| Colon carcinoma | Tissue | Contribute to disease progression | (Volp et al., 2006) |
| Gastrointestinal stromal tumors | Tissue | Contribute to disease progression | (Choi et al., 2003) |
| Colorectal | Macrophages in the lymph nodes of colorectal cancer | Contribute to disease progress | (Moriwaka et al., 2010) |
| Ear, Nose, and Throat | |||
| Laryngeal squamous cell carcinoma | Tissue | HMGB1 contribute to disease development and may be an independent prognostic factor | (Tang et al., 2013b) |
| Laryngeal squamous cell carcinoma (LSCC) | Tissue | HMGB1 may become a valuable marker for the prediction of prognosis in patients with LSCC | (Liu et al., 2012d) |
| Laryngeal squamous cell carcinoma (LSCC) | Tissue | HMGB1 might play a critical role in the initiation and progression of LSCC | (Liu et al., 2011e) |
| Squamous-cell carcinoma of the head and neck (SCCHN) | Tissue | HMGB1 may contribute to the malignant progression of SCCHN, and present as a novel prognostic marker and a potential therapeutic target for patients with SCCHN | (Liu et al., 2010d) |
| Sasopharyngeal carcinoma (NPC) | Tissue | Predictor for clinical outcome | (Wu et al., 2008) |
| Laryngeal squamous cell carcinoma (LSCC) | Tissue | Correlated with tumor stage | (Guo et al., 2012) |
| Chronic rhinosinusitis with nasal polyposis | Tissue | HMGB1 may play a crucial role in the pathogenesis of chronic rhinosinusitis with nasal polyps | (Bellussi et al., 2012) |
| Chronic rhinosinusitis | Paranasal sinus mucosa | A possible contribution of HMGB1in the pathophysiology of CRS | (Hong et al., 2013) |
| Head and neck cancer | Serum and tumor cells | Contribute to disease progression | (Wild et al., 2012b) |
| Ophthalmology | |||
| Conjunctivitis and Blepharitis | Tears | Contribute to disease progression | (Cavone et al., 2011a) |
| Children affected by vernal keratoconjunctivitis | Serum | Contribute to disease progression | (Zicari et al., 2013) |
| Proliferative vitreoretinopathy | Stromal cells | The HMGB1/RAGE/OPN/Egr-1 pathway may be involved in inflammatory, angiogenic and fibrotic responses in proliferative vitreoretinal disorders | (El-Asrar et al., 2011a) |
| Endophthalmitis | Tissue | Contribute to disease progression | (Arimura et al., 2008) |
| Hematology | |||
| T-cell lymphoma | Tissue | Elevated expression of HMGB1 may be an important biomarker for the development and progression of T-cell lymphoma | (Mao et al., 2012) |
| Children with acute lymphocytic leukemia | Serum | Predictor of prognosis | (Kang et al., 2007) |
| Disseminated intravascular coagulation(D IC) | Plasma | A potentially suitable prognostic marker of DIC | (Hatada et al., 2005) |
| Chronic idiopathic neutropenia | Bone marrow cells | Contribute to disease progression | (Velegraki et al., 2012) |
| Non-Hodgkin lymphoma | Tissue | An interesting therapeutic target as well | (Meyer et al., 2008) |
| Nervous system | |||
| Multiple sclerosis (MS) | Cerebrospinal fluids | HMGB1 in the CNS is a useful biomarker and may contribute to the chronicity of neuroinflammation | (Andersson et al., 2008) |
| Neuromyelitis optica (NMO) | Cerebrospinal fluids | HMGB1 could play a key role in central nervous system inflammation in NMO patients | (Uzawa et al., 2013a) |
| Ischemic stroke | Plasma | Plasma HMGB1 level represents a novel biomarker for predicting outcomes of ischemic stroke | (Huang et al., 2013c) |
| Aneurysmal subarachnoid hemorrhage | Plasma | HMGB1 level is a useful, complementary tool to predict functional outcome and mortality after aneurysmal subarachnoid hemorrhage | (Zhu et al., 2012b) |
| Traumatic brain injury (TBI) | Plasma | Plasma HMGB1 concentration emerges as a novel biomarker for predicting clinical outcomes of TBI | (Wang et al., 2012b) |
| Acute cerebral infarction (ACI) | Serum | Might be helpful to evaluate the severity and prognosis of ACI | (Zhou et al., 2012a) |
| Neonates with hypoxic-ischemic encephalopathy | The umbilical artery HMG B1 | HMGB1 is a useful index of the inhibition of early stage inflammation. | (Nakamura et al., 2013) |
| Traumatic brain injury | Tissue | Might be a therapeutic target | (Gao et al., 2012) |
| Pediatric traumatic brain injury | Cerebrospinal fluids(CSF) | These data are also consistent with the designation of HMGB1 as a "danger signal” | (Au et al., 2012) |
| Intracranial inflammatory lesions | cytoplasm collapse | Contribute to disease progression | (Hirano et al., 2012) |
| Febrile seizure | Serum | Might be a therapeutic target | (Choi et al., 2011) |
| Several neurological diseases | Cerebrospinal fluids(CSF) | HMGB1 is a poor disease marker for acute encephalopathy | (Asano et al., 2011) |
| Acute intracerebral hemorrhage | Serum | Contribute to disease progression | (Zhou et al., 2010) |
| Stroke | Serum | Serum HMGB1 release are possible mediators in stroke induced activation of T cells | (Vogelgesang et al., 2010) |
| Subarachnoid hemorrhage (SAH) | Elisa | HMGB1 might play a key role in the inflammatory response in the CNS of SAH patients | (Nakahara et al., 2009) |
| Pediatric patients with meningitis | Spinal fluid | Contribute to disease progression | (Tang et al., 2008a) |
| Cerebral and myocardial ischemia | Serum | Systemic HMGB1 levels are elevated in human ischemic disease | (Goldstein et al., 2006) |
| Infectious diseases | |||
| Acute liver failure | Serum | Correlate with disease pathogenesis | (Allonso et al., 2012) |
| Sepsis and septic shock | Serum | HMGB1is indeed a downstream mediator of inflammation | (Sunden-Cullberg et al., 2005) |
| 2009 pandemic H1N1 | Serum | Correlate with disease pathogenesis | (Momonaka et al., 2013) |
| Brucellosis | Serum | May be diagnostic markers for brucellosis | (Ayarci et al., 2013) |
| HIV-1 | Serum | Contribute to disease progression | (Troseid et al., 2010) |
| Community acquired infections and bacteraemia | Serum | Whereas LBP, IL-6 and CRP seem to be good markers to detect patients with bacteraemia, HMGB1 seem to be of minor importance | (Pavare et al., 2010) |
| Severe infection | Plasma | Plasma HMGB1 was higher in HIV-infected children than in HIV-uninfected children | (Carrol et al., 2009) |
| Severe infection | Serum | Correlate with disease pathogenesis | (van Zoelen et al., 2007) |
| Multiple organ dysfunction syndrome, coupled plasma filtration adsorption (CPFA) | Serum | Predictor of therapy effect | (Hu et al., 2012a) |
| Sepsis-induced immunosuppression | Serum | Used for monitoring of monocytic function in immunostimulatory trials | (Unterwalder et al., 2010) |
| Septic shock | Serum | Predictor of therapy effect | (Sakamoto et al., 2007) |
| Pulmonary tuberculosis | Lung tissue and serum | The measurement of serum HMGB1 is useful to evaluate the severity of disease | (Yang and Yang, 2013) |
| HIV-infection | Plasma | Contribute to disease progression | (Troseid et al., 2013) |
| Malaria | Serum | Could offer a potential target for therapeutic intervention | (Angeletti et al., 2013) |
| Severe and fatal Plasmodium falciparum malaria | Plasma | An informative prognostic marker of disease severity | (Higgins et al., 2013) |
| M. tuberculosis infection | Serum | HMGB1 is secreted during active and latent tuberculosis in the highest amounts compared to other lung diseases | (Magrys et al., 2013) |
| 2009 H1N1 influenza virus | Serum | HMGB1 could play an important role in the pathogenesis of severe pneumonia | (Ito et al., 2011) |
| Sepsis in severely burned patients. | Plasma | Dynamic measurements of circulating HMGB1 levels should be helpful to monitor the disease course and judge the prognosis of burned patients | (Dong et al., 2007) |
| Suspected community-acquired infections and sepsis | Serum | Levels of HMGB1 correlated only very weakly to other pro-inflammatory markers and did not correlate to the anti-inflammatory marker sCD163 | (Gaini et al., 2007) |
| Septic shock | Serum | Contribute to disease progression | (Nakamura et al., 2011c) |
| Chronic hepatitis B | Serum | Serve as a therapeutic marker | (Wang et al., 2010d) |
| Severely burned patients | Serum | Correlated with disease progression | (Huang et al., 2011b) |
| Bacteraemia | Serum | Neither HMGB1 nor any of the proinflammatory markers were elevated in fatal cases compared to survivors | (Gaini et al., 2008) |
| Severe sepsis | Serum | Serum HMGB1 concentrations were elevated in patients with severe sepsis | (Karlsson et al., 2008) |
| Septic shock | Serum | Correlate with disease stage | (Gibot et al., 2007) |
| HEV | Serum | Contribute to disease progression | (Majumdar et al., 2013) |
| Falciparum malaria | Serum | Contribute to disease progression | (Alleva et al., 2005) |
| Severe sepsis and septic shock | Serum | Contribute to disease progression | (Sasahira et al., 2005a) |
| Hepatitis B | Serum | HMGB1 is a noninvasive, repeatable, and convenient marker for distinguishing advanced fibrosis from low fibrosis in chronic HBV patients | (Albayrak et al., 2010) |
| Chronic hepatitis B (HBV) | Serum | HMGB1 may play a key role in the pathogenesis of chronic severe hepatitis B and liver failure | (Liu et al., 2007b) |
| HBV-related ACLF | Serum | HMGB1 may be useful as a prognostic marker for development of ACLF | (Duan et al., 2013) |
| Dermatology | |||
| Recessive dystrophic epidermolys is bullosa | Serum | HMGB1 levels may represent a new biomarker reflecting disease severity | (Petrof et al., 2013) |
| Psoriasis vulgaris (PV) and atopic dermatitis (AD) | Serum | HMGB1 might be involved in the pathogenesis of PV | (Chen et al., 2013d) |
| Epidermal tumors | Seborrheic keratosis | They also indicate that the TLR4 signaling pathway, rather than HMGB1, may be the principal mediator of inflammation in high-grade malignant epidermal tumors | (Weng et al., 2013) |
| Stevens-Johnson Syndrome | Serum | Would be a useful diagnostic tool | (Nakajima et al., 2011) |
| Psychiatry | |||
| Autistic disorder | Serum | Compared with healthy subjects, serum levels of HMGB1 were significantly higher in patients with autistic disorder | (Emanuele et al., 2010) |
| Autism | Serum | EGF levels correlated with HMGB1 levels but not the other tested putative biomarkers | (Russo, 2013) |
| Endocrine system | |||
| Type 1 and 2 diabetes mellitus | Serum | Reflect endothelial dysfunction developing in diabetes | (Skrha et al., 2012) |
| Proliferative diabetic retinopathy (PDR) | Serum | HMGB1 regulates the angiogenesis in PDR | (Abu El-Asrar et al., 2012) |
| Proliferative diabetic retinopathy (PDR) | Serum | Contribute disease progression | (El-Asrar et al., 2011b) |
| Proliferative diabetic retinopathy | Serum | Contribute disease progression | (Abu El-Asrar et al., 2013) |
| Type 1 diabetes | Serum | Associated with a higher risk of all-cause mortality | (Nin et al., 2012a) |
| Type 1 diabetes | Serum | Contribute disease progression | (Nin et al., 2012b) |
| Type 2 diabetes | Serum | Contribute disease progression | (Dasu et al., 2010) |
| Type 1 diabetes | Serum | Contribute disease progression | (Devaraj et al., 2009) |
| Gynecology and obstetrics | |||
| Normal pregnancy and preeclampsia | Serum | Serum | (Naruse et al., 2012) |
| Preterm parturition | Amniotic fluid | Contribute disease progression | (Romero et al., 2011) |
| Early neonates | Plasma | Contribute disease progression | (Nakamura et al., 2012b) |
| Preeclampsia | Placental tissue | Contribute disease progression | (Wang et al., 2011a) |
| Inflammation-Induced Preterm Birth and Fetal Tissue Injury | Fetal circulation | Important mediators of cellular injury in fetuses delivered in the setting of inflammation-induced preterm birth | (Buhimschi et al., 2009) |
| Preterm and term cervix | Tissue | Contribute disease progression | (Dubicke et al., 2010) |
| Pre-eclampsia | Placenta | Contribute disease progression | (Gao et al., 2008) |
| Human endometrium | Endometrium | HMGB1 is expressed in the human endometrium, and its expression is modulated by E2, progesterone, and nitric oxide | (Zicari et al., 2008) |
| Human term placental | Placenta | Contribute disease progression | (Holmlund et al., 2007) |
| Chondrosarcoma | Tissue | The numbers of cells positive for HMGB1 expression are positively associated with histologic grade | (Takeuchi et al., 2007) |
| Breast cancer after the initial dose of epirubicin/doc etaxel | Plasma | HMGB1 could be a promising biomarker to predict the final response to therapy in breast cancer patients | (Arnold et al., 2013) |
| Breast cancer patients during neoadjuvant chemotherapy | Serum | Valuable for the diagnosis and early estimation of response to n therapy | (Stoetzer et al., 2013) |
| Ovarian cancer | Tissue | HMGB1 may serve as a new biomarker and a therapeutic target for ovarian cancer in the future | (Chen et al., 2012a) |
| Human epithelial ovarian cancer | Tissue | Contribute to disease progression and serve as a therapeutic target for ovarian cancer | (Zhang et al., 2013e) |
| Chorioamnionitis | Amniotic fluid | HMGB1are engaged in the process of clinical chorioamnionitis at term | (Romero et al., 2012) |
| Department of stomatology | |||
| Periodontitis | Tissue | HMGB1 may be a potential target for the therapy of periodontitis | (Xie et al., 2011) |
| Metabolic disorders | |||
| Obese children | Serum | HMGB1 may be an important diagnostic marker for obesity-related complications | (Arrigo et al., 2013) |
| Hyperlipidemia | Serum | Serum HMGB1 levels are increased in patients with hyperlipidemia which could be reduced by atorvastatin | (Jin et al., 2012) |
| Trauma | |||
| Severe trauma | Serum | Serum HMGB1 of severe trauma patients can be used for the clinical indicator of prognosis | (Dang et al., 2011) |
| Multiple trauma | Serum | HMG-1 can be used as a warning indicator of the onset of MODS | (Fei et al., 2005) |
| Surgical/anesthesia trauma | Serum | Contribute to disease progression | (Manganelli et al., 2010) |
| Mechanical trauma | Plasma | A potential target for future therapeutics. | (Peltz et al., 2009) |
| Burn trauma | Plasma | Contribute to disease progression | (Lantos et al., 2010) |
| Extensively burned patient | Plasma | Might be involved in the pathogenesis of suppression of T cell-mediated immunity in these patients | (Dong et al., 2008) |
| Emergencies | |||
| Emergent diseases | Serum | HMGB-1 is not significantly sensitive or specific for diagnosis of sepsis | (Gamez-Diaz et al., 2011) |
| Other | |||
| Human malignant tumors | Tissue | Could have a prognostic meaning in carcinogenesis | (Kostova et al., 2010) |
| Cancerous and Inflammatory Effusions | Pleural and peritoneal effusions | A possible target for treatment in advanced cancer as well | (Winter et al., 2009) |
15 Concluding Remarks
In 1973, the discovery of HMGB1 as a non-histone chromosomal protein was reported. Nuclear HMGB1 regulates a number of DNA-related events such as replication, recombination, repair, and transcription (Goodwin et al., 1973). In 1999, with the discovery of HMGB1 as a late mediator of sepsis, a new research field was born (Wang et al., 1999). During the past 15 years, a number of HMGB1 receptors and posttranslational modifications have been identified, which enhance or diminish extracellular HMGB1activity in multiple cellular processes (Bianchi, 2009; Yang et al., 2013c). In addition to its receptors, HMGB1 endocytic uptake plays an important role in mediated HMGB1 activity and degradation (Kang et al., 2014; Xu et al., 2014; Zhang et al., 2012e). Intracellular and extracellular HMGB1 play significantly different roles in inflammation, injury, and cancer. A new function for HMGB1 is as an autophagy regulator, which has been linked to sterile inflammation, infection, neurodegenerative disease, and cancer (Kang et al., 2011c; Zhang et al., 2013d). These properties of HMGB1 make it an attractive biomarker and therapeutic target (Andersson and Tracey, 2011; Sims et al., 2010). Numerous strategies are being employed to inhibit HMGB1 release and activity in inflammation-associated diseases (Musumeci et al., 2014; Wang et al., 2014b). Several genetic animal models of HMGB1 have recently been created to examine the physiological and pathological roles of HMGB1 in health and disease. The phenotype of HMGB1 conditional knockout mice is complex and even paradoxical (Ge et al., 2014; Huang et al., 2013a, 2014; Huebener et al., 2014; Kang et al., 2013b; Tang et al., 2014; Yanai et al., 2013). Future work investigating the details of HMGB1 location, structure, modification, and partners will uncover the secrets of HMGB1’s multiple functions.
Acknowledgments
We thank Christine Heiner (Department of Surgery, University of Pittsburgh) for her critical reading of the manuscript. This work was supported by the National Institutes of Health (R01CA160417 to D.T.; R01CA181450 to H.J.Z/M.T.L; R01AT005076 and R01GM063075 to H.W.), a grant from the National Natural Sciences Foundation of China (31171328 to L.C.) and a 2013 Pancreatic Cancer Action Network-AACR Career Development Award (Grant Number 13-20-25-TANG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273–1275. [PubMed] [Google Scholar]
- Abarbanell AM, Hartley JA, Herrmann JL, Weil BR, Wang Y, Manukyan MC, Poynter JA, Meldrum DR. Exogenous high-mobility group box 1 improves myocardial recovery after acute global ischemia/reperfusion injury. Surgery. 2011;149(3):329–335. doi: 10.1016/j.surg.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Abdul-Razzak KK, Denton ML, Cox DJ, Reeck GR. Isolation and characterization of folded fragments released by Staphylococcal aureus proteinase from the non-histone chromosomal protein HMG-1. Biochim Biophys Acta. 1989;996(1–2):125–131. doi: 10.1016/0167-4838(89)90104-0. [DOI] [PubMed] [Google Scholar]
- Abdulahad DA, Westra J, Bijzet J, Dolff S, van Dijk MC, Limburg PC, Kallenberg CG, Bijl M. Urine levels of HMGB1 in Systemic Lupus Erythematosus patients with and without renal manifestations. Arthritis research & therapy. 2012;14(4):R184. doi: 10.1186/ar4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulahad DA, Westra J, Bijzet J, Limburg PC, Kallenberg CG, Bijl M. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(3):R71. doi: 10.1186/ar3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulahad DA, Westra J, Reefman E, Zuidersma E, Bijzet J, Limburg PC, Kallenberg CG, Bijl M. High mobility group box1 (HMGB1) in relation to cutaneous inflammation in systemic lupus erythematosus (SLE) Lupus. 2013;22(6):597–606. doi: 10.1177/0961203313483377. [DOI] [PubMed] [Google Scholar]
- Abe S, Hayashi H, Seo Y, Matsuda K, Kamio K, Saito Y, Usuki J, Azuma A, Kudo S, Gemma A. Reduction in serum high mobility group box-1 level by polymyxin B-immobilized fiber column in patients with idiopathic pulmonary fibrosis with acute exacerbation. Blood Purif. 2011;32(4):310–316. doi: 10.1159/000330325. [DOI] [PubMed] [Google Scholar]
- Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, Uchimura T, Ida N, Yamazaki Y, Yamada S, Yamamoto Y, Yamamoto H, Iino S, Taniguchi N, Maruyama I. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115(5):1267–1274. doi: 10.1172/JCI22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham AB, Bronstein R, Chen EI, Koller A, Ronfani L, Maletic-Savatic M, Tsirka SE. Members of the high mobility group B protein family are dynamically expressed in embryonic neural stem cells. Proteome Sci. 2013a;11(1):18. doi: 10.1186/1477-5956-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham AB, Bronstein R, Reddy AS, Maletic-Savatic M, Aguirre A, Tsirka SE. Aberrant Neural Stem Cell Proliferation and Increased Adult Neurogenesis in Mice Lacking Chromatin Protein HMGB2. PLoS ONE. 2013b;8(12):e84838. doi: 10.1371/journal.pone.0084838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165(6):2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- Abu El-Asrar AM, Nawaz MI, Kangave D, Abouammoh M, Mohammad G. High-mobility group box-1 and endothelial cell angiogenic markers in the vitreous from patients with proliferative diabetic retinopathy. Mediators of inflammation. 2012;2012:697489. doi: 10.1155/2012/697489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu El-Asrar AM, Nawaz MI, Siddiquei MM, Al-Kharashi AS, Kangave D, Mohammad G. High-mobility group box-1 induces decreased brain-derived neurotrophic factor-mediated neuroprotection in the diabetic retina. Mediators of inflammation. 2013;2013:863036. doi: 10.1155/2013/863036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achouiti A, van der Meer AJ, Florquin S, Yang H, Tracey KJ, van 't Veer C, de Vos AF, van der Poll T. High-mobility group box 1 and the receptor for advanced glycation end products contribute to lung injury during Staphylococcus aureus pneumonia. Crit Care. 2013;17(6):R296. doi: 10.1186/cc13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adilakshmi T, Ness-Myers J, Madrid-Aliste C, Fiser A, Tapinos N. A nuclear variant of ErbB3 receptor tyrosine kinase regulates ezrin distribution and Schwann cell myelination. J Neurosci. 2011;31(13):5106–5119. doi: 10.1523/JNEUROSCI.5635-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjaye J, Huntriss J, Herwig R, BenKahla A, Brink TC, Wierling C, Hultschig C, Groth D, Yaspo ML, Picton HM, Gosden RG, Lehrach H. Primary differentiation in the human blastocyst: comparative molecular portraits of inner cell mass and trophectoderm cells. Stem Cells. 2005;23(10):1514–1525. doi: 10.1634/stemcells.2005-0113. [DOI] [PubMed] [Google Scholar]
- Adlesic M, Verdrengh M, Bokarewa M, Dahlberg L, Foster SJ, Tarkowski A. Histamine in rheumatoid arthritis. Scand J Immunol. 2007;65(6):530–537. doi: 10.1111/j.1365-3083.2007.01938.x. [DOI] [PubMed] [Google Scholar]
- af Klint E, Grundtman C, Engstrom M, Catrina AI, Makrygiannakis D, Klareskog L, Andersson U, Ulfgren AK. Intraarticular glucocorticoid treatment reduces inflammation in synovial cell infiltrations more efficiently than in synovial blood vessels. Arthritis Rheum. 2005;52(12):3880–3889. doi: 10.1002/art.21488. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Qamar A, Sharma V, Sharma A. Abdominal aortic aneurysm: A comprehensive review. Exp Clin Cardiol. 2011;16(1):11–15. [PMC free article] [PubMed] [Google Scholar]
- Agnello D, Wang H, Yang H, Tracey KJ, Ghezzi P. HMGB-1, a DNA-binding protein with cytokine activity, induces brain TNF and IL-6 production, and mediates anorexia and taste aversion. Cytokine. 2002;18(4):231–236. doi: 10.1006/cyto.2002.0890. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Schatz DG. RAG1 and RAG2 form a stable postcleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell. 1997;89(1):43–53. doi: 10.1016/s0092-8674(00)80181-6. [DOI] [PubMed] [Google Scholar]
- Agresti A, Bianchi ME. HMGB proteins and gene expression. Curr Opin Genet Dev. 2003;13(2):170–178. doi: 10.1016/s0959-437x(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Agresti A, Lupo R, Bianchi ME, Muller S. HMGB1 interacts differentially with members of the Rel family of transcription factors. Biochem Biophys Res Commun. 2003;302(2):421–426. doi: 10.1016/s0006-291x(03)00184-0. [DOI] [PubMed] [Google Scholar]
- Agresti A, Scaffidi P, Riva A, Caiolfa VR, Bianchi ME. GR and HMGB1 interact only within chromatin and influence each other's residence time. Mol Cell. 2005;18(1):109–121. doi: 10.1016/j.molcel.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Aguilera R, Saffie C, Tittarelli A, Gonzalez FE, Ramirez M, Reyes D, Pereda C, Hevia D, Garcia T, Salazar L, Ferreira A, Hermoso M, Mendoza-Naranjo A, Ferrada C, Garrido P, Lopez MN, Salazar-Onfray F. Heat-shock induction of tumor-derived danger signals mediates rapid monocyte differentiation into clinically effective dendritic cells. Clin Cancer Res. 2011;17(8):2474–2483. doi: 10.1158/1078-0432.CCR-10-2384. [DOI] [PubMed] [Google Scholar]
- Ahn JK, Cha HS, Bae EK, Lee J, Koh EM. Extracellular high-mobility group box 1 is increased in patients with Behcet's disease with intestinal involvement. Journal of Korean medical science. 2011;26(5):697–700. doi: 10.3346/jkms.2011.26.5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aidinis V, Bonaldi T, Beltrame M, Santagata S, Bianchi ME, Spanopoulou E. The RAG1 homeodomain recruits HMG1 and HMG2 to facilitate recombination signal sequence binding and to enhance the intrinsic DNA-bending activity of RAG1-RAG2. Mol Cell Biol. 1999;19(10):6532–6542. doi: 10.1128/mcb.19.10.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aits S, Jaattela M. Lysosomal cell death at a glance. J Cell Sci. 2013;126(Pt 9):1905–1912. doi: 10.1242/jcs.091181. [DOI] [PubMed] [Google Scholar]
- Aizawa S, Nishino H, Saito K, Kimura K, Shirakawa H, Yoshida M. Stimulation of transcription in cultured cells by high mobility group protein 1: essential role of the acidic carboxyl-terminal region. Biochemistry. 1994;33(49):14690–14695. doi: 10.1021/bi00253a006. [DOI] [PubMed] [Google Scholar]
- Akaike H, Kono K, Sugai H, Takahashi A, Mimura K, Kawaguchi Y, Fujii H. Expression of high mobility group box chromosomal protein-1 (HMGB-1) in gastric cancer. Anticancer Res. 2007;27(1A):449–457. [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Alami-Ouahabi N, Veilleux S, Meistrich ML, Boissonneault G. The testis-specific high-mobility-group protein, a phosphorylation-dependent DNA-packaging factor of elongating and condensing spermatids. Mol Cell Biol. 1996;16(7):3720–3729. doi: 10.1128/mcb.16.7.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albayrak A, Uyanik MH, Cerrah S, Altas S, Dursun H, Demir M, Uslu H. Is HMGB1 a new indirect marker for revealing fibrosis in chronic hepatitis and a new therapeutic target in treatment? Viral Immunol. 2010;23(6):633–638. doi: 10.1089/vim.2010.0080. [DOI] [PubMed] [Google Scholar]
- Albayrak Y, Albayrak A, Celik M, Gelincik I, Demiryilmaz I, Yildirim R, Ozogul B. High mobility group box protein-1 (HMGB-1) as a new diagnostic marker in patients with acute appendicitis. Scandinavian journal of trauma, resuscitation and emergency medicine. 2011;19:27. doi: 10.1186/1757-7241-19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleporou-Marinou V, Drosos Y, Ninios Y, Agelopoulou B, Patargias T. High mobility group-like proteins of the insect Plodia interpunctella. Biochem Genet. 2003;41(1–2):39–46. doi: 10.1023/a:1020922512440. [DOI] [PubMed] [Google Scholar]
- Alexandrova EA, Beltchev BG. Differences between HMG1 proteins isolated from normal and tumour cells. Biochim Biophys Acta. 1987;915(3):399–405. doi: 10.1016/0167-4838(87)90026-4. [DOI] [PubMed] [Google Scholar]
- Alexandrova EA, Beltchev BG. Acetylated HMG1 protein interacts specifically with homologous DNA polymerase alpha in vitro. Biochem Biophys Res Commun. 1988;154(3):918–927. doi: 10.1016/0006-291x(88)90227-6. [DOI] [PubMed] [Google Scholar]
- Alexandrova EA, Marekov LN, Beltchev BG. Involvement of protein HMG1 in DNA replication. FEBS Lett. 1984;178(1):153–155. doi: 10.1016/0014-5793(84)81260-0. [DOI] [PubMed] [Google Scholar]
- Ali I, Gruenloh S, Gao Y, Clough A, Falck JR, Medhora M, Jacobs ER. Protection by 20-5,14-HEDGE against surgically induced ischemia reperfusion lung injury in rats. Ann Thorac Surg. 2012;93(1):282–288. doi: 10.1016/j.athoracsur.2011.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat Immunol. 2010;11(10):945–952. doi: 10.1038/ni.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliahmad P, Kaye J. Development of all CD4 T lineages requires nuclear factor TOX. J Exp Med. 2008;205(1):245–256. doi: 10.1084/jem.20071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliahmad P, Seksenyan A, Kaye J. The many roles of TOX in the immune system. Curr Opin Immunol. 2012;24(2):173–177. doi: 10.1016/j.coi.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva LM, Yang H, Tracey KJ, Clark IA. High mobility group box 1 (HMGB1) protein: possible amplification signal in the pathogenesis of falciparum malaria. Trans R Soc Trop Med Hyg. 2005;99(3):171–174. doi: 10.1016/j.trstmh.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Allonso D, Belgrano FS, Calzada N, Guzman MG, Vazquez S, Mohana-Borges R. Elevated serum levels of high mobility group box 1 (HMGB1) protein in dengue-infected patients are associated with disease symptoms and secondary infection. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2012;55(3):214–219. doi: 10.1016/j.jcv.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Alves JN, Pires KM, Lanzetti M, Barroso MV, Benjamim CF, Costa CA, Resende AC, Santos JC, Ribeiro ML, Porto LC, Valenca SS. Critical role for CCR2 and HMGB1 in induction of experimental endotoxic shock. Arch Biochem Biophys. 2013;537(1):72–81. doi: 10.1016/j.abb.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Amano T, Leu K, Yoshizato K, Shi YB. Thyroid hormone regulation of a transcriptional coactivator in Xenopus laevis: implication for a role in postembryonic tissue remodeling. Dev Dyn. 2002;223(4):526–535. doi: 10.1002/dvdy.10075. [DOI] [PubMed] [Google Scholar]
- Ammar R, Torti D, Tsui K, Gebbia M, Durbic T, Bader GD, Giaever G, Nislow C. Chromatin is an ancient innovation conserved between Archaea and Eukarya. eLife. 2012;1:e00078. doi: 10.7554/eLife.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An W, van Holde K, Zlatanova J. The non-histone chromatin protein HMG1 protects linker DNA on the side opposite to that protected by linker histones. J Biol Chem. 1998;273(41):26289–26291. doi: 10.1074/jbc.273.41.26289. [DOI] [PubMed] [Google Scholar]
- Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, Bruce JN, Kane LP, Kuchroo VK, Hafler DA. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318(5853):1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- Andersson A, Covacu R, Sunnemark D, Danilov AI, Dal Bianco A, Khademi M, Wallstrom E, Lobell A, Brundin L, Lassmann H, Harris RA. Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. Journal of leukocyte biology. 2008;84(5):1248–1255. doi: 10.1189/jlb.1207844. [DOI] [PubMed] [Google Scholar]
- Andersson U, Erlandsson-Harris H. HMGB1 is a potent trigger of arthritis. J Intern Med. 2004;255(3):344–350. doi: 10.1111/j.1365-2796.2003.01303.x. [DOI] [PubMed] [Google Scholar]
- Andersson U, Harris HE. The role of HMGB1 in the pathogenesis of rheumatic disease. Biochim Biophys Acta. 2010;1799(1–2):141–148. doi: 10.1016/j.bbagrm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Andersson U, Tracey KJ. HMGB1 in sepsis. Scand J Infect Dis. 2003;35(9):577–584. doi: 10.1080/00365540310016286. [DOI] [PubMed] [Google Scholar]
- Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192(4):565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbaurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117(25):3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- Andrassy M, Volz HC, Riedle N, Gitsioudis G, Seidel C, Laohachewin D, Zankl AR, Kaya Z, Bierhaus A, Giannitsis E, Katus HA, Korosoglou G. HMGB1 as a predictor of infarct transmurality and functional recovery in patients with myocardial infarction. Journal of internal medicine. 2011;270(3):245–253. doi: 10.1111/j.1365-2796.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- Andrews AJ, Luger K. Nucleosome structure(s) and stability: variations on a theme. Annual review of biophysics. 2011;40:99–117. doi: 10.1146/annurev-biophys-042910-155329. [DOI] [PubMed] [Google Scholar]
- Aneja RK, Tsung A, Sjodin H, Gefter JV, Delude RL, Billiar TR, Fink MP. Preconditioning with high mobility group box 1 (HMGB1) induces lipopolysaccharide (LPS) tolerance. J Leukoc Biol. 2008 doi: 10.1189/jlb.0108030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeletti D, Kiwuwa MS, Byarugaba J, Kironde F, Wahlgren M. Elevated levels of high-mobility group box-1 (HMGB1) in patients with severe or uncomplicated Plasmodium falciparum malaria. The American journal of tropical medicine and hygiene. 2013;88(4):733–735. doi: 10.4269/ajtmh.12-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Critical care medicine. 2007;35(4):1061–1067. doi: 10.1097/01.CCM.0000259534.68873.2A. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Dear JW, Starkey-Lewis P, Platt V, Coyle J, Masson M, Thanacoody RH, Gray AJ, Webb DJ, Moggs JG, Bateman DN, Goldring CE, Park BK. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013 doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine DJ, Harris HE, Andersson U, Tracey KJ, Bianchi ME. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol Med. 2014 doi: 10.2119/molmed.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. Journal of Hepatology. 2012a;56(5):1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. Journal of hepatology. 2012b;56(5):1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Antoine DJ, Williams DP, Kipar A, Jenkins RE, Regan SL, Sathish JG, Kitteringham NR, Park BK. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112(2):521–531. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med. 2010;16(11–12):479–490. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008;68(11):4026–4030. doi: 10.1158/0008-5472.CAN-08-0427. [DOI] [PubMed] [Google Scholar]
- Apetrei NS, Calugaru A, Kerek F, Panteli M, Rasit I, Cremer L, Szegli G, Lupu AR. A highly purified vegetal fraction able to modulate HMGB1 and to attenuate septic shock in mice. Roum Arch Microbiol Immunol. 2011;70(3):114–123. [PubMed] [Google Scholar]
- Aravind L, Landsman D. AT-hook motifs identified in a wide variety of DNA-binding proteins. Nucleic Acids Res. 1998;26(19):4413–4421. doi: 10.1093/nar/26.19.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends MJ, Morris RG, Wyllie AH. Apoptosis. The role of the endonuclease. Am J Pathol. 1990;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Arimura N, Ki IY, Hashiguchi T, Sakamoto T, Maruyama I. High-mobility group box 1 protein in endophthalmitis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2008;246(7):1053–1058. doi: 10.1007/s00417-008-0827-2. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Tai AK, Manfioletti G, Clifford C, Jay G, Ono SJ. Transgenic mice expressing a truncated form of the high mobility group I-C protein develop adiposity and an abnormally high prevalence of lipomas. J Biol Chem. 2000;275(19):14394–14400. doi: 10.1074/jbc.m000564200. [DOI] [PubMed] [Google Scholar]
- Arnold T, Michlmayr A, Baumann S, Burghuber C, Pluschnig U, Bartsch R, Steger G, Gnant M, Bergmann M, Bachleitner-Hofmann T, Oehler R. Plasma HMGB-1 after the initial dose of epirubicin/docetaxel in cancer. European journal of clinical investigation. 2013;43(3):286–291. doi: 10.1111/eci.12043. [DOI] [PubMed] [Google Scholar]
- Arrigo T, Chirico V, Salpietro V, Munafo C, Ferrau V, Gitto E, Lacquaniti A, Salpietro C. High-mobility group protein B1: a new biomarker of metabolic syndrome in obese children. European journal of endocrinology / European Federation of Endocrine Societies. 2013;168(4):631–638. doi: 10.1530/EJE-13-0037. [DOI] [PubMed] [Google Scholar]
- Asano T, Ichiki K, Koizumi S, Kaizu K, Hatori T, Mashiko K, Sakamoto Y, Miyasho T, Fujino O, Fukunaga Y. High mobility group box 1 in cerebrospinal fluid from several neurological diseases at early time points. The International journal of neuroscience. 2011;121(8):480–484. doi: 10.3109/00207454.2011.580868. [DOI] [PubMed] [Google Scholar]
- Ashar HR, Fejzo MS, Tkachenko A, Zhou X, Fletcher JA, Weremowicz S, Morton CC, Chada K. Disruption of the architectural factor HMGI-C: DNA-binding AT hook motifs fused in lipomas to distinct transcriptional regulatory domains. Cell. 1995;82(1):57–65. doi: 10.1016/0092-8674(95)90052-7. [DOI] [PubMed] [Google Scholar]
- Assenberg R, Webb M, Connolly E, Stott K, Watson M, Hobbs J, Thomas JO. A critical role in structure-specific DNA binding for the acetylatable lysine residues in HMGB1. Biochem J. 2008;411(3):553–561. doi: 10.1042/BJ20071613. [DOI] [PubMed] [Google Scholar]
- Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, Oates JA, Peck CC, Schooley RT, Spilker BA, Woodcock J, Zeger SL, Grp BDW. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clinical Pharmacology & Therapeutics. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Au AK, Aneja RK, Bell MJ, Bayir H, Feldman K, Adelson PD, Fink EL, Kochanek PM, Clark RS. Cerebrospinal fluid levels of high-mobility group box 1 and cytochrome C predict outcome after pediatric traumatic brain injury. Journal of neurotrauma. 2012;29(11):2013–2021. doi: 10.1089/neu.2011.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos AM, Kiefer K, Tian J, Christensen S, Shlomchik M, Coyle AJ, Marshak-Rothstein A. RAGE-independent autoreactive B cell activation in response to chromatin and HMGB1/DNA immune complexes. Autoimmunity. 2010;43(1):103–110. doi: 10.3109/08916930903384591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayarci AO, Yilmaz E, Sigirli D, Budak F, Goral G, Oral HB. Diagnostic value of serum concentrations of high-mobility group-box protein 1 and soluble hemoglobin scavenger receptor in brucellosis. Microbiology and immunology. 2013;57(2):150–158. doi: 10.1111/1348-0421.12016. [DOI] [PubMed] [Google Scholar]
- Aychek T, Miller K, Sagi-Assif O, Levy-Nissenbaum O, Israeli-Amit M, Pasmanik-Chor M, Jacob-Hirsch J, Amariglio N, Rechavi G, Witz IP. E-selectin regulates gene expression in metastatic colorectal carcinoma cells and enhances HMGB1 release. Int J Cancer. 2008;123(8):1741–1750. doi: 10.1002/ijc.23375. [DOI] [PubMed] [Google Scholar]
- Bae JS. Effects of lower concentration thrombin on high-mobility group box 1 protein-mediated inflammatory responses. Inflammation. 2012;35(3):1078–1086. doi: 10.1007/s10753-011-9414-5. [DOI] [PubMed] [Google Scholar]
- Bae JS, Rezaie AR. Activated protein C inhibits high mobility group box 1 signaling in endothelial cells. Blood. 2011;118(14):3952–3959. doi: 10.1182/blood-2011-06-360701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C, Isenberg I, Goodwin GH, Johns EW. Physical studies of the nonhistone chromosomal proteins HMG-U and HMG-2. Biochemistry. 1976;15(8):1645–1649. doi: 10.1021/bi00653a009. [DOI] [PubMed] [Google Scholar]
- Balani P, Boulaire J, Zhao Y, Zeng J, Lin J, Wang S. High mobility group box2 promoter-controlled suicide gene expression enables targeted glioblastoma treatment. Mol Ther. 2009;17(6):1003–1011. doi: 10.1038/mt.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramani M, Day BW, Schoen RE, Getzenberg RH. Altered expression and localization of creatine kinase B, heterogeneous nuclear ribonucleoprotein F, and high mobility group box 1 protein in the nuclear matrix associated with colon cancer. Cancer Res. 2006;66(2):763–769. doi: 10.1158/0008-5472.CAN-05-3771. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam S, Comstock CE, Ertel A, Jeong KW, Stallcup MR, Addya S, McCue PA, Ostrander WF, Jr, Augello MA, Knudsen KE. Aberrant BAF57 signaling facilitates prometastatic phenotypes. Clin Cancer Res. 2013;19(10):2657–2667. doi: 10.1158/1078-0432.CCR-12-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Homig-Holzel C, Reuten R, Schadow B, Weighardt H, Wenzel D, Helfrich I, Schadendorf D, Bloch W, Bianchi ME, Lugassy C, Barnhill RL, Koch M, Fleischmann BK, Forster I, Kastenmuller W, Kolanus W, Holzel M, Gaffal E, Tuting T. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014 doi: 10.1038/nature13111. [DOI] [PubMed] [Google Scholar]
- Baldassarre G, Fedele M, Battista S, Vecchione A, Klein-Szanto AJ, Santoro M, Waldmann TA, Azimi N, Croce CM, Fusco A. Onset of natural killer cell lymphomas in transgenic mice carrying a truncated HMGI-C gene by the chronic stimulation of the IL-2 and IL-15 pathway. Proc Natl Acad Sci U S A. 2001;98(14):7970–7975. doi: 10.1073/pnas.141224998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balosso S, Liu J, Bianchi ME, Vezzani A. Disulfide-Containing High Mobility Group Box-1 Promotes N-Methyl-d-Aspartate Receptor Function and Excitotoxicity by Activating Toll-Like Receptor 4-Dependent Signaling in Hippocampal Neurons. Antioxid Redox Signal. 2014 doi: 10.1089/ars.2013.5349. [DOI] [PubMed] [Google Scholar]
- Balsamo M, Zambello R, Teramo A, Pedrazzi M, Sparatore B, Scordamaglia F, Pende D, Mingari MC, Moretta L, Moretta A, Semenzato G, Vitale M. Analysis of NK cell/DC interaction in NK-type lymphoproliferative disease of granular lymphocytes (LDGL): role of DNAM-1 and NKp30. Exp Hematol. 2009;37(10):1167–1175. doi: 10.1016/j.exphem.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, Dematteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2009 doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51(2):621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, de Freitas A, Friggeri A, Zmijewski JW, Liu G, Abraham E. Intracellular HMGB1 Negatively Regulates Efferocytosis. J Immunol. 2011;187(9):4686–4694. doi: 10.4049/jimmunol.1101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Friggeri A, Liu G, Abraham E. The C-terminal acidic tail is responsible for the inhibitory effects of HMGB1 on efferocytosis. J Leukoc Biol. 2010;88(5):973–979. doi: 10.1189/jlb.0510262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Kundu TK. The acidic C-terminal domain and A-box of HMGB-1 regulates p53-mediated transcription. Nucleic Acids Res. 2003;31(12):3236–3247. doi: 10.1093/nar/gkg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao G, Qiao Q, Zhao H, He X. Prognostic value of HMGB1 overexpression in resectable gastric adenocarcinomas. World J Surg Oncol. 2010;8:52. doi: 10.1186/1477-7819-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nature reviews. Cancer. 2002;2(12):897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- Barkauskaite V, Ek M, Popovic K, Harris HE, Wahren-Herlenius M, Nyberg F. Translocation of the novel cytokine HMGB1 to the cytoplasm and extracellular space coincides with the peak of clinical activity in experimentally UV-induced lesions of cutaneous lupus erythematosus. Lupus. 2007;16(10):794–802. doi: 10.1177/0961203307081895. [DOI] [PubMed] [Google Scholar]
- Barkess G, Postnikov Y, Campos CD, Mishra S, Mohan G, Verma S, Bustin M, West KL. The chromatin-binding protein HMGN3 stimulates histone acetylation and transcription across the Glyt1 gene. Biochem J. 2012;442(3):495–505. doi: 10.1042/BJ20111502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlan AU, Griffin TM, McGuire KA, Wiethoff CM. Adenovirus membrane penetration activates the NLRP3 inflammasome. J Virol. 2011;85(1):146–155. doi: 10.1128/JVI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnay-Verdier S, Gaillard C, Messmer M, Borde C, Gibot S, Marechal V. PCA-ELISA: a sensitive method to quantify free and masked forms of HMGB1. Cytokine. 2011;55(1):4–7. doi: 10.1016/j.cyto.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. 2013;12(7):543–559. doi: 10.1038/nrd4025. [DOI] [PubMed] [Google Scholar]
- Barqasho B, Nowak P, Abdurahman S, Walther-Jallow L, Sonnerborg A. Implications of the release of high-mobility group box 1 protein from dying cells during human immunodeficiency virus type 1 infection in vitro. J Gen Virol. 2010;91(Pt 7):1800–1809. doi: 10.1099/vir.0.016915-0. [DOI] [PubMed] [Google Scholar]
- Basta G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis. 2008;196(1):9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Battista S, Fedele M, Martinez Hoyos J, Pentimalli F, Pierantoni GM, Visone R, De Martino I, Croce CM, Fusco A. High-mobility-group A1 (HMGA1) proteins down-regulate the expression of the recombination activating gene 2 (RAG2) Biochem J. 2005;389(Pt 1):91–97. doi: 10.1042/BJ20041607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista S, Pentimalli F, Baldassarre G, Fedele M, Fidanza V, Croce CM, Fusco A. Loss of Hmga1 gene function affects embryonic stem cell lympho-hematopoietic differentiation. Faseb J. 2003;17(11):1496–1498. doi: 10.1096/fj.02-0977fje. [DOI] [PubMed] [Google Scholar]
- Bauer EM, Shapiro R, Billiar TR, Bauer PM. High mobility group Box 1 inhibits human pulmonary artery endothelial cell migration via a Toll-like receptor 4- and interferon response factor 3-dependent mechanism(s) J Biol Chem. 2013;288(2):1365–1373. doi: 10.1074/jbc.M112.434142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Jr, Chauhan S, Woodson SA, Kallenbach NR. Interactions of recombinant HMGB proteins with branched RNA substrates. Biochem Biophys Res Commun. 2008;377(1):262–267. doi: 10.1016/j.bbrc.2008.09.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellussi LM, Chen L, Chen D, Passali FM, Passali D. The role of High Mobility Group Box 1 chromosomal protein in the pathogenesis of chronic sinusitis and nasal polyposis. Acta otorhinolaryngologica Italica: organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale. 2012;32(6):386–392. [PMC free article] [PubMed] [Google Scholar]
- Bellussi LM, Iosif C, Sarafoleanu C, Jianu E, Duda R, Panaitescu E, Passali FM, Passali D. Are HMGB1 protein expression and secretion markers of upper airways inflammatory diseases? Journal of biological regulators and homeostatic agents. 2013;27(3):791–804. [PubMed] [Google Scholar]
- Berasi SP, Xiu M, Yee AS, Paulson KE. HBP1 repression of the p47phox gene: cell cycle regulation via the NADPH oxidase. Mol Cell Biol. 2004;24(7):3011–3024. doi: 10.1128/MCB.24.7.3011-3024.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernales S, Schuck S, Walter P. ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy. 2007;3(3):285–287. doi: 10.4161/auto.3930. [DOI] [PubMed] [Google Scholar]
- Bernues J, Espel E, Querol E. Identification of the core-histone-binding domains of HMG1 and HMG2. Biochim Biophys Acta. 1986;866(4):242–251. doi: 10.1016/0167-4781(86)90049-7. [DOI] [PubMed] [Google Scholar]
- Bernues J, Querol E, Martinez P, Barris A, Espel E, Lloberas J. Detection by chemical cross-linking of interaction between high mobility group protein 1 and histone oligomers in free solution. J Biol Chem. 1983;258(18):11020–11024. [PubMed] [Google Scholar]
- Bianchi ME. Interaction of a protein from rat liver nuclei with cruciform DNA. Embo J. 1988;7(3):843–849. doi: 10.1002/j.1460-2075.1988.tb02883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME. HMGB1 loves company. J Leukoc Biol. 2009;86(3):573–576. doi: 10.1189/jlb.1008585. [DOI] [PubMed] [Google Scholar]
- Bianchi ME, Agresti A. HMG proteins: dynamic players in gene regulation and differentiation. Curr Opin Genet Dev. 2005;15(5):496–506. doi: 10.1016/j.gde.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Bianchi ME, Beltrame M. Flexing DNA: HMG-box proteins and their partners. Am J Hum Genet. 1998;63(6):1573–1577. doi: 10.1086/302170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME, Beltrame M, Paonessa G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science. 1989;243(4894 Pt 1):1056–1059. doi: 10.1126/science.2922595. [DOI] [PubMed] [Google Scholar]
- Bianchi ME, Falciola L, Ferrari S, Lilley DM. The DNA binding site of HMG1 protein is composed of two similar segments (HMG boxes), both of which have counterparts in other eukaryotic regulatory proteins. Embo J. 1992;11(3):1055–1063. doi: 10.1002/j.1460-2075.1992.tb05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 2005;83(11):876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- Birger Y, Catez F, Furusawa T, Lim JH, Prymakowska-Bosak M, West KL, Postnikov YV, Haines DC, Bustin M. Increased tumorigenicity and sensitivity to ionizing radiation upon loss of chromosomal protein HMGN1. Cancer Res. 2005;65(15):6711–6718. doi: 10.1158/0008-5472.CAN-05-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birger Y, Davis J, Furusawa T, Rand E, Piatigorsky J, Bustin M. A role for chromosomal protein HMGN1 in corneal maturation. Differentiation. 2006;74(1):19–29. doi: 10.1111/j.1432-0436.2006.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birger Y, Ito Y, West KL, Landsman D, Bustin M. HMGN4, a newly discovered nucleosome-binding protein encoded by an intronless gene. DNA Cell Biol. 2001;20(5):257–264. doi: 10.1089/104454901750232454. [DOI] [PubMed] [Google Scholar]
- Birger Y, West KL, Postnikov YV, Lim JH, Furusawa T, Wagner JP, Laufer CS, Kraemer KH, Bustin M. Chromosomal protein HMGN1 enhances the rate of DNA repair in chromatin. Embo J. 2003;22(7):1665–1675. doi: 10.1093/emboj/cdg142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscetti F, Ghirlanda G, Flex A. Therapeutic potential of high mobility group box-1 in ischemic injury and tissue regeneration. Curr Vasc Pharmacol. 2011;9(6):677–681. doi: 10.2174/157016111797484125. [DOI] [PubMed] [Google Scholar]
- Biscetti F, Straface G, De Cristofaro R, Lancellotti S, Rizzo P, Arena V, Stigliano E, Pecorini G, Egashira K, De Angelis G, Ghirlanda G, Flex A. High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism. Diabetes. 2010;59(6):1496–1505. doi: 10.2337/db09-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitto A, Barone M, David A, Polito F, Familiari D, Monaco F, Giardina M, David T, Messina R, Noto A, Di Stefano V, Altavilla D, Bonaiuto A, Minutoli L, Guarini S, Ottani A, Squadrito F, Venuti FS. High mobility group box-1 expression correlates with poor outcome in lung injury patients. Pharmacological research : the official journal of the Italian Pharmacological Society. 2010;61(2):116–120. doi: 10.1016/j.phrs.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Boffa LC, Bolognesi C. Methylating agents: their target amino acids in nuclear proteins. Carcinogenesis. 1985;6(9):1399–1401. doi: 10.1093/carcin/6.9.1399. [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Langst G, Strohner R, Becker PB, Bianchi ME. The DNA chaperone HMGB1 facilitates ACF/CHRAC-dependent nucleosome sliding. Embo J. 2002;21(24):6865–6873. doi: 10.1093/emboj/cdf692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. Embo J. 2003;22(20):5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno G, Raiteri L, Milanese M, Zappettini S, Melloni E, Pedrazzi M, Passalacqua M, Tacchetti C, Usai C, Sparatore B. The high-mobility group box 1 cytokine induces transporter-mediated release of glutamate from glial subcellular particles (gliosomes) prepared from in situ-matured astrocytes. Int Rev Neurobiol. 2007;82:73–93. doi: 10.1016/S0074-7742(07)82004-6. [DOI] [PubMed] [Google Scholar]
- Bonne-Andrea C, Harper F, Puvion E, Delpech M, De Recondo AM. Nuclear accumulation of HMG1 protein is correlated to DNA synthesis. Biol Cell. 1986;58(3):185–194. doi: 10.1111/j.1768-322x.1986.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Bonne-Andrea C, Harper F, Sobczak J, De Recondo AM. Rat liver HMG1: a physiological nucleosome assembly factor. Embo J. 1984a;3(5):1193–1199. doi: 10.1002/j.1460-2075.1984.tb01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne-Andrea C, Harper F, Sobczak J, De Recondo AM. The role of HMG1 protein in nucleosome assembly and in chromatin replication. Adv Exp Med Biol. 1984b;179:479–488. doi: 10.1007/978-1-4684-8730-5_49. [DOI] [PubMed] [Google Scholar]
- Bonne C, Duguet M, de Recondo AM. Rat liver DNA binding proteins: physiological variations. FEBS Lett. 1979;106(2):292–296. doi: 10.1016/0014-5793(79)80517-7. [DOI] [PubMed] [Google Scholar]
- Bonne C, Sautiere P, Duguet M, de Recondo AM. Identification of a single-stranded DNA binding protein from rat liver with high mobility group protein 1. J Biol Chem. 1982;257(6):2722–2725. [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Melvin V, Prendergast P, Altmann M, Ronfani L, Bianchi ME, Taraseviciene L, Nordeen SK, Allegretto EA, Edwards DP. High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Mol Cell Biol. 1998;18(8):4471–4487. doi: 10.1128/mcb.18.8.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrmann L, Kim I, Schultheiss D, Rogalla P, Bullerdiek J. Regulation of the expression of HMG1, a co-activator of the estrogen receptor. Anticancer Res. 2001;21(1A):301–305. [PubMed] [Google Scholar]
- Bossaller L, Rothe A. Monoclonal antibody treatments for rheumatoid arthritis. Expert Opin Biol Ther. 2013;13(9):1257–1272. doi: 10.1517/14712598.2013.811230. [DOI] [PubMed] [Google Scholar]
- Bottger M, Vogel F, Platzer M, Kiessling U, Grade K, Strauss M. Condensation of vector DNA by the chromosomal protein HMG1 results in efficient transfection. Biochim Biophys Acta. 1988;950(2):221–228. doi: 10.1016/0167-4781(88)90014-0. [DOI] [PubMed] [Google Scholar]
- Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410(6832):1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- Branzk N, Papayannopoulos V. Molecular mechanisms regulating NETosis in infection and disease. Semin Immunopathol. 2013;35(4):513–530. doi: 10.1007/s00281-013-0384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breikers G, van Breda SG, Bouwman FG, van Herwijnen MH, Renes J, Mariman EC, Kleinjans JC, van Delft JH. Potential protein markers for nutritional health effects on colorectal cancer in the mouse as revealed by proteomics analysis. Proteomics. 2006;6(9):2844–2852. doi: 10.1002/pmic.200500067. [DOI] [PubMed] [Google Scholar]
- Brezniceanu ML, Volp K, Bosser S, Solbach C, Lichter P, Joos S, Zornig M. HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. Faseb J. 2003;17(10):1295–1297. doi: 10.1096/fj.02-0621fje. [DOI] [PubMed] [Google Scholar]
- Brickman JM, Adam M, Ptashne M. Interactions between an HMG-1 protein and members of the Rel family. Proc Natl Acad Sci U S A. 1999;96(19):10679–10683. doi: 10.1073/pnas.96.19.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol. 2012;198(5):773–783. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briolay A, Rousset B, Roux B. Nuclear proteins interacting with DNA and tubulin. Study of the interaction of the High Mobility Group protein 1 with tubulin. Biochim Biophys Acta. 1994;1219(1):39–46. doi: 10.1016/0167-4781(94)90244-5. [DOI] [PubMed] [Google Scholar]
- Brown E, Goodwin GH, Mayes EL, Hastings JR, Johns EW. Heterogeneity of proteins resembling high-mobility-group protein HMG-T in trout testes nuclei. Biochem J. 1980;191(2):661–664. doi: 10.1042/bj1910661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchfeld A, Qureshi AR, Lindholm B, Barany P, Yang L, Stenvinkel P, Tracey KJ. High Mobility Group Box Protein-1 correlates with renal function in chronic kidney disease (CKD) Mol Med. 2008;14(3–4):109–115. doi: 10.2119/2007-00107.Bruchfeld. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchfeld A, Wendt M, Bratt J, Qureshi AR, Chavan S, Tracey KJ, Palmblad K, Gunnarsson I. High-mobility group box-1 protein (HMGB1) is increased in antineutrophilic cytoplasmatic antibody (ANCA)-associated vasculitis with renal manifestations. Mol Med. 2011;17(1–2):29–35. doi: 10.2119/molmed.2010.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn SL, Pil PM, Essigmann JM, Housman DE, Lippard SJ. Isolation and characterization of human cDNA clones encoding a high mobility group box protein that recognizes structural distortions to DNA caused by binding of the anticancer agent cisplatin. Proc Natl Acad Sci U S A. 1992;89(6):2307–2311. doi: 10.1073/pnas.89.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi CS, Baumbusch MA, Dulay AT, Oliver EA, Lee S, Zhao G, Bhandari V, Ehrenkranz RA, Weiner CP, Madri JA, Buhimschi IA. Characterization of RAGE, HMGB1, and S100beta in inflammation-induced preterm birth and fetal tissue injury. The American journal of pathology. 2009;175(3):958–975. doi: 10.2353/ajpath.2009.090156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999;19(8):5237–5246. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M. Revised nomenclature for high mobility group (HMG) chromosomal proteins. Trends Biochem Sci. 2001;26(3):152–153. doi: 10.1016/s0968-0004(00)01777-1. [DOI] [PubMed] [Google Scholar]
- Bustin M, Lehn DA, Landsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta. 1990;1049(3):231–243. doi: 10.1016/0167-4781(90)90092-g. [DOI] [PubMed] [Google Scholar]
- Bustin M, Neihart NK. Antibodies against chromosomal HMG proteins stain the cytoplasm of mammalian cells. Cell. 1979;16(1):181–189. doi: 10.1016/0092-8674(79)90199-5. [DOI] [PubMed] [Google Scholar]
- Bustin M, Neihart NK, Fagan JB. mRNA of chromosomal proteins HMG-1 and HMG-2 are polyadenylated. Biochem Biophys Res Commun. 1981;101(3):893–897. doi: 10.1016/0006-291x(81)91833-7. [DOI] [PubMed] [Google Scholar]
- Bustin M, Soares N. Differential binding of chromosomal proteins HMG1 and HMG2 to superhelical DNA. Biochem Biophys Res Commun. 1985;133(2):633–640. doi: 10.1016/0006-291x(85)90952-0. [DOI] [PubMed] [Google Scholar]
- Cabart P, Kalousek I, Jandova D, Hrkal Z. Differential expression of nuclear HMG1, HMG2 proteins and H1(zero) histone in various blood cells. Cell Biochem Funct. 1995;13(2):125–133. doi: 10.1002/cbf.290130209. [DOI] [PubMed] [Google Scholar]
- Cai C, Cao Z, Loughran PA, Kim S, Darwiche S, Korff S, Billiar TR. Mast cells play a critical role in the systemic inflammatory response and end-organ injury resulting from trauma. J Am Coll Surg. 2011;213(5):604–615. doi: 10.1016/j.jamcollsurg.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Gill R, Eum HA, Cao Z, Loughran PA, Darwiche S, Edmonds RD, Menzel CL, Billiar TR. Complement factor 3 deficiency attenuates hemorrhagic shock-related hepatic injury and systemic inflammatory response syndrome. Am J Physiol Regul Integr Comp Physiol. 2010;299(5):R1175–R1182. doi: 10.1152/ajpregu.00282.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Shi X, Korff S, Zhang J, Loughran PA, Ruan X, Zhang Y, Liu L, Billiar TR. CD14 Contributes to Warm Hepatic Ischemia-Reperfusion Injury in Mice. Shock. 2013 doi: 10.1097/SHK.0b013e318299d1a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Hu X, Yi B, Zhang T, Wen Z. Glucagon-like peptide-1 receptor agonist protects against hyperglycemia-induced cardiocytes injury by inhibiting high mobility group box 1 expression. Mol Biol Rep. 2012;39(12):10705–10711. doi: 10.1007/s11033-012-1961-9. [DOI] [PubMed] [Google Scholar]
- Calogero S, Grassi F, Aguzzi A, Voigtlander T, Ferrier P, Ferrari S, Bianchi ME. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet. 1999;22(3):276–280. doi: 10.1038/10338. [DOI] [PubMed] [Google Scholar]
- Campana L, Bosurgi L, Bianchi ME, Manfredi AA, Rovere-Querini P. Requirement of HMGB1 for stromal cell-derived factor-1/CXCL12-dependent migration of macrophages and dendritic cells. J Leukoc Biol. 2009;86(3):609–615. doi: 10.1189/jlb.0908576. [DOI] [PubMed] [Google Scholar]
- Campbell PA, Rudnicki MA. Oct4 interaction with Hmgb2 regulates Akt signaling and pluripotency. Stem Cells. 2013;31(6):1107–1120. doi: 10.1002/stem.1365. [DOI] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–599. doi: 10.1146/annurev.genet.032608.103928. [DOI] [PubMed] [Google Scholar]
- Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci U S A. 2003;100(8):4754–4759. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo M, Puigdomenech P, Palau J. DNA and histone H1 interact with different domains of HMG 1 and 2 proteins. Embo J. 1983;2(10):1759–1764. doi: 10.1002/j.1460-2075.1983.tb01654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo M, Puigdomenech P, Tancredi T, Palau J. Interaction between domains in chromosomal protein HMG-1. Embo J. 1984;3(6):1255–1261. doi: 10.1002/j.1460-2075.1984.tb01960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal J, Pan P, Dhupar R, Ross M, Nakao A, Lotze M, Billiar T, Geller D, Tsung A. Cisplatin prevents high mobility group box 1 release and is protective in a murine model of hepatic ischemia/reperfusion injury. Hepatology. 2009;50(2):565–574. doi: 10.1002/hep.23021. [DOI] [PubMed] [Google Scholar]
- Carneiro VC, de Moraes Maciel R, de Abreu da Silva IC, da Costa RF, Paiva CN, Bozza MT, Fantappie MR. The extracellular release of Schistosoma mansoni HMGB1 nuclear protein is mediated by acetylation. Biochem Biophys Res Commun. 2009;390(4):1245–1249. doi: 10.1016/j.bbrc.2009.10.129. [DOI] [PubMed] [Google Scholar]
- Carrol ED, Mankhambo LA, Jeffers G, Parker D, Guiver M, Newland P, Banda DL, Molyneux EM, Heyderman RS, Molyneux ME, Hart CA. The diagnostic and prognostic accuracy of five markers of serious bacterial infection in Malawian children with signs of severe infection. PLoS ONE. 2009;4(8):e6621. doi: 10.1371/journal.pone.0006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary PD, Crane-Robinson C, Bradbury EM, Javaherian K, Goodwin GH, Johns EW. Conformational studies of two non-histone chromosomal proteins and their interactions with DNA. Eur J Biochem. 1976;62(3):583–590. doi: 10.1111/j.1432-1033.1976.tb10193.x. [DOI] [PubMed] [Google Scholar]
- Cary PD, Turner CH, Leung I, Mayes E, Crane-Robinson C. Conformation and domain structure of the non-histone chromosomal proteins HMG 1 and 2. Domain interactions. Eur J Biochem. 1984;143(2):323–330. doi: 10.1111/j.1432-1033.1984.tb08375.x. [DOI] [PubMed] [Google Scholar]
- Cassetta L, Fortunato O, Adduce L, Rizzi C, Hering J, Rovere-Querini P, Bianchi ME, Alfano M, Poli G. Extracellular high mobility group box-1 inhibits R5 and X4 HIV-1 strains replication in mononuclear phagocytes without induction of chemokines and cytokines. Aids. 2009;23(5):567–577. doi: 10.1097/QAD.0b013e328325a47e. [DOI] [PubMed] [Google Scholar]
- Casula M, Iyer AM, Spliet WG, Anink JJ, Steentjes K, Sta M, Troost D, Aronica E. Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience. 2011;179:233–243. doi: 10.1016/j.neuroscience.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Catena R, Escoffier E, Caron C, Khochbin S, Martianov I, Davidson I. HMGB4, a novel member of the HMGB family, is preferentially expressed in the mouse testis and localizes to the basal pole of elongating spermatids. Biol Reprod. 2009;80(2):358–366. doi: 10.1095/biolreprod.108.070243. [DOI] [PubMed] [Google Scholar]
- Catez F, Brown DT, Misteli T, Bustin M. Competition between histone H1 and HMGN proteins for chromatin binding sites. EMBO Rep. 2002;3(8):760–766. doi: 10.1093/embo-reports/kvf156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato L, Stott K, Watson M, Thomas JO. The interaction of HMGB1 and linker histones occurs through their acidic and basic tails. J Mol Biol. 2008;384(5):1262–1272. doi: 10.1016/j.jmb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Cavanaugh AH, Hempel WM, Taylor LJ, Rogalsky V, Todorov G, Rothblum LI. Activity of RNA polymerase I transcription factor UBF blocked by Rb gene product. Nature. 1995;374(6518):177–180. doi: 10.1038/374177a0. [DOI] [PubMed] [Google Scholar]
- Cavone L, Muzzi M, Mencucci R, Sparatore B, Pedrazzi M, Moroni F, Chiarugi A. 18beta-glycyrrhetic acid inhibits immune activation triggered by HMGB1, a pro-inflammatory protein found in the tear fluid during conjunctivitis and blepharitis. Ocular immunology and inflammation. 2011a;19(3):180–185. doi: 10.3109/09273948.2010.538121. [DOI] [PubMed] [Google Scholar]
- Cavone L, Muzzi M, Mencucci R, Sparatore B, Pedrazzi M, Moroni F, Chiarugi A. 18beta-glycyrrhetic acid inhibits immune activation triggered by HMGB1, a pro-inflammatory protein found in the tear fluid during conjunctivitis and blepharitis. Ocul Immunol Inflamm. 2011b;19(3):180–185. doi: 10.3109/09273948.2010.538121. [DOI] [PubMed] [Google Scholar]
- Celona B, Weiner A, Di Felice F, Mancuso FM, Cesarini E, Rossi RL, Gregory L, Baban D, Rossetti G, Grianti P, Pagani M, Bonaldi T, Ragoussis J, Friedman N, Camilloni G, Bianchi ME, Agresti A. Substantial histone reduction modulates genomewide nucleosomal occupancy and global transcriptional output. PLoS Biol. 2011;9(6):e1001086. doi: 10.1371/journal.pbio.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Bhatt KH, Sodhi A. Ultraviolet B induces high mobility group box 1 release from mouse peritoneal macrophages in vitro via caspase-1 mediated secretion pathway. Immunobiology. 2013;218(2):135–144. doi: 10.1016/j.imbio.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Chang Y, Huang X, Liu Z, Han G, Huang L, Xiong YC, Wang Z. Dexmedetomidine inhibits the secretion of high mobility group box 1 from lipopolysaccharide-activated macrophages in vitro. J Surg Res. 2013;181(2):308–314. doi: 10.1016/j.jss.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Chao YB, Scovell WM, Yan SB. High mobility group protein, HMG-1, contains insignificant glycosyl modification. Protein Sci. 1994;3(12):2452–2454. doi: 10.1002/pro.5560031230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau KY, Lam HY, Lee KL. Estrogen treatment induces elevated expression of HMG1 in MCF-7 cells. Exp Cell Res. 1998;241(1):269–272. doi: 10.1006/excr.1998.4052. [DOI] [PubMed] [Google Scholar]
- Chau KY, Patel UA, Lee KL, Lam HY, Crane-Robinson C. The gene for the human architectural transcription factor HMGI-C consists of five exons each coding for a distinct functional element. Nucleic Acids Res. 1995;23(21):4262–4266. doi: 10.1093/nar/23.21.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaung WW, Wu R, Ji Y, Dong W, Wang P. Mitochondrial transcription factor A is a proinflammatory mediator in hemorrhagic shock. Int J Mol Med. 2012;30(1):199–203. doi: 10.3892/ijmm.2012.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P, Zeiher AM, Chavakis T, Dimmeler S. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res. 2007;100(2):204–212. doi: 10.1161/01.RES.0000257774.55970.f4. [DOI] [PubMed] [Google Scholar]
- Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198(10):1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CG, Cui L, Tang P, Yu ZT. Clinical significance of serum high-mobility group box 1 detection in esophageal squamous cell carcinoma. Zhonghua wei chang wai ke za zhi = Chinese journal of gastrointestinal surgery. 2013a;16(9):838–841. [PubMed] [Google Scholar]
- Chen D, Bi A, Dong X, Jiang Y, Rui B, Liu J, Yin Z, Luo L. Luteolin exhibits anti-inflammatory effects by blocking the activity of heat shock protein 90 in macrophages. Biochem Biophys Res Commun. 2014a;443(1):326–332. doi: 10.1016/j.bbrc.2013.11.122. [DOI] [PubMed] [Google Scholar]
- Chen D, Mao M, Bellussi LM, Passali D, Chen L. Increase of high mobility group box chromosomal protein 1 in eosinophilic chronic rhinosinusitis with nasal polyps. International forum of allergy & rhinology. 2014b doi: 10.1002/alr.21294. [DOI] [PubMed] [Google Scholar]
- Chen G, Li J, Ochani M, Rendon-Mitchell B, Qiang X, Susarla S, Ulloa L, Yang H, Fan S, Goyert SM, Wang P, Tracey KJ, Sama AE, Wang H. Bacterial endotoxin stimulates macrophages to release HMGB1 partly through CD14- and TNF-dependent mechanisms. J Leukoc Biol. 2004;76(5):994–1001. doi: 10.1189/jlb.0404242. [DOI] [PubMed] [Google Scholar]
- Chen G, Li J, Qiang X, Czura CJ, Ochani M, Ochani K, Ulloa L, Yang H, Tracey KJ, Wang P, Sama AE, Wang H. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine:an additional mechanism for its therapeutic effects in experimental sepsis. J Lipid Res. 2005;46(4):623–627. doi: 10.1194/jlr.C400018-JLR200. [DOI] [PubMed] [Google Scholar]
- Chen GE, Wu H, Ma J, Chadban SJ, Sharland A. Toll-like receptor 4 engagement contributes to expression of NKG2D ligands by renal tubular epithelial cells. Nephrol Dial Transplant. 2011a;26(12):3873–3881. doi: 10.1093/ndt/gfr234. [DOI] [PubMed] [Google Scholar]
- Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323(5922):1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HG, Xie KL, Han HZ, Wang WN, Liu DQ, Wang GL, Yu YH. Heme oxygenase-1 mediates the anti-inflammatory effect of molecular hydrogen in LPS-stimulated RAW 264.7 macrophages. Int J Surg. 2013b;11(10):1060–1066. doi: 10.1016/j.ijsu.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Chen J, John R, Richardson JA, Shelton JM, Zhou XJ, Wang Y, Wu QQ, Hartono JR, Winterberg PD, Lu CY. Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney Int. 2011b;79(3):288–299. doi: 10.1038/ki.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xi B, Zhao Y, Yu Y, Zhang J, Wang C. High-mobility group protein B1 (HMGB1) is a novel biomarker for human ovarian cancer. Gynecol Oncol. 2012a;126(1):109–117. doi: 10.1016/j.ygyno.2012.03.051. [DOI] [PubMed] [Google Scholar]
- Chen P, Wang XL, Ma ZS, Xu Z, Jia B, Ren J, Hu YX, Zhang QH, Ma TG, Yan BD, Yan QZ, Li YL, Li Z, Yu JY, Gao R, Fan N, Li B, Yang JL. Knockdown of HMGN5 expression by RNA interference induces cell cycle arrest in human lung cancer cells. Asian Pac J Cancer Prev. 2012b;13(7):3223–3228. doi: 10.7314/apjcp.2012.13.7.3223. [DOI] [PubMed] [Google Scholar]
- Chen R, Hou W, Zhang Q, Kang R, Fan XG, Tang D. Emerging role of high-mobility group box 1 (HMGB1) in liver diseases. Mol Med. 2013c;19:357–366. doi: 10.2119/molmed.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RC, Yi PP, Zhou RR, Xiao MF, Huang ZB, Tang DL, Huang Y, Fan XG. The role of HMGB1-RAGE axis in migration and invasion of hepatocellular carcinoma cell lines. Mol Cell Biochem. 2014c doi: 10.1007/s11010-014-1978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Guo ZP, Li L, Wang L, Jia RZ, Cao N, Qin S, Li MM. Increased HMGB1 serum levels and altered HMGB1 expression in patients with psoriasis vulgaris. Archives of dermatological research. 2013d;305(3):263–267. doi: 10.1007/s00403-013-1330-0. [DOI] [PubMed] [Google Scholar]
- Chen X, Wan Y, Zhou T, Li J, Wei Y. Ursolic acid attenuates lipopolysaccharide-induced acute lung injury in a mouse model. Immunotherapy. 2013e;5(1):39–47. doi: 10.2217/imt.12.144. [DOI] [PubMed] [Google Scholar]
- Cheng BQ, Jia CQ, Liu CT, Lu XF, Zhong N, Zhang ZL, Fan W, Li YQ. Serum high mobility group box chromosomal protein 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2008;40(6):446–452. doi: 10.1016/j.dld.2007.11.024. [DOI] [PubMed] [Google Scholar]
- Cheng BQ, Liu CT, Li WJ, Fan W, Zhong N, Zhang Y, Jia XQ, Zhang SZ. Ethyl pyruvate improves survival and ameliorates distant organ injury in rats with severe acute pancreatitis. Pancreas. 2007;35(3):256–261. doi: 10.1097/MPA.0b013e318064678a. [DOI] [PubMed] [Google Scholar]
- Cheng P, Dai W, Wang F, Lu J, Shen M, Chen K, Li J, Zhang Y, Wang C, Yang J, Zhu R, Zhang H, Zheng Y, Guo CY, Xu L. Ethyl pyruvate inhibits proliferation and induces apoptosis of hepatocellular carcinoma via regulation of the HMGB1-RAGE and AKT pathways. Biochem Biophys Res Commun. 2014;443(4):1162–1168. doi: 10.1016/j.bbrc.2013.12.064. [DOI] [PubMed] [Google Scholar]
- Cheng P, Ni Z, Dai X, Wang B, Ding W, Rae Smith A, Xu L, Wu D, He F, Lian J. The novel BH-3 mimetic apogossypolone induces Beclin-1- and ROS-mediated autophagy in human hepatocellular carcinoma [corrected] cells. Cell Death Dis. 2013;4:e489. doi: 10.1038/cddis.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Dai LL, Cao DF, Wu QG, Song YN, Kang Y, Xia J, Si JM, Chen CY. Changes of HMGB1 and RAGE in induced sputum from patients with bronchial asthma. Zhonghua yi xue za zhi. 2011a;91(22):1538–1542. [PubMed] [Google Scholar]
- Cheng Z, Kang Y, Wu QG, Dai LL, Song YN, Xia J, Si JM, Chen CY. Levels of HMGB1 in induced sputum from patients with asthma and chronic obstructive pulmonary disease. Zhonghua yi xue za zhi. 2011b;91(42):2981–2984. [PubMed] [Google Scholar]
- Chiang HS, Maric M. Lysosomal thiol reductase negatively regulates autophagy by altering glutathione synthesis and oxidation. Free Radic Biol Med. 2011;51(3):688–699. doi: 10.1016/j.freeradbiomed.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita, Yoichiro Fujioka H, Ohba Y, Gorman J, Colgan J, Hirashima M, Uede T, Yagita H, Jinushi M. Tumor-infiltrating dendritic cells suppress nucleic acid-mediated innate immune responses through TIM-3-HMGB1 interactions. Nat Immunol. 2012a doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, Hirashima M, Uede T, Takaoka A, Yagita H, Jinushi M. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012b;13(9):832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieffi P, Battista S, Barchi M, Di Agostino S, Pierantoni GM, Fedele M, Chiariotti L, Tramontano D, Fusco A. HMGA1 and HMGA2 protein expression in mouse spermatogenesis. Oncogene. 2002;21(22):3644–3650. doi: 10.1038/sj.onc.1205501. [DOI] [PubMed] [Google Scholar]
- Cho HI, Kim KM, Kwak JH, Lee SK, Lee SM. Protective mechanism of anethole on hepatic ischemia/reperfusion injury in mice. J Nat Prod. 2013;76(9):1717–1723. doi: 10.1021/np4004323. [DOI] [PubMed] [Google Scholar]
- Choi J, Min HJ, Shin JS. Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J Neuroinflammation. 2011;8:135. doi: 10.1186/1742-2094-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YR, Kim H, Kang HJ, Kim NG, Kim JJ, Park KS, Paik YK, Kim HO. Overexpression of high mobility group box 1 in gastrointestinal stromal tumors with KIT mutation. Cancer Res. 2003;63(9):2188–2193. [PubMed] [Google Scholar]
- Chorny A, Anderson P, Gonzalez-Rey E, Delgado M. Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. J Immunol. 2008;180(12):8369–8377. doi: 10.4049/jimmunol.180.12.8369. [DOI] [PubMed] [Google Scholar]
- Chorny A, Delgado M. Neuropeptides rescue mice from lethal sepsis by downregulating secretion of the late-acting inflammatory mediator high mobility group box 1. Am J Pathol. 2008;172(5):1297–1307. doi: 10.2353/ajpath.2008.070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuangui C, Peng T, Zhentao Y. The expression of high mobility group box 1 is associated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Pathology oncology research : POR. 2012;18(4):1021–1027. doi: 10.1007/s12253-012-9539-3. [DOI] [PubMed] [Google Scholar]
- Chung HW, Lee SG, Kim H, Hong DJ, Chung JB, Stroncek D, Lim JB. Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J Transl Med. 2009;7:38. doi: 10.1186/1479-5876-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HW, Lim JB, Jang S, Lee KJ, Park KH, Song SY. Serum high mobility group box-1 is a powerful diagnostic and prognostic biomarker for pancreatic ductal adenocarcinoma. Cancer science. 2012;103(9):1714–1721. doi: 10.1111/j.1349-7006.2012.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KY, Park JJ, Kim YS. The role of high-mobility group box-1 in renal ischemia and reperfusion injury and the effect of ethyl pyruvate. Transplant Proc. 2008;40(7):2136–2138. doi: 10.1016/j.transproceed.2008.06.040. [DOI] [PubMed] [Google Scholar]
- Cirillo P, Giallauria F, Pacileo M, Petrillo G, D'Agostino M, Vigorito C, Chiariello M. Increased high mobility group box-1 protein levels are associated with impaired cardiopulmonary and echocardiographic findings after acute myocardial infarction. J Card Fail. 2009;15(4):362–367. doi: 10.1016/j.cardfail.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Ciucci A, Gabriele I, Percario ZA, Affabris E, Colizzi V, Mancino G. HMGB1 and cord blood: its role as immuno-adjuvant factor in innate immunity. PLoS ONE. 2011;6(8):e23766. doi: 10.1371/journal.pone.0023766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerigues V, Guillen MI, Gomar F, Alcaraz MJ. Haem oxygenase-1 counteracts the effects of interleukin-1beta on inflammatory and senescence markers in cartilage-subchondral bone explants from osteoarthritic patients. Clin Sci (Lond) 2012;122(5):239–250. doi: 10.1042/CS20100519. [DOI] [PubMed] [Google Scholar]
- Cleynen I, Van de Ven WJ. The HMGA proteins: a myriad of functions (Review) Int J Oncol. 2008;32(2):289–305. [PubMed] [Google Scholar]
- Cockerill PN, Goodwin GH. Demonstration of an S1-nuclease sensitive site near the human beta-globin gene, and its protection by HMG 1 and 2. Biochem Biophys Res Commun. 1983;112(2):547–554. doi: 10.1016/0006-291x(83)91499-7. [DOI] [PubMed] [Google Scholar]
- Cockerill PN, Goodwin GH, Cary PD, Turner C, Johns EW. Comparisons of the structures of the chromosomal high mobility group proteins HMG1 and HMG2 prepared under conditions of neutral and acidic pH. Biochim Biophys Acta. 1983;745(1):70–81. doi: 10.1016/0167-4838(83)90171-1. [DOI] [PubMed] [Google Scholar]
- Coffey JC, Wang JH, Kelly R, Romics L, Jr, O'Callaghan A, Fiuza C, Redmond HP. Tolerization with BLP down-regulates HMGB1 a critical mediator of sepsis-related lethality. J Leukoc Biol. 2007;82(4):906–914. doi: 10.1189/jlb.0806504. [DOI] [PubMed] [Google Scholar]
- Conti L, Lanzardo S, Arigoni M, Antonazzo R, Radaelli E, Cantarella D, Calogero RA, Cavallo F. The noninflammatory role of high mobility group box 1/Toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. Faseb J. 2013;27(12):4731–4744. doi: 10.1096/fj.13-230201. [DOI] [PubMed] [Google Scholar]
- Copley MR, Babovic S, Benz C, Knapp DJ, Beer PA, Kent DG, Wohrer S, Treloar DQ, Day C, Rowe K, Mader H, Kuchenbauer F, Humphries RK, Eaves CJ. The Lin28b-let-7-Hmga2 axis determines the higher self-renewal potential of fetal haematopoietic stem cells. Nat Cell Biol. 2013;15(8):916–925. doi: 10.1038/ncb2783. [DOI] [PubMed] [Google Scholar]
- Costello DA, Watson MB, Cowley TR, Murphy N, Murphy Royal C, Garlanda C, Lynch MA. Interleukin-1alpha and HMGB1 mediate hippocampal dysfunction in SIGIRR-deficient mice. J Neurosci. 2011;31(10):3871–3879. doi: 10.1523/JNEUROSCI.6676-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E, Saudan P, Winocour E, Pizer L, Beard P. High mobility group chromosomal protein 1 binds to the adeno-associated virus replication protein (Rep) and promotes Rep-mediated site-specific cleavage of DNA, ATPase activity and transcriptional repression. Embo J. 1997;16(19):5943–5954. doi: 10.1093/emboj/16.19.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore SF, Tattersall P. High-mobility group 1/2 proteins are essential for initiating rolling-circle-type DNA replication at a parvovirus hairpin origin. J Virol. 1998;72(11):8477–8484. doi: 10.1128/jvi.72.11.8477-8484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS ONE. 2009;4(10):e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa MP, Nickol JM, Bustin M. Developmental changes in the expression of high mobility group chromosomal proteins. J Biol Chem. 1991;266(5):2712–2714. [PubMed] [Google Scholar]
- Cuddapah S, Schones DE, Cui K, Roh TY, Barski A, Wei G, Rochman M, Bustin M, Zhao K. Genomic profiling of HMGN1 reveals an association with chromatin at regulatory regions. Mol Cell Biol. 2011;31(4):700–709. doi: 10.1128/MCB.00740-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui XS, Shen XH, Kim NH. High mobility group box 1 (HMGB1) is implicated in preimplantation embryo development in the mouse. Mol Reprod Dev. 2008;75(8):1290–1299. doi: 10.1002/mrd.20694. [DOI] [PubMed] [Google Scholar]
- Curran CS, Bertics PJ. Human eosinophils express RAGE, produce RAGE ligands, exhibit PKC-delta phosphorylation and enhanced viability in response to the RAGE ligand, S100B. Int Immunol. 2011;23(12):713–728. doi: 10.1093/intimm/dxr083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, Muhammad AK, Clark MC, Arditi M, Comin-Anduix B, Ribas A, Lowenstein PR, Castro MG. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6(1):e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agati V, Schmidt AM. RAGE and the pathogenesis of chronic kidney disease. Nat Rev Nephrol. 2010;6(6):352–360. doi: 10.1038/nrneph.2010.54. [DOI] [PubMed] [Google Scholar]
- Dahlhaus M, Schult C, Lange S, Freund M, Junghanss C. MicroRNA 181a influences the expression of HMGB1 and CD4 in acute Leukemias. Anticancer Res. 2013;33(2):445–452. [PubMed] [Google Scholar]
- Dai S, Sodhi C, Cetin S, Richardson W, Branca M, Neal MD, Prindle T, Ma C, Shapiro RA, Li B, Wang JH, Hackam DJ. Extracellular high mobility group box-1 (HMGB1) inhibits enterocyte migration via activation of Toll-like receptor-4 and increased cell-matrix adhesiveness. J Biol Chem. 2010;285(7):4995–5002. doi: 10.1074/jbc.M109.067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Wong B, Yen YM, Oettinger MA, Kwon J, Johnson RC. Determinants of HMGB proteins required to promote RAG1/2-recombination signal sequence complex assembly and catalysis during V(D)J recombination. Mol Cell Biol. 2005;25(11):4413–4425. doi: 10.1128/MCB.25.11.4413-4425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang W, Zhang X, Yao YM, Su Q, Guo YF, Sun RJ, Qin YH, Ma JX, Zhao XD. Effect of early intensive insulin therapy on high mobility group box 1 protein levels and prognosis of patients with severe trauma. Zhongguo wei zhong bing ji jiu yi xue = Chinese critical care medicine = Zhongguo weizhongbing jijiuyixue. 2011;23(3):173–175. [PubMed] [Google Scholar]
- Das D, Peterson RC, Scovell WM. High mobility group B proteins facilitate strong estrogen receptor binding to classical and half-site estrogen response elements and relax binding selectivity. Mol Endocrinol. 2004;18(11):2616–2632. doi: 10.1210/me.2004-0125. [DOI] [PubMed] [Google Scholar]
- Das D, Scovell WM. The binding interaction of HMG-1 with the TATA-binding protein/TATA complex. J Biol Chem. 2001;276(35):32597–32605. doi: 10.1074/jbc.M011792200. [DOI] [PubMed] [Google Scholar]
- Das UN. Is multiple sclerosis a proresolution deficiency disorder? Nutrition. 2012;28(10):951–958. doi: 10.1016/j.nut.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Daston MM, Ratner N. Amphoterin (P30, HMG-1) and RIP are early markers of oligodendrocytes in the developing rat spinal cord. J Neurocytol. 1994;23(5):323–332. doi: 10.1007/BF01188500. [DOI] [PubMed] [Google Scholar]
- Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes care. 2010;33(4):861–868. doi: 10.2337/dc09-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, Prevost MC, Leber B, Andrews D, Penninger J, Kroemer G. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. Faseb J. 2000;14(5):729–739. [PubMed] [Google Scholar]
- Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol. 2013;201(4):613–629. doi: 10.1083/jcb.201206006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave SH, Tilstra JS, Matsuoka K, Li F, DeMarco RA, Beer-Stolz D, Sepulveda AR, Fink MP, Lotze MT, Plevy SE. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol. 2009;86(3):633–643. doi: 10.1189/jlb.1008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K, Banerjee S, Friggeri A, Bell C, Abraham E, Zerfaoui M. Poly(ADP-ribosyl)ation of high mobility group box 1 (HMGB1) protein enhances inhibition of efferocytosis. Mol Med. 2012a;18:359–369. doi: 10.2119/molmed.2011.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K, Banerjee S, Friggeri A, Bell C, Abraham E, Zerfaoui M. Poly(ADP-Ribosyl)ation of High Mobility Group Box 1 (HMGB1) Protein Enhances Inhibition of Efferocytosis. Mol Med. 2012b;18(1):359–369. doi: 10.2119/molmed.2011.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- de Abreu da Silva IC, Carneiro VC, Maciel Rde M, da Costa RF, Furtado DR, de Oliveira FM, da Silva-Neto MA, Rumjanek FD, Fantappie MR. CK2 phosphorylation of Schistosoma mansoni HMGB1 protein regulates its cellular traffic and secretion but not its DNA transactions. PLoS ONE. 2011;6(8):e23572. doi: 10.1371/journal.pone.0023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino I, Visone R, Fedele M, Petrocca F, Palmieri D, Martinez Hoyos J, Forzati F, Croce CM, Fusco A. Regulation of microRNA expression by HMGA1 proteins. Oncogene. 2009;28(11):1432–1442. doi: 10.1038/onc.2008.495. [DOI] [PubMed] [Google Scholar]
- De Mori R, Straino S, Di Carlo A, Mangoni A, Pompilio G, Palumbo R, Bianchi ME, Capogrossi MC, Germani A. Multiple effects of high mobility group box protein 1 in skeletal muscle regeneration. Arterioscler Thromb Vasc Biol. 2007;27(11):2377–2383. doi: 10.1161/ATVBAHA.107.153429. [DOI] [PubMed] [Google Scholar]
- de Oliveira FM, de Abreu da Silva IC, Rumjanek FD, Dias-Neto E, Guimaraes PE, Verjovski-Almeida S, Stros M, Fantappie MR. Cloning the genes and DNA binding properties of High Mobility Group B1 (HMGB1) proteins from the human blood flukes Schistosoma mansoni and Schistosoma japonicum. Gene. 2006;377:33–45. doi: 10.1016/j.gene.2006.03.001. [DOI] [PubMed] [Google Scholar]
- de Silva S, Lotta LT, Jr, Burris CA, Bowers WJ. Virion-associated cofactor high-mobility group DNA-binding protein-1 facilitates transposition from the herpes simplex virus/Sleeping Beauty amplicon vector platform. Hum Gene Ther. 2010;21(11):1615–1622. doi: 10.1089/hum.2010.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza A, Westra J, Bijzet J, Limburg PC, Stegeman CA, Bijl M, Kallenberg CG. Is serum HMGB1 a biomarker in ANCA-associated vasculitis? Arthritis Res Ther. 2013a;15(5):R104. doi: 10.1186/ar4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza AW, Westra J, Bijzet J, Limburg PC, Stegeman CA, Bijl M, Kallenberg CG. Is serum HMGB1 a biomarker in ANCA-associated vasculitis? Arthritis Res Ther. 2013b;15(5):R104. doi: 10.1186/ar4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza AW, Westra J, Limburg PC, Bijl M, Kallenberg CG. HMGB1 in vascular diseases: Its role in vascular inflammation and atherosclerosis. Autoimmun Rev. 2012;11(12):909–917. doi: 10.1016/j.autrev.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gelinas C, Fan Y, Nelson DA, Jin S, White E. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10(1):51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152(6):1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degryse B, de Virgilio M. The nuclear protein HMGB1, a new kind of chemokine? FEBS Lett. 2003;553(1–2):11–17. doi: 10.1016/s0014-5793(03)01027-5. [DOI] [PubMed] [Google Scholar]
- Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Dejean E, Foisseau M, Lagarrigue F, Lamant L, Prade N, Marfak A, Delsol G, Giuriato S, Gaits-Iacovoni F, Meggetto F. ALK+ALCLs induce cutaneous, HMGB-1-dependent IL-8/CXCL8 production by keratinocytes through NF-kappaB activation. Blood. 2012;119(20):4698–4707. doi: 10.1182/blood-2011-10-386011. [DOI] [PubMed] [Google Scholar]
- Delucchi F, Berni R, Frati C, Cavalli S, Graiani G, Sala R, Chaponnier C, Gabbiani G, Calani L, Del Rio D, Bocchi L, Lagrasta C, Quaini F, Stilli D. Resveratrol treatment reduces cardiac progenitor cell dysfunction and prevents morpho-functional ventricular remodeling in type-1 diabetic rats. PLoS ONE. 2012;7(6):e39836. doi: 10.1371/journal.pone.0039836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco RA, Fink MP, Lotze MT. Monocytes promote natural killer cell interferon gamma production in response to the endogenous danger signal HMGB1. Mol Immunol. 2005;42(4):433–444. doi: 10.1016/j.molimm.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Deneault E, Cellot S, Faubert A, Laverdure JP, Frechette M, Chagraoui J, Mayotte N, Sauvageau M, Ting SB, Sauvageau G. A functional screen to identify novel effectors of hematopoietic stem cell activity. Cell. 2009;137(2):369–379. doi: 10.1016/j.cell.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng CQ, Deng GH, Wang YM. HMGB1 gene polymorphisms in patients with chronic hepatitis B virus infection. World J Gastroenterol. 2013;19(31):5144–5149. doi: 10.3748/wjg.v19.i31.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Dasu MR, Park SH, Jialal I. Increased levels of ligands of Toll-like receptors 2 and 4 in type 1 diabetes. Diabetologia. 2009;52(8):1665–1668. doi: 10.1007/s00125-009-1394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhupar R, Klune JR, Evankovich J, Cardinal J, Zhang M, Ross M, Murase N, Geller DA, Billiar TR, Tsung A. Interferon regulatory factor 1 mediates acetylation and release of high mobility group box 1 from hepatocytes during murine liver ischemia-reperfusion injury. Shock. 2011;35(3):293–301. doi: 10.1097/SHK.0b013e3181f6aab0. [DOI] [PubMed] [Google Scholar]
- Di Agostino S, Fedele M, Chieffi P, Fusco A, Rossi P, Geremia R, Sette C. Phosphorylation of high-mobility group protein A2 by Nek2 kinase during the first meiotic division in mouse spermatocytes. Mol Biol Cell. 2004;15(3):1224–1232. doi: 10.1091/mbc.E03-09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cello F, Hillion J, Hristov A, Wood LJ, Mukherjee M, Schuldenfrei A, Kowalski J, Bhattacharya R, Ashfaq R, Resar LM. HMGA2 participates in transformation in human lung cancer. Mol Cancer Res. 2008;6(5):743–750. doi: 10.1158/1541-7786.MCR-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H, Kang Z, Han F, Jiang W. Astilbin protects diabetic rat heart against ischemia-reperfusion injury via blockade of HMGB1-dependent NF-kappaB signaling pathway. Food Chem Toxicol. 2014;63:104–110. doi: 10.1016/j.fct.2013.10.045. [DOI] [PubMed] [Google Scholar]
- Diffley JF, Stillman B. A close relative of the nuclear, chromosomal high-mobility group protein HMG1 in yeast mitochondria. Proc Natl Acad Sci U S A. 1991;88(17):7864–7868. doi: 10.1073/pnas.88.17.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley JF, Stillman B. DNA binding properties of an HMG1-related protein from yeast mitochondria. J Biol Chem. 1992;267(5):3368–3374. [PubMed] [Google Scholar]
- Dimov SI, Alexandrova EA, Beltchev BG. Differences between some properties of acetylated and nonacetylated forms of HMG1 protein. Biochem Biophys Res Commun. 1990;166(2):819–826. doi: 10.1016/0006-291x(90)90883-o. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12(11):404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- Dinarvand P, Hassanian SM, Qureshi SH, Manithody C, Eissenberg JC, Yang L, Rezaie AR. Polyphosphate amplifies proinflammatory responses of nuclear proteins through interaction with receptor for advanced glycation end products and P2Y1 purinergic receptor. Blood. 2014;123(6):935–945. doi: 10.1182/blood-2013-09-529602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding HF, Bustin M, Hansen U. Alleviation of histone H1-mediated transcriptional repression and chromatin compaction by the acidic activation region in chromosomal protein HMG-14. Mol Cell Biol. 1997;17(10):5843–5855. doi: 10.1128/mcb.17.10.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding HS, Yang J. High mobility group box-1 and cardiovascular diseases. Saudi Med J. 2010;31(5):486–489. [PubMed] [Google Scholar]
- Dintilhac A, Bernues J. HMGB1 interacts with many apparently unrelated proteins by recognizing short amino acid sequences. J Biol Chem. 2002;277(9):7021–7028. doi: 10.1074/jbc.M108417200. [DOI] [PubMed] [Google Scholar]
- Ditsworth D, Zong WX, Thompson CB. Activation of poly(ADP)-ribose polymerase (PARP-1) induces release of the pro-inflammatory mediator HMGB1 from the nucleus. J Biol Chem. 2007;282(24):17845–17854. doi: 10.1074/jbc.M701465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- Domingos PM, Obukhanych TV, Altmann CR, Hemmati-Brivanlou A. Cloning and developmental expression of Baf57 in Xenopus laevis. Mech Dev. 2002;116(1–2):177–181. doi: 10.1016/s0925-4773(02)00129-6. [DOI] [PubMed] [Google Scholar]
- Dong N, Jin BQ, Yao YM, Yu Y, Cao YJ, He LX, Chai JK, Sheng ZY. [Change in T cell-mediated immunity and its relationship with high mobility group box-1 protein levels in extensively burned patients] Zhonghua wai ke za zhi [Chinese journal of surgery] 2008;46(10):759–762. [PubMed] [Google Scholar]
- Dong N, Yao YM, Yu Y, Gu CY, Lei SH, Sheng ZY. [Changes in plasma high mobility group box-1 protein levels and its relationship with sepsis in severely burned patients]. Zhongguo yi xue ke xue yuan xue bao. Acta Academiae Medicinae Sinicae. 2007;29(4):466–470. [PubMed] [Google Scholar]
- Dong Xda E, Ito N, Lotze MT, Demarco RA, Popovic P, Shand SH, Watkins S, Winikoff S, Brown CK, Bartlett DL, Zeh HJ., 3rd High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J Immunother. 2007;30(6):596–606. doi: 10.1097/CJI.0b013e31804efc76. [DOI] [PubMed] [Google Scholar]
- Dong YD, Cui L, Peng CH, Cheng DF, Han BS, Huang F. Expression and clinical significance of HMGB1 in human liver cancer: Knockdown inhibits tumor growth and metastasis in vitro and in vivo. Oncol Rep. 2013;29(1):87–94. doi: 10.3892/or.2012.2070. [DOI] [PubMed] [Google Scholar]
- Dormoy-Raclet V, Cammas A, Celona B, Lian XJ, van der Giessen K, Zivojnovic M, Brunelli S, Riuzzi F, Sorci G, Wilhelm BT, Di Marco S, Donato R, Bianchi ME, Gallouzi IE. HuR and miR-1192 regulate myogenesis by modulating the translation of HMGB1 mRNA. Nature communications. 2013;4:2388. doi: 10.1038/ncomms3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downard CD, Grant SN, Matheson PJ, Guillaume AW, Debski R, Fallat ME, Garrison RN. Altered intestinal microcirculation is the critical event in the development of necrotizing enterocolitis. J Pediatr Surg. 2011;46(6):1023–1028. doi: 10.1016/j.jpedsurg.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Downs JA. Chromatin structure and DNA double-strand break responses in cancer progression and therapy. Oncogene. 2007;26(56):7765–7772. doi: 10.1038/sj.onc.1210874. [DOI] [PubMed] [Google Scholar]
- Dragomir AC, Laskin JD, Laskin DL. Macrophage activation by factors released from acetaminophen-injured hepatocytes: Potential role of HMGB1. Toxicol Appl Pharm. 2011;253(3):170–177. doi: 10.1016/j.taap.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du D, Yan J, Ren J, Lv H, Li Y, Xu S, Wang Y, Ma S, Qu J, Tang W, Hu Z, Yu S. Synthesis, biological evaluation, and molecular modeling of glycyrrhizin derivatives as potent high-mobility group box-1 inhibitors with anti-heart-failure activity in vivo. J Med Chem. 2013;56(1):97–108. doi: 10.1021/jm301248y. [DOI] [PubMed] [Google Scholar]
- Du X, Hu X, Wei J. Postconditioning with rosuvastatin reduces myocardial ischemia-reperfusion injury by inhibiting high mobility group box 1 protein expression. Experimental and therapeutic medicine. 2014;7(1):117–120. doi: 10.3892/etm.2013.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Wang CY, Chen J, Gong Q, Zhu P, Zheng F, Tan Z, Gong F, Fang M. High-mobility group box 1 promotes early acute allograft rejection by enhancing IL-6-dependent Th17 alloreactive response. Lab Invest. 2011;91(1):43–53. doi: 10.1038/labinvest.2010.141. [DOI] [PubMed] [Google Scholar]
- Duan XZ, Hu JH, Li C, Liu FF, Liu XY, Tong JJ, Xin SJ. [Relation between serum levels of high mobility group box 1 and hepatitis B virus-related acute-on-chronic liver failure] Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese journal of hepatology. 2013;21(6):434–437. doi: 10.3760/cma.j.issn.1007-3418.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Dubicke A, Andersson P, Fransson E, Andersson E, Sioutas A, Malmstrom A, Sverremark-Ekstrom E, Ekman-Ordeberg G. High-mobility group box protein 1 and its signalling receptors in human preterm and term cervix. J Reprod Immunol. 2010;84(1):86–94. doi: 10.1016/j.jri.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Duguet M, Soussi T, Rossignol JM, Mechali M, De Recondo AM. Stimulation of rat liver alpha- and beta-type DNA polymerases by an homologous DNA-unwinding protein. FEBS Lett. 1977;79(1):160–164. doi: 10.1016/0014-5793(77)80374-8. [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Baruah P, Bianchi ME, Manfredi AA, Rovere-Querini P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur J Immunol. 2005a;35(7):2184–2190. doi: 10.1002/eji.200526066. [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol. 2005b;26(7):381–387. doi: 10.1016/j.it.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005c;174(12):7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol. 2007;81(1):84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- Duncan JA, Gao X, Huang MT, O'Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182(10):6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WA, Jr, Cregg JM, Kiel JA, van der Klei IJ, Oku M, Sakai Y, Sibirny AA, Stasyk OV, Veenhuis M. Pexophagy: the selective autophagy of peroxisomes. Autophagy. 2005;1(2):75–83. doi: 10.4161/auto.1.2.1737. [DOI] [PubMed] [Google Scholar]
- Dupont N, Jiang S, Pilli M, Ornatowski W, Bhattacharya D, Deretic V. Autophagybased unconventional secretory pathway for extracellular delivery of IL-1beta. Embo J. 2011;30(23):4701–4711. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer MA, Hayes PJ, Baron MH. The HMG domain protein SSRP1/PREIIBF is involved in activation of the human embryonic beta-like globin gene. Mol Cell Biol. 1998;18(5):2617–2628. doi: 10.1128/mcb.18.5.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw WC, Mackay AM. Role of nonhistone proteins in the chromosomal events of mitosis. Faseb J. 1994;8(12):947–956. doi: 10.1096/fasebj.8.12.8088460. [DOI] [PubMed] [Google Scholar]
- Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- Eguchi T, Nomura Y, Hashiguchi T, Masuda K, Arata M, Hazeki D, Ueno K, Nishi J, Kawano Y, Maruyama I. An elevated value of high mobility group box 1 is a potential marker for poor response to high-dose of intravenous immunoglobulin treatment in patients with Kawasaki syndrome. Pediatr Infect Dis J. 2009;28(4):339–341. doi: 10.1097/INF.0b013e31818ffe60. [DOI] [PubMed] [Google Scholar]
- Einck L, Bustin M. The intracellular distribution and function of the high mobility group chromosomal proteins. Exp Cell Res. 1985;156(2):295–310. doi: 10.1016/0014-4827(85)90539-7. [DOI] [PubMed] [Google Scholar]
- Einck L, Soares N, Bustin M. Localization of HMG chromosomal proteins in the nucleus and cytoplasm by microinjection of functional antibody fragments into living fibroblasts. Exp Cell Res. 1984;152(2):287–301. doi: 10.1016/0014-4827(84)90631-1. [DOI] [PubMed] [Google Scholar]
- Eisenhut M. Mediators of cellular stress response in bacterial meningitis. Crit Care Med. 2008;36(1):365–366. doi: 10.1097/01.CCM.0000295270.81168.CB. [DOI] [PubMed] [Google Scholar]
- El-Asrar AM, Missotten L, Geboes K. Expression of high-mobility groups box-1/receptor for advanced glycation end products/osteopontin/early growth response-1 pathway in proliferative vitreoretinal epiretinal membranes. Molecular vision. 2011a;17:508–518. [PMC free article] [PubMed] [Google Scholar]
- El-Asrar AM, Nawaz MI, Kangave D, Geboes K, Ola MS, Ahmad S, Al-Shabrawey M. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Molecular vision. 2011b;17:1829–1838. [PMC free article] [PubMed] [Google Scholar]
- El Gazzar M. HMGB1 modulates inflammatory responses in LPS-activated macrophages. Inflamm Res. 2007;56(4):162–167. doi: 10.1007/s00011-006-6112-0. [DOI] [PubMed] [Google Scholar]
- El Gazzar M, Yoza BK, Chen X, Garcia BA, Young NL, McCall CE. Chromatin-specific remodeling by HMGB1 and linker histone H1 silences proinflammatory genes during endotoxin tolerance. Mol Cell Biol. 2009;29(7):1959–1971. doi: 10.1128/MCB.01862-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mezayen R, El Gazzar M, Seeds MC, McCall CE, Dreskin SC, Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett. 2007;111(1):36–44. doi: 10.1016/j.imlet.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov I, Pelovsky P, Ugrinova I, Takahashi M, Pasheva E. The DNA binding and bending activities of truncated tail-less HMGB1 protein are differentially affected by Lys-2 and Lys-81 residues and their acetylation. Int J Biol Sci. 2011;7(6):691–699. doi: 10.7150/ijbs.7.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgamal OA, Park JK, Gusev Y, Azevedo-Pouly AC, Jiang J, Roopra A, Schmittgen TD. Tumor suppressive function of mir-205 in breast cancer is linked to HMGB3 regulation. PLoS ONE. 2013;8(10):e76402. doi: 10.1371/journal.pone.0076402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin SC, Weintraub H. Chromosomal proteins and chromatin structure. Annu Rev Biochem. 1975;44:725–774. doi: 10.1146/annurev.bi.44.070175.003453. [DOI] [PubMed] [Google Scholar]
- Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, Lotze MT. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13(10):2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- Ellwood KB, Yen YM, Johnson RC, Carey M. Mechanism for specificity by HMG-1 in enhanceosome assembly. Mol Cell Biol. 2000;20(12):4359–4370. doi: 10.1128/mcb.20.12.4359-4370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele E, Boso M, Brondino N, Pietra S, Barale F, Ucelli di Nemi S, Politi P. Increased serum levels of high mobility group box 1 protein in patients with autistic disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2010;34(4):681–683. doi: 10.1016/j.pnpbp.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Enokido Y, Yoshitake A, Ito H, Okazawa H. Age-dependent change of HMGB1 and DNA double-strand break accumulation in mouse brain. Biochem Biophys Res Commun. 2008;376(1):128–133. doi: 10.1016/j.bbrc.2008.08.108. [DOI] [PubMed] [Google Scholar]
- Enomoto N, Suda T, Uto T, Kato M, Kaida Y, Ozawa Y, Miyazaki H, Kuroishi S, Hashimoto D, Naito T, Fujisawa T, Matsui T, Inui N, Nakamura Y, Sato J, Mizuguchi T, Kato A, Chida K. Possible therapeutic effect of direct haemoperfusion with a polymyxin B immobilized fibre column (PMX-DHP) on pulmonary oxygenation in acute exacerbations of interstitial pneumonia. Respirology. 2008;13(3):452–460. doi: 10.1111/j.1440-1843.2008.01290.x. [DOI] [PubMed] [Google Scholar]
- Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, Wang H, Yang H, Sharma L, Phan BD, Javdan M, Chavan SS, Miller EJ, Tracey KJ, Mantell LL. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas Aeruginosa pneumonia in cystic fibrosis. Mol Med. 2012;18:477–485. doi: 10.2119/molmed.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escamilla-Powers JR, Daniel CJ, Farrell A, Taylor K, Zhang X, Byers S, Sears R. The tumor suppressor protein HBP1 is a novel c-myc-binding protein that negatively regulates c-myc transcriptional activity. J Biol Chem. 2010;285(7):4847–4858. doi: 10.1074/jbc.M109.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito E, Genovese T, Caminiti R, Bramanti P, Meli R, Cuzzocrea S. Melatonin reduces stress-activated/mitogen-activated protein kinases in spinal cord injury. J Pineal Res. 2009;46(1):79–86. doi: 10.1111/j.1600-079X.2008.00633.x. [DOI] [PubMed] [Google Scholar]
- Esposito F, Tornincasa M, Federico A, Chiappetta G, Pierantoni GM, Fusco A. High-mobility group A1 protein inhibits p53-mediated intrinsic apoptosis by interacting with Bcl-2 at mitochondria. Cell Death Dis. 2012;3:e383. doi: 10.1038/cddis.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Eun SY, Seo J, Park SW, Lee JH, Chang KC, Kim HJ. LPS potentiates nucleotide-induced inflammatory gene expression in macrophages via the upregulation of P2Y2 receptor. Int Immunopharmacol. 2014;18(2):270–276. doi: 10.1016/j.intimp.2013.11.026. [DOI] [PubMed] [Google Scholar]
- Evankovich J, Cho SW, Zhang R, Cardinal J, Dhupar R, Zhang L, Klune JR, Zlotnicki J, Billiar T, Tsung A. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem. 2010;285(51):39888–39897. doi: 10.1074/jbc.M110.128348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fages C, Nolo R, Huttunen HJ, Eskelinen E, Rauvala H. Regulation of cell migration by amphoterin. J Cell Sci. 2000;113(Pt 4):611–620. doi: 10.1242/jcs.113.4.611. [DOI] [PubMed] [Google Scholar]
- Fahmueller YN, Nagel D, Hoffmann RT, Tatsch K, Jakobs T, Stieber P, Holdenrieder S. Immunogenic cell death biomarkers HMGB1, RAGE, and DNAse indicate response to radioembolization therapy and prognosis in colorectal cancer patients. International journal of cancer. Journal international du cancer. 2013;132(10):2349–2358. doi: 10.1002/ijc.27894. [DOI] [PubMed] [Google Scholar]
- Falciola L, Murchie AI, Lilley DM, Bianchi M. Mutational analysis of the DNA binding domain A of chromosomal protein HMG1. Nucleic Acids Res. 1994;22(3):285–292. doi: 10.1093/nar/22.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falciola L, Spada F, Calogero S, Langst G, Voit R, Grummt I, Bianchi ME. High mobility group 1 protein is not stably associated with the chromosomes of somatic cells. J Cell Biol. 1997;137(1):19–26. doi: 10.1083/jcb.137.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z, Beresford PJ, Zhang D, Lieberman J. HMG2 interacts with the nucleosome assembly protein SET and is a target of the cytotoxic T-lymphocyte protease granzyme A. Mol Cell Biol. 2002;22(8):2810–2820. doi: 10.1128/MCB.22.8.2810-2820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Ang B, Xu X, Huang X, Wu Y, Sun Y, Wang W, Li N, Cao X, Wan T. TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell Mol Immunol. 2013a doi: 10.1038/cmi.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Liu A, Dahmen U, Dirsch O. Dual role of chloroquine in liver ischemia reperfusion injury: reduction of liver damage in early phase, but aggravation in late phase. Cell Death Dis. 2013b;4:e694. doi: 10.1038/cddis.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Pan HC, Lin SL, Zhang WQ, Rauvala H, Schachner M, Shen YQ. HMGB1 Contributes to Regeneration After Spinal Cord Injury in Adult Zebrafish. Mol Neurobiol. 2014;49(1):472–483. doi: 10.1007/s12035-013-8533-4. [DOI] [PubMed] [Google Scholar]
- Fang P, Schachner M, Shen YQ. HMGB1 in development and diseases of the central nervous system. Mol Neurobiol. 2012;45(3):499–506. doi: 10.1007/s12035-012-8264-y. [DOI] [PubMed] [Google Scholar]
- Fang WH, Yao YM, Shi ZG, Yu Y, Wu Y, Lu LR, Sheng ZY. The significance of changes in high mobility group-1 protein mRNA expression in rats after thermal injury. Shock. 2002;17(4):329–333. doi: 10.1097/00024382-200204000-00016. [DOI] [PubMed] [Google Scholar]
- Fang X, Zheng P, Tang J, Liu Y. CD24: from A to Z. Cell Mol Immunol. 2010;7(2):100–103. doi: 10.1038/cmi.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele M, Battista S, Kenyon L, Baldassarre G, Fidanza V, Klein-Szanto AJ, Parlow AF, Visone R, Pierantoni GM, Outwater E, Santoro M, Croce CM, Fusco A. Overexpression of the HMGA2 gene in transgenic mice leads to the onset of pituitary adenomas. Oncogene. 2002;21(20):3190–3198. doi: 10.1038/sj.onc.1205428. [DOI] [PubMed] [Google Scholar]
- Fedele M, Berlingieri MT, Scala S, Chiariotti L, Viglietto G, Rippel V, Bullerdiek J, Santoro M, Fusco A. Truncated and chimeric HMGI-C genes induce neoplastic transformation of NIH3T3 murine fibroblasts. Oncogene. 1998;17(4):413–418. doi: 10.1038/sj.onc.1201952. [DOI] [PubMed] [Google Scholar]
- Fedele M, Fidanza V, Battista S, Pentimalli F, Klein-Szanto AJ, Visone R, De Martino I, Curcio A, Morisco C, Del Vecchio L, Baldassarre G, Arra C, Viglietto G, Indolfi C, Croce CM, Fusco A. Haploinsufficiency of the Hmga1 gene causes cardiac hypertrophy and myelo-lymphoproliferative disorders in mice. Cancer Res. 2006;66(5):2536–2543. doi: 10.1158/0008-5472.CAN-05-1889. [DOI] [PubMed] [Google Scholar]
- Fedele M, Fusco A. HMGA and cancer. Biochim Biophys Acta. 2010;1799(1–2):48–54. doi: 10.1016/j.bbagrm.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Fedele M, Pentimalli F, Baldassarre G, Battista S, Klein-Szanto AJ, Kenyon L, Visone R, De Martino I, Ciarmiello A, Arra C, Viglietto G, Croce CM, Fusco A. Transgenic mice overexpressing the wild-type form of the HMGA1 gene develop mixed growth hormone/prolactin cell pituitary adenomas and natural killer cell lymphomas. Oncogene. 2005;24(21):3427–3435. doi: 10.1038/sj.onc.1208501. [DOI] [PubMed] [Google Scholar]
- Fei J, Yu HJ, Zhou J, Huang XK, Liang HP, Jiang YG. [Study on high mobility group-1 protein in patients with multiple trauma] Zhongguo wei zhong bing ji jiu yi xue = Chinese critical care medicine = Zhongguo weizhongbing jijiuyixue. 2005;17(5):273–275. [PubMed] [Google Scholar]
- Fei J, Wang W, Kwiecinski J, Josefsson E, Pullerits R, Jonsson IM, Magnusson M, Jin T. The combination of a tumor necrosis factor inhibitor and antibiotic alleviates staphylococcal arthritis and sepsis in mice. J Infect Dis. 2011;204(3):348–357. doi: 10.1093/infdis/jir266. [DOI] [PubMed] [Google Scholar]
- Feldman P, Due MR, Ripsch MS, Khanna R, White FA. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J Neuroinflammation. 2012;9:180. doi: 10.1186/1742-2094-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Zhu M, Zhang M, Gu J, Jia X, Tan X, Gao C, Zhu Q. The protection of 4,4'-diphenylmethane-bis(methyl) carbamate from Cortex Mori on advanced glycation end productinduced endothelial dysfunction: via inhibiting AGE formation or blocking AGEs-RAGE axis? Fitoterapia. 2013;89:239–249. doi: 10.1016/j.fitote.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Ferguson M, Henry PA, Currie RA. Histone deacetylase inhibition is associated with transcriptional repression of the Hmga2 gene. Nucleic Acids Res. 2003;31(12):3123–3133. doi: 10.1093/nar/gkg403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferhani N, Letuve S, Kozhich A, Thibaudeau O, Grandsaigne M, Maret M, Dombret MC, Sims GP, Kolbeck R, Coyle AJ, Aubier M, Pretolani M. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2010;181(9):917–927. doi: 10.1164/rccm.200903-0340OC. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Harley VR, Pontiggia A, Goodfellow PN, Lovell-Badge R, Bianchi ME. SRY, like HMG1, recognizes sharp angles in DNA. Embo J. 1992;11(12):4497–4506. doi: 10.1002/j.1460-2075.1992.tb05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Ronfani L, Calogero S, Bianchi ME. The mouse gene coding for high mobility group 1 protein (HMG1) J Biol Chem. 1994;269(46):28803–28808. [PubMed] [Google Scholar]
- Feser J, Truong D, Das C, Carson JJ, Kieft J, Harkness T, Tyler JK. Elevated histone expression promotes life span extension. Mol Cell. 2010;39(5):724–735. doi: 10.1016/j.molcel.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtinger-Schepman AM, van der Veer JL, den Hartog JH, Lohman PH, Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101(7):2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- Fontanini A, Foti C, Potu H, Crivellato E, Maestro R, Bernardi P, Demarchi F, Brancolini C. The Isopeptidase Inhibitor G5 Triggers a Caspase-independent Necrotic Death in Cells Resistant to Apoptosis: A COMPARATIVE STUDY WITH THE PROTEASOME INHIBITOR BORTEZOMIB. J Biol Chem. 2009;284(13):8369–8381. doi: 10.1074/jbc.M806113200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Chiefari E, Fedele M, Iuliano R, Brunetti L, Paonessa F, Manfioletti G, Barbetti F, Brunetti A, Croce CM, Fusco A. Lack of the architectural factor HMGA1 causes insulin resistance and diabetes in humans and mice. Nat Med. 2005;11(7):765–773. doi: 10.1038/nm1254. [DOI] [PubMed] [Google Scholar]
- Friedmann M, Holth LT, Zoghbi HY, Reeves R. Organization, inducible-expression and chromosome localization of the human HMG-I(Y) nonhistone protein gene. Nucleic Acids Res. 1993;21(18):4259–4267. doi: 10.1093/nar/21.18.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggeri A, Banerjee S, Biswas S, de Freitas A, Liu G, Bierhaus A, Abraham E. Participation of the receptor for advanced glycation end products in efferocytosis. J Immunol. 2011;186(11):6191–6198. doi: 10.4049/jimmunol.1004134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggeri A, Yang Y, Banerjee S, Park YJ, Liu G, Abraham E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin. Am J Physiol Cell Physiol. 2010;299(6):C1267–C1276. doi: 10.1152/ajpcell.00152.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spisek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71(14):4821–4833. doi: 10.1158/0008-5472.CAN-11-0950. [DOI] [PubMed] [Google Scholar]
- Fucikova J, Moserova I, Truxova I, Hermanova I, Vancurova I, Partlova S, Fialova A, Sojka L, Cartron PF, Houska M, Rob L, Bartunkova J, Spisek R. Article title: High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int J Cancer. 2014 doi: 10.1002/ijc.28766. [DOI] [PubMed] [Google Scholar]
- Fuentes E, Rojas A, Palomo I. Role of multiligand/RAGE axis in platelet activation. Thromb Res. 2014;133(3):308–314. doi: 10.1016/j.thromres.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Nakano T, Hayakawa K, Irie K, Akitake Y, Sakamoto Y, Mishima K, Muroi C, Yonekawa Y, Banno F, Kokame K, Miyata T, Nishio K, Okuchi K, Iwasaki K, Fujiwara M, Siesjo BK. ADAMTS13 gene deletion enhances plasma high-mobility group box1 elevation and neuroinflammation in brain ischemia-reperfusion injury. Neurol Sci. 2012;33(5):1107–1115. doi: 10.1007/s10072-011-0913-9. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Furuta A, Kikuchi H, Aizawa S, Hatanaka Y, Konya C, Uchida K, Yoshimura A, Tamai Y, Wada K, Kabuta T. Discovery of a novel type of autophagy targeting RNA. Autophagy. 2013;9(3) doi: 10.4161/auto.23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami A, Adachi H, Yamagishi S, Matsui T, Ueda S, Nakamura K, Enomoto M, Otsuka M, Kumagae S, Nanjo Y, Kumagai E, Esaki E, Murayama K, Hirai Y, Imaizumi T. Factors associated with serum high mobility group box 1 HMGB1) levels in a general population. Metabolism. 2009;58(12):1688–1693. doi: 10.1016/j.metabol.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Funayama A, Shishido T, Netsu S, Narumi T, Kadowaki S, Takahashi H, Miyamoto T, Watanabe T, Woo CH, Abe J, Kuwahara K, Nakao K, Takeishi Y, Kubota I. Cardiac nuclear high mobility group box 1 prevents the development of cardiac hypertrophy and heart failure. Cardiovasc Res. 2013;99(4):657–664. doi: 10.1093/cvr/cvt128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlani D, Donndorf P, Westien I, Ugurlucan M, Pittermann E, Wang W, Li W, Vollmar B, Steinhoff G, Kaminski A, Ma N. HMGB-1 induces c-kit+ cell microvascular rolling and adhesion via both toll-like receptor-2 and toll-like receptor-4 of endothelial cells. J Cell Mol Med. 2012;16(5):1094–1105. doi: 10.1111/j.1582-4934.2011.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuita K, Murata S, Jee J, Ichikawa S, Matsuda A, Kojima C. NMR studies of DNA recognition mechanism of HMGB1 protein. Nucleic Acids Symp Ser (Oxf) 2009;53:89–90. doi: 10.1093/nass/nrp045. [DOI] [PubMed] [Google Scholar]
- Furuita K, Murata S, Jee JG, Ichikawa S, Matsuda A, Kojima C. Structural feature of bent DNA recognized by HMGB1. J Am Chem Soc. 2011;133(15):5788–5790. doi: 10.1021/ja2013399. [DOI] [PubMed] [Google Scholar]
- Furusawa T, Cherukuri S. Developmental function of HMGN proteins. Biochim Biophys Acta. 2010;1799(1–2):69–73. doi: 10.1016/j.bbagrm.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa T, Lim JH, Catez F, Birger Y, Mackem S, Bustin M. Down-regulation of nucleosomal binding protein HMGN1 expression during embryogenesis modulates Sox9 expression in chondrocytes. Mol Cell Biol. 2006;26(2):592–604. doi: 10.1128/MCB.26.2.592-604.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco A, Fedele M. Roles of HMGA proteins in cancer. Nat Rev Cancer. 2007;7(12):899–910. doi: 10.1038/nrc2271. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A, Rowe SM, Matthew H, Blalock JE. Proline-Glycine-Proline (PGP) and High Mobility Group Box Protein-1 (HMGB1): Potential Mediators of Cystic Fibrosis Airway Inflammation. Open Respir Med J. 2010;4:32–38. doi: 10.2174/1874306401004010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard C, Borde C, Gozlan J, Marechal V, Strauss F. A high-sensitivity method for detection and measurement of HMGB1 protein concentration by high-affinity binding to DNA hemicatenanes. PLoS ONE. 2008;3(8):e2855. doi: 10.1371/journal.pone.0002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard C, Strauss F. Association of poly(CA).poly(TG) DNA fragments into four-stranded complexes bound by HMG1 and 2. Science. 1994;264(5157):433–436. doi: 10.1126/science.8153633. [DOI] [PubMed] [Google Scholar]
- Gaillard C, Strauss F. High affinity binding of proteins HMG1 and HMG2 to semicatenated DNA loops. BMC Mol Biol. 2000;1:1. doi: 10.1186/1471-2199-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaini S, Pedersen SS, Koldkaer OG, Pedersen C, Moestrup SK, Moller HJ. New immunological serum markers in bacteraemia: anti-inflammatory soluble CD163, but not proinflammatory high mobility group-box 1 protein, is related to prognosis. Clinical and experimental immunology. 2008;151(3):423–431. doi: 10.1111/j.1365-2249.2007.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaini S, Pedersen SS, Koldkjaer OG, Pedersen C, Moller HJ. High mobility group box-1 protein in patients with suspected community-acquired infections and sepsis: a prospective study. Critical care (London, England) 2007;11(2):R32. doi: 10.1186/cc5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19(1):107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamez-Diaz LY, Enriquez LE, Matute JD, Velasquez S, Gomez ID, Toro F, Ospina S, Bedoya V, Arango CM, Valencia ML, De La Rosa G, Gomez CI, Garcia A, Patino PJ, Jaimes FA. Diagnostic accuracy of HMGB-1, sTREM-1, and CD64 as markers of sepsis in patients recently admitted to the emergency department. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2011;18(8):807–815. doi: 10.1111/j.1553-2712.2011.01113.x. [DOI] [PubMed] [Google Scholar]
- Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci. 2011a;31(3):1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Liu WH, Luan NN, Feng C, Shang T. [Correlation between the expression of high mobility group box 1 and receptor for advanced glycation end products and the onset of pre-eclampsia] Zhonghua fu chan ke za zhi. 2008;43(10):746–750. [PubMed] [Google Scholar]
- Gao M, Hu Z, Zheng Y, Zeng Y, Shen X, Zhong D, He F. Peroxisome proliferator-activated receptor gamma agonist troglitazone inhibits high mobility group box 1 expression in endothelial cells via suppressing transcriptional activity of nuclear factor kappaB and activator protein 1. Shock. 2011b;36(3):228–234. doi: 10.1097/SHK.0b013e318225b29a. [DOI] [PubMed] [Google Scholar]
- Gao TL, Yuan XT, Yang D, Dai HL, Wang WJ, Peng X, Shao HJ, Jin ZF, Fu ZJ. Expression of HMGB1 and RAGE in rat and human brains after traumatic brain injury. The journal of trauma and acute care surgery. 2012;72(3):643–649. doi: 10.1097/TA.0b013e31823c54a6. [DOI] [PubMed] [Google Scholar]
- Garcia-Arnandis I, Guillen MI, Castejon MA, Gomar F, Alcaraz MJ. Haem oxygenase-1 down-regulates high mobility group box 1 and matrix metalloproteinases in osteoarthritic synoviocytes. Rheumatology (Oxford) 2010a;49(5):854–861. doi: 10.1093/rheumatology/kep463. [DOI] [PubMed] [Google Scholar]
- Garcia-Arnandis I, Guillen MI, Gomar F, Pelletier JP, Martel-Pelletier J, Alcaraz MJ. High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1beta in osteoarthritic synoviocytes. Arthritis Res Ther. 2010b;12(4):R165. doi: 10.1186/ar3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3(73):73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3(10):995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariboldi M, De Gregorio L, Ferrari S, Manenti G, Pierotti MA, Bianchi ME, Dragani TA. Mapping of the Hmg1 gene and of seven related sequences in the mouse. Mamm Genome. 1995;6(9):581–585. doi: 10.1007/BF00352361. [DOI] [PubMed] [Google Scholar]
- Gdynia G, Keith M, Kopitz J, Bergmann M, Fassl A, Weber AN, George J, Kees T, Zentgraf HW, Wiestler OD, Schirmacher P, Roth W. Danger signaling protein HMGB1 induces a distinct form of cell death accompanied by formation of giant mitochondria. Cancer Res. 2010;70(21):8558–8568. doi: 10.1158/0008-5472.CAN-10-0204. [DOI] [PubMed] [Google Scholar]
- Ge H, Roeder RG. The high mobility group protein HMG1 can reversibly inhibit class II gene transcription by interaction with the TATA-binding protein. J Biol Chem. 1994;269(25):17136–17140. [PubMed] [Google Scholar]
- Ge WS, Wu JX, Fan JG, Wang YJ, Chen YW. Inhibition of high-mobility group box 1 expression by siRNA in rat hepatic stellate cells. World J Gastroenterol. 2011;17(36):4090–4098. doi: 10.3748/wjg.v17.i36.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Antoine DJ, Lu Y, Arriazu E, Leung TM, Klepper AL, Branch AD, Fiel MI, Nieto N. High-Mobility Group Box-1 (HMGB1) Participates in the Pathogenesis of Alcoholic Liver Disease (ALD) J Biol Chem. 2014 doi: 10.1074/jbc.M114.552141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gea-Sorli S, Closa D. Role of macrophages in the progression of acute pancreatitis. World journal of gastrointestinal pharmacology and therapeutics. 2010;1(5):107–111. doi: 10.4292/wjgpt.v1.i5.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, Hess J, Angel P. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205(2):275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu Rev Biochem. 2002;71:101–132. doi: 10.1146/annurev.biochem.71.090501.150203. [DOI] [PubMed] [Google Scholar]
- Genschel J, Modrich P. Functions of MutLalpha, replication protein A (RPA), and HMGB1 in 5'-directed mismatch repair. J Biol Chem. 2009;284(32):21536–21544. doi: 10.1074/jbc.M109.021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlitz G. HMGNs, DNA repair and cancer. Biochim Biophys Acta. 2010;1799(1–2):80–85. doi: 10.1016/j.bbagrm.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlitz G, Hock R, Ueda T, Bustin M. The dynamics of HMG protein-chromatin interactions in living cells. Biochem Cell Biol. 2009;87(1):127–137. doi: 10.1139/O08-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germani A, Limana F, Capogrossi MC. Pivotal advances: high-mobility group box 1 protein--a cytokine with a role in cardiac repair. J Leukoc Biol. 2007;81(1):41–45. doi: 10.1189/jlb.0306165. [DOI] [PubMed] [Google Scholar]
- Giallauria F, Cirillo P, D'Agostino M, Petrillo G, Vitelli A, Pacileo M, Angri V, Chiariello M, Vigorito C. Effects of exercise training on high-mobility group box-1 levels after acute myocardial infarction. Journal of cardiac failure. 2011;17(2):108–114. doi: 10.1016/j.cardfail.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Giallauria F, Cirillo P, Lucci R, Pacileo M, D'Agostino M, Maietta P, Vitelli A, Chiariello M, Vigorito C. Autonomic dysfunction is associated with high mobility group box-1 levels in patients after acute myocardial infarction. Atherosclerosis. 2010;208(1):280–284. doi: 10.1016/j.atherosclerosis.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavara S, Kosmidou E, Hande MP, Bianchi ME, Morgan A, d'Adda di Fagagna F, Jackson SP. Yeast Nhp6A/B and mammalian Hmgb1 facilitate the maintenance of genome stability. Curr Biol. 2005;15(1):68–72. doi: 10.1016/j.cub.2004.12.065. [DOI] [PubMed] [Google Scholar]
- Gibb CL, Cheng W, Morozov VN, Kallenbach NR. Effect of nuclear protein HMG1 on in vitro slippage synthesis of the tandem repeat dTG × dCA. Biochemistry. 1997;36(18):5418–5424. doi: 10.1021/bi962037v. [DOI] [PubMed] [Google Scholar]
- Gibot S, Massin F, Cravoisy A, Barraud D, Nace L, Levy B, Bollaert PE. High-mobility group box 1 protein plasma concentrations during septic shock. Intensive care medicine. 2007;33(8):1347–1353. doi: 10.1007/s00134-007-0691-2. [DOI] [PubMed] [Google Scholar]
- Giese K, Amsterdam A, Grosschedl R. DNA-binding properties of the HMG domain of the lymphoid-specific transcriptional regulator LEF-1. Genes Dev. 1991;5(12B):2567–2578. doi: 10.1101/gad.5.12b.2567. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M, Velusamy R, He YX, Ramaswamy K. Cloning and characterization of a high mobility group box 1 (HMGB1) homologue protein from Schistosoma mansoni. Mol Biochem Parasitol. 2006;145(2):137–146. doi: 10.1016/j.molbiopara.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Goldstein RS. High mobility group box-1 protein as a tumor necrosis factor-independent therapeutic target in rheumatoid arthritis. Arthritis Res Ther. 2008;10(3):111. doi: 10.1186/ar2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RS, Bruchfeld A, Yang L, Qureshi AR, Gallowitsch-Puerta M, Patel NB, Huston BJ, Chavan S, Rosas-Ballina M, Gregersen PK, Czura CJ, Sloan RP, Sama AE, Tracey KJ. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med. 2007;13(3–4):210–215. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RS, Gallowitsch-Puerta M, Yang L, Rosas-Ballina M, Huston JM, Czura CJ, Lee DC, Ward MF, Bruchfeld AN, Wang H, Lesser ML, Church AL, Litroff AH, Sama AE, Tracey KJ. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock (Augusta, Ga.) 2006;25(6):571–574. doi: 10.1097/01.shk.0000209540.99176.72. [DOI] [PubMed] [Google Scholar]
- Gong G, Yuan LB, Hu L, Wu W, Yin L, Hou JL, Liu YH, Zhou LS. Glycyrrhizin attenuates rat ischemic spinal cord injury by suppressing inflammatory cytokines and HMGB1. Acta Pharmacol Sin. 2012;33(1):11–18. doi: 10.1038/aps.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Yin H, Fang M, Xiang Y, Yuan CL, Zheng GY, Yang H, Xiong P, Chen G, Gong FL, Zheng F. Heme oxygenase-1 upregulation significantly inhibits TNF-alpha and Hmgb1 releasing and attenuates lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2008;8(6):792–798. doi: 10.1016/j.intimp.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Gong QA, Zhang H, Li JH, Duan LH, Zhong S, Kong XL, Zheng F, Tan Z, Xiong P, Chen G, Fang M, Gong FL. High-mobility group box 1 exacerbates concanavalin A-induced hepatic injury in mice. J Mol Med-Jmm. 2010a;88(12):1289–1298. doi: 10.1007/s00109-010-0681-7. [DOI] [PubMed] [Google Scholar]
- Gong W, Li Y, Chao F, Huang G, He F. Amino acid residues 201–205 in C-terminal acidic tail region plays a crucial role in antibacterial activity of HMGB1. J Biomed Sci. 2009;16:83. doi: 10.1186/1423-0127-16-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Zheng Y, Chao F, Li Y, Xu Z, Huang G, Gao X, Li S, He F. The anti-inflammatory activity of HMGB1 A box is enhanced when fused with C-terminal acidic tail. J Biomed Biotechnol. 2010b;2010:915234. doi: 10.1155/2010/915234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Cao Y, Song L, Zhou J, Wang C, Wu B. HMGB3 characterization in gastric cancer. Genet Mol Res. 2013;12(4):6032–6039. doi: 10.4238/2013.December.2.1. [DOI] [PubMed] [Google Scholar]
- Goodwin GH, Brown E, Walker JM, Johns EW. The isolation of three new high mobility group nuclear proteins. Biochim Biophys Acta. 1980;623(2):329–338. doi: 10.1016/0005-2795(80)90260-3. [DOI] [PubMed] [Google Scholar]
- Goodwin GH, Johns EW. Isolation and characterisation of two calf-thymus chromatin non-histone proteins with high contents of acidic and basic amino acids. Eur J Biochem. 1973;40(1):215–219. doi: 10.1111/j.1432-1033.1973.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Goodwin GH, Johns EW. Are the high mobility group non-histone chromosomal proteins associated with 'active' chromatin? Biochim Biophys Acta. 1978;519(1):279–284. doi: 10.1016/0005-2787(78)90081-3. [DOI] [PubMed] [Google Scholar]
- Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38(1):14–19. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- Goto T, Hussein MH, Kato S, Daoud GA, Kato T, Kakita H, Mizuno H, Imai M, Ito T, Kato I, Suzuki S, Okada N, Togari H, Okada H. Endothelin receptor antagonist attenuates inflammatory response and prolongs the survival time in a neonatal sepsis model. Intensive Care Med. 2010;36(12):2132–2139. doi: 10.1007/s00134-010-2040-0. [DOI] [PubMed] [Google Scholar]
- Gougeon ML, Bras M. Natural killer cells, dendritic cells, and the alarmin high-mobility group box 1 protein: a dangerous trio in HIV-1 infection? Curr Opin HIV AIDS. 2011;6(5):364–372. doi: 10.1097/COH.0b013e328349b089. [DOI] [PubMed] [Google Scholar]
- Gougeon ML, Melki MT, Saidi H. HMGB1, an alarmin promoting HIV dissemination and latency in dendritic cells. Cell Death Differ. 2012;19(1):96–106. doi: 10.1038/cdd.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goula AV, Berquist BR, Wilson DM, 3rd, Wheeler VC, Trottier Y, Merienne K. Stoichiometry of base excision repair proteins correlates with increased somatic CAG instability in striatum over cerebellum in Huntington's disease transgenic mice. PLoS Genet. 2009;5(12):e1000749. doi: 10.1371/journal.pgen.1000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasser KD. Plant chromosomal high mobility group (HMG) proteins. Plant J. 1995;7(2):185–192. doi: 10.1046/j.1365-313x.1995.7020185.x. [DOI] [PubMed] [Google Scholar]
- Grasser KD, Grill S, Duroux M, Launholt D, Thomsen MS, Nielsen BV, Nielsen HK, Merkle T. HMGB6 from Arabidopsis thaliana specifies a novel type of plant chromosomal HMGB protein. Biochemistry. 2004;43(5):1309–1314. doi: 10.1021/bi035931c. [DOI] [PubMed] [Google Scholar]
- Grasser KD, Launholt D, Grasser M. High mobility group proteins of the plant HMGB family: dynamic chromatin modulators. Biochim Biophys Acta. 2007;1769(5–6):346–357. doi: 10.1016/j.bbaexp.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Grasser KD, Teo SH, Lee KB, Broadhurst RW, Rees C, Hardman CH, Thomas JO. DNA-binding properties of the tandem HMG boxes of high-mobility-group protein 1 (HMG1) Eur J Biochem. 1998;253(3):787–795. doi: 10.1046/j.1432-1327.1998.2530787.x. [DOI] [PubMed] [Google Scholar]
- Grasso D, Ropolo A, Lo Re A, Boggio V, Molejon MI, Iovanna JL, Gonzalez CD, Urrutia R, Vaccaro MI. Zymophagy, a novel selective autophagy pathway mediated by VMP1-USP9x-p62, prevents pancreatic cell death. J Biol Chem. 2011;286(10):8308–8324. doi: 10.1074/jbc.M110.197301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9(5):353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griess EA, Rensing SA, Grasser KD, Maier UG, Feix G. Phylogenetic relationships of HMG box DNA-binding domains. J Mol Evol. 1993;37(2):204–210. doi: 10.1007/BF02407357. [DOI] [PubMed] [Google Scholar]
- Griffin KL, Fischer BM, Kummarapurugu AB, Zheng S, Kennedy TP, Rao NV, Foster WM, Voynow JA. 2-O, 3-O-Desulfated Heparin Inhibits Neutrophil Elastase-Induced HMGB-1 Secretion and Airway Inflammation. Am J Respir Cell Mol Biol. 2013 doi: 10.1165/rcmb.2013-0338RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronostajski RM. Roles of the NFI/CTF gene family in transcription and development. Gene. 2000;249(1–2):31–45. doi: 10.1016/s0378-1119(00)00140-2. [DOI] [PubMed] [Google Scholar]
- Grosschedl R, Giese K, Pagel J. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994;10(3):94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Grover A, Taylor J, Troudt J, Keyser A, Sommersted K, Schenkel A, Izzo AA. Mycobacterial infection induces the secretion of high-mobility group box 1 protein. Cell Microbiol. 2008;10(6):1390–1404. doi: 10.1111/j.1462-5822.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- Grover A, Troudt J, Foster C, Basaraba R, Izzo A. High Mobility group box 1 (HMGB1) acts as an adjuvant for tuberculosis subunit vaccines. Immunology. 2013 doi: 10.1111/imm.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundtman C, Bruton J, Yamada T, Ostberg T, Pisetsky DS, Harris HE, Andersson U, Lundberg IE, Westerblad H. Effects of HMGB1 on in vitro responses of isolated muscle fibers and functional aspects in skeletal muscles of idiopathic inflammatory myopathies. Faseb J. 2010;24(2):570–578. doi: 10.1096/fj.09-144782. [DOI] [PubMed] [Google Scholar]
- Grundy GJ, Ramon-Maiques S, Dimitriadis EK, Kotova S, Biertumpfel C, Heymann JB, Steven AC, Gellert M, Yang W. Initial stages of V(D)J recombination: the organization of RAG1/2 and RSS DNA in the postcleavage complex. Mol Cell. 2009;35(2):217–227. doi: 10.1016/j.molcel.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu XJ, Xu J, Ma BY, Chen G, Gu PY, Wei D, Hu WX. Effect of glycyrrhizin on traumatic brain injury in rats and its mechanism. Chin J Traumatol. 2014;17(1):1–7. [PubMed] [Google Scholar]
- Gu Z, Akiyama K, Ma X, Zhang H, Feng X, Yao G, Hou Y, Lu L, Gilkeson GS, Silver RM, Zeng X, Shi S, Sun L. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus. 2010;19(13):1502–1514. doi: 10.1177/0961203310373782. [DOI] [PubMed] [Google Scholar]
- Guazzi S, Strangio A, Franzi AT, Bianchi ME. HMGB1, an architectural chromatin protein and extracellular signalling factor, has a spatially and temporally restricted expression pattern in mouse brain. Gene Expr Patterns. 2003;3(1):29–33. doi: 10.1016/s1567-133x(02)00093-5. [DOI] [PubMed] [Google Scholar]
- Guerin R, Arseneault G, Dumont S, Rokeach LA. Calnexin is involved in apoptosis induced by endoplasmic reticulum stress in the fission yeast. Mol Biol Cell. 2008;19(10):4404–4420. doi: 10.1091/mbc.E08-02-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermah M, Palhan VB, Tackett AJ, Chait BT, Roeder RG. Synergistic functions of SII and p300 in productive activator-dependent transcription of chromatin templates. Cell. 2006;125(2):275–286. doi: 10.1016/j.cell.2006.01.055. [DOI] [PubMed] [Google Scholar]
- Guillet F, Tournefier A, Denoulet P, Capony JP, Kerfourn F, Charlemagne J. High levels of HMG1–2 protein expression in the cytoplasm and nucleus of hydrocortisone sensitive amphibian thymocytes. Biol Cell. 1990;69(3):153–160. doi: 10.1016/0248-4900(90)90341-y. [DOI] [PubMed] [Google Scholar]
- Guo HF, Liu SX, Zhang YJ, Liu QJ, Hao J, Gao LX. High mobility group box 1 induces synoviocyte proliferation in rheumatoid arthritis by activating the signal transducer and activator transcription signal pathway. Clin Exp Med. 2011;11(2):65–74. doi: 10.1007/s10238-010-0116-3. [DOI] [PubMed] [Google Scholar]
- Guo J, Friedman SL. Hepatic fibrogenesis. Seminars in liver disease. 2007;27(4):413–426. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- Guo Y, Liu Y, Tan PQ, Li G, Su ZW, Tian YQ, Qiu YZ. [Expressions and clinical significance of high mobility group box-1 mRNA and protein in laryngeal squamous cell carcinoma tissues and serum] Zhonghua er bi yan hou tou jing wai ke za zhi = Chinese journal of otorhinolaryngology head and neck surgery. 2012;47(6):487–490. [PubMed] [Google Scholar]
- Ha T, Xia Y, Liu X, Lu C, Liu L, Kelley J, Kalbfleisch J, Kao RL, Williams DL, Li C. Glucan phosphate attenuates myocardial HMGB1 translocation in severe sepsis through inhibiting NF-kappaB activation. Am J Physiol Heart Circ Physiol. 2011a;301(3):H848–H855. doi: 10.1152/ajpheart.01007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TK, Her NG, Lee MG, Ryu BK, Lee JH, Han J, Jeong SI, Kang MJ, Kim NH, Kim HJ, Chi SG. Caveolin-1 increases aerobic glycolysis in colorectal cancers by stimulating HMGA1-mediated GLUT3 transcription. Cancer Res. 2012a;72(16):4097–4109. doi: 10.1158/0008-5472.CAN-12-0448. [DOI] [PubMed] [Google Scholar]
- Ha YM, Ham SA, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Park MK, Chang KC. beta(1)-adrenergic receptor-mediated HO-1 induction, via PI3K and p38 MAPK, by isoproterenol in RAW 264.7 cells leads to inhibition of HMGB1 release in LPS-activated RAW 264.7 cells and increases in survival rate of CLP-induced septic mice. Biochem Pharmacol. 2011b;82(7):769–777. doi: 10.1016/j.bcp.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Ha YM, Kim MY, Park MK, Lee YS, Kim YM, Kim HJ, Lee JH, Chang KC. Higenamine reduces HMGB1 during hypoxia-induced brain injury by induction of heme oxygenase-1 through PI3K/Akt/Nrf-2 signal pathways. Apoptosis. 2012b;17(5):463–474. doi: 10.1007/s10495-011-0688-8. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Hasegawa A, Asai N, Noguchi T. High-dose intravenous immunoglobulin G improves systemic inflammation in a rat model of CLP-induced sepsis. Intensive Care Med. 2008a;34(10):1812–1819. doi: 10.1007/s00134-008-1161-1. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Hasegawa A, Asai N, Noguchi T. Hyperglycemia contributes to cardiac dysfunction in a lipopolysaccharide-induced systemic inflammation model. Crit Care Med. 2009a;37(7):2223–2227. doi: 10.1097/CCM.0b013e3181a007c6. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Hasegawa A, Asai N, Uchida T, Noguchi T. Dendritic cell activation in response to ischemia-reperfusion injury of the small intestine. Surg Today. 2010a;40(2):137–145. doi: 10.1007/s00595-009-4033-6. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Hasegawa A, Koga H, Noguchi T. Effects of hyperglycemia and insulin therapy on high mobility group box 1 in endotoxin-induced acute lung injury in a rat model. Crit Care Med. 2008b;36(8):2407–2413. doi: 10.1097/CCM.0b013e318180b3ba. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Hasegawa A, Kudo K, Kusaka J, Oyama Y, Noguchi T. Infusion of a glucose solution reduces autophagy in the liver after LPS-induced systemic inflammation. Inflammation. 2012;35(1):249–258. doi: 10.1007/s10753-011-9311-y. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Hasegawa A, Oyama M, Imatomi R, Uchida T, Noguchi T. Adenosine diphosphate receptor antagonist clopidogrel sulfate attenuates LPS-induced systemic inflammation in a rat model. Shock. 2011a;35(3):289–292. doi: 10.1097/SHK.0b013e3181f48987. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Hidaka S, Hasegawa A, Koga H, Noguchi T. Antagonist of the type-1 ANG II receptor prevents against LPS-induced septic shock in rats. Intensive Care Med. 2009b;35(8):1471–1478. doi: 10.1007/s00134-009-1545-x. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Hidaka S, Hasegawa A, Noguchi T. Neutrophil elastase inhibitor (sivelestat) reduces the levels of inflammatory mediators by inhibiting NF-kB. Inflamm Res. 2009c;58(4):198–203. doi: 10.1007/s00011-008-8131-5. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Hidaka S, Hishiyama S, Noguchi T. Danaparoid sodium inhibits systemic inflammation and prevents endotoxin-induced acute lung injury in rats. Crit Care. 2008c;12(2):R43. doi: 10.1186/cc6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Maeda H, Noguchi T. Landiolol, an ultrashort-acting beta1-adrenoceptor antagonist, has protective effects in an LPS-induced systemic inflammation model. Shock. 2009d;31(5):515–520. doi: 10.1097/SHK.0b013e3181863689. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Matsumoto S, Noguchi T. High dose antithrombin III inhibits HMGB1 and improves endotoxin-induced acute lung injury in rats. Intensive Care Med. 2008d;34(2):361–367. doi: 10.1007/s00134-007-0887-5. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Matumoto S, Hidaka S, Noguchi T. Effects of an angiotensin-converting enzyme inhibitor on the inflammatory response in in vivo and in vitro models. Crit Care Med. 2009e;37(2):626–633. doi: 10.1097/CCM.0b013e3181958d91. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Noguchi T. Nafamostat mesilate inhibits the expression of HMGB1 in lipopolysaccharide-induced acute lung injury. J Anesth. 2007;21(2):164–170. doi: 10.1007/s00540-006-0468-8. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Shingu C, Matsumoto S, Uchida T, Nishida T, Mizunaga S, Saikawa T, Noguchi T. Danaparoid sodium attenuates the effects of heat stress. J Surg Res. 2011b;171(2):762–768. doi: 10.1016/j.jss.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Shingu C, Matsumoto S, Uchida T, Noguchi T. Antithrombin III prevents cerulein-induced acute pancreatitis in rats. Pancreas. 2009f;38(7):746–751. doi: 10.1097/MPA.0b013e3181aba9fa. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Shingu C, Matsumoto S, Uchida T, Noguchi T. High-dose antithrombin III prevents heat stroke by attenuating systemic inflammation in rats. Inflamm Res. 2010b;59(7):511–518. doi: 10.1007/s00011-009-0155-y. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Togo K, Noguchi T. A neutrophil elastase inhibitor, sivelestat, reduces lung injury following endotoxin-induced shock in rats by inhibiting HMGB1. Inflammation. 2008e;31(4):227–234. doi: 10.1007/s10753-008-9069-z. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Uchida T, Hasegawa A, Asai N, Noguchi T. Danaparoid sodium prevents cerulein-induced acute pancreatitis in rats. Shock. 2009g;32(1):94–99. doi: 10.1097/SHK.0b013e31818ec2c2. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Uchino T, Noguchi T. High mobility group box 1 induces a negative inotropic effect on the left ventricle in an isolated rat heart model of septic shock: a pilot study. Circ J. 2008f;72(6):1012–1017. doi: 10.1253/circj.72.1012. [DOI] [PubMed] [Google Scholar]
- Hah N, Kolkman A, Ruhl DD, Pijnappel WW, Heck AJ, Timmers HT, Kraus WL. A role for BAF57 in cell cycle-dependent transcriptional regulation by the SWI/SNF chromatin remodeling complex. Cancer Res. 2010;70(11):4402–4411. doi: 10.1158/0008-5472.CAN-09-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, Torikai M, Kuwazuru A, Tanaka M, Horai N, Fukuda T, Yamada S, Nagayama S, Hashiguchi K, Sunahara N, Fukuzaki K, Nagata R, Komiya S, Maruyama I, Abeyama K. Extracellular high mobility group box chromosomal protein 1 is a coupling factor for hypoxia and inflammation in arthritis. Arthritis Rheum. 2008;58(9):2675–2685. doi: 10.1002/art.23729. [DOI] [PubMed] [Google Scholar]
- Hamana K, Kawada K. Release of nucleosomes from nuclei by bleomycin-induced DNA strand scission. Biochem Int. 1989;18(5):971–979. [PubMed] [Google Scholar]
- Han J, Zhong J, Wei W, Wang Y, Huang Y, Yang P, Purohit S, Dong Z, Wang MH, She JX, Gong F, Stern DM, Wang CY. Extracellular high-mobility group box 1 acts as an innate immune mediator to enhance autoimmune progression and diabetes onset in NOD mice. Diabetes. 2008;57(8):2118–2127. doi: 10.2337/db07-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Kim K, Lee M. A high mobility group B-1 box A peptide combined with an artery wall binding peptide targets delivery of nucleic acids to smooth muscle cells. J Cell Biochem. 2009;107(1):163–170. doi: 10.1002/jcb.22112. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hanspal M, Hanspal JS. The association of erythroblasts with macrophages promotes erythroid proliferation and maturation: a 30-kD heparin-binding protein is involved in this contact. Blood. 1994;84(10):3494–3504. [PubMed] [Google Scholar]
- Hao J, Zhang YJ, Lv X, Xu N, Liu QJ, Zhao S, Feng XJ, Xing LL, Kang PP, Li GY, Liu SX. IFN-gamma induces lipogenesis in mouse mesangial cells via the JAK2/STAT1 pathway. Am J Physiol Cell Physiol. 2013;304(8):C760–C767. doi: 10.1152/ajpcell.00352.2012. [DOI] [PubMed] [Google Scholar]
- Haque A, Kunimoto F, Narahara H, Okawa M, Hinohara H, Kurabayashi M, Saito S. High mobility group box 1 levels in on and off-pump cardiac surgery patients. International heart journal. 2011;52(3):170–174. doi: 10.1536/ihj.52.170. [DOI] [PubMed] [Google Scholar]
- Haraba R, Suica VI, Uyy E, Ivan L, Antohe F. Hyperlipidemia stimulates the extracellular release of the nuclear high mobility group box 1 protein. Cell Tissue Res. 2011a;346(3):361–368. doi: 10.1007/s00441-011-1277-4. [DOI] [PubMed] [Google Scholar]
- Haraba R, Uyy E, Suica VI, Ivan L, Antohe F. Fluvastatin reduces the high mobility group box 1 protein expression in hyperlipidemia. Int J Cardiol. 2011b;150(1):105–107. doi: 10.1016/j.ijcard.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Harada O, Suga T, Suzuki T, Nakamoto K, Kobayashi M, Nomiyama T, Nadano D, Ohyama C, Fukuda MN, Nakayama J. The role of trophinin, an adhesion molecule unique to human trophoblasts, in progression of colorectal cancer. Int J Cancer. 2007;121(5):1072–1078. doi: 10.1002/ijc.22821. [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Takasaki K, Naito T, Hayakawa K, Katsurabayashi S, Mishima K, Iwasaki K, Fujiwara M. Cerebroprotective action of telmisartan by inhibition of macrophages/microglia expressing HMGB1 via a peroxisome proliferator-activated receptor gamma-dependent mechanism. Neurosci Lett. 2009;464(3):151–155. doi: 10.1016/j.neulet.2009.08.043. [DOI] [PubMed] [Google Scholar]
- Hardman CH, Broadhurst RW, Raine AR, Grasser KD, Thomas JO, Laue ED. Structure of the A-domain of HMG1 and its interaction with DNA as studied by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry. 1995;34(51):16596–16607. doi: 10.1021/bi00051a007. [DOI] [PubMed] [Google Scholar]
- Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118(1):183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley VR, Goodfellow PN. The biochemical role of SRY in sex determination. Mol Reprod Dev. 1994;39(2):184–193. doi: 10.1002/mrd.1080390211. [DOI] [PubMed] [Google Scholar]
- Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8(4):195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Whitehouse A. Kaposi's sarcoma-associated herpesvirus (KSHV) Rta and cellular HMGB1 proteins synergistically transactivate the KSHV ORF50 promoter. FEBS Lett. 2008;582(20):3080–3084. doi: 10.1016/j.febslet.2008.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartlerode AJ, Guan Y, Rajendran A, Ura K, Schotta G, Xie A, Shah JV, Scully R. Impact of histone H4 lysine 20 methylation on 53BP1 responses to chromosomal double strand breaks. PLoS ONE. 2012;7(11):e49211. doi: 10.1371/journal.pone.0049211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa A, Iwasaka H, Hagiwara S, Asai N, Nishida T, Noguchi T. Alternate day calorie restriction improves systemic inflammation in a mouse model of sepsis induced by cecal ligation and puncture. J Surg Res. 2012;174(1):136–141. doi: 10.1016/j.jss.2010.11.883. [DOI] [PubMed] [Google Scholar]
- Hasegawa A, Iwasaka H, Hagiwara S, Koga H, Hasegawa R, Kudo K, Kusaka J, Noguchi T. Anti-inflammatory effects of perioperative intensive insulin therapy during cardiac surgery with cardiopulmonary bypass. Surg Today. 2011;41(10):1385–1390. doi: 10.1007/s00595-010-4458-y. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Ishii J, Kitagawa F, Yamada S, Hattori K, Okumura M, Naruse H, Motoyama S, Matsui S, Tanaka I, Izawa H, Maruyama I, Nomura M, Ozaki Y. Circulating high-mobility group box 1 and cardiovascular mortality in unstable angina and non-ST-segment elevation myocardial infarction. Atherosclerosis. 2012;221(2):490–495. doi: 10.1016/j.atherosclerosis.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Hassa PO, Haenni SS, Elser M, Hottiger MO. Nuclear ADP-ribosylation reactions in mammalian cells: where are we today and where are we going? Microbiol Mol Biol Rev. 2006;70(3):789–829. doi: 10.1128/MMBR.00040-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatada T, Wada H, Nobori T, Okabayashi K, Maruyama K, Abe Y, Uemoto S, Yamada S, Maruyama I. Plasma concentrations and importance of High Mobility Group Box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thrombosis and haemostasis. 2005;94(5):975–979. doi: 10.1160/TH05-05-0316. [DOI] [PubMed] [Google Scholar]
- Hataji K, Watanabe T, Oowada S, Nagaya M, Kamibayashi M, Murakami E, Kawakami H, Ishiuchi A, Kumai T, Nakano H, Kobayashi S, Otsubo T. Effects of a calcium-channel blocker (CV159) on hepatic ischemia/reperfusion injury in rats: evaluation with selective NO/pO2 electrodes and an electron paramagnetic resonance spin-trapping method. Biol Pharm Bull. 2010;33(1):77–83. doi: 10.1248/bpb.33.77. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Irie K, Hazekawa M, Mishima S, Fujioka M, Orito K, Egashira N, Katsurabayashi S, Takasaki K, Iwasaki K, Fujiwara M. Cannabidiol prevents a post-ischemic injury progressively induced by cerebral ischemia via a high-mobility group box1-inhibiting mechanism. Neuropharmacology. 2008a;55(8):1280–1286. doi: 10.1016/j.neuropharm.2008.06.040. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Hazekawa M, Mishima S, Fujioka M, Orito K, Egashira N, Iwasaki K, Fujiwara M. Delayed treatment with minocycline ameliorates neurologic impairment through activated microglia expressing a high-mobility group box1-inhibiting mechanism. Stroke. 2008b;39(3):951–958. doi: 10.1161/STROKEAHA.107.495820. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Nakano T, Irie K, Higuchi S, Fujioka M, Orito K, Iwasaki K, Jin G, Lo EH, Mishima K, Fujiwara M. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2010;30(4):871–882. doi: 10.1038/jcbfm.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Nagafuchi H, Ito I, Hirota K, Yoshida M, Ozaki S. Lupus antibodies to the HMGB1 chromosomal protein: epitope mapping and association with disease activity. Mod Rheumatol. 2009;19(3):283–292. doi: 10.1007/s10165-009-0151-7. [DOI] [PubMed] [Google Scholar]
- He GZ, Zhou KG, Zhang R, Wang YK, Chen XF. Impact of intestinal ischemia/reperfusion and lymph drainage on distant organs in rats. World J Gastroenterol. 2012a;18(48):7271–7278. doi: 10.3748/wjg.v18.i48.7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Kubo H, Morimoto K, Fujino N, Suzuki T, Takahasi T, Yamada M, Yamaya M, Maekawa T, Yamamoto Y, Yamamoto H. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 2011;12(4):358–364. doi: 10.1038/embor.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Liang CH, Lippard SJ. Steroid hormones induce HMG1 overexpression and sensitize breast cancer cells to cisplatin and carboplatin. Proc Natl Acad Sci U S A. 2000;97(11):5768–5772. doi: 10.1073/pnas.100108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, You H, Li XM, Liu TH, Wang P, Wang BE. HMGB1 Promotes the Synthesis of Pro-IL-1beta and Pro-IL-18 by Activation of p38 MAPK and NF-kappaB Through Receptors for Advanced Glycation End-products in Macrophages. Asian Pac J Cancer Prev. 2012b;13(4):1365–1370. doi: 10.7314/apjcp.2012.13.4.1365. [DOI] [PubMed] [Google Scholar]
- He W, Tang B, Yang D, Li Y, Song W, Cheang T, Chen X, Li Y, Chen L, Zhan W, Li W, He Y. Double-positive expression of high-mobility group box 1 and vascular endothelial growth factor C indicates a poorer prognosis in gastric cancer patients. World journal of surgical oncology. 2013;11:161. doi: 10.1186/1477-7819-11-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zha J, Wang Y, Liu W, Yang X, Yu P. Tissue damage-associated 'danger signals' influence T cell responses that promote the progression of pre-neoplasia to cancer. Cancer Res. 2012c doi: 10.1158/0008-5472.CAN-12-2704. [DOI] [PubMed] [Google Scholar]
- He Z, Shotorbani SS, Jiao Z, Su Z, Tong J, Liu Y, Shen P, Ma J, Gao J, Wang T, Xia S, Shao Q, Wang S, Xu H. HMGB1 promotes the differentiation of Th17 via up-regulating TLR2 and IL-23 of CD14+ monocytes from patients with rheumatoid arthritis. Scand J Immunol. 2012d;76(5):483–490. doi: 10.1111/j.1365-3083.2012.02759.x. [DOI] [PubMed] [Google Scholar]
- Hegyi P, Pandol S, Venglovecz V, Rakonczay Z., Jr The acinar-ductal tango in the pathogenesis of acute pancreatitis. Gut. 2011;60(4):544–552. doi: 10.1136/gut.2010.218461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans J, Buller NV, Hoff E, Dihal AA, van der Poll T, van Zoelen MA, Bierhaus A, Biemond I, Hardwick JC, Hommes DW, Muncan V, van den Brink GR. Rage signalling promotes intestinal tumourigenesis. Oncogene. 2012 doi: 10.1038/onc.2012.119. [DOI] [PubMed] [Google Scholar]
- Henes FO, Chen Y, Bley TA, Fabel M, Both M, Herrmann K, Csernok E, Gross WL, Moosig F. Correlation of serum level of high mobility group box 1 with the burden of granulomatous inflammation in granulomatosis with polyangiitis (Wegener's) Annals of the rheumatic diseases. 2011;70(11):1926–1929. doi: 10.1136/ard.2010.146456. [DOI] [PubMed] [Google Scholar]
- Herter J, Zarbock A. Integrin Regulation during Leukocyte Recruitment. J Immunol. 2013;190(9):4451–4457. doi: 10.4049/jimmunol.1203179. [DOI] [PubMed] [Google Scholar]
- Herzog C, Lorenz A, Gillmann HJ, Chowdhury A, Larmann J, Harendza T, Echtermeyer F, Muller M, Schmitz M, Stypmann J, Seidler DG, Damm M, Stehr SN, Koch T, Wollert KC, Conway EM, Theilmeier G. Thrombomodulin's lectin-like domain reduces myocardial damage by interfering with HMGB1-mediated TLR2 signalling. Cardiovasc Res. 2014;101(3):400–410. doi: 10.1093/cvr/cvt275. [DOI] [PubMed] [Google Scholar]
- Hidaka S, Iwasaka H, Hagiwara S, Noguchi T. Gabexate mesilate inhibits the expression of HMGB1 in lipopolysaccharide-induced acute lung injury. J Surg Res. 2011;165(1):142–150. doi: 10.1016/j.jss.2009.05.039. [DOI] [PubMed] [Google Scholar]
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- Higgins SJ, Xing K, Kim H, Kain DC, Wang F, Dhabangi A, Musoke C, Cserti-Gazdewich CM, Tracey KJ, Kain KC, Liles WC. Systemic release of high mobility group box 1 (HMGB1) protein is associated with severe and fatal Plasmodium falciparum malaria. Malaria journal. 2013;12:105. doi: 10.1186/1475-2875-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DA, Reeves R. Competition between HMG-I(Y), HMG-1 and histone H1 on four-way junction DNA. Nucleic Acids Res. 1997;25(17):3523–3531. doi: 10.1093/nar/25.17.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillion J, Dhara S, Sumter TF, Mukherjee M, Di Cello F, Belton A, Turkson J, Jaganathan S, Cheng L, Ye Z, Jove R, Aplan P, Lin YW, Wertzler K, Reeves R, Elbahlouh O, Kowalski J, Bhattacharya R, Resar LM. The high-mobility group A1a/signal transducer and activator of transcription-3 axis: an achilles heel for hematopoietic malignancies? Cancer Res. 2008;68(24):10121–10127. doi: 10.1158/0008-5472.CAN-08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano H, Yoshioka T, Yunoue S, Fujio S, Yonezawa H, Niiro T, Habu M, Oyoshi T, Sugata S, Kamezawa T, Arimura H, Hanaya R, Tokimura H, Tokudome M, Arita K. TLR4, IL-6, IL-18, MyD88 and HMGB1 are highly expressed in intracranial inflammatory lesions and the IgG4/IgG ratio correlates with TLR4 and IL-6. Neuropathology : official journal of the Japanese Society of Neuropathology. 2012;32(6):628–637. doi: 10.1111/j.1440-1789.2012.01310.x. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Takeuchi H, Suda K, Hagiwara T, Miyasho T, Kawamura Y, Yamada S, Oyama T, Takahashi T, Wada N, Saikawa Y, Ichihara A, Kitagawa Y. (Pro)renin receptor blocker improves survival of rats with sepsis. J Surg Res. 2014;186(1):269–277. doi: 10.1016/j.jss.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Kurobe H, Higashida M, Fukuda D, Shimabukuro M, Tanaka K, Higashikuni Y, Kitagawa T, Sata M. HMGB1 plays a critical role in vascular inflammation and lesion formation via toll-like receptor 9. Atherosclerosis. 2013;231(2):227–233. doi: 10.1016/j.atherosclerosis.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Hoarau JJ, Krejbich-Trotot P, Jaffar-Bandjee MC, Das T, Thon-Hon GV, Kumar S, Neal JW, Gasque P. Activation and control of CNS innate immune responses in health and diseases: a balancing act finely tuned by neuroimmune regulators (NIReg) CNS Neurol Disord Drug Targets. 2011;10(1):25–43. doi: 10.2174/187152711794488601. [DOI] [PubMed] [Google Scholar]
- Hock R, Furusawa T, Ueda T, Bustin M. HMG chromosomal proteins in development and disease. Trends Cell Biol. 2007;17(2):72–79. doi: 10.1016/j.tcb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JS, Locker D, Villani G, Leng M. HMG1 protein inhibits the translesion synthesis of the major DNA cisplatin adduct by cell extracts. J Mol Biol. 1997;270(4):539–543. doi: 10.1006/jmbi.1997.1143. [DOI] [PubMed] [Google Scholar]
- Hohne C, Wenzel M, Angele B, Hammerschmidt S, Hacker H, Klein M, Bierhaus A, Sperandio M, Pfister HW, Koedel U. High mobility group box 1 prolongs inflammation and worsens disease in pneumococcal meningitis. Brain. 2013;136(Pt 6):1746–1759. doi: 10.1093/brain/awt064. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S, Nagel D, Schalhorn A, Heinemann V, Wilkowski R, von Pawel J, Raith H, Feldmann K, Kremer AE, Muller S, Geiger S, Hamann GF, Seidel D, Stieber P. Clinical relevance of circulating nucleosomes in cancer. Ann N Y Acad Sci. 2008;1137:180–189. doi: 10.1196/annals.1448.012. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S, Stieber P. Clinical use of circulating nucleosomes. Crit Rev Clin Lab Sci. 2009;46(1):1–24. doi: 10.1080/10408360802485875. [DOI] [PubMed] [Google Scholar]
- Holmlund U, Wahamaa H, Bachmayer N, Bremme K, Sverremark-Ekstrom E, Palmblad K. The novel inflammatory cytokine high mobility group box protein 1 (HMGB1) is expressed by human term placenta. Immunology. 2007;122(3):430–437. doi: 10.1111/j.1365-2567.2007.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SM, Cho JS, Um JY, Shin JM, Park IH, Lee SH, Lee SH, Lee HM. Increased expression of high-mobility group protein B1 in chronic rhinosinusitis. American journal of rhinology & allergy. 2013;27(4):278–282. doi: 10.2500/ajra.2013.27.3909. [DOI] [PubMed] [Google Scholar]
- Hong SM, Park JY, Hruban RH, Goggins M. Molecular signatures of pancreatic cancer. Archives of pathology & laboratory medicine. 2011;135(6):716–727. doi: 10.5858/2010-0566-ra.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe G, Talcott KE, Bhattacharya SK, Crabb JW, Sears JE. Molecular basis for the redox control of nuclear transport of the structural chromatin protein Hmgb1. Exp Cell Res. 2006;312(18):3526–3538. doi: 10.1016/j.yexcr.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Hoshina T, Kusuhara K, Ikeda K, Mizuno Y, Saito M, Hara T. High mobility group box 1 (HMGB1) and macrophage migration inhibitory factor (MIF) in Kawasaki disease. Scand J Rheumatol. 2008;37(6):445–449. doi: 10.1080/03009740802144143. [DOI] [PubMed] [Google Scholar]
- Hou C, Zhao H, Liu L, Li W, Zhou X, Lv Y, Shen X, Liang Z, Cai S, Zou F. High mobility group protein B1 (HMGB1) in Asthma: comparison of patients with chronic obstructive pulmonary disease and healthy controls. Mol Med. 2011a;17(7–8):807–815. doi: 10.2119/molmed.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou CH, Fong YC, Tang CH. HMGB-1 induces IL-6 production in human synovial fibroblasts through c-Src, Akt and NF-kappaB pathways. J Cell Physiol. 2011b;226(8):2006–2015. doi: 10.1002/jcp.22541. [DOI] [PubMed] [Google Scholar]
- Hou W, Zhang Q, Yan Z, Chen R, Zeh Iii HJ, Kang R, Lotze MT, Tang D. Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell Death Dis. 2013;4:e966. doi: 10.1038/cddis.2013.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hreggvidsdottir HS, Lundberg AM, Aveberger AC, Klevenvall L, Andersson U, Harris HE. High mobility group box protein 1 (HMGB1)-partner molecule complexes enhance cytokine production by signaling through the partner molecule receptor. Mol Med. 2012;18:224–230. doi: 10.2119/molmed.2011.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hreggvidsdottir HS, Ostberg T, Wahamaa H, Schierbeck H, Aveberger AC, Klevenvall L, Palmblad K, Ottosson L, Andersson U, Harris HE. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86(3):655–662. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Adsay NV. Molecular classification of neoplasms of the pancreas. Hum Pathol. 2009;40(5):612–623. doi: 10.1016/j.humpath.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Hu D, Sun S, Zhu B, Mei Z, Wang L, Zhu S, Zhao W. Effects of coupled plasma filtration adsorption on septic patients with multiple organ dysfunction syndrome. Renal failure. 2012a;34(7):834–839. doi: 10.3109/0886022X.2012.684553. [DOI] [PubMed] [Google Scholar]
- Hu G, Zhang Y, Jiang H, Hu X. Exendin-4 attenuates myocardial ischemia and reperfusion injury by inhibiting high mobility group box 1 protein expression. Cardiol J. 2013;20(6):600–604. doi: 10.5603/CJ.2013.0159. [DOI] [PubMed] [Google Scholar]
- Hu X, Cui B, Zhou X, Xu C, Lu Z, Jiang H. Ethyl pyruvate reduces myocardial ischemia and reperfusion injury by inhibiting high mobility group box 1 protein in rats. Mol Biol Rep. 2012b;39(1):227–231. doi: 10.1007/s11033-011-0730-5. [DOI] [PubMed] [Google Scholar]
- Hu X, Jiang H, Bai Q, Zhou X, Xu C, Lu Z, Cui B, Wen H. Increased serum HMGB1 is related to the severity of coronary artery stenosis. Clinica chimica acta; international journal of clinical chemistry. 2009;406(1–2):139–142. doi: 10.1016/j.cca.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhou X, He B, Xu C, Wu L, Cui B, Wen H, Lu Z, Jiang H. Minocycline protects against myocardial ischemia and reperfusion injury by inhibiting high mobility group box 1 protein in rats. Eur J Pharmacol. 2010;638(1–3):84–89. doi: 10.1016/j.ejphar.2010.03.059. [DOI] [PubMed] [Google Scholar]
- Hu YM, Pai MH, Yeh CL, Hou YC, Yeh SL. Glutamine administration ameliorates sepsis-induced kidney injury by downregulating the high-mobility group box protein-1-mediated pathway in mice. Am J Physiol Renal Physiol. 2012c;302(1):F150–F158. doi: 10.1152/ajprenal.00246.2011. [DOI] [PubMed] [Google Scholar]
- Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, Liao X, Billiar T, Xu J, Esmon CT, Tsung A. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology. 2011a;54(3):999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Nace GW, McDonald KA, Tai S, Klune JR, Rosborough BR, Ding Q, Loughran P, Zhu X, Beer-Stolz D, Chang EB, Billiar T, Tsung A. Hepatocyte specific HMGB1 deletion worsens the injury in liver ischemia/reperfusion: A role for intracellular HMGB1 in cellular protection. Hepatology. 2013a doi: 10.1002/hep.26976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Nace GW, McDonald KA, Tai S, Klune JR, Rosborough BR, Ding Q, Loughran P, Zhu X, Beer-Stolz D, Chang EB, Billiar T, Tsung A. Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: a role for intracellular high-mobility group box 1 in cellular protection. Hepatology. 2014;59(5):1984–1997. doi: 10.1002/hep.26976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Liu B, Yang C, Chen H, Eunice D, Yuan Z. Acute hyperglycemia worsens ischemic stroke-induced brain damage via high mobility group box-1 in rats. Brain Res. 2013b;1535:148–155. doi: 10.1016/j.brainres.2013.08.057. [DOI] [PubMed] [Google Scholar]
- Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R, Vernon P, Cao L, Tang D. HMGB1 promotes drug resistance in osteosarcoma. Cancer Res. 2012a;72(1):230–238. doi: 10.1158/0008-5472.CAN-11-2001. [DOI] [PubMed] [Google Scholar]
- Huang JC, Zamble DB, Reardon JT, Lippard SJ, Sancar A. HMG-domain proteins specifically inhibit the repair of the major DNA adduct of the anticancer drug cisplatin by human excision nuclease. Proc Natl Acad Sci U S A. 1994;91(22):10394–10398. doi: 10.1073/pnas.91.22.10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JM, Hu J, Chen N, Hu ML. Relationship between plasma high-mobility group box-1 levels and clinical outcomes of ischemic stroke. Journal of critical care. 2013c;28(5):792–797. doi: 10.1016/j.jcrc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Huang LF, Yao YM, Dong N, Yu Y, He LX, Sheng ZY. Association of high mobility group box-1 protein levels with sepsis and outcome of severely burned patients. Cytokine. 2011b;53(1):29–34. doi: 10.1016/j.cyto.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Huang LF, Yao YM, Li JF, Zhang SW, Li WX, Dong N, Yu Y, Sheng ZY. The effect of Astragaloside IV on immune function of regulatory T cell mediated by high mobility group box 1 protein in vitro. Fitoterapia. 2012b;83(8):1514–1522. doi: 10.1016/j.fitote.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Huang QX, Wang GB, Sun NF, Wang CY. [Inhibitory effects of high mobility group box 1 antisense nucleotide on invasion of human pancreatic cancer cell line PCNA-1] Ai Zheng. 2004;23(9):1036–1040. [PubMed] [Google Scholar]
- Huang W, Liu Y, Li L, Zhang R, Liu W, Wu J, Mao E, Tang Y. HMGB1 increases permeability of the endothelial cell monolayer via RAGE and Src family tyrosine kinase pathways. Inflammation. 2012c;35(1):350–362. doi: 10.1007/s10753-011-9325-5. [DOI] [PubMed] [Google Scholar]
- Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51(2):119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Huang Y, Xie K, Li J, Xu N, Gong G, Wang G, Yu Y, Dong H, Xiong L. Beneficial effects of hydrogen gas against spinal cord ischemia-reperfusion injury in rabbits. Brain Res. 2011c;1378:125–136. doi: 10.1016/j.brainres.2010.12.071. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yin H, Han J, Huang B, Xu J, Zheng F, Tan Z, Fang M, Rui L, Chen D, Wang S, Zheng X, Wang CY, Gong F. Extracellular hmgb1 functions as an innate immune-mediator implicated in murine cardiac allograft acute rejection. Am J Transplant. 2007;7(4):799–808. doi: 10.1111/j.1600-6143.2007.01734.x. [DOI] [PubMed] [Google Scholar]
- Huang YH, Wang PW, Tiao MM, Chou MH, Du YY, Huang CC, Chuang JH. Glucocorticoid modulates high-mobility group box 1 expression and Toll-like receptor activation in obstructive jaundice. J Surg Res. 2011d;170(1):e47–e55. doi: 10.1016/j.jss.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Huebener P, Gwak GY, Pradere JP, Quinzii CM, Friedman R, Lin CS, Trent CM, Mederacke I, Zhao E, Dapito DH, Lin Y, Goldberg IJ, Czaja MJ, Schwabe RF. High-mobility group box 1 is dispensable for autophagy, mitochondrial quality control, and organ function in vivo. Cell Metab. 2014;19(3):539–547. doi: 10.1016/j.cmet.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Yao Y, Wang S, Yu Y, Dong N, Li H, Sheng Z. Inhibition of Janus kinase 2 and signal transduction and activator of transcription 3 protect against cecal ligation and puncture-induced multiple organ damage and mortality. J Trauma. 2009;66(3):859–865. doi: 10.1097/TA.0b013e318164d05f. [DOI] [PubMed] [Google Scholar]
- Hung YH, Chen LM, Yang JY, Yang WY. Spatiotemporally controlled induction of autophagy-mediated lysosome turnover. Nature communications. 2013;4:2111. doi: 10.1038/ncomms3111. [DOI] [PubMed] [Google Scholar]
- Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, Ashok M, Goldstein RS, Chavan S, Pavlov VA, Metz CN, Yang H, Czura CJ, Wang H, Tracey KJ. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35(12):2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- Huston JM, Wang H, Ochani M, Ochani K, Rosas-Ballina M, Gallowitsch-Puerta M, Ashok M, Yang L, Tracey KJ, Yang H. Splenectomy protects against sepsis lethality and reduces serum HMGB1 levels. J Immunol. 2008;181(5):3535–3539. doi: 10.4049/jimmunol.181.5.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison ER, Kawamoto EM, Taub DD, Lal A, Abdelmohsen K, Zhang Y, Wood WH, 3rd, Lehrmann E, Camandola S, Becker KG, Gorospe M, Mattson MP. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia. 2013;61(7):1018–1028. doi: 10.1002/glia.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutten S, Kehlenbach RH. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol. 2007;17(4):193–201. doi: 10.1016/j.tcb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002;62(16):4805–4811. [PubMed] [Google Scholar]
- Hwa JS, Jin YC, Lee YS, Ko YS, Kim YM, Shi LY, Kim HJ, Lee JH, Ngoc TM, Bae KH, Kim YS, Chang KC. 2-methoxycinnamaldehyde from Cinnamomum cassia reduces rat myocardial ischemia and reperfusion injury in vivo due to HO-1 induction. J Ethnopharmacol. 2012;139(2):605–615. doi: 10.1016/j.jep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Hwaiz R, Hasan Z, Rahman M, Zhang S, Palani K, Syk I, Jeppsson B, Thorlacius H. Rac1 signaling regulates sepsis-induced pathologic inflammation in the lung via attenuation of Mac-1 expression and CXC chemokine formation. J Surg Res. 2013;183(2):798–807. doi: 10.1016/j.jss.2013.02.045. [DOI] [PubMed] [Google Scholar]
- Hwang CS, Liu GT, Chang MD, Liao IL, Chang HT. Elevated serum autoantibody against high mobility group box 1 as a potent surrogate biomarker for amyotrophic lateral sclerosis. Neurobiol Dis. 2013;58:13–18. doi: 10.1016/j.nbd.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Hwang JS, Kang ES, Ham SA, Yoo T, Lee H, Paek KS, Park C, Kim JH, Lim DS, Seo HG. Activation of peroxisome proliferator-activated receptor gamma by rosiglitazone inhibits lipopolysaccharide-induced release of high mobility group box 1. Mediators Inflamm. 2012;2012:352–807. doi: 10.1155/2012/352807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2(4):277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009;122(Pt 18):3203–3208. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]
- Ilmakunnas M, Tukiainen EM, Rouhiainen A, Rauvala H, Arola J, Nordin A, Makisalo H, Hockerstedt K, Isoniemi H. High mobility group box 1 protein as a marker of hepatocellular injury in human liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008;14(10):1517–1525. doi: 10.1002/lt.21573. [DOI] [PubMed] [Google Scholar]
- Imamura T, Izumi H, Nagatani G, Ise T, Nomoto M, Iwamoto Y, Kohno K. Interaction with p53 enhances binding of cisplatin-modified DNA by high mobility group 1 protein. J Biol Chem. 2001;276(10):7534–7540. doi: 10.1074/jbc.M008143200. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kawahara K, Biswas KK, Ando K, Mitsudo K, Nobuyoshi M, Maruyama I. HMGB1 expression by activated vascular smooth muscle cells in advanced human atherosclerosis plaques. Cardiovasc Pathol. 2007;16(3):136–143. doi: 10.1016/j.carpath.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Iori V, Maroso M, Rizzi M, Iyer AM, Vertemara R, Carli M, Agresti A, Antonelli A, Bianchi ME, Aronica E, Ravizza T, Vezzani A. Receptor for Advanced Glycation Endproducts is upregulated in temporal lobe epilepsy and contributes to experimental seizures. Neurobiol Dis. 2013;58:102–114. doi: 10.1016/j.nbd.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Bidney DL, Reeck GR, Neihart NK, Bustin M. High mobility group chromosomal proteins isolated from muclei and cytosol of cultured hepatoma cells are similar. Biochemistry. 1980;19(19):4466–4471. doi: 10.1021/bi00560a013. [DOI] [PubMed] [Google Scholar]
- Ito I, Fukazawa J, Yoshida M. Post-translational methylation of high mobility group box 1 (HMGB1) causes its cytoplasmic localization in neutrophils. J Biol Chem. 2007a;282(22):16336–16344. doi: 10.1074/jbc.M608467200. [DOI] [PubMed] [Google Scholar]
- Ito N, DeMarco RA, Mailliard RB, Han J, Rabinowich H, Kalinski P, Stolz DB, Zeh HJ, 3rd, Lotze MT. Cytolytic cells induce HMGB1 release from melanoma cell lines. J Leukoc Biol. 2007b;81(1):75–83. doi: 10.1189/jlb.0306169. [DOI] [PubMed] [Google Scholar]
- Ito T, Kawahara K, Nakamura T, Yamada S, Abeyama K, Hashiguchi T, Maruyama I. High-mobility group box 1 protein promotes development of microvascular thrombosis in rats. J Thromb Haemost. 2007c;5(1):109–116. doi: 10.1111/j.1538-7836.2006.02255.x. [DOI] [PubMed] [Google Scholar]
- Ito T, Kawahara K, Okamoto K, Yamada S, Yasuda M, Imaizumi H, Nawa Y, Meng X, Shrestha B, Hashiguchi T, Maruyama I. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol. 2008;28(10):1825–1830. doi: 10.1161/ATVBAHA.107.150631. [DOI] [PubMed] [Google Scholar]
- Ito Y, Bustin M. Immunohistochemical localization of the nucleosome-binding protein HMGN3 in mouse brain. J Histochem Cytochem. 2002;50(9):1273–1275. doi: 10.1177/002215540205000914. [DOI] [PubMed] [Google Scholar]
- Ito Y, Torii Y, Ohta R, Imai M, Hara S, Kawano Y, Matsubayashi T, Inui A, Yoshikawa T, Nishimura N, Ozaki T, Morishima T, Kimura H. Increased levels of cytokines and high-mobility group box 1 are associated with the development of severe pneumonia, but not acute encephalopathy, in 2009 H1N1 influenza-infected children. Cytokine. 2011;56(2):180–187. doi: 10.1016/j.cyto.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Itoh T, Iwahashi S, Kanak MA, Shimoda M, Takita M, Chujo D, Tamura Y, Rahman AM, Chung WY, Onaca N, Coates PT, Dennison AR, Naziruddin B, Levy MF, Matsumoto S. Elevation of High-Mobility Group Box 1 after Clinical Autologous Islet Transplantation and Its Inverse Correlation with Outcomes. Cell Transplant. 2012a doi: 10.3727/096368912X658980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Sugimoto K, Takita M, Shimoda M, Chujo D, SoRelle JA, Naziruddin B, Levy MF, Matsumoto S. Low temperature condition prevents hypoxia-induced islet cell damage and HMGB1 release in a mouse model. Cell Transplant. 2012b;21(7):1361–1370. doi: 10.3727/096368912X637514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Takita M, SoRelle JA, Shimoda M, Sugimoto K, Chujo D, Qin H, Naziruddin B, Levy MF, Matsumoto S. Correlation of released HMGB1 levels with the degree of islet damage in mice and humans and with the outcomes of islet transplantation in mice. Cell Transplant. 2012c;21(7):1371–1381. doi: 10.3727/096368912X640592. [DOI] [PubMed] [Google Scholar]
- Itou J, Taniguchi N, Oishi I, Kawakami H, Lotz M, Kawakami Y. HMGB factors are required for posterior digit development through integrating signaling pathway activities. Dev Dyn. 2011;240(5):1151–1162. doi: 10.1002/dvdy.22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, Chu WM. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110(6):1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivics Z, Hackett PB, Plasterk RH, Izsvak Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91(4):501–510. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- Ivics Z, Kaufman CD, Zayed H, Miskey C, Walisko O, Izsvak Z. The Sleeping Beauty transposable element: evolution, regulation and genetic applications. Curr Issues Mol Biol. 2004;6(1):43–55. [PubMed] [Google Scholar]
- Iwata Y, Furuichi K, Sakai N, Yamauchi H, Shinozaki Y, Zhou H, Kurokawa Y, Toyama T, Kitajima S, Okumura T, Yamada S, Maruyama I, Matsushima K, Kaneko S, Wada T. Dendritic cells contribute to autoimmune kidney injury in MRL-Faslpr mice. J Rheumatol. 2009;36(2):306–314. doi: 10.3899/jrheum.080318. [DOI] [PubMed] [Google Scholar]
- Izuishi K, Tsung A, Jeyabalan G, Critchlow ND, Li J, Tracey KJ, Demarco RA, Lotze MT, Fink MP, Geller DA, Billiar TR. Cutting edge: high-mobility group box 1 preconditioning protects against liver ischemia-reperfusion injury. J Immunol. 2006;176(12):7154–7158. doi: 10.4049/jimmunol.176.12.7154. [DOI] [PubMed] [Google Scholar]
- Izuta S, Ueki M, Ueno M, Nishina K, Shiozawa S, Maekawa N. T-5224, a selective inhibitor of c-Fos/activator protein-1, attenuates lipopolysaccharide-induced liver injury in mice. Biotechnol Lett. 2012;34(12):2175–2182. doi: 10.1007/s10529-012-1022-4. [DOI] [PubMed] [Google Scholar]
- Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23(5):687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- Jain A, Akanchha S, Rajeswari MR. Stabilization of purine motif DNA triplex by a tetrapeptide from the binding domain of HMGBI protein. Biochimie. 2005;87(8):781–790. doi: 10.1016/j.biochi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Kim YM, Tsoyi K, Park EJ, Lee YS, Kim HJ, Lee JH, Joe Y, Chung HT, Chang KC. Ethyl pyruvate induces heme oxygenase-1 through p38 mitogen-activated protein kinase activation by depletion of glutathione in RAW 264.7 cells and improves survival in septic animals. Antioxid Redox Signal. 2012;17(6):878–889. doi: 10.1089/ars.2011.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaouen S, de Koning L, Gaillard C, Muselikova-Polanska E, Stros M, Strauss F. Determinants of specific binding of HMGB1 protein to hemicatenated DNA loops. J Mol Biol. 2005;353(4):822–837. doi: 10.1016/j.jmb.2005.08.073. [DOI] [PubMed] [Google Scholar]
- Jaulmes A, Thierry S, Janvier B, Raymondjean M, Marechal V. Activation of sPLA2-IIA and PGE2 production by high mobility group protein B1 in vascular smooth muscle cells sensitized by IL-1beta. Faseb J. 2006;20(10):1727–1729. doi: 10.1096/fj.05-5514fje. [DOI] [PubMed] [Google Scholar]
- Javaherian K, Liu JF, Wang JC. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science. 1978;199(4335):1345–1346. doi: 10.1126/science.628842. [DOI] [PubMed] [Google Scholar]
- Javaherian K, Sadeghi M, Liu LF. Nonhistone proteins HMG1 and HMG2 unwind DNA double helix. Nucleic Acids Res. 1979;6(11):3569–3580. doi: 10.1093/nar/6.11.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman L, Moorthy NC, Murthy KG, Manley JL, Bustin M, Prives C. High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev. 1998;12(4):462–472. doi: 10.1101/gad.12.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon HM, Lee SY, Ju MK, Kim CH, Park HG, Kang HS. Early growth response 1 regulates glucose deprivation-induced necrosis. Oncol Rep. 2013;29(2):669–675. doi: 10.3892/or.2012.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji MH, Zhu XL, Liu FF, Li GM, Tian M, Wu J, Fan YX, Li N, Yang JJ. Alpha 2A-adrenoreceptor blockade improves sepsis-induced acute lung injury accompanied with depressed high mobility group box-1 levels in rats. Cytokine. 2012a;60(3):639–645. doi: 10.1016/j.cyto.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Ji SQ, Yao L, Zhang XY, Li XS, Zhou LQ. Knockdown of the nucleosome binding protein 1 inhibits the growth and invasion of clear cell renal cell carcinoma cells in vitro and in vivo. J Exp Clin Cancer Res. 2012b;31:22. doi: 10.1186/1756-9966-31-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Little AJ, Banerjee JK, Hao B, Oltz EM, Krangel MS, Schatz DG. Promoters, enhancers, and transcription target RAG1 binding during V(D)J recombination. J Exp Med. 2010;207(13):2809–2816. doi: 10.1084/jem.20101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Clear A, Liu FT, Matthews J, Uddin N, McCarthy A, Hoxha E, Durance C, Iqbal S, Gribben JG. Extracellular HMGB1 promotes differentiation of nurse-like cells in chronic lymphocytic leukemia. Blood. 2014a doi: 10.1182/blood-2013-10-529610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Xue R, Liu G, Li L, Yang J, Pi G, Ma S, Kan Q. HMGB1 Is Involved in the Protective Effect of the PPAR alpha Agonist Fenofibrate against Cardiac Hypertrophy. PPAR Res. 2014b;2014:541394. doi: 10.1155/2014/541394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Liao R. The paradoxical role of inflammation in cardiac repair and regeneration. J Cardiovasc Transl Res. 2010;3(4):410–416. doi: 10.1007/s12265-010-9193-7. [DOI] [PubMed] [Google Scholar]
- Jiang G, Sun D, Yang H, Lu Q, Kaplan HJ, Shao H. HMGB1 is an early and critical mediator in an animal model of uveitis induced by IRBP-specific T cells. J Leukoc Biol. 2013 doi: 10.1189/jlb.0613337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Fu F, Tian J, Zhu H, Hou J. Curculigoside A attenuates experimental cerebral ischemia injury in vitro and vivo. Neuroscience. 2011;192:572–579. doi: 10.1016/j.neuroscience.2011.06.079. [DOI] [PubMed] [Google Scholar]
- Jiang W, Li J, Gallowitsch-Puerta M, Tracey KJ, Pisetsky DS. The effects of CpG DNA on HMGB1 release by murine macrophage cell lines. J Leukoc Biol. 2005;78(4):930–936. doi: 10.1189/jlb.0405208. [DOI] [PubMed] [Google Scholar]
- Jiang W, Pisetsky DS. The role of IFN-alpha and nitric oxide in the release of HMGB1 by RAW 264.7 cells stimulated with polyinosinic-polycytidylic acid or lipopolysaccharide. J Immunol. 2006;177(5):3337–3343. doi: 10.4049/jimmunol.177.5.3337. [DOI] [PubMed] [Google Scholar]
- Jiang W, Pisetsky DS. Expression of high mobility group protein 1 in the sera of patients and mice with systemic lupus erythematosus. Ann Rheum Dis. 2008a;67(5):727–728. doi: 10.1136/ard.2007.074484. [DOI] [PubMed] [Google Scholar]
- Jiang W, Pisetsky DS. The induction of HMGB1 release from RAW 264.7 cells by transfected DNA. Mol Immunol. 2008b;45(7):2038–2044. doi: 10.1016/j.molimm.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Wang Z, Li X, Fan X, Duan Y. High-mobility group box 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Pathol Oncol Res. 2012a;18(2):293–298. doi: 10.1007/s12253-011-9442-3. [DOI] [PubMed] [Google Scholar]
- Jiang W, Wang Z, Li X, Li J, Huang Y, Fan X, Duan Y. Reduced high-mobility group box 1 expression induced by RNA interference inhibits the bioactivity of hepatocellular carcinoma cell line HCCLM3. Dig Dis Sci. 2012b;57(1):92–98. doi: 10.1007/s10620-011-1944-z. [DOI] [PubMed] [Google Scholar]
- Jiang WL, Fu FH, Xu BM, Tian JW, Zhu HB, Jian H. Cardioprotection with forsythoside B in rat myocardial ischemia-reperfusion injury: relation to inflammation response. Phytomedicine. 2010;17(8–9):635–639. doi: 10.1016/j.phymed.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Jiang WL, Xu Y, Zhang SP, Zhu HB, Hou J. Tricin 7-glucoside protects against experimental cerebral ischemia by reduction of NF-kappaB and HMGB1 expression. Eur J Pharm Sci. 2012c;45(1–2):50–57. doi: 10.1016/j.ejps.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Jiang WL, Yong X, Zhang SP, Zhu HB, Jian H. Forsythoside B protects against experimental sepsis by modulating inflammatory factors. Phytother Res. 2012d;26(7):981–987. doi: 10.1002/ptr.3668. [DOI] [PubMed] [Google Scholar]
- Jiang WL, Zhang SP, Zhu HB, Hou J. Cardioprotection of Asperosaponin X on experimental myocardial ischemia injury. Int J Cardiol. 2012e;155(3):430–436. doi: 10.1016/j.ijcard.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Wang HC, Fan SJ. Growth suppression and radiosensitivity increase by HMGB1 in breast cancer. Acta Pharmacol Sin. 2007;28(12):1957–1967. doi: 10.1111/j.1745-7254.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- Jin D, Wu Y, Zhao L, Guo J, Zhang K, Chen Z. Atorvastatin reduces serum HMGB1 levels in patients with hyperlipidemia. Experimental and therapeutic medicine. 2012;4(6):1124–1126. doi: 10.3892/etm.2012.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YC, Kim SW, Cheng F, Shin JH, Park JK, Lee S, Lee JE, Han PL, Lee M, Kim KK, Choi H, Lee JK. The effect of biodegradable gelatin microspheres on the neuroprotective effects of high mobility group box 1 A box in the postischemic brain. Biomaterials. 2011;32(3):899–908. doi: 10.1016/j.biomaterials.2010.09.054. [DOI] [PubMed] [Google Scholar]
- Jinushi M. Regulatory mechanisms of nucleic acid-mediated innate immune responses in the tumor microenvironment. Oncoimmunology. 2012;1(9):1632–1634. doi: 10.4161/onci.21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo IJ, Bae GS, Park KC, Choi SB, Jung WS, Jung SY, Cho JH, Choi MO, Song HJ, Park SJ. Scolopendra subspinipes mutilans protected the cerulein-induced acute pancreatitis by inhibiting high-mobility group box protein-1. World J Gastroenterol. 2013;19(10):1551–1562. doi: 10.3748/wjg.v19.i10.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jog NR, Dinnall JA, Gallucci S, Madaio MP, Caricchio R. Poly(ADP-ribose) polymerase-1 regulates the progression of autoimmune nephritis in males by inducing necrotic cell death and modulating inflammation. J Immunol. 2009;182(11):7297–7306. doi: 10.4049/jimmunol.0803565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Cook SA, Davisson MT. Chromosomal localization of the murine gene and two related sequences encoding high-mobility-group I and Y proteins. Genomics. 1992;12(3):503–509. doi: 10.1016/0888-7543(92)90441-t. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Lehn DA, Reeves R. Alternative processing of mRNAs encoding mammalian chromosomal high-mobility-group proteins HMG-I and HMG-Y. Mol Cell Biol. 1989;9(5):2114–2123. doi: 10.1128/mcb.9.5.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SR, Sarpong YC, Peterson RC, Scovell WM. Nucleosome dynamics: HMGB1 relaxes canonical nucleosome structure to facilitate estrogen receptor binding. Nucleic Acids Res. 2012;40(20):10161–10171. doi: 10.1093/nar/gks815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan N, Jobart-Malfait A, Dos Reis G, Quignon F, Piolot T, Klein C, Tramier M, Coppey-Moisan M, Marechal V. Live-cell imaging reveals multiple interactions between Epstein-Barr virus nuclear antigen 1 and cellular chromatin during interphase and mitosis. J Virol. 2012;86(9):5314–5329. doi: 10.1128/JVI.06303-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jube S, Rivera ZS, Bianchi ME, Powers A, Wang E, Pagano I, Pass HI, Gaudino G, Carbone M, Yang H. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012;72(13):3290–3301. doi: 10.1158/0008-5472.CAN-11-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun MS, Kim HS, Kim YM, Kim HJ, Park EJ, Lee JH, Lee KR, Kim YS, Chang KC. Ethanol extract of Prunella vulgaris var. lilacina inhibits HMGB1 release by induction of heme oxygenase-1 in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Phytother Res. 2012;26(4):605–612. doi: 10.1002/ptr.3613. [DOI] [PubMed] [Google Scholar]
- Jung JH, Park JH, Jee MH, Keum SJ, Cho MS, Yoon SK, Jang SK. Hepatitis C virus infection is blocked by HMGB1 released from virus-infected cells. J Virol. 2011;85(18):9359–9368. doi: 10.1128/JVI.00682-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Lippard SJ. Nature of full-length HMGB1 binding to cisplatin-modified DNA. Biochemistry. 2003;42(9):2664–2671. doi: 10.1021/bi026972w. [DOI] [PubMed] [Google Scholar]
- Kaczorowski DJ, Nakao A, Vallabhaneni R, Mollen KP, Sugimoto R, Kohmoto J, Zuckerbraun BS, McCurry KR, Billiar TR. Mechanisms of Toll-like receptor 4 (TLR4)-mediated inflammation after cold ischemia/reperfusion in the heart. Transplantation. 2009a;87(10):1455–1463. doi: 10.1097/TP.0b013e3181a36e5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski DJ, Tsung A, Billiar TR. Innate immune mechanisms in ischemia/reperfusion. Front Biosci (Elite Ed) 2009b;1:91–98. doi: 10.2741/E10. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Morishita A, Niki T, Hara J, Sato M, Tani J, Miyoshi H, Yoneyama H, Masaki T, Hattori T, Matsukawa A, Hirashima M. Galectin-9 prolongs the survival of septic mice by expanding tim-3-expressing natural killer T cells and PDCA-1+ CD11c+ macrophages. Crit Care. 2013;17(6):R284. doi: 10.1186/cc13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinina N, Agrotis A, Antropova Y, DiVitto G, Kanellakis P, Kostolias G, Ilyinskaya O, Tararak E, Bobik A. Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol. 2004;24(12):2320–2325. doi: 10.1161/01.ATV.0000145573.36113.8a. [DOI] [PubMed] [Google Scholar]
- Kalinowska-Herok M, Widlak P. High mobility group proteins stimulate DNA cleavage by apoptotic endonuclease DFF40/CAD due to HMG-box interactions with DNA. Acta Biochim Pol. 2008;55(1):21–26. [PubMed] [Google Scholar]
- Kambas K, Mitroulis I, Apostolidou E, Girod A, Chrysanthopoulou A, Pneumatikos I, Skendros P, Kourtzelis I, Koffa M, Kotsianidis I, Ritis K. Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis. PLoS ONE. 2012;7(9):e45427. doi: 10.1371/journal.pone.0045427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska D, Koscielska-Kasprzak K, Drulis-Fajdasz D, Halon A, Polak W, Chudoba P, Janczak D, Mazanowska O, Patrzalek D, Klinger M. Kidney ischemic injury genes expressed after donor brain death are predictive for the outcome of kidney transplantation. Transplantation proceedings. 2011;43(8):2891–2894. doi: 10.1016/j.transproceed.2011.08.062. [DOI] [PubMed] [Google Scholar]
- Kamo N, Ke B, Ghaffari AA, Shen XD, Busuttil RW, Cheng G, Kupiec-Weglinski JW. ASC/caspase-1/IL-1beta signaling triggers inflammatory responses by promoting HMGB1 induction in liver ischemia/reperfusion injury. Hepatology. 2013;58(1):351–362. doi: 10.1002/hep.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakoudi-Tsakalidou F, Farmaki E, Tzimouli V, Taparkou A, Paterakis G, Trachana M, Pratsidou-Gertsi P, Nalbanti P, Papachristou F. Simultaneous changes in serum HMGB1 and IFN-alpha levels and in LAIR-1 expression on plasmatoid dendritic cells of patients with juvenile SLE.. New therapeutic options? Lupus. 2014 doi: 10.1177/0961203313519157. [DOI] [PubMed] [Google Scholar]
- Kanazawa H, Tochino Y, Asai K, Ichimaru Y, Watanabe T, Hirata K. Validity of HMGB1 measurement in epithelial lining fluid in patients with COPD. Eur J Clin Invest. 2012;42(4):419–426. doi: 10.1111/j.1365-2362.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- Kanellakis P, Agrotis A, Kyaw TS, Koulis C, Ahrens I, Mori S, Takahashi HK, Liu K, Peter K, Nishibori M, Bobik A. High-mobility group box protein 1 neutralization reduces development of diet-induced atherosclerosis in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31(2):313–319. doi: 10.1161/ATVBAHA.110.218669. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Lee H, Choi HJ, Youn JH, Shin JS, Ahn YH, Yoo JS, Paik YK, Kim H. Non-histone nuclear factor HMGB1 is phosphorylated and secreted in colon cancers. Lab Invest. 2009;89(8):948–959. doi: 10.1038/labinvest.2009.47. [DOI] [PubMed] [Google Scholar]
- Kang JW, Koh EJ, Lee SM. Melatonin protects liver against ischemia and reperfusion injury through inhibition of toll-like receptor signaling pathway. J Pineal Res. 2011a;50(4):403–411. doi: 10.1111/j.1600-079X.2011.00858.x. [DOI] [PubMed] [Google Scholar]
- Kang R, Livesey KM, Zeh HJ, 3rd, Lotze MT, Tang D. HMGB1 as an autophagy sensor in oxidative stress. Autophagy. 2011b;7(8):904–906. doi: 10.4161/auto.7.8.15704. [DOI] [PubMed] [Google Scholar]
- Kang R, Livesey KM, Zeh HJ, 3rd, Loze MT, Tang D. Metabolic regulation by HMGB1-mediated autophagy and mitophagy. Autophagy. 2011c;7(10) doi: 10.4161/auto.7.10.16753. [DOI] [PubMed] [Google Scholar]
- Kang R, Loux T, Tang D, Schapiro NE, Vernon P, Livesey KM, Krasinskas A, Lotze MT, Zeh HJ., 3rd The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proc Natl Acad Sci U S A. 2012a;109(18):7031–7036. doi: 10.1073/pnas.1113865109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Loux T, Tang D, Schapiro NE, Vernon P, Livesey KM, Krasinskas A, Lotze MT, Zeh HJ., 3rd The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proc Natl Acad Sci U S A. 2012b doi: 10.1073/pnas.1113865109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Tang D. Autophagy in pancreatic cancer pathogenesis and treatment. Am J Cancer Res. 2012;2(4):383–396. [PMC free article] [PubMed] [Google Scholar]
- Kang R, Tang D, Livesey KM, Schapiro NE, Lotze MT, Zeh HJ., 3rd The Receptor for Advanced Glycation End-products (RAGE) protects pancreatic tumor cells against oxidative injury. Antioxid Redox Signal. 2011d;15(8):2175–2184. doi: 10.1089/ars.2010.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Tang D, Loze MT, Zeh HJ. Apoptosis to autophagy switch triggered by the MHC class III-encoded receptor for advanced glycation endproducts (RAGE) Autophagy. 2011e;7(1):91–93. doi: 10.1038/cdd.2009.149. [DOI] [PubMed] [Google Scholar]
- Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P, Bierhaus A, Lotze MT, Zeh HJ. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17(4):666–676. doi: 10.1038/cdd.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Tang D, Schapiro NE, Loux T, Livesey KM, Billiar TR, Wang H, Van Houten B, Lotze MT, Zeh HJ. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene. 2013a doi: 10.1038/onc.2012.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Tang D, Schapiro NE, Loux T, Livesey KM, Billiar TR, Wang H, Van Houten B, Lotze MT, Zeh HJ. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene. 2014;33(5):567–577. doi: 10.1038/onc.2012.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Tang DL, Cao LZ, Yu Y, Zhang GY, Xiao XZ. [High mobility group box 1 is increased in children with acute lymphocytic leukemia and stimulates the release of tumor necrosis factor-alpha in leukemic cell]. Zhonghua er ke za zhi. Chinese journal of pediatrics. 2007;45(5):329–333. [PubMed] [Google Scholar]
- Kang R, Zhang Q, Hou W, Yan Z, Chen R, Bonaroti J, Bansal P, Billiar TR, Tsung A, Wang Q, Bartlett DL, Whitcomb DC, Chang EB, Zhu X, Wang H, Lu B, Tracey KJ, Cao L, Fan XG, Lotze MT, Zeh HJ, 3rd, Tang D. Intracellular Hmgb1 Inhibits Inflammatory Nucleosome Release and Limits Acute Pancreatitis in Mice. Gastroenterology. 2013b doi: 10.1053/j.gastro.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Zhang Q, Zeh HJ, 3rd, Lotze MT, Tang D. HMGB1 in cancer: good, bad, or both? Clin Cancer Res. 2013c;19(15):4046–4057. doi: 10.1158/1078-0432.CCR-13-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang ZC, Jiang WL, Xu Y, Zhu HB, Hou J. Cardioprotection with 8-O-acetyl shanzhiside methylester on experimental myocardial ischemia injury. Eur J Pharm Sci. 2012c;47(1):124–130. doi: 10.1016/j.ejps.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Kanki T, Ohgaki K, Gaspari M, Gustafsson CM, Fukuoh A, Sasaki N, Hamasaki N, Kang D. Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol Cell Biol. 2004;24(22):9823–9834. doi: 10.1128/MCB.24.22.9823-9834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao YH, Jawan B, Goto S, Hung CT, Lin YC, Nakano T, Hsu LW, Lai CY, Tai MH, Chen CL. High-mobility group box 1 protein activates hepatic stellate cells in vitro. Transplant Proc. 2008;40(8):2704–2705. doi: 10.1016/j.transproceed.2008.07.055. [DOI] [PubMed] [Google Scholar]
- Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, Sen ZD, Dalkara T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339(6123):1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- Karlsson S, Pettila V, Tenhunen J, Laru-Sompa R, Hynninen M, Ruokonen E. HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive care medicine. 2008;34(6):1046–1053. doi: 10.1007/s00134-008-1032-9. [DOI] [PubMed] [Google Scholar]
- Kasai N, Tsunaka Y, Ohki I, Hirose S, Morikawa K, Tate S. Solution structure of the HMG-box domain in the SSRP1 subunit of FACT. J Biomol NMR. 2005;32(1):83–88. doi: 10.1007/s10858-005-3662-3. [DOI] [PubMed] [Google Scholar]
- Kasparkova J, Delalande O, Stros M, Elizondo-Riojas MA, Vojtiskova M, Kozelka J, Brabec V. Recognition of DNA interstrand cross-link of antitumor cisplatin by HMGB1 protein. Biochemistry. 2003;42(5):1234–1244. doi: 10.1021/bi026695t. [DOI] [PubMed] [Google Scholar]
- Kassim M, Yusoff KM, Ong G, Sekaran S, Yusof MY, Mansor M. Gelam honey inhibits lipopolysaccharide-induced endotoxemia in rats through the induction of heme oxygenase-1 and the inhibition of cytokines, nitric oxide, and high-mobility group protein B1. Fitoterapia. 2012;83(6):1054–1059. doi: 10.1016/j.fitote.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Kato S, Hussein MH, Kakita H, Goto T, Daoud GA, Kato T, Sugiura T, Nobata M, Nakajima Y, Endo T, Mizuno K, Ito T, Kato I, Suzuki S, Togari H. Edaravone, a novel free radical scavenger, reduces high-mobility group box 1 and prolongs survival in a neonatal sepsis model. Shock. 2009;32(6):586–592. doi: 10.1097/SHK.0b013e3181a2b886. [DOI] [PubMed] [Google Scholar]
- Kawahara K, Hashiguchi T, Masuda K, Saniabadi AR, Kikuchi K, Tancharoen S, Ito T, Miura N, Morimoto Y, Biswas KK, Nawa Y, Meng X, Oyama Y, Takenouchi K, Shrestha B, Sameshima H, Shimizu T, Adachi T, Adachi M, Maruyama I. Mechanism of HMGB1 release inhibition from RAW264.7 cells by oleanolic acid in Prunus mume Sieb. et Zucc. Int J Mol Med. 2009;23(5):615–620. doi: 10.3892/ijmm_00000172. [DOI] [PubMed] [Google Scholar]
- Kawahara K, Setoyama K, Kikuchi K, Biswas KK, Kamimura R, Iwata M, Ito T, Morimoto Y, Hashiguchi T, Takao S, Maruyama I. HMGB1 release in co-cultures of porcine endothelial and human T cells. Xenotransplantation. 2007;14(6):636–641. doi: 10.1111/j.1399-3089.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- Kawahara N, Tanaka T, Yokomizo A, Nanri H, Ono M, Wada M, Kohno K, Takenaka K, Sugimachi K, Kuwano M. Enhanced coexpression of thioredoxin and high mobility group protein 1 genes in human hepatocellular carcinoma and the possible association with decreased sensitivity to cisplatin. Cancer Res. 1996;56(23):5330–5333. [PubMed] [Google Scholar]
- Kawakami M, Narumoto O, Matsuo Y, Horiguchi K, Horiguchi S, Yamashita N, Sakaguchi M, Lipp M, Nagase T. The role of CCR7 in allergic airway inflammation induced by house dust mite exposure. Cell Immunol. 2012;275(1–2):24–32. doi: 10.1016/j.cellimm.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Kawano A, Tsukimoto M, Mori D, Noguchi T, Harada H, Takenouchi T, Kitani H, Kojima S. Regulation of P2X7-dependent inflammatory functions by P2X4 receptor in mouse macrophages. Biochem Biophys Res Commun. 2012;420(1):102–107. doi: 10.1016/j.bbrc.2012.02.122. [DOI] [PubMed] [Google Scholar]
- Kawase T, Sato K, Ueda T, Yoshida M. Distinct domains in HMGB1 are involved in specific intramolecular and nucleosomal interactions. Biochemistry. 2008;47(52):13991–13996. doi: 10.1021/bi8013449. [DOI] [PubMed] [Google Scholar]
- Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29(1):21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Zeng X, Wang Y, Zhang QH, Kapoor M, Shu H, Goodman R, Lozano G, Zhao Y, Lu H. A DNA damage-induced p53 serine 392 kinase complex contains CK2, hSpt16, and SSRP1. Mol Cell. 2001;7(2):283–292. doi: 10.1016/s1097-2765(01)00176-9. [DOI] [PubMed] [Google Scholar]
- Kennedy CL, Smith DJ, Lyras D, Chakravorty A, Rood JI. Programmed cellular necrosis mediated by the pore-forming alpha-toxin from Clostridium septicum. PLoS Pathog. 2009;5(7):e1000516. doi: 10.1371/journal.ppat.1000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew RR, Penzo M, Habiel DM, Marcu KB. The IKKalpha-dependent NF-kappaB p52/RelB noncanonical pathway is essential to sustain a CXCL12 autocrine loop in cells migrating in response to HMGB1. J Immunol. 2012;188(5):2380–2386. doi: 10.4049/jimmunol.1102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijanka G, Hector S, Kay EW, Murray F, Cummins R, Murphy D, MacCraith BD, Prehn JH, Kenny D. Human IgG antibody profiles differentiate between symptomatic patients with and without colorectal cancer. Gut. 2010;59(1):69–78. doi: 10.1136/gut.2009.178574. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Kawahara K, Biswas KK, Ito T, Tancharoen S, Morimoto Y, Matsuda F, Oyama Y, Takenouchi K, Miura N, Arimura N, Nawa Y, Meng X, Shrestha B, Arimura S, Iwata M, Mera K, Sameshima H, Ohno Y, Maenosono R, Yoshida Y, Tajima Y, Uchikado H, Kuramoto T, Nakayama K, Shigemori M, Hashiguchi T, Maruyama I. Minocycline attenuates both OGD-induced HMGB1 release and HMGB1-induced cell death in ischemic neuronal injury in PC12 cells. Biochem Biophys Res Commun. 2009a;385(2):132–136. doi: 10.1016/j.bbrc.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Kawahara K, Tancharoen S, Matsuda F, Morimoto Y, Ito T, Biswas KK, Takenouchi K, Miura N, Oyama Y, Nawa Y, Arimura N, Iwata M, Tajima Y, Kuramoto T, Nakayama K, Shigemori M, Yoshida Y, Hashiguchi T, Maruyama I. The free radical scavenger edaravone rescues rats from cerebral infarction by attenuating the release of high-mobility group box-1 in neuronal cells. J Pharmacol Exp Ther. 2009b;329(3):865–874. doi: 10.1124/jpet.108.149484. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Tancharoen S, Ito T, Morimoto-Yamashita Y, Miura N, Kawahara K, Maruyama I, Murai Y, Tanaka E. Potential of the angiotensin receptor blockers (ARBs) telmisartan, irbesartan, and candesartan for inhibiting the HMGB1/RAGE axis in prevention and acute treatment of stroke. Int J Mol Sci. 2013;14(9):18899–18924. doi: 10.3390/ijms140918899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen ME, Englert JA, Stolz DB, Song M, Han Y, Delude RL, Kellum JA, Fink MP. The phase 2 enzyme inducers ethacrynic acid, DL-sulforaphane, and oltipraz inhibit lipopolysaccharide-induced high-mobility group box 1 secretion by RAW 264.7 cells. J Pharmacol Exp Ther. 2006;316(3):1070–1079. doi: 10.1124/jpet.105.092841. [DOI] [PubMed] [Google Scholar]
- Kim DE, Min KJ, Kim JS, Kwon TK. High-mobility group box-1 protein induces mucin 8 expression through the activation of the JNK and PI3K/Akt signal pathways in human airway epithelial cells. Biochem Biophys Res Commun. 2012a;421(3):436–441. doi: 10.1016/j.bbrc.2012.03.131. [DOI] [PubMed] [Google Scholar]
- Kim E, Lu HC, Zoghbi HY, Song JJ. Structural basis of protein complex formation and reconfiguration by polyglutamine disease protein Ataxin-1 and Capicua. Genes Dev. 2013a;27(6):590–595. doi: 10.1101/gad.212068.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Park EJ, Park SW, Kim HJ, Chang KC. A tetrahydroisoquinoline alkaloid THI-28 reduces LPS-induced HMGB1 and diminishes organ injury in septic mice through p38 and PI3K/Nrf2/HO-1 signals. Int Immunopharmacol. 2013b;17(3):684–692. doi: 10.1016/j.intimp.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Kim ID, Lee JK. HMGB1-Binding Heptamer Confers Anti-Inflammatory Effects in Primary Microglia Culture. Experimental neurobiology. 2013;22(4):301–307. doi: 10.5607/en.2013.22.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ID, Lim CM, Kim JB, Nam HY, Nam K, Kim SW, Park JS, Lee JK. Neuroprotection by biodegradable PAMAM ester (e-PAM-R)-mediated HMGB1 siRNA delivery in primary cortical cultures and in the postischemic brain. J Control Release. 2010a;142(3):422–430. doi: 10.1016/j.jconrel.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Kim ID, Shin JH, Kim SW, Choi S, Ahn J, Han PL, Park JS, Lee JK. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol Ther. 2012b;20(4):829–839. doi: 10.1038/mt.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ID, Shin JH, Lee HK, Jin YC, Lee JK. Intranasal delivery of HMGB1-binding heptamer peptide confers a robust neuroprotection in the postischemic brain. Neurosci Lett. 2012c;525(2):179–183. doi: 10.1016/j.neulet.2012.07.040. [DOI] [PubMed] [Google Scholar]
- Kim J, Sohn E, Kim CS, Jo K, Kim JS. The role of high-mobility group box-1 protein in the development of diabetic nephropathy. Am J Nephrol. 2011a;33(6):524–529. doi: 10.1159/000327992. [DOI] [PubMed] [Google Scholar]
- Kim JB, Lim CM, Yu YM, Lee JK. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J Neurosci Res. 2008a;86(5):1125–1131. doi: 10.1002/jnr.21555. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26(24):6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim SJ, Lee IS, Lee MS, Uematsu S, Akira S, Oh KI. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J Immunol. 2009;182(4):2458–2466. doi: 10.4049/jimmunol.0801364. [DOI] [PubMed] [Google Scholar]
- Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L958–L965. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- Kim K, Han JS, Kim HA, Lee M. Expression, purification and characterization of TAThigh mobility group box-1A peptide as a carrier of nucleic acids. Biotechnol Lett. 2008b;30(8):1331–1337. doi: 10.1007/s10529-008-9695-4. [DOI] [PubMed] [Google Scholar]
- Kim K, Jung N, Lee K, Choi J, Kim S, Jun J, Kim E, Kim D. Dietary omega-3 polyunsaturated fatty acids attenuate hepatic ischemia/reperfusion injury in rats by modulating toll-like receptor recruitment into lipid rafts. Clin Nutr. 2013c;32(5):855–862. doi: 10.1016/j.clnu.2012.11.026. [DOI] [PubMed] [Google Scholar]
- Kim S, Kim SY, Pribis JP, Lotze M, Mollen KP, Shapiro R, Loughran P, Scott MJ, Billiar TR. Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med. 2013d;19:88–98. doi: 10.2119/molmed.2012.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Wolyniak MJ, Staab JF, Sundstrom P. A 368-base-pair cis-acting HWP1 promoter region, HCR, of Candida albicans confers hypha-specific gene regulation and binds architectural transcription factors Nhp6 and Gcf1p. Eukaryot Cell. 2007;6(4):693–709. doi: 10.1128/EC.00341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Jin Y, Shin JH, Kim ID, Lee HK, Park S, Han PL, Lee JK. Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol Dis. 2012d;46(1):147–156. doi: 10.1016/j.nbd.2011.12.056. [DOI] [PubMed] [Google Scholar]
- Kim SW, Lim CM, Kim JB, Shin JH, Lee S, Lee M, Lee JK. Extracellular HMGB1 released by NMDA treatment confers neuronal apoptosis via RAGE-p38 MAPK/ERK signaling pathway. Neurotox Res. 2011b;20(2):159–169. doi: 10.1007/s12640-010-9231-x. [DOI] [PubMed] [Google Scholar]
- Kim TH, Ku SK, Bae JS. Inhibitory effects of kaempferol-3-O-sophoroside on HMGB1-mediated proinflammatory responses. Food Chem Toxicol. 2012e;50(3–4):1118–1123. doi: 10.1016/j.fct.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Kim TH, Ku SK, Bae JS. Anti-inflammatory activities of isorhamnetin-3-O-galactoside against HMGB1-induced inflammatory responses in both HUVECs and CLP-induced septic mice. J Cell Biochem. 2013e;114(2):336–345. doi: 10.1002/jcb.24361. [DOI] [PubMed] [Google Scholar]
- Kim TH, Ku SK, Bae JS. Persicarin is anti-inflammatory mediator against HMGB1-induced inflammatory responses in HUVECs and in CLP-induced sepsis mice. J Cell Physiol. 2013f;228(4):696–703. doi: 10.1002/jcp.24214. [DOI] [PubMed] [Google Scholar]
- Kim TH, Ku SK, Lee T, Bae JS. Vascular barrier protective effects of phlorotannins on HMGB1-mediated proinflammatory responses in vitro and in vivo. Food Chem Toxicol. 2012f;50(6):2188–2195. doi: 10.1016/j.fct.2012.03.082. [DOI] [PubMed] [Google Scholar]
- Kim TH, Yoon SJ, Lee SM. Genipin attenuates sepsis by inhibiting Toll-like receptor signaling. Mol Med. 2012g;18:455–465. doi: 10.2119/molmed.2011.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TS, Gorski SA, Hahn S, Murphy KM, Braciale TJ. Distinct Dendritic Cell Subsets Dictate the Fate Decision between Effector and Memory CD8(+) T Cell Differentiation by a CD24-Dependent Mechanism. Immunity. 2014;40(3):400–413. doi: 10.1016/j.immuni.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Choi CY, Kim Y. Covalent modification of the homeodomain-interacting protein kinase 2 (HIPK2) by the ubiquitin-like protein SUMO-1. Proc Natl Acad Sci U S A. 1999;96(22):12350–12355. doi: 10.1073/pnas.96.22.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Kwon JS, Cho YK, Jeong MH, Cho JG, Park JC, Kang JC, Ahn Y. Curcumin reduces the cardiac ischemia-reperfusion injury: involvement of the toll-like receptor 2 in cardiomyocytes. J Nutr Biochem. 2012h;23(11):1514–1523. doi: 10.1016/j.jnutbio.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Kim YS, Lee YM, Park JS, Lee SK, Kim EC. SIRT1 modulates high-mobility group box 1-induced osteoclastogenic cytokines in human periodontal ligament cells. J Cell Biochem. 2010b;111(5):1310–1320. doi: 10.1002/jcb.22858. [DOI] [PubMed] [Google Scholar]
- Kimura K, Katoh N, Sakurada K, Kubo S. Phosphorylation of high mobility group 1 protein by phospholipid-sensitive Ca2+-dependent protein kinase from pig testis. Biochem J. 1985;227(1):271–276. doi: 10.1042/bj2270271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RS, Newmark PA. The cell biology of regeneration. J Cell Biol. 2012;196(5):553–562. doi: 10.1083/jcb.201105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskinis E, Garcia-Pedrero JM, Villaronga MA, Parker MG, Belandia B. Identification of BAF57 mutations in human breast cancer cell lines. Breast Cancer Res Treat. 2006;98(2):191–198. doi: 10.1007/s10549-005-9149-9. [DOI] [PubMed] [Google Scholar]
- Kitahara T, Takeishi Y, Harada M, Niizeki T, Suzuki S, Sasaki T, Ishino M, Bilim O, Nakajima O, Kubota I. High-mobility group box 1 restores cardiac function after myocardial infarction in transgenic mice. Cardiovasc Res. 2008;80(1):40–46. doi: 10.1093/cvr/cvn163. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Holmes GL. Taming TLR4 may ease seizures. Nat Med. 2010;16(4):369–370. doi: 10.1038/nm0410-369. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HK, Hsu WH, Hsieh CC, Lien TC, Lee TS, Kou YR. High expression of high-mobility group box 1 in the blood and lungs is associated with the development of chronic obstructive pulmonary disease in smokers. Respirology (Carlton, Vic.) 2013 doi: 10.1111/resp.12209. [DOI] [PubMed] [Google Scholar]
- Ko YB, Kim BR, Nam SL, Yang JB, Park SY, Rho SB. High-mobility group box 1 (HMGB1) protein regulates tumor-associated cell migration through the interaction with BTB domain. Cell Signal. 2014;26(4):777–783. doi: 10.1016/j.cellsig.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Kocsis AK, Szabolcs A, Hofner P, Takacs T, Farkas G, Boda K, Mandi Y. Plasma concentrations of high-mobility group box protein 1, soluble receptor for advanced glycation end-products and circulating DNA in patients with acute pancreatitis. Pancreatology. 2009;9(4):383–391. doi: 10.1159/000181172. [DOI] [PubMed] [Google Scholar]
- Koga H, Hagiwara S, Inomata M, Kusaka J, Asai N, Oyama M, Kita K, Kashima K, Yokoi I, Noguchi T. Vitamin E derivative ETS-GS reduces liver ischemia-reperfusion injury in rats. J Surg Res. 2012;175(1):118–122. doi: 10.1016/j.jss.2011.02.045. [DOI] [PubMed] [Google Scholar]
- Koga Y, Fujita M, Tsuruta R, Koda Y, Nakahara T, Yagi T, Aoki T, Kobayashi C, Izumi T, Kasaoka S, Yuasa M, Maekawa T. Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol Res. 2010;32(9):925–932. doi: 10.1179/016164110X12645013515133. [DOI] [PubMed] [Google Scholar]
- Kohka Takahashi H, Sadamori H, Liu K, Wake H, Mori S, Yoshino T, Yamamoto Y, Yamamoto H, Nishibori M. Role of cell-cell interactions in high mobility group box 1 cytokine activity in human peripheral blood mononuclear cells and mouse splenocytes. Eur J Pharmacol. 2013;701(1–3):194–202. doi: 10.1016/j.ejphar.2012.11.058. [DOI] [PubMed] [Google Scholar]
- Kohles N, Nagel D, Jungst D, Stieber P, Holdenrieder S. Predictive value of immunogenic cell death biomarkers HMGB1, sRAGE, and DNase in liver cancer patients receiving transarterial chemoembolization therapy. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2012;33(6):2401–2409. doi: 10.1007/s13277-012-0504-2. [DOI] [PubMed] [Google Scholar]
- Kohlstaedt LA, Cole RD. Effect of pH on interactions between DNA and high-mobility group protein HMG1. Biochemistry. 1994a;33(42):12702–12707. doi: 10.1021/bi00208a022. [DOI] [PubMed] [Google Scholar]
- Kohlstaedt LA, Cole RD. Specific interaction between H1 histone and high mobility protein HMG1. Biochemistry. 1994b;33(2):570–575. doi: 10.1021/bi00168a023. [DOI] [PubMed] [Google Scholar]
- Kohlstaedt LA, King DS, Cole RD. Native state of high mobility group chromosomal proteins 1 and 2 is rapidly lost by oxidation of sulfhydryl groups during storage. Biochemistry. 1986;25(16):4562–4565. doi: 10.1021/bi00364a016. [DOI] [PubMed] [Google Scholar]
- Kohlstaedt LA, Sung EC, Fujishige A, Cole RD. Non-histone chromosomal protein HMG1 modulates the histone H1-induced condensation of DNA. J Biol Chem. 1987;262(2):524–526. [PubMed] [Google Scholar]
- Kohno T, Anzai T, Kaneko H, Sugano Y, Shimizu H, Shimoda M, Miyasho T, Okamoto M, Yokota H, Yamada S, Yoshikawa T, Okada Y, Yozu R, Ogawa S, Fukuda K. High-mobility group box 1 protein blockade suppresses development of abdominal aortic aneurysm. Journal of cardiology. 2012;59(3):299–306. doi: 10.1016/j.jjcc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Kohno T, Anzai T, Naito K, Miyasho T, Okamoto M, Yokota H, Yamada S, Maekawa Y, Takahashi T, Yoshikawa T, Ishizaka A, Ogawa S. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovascular research. 2009;81(3):565–573. doi: 10.1093/cvr/cvn291. [DOI] [PubMed] [Google Scholar]
- Kohno T, Anzai T, Shimizu H, Kaneko H, Sugano Y, Yamada S, Yoshikawa T, Ishizaka A, Yozu R, Ogawa S. Impact of serum high-mobility group box 1 protein elevation on oxygenation impairment after thoracic aortic aneurysm repair. Heart and vessels. 2011;26(3):306–312. doi: 10.1007/s00380-010-0056-6. [DOI] [PubMed] [Google Scholar]
- Kojima D, Mera T, Nishinakamura H, Itoh T, Ogata T, Matsuoka N, Kodama S, Yasunami Y. Prevention of high-mobility group box 1-mediated early loss of transplanted mouse islets in the liver by antithrombin III. Transplantation. 2012a;93(10):983–988. doi: 10.1097/TP.0b013e31824d3508. [DOI] [PubMed] [Google Scholar]
- Kojima M, Tanabe M, Shinoda M, Yamada S, Miyasho T, Suda K, Hibi T, Obara H, Itano O, Kawachi S, Kitajima M, Maruyama I, Kitagawa Y. Role of high mobility group box chromosomal protein 1 in ischemia-reperfusion injury in the rat small intestine. J Surg Res. 2012b;178(1):466–471. doi: 10.1016/j.jss.2012.01.044. [DOI] [PubMed] [Google Scholar]
- Kokkola R, Sundberg E, Ulfgren AK, Palmblad K, Li J, Wang H, Ulloa L, Yang H, Yan XJ, Furie R, Chiorazzi N, Tracey KJ, Andersson U, Harris HE. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 2002;46(10):2598–2603. doi: 10.1002/art.10540. [DOI] [PubMed] [Google Scholar]
- Kong X, Yuan H, Wu X, Zhang J, Zhou H, Wang M, Liu Y, Jin X. High-mobility-group box protein 1A box reduces development of sodium laurate-induced thromboangiitis obliterans in rats. J Vasc Surg. 2013;57(1):194–204. doi: 10.1016/j.jvs.2012.06.083. [DOI] [PubMed] [Google Scholar]
- Kono Y, Inomata M, Hagiwara S, Shiraishi N, Noguchi T, Kitano S. A newly synthetic vitamin E derivative, E-Ant-S-GS, attenuates lung injury caused by cecal ligation and puncture-induced sepsis in rats. Surgery. 2012;151(3):420–426. doi: 10.1016/j.surg.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Korbelik M, Zhang W, Merchant S. Involvement of damage-associated molecular patterns in tumor response to photodynamic therapy: surface expression of calreticulin and high-mobility group box-1 release. Cancer Immunol Immunother. 2011;60(10):1431–1437. doi: 10.1007/s00262-011-1047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Clevers H. Two members of the Tcf family implicated in Wnt/beta-catenin signaling during embryogenesis in the mouse. Mol Cell Biol. 1998;18(3):1248–1256. doi: 10.1128/mcb.18.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblit B, Masmas T, Petersen SL, Madsen HO, Heilmann C, Schejbel L, Sengelov H, Muller K, Garred P, Vindelov L. Association of HMGB1 polymorphisms with outcome after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16(2):239–252. doi: 10.1016/j.bbmt.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kornblit B, Munthe-Fog L, Madsen HO, Strom J, Vindelov L, Garred P. Association of HMGB1 polymorphisms with outcome in patients with systemic inflammatory response syndrome. Crit Care. 2008;12(3):R83. doi: 10.1186/cc6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblit B, Munthe-Fog L, Petersen SL, Madsen HO, Vindelov L, Garred P. The genetic variation of the human HMGB1 gene. Tissue Antigens. 2007;70(2):151–156. doi: 10.1111/j.1399-0039.2007.00854.x. [DOI] [PubMed] [Google Scholar]
- Korner U, Bustin M, Scheer U, Hock R. Developmental role of HMGN proteins in Xenopus laevis. Mech Dev. 2003;120(10):1177–1192. doi: 10.1016/j.mod.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Kosai K, Seki M, Yanagihara K, Nakamura S, Kurihara S, Izumikawa K, Kakeya H, Yamamoto Y, Tashiro T, Kohno S. Elevated levels of high mobility group box chromosomal protein-1 (HMGB-1) in sera from patients with severe bacterial pneumonia coinfected with influenza virus. Scand J Infect Dis. 2008;40(4):338–342. doi: 10.1080/00365540701660486. [DOI] [PubMed] [Google Scholar]
- Kostova N, Zlateva S, Ugrinova I, Pasheva E. The expression of HMGB1 protein and its receptor RAGE in human malignant tumors. Molecular and cellular biochemistry. 2010;337(1–2):251–258. doi: 10.1007/s11010-009-0305-0. [DOI] [PubMed] [Google Scholar]
- Kraft C, Deplazes A, Sohrmann M, Peter M. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat Cell Biol. 2008;10(5):602–610. doi: 10.1038/ncb1723. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9(12):1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohn NM, Yanagisawa S, Grasser KD. Specificity of the stimulatory interaction between chromosomal HMGB proteins and the transcription factor Dof2 and its negative regulation by protein kinase CK2-mediated phosphorylation. J Biol Chem. 2002;277(36):32438–32444. doi: 10.1074/jbc.M203814200. [DOI] [PubMed] [Google Scholar]
- Kruger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M, Kramer BK, Colvin RB, Heeger PS, Murphy BT, Schroppel B. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106(9):3390–3395. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger B, Yin N, Zhang N, Yadav A, Coward W, Lal G, Zang W, P SH, Bromberg JS, Murphy B, Schroppel B. Islet-expressed TLR2 and TLR4 sense injury and mediate early graft failure after transplantation. Eur J Immunol. 2010;40(10):2914–2924. doi: 10.1002/eji.201040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynetskaia N, Xie H, Vucetic S, Obradovic Z, Krynetskiy E. High mobility group protein B1 is an activator of apoptotic response to antimetabolite drugs. Mol Pharmacol. 2008;73(1):260–269. doi: 10.1124/mol.107.041764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynetskaia NF, Phadke MS, Jadhav SH, Krynetskiy EY. Chromatin-associated proteins HMGB1/2 and PDIA3 trigger cellular response to chemotherapy-induced DNA damage. Mol Cancer Ther. 2009;8(4):864–872. doi: 10.1158/1535-7163.MCT-08-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynetski EY, Krynetskaia NF, Bianchi ME, Evans WE. A nuclear protein complex containing high mobility group proteins B1 and B2, heat shock cognate protein 70, ERp60, and glyceraldehyde-3-phosphate dehydrogenase is involved in the cytotoxic response to DNA modified by incorporation of anticancer nucleoside analogues. Cancer Res. 2003;63(1):100–106. [PubMed] [Google Scholar]
- Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- Ku SK, Kim JA, Bae JS. Vascular barrier protective effects of piperlonguminine in vitro and in vivo. Inflamm Res. 2014 doi: 10.1007/s00011-014-0708-6. [DOI] [PubMed] [Google Scholar]
- Ku SK, Lee IC, Kim JA, Bae JS. Anti-septic Effects of Pellitorine in HMGB1-Induced Inflammatory Responses In Vitro and In Vivo. Inflammation. 2013 doi: 10.1007/s10753-013-9745-5. [DOI] [PubMed] [Google Scholar]
- Kuang X, Huang Y, Gu HF, Zu XY, Zou WY, Song ZB, Guo QL. Effects of intrathecal epigallocatechin gallate, an inhibitor of Toll-like receptor 4, on chronic neuropathic pain in rats. Eur J Pharmacol. 2012;676(1–3):51–56. doi: 10.1016/j.ejphar.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Kuchler R, Schroeder BO, Jaeger SU, Stange EF, Wehkamp J. Antimicrobial activity of high-mobility-group box 2: a new function to a well-known protein. Antimicrob Agents Chemother. 2013;57(10):4782–4793. doi: 10.1128/AAC.00805-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo D, Toyama M, Aoyagi T, Akahori Y, Yamamoto H, Ishii K, Kanno E, Maruyama R, Kaku M, Kushimoto S, Kawakami K. Involvement of high mobility group box 1 and the therapeutic effect of recombinant thrombomodulin in a mouse model of severe acute respiratory distress syndrome. Clin Exp Immunol. 2013;173(2):276–287. doi: 10.1111/cei.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl L, Rechsteiner M, Wu L. Relationship between the structure of chromosomal protein HMG1 and its accumulation in the cell nucleus. J Biol Chem. 1985;260(18):10361–10368. [PubMed] [Google Scholar]
- Kuehl L, Salmond B, Tran L. Concentrations of high-mobility-group proteins in the nucleus and cytoplasm of several rat tissues. J Cell Biol. 1984;99(2):648–654. doi: 10.1083/jcb.99.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler JE, Deng T, Bustin M. The HMGN family of chromatin-binding proteins: dynamic modulators of epigenetic processes. Biochim Biophys Acta. 2012;1819(7):652–656. doi: 10.1016/j.bbagrm.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler JE, Horsch M, Huang D, Furusawa T, Rochman M, Garrett L, Becker L, Bohla A, Holter SM, Prehn C, Rathkolb B, Racz I, Aguilar-Pimentel JA, Adler T, Adamski J, Beckers J, Busch DH, Eickelberg O, Klopstock T, Ollert M, Stoger T, Wolf E, Wurst W, Yildirim AO, Zimmer A, Gailus-Durner V, Fuchs H, Hrabe de Angelis M, Garfinkel B, Orly J, Ovcharenko I, Bustin M. High mobility group N proteins modulate the fidelity of the cellular transcriptional profile in a tissue- and variant-specific manner. J Biol Chem. 2013;288(23):16690–16703. doi: 10.1074/jbc.M113.463315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Singal A, Rizvi MM, Chauhan VS. High mobility group box (HMGB) proteins of Plasmodium falciparum: DNA binding proteins with pro-inflammatory activity. Parasitol Int. 2008;57(2):150–157. doi: 10.1016/j.parint.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Armenteros-Monterroso E, East P, Chakravorty P, Matthews N, Winslow MM, Downward J. HMGA2 functions as a competing endogenous RNA to promote lung cancer progression. Nature. 2014;505(7482):212–217. doi: 10.1038/nature12785. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kuniyasu H, Chihara Y, Kondo H. Differential effects between amphoterin and advanced glycation end products on colon cancer cells. Int J Cancer. 2003a;104(6):722–727. doi: 10.1002/ijc.11016. [DOI] [PubMed] [Google Scholar]
- Kuniyasu H, Chihara Y, Takahashi T. Co-expression of receptor for advanced glycation end products and the ligand amphoterin associates closely with metastasis of colorectal cancer. Oncology reports. 2003b;10(2):445–448. [PubMed] [Google Scholar]
- Kuniyasu H, Oue N, Wakikawa A, Shigeishi H, Matsutani N, Kuraoka K, Ito R, Yokozaki H, Yasui W. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196(2):163–170. doi: 10.1002/path.1031. [DOI] [PubMed] [Google Scholar]
- Kuniyasu H, Sasaki T, Sasahira T, Ohmori H, Takahashi T. Depletion of tumor-infiltrating macrophages is associated with amphoterin expression in colon cancer. Pathobiology. 2004;71(3):129–136. doi: 10.1159/000076467. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Furusawa T, Ueda T, Bustin M. The nucleosome binding protein HMGN3 is expressed in pancreatic alpha-cells and affects plasma glucagon levels in mice. J Cell Biochem. 2010;109(1):49–57. doi: 10.1002/jcb.22377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa Y, Takakusagi Y, Kusayanagi T, Kuramochi K, Imai T, Hirayama T, Ito I, Yoshida M, Sakaguchi K, Sugawara F. Identification and characterization of the direct interaction between methotrexate (MTX) and high-mobility group box 1 (HMGB1) protein. PLoS ONE. 2013;8(5):e63073. doi: 10.1371/journal.pone.0063073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusume A, Sasahira T, Luo Y, Isobe M, Nakagawa N, Tatsumoto N, Fujii K, Ohmori H, Kuniyasu H. Suppression of dendritic cells by HMGB1 is associated with lymph node metastasis of human colon cancer. Pathobiology. 2009;76(4):155–162. doi: 10.1159/000218331. [DOI] [PubMed] [Google Scholar]
- Kutsuna S, Tsuruta R, Fujita M, Todani M, Yagi T, Ogino Y, Igarashi M, Takahashi K, Izumi T, Kasaoka S, Yuasa M, Maekawa T. Cholinergic agonist physostigmine suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in rats with forebrain ischemia/reperfusion. Brain Res. 2010;1313:242–249. doi: 10.1016/j.brainres.2009.11.077. [DOI] [PubMed] [Google Scholar]
- Kwak KJ, Kim JY, Kim YO, Kang H. Characterization of transgenic Arabidopsis plants overexpressing high mobility group B proteins under high salinity, drought or cold stress. Plant Cell Physiol. 2007;48(2):221–231. doi: 10.1093/pcp/pcl057. [DOI] [PubMed] [Google Scholar]
- Kwon JH, Kim J, Park JY, Hong SM, Park CW, Hong SJ, Park SY, Choi YJ, Do IG, Joh JW, Kim DS, Choi KY. Overexpression of high-mobility group box 2 is associated with tumor aggressiveness and prognosis of hepatocellular carcinoma. Clin Cancer Res. 2010a;16(22):5511–5521. doi: 10.1158/1078-0432.CCR-10-0825. [DOI] [PubMed] [Google Scholar]
- Kwon WY, Suh GJ, Kim KS, Jo YH, Lee JH, Kim K, Jung SK. Glutamine attenuates acute lung injury by inhibition of high mobility group box protein-1 expression during sepsis. Br J Nutr. 2010b;103(6):890–898. doi: 10.1017/S0007114509992509. [DOI] [PubMed] [Google Scholar]
- Labazi M, Jaafar L, Flores-Rozas H. Modulation of the DNA-binding activity of Saccharomyces cerevisiae MSH2-MSH6 complex by the high-mobility group protein NHP6A, in vitro. Nucleic Acids Res. 2009;37(22):7581–7589. doi: 10.1093/nar/gkp649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackman RL, Cresswell P. Exposure of the promonocytic cell line THP-1 to Escherichia coli induces IFN-gamma-inducible lysosomal thiol reductase expression by inflammatory cytokines. J Immunol. 2006;177(7):4833–4840. doi: 10.4049/jimmunol.177.7.4833. [DOI] [PubMed] [Google Scholar]
- Lackman RL, Jamieson AM, Griffith JM, Geuze H, Cresswell P. Innate immune recognition triggers secretion of lysosomal enzymes by macrophages. Traffic. 2007;8(9):1179–1189. doi: 10.1111/j.1600-0854.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Lai PF, Cheng CF, Lin H, Tseng TL, Chen HH, Chen SH. ATF3 Protects against LPS-Induced Inflammation in Mice via Inhibiting HMGB1 Expression. Evid Based Complement Alternat Med. 2013;2013:716481. doi: 10.1155/2013/716481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird MD, Shields JS, Sukumari-Ramesh S, Kimbler DE, Fessler RD, Shakir B, Youssef P, Yanasak N, Vender JR, Dhandapani KM. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia. 2014;62(1):26–38. doi: 10.1002/glia.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laliena A, San Miguel B, Crespo I, Alvarez M, Gonzalez-Gallego J, Tunon MJ. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J Pineal Res. 2012;53(3):270–278. doi: 10.1111/j.1600-079X.2012.00995.x. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol. 2011;187(2):597–602. doi: 10.4049/jimmunol.1100229. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol. 2010;185(7):4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman D, Bustin M. A signature for the HMG-1 box DNA-binding proteins. Bioessays. 1993;15(8):539–546. doi: 10.1002/bies.950150807. [DOI] [PubMed] [Google Scholar]
- Lang CH, Silvis C, Deshpande N, Nystrom G, Frost RA. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1beta,-6, and high-mobility-group protein-1 in skeletal muscle. Shock. 2003;19(6):538–546. doi: 10.1097/01.shk.0000055237.25446.80. [DOI] [PubMed] [Google Scholar]
- Lange SS, Mitchell DL, Vasquez KM. High mobility group protein B1 enhances DNA repair and chromatin modification after DNA damage. Proc Natl Acad Sci U S A. 2008;105(30):10320–10325. doi: 10.1073/pnas.0803181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange SS, Reddy MC, Vasquez KM. Human HMGB1 directly facilitates interactions between nucleotide excision repair proteins on triplex-directed psoralen interstrand crosslinks. DNA Repair (Amst) 2009;8(7):865–872. doi: 10.1016/j.dnarep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange SS, Vasquez KM. HMGB1: the jack-of-all-trades protein is a master DNA repair mechanic. Mol Carcinog. 2009;48(7):571–580. doi: 10.1002/mc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantos J, Foldi V, Roth E, Weber G, Bogar L, Csontos C. Burn trauma induces early HMGB1 release in patients: its correlation with cytokines. Shock. 2010;33(6):562–567. doi: 10.1097/SHK.0b013e3181cd8c88. [DOI] [PubMed] [Google Scholar]
- Lanuszewska J, Widlak P. High mobility group 1 and 2 proteins bind preferentially to DNA that contains bulky adducts induced by benzo[a]pyrene diol epoxide and N-acetoxy-acetylaminofluorene. Cancer Lett. 2000;158(1):17–25. doi: 10.1016/s0304-3835(00)00517-6. [DOI] [PubMed] [Google Scholar]
- Lau A, Wang S, Jiang J, Haig A, Pavlosky A, Linkermann A, Zhang ZX, Jevnikar AM. RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival. Am J Transplant. 2013;13(11):2805–2818. doi: 10.1111/ajt.12447. [DOI] [PubMed] [Google Scholar]
- Laudet V, Stehelin D, Clevers H. Ancestry and diversity of the HMG box superfamily. Nucleic Acids Res. 1993;21(10):2493–2501. doi: 10.1093/nar/21.10.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launholt D, Merkle T, Houben A, Schulz A, Grasser KD. Arabidopsis chromatin-associated HMGA and HMGB use different nuclear targeting signals and display highly dynamic localization within the nucleus. Plant Cell. 2006;18(11):2904–2918. doi: 10.1105/tpc.106.047274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc PM, Doggett TA, Choi J, Hancock MA, Durocher Y, Frank F, Nagar B, Ferguson TA, Saleh M. An Immunogenic Peptide in the A-box of HMGB1 Reverses Apoptosis-Induced Tolerance Through RAGE. J Biol Chem. 2014 doi: 10.1074/jbc.M113.541474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc P, Wahamaa H, Idborg H, Jakobsson PJ, Harris HE, Korotkova M. IL-1beta/HMGB1 complexes promote The PGE2 biosynthesis pathway in synovial fibroblasts. Scand J Immunol. 2013;77(5):350–360. doi: 10.1111/sji.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford JG, Lo B, Kislan MM, Thomas JM, Evans K, Cain DW, Kraft M, Williams KL, Wright JR. Surfactant protein-A inhibits mycoplasma-induced dendritic cell maturation through regulation of HMGB-1 cytokine activity. J Immunol. 2010;185(7):3884–3894. doi: 10.4049/jimmunol.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Bae J, Kim YK, Gil M, Lee JY, Park CS, Lee KJ. Inhibitory effects of berberine on lipopolysaccharide-induced inducible nitric oxide synthase and the high-mobility group box 1 release in macrophages. Biochem Biophys Res Commun. 2013a;431(3):506–511. doi: 10.1016/j.bbrc.2012.12.143. [DOI] [PubMed] [Google Scholar]
- Lee D, Lee KH, Park H, Kim SH, Jin T, Cho S, Chung JH, Lim S, Park S. The effect of soluble RAGE on inhibition of angiotensin II-mediated atherosclerosis in apolipoprotein E deficient mice. PLoS ONE. 2013b;8(8):e69669. doi: 10.1371/journal.pone.0069669. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lee H, Park M, Shin N, Kim G, Kim YG, Shin JS, Kim H. High mobility group box-1 is phosphorylated by protein kinase C zeta and secreted in colon cancer cells. Biochem Biophys Res Commun. 2012a;424(2):321–326. doi: 10.1016/j.bbrc.2012.06.116. [DOI] [PubMed] [Google Scholar]
- Lee H, Shin N, Song M, Kang UB, Yeom J, Lee C, Ahn YH, Yoo JS, Paik YK, Kim H. Analysis of nuclear high mobility group box 1 (HMGB1)-binding proteins in colon cancer cells: clustering with proteins involved in secretion and extranuclear function. J Proteome Res. 2010a;9(9):4661–4670. doi: 10.1021/pr100386r. [DOI] [PubMed] [Google Scholar]
- Lee H, Song M, Shin N, Shin CH, Min BS, Kim HS, Yoo JS, Kim H. Diagnostic significance of serum HMGB1 in colorectal carcinomas. PloS one. 2012b;7(4):e34318. doi: 10.1371/journal.pone.0034318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Hypoxia-inducible Factor-1 (HIF-1)-independent hypoxia response of the small heat shock protein hsp-16.1 gene regulated by chromatin-remodeling factors in the nematode Caenorhabditis elegans. J Biol Chem. 2013;288(3):1582–1589. doi: 10.1074/jbc.M112.401554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MN, Roy M, Ong SE, Mertins P, Villani AC, Li W, Dotiwala F, Sen J, Doench JG, Orzalli MH, Kramnik I, Knipe DM, Lieberman J, Carr SA, Hacohen N. Identification of regulators of the innate immune response to cytosolic DNA and retroviral infection by an integrative approach. Nat Immunol. 2013c;14(2):179–185. doi: 10.1038/ni.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee OH, Kim J, Kim JM, Lee H, Kim EH, Bae SK, Choi Y, Nam HS, Heo JH. Decreased expression of sirtuin 6 is associated with release of high mobility group box-1 after cerebral ischemia. Biochem Biophys Res Commun. 2013d;438(2):388–394. doi: 10.1016/j.bbrc.2013.07.085. [DOI] [PubMed] [Google Scholar]
- Lee SY, Jeon HM, Kim CH, Jeong EK, Ju MK, Park SY, Jung SY, Kim YJ, Lim SC, Han SI, Kang HS. CuZnSOD and MnSOD inhibit metabolic stress-induced necrosis and multicellular tumour spheroid growth. Int J Oncol. 2010b;37(1):195–202. doi: 10.3892/ijo_00000667. [DOI] [PubMed] [Google Scholar]
- Lee SY, Jeon HM, Kim CH, Ju MK, Bae HS, Park HG, Lim SC, Han SI, Kang HS. Homeobox gene Dlx-2 is implicated in metabolic stress-induced necrosis. Mol Cancer. 2011a;10:113. doi: 10.1186/1476-4598-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Kim TH, Ku SK, Min KJ, Lee HS, Kwon TK, Bae JS. Barrier protective effects of withaferin A in HMGB1-induced inflammatory responses in both cellular and animal models. Toxicol Appl Pharmacol. 2012c;262(1):91–98. doi: 10.1016/j.taap.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Lee W, Ku SK, Bae JW, Bae JS. Inhibitory effects of lycopene on HMGB1-mediated pro-inflammatory responses in both cellular and animal models. Food Chem Toxicol. 2012d;50(6):1826–1833. doi: 10.1016/j.fct.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Lee W, Ku SK, Kim JA, Lee T, Bae JS. Inhibitory effects of epi-sesamin on HMGB1-induced vascular barrier disruptive responses in vitro and in vivo. Toxicol Appl Pharmacol. 2013e;267(3):201–208. doi: 10.1016/j.taap.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Lee W, Ku SK, Kim TH, Bae JS. Emodin-6-O-beta-D-glucoside inhibits HMGB1-induced inflammatory responses in vitro and in vivo. Food Chem Toxicol. 2013f;52:97–104. doi: 10.1016/j.fct.2012.10.061. [DOI] [PubMed] [Google Scholar]
- Lee W, Ku SK, Lee YM, Bae JS. Anti-septic effects of glyceollins in HMGB1-induced inflammatory responses in vitro and in vivo. Food Chem Toxicol. 2014;63:1–8. doi: 10.1016/j.fct.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40(1):6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- Lee Y, Fryer JD, Kang H, Crespo-Barreto J, Bowman AB, Gao Y, Kahle JJ, Hong JS, Kheradmand F, Orr HT, Finegold MJ, Zoghbi HY. ATXN1 protein family and CIC regulate extracellular matrix remodeling and lung alveolarization. Dev Cell. 2011b;21(4):746–757. doi: 10.1016/j.devcel.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee HE, Shin JM, Sohn KC, Im M, Kim CD, Seo YJ, Lee YH, Lee JH. Clinical significance of serum high-mobility group box 1 level in alopecia areata. Journal of the American Academy of Dermatology. 2013g;69(5):742–747. doi: 10.1016/j.jaad.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Leelahavanichkul A, Huang Y, Hu X, Zhou H, Tsuji T, Chen R, Kopp JB, Schnermann J, Yuen PS, Star RA. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney Int. 2011;80(11):1198–1211. doi: 10.1038/ki.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans JC, Butter LM, Pulskens WP, Teske GJ, Claessen N, van der Poll T, Florquin S. The role of Toll-like receptor 2 in inflammation and fibrosis during progressive renal injury. PLoS ONE. 2009;4(5):e5704. doi: 10.1371/journal.pone.0005704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehming N, Le Saux A, Schuller J, Ptashne M. Chromatin components as part of a putative transcriptional repressing complex. Proc Natl Acad Sci U S A. 1998;95(13):7322–7326. doi: 10.1073/pnas.95.13.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehming N, Thanos D, Brickman JM, Ma J, Maniatis T, Ptashne M. An HMG-like protein that can switch a transcriptional activator to a repressor. Nature. 1994;371(6493):175–179. doi: 10.1038/371175a0. [DOI] [PubMed] [Google Scholar]
- Lehtonen S, Lehtonen E. HMG-17 is an early marker of inductive interactions in the developing mouse kidney. Differentiation. 2001;67(4–5):154–163. doi: 10.1046/j.1432-0436.2001.670407.x. [DOI] [PubMed] [Google Scholar]
- Lepp WA, Martinez P. Solid-phase enzyme immunoassay for the detection of HMG nonhistone proteins in their native structure. J Immunoassay. 1989;10(4):449–465. doi: 10.1080/01971528908053252. [DOI] [PubMed] [Google Scholar]
- Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120(2):159–162. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP, Vodovotz Y, Billiar TR. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293(4):R1538–R1544. doi: 10.1152/ajpregu.00272.2007. [DOI] [PubMed] [Google Scholar]
- Li C, Gao Y, Xing Y, Zhu H, Shen J, Tian J. Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia-reperfusion injury in rats via regulating the inflammation response. Food Chem Toxicol. 2011a;49(9):2090–2095. doi: 10.1016/j.fct.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Li DQ, Hou YF, Wu J, Chen Y, Lu JS, Di GH, Ou ZL, Shen ZZ, Ding J, Shao ZM. Gene expression profile analysis of an isogenic tumour metastasis model reveals a functional role for oncogene AF1Q in breast cancer metastasis. Eur J Cancer. 2006a;42(18):3274–3286. doi: 10.1016/j.ejca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Li F, Chen Z, Pan Q, Fu S, Lin F, Ren H, Han H, Billiar TR, Sun F, Li Q. The protective effect of PNU-282987, a selective alpha7 nicotinic acetylcholine receptor agonist, on the hepatic ischemia-reperfusion injury is associated with the inhibition of high-mobility group box 1 protein expression and nuclear factor kappaB activation in mice. Shock. 2013a;39(2):197–203. doi: 10.1097/SHK.0b013e31827aa1f6. [DOI] [PubMed] [Google Scholar]
- Li F, Yang N, Zhang L, Tan H, Huang B, Liang Y, Chen M, Yu X. Increased expression of toll-like receptor 2 in rat diabetic nephropathy. Am J Nephrol. 2010a;32(2):179–186. doi: 10.1159/000317023. [DOI] [PubMed] [Google Scholar]
- Li G, Liang X, Lotze MT. HMGB1: The Central Cytokine for All Lymphoid Cells. Frontiers in immunology. 2013b;4:68. doi: 10.3389/fimmu.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Bian C, Liao L, Li J, Zhao RC. miR-17-5p promotes human breast cancer cell migration and invasion through suppression of HBP1. Breast Cancer Res Treat. 2011b;126(3):565–575. doi: 10.1007/s10549-010-0954-4. [DOI] [PubMed] [Google Scholar]
- Li J, Dong Y, Chen H, Han H, Yu Y, Wang G, Zeng Y, Xie K. Protective effects of hydrogen-rich saline in a rat model of permanent focal cerebral ischemia via reducing oxidative stress and inflammatory cytokines. Brain Res. 2012a;1486:103–111. doi: 10.1016/j.brainres.2012.09.031. [DOI] [PubMed] [Google Scholar]
- Li J, Kokkola R, Tabibzadeh S, Yang R, Ochani M, Qiang X, Harris HE, Czura CJ, Wang H, Ulloa L, Warren HS, Moldawer LL, Fink MP, Andersson U, Tracey KJ, Yang H. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med. 2003;9(1–2):37–45. [PMC free article] [PubMed] [Google Scholar]
- Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, Chan FK, Wu H. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012b;150(2):339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Qu X, Schmidt AM. Sp1-binding elements in the promoter of RAGE are essential for amphoterin-mediated gene expression in cultured neuroblastoma cells. J Biol Chem. 1998;273(47):30870–30878. doi: 10.1074/jbc.273.47.30870. [DOI] [PubMed] [Google Scholar]
- Li J, Wang FP, She WM, Yang CQ, Li L, Tu CT, Wang JY, Jiang W. Enhanced high-mobility group box 1 (HMGB1) modulates regulatory T cells (Treg)/T helper 17 (Th17) balance via toll-like receptor (TLR)-4-interleukin (IL)-6 pathway in patients with chronic hepatitis B. Journal of viral hepatitis. 2014a;21(2):129–140. doi: 10.1111/jvh.12152. [DOI] [PubMed] [Google Scholar]
- Li J, Xie H, Wen T, Liu H, Zhu W, Chen X. Expression of high mobility group box chromosomal protein 1 and its modulating effects on downstream cytokines in systemic lupus erythematosus. The Journal of rheumatology. 2010b;37(4):766–775. doi: 10.3899/jrheum.090663. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang Y, Xiang Z, Xiao S, Yu F, Yu Z. High mobility group box 1 can enhance NF-kappaB activation and act as a pro-inflammatory molecule in the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2013c;35(1):63–70. doi: 10.1016/j.fsi.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao S, Yi M, Hu X, Xie H, Zhu W, Chen M. Activation of JAK-STAT1 signal transduction pathway in lesional skin and monocytes from patients with systemic lupus erythematosus. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011c;36(2):109–115. doi: 10.3969/j.issn.1672-7347.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Li L, Chen L, Hu L, Liu Y, Sun HY, Tang J, Hou YJ, Chang YX, Tu QQ, Feng GS, Shen F, Wu MC, Wang HY. Nuclear factor high-mobility group box1 mediating the activation of Toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology. 2011d;54(5):1620–1630. doi: 10.1002/hep.24552. [DOI] [PubMed] [Google Scholar]
- Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412(6842):95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- Li M, Song L, Gao X, Chang W, Qin X. Toll-like receptor 4 on islet beta cells senses expression changes in high-mobility group box 1 and contributes to the initiation of type 1 diabetes. Exp Mol Med. 2012c;44(4):260–267. doi: 10.3858/emm.2012.44.4.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Bledsoe G, Yang ZR, Fan H, Chao L, Chao J. Human kallistatin administration reduces organ injury and improves survival in a mouse model of polymicrobial sepsis. Immunology. 2014b doi: 10.1111/imm.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bao HG, Han L, Liu L, Wang X. Effects of adiponectin on mortality and its mechanism in a sepsis mouse model. J Invest Surg. 2012d;25(4):214–219. doi: 10.3109/08941939.2011.624257. [DOI] [PubMed] [Google Scholar]
- Li S, Luo C, Yin C, Peng C, Han R, Zhou J, He Q. Endogenous HMGB1 is required in endotoxin tolerance. J Surg Res. 2013d;185(1):319–328. doi: 10.1016/j.jss.2013.05.062. [DOI] [PubMed] [Google Scholar]
- Li T, Gui Y, Yuan T, Liao G, Bian C, Jiang Q, Huang S, Liu B, Wu D. Overexpression of high mobility group box 1 with poor prognosis in patients after radical prostatectomy. BJU international. 2012e;110(11 Pt C):E1125–E1130. doi: 10.1111/j.1464-410X.2012.11277.x. [DOI] [PubMed] [Google Scholar]
- Li T, Zuo X, Zhou Y, Wang Y, Zhuang H, Zhang L, Zhang H, Xiao X. The vagus nerve and nicotinic receptors involve inhibition of HMGB1 release and early pro-inflammatory cytokines function in collagen-induced arthritis. J Clin Immunol. 2010c;30(2):213–220. doi: 10.1007/s10875-009-9346-0. [DOI] [PubMed] [Google Scholar]
- Li W, Ashok M, Li J, Yang H, Sama AE, Wang H. A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1. PLoS ONE. 2007a;2(11):e1153. doi: 10.1371/journal.pone.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li J, Ashok M, Wu R, Chen D, Yang L, Yang H, Tracey KJ, Wang P, Sama AE, Wang H. A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1. J Immunol. 2007b;178(6):3856–3864. doi: 10.4049/jimmunol.178.6.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li J, Sama AE, Wang H. Carbenoxolone blocks endotoxin-induced protein kinase R (PKR) activation and high mobility group box 1 (HMGB1) release. Mol Med. 2013e;19:203–211. doi: 10.2119/molmed.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Sama AE, Wang H. Role of HMGB1 in cardiovascular diseases. Curr Opin Pharmacol. 2006b;6(2):130–135. doi: 10.1016/j.coph.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wu K, Zhao E, Shi L, Li R, Zhang P, Yin Y, Shuai X, Wang G, Tao K. HMGB1 recruits myeloid derived suppressor cells to promote peritoneal dissemination of colon cancer after resection. Biochem Biophys Res Commun. 2013f;436(2):156–161. doi: 10.1016/j.bbrc.2013.04.109. [DOI] [PubMed] [Google Scholar]
- Li W, Zhu S, Li J, Assa A, Jundoria A, Xu J, Fan S, Eissa NT, Tracey KJ, Sama AE, Wang H. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem Pharmacol. 2011e;81(9):1152–1163. doi: 10.1016/j.bcp.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhu S, Li J, Huang Y, Zhou R, Fan X, Yang H, Gong X, Eissa NT, Jahnen-Dechent W, Wang P, Tracey KJ, Sama AE, Wang H. A hepatic protein, fetuin-A, occupies a protective role in lethal systemic inflammation. PLoS ONE. 2011f;6(2):e16945. doi: 10.1371/journal.pone.0016945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang LK, Wang LW, Han XQ, Yang F, Gong ZJ. Cisplatin Protects against Acute Liver Failure by Inhibiting Nuclear HMGB1 Release. Int J Mol Sci. 2013g;14(6):11224–11237. doi: 10.3390/ijms140611224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gong W, Zhang L, Huang G, Li J, Shen X, He F. Expression and purification of the fusion protein HMGB1Abox-TMD1, a novel HMGB1 antagonist. Biochemistry (Mosc) 2010d;75(4):466–471. doi: 10.1134/s0006297910040103. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang C, Zhao M, Liang G, Xiao R, Yung S, Chan TM, Lu Q. A possible role of HMGB1 in DNA demethylation in CD4+ T cells from patients with systemic lupus erythematosus. Clin Dev Immunol. 2013h;2013:206298. doi: 10.1155/2013/206298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang LX, Pang P, Cui Z, Aung S, Haley D, Fox BA, Urba WJ, Hu HM. Tumor-derived autophagosome vaccine: mechanism of cross-presentation and therapeutic efficacy. Clin Cancer Res. 2011g;17(22):7047–7057. doi: 10.1158/1078-0432.CCR-11-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xiang M, Yuan Y, Xiao G, Zhang J, Jiang Y, Vodovotz Y, Billiar TR, Wilson MA, Fan J. Hemorrhagic shock augments lung endothelial cell activation: role of temporal alterations of TLR4 and TLR2. Am J Physiol Regul Integr Comp Physiol. 2009;297(6):R1670–R1680. doi: 10.1152/ajpregu.00445.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ramanujan K, Ma Y, Kirsch DG, Glass DJ. Oncogenic NRAS, required for pathogenesis of embryonic rhabdomyosarcoma, relies upon the HMGA2-IGF2BP2 pathway. Cancer Res. 2013i;73(10):3041–3050. doi: 10.1158/0008-5472.CAN-12-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Li X, Zhang X, Lv Z, He G, Zhao W, Ren X, Li Y, Bian X, Liao W, Liu W, Yang G, Ding Y. MicroRNA-137, an HMGA1 target, suppresses colorectal cancer cell invasion and metastasis in mice by directly targeting FMNL2. Gastroenterology. 2013a;144(3):624–635. e624. doi: 10.1053/j.gastro.2012.11.033. [DOI] [PubMed] [Google Scholar]
- Liang X, Chavez AR, Schapiro NE, Loughran P, Thorne SH, Amoscato AA, Zeh HJ, Beer-Stolz D, Lotze MT, de Vera ME. Ethyl pyruvate administration inhibits hepatic tumor growth. Journal of leukocyte biology. 2009;86(3):599–607. doi: 10.1189/jlb.0908578. [DOI] [PubMed] [Google Scholar]
- Liang X, Wang RS, Wang F, Liu S, Guo F, Sun L, Wang YJ, Sun YX, Chen XL. Sodium butyrate protects against severe burn-induced remote acute lung injury in rats. PLoS ONE. 2013b;8(7):e68786. doi: 10.1371/journal.pone.0068786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau SS, Jazag A, Ito K, Whang EE. Overexpression of HMGA1 promotes anoikis resistance and constitutive Akt activation in pancreatic adenocarcinoma cells. Br J Cancer. 2007;96(6):993–1000. doi: 10.1038/sj.bjc.6603654. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liau SS, Rocha F, Matros E, Redston M, Whang E. High mobility group AT-hook 1 (HMGA1) is an independent prognostic factor and novel therapeutic target in pancreatic adenocarcinoma. Cancer. 2008;113(2):302–314. doi: 10.1002/cncr.23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichota J, Grasser KD. Differential chromatin association and nucleosome binding of the maize HMGA, HMGB, and SSRP1 proteins. Biochemistry. 2001;40(26):7860–7867. doi: 10.1021/bi010548y. [DOI] [PubMed] [Google Scholar]
- Lildballe DL, Pedersen DS, Kalamajka R, Emmersen J, Houben A, Grasser KD. The expression level of the chromatin-associated HMGB1 protein influences growth, stress tolerance, and transcriptome in Arabidopsis. J Mol Biol. 2008;384(1):9–21. doi: 10.1016/j.jmb.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hammerling GG, Nawroth PP, Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113(11):1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley DM, Clegg RM. The structure of the four-way junction in DNA. Annu Rev Biophys Biomol Struct. 1993;22:299–328. doi: 10.1146/annurev.bb.22.060193.001503. [DOI] [PubMed] [Google Scholar]
- Lilljebjorn H, Heidenblad M, Nilsson B, Lassen C, Horvat A, Heldrup J, Behrendtz M, Johansson B, Andersson A, Fioretos T. Combined high-resolution array-based comparative genomic hybridization and expression profiling of ETV6/RUNX1-positive acute lymphoblastic leukemias reveal a high incidence of cryptic Xq duplications and identify several putative target genes within the commonly gained region. Leukemia. 2007;21(10):2137–2144. doi: 10.1038/sj.leu.2404879. [DOI] [PubMed] [Google Scholar]
- Lim JH, Catez F, Birger Y, West KL, Prymakowska-Bosak M, Postnikov YV, Bustin M. Chromosomal protein HMGN1 modulates histone H3 phosphorylation. Mol Cell. 2004;15(4):573–584. doi: 10.1016/j.molcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Lim JH, West KL, Rubinstein Y, Bergel M, Postnikov YV, Bustin M. Chromosomal protein HMGN1 enhances the acetylation of lysine 14 in histone H3. Embo J. 2005;24(17):3038–3048. doi: 10.1038/sj.emboj.7600768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SC, Kim SM, Choi JE, Kim CH, Duong HQ, Han SI, Kang HS. Sodium salicylate switches glucose depletion-induced necrosis to autophagy and inhibits high mobility group box protein 1 release in A549 lung adenocarcinoma cells. Oncol Rep. 2008;19(5):1165–1171. [PubMed] [Google Scholar]
- Limana F, Esposito G, D'Arcangelo D, Di Carlo A, Romani S, Melillo G, Mangoni A, Bertolami C, Pompilio G, Germani A, Capogrossi MC. HMGB1 attenuates cardiac remodelling in the failing heart via enhanced cardiac regeneration and miR-206-mediated inhibition of TIMP-3. PLoS ONE. 2011;6(6):e19845. doi: 10.1371/journal.pone.0019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limana F, Esposito G, Fasanaro P, Foglio E, Arcelli D, Voellenkle C, Di Carlo A, Avitabile D, Martelli F, Russo MA, Pompilio G, Germani A, M CC. Transcriptional profiling of HMGB1-induced myocardial repair identifies a key role for Notch signaling. Mol Ther. 2013;21(10):1841–1851. doi: 10.1038/mt.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G, Leoni O, Palumbo R, Battistini L, Rastaldo R, Muller S, Pompilio G, Anversa P, Bianchi ME, Capogrossi MC. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res. 2005;97(8):e73–e83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- Lin L, Zhong K, Sun Z, Wu G, Ding G. Receptor for advanced glycation end products (RAGE) partially mediates HMGB1-ERKs activation in clear cell renal cell carcinoma. Journal of cancer research and clinical oncology. 2012a;138(1):11–22. doi: 10.1007/s00432-011-1067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Yiu WH, Li RX, Wu HJ, Wong DW, Chan LY, Leung JC, Lai KN, Tang SC. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int. 2013;83(5):887–900. doi: 10.1038/ki.2013.11. [DOI] [PubMed] [Google Scholar]
- Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS, Chan KW, Lai KN, Tang SC. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. 2012b;23(1):86–102. doi: 10.1681/ASN.2010111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Yang XP, Fang D, Ren X, Zhou H, Fang J, Liu X, Zhou S, Wen F, Yao X, Wang JM, Su SB. High-mobility group box-1 mediates toll-like receptor 4-dependent angiogenesis. Arterioscler Thromb Vasc Biol. 2011;31(5):1024–1032. doi: 10.1161/ATVBAHA.111.224048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Sammy F, Yang H, Thundivalappil S, Hellman J, Tracey KJ, Warren HS. Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. J Immunol. 2012c;189(4):2017–2022. doi: 10.4049/jimmunol.1103623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TY, Tseng SH, Li SJ, Chen JC, Shieh JS, Chen Y. Tetrandrine increased the survival rate of mice with lipopolysaccharide-induced endotoxemia. J Trauma. 2009;66(2):411–417. doi: 10.1097/TA.0b013e31815ebae9. [DOI] [PubMed] [Google Scholar]
- Lin X, Yang H, Sakuragi T, Hu M, Mantell LL, Hayashi S, Al-Abed Y, Tracey KJ, Ulloa L, Miller EJ. Alpha-chemokine receptor blockade reduces high mobility group box 1 protein-induced lung inflammation and injury and improves survival in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289(4):L583–L590. doi: 10.1152/ajplung.00091.2005. [DOI] [PubMed] [Google Scholar]
- Lindersson EK, Hojrup P, Gai WP, Locker D, Martin D, Jensen PH. alpha-Synuclein filaments bind the transcriptional regulator HMGB-1. Neuroreport. 2004;15(18):2735–2739. [PubMed] [Google Scholar]
- Lindstrom O, Tukiainen E, Kylanpaa L, Mentula P, Rouhiainen A, Puolakkainen P, Rauvala H, Repo H. Circulating levels of a soluble form of receptor for advanced glycation end products and high-mobility group box chromosomal protein 1 in patients with acute pancreatitis. Pancreas. 2009;38(8):e215–e220. doi: 10.1097/MPA.0b013e3181bb59a7. [DOI] [PubMed] [Google Scholar]
- Ling Y, Yang ZY, Yin T, Li L, Yuan WW, Wu HS, Wang CY. Heparin changes the conformation of high-mobility group protein 1 and decreases its affinity toward receptor for advanced glycation endproducts in vitro. Int Immunopharmacol. 2011;11(2):187–193. doi: 10.1016/j.intimp.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Link KA, Balasubramaniam S, Sharma A, Comstock CE, Godoy-Tundidor S, Powers N, Cao KH, Haelens A, Claessens F, Revelo MP, Knudsen KE. Targeting the BAF57 SWI/SNF subunit in prostate cancer: a novel platform to control androgen receptor activity. Cancer Res. 2008;68(12):4551–4558. doi: 10.1158/0008-5472.CAN-07-6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link KA, Burd CJ, Williams E, Marshall T, Rosson G, Henry E, Weissman B, Knudsen KE. BAF57 governs androgen receptor action and androgen-dependent proliferation through SWI/SNF. Mol Cell Biol. 2005;25(6):2200–2215. doi: 10.1128/MCB.25.6.2200-2215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou TG, Adler FR, Keogh RH, Li Y, Jensen JL, Walsh W, Packer K, Clark T, Carveth H, Chen J, Rogers SL, Lane C, Moore J, Sturrock A, Paine R, 3rd, Cox DR, Hoidal JR. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PLoS ONE. 2012;7(8):e42748. doi: 10.1371/journal.pone.0042748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, Szabo G. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol. 2013;94(1):171–182. doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AJ, Corbett E, Ortega F, Schatz DG. Cooperative recruitment of HMGB1 during V(D)J recombination through interactions with RAG1 and DNA. Nucleic Acids Res. 2013;41(5):3289–3301. doi: 10.1093/nar/gks1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Dirsch O, Fang H, Sun J, Jin H, Dong W, Dahmen U. HMGB1 in ischemic and non-ischemic liver after selective warm ischemia/reperfusion in rat. Histochem Cell Biol. 2011a;135(5):443–452. doi: 10.1007/s00418-011-0802-6. [DOI] [PubMed] [Google Scholar]
- Liu A, Fang H, Dirsch O, Jin H, Dahmen U. Oxidation of HMGB1 causes attenuation of its pro-inflammatory activity and occurs during liver ischemia and reperfusion. PLoS ONE. 2012a;7(4):e35379. doi: 10.1371/journal.pone.0035379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Fang H, Yang Y, Sun J, Fan H, Liu S, Dirsch O, Dahmen U. The fibrin-derived peptide bbeta15–42 attenuates liver damage in a rat model of liver ischemia/reperfusion injury. Shock. 2013a;39(4):397–403. doi: 10.1097/SHK.0b013e31828c2b75. [DOI] [PubMed] [Google Scholar]
- Liu A, Fang H, Yang Y, Sun J, Hua F, Liu S, Dirsch O, Dahmen U. The Fibrin-derived Peptide Bss15–42 Attenuates the Liver Damage in a Rat Model of Liver Ischemia Reperfusion Injury. Shock (Augusta, Ga.) 2013b doi: 10.1097/SHK.0b013e31828c2b75. [DOI] [PubMed] [Google Scholar]
- Liu F, Chau KY, Arlotta P, Ono SJ. The HMG I proteins: dynamic roles in gene activation, development, and tumorigenesis. Immunol Res. 2001;24(1):13–29. doi: 10.1385/IR:24:1:13. [DOI] [PubMed] [Google Scholar]
- Liu F, Zhang Y, Peng Z, Gao H, Xu L, Chen M. High expression of high mobility group box 1 (hmgb1) predicts poor prognosis for hepatocellular carcinoma after curative hepatectomy. J Transl Med. 2012b;10:135. doi: 10.1186/1479-5876-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Wang J, Park YJ, Tsuruta Y, Lorne EF, Zhao X, Abraham E. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J Immunol. 2008a;181(6):4240–4246. doi: 10.4049/jimmunol.181.6.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yao YM, Yu Y, Dong N, Yin HN, Sheng ZY. Role of Janus kinase/signal transducer and activator of transcription pathway in regulation of expression and inflammation-promoting activity of high mobility group box protein 1 in rat peritoneal macrophages. Shock. 2007a;27(1):55–60. doi: 10.1097/01.shk.0000233197.40989.31. [DOI] [PubMed] [Google Scholar]
- Liu HB, Fan XG, Huang JJ, Li N, Peng JP, Li SL, Wang H. Serum level of HMGB1 in patients with hepatitis B and its clinical significance. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chinese journal of hepatology. 2007b;15(11):812–815. [PubMed] [Google Scholar]
- Liu J, Liu Y, Zhang H, Chen G, Wang K, Xiao X. KLF4 promotes the expression, translocation, and releas eof HMGB1 in RAW264.7 macrophages in response to LPS. Shock. 2008b;30(3):260–266. doi: 10.1097/shk.0b013e318162bef7. [DOI] [PubMed] [Google Scholar]
- Liu J, Schiltz JF, Ashar HR, Chada KK. Hmga1 is required for normal sperm development. Mol Reprod Dev. 2003;66(1):81–89. doi: 10.1002/mrd.10323. [DOI] [PubMed] [Google Scholar]
- Liu K, Huang J, Xie M, Yu Y, Zhu S, Kang R, Cao L, Tang D, Duan X. MIR34A regulates autophagy and apoptosis by targeting HMGB1 in the retinoblastoma cell. Autophagy. 2014a;10(3) doi: 10.4161/auto.27418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T, Nishibori M. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. Faseb J. 2007c;21(14):3904–3916. doi: 10.1096/fj.07-8770com. [DOI] [PubMed] [Google Scholar]
- Liu L, Wang S, Zhang Q, Ding Y. Identification of potential genes/proteins regulated by Tiam1 in colorectal cancer by microarray analysis and proteome analysis. Cell Biol Int. 2008c;32(10):1215–1222. doi: 10.1016/j.cellbi.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Liu L, Yang M, Kang R, Wang Z, Zhao Y, Yu Y, Xie M, Yin X, Livesey KM, Lotze MT, Tang D, Cao L. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia. 2011b;25(1):23–31. doi: 10.1038/leu.2010.225. [DOI] [PubMed] [Google Scholar]
- Liu L, Yang M, Kang R, Wang Z, Zhao Y, Yu Y, Xie M, Yin X, Livesey KM, Lotze MT, Tang D, Cao L. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2011c;25(1):23–31. doi: 10.1038/leu.2010.225. [DOI] [PubMed] [Google Scholar]
- Liu M, Yu Y, Jiang H, Zhang L, Zhang PP, Yu P, Jia JG, Chen RZ, Zou YZ, Ge JB. Simvastatin suppresses vascular inflammation and atherosclerosis in ApoE(−/−) mice by downregulating the HMGB1-RAGE axis. Acta Pharmacol Sin. 2013c;34(6):830–836. doi: 10.1038/aps.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ML, Dong HY, Zhang B, Zheng WS, Zhao PT, Liu Y, Niu W, Xu DQ, Li ZC. Insulin reduces LPS-induced lethality and lung injury in rats. Pulm Pharmacol Ther. 2012c;25(6):472–477. doi: 10.1016/j.pupt.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Liu PL, Tsai JR, Hwang JJ, Chou SH, Cheng YJ, Lin FY, Chen YL, Hung CY, Chen WC, Chen YH, Chong IW. High-mobility group box 1-mediated matrix metalloproteinase-9 expression in non-small cell lung cancer contributes to tumor cell invasiveness. Am J Respir Cell Mol Biol. 2010a;43(5):530–538. doi: 10.1165/rcmb.2009-0269OC. [DOI] [PubMed] [Google Scholar]
- Liu QY, Yao YM, Yan YH, Dong N, Sheng ZY. High mobility group box 1 protein suppresses T cell-mediated immunity via CD11c(low)CD45RB(high) dendritic cell differentiation. Cytokine. 2011d;54(2):205–211. doi: 10.1016/j.cyto.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Liu S, Stolz DB, Sappington PL, Macias CA, Killeen ME, Tenhunen JJ, Delude RL, Fink MP. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am J Physiol Cell Physiol. 2006;290(4):C990–C999. doi: 10.1152/ajpcell.00308.2005. [DOI] [PubMed] [Google Scholar]
- Liu W, Deng J, Xu J, Wang H, Yuan M, Liu N, Jiang Y, Liu J. High-mobility group box 1 (HMGB1) downregulates cardiac transient outward potassium current (Ito) through downregulation of Kv4.2 and Kv4.3 channel transcripts and proteins. J Mol Cell Cardiol. 2010b;49(3):438–448. doi: 10.1016/j.yjmcc.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Liu Y, Prasad R, Beard WA, Hou EW, Horton JK, McMurray CT, Wilson SH. Coordination between polymerase beta and FEN1 can modulate CAG repeat expansion. J Biol Chem. 2009;284(41):28352–28366. doi: 10.1074/jbc.M109.050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Prasad R, Wilson SH. HMGB1: roles in base excision repair and related function. Biochim Biophys Acta. 2010c;1799(1–2):119–130. doi: 10.1016/j.bbagrm.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Qiu Y, Zhang X, Tian Y, Huang D, Zhou X, Tan P, Yu C, Qi L, Xiao J. The expression and correlation of HMGB1 and VEGF protein in laryngeal squamous cell carcinoma. Lin chuang er bi yan hou tou jing wai ke za zhi = Journal of clinical otorhinolaryngology, head, and neck surgery. 2011e;25(6):265–269. [PubMed] [Google Scholar]
- Liu Y, Xie C, Zhang X, Huang D, Zhou X, Tan P, Qi L, Hu G, Tian Y, Qiu Y. Elevated expression of HMGB1 in squamous-cell carcinoma of the head and neck and its clinical significance. European journal of cancer (Oxford, England: 1990) 2010d;46(16):3007–3015. doi: 10.1016/j.ejca.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xie CL, Qiu YZ, Tian YQ, Zhang X, Huang DH, Zhou XJ, Tan PQ, Yu CY, Qi L, Li B, Xiao JY. Expression of HMGB1 protein in laryngeal squamous cell carcinoma and its clinical significance. Zhonghua zhong liu za zhi [Chinese journal of oncology] 2012d;34(2):132–136. doi: 10.3760/cma.j.issn.0253-3766.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang L, Tao K, Vizcaychipi MP, Lloyd DM, Sun X, Irwin MG, Ma D, Yu W. Protective effects of hydrogen enriched saline on liver ischemia reperfusion injury by reducing oxidative stress and HMGB1 release. BMC Gastroenterol. 2014b;14(1):12. doi: 10.1186/1471-230X-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Falo LD, Jr, You Z. Knockdown of HMGB1 in tumor cells attenuates their ability to induce regulatory T cells and uncovers naturally acquired CD8 T cell-dependent antitumor immunity. J Immunol. 2011f;187(1):118–125. doi: 10.4049/jimmunol.1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang J, Huang X, Huang L, Li S, Wang Z. Magnesium sulfate inhibits the secretion of high mobility group box 1 from lipopolysaccharide-activated RAW264.7 macrophages in vitro. J Surg Res. 2013d;179(1):e189–e195. doi: 10.1016/j.jss.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Livesey K, Kang R, Vernon P, Buchser W, Loughran P, Watkins SC, Zhang L, Manfredi JJ, Zeh HJ, Li L, Lotze M, Tang D. p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Res. 2012a;72(8):1996–2005. doi: 10.1158/0008-5472.CAN-11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey KM, Kang R, Vernon P, Buchser W, Loughran P, Watkins SC, Zhang L, Manfredi JJ, Zeh HJ, 3rd, Li L, Lotze MT, Tang D. p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Res. 2012b;72(8):1996–2005. doi: 10.1158/0008-5472.CAN-11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey KM, Kang R, Vernon P, Buchser W, Loughran P, Watkins SC, Zhang L, Manfredi JJ, Zeh HJ, 3rd, Li L, Lotze MT, Tang D. p53/HMGB1 Complexes Regulate Autophagy and Apoptosis. Cancer Res. 2012c doi: 10.1158/0008-5472.CAN-11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey KM, Kang R, Zeh HJ, 3rd, Lotze MT, Tang D. Direct molecular interactions between HMGB1 and TP53 in colorectal cancer. Autophagy. 2012d;8(5):846–848. doi: 10.4161/auto.19891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Coco D, Veglianese P, Allievi E, Bendotti C. Distribution and cellular localization of high mobility group box protein 1 (HMGB1) in the spinal cord of a transgenic mouse model of ALS. Neurosci Lett. 2007;412(1):73–77. doi: 10.1016/j.neulet.2006.10.063. [DOI] [PubMed] [Google Scholar]
- Lotfi R, Herzog GI, DeMarco RA, Beer-Stolz D, Lee JJ, Rubartelli A, Schrezenmeier H, Lotze MT. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J Immunol. 2009;183(8):5023–5031. doi: 10.4049/jimmunol.0900504. [DOI] [PubMed] [Google Scholar]
- Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. 2007;30(1):16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- Lotfi S, Rabbani-Chadegani A, Ghadam P. Evidence for the binding affinity of daunomycin to HMGB1 protein in chromatin and in solution. Int J Biol Macromol. 2013;52:206–211. doi: 10.1016/j.ijbiomac.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Lotze MT, DeMarco RA. Dealing with death: HMGB1 as a novel target for cancer therapy. Curr Opin Investig Drugs. 2003;4(12):1405–1409. [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5(4):331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Loukili N, Rosenblatt-Velin N, Li J, Clerc S, Pacher P, Feihl F, Waeber B, Liaudet L. Peroxynitrite induces HMGB1 release by cardiac cells in vitro and HMGB1 upregulation in the infarcted myocardium in vivo. Cardiovasc Res. 2011;89(3):586–594. doi: 10.1093/cvr/cvq373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Antoine DJ, Kwan K, Lundback P, Wahamaa H, Schierbeck H, Robinson M, Van Zoelen MA, Yang H, Li J, Erlandsson-Harris H, Chavan SS, Wang H, Andersson U, Tracey KJ. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Lee J, Nie X, Li M, Morozov YI, Venkatesh S, Bogenhagen DF, Temiakov D, Suzuki CK. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease. Mol Cell. 2013a;49(1):121–132. doi: 10.1016/j.molcel.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012a doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012b;488(7413):670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Sodhi CP, Hackam DJ. Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology. 2013b doi: 10.1016/j.pathophys.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan ZG, Ma XC, Zhang H, Zhang C, Guo RX. Protective effect of ethyl pyruvate on pancreas injury in rats with severe acute pancreatitis. J Surg Res. 2013a;181(1):76–84. doi: 10.1016/j.jss.2012.05.066. [DOI] [PubMed] [Google Scholar]
- Luan ZG, Naranpurev M, Ma XC. Treatment of Low Molecular Weight Heparin Inhibits Systemic Inflammation and Prevents Endotoxin-Induced Acute Lung Injury in Rats. Inflammation. 2014 doi: 10.1007/s10753-014-9812-6. [DOI] [PubMed] [Google Scholar]
- Luan ZG, Zhang H, Ma XC, Zhang C, Guo RX. Role of high-mobility group box 1 protein in the pathogenesis of intestinal barrier injury in rats with severe acute pancreatitis. Pancreas. 2010;39(2):216–223. doi: 10.1097/MPA.0b013e3181bab5c5. [DOI] [PubMed] [Google Scholar]
- Luan ZG, Zhang H, Ma XC, Zhang C, Guo RX. Therapeutic treatment with ethyl pyruvate attenuates the severity of liver injury in rats with severe acute pancreatitis. Pancreas. 2012;41(5):729–737. doi: 10.1097/MPA.0b013e31823cd3ef. [DOI] [PubMed] [Google Scholar]
- Luan ZG, Zhang J, Yin XH, Ma XC, Guo RX. Ethyl pyruvate significantly inhibits tumour necrosis factor-alpha, interleukin-1beta and high mobility group box 1 releasing and attenuates sodium taurocholate-induced severe acute pancreatitis associated with acute lung injury. Clin Exp Immunol. 2013b;172(3):417–426. doi: 10.1111/cei.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum HK, Lee KL. The human HMGB1 promoter is modulated by a silencer and an enhancer-containing intron. Biochim Biophys Acta. 2001;1520(1):79–84. doi: 10.1016/s0167-4781(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Lund T, Holtlund J, Fredriksen M, Laland SG. On the presence of two new high mobility group-like proteins in HeLa S3 cells. FEBS Lett. 1983;152(2):163–167. doi: 10.1016/0014-5793(83)80370-6. [DOI] [PubMed] [Google Scholar]
- Lund T, Holtlund J, Laland SG. On the phosphorylation of low molecular mass HMG (high mobility group) proteins in Ehrlich ascites cells. FEBS Lett. 1985;180(2):275–279. doi: 10.1016/0014-5793(85)81085-1. [DOI] [PubMed] [Google Scholar]
- Luo Y, Chihara Y, Fujimoto K, Sasahira T, Kuwada M, Fujiwara R, Fujii K, Ohmori H, Kuniyasu H. High mobility group box 1 released from necrotic cells enhances regrowth and metastasis of cancer cells that have survived chemotherapy. Eur J Cancer. 2013;49(3):741–751. doi: 10.1016/j.ejca.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Luo Y, Yoneda J, Ohmori H, Sasaki T, Shimbo K, Eto S, Kato Y, Miyano H, Kobayashi T, Sasahira T, Chihara Y, Kuniyasu H. Cancer usurps skeletal muscle as an energy repository. Cancer Res. 2014;74(1):330–340. doi: 10.1158/0008-5472.CAN-13-1052. [DOI] [PubMed] [Google Scholar]
- Luo Y, Zhang B, Xu DQ, Liu Y, Dong MQ, Zhao PT, Li ZC. Protective effect of bicyclol on lipopolysaccharide-induced acute lung injury in mice. Pulm Pharmacol Ther. 2011;24(2):240–246. doi: 10.1016/j.pupt.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Lutz W, Stetkiewicz J. High mobility group box 1 protein as a late-acting mediator of acute lung inflammation. Int J Occup Med Environ Health. 2004;17(2):245–254. [PubMed] [Google Scholar]
- Lv B, Wang H, Tang Y, Fan Z, Xiao X, Chen F. High-mobility group box 1 protein induces tissue factor expression in vascular endothelial cells via activation of NF-kappaB and Egr-1. Thromb Haemost. 2009;102(2):352–359. doi: 10.1160/TH08-11-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science. 2000;287(5462):2486–2492. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- Ma CY, Jiao YL, Zhang J, Yang QR, Zhang ZF, Shen YJ, Chen ZJ, Zhao YR. Elevated plasma level of HMGB1 is associated with disease activity and combined alterations with IFN-alpha and TNF-alpha in systemic lupus erythematosus. Rheumatol Int. 2012a;32(2):395–402. doi: 10.1007/s00296-010-1636-6. [DOI] [PubMed] [Google Scholar]
- Ma L, Kim SJ, Oh KI. Calcium/Calmodulin-Dependent Protein Kinase is Involved in the Release of High Mobility Group Box 1 Via the Interferon-beta Signaling Pathway. Immune Netw. 2012b;12(4):148–154. doi: 10.4110/in.2012.12.4.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciotta S, Meregalli M, Cassinelli L, Parolini D, Farini A, Fraro GD, Gandolfi F, Forcato M, Ferrari S, Gabellini D, Bicciato S, Cossu G, Torrente Y. Hmgb3 is regulated by microRNA-206 during muscle regeneration. PLoS ONE. 2012;7(8):e43464. doi: 10.1371/journal.pone.0043464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Hikiba Y, Shibata W, Ohmae T, Yanai A, Ogura K, Yamada S, Omata M. Essential roles of high-mobility group box 1 in the development of murine colitis and colitisassociated cancer. Biochem Biophys Res Commun. 2007;360(2):394–400. doi: 10.1016/j.bbrc.2007.06.065. [DOI] [PubMed] [Google Scholar]
- Maeda T, Ozaki M, Kobayashi Y, Kiguchi N, Kishioka S. HMGB1 as a potential therapeutic target for neuropathic pain. J Pharmacol Sci. 2013;123(4):301–305. doi: 10.1254/jphs.13r08cp. [DOI] [PubMed] [Google Scholar]
- Magna M, Pisetsky DS. The Role of HMGB1 in the Pathogenesis of Inflammatory and Autoimmune Diseases. Mol Med. 2014 doi: 10.2119/molmed.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrys A, Paluch-Oles J, Koziol-Montewka M, Zaborowski T, Milanowski J, Maciejewska B. Evaluation of high-mobility group box 1 protein concentration in serum of patients with M. tuberculosis infection. Immunological investigations. 2013;42(1):49–60. doi: 10.3109/08820139.2012.723769. [DOI] [PubMed] [Google Scholar]
- Maher JF, Nathans D. Multivalent DNA-binding properties of the HMG-1 proteins. Proc Natl Acad Sci U S A. 1996;93(13):6716–6720. doi: 10.1073/pnas.93.13.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maicas N, Ferrandiz ML, Devesa I, Motterlini R, Koenders MI, van den Berg WB, Alcaraz MJ. The CO-releasing molecule CORM-3 protects against articular degradation in the K/BxN serum transfer arthritis model. Eur J Pharmacol. 2010;634(1–3):184–191. doi: 10.1016/j.ejphar.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Maitra A, Hruban RH. Pancreatic cancer. Annual review of pathology. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar M, Ratho R, Chawla Y, Singh MP. High levels of circulating HMGB1 as a biomarker of acute liver failure in patients with viral hepatitis E. Liver international : official journal of the International Association for the Study of the Liver. 2013;33(9):1341–1348. doi: 10.1111/liv.12197. [DOI] [PubMed] [Google Scholar]
- Makiguchi K, Chida Y, Yoshida M, Shimura K. Mg2+-dependent unwinding of DNA by nonhistone chromosomal protein HMG(1 + 2) from pig thymus as determined by DNA melting temperature analysis. J Biochem. 1984;95(2):423–429. doi: 10.1093/oxfordjournals.jbchem.a134623. [DOI] [PubMed] [Google Scholar]
- Malarkey CS, Churchill ME. The high mobility group box: the ultimate utility player of a cell. Trends Biochem Sci. 2012;37(12):553–562. doi: 10.1016/j.tibs.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malina J, Kasparkova J, Natile G, Brabec V. Recognition of major DNA adducts of enantiomeric cisplatin analogs by HMG box proteins and nucleotide excision repair of these adducts. Chem Biol. 2002;9(5):629–638. doi: 10.1016/s1074-5521(02)00134-5. [DOI] [PubMed] [Google Scholar]
- Manfredi AA, Capobianco A, Bianchi ME, Rovere-Querini P. Regulation of dendritic-and T-cell fate by injury-associated endogenous signals. Crit Rev Immunol. 2009;29(1):69–86. doi: 10.1615/critrevimmunol.v29.i1.30. [DOI] [PubMed] [Google Scholar]
- Manfredi AA, Capobianco A, Esposito A, De Cobelli F, Canu T, Monno A, Raucci A, Sanvito F, Doglioni C, Nawroth PP, Bierhaus A, Bianchi ME, Rovere-Querini P, Del Maschio A. Maturing dendritic cells depend on RAGE for in vivo homing to lymph nodes. J Immunol. 2008;180(4):2270–2275. doi: 10.4049/jimmunol.180.4.2270. [DOI] [PubMed] [Google Scholar]
- Manganelli V, Signore M, Pacini I, Misasi R, Tellan G, Garofalo T, Lococo E, Chirletti P, Sorice M, Delogu G. Increased HMGB1 expression and release by mononuclear cells following surgical/anesthesia trauma. Critical care (London, England) 2010;14(6):R197. doi: 10.1186/cc9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab. 2014;25(1):15–22. doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao XJ, Wang GF, Chen ZJ, Wang LN, Zhang JB, Wang HL. Expression of HMGB1 and its clinical significance in T-cell lymphoma. Asian Pacific journal of cancer prevention : APJCP. 2012;13(11):5569–5571. doi: 10.7314/apjcp.2012.13.11.5569. [DOI] [PubMed] [Google Scholar]
- Mao YW, Tseng HW, Liang WL, Chen IS, Chen ST, Lee MH. Anti-inflammatory and free radial scavenging activities of the constituents isolated from Machilus zuihoensis. Molecules. 2011;16(11):9451–9466. doi: 10.3390/molecules16119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Di Carlo A, Facchiano F, Senatore C, De Cristofaro R, Luzi A, Federici M, Romani M, Napolitano M, Capogrossi MC, Germani A. High mobility group box 1 is a novel substrate of dipeptidyl peptidase-IV. Diabetologia. 2012;55(1):236–244. doi: 10.1007/s00125-011-2213-6. [DOI] [PubMed] [Google Scholar]
- Mardente S, Mari E, Consorti F, Di Gioia C, Negri R, Etna M, Zicari A, Antonaci A. HMGB1 induces the overexpression of miR-222 and miR-221 and increases growth and motility in papillary thyroid cancer cells. Oncol Rep. 2012;28(6):2285–2289. doi: 10.3892/or.2012.2058. [DOI] [PubMed] [Google Scholar]
- Marekov LN, Demirov DG, Beltchev BG. Isolation of high-mobility-group proteins HMG1 and HMG2 in non denaturing conditions and comparison of their properties with those of acid-extracted proteins. Biochim Biophys Acta. 1984;789(1):63–68. doi: 10.1016/0167-4838(84)90061-x. [DOI] [PubMed] [Google Scholar]
- Marmillot P, Scovell W. Enhancement of transcription factor, USF, binding to the adenovirus major late promoter: effect of dithiothreitol and high mobility group protein-1. Biochim Biophys Acta. 1998;1395(2):228–236. doi: 10.1016/s0167-4781(97)00153-x. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16(4):413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Bianchi ME, Vezzani A. Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: the importance of IL-1beta and high-mobility group box 1. J Intern Med. 2011;270(4):319–326. doi: 10.1111/j.1365-2796.2011.02431.x. [DOI] [PubMed] [Google Scholar]
- Marrugo J, Marsh DG, Ghosh B. The conserved lymphokine element-0 in the IL5 promoter binds to a high mobility group-1 protein. Mol Immunol. 1996;33(14):1119–1125. doi: 10.1016/s0161-5890(96)00073-9. [DOI] [PubMed] [Google Scholar]
- Martinez Hoyos J, Ferraro A, Sacchetti S, Keller S, De Martino I, Borbone E, Pallante P, Fedele M, Montanaro D, Esposito F, Cserjesi P, Chiariotti L, Troncone G, Fusco A. HAND1 gene expression is negatively regulated by the High Mobility Group A1 proteins and is drastically reduced in human thyroid carcinomas. Oncogene. 2009;28(6):876–885. doi: 10.1038/onc.2008.438. [DOI] [PubMed] [Google Scholar]
- Mathew CG, Goodwin GH, Johns EW. Studies on the association of the high mobility group non-histone chromatin proteins with isolated nucleosomes. Nucleic Acids Res. 1979;6(1):167–179. doi: 10.1093/nar/6.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21(11):1367–1381. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Hayashi Y, Omori H, Honda T, Daito T, Horie M, Ikuta K, Fujino K, Nakamura S, Schneider U, Chase G, Yoshimori T, Schwemmle M, Tomonaga K. Bornavirus closely associates and segregates with host chromosomes to ensure persistent intranuclear infection. Cell Host Microbe. 2012;11(5):492–503. doi: 10.1016/j.chom.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Matsuoka N, Itoh T, Watarai H, Sekine-Kondo E, Nagata N, Okamoto K, Mera T, Yamamoto H, Yamada S, Maruyama I, Taniguchi M, Yasunami Y. High-mobility group box 1 is involved in the initial events of early loss of transplanted islets in mice. J Clin Invest. 2010;120(3):735–743. doi: 10.1172/JCI41360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteucci E, Giampietro O. Dipeptidyl peptidase-4 (CD26): knowing the function before inhibiting the enzyme. Curr Med Chem. 2009;16(23):2943–2951. doi: 10.2174/092986709788803114. [DOI] [PubMed] [Google Scholar]
- Maugeri N, Franchini S, Campana L, Baldini M, Ramirez GA, Sabbadini MG, Rovere-Querini P, Manfredi AA. Circulating platelets as a source of the damage-associated molecular pattern HMGB1 in patients with systemic sclerosis. Autoimmunity. 2012a;45(8):584–587. doi: 10.3109/08916934.2012.719946. [DOI] [PubMed] [Google Scholar]
- Maugeri N, Franchini S, Campana L, Baldini M, Ramirez GA, Sabbadini MG, Rovere-Querini P, Manfredi AA. Circulating platelets as a source of the damage-associated molecular pattern HMGB1 in patients with systemic sclerosis. Autoimmunity. 2012b doi: 10.3109/08916934.2012.719946. [DOI] [PubMed] [Google Scholar]
- Maugeri N, Rovere-Querini P, Baldini M, Baldissera E, Sabbadini MG, Bianchi ME, Manfredi AA. Oxidative Stress Elicits Platelet/Leukocyte Inflammatory Interactions via HMGB1: A Candidate for Microvessel Injury in Sytemic Sclerosis. Antioxid Redox Signal. 2014 doi: 10.1089/ars.2013.5298. [DOI] [PubMed] [Google Scholar]
- Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Maroso M, Iori V, Vezzani A, Carli M. High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and Receptor for Advanced Glycation End Products. Exp Neurol. 2011;232(2):143–148. doi: 10.1016/j.expneurol.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur PK, Siveke JT. Genetically engineered mouse models of pancreatic cancer: unravelling tumour biology and progressing translational oncology. Gut. 2011 doi: 10.1136/gutjnl-2011-300756. [DOI] [PubMed] [Google Scholar]
- McDonnell M, Liang Y, Noronha A, Coukos J, Kasper DL, Farraye FA, Ganley-Leal LM. Systemic Toll-like receptor ligands modify B-cell responses in human inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(1):298–307. doi: 10.1002/ibd.21424. [DOI] [PubMed] [Google Scholar]
- McKinney K, Prives C. Efficient specific DNA binding by p53 requires both its central and C-terminal domains as revealed by studies with high-mobility group 1 protein. Mol Cell Biol. 2002;22(19):6797–6808. doi: 10.1128/MCB.22.19.6797-6808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki MT, Saidi H, Dufour A, Olivo-Marin JC, Gougeon ML. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk--a pivotal role of HMGB1. PLoS Pathog. 2010;6(4):e1000862. doi: 10.1371/journal.ppat.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni E, Sparatore B, Patrone M, Pessino A, Passalacqua M, Pontremoli S. Extracellular release of the 'differentiation enhancing factor', a HMG1 protein type, is an early step in murine erythroleukemia cell differentiation. FEBS Lett. 1995a;368(3):466–470. doi: 10.1016/0014-5793(95)00716-m. [DOI] [PubMed] [Google Scholar]
- Melloni E, Sparatore B, Patrone M, Pessino A, Passalacqua M, Pontremoli S. Identity in molecular structure between "differentiation enhancing factor" of murine erythroleukemia cells and the 30 kD heparin-binding protein of developing rat brain. Biochem Biophys Res Commun. 1995b;210(1):82–89. doi: 10.1006/bbrc.1995.1630. [DOI] [PubMed] [Google Scholar]
- Melvin VS, Harrell C, Adelman JS, Kraus WL, Churchill M, Edwards DP. The role of the C-terminal extension (CTE) of the estrogen receptor alpha and beta DNA binding domain in DNA binding and interaction with HMGB. J Biol Chem. 2004;279(15):14763–14771. doi: 10.1074/jbc.M313335200. [DOI] [PubMed] [Google Scholar]
- Melvin VS, Roemer SC, Churchill ME, Edwards DP. The C-terminal extension (CTE) of the nuclear hormone receptor DNA binding domain determines interactions and functional response to the HMGB-1/-2 co-regulatory proteins. J Biol Chem. 2002;277(28):25115–25124. doi: 10.1074/jbc.M110400200. [DOI] [PubMed] [Google Scholar]
- Meng E, Guo Z, Wang H, Jin J, Wang J, Wu C, Wang L. High mobility group box 1 protein inhibits the proliferation of human mesenchymal stem cells and promotes their migration and differentiation along osteoblastic pathway. Stem Cells Dev. 2008;17(4):805–813. doi: 10.1089/scd.2007.0276. [DOI] [PubMed] [Google Scholar]
- Merenmies J, Pihlaskari R, Laitinen J, Wartiovaara J, Rauvala H. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem. 1991;266(25):16722–16729. [PubMed] [Google Scholar]
- Merkle T, Grasser KD. Unexpected mobility of plant chromatin-associated HMGB proteins. Plant Signal Behav. 2011;6(6):878–880. doi: 10.4161/psb.6.6.15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173(1):307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Metzeler KH, Heilmeier B, Edmaier KE, Rawat VP, Dufour A, Dohner K, Feuring-Buske M, Braess J, Spiekermann K, Buchner T, Sauerland MC, Dohner H, Hiddemann W, Bohlander SK, Schlenk RF, Bullinger L, Buske C. High expression of lymphoid enhancer-binding factor-1 (LEF1) is a novel favorable prognostic factor in cytogenetically normal acute myeloid leukemia. Blood. 2012;120(10):2118–2126. doi: 10.1182/blood-2012-02-411827. [DOI] [PubMed] [Google Scholar]
- Meyer A, Staratschek-Jox A, Springwald A, Wenk H, Wolf J, Wickenhauser C, Bullerdiek J. Non-Hodgkin lymphoma expressing high levels of the danger-signalling protein HMGB1. Leukemia & lymphoma. 2008;49(6):1184–1189. doi: 10.1080/10428190802064909. [DOI] [PubMed] [Google Scholar]
- Miksa M, Wu R, Dong W, Komura H, Amin D, Ji Y, Wang Z, Wang H, Ravikumar TS, Tracey KJ, Wang P. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected] J Immunol. 2009;183(9):5983–5990. doi: 10.4049/jimmunol.0802994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovich A, Travis A, Grosschedl R, Francke U. Gene for lymphoid enhancer-binding factor 1 (LEF1) mapped to human chromosome 4 (q23–q25) and mouse chromosome 3 near Egf. Genomics. 1991;11(4):1040–1048. doi: 10.1016/0888-7543(91)90030-i. [DOI] [PubMed] [Google Scholar]
- Milev P, Chiba A, Haring M, Rauvala H, Schachner M, Ranscht B, Margolis RK, Margolis RU. High affinity binding and overlapping localization of neurocan and phosphacan/protein-tyrosine phosphatase-zeta/beta with tenascin-R, amphoterin, and the heparin-binding growth-associated molecule. J Biol Chem. 1998;273(12):6998–7005. doi: 10.1074/jbc.273.12.6998. [DOI] [PubMed] [Google Scholar]
- Miller JM, Thompson JK, Macpherson MB, Beuschel SL, Westbom CM, Sayan M, Shukla A. Curcumin: A Double Hit on Malignant Mesothelioma. Cancer Prev Res (Phila) 2014 doi: 10.1158/1940-6207.CAPR-13-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HJ, Ko EA, Wu J, Kim ES, Kwon MK, Kwak MS, Choi JE, Lee JE, Shin JS. Chaperone-like activity of high-mobility group box 1 protein and its role in reducing the formation of polyglutamine aggregates. J Immunol. 2013;190(4):1797–1806. doi: 10.4049/jimmunol.1202472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitkova E, Ugrinova I, Pashev IG, Pasheva EA. The inhibitory effect of HMGB-1 protein on the repair of cisplatin-damaged DNA is accomplished through the acidic domain. Biochemistry. 2005;44(15):5893–5898. doi: 10.1021/bi047712c. [DOI] [PubMed] [Google Scholar]
- Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, Melloni E, Presta M. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol. 2006;176(1):12–15. doi: 10.4049/jimmunol.176.1.12. [DOI] [PubMed] [Google Scholar]
- Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT, Ritis K. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS ONE. 2011;6(12):e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsouras K, Wong B, Arayata C, Johnson RC, Carey M. The DNA architectural protein HMGB1 displays two distinct modes of action that promote enhanceosome assembly. Mol Cell Biol. 2002;22(12):4390–4401. doi: 10.1128/MCB.22.12.4390-4401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal D, Saccheri F, Venereau E, Pusterla T, Bianchi ME, Rescigno M. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. Embo J. 2010;29(13):2242–2252. doi: 10.1038/emboj.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed OA, Bustin M, Clarke HJ. High-mobility group proteins 14 and 17 maintain the timing of early embryonic development in the mouse. Dev Biol. 2001;229(1):237–249. doi: 10.1006/dbio.2000.9942. [DOI] [PubMed] [Google Scholar]
- Moisy D, Avilov SV, Jacob Y, Laoide BM, Ge X, Baudin F, Naffakh N, Jestin JL. HMGB1 protein binds to influenza virus nucleoprotein and promotes viral replication. J Virol. 2012;86(17):9122–9133. doi: 10.1128/JVI.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moleri S, Cappellano G, Gaudenzi G, Cermenati S, Cotelli F, Horner DS, Beltrame M. The HMGB protein gene family in zebrafish: Evolution and embryonic expression patterns. Gene Expr Patterns. 2011;11(1–2):3–11. doi: 10.1016/j.gep.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, Bianchi ME. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14(4):431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Momonaka H, Hasegawa S, Matsushige T, Inoue H, Kajimoto M, Okada S, Nakatsuka K, Morishima T, Ichiyama T. High mobility group box 1 in patients with 2009 pandemic H1N1 influenza-associated encephalopathy. Brain & development. 2013 doi: 10.1016/j.braindev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- Morinaga Y, Yanagihara K, Nakamura S, Hasegawa H, Seki M, Izumikawa K, Kakeya H, Yamamoto Y, Yamada Y, Kohno S, Kamihira S. Legionella pneumophila induces cathepsin B-dependent necrotic cell death with releasing high mobility group box1 in macrophages. Respir Res. 2010;11:158. doi: 10.1186/1465-9921-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita A, Zaidi MR, Mitoro A, Sankarasharma D, Szabolcs M, Okada Y, D'Armiento J, Chada K. HMGA2 is a driver of tumor metastasis. Cancer Res. 2013;73(14):4289–4299. doi: 10.1158/0008-5472.CAN-12-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, Hasunuma R, Kawaguchi K, Adachi Y, Tanaka S, Kumazawa Y. Limitation of polymyxin B on suppression of endotoxin shock induced by Salmonella infection in mice. Biol Pharm Bull. 2004;27(11):1840–1843. doi: 10.1248/bpb.27.1840. [DOI] [PubMed] [Google Scholar]
- Moriwaka Y, Luo Y, Ohmori H, Fujii K, Tatsumoto N, Sasahira T, Kuniyasu H. HMGB1 attenuates anti-metastatic defense of the lymph nodes in colorectal cancer. Pathobiology : journal of immunopathology, molecular and cellular biology. 2010;77(1):17–23. doi: 10.1159/000272950. [DOI] [PubMed] [Google Scholar]
- Morris JPt, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010a;10(10):683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JPt, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nature reviews. Cancer. 2010b;10(10):683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B, Bekos C, Zimprich F, Nickl S, Klepetko W, Ankersmit J. The receptor for advanced glycation endproducts and its ligands in patients with myasthenia gravis. Biochem Biophys Res Commun. 2012;420(1):96–101. doi: 10.1016/j.bbrc.2012.02.121. [DOI] [PubMed] [Google Scholar]
- Mosevitsky MI, Novitskaya VA, Iogannsen MG, Zabezhinsky MA. Tissue specificity of nucleo-cytoplasmic distribution of HMG1 and HMG2 proteins and their probable functions. Eur J Biochem. 1989;185(2):303–310. doi: 10.1111/j.1432-1033.1989.tb15116.x. [DOI] [PubMed] [Google Scholar]
- Motoyama K, Inoue H, Nakamura Y, Uetake H, Sugihara K, Mori M. Clinical significance of high mobility group A2 in human gastric cancer and its relationship to let-7 microRNA family. Clin Cancer Res. 2008;14(8):2334–2340. doi: 10.1158/1078-0432.CCR-07-4667. [DOI] [PubMed] [Google Scholar]
- Mouri F, Tsukada J, Mizobe T, Higashi T, Yoshida Y, Minami Y, Izumi H, Kominato Y, Kohno K, Tanaka Y. Intracellular HMGB1 transactivates the human IL1B gene promoter through association with an Ets transcription factor PU.1. Eur J Haematol. 2008;80(1):10–19. doi: 10.1111/j.1600-0609.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28(46):12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Bianchi ME, Knapp S. Thermodynamics of HMGB1 interaction with duplex DNA. Biochemistry. 2001;40(34):10254–10261. doi: 10.1021/bi0100900. [DOI] [PubMed] [Google Scholar]
- Muller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med. 2004;255(3):332–343. doi: 10.1111/j.1365-2796.2003.01296.x. [DOI] [PubMed] [Google Scholar]
- Murakami T, Obata T, Kuwahara-Arai K, Tamura H, Hiramatsu K, Nagaoka I. Antimicrobial cathelicidin polypeptide CAP11 suppresses the production and release of septic mediators in D-galactosamine-sensitized endotoxin shock mice. Int Immunol. 2009;21(8):905–912. doi: 10.1093/intimm/dxp057. [DOI] [PubMed] [Google Scholar]
- Murakami T, Suzuki K, Tamura H, Nagaoka I. Suppressive action of resolvin D1 on the production and release of septic mediators in D-galactosamine-sensitized endotoxin shock mice. Experimental and therapeutic medicine. 2011;2(1):57–61. doi: 10.3892/etm.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Matsumoto H, Roh M, Giani A, Kataoka K, Morizane Y, Kayama M, Thanos A, Nakatake S, Notomi S, Hisatomi T, Ikeda Y, Ishibashi T, Connor KM, Miller JW, Vavvas DG. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ. 2014;21(2):270–277. doi: 10.1038/cdd.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci D, Bucci EM, Roviello GN, Sapio R, Valente M, Moccia M, Bianchi ME, Pedone C. DNA-based strategies for blocking HMGB1 cytokine activity: design, synthesis and preliminary in vitro/in vivo assays of DNA and DNA-like duplexes. Mol Biosyst. 2011;7(5):1742–1752. doi: 10.1039/c1mb05009e. [DOI] [PubMed] [Google Scholar]
- Musumeci D, Roviello GN, Moccia M, Pedone C, Bucci EM, Sapio R, Valente M, Fumero S. Bent oligonucleotide duplexes as HMGB1 inhibitors: a comparative study. Nucleosides Nucleotides Nucleic Acids. 2007;26(10–12):1447–1450. doi: 10.1080/15257770701542330. [DOI] [PubMed] [Google Scholar]
- Musumeci D, Roviello GN, Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther. 2014;141(3):347–357. doi: 10.1016/j.pharmthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Nace GW, Huang H, Klune JR, Eid RE, Rosborough BR, Korff S, Li S, Shapiro RA, Stolz DB, Sodhi CP, Hackam DJ, Geller DA, Billiar TR, Tsung A. Cellular-specific role of toll-like receptor 4 in hepatic ischemia-reperfusion injury in mice. Hepatology. 2013;58(1):374–387. doi: 10.1002/hep.26346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadatani Y, Watanabe T, Tanigawa T, Ohkawa F, Takeda S, Higashimori A, Sogawa M, Yamagami H, Shiba M, Watanabe K, Tominaga K, Fujiwara Y, Takeuchi K, Arakawa T. High-mobility group box 1 inhibits gastric ulcer healing through Toll-like receptor 4 and receptor for advanced glycation end products. PLoS ONE. 2013;8(11):e80130. doi: 10.1371/journal.pone.0080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaki S, Yamamoto M, Yumoto Y, Shirakawa H, Yoshida M, Teraoka H. Non-histone chromosomal proteins HMG1 and 2 enhance ligation reaction of DNA double-strand breaks. Biochem Biophys Res Commun. 1998;246(1):137–141. doi: 10.1006/bbrc.1998.8589. [DOI] [PubMed] [Google Scholar]
- Nagata S, Nagase H, Kawane K, Mukae N, Fukuyama H. Degradation of chromosomal DNA during apoptosis. Cell Death Differ. 2003;10(1):108–116. doi: 10.1038/sj.cdd.4401161. [DOI] [PubMed] [Google Scholar]
- Nagatani G, Nomoto M, Takano H, Ise T, Kato K, Imamura T, Izumi H, Makishima K, Kohno K. Transcriptional activation of the human HMG1 gene in cisplatin-resistant human cancer cells. Cancer Res. 2001;61(4):1592–1597. [PubMed] [Google Scholar]
- Nagato M, Okamoto K, Abe Y, Higure A, Yamaguchi K. Recombinant human soluble thrombomodulin decreases the plasma high-mobility group box-1 protein levels, whereas improving the acute liver injury and survival rates in experimental endotoxemia. Crit Care Med. 2009;37(7):2181–2186. doi: 10.1097/CCM.0b013e3181a55184. [DOI] [PubMed] [Google Scholar]
- Naghavi MH, Nowak P, Andersson J, Sonnerborg A, Yang H, Tracey KJ, Vahlne A. Intracellular high mobility group B1 protein (HMGB1) represses HIV-1 LTR-directed transcription in a promoter- and cell-specific manner. Virology. 2003;314(1):179–189. doi: 10.1016/s0042-6822(03)00453-7. [DOI] [PubMed] [Google Scholar]
- Nagpal S, Ghosn C, DiSepio D, Molina Y, Sutter M, Klein ES, Chandraratna RA. Retinoid-dependent recruitment of a histone H1 displacement activity by retinoic acid receptor. J Biol Chem. 1999;274(32):22563–22568. doi: 10.1074/jbc.274.32.22563. [DOI] [PubMed] [Google Scholar]
- Najima Y, Yahagi N, Takeuchi Y, Matsuzaka T, Sekiya M, Nakagawa Y, Amemiya-Kudo M, Okazaki H, Okazaki S, Tamura Y, Iizuka Y, Ohashi K, Harada K, Gotoda T, Nagai R, Kadowaki T, Ishibashi S, Yamada N, Osuga J, Shimano H. High mobility group protein-B1 interacts with sterol regulatory element-binding proteins to enhance their DNA binding. J Biol Chem. 2005;280(30):27523–27532. doi: 10.1074/jbc.m414549200. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Tsuruta R, Kaneko T, Yamashita S, Fujita M, Kasaoka S, Hashiguchi T, Suzuki M, Maruyama I, Maekawa T. High-mobility group box 1 protein in CSF of patients with subarachnoid hemorrhage. Neurocritical care. 2009;11(3):362–368. doi: 10.1007/s12028-009-9276-y. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Watanabe H, Tohyama M, Sugita K, Iijima M, Hashimoto K, Tokura Y, Nishimura Y, Doi H, Tanioka M, Miyachi Y, Kabashima K. High-mobility group box 1 protein (HMGB1) as a novel diagnostic tool for toxic epidermal necrolysis and Stevens-Johnson syndrome. Arch Dermatol. 2011;147(9):1110–1112. doi: 10.1001/archdermatol.2011.239. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Mori Y, Hagiwara K, Suzuki T, Sakakibara T, Kikuchi T, Igarashi T, Ebina M, Abe T, Miyazaki J, Takai T, Nukiwa T. Increased susceptibility to LPS-induced endotoxin shock in secretory leukoprotease inhibitor (SLPI)-deficient mice. J Exp Med. 2003;197(5):669–674. doi: 10.1084/jem.20021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Fujiwara N, Sato E, Kawagoe Y, Ueda Y, Yamada S, Koide H. Effect of polymyxin B-immobilized fiber hemoperfusion on serum high mobility group box-1 protein levels and oxidative stress in patients with acute respiratory distress syndrome. ASAIO journal (American Society for Artificial Internal Organs: 1992) 2009a;55(4):395–399. doi: 10.1097/MAT.0b013e3181a5290f. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Kawagoe Y, Ueda Y, Yamada S, Koide H. Hemoperfusion treatment in a septic shock patient with autosomal dominant polycystic kidney disease and increased HMGB1 protein levels. Blood Purif. 2011a;32(2):139–142. doi: 10.1159/000325731. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Maeda S, Yamagishi S. Increased levels of soluble receptor for advanced glycation end products (sRAGE) and high mobility group box 1 (HMGB1) are associated with death in patients with acute respiratory distress syndrome. Clin Biochem. 2011b;44(8–9):601–604. doi: 10.1016/j.clinbiochem.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Maeda S, Yamagishi S. Suppression of high-mobility group box-1 and receptor for advanced glycation end-product axis by polymyxin B-immobilized fiber hemoperfusion in septic shock patients. J Crit Care. 2011c;26(6):546–549. doi: 10.1016/j.jcrc.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Ueda Y, Suzuki T, Yamada S, Takeuchi M, Fukami K, Ueda S, Adachi H, Matsui T, Okuda S, Yamagishi S. Positive association of serum levels of advanced glycation end products and high mobility group box-1 with asymmetric dimethylarginine in nondiabetic chronic kidney disease patients. Metabolism. 2009b;58(11):1624–1628. doi: 10.1016/j.metabol.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Yamada S, Ueda Y, Koide H. Changes in urinary albumin excretion, inflammatory and oxidative stress markers in ADPKD patients with hypertension. Am J Med Sci. 2012a;343(1):46–51. doi: 10.1097/MAJ.0b013e31821f0552. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yamada S, Yoshioka T. Measurement of plasma concentration of high mobility group box1 (HMGB1) in early neonates and evaluation of its usefulness. Clinica chimica acta; international journal of clinical chemistry. 2012b;413(1–2):237–239. doi: 10.1016/j.cca.2011.09.035. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yamada S, Yoshioka T. Brain hypothermic therapy dramatically decreases elevated blood concentrations of high mobility group box 1 in neonates with hypoxic-ischemic encephalopathy. Disease markers. 2013;35(5):327–330. doi: 10.1155/2013/327604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, Yagita H, Okumura K. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113(16):3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- Namiki Y, Takahashi T, Ohno T. Gene transduction for disseminated intraperitoneal tumor using cationic liposomes containing non-histone chromatin proteins: cationic liposomal gene therapy of carcinomatosa. Gene Ther. 1998;5(2):240–246. doi: 10.1038/sj.gt.3300577. [DOI] [PubMed] [Google Scholar]
- Naruse K, Sado T, Noguchi T, Tsunemi T, Yoshida S, Akasaka J, Koike N, Oi H, Kobayashi H. Peripheral RAGE (receptor for advanced glycation endproducts)-ligands in normal pregnancy and preeclampsia: novel markers of inflammatory response. Journal of reproductive immunology. 2012;93(2):69–74. doi: 10.1016/j.jri.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Naumnik W, Nilklinska W, Ossolinska M, Chyczewska E. Serum levels of HMGB1, survivin, and VEGF in patients with advanced non-small cell lung cancer during chemotherapy. Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society. 2009;47(4):703–709. doi: 10.2478/v10042-009-0025-z. [DOI] [PubMed] [Google Scholar]
- Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119(16):3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth MJ, Cline AP, Anderson SM, Garrett-Beal LJ, Bodine DM. Hmgb3 deficiency deregulates proliferation and differentiation of common lymphoid and myeloid progenitors. Blood. 2005;105(2):627–634. doi: 10.1182/blood-2004-07-2551. [DOI] [PubMed] [Google Scholar]
- Nemeth MJ, Curtis DJ, Kirby MR, Garrett-Beal LJ, Seidel NE, Cline AP, Bodine DM. Hmgb3: an HMG-box family member expressed in primitive hematopoietic cells that inhibits myeloid and B-cell differentiation. Blood. 2003;102(4):1298–1306. doi: 10.1182/blood-2002-11-3541. [DOI] [PubMed] [Google Scholar]
- Nemeth MJ, Kirby MR, Bodine DM. Hmgb3 regulates the balance between hematopoietic stem cell self-renewal and differentiation. Proc Natl Acad Sci U S A. 2006;103(37):13783–13788. doi: 10.1073/pnas.0604006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ner SS, Churchill ME, Searles MA, Travers AA. dHMG-Z, a second HMG-1-related protein in Drosophila melanogaster. Nucleic Acids Res. 1993;21(18):4369–4371. doi: 10.1093/nar/21.18.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ner SS, Travers AA. HMG-D, the Drosophila melanogaster homologue of HMG 1 protein, is associated with early embryonic chromatin in the absence of histone H1. Embo J. 1994;13(8):1817–1822. doi: 10.1002/j.1460-2075.1994.tb06450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni YF, Tian F, Lu ZF, Yang GD, Fu HY, Wang J, Yan XL, Zhao YC, Wang YJ, Jiang T. Protective effect of nicotine on lipopolysaccharide-induced acute lung injury in mice. Respiration. 2011;81(1):39–46. doi: 10.1159/000319151. [DOI] [PubMed] [Google Scholar]
- Ni Z, Dai X, Wang B, Ding W, Cheng P, Xu L, Lian J, He F. Natural Bcl-2 inhibitor (−)-gossypol induces protective autophagy via reactive oxygen species-high mobility group box 1 pathway in Burkitt lymphoma. Leuk Lymphoma. 2013;54(10):2263–2268. doi: 10.3109/10428194.2013.775437. [DOI] [PubMed] [Google Scholar]
- Niemeyer CC, Foerster-Ziober A, Flytzanis CN. Purification of a high-mobility-group 1 sea-urchin protein and cloning of cDNAs. Gene. 1995;164(2):211–218. doi: 10.1016/0378-1119(95)00410-8. [DOI] [PubMed] [Google Scholar]
- Nightingale K, Dimitrov S, Reeves R, Wolffe AP. Evidence for a shared structural role for HMG1 and linker histones B4 and H1 in organizing chromatin. Embo J. 1996;15(3):548–561. [PMC free article] [PubMed] [Google Scholar]
- Nimura K, Ura K, Shiratori H, Ikawa M, Okabe M, Schwartz RJ, Kaneda Y. A histone H3 lysine 36 trimethyltransferase links Nkx2–5 to Wolf-Hirschhorn syndrome. Nature. 2009;460(7252):287–291. doi: 10.1038/nature08086. [DOI] [PubMed] [Google Scholar]
- Nin JW, Ferreira I, Schalkwijk CG, Jorsal A, Prins MH, Parving HH, Tarnow L, Rossing P, Stehouwer CD. Higher plasma high-mobility group box 1 levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12 year follow-up study. Diabetologia. 2012a;55(9):2489–2493. doi: 10.1007/s00125-012-2622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nin JW, Ferreira I, Schalkwijk CG, Prins MH, Chaturvedi N, Fuller JH, Stehouwer CD. Serum high-mobility group box-1 levels are positively associated with micro- and macroalbuminuria but not with cardiovascular disease in type 1 diabetes: the EURODIAB Prospective Complications Study. European journal of endocrinology / European Federation of Endocrine Societies. 2012b;166(2):325–332. doi: 10.1530/EJE-11-0662. [DOI] [PubMed] [Google Scholar]
- Ninios YP, Sekeri-Pataryas KE, Sourlingas TG. Histone H1 subtype preferences of DFF40 and possible nuclear localization of DFF40/45 in normal and trichostatin A-treated NB4 leukemic cells. Apoptosis. 2010;15(2):128–138. doi: 10.1007/s10495-009-0418-7. [DOI] [PubMed] [Google Scholar]
- Nissen MS, Langan TA, Reeves R. Phosphorylation by cdc2 kinase modulates DNA binding activity of high mobility group I nonhistone chromatin protein. J Biol Chem. 1991;266(30):19945–19952. [PubMed] [Google Scholar]
- Nowak P, Barqasho B, Sonnerborg A. Elevated plasma levels of high mobility group box protein 1 in patients with HIV-1 infection. Aids. 2007;21(7):869–871. doi: 10.1097/QAD.0b013e3280b079b6. [DOI] [PubMed] [Google Scholar]
- Nowak P, Barqasho B, Treutiger CJ, Harris HE, Tracey KJ, Andersson J, Sonnerborg A. HMGB1 activates replication of latent HIV-1 in a monocytic cell-line, but inhibits HIV-1 replication in primary macrophages. Cytokine. 2006;34(1–2):17–23. doi: 10.1016/j.cyto.2006.03.010. [DOI] [PubMed] [Google Scholar]
- O'Donnell PW, Haque A, Klemsz MJ, Kaplan MH, Blum JS. Cutting edge: induction of the antigen-processing enzyme IFN-gamma-inducible lysosomal thiol reductase in melanoma cells Is STAT1-dependent but CIITA-independent. J Immunol. 2004;173(2):731–735. doi: 10.4049/jimmunol.173.2.731. [DOI] [PubMed] [Google Scholar]
- O'Mahony DJ, Rothblum LI. Identification of two forms of the RNA polymerase I transcription factor UBF. Proc Natl Acad Sci U S A. 1991;88(8):3180–3184. doi: 10.1073/pnas.88.8.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan RJ, Kubicek S, Schreiber SL, Karlseder J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat Struct Mol Biol. 2010;17(10):1218–1225. doi: 10.1038/nsmb.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Tsuruta R, Fujita M, Kaneda K, Kawamura Y, Izumi T, Kasaoka S, Maruyama I, Maekawa T. Prediction of the neurological outcome with intrathecal high mobility group box 1 and S100B in cardiac arrest victims: a pilot study. Resuscitation. 2012;83(8):1006–1012. doi: 10.1016/j.resuscitation.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12(8):695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Aizawa S, Shirakawa H, Yoshida M. Stimulation of transcription accompanying relaxation of chromatin structure in cells overexpressing high mobility group 1 protein. J Biol Chem. 1995;270(16):9272–9280. doi: 10.1074/jbc.270.16.9272. [DOI] [PubMed] [Google Scholar]
- Ogiku M, Kono H, Hara M, Tsuchiya M, Fujii H. Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by Kupffer cells after ischemia-reperfusion in rats. J Pharmacol Exp Ther. 2011;339(1):93–98. doi: 10.1124/jpet.111.182592. [DOI] [PubMed] [Google Scholar]
- Ogiku M, Kono H, Hara M, Tsuchiya M, Fujii H. Interleukin-17A plays a pivotal role in polymicrobial sepsis according to studies using IL-17A knockout mice. J Surg Res. 2012;174(1):142–149. doi: 10.1016/j.jss.2010.11.901. [DOI] [PubMed] [Google Scholar]
- Oh YJ, Youn JH, Ji Y, Lee SE, Lim KJ, Choi JE, Shin JS. HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J Immunol. 2009;182(9):5800–5809. doi: 10.4049/jimmunol.0801873. [DOI] [PubMed] [Google Scholar]
- Oh YJ, Youn JH, Min HJ, Kim DH, Lee SS, Choi IH, Shin JS. CKD712, (S)-1-(alpha-naphthylmethyl)-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline, inhibits the lipopolysaccharide-stimulated secretion of HMGB1 by inhibiting PI3K and classical protein kinase C. Int Immunopharmacol. 2011;11(9):1160–1165. doi: 10.1016/j.intimp.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Ohndorf UM, Rould MA, He Q, Pabo CO, Lippard SJ. Basis for recognition of cisplatin-modified DNA by high-mobility-group proteins. Nature. 1999;399(6737):708–712. doi: 10.1038/21460. [DOI] [PubMed] [Google Scholar]
- Ohnishi M, Katsuki H, Fukutomi C, Takahashi M, Motomura M, Fukunaga M, Matsuoka Y, Isohama Y, Izumi Y, Kume T, Inoue A, Akaike A. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology. 2011;61(5–6):975–980. doi: 10.1016/j.neuropharm.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Oishi K, Hagiwara S, Koga S, Kawabe S, Uno T, Iwasaka H, Noguchi T. The vitamin E derivative, EPC-K1, suppresses inflammation during hepatic ischemia-reperfusion injury and exerts hepatoprotective effects in rats. J Surg Res. 2012;176(1):164–170. doi: 10.1016/j.jss.2011.03.080. [DOI] [PubMed] [Google Scholar]
- Okano M, Kano S, Munakata H, Ohtsuki K. Biochemical characterization of cholesterol-3-sulfate as the sole effector for the phosphorylation of HMG1 by casein kinase I in vitro. Biochem Biophys Res Commun. 2001;281(5):1325–1330. doi: 10.1006/bbrc.2001.4514. [DOI] [PubMed] [Google Scholar]
- Oktayoglu P, Em S, Tahtasiz M, Bozkurt M, Ucar D, Yazmalar L, Nas K, Yardimeden I, Cevik F, Celik Y, Mete N. Elevated serum levels of high mobility group box protein 1 (HMGB1) in patients with ankylosing spondylitis and its association with disease activity and quality of life. Rheumatology international. 2013;33(5):1327–1331. doi: 10.1007/s00296-012-2578-y. [DOI] [PubMed] [Google Scholar]
- Okuyama H, Endo H, Akashika T, Kato K, Inoue M. Downregulation of c-MYC protein levels contributes to cancer cell survival under dual deficiency of oxygen and glucose. Cancer Res. 2010;70(24):10213–10223. doi: 10.1158/0008-5472.CAN-10-2720. [DOI] [PubMed] [Google Scholar]
- Onate SA, Prendergast P, Wagner JP, Nissen M, Reeves R, Pettijohn DE, Edwards DP. The DNA-bending protein HMG-1 enhances progesterone receptor binding to its target DNA sequences. Mol Cell Biol. 1994;14(5):3376–3391. doi: 10.1128/mcb.14.5.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterwegel M, van de Wetering M, Timmerman J, Kruisbeek A, Destree O, Meijlink F, Clevers H. Differential expression of the HMG box factors TCF-1 and LEF-1 during murine embryogenesis. Development. 1993;118(2):439–448. doi: 10.1242/dev.118.2.439. [DOI] [PubMed] [Google Scholar]
- Oozawa S, Mori S, Kanke T, Takahashi H, Liu K, Tomono Y, Asanuma M, Miyazaki I, Nishibori M, Sano S. Effects of HMGB1 on ischemia-reperfusion injury in the rat heart. Circ J. 2008;72(7):1178–1184. doi: 10.1253/circj.72.1178. [DOI] [PubMed] [Google Scholar]
- Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, Chavakis T. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. Embo J. 2007;26(4):1129–1139. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orphanides G, Wu WH, Lane WS, Hampsey M, Reinberg D. The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature. 1999;400(6741):284–288. doi: 10.1038/22350. [DOI] [PubMed] [Google Scholar]
- Oshima G, Shinoda M, Tanabe M, Ebinuma H, Nishiyama R, Takano K, Yamada S, Miyasho T, Masugi Y, Matsuda S, Suda K, Fukunaga K, Matsubara K, Hibi T, Yagi H, Hayashida T, Yamagishi Y, Obara H, Itano O, Takeuchi H, Kawachi S, Saito H, Hibi T, Maruyama I, Kitagawa Y. Increased plasma levels of high mobility group box 1 in patients with acute liver failure. European surgical research. Europaische chirurgische Forschung. Recherches chirurgicales europeennes. 2012;48(3):154–162. doi: 10.1159/000338363. [DOI] [PubMed] [Google Scholar]
- Ostberg T, Kawane K, Nagata S, Yang H, Chavan S, Klevenvall L, Bianchi ME, Harris HE, Andersson U, Palmblad K. Protective targeting of high mobility group box chromosomal protein 1 in a spontaneous arthritis model. Arthritis Rheum. 2010;62(10):2963–2972. doi: 10.1002/art.27590. [DOI] [PubMed] [Google Scholar]
- Ostberg T, Wahamaa H, Palmblad K, Ito N, Stridh P, Shoshan M, Lotze MT, Harris HE, Andersson U. Oxaliplatin retains HMGB1 intranuclearly and ameliorates collagen type II-induced arthritis. Arthritis Res Ther. 2008;10(1):R1. doi: 10.1186/ar2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otoshi K, Kikuchi S, Kato K, Sekiguchi M, Konno S. Anti-HMGB1 neutralization antibody improves pain-related behavior induced by application of autologous nucleus pulposus onto nerve roots in rats. Spine (Phila Pa 1976) 2011;36(11):E692–E698. doi: 10.1097/BRS.0b013e3181ecd675. [DOI] [PubMed] [Google Scholar]
- Oue N, Aung PP, Mitani Y, Kuniyasu H, Nakayama H, Yasui W. Genes involved in invasion and metastasis of gastric cancer identified by array-based hybridization and serial analysis of gene expression. Oncology. 2005;69(Suppl 1):17–22. doi: 10.1159/000086627. [DOI] [PubMed] [Google Scholar]
- Overbye A, Fengsrud M, Seglen PO. Proteomic analysis of membrane-associated proteins from rat liver autophagosomes. Autophagy. 2007;3(4):300–322. doi: 10.4161/auto.3910. [DOI] [PubMed] [Google Scholar]
- Oyama Y, Hashiguchi T, Taniguchi N, Tancharoen S, Uchimura T, Biswas KK, Kawahara K, Nitanda T, Umekita Y, Lotz M, Maruyama I. High-mobility group box-1 protein promotes granulomatous nephritis in adenine-induced nephropathy. Lab Invest. 2010;90(6):853–866. doi: 10.1038/labinvest.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo R, Bianchi ME. High mobility group box 1 protein, a cue for stem cell recruitment. Biochem Pharmacol. 2004;68(6):1165–1170. doi: 10.1016/j.bcp.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Palumbo R, De Marchis F, Pusterla T, Conti A, Alessio M, Bianchi ME. Src family kinases are necessary for cell migration induced by extracellular HMGB1. J Leukoc Biol. 2009;86(3):617–623. doi: 10.1189/jlb.0908581. [DOI] [PubMed] [Google Scholar]
- Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB, Bianchi ME. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol. 2007;179(1):33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164(3):441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palvimo J, Linnala-Kankkunen A. Identification of sites on chromosomal protein HMG-I phosphorylated by casein kinase II. FEBS Lett. 1989;257(1):101–104. doi: 10.1016/0014-5793(89)81796-x. [DOI] [PubMed] [Google Scholar]
- Palvimo J, Mahonen A, Maenpaa PH. Phosphorylation of high-mobility-group chromatin proteins by protein kinase C from rat brain. Biochim Biophys Acta. 1987;931(3):376–383. doi: 10.1016/0167-4889(87)90229-1. [DOI] [PubMed] [Google Scholar]
- Pan HF, Wu GC, Li WP, Li XP, Ye DQ. High Mobility Group Box 1: a potential therapeutic target for systemic lupus erythematosus. Mol Biol Rep. 2010;37(3):1191–1195. doi: 10.1007/s11033-009-9485-7. [DOI] [PubMed] [Google Scholar]
- Pan K, Chen Y, Roth M, Wang W, Wang S, Yee AS, Zhang X. HBP1-mediated transcriptional regulation of DNA methyltransferase 1 and its impact on cell senescence. Mol Cell Biol. 2013;33(5):887–903. doi: 10.1128/MCB.00637-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Xiao R, Liu X, Li Q. Identification and characterization of the lamprey high-mobility group box 1 gene. PLoS ONE. 2012;7(4):e35755. doi: 10.1371/journal.pone.0035755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M, Budick-Harmelin N, Tirosh B, Tirosh O. Antioxidant defense in hepatic ischemia-reperfusion injury is regulated by damage-associated molecular pattern signal molecules. Free Radic Biol Med. 2008;45(8):1073–1083. doi: 10.1016/j.freeradbiomed.2008.06.029. [DOI] [PubMed] [Google Scholar]
- Park EJ, Jang HJ, Tsoyi K, Kim YM, Park SW, Kim HJ, Lee JH, Chang KC. The heme oxygenase-1 inducer THI-56 negatively regulates iNOS expression and HMGB1 release in LPS-activated RAW 264.7 cells and CLP-induced septic mice. PLoS ONE. 2013;8(10):e76293. doi: 10.1371/journal.pone.0076293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, Abraham E. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284(4):C870–C879. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290(3):C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Park S, Lippard SJ. Redox state-dependent interaction of HMGB1 and cisplatin-modified DNA. Biochemistry. 2011;50(13):2567–2574. doi: 10.1021/bi2000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lippard SJ. Binding interaction of HMGB4 with cisplatin-modified DNA. Biochemistry. 2012;51(34):6728–6737. doi: 10.1021/bi300649v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YE, Hayashi YK, Bonne G, Arimura T, Noguchi S, Nonaka I, Nishino I. Autophagic degradation of nuclear components in mammalian cells. Autophagy. 2009;5(6):795–804. doi: 10.4161/auto.8901. [DOI] [PubMed] [Google Scholar]
- Parkkinen J, Raulo E, Merenmies J, Nolo R, Kajander EO, Baumann M, Rauvala H. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J Biol Chem. 1993;268(26):19726–19738. [PubMed] [Google Scholar]
- Parkkinen J, Rauvala H. Interactions of plasminogen and tissue plasminogen activator (t-PA) with amphoterin. Enhancement of t-PA-catalyzed plasminogen activation by amphoterin. J Biol Chem. 1991;266(25):16730–16735. [PubMed] [Google Scholar]
- Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, Hudson L, Lin X, Patel N, Johnson SM, Chavan S, Goldstein RS, Czura CJ, Miller EJ, Al-Abed Y, Tracey KJ, Pavlov VA. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14(9–10):567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pash JM, Bhorjee JS, Patterson BM, Bustin M. Persistence of chromosomal proteins HMG-14/-17 in myotubes following differentiation-dependent reduction of HMG mRNA. J Biol Chem. 1990;265(8):4197–4199. [PubMed] [Google Scholar]
- Pasheva E, Sarov M, Bidjekov K, Ugrinova I, Sarg B, Lindner H, Pashev IG. In vitro acetylation of HMGB-1 and-2 proteins by CBP: the role of the acidic tail. Biochemistry. 2004;43(10):2935–2940. doi: 10.1021/bi035615y. [DOI] [PubMed] [Google Scholar]
- Pasheva EA, Pashev IG, Favre A. Preferential binding of high mobility group 1 protein to UV-damaged DNA. Role of the COOH-terminal domain. J Biol Chem. 1998;273(38):24730–24736. doi: 10.1074/jbc.273.38.24730. [DOI] [PubMed] [Google Scholar]
- Passalacqua M, Zicca A, Sparatore B, Patrone M, Melloni E, Pontremoli S. Secretion and binding of HMG1 protein to the external surface of the membrane are required for murine erythroleukemia cell differentiation. FEBS Lett. 1997;400(3):275–279. doi: 10.1016/s0014-5793(96)01402-0. [DOI] [PubMed] [Google Scholar]
- Patrick SM, Henkels KM, Turchi JJ. High-mobility group 1 protein inhibits helicase catalyzed displacement of cisplatin-damaged DNA. Biochim Biophys Acta. 1997;1354(3):279–290. doi: 10.1016/s0167-4781(97)00136-x. [DOI] [PubMed] [Google Scholar]
- Patrick SM, Turchi JJ. Human replication protein A preferentially binds cisplatin-damaged duplex DNA in vitro. Biochemistry. 1998;37(24):8808–8815. doi: 10.1021/bi9730590. [DOI] [PubMed] [Google Scholar]
- Patrone M, Pessino A, Passalacqua M, Sparatore B, Melloni E, Pontremoli S. Correlation between levels of delta protein kinase C and resistance to differentiation in murine erythroleukemia cells. Biochem Biophys Res Commun. 1996;220(1):26–30. doi: 10.1006/bbrc.1996.0350. [DOI] [PubMed] [Google Scholar]
- Pauling L, Corey RB, Branson HR. The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proc Natl Acad Sci U S A. 1951;37(4):205–211. doi: 10.1073/pnas.37.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Haykinson MJ, Johnson RC. The nonspecific DNA-binding and -bending proteins HMG1 and HMG2 promote the assembly of complex nucleoprotein structures. Genes Dev. 1993;7(8):1521–1534. doi: 10.1101/gad.7.8.1521. [DOI] [PubMed] [Google Scholar]
- Paull TT, Johnson RC. DNA looping by Saccharomyces cerevisiae high mobility group proteins NHP6A/B. Consequences for nucleoprotein complex assembly and chromatin condensation. J Biol Chem. 1995;270(15):8744–8754. doi: 10.1074/jbc.270.15.8744. [DOI] [PubMed] [Google Scholar]
- Pavare J, Grope I, Kalnins I, Gardovska D. High-mobility group box-1 protein, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in children with community acquired infections and bacteraemia: a prospective study. BMC infectious diseases. 2010;10:28. doi: 10.1186/1471-2334-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, Larosa GJ, Miller EJ, Tracey KJ, Al-Abed Y. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35(4):1139–1144. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- Paweletz N. Walther Flemming: pioneer of mitosis research. Nat Rev Mol Cell Biol. 2001;2(1):72–75. doi: 10.1038/35048077. [DOI] [PubMed] [Google Scholar]
- Pedersen DS, Grasser KD. The role of chromosomal HMGB proteins in plants. Biochim Biophys Acta. 2010;1799(1–2):171–174. doi: 10.1016/j.bbagrm.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Pedrazzi M, Averna M, Sparatore B, Patrone M, Salamino F, Marcoli M, Maura G, Cervetto C, Frattaroli D, Pontremoli S, Melloni E. Potentiation of NMDA receptor-dependent cell responses by extracellular high mobility group box 1 protein. PLoS ONE. 2012;7(8):e44518. doi: 10.1371/journal.pone.0044518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzi M, Patrone M, Passalacqua M, Ranzato E, Colamassaro D, Sparatore B, Pontremoli S, Melloni E. Selective proinflammatory activation of astrocytes by high-mobility group box 1 protein signaling. J Immunol. 2007;179(12):8525–8532. doi: 10.4049/jimmunol.179.12.8525. [DOI] [PubMed] [Google Scholar]
- Pei H, Wu X, Liu T, Yu K, Jelinek DF, Lou Z. The histone methyltransferase MMSET regulates class switch recombination. J Immunol. 2013;190(2):756–763. doi: 10.4049/jimmunol.1201811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado JR, Quiros PM, Pulido MR, Marino G, Martinez-Chantar ML, Vazquez-Martinez R, Freije JM, Lopez-Otin C, Malagon MM. Proteomic profiling of adipose tissue from Zmpste24−/− mice, a model of lipodystrophy and premature aging, reveals major changes in mitochondrial function and vimentin processing. Mol Cell Proteomics. 2011;10(11) doi: 10.1074/mcp.M111.008094. M111 008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelovsky P, Pashev IG, Pasheva E. Interplay between in vitro acetylation and phosphorylation of tailless HMGB1 protein. Biochem Biophys Res Commun. 2009;380(1):138–142. doi: 10.1016/j.bbrc.2009.01.056. [DOI] [PubMed] [Google Scholar]
- Peltz ED, Moore EE, Eckels PC, Damle SS, Tsuruta Y, Johnson JL, Sauaia A, Silliman CC, Banerjee A, Abraham E. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock (Augusta, Ga.) 2009;32(1):17–22. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena G, Cai B, Deitch EA, Ulloa L. JAK2 inhibition prevents innate immune responses and rescues animals from sepsis. J Mol Med (Berl) 2010;88(8):851–859. doi: 10.1007/s00109-010-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold SA, Coughlan MT, Patel SK, Srivastava PM, Sourris KC, Steer D, Webster DE, Thomas MC, MacIsaac RJ, Jerums G, Burrell LM, Cooper ME, Forbes JM. Circulating high-molecular-weight RAGE ligands activate pathways implicated in the development of diabetic nephropathy. Kidney Int. 2010;78(3):287–295. doi: 10.1038/ki.2010.134. [DOI] [PubMed] [Google Scholar]
- Peng KF, Wu XF, Zhao HW, Sun Y. Advanced oxidation protein products induce monocyte chemoattractant protein-1 expression via p38 mitogen-activated protein kinase activation in rat vascular smooth muscle cells. Chin Med J (Engl) 2006;119(13):1088–1093. [PubMed] [Google Scholar]
- Peng RQ, Wu XJ, Ding Y, Li CY, Yu XJ, Zhang X, Pan ZZ, Wan DS, Zheng LM, Zeng YX, Zhang XS. Co-expression of nuclear and cytoplasmic HMGB1 is inversely associated with infiltration of CD45RO+ T cells and prognosis in patients with stage IIIB colon cancer. BMC cancer. 2010;10:496. doi: 10.1186/1471-2407-10-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentimalli F, Palmieri D, Pacelli R, Garbi C, Cesari R, Martin E, Pierantoni GM, Chieffi P, Croce CM, Costanzo V, Fedele M, Fusco A. HMGA1 protein is a novel target of the ATM kinase. Eur J Cancer. 2008;44(17):2668–2679. doi: 10.1016/j.ejca.2008.07.033. [DOI] [PubMed] [Google Scholar]
- Penzo M, Molteni R, Suda T, Samaniego S, Raucci A, Habiel DM, Miller F, Jiang HP, Li J, Pardi R, Palumbo R, Olivotto E, Kew RR, Bianchi ME, Marcu KB. Inhibitor of NF-kappa B kinases alpha and beta are both essential for high mobility group box 1-mediated chemotaxis [corrected] J Immunol. 2010;184(8):4497–4509. doi: 10.4049/jimmunol.0903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Carrion MD, Cena V. Knocking down HMGB1 using dendrimer-delivered siRNA unveils its key role in nmda-induced autophagy in rat cortical neurons. Pharm Res. 2013;30(10):2584–2595. doi: 10.1007/s11095-013-1049-9. [DOI] [PubMed] [Google Scholar]
- Perez-Martin J, Johnson AD. The C-terminal domain of Sin1 interacts with the SWI-SNF complex in yeast. Mol Cell Biol. 1998a;18(7):4157–4164. doi: 10.1128/mcb.18.7.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martin J, Johnson AD. Mutations in chromatin components suppress a defect of Gcn5 protein in Saccharomyces cerevisiae. Mol Cell Biol. 1998b;18(2):1049–1054. doi: 10.1128/mcb.18.2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrella MA, Pellacani A, Wiesel P, Chin MT, Foster LC, Ibanez M, Hsieh CM, Reeves R, Yet SF, Lee ME. High mobility group-I(Y) protein facilitates nuclear factor-kappaB binding and transactivation of the inducible nitric-oxide synthase promoter/enhancer. J Biol Chem. 1999;274(13):9045–9052. doi: 10.1074/jbc.274.13.9045. [DOI] [PubMed] [Google Scholar]
- Peter K, Bobik A. HMGB1 signals danger in acute coronary syndrome: emergence of a new risk marker for cardiovascular death? Atherosclerosis. 2012;221(2):317–318. doi: 10.1016/j.atherosclerosis.2011.11.039. [DOI] [PubMed] [Google Scholar]
- Peterson EB, Mastrangelo MA, Federoff HJ, Bowers WJ. Neuronal specificity of HSV/sleeping beauty amplicon transduction in utero is driven primarily by tropism and cell type composition. Mol Ther. 2007;15(10):1848–1855. doi: 10.1038/sj.mt.6300267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A, Ragu C, Della-Valle V, Mozziconacci MJ, Lafage-Pochitaloff M, Soler G, Schluth C, Radford I, Ottolenghi C, Bernard OA, Penard-Lacronique V, Romana SP. NUP98-HMGB3: a novel oncogenic fusion. Leukemia. 2010;24(3):654–658. doi: 10.1038/leu.2009.241. [DOI] [PubMed] [Google Scholar]
- Petrof G, Abdul-Wahab A, Proudfoot L, Pramanik R, Mellerio JE, McGrath JA. Serum levels of high mobility group box 1 correlate with disease severity in recessive dystrophic epidermolysis bullosa. Experimental dermatology. 2013;22(6):433–435. doi: 10.1111/exd.12152. [DOI] [PubMed] [Google Scholar]
- Phair RD, Scaffidi P, Elbi C, Vecerova J, Dey A, Ozato K, Brown DT, Hager G, Bustin M, Misteli T. Global nature of dynamic protein-chromatin interactions in vivo: three-dimensional genome scanning and dynamic interaction networks of chromatin proteins. Mol Cell Biol. 2004;24(14):6393–6402. doi: 10.1128/MCB.24.14.6393-6402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pil PM, Chow CS, Lippard SJ. High-mobility-group 1 protein mediates DNA bending as determined by ring closures. Proc Natl Acad Sci U S A. 1993;90(20):9465–9469. doi: 10.1073/pnas.90.20.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pil PM, Lippard SJ. Specific binding of chromosomal protein HMG1 to DNA damaged by the anticancer drug cisplatin. Science. 1992;256(5054):234–237. doi: 10.1126/science.1566071. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. Cell death in the pathogenesis of immune-mediated diseases: the role of HMGB1 and DAMP-PAMP complexes. Swiss Med Wkly. 2011;141:w13256. doi: 10.4414/smw.2011.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisetsky DS. HMGB1: a dangerous player in lupus pathogenesis. J Rheumatol. 2010;37(4):689–691. doi: 10.3899/jrheum.091459. [DOI] [PubMed] [Google Scholar]
- Pisetsky DS. HMGB1: a smoking gun in lupus nephritis? Arthritis Res Ther. 2012a;14(2):112. doi: 10.1186/ar3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisetsky DS. The origin and properties of extracellular DNA: from PAMP to DAMP. Clin Immunol. 2012b;144(1):32–40. doi: 10.1016/j.clim.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisetsky DS, Erlandsson-Harris H, Andersson U. High-mobility group box protein 1 (HMGB1): an alarmin mediating the pathogenesis of rheumatic disease. Arthritis Res Ther. 2008;10(3):209. doi: 10.1186/ar2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisetsky DS, Gauley J, Ullal AJ. HMGB1 and microparticles as mediators of the immune response to cell death. Antioxid Redox Signal. 2011;15(8):2209–2219. doi: 10.1089/ars.2010.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistoia V, Raffaghello L. Damage-associated molecular patterns (DAMPs) and mesenchymal stem cells: a matter of attraction and excitement. Eur J Immunol. 2011;41(7):1828–1831. doi: 10.1002/eji.201141724. [DOI] [PubMed] [Google Scholar]
- Polanska E, Dobsakova Z, Dvorackova M, Fajkus J, Stros M. HMGB1 gene knockout in mouse embryonic fibroblasts results in reduced telomerase activity and telomere dysfunction. Chromosoma. 2012;121(4):419–431. doi: 10.1007/s00412-012-0373-x. [DOI] [PubMed] [Google Scholar]
- Polanska E, Pospisilova S, Stros M. Binding of Histone H1 to DNA Is Differentially Modulated by Redox State of HMGB1. PLoS ONE. 2014;9(2):e89070. doi: 10.1371/journal.pone.0089070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic K, Ek M, Espinosa A, Padyukov L, Harris HE, Wahren-Herlenius M, Nyberg F. Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum. 2005;52(11):3639–3645. doi: 10.1002/art.21398. [DOI] [PubMed] [Google Scholar]
- Popovic PJ, DeMarco R, Lotze MT, Winikoff SE, Bartlett DL, Krieg AM, Guo ZS, Brown CK, Tracey KJ, Zeh HJ., 3rd High mobility group B1 protein suppresses the human plasmacytoid dendritic cell response to TLR9 agonists. J Immunol. 2006;177(12):8701–8707. doi: 10.4049/jimmunol.177.12.8701. [DOI] [PubMed] [Google Scholar]
- Porto A, Palumbo R, Pieroni M, Aprigliano G, Chiesa R, Sanvito F, Maseri A, Bianchi ME. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. Faseb J. 2006;20(14):2565–2566. doi: 10.1096/fj.06-5867fje. [DOI] [PubMed] [Google Scholar]
- Potier MC, Rivals I, Mercier G, Ettwiller L, Moldrich RX, Laffaire J, Personnaz L, Rossier J, Dauphinot L. Transcriptional disruptions in Down syndrome: a case study in the Ts1Cje mouse cerebellum during post-natal development. J Neurochem. 2006;97(Suppl 1):104–109. doi: 10.1111/j.1471-4159.2005.03624.x. [DOI] [PubMed] [Google Scholar]
- Pouwels SD, Heijink IH, Ten Hacken NH, Vandenabeele P, Krysko DV, Nawijn MC, van Oosterhout AJ. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol. 2014;7(2):215–226. doi: 10.1038/mi.2013.77. [DOI] [PubMed] [Google Scholar]
- Prasad R, Liu Y, Deterding LJ, Poltoratsky VP, Kedar PS, Horton JK, Kanno S, Asagoshi K, Hou EW, Khodyreva SN, Lavrik OI, Tomer KB, Yasui A, Wilson SH. HMGB1 is a cofactor in mammalian base excision repair. Mol Cell. 2007;27(5):829–841. doi: 10.1016/j.molcel.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Thakur MK. In vitro acetylation of the liver HMG non-histone proteins and its modulation by spermine and dexamethasone during aging of rats. Mol Biol Rep. 1988;13(4):221–224. doi: 10.1007/BF00788174. [DOI] [PubMed] [Google Scholar]
- Prasad S, Thakur MK. Distribution of high mobility group proteins in different tissues of rats during aging. Biochem Int. 1990a;20(4):687–695. [PubMed] [Google Scholar]
- Prasad S, Thakur MK. Effects of spermine and sodium butyrate on the in vitro phosphorylation of HMG non-histone proteins of the liver of young and old rats. Arch Gerontol Geriatr. 1990b;10(3):231–238. doi: 10.1016/0167-4943(90)90024-z. [DOI] [PubMed] [Google Scholar]
- Pullerits R, Brisslert M, Jonsson IM, Tarkowski A. Soluble receptor for advanced glycation end products triggers a proinflammatory cytokine cascade via beta2 integrin Mac-1. Arthritis Rheum. 2006;54(12):3898–3907. doi: 10.1002/art.22217. [DOI] [PubMed] [Google Scholar]
- Pullerits R, Jonsson IM, Kollias G, Tarkowski A. Induction of arthritis by high mobility group box chromosomal protein 1 is independent of tumour necrosis factor signalling. Arthritis Res Ther. 2008;10(3):R72. doi: 10.1186/ar2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullerits R, Jonsson IM, Verdrengh M, Bokarewa M, Andersson U, Erlandsson-Harris H, Tarkowski A. High mobility group box chromosomal protein 1, a DNA binding cytokine, induces arthritis. Arthritis Rheum. 2003;48(6):1693–1700. doi: 10.1002/art.11028. [DOI] [PubMed] [Google Scholar]
- Pullerits R, Urbonaviciute V, Voll RE, Forsblad-D'Elia H, Carlsten H. Serum levels of HMGB1 in postmenopausal patients with rheumatoid arthritis: associations with proinflammatory cytokines, acute-phase reactants, and clinical disease characteristics. J Rheumatol. 2011;38(7):1523–1525. doi: 10.3899/jrheum.110091. [DOI] [PubMed] [Google Scholar]
- Pusterla T, de Marchis F, Palumbo R, Bianchi ME. High mobility group B2 is secreted by myeloid cells and has mitogenic and chemoattractant activities similar to high mobility group B1. Autoimmunity. 2009;42(4):308–310. doi: 10.1080/08916930902831845. [DOI] [PubMed] [Google Scholar]
- Qi ML, Tagawa K, Enokido Y, Yoshimura N, Wada Y, Watase K, Ishiura S, Kanazawa I, Botas J, Saitoe M, Wanker EE, Okazawa H. Proteome analysis of soluble nuclear proteins reveals that HMGB1/2 suppress genotoxic stress in polyglutamine diseases. Nat Cell Biol. 2007;9(4):402–414. doi: 10.1038/ncb1553. [DOI] [PubMed] [Google Scholar]
- Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, Yang H. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203(7):1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Chen Y, Wang W, Wang Z, Tang G, Zhang P, He Z, Liu Y, Dai SM, Shen Q. HMGB1-LPS complex promotes transformation of osteoarthritis synovial fibroblasts to a rheumatoid arthritis synovial fibroblast-like phenotype. Cell Death Dis. 2014;5:e1077. doi: 10.1038/cddis.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin YH, Dai SM, Tang GS, Zhang J, Ren D, Wang ZW, Shen Q. HMGB1 enhances the proinflammatory activity of lipopolysaccharide by promoting the phosphorylation of MAPK p38 through receptor for advanced glycation end products. J Immunol. 2009;183(10):6244–6250. doi: 10.4049/jimmunol.0900390. [DOI] [PubMed] [Google Scholar]
- Qing X, Pitashny M, Thomas DB, Barrat FJ, Hogarth MP, Putterman C. Pathogenic anti-DNA antibodies modulate gene expression in mesangial cells: involvement of HMGB1 in anti-DNA antibody-induced renal injury. Immunol Lett. 2008;121(1):61–73. doi: 10.1016/j.imlet.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28(5):927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- Qu J, Yan R, Chen J, Xu T, Zhou J, Wang M, Chen C, Yan Y, Lu Y. HMGN5: a potential oncogene in gliomas. J Neurooncol. 2011;104(3):729–736. doi: 10.1007/s11060-011-0558-9. [DOI] [PubMed] [Google Scholar]
- Rabadi MM, Ghaly T, Goligorksy MS, Ratliff BB. HMGB1 in renal ischemic injury. Am J Physiol Renal Physiol. 2012;303(6):F873–F885. doi: 10.1152/ajprenal.00092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33(1):9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin JN, Gonzalez-Rivera C, Ivie SE, McClain MS, Cover TL. Helicobacter pylori VacA induces programmed necrosis in gastric epithelial cells. Infect Immun. 2011;79(7):2535–2543. doi: 10.1128/IAI.01370-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos L, Pena G, Cai B, Deitch EA, Ulloa L. Mast cell stabilization improves survival by preventing apoptosis in sepsis. J Immunol. 2010;185(1):709–716. doi: 10.4049/jimmunol.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsgaard L, Englert JM, Manni ML, Milutinovic PS, Gefter J, Tobolewski J, Crum L, Coudriet GM, Piganelli J, Zamora R, Vodovotz Y, Enghild JJ, Oury TD. Lack of the receptor for advanced glycation end-products attenuates E. coli pneumonia in mice. PLoS ONE. 2011;6(5):e20132. doi: 10.1371/journal.pone.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzato E, Patrone M, Pedrazzi M, Burlando B. HMGb1 promotes scratch wound closure of HaCaT keratinocytes via ERK1/2 activation. Mol Cell Biochem. 2009;332(1–2):199–205. doi: 10.1007/s11010-009-0192-4. [DOI] [PubMed] [Google Scholar]
- Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) Faseb J. 2008;22(10):3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- Rausch MP, Hastings KT. GILT modulates CD4+ T-cell tolerance to the melanocyte differentiation antigen tyrosinase-related protein 1. J Invest Dermatol. 2012;132(1):154–162. doi: 10.1038/jid.2011.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauvala H, Pihlaskari R. Isolation and some characteristics of an adhesive factor of brain that enhances neurite outgrowth in central neurons. J Biol Chem. 1987;262(34):16625–16635. [PubMed] [Google Scholar]
- Reddy MC, Christensen J, Vasquez KM. Interplay between human high mobility group protein 1 and replication protein A on psoralen-cross-linked DNA. Biochemistry. 2005;44(11):4188–4195. doi: 10.1021/bi047902n. [DOI] [PubMed] [Google Scholar]
- Reeves R. Structure and function of the HMGI(Y) family of architectural transcription factors. Environ Health Perspect. 2000;108(Suppl 5):803–809. doi: 10.1289/ehp.00108s5803. [DOI] [PubMed] [Google Scholar]
- Reeves R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene. 2001;277(1–2):63–81. doi: 10.1016/s0378-1119(01)00689-8. [DOI] [PubMed] [Google Scholar]
- Reeves R. HMGA proteins: flexibility finds a nuclear niche? Biochem Cell Biol. 2003;81(3):185–195. doi: 10.1139/o03-044. [DOI] [PubMed] [Google Scholar]
- Reeves R, Beckerbauer L. HMGI/Y proteins: flexible regulators of transcription and chromatin structure. Biochim Biophys Acta. 2001;1519(1–2):13–29. doi: 10.1016/s0167-4781(01)00215-9. [DOI] [PubMed] [Google Scholar]
- Reggiori F, Komatsu M, Finley K, Simonsen A. Autophagy: more than a nonselective pathway. Int J Cell Biol. 2012;2012:219625. doi: 10.1155/2012/219625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18(4):581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Sun R, Wang S. Role of inducible nitric oxide synthase expressed by alveolar macrophages in high mobility group box 1--induced acute lung injury. Inflamm Res. 2006;55(5):207–215. doi: 10.1007/s00011-006-0072-2. [DOI] [PubMed] [Google Scholar]
- Ren PC, Zhang Y, Zhang XD, An LJ, Lv HG, He J, Gao CJ, Sun XD. High-mobility group box 1 contributes to mechanical allodynia and spinal astrocytic activation in a mouse model of type 2 diabetes. Brain Res Bull. 2012;88(4):332–337. doi: 10.1016/j.brainresbull.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G, Sama AE, Tracey KJ. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol. 2003;170(7):3890–3897. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- Ribeiro FS, de Abreu da Silva IC, Carneiro VC, Belgrano Fdos S, Mohana-Borges R, de Andrade Rosa I, Benchimol M, Souza NR, Mesquita RD, Sorgine MH, Gazos-Lopes F, Vicentino AR, Wu W, de Moraes Maciel R, da Silva-Neto MA, Fantappie MR. The dengue vector Aedes aegypti contains a functional high mobility group box 1 (HMGB1) protein with a unique regulatory C-terminus. PLoS ONE. 2012;7(7):e40192. doi: 10.1371/journal.pone.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Kohl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14(5):551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riuzzi F, Sorci G, Donato R. RAGE expression in rhabdomyosarcoma cells results in myogenic differentiation and reduced proliferation, migration, invasiveness, and tumor growth. Am J Pathol. 2007;171(3):947–961. doi: 10.2353/ajpath.2007.070049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert SM, Sjodin H, Fink MP, Aneja RK. Preconditioning with high mobility group box 1 (HMGB1) induces lipoteichoic acid (LTA) tolerance. J Immunother. 2010;33(7):663–671. doi: 10.1097/CJI.0b013e3181dcd111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AB, Klungland A, Rognes T, Leiros I. DNA repair in mammalian cells: Base excision repair: the long and short of it. Cell Mol Life Sci. 2009;66(6):981–993. doi: 10.1007/s00018-009-8736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson AP, Caldis MW, Harp CT, Goings GE, Miller SD. High-mobility group box 1 protein (HMGB1) neutralization ameliorates experimental autoimmune encephalomyelitis. J Autoimmun. 2013;43:32–43. doi: 10.1016/j.jaut.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman M, Postnikov Y, Correll S, Malicet C, Wincovitch S, Karpova TS, McNally JG, Wu X, Bubunenko NA, Grigoryev S, Bustin M. The interaction of NSBP1/HMGN5 with nucleosomes in euchromatin counteracts linker histone-mediated chromatin compaction and modulates transcription. Mol Cell. 2009;35(5):642–656. doi: 10.1016/j.molcel.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman M, Taher L, Kurahashi T, Cherukuri S, Uversky VN, Landsman D, Ovcharenko I, Bustin M. Effects of HMGN variants on the cellular transcription profile. Nucleic Acids Res. 2011;39(10):4076–4087. doi: 10.1093/nar/gkq1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenfeller P, Ring J, Muschett V, Beranek A, Buettner S, Carmona-Gutierrez D, Eisenberg T, Khoury C, Rechberger G, Kohlwein SD, Kroemer G, Madeo F. Fatty acids trigger mitochondrion-dependent necrosis. Cell Cycle. 2010;9(14):2836–2842. doi: 10.4161/cc.9.14.12267. [DOI] [PubMed] [Google Scholar]
- Romani M, Rodman TC, Vidali G, Bustin M. Serological analysis of species specificity in the high mobility group chromosomal proteins. J Biol Chem. 1979;254(8):2918–2922. [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2011;24(12):1444–1455. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(6):558–567. doi: 10.3109/14767058.2011.599083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romine LE, Wood JR, Lamia LA, Prendergast P, Edwards DP, Nardulli AM. The high mobility group protein 1 enhances binding of the estrogen receptor DNA binding domain to the estrogen response element. Mol Endocrinol. 1998;12(5):664–674. doi: 10.1210/mend.12.5.0111. [DOI] [PubMed] [Google Scholar]
- Ronfani L, Ferraguti M, Croci L, Ovitt CE, Scholer HR, Consalez GG, Bianchi ME. Reduced fertility and spermatogenesis defects in mice lacking chromosomal protein Hmgb2. Development. 2001;128(8):1265–1273. doi: 10.1242/dev.128.8.1265. [DOI] [PubMed] [Google Scholar]
- Rong J, Ye S, Liang MY, Chen GX, Liu H, Zhang JX, Wu ZK. Receptor for advanced glycation end products involved in lung ischemia reperfusion injury in cardiopulmonary bypass attenuated by controlled oxygen reperfusion in a canine model. Asaio J. 2013;59(3):302–308. doi: 10.1097/MAT.0b013e318290504e. [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15(7–8):195–202. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini A, Zacheo A, Mocini D, Totta P, Facchiano A, Castoldi R, Sordini P, Pompilio G, Abeni D, Capogrossi MC, Germani A. HMGB1-stimulated human primary cardiac fibroblasts exert a paracrine action on human and murine cardiac stem cells. J Mol Cell Cardiol. 2008;44(4):683–693. doi: 10.1016/j.yjmcc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Kruger I, Thompson LH, Lobrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol. 2003;23(16):5706–5715. doi: 10.1128/MCB.23.16.5706-5715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhiainen A, Kuja-Panula J, Wilkman E, Pakkanen J, Stenfors J, Tuominen RK, Lepantalo M, Carpen O, Parkkinen J, Rauvala H. Regulation of monocyte migration by amphoterin (HMGB1) Blood. 2004;104(4):1174–1182. doi: 10.1182/blood-2003-10-3536. [DOI] [PubMed] [Google Scholar]
- Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin) J Leukoc Biol. 2007;81(1):49–58. doi: 10.1189/jlb.0306200. [DOI] [PubMed] [Google Scholar]
- Roussel BD, Mysiorek C, Rouhiainen A, Jullienne A, Parcq J, Hommet Y, Culot M, Berezowski V, Cecchelli R, Rauvala H, Vivien D, Ali C. HMGB-1 promotes fibrinolysis and reduces neurotoxicity mediated by tissue plasminogen activator. J Cell Sci. 2011;124(Pt 12):2070–2076. doi: 10.1242/jcs.084392. [DOI] [PubMed] [Google Scholar]
- Rowe SM, Jackson PL, Liu G, Hardison M, Livraghi A, Solomon GM, McQuaid DB, Noerager BD, Gaggar A, Clancy JP, O'Neal W, Sorscher EJ, Abraham E, Blalock JE. Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am J Respir Crit Care Med. 2008;178(8):822–831. doi: 10.1164/rccm.200712-1894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell JP, Simpson KL, Stott K, Watson M, Thomas JO. HMGB1-Facilitated p53 DNA Binding Occurs via HMG-Box/p53 Transactivation Domain Interaction, Regulated by the Acidic Tail. Structure. 2012;20(12):2014–2024. doi: 10.1016/j.str.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Roy A, Ganesh G, Sippola H, Bolin S, Sawesi O, Dagalv A, Schlenner SM, Feyerabend T, Rodewald HR, Kjellen L, Hellman L, Abrink M. Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation. J Biol Chem. 2014;289(1):237–250. doi: 10.1074/jbc.M112.435156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein YR, Furusawa T, Lim JH, Postnikov YV, West KL, Birger Y, Lee S, Nguyen P, Trepel JB, Bustin M. Chromosomal protein HMGN1 modulates the expression of N-cadherin. Febs J. 2005;272(22):5853–5863. doi: 10.1111/j.1742-4658.2005.04980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russanova VR, Ando I. A study on the amount of high-mobility-group chromatin proteins in T-cells at different stages of differentiation. Biochim Biophys Acta. 1985;825(4):405–410. doi: 10.1016/0167-4781(85)90068-5. [DOI] [PubMed] [Google Scholar]
- Russo AJ. Decreased Epidermal Growth Factor (EGF) Associated with HMGB1 and Increased Hyperactivity in Children with Autism. Biomarker insights. 2013;8:35–41. doi: 10.4137/BMI.S11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadofsky MJ. The RAG proteins in V(D)J recombination: more than just a nuclease. Nucleic Acids Res. 2001;29(7):1399–1409. doi: 10.1093/nar/29.7.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu D, Debnath P, Takayama Y, Iwahara J. Redox properties of the A-domain of the HMGB1 protein. FEBS Lett. 2008;582(29):3973–3978. doi: 10.1016/j.febslet.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi H, Melki MT, Gougeon ML. HMGB1-dependent triggering of HIV-1 replication and persistence in dendritic cells as a consequence of NK-DC cross-talk. PLoS ONE. 2008;3(10):e3601. doi: 10.1371/journal.pone.0003601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai E, Shimada-Sugawara M, Nishishita K, Fukuma Y, Naito M, Okamoto K, Nakayama K, Tsukuba T. Suppression of RANKL-dependent heme oxygenase-1 is required for high mobility group box 1 release and osteoclastogenesis. J Cell Biochem. 2012;113(2):486–498. doi: 10.1002/jcb.23372. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Mashiko K, Matsumoto H, Hara Y, Kutsukata N, Takei K, Ueno Y, Tomita Y, Yamamoto Y. Effect of direct hemoperfusion with a polymyxin B immobilized fiber column on high mobility group box-1 (HMGB-1) in severe septic shock: report of a case. Asaio J. 2006;52(6):e37–e39. doi: 10.1097/01.mat.0000248996.22865.ce. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Mashiko K, Matsumoto H, Hara Y, Kutsukata N, Yamamoto Y. Relationship between effect of polymyxin B-immobilized fiber and high-mobility group box-1 protein in septic shock patients. ASAIO journal (American Society for Artificial Internal Organs: 1992) 2007;53(3):324–328. doi: 10.1097/MAT.0b013e3180340301. [DOI] [PubMed] [Google Scholar]
- Salmivirta M, Rauvala H, Elenius K, Jalkanen M. Neurite growth-promoting protein (amphoterin, p30) binds syndecan. Exp Cell Res. 1992;200(2):444–451. doi: 10.1016/0014-4827(92)90194-d. [DOI] [PubMed] [Google Scholar]
- Salpietro C, Cuppari C, Grasso L, Tosca MA, Miraglia Del Giudice M, La Rosa M, Marseglia GL, Salpietro A, Ciprandi G. Nasal high-mobility group box-1 protein in children with allergic rhinitis. International archives of allergy and immunology. 2013;161(2):116–121. doi: 10.1159/000345246. [DOI] [PubMed] [Google Scholar]
- Sampson EM, Haque ZK, Ku MC, Tevosian SG, Albanese C, Pestell RG, Paulson KE, Yee AS. Negative regulation of the Wnt-beta-catenin pathway by the transcriptional repressor HBP1. Embo J. 2001;20(16):4500–4511. doi: 10.1093/emboj/20.16.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford AN, Dietzmann K, Sullivan KE. Apoptotic cells, autoantibodies, and the role of HMGB1 in the subcellular localization of an autoantigen. J Autoimmun. 2005;25(4):264–271. doi: 10.1016/j.jaut.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Sapojnikova N, Maman J, Myers FA, Thorne AW, Vorobyev VI, Crane-Robinson C. Biochemical observation of the rapid mobility of nuclear HMGB1. Biochim Biophys Acta. 2005;1729(1):57–63. doi: 10.1016/j.bbaexp.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123(3):790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- Sarai N, Nimura K, Tamura T, Kanno T, Patel MC, Heightman TD, Ura K, Ozato K. WHSC1 links transcription elongation to HIRA-mediated histone H3.3 deposition. Embo J. 2013;32(17):2392–2406. doi: 10.1038/emboj.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasahira T, Akama Y, Fujii K, Kuniyasu H. Expression of receptor for advanced glycation end products and HMGB1/amphoterin in colorectal adenomas. Virchows Arch. 2005a;446(4):411–415. doi: 10.1007/s00428-005-1210-x. [DOI] [PubMed] [Google Scholar]
- Sasahira T, Kirita T, Bhawal UK, Ikeda M, Nagasawa A, Yamamoto K, Kuniyasu H. The expression of receptor for advanced glycation end products is associated with angiogenesis in human oral squamous cell carcinoma. Virchows Arch. 2007;450(3):287–295. doi: 10.1007/s00428-006-0359-2. [DOI] [PubMed] [Google Scholar]
- Sasahira T, Sasaki T, Kuniyasu H. Interleukin-15 and transforming growth factor alpha are associated with depletion of tumor-associated macrophages in colon cancer. J Exp Clin Cancer Res. 2005b;24(1):69–74. [PubMed] [Google Scholar]
- Sato F, Maruyama S, Hayashi H, Sakamoto I, Yamada S, Uchimura T, Morita Y, Ito Y, Yuzawa Y, Maruyama I, Matsuo S. High mobility group box chromosomal protein 1 in patients with renal diseases. Nephron Clin Pract. 2008;108(3):c194–c201. doi: 10.1159/000118942. [DOI] [PubMed] [Google Scholar]
- Satoh A, Shimosegawa T, Kimura K, Moriizumi S, Masamune A, Koizumi M, Toyota T. Nitric oxide is overproduced by peritoneal macrophages in rat taurocholate pancreatitis: the mechanism of inducible nitric oxide synthase expression. Pancreas. 1998;17(4):402–411. doi: 10.1097/00006676-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12(47):7666–7670. doi: 10.3748/wjg.v12.i47.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Schaper F, Westra J, Bijl M. Recent developments on the role of High Mobility Group Box 1 in Systemic Lupus Erythematosus. Mol Med. 2014 doi: 10.2119/molmed.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierbeck H, Pullerits R, Pruunsild C, Fischer M, Holzinger D, Laestadius A, Sundberg E, Harris HE. HMGB1 levels are increased in patients with juvenile idiopathic arthritis, correlate with early onset of disease, and are independent of disease duration. The Journal of rheumatology. 2013;40(9):1604–1613. doi: 10.3899/jrheum.120987. [DOI] [PubMed] [Google Scholar]
- Schierbeck H, Wahamaa H, Andersson U, Harris HE. Immunomodulatory drugs regulate HMGB1 release from activated human monocytes. Mol Med. 2010;16(9–10):343–351. doi: 10.2119/molmed.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, Uguccioni M. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209(3):551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlueter C, Weber H, Meyer B, Rogalla P, Roser K, Hauke S, Bullerdiek J. Angiogenetic signaling through hypoxia: HMGB1: an angiogenetic switch molecule. Am J Pathol. 2005;166(4):1259–1263. doi: 10.1016/S0002-9440(10)62344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp G, Santori F, Carles C, Riva M, Grummt I. The HMG box-containing nucleolar transcription factor UBF interacts with a specific subunit of RNA polymerase I. Embo J. 1994;13(1):190–199. doi: 10.1002/j.1460-2075.1994.tb06248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Schrumpfova PP, Fojtova M, Mokros P, Grasser KD, Fajkus J. Role of HMGB proteins in chromatin dynamics and telomere maintenance in Arabidopsis thaliana. Curr Protein Pept Sci. 2011;12(2):105–111. doi: 10.2174/138920311795684922. [DOI] [PubMed] [Google Scholar]
- Schulman IG, Wang T, Wu M, Bowen J, Cook RG, Gorovsky MA, Allis CD. Macronuclei and micronuclei in Tetrahymena thermophila contain high-mobility-group-like chromosomal proteins containing a highly conserved eleven-amino-acid putative DNA-binding sequence. Mol Cell Biol. 1991;11(1):166–174. doi: 10.1128/mcb.11.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze J, Zierath D, Tanzi P, Cain K, Shibata D, Dressel A, Becker K. Severe stroke induces long-lasting alterations of high-mobility group box 1. Stroke. 2013;44(1):246–248. doi: 10.1161/STROKEAHA.112.676072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeler BJ, Horton MJ, Szego CM, DeLange RJ. Monoclonal antibody toward lysosomal cathepsin B cross-reacts preferentially with distinct histone classes. Int J Biochem. 1988;20(10):1089–1106. doi: 10.1016/0020-711x(88)90254-6. [DOI] [PubMed] [Google Scholar]
- Seeler JS, Marchio A, Sitterlin D, Transy C, Dejean A. Interaction of SP100 with HP1 proteins: a link between the promyelocytic leukemia-associated nuclear bodies and the chromatin compartment. Proc Natl Acad Sci U S A. 1998;95(13):7316–7321. doi: 10.1073/pnas.95.13.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall AM, Goodman SD, Nash HA. Architectural elements in nucleoprotein complexes: interchangeability of specific and non-specific DNA binding proteins. Embo J. 1994;13(19):4536–4548. doi: 10.1002/j.1460-2075.1994.tb06775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106(2):609–616. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- Semino C, Ceccarelli J, Lotti LV, Torrisi MR, Angelini G, Rubartelli A. The maturation potential of NK cell clones toward autologous dendritic cells correlates with HMGB1 secretion. J Leukoc Biol. 2007;81(1):92–99. doi: 10.1189/jlb.0306172. [DOI] [PubMed] [Google Scholar]
- Sessa L, Gatti E, Zeni F, Antonelli A, Catucci A, Koch M, Pompilio G, Fritz G, Raucci A, Bianchi ME. The Receptor for Advanced Glycation End-Products (RAGE) Is Only Present in Mammals, and Belongs to a Family of Cell Adhesion Molecules (CAMs) PLoS ONE. 2014;9(1):e86903. doi: 10.1371/journal.pone.0086903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedin SM, Pehrson JR, Cole RD. Loss of chromosomal high mobility group proteins HMG1 and HMG2 when mouse neuroblastoma and Friend erythroleukemia cells become committed to differentiation. Proc Natl Acad Sci U S A. 1981;78(10):5988–5992. doi: 10.1073/pnas.78.10.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180(4):2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- Shang GH, Jia CQ, Tian H, Xiao W, Li Y, Wang AH, Dong L, Lin DJ. Serum high mobility group box protein 1 as a clinical marker for non-small cell lung cancer. Respiratory medicine. 2009;103(12):1949–1953. doi: 10.1016/j.rmed.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Sharma A, Ray R, Rajeswari MR. Overexpression of high mobility group (HMG) B1 and B2 proteins directly correlates with the progression of squamous cell carcinoma in skin. Cancer Invest. 2008;26(8):843–851. doi: 10.1080/07357900801954210. [DOI] [PubMed] [Google Scholar]
- Sharma L, Wu J, Patel V, Sitapara R, Rao NV, Kennedy TP, Mantell LL. Partially-desulfated heparin improves survival in Pseudomonas pneumonia by enhancing bacterial clearance and ameliorating lung injury. J Immunotoxicol. 2013 doi: 10.3109/1547691X.2013.839587. [DOI] [PubMed] [Google Scholar]
- Sharman AC, Hay-Schmidt A, Holland PW. Cloning and analysis of an HMG gene from the lamprey Lampetra fluviatilis: gene duplication in vertebrate evolution. Gene. 1997;184(1):99–105. doi: 10.1016/s0378-1119(96)00580-x. [DOI] [PubMed] [Google Scholar]
- Sheflin LG, Fucile NW, Spaulding SW. The specific interactions of HMG 1 and 2 with negatively supercoiled DNA are modulated by their acidic C-terminal domains and involve cysteine residues in their HMG 1/2 boxes. Biochemistry. 1993;32(13):3238–3248. doi: 10.1021/bi00064a005. [DOI] [PubMed] [Google Scholar]
- Shen M, Lu J, Cheng P, Lin C, Dai W, Wang F, Wang C, Zhang Y, Chen K, Xu L, Zhou Y, Guo C. Ethyl pyruvate pretreatment attenuates concanavalin a-induced autoimmune hepatitis in mice. PLoS ONE. 2014;9(2):e87977. doi: 10.1371/journal.pone.0087977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Lu J, Dai W, Wang F, Xu L, Chen K, He L, Cheng P, Zhang Y, Wang C, Wu D, Yang J, Zhu R, Zhang H, Zhou Y, Guo C. Ethyl pyruvate ameliorates hepatic ischemia-reperfusion injury by inhibiting intrinsic pathway of apoptosis and autophagy. Mediators Inflamm. 2013;2013:461–536. doi: 10.1155/2013/461536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Hong L, Sun H, Shi M, Song Y. The expression of high-mobility group protein box 1 correlates with the progression of non-small cell lung cancer. Oncol Rep. 2009a;22(3):535–539. doi: 10.3892/or_00000468. [DOI] [PubMed] [Google Scholar]
- Shen Y, Peng H, Deng J, Wen Y, Luo X, Pan S, Wu C, Feng M. High mobility group box 1 protein enhances polyethylenimine mediated gene delivery in vitro. Int J Pharm. 2009b;375(1–2):140–147. doi: 10.1016/j.ijpharm.2009.03.040. [DOI] [PubMed] [Google Scholar]
- Shen Y, Peng H, Pan S, Feng M, Wen Y, Deng J, Luo X, Wu C. Interaction of DNA/nuclear protein/polycation and the terplexes for gene delivery. Nanotechnology. 2010;21(4):045102. doi: 10.1088/0957-4484/21/4/045102. [DOI] [PubMed] [Google Scholar]
- Sheng X, Du X, Zhang X, Li D, Lu C, Li Q, Ma Z, Song Q, Wang C. Clinical value of serum HMGB1 levels in early detection of recurrent squamous cell carcinoma of uterine cervix: comparison with serum SCCA, CYFRA21-1, and CEA levels. Croatian medical journal. 2009;50(5):455–464. doi: 10.3325/cmj.2009.50.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AV, Thirugnanam S, Dakshinamoorthy G, Samykutty A, Zheng G, Chen A, Bosland MC, Kajdacsy-Balla A, Gnanasekar M. 18alpha-glycyrrhetinic acid targets prostate cancer cells by down-regulating inflammation-related genes. Int J Oncol. 2011;39(3):635–640. doi: 10.3892/ijo.2011.1061. [DOI] [PubMed] [Google Scholar]
- Shi Y, Sandoghchian Shotorbani S, Su Z, Liu Y, Tong J, Zheng D, Chen J, Liu Y, Xu Y, Jiao Z, Wang S, Lu L, Huang X, Xu H. Enhanced HMGB1 expression may contribute to Th17 cells activation in rheumatoid arthritis. Clinical & developmental immunology. 2012a;2012:295081. doi: 10.1155/2012/295081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Sandoghchian Shotorbani S, Su Z, Liu Y, Tong J, Zheng D, Chen J, Xu Y, Jiao Z, Wang S, Lu L, Huang X, Xu H. Enhanced HMGB1 expression may contribute to Th17 cells activation in rheumatoid arthritis. Clin Dev Immunol. 2012b;2012:295081. doi: 10.1155/2012/295081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki M, Sasaki M, Miura M, Mizukoshi K, Ueno H, Hashimoto S, Tanaka Y, Amaya F. Induction of high mobility group box-1 in dorsal root ganglion contributes to pain hypersensitivity after peripheral nerve injury. Pain. 2010;149(3):514–521. doi: 10.1016/j.pain.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Shibusawa K, Murakami T, Yomogida S, Tamura H, Nagaoka I. Antimicrobial cathelicidin peptide CAP11 suppresses HMGB1 release from lipopolysaccharide-stimulated mononuclear phagocytes via the prevention of necrotic cell death. Int J Mol Med. 2009;23(3):341–346. doi: 10.3892/ijmm_00000137. [DOI] [PubMed] [Google Scholar]
- Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, Sekiya T, Ooboshi H, Kitazono T, Yanagawa T, Ishii T, Takahashi H, Mori S, Nishibori M, Kuroda K, Akira S, Miyake K, Yoshimura A. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med. 2012;18(6):911–917. doi: 10.1038/nm.2749. [DOI] [PubMed] [Google Scholar]
- Shim EJ, Chun E, Lee HS, Bang BR, Kim TW, Cho SH, Min KU, Park HW. The role of high-mobility group box-1 (HMGB1) in the pathogenesis of asthma. Clin Exp Allergy. 2012;42(6):958–965. doi: 10.1111/j.1365-2222.2012.03998.x. [DOI] [PubMed] [Google Scholar]
- Shin JH, Lee HK, Lee HB, Jin Y, Lee JK. Ethyl pyruvate inhibits HMGB1 phosphorylation and secretion in activated microglia and in the postischemic brain. Neurosci Lett. 2014;558:159–163. doi: 10.1016/j.neulet.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Shingu C, Hagiwara S, Iwasaka H, Matsumoto S, Koga H, Yokoi I, Noguchi T. EPCK1, a vitamin C and E analogue, reduces endotoxin-induced systemic inflammation in mice. J Surg Res. 2011;171(2):719–725. doi: 10.1016/j.jss.2010.03.040. [DOI] [PubMed] [Google Scholar]
- Shiota M, Izumi H, Miyamoto N, Onitsuka T, Kashiwagi E, Kidani A, Hirano G, Takahashi M, Ono M, Kuwano M, Naito S, Sasaguri Y, Kohno K. Ets regulates peroxiredoxin1 and 5 expressions through their interaction with the high-mobility group protein B1. Cancer Sci. 2008;99(10):1950–1959. doi: 10.1111/j.1349-7006.2008.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa H, Landsman D, Postnikov YV, Bustin M. NBP-45, a novel nucleosomal binding protein with a tissue-specific and developmentally regulated expression. J Biol Chem. 2000;275(9):6368–6374. doi: 10.1074/jbc.275.9.6368. [DOI] [PubMed] [Google Scholar]
- Shrivastava P, Bhatia M. Essential role of monocytes and macrophages in the progression of acute pancreatitis. World J Gastroenterol. 2010;16(32):3995–4002. doi: 10.3748/wjg.v16.i32.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Sikdar N, Banerjee S, Zhang H, Smith S, Myung K. Spt2p defines a new transcription-dependent gross chromosomal rearrangement pathway. PLoS Genet. 2008;4(12):e1000290. doi: 10.1371/journal.pgen.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Arcaroli J, He Q, Svetkauskaite D, Coldren C, Nick JA, Poch K, Park JS, Banerjee A, Abraham E. HMGB1 and LPS induce distinct patterns of gene expression and activation in neutrophils from patients with sepsis-induced acute lung injury. Intensive Care Med. 2007;33(10):1829–1839. doi: 10.1007/s00134-007-0748-2. [DOI] [PubMed] [Google Scholar]
- Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Singh J, Dixon GH. High mobility group proteins 1 and 2 function as general class II transcription factors. Biochemistry. 1990;29(26):6295–6302. doi: 10.1021/bi00478a026. [DOI] [PubMed] [Google Scholar]
- Singh M, D'Silva L, Holak TA. DNA-binding properties of the recombinant high-mobility-group-like AT-hook-containing region from human BRG1 protein. Biol Chem. 2006;387(10–11):1469–1478. doi: 10.1515/BC.2006.184. [DOI] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu YS, Li L, Leung MF, Lee KL, Li P. Polyethylenimine-based amphiphilic core-shell nanoparticles: study of gene delivery and intracellular trafficking. Biointerphases. 2012;7(1–4):16. doi: 10.1007/s13758-011-0016-4. [DOI] [PubMed] [Google Scholar]
- Skrha J, Jr, Kalousova M, Svarcova J, Muravska A, Kvasnicka J, Landova L, Zima T, Skrha J. Relationship of soluble RAGE and RAGE ligands HMGB1 and EN-RAGE to endothelial dysfunction in type 1 and type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2012;120(5):277–281. doi: 10.1055/s-0031-1283161. [DOI] [PubMed] [Google Scholar]
- Sloots A, Wels WS. Recombinant derivatives of the human high-mobility group protein HMGB2 mediate efficient nonviral gene delivery. Febs J. 2005;272(16):4221–4236. doi: 10.1111/j.1742-4658.2005.04834.x. [DOI] [PubMed] [Google Scholar]
- Smerdon MJ, Isenberg I. Interactions between the subfractons of calf thymus H1 and nonhistone chromosomal proteins HMG1 and HMG2. Biochemistry. 1976;15(19):4242–4247. doi: 10.1021/bi00664a017. [DOI] [PubMed] [Google Scholar]
- Soldevilla B, Diaz R, Silva J, Campos-Martin Y, Munoz C, Garcia V, Garcia JM, Pena C, Herrera M, Rodriguez M, Gomez I, Mohamed N, Marques MM, Bonilla F, Dominguez G. Prognostic impact of DeltaTAp73 isoform levels and their target genes in colon cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(18):6029–6039. doi: 10.1158/1078-0432.CCR-10-2388. [DOI] [PubMed] [Google Scholar]
- Somervaille TC, Matheny CJ, Spencer GJ, Iwasaki M, Rinn JL, Witten DM, Chang HY, Shurtleff SA, Downing JR, Cleary ML. Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell. 2009;4(2):129–140. doi: 10.1016/j.stem.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JX, Lu JH, Liu LF, Chen LL, Durairajan SS, Yue Z, Zhang HQ, Li M. HMGB1 is involved in autophagy inhibition caused by SNCA/alpha-synuclein overexpression: a process modulated by the natural autophagy inducer corynoxine B. Autophagy. 2014;10(1):144–154. doi: 10.4161/auto.26751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MJ, Hwang S, Wong W, Round J, Martinez-Guzman D, Turpaz Y, Liang J, Wong B, Johnson RC, Carey M, Sun R. The DNA architectural protein HMGB1 facilitates RTAmediated viral gene expression in gamma-2 herpesviruses. J Virol. 2004;78(23):12940–12950. doi: 10.1128/JVI.78.23.12940-12950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N, Liu B, Wu JL, Zhang RF, Duan L, He WS, Zhang CM. Prognostic value of HMGB3 expression in patients with non-small cell lung cancer. Tumour Biol. 2013;34(5):2599–2603. doi: 10.1007/s13277-013-0807-y. [DOI] [PubMed] [Google Scholar]
- Sorci G, Riuzzi F, Arcuri C, Giambanco I, Donato R. Amphoterin stimulates myogenesis and counteracts the antimyogenic factors basic fibroblast growth factor and S100B via RAGE binding. Mol Cell Biol. 2004;24(11):4880–4894. doi: 10.1128/MCB.24.11.4880-4894.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreide K. The role of high-mobility group box-1 (HMGB-1) in the management of suspected acute appendicitis: useful diagnostic biomarker or just another blind alley? Scandinavian journal of trauma, resuscitation and emergency medicine. 2011;19:28. doi: 10.1186/1757-7241-19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen MV, Pedersen S, Mogelvang R, Skov-Jensen J, Flyvbjerg A. Plasma high-mobility group box 1 levels predict mortality after ST-segment elevation myocardial infarction. JACC. Cardiovascular interventions. 2011a;4(3):281–286. doi: 10.1016/j.jcin.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Sorensen MV, Pedersen S, Mogelvang R, Skov-Jensen J, Flyvbjerg A. Plasma high-mobility group box 1 levels predict mortality after ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2011b;4(3):281–286. doi: 10.1016/j.jcin.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, Thomas M, Forbes JM, Nawroth PP, Bierhaus A, Cooper ME, Jandeleit-Dahm KA. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57(9):2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparatore B, Melloni E, Patrone M, Passalacqua M, Pontremoli S. A 6 kDa protein homologous to the N-terminus of the HMG1 protein promoting stimulation of murine erythroleukemia cell differentiation. FEBS Lett. 1996a;386(2–3):95–98. doi: 10.1016/0014-5793(96)00418-8. [DOI] [PubMed] [Google Scholar]
- Sparatore B, Passalacqua M, Patrone M, Melloni E, Pontremoli S. Extracellular high-mobility group 1 protein is essential for murine erythroleukaemia cell differentiation. Biochem J. 1996b;320(Pt 1):253–256. doi: 10.1042/bj3200253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparatore B, Passalacqua M, Patrone M, Pessino A, Melloni E, Pontremoli S. Differentiation of HL60 promyelocytic cells is promoted by a 'differentiation enhancing factor' produced by erythroleukemia cells. FEBS Lett. 1993a;334(2):198–202. doi: 10.1016/0014-5793(93)81711-8. [DOI] [PubMed] [Google Scholar]
- Sparatore B, Patrone M, Passalacqua M, Pedrazzi M, Gaggero D, Pontremoli S, Melloni E. Extracellular processing of amphoterin generates a peptide active on erythroleukaemia cell differentiation. Biochem J. 2001;357(Pt 2):569–574. doi: 10.1042/0264-6021:3570569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparatore B, Patrone M, Salamino F, Passalacqua M, Melloni E, Pontremoli S. A vincristine-resistant murine erythroleukemia cell line secretes a differentiation enhancing factor. Biochem Biophys Res Commun. 1990;173(1):156–163. doi: 10.1016/s0006-291x(05)81035-6. [DOI] [PubMed] [Google Scholar]
- Sparatore B, Pedrazzi M, Passalacqua M, Gaggero D, Patrone M, Pontremoli S, Melloni E. Stimulation of erythroleukaemia cell differentiation by extracellular high-mobility group-box protein 1 is independent of the receptor for advanced glycation end-products. Biochem J. 2002;363(Pt 3):529–535. doi: 10.1042/0264-6021:3630529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparatore B, Pessino A, Patrone M, Passalacqua M, Melloni E, Pontremoli S. Role of delta-PKC on the differentiation process of murine erythroleukemia cells. Biochem Biophys Res Commun. 1993b;193(1):220–227. doi: 10.1006/bbrc.1993.1612. [DOI] [PubMed] [Google Scholar]
- Spencer JA, Baron MH, Olson EN. Cooperative transcriptional activation by serum response factor and the high mobility group protein SSRP1. J Biol Chem. 1999;274(22):15686–15693. doi: 10.1074/jbc.274.22.15686. [DOI] [PubMed] [Google Scholar]
- Spiker S. High-mobility group chromosomal proteins of wheat. J Biol Chem. 1984;259(19):12007–12013. [PubMed] [Google Scholar]
- Spiker S, Mardian JK, Isenberg I. Chomosomal HMG proteins occur in three eukaryotic kingdoms. Biochem Biophys Res Commun. 1978;82(1):129–135. doi: 10.1016/0006-291x(78)90586-7. [DOI] [PubMed] [Google Scholar]
- Splichalova A, Splichal I, Chmelarova P, Trebichavsky I. Alarmin HMGB1 is released in the small intestine of gnotobiotic piglets infected with enteric pathogens and its level in plasma reflects severity of sepsis. Journal of clinical immunology. 2011;31(3):488–497. doi: 10.1007/s10875-010-9505-3. [DOI] [PubMed] [Google Scholar]
- Srikrishna G, Huttunen HJ, Johansson L, Weigle B, Yamaguchi Y, Rauvala H, Freeze HH. N -Glycans on the receptor for advanced glycation end products influence amphoterin binding and neurite outgrowth. J Neurochem. 2002;80(6):998–1008. doi: 10.1046/j.0022-3042.2002.00796.x. [DOI] [PubMed] [Google Scholar]
- Steer SA, Scarim AL, Chambers KT, Corbett JA. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med. 2006;3(2):e17. doi: 10.1371/journal.pmed.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein GS, Spelsberg TC, Kleinsmith LJ. Nonhistone chromosomal proteins and gene regulation. Science. 1974;183(4127):817–824. doi: 10.1126/science.183.4127.817. [DOI] [PubMed] [Google Scholar]
- Steinberg BE, Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci STKE. 2007;2007(379):e11. doi: 10.1126/stke.3792007pe11. [DOI] [PubMed] [Google Scholar]
- Stemmer C, Leeming DJ, Franssen L, Grimm R, Grasser KD. Phosphorylation of maize and Arabidopsis HMGB proteins by protein kinase CK2alpha. Biochemistry. 2003;42(12):3503–3508. doi: 10.1021/bi027350d. [DOI] [PubMed] [Google Scholar]
- Stemmer C, Schwander A, Bauw G, Fojan P, Grasser KD. Protein kinase CK2 differentially phosphorylates maize chromosomal high mobility group B (HMGB) proteins modulating their stability and DNA interactions. J Biol Chem. 2002;277(2):1092–1098. doi: 10.1074/jbc.M109503200. [DOI] [PubMed] [Google Scholar]
- Sterner R, Vidali G, Allfrey VG. Studies of acetylation and deacetylation in high mobility group proteins. Identification of the sites of acetylation in HMG-1. J Biol Chem. 1979;254(22):11577–11583. [PubMed] [Google Scholar]
- Sterner R, Vidali G, Heinrikson RL, Allfrey VG. Postsynthetic modification of high mobility group proteins. Evidence that high mobility group proteins are acetylated. J Biol Chem. 1978;253(21):7601–7604. [PubMed] [Google Scholar]
- Stoetzer OJ, Fersching DM, Salat C, Steinkohl O, Gabka CJ, Hamann U, Braun M, Feller AM, Heinemann V, Siegele B, Nagel D, Holdenrieder S. Circulating immunogenic cell death biomarkers HMGB1 and RAGE in breast cancer patients during neoadjuvant chemotherapy. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34(1):81–90. doi: 10.1007/s13277-012-0513-1. [DOI] [PubMed] [Google Scholar]
- Stott K, Tang GS, Lee KB, Thomas JO. Structure of a complex of tandem HMG boxes and DNA. J Mol Biol. 2006;360(1):90–104. doi: 10.1016/j.jmb.2006.04.059. [DOI] [PubMed] [Google Scholar]
- Stros M. Binding of non-histone chromosomal protein HMG1 to histone H3 in nucleosomes detected by photochemical cross-linking. Biochem Biophys Res Commun. 1987;147(1):301–308. doi: 10.1016/s0006-291x(87)80121-3. [DOI] [PubMed] [Google Scholar]
- Stros M. DNA bending by the chromosomal protein HMG1 and its high mobility group box domains. Effect of flanking sequences. J Biol Chem. 1998;273(17):10355–10361. [PubMed] [Google Scholar]
- Stros M. Two mutations of basic residues within the N-terminus of HMG-1 B domain with different effects on DNA supercoiling and binding to bent DNA. Biochemistry. 2001;40(15):4769–4779. doi: 10.1021/bi002741i. [DOI] [PubMed] [Google Scholar]
- Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta. 2010;1799(1–2):101–113. doi: 10.1016/j.bbagrm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Stros M, Bacikova A, Polanska E, Stokrova J, Strauss F. HMGB1 interacts with human topoisomerase IIalpha and stimulates its catalytic activity. Nucleic Acids Res. 2007a;35(15):5001–5013. doi: 10.1093/nar/gkm525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stros M, Bernues J, Querol E. Calcium modulates the binding of high-mobility-group protein 1 to DNA. Biochem Int. 1990;21(5):891–899. [PubMed] [Google Scholar]
- Stros M, Dixon GH. A retropseudogene for non-histone chromosomal protein HMG-1. Biochim Biophys Acta. 1993;1172(1–2):231–235. doi: 10.1016/0167-4781(93)90303-u. [DOI] [PubMed] [Google Scholar]
- Stros M, Kolibalova A. Interaction of non-histone proteins HMG1 and HMG2 with core histones in nucleosomes and core particles revealed by chemical cross-linking. Eur J Biochem. 1987;162(1):111–118. doi: 10.1111/j.1432-1033.1987.tb10549.x. [DOI] [PubMed] [Google Scholar]
- Stros M, Launholt D, Grasser KD. The HMG-box: a versatile protein domain occurring in a wide variety of DNA-binding proteins. Cell Mol Life Sci. 2007b;64(19–20):2590–2606. doi: 10.1007/s00018-007-7162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stros M, Muselikova-Polanska E, Pospisilova S, Strauss F. High-affinity binding of tumor-suppressor protein p53 and HMGB1 to hemicatenated DNA loops. Biochemistry. 2004;43(22):7215–7225. doi: 10.1021/bi049928k. [DOI] [PubMed] [Google Scholar]
- Stros M, Muselikova E. A role of basic residues and the putative intercalating phenylalanine of the HMG-1 box B in DNA supercoiling and binding to four-way DNA junctions. J Biol Chem. 2000;275(46):35699–35707. doi: 10.1074/jbc.M007167200. [DOI] [PubMed] [Google Scholar]
- Stros M, Nishikawa S, Dixon GH. cDNA sequence and structure of a gene encoding trout testis high-mobility-group-1 protein. Eur J Biochem. 1994a;225(2):581–591. doi: 10.1111/j.1432-1033.1994.00581.x. [DOI] [PubMed] [Google Scholar]
- Stros M, Ozaki T, Bacikova A, Kageyama H, Nakagawara A. HMGB1 and HMGB2 cell-specifically down-regulate the p53- and p73-dependent sequence-specific transactivation from the human Bax gene promoter. J Biol Chem. 2002;277(9):7157–7164. doi: 10.1074/jbc.M110233200. [DOI] [PubMed] [Google Scholar]
- Stros M, Polanska E, Struncova S, Pospisilova S. HMGB1 and HMGB2 proteins upregulate cellular expression of human topoisomerase IIalpha. Nucleic Acids Res. 2009;37(7):2070–2086. doi: 10.1093/nar/gkp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stros M, Reich J, Kolibalova A. Calcium binding to HMG1 protein induces DNA looping by the HMG-box domains. FEBS Lett. 1994b;344(2–3):201–206. doi: 10.1016/0014-5793(94)00364-5. [DOI] [PubMed] [Google Scholar]
- Stros M, Stokrova J, Thomas JO. DNA looping by the HMG-box domains of HMG1 and modulation of DNA binding by the acidic C-terminal domain. Nucleic Acids Res. 1994c;22(6):1044–1051. doi: 10.1093/nar/22.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stros M, Vorlickova M. Non-histone chromosomal protein HMG1 reduces the histone H5-induced changes in c.d. spectra of DNA: the acidic C-terminus of HMG1 is necessary for binding to H5. Int J Biol Macromol. 1990;12(5):282–288. doi: 10.1016/0141-8130(90)90014-2. [DOI] [PubMed] [Google Scholar]
- Stumbo AC, Cortez E, Rodrigues CA, Henriques M, Porto LC, Barbosa HS, Carvalho L. Mitochondrial localization of non-histone protein HMGB1 during human endothelial cell-Toxoplasma gondii infection. Cell Biol Int. 2008;32(2):235–238. doi: 10.1016/j.cellbi.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Su FF, Shi MQ, Guo WG, Liu XT, Wang HT, Lu ZF, Zheng QS. High-mobility group box 1 induces calcineurin-mediated cell hypertrophy in neonatal rat ventricular myocytes. Mediators Inflamm. 2012;2012:805149. doi: 10.1155/2012/805149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Sun C, Zhou C, Liu Y, Zhu H, Sandoghchian S, Zheng D, Peng T, Zhang Y, Jiao Z, Wang S, Xu H. HMGB1 blockade attenuates experimental autoimmune myocarditis and suppresses Th17-cell expansion. Eur J Immunol. 2011;41(12):3586–3595. doi: 10.1002/eji.201141879. [DOI] [PubMed] [Google Scholar]
- Suda K, Kitagawa Y, Ozawa S, Miyasho T, Okamoto M, Saikawa Y, Ueda M, Yamada S, Tasaka S, Funakoshi Y, Hashimoto S, Yokota H, Maruyama I, Ishizaka A, Kitajima M. Neutrophil elastase inhibitor improves postoperative clinical courses after thoracic esophagectomy. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / I.S.D.E. 2007;20(6):478–486. doi: 10.1111/j.1442-2050.2007.00699.x. [DOI] [PubMed] [Google Scholar]
- Suda K, Kitagawa Y, Ozawa S, Saikawa Y, Ueda M, Abraham E, Kitajima M, Ishizaka A. Serum concentrations of high-mobility group box chromosomal protein 1 before and after exposure to the surgical stress of thoracic esophagectomy: a predictor of clinical course after surgery? Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / I.S.D.E. 2006;19(1):5–9. doi: 10.1111/j.1442-2050.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- Sugita H, Yamaguchi Y, Ikei S, Yamada S, Ogawa M. Enhanced expression of cytokine-induced neutrophil chemoattractant (CINC) by bronchoalveolar macrophages in cerulein-induced pancreatitis rats. Dig Dis Sci. 1997;42(1):154–160. doi: 10.1023/a:1018809810561. [DOI] [PubMed] [Google Scholar]
- Sukkar MB, Wood LG, Tooze M, Simpson JL, McDonald VM, Gibson PG, Wark PA. Soluble RAGE is deficient in neutrophilic asthma and COPD. The European respiratory journal. 2012;39(3):721–729. doi: 10.1183/09031936.00022011. [DOI] [PubMed] [Google Scholar]
- Sundberg E, Fasth AE, Palmblad K, Harris HE, Andersson U. High mobility group box chromosomal protein 1 acts as a proliferation signal for activated T lymphocytes. Immunobiology. 2009;214(4):303–309. doi: 10.1016/j.imbio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Sundberg E, Grundtman C, Af Klint E, Lindberg J, Ernestam S, Ulfgren AK, Harris HE, Andersson U. Systemic TNF blockade does not modulate synovial expression of the proinflammatory mediator HMGB1 in rheumatoid arthritis patients--a prospective clinical study. Arthritis research & therapy. 2008;10(2):R33. doi: 10.1186/ar2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Critical care medicine. 2005;33(3):564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397(6718):441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Sutrias-Grau M, Bianchi ME, Bernues J. High mobility group protein 1 interacts specifically with the core domain of human TATA box-binding protein and interferes with transcription factor IIB within the pre-initiation complex. J Biol Chem. 1999;274(3):1628–1634. doi: 10.1074/jbc.274.3.1628. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T, Kono K. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer research. 2012;72(16):3967–3976. doi: 10.1158/0008-5472.CAN-12-0851. [DOI] [PubMed] [Google Scholar]
- Swanson PC. Fine structure and activity of discrete RAG-HMG complexes on V(D)J recombination signals. Mol Cell Biol. 2002a;22(5):1340–1351. doi: 10.1128/mcb.22.5.1340-1351.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson PC. A RAG-1/RAG-2 tetramer supports 12/23-regulated synapsis, cleavage, and transposition of V(D)J recombination signals. Mol Cell Biol. 2002b;22(22):7790–7801. doi: 10.1128/MCB.22.22.7790-7801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata C, Kanemura S, Tabata R, Masachika E, Shibata E, Otsuki T, Nishizaki T, Nakano T. Serum HMGB1 as a diagnostic marker for malignant peritoneal mesothelioma. Journal of clinical gastroenterology. 2013a;47(8):684–688. doi: 10.1097/MCG.0b013e318297fa65. [DOI] [PubMed] [Google Scholar]
- Tabata C, Shibata E, Tabata R, Kanemura S, Mikami K, Nogi Y, Masachika E, Nishizaki T, Nakano T. Serum HMGB1 as a prognostic marker for malignant pleural mesothelioma. BMC cancer. 2013b;13:205. doi: 10.1186/1471-2407-13-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadie JM, Bae HB, Banerjee S, Zmijewski JW, Abraham E. Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing. Am J Physiol Cell Physiol. 2012a;302(1):C249–C256. doi: 10.1152/ajpcell.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadie JM, Bae HB, Deshane JS, Bell CP, Lazarowski ER, Chaplin DD, Thannickal VJ, Abraham E, Zmijewski JW. Toll-like receptor 4 engagement inhibits adenosine 5'-monophosphate-activated protein kinase activation through a high mobility group box 1 protein-dependent mechanism. Mol Med. 2012b;18:659–668. doi: 10.2119/molmed.2011.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadie JM, Bae HB, Jiang S, Park DW, Bell CP, Yang H, Pittet JF, Tracey K, Thannickal VJ, Abraham E, Zmijewski JW. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am J Physiol Lung Cell Mol Physiol. 2013;304(5):L342–L349. doi: 10.1152/ajplung.00151.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafani M, Schito L, Pellegrini L, Villanova L, Marfe G, Anwar T, Rosa R, Indelicato M, Fini M, Pucci B, Russo MA. Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa}B. Carcinogenesis. 2011;32(8):1167–1175. doi: 10.1093/carcin/bgr101. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405(6784):354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- Taira T, Matsuyama W, Mitsuyama H, Kawahara KI, Higashimoto I, Maruyama I, Osame M, Arimura K. Increased serum high mobility group box-1 level in Churg-Strauss syndrome. Clin Exp Immunol. 2007;148(2):241–247. doi: 10.1111/j.1365-2249.2007.03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Takahara PM, Rosenzweig AC, Frederick CA, Lippard SJ. Crystal structure of double-stranded DNA containing the major adduct of the anticancer drug cisplatin. Nature. 1995;377(6550):649–652. doi: 10.1038/377649a0. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Sadamori H, Teshigawara K, Niwa A, Liu K, Wake H, Mori S, Yoshino T, Nishibori M. Histamine inhibits high mobility group box 1-induced adhesion molecule expression on human monocytes. Eur J Pharmacol. 2013a;718(1–3):305–313. doi: 10.1016/j.ejphar.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Fukushima S, Yamahara K, Yashiro K, Shintani Y, Coppen SR, Salem HK, Brouilette SW, Yacoub MH, Suzuki K. Modulated inflammation by injection of high-mobility group box 1 recovers post-infarction chronically failing heart. Circulation. 2008;118(14 Suppl):S106–S114. doi: 10.1161/CIRCULATIONAHA.107.757443. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Katsuta S, Tamura Y, Nagase N, Suzuki K, Nomura M, Tomatsu S, Miyamoto K, Kobayashi S. Bone-targeting endogenous secretory receptor for advanced glycation end products rescues rheumatoid arthritis. Mol Med. 2013b;19:183–194. doi: 10.2119/molmed.2012.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Hori T, Cooper TK, Liao J, Desai N, Serfass JM, Young MM, Park S, Izu Y, Wang HG. Bif-1 haploinsufficiency promotes chromosomal instability and accelerates Myc-driven lymphomagenesis via suppression of mitophagy. Blood. 2013c doi: 10.1182/blood-2012-10-459826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata R, Ono S, Tsujimoto H, Hiraki S, Kimura A, Kinoshita M, Miyazaki H, Saitoh D, Hase K. Postoperative serum concentrations of high mobility group box chromosomal protein-1 correlates to the duration of SIRS and pulmonary dysfunction following gastrointestinal surgery. The Journal of surgical research. 2011;170(1):e135–e140. doi: 10.1016/j.jss.2011.04.040. [DOI] [PubMed] [Google Scholar]
- Takaishi H, Kanai T, Nakazawa A, Sugata F, Nikai A, Yoshizawa S, Hamamoto Y, Funakoshi S, Yajima T, Iwao Y, Takemura M, Ozaki S, Hibi T. Anti-high mobility group box 1 and box 2 non-histone chromosomal proteins (HMGB1/HMGB2) antibodies and anti-Saccharomyces cerevisiae antibodies (ASCA): accuracy in differentially diagnosing UC and CD and correlation with inflammatory bowel disease phenotype. J Gastroenterol. 2012;47(9):969–977. doi: 10.1007/s00535-012-0566-3. [DOI] [PubMed] [Google Scholar]
- Takamiya R, Hung CC, Hall SR, Fukunaga K, Nagaishi T, Maeno T, Owen C, Macias AA, Fredenburgh LE, Ishizaka A, Blumberg RS, Baron RM, Perrella MA. High-mobility group box 1 contributes to lethality of endotoxemia in heme oxygenase-1-deficient mice. Am J Respir Cell Mol Biol. 2009;41(2):129–135. doi: 10.1165/rcmb.2008-0331OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata K, Kitamura Y, Kakimura J, Shibagaki K, Tsuchiya D, Taniguchi T, Smith MA, Perry G, Shimohama S. Role of high mobility group protein-1 (HMG1) in amyloid-beta homeostasis. Biochem Biophys Res Commun. 2003;301(3):699–703. doi: 10.1016/s0006-291x(03)00024-x. [DOI] [PubMed] [Google Scholar]
- Takata K, Takada T, Ito A, Asai M, Tawa M, Saito Y, Ashihara E, Tomimoto H, Kitamura Y, Shimohama S. Microglial Amyloid-beta1–40 Phagocytosis Dysfunction Is Caused by High-Mobility Group Box Protein-1: Implications for the Pathological Progression of Alzheimer's Disease. International journal of Alzheimer's disease. 2012;2012:685–739. doi: 10.1155/2012/685739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi K, Mishima Y, Kominami R. Difference in HMG1-induced DNA bending among microsatellites. DNA Res. 1997;4(3):241–247. doi: 10.1093/dnares/4.3.241. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Yamamoto Y, Tsuneyama K, Cheng C, Yonekura H, Watanabe T, Shimizu K, Tomita K, Yamamoto H, Tsuchiya H. Endogenous secretory receptor for advanced glycation endproducts as a novel prognostic marker in chondrosarcoma. Cancer. 2007;109(12):2532–2540. doi: 10.1002/cncr.22731. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Sakazume K, Tonooka A, Zaitsu M, Takeshima Y, Mikami K, Uekusa T. Cytosolic HMGB1 expression in human renal clear cell cancer indicates higher pathological T classifications and tumor grades. Urology journal. 2013;10(3):960–965. [PubMed] [Google Scholar]
- Tamai K, Yamazaki T, Chino T, Ishii M, Otsuru S, Kikuchi Y, Iinuma S, Saga K, Nimura K, Shimbo T, Umegaki N, Katayama I, Miyazaki J, Takeda J, McGrath JA, Uitto J, Kaneda Y. PDGFRalpha-positive cells in bone marrow are mobilized by high mobility group box 1 (HMGB1) to regenerate injured epithelia. Proc Natl Acad Sci U S A. 2011;108(16):6609–6614. doi: 10.1073/pnas.1016753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Chiba Y, Tanioka T, Shimizu N, Shinozaki S, Yamada M, Kaneki K, Mori S, Araki A, Ito H, Kaneki M. NO donor induces Nec-1-inhibitable, but RIP1-independent, necrotic cell death in pancreatic beta-cells. FEBS Lett. 2011;585(19):3058–3064. doi: 10.1016/j.febslet.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Yamaguchi K, Ishikura H, Tsubota M, Sekiguchi F, Seki Y, Tsujiuchi T, Murai A, Umemura T, Kawabata A. Bladder pain relief by HMGB1 neutralization and soluble thrombomodulin in mice with cyclophosphamide-induced cystitis. Neuropharmacology. 2014;79:112–118. doi: 10.1016/j.neuropharm.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Fujita M, Tsuruta R, Fujimoto K, Aki HS, Kumagai K, Aoki T, Kobayashi A, Izumi T, Kasaoka S, Yuasa M, Maekawa T. Urinary trypsin inhibitor suppresses excessive generation of superoxide anion radical, systemic inflammation, oxidative stress, and endothelial injury in endotoxemic rats. Inflamm Res. 2010;59(8):597–606. doi: 10.1007/s00011-010-0166-8. [DOI] [PubMed] [Google Scholar]
- Tang CW, Feng WM, Du HM, Bao Y, Zhu M. Delayed administration of D-Ala2-D-Leu5-enkephalin, a delta-opioid receptor agonist, improves survival in a rat model of sepsis. Tohoku J Exp Med. 2011a;224(1):69–76. doi: 10.1620/tjem.224.69. [DOI] [PubMed] [Google Scholar]
- Tang D, Billiar TA, Lotze MT. A Janus Tale of Two Active HMGB1 Redox States. Mol Med. 2012;18:1360–1362. doi: 10.2119/molmed.2012.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Cao L, Zhang G, Yu Y, Xiao W, Wang H, Xiao X. A pilot study to detect high mobility group box 1 and heat shock protein 72 in cerebrospinal fluid of pediatric patients with meningitis. Crit Care Med. 2008a;36(1):291–295. doi: 10.1097/01.CCM.0000295316.86942.CE. [DOI] [PubMed] [Google Scholar]
- Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, Zeh HJ, Lotze MT. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010a;29(38):5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ, 3rd, Lotze MT. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010b;190(5):881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ, 3rd, Lotze MT. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010c;190(5):881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Livesey KM, Kroemer G, Billiar TR, Van Houten B, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metabolism. 2011b;13(6):701–711. doi: 10.1016/j.cmet.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Livesey KM, Kroemer G, Billiar TR, Van Houten B, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011c;13(6):701–711. doi: 10.1016/j.cmet.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Livesey KM, Zeh HJ, 3rd, Lotze MT. High mobility group box 1 (HMGB1) activates an autophagic response to oxidative stress. Antioxid Redox Signal. 2011d;15(8):2185–2195. doi: 10.1089/ars.2010.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Van Houten B, Zeh HJ, Billiar TR, Lotze MT. HMGB1 Phenotypic Role Revealed with Stress. Mol Med. 2014 doi: 10.2119/molmed.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Xiao W, Jiang L, Liu M, Shi Y, Wang K, Wang H, Xiao X. Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release. J Immunol. 2007a;178(11):7376–7384. doi: 10.4049/jimmunol.178.11.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Xiao W, Jiang L, Liu M, Shi Y, Wang K, Wang H, Xiao X. Nuclear Heat Shock Protein 72 as a Negative Regulator of Oxidative Stress (Hydrogen Peroxide)-Induced HMGB1 Cytoplasmic Translocation and Release. J Immunol. 2007b;178(11):7376–7384. doi: 10.4049/jimmunol.178.11.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Xiao W, Wang H, Calderwood SK, Xiao X. The Anti-inflammatory Effects of Heat Shock Protein 72 Involve Inhibition of High-Mobility-Group Box 1 Release and Proinflammatory Function in Macrophages. J Immunol. 2007c;179(2):1236–1244. doi: 10.4049/jimmunol.179.2.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Xiao W, Wang H, Calderwood SK, Xiao X. The anti-inflammatory effects of heat shock protein 72 involve inhibition of high-mobility-group box 1 release and proinflammatory function in macrophages. J Immunol. 2007d;179(2):1236–1244. doi: 10.4049/jimmunol.179.2.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Xiao W, Zhang H, Lotze MT, Wang H, Xiao X. Quercetin prevents LPS-induced high-mobility group box 1 release and proinflammatory function. Am J Respir Cell Mol Biol. 2009;41(6):651–660. doi: 10.1165/rcmb.2008-0119OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010d;1799(1–2):131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011e;14(7):1315–1335. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Lotze MT. Tumor immunity times out: TIM-3 and HMGB1. Nat Immunol. 2012;13(9):808–810. doi: 10.1038/ni.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Loze MT, Zeh HJ, Kang R. The redox protein HMGB1 regulates cell death and survival in cancer treatment. Autophagy. 2010e;6(8):1181–1183. doi: 10.4161/auto.6.8.13367. [DOI] [PubMed] [Google Scholar]
- Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, Xiao X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol. 2007e;81(3):741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Wang YC, Li YI, Lin HC, Manzanero S, Hsieh YH, Phipps S, Hu CJ, Chiou HY, Huang YS, Yang WS, Mattson MP, Arumugam TV, Jeng JS. Functional role of soluble receptor for advanced glycation end products in stroke. Arterioscler Thromb Vasc Biol. 2013a;33(3):585–594. doi: 10.1161/ATVBAHA.112.300523. [DOI] [PubMed] [Google Scholar]
- Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, Ho SM. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology. 2008b;149(12):5922–5931. doi: 10.1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Shen X, Qian X, Gao X. Expressions of HMGB1, MMP-2 and MMP-9 and prognostic alue in human laryngeal carcinoma. Lin chuang er bi yan hou tou jing wai ke za zhi = Journal of clinical otorhinolaryngology, head, and neck surgery. 2013b;27(4):181–187. [PubMed] [Google Scholar]
- Tang Y, Lv B, Wang H, Xiao X, Zuo X. PACAP inhibit the release and cytokine activity of HMGB1 and improve the survival during lethal endotoxemia. Int Immunopharmacol. 2008c;8(12):1646–1651. doi: 10.1016/j.intimp.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Taniguchi N, Carames B, Hsu E, Cherqui S, Kawakami Y, Lotz M. Expression patterns and function of chromatin protein HMGB2 during mesenchymal stem cell differentiation. J Biol Chem. 2011;286(48):41489–41498. doi: 10.1074/jbc.M111.236984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N, Carames B, Kawakami Y, Amendt BA, Komiya S, Lotz M. Chromatin protein HMGB2 regulates articular cartilage surface maintenance via beta-catenin pathway. Proc Natl Acad Sci U S A. 2009a;106(39):16817–16822. doi: 10.1073/pnas.0904414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N, Carames B, Ronfani L, Ulmer U, Komiya S, Bianchi ME, Lotz M. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009b;106(4):1181–1186. doi: 10.1073/pnas.0806062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K, Matsunaga S, Nakajima T, Komiya S, Maruyama I. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003;48(4):971–981. doi: 10.1002/art.10859. [DOI] [PubMed] [Google Scholar]
- Tanuma S, Johnson GS. ADP-ribosylation of nonhistone high mobility group proteins in intact cells. J Biol Chem. 1983;258(7):4067–4070. [PubMed] [Google Scholar]
- Tanuma S, Kawashima K, Endo H. Comparison of ADP-ribosylation of chromosomal proteins between intact and broken cells. Biochem Biophys Res Commun. 1985a;127(3):896–902. doi: 10.1016/s0006-291x(85)80028-0. [DOI] [PubMed] [Google Scholar]
- Tanuma S, Kawashima K, Endo H. Acceptor proteins for (ADP-ribose)n in the HeLa S3 cell cycle. J Biochem. 1986;99(3):915–922. doi: 10.1093/oxfordjournals.jbchem.a135553. [DOI] [PubMed] [Google Scholar]
- Tanuma S, Yagi T, Johnson GS. Endogenous ADP ribosylation of high mobility group proteins 1 and 2 and histone H1 following DNA damage in intact cells. Arch Biochem Biophys. 1985b;237(1):38–42. doi: 10.1016/0003-9861(85)90251-6. [DOI] [PubMed] [Google Scholar]
- Tasaka S, Kobayashi S, Kamata H, Kimizuka Y, Fujiwara H, Funatsu Y, Mizoguchi K, Ishii M, Takeuchi T, Hasegawa N. Cytokine profiles of bronchoalveolar lavage fluid in patients with pneumocystis pneumonia. Microbiol Immunol. 2010;54(7):425–433. doi: 10.1111/j.1348-0421.2010.00229.x. [DOI] [PubMed] [Google Scholar]
- Teo SH, Grasser KD, Hardman CH, Broadhurst RW, Laue ED, Thomas JO. Two mutations in the HMG-box with very different structural consequences provide insights into the nature of binding to four-way junction DNA. Embo J. 1995a;14(15):3844–3853. doi: 10.1002/j.1460-2075.1995.tb00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo SH, Grasser KD, Thomas JO. Differences in the DNA-binding properties of the HMG-box domains of HMG1 and the sex-determining factor SRY. Eur J Biochem. 1995b;230(3):943–950. doi: 10.1111/j.1432-1033.1995.tb20640.x. [DOI] [PubMed] [Google Scholar]
- Terada K, Kitayama A, Kanamoto T, Ueno N, Furukawa T. Nucleosome regulator Xhmgb3 is required for cell proliferation of the eye and brain as a downstream target of Xenopus rax/Rx1. Dev Biol. 2006;291(2):398–412. doi: 10.1016/j.ydbio.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon JP, Jooste V, van Endert P, Ducreux M, Zitvogel L, Piard F, Kroemer G. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- Tessari MA, Gostissa M, Altamura S, Sgarra R, Rustighi A, Salvagno C, Caretti G, Imbriano C, Mantovani R, Del Sal G, Giancotti V, Manfioletti G. Transcriptional activation of the cyclin A gene by the architectural transcription factor HMGA2. Mol Cell Biol. 2003;23(24):9104–9116. doi: 10.1128/MCB.23.24.9104-9116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh HA. The role of HMGB1 in ischemia-reperfusion injury in the rat small intestine. J Surg Res. 2013;183(1):96–97. doi: 10.1016/j.jss.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Shih HH, Mendelson KG, Sheppard KA, Paulson KE, Yee AS. HBP1: a HMG box transcriptional repressor that is targeted by the retinoblastoma family. Genes Dev. 1997;11(3):383–396. doi: 10.1101/gad.11.3.383. [DOI] [PubMed] [Google Scholar]
- Thakur MK, Prasad S. ADP-ribosylation of HMG proteins and its modulation by different effectors in the liver of aging rats. Mech Ageing Dev. 1990;53(1):91–100. doi: 10.1016/0047-6374(90)90037-g. [DOI] [PubMed] [Google Scholar]
- Thakur MK, Prasad S. Analysis of age-associated alteration in the synthesis of HMG nonhistone proteins of the rat liver. Mol Biol Rep. 1991;15(1):19–24. doi: 10.1007/BF00369896. [DOI] [PubMed] [Google Scholar]
- Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425(6960):851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirugnanam S, Munirathinam G, Veerapathran A, Dakshinamoorthy G, Reddy MV, Ramaswamy K. Cloning and characterization of high mobility group box protein 1 (HMGB1) of Wuchereria bancrofti and Brugia malayi. Parasitol Res. 2012;111(2):619–627. doi: 10.1007/s00436-012-2878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JO. HMG1 and 2: architectural DNA-binding proteins. Biochem Soc Trans. 2001;29(Pt 4):395–401. doi: 10.1042/bst0290395. [DOI] [PubMed] [Google Scholar]
- Thomas JO, Stott K. H1 and HMGB1: modulators of chromatin structure. Biochem Soc Trans. 2012;40(2):341–346. doi: 10.1042/BST20120014. [DOI] [PubMed] [Google Scholar]
- Thomas JO, Travers AA. HMG1 and 2, and related 'architectural' DNA-binding proteins. Trends Biochem Sci. 2001;26(3):167–174. doi: 10.1016/s0968-0004(01)01801-1. [DOI] [PubMed] [Google Scholar]
- Thongsroy J, Matangkasombut O, Thongnak A, Rattanatanyong P, Jirawatnotai S, Mutirangura A. Replication-independent endogenous DNA double-strand breaks in Saccharomyces cerevisiae model. PLoS ONE. 2013;8(8):e72706. doi: 10.1371/journal.pone.0072706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2009;16(1):175–183. doi: 10.1038/cdd.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-beta employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174(2):175–183. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8(5):487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- Tiszlavicz Z, Nemeth B, Fulop F, Vecsei L, Tapai K, Ocsovszky I, Mandi Y. Different inhibitory effects of kynurenic acid and a novel kynurenic acid analogue on tumour necrosis factor-alpha (TNF-alpha) production by mononuclear cells, HMGB1 production by monocytes and HNP1–3 secretion by neutrophils. Naunyn Schmiedebergs Arch Pharmacol. 2011;383(5):447–455. doi: 10.1007/s00210-011-0605-2. [DOI] [PubMed] [Google Scholar]
- Tkachuk DC, Kohler S, Cleary ML. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992;71(4):691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- Tong HS, Tang YQ, Chen Y, Qiu JM, Wen Q, Su L. Early elevated HMGB1 level predicting the outcome in exertional heatstroke. The Journal of trauma. 2011;71(4):808–814. doi: 10.1097/TA.0b013e318220b957. [DOI] [PubMed] [Google Scholar]
- Tong W, Wang W, Huang J, Ren N, Wu SX, Li YQ. Spinal high-mobility group box 1 contributes to mechanical allodynia in a rat model of bone cancer pain. Biochem Biophys Res Commun. 2010;395(4):572–576. doi: 10.1016/j.bbrc.2010.04.086. [DOI] [PubMed] [Google Scholar]
- Topalova D, Ugrinova I, Pashev IG, Pasheva EA. HMGB1 protein inhibits DNA replication in vitro: a role of the acetylation and the acidic tail. Int J Biochem Cell Biol. 2008;40(8):1536–1542. doi: 10.1016/j.biocel.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Totsingan F, Bell AJ., Jr Interaction of HMG proteins and H1 with hybrid PNA-DNA junctions. Protein Sci. 2013;22(11):1552–1562. doi: 10.1002/pro.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Travers AA. Priming the nucleosome: a role for HMGB proteins? EMBO Rep. 2003;4(2):131–136. doi: 10.1038/sj.embor.embor741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutiger CJ, Mullins GE, Johansson AS, Rouhiainen A, Rauvala HM, Erlandsson-Harris H, Andersson U, Yang H, Tracey KJ, Andersson J, Palmblad JE. High mobility group 1 B-box mediates activation of human endothelium. J Intern Med. 2003;254(4):375–385. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- Troseid M, Lind A, Nowak P, Barqasho B, Heger B, Lygren I, Pedersen KK, Kanda T, Funaoka H, Damas JK, Kvale D. Circulating levels of HMGB1 are correlated strongly with MD2 in HIV-infection: possible implication for TLR4-signalling and chronic immune activation. Innate immunity. 2013;19(3):290–297. doi: 10.1177/1753425912461042. [DOI] [PubMed] [Google Scholar]
- Troseid M, Nowak P, Nystrom J, Lindkvist A, Abdurahman S, Sonnerborg A. Elevated plasma levels of lipopolysaccharide and high mobility group box-1 protein are associated with high viral load in HIV-1 infection: reduction by 2-year antiretroviral therapy. Aids. 2010;24(11):1733–1737. doi: 10.1097/QAD.0b013e32833b254d. [DOI] [PubMed] [Google Scholar]
- Troseid M, Sonnerborg A, Nowak P. High mobility group box protein-1 in HIV-1 infection. Curr HIV Res. 2011;9(1):6–10. doi: 10.2174/157016211794582632. [DOI] [PubMed] [Google Scholar]
- Tsao PN, Wei SC, Huang MT, Lee MC, Chou HC, Chen CY, Hsieh WS. Lipopolysaccharide-induced Notch signaling activation through JNK-dependent pathway regulates inflammatory response. J Biomed Sci. 2011;18:56. doi: 10.1186/1423-0127-18-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng TL, Chen MF, Tsai MJ, Hsu YH, Chen CP, Lee TJ. Oroxylin-A rescues LPS-induced acute lung injury via regulation of NF-kappaB signaling pathway in rodents. PLoS ONE. 2012;7(10):e47403. doi: 10.1371/journal.pone.0047403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoyi K, Jang HJ, Kim JW, Chang HK, Lee YS, Pae HO, Kim HJ, Seo HG, Lee JH, Chung HT, Chang KC. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxid Redox Signal. 2011a;14(11):2057–2070. doi: 10.1089/ars.2010.3555. [DOI] [PubMed] [Google Scholar]
- Tsoyi K, Jang HJ, Nizamutdinova IT, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice. Br J Pharmacol. 2011b;162(7):1498–1508. doi: 10.1111/j.1476-5381.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Heme-oxygenase-1 induction and carbon monoxide-releasing molecule inhibit lipopolysaccharide (LPS)-induced high-mobility group box 1 release in vitro and improve survival of mice in LPS- and cecal ligation and puncture-induced sepsis model in vivo. Mol Pharmacol. 2009;76(1):173–182. doi: 10.1124/mol.109.055137. [DOI] [PubMed] [Google Scholar]
- Tsoyi K, Nizamutdinova IT, Jang HJ, Mun L, Kim HJ, Seo HG, Lee JH, Chang KC. Carbon monoxide from CORM-2 reduces HMGB1 release through regulation of IFN-beta/JAK2/STAT-1/INOS/NO signaling but not COX-2 in TLR-activated macrophages. Shock. 2010;34(6):608–614. doi: 10.1097/SHK.0b013e3181e46f15. [DOI] [PubMed] [Google Scholar]
- Tsujii S, Okabayashi T, Shiga M, Takezaki Y, Sugimoto T, Kobayashi M, Hanazaki K. The effect of the neutrophil elastase inhibitor sivelestat on early injury after liver resection. World journal of surgery. 2012;36(5):1122–1127. doi: 10.1007/s00268-012-1501-8. [DOI] [PubMed] [Google Scholar]
- Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007a;204(12):2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201(7):1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsung A, Zheng N, Jeyabalan G, Izuishi K, Klune JR, Geller DA, Lotze MT, Lu L, Billiar TR. Increasing numbers of hepatic dendritic cells promote HMGB1-mediated ischemia-reperfusion injury. J Leukoc Biol. 2007b;81(1):119–128. doi: 10.1189/jlb.0706468. [DOI] [PubMed] [Google Scholar]
- Tsuzuki S, Seto M. TEL (ETV6)-AML1 (RUNX1) initiates self-renewing fetal pro-B cells in association with a transcriptional program shared with embryonic stem cells in mice. Stem Cells. 2013;31(2):236–247. doi: 10.1002/stem.1277. [DOI] [PubMed] [Google Scholar]
- Tu CT, Yao QY, Xu BL, Zhang SC. Curcumin protects against concanavalin A-induced hepatitis in mice through inhibiting the cytoplasmic translocation and expression of high mobility group box 1. Inflammation. 2013;36(1):206–215. doi: 10.1007/s10753-012-9536-4. [DOI] [PubMed] [Google Scholar]
- Ueda T, Catez F, Gerlitz G, Bustin M. Delineation of the protein module that anchors HMGN proteins to nucleosomes in the chromatin of living cells. Mol Cell Biol. 2008;28(9):2872–2883. doi: 10.1128/MCB.02181-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Chou H, Kawase T, Shirakawa H, Yoshida M. Acidic C-tail of HMGB1 is required for its target binding to nucleosome linker DNA and transcription stimulation. Biochemistry. 2004;43(30):9901–9908. doi: 10.1021/bi035975l. [DOI] [PubMed] [Google Scholar]
- Ueda T, Furusawa T, Kurahashi T, Tessarollo L, Bustin M. The nucleosome binding protein HMGN3 modulates the transcription profile of pancreatic beta cells and affects insulin secretion. Mol Cell Biol. 2009;29(19):5264–5276. doi: 10.1128/MCB.00526-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Yoshida M. HMGB proteins and transcriptional regulation. Biochim Biophys Acta. 2010;1799(1–2):114–118. doi: 10.1016/j.bbagrm.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, Soejima J, Koh H, Ishizaka A. Contributions of high mobility group box protein in experimental and clinical acute lung injury. American journal of respiratory and critical care medicine. 2004;170(12):1310–1316. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- Ugrinova I, Mitkova E, Moskalenko C, Pashev I, Pasheva E. DNA Bending versus DNA End Joining Activity of HMGB1 Protein Is Modulated in Vitro by Acetylation. Biochemistry. 2007 doi: 10.1021/bi0614479. [DOI] [PubMed] [Google Scholar]
- Ugrinova I, Pashev IG, Pasheva EA. Nucleosome binding properties and Co-remodeling activities of native and in vivo acetylated HMGB-1 and HMGB-2 proteins. Biochemistry. 2009a;48(27):6502–6507. doi: 10.1021/bi9004304. [DOI] [PubMed] [Google Scholar]
- Ugrinova I, Pashev IG, Pasheva EA. Cyclin-dependent kinase 5 phosphorylates mammalian HMGB1 protein only if acetylated. J Biochem. 2011;149(5):563–568. doi: 10.1093/jb/mvr005. [DOI] [PubMed] [Google Scholar]
- Ugrinova I, Pasheva EA, Armengaud J, Pashev IG. In vivo acetylation of HMG1 protein enhances its binding affinity to distorted DNA structures. Biochemistry. 2001;40(48):14655–14660. doi: 10.1021/bi0113364. [DOI] [PubMed] [Google Scholar]
- Ugrinova I, Zlateva S, Pashev IG, Pasheva EA. Native HMGB1 protein inhibits repair of cisplatin-damaged nucleosomes in vitro. Int J Biochem Cell Biol. 2009b;41(7):1556–1562. doi: 10.1016/j.biocel.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Ugrinova I, Zlateva S, Pasheva E. The effect of PKC phosphorylation on the "architectural" properties of HMGB1 protein. Mol Biol Rep. 2012;39(11):9947–9953. doi: 10.1007/s11033-012-1863-x. [DOI] [PubMed] [Google Scholar]
- Ulfgren AK, Grundtman C, Borg K, Alexanderson H, Andersson U, Harris HE, Lundberg IE. Down-regulation of the aberrant expression of the inflammation mediator high mobility group box chromosomal protein 1 in muscle tissue of patients with polymyositis and dermatomyositis treated with corticosteroids. Arthritis and rheumatism. 2004;50(5):1586–1594. doi: 10.1002/art.20220. [DOI] [PubMed] [Google Scholar]
- Ullah MA, Loh Z, Gan WJ, Zhang V, Yang H, Li JH, Yamamoto Y, Schmidt AM, Armour CL, Hughes JM, Phipps S, Sukkar MB. Receptor for advanced glycation end products and its ligand high-mobility group box-1 mediate allergic airway sensitization and airway inflammation. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2013.12.1035. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99(19):12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unlu S, Tang S, Wang EN, Martinez I, Tang D, Bianchi ME, Zeh HJ, 3rd, Lotze MT. Damage Associated Molecular Pattern Molecule-Induced microRNAs (DAMPmiRs) in Human Peripheral Blood Mononuclear Cells. PLoS ONE. 2012;7(6):e38899. doi: 10.1371/journal.pone.0038899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterwalder N, Meisel C, Savvatis K, Hammoud B, Fotopoulou C, Volk HD, Reinke P, Schefold JC. High-mobility group box-1 protein serum levels do not reflect monocytic function in patients with sepsis-induced immunosuppression. Mediators of inflammation. 2010;2010:745724. doi: 10.1155/2010/745724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura K, Nightingale K, Wolffe AP. Differential association of HMG1 and linker histones B4 and H1 with dinucleosomal DNA: structural transitions and transcriptional repression. Embo J. 1996;15(18):4959–4969. [PMC free article] [PubMed] [Google Scholar]
- Uramoto H, Izumi H, Nagatani G, Ohmori H, Nagasue N, Ise T, Yoshida T, Yasumoto K, Kohno K. Physical interaction of tumour suppressor p53/p73 with CCAAT-binding transcription factor 2 (CTF2) and differential regulation of human high-mobility group 1 (HMG1) gene expression. Biochem J. 2003;371(Pt 2):301–310. doi: 10.1042/BJ20021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, Kalden JR, Schett G, Rovere-Querini P, Herrmann M, Voll RE. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205(13):3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbonaviciute V, Furnrohr BG, Weber C, Haslbeck M, Wilhelm S, Herrmann M, Voll RE. Factors masking HMGB1 in human serum and plasma. J Leukoc Biol. 2007;81(1):67–74. doi: 10.1189/jlb.0306196. [DOI] [PubMed] [Google Scholar]
- Urbonaviciute V, Starke C, Pirschel W, Pohle S, Frey S, Daniel C, Amann K, Schett G, Herrmann M, Voll RE. Toll-like receptor 2 is required for autoantibody production and development of renal disease in pristane-induced lupus. Arthritis Rheum. 2013;65(6):1612–1623. doi: 10.1002/art.37914. [DOI] [PubMed] [Google Scholar]
- Urbonaviciute V, Voll RE. High-mobility group box 1 represents a potential marker of disease activity and novel therapeutic target in systemic lupus erythematosus. J Intern Med. 2011;270(4):309–318. doi: 10.1111/j.1365-2796.2011.02432.x. [DOI] [PubMed] [Google Scholar]
- Uzawa A, Mori M, Masuda S, Muto M, Kuwabara S. CSF high-mobility group box 1 is associated with intrathecal inflammation and astrocytic damage in neuromyelitis optica. Journal of neurology, neurosurgery, and psychiatry. 2013a;84(5):517–522. doi: 10.1136/jnnp-2012-304039. [DOI] [PubMed] [Google Scholar]
- Uzawa A, Mori M, Taniguchi J, Masuda S, Muto M, Kuwabara S. Anti-high mobility group box 1 monoclonal antibody ameliorates experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2013b;172(1):37–43. doi: 10.1111/cei.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T, Beltrame M, Ferrari S, Bianchi ME. Hmg4, a new member of the Hmg1/2 gene family. Genomics. 1998;49(2):247–252. doi: 10.1006/geno.1998.5214. [DOI] [PubMed] [Google Scholar]
- Valdes-Ferrer SI, Rosas-Ballina M, Olofsson PS, Lu B, Dancho ME, Ochani M, Li JH, Scheinerman JA, Katz DA, Levine YA, Hudson LK, Yang H, Pavlov VA, Roth J, Blanc L, Antoine DJ, Chavan SS, Andersson U, Diamond B, Tracey KJ. HMGB1 mediates splenomegaly and expansion of splenic CD11b+ Ly-6C(high) inflammatory monocytes in murine sepsis survivors. J Intern Med. 2013;274(4):381–390. doi: 10.1111/joim.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallone D, Battista S, Pierantoni GM, Fedele M, Casalino L, Santoro M, Viglietto G, Fusco A, Verde P. Neoplastic transformation of rat thyroid cells requires the junB and fra-1 gene induction which is dependent on the HMGI-C gene product. Embo J. 1997;16(17):5310–5321. doi: 10.1093/emboj/16.17.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1) Angiogenesis. 2008;11(1):91–99. doi: 10.1007/s10456-008-9093-5. [DOI] [PubMed] [Google Scholar]
- van Beijnum JR, Dings RP, van der Linden E, Zwaans BM, Ramaekers FC, Mayo KH, Griffioen AW. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108(7):2339–2348. doi: 10.1182/blood-2006-02-004291. [DOI] [PubMed] [Google Scholar]
- van Beijnum JR, Nowak-Sliwinska P, van den Boezem E, Hautvast P, Buurman WA, Griffioen AW. Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1. Oncogene. 2012 doi: 10.1038/onc.2012.49. [DOI] [PubMed] [Google Scholar]
- van Beijnum JR, Nowak-Sliwinska P, van den Boezem E, Hautvast P, Buurman WA, Griffioen AW. Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1. Oncogene. 2013;32(3):363–374. doi: 10.1038/onc.2012.49. [DOI] [PubMed] [Google Scholar]
- Van den Broeck D, Van der Straeten D, Van Montagu M, Caplan A. A group of chromosomal proteins is specifically released by spermine and loses DNA-binding activity upon phosphorylation. Plant Physiol. 1994;106(2):559–566. doi: 10.1104/pp.106.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8(22):2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- van Houte LP, Chuprina VP, van der Wetering M, Boelens R, Kaptein R, Clevers H. Solution structure of the sequence-specific HMG box of the lymphocyte transcriptional activator Sox-4. J Biol Chem. 1995;270(51):30516–30524. doi: 10.1074/jbc.270.51.30516. [DOI] [PubMed] [Google Scholar]
- Van Opdenbosch N, Gurung P, Vande Walle L, Fossoul A, Kanneganti TD, Lamkanfi M. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nature communications. 2014;5:3209. doi: 10.1038/ncomms4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zoelen MA, Ishizaka A, Wolthuls EK, Choi G, van der Poll T, Schultz MJ. Pulmonary levels of high-mobility group box 1 during mechanical ventilation and ventilator-associated pneumonia. Shock. 2008;29(4):441–445. doi: 10.1097/SHK.0b013e318157eddd. [DOI] [PubMed] [Google Scholar]
- van Zoelen MA, Laterre PF, van Veen SQ, van Till JW, Wittebole X, Bresser P, Tanck MW, Dugernier T, Ishizaka A, Boermeester MA, van der Poll T. Systemic and local high mobility group box 1 concentrations during severe infection. Critical care medicine. 2007;35(12):2799–2804. doi: 10.1097/01.CCM.0000287588.69000.97. [DOI] [PubMed] [Google Scholar]
- van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, van der Poll T. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31(3):280–284. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11(10):700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- Varga-Weisz P, van Holde K, Zlatanova J. Competition between linker histones and HMG1 for binding to four-way junction DNA: implications for transcription. Biochem Biophys Res Commun. 1994;203(3):1904–1911. doi: 10.1006/bbrc.1994.2410. [DOI] [PubMed] [Google Scholar]
- Velegraki M, Koutala H, Tsatsanis C, Papadaki HA. Increased levels of the high mobility group box 1 protein sustain the inflammatory bone marrow microenvironment in patients with chronic idiopathic neutropenia via activation of toll-like receptor 4. Journal of clinical immunology. 2012;32(2):312–322. doi: 10.1007/s10875-011-9620-9. [DOI] [PubMed] [Google Scholar]
- Velegraki M, Papakonstanti E, Mavroudi I, Psyllaki M, Tsatsanis C, Oulas A, Iliopoulos I, Katonis P, Papadaki HA. Impaired clearance of apoptotic cells leads to HMGB1 release in the bone marrow of patients with myelodysplastic syndromes and induces TLR4-mediated cytokine production. Haematologica. 2013;98(8):1206–1215. doi: 10.3324/haematol.2012.064642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L, Shams SS, Yang H, Varani L, Andersson U, Tracey KJ, Bachi A, Uguccioni M, Bianchi ME. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209(9):1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374(6517):70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- Vernon PJ, Loux TJ, Schapiro NE, Kang R, Muthuswamy R, Kalinski P, Tang D, Lotze MT, Zeh HJ., 3rd The receptor for advanced glycation end products promotes pancreatic carcinogenesis and accumulation of myeloid-derived suppressor cells. J Immunol. 2013;190(3):1372–1379. doi: 10.4049/jimmunol.1201151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier CS, Roodi N, Yee CJ, Bailey LR, Jensen RA, Bustin M, Parl FF. High-mobility group (HMG) protein HMG-1 and TATA-binding protein-associated factor TAF(II)30 affect estrogen receptor-mediated transcriptional activation. Mol Endocrinol. 1997;11(8):1009–1019. doi: 10.1210/mend.11.8.9962. [DOI] [PubMed] [Google Scholar]
- Verrijdt G, Haelens A, Schoenmakers E, Rombauts W, Claessens F. Comparative analysis of the influence of the high-mobility group box 1 protein on DNA binding and transcriptional activation by the androgen, glucocorticoid, progesterone and mineralocorticoid receptors. Biochem J. 2002;361(Pt 1):97–103. doi: 10.1042/0264-6021:3610097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011;25(7):1281–1289. doi: 10.1016/j.bbi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Vezzoli M, Castellani P, Corna G, Castiglioni A, Bosurgi L, Monno A, Brunelli S, Manfredi AA, Rubartelli A, Rovere-Querini P. High-mobility group box 1 release and redox regulation accompany regeneration and remodeling of skeletal muscle. Antioxid Redox Signal. 2011;15(8):2161–2174. doi: 10.1089/ars.2010.3341. [DOI] [PubMed] [Google Scholar]
- Vicentino AR, Carneiro VC, Amarante Ade M, Benjamim CF, de Aguiar AP, Fantappie MR. Evaluation of 3-(3-chloro-phenyl)-5-(4-pyridyl)-4,5-dihydroisoxazole as a novel anti-inflammatory drug candidate. PLoS ONE. 2012;7(6):e39104. doi: 10.1371/journal.pone.0039104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- Visone R, Iuliano R, Palmieri D, Server IN, Chiappetta G, De Martino I, Fedele M, Costinean S, Oberyszyn TM, Kusewitt DF, Croce CM, Fusco A. Hmga1 null mice are less susceptible to chemically induced skin carcinogenesis. Eur J Cancer. 2008;44(2):318–325. doi: 10.1016/j.ejca.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Vitali R, Palone F, Cucchiara S, Negroni A, Cavone L, Costanzo M, Aloi M, Dilillo A, Stronati L. Dipotassium Glycyrrhizate Inhibits HMGB1-Dependent Inflammation and Ameliorates Colitis in Mice. PLoS ONE. 2013;8(6):e66527. doi: 10.1371/journal.pone.0066527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali R, Stronati L, Negroni A, Di Nardo G, Pierdomenico M, del Giudice E, Rossi P, Cucchiara S. Fecal HMGB1 is a novel marker of intestinal mucosal inflammation in pediatric inflammatory bowel disease. The American journal of gastroenterology. 2011;106(11):2029–2040. doi: 10.1038/ajg.2011.231. [DOI] [PubMed] [Google Scholar]
- Vogelgesang A, May VE, Grunwald U, Bakkeboe M, Langner S, Wallaschofski H, Kessler C, Broker BM, Dressel A. Functional status of peripheral blood T-cells in ischemic stroke patients. PloS one. 2010;5(1):e8718. doi: 10.1371/journal.pone.0008718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll RE, Urbonaviciute V, Furnrohr B, Herrmann M, Kalden JR. The role of high-mobility group box 1 protein in the pathogenesis of autoimmune diseases. Curr Rheumatol Rep. 2008a;10(5):341–342. doi: 10.1007/s11926-008-0054-1. [DOI] [PubMed] [Google Scholar]
- Voll RE, Urbonaviciute V, Herrmann M, Kalden JR. High mobility group box 1 in the pathogenesis of inflammatory and autoimmune diseases. Isr Med Assoc J. 2008b;10(1):26–28. [PubMed] [Google Scholar]
- Volp K, Brezniceanu ML, Bosser S, Brabletz T, Kirchner T, Gottel D, Joos S, Zornig M. Increased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomas. Gut. 2006;55(2):234–242. doi: 10.1136/gut.2004.062729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz HC, Kaya Z, Katus HA, Andrassy M. The role of HMGB1/RAGE in inflammatory cardiomyopathy. Semin Thromb Hemost. 2010a;36(2):185–194. doi: 10.1055/s-0030-1251503. [DOI] [PubMed] [Google Scholar]
- Volz HC, Laohachewin D, Schellberg D, Wienbrandt AR, Nelles M, Zugck C, Kaya Z, Katus HA, Andrassy M. HMGB1 is an independent predictor of death and heart transplantation in heart failure. Clin Res Cardiol. 2012;101(6):427–435. doi: 10.1007/s00392-011-0409-x. [DOI] [PubMed] [Google Scholar]
- Volz HC, Seidel C, Laohachewin D, Kaya Z, Muller OJ, Pleger ST, Lasitschka F, Bianchi ME, Remppis A, Bierhaus A, Katus HA, Andrassy M. HMGB1: the missing link between diabetes mellitus and heart failure. Basic Res Cardiol. 2010b;105(6):805–820. doi: 10.1007/s00395-010-0114-3. [DOI] [PubMed] [Google Scholar]
- Waga S, Mizuno S, Yoshida M. Nonhistone protein HMG1 removes the transcriptional block caused by left-handed Z-form segment in a supercoiled DNA. Biochem Biophys Res Commun. 1988;153(1):334–339. doi: 10.1016/s0006-291x(88)81227-0. [DOI] [PubMed] [Google Scholar]
- Waga S, Mizuno S, Yoshida M. Nonhistone proteins HMG1 and HMG2 suppress the nucleosome assembly at physiological ionic strength. Biochim Biophys Acta. 1989;1007(2):209–214. doi: 10.1016/0167-4781(89)90041-9. [DOI] [PubMed] [Google Scholar]
- Wagner CR, Hamana K, Elgin SC. A high-mobility-group protein and its cDNAs from Drosophila melanogaster. Mol Cell Biol. 1992;12(5):1915–1923. doi: 10.1128/mcb.12.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahafu W, He ZS, Zhang XY, Zhang CJ, Yao K, Hao H, Song G, He Q, Li XS, Zhou LQ. The nucleosome binding protein NSBP1 is highly expressed in human bladder cancer and promotes the proliferation and invasion of bladder cancer cells. Tumour Biol. 2011;32(5):931–939. doi: 10.1007/s13277-011-0195-0. [DOI] [PubMed] [Google Scholar]
- Wahamaa H, Schierbeck H, Hreggvidsdottir HS, Palmblad K, Aveberger AC, Andersson U, Harris HE. High mobility group box protein 1 in complex with lipopolysaccharide or IL-1 promotes an increased inflammatory phenotype in synovial fibroblasts. Arthritis Res Ther. 2011;13(4):R136. doi: 10.1186/ar3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahamaa H, Vallerskog T, Qin S, Lunderius C, LaRosa G, Andersson U, Harris HE. HMGB1-secreting capacity of multiple cell lineages revealed by a novel HMGB1 ELISPOT assay. J Leukoc Biol. 2007;81(1):129–136. doi: 10.1189/jlb.0506349. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Wang S, Hnilica LS. Immunospecificity of nonhistone proteins in chromatin. Biochemistry. 1974;13(5):1027–1032. doi: 10.1021/bi00702a030. [DOI] [PubMed] [Google Scholar]
- Wake H, Mori S, Liu K, Takahashi HK, Nishibori M. High mobility group box 1 complexed with heparin induced angiogenesis in a matrigel plug assay. Acta Med Okayama. 2009a;63(5):249–262. doi: 10.18926/AMO/31845. [DOI] [PubMed] [Google Scholar]
- Wake H, Mori S, Liu K, Takahashi HK, Nishibori M. Histidine-rich glycoprotein inhibited high mobility group box 1 in complex with heparin-induced angiogenesis in matrigel plug assay. Eur J Pharmacol. 2009b;623(1–3):89–95. doi: 10.1016/j.ejphar.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Wan R, Guo R, Chen C, Jin L, Zhu C, Zhang Q, Xu Y, Li S. Urocortin increased LPS-induced endothelial permeability by regulating the cadherin-catenin complex via corticotrophin-releasing hormone receptor 2. J Cell Physiol. 2013;228(6):1295–1303. doi: 10.1002/jcp.24286. [DOI] [PubMed] [Google Scholar]
- Wang B, Koga K, Osuga Y, Hirata T, Saito A, Yoshino O, Hirota Y, Harada M, Takemura Y, Fujii T, Taketani Y. American journal of reproductive immunology. 2. Vol. 66. New York, NY: 2011a. High mobility group box 1 (HMGB1) levels in the placenta and in serum in preeclampsia; pp. 143–148. (1989) [DOI] [PubMed] [Google Scholar]
- Wang CM, Jiang M, Wang HJ. Effect of NFkappaB inhibitor on highmobility group protein B1 expression in a COPD rat model. Mol Med Rep. 2013a;7(2):499–502. doi: 10.3892/mmr.2012.1181. [DOI] [PubMed] [Google Scholar]
- Wang FP, Li L, Li J, Wang JY, Wang LY, Jiang W. High mobility group box-1 promotes the proliferation and migration of hepatic stellate cells via TLR4-dependent signal pathways of PI3K/Akt and JNK. PLoS ONE. 2013b;8(5):e64373. doi: 10.1371/journal.pone.0064373. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang G, Liu L, Zhang Y, Han D, Lu J, Xu J, Xie X, Wu Y, Zhang D, Ke R, Li S, Zhu Y, Feng W, Li M. Activation of PPARgamma attenuates LPS-induced acute lung injury by inhibition of HMGB1-RAGE levels. Eur J Pharmacol. 2014a;726C:27–32. doi: 10.1016/j.ejphar.2014.01.030. [DOI] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wang H, Li W, Goldstein R, Tracey KJ, Sama AE. HMGB1 as a potential therapeutic target. Novartis Foundation symposium. 2007a;280:73–85. discussion 85–91, 160–164. [PubMed] [Google Scholar]
- Wang H, Li W, Li J, Rendon-Mitchell B, Ochani M, Ashok M, Yang L, Yang H, Tracey KJ, Wang P, Sama AE. The aqueous extract of a popular herbal nutrient supplement, Angelica sinensis, protects mice against lethal endotoxemia and sepsis. J Nutr. 2006;136(2):360–365. doi: 10.1093/jn/136.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li W, Zhu S, Li J, D'Amore J, Ward MF, Yang H, Wu R, Jahnen-Dechent W, Tracey KJ, Wang P, Sama AE. Peripheral administration of fetuin-A attenuates early cerebral ischemic injury in rats. J Cereb Blood Flow Metab. 2010a;30(3):493–504. doi: 10.1038/jcbfm.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004a;10(11):1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu D. Baicalin Inhibits High-Mobility Group Box 1 Release and Improves Survival in Experimental Sepsis. Shock. 2014 doi: 10.1097/SHK.0000000000000122. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang K, Wang C, Xu F, Zhong X, Qiu W, Hu X. Cerebrospinal fluid high-mobility group box protein 1 in neuromyelitis optica and multiple sclerosis. Neuroimmunomodulation. 2013c;20(2):113–118. doi: 10.1159/000345994. [DOI] [PubMed] [Google Scholar]
- Wang H, Ward MF, Sama AE. Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock. 2009a;32(4):348–357. doi: 10.1097/SHK.0b013e3181a551bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ward MF, Sama AE. Targeting HMGB1 in the treatment of sepsis. Expert Opin Ther Targets. 2014b;18(3):257–268. doi: 10.1517/14728222.2014.863876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004b;255(3):320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhu S, Zhou R, Li W, Sama AE. Therapeutic potential of HMGB1-targeting agents in sepsis. Expert Rev Mol Med. 2008a;10:e32. doi: 10.1017/S1462399408000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Xing YQ, Xu YX, Rong F, Lei WF, Zhang WH. The protective effect of lidocaine on septic rats via the inhibition of high mobility group box 1 expression and NF-kappaB activation. Mediators Inflamm. 2013d;2013:570370. doi: 10.1155/2013/570370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Zhang WH, Lei WF, Zhou CQ, Ye T. The inhibitory effect of lidocaine on the release of high mobility group box 1 in lipopolysaccharide-stimulated macrophages. Anesth Analg. 2011b;112(4):839–844. doi: 10.1213/ANE.0b013e31820dca9f. [DOI] [PubMed] [Google Scholar]
- Wang J, Hu X, Jiang H. Nrf-2-HO-1-HMGB1 axis: An important therapeutic approach for protection against myocardial ischemia and reperfusion injury. Int J Cardiol. 2014c;172(1):223–224. doi: 10.1016/j.ijcard.2013.12.273. [DOI] [PubMed] [Google Scholar]
- Wang J, Tochio N, Takeuchi A, Uewaki J, Kobayashi N, Tate S. Redox-sensitive structural change in the A-domain of HMGB1 and its implication for the binding to cisplatin modified DNA. Biochem Biophys Res Commun. 2013e;441(4):701–706. [PubMed] [Google Scholar]
- Wang J, Tochio N, Takeuchi A, Uewaki JI, Kobayashi N, Tate SI. Redox-sensitive structural change in the A-domain of HMGB1 and its implication for the binding to cisplatin modified DNA. Biochem Biophys Res Commun. 2013f [PubMed] [Google Scholar]
- Wang J, Yang H, Hu X, Fu W, Xie J, Zhou X, Xu W, Jiang H. Dobutamine-mediated heme oxygenase-1 induction via PI3K and p38 MAPK inhibits high mobility group box 1 protein release and attenuates rat myocardial ischemia/reperfusion injury in vivo. J Surg Res. 2013g;183(2):509–516. doi: 10.1016/j.jss.2013.02.051. [DOI] [PubMed] [Google Scholar]
- Wang KC, Tsai CP, Lee CL, Chen SY, Chin LT, Chen SJ. Elevated plasma high-mobility group box 1 protein is a potential marker for neuromyelitis optica. Neuroscience. 2012a;226:510–516. doi: 10.1016/j.neuroscience.2012.08.041. [DOI] [PubMed] [Google Scholar]
- Wang KY, Yu GF, Zhang ZY, Huang Q, Dong XQ. Plasma high-mobility group box 1 levels and prediction of outcome in patients with traumatic brain injury. Clinica chimica acta; international journal of clinical chemistry. 2012b;413(21–22):1737–1741. doi: 10.1016/j.cca.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Wang L, Heidt DG, Lee CJ, Yang H, Logsdon CD, Zhang L, Fearon ER, Ljungman M, Simeone DM. Oncogenic function of ATDC in pancreatic cancer through Wnt pathway activation and beta-catenin stabilization. Cancer Cell. 2009b;15(3):207–219. doi: 10.1016/j.ccr.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang X, Liu L, Cui L, Yang R, Li M, Du W. Tanshinone II A down-regulates HMGB1, RAGE, TLR4, NF-kappaB expression, ameliorates BBB permeability and endothelial cell function, and protects rat brains against focal ischemia. Brain Res. doi: 10.1016/j.brainres.2009.12.046. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang X, Liu L, Cui L, Yang R, Li M, Du W. Tanshinone II A down-regulates HMGB1, RAGE, TLR4, NF-kappaB expression, ameliorates BBB permeability and endothelial cell function, and protects rat brains against focal ischemia. Brain Res. 2010b;1321:143–151. doi: 10.1016/j.brainres.2009.12.046. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang X, Liu L, Yang R, Cui L, Li M. Atorvastatin protects rat brains against permanent focal ischemia and downregulates HMGB1, HMGB1 receptors (RAGE and TLR4), NF-kappaB expression. Neurosci Lett. 2010c;471(3):152–156. doi: 10.1016/j.neulet.2010.01.030. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Lu L, Zhang FR, Chen QJ, De Caterina R, Shen WF. Increased serum high-mobility group box-1 and cleaved receptor for advanced glycation endproducts levels and decreased endogenous secretory receptor for advanced glycation endproducts levels in diabetic and non-diabetic patients with heart failure. European journal of heart failure. 2011c;13(4):440–449. doi: 10.1093/eurjhf/hfq231. [DOI] [PubMed] [Google Scholar]
- Wang LL, Meng QH, Jiao Y, Xu JY, Ge CM, Zhou JY, Rosen EM, Wang HC, Fan SJ. High-mobility group boxes mediate cell proliferation and radiosensitivity via retinoblastoma-interaction-dependent and -independent mechanisms. Cancer Biother Radiopharm. 2012c;27(5):329–335. doi: 10.1089/cbr.2012.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LW, Chen H, Gong ZJ. High mobility group box-1 protein inhibits regulatory T cell immune activity in liver failure in patients with chronic hepatitis B. Hepatobiliary & pancreatic diseases international : HBPD INT. 2010d;9(5):499–507. [PubMed] [Google Scholar]
- Wang M, Wang L, Guo Y, Zhou Z, Yi Q, Zhang D, Zhang H, Liu R, Song L. A high mobility group box 1 (HMGB1) gene from Chlamys farreri and the DNA-binding ability and pro-inflammatory activity of its recombinant protein. Fish Shellfish Immunol. 2014d;36(2):393–400. doi: 10.1016/j.fsi.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Wang M, Yue Y, Dong C, Li X, Xu W, Xiong S. Mucosal immunization with high-mobility group box 1 in chitosan enhances DNA vaccine-induced protection against coxsackievirus B3-induced myocarditis. Clin Vaccine Immunol. 2013h;20(11):1743–1751. doi: 10.1128/CVI.00466-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Guo QL, Ye Z, Xia PP, Wang E, Yuan YJ. Preconditioning of intravenous parecoxib attenuates focal cerebral ischemia/reperfusion injury in rats. Chin Med J (Engl) 2011d;124(13):2004–2008. [PubMed] [Google Scholar]
- Wang N, Min X, Li D, He P, Zhao L. Geranylgeranylacetone protects against myocardial ischemia and reperfusion injury by inhibiting high-mobility group box 1 protein in rats. Mol Med Rep. 2012d;5(2):521–524. doi: 10.3892/mmr.2011.666. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ding Q, Zhou Y, Gou X, Hou L, Chen S, Zhu Z, Xiong L. Ethyl pyruvate attenuates spinal cord ischemic injury with a wide therapeutic window through inhibiting high-mobility group box 1 release in rabbits. Anesthesiology. 2009c;110(6):1279–1286. doi: 10.1097/ALN.0b013e3181a160d6. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zeng M, Wang W, Tang J. The HMGB1 acidic tail regulates HMGB1 DNA binding specificity by a unique mechanism. Biochem Biophys Res Commun. 2007b;360(1):14–19. doi: 10.1016/j.bbrc.2007.05.130. [DOI] [PubMed] [Google Scholar]
- Wang RK, Zhang QQ, Pan YD, Guo QL. Etanercept decreases HMGB1 expression in dorsal root ganglion neuron cells in a rat chronic constriction injury model. Experimental and therapeutic medicine. 2013i;5(2):581–585. doi: 10.3892/etm.2012.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Schmaderer C, Kiss E, Schmidt C, Bonrouhi M, Porubsky S, Gretz N, Schaefer L, Kirschning CJ, Popovic ZV, Grone HJ. Recipient Toll-like receptors contribute to chronic graft dysfunction by both MyD88- and TRIF-dependent signaling. Dis Model Mech. 2010e;3(1–2):92–103. doi: 10.1242/dmm.003533. [DOI] [PubMed] [Google Scholar]
- Wang W, Bansal S, Falk S, Ljubanovic D, Schrier R. Ghrelin protects mice against endotoxemia-induced acute kidney injury. Am J Physiol Renal Physiol. 2009d;297(4):F1032–F1037. doi: 10.1152/ajprenal.00044.2009. [DOI] [PubMed] [Google Scholar]
- Wang W, Jiang H, Zhu H, Zhang H, Gong J, Zhang L, Ding Q. Overexpression of high mobility group box 1 and 2 is associated with the progression and angiogenesis of human bladder carcinoma. Oncology letters. 2013j;5(3):884–888. doi: 10.3892/ol.2012.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WK, Wang B, Lu QH, Zhang W, Qin WD, Liu XJ, Liu XQ, An FS, Zhang Y, Zhang MX. Inhibition of high-mobility group box 1 improves myocardial fibrosis and dysfunction in diabetic cardiomyopathy. Int J Cardiol. 2014e doi: 10.1016/j.ijcard.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Wang WK, Wang B, Lu QH, Zhang W, Qin WD, Liu XJ, Liu XQ, An FS, Zhang Y, Zhang MX. Inhibition of high-mobility group box 1 improves myocardial fibrosis and dysfunction in diabetic cardiomyopathy. Int J Cardiol. 2014f;172(1):202–212. doi: 10.1016/j.ijcard.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Wang X, Bu HF, Zhong W, Asai A, Zhou Z, Tan XD. MFG-E8 and HMGB1 are involved in the mechanism underlying alcohol-induced impairment of macrophage efferocytosis. Mol Med. 2013k;19:170–182. doi: 10.2119/molmed.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sun R, Wei H, Tian Z. High-mobility group box 1 (HMGB1)-Toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: Interaction of gammadelta T cells with macrophages. Hepatology. 2013l;57(1):373–384. doi: 10.1002/hep.25982. [DOI] [PubMed] [Google Scholar]
- Wang XF, Sun R, Wei HM, Tian ZG. High-mobility group box 1 (HMGB1)-toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: Interaction of gamma delta T cells with macrophages. Hepatology. 2013m;57(1):373–384. doi: 10.1002/hep.25982. [DOI] [PubMed] [Google Scholar]
- Wang XY, Dong WP, Bi SH, Pan ZG, Yu H, Wang XW, Ma T, Wang J, Zhang WD. Protective effects of osthole against myocardial ischemia/reperfusion injury in rats. Int J Mol Med. 2013n;32(2):365–372. doi: 10.3892/ijmm.2013.1386. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wu L, You W, Ji C, Chen G. Melatonin alleviates secondary brain damage and neurobehavioral dysfunction after experimental subarachnoid hemorrhage: possible involvement of TLR4-mediated inflammatory pathway. J Pineal Res. 2013o;55(4):399–408. doi: 10.1111/jpi.12087. [DOI] [PubMed] [Google Scholar]
- Wang ZT, Yao YM, Sheng ZY. Effect of high mobility group box 1 protein on proliferation and apoptosis and balance between Th1/Th2 and Tc1/Tc2 of lymphocytes in vitro. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2008b;24(4):324–327. [PubMed] [Google Scholar]
- Watanabe F, Shirakawa H, Yoshida M, Tsukada K, Teraoka H. Stimulation of DNA-dependent protein kinase activity by high mobility group proteins 1 and 2. Biochem Biophys Res Commun. 1994;202(2):736–742. doi: 10.1006/bbrc.1994.1992. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Yamashita K, Kamachi H, Kuraya D, Koshizuka Y, Shibasaki S, Asahi Y, Ono H, Emoto S, Ogura M, Yoshida T, Ozaki M, Umezawa K, Matsushita M, Todo S. Efficacy of DHMEQ, a NF-kappaB inhibitor, in islet transplantation: II. Induction DHMEQ treatment ameliorates subsequent alloimmune responses and permits long-term islet allograft acceptance. Transplantation. 2013;96(5):454–462. doi: 10.1097/TP.0b013e31829b077f. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Ueda Y, Akaboshi S, Hino Y, Sekita Y, Nakao M. HMGA2 maintains oncogenic RAS-induced epithelial-mesenchymal transition in human pancreatic cancer cells. Am J Pathol. 2009;174(3):854–868. doi: 10.2353/ajpath.2009.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Asai K, Fujimoto H, Tanaka H, Kanazawa H, Hirata K. Increased levels of HMGB-1 and endogenous secretory RAGE in induced sputum from asthmatic patients. Respir Med. 2011;105(4):519–525. doi: 10.1016/j.rmed.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kubota S, Nagaya M, Ozaki S, Nagafuchi H, Akashi K, Taira Y, Tsukikawa S, Oowada S, Nakano S. The role of HMGB-1 on the development of necrosis during hepatic ischemia and hepatic ischemia/reperfusion injury in mice. J Surg Res. 2005;124(1):59–66. doi: 10.1016/j.jss.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Waterer GW. High-mobility group box 1 (HMGB1) as a potential therapeutic target in sepsis--more questions than answers. Crit Care Med. 2007;35(4):1205–1206. doi: 10.1097/01.CCM.0000259171.54501.B2. [DOI] [PubMed] [Google Scholar]
- Watson DC, Peters EH, Dixon GH. The purification, characterization and partial sequence determination of a trout testis non-histone protein, HMG-T. Eur J Biochem. 1977;74(1):53–60. doi: 10.1111/j.1432-1033.1977.tb11365.x. [DOI] [PubMed] [Google Scholar]
- Webb M, Thomas JO. Structure-specific binding of the two tandem HMG boxes of HMG1 to four-way junction DNA is mediated by the A domain. J Mol Biol. 1999;294(2):373–387. doi: 10.1006/jmbi.1999.3150. [DOI] [PubMed] [Google Scholar]
- Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27(6):1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Burenkova O, Lippard SJ. Cisplatin sensitivity in Hmbg1−/− and Hmbg1+/+ mouse cells. J Biol Chem. 2003;278(3):1769–1773. doi: 10.1074/jbc.M210562200. [DOI] [PubMed] [Google Scholar]
- Wei W, Zhu Y, Wang J, Guo M, Li Y, Li J. Six-month feeding of low-dose fish oil decreases vascular expression of high mobility group box1 and receptor for advanced glycation end-products in rat chronic allograft vasculopathy. Transplant Proc. 2013;45(5):1771–1775. doi: 10.1016/j.transproceed.2013.01.057. [DOI] [PubMed] [Google Scholar]
- Weiner LM, Lotze MT. Tumor-Cell Death, Autophagy, and Immunity. New England Journal of Medicine. 2012;366(12):1156–1158. doi: 10.1056/NEJMcibr1114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir HM, Kraulis PJ, Hill CS, Raine AR, Laue ED, Thomas JO. Structure of the HMG box motif in the B-domain of HMG1. Embo J. 1993;12(4):1311–1319. doi: 10.1002/j.1460-2075.1993.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Huang JK, Johnson BH, Reeck GR. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res. 1989;17(3):1197–1214. doi: 10.1093/nar/17.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Xu L, Chen X, Xu W, Yin Z, Gao X, Xiong S. Autoantibody induction by DNA-containing immune complexes requires HMGB1 with the TLR2/microRNA-155 pathway. J Immunol. 2013;190(11):5411–5422. doi: 10.4049/jimmunol.1203301. [DOI] [PubMed] [Google Scholar]
- Wend P, Runke S, Wend K, Anchondo B, Yesayan M, Jardon M, Hardie N, Loddenkemper C, Ulasov I, Lesniak MS, Wolsky R, Bentolila LA, Grant SG, Elashoff D, Lehr S, Latimer JJ, Bose S, Sattar H, Krum SA, Miranda-Carboni GA. WNT10B/beta-catenin signalling induces HMGA2 and proliferation in metastatic triple-negative breast cancer. EMBO Mol Med. 2013;5(2):264–279. doi: 10.1002/emmm.201201320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng H, Deng Y, Xie Y, Liu H, Gong F. Expression and significance of HMGB1, TLR4 and NF-kappaB p65 in human epidermal tumors. BMC cancer. 2013;13:311. doi: 10.1186/1471-2407-13-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng TI, Wu HY, Chen BL, Liu SH. Honokiol attenuates the severity of acute pancreatitis and associated lung injury via acceleration of acinar cell apoptosis. Shock. 2012;37(5):478–484. doi: 10.1097/SHK.0b013e31824653be. [DOI] [PubMed] [Google Scholar]
- Werner MH, Huth JR, Gronenborn AM, Clore GM. Molecular basis of human 46X,Y sex reversal revealed from the three-dimensional solution structure of the human SRY-DNA complex. Cell. 1995;81(5):705–714. doi: 10.1016/0092-8674(95)90532-4. [DOI] [PubMed] [Google Scholar]
- West KL, Ito Y, Birger Y, Postnikov Y, Shirakawa H, Bustin M. HMGN3a and HMGN3b, two protein isoforms with a tissue-specific expression pattern, expand the cellular repertoire of nucleosome-binding proteins. J Biol Chem. 2001;276(28):25959–25969. doi: 10.1074/jbc.M101692200. [DOI] [PubMed] [Google Scholar]
- Whilding LM, Archibald KM, Kulbe H, Balkwill FR, Oberg D, McNeish IA. Vaccinia virus induces programmed necrosis in ovarian cancer cells. Mol Ther. 2013;21(11):2074–2086. doi: 10.1038/mt.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354(20):2142–2150. doi: 10.1056/NEJMcp054958. [DOI] [PubMed] [Google Scholar]
- Whitman BA, Knapp DJ, Werner DF, Crews FT, Breese GR. The cytokine mRNA increase induced by withdrawal from chronic ethanol in the sterile environment of brain is mediated by CRF and HMGB1 release. Alcohol Clin Exp Res. 2013;37(12):2086–2097. doi: 10.1111/acer.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibisono D, Csernok E, Lamprecht P, Holle JU, Gross WL, Moosig F. Serum HMGB1 levels are increased in active Wegener's granulomatosis and differentiate between active forms of ANCA-associated vasculitis. Annals of the rheumatic diseases. 2010;69(10):1888–1889. doi: 10.1136/ard.2009.119172. [DOI] [PubMed] [Google Scholar]
- Widlak P, Garrard WT. Discovery, regulation, and action of the major apoptotic nucleases DFF40/CAD and endonuclease G. J Cell Biochem. 2005;94(6):1078–1087. doi: 10.1002/jcb.20409. [DOI] [PubMed] [Google Scholar]
- Wild CA, Bergmann C, Fritz G, Schuler P, Hoffmann TK, Lotfi R, Westendorf A, Brandau S, Lang S. HMGB1 conveys immunosuppressive characteristics on regulatory and conventional T cells. Int Immunol. 2012a;24(8):485–494. doi: 10.1093/intimm/dxs051. [DOI] [PubMed] [Google Scholar]
- Wild CA, Brandau S, Lotfi R, Mattheis S, Gu X, Lang S, Bergmann C. HMGB1 is overexpressed in tumor cells and promotes activity of regulatory T cells in patients with head and neck cancer. Oral Oncol. 2012b;48(5):409–416. doi: 10.1016/j.oraloncology.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Wilkinson B, Chen JY, Han P, Rufner KM, Goularte OD, Kaye J. TOX: an HMG box protein implicated in the regulation of thymocyte selection. Nat Immunol. 2002;3(3):272–280. doi: 10.1038/ni767. [DOI] [PubMed] [Google Scholar]
- Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, Duncan JA, Ting JP. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol. 2009;183(3):2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham SB, Bergstralh DT, O'Connor W, Morrison AC, Taxman DJ, Duncan JA, Barnoy S, Venkatesan MM, Flavell RA, Deshmukh M, Hoffman HM, Ting JP. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe. 2007;2(3):147–159. doi: 10.1016/j.chom.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter N, Meyer A, Richter A, Krisponeit D, Bullerdiek J. Elevated levels of HMGB1 in cancerous and inflammatory effusions. Anticancer Res. 2009;29(12):5013–5017. [PubMed] [Google Scholar]
- Wisniewski JR, Schulze E. Insect proteins homologous to mammalian high mobility group protein 1. Characterization and DNA-binding properties. J Biol Chem. 1992;267(24):17170–17177. [PubMed] [Google Scholar]
- Wisniewski JR, Schulze E. High affinity interaction of dipteran high mobility group (HMG) proteins 1 with DNA is modulated by COOH-terminal regions flanking the HMG box domain. J Biol Chem. 1994;269(14):10713–10719. [PubMed] [Google Scholar]
- Wisniewski JR, Schulze E, Sapetto B. DNA binding and nuclear translocation of insect high-mobility-group- protein-1 (HMG1) proteins are inhibited by phosphorylation. Eur J Biochem. 1994;225(2):687–693. doi: 10.1111/j.1432-1033.1994.00687.x. [DOI] [PubMed] [Google Scholar]
- Wisniewski JR, Szewczuk Z, Petry I, Schwanbeck R, Renner U. Constitutive phosphorylation of the acidic tails of the high mobility group 1 proteins by casein kinase II alters their conformation, stability, and DNA binding specificity. J Biol Chem. 1999;274(29):20116–20122. [PubMed] [Google Scholar]
- Wittemann B, Neuer G, Michels H, Truckenbrodt H, Bautz FA. Autoantibodies to nonhistone chromosomal proteins HMG-1 and HMG-2 in sera of patients with juvenile rheumatoid arthritis. Arthritis Rheum. 1990;33(9):1378–1383. doi: 10.1002/art.1780330910. [DOI] [PubMed] [Google Scholar]
- Wittwer C, Boeck S, Heinemann V, Haas M, Stieber P, Nagel D, Holdenrieder S. Circulating nucleosomes and immunogenic cell death markers HMGB1, sRAGE and DNAse in patients with advanced pancreatic cancer undergoing chemotherapy. Int J Cancer. 2013a;133(11):2619–2630. doi: 10.1002/ijc.28294. [DOI] [PubMed] [Google Scholar]
- Wittwer C, Boeck S, Heinemann V, Haas M, Stieber P, Nagel D, Holdenrieder S. Circulating nucleosomes and immunogenic cell death markers HMGB1, sRAGE and DNAse in patients with advanced pancreatic cancer undergoing chemotherapy. International Journal of Cancer. 2013b;133(11):2619–2630. doi: 10.1002/ijc.28294. [DOI] [PubMed] [Google Scholar]
- Wiwanitkit V. Expression of HMG1 and metastasis of colorectal cancer. Int J Colorectal Dis. 2010;25(5):661. doi: 10.1007/s00384-009-0844-6. [DOI] [PubMed] [Google Scholar]
- Wolf M, Lossdorfer S, Abuduwali N, Jager A. Potential role of high mobility group box protein 1 and intermittent PTH (1–34) in periodontal tissue repair following orthodontic tooth movement in rats. Clinical oral investigations. 2012 doi: 10.1007/s00784-012-0777-2. [DOI] [PubMed] [Google Scholar]
- Wolf M, Lossdorfer S, Craveiro R, Gotz W, Jager A. Regulation of macrophage migration and activity by high-mobility group box 1 protein released from periodontal ligament cells during orthodontically induced periodontal repair: an in vitro and in vivo experimental study. Journal of orofacial orthopedics = Fortschritte der Kieferorthopadie : Organ/official journal Deutsche Gesellschaft fur Kieferorthopadie. 2013a;74(5):420–434. doi: 10.1007/s00056-013-0167-7. [DOI] [PubMed] [Google Scholar]
- Wolf M, Lossdorfer S, Craveiro R, Jager A. High-mobility group box protein-1 released by human-periodontal ligament cells cells modulates macrophage migration and activity in vitro. Innate Immun. 2013b doi: 10.1177/1753425913505121. [DOI] [PubMed] [Google Scholar]
- Wolf M, Lossdorfer S, Kupper K, Jager A. Regulation of high mobility group box protein 1 expression following mechanical loading by orthodontic forces in vitro and in vivo. European journal of orthodontics. 2013c doi: 10.1093/ejo/cjt037. [DOI] [PubMed] [Google Scholar]
- Wolfson RK, Chiang ET, Garcia JG. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res. 2011;81(2):189–197. doi: 10.1016/j.mvr.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson RK, Mapes B, Garcia JG. Excessive mechanical stress increases HMGB1 expression in human lung microvascular endothelial cells via STAT3. Microvasc Res. 2013 doi: 10.1016/j.mvr.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LC, Sharpe DJ, Wong SS. High-mobility group and other nonhistone substrates for nuclear histone N-acetyltransferase. Biochem Genet. 1991;29(9–10):461–475. doi: 10.1007/BF02399688. [DOI] [PubMed] [Google Scholar]
- Wu C, Sun H, Wang H, Chi J, Liu Q, Guo H, Gong J. Evaluation of high mobility group box 1 protein as a presurgical diagnostic marker reflecting the severity of acute appendicitis. Scandinavian journal of trauma, resuscitation and emergency medicine. 2012a;20:61. doi: 10.1186/1757-7241-20-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Ding Y, Wang S, Zhang Q, Liu L. Increased expression of high mobility group box 1 (HMGB1) is associated with progression and poor prognosis in human nasopharyngeal carcinoma. The Journal of pathology. 2008;216(2):167–175. doi: 10.1002/path.2391. [DOI] [PubMed] [Google Scholar]
- Wu F, Zhao ZH, Ding ST, Wu HH, Lu JJ. High mobility group box 1 protein is methylated and transported to cytoplasm in clear cell renal cell carcinoma. Asian Pac J Cancer Prev. 2013a;14(10):5789–5795. doi: 10.7314/apjcp.2013.14.10.5789. [DOI] [PubMed] [Google Scholar]
- Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117(10):2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, Chadban SJ. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010;21(11):1878–1890. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Steenstra R, de Boer EC, Zhao CY, Ma J, van der Stelt JM, Chadban SJ. Preconditioning with recombinant high-mobility group box 1 protein protects the kidney against ischemia-reperfusion injury in mice. Kidney Int. 2013b doi: 10.1038/ki.2013.475. [DOI] [PubMed] [Google Scholar]
- Wu J, Li J, Salcedo R, Mivechi NF, Trinchieri G, Horuzsko A. The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res. 2012b;72(16):3977–3986. doi: 10.1158/0008-5472.CAN-12-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Zhang W, Pwee KH, Kumar PP. Cloning and characterization of rice HMGB1 gene. Gene. 2003;312:103–109. doi: 10.1016/s0378-1119(03)00605-x. [DOI] [PubMed] [Google Scholar]
- Wu R, Dong W, Qiang X, Wang H, Blau SA, Ravikumar TS, Wang P. Orexigenic hormone ghrelin ameliorates gut barrier dysfunction in sepsis in rats. Crit Care Med. 2009;37(8):2421–2426. doi: 10.1097/CCM.0b013e3181a557a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Mi Y, Yang H, Hu A, Zhang Q, Shang C. The activation of HMGB1 as a progression factor on inflammation response in normal human bronchial epithelial cells through RAGE/JNK/NF-kappaB pathway. Mol Cell Biochem. 2013c;380(1–2):249–257. doi: 10.1007/s11010-013-1680-0. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang K, Zhao L, Guo J, Hu X, Chen Z. Increased serum HMGB1 is related to oxidative stress in patients with atrial fibrillation. The Journal of international medical research. 2013d;41(6):1796–1802. doi: 10.1177/0300060513503917. [DOI] [PubMed] [Google Scholar]
- Xiang M, Shi X, Li Y, Xu J, Yin L, Xiao G, Scott MJ, Billiar TR, Wilson MA, Fan J. Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J Immunol. 2011;187(9):4809–4817. doi: 10.4049/jimmunol.1102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Benson KF, Chada K. Mini-mouse: disruption of the pygmy locus in a transgenic insertional mutant. Science. 1990;247(4945):967–969. doi: 10.1126/science.2305264. [DOI] [PubMed] [Google Scholar]
- Xiang YY, Wang DY, Tanaka M, Suzuki M, Kiyokawa E, Igarashi H, Naito Y, Shen Q, Sugimura H. Expression of high-mobility group-1 mRNA in human gastrointestinal adenocarcinoma and corresponding non-cancerous mucosa. Int J Cancer. 1997;74(1):1–6. doi: 10.1002/(sici)1097-0215(19970220)74:1<1::aid-ijc1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Xiao DM, Pak JH, Wang X, Sato T, Huang FL, Chen HC, Huang KP. Phosphorylation of HMG-I by protein kinase C attenuates its binding affinity to the promoter regions of protein kinase C gamma and neurogranin/RC3 genes. J Neurochem. 2000;74(1):392–399. doi: 10.1046/j.1471-4159.2000.0740392.x. [DOI] [PubMed] [Google Scholar]
- Xiao J, Ding Y, Huang J, Li Q, Liu Y, Ni W, Zhang Y, Zhu Y, Chen L, Chen B. The Association of HMGB1 Gene with the Prognosis of HCC. PLoS ONE. 2014;9(2):e89097. doi: 10.1371/journal.pone.0089097. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xie J, Hodgkinson JW, Li C, Kovacevic N, Belosevic M. Identification and functional characterization of the goldfish (Carassius auratus L.) high mobility group box 1 (HMGB1) chromatin-binding protein. Dev Comp Immunol. 2014;44(1):245–253. doi: 10.1016/j.dci.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Xie K, Yu Y, Huang Y, Zheng L, Li J, Chen H, Han H, Hou L, Gong G, Wang G. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. Shock. 2012;37(5):548–555. doi: 10.1097/SHK.0b013e31824ddc81. [DOI] [PubMed] [Google Scholar]
- Xie K, Yu Y, Pei Y, Hou L, Chen S, Xiong L, Wang G. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock. 2010;34(1):90–97. doi: 10.1097/SHK.0b013e3181cdc4ae. [DOI] [PubMed] [Google Scholar]
- Xie P, Deng LX, Gong P, Ding Y, Tang XH. Expression of HMGB1 and HMGN2 in gingival tissues, GCF and PICF of periodontitis patients and peri-implantitis. Brazilian journal of microbiology : [publication of the Brazilian Society for Microbiology] 2011;42(3):1213–1219. doi: 10.1590/S1517-838220110003000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong XX, Gu LJ, Shen J, Kang XH, Zheng YY, Yue SB, Zhu SM. Probenecid protects against transient focal cerebral ischemic injury by inhibiting HMGB1 release and attenuating AQP4 expression in mice. Neurochem Res. 2014;39(1):216–224. doi: 10.1007/s11064-013-1212-z. [DOI] [PubMed] [Google Scholar]
- Xiu M, Kim J, Sampson E, Huang CY, Davis RJ, Paulson KE, Yee AS. The transcriptional repressor HBP1 is a target of the p38 mitogen-activated protein kinase pathway in cell cycle regulation. Mol Cell Biol. 2003;23(23):8890–8901. doi: 10.1128/MCB.23.23.8890-8901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Young J, Song D, Esko JD. Heparan sulfate is essential for high mobility group protein 1 (HMGB1) signaling by the receptor for advanced glycation end products (RAGE) J Biol Chem. 2011a;286(48):41736–41744. doi: 10.1074/jbc.M111.299685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DQ, Gao C, Niu W, Li Y, Wang YX, Gao CJ, Ding Q, Yao LN, Chai W, Li ZC. Sodium hydrosulfide alleviates lung inflammation and cell apoptosis following resuscitated hemorrhagic shock in rats. Acta Pharmacol Sin. 2013a;34(12):1515–1525. doi: 10.1038/aps.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Su Z, Wu J, Yang M, Penninger JM, Martin CM, Kvietys PR, Rui T. The alarmin cytokine, high mobility group box 1, is produced by viable cardiomyocytes and mediates the lipopolysaccharide-induced myocardial dysfunction via a TLR4/phosphatidylinositol 3-kinase gamma pathway. J Immunol. 2010;184(3):1492–1498. doi: 10.4049/jimmunol.0902660. [DOI] [PubMed] [Google Scholar]
- Xu H, Yao Y, Su Z, Yang Y, Kao R, Martin CM, Rui T. Endogenous HMGB1 contributes to ischemia-reperfusion-induced myocardial apoptosis by potentiating the effect of TNF-α/JNK. Am J Physiol Heart Circ Physiol. 2011b;300(3):H913–H921. doi: 10.1152/ajpheart.00703.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Jiang Y, Wang J, Shi X, Liu Q, Liu Z, Li Y, Scott MJ, Xiao G, Li S, Fan L, Billiar TR, Wilson MA, Fan J. Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Bao H, Si Y, Wang X. Effects of dexmedetomidine on early and late cytokines during polymicrobial sepsis in mice. Inflamm Res. 2013b;62(5):507–514. doi: 10.1007/s00011-013-0604-5. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jiang WL, Zhang SP, Zhu HB, Hou J. Protocatechuic aldehyde protects against experimental sepsis in vitro and in vivo. Basic Clin Pharmacol Toxicol. 2012;110(4):384–389. doi: 10.1111/j.1742-7843.2011.00827.x. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ueda T, Sato K, Yoshida M. ATP-dependent chromatin structural modulation by multiprotein complex including HMGB1. J Biochem. 2004;135(1):149–153. doi: 10.1093/jb/mvh017. [DOI] [PubMed] [Google Scholar]
- Yamada S, Inoue K, Yakabe K, Imaizumi H, Maruyama I. High mobility group protein 1 (HMGB1) quantified by ELISA with a monoclonal antibody that does not cross-react with HMGB2. Clin Chem. 2003;49(9):1535–1537. doi: 10.1373/49.9.1535. [DOI] [PubMed] [Google Scholar]
- Yamada S, Yakabe K, Ishii J, Imaizumi H, Maruyama I. New high mobility group box 1 assay system. Clin Chim Acta. 2006;372(1–2):173–178. doi: 10.1016/j.cca.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Fujii T, Ishijima R, Tachibana H, Yokoue N, Takasawa R, Tanuma S. DR396, an apoptotic DNase gamma inhibitor, attenuates high mobility group box 1 release from apoptotic cells. Bioorg Med Chem. 2011a;19(1):168–171. doi: 10.1016/j.bmc.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Fujii T, Ishijima R, Tachibana H, Yokoue N, Takasawa R, Tanuma S. The release of high mobility group box 1 in apoptosis is triggered by nucleosomal DNA fragmentation. Arch Biochem Biophys. 2011b;506(2):188–193. doi: 10.1016/j.abb.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ono T, Ito T, Yamanoi A, Maruyama I, Tanaka T. Hemoperfusion with a high-mobility group box 1 adsorption column can prevent the occurrence of hepatic ischemia-reperfusion injury in rats. Crit Care Med. 38(3):879–885. doi: 10.1097/CCM.0b013e3181c58951. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ono T, Ito T, Yamanoi A, Maruyama I, Tanaka T. Hemoperfusion with a high-mobility group box 1 adsorption column can prevent the occurrence of hepatic ischemia-reperfusion injury in rats. Crit Care Med. 2010;38(3):879–885. doi: 10.1097/CCM.0b013e3181c58951. [DOI] [PubMed] [Google Scholar]
- Yamanaka S, Katayama E, Yoshioka K, Nagaki S, Yoshida M, Teraoka H. Nucleosome linker proteins HMGB1 and histone H1 differentially enhance DNA ligation reactions. Biochem Biophys Res Commun. 2002;292(1):268–273. doi: 10.1006/bbrc.2002.6647. [DOI] [PubMed] [Google Scholar]
- Yamato M, Minematsu Y, Fujii J, Mori K, Minato T, Miyagawa S, Fujimura R, Morikage N, Arata Y, Nakano C, Wada A, Ito T. Effective combination therapy of polymyxin-B direct hemoperfusion and recombinant thrombomodulin for septic shock accompanied by disseminated intravascular coagulation: a historical controlled trial. Ther Apher Dial. 2013;17(5):472–476. doi: 10.1111/1744-9987.12112. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Hannani D, Poirier-Colame V, Ladoire S, Locher C, Sistigu A, Prada N, Adjemian S, Catani JP, Freudenberg M, Galanos C, Andre F, Kroemer G, Zitvogel L. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2014;21(1):69–78. doi: 10.1038/cdd.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HX, Wu HP, Zhang HL, Ashton C, Tong C, Wu H, Qian QJ, Wang HY, Ying QL. p53 promotes inflammation-associated hepatocarcinogenesis by inducing HMGB1 release. J Hepatol. 2013a doi: 10.1016/j.jhep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HX, Wu HP, Zhang HL, Ashton C, Tong C, Wu H, Qian QJ, Wang HY, Ying QL. p53 promotes inflammation-associated hepatocarcinogenesis by inducing HMGB1 release. J Hepatol. 2013b;59(4):762–768. doi: 10.1016/j.jhep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Ding Y, Sun F, Lu Z, Xue L, Liu X, Shuai M, Fang C, Wang Y, Cheng H, Zhou L, Zheng MH, Zheng S. Pretreatment of cisplatin in recipients attenuates post-transplantation pancreatitis in murine model. Int J Biol Sci. 2012a;8(3):298–309. doi: 10.7150/ijbs.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yan W, Chang Y, Liang X, Cardinal JS, Huang H, Thorne SH, Monga SP, Geller DA, Lotze MT, Tsung A. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012b;55(6):1863–1875. doi: 10.1002/hep.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX, Lu L, Peng WH, Wang LJ, Zhang Q, Zhang RY, Chen QJ, Shen WF. Increased serum HMGB1 level is associated with coronary artery disease in nondiabetic and type 2 diabetic patients. Atherosclerosis. 2009;205(2):544–548. doi: 10.1016/j.atherosclerosis.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Yanagisawa BL, Resar LM. Hitting the bull's eye: targeting HMGA1 in cancer stem cells. Expert Rev Anticancer Ther. 2013 doi: 10.1586/14737140.2013.859988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagishita M. Function of proteoglycans in the extracellular matrix. Acta pathologica japonica. 1993;43(6):283–293. doi: 10.1111/j.1440-1827.1993.tb02569.x. [DOI] [PubMed] [Google Scholar]
- Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, Taniguchi T. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462(7269):99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- Yanai H, Chiba S, Ban T, Nakaima Y, Onoe T, Honda K, Ohdan H, Taniguchi T. Suppression of immune responses by nonimmunogenic oligodeoxynucleotides with high affinity for high-mobility group box proteins (HMGBs) Proc Natl Acad Sci U S A. 2011;108(28):11542–11547. doi: 10.1073/pnas.1108535108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai H, Matsuda A, An J, Koshiba R, Nishio J, Negishi H, Ikushima H, Onoe T, Ohdan H, Yoshida N, Taniguchi T. Conditional ablation of HMGB1 in mice reveals its protective function against endotoxemia and bacterial infection. Proc Natl Acad Sci U S A. 2013;110(51):20699–20704. doi: 10.1073/pnas.1320808110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaneva J, Leuba SH, van Holde K, Zlatanova J. The major chromatin protein histone H1 binds preferentially to cis-platinum-damaged DNA. Proc Natl Acad Sci U S A. 1997;94(25):13448–13451. doi: 10.1073/pnas.94.25.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Peng L, Su J. Two HMGB1 genes from grass carp Ctenopharyngodon idella mediate immune responses to viral/bacterial PAMPs and GCRV challenge. Dev Comp Immunol. 2013a;39(3):133–146. doi: 10.1016/j.dci.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81(1):59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- Yang D, Postnikov YV, Li Y, Tewary P, de la Rosa G, Wei F, Klinman D, Gioannini T, Weiss JP, Furusawa T, Bustin M, Oppenheim JJ. High-mobility group nucleosomebinding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses. J Exp Med. 2012a;209(1):157–171. doi: 10.1084/jem.20101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EJ, Ku SK, Lee W, Lee S, Lee T, Song KS, Bae JS. Barrier protective effects of rosmarinic acid on HMGB1-induced inflammatory responses in vitro and in vivo. J Cell Physiol. 2013b;228(5):975–982. doi: 10.1002/jcp.24243. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Lee W, Ku SK, Song KS, Bae JS. Anti-inflammatory activities of oleanolic acid on HMGB1 activated HUVECs. Food Chem Toxicol. 2012b;50(5):1288–1294. doi: 10.1016/j.fct.2012.02.026. [DOI] [PubMed] [Google Scholar]
- Yang F, Li X, Wang LK, Wang LW, Han XQ, Zhang H, Gong ZJ. Inhibitions of NFkappaB and TNF-alpha Result in Differential Effects in Rats with Acute on Chronic Liver Failure Induced by D-Gal and LPS. Inflammation. 2014a doi: 10.1007/s10753-013-9805-x. [DOI] [PubMed] [Google Scholar]
- Yang GL, Zhang LH, Bo JJ, Huo XJ, Chen HG, Cao M, Liu DM, Huang YR. Increased expression of HMGB1 is associated with poor prognosis in human bladder cancer. J Surg Oncol. 2012c;106(1):57–61. doi: 10.1002/jso.23040. [DOI] [PubMed] [Google Scholar]
- Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013c;93(6):865–873. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010a;107(26):11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lundback P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, Al-Abed Y, Andersson U, Tracey KJ, Antoine DJ. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1) Mol Med. 2012d;18(1):250–259. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004a;101(1):296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004b;101(1):296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Rivera Z, Jube S, Nasu M, Bertino P, Goparaju C, Franzoso G, Lotze MT, Krausz T, Pass HI, Bianchi ME, Carbone M. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci U S A. 2010b;107(28):12611–12616. doi: 10.1073/pnas.1006542107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Huang C, Jiang H, Ding J. Statins attenuate high mobility group box-1 protein induced vascular endothelial activation : a key role for TLR4/NF-kappaB signaling pathway. Mol Cell Biochem. 2010c;345(1–2):189–195. doi: 10.1007/s11010-010-0572-9. [DOI] [PubMed] [Google Scholar]
- Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W, Kang R, Lotze M, Billiar T, Wang H, Cao L, Tang D. PKM2 Regulates the Warburg Effect and Promotes HMGB1 Release in Sepsis. Nature communications. 2014b doi: 10.1038/ncomms5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Yu Y, Kang R, Yang M, Xie M, Wang Z, Tang D, Zhao M, Liu L, Zhang H, Cao L. Up-regulated autophagy by endogenous high mobility group box-1 promotes chemoresistance in leukemia cells. Leuk Lymphoma. 2012e;53(2):315–322. doi: 10.3109/10428194.2011.616962. [DOI] [PubMed] [Google Scholar]
- Yang M, Cao L, Xie M, Yu Y, Kang R, Yang L, Zhao M, Tang D. Chloroquine inhibits HMGB1 inflammatory signaling and protects mice from lethal sepsis. Biochem Pharmacol. 2013d;86(3):410–418. doi: 10.1016/j.bcp.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Guo L, Duan ZJ, Tepper CG, Xue L, Chen X, Kung HJ, Gao AC, Zou JX, Chen HW. Histone methyltransferase NSD2/MMSET mediates constitutive NF-kappaB signaling for cancer cell proliferation, survival, and tumor growth via a feed-forward loop. Mol Cell Biol. 2012f;32(15):3121–3131. doi: 10.1128/MCB.00204-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Liu X, Yao Z, Mao S, Wei Q, Chang Y. Penehyclidine hydrochloride inhibits the release of high-mobility group box 1 in lipopolysaccharide-activated RAW264.7 cells and cecal ligation and puncture-induced septic mice. J Surg Res. 2014c;186(1):310–317. doi: 10.1016/j.jss.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Yang R, Miki K, Oksala N, Nakao A, Lindgren L, Killeen ME, Mennander A, Fink MP, Tenhunen J. Bile high-mobility group box 1 contributes to gut barrier dysfunction in experimental endotoxemia. Am J Physiol Regul Integr Comp Physiol. 2009a;297(2):R362–R369. doi: 10.1152/ajpregu.00184.2009. [DOI] [PubMed] [Google Scholar]
- Yang R, Shaufl AL, Killeen ME, Fink MP. Ethyl pyruvate ameliorates liver injury secondary to severe acute pancreatitis. J Surg Res. 2009b;153(2):302–309. doi: 10.1016/j.jss.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Yang R, Zhang S, Cotoia A, Oksala N, Zhu S, Tenhunen J. High mobility group B1 impairs hepatocyte regeneration in acetaminophen hepatotoxicity. BMC Gastroenterol. 2012g;12:45. doi: 10.1186/1471-230X-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Xu L, Yang T, Wang F. High-mobility group box-1 and its role in angiogenesis. J Leukoc Biol. 2014d doi: 10.1189/jlb.0713412. [DOI] [PubMed] [Google Scholar]
- Yang W, Yamada M, Tamura Y, Chang K, Mao J, Zou L, Feng Y, Kida K, Scherrer-Crosbie M, Chao W, Ichinose F, Yu YM, Fischman AJ, Tompkins RG, Yao S, Kaneki M. Farnesyltransferase inhibitor FTI-277 reduces mortality of septic mice along with improved bacterial clearance. J Pharmacol Exp Ther. 2011;339(3):832–841. doi: 10.1124/jpet.111.183558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XM, Yang H. Expression of high mobility group box-1 in the lung tissue and serum of patients with pulmonary tuberculosis. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases. 2013;36(7):497–500. [PubMed] [Google Scholar]
- Yang Y, Wang J, Yang Q, Wu S, Yang Z, Zhu H, Zheng M, Liu W, Wu W, He J, Chen Z. Shikonin inhibits the lipopolysaccharide-induced release of HMGB1 in RAW264.7 cells via IFN and NF-kappaB signaling pathways. Int Immunopharmacol. 2014e;19(1):81–87. doi: 10.1016/j.intimp.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Yang Z, Deng Y, Su D, Tian J, Gao Y, He Z, Wang X. TLR4 as receptor for HMGB1-mediated acute lung injury after liver ischemia/reperfusion injury. Lab Invest. 2013e;93(7):792–800. doi: 10.1038/labinvest.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010a;12(9):814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010b;22(2):124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZY, Ling Y, Yin T, Tao J, Xiong JX, Wu HS, Wang CY. Delayed ethyl pyruvate therapy attenuates experimental severe acute pancreatitis via reduced serum high mobility group box 1 levels in rats. World J Gastroenterol. 2008;14(28):4546–4550. doi: 10.3748/wjg.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, Brownlee M. Hyperglycemia-Induced Reactive Oxygen Species Increase Expression of RAGE and RAGE Ligands. Diabetes. 2009 doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59(1):249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HC, Zhao AP, Han QF, Wu L, Yao DK, Wang LX. Correlation between serum high-mobility group box-1 levels and high-sensitivity C-reactive protein and troponin I in patients with coronary artery disease. Experimental and therapeutic medicine. 2013;6(1):121–124. doi: 10.3892/etm.2013.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Zhao G, Yang H, Hong X, Bie L, Liu G. Overexpression of high-mobility group box 1 correlates with tumor progression and poor prognosis in human colorectal carcinoma. J Cancer Res Clin Oncol. 2010;136(5):677–684. doi: 10.1007/s00432-009-0706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell AT, Oh S, Reinberg D, Lippard SJ. Interaction of FACT, SSRP1, and the high mobility group (HMG) domain of SSRP1 with DNA damaged by the anticancer drug cisplatin. J Biol Chem. 2001;276(28):25736–25741. doi: 10.1074/jbc.M101208200. [DOI] [PubMed] [Google Scholar]
- Yaser AM, Huang Y, Zhou RR, Hu GS, Xiao MF, Huang ZB, Duan CJ, Tian W, Tang DL, Fan XG. The Role of Receptor for Advanced Glycation End Products (RAGE) in the Proliferation of Hepatocellular Carcinoma. Int J Mol Sci. 2012;13(5):5982–5997. doi: 10.3390/ijms13055982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda N, Goto K, Yamamoto S, Hidaka S, Hagiwara S, Noguchi T. Removal of 17 cytokines, HMGB1, and albumin by continuous hemofiltration using a cellulose triacetate membrane: an ex vivo study. J Surg Res. 2012;176(1):226–231. doi: 10.1016/j.jss.2011.07.047. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Ueda T, Shinzeki M, Sawa H, Nakajima T, Takeyama Y, Kuroda Y. Increase of high-mobility group box chromosomal protein 1 in blood and injured organs in experimental severe acute pancreatitis. Pancreas. 2007;34(4):487–488. doi: 10.1097/MPA.0b013e31804154e4. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33(4):359–363. doi: 10.1097/01.mpa.0000236741.15477.8b. [DOI] [PubMed] [Google Scholar]
- Ye HH, Hua R, Yu L, Wu KJ, Fei SJ, Qin X, Song Y, Cao JL, Zhang YM. Abnormal expression of Toll-like receptor 4 is associated with susceptibility to ethanol-induced gastric mucosal injury in mice. Dig Dis Sci. 2013a;58(10):2826–2839. doi: 10.1007/s10620-013-2727-5. [DOI] [PubMed] [Google Scholar]
- Ye HH, Wu KJ, Fei SJ, Zhang XW, Liu HX, Zhang JL, Zhang YM. Propofol participates in gastric mucosal protection through inhibiting the toll-like receptor-4/nuclear factor kappa-B signaling pathway. Clinics and research in hepatology and gastroenterology. 2013b;37(1):e3–e15. doi: 10.1016/j.clinre.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Ye X, Chopp M, Liu X, Zacharek A, Cui X, Yan T, Roberts C, Chen J. Niaspan reduces high-mobility group box 1/receptor for advanced glycation endproducts after stroke in type-1 diabetic rats. Neuroscience. 2011;190:339–345. doi: 10.1016/j.neuroscience.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JL, Hsu JH, Liang JC, Chen IJ, Liou SF. Lercanidipine and labedipinedilol--A attenuate lipopolysaccharide/interferon-gamma-induced inflammation in rat vascular smooth muscle cells through inhibition of HMGB1 release and MMP-2, 9 activities. Atherosclerosis. 2013;226(2):364–372. doi: 10.1016/j.atherosclerosis.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol. 2011;13(7):762–770. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi WJ, Yang J, Li C, Wang HY, Liu CW, Tao L, Cheng SX, Zhuo RX, Zhang XZ. Enhanced nuclear import and transfection efficiency of TAT peptide-based gene delivery systems modified by additional nuclear localization signals. Bioconjug Chem. 2012;23(1):125–134. doi: 10.1021/bc2005472. [DOI] [PubMed] [Google Scholar]
- Yie J, Liang S, Merika M, Thanos D. Intra- and intermolecular cooperative binding of high-mobility-group protein I(Y) to the beta-interferon promoter. Mol Cell Biol. 1997;17(7):3649–3662. doi: 10.1128/mcb.17.7.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K, Gribbin E, Wang H. Interferon-gamma inhibition attenuates lethality after cecal ligation and puncture in rats: implication of high mobility group box-1. Shock. 2005;24(4):396–401. doi: 10.1097/01.shk.0000175556.03300.c6. [DOI] [PubMed] [Google Scholar]
- Yoo H, Ku SK, Baek YD, Bae JS. Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflamm Res. 2014;63(3):197–206. doi: 10.1007/s00011-013-0689-x. [DOI] [PubMed] [Google Scholar]
- Yoshida M. Mg2+-, Ca2+-dependent unwinding of DNA by poly-L-glutamic acid. Biochem Biophys Res Commun. 1983;116(1):217–221. doi: 10.1016/0006-291x(83)90403-5. [DOI] [PubMed] [Google Scholar]
- Yoshida M. High glutamic and aspartic region in nonhistone protein HMG(1+2) unwinds DNA double helical structure. J Biochem. 1987;101(1):175–180. doi: 10.1093/oxfordjournals.jbchem.a121888. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Makiguchi K, Chida Y, Shimura K. Unwinding of DNA by nonhistone protein HMG1 and HMG2. Nucleic Acids Symp Ser. 1984;(15):181–184. [PubMed] [Google Scholar]
- Yoshikawa T, Takeuchi H, Suda K, Miyasho T, Yamada S, Okamoto M, Kawamura Y, Maruyama I, Kitajima M, Kitagawa Y. High-dose immunoglobulin preparations improve survival in a CLP-induced rat model of sepsis. Langenbecks Arch Surg. 2012;397(3):457–465. doi: 10.1007/s00423-011-0878-4. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Saito K, Tanabe T, Yamamoto A, Ando Y, Nakamura Y, Shirakawa H, Yoshida M. Differences in DNA recognition and conformational change activity between boxes A and B in HMG2 protein. Biochemistry. 1999;38(2):589–595. doi: 10.1021/bi981834l. [DOI] [PubMed] [Google Scholar]
- Yoshizaki A, Komura K, Iwata Y, Ogawa F, Hara T, Muroi E, Takenaka M, Shimizu K, Hasegawa M, Fujimoto M, Sato S. Clinical significance of serum HMGB-1 and sRAGE levels in systemic sclerosis: association with disease severity. Journal of clinical immunology. 2009;29(2):180–189. doi: 10.1007/s10875-008-9252-x. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JH, Kwak MS, Wu J, Kim ES, Ji Y, Min HJ, Yoo JH, Choi JE, Cho HS, Shin JS. Identification of lipopolysaccharide-binding peptide regions within HMGB1 and their effects on subclinical endotoxemia in a mouse model. Eur J Immunol. 2011;41(9):2753–2762. doi: 10.1002/eji.201141391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JH, Oh YJ, Kim ES, Choi JE, Shin JS. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-alpha production in human monocytes. J Immunol. 2008;180(7):5067–5074. doi: 10.4049/jimmunol.180.7.5067. [DOI] [PubMed] [Google Scholar]
- Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006;177(11):7889–7897. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- Yu H, Schwarzer K, Forster M, Kniemeyer O, Forsbach-Birk V, Straube E, Rodel J. Role of high-mobility group box 1 protein and poly(ADP-ribose) polymerase 1 degradation in Chlamydia trachomatis-induced cytopathicity. Infect Immun. 2010;78(7):3288–3297. doi: 10.1128/IAI.01404-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Zheng L, Yin L, Xu L, Qi Y, Han X, Xu Y, Liu K, Peng J. Protective effects of the total saponins from Dioscorea nipponica Makino against carbon tetrachloride-induced liver injury in mice through suppression of apoptosis and inflammation. Int Immunopharmacol. 2014;19(2):233–244. doi: 10.1016/j.intimp.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Yu J, Mallon MA, Zhang W, Freimuth RR, Marsh S, Watson MA, Goodfellow PJ, McLeod HL. DNA repair pathway profiling and microsatellite instability in colorectal cancer. Clin Cancer Res. 2006a;12(17):5104–5111. doi: 10.1158/1078-0432.CCR-06-0547. [DOI] [PubMed] [Google Scholar]
- Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006b;26(2):174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- Yu M, Wang J, Li W, Yuan YZ, Li CY, Qian XH, Xu WX, Zhan YQ, Yang XM. Proteomic screen defines the hepatocyte nuclear factor 1alpha-binding partners and identifies HMGB1 as a new cofactor of HNF1alpha. Nucleic Acids Res. 2008;36(4):1209–1219. doi: 10.1093/nar/gkm1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SH, Spring TG. The interaction of nonhistone chromosomal proteins HMG1 and HMG2 with subfractions of H1 histone immobilized on agarose. Biochim Biophys Acta. 1977;492(1):20–28. doi: 10.1016/0005-2795(77)90210-0. [DOI] [PubMed] [Google Scholar]
- Yu SS, Li HJ, Goodwin GH, Johns EW. Interaction of non-histone chromosomal proteins HMG1 and HMG2 with DNA. Eur J Biochem. 1977;78(2):497–502. doi: 10.1111/j.1432-1033.1977.tb11762.x. [DOI] [PubMed] [Google Scholar]
- Yu X, Xing C, Pan Y, Ma H, Zhang J, Li W. IGF-1 alleviates ox-LDL-induced inflammation via reducing HMGB1 release in HAECs. Acta Biochim Biophys Sin (Shanghai) 2012a;44(9):746–751. doi: 10.1093/abbs/gms059. [DOI] [PubMed] [Google Scholar]
- Yu Y, Liu M, Zhang L, Cao Q, Zhang P, Jiang H, Zou Y, Ge J. Heat shock transcription factor 1 inhibits H(2)O(2)-induced cardiomyocyte death through suppression of high-mobility group box 1. Mol Cell Biochem. 2012b;364(1–2):263–269. doi: 10.1007/s11010-012-1226-x. [DOI] [PubMed] [Google Scholar]
- Yuan F, Gu L, Guo S, Wang C, Li GM. Evidence for involvement of HMGB1 protein in human DNA mismatch repair. J Biol Chem. 2004;279(20):20935–20940. doi: 10.1074/jbc.M401931200. [DOI] [PubMed] [Google Scholar]
- Yuan H, Jin X, Sun J, Li F, Feng Q, Zhang C, Cao Y, Wang Y. Protective effect of HMGB1 a box on organ injury of acute pancreatitis in mice. Pancreas. 2009;38(2):143–148. doi: 10.1097/MPA.0b013e31818166b4. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Chen J, Zhang Y, Peng Y. Construction and characterization of the HMGB1 mutant as a competitive antagonist to HMGB1 induced cytokines release. Biochem Biophys Res Commun. 2008;372(4):703–707. doi: 10.1016/j.bbrc.2008.05.115. [DOI] [PubMed] [Google Scholar]
- Yumoto M, Nishida O, Moriyama K, Shimomura Y, Nakamura T, Kuriyama N, Hara Y, Yamada S. In vitro evaluation of high mobility group box 1 protein removal with various membranes for continuous hemofiltration. Ther Apher Dial. 2011;15(4):385–393. doi: 10.1111/j.1744-9987.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- Yumoto Y, Shirakawa H, Yoshida M, Suwa A, Watanabe F, Teraoka H. High mobility group proteins 1 and 2 can function as DNA-binding regulatory components for DNA-dependent protein kinase in vitro. J Biochem. 1998;124(3):519–527. doi: 10.1093/oxfordjournals.jbchem.a022143. [DOI] [PubMed] [Google Scholar]
- Yun N, Eum HA, Lee SM. Protective role of heme oxygenase-1 against liver damage caused by hepatic ischemia and reperfusion in rats. Antioxid Redox Signal. 2010;13(10):1503–1512. doi: 10.1089/ars.2009.2873. [DOI] [PubMed] [Google Scholar]
- Yusein-Myashkova S, Ugrinova I, Pasheva E. Non-histone protein HMGB1 inhibits the repair of cisplatin damaged DNA in NIH-3T3 murine fibroblasts. BMB Rep. 2013 doi: 10.5483/BMBRep.2016.49.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi MR, Okada Y, Chada KK. Misexpression of full-length HMGA2 induces benign mesenchymal tumors in mice. Cancer Res. 2006;66(15):7453–7459. doi: 10.1158/0008-5472.CAN-06-0931. [DOI] [PubMed] [Google Scholar]
- Zakiyanov O, Kriha V, Vachek J, Zima T, Tesar V, Kalousova M. Placental growth factor, pregnancy-associated plasma protein-A, soluble receptor for advanced glycation end products, extracellular newly identified receptor for receptor for advanced glycation end products binding protein and high mobility group box 1 levels in patients with acute kidney injury: a cross sectional study. BMC nephrology. 2013a;14:245. doi: 10.1186/1471-2369-14-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiyanov O, VZ KI, Vachek J, Zima T, Tesa V, Kalousova M. Placental growth factor, pregnancy-associated plasma protein-A, soluble receptor for advanced glycation end products, extracellular newly identified receptor for receptor for advanced glycation end products binding protein and high mobility group box 1 levels in patients with acute kidney injury: a cross sectional study. BMC nephrology. 2013b;14(1):245. doi: 10.1186/1471-2369-14-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaldivar MM, Berres ML, Sahin H, Nellen A, Heinrichs D, Schmitz P, Gassler N, Streetz KL, Trautwein C, Wasmuth HE. The chemokine receptor CXCR3 limits injury after acute toxic liver damage. Lab Invest. 2012;92(5):724–734. doi: 10.1038/labinvest.2012.48. [DOI] [PubMed] [Google Scholar]
- Zamora R, Grishin A, Wong C, Boyle P, Wang J, Hackam D, Upperman JS, Tracey KJ, Ford HR. High-mobility group box 1 protein is an inflammatory mediator in necrotizing enterocolitis: protective effect of the macrophage deactivator semapimod. Am J Physiol Gastrointest Liver Physiol. 2005;289(4):G643–G652. doi: 10.1152/ajpgi.00067.2005. [DOI] [PubMed] [Google Scholar]
- Zandarashvili L, Sahu D, Lee K, Lee YS, Singh P, Rajarathnam K, Iwahara J. Real-time kinetics of high-mobility group box 1 (HMGB1) oxidation in extracellular fluids studied by in situ protein NMR spectroscopy. J Biol Chem. 2013;288(17):11621–11627. doi: 10.1074/jbc.M113.449942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappavigna V, Falciola L, Helmer-Citterich M, Mavilio F, Bianchi ME. HMG1 interacts with HOX proteins and enhances their DNA binding and transcriptional activation. Embo J. 1996;15(18):4981–4991. [PMC free article] [PubMed] [Google Scholar]
- Zayed H, Izsvak Z, Khare D, Heinemann U, Ivics Z. The DNA-bending protein HMGB1 is a cellular cofactor of Sleeping Beauty transposition. Nucleic Acids Res. 2003;31(9):2313–2322. doi: 10.1093/nar/gkg341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayed H, Izsvak Z, Walisko O, Ivics Z. Development of hyperactive sleeping beauty transposon vectors by mutational analysis. Mol Ther. 2004;9(2):292–304. doi: 10.1016/j.ymthe.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Zeng L, Zhang AQ, Gu W, Chen KH, Jiang DP, Zhang LY, Du DY, Hu P, Huang SN, Wang HY, Jiang JX. Clinical relevance of single nucleotide polymorphisms of the high mobility group box 1 protein gene in patients with major trauma in southwest China. Surgery. 2012;151(3):427–436. doi: 10.1016/j.surg.2011.07.075. [DOI] [PubMed] [Google Scholar]
- Zeng S, Dun H, Ippagunta N, Rosario R, Zhang QY, Lefkowitch J, Yan SF, Schmidt AM, Emond JC. Receptor for advanced glycation end product (RAGE)-dependent modulation of early growth response-1 in hepatic ischemia/reperfusion injury. J Hepatol. 2009;50(5):929–936. doi: 10.1016/j.jhep.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Zeng SX, Dai MS, Keller DM, Lu H. SSRP1 functions as a co-activator of the transcriptional activator p63. Embo J. 2002;21(20):5487–5497. doi: 10.1093/emboj/cdf540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterstrom CK, Bergman T, Rynnel-Dagoo B, Erlandsson Harris H, Soder O, Andersson U, Boman HG. High mobility group box chromosomal protein 1 (HMGB1) is an antibacterial factor produced by the human adenoid. Pediatr Res. 2002;52(2):148–154. doi: 10.1203/00006450-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Zetterstrom CK, Jiang W, Wahamaa H, Ostberg T, Aveberger AC, Schierbeck H, Lotze MT, Andersson U, Pisetsky DS, Erlandsson Harris H. Pivotal advance: inhibition of HMGB1 nuclear translocation as a mechanism for the anti-rheumatic effects of gold sodium thiomalate. J Leukoc Biol. 2008;83(1):31–38. doi: 10.1189/jlb.0507323. [DOI] [PubMed] [Google Scholar]
- Zetterstrom CK, Strand ML, Soder O. The high mobility group box chromosomal protein 1 is expressed in the human and rat testis where it may function as an antibacterial factor. Hum Reprod. 2006;21(11):2801–2809. doi: 10.1093/humrep/del256. [DOI] [PubMed] [Google Scholar]
- Zhai CL, Zhang MQ, Zhang Y, Xu HX, Wang JM, An GP, Wang YY, Li L. Glycyrrhizin protects rat heart against ischemia-reperfusion injury through blockade of HMGB1-dependent phospho-JNK/Bax pathway. Acta Pharmacol Sin. 2012;33(12):1477–1487. doi: 10.1038/aps.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan H, Li S, Sun J, Liu R, Yan F, Niu B, Zhang H, Wang X. Lettuce glycoside B ameliorates cerebral ischemia reperfusion injury by increasing nerve growth factor and neurotrophin-3 expression of cerebral cortex in rats. Indian J Pharmacol. 2014;46(1):63–68. doi: 10.4103/0253-7613.125171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Z, Li Q, Wu P, Ye Y, Tseng HY, Zhang L, Zhang XD. Autophagy-mediated HMGB1 release antagonizes apoptosis of gastric cancer cells induced by vincristine via transcriptional regulation of Mcl-1. Autophagy. 2012;8(1):109–121. doi: 10.4161/auto.8.1.18319. [DOI] [PubMed] [Google Scholar]
- Zhang B, Liu ZY, Li YY, Luo Y, Liu ML, Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG, Li ZC. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur J Pharm Sci. 2011a;44(5):573–579. doi: 10.1016/j.ejps.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Zhang BX, Li N, Zhang ZP, Liu HB, Zhou RR, Zhong BY, Zou MX, Dai XH, Xiao MF, Liu XQ, Fan XG. Protective effect of Acanthopanax gracilistylus-extracted Acankoreanogenin A on mice with fulminant hepatitis. Int Immunopharmacol. 2011b;11(8):1018–1023. doi: 10.1016/j.intimp.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Zhang C, Ge S, Hu C, Yang N, Zhang J. MiRNA-218, a new regulator of HMGB1, suppresses cell migration and invasion in non-small cell lung cancer. Acta Biochim Biophys Sin (Shanghai) 2013a;45(12):1055–1061. doi: 10.1093/abbs/gmt109. [DOI] [PubMed] [Google Scholar]
- Zhang CG, Wang H, Niu ZG, Zhang JJ, Yin MM, Gao ZT, Hu LH. Tax is involved in up-regulation of HMGB1 expression levels by interaction with C/EBP. Asian Pac J Cancer Prev. 2013b;14(1):359–365. doi: 10.7314/apjcp.2013.14.1.359. [DOI] [PubMed] [Google Scholar]
- Zhang G, Chen F, Cao Y, See WA. Contributors to HMGB1 release by urothelial carcinoma cells in response to bacillus Calmette-Guerin. J Urol. 2013c;190(4):1398–1403. doi: 10.1016/j.juro.2013.03.123. [DOI] [PubMed] [Google Scholar]
- Zhang G, Kobayashi T, Kamitani W, Komoto S, Yamashita M, Baba S, Yanai H, Ikuta K, Tomonaga K. Borna disease virus phosphoprotein represses p53-mediated transcriptional activity by interference with HMGB1. J Virol. 2003;77(22):12243–12251. doi: 10.1128/JVI.77.22.12243-12251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kou YB, Zhu JS, Chen WX, Li S. Knockdown of HMGB1 inhibits growth and invasion of gastric cancer cells through the NF-kappaB pathway in vitro and in vivo. Int J Oncol. 2014a;44(4):1268–1276. doi: 10.3892/ijo.2014.2285. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang K, Zhang J, Liu SS, Dai L, Zhang JY. Using proteomic approach to identify tumor-associated proteins as biomarkers in human esophageal squamous cell carcinoma. Journal of proteome research. 2011c;10(6):2863–2872. doi: 10.1021/pr200141c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhu JS, Zhou Z, Chen WX, Chen NW. Inhibitory effects of ethyl pyruvate administration on human gastric cancer growth via regulation of the HMGB1-RAGE and Akt pathways in vitro and in vivo. Oncol Rep. 2012a;27(5):1511–1519. doi: 10.3892/or.2012.1623. [DOI] [PubMed] [Google Scholar]
- Zhang K, Gao H, Wu X, Wang J, Zhou W, Sun G, Wang Y, Mu B, Kim C, Chu P, Ho DM, Ann DK, Wong TT, Yen Y. Frequent overexpression of HMGA2 in human atypical teratoid/rhabdoid tumor and its correlation with let-7a3/let-7b miRNA. Clin Cancer Res. 2014b doi: 10.1158/1078-0432.CCR-13-1452. [DOI] [PubMed] [Google Scholar]
- Zhang L, Bukulin M, Kojro E, Roth A, Metz VV, Fahrenholz F, Nawroth PP, Bierhaus A, Postina R. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem. 2008a;283(51):35507–35516. doi: 10.1074/jbc.M806948200. [DOI] [PubMed] [Google Scholar]
- Zhang LT, Yao YM, Lu JQ, Yan XJ, Yu Y, Sheng ZY. Sodium butyrate prevents lethality of severe sepsis in rats. Shock. 2007;27(6):672–677. doi: 10.1097/SHK.0b013e31802e3f4c. [DOI] [PubMed] [Google Scholar]
- Zhang LT, Yao YM, Lu JQ, Yan XJ, Yu Y, Sheng ZY. Recombinant bactericidal/permeability-increasing protein inhibits endotoxin-induced high-mobility group box 1 protein gene expression in sepsis. Shock. 2008b;29(2):278–284. doi: 10.1097/shk.0b013e31811ff581. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Kang R, Zeh HJ, 3rd, Lotze MT, Tang D. DAMPs and autophagy: cellular adaptation to injury and unscheduled cell death. Autophagy. 2013d;9(4):451–458. doi: 10.4161/auto.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Wang Y. HMG modifications and nuclear function. Biochim Biophys Acta. 2010;1799(1–2):28–36. doi: 10.1016/j.bbagrm.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Luo L, Wang Y, Rahman M, Lepsenyi M, Syk I, Jeppsson B, Thorlacius H. Simvastatin protects against T cell immune dysfunction in abdominal sepsis. Shock. 2012b;38(5):524–531. doi: 10.1097/SHK.0b013e31826fb073. [DOI] [PubMed] [Google Scholar]
- Zhang T, Hu X, Cai Y, Yi B, Wen Z. Metformin protects against hyperglycemia-induced cardiomyocytes injury by inhibiting the expressions of receptor for advanced glycation end products and high mobility group box 1 protein. Mol Biol Rep. 2014c;41(3):1335–1340. doi: 10.1007/s11033-013-2979-3. [DOI] [PubMed] [Google Scholar]
- Zhang W, Tian J, Hao Q. HMGB1 combining with tumor-associated macrophages enhanced lymphangiogenesis in human epithelial ovarian cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013e doi: 10.1007/s13277-013-1288-8. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang LW, Wang LK, Li X, Zhang H, Luo LP, Song JC, Gong ZJ. Betaine protects against high-fat-diet-induced liver injury by inhibition of high-mobility group box 1 and Toll-like receptor 4 expression in rats. Dig Dis Sci. 2013f;58(11):3198–3206. doi: 10.1007/s10620-013-2775-x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Guo L, Collage RD, Stripay JL, Tsung A, Lee JS, Rosengart MR. Calcium/calmodulin-dependent protein kinase (CaMK) Ialpha mediates the macrophage inflammatory response to sepsis. J Leukoc Biol. 2011d;90(2):249–261. doi: 10.1189/jlb.0510286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kim J, Ruthazer R, McDevitt MA, Wazer DE, Paulson KE, Yee AS. The HBP1 transcriptional repressor participates in RAS-induced premature senescence. Mol Cell Biol. 2006;26(22):8252–8266. doi: 10.1128/MCB.00604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang H, Wang J. Expression of HMGB1 and NF-kappaB p65 and its significance in non-small cell lung cancer. Contemp Oncol (Pozn) 2013g;17(4):350–355. doi: 10.5114/wo.2013.35291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wheeler D, Tang Y, Guo L, Shapiro RA, Ribar TJ, Means AR, Billiar TR, Angus DC, Rosengart MR. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J Immunol. 2008c;181(7):5015–5023. doi: 10.4049/jimmunol.181.7.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XJ, Luan ZG, Ma XC. shRNAs targeting high-mobility group box-1 inhibit E-selectin expression via homeobox A9 in human umbilical vein endothelial cells. Mol Med Rep. 2013h;7(4):1251–1256. doi: 10.3892/mmr.2013.1314. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Guo ZQ, Ji SQ, Zhang M, Jiang N, Li XS, Zhou LQ. Small interfering RNA targeting HMGN5 induces apoptosis via modulation of a mitochondrial pathway and Bcl-2 family proteins in prostate cancer cells. Asian J Androl. 2012c;14(3):487–492. doi: 10.1038/aja.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cheng Y, Ren X, Zhang L, Yap KL, Wu H, Patel R, Liu D, Qin ZH, Shih IM, Yang JM. NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene. 2012d;31(8):1055–1064. doi: 10.1038/onc.2011.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li W, Zhu S, Jundoria A, Li J, Yang H, Fan S, Wang P, Tracey KJ, Sama AE, Wang H. Tanshinone IIA sodium sulfonate facilitates endocytic HMGB1 uptake. Biochem Pharmacol. 2012e;84(11):1492–1500. doi: 10.1016/j.bcp.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang JW, Ren X, Yang JM. NAC1 and HMGB1 enter a partnership for manipulating autophagy. Autophagy. 2011e;7(12):1557–1558. doi: 10.4161/auto.7.12.17910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yao YM, Huang LF, Dong N, Yu Y, Sheng ZY. The potential effect and mechanism of high-mobility group box 1 protein on regulatory T cell-mediated immunosuppression. J Interferon Cytokine Res. 2011f;31(2):249–257. doi: 10.1089/jir.2010.0019. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yuan F, Presnell SR, Tian K, Gao Y, Tomkinson AE, Gu L, Li GM. Reconstitution of 5'-directed human mismatch repair in a purified system. Cell. 2005;122(5):693–705. doi: 10.1016/j.cell.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Zhang JR, Wang ZG. Mycophenolate mofetil affects monocyte Toll-like receptor 4 signaling during mouse renal ischemia/reperfusion injury. Chin Med J (Engl) 2013i;126(7):1224–1229. [PubMed] [Google Scholar]
- Zhang Z, Lin C, Peng L, Ouyang Y, Cao Y, Wang J, Friedman SL, Guo J. High mobility group box 1 activates Toll like receptor 4 signaling in hepatic stellate cells. Life Sci. 2012f;91(5–6):207–212. doi: 10.1016/j.lfs.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wu Y, Zhao Y, Xiao X, Liu J, Zhou X. Dynamic changes in HMGB1 levels correlate with inflammatory responses during cardiopulmonary bypass. Experimental and therapeutic medicine. 2013j;5(5):1523–1527. doi: 10.3892/etm.2013.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhu Y, Lai Y, Wu X, Feng Z, Yu Y, Bast RC, Jr, Wan X, Xi X, Feng Y. Follicle-stimulating hormone inhibits apoptosis in ovarian cancer cells by regulating the OCT4 stem cell signaling pathway. Int J Oncol. 2013k;43(4):1194–1204. doi: 10.3892/ijo.2013.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW, Zhang QY, Zhou MT, Liu NX, Chen TK, Zhu YF, Wu L. Antioxidant inhibits HMGB1 expression and reduces pancreas injury in rats with severe acute pancreatitis. Dig Dis Sci. 2010;55(9):2529–2536. doi: 10.1007/s10620-009-1073-0. [DOI] [PubMed] [Google Scholar]
- Zhao H, Watts HR, Chong M, Huang H, Tralau-Stewart C, Maxwell PH, Maze M, George AJ, Ma D. Xenon treatment protects against cold ischemia associated delayed graft function and prolongs graft survival in rats. Am J Transplant. 2013;13(8):2006–2018. doi: 10.1111/ajt.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Yang M, Yang L, Yu Y, Xie M, Zhu S, Kang R, Tang D, Jiang Z, Yuan W, Wu X, Cao L. HMGB1 regulates autophagy through increasing transcriptional activities of JNK and ERK in human myeloid leukemia cells. BMB Rep. 2011a;44(9):601–606. doi: 10.5483/bmbrep.2011.44.9.601. [DOI] [PubMed] [Google Scholar]
- Zhao X, Kuja-Panula J, Rouhiainen A, Chen YC, Panula P, Rauvala H. High mobility group box-1 (HMGB1; amphoterin) is required for zebrafish brain development. J Biol Chem. 2011b;286(26):23200–23213. doi: 10.1074/jbc.M111.223834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Cheng W, Gibb CL, Gupta G, Kallenbach NR. HMG box proteins interact with multiple tandemly repeated (GCC)n (GGC)m DNA sequences. J Biomol Struct Dyn. 1996;14(2):235–238. doi: 10.1080/07391102.1996.10508113. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Oh S, Li D, Ni D, Pirooz SD, Lee JH, Yang S, Lee JY, Ghozalli I, Costanzo V, Stark JM, Liang C. A Dual Role for UVRAG in Maintaining Chromosomal Stability Independent of Autophagy. Dev Cell. 2012 doi: 10.1016/j.devcel.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Hu B, Wu D. Sequence of a cDNA encoding turtle high mobility group 1 protein. Genetika. 2005;41(7):925–930. [PubMed] [Google Scholar]
- Zhou JG, Dong JY, Zhang LH, Wang J. Expression of high mobility group box chromosomal protein 1 in mice with lupus nephritis. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2011a;40(2):200–206. doi: 10.3785/j.issn.1008-9292.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Zhou JR, Zhang LD, Wei HF, Wang X, Ni HL, Yang F, Zhang T, Jiang CL. Neuropeptide Y induces secretion of high-mobility group box 1 protein in mouse macrophage via PKC/ERK dependent pathway. J Neuroimmunol. 2013a;260(1–2):55–59. doi: 10.1016/j.jneuroim.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Zhou L, Wang X, Xue W, Xie K, Huang Y, Chen H, Gong G, Zeng Y. Beneficial effects of hydrogen-rich saline against spinal cord ischemia-reperfusion injury in rabbits. Brain Res. 2013b;1517:150–160. doi: 10.1016/j.brainres.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Zhou P, Li YQ, Li WD, Han TS, Yang SJ, Yao YM, Zhang YY, Yu Y. Changes in serum high mobility group box-1 protein and high-sensitivity C-reactive protein in patients with acute cerebral infarction and their clinical significance. Zhongguo wei zhong bing ji jiu yi xue = Chinese critical care medicine = Zhongguo weizhongbing jijiuyixue. 2012a;24(5):265–268. [PubMed] [Google Scholar]
- Zhou RR, Liu HB, Peng JP, Huang Y, Li N, Xiao MF, Wang H, Fan XG. High mobility group box chromosomal protein 1 in acute-on-chronic liver failure patients and mice with ConA-induced acute liver injury. Experimental and molecular pathology. 2012b;93(2):213–219. doi: 10.1016/j.yexmp.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Zhou RR, Zhao SS, Zou MX, Zhang P, Zhang BX, Dai XH, Li N, Liu HB, Wang H, Fan XG. HMGB1 cytoplasmic translocation in patients with acute liver failure. BMC gastroenterology. 2011b;11:21. doi: 10.1186/1471-230X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou RR, Zhao SS, Zou MX, Zhang P, Zhang BX, Dai XH, Li N, Liu HB, Wang HC, Fan XG. HMGB1 cytoplasmic translocation in patients with acute liver failure. Bmc Gastroenterology. 2011c;11 doi: 10.1186/1471-230X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou WF, Chen Q, Jin MF, Ji ZH, Zhang MZ, Li HM, Liu FJ, Ji W. The diagnostic accuracy of high-mobility group box 1 protein and twelve other markers in discriminating bacterial, viral and co-infected bronchial pneumonia in Han children. Microbiology and immunology. 2011d;55(4):279–288. doi: 10.1111/j.1348-0421.2011.00306.x. [DOI] [PubMed] [Google Scholar]
- Zhou X, Benson KF, Ashar HR, Chada K. Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature. 1995;376(6543):771–774. doi: 10.1038/376771a0. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jiang YQ, Wang WX, Zhou ZX, Wang YG, Yang L, Ji YL. HMGB1 and RAGE levels in induced sputum correlate with asthma severity and neutrophil percentage. Hum Immunol. 2012c;73(11):1171–1174. doi: 10.1016/j.humimm.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xiong KL, Lin S, Zhong Q, Lu FL, Liang H, Li JC, Wang JZ, Yang QW. Elevation of high-mobility group protein box-1 in serum correlates with severity of acute intracerebral hemorrhage. Mediators of inflammation. 2010;2010 doi: 10.1155/2010/142458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wang L, Ruan Y, Zhou L, Zhang D, Min Z, Xie J, Yu M, Gu J. An efficient delivery of DAMPs on the cell surface by the unconventional secretion pathway. Biochem Biophys Res Commun. 2011a;404(3):790–795. doi: 10.1016/j.bbrc.2010.12.061. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zhang Y, Hu X, Yi C, Zhong S, Wang Y, Yang F. The effects of high-dose qinggan huoxue recipe on acute liver failure induced by d-galactosamine in rats. Evid Based Complement Alternat Med. 2013a;2013:905715. doi: 10.1155/2013/905715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Duan L, Chen J, Xiong A, Xu Q, Zhang H, Zheng F, Tan Z, Gong F, Fang M. Gene silencing of NALP3 protects against liver ischemia-reperfusion injury in mice. Hum Gene Ther. 2011b;22(7):853–864. doi: 10.1089/hum.2010.145. [DOI] [PubMed] [Google Scholar]
- Zhu P, Xie L, Ding HS, Gong Q, Yang J, Yang L. High mobility group box 1 and kidney diseases (Review) Int J Mol Med. 2013b;31(4):763–768. doi: 10.3892/ijmm.2013.1286. [DOI] [PubMed] [Google Scholar]
- Zhu S, Ashok M, Li J, Li W, Yang H, Wang P, Tracey KJ, Sama AE, Wang H. Spermine protects mice against lethal sepsis partly by attenuating surrogate inflammatory markers. Mol Med. 2009;15(7–8):275–282. doi: 10.2119/molmed.2009.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Deng S, Ma Q, Zhang T, Jia C, Zhuo D, Yang F, Wei J, Wang L, Dykxhoorn DM, Hare JM, Goldschmidt-Clermont PJ, Dong C. MicroRNA-10A* and MicroRNA-21 modulate endothelial progenitor cell senescence via suppressing high-mobility group A2. Circ Res. 2013c;112(1):152–164. doi: 10.1161/CIRCRESAHA.112.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Li W, Li J, Jundoria A, Sama AE, Wang H. It Is Not Just Folklore: The Aqueous Extract of Mung Bean Coat Is Protective against Sepsis. Evid Based Complement Alternat Med. 2012a;2012:498467. doi: 10.1155/2012/498467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XD, Chen JS, Zhou F, Liu QC, Chen G, Zhang JM. Relationship between plasma high mobility group box-1 protein levels and clinical outcomes of aneurysmal subarachnoid hemorrhage. Journal of neuroinflammation. 2012b;9:194. doi: 10.1186/1742-2094-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicari A, Centonze C, Realacci M, Buchetti B, Pietropolli A, Ticconi C. Estradiol 17-beta and progesterone modulate inducible nitric oxide synthase and high mobility group box 1 expression in human endometrium. Reproductive sciences (Thousand Oaks, Calif.) 2008;15(6):559–566. doi: 10.1177/1933719107312560. [DOI] [PubMed] [Google Scholar]
- Zicari AM, Zicari A, Nebbioso M, Mari E, Celani C, Lollobrigida V, Marcelli AC, Occasi F, Duse M. High-mobility group box-1 (HMGB-1) and serum soluble receptor for advanced glycation end products (sRAGE) in children affected by vernal keratoconjunctivitis. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2013 doi: 10.1111/pai.12142. [DOI] [PubMed] [Google Scholar]
- Zickert A, Palmblad K, Sundelin B, Chavan S, Tracey KJ, Bruchfeld A, Gunnarsson I. Renal expression and serum levels of high mobility group box 1 protein in lupus nephritis. Arthritis Res Ther. 2012;14(1):R36. doi: 10.1186/ar3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J, Maher LJ., 3rd Transient HMGB protein interactions with B-DNA duplexes and complexes. Biochem Biophys Res Commun. 2008;371(1):79–84. doi: 10.1016/j.bbrc.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Volkel D, Pable S, Lindner T, Kramberger F, Bahrami S, Scheiflinger F. Native versus recombinant high-mobility group B1 proteins: functional activity in vitro. Inflammation. 2004;28(4):221–229. doi: 10.1023/b:ifla.0000049047.61014.e3. [DOI] [PubMed] [Google Scholar]
- Zlatanova J, Leuba SH, van Holde K. Chromatin structure revisited. Crit Rev Eukaryot Gene Expr. 1999;9(3–4):245–255. doi: 10.1615/critreveukargeneexpr.v9.i3-4.90. [DOI] [PubMed] [Google Scholar]
- Zlatanova J, Van Holde K. Histone H1 and transcription: still an enigma? J Cell Sci. 1992;103(Pt 4):889–895. doi: 10.1242/jcs.103.4.889. [DOI] [PubMed] [Google Scholar]
- Zlatanova J, van Holde K. Binding to four-way junction DNA: a common property of architectural proteins? Faseb J. 1998a;12(6):421–431. [PubMed] [Google Scholar]
- Zlatanova J, van Holde K. Linker histones versus HMG1/2: a struggle for dominance? Bioessays. 1998b;20(7):584–588. doi: 10.1002/(SICI)1521-1878(199807)20:7<584::AID-BIES10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Zong M, Bruton JD, Grundtman C, Yang H, Li JH, Alexanderson H, Palmblad K, Andersson U, Harris HE, Lundberg IE, Westerblad H. TLR4 as receptor for HMGB1 induced muscle dysfunction in myositis. Ann Rheum Dis. 2013;72(8):1390–1399. doi: 10.1136/annrheumdis-2012-202207. [DOI] [PubMed] [Google Scholar]
- Zong M, Jorholt J, Winter J, Lindroos E, Harris HE, Lundberg IE. A8.24 autophagy may contribute to glucocorticoid resistance in myositis patients by maitaining muscle T cells homeostasis. Ann Rheum Dis. 2014;73(Suppl 1):A85–A86. [Google Scholar]
- Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18(11):1272–1282. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20(1):1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- Zou J, Kawai T, Tsuchida T, Kozaki T, Tanaka H, Shin KS, Kumar H, Akira S. Poly IC triggers a cathepsin D- and IPS-1-dependent pathway to enhance cytokine production and mediate dendritic cell necroptosis. Immunity. 2013;38(4):717–728. doi: 10.1016/j.immuni.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Zuo XX, Zhou YO, Gong YH, Wang YP, Tang DL, Xiao XZ. Expression of high mobility group box chromosomal protein 1 in peripheral blood of patients with rheumatoid arthritis. Zhonghua nei ke za zhi [Chinese journal of internal medicine] 2007;46(7):547–550. [PubMed] [Google Scholar]
- Zurolo E, Iyer A, Maroso M, Carbonell C, Anink JJ, Ravizza T, Fluiter K, Spliet WG, van Rijen PC, Vezzani A, Aronica E. Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain. 2011;134(Pt 4):1015–1032. doi: 10.1093/brain/awr032. [DOI] [PubMed] [Google Scholar]