Abstract
The origins of schizophrenia have eluded clinicians and researchers since Kraepelin and Bleuler began documenting their findings. However, large clinical research efforts in recent decades have identified numerous genetic and environmental risk factors for schizophrenia. The combined data strongly support the neurodevelopmental hypothesis of schizophrenia and underscore the importance of the common converging effects of diverse insults. In this review, we discuss the evidence that genetic and environmental risk factors that predispose to schizophrenia disrupt the development and normal functioning of the GABAergic system.
INTRODUCTION
Schizophrenia is a devastating neuropsychiatric disorder that affects approximately 1% of the population. Its core symptoms fall into three domains: positive symptoms such as psychosis, negative symptoms such as poor social function, and cognitive symptoms such as deficient working memory and attention. Onset is typically in late adolescence or early adulthood, but signs of dysfunction can be seen in an earlier prodromal phase (Lewis and Levitt, 2002). Based on the clinical progression and the 64–81% heritability of the disorder (Giusti-Rodriguez and Sullivan, 2013), a hypothesis emerged that schizophrenia’s origins could be found early in development, long before the onset of symptoms (Weinberger, 1987). For more than 35 years, clinicians and scientists have searched for the biological foundations of an altered developmental trajectory that leads to the specific disease symptoms, but our understanding of this process is far from complete.
Accumulating evidence from clinical, genetic, and epidemiologic studies over the past several decades supports the neurodevelopmental origin of schizophrenia and has begun to identify specific disturbances of brain development that might be pivotal for the emergence of the disease (Lewis and Levitt, 2002; Marenco and Weinberger, 2000; Rapoport et al, 2012). The data have identified numerous factors that increase risk of diagnosis (to be discussed), yet it is clear that none of these pathological processes alone can be identified as a singular cause of the disorder.
The genetics of schizophrenia are extremely complex. Over the past two decades, genetic studies of candidate genes implicated multiple disease-predisposing DNA sequence variants in disrupted-in-schizophrenia 1 (DISC1), neuregulin 1 (NRG1), catechol-O-methyl transferase (COMT), regulator of G-protein signaling 4 (RGS4), metabotropic glutamate receptor 3 (GRM3), dysbindin (DTNBP1), G72, and other sequences (Harrison and Weinberger, 2005), yet replications of these findings were quite inconsistent from cohort to cohort. More recently, genome-wide association studies (GWAS) analyzing DNA from tens of thousands of patients with schizophrenia have been identified between one and ‘several thousands of common alleles of very small effect’ associated with diagnosis (Aberg et al, 2013; Cross-Disorder Group of the Psychiatric Genomics C, Genetic Risk Outcome of Psychosis C, 2013; McAllister, 2014; Purcell et al, 2009; Ripke et al, 2013; Shi et al, 2009; Stefansson et al, 2009), yet these findings showed only a modest overlap with the outcomes of the candidate gene studies. With the expansion of the patient cohorts and development of more sophisticated analytical approaches, the newest GWAS data argue that diverse common alleles accumulate within a pathway and reach a threshold for susceptibility that leads to disease (Horvath and Mirnics, 2014b). Furthermore, it also appears that rare copy number variants with potentially large effect sizes might have an important role in predisposing to schizophrenia. These deletions or duplications of chromosomal regions often span multiple genes and can either increase risk or protect from diagnosis, presumably by altering the ‘dosing’ of the genes contained within the variant region (Grozeva et al, 2010; International Schizophrenia C, 2008; Stefansson et al, 2008). For example, 22q11 hemideletion is associated with high rates of schizophrenia (Gothelf et al, 1997; Karayiorgou et al, 2010; Murphy et al, 1999), whereas 22q11 duplication may protect against schizophrenia diagnosis (Rees et al, 2014), suggesting that expression levels of specific genes are critical to normal and pathological brain development.
Epidemiologic studies similarly identified various environmental disturbances that confer increased brain disease risk, but do not singularly cause the disorder (Sullivan et al, 2003; Tsuang, 2000; van Os et al, 2010). There is evidence that prenatal maternal immune activation (MIA) (Brown and Derkits, 2010), perinatal hypoxia (Cannon et al, 2002; Schmidt-Kastner et al, 2012), adolescent cannabis use (Arseneault et al, 2004; Henquet et al, 2008), stress (Norman and Malla, 1993), obstetric complications (Dalman et al, 1999), urbanicity (Vassos et al, 2012), migrant status (Cantor-Graae and Selten, 2005), advanced paternal age (Malaspina, 2001), and others (Brown, 2011; Tandon et al, 2008) interact with predisposing genetics to increase risk for illness.
Therefore, genetic and environmental influences alone may confer risk for schizophrenia, but it appears that a combination of multiple factors is necessary for disease manifestation in most cases (Giusti-Rodriguez and Sullivan, 2013; Lewis and Levitt, 2002; Mowry and Gratten, 2013; Sullivan et al, 2003; Tsuang, 2000). Supported by expanded basic science efforts and increasingly sophisticated animal models, a unifying concept has emerged stating that many of these disparate risk factors converge onto common dysfunctional pathways and lead to illness (Chen et al, 2013; de Jong et al, 2012; Horvath and Mirnics, 2009, 2014b; Mirnics et al, 2006). In this review, we discuss the concept that GABA system development is a major convergence point for genetic and environmental susceptibility factors for schizophrenia.
CORTICAL DEVELOPMENT
Early in mammalian development, the telencephalon emerges from the anterior portion of the neural tube and develops into the cortex and hippocampus. The plethora of cell types in the adult brain arise from progenitor pools in distinct subregions of the developing brain that are established as early as the morphogenic patterning of the neural tube (Bystron et al, 2008; Marin and Rubenstein, 2003). Progenitors in the subventricular zone that produce projection neurons express diverse combinations of transcription factors that guide them from proliferation through differentiation into unique types of cells (Greig et al, 2013; Molyneaux et al, 2007). Similarly, GABAergic interneuron types are predetermined based on unique transcription factor combinations in different progenitor types in the subpallial ganglionic eminences (Flames et al, 2007; Wonders and Anderson, 2006). However, migration patterns of glutamatergic projection neurons and GABAergic interneurons are quite different: while projection neurons migrate radially and remain in the general area where they were born, interneurons have a predominantly tangential migration, and integrate into regions far away from their origins (Bystron et al, 2008; Guo and Anton, 2014; Marin and Rubenstein, 2003; Nadarajah and Parnavelas, 2002).
Cajal–Retzius cells are interneurons that are among the first-born cells in the developing cortex and hippocampus. At the beginning of cortical expansion, they migrate into the cortical preplate (Bielle et al, 2005) and coordinate the organization of radial glia and migrating projection neurons by secreting reelin (RELN) (Tissir and Goffinet, 2003). RELN signals new projection neurons migrating along radial glia to bypass earlier-born neurons and layer in an ‘inside-out’ manner (Hashimoto-Torii et al, 2008; Tissir and Goffinet, 2003). The radial glia, meanwhile, are maintained by NRG1/ErbB signaling and even transient disruptions of NRG1 signaling cause these cells to differentiate prematurely, which could have a profound impact on radial migration (Schmid et al, 2003). GABAergic interneurons destined for the cortex, however, migrate tangentially over much longer distances from the subpallium, avoid the striatum via semaphorin 3A- and 3F-mediated chemorepulsion (Marin et al, 2001b), and settle into final positions after the migration of projection cells is compete (Bartolini et al, 2013; Pla et al, 2006). NRG1/ErbB interactions are necessary for GABAergic cell migration as neuregulin acts as both a local and long-range attractant cue for migrating interneurons and dysfunction of either ligand or receptor leads to deficits in cell migration and cortical patterning (Flames et al, 2004). Once they reach the cortex, interneuron populations tend to cluster together in specific layers (Ciceri et al, 2013), likely via cell type-specific expression of cell adhesion molecules including ErbB4 (Fazzari et al, 2010). Thus, interneuron integration into cortical lamina appears to be a tightly regulated spatial–temporal process.
Neuronal differentiation, migration, and integration are managed by a number of well-studied molecular processes. Brain-derived neurotrophic factor (BDNF) and other neurotrophins promote cellular migration, synaptogenesis, dendritic extension, and synaptic maintenance throughout life (Behar et al, 1997; Huang and Reichardt, 2001; Marin and Rubenstein, 2001a; Park and Poo, 2013). Altering levels of BDNF in the developing brain can disrupt the migration of both projection neurons and interneurons (Knusel et al, 1994; Polleux et al, 2002; Woo and Lu, 2006) and their coordinated extension and retraction of dendrites (McAllister et al, 1997). In addition to establishing cortical circuits, these functions of BDNF continue to be critical when synapses are strengthened or pruned later in development (Gorski et al, 2003; Vicario-Abejon et al, 2002) at a critical time for susceptibility for psychiatric disease (Paus et al, 2008). Therefore, maintaining appropriate local levels of BDNF and other neurotrophins is critical at multiple points in development from neural progenitor stages to adolescent brain maturation.
RELN, NRG1/ErbB4, and BDNF are critical for the establishment of GABAergic circuitry. Defects in these pathways early in development could have cascading consequences due to GABA’s own role as a trophic factor during cortical development (Owens and Kriegstein, 2002).
DEVELOPMENT OF THE GABAergic SYSTEM
GABA is the major inhibitory neurotransmitter in the brain; however, GABAA receptor activation is excitatory before birth (Ben-Ari, 2002). Tonic GABA release early in development (Cellot and Cherubini, 2013; Manent et al, 2005) stimulates and guides the migration of new projection neurons in a receptor type-dependent manner (Owens and Kriegstein, 2002). GABAA receptors are expressed on neural progenitors in the proliferative zone where GABA signaling promotes cell cycle exit and migration (LoTurco et al, 1995). GABAB and GABAC stimulation maintains migration through the cortical plate (Behar et al, 2001), whereas additional GABAA activation provides a stop signal (Behar et al, 2000). This entire process is highly orchestrated by dynamic expression of receptors during migration (Maric et al, 2001). Once in place, continued GABAA stimulation signals new projection neurons to extend processes (Barbin et al, 1993; Marty et al, 1996) and integrate into developing circuitry through new synaptic contacts (Wang and Kriegstein, 2008) while also regulating the maturation of inhibitory contacts (Wu et al, 2012). Increasing potassium chloride cotransporter 2 (KCC2) expression around the time of birth switches the chloride gradient from depolarizing to hyperpolarizing; however, there is evidence that local chloride gradients may be different at certain interneuron synapses and in disease states (Arion and Lewis, 2011; Hyde et al, 2011). These processes continue to guide migration and integration in the dentate gyrus of the hippocampus where neurogenesis continues into adulthood (Ge et al, 2006).
The final product of development is a diverse population of interneurons, each serving a different function. Mature interneurons can be distinguished by their molecular content, electrical properties, synaptic targets and laminar distributions (Ascoli et al, 2008; DeFelipe et al, 2013; Markram et al, 2004). Interneuron cell types typically contain either the calcium binding proteins, such as parvalbumin (PV), calretinin (CR), or calbindin (CB), or the neuropeptides, such as cholecystokinin (CCK), neuropeptide Y (NPY), somatostatin (SST), or vasointestinal peptide (VIP), but occasionally contain more than one of these markers (Ascoli et al, 2008; Markram et al, 2004). PV+ cells are fast-spiking basket and chandelier cells that innervate pyramidal cell soma and axon initial segments, respectively (Markram et al, 2004), as well as other interneuron populations (Lovett-Barron et al, 2012). Another type of basket cells, containing CCK, also form perisomatic contacts onto pyramidal cells; however, they are distinguished from the PV+ variety by their slower, accommodating firing patterns (Hefft and Jonas, 2005) that integrate neuromodulatory information with faster network activity (Freund, 2003; Varga et al, 2009). Interconnected networks of NPY+ neurogliaform cells mediate regional tonic inhibition through extrasynaptic volume transmission in multiple cortical and subcortical regions (Manko et al, 2012; Olah et al, 2009; Price et al, 2005). Martinotti cells, containing CR, CB, SST, NPY, and/or CCK, span both cortical lamina and cortical columns and synapse onto pyramidal cell tuft dendrites in layer I (Markram et al, 2004). Bipolar and double bouquet cells are found in multiple cortical lamina and primarily synapse onto pyramidal cell dendrites and other interneurons and express some combination of CCK, CR, CB, or VIP, whereas bitufted cells are similar in their synaptic contacts and function, but can also express NPY or SST (Markram et al, 2004). A subset of these cells expressing VIP are particularly interesting because they regulate PV+ and SST+ interneurons to disinhibit cortical circuits (Pi et al, 2013). While these are common generalized examples, interneuron cell types are, in fact, so diverse that they can be further subdivided using numerous features (Ascoli et al, 2008). For example, 21 types of interneurons regulate the function of only three types of glutamatergic cells in the hippocampus (Klausberger and Somogyi, 2008). This diversity and integration within networks highlights the importance of coordinated interneuron development and function in multiple brain regions.
Brain development requires precise coordination and timing of many contributing molecular systems. Any disruption could offset, alter, or cease these coordinated processes with immediate, delayed, or cascading consequences on brain function and alter the trajectory of development (Lewis and Levitt, 2002). Depending on the timing of the insult, common disruptions could have transient effects or marked and compounding consequences that lead to chronic disability (Horvath and Mirnics, 2014a; Insel, 2010; Lewis and Levitt, 2002). Furthermore, the individual genetic makeup appears to be critical: the same insult can have minimal effect or a large effect in two different individuals, and this will largely depend on the disease-predisposing sequence variants in their genome (Horvath and Mirnics, 2014b).
ANATOMICAL AND HISTOLOGICAL FINDINGS SUGGEST NEURODEVELOPMENTAL DYSFUNCTION OF CELL MIGRATION AND SYNAPTIC INTEGRATION IN SCHIZOPHRENIA
In addition to the clinical progression of symptoms across adolescent and adult development (Insel, 2010; Lewis and Lieberman, 2000), clues connecting neurodevelopmental dysfunction with schizophrenia can be found in post-mortem brain tissue from patients with the disorder. Perhaps, the best documented anatomical findings are reduced cortical thickness and enlarged ventricles (Harrison, 1999). However, the lack of degenerative pathology suggests that these findings are due to abnormalities in cellular migration and/or neuronal arborization and synaptic function (Folsom and Fatemi, 2013; Harrison, 1999; Marenco et al, 2000; Selemon and Goldman-Rakic, 1999). Several cellular and molecular findings support this view and provide evidence for neurodevelopmental abnormalities long before the onset of illness.
Differences in cellular distribution are often attributed to altered neuronal migration early in brain development (Metin et al, 2008). Altered neuronal densities were reported in the prefrontal cortex (Akbarian et al, 1996; Anderson et al, 1996; Connor et al, 2009; Daviss and Lewis, 1995; Ikeda et al, 2004; Joshi et al, 2012; Morris et al, 2008; Rajkowska et al, 1998; Selemon et al, 1995, 1998, 2003; Yang et al, 2011), auditory cortex (Dorph-Petersen et al, 2009), cingulate cortex (Benes, 1991, 1993; Brune et al, 2010; Connor et al, 2009), entorhinal cortex (Arnold et al, 1991; Falkai et al, 2000; Jakob and Beckmann, 1986; Kovalenko et al, 2003; Wang et al, 2011), fusiform cortex (Di Rosa et al, 2009), occipital cortex (Selemon et al, 1995), parietal cortex (Chance et al, 2005), visual cortex (Dorph-Petersen et al, 2007), thalamus (Young et al, 2000), hypothalamus (Bernstein et al, 1998), striatum (Kreczmanski et al, 2007), amygdala (Kreczmanski et al, 2007), and hippocampus (Konradi et al, 2011) in post-mortem brain tissue from schizophrenic patients. Interestingly, many of these reports show specific defects in GABAergic interneuron density or distribution (Benes et al, 1991; Chance et al, 2005; Daviss and Lewis, 1995; Di Rosa et al, 2009; Ikeda et al, 2004; Joshi et al, 2012; Konradi et al, 2011; Morris et al, 2008; Wang et al, 2011; Yang et al, 2011). While the majority of studies report decreased cell densities, others argue the opposite or find no change (Beasley et al, 2009; Cullen et al, 2006; Heckers et al, 1991; Pennington et al, 2008; Smiley et al, 2012).
Interstitial white matter neurons (IWMNs) have also been particularly well studied. IWMNs are neurons in white matter tracts that remain from the early cortical subplate zone (Chun and Shatz, 1989) or GABAergic interneurons from the ganglionic eminences (Anderson et al, 2001). The density of these cells typically declines during development as migration is completed and the subplate disappears (Connor et al, 2009; Kostovic and Rakic, 1990; Meyer et al, 1992). Several studies have reported changes in the distribution of superficial and/or deep IWMNs in the cortices of subjects with schizophrenia. However, like the cell density studies, these data do not form a consensus. Some studies report increased density in the superficial white matter (Anderson et al, 1996; Connor et al, 2009; Eastwood and Harrison, 2005; Joshi et al, 2012; Kirkpatrick et al, 1999, 2003; Yang et al, 2011), whereas others report decreased density in superficial, but increased or variable density in deep white matter (Akbarian et al, 1993a, 1993b, 1996). Variation in patient populations, brain regions, or methodologies (such as particular molecular markers used to identify cells) may account for these discrepancies (Connor et al, 2009; Eastwood and Harrison, 2005; Harrison, 1999; Heckers, 1997; Meyer et al, 1992). Regardless of the reported differences, the most likely explanation for the displacement of IWMNs is that cellular migration of GABAergic interneurons and/or their cell death are disrupted very early in development.
In summary, the anatomical and histological findings in schizophrenia suggest that altered cellular migration and synaptic formation are an important part of the disease process, and that GABA system-associated genes are particularly affected in this cascade of deleterious events. This view is also supported by molecular studies in post-mortem tissue from patients with schizophrenia and mechanistic studies in animal models (to be discussed).
GENE EFFECTS CONVERGE ONTO GABA SYSTEM DEVELOPMENT
In addition to cellular evidence, changes in the expression of genes with known importance for developmental processes—including cellular migration, synaptogenesis, synaptic maintenance, cell signaling, glia, immune regulation, and mitochondrial function—have been found in post-mortem tissue from patients with schizophrenia (Arion et al, 2007, 2010; Clay et al, 2010; Hakak et al, 2001; Harrison and Weinberger, 2005; Horvath and Mirnics, 2014a, 2014b; Jaaro-Peled et al, 2009; Lewis et al, 2005; McGlashan and Hoffman, 2000; Middleton et al, 2002; Mirnics et al, 2000, 2001b; Mirnics and Pevsner, 2004; Roussos et al, 2012). Importantly, multiple studies report expression changes in GABA system-related transcripts, including altered expression of GABA-synthesizing enzymes, glutamic acid decarboxylase 1 and 2 (GAD1 and GAD2, discussed in the next section), interneuron-expressed proteins and neuropeptide genes (PV, CCK, NPY, SST, and CB) (Hashimoto et al, 2003, 2008a; Hoftman et al, 2013; Iritani et al, 2000; Kuromitsu et al, 2001; Maldonado-Aviles et al, 2009; Mellios et al, 2009; Volk et al, 2012), GABA receptor subunits (GABRA1–2, GABRA4–6, and GABRD) (Benes et al, 1992; Hashimoto et al, 2008a, 2008b; Hoftman et al, 2013; Maldonado-Aviles et al, 2009; Volk et al, 2002b), and interneuron development- and maintenance-related mRNAs (GABA transporter 1, sodium potassium chloride cotransporter 1 (NKCC1), and KCC2) (Arion et al, 2011; Fish et al, 2011; Hashimoto et al, 2008a, 2008b; Hoftman et al, 2013; Hyde et al, 2011; Volk et al, 2002b). Of these, the current review will focus primarily on the GAD1 deficit and its relationships with RELN, BDNF, NRG1, and DISC1.
Deficiencies in GAD1 expression, the enzyme responsible for producing the majority of the GABA in the brain, are commonly found in many brain regions in post-mortem tissue from patients with schizophrenia (Akbarian and Huang, 2006; Akbarian et al, 1995; Costa et al, 2004; Curley et al, 2011; Fatemi et al, 2005; Guidotti et al, 2000a; Hashimoto et al, 2003, 2008a, 2008b; Huang and Akbarian, 2007; Impagnatiello et al, 1998; Kalkman and Loetscher, 2003; Knable et al, 2002; Lewis et al, 2005; Mirnics et al, 2000; Thompson Ray et al, 2011; Volk et al, 2000; Volk and Lewis, 2002a). Interestingly, GAD1 mRNA was not detectable in approximately 30% of GABAergic interneurons in the cortex of post-mortem brains from individuals with schizophrenia (Akbarian et al, 1995; Volk et al, 2000), whereas cells with detectable GAD1 appeared to have normal levels (Volk et al, 2000), suggesting dysregulation of GABAergic gene expression is cell type-specific. However, this does not mean that the majority of interneurons are unaffected by the disease process. Reductions of interneuronal-expressed genes NPY, SST, CCK, and PV have been found repeatedly in the cortex of subjects with schizophrenia in post-mortem studies (Hashimoto et al, 2003, 2008a; Hoftman et al, 2013; Ikeda et al, 2004; Iritani et al, 2000; Kuromitsu et al, 2001; Maldonado-Aviles et al, 2009; Mellios et al, 2009; Volk et al, 2012). Deleting GAD1 in animal models causes catastrophic effects on development by almost completely reducing brain GABA content and is not compatible with life (Asada et al, 1997). However, GAD1 suppression in limited periods of development or in restricted cell types has multiple consequences. Disrupting GABA signaling during early development alters cellular migration and cortical architecture in cell type-dependent ways (Aronne et al, 2011; Cuzon et al, 2008; Haas et al, 2013; Manent et al, 2007; Thompson et al, 2009; Wu et al, 2012). PV+ interneurons are selectively disrupted by exogenous GABA potentiation (Haas et al, 2013; Levav-Rabkin et al, 2010). During adolescence, when cells have finished migrating and cortical circuits are maturing, GAD1 suppression decreases axonal branching in PV+ cells in a cell autonomous manner (Chattopadhyaya et al, 2007) and increases pyramidal cell activity (Lazarus et al, 2013). Adult mice with GAD1 gene expression deficits in restricted interneuron populations have distinct molecular and behavioral dysfunction depending on the affected cell type (Brown et al, 2013; Kvitsiani et al, 2013; Schmidt et al, 2013). These data provide functional context to post-mortem studies that consistently implicate diverse interneuron cell types in schizophrenia (Hashimoto et al, 2003, 2008a; Hoftman et al, 2013; Iritani et al, 2000; Kuromitsu et al, 2001; Maldonado-Aviles et al, 2009; Mellios et al, 2009; Morris et al, 2008; Volk et al, 2012) and suggest that GABAergic gene expression deficits seen in post-mortem studies of patients with schizophrenia actively contribute to important aspects of brain development and behavior (Lewis et al, 2005; Marin, 2012; Schmidt and Mirnics, 2012).
As mentioned previously, RELN is critical for the migration and laminar organization of the cortex and hippocampus. RELN is expressed in Cajal–Retzius cells during early development and from many GABAergic cells in multiple cortical layers shortly after birth (Alcantara et al, 2006). Brain tissue from schizophrenic patients also consistently report decreased expression of the RELN gene (Eastwood and Harrison, 2006; Fatemi et al, 2000, 2001; Folsom and Fatemi, 2013; Guidotti et al, 2000a; Habl et al, 2012; Impagnatiello et al, 1998; Maloku et al, 2010; Ruzicka et al, 2007), which is likely the result of altered genetic and/or epigenetic regulation (Costa et al, 2003; Grayson et al, 2005, 2006; Tochigi et al, 2008; Veldic et al, 2004, 2007). While the RELN deficiency observed in post-mortem tissue clearly does not impact cortical architecture to the same degree as total RELN loss during cortical development, it is likely that even a small reduction of RELN would affect synaptic integration during development and/or synaptic stability and plasticity in adulthood (Frotscher, 2010). It is also likely that the ontogeny of this deficit varies from patient-to-patient. RELN was initially discovered as a mutation affecting cortical development and behavior in reeler mice (reviewed by (Folsom and Fatemi, 2013; Lambert de Rouvroit and Goffinet, 1998; Tissir and Goffinet, 2003) and has been studied extensively in other systems and clinical populations. In addition to being a necessary component of cortical development, RELN also has a role in stabilizing neurons and synapses throughout life (Abraham and Meyer, 2003; Frotscher, 2010; Guidotti et al, 2000b). It is expressed by GABAergic interneurons and the expression of the GAD1 and RELN genes is tightly coordinated by a common epigenetic mechanism (Costa et al, 2004; Grayson et al, 2005, 2006; Guidotti et al, 2000a; Impagnatiello et al, 1998; Kundakovic et al, 2009; Maloku et al, 2010; Noh et al, 2005; Pesold et al, 1999; Rodriguez et al, 2002; Ruzicka et al, 2007; Tochigi et al, 2008; Veldic et al, 2004, 2007). In addition, rodent models show that RELN deficiency alone can result in downstream reductions of both GAD1 (Kutiyanawalla et al, 2012; Nullmeier et al, 2011; Pascual et al, 2004; Takayama, 1994) and BDNF (Pillai and Mahadik, 2008). Thus, it appears that RELN and GABAergic deficits in schizophrenia are tightly linked.
A similar decrease in BDNF has been observed consistently in several studies (Hashimoto et al, 2005; Mellios et al, 2009; Thompson Ray et al, 2011; Toyooka et al, 2002; Weickert et al, 2003). Genetic variants of the BDNF gene associated with schizophrenia (Neves-Pereira et al, 2005) produce progressive cortical and hippocampal structural changes, as well as behavioral impairment (Egan et al, 2003a; Pezawas et al, 2004). A genetic variant of BDNF associated with increased risk for psychiatric disorders including schizophrenia (Egan et al, 2003b; Gratacos et al, 2007) is linked to reduced cortical and hippocampal volumes and impaired learning and memory, presumably by interfering with the development and maintenance of neurons and synapses (Egan et al, 2003b; Eisenberg et al, 2013; Hariri et al, 2003; Pezawas et al, 2004; Szeszko et al, 2005; Tost et al, 2013). However, these findings are not always replicated and more studies are needed to clarify the mechanisms of the Val66Met allele and psychiatric illness (Kanazawa et al, 2007). Animal studies show that BDNF is also vital for developing GABAergic circuitry, controlling everything from interneuron migration to establishing synaptic contacts (Danglot et al, 2006; Ikeda et al, 2006; Ohba et al, 2005; Yamada et al, 2002), positioning and activating RELN-secreting Cajal–Retzius cells (Alcantara et al, 2006; Ringstedt et al, 1998), regulating GABA release probability (Ohba et al, 2005), and expressing GAD1 (Huang et al, 1999; Ohba et al, 2005; Yamada et al, 2002). Conversely, GABA regulates BDNF through activity-dependent processes that switch from inducing to inhibiting BDNF gene expression around the same time GABA signaling switches from excitatory to inhibitory (Berninger et al, 1995). PV-expressing (PV+) interneurons were hypothesized to be the main target of BDNF-dependent processes (Hashimoto et al, 2005; Lewis et al, 2005) because PV+ interneurons express the BDNF receptor TrkB (Cellerino et al, 1996); however, the differentiation of NPY+ interneurons in vitro is also BDNF-dependent (Marty et al, 1996) and in vivo rodent studies demonstrate that BDNF is necessary for the expression of NPY and SST in the absence of any changes in PV, GAD1, or GAD2 (Glorioso et al, 2006). These results closely mirror post-mortem studies of schizophrenia that show tight correlations between NPY, SST, and BDNF gene expression (Hashimoto et al, 2008b; Mellios et al, 2009). It is possible, based on the GABAergic regulation of BDNF, that the developmental time points of in vitro and in vivo measurements in model systems could affect the interpretation of these and other results due to the changing influence of GABA signaling on activity-dependent processes across development. However, this prospect also highlights the very interesting possibility that risk factors for schizophrenia have different and even opposing consequences depending on the specific timing of the insult. Regardless, it is clear that BDNF and GABA systems interact extensively and deficits in either system may affect the other to a large degree, particularly during development.
NRG1 and its receptor ErbB4 have both been implicated in genetic susceptibility for schizophrenia in candidate gene studies (Harrison and Law, 2006; Mei and Xiong, 2008; Rico and Marin, 2011; Stefansson et al, 2004). NRG1 mRNA is increased in the brains of schizophrenic patients along with its receptor ErbB4 (Chong et al, 2008; Harrison and Law, 2006; Hashimoto et al, 2004; Law et al, 2006, 2007) along with increased NRG1 protein intracellular domain (Chong et al, 2008) but decreased C-terminal fragment (Barakat et al, 2010), indicating abnormal proteolytic cleavage and dysfunctional NRG1 signaling. Animal studies have elaborated the importance of NRG1/ErbB in GABAergic interneuron migration and provided support for translatability of the findings. ErbB4 shows conserved interneuron-specific expression in mice, rats, monkeys, and humans (Neddens et al, 2011) and NRG1/ErbB4 signaling is necessary for the development of inhibitory circuits (Del Pino et al, 2013; Fazzari et al, 2010). Neddens et al (2011) also showed that ErbB4 expression was restricted to cells that express interneuron subclass markers PV, CCK, or CR, but not those that express CB, which is particularly interesting since CB interneurons are also those that appear to be unaffected in schizophrenia (Lewis et al, 2005). NRG1/ErbB4 signaling also appears to have distinct functions in development and maintenance of cortical circuitry. ErbB4 associates with GABAAα1 subunit-containing GABA receptors expressed on interneurons and NRG1/ErbB4 signaling reduces their surface expression (Mitchell et al, 2013), which likely contributes to increased excitability of interneurons by NRG1 (Li et al, 2012) and partially explains the mechanism behind increased GABA release and decreased pyramidal cell activity after NRG1 application (Wen et al, 2010). Fast-spiking PV+ interneurons are necessary for the generation of gamma oscillations and it is possible that these pathways underlie deficient oscillatory activity in schizophrenia (Hou et al, 2013; Lewis et al, 2012; Uhlhaas and Singer, 2010). However, ERBB4 deletion in mice can lead to either impaired or increased gamma oscillations depending on the timing of the deletion. Genomic ERBB4 deletion was accompanied by a ∼30% reduction in the number of PV+ interneurons and lead to decreased oscillatory activity (Fisahn et al, 2009), whereas conditional deletion in postmitotic interneurons, albeit with residual expression due to low receptor turnover, displayed normal PV+ cell numbers and lead to increased oscillatory power (Del Pino et al, 2013), suggesting that reduced gamma oscillations in schizophrenia might arise from insults very early in development. Despite the evidence in favor of a common NRG1/ErbB4 signaling/PV+ interneuron dysfunction phenotype, restricting ERBB4 deletion to PV+ interneurons did not account for all of the NRG1/ErbB4-associated behavioral abnormalities due to the presence of a large number of NRG1+/PV− cells in the amygdala (Shamir et al, 2012). This contrast is a quintessential example of how similar genetic insults lead to divergent phenotypes depending on their developmental timing and cell type-specific expression, as well as brain region-specific differences, and highlights the importance of GABAergic cell type-specific effects of genetic manipulations.
DISC1 was identified as a schizophrenia susceptibility gene in a pedigree of a Scottish family carrying a translocation that was associated with major mental illness (Muir et al, 2008; St Clair et al, 1990). Subsequent genetic and biological research has clarified the function of DISC1 and its importance in development. DISC1 associates with proteins that regulate microtubules and is necessary for normal cell migration and neurite outgrowth (Brandon and Sawa, 2011; Kamiya et al, 2005). It is also important for synaptic integration in the dentate gyrus in adulthood as DISC1 knockdown produces abnormalities in neuronal positioning and synaptic contacts (Duan et al, 2007). These findings support the role of DISC1 in developing synaptic connections in the cortex and hippocampus in schizophrenia, which have been elaborated in mice (Jaaro-Peled, 2009). Of particular interest for this review, activity-dependent GABAergic stimulation during early cortical development and during adult neurogenesis in the hippocampus is critical for the DISC1-dependent regulation of neurite outgrowth and synaptic integration (Duan et al, 2007; Kim et al, 2012). This interaction between DISC1 and GABAergic systems is thought to underlie alterations in cortical volumes (Brauns et al, 2011; Duff et al, 2013; Mata et al, 2010; Trost et al, 2013) and hippocampal function (Callicott et al, 2013) in patients with DISC1 risk alleles. Furthermore, the codependence of DISC1 and GABA in this period of development represents a point of convergence with other risk factors including NRG1 (Mata et al, 2010; Wood et al, 2009) and environmental exposures. Importantly, a dominant-negative DISC1 mutation had differential effects on the brain and behavior depending on the specific timing of its expression during development (Ayhan et al, 2011), which reinforces the importance of the timing of developmental insults.
While extremely informative and irreplaceable, post-mortem research cannot determine if, when, or how specific gene expression deficits, environmental insults, or gene × environment interactions (G × E) incite their principal and cascading effects. Yet, the question of the developmental pathophysiological cascade is critical for understanding the disease: diverse genetic predispositions and various environmental insults, when combined, give rise to a set of common phenotypic manifestations that we classify as schizophrenia. Thus, understanding the convergence process that leads from etiological diversity to phenotypic similarity must be pursued through various in vitro and in vivo animal models, which has a potential for direct furthering clinical research and drug discovery (Harrison and Weinberger, 2005; Horvath et al, 2011; Horvath and Mirnics, 2009, 2014a; Levitt et al, 2006; Lewis and Mirnics, 2006; Mirnics et al, 2001b, 2006).
ENVIRONMENTAL INSULTS DISRUPT GABAergic SYSTEM DEVELOPMENT
The combination of anatomical, histological, and molecular findings in post-mortem tissue of subjects with schizophrenia is consistent with early neurodevelopmental disturbances. Importantly, gene expression is one of the initial points of interaction between genes and environment: cell signaling pathways initiated by environmental events appear to converge on transcriptional regulators to induce or inhibit the expression of specific genes (Harrison and Weinberger, 2005; Horvath et al, 2011; Horvath and Mirnics, 2009, 2014a, 2014b; Levitt et al, 2006; Lewis and Mirnics, 2006; Mirnics et al, 2001b, 2006). Therefore, while gene expression changes can indicate either genetic or environmental disruptions, in the context of schizophrenia they likely represent a sum of G × E interactions. As mentioned previously, genetic susceptibility alone cannot account for the risk of schizophrenia diagnosis. The cumulative and interactive effects of genetic and environmental factors represent the remainder of the risk. Environmental factors exert their influences directly by affecting specific cellular processes (eg, toxins, fast cell signaling events, etc) or indirectly by manipulating the expression of genes (eg, hormones, drugs, immune system activation, modulatory cell signaling events, etc). The interaction between genetics and environment, through which a genetic predisposition is revealed, can explain how individuals with identical genetic makeup (ie, monozygotic twins) differ in subtle aspects of their appearance or personality, and in some cases in drastic aspects of their physical and mental health. For example, concordance rate for schizophrenia diagnosis in monozygotic twins is only about 50% (Cardno and Gottesman, 2000), suggesting that the remainder of risk for psychosis is attributable to other factors including environmental exposures. Animal models have been used to determine the mechanisms behind these environmental insults, G × E interactions, and brain development.
As mentioned previously, prenatal MIA, stress, cannabis use, and others have been established as environmental risk factors for schizophrenia (van Os et al, 2010). However, determining causality can be difficult because of the protracted amount of time between insult and diagnosis (Lewis and Levitt, 2002). Immune system activation has been implicated as a risk factor for schizophrenia (Horvath and Mirnics, 2014a) and the major histocompatibility complex is the most prominent signal in GWAS studies (McAllister, 2014; Stefansson et al, 2009). MIA in rats and mice causes dysfunction of GABAergic circuitry in the hippocampus, amygdala, and cortex (Canetta and Brown, 2012; Meyer, 2014). GABA content decreases (Bitanihirwe et al, 2010), GAD1 gene expression decreases (Deslauriers et al, 2013; Richetto et al, 2013), and GABA receptor subunit expression increases (Nyffeler et al, 2006) following immune activation during prenatal development. These effects appear to affect specifically PV+ interneurons (Ducharme et al, 2012; Ibi et al, 2010; Piontkewitz et al, 2012), although effects on other interneuronal cell types cannot be excluded. Rodent models have pinpointed interleukin-6 (IL-6) as the critical factor leading to molecular and behavioral dysfunction (Garbett et al, 2012; Smith et al, 2007), suggesting that modulating the IL-6 pathway for therapeutic development may be beneficial. Interestingly, a schizophrenia-associated missense mutation in the NRG1 gene leads to increased IL-6 gene expression and protein secretion in humans (Marballi et al, 2010). Furthermore, interferon-induced transmembrane protein 3 (IFITM3) expression in astroglia appears to be involved in mediating this MIA-IL-6 response (Ibi et al, 2013), which is interesting considering IFITM3 is increased in schizophrenia and negatively correlated with GABAergic gene expression (Horvath and Mirnics, 2014a; Siegel et al, 2013). Furthermore, the delayed molecular and behavioral effects of MIA in adulthood can be revealed in at-risk genotypes as mice with mutant forms of DISC1 display additional phenotypes after in utero exposure to polyinosinic:polycytidylic acid (poly I:C), a double-stranded RNA viral mimetic and cytokine inducer (Abazyan et al, 2010; Ibi et al, 2010; Lipina et al, 2013). The immune system, GABAergic systems, and schizophrenia risk genes may be an important point of G × E interaction in schizophrenia. This concept is further supported by the previously mentioned influences of NRG1/ErbB4 and immune activation on the migration/function of PV+ interneurons and the interaction between DISC1 and GABA on neuronal migration and synaptic formation.
Stress is also an important risk factor for schizophrenia (Weinberger, 1987) and animal models provide evidence that it potentiates the effects of other disease-predisposing factors. Stress during adulthood compounds the effects of in utero MIA exposure on GABAergic gene expression, including decreased expression of GAD1, and leads to dysfunctional behavior (Deslauriers et al, 2013; Giovanoli et al, 2013). Chronic social stress interacts with NRG1 deficiency to change inflammatory cytokine and BDNF gene expression (Desbonnet et al, 2012). Early life stress (Roth et al, 2009) or chronic social defeat stress (Tsankova et al, 2006) increases persistent methylation and decreases BDNF gene expression in rodents. While this result mirrors the decreased BDNF expression seen in patients, the Val66Met variant associated with psychosis is accompanied by less BDNF methylation in the PFC (Mill et al, 2008). Owing to the brain region- and promoter-specific nature of these effects (Wong et al, 2010), and differences in rodent and human neuroanatomy, more evidence will be required to understand the interaction between Val66Met, BDNF epigenetics, stress, and psychosis (Boulle et al, 2011). Chronic stress also interacts with the cannabinoid system to sensitize the effects of cannabinoids and shift cannabinoid-mediated control of plasticity from projection neurons to GABAergic cells (Patel et al, 2009; Reich et al, 2013). CCK+ interneurons are the only interneuron cell type that expresses the cannabinoid receptor CNR1 (Eggan et al, 2010), which silences CCK+ interneurons (Losonczy et al, 2004) and disrupts the hippocampus (Hajos et al, 2000; Katona et al, 1999) and amygdala (Katona et al, 2001; Tan et al, 2010) function. Furthermore, GAD1 suppression in CCK+/CNR1+ interneurons leads to dysfunctional amygdala-dependent behavior and aminergic signaling (Brown et al, 2013; Schmidt et al, 2013). Finally, mild stress during development results in epigenetic-mediated reduction of dopaminergic cell function in DISC1 mutant mice (Niwa et al, 2013), whereas the loss of DISC1 in the frontal cortex of adult rats increased stress sensitivity and resulted in cognitive impairments that were not observed in rats with normal DISC1 expression (Gamo et al, 2013). These results establish an important G × E interaction between DISC1 and stress and reinforce the importance of developmental timing of this interaction.
In this manner, animal models provide the opportunity for linking data and understanding dynamically and reciprocally regulated functional and molecular networks. It is likely that immune activation, stress, and/or repeated cannabis exposure interact with genetic and/or molecular dysfunction, including GABA system genes, NRG1, DISC1, and others, to impair GABAergic circuitry to a greater degree than any aspect alone and lead to behavioral abnormalities.
FUTURE RESEARCH DIRECTIONS
Since the initial description of the neurodevelopmental hypothesis of schizophrenia, data from epidemiological, clinical, post-mortem, and animal model studies continue to support and extend its premise (Brandon and Sawa, 2011; Brown, 2011; Horvath and Mirnics, 2014a, 2014b; Lewis and Levitt, 2002; Lewis and Mirnics, 2006; Michel et al, 2012; Mirnics et al, 2000, 2001b, 2006; Mirnics and Lewis, 2001a; Rapoport et al, 2012; Schmidt and Mirnics, 2012). Concurrently, GABAergic dysfunction has become recognized as a hallmark feature of the disorder. The number of studies reporting GABAergic dysfunction in the post-mortem brain of subjects with schizophrenia and the percentage of patients with GAD1 deficits in these studies far surpasses the accountability of GAD1 genetic variation. Rather, human data and animal models strongly argue that environmental insults, especially through immune system changes, converge with genetic susceptibility to alter GABAergic development and function, and contribute to behavioral impairment. However several questions remain unanswered and warrant further study.
First, GAD1, RELN, BDNF, NRG1, DISC1, and other genes with important developmental functions are regulated by activity-dependent processes, and this makes the expression changes found in post-mortem tissue from patients challenging to interpret. These alterations are either caused by specific disruptions of signaling events and transcriptional processes specific for each gene (Horvath and Mirnics, 2014b; Mirnics et al, 2001b) or are possibly adaptations to generic decreases in synaptic activity. As GABA is excitatory early in development and inhibitory after birth, changes in GABA system function should have opposing consequences on activity-dependent gene expression depending on when they occur. Recent studies showing that NKCC1 and KCC2, the ion transporters responsible for the excitatory/inhibitory switch, are dysregulated in schizophrenia, further complicating the possible interpretational framework (Arion et al, 2011; Hyde et al, 2011; Tao et al, 2012). More studies are needed to determine how the specific developmental timing of environmental insults interact with genetic susceptibility, and we need a better understanding how altered chloride transporter expression in the adult brain impacts schizophrenia-relevant behaviors.
Second, the GABA system is incredibly diverse, making it difficult to determine what are the effects of specific GABAergic system deficits at the level of synaptic circuitry (Ascoli et al, 2008; DeFelipe et al, 2013). It appears that schizophrenia is characterized by dysfunction of multiple interneuronal cell types (Lewis et al, 2005). These deficits presumably interact at a level of neuronal networks, giving rise to complex behavioral phenotypes, yet we study them in isolation. For example, PV+ chandelier cells regulate the output of cortical pyramidal cells across multiple areas (Markram et al, 2004; Woodruff et al, 2010). Their ‘fast-spiking’ activity is determined by their glutamatergic innervation and by expression of P/Q-type voltage-gated calcium channels, which cluster at synaptic active zones and support rapid vesicular release (Hefft and Jonas, 2005). However, this makes it difficult to determine whether PV+ interneurons are inherently dysfunctional in the pathophysiology of schizophrenia or whether activity-dependent deficits driven by the glutamatergic system are a more proximal disturbance. Since PV+ interneurons receive dense glutamatergic projections (Hefft and Jonas, 2005), glutamate system dysregulation in schizophrenia (Javitt, 2012) could preferentially target them even in the absence of primary GABAergic disturbances. The issue is also complicated by evidence that at least some PV+ GABAergic synapses may actually be excitatory owing to atypical local chloride gradients at axon initial segments (Woodruff et al, 2010). Furthermore, the activity of PV+ cells is modulated by the inhibitory action of nearby CCK+ GABAergic interneurons (Karson et al, 2009). Therefore, GAD1 suppression in CCK+ interneurons could actually result in a net increase of inhibitory tone at the circuit level by disinhibiting PV+ cells (Freund and Katona, 2007), suggesting that disturbances in multiple interneuronal sub-populations might have complex, and often unexpected behavioral consequences.
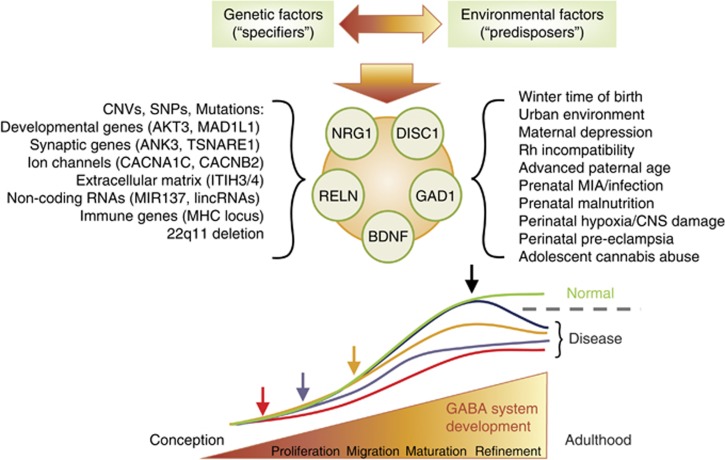
Third, GABAergic dysfunction is not unique to schizophrenia. Nearly all neuropsychiatric disorders include dysfunctional GABA system components: schizophrenia (Hashimoto et al, 2008a), bipolar disorder (Fatemi et al, 2013; Guidotti et al, 2000a), anxiety (Mohler, 2012), depression (Gao et al, 2013; Thompson Ray et al, 2011), panic disorder (Malizia et al, 1998), posttraumatic stress disorder (Geuze et al, 2008), attention deficit hyperactivity disorder (Edden et al, 2012), autism (Fatemi et al, 2010, 2002), Rett syndrome (Blue et al, 1999; Chao et al, 2010), epilepsy (Kang and Macdonald, 2009), and others (Marin, 2012). There is also some overlap in environmental risk factors such as immune system activation during development in schizophrenia and autism (Michel et al, 2012; Patterson, 2009). This raises three intriguing possibilities. First, common insults like MIA might lead to multiple divergent phenotypes depending on the specific timing of the insult during development (Lewis and Levitt, 2002). No where is this potential more evident than in the GABA system where GABA receptor activation has opposite effects on neural activity before and after birth. For example, GABAergic excitation is necessary for DISC1-dependent regulation of neural development (Duan et al, 2007; Kim et al, 2012) and NRG1 regulates the expression and activity of GABA receptors and interneuron activity (Li et al, 2012; Mitchell et al, 2013; Wen et al, 2010). Therefore, any environmental exposure affecting GAD1, DISC1, or NRG1 might have opposite effects depending on the developmental time point of its introduction. The second possibility is that neuropsychiatric patients are inherently heterogeneous and developmental risk factors common to multiple disorders are found in overlapping parts of the spectrum (Adam, 2013; Insel, 2010). In this context, specific GABAergic deficits reported in multiple disorders may also represent this overlap. This option may be informed by new research initiatives seeking to identify biology underlying specific symptom domains (Cuthbert and Insel, 2013) and subsequent stratification of future clinical studies. A third possible explanation is that environmental factors might predispose to altered trajectory of brain development, but individual genetic susceptibility defines the phenotype (and ultimately the diagnosis). We favor this last explanation (Horvath and Mirnics, 2014b), as MIA and immune system activation predispose to both autism and schizophrenia (Michel et al, 2012; Patterson, 2009), and early stress predisposes to a host of psychiatric disorders (Chrousos and Gold, 1992; Corcoran et al, 2003; O'Donnell, 2012; Walker et al, 2008). However, symptoms of autism emerge very early in life while schizophrenia onset is typically during late adolescence or early adulthood. Therefore, one might argue that the different genetic susceptibilities will define the disease phenotype in a G × E manner, in which the environment can be considered a ‘disease-predisposer’, and the genetic susceptibility is the ‘disease-specifier’ (Figure 1). This view has some support from animal studies, where MIA, in conjunction with a schizophrenia-susceptibility genotype (such as DISC1), mirrors the late-onset behavioral abnormalities observed in schizophrenia (Abazyan et al, 2010; Ibi et al, 2010).
Figure 1.
Genetic factors and environmental influences jointly alter normal interneuronal development. Copy number variants (CNVs), single-nucleotide polymorphisms (SNPs), and mutations interact with a host of environmental factors. Their effects summate, and jointly regulate the expression of brain-derived neurotrophic factor (BDNF), reelin (RELN), neuregulin 1 (NRG1), disrupted-in-schizophrenia 1 (DISC1) and glutamic acid decarboxylase 1 (GAD1). This interaction occurs on a developmental timeline, and alters the typical developmental trajectory of interneurons. Depending on the insults and their timing, the gene × environment (G × E) interaction can disrupt the developmental trajectory at multiple developmental time points (arrows) and might alter cell proliferation, migration, maturation, integration into cortical circuits, or refinement of GABAergic synaptic connections. Such mechanism might explain the variability of GABAergic disturbances seen across the patient cohorts. Regardless of the timing of the insult and the exact time point where the developmental trajectory starts deviating from the typical developmental curve, the end result might be similar—a dysfunctional GABAergic circuitry, which contributes to the emergence of the disease symptoms. Disease threshold is indicated by the dashed gray line. CNS, central nervous system; GABA, gamma-aminobutyric acid; MIA, maternal immune activation.
Finally, investigation of the pathophysiology of schizophrenia with animal models will remain a major challenge. The mouse brain is different from the human brain and rodents do not get schizophrenia. Furthermore, the utility of animal models in schizophrenia research is limited by the specificity of each hypothesis under examination. Yet, this research will continue to be essential for our understanding of brain function and behavior. Rodent models might not be ideal for determining causal paths to psychosis, but they are extremely valuable when asking well-defined questions about development programs in the brain, the functions of genes, and many other questions. In this context, researchers can assess whether risk factors identified by human genetic and epidemiological studies alter the brain or species-relevant behavior and whether combinations of these risk factors interact as part of larger malfunctioning systems. Taking an ‘apples to apples’ approach removes confusion associated with attempts to ‘diagnose’ the behavior of rodents and facilitates fundamental research. In the absence of anthropomorphism, manipulating gene expression in mice has provided a wealth of data regarding the roles GAD1, RELN, BDNF, NRG1, DISC1, and other genes have in development and behavior. In fact, our understanding of the developmental importance of the GABAergic system comes largely from transgenic mouse research. Simply put, while there are no (and perhaps never will be) ‘true’ rodent models of schizophrenia or ‘schizophrenic mice’, a combined use of transgenic technology and environmental challenges in rodent models is essential for understanding genes, cognition, and mental disorders (Brown et al, 2013; Garbett et al, 2010, 2012; Jaaro-Peled et al, 2009; Levitt, 2005; Papaleo et al, 2012; Schmidt et al, 2013; Schmidt and Mirnics, 2012; Smith et al, 2007).
As the field moves toward more complex assessments of the impact of genetic and environmental factors on normal and abnormal brain development, it will be increasingly important to thoughtfully consider and report the precise timing and cell type specificity of the findings. Despite these challenges, we can be hopeful that the wealth of information provided by such studies will identify the biological foundations of specific behavioral dysfunctions. Only then can we build the path to new treatment options, and perhaps arrive to the long-coveted concept of personalized medicine for psychiatric disorders.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
KM’s work is funded by NIH Grants R01 MH093332, R01 MH079299, R01 MH067234, and P30 HD015052. MJS’ work was partially supported by a Vanderbilt Brain Institute Scholar Award.
References
- Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F et al (2010). Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry 68: 1172–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg KA, Liu Y, Bukszar J, McClay JL, Khachane AN, Andreassen OA et al (2013). A comprehensive family-based replication study of schizophrenia genes. JAMA Psychiatry 70: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham H, Meyer G (2003). Reelin-expressing neurons in the postnatal and adult human hippocampal formation. Hippocampus 13: 715–727. [DOI] [PubMed] [Google Scholar]
- Adam D (2013). Mental health: on the spectrum. Nature 496: 416–418. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Bunney WE Jr, Potkin SG, Wigal SB, Hagman JO, Sandman CA et al (1993. a). Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry 50: 169–177. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huang HS (2006). Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev 52: 293–304. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE Jr et al (1995). Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry 52: 258–266. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE Jr, Jones EG (1996). Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry 53: 425–436. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Vinuela A, Kim JJ, Potkin SG, Bunney WE Jr, Jones EG (1993. b). Distorted distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Arch Gen Psychiatry 50: 178–187. [DOI] [PubMed] [Google Scholar]
- Alcantara S, Pozas E, Ibanez CF, Soriano E (2006). BDNF-modulated spatial organization of Cajal–Retzius and GABAergic neurons in the marginal zone plays a role in the development of cortical organization. Cereb Cortex 16: 487–499. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL (2001). Distinct cortical migrations from the medial and lateral ganglionic eminences. Development 128: 353–363. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Volk DW, Lewis DA (1996). Increased density of microtubule associated protein 2-immunoreactive neurons in the prefrontal white matter of schizophrenic subjects. Schizophr Res 19: 111–119. [DOI] [PubMed] [Google Scholar]
- Arion D, Horvath S, Lewis DA, Mirnics K (2010). Infragranular gene expression disturbances in the prefrontal cortex in schizophrenia: signature of altered neural development? Neurobiol Dis 37: 738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arion D, Lewis DA (2011). Altered expression of regulators of the cortical chloride transporters NKCC1 and KCC2 in schizophrenia. Arch Gen Psychiatry 68: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arion D, Unger T, Lewis DA, Levitt P, Mirnics K (2007). Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry 62: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR (1991). Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry 48: 625–632. [DOI] [PubMed] [Google Scholar]
- Aronne MP, Guadagnoli T, Fontanet P, Evrard SG, Brusco A (2011). Effects of prenatal ethanol exposure on rat brain radial glia and neuroblast migration. Exp Neurol 229: 364–371. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Witton J, Murray RM (2004). Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry 184: 110–117. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N et al (1997). Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA 94: 6496–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A et al (2008). Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci 9: 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN et al (2011). Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry 16: 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat A, Dean B, Scarr E, Evin G (2010). Decreased neuregulin 1 C-terminal fragment in Brodmann’s area 6 of patients with schizophrenia. Schizophr Res 124: 200–207. [DOI] [PubMed] [Google Scholar]
- Barbin G, Pollard H, Gaiarsa JL, Ben-Ari Y (1993). Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci Lett 152: 150–154. [DOI] [PubMed] [Google Scholar]
- Bartolini G, Ciceri G, Marin O (2013). Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron 79: 849–864. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Honavar M, Everall IP, Cotter D (2009). Two-dimensional assessment of cytoarchitecture in the superior temporal white matter in schizophrenia, major depressive disorder and bipolar disorder. Schizophr Res 115: 156–162. [DOI] [PubMed] [Google Scholar]
- Behar TN, Dugich-Djordjevic MM, Li YX, Ma W, Somogyi R, Wen X et al (1997). Neurotrophins stimulate chemotaxis of embryonic cortical neurons. Eur J Neurosci 9: 2561–2570. [DOI] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL (2000). GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex 10: 899–909. [DOI] [PubMed] [Google Scholar]
- Behar TN, Smith SV, Kennedy RT, McKenzie JM, Maric I, Barker JL (2001). GABA(B) receptors mediate motility signals for migrating embryonic cortical cells. Cereb Cortex 11: 744–753. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y (2002). Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci 3: 728–739. [DOI] [PubMed] [Google Scholar]
- Benes FM (1993). Neurobiological investigations in cingulate cortex of schizophrenic brain. Schizophr Bull 19: 537–549. [DOI] [PubMed] [Google Scholar]
- Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL (1991). Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 48: 996–1001. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Alsterberg G, Bird ED, SanGiovanni JP (1992). Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. J Neurosci 12: 924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger B, Marty S, Zafra F, da Penha Berzaghi M, Thoenen H, Lindholm D (1995). GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development 121: 2327–2335. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Stanarius A, Baumann B, Henning H, Krell D, Danos P et al (1998). Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience 83: 867–875. [DOI] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Neme N, Vigneau S, Sigrist M, Arber S et al (2005). Multiple origins of Cajal–Retzius cells at the borders of the developing pallium. Nat Neurosci 8: 1002–1012. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U (2010). Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology 35: 2462–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue ME, Naidu S, Johnston MV (1999). Altered development of glutamate and GABA receptors in the basal ganglia of girls with Rett syndrome. Exp Neurol 156: 345–352. [DOI] [PubMed] [Google Scholar]
- Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J et al (2011). Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatry 17: 584–596. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Sawa A (2011). Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci 12: 707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauns S, Gollub RL, Roffman JL, Yendiki A, Ho BC, Wassink TH et al (2011). DISC1 is associated with cortical thickness and neural efficiency. NeuroImage 57: 1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS (2011). The environment and susceptibility to schizophrenia. Prog Neurobiol 93: 23–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ (2010). Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167: 261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Horvath S, Garbett KA, Schmidt MJ, Everheart M, Gellert L et al (2013). The role of cannabinoid 1 receptor expressing interneurons in behavior. Neurobiol Dis 63C: 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune M, Schobel A, Karau R, Benali A, Faustmann PM, Juckel G et al (2010). Von Economo neuron density in the anterior cingulate cortex is reduced in early onset schizophrenia. Acta Neuropathol 119: 771–778. [DOI] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P (2008). Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci 9: 110–122. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Feighery EL, Mattay VS, White MG, Chen Q, Baranger DA et al (2013). DISC1 and SLC12A2 interaction affects human hippocampal function and connectivity. J Clin Invest 123: 2961–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta SE, Brown AS (2012). Prenatal infection, maternal immune activation, and risk for schizophrenia. Transl Neurosci 3: 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Rosso IM, Huttunen M, Lonnqvist J, Pirkola T et al (2002). Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry 59: 35–41. [DOI] [PubMed] [Google Scholar]
- Cantor-Graae E, Selten JP (2005). Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry 162: 12–24. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Gottesman II (2000). Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet 97: 12–17. [PubMed] [Google Scholar]
- Cellerino A, Maffei L, Domenici L (1996). The distribution of brain-derived neurotrophic factor and its receptor trkB in parvalbumin-containing neurons of the rat visual cortex. Eur J Neurosci 8: 1190–1197. [DOI] [PubMed] [Google Scholar]
- Cellot G, Cherubini E (2013). Functional role of ambient GABA in refining neuronal circuits early in postnatal development. Front Neural Circuits 7: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance SA, Walker M, Crow TJ (2005). Reduced density of calbindin-immunoreactive interneurons in the planum temporale in schizophrenia. Brain Res 1046: 32–37. [DOI] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J et al (2010). Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y et al (2007). GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron 54: 889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Cheng L, Grennan K, Pibiri F, Zhang C, Badner JA et al (2013). Two gene co-expression modules differentiate psychotics and controls. Mol Psychiatry 18: 1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong VZ, Thompson M, Beltaifa S, Webster MJ, Law AJ, Weickert CS (2008). Elevated neuregulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. Schizophr Res 100: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW (1992). The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267: 1244–1252. [PubMed] [Google Scholar]
- Chun JJM, Shatz CJ (1989). Interstitial-cells of the adult neocortical white matter are the remnant of the early generated subplate neuron population. J Comp Neurol 282: 555–569. [DOI] [PubMed] [Google Scholar]
- Ciceri G, Dehorter N, Sols I, Huang ZJ, Maravall M, Marin O (2013). Lineage-specific laminar organization of cortical GABAergic interneurons. Nat Neurosci 16: 1199–1210. [DOI] [PubMed] [Google Scholar]
- Clay HB, Sillivan S, Konradi C (2010). Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci 29: 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CM, Guo Y, Akbarian S (2009). Cingulate white matter neurons in schizophrenia and bipolar disorder. Biol Psychiatry 66: 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L et al (2003). The stress cascade and schizophrenia: etiology and onset. Schizophr Bull 29: 671–692. [DOI] [PubMed] [Google Scholar]
- Costa E, Davis JM, Dong E, Grayson DR, Guidotti A, Tremolizzo L et al (2004). A GABAergic cortical deficit dominates schizophrenia pathophysiology. Crit Rev Neurobiol 16: 1–23. [DOI] [PubMed] [Google Scholar]
- Costa E, Grayson DR, Mitchell CP, Tremolizzo L, Veldic M, Guidotti A (2003). GABAergic cortical neuron chromatin as a putative target to treat schizophrenia vulnerability. Crit Rev Neurobiol 15: 121–142. [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics C, Genetic Risk Outcome of Psychosis C (2013). Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381: 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen TJ, Walker MA, Eastwood SL, Esiri MM, Harrison PJ, Crow TJ (2006). Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. Br J Psychiatry 188: 26–31. [DOI] [PubMed] [Google Scholar]
- Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN et al (2011). Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry 168: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR (2013). Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 11: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PW, Yanagawa Y, Obata K, Yeh HH (2008). Ethanol consumption during early pregnancy alters the disposition of tangentially migrating GABAergic interneurons in the fetal cortex. J Neurosci 28: 1854–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalman C, Allebeck P, Cullberg J, Grunewald C, Koster M (1999). Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch Gen Psychiatry 56: 234–240. [DOI] [PubMed] [Google Scholar]
- Danglot L, Triller A, Marty S (2006). The development of hippocampal interneurons in rodents. Hippocampus 16: 1032–1060. [DOI] [PubMed] [Google Scholar]
- Daviss SR, Lewis DA (1995). Local circuit neurons of the prefrontal cortex in schizophrenia: selective increase in the density of calbindin-immunoreactive neurons. Psychiatry Res 59: 81–96. [DOI] [PubMed] [Google Scholar]
- de Jong S, Boks MP, Fuller TF, Strengman E, Janson E, de Kovel CG et al (2012). A gene co-expression network in whole blood of schizophrenia patients is independent of antipsychotic-use and enriched for brain-expressed genes. PLoS One 7: e39498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S et al (2013). New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci 14: 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pino I, Garcia-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, Martinez de Lagran M et al (2013). Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron 79: 1152–1168. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, O'Tuathaigh C, Clarke G, O'Leary C, Petit E, Clarke N et al (2012). Phenotypic effects of repeated psychosocial stress during adolescence in mice mutant for the schizophrenia risk gene neuregulin-1: a putative model of gene × environment interaction. Brain Behav Immun 26: 660–671. [DOI] [PubMed] [Google Scholar]
- Deslauriers J, Larouche A, Sarret P, Grignon S (2013). Combination of prenatal immune challenge and restraint stress affects prepulse inhibition and dopaminergic/GABAergic markers. Progr Neuro-psychopharmacol Biol Psychiatry 45: 156–164. [DOI] [PubMed] [Google Scholar]
- Di Rosa E, Crow TJ, Walker MA, Black G, Chance SA (2009). Reduced neuron density, enlarged minicolumn spacing and altered ageing effects in fusiform cortex in schizophrenia. Psychiatry Res 166: 102–115. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Delevich KM, Marcsisin MJ, Zhang W, Sampson AR, Gundersen HJ et al (2009). Pyramidal neuron number in layer 3 of primary auditory cortex of subjects with schizophrenia. Brain Res 1285: 42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA (2007). Primary visual cortex volume and total neuron number are reduced in schizophrenia. J Comp Neurol 501: 290–301. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y et al (2007). Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130: 1146–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme G, Lowe GC, Goutagny R, Williams S (2012). Early alterations in hippocampal circuitry and theta rhythm generation in a mouse model of prenatal infection: implications for schizophrenia. PLoS One 7: e29754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff BJ, Macritchie KA, Moorhead TW, Lawrie SM, Blackwood DH (2013). Human brain imaging studies of DISC1 in schizophrenia, bipolar disorder and depression: a systematic review. Schizophr Res 147: 1–13. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ (2005). Interstitial white matter neuron density in the dorsolateral prefrontal cortex and parahippocampal gyrus in schizophrenia. Schizophr Res 79: 181–188. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ (2006). Cellular basis of reduced cortical reelin expression in schizophrenia. Am J Psychiatry 163: 540–542. [DOI] [PubMed] [Google Scholar]
- Edden RA, Crocetti D, Zhu H, Gilbert DL, Mostofsky SH (2012). Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 69: 750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A et al (2003. a). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257–269. [DOI] [PubMed] [Google Scholar]
- Egan MF, Weinberger DR, Lu B (2003. b). Schizophrenia, III: brain-derived neurotropic factor and genetic risk. Am J Psychiatry 160: 1242. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA (2010). Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience 169: 1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DP, Ianni AM, Wei SM, Kohn PD, Kolachana B, Apud J et al (2013). Brain-derived neurotrophic factor (BDNF) Val(66)Met polymorphism differentially predicts hippocampal function in medication-free patients with schizophrenia. Mol Psychiatry 18: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkai P, Schneider-Axmann T, Honer WG (2000). Entorhinal cortex pre-alpha cell clusters in schizophrenia: quantitative evidence of a developmental abnormality. Biol Psychiatry 47: 937–943. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, McMenomy T (2000). Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry 5: 571. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Rooney RJ, Thuras PD (2013). Expression of GABAA alpha2-, beta1- and epsilon-receptors are altered significantly in the lateral cerebellum of subjects with schizophrenia, major depression and bipolar disorder. Transl Psychiatry 3: e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR (2002). Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry 52: 805–810. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Kroll JL, Stary JM (2001). Altered levels of Reelin and its isoforms in schizophrenia and mood disorders. NeuroReport 12: 3209–3215. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Rooney RJ, Patel DH, Thuras PD (2010). mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord 40: 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E (2005). GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res 72: 109–122. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K et al (2010). Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature 464: 1376–1380. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Neddens J, Yan L, Buonanno A (2009). Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex 19: 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish KN, Sweet RA, Lewis DA (2011). Differential distribution of proteins regulating GABA synthesis and reuptake in axon boutons of subpopulations of cortical interneurons. Cerebral Cortex 21: 2450–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C et al (2004). Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron 44: 251–261. [DOI] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O (2007). Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci 27: 9682–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom TD, Fatemi SH (2013). The involvement of Reelin in neurodevelopmental disorders. Neuropharmacology 68: 122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF (2003). Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci 26: 489–495. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I (2007). Perisomatic inhibition. Neuron 56: 33–42. [DOI] [PubMed] [Google Scholar]
- Frotscher M (2010). Role for Reelin in stabilizing cortical architecture. Trends Neurosci 33: 407–414. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, Duque A, Paspalas CD, Kata A, Fine R, Boven L et al (2013). Role of disrupted in schizophrenia 1 (DISC1) in stress-induced prefrontal cognitive dysfunction. Transl Psychiatry 3: e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SF, Klomp A, Wu JL, Swaab DF, Bao AM (2013). Reduced GAD(65/67) immunoreactivity in the hypothalamic paraventricular nucleus in depression: a postmortem study. J Affect Disord 149: 422–425. [DOI] [PubMed] [Google Scholar]
- Garbett KA, Horvath S, Ebert PJ, Schmidt MJ, Lwin K, Mitchell A et al (2010). Novel animal models for studying complex brain disorders: BAC-driven miRNA-mediated in vivo silencing of gene expression. Mol Psychiatry 15: 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett KA, Hsiao EY, Kalman S, Patterson PH, Mirnics K (2012). Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl Psychiatry 2: e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H (2006). GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, van Berckel BN, Lammertsma AA, Boellaard R, de Kloet CS, Vermetten E et al (2008). Reduced GABAA benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Mol Psychiatry 13: 74–83 73. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R et al (2013). Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339: 1095–1099. [DOI] [PubMed] [Google Scholar]
- Giusti-Rodriguez P, Sullivan PF (2013). The genomics of schizophrenia: update and implications. J Clin Invest 123: 4557–4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso C, Sabatini M, Unger T, Hashimoto T, Monteggia LM, Lewis DA et al (2006). Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry 11: 633–648. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Zeiler SR, Tamowski S, Jones KR (2003). Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci 23: 6856–6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Frisch A, Munitz H, Rockah R, Aviram A, Mozes T et al (1997). Velocardiofacial manifestations and microdeletions in schizophrenic inpatients. Am J Med Genet 72: 455–461. [PubMed] [Google Scholar]
- Gratacos M, Gonzalez JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X (2007). Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case–control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol Psychiatry 61: 911–922. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Chen Y, Costa E, Dong E, Guidotti A, Kundakovic M et al (2006). The human reelin gene: transcription factors (+), repressors (−) and the methylation switch (+/−) in schizophrenia. Pharmacol Ther 111: 272–286. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A et al (2005). Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci USA 102: 9341–9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD (2013). Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci 14: 755–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozeva D, Kirov G, Ivanov D, Jones IR, Jones L, Green EK et al (2010). Rare copy number variants: a point of rarity in genetic risk for bipolar disorder and schizophrenia. Arch Gen Psychiatry 67: 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR et al (2000. a). Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry 57: 1061–1069. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Pesold C, Costa E (2000. b). New neurochemical markers for psychosis: a working hypothesis of their operation. Neurochem Res 25: 1207–1218. [DOI] [PubMed] [Google Scholar]
- Guo J, Anton ES (2014). Decision making during interneuron migration in the developing cerebral cortex. Trends Cell Biol pii: S0962-8924(13)00224-9. doi:10.1016/j.tcb.2013.12.001. (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- Haas M, Qu Z, Kim TH, Vargas E, Campbell K, Petrou S et al (2013). Perturbations in cortical development and neuronal network excitability arising from prenatal exposure to benzodiazepines in mice. Eur J Neurosci 37: 1584–1593. [DOI] [PubMed] [Google Scholar]
- Habl G, Schmitt A, Zink M, von Wilmsdorff M, Yeganeh-Doost P, Jatzko A et al (2012). Decreased reelin expression in the left prefrontal cortex (BA9) in chronic schizophrenia patients. Neuropsychobiology 66: 57–62. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I et al (2000). Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci 12: 3239–3249. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD et al (2001). Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA 98: 4746–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF et al (2003). Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci 23: 6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ (1999). The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain 122(Part 4): 593–624. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ (2006). Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry 60: 132–140. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR (2005). Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol psychiatry 10: 40–68 image 45. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Torii K, Torii M, Sarkisian MR, Bartley CM, Shen J, Radtke F et al (2008). Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron 60: 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR (2004). Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry 9: 299–307. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW et al (2008. a). Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 13: 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA (2008. b). Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry 165: 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN et al (2005). Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci 25: 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z et al (2003). Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci 23: 6315–6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S (1997). Neuropathology of schizophrenia: cortex, thalamus, basal ganglia, and neurotransmitter-specific projection systems. Schizophr Bull 23: 403–421. [DOI] [PubMed] [Google Scholar]
- Heckers S, Heinsen H, Geiger B, Beckmann H (1991). Hippocampal neuron number in schizophrenia. A stereological study. Arch Gen Psychiatry 48: 1002–1008. [DOI] [PubMed] [Google Scholar]
- Hefft S, Jonas P (2005). Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci 8: 1319–1328. [DOI] [PubMed] [Google Scholar]
- Henquet C, Di Forti M, Morrison P, Kuepper R, Murray RM (2008). Gene–environment interplay between cannabis and psychosis. Schizophr Bull 34: 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA (2013). Altered cortical expression of GABA-related genes in schizophrenia: illness progression vs developmental disturbance. Schizophr Bull (Epub ahead of print) PMID:24361861. [DOI] [PMC free article] [PubMed]
- Horvath S, Janka Z, Mirnics K (2011). Analyzing schizophrenia by DNA microarrays. Biol Psychiatry 69: 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Mirnics K (2009). Breaking the gene barrier in schizophrenia. Nat Med 15: 488–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Mirnics K (2014. a). Immune system disturbances in schizophrenia. Biol Psychiatry 75: 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Mirnics K (2014. b). Schizophrenia as a disorder of molecular pathways. Biol Psychiatry pii: S0006-3223(14)00007-9. doi:10.1016/j.biopsych.2014.01.001. (Epub ahead of print) PMID:24507510. [DOI] [PMC free article] [PubMed]
- Hou XJ, Ni KM, Yang JM, Li XM (2013). Neuregulin 1/ErbB4 enhances synchronized oscillations of prefrontal cortex neurons via inhibitory synapses. Neuroscience 261: 107–117. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF (2001). Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Akbarian S (2007). GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PLoS One 2: e809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF et al (1999). BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98: 739–755. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE et al (2011). Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci 31: 11088–11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, Nagai T, Koike H, Kitahara Y, Mizoguchi H, Niwa M et al (2010). Combined effect of neonatal immune activation and mutant DISC1 on phenotypic changes in adulthood. Behav Brain Res 206: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, Nagai T, Nakajima A, Mizoguchi H, Kawase T, Tsuboi D et al (2013). Astroglial IFITM3 mediates neuronal impairments following neonatal immune challenge in mice. Glia 61: 679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Ikeda K, Iritani S, Ueno H, Niizato K (2004). Distribution of neuropeptide Y interneurons in the dorsal prefrontal cortex of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 28: 379–383. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Tamura N, Matsuki N, Yamada MK (2006). Conserved role of brain-derived neurotrophic factor in Val66Met: target-selective reinforcement of GABAergic synapses. NeuroReport 17: 1847–1851. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG et al (1998). A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci USA 95: 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR (2010). Rethinking schizophrenia. Nature 468: 187–193. [DOI] [PubMed] [Google Scholar]
- International Schizophrenia C (2008). Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature 455: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iritani S, Kuroki N, Niizato K, Ikeda K (2000). Morphological changes in neuropeptide Y-positive fiber in the hippocampal formation of schizophrenics. Progr Neuro-Psychopharmacol Biol Psychiatry 24: 241–249. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H (2009). Gene models of schizophrenia: DISC1 mouse models. Prog Brain Res 179: 75–86. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A (2009). Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci 32: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob H, Beckmann H (1986). Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm 65: 303–326. [DOI] [PubMed] [Google Scholar]
- Javitt DC (2012). Twenty-five years of glutamate in schizophrenia: are we there yet? Schizophr Bull 38: 911–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D, Fung SJ, Rothwell A, Weickert CS (2012). Higher gamma-aminobutyric acid neuron density in the white matter of orbital frontal cortex in schizophrenia. Biol Psychiatry 72: 725–733. [DOI] [PubMed] [Google Scholar]
- Kalkman HO, Loetscher E (2003). GAD(67): the link between the GABA-deficit hypothesis and the dopaminergic- and glutamatergic theories of psychosis. J Neural Transm 110: 803–812. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y et al (2005). A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol 7: 1167–1178. [DOI] [PubMed] [Google Scholar]
- Kanazawa T, Glatt SJ, Kia-Keating B, Yoneda H, Tsuang MT (2007). Meta-analysis reveals no association of the Val66Met polymorphism of brain-derived neurotrophic factor with either schizophrenia or bipolar disorder. Psychiatr Genet 17: 165–170. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Macdonald RL (2009). Making sense of nonsense GABA(A) receptor mutations associated with genetic epilepsies. Trends Mol Med 15: 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ, Gogos JA (2010). 22q11.2 Microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci 11: 402–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson MA, Tang AH, Milner TA, Alger BE (2009). Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci 29: 4140–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N et al (2001). Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci 21: 9506–9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K et al (1999). Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19: 4544–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Liu CY, Zhang F, Duan X, Wen Z, Song J et al (2012). Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell 148: 1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Conley RC, Kakoyannis A, Reep RL, Roberts RC (1999). Interstitial cells of the white matter in the inferior parietal cortex in schizophrenia: an unbiased cell-counting study. Synapse 34: 95–102. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias NC, Conley RR, Roberts RC (2003). Interstitial cells of the white matter in the dorsolateral prefrontal cortex in deficit and nondeficit schizophrenia. J Nerv Ment Dis 191: 563–567. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P (2008). Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321: 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Bartko JJ, Webster MJ, Torrey EF (2002). Molecular abnormalities in the major psychiatric illnesses: classification and regression tree (CRT) analysis of post-mortem prefrontal markers. Mol Psychiatry 7: 392–404. [DOI] [PubMed] [Google Scholar]
- Knusel B, Rabin SJ, Hefti F, Kaplan DR (1994). Regulated neurotrophin receptor responsiveness during neuronal migrationand early differentiation. J Neurosci 14(Part 2): 1542–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H et al (2011). Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res 131: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I, Rakic P (1990). Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol 297: 441–470. [DOI] [PubMed] [Google Scholar]
- Kovalenko S, Bergmann A, Schneider-Axmann T, Ovary I, Majtenyi K, Havas L et al (2003). Regio entorhinalis in schizophrenia: more evidence for migrational disturbances and suggestions for a new biological hypothesis. Pharmacopsychiatry 36(Suppl 3): S158–S161. [DOI] [PubMed] [Google Scholar]
- Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N et al (2007). Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain 130(Part 3): 678–692. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Guidotti A, Grayson DR (2009). The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Mol Pharmacol 75: 342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromitsu J, Yokoi A, Kawai T, Nagasu T, Aizawa T, Haga S et al (2001). Reduced neuropeptide Y mRNA levels in the frontal cortex of people with schizophrenia and bipolar disorder. Brain Res Gene Expr Patterns 1: 17–21. [DOI] [PubMed] [Google Scholar]
- Kutiyanawalla A, Promsote W, Terry A, Pillai A (2012). Cysteamine treatment ameliorates alterations in GAD67 expression and spatial memory in heterozygous reeler mice. Int J Neuropsychopharmacol 15: 1073–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A (2013). Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature 498: 363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert de Rouvroit C, Goffinet AM (1998). The reeler mouse as a model of brain development. Adv Anat Embryol Cell Biol 150: 1–106. [PubMed] [Google Scholar]
- Law AJ, Kleinman JE, Weinberger DR, Weickert CS (2007). Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet 16: 129–141. [DOI] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R et al (2006). Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5' SNPs associated with the disease. Proc Natl Acad Sci USA 103: 6747–6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus MS, Krishnan K, Huang ZJ (2013). GAD67 deficiency in parvalbumin interneurons produces deficits in inhibitory transmission and network disinhibition in mouse prefrontal cortex. Cereb Cortex (Epub ahead of print) PMID:24275833. [DOI] [PMC free article] [PubMed]
- Levav-Rabkin T, Melamed O, Clarke G, Farber M, Cryan JF, Dinan TG et al (2010). A sensitive period of mice inhibitory system to neonatal GABA enhancement by vigabatrin is brain region dependent. Neuropsychopharmacology 35: 1138–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P (2005). Disruption of interneuron development. Epilepsia 46(Suppl 7): 22–28. [DOI] [PubMed] [Google Scholar]
- Levitt P, Ebert P, Mirnics K, Nimgaonkar VL, Lewis DA (2006). Making the case for a candidate vulnerability gene in schizophrenia: convergent evidence for regulator of G-protein signaling 4 (RGS4). Biol Psychiatry 60: 534–537. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW (2012). Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW (2005). Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6: 312–324. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P (2002). Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 25: 409–432. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA (2000). Catching up on schizophrenia: natural history and neurobiology. Neuron 28: 325–334. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Mirnics K (2006). Transcriptome alterations in schizophrenia: disturbing the functional architecture of the dorsolateral prefrontal cortex. Progr Brain Res 158: 141–152. [DOI] [PubMed] [Google Scholar]
- Li KX, Lu YM, Xu ZH, Zhang J, Zhu JM, Zhang JM et al (2012). Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci 15: 267–273. [DOI] [PubMed] [Google Scholar]
- Lipina TV, Zai C, Hlousek D, Roder JC, Wong AH (2013). Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice. J Neurosci 33: 7654–7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Biro AA, Nusser Z (2004). Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci USA 101: 1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR (1995). GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 15: 1287–1298. [DOI] [PubMed] [Google Scholar]
- Lovett-Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun XH et al (2012). Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci 15: 423–430. [DOI] [PubMed] [Google Scholar]
- Malaspina D (2001). Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizophr Bull 27: 379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Aviles JG, Curley AA, Hashimoto T, Morrow AL, Ramsey AJ, O'Donnell P et al (2009). Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry 166: 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malizia AL, Cunningham VJ, Bell CJ, Liddle PF, Jones T, Nutt DJ (1998). Decreased brain GABA(A)–benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry 55: 715–720. [DOI] [PubMed] [Google Scholar]
- Maloku E, Covelo IR, Hanbauer I, Guidotti A, Kadriu B, Hu Q et al (2010). Lower number of cerebellar Purkinje neurons in psychosis is associated with reduced reelin expression. Proc Natl Acad Sci USA 107: 4407–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L et al (2005). A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci 25: 4755–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent JB, Jorquera I, Mazzucchelli I, Depaulis A, Perucca E, Ben-Ari Y et al (2007). Fetal exposure to GABA-acting antiepileptic drugs generates hippocampal and cortical dysplasias. Epilepsia 48: 684–693. [DOI] [PubMed] [Google Scholar]
- Manko M, Bienvenu TC, Dalezios Y, Capogna M (2012). Neurogliaform cells of amygdala: a source of slow phasic inhibition in the basolateral complex. J Physiol 590(Part 22): 5611–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marballi K, Quinones MP, Jimenez F, Escamilla MA, Raventos H, Soto-Bernardini MC et al (2010). In vivo and in vitro genetic evidence of involvement of neuregulin 1 in immune system dysregulation. J Mol Med (Berl) 88: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco S, Weinberger DR (2000). The neurodevelopmental hypothesis of schizophrenia: following a trail of evidence from cradle to grave. Dev Psychopathol 12: 501–527. [DOI] [PubMed] [Google Scholar]
- Maric D, Liu QY, Maric I, Chaudry S, Chang YH, Smith SV et al (2001). GABA expression dominates neuronal lineage progression in the embryonic rat neocortex and facilitates neurite outgrowth via GABA(A) autoreceptor/Cl− channels. J Neurosci 21: 2343–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O (2012). Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 13: 107–120. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL (2001. a). A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci 2: 780–790. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL (2003). Cell migration in the forebrain. Annu Rev Neurosci 26: 441–483. [DOI] [PubMed] [Google Scholar]
- Marin O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL (2001. b). Sorting of striatal and cortical interneurons regulated by semaphorin–neuropilin interactions. Science 293: 872–875. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C (2004). Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807. [DOI] [PubMed] [Google Scholar]
- Marty S, Berninger B, Carroll P, Thoenen H (1996). GABAergic stimulation regulates the phenotype of hippocampal interneurons through the regulation of brain-derived neurotrophic factor. Neuron 16: 565–570. [DOI] [PubMed] [Google Scholar]
- Mata I, Perez-Iglesias R, Roiz-Santianez R, Tordesillas-Gutierrez D, Gonzalez-Mandly A, Berja A et al (2010). Additive effect of NRG1 and DISC1 genes on lateral ventricle enlargement in first episode schizophrenia. NeuroImage 53: 1016–1022. [DOI] [PubMed] [Google Scholar]
- McAllister AK (2014). Major histocompatibility complex I in brain development and schizophrenia. Biol Psychiatry 75: 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC (1997). Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron 18: 767–778. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE (2000). Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry 57: 637–648. [DOI] [PubMed] [Google Scholar]
- Mei L, Xiong WC (2008). Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci 9: 437–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S (2009). Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry 65: 1006–1014. [DOI] [PubMed] [Google Scholar]
- Metin C, Vallee RB, Rakic P, Bhide PG (2008). Modes and mishaps of neuronal migration in the mammalian brain. J Neurosci 28: 11746–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Wahle P, Castaneyra-Perdomo A, Ferres-Torres R (1992). Morphology of neurons in the white matter of the adult human neocortex. Exp Brain Res 88: 204–212. [DOI] [PubMed] [Google Scholar]
- Meyer U (2014). Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry 75: 307–315. [DOI] [PubMed] [Google Scholar]
- Michel M, Schmidt MJ, Mirnics K (2012). Immune system gene dysregulation in autism and schizophrenia. Dev Neurobiol 72: 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Mirnics K, Pierri JN, Lewis DA, Levitt P (2002). Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J Neurosci 22: 2718–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L et al (2008). Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet 82: 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirnics K, Levitt P, Lewis DA (2006). Critical appraisal of DNA microarrays in psychiatric genomics. Biol Psychiatry 60: 163–176. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Lewis DA (2001. a). Genes and subtypes of schizophrenia. Trends Mol Med 7: 281–283. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Lewis DA, Levitt P (2001. b). Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci 24: 479–486. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P (2000). Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron 28: 53–67. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Pevsner J (2004). Progress in the use of microarray technology to study the neurobiology of disease. Nat Neurosci 7: 434–439. [DOI] [PubMed] [Google Scholar]
- Mitchell RM, Janssen MJ, Karavanova I, Vullhorst D, Furth K, Makusky A et al (2013). ErbB4 reduces synaptic GABAA currents independent of its receptor tyrosine kinase activity. Proc Natl Acad Sci USA 110: 19603–19608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H (2012). The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 62: 42–53. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD (2007). Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci 8: 427–437. [DOI] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA (2008). Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex 18: 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowry BJ, Gratten J (2013). The emerging spectrum of allelic variation in schizophrenia: current evidence and strategies for the identification and functional characterization of common and rare variants. Mol Psychiatry 18: 38–52. [DOI] [PubMed] [Google Scholar]
- Muir WJ, Pickard BS, Blackwood DH (2008). Disrupted-in-schizophrenia-1. Curr Psychiatry Rep 10: 140–147. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ (1999). High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry 56: 940–945. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Parnavelas JG (2002). Modes of neuronal migration in the developing cerebral cortex. Nat Rev Neurosci 3: 423–432. [DOI] [PubMed] [Google Scholar]
- Neddens J, Fish KN, Tricoire L, Vullhorst D, Shamir A, Chung W et al (2011). Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: implications for schizophrenia. Biol Psychiatry 70: 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves-Pereira M, Cheung JK, Pasdar A, Zhang F, Breen G, Yates P et al (2005). BDNF gene is a risk factor for schizophrenia in a Scottish population. Mol Psychiatry 10: 208–212. [DOI] [PubMed] [Google Scholar]
- Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y et al (2013). Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science 339: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh JS, Sharma RP, Veldic M, Salvacion AA, Jia X, Chen Y et al (2005). DNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures. Proc Natl Acad Sci USA 102: 1749–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RM, Malla AK (1993). Stressful life events and schizophrenia. I: a review of the research. Br J Psychiatry 162: 161–166. [DOI] [PubMed] [Google Scholar]
- Nullmeier S, Panther P, Dobrowolny H, Frotscher M, Zhao S, Schwegler H et al (2011). Region-specific alteration of GABAergic markers in the brain of heterozygous reeler mice. Eur J Neurosci 33: 689–698. [DOI] [PubMed] [Google Scholar]
- Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I (2006). Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience 143: 51–62. [DOI] [PubMed] [Google Scholar]
- O'Donnell P (2012). Cortical interneurons, immune factors and oxidative stress as early targets for schizophrenia. Eur J Neurosci 35: 1866–1870. [DOI] [PubMed] [Google Scholar]
- Ohba S, Ikeda T, Ikegaya Y, Nishiyama N, Matsuki N, Yamada MK (2005). BDNF locally potentiates GABAergic presynaptic machineries: target-selective circuit inhibition. Cereb Cortex 15: 291–298. [DOI] [PubMed] [Google Scholar]
- Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P et al (2009). Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature 461: 1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR (2002). Is there more to GABA than synaptic inhibition? Nat Rev Neurosci 3: 715–727. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Lipska BK, Weinberger DR (2012). Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology 62: 1204–1220. [DOI] [PubMed] [Google Scholar]
- Park H, Poo MM (2013). Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14: 7–23. [DOI] [PubMed] [Google Scholar]
- Pascual M, Perez-Sust P, Soriano E (2004). The GABAergic septohippocampal pathway in control and reeler mice: target specificity and termination onto Reelin-expressing interneurons. Mol Cell Neurosci 25: 679–691. [DOI] [PubMed] [Google Scholar]
- Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG (2009). Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology 34: 2699–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH (2009). Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 204: 313–321. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN (2008). Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9: 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington K, Dicker P, Hudson L, Cotter DR (2008). Evidence for reduced neuronal somal size within the insular cortex in schizophrenia, but not in affective disorders. Schizophr Res 106: 164–171. [DOI] [PubMed] [Google Scholar]
- Pesold C, Liu WS, Guidotti A, Costa E, Caruncho HJ (1999). Cortical bitufted, horizontal, and Martinotti cells preferentially express and secrete reelin into perineuronal nets, nonsynaptically modulating gene expression. Proc Natl Acad Sci USA 96: 3217–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE et al (2004). The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci 24: 10099–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A (2013). Cortical interneurons that specialize in disinhibitory control. Nature 503: 521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Mahadik SP (2008). Increased truncated TrkB receptor expression and decreased BDNF/TrkB signaling in the frontal cortex of reeler mouse model of schizophrenia. Schizophr Res 100: 325–333. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Bernstein HG, Dobrowolny H, Bogerts B, Weiner I, Keilhoff G (2012). Effects of risperidone treatment in adolescence on hippocampal neurogenesis, parvalbumin expression, and vascularization following prenatal immune activation in rats. Brain Behav Immun 26: 353–363. [DOI] [PubMed] [Google Scholar]
- Pla R, Borrell V, Flames N, Marin O (2006). Layer acquisition by cortical GABAergic interneurons is independent of Reelin signaling. J Neurosci 26: 6924–6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A (2002). Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development 129: 3147–3160. [DOI] [PubMed] [Google Scholar]
- Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, Shigemoto R et al (2005). Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci 25: 6775–6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF et al (2009). Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460: 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS (1998). Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry 55: 215–224. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N (2012). Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry 17: 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees E, Kirov G, Sanders A, Walters JT, Chambert KD, Shi J et al (2014). Evidence that duplications of 22q11.2 protect against schizophrenia. Mol Psychiatry 19: 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich CG, Mihalik GR, Iskander AN, Seckler JC, Weiss MS (2013). Adolescent chronic mild stress alters hippocampal CB1 receptor-mediated excitatory neurotransmission and plasticity. Neuroscience 253: 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetto J, Calabrese F, Riva MA, Meyer U (2013). Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. Schizophr Bull 40: 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B, Marin O (2011). Neuregulin signaling, cortical circuitry development and schizophrenia. Curr Opin Genet Dev 21: 262–270. [DOI] [PubMed] [Google Scholar]
- Ringstedt T, Linnarsson S, Wagner J, Lendahl U, Kokaia Z, Arenas E et al (1998). BDNF regulates reelin expression and Cajal–Retzius cell development in the cerebral cortex. Neuron 21: 305–315. [DOI] [PubMed] [Google Scholar]
- Ripke S, O'Dushlaine C, Chambert K, Moran JL, Kahler AK, Akterin S et al (2013). Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet 45: 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MA, Caruncho HJ, Costa E, Pesold C, Liu WS, Guidotti A (2002). In Patas monkey, glutamic acid decarboxylase-67 and reelin mRNA coexpression varies in a manner dependent on layers and cortical areas. J Comp Neurol 451: 279–288. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD (2009). Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry 65: 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Katsel P, Davis KL, Siever LJ, Haroutunian V (2012). A system-level transcriptomic analysis of schizophrenia using postmortem brain tissue samples. Arch Gen Psychiatry 69: 1205–1213. [DOI] [PubMed] [Google Scholar]
- Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A (2007). Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry 12: 385–397. [DOI] [PubMed] [Google Scholar]
- Schmid RS, McGrath B, Berechid BE, Boyles B, Marchionni M, Sestan N et al (2003). Neuregulin 1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc Natl Acad Sci USA 100: 4251–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, van Os J, Esquivel G, Steinbusch HW, Rutten BP (2012). An environmental analysis of genes associated with schizophrenia: hypoxia and vascular factors as interacting elements in the neurodevelopmental model. Mol Psychiatry 17: 1194–1205. [DOI] [PubMed] [Google Scholar]
- Schmidt MJ, Horvath S, Ebert P, Norris JL, Seeley EH, Brown J et al (2013). Modulation of behavioral networks by selective interneuronal inactivation. Mol Psychiatry 19: 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MJ, Mirnics K (2012). Modeling interneuron dysfunction in schizophrenia. Dev Neurosci 34: 152–158. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS (1999). The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 45: 17–25. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Mrzljak J, Kleinman JE, Herman MM, Goldman-Rakic PS (2003). Regional specificity in the neuropathologic substrates of schizophrenia: a morphometric analysis of Broca’s area 44 and area 9. Arch Gen Psychiatry 60: 69–77. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS (1995). Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry 52: 805–818 discussion 819–820. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS (1998). Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol 392: 402–412. [PubMed] [Google Scholar]
- Shamir A, Kwon OB, Karavanova I, Vullhorst D, Leiva-Salcedo E, Janssen MJ et al (2012). The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci 32: 2988–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I et al (2009). Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 460: 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel BI, Sengupta EJ, Edelson JR, Lewis DA, Volk DW (2013). Elevated viral restriction factor levels in cortical blood vessels in schizophrenia. Biol Psychiatry pii: S0006-3223(13)00860-3. doi:10.1016/j.biopsych.2013.09.019. (Epub ahead of print) PMID:24209773. [DOI] [PMC free article] [PubMed]
- Smiley JF, Konnova K, Bleiwas C (2012). Cortical thickness, neuron density and size in the inferior parietal lobe in schizophrenia. Schizophr Res 136: 43–50. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH (2007). Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27: 10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G et al (1990). Association within a family of a balanced autosomal translocation with major mental illness. Lancet 336: 13–16. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D et al (2009). Common variants conferring risk of schizophrenia. Nature 460: 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S et al (2008). Large recurrent microdeletions associated with schizophrenia. Nature 455: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Steinthorsdottir V, Thorgeirsson TE, Gulcher JR, Stefansson K (2004). Neuregulin 1 and schizophrenia. Ann Med 36: 62–71. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC (2003). Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry 60: 1187–1192. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S et al (2005). Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry 10: 631–636. [DOI] [PubMed] [Google Scholar]
- Takayama C (1994). Altered distribution of inhibitory synaptic terminals in reeler cerebellum with special reference to malposition of GABAergic neurons. Neurosci Res 20: 239–250. [DOI] [PubMed] [Google Scholar]
- Tan H, Lauzon NM, Bishop SF, Bechard MA, Laviolette SR (2010). Integrated cannabinoid CB1 receptor transmission within the amygdala–prefrontal cortical pathway modulates neuronal plasticity and emotional memory encoding. Cereb Cortex 20: 1486–1496. [DOI] [PubMed] [Google Scholar]
- Tandon R, Keshavan MS, Nasrallah HA (2008). Schizophrenia, ‘just the facts’ what we know in 2008. 2. Epidemiology and etiology. Schizophr Res 102: 1–18. [DOI] [PubMed] [Google Scholar]
- Tao R, Li C, Newburn EN, Ye T, Lipska BK, Herman MM et al (2012). Transcript-specific associations of SLC12A5 (KCC2) in human prefrontal cortex with development, schizophrenia, and affective disorders. J Neurosci 32: 5216–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD (2009). Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci 10: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Ray M, Weickert CS, Wyatt E, Webster MJ (2011). Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci 36: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM (2003). Reelin and brain development. Nat Rev Neurosci 4: 496–505. [DOI] [PubMed] [Google Scholar]
- Tochigi M, Iwamoto K, Bundo M, Komori A, Sasaki T, Kato N et al (2008). Methylation status of the reelin promoter region in the brain of schizophrenic patients. Biol Psychiatry 63: 530–533. [DOI] [PubMed] [Google Scholar]
- Tost H, Alam T, Geramita M, Rebsch C, Kolachana B, Dickinson D et al (2013). Effects of the BDNF Val66Met polymorphism on white matter microstructure in healthy adults. Neuropsychopharmacology 38: 525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K, Asama K, Watanabe Y, Muratake T, Takahashi M, Someya T et al (2002). Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res 110: 249–257. [DOI] [PubMed] [Google Scholar]
- Trost S, Platz B, Usher J, Scherk H, Wobrock T, Ekawardhani S et al (2013). DISC1 (disrupted-in-schizophrenia 1) is associated with cortical grey matter volumes in the human brain: a voxel-based morphometry (VBM) study. J Psychiatr Res 47: 188–196. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9: 519–525. [DOI] [PubMed] [Google Scholar]
- Tsuang M (2000). Schizophrenia: genes and environment. Biol Psychiatry 47: 210–220. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W (2010). Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11: 100–113. [DOI] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP (2010). The environment and schizophrenia. Nature 468: 203–212. [DOI] [PubMed] [Google Scholar]
- Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A et al (2009). Fast synaptic subcortical control of hippocampal circuits. Science 326: 449–453. [DOI] [PubMed] [Google Scholar]
- Vassos E, Pedersen CB, Murray RM, Collier DA, Lewis CM (2012). Meta-analysis of the association of urbanicity with schizophrenia. Schizophr Bull 38: 1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR et al (2004). DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci USA 101: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Kadriu B, Maloku E, Agis-Balboa RC, Guidotti A, Davis JM et al (2007). Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr Res 91: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario-Abejon C, Owens D, McKay R, Segal M (2002). Role of neurotrophins in central synapse formation and stabilization. Nat Rev Neurosci 3: 965–974. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA (2000). Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry 57: 237–245. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA (2002. a). Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction. Physiol Behav 77: 501–505. [DOI] [PubMed] [Google Scholar]
- Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y et al (2012). Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry 169: 1082–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA (2002. b). Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex 12: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K (2008). Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol 4: 189–216. [DOI] [PubMed] [Google Scholar]
- Wang AY, Lohmann KM, Yang CK, Zimmerman EI, Pantazopoulos H, Herring N et al (2011). Bipolar disorder type 1 and schizophrenia are accompanied by decreased density of parvalbumin- and somatostatin-positive interneurons in the parahippocampal region. Acta Neuropathol 122: 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Kriegstein AR (2008). GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J Neurosci 28: 5547–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE (2003). Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry 8: 592–610. [DOI] [PubMed] [Google Scholar]
- Weinberger DR (1987). Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44: 660–669. [DOI] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ et al (2010). Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci USA 107: 1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA (2006). The origin and specification of cortical interneurons. Nat Rev Neurosci 7: 687–696. [DOI] [PubMed] [Google Scholar]
- Wong J, Hyde TM, Cassano HL, Deep-Soboslay A, Kleinman JE, Weickert CS (2010). Promoter specific alterations of brain-derived neurotrophic factor mRNA in schizophrenia. Neuroscience 169: 1071–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Lu B (2006). Regulation of cortical interneurons by neurotrophins: from development to cognitive disorders. Neuroscientist 12: 43–56. [DOI] [PubMed] [Google Scholar]
- Wood JD, Bonath F, Kumar S, Ross CA, Cunliffe VT (2009). Disrupted-in-schizophrenia 1 and neuregulin 1 are required for the specification of oligodendrocytes and neurones in the zebrafish brain. Hum Mol Genet 18: 391–404. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Anderson SA, Yuste R (2010). The enigmatic function of chandelier cells. Front Neurosci 4: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Fu Y, Knott G, Lu J, Di Cristo G, Huang ZJ (2012). GABA signaling promotes synapse elimination and axon pruning in developing cortical inhibitory interneurons. J Neurosci 32: 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N et al (2002). Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci 22: 7580–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Fung SJ, Rothwell A, Tianmei S, Weickert CS (2011). Increased interstitial white matter neuron density in the dorsolateral prefrontal cortex of people with schizophrenia. Biol Psychiatry 69: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Manaye KF, Liang C, Hicks PB, German DC (2000). Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry 47: 944–953. [DOI] [PubMed] [Google Scholar]