Abstract
The msp2 and p44 genes encode polymorphic major outer membrane proteins that are considered unique to the intraerythrocytic agent of Anaplasma marginale and the intragranulocytic agent of Anaplasma phagocytophilum, respectively. In the present study, however, we found an msp2 gene in A. phagocytophilum that was remarkably conserved among A. phagocytophilum strains from human granulocytic anaplasmosis (HGA) patients, ticks, and a horse from various regions in the United States, but the gene was different in a sheep isolate from the United Kingdom. The msp2 gene in the A. phagocytophilum strain HZ genome was a single-copy gene and was located downstream of two Ehrlichia chaffeensis omp-1 homologs and a decarboxylase gene (ubiD). The msp2 gene was expressed by A. phagocytophilum in the blood from HGA patients NY36 and NY37 and by A. phagocytophilum isolates from these patients cultured in HL-60 cells at 37°C. The msp2 gene was also expressed in a DBA/2 mouse infected by attaching ticks infected with strain NTN-1 and in a horse experimentally infected by attaching strain HZ-infected ticks. However, the transcript of the msp2 gene was undetectable in A. phagocytophilum strain HZ in SCID mice and Ixodes scapularis ticks infected with strain NTN-1. These results indicate that msp2 is functional in various strains of A. phagocytophilum, and relative expression ratios of msp2 to p44 vary in different infected hosts. These findings may be important in understanding roles that Msp2 proteins play in granulocytic ehrlichia infection and evolution of the polymorphic major outer membrane protein gene families in Anaplasma species.
Polymorphic multigene families that encode major surface antigens are found in several vector-borne pathogens and confer antigenic variation (5). Thus, analysis of polymorphic multigene families among closely related species and strains is important in understanding the pathogenesis, host adaptation, and evolution of these pathogens. The human obligate intragranulocytic agent Anaplasma phagocytophilum and the bovine obligate intraerythrocytic agent Anaplasma marginale have polymorphic multigene families encoding the major outer membrane proteins P44 and Msp2, respectively (24, 29). P44 and Msp2 proteins are homologous yet distinct groups of proteins. The emergence of new P44 and Msp2 variants in different environments and in different infection stages are considered important for the survival and persistence of these obligatory intracellular bacteria in mammalian and tick hosts (4, 9, 13, 16, 17, 20, 21, 29, 30). Variation of msp2 expression from a single unique polycistronic expression locus involves a combinatorial gene conversion mechanism in A. marginale (3), and a similar polycistronic p44 expression locus was found in the A. phagocytophilum genome, although the expression mechanism of p44 at this locus may not be identical to that of msp2 (4, 20). Both expression loci are flanked by homologs of omp-1 genes which encode polymorphic major outer membrane proteins among Ehrlichia spp. in the family Anaplasmataceae (3, 4, 20). These results suggest that msp2, p44, and omp-1 may have coevolved from a common ancestral origin as they diverged to infect different cell types in mammals and multiple tick species. Therefore, we examined whether these three genes coexist in the same genome. In the present study we found an msp2 gene in all A. phagocytophilum strains. Thus, the A. phagocytophilum genome carries msp2, p44, and omp-1. Due to reported genetic polymorphisms (12) and biological differences among A. phagocytophilum strains, we compared the msp2 gene and its expression locus from various strains isolated from different hosts and from diverse geographic regions. In order to understand the role of msp2 in granulocytic A. phagocytophilum infection, we further determined expression of msp2 in different hosts. The p44 genes of A. phagocytophilum have been referred to as msp2 by some researchers. In the present study we propose to continue calling them p44 genes to distinguish them from msp2 genes coexisting in the same A. phagocytophilum genome until we learn more about these intriguing gene clusters.
MATERIALS AND METHODS
Cultivation and purification.
All A. phagocytophilum strains used in this study are described in Table 1. All strains, except the Old Sourhope (OS) strain, were cultured in HL-60 cells (human promyelocytic leukemia cell line) at 37°C as previously described (2, 21, 26, 31). A. phagocytophilum strains HZ, LL, NY-31, NY-36, and NY-37 purified from infected cells by Sephacryl S-1000 (Amersham Pharmacia Biotech, Piscataway, N.J.) column chromatography were used for the preparation of genomic DNA as previously described (25). Strains Trustom, Gaillard, and AVK-HLPA1 were isolated from questing Ixodes scapularis ticks. The Trustom and Gaillard strains were initially passaged through BALB/c mice, and the AVK-HLPA1 strain was initially passed through a dog. Infected mouse or dog blood was used to obtain the isolate in HL-60 cells.
TABLE 1.
Strains used in msp2 locus PCR
| Isolate | Geographical origin and source | Reference |
|---|---|---|
| HZ | New York, HGA patient | 26 |
| LL | New York, HGA patient | 31 |
| NY-31 | New York, HGA patient | 21 |
| NY-36 | New York, HGA patient | 21 |
| NY-37 | New York, HGA patient | 21 |
| MN-2 | Minnesota, HGA patient | 15 |
| MN-9 | Minnesota, HGA patient | 15 |
| Trustom | Rhode Island, tick | This study |
| AVK-HLPA1 | Pennsylvania, tick | This study |
| Gaillard | Connecticut, tick | This study |
| MRK | California, horse | 2 |
| OS | Scotland, United Kingdom, Sheep | 14 |
A. phagocytophilum specimens from HGA patients, sheep, SCID mice, a DBA/2 mouse, ticks, and a horse.
The buffy-coat specimens were prepared from the blood of two patients (NY36 and NY37) suspected of having human granulocytic anaplasmosis (HGA) based upon their clinical presentation to the Westchester Medical Center in June and July of 2000 (21). Parts of the buffy-coat specimens were stored in RNALater reagent (Ambion, Inc., Austin, Tex.). The blood sample of A. phagocytophilum strain OS was obtained from a sheep 5 days after primary infection (14). The blood samples from two 4-week-old ICR strain SCID male mice (Taconic Farm Inc., Germantown, N.Y.) were obtained 5 and 15 days after intraperitoneal inoculation with the HZ strain of A. phagocytophilum. A blood specimen was also obtained from a horse (EQ005) at day 22 after attaching 89 I. scapularis adults which were infected as nymphs by feeding on ICR strain SCID mice infected with the HZ strain (X. Wang, Y. Rikihisa, Y. Kumagai, and N. Zhi, Abstr. 104th Gen. Meet. Am. Soc. Microbiol., abstr. D-187, 2004). The horse and SCID mice peripheral blood leukocytes were prepared as described elsewhere (18). Infected tick specimens were prepared as previously described (13). Pooled salivary gland samples from five strain NTN-1-infected nymphal ticks removed after attaching to naïve DBA/2 mice as well as blood specimens obtained from one DBA/2 mouse collected 10 days after the attachment of these infected ticks were processed as described elsewhere (13).
Transcriptional analysis.
Reverse transcription (RT)-PCR was performed by the procedure described previously (21). Total RNA was extracted from 5 × 106 A. phagocytophilum-infected HL-60 cells (90 to 100% infection) and from the specimens of HGA patients, mice, a horse, and ticks by using the RNeasy Mini kit (QIAGEN, Valencia, Calif.). After DNase I treatment, the isolated RNA (5 μg) was heated at 70°C for 10 min. Samples were then subjected to RT at 42°C for 50 min in a 20-μl reaction mixture containing 0.5 mM concentrations of each deoxynucleotide triphosphate, 200 U of SuperScript II reverse transcriptase (Invitrogen, Carlsbad, Calif.), 200 ng of random hexamers, and 3 mM MgCl2. PCR was performed in a 50-μl reaction mixture containing 4 μl of the cDNA product, 10 pmol of each primer (primer sets 1 to 6 in Table 2 and Fig. 1), 0.2 mM concentrations of each deoxynucleotide triphosphate, 5 U of Taq DNA polymerase, and 1.5 mM MgCl2. PCR conditions included 3 min of denaturation at 94°C followed by 35 cycles consisting of 1 min of denaturation at 94°C, 1 min of annealing at 54°C, and 1 min of extension at 72°C. PCR products were purified from a gel and were cloned into a pCR II vector or a pCR-XL-TOPO vector (Invitrogen). Twenty cDNA clones were randomly selected from the transformants and were sequenced on an ABI 373XL Stretch DNA sequencer with a ABI PRISM BigDye Terminator Cycle Sequencing Reaction kit. DNA-PCR was performed (primer sets 1 to 6 and 9 to 13 in Table 2 and Fig. 1) under the same conditions as those described above for RT-PCR.
TABLE 2.
Oligonucleotide primers used in PCR and 5′RACE
| Primer set no. | Target | Purpose | 5′ Primer (5′RACE or genomic walking primer 2) | 3′ Primer (5′RACE or genomic walking primer 1) | RT primer |
|---|---|---|---|---|---|
| 1 | msp2 | RT-PCR, DNA PCR | 5′CCGGCGCATGTGTAAGGTGAAA3′ | 5′GGTTACATAAGGGCCGCAAAGGTG3′ | Random hexamer |
| 2 | ubiD | RT-PCR, DNA PCR | 5′CTGACGAAGGAATGACAATACAC3′ | 5′TGGGGGCTGATCCTGCTACAA3′ | Random hexamer |
| 3 | omp-1A | RT-PCR, DNA PCR | 5′GCAACACAGGATCTACAGGA3′ | 5′TGAGCAGCACGGGGACAG3′ | Random hexamer |
| 4 | omp-1B | RT-PCR, DNA PCR | 5′TGTACCATTCCAGGCATTTTCTCA3′ | 5′CCGTGGGTTAGCTTCCGTGTTT3′ | Random hexamer |
| 5 | omp-1B-msp2 | RT-PCR, DNA PCR | 5′GCAACACAGGATCTACAGGA3′ | 5′CGGCCCTTATGTAACCCAGATTTT3′ | Random hexamer |
| 6 | omp-1A-msp2 | RT-PCR, DNA PCR | 5′TGTACCATTCCAGGCATTTTCTCA3′ | 5′CGGCCCTTATGTAACCCAGATTTT3′ | Random hexamer |
| 7 | omp-1A | 5′RACE | 5′CAGCCCTAAATGTACGCAAACGC3′ | 5′AACTAGCACCACCCTTTATTTGGTA3′ | 5′AGATCATGATAATAGATGCC3′ |
| 8 | omp-1B | 5′RACE | 5′AATTCCTCTTTATAATACGCATCGAAT3′ | 5′TATTCTGAAGTTATTTATTGCGTAACCA3′ | 5′AACTCACTGTTCATTCCC3′ |
| 9 | msp2 full length | DNA PCR | 5′TTATGATTAGGCCTTTGGGCATG3′ | 5′TCAGAAAGATACACGTGCGCCC3′ | |
| 10 | omp-1A-1B | DNA PCR | 5′CATCGTTACGCAGCACCAAGACAT3′ | 5′CCGTGGGTTAGCTTCCGTGTTT3′ | |
| 11 | omp-1A-ubiD | DNA PCR | 5′TGTACCATTCCAGGCATTTTCTCA3′ | 5′TGGGGGCTGATCCTGCTACAA3′ | |
| 12 | ubiD-msp2 | DNA PCR | 5′CTGACGAAGGAATGACAATACAC3′ | 5′GGTTACATAAGGGCCGCAAAGGTG3′ | |
| 13 | p44 | DNA PCR | 5′TGATGTCAGGGCTCATGATG3′ | 5′AGAAGATCATAACAAGCATTG3′ |
FIG. 1.
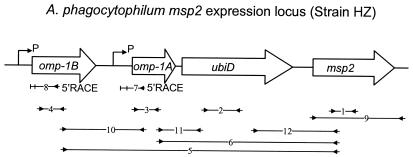
Schematic representation of the genomic region including the msp2 expression locus of A. phagocytophilum strain HZ. The ORFs in the expression locus are shown as white arrows indicating the orientations. Lines with facing arrows at each end indicate the regions of RT-PCR in the transcriptional analysis. Numbers in small boxes correspond to primer set numbers in Table 2. Bent arrows located upstream of omp-1B and omp-1A represents the putative transcriptional start site for polycistronic transcription linked to the expression locus, which was deduced by 5′RACE analysis (indicated by an arrow labeled 5′RACE).
DNA PCR.
Total DNA was extracted from 5 × 106 A. phagocytophilum-infected HL-60 cells by using a QIAamp DNA Mini kit (QIAGEN). The OS strain DNA was extracted from blood of an experimentally infected sheep 5 days after primary infection.
5′RACE.
The 5′ rapid amplification of cDNA ends (5′RACE) experiment was performed according to the protocol provided by the manufacturer (Invitrogen). DNase I-treated total RNA (3 μg) was reverse transcribed with Superscript II and gene-specific primers at 42°C for 50 min (primer sets 7 and 8 in Table 2 and Fig. 1). The cDNA was tailed by using terminal transferase to add cytidine residues at the 3′ end and then was amplified by PCR with a second gene-specific primer and an oligo(dG)-linked amplification primer. The PCR conditions included 35 cycles of 1 min of denaturation at 94°C, 1 min of annealing at 54°C, and 1 min of extension at 72°C. Primary PCR products were further amplified by a nested gene-specific primer and the amplification primer without an oligo(dG) anchor. The secondary PCR products were purified and cloned. Inserts of 20 clones were sequenced for each sample.
Sequence analysis.
The protein signal sequence was analyzed with the SignalP program (www.cbs.dtu.dk). Sequence assembling, alignments, and analysis were done with SeqMan, MegAlign, and MapDraw tools in the DNAStar program (DNAStar, Madison, Wis.). Phylogenic analysis was performed with the PHYLIP software package (version 3.6).
The A. phagocytophilum HZ genome sequence database is located at www.tigr.org. The A. marginale St. Maries genome sequence database is at www.vetmed.wsu.edu.
Nucleotide sequence accession numbers.
GenBank accession numbers of newly identified msp2 genes are the following: strain NY-31, AY541007; NY-36, AY541006; NY-37, AY541005; OS, AY541004; Trustom, AY541003; AVK-HLPA1, AY541002; Gaillard, AY541001; MN-2, AY541000; MN-9, AY540999; MRK, AY568557; and LL, AY568558.
RESULTS
Relationship of proteins encoded by A. phagocytophilum msp2 and p44 and A. marginale msp2 superfamilies.
By searching the A. phagocytophilum HZ strain genome database, we identified a 1,098-bp open reading frame (ORF) with a deduced amino acid sequence that showed 28 to 33% identity and 43 to 47% similarity to the Msp2 proteins of A. marginale and Anaplasma ovis (BlastP E values of 1e-40 to 1e-25). Unlike A. marginale msp2 or A. phagocytophilum p44 genes, only a single msp2 homolog was found in the genome of A. phagocytophilum HZ. The ORF had a universal translational start codon (AUG), and after removing a cleavable signal peptide of 25 amino acid residues it encoded a 38-kDa predicted mature membrane protein by PSORT analysis (http://psort.nibb.ac.jp) with a high antigenic index by Protean analysis (DNAStar). The sequence alignment showed that A. phagocytophilum Msp2 lacked the N- and C-terminal conserved sequences of P44 and the five conserved amino acid residues in the central hypervariable region invariably found in all P44 proteins (21). To characterize the relationship of the protein encoded by this msp2 homolog among the proteins encoded by msp2 and p44 genes in the two Anaplasma species, we constructed a phylogenetic tree based on the deduced amino acid sequence of 12 different full-length P44 proteins from A. phagocytophilum and 16 complete msp2 genes from A. marginale. These p44 paralogs include seven p44 paralogs randomly selected from full-length p44 genes, each from a different genomic locus, including the p44-18 gene in the polycistronic p44 expression locus in the A. phagocytophilum strain HZ genome and five p44 genes in strains HZ, NY18, HGE2, and Webster and in HGA patients (4, 21). The 16 full-length msp2 genes included 5 msp2 genes detected in the St. Maries strain (28), 2 from the South Idaho strain (27), 1 from the Florida strain (3), 1 from the Idaho7/17 strain (3), and 7 additional msp2 homologs from the St. Maries strain that were newly identified in the present study by searching the A. marginale St. Maries genome sequence database (www.vetmed.wsu.edu). These seven msp2 paralogs were found at sites other than the polycistronic msp2 expression locus and had 17 to 50% predicted amino acid identities with msp2 genes identified in previous studies of A. marginale (3, 6-8, 24, 27, 28) and also had 12 to 20% predicted amino acid identities with Msp3 proteins identified in previous studies (1). These new msp2 genes appear to encode full-length proteins, with a range of predicted molecular sizes between 30 to 41 kDa if AUG was used as the start codon. Recently, Brayton et al. reported that other than the seven previously identified pseudogenes of msp2 and msp3, the msp2-msp3 superfamily includes 14 additional newly identified genes (K. A. Brayton, G. H. Palmer, and D. F. Knowles, Jr., Abstr. Am. Soc. Rickettsiol. 18th Meet., abstr. 15, 2003, and reference 7). Overall these 30 predicted protein sequences were clustered into two groups: A. phagocytophilum P44 and A. marginale Msp2 (Fig. 2). The P44 group had 73 to 90% predicted amino acid identities among themselves. The A. phagocytophilum HZ strain Msp2 belonged to the A. marginale Msp2 group (Fig. 2).
FIG. 2.
Phylogram of Msp2 and P44 proteins in A. phagocytophilum and A. marginale. The amino acid sequences were aligned with the Protpars program, and the tree was constructed by using the neighbor-joining method of PHYLIP software package 3.6. P44 and Msp2 protein sequences were obtained by BLAST searching the A. phagocytophilum genome, the A. marginale genome, and the GenBank databases using A. phagocytophilum Msp2 (A. phagocytophilum HZ and A. phagocytophilum OS, boldfaced with shaded background). P44 and Msp2 groups are surrounded by dotted lines. Strain names are shown in parentheses. Msp2-SGV1 (AY107766) and Msp2-SGV2 (AY107767) are from A. marginale strain South Idaho (S.ID). Msp2 (AF200927) is from A. marginale strain Idaho7/17 (ID 7/17). Msp2 (AF200925) is from A. marginale strain Florida (FL). Msp2-SGV1, Msp2-SGV2, Msp2-SGV8, Msp2-SGV9, and Msp2-SGV10 (GenBank accession numbers AF290590, AF290591, AF290597, AF290598, and AF290599, respectively) are from A. marginale strain St. Maries (StM). Msp2 proteins from A. marginale strain St. Maries without GenBank accession numbers are referenced by the contig number with the first nucleotide position in the contig (www.vetmed.wsu.edu). P44-2, P44-7, P44-14, P44-18, P44-32, P44-49, and P44-53 are from the HZ genome (www.tigr.org). P44-12 (AF135260 and AY164491) and P44-21 (AF421831 and AY164490) are from strains HZ, NY-18, and Webster (4, 29). P44-20 (AF414591 and AY164492) is from strain HZ and strain HGE2 (4, 30). P44-63 (AY164493) is from HGA patient 2 (P) (4). P44-33 (AY164494) is also from HGA patient 2 (P) (4). Msp1b-StM of A. marginale St. Maries strain is used as an outgroup species.
Transcription of A. phagocytophilum msp2.
Similar to the p44 and msp2 polycistronic expression loci, analysis of the A. phagocytophilum HZ genome sequencing data revealed two omp-1 homologues, omp-1A (BlastP E value of 3e-13) and omp-1B (BlastP E value of 6e-20), upstream of the A. phagocytophilum msp2. The omp-1A gene was 579 bp in length if a common translational start codon (AUG) was used, and omp-1B was 861 bp in length and was located 477 bp upstream of omp-1A (Fig. 1). A putative signal peptide for Omp-1B was found at amino acid positions 1 to 31. No signal peptide was detected for Omp-1A. By use of a National Center for Biotechnology domain search, a 1,494-bp ORF encoding a 56-kDa protein was located 285 bp upstream of msp2 and was identified as ubiD, a 3-polyprenyl-4-hydroxybenzote decarboxylase gene (BlastP E value of 4e-165 to unknown genes of Rickettsia prowazekii RP821 and R. conorii RC1271). The intergenic space between omp-1A and ubiD was 84 bp. The ORF upstream of the ubiD homolog in the R. prowazekii genome (RP820) is a homolog of the poly-β-hydroxybutyrate synthase gene of Azospirillum brasilense by BLAST search with BlastP E values of e-136. The upstream ORF (RC1270) of ubiD in R. conorii is annotated as an unknown sequence. The downstream ORFs of the ubiD homolog in the R. prowazekii genome (RP822) and in R. conorii (RC1272) are annotated as unknown ORFs which had high similarity with a peptidyl-prolyl cis-trans isomerase protein of Sinorhizobium meliloti, with BlastP E values of 8e-29 and e-30, respectively.
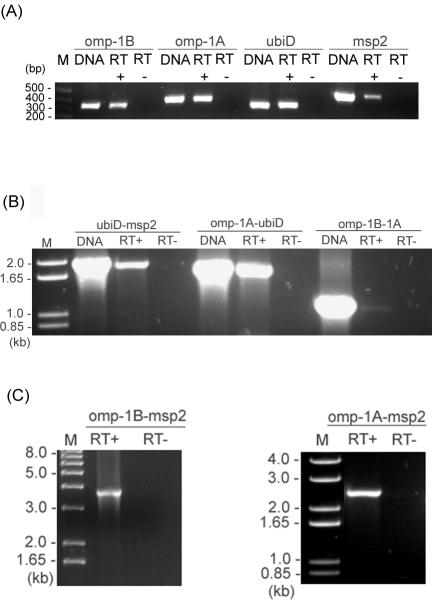
Previous studies showed that the msp2 multigene family is expressed from a single polycistronic expression locus in A. marginale (3). Both the polycistronic p44 expression locus of A. phagocytophilum and the msp2 expression locus of A. marginale have a 5′-upstream transcriptional regulator gene, tr1 (A. F. Barbet, P. F. M. Meeus, A. R. Alleman, A. Moreland, and A. M. Lundgren, Abstr. Am. Soc. Rickettsiol. 18th Meet., abstr. 30, 2003, and reference 20). However, tr1 was not found in the msp2 locus of A. phagocytophilum. To analyze the transcription at the A. phagocytophilum msp2 locus, first we examined the transcription of each gene in the locus by RT-PCR using gene-specific primers (primer sets 1 to 4 in Table 2 and Fig. 1). The RT-PCR result showed that all four genes were transcriptionally active in strain HZ (Fig. 3A). omp-1B, omp-1A, ubiD, and msp2 were found in the same transcriptional orientation, and their intergenic spaces were relatively short, indicating that these genes may be polycistronically transcribed. To examine this possibility, we used RT-PCR to find transcripts of three sets of two genes adjacent to the msp2 expression locus, including their intergenic spaces (primer sets 10 to 12 in Table 2 and Fig. 1). As shown in Fig. 3B, omp-1B-omp-1A, omp-1A-ubiD, and ubiD-msp2 regions were cotranscribed, including the intergenic spaces. The transcripts between omp-1B-msp2 and omp-1A-msp2 were examined by RT-PCR using gene-specific primers (Fig. 3C; the primers used are primer sets 5 and 6 in Table 2 and Fig. 1). The amplicons were cloned and sequenced, and the result confirmed the polycistronic transcription at this genomic locus.
FIG. 3.
RT-PCR analysis of polycistronic transcription linked to the msp2 expression locus in strain HZ. (A) RT-PCR was used for transcriptional analysis of four ORFs. (B) RT-PCR was used for transcriptional analysis of four sets of two adjacent genes and their intergenic spaces in the msp2 locus, as indicated below each panel. (C) RT-PCR was used for amplification of the regions omp-1A to msp2 and omp-1B to msp2. The DNA template control included 0.1 ng of genomic DNA from the purified HZ strain and shows the intensity and specificity of the bands detected with each pair of primers (lane labeled DNA). Primers used include sets 1 to 4, as shown in Table 2 and Fig. 1. RT+ and RT− indicate the presence and absence of reverse transcriptase, respectively. M, molecular size markers.
Characterization of the promoter regions for polycistronic transcription of the msp2 locus in strain HZ in cell culture.
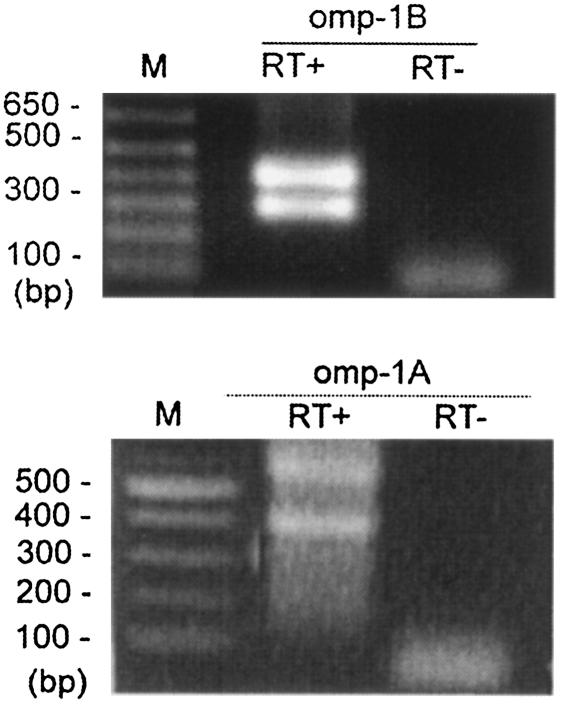
To identify the promoter region for the polycistronic transcription of the msp2 locus, we analyzed the transcriptional initiation sites of omp-1B and omp-1A by 5′RACE. With the addition of a polymeric dC tail at the 3′ end of the cDNA, the major 5′RACE product was detected as double bands (Fig. 4) for both omp-1B and omp-1A by 5′RACE. From sequence analysis of omp-1B 5′RACE products (GTTTATTCCTTAAAAACGCTGTCCTGCAGATTAGGGGGCACGGT), the initiation site for polycistronic transcription was determined to be an adenine (in boldface) located 34 bp upstream from the translational start codon AUG of omp-1B. The putative −10 and −35 promoter regions (underlined), which resemble the σ70 type consensus promoter sequences of Escherichia coli (23), were found upstream of the initiation site. The second transcriptional start site was an A which was located 49-bp downstream of the first translational start site, AUG. There was no universal translational start codon and no significant promoter could be identified from the second transcriptional start site. However, a less common alternate start codon, UUG (which encodes a leucine residue), was found 16 bp downstream of the second transcriptional start site (Fig. 4) (19).
FIG. 4.
5′RACE analysis of the promoter region located upstream from omp-1A and omp-1B in strain HZ. Primer sets 7 and 8, described in Table 2, were used in 5′RACE. RT+ and RT− indicate the presence and absence of reverse transcriptase, respectively. M, molecular size markers.
The intergenic space between omp-1A and omp-1B was relatively long, 477 bp, and the level of cotranscription of omp-1A and omp-1B was weak (Fig. 3B), suggesting the possibility of a promoter region in the intergenic space. To test this possibility, 5′RACE was performed from the omp-1A gene. The 5′RACE results showed two possible transcriptional start sites from omp-1A (Fig. 4). The first one started at a thymine which was 152-bp downstream of omp-1B. A putative σ70-type promoter (−35 and −10; underlined in the following sequence) was found upstream of the thymine (in boldface): TGACTTGTAGTGCATACACGCTAGAATGCGTACTTG. No common translational start codon (AUG) was found close to the transcriptional start site. However, a less common start codon (UUG) was found 17 bp downstream of the thymine. The codons UUG and GUG are used as start codons in >10% of E. coli genes (19). The second transcriptional start site begins at a guanine which is 185 bp downstream of the thymine. No significant promoter sequence could be found upstream of the second start site.
msp2 expression by A. phagocytophilum in patients, mice, horse, and ticks.
Expression of msp2 was examined by RT-PCR (primer set 1 in Table 2 and Fig. 1). RT-minus negative controls, p44 RT-PCR positive controls (primer set 13 in Table 2), and msp2 DNA PCR were included in the assays. In all specimens, RT-minus PCR was negative and p44 genes were transcribed (Fig. 5). msp2 was expressed by A. phagocytophilum in the blood of both HGA patients NY36 and NY37 and also their isolates NY-36 and NY-37 in HL-60 cells cultured at 37°C (Fig. 5A). It was also expressed in an immunocompetent DBA/2 mouse infected with the tick isolate NTN-1 and in a horse infected by feeding of strain HZ-infected ticks (Fig. 5B). However, msp2 expression could not be detected in the specimens from SCID mice or ticks infected with strains HZ and NTN-1 (Fig. 5B).
FIG. 5.
RT-PCR analysis of msp2 gene expression in the msp2 expression locus. (A) msp2 gene expression in the blood of HGA patients NY36 and NY37 and their corresponding isolates cultured in HL-60 cells. (B) msp2 gene expression in a horse, ticks, a DBA/2 mouse, and SCID mice. RT+ and RT− indicate the presence and absence of reverse transcriptase, respectively. DNA template control was from the same amount of peripheral blood leukocyte specimen that was used for RNA extraction. p44 RT-PCR was performed with the same tick RNA samples.
msp2 sequence comparison in A. phagocytophilum isolates from different hosts from different geographic regions.
To compare the msp2 genes in different A. phagocytophilum isolates, we used PCR with msp2-specific primers (set 9 in Table 2 and Fig. 1) to amplify the gene from the isolates shown in Table 1. msp2 was universally detected in all strains, including human isolates LL, NY-31, NY-36, and NY-37 (New York), MN-2 and MN-9 (Minnesota), sheep isolate OS (Scotland, The United Kingdom), a horse isolate MRK (California), and three tick isolates: Trustom (Rhode Island), AVK-HLPA1 (Pennsylvania), and Gaillard (Connecticut). These PCR products were cloned and sequenced and showed that msp2 was remarkably conserved within strains from the United States. The base sequence was identical in four human isolates and three tick isolates from northeastern states and in two human isolates from Minnesota. The msp2 sequences of the horse isolate MRK from California and two human isolates from Minnesota each differed from the HZ strain msp2 by a single nucleotide. This one-base change still specified the same amino acid, thus, Msp2 proteins of all human and tick isolates were identical at the amino acid level in the United States. Msp2 of the horse isolate MRK was one amino acid residue different from other Msp2 protein sequences. In contrast, the msp2 sequence of the sheep isolate OS from the United Kingdom showed 89% identity (74 bases different) at the nucleotide level and 93% identity at the amino acid sequence level to msp2 of strain HZ. Amino acid sequence comparison of the Msp2 proteins from OS and HZ strains showed that the variable region was between residues 184 and 221 of the OS strain protein.
The A. phagocytophilum msp2 genomic loci, however, appear to be diverse among different isolates. By gene-specific PCR, msp2 and ubiD were detected in all 12 isolates (primer sets 1 and 2 in Table 2 and Fig. 1). However, we could not coamplify ubiD and msp2, including the intergenic space (primer set 12 in Table 2 and Fig. 1), in 10 isolates. By gene-specific PCR (primer sets 3 and 4 in Table 2 and Fig. 1), omp-1A and omp-1B were detected in two 1995 human isolates (LL and HZ) from New York (26, 31) and two Minnesota strains (NM-2 and MN-9) (15), but neither of these two tandem genes was detected in the six remaining strains. The region of the omp-1A gene that was amplified from MN-2 and MN-9 using gene-specific primers was slightly larger than the amplicon from strain HZ. These results suggest that the sequences upstream of msp2 in A. phagocytophilum strains are rapidly changing.
DISCUSSION
A. phagocytophilum isolates have been shown to cause diseases in a wide range of mammalian species, including sheep, goats, horses, dogs, and humans. The life cycle of A. phagocytophilum involves a complex interaction between its natural host(s) or reservoir(s) and its tick vector(s) that progress through multiple life stages and may transmit infection to animals and humans. This requires that the bacterium has the ability to adapt to these multiple environments and environmental pressures. The present study identified and characterized the msp2 gene of A. phagocytophilum. The msp2 gene was found conserved among 12 strains of A. phagocytophilum. However, the upstream genes in the operon were not conserved, suggesting that this single msp2 is important, but the remaining genes are dispensable at this genomic locus for A. phagocytophilum strains. Conservation of A. phagocytophilum msp2 sequences within isolates from the United States is remarkable considering the diversity of msp2 sequences found in A. marginale strains. The A. phagocytophilum msp2 gene shows conservation that is comparable to that of the 16S rRNA gene and much higher than that of the ank gene among A. phagocytophilum isolates from the United States (10, 11, 22)
Previous studies showed that the msp2 homologous genes, collectively referred to as p44, are diversely expressed in mammals, ticks, and humans (21, 30). In the present study, we investigated msp2 expression in ticks, in immunocompetent mice and a horse, in immunocompromised SCID mice, in acutely infected human patients, and in a human promyelocytic leukemia cell line (HL-60) culture maintained at 37°C. Our results showed that msp2 was expressed in the blood of two HGA patients infected with strains NY-36 and NY-37 during acute stages of HGA (21). After the organisms were isolated (in the year 2000), msp2 continued to be expressed when A. phagocytophilum was cultivated in HL-60 cells at 37°C for more than 20 passages. msp2 of the HZ strain, first isolated from an HGA patient in 1995, continued to be expressed in HL-60 cell culture for more than 100 passages. msp2 was expressed in an immune-competent DBA/2 mouse infected by tick transmission of strain NTN-1 and in a horse after tick transmission of strain HZ. However, msp2 expression could not be detected in the blood of infected SCID mice or in that of ticks infected with strain HZ or strain NTN-1, although all these specimens were positive for A. phagocytophilum p44 by RT-PCR. Although more studies are necessary, this result suggests that differential msp2 expression is associated with host adaptive immunity. The polycistronic expression locus does not appear to be essential for msp2, because the sequences upstream of msp2 in A. phagocytophilum strains are variable among strains.
All strains of A. phagocytophilum studied have msp2, p44, and omp-1 genes, whereas A. marginale has only msp2 and omp-1 genes and lacks the p44 genes; Ehrlichia spp. have only omp-1 genes. Therefore, it may be possible that msp2, p44, and omp-1 genes arose and coevolved within the same genome by gene duplication. A. marginale may have lost its p44 genes and duplicated msp2 genes, and Ehrlichia spp. may have duplicated omp-1 genes extensively and lost both p44 and msp2 genes. On the other hand, A. phagocytophilum may have duplicated the p44 genes extensively (>80 copies) while the msp2 polycistronic expression locus has been in the process of degeneration. It is tempting to speculate that these duplications of particular groups of genes encoding major variable outer membrane proteins may have facilitated their adaptation in different host cell types (erythrocytes, granulocytes, and monocytes), in different host mammal species, and in different tick species. We could not rule out the possibility of horizontal gene transmission between A. marginale and A. phagocytophilum, and coinfection of a deer with Anaplasma centrale and A. phagocytophilum has recently been reported (M. Kawahara, K. Tahara, A. Itagaki, E. Isogai, T. Tajima, Q. Lin, and Y. Rikihisa, Abstr. 104th Gen. Meet. Am. Soc. Microbiol., abstr. Y-009, 2004).
Acknowledgments
We thank Ning Zhi for excellent assistance in tick and horse experiments.
This research was supported by grant R01AI47407 from the National Institutes of Health (NIH). The genome of A. phagocytophilum HZ was sequenced at The Institute for Genomic Research. The sequencing project was supported by NIH grant R01 AI47885 to Y.R. The A. marginale genome sequencing program at Washington State University was supported by USDA/ARS (CRIS# 5348-32000-016-00D) under the direction of Don Knowles, and support for this project was continued with a grant from the USDA (CSREES# 2001-52100-11342) awarded to Kelly Brayton and Guy Palmer.
Editor: J. T. Barbieri
REFERENCES
- 1.Alleman, A. R., G. H. Palmer, T. C. McGuire, T. F. McElwain, L. E. Perryman, and A. F. Barbet. 1997. Anaplasma marginale major surface protein 3 is encoded by a polymorphic, multigene family. Infect. Immun. 65:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asanovich, K. M., J. S. Bakken, J. E. Madigan, M. Aguero-Rosenfeld, G. P. Wormser, and J. S. Dumler. 1997. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J. Infect. Dis. 176:1029-1034. [DOI] [PubMed] [Google Scholar]
- 3.Barbet, A. F., A. Lundgren, J. Yi, F. R. Rurangirwa, and G. H. Palmer. 2000. Antigenic variation of Anaplasma marginale by expression of MSP2 mosaics. Infect. Immun. 68:6133-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbet, A. F., P. F. M. Meeus, M. Belanger, M. V. Bowie, J. Yi, A. M. Lundgren, A. R. Alleman, S. J. Wong, F. K. Chu, U. G. Munderloh, and S. D. Jauron. 2003. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect. Immun. 71:1706-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour, A. G., and B. I. Restrepo. 2000. Antigenic variation in vector-borne pathogens. Emerg. Infect. Dis. 6:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brayton, K. A., D. P. Knowles, T. C. McGuire, and G. H. Palmer. 2001. Efficient use of a small genome to generate antigenic diversity in tick-borne ehrlichial pathogens. Proc. Natl. Acad. Sci. USA 98:4130-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brayton, K. A., P. F. Meeus, A. F. Barbet, and G. H. Palmer. 2003. Simultaneous variation of the immunodominant outer membrane proteins, MSP2 and MSP3, during Anaplasma marginale persistence in vivo. Infect. Immun. 71:6627-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brayton, K. A., G. H. Palmer, A. Lundgren, J. Yi, and A. F. Barbet. 2002. Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Mol. Microbiol. 43:1151-1159. [DOI] [PubMed] [Google Scholar]
- 9.Caspersen, K., J. H. Park, S. Patil, and J. S. Dumler. 2002. Genetic variability and stability of Anaplasma phagocytophilum msp2 (p44). Infect. Immun. 70:1230-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chae, J. S., J. E. Foley, J. S. Dumler, and J. E. Madigan. 2000. Comparison of the nucleotide sequences of 16S rRNA, 444 Ep-ank, and groESL heat shock operon genes in naturally occurring Ehrlichia equi and human granulocytic ehrlichiosis agent isolates from northern California. J. Clin. Microbiol. 38:1364-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumler, J. S., K. M. Asanovich, and J. S. Bakken. 2003. Analysis of genetic identity of North American Anaplasma phagocytophilum strains by pulsed-field gel electrophoresis. J. Clin. Microbiol. 41:3392-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felek, S., S. Telford III, R. C. Falco, and Y. Rikihisa. 2004. Sequence analysis of p44 homologs expressed by Anaplasma phagocytophilum in infected ticks feeding on naive hosts and in mice infected by tick attachment. Infect. Immun. 72:659-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster, W. N., and A. E. Cameron. 1970. Observations on ovine strains of tick-borne fever. J. Comp. Pathol. 80:429-436. [DOI] [PubMed] [Google Scholar]
- 15.Goodman, J. L., C. Nelson, B. Vitale, J. E. Madigan, J. S. Dumler, T. J. Kurtti, and U. G. Munderloh. 1996. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N. Engl. J. Med. 334:209-215. [DOI] [PubMed] [Google Scholar]
- 16.Ijdo, J. W., C. Wu, S. R. Telford III, and E. Fikrig. 2002. Differential expression of the p44 gene family in the agent of human granulocytic ehrlichiosis. Infect. Immun. 70:5295-5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jauron, S. D., C. M. Nelson, V. Fingerle, M. D. Ravyn, J. L. Goodman, R. C. Johnson, R. Lobentanzer, B. Wilske, and U. G. Munderloh. 2001. Host cell-specific expression of a p44 epitope by the human granulocytic ehrlichiosis agent. J. Infect. Dis. 184:1445-1450. [DOI] [PubMed] [Google Scholar]
- 18.Kim, H. Y., and Y. Rikihisa. 2000. Expression of interleukin-1β, tumor necrosis factor alpha, and interleukin-6 in human peripheral blood leukocytes exposed to human granulocytic ehrlichiosis agent or recombinant major surface protein P44. Infect. Immun. 68:3394-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozak, M. 1999. Initiation of translation in prokaryotes and eukaryotes. Gene 234:187-208. [DOI] [PubMed] [Google Scholar]
- 20.Lin, Q., Y. Rikihisa, N. Ohashi, and N. Zhi. 2003. Mechanisms of variable p44 expression by Anaplasma phagocytophilum. Infect. Immun. 71:5650-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, Q., N. Zhi, N. Ohashi, H. W. Horowitz, M. E. Aguero-Rosenfeld, J. Raffalli, G. P. Wormser, and Y. Rikihisa. 2002. Analysis of sequences and loci of p44 homologs expressed by Anaplasma phagocytophilum in acutely infected patients. J. Clin. Microbiol. 40:2981-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massung, R. F., J. H. Owens, D. Ross, K. D. Reed, M. Petrovec, A. Bjoersdorff, R. T. Coughlin, G. A. Beltz, and C. I. Murphy. 2000. Sequence analysis of the ank gene of granulocytic ehrlichiae. J. Clin. Microbiol. 38:2917-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliver, D. 1985. Protein secretion in Escherichia coli. Annu. Rev. Microbiol. 39:615-648. [DOI] [PubMed] [Google Scholar]
- 24.Palmer, G. H., G. Eid, A. F. Barbet, T. C. McGuire, and T. F. McElwain. 1994. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect. Immun. 62:3808-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rikihisa, Y., S. A. Ewing, J. C. Fox, A. G. Siregar, F. H. Pasaribu, and M. B. Malole. 1992. Analyses of Ehrlichia canis and a canine granulocytic Ehrlichia infection. J. Clin. Microbiol. 30:143-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rikihisa, Y., N. Zhi, G. P. Wormser, B. Wen, H. W. Horowitz, and K. E. Hechemy. 1997. Ultrastructural and antigenic characterization of a granulocytic ehrlichiosis agent directly isolated and stably cultivated from a patient in New York state. J. Infect. Dis. 175:210-213. [DOI] [PubMed] [Google Scholar]
- 27.Rurangirwa, F. R., D. Stiller, D. M. French, and G. H. Palmer. 1999. Restriction of major surface protein 2 (MSP2) variants during tick transmission of the ehrlichia Anaplasma marginale. Proc. Natl. Acad. Sci. USA 96:3171-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rurangirwa, F. R., D. Stiller, and G. H. Palmer. 2000. Strain diversity in major surface protein 2 expression during tick transmission of Anaplasma marginale. Infect. Immun. 68:3023-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhi, N., N. Ohashi, and Y. Rikihisa. 1999. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J. Biol. Chem. 274:17828-17836. [DOI] [PubMed] [Google Scholar]
- 30.Zhi, N., N. Ohashi, T. Tajima, J. Mott, R. W. Stich, D. Grover, S. R. Telford III, Q. Lin, and Y. Rikihisa. 2002. Transcript heterogeneity of the p44 multigene family in a human granulocytic ehrlichiosis agent transmitted by ticks. Infect. Immun. 70:1175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhi, N., Y. Rikihisa, H. Y. Kim, G. P. Wormser, and H. W. Horowitz. 1997. Comparison of major antigenic proteins of six strains of the human granulocytic ehrlichiosis agent by Western immunoblot analysis. J. Clin. Microbiol. 35:2606-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]