Abstract
Sensory cues that predict reward or punishment are fundamental drivers of animal behavior. For example, attractive odors of palatable food or a potential mate predict reward while aversive odors of pathogen-laced food or a predator predict punishment. Aversive and attractive odors can be detected by intermingled sensory neurons that express highly related olfactory receptors and display similar central projections. These findings raise basic questions of how innate odor valence is extracted from olfactory circuits, how such circuits are developmentally endowed and modulated by state, and the relationship between innate and learned odor responses. Here, we review odors, receptors, and neural circuits associated with stimulus valence, discussing salient principles derived from studies on nematodes, insects, and vertebrates. Understanding the organization of neural circuitry that mediates odor aversion and attraction will provide key insights into how the brain functions.
Introduction
Our five basic external senses- touch, taste, vision, hearing, and smell- and our internal sensory systems that regulate bodily homeostasis are strong drivers of behavior. For example, mice exhibit fear responses to the smell of a cat, the sight of a looming hawk, or the sound of an unknown animal rustling in nearby leaves. Anatomically distinct brain regions initially process smell, sight, and sound, yet responding neural pathways can converge on similar control centers to execute a common behavior like fear. Conversely, other sensory stimuli- like the smells of mates, food, or offspring- will evoke different behaviors related to reproduction, feeding, or parental care. So within a sensory system, neural pathways that are anatomically quite similar can diverge centrally for execution of specific responses. Understanding how input from different sensory systems might converge, whereas inputs within a sensory system might diverge, presents an important challenge for study. In general, the routing logic at the interface of sensory systems and descending motor programs that enables execution of stimulus-appropriate behaviors remains poorly defined.
Here, we discuss recent advances in understanding the molecular basis of odor attraction and aversion behavior as a model for deciphering how a sensory system can evoke divergent responses. Olfaction is a powerful model system for unraveling the molecular basis of behavior- many species rely on their sense of smell for survival, and olfactory circuits are highly streamlined, using a small number of synaptic connections to convert sensory inputs into behavioral outputs [1, 2]. Our hope is that principles gleaned from understanding odor aversion and attraction will shed light on how the olfactory system can drive other divergent behaviors, like responses to pheromones that evoke or inhibit sexual behavior, aggression, and parental care [3]. We take an integrative analysis, describing odors, receptors, and neural circuits associated with aversion and attraction across species, with a focused discussion of model organisms where advances have been numerous.
Odor valence in the nematode C. elegans
Attractive and aversive odors to C. elegans
The nematode C. elegans, with its simple nervous system, provides one powerful model for mechanistic dissection of odor aversion and attraction behavior. Like other animals, C. elegans uses its olfactory system for social behavior, foraging, and pathogen avoidance [4]. Many attractants are essential nutrients, minerals, and food-associated odors that signal the presence of nearby bacterial prey [5]. Chemotaxis towards water-soluble attractants involves a characteristic movement pattern similar to the bacterial random walk, where animals pirouette and change direction at a frequency inversely related to changes in attractant concentration [6]. In addition, social cues such as pheromones can be attractive [7]. C. elegans releases and detects pheromone blends containing structurally related glycolipids called ascarosides [8–10]. Ascarosides released by C. elegans hermaphrodites attract males, and depending on concentration and the social nature of the strain, cause aggregation or dispersal behavior in other hermaphrodites [11, 12]. C. elegans also displays long-range chemotaxis behavior towards chemically diverse airborne stimuli [13], including an odor diacetyl (2,3-butanedione) for which the first nematode chemosensory receptor was identified (see below) [14].
C. elegans displays stereotyped avoidance behaviors to carbon dioxide and many volatile odors, including long chain alcohols and ketones [13, 15, 16]. Detection of pathogen-derived cues, like the lipopeptide serrawettin W2 produced during Serratia marcescens swarming behavior or biofilm-promoting metabolites from Pseudomonas aeruginosa, enables avoidance of pathogen-infested bacterial lawns [17, 18]. Odors of other pathogenic bacteria are not innately aversive, but associated illness causes learned odor avoidance through a serotonin-mediated mechanism [19]. Other stimuli also evoke olfactory learning in C. elegans, such as nutrient abundance or scarcity [4, 20], with distinct neural circuits mediating innate and acquired responses (see below) [21].
C. elegans olfactory receptors and sensory neurons
C. elegans contains three pairs of chemosensory neurons that play a major role in odor detection- AWA, AWB, and AWC neurons (Figure 1A)- as well as other sensory neurons that detect water-soluble chemicals, pheromones, oxygen, carbon dioxide, and nociceptive stimuli [4]. AWA, AWB, and AWC sensory neurons detect odors using hundreds of co-expressed chemosensory G Protein-Coupled Receptors (GPCRs) [4]. Each sensory neuron expresses a large and unique receptor repertoire, suggesting an ability to detect chemically diverse stimuli [4]. Activation of any chemosensory GPCR within a particular neuron likely has the capacity to trigger common signaling pathways, neural circuits, and behavioral responses. Laser microbeam-induced ablation of AWA and AWC neurons eliminated several odor attraction responses, while ablation of AWB neurons eliminated several odor aversion responses [13, 22]. These and other neurons have also been genetically linked to attraction and aversion behaviors by their response properties and cell-type specific rescue of key sensory signaling molecules [23, 24].
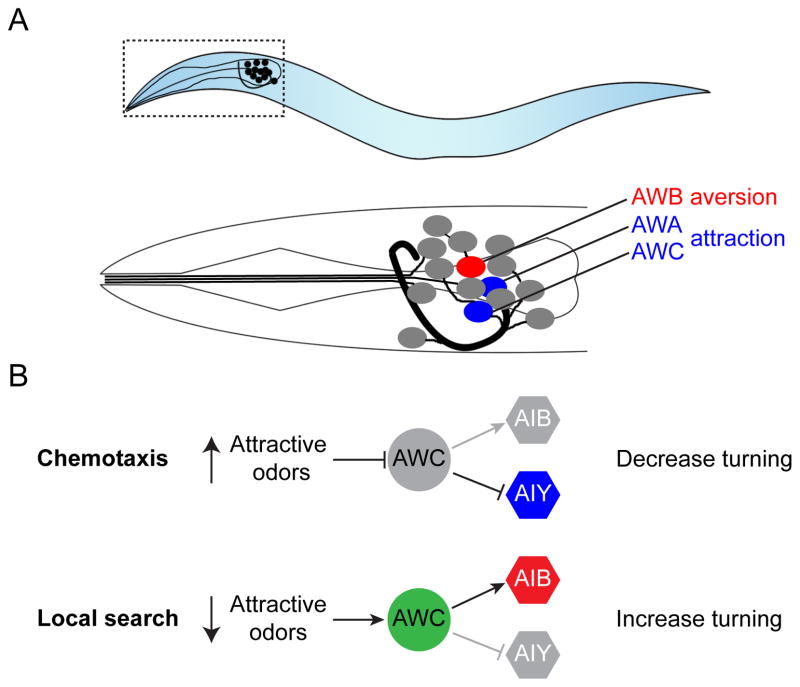
Figure 1. Aversion and attraction in C. elegans.
(A) Location of olfactory sensory neurons (AWA, AWB, AWC) in the worm head. Each AWA, AWB, and AWC neuron pair extends sensory cilia that are embedded in the sheath near the amphid pore. Image adapted from [134]. (B) AWC neurons are inhibited by attractive odors, and control turning rate by gating the activity of AIB and AIY interneurons through glutamate release.
One C. elegans chemosensory receptor, Odr-10, mediates attraction to the volatile odor diacetyl [14]. Mutant animals lacking Odr-10 have a specific chemotaxis deficit to diacetyl but not other chemicals. Odr-10 is expressed in AWA neurons that mediate attraction responses, but interestingly, misexpression of Odr-10 in AWB neurons instead of AWA neurons reverses the valence of the odor response, causing worms to avoid diacetyl [22]. These findings elegantly demonstrate that the valence of a C. elegans odor response is guided by an intrinsic property of the responding neuron, such as its connectivity or synaptic release properties, that is independent of the responding receptor. It should be noted that response valence directed by a particular sensory neuron can be reversibly encoded; a presynaptic signaling pathway in AWC axons can switch these neurons from specifying attraction to aversion behavior [25].
Higher-order processing of odor valence in C. elegans
The complete wiring diagram of the C. elegans nervous system has revealed potential avenues for information flow during odor aversion and attraction behavior. In only a few synapses- from sensory neuron to sensory interneuron to command neuron to motor neuron- an odor can drive a behavioral response [26]. For example, AWC neurons mediate either attractive chemotaxis behavior or local search behavior by gating the relative activity of different interneurons (Figure 1B) [23]. AWC neurons detect several attractive odors, and respond by hyperpolarizing and decreasing neurotransmitter release [23]. AWC neurons form excitatory glutamatergic synapses with AIB interneurons that promote turning behavior and inhibitory glutamatergic synapses with AIY interneurons that restrict turning behavior [23]. Thus, an attractive odor will restrict turning behavior by removing sensory neuron-mediated inhibition of AIY interneurons. Conversely, abrupt removal of an attractive food odor source activates AWC neurons and AIB interneurons, causing the opposite response: a dramatic increase in turning frequency characteristic of local search behavior.
Despite the apparent simplicity of the neural wiring diagram in C. elegans, the flow of information can be strikingly complex and dynamic, with neurons changing roles and synapses changing strength depending on experience and state [21, 24, 27]. Olfactory information can be controllably routed by modulating the response or release properties of neurons throughout the circuit [19, 25, 27–29], and new neurons can be recruited to the circuit during odor learning [21]. Considering sensory neurons alone, output can be changed by odor adaptation, odor sensitization, feedback from neuromodulators such as biogenic amines and insulin-like peptides, and gap junctions that function as part of a hub-and-spoke circuit (see below) [12, 20, 27–29]. Neuromodulators can impact sensory neuron responses, synaptic strength, and perhaps even the basic logic gate (or shift in behavioral salience) of a synapse [24, 25, 28]. Dynamic modulation of competing sensory neuron inputs to a particular interneuron can create a so-called push-pull circuitry motif, with the relative synaptic strengths instructing behavioral outcome [24]. The same neuromodulator can also be used in different contexts; for example, serotonin released from different neurons can either signal the rewarding presence of food or the aversive effects of pathogen-induced illness [19, 20]. Furthermore, the function of a single sensory neuron type can be flipped. Mutations in a presynaptic signaling pathway involving guanylate cyclase and diacylglycerol reverses the polarity of the AWC neuron-mediated behavioral response, and perhaps toggling of this pathway naturally underlies a state-dependent change in odor response [25].
Electrical coupling, as occurs in the hub-and-spoke circuit motif, provides another potential mechanism for dynamic control of sensory information [12]. The RMG neuron is an interneuron at the center of a hub-and-spoke circuit that controls the choice between pheromone-evoked attraction (social aggregation) or aversion (dispersal) [12]. The RMG neuron is electrically coupled to multiple sensory neurons that receive input about hermaphrodite population density- for example through detection of ascarosides or oxygen. Activity in the RMG neuron is broadly transmitted through these sensory neurons, helping to amplify and coordinate weak responses. Social strains have mutations in a receptor for an inhibitory neuromodulator Npr-1, causing a boost in hub-and-spoke output [12, 30]. Npr-1 signaling possibly restricts RMG function by dampening basal activity or by reducing the extent of electrical coupling. Signaling through hub-and-spoke circuitry can be integrated with other neuromodulator effects, enabling complex sex- and state-specific changes in neuron output [24]. The many opportunities for dynamic circuit modulation in C. elegans odor responses indicate that a full understanding of nervous system structure through a connectivity map provides only a starting point for understanding how the flow of sensory information through neural circuits can be flexibly routed to evoke behaviors that vary with state and experience [31].
Odor valence in insects: flies and mosquitoes
Attractive and aversive insect odors
Odors that attract or repel insects provide tools to thwart agricultural pests and potentially disease vectors [32, 33]. Here, we focus on insect model systems that have enabled genetic access to the olfactory system: the fruit fly, Drosophila melanogaster [34–37], and more recently the mosquito Aedes aegypti [38, 39].
Drosophila display innate attraction to food-related odors, including farnesol in citrus fruit peels, amines associated with protein breakdown, and complex odor mixtures emitted from apple cider vinegar, yeast, and natural blends of spoiled and unspoiled fruit [35, 40–42]. It should be noted that the valence of odor responses can be concentration-dependent; increasing odor levels can recruit additional olfactory receptors, change central odor representations, and even alter perceived valence [35]. Attraction to food odors is also state-dependent, with behavioral responses enhanced by food deprivation [43, 44]. Mosquitoes and flies display different feeding preferences; mosquitoes generally feed on nectar, with females about to lay eggs also feeding on blood. Female mosquitoes locate human targets using multiple sensory inputs, including sweat and skin odors, carbon dioxide in breath, and non-olfactory stimuli such as heat and visual cues [33, 38, 39]. Whereas mosquitoes are attracted to carbon dioxide (0.1% above ambient), Drosophila display a different and complex response. Walking flies avoid elevated carbon dioxide, but flies in flight track a carbon dioxide plume, suggesting state-dependent responses [46]. Carbon dioxide may function as an alarm signal released by stressed flies and/or indicate fruit palatability [36, 45]. Flies also avoid geosmin, an odor produced by harmful microbes, high concentrations of acids, and various other volatiles [34, 47, 48]. Both mosquitoes and flies innately avoid the widely used insect repellant DEET (N,N-diethyl-3-methylbenzamide), and other chemicals that target DEET-activated sensory neurons [33].
Attractive sex pheromones have been identified from thousands of insect species, including agricultural pests that are effectively controlled by luring to pheromone-laced traps [32]. The first pheromone identified in any species was bombykol, a fatty acid derivative produced by the female silkworm which is a powerful long-range attractant for conspecific males [50]. Similar far-acting attractants have not been identified in the fly, but the male sex pheromone 11-cis-vaccenyl acetate (cVA) controls courtship and male aggression, providing a powerful tool for study of sexually dimorphic neural circuitry [2, 51–54].
Insect olfactory receptors and sensory neurons
Drosophila has ~2600 olfactory sensory neurons located in sensilla of the antenna and maxillary palp. (Figure 2A; for a recent review of Drosophila olfaction, see [55]). Drosophila sensory neurons are bipolar neurons containing both sensory dendrites that detect odors and long axons that transmit information to the antennal lobe of the brain [55]. In the antennal lobe, sensory neurons communicate with second-order projection neurons at specialized structures termed glomeruli [55]. Olfactory sensory neurons detect odors using large families of chemosensory receptors: odorant receptors (ORs), ionotropic receptors (IRs), and gustatory receptors (GRs) [37, 56–59]. Drosophila ORs, IRs, and GRs are not GPCRs, but instead are heteromeric ligand-gated ion channels [56, 60–62]. Sensory neurons typically express one or a few IRs, ORs or GRs, and ORs are usually expressed together with an obligate co-receptor (Or83b or ORCO) [37]. Sensory neurons containing the same receptor target dedicated glomeruli in the fly brain, with the small size of the Drosophila olfactory system enabling a nearly comprehensive assignment of antennal lobe glomeruli and the sensory neurons that innervate them [63, 64].
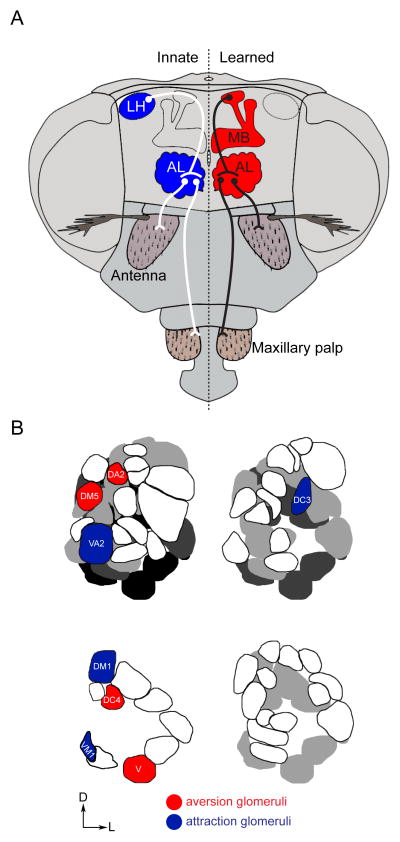
Figure 2. Anatomical organization of fly olfactory circuits.
(A) Distinct olfactory circuits mediate innate (blue) and learned (red) odor responses. Image adapted from [76]. (B) Glomeruli in the fly antennal lobe that mediate aversion (red) and attraction (blue) are intermingled and can be adjacent. Depicted glomeruli respond to vinegar (low threshold: VA2, DM1; high threshold: DM5), farnesol (DC3), amines (VM1), geosmin (DA2), acids (DC4), and CO2 (V). Anterior to posterior antennal lobe representations are depicted clockwise from the top left. Image adapted from [63, 64].
Several insect ORs and IRs are required for aversion and attraction responses to particular odors. Fly attraction to apple cider vinegar involves Or42b and Or92a, while high vinegar concentrations recruit an additional low affinity receptor, Or85a, which mediates dominant aversion responses [35]. Specific fly olfactory receptors also mediate attraction to amines (IR92a) [42], attraction to farnesol (Or83c) [41], aversion to acids (IR64a) [34, 47], and aversion to the microbe-associated odor geosmin (Or56a) [48]. Fruit-associated odors influence oviposition and male courtship through specific olfactory receptors (OR19a, IR84a), presumably to promote egg laying on preferred energy-rich substrates [65, 66]. In fly larva, two different olfactory receptors (Or42a, Or42b) mediate behavioral attraction to low and high concentrations of ethyl acetate [67]. Flies and mosquitoes display different behavioral responses to carbon dioxide (0.1–1.0% above ambient), but use orthologous receptors for detection, suggesting species-specific differences in receptor-associated neural circuitry [36, 39]. The repellant DEET activates multiple chemosensory receptors, including an IR, an OR, and a GR, and may act as a ‘confusant’ that distorts other receptor-ligand interactions [33, 38]. Aversive and attractive odor responses are mediated by intermingled and often adjacent glomeruli in the antennal lobe (Figure 2B), although some spatial organization of odor valence has been proposed [68]. Together, these findings indicate that neurons with related sensory receptors and similar projections to the olfactory bulb can generate opposing behaviors in the fly.
Higher-order processing of odor valence in insects
Each of the ~50 antennal lobe glomeruli is innervated by on average 4 projection neurons, with most projection neurons innervating a single glomerulus [69, 70]. Projection neurons generally respond to the same odors as their connected sensory neurons, with response gain and kinetics modulated by local circuits of the antennal lobe [55]. Projection neurons transmit olfactory information to two higher order olfactory nuclei, the lateral horn and the mushroom body, which are thought to mediate innate and learned odor responses respectively.
Roles for the lateral horn in innate behaviors- including attraction, aversion, and pheromone responses- have largely been inferred by observing the residual olfactory behaviors that persist after mushroom body ablation [71, 72]. The lateral horn receives input from antennal lobe projection neurons, and this input has been systematically mapped using single neuron genetic approaches [69, 70, 73, 74]. The axons of projection neurons are highly stereotyped and regionalized within the lateral horn, with those of a given glomerulus typically clustering spatially. (There are exceptions, such as the divergently innervating projection neurons from the CO2-responsive glomerulus [42].) In contrast, input from different glomeruli is more variably distributed, with projection neurons from neighboring glomeruli sometimes targeting quite distant locations [69, 70]. The lateral horn is proposed to contain multiple input zones, including dedicated zones for processing pheromones and attractive food odors [74]. Projections from individual glomeruli linked to odor attraction (amines) and aversion (acids and CO2) target topographically distinct regions of the lateral horn, raising the suggestion that valence is spatially encoded in the lateral horn [42]. Whether a lateral horn map based on ethological salience will be completely general is unclear, as projection neurons responsive to the food-associated odor farnesol innervate the putative pheromone-response zone [41].
Third-order lateral horn neurons can be classified based on their anatomy and breadth of response properties [73, 75]. For example, one group of lateral horn neurons responds broadly to odors that activate several invariant glomeruli, perhaps allowing for channeling of ecologically related odors, such as food odors, into a common hard-wired response [75]. In contrast, a second group of lateral horn neurons receives input from one glomerulus but responds more narrowly due to stereotyped inhibitory input from other coactivated glomeruli [75]. Thus, third-order neurons of the lateral horn can have either broader or narrower response fields than their connected projection neurons. How do lateral horn neurons evoke particular innate behaviors? One possibility is that different classes of lateral horn neurons couple to different descending motor programs. Experiments that traced an entire pheromone-responsive circuit from input to output revealed a class of highly tuned pheromone-responsive lateral horn neurons with sexually dimorphic projections, including to male-specific descending neurons that enter the ventral nerve cord [2]. Identifying other classes of lateral horn output neurons that evoke either innate aversion or attraction would provide a foundation for understanding how these behaviors are extracted from particular sensory inputs.
The mushroom body is the major site of associative olfactory learning in insects [72, 76, 77]. The fly mushroom body contains ~2500 intrinsic third-order neurons called Kenyon cells that together receive sensory input from ~200 projection neurons. (Other insects can have far more Kenyon cells; for example, the honeybee has 170,000 [77]). Projection neurons send diffuse collaterals across the mushroom body calyx, with extensive intermingling of projection neurons from different glomeruli [69, 70, 73]. Each Kenyon cell forms synapses with incoming projection neurons at the calyx, with Kenyon cell dendrites displaying a characteristic claw-like morphology (termed a dendritic claw) that envelops the axon of an incoming projection neuron [78]. Each Kenyon cell has on average 7 dendritic claws [79], with each claw connecting to a different projection neuron and responding to different odors [78]. Different dendritic claws of the same Kenyon cell receive input from random projection neurons, with little or no assignment bias due to the location, response properties, or ethological salience of projection neurons, or due to the class of the responding Kenyon cell [79]. Kenyon cells require activation of multiple claws to fire, with convergent input from multiple claws summed to drive Kenyon cells to spike threshold [78]. This anatomical organization and requirement for coincidence detection is consistent with the sparse and distributed pattern of odor-evoked activity observed across the Kenyon cell population [80, 81]. These findings are consistent with a primary role for the mushroom body in associative learning; it is difficult to imagine how innate responses could be specifically encoded through a random wiring architecture of unsupervised design. (See the Text Box for a discussion of mushroom body-mediated olfactory learning.)
Innate and learned odor responses can be further modulated by state and sex to ensure situation-appropriate display of attraction or aversion. For example, hungry flies display increased attraction to food odors, an effect due at least in part to neuropeptide modulation of neurons throughout olfactory circuits [43, 44]. In sensory neurons, starvation-induced decreases in insulin promote expression of the short Neuropeptide F (sNPF) receptor, through which sNPF induces presynaptic facilitation, potentiation of glomerular responses, and enhanced food-seeking behavior [44]. A related neuropeptide, Drosophila Neuropeptide F, gates activity of mushroom body-innervating dopamine neurons important for hunger state-dependent memory retrieval [43]. Olfactory circuits also differentially route pheromone input to evoke sex-specific behaviors [2]. Sexually dimorphic neural circuits develop under control of the transcription factor FruM, which is expressed in and/or shapes the architecture of first-order sensory neurons, second-order projection neurons, third-order lateral horn neurons, and fourth-order descending neurons of a pheromone-response pathway [2]. It seems that in Drosophila, as in C. elegans, state- and sex-dependent behaviors are produced through multi-tiered control of neurons at multiple levels in the circuit.
Odor valence in mice
Attractive and aversive mouse odors
Mice, like other species discussed, display attraction to food and mates as well as aversion to predators and pathogens. Aversion and attraction responses in mice, as in insects and nematodes, are strongly guided by learning. Mice display learned attraction to odors associated with energy-rich meals, through integration with taste or internal sensory pathways, and learned aversion to odors conditioned by post-ingestive illness. Mice even locate their first meal, milk, by chemotaxis towards maternal odors learned in utero and during parturition [82]. Mice also use olfactory cues for social behavior, displaying robust attraction to scent marks, pheromone-rich urine deposits used for territorial marking and mate attraction. Many odors present in mouse urine are attractive, including trimethylamine, methylthio-methylthiol (MTMT), (Z)-5-tetradecen-1-ol, dehydro-exo-brevicomin, farnesenes, and a urinary protein darcin [83–87]. Neonatal odors can be attractive to parents but neutral (mice) or aversive (rat) to sexually naive animals, with associated neural circuitry modulated by pregnancy hormones and experience [88]. Predator odors are aversive to mice upon first exposure, suggesting a hard-wired response. Predator odors avoided by mice include lipocalin proteins, the carnivore odor 2-phenylethylamine, and the fox odor 2,5-dihydro-2,4,5-trimethylthiazoline (TMT) [89–91]. Conversely, certain prey odors such as skunk odor thiols and the mouse odor trimethylamine repel would-be predators [84], and mice reportedly release alarm pheromones to warn nearby conspecifics of danger [92]. Mice also avoid carbon dioxide, spoiled food odors such as isoamylamine, aliphatic acids, and aliphatic aldehydes, and the death-associated odor cadaverine [84, 90, 93–95].
Each of these innate aversion responses can be flexible across species: for example, the carnivore odor 2-phenylethylamine is aversive to mice but is proposed to function as a sex pheromone in tigers [89, 96]. Carrion odors provide aversive pathogen-related danger signals to some animals, but attractive food-associated cues to scavengers like vultures and burying beetles [95, 97]. Finally, trimethylamine is an attractive scent odor constituent in mice but repels rats (who eat mice) and humans [84]. Such species-specific behaviors suggest that the olfactory system is capable of morphing on an evolutionary time scale to change the valence of specific odor responses. Moreover, we can take advantage of species-specific behaviors to design bait traps and repellants tailored to the olfactory systems of particular pests. The identification of aversive and attractive odors in mice and other species provides a framework for understanding responding neural pathways.
Mouse olfactory receptors and sensory neurons
Mice have ~5,000,000 olfactory sensory neurons located in the main olfactory epithelium, with additional chemosensory neurons located in the vomeronasal organ, grueneberg ganglion, and septal organ [3]. As in the fly, mouse olfactory sensory neurons are bipolar neurons, containing both sensory dendrites and long axons that innervate glomeruli in the olfactory bulb. Sensory neurons detect odors using >1,500 receptors from five evolutionarily distinct GPCR families: odorant receptors (ORs) and trace amine-associated receptors (TAARs) in the main olfactory epithelium, and vomeronasal receptors (V1Rs, V2Rs) and formyl peptide receptors (FPRs) in the vomeronasal organ [98–105]. In addition, rare sensory neurons detect odors using a membrane-associated guanylate cyclase [106].
Large-scale ablation of >100 glomeruli throughout the dorsal olfactory bulb (ΔD mutants) produced striking deficits in aversion responses to leopard urine, the fox odor TMT, 2-methylbutyric acid, isoamylamine, and other odors [90]. Interestingly, despite the absence of innate aversion to TMT and 2-methylbutyric acid, ΔD mutant animals could still detect these cues and learn to like or dislike them using other glomeruli. These findings indicate that TMT and 2-methylbutyric acid can activate multiple glomeruli with only specific glomeruli mediating innate responses.
Which olfactory sensory neurons mediate innate responses and how do they develop appropriate circuit connectivity? Only a few mouse olfactory receptors have been linked to aversion and attraction behavior. Certain receptors detect attractants, including trimethylamine (TAAR5), (Z)-5-tetradecen-1-ol (Olfr288), and MTMT (Olfr1509), and other receptors detect repellants, including the carnivore odor 2-phenylethylamine (TAAR4), hexanal (many ORs), hexanoic acid (many ORs), N-methylpiperidine (TAAR8c, TAAR9), and isoamylamine (TAAR3) [84, 87, 89, 107–109]. Knockout of mouse Taar4 eliminates 2-phenylethylamine aversion while knockout of mouse Taar5 eliminates trimethylamine attraction [84, 93]. TAAR4 and TAAR5 are encoded by adjacent genes and neurons that express these receptors project axons to adjacent glomeruli in the brain (Figure 3B) [110]; nevertheless, these receptors mediate behavioral responses of opposing valence in mouse. Interestingly, neurons initially committed to express a Taar5 knockout allele can switch receptor choice, with some neurons secondarily expressing Taar4 [110]. This observation suggests that the commitment of a sensory neuron to couple to aversion or attraction circuitry may arise relatively late in development, seemingly after receptor choice has stabilized; alternatively, it is possible that TAAR5-mediated attraction involves a learned override of a developmentally established aversion circuit.
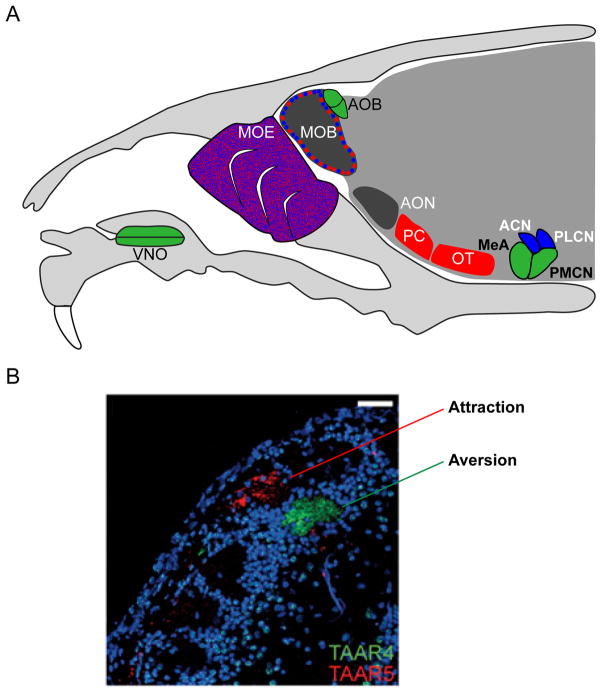
Figure 3. Olfactory pathways in the mouse.
(A) The main olfactory system involves different brain nuclei that may mediate innate (blue) and learned (red) responses. Signals from the main olfactory epithelium (MOE) are transmitted to the main olfactory bulb (MOB) and then to several brain regions including the anterior olfactory nucleus (AON), piriform cortex (PC), olfactory tubercle (OT), posterolateral cortical amygdaloid nucleus (PLCN), and anterior cortical nucleus (ACN). In the accessory olfactory pathway (green), signals from the VNO are sent to the accessory olfactory bulb (AOB) and then to the medial amygdala (MeA) and posteromedial cortical amygdalaoid nucleus (PMCN). (B) The adjacent projections of TAAR4 (green) and TAAR5 (red) sensory neurons were revealed by antibody staining [110].
Higher-order processing of odor valence in mice
Olfactory information in mice, like in flies, is processed in parallel by multiple brain regions. Sensory neurons from the main olfactory epithelium and vomeronasal organ target the main and accessory olfactory bulbs (MOB and AOB) respectively, which then couple to strikingly divergent pathways deeper in the brain (Figure 3A) [1]. MOB projection neurons (termed mitral or tufted cells) provide dense innervation of several cortical and limbic areas including the piriform cortex, cortical amygdala, and olfactory tubercle. In contrast, AOB mitral cells directly target limbic system nuclei, including the vomeronasal amygdala and bed nucleus of the stria terminalis (BNST). Despite these anatomical differences, both systems can mediate aversion and attraction behaviors [84, 86, 89, 91].
Responses to predator odors are arguably the best studied innate odor-driven mouse behaviors [111]. Different classes of predator odors (volatile vs non-volatile) are detected by the main olfactory epithelium (TMT, 2-phenylethylamine, crude predator urine) and vomeronasal organ (lipocalins, crude cat dander or collar odors) [89–91, 111]. These different predator odor classes activate non-overlapping neural pathways, as determined by focal lesion analysis and patterns of immediate early gene expression. Aversion to TMT requires several limbic system nuclei, including the medial BNST and the lateral septum, while the spoiled food odor 2-methylbutyric acid activates neurons in the adjacent lateral BNST [90, 112]. VNO-activating predator odors activate a different neural network termed the medial hypothalamic defensive system, composed of the medial amygdala, the dorsomedial region of the ventromedial hypothalamus, the premamillary nucleus, periaqueductal grey, and other brain regions [113]. Both main and accessory olfactory pathways (and predator-derived visual and auditory inputs) ultimately engage common effectors in the limbic system such as the hypothalamic-pituitary-adrenal axis which coordinates systemic stress responses.
The routing logic between sensory inputs and brain centers that control innate responses remains poorly defined in the mouse. Dense, stereotyped projections from the MOB arise in the cortical amygdala, which may function in innate odor responses [114–116]. Projections from the AOB densely innervate the nearby vomeronasal amygdala, which may serve a similar organizing role. The odors of males, females, juveniles, and predators activate neuron subsets in the medial amydala [117–120], suggesting that the vomeronasal system contains parallel processing streams relevant for different behaviors. One model is that the medial amygdala acts as a hub, receiving inputs from a large number of different vomeronasal receptors, and funneling information selectively to evoke appropriate behavioral outcomes. It will be exciting to understand the diversity of medial amygdala neuron subtypes, their roles in different innate behaviors, and whether (and how) they display stereotyped connections with specific classes of projection neurons.
Hormone signaling can sculpt olfactory circuits to generate sex- and state-dependent rodent behaviors. In the rat, neonatal odors evoke different responses in mothers and sexually naive females [88]. A change in neonatal odor valence from aversion to attraction has been attributed to the ordered release of pregnancy hormones, which in turn, cause a re-routing of amygdala outputs. Sex hormone signaling also shapes the architecture and function of olfactory circuits during a perinatal critical period and at puberty [121]. Testosterone produced during a perinatal surge is converted locally in the brain into estradiol, which perhaps counterintuitively, functions as an organizing signal to masculinize neural circuits. Higher-order olfactory nuclei, including the medial amygdala, ventromedial hypothalamus, and other areas, display sexual dimorphisms in neuron number and projections, spine density, neurotransmitter usage, and gene expression programs. It will be interesting to understand how hormone-responsive signaling pathways function at a molecular and cellular level to mold olfactory neural circuits and change behaviors.
Finally, odor aversion and attraction responses can be crafted in the mouse by experience. Associative olfactory learning can involve the piriform cortex, a large third-order olfactory nucleus that shares many similarities with the insect mushroom body. The piriform cortex is a three-layered paleocortex that receives dense and direct MOB input [114–116, 122]. The axons of incoming mitral and tufted cells innervate the superficial layer (layer 1), where they form synapses with third-order pyramidal neurons whose cell bodies are predominantly located in layer 2. Inputs from individual glomeruli are broadly dispersed across the piriform cortex, with little or no bias based on glomerulus position in the olfactory bulb [114–116, 122]. Individual piriform pyramidal cells are coincidence detectors, contacting mitral cells from several spatially uncorrelated glomeruli and requiring multiple active inputs to fire [122]. Individual odors activate 3–15% of piriform pyramidal cells [122, 123], suggesting that odor identity is encoded by neural activity patterns across cells rather than by rare neurons that function as ‘grandmother’ cells. Interestingly, activation of a stochastic ensemble of ~500 piriform pyramidal cells, arbitrarily induced to express channelrhodopsin by viral infection, can elicit either attraction or aversion behavior following training [124]. Both aversion and attraction could be similarly entrained by ensembles in different locations of the piriform cortex, suggesting valence not to be topographically encoded. How can neural ensembles be entrained to evoke divergent behaviors? One model is that there are different classes of piriform pyramidal cells relevant for aversion or attraction; alternatively, all piriform pyramidal cells may display equal and diverse synaptic contacts to output neurons involved in aversion, attraction, and presumably other behaviors, with the relative strengths of different output synapses gated by experience to allow for flexible information flow. The latter model immediately poses the question of how much functional diversity exists among fourth-order neurons. Higher order brain regions that receive input from the piriform cortex, including the ventral striatum, olfactory tubercle, and amygdala, can assign odor valence through dopamine-dependent learning [125, 126]. It is intriguing to consider that olfactory learning in both the fly and mouse may involve dopamine-dependent strengthening of the third synapse in the circuit.
Concluding thoughts
Recent work has identified odors and receptors that evoke aversion and attraction behavior in several species, providing an important framework for understanding how associated neural circuits are organized and modulated by state, sex, and experience. The olfactory systems of nematodes, insects, and vertebrates are charged with similar tasks- to find food and mates and avoid predators and pathogens. Unique olfactory system features arose in each lineage: for example, vertebrates and nematodes predominantly sense odors with GPCRs, while insects use ion channels. Also, in nematodes, individual olfactory sensory neurons express large repertoires of chemosensory receptors while in insects and vertebrates, individual olfactory sensory neurons express one or a small number of receptors. Nevertheless, many common principles have emerged. In each model organism, receptors and sensory neurons have been identified that specify innate aversion or attraction behavior. Furthermore, in flies and mice, olfactory sensory neurons form synapses at analogous brain structures termed glomeruli, and odor inputs are routed to different higher-order olfactory centers involved in innate and learned responses. Third-order neurons within learning centers (mushroom body and piriform cortex) display sparse odor responses, a distributed connectivity to projection neurons, and sensitivity to neuromodulators such as dopamine. A comparative analysis of olfactory physiology across model systems has revealed general principles of neural coding, with advances in each model system synergistically guiding progress in others.
Yet, basic questions remain about how neural circuits differentially process aversive and attractive odors. These opposing behaviors can be generated by intermingled sensory neurons with related olfactory receptors and similar central projections, pushing the question of how valence is encoded to higher order synapses in the circuit. We will be required to understand the functional diversity of second-order projection neurons, third-order neurons in the lateral horn, mushroom body, cortical amygdala, and piriform cortex, neuromodulatory neurons in learning centers, and fourth-order neurons that export olfactory information from brain nuclei that process innate and learned odor responses. At each level of the circuit, we will need to understand the diversity of neuron projections, responses, and behavioral roles. Do specific higher order neurons specify aversion, attraction, or even more complicated behaviors such as aggression, mating, or fear? If so, where in the circuit does behavioral specification occur, and what are the rules that govern connectivity to appropriate sensory neurons? Understanding the structure of odor aversion and attraction circuitry, and how information may flow through it with state-dependent dynamics, will provide insights into how a sensory system can evoke divergent behaviors, and more generally, into how the nervous system functions.
A model for olfactory learning in the mushroom body.
What are the mechanisms of associative attraction and aversion learning in the mushroom body? One compelling model is that learning is regulated at the synapse between Kenyon cells that report odor identity, and mushroom body output neurons that report behavioral significance [76, 77]. Synapses between Kenyon cells and output neurons would be strengthened by learning, via neuromodulators that provide signals of unconditioned reward or punishment and whose release occurs coincidentally with Kenyon cell activity. The same neurotransmitter, dopamine, provides an unconditioned signal for both reward (sugar) and punishment (electric shock) [127–131]. Dopamine neurons that mediate attraction and aversion learning are largely but not exclusively located in different neuron clusters extrinsic to the mushroom body, and innervate spatially segregated axonal compartments of different Kenyon cell classes [130, 131]. It is possible that a single Kenyon cell couples to different readouts through different synapses; alternatively, recent evidence indicates that aversion and attraction may be driven by non-overlapping Kenyon cell populations [132]. In the latter model, it is important that odors are represented across each subtype of Kenyon cell. Either way, output neurons must be in tight opposition to particular dopamine neurons to ensure appropriate responses- this is appreciated for one class of mushroom body output neuron (MB-V2 neurons) that mediates aversion but not appetitive memory performance [133]. Interestingly, MB-V2 neurons send projections to the lateral horn, raising the possibility that execution of learned odor responses involves recruitment of circuitry components underlying innate odor responses [133]. A comprehensive understanding of the diversity of output neurons and associated neuromodulatory neurons available to Kenyon cells may inform on the repertoire of learnable fly behaviors.
Acknowledgments
We thank David Strochlic and Erika Williams for comments on the manuscript, and an NIH grant (RO1DC013289 to SDL) for support.
References
- 1.Dulac C, Wagner S. Genetic analysis of brain circuits underlying pheromone signaling. Annu Rev Genet. 2006;40:449–467. doi: 10.1146/annurev.genet.39.073003.093937. [DOI] [PubMed] [Google Scholar]
- 2.Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- 3.Liberles SD. Mammalian pheromones. Annu Rev Physiol. 2014;76:151–175. doi: 10.1146/annurev-physiol-021113-170334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bargmann CI. Chemosensation in C. elegans. WormBook. 2006:1–29. doi: 10.1895/wormbook.1.123.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- 6.Pierce-Shimomura JT, Dores M, Lockery SR. Analysis of the effects of turning bias on chemotaxis in C. elegans. J Exp Biol. 2005;208:4727–4733. doi: 10.1242/jeb.01933. [DOI] [PubMed] [Google Scholar]
- 7.Edison AS. Caenorhabditis elegans pheromones regulate multiple complex behaviors. Curr Opin Neurobiol. 2009;19:378–388. doi: 10.1016/j.conb.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim YH, Kim K, Paik YK. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PE, Malik RU, Edison AS, Sternberg PW, Schroeder FC. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 11.Simon JM, Sternberg PW. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:1598–1603. doi: 10.1073/pnas.032225799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- 14.Sengupta P, Chou JH, Bargmann CI. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell. 1996;84:899–909. doi: 10.1016/s0092-8674(00)81068-5. [DOI] [PubMed] [Google Scholar]
- 15.Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2008;105:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bretscher AJ, Busch KE, de Bono M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2008;105:8044–8049. doi: 10.1073/pnas.0707607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meisel JD, Panda O, Mahanti P, Schroeder FC, Kim DH. Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell. 2014;159:267–280. doi: 10.1016/j.cell.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pradel E, Zhang Y, Pujol N, Matsuyama T, Bargmann CI, Ewbank JJ. Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:2295–2300. doi: 10.1073/pnas.0610281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- 20.Chao MY, Komatsu H, Fukuto HS, Dionne HM, Hart AC. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci U S A. 2004;101:15512–15517. doi: 10.1073/pnas.0403369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha HI, Hendricks M, Shen Y, Gabel CV, Fang-Yen C, Qin Y, Colon-Ramos D, Shen K, Samuel AD, Zhang Y. Functional organization of a neural network for aversive olfactory learning in Caenorhabditis elegans. Neuron. 2010;68:1173–1186. doi: 10.1016/j.neuron.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Troemel ER, Kimmel BE, Bargmann CI. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell. 1997;91:161–169. doi: 10.1016/s0092-8674(00)80399-2. [DOI] [PubMed] [Google Scholar]
- 23.Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, Bargmann CI. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450:63–70. doi: 10.1038/nature06292. [DOI] [PubMed] [Google Scholar]
- 24.Jang H, Kim K, Neal SJ, Macosko E, Kim D, Butcher RA, Zeiger DM, Bargmann CI, Sengupta P. Neuromodulatory state and sex specify alternative behaviors through antagonistic synaptic pathways in C. elegans. Neuron. 2012;75:585–592. doi: 10.1016/j.neuron.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsunozaki M, Chalasani SH, Bargmann CI. A behavioral switch: cGMP and PKC signaling in olfactory neurons reverses odor preference in C. elegans. Neuron. 2008;59:959–971. doi: 10.1016/j.neuron.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faumont S, Lindsay TH, Lockery SR. Neuronal microcircuits for decision making in C. elegans. Curr Opin Neurobiol. 2012;22:580–591. doi: 10.1016/j.conb.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, Hendricks M, Cornils A, Maier W, Alcedo J, Zhang Y. Two insulin-like peptides antagonistically regulate aversive olfactory learning in C. elegans. Neuron. 2013;77:572–585. doi: 10.1016/j.neuron.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci. 2010;13:615–621. doi: 10.1038/nn.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris GP, Hapiak VM, Wragg RT, Miller SB, Hughes LJ, Hobson RJ, Steven R, Bamber B, Komuniecki RW. Three distinct amine receptors operating at different levels within the locomotory circuit are each essential for the serotonergic modulation of chemosensation in Caenorhabditis elegans. J Neurosci. 2009;29:1446–1456. doi: 10.1523/JNEUROSCI.4585-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 31.Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- 32.Jurenka R. Insect pheromone biosynthesis. Top Curr Chem. 2004;239:97–132. doi: 10.1007/b95450. [DOI] [PubMed] [Google Scholar]
- 33.Kain P, Boyle SM, Tharadra SK, Guda T, Pham C, Dahanukar A, Ray A. Odour receptors and neurons for DEET and new insect repellents. Nature. 2013;502:507–512. doi: 10.1038/nature12594. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, Suh GS. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–223. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 37.Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- 38.DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, Vosshall LB. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498:487–491. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell. 2014;156:1060–1071. doi: 10.1016/j.cell.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruebenbauer A, Schlyter F, Hansson BS, Lofstedt C, Larsson MC. Genetic variability and robustness of host odor preference in Drosophila melanogaster. Curr Biol. 2008;18:1438–1443. doi: 10.1016/j.cub.2008.08.062. [DOI] [PubMed] [Google Scholar]
- 41.Ronderos DS, Lin CC, Potter CJ, Smith DP. Farnesol-detecting olfactory neurons in Drosophila. J Neurosci. 2014;34:3959–3968. doi: 10.1523/JNEUROSCI.4582-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Min S, Ai M, Shin SA, Suh GS. Dedicated olfactory neurons mediating attraction behavior to ammonia and amines in Drosophila. Proc Natl Acad Sci U S A. 2013;110:E1321–1329. doi: 10.1073/pnas.1215680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faucher C, Forstreuter M, Hilker M, de Bruyne M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol. 2006;209:2739–2748. doi: 10.1242/jeb.02297. [DOI] [PubMed] [Google Scholar]
- 46.Wasserman S, Salomon A, Frye MA. Drosophila tracks carbon dioxide in flight. Curr Biol. 2013;23:301–306. doi: 10.1016/j.cub.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silbering AF, Rytz R, Grosjean Y, Abuin L, Ramdya P, Jefferis GS, Benton R. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 2011;31:13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stensmyr MC, Dweck HK, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 49.Lee Y, Kim SH, Montell C. Avoiding DEET through insect gustatory receptors. Neuron. 2010;67:555–561. doi: 10.1016/j.neuron.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butenandt A, Beckmann R, Stamm D, Hecker E. Ü ber den Sexuallockstoff des Seidenspinners Bombyx mori. Reindarstellung und Konstitution. Z Naturforsch. 1959;14b:283–284. [Google Scholar]
- 51.Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- 52.Kohl J, Ostrovsky AD, Frechter S, Jefferis GS. A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell. 2013;155:1610–1623. doi: 10.1016/j.cell.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–231. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson RI. Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci. 2013;36:217–241. doi: 10.1146/annurev-neuro-062111-150533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- 58.Gao Q, Chess A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics. 1999;60:31–39. doi: 10.1006/geno.1999.5894. [DOI] [PubMed] [Google Scholar]
- 59.Scott K, Brady R, Jr, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 60.Sato K, Pellegrino M, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 61.Sato K, Tanaka K, Touhara K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc Natl Acad Sci U S A. 2011;108:11680–11685. doi: 10.1073/pnas.1019622108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 63.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 64.Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 65.Grosjean Y, Rytz R, Farine JP, Abuin L, Cortot J, Jefferis GS, Benton R. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011;478:236–240. doi: 10.1038/nature10428. [DOI] [PubMed] [Google Scholar]
- 66.Dweck HK, Ebrahim SA, Kromann S, Bown D, Hillbur Y, Sachse S, Hansson BS, Stensmyr MC. Olfactory preference for egg laying on citrus substrates in Drosophila. Curr Biol. 2013;23:2472–2480. doi: 10.1016/j.cub.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 67.Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59:110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knaden M, Strutz A, Ahsan J, Sachse S, Hansson BS. Spatial representation of odorant valence in an insect brain. Cell Rep. 2012;1:392–399. doi: 10.1016/j.celrep.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- 70.Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- 71.Heimbeck G, Bugnon V, Gendre N, Keller A, Stocker RF. A central neural circuit for experience-independent olfactory and courtship behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:15336–15341. doi: 10.1073/pnas.011314898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka NK, Awasaki T, Shimada T, Ito K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr Biol. 2004;14:449–457. doi: 10.1016/j.cub.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 74.Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, Jr, Luo L. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fisek M, Wilson RI. Stereotyped connectivity and computations in higher-order olfactory neurons. Nat Neurosci. 2014;17:280–288. doi: 10.1038/nn.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perisse E, Burke C, Huetteroth W, Waddell S. Shocking revelations and saccharin sweetness in the study of Drosophila olfactory memory. Curr Biol. 2013;23:R752–763. doi: 10.1016/j.cub.2013.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 78.Gruntman E, Turner GC. Integration of the olfactory code across dendritic claws of single mushroom body neurons. Nat Neurosci. 2013;16:1821–1829. doi: 10.1038/nn.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caron SJ, Ruta V, Abbott LF, Axel R. Random convergence of olfactory inputs in the Drosophila mushroom body. Nature. 2013;497:113–117. doi: 10.1038/nature12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Honegger KS, Campbell RA, Turner GC. Cellular-resolution population imaging reveals robust sparse coding in the Drosophila mushroom body. J Neurosci. 2011;31:11772–11785. doi: 10.1523/JNEUROSCI.1099-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murthy M, Fiete I, Laurent G. Testing odor response stereotypy in the Drosophila mushroom body. Neuron. 2008;59:1009–1023. doi: 10.1016/j.neuron.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Logan DW, Brunet LJ, Webb WR, Cutforth T, Ngai J, Stowers L. Learned recognition of maternal signature odors mediates the first suckling episode in mice. Curr Biol. 2012;22:1998–2007. doi: 10.1016/j.cub.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jemiolo B, Xie TM, Novotny M. Socio-sexual olfactory preference in female mice: attractiveness of synthetic chemosignals. Physiol Behav. 1991;50:1119–1122. doi: 10.1016/0031-9384(91)90570-e. [DOI] [PubMed] [Google Scholar]
- 84.Li Q, Korzan WJ, Ferrero DM, Chang RB, Roy DS, Buchi M, Lemon JK, Kaur AW, Stowers L, Fendt M, et al. Synchronous evolution of an odor biosynthesis pathway and behavioral response. Curr Biol. 2013;23:11–20. doi: 10.1016/j.cub.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- 86.Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 2010;8:75. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoshikawa K, Nakagawa H, Mori N, Watanabe H, Touhara K. An unsaturated aliphatic alcohol as a natural ligand for a mouse odorant receptor. Nat Chem Biol. 2013;9:160–162. doi: 10.1038/nchembio.1164. [DOI] [PubMed] [Google Scholar]
- 88.Numan M, Fleming AS, Levy F. Maternal behavior. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3. Vol. 2. St. Louis, MO: Elsevier Academic Press; 2006. pp. 1921–1993. [Google Scholar]
- 89.Ferrero DM, Lemon JK, Fluegge D, Pashkovski SL, Korzan WJ, Datta SR, Spehr M, Fendt M, Liberles SD. Detection and avoidance of a carnivore odor by prey. Proc Natl Acad Sci U S A. 2011;108:11235–11240. doi: 10.1073/pnas.1103317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- 91.Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brechbuhl J, Moine F, Klaey M, Nenniger-Tosato M, Hurni N, Sporkert F, Giroud C, Broillet MC. Mouse alarm pheromone shares structural similarity with predator scents. Proc Natl Acad Sci U S A. 2013;110:4762–4767. doi: 10.1073/pnas.1214249110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dewan A, Pacifico R, Zhan R, Rinberg D, Bozza T. Non-redundant coding of aversive odours in the main olfactory pathway. Nature. 2013 doi: 10.1038/nature12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- 95.Hussain A, Saraiva LR, Ferrero DM, Ahuja G, Krishna VS, Liberles SD, Korsching SI. High-affinity olfactory receptor for the death-associated odor cadaverine. Proc Natl Acad Sci U S A. 2013;110:19579–19584. doi: 10.1073/pnas.1318596110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brahmachary RL, Dutta J. Phenylethylamine as a biochemical marker of tiger. Z Naturforsch C. 1979;34:632–633. doi: 10.1515/znc-1979-7-824. [DOI] [PubMed] [Google Scholar]
- 97.DeVault TL, Rhodes OE, Shivik JA. Scavenging by vertebrates: behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestial ecosystems. Oikos. 2003;102:225–234. [Google Scholar]
- 98.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 99.Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 100.Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- 101.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 102.Liberles SD, Horowitz LF, Kuang D, Contos JJ, Wilson KL, Siltberg-Liberles J, Liberles DA, Buck LB. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc Natl Acad Sci U S A. 2009;106:9842–9847. doi: 10.1073/pnas.0904464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell. 1997;90:775–784. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- 104.Riviere S, Challet L, Fluegge D, Spehr M, Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459:574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- 105.Ryba NJ, Tirindelli R. A new multigene family of putative pheromone receptors. Neuron. 1997;19:371–379. doi: 10.1016/s0896-6273(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 106.Fulle HJ, Vassar R, Foster DC, Yang RB, Axel R, Garbers DL. A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons. Proc Natl Acad Sci U S A. 1995;92:3571–3575. doi: 10.1073/pnas.92.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duan X, Block E, Li Z, Connelly T, Zhang J, Huang Z, Su X, Pan Y, Wu L, Chi Q, et al. Crucial role of copper in detection of metal-coordinating odorants. Proc Natl Acad Sci U S A. 2012;109:3492–3497. doi: 10.1073/pnas.1111297109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferrero DM, Wacker D, Roque MA, Baldwin MW, Stevens RC, Liberles SD. Agonists for 13 trace amine-associated receptors provide insight into the molecular basis of odor selectivity. ACS Chem Biol. 2012;7:1184–1189. doi: 10.1021/cb300111e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD. Odor coding by a Mammalian receptor repertoire. Sci Signal. 2009;2:ra9. doi: 10.1126/scisignal.2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnson MA, Tsai L, Roy DS, Valenzuela DH, Mosley C, Magklara A, Lomvardas S, Liberles SD, Barnea G. Neurons expressing trace amine-associated receptors project to discrete glomeruli and constitute an olfactory subsystem. Proc Natl Acad Sci U S A. 2012;109:13410–13415. doi: 10.1073/pnas.1206724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–1144. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 112.Fendt M, Endres T. 2,3,5-Trimethyl-3-thiazoline (TMT), a component of fox odor - just repugnant or really fear-inducing? Neurosci Biobehav Rev. 2008;32:1259–1266. doi: 10.1016/j.neubiorev.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 113.Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- 114.Ghosh S, Larson SD, Hefzi H, Marnoy Z, Cutforth T, Dokka K, Baldwin KK. Sensory maps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature. 2011;472:217–220. doi: 10.1038/nature09945. [DOI] [PubMed] [Google Scholar]
- 115.Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011;472:191–196. doi: 10.1038/nature09714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bergan JF, Ben-Shaul Y, Dulac C. Sex-specific processing of social cues in the medial amygdala. Elife. 2014;3:e02743. doi: 10.7554/eLife.02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 119.Ferrero DM, Moeller LM, Osakada T, Horio N, Li Q, Roy DS, Cichy A, Spehr M, Touhara K, Liberles SD. A juvenile mouse pheromone inhibits sexual behaviour through the vomeronasal system. Nature. 2013;502:368–371. doi: 10.1038/nature12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- 121.Manoli DS, Fan P, Fraser EJ, Shah NM. Neural control of sexually dimorphic behaviors. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davison IG, Ehlers MD. Neural circuit mechanisms for pattern detection and feature combination in olfactory cortex. Neuron. 2011;70:82–94. doi: 10.1016/j.neuron.2011.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 124.Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R. Driving opposing behaviors with ensembles of piriform neurons. Cell. 2011;146:1004–1015. doi: 10.1016/j.cell.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- 126.Setlow B, Schoenbaum G, Gallagher M. Neural encoding in ventral striatum during olfactory discrimination learning. Neuron. 2003;38:625–636. doi: 10.1016/s0896-6273(03)00264-2. [DOI] [PubMed] [Google Scholar]
- 127.Aso Y, Siwanowicz I, Bracker L, Ito K, Kitamoto T, Tanimoto H. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenbock G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu C, Placais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 131.Waddell S. Reinforcement signalling in Drosophila; dopamine does it all after all. Curr Opin Neurobiol. 2013;23:324–329. doi: 10.1016/j.conb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Perisse E, Yin Y, Lin AC, Lin S, Huetteroth W, Waddell S. Different kenyon cell populations drive learned approach and avoidance in Drosophila. Neuron. 2013;79:945–956. doi: 10.1016/j.neuron.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sejourne J, Placais PY, Aso Y, Siwanowicz I, Trannoy S, Thoma V, Tedjakumala SR, Rubin GM, Tchenio P, Ito K, et al. Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nat Neurosci. 2011;14:903–910. doi: 10.1038/nn.2846. [DOI] [PubMed] [Google Scholar]
- 134.Ludewig AH, Schroeder FC. Ascaroside signaling in C. elegans. WormBook. 2013:1–22. doi: 10.1895/wormbook.1.155.1. [DOI] [PMC free article] [PubMed] [Google Scholar]