Abstract
The AGC kinase Sch9 regulates filamentation in Candida albicans. Here, we show that Sch9 binding is most enriched at the centromeres in C. albicans, but not in Saccharomyces cerevisiae. Deletion of CaSch9 leads to a 150- to 750-fold increase in chromosome loss. Thus, we report a previously unknown role of Sch9 in chromosome segregation.
Keywords: kinase, Sch9, centromere, chromosome segregation, kinetochore
TARGET of rapamycin complex 1 (TORC1) is a major regulator of cell growth and a nutrient sensor in all eukaryotic cells. In the pathogenic yeast Candida albicans, the AGC kinase Sch9, one of the direct downstream targets of TORC1, represses filamentation in hypoxia and under high CO2 conditions. Sch9 performs distinct functions in growth and morphogenesis depending on the availability of O2 and CO2 (Stichternoth et al. 2011). Earlier studies indicated that absence of Sch9 increases the chronological life span of Saccharomyces cerevisiae (Fabrizio et al. 2001). However, sch9 mutant cells of C. albicans have a reduced longevity only under normoxic conditions and not under hypoxic conditions (Stichternoth et al. 2011).
In this study, we sought to determine the genomic binding sites of the HA-tagged Sch9 protein by ChIP on chip (ChIP-chip) experiments under normoxia as well as hypoxia with and without elevated CO2 levels in C. albicans. Remarkably, the major binding peaks of Sch9 coincided with the centromere (CEN) regions. Centromeric Sch9 binding was observed under normoxia (Figure 1A) as well as under hypoxia with or without 6% CO2 (Figure 1B). Under all conditions, a few reproducible Sch9 binding peaks occurred outside the CEN regions as well (not shown). The ChIP-chip data were validated by semiquantitative (data not shown) and quantitative PCR (qPCR) analysis using CEN5- and CEN7-specific primers (Figure 1C).
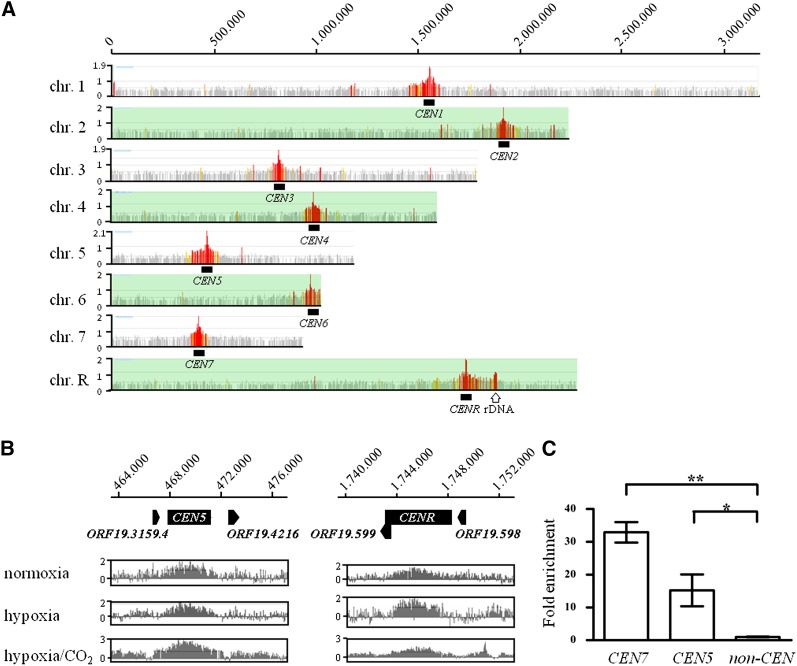
Figure 1.
Genomic localization of the Sch9 kinase. The ChIP-chip procedure was carried out essentially as described previously (Lassak et al. 2011; Schaekel et al. 2013). C. albicans genomic tiling microarrays (NimbleGen) were probed pair-wise by immunoprecipitated chromatin of a strain expressing HA-tagged Sch9 (AF1006) and the corresponding control strain (CAS1). Two independent cultures were assayed for each combination of strains. (A) An overview of Sch9 binding. Significant binding peaks were calculated by the NimbleScan software (NimbleGen) and color-coded according to their FDR values in red [false discovery rate (FDR) ≤ 0.05], orange (FDR ≤ 0.1), yellow (FDR 0.1–0.2), and gray (FDR > 0.2). Significant Sch9-binding peaks were detected at centromeres by genomic ChIP-chip on all C. albicans chromosomes. In addition, a significant peak occurred at the rDNA locus (open arrow). (B) Examples for centromeric binding of Sch9 at CEN5 and CENR. Scaled log2 ratios of cells grown in normoxia and hypoxia with or without 6% CO2 are shown. Note that Sch9 enrichment was obtained for cells grown under normoxia or hypoxia conditions. (C) Enrichment at CEN7, CEN5, and the noncentromeric region was analyzed using qPCR. qPCR analysis reveals the mean fold enrichment of Sch9 at the centromeres obtained in two independent ChIP experiments (±SD) relative to the no-tag control and normalized to the input samples. The calculation was done with two biological replicates (two ChIP samples), and each measurement was performed in triplicate. Significant difference was observed in Sch9 recruitment at the CEN5 and CEN7 region (P < 0.05) and (P < 0.01), respectively (shown by asterisks).
Enrichment of Sch9 binding at CEN regions led us to examine its possible role in the stability of the kinetochore, a multiprotein complex formed on the CEN DNA. The centromere–kinetochore complex plays a central role in the microtubule–kinetochore-mediated process of chromosome segregation. First, we analyzed the nuclear morphology in wild-type (CAI4) and mutant cells (CAS1 and CCS3) (strain construction, Southern confirmation, and genotype of strains are described in the Supporting Information, File S1, Figure S1 and Table S1, respectively). Except for a marginal increase in proportion of large-budded cells (at G2/M stage) with the unsegregated nucleus in the sch9 mutant cells (CAS1 and CCS3) as compared to the wild type (CAI4), no significant difference was evident (Figure S2). A marginal increase observed in the proportion of large-budded mutant cells having an unsegregated nuclear mass as compared to wild type is insignificant, since the wild-type cells also showed unsegregated DNA mass, as expected, during the pre-anaphase stage of the cell cycle. Moreover, a significant delay in G1 in the sch9 mutant added to the complexity of analysis. Like S. cerevisiae, centromeres are clustered throughout the cell cycle in C. albicans (Roy et al. 2011; Sanyal and Carbon 2002; Thakur and Sanyal 2012). Depletion of an essential kinetochore protein leads to centromere declustering and delocalization of the centromere-specific histone Cse4 in C. albicans (Thakur and Sanyal 2012). However, we found neither centromere declustering nor any significant change in the centromeric histone Cse4 levels at the kinetochore (Cse4-GFP intensity) in wild-type (strain 8675) and sch9 mutant (strain 8675T) strains (Figure 2A). To further investigate the role of Sch9 in Cse4 localization at the centromeres, we performed Cse4-ChIP assays with wild-type (J200) and mutant cells (J200T). We analyzed enrichment of Cse4 at CEN5 and CEN7 regions both by semiquantitative (Figure S3) and qPCR (Figure 2B) (primer sequences are listed in Table S2). Cse4 binding was found to be similar at the centromeres in the presence or absence of Sch9. Thus, Sch9 does not seem to play a direct role in Cse4-mediated kinetochore integrity in C. albicans.
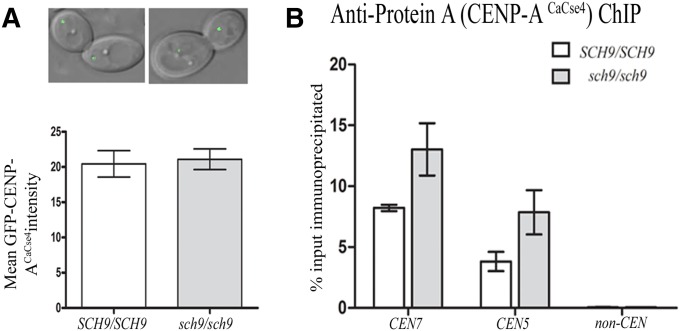
Figure 2.
Sch9 is not required for Cse4-mediated kinetochore stability. (A) Microscopy images showing Cse4-GFP signal intensities in wild-type 8675 (CSE4-GFP/CSE4;SCH9/SCH9) and mutant 8675T (CSE4-GFP/CSE4;sch9/sch9). C. albicans wild-type and mutant strains where CSE4 is GFP-tagged were grown overnight at 30° under normoxic conditions in YPDU (1% yeast extract, 2% peptone, 2% dextrose supplemented with 10mg/100ml uridine) and washed with water, and images were taken using a confocal laser-scanning microscope (LSM 510 META, Carl Zeiss). The brightest GFP signal in each cell was determined using the Image J software as described before (Roy et al. 2011). Briefly, an equal area from each cell was selected. The average pixel intensity was measured and corrected for the background by subtracting the lowest pixel intensity value in the field from the average. Then the mean GFP intensity was measured using the Image J software and the graph was plotted using Graph Pad Prism. Measurement was taken from 45 cells in each case. The experiment was performed twice. Standard error of mean (t-test) was used to calculate statistical significance (P < 0.05). For strain construction (see Supporting Information). (B) Cse4 localization at the centromere is not affected by absence of Sch9. Standard ChIP assays were performed on strains CAKS102 (CSE4-TAP/CSE4;SCH9/SCH9) and J200T (CSE4-TAP/Cse4; sch9/sch9) (grown at 30° under normoxic conditions) using anti-Protein A antibodies. Enrichment at CEN7, CEN5, and the noncentromeric region was analyzed by qPCR. PCR using total DNA (T) or ChIP DNA fractions with (+) or without (−) antibodies was performed. qPCR analysis revealed the enrichment of Cse4 at the centromere as a percentage of the total chromatin input, and values were plotted as mean of triplicates ±SD. No significant difference was observed in CaCse4 recruitment at the CEN5 and CEN7 region (P > 0.05). Percentage input was calculated as 100*2^[adjusted input − Ct(IP)] (Mukhopadhyay et al. 2008).
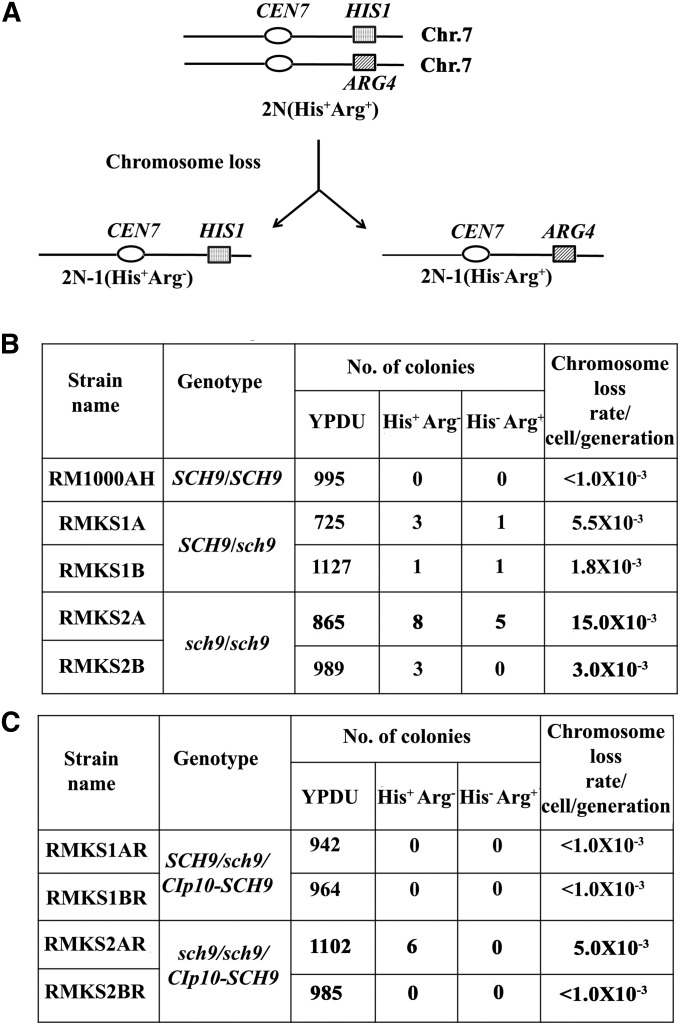
Many kinetochore proteins play crucial but nonessential roles in the process of chromosome segregation (Sanyal et al. 1998; Ortiz et al. 1999; Poddar et al. 1999; Ghosh et al. 2001; Measday et al. 2002). The centromeric localization of Sch9 prompted us to examine whether Sch9 plays a role in high-fidelity chromosome segregation. One or both of the alleles of SCH9 were deleted from the diploid genome of the C. albicans wild-type strain RM1000AH, which was previously used to study chromosome loss (Sanyal et al. 2004). Each homolog of chromosome 7 is marked by the auxotrophic marker HIS1 or ARG4 in RM1000AH (Figure 3A). The strains were confirmed by Southern blot analysis (Figure S1). We previously reported that the natural rate of loss of a chromosome in wild-type C. albicans (SN148) is <5 × 10−4/cell/generation (Mitra et al. 2014). Two independent null (sch9/sch9) mutant strains (RMKS2A and RMKS2B) exhibited a 150- to 750-fold increase in chromosome loss as compared to the spontaneous rate of loss of a chromosome (Figure 3B). Even heterozygous (SCH9/sch9) mutants of SCH9 (RMKS1A and RMKS1B) exihibited chromosome loss at a rate higher than the wild type (Figure 3B). This high rate of chromosome loss is comparable to the loss rate exhibited by several S. cerevisiae kinetochore mutants. The role of Sch9 in chromosome segregation was further validated by re-integrating the SCH9 ORF, including its native promoter and terminator sequences at the RPS10 locus. While the chromosome loss was completely suppressed in re-integrants (RMKS1AR and RMKS1BR) generated in the SCH9/sch9 mutant background (RMKS1A and RMKS1B), a reduced rate of loss was observed in re-integrants (RMKS2AR and RMKS2BR) in the sch9/sch9 null mutant background (RMKS2A and RMKS2B) (Figure 3C). While this assay measures the loss of heterozygosity, the loss of an unlinked single nucleotide polymorphism (SNP) on chromosome 7 along with the loss of a marker gene on the same chromosome could determine the loss of the entire chromosome. We observed that SNPs on chromosome 7 in the strains used in this study are absent, as compared to another C. albicans strain reported previously (Forche et al. 2009). However, binding of Sch9 to all centromeres combined with a higher rate of loss of the marker gene by the homozygous and heterozygous mutants led us to conclude that absence of Sch9 indeed increases the rate of chromosome loss. This confirms the critical role of Sch9 in the high-fidelity process of chromosome segregation.
Figure 3.
Chromosome loss assay. (A) Schematic of chromosome loss assay. (B and C) The chromosome loss assay was performed with two independent transformants of both mutants and revertants, as described before (Sanyal et al. 2004). The numbers indicate the summation of colonies patched in independent experiments. Briefly, the strains were grown for ∼20 generations on YPDU medium at 30° under normoxic conditions. Subsequently, ∼1000 cells were plated on YPDU agar plates for each transformant and incubated at 30° for 2 days. The single colonies were patched on YPDU, SD minimal medium (SD) without arginine (CM−arg), and SD without histidine (CM−his). The chromosome loss rate was calculated by the number of colonies that were unable to grow on selective media divided by the total number of colonies grown on nonselective media.
To verify if centromere DNA binding of the Sch9 kinase also occurs in other yeast species outside the Candida-specific CTG clade, we carried out a genome-wide ChIP-chip experiment to localize HA-tagged ScSch9 (Pascual-Ahuir and Proft 2007) in S. cerevisiae. No detectable binding of ScSch9 to any centromere region was found. However, as in C. albicans, the ribosomal DNA (rDNA) locus showed significant ScSch9 binding in S. cerevisiae (data not shown). We conclude that Sch9 binding to centromeres is not a general feature among Hemiascomycetes fungi and may have arisen specifically in C. albicans, a member of the CTG clade, while binding to rDNA remained conserved. It would be tempting to speculate that the association of Sch9 with centromeres might have arisen specifically in the CTG clade. Nevertheless, Sch9 is important for growth in both S. cerevisiae and C. albicans (Pascual-Ahuir and Proft 2007; Stichternoth et al. 2011).
Rapid fungal growth requires effective biosynthetic, metabolic, and regulatory activities of cells. Nutrient abundance is signaled by the Tor1 pathway via the Sch9 AGC kinase. Thus, the remarkable strong binding of Sch9 to centromeres could be related to effective chromosomal replication. Incidentally, deletion of a centromere-proximal replication origin leads to a moderate increase in chromosome loss (Mitra et al. 2014). In addition, phospho-regulation of kinetochore proteins by kinases (such as Aurora B kinase/polo-like kinase 1) has been shown to be critical for proper chromosome segregation (Shang et al. 2003; McKinley and Cheeseman 2014). Unlike short 125-bp, genetically determined, sequence-specific point centromeres of S. cerevisiae, C. albicans chromosomes contain unique sequence-independent epigenetically specified regional centromeres (Sanyal et al. 2004; Baum et al. 2006; Thakur and Sanyal 2013). While the targets of this AGC kinase Sch9 are largely unknown, centromere binding of this protein selectively in C. albicans but not in S. cerevisiae provides new insights of functional evolution of a protein in organisms having different types of centromeres.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Department of Biotechnology, Government of India, and intramural funding from the Jawaharlal Nehru Centre for Advanced Scientific Research (to K.S.) and by grants from the Jürgen Manchot Stiftung Düsseldorf (to A.S. and L.v.W.) and the ERA-NET PathoGenoMics project OXYstress (to J.F.E.). N.V. and R.S. are supported by fellowships from the Council of Scientific and Industrial Research and University Grants Commission (Government of India), respectively.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.173542/-/DC1.
ChIP-chip data have been deposited at http://www.candidagenome.org/download/systematic_results/Chakraborty_2014/.
Communicating editor: A. P. Mitchell
Literature Cited
- Baum M., Sanyal K., Mishra P. K., Thaler N., Carbon J., 2006. Formation of functional centromeric chromatin is specified epigenetically in Candida albicans. Proc. Natl. Acad. Sci. USA 103: 14877–14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P., Pozza F., Pletcher S. D., Gendron C. M., Longo V. D., 2001. Regulation of longevity and stress resistance by Sch9 in yeast. Science 292: 288–290. [DOI] [PubMed] [Google Scholar]
- Forche A., Steinbach M., Berman J., 2009. Efficient and rapid identification of Candida albicans allelic status using SNP-RFLP. FEMS Yeast Res. 9: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. K., Poddar A., Hajra S., Sanyal K., Sinha P., 2001. The IML3/MCM19 gene of Saccharomyces cerevisiae is required for a kinetochore-related process during chromosome segregation. Mol. Genet. Genomics 265: 249–257. [DOI] [PubMed] [Google Scholar]
- Lassak T., Schneider E., Bussmann M., Kurtz D., Manak J. R., et al. , 2011. Target specificity of the Candida albicans Efg1 regulator. Mol. Microbiol. 82: 602–618. [DOI] [PubMed] [Google Scholar]
- McKinley K. L., Cheeseman I. M., 2014. Polo-like kinase 1 licenses CENP-A deposition at centromeres. Cell 158: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measday V., Hailey D. W., Pot I., Givan S. A., Hyland K. M., et al. , 2002. Ctf3p, the Mis6 budding yeast homolog, interacts with Mcm22p and Mcm16p at the yeast outer kinetochore. Genes Dev. 16: 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S., Gomez-Raja J., Larriba G., Dubey D. D., Sanyal K., 2014. Rad51-Rad52 mediated maintenance of centromeric chromatin in Candida albicans. PLoS Genet. 10: e1004344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A., Deplancke B., Walhout A. J., Tissenbaum H. A., 2008. Chromatin immunoprecipitation (ChIP) coupled to detection by quantitative real-time PCR to study transcription factor binding to DNA in Caenorhabditis elegans. Nat. Protoc. 3: 698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J., Stemmann O., Rank S., Lechner J., 1999. A putative protein complex consisting of Ctf19, Mcm21, and Okp1 represents a missing link in the budding yeast kinetochore. Genes Dev. 13: 1140–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Ahuir A., Proft M., 2007. Control of stress-regulated gene expression and longevity by the Sch9 protein kinase. Cell Cycle 6: 2445–2447. [DOI] [PubMed] [Google Scholar]
- Poddar A., Roy N., Sinha P., 1999. MCM21 and MCM22, two novel genes of the yeast Saccharomyces cerevisiae are required for chromosome transmission. Mol. Microbiol. 31: 349–360. [DOI] [PubMed] [Google Scholar]
- Roy B., Burrack L. S., Lone M. A., Berman J., Sanyal K., 2011. CaMtw1, a member of the evolutionarily conserved Mis12 kinetochore protein family, is required for efficient inner kinetochore assembly in the pathogenic yeast Candida albicans. Mol. Microbiol. 80: 14–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal K., Carbon J., 2002. The CENP-A homolog CaCse4p in the pathogenic yeast Candida albicans is a centromere protein essential for chromosome transmission. Proc. Natl. Acad. Sci. USA 99: 12969–12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal K., Ghosh S. K., Sinha P., 1998. The MCM16 gene of the yeast Saccharomyces cerevisiae is required for chromosome segregation. Mol. Gen. Genet. 260: 242–250. [DOI] [PubMed] [Google Scholar]
- Sanyal K., Baum M., Carbon J., 2004. Centromeric DNA sequences in the pathogenic yeast Candida albicans are all different and unique. Proc. Natl. Acad. Sci. USA 101: 11374–11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaekel A., Desai P. R., Ernst J. F., 2013. Morphogenesis-regulated localization of protein kinase A to genomic sites in Candida albicans. BMC Genomics 14: 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C., Hazbun T. R., Cheeseman I. M., Aranda J., Fields S., et al. , 2003. Kinetochore protein interactions and their regulation by the Aurora kinase Ipl1p. Mol. Biol. Cell 14: 3342–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stichternoth C., Fraund A., Setiadi E., Giasson L., Vecchiarelli A., et al. , 2011. Sch9 kinase integrates hypoxia and CO2 sensing to suppress hyphal morphogenesis in Candida albicans. Eukaryot. Cell 10: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur J., Sanyal K., 2012. A coordinated interdependent protein circuitry stabilizes the kinetochore ensemble to protect CENP-A in the human pathogenic yeast Candida albicans. PLoS Genet. 8: e1002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur J., Sanyal K., 2013. Efficient neocentromere formation is suppressed by gene conversion to maintain centromere function at native physical chromosomal loci in Candida albicans. Genome Res. 23: 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.