Abstract
Protein kinases represent one of the largest families of genes found in eukaryotes. Kinases mediate distinct cellular processes ranging from proliferation, differentiation, survival, and apoptosis. Ligand-mediated activation of receptor kinases can lead to the production of endogenous H2O2 by membrane-bound NADPH oxidases. In turn, H2O2 can be utilized as a secondary messenger in signal transduction pathways. This review presents an overview of the molecular mechanisms involved in redox regulation of protein kinases and its effects on signaling cascades. In the first half, we will focus primarily on receptor tyrosine kinases (RTKs), whereas the latter will concentrate on downstream non-receptor kinases involved in relaying stimulant response. Select examples from the literature are used to highlight the functional role of H2O2 regarding kinase activity, as well as the components involved in H2O2 production and regulation during cellular signaling. In addition, studies demonstrating direct modulation of protein kinases by H2O2 through cysteine oxidation will be emphasized. Identification of these redox-sensitive residues may help uncover signaling mechanisms conserved within kinase subfamilies. In some cases, these residues can even be exploited as targets for the development of new therapeutics. Continued efforts in this field will further basic understanding of kinase redox regulation, and delineate the mechanisms involved in physiologic and pathological H2O2 responses.
Keywords: Cysteine, redox regulation, protein oxidation, hydrogen peroxide, NADPH oxidase, growth factor signaling, protein kinases
Introduction
The response to extracellular stimuli relies on an integrated network of signal transduction pathways that function in a highly coordinated manner. Crosstalk between these networks is modulated in part by phosphorylation of target proteins by protein kinases and the complementary actions of protein phosphatases (Chiarugi and Buricchi, 2007). Initiation of signaling cascades occurs through the activation of receptor tyrosine kinases (RTKs) upon stimulation with growth factors and other ligands. RTKs relay signals downstream through activation of effector proteins, such as non-receptor kinases. Protein kinases (receptor and non-receptor) are among one of the largest families of genes found within eukaryotes. For example, the human genome contains ∼ 518 kinase genes, constituting about 1.7% of all human genes (Manning et al., 2002). Kinases are critical enzymes involved in mediating a number of fundamental processes including the cell cycle, proliferation, differentiation, metabolism, migration, and survival (Schlessinger, 2000, Hubbard and Till, 2000). Perturbation of physiologic kinase signaling due to mutations and other genetic alterations can result in aberrant kinase activity and malignant transformation, as characterized in a number of human disease states (Blume-Jensen and Hunter, 2001).
During cellular signaling, kinase activity can also be modulated by additional mechanisms in conjunction with phosphorylation. A wealth of evidence now implicates the regulatory role of reactive oxygen species (ROS) in signal transduction networks (Rhee, 2006, D'Autreaux and Toledano, 2007, Dickinson and Chang, 2011). Controlled production of endogenous hydrogen peroxide (H2O2) can be induced when stimulants bind to their respective receptors (i.e. RTKs) (Finkel, 2011). In turn, H2O2 modulates downstream pathways by reacting with specific protein targets through oxidative modification of key cysteine residues. Protein kinases represent a large number of candidates that are regulated by and utilize H2O2 as secondary messengers (Nakashima et al., 2002, Aslan and Ozben, 2003). For more detailed discussion of general concepts underlying redox signaling, the interested reader is referred to the following extensive reviews for additional information on these topics (D'Autreaux and Toledano, 2007, Dickinson and Chang, 2011, Giorgio et al., 2007, Lambeth, 2004, Stone and Yang, 2006, Mamathambika and Bardwell, 2008, Go and Jones, 2013, Depuydt et al., 2011). Abnormal H2O2 production can result in promiscuous damage of biomolecules, and is prevalent in aging and disease states such as human cancers, neurodegenerative disorders, and diabetes (Finkel et al., 2007, Barnham et al., 2004, Lowell and Shulman, 2005).
In this review, we provide an overview of the molecular mechanisms involved in redox regulation of protein kinases and its effects on downstream signaling cascades. We begin by highlighting key examples of receptor kinases characterized by stimulant-induced H2O2 production as well as non-receptor kinases. Components involved in the production and modulation of H2O2 levels during kinase signaling will be addressed. In addition, we discuss the functional role of H2O2-mediated signaling with regards to kinase activity. Finally, we shift our focus to examples from the literature that demonstrates direct modulation of protein kinases by H2O2 occurs through cysteine oxidation of key residues. Depending on the kinase, direct oxidation can result in activation or deactivation. Continued efforts geared towards identifying protein targets of signal-derived H2O2 will further our mechanistic understanding of redox-based kinase signaling, and may lead to the development of new therapeutic strategies.
Sections
Evidence for Redox Regulation of Receptor Tyrosine Kinases
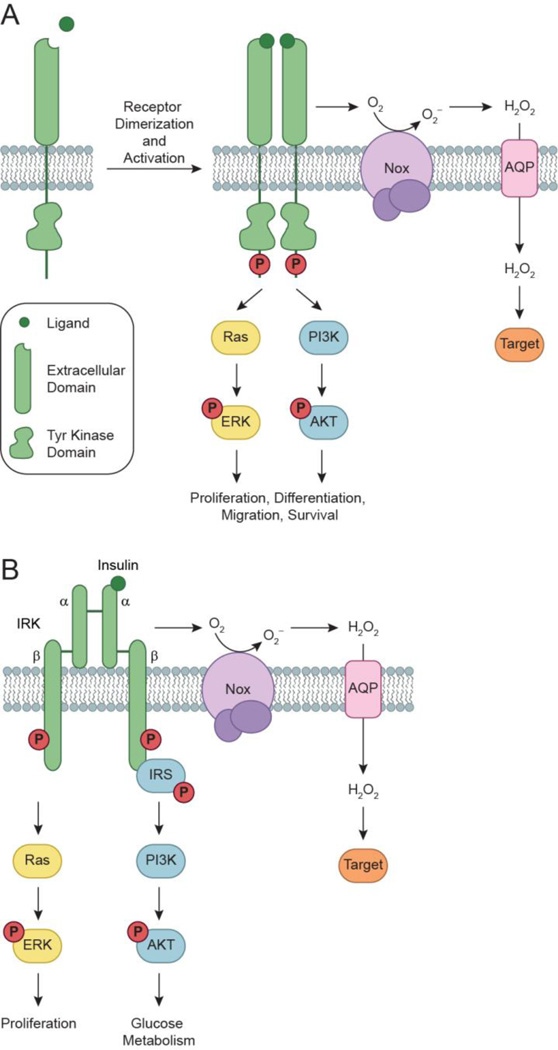
RTKs play an important role in recognition and response to stimulant binding. These enzymes are composed of an extracellular ligand binding domain, a transmembrane domain, and an intracellular domain containing a conserved tyrosine kinase core and additional regulatory sequences. The RTKs covered in this review, with the exception of the insulin receptor kinase (IRK), exist as monomers in the cellular membrane. In a simplified model (Fig. 1a), the receptor-ligand interaction induces receptor dimerization (homo- or hetero-), followed by autophosphorylation of key residues located within the tyrosine kinase core to provide docking sites for binding proteins. Once activated, the RTK relays the signal downstream through non-receptor kinases to mediate a number of biological processes.
Figure 1.
Activation of RTKs and downstream signaling cascades. (a) Growth factors bind to receptor tyrosine kinases (RTKs) to induce receptor dimerization, followed by autophosphorylation of key tyrosine (Tyr) residues (red circles) located within its cytoplasmic domain. In turn, these phosphorylated Tyr residues serve as docking sites for associating proteins to activate a number of downstream signaling cascades. Two such pathways, Ras/ERK and PI3K/AKT, are shown here for simplicity. Ligand-receptor interactions also trigger the assembly and activation of NADPH oxidase (Nox) complexes, followed by subsequent production of H2O2 through spontaneous dismutation or action of superoxide dismutase (SOD). Once formed, endogenous H2O2 may pass through specific aquaporin (AQP) channels and/or diffuse across the membrane to reach the intracellular cytosol. Transient increases in H2O2 lead to the oxidation of localized redox targets. (b) Unlike other RTKs, insulin receptor kinase (IRK) exists as a heterotetrameric receptor composed of two extracellular α subunits and two transmembrane β subunits. Binding of insulin to IRK α subunits induces a conformational change in its quaternary structure to enable ATP binding, receptor autophosphorylation, and production of Nox-derived H2O2. Once activated, IRK recruits members of the insulin receptor substrate (IRS) protein family to initiate glucose metabolism through the PI3K/AKT pathway. Insulin signaling also has mitogenic effects that are mediated through Ras/ERK.
Beginning in the 1990s, several groups observed that growth factor stimulation resulted in rapid bursts of intracellular H2O2. Contrary to the preconceived notion that once considered ROS as toxic byproducts of aerobic respiration, ensuing efforts have established that these species can act as secondary messengers. In a landmark study by Finkel and colleagues, platelet-derived growth factor (PDGF)-induced H2O2 production was shown to have downstream effects on global Tyr phosphorylation levels, activation of MAPK pathways, DNA synthesis, and chemotaxis (Sundaresan et al., 1995). Thereafter, epidermal growth factor (EGF) was reported to induce increased endogenous ROS levels upon receptor binding (Bae et al., 1997). Inhibition of ROS production by peroxide-detoxifying enzymes and chemical inhibitors blocked normal Tyr signaling triggered by growth factor stimulation. These observations, along with additional growth factors (Table 1), suggest that RTK stimulation may utilize redox-based mechanisms during signal transduction in parallel to protein phosphorylation.
Table 1.
Growth factors (that induce ROS production.
| Growth factor | Organism* | ROS source† | Effect of stimulant | Reference |
|---|---|---|---|---|
| PDGF | M, R | Nox | Proliferation, migration | Sundaresan et al., 1995 |
| Sundaresan et al., 1996 | ||||
| EGF | H | Nox | Proliferation | Bae et al., 1997 |
| Miller et a!., 2007 | ||||
| VEGF | H, P | L, Nox | Proliferation, migration, angiogenesis | Colavitti et al., 2002 |
| Ushio-Fukai et al., 2002 | ||||
| FGF | B | Nox | Proliferation | Lo&Cruz, 1995 |
| Insulin | M, R | Nox | Glucose metabolism, glucose uptake/transport | May & de Haen, 1979b |
| Mahadev et al., 2001b |
B, bovine; H, human; M, mouse; P, pig; R, rat.
L, lipoxygenase; ND, not determined.
Downstream Signaling Pathways Mediated by RTKs
Ligand stimulation of RTKs activates a number of signaling routes that include, but are not limited to the following: 1) Ras/mitogen activated protein kinase (MAPK) pathway, 2) phosphatidylinositol 3’ kinase (PI3K)/Akt pathway, or 3) phospholipase C-γ (PLC-γ). RTKs essentially share similar transduction machinery to elicit, in many cases, different cellular responses. In the Ras/MAPK pathway, signaling is triggered in a sequential order (Ras-Raf-MEK-Erk1/2). This pathway is initiated by receptor recruitment of adaptor protein SHC, growth factor receptor-bound protein 2 (Grb2), and a guanine nucleotide exchange protein (SOS) to form a tertiary complex. SOS facilitates GDP exchange to activate the small GTPase Ras. Ras completes the relay by stimulating a kinase cascade through activation of Raf (MAP3K), MEK, and Erk1/2 (MAPK). Upon activation, Erk1/2 (extracellular regulated kinase 1/2) translocates to the nucleus whereby it phosphorylates substrates (∼ 200 known targets to date) ranging from transcription factors, other kinases and phosphatases, and cytoskeletal elements (Lu and Xu, 2006, Wortzel and Seger, 2011). Small fractions of Erk1/2 may localize to other subcellular compartments such as the mitochondria, Golgi apparatus, or cell membrane to dictate signal specificity. Erk1/2 regulates cellular proliferation, differentiation, and survival, whereas other MAPKs such as JNK (Jun N-terminal kinase) and p38 function mainly as stress-activated kinases involved in inflammatory response and apoptosis (Mebratu and Tesfaigzi, 2009, Matsuzawa and Ichijo, 2005). MAPK pathways are known to be subject to redox regulation, albeit mainly through indirect mechanisms occurring upstream (McCubrey et al., 2006).
The PI3K/Akt pathway represents a common route activated by RTKs susceptible to redox-based modulation (Leslie, 2006). Upon stimulation, PI3K increases the intracellular levels of phosphatidylinositol (3,4,5) triphosphate (PIP3). PI3K phosphorylates phosphatidylinositol (4,5) bisphosphate (PIP2) at its 3-hydroxyl position to generate PIP3. PIP3 acts as a lipid secondary messenger to activate Akt (also known as PKB, protein kinase B) by binding to its pleckstrin homology (PH) domain (Stokoe et al., 1997, Franke et al., 1997). This results in translocation of Akt to the membrane, wherein it undergoes phosphorylation by PDK1 (phosphoinositide dependent kinase 1) and the mTOR (mammalian target of rapamycin)-rictor kinase complex to enable full activation. Akt phosphorylates a number of targets (∼ 100 known to date) to mediate a vast range of biological processes involved in pro-survival events (Manning and Cantley, 2007). PI3K/Akt activity is negatively regulated by a dual protein and lipid phosphatase, PTEN (phosphatase and tensin homologue) (Leslie et al., 2003). PTEN modulates PI3K activity by hydrolyzing the 3-phosphate group from PIP3, and thereby functions as a crucial tumor suppressor in this pathway (Stambolic et al., 1998, Cantley and Neel, 1999).
Sources of Reactive Oxygen Species (ROS)
Biologically relevant ROS include superoxide (O2•−), hydrogen peroxide (H2O2), and the hydroxyl radical (•OH). The half-lives of superoxide (t1/2 = 10−6 s) and hydroxyl radicals (t1/2 = 10−9 s) are considerably shorter when compared to hydrogen peroxide (t1/2 = 10−5 s) (Giorgio et al., 2007). Superoxide can spontaneously dismutate to H2O2 (∼ 105 M−1 s−1), a process greatly enhanced by superoxide dismutases (SODs) (McCord and Fridovich, 1969, Hsu et al., 1996). SODs catalyze the conversion of superoxide into H2O2 and O2 (∼ 7 × 109 M−1 s−1) to maintain steady-state levels of superoxide (∼ 10−10 M) (Fridovich, 1995, Gardner et al., 1995). Reaction of H2O2 with trace metal ions (Fe2+ or Cu2+) through Fenton or Haber-Weiss chemistry generates •OH (in vivo concentration of 10−15 M) (Jacob and Winyard, 2009). H2O2 is the most abundant form of ROS (in vivo concentration of 10−7 M), and considered to be the most stable species generated in response to biological stimuli. Its rapid production is selectively perceived by downstream targets, and subsequently undergoes controlled degradation by antioxidant defense systems. The uncharged nature and relative stability of H2O2 permits it to freely diffuse across membranes to participate as a messenger in signal transduction. More recently, evidence has demonstrated that aquaporin channels can facilitate translocation of H2O2 across membranes (Bienert et al., 2006, Bienert et al., 2007, Miller et al., 2010).
Spatial and temporal production of H2O2 allows specificity during cellular signaling. The NADPH oxidase (Nox) family of enzymes represents a major source of endogenous ROS generated during redox-mediated kinase signaling. Nox enzymes produce ROS by translocating an electron from reduced nicotinamide adenine dinucleotide phosphate (NADPH) across the cell membrane (Lambeth, 2004). The prototypical Nox enzyme, Nox2 (also known as gp91phox), was initially thought to be confined to phagocytes to protect against microbial invasion by generating millimolar quantities of H2O2. Other Nox homologues (Nox1–5 and Duox1–2) have since been identified in nonphagocytes and exhibit differential subcellular localization and cell- and tissue-specific expression patterns (Suh et al., 1999, Cheng et al., 2001, De Deken et al., 2000). Activation of Nox complexes requires binding of flavin adenine dinucleotide (FAD) cofactors, association of cytoplasmic coactivator proteins (Nox1–4, Duox1–2), and/or calcium to its intracellular domain (Nox5, Duox1–2).
Mitochondrial-derived oxidants represent another major source of intracellular ROS. Formation of ROS occurs when electrons leak prematurely from the electron transport chain (ETC) and result in the incomplete reduction of O2 and generation of superoxide (Raha and Robinson, 2000, St-Pierre et al., 2002). This process was believed to be an inevitable repercussion of aerobic existence, but cellular stimuli have been shown to initiate controlled mitochondrial ROS production (i.e. redox enzyme p66Shc) (Migliaccio et al., 1999, Giorgio et al., 2005). It is important to note that other enzymes such as xanthine oxidase, cytochrome P-450, cyclooxygenases, and lipoxygenases can also serve as critical sources of ROS within the cellular context (Finkel, 2011).
Reactive nitrogen species (RNS) may also act as biologically relevant signaling molecules. Reaction of cysteine thiols with RNS, such as nitric oxide (NO), generates nitrosothiols (SNO). This process, known as S-nitrosylation, is reversible and participates in cellular signaling (Hess and Stamler, 2012). Evidence supporting S-nitrosylation during kinase signaling will be referenced appropriately within their respective sections.
Regulation of ROS Levels During Signal Transduction
The concentration of H2O2 must increase rapidly above a certain threshold, ranging from low nanomolar to low micromolar levels, and remain elevated long enough for it to oxidize its effectors in order to serve as an efficient signaling molecule (Stone and Yang, 2006). Signal duration can be mediated through the collective efforts of antioxidant defense systems. Enzymes such as catalase, peroxiredoxins (Prxs), and glutathione peroxidases (Gpxs) contribute by catalyzing the dismutation (catalase) or reduction (Prxs, Gpxs) of H2O2 (Hall et al., 2009, Klomsiri et al., 2011). Peroxiredoxins, namely 2-Cys Prxs, exist as homodimers and contain a peroxidatic cysteine. This residue becomes oxidized upon reaction with H2O2 to a sulfenic acid, and condenses with a resolving cysteine in an adjoining subunit to form an intermolecular disulfide. Glutathione peroxidases (i.e. in higher eukaryotes) contain a selenocysteine that is oxidized by H2O2 to SeOH, and forms a disulfide with glutathione. Disulfide bonds are reduced by buffering systems such as thioredoxin/thioredoxin reductase (Trx/TrxR) (for Prxs) or glutathione/glutaredoxin (GSH/Grx) (for Gpxs) with the aid of reducing equivalents provided by NADPH. Completion of the catalytic cycle enables these enzymes to maintain intracellular H2O2 levels as deemed necessary.
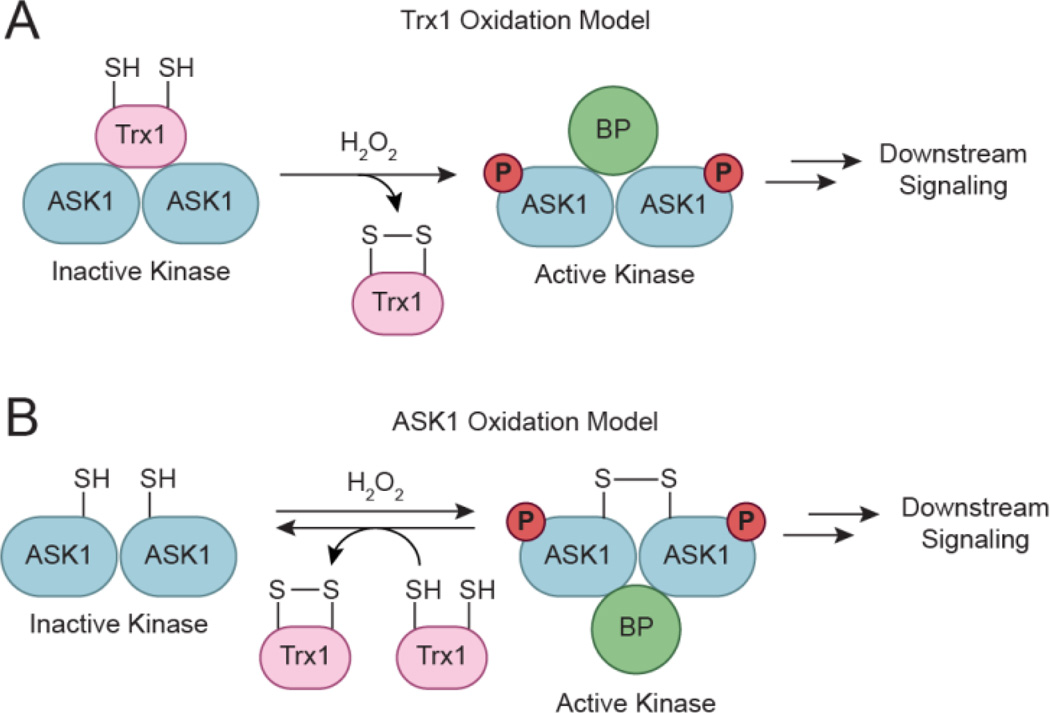
Coordination of Reversible Tyrosine Phosphorylation through Redox-Dependent Signaling
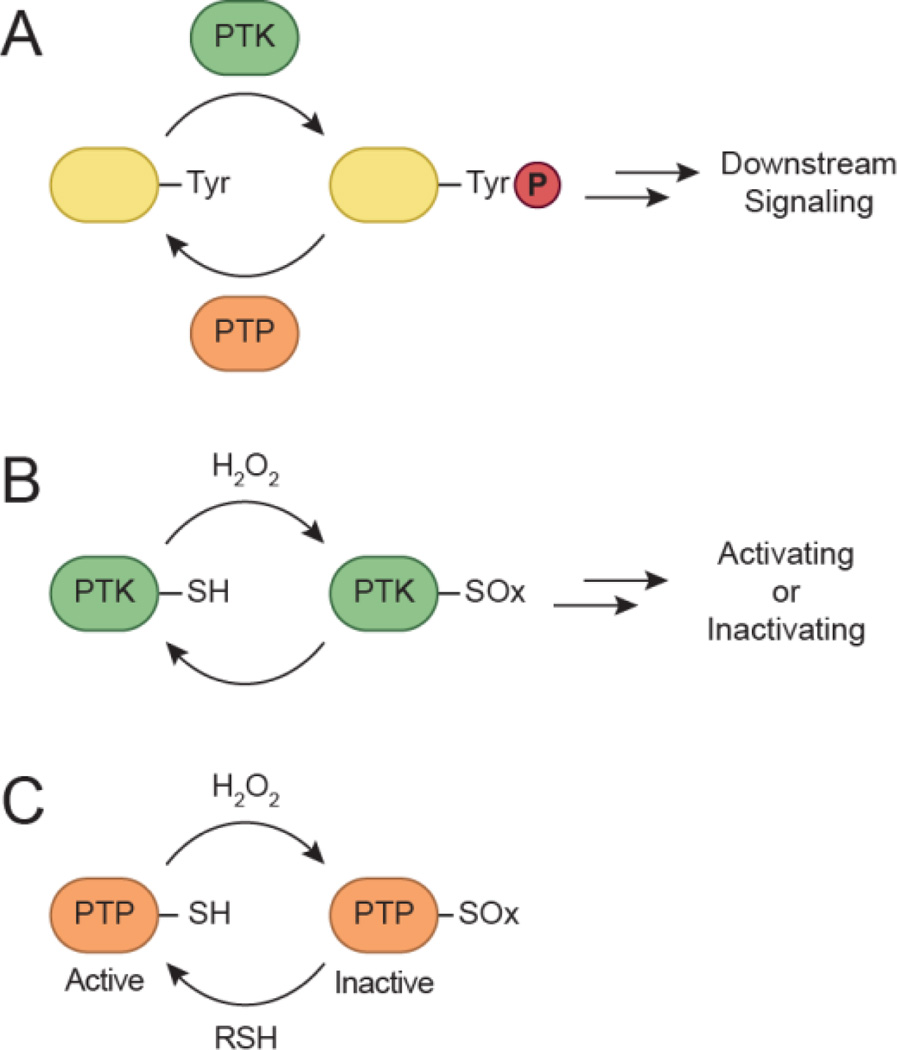
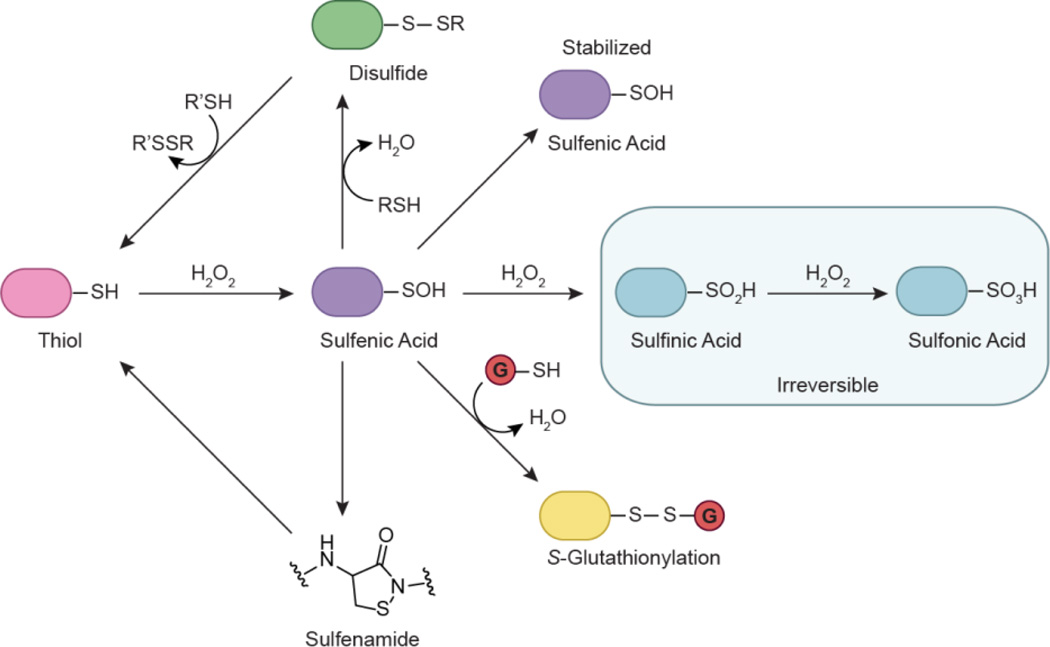
The distinct but complementary function of kinases and phosphatases represents a finely tuned process wherein crosstalk occurs between separate but interrelated networks (Fig. 2a). Redox-based mechanisms coordinate enzymatic activity between the two groups (Fig. 2b, 2c). Localized changes in redox homeostasis can be sensed through oxidative modification of cysteine residues within specific protein targets. The initial product of the reaction between H2O2 and a thiolate (SH) is sulfenic acid (SOH) (Fig. 3). This reversible modification, also known as sulfenylation, can be directly reduced back to the thiol or indirectly by disulfide bonds. Alternatively, sulfenic acids can be stabilized by the protein microenvironment or undergo additional modifications. Other relevant oxoforms include disulfides, sulfenamides, nitrosothiols (SNO), sulfinic (SO2H), and sulfonic (SO3H) acids. The reversibility of sulfenic acid represents a mechanism whereby redox-based signaling can be regulated, akin to phosphorylation. In addition, disulfide exchange and bond formation can also play an important role in maintaining specificity during redox signaling (Mamathambika and Bardwell, 2008, Go and Jones, 2013, Depuydt et al., 2011). The utility and functional role of reversible cysteine oxidation has been evidenced by an increasing number of studies (Reddie and Carroll, 2008, Paulsen and Carroll, 2010, Jacob et al., 2012). In particular, protein tyrosine phosphatases (PTPs) were originally implicated as targets of signal-induced H2O2 production due to early studies by Denu and Tanner (Denu and Tanner, 1998). The PTP superfamily contains a signature motif, (I/V)-H-C-X-X-G-X-X-R-(S/T), which includes a conserved cysteine that functions as a nucleophile during catalysis. The susceptibility of certain residues to oxidation is inherently dependent on its nucleophilicity, and provides a basis for specificity during redox-mediated signaling. Thiolate anions are intrinsically more reactive than their protonated counterparts (Winterbourn and Metodiewa, 1999), exhibiting enhanced reactivity towards H2O2 ranging across seven orders of magnitude (1–107 M−1 s−1) (Winterbourn, 2008). Free cysteines are typically protonated at physiological pH, and have a pKa of ∼ 8.5. Protein microenvironments can profoundly influence the pKa of cysteine thiols and in some cases, decrease the pKa to as low as 3.5 (Salsbury et al., 2008, Banerjee, 2008). These factors include metal coordination, residue accessibility, proximity to the oxidant source, and stabilization of the thiolate anion by electrostatic interactions from neighboring residues. The catalytic cysteine of PTPs is characterized by a low pKa value ranging from 4.6 to 5.5 owing to the unique chemical environment of its active site, which renders it susceptible to oxidative inactivation (Zhang and Dixon, 1993, Lohse et al., 1997). Although PTPs were initially suggested to be the sole targets of ligand-induced ROS production, attention has also shifted towards protein kinases. In the following sections, biochemical studies and evidence for redox regulation will be presented for select kinase examples.
Figure 2.
Model for redox-dependent signal transduction. (a) Protein tyrosine kinases (PTKs) catalyze the transfer of γ-phosphoryl groups from ATP to tyrosine hydroxyls of proteins, whereas protein tyrosine phosphatases (PTPs) remove phosphate groups from phosphorylated tyrosine residues. (b) Regulatory cysteines in protein kinases can undergo oxidation/reduction to modulate their function. Depending on the kinase, redox modifications can stimulate or inhibit enzymatic function. (c) PTPs function in a coordinated manner with PTKs to control signaling pathways to regulate a diverse array of cellular processes. Oxidation of the conserved active site cysteine residue in PTPs inactivates these enzymes, and can be restored by reducing the oxidized residue back to its thiol form. SOx: oxidized cysteine.
Figure 3.
Oxidative modification of cysteine residues by hydrogen peroxide (H2O2). The initial reaction product of a thiolate with H2O2 yields sulfenic acid (RSOH). This modification, also known as sulfenylation, is reversible and can be directly reduced back to the thiol form or indirectly through disulfide bond formation. Sulfenic acids can be stabilized by the protein microenvironment and/or undergo subsequent modification. For example, sulfenic acids can condense with a second cysteine in the same or different protein to generate disulfide bonds. Alternatively, reaction with the low molecular weight thiol glutathione (GSH, red circle) affords a mixed disulfide through a process known as S-glutathionylation. In some proteins, such as PTP1B, nucleophilic attack of a backbone amide on RSOH results in sulfenamide formation. Sulfenyl groups can also oxidize further to the sulfinic (RSO2H) and/or sulfonic (RSO3H) acid forms under conditions of high oxidative stress.
Receptor Tyrosine Kinases
Platelet-Derived Growth Factor Receptor (PDGFR)
PDGF exists as a homo- or heterodimer connected by disulfide bonds, and is composed from a combination of four isoforms (PDGF-A, PDGF-B, PDGF-C, and PDGF-D). PDGF initiates signal transduction by binding to its receptor, PDGFR (−α or −β forms). Activation of PDGFR engages signaling routes such as Ras/MAPK, PI3K/Akt, or binding of PLC-γ to elicit mitogenic and anti-apoptotic effects. For example, PDGF plays crucial roles in embryonic development, blood vessel formation, and wound response (Heldin and Westermark, 1999). PDGF-mediated signaling specificity in various cell types (i.e. fibroblasts, smooth muscle cells) is achieved by different ligand-receptor combinations (Andrae et al., 2008). Therefore, discrepancies in the literature may actually reflect the diverse signaling nature of these isoforms. For the purpose of this section, ligand and receptor isoforms will be referenced when deemed necessary. In particular, the majority of work discussed regarding PDGFR redox regulation is comprised of observations obtained from PDGF-BB/ PDGFR-β interactions.
The biological relevance of growth factor-induced H2O2 production was first highlighted in vascular smooth muscle cells (VSMCs) (Sundaresan et al., 1995). Endogenous ROS levels peaked rapidly upon PDGF stimulation, and were inhibited by enzymatic and chemical H2O2 scavengers. Transient H2O2 increases were also accompanied by elevated Tyr phosphorylation levels and activation of the MAPK pathway. PDGF stimulation of adipocytes has been shown to induce intracellular H2O2, originating from a membrane-bound Nox complex (Krieger-Brauer and Kather, 1992). Signal-derived H2O2 subsequently participates in facilitation and enhancement of adipose differentiation, albeit this observation is isoform-specific (PDGF-AA) (Krieger-Brauer and Kather, 1995).
Many research efforts have been devoted to elucidating the molecular mechanisms that produce and regulate endogenous H2O2 during cellular signaling. Activation of PI3K, a downstream effector of PDGFR, is required for growth factor-mediated production of H2O2 (Bae et al., 2000). Mutation of PI3K binding sites (Tyr740 and Tyr751) or exposure to PI3K inhibitors (LY294002 and wortmannin) failed to induce H2O2 production upon ligand stimulation. These results and others (Kwon et al., 2004) suggest that PIP3, the product of activated PI3K, is essential for redox-based PDGF signaling. Additionally, expression of a dominant negative (DN) mutant of the small GTPase Rac1 (Rac1N17) blocks increases in H2O2 and corroborates earlier observations in fibroblasts (Sundaresan et al., 1996). Rac1 is one of several subunits comprising the Nox enzyme complex, and contains an effector region (residues 124–135) required for Nox activation. Deletion of this region does not interfere with its functional abilities, but abrogates Rac1-mediated superoxide production through Nox (Joneson and Bar-Sagi, 1998). PIP3 stimulates Rac1 activation through guanine nucleotide exchange factors (GEFs). Therefore, it appears that Rac1 activation of Nox is inherently dependent on PI3K production of PIP3, which has also been demonstrated with regards to EGF-mediated signaling (Park et al., 2004a). Nox complexes serve as the main oxidant source during PDGF signaling, as established by chemical inhibition and enzyme overexpression studies (Lassegue et al., 2001, Chiarugi et al., 2001).
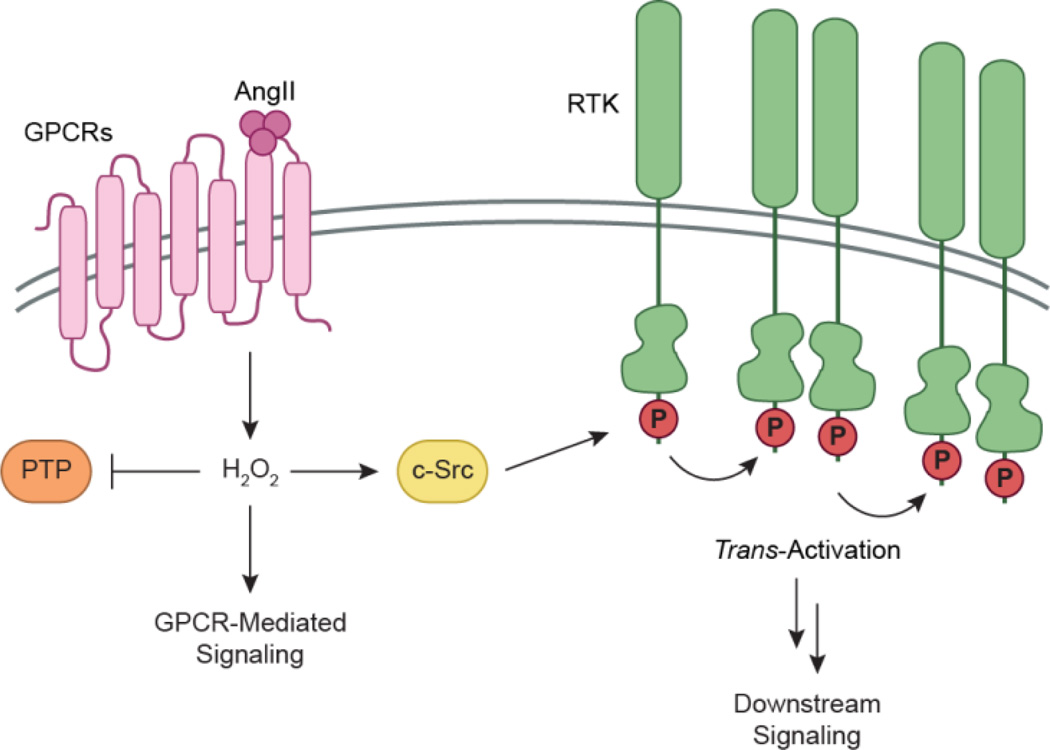
PDGFR activation can also occur through an alternate mechanism known as trans-activation, and is specific to the β-receptor isoform (Saito and Berk, 2001, Heeneman et al., 2000). In this scenario, angiotensin II (AngII) binds to GPCRs (G protein-coupled receptors) to trigger H2O2 production and mobilization of Ca2+ from intracellular stores. In turn, H2O2 activates additional kinases such as c-Src and PKC (protein kinase C) to promote RTK phosphorylation (Saito et al., 2002). The underlying mechanisms regarding H2O2 production during these events remains unclear, although some evidence indicates that mitochondrial function may play a role (Chen et al., 2004). Trans-activation represents an additional level whereby alternate stimulants can initiate signaling of ligand-inaccessible RTKs in lieu of traditional ligand-receptor interactions (Fig. 4).
Figure 4.
Trans-activation of PDGFR and EGFR. Alternative stimulants such as angiotensin II (AngII) can initiate activation of ligand-inaccessible RTKs in lieu of traditional ligand-receptor interactions. In this scenario, AngII binds to GPCRs to promote endogenous H2O2 production and activation of redox regulated PTKs such as c-Src. Src promotes phosphorylation of PDGFR or EGFR, and neighboring receptors can be activated in a lateral-based mechanism. Additionally, concurrent PTP inactivation has also been suggested to promote RTK trans-activation.
PDGFR activity is modulated by a number of PTPs, such as low molecular weight PTP (LMW-PTP) (Chiarugi et al., 1995) and SHP-2 (Meng et al., 2002). LMW-PTP is characterized by a unique feature among the PTP family whereby it contains two cysteine residues, Cys12 and Cys17, located in its catalytic pocket (Caselli et al., 1998). Upon PDGF stimulation, endogenous H2O2 inactivates LMW-PTP through disulfide bond formation to enable increased receptor activity (Chiarugi et al., 2001). GSH depletion by treatment with buthionine sulfoxide (BSO), an inhibitor of γ-glutamylcysteine synthetase, severely impairs rescue of oxidized LMW-PTP, and suggests phosphatase reactivation proceeds through a GSH-dependent process. Glutaredoxin (Grx) was later identified as a component of GSH-dependent modulation of LMW-PTP (Kanda et al., 2006). LMW-PTP mutant C17A is unable to recover from oxidative inactivation, and suggests Cys17 forms a disulfide bond with Cys12 to protect the catalytic cysteine from hyperoxidation (i.e. sulfinic or sulfonic acids). Follow-up studies imply PTPs may regulate the duration of PDGFR signal by preferentially targeting membrane-exposed receptors, whereas the endosomal receptor pool has been shown to remain phosphorylated for up to 2 h (Chiarugi et al., 2002).
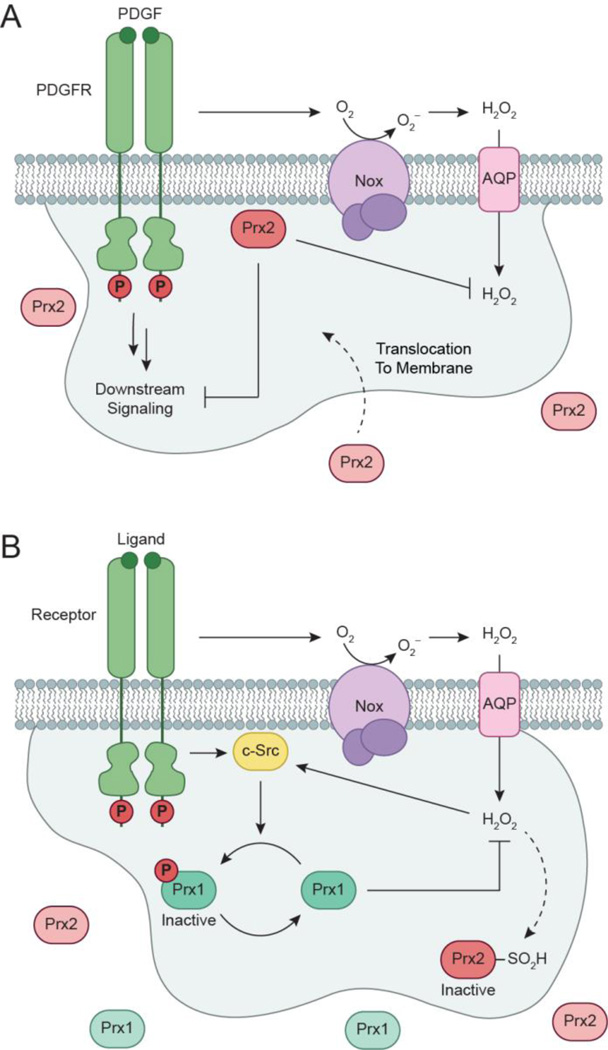
The extent of growth factor signaling can also be controlled by antioxidant defense systems. In particular, PrxII functions as a negative regulator of PDGF signaling (Choi et al., 2005). PrxII deficient mouse embryonic fibroblast (MEF) cells exhibit 2-fold increases in H2O2 production and enhanced activation of PDGFR and PLC-γ1. Co-immunoprecipitation demonstrates that active PrxII is recruited to the receptor upon ligand stimulation. Translocated PrxII relieves oxidative inactivation of membrane-associated PTPs by eliminating localized H2O2 production within the PDGFR microenvironment. These results demonstrate PrxII regulates site-selective amplification of PDGFR phosphorylation through endogenous H2O2 (Fig. 5a). Alternatively, mechanisms have evolved to allow localized H2O2 accumulation during signaling. Prxs are extremely efficient at H2O2 elimination, and react with H2O2 at second-order rate constants ranging from 105 – 108 M−1 s−1 (Parsonage et al., 2008, Peskin et al., 2007). Therefore, it is intriguing how H2O2 can accumulate within the cytosol long enough to modulate its effectors. Recent studies have discovered phosphorylation of Tyr194 inactivates PrxI (Woo et al., 2010). PrxI phosphorylation is induced in several cell types (NIH 3T3, A431, Ramos B cells, Jurkat T cells) upon activation of various receptors (PDGFR, EGFR, BCR, and TCR), and found to be localized to membrane microdomains. Nox1 deficient mice exhibit a 60% reduction in PrxI phosphorylation, and suggests Nox1-derived H2O2 induces inactivation of PrxI and PTPs to promote localized H2O2 accumulation. Knockdown c-Src siRNA experiments decrease PrxI inactivation by 25–50%, thereby identifying c-Src as the partially responsible kinase. This study provides a key contribution that demonstrates localized PrxI inactivation enables transient H2O2 accumulation near the membrane, while simultaneously preventing toxic ROS increases elsewhere during signaling (Fig. 5b). Additionally, the two aforementioned studies highlight isoform-specific Prx responses involved in maintaining growth factor-induced H2O2 production.
Figure 5.
Isoform-specific roles of peroxiredoxin (Prx) during redox-based PDGFR signaling. (a) PrxII functions as a negative regulator of PDGF signaling. Upon growth factor stimulation, active PrxII is recruited to the membrane and serves to relieve oxidative inactivation of membrane-associated PTPs by eliminating localized H2O2 production within the PDGFR microenvironment. (b) Receptor activation can also induce localized phosphorylation and inactivation of PrxI by PTKs, such as the redox-regulated cytoplasmic Src (c-Src). Deactivation of PrxI reduces the redox-buffering capacity adjacent to the cellular membrane, allowing for transient and localized increases in H2O2 for signal transduction. Additionally, increased H2O2 concentrations can also inactivate Prx2 by oxidation of its catalytic cysteine to sulfinic acid.
Until now, we have only discussed indirect mechanisms related to redox-based modulation of PDGFR. Currently, there is little evidence to support direct modification of PDGFR through cysteine oxidation. In an early study, PDGF stimulation was reported to induce covalent receptor dimerization through disulfide bonds within the extracellular domain (Li and Schlessinger, 1991). Unfortunately, the identification of residues responsible for PDGFR dimerization has not been reported. A separate study examined the functional role of conserved cysteine residues located in the cytoplasmic domain of PDGFR. Treatment with the sulfhydryl-specific alkylating agent, N-ethylmaleimide (NEM), inhibits kinase activity and suggests the receptor contains critical cysteine residues located within its kinase domain (Lee et al., 2004). MS analysis identified two residues, Cys822 and Cys940, deemed necessary for receptor activity by in vitro assays using Ser mutants. Cys822 is located in the catalytic loop at the enzyme active site, whereas Cys940 is located in the C-lobe of the kinase domain. These residues do not participate in disulfide bond formation, as determined by non-reducing gel analysis. Additionally, they do not interfere with ATP substrate binding as is observed in other RTKs (i.e. EGFR). Proteolytic cleavage assays demonstrate Cys940 affects receptor activity by inducing a conformational change, but the mechanism by which this proceeds remains unclear. Although the preceding studies indicate PDGFR may require specific cysteines for functional activity, it remains unknown whether the receptor contains cysteine residues that are directly modulated by H2O2.
Epidermal Growth Factor Receptor (EGFR)
Epidermal growth factor receptor (EGFR) was the first RTK to be identified, and has since been widely characterized in physiologic and pathological settings (Cohen et al., 1980, Carpenter et al., 1978, Cohen, 2008). EGFR, also known as erbB1 (or HER1), is grouped into a subfamily that consists of three other closely related receptors: erbB2 (HER2), erbB3 (HER3), and erbB4 (HER4). EGFR forms a homo- or heterodimer followed by autophosphorylation of key residues upon stimulation (Schlessinger, 2002). Once activated, EGFR relays the signal through one of its two major pro-survival signaling routes, Ras/MAPK or PI3K/Akt. The erbB family of receptors is activated by various ligand-receptor combinations to achieve signaling specificity in different cell types and signaling cascades (Yarden and Sliwkowski, 2001). Therefore, we will mainly focus on effects resulting from the EGF-EGFR interaction for the purpose of this review. EGFR and other erbB family members have been found to be mutated or amplified in human cancers such as breast and lung, making them attractive targets for the development of therapeutic strategies (Macias et al., 1987, Herbst, 2004).
Culminating evidence has established that EGFR is modulated by and utilizes H2O2 as a secondary messenger during cellular signaling (Finkel, 2012, Truong and Carroll, 2012). Early reports demonstrate exogenous H2O2 increases [32P] phosphate incorporation, albeit the signal is half of what is observed in EGF-stimulated cells (Gamou and Shimizu, 1995). Tryptic phosphopeptide mapping attributes this difference to preferential enhancement of EGFR Tyr phosphorylation by H2O2, whereas EGF triggers a combination of Ser/Thr and Tyr receptor phosphorylation. In a landmark study, EGF was reported to increase endogenous H2O2 levels upon receptor stimulation in cells overexpressing EGFR (A431) (Bae et al., 1997). Catalase incorporation abolishes EGF-induced H2O2 production and blunts increased Tyr phosphorylation of EGFR and PLC-γ1, a well-characterized target of EGFR. Additionally, assays with mutated EGFR receptors demonstrate EGF-dependent H2O2 formation requires intrinsic kinase activity, but not necessarily its autophosphorylation sites. Subsequent reports have shown H2O2 activates EGFR kinase and markedly enhances receptor half-life in conjunction with the native ligand (Goldkorn et al., 1998). From these results, it is apparent that H2O2 modulates EGFR activation and influences downstream signaling. Other studies suggest S-nitrosylation may regulate EGFR activity, albeit with contradictory results (Murillo-Carretero et al., 2009, Lam et al., 2010, Switzer et al., 2012). Additionally, EGFR is the only other RTK besides PDGFR known to undergo trans-activation by AngII, and proceeds through H2O2-dependent c-Src activation (Ushio-Fukai et al., 2001, Chen et al., 2001a, Giannoni et al., 2008). Neighboring EGFR kinases may be activated in a lateral-based mechanism, and concurrent PTP inactivation has been suggested to promote EGFR trans-activation (Reynolds et al., 2003). (Fig. 4)
PTPs were one of the first redox-based targets identified in EGF signaling. EGFR was originally identified as an in vivo substrate of PTP1B using a substrate-trapping mutant technique (Flint et al., 1997). These mutants retain their ability to bind substrates in a cellular context, but lack catalytic functionality to enable stabilization of enzyme-substrate complexes for isolation purposes. PTP1B is localized exclusively on the cytoplasmic face of the endoplasmic reticulum (ER), and serves to dephosphorylate activated EGFR that has been internalized and transported for endocytosis (Haj et al., 2003, Haj et al., 2002). Signal-derived H2O2 inactivates PTP1B to enable equilibrium between EGFR and PTP1B activity (Lee et al., 1998). PTP1B exhibits decreased time-dependent incorporation of radiolabeled iodoacetic acid (IAA) in A431 cells when stimulated with EGF. IAA is a sulfhydryl-modifying reagent known to react with the catalytic cysteine of PTP1B (Cys215). PTEN is another PTP known to maintain a close relationship with EGFR, and functions as a negative regulator of the PI3K/Akt pathway. Recent evidence implicates PTEN regulates EGFR by targeting the receptor for degradation through ubiquitylation (Vivanco et al., 2010). EGFR ubiquitylation is mediated by the Cbl family of E3 ubiquitin ligases, which bind to the activated receptor to form a complex stabilized by the phosphatase. PTEN contains five cysteine residues within its catalytic domain and undergoes reversible inactivation by H2O2. Mutation of these residues reveals Cys124 is oxidized upon exposure to exogenous H2O2, and forms an intramolecular disulfide with Cys71 that can be rescued by Trx (Lee et al., 2002). Subsequent work has shown that EGF-induced H2O2 inactivates PTEN similarly to PTP1B (Kwon et al., 2004). Collectively, these studies provide evidence that growth factor activation of RTKs may not be sufficient to increase the steady-state level of protein phosphorylation during signaling, and that concomitant inactivation of PTPs is also required. Interestingly, Nox1 overexpression induces increased PIP3 levels (Kwon et al., 2004) and is a component involved in EGFR activation of PI3K (Park et al., 2004a). PTEN inactivation represents a positive feedback loop that promotes continued accumulation of intracellular PIP3 during EGF signaling.
EGF-induced H2O2 is produced and regulated by distinct components. Multiple Nox isoforms mediate H2O2 production within EGF signaling cascades. For example, a positive feedback loop due to sequential activation of PI3K-βPix-Rac1-Nox1 is involved in endogenous H2O2 production (Park et al., 2004a). In this loop, EGF stimulation activates PI3K to elicit increased PIP3 production. These lipid products activate βPix by interacting with its PH domain, which then catalyzes GDP exchange to activate Rac1. Finally, Rac1 directly binds to Nox1 to promote NADPH electron transfer and H2O2 generation. βPix siRNA-based knockdown experiments block EGF-induced H2O2 production and Rac1 activation. A separate study reports Nox1 is negatively modulated during EGF signaling by phosphorylation of Ser282 of Nox activator 1 (NOXA1, a Nox1 subunit) by Erk1/2 and Ser172 by PKC to prevent hyperactivation (Oh et al., 2010, Kroviarski et al., 2010). Nox4 is another isoform that regulates EGFR in a spatially dependent manner through PTP1B inactivation (Chen et al., 2008). Confocal microscopy and cellular fractionation experiments with the ER marker protein GRP78 confirmed ER localization of Nox4. Upon EGF stimulation, co-localization of Nox4 and PTP1B to the ER is required for phosphatase inactivation in aortic endothelial cells. Nox4 siRNA knockdown increases reduced PTP1B levels, indicating Nox4 serves as the primary oxidant source for PTP1B oxidation within the ER and consequently allows Nox4 to spatially modulate the duration of EGFR signaling. These results were validated by PTP1B substrate-trapping mutants, which were attenuated in their ability to effectively bind EGFR due to Nox4 overexpression.
As described earlier, SODs catalyze the conversion of superoxide into H2O2 to maintain steady-state levels. Treatment with SOD1 inhibitor ATN-224 decreases intracellular H2O2 levels and attenuates EGFR activation and downstream targets (Juarez et al., 2008). Furthermore, SOD1 was shown to mediate transient oxidation of PTPs such as PTP1B and PTEN. Separate studies demonstrate H2O2 utilized during EGF signaling is generated extracellularly at the receptor-ligand interface, and permeates across the membrane to facilitate kinase activation (DeYulia et al., 2005, DeYulia and Carcamo, 2005). In vitro assays with recombinant protein containing only the extracellular EGFR binding domain provides evidence that this domain is sufficient to induce H2O2 production upon ligand binding. Once generated, H2O2 can freely diffuse across membranes or be transported through aquaporin channels to elicit signaling events (Miller et al., 2010). Thereafter, buffering systems and peroxide-detoxifying enzymes such as Prx and Gpx mediate signal duration by maintaining appropriate H2O2 levels (Halvey et al., 2005, Kang et al., 1998, Handy et al., 2009).
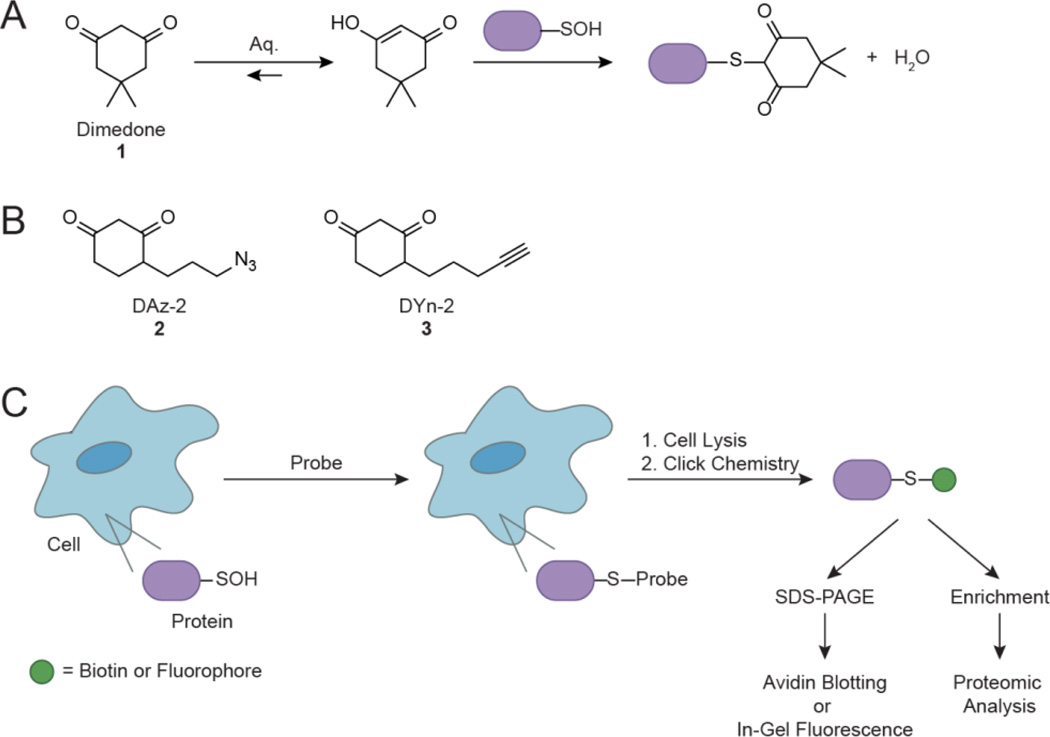
Mounting evidence has established redox-based modulation of EGFR activity, albeit mainly through indirect mechanisms. Although EGFR contains ∼ 50 cysteine residues, 9 of which are located within its intracellular domain, none of these have been implicated to undergo oxidative modification until recently (Paulsen et al., 2012). Early work demonstrates EGFR autophosphorylation is inactivated by NEM alkylation (Buhrow et al., 1982, Clark and Konstantopoulos, 1993). NEM treatment blocks reaction of a nucleotide analog [(p-fluorosulfonyl)-benzoyl]-5’ adenosine (5’-FSBA) with the kinase active site, suggesting alkylation may exert an inhibitory effect by reacting with a cysteine residue located in the ATP binding site (Buhrow et al., 1982). These observations were corroborated in separate studies whereby co-incubation of EGFR with AMP-PNP, a hydrolysis-resistant ATP analog, blocks receptor inactivation by NEM (Woltjer and Staros, 1997). Although these studies alluded that a cysteine residue may play a role in redox-based EGFR signaling, specific modification sites were not identified. Sulfhydryl alkylating agents represent an indirect method to detect cysteine modifications because they readily react with reduced thiols, but are unable to modify other cysteine oxoforms important to signaling such as sulfenic acids. Therefore, the detection of relevant cysteine modifications represents a tremendous need for the development of tools geared towards this goal. Techniques based on chemical probes (Poole et al., 2005, Poole et al., 2007, Reddie et al., 2008, Leonard et al., 2009, Seo and Carroll, 2011, Truong et al., 2011) and antibodies (Seo and Carroll, 2009, Maller et al., 2011) have recently been developed to detect sulfenic acid formation. In particular, cell permeable probes enable direct trapping of sulfenylated proteins within their native cellular environment. These probes are functionalized with an azide or alkyne reporter group (Reddie et al., 2008, Leonard et al., 2009, Paulsen et al., 2012), and utilize a cyclic β-diketone warhead chemically selective for sulfenic acids (Benitez and Allison, 1974) (Fig. 6a, 6b). Bioorthogonal biotin or fluorescent reporter tags can be appended post-lysis for detection via the Staudinger ligation (Saxon and Bertozzi, 2000) or Huisgen [3+2] cycloaddition (i.e. click chemistry) (Rostovtsev et al., 2002). Overall, this general approach provides a facile method to profile protein sulfenylation in a cellular context (Fig. 6c).
Figure 6.
General strategy for detecting protein sulfenic acids in cells. (a) Chemoselective reaction between 5,5-dimethyl-1,3-cyclohexanedione (dimedone, 1) and sulfenic acid. (b) Azide and alkyne-functionalized small-molecule probes for trapping and tagging protein sulfenic acids include DAz-2 (2) and DYn-2 (3). (c) Detection of protein sulfenic acids in living cells. Target cells are incubated with cell-permeable probes to trap and tag protein sulfenic acids in situ. After labeling, cell lysates are prepared and tagged proteins are bioorthogonally ligated to biotin or fluorescent reporter tags via click chemistry to enable detection by Western blot or in-gel fluorescence. Alternatively, biotinylated proteins can be enriched for proteomic analysis.
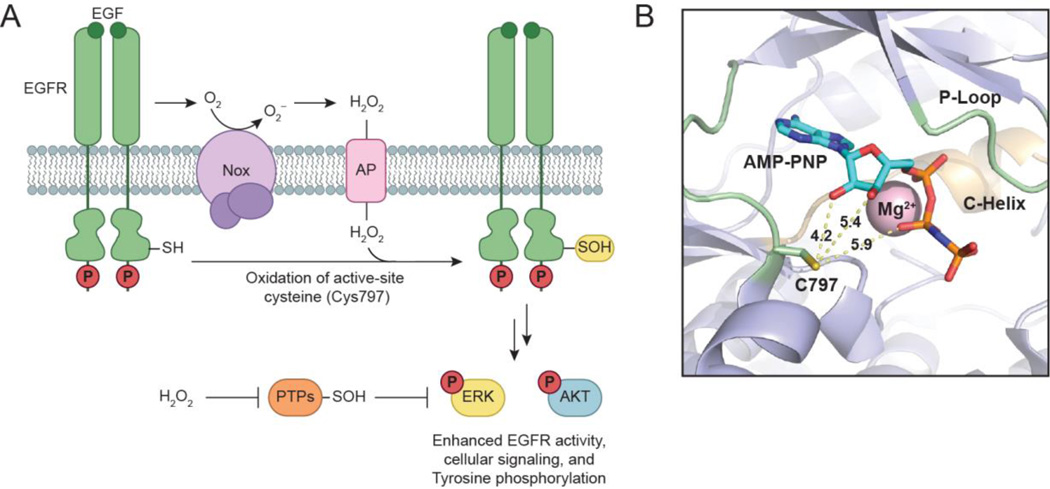
Overexpression of EGFR and HER2 in breast cancer cell lines is correlated to high H2O2 levels and increased global protein sulfenylation (Seo and Carroll, 2009). In more recent work, the functional role of protein sulfenylation during EGFR signaling was explored in detail (Fig. 7a). Sulfenic acid formation was profiled in A431 cells upon ligand stimulation, and captured with cell-permeable probe DYn-2 (Paulsen et al., 2012). Ensuing analysis revealed EGF stimulation induces dynamic and global increases in protein sulfenylation. This occurs in a dose- and time-dependent manner relative to EGF, and is accompanied by Nox2-derived H2O2 production. DYn-2 was used in a targeted approach to confirm sulfenylation of three phosphatases involved in EGFR signaling: SHP2, PTEN, and PTP1B. Immunoprecipitation demonstrated each PTP was sulfenylated in an EGF-dependent manner in cells. Moreover, individual PTPs displayed distinct oxidation profiles as a function of growth factor concentration. PTP sensitivity to EGF-mediated oxidation suggests this observation may be related to subcellular protein localization, and is supported by previous work (Chen et al., 2008). Finally, EGFR itself was shown to be activated and directly modified upon EGF stimulation, peaking at low concentrations (4 ng/ml). The EGFR kinase domain contains six cysteine residues, one of which is a conserved cysteine (Cys797) that resides in the ATP binding site (Fig. 7b). This residue is selectively targeted by irreversible EGFR inhibitors (i.e. afatinib) currently undergoing clinical trials for breast and non-small cell lung cancers (Singh et al., 2010, Singh et al., 2011). Treatment with irreversible EGFR inhibitors (afatinib, canertinib, or pelitinib) abrogates the ability of DYn-2 to detect EGFR oxidation, and suggests Cys797 may be susceptible to oxidation as evidenced by signal loss of EGFR sulfenylation. Cys797 was identified as the site of EGFR modification by MS analysis. These results demonstrate EGFR Cys797 is a direct target of growth factor-induced H2O2, and that EGF signaling induces dynamic sulfenic acid formation within EGFR signaling pathways (Paulsen et al., 2012). Phosphorylation and sulfenylation appear to work in parallel with each other to regulate receptor kinase activity during EGF signaling.
Figure 7.
Model for H2O2-dependent regulation of EGFR activation. (a) Binding of EGF to the receptor induces production of H2O2 through Nox2. Nox-derived H2O2 directly modifies EGFR to sulfenic acid at a conserved cysteine residue (Cys797) located in its active site, which enhances the receptor’s intrinsic tyrosine kinase activity. Endogenous H2O2 can also oxidize and deactivate localized PTPs, leading to a net increase in EGFR phosphorylation and activation of downstream signaling cascades. (b) Crystal structure of the EGFR kinase domain (PDB 3GT8) bound to AMP-PNP, a hydrolysis resistant ATP analog, and Mg2+. The yellow dashed lines and accompanying numbers indicate the distance (Å) between the γ-sulfur atom of Cys797 and key substrate functional groups. It is important to note that Cys797 can adopt different rotamers, and sulfenylation of this residue may enhance its ability to participate in electrostatic and hydrogen bonding interactions with its substrate.
Vascular Endothelial Growth Factor Receptor (VEGFR)
Angiogenesis is the physiological process whereby new blood vessels are formed from pre-existing vasculature. This is a vital process involved in embryonic development, skeletal growth, wound repair, and reproductive functions (Ferrara et al., 2003). A wide range of diseases including chronic inflammation, vascular ischemia, and atherosclerosis have been linked to angiogenesis (Olsson et al., 2006). Vascular endothelial growth factor (VEGF) is a key angiogenic growth factor that stimulates cellular proliferation, migration, and tube formation of endothelial cells (ECs). The biological effects of VEGF are mediated by three RTKs, VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk1), and VEGFR-3, which differ considerably in their signaling properties. VEGFR activation initiates downstream signaling through Ras/MAPK, PI3K/Akt, PLC-γ, and Src family of kinases to induce endothelial growth and mediate pro-survival effects. For the purpose of this section, we will focus primarily on VEGFR-2 due to its involvement in VEGF-mediated redox signaling (Ushio-Fukai, 2007).
Growth factor-induced H2O2 production modulates RTK activity, as discussed in preceding sections. Interestingly, H2O2 treatment of ECs has been shown to upregulate gene expression of VEGF (Chua et al., 1998, Kuroki et al., 1996). Further analysis with inhibitors of protein (cycloheximide) and RNA synthesis (actinomycin D) demonstrate induction of VEGF mRNA levels by H2O2 is inhibited by actinomycin D, indicating that de novo RNA synthesis is required for this response. In a later study, H2O2 was also found to elicit increased VEGFR-2 mRNA levels (Gonzalez-Pacheco et al., 2006). The biological relevance of these observations was explored in detail, and suggests upregulation of VEGF and VEGFR-2 functions primarily to protect ECs against oxidative injury. Additionally, VEGF-mediated signaling is also accompanied by bursts of intracellular ROS (Colavitti et al., 2002). Akin to its RTK counterparts, VEGF-induced H2O2 production increases receptor autophosphorylation and activation of downstream targets such as Erk1/2, Akt, and PLC-γ1. Phosphatase inhibition with vanadate restores Tyr phosphorylation levels during VEGF stimulation, and reinforces the idea that oxidative inactivation of PTPs such as PTP1B (Nakamura et al., 2008) and DEP-1 (Grazia Lampugnani et al., 2003) promotes kinase activity. Similar to results observed with PDGFR and EGFR, activation of PI3K was shown to be essential for VEGF-derived H2O2 production using PI3K inhibitors (wortmannin) and Rac1N17.
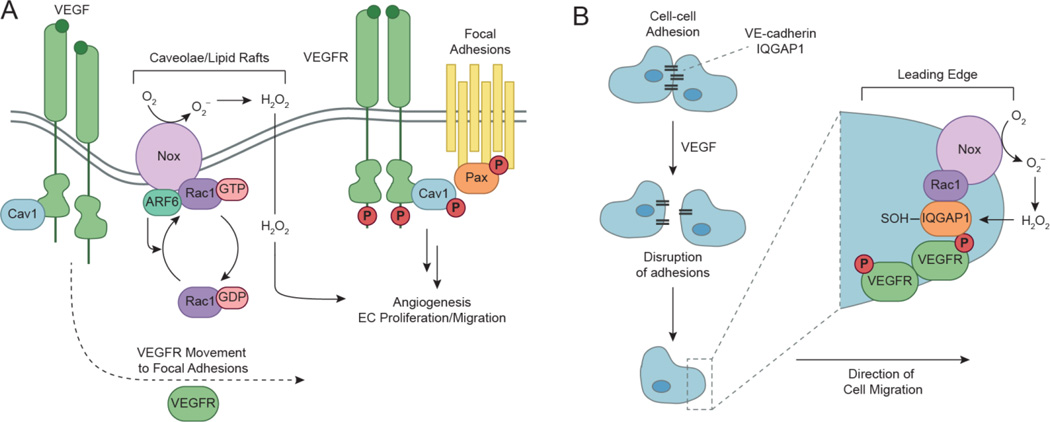
Nox enzymes serve as the major source of endothelial H2O2, and are required for EC proliferation and migration (Babior, 2000, Gorlach et al., 2000, Abid et al., 2000). Therefore, efforts quickly focused on elucidating the potential role of Nox-derived H2O2 during VEGF-mediated signaling and angiogenesis (Ushio-Fukai et al., 2002). Treatment of ECs with various Nox inhibitors (DPI, apocynin, and AEBSF) and gp91phox antisense oligonucleotides attenuate receptor autophosphorylation, VEGF-stimulated proliferation and migration, and H2O2 production. Rac1N17 overexpression results in similar decreases, indicating Nox2 activation is Rac1-dependent. Evidence suggests that Nox-derived H2O2 during VEGF signaling is spatially and temporally controlled by various components within discrete subcellular compartments including caveolae/lipid rafts, focal adhesions/complexes, and at the leading edge (Ushio-Fukai, 2006). Caveolae are flask-shaped membrane microdomains primarily composed of protein caveolae (i.e. caveolin-1), and are subsets of lipid rafts that compartmentalize signaling components such as RTKs, GPCRs, and Rac1. Caveolin-1 negatively modulates VEGFR-2 by binding to the receptor (Labrecque et al., 2003). VEGF stimulation promotes Tyr phosphorylation of caveolin-1, and triggers its dissociation from VEGFR-2. Once released, activated VEGFR-2 localizes with p-caveolin-1 at focal adhesions/complexes to initiate signaling. Focal adhesions are dynamic protein complexes that mediate cell anchorage and motility with respect to the extracellular matrix (ECM). A later study identified that colocalization of VEGFR-2 with Rac1 and the small GTPase ARF6 (ADP-ribosylation factor 6) occurs in caveolae/lipid rafts (Ikeda et al., 2005a). Expression of DN ARF6 mutant (T27N) inhibits Rac1 activation, Tyr phosphorylation of caveolin-1, and translocation of VEGFR-2 from caveolae/lipid rafts. Additionally, ARF6 (T27N) attenuates VEGF-induced H2O2 production and suggests ARF6 mediates recruitment of activated VEGFR-2 to focal adhesions/complexes through Rac1-dependent Nox activation (Fig. 8a). Alternatively, Nox1 overexpression has been shown to promote tumor angiogenesis through upregulation of VEGF and VEGFR-2 (Arbiser et al., 2002).
Figure 8.
Spatial and temporal modulation of VEGFR2 signaling occurs in discrete subcellular compartments. (a) In the basal state, caveolin-1 (Cav1) negatively modulates VEGFR2 by binding to the receptor in caveolae/lipid rafts. VEGF initiates activation of VEGFR2 by promoting dissociation of Cav1 from the receptor. Once released, activated VEGFR2 localizes with phosphorylated Cav1 and paxillin (Pax) at focal adhesions/complexes to initiate downstream signaling. Growth factor stimulation also recruits small GTPases, ARF6 and Rac1, to activate Nox complexes located in caveolae/lipid rafts. Localized production of H2O2 acts as a secondary messenger during VEGF signaling to promote angiogenesis, proliferation, and migration in endothelial cells (ECs). (b) Endothelial migration is a key event that occurs during angiogenesis in ECs. Cell-cell adhesions are mediated by interactions between VE-cadherin and IQGAP1, which are disrupted upon VEGF stimulation to initiate the migration process. During active migration, IQGAP1 functions as a scaffolding protein to recruit signaling components such as activated VEGFR2, Nox, and Rac1 to the leading edge. Additionally, Nox-derived H2O2 induces localized sulfenic acid formation in IQGAP1 to promote directional migration events. Adapted from Ushio-Fukai, 2007.
VEGF signaling can also be modulated by extracellular SOD (ecSOD). ecSOD is the major SOD found in the vascular extracellular space, and anchors to the EC surface by binding to heparin sulfate proteoglycans (HSPGs) through its heparin-binding domain (HBD). Extracellular H2O2 derived from ecSOD enhances VEGFR-2 autophosphorylation, and functions in a HBD-dependent manner (Oshikawa et al., 2010). As discussed earlier, VEGFR activation initially occurs in caveolin-enriched lipid rafts where Nox complex subunits are localized within ECs. Sucrose gradient fractionation with ecSOD and ecSOD-ΔHBD expressing cells demonstrate that the HBD is required for ecSOD localization within caveolin-enriched lipid rafts. Additionally, PTPs involved in regulating VEGFR-2 activity such as PTP-1B and DEP-1 were oxidatively inactivated by ecSOD-derived H2O2. These PTPs were only inactivated in caveolae/lipid rafts, thereby indicating ecSOD promotes VEGF-induced H2O2 production and receptor autophosphorylation in a localized manner.
Endothelial migration is essential for the formation of new blood vessels. Cell-cell adhesion is mediated primarily by VE-cadherin, and requires co-localization of IQGAP1 in quiescent ECs (Yamaoka-Tojo et al., 2006). VEGF initiates endothelial migration by reducing IQGAP1/VE-cadherin interactions to disengage cellular contacts. IQGAP1 is a VEGFR-2 binding protein originally identified in a library screen, and preferentially binds to the activated receptor at the leading edge in actively migrating cells (Yamaoka-Tojo et al., 2004). IQGAP1 functions as a scaffolding protein that controls cellular motility and morphogenesis by directly interacting with cytoskeletal and cell adhesion elements, in addition to small GTPases such as Rac1. Co-immunoprecipitation demonstrates VEGF stimulation rapidly promotes recruitment of Rac1 to IQGAP1, and initiates complex formation with VEGFR-2. IQGAP1 siRNA knockdown inhibits VEGF-induced H2O2 production and activation of downstream pathways such as PI3K/Akt. In a wound scratch assay, Nox2 translocates to the leading edge and co-localizes with IQGAP1 upon VEGF stimulation (Ikeda et al., 2005b). Collectively, these studies highlight the crucial role of IQGAP1 in endothelial migration during redox-mediated VEGFR signaling (Fig. 8b). A recent study using a dimedone-based trapping reagent (DCP-Bio1) demonstrates IQGAP1 undergoes localized sulfenic acid formation at the leading edge induced by VEGF-mediated H2O2 generation (Kaplan et al., 2011). Sulfenyl modification of IQGAP1 positively impacts its ability to promote directional migration events, and was also observed in hindlimb ischemia models.
VEGFR-2 can also undergo direction modulation through cysteine oxidation. During VEGF signaling, Nox2-derived H2O2 selectively exerts its effects on the PI3K/Akt pathway by inducing c-Src activation (Abid et al., 2007). A cysteinyl labeling strategy highly sensitive to sulfenic acid detection (Boivin et al., 2008) was used to examine potential oxidation of VEGFR-2 and c-Src during VEGF signaling. Both kinases were identified to contain redox-active cysteines, and require Nox-2 derived H2O2 to elicit downstream signaling. This study does not identify the specific residues responsible for these observations, although there have been numerous reports of redox-sensitive cysteines present in c-Src (discussed later). Interestingly, a separate study reports VEGFR-2 activity is negatively modulated by an intramolecular disulfide bond between Cys1209 (catalytic) and Cys1199 (resolving) in its C-terminal tail (Kang et al., 2011). PrxII preserves VEGF signaling in vascular ECs by rescuing VEGFR-2 from oxidative inactivation, as shown by PrxII siRNA knockdown. Incorporation of cytosolic and membrane-targeted catalase effectively abrogates VEGF-induced H2O2 production; however, VEGFR-2 phosphorylation is only restored in the presence of membrane-targeted catalase. This suggests that PrxII, a cytosolic enzyme, must translocate to the cellular membrane to positively modulate VEGFR-2 activity. PrxII has been previously identified to be distributed within caveolae structures in vascular ECs, which coincides with known locations of subcellular VEGFR-2 signaling (Woo et al., 2010). Sucrose gradient fractionation demonstrates co-localization of VEGFR-2 and PrxII occurs only within caveolae-enriched fractions. Additionally, oxidatively inactivated VEGFR-2 was only found in caveolae fractions in PrxII knockdown cells. These findings reinforce the idea that redox modulation of VEGFR-2 signaling occurs in a highly localized manner in ECs and contrasting effects of H2O2 on VEGFR-2 activity may be due to subcellular factors.
Insulin Receptor Kinase (IRK)
Insulin is the major hormone responsible for critical energy functions such as glucose and lipid metabolism. Insulin activates insulin receptor kinase (IRK), which is a heterotetrameric receptor composed of two extracellular α subunits and two transmembrane β subunits. Binding of insulin to the IRK extracellular domain induces a conformational change in its quaternary structure that enables ATP binding and leads to increased receptor autophosphorylation (Youngren, 2007). Once activated, IRK elicits Tyr phosphorylation of various cytosolic docking proteins, most notably adaptor protein SHC and members of the insulin receptor substrate (IRS) protein family (Kido et al., 2001). Insulin signaling has mitogenic effects that are primarily associated with the Ras/MAPK pathway through SHC (Avruch, 1998). Unlike other RTKs previously discussed in this review, IRK utilizes IRS docking proteins to initiate signal transduction (Fig. 1b). Activated IRS proteins recruit effectors to relay downstream signaling through the PI3K/Akt pathway and GLUT4, which play major roles in insulin function. For example, Akt activation induces glycogen synthesis through inhibition of GSK-3 (glycogen synthase kinase-3) (Cross et al., 1995). Additionally, the PI3K/Akt pathway mediates insulin-induced glucose uptake by translocation of GLUT4 vesicles to the cellular membrane (Kohn et al., 1996, Ueki et al., 1998). Although RTKs mediate long-term events such as cellular differentiation and mitogenesis, the primary function of IRK is the acute regulation of glucose metabolism in muscle and adipose cells. Despite these differences, RTKs and IRK essentially share the same transduction machinery to elicit their respective responses.
For many years, exogenous H2O2 has been recognized to mimic the effects of insulin (i.e. glucose transport) in adipocytes (May and de Haen, 1979a). Insulin-induced production of intracellular H2O2 was first observed in rat adipocytes (May and de Haen, 1979b). These observations were coupled to increased glucose metabolism, and suggest H2O2 acts as a secondary messenger to mediate the observed effects of insulin. H2O2 can enhance or reduce insulin responsiveness, depending on the concentration and duration of the oxidant. Prolonged exposure to H2O2 can impair insulin action and trigger insulin resistance, a characteristic feature of type 2 diabetes (Rudich et al., 1998, Lin et al., 2005, Houstis et al., 2006). Under normal conditions, insulin-induced H2O2 mediates early signaling events during the insulin action response. Akin to previously discussed RTKs, endogenous H2O2 enhances activation of IRK and IRS proteins through Tyr phosphorylation of key residues (Mahadev et al., 2001b). Concomitant inactivation of PTPs such as PTP1B and PTEN has similarly been shown to propagate kinase activity during insulin signaling in hepatoma and adipose cells (Nakashima et al., 2000). Follow-up studies have also demonstrated the role of insulin-dependent H2O2 generation with regards to distal insulin response events (Mahadev et al., 2001a). Signal-derived H2O2 blocked with chemical inhibitors attenuated response events associated with the PI3K/Akt pathway, such as glucose uptake and GLUT4 translocation. It is interesting to note that unlike PDGF (Bae et al., 2000), insulin-induced H2O2 production is independent of PI3K activation. Treatment of 3T3-L1 adipocytes with PI3K inhibitors (i.e. wortmannin or LY294002) does not block H2O2 production upon insulin stimulation, and most likely proceeds through a different mechanism than those identified in other RTKs.
Insulin-induced H2O2 production was originally linked to a membrane-bound complex identified in adipocytes with enzymatic characteristics consistent with Nox activity (Krieger-Brauer and Kather, 1992, Krieger-Brauer et al., 1997). Northern blot analysis and reverse-transcriptase PCR (RT-PCR) identified Nox4 as the isoform responsible for H2O2 production within the insulin action pathway (Mahadev et al., 2004). Expression of Nox4 deletion constructs lacking NADPH or FAD/NADPH cofactor binding domains functioned in a dominant-negative manner in differentiated adipocytes. These constructs attenuate insulin-derived H2O2 production, Tyr phosphorylation of IRK and IRS proteins, PI3K/Akt pathway activation, and subsequent insulin response events. Collectively, these results provide crucial evidence linking H2O2 production to insulin-mediated signal transduction. Additionally, co-expression of PTP1B and Nox4 reverses phosphatase activity and increases Tyr phosphorylation. PTP1B plays an important role in downregulation of insulin signaling and is an established therapeutic target for type 2 diabetes (Saltiel and Kahn, 2001). PTP1B knockout mice are healthy and exhibit heightened insulin sensitivity, enhanced glucose and insulin tolerance, and are resistant to diet-induced obesity (Elchebly et al., 1999, Klaman et al., 2000). In particular, this has stimulated therapeutic-based approaches that target and recognize the inactive form of PTPs (Haque et al., 2011, Leonard et al., 2011).
Accumulating evidence has speculated that direct modulation of IRK occurs upon H2O2 exposure, but the functional details of this process are not fully characterized (Mukherjee et al., 1978, Wilden and Pessin, 1987). Iodoacetamide (IAM) treatment increases the catalytic activity of the IRK cytoplasmic domain, whereas another alkylating agent, NEM, inhibits the receptor (Clark and Konstantopoulos, 1993). Regardless, both sulfhydryl agents strongly indicate that functional activity of IRK can be mediated by oxidative modification of one or more cysteine residues located within the IRK β chains. In a separate study, inhibitors of glutathione synthetase (BSO) or glutathione reductase (BCNU, 1,3-bis-(2-chloroethyl)-1-nitrosourea) were used to modulate intracellular GSH levels in IRK transfected CHO cells (Schmid et al., 1998). Both inhibitors increase the GSSG/GSH ratio to induce a moderate shift to mildly oxidative conditions, and lead to structural and functional changes in the IRK β-chain. Additionally, decreased sulfhydryl groups were observed in IRK β-chains in the absence of detectable Tyr phosphorylation. These results suggest IRK may proceed through a “redox-priming stage”, wherein specific cysteine residues undergo oxidative modification prior to full activation through receptor autophosphorylation. Later studies with isolated IRK from transfected CHO cells provide a functional role for redox-based receptor modulation (Schmitt et al., 2005). IRK autophosphorylation is inhibited by binding of ADP within its catalytic active site, which serves to regulate kinase activity. Exposure to H2O2 induces oxidation of two residues (Cys1245 and Cys1308), nucleotide release, and reverses receptor inhibition. It is important to note that direct redox modulation of IRK can promote insulin signaling, but may also contribute to obesity. In muscle cells, cytoplasmic creatine kinase mediates the rapid removal of ADP to promote IRK activation (Stockler-Ipsiroglu, 1997). This process does not occur in adipocytes, and represents a potentially advantageous mechanism to modulate IRK activity in muscle cells. This advantage may be decreased if ADP conversion in adipocytes is elevated due to increased H2O2 levels.
Fibroblast Growth Factor Receptor (FGFR)
Fibroblast growth factors (FGF) and their receptors (FGFRs) regulate developmental signaling pathways and are expressed in many different cell types (Turner and Grose, 2010). The mammalian FGF family is composed of 18 distinct ligands, and exerts its actions through 4 highly conserved transmembrane RTKs (FGFR1, FGFR2, FGFR3, and FGFR4). Upon stimulation, FGFR undergoes receptor dimerization and Tyr autophosphorylation within its intracellular kinase domain. These residues act as docking sites for adaptor proteins and lead the activation of four key downstream pathways: 1) Ras/MAPK pathway, 2) PI3K/Akt pathway, 3) STAT (signal transducer and activator of transcription), and 4) PLC-γ.
The molecular mechanisms underlying redox regulation of FGFR remains the least characterized amongst other RTKs. Initial studies demonstrate that basic FGF (bFGF) induces H2O2 production upon stimulation in chondrocytes (Lo and Cruz, 1995). FGF signaling promotes early response genes such as c-fos and c-jun, which dimerize to form the AP-1 transcription factor. Exposure to exogenous H2O2 recapitulates these observations in chondrocytes and other cell types (Devary et al., 1991, Nose et al., 1991). Additionally, cells treated with DPI exhibit decreased H2O2 levels upon growth factor stimulation. These results suggest that a Nox complex may serve as the oxidant source during FGF signaling, albeit further studies are necessary to confirm these observations. In an isoform-specific study with PDGF and FGF, endogenous H2O2 facilitates adipose differentiation in 3T3-L1 adipocytes (Krieger-Brauer and Kather, 1995). Alternatively, FGF stimulation may generate H2O2 through mechanisms independent of Nox complexes. In lung fibroblasts, Nox activity was measured and remained unchanged during growth factor stimulation. Expression of a dominant-negative Ras mutant (RasN17) diminishes signal-induced H2O2 production and suggests it may proceed through a Ras-dependent mechanism (Thannickal et al., 2000).
A more recent study identifies direct oxidation of cysteine residues in FGFR. These residues were first characterized in cytoplasmic Src (c-Src) using cysteine mutants to distinguish decreased responses to H2O2 exposure (Kemble and Sun, 2009). Mutation of c-Src Cys277 abolishes its sensitivity to H2O2 during in vitro assays, and maintains the kinase in a constitutively active form. Gel analysis reveals oxidation of Cys277 inactivates c-Src by forming homodimers connected by a disulfide bond. Cys277 is located within a glycine rich loop (GQGCFG, Gly loop), which participates in catalysis by interacting with the γ-phosphate of ATP. The Gly loop is a universally conserved signature motif present among all protein kinases, but intervening residues can be variable (Taylor et al., 1992). Sequence analysis reveals that only a small subset of protein kinases (∼ 8) contains a homologous residue at an equivalent position to c-Src Cys277. These include 3 of 10 Src family kinase members (Src, Yes, Fgr) and all 4 FGFR family members. To confirm its significance, the equivalent residue in FGFR1 (Cys488) was mutated and subjected to similar assays to assess its functional role. C488A attenuates the ability of FGFR1 to respond to H2O2 and inactivates the kinase, as observed with c-Src. This mutation does not completely abolish FGFR1 dimerization and suggests that there may be other contributing factors. Although this study identifies a conserved redox active cysteine found in Src and FGFR family members, the cellular significance of this modification currently remains unknown and presents a potential regulatory mechanism unique to a small subset of kinases.
Non-Receptor Kinases
Akt (also named Protein Kinase B, PKB)
The serine/threonine kinase Akt is a central node downstream of growth factors, cytokines, and other cellular stimuli (Manning and Cantley, 2007). As described earlier, Akt can be activated by a wide range of stimulants, and is dependent on upstream production of lipid products by PI3K. Negative regulation of Akt is maintained through direct dephosphorylation by protein phosphatase 2A (PP2A) (Kageyama et al., 2002), or phosphatase activity of PTEN towards PIP3 (Seo et al., 2005). Akt has three isoforms (Akt1, Akt2, and Akt3), and achieves signaling specificity through tissue-specific expression (Gonzalez and McGraw, 2009a). Akt1 is the predominantly expressed isoform in a majority of tissues, whereas Akt2 is found to be highly enriched within insulin targeted-tissues (Chan et al., 1999, Gonzalez and McGraw, 2009b). In particular, Akt2-deficient mice are insulin resistant and display a diabetic-like phenotype (Cho et al., 2001a). Akt1-null mice have normal glucose tolerance, but exhibit severe growth retardation (Cho et al., 2001b, Chen et al., 2001b). These mice models demonstrate Akt isoforms are not functionally redundant and are differentially expressed based on substrate preference and subcellular distribution. Aberrant gain or loss of Akt function underlies the pathological states in a variety of diseases, and represents a prominent target for the development of therapeutic strategies (Engelman et al., 2006).
Signal-mediated production of endogenous H2O2 has been shown to increase Akt activation in numerous cell types and is modulated by a various signaling components (Ushio-Fukai et al., 1999). Exposure to PI3K inhibitors (wortmannin and LY294002) strongly attenuates Akt phosphorylation levels and indicates upstream PI3K activation is required for redox-based modulation of Akt (Shaw et al., 1998). Studies report that H2O2 can induce PIP3 levels in cells overexpressing Nox1 (Kwon et al., 2004), or accumulate PIP2 under conditions of oxidative stress (Van der Kaay et al., 1999). These lipids bind to Akt to increase its activity and propagate downstream events. H2O2 activation of Akt is also mediated in part by Src kinase (Esposito et al., 2003). The detailed mechanism underlying this relationship has not been completely delineated, but may be due to Src-mediated interference of PTEN phosphatase activity (Lu et al., 2003). Additionally, EGFR-dependent activation of Akt enhances cell survival during H2O2-induced apoptosis (Wang et al., 2000). Overexpression of constitutively active Akt in HeLa and NIH 3T3 fibroblasts results in approximately 40% fewer apoptotic cells when compared to control cells. These results indicate Akt activity may be elevated under conditions of stress to promote cell survival, parallel to observations obtained with Erk1/2 (Guyton et al., 1996). Interestingly, Akt nitrosylation inactivates the kinase and is suggested to contribute to redox-based insulin resistance as well (Carvalho-Filho et al., 2005, Yasukawa et al., 2005).
Redox modulation of multiple cysteine residues have been identified and shown to regulate Akt kinase activity. Treatment with NEM blocks PDGF-induced Akt activation without affecting PI3K function (Yellaturu et al., 2002). Additionally, NEM inhibits phosphorylation of several Akt downstream effectors, which includes the p70S6K, 4E–BP1, BAD, and FKHR family of transcription factors. These results suggest that sulfhydryl alkylation interferes with the PI3K/Akt pathway at the Akt level. Crystal structures reveal Akt2 forms an intramolecular disulfide bond (Cys297 and Cys311) within its activation loop, which inactivates the kinase (Huang et al., 2003). These residues are conserved in all three Akt isoforms, but do not significantly alter kinase activity in other isoforms. Inactive Akt2 can be rescued by glutaredoxin (Grx) through reduction of this disulfide to protect cells against apoptosis (Murata et al., 2003). Grx overexpression suppresses recruitment of PP2A to Akt to sustain phosphatase activity and promote cell survival. A more recent study identifies a third cysteine residue responsible for isoform-specific redox regulation of Akt (Wani et al., 2011). Using DCP-Bio1, the authors observed that Akt2 forms a sulfenic acid in response to PDGF stimulation that inactivates the kinase. MS analysis coupled with dimedone labeling and H2O2-treated Akt2 identified a sulfenyl modification at Akt2 Cys124. This residue is located in the Akt2 linker region, is not conserved in other isoforms, and is partially responsible for oxidative inactivation of Akt2. Parallel H2O2 treatment of NIH 3T3 cells induces indiscriminate Tyr phosphorylation of Akt1 and Akt2, but eventually leads to oxidative inactivation of only Akt2. Additionally, glucose uptake was decreased in WT Akt2, and was unperturbed in an Akt2 C124S mutant. Although H2O2 induces Akt activation in many scenarios, it appears that oxidative modification of these three cysteine residues may serve to control the amplitude of Akt2 during signaling.
MAPK (Erk1/2, JNK, p38)
MAPKs are serine/threonine specific kinases that are modulated by a number of receptor-ligand interactions (Chen et al., 1995, Lo et al., 1996, Kamata et al., 2005, Ushio-Fukai et al., 1998). Although MAP kinases are composed of five subfamilies, we will focus mainly on evidence implicating redox modulation of Erk1/2, JNK, and p38. Activation of MAPKs proceeds through a three-tiered cascade that begins with a MAP kinase kinase kinase (MEKK or MAP3K), MAP kinase kinase (MEK or MAP2K), and ends with MAPK to elicit its respective effectors. MAPKs have distinct functional roles; Erk1/2 mediates pro-survival events, whereas JNK and p38 are mainly involved in stress-related responses (Roberts and Der, 2007, Wagner and Nebreda, 2009). The components involved in redox regulation of MAPKs have been covered extensively in other reviews (McCubrey et al., 2006). Therefore, specific examples emphasizing the functional role of cysteine modifications in MAPK pathways will be highlighted for the purpose of the following section.
Early studies demonstrate that Erk1/2 is activated in response to H2O2 and enhances cell survival following oxidant injury (Guyton et al., 1996, Wang et al., 1998). H2O2 treatment increases Erk1/2 phosphorylation levels 10–20 fold, and moderately for JNK and p38 (∼ 3–5 fold). RasN17 expression diminishes H2O2-induced Erk1/2 activation, and renders cells more sensitive to apoptosis when compared to WT controls. Ras can undergo S-nitrosylation at Cys118, and represents one mechanism by which redox regulation of Erk1/2 can occur upstream (Lander et al., 1996). Additionally, overexpression of constitutively active MEK promotes increased cellular resistance to H2O2 toxicity. These results demonstrate stimulant-induced H2O2 production appears to affect protein targets upstream of Erk1/2 to initiate kinase activity.
Distinct mechanisms have evolved to regulate redox-based responses of MAPK family members. For example, c-Src mediates JNK activation by promoting Cas-Crk complex formation in response to H2O2 (Yoshizumi et al., 2000). Cas is a c-Src substrate known to trigger JNK signaling (Dolfi et al., 1998). Furthermore, JNK is inhibited in Src-deficient mice or when treated with Src inhibitors (PP2). This mechanism is unique to JNK, and is not observed with respect to Erk1/2 or p38. Alternatively, Prxs represent another regulatory component involved in JNK signaling. Although the major role of Prxs is to catalyze H2O2 decomposition, one study provides mechanistic evidence demonstrating direct JNK activation by Prx (Veal et al., 2004). Sty1, a JNK homologue found in fission yeast, forms a H2O2-induced intermolecular disulfide bond with Tpx1 (2-Cys Prx). This bond activates Sty1, and forms between the catalytic cysteine of Tpx1 (Cys48) and Sty1 (Cys35). Both residues are conserved in their mammalian counterparts, and suggest similar mechanisms may exist in other eukaryotes. In macrophages, JNK is negatively modulated by S-nitrosylation at Cys116 (Park et al., 2000).
Additionally, upstream elements of MAPKs are also subject to direct redox regulation. The apoptosis signal-regulating kinase (ASK1)/Trx1 system is a well-known complex explored by many researchers. ASK1 is a MAP3K responsible for activating JNK and p38 pathways, and is required for tumor necrosis factor-α (TNF-α) mediated apoptosis (Ichijo et al., 1997). Two models have been proposed to describe H2O2-induced ASK1 activation. In the first model (Fig. 9a), Trx1 sequesters ASK1 in an inactive complex and undergoes intramolecular disulfide formation upon TNF-α or H2O2 stimulation. This releases ASK1, permits oligomerization, and leads to formation of the active complex (Saitoh et al., 1998, Liu et al., 2000). However, a later study (Fig. 9b) demonstrates stable ASK1 oligomerization and activation is mediated by disulfide bond formation between ASK1 monomers (Nadeau et al., 2007). This alternate model suggests Trx1 negatively regulates ASK1 by maintaining its reduced state. Oxidation of ASK1 Cys250 is essential for H2O2-dependent activation of JNK, albeit the exact details underlying this modification are unknown (Nadeau et al., 2009). ASK1 C250A mutants attenuate Trx1 association, but do not activate JNK. These results suggest simple dissociation of Trx1 is not sufficient to promote downstream signaling and that oxidative modification of C250 may induce conformational changes in ASK1 to prompt pathway activation. Contrastingly, S-nitrosylation of ASK1 (Cys869) inactivates the kinase by interfering with MAP2K binding (Park et al., 2004b).
Figure 9.
Two proposed models for redox-based activation of ASK1. (a) Trx1 oxidation model. In the cell, ASK1 assembles into multimers that interact with Trx1. Association of Trx1 with ASK1 sequesters the kinase in an inactive conformation that is released upon oxidation of Trx1 by H2O2. Once released, activated ASK1 interacts with additional binding proteins (BPs) to initiate downstream signaling. (b) ASK1 oxidation model. In this second model, H2O2 induces intermolecular disulfide bond formation between ASK1 monomers to promote kinase activation and multimerization. Trx1 is suggested to negatively regulate ASK1 signaling by maintaining the kinase in its reduced state.
MEKK1 is another MAP3K responsible for JNK activation, and forms a glutathione adduct at Cys1238 upon H2O2 treatment (Cross and Templeton, 2004). This residue is located in the ATP binding domain, and represents one of many mechanisms utilized to obtain signaling specificity in MAPK pathways. Interestingly, MKK6 (MAP2K activator of p38) was recently identified to form an intramolecular disulfide bond (Cys109 and Cys196) (Diao et al., 2010). Bond formation negatively regulates p38 by interfering with its ATP binding ability. Sequence alignment of MKK6 reveals that these two residues are conserved in all 7 human MAP2Ks. Similar experiments demonstrates that other MAP2Ks (MKK1 and MKK4) undergo oxidative inactivation analogous to MKK6. Another study demonstrates p38 may also contain redox-sensitive cysteines (Cys119 and Cys162) that reversibly inactivate the kinase upon oxidation (Templeton et al., 2010). Non-reducing gel analysis suggests these residues do not form an intramolecular disulfide, but the exact identity of this modification remains unclear and further studies are necessary to probe its functional relevance. Although many studies have reported H2O2-induced activation of MAPK pathways, redox-based inactivation of upstream components also serves to modulate MAPK signal duration.
c-Src
Cytoplasmic Src (c-Src) is a tyrosine kinase and a key regulator of proliferation, survival, cytoskeletal organization, and integrin-dependent signaling responses (Thomas and Brugge, 1997, Ma and Huang, 2002). Abnormal expression or hyperactivation of c-Src is present in several human malignancies and has been linked to the development of human cancers (Yeatman, 2004). As mentioned previously, c-Src interacts with many ligand-activated RTKs and affects a number of substrates that include adaptor proteins, focal-adhesion proteins, and transcription factors. The Src family of kinases (SFKs) is composed of nine members and can be mostly grouped into two subfamilies, namely the Src-related (Src, Yes, Fyn, Fgr) and the Lyn-related (Lyn, Hck, Lck, Blk) kinases. c-Src and other SFK members share a conserved domain architecture composed of an N-terminal region with high variability among family members to enable distinct functionality, followed by SH3, SH2, and tyrosine kinase (SH1) domains flanked by a C-terminal tail (Fig. 10). c-Src contains two residues (Tyr416 and Tyr527) endowed with opposing regulatory mechanisms. Phosphorylation of C-terminal Tyr527 locks Src in an inactive conformation by binding to its SH2 domain, and is further clinched by interactions between the SH2 and SH3 domains (Xu et al., 1997). In this conformation, the activation loop (A-loop) adopts a compacted structure that precludes ATP access and kinase activation through Tyr416. Concurrent dephosphorylation of Tyr527 and disruption of SH2/SH3 interactions induces an open conformation to enable Tyr416 phosphorylation, leading to full activation of c-Src.
Figure 10.
Regulation of c-Src. Inactive c-Src exhibits a “closed” conformation, characterized by binding of phosphorylated Tyr527 to its SH2 domain and interactions between its SH2/SH3 domains. Growth factors and cytokines initiate c-Src activation by promoting concurrent dephosphorylation of Tyr527 and disruption of SH2/SH3 interactions to induce an “open” conformation for Tyr416 autophosphorylation. In the late phase of activation, signal-derived H2O2 mediates the formation of an intramolecular disulfide bond between c-Src Cys245 and Cys487. Bond formation promotes kinase activation, Src-mediated cell adhesion, and cytoskeletal reorganization events. Adapted from Giannoni et al., 2010.
Studies reporting H2O2-induced Src activation are typically accompanied by increased Tyr416 phosphorylation (Li et al., 2008, Krasnowska et al., 2008), whereas inactivation is invariably accompanied by increased Tyr527 phosphorylation (Cunnick et al., 1998, Tang et al., 2005). These complications make it difficult to ascertain whether these effects are due to direct or indirect regulatory mechanisms (Sun and Kemble, 2009, Giannoni et al., 2010). c-Src is involved in mediating redox-based responses of many signaling components within the Ras/MAPK or PI3K/Akt pathways, and Nox complexes. Suppression of c-Src activity with chemical inhibitors, antisense oligonucleotides, or Src knockout mice interferes with the ability of these pathways to effectively respond to oxidants during signaling and conditions of oxidative stress (Gianni et al., 2008, Mehdi et al., 2005, Abe et al., 1997). Negative regulation of Src is due to phosphorylation of Tyr527 by Csk (C-terminal Src kinase). Csk is unique among other tyrosine kinases because it only phosphorylates one class of substrate, the conserved Tyr residue located within the C-terminal tail of SFKs (Sondhi et al., 1998). Interestingly, evidence suggests the relationship between Src and Csk may be subject to redox regulation. The SH2 domain of Csk contains two cysteine residues (Cys122 and Cys164) that form a unique intramolecular disulfide bond, and is not present in other known SH2 domains (Mills et al., 2007). Bond formation induces a conformational change and is correlated to decreased Csk catalytic activity. Therefore, this disulfide may serve as a regulatory mechanism to suppress Src inhibition through Csk-mediated phosphorylation. Crystal structures of the Csk-Src complex reveal that disulfide formation occurs between Src Cys277 and Csk Cys290 (Levinson et al., 2008). This does not form within the same kinase-substrate complex, but rather, is composed from Src and Csk in two separate heterodimers. Structural analysis demonstrates this disulfide is crucial in positioning the C-terminal tail of c-Src near the Csk active site to promote Tyr527 phosphorylation. Many Tyr kinases contain a canonical substrate binding site allowing for phosphorylation of several effectors that is destabilized in Csk. Consequently, Csk solely phosphorylates SFK members due to this. The two aforementioned studies demonstrate different functional roles that disulfide bond formation plays in modulating Src activation.
Cellular studies provide evidence that cysteine oxidation can directly affect c-Src activity, albeit with contradictory conclusions. Evidence for both cases will be presented, but it is important to note contrasting results may be due to the cell types used or sample manipulation. An initial study reports treatment of NIH 3T3 fibroblasts or immunoprecipitated c-Src with exogenous NO donors (i.e. SNAP) promotes kinase activation through S-nitrosylation (Akhand et al., 1999). The sulfhydryl residues responsible for this were not identified, but seemingly enhanced Tyr416 autophosphorylation. More recent work identifies c-Src Cys498, a conserved residue in SFK members, as the target of NO-mediated kinase activation (Rahman et al., 2010). H2O2 activation of c-Src has also been reported to occur during cell adhesion events. Integrin ligation elicits production of H2O2 by lipoxygenases (5-LipOX) to induce c-Src activation through an intramolecular disulfide bond (Cys245 and Cys487) (Giannoni et al., 2005). These residues are respectively located within the SH2 and kinase domains of c-Src. During cell adhesion, evidence suggests c-Src proceeds through a biphasic activation process that begins with an early phase characterized by formation of focal adhesions and Tyr527 dephosphorylation. A late phase quickly ensues and is distinguished by lipoxygenase-derived H2O2 and phosphorylation of Tyr416. c-Src cysteine mutants demonstrate that disulfide bond formation is required to induce Tyr416 hyperactivation. These combined events demonstrate H2O2 promotes kinase activation and Src-mediated cell adhesion and cytoskeletal reorganization (Fig. 10). Lyn, another SFK member, contains a single cysteine residue (Cys466) that functions as a redox senor to mediate leukocyte wound attraction in zebrafish (Yoo et al., 2011). Alternatively, H2O2 has also been shown to suppress Src activation in other studies. As discussed in detail in the FGFR section, a conserved cysteine residue (Cys277) originally identified in c-Src promotes kinase inactivation upon oxidative modification. The significance of this residue, homologous in SFK and FGFR members, was similarly demonstrated with FGFR1. Therefore, it is reasonable to conclude that opposing regulatory redox mechanisms exist to modulate c-Src activity akin to its phosphorylation sites.
IKK (Inhibitory κB kinase)
Inhibitory κB kinase (IKK) is a serine/threonine kinase primarily responsible for activation of transcription factor nuclear factor-κB (NF-κB). IKK is composed of two catalytic subunits, IKKα and IKKβ, and regulatory subunit IKKγ. Stimulation with cytokines such as TNFα (tumor necrosis factor α) or interleukins induces Ser phosphorylation of IKKα and IKKβ subunits by upstream kinases (i.e. MEKK1, Akt). The activated IKK complex phosphorylates inhibitory κB (IκB) proteins, which maintain and sequester NF-κB in its latent form in the cytoplasm. Phosphorylation fosters inducible degradation of IκB proteins and unmasks the nuclear localization signal (NLS) of NF-κB to promote nuclear translocation (Fig. 11). NF-κB binds to recognition motifs and activates gene transcription of over ∼100 target genes that mediate inflammatory response, proliferation, and survival. NF-κB can be directly activated by ROS during TNFα signaling and has been extensively analyzed in a number of studies (Pantano et al., 2006).
Figure 11.
IKK activation of NF-κB. Stimulation with cytokines (i.e. TNFα or interleukins) triggers Ser phosphorylation of the IKK complex by upstream kinases such as MEKK1 or Akt. Once activated, IKK phosphorylates IκB proteins, which negatively regulate NF-κB and maintain the latent transcription factor in the cytoplasm. Phosphorylation of IκB unmasks the nuclear localization signal of NF-κB, and promotes nuclear translocation. Once NF-κB is released, IκB proteins are subsequently degraded by proteasomes. In addition, IKK can be inactivated by endogenously produced H2O2 or NO through direct modulation of Cys179.
ROS can also modulate activity of upstream components within the NF-κB pathway. For example, IKK becomes inactivated upon stimulation with NO or H2O2. Exposure of epithelial cells with exogenous NO donor SNAP inhibits IKK kinase activity, but does not interfere with enzyme activation (Reynaert et al., 2004). These results suggest NO directly modifies IKK rather than acting upon upstream components. MS analysis identified IKKβ Cys179 as the major target for S-nitrosylation leading to kinase inactivation. Repression of endogenous NO using NO synthase (NOS) inhibitors relieved SNO-based IKK complex inactivation in Jurkat T cells. Cys179 is strategically located between the two Ser residues (Ser177 and Ser181) required for IKKβ activation by TNFα. Therefore, it is probable that IKK phosphorylation at its Ser residues may induce a negatively charged environment surrounding Cys179 to promote its susceptibility to S-nitrosylation. Interestingly, IKKβ Cys179 undergoes inactivation upon exposure to environmental toxins (Kapahi et al., 2000). IKK inhibition due to H2O2-induced oxidation is also observed in TNFα stimulated epithelial cells (Korn et al., 2001). MS analysis and dimedone-based experiments reveal that IKKβ Cys179 proceeds through a sulfenic acid intermediate to form a glutathione adduct (Reynaert et al., 2006). S-glutathionylation of IKKβ Cys179 is reversed by Grx to restore intrinsic kinase activity. Collectively, these results demonstrate S-glutathionylation is a physiologically relevant mechanism that controls the magnitude of NF-κB activation. Multiple studies have identified a number of Cys179 modification states due to different oxidant sources, indicating redox regulation of the IKK complex functions in a complex manner. In contrast, studies have also reported H2O2-mediated activation of IKK; however, it is important to note this may be due to different cell types or oxidant concentration utilized in the studies (Jaspers et al., 2001, Kamata et al., 2002).
Future Perspectives
Although PTPs were originally identified as the main targets of signal-derived H2O2, direct modulation of protein kinases by reversible cysteine oxidation has been identified in a continuously growing number of examples (Table 2). For instance, S-glutathionylation of c-Abl, a non-receptor Tyr kinase, serves to regulate kinase activity under conditions of oxidation stress (Leonberg and Chai, 2007). Specific cysteine residues in two other kinases, ataxia-telangiectasia mutated (ATM) kinase and pyruvate kinase M2 (PKM2), have recently been identified and exhibit distinct functional responses to oxidation. ATM is activated by DNA double-strand breaks (DSBs) and orchestrates signaling pathways that initiate DNA damage response. Upon exposure to H2O2, ATM is activated through an intermolecular disulfide at Cys2991 (Guo et al., 2010b, Guo et al., 2010a). Redox-based ATM activation occurs separately from DNA damage-based kinase activation, and represents a branch point wherein ATM can also act to modulate global cellular responses to oxidative stress. Moreover, oxidation of PKM2 at Cys358 was shown to inactivate the kinase in A549 lung cancer cells (Anastasiou et al., 2011). PKM2 inactivation diverts glucose flux into the pentose phosphate pathway and results in increased generation of reducing equivalents (NADPH) for the detoxification of ROS through Prx, GSH, and Trx systems. These recent discoveries demonstrate the likelihood that other kinases may rely on redox-based mechanisms that remain to be unraveled.
Table 2.
Examples of redox-regulated protein kinases.
| Kinase | Oxidant/source* | Residue/location | Oxoform† | Effect of modification | Reference |
|---|---|---|---|---|---|
| PDGFR | NA | Cys822 (catalytic loop) Cys940 (C-terminal kinase domain) |
NA | Activation, initiates kinase activity | Lee et al., 2004 |
| EGFR | H2O2 (Nox) | Cys797 (ATP-binding site) | Sulfenic acid | Activation, facilitates EGF signaling | Paulsen et al., 2012 |
| VEGFR | H2O2 (Nox) | Cys1209 (C-terminal tail) | Disulfide (intra) | Inactivation | Kang et al., 2011 |
| Cys1199(C-terminal tail) | |||||
| IRK | H2O2 (exo) | Cys1245 (kinase domain) | Disulfide (intra) or S-glulathionylation |
Activation, promotes release of ADP from kinase domain |
Schmitt et al., 2005 |
| Cys1308 (kinase domain) | |||||
| FGFR | H2O2 (exo) | Cys488 (Gly loop in catalytic domain) | Disulfide (inter) | Inactivation | Kemble & Sun, 2009 |
| Akt | H2O2 (exo) | Cys297 (activation loop) | Disulfide (intra) | Inactivation | Huang et al., 2003 |
| Cys311 (activation loop) | Murata et al., 2003 | ||||
| H2O2 (PDGF-induced] | Cys124 (linker region) | Sulfenic acid | Inactivation, inhibits glucose uptake | Wani et al., 2011 | |
| Sty 1 (JNK yeast | H2O2 (exo) | Cys35 | Disulfide (inter) with Tpxl | Activation | Veal et al., 2004 |
| homolog) | (2 Cys-Prx yeast homolog) | ||||
| JNK | NO (NOS) | Cys116 | S-nitrosylation | Inactivation, anti-apoptotic | Park et al., 2000 |
| ASKI | NO (exo), NO (NOS) | Cys869 (kinase domain) | S-nitrosylation | Inactivation, inhibits binding to MKK3/MMK6 |
Park et al., 2004b |
| Cys22 and/or Cys30, Cys250, Cys835, Cys1052, Cys1361 |
Disulfide (inter), niultimediation | Activation, induction of apoplosis and JNK signaling |
Nadeau et al., 2007 | ||
| Nadeau el al. 2009 | |||||
| MEKK1 | H2O2 (exo) | Cys1238 (catalytic domain) | S-glutathionylation | Inactivation | Cross & Templeton. 2004 |
| MKK6 | H2O2(exo) | Cys109 | Disulfide (intra) | Inactivation, inhibits ATP binding | Diao et al., 2010 |
| Cys196 | |||||
| p38 | H2O2 (exo) | Cys119 | ND | Inactivation | Templeton et al., 2010 |
| Cys162 | |||||
| c-Src | NO (NOS) | Cys498 (C-terminal) | S-nitrosylation | Activation | Rahman et al., 2010 |
| H2O2 (L) | Cys245 | Disulfide (intra) | Activation, promotes proliferation and cell adhesion |
Giannoni et al., 2005 | |
| Cys487 | |||||
| H2O2 (exo) | Cys277 (Gly loop in catalytic domain) | Disulfide (inter) | Inactivation | Kemble & Sun, 2009 | |
| Lyn | H2O2(Nox) | Cys466 (C-terminal kinase domain) | ND | Activation, leukocyte wound attraction | Yoo et al., 2011 |
| IKK | NO (NOS) | Cys179 (IKKβ subunit) | S-nitrosylation | Inactivation, represses NF-κB | Reynaert et al., 2004 |
| H2O2 (TNFα-induced) | Cys179 (IKKβ subunit) | S-glutathionylation | Inactivation, represses NF-κB | Reynaert et al., 2006 | |
| ATM | H2O2 (exo) | Cys299l (FATC domain) | Disulfide (inter) | Activation, redox sensor | Guo et al., 2010 |
| PKM2 | H2O2 | Cys358 | Disulfide (intra) | Inactivation, generates reducing potential for ROS detoxification |
Anastasiou et al., 2011 |
Exo, exogenous: L, lipoxygenase: NA, not applicable.
Intra, intramolecular: inter, intermolecular: ND, not determined.
The catalytic cysteine of PTPs serves as a well-established regulatory switch during H2O2-mediated signaling to promote kinase activation and downstream signaling in a controlled manner. Therefore, it is intriguing that similar mechanisms may regulate PTKs containing conserved cysteine residues. Eight protein kinases, which include SFK and FGFR members, share a cysteine residue located within a conserved glycine-rich loop (Kemble and Sun, 2009). This residue causes kinase inactivation in vitro, albeit the cellular function of this modification remains unknown. Of the ∼95 receptor and non-receptor PTKs in the human genome, nine additional members harbor a cysteine residue that corresponds to EGFR Cys797, including two erbB family members (HER2 and HER4) (Fig. 12). Cys797 and its structural homologues serve as the N-terminal end of an alpha helix known as the Ncap position. Interestingly, cysteine is the most sparsely occurring Ncap residue in proteins and comprises less than 1% of these positions (Penel et al., 1999). Interaction of a cysteine residue with the helical dipole within an Ncap context reduces the pKa of the residue, which inherently increases its reactivity (Anderson and Sauer, 2003). Efforts focused on delineating the molecular mechanisms by which protein kinases are directly modulated by H2O2 could uncover general signaling mechanisms that regulate kinases and their structural homologues.
Figure 12.
Abbreviated sequence alignment of EGFR and nine additional kinases that harbor a cysteine residue structurally homologous to EGFR Cys797. This group includes two erbB family members, HER2 and HER4.
Aberrant kinase activation has been identified in a number of human pathologies, as referenced for each kinase within its respective section. This has stimulated efforts to develop therapeutic strategies such as antibodies and reversible inhibitors to target specific kinase classes. Many reversible inhibitors attenuate kinase activation by interfering with the ATP binding site. Additionally, irreversible inhibitors have also been developed for certain kinases (Zhang et al., 2012, Kwarcinski et al., 2012). For example, EGFR is mutated or amplified in a number of human carcinomas such as breast and lung cancers. This has motivated the development of a class of selective kinase inhibitors that covalently modify EGFR Cys797 (Singh et al., 1997, Liu et al., 2013). Several of these irreversible EGFR inhibitors are currently undergoing preclinical or clinical trials (Singh et al., 2010, Singh et al., 2011). Overexpression of EGFR and HER2 is correlated to increased H2O2 levels and global protein oxidation in breast cancer cell lines (Seo and Carroll, 2009). When coupled to recent evidence that identifies sulfenic acid modification of EGFR Cys797, these findings raise several fundamental questions regarding cysteine modifications versus thiol-targeted covalent inhibitors. The aforementioned irreversible EGFR inhibitors contain an acrylamide moiety that undergoes Michael addition with Cys797 in its thiol form, but are not capable of reacting with sulfenic acid or disulfide states. Oxidative modification of Cys797 could impact the potency of these inhibitors, particularly in cancers characterized by high levels of stress and aberrant H2O2 levels. Therefore, the sulfenyl form of EGFR Cys797 could be exploited to develop new classes of irreversible inhibitors that incorporate nucleophilic warheads akin to chemical probes recently developed for PTPs (Leonard et al., 2011). If successful, redox-based EGFR inhibitors would exhibit enhanced selectively for cancerous cells exhibiting high levels of oxidative stress, and may reduce the toxicity caused by acrylamide-based compounds. Analogous approaches could be employed to generate nucleophilic warheads for other classes of redox-modulated protein families as well.
Conclusions
The role of protein kinases in physiologic and pathological settings represents an active and exciting area of research. The collective efforts of several individuals have contributed tremendously towards uncovering the underlying mechanisms involved in redox-regulation of protein kinases. These studies have expanded our knowledge and demonstrate that kinases may be modulated both directly and indirectly upon stimulant-induced H2O2 production. The continued efforts to dissect kinase function will further our basic understanding of kinase redox-regulation, uncover new protein targets, and could lead to the development of new redox-based strategies for therapeutics.
Acknowledgements
Declaration of Interest
The authors acknowledge funding from the Camille Henry Dreyfus Teacher Scholar Award (to K.S.C.) and the National Institutes of Health grant GM102187 (to K.S.C.).
References
- Abe J, Takahashi M, Ishida M, Lee JD, Berk BC. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J Biol Chem. 1997;272:20389–20394. doi: 10.1074/jbc.272.33.20389. [DOI] [PubMed] [Google Scholar]
- Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000;486:252–256. doi: 10.1016/s0014-5793(00)02305-x. [DOI] [PubMed] [Google Scholar]
- Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem. 2007;282:35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- Akhand AA, Pu M, Senga T, Kato M, Suzuki H, Miyata T, Hamaguchi M, Nakashima I. Nitric oxide controls src kinase activity through a sulfhydryl group modification-mediated Tyr-527-independent and Tyr-416-linked mechanism. J Biol Chem. 1999;274:25821–25826. doi: 10.1074/jbc.274.36.25821. [DOI] [PubMed] [Google Scholar]
- Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson TA, Sauer RT. Role of an N(cap) residue in determining the stability and operator-binding affinity of Arc repressor. Biophys Chem. 2003;100:341–350. doi: 10.1016/s0301-4622(02)00291-0. [DOI] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbiser JL, Petros J, Klafter R, Govindajaran B, Mclaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan M, Ozben T. Oxidants in receptor tyrosine kinase signal transduction pathways. Antioxid Redox Signal. 2003;5:781–788. doi: 10.1089/152308603770380089. [DOI] [PubMed] [Google Scholar]
- Avruch J. Insulin signal transduction through protein kinase cascades. Mol Cell Biochem. 1998;182:31–48. [PubMed] [Google Scholar]
- Babior BM. The NADPH oxidase of endothelial cells. IUBMB Life. 2000;50:267–269. doi: 10.1080/713803730. [DOI] [PubMed] [Google Scholar]
- Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauskas A, Rhee SG. Platelet-derived growth factor-induced H(2)O(2) production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem. 2000;275:10527–10531. doi: 10.1074/jbc.275.14.10527. [DOI] [PubMed] [Google Scholar]
- Banerjee R, editor. Redox Biochemistry. Hoboken, NJ: John Wiley & Sons; 2008. [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Benitez LV, Allison WS. The inactivation of the acyl phosphatase activity catalyzed by the sulfenic acid form of glyceraldehyde 3-phosphate dehydrogenase by dimedone and olefins. J Biol Chem. 1974;249:6234–6243. [PubMed] [Google Scholar]
- Bienert GP, Moller AL, Kristiansen KA, Schulz A, Moller IM, Schjoerring JK, Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- Boivin B, Zhang S, Arbiser JL, Zhang ZY, Tonks NK. A modified cysteinyl-labeling assay reveals reversible oxidation of protein tyrosine phosphatases in angiomyolipoma cells. Proc Natl Acad Sci U S A. 2008;105:9959–9964. doi: 10.1073/pnas.0804336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrow SA, Cohen S, Staros JV. Affinity labeling of the protein kinase associated with the epidermal growth factor receptor in membrane vesicles from A431 cells. J Biol Chem. 1982;257:4019–4022. [PubMed] [Google Scholar]
- Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G, King L, Jr, Cohen S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature. 1978;276:409–410. doi: 10.1038/276409a0. [DOI] [PubMed] [Google Scholar]
- Carvalho-Filho MA, Ueno M, Hirabara SM, Seabra AB, Carvalheira JB, De Oliveira MG, Velloso LA, Curi R, Saad MJ. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes. 2005;54:959–967. doi: 10.2337/diabetes.54.4.959. [DOI] [PubMed] [Google Scholar]
- Caselli A, Marzocchini R, Camici G, Manao G, Moneti G, Pieraccini G, Ramponi G. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J Biol Chem. 1998;273:32554–32560. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Thomas SR, Albano A, Murphy MP, Keaney JF., Jr Mitochondrial function is required for hydrogen peroxide-induced growth factor receptor transactivation and downstream signaling. J Biol Chem. 2004;279:35079–35086. doi: 10.1074/jbc.M404859200. [DOI] [PubMed] [Google Scholar]
- Chen K, Vita JA, Berk BC, Keaney JF., Jr c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves SRC-dependent epidermal growth factor receptor transactivation. J Biol Chem. 2001a;276:16045–16050. doi: 10.1074/jbc.M011766200. [DOI] [PubMed] [Google Scholar]
- Chen Q, Olashaw N, Wu J. Participation of reactive oxygen species in the lysophosphatidic acid-stimulated mitogen-activated protein kinase kinase activation pathway. J Biol Chem. 1995;270:28499–28502. doi: 10.1074/jbc.270.48.28499. [DOI] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001b;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Cao Z, Xu X, Van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Buricchi F. Protein tyrosine phosphorylation and reversible oxidation: two cross-talking posttranslation modifications. Antioxid Redox Signal. 2007;9:1–24. doi: 10.1089/ars.2007.9.1. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Cirri P, Raugei G, Camici G, Dolfi F, Berti A, Ramponi G. PDGF receptor as a specific in vivo target for low M(r) phosphotyrosine protein phosphatase. FEBS Lett. 1995;372:49–53. doi: 10.1016/0014-5793(95)00947-8. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Cirri P, Taddei ML, Talini D, Doria L, Fiaschi T, Buricchi F, Giannoni E, Camici G, Raugei G, Ramponi G. New perspectives in PDGF receptor downregulation: the main role of phosphotyrosine phosphatases. J Cell Sci. 2002;115:2219–2232. doi: 10.1242/jcs.115.10.2219. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Fiaschi T, Taddei ML, Talini D, Giannoni E, Raugei G, Ramponi G. Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J Biol Chem. 2001;276:33478–33487. doi: 10.1074/jbc.M102302200. [DOI] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001a;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001b;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, Bae YS, Lee BI, Rhee SG, Kang SW. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- Chua CC, Hamdy RC, Chua BH. Upregulation of vascular endothelial growth factor by H2O2 in rat heart endothelial cells. Free Radic Biol Med. 1998;25:891–897. doi: 10.1016/s0891-5849(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Clark S, Konstantopoulos N. Sulphydryl agents modulate insulin- and epidermal growth factor (EGF)-receptor kinase via reaction with intracellular receptor domains: differential effects on basal versus activated receptors. Biochem J 292. 1993;(Pt 1):217–223. doi: 10.1042/bj2920217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Origins of growth factors: NGF and EGF. J Biol Chem. 2008;283:33793–33797. doi: 10.1074/jbc.X800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Carpenter G, King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980;255:4834–4842. [PubMed] [Google Scholar]
- Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Cross JV, Templeton DJ. Oxidative stress inhibits MEKK1 by site-specific glutathionylation in the ATP-binding domain. Biochem J. 2004;381:675–683. doi: 10.1042/BJ20040591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnick JM, Dorsey JF, Standley T, Turkson J, Kraker AJ, Fry DW, Jove R, Wu J. Role of tyrosine kinase activity of epidermal growth factor receptor in the lysophosphatidic acid-stimulated mitogen-activated protein kinase pathway. J Biol Chem. 1998;273:14468–14475. doi: 10.1074/jbc.273.23.14468. [DOI] [PubMed] [Google Scholar]
- D'autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- Depuydt M, Messens J, Collet JF. How proteins form disulfide bonds. Antioxid Redox Signal. 2011;15:49–66. doi: 10.1089/ars.2010.3575. [DOI] [PubMed] [Google Scholar]
- Devary Y, Gottlieb RA, Lau LF, Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991;11:2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyulia GJ, Jr, Carcamo JM. EGF receptor-ligand interaction generates extracellular hydrogen peroxide that inhibits EGFR-associated protein tyrosine phosphatases. Biochem Biophys Res Commun. 2005;334:38–42. doi: 10.1016/j.bbrc.2005.06.056. [DOI] [PubMed] [Google Scholar]
- Deyulia GJ, Jr, Carcamo JM, Borquez-Ojeda O, Shelton CC, Golde DW. Hydrogen peroxide generated extracellularly by receptor-ligand interaction facilitates cell signaling. Proc Natl Acad Sci U S A. 2005;102:5044–5049. doi: 10.1073/pnas.0501154102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao Y, Liu W, Wong CC, Wang X, Lee K, Cheung PY, Pan L, Xu T, Han J, Yates JR, 3rd, Zhang M, Wu Z. Oxidation-induced intramolecular disulfide bond inactivates mitogen-activated protein kinase kinase 6 by inhibiting ATP binding. Proc Natl Acad Sci U S A. 2010;107:20974–20979. doi: 10.1073/pnas.1007225107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7:504–511. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfi F, Garcia-Guzman M, Ojaniemi M, Nakamura H, Matsuda M, Vuori K. The adaptor protein Crk connects multiple cellular stimuli to the JNK signaling pathway. Proc Natl Acad Sci U S A. 1998;95:15394–15399. doi: 10.1073/pnas.95.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Esposito F, Chirico G, Montesano Gesualdi N, Posadas I, Ammendola R, Russo T, Cirino G, Cimino F. Protein kinase B activation by reactive oxygen species is independent of tyrosine kinase receptor phosphorylation and requires SRC activity. J Biol Chem. 2003;278:20828–20834. doi: 10.1074/jbc.M211841200. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, Lecouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci Signal. 2012;5:pe10. doi: 10.1126/scisignal.2002943. [DOI] [PubMed] [Google Scholar]
- Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Tiganis T, Barford D, Tonks NK. Development of "substrate-trapping" mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 1997;94:1680–1685. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Gamou S, Shimizu N. Hydrogen peroxide preferentially enhances the tyrosine phosphorylation of epidermal growth factor receptor. FEBS Lett. 1995;357:161–164. doi: 10.1016/0014-5793(94)01335-x. [DOI] [PubMed] [Google Scholar]
- Gardner PR, Raineri I, Epstein LB, White CW. Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem. 1995;270:13399–13405. doi: 10.1074/jbc.270.22.13399. [DOI] [PubMed] [Google Scholar]
- Gianni D, Bohl B, Courtneidge SA, Bokoch GM. The involvement of the tyrosine kinase c-Src in the regulation of reactive oxygen species generation mediated by NADPH oxidase-1. Mol Biol Cell. 2008;19:2984–2994. doi: 10.1091/mbc.E08-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni E, Buricchi F, Grimaldi G, Parri M, Cialdai F, Taddei ML, Raugei G, Ramponi G, Chiarugi P. Redox regulation of anoikis: reactive oxygen species as essential mediators of cell survival. Cell Death Differ. 2008;15:867–878. doi: 10.1038/cdd.2008.3. [DOI] [PubMed] [Google Scholar]
- Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni E, Taddei ML, Chiarugi P. Src redox regulation: again in the front line. Free Radic Biol Med. 2010;49:516–527. doi: 10.1016/j.freeradbiomed.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- Go YM, Jones DP. Thiol/disulfide redox states in signaling and sensing. Crit Rev Biochem Mol Biol. 2013 doi: 10.3109/10409238.2013.764840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldkorn T, Balaban N, Matsukuma K, Chea V, Gould R, Last J, Chan C, Chavez C. EGF-Receptor phosphorylation and signaling are targeted by H2O2 redox stress. Am J Respir Cell Mol Biol. 1998;19:786–798. doi: 10.1165/ajrcmb.19.5.3249. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Pacheco FR, Deudero JJ, Castellanos MC, Castilla MA, Alvarez-Arroyo MV, Yague S, Caramelo C. Mechanisms of endothelial response to oxidative aggression: protective role of autologous VEGF and induction of VEGFR2 by H2O2. Am J Physiol Heart Circ Physiol. 2006;291:H1395–H1401. doi: 10.1152/ajpheart.01277.2005. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Mcgraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009a;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, Mcgraw TE. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc Natl Acad Sci U S A. 2009b;106:7004–7009. doi: 10.1073/pnas.0901933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- Grazia Lampugnani M, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Deshpande R, Paull TT. ATM activation in the presence of oxidative stress. Cell Cycle. 2010a;9:4805–4811. doi: 10.4161/cc.9.24.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010b;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- Haj FG, Markova B, Klaman LD, Bohmer FD, Neel BG. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J Biol Chem. 2003;278:739–744. doi: 10.1074/jbc.M210194200. [DOI] [PubMed] [Google Scholar]
- Haj FG, Verveer PJ, Squire A, Neel BG, Bastiaens PI. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science. 2002;295:1708–1711. doi: 10.1126/science.1067566. [DOI] [PubMed] [Google Scholar]
- Hall A, Karplus PA, Poole LB. Typical 2-Cys peroxiredoxins--structures, mechanisms and functions. FEBS J. 2009;276:2469–2477. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvey PJ, Watson WH, Hansen JM, Go YM, Samali A, Jones DP. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signalling. Biochem J. 2005;386:215–219. doi: 10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy DE, Lubos E, Yang Y, Galbraith JD, Kelly N, Zhang YY, Leopold JA, Loscalzo J. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J Biol Chem. 2009;284:11913–11921. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Andersen JN, Salmeen A, Barford D, Tonks NK. Conformation-sensing antibodies stabilize the oxidized form of PTP1B and inhibit its phosphatase activity. Cell. 2011;147:185–198. doi: 10.1016/j.cell.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeneman S, Haendeler J, Saito Y, Ishida M, Berk BC. Angiotensin II induces transactivation of two different populations of the platelet-derived growth factor beta receptor. Key role for the p66 adaptor protein Shc. J Biol Chem. 2000;275:15926–15932. doi: 10.1074/jbc.M909616199. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Hess DT, Stamler JS. Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem. 2012;287:4411–4418. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Hsu JL, Hsieh Y, Tu C, O'connor D, Nick HS, Silverman DN. Catalytic properties of human manganese superoxide dismutase. J Biol Chem. 1996;271:17687–17691. doi: 10.1074/jbc.271.30.17687. [DOI] [PubMed] [Google Scholar]
- Huang X, Begley M, Morgenstern KA, Gu Y, Rose P, Zhao H, Zhu X. Crystal structure of an inactive Akt2 kinase domain. Structure. 2003;11:21–30. doi: 10.1016/s0969-2126(02)00937-1. [DOI] [PubMed] [Google Scholar]
- Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu Rev Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, Ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Ushio-Fukai M, Zuo L, Tojo T, Dikalov S, Patrushev NA, Alexander RW. Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2005a;96:467–475. doi: 10.1161/01.RES.0000158286.51045.16. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, Ushio-Fukai M. IQGAP1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol. 2005b;25:2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- Jacob C, Battaglia E, Burkholz T, Peng D, Bagrel D, Montenarh M. Control of oxidative posttranslational cysteine modifications: from intricate chemistry to widespread biological and medical applications. Chem Res Toxicol. 2012;25:588–604. doi: 10.1021/tx200342b. [DOI] [PubMed] [Google Scholar]
- Jacob C, Winyard PG, editors. Redox Signaling and Regulation in Biology and Medicine. Weinheim: Wiley-VCH; 2009. [Google Scholar]
- Jaspers I, Zhang W, Fraser A, Samet JM, Reed W. Hydrogen peroxide has opposing effects on IKK activity and IkappaBalpha breakdown in airway epithelial cells. Am J Respir Cell Mol Biol. 2001;24:769–777. doi: 10.1165/ajrcmb.24.6.4344. [DOI] [PubMed] [Google Scholar]
- Joneson T, Bar-Sagi D. A Rac1 effector site controlling mitogenesis through superoxide production. J Biol Chem. 1998;273:17991–17994. doi: 10.1074/jbc.273.29.17991. [DOI] [PubMed] [Google Scholar]
- Juarez JC, Manuia M, Burnett ME, Betancourt O, Boivin B, Shaw DE, Tonks NK, Mazar AP, Donate F. Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc Natl Acad Sci U S A. 2008;105:7147–7152. doi: 10.1073/pnas.0709451105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama K, Ihara Y, Goto S, Urata Y, Toda G, Yano K, Kondo T. Overexpression of calreticulin modulates protein kinase B/Akt signaling to promote apoptosis during cardiac differentiation of cardiomyoblast H9c2 cells. J Biol Chem. 2002;277:19255–19264. doi: 10.1074/jbc.M112377200. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kamata H, Manabe T, Oka S, Kamata K, Hirata H. Hydrogen peroxide activates IkappaB kinases through phosphorylation of serine residues in the activation loops. FEBS Lett. 2002;519:231–237. doi: 10.1016/s0014-5793(02)02712-6. [DOI] [PubMed] [Google Scholar]
- Kanda M, Ihara Y, Murata H, Urata Y, Kono T, Yodoi J, Seto S, Yano K, Kondo T. Glutaredoxin modulates platelet-derived growth factor-dependent cell signaling by regulating the redox status of low molecular weight protein-tyrosine phosphatase. J Biol Chem. 2006;281:28518–28528. doi: 10.1074/jbc.M604359200. [DOI] [PubMed] [Google Scholar]
- Kang DH, Lee DJ, Lee KW, Park YS, Lee JY, Lee SH, Koh YJ, Koh GY, Choi C, Yu DY, Kim J, Kang SW. Peroxiredoxin II is an essential antioxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells. Mol Cell. 2011;44:545–558. doi: 10.1016/j.molcel.2011.08.040. [DOI] [PubMed] [Google Scholar]
- Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Takahashi T, Natoli G, Adams SR, Chen Y, Tsien RY, Karin M. Inhibition of NF-kappa B activation by arsenite through reaction with a critical cysteine in the activation loop of Ikappa B kinase. J Biol Chem. 2000;275:36062–36066. doi: 10.1074/jbc.M007204200. [DOI] [PubMed] [Google Scholar]
- Kaplan N, Urao N, Furuta E, Kim SJ, Razvi M, Nakamura Y, Mckinney RD, Poole LB, Fukai T, Ushio-Fukai M. Localized cysteine sulfenic acid formation by vascular endothelial growth factor: role in endothelial cell migration and angiogenesis. Free Radic Res. 2011;45:1124–1135. doi: 10.3109/10715762.2011.602073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble DJ, Sun G. Direct and specific inactivation of protein tyrosine kinases in the Src and FGFR families by reversible cysteine oxidation. Proc Natl Acad Sci U S A. 2009;106:5070–5075. doi: 10.1073/pnas.0806117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido Y, Nakae J, Accili D. Clinical review 125: The insulin receptor and its cellular targets. J Clin Endocrinol Metab. 2001;86:972–979. doi: 10.1210/jcem.86.3.7306. [DOI] [PubMed] [Google Scholar]
- Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B–deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomsiri C, Karplus PA, Poole LB. Cysteine-based redox switches in enzymes. Antioxid Redox Signal. 2011;14:1065–1077. doi: 10.1089/ars.2010.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- Korn SH, Wouters EF, Vos N, Janssen-Heininger YM. Cytokine-induced activation of nuclear factor-kappa B is inhibited by hydrogen peroxide through oxidative inactivation of IkappaB kinase. J Biol Chem. 2001;276:35693–35700. doi: 10.1074/jbc.M104321200. [DOI] [PubMed] [Google Scholar]
- Krasnowska EK, Pittaluga E, Brunati AM, Brunelli R, Costa G, De Spirito M, Serafino A, Ursini F, Parasassi T. N-acetyl-l-cysteine fosters inactivation and transfer to endolysosomes of c-Src. Free Radic Biol Med. 2008;45:1566–1572. doi: 10.1016/j.freeradbiomed.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Krieger-Brauer HI, Kather H. Human fat cells possess a plasma membrane-bound H2O2-generating system that is activated by insulin via a mechanism bypassing the receptor kinase. J Clin Invest. 1992;89:1006–1013. doi: 10.1172/JCI115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Brauer HI, Kather H. Antagonistic effects of different members of the fibroblast and platelet-derived growth factor families on adipose conversion and NADPH-dependent H2O2 generation in 3T3 L1-cells. Biochem J. 1995;307(Pt 2):549–556. doi: 10.1042/bj3070549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Brauer HI, Medda PK, Kather H. Insulin-induced activation of NADPH-dependent H2O2 generation in human adipocyte plasma membranes is mediated by Galphai2. J Biol Chem. 1997;272:10135–10143. doi: 10.1074/jbc.272.15.10135. [DOI] [PubMed] [Google Scholar]
- Kroviarski Y, Debbabi M, Bachoual R, Perianin A, Gougerot-Pocidalo MA, El-Benna J, Dang PM. Phosphorylation of NADPH oxidase activator 1 (NOXA1) on serine 282 by MAP kinases and on serine 172 by protein kinase C and protein kinase A prevents NOX1 hyperactivation. FASEB J. 2010;24:2077–2092. doi: 10.1096/fj.09-147629. [DOI] [PubMed] [Google Scholar]
- Kuroki M, Voest EE, Amano S, Beerepoot LV, Takashima S, Tolentino M, Kim RY, Rohan RM, Colby KA, Yeo KT, Adamis AP. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996;98:1667–1675. doi: 10.1172/JCI118962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwarcinski FE, Fox CC, Steffey ME, Soellner MB. Irreversible Inhibitors of c-Src Kinase That Target a Nonconserved Cysteine. ACS Chem Biol. 2012 doi: 10.1021/cb300337u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Beliveau R. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell. 2003;14:334–347. doi: 10.1091/mbc.E02-07-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Yuan Y, Isaac J, Babu CV, Meller J, Ho SM. Comprehensive identification and modified-site mapping of S-nitrosylated targets in prostate epithelial cells. PLoS One. 2010;5:e9075. doi: 10.1371/journal.pone.0009075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lander HM, Milbank AJ, Tauras JM, Hajjar DP, Hempstead BL, Schwartz GD, Kraemer RT, Mirza UA, Chait BT, Burk SC, Quilliam LA. Redox regulation of cell signalling. Nature. 1996;381:380–381. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- Lee JW, Kim JE, Park EJ, Kim JH, Lee CH, Lee SR, Kwon J. Two conserved cysteine residues are critical for the enzymic function of the human platelet-derived growth factor receptor-beta: evidence for different roles of Cys-822 and Cys-940 in the kinase activity. Biochem J. 2004;382:631–639. doi: 10.1042/BJ20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Leonard SE, Garcia FJ, Goodsell DS, Carroll KS. Redox-based probes for protein tyrosine phosphatases. Angew Chem Int Ed Engl. 2011;50:4423–4427. doi: 10.1002/anie.201007871. [DOI] [PubMed] [Google Scholar]
- Leonard SE, Reddie KG, Carroll KS. Mining the thiol proteome for sulfenic acid modifications reveals new targets for oxidation in cells. ACS Chem Biol. 2009;4:783–799. doi: 10.1021/cb900105q. [DOI] [PubMed] [Google Scholar]
- Leonberg AK, Chai YC. The functional role of cysteine residues for c-Abl kinase activity. Mol Cell Biochem. 2007;304:207–212. doi: 10.1007/s11010-007-9501-y. [DOI] [PubMed] [Google Scholar]
- Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal. 2006;8:1765–1774. doi: 10.1089/ars.2006.8.1765. [DOI] [PubMed] [Google Scholar]
- Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson NM, Seeliger MA, Cole PA, Kuriyan J. Structural basis for the recognition of c-Src by its inactivator Csk. Cell. 2008;134:124–134. doi: 10.1016/j.cell.2008.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang Y, Marden JJ, Banfi B, Engelhardt JF. Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury. Biochem J. 2008;411:531–541. doi: 10.1042/BJ20071534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Schlessinger J. Platelet-derived growth factor (PDGF)-induced disulfide-linked dimerization of PDGF receptor in living cells. Mol Cell Biol. 1991;11:3756–3761. doi: 10.1128/mcb.11.7.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, Rajala MW, Du X, Rollman B, Li W, Hawkins M, Barzilai N, Rhodes CJ, Fantus IG, Brownlee M, Scherer PE. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem. 2005;280:4617–4626. doi: 10.1074/jbc.M411863200. [DOI] [PubMed] [Google Scholar]
- Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sabnis Y, Zhao Z, Zhang T, Buhrlage SJ, Jones LH, Gray NS. Developing irreversible inhibitors of the protein kinase cysteinome. Chem Biol. 2013;20:146–159. doi: 10.1016/j.chembiol.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YY, Cruz TF. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J Biol Chem. 1995;270:11727–11730. doi: 10.1074/jbc.270.20.11727. [DOI] [PubMed] [Google Scholar]
- Lo YY, Wong JM, Cruz TF. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.26.15703. [DOI] [PubMed] [Google Scholar]
- Lohse DL, Denu JM, Santoro N, Dixon JE. Roles of aspartic acid-181 and serine-222 in intermediate formation and hydrolysis of the mammalian protein-tyrosine-phosphatase PTP1. Biochemistry. 1997;36:4568–4575. doi: 10.1021/bi963094r. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, Mcmurray JS, Fang X, Yung WK, Siminovitch KA, Mills GB. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J Biol Chem. 2003;278:40057–40066. doi: 10.1074/jbc.M303621200. [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58:621–631. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- Ma YC, Huang XY. Novel regulation and function of Src tyrosine kinase. Cell Mol Life Sci. 2002;59:456–462. doi: 10.1007/s00018-002-8438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias A, Azavedo E, Hagerstrom T, Klintenberg C, Perez R, Skoog L. Prognostic significance of the receptor for epidermal growth factor in human mammary carcinomas. Anticancer Res. 1987;7:459–464. [PubMed] [Google Scholar]
- Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadev K, Wu X, Zilbering A, Zhu L, Lawrence JT, Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem. 2001a;276:48662–48669. doi: 10.1074/jbc.M105061200. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001b;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- Maller C, Schroder E, Eaton P. Glyceraldehyde 3-phosphate dehydrogenase is unlikely to mediate hydrogen peroxide signaling: studies with a novel anti-dimedone sulfenic acid antibody. Antioxid Redox Signal. 2011;14:49–60. doi: 10.1089/ars.2010.3149. [DOI] [PubMed] [Google Scholar]
- Mamathambika BS, Bardwell JC. Disulfide-linked protein folding pathways. Annu Rev Cell Dev Biol. 2008;24:211–235. doi: 10.1146/annurev.cellbio.24.110707.175333. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signal. 2005;7:472–481. doi: 10.1089/ars.2005.7.472. [DOI] [PubMed] [Google Scholar]
- May JM, De Haen C. The insulin-like effect of hydrogen peroxide on pathways of lipid synthesis in rat adipocytes. J Biol Chem. 1979a;254:9017–9021. [PubMed] [Google Scholar]
- May JM, De Haen C. Insulin-stimulated intracellular hydrogen peroxide production in rat epididymal fat cells. J Biol Chem. 1979b;254:2214–2220. [PubMed] [Google Scholar]
- Mccord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Mccubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi MZ, Pandey NR, Pandey SK, Srivastava AK. H2O2-induced phosphorylation of ERK1/2 and PKB requires tyrosine kinase activity of insulin receptor and c-Src. Antioxid Redox Signal. 2005;7:1014–1020. doi: 10.1089/ars.2005.7.1014. [DOI] [PubMed] [Google Scholar]
- Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Miller EW, Dickinson BC, Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci U S A. 2010;107:15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JE, Whitford PC, Shaffer J, Onuchic JN, Adams JA, Jennings PA. A novel disulfide bond in the SH2 Domain of the C-terminal Src kinase controls catalytic activity. J Mol Biol. 2007;365:1460–1468. doi: 10.1016/j.jmb.2006.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee SP, Lane RH, Lynn WS. Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol. 1978;27:2589–2594. doi: 10.1016/0006-2952(78)90332-5. [DOI] [PubMed] [Google Scholar]
- Murata H, Ihara Y, Nakamura H, Yodoi J, Sumikawa K, Kondo T. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem. 2003;278:50226–50233. doi: 10.1074/jbc.M310171200. [DOI] [PubMed] [Google Scholar]
- Murillo-Carretero M, Torroglosa A, Castro C, Villalobo A, Estrada C. S-Nitrosylation of the epidermal growth factor receptor: a regulatory mechanism of receptor tyrosine kinase activity. Free Radic Biol Med. 2009;46:471–479. doi: 10.1016/j.freeradbiomed.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Nadeau PJ, Charette SJ, Landry J. REDOX reaction at ASK1-Cys250 is essential for activation of JNK and induction of apoptosis. Mol Biol Cell. 2009;20:3628–3637. doi: 10.1091/mbc.E09-03-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau PJ, Charette SJ, Toledano MB, Landry J. Disulfide Bond-mediated multimerization of Ask1 and its reduction by thioredoxin-1 regulate H(2)O(2)-induced c-Jun NH(2)-terminal kinase activation and apoptosis. Mol Biol Cell. 2007;18:3903–3913. doi: 10.1091/mbc.E07-05-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Patrushev N, Inomata H, Mehta D, Urao N, Kim HW, Razvi M, Kini V, Mahadev K, Goldstein BJ, Mckinney R, Fukai T, Ushio-Fukai M. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res. 2008;102:1182–1191. doi: 10.1161/CIRCRESAHA.107.167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima I, Kato M, Akhand AA, Suzuki H, Takeda K, Hossain K, Kawamoto Y. Redox-linked signal transduction pathways for protein tyrosine kinase activation. Antioxid Redox Signal. 2002;4:517–531. doi: 10.1089/15230860260196326. [DOI] [PubMed] [Google Scholar]
- Nakashima N, Sharma PM, Imamura T, Bookstein R, Olefsky JM. The tumor suppressor PTEN negatively regulates insulin signaling in 3T3-L1 adipocytes. J Biol Chem. 2000;275:12889–12895. doi: 10.1074/jbc.275.17.12889. [DOI] [PubMed] [Google Scholar]
- Nose K, Shibanuma M, Kikuchi K, Kageyama H, Sakiyama S, Kuroki T. Transcriptional activation of early-response genes by hydrogen peroxide in a mouse osteoblastic cell line. Eur J Biochem. 1991;201:99–106. doi: 10.1111/j.1432-1033.1991.tb16261.x. [DOI] [PubMed] [Google Scholar]
- Oh H, Jung HY, Kim J, Bae YS. Phosphorylation of serine282 in NADPH oxidase activator 1 by Erk desensitizes EGF-induced ROS generation. Biochem Biophys Res Commun. 2010;394:691–696. doi: 10.1016/j.bbrc.2010.03.053. [DOI] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Oshikawa J, Urao N, Kim HW, Kaplan N, Razvi M, Mckinney R, Poole LB, Fukai T, Ushio-Fukai M. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS One. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantano C, Reynaert NL, Van Der Vliet A, Janssen-Heininger YM. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal. 2006;8:1791–1806. doi: 10.1089/ars.2006.8.1791. [DOI] [PubMed] [Google Scholar]
- Park HS, Huh SH, Kim MS, Lee SH, Choi EJ. Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc Natl Acad Sci U S A. 2000;97:14382–14387. doi: 10.1073/pnas.97.26.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Lee SH, Park D, Lee JS, Ryu SH, Lee WJ, Rhee SG, Bae YS. Sequential activation of phosphatidylinositol 3-kinase, beta Pix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol. 2004a;24:4384–4394. doi: 10.1128/MCB.24.10.4384-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Yu JW, Cho JH, Kim MS, Huh SH, Ryoo K, Choi EJ. Inhibition of apoptosis signal-regulating kinase 1 by nitric oxide through a thiol redox mechanism. J Biol Chem. 2004b;279:7584–7590. doi: 10.1074/jbc.M304183200. [DOI] [PubMed] [Google Scholar]
- Parsonage D, Karplus PA, Poole LB. Substrate specificity and redox potential of AhpC, a bacterial peroxiredoxin. Proc Natl Acad Sci U S A. 2008;105:8209–8214. doi: 10.1073/pnas.0708308105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen CE, Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 2010;5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen CE, Truong TH, Garcia FJ, Homann A, Gupta V, Leonard SE, Carroll KS. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat Chem Biol. 2012;8:57–64. doi: 10.1038/nchembio.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penel S, Hughes E, Doig AJ. Side-chain structures in the first turn of the alpha-helix. J Mol Biol. 1999;287:127–143. doi: 10.1006/jmbi.1998.2549. [DOI] [PubMed] [Google Scholar]
- Peskin AV, Low FM, Paton LN, Maghzal GJ, Hampton MB, Winterbourn CC. The high reactivity of peroxiredoxin 2 with H(2)O(2) is not reflected in its reaction with other oxidants and thiol reagents. J Biol Chem. 2007;282:11885–11892. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- Poole LB, Klomsiri C, Knaggs SA, Furdui CM, Nelson KJ, Thomas MJ, Fetrow JS, Daniel LW, King SB. Fluorescent and affinity-based tools to detect cysteine sulfenic acid formation in proteins. Bioconjug Chem. 2007;18:2004–2017. doi: 10.1021/bc700257a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole LB, Zeng BB, Knaggs SA, Yakubu M, King SB. Synthesis of chemical probes to map sulfenic acid modifications on proteins. Bioconjug Chem. 2005;16:1624–1628. doi: 10.1021/bc050257s. [DOI] [PubMed] [Google Scholar]
- Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Senga T, Ito S, Hyodo T, Hasegawa H, Hamaguchi M. S-nitrosylation at cysteine 498 of c-Src tyrosine kinase regulates nitric oxide-mediated cell invasion. J Biol Chem. 2010;285:3806–3814. doi: 10.1074/jbc.M109.059782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddie KG, Carroll KS. Expanding the functional diversity of proteins through cysteine oxidation. Curr Opin Chem Biol. 2008;12:746–754. doi: 10.1016/j.cbpa.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Reddie KG, Seo YH, Muse Iii WB, Leonard SE, Carroll KS. A chemical approach for detecting sulfenic acid-modified proteins in living cells. Mol Biosyst. 2008;4:521–531. doi: 10.1039/b719986d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, Van Der Vliet A, Janssen-Heininger YM. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaert NL, Van Der Vliet A, Guala AS, Mcgovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci U S A. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AR, Tischer C, Verveer PJ, Rocks O, Bastiaens PI. EGFR activation coupled to inhibition of tyrosine phosphatases causes lateral signal propagation. Nat Cell Biol. 2003;5:447–453. doi: 10.1038/ncb981. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes. 1998;47:1562–1569. doi: 10.2337/diabetes.47.10.1562. [DOI] [PubMed] [Google Scholar]
- Saito S, Frank GD, Mifune M, Ohba M, Utsunomiya H, Motley ED, Inagami T, Eguchi S. Ligand-independent trans-activation of the platelet-derived growth factor receptor by reactive oxygen species requires protein kinase C-delta and c-Src. J Biol Chem. 2002;277:44695–44700. doi: 10.1074/jbc.M208332200. [DOI] [PubMed] [Google Scholar]
- Saito Y, Berk BC. Transactivation: a novel signaling pathway from angiotensin II to tyrosine kinase receptors. J Mol Cell Cardiol. 2001;33:3–7. doi: 10.1006/jmcc.2000.1272. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salsbury FR, Jr, Knutson ST, Poole LB, Fetrow JS. Functional site profiling and electrostatic analysis of cysteines modifiable to cysteine sulfenic acid. Protein Sci. 2008;17:299–312. doi: 10.1110/ps.073096508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- Schmid E, El Benna J, Galter D, Klein G, Droge W. Redox priming of the insulin receptor beta-chain associated with altered tyrosine kinase activity and insulin responsiveness in the absence of tyrosine autophosphorylation. FASEB J. 1998;12:863–870. doi: 10.1096/fasebj.12.10.863. [DOI] [PubMed] [Google Scholar]
- Schmitt TL, Hotz-Wagenblatt A, Klein H, Droge W. Interdependent regulation of insulin receptor kinase activity by ADP and hydrogen peroxide. J Biol Chem. 2005;280:3795–3801. doi: 10.1074/jbc.M410352200. [DOI] [PubMed] [Google Scholar]
- Seo JH, Ahn Y, Lee SR, Yeol Yeo C, Chung Hur K. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol Biol Cell. 2005;16:348–357. doi: 10.1091/mbc.E04-05-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo YH, Carroll KS. Profiling protein thiol oxidation in tumor cells using sulfenic acid-specific antibodies. Proc Natl Acad Sci U S A. 2009;106:16163–16168. doi: 10.1073/pnas.0903015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo YH, Carroll KS. Quantification of protein sulfenic acid modifications using isotope-coded dimedone and iododimedone. Angew Chem Int Ed Engl. 2011;50:1342–1345. doi: 10.1002/anie.201007175. [DOI] [PubMed] [Google Scholar]
- Shaw M, Cohen P, Alessi DR. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J. 1998;336(Pt 1):241–246. doi: 10.1042/bj3360241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Dobrusin EM, Fry DW, Haske T, Whitty A, Mcnamara DJ. Structure-based design of a potent, selective, and irreversible inhibitor of the catalytic domain of the erbB receptor subfamily of protein tyrosine kinases. J Med Chem. 1997;40:1130–1135. doi: 10.1021/jm960380s. [DOI] [PubMed] [Google Scholar]
- Singh J, Petter RC, Baillie TA, Whitty A. The resurgence of covalent drugs. Nat Rev Drug Discov. 2011;10:307–317. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- Singh J, Petter RC, Kluge AF. Targeted covalent drugs of the kinase family. Curr Opin Chem Biol. 2010;14:475–480. doi: 10.1016/j.cbpa.2010.06.168. [DOI] [PubMed] [Google Scholar]
- Sondhi D, Xu W, Songyang Z, Eck MJ, Cole PA. Peptide and protein phosphorylation by protein tyrosine kinase Csk: insights into specificity and mechanism. Biochemistry. 1998;37:165–172. doi: 10.1021/bi9722960. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, De La Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Stockler-Ipsiroglu S. Creatine deficiency syndromes: a new perspective on metabolic disorders and a diagnostic challenge. J Pediatr. 1997;131:510–511. [PubMed] [Google Scholar]
- Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, Holmes AB, Mccormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- Sun G, Kemble DJ. To C or not to C: direct and indirect redox regulation of Src protein tyrosine kinase. Cell Cycle. 2009;8:2353–2355. doi: 10.4161/cc.8.15.9225. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Sulciner DJ, Gutkind JS, Irani K, Goldschmidt-Clermont PJ, Finkel T. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem J. 1996;318(Pt 2):379–382. doi: 10.1042/bj3180379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer CH, Glynn SA, Cheng RY, Ridnour LA, Green JE, Ambs S, Wink DA. S-Nitrosylation of EGFR and Src Activates an Oncogenic Signaling Network in Human Basal-Like Breast Cancer. Mol Cancer Res. 2012;10:1203–1215. doi: 10.1158/1541-7786.MCR-12-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Hao Q, Rutherford SA, Low B, Zhao ZJ. Inactivation of SRC family tyrosine kinases by reactive oxygen species in vivo. J Biol Chem. 2005;280:23918–23925. doi: 10.1074/jbc.M503498200. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Knighton DR, Zheng J, Ten Eyck LE, Sowadski JM. Structural framework for the protein kinase family. Annu Rev Cell Biol. 1992;8:429–462. doi: 10.1146/annurev.cb.08.110192.002241. [DOI] [PubMed] [Google Scholar]
- Templeton DJ, Aye MS, Rady J, Xu F, Cross JV. Purification of reversibly oxidized proteins (PROP) reveals a redox switch controlling p38 MAP kinase activity. PLoS One. 2010;5:e15012. doi: 10.1371/journal.pone.0015012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal VJ, Day RM, Klinz SG, Bastien MC, Larios JM, Fanburg BL. Ras-dependent and -independent regulation of reactive oxygen species by mitogenic growth factors and TGF-beta1. FASEB J. 2000;14:1741–1748. doi: 10.1096/fj.99-0878com. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Truong TH, Carroll KS. Redox Regulation of Epidermal Growth Factor Receptor Signaling through Cysteine Oxidation. Biochemistry. 2012;51:9954–9965. doi: 10.1021/bi301441e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong TH, Garcia FJ, Seo YH, Carroll KS. Isotope-coded chemical reporter and acid-cleavable affinity reagents for monitoring protein sulfenic acids. Bioorg Med Chem Lett. 2011;21:5015–5020. doi: 10.1016/j.bmcl.2011.04.115. [DOI] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Ueki K, Yamamoto-Honda R, Kaburagi Y, Yamauchi T, Tobe K, Burgering BM, Coffer PJ, Komuro I, Akanuma Y, Yazaki Y, Kadowaki T. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2007;9:731–739. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:489–495. doi: 10.1161/01.atv.21.4.489. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- Van Der Kaay J, Beck M, Gray A, Downes CP. Distinct phosphatidylinositol 3-kinase lipid products accumulate upon oxidative and osmotic stress and lead to different cellular responses. J Biol Chem. 1999;274:35963–35968. doi: 10.1074/jbc.274.50.35963. [DOI] [PubMed] [Google Scholar]
- Veal EA, Findlay VJ, Day AM, Bozonet SM, Evans JM, Quinn J, Morgan BA. A 2-Cys peroxiredoxin regulates peroxide-induced oxidation and activation of a stress-activated MAP kinase. Mol Cell. 2004;15:129–139. doi: 10.1016/j.molcel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Rohle D, Versele M, Iwanami A, Kuga D, Oldrini B, Tanaka K, Dang J, Kubek S, Palaskas N, Hsueh T, Evans M, Mulholland D, Wolle D, Rajasekaran S, Rajasekaran A, Liau LM, Cloughesy TF, Dikic I, Brennan C, Wu H, Mischel PS, Perera T, Mellinghoff IK. The phosphatase and tensin homolog regulates epidermal growth factor receptor (EGFR) inhibitor response by targeting EGFR for degradation. Proc Natl Acad Sci U S A. 2010;107:6459–6464. doi: 10.1073/pnas.0911188107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333(Pt 2):291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mccullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- Wani R, Qian J, Yin L, Bechtold E, King SB, Poole LB, Paek E, Tsang AW, Furdui CM. Isoform-specific regulation of Akt by PDGF-induced reactive oxygen species. Proc Natl Acad Sci U S A. 2011;108:10550–10555. doi: 10.1073/pnas.1011665108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden PA, Pessin JE. Differential sensitivity of the insulin-receptor kinase to thiol and oxidizing agents in the absence and presence of insulin. Biochem J. 1987;245:325–331. doi: 10.1042/bj2450325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med. 1999;27:322–328. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Woltjer RL, Staros JV. Effects of sulfhydryl modification reagents on the kinase activity of the epidermal growth factor receptor. Biochemistry. 1997;36:9911–9916. doi: 10.1021/bi963007v. [DOI] [PubMed] [Google Scholar]
- Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Wortzel I, Seger R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes Cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- Yamaoka-Tojo M, Tojo T, Kim HW, Hilenski L, Patrushev NA, Zhang L, Fukai T, Ushio-Fukai M. IQGAP1 mediates VE-cadherin-based cell-cell contacts and VEGF signaling at adherence junctions linked to angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1991–1997. doi: 10.1161/01.ATV.0000231524.14873.e7. [DOI] [PubMed] [Google Scholar]
- Yamaoka-Tojo M, Ushio-Fukai M, Hilenski L, Dikalov SI, Chen YE, Tojo T, Fukai T, Fujimoto M, Patrushev NA, Wang N, Kontos CD, Bloom GS, Alexander RW. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species--dependent endothelial migration and proliferation. Circ Res. 2004;95:276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;280:7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- Yellaturu CR, Bhanoori M, Neeli I, Rao GN. N-Ethylmaleimide inhibits platelet-derived growth factor BB-stimulated Akt phosphorylation via activation of protein phosphatase 2A. J Biol Chem. 2002;277:40148–40155. doi: 10.1074/jbc.M206376200. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109–112. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizumi M, Abe J, Haendeler J, Huang Q, Berk BC. Src and Cas mediate JNK activation but not ERK1/2 and p38 kinases by reactive oxygen species. J Biol Chem. 2000;275:11706–11712. doi: 10.1074/jbc.275.16.11706. [DOI] [PubMed] [Google Scholar]
- Youngren JF. Regulation of insulin receptor function. Cell Mol Life Sci. 2007;64:873–891. doi: 10.1007/s00018-007-6359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Inesta-Vaquera F, Niepel M, Zhang J, Ficarro SB, Machleidt T, Xie T, Marto JA, Kim N, Sim T, Laughlin JD, Park H, Lograsso PV, Patricelli M, Nomanbhoy TK, Sorger PK, Alessi DR, Gray NS. Discovery of potent and selective covalent inhibitors of JNK. Chem Biol. 2012;19:140–154. doi: 10.1016/j.chembiol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Dixon JE. Active site labeling of the Yersinia protein tyrosine phosphatase: the determination of the pKa of the active site cysteine and the function of the conserved histidine 402. Biochemistry. 1993;32:9340–9345. doi: 10.1021/bi00087a012. [DOI] [PubMed] [Google Scholar]