Abstract
Kaposi’s sarcoma-associated herpesvirus (KSHV) belongs to the gamma herpesvirus family and is the causative agent of various lymphoproliferative diseases in humans. KSHV, like other herpesviruses, establishes life-long latent infection with the expression of a limited number of viral genes. Expression of these genes is tightly regulated by both the viral and cellular factors. Recent advancements in identifying the expression profiles of viral transcripts, using tilling arrays and next generation sequencing have identified additional coding and non-coding transcripts in the KSHV genome. Determining the functions of these transcripts will provide a better understanding of the mechanisms utilized by KSHV in altering cellular pathways involved in promoting cell growth and tumorigenesis. Replication of the viral genome is critical in maintaining the existing copies of the viral episomes during both latent and lytic phases of the viral life cycle. The replication of the viral episome is facilitated by viral components responsible for recruiting chromatin modifying enzymes and replication factors for altering the chromatin complexity and replication initiation functions, respectively. Importantly, chromatin modification of the viral genome plays a crucial role in determining whether the viral genome will persist as latent episome or undergo lytic reactivation. Additionally, chromatinization of the incoming virion DNA, which lacks chromatin structure, in the target cells during primary infection, helps in establishing latent infection. Here, we discuss the recent advancements on our understating of KSHV genome chromatinization and the consequences of chromatin modifications on viral life cycle.
Keywords: KSHV, oncogenic virus, LANA, RTA, epigenetics, viral chromatin, histone modifications, DNA methylation, PAN RNA, de novo infection
1. Introduction
Kaposi’s Sarcoma (KS), first described in 1872 by the Hungarian dermatologist Moritz Kaposi, is defined as a multiple idiopathic sarcoma of the skin. The causative agent of Kaposi’s sarcoma was identified as the human herpesvirus 8 (HHV8), or Kaposi’s sarcoma-associated herpesvirus (KSHV) from the tissue biopsies of AIDS-associated KS by Chang and Moore in 1994, using the representational difference analysis approach [1]. KSHV is an oncogenic γ-herpesvirus that establishes life-long persistent infection and causes tumors in immunosuppressed individuals; particularly, in transplant recipients and patients infected with HIV. Since its initial discovery in KS lesions, KSHV has been tightly linked with endothelial tumors, Kaposi’s sarcoma and two B-cell lymphoproliferative disorders, primary effusion lymphoma (PEL), also known as body cavity-based lymphoma [2], and a plasmablastic variant of multicentric Castleman’s disease (MCD) [3]. Additionally, KSHV has been linked to different lymphomas, including Burkitt’s lymphoma, Germinotropic Lymphoproliferative Disorder (GLD), multiple myeloma, angio-sarcomas, malignant skin tumors, angio-immunoblastic lymphoma and primary pulmonary hypertension [4,5,6]. There have also been reports of a new KSHV/HHV8-associated germinotropic lymphoproliferative disorder in HIV-seronegative individuals [7].
1.1. Clinical Diseases Associated with KSHV Infection
1.1.1. Kaposi’s Sarcoma (KS)
Kaposiʼs sarcoma is the first tumor to be associated with HIV infection and remains the most common cancer in Sub‐Saharan Africa and the second most common cancer in HIV‐infected patients [8]. KS is a highly vascular, non-classical tumor of endothelial lymphatic origin clinically characterized by dark red, brown or purple patches or plaques found cutaneously, mucosally or viscerally [9]. KS lesions are characterized by the presence of spindle shaped, poorly differentiated and highly proliferative cells and by infiltration of inflammatory cells and extensive neo-angiogenesis [10]. Several studies indicate that KS spindle cells are of endothelial lineage as they express the vascular endothelial cell markers CD31, CD34, CD36 [11]. More recently, KS spindle cells are thought to be of lymphatic endothelial cell (LECs) origin because they express LYVE-1, VEGFR-3 and podoplanin, markers of the lymphatic endothelium, making it difficult to identify the precursor cell type [12,13]. KSHV is required for the development of KS with greater than 95% of KS lesions harboring KSHV viral DNA in latent phase [14]. The role of KSHV in KS development is complex and involves both latent and lytic genes, many of which are pirated versions of cellular genes. The virus is found in all epidemiologic-clinical forms of the disease, including classic, African (endemic), HIV-associated (epidemic) and iatrogenic (transplant associated) KS (reviewed in [15]). Classic KS (indolent form) usually is present as lesions in the lower and upper extremities without the involvement of lymph nodes and internal organs [16]. Endemic KS, on the other hand, can be indolent or aggressive and transplant-related that represents a relatively indolent and chronic condition with a rapidly progressive course involving lymph nodes, mucosa and inner organs [17]. HIV-related KS is the most frequent and aggressive form that includes the lymph nodes and visceral spreading, though the role of HIV infection in KS development is not very well understood [18]. Since the introduction of highly active antiretroviral therapy (HAART), there has been a decline in KS incidence. However, Kaposiʼs sarcoma continues to be diagnosed in HIV-infected patients.
1.1.2. Primary Effusion Lymphoma (PEL)
PEL, also referred to as body cavity-based lymphoma (BCBL), is an aggressive form of non-Hodgkin’s B-cell lymphoma and is linked to KSHV infection and commonly found in late stage immunocompromised AIDS patients [19]. Evidence for the contributing role of KSHV in PEL development is attributed to detection of approximately 50–150 copies of KSHV latent genomes per cell with less than 1% of cells entering the lytic cycle [20,21]. Additionally, PEL cell survival has been shown to depend on the expression of CD138/syndecan-1, the KSHV latent genes (primarily ORF73, LANA) and viral microRNAs, highlighting the association between KSHV infection and PEL development [22]. Approximately 50% of PEL patients are KSHV-positive and found to be co-infected with Epstein-Barr virus (EBV), although cases of KSHV and EBV-negative PEL have also been reported [23,24]. Consistent growth of PEL cell lines in culture and easy induction of infectious KSHV virions release have made them a valuable in vitro infection model for understanding the molecular mechanisms of KSHV-related oncogenesis, in terms of cell transformation, signaling, cell growth, cell survival, angiogenesis and host-immune invasion, though how KSHV directly contributes to this B-cell malignancy is yet to be known [25].
1.1.3. Multicentric Castleman’s Disease
The plasmablastic variant of Multicentric Castleman’s disease (MCD) containing large plasmablastic cells is frequently linked to KSHV infection and is usually characterized by lymphadenopathy and immune deregulation [14,26]. MCD is a lymphoproliferative disorder that is often diagnosed in HIV-infected patients. KSHV co-infection is predominantly observed in the immunoblastic B cells within the mantle zone of germinal centers of lymph nodes in almost all HIV-seropositive MCD cases and in less than 40% HIV-seronegative MCD cases [27]. Interestingly, in contrast to KS and PEL, KSHV infection in MCD is mostly lytic and thought to be driven by an elevated levels of cytokines, interleukin-6 and 10 (IL-6, IL-10) and the vascular endothelial growth factor (VEGF) [28]. Additionally, so far the co-infection of KSHV with EBV has not been detected in MCD plasmablasts [29].
1.2. Epidemiology and KSHV Transmission
Epidemiology studies on KSHV have identified eight distinctive subtypes based on the sequence analysis of a well-conserved minor capsid gene, ORF26 [30]. Though large parts of the KSHV genome are conserved in these variants, several regions were shown to be highly variable, including the K1 and K15 gene regions. The sequence variability of the K1 gene has led to the identification of seven major viral subtypes, namely A, B, C, D, E, F and G [31,32]. General nucleotide differences between these subtypes are only 3%, however up to 60% sequence variation is observed in the two-hypervariable regions (VR1 and VR2) of the K1 gene [33]. Only two of the KSHV subtypes, A and C have diversity in the region surrounding K15 with variants designated as P (or prototype), M (or minority), N and Q. These variations are thought to arise from a recombination event with an unknown progenitor herpesvirus [34,35].
KSHV prevalence is found to correlate with geographic location. For instance, subgroup A1–4 and subtype C are predominant among individuals in North Europe, the United States and in some regions of Asia and Middle East. Subgroup B1–4 is located primarily in sub-Saharan Africa while D and E strains are found principally in Australia, the Pacific and Brazilian Amerindians [36,37,38]. Several serology studies have indicated that KSHV infection is widespread in sub-Saharan Africa and the Amazon basin where greater than 50% population is infected [39]. Intermediate levels of KSHV prevalence are seen in Mediterranean, Middle East and the Caribbean regions where 4%–45% population is tested as KSHV-seropositive. Lower levels of viral infection occur in Northern Europe and North America with KSHV seropositivity ranging from 3% to 10% [40,41].
Although, the transmission modes and the risk factors for KSHV infection are not well understood, the virus is reported to transmit through both sexual and non-sexual transmission routes [42,43]. In low prevalence areas, a direct link has been reported between the number of sexual partners and the risk of KSHV infection, indicating sexual transmission as the predominant transmission route [44]. Also, seroprevalence is lower for women and children than in men. The epidemiology differs significantly in the high prevalence areas with equal seroprevalence amongst children, adult men and women highlighting non-sexual routes of virus transmission. Studies in Italy, northern Sweden and Uganda suggest that the KSHV virions are mostly transmitted via saliva and sometimes through water and insect bite indicating KSHV transmission is dependent on a combination of both environmental and genetic factors [45,46,47].
1.3. KSHV Genome and Its Chromatinization
KSHV has a double stranded DNA (dsDNA) genome of 165–170 kb consisting of long unique coding region, which is ~140 kb in length and is flanked with highly GC-rich 801 bp long terminal repeat sequences (TRs) that encodes for nearly 86 viral open-reading frames (vORFs), 12 miRNAs and a number of non-coding RNAs (ncRNAs) and antisense RNAs [48,49]. The viral genes encoded by KSHV can be classified into three categories: (1) Herpesviruses-common genes; (2) KSHV-unique genes, and (3) Cellular-homologous genes, which may include categories (1) and (2) [50]. Studies on KSHV virion-associated proteins indicate that the virus particle is formed through several highly specific protein-protein interactions among capsid, tegument proteins and glycoproteins [51].
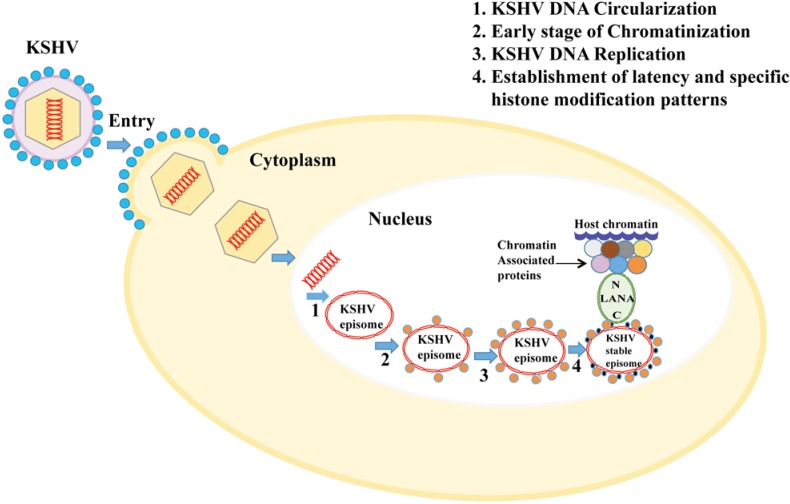
Each KSHV virion consists of a linear dsDNA duplex. The virion particle binds to the host cell surface receptor and penetrates into the host cell cytoplasm by a complex multistep process (reviewed in [52]). Virion capsids are then transported to the nuclear pore with concominant release of linear viral DNA. During de novo infection (i.e., entry of the virion in the target cells) and establishment of latency, the incoming linear dsDNA is circularized using the cellular enzymatic machinery to generate a closed-circular DNA, which is further maintained in a circular episomal form tightly packed as nucleosome (Figure 1). Encapsidated virion DNA is generally naïve and shows no chromatin structure, however, the resulting circular episome gets chromatinized due to its association with cellular histones in order to ensure: (1) protection of viral DNA ends to escape the host DNA damage response; (2) stable maintenance, replication and segregation of the viral genome to daughter cells during mitosis; (3) successful completion of viral life cycle; and (4) the regulation of viral gene expression (reviewed in [53]).
Figure 1.
The chromatinization and maintenance of the KSHV genome following de novo infection: After the KSHV virion attaches to the host cell, the viral capsid enters the cytoplasm, followed by the ejection of viral DNA into the nucleus. Subsequently, the linear viral genome is circularized into an extrachromosomal episome to avoid detection by the host DNA damage response; (2) Circularized genome is further chromatinized using cellular histones and histone modifying factors resulting in a stable episome; (3) Viral genome, which is being maintained as multicopy chromatinized episome then replicates along with the cellular genome; (4) For the stable persistence and segregation of KSHV epigenome during latency, KSHV latent protein LANA binds to the TR region of the viral genome and tethers the viral genome to the host chromosome through its amino-terminus via interaction with histones and cellular chromatin associated proteins.
Though the processes of viral genome circularization and chromatinization are poorly understood, there are key regulatory steps in the life cycle of many viruses, which are crucial for the establishment and persistence of the viral quiescence or latency [53,54]. The viral genome upon entry into the host cell nucleus must adopt a structure similar to the host genome and interact with cellular chromatin. The chromatinized viral DNA is influenced by the same epigenetic factors as the cellular DNA thereby generating the so-called “viral epigenome”, which significantly impacts both latency and lytic reactivation of the viral genome. Additionally, studies have indicated that viruses that enter and hide in the host nucleus have co-evolved with numerous cellular chromatin modulation mechanisms to ensure their survival and propagation [54].
Recent studies have uncovered a wealth of information regarding the dynamic aspects of chromatin structure and the mechanisms whereby chromatin modifications regulate the viral gene expression and viral-host chromatin interactions. Importantly, chromatin and epigenetic modulation of KSHV genome represents a novel antiviral target for blocking virus-mediated tumorigenesis. This compilation review summarizes some of the emerging concepts that will describe in the detail our current knowledge of chromatin assembly and remodeling factors, and epigenetic alterations, including DNA methylation, post-translational histone modifications, and nucleosome occupancy of KSHV genome, as the master controllers of KSHV’s biphasic life cycle, gene expression pattern and associated pathogenesis.
2. Chromatin Regulation and Gene Expression Pattern
The eukaryotic DNA is wrapped around histone proteins to form nucleosomes, the fundamental repeating units of chromatin. The nucleosome consists of an octameric histone core containing two H2A-H2B dimers and one H3-H4 tetramer with 147-bp segment of DNA wrapped in 1.65 turns and ~50 bp of free DNA separating the neighboring nucleosomes [55]. Together with linker histone H1, nucleosomes can compact to form condensed chromatin. The histone tails that protrudes out from the nucleosome undergoes different posttranslational modifications such as lysine acetylation, lysine and arginine methylation, serine, tyrosine and threonine phosphorylation and lysine ubiquitination to provide coding mechanism for protein recognition and signaling (reviewed in [56,57]).
Acetylation of histone tails, carried out by histone acetyltransferases (HAT’s) results in the relaxation of the chromatin structure through increased charge repulsions and is primarily associated with active gene expression [58]. Higher-order folding of the nucleosomal DNA can give rise to either less condensed, actively transcribed euchromatin or to highly condensed, transcriptionally silent heterochromatin and histone modifications can be found in both varieties of the chromatin. Euchromatin state is characterized by high levels of histone acetylation and methylation of lysine residues at 4, 36 and 79 of histone H3. Alternatively, the heterochromatin has low levels of acetylation and high levels of methylation of lysine residues at 9 and 27 of histone H3. Genes that are transcriptionally active possess high levels of H3K4me3, H3K27ac, H2BK5ac and H4K20me1 histone marks in their promoter region and H3K79me1 and H4K20me1 along the gene body [59]. These patterns of histone modifications that trigger activation or silencing of the gene can potentially be transmitted to the daughter cells and thus referred to as sequence-independent heritable changes of the genome (epigenome). Several well defined “epigenomic marks” on chromatin range from chemical modifications on DNA, histone proteins and 3D chromatin organization.
Structure of chromatin is highly variable and dynamic and their structures vary among different cell types. Analysis of the chromatin structure at the genomic level leads to characterization of the landscape of the epigenome that helps to define the gene expression pattern of any cell. Advances in next-generation sequencing technologies have enabled a high-resolution genome-wide investigation of the viral and cellular epigenomic landscape in various cell types. Powerful sequencing-based methods are used as excellent tools to interrogate the interplay between genomic locations of open chromatin regions, DNA-binding factors, nucleosomes and chromatin conformations at a single nucleotide resolution [60,61].
Chromatin Immunoprecipitation (ChIP) analysis of DNA-histone complexes is one of the efficient methods for the elucidation of chromatin structure and histone modifications. ChIP assays combined with DNA microarrays (ChIP-on-Chip) or high-throughput sequencing of purified DNA fragments (ChIP-seq) and chromatin conformation capture assays (3C) are primarily used to characterize endogenous chromatin structure together with mapping the genomic locations of DNA-associated proteins. In the context of the KSHV genome, prior studies conducted on KSHV infected cell lines at nucleotide resolution using ChIP-seq established the presence of histones, DNA methylation, DNA-protein interactions such as RNA polymerase II binding, LANA and CTCF-cohesin occupancy and nucleosome depletion on the latent KSHV episome [62,63,64]. More recently FAIRE (Formaldehyde-Assisted Isolation of Regulatory Elements) has been utilized as an alternative and robust approach than antibody-dependent ChIP-sequencing, to depict several regions of open chromatin in the latent KSHV genome. FAIRE, followed by next-generation sequencing (FAIRE-seq), together with previously identified nucleosome depletion and histone modification data, has led to the identification of the regulatory elements in KSHV genome, adding to our existing knowledge on the whole genome landscape of latent KSHV chromatin [65].
3. KSHV Latent Gene Expression Profile
During latent infection, the KSHV genome persists in a relatively quiescent state as multicopy chromatinized “minichromosomes or episomes” that transcribes and replicates simultaneously with the cellular chromosome thereby evades host’s immune surveillance [66]. Major latent genes are located between ORF 69 and K14 in the KSHV genome in the latency locus with their gene expression under the control of a few promoters, whereas the other viral genes are silenced during latency (reviewed in [67]). Viral latency is, however, reversible and can be periodically reactivated into lytic replication by specific environmental and physiological stimuli, thereby expressing all of the viral lytic genes in an ordered cascade resulting in the production of viral progeny. Both latent and lytic cycle replications are essential for the long-term persistence of the virus, and the gene products of these transcriptional programs contribute to KSHV-induced pathogenesis [49,67].
Majority of the KS biopsy samples and PEL cell lines exhibits latent gene expression profiles, thus provides a remarkable tumor model for better understanding of the mechanisms that regulate the expression of viral genes during latency. These latency models express a restricted yet variable pattern of viral latency-associated genes that modulate both viral and cellular gene expressions to establish and maintain latency. KSHV latent genes or transcripts include the latency-associated nuclear antigen LANA encoded by ORF73, viral cyclinD homologue, v-Cyclin encoded by ORF72, a viral homologue of a FLICE-inhibitory protein v-FLIP encoded by ORF71, Kaposin encoded by K12, and a cluster of 12 viral microRNAs [68,69,70,71,72], mRNA of several other viral genes including viral G protein-coupled receptor v-GPCR encoded by ORF74, K1, vIL-6 and ORF59 encoded by ORF59 have also been detected in most KS and PEL-tumor models [14]. Together, these latency-associated viral proteins are required for constant latent infection and survival of the infected cell.
For most of the KSHV positive PEL-derived cell lines studied so far the expression of the major viral latent proteins: LANA, v-Cyclin and v-FLIP is limited to a single multi-cistronic latent transcript [66]. LANA, functional orthologue of Epstein-Barr virus encoded EBNA1, binds to multiple sites on the KSHV genome, preferentially in the TR region, which helps in tethering of the viral genome to the host chromosome and maintenance of the replicated episomal copies after duplication of the tumor cells during latency [73]. The v-Cyclin and v-FLIP play key roles in host cell proliferation and survival [74,75,76]. The Kaposin transcript is expressed either from promoters downstream of LANA or the ones within the repeat region upstream of Kaposin [77]. Along with ORF74, K14 is expressed as a bi-cistronic transcript from the promoter downstream of LANA-v-Cyclin-v-FLIP RNA [78]. The ORF74-K14 transcript initiates from the 5' UTR of the LANA-v-Cyclin-v-FLIP and is expressed in latently infected cells along with LANA-v-Cyclin-v-FLIP gene expression [79]. More sensitive methods using micro-arrays and proteomics have identified several viral transcripts and peptide motifs that further provide valuable knowledge regarding viral latent gene expression [80].
Latency-associated Nuclear Antigen (LANA): LANA, a large nuclear protein of 220–230 kDa with DNA binding and chromatin binding domains in carboxy and amnio terminal domains, respectively is encoded by ORF73 and is critical for the establishment of latent infection [73,81,82]. LANA, a multifunctional protein can regulate transcription of cellular and viral genes through activation or repression of various cellular and viral promoters [83]. Additionally, LANA has also been shown to deregulate the expressions many oncogenes (by stabilizing) and tumor suppressors (by degrading) to induce tumor growth and these includes c-Myc, p53, hypoxia-inducible factor 1 (HIF-1), glycogen synthase kinase 3 (GSK3), von Hippel-Lindau protein (pVHL) and β-catenin [84,85,86,87,88,89]. LANA also controls lytic origin dependent DNA synthesis by interacting with origin binding protein (OBP), K-bZIP [90]. LANA promotes tumorigenesis and cell growth by inducing chromosome instability and upregulation of cellular IAP expression in KSHV-infected cells [91]. LANA plays a critical role in maintaining the KSHV genome in infected cells by ensuring faithful segregation of the newly synthesized viral genome into the divided cells by interacting with cellular proteins, Bub-1 and CENP-F [92]. Additionally, LANA inhibits interleukin-4 (IL-4)-mediated STAT6 phosphorylation, which helps in blocking apoptosis to maintain latency [93].
The role of LANA in the persistence of the KSHV genome was identified by using the 33-kb left-end of KSHV in a persistence assay in BJAB cells expressing LANA [73]. Additional studies identified the LANA binding sites, named LBS1 in the TR region of the KSHV genome [81,94,95]. Minimal LANA binding sequence in TR was identified by in vitro binding assays to be a 13 bp sequence within the TR [95]. Additional studies identified another LANA binding site (LBS2), which has lower affinity than LBS1 and is located right next to the first LANA binding site (LBS1) in the TRs [96]. The first LANA binding site lies between positions 571 and 589 of the 801bp long TR and is termed as LANA binding sequence 1 (LBS1) [96]. Binding of LANA to these two LANA Binding Sequences (LBS1/2) of the TR creates DNA bending and suppresses transcriptional activities when fused to a reporter plasmid [94,97].
The DNA binding domain of LANA is mapped to the C-terminal domain between amino acids 996 and 1139 [98]. Scanning of this region for identifying the exact residues in DNA binding by generating substitution mutants determined that amino acids between 1007 and 10021aa may be the DNA contact residues as the mutants of this region abolished DNA binding as well as replication and episome persistence abilities of LANA [98]. The amino terminal domain of LANA binds to the host chromatin through nucleosomal histones and tethers the viral genome bound to the C-terminus of LANA to the host chromosomes. Along with histones various other chromatin-associated proteins including RING3, which colocalizes to heterochromatin and heterochromatin proteins 1 (HP1) was also detected as LANA binding proteins by various biochemical assays [99]. A model for tethering of LANA to the host chromatin either through N-terminus binding proteins such as linker histone H1, core histones H2A/H2B, budding uninhibited by benzimidazole 1 (Bub1) and centromeric protein F (CENP-F) or C-terminus binding proteins including 43-kDa protein DEK, methyl CpG binding protein (MeCP2), bromodomain proteins Brd2/Brd4, nuclear mitotic apparatus protein CENP-F and Bub1 has been proposed [100].
A number of studies have demonstrated the role of LANA in modulating the activities of various cellular pathways by directly interacting with the major players of the pathways or complexing with additional proteins to favor cell growth (reviewed in [101]). LANA does not seem to possess enzymatic activities required for DNA replication but is critical for the replication of TR-containing plasmids [102]. LANA achieves this by recruiting host cellular replication factors to the TR in a cell cycle dependent manner (reviewed in [101,103]. Studies from others as well as our lab have shown the involvement of host cellular replication proteins in the replication of KSHV episomal DNA [104,105,106,107]. The replication of host genome occurs in a very precise manner in order to maintain genetic integrity, which is achieved by ensuring that no segment of DNA replicates more than once per cell cycle [108]. Replication process starts during the late G1 and early S phase by licensing of the replication origin sites by sequential loading of ORCs, cdc6, Cdt1, and the heterohexameric complex MCM2-7 (minichromosome maintenance proteins 2 to 7) to the origins to be used during the S phase [109]. Upon loading of these licensing factors, origins are licensed to form a pre-replicative complex (pre-RC) for replication initiation [107,110].
LANA has also been shown to upregulate the proteins important for the immortalization of infected cells, which includes modulation of hTERT and E2F1 responsive promoters [111,112]. LANA also promotes cell cycle progression through redistribution of the cellular pool of glycogen synthase kinase-3 beta (GSK-3b) to the nucleus, which leads to an accumulation of β-catenin in the cytoplasm. Increased levels of β-catenin in the cytoplasm are translocated to the nucleus, which in turns upregulates the β-catenin responsive, Lef-Tcf promoters. Increased levels of Lef-Tcf enhance the transcription of S-phase entry genes such as MYC, JUN and CCND1 (Cyclin D1), which drive cell cycle progression [85]. Additionally, LANA modulates apoptotic pathways as the LANA expressing cells are resistant to p53-dependent apoptosis but not the p53-independent apoptosis confirming specificity to p53 [112]. LANA also modulates the activity of another tumor suppressor, pRb, which was determined by the fact that expression of LANA overcame the flat-cell (growth arrested) phenotype in RB1 negative cells. Saos2 (deletion mutant of RB1) cells enter cell cycle arrest upon exogenous expression of pRb, but the interaction of LANA with pRb overcomes this phenotype, suggesting re-entry of these cells into the S-phase of cell cycle. LANA expressing cells inhibits pRb and p53 pathways that enable these cells to circumvent the G1/S checkpoint and the apoptotic pathway, respectively, which leads to the immortalization and tumorigenesis of LANA expressing cells [113].
v-Cyclin: It is encoded by ORF72 and is the homologue of cellular D-cyclin which acts like a constitutive activator of cellular cyclin-dependent kinase 6 (CDK6) to regulate cellular proliferation and viral replication [114]. The v-Cyclin-CDK6 complex can phosphorylate its cellular counterpart pRb protein, Histones H1, CDK inhibitor (cdki) and p27 (Kip1) [115]. The exact role of this viral protein in regulating KSHV life cycle is not fully understood but studies indicate that v-Cyclin-CDK6 complex mediated phosphorylation of nucleophosmin (NPM) facilitates NPM-LANA interaction and recruitment of HDAC1 to promote KSHV latency [116]. Additionally, KSHV v-Cyclin shares close functional relationship with murine gammaherpesvirus 68 (MHV68) v-Cyclin that is known to mediate efficient lytic reactivation from latency [117].
v-FLIP/K13: ORF71 encodes the KSHV homologue of cellular FLICE like inhibitory protein v-FLIP, also known as K13. KSHV v-FLIP is likely to contribute to latency by promoting cell survival [74,118]. The v-FLIP can block apoptosis by binding to the inhibitor of kB-kinase γ (IKK γ), leading to the activation of the NF-kB pathway [119]. NF-kB pathway activation by v-FLIP has been linked to KSHV lytic replication as the KSHV mutant deficient in v-FLIP inhibits ORF50/RTA lytic gene expression [120]. Additionally, v-FLIP regulates the activation of a key cellular survival pathway to promote cell proliferation and survival during latency (reviewed in [121]).
Kaposins: The Kaposin locus (K12) and surrounding direct repeat regions DR1 and DR2 encode for 3 proteins, namely, Kaposin A, B and C [122]. Kaposin A is a hydrophobic latent protein with transforming potential in Rat-1 fibroblasts whereas Kaposin B is a small soluble nuclear protein, which affects signaling by binding to a mitogen-activated protein (MAP) kinase 2 (MK2) [123]. Kaposin C is a trans-membrane protein, however the biological function of this protein is not yet known [122]. All of these proteins are shown to contribute to the inflammatory microenvironment of KS [122].
LANA2/vIRF-3: The KSHV genome encodes four viral homologues of cellular interferon regulatory protein (vIRFs) to counteract the interferon system (IFN) for evading the host’s immune response. One such protein predominantly expressed during KSHV latency is K10.5/LANA2 that is considered as a KSHV defense protein against IFN [124]. LANA2 is exclusively expressed in both PEL and MCD cell lines and has been linked to the disruption of cellular IRF-7, IRF-3 and IRF-5 mediated signaling and cellular PKR signaling [125]. LANA2 also inhibits p53 and NF-kB, which suggests its role in KSHV infection and pathogenesis [126].
Viral microRNAs: KSHV encodes 12 pre-miRNAs, of which 10 miRNA (miR-K1-9 and -K11) are encoded between the kaposin and ORF71/K13 region while the other two miRNAs, miR-K10 and -K12 are located in the coding and 3' untranslated region of K12, respectively [127,128,129,130,131]. All miRNAs are expressed in cells latently infected with KSHV and regulate latency while suppressing lytic reactivation of the virus [132,133,134,135]. Among these miRNAs, miR-K1 represses the expression of IκBα-an inhibitor of the pro-survival NF-κB pathway and inhibits the activation of lytic viral promoters. Since the NF-κB pathway is involved in cell growth and survival, miR-K1 might directly affect KSHV-mediated pathogenesis [136,137]. KSHV miR-K10 targets TNF-like weak inducer of apoptosis (TWEAKR) and is shown to protect cell from apoptosis and suppress pro-inflammatory responses, which might contribute to KSHV latent infection [136,138]. Several other miRNAs such as miR-K3, -K4, -K5 and -K9 target nuclear factor I/B, Rbl2 protein, Bcl-2 associated factor, BCLAF1 and activates the 3'UTR region of RTA protein respectively, to inhibit cell death and regulate lytic replication [139].
Chromatin Organization of KSHV Genome during Latent Infection
LANA-dependent DNA Replication and Epigenetic Modifications at the KSHV TR: KSHV LANA, a DNA binding nuclear protein acts as an initiator of DNA replication by specifically binding to a ~60 bp motif in the KSHV TRs [102]. A DNA-LANA protein complex is formed between LANA, trans-acting protein, and the LANA binding, cis-elements of KSHV TR [94,95,140]. Two or more copies of the TR are required for maintenance of the plasmid while a single copy of TR and the minimal replicator element is sufficient to initiate DNA replication [98,107,141,142]. Despite the absence of any enzymatic activity in LANA required for DNA replication, LANA supports DNA replication via its interaction with the host cellular replication factors including the origin recognition complexes (ORCs), Topoismerase IIβ, replication protein A (RPA) and proliferating cell nuclear antigen (PCNA) [104,106,110]. A recent study has demonstrated that LANA mediates KSHV DNA replication and virus persistence by recruiting replication factor C (RFC), the DNA polymerase clamp, proliferating cell nuclear antigen (PCNA) loader. LANA mutant with deleted RFC binding domain was found to have a negative impact on LANA-mediated DNA replication and episome persistence [110]. However, the detailed mechanism by which LANA interacts with ORCs and other replication proteins to initiate replication needs to be explored. Although, the LANA-dependent TR replication is believed to be the primary site of replication initiation but, KSHV DNA synthesis can also occur outside of the TR by the autonomously replicating element in the long unique region (LUR) of the viral genome.
During the G1/S transition phases of the cell cycle, chromatin structure at the TR (nucleosomes and two LANA binding sites) is modified to make the viral DNA more accessible to the host cell DNA replication machinery. Two independent new studies [143,144] have utilized chromatin immunoprecipitation data together with KSHV specific microarrays (CHIP-on-chip) to generate high-resolution profiles of the chromatin structure of the entire KSHV episomes in latently-infected cell lines, providing novel genome-wide sequencing of the epigenetic landscape of KSHV genome. Characteristic DNA methylation patterns were found throughout the KSHV genome, though KSHV is not subjected to extensive DNA methylation. Various peaks of CpG methylation were observed with remarkable similarity among different cell lines, ranging from PELs and infected SLKs. In all cases, regions immediately upstream of LANA and several locations including K9, ORF45/50, K7 and ORF8 were deprived of CpG methylation [143,144].
Studies so far clearly show that latent KSHV genome is extensively chromatinized with both the activating (H3ac and H3K4me3) and repressive (H3K9me3 and H3K27me3) histone marks among them the Latency-associated genes possess activating histone marks as well as colocalize with transcriptionally active RNA polymerase II (RNAPII). Immediate early (IE) and early genes (E) have bivalent chromatin marks (acH3, H3K4me3 and H3K27me3) or active chromatin marks (acH3/H3K4me3-rich) whereas all of the late genes have H3K9me3- and H3K27me3-marked heterochromatin. Also, two of the chromatin modifying enzymes: EZH2 (H3K27me3 histone methyltransferase) of the Polycomb Repressive Complex 2 (PRC2) family and JMJD2A (H3K9me3 histone demethylase) have been shown to associate with the latent KSHV genome [143,145,146]. EZH2, which ubiquitously binds on the viral genome represses the lytic gene expression program during latency and JMJD2 that binds to the acH3/H3K4me3 chromatin regions of KSHV genome, guards the methylation of H3K9. Overall, the whole KSHV genome epigenetic analysis indicates that KSHV genome chromatinization regulates the expression of latent and lytic genes in a systematic manner.
Additionally, the DNA replication is governed by various epigenetic modifications including DNA methylation, post-synthesis histone modification patterns and nucleosome occupancy [147]. DNA methylation slowly builds up over the entire KSHV genome following the de novo infection and typically represses the viral gene expression [148] (Figure 2). For KSHV, DNA methylation does not exist at transcriptionally active LANA promoters, however it is found at several transcriptionally silent regions [53]. Though the factors governing the locus-specific methylation of DNA are not clearly understood, it is predicted that some sites are methylated due to a lack of transcriptional activity whereas others lack DNA methylation due to the inhibitory effects by DNA-binding proteins. Chromatin immunoprecipitation assays suggest that KSHV TR is associated with stable positioned nucleosomes that are further subjected to cell cycle regulation and nucleosome remodeling [104].
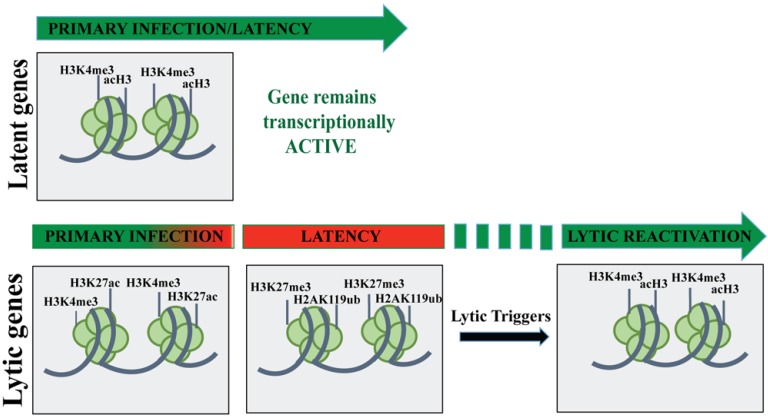
Figure 2.
The chromatin landscape of the KSHV genome during various phases of viral life cycle. Following primary infection, promoters of the key latent genes get epigenetically modified with activating histone marks (H3K4me3/acH3) resulting in the expression of those genes throughout the viral life cycle. In contrast, KSHV lytic gene promoters possess either H3K27Ac/H3K4Me3-rich, active chromatin or H3Ac/H3K4Me3/H3K27Me3-rich bivalent chromatin. During latency, the lytic gene promoters are epigenetically modified with H3K9Me3/H3K27Me3 marks to form a heterochromatin. However, upon reactivation these repressive histone marks are enzymatically removed and gets modified with the activating histone marks (H3K4Me3/H3Ac).
CTCF-cohesin Binding and Higher-order Chromatin Conformation: The association of architectural proteins, cellular chromatin boundary factor (CTCF) and cohesin plays an integral role in sister-chromatid cohesion and chromosome segregation during mitosis [149]. CTCF/cohesin binds to the first intron of the LANA-vCyclin-vFLIP multicistronic latent transcript in KSHV, nearly 15 kb from the TR [150]. Depletion of the CTCF, cohesin or the CTCF-cohesin binding sites in the KSHV bacmid leads to dysregulated gene expression [150,151]. This suggests that CTCF-cohesin complex provides a chromosome-organizing center that facilitates stable episome maintenance [150]. Genome-wide ChIP analysis combined with next generation sequencing of the KSHV genome indicate cohesin binding sites are colocalized with CTCF binding sites at several sites throughout the latent KSHV genomes [62,150]. These studies support that the DNA-binding factor CTCF/CTCF cluster is important for the control of latent transcription. A recent study further demonstrated that CTCF and cohesion binding on the viral genome dynamically changes during viral reactivation and depletion of these chromatin insulators positively regulate the transcription of lytic genes [152]. The molecular functions of these CTCF-cohesin clusters are beginning to understand and it is likely that during mitotic cell division these clusters correlate with chromosome for segregation and gene transcription [153].
Higher order DNA structure is likely to be a heritable epigenetic regulatory factor. Higher order DNA structures like those involving DNA looping have been identified for the CTCF binding sites in the KSHV LANA promoter region [154]. A loop was formed with the 3' end of the KSHV latency transcripts ending at the K12 gene suggesting that the entire latency transcription area is restrained to a DNA loop mediated in part by CTCF binding. Using the viral genome-wide chromatin conformation capture method (3C), chromatin conformation of the latent EBV and KSHV genomes has been depicted recently and CTCF/cohesin binding sites have been found to be physically linked to other regions of the viral genomes via extensive DNA-loop formation [150]. For KSHV, a long DNA loop is found between the LANA and RTA control regions and these loops are stabilized by cohesin subunits primarily SMC1, SMC3 and Rad21 and SA1/SA2 [62]. Depletion of cohesin subunits disrupts the DNA loop between the latency and lytic promoter regions and a robust reactivation of lytic cycle gene transcription, indicating an important role of chromatin organizing factors and chromosome conformation in maintenance of stable gene programs during KSHV latent infection [150].
Recently, MAPit (Methylation Accessibility Probing for individual templates) single-molecule footprinting assays were employed to characterize several chromatin states at selected loci within mammalian nuclei [155,156]. The chromatin structures of the latency promoter and immediate early lytic genes, RTA and K2 promoter were investigated. The results indicated a heterogeneous chromatin structure with both fully closed and open conformations being present at investigated promoter regions in the KSHV genome. In addition, the epigenetic drift, i.e., imperfect maintenance of the chromatin states was found to coordinate the latent and lytic gene control [156].
4. KSHV Lytic Gene Expression Profile
Within the predominantly latent population of KS spindle cells a few percent of the latent cells express markers of lytic replication [157,158]. During lytic DNA replication, a linear form of dsDNA molecule is generated and one copy of viral DNA is packaged per virion. For KSHV, viral lytic genes promote cell proliferation, survival and angiogenesis leading to onset and progression of KS lesions [15,159].
During lytic phase, viral gene expression is time-controlled and tightly regulated in order to allow a systematic synthesis of viral gene products. Lytic genes are widely distributed across the whole KSHV genome with their expression been controlled by several different promoters. Genes expressed during the lytic cycle can be grouped according to their timing and expression in response to the protein synthesis/DNA replication inhibitors as immediate early (IE), early (E) and late (L) genes [160,161,162,163]. IE genes include RTA/ORF50, ORF45 and K4.2, the primary genes expressed during lytic replication and encode regulatory proteins for viral replication. The latent to lytic switch of KSHV is regulated by the Replication and Transcription Activator, RTA, a 110 kDa protein, encoded by the ORF50 gene, which is capable of inducing the cascade of lytic gene expression including viral microphage inflammatory protein-I, viral interleukin 6 (vIL-6), ORF59, ORF65 and K8.1 and the production of DNase-resistant encapsidated viral DNA. Interestingly, the over-expression of RTA protein alone is necessary and sufficient to disrupt KSHV latency and initiate the lytic replication cascade [164,165,166,167]. Many early genes (E) have enzymatic functions (ORF59), regulation of gene expression (MTA protein), modulation of the immune system (MIR1/2) and selective accelerated turnover of host mRNA (ORF37) [168,169,170]. The expression of early genes (E) is controlled by the IE genes and include the polyadenylated nuclear RNA (PAN RNA), Kaposin, ORF57, k-bZIP (K8), K5, K9, K14, K15 ORF6, ORF21 and ORF74 [15], followed by expression of the late genes including major capsid protein (MCP) encoded by ORF25 and the small viral capsid (sVCA). Late genes are transcribed following DNA replication and they are the structural genes for virus assembly [160,161].
Recently, anem’s group performed a comprehensive genome analysis of transcriptional and translational profiles of the KSHV genome during the productive/lytic infection in epithelial iSLK-219 cell line using a combination of mRNA-sequencing (mRNA-seq) and ribosome profiling (Ribo-seq) [171]. This showed that ribosomes promptly bind to the transcripts of lytic genes during reactivation, suggesting that they are regulated at the transcriptional level. These modern approaches also revealed a wealth of additional information including occupancy of ribosomes on viral non coding RNA, numerous small open reading frames (ORFs), alternative splicing sites and alternative translation initiation sites to expand the coding potential of the viral genome. These new features of the KSHV genome led to a new annotation, which is termed, KSHV 2.0 [171].
4.1. Chromatin Organization of the KSHV Genome during Lytic Reactivation
The Lytic reactivation is regulated by alteration in the histone modifications of the viral genome including acetylation of core histone tails by histone acetyltransferases (HATs) and making the chromatin transcriptionally active. On the other hand, deacetylation of histone tails by histone acetyltransferases (HDACs) condenses the chromatin to make it transcriptionally silent [172]. During latency, HDACs attach to the RTA promoter resulting in hypoacetylation of histones and an inactive promoter [173,174]. However, the RTA promoter can be activated by physiological conditions, such as hypoxia, or in latently infected cell cultures by treatment with HDAC inhibitors, sodium butyrate (NaB) or the HAT inducer tetradecanoylphorbol acetate (TPA) leading to hyperacetylation of histones [175]. Additionally, DNA methylation plays an important role in controlling lytic reactivation as the inhibitor of enzyme DNA methyltransferases, 5-azacytidine (5-AzaC) triggers the KSHV lytic cycle [176]. The KSHV genome is subjected to methylation at CpG dinucleotides and shows CpG suppression at the RTA promoter during latency [144]. Together, these facts indicate that epigenetic modifications play an important role in the lytic reactivation process.
KSHV viral long non-coding PAN RNA and gene regulation: Among the early gene transcripts, PAN RNA is the most abundant lytic cycle transcript of KSHV [158,161]. New insights suggest an important role for long non-coding RNAs (lncRNAs) in the regulation of gene expression patterns via modulation of the lytic switch [177]. Long non-coding RNAs often referred to as “junk-regions” are RNAs which are typically longer than 200 bases that do not code for any protein [178]. KSHV expresses an unusual ncRNA called Polyadenylated nuclear non-coding RNA, abbreviated as PAN RNA in the nucleus during lytic induction that down regulates the expression of many immunomodulatory genes [176,179]. PAN RNA is transcribed by Pol II, is capped at its 5' end and ends with a 3' polyadenylate tail [180]. The expression of PAN RNA appears to be tightly regulated by RTA through a cis-acting RTA Response Element (RRE) present in the PAN RNA promoter region and by ORF57, which further stabilizes PAN RNA [181,182,183]. Although PAN RNA interacts with several viral and cellular encoded proteins to suppress gene expression, the exact role of PAN RNA in virus replication and KSHV growth is yet to be elucidated owing to the difficulty of generating a recombinant virus lacking the PAN RNA.
In order to elucidate the role of PAN RNA in the KSHV life cycle, recent studies on a recombinant BACmid with a deleted PAN RNA locus showed decreased RTA expression in the induced cells at both the early and late induction time points [177]. The RTA promoter is enriched in both activating (H3K4me3) and repressive (H3K27me3) histone marks. As a result, the chromatin of RTA promoter is often referred to as a bivalent promoter [144]. H3K27me3 is deposited by one of the Polycomb-group proteins, namely Polycomb Repressive Complex 2 (PRC2) that consists of subunit: EZH2, SUZ12, EED and the histone binding proteins RbAp48/46. Upon reactivation, chromatin remodeling proteins, JMJD3 and UTX H3K27me3 histone demethylases and H3K4me3 histone methyltransferase are recruited to the RTA promoter via KSHV-encoded Polyadenylated nuclear non-coding PAN RNA that disrupts polycomb mediated chromatin repression [177]. RTA binds to its own promoter through the cellular transcription factor CBF1 and recruits histone acetyltransferases (CBP/p300) and chromatin remodeling factors (SWI/SNF2) to modify the viral chromatin structure to a transcriptionally active state allowing a complete cycle of viral reactivation (Figure 3). RNA ChIP assays show that PAN RNA interacts with histone H3K27 demethylases JMJD3, UTX and methylases MLL2 to reverse the Polycomb-mediated repression of viral IE RTA transcripts through an interaction with the viral genome [177]. These studies further establish that PAN RNA is a multifunctional regulatory transcript that controls KSHV gene expression by mediating chromatin-modulations of the KSHV repressed genome.
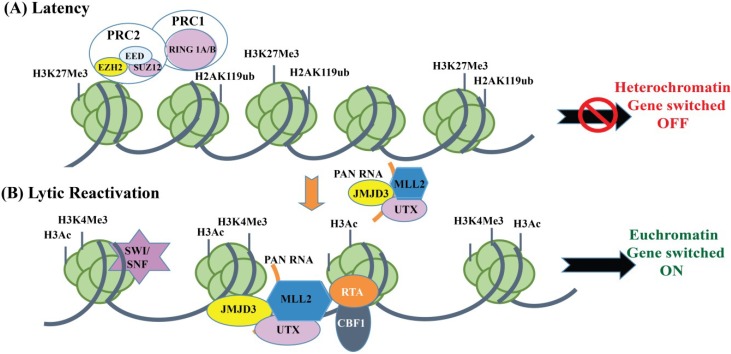
Figure 3.
Polycomb repressive complexes (PRC1 and PRC2) and Polyadenylated RNA-mediated control of KSHV RTA promoter during latency and lytic reactivation. (A) During latency, the histone methyltransferase EZH2 subunit of PRC2 and RING 1A/B subunit of PRC1 bind to the KSHV genome and colocalizes with corresponding repressive histone marks H3K27me3 and H2AK119ub, respectively, leading to silencing of the target genes; (B) Lytic reactivation, triggered by the dissociation of PRC2 and PRC1 from the viral genome or inhibition of EZH2 by chemical compounds, leads to the enrichment of activating histone marks on the viral genome and the activation of gene transcription. PAN RNA, which is highly expressed during lytic reactivation, recruits several cellular and viral chromatin factors (MLL2, JMJD3 and UTX) to the RTA promoter and triggers the expression of immediate early, RTA protein.
4.2. Chromatin Organization of KSHV Genome during de Novo Infection
PEL cell lines are latently infected by KSHV and can be induced to trigger lytic cycle and produce infectious virions. However, PEL cells being lymphoid in nature do not support de novo KSHV infection, viral replication or serial propagation. Similarly, many other standard established cell lines such as 293 cells have been reported to support a very low level of KSHV infection with limited release of infectious virus [184,185]. These limitations have impacted the development of systems for the genetic analysis of KSHV and in turn a deeper understanding of early events of KSHV infection.
The process of KSHV de novo or primary infection involves attachment of the viral envelope proteins to host cell receptors and entry in the target cells by a multistep complex process [186]. Several transmembrane glycoproteins, encoded by KSHV have been found to be involved in the attachment and entry of KSHV in the target cells including gB (ORF8), gH (ORF22), gL (ORF 47), gM (ORF39) and gN (ORF53) [187,188,189]. Following the entry of viral genome into the nucleus, the viral genome undergoes extensive modification including circularization and chromatinization to escape the host cell defenses [53,190]. KSHV encoded ORF75 protein, which belongs a formylglycineamide ribotide amidotransferase (FGARAT), blocks cellular defenses by antagonizing the ND10 (nuclear domain 10) components [190]. Since chromatin association of the genome restricts the access of transcription factors to the viral promoter regions, modifications of the viral chromatin play an integral role in controlling viral gene expression [191].
Two independent studies have used ChIP-on-chip assays to provide the first unbiased and genome-wide views of the latent KSHV chromatin in infected BCBL-1 and SLK cells indicating a uniform distribution of total histone H3 and genomic localization of its modified forms, i.e., activated H3K4me3 and acetylated H3K9 (acH3) and repressive histone marks [143,144]. Latent genes, IE and E lytic genes are found to be rich in H3K4me3/H3K9 (acH3) during latency and reactivation whereas the genomic regions of KSHV that encode for many late lytic genes display high levels of H3K9me3 /H3K27me3 during latency and early lytic reactivation [67].
In order to provide an overall description of the pre-latency phase of KSHV infection, a comprehensive epigenetic study was performed by Dr. Jae Jung’s group on SLK cells using ChIP-on-chip and FAIRE analysis [192]. During the onset of infection, the KSHV epigenome develops a transcriptionally active chromatin structure (euchromatin) with a high level of activating histone marks H3K4me3 and H3K27ac, accompanied by the temporary induction of a limited number of lytic genes, which further increase the activating histone marks on the viral genome. Between 24 and 72 h post-infection, levels of activating histone marks decrease due to the Polycomb group protein (PcG protein)-mediated increase in the amount of repressive histone marks H3K27me3 and H2AK119ub, thereby inhibiting lytic gene expression. PcG proteins are cellular transcription silencing proteins that form enzymatic complexes with cellular Polycomb Repressive Complex 1 (PRC1) and PRC2 [193]. De novo acquisition of H3K27me3 marks is catalyzed by EZH2, which together with SUZ12 and EED form the subunits of the PRC2 complex. Both PRC1 and PRC2 are recruited to the KSHV genome leading to the temporally ordered biphasic euchromatin to heterochromatin transition in SLK and TIME cells, following de novo infection [192]. In contrast, KSHV is proposed to exist in a transcriptionally active euchromatin form in oral epithelial cells, resulting in efficient and robust lytic gene expression. Thus, the differential epigenetic modification of the KSHV genome in distinct cell types is a potential determining factor for latent infection versus lytic replication [192].
During the past years, components of distinct nuclear compartments called Promyelocytic leukemia nuclear bodies (PML-NB) have been found to regulate viral chromatin and viral gene expression [194]. PML-NB, also called nuclear domain 10 (ND10s) are nuclear multi-protein complexes that are 0.2–1.0 µm in size and include several subunits like Daxx (Death domain associated protein, Sp100 (speckled protein of 100 kDa), SUMO (small ubiquitin-related modifier) and 53 kDa protein associated with the nuclear matrix [195,196]. ND10s are the mediators of the innate antiviral defense mechanism and many viruses have developed strategies to counteract repressive properties of ND10s [196,197]. A systematic study by Grundhoff’s group reported the role of ND10s and its core components on the establishment of KSHV latency during the early infection phase in de novo infected SLK, established from tumor biopsy of oral mucosa, cells [148]. The KSHV episome/LANA in iSLK cells is found not to directly or transiently interact with ND10s during the establishment of latency (between 24 and 48 h post infection) i.e., the time period when H3K27me3 marks accumulate on the KSHV episome. However, KSHV infection is reported to influence relocalization of ND10s components especially Sp100 protein, which is efficiently and permanently relocalized from nucleoplasmic and chromatin-associated fractions into the insoluble matrix by LANA, which induces SUMOylation of Sp100. Depletion of ND10s core components, Sp100, PML or Daxx did not interfere with latency establishment, though depletion of Sp100 accelerates the occupancy of the repressive histone mark H3K27me3 on viral episomes indicating that Sp100 acts as a negative regulator of PRC2 recruitment onto the KSHV genome [148].
5. Conclusions
KSHV is a complex and sophisticated oncogenic virus that developed numerous regulatory mechanisms to modulate the host-cell proliferation, apoptosis and host immune evasion, enabling the prolonged survival of the infected cell with the following latent infection and lytic reactivation. Despite the enormous wealth of information available about the mechanisms of how gammaherpesviruses persist in tumor cells, we are far from fully understanding the mechanism of latency establishment by KSHV, and the key cellular and viral factors that are responsible for restricted lytic gene expression during primary infection. Undoubtedly, the chromatin remodeling and epigenetic modifications of both viral and host genomes play crucial roles in determining the expression pattern of genes, that will lead to abortive or persistent infection. These epigenetic modifications include DNA methylation, histones post-translational modifications and higher-order chromosome conformations, including long intervening DNA loops. More importantly, prior to the chromatinization of incoming viral genomes, function of the cellular chromatin modifying enzymes is deregulated by a combination of viral proteins in order to re-program the cellular gene expression profiles and generate an altered chromatin landscape of KSHV genome favoring latency establishment [198,199]. Although the advances in technological development has enabled extremely high-resolution whole-epigenome analysis of KSHV on a large scale and provided us with a better picture of the chromatin regulation of the KSHV latent and lytic genomes, much remains to be discovered for understanding the complex orchestration of various epigenetic players that regulate KSHV chromatin remodeling by site-specific recruitment of histone-modifying machinery during various stages of viral infection. Therefore, further investigations of significant cellular and viral factors along with most important epigenetic regulatory factors, including histone-modifying enzymes, HDAC inhibitors and demethylating agents that alter the chromatin status of KSHV towards the formation of unique latent epigenomes need to be performed in order (1) to generate a detailed and complete epigenomic map for KSHV; and (2) to provide novel therapeutic strategies that can be exploited for controlling KSHV infection and KSHV-driven carcinogenesis.
Acknowledgments
We sincerely apologize to the authors whose important work could not be cited here owing to space limitations. This work was supported by the public health grants from NIH (CA174459 and AI105000) to Subhash C. Verma and CA137894, CA171979, CA174439, CA177423, P30-DK-050306 and P01-CA-174439 to Erle S. Robertson.
Author Contributions
This review was conceptualized and written through the combined efforts of all the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chang Y., Cesarman E., Pessin M.S., Lee F., Culpepper J., Knowles D.M., Moore P.S. Identification of herpesvirus-like DNA sequences in aids-associated Kaposiʼs sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Cesarman E., Chang Y., Moore P.S., Said J.W., Knowles D.M. Kaposiʼs sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 3.Soulier J., Grollet L., Oksenhendler E., Cacoub P., Cazals-Hatem D., Babinet P., dʼAgay M.F., Clauvel J.P., Raphael M., Degos L., et al. Kaposiʼs sarcoma-associated herpesvirus-like DNA sequences in multicentric castlemanʼs disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 4.Deloose S.T., Smit L.A., Pals F.T., Kersten M.J., van Noesel C.J., Pals S.T. High incidence of Kaposi sarcoma-associated herpesvirus infection in HIV-related solid immunoblastic/plasmablastic diffuse large B-cell lymphoma. Leukemia. 2005;19:851–855. doi: 10.1038/sj.leu.2403709. [DOI] [PubMed] [Google Scholar]
- 5.Cool C.D., Rai P.R., Yeager M.E., Hernandez-Saavedra D., Serls A.E., Bull T.M., Geraci M.W., Brown K.K., Routes J.M., Tuder R.M., et al. Expression of human herpesvirus 8 in primary pulmonary hypertension. N. Engl. J. Med. 2003;349:1113–1122. doi: 10.1056/NEJMoa035115. [DOI] [PubMed] [Google Scholar]
- 6.Carbone A., Gloghini A., Vaccher E., Cerri M., Gaidano G., Dalla-Favera R., Tirelli U. Kaposiʼs sarcoma-associated herpesvirus/human herpesvirus type 8-positive solid lymphomas: A tissue-based variant of primary effusion lymphoma. J. Mol. Diagn. 2005;7:17–27. doi: 10.1016/S1525-1578(10)60004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du M.Q., Diss T.C., Liu H., Ye H., Hamoudi R.A., Cabecadas J., Dong H.Y., Harris N.L., Chan J.K., Rees J.W., et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood. 2002;100:3415–3418. doi: 10.1182/blood-2002-02-0487. [DOI] [PubMed] [Google Scholar]
- 8.Gbabe O.F., Okwundu C.I., Dedicoat M., Freeman E.E. Treatment of severe or progressive Kaposiʼs sarcoma in HIV-infected adults. Cochrane Database Syst. Rev. 2014;8:CD003256. doi: 10.1002/14651858.CD003256.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Cornali E., Zietz C., Benelli R., Weninger W., Masiello L., Breier G., Tschachler E., Albini A., Sturzl M. Vascular endothelial growth factor regulates angiogenesis and vascular permeability in Kaposiʼs sarcoma. Am. J. Pathol. 1996;149:1851–1869. [PMC free article] [PubMed] [Google Scholar]
- 10.Gessain A., Duprez R. Spindle cells and their role in Kaposiʼs sarcoma. Int. J. Biochem. Cell Biol. 2005;37:2457–2465. doi: 10.1016/j.biocel.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Gasperini P., Espigol-Frigole G., McCormick P.J., Salvucci O., Maric D., Uldrick T.S., Polizzotto M.N., Yarchoan R., Tosato G. Kaposi sarcoma herpesvirus promotes endothelial-to-mesenchymal transition through notch-dependent signaling. Cancer Res. 2012;72:1157–1169. doi: 10.1158/0008-5472.CAN-11-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H.W., Trotter M.W., Lagos D., Bourboulia D., Henderson S., Makinen T., Elliman S., Flanagan A.M., Alitalo K., Boshoff C. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 2004;36:687–693. doi: 10.1038/ng1384. [DOI] [PubMed] [Google Scholar]
- 13.Hong Y.K., Foreman K., Shin J.W., Hirakawa S., Curry C.L., Sage D.R., Libermann T., Dezube B.J., Fingeroth J.D., Detmar M. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 2004;36:683–685. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- 14.Wen K.W., Damania B. Kaposi sarcoma-associated herpesvirus (KSHV): Molecular biology and oncogenesis. Cancer Lett. 2010;289:140–150. doi: 10.1016/j.canlet.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dourmishev L.A., Dourmishev A.L., Palmeri D., Schwartz R.A., Lukac D.M. Molecular genetics of Kaposiʼs sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol Mol. Biol. Rev. 2003;67:175–212. doi: 10.1128/MMBR.67.2.175-212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantanowitz L., Dezube B.J. Kaposi sarcoma in unusual locations. BMC cancer. 2008;8:190. doi: 10.1186/1471-2407-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeVita V.T., Lawrence T.S., Rosenberg S.A. Cancer: Principles and Practice of Oncology. 8th ed. Volume 2. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2008. p. 3151. [Google Scholar]
- 18.Wool G.M. Aids-related malignancies. Oncologist. 1998;3:279–283. [PubMed] [Google Scholar]
- 19.Ablashi D.V., Chatlynne L.G., Whitman J.E., Jr., Cesarman E. Spectrum of Kaposiʼs sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin. Microbiol. Rev. 2002;15:439–464. doi: 10.1128/CMR.15.3.439-464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nador R.G., Cesarman E., Chadburn A., Dawson D.B., Ansari M.Q., Sald J., Knowles D.M. Primary effusion lymphoma: A distinct clinicopathologic entity associated with the Kaposiʼs sarcoma-associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 21.Edelman D.C. Human herpesvirus 8—A novel human pathogen. Virol. J. 2005;2:78. doi: 10.1186/1743-422X-2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haecker I., Gay L.A., Yang Y., Hu J., Morse A.M., McIntyre L.M., Renne R. Ago HITS-clip expands understanding of Kaposiʼs sarcoma-associated herpesvirus miRNA function in primary effusion lymphomas. PLOS Pathog. 2012;8:e1002884. doi: 10.1371/journal.ppat.1002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan W., Bubman D., Chadburn A., Harrington W.J., Jr., Cesarman E., Knowles D.M. Distinct subsets of primary effusion lymphoma can be identified based on their cellular gene expression profile and viral association. J. Virol. 2005;79:1244–1251. doi: 10.1128/JVI.79.2.1244-1251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueda K., Ohsaki E., Nakano K., Zheng X. Characterization of Kaposiʼs sarcoma-associated herpesvirus-related lymphomas by DNA microarray analysis. Leuk. Res. Treat. 2011;2011:726964. doi: 10.4061/2011/726964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dittmer D.P., Damania B. Kaposi sarcoma associated herpesvirus pathogenesis (KSHV)—An update. Curr. Opin. Virol. 2013;3:238–244. doi: 10.1016/j.coviro.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curioni S., DʼAmico M., Quartagno R., Martino S., DellʼAntonio G., Cusi D. Castlemanʼs disease with nephrotic syndrome, amyloidosis and autoimmune manifestations. Nephrol. Dial. Transplant. 2001;16:1475–1478. doi: 10.1093/ndt/16.7.1475. [DOI] [PubMed] [Google Scholar]
- 27.Dupin N., Fisher C., Kellam P., Ariad S., Tulliez M., Franck N., van Marck E., Salmon D., Gorin I., Escande J.P., et al. Distribution of human herpesvirus-8 latently infected cells in kaposiʼs sarcoma, multicentric castlemanʼs disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA. 1999;96:4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbone A., Cesarman E., Spina M., Gloghini A., Schulz T.F. HIV-associated lymphomas and gamma-herpesviruses. Blood. 2009;113:1213–1224. doi: 10.1182/blood-2008-09-180315. [DOI] [PubMed] [Google Scholar]
- 29.Nishi J., Arimura K., Utsunomiya A., Yonezawa S., Kawakami K., Maeno N., Ijichi O., Ikarimoto N., Nakata M., Kitajima I., et al. Expression of vascular endothelial growth factor in sera and lymph nodes of the plasma cell type of castlemanʼs disease. Br. J. Haematol. 1999;104:482–485. doi: 10.1046/j.1365-2141.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- 30.Zong J.C., Kajumbula H., Boto W., Hayward G.S. Evaluation of global clustering patterns and strain variation over an extended ORF26 gene locus from Kaposiʼs sarcoma herpesvirus. J. Clin. Virol. 2007;40:19–25. doi: 10.1016/j.jcv.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayward G.S. KSHV strains: The origins and global spread of the virus. Semin. Cancer Biol. 1999;9:187–199. doi: 10.1006/scbi.1998.0116. [DOI] [PubMed] [Google Scholar]
- 32.Hayward G.S., Zong J.C. Modern evolutionary history of the human KSHV genome. Curr. Top. Microbiol. Immunol. 2007;312:1–42. doi: 10.1007/978-3-540-34344-8_1. [DOI] [PubMed] [Google Scholar]
- 33.Stebbing J., Bourboulia D., Johnson M., Henderson S., Williams I., Wilder N., Tyrer M., Youle M., Imami N., Kobu T., et al. Kaposiʼs sarcoma-associated herpesvirus cytotoxic T lymphocytes recognize and target darwinian positively selected autologous K1 epitopes. J. Virol. 2003;77:4306–4314. doi: 10.1128/JVI.77.7.4306-4314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole L.J., Zong J.C., Ciufo D.M., Alcendor D.J., Cannon J.S., Ambinder R., Orenstein J.M., Reitz M.S., Hayward G.S. Comparison of genetic variability at multiple loci across the genomes of the major subtypes of Kaposiʼs sarcoma-associated herpesvirus reveals evidence for recombination and for two distinct types of open reading frame K15 alleles at the right-hand end. J. Virol. 1999;73:6646–6660. doi: 10.1128/jvi.73.8.6646-6660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zong J., Ciufo D.M., Viscidi R., Alagiozoglou L., Tyring S., Rady P., Orenstein J., Boto W., Kalumbuja H., Romano N., et al. Genotypic analysis at multiple loci across Kaposiʼs sarcoma herpesvirus (KSHV) DNA molecules: Clustering patterns, novel variants and chimerism. J. Clin. Virol. 2002;23:119–148. doi: 10.1016/S1386-6532(01)00205-0. [DOI] [PubMed] [Google Scholar]
- 36.Cook P.M., Whitby D., Calabro M.L., Luppi M., Kakoola D.N., Hjalgrim H., Ariyoshi K., Ensoli B., Davison A.J., Schulz T.F. Variability and evolution of Kaposiʼs sarcoma-associated herpesvirus in Europe and Africa. International collaborative group. AIDS. 1999;13:1165–1176. doi: 10.1097/00002030-199907090-00004. [DOI] [PubMed] [Google Scholar]
- 37.Meng Y.X., Spira T.J., Bhat G.J., Birch C.J., Druce J.D., Edlin B.R., Edwards R., Gunthel C., Newton R., Stamey F.R., et al. Individuals from North America, Australasia, and Africa are infected with four different genotypes of human herpesvirus 8. Virology. 1999;261:106–119. doi: 10.1006/viro.1999.9853. [DOI] [PubMed] [Google Scholar]
- 38.Biggar R.J., Whitby D., Marshall V., Linhares A.C., Black F. Human herpesvirus 8 in Brazilian amerindians: A hyperendemic population with a new subtype. J. Infect. Dis. 2000;181:1562–1568. doi: 10.1086/315456. [DOI] [PubMed] [Google Scholar]
- 39.Engels E.A., Sinclair M.D., Biggar R.J., Whitby D., Ebbesen P., Goedert J.J., Gastwirth J.L. Latent class analysis of human herpesvirus 8 assay performance and infection prevalence in sub-Saharan Africa and Malta. Int. J. Cancer. 2000;88:1003–1008. doi: 10.1002/1097-0215(20001215)88:6<1003::AID-IJC26>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Serraino D., Toma L., Andreoni M., Butto S., Tchangmena O., Sarmati L., Monini P., Franceschi S., Ensoli B., Rezza G. A seroprevalence study of human herpesvirus type 8 (HHV8) in Eastern and Central Africa and in the mediterranean area. Eur. J. Epidemiol. 2001;17:871–876. doi: 10.1023/A:1015678312153. [DOI] [PubMed] [Google Scholar]
- 41.Pellett P.E., Wright D.J., Engels E.A., Ablashi D.V., Dollard S.C., Forghani B., Glynn S.A., Goedert J.J., Jenkins F.J., Lee T.H., et al. Multicenter comparison of serologic assays and estimation of human herpesvirus 8 seroprevalence among us blood donors. Transfusion. 2003;43:1260–1268. doi: 10.1046/j.1537-2995.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- 42.Vitale F., Viviano E., Perna A.M., Bonura F., Mazzola G., Ajello F., Romano N. Serological and virological evidence of non-sexual transmission of human herpesvirus type 8 (HHV8) Epidemiol. Infect. 2000;125:671–675. doi: 10.1017/S0950268800004726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mbulaiteye S., Marshall V., Bagni R.K., Wang C.D., Mbisa G., Bakaki P.M., Owor A.M., Ndugwa C.M., Engels E.A., Katongole-Mbidde E., et al. Molecular evidence for mother-to-child transmission of Kaposi sarcoma-associated herpesvirus in uganda and K1 gene evolution within the host. J. Infect. Dis. 2006;193:1250–1257. doi: 10.1086/503052. [DOI] [PubMed] [Google Scholar]
- 44.Mesri E.A., Cesarman E., Boshoff C. Kaposiʼs sarcoma and its associated herpesvirus. Nat. Rev. Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coluzzi M., Manno D., Guzzinati S., Tognazzo S., Zambon P., Arca B., Costantini C., Ascoli V. The bloodsucking arthropod bite as possible cofactor in the transmission of human herpesvirus-8 infection and in the expression of Kaposiʼs sarcoma disease. Parassitologia. 2002;44:123–129. [PubMed] [Google Scholar]
- 46.Ascoli V., Facchinelli L., Valerio L., Manno D., Coluzzi M. Kaposiʼs sarcoma, human herpesvirus 8 infection and the potential role of promoter-arthropod bites in Northern Sweden. J. Med. Virol. 2006;78:1452–1455. doi: 10.1002/jmv.20718. [DOI] [PubMed] [Google Scholar]
- 47.Mbulaiteye S.M., Biggar R.J., Pfeiffer R.M., Bakaki P.M., Gamache C., Owor A.M., Katongole-Mbidde E., Ndugwa C.M., Goedert J.J., Whitby D., et al. Water, socioeconomic factors, and human herpesvirus 8 infection in ugandan children and their mothers. J. Acquir. Immune Defic. Syndr. 2005;38:474–479. doi: 10.1097/01.qai.0000132495.89162.c0. [DOI] [PubMed] [Google Scholar]
- 48.Renne R., Lagunoff M., Zhong W., Ganem D. The size and conformation of Kaposiʼs sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J. Virol. 1996;70:8151–8154. doi: 10.1128/jvi.70.11.8151-8154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russo J.J., Bohenzky R.A., Chien M.C., Chen J., Yan M., Maddalena D., Parry J.P., Peruzzi D., Edelman I.S., Chang Y., et al. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc. Natl. Acad. Sci. USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong E.L., Damania B. Linking KSHV to human cancer. Curr. Oncol. Rep. 2005;7:349–356. doi: 10.1007/s11912-005-0061-6. [DOI] [PubMed] [Google Scholar]
- 51.Zhu F.X., Chong J.M., Wu L., Yuan Y. Virion proteins of Kaposiʼs sarcoma-associated herpesvirus. J. Virol. 2005;79:800–811. doi: 10.1128/JVI.79.2.800-811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spear P.G., Longnecker R. Herpesvirus entry: An update. J. Virol. 2003;77:10179–10185. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lieberman P.M. Keeping it quiet: Chromatin control of gammaherpesvirus latency. Nat. Rev. Microbiol. 2013;11:863–875. doi: 10.1038/nrmicro3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lieberman P.M. Chromatin regulation of virus infection. Trends Microbiol. 2006;14:132–140. doi: 10.1016/j.tim.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Luger K., Mader A.W., Richmond R.K., Sargent D.F., Richmond T.J. Crystal structure of the nucleosome core particle at 2.8 a resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 56.Choudhuri S., Cui Y., Klaassen C.D. Molecular targets of epigenetic regulation and effectors of environmental influences. Toxicol. Appl. Pharmacol. 2010;245:378–393. doi: 10.1016/j.taap.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campos E.I., Reinberg D. Histones: Annotating chromatin. Annu. Rev. Genet. 2009;43:559–599. doi: 10.1146/annurev.genet.032608.103928. [DOI] [PubMed] [Google Scholar]
- 58.Knipe D.M., Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nat. Rev. Microbiol. 2008;6:211–221. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 59.Karlic R., Chung H.R., Lasserre J., Vlahovicek K., Vingron M. Histone modification levels are predictive for gene expression. Proc. Natl. Acad. Sci. USA. 2010;107:2926–2931. doi: 10.1073/pnas.0909344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirst M., Marra M.A. Next generation sequencing based approaches to epigenomics. Brief. Funct. Genomics. 2010;9:455–465. doi: 10.1093/bfgp/elq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarda S., Hannenhalli S. Next-generation sequencing and epigenomics research: A hammer in search of nails. Genomics Inf. 2014;12:2–11. doi: 10.5808/GI.2014.12.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen H.S., Wikramasinghe P., Showe L., Lieberman P.M. Cohesins repress Kaposiʼs sarcoma-associated herpesvirus immediate early gene transcription during latency. J. Virol. 2012;86:9454–9464. doi: 10.1128/JVI.00787-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang H., Cho H., Sung G.H., Lieberman P.M. CTCF regulates Kaposiʼs sarcoma-associated herpesvirus latency transcription by nucleosome displacement and RNA polymerase programming. J. Virol. 2013;87:1789–1799. doi: 10.1128/JVI.02283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang H., Lieberman P.M. Cell cycle control of Kaposiʼs sarcoma-associated herpesvirus latency transcription by CTCF-cohesin interactions. J. Virol. 2009;83:6199–6210. doi: 10.1128/JVI.00052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hilton I.B., Simon J.M., Lieb J.D., Davis I.J., Damania B., Dittmer D.P. The open chromatin landscape of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2013;87:11831–11842. doi: 10.1128/JVI.01685-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greene W., Kuhne K., Ye F., Chen J., Zhou F., Lei X., Gao S.J. Molecular biology of KSHV in relation to AIDS-associated oncogenesis. Cancer Treat. Res. 2007;133:69–127. doi: 10.1007/978-0-387-46816-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toth Z., Brulois K., Jung J.U. The chromatin landscape of Kaposi’s sarcoma-associated herpesvirus. Viruses. 2013;5:1346–1373. doi: 10.3390/v5051346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dittmer D., Lagunoff M., Renne R., Staskus K., Haase A., Ganem D. A cluster of latently expressed genes in Kaposiʼs sarcoma-associated herpesvirus. J. Virol. 1998;72:8309–8315. doi: 10.1128/jvi.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sturzl M., Hohenadl C., Zietz C., Castanos-Velez E., Wunderlich A., Ascherl G., Biberfeld P., Monini P., Browning P.J., Ensoli B. Expression of K13/V-FLIP gene of human herpesvirus 8 and apoptosis in Kaposiʼs sarcoma spindle cells. J. Natl. Cancer Inst. 1999;91:1725–1733. doi: 10.1093/jnci/91.20.1725. [DOI] [PubMed] [Google Scholar]
- 70.Davis M.A., Sturzl M.A., Blasig C., Schreier A., Guo H.G., Reitz M., Opalenik S.R., Browning P.J. Expression of human herpesvirus 8-encoded cyclin D in Kaposiʼs sarcoma spindle cells. J. Natl. Cancer Inst. 1997;89:1868–1874. doi: 10.1093/jnci/89.24.1868. [DOI] [PubMed] [Google Scholar]
- 71.Muralidhar S., Veytsmann G., Chandran B., Ablashi D., Doniger J., Rosenthal L.J. Characterization of the human herpesvirus 8 (Kaposiʼs sarcoma-associated herpesvirus) oncogene, kaposin (ORF K12) J. Clin. Virol. 2000;16:203–213. doi: 10.1016/S1386-6532(99)00081-5. [DOI] [PubMed] [Google Scholar]
- 72.Cai X., Lu S., Zhang Z., Gonzalez C.M., Damania B., Cullen B.R. Kaposiʼs sarcoma-associated herpesvirus expresses an array of viral micrornas in latently infected cells. Proc. Natl. Acad. Sci. USA. 2005;102:5570–5575. doi: 10.1073/pnas.0408192102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ballestas M.E., Chatis P.A., Kaye K.M. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284:641–644. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 74.Ye F.C., Zhou F.C., Xie J.P., Kang T., Greene W., Kuhne K., Lei X.F., Li Q.H., Gao S.J. Kaposiʼs sarcoma-associated herpesvirus latent gene vFLIP inhibits viral lytic replication through NF-kappaB-mediated suppression of the AP-1 pathway: A novel mechanism of virus control of latency. J. Virol. 2008;82:4235–4249. doi: 10.1128/JVI.02370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thome M., Schneider P., Hofmann K., Fickenscher H., Meinl E., Neipel F., Mattmann C., Burns K., Bodmer J.L., Schroter M., et al. Viral flice-inhibitory proteins (FLIPS) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 76.Godden-Kent D., Talbot S.J., Boshoff C., Chang Y., Moore P., Weiss R.A., Mittnacht S. The cyclin encoded by Kaposiʼs sarcoma-associated herpesvirus stimulates CDK6 to phosphorylate the retinoblastoma protein and histone H1. J. Virol. 1997;71:4193–4198. doi: 10.1128/jvi.71.6.4193-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li H., Komatsu T., Dezube B.J., Kaye K.M. The Kaposiʼs sarcoma-associated herpesvirus K12 transcript from a primary effusion lymphoma contains complex repeat elements, is spliced, and initiates from a novel promoter. J. Virol. 2002;76:11880–11888. doi: 10.1128/JVI.76.23.11880-11888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bieleski L., Talbot S.J. Kaposiʼs sarcoma-associated herpesvirus vcyclin open reading frame contains an internal ribosome entry site. J. Virol. 2001;75:1864–1869. doi: 10.1128/JVI.75.4.1864-1869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tempera I., Lieberman P.M. Chromatin organization of gammaherpesvirus latent genomes. Biochim. Biophys. Acta. 2010;1799:236–245. doi: 10.1016/j.bbagrm.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maxwell K.L., Frappier L. Viral proteomics. Microbiol. Mol. Biol. Rev. 2007;71:398–411. doi: 10.1128/MMBR.00042-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cotter M.A., 2nd, Robertson E.S. The latency-associated nuclear antigen tethers the Kaposiʼs sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology. 1999;264:254–264. doi: 10.1006/viro.1999.9999. [DOI] [PubMed] [Google Scholar]
- 82.Si H., Verma S.C., Lampson M.A., Cai Q., Robertson E.S. Kaposiʼs sarcoma-associated herpesvirus-encoded LANA can interact with the nuclear mitotic apparatus protein to regulate genome maintenance and segregation. J. Virol. 2008;82:6734–6746. doi: 10.1128/JVI.00342-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelley-Clarke B., de Leon-Vazquez E., Slain K., Barbera A.J., Kaye K.M. Role of Kaposiʼs sarcoma-associated herpesvirus C-terminal LANA chromosome binding in episome persistence. J. Virol. 2009;83:4326–4337. doi: 10.1128/JVI.02395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fujimuro M., Hayward S.D. The latency-associated nuclear antigen of Kaposiʼs sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3beta. J. Virol. 2003;77:8019–8030. doi: 10.1128/JVI.77.14.8019-8030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujimuro M., Wu F.Y., ApRhys C., Kajumbula H., Young D.B., Hayward G.S., Hayward S.D. A novel viral mechanism for dysregulation of beta-catenin in Kaposi’s sarcoma-associated herpesvirus latency. Nat. Med. 2003;9:300–306. doi: 10.1038/nm829. [DOI] [PubMed] [Google Scholar]
- 86.Cai Q., Lan K., Verma S.C., Si H., Lin D., Robertson E.S. Kaposiʼs sarcoma-associated herpesvirus latent protein LANA interacts with HIF-1 alpha to upregulate RTA expression during hypoxia: Latency control under low oxygen conditions. J. Virol. 2006;80:7965–7975. doi: 10.1128/JVI.00689-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J., Martin H.J., Liao G., Hayward S.D. The Kaposiʼs sarcoma-associated herpesvirus LANA protein stabilizes and activates C-myc. J. Virol. 2007;81:10451–10459. doi: 10.1128/JVI.00804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai Q., Murakami M., Si H., Robertson E.S. A potential alpha-helix motif in the amino terminus of LANA encoded by Kaposiʼs sarcoma-associated herpesvirus is critical for nuclear accumulation of HIF-1alpha in normoxia. J. Virol. 2007;81:10413–10423. doi: 10.1128/JVI.00611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cai Q.L., Knight J.S., Verma S.C., Zald P., Robertson E.S. Ec5s ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and P53 tumor suppressors. PLOS Pathog. 2006;2:e116. doi: 10.1371/journal.ppat.0020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rossetto C., Yamboliev I., Pari G.S. Kaposiʼs sarcoma-associated herpesvirus/human herpesvirus 8 K-BZIP modulates latency-associated nuclear protein-mediated suppression of lytic origin-dependent DNA synthesis. J. Virol. 2009;83:8492–8501. doi: 10.1128/JVI.00922-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu J., Verma S.C., Murakami M., Cai Q., Kumar P., Xiao B., Robertson E.S. Latency-associated nuclear antigen of Kaposiʼs sarcoma-associated herpesvirus (KSHV) upregulates survivin expression in KSHV-associated B-lymphoma cells and contributes to their proliferation. J. Virol. 2009;83:7129–7141. doi: 10.1128/JVI.00397-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao B., Verma S.C., Cai Q., Kaul R., Lu J., Saha A., Robertson E.S. BUB1 and CENP-F can contribute to Kaposiʼs sarcoma-associated herpesvirus genome persistence by targeting LANA to kinetochores. J. Virol. 2010;84:9718–9732. doi: 10.1128/JVI.00713-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cai Q., Verma S.C., Choi J.Y., Ma M., Robertson E.S. Kaposiʼs sarcoma-associated herpesvirus inhibits interleukin-4-mediated STAT6 phosphorylation to regulate apoptosis and maintain latency. J. Virol. 2010;84:11134–11144. doi: 10.1128/JVI.01293-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garber A.C., Shu M.A., Hu J., Renne R. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposiʼs sarcoma-associated herpesvirus. J. Virol. 2001;75:7882–7892. doi: 10.1128/JVI.75.17.7882-7892.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cotter M.A., 2nd, Subramanian C., Robertson E.S. The Kaposiʼs sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxy-terminus. Virology. 2001;291:241–259. doi: 10.1006/viro.2001.1202. [DOI] [PubMed] [Google Scholar]
- 96.Garber A.C., Hu J., Renne R. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 2002;277:27401–27411. doi: 10.1074/jbc.M203489200. [DOI] [PubMed] [Google Scholar]
- 97.Wong L.Y., Wilson A.C. Kaposiʼs sarcoma-associated herpesvirus latency-associated nuclear antigen induces a strong bend on binding to terminal repeat DNA. J. Virol. 2005;79:13829–13836. doi: 10.1128/JVI.79.21.13829-13836.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Komatsu T., Ballestas M.E., Barbera A.J., Kelley-Clarke B., Kaye K.M. KSHV LANA1 binds DNA as an oligomer and residues N-terminal to the oligomerization domain are essential for DNA binding, replication, and episome persistence. Virology. 2004;319:225–236. doi: 10.1016/j.virol.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 99.Lim C., Lee D., Seo T., Choi C., Choe J. Latency-associated nuclear antigen of Kaposiʼs sarcoma-associated herpesvirus functionally interacts with heterochromatin protein 1. J. Biol. Chem. 2003;278:7397–7405. doi: 10.1074/jbc.M211912200. [DOI] [PubMed] [Google Scholar]
- 100.Verma S.C., Cai Q., Kreider E., Lu J., Robertson E.S. Comprehensive analysis of LANA interacting proteins essential for viral genome tethering and persistence. PLOS ONE. 2013;8:e74662. doi: 10.1371/journal.pone.0074662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cai Q., Verma S.C., Lu J., Robertson E.S. Molecular biology of Kaposiʼs sarcoma-associated herpesvirus and related oncogenesis. Adv. Virus Res. 2010;78:87–142. doi: 10.1016/B978-0-12-385032-4.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu J., Garber A.C., Renne R. The latency-associated nuclear antigen of Kaposiʼs sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 2002;76:11677–11687. doi: 10.1128/JVI.76.22.11677-11687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Verma S.C., Lan K., Robertson E. Structure and function of latency-associated nuclear antigen. Curr. Top. Microbiol. Immunol. 2007;312:101–136. doi: 10.1007/978-3-540-34344-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stedman W., Deng Z., Lu F., Lieberman P.M. Orc, mcm, and histone hyperacetylation at the Kaposi’s sarcoma-associated herpesvirus latent replication origin. J. Virol. 2004;78:12566–12575. doi: 10.1128/JVI.78.22.12566-12575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Purushothaman P., McDowell M.E., McGuinness J., Salas R., Rumjahn S.M., Verma S.C. Kaposiʼs sarcoma-associated herpesvirus-encoded LANA recruits topoisomerase iibeta for latent DNA replication of the terminal repeats. J. Virol. 2012;86:9983–9994. doi: 10.1128/JVI.00839-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Verma S.C., Choudhuri T., Kaul R., Robertson E.S. Latency-associated nuclear antigen (LANA) of Kaposi’s sarcoma-associated herpesvirus interacts with origin recognition complexes at the LANA binding sequence within the terminal repeats. J. Virol. 2006;80:2243–2256. doi: 10.1128/JVI.80.5.2243-2256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Verma S.C., Choudhuri T., Robertson E.S. The minimal replicator element of the Kaposiʼs sarcoma-associated herpesvirus terminal repeat supports replication in a semiconservative and cell-cycle-dependent manner. J. Virol. 2007;81:3402–3413. doi: 10.1128/JVI.01607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nishitani H., Lygerou Z. Control of DNA replication licensing in a cell cycle. Genes Cells. 2002;7:523–534. doi: 10.1046/j.1365-2443.2002.00544.x. [DOI] [PubMed] [Google Scholar]
- 109.Bell S.P., Dutta A. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 110.Sun Q., Tsurimoto T., Juillard F., Li L., Li S., de Leon Vazquez E., Chen S., Kaye K. Kaposiʼs sarcoma-associated herpesvirus LANA recruits the DNA polymerase clamp loader to mediate efficient replication and virus persistence. Proc. Natl. Acad. Sci. USA. 2014;111:11816–11821. doi: 10.1073/pnas.1404219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Verma S.C., Borah S., Robertson E.S. Latency-associated nuclear antigen of Kaposiʼs sarcoma-associated herpesvirus up-regulates transcription of human telomerase reverse transcriptase promoter through interaction with transcription factor SP1. J. Virol. 2004;78:10348–10359. doi: 10.1128/JVI.78.19.10348-10359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Friborg J., Jr., Kong W., Hottiger M.O., Nabel G.J. P53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889–894. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 113.Radkov S.A., Kellam P., Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene HRAS transforms primary rat cells. Nat. Med. 2000;6:1121–1127. doi: 10.1038/80459. [DOI] [PubMed] [Google Scholar]
- 114.Van Dross R., Yao S., Asad S., Westlake G., Mays D.J., Barquero L., Duell S., Pietenpol J.A., Browning P.J. Constitutively active K-cyclin/CDK6 kinase in Kaposi sarcoma-associated herpesvirus-infected cells. J. Natl. Cancer Inst. 2005;97:656–666. doi: 10.1093/jnci/dji113. [DOI] [PubMed] [Google Scholar]
- 115.Direkze S., Laman H. Regulation of growth signalling and cell cycle by Kaposiʼs sarcoma-associated herpesvirus genes. Int. J. Exp. Pathol. 2004;85:305–319. doi: 10.1111/j.0959-9673.2004.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sarek G., Jarviluoma A., Moore H.M., Tojkander S., Vartia S., Biberfeld P., Laiho M., Ojala P.M. Nucleophosmin phosphorylation by v-cyclin-CDK6 controls KSHV latency. PLOS Pathog. 2010;6:e1000818. doi: 10.1371/journal.ppat.1000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liang X., Paden C.R., Morales F.M., Powers R.P., Jacob J., Speck S.H. Murine gamma-herpesvirus immortalization of fetal liver-derived B cells requires both the viral cyclin D homolog and latency-associated nuclear antigen. PLOS Pathog. 2011;7:e1002220. doi: 10.1371/journal.ppat.1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guasparri I., Keller S.A., Cesarman E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J. Exp. Med. 2004;199:993–1003. doi: 10.1084/jem.20031467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Israel A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harbor Perspect. Biol. 2010;2:a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Field N., Low W., Daniels M., Howell S., Daviet L., Boshoff C., Collins M. KSHV vFLIP binds to IKK-gamma to activate IKK. J. Cell Sci. 2003;116:3721–3728. doi: 10.1242/jcs.00691. [DOI] [PubMed] [Google Scholar]
- 121.Ye F., Lei X., Gao S.J. Mechanisms of Kaposiʼs sarcoma-associated herpesvirus latency and reactivation. Adv. Virol. 2011;2011 doi: 10.1155/2011/193860. Article ID 193860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sadler R., Wu L., Forghani B., Renne R., Zhong W., Herndier B., Ganem D. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposiʼs sarcoma-associated herpesvirus. J. Virol. 1999;73:5722–5730. doi: 10.1128/jvi.73.7.5722-5730.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McCormick C., Ganem D. The kaposin B protein of KSHV activates the P38/MK2 pathway and stabilizes cytokine mrnas. Science. 2005;307:739–741. doi: 10.1126/science.1105779. [DOI] [PubMed] [Google Scholar]
- 124.Kanno T., Sato Y., Sata T., Katano H. Expression of Kaposiʼs sarcoma-associated herpesvirus-encoded K10/10.1 protein in tissues and its interaction with poly(A)-binding protein. Virology. 2006;352:100–109. doi: 10.1016/j.virol.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 125.Esteban M., Garcia M.A., Domingo-Gil E., Arroyo J., Nombela C., Rivas C. The latency protein LANA2 from Kaposiʼs sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNase l activation. J. Gen. Virol. 2003;84:1463–1470. doi: 10.1099/vir.0.19014-0. [DOI] [PubMed] [Google Scholar]
- 126.Rivas C., Thlick A.E., Parravicini C., Moore P.S., Chang Y. Kaposiʼs sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 2001;75:429–438. doi: 10.1128/JVI.75.1.429-438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lin Y.T., Kincaid R.P., Arasappan D., Dowd S.E., Hunicke-Smith S.P., Sullivan C.S. Small RNA profiling reveals antisense transcription throughout the KSHV genome and novel small RNAs. RNA. 2010;16:1540–1558. doi: 10.1261/rna.1967910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cai X., Cullen B.R. Transcriptional origin of Kaposiʼs sarcoma-associated herpesvirus micrornas. J. Virol. 2006;80:2234–2242. doi: 10.1128/JVI.80.5.2234-2242.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grundhoff A., Sullivan C.S., Ganem D. A combined computational and microarray-based approach identifies novel micrornas encoded by human gamma-herpesviruses. RNA. 2006;12:733–750. doi: 10.1261/rna.2326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pfeffer S., Sewer A., Lagos-Quintana M., Sheridan R., Sander C., Grasser F.A., van Dyk L.F., Ho C.K., Shuman S., Chien M., et al. Identification of micrornas of the herpesvirus family. Nat. Methods. 2005;2:269–276. doi: 10.1038/nmeth746. [DOI] [PubMed] [Google Scholar]
- 131.Samols M.A., Hu J., Skalsky R.L., Renne R. Cloning and identification of a microrna cluster within the latency-associated region of Kaposiʼs sarcoma-associated herpesvirus. J. Virol. 2005;79:9301–9305. doi: 10.1128/JVI.79.14.9301-9305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Feldman E.R., Kara M., Coleman C.B., Grau K.R., Oko L.M., Krueger B.J., Renne R., van Dyk L.F., Tibbetts S.A. Virus-encoded micrornas facilitate gammaherpesvirus latency and pathogenesis in vivo. mBio. 2014;5 doi: 10.1128/mBio.00981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lei X., Bai Z., Ye F., Huang Y., Gao S.J. Regulation of herpesvirus lifecycle by viral micrornas. Virulence. 2010;1:433–435. doi: 10.4161/viru.1.5.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu C.C., Li Z., Chu C.Y., Feng J., Feng J., Sun R., Rana T.M. Micrornas encoded by Kaposiʼs sarcoma-associated herpesvirus regulate viral life cycle. EMBO Rep. 2010;11:784–790. doi: 10.1038/embor.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Plaisance-Bonstaff K., Choi H.S., Beals T., Krueger B.J., Boss I.W., Gay L.A., Haecker I., Hu J., Renne R. Kshv mirnas decrease expression of lytic genes in latently infected PEL and endothelial cells by targeting host transcription factors. Viruses. 2014;6:4005–4023. doi: 10.3390/v6104005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moody R., Zhu Y., Huang Y., Cui X., Jones T., Bedolla R., Lei X., Bai Z., Gao S.J. KSHV micrornas mediate cellular transformation and tumorigenesis by redundantly targeting cell growth and survival pathways. PLOS Pathog. 2013;9:e1003857. doi: 10.1371/journal.ppat.1003857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lei X., Bai Z., Ye F., Xie J., Kim C.G., Huang Y., Gao S.J. Regulation of NF-kappaB inhibitor ikappabalpha and viral replication by a KSHV microrna. Nat. Cell Biol. 2010;12:193–199. doi: 10.1038/ncb2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suffert G., Malterer G., Hausser J., Viiliainen J., Fender A., Contrant M., Ivacevic T., Benes V., Gros F., Voinnet O., et al. Kaposiʼs sarcoma herpesvirus micrornas target caspase 3 and regulate apoptosis. PLOS Pathog. 2011;7:e1002405. doi: 10.1371/journal.ppat.1002405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qin Z., Jakymiw A., Findlay V., Parsons C. KSHV-encoded micrornas: Lessons for viral cancer pathogenesis and emerging concepts. Int. J. Cell Biol. 2012;2012:603961. doi: 10.1155/2012/603961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ballestas M.E., Kaye K.M. Kaposiʼs sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through CIS-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 2001;75:3250–3258. doi: 10.1128/JVI.75.7.3250-3258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hu J., Renne R. Characterization of the minimal replicator of Kaposiʼs sarcoma-associated herpesvirus latent origin. J. Virol. 2005;79:2637–2642. doi: 10.1128/JVI.79.4.2637-2642.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Skalsky R.L., Hu J., Renne R. Analysis of viral cis elements conferring Kaposiʼs sarcoma-associated herpesvirus episome partitioning and maintenance. J. Virol. 2007;81:9825–9837. doi: 10.1128/JVI.00842-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Toth Z., Maglinte D.T., Lee S.H., Lee H.R., Wong L.Y., Brulois K.F., Lee S., Buckley J.D., Laird P.W., Marquez V.E., et al. Epigenetic analysis of KSHV latent and lytic genomes. PLOS Pathog. 2010;6:e1001013. doi: 10.1371/journal.ppat.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gunther T., Grundhoff A. The epigenetic landscape of latent Kaposi sarcoma-associated herpesvirus genomes. PLOS Pathog. 2010;6:e1000935. doi: 10.1371/journal.ppat.1000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.He M., Zhang W., Bakken T., Schutten M., Toth Z., Jung J.U., Gill P., Cannon M., Gao S.J. Cancer angiogenesis induced by Kaposi sarcoma-associated herpesvirus is mediated by EZH2. Cancer Res. 2012;72:3582–3592. doi: 10.1158/0008-5472.CAN-11-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chang P.C., Fitzgerald L.D., Hsia D.A., Izumiya Y., Wu C.Y., Hsieh W.P., Lin S.F., Campbell M., Lam K.S., Luciw P.A., et al. Histone demethylase JMJD2A regulates Kaposiʼs sarcoma-associated herpesvirus replication and is targeted by a viral transcriptional factor. J. Virol. 2011;85:3283–3293. doi: 10.1128/JVI.02485-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Paschos K., Allday M.J. Epigenetic reprogramming of host genes in viral and microbial pathogenesis. Trends Microbiol. 2010;18:439–447. doi: 10.1016/j.tim.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gunther T., Schreiner S., Dobner T., Tessmer U., Grundhoff A. Influence of ND10 components on epigenetic determinants of early KSHV latency establishment. PLOS Pathog. 2014;10:e1004274. doi: 10.1371/journal.ppat.1004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Merkenschlager M., Odom D.T. CTCF and Cohesin: Linking gene regulatory elements with their targets. Cell. 2013;152:1285–1297. doi: 10.1016/j.cell.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 150.Kang H., Wiedmer A., Yuan Y., Robertson E., Lieberman P.M. Coordination of KSHV latent and lytic gene control by CTCF-cohesin mediated chromosome conformation. PLOS Pathog. 2011;7:e1002140. doi: 10.1371/journal.ppat.1002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stedman W., Kang H., Lin S., Kissil J.L., Bartolomei M.S., Lieberman P.M. Cohesins localize with CTCF at the KSHV latency control region and at cellular C-myc and H19/IGF2 insulators. EMBO J. 2008;27:654–666. doi: 10.1038/emboj.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li D.J., Verma D., Mosbruger T., Swaminathan S. CTCF and RAD21 act as host cell restriction factors for Kaposiʼs sarcoma-associated herpesvirus (KSHV) lytic replication by modulating viral gene transcription. PLOS Pathog. 2014;10:e1003880. doi: 10.1371/journal.ppat.1003880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Losada A. Cohesin in cancer: Chromosome segregation and beyond. Nat. Rev. Cancer. 2014;14:389–393. doi: 10.1038/nrc3743. [DOI] [PubMed] [Google Scholar]
- 154.Chen H.S., Lu F., Lieberman P.M. Epigenetic regulation of EBV and KSHV latency. Curr. Opin. Virol. 2013;3:251–259. doi: 10.1016/j.coviro.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Darst R.P., Haecker I., Pardo C.E., Renne R., Kladde M.P. Epigenetic diversity of Kaposiʼs sarcoma-associated herpesvirus. Nucl. Acids Res. 2013;41:2993–3009. doi: 10.1093/nar/gkt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Darst R.P., Nabilsi N.H., Pardo C.E., Riva A., Kladde M.P. DNA methyltransferase accessibility protocol for individual templates by deep sequencing. Methods Enzymol. 2012;513:185–204. doi: 10.1016/B978-0-12-391938-0.00008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Staskus K.A., Zhong W., Gebhard K., Herndier B., Wang H., Renne R., Beneke J., Pudney J., Anderson D.J., Ganem D., et al. Kaposiʼs sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhong W., Wang H., Herndier B., Ganem D. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA. 1996;93:6641–6646. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cavallin L.E., Goldschmidt-Clermont P., Mesri E.A. Molecular and cellular mechanisms of KSHV oncogenesis of Kaposiʼs sarcoma associated with HIV/AIDS. PLOS Pathog. 2014;10:e1004154. doi: 10.1371/journal.ppat.1004154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Honess R.W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun R., Lin S.F., Staskus K., Gradoville L., Grogan E., Haase A., Miller G. Kinetics of Kaposiʼs sarcoma-associated herpesvirus gene expression. J. Virol. 1999;73:2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhu F.X., Cusano T., Yuan Y. Identification of the immediate-early transcripts of Kaposiʼs sarcoma-associated herpesvirus. J. Virol. 1999;73:5556–5567. doi: 10.1128/jvi.73.7.5556-5567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jenner R.G., Alba M.M., Boshoff C., Kellam P. Kaposiʼs sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 2001;75:891–902. doi: 10.1128/JVI.75.2.891-902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lukac D.M., Kirshner J.R., Ganem D. Transcriptional activation by the product of open reading frame 50 of Kaposiʼs sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 1999;73:9348–9361. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lukac D.M., Renne R., Kirshner J.R., Ganem D. Reactivation of Kaposiʼs sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- 166.Sun R., Lin S.F., Gradoville L., Yuan Y., Zhu F., Miller G. A viral gene that activates lytic cycle expression of Kaposiʼs sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu Y., AuCoin D.P., Huete A.R., Cei S.A., Hanson L.J., Pari G.S. A Kaposiʼs sarcoma-associated herpesvirus/human herpesvirus 8 ORF50 deletion mutant is defective for reactivation of latent virus and DNA replication. J. Virol. 2005;79:3479–3487. doi: 10.1128/JVI.79.6.3479-3487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Glaunsinger B., Ganem D. Lytic KSHV infection inhibits host gene expression by accelerating global mrna turnover. Mol. Cell. 2004;13:713–723. doi: 10.1016/S1097-2765(04)00091-7. [DOI] [PubMed] [Google Scholar]
- 169.Majerciak V., Yamanegi K., Zheng Z.M. Gene structure and expression of Kaposiʼs sarcoma-associated herpesvirus ORF56, ORF57, ORF58, and ORF59. J. Virol. 2006;80:11968–11981. doi: 10.1128/JVI.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Goto E., Ishido S., Sato Y., Ohgimoto S., Ohgimoto K., Nagano-Fujii M., Hotta H. C-mir, a human E3 ubiquitin ligase, is a functional homolog of herpesvirus proteins MIR1 and MIR2 and has similar activity. J. Biol. Chem. 2003;278:14657–14668. doi: 10.1074/jbc.M211285200. [DOI] [PubMed] [Google Scholar]
- 171.Arias C., Weisburd B., Stern-Ginossar N., Mercier A., Madrid A.S., Bellare P., Holdorf M., Weissman J.S., Ganem D. KSHV 2.0: A comprehensive annotation of the Kaposiʼs sarcoma-associated herpesvirus genome using next-generation sequencing reveals novel genomic and functional features. PLOS Pathog. 2014;10:e1003847. doi: 10.1371/journal.ppat.1003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen W.Y., Townes T.M. Molecular mechanism for silencing virally transduced genes involves histone deacetylation and chromatin condensation. Proc. Natl. Acad. Sci. USA. 2000;97:377–382. doi: 10.1073/pnas.97.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen J., Ueda K., Sakakibara S., Okuno T., Parravicini C., Corbellino M., Yamanishi K. Activation of latent Kaposiʼs sarcoma-associated herpesvirus by demethylation of the promoter of the lytic transactivator. Proc. Natl. Acad. Sci. USA. 2001;98:4119–4124. doi: 10.1073/pnas.051004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lu F., Zhou J., Wiedmer A., Madden K., Yuan Y., Lieberman P.M. Chromatin remodeling of the Kaposiʼs sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency. J. Virol. 2003;77:11425–11435. doi: 10.1128/JVI.77.21.11425-11435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Morris T.L., Arnold R.R., Webster-Cyriaque J. Signaling cascades triggered by bacterial metabolic end products during reactivation of Kaposiʼs sarcoma-associated herpesvirus. J. Virol. 2007;81:6032–6042. doi: 10.1128/JVI.02504-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pantry S.N., Medveczky P.G. Epigenetic regulation of Kaposiʼs sarcoma-associated herpesvirus replication. Semin. Cancer Biol. 2009;19:153–157. doi: 10.1016/j.semcancer.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rossetto C.C., Pari G. KSHV PAN RNA associates with demethylases UTX and JMJD3 to activate lytic replication through a physical interaction with the virus genome. PLOS Pathog. 2012;8:e1002680. doi: 10.1371/journal.ppat.1002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Groen J.N., Morris K.V. Chromatin, non-coding RNAs, and the expression of HIV. Viruses. 2013;5:1633–1645. doi: 10.3390/v5071633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Majerciak V., Ni T., Yang W., Meng B., Zhu J., Zheng Z.M. A viral genome landscape of RNA polyadenylation from KSHV latent to lytic infection. PLOS Pathog. 2013;9:e1003749. doi: 10.1371/journal.ppat.1003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Steitz J., Borah S., Cazalla D., Fok V., Lytle R., Mitton-Fry R., Riley K., Samji T. Noncoding RNPs of viral origin. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a005165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Song M.J., Brown H.J., Wu T.T., Sun R. Transcription activation of polyadenylated nuclear rna by RTA in human herpesvirus 8/Kaposiʼs sarcoma-associated herpesvirus. J. Virol. 2001;75:3129–3140. doi: 10.1128/JVI.75.7.3129-3140.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sahin B.B., Patel D., Conrad N.K. Kaposiʼs sarcoma-associated herpesvirus ORF57 protein binds and protects a nuclear noncoding RNA from cellular RNA decay pathways. PLOS Pathog. 2010;6:e1000799. doi: 10.1371/journal.ppat.1000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Massimelli M.J., Majerciak V., Kruhlak M., Zheng Z.M. Interplay between polyadenylate-binding protein 1 and Kaposiʼs sarcoma-associated herpesvirus ORF57 in accumulation of polyadenylated nuclear RNA, a viral long noncoding RNA. J. Virol. 2013;87:243–256. doi: 10.1128/JVI.01693-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Blauvelt A., Herndier B.G., Orenstein J.M. Propagation of a human herpesvirus from AIDS-associated Kaposi’s sarcoma. N. Engl. J. Med. 1997;336:1837–1839. doi: 10.1056/NEJM199706193362517. [DOI] [PubMed] [Google Scholar]
- 185.Renne R., Blackbourn D., Whitby D., Levy J., Ganem D. Limited transmission of Kaposiʼs sarcoma-associated herpesvirus in cultured cells. J. Virol. 1998;72:5182–5188. doi: 10.1128/jvi.72.6.5182-5188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Veettil M.V., Bandyopadhyay C., Dutta D., Chandran B. Interaction of KSHV with host cell surface receptors and cell entry. Viruses. 2014;6:4024–4046. doi: 10.3390/v6104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Luna R.E., Zhou F., Baghian A., Chouljenko V., Forghani B., Gao S.J., Kousoulas K.G. Kaposiʼs sarcoma-associated herpesvirus glycoprotein K8.1 is dispensable for virus entry. J. Virol. 2004;78:6389–6398. doi: 10.1128/JVI.78.12.6389-6398.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chandran B., Bloomer C., Chan S.R., Zhu L., Goldstein E., Horvat R. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology. 1998;249:140–149. doi: 10.1006/viro.1998.9316. [DOI] [PubMed] [Google Scholar]
- 189.Koyano S., Mar E.C., Stamey F.R., Inoue N. Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J. Gen. Virol. 2003;84:1485–1491. doi: 10.1099/vir.0.18941-0. [DOI] [PubMed] [Google Scholar]
- 190.Full F., Jungnickl D., Reuter N., Bogner E., Brulois K., Scholz B., Sturzl M., Myoung J., Jung J.U., Stamminger T., et al. Kaposiʼs sarcoma associated herpesvirus tegument protein ORF75 is essential for viral lytic replication and plays a critical role in the antagonization of ND10-instituted intrinsic immunity. PLOS Pathog. 2014;10:e1003863. doi: 10.1371/journal.ppat.1003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chandran B. Early events in Kaposiʼs sarcoma-associated herpesvirus infection of target cells. J. Virol. 2010;84:2188–2199. doi: 10.1128/JVI.01334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Toth Z., Brulois K., Lee H.R., Izumiya Y., Tepper C., Kung H.J., Jung J.U. Biphasic euchromatin-to-heterochromatin transition on the KSHV genome following de novo infection. PLOS Pathog. 2013;9:e1003813. doi: 10.1371/journal.ppat.1003813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Morey L., Helin K. Polycomb group protein-mediated repression of transcription. Trends Biochem. Sci. 2010;35:323–332. doi: 10.1016/j.tibs.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 194.Lallemand-Breitenbach V., Zhu J., Puvion F., Koken M., Honore N., Doubeikovsky A., Duprez E., Pandolfi P.P., Puvion E., Freemont P., et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11s proteasome recruitment, and AS2O3-induced PML or PML/retinoic acid receptor alpha degradation. J. Exp. Med. 2001;193:1361–1371. doi: 10.1084/jem.193.12.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chan J.Y., Li L., Fan Y.H., Mu Z.M., Zhang W.W., Chang K.S. Cell-cycle regulation of DNA damage-induced expression of the suppressor gene PML. Biochem. Biophys. Res. Commun. 1997;240:640–646. doi: 10.1006/bbrc.1997.7692. [DOI] [PubMed] [Google Scholar]
- 196.Ishov A.M., Sotnikov A.G., Negorev D., Vladimirova O.V., Neff N., Kamitani T., Yeh E.T., Strauss J.F., 3rd, Maul G.G. PML is critical for ND10 formation and recruits the PML-interacting protein DAXX to this nuclear structure when modified by SUMO-1. J. Cell Biol. 1999;147:221–234. doi: 10.1083/jcb.147.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kyratsous C.A., Silverstein S.J. Components of nuclear domain 10 bodies regulate varicella-zoster virus replication. J. Virol. 2009;83:4262–4274. doi: 10.1128/JVI.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gregory S.M., Davis B.K., West J.A., Taxman D.J., Matsuzawa S., Reed J.C., Ting J.P., Damania B. Discovery of a viral NLR homolog that inhibits the inflammasome. Science. 2011;331:330–334. doi: 10.1126/science.1199478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kerur N., Veettil M.V., Sharma-Walia N., Bottero V., Sadagopan S., Otageri P., Chandran B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]