Abstract
Membranes constitute a meeting point for lipids and proteins. Not only do they define the entity of cells and cytosolic organelles but they also display a wide variety of important functions previously ascribed to the activity of proteins alone. Indeed, lipids have commonly been considered a mere support for the transient or permanent association of membrane proteins, while acting as a selective cell/organelle barrier. However, mounting evidence demonstrates that lipids themselves regulate the location and activity of many membrane proteins, as well as defining membrane microdomains that serve as spatio-temporal platforms for interacting signalling proteins. Membrane lipids are crucial in the fission and fusion of lipid bilayers and they also act as sensors to control environmental or physiological conditions. Lipids and lipid structures participate directly as messengers or regulators of signal transduction. Moreover, their alteration has been associated with the development of numerous diseases. Proteins can interact with membranes through lipid co-/post-translational modifications, and electrostatic and hydrophobic interactions, van der Waals forces and hydrogen bonding are all involved in the associations among membrane proteins and lipids. The present study reviews these interactions from the molecular and biomedical point of view, and the effects of their modulation on the physiological activity of cells, the aetiology of human diseases and the design of clinical drugs. In fact, the influence of lipids on protein function is reflected in the possibility to use these molecular species as targets for therapies against cancer, obesity, neurodegenerative disorders, cardiovascular pathologies and other diseases, using a new approach called membrane-lipid therapy.
Keywords: lipid bilayer, lipid composition-structure, membrane lipid organization, membrane ion channel, membrane receptor, GPCR, G protein, PKC, cell signalling, heat-shock protein, transmembrane protein, peripheral protein, protein-lipid interactions
Introduction
Most cell functions occur in or around membranes. Membranes not only define the cell's boundary but they also create cytoplasmic compartments into which certain activities can be segregated or to make them more efficient. Membrane proteins have been attributed with the most important roles in membranes, although lipids have also been acknowledged as key elements in numerous processes. The present review shows how membrane lipids, and the structures they form, participate and regulate numerous important cellular activities.
Membrane-spanning (integral, intrinsic) proteins are permanently embedded in the lipid bilayer (Fig. 1). In many cases, the type of lipids that interact with amino acids in the hydrophobic environment of the membrane core and those at the interface are more or less defined, and to a certain extent regulated by the features of the protein. Transmembrane proteins also influence lipid structure in the membrane. Therefore, it is not surprising that changes in the lipid environment of membranes regulate or alter the function of intrinsic membrane proteins (see below). Indeed, in regions rich in a given type of receptor (e.g. synaptosomes, receptor clusters, etc.) these protein-lipid interactions play important roles in both directions.
1.
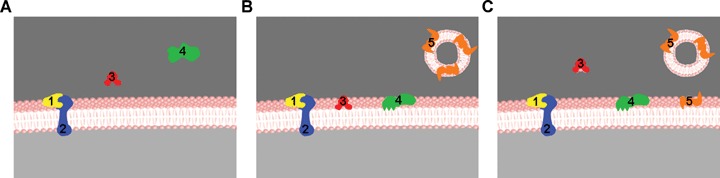
A simplified drawing representing the various interactions of proteins with lipid bilayers as a function of time:1, a peripheral or extrinsic protein; 2, an integral or intrinsic protein; 3, a non-permanent protein that interacts reversibly with the membrane, a lipid-transfer protein in this particular example; 4, a non-permanent protein that becomes irreversibly bound to the bilayer once it interacts with it; 5, a non-permanent protein reversibly bound to a secretion vesicle and then transferred to a target membrane. A, B and C correspond to consecutive stages in the interaction process.
On the other hand, peripheral (extrinsic) proteins also regulate and are regulated by membrane lipids. G proteins provide a good example of how proteins can affect membrane composition and structure [1]. Like integral proteins, peripheral proteins may regulate membrane composition and structure, and many of these proteins undergo co-/post-translational modifications, that include the addition or removal of fatty acids or isoprenoid moieties. Recent studies show that these lipid modifications, and the surrounding amino acids are not only involved in the interaction with membranes but that they also regulate: (i) membrane lipid structure; (ii) the formation of lipid domains in membranes and (iii) clustering of G protein peptides [2, 3].
Membrane lipids participate in the interaction of proteins with the cell barrier [4, 5]. They also regulate the distribution and localization of peripheral proteins to membrane domains where they can interact with other signalling proteins [6]. In this context, heterotrimeric and dimeric G proteins prefer hexagonal (HII)-prone membranes, whereas monomeric Gαi proteins prefer lamellar regions of the membrane. Indeed, both peptide and lipid moieties of these proteins are involved in the reciprocal regulation of structural and functional aspects of the membrane [1, 6–9].
Membranes are formed by a matrix of lipids whose structure and composition is far from simple. First, the number of different lipid molecules found in the plasma membrane of a cell can exceed 1000. Second, the high number of structures formed by lipids in vitro indicates that the structural properties of membranes can vary greatly in vivo. Third, the interaction of lipid molecules to form membranes is not determined by covalent bonds, like that of amino acids in proteins or bases in nucleic acids. In turn, membrane lipids participate in dynamic interactions that facilitate changes in their relative position in membranes, membrane thickness, surface packing, lateral and rotational mobility and other properties that complicate the study of membrane structure. Fourth, in most membranes different regions and domains with defined lipid and protein compositions usually coexist and similar domains are not always entirely equal (e.g. two lipid rafts may differ in their size or transient nature, as well as in the proportions of lipids and proteins). Finally, membrane proteins and lipids may both be subjected to regulatory processes in response to pathophysiological situations or nutritional/pharmacological interventions, which in turn may alter the activity and functions of the membrane.
Membranes constitute a meeting point for lipids and proteins, both fulfilling prominent roles in certain cellular processes, equally relevant in many cases. Thus, a given organism may respond to environmental situations not only by regulating protein expression, but also by modulating membrane lipid levels. For example, there are dramatic changes in membrane lipid composition in the brains of fish living in rivers whose temperature varies from 20°C in the summer to 4°C in the winter [10]. If this were not the case, the physicochemical properties of their membranes would not be appropriate to allow cell signalling or to facilitate other important physiological functions. Indeed, all organisms are exposed to stressful conditions such as elevated temperatures and irradiation, as well as physiological stress such as rapid cellular proliferation, oxidative stress due to metabolic reactions, or pathophysiological stress due to infection and inflammation. If unmitigated, such stressful conditions can lead to membrane disintegration, protein misfolding and aggregation, cellular dysfunction and cell death. Significantly, recent studies strongly suggest that plasma membranes play a critical role in sensing and responding to most stress stimuli, particularly through the activation of specific signal transduction pathways that function in membrane and protein homeostasis [11, 12]. In addition, many diseases are associated with alterations in membrane lipid levels (see sections below). Therefore, therapeutic approaches based on their regulation appear to be useful novel clinical alternatives to other pharmacological strategies [9]. In addition, most drugs currently under development are targeted at G protein-coupled receptors (GPCRs), a ubiquitous family of membrane receptors that control a great number of cellular and physiological functions, whose activity is controlled by their lipid environment (see below). Therefore, membranes also constitute meeting points for therapies that, through regulation of membrane lipids and/or proteins, reverse cellular malfunctions. This fact further shows the relevance of membranes in the control of cell functions, their homeostasis, communication and responses to environmental and pathophysiological situations.
Membrane lipid composition
Biological membranes consist of a lipid bilayer to which proteins and carbohydrates may be associated or covalently linked. Recent advances have provided new perspectives from which the roles of membrane lipids in cells can be evaluated, having evolved from a simple physical barrier to a critical component in cell signalling and other cellular processes.
Membrane lipids can be classified into three main groups: glycerol-based lipids, cholesterol and ceramide-based sphingolipids. Glycerol-based lipids can be divided into two broad categories: glycosylglycerides and phospholipids. Glycosylglycerides form a highly complex lipid family in which the sn-3 position of the glycerol backbone is esterified to a glycosyl moiety (e.g. galactose, glucose, etc.). They are the most abundant membrane glycerolipids; however, this lipid family is beyond the scope of this review and we will focus our attention on phospholipids. In phospholipids, while their sn-1 and sn-2 positions are esterified to a fatty acid, the sn-3 position is esterified to a phosphate group that in turn is also esterified to a polar headgroup. Although the fatty acid moiety greatly influences their physicochemical properties, these phospholipids are usually classified according to their polar headgroup (Table 1). Cholesterol contains a hydroxyl group that interacts with the phosphate head of phospholipids, whereas the bulky steroid region interacts with phospholipid acyl chains. Among other important physical properties of membranes, these interactions regulate membrane fluidity, membrane packing, non-lamellar phase propensity and the formation of microdomains. Finally, sphingolipids are defined by the presence of a sphingoid-base backbone (i.e. 2-aminoalk[ane or ene]1,3-diol with 2S,3R stereochemistry). The main feature that allows the formation of an impermeable lipid bilayer is the amphipathic nature of these molecules, resulting in a highly hydrophobic core and hydrophilic surface, the landmark of biological and model membranes.
1.
Glycerophospholipid classification according to their polar headgroup
| Glycerophospholipid | Headgroup | Formula of headgroup |
|---|---|---|
| Phosphatidic acid | - | -H |
| Phosphatidylethanolamine | Ethanolamine | -CH2-CH2-NH3+ |
| Phosphatidylcholine | Choline | -CH2-CH2-N +(CH3)3 |
| Phosphatidylserine | Serine | -CH2-CH2(COO)−-NH3+ |
| Phospatidylglycerol | Glycerol | -CH2-CH(OH)- CH2OH |
| Phosphatidylinositol 4,5-bisphosphate | Myo-inositol 4,5-bisphosphate | 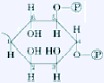 |
| Cardiolipin | Phosphatidylglycerol | 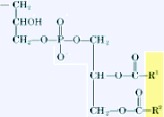 |
Membrane lipid structure
Most phospholipids spontaneously form lipid bilayers in aqueous environments with a pH and ionic strength similar to that of biological systems. However, certain lipids can organize into non-lamellar structures under physiological or non-physiological conditions. Moreover, lipids may not only display different phases under different conditions (lipid mesomorphism), but membranes lipids may also show distinct finite structures within cell membranes (membrane microdomains). This lipid mesomorphism has been mainly studied in vitro, although non-lamellar structures have also been observed in vivo[13]. Thus, although lipids usually organize into bilayers their structural versatility implies that the nature of the membranes that form may be diverse.
Membranes are made up of molecules that to some extent preserve their individual characteristics and hence, the particular structure of these molecular bricks influences the structural properties of the membrane. In this context, phospholipids with a bulky polar head, such as phosphatidylcholine (PC), have a cylindrical molecular or effective shape and they tend to associate with other cylinder-like phospholipids to form planar structures [14, 15]. Other lipids might be prone to form non-bilayer structures. Cone-shaped lipids with bulky polar heads such as lysophosphatidylcholine (LPC), or truncated cone-shaped lipids with small headgroups such as phosphatidylethanolamine (PE), may form spherical micelles or tubular structures with positive (HI) or negative curvature (HII), respectively. Although these lipids form non-bilayer structures in membranes, the roles of which in general remain to be determined [13], in the last few years some functions have been attributed to non-bilayer prone lipids in planar structures (lipid bilayers). Indeed, these lipids appear to participate in the interaction of several proteins, such as, e.g. protein kinase C (PKC) with membranes [4]. Non-lamellar-prone membranes also favour the binding of heterotrimeric G proteins and Gβγ dimers, as well as displaying a lower binding affinity for Gα monomers [4–6]. The influence of non-lamellar-prone lipids in facilitating or regulating the docking of amphitropic membrane proteins may have originated from the interaction of a protein with membrane fatty acyl chains leaving the membrane plane, or through the insertion of a protein's hydrophobic domain into a bilayer with ‘loose' surface packing (Fig. 2) [16]. This is possibly due to the presence of HII-prone lipids, which generate ‘frustrated’ bilayers (lɛ phase) [15], which may be stabilized by interactions with proteins or lipids (Fig. 2) [9, 16]. Non-lamellar-prone lipids also participate in the formation of the cleavage furrow during cell cytokinesis [17], as well as in other membrane fission and fusion processes. Finally, membrane lipid heterogeneity is responsible for the distinct membrane regions, domains and microdomains that form the spatial organization of, which it is related to, the specific activity at the membrane.
2.
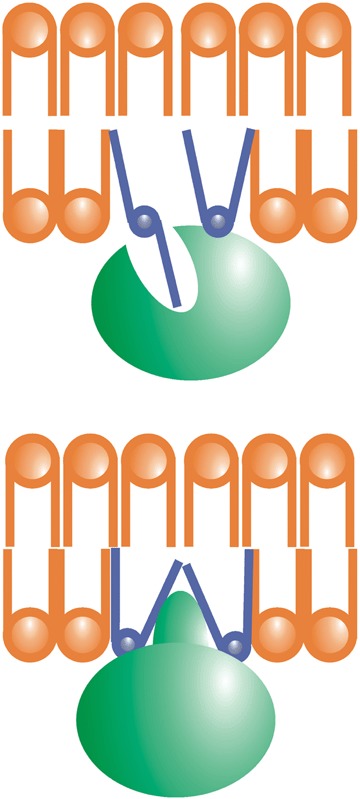
Non-lamellar-prone lipids with a small polar head-group (e.g. phosphatidylethanolamine [PE], blue) induce the formation of non-lamellar-prone regions. These bilayers, with a frustrated (lɛ) lamellar phase, can be stabilized by proteins (green) or other lamellar-prone lipids (orange). The loose packing of these bilayers allows some acyl chains to exit the membrane plane and become located in hydrophobic protein sockets (upper scheme). Hydrophobic protein domains, which may correspond to amino acid sequences or lipid modifications, may also be inserted into the membrane. Therefore, non-lamellar-prone lipids facilitate the docking of amphitropic proteins to the membrane. One of these lipids, PE, is abundant in the inner monolayer of the plasma membrane where most peripheral proteins are found.
Membrane lipid organization
The specific types of lipid species and their levels in membranes appear to be regulated exquisitely, whereby general patterns emerge that are associated with the type of cell and organelles studied. Each membrane type is highly specialized and its different attributes are determined by specific membrane proteins and lipids. Thus, a given membrane has a stable and specific lipid composition, and although there are many combinations of lipid types and proportions, changes in composition only occur under certain pathological or physiological situations. The wide variety of lipid compositions is shown in Table 2 and the differences observed in the lipid composition of the various membrane types listed, have important consequences on lipid organization in the membrane [18].
2.
Lipid composition of various types of membranes
| Percentage of phospholipids | Cholesterol (μg/mg protein) | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC | PE | PS | PI | PA | CL | LGP* | SM | ||||||||||||||||||||||||||||||||||||
| Rectal gland plasma membrane | 50.4 | 35.5 | 8.4 | <1 | – | – | – | 5.7 | N.D. | ||||||||||||||||||||||||||||||||||
| Brush border membrane | 33.3 | 35.6 | 7.4 | 8.2 | 1.2 | N.D. | 4.1 | 10.3 | 50 | ||||||||||||||||||||||||||||||||||
| Cholinergic receptor membranes | 37 | 40.5 | 17 | – | <1 | – | <1 | <1 | 135 | ||||||||||||||||||||||||||||||||||
| Plasma membrane | 39 | 23 | 9 | 8 | 1 | 1 | 2 | 16 | 128 | ||||||||||||||||||||||||||||||||||
| Mitochondria | 40 | 35 | 1 | 5 | – | 18 | 1 | 1 | 3 | ||||||||||||||||||||||||||||||||||
| Micorsome | 58 | 22 | 2 | 10 | 1 | 1 | 11 | 1 | 14 | ||||||||||||||||||||||||||||||||||
| Lysosomes | 40 | 14 | 2 | 5 | 1 | 1 | 7 | 20 | 38 | ||||||||||||||||||||||||||||||||||
| Nuclear membrane | 55 | 13 | 3 | 10 | 2 | 4 | 3 | 3 | 38 | ||||||||||||||||||||||||||||||||||
| Golgi membrane | 50 | 20 | 6 | 12 | <1 | 1 | 3 | 8 | 78 | ||||||||||||||||||||||||||||||||||
| Sarcoplasmic reticulum | 72.7 | 13.5 | 1.8 | 8.7 | <1 | <1 | – | 1 | 12 | ||||||||||||||||||||||||||||||||||
Values for lysoglycerophospholipids (LGP) in excess of a few percent should be viewed with caution. High LGP contents of are probably the result of phospholipid degradation during preparation of the material.
Adapted from [18].
The fluid mosaic model proposed that membranes were formed by a fluid bilayer in which proteins and lipids could move freely [19], contributing greatly to our concept of a cell membrane. Initially, the mosaic model depicted by Singer and Nicolson referred to the random behaviour of the different proteins in the membrane bilayer as if in a ‘sea of lipids’. Today, the extended fluid mosaic model contemplates additional structural and functional restraints on membrane organization [5, 20]. In this context, most biological membranes are asymmetrical, both laterally and in cross-section. The cross-sectional asymmetry reflects the lipid composition of each leaflet (Fig. 3) [21]. The external leaflet of the plasma membrane (exoplasmic, E face) and the lumenal leaflets of internal organelles are highly enriched in choline-containing lipids such as PC and sphingomyelin (SM). In contrast, the cytoplasmic leaflet (protoplasmic, P face) is rich in amine-containing glycerophospho-lipids such as PE and phosphatidylserine (PS). Other minor phospholipids, such as phosphatidic acid (PA), phosphatidylinositol (PI), phosphatidylinositol-4-monophosphate (PIP) and phosphatidylinositol-4, 5-biphosphate (PIP2), are also enriched on the cytoplasmic face of the membrane where they participate in cell signalling [22, 23]. Interestingly, the membrane of the endoplasmic reticulum shows a symmetric lipid distribution and it primarily contains unsaturated glycerolipids that provide flexibility, and that facilitate the incorporation of newly synthesized proteins [24]. This is a clear example of how the same components may combine differently to yield different lipid organizations with features that adjust to their specific needs. Membrane heterogeneity is further achieved by lateral asymmetry in which membrane regions (basal, lateral, apical), and different specialized membrane regions or microdomains (lipid rafts, caveolae, coated pits, synaptosomes, etc.) [9, 25 and references therein] extend the mosaic nature of membranes. The formation of these domains in part results from the non-ideal mixing of lipids in membranes and in some cases, it is enhanced by the participation of certain cytoskeletal structures that underpin the lipid bilayer and that restrict the traffic of proteins and lipids.
3.
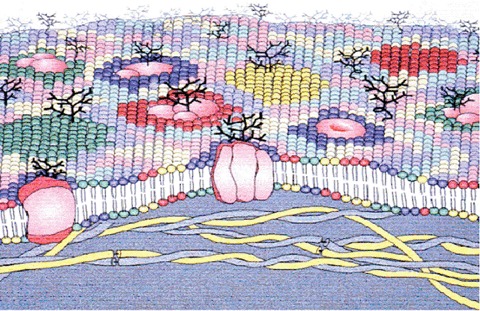
Schematic illustration of a biomembrane, depicting membrane lipid asymmetry as well as microdomains enriched in particular lipids and those induced by membrane proteins.
Cells possess complex mechanisms to control the specific lipid composition of membrane, imposing directionality and selectivity to the mobilization of lipids. Several specialized enzymes, such as flippases, floppases and scramblases, are responsible for maintaining this precise membrane lipid distribution [24, 26]. Indeed, the heterogeneous distribution of lipids is critical for the correct physiological homeostasis of cells. Accordingly, the loss of transmembrane lipid asymmetry and the concomitant exposure of PS to the external milieu occurs upon induction of programmed cell death (apoptosis) [27], or on platelet activation and aggregation [28]. Externalization of PS changes the cell surface charge, as its negative net charge at physiological pH alters the electric properties of the external leaflet, mainly influenced by the glycocalix. The net result of this process is an alteration of cell–cell interactions that might be involved in the conversion to a procoagulating state [29], increased adhesion and aggregation [30] and recognition by phagocytic cells [31]. Although these processes are essential for normal cell development and homeostasis, unregulated loss of PS asymmetry may contribute to the development of heart disease, stroke and diabetes [30].
The organization of specific domains is an important aspect of membrane structure that has recently received increasing attention. Because lipids have significant lateral mobility, one might expect that they would be homogenously distributed in most membranes. However, both model and biological membranesexhibit non-ideal mixing in systems containing two or more elements [32, 33]. The physicochemical forces involved in this lateral segregation into microdomains are discussed below (Fig. 3). Although various different domains are known to exist, research has focused on lipid rafts for many years. From a practical point of view, a lipid raft can be defined as a single, detergent-insoluble glycolipid-enriched (DIG) membrane fraction, with a high content of cholesterol, glycosphingolipids, SM and proteins. Although some authors also consider caveolae to be lipid rafts, others consider them as a different kind of cholesterol-free sphingolipid containing microdomain with a different protein and lipid composition. Thus, whereas lipid rafts are planar domains with high levels of glycosylphosphatidylinositol (GPI)-anchored proteins and deficient in caveolin, caveolae are cell surface invaginations stabilized by structural proteins such as caveolins and deficient in GPI-anchored proteins [34].
Although still controversial, studies using fluorescence resonance techniques have estimated rafts to have a mean diameter between 30 to 50 nm [35] and several hundred micrometers [36]. It was earlier suggested that rafts moved within the exofacial leaflet of the membrane bilayer [37], although current evidence suggests that rafts also extend through with the cytofacial leaflet due to the organization of lipids associated with the presence of membrane signalling proteins such as rho-A, fyn and the interleukin receptor IL2R-β[38–40]. GPI-anchored proteins are very abundant in rafts [41, 42] but they do not span the membrane bilayer and therefore, they probably have little effect on the organization of the cytosolic leaflet. On the other hand, rafts contain unusual amounts of ethanolamine plasmalogens, PS [43] and the GM3 ganglioside [41].
Caveolae are cell surface invaginations that are usually smaller than lipid rafts, the diameter of their opening at the cell surface typically ranges from 60 to 80 nm. Caveolae contain more free cholesterol than lipid rafts (with respect to sphingolipids) [41, 43], whereas GM3 is practically undetectable [41]. The main feature of caveolae is the presence of caveolin-1, a structural protein present in the form of high molecular weight oligomers, while a second caveolin (caveolin-2) is also often present. When compared to rafts, GPI-anchored proteins are largely or completely absent from caveolin-containing microdomains [41, 42]. Significantly, a wide variety of receptors and signalling proteins have been co-purified with caveolins: receptor kinases, platelet-derived growth factor receptor (PDGF-R), insulin receptor, shc, h-Ras, etc. Protein kinase A, adenyl cyclase and several iso-forms of PKC have also been recovered from sub-cellular fractions of caveolae [44].
The formation of microdomains within the membrane allows the selective incorporation or exclusion of specific proteins, providing a mechanism to govern protein–protein and protein–lipid interactions [45]. Numerous studies have implicated rafts in the compartmentalization, modulation and integration of signalling events, providing platforms for the assembly of cell surface receptors and their downstream signalling proteins [46]. Along these lines, SNAREs (soluble N-ethyl-maleimide sensitive factor attachment protein receptor) are known to concentrate in cholesterol-dependent microdomains that define docking and fusion sites for the exocytosis and release of neurotransmitters, hormones, enzymes and other proteins or small molecules [47]. Caveolae have also been implicated in clathrin-independent endocytosis of GPI-anchored proteins and glycosphingolipid-binding toxins [48, 49].
Why so many different lipids?
Lipids are by far the chemically most diverse class of biomolecules, with an average portfolio of a eukaryote cell being comprised approximately 1300–1500 different species. Moreover, different cell types, such as liver parenchymal cells and brain cells have different lipid compositions, as do different cellular organelles. Although some of this diversity is likely to result from diet-induced variation, the overall patterns are actively maintained by the cells, and, accordingly, require significant metabolic energy input and the presence of a large collection of enzymes and transfer proteins, responsible for the active control of lipid compositions and dynamic distribution within a cell. The above, together with the discovery of bioactive lipids (such as platelet activating factor, PAF), and recognition of the key involvement of lipids (e.g. PI, diacylglycerol [DAG] and ceramide) in cellular signalling cascades readily makes obsolete the view that lipids are just mere building blocks for making impermeable membranes to provide the cell with distinct compartments.
There are now extensive efforts to establish spatio-temporal, organelle level compositional patterns of lipids and to correlate these to the physiological states of cells, approaching cell behaviour from the point of view of lipidomics. With the emergence of these data it becomes mandatory to understand the mechanisms pertaining to the biological activities of lipids. Lipids represent the paradigm for molecular self-assembly, mainly driven by their amphiphilicity, and biophysical studies conducted during the last four decades or so have demonstrated these assemblies to form, depending on the lipid in question, a range of different phases, sensitive to factors including temperature, pressure, ions, hydration, small molecules (e.g. metabolites and drugs) and pH. Exploration of the behaviour of lipid mixtures, consisting of only two to three species has further shown membranes to possess a rich scale of 2- and 3-D organization on different length- and timescales. Taken the complexity of biomembrane compositions and the fact, that they are at thermodynamic non-equilibrium (because of membrane potential, for instance), it is obvious that we are still in the early-phases in lacking a true, molecular and system level understanding of biomembranes and the roles played by different lipids. Regarding the latter, the effects of lipids can be divided into two fundamentally different categories. The first type is (i) the recognition and binding of lipids as individual molecules by specific proteins, accommodating lipids as ligands. A good example is the PAF receptor, that belongs to the GPCR family [1]. This mode of action of PAF represents a ‘classical’ protein–small molecule interactions and does not assign any role to the collective membrane properties of the lipid agonist. The other, much more poorly understood area relates exactly to the latter, (ii) lipid bilayer biophysical properties and the impact on these by different lipids, further influencing membrane and membrane protein functions, as well as 2- and 3-D organization of the membrane, including its proteins [50, 51]. A good example is provided by DAG, which can constitute up to 10 mol% of the membrane lipids in transformed cells. Although tentative scenarios have been forwarded to explain the coupling between the physiological state of the cancer cell and the physical properties, such as imparted on cellular membranes by DAG [15, 52, 53], we are still missing conclusive mechanisms. Nevertheless, it is clear that such a high content of DAG necessarily has profound effects on the membranes of transformed cells, including the functions of membrane proteins such as PKC, activated by this lipid. Accordingly, to avoid lipidomics to remain mere cataloguing it is essential to be able to connect the individual lipid species to the overall collective bio-physical properties of membranes and their involvement in cellular physiology and pathophysiology, in addition to the recognition of possible novel receptors and effectors for specific lipids. It is the former, biophysical approach on lipidomics, which is outlined and discussed below. This approach thus provides a partial answer to the question on the chemical diversity of lipid structures. For instance, the different acidic phospholipid species as well as the cationic lipid sphingosine allow complex regulation of the protonation behaviour by surface change density, affecting electrostatic interactions on membrane surfaces. Moreover, these lipids also differ in their affinity for divalent metal cations, such as Ca2+, thus imparting sensitive to the latter to particular membranes. As membrane electrostatics are further intimately coupled to factors such as acyl chain saturation controlling lipid phase state and lateral organization – these already seemingly simple chemical variations created a rich scale of structures, sensitive to specific environmental variables and controlling membrane protein functions in a highly cooperative manner.
Lipid mixing and demixing
Lipids impact not only the bulk biophysical properties, such as rates of lateral diffusion, but also the dynamic organization of these assemblies. A timely example is the current surge of interest in lipids and their involvement in the lateral heterogeneity and microdomains in biomembranes (Fig. 3), together with the recognition of the large variety of biomembrane functions controlled by this dynamic organization [51, 54]. Membrane microdomains (‘rafts’) were discovered already in the 1970s and early 1980s using different approaches [55–63] and, importantly, were attributed to the physical properties and organization of lipid mixtures [14, 58, 59]. In this regard, the dependence of the lipid phase transition temperatures on the extent of acyl chain unsaturation and chain lengths as determinants of lipid–lipid and lipid–protein interactions in driving lateral demixing are of importance (e.g. myristoylated versus palmitoylated proteins, [54]). Further along these lines of particular current interest are SM and cholesterol, which have been shown to segregate form domains in cells [60] as well as in model membranes [64, 65]. The driving forces for the phase separation in this lipid mixture are still controversial. One possibility is hydrophobic mismatch, which has been demonstrated to drive the formation of microdomains in reconstituted membranes [66, 67]. Accordingly, with the phospholipid acyl chain order augmenting due to cholesterol and thus causing an increase in the bilayer thickness, phase separation would take place, with the formation of the cholesterol-enriched liquid ordered lo phase [68] decreasing the free energy penalty due to hydrophobic mismatch (line tension) between these and the liquid disordered (ld) cholesterol-poor regions [69]. Because of the dynamic nature of the lo phase and fast exchange between the lo and ld domains, these structures can be expected to have a major impact on the lateral organization of integral membrane proteins.
Another example of lipid-driven membrane demix-ing is provided by ceramide, which is involved in cellular signalling of apoptosis, programmed cell death [70]. This lipid has a pronounced tendency for self-association, caused by intermolecular hydrogen bonding, further promoted by the weak hydration of its headgroup and tight packing of the saturated hydrocarbon chains. The segregated ceramide-enriched phases have significantly elevated chain melting temperatures and are crystalline in nature at physiological temperature [71]. Accordingly, the properties of ceramide- and cholesterol-induced phases are distinctively different and they can be expected to have very different impacts on the lateral organization of membrane proteins, for instance. The tight packing of ceramide further manifests another important property characterizing membranes, bending rigidity, pertaining to the energy required to change the shape (curvature) of a membrane. Although membranes formed by unsaturated PCs, for instance, are very soft, with intense fluctuations in their shape caused by thermal energy, membranes enriched in ceramide are rigid. Ceramide illustrates also another important property of lipids, spontaneous curvature. More specifically, depending on their shapes [14] and more exactly, on their effective shapes [52], the curvature of the surfaces formed by lipids can be negative, zero or positive. Because of their small size and tight packing of the ceramide headgroups by intermolecular hydrogen bonding, ceramide-enriched membrane domains have negative spontaneous curvature, ultimately favouring the formation of the inverted hexagonal (HII) phase (Fig. 4). The negative spontaneous curvature of the ceramide-enriched membrane domains, together with their high bending rigidity, have interesting consequences, such as those given below. Ceramide can be enzymatically generated in membranes from SM, which has a strongly hydrated phosphocholine headgroup attached to ceramide. In contrast to ceramide, SM readily mixes at physiological temperatures with liquid disordered, unsaturated PCs. However, upon the conversion of SM to ceramide by sphingomyelinase (SMase), the reaction product segregates into microdomains [72] with negative spontaneous curvature and high bending rigidity. As a consequence, these microdomains start bending the membrane, ultimately causing shedding of ceramide-enriched vesicles from the original bilayer membrane [73, 74]. Accordingly, the formation of ceramide in biomembranes can be anticipated to cause lateral segregation of membrane constituents and be responsible for the membrane blebbing in apoptotic cells, in essence causing both 2-D as well as 3-D reorganization of membrane with its embedded proteins [75].
4.
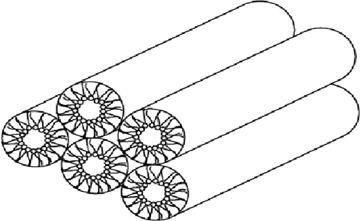
Inverted HII hexagonal phase composed of water-filled tubes with the lipid acyl chains pointing outwards. Different cellular membranes with a planar geometry invariably contain a variety of lipids and would therefore form such a phase. The presence of these lipids imparts frustration to the membrane, with a high packing density in the hydrocarbon region of the bilayer. Adapted from [51].
Lateral pressure
Intimately related to membrane spontaneous curvature is the lateral pressure profile [76]. This concept is highly useful in understanding of several characteristics of membranes. In brief, approaching the surface of a PC bilayer from the water phase and recording the prevailing forces acting on the lipid assembly, there is first a zone with repulsive potential between the strongly hydrated headgroups, such as for PCs and SMs (Fig. 5). Adjacent to the above there is a zone where the hydrophobic effect manifests as interfacial tension, with hydrophobicity of the lipid hydrocarbon chains restricting their contacts with water molecules. Whenever thermal motion and repulsive interactions increased the exposure of the acyl chains to water, its entropy decreased. The resulting tension balances not only the steric repulsion between the headgroups but also the repulsion between the acyl chains, prevailing in the hydrocarbon region of the bilayer. Because of these forces are acting on very narrow zones, the pressure can be considerable, estimated to be hundreds of atmospheres.
5.
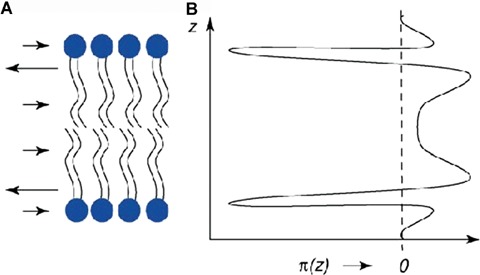
Lateral pressure profile for a lipid bilayer (left), with surface tension being balanced by steric repulsion between the head-groups and acyl chains. See text for details. Adapted from [51].
The lateral pressure profile can be influenced in a number of ways, e.g. by small membrane partitioning molecules as well as by the lipid composition. Introducing a lipid with a large headgroup for example, augments repulsion at this level and therefore reduces the entropic pressure between the chains. Consequently, the acyl chain order and membrane thickness decrease. Introducing a lipid with a small headgroup, such as unsaturated PE (e.g. palmitoyl oleoil phosphatidylethanolamine [POPE]) has the opposite effect, with the contribution of the repulsion between the chains in counteracting the interfacial tension becoming more significant. Under these conditions, with moderate contents of POPE, the membrane remains lamellar, yet its free energy increases as the free energy minimum would require negative spontaneous curvature. Such membranes are defined as frustrated phases and this can be expected to have interesting consequences for lipid–protein interactions [15]. It has been demonstrated that it is this frustration, which activates PKC by inverted hexagonal (HII) phase (Figs. 2 and 4) forming lipids (with high negative spontaneous curvature), not the formation of this phase per se (for a review, see [15]). High internal pressure within the bilayer can further result in a novel type of lipid–protein interaction, extended lipid anchorage, with one chain of a membrane embedded lipid extending out from the membrane and accommodating into a hydrophobic cavity of a protein, thus attaching the latter to the membrane surface without protein intercalation into the bilayer [77, 78].
Unfortunately, there are no direct experimental techniques available for the quantitative assessment of the lateral pressure profile. Interestingly, recent computer simulations suggest cholesterol to have a profound impact [79]. As the lateral pressure profile should profoundly influence both lipid–lipid and lipid–protein interaction potentials, it is likely that this characteristic property of membranes is under stringent control, as is evidenced by the crucial role of the control of the membrane contents of lipids with negative spontaneous curvature, required for the proper growth of microorganisms [15, 52, 53].
Lastly, it is worth mentioning the difference between the lateral pressure profile and equilibrium lateral pressure, the latter simply being the numeric value where the forces are at equilibrium for a tension-free bilayer. Both theoretical and experimental studies (including relative efficiencies of different phospholipases A2 in hydrolyzing erythrocyte phospholipids and comparison with their action on lipid monolayers at varying lipid lateral packing densities) have yielded an estimate of approximately 30–35 mN/m for the equilibrium lateral pressure of biomembranes. Although this parameter is unlikely to vary significantly with lipid compositions found in membranes, their susceptibility to osmotic forces (membrane stretching) has been shown to vary markedly depending on the lipids present [80, 81]. Reflecting lipid acyl chain compositions, for instance, the extent of area increase caused by equal osmotic pressure gradients across a bilayer should greatly increase upon increasing cis-unsatu-ration and shorter chain lengths. As osmotic forces are tightly controlled in cells and regulate proteins such as stretch-sensitive ion channels and phospholipases, these issues would deserve to be investigated in more detail. Of the latter group of enzymes, phospholipase A2 represents the rate-limiting step in the synthesis of prostaglandins from arachidonic acid, making it a key player in the control of inflammation and thus emphasizing the importance of understanding the biophysics governing biomembrane functional properties. The control of this enzyme by osmotic stretching of the membrane provides an example how a physical property of a lipid bilayer is converted directly into a biochemical signal [82].
Surface electrostatics
From the point of physical chemistry the importance of electrostatics to the assembly of membranes is obvious. Thoroughly studied examples are cationic proteins or proteins with clusters of cationic residues, avidly associating with the negatively charged acidic phospholipids, such as PS and phosphatidylglycerol, PA and cardiolipin. In addition to pure Coulombic attraction specific interactions between particular lipid structures and proteins are expected. The surface electrostatics can be coupled with phospholipid phase behaviour. Accordingly, phase separation of anionic lipids into microdomains with high enough negative surface charge density can be used to control the membrane association of cationic proteins [83]. It is also worth noticing that electrostatics in biomembranes is, in general, dominated by the above negatively charged lipids. Yet, also cationic lipids are found, the most abundant being sphingosine [84] and studies with model membranes have shown its association with acidic phospholipids to regulate peripheral lipid–protein as well as lipid–small molecule interactions [85]. More detailed studies on model membranes have demonstrated the interplay of surface charge density and the dissociation behaviour of the acidic phospholipid headgroup to exert a pronounced impact on lipid–protein interactions evident as distinctly different binding modes of cytochrome c to protonated and deprotonated phospholipid [86, 87].
The importance of understanding in detail the role of electrostatics in lipid–protein interactions is exemplified by studies on the mechanisms of action the so-called antimicrobial peptides (AMPs), constituting the first line of defense of multicellular eukaryotes against invading microbes. More specifically, whereas other modes of action are also involved, it is now thought that one of the principal targets for these short, cationic and amphiphilic peptides are acidic phospholipids in the outer surface of bacteria. Following the association of AMPs to the surface, charge neutralization allows for them to aggregate, leading to the formation of membrane-permeabilizing structures. In this regard, it is of interest that AMPs and acidic phospholipids form Congo red staining fibres, the diagnostic hallmark of amyloids [88, 89]. In the absence of lipids, the formation of these amyloids in vitro is generally rather slow and requires slightly acidic pH and low dielectricity (e.g. 30% trifluoroethanol). Accordingly, the relevance of these conditions to the in vivo misfolding and emergence of amyloid has been questioned. It should be noted that surface pH of approximately 5.2 has been estimated for membranes containing 20 mol% acidic phospholipids. Simultaneously, the membranes also provide a low dielectricity as well as a highly anisotropic environment, which causes a strong alignment of associated peptides and proteins [90]. In keeping with the above, a fast formation of amyloid fibres in the presence of acidic phospholipid containing liposomes has been demonstrated in vitro for a number of cationic peptides and proteins. It is of particular interest that these include, in addition to AMPs, proteins (for instance histone H1, cytochrome c, α-lactalbumin, lysozyme and endostatin) that are cytotoxic and trigger apoptosis in eukaryotes. Accordingly, it has been suggested that the formation of amyloid ‘protofibrils’would underlie the mechanism of toxicity of these peptides and proteins, in addition to possible other modes of action [86, 88–91]. Taking into account that amyloid formation is directly implicated in major diseases such as type 2 diabetes, Alzheimer's, Parkinson's and prion diseases, it is obvious that development of detailed understanding of the molecular level mechanisms involved would be of paramount medical importance.
Role of lipids in cell function
We are still far from formulating a general hypothesis to explain the variety of lipids found in membranes because most membrane functions that we are aware of could be fulfilled with just a few lipid species. However, the ever-increasing number of studies providing information regarding the different functions of specific phospholipids is helping us to understand why membranes are formed by hundreds of different lipid molecules. Phosphoinositides (PIs) are important in cell signalling and vesicle formation, key events in neurotransmission and in the transit of vesicles from the endoplasmic reticulum to Golgi [92]. Moreover, PIs participate in a coordinated manner with PE during cell division, whereby changes in PIP2 production inside the cleavage furrow occur concomitant with the accumulation of PE in this structure during cytokinesis [93, 94]. This type of association between lipids could be considered as a unique membrane domain that arises transiently to help the cytoskeletal machinery produce two daughter cells. On the other hand, nuclear PS and PI are involved in cell cycle regulation because they stimulate the synthesis of DNA polymerase α[95, 96]. Finally, the presence of anionic phospholipids, in particular PS, is required for the assembly of the pro-thrombinase complex on membrane surfaces during platelet activation [28] and as mentioned above, exposure of PS at the exofacial leaflet occurs in the initial steps of apoptosis and blood coagulation [97, 98]. These lipids also participate in more complex activities, such as the docking of peripheral proteins to membranes [1, 4, 99]. Cholesterol, SM and gangliosides also participate in the formation of lipid domains. In a similar fashion, membranes have a high number of different fatty acid moieties and although thicker microdomains may require long saturated fatty acid moieties, thinner domains or some membrane proteins may need shorter or unsaturated acyl chains. Likewise, the transmembrane regions of integral proteins may have specific lipid requirements or at least display certain preferences.
An additional degree of complexity in membrane lipid functions lies in the metabolic relationship between phospholipid species. There is a relatively large number of phospholipases and phosphatases that specifically participate in the interconversion of phospholipids and that in turn, can modulate the activity of those enzymes. The cascade of phospholipase D (PLD) activation is a clear example of this complexity (Fig. 6) [100]. The initial step of the cascade involves the agonist-induced and GPCR-mediated activation of phospholipase C (PLC) to hydrolyze phosphatidylinositol (PIP2) into DAG and IP3. The former activates PKC and the latter induces the release of Ca2+ into the cytosol through IP3-activated channels, which also activates PKC [101]. Interestingly, mammalian PLDs also require PIP2 as an essential cofactor for their enzymatic activity [102]. PLD is present in Golgi membranes [103] and it hydrolyzes PC to PA, a downstream effector of the small guanosine triphosphate (GTP)-binding protein ademine diphosphate (ADP)-ribosylation factor (ARF-1) [104]. Finally, PA can also be converted to DAG by PA phosphatases, whose activity is involved in both lipid metabolism and glycerolipid signalling [105]. The complexity of the signalling and metabolic pathways in which phospholipids participate, as well as the cross-talk between these cascades, emphasizes the existence of highly sophisticated regulatory mechanisms that remain to be fully understood. Together, these studies demonstrate the role of membrane lipids in a large variety of cellular functions and emphasize the close relationship between membrane lipid composition and function. In addition, the number of existing human pathologies related to alterations in lipid metabolism is evidence of the importance of membrane lipids and their role in signalling pathways (Table 3).
6.
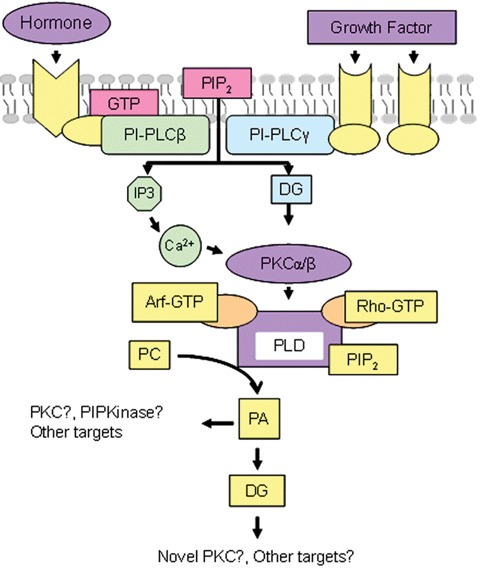
Phospholipase cascade of PLD activation and amplification of diacylglycerol production. Abbreviations: PI-PLC, phosphatidylinositol-specific phospholipase C; DG, diacylglyerol; PC, phosphatidylcholine; PA, phosphatidic acid; PKC, protein kinase C; PIP2, PI-4,5-bisphosphate and PIP kinase, PI phosphate kinase. Adapted from [100].
3.
Human pathologies and lipid abnormalities
| Disease | Membrane abnormality | Proposed molecular mechanisms |
|---|---|---|
| Cardiovascular (Hypertension) | Changes in membrane phospholipid and cholesterol levels, changes in fatty acid levels | Regulation of the membrane structure with concomitant alteration of membrane signalling, protein localization and activity |
| Cardiovascular (Sudden Cardiac death) | Changes in membrane levels of saturated and unsaturated fatty acids | Alterations in δ-6-desaturase activity in the coronary artery wall |
| Cardiovascular (Cardiac hypertrophy) | Changes in membrane levels of triacylglycerol species and other lipids | Changes in cell signalling and impaired triacylglycerol availability |
| Cancer (pathologic proliferation) | Changes in membrane fatty acid levels | Altered cell structure and function (including cell proliferation) |
| Cancer (multidrug resistance) | Alterations in the levels of phospholipid species (PS*) | Reduced drug intake and facilitated drug removal from cancer cells |
| Respiratory pathologies | Changes in the lipid composition of membrane microdomains | Alterations in mechanotransduction and other signalling processes |
| Renal Pathologies | Increased lipid peroxidation and augmented proportions of saturated fatty acids caused by haemodialysis | Increased cellular oxidative stress |
| Alzheimer's disease, Aging and neurodegeneration | Reduced levels of PUFA† in brain cell membranes | Altered expression of transthyretin and other genes related to learning, cognitive and integrative functions |
| Inflammation, autoimmune and related diseases | Release of pro-inflammatory lipids from membranes | Formation of eicosanoids from arachidonic acid, changes in membrane fluidity, changes in membrane lipid–protein interactions |
| Infectious diseases | Increased ceramide-enriched membrane domains | Modified membrane lipid domains act as platforms for a wide variety of virus, bacteria and parasite infections |
| Schizophrenia | Decreased proportion of PUFA† in membrane phospholipids | Myelin-related and neurotransmitter signalling dysfunctions |
| Obesity | Changes in membrane lipids | Alterations in membrane protein function |
| Alcohol-induced fetal damage | Changes in cell membrane composition | Various cell functions alterations |
| Coagulation (Scott Syndrome) | Defective PS* flip-flop translocation in membranes | Impaired interaction of coagulation factors and blood cell membranes |
| Triose Phosphate Isomerase deficiency | Lack of symptoms is associated with modification of membrane lipids and lipid fluidity | Changes in membrane protein–lipid interactions and in the activity of certain enzymes |
Adapted from [9].
PS, phosphatidylserine and †PUFA, polyunsaturated fatty acids.
Lipid influence in transmembrane protein function
The previous paragraphs have been focused on the lipid composition of membranes and how lipids determine the properties of membranes, defining the formation of different types of membrane structures and microdomains. Lipids, and the structures they form, participate in the interaction of proteins with membranes, delineating their functions and modulating their structure. The next paragraphs will describe the interactions between lipids and both peripheral and transmembrane proteins and how lipids intervene in cell functions controlled by such proteins.
Transmembrane proteins are highly diverse but have one property in common: they contain one or more hydrophobic regions that transverse the membrane bilayer and therefore these proteins become intimately exposed to membrane lipids. Such exposure results in a variety of lipid–protein interactions that most frequently reveal themselves as highly relevant to the membrane protein functioning. The observed effects may sometimes be caused by a lipid-induced misfolding or misassembly of the protein within the membrane but more frequently, effects of lipids on the function of properly folded and assembled transmembrane proteins have been documented. Nonetheless, despite the extensive information obtained on the functional dependence of many membrane proteins by its surrounding lipids [106, 107], this has been a controversial issue over the years and the mechanisms by which such membrane protein modulation is exerted by the different lipid classes still remain unclear. Two general cases could be considered. On the one hand, when the interaction with the lipid is less specific, there are still lipid-associated parameters defining physical attributes of the biological membrane, which are known to modulate membrane proteins. These include lateral pressure [76, 108, 109], membrane fluidity [110], bilayer thickness [111] surface charge distribution [112] or the segregation of membrane microdomains or ‘rafts’[113], all of which may affect the structure and/or the function of intrinsic membrane proteins. On the other hand, when there is a sufficiently high lipid specificity in the interaction, there could be more direct effects through binding of lipids to defined sites on the transmembrane portion of the protein [114–118], which has led to postulate a possible role of certain lipids as peculiar ‘allosteric’effectors of the proteins.
In an attempt to illustrate some of these phenomena we have focused on reviewing the subject with regard to ion channels and receptors, two classes of very important integral membrane proteins, which usually allow for very precise monitoring of their functional activities and in some cases, have their structure solved at high resolution.
A prokaryotic potassium channel: KcsA
One bacterial K-channel that has been identified, cloned [119] and its structure determined by X-ray crystallography [120] is the KcsA channel from the Gram-positive soil bacterium Streptomyces lividans. KcsA is a homotetramer, each subunit containing 160 residues with two transmembrane α helices separated by a P-loop, and cytoplasmic N- and C-terminal domains.
Previous studies have shown that specific membrane lipids have a strong influence on KCSA assembly and stability [109, 121]. In particular, the presence of anionic lipids like PA or PG influence the stability and folding properties of the potassium channel KCSA [122]. This lipid specificity is also observed in lipid-binding studies [123]. Moreover, the high-resolution structure of KcsA shows tightly bound lipids within the crystal; one modelled as nonan-1-ol and the other located between transmembrane α-helices at monomer–monomer interfaces, modelled as a DAG with one C14 and one C9 chain. Because purified KcsA contains approximately 0.7 phosphatidylglycerol molecules per KcsA monomer, the lipid modelled from the X-ray as a DAG is probably a phosphatidylglycerol whose headgroup is too mobile to be resolved. More recently, such lipids have been found essential for full refolding and tetramerization of unfolded KcsA in vitro[124].
Functional studies with KcsA reconstituted into liposomes suggest that a functional channel could only be obtained in the presence of anionic phospholipids, with little or no specificity for the different lipid classes, which could either be phosphatidylglycerol, PS or cardiolipin [125]. More recently, Lee and coworkers have shown positive cooperativity between the open channel probability and the PG content [126].
The functional and structural results mentioned above indicate that the modulation of KcsA by membrane lipids occurs through specific interactions between the protein and tightly bound PG. Interestingly, there are reports on the detection on lipid bound in the crystal structures of other membrane proteins [127–129] and, therefore, it is likely that such proteins might be regulated by lipid in a manner similar to that of KcsA.
Mechanosensitive channels: Msc
The mechanosensitive channels are a structurally heterogeneous protein family widely distributed in archaea, prokaryotes and eukaryotes. These channels are attractive models to study lipid–protein interactions because their function is to couple tension in the lipid membrane to protein conformation [130]. Their topology is very diverse including two pore domain channels, that conduct potassium [131, 132] or sodium ions [133], the TRP (transient receptor potential) channels, such as TRPC1 and TRPY, that are non-selective cation channels [134] and the prokaryotic Msc channels that conduct potassium ions [135].
Bacterial mechanosensitive channels are activated by increasing tension in the lipid bilayer of the cytoplasmic membrane, where they transiently create large pores in a controlled manner. When reconstituted into a bilayer system, the mechanosensitive channel can be opened from its closed state(s) by the addition of LPC to one monolayer of the bilayer. This changes the bilayer curvature and increases the tension, which results in tilting the transmembrane helices and lowering the threshold for channel opening [136]. In similar studies using phospholipids with different acyl chain lengths to change the bilayer hydrophobic thickness, it is observed that thicker bilayers tend to stabilize the closed state of the channel, whereas thinner ones favour channel openings [137]. From these observations it follows that the opened and closed states of the channel are likely to differ in its hydrophobic thickness, such that changing the bilayer thickness should accommodate better to one of such states, and stabilize it accordingly.
In addition to the need of a good hydrophobic matching between the Msc protein and the lipid for channel function, it has been shown that there is a chain-length dependence of the lipid binding to the protein that is different in the two sides of the membrane. Such differences imply that the hydrophobic matching needed for Msc channel function likely involves bending of transmembrane α-helices, rather than simple tilting [138]. Therefore, in Msc channels and similar cases [139, 140] it seems that certain bilayer properties, such as the intrinsic curvature or the extent of hydrophobic mismatch, may influence protein function directly, without any specific interaction arising from a particular lipid class.
A voltage-gated potassium channel: KvAP
Voltaged-gated ionic channels (VGICs) are membrane proteins that transiently open a pore through the lipid membrane in response to changes in membrane potential. Kv channels are VGICs and play a critical role in a wide variety of physiological processes, including the regulation of heart rate, muscle contraction, neurotransmitter release, neuronal excitability, insulin secretion, epithelial electrolyte transport, cell volume regulation and cell proliferation. Gating of the Kv channels in response to membrane potential has been correlated with the movement of the positively charged amphipatic S4 transmembrane segment. In the case of KvAP, a prokaryotic voltage-gated channel, diverse studies have demonstrated that the phospholipid membrane provides an appropriate environment for the energetic stability and operation of the voltage-sensing machinery, perhaps by contributing stabilizing interactions between positively charged voltage sensor residues and negatively charged lipid phosphodiester groups [141]. Therefore in the Kv channels and similar cases [142, 143] it seems that a bilayer feature, i.e. the charge provided by the phospholipid headgroups, influences protein function by controlling electrostatic interactions at the lipid–protein interface.
Nicotinic acetylcholine receptor (nAcChR)
Ligand-gated ionic channels are membrane proteins that transiently open a pore through the lipid membrane in response to neurotransmitter binding. The nAcChR is one of the best-understood members of this family. The nAcChRs present in the neuromuscular synapses are heteropentamers comprised four different but highly homologous subunits (for reviews see references in [144]). Each subunit contains an extracellular N-terminal domain (which include the ACh binding sites), four hydrophobic transmembrane domains (M1–M4) and a small intracellular C-terminal domain. Upon activation by agonist, nAcChRs transiently open a cationic channel responsible for the initiation of postsynaptic membrane depolarization.
Extensive biochemical studies have demonstrated that the ability of the nAcChR to support ion channel function requires the presence of specific lipids. These effects in nAcChR function may be exerted through binding to specific sites of the protein or by modification of bilayer physical properties. Previous results have demonstrated that membrane lipids interact differentially with nAcChR. For example, sterol, PA and fatty acid spin labels have a relative high affinity for nAcChR compared with other spin-labelled phospholipids [145].
Additionally, several lines of evidence demonstrate the existence of more specific distinct lipid-binding sites, namely non-annular sites. McNamee and Lee used brominated lipids to partially quench the intrinsic fluorescence of the nAcChR to monitor contacts with the surrounding lipid in reconstituted membranes. They found that receptor quenching by PC was independent of the presence of cholesterol, but there is an additive quenching due to brominated cholesterol derivatives [118]. These results argue strongly for independent binding sites for cholesterol and phospholipids.
Although cholesterol may affect the nAcChR directly, it definitely has profound effects on structure of the membrane environment, most notably by changing membrane order or fluidity. In earlier studies both the agonist affinity and ion flux seemed to require an optimal fluidity [110]. However subsequent studies showed that although the ion flux activity of the nAcChR was strongly influenced by lipid composition [116], there was no correlation with membrane fluidity, as measured by steady state anisotropy of membrane probes [146]. Measurements of membrane fluidity showed that cholesterol further ordered membranes containing PC and PA, but another sterol, like androstanol, did not, although either one of the two sterols supported similar ion fluxes. Thus, in this case cholesterol exerts its effect on the nAcChR through direct interaction with the protein [115].
With respect to PA, in vitro studies with nAcChR reconstituted in lipid vesicles of controlled composition show that PA is among those phospholipids that bind the protein with a higher affinity, and it is also most effective in preserving nAcChR function [118, 145, 147], possibly through a stabilization of the resting versus the desensitized state of the protein [148]. Moreover, in PA-containing membrane, nAcChR leads to a dramatic increase in both the lateral packing densities and the gel-to-liquid crystal phase-transition temperatures of the reconstituted lipid bilayers [148, 149]. This strong interaction leads to the segregation of a PA-enriched domain from a complex mixture [149, 150].
The formation of a PA domain is the most likely explanation for the modulatory effects observed in vivo upon PA enrichment of oocyte membranes [151]. Moreover, similar PA domains critical for budding of viral particles, have been reported in vivo as the result of interaction between the host membrane and envelope viral proteins [152].
Regardless of the in vivo possible relevance, lipid modulation of the nAcChR seems to be exerted both through specific interactions of cholesterol with non-annular protein sites and by the protein-induced segregation of a PA domains optimal for protein functioning.
G protein-coupled receptors
GPCRs regulate a wide range of cellular processes, including the senses of taste, smell and vision, and control a myriad of intracellular signalling systems in response to external stimuli. These transmembrane proteins interact with extracellular signals, usually through the binding of small signalling molecules, which induce a change in the conformation of the receptor. Such change is transmitted to the cytoplasmic face of the protein and enables a coupling with an intracellular heterotrimeric G protein (GTP-binding protein). The intracellular G protein, in turn, acts as a signal transducer that regulates the activity of ‘effector proteins’ (e.g. adenylyl cyclase, guanylyl cyclase, PLC, potassium channels, etc.). Diseases such as certain forms of blindness, obesity, inflammation, depression, cancer and hypertension, among others, can be linked to malfunctions of GPCRs.
Rhodopsin, perhaps the best-characterized GPCR and the only one to have its structure solved at high resolution, is modulated by membrane lipids. This protein contains a membrane surface recognition domain that adopts an amphiphilic helical structure as a function of membrane composition, PS being most active in this regard. Such structural change mediated by membrane phospholipids may also contribute significantly to the optimal kinetic functioning of this prototypical G protein [153]. Also, it has been demonstrated that changing the bilayer composition produces changes in the rates of formation of both Metarhodopsin II and Metarhodopsin II-Gt complexes, demonstrating that the diffusion of receptor and G protein are sensitive to the lipid composition of the membrane [154].
Similarly, the angiotensin II receptor, which is also a GPCR, has a carboxy terminus that associates with the cytoplasmic face of the cell membrane via a high-affinity, anionic phospholipid-specific tethering that serves to increase the amphipathic helicity of this region [155]. Therefore, such associations with anionic phospholipids seem common in GPCRs and, as in the case of rhodopsin, it is possible that they might be relevant for receptor function. Many GPCRs have a lipid modification in the carboxy-terminal region that creates a fourth intracellular loop in their structure. In this context, palmitoylation/depalmitoylation regulates the coupling of the endothelin B receptor to different Gα proteins subtypes [156]. Studies on 5-HT4a serotonin receptor and the luteinizing hormone receptor in palmitoylation-deficient mutant mice, further support the importance of receptor palmitoylation as a regulator of GPCR-mediated signal transduction [157, 158], further demonstrating the relevance of protein–lipid interaction in the function of these integral proteins.
As indicated above, most drugs under pharmaceutical development are targeted at GPCRs, because of the great variety of functions they control and their involvement either in the aetiology of diseases or in reversion of pathological states. This fact highlights the relevance of these membrane receptors and the control of cell signalling, exerted at the plasma membrane, in the cell's physiology and human therapy.
Other examples
Table 4 shows other documented cases in which the activities of integral proteins (ion channels and transmembrane receptors) have been shown to be dependent on membrane lipids. Again, such examples include cases where lipid–protein interactions seem very specific, along with others in which the general properties of the lipid bilayer seem the determinant factor that influences protein function.
4.
Additional examples of interactions between ion channel or receptor proteins and membrane lipids
| Membrane protein | Observed lipid effect | References |
|---|---|---|
| Kv 2.1 | Cholesterol depletion favours inactivation | [329] |
| Kv 1.3 | Ceramide inhibits channel activity | [330] |
| Kv 1.5 | Targeted to caveola lipid rafts | [329] |
| Ca2+ activated K+ channels | Requires anionic phospholipids for channel activation Modulated by lipid bilayer thickness | [140] |
| KATP channel | Activation proportional to number of negative charges on the lipid head group | [331] |
| TREK | Activation by lysophospholipids as a function of acyl chain length and polar head group size | [332] |
| ENaC | Anionic phospholipids may mediate their regulation | [143] |
| TRP Ca2+ channels | The existence of a lipid domain modulated the activity of the channel | [333] |
| NMDA | Correlation between membrane tension (induced by either mechanical or chemical stimuli) and internal Mg2+ block | [334] |
| α7 nNAcChR | Target to lipid rafts in the somatic spines of ciliary neurons | [335] |
Non-permanent proteins in membranes
Association processes of proteins with membranes are not limited to constitutive membrane proteins, but rather include as well those that translocate or insert into a membrane at a specific juncture in their biological functions. The latter proteins are generally soluble and do not fold and assemble into membranes in a constitutive way. They develop instead the ability to insert and/or translocate into membranes under specific conditions and/or when exposed to lipid bilayers [159]. Thus in the living cell, a number of membrane proteins are permanently bound to the lipid bilayer, either as integral or as peripheral proteins (see above), whereas others will contact the membrane only under certain conditions, thereby remaining membrane bound, or returning promptly to the aqueous medium to which they belong (Table 5). This section deals with this kind of proteins that interact briefly with the cell membrane and with those that, becoming only occasionally in contact with the membrane, are irreversibly bound to it when they do. Both one and the other will interact in a more or less specific way with the bilayer and will cause some degree of bilayer perturbation. In both cases the nature of the interaction/perturbation will be directly related to the physiological or pathological role of the proteins. This heterogeneous group of molecules has been designated as non-permanent membrane proteins [160]. Figure 1 depicts a schematic representation of the various kinds of permanent and non-permanent membrane proteins.
5.
A classification of non-permanent membrane proteins (modified from [160])
| Type | Examples | References |
|---|---|---|
| (A) According to the reversibility of the membrane contact | ||
| (1) Proteins that interact reversibly with the membrane | Lipid transfer proteins Ceramide-activated protein kinase | [168, 179, 181] |
| (2) Proteins with very long-lived (irreversible) contacts | Bacterial toxins (e.g. aerolysin) Blood coagulation factors. | [187, 197] |
| (B) According to the nature (strength) of the interaction | ||
| (1) Proteins that interact weakly with the membrane (extrinsic-like) | TrwD Protein kinases C | [176] |
| (2) Proteins that interact strongly with the membrane: | ||
| (2.1) Without covalent modification of the lipid (intrinsic like) | RTX toxins Complement proteins | [196] |
| (2.2) With covalent modification of the lipid | Phospholipases Enzymes of lipid metabolism | [182] |
The subject of proteins that can exist either free or membrane bound has been studied in the past by several workers. Wilson [161] called them ‘ambiquitous proteins’, and was perhaps the first to present, in a systematic way, the idea that variation in intracellular distribution may represent a regulatory mechanism to suit changing metabolic needs. Burn [162] introduced the concept of ‘amphitropic proteins’ to encompass the wide group of proteins that associate reversibly with membranes under certain physiological conditions. Later, Nelsestuen and coworkers exemplified in protein kinases C and the annexins the paradigm of proteins that are found either in soluble or membrane-bound forms – their change in location having important physiological consequences [163]. Also among the precedents of this concept, the work by Wimley and White [164] should be mentioned. The latter authors achieved a quantitative description of the partitioning of peptides into membrane interfaces, by constructing an ‘interfacial hydrophobicity’ scale that has found important applications afterwards. Wimley et al.[165] defined as ‘non-constitutive’ the soluble proteins that exert a biological function by undergoing transient bilayer insertion.
Non-permanent membrane proteins may be classified either according to the reversibility of the membrane contact, or to the nature (strength) of their interaction with the host membrane (Table 5).
Proteins that interact reversibly with the bilayers
These proteins have in common that, within a timescale compatible with the turnover time of membrane components (up to tens of minutes), the protein binds the membrane, and then comes back to the aqueous medium. This group encompasses a large variety of proteins, with widely diverging kinetics of membrane binding. They are often, but not always, proteins with specific lipid-binding sites. At the limit of the fast exchange we should mention the ceramide-activated protein phosphatases 1 and 2A [166]. In fact, these proteins have never been isolated in a membrane-bound form, but they contain a ceramide-binding site in their catalytic subunit, and strong experimental evidence points towards a direct interaction with ceramide [167].
Other ceramide-binding proteins are known to exist transiently in the membrane-bound form, such as ceramide-activated protein kinase [168], some isoenzymes of PKC [169, 170] or c-Raf-1 [171]. It has been proposed that ceramide binds proteins in this group through their cysteine-rich domains [172]. Recently, ceramide-1-phosphate has been shown to bind a cytosolic phospholipase A2 via interaction with its C2 domain [173].
A large number of proteins are known that bind transiently the cell membranes, and have a specific binding site for DAG (see [174, 175] for reviews). Chief among these are the PKC isoenzymes belonging to the so-called ‘conventional’ and ‘novel’ families. In these enzymes, that otherwise are found in the cytosol, DAG binding induces their docking to membrane, in addition to causing protein activation. The movement from cytosol to membrane is called translocation and constitutes an important event in signal [176].
Some heat-shock protein (Hsps) are associated with membranes, although they do not contain transmembrane domain or signal sequences, thus they can also integrate the category of non-permanent membrane proteins (see below). Recent studies indicate that these proteins play an important role in membrane quality control and thereby potentially contribute to the maintenance of membrane integrity, especially under stress conditions [177]. The bacterial Min protein system prevents the aberrant localization of the cell division machinery at the cell poles. Min happens to be a non-permanent, or amphitropic protein, that binds anionic phospholipids and undergoes dynamic oligomerization on the membrane surface [178]. In this line, membrane lipid reorganization has been shown to play an important role.
Another important group of proteins that bind the cell membranes transiently is constituted by the so-called ‘lipid transfer proteins’[179]. These are intracellular proteins with the capacity to bind a lipid from one membrane and release it to a different one. Some recent results have revealed their role as mediators between lipid metabolism and cell functions. For example, the Sec14-superfamily of PI transfer proteins are important in linking phospholipid metabolism, membrane traffic and intracellular signalling networks [180], and the ceramide transport protein transports the sphingolipid to the trans-Golgi apparatus, where it is converted into ceramide-1-phosphate, in turn an activator in the synthesis of prostaglandins [181].
Several enzymes involved in phospholipid metabolism are, by conventional standards, cytosolic, although they exert their catalytic properties in the membrane-bound state. The various phospholipases are often good examples of cytosolic or extracellular proteins that become transiently docked to membranes to perform their catalytic roles. All of the above enzymes possess lipid-binding sites, either regulatory or catalytic, thus their transient binding to membranes must be mediated by those specific sites. This is not the case, however, of the transiently membrane-bound CTP: phosphocholine cytidyltransferase, an important enzyme in the synthesis of PC whose substrates and products are water soluble, and has no specific lipid-binding site [182]. Studies in which membrane binding and activity of the purified enzyme were measured in lipid vesicles [183] showed that membrane binding of the cytidyltransferase required anionic lipids.
Bacteriophage M13 offers an interesting and rather unique example of a protein that becomes inserted into the cell membrane in the way of the integral proteins, yet insertion is reversible. During viral replication the major coat protein of M13 (protein VIII) accumulates in the host cell membrane, in the form of an integral protein [184]. Inside the cell, the newly synthesized phage DNA is protected transiently by protein V. Viral extrusion occurs without lysis of the host cell and, simultaneously, protein V is released to the cytoplasm and protein VIII is taken up from the membrane. Protein VIII appears to exist in two conformations, α, or viral form, and β, or membrane form [184].
Proteins that interact irreversibly with the bilayers
In some cases, proteins that are not permanent constituents of the membrane become associated with it in an irreversible way. This occurs most often with proteins that are not encoded by the own cell genome, i.e. proteins from parasitic or toxic organ-isms. To mention a few examples, this is the case of equinatoxin II from the sea anemone Actinia equina[185], α-haemolysin from Escherichia coli[186], or the variety of ‘poreforming protein toxins’ reviewed by Parker and Feil [187].
In all these cases, after insertion, the proteins behave exactly like any other integral protein in the cell membrane. Note that the toxins are released as soluble proteins to the aqueous medium, and the mechanism by which they undergo the transition from soluble (water soluble) to integral (lipid soluble) proteins is a fascinating mystery, and a difficult one to unravel.
The above concepts can be illustrated with the example of aerolysin, a 47-kD channel-forming protein that contributes to the pathogenicity of Aeromonas hydrophila, a bacterium associated with intestinal and deep wound infections [188]. The toxin is secreted in the form of an inactive precursor called proaerolysin that can be proteolytically processed to aerolysin by a number of proteases including trypsin and furin. The active form of the toxin is a water-soluble molecule that can spontaneously oligomerize, producing heptamers that may then insert into lipid bilayers, giving rise to discrete hydrophilic channels. On the basis of the crystal structure of proaerolysin [189], which reveals extensive β-structure, it seems likely that the oligomeric form of the toxin contains an amphipathic β-barrel analogous to that observed in the heptamer of Staphylococcus aureusα-toxin [190]. Aerolysin has no apparent affinity for lipids until it has oligomerized, but the oligomer contains an exposed hydrophobic surface, presumably the outside of the amphiphathic barrel, and can insert directly from solution. How such a large structure as the barrel penetrates a lipid bilayer is a largely unexplored puzzle. Alonso et al.[191] have proposed that the aerolysin oligomer may overcome the barrier of the polar interfacial region by destabilizing the bilayer locally, causing the formation of non-lamellar structures. Using liposomes as the host membranes, those authors found that the inclusion of lipids that facilitate the lamellar-to-inverted hexagonal phase transition enhanced aerolysin insertion, whereas the opposite occurred when lipids that stabilize the lamellar phase were present.
An additional aspect that complicates the taxonomy of non-permanent membrane proteins is that there are examples of proteins that can bind lipid bilayers either reversibly or irreversibly, depending on the composition and physical properties of the bilayer. This is the case of E. coliα-haemolysin that binds reversibly bilayers in the gel state and irreversibly those in the liquid-crystalline state [192, 193]. Also, recent data [194] suggest that a fraction of α-haemolysin is secreted from the bacterium bound to outer membrane vesicles, from which the toxin is transferred to the target cells, thus α-haemolysin is reversibly bound to the secretion vesicles before becoming irreversibly inserted into the target membrane. Equinatoxin II binds reversibly pure PC bilayers, but irreversible insertion occurs when SM is incorporated in the bilayer composition [185, 195]. However, binding to cell membranes is almost always irreversible, and this is why these toxins are best classified within the group of non-permanent membrane proteins that become irreversibly bound.
In the previous paragraphs we have seen examples of bacterial or viral proteins that become part of the host cell membrane. The opposite may occur as well, when the host tries to attack an unwanted parasite, as in the case of complement-mediated bacterial killing. Complement proteins, of which more than 20 are known, exist in blood as part of the innate immune system. Proteins 5b and 9 of the complement system form the so-called ‘membrane attack complex’, that binds the outer membrane of Gram-negative bacteria. The bactericidal activity of complement is dependent upon C9, but currently it is not understood how this protein translocates across the periplasm and dissipates the potential across the inner membrane [196].
In some cases, proteins encoded by the same organism may bind irreversibly its cell membranes. This is the case of several proteins involved in blood coagulation, e.g. factor X [197], factor VIII [198] or factor V [199]. To mention but one example, factor V circulates in plasma as a single chain procofactor (330 kD), from which derive the two chains, respectively, 94 and 74 kD, of the active form, factor Va. Factor Va facilitates activation of prothrombin to α-thrombin by factor Xa. For this purpose, the serine protease factor Xa and factor Va assemble on a membrane surface in the presence of calcium ions. Insertion of factor Va in lipid bilayers containing neutral and acidic phospholipids has been studied by Majumder et al.[200]. Membrane association of factor Va appears to be a complex process involving both chains of the protein, changes in lipid packing, and in protein conformation. Interestingly, membrane binding also facilitates proteolytic degradation of factor Va [199].
Non-permanent membrane proteins can also be classified according to the nature (strength) of their interaction with the host membrane (Table 5).
Proteins that interact weakly with the membrane
These are proteins that remain membrane-bound through non-covalent forces other than hydrophobic interactions. Electrostatic and polar forces are the most relevant in this case. This group of proteins overlaps almost exactly with those that bind reversibly the cell membranes: many ceramide- and DAG-activated proteins involved in cell signalling belong to it. However, phospholipases and other enzymes of lipid metabolism that induce covalent modification of membrane lipids should not be included here, because for the most part they bind the membrane through strong, through transient, hydrophobic forces.
Proteins that interact strongly with the membrane
The proteins in this group are bound to the membrane mainly, but not exclusively, through hydrophobic forces. This does not mean that hydrophobic forces are particularly strong. When hydrophobic interactions are individually considered they are actually rather weak (of the order of 5 kJ/mol), their strength coming from the fact that, within the non-polar membrane matrix, a multitude of hydrophobic interactions join their forces providing an overall strong bond for the incoming protein. Among the non-permanent proteins that interact strongly with the membrane, an important distinction must be made between:
-
1
Proteins whose interaction does not lead to covalent modification of the membrane lipids and
-
2
Proteins whose interaction with membranes does lead to covalent modification of the lipids.
In other words, strongly bound non-permanent membrane proteins may or may not have an enzyme activity on the lipids. Among the proteins devoid of enzyme activity on lipids are the toxins mentioned above: α-haemolysin, equinatoxin II, aerolysin and many others, but note that there are bacterial toxins with phospholipase activity. The latter, together with the physiological phospholipases (including SMase) and other enzymes of lipid metabolism constitute the second subgroup.
From a thermodynamic point of view, cell membranes constitute an open system, far from equilibrium, in constant exchange of matter and energy with their environment. Molecules as important (qualitatively and quantitatively) as proteins may become in contact with the membranes, either for short or for long periods. The term ‘non-permanent proteins’has been suggested [160] to encompass the variety of such molecules that, at some stage, come to interact with any of the cell membranes. Some of them are originated by the own cell genome, others arise from a foreign genome. New methods to study the interaction of the membrane with these ‘visitors’, particularly with those that favour very short visits, will have to be developed. Our view of the structure and dynamics of cell membranes will have to be gradually broadened, to encompass the increasing number of proteins that, being only transitorily part of the membrane, are not less worthy of attention than the more permanent ones. The important observation has been made [201] that non-permanent proteins can generate large local tensions in the membrane, that frequently relax into a variety of physical effects along lateral and transverse planes of the membrane. In turn these effects can mediate molecular and structural changes modifying membrane topography and dynamics, and ultimately membrane biochemical reactivity.
In the next paragraphs, we will review the molecular bases of the interaction of specific proteins with membranes as examples of the general principles depicted in this section.
G proteins and their interactions with membranes
Interactions with lipids regulate the localization and activity of many amphitropic proteins. These proteins can interact with various types of membrane lipid species or lipid structures in defined membrane microdomains, depending on their transient structural status, on their tertiary structure or on the modifications in the number of oligomeric subunits in the complexes. Therefore, the messages they propagate into the cell will somehow depend on these protein–lipid interactions. Here, we shall review the interactions of several types of amphitropic proteins with membranes and the role these interactions play in the cell's physiology.
GPCRs constitute the largest gene family of membrane receptors (see above sections) and they are involved in controlling a wide number of cellular and physiological processes, such as: vision, taste, smell, blood pressure, metabolism, cell proliferation, body weight, neurotransmitter release, affective status, etc. [202, 203]. Agonist-mediated activation of these receptors propagates signal transduction through heterotrimeric G proteins. Hence, the wide range of receptor types and the relevance of the functions they control, makes via G protein signalling one of the most important cell signalling pathways in the cell (for a review, see [202, 203]). GPCRs undergo conformational changes upon receptor activation that activate the associated heterotrimeric G proteins. However, recent evidence indicates that some GPCRs are able to trigger signalling events in cells without the intervention of G proteins [204, 205], and that G proteins can be activated by means of GPCR-independent activators of G protein signalling proteins (AGS proteins) [206, 207]. There are at least 16 types of Gα subunits, 5 of Gβ subunits and 12 types of Gγ subunits encoded by the G protein gene family [208]. Gα subunits can be divided at least into four structurally related categories: Gαi/o, Gαs, Gαq/11 and Gα12/13, although other classifications based on their functional properties have been also proposed. Among all the G proteins, the GTPase domain is highly conserved whereas the helical domain that directs the coupling with the GPCRs varies [208].
The association between an extracellular agonist ligand and the GPCR molecule induces the productive coupling to intracellular heterotrimeric (Gαβγ) G proteins. This coupling promotes the exchange of guanosine diphosphate (GDP) for GTP on the Gα-subunit and the Gα subunit then dissociates from the Gβγ dimer, the GPCR moving to other membrane domains. Both, Gα and Gβγ proteins exert their action by modulating the activity of different intracellular effectors and kinases, the latter being responsible for receptor phosphorylation and inactivation. While the GPCR remains active, several G protein molecules can be activated by a single receptor molecule, resulting in the amplification of the incoming message. For effective signal amplification, a large number of G proteins must be available near to the receptor molecule, which is achieved by specific protein–lipid interactions. Termination of Gα signalling activity occurs when the γ-phosphate of GTP is removed by the intrinsic GTPase activity of the Gα-subunit, leaving GDP in the nucleotide-binding domain of Gα. As a result, the Gα-GDP re-associates with the Gβγ complex and the G protein trimer is again ready to be activated by a receptor molecule [209]. Activated Gα monomers regulate the activity of effector proteins, such as adenylyl cyclase, PLC, K+ channels, etc., which in turn control the cytosolic levels of second messengers. Most effector proteins stimulated or inhibited by G protein subunits are to some extent membrane-bound proteins and therefore, they are associated with lipids. In this context, G protein–lipid interactions may also play a role in driving G protein subunits to specific membrane domains where their protein targets reside. G protein-mediated signalling is further regulated by the binding of regulators of G protein signalling (RGS) to Gα subunits. RGS proteins constitute a large group of proteins that stimulate the GTPase activity of Gα subunits [209, 210]. In the past few years, it has become clear that the role of RGS in G protein signalling is not the mere inactivation of Gα subunit but rather, they act as mediators of signalling linking G proteins with signal transduction pathways that influence cell motility, intracellular trafficking and important responses such as cell growth and differentiation [210].
G proteins bind tightly yet reversibly to cell membranes, and lipid modifications to their α and γ subunits assist in their membrane docking [1]. Indeed, both lipid–lipid and protein–lipid interactions participate in the binding of G proteins to membranes [3, 99]. Gα subunits are modified at their N-termini by myristoylation and/or palmitoylation, whereas farnesyl or geranylgeranyl isoprenoid moieties can be added to the C-termini of γ subunits. Myristoylation occurs co-translationally on glycine residues of Gαi proteins [211, 212], whereas other Gα proteins (with few exceptions) are post-translationally and reversibly palmitoylated on cysteine residues [211, 212]. The regulation of the state of palmitoylation by GPCR-mediated activation influences the cellular localization of Gα subunits and the propagation of signals through these membrane receptors [8, 213–216].
All 12 known Gγ proteins are isoprenylated on carboxy-terminal cysteine residues via a thioether bond. Either a farnesyl moiety on the Gγ1, Gγ9 and Gγ11 subunits (CVIS motif) or a geranylgeranyl moiety on the Gγ2, Gγ3, Gγ4, Gγ5, Gγ7, Gγ8, Gγ10, Gγ12 and Gγ13 subunits (CAIL motif) is recognized by the corresponding enzyme [1]. Isoprenoids bind with high affinity tonon-lamellar-prone lipids (e.g. PE) and increase their hexagonal (HII) phase propensity [2]. This lipid modification is involved not only in G protein–membrane interactions but also in the formation of non-lamellar-prone membrane domains [2, 3]. Moreover, regions with high non-lamellar-phase propensity are important in the activity of the various forms of G proteins produced during their cycle of activity: Both Gαβγ heterotrimers and Gβγ heterodimers prefer non-lamellar prone domains (PE-rich), whereas Gα subunits prefer lamellar regions (e.g. lipid rafts) [6]. The behaviour of these proteins would explain the accumulation of heterotrimeric G protein molecules near receptors, where they are needed in a molar excess to amplify GPCR-mediated signals. Similarly, these preferences explain how Gα subunits leave the receptor microdomain upon activation and dissociation from the Gβγ complex, facilitating their encounters with signalling effectors found in other membrane domains. This model also explains why Gβγ dimers remain around GPCRs where they may recruit GRK to phosphorylate and inactivate the receptor protein. Thus, lipid structures fulfil an active role in signal propagation, generating first a pool of Gαβγ proteins near GPCRs, and later defining the localization of Gα and Gβγ proteins to different membrane regions [6].
In addition to lipid–lipid interactions, G proteins are also associated with membranes via electrostatic interactions between specific amino acids and membrane phospholipids. The inner leaflet of the membrane contains relatively large amounts of PS, so that protein–lipid interactions between this anionic phospholipid and the cationic amino acids from G proteins may also participate in the docking of this transducer to membranes [3, 99].
Although GPCRs have gathered the attention of academia and industry to delineate therapies, G proteins themselves have not been on the focus of the design of therapeutic drugs. This is in part due to the fact that small-molecule ligands for GPCRs differ among receptor types and specific molecules with high subtype recognition have been or can be synthesized, whereas all G proteins bind the same ligands: GDP, GTP and their synthetic analogues. However, G proteins show a greater variation in their membrane–lipid interactions. Moreover, a given Gα protein interacts differentially with membranes in its monomeric and trimeric forms or when it has or lacks a palmitic acid moiety, which results in different activity states. This possibility to regulate G protein activity through regulation of their lipid environment has been recently used to develop drugs for treatment of cancer, obesity, hypertension, etc. [2, 3, 9, 16].
Small monomeric G proteins: the Ras and Ras-like family
Many growth factor receptors activate Ras and Ras-like proteins, most of them belonging to the tyrosine kinase receptor family. Mammalian Ras proteins (H-Ras, K-Ras4A, K-Ras4B, N-Ras) have a molecular weight of 21 kD and except for K-Ras4B (188 amino acids), they contain 189 amino acids. The N-terminal 85 residues of the four members are identical and their similarity remains as high as 90% over the following 80 residues. Accordingly, the principal source of divergence among Ras isoforms is restricted to the 24 C-terminal amino acids, which display less than 15% homology [217]. This diversity is related to the different membrane anchors used by the Ras isoforms to interact with the plasma membrane. H-Ras forms transient interactions with lipid rafts when bound to GDP, whereas when bound to GTP it aggregates with cholesterol-insensitive, galectin-1-dependent, non-raft domains. On the other hand, K-Ras is clustered in cholesterol-insensitive, non-raft domains that differ from the activated H-Ras microdomains [218, 219]. H-Ras, N-Ras and K-Ras4A are palmitoylated on cysteine residues in the hypervariable domain, and they are isoprenylated at their C-terminal cysteine (Cys186). In contrast, K-Ras4B is not palmitoylated but rather it is targeted to membranes by the combination of a polybasic domain and isoprenylation. Polyisoprenylated but non-palmitoylated H-Ras protein is biologically fully active and it associates weakly with cell membranes. However, palmitoylation increases the membrane binding of H-Ras and enhances its transforming activity [220].
The Ras-like small GTPase family is made up of several members, such as Ras, Rap1, Rap2, R-Ras, Ra1, Rheb, M-Ras and TC21 [217]. Ras-like proteins play important roles in various cellular signal transduction pathways, regulating differentiation and proliferation through their interaction with several signal transduction proteins known as Ras effectors. These effectors include a large number of protein kinases, lipid kinases and guanine nucleotide exchange factors, such as SOS and CD25 [218–224].
Like the γ subunit of heterotrimeric G proteins, Ras and Ras-like proteins contain a CAAX isoprenylation motif at their carboxy terminus (C for cysteine, A for aliphatic and X for any amino acid, see above) [81, 225]. This post-translational modification favours the anchoring of proteins to membranes and modifies the structural properties of membranes. In this way, non-lamellar-prone regions are generated that further increase the affinity of this lipid for membranes, and the segregation of membrane microdomains enriched in isoprenoids [2, 3, 226–228]. Prenylation of Ras and related proteins is a complex process, frequently followed by proteolytic cleavage by zinc metalloproteases such as AFC1 and RCE1 [229, 230]. This process removes the last three amino acid residues, altering the isoprenyl-modified cysteine and making this C-terminal lipid modification permanent.
Several proteins of this family are also palmitoylated at 1 or 2 cysteines near the farnesylated carboxy-terminus [231]. In recent years, the post-translational lipid modification of Ras and other membrane-associated proteins has been associated with processes other than membrane anchorage. Lipid modifications, such as isoprenylation and N- and S-acylation also play important roles as specific recognition elements for protein–protein interactions, as well as hydrophobic switches that permit the temporal regulation of docking to sub-compartments like lipid rafts, caveolae [232] and other cellular locations [233]. Palmitoylation augments the affinity of Ras proteins for membranes and activates the mitogen-activated protein kinase (MAPK) pathway [234]. In contrast to farnesylation, palmitoylation of Ras and Ras-like proteins is reversible, reflecting its connection to regulatory phenomena [228, 234]. Ras activation is the first step in the MAPK pathways, an important and conserved signal transduction mechanisms in eukaryotes [235, 236], exemplified by the number of different and interconnected MAPK signal transduction pathways that coexist within cells [235, 236]. In this context, Ras activates Raf (MAP kinase kinase kinase, MAPKKK) through interactions that occur at the plasma membrane. Activated (phosphorylated) Raf activates MAPKK (MEK), which in turn activates MAPK (also called extracellular signal-regulated kinase, ERK). The transmission of intracellular signals is then produced by sequential phosphorylation (and activation) of the components specific to any respective cascade [235, 236]. In mammals, a number of MAPK pathways coexist, including the ERK1/2, c-Jun N-terminal kinase (JNK) and p38 MAPK cascades [237, 238]. These signalling pathways regulate critical events in the cell, such as proliferation, apoptosis and the cell's response to a variety of stimuli. Because the first steps in propagating signals via these cascades are associated with membranes, protein–lipid interactions are crucial in the regulation of distinct cellular activities.
Mutations of Ras proteins have been found in about one third of all human cancers. Because their tumourigenic potential is lost if their interactions with membrane lipids are impaired, some therapies have aimed inhibition of Ras-membrane interactions. However, cancer cells can bypass farnesyl transferase inhibition through addition of a geranylgeraniol moiety to the C-terminal cysteine that it is usually farnesylated. In any case, future treatments involving combined farnesyl and geranylgeranyl transferase inhibition or even alternative ways to control Ras-lipid interactions through other membrane-lipid therapy approaches might be of clinical interest.
Protein kinase C
Since its identification in the bovine cerebellum [239], membrane-associated PKC isozymes have been shown to be fundamental signal transduction molecules, involved in a huge variety of events including cell-cycle regulation, cellular survival, malignant transformation and apoptosis [240]. PKC isoforms can be categorized into three groups depending on the cofactors that regulate their activity: conventional (c) PKC isoforms (α, βI, βII and γ) that require Ca2+ and DAG; novel (n) PKC isoforms (δ, ɛ, ζ, and μ) that are only activated by DAG; and atypical (a) PKC isoforms (χ, ι and its murine homologue, λ) that do not require Ca2+ or DAG. The distinct PKC isoforms exert different responses depending on the cellular context, highlighting the necessity to understand the signalling events controlled by PKCs in each cell type [241].
A common feature of all PKC isoforms is that their activation is dependent on establishing a close interaction with membrane lipids. Indeed, current models for the interaction of PKC with phorbol esters consider lipids to be essential cofactors of the enzyme [242, 243]. PKC can interact with different lipids in a variety of ways although the regulatory region of (c) PKCs contains two membrane-targeting domains, C1 and C2. Whereas the C1 domain is composed of two cysteine-rich zinc finger motifs that bind DAG and phorbol esters (C1a and C1b), the C2 domain is responsible for Ca2+-dependent membrane binding [244]. DAG is a by-product of the digestion of PI by PLC and it activates this kinase both through specific protein–lipid interactions and through the induction of non-lamellar phases [175]. In fact, non-lamellar (HII) phase propensity has been shown to be involved in the translocation of PKC from the cytosol to membranes, a phenomenon associated with enzyme activation [4, 245]. On the other hand, some studies suggest that a specific interaction between PKC and PS occurs [246–248], although other studies have indicated that different anionic phospho-lipids [249], or even neutral phospholipids like PC [250], can also activate PKC. These works demonstrate the importance of PKC–lipid interactions in the activity of this enzyme, and have been related to the therapeutic activity of some drugs against cancer, such as Minerval [9, 245].
It has been proposed that substrate binding can induce the translocation of PKCα to the membrane and that removal of its pseudosubstrate domain may be coupled to a conformational change that results in exposure of hydrophobic groups [251]. Alternatively, myristoylation of PKC substrates promotes their attachment to the membrane, and the associated enzyme-substrate co-localization would be reflected in more efficient catalysis [252–254]. In this regard, myristoylated peptides mimicking the pseudosubstrate regions of a number of PKC isoforms have been used as specific and efficient enzyme inhibitors [252–255]. These studies highlight the relevance of protein–lipid interactions in co-localizing proteins that must physically interact to yield the productive propagation of signals. Thus, lipids also regulate protein activity by modulating their cellular localization.
In addition to membrane phospholipids, cis-unsaturated fatty acids such as arachidonic, linoleic and oleic acid can also activate PKC independently from DAG [250, 256, 257]. These lipids increase the non-lamellar (HII) phase propensity of membranes [258], which in turn favours the translocation of PKC to membranes [4]. In this regard, the 18:1/22:6 species of PE but not those of PC cause an increase in the rate of histone phosphorylation by PKC beyond that caused by other less unsaturated PEs [259]. Similarly, in model systems of pure membrane-forming phospholipids and purified PKCα, non-lamellar-prone membranes of dioleoyl phosphatidylethanolamine have a greater capacity to bind PKCα than membranes containing only the lamellar-forming phospholipid dioleoyl phosphatidylcholine [245]. The importance of this phenomenon has been highlighted by the development of a new anti-cancer drug (Minerval) that increases PKC binding to natural and model membranes containing PE [245].
Membrane microdomains and lipid mediators in the control of the heat shock protein response
Induced by a wide range of stressors, ranging from temperature stress to hypoxia, inflammation, infections or environmental pollutants, stress proteins, also termed Hsps, play key roles in all living systems [260]. Their major conserved classes are grouped according to their molecular weights (Hsp100, Hsp90, Hsp70, Hsp60 and the ‘small Hsps’, sHsps) and some Hsps are encoded by more than one gene [261]. Acting as molecular chaperones, Hsps are able to recognize unfolded and/or damaged proteins and further sort them for repair (refolding) or proteolysis. Hsps can regulate the life or death of cells by directly modulating certain apoptotic signalling events, or indirectly, by participating in antigen processing [261]. Hsps reside not only in the cytosol, but also in the plasma membrane, lysosomes, nucleus and mitochondria. The glucose-regulated Hsp prologues (Grps) reside in the ER. Surprisingly, some Hsps are localized in the extracellular space [261].
Stress sensing and signalling: the membrane sensor theory
Due to their multiple and vital functions briefly highlighted above, Hsps play fundamental roles in the aetiology of several human diseases [262]. Aberrantly high levels of either the overall array of Hsps, or certain Hsp classes are characteristic in different cancer cells and the converse situation applies typically for type 2 diabetes, neurodegeneration, cardiovascular diseases or aging [11, 16]. In certain cancers, development and metastatic potential favours tumours that express a higher level of sHsps and a lower level of Hsp70 in their plasma membranes [263]. Accordingly, it is of key importance to understand the mechanism whereby cells can elicit a Hsp response and regulate the cellular translocation and membrane association of various Hsp classes [11, 16]. As a commonly accepted paradigm, it was earlier suggested that stress-induced protein denaturation serves as a primary stress-sensing machinery that triggers Hsp gene expression [264, 265]. During the past decade, a new, but not exclusive model, the ‘membrane sensor’model, has emerged, which predicts the existence of a membrane-associated stress sensing and signalling mechanism from prokaryotes to mammalian cells [12, 266–273]. In favour of this model, the exposure of mammalian cells to various membrane fluidizers, or compounds with the ability to interact with certain membrane lipids, substantially modulate Hsp expression without inducing protein unfolding [266]. It was recently documented in a cellular melanoma model that changes in the physical state (fluidity) and the concomitant destabilization/reorganization of cholesterol-rich membrane microdomains may indeed serve as such a ‘molecular switch’ and is sufficient for the operation of these ‘cellular thermometers’[11, 16, 270]. Thus, although many inducers/silencers of the Hsp response may function through a protein-unfolding pathway, some inducers/silencers may work through a distinct mechanism.
The plasma membrane, which is the barrier to the external environment, is well suited for sensing stress and acts as an important regulatory interface. As detailed elsewhere, even subtle alterations in its lipids (by causing ‘membrane defects’) may influence membrane-initiated stress-signalling processes by changing the global fluidity, the membrane thickness, the local organization of microdomains and thereby the clustering of receptors or other proteins localized in the plasma membrane [11, 16, 262].
Hsp signalling in cancer and diabetes
We recently suggested that, in such prominent disease states as insulin-resistant diabetes and cancer, where the directions of Hsp dysregulation and membrane fluidity run in parallel but opposite manners, there must exist a conserved signalling cascade which is uniformly controlled by membrane hyper-structures and ultimately affects the level of Hsp expression as well [11]. Signalling from the transmembrane growth factor receptors to Hsp genes ful-fils such a criterion. PI3K, Akt and GSK3, the latter as a negative regulator of HSF1 activation [274], are central components of such a signalling cascade. Insulin resistance and type 2 diabetes are known to be associated with low Hsp levels, and with decreased PI3K and enhanced GSK3 activities (see references in [275]). Correction of a low Hsp state improves insulin resistance [275]. A close chemical relative of the membrane-intercalating drug candidate, the hydroxylamine bimoclomol [276, 277], BRX-220, has been reported to improve insulin sensitivity of both Zucker diabetic fatty rats and streptozotocin-diabetic rats [278]. Another hydroxylamine analogue, the Hsp co-inducer BGP-15, has also successfully passed a phase IIa human clinical trial as an insulin-sensitizer compound [279]. Using BGP-15 to achieve an elevation of Hsp72 protein, protection against diet or obesity-induced hyperglycaemia, hyper-insulinemia, glucose intolerance and insulin resistance was observed. This protection was tightly associated with the prevention of JNK phosphorylation [280].
As documented by Khaleque et al.[281], Hsp elevation in tumour cells can be induced by the malignant growth factor heregulin β1 (HRGβ1), which causes homo- and heterodimerization between each member of the four ErbB receptor molecules via ‘horizontal signalling’ in the plane of the plasma membrane. The formation of raft-associated growth factor receptor dimers is followed by tyrosine phosphorylation of their intracellular domains. The major downstream signalling pathways include the Ras-Raf1-Mek-ERK and PI3K-PDK1-Akt pathways [281]. HRGβ1 appears to be linked to Hsp expression by its activation of HSF1 through inhibition of the constitutive kinase GSK3. It should be noted that the transmission of a proper signal from the cell surface to Hsp genes is uniformly dependent on precise regulation by the lipid composition of the membranes. The localization of growth factor receptors to distinct microdomains possessing a well-balanced ratio of inner and outer leaflet lipids appears to modulate both their ligand binding and tyrosine kinase activities [282]. Moreover, the major signal termination mechanism, i.e. the lateral movement of dimerized receptors in the plane of the plasma membrane and the delivery of activated receptor-containing endosomes, is totally lipid dependent [283, 284].
The role of membrane microdomains
The exact role played by lipid microdomains in membrane-directed stress sensing and signalling is far from clear. The related studies have been hampered by the lack of suitable physical methods for the visualization of membrane microdomains in intact cells [20, 285]. Membrane structures in mammalian cells display an enormous variety of lipids, functions, localizations, associations and intimate links with neighbouring membranes, and this makes their study extremely difficult. It is tempting to speculate that one of the major roles of the more than one thousand lipid molecular species in mammalian membranes is to provide an on–off switch for signalling events at the membrane level ([286] and see references within). Membrane lipids are among the molecules that adapt best in response to various environmental perturbations. Even subtle changes in the compositions of acyl chains or head groups can alter the packing arrangements of lipids within a bilayer. As a chain reaction, altered lipid packing properties change the balance between bilayer and non-bilayer lipids, affect the bilayer stability and fluidity, and ultimately alters the lipid–protein interactions and microdomain organizations. External factors, including temperature, chemicals, ions, radiation, pressure, nutrients, the growth phase of cultured cells, etc., are all capable of changing the membrane packing, order and lipid composition (see references in [286]). Our understanding of the plasma membrane has changed considerably as our knowledge of membrane microdomains, rafts, has expanded.
We should point out that the ‘lipid building blocks’ of cell membranes can also serve as a source of those mediators which can be involved in the activation or attenuation of Hsp signalling pathways. Lipids, lipid kinases and lipid phosphatases have not received the same amount of attention as proteins in studies of signal transduction [287]. Thus, stresses and clinical conditions can induce alterations in the raft organization, but also give rise to changes in the metabolism of membrane lipids in producing a unique set of lipid mediators with the potential of retailoring the pre-existing Hsp profile [11, 16, 20, 262, 286].
Lipid mediators of the stress response
Many of the lipid mediators (e.g. leukotrienes, prostaglandins and certain lysophospholipids) leave the host cells and bind to GPCRs in the surface membrane of the same or neighbouring cells. Other lipid signals converge on PLC, PIs or Ca2+. As highlighted in Fig. 7 the typical non-raft phospholipids and raft lipid classes (i.e. cholesterol and SM) can equally serve as stress-response modulating factors. Phospholipids are reservoirs of arachidonic acid. Phospholipase A2 is known to be activated by different stressors, and the concomitant release of arachidonic acid can stimulate heat shock factor1 (HSF1)-DNA binding, increase the phosphorylation of HSF1 and ultimately up-regulate some Hsps [288]. On the other hand, arachidonic acid is used in the synthesis of prostaglandins via cyclooxygenases, some of which are also potent Hsp inducers ([289] and see references in [16, 287]). Moreover, certain lipoxygenase products of arachidonic acid, such as 12-hydroxyeicosatetraenoic acid, have also been shown to induce the expression of individual Hsps inhuman leucocytes [290]. Upon exposure to environmental stress, cholesterol can rapidly transform to cholesteryl glucoside, and its production is followed by the activation of certain protein kinases also engaged in the induction of Hsps [291]. Similar to cholesterol, SM may play both structural and functional roles as a raft lipid, and in parallel it takes part in the generation and transduction of Hsp signals. Within the large SM family of lipid mediators, the reactions are reversible and the lipids are intercon-vertible. Ceramide and sphingosine can be released by the sequential cleavage of SM under various stress conditions [292]. Ceramide, ceramide-1-phosphate, sphingosine and sphingosine-1-phosphate have all been shown to carry out second messenger functions linked ultimately to refinement of the cellular Hsp pattern [293].
7.
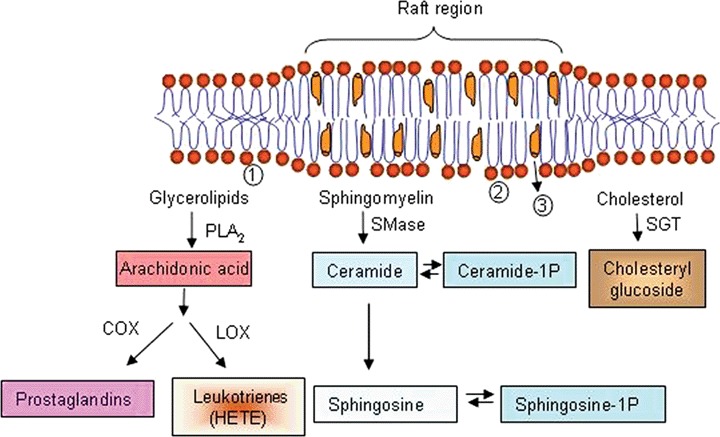
Some typical lipid mediators capable of altering the Hsp response. The parental molecules of a variety of lipid mediators (boxes) are:(1) glycerolipids of the bulk membrane; constituents of rafts such as (2) SM and (3) cholesterol. PLA2: phospholipase A2; SMase: sphingomyelinase; SGT: sterol glucosyltransferase; COX: cyclooxygenase and LOX: lipoxygenase.
In conclusion, the above observations indicate that Hsp expression in response to various stressors is regulated by differential control mechanisms rather than by a uniform mechanism. It appears that one type of signal in the multistep activation pathway of HSF might arise from the exposure of hydrophobic domains of cytosolic proteins and in such cases typically the entire array of stress proteins is induced. There must exist, however, ‘alternative’ sensing-signalling pathways, which cause the expression of varying Hsp profiles obtained with different stressors. We propose that changes in the composition and microdomain organization of membranes offer the solution to the problem and represent a new theory: subtle changes of membrane lipids can cause remarkable alterations in the expression pattern of Hsps. Changes in lipid composition and membrane microheterogeneity during stress or disease states could trigger selective stress signalling responses either through global effects on the physical state of the membrane matrix, or via specific chemical interactions of boundary lipids with membrane proteins.
Various membrane imaging technologies, real-time detection and the monitoring of rafts and combination of these technologies with lipidomics and computational methods may allow the identification and characterization of the hypothetical stress-sensory membrane domains. Hsps could be induced or inhibited by drugs that specifically change the activity of such sensory lipid–protein membrane hyperstructures according to the principles of membrane-lipid therapy [9, 11].
A subpopulation of Hsps can interact with and translocate through membranes
The presence of Hsps in membranes and lipid rafts is widely documented. On the basis of prokaryotic models, we suggested earlier that a lipid-selective association of a subpopulation of Hsps (GroEL and sHsps) with membranes, leading to increased molecular order, may in turn result in down-regulation of Hsp gene expression [177, 269, 294–297]. Such hypothetical ‘cross-talk’ between the membrane primary stress sensors (see above) and Hsps suggests a feedback loop mechanism in the regulation of Hsp genes [11, 16]. In addition, interactions between certain amphitropic Hsps and specific lipid domains can remodel the pre-existing architecture and physical order of membranes. In parallel with the microdomain reorganization and thermal stabilization, we have shown that highly saturated ‘heat shock lipids’ can accumulate in membranes and exhibit a preferential interaction with sHsps [298]. Non-bilayer-forming lipids are known to be important controlling factors of cell signalling because they regulate the localization (and thereby activity) of key signalling proteins such as PKC and G proteins [4, 9]. It has been demonstrated that, in membranes composed of non-lamellar lipids, sHsps inhibit the formation of inverted hexagonal structures and are thereby important determinants of the membrane lipid secondary structures. Evidence from FTIR and DSC studies has indicated that the interactions of sHsps are preferential by/with anionic lipids and affect both the polar headgroup region and the hydrophobic core [177, 295, 297]. We inferred from these results that the association between sHsps and membranes may constitute a general mechanism that preserves the membrane integrity under fluctuating, stressful conditions. Moreover, we reasoned that the specific Hsp–lipid interactions may serve as an unrecognized means for the spatial separation and distinct compartmentalization of Hsps to lipid domains which are thought to be involved in various stress signalling pathways [11, 16, 286].
Hsp90 in eukaryotic membranes
The presence of Hsps in plasma membranes and lipid rafts of mammalian cells is widely documented. Much less is known, however, about the mechanism controlling the translocation of Hsps to membranes and the modes of their interactions with membrane lipids, membrane proteins or both. It has been shown that Hsp90 is an iron-binding protein associated with the plasma membrane of HeLa cells [299]. Moreover, the interactions of STAT3 with caveolin-1 and Hsp90 in plasma membrane rafts have been revealed to play a role in the preservation of cytokine signalling during fever [300]. Further, the interaction of Hsp90 with the heterotrimeric G protein Gα12 has been demonstrated to target Gα12 to lipid rafts [301]. Hsp90 is present in membrane microdomains, together with CD14 and other molecules, following lipopolysaccharide-induced cell activation. Lipid raft integrity is essential for the process [302]. In fact, treatment with the Hsp90 inhibitor geldanamycin ameliorates the response to LPS in murine macrophages by decreasing CD14 surface expression, presumably due to the improper folding and arrest of CD14 within the endplasmic reticulum [303]. ErbB2, a member of the EGF receptor family of tyrosine kinases is overexpressed on many tumour cells of epithelial origin and colocalized with Hsp90 in plasma membrane. It is the molecular target of trastuzumab (Herceptin), the first humanized antibody used in the therapy of solid tumours.
Hsp70 in cell membranes
Direct and specific interactions of Hsc70 (the constitutive form of the 70 kD Hsp) and Hsp70 with membrane lipids have been suggested to play a role in the folding of membrane proteins and the translocation of polypeptides across membranes [304]. The interactions of Hsp70 with lipids are additionally indicated by their intrinsic capacity to open ion conductance channels or by aggregating liposomes. The interactions of Hsp70 with lipids are highly dependent on the presence of PS. In line with this, Hsp70 expresses toxicity towards cells presenting PS on their surface [305]. The possible role of Hsps as enhancers of endocytosis has been speculated to be part of the cellular stress response for rapid remodelling of the plasma membrane [306].
Anti-inflammatory drugs cause the differential up-regulation of cytosolic and membrane-bound Hsp70 in tumour cells [307]. It has been suggested that such an increase in membrane-bound Hsp70 corresponds to an enhanced sensitivity to granzyme B-induced apoptosis and natural killer cell-mediated killing. This finding provided a biological rationale for combining anti-inflammatory drugs with immunotherapy in cancer treatment. Moreover, the cell surface-bound Hsp70 has been postulated to mediate perforin-independent apoptosis by the specific binding and uptake of granzyme B [308]. Su et al. demonstrated, that constitutive Hsp70 (Hsc70) attenuates hydrogen peroxide-induced membrane lipid peroxidation [309]. The finding that Hsc70 reduces lipid peroxidation suggests that this protein (and maybe other Hsp members like sHsps) may act through a general mechanism to interfere with the reactions of lipid oxi-dation. The fact, that Hsp70 is capable to form complexes with the acidic glyco- and phospholipids further implicates that Hsp70 plays various, hitherto unidentified and lipid-mediated functions on the membrane surfaces [310].
Hsp27-membrane interactions
It has been shown that Hsp27, which associates with membranes via specific lipid interactions [297] has a potent protective effect against α-synuclein-induced cell death in mammalian neuronal cells [311] where the association of α-synuclein with membranes leads to disruption of the membrane bilayer structure. After preconditioning of rat heart (a single episode of 5 min. global ischaemia followed by 5 min. of reperfusion), HSP27 redistributed from the cytosol to the sarcomere and recovery of the contractile function was significantly enhanced, which suggests that translocation of HSP27 to the sarcomere may be involved in the cardioprotective mechanism afforded by ischaemic preconditioning in rat heart [312]. In contrast with Hsp27, Hsp70 does not exert such an action.
Secreted Hsps
Despite lacking a secretory signal, some Hsps are released from cells through physiological secretory mechanisms [261]. Thus, extracellular stress proteins including glucose-regulated proteins are emerging as important mediators of intercellular signalling and transport. Among others, immunological ‘danger signals’, physical trauma or behavioural stress are capable of triggering the release of these proteins from cells [313]. Evidence has been presented that the ‘leaderless'stress proteins may be secreted into the circulation via lipid raft-, granule- or exosome-mediated exocytosis, for instance in haematopoietic or tumour cells. Hsp70 surface-positive tumour exo-somes are able to stimulate the cytolytic and migratory capacity of resting natural killer cells [314]. Hsp70 is released from prostate carcinoma cells through an active secretory mechanism that has been documented to involve the endolysosomal compartments, and is taken up by other cells ([313] and see references within). It has been demonstrated that neither the common secretory pathway nor the lipid raft-mediated pathway is involved, however, in the release of Hsp70 from human mononuclear cells, perhaps the best-studied model of Hsp secretion [261]. In stressed Caco-2 cells, the amount of Hsp70 is increased specifically in the lipid rafts and this is correlated with the robust stimulation of Hsp70 release. Manipulation of the lipid composition of rafts resulted in a concomitant modulation of Hsp70 release, suggesting that in this case the lipid raft may represent a cellular mechanism for membrane delivery and the release of Hsps [315].
Table 6 illustrates this newly acknowledged dual function of Hsps: while maintaining cellular homeostasis intracellularly during protein- and/or membrane-damaging stresses, they may protect cells also beyond their borders. We will next highlight a few of the representative cases listed in Table 6.
6.
Hsps in membranes and in the extracellular space
| HSP | Localization | Tissue/cell type | Conditions | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hsp47 | Basement membrane | Muscle | Muscular dystrophy | [316] | |||||||||
| Hsp60 | Plasma membrane | Human endothel | After stress | [336] | |||||||||
| Cardiac myocyte | Hypoxia | [317] | |||||||||||
| Exosomes, attached to the membrane | Rat adult cardiac myocytes | Unstressed, hypoxia | [318] | ||||||||||
| Exosomes | Mouse B lymphoma cells | Unstressed, heat shocked | [340] | ||||||||||
| Hsp70 | Plasma membrane | Rat heart | Insulin treatment | [319] | |||||||||
| Exosomes (independent of rafts) | PBMC, 3T3-L1, L6 myotubes, B cells | Heat shock | [337] | ||||||||||
| Extracellular space, (unconventional release) | In many tumour cells | Unstressed, heat shocked During necrotic cell death | [321] | ||||||||||
| Cell death | [338] | ||||||||||||
| Under pathological conditions of normal cells | [339] | ||||||||||||
| Lysosomal membrane | Human tumour cells | Cytokines, anticancer drugs, irradiation, oxidative stress, photolysis. | [320] | ||||||||||
| Hsp90 | B cells | Unstressed, heat shocked | [321] | ||||||||||
| Mouse B lymphoma cells | Unstressed, heat- shocked | [340] | |||||||||||
| H460 cells | γ-irradiation | [341] | |||||||||||
| Exosomes | Human mesothelioma cells | Unstressed | [342] | ||||||||||
| α-B crystalline | Plasma membrane | DRM myotubes | Dexamethasone treatment | [343] | |||||||||
| Bovine Ocular lens | Increased upon mutation | [344] | |||||||||||
| HSPB1 (Hsp27) | Outer mitochondrial membrane | U937 cells | During apoptosis | [324] | |||||||||
| Plasma membrane | |||||||||||||
| Bovine endothelial cells | Overexpressed human Hsp27 | [322] | |||||||||||
| Dexamethasone treatment | |||||||||||||
| DRM myotubes | [343] | ||||||||||||
| Exosomes | Unstressed, heat- shocked | ||||||||||||
| B cells | [321] | ||||||||||||
| HSPB2 | Outer mitochondrial membrane | C2C12 cells, KNS-81 cells | Under normal conditions | [323] | |||||||||
Hsp47, a collagen-specific molecular chaperone, is involved in the processing and secretion of procollagens, and its expression is increased in various fibrotic diseases. The overexpressed Hsp47 has been observed in the muscle membrane only in the case of active inflammatory myopathy. In particular, Hsp47 is strongly expressed in the membrane of regenerating fibres, implying that it may be involved in the repair or regeneration of muscle fibres in addition to the fibrotic change in the connective tissue [316].
Other studies have reported that hypoxia results in disassociation of the Hsp60-Bax complex, with translocation of cytosolic Hsp60 to the plasma membrane and Bax to the mitochondria [317]. The interaction between Hsp60 and Bax may be critical in preventing apoptosis in the normal cell. The surface presentation of Hsp60 on the myocyte combined with serum antibodies to this protein, may be one mechanism fuelling the downward spiral in heart failure [318]. It is possible that membrane Hsp60 may be recognized by macrophages and thus mark the myocyte for destruction. Whether translocation of Hsp60 to the membrane might possibly stabilize its structure and exert a protective effect is unknown, as is the mechanism(s) controlling this translocation. Hsp60 is released via exosomes and within the exo-some Hsp60 is tightly attached to the exosome membrane. Hsp60 is released from adult cardiac myocytes in both the basal state and following mild stress through an exosome-mediated process. Lipid rafts participate in this process, as inhibition of lipid raft formation reduces the release of Hsp60 [318].
Insulin-treated hearts display elevated levels of Hsp70, particularly in the membrane fraction. In contrast, heat-shocked hearts exhibit elevated levels of Hsp70 in the cytosol, membrane and pellet fractions. After insulin treatment, Hsp70 is mostly colocalized to the plasma membrane with dystrophin. In contrast, after heat shock, Hsp70 is localized mainly between the cardiomyocytes in apparently vascular or perivascular elements. The localization of Hsp70 is dependent on the inducing stimulus of either heat shock or insulin treatment. The cell membrane versus vascular localization of Hsp70 suggests the interesting possibility of functionally distinct roles for Hsp70 in the heart, depending on whether it is induced by insulin or heat shock treatment [319].
It was reported earlier, that Hsp70 acts as a potent survival protein whose depletion triggers massive caspase-independent tumour cell death [320]. This Hsp70-mediated protection against various death stimuli in Hsp70-expressing human tumour cells and in immortalized Hsp70 transgenic murine fibroblasts occurs at the level of the lysosomal permeabilization. The cell death induced by Hsp70 depletion is preceded by the release of lysosomal enzymes into the cytosol and inhibited by pharmacological inhibitors of lysosomal cysteine proteases. However, Hsp70 does not inhibit cytochrome c-induced, apoptosome-dependent caspase activation in vitro and Fas ligand-induced, caspase-dependent apoptosis in immortalized fibroblasts. Immunoelectron microscopy has revealed that endosomal and lysosomal membranes of tumour cells contain Hsp70. The permeabi-lization of purified endosomes and lysosomes bydigitonin fails to release Hsp70, suggesting that it is physically associated with the membranes. Finally, Hsp70-positive lysosomes display increased size and resistance to chemical and physical membrane destabilization. Taken together, these data identify Hsp70 as a ‘survival protein’that functions by inhibiting the death-associated permeabilization of lyso-somes [320].
As outlined above, the extracellular factors that regulate the quantity and phenotype of the exo-somes produced are poorly understood, and the properties of the exosomes that dictate their immune functions are not yet clear. Studies on the effects of cellular stress on the exosomes produced by B-lymphoblastoid cell lines have revealed that, under steady-state conditions, the exosomes are positive for Hsp27, Hsc70, Hsp70 and Hsp90. Exposure of cells to heat stress results in a marked increase in these Hsps, whereas the expressions of other stress proteins, such as Hsp60 and Grp96, remain negative [321]. It has also been elucidated that Hsps are located within the exosome lumen, rather than at the exo-some surface, suggesting that such exosomes may not interact with target cells through cell-surface Hsp receptors. It has been concluded that specific alterations in exosome phenotype are a hitherto unknown component of the cellular response to environmental stress, and their extracellular function does not involve the direct activation of dendritic cells [321].
To investigate the putative association of Hsp27 (known at that time as ‘barbed-end microfilament capping protein’) with plasma membranes, bovine endothelial cells expressing the human wild-type or a mutant non-phosphorylatable 27-kD Hsp were subjected to subcellular fractionation and subsequent immunoblot analysis [322]. The 25-kD endogenous bovine homologue and both exogenous gene products partitioned with cytosolic or plasma membrane components, indicating that phosphorylation is not required for membrane association. Phorbol ester treatment results in phosphorylation of only membrane-associated 25-kD and wild-type 27-kD Hsp and does not induce redistribution [322].
The sHsp HspB2 colocalizes with mitochondria in differentiated C2C12 cells, KNS-81 glioma cells and NIH3T3 cells transfected with a HspB2 [323]. Together with the fact that HspB2 does not possess a mitochondrial targeting signal sequence, these results suggest that HspB2 is not located inside the mitochondria. Instead, it is attached to the surface of the mitochondria by weak interactions with some outer membrane components. The amount of HspB2 in the mitochondrial fraction increases when the cells are subjected to mild heat shock, suggesting that this association can be altered by stress conditions.
Association of another sHsp family member, Hsp27, occurs with the mitochondrial outer membrane in U937 cells. It has been suggested that this pool of Hsp27 negatively regulates cell death by interacting with cytochrome c released from the mitochondria into the cytosol in apoptotic pathways [324].
In conclusion, we assume that aging and various pathological states which lead to abnormal alterations in the lipid metabolism give rise to abnormal global (fluidity, surface charge, permeability, etc.) and specific (raft remodelling) changes within membranes. The formation of ‘defects’ in response to stress within the membrane microdomains is causally linked to the dysregulated expression and, at least in part, to the abnormal intra or extracellular localization of specific Hsps. In this context, membrane microdomains represent new therapeutic targets. In fact, a targeted and distinct reorganization of membrane microdomains with drug candidates acting according to the principles of ‘membrane-lipid therapy’[9] best represented by the lipid-interacting hydoxylamines, has been shown to be coupled with the simultaneous normalization of the dysregulated expression and cellular localization of Hsps in such prominent disease states as type 2 diabetes and amyotrophic lateral sclerosis.
Concluding remarks
Membrane lipids, and the structures they form, not only play structural roles but also participate actively in numerous cellular processes. Hundreds of lipids form complex and heterogeneous membranes, where stable (e.g. synaptosomes) or dynamic (e.g. caveolae) microdomains define the structural and functional properties of a given bilayer. Membranes are the meeting point of many proteins and lipids, and they are the structures where most cellular activities take place. Lipids are not simply the physical scaffold for membrane proteins, forming barriers that isolate and define cells and organelles, but rather, they are also active in many cellular functions. In this context, they regulate the co-localization of proteins to receive and propagate messages that regulate hormone and neurotransmitter release, protein secretion, the cell division cycle, cell differentiation, contractility, gene expression, etc. In addition, certain membrane lipids act as ligands or subrates of signalling proteins and enzymes. Therefore, protein–lipid and lipid–lipid interactions have a great impact in the physiology of cells. Similar to regulation of gene expression, the presence and levels of lipids species can change in response to diet, physiological and environmental conditions. In this context, in several human pathologies there have been described changes in membrane lipids, that have been associated either with adaptive responses or with the aetiology of the disease [9, 325, 326] (Table 3). This fact highlights the prominent role of lipids in cells and suggests that they may also be appropriate targets for therapeutic interventions. Currently, most clinical drugs are targeted to proteins. Moreover, over a half the drugs currently under development are directed against GPCRs [327] and many others are designed to modulate the activity of other membrane proteins. Because the activity of membrane proteins can be regulated by lipids, it is feasible that lipid treatments could modulate the activities of these proteins and/or associated signalling mechanisms. This approach, called membrane-lipid therapy [9], has been used to develop molecules with low toxicity and high efficacy, to treat cancer, cardiovascular diseases, obesity, etc. In this sense, interventions on the composition and structure of membranes may impinge on the physical and structural properties of the lipid bilayer and concomitantly on the interaction and activity of signalling proteins with lipids or with other membrane proteins. Moreover, whereas the cis-MUFA oleic acid regulates membrane structure and cell signalling, its trans-isomer, elaidic acid, and its saturated fatty acid analogue, stearic acid, do not regulate neither lipid structure nor GPCR-mediated function [328]. This fact is only one example of the many available showing the relationship between lipid structure and cell activity and it might be related to the beneficial (e.g.cis-MUFA) and detrimental (e.g.trans-MUFA and saturated fats) effects that lipids have on human health. Our current understanding of lipids, the structures they form, their roles in cells, and the physicochemical and biological properties of membranes are the result of many years of research. The following years present a thrilling scenario for the study of membranes, in which many issues that remain poorly understood are likely to be successfully resolved, thanks in part to new discoveries and the development of new experimental approaches.
Acknowledgments
The present work was financed in part by Grants SAF2003–00232, SAF2004–05249 and BFU2007–61071 (Ministerio de Educación y Ciencia, Spain); and Grants PRIB-2004–10131, PCTIB-2005GC4–07 and PROGECIC-3C (Govern de les Illes Balears, Spain). Grants RET OMFB 00067/2005 and OTKA 68379 (Hungary) to I.H. and L.V., the Finnish Academy of Sciences, the Marathon Foundation and the Sigrid Juslius Foundation also financed in part the present study.
References
- 1.Escribá PV, Wedegaertner PB, Goñi FM, Vögler O. Lipid-protein interactions in GPCR-associated signaling. Biochim Biophys Acta. 2007;1768:836–52. doi: 10.1016/j.bbamem.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Funari SS, Prades J, Escribá PV, Barceló F. Farnesol and geranylgeraniol modulate the structural properties of phosphatidylethanolamine model membranes. Mol Membr Biol. 2005;22:303–11. doi: 10.1080/09687860500135411. [DOI] [PubMed] [Google Scholar]
- 3.Barceló F, Prades J, Encinar JA, Funari SS, Vögler O, Gonzalez-Ros JM, Escribá PV. Interaction of the C-terminal region of the G{gamma} protein with model membranes. Biophys J. 2007;93:2530–41. doi: 10.1529/biophysj.106.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escribá PV, Sastre M, García-Sevilla JA. Disruption of cellular signaling pathways by daunomycin through destabilization of nonlamellar membrane structures. Proc Natl Acad Sci USA. 1995;92:7595–9. doi: 10.1073/pnas.92.16.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Escribá PV, Ozaita A, Ribas C, Miralles A, Fodor E, Farkas T, García-Sevilla JA. Role of lipid polymorphism in G protein-membrane interactions: nonlamellar-prone phospholipids and peripheral protein binding to membranes. Proc Natl Acad Sci USA. 1997;94:11375–80. doi: 10.1073/pnas.94.21.11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vögler O, Casas J, Capó D, Nagy T, Borchert G, Martorell G, Escribá PV. The Gbetagamma dimer drives the interaction of heterotrimeric Gi proteins with nonlamellar membrane structures. J Biol Chem. 2004;279:36540–5. doi: 10.1074/jbc.M402061200. [DOI] [PubMed] [Google Scholar]
- 7.Wedegaertner PB, Bourne HR, Von Zastrow M. Activation-induced subcellular redistribution of Gs alpha. Mol Biol Cell. 1996;7:1225–33. doi: 10.1091/mbc.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novotny J, Durchankova D, Ward RJ, Carrillo JJ, Svoboda P, Milligan G. Functional interactions between the alpha1b-adrenoceptor and Galpha11 are compromised by de-palmitoylation of the G protein but not of the receptor. Cell Signal. 2006;18:1244–51. doi: 10.1016/j.cellsig.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Escribá PV. Membrane-lipid therapy: a new approach in molecular medicine. Trends Mol Med. 2006;12:34–43. doi: 10.1016/j.molmed.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Buda C, Dey I, Balogh N, Horvath LI, Maderspach K, Juhasz M, Yeo YK, Farkas T. Structural order of membranes and composition of phospholipids in fish brain cells during thermal acclimatization. Proc Natl Acad Sci USA. 1994;91:8234–8. doi: 10.1073/pnas.91.17.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vigh L, Horvath I, Maresca B, Harwood JL. Can the stress protein response be controlled by ‘membrane-lipid therapy’? Trends Biochem Sci. 2007;32:357–63. doi: 10.1016/j.tibs.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Vigh L, Maresca B, Harwood JL. Does the membrane's physical state control the expression of heat shock and other genes. Trends Biochem Sci. 1998;23:369–74. doi: 10.1016/s0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]
- 13.Borovyagin VL, Sabelnikov AG. Lipid polymorphism of model and cellular membranes as revealed by electron microscopy. Electron Microsc Rev. 1989;2:75–115. doi: 10.1016/0892-0354(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 14.Israelachvili JN, Marcelja S, Horn RG. Physical principles of membrane organization. Q Rev Biophys. 1980;13:121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- 15.Kinnunen PKJ. On the molecular level mechanisms of peripheral protein–membrane interactions induced by lipids forming inverted non-lamellar phases. Chem Phys Lipids. 1996;81:151–66. [Google Scholar]
- 16.Vigh L, Escribá PV, Sonnleitner A, Sonnleitner M, Piotto S, Maresca B, Horvath I, Harwood JL. The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog Lipid Res. 2005;44:303–44. doi: 10.1016/j.plipres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Emoto K, Kobayashi T, Yamaji A, Aizawa H, Yahara I, Inoue K, Umeda M. Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc Natl Acad Sci USA. 1996;93:12867–72. doi: 10.1073/pnas.93.23.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeagle PL. The structure of biological membranes. 2. Boca Raton, New York: CRC Press; 2005. [Google Scholar]
- 19.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–31. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 20.Vereb G, Szollosi J, Matko J, Nagy P, Farkas T, Vigh L, Matyus L, Waldmann TA, Damjanovich S. Dynamic, yet structured: the cell membrane three decades after the Singer-Nicolson model. Proc Natl Acad Sci USA. 2003;100:8053–8. doi: 10.1073/pnas.1332550100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verkleij AJ, Zwaal RF, Roelofsen B, Comfurius P, Kastelijn D, Van Deenen LL. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta. 1973;323:178–93. doi: 10.1016/0005-2736(73)90143-0. [DOI] [PubMed] [Google Scholar]
- 22.Bretscher MS. Phosphatidyl-ethanolamine: differential labelling in intact cells and cell ghosts of human erythrocytes by a membrane-impermeable reagent. J Mol Biol. 1972;71:523–8. doi: 10.1016/s0022-2836(72)80020-2. [DOI] [PubMed] [Google Scholar]
- 23.Gascard P, Tran D, Sauvage M, Sulpice JC, Fukami K, Takenawa T, Claret M, Giraud F. Asymmetric distribution of phosphoinositides and phosphatidic acid in the human erythrocyte membrane. Biochim Biophys Acta. 1991;1069:27–36. doi: 10.1016/0005-2736(91)90100-m. [DOI] [PubMed] [Google Scholar]
- 24.Holthuis JC, Van Meer G, Huitema K. Lipid microdomains, lipid translocation and the organization of intracellular membrane transport. Mol Membr Biol. 2003;20:231–41. [PubMed] [Google Scholar]
- 25.Op den Kamp JA. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- 26.Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–42. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–16. [PubMed] [Google Scholar]
- 28.Bevers EM, Comfurius P, Dekkers DW, Zwaal RF. Lipid translocation across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1999;1439:317–30. doi: 10.1016/s1388-1981(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 29.Lubin B, Chiu D, Roelofsen B, Van Deenen LL. Abnormal membrane phospholipid asymmetry in sickle erythrocytes and its pathophysiologic significance. Prog Clin Biol Res. 1981;56:171–93. [PubMed] [Google Scholar]
- 30.Wali RK, Jaffe S, Kumar D, Kalra VK. Alterations in organization of phospholipids in erythrocytes as factor in adherence to endothelial cells in diabetes mellitus. Diabetes. 1988;37:104–11. doi: 10.2337/diab.37.1.104. [DOI] [PubMed] [Google Scholar]
- 31.Fadok VA, Bratton DL, Frasch SC, Warner ML, Henson PM. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998;5:551–62. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- 32.Mabrey S, Mateo PL, Sturtevant JM. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978;17:2464–8. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- 33.Marguet D, Lenne PF, Rigneault H, He HT. Dynamics in the plasma membrane: how to combine fluidity and order. EMBO J. 2006;25:3446–57. doi: 10.1038/sj.emboj.7601204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fielding CJ, Fielding PE. Relationship between cholesterol trafficking and signaling in rafts and caveolae. Biochim Biophys Acta. 2003;1610:219–28. doi: 10.1016/s0005-2736(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 35.Schutz GJ, Kada G, Pastushenko VP, Schindler H. Properties of lipid microdomains in a muscle cell membrane visualized by single molecule microscopy. EMBO J. 2000;19:892–901. doi: 10.1093/emboj/19.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simons K, Van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 38.Lou Z, Billadeau DD, Savoy DN, Schoon RA, Leibson PJ. A role for a RhoA/ROCK/LIM-kinase pathway in the regulation of cytotoxic lymphocytes. J Immunol. 2001;167:5749–57. doi: 10.4049/jimmunol.167.10.5749. [DOI] [PubMed] [Google Scholar]
- 39.Marmor MD, Julius M. Role for lipid rafts in regulating interleukin-2 receptor signaling. Blood. 2001;98:1489–97. doi: 10.1182/blood.v98.5.1489. [DOI] [PubMed] [Google Scholar]
- 40.Villalba M, Bi K, Rodriguez F, Tanaka Y, Schoenberger S, Altman A. Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J Cell Biol. 2001;155:331–8. doi: 10.1083/jcb.200107080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwabuchi K, Handa K, Hakomori S. Separation of “glycosphingolipid signaling domain” from caveolincontaining membrane fraction in mouse melanoma B16 cells and its role in cell adhesion coupled with signaling. J Biol Chem. 1998;273:33766–73. doi: 10.1074/jbc.273.50.33766. [DOI] [PubMed] [Google Scholar]
- 42.Abrami L, Fivaz M, Kobayashi T, Kinoshita T, Parton RG, Van Der Goot FG. Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains. J Biol Chem. 2001;276:30729–36. doi: 10.1074/jbc.M102039200. [DOI] [PubMed] [Google Scholar]
- 43.Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–88. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 44.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishitsuka R, Sato SB, Kobayashi T. Imaging lipid rafts. J Biochem. 2005;137:249–54. doi: 10.1093/jb/mvi041. [DOI] [PubMed] [Google Scholar]
- 46.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–9. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 47.Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–13. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puri V, Watanabe R, Dominguez M, Sun X, Wheatley CL, Marks DL, Pagano RE. Cholesterol modulates membrane traffic along the endocytic pathway in sphingolipid-storage diseases. Nat Cell Biol. 1999;1:386–8. doi: 10.1038/14084. [DOI] [PubMed] [Google Scholar]
- 49.Nichols BJ, Kenworthy AK, Polishchuk RS, Lodge R, Roberts TH, Hirschberg K, Phair RD, Lippincott-Schwartz J. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J Cell Biol. 2001;153:529–41. doi: 10.1083/jcb.153.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mouritsen OG, Kinnunen PJK. Role of lipid organization and dynamics for membrane functionality. In: Merz KM Jr, Roux B, editors. Biological membranes. A molecular perspective from computation to experiment. Boston: Birkhäuser; 1996. pp. 463–502. [Google Scholar]
- 51.Janmey PA, Kinnunen PK. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006;16:538–46. doi: 10.1016/j.tcb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Kinnunen PKJ. On the mechanisms of the lamellar → hexagonal HII phase transition and the biological significance of the HII propensity. In: Lasic D, Arenholz Y, editors. Nonmedical applications of liposomes. Boca Raton, FL: CRC Press; 1996. pp. 153–71. [Google Scholar]
- 53.Kinnunen PKJ. Membrane negative spontaneous curvature as an ancient signal for cell growth. In: Mouritsen OG, Andersen OS, editors. Search of a new biomembrane model. Copenhangen: Biologiske Skrifter; 1998. pp. 175–8. [Google Scholar]
- 54.Kinnunen PK. On the principles of functional ordering in biological membranes. Chem Phys Lipids. 1991;57:375–99. doi: 10.1016/0009-3084(91)90087-r. [DOI] [PubMed] [Google Scholar]
- 55.Horwitz AF, Hatten ME, Burger MM. Membrane fatty acid replacements and their effect on growth and lectin-induced agglutinability. Proc Natl Acad Sci USA. 1974;71:3115–9. doi: 10.1073/pnas.71.8.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mavis RD, Vagelos PR. The effect of phospholipid fatty acid composition in membranous enzymes in Escherichia coli. J Biol Chem. 1972;247:652–9. [PubMed] [Google Scholar]
- 57.Steim JM, Tourtellotte ME, Reinert JC, McElhaney RN, Rader RL. Calorimetric evidence for the liquid-crystalline state of lipids in a biomembrane. Proc Natl Acad Sci USA. 1969;63:104–9. doi: 10.1073/pnas.63.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stier A, Sackmann E. Spin labels as enzyme substrates. Heterogeneous lipid distribution in liver microsomal membranes. Biochim Biophys Acta. 1973;311:400–8. doi: 10.1016/0005-2736(73)90320-9. [DOI] [PubMed] [Google Scholar]
- 59.Trauble H, Overath P. The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim Biophys Acta. 1973;307:491–512. doi: 10.1016/0005-2736(73)90296-4. [DOI] [PubMed] [Google Scholar]
- 60.Goodsaid-Zalduondo F, Rintoul DA, Carlson JC, Hansel W. Luteolysis-induced changes in phase composition and fluidity of bovine luteal cell membranes. Proc Natl Acad Sci USA. 1982;79:4332–6. doi: 10.1073/pnas.79.14.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoover RL, Dawidowicz EA, Robinson JM, Karnovsky MJ. Role of cholesterol in the capping of surface immunoglobulin receptors on murine lymphocytes. J Cell Biol. 1983;97:73–80. doi: 10.1083/jcb.97.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karnovsky MJ, Kleinfeld AM, Hoover RL, Klausner RD. The concept of lipid domains in membranes. J Cell Biol. 1982;94:1–6. doi: 10.1083/jcb.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klausner RD, Kleinfeld AM, Hoover RL, Karnovsky MJ. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem. 1980;255:1286–95. [PubMed] [Google Scholar]
- 64.Estep TN, Mountcastle DB, Barenholz Y, Biltonen RL, Thompson TE. Thermal behavior of synthetic sphingomyelin-cholesterol dispersions. Biochemistry. 1979;18:2112–7. doi: 10.1021/bi00577a042. [DOI] [PubMed] [Google Scholar]
- 65.Sankaram MB, Thompson TE. Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry. 1990;29:10670–5. doi: 10.1021/bi00499a014. [DOI] [PubMed] [Google Scholar]
- 66.Lehtonen JY, Holopainen JM, Kinnunen PK. Evidence for the formation of microdomains in liquid crystalline large unilamellar vesicles caused by hydrophobic mismatch of the constituent phospholipids. Biophys J. 1996;70:1753–60. doi: 10.1016/S0006-3495(96)79738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lehtonen JY, Kinnunen PK. Evidence for phospholipid microdomain formation in liquid crystalline lipo-somes reconstituted with Escherichia coli lactose permease. Biophys J. 1997;72:1247–57. doi: 10.1016/S0006-3495(97)78771-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ipsen JH, Karlstrom G, Mouritsen OG, Wennerstrom H, Zuckermann MJ. Phase equilibria in the phosphatidylcholinecholesterol system. Biochim Biophys Acta. 1987;905:162–72. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 69.Holopainen JM, Metso AJ, Mattila JP, Jutila A, Kinnunen PK. Evidence for the lack of a specific interaction between cholesterol and sphingomyelin. Biophys J. 2004;86:1510–20. doi: 10.1016/S0006-3495(04)74219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goñi FM, Alonso A. Biophysics of sphingolipids I. Membrane properties of sphingosine, ceramides and other simple sphingolipids. Biochim Biophys Acta. 2006;1758:1902–21. doi: 10.1016/j.bbamem.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 71.Holopainen JM, Lehtonen JY, Kinnunen PK. Lipid microdomains in dimyristoylphosphatidylcholine-ceramide liposomes. Chem Phys Lipids. 1997;88:1–13. doi: 10.1016/s0009-3084(97)00040-6. [DOI] [PubMed] [Google Scholar]
- 72.Holopainen JM, Subramanian M, Kinnunen PK. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry. 1998;37:17562–70. doi: 10.1021/bi980915e. [DOI] [PubMed] [Google Scholar]
- 73.Holopainen JM, Medina OP, Metso AJ, Kinnunen PK. Sphingomyelinase activity associated with human plasma low density lipoprotein. J Biol Chem. 2000;275:16484–9. doi: 10.1074/jbc.275.22.16484. [DOI] [PubMed] [Google Scholar]
- 74.Nurminen TA, Holopainen JM, Zhao H, Kinnunen PK. Observation of topical catalysis by sphingomyelinase coupled to microspheres. J Am Chem Soc. 2002;124:12129–34. doi: 10.1021/ja017807r. [DOI] [PubMed] [Google Scholar]
- 75.Kinnunen PKJ, Holopainen JM. Ceramide in apoptosis: possible biophysical foundation of action. In: Futerman AH, editor. Ceramide signaling. Molecular biology intellingence unit 21. New York: Landes Bioscience; 2002. pp. 9–19. [Google Scholar]
- 76.Cantor RS. The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Biochemistry. 1997;36:2339–44. doi: 10.1021/bi9627323. [DOI] [PubMed] [Google Scholar]
- 77.Kinnunen PK, Koiv A, Lehtonen JY, Rytomaa M, Mustonen P. Lipid dynamics and peripheral interactions of proteins with membrane surfaces. Chem Phys Lipids. 1994;73:181–207. doi: 10.1016/0009-3084(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 78.Tuominen EK, Wallace CJ, Kinnunen PK. Phospholipid-cytochrome c interaction: evidence for the extended lipid anchorage. J Biol Chem. 2002;277:8822–6. doi: 10.1074/jbc.M200056200. [DOI] [PubMed] [Google Scholar]
- 79.Patra M. Lateral pressure profiles in cholesterol-DPPC bilayers. Eur Biophys J. 2005;35:79–88. doi: 10.1007/s00249-005-0011-0. [DOI] [PubMed] [Google Scholar]
- 80.Lehtonen JY, Kinnunen PK. Phospholipase A2 as a mechanosensor. Biophys J. 1995;68:1888–94. doi: 10.1016/S0006-3495(95)80366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marshall CJ. Ras effectors. Curr Opin Cell Biol. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- 82.Kinnunen PK. Lipid bilayers as osmotic response elements. Cell Physiol Biochem. 2000;10:243–50. doi: 10.1159/000016360. [DOI] [PubMed] [Google Scholar]
- 83.Mustonen P, Virtanen JA, Somerharju PJ, Kinnunen PK. Binding of cytochrome c to liposomes as revealed by the quenching of fluorescence from pyrene-labeled phospholipids. Biochemistry. 1987;26:2991–7. doi: 10.1021/bi00385a006. [DOI] [PubMed] [Google Scholar]
- 84.Kinnunen PK, Rytomaa M, Koiv A, Lehtonen J, Mustonen P, Aro A. Sphingosine-mediated membrane association of DNA and its reversal by phosphatidic acid. Chem Phys Lipids. 1993;66:75–85. doi: 10.1016/0009-3084(93)90033-y. [DOI] [PubMed] [Google Scholar]
- 85.Mustonen P, Lehtonen J, Koiv A, Kinnunen PK. Effects of sphingosine on peripheral membrane interactions: comparison of adriamycin, cytochrome c, and phospholipase A2. Biochemistry. 1993;32:5373–80. doi: 10.1021/bi00071a012. [DOI] [PubMed] [Google Scholar]
- 86.Gorbenko GP, Molotkovsky JG, Kinnunen PK. Cytochrome C interaction with cardiolipin/phosphatidylcholine model membranes: effect of cardiolipin protonation. Biophys J. 2006;90:4093–103. doi: 10.1529/biophysj.105.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rytomaa M, Kinnunen PK. Evidence for two distinct acidic phospholipid-binding sites in cytochrome c. J Biol Chem. 1994;269:1770–4. [PubMed] [Google Scholar]
- 88.Zhao H, Jutila A, Nurminen T, Wickstrom SA, Keski-Oja J, Kinnunen PK. Binding of endostatin to phosphatidylserine-containing membranes and formation of amyloid-like fibers. Biochemistry. 2005;44:2857–63. doi: 10.1021/bi048510j. [DOI] [PubMed] [Google Scholar]
- 89.Zhao H, Sood R, Jutila A, Bose S, Fimland G, Nissen-Meyer J, Kinnunen PK. Interaction of the antimicrobial peptide pheromone Plantaricin A with model membranes: implications for a novel mechanism of action. Biochim Biophys Acta. 2006;1758:1461–74. doi: 10.1016/j.bbamem.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 90.Gorbenko GP, Kinnunen PK. The role of lipid-protein interactions in amyloid-type protein fibril formation. Chem Phys Lipids. 2006;141:72–82. doi: 10.1016/j.chemphyslip.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 91.Zhao H, Tuominen EK, Kinnunen PK. Formation of amyloid fibers triggered by phosphatidylserine-containing membranes. Biochemistry. 2004;43:10302–7. doi: 10.1021/bi049002c. [DOI] [PubMed] [Google Scholar]
- 92.Rohrbough J, Broadie K. Lipid regulation of the synaptic vesicle cycle. Nat Rev Neurosci. 2005;6:139–50. doi: 10.1038/nrn1608. [DOI] [PubMed] [Google Scholar]
- 93.Emoto K, Inadome H, Kanaho Y, Narumiya S, Umeda M. Local change in phospholipid composition at the cleavage furrow is essential for completion of cytokinesis. J Biol Chem. 2005;280:37901–7. doi: 10.1074/jbc.M504282200. [DOI] [PubMed] [Google Scholar]
- 94.Emoto K, Umeda M. An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J Cell Biol. 2000;149:1215–24. doi: 10.1083/jcb.149.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manzoli FA, Capitani S, Mazzotti G, Barnabei O, Maraldi NM. Role of chromatin phospholipids on template availability and ultrastructure of isolated nuclei. Adv Enzyme Regul. 1982;20:247–62. doi: 10.1016/0065-2571(82)90019-x. [DOI] [PubMed] [Google Scholar]
- 96.Sylvia V, Curtin G, Norman J, Stec J, Busbee D. Activation of a low specific activity form of DNA polymerase alpha by inositol-1,4-bisphosphate. Cell. 1988;54:651–8. doi: 10.1016/s0092-8674(88)80009-6. [DOI] [PubMed] [Google Scholar]
- 97.Mann KG, Jenny RJ, Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Annu Rev Biochem. 1988;57:915–56. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- 98.Comfurius P, Smeets EF, Willems GM, Bevers EM, Zwaal RF. Assembly of the prothrombinase complex on lipid vesicles depends on the stereochemical configuration of the polar headgroup of phosphatidylserine. Biochemistry. 1994;33:10319–24. doi: 10.1021/bi00200a012. [DOI] [PubMed] [Google Scholar]
- 99.Hessel E, Heck M, Muller P, Herrmann A, Hofmann KP. Signal transduction in the visual cascade involves specific lipid-protein interactions. J Biol Chem. 2003;278:22853–60. doi: 10.1074/jbc.M302747200. [DOI] [PubMed] [Google Scholar]
- 100.Vance DE, Vance JE. Biochemistry of lipids, lipoproteins and membranes. 4. Amsterdam: Elsevier; 2004. [Google Scholar]
- 101.Hisatsune C, Nakamura K, Kuroda Y, Nakamura T, Mikoshiba K. Amplification of Ca2+ signaling by diacylglycerol-mediated inositol 1,4,5-trisphosphate production. J Biol Chem. 2005;280:11723–30. doi: 10.1074/jbc.M409535200. [DOI] [PubMed] [Google Scholar]
- 102.Pertile P, Liscovitch M, Chalifa V, Cantley LC. Phosphatidylinositol 4,5-bisphosphate synthesis is required for activation of phospholipase D in U937 cells. J Biol Chem. 1995;270:5130–5. doi: 10.1074/jbc.270.10.5130. [DOI] [PubMed] [Google Scholar]
- 103.Ktistakis NT, Brown HA, Sternweis PC, Roth MG. Phospholipase D is present on Golgi-enriched membranes and its activation by ADP ribosylation factor is sensitive to brefeldin A. Proc Natl Acad Sci USA. 1995;92:4952–6. doi: 10.1073/pnas.92.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cockcroft S, Thomas GM, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty NF, Truong O, Hsuan JJ. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994;263:523–6. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- 105.Waggoner DW, Xu J, Singh I, Jasinska R, Zhang QX, Brindley DN. Structural organization of mammalian lipid phosphate phosphatases: implications for signal transduction. Biochim Biophys Acta. 1999;1439:299–316. doi: 10.1016/s1388-1981(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 106.Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta. 2004;1666:62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 107.Palsdottir H, Hunte C. Lipids in membrane protein structures. Biochim Biophys Acta. 2004;1666:2–18. doi: 10.1016/j.bbamem.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 108.De Kruijff B. Lipid polymorphism and biomembrane function. Curr Opin Chem Biol. 1997;1:564–9. doi: 10.1016/s1367-5931(97)80053-1. [DOI] [PubMed] [Google Scholar]
- 109.Van Den Brink-van der Laan E, Chupin V, Killian JA, De Kruijff B. Stability of KcsA tetramer depends on membrane lateral pressure. Biochemistry. 2004;43:4240–50. doi: 10.1021/bi036129d. [DOI] [PubMed] [Google Scholar]
- 110.Fong TM, McNamee MG. Correlation between acetylcholine receptor function and structural properties of membranes. Biochemistry. 1986;25:830–40. doi: 10.1021/bi00352a015. [DOI] [PubMed] [Google Scholar]
- 111.Jensen MO, Mouritsen OG. Lipids do influence protein function-the hydrophobic matching hypothesis revisited. Biochim Biophys Acta. 2004;1666:205–26. doi: 10.1016/j.bbamem.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 112.Turnheim K, Gruber J, Wachter C, Ruiz-Gutierrez V. Membrane phospholipid composition affects function of potassium channels from rabbit colon epithelium. Am J Physiol. 1999;277:C83–90. doi: 10.1152/ajpcell.1999.277.1.C83. [DOI] [PubMed] [Google Scholar]
- 113.Martens JR, O’Connell K, Tamkun M. Targeting of ion channels to membrane microdomains: localization of KV channels to lipid rafts. Trends Pharmacol Sci. 2004;25:16–21. doi: 10.1016/j.tips.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 114.Blanton MP, Wang HH. Photoaffinity labeling of the Torpedo californica nicotinic acetylcholine receptor with an aryl azide derivative of phosphatidylserine. Biochemistry. 1990;29:1186–94. doi: 10.1021/bi00457a014. [DOI] [PubMed] [Google Scholar]
- 115.Fernandez AM, Fernandez-Ballester G, Ferragut JA, Gonzalez-Ros JM. Labeling of the nicotinic acetylcholine receptor by a photoactivatable steroid probe: effects of cholesterol and cholinergic ligands. Biochim Biophys Acta. 1993;1149:135–44. doi: 10.1016/0005-2736(93)90034-w. [DOI] [PubMed] [Google Scholar]
- 116.Fernandez-Ballester G, Castresana J, Fernandez AM, Arrondo JL, Ferragut JA, Gonzalez-Ros JM. A role for cholesterol as a structural effector of the nico-tinic acetylcholine receptor. Biochemistry. 1994;33:4065–71. doi: 10.1021/bi00179a035. [DOI] [PubMed] [Google Scholar]
- 117.Fong TM, McNamee MG. Stabilization of acetylcholine receptor secondary structure by cholesterol and negatively charged phospholipids in membranes. Biochemistry. 1987;26:3871–80. doi: 10.1021/bi00387a020. [DOI] [PubMed] [Google Scholar]
- 118.Jones OT, McNamee MG. Annular and nonannular binding sites for cholesterol associated with the nico-tinic acetylcholine receptor. Biochemistry. 1988;27:2364–74. doi: 10.1021/bi00407a018. [DOI] [PubMed] [Google Scholar]
- 119.Schrempf H, Schmidt O, Kummerlen R, Hinnah S, Muller D, Betzler M, Steinkamp T, Wagner R. A prokaryotic potassium ion channel with two predicted transmembrane segments from Streptomyces lividans. EMBO J. 1995;14:5170–8. doi: 10.1002/j.1460-2075.1995.tb00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 121.Van Dalen A, Hegger S, Killian JA, De Kruijff B. Influence of lipids on membrane assembly and stability of the potassium channel KcsA. FEBS Lett. 2002;525:33–8. doi: 10.1016/s0014-5793(02)03061-2. [DOI] [PubMed] [Google Scholar]
- 122.Raja M, Spelbrink RE, De Kruijff B, Killian JA. Phosphatidic acid plays a special role in stabilizing and folding of the tetrameric potassium channel KcsA. FEBS Lett. 2007;581:5715–22. doi: 10.1016/j.febslet.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 123.Demmers JA, Van Dalen A, De Kruijff B, Heck AJ, Killian JA. Interaction of the K+ channel KcsA with membrane phospholipids as studied by ESI mass spectrometry. FEBS Lett. 2003;541:28–32. doi: 10.1016/s0014-5793(03)00282-5. [DOI] [PubMed] [Google Scholar]
- 124.Barrera FN, Renert ML, Poveda JA, De Kruijff B, Killian JA, González-Ros JM. Protein self-assembly and lipid binding in the folding of the potassium channel KcsA. Biochemistry. 2008;47:2123–33. doi: 10.1021/bi700778c. [DOI] [PubMed] [Google Scholar]
- 125.Heginbotham L, Kolmakova-Partensky L, Miller C. Functional reconstitution of a prokaryotic K+ channel. J Gen Physiol. 1998;111:741–9. doi: 10.1085/jgp.111.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marius P, Zagnoni M, Sandison ME, East JM, Morgan H, Lee AG. Binding of anionic lipids to at least three nonannular sites on the potassium channel KcsA is required for channel opening. Biophys J. 2008;94:1689–98. doi: 10.1529/biophysj.107.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Belrhali H, Nollert P, Royant A, Menzel C, Rosenbusch JP, Landau EM, Pebay-Peyroula E. Protein, lipid and water organization in bacteriorhodopsin crystals: a molecular view of the purple membrane at 1.9 A resolution. Structure. 1999;7:909–17. doi: 10.1016/s0969-2126(99)80118-x. [DOI] [PubMed] [Google Scholar]
- 128.Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trezeguet V, Lauquin GJ, Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]
- 129.Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–4. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- 130.Blount P. Molecular mechanisms of mechanosensation: big lessons from small cells. Neuron. 2003;37:731–4. doi: 10.1016/s0896-6273(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 131.Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–90. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Perozo E. Gating prokaryotic mechanosensitive channels. Nat Rev Mol Cell Biol. 2006;7:109–19. doi: 10.1038/nrm1833. [DOI] [PubMed] [Google Scholar]
- 133.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–67. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 134.Corey DP. New TRP channels in hearing and mechanosensation. Neuron. 2003;39:585–8. doi: 10.1016/s0896-6273(03)00505-1. [DOI] [PubMed] [Google Scholar]
- 135.Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413:194–202. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- 136.Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 137.Perozo E, Cortes DM, Sompornpisut P, Kloda A, Martinac B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 2002;418:942–8. doi: 10.1038/nature00992. [DOI] [PubMed] [Google Scholar]
- 138.Powl AM, East JM, Lee AG. Different effects of lipid chain length on the two sides of a membrane and the lipid annulus of MscL. Biophys J. 2007;93:113–22. doi: 10.1529/biophysj.107.105130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martinac B, Hamill OP. Gramicidin A channels switch between stretch activation and stretch inactivation depending on bilayer thickness. Proc Natl Acad Sci USA. 2002;99:4308–12. doi: 10.1073/pnas.072632899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yuan C, O’Connell RJ, Jacob RF, Mason RP, Treistman SN. Regulation of the gating of BKCa channel by lipid bilayer thickness. J Biol Chem. 2007;282:7276–86. doi: 10.1074/jbc.M607593200. [DOI] [PubMed] [Google Scholar]
- 141.Schmidt D, Jiang QX, MacKinnon R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature. 2006;444:775–9. doi: 10.1038/nature05416. [DOI] [PubMed] [Google Scholar]
- 142.Johnson JE, Cornell RB. Amphitropic proteins: regulation by reversible membrane interactions. Mol Membr Biol. 1999;16:217–35. doi: 10.1080/096876899294544. [DOI] [PubMed] [Google Scholar]
- 143.Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC) J Biol Chem. 2002;277:7641–4. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- 144.Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3:102–14. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- 145.Ellena JF, Blazing MA, McNamee MG. Lipid-protein interactions in reconstituted membranes containing acetylcholine receptor. Biochemistry. 1983;22:5523–35. doi: 10.1021/bi00293a012. [DOI] [PubMed] [Google Scholar]
- 146.Sunshine C, McNamee MG. Lipid modulation of nicotinic acetylcholine receptor function: the role of membrane lipid composition and fluidity. Biochim Biophys Acta. 1994;1191:59–64. doi: 10.1016/0005-2736(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 147.Fernandez-Carvajal AM, Encinar JA, Poveda JA, De Juan E, Martínez-Pinna J, Ivorra I, Ferragut JA, Morales A, Gonzalez-Ros JM. Structural and functional changes induced in the nicotinic acetylcholine receptor by membrane phospholipids. J Mol Neurosci. 2006;30:121–4. doi: 10.1385/JMN:30:1:121. [DOI] [PubMed] [Google Scholar]
- 148.DaCosta CJ, Ogrel AA, McCardy EA, Blanton MP, Baenziger JE. Lipid-protein interactions at the nicotinic acetylcholine receptor. A functional coupling between nicotinic receptors and phosphatidic acid-containing lipid bilayers. J Biol Chem. 2002;277:201–8. doi: 10.1074/jbc.M108341200. [DOI] [PubMed] [Google Scholar]
- 149.Wenz JJ, Barrantes FJ. Nicotinic acetylcholine receptor induces lateral segregation of phosphatidic acid and phosphatidylcholine in reconstituted membranes. Biochemistry. 2005;44:398–410. doi: 10.1021/bi048026g. [DOI] [PubMed] [Google Scholar]
- 150.Poveda JA, Encinar JA, Fernandez AM, Mateo CR, Ferragut JA, Gonzalez-Ros JM. Segregation of phosphatidic acid-rich domains in reconstituted acetylcholine receptor membranes. Biochemistry. 2002;41:12253–62. doi: 10.1021/bi0200099. [DOI] [PubMed] [Google Scholar]
- 151.Morales A, De Juan E, Fernandez-Carvajal AM, Martínez-Pinna J, Poveda JA, Encinar JA, Ivorra I, Gonzalez-Ros JM. Nicotinic acetylcholine receptor properties are modulated by surrounding lipids: an in vivo study. J Mol Neurosci. 2006;30:5–6. doi: 10.1385/JMN:30:1:5. [DOI] [PubMed] [Google Scholar]
- 152.Luan P, Yang L, Glaser M. Formation of membrane domains created during the budding of vesicular stomatitis virus. A model for selective lipid and protein sorting in biological membranes. Biochemistry. 1995;34:9874–83. doi: 10.1021/bi00031a008. [DOI] [PubMed] [Google Scholar]
- 153.Krishna AG, Menon ST, Terry TJ, Sakmar TP. Evidence that helix 8 of rhodopsin acts as a membrane-dependent conformational switch. Biochemistry. 2002;41:8298–309. doi: 10.1021/bi025534m. [DOI] [PubMed] [Google Scholar]
- 154.Mitchell DC, Niu SL, Litman BJ. Optimization of receptor-G protein coupling by bilayer lipid composition I: kinetics of rhodopsin-transducin binding. J Biol Chem. 2001;276:42801–6. doi: 10.1074/jbc.M105772200. [DOI] [PubMed] [Google Scholar]
- 155.Mozsolits H, Unabia S, Ahmad A, Morton CJ, Thomas WG, Aguilar MI. Electrostatic and hydrophobic forces tether the proximal region of the angiotensin II receptor (AT1A) carboxyl terminus to anionic lipids. Biochemistry. 2002;41:7830–40. doi: 10.1021/bi0121813. [DOI] [PubMed] [Google Scholar]
- 156.Okamoto Y, Ninomiya H, Tanioka M, Sakamoto A, Miwa S, Masaki T. Palmitoylation of human endothelinB. Its critical role in G protein coupling and a differential requirement for the cytoplasmic tail by G protein subtypes. J Biol Chem. 1997;272:21589–96. doi: 10.1074/jbc.272.34.21589. [DOI] [PubMed] [Google Scholar]
- 157.Munshi UM, Clouser CL, Peegel H, Menon KM. Evidence that palmitoylation of carboxyl terminus cysteine residues of the human luteinizing hormone receptor regulates postendocytic processing. Mol Endocrinol. 2005;19:749–58. doi: 10.1210/me.2004-0335. [DOI] [PubMed] [Google Scholar]
- 158.Ponimaskin E, Dumuis A, Gaven F, Barthet G, Heine M, Glebov K, Richter DW, Oppermann M. Palmitoylation of the 5-hydroxytryptamine4a receptor regulates receptor phosphorylation, desensitization, and beta-arrestin-mediated endocytosis. Mol Pharmacol. 2005;67:1434–43. doi: 10.1124/mol.104.008748. [DOI] [PubMed] [Google Scholar]
- 159.Minetti CA, Remeta DP. Energetics of membrane protein folding and stability. Arch Biochem Biophys. 2006;453:32–53. doi: 10.1016/j.abb.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 160.Goñi FM. Non-permanent proteins in membranes: when proteins come as visitors. Mol Membr Biol. 2002;19:237–45. doi: 10.1080/0968768021000035078. [DOI] [PubMed] [Google Scholar]
- 161.Wilson JE. Ambiquitous enzymes: variation in intracellular distribution as a regulatory mechanism. Trends Biochem Sci. 1978;3:124–5. [Google Scholar]
- 162.Burn P. Amphitropic proteins: a new class of membrane proteins. Trends Biochem Sci. 1988;13:79–83. doi: 10.1016/0968-0004(88)90043-6. [DOI] [PubMed] [Google Scholar]
- 163.Bazzi MD, Nelsestuen GL. Protein kinase C and annexins: unusual calcium response elements in the cell. Cell Signal. 1993;5:357–65. doi: 10.1016/0898-6568(93)90075-w. [DOI] [PubMed] [Google Scholar]
- 164.Wimley WC, White SH. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat Struct Biol. 1996;3:842–8. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- 165.Wimley WC, Hristova K, Ladokhin AS, Silvestro L, Axelsen PH, White SH. Folding of beta-sheet membrane proteins: a hydrophobic hexapeptide model. J Mol Biol. 1998;277:1091–110. doi: 10.1006/jmbi.1998.1640. [DOI] [PubMed] [Google Scholar]
- 166.Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B, Hannun YA. De novo ceramide regulates the alternative splicing of cas-pase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 2002;277:12587–95. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- 167.Plummer G, Perreault KR, Holmes CF, Posse De Chaves EI. Activation of serine/threonine protein phosphatase-1 is required for ceramide-induced survival of sympathetic neurons. Biochem J. 2005;385:685–93. doi: 10.1042/BJ20040929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yan F, Polk DB. Kinase suppressor of ras is necessary for tumor necrosis factor alpha activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in intestinal epithelial cells. Cancer Res. 2001;61:963–9. [PubMed] [Google Scholar]
- 169.Diaz-Meco MT, Municio MM, Frutos S, Sanchez P, Lozano J, Sanz L, Moscat J. The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell. 1996;86:777–86. doi: 10.1016/s0092-8674(00)80152-x. [DOI] [PubMed] [Google Scholar]
- 170.Kashiwagi M, Ohba M, Chida K, Kuroki T. Protein kinase C eta (PKC eta): its involvement in ker-atinocyte differentiation. J Biochem. 2002;132:853–7. doi: 10.1093/oxfordjournals.jbchem.a003297. [DOI] [PubMed] [Google Scholar]
- 171.Zhou M, Horita DA, Waugh DS, Byrd RA, Morrison DK. Solution structure and functional analysis of the cysteine-rich C1 domain of kinase suppressor of Ras (KSR) J Mol Biol. 2002;315:435–46. doi: 10.1006/jmbi.2001.5263. [DOI] [PubMed] [Google Scholar]
- 172.Van Blitterswijk WJ. Hypothesis: ceramide conditionally activates atypical protein kinases C, Raf-1 and KSR through binding to their cysteine-rich domains. Biochem J. 1998;331:679–80. [PMC free article] [PubMed] [Google Scholar]
- 173.Stahelin RV, Subramanian P, Vora M, Cho W, Chalfant CE. Ceramide-1-phosphate binds group IVA cytosolic phospholipase a2 via a novel site in the C2 domain. J Biol Chem. 2007;282:20467–74. doi: 10.1074/jbc.M701396200. [DOI] [PubMed] [Google Scholar]
- 174.Gómez-Fernández JC, Corbalán-Garca S. Diacylglycerols, multivalent membrane modulators. Chem Phys Lipids. 2007;148:1–25. doi: 10.1016/j.chemphyslip.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 175.Goñi FM, Alonso A. Structure and functional properties of diacylglycerols in membranes. Prog Lipid Res. 1999;38:1–48. doi: 10.1016/s0163-7827(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 176.Jaken S, Parker PJ. Protein kinase C binding partners. Bioessays. 2000;22:245–54. doi: 10.1002/(SICI)1521-1878(200003)22:3<245::AID-BIES6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 177.Nakamoto H, Vigh L. The small heat shock proteins and their clients. Cell Mol Life Sci. 2007;64:294–306. doi: 10.1007/s00018-006-6321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mileykovskaya E, Dowhan W. Role of membrane lipids in bacterial division-site selection. Curr Opin Microbiol. 2005;8:135–42. doi: 10.1016/j.mib.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 179.Wirtz KW. Phospholipid transfer proteins revisited. Biochem J. 1997;324:353–60. doi: 10.1042/bj3240353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mousley CJ, Tyeryar KR, Vincent-Pope P, Bankaitis VA. The Sec14-superfamily and the regulatory interface between phospholipid metabolism and membrane trafficking. Biochim Biophys Acta. 2007;1771:727–36. doi: 10.1016/j.bbalip.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lamour NF, Stahelin RV, Wijesinghe DS, Maceyka M, Wang E, Allegood JC, Merrill AH, Jr, Cho W, Chalfant CE. Ceramide kinase uses ceramide provided by ceramide transport protein: localization to organelles of eicosanoid synthesis. J Lipid Res. 2007;48:1293–304. doi: 10.1194/jlr.M700083-JLR200. [DOI] [PubMed] [Google Scholar]
- 182.Davies SM, Epand RM, Kraayenhof R, Cornell RB. Regulation of CTP: phosphocholine cytidylyltransferase activity by the physical properties of lipid membranes: an important role for stored curvature strain energy. Biochemistry. 2001;40:10522–31. doi: 10.1021/bi010904c. [DOI] [PubMed] [Google Scholar]
- 183.Arnold RS, DePaoli-Roach AA, Cornell RB. Binding of CTP: phosphocholine cytidylyltransferase to lipid vesicles: diacylglycerol and enzyme dephosphorylation increase the affinity for negatively charged membranes. Biochemistry. 1997;36:6149–56. doi: 10.1021/bi970023z. [DOI] [PubMed] [Google Scholar]
- 184.Stopar D, Spruijt RB, Wolfs CJ, Hemminga MA. Structural characterization of bacteriophage M13 solubilization by amphiphiles. Biochim Biophys Acta. 2002;1594:54–63. doi: 10.1016/s0167-4838(01)00281-3. [DOI] [PubMed] [Google Scholar]
- 185.Barlic A, Gutierrez-Aguirre I, Caaveiro JM, Cruz A, Ruiz-Arguello MB, Perez-Gil J, Gonzalez-Manas JM. Lipid phase coexistence favors membrane insertion of equinatoxin-II, a pore-forming toxin from Actinia equina. J Biol Chem. 2004;279:34209–16. doi: 10.1074/jbc.M313817200. [DOI] [PubMed] [Google Scholar]
- 186.Sanchez-Magraner L, Viguera AR, García-Pacios M, Garcillan MP, Arrondo JL, De La Cruz F, Goñi FM, Ostolaza H. The calcium-binding C-terminal domain of Escherichia coli alpha-hemolysin is a major determinant in the surface-active properties of the protein. J Biol Chem. 2007;282:11827–35. doi: 10.1074/jbc.M700547200. [DOI] [PubMed] [Google Scholar]
- 187.Parker MW, Feil SC. Pore-forming protein toxins: from structure to function. Prog Biophys Mol Biol. 2005;88:91–142. doi: 10.1016/j.pbiomolbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 188.Buckley JT. The channel-forming toxin aerolysin. In: Alouf JE, John H, Freer JH, editors. The comprehensive sourcebook of bacterial protein toxins. New York: Academic Press; 1999. pp. 362–72. [Google Scholar]
- 189.Parker MW, Buckley JT, Postma JP, Tucker AD, Leonard K, Pattus F, Tsernoglou D. Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states. Nature. 1994;367:292–5. doi: 10.1038/367292a0. [DOI] [PubMed] [Google Scholar]
- 190.Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–66. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 191.Alonso A, Goñi FM, Buckley JT. Lipids favoring inverted phase enhance the ability of aerolysin to permeabilize liposome bilayers. Biochemistry. 2000;39:14019–24. doi: 10.1021/bi001739o. [DOI] [PubMed] [Google Scholar]
- 192.Bakas L, Ostolaza H, Vaz WL, Goñi FM. Reversible adsorption and nonreversible insertion of Escherichia coli alpha-hemolysin into lipid bilayers. Biophys J. 1996;71:1869–76. doi: 10.1016/S0006-3495(96)79386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sanchez-Magraner L, Cortajarena AL, Goñi FM, Ostolaza H. Membrane insertion of Escherichia coli alpha-hemolysin is independent from membrane lysis. J Biol Chem. 2006;281:5461–7. doi: 10.1074/jbc.M512897200. [DOI] [PubMed] [Google Scholar]
- 194.Balsalobre C, Silvan JM, Berglund S, Mizunoe Y, Uhlin BE, Wai SN. Release of the type I secreted alpha-haemolysin via outer membrane vesicles from Escherichia coli. Mol Microbiol. 2006;59:99–112. doi: 10.1111/j.1365-2958.2005.04938.x. [DOI] [PubMed] [Google Scholar]
- 195.Caaveiro JM, Echabe I, Gutierrez-Aguirre I, Nieva JL, Arrondo JL, Gonzalez-Manas JM. Differential interaction of equinatoxin II with model membranes in response to lipid composition. Biophys J. 2001;80:1343–53. doi: 10.1016/S0006-3495(01)76107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang Y, Bjes ES, Esser AF. Molecular aspects of complement-mediated bacterial killing. Periplasmic conversion of C9 from a protoxin to a toxin. J Biol Chem. 2000;275:4687–92. doi: 10.1074/jbc.275.7.4687. [DOI] [PubMed] [Google Scholar]
- 197.Wu JR, Zhou C, Majumder R, Powers DD, Weinreb G, Lentz BR. Role of procoagulant lipids in human prothrombin activation. 1. Prothrombin activation by factor X(a) in the absence of factor V(a) and in the absence and presence of membranes. Biochemistry. 2002;41:935–49. doi: 10.1021/bi0116893. [DOI] [PubMed] [Google Scholar]
- 198.Brinkman HJ, Mertens K, Van Mourik JA. Phospholipid-binding domain of factor VIII is involved in endothelial cell-mediated activation of factor X by factor IXa. Arterioscler Thromb Vasc Biol. 2002;22:511–6. doi: 10.1161/hq0302.105359. [DOI] [PubMed] [Google Scholar]
- 199.Kalafatis M, Mann KG. The role of the membrane in the inactivation of factor Va by plasmin. Amino acid region 307–348 of factor V plays a critical role in factor Va cofactor function. J Biol Chem. 2001;276:18614–23. doi: 10.1074/jbc.M007134200. [DOI] [PubMed] [Google Scholar]
- 200.Majumder R, Quinn-Allen MA, Kane WH, Lentz BR. The phosphatidylserine binding site of the factor Va C2 domain accounts for membrane binding but does not contribute to the assembly or activity of a human factor Xa-factor Va complex. Biochemistry. 2005;44:711–8. doi: 10.1021/bi047962t. [DOI] [PubMed] [Google Scholar]
- 201.Maggio B, Rosetti CM, Borioli GA, Fanani ML, Del Boca M. Protein-mediated surface structuring in biomembranes. Braz J Med Biol Res. 2005;38:1735–48. doi: 10.1590/s0100-879x2005001200002. [DOI] [PubMed] [Google Scholar]
- 202.Malbon CC, Berrios M, Guest SJ, Hadcock JR, Morris GM, Galvin-Parton PA, Wang HY. Signal transduction via G-protein-linked receptors: physiological regulation from the plasma membrane to the genome. Chin J Physiol. 1991;34:105–20. [PubMed] [Google Scholar]
- 203.Morris AJ, Malbon CC. Physiological regulation of G protein-linked signaling. Physiol Rev. 1999;79:1373–430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- 204.Duchene J, Chauhan SD, Lopez F, Pecher C, Esteve JP, Girolami JP, Bascands JL, Schanstra JP. Direct protein-protein interaction between PLCgamma1 and the bradykinin B2 receptor–importance of growth conditions. Biochem Biophys Res Commun. 2005;326:894–900. doi: 10.1016/j.bbrc.2004.11.126. [DOI] [PubMed] [Google Scholar]
- 205.Duchene J, Schanstra JP, Pecher C, Pizard A, Susini C, Esteve JP, Bascands JL, Girolami JP. A novel protein-protein interaction between a G protein-coupled receptor and the phosphatase SHP-2 is involved in bradykinin-induced inhibition of cell proliferation. J Biol Chem. 2002;277:40375–83. doi: 10.1074/jbc.M202744200. [DOI] [PubMed] [Google Scholar]
- 206.Cismowski MJ, Takesono A, Ma C, Lizano JS, Xie X, Fuernkranz H, Lanier SM, Duzic E. Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nat Biotechnol. 1999;17:878–83. doi: 10.1038/12867. [DOI] [PubMed] [Google Scholar]
- 207.Takesono A, Cismowski MJ, Ribas C, Bernard M, Chung P, Hazard S, 3rd, Duzic E, Lanier SM. Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem. 1999;274:33202–5. doi: 10.1074/jbc.274.47.33202. [DOI] [PubMed] [Google Scholar]
- 208.Downes GB, Gautam N. The G protein subunit gene families. Genomics. 1999;62:544–52. doi: 10.1006/geno.1999.5992. [DOI] [PubMed] [Google Scholar]
- 209.Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr Rev. 2003;24:765–81. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 210.Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–59. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- 211.Chen CA, Manning DR. Regulation of G proteins by covalent modification. Oncogene. 2001;20:1643–52. doi: 10.1038/sj.onc.1204185. [DOI] [PubMed] [Google Scholar]
- 212.Wedegaertner PB. Lipid modifications and membrane targeting of G alpha. Biol Signals Recept. 1998;7:125–35. doi: 10.1159/000014538. [DOI] [PubMed] [Google Scholar]
- 213.Allen JA, Yu JZ, Donati RJ, Rasenick MM. Beta-adrenergic receptor stimulation promotes G alpha s internalization through lipid rafts: a study in living cells. Mol Pharmacol. 2005;67:1493–504. doi: 10.1124/mol.104.008342. [DOI] [PubMed] [Google Scholar]
- 214.Hynes TR, Mervine SM, Yost EA, Sabo JL, Berlot CH. Live cell imaging of Gs and the beta2-adrenergic receptor demonstrates that both alphas and beta1gamma7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the beta2-adrenergic receptor. J Biol Chem. 2004;279:44101–12. doi: 10.1074/jbc.M405151200. [DOI] [PubMed] [Google Scholar]
- 215.Wedegaertner PB, Chu DH, Wilson PT, Levis MJ, Bourne HR. Palmitoylation is required for signaling functions and membrane attachment of Gq alpha and Gs alpha. J Biol Chem. 1993;268:25001–8. [PubMed] [Google Scholar]
- 216.Yu JZ, Rasenick MM. Real-time visualization of a fluorescent G(alpha)(s): dissociation of the activated G protein from plasma membrane. Mol Pharmacol. 2002;61:352–9. doi: 10.1124/mol.61.2.352. [DOI] [PubMed] [Google Scholar]
- 217.Lowy DR, Willumsen BM. Function and regulation of ras. Annu Rev Biochem. 1993;62:851–91. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- 218.Prior IA, Harding A, Yan J, Sluimer J, Parton RG, Hancock JF. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol. 2001;3:368–75. doi: 10.1038/35070050. [DOI] [PubMed] [Google Scholar]
- 219.Prior IA, Muncke C, Parton RG, Hancock JF. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J Cell Biol. 2003;160:165–70. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hancock JF, Magee AI, Childs JE, Marshall CJ. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989;57:1167–77. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 221.Crespo P, Leon J. Ras proteins in the control of the cell cycle and cell differentiation. Cell Mol Life Sci. 2000;57:1613–36. doi: 10.1007/PL00000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bollag G, McCormick F. Regulators and effectors of ras proteins. Annu Rev Cell Biol. 1991;7:601–32. doi: 10.1146/annurev.cb.07.110191.003125. [DOI] [PubMed] [Google Scholar]
- 223.Herrmann C, Nassar N. Ras and its effectors. Prog Biophys Mol Biol. 1996;66:1–41. doi: 10.1016/s0079-6107(96)00015-6. [DOI] [PubMed] [Google Scholar]
- 224.Joneson T, Bar-Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med. 1997;75:587–93. doi: 10.1007/s001090050143. [DOI] [PubMed] [Google Scholar]
- 225.Katz ME, McCormick F. Signal transduction from multiple Ras effectors. Curr Opin Genet Dev. 1997;7:75–9. doi: 10.1016/s0959-437x(97)80112-8. [DOI] [PubMed] [Google Scholar]
- 226.Rodriguez-Viciana P, McCormick F. Characterization of interactions between ras family GTPases and their effectors. Methods Enzymol. 2005;407:187–94. doi: 10.1016/S0076-6879(05)07016-3. [DOI] [PubMed] [Google Scholar]
- 227.Wittinghofer A. Signal transduction via Ras. Biol Chem. 1998;379:933–7. [PubMed] [Google Scholar]
- 228.Mumby SM. Reversible palmitoylation of signaling proteins. Curr Opin Cell Biol. 1997;9:148–54. doi: 10.1016/s0955-0674(97)80056-7. [DOI] [PubMed] [Google Scholar]
- 229.Glomset JA, Farnsworth CC. Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu Rev Cell Biol. 1994;10:181–205. doi: 10.1146/annurev.cb.10.110194.001145. [DOI] [PubMed] [Google Scholar]
- 230.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–69. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 231.Akopyan TN, Couedel Y, Orlowski M, Fournie-Zaluski MC, Roques BP. Proteolytic processing of farnesylated peptides: assay and partial purification from pig brain membranes of an endopeptidase which has the characteristics of E.C. 3.4.24.15. Biochem Biophys Res Commun. 1994;198:787–94. doi: 10.1006/bbrc.1994.1113. [DOI] [PubMed] [Google Scholar]
- 232.Boyartchuk VL, Ashby MN, Rine J. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science. 1997;275:1796–800. doi: 10.1126/science.275.5307.1796. [DOI] [PubMed] [Google Scholar]
- 233.Milligan G, Parenti M, Magee AI. The dynamic role of palmitoylation in signal transduction. Trends Biochem Sci. 1995;20:181–7. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- 234.Pechlivanis M, Kuhlmann J. Hydrophobic modifications of Ras proteins by isoprenoid groups and fatty acids–more than just membrane anchoring. Biochim Biophys Acta. 2006;1764:1914–31. doi: 10.1016/j.bbapap.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 235.Dudler T, Gelb MH. Palmitoylation of Ha-Ras facilitates membrane binding, activation of downstream effectors, and meiotic maturation in Xenopus oocytes. J Biol Chem. 1996;271:11541–7. doi: 10.1074/jbc.271.19.11541. [DOI] [PubMed] [Google Scholar]
- 236.Nomura K, Kanemura H, Satoh T, Kataoka T. Identification of a novel domain of Ras and Rap1 that directs their differential subcellular localizations. J Biol Chem. 2004;279:22664–73. doi: 10.1074/jbc.M314169200. [DOI] [PubMed] [Google Scholar]
- 237.Elion EA. Routing MAP kinase cascades. Science. 1998;281:1625–6. doi: 10.1126/science.281.5383.1625. [DOI] [PubMed] [Google Scholar]
- 238.Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–44. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Takai Y, Kishimoto A, Inoue M, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977;252:7603–9. [PubMed] [Google Scholar]
- 240.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–94. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 241.Grossoni VC, Falbo KB, Kazanietz MG, De Kier Joffe ED, Urtreger AJ. Protein kinase C delta enhances proliferation and survival of murine mammary cells. Mol Carcinog. 2007;46:381–90. doi: 10.1002/mc.20287. [DOI] [PubMed] [Google Scholar]
- 242.Kazanietz MG, Barchi JJ, Jr, Omichinski JG, Blumberg PM. Low affinity binding of phorbol esters to protein kinase C and its recombinant cysteine-rich region in the absence of phospholipids. J Biol Chem. 1995;270:14679–84. doi: 10.1074/jbc.270.24.14679. [DOI] [PubMed] [Google Scholar]
- 243.Nelsestuen GL, Bazzi MD. Activation and regulation of protein kinase C enzymes. J Bioenerg Biomembr. 1991;23:43–61. doi: 10.1007/BF00768838. [DOI] [PubMed] [Google Scholar]
- 244.Medkova M, Cho W. Interplay of C1 and C2 domains of protein kinase C-alpha in its membrane binding and activation. J Biol Chem. 1999;274:19852–61. doi: 10.1074/jbc.274.28.19852. [DOI] [PubMed] [Google Scholar]
- 245.Martnez J, Vögler O, Casas J, Barceló F, Alemany R, Prades J, Nagy T, Baamonde C, Kasprzyk PG, Teres S, Saus C, Escribá PV. Membrane structure modulation, protein kinase C alpha activation, and anticancer activity of Minerval. Mol Pharmacol. 2005;67:531–40. doi: 10.1124/mol.104.000778. [DOI] [PubMed] [Google Scholar]
- 246.Kazanietz MG, Krausz KW, Blumberg PM. Differential irreversible insertion of protein kinase C into phospholipid vesicles by phorbol esters and related activators. J Biol Chem. 1992;267:20878–86. [PubMed] [Google Scholar]
- 247.Konig B, DiNitto PA, Blumberg PM. Stoichiometric binding of diacylglycerol to the phorbol ester receptor. J Cell Biochem. 1985;29:37–44. doi: 10.1002/jcb.240290105. [DOI] [PubMed] [Google Scholar]
- 248.Bell RM, Burns DJ. Lipid activation of protein kinase C. J Biol Chem. 1991;266:4661–4. [PubMed] [Google Scholar]
- 249.Mosior M, Golini ES, Epand RM. Chemical specificity and physical properties of the lipid bilayer in the regulation of protein kinase C by anionic phospholipids: evidence for the lack of a specific binding site for phosphatidylserine. Proc Natl Acad Sci USA. 1996;93:1907–12. doi: 10.1073/pnas.93.5.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chen SG, Kulju D, Halt S, Murakami K. Phosphatidylcholine-dependent protein kinase C activation. Effects of cis-fatty acid and diacylglycerol on synergism, autophosphorylation and Ca(2+)-dependency. Biochem J. 1992;284:221–6. doi: 10.1042/bj2840221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bruins RH, Epand RM. Substrate-induced translocation of PKC-alpha to the membrane. Arch Biochem Biophys. 1995;324:216–22. doi: 10.1006/abbi.1995.0033. [DOI] [PubMed] [Google Scholar]
- 252.Aderem AA. Protein myristoylation as an intermediate step during signal transduction in macrophages: its role in arachidonic acid metabolism and in responses to interferon gamma. J Cell Sci Suppl. 1988;9:151–67. doi: 10.1242/jcs.1988.supplement_9.8. [DOI] [PubMed] [Google Scholar]
- 253.Aderem AA, Albert KA, Keum MM, Wang JK, Greengard P, Cohn ZA. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature. 1988;332:362–4. doi: 10.1038/332362a0. [DOI] [PubMed] [Google Scholar]
- 254.Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991;351:320–2. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- 255.Harris TE, Persaud SJ, Jones PM. Pseudosubstrate peptide inhibitors of beta-cell protein kinases: altered selectivity after myristoylation. Mol Cell Endocrinol. 1999;155:61–8. doi: 10.1016/s0303-7207(99)00114-8. [DOI] [PubMed] [Google Scholar]
- 256.Hansson A, Serhan CN, Haeggstrom J, Ingelman-Sundberg M, Samuelsson B. Activation of protein kinase C by lipoxin A and other eicosanoids. Intracellular action of oxygenation products of arachidonic acid. Biochem Biophys Res Commun. 1986;134:1215–22. doi: 10.1016/0006-291x(86)90380-3. [DOI] [PubMed] [Google Scholar]
- 257.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–14. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 258.Funari SS, Barceló F, Escribá PV. Effects of oleic acid and its congeners, elaidic and stearic acids, on the structural properties of phosphatidylethanolamine membranes. J Lipid Res. 2003;44:567–75. doi: 10.1194/jlr.M200356-JLR200. [DOI] [PubMed] [Google Scholar]
- 259.Giorgione J, Epand RM, Buda C, Farkas T. Role of phospholipids containing docosahexaenoyl chains in modulating the activity of protein kinase C. Proc Natl Acad Sci USA. 1995;92:9767–70. doi: 10.1073/pnas.92.21.9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kultz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–57. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- 261.Kim HP, Morse D, Choi AM. Heat-shock proteins: new keys to the development of cytoprotective therapies. Expert Opin Ther Targets. 2006;10:759–69. doi: 10.1517/14728222.10.5.759. [DOI] [PubMed] [Google Scholar]
- 262.Soti C, Nagy E, Giricz Z, Vigh L, Csermely P, Ferdinandy P. Heat shock proteins as emerging therapeutic targets. Br J Pharmacol. 2005;146:769–80. doi: 10.1038/sj.bjp.0706396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bausero MA, Page DT, Osinaga E, Asea A. Surface expression of Hsp25 and Hsp72 differentially regulates tumor growth and metastasis. Tumour Biol. 2004;25:243–51. doi: 10.1159/000081387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ananthan J, Goldberg AL, Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986;232:522–4. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- 265.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–96. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 266.Balogh G, Horvath I, Nagy E, Hoyk Z, Benko S, Bensaude O, Vigh L. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005;272:6077–86. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- 267.Carratu L, Franceschelli S, Pardini CL, Kobayashi GS, Horvath I, Vigh L, Maresca B. Membrane lipid perturbation modifies the set point of the temperature of heat shock response in yeast. Proc Natl Acad Sci USA. 1996;93:3870–5. doi: 10.1073/pnas.93.9.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chatterjee MT, Khalawan SA, Curran BP. Cellular lipid composition influences stress activation of the yeast general stress response element (STRE) Microbiology. 2000;146:877–84. doi: 10.1099/00221287-146-4-877. [DOI] [PubMed] [Google Scholar]
- 269.Horvath I, Glatz A, Varvasovszki V, Torok Z, Pali T, Balogh G, Kovacs E, Nadasdi L, Benko S, Joo F, Vigh L. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene”. Proc Natl Acad Sci USA. 1998;95:3513–8. doi: 10.1073/pnas.95.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Nagy E, Balogi Z, Gombos I, Akerfelt M, Bjorkbom A, Balogh G, Torok Z, Maslyanko A, Fiszer-Kierzkowska A, Lisowska K, Slotte PJ, Sistonen L, Horvath I, Vigh L. Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. Proc Natl Acad Sci USA. 2007;104:7945–50. doi: 10.1073/pnas.0702557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Shigapova N, Torok Z, Balogh G, Goloubinoff P, Vigh L, Horvath I. Membrane fluidization triggers membrane remodeling which affects the thermotolerance in Escherichia coli. Biochem Biophys Res Commun. 2005;328:1216–23. doi: 10.1016/j.bbrc.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 272.Vigh L, Horvath I, Thompson GA., Jr Recovery of Dunaliella salina cells following hydrogenation of lipids in specific membranes by a homogeneous palladium catalyst. Biochim Biophys Acta. 1988;937:42–50. doi: 10.1016/0005-2736(88)90225-8. [DOI] [PubMed] [Google Scholar]
- 273.Vigh L, Los DA, Horvath I, Murata N. The primary signal in the biological perception of temperature: Pd-catalyzed hydrogenation of membrane lipids stimulated the expression of the desA gene in Synechocystis PCC6803. Proc Natl Acad Sci USA. 1993;90:9090–4. doi: 10.1073/pnas.90.19.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bijur GN, Jope RS. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3beta in the regulation of HSF-1 activity. J Neurochem. 2000;75:2401–8. doi: 10.1046/j.1471-4159.2000.0752401.x. [DOI] [PubMed] [Google Scholar]
- 275.Hooper PL, Hooper JJ. Loss of defense against stress: diabetes and heat shock proteins. Diabetes Technol Ther. 2005;7:204–8. doi: 10.1089/dia.2005.7.204. [DOI] [PubMed] [Google Scholar]
- 276.Torok Z, Tsvetkova NM, Balogh G, Horvath I, Nagy E, Penzes Z, Hargitai J, Bensaude O, Csermely P, Crowe JH, Maresca B, Vigh L. Heat shock protein coinducers with no effect on protein denaturation specifically modulate the membrane lipid phase. Proc Natl Acad Sci USA. 2003;100:3131–6. doi: 10.1073/pnas.0438003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Vigh L, Literati PN, Horvath I, Torok Z, Balogh G, Glatz A, Kovacs E, Boros I, Ferdinandy P, Farkas B, Jaszlits L, Jednakovits A, Koranyi L, Maresca B. Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat Med. 1997;3:1150–4. doi: 10.1038/nm1097-1150. [DOI] [PubMed] [Google Scholar]
- 278.Kurthy M, Mogyorosi T, Nagy K, Kukorelli T, Jednakovits A, Talosi L, Biro K. Effect of BRX-220 against peripheral neuropathy and insulin resistance in diabetic rat models. Ann N Y Acad Sci. 2002;967:482–9. doi: 10.1111/j.1749-6632.2002.tb04306.x. [DOI] [PubMed] [Google Scholar]
- 279.Kolonics A. BGP-15, a new type of insuline sensitizer. Diabetes. 2006;55:A483. [Google Scholar]
- 280.Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PD, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2008;105:1739–44. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Khaleque MA, Bharti A, Sawyer D, Gong J, Benjamin IJ, Stevenson MA, Calderwood SK. Induction of heat shock proteins by heregulin beta1 leads to protection from apoptosis and anchorage-independent growth. Oncogene. 2005;24:6564–73. doi: 10.1038/sj.onc.1208798. [DOI] [PubMed] [Google Scholar]
- 282.Pike LJ, Han X, Gross RW. Epidermal growth factor receptors are localized to lipid rafts that contain a balance of inner and outer leaflet lipids: a shotgun lipidomics study. J Biol Chem. 2005;280:26796–804. doi: 10.1074/jbc.M503805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gur G, Yarden Y. Enlightened receptor dynamics. Nat Biotechnol. 2004;22:169–70. doi: 10.1038/nbt0204-169. [DOI] [PubMed] [Google Scholar]
- 284.Kooijman EE, Chupin V, Fuller NL, Kozlov MM, De Kruijff B, Burger KN, Rand PR. Spontaneous curvature of phosphatidic acid and lysophosphatidic acid. Biochemistry. 2005;44:2097–102. doi: 10.1021/bi0478502. [DOI] [PubMed] [Google Scholar]
- 285.Hinderliter A, Biltonen RL, Almeida PF. Lipid modulation of protein-induced membrane domains as a mechanism for controlling signal transduction. Biochemistry. 2004;43:7102–10. doi: 10.1021/bi036334t. [DOI] [PubMed] [Google Scholar]
- 286.Vígh L, Török Zs, Balogh G, Glatz A, Piotto S, Horváth I. Membrane-regulated stress response. In: Csermely P, Vgh L, editors. A Theoretical and practical approach in molecular aspects of the stress response: chaperones, membranes and networks. Austin, TX: Landes Bioscience and Springer+Bisoness Media; 2007. pp. 114–42. [Google Scholar]
- 287.Eyster KM. The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Adv Physiol Educ. 2007;31:5–16. doi: 10.1152/advan.00088.2006. [DOI] [PubMed] [Google Scholar]
- 288.Jurivich DA, Pangas S, Qiu L, Welk JF. Phospholipase A2 triggers the first phase of the thermal stress response and exhibits cell-type specificity. J Immunol. 1996;157:1669–77. [PubMed] [Google Scholar]
- 289.Ohtsuka K, Kawashima D, Gu Y, Saito K. Inducers and co-inducers of molecular chaperones. Int J Hyperthermia. 2005;21:703–11. doi: 10.1080/02656730500384248. [DOI] [PubMed] [Google Scholar]
- 290.Koller M, Konig W. 12-Hydroxyeicosatetraenoic acid (12-HETE) induces heat shock proteins in human leukocytes. Biochem Biophys Res Commun. 1991;175:804–9. doi: 10.1016/0006-291x(91)91636-q. [DOI] [PubMed] [Google Scholar]
- 291.Kunimoto S, Murofushi W, Kai H, Ishida Y, Uchiyama A, Kobayashi T, Kobayashi S, Murofushi H, Murakami-Murofushi K. Steryl glucoside is a lipid mediator in stress-responsive signal transduction. Cell Struct Funct. 2002;27:157–62. doi: 10.1247/csf.27.157. [DOI] [PubMed] [Google Scholar]
- 292.Jenkins GM. The emerging role for sphingolipids in the eukaryotic heat shock response. Cell Mol Life Sci. 2003;60:701–10. doi: 10.1007/s00018-003-2239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Mathieson FA, Nixon GF. Sphingolipids differentially regulate mitogen-activated protein kinases and intracellular Ca2+ in vascular smooth muscle: effects on CREB activation. Br J Pharmacol. 2006;147:351–9. doi: 10.1038/sj.bjp.0706600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kovacs E, Török Z, Horvath I, Vigh L. Heat stress induces association of the GroEL-analog chaperonin with thylakoid membranes in cyanobacterium, Synechocystis PCC 6803. Plant Physiol Biochem. 1994;32:285–93. [Google Scholar]
- 295.Torok Z, Goloubinoff P, Horvath I, Tsvetkova NM, Glatz A, Balogh G, Varvasovszki V, Los DA, Vierling E, Crowe JH, Vigh L. Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc Natl Acad Sci USA. 2001;98:3098–103. doi: 10.1073/pnas.051619498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Torok Z, Horvath I, Goloubinoff P, Kovacs E, Glatz A, Balogh G, Vigh L. Evidence for a lipochaperonin: association of active protein-folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc Natl Acad Sci USA. 1997;94:2192–7. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tsvetkova NM, Horvath I, Torok Z, Wolkers WF, Balogi Z, Shigapova N, Crowe LM, Tablin F, Vierling E, Crowe JH, Vigh L. Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci USA. 2002;99:13504–9. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Balogi Z, Torok Z, Balogh G, Josvay K, Shigapova N, Vierling E, Vigh L, Horvath I. “Heat shock lipid” in cyanobacteria during heat/light-acclimation. Arch Biochem Biophys. 2005;436:346–54. doi: 10.1016/j.abb.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 299.Kovár J, Stýbrová H, Novák P, Ehrlichová M, Truksa J, Koc M, Kriegerbecková K, Scheiber-Mojdehkar B, Goldenberg H. Heat shock protein 90 recognized as an iron-binding protein associated with the plasma membrane of HeLa cells. Cell Physiol Biochem. 2004;14:41–46. doi: 10.1159/000076925. [DOI] [PubMed] [Google Scholar]
- 300.Shah M, Patel K, Fried VA, Sehgal PB. Interactions of STAT3 with caveolin-1 and heat shock protein 90 in plasma membrane raft and cytosolic complexes. Preservation of cytokine signaling during fever. J Biol Chem. 2002;277:45662–9. doi: 10.1074/jbc.M205935200. [DOI] [PubMed] [Google Scholar]
- 301.Waheed AA, Jones TL. Hsp90 interactions and acylation target the G protein Galpha 12 but not Galpha 13 to lipid rafts. J Biol Chem. 2002;277:32409–12. doi: 10.1074/jbc.C200383200. [DOI] [PubMed] [Google Scholar]
- 302.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–11. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 303.Vega VL, De Maio A. Geldanamycin treatment ameliorates the response to LPS in murine macrophages by decreasing CD14 surface expression. Mol Biol Cell. 2003;14:764–73. doi: 10.1091/mbc.E02-08-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Arispe N, Doh M, De Maio A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones. 2002;7:330–8. doi: 10.1379/1466-1268(2002)007<0330:lidtca>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Arispe N, Doh M, Simakova O, Kurganov B, De Maio A. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEBJ. 2004;18:1636–45. doi: 10.1096/fj.04-2088com. [DOI] [PubMed] [Google Scholar]
- 306.Vega VL, De Maio A. Increase in phagocytosis after geldanamycin treatment or heat shock: role of heat shock proteins. J Immunol. 2005;175:5280–7. doi: 10.4049/jimmunol.175.8.5280. [DOI] [PubMed] [Google Scholar]
- 307.Gehrmann M, Brunner M, Pfister K, Reichle A, Kremmer E, Multhoff G. Differential up-regulation of cytosolic and membrane-bound heat shock protein 70 in tumor cells by anti-inflammatory drugs. Clin Cancer Res. 2004;10:3354–64. doi: 10.1158/1078-0432.CCR-03-0382. [DOI] [PubMed] [Google Scholar]
- 308.Gross C, Koelch W, DeMaio A, Arispe N, Multhoff G. Cell surface-bound heat shock protein 70 (Hsp70) mediates perforin-independent apoptosis by specific binding and uptake of granzyme B. J Biol Chem. 2003;278:41173–81. doi: 10.1074/jbc.M302644200. [DOI] [PubMed] [Google Scholar]
- 309.Su CY, Chong KY, Edelstein K, Lille S, Khardori R, Lai CC. Constitutive hsp70 attenuates hydrogen peroxide-induced membrane lipid peroxidation. Biochem Biophys Res Commun. 1999;265:279–84. doi: 10.1006/bbrc.1999.1649. [DOI] [PubMed] [Google Scholar]
- 310.Harada Y, Sato C, Kitajima K. Complex formation of 70-kDa heat shock protein with acidic glycolipids and phospholipids. Biochem Biophys Res Commun. 2007;353:655–60. doi: 10.1016/j.bbrc.2006.12.068. [DOI] [PubMed] [Google Scholar]
- 311.Zourlidou A, Payne Smith MD, Latchman DS. HSP27 but not HSP70 has a potent protective effect against alpha-synuclein-induced cell death in mammalian neuronal cells. J Neurochem. 2004;88:1439–48. doi: 10.1046/j.1471-4159.2003.02273.x. [DOI] [PubMed] [Google Scholar]
- 312.Sakamoto K, Urushidani T, Nagao T. Translocation of HSP27 to sarcomere induced by ischemic preconditioning in isolated rat hearts. Biochem Biophys Res Commun. 2000;269:137–42. doi: 10.1006/bbrc.2000.2233. [DOI] [PubMed] [Google Scholar]
- 313.Calderwood SK, Mambula SS, Gray PJ, Jr, Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581:3689–94. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 314.Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–47. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem. 2003;278:21601–6. doi: 10.1074/jbc.M302326200. [DOI] [PubMed] [Google Scholar]
- 316.Higuchi I, Hashiguchi A, Matsuura E, Higashi K, Shiraishi T, Hirata N, Arimura K, Osame M. Different pattern of HSP47 expression in skeletal muscle of patients with neuromuscular diseases. Neuromuscul Disord. 2007;17:221–6. doi: 10.1016/j.nmd.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 317.Gupta S, Knowlton AA. Cytosolic heat shock protein 60, hypoxia, and apoptosis. Circulation. 2002;106:2727–33. doi: 10.1161/01.cir.0000038112.64503.6e. [DOI] [PubMed] [Google Scholar]
- 318.Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292:H3052–6. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 319.Li G, Ali IS, Currie RW. Insulin induces myocardial protection and Hsp70 localization to plasma membranes in rat hearts. Am J Physiol Heart Circ Physiol. 2006;291:H1709–21. doi: 10.1152/ajpheart.00201.2006. [DOI] [PubMed] [Google Scholar]
- 320.Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Hoyer-Hansen M, Weber E, Multhoff G, Rohde M, Jaattela M. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004;200:425–35. doi: 10.1084/jem.20040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–8. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 322.Piotrowicz RS, Levin EG. Basolateral membrane-associated 27-kDa heat shock protein and microfilament polymerization. J Biol Chem. 1997;272:25920–7. doi: 10.1074/jbc.272.41.25920. [DOI] [PubMed] [Google Scholar]
- 323.Nakagawa M, Tsujimoto N, Nakagawa H, Iwaki T, Fukumaki Y, Iwaki A. Association of HSPB2, a member of the small heat shock protein family, with mitochondria. Exp Cell Res. 2001;271:161–8. doi: 10.1006/excr.2001.5362. [DOI] [PubMed] [Google Scholar]
- 324.Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–52. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 325.Escribá PV, Ferrer-Montiel AV, Ferragut JA, Gonzalez-Ros JM. Role of membrane lipids in the interaction of daunomycin with plasma membranes from tumor cells: implications in drug-resistance phenomena. Biochemistry. 1990;29:7275–82. doi: 10.1021/bi00483a017. [DOI] [PubMed] [Google Scholar]
- 326.Hollan S, Magocsi M, Fodor E, Horanyi M, Harsanyi V, Farkas T. Search for the pathogenesis of the differing phenotype in two compound heterozy-gote Hungarian brothers with the same genotypic triosephosphate isomerase deficiency. Proc Natl Acad Sci USA. 1997;94:10362–6. doi: 10.1073/pnas.94.19.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Swindells MB, Overington JP. Prioritizing the proteome: identifying pharmaceutically relevant targets. Drug Discov Today. 2002;7:516–21. doi: 10.1016/s1359-6446(02)02250-x. [DOI] [PubMed] [Google Scholar]
- 328.Yang Q, Alemany R, Casas J, Kitajka K, Lanier SM, Escribá PV. Influence of the membrane lipid structure on signal processing via G protein-coupled receptors. Mol Pharmacol. 2005;68:210–7. doi: 10.1124/mol.105.011692. [DOI] [PubMed] [Google Scholar]
- 329.Martens JR, Sakamoto N, Sullivan SA, Grobaski TD, Tamkum MM. Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations. Targeting of Kv 1.5 to caveolae. J Biol Chem. 2001;276:8409–14. doi: 10.1074/jbc.M009948200. [DOI] [PubMed] [Google Scholar]
- 330.Gulbins E, Szabo I, Baltzer K, Lang F. Ceramide-induced inhibition of T lymphocyte voltage-gated potassium channel is mediated by tyrosine kinases. Proc Nafl Acad Sci USA. 1997;94:7661–6. doi: 10.1073/pnas.94.14.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem. 1997;272:5388–95. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- 332.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAKK. J Biol Chem. 2000;275:10128–33. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- 333.Cannon B, Hermansson M, Gyorke S, Somerharju P, Virtanen JA, Cheng KH. Regulation of calcium channel activity by lipid domain formation in planar lipid bilayers. Biophys. J. 2003;85:933–42. doi: 10.1016/S0006-3495(03)74532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Kloda A, Lua L, Hall R, Adams DJ, Martinac B. Liposome reconstitution and modulation of recombinant N-methyl-D-aspartate receptor channels by membrane stretch. Proc Natl Acad Sci USA. 2007;104:1540–5. doi: 10.1073/pnas.0609649104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Brusés JL, Chauvet N, Rutishauer U. Membrane lipid rafts are necessary for the maintenance of the α7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons. J Neurosci. 2001;21:504–12. doi: 10.1523/JNEUROSCI.21-02-00504.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pfister G, Stroh CM, Perschinka H, Kind M, Knoflach M, Hinterdorfer P, Wick G. Detection of HSP60 on the membrane surface of stressed human endothelial cells by atomic force and confocal microscopy. J Cell Sci. 2005;118:1587–94. doi: 10.1242/jcs.02292. [DOI] [PubMed] [Google Scholar]
- 337.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70. J Biol Chem. 2005;280:23349–55. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 338.Mambula SS, Calderwood SK. Heat induced release of Hsp70 from prostate carcinoma cells involves both active secretion and passive release from necrotic cells. Int J Hyperthermia. 2006;22:575–85. doi: 10.1080/02656730600976042. [DOI] [PubMed] [Google Scholar]
- 339.Pockley AG. Heat shock proteins, inflammation, and cardiovascular disease. Circulation. 2002;105:1012–17. doi: 10.1161/hc0802.103729. [DOI] [PubMed] [Google Scholar]
- 340.Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, Cao X. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol. 2006;36:1598–607. doi: 10.1002/eji.200535501. [DOI] [PubMed] [Google Scholar]
- 341.Yu X, Harris SL, Levine AJ. The regulation of exo-some secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 342.Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Larnbrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol. 2004;164:1807–15. doi: 10.1016/S0002-9440(10)63739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Nédellec P, Edling Y, Perret E, Fardeau M, Vicart P. Glucocorticoid treatment induces expression of small heat shock proteins in human satellite cell populations: consequences for a desmin-related myopathy involving the R120G alpha B-crystallin mutation. Neuromuscul Disord. 2002;12:457–65. doi: 10.1016/s0960-8966(01)00306-6. [DOI] [PubMed] [Google Scholar]
- 344.Cobb BA, Petrash JM. Structural and Functional Changes in the RA-crystallin R116C mutant in hereditary cataracts. Biochemistry. 2000;39:15791–8. doi: 10.1021/bi001453j. [DOI] [PMC free article] [PubMed] [Google Scholar]


