Highlight
In Fraxinus excelsior the sucrose transporter FeSUT1 is located in the phloem and involved in apoplastic sucrose loading and retrieval. The expression of FeSUT1 is higher under low sucrose conditions.
Key words: Immunolocalization, Fraxinus excelsior, heterologous expression, phloem loading, sucrose transporter.
Abstract
Trees are generally assumed to be symplastic phloem loaders. A typical feature for most wooden species is an open minor vein structure with symplastic connections between mesophyll cells and phloem cells, which allow sucrose to move cell-to-cell through the plasmodesmata into the phloem. Fraxinus excelsior (Oleaceae) also translocates raffinose family oligosaccharides in addition to sucrose. Sucrose concentration was recently shown to be higher in the phloem sap than in the mesophyll cells. This suggests the involvement of apoplastic steps and the activity of sucrose transporters in addition to symplastic phloem-loading processes. In this study, the sucrose transporter FeSUT1 from F. excelsior was analysed. Heterologous expression in baker’s yeast showed that FeSUT1 mediates the uptake of sucrose. Immunohistochemical analyses revealed that FeSUT1 was exclusively located in phloem cells of minor veins and in the transport phloem of F. excelsior. Further characterization identified these cells as sieve elements and possibly ordinary companion cells but not as intermediary cells. The localization and expression pattern point towards functions of FeSUT1 in phloem loading of sucrose as well as in sucrose retrieval. FeSUT1 is most likely responsible for the observed sucrose gradient between mesophyll and phloem. The elevated expression level of FeSUT1 indicated an increased apoplastic carbon export activity from the leaves during spring and late autumn. It is hypothesized that the importance of apoplastic loading is high under low-sucrose conditions and that the availability of two different phloem-loading mechanisms confers advantages for temperate woody species like F. excelsior.
Introduction
Deciduous trees are highly integrated systems of different source tissues and of competing carbohydrate sinks. Whereas the major sinks in herbaceous annuals are generative organs and roots, the major sinks in trees include wood tissue that exhibits annual cycles of carbohydrate storage and remobilization for growth. Plants produce a variety of sugars and while one portion of the soluble carbohydrates is synthesized for carbon storage purposes another portion mainly functions as transport vehicles for energy and carbon. Indeed, as much as 50–70% of the photosynthetically fixed CO2 in mature leaves of herbal plants is exported from the leaf during the light period (Riens et al., 1994). Most of the carbohydrates move to sink tissues through the phloem. Sugar production, phloem loading, and delivery through the phloem are different options that can be regulated to cope with environmental and seasonal changes. This is especially true for woody species because of their long lifespan and exposure to potentially extreme climate changes during annual cycles in temperate forests (De Schepper et al., 2013). However, the regulation processes involved in carbon partitioning in trees are far from understood because most research regarding phloem loading as a regulatory step in carbohydrate delivery has been performed in herbaceous species.
Mechanisms of phloem loading
There are currently three major mechanisms of phloem loading discussed:
(i) The active apoplastic phloem-loading mechanism is associated with a closed minor vein type (type 2) (Gamalei, 1991). Typical for closed minor veins are sieve element-companion cell complexes (SE-CCC), which are not connected to the neighbouring cells by plasmodesmata. Sucrose diffuses symplastically through plasmodesmata from mesophyll cells (MCs) into bundle sheath cells (BSCs) or phloem parenchyma cells (Schulz, 2015). From these cells sucrose will be released to the apoplast by SWEET proteins (Chen et al., 2012) and is taken up into phloem companion cells (CC) by proton-coupled transporters (Giaquinta, 1977; Riesmeier et al., 1994; Stadler et al., 1995; Sauer, 2007). This process results in a higher concentration of sucrose in the SE-CCC than in the surrounding cells.
(ii) The active symplastic phloem-loading mechanism, known as the ‘polymer-trap’ mechanism, is associated with an open minor vein anatomy (type 1) (Gamalei, 1991), the presence of so-called intermediary cells (ICs), and the transport of raffinose family oligosaccharides (RFOs). Sucrose moves from BSCs through branched plasmodesmata into the ICs, which are specialized companion cells in the minor vein phloem. Within the ICs sucrose and galactinol are used as substrates for the synthesis of RFOs (Turgeon et al., 1993; Voitsekhovskaja et al., 2009). According to the hypothesis, the plasmodesmata fields at the BSC–IC interface act as filters that allow the disaccharide sucrose to pass, but reject the larger RFOs (Turgeon et al., 1993; Liesche and Schulz, 2013). The movement of sucrose is maintained by the constant synthesis of RFOs (Voitsekhovskaja et al., 2009) and the process results in high concentrations of RFOs in the phloem.
Minor veins that belong to the open type 1 group are heterogeneous with respect to the CCs. Hence, this group was further divided into type 1 (IC) and type I (CC) (Davidson et al., 2011). Minor veins of type I (IC) contain ICs with abundant branched plasmodesmata on the IC side that are typical for RFO loaders, whereas the minor veins of type 1 (CC) contain ‘ordinary’ CCs. The CCs of type 1 (CC) have symmetrically branched or simple plasmodesmata on the MC or BSC interface with a higher frequency compared to CCs from type 2 minor veins (Davidson et al., 2011).
(iii) The passive loading pathway is associated with minor vein type 1 (CC). Passive transport of sucrose into the phloem is characterized by a symplastic flux of sucrose that relies on a higher concentration of sugars in the MCs than in the SE-CCC. Owing to high plasmodesmatal connectivity between cells of the phloem and the surrounding cells in several tree species, it was assumed that trees are mostly passive phloem loaders (Davidson et al., 2011). Indeed, plasmolysis and autoradiography experiments with 14C-sugars showed no accumulation of photoassimilates in minor veins of Salix babylonica L. (Turgeon and Medville, 1998), supporting the hypothesis of passive phloem loading in trees. In Populus tremula x alba (grey poplar), the expression of yeast invertase in the cell walls of transgenic plants did not inhibit phloem transport (Zhang et al., 2014) and this result was discussed in relation to phloem loading of sucrose through plasmodesmata in poplar. However, in other tree species with an open minor vein type 1 (CC), like Liriodendron tulipifera or Liquidambar styraciflua, active apoplastic phloem loading has been shown to take place (Goggin et al., 2001; Turgeon and Medville, 2004). In Quercus robur (oak), sucrose was more concentrated in the phloem sap than in the cytosol of MCs, which excludes the simple diffusion of sucrose in the direction of the phloem (Öner-Sieben and Lohaus, 2014).
Apparently, phloem-loading strategies are not necessarily predictable based on minor vein structure. Moreover, recent studies have shown that more than one phloem-loading mechanism could exist in parallel in a plant species or in a single minor vein, respectively (Braun and Slewinski, 2009; Voitsekhovskaja et al., 2009; Gil et al., 2011). Slewinski et al. (2013) have discussed that all plant species probably make use of multiple loading strategies.
The role of sucrose transporters
Sucrose transporters (SUTs) play an important role in apoplastic phloem loading in higher plants (Sauer, 2007; Reinders et al., 2012). Based on sequence homology and biochemical activity, SUTs have been divided into different groups, types, clades, or sub-families, depending on the authors chosen nomenclature (Sauer, 2007; Braun and Slewinski, 2009; Reinders et al., 2012). Here, the nomenclature of Sauer (2007) is used. Proton-coupled Group I (monocot) and Group II (dicot) SUTs in the plasmamembrane of phloem cells mediate apoplastic phloem loading with sucrose or sucrose retrieval from the apoplast. Experimental evidence shows that suppression of these SUTs leads to several phenotypical aberrations in different species, such as stunted growth, sterility, and high accumulation of sugars in leaves (Riesmeier et al., 1994; Gottwald et al., 2000). Each angiosperm genome appears to have at least one sequence of the probably ancestral Group III transporter that contains an enlarged central cytoplasmatic loop (Reinders et al., 2012). SUTs of Group IV are mainly found in the tonoplast of MCs, where they are apparently involved in sucrose efflux out of the vacuole into the cytosol (Endler et al., 2006; Frost et al., 2012; Schneider et al., 2012).
Owing to the important role of SUT proteins in carbon allocation, SUT gene expression is tightly regulated on several levels (Ainsworth and Bush, 2011; Liesche et al. 2011). A great variety of factors, such as light, temperature, sugar concentrations, hormones, or pathogens, have an impact on SUT expression (Shakya and Sturm, 1998; Decourteix et al., 2006; Gil et al., 2011). The plasticity of phloem-loading activity has been demonstrated in response to defoliation in Lolium perenne (Berthier et al., 2009). Most of the information on SUT localization, regulation, and function has been derived from studies on herbaceous plants. Only a few transporters have been described in trees so far, e.g. in Juglans regia L. (walnut), Betula pendula (birch), Hevea brasiliensis (para rubber tree), or poplar (Decourteix et al., 2006; Wright et al., 2000; Tang et al., 2010; Payyavula et al., 2011).
Fraxinus excelsior has an open minor vein type 1 (IC) (Öner-Sieben and Lohaus, 2014), and it is thus categorized as an active symplastic phloem loader. It also contains two types of CCs in the minor veins, ICs and one or two ordinary CCs, similar to other symplastic loaders (Turgeon et al., 1993; Voitsekhovskaja et al., 2009; Öner-Sieben and Lohaus, 2014). Recent studies on F. excelsior revealed that, besides high amounts of RFOs, sucrose was present in high concentrations in the phloem sap as well. Moreover, a significant difference in sucrose concentration was observed, with higher concentration in the phloem than in the cytoplasm of MCs.
Based on these results it was assumed that phloem loading of sucrose needs to have an active component in addition to symplastic diffusion (Öner-Sieben and Lohaus, 2014).
In the present study, FeSUT1 from F. excelsior was expressed in yeast and functionally characterized. Immunohistochemical analyses were performed on sections of leaf tissues to determine whether FeSUT1 may be involved in phloem loading. Moreover, the study focused on the tissue-specific sugar contents and FeSUT1 expression in F. excelsior during the course of the year and under different light-dark regimes, especially in leaves.
Materials and methods
Plant material and experimental design
For the experiments, 30-year-old trees from a small forest near the campus (51° 14′ 42″ N, 7° 9′ 5″ E) and 3-year-old saplings that were grown in 5L pots in an open greenhouse were used. Pots were filled with soil that was dug out from a mixed forest (near Wuppertal, Germany). Plant material was obtained from a nursery (Baumschule Selders, Hilden, Germany). Sidewalls of the greenhouse were made of wire to allow free airflow. Experiments were designed as follows: (A) For immunolocalization of FeSUT1, leaves were taken from three 30-year-old trees. Leaves were taken at about 2–3 m height after about 6h of daylight. (B) Tissue-specific samples were taken from three 3-year-old saplings grown in 5L pots. Samples were taken in winter (9 January 2013; T = −4°C), in spring (25 April 2013; T = 18°C), and in summer (25 July 2013, T = 29°C), always in the second half of the light period. Samples were stored at −80°C until analysis. (C) For seasonal monitoring, leaves of three 30-year-old trees were sampled every month over a course of 3 years from 2009 to 2012. Leaves were taken at about 2–3 m height after 6–8h of daylight and stored at −80°C until analysis. (D) For light and dark experiments, leaves were taken from three 3-year-old saplings. Experiments were conducted on the 18–20 July 2012 with plants in the greenhouse described above. The first set of leaves was taken in the second half of the light period. After the end of the illumination period three plants were translocated in a completely dark room. Leaf samples were taken after 5h, 17h, and 43h of darkness.
Expression of FeSUT1 in Saccharomyces cerevisiae and uptake experiments
The shuttle vector NEV-E (Sauer and Stolz, 1994) was used for heterologous expression of FeSUT1 in Saccharomyces cerevisiae. FeSUT1 was cloned by reverse transcription PCR and RACE from leaves of F. excelsior (Öner-Sieben and Lohaus, 2014). To enhance the expression, the 5′-untranslated region of PmSUC2 (5′-AAGCTTGTAAAAGAA-3′; Gahrtz et al., 1994; Sauer and Stolz, 1994) was introduced into the full-length cDNA sequence of FeSUT1 that was subsequently cloned into the EcoRI site of the vector. The yeast strain SEY2102 (Emr et al., 1983) was transformed with plasmids harbouring inserts in sense or antisense orientation. Uptake was analysed as described (Gahrtz et al., 1994).
Preparation of plasma membranes and SDS-polyacrylamide gel electrophoresis
Isolation of yeast plasma membrane proteins and SDS-polyacrylamide gel electrophoresis are described elsewhere (Laemmli, 1970).
Production and purification of anti-FeSUT1 antiserum
A region within the central loop of the protein was chosen for immunization. The corresponding peptide sequence QAEPPENIGHGVVK was synthesized, coupled to keyhole limpet haemocyanin, and used to immunize three rabbits (Pineda Antikörperservice, Berlin, Germany). The crude anti-FeSUT1 antisera were purified by adsorption to the synthetic FeSUT1 peptide coupled to keyhole limpet haemocyanin, which had been immobilized on nitrocellulose membranes (Sauer and Stadler, 1993; Schmitt et al., 2008).
Tissue preparation, immunohistochemistry, and fluorescence microscopy
The plant tissue was immersed in fixative (75% (v/v) ethanol p.A., 25% (v/v) glacial acidic acid) for 90min at 4°C. Embedding was carried out as described earlier (Stadler and Sauer, 1996; Schmitt et al., 2008). All immunohistochemical analyses were performed with purified Anti-FeSUT1 antiserum (diluted 1:4 in blocking buffer). Anti-rabbit IgG Alexa Fluor®488 (Thermo Fischer Scientific, Waltham, MA, USA) was used as secondary antibody. Callose was stained with 0.1% aniline blue (Water Blue; Fluka, Buchs, Switzerland) in TBS for 5min. Aniline blue fluorescence was detected with an excitation light of 365nm using a conventional fluorescence microscope (Axioskop; Carl Zeiss, Jena, Germany). Overlay images of Alexa Fluor®488 and aniline blue fluorescence were created using the Adobe Photoshop CS6 software. All other fluorescence and phase-contrast images were created at the confocal laser microscopes TCS SPII and TCS SP5 (Leica Microsystems, Wetzlar, Germany).
Extraction of soluble carbohydrates from tissue and sugar analyses
Tissues were ground to a fine powder with pestle and mortar in liquid nitrogen. Water-chloroform-methanol extracts were prepared according to Riens et al. (1991). Sugars and sugar alcohols in tissue extracts were analysed by HPLC according to Öner-Sieben and Lohaus (2014).
RNA isolation and quantitative reverse transcription PCR
RNA from different tissues was isolated using a modified protocol from Chang et al. (1993). Synthesis of cDNA was performed using the RevertAidTM First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, St. Leon-Rot, Germany) with oligo(dT)18 primers. Quantitative real-time PCR (qPCR) analyses were performed using a Maxima SYBR Green qPCR Master Mix, (Thermo Fisher Scientific) and a Mx3005P qPCR System (Agilent Technologies, Inc., Waldbronn, Germany) with the software MxPro Version 4.10 (cycle protocol: 10 s 95°C denaturation; 60°C combined annealing and elongation). Slopes of standard curves of 2-fold dilutions were used to determine the efficiencies of PCR reactions. The following primer sets with corresponding amplicon lengths of 235bp (actin) and 127bp (FeSUT1) were used: Actin forward: AGA GAT TCC GTT GCC CAG AA; Actin reverse: GCC ACA ACC TTA ATC TTC ATG C; FeSUT1 forward: GCT CTC CTT GTT GAC TCC; FeSUT1 reverse: ATT GTC ACT GTA GTA GCC A. Relative initial mRNA concentrations of FeSUT1 (GenBank accession number: KF736981) in F. excelsior were estimated by normalizing expression levels to the housekeeping gene actin (AM063027), which was used as a control gene in previous studies (Chen et al., 2013; Li et al., 2013). The first sample of each experiment was used as a calibrator, which was set to one, and further samples are given as relative expression levels to the calibrator.
Statistical analysis
To estimate the probability of the mean values of the sampled tissues, a Student’s t-test with P = 0.05 was performed. Because values were obtained from samples of the same plants at different time points of a certain experiment or development a paired t-test was conducted.
Results
Characterization of FeSUT1
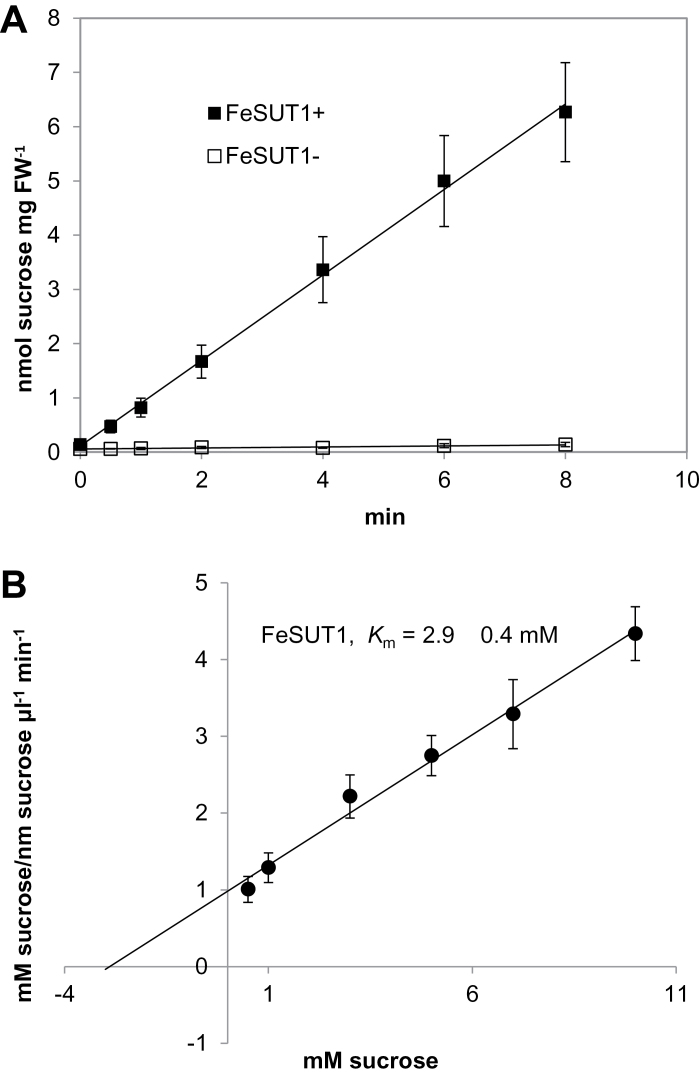
Recent studies identified a Group II SUT from source leaves of F. excelsior (Öner-Sieben and Lohaus, 2014; Supplementary Fig. S1). This transport protein was examined to see if it is involved in the observed accumulation of sucrose in the phloem. To evaluate the properties of FeSUT1, the transporter was analysed in a yeast strain previously used for functional analysis of plant SUTs (Gahrtz et al., 1994; Sauer and Stolz, 1994). The FeSUT1 cDNA was cloned in sense and antisense direction into the shuttle vector NEV-E and the resulting constructs were used to transform the yeast strain SEY2102. Yeasts containing the sense plasmid constructs (FeSUT1+) transported 14C-sucrose at high rates whereas sucrose uptake in the negative control (FeSUT1−) was negligibly low (Fig. 1A). This result proved that FeSUT1 is in fact a functional sucrose transport protein. Kinetic analyses of 14C-sucrose uptake by yeast cells expressing FeSUT1 revealed an apparent K m-value for sucrose of 2.9±0.4mM at pH 5.5 (Fig. 1B).
Fig. 1.
(A) Uptake of 14C-sucrose into SEY2102 yeast cells that expressed FeSUT1. The exogenous sucrose concentration was 1mM and measurements were conducted three times with three different transformants. (B) Hanes-Woolf plot for the calculation of the K m-value of FeSUT1. The initial sucrose concentrations to the reaction velocities are plotted against the sucrose concentrations.
Specificity of the anti-FeSUT1 antiserum
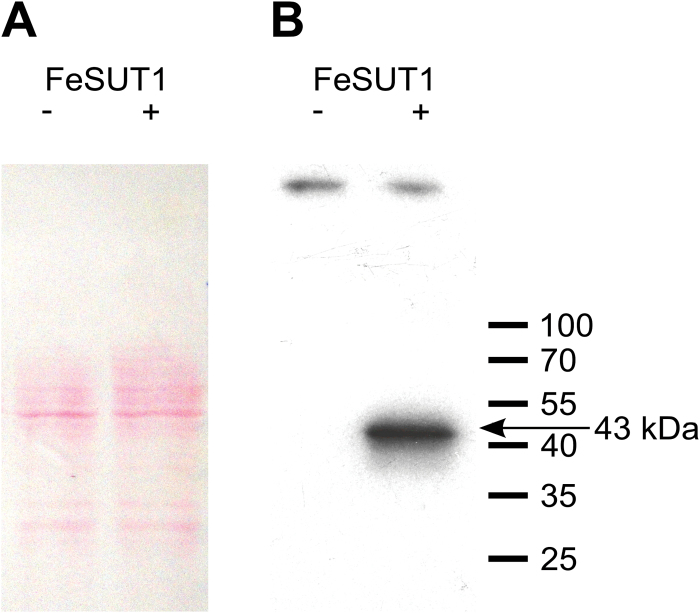
Three polyclonal antisera against a part of the central loop of FeSUT1 were raised in rabbits. The specificity of the antisera was confirmed by western blot analyses using plasma membrane proteins from FeSUT1-expressing yeast cells. All three anti-FeSUT1-antisera resulted in similar signals, but the antiserum of rabbit 1 showed the strongest reaction. It recognized a polypeptide with an apparent molecular weight of 43kDa (FeSUT1 in sense direction, FeSUT1+ in Fig. 2B). No bands were present in the negative control (FeSUT1 in antisense direction, FeSUT1− in Fig. 2B) although similar protein amounts were blotted (Fig. 2A). The apparent molecular weight (43kDa) was lower than the calculated molecular weight (54.2kDa) of FeSUT1, which was also shown for other lipophilic membrane proteins (Beyreuther et al., 1980; Sauer and Tanner, 1984).
Fig. 2.
Western blot analysis of anti-FeSUT1 antiserum on isolated crude membrane protein extracts from yeast cells expressing FeSUT1 in sense (lane +) and in antisense direction (lane −). (A) Successful blotting was verified by Ponceau S staining. (B) The anti-FeSUT1-antiserum showed a strong specific band at 43kDa on membrane protein extracts from transgenic yeast that expressed FeSUT1 (lane +). The negative control showed no reactions (lane −). (This figure is available in colour at JXB online.)
Immunolocalization of FeSUT1
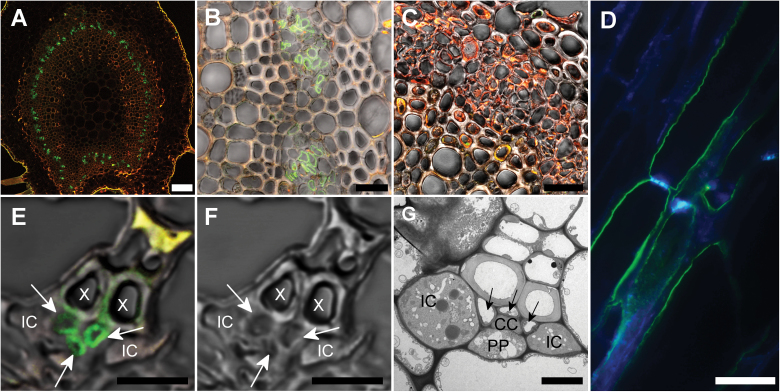
To localize FeSUT1 on the cellular level, anti-FeSUT1-antiserum was used on sections from leaves of F. excelsior. Figure 3A, B shows cross-sections of the midrib of F. excelsior with anti-FeSUT1-antiserum-derived fluorescence signal in the transport phloem. Pre-immune serum was used as a negative control (Fig. 3C). Autofluorescence of cells containing phenolic compounds is shown in yellow or red.
Fig. 3.
Localization of FeSUT1 in midribs and minor veins of F. excelsior leaves by fluorescence detection with anti-FeSUT1 antibody. Leaves were taken from ~30-year-old trees. Yellow or red staining shows autofluorescence of phenolics in lignified cells, green staining shows FeSUT1 localization. Specific anti-FeSUT1 antibody reaction is indicated by green Alexa Fluor® labelling. (A) Overview of a cross section of a leaf midrib treated with anti-FeSUT1-antiserum. Labelling was seen in the circularly arranged transport phloem. (B) Higher magnification of the transport phloem in the leaf midrib imaged in phase-contrast. Irregular shaped cells of the phloem were stained. (C) Section of the midrib incubated with antiserum prior to immunization as negative control imaged in phase-contrast. (D) Longitudinal sections of a vascular bundle of the midrib. Green staining shows FeSUT1 localization. Blue labelling shows detection of callose in sieve plates with aniline blue. (E) Phase-contrast image of the FeSUT1-localization in the minor veins of F. excelsior. Labelled cells (arrow) are SEs or CCs rather than ICs. (F) Same minor vein image without fluorescence light. (G) Electron micrograph of a minor vein of F. excelsior highlighting the regular arrangement of cells. SEs (arrowhead) and CCs are located in the central part of the minor veins whereas the ICs are laterally localized. Scale bars = (A) 100 µm; (B, C) 25 µm; (D) 20 µm; (E, F) 7.5 µm; (G) 5 µm. PP, phloem parenchyma cell; X, xylem.
To further confirm the identity of the labelled cells, longitudinal sections of the midrib of F. excelsior leaves were stained simultaneously with anti-FeSUT1 antibody and the callose/sieve plate-marker aniline blue. The resulting images showed that the cells that contained a sieve plate were also labelled with anti-FeSUT1-antiserum (Fig. 3D). However, in some of the cells labelled with anti-FeSUT1-antiserum no sieve plate was visible. For several species of Solanaceae it has been shown that some anti-SUT antibodies can bind to unknown, non-SUT epitopes in sieve elements (SEs) and this unspecific binding was not seen with purified antisera (Schmitt et al., 2008). Based on that observation purified anti-FeSUT1 antisera were used for all experiments.
The immunolocalization of FeSUT1 in minor veins was investigated in cross sections of F. excelsior leaflets (Fig. 3E). In the minor vein of F. excelsior two types of CCs were present (Öner-Sieben and Lohaus, 2014). The minor vein contained two laterally positioned ICs with plasmodesmata fields that connect them to adjacent BSCs side. However, one or two ordinary CCs without this kind of plasmodesmata field were found in the central part of the minor vein. In addition three or four SEs were present in the central part of the minor vein adjacenct to xylem cells. After treatment with anti-FeSUT1 antibody, green fluorescence was seen in the inner part of the minor vein phloem. To further characterize the cell type, electron micrographs of minor veins from the same order were examined (Fig. 3G). Based on their position the anti-FeSUT1-antiserum-marked cells could be SEs and CCs but not the laterally positioned ICs.
Tissue-specific sugar composition and expression of FeSUT1 in winter, spring, and summer
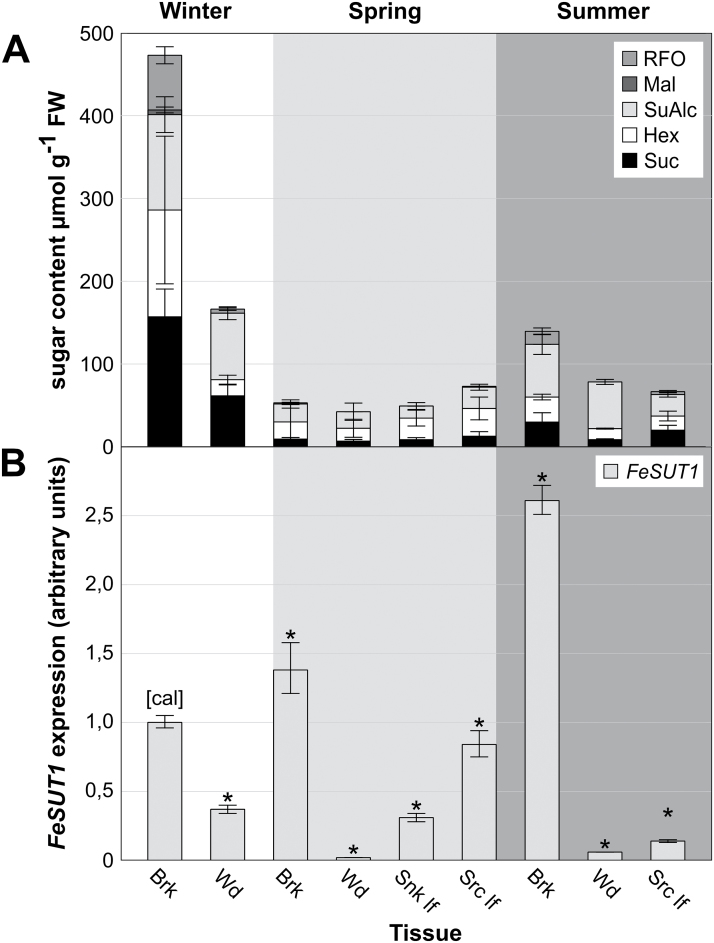
After the localization of FeSUT1 in phloem cells, environmental conditions that might influence FeSUT1 expression were analysed. Sugar and sugar alcohol contents as well as mRNA levels of FeSUT1 in different tissues of F. excelsior during different seasons were measured. The overall sugar and sugar alcohol contents were highest in bark [up to 500 µmol g−1 fresh weight (FW)] and wood in winter (Fig. 4A). Because data for sugar content and SUT expression refer to FW, and wood contains less symplast per gram FW than bark or leaves, the values for wood would be even higher if the data referred to the symplast. However, values were given per gram FW to ensure comparability with other published data (e.g. Ferner et al., 2012). In the stem tissues the main sugars were sucrose, hexoses, and RFOs (Fig. 4A). Mannitol was by far the dominant sugar alcohol (more than 90% at the total content of sugar alcohols; data not shown). In spring, sugar and sugar alcohol contents were much lower (below about 80 µmol g−1 FW) in all tissues (bark, wood, sink leaves, and source leaves). Sink leaves and source leaves were defined by size and weight. ‘Source leaves’ in spring were identified as those 80–100% of the size and weight of mature leaves in summer, whereas ‘sink leaves’ had only about 10% of those values. In summer, sugar and sugar alcohol contents in bark and wood were higher than in spring (Fig. 4A) whereas the contents in source leaves were similar or slightly lower.
Fig. 4.
Tissue-specific sugar composition and expression of FeSUT1 in F. excelsior. Samples were taken from three 3-year-old saplings grown in an open greenhouse. (A) Tissue-specific sugar and sugar alcohol content during winter, spring, and summer in F. excelsior. (B) Tissue-specific expression levels of FeSUT1 during winter, spring, and summer. Expression levels of FeSUT1 were normalized to actin and values are given as relative expression levels to the first sample of the measurement (calibrator [cal]). Student’s t-test was performed with P ≤ 0.05 to test for significance of changes compared to calibrator [cal] (asterisk denotes significant difference). Brk, bark; Hex, hexoses; Mal, maltose; RFO, raffinose-oligosaccharide family sugars; Snk lf, sink leaf; Src lf, source leaf; SuAlc, sugar alcohols; Suc, sucrose; Wd, wood.
qPCR assays were normalized to actin, which was used as a control gene in other studies in Fraxinus (Chen et al., 2013; Li et al., 2013). Expression values of FeSUT1 are given relative to the expression in the first collected bark sample in spring. Significant differences between the mean values of samples and the calibrator of each experiment were calculated using Student’s t-test with P = 0.05. FeSUT1 expression showed a strong tissue-specific variation with highest expression levels in bark (Fig. 4B). In bark tissue the expression level of FeSUT1 increased from winter to spring and was highest in summer. The lowest expression levels were found in wood during the whole season (Fig. 4B). The expression level of FeSUT1 in source leaves decreased to about one sixth from spring to summer (Fig. 4B).
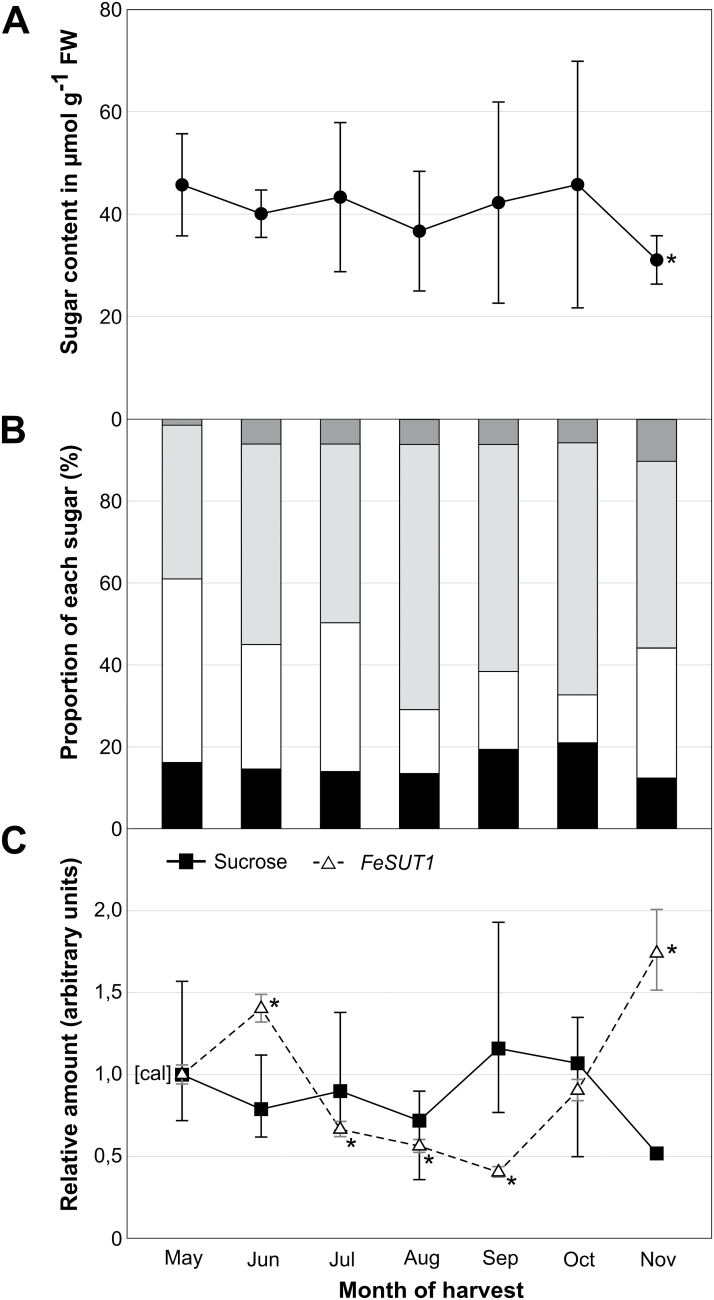
Sugar composition and FeSUT1 expression in leaves during growing season
Leaves of ~30-year-old trees contained similar amounts of sugars and sugar alcohols between May and October (40 to 50 µmol g FW−1; Fig. 5A). Only in late autumn (November) did sugar content decrease. The high values of standard deviation (SD) are due to the fact that samples were taken from three different plants in their natural environment over a course of 3 years (always at the same time of the day (2 p.m.) and the same date of the month without regard to outside temperature, humidity, or hours of sunshine). The distribution of sugars and sugar alcohols varied during the growing season. Sugar alcohols (mainly mannitol) were the dominant component in leaves of F. excelsior for most of the year, followed by hexoses (hex) and sucrose (Fig. 5B). RFOs started with 1.5% in May and showed higher proportions in summer and autumn (Fig. 5B).
Fig. 5.
Sugar and sugar alcohol content as well as FeSUT1 expression in leaves of F. excelsior during growing season. Samples were taken over a period of 3 years (2010–2012) from three individual trees from a 25- to 30-year-old forest. (A) Total sugar and sugar alcohol content in leaves. (B) Relative amounts of sugar and sugar alcohol at the total content. (C) Relative sucrose content (■, closed squares, continuous line) and relative expression of FeSUT1 (Δ, open triangles, broken line). Expression levels of FeSUT1 were normalized to actin and values are given as relative expression levels to the first sample of the measurement (calibrator [cal]). Student’s t-test was performed with P ≤ 0.05 to test for significance of changes compared to calibrator [cal].
FeSUT1 was expressed constantly during the whole growing season (Fig. 5C). Relative FeSUT1 expression was higher in spring and early summer (May, June) and lower in summer and early autumn (July, August, September). Similar results were also shown for leaves of 3-year-old saplings (see Fig. 4B). In the second half of autumn, the expression level increased again (Fig. 5C). Sucrose content was similar in spring and summer and increased in early autumn (September and October). In leaves from November (leaves had shown strong signs of senescence), sucrose content decreased whereas expression levels of FeSUT1 increased about 4-fold from September to November (Fig. 5C).
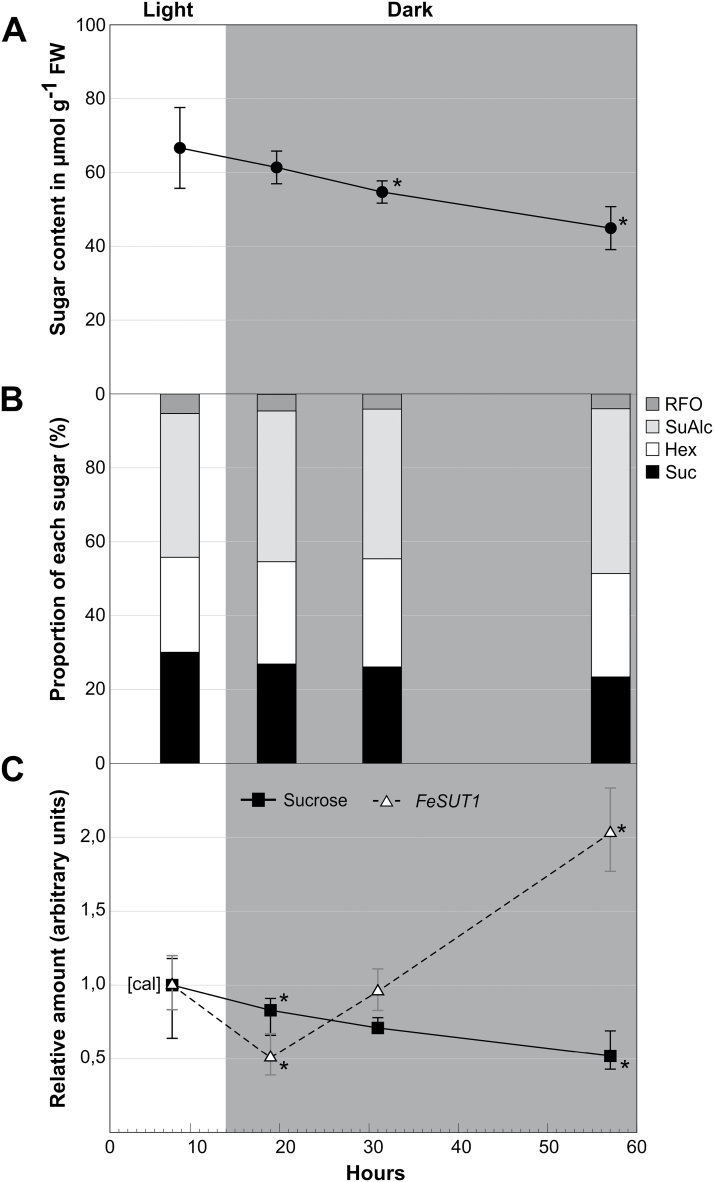
Sugar composition and FeSUT1 expression under different light-dark regimes
After transferring plants into continuous darkness, sugar and sugar alcohol content in leaves started to decline (Fig. 6A). Nevertheless, the sugar content in leaves after about 2 days of darkness was still two-thirds of the values found in illuminated leaves. The sugar and sugar alcohol composition also changed slightly during the long dark period. The proportion of sugar alcohol (mainly mannitol) increased whereas the proportion of RFOs and sucrose decreased (Fig. 6B). The expression of FeSUT1 compared with sucrose content in F. excelsior is shown in Fig. 6C. Like the whole sugar content, sucrose content decreased after illumination stopped. In contrast, expression of FeSUT1 increased strongly during the long dark period (Fig. 6C).
Fig. 6.
Sugar and sugar alcohol content as well as FeSUT1 expression in leaves of F. excelsior in light and during a prolonged dark period. Experiments were performed with three 3-year-old saplings. Leaves were taken in the second half of the normal light period and after 5, 17, and 43h of continuous darkness. (A) Total sugar and sugar alcohol content in leaves. (B) Relative amounts of sugar and sugar alcohol at the total content. (C) Relative sucrose content (■, closed squares, continuous line) and relative expression of FeSUT1 (Δ, open triangles, broken line). Expression levels of FeSUT1 were normalized to actin and values are given as relative expression levels to the first sample of the measurement (calibrator [cal]). Student’s t-test was performed with P ≤ 0.05 to test for significance of changes compared to calibrator [cal]. Hex, hexoses; Mal, maltose; RFO, raffinose-oligosaccharide family sugars; SuAlc, sugar alcohols; Suc, sucrose.
Discussion
Comparison of biochemical characteristics of FeSUT1 and other SUTs
FeSUT1 described in the present study was isolated from source leaf RNAs of F. excelsior and identified as a Group II SUT (Öner-Sieben and Lohaus, 2014). Data about enzymatic features are primarily available for SUTs from herbs and according to the best available knowledge only one tree-specific Group II SUT from Hevea brasiliensis (HbSUT3; Tang et al., 2010) has been characterized, in yeast. The apparent K m value for sucrose of FeSUT1 was about 2.9mM and similar K m values have been reported for other SUTs (Table 1). Group II SUTs have high substrate affinities with K m values between 1 and 2mM (Table 1). Most Group III and IV SUTs have considerably lower substrate affinities and a greater variability regarding the measured K m values (Table 1). FeSUT1 of F. excelsior showed great similarities, both structurally and biochemically, to AmSUT1 from Alonsoa meridionalis, which had a K m value of 1.8mM. Both species are members of the order Lamiales and they are characterized as putative mixed apoplastic and symplastic phloem loaders (Knop et al., 2001; 2004; Voitsekhovskaja et al., 2009; Öner-Sieben and Lohaus, 2014).
Table 1.
Km values of FeSUT1 for sucrose compared with values of other SUTs
| Species | Transporter | Group | K m | Protein-ID | Reference |
|---|---|---|---|---|---|
| Hordeum vulgare | HvSUT1 | I | 7.5 mM | CAJ20123 | Weschke et al., 2000 |
| Fraxinus excelsior | FeSUT1 | II | 2.9±0.4 mM | AHB33870 | this paper |
| Asarina barclaiana | AbSUT1 | II | 0.5 mM | AAF04294 | Knop et al., 2001 |
| Alonsoa meridionalis | AmSUT1 | II | 1.8 mM | AAF04295 | Knop et al., 2004 |
| Arabidopsis thaliana | AtSUC1 | II | 0.5 mM | CAA53147 | Sauer and Stolz, 1994 |
| Arabidopsis thaliana | AtSUC2 | II | 0.77 mM | CAA53150 | Sauer and Stolz, 1994 |
| Medicago truncatula | MtSUT1 | II | 1.7 mM | AFM28284 | Doidy et al., 2012 |
| Plantago major | PmSUC2 | II | 1 mM | CAA53390 | Gahrtz et al., 1994 |
| Phaseolus vulgaris | PvSUT1 | II | 8.5±0.7 mM | ABB30164 | Zhou et al., 2007 |
| Ricinus communis | RcScr1 | II | 2 mM | CAA83436 | Weig and Komor, 1995 |
| Solanum tuberosum | StSUT1 | II | 1 mM | CAA48915 | Riesmeier et al., 1993 |
| Spinacia oleracea | SoSUT1 | II | 1.5 mM | CAA47604 | Riesmeier et al., 1992 |
| Arabidopsis thaliana | AtSUT2/AtSUC3 | III | 11.7 mM | CAB92307 | Meyer et al., 2000 |
| Plantago major | PmSUC3 | III | 5.5±1.1 mM | CAD58887 | Barth et al., 2003 |
| Arabidopsis thaliana | AtSUT4 | IV | 11.6±0.6 mM | AAL59915 | Weise et al., 2000 |
| Daucus carota | DcSUT1a | IV | 0.5 mM | CAA76367 | Shakya and Sturm, 1998 |
| Hordeum vulgare | HvSUT2 | IV | 5 mM | CAB75881 | Weschke et al., 2000 |
| Lotus japonicus | LjSUT4 | IV | 12.9 mM | CAD61275 | Flemetakis et al., 2003 |
| Oryza sativa | OsSUT2 | IV | 1.86±0.38 mM | BAC67163 | Eom et al., 2011 |
| Solanum tuberosum | StSUT4 | IV | 6.0±1.2 mM | AAG25923 | Weise et al., 2000 |
FeSUT1 is localized in the collection and transport phloem of F. excelsior
In order to function in phloem loading, FeSUT1 should be expressed in phloem tissue. To verify this hypothesis, immunolocalization experiments were conducted on tissue sections of F. excelsior. The purified anti-FeSUT1-antiserum marked cells of both the transport and the collection phloem (Fig. 3). SUTs that were localized in the phloem have been described in several herbaceous species like Plantago major, Arabidopsis thaliana, Solanum tuberosum, Alonsoa meridionalis, and Triticum aestivum (Stadler et al., 1995; Stadler and Sauer, 1996; Kühn et al., 1997; Knop et al., 2004; Aoki et al., 2004; Schmitt et al., 2008). In herbaceous plant species, like P. major and A. thaliana, SUTs have been identified to be localized in CCs (Stadler et al., 1995; Stadler and Sauer; 1996). However, in solanaceous species, SUTs have been localized in both CCs as well as SEs (Kühn et al., 1997; Schmitt et al., 2008). Results for the woody species F. excelsior have clearly shown the localization of FeSUT1 in SEs (Fig. 3). Depending on plant species or plant tissues, SUTs could be found in different cell types of the phloem, SEs, and/or CCs, which may reflect the necessity to deploy transporters in response to local, temporal, or physiological needs of the plant.
SUTs are probably involved in the primary phloem loading with sucrose and in the retrieval of sucrose that leaks out of the sieve tubes into the lateral tissue (van Bel, 2003). While some of the leaked sucrose is used to supply the surrounding tissue with carbohydrates, most is transported back into the phloem to maintain the strong concentration gradient that drives the bulk flow (Minchin and Thorpe, 1987; Gould et al., 2012). The loss of sucrose is probably due to diffusion and driven by the very high concentration itself (Kedem and Katchalsky, 1958). The sugar concentrations in the phloem sap of F. excelsior are also very high (sucrose 0.4M and RFOs 0.6M; Öner-Sieben and Lohaus, 2014), thus leakage of sucrose is likely. In addition the height of trees is normally about 100-fold larger than that of herbaceous plants (i.e. 30–50 m in relation to 30–50cm). Therefore, the distance between phloem loading and phloem unloading in trees is very often exceptionally large and retrieval of sucrose into the phloem to maintain the concentration gradient should be very important. In contrast, at least for small herbaceous plants, the retrieval function is not essential as shown for Arabidopsis (Srivastava et al., 2008). In general, the SEs of the transport phloem are much larger than the CCs in this tissue (van Bel, 2003; De Schepper et al., 2013). The direct contact of the large membrane surface of SEs with the apoplastic interface requires a set of uptake devices (van Bel, 2003). Retrieval of sucrose into the larger SEs can occur with a greater extent than into the smaller CCs, which would explain the presence of SUTs at the interface between SEs and apoplast (Fig. 3). Probably the localization of the sucrose carrier in the phloem is related to the relevance of sucrose retrieval for the respective species. Owing to the major importance of a retrieval mechanism for trees and its minor importance for herbaceous species, the sucrose carrier is localized to SEs in trees whereas it is localized predominately in CCs in herbaceous species. Maybe other factors like annual cycles also contribute to the different locations of SUTs in the transport phloem.
In addition to the transport phloem, FeSUT1 was also localized in the phloem cells of minor veins of F. excelsior, which confirmed the hypothesis that FeSUT1 functions in phloem loading. The structure of the minor veins of F. excelsior with two types of CCs (Öner-Sieben and Lohaus, 2014) is typical for RFO-translocating and putative symplastic phloem-loading species (Fisher, 1986; Hoffmann-Thoma et al., 2001; Knop et al., 2004). FeSUT1 was confined to SEs and possibly CCs but not to ICs (Fig. 3), even though ICs need to import a high amount of sucrose because the synthesis of RFOs for phloem transport occurs in ICs. Voitsekhovskaja et al. (2009) showed that the gene for stachyose-synthase AmSTS1 was expressed exclusively in the ICs of Alonsoa meridionalis. Therefore, the fact that FeSUT1 was not localized in ICs indicates that the sucrose, which is necessary for RFO synthesis in ICs, was probably imported symplastically. FeSUT1 was located in SEs and probably CCs and in these cell types the transporter was most likely involved in active sucrose uptake from the apoplast. The SUT AmSUT1 has similar functions in A. meridionalis (Voitsekhovskaja et al., 2009). That means two types of SE-CC complexes (SE-IC and SE-CC) within the same minor vein load or produce different carbohydrates and use contrasting mechanisms for their delivery into the phloem. It has been hypothesized that the phloem turgor regulates the conductance of the plasmodesmata leading into the CCs or ICs (Oparka and Prior, 1992; De Schepper et al., 2013). Therefore phloem pressure could be one possibility regulating the extent of symplastic and apoplastic phloem loading.
A shift in the phloem-loading mechanism occurs during growing season
Sugar transport is a dynamic process that is tightly regulated by environmental factors and by the physiological needs of the plants. Several factors, e.g. light (Kühn et al., 2003), freeze-thaw cycles (Decourteix et al., 2006), or salt stress (Noiraud et al., 2000), have an impact on SUT expression. To get insight into possible mechanisms that regulate the expression of FeSUT1, qPCR assays were conducted and sugar contents were measured. The tissue-specific expression levels showed that FeSUT1 was expressed in all tissues and throughout all measurements but it was predominantly expressed in bark (Fig. 4B). Bark contains the secondary phloem, which is primarily involved in assimilate transport from source to sink tissues. Interestingly, in winter the expression level of FeSUT1 was also relatively high in wood indicating that transport processes are essential for basal physiological function during dormancy. SUT expression in wood has also been reported for other trees and it was even increased after freeze conditions in walnut (Decourteix et al., 2006). During the transition from dormancy to the vegetative state in spring and summer the expression of FeSUT1 increased in bark tissue while it declined in wood (Fig. 4B). A possible explanation could be that in spring the retrieval and transport of sucrose became more important and FeSUT1 expression was up-regulated in the transport phloem. Seasonal redistributions of sucrose in stems have also been reported in other tree species, e.g. Populus (Sauter, 1988).
In leaves, FeSUT1 expression was higher in spring than in summer (Fig. 4B). This indicates that apoplastic loading might be more important in spring than in summer. The analysis of the sugar and sugar alcohol content in the respective tissues gave another hint towards the presence of a possible shift of phloem-loading mechanisms. RFOs were nearly absent in spring but were detectable in bark and source leaf tissue in summer (Figs. 4A, 5B), meaning that the synthesis of RFOs was also up-regulated from spring to summer. The synthesis of RFOs is restricted to the ICs (Voitsekhovskaja et al., 2009; Öner-Sieben and Lohaus, 2014) and corresponds to the ‘polymer trap’ hypothesis that sucrose diffuses symplastically into the ICs. The data suggest that apoplastic loading becomes less important when the polymer trapping mechanisms get established during the transition from the initial growth period in spring to a more vegetative state in summer.
Apoplastic phloem loading increases during periods of lower sucrose content
The data indicate that FeSUT1 expression is induced whenever the availability of sucrose is low. The overall sugar and sugar alcohol content as well as the sucrose content declined in November, when senescence of the leaves was far advanced (Fig. 5A, C). During this period the expression of FeSUT1 reached its seasonal high (Fig. 5C). Deciduous species store high concentrations of soluble carbohydrates in the stem during winter as an antifreeze agent and as energy store for bud break in spring (Sauter, 1988). The elevated expression level of FeSUT1 indicates an increased apoplastic carbon export activity from the leaves during late autumn.
Similar results were obtained for plants transferred into darkness (Fig 6). In leaves of these plants the overall sugar and sugar alcohol content as well as the sucrose content decreased during darkness (Fig. 6A, C). The decline in F. excelsior was less distinct than in herbaceous plants (Riens et al., 1994). Even after about 2 days of permanent darkness, 68% of the sugar and sugar alcohol content measured in the second half of the light period was still present (Fig. 6A). Again, the expression of FeSUT1 increased when sucrose availability declined (Fig. 6C).
The observed increase in FeSUT1 expression indicates that apoplastic phloem loading is induced when the sucrose concentration is low. The symplastic movement of sucrose from the MCs or BSCs into the ICs of the phloem requires higher sugar concentration in the MCs than in the phloem. It is hypothesized that the sugar concentration in MCs falls below the critical concentration for this kind of movement under conditions of senescence or longer dark periods. Apoplastic phloem loading mediated by FeSUT1 could be necessary to maintain the transport of sucrose into the phloem. It has not been investigated so far if apoplastic phloem loading can occur efficiently in open minor veins. It would be interesting to study a possible plasmodesmatal closure between MCs and SE-CCCs during that time of the year.
Flexibility in processes of sucrose transport has also been shown for some other species, like Cucumis melo and Populus tremula x alba. A shift from symplastic to apoplastic loading was reported for melon after infection with cucumber mosaic virus (Gil et al., 2011). The tree species Populus tremula x alba is associated with the passive phloem-loading mode. Several SUTs have been cloned from this species (Payyavula et al., 2011). PtaSUT4 is localized in the tonoplast of MCs and could facilitate symplastic loading of the minor vein companion cells by modulating cytosolic sucrose concentrations in the nearby mesophyll. PtaSUT3 is a Group II SUT which is associated with apoplastic transport and transcripts were observed in minor veins of source leaves. By down-regulating the tonoplast PtaSUT4 the level of the Group II PtaSUT3 was increased in mature leaves of poplar (Payyavula et al., 2011). Such morphological and gene expression data in particular point to the heterogeneity in SE-CCCs in minor veins and phloem-loading strategies. These different mechanisms may confer advantages under various environmental conditions.
Conclusion
Heterologous expression of FeSUT1 in yeast showed that it codes for a functional SUT. The K m value of FeSUT1 was typical for Group II SUTs. Immunolocalization of FeSUT1 revealed that this transporter is expressed in the transport phloem and in the minor veins of F. excelsior. In the transport phloem FeSUT1 is involved in the retrieval of sucrose into the large SEs. In minor veins phloem loading of sucrose in F. excelsior is at least partly mediated by the activity of FeSUT1 in addition to symplastic phloem loading. Probably during different growing stages or seasons, the proportion of apoplastic and symplastic phloem loading varies depending on the physiological requirements or environmental conditions. This feature could provide flexibility in regard to environmental changes.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Sequence of the full-length cDNA of FeSUT1 encoding the Group II SUT FeSUT1 from F. excelsior.
Acknowledgements
The authors would like to thank Kira Tiedge for critical reading of the manuscript and the reviewers for the helpful comments.
Glossary
Abbreviations:
- BSC
bundle sheath cell
- CC
companion cell
- IC
intermediary cell
- FW
fresh weight
- MC
mesophyll cell
- qPCR
quantitative polymerase chain reaction
- RFO
raffinose oligosaccharide
- SE
sieve element
- SE-CCC
sieve element-companion cell complex
- SUT
sucrose transporter.
References
- Ainsworth E, Bush D. 2011. Carbohydrate export from the leaf: a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiology 155, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki G N, Scofield X-D, Wang J W, Patrick C E, Offler R T, Furbank 2004. Expression and localisation analysis of the wheat sucrose transporter TaSUT1 in vegetative tissues. Planta 219, 176–184. [DOI] [PubMed] [Google Scholar]
- Barth I, Meyer S, Sauer N. 2003. PmSUC3: characterization of a SUT2/SUC3-type sucrose transporter from Plantago major . Plant Cell 15, 1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier A, Desclos M, Amiard V, Morvan-Bertrand A, Demmig-Adams B, Adams W, Turgeon R, Prud’homme M, Noiraud-Romy N. 2009. Activation of sucrose transport in defoliated Lolium perenne L.: an example of apoplastic phloem loading plasticity. Plant and Cell Physiology 50, 1329–1344. [DOI] [PubMed] [Google Scholar]
- Beyreuther K, Bieseler B, Ehring R, Griesser HW, Mieschendahl M, Müller-Hill B, Triesch I. 1980. Investigation of structure and function of lactose permease of Escherichia coli . Biochemical Society Transaction 6, 675–676. [DOI] [PubMed] [Google Scholar]
- Braun DM, Slewinski TL. 2009. Genetic control of carbon partitioning in grasses: roles of sucrose transporters and tie-dyed loci in phloem loading. Plant Physiology 149, 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. 1993. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter 11, 113–116. [Google Scholar]
- Chen G, Xing Z, Pan W, Bai L, Ye J, Ma D, Wei Z, Fan J, Guo Z. 2013. Cloning of a novel stearoyl-acyl desaturase gene from white ash (Fraxinus americana) and evolution analysis with those from other plants. African Journal of Biotechnology 10, 18185–18193. [Google Scholar]
- Chen L, Qu X, Hou B, Sosso D, Osorio S, Fernie A, Frommer W. 2012. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335, 207–211. [DOI] [PubMed] [Google Scholar]
- Davidson A, Keller F, Turgeon R. 2011. Phloem loading, plant growth form, and climate. Protoplasma 248, 153–163. [DOI] [PubMed] [Google Scholar]
- Decourteix M, Alves G, Brunel N, Améglio T, Guillio A, Lemoine R, Pétel G, Sakr S. 2006. JrSUT1, a putative xylem sucrose transporter, could mediate sucrose influx into xylem parenchyma cells and be up-regulated by freeze-thaw cycles over the autumn-winter period in walnut tree (Juglans regia L.). Plant, Cell and Environment 29, 36–47. [DOI] [PubMed] [Google Scholar]
- De Schepper V, De Swaef T, Bauweraerts I, Steppe K. 2013. Phloem transport: a review of mechanisms and controls. Journal of Experimental Botany 64, 4839–4850. [DOI] [PubMed] [Google Scholar]
- Doidy J, van Tuinen D, Lamotte O, Corneillat M, Alcaraz G, Wipf D. 2012. The Medicago truncatula sucrose transporter family: characterization and implication of key members in carbon partitioning towards arbuscular mycorrhizal fungi. Molecular Plant 5, 1346–1358. [DOI] [PubMed] [Google Scholar]
- Emr S, Schekman R, Flessel M, Thorner J. 1983. An Mf Alpha-1-suc2 (alpha-factor-invertase) gene fusion for study of protein localization and gene-expression in yeast. Proceedings of the National Academy of Sciences USA 80, 7080–7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, Peters S, Keller F, Baginsky S, Martinoia E, Schmidt U. 2006. Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiology 141, 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom J, Cho J, Reinders A, et al. 2011. Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiology 157, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferner E, Rennenberg H, Kreuzwieser J. 2012. Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiology 32, 135–145. [DOI] [PubMed] [Google Scholar]
- Fisher D. 1986. Ultrastructure, plasmodesmatal frequency, and solute concentration in green areas of variegated Coleus blumei Benth. leaves. Planta 169, 141–152. [DOI] [PubMed] [Google Scholar]
- Flemetakis E, Dimou M, Cotzur D, Efrose R, Aivalakis G, Colebatch G, Udvardi M, Katinakis P. 2003. A sucrose transporter, LjSUT4, is up-regulated during Lotus japonicus nodule development. Journal of Experimental Botany 54, 1789–1791. [DOI] [PubMed] [Google Scholar]
- Frost C, Nyamdari B, Tsai C, Harding S. 2012. The tonoplast-localized sucrose transporter in Populus (PtaSUT4) regulates whole-plant water relations, responses to water stress, and photosynthesis. PLoS One 7, e44467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahrtz M, Stolz J, Sauer N. 1994. A phloem-specific sucrose-H+ symporter from Plantago major L. supports the model of apoplastic phloem loading. The Plant Journal 6, 697–706. [DOI] [PubMed] [Google Scholar]
- Gamalei Y. 1991. Phloem loading and its development related to plant evolution from trees to herbs. Trees - Structure and Function 5, 50–64. [Google Scholar]
- Giaquinta R. 1977. Sucrose hydrolysis in relation to phloem translocation in Beta vulgaris . Plant Physiology 60, 339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil L, Yaron I, Shalitin D, Sauer N, Turgeon R, Wolf S. 2011. Sucrose transporter plays a role in phloem loading in CMV-infected melon plants that are defined as symplastic loaders. The Plant Journal 66, 366–374. [DOI] [PubMed] [Google Scholar]
- Goggin FL, Medville R, Turgeon R. 2001. Phloem loading in the tulip tree. Mechanisms and evolutionary implications. Plant Physiology 125, 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald RR, Krysan PJ, Young JC, Evert RF, Sussman MR. 2000. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proceedings of the National Academy of Sciences USA 97, 13979–13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould N, Thorpe MR, Pritchard J, Christeller JT, Williams LE, Roeb G, Schurr U, Minchin PEH. 2012. AtSUC2 has a role for sucrose retrieval along the phloem pathway: evidence from carbon-11 tracer studies. Plant Science 188–189, 97–101. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Thoma G, van Bel A, Ehlers K. 2001. Ultrastructure of minor-vein phloem and assimilate export in summer and winter leaves of the symplasmically loading evergreens Ajuga reptans L., Aucuba japonica Thunb., and Hedera helix L . Planta 212, 231–242. [DOI] [PubMed] [Google Scholar]
- Kedem O, Katchalsky A. 1958. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochemica et Biophysica Acta 27, 229–246. [DOI] [PubMed] [Google Scholar]
- Knop C, Stadler R, Sauer N, Lohaus G. 2004. AmSUT1, a sucrose transporter in collection and transport phloem of the putative symplastic phloem loader Alonsoa meridionalis . Plant Physiology 134, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop C, Voitsekhovskaja O, Lohaus G. 2001. Sucrose transporters in two members of the Scrophulariaceae with different types of transport sugar. Planta 213, 80–91. [DOI] [PubMed] [Google Scholar]
- Kühn C. 2003. A comparison of the sucrose transporter systems of different plant species. Plant Biology 5, 215–232. [Google Scholar]
- Kühn C, Franceschi V, Schulz A, Lemoine R, Frommer W. 1997. Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275, 1298–1300. [DOI] [PubMed] [Google Scholar]
- Kühn C, Hajirezaei M, Fernie A, Rössner-Tunali U, Czechowski T, Hirner B, Frommer W. 2003. The sucrose transporter StSUT1 localizes to sieve elements in potato tuber phloem and influences tuber physiology and development. Plant Physiology 131, 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Li T, Peng Z, Bi Y, Fan Z. 2013. Molecular cloning and expression analysis of FvMYB1 from Fraxinus velutina Torr. Turkish Journal of Agriculture and Forestry 37, 517–526. [Google Scholar]
- Liesche J, Krügel U, He H, Chincinska I, Hackel A, Kühn C. 2011. Sucrose transporter regulation at the transcriptional, post-transcriptional and post-translational level. Journal of Plant Physiology 168, 1426–1433. [DOI] [PubMed] [Google Scholar]
- Liesche J, Schulz A. 2013. Modeling the parameters for plasmodesmal sugar filtering in active symplasmic phloem loaders. Frontiers in Plant Science 4, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Melzer M, Truernit E, Hümmer C, Besenbeck R, Stadler R, Sauer N. 2000. AtSUC3, a gene encoding a new Arabidopsis sucrose transporter, is expressed in cells adjacent to the vascular tissue and in a carpel cell layer. The Plant Journal 24, 869–882. [DOI] [PubMed] [Google Scholar]
- Minchin PEH, Thorpe MR. 1987. Measurement of unloading and reloading of photo-assimilate within the stem of bean. Journal of Experimental Botany 38, 211–220. [Google Scholar]
- Noiraud N, Delrot S, Lemoine R. 2000. The sucrose transporter of celery. Identification and expression during salt stress. Plant Physiology 122, 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öner-Sieben S, Lohaus G. 2014. Apoplastic and symplastic phloem loading in Quercus robur and Fraxinus excelsior . Journal of Experimental Botany 65, 1905–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparka KJ, Prior DAM. 1992. Direct evidence for pressure-generated closure of plasmodesmata. The Plant Journal 2, 741–750. [Google Scholar]
- Payyavula R, Tay K, Tsai C, Harding S. 2011. The sucrose transporter family in Populus: The importance of a tonoplast PtaSUT4 to biomass and carbon partitioning. The Plant Journal 65, 757–770. [DOI] [PubMed] [Google Scholar]
- Reinders A, Sivitz A, Ward J. 2012. Evolution of plant sucrose uptake transporters. Frontiers in Plant Science 3, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riens B, Lohaus G, Heineke D, Heldt H. 1991. Amino acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the phloem sap of spinach leaves. Plant Physiology 97, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riens B, Lohaus G, Winter H, Heldt H. 1994. Production and diurnal utilization of assimilates in leaves of spinach (Spinacia oleracea L.) and barley (Hordeum vulgare L.). Planta 192, 497–501. [Google Scholar]
- Riesmeier J, Willmitzer L, Frommer W. 1994. Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO Journal 13, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier J, Hirner B, Frommer W. 1993. Potato sucrose transporter expression in minor veins indicates a role in phloem loading. The Plant Cell 5, 1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier J, Willmitzer L, Frommer W. 1992. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO Journal 11, 4705–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N. 2007. Molecular physiology of higher plant sucrose transporters. FEBS Letters 581, 2309–2317. [DOI] [PubMed] [Google Scholar]
- Sauer N, Stadler R. 1993. A sink-specific H+/monosaccharide co-transporter from Nicotiana tabacum: cloning and heterologous expression in baker’s yeast. The Plant Journal 4, 601–610. [DOI] [PubMed] [Google Scholar]
- Sauer N, Stolz J. 1994. SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker’s yeast and identification of the histidine-tagged protein. The Plant Journal 6, 67–77. [DOI] [PubMed] [Google Scholar]
- Sauer N, Tanner W. 1984. Partial purification and characterization of inducible transport proteins of Chlorella . Zeitschrift Pflanzenphysiologie 114, 367–375. [Google Scholar]
- Sauter JJ. 1988. Temperature-induced changes in starch and sugars in the stems of Populus x canademis ‘robusta’. Journal of Plant Physiology 132, 608–612. [Google Scholar]
- Schmitt B, Stadler R, Sauer N. 2008. Immunolocalization of solanaceous SUT1 proteins in companion cells and xylem parenchyma: new perspectives for phloem loading and transport. Plant Physiology 148, 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Hulpke S, Schulz A, Yaron I, Höll J, Imlau A, Schmitt B, Batz S, Wolf S, Hedrich R, Sauer N. 2012. Vacuoles release sucrose via tonoplast-localised SUC4-type transporters. Plant Biology 14, 325–336. [DOI] [PubMed] [Google Scholar]
- Schulz A. 2015. Diffusion or bulk flow: how plasmodesmata facilitate pre-phloem transport of assimilates. Journal of Plant Research 128, 49–61. [DOI] [PubMed] [Google Scholar]
- Shakya R, Sturm A. 1998. Characterization of source- and sink-specific sucrose/H+ symporters from carrot. Plant Physiology 118, 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slewinski T, Zhang C, Turgeon R. 2013. Structural and functional heterogeneity in phloem loading and transport. Frontiers in Plant Science 4, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AC, Ganesan S, Ismail IO, Ayre BG. 2008. Functional characterization of the Arabidopsis AtSUC2 Sucrose/H+ symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiology 148, 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Brandner J, Schulz A, Gahrtz M, Sauer N. 1995. Phloem loading by the PmSUC2 sucrose carrier from Plantago major occurs into companion cells. The Plant Cell 7, 1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Sauer N. 1996. The Arabidopsis thaliana AtSUC2 gene is specifically expressed in companion cells. Botanica Acta 109, 299–306. [Google Scholar]
- Tang C, Huang D, Yang J, Liu S, Sakr S, Li H, Zhou Y, Qin Y. 2010. The sucrose transporter HbSUT3 plays an active role in sucrose loading to laticifer and rubber productivity in exploited trees of Hevea brasiliensis (para rubber tree). Plant, Cell and Environment 33, 1708–1720. [DOI] [PubMed] [Google Scholar]
- Turgeon R, Beebe D, Gowan E. 1993. The intermediary cell: Minor-vein anatomy and raffinose oligosaccharide synthesis in the Scrophulariaceae. Planta 191, 446–456. [Google Scholar]
- Turgeon R, Medville R. 2004. Phloem loading. A reevaluation of the relationship between plasmodesmatal frequencies and loading strategies. Plant Physiology 136, 3795–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Medville R. 1998. The absence of phloem loading in willow leaves. Proceedings of the National Academy of Sciences USA 95, 12055–12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bel A. 2003. The phloem, a miracle of ingenuity. Plant, Cell and Environment 26, 125–149. [Google Scholar]
- Voitsekhovskaja O, Rudashevskaya E, Demchenko K, Pakhomova M, Batashev D, Gamalei Y, Lohaus G, Pawlowski K. 2009. Evidence for functional heterogeneity of sieve element-companion cell complexes in minor vein phloem of Alonsoa meridionalis . Journal of Experimental Botany 60, 1873–1883. [DOI] [PubMed] [Google Scholar]
- Weig A, Komor E. 1995. An active sucrose carrier (Scr1) that is predominantly expressed in the seedling of Ricinus communis L. Journal of Plant Physiology 147, 685–690. [Google Scholar]
- Weise A, Barker L, Kühn C, Lalonde S, Buschmann H, Frommer W, Ward J. 2000. A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 12, 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschke W, Panitz R, Sauer N, Wang Q, Neubohn B, Weber H, Wobus U. 2000. Sucrose transport into barley seeds: molecular characterization of two transporters and implications for seed development and starch accumulation. The Plant Journal 21, 455–467. [DOI] [PubMed] [Google Scholar]
- Wright D, Scholes J, Read D, Rolfe S. 2000. Changes in carbon allocation and expression of carbon transporter genes in Betula pendula Roth. colonized by the ectomycorrhizal fungus Paxillus involutus (Batsch) Fr. Plant, Cell and Environment 23, 39–49. [Google Scholar]
- Zhang C, Han L, Slewinski T, Sun J, Zhang J, Wang Z, Turgeon R. 2014. Symplastic phloem loading in poplar. Plant Physiology 166, 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Qu H, Dibley K, Offler C, Patrick J. 2007. A suite of sucrose transporters expressed in coats of developing legume seeds includes novel pH-independent facilitators. The Plant Journal 49, 750–764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.