Abstract
Conventional bioreactors are used to enhance extracellular matrix (ECM) production and mechanical strength of tissue-engineered vessels (TEVs) by applying circumferential strain, which is uniaxial stretching. However, the resulting TEVs still suffer from inadequate mechanical properties, where rupture strengths and compliance values are still very different from native arteries. The biomechanical milieu of native arteries consists of both circumferential and axial loading. Therefore, to better simulate the physiological stresses acting on native arteries, we built a novel bioreactor system to enable biaxial stretching of engineered arteries during culture. This new bioreactor system allows for independent control of circumferential and axial stretching parameters, such as displacement and beat rate. The assembly and setup processes for this biaxial bioreactor system are reliable with a success rate greater than 75% for completion of long-term sterile culture. This bioreactor also supports side-by-side assessments of TEVs that are cultured under three types of mechanical conditions (static, uniaxial, and biaxial), all within the same biochemical environment. Using this bioreactor, we examined the impact of biaxial stretching on arterial wall remodeling of TEVs. Biaxial TEVs developed the greatest wall thickness compared with static and uniaxial TEVs. Unlike uniaxial loading, biaxial loading led to undulated collagen fibers that are commonly found in native arteries. More importantly, the biaxial TEVs developed the most mature elastin in the ECM, both qualitatively and quantitatively. The presence of mature extracellular elastin along with the undulated collagen fibers may contribute to the observed vascular compliance in the biaxial TEVs. The current work shows that biaxial stretching is a novel and promising means to improve TEV generation. Furthermore, this novel system allows us to optimize biomechanical conditioning by unraveling the interrelationships among the applied mechanical stress, the resulting ECM properties, and the mechanics of TEVs.
Introduction
The impact of mechanical cues on structural changes in extracellular matrix (ECM), as well as the effect of the resulting ECM architecture on the mechanical properties of tissue-engineered vessels (TEVs), has yet to be fully elucidated. A bioreactor is a biomimetic system devised to efficiently control and manipulate the chemomechanical environment of engineered tissues. Thus, a bioreactor is an excellent tool to examine how ECM remodels in response to applied biochemical and biomechanical cues in developing engineered vessels.
Previously, a pulsatile bioreactor was designed to apply cyclic circumferential stretch (uniaxial stretch) to developing TEVs.1 Application of this mechanical conditioning results in implantable arterial grafts with robust mechanical strength.1,2 This bioreactor was also used to analyze the ultrastructural collagen matrix of TEVs cultured under pulsatile forces.3 This study showed that the ECM properties and the mechanical properties of circumferentially stretched TEVs (uniaxially stretched) were significantly different from those of native arteries in various respects. For example, the burst pressure of the uniaxial TEVs was measured to be 107±14 kPa compared with 443±55 kPa of native arteries.4 Native arteries have significantly more circumferential and fewer axial collagen fibrils than TEVs, which may contribute to high burst strength of native arteries.3 In efforts to understand the causes for these differences between native and engineered arteries, we noted that circumferential stretch is not the only mechanical force exerted in vivo. In fact, most arteries are prestretched axially in situ by a tension that is sustained by the tethering of arteries to perivascular connective tissues.5,6 Arteries that are under this axial tension can be prestretched up to 40–65% of their unloaded length.7 Therefore, to better simulate the physiologic environment of native arteries, we built a biaxial bioreactor to exert axial as well as circumferential loading on TEVs in this study.
The effect of axial loading on ECM growth and remodeling of native arteries has been examined previously by several groups. For example, an ex vivo perfusion system has been used to study the biaxial mechanical properties of explanted arteries.5,8,9 A constant axial stretch results in reestablishment of vascular homeostasis through remodeling of tissue growth (wall thickness, wall stress, and vessel length) as well as a change in both circumferential and axial mechanical properties.5,8–10
However, only recently have studies begun to focus on the effect of biaxial stretching on ECM growth and remodeling in engineered vessels. These studies led to the development of bioreactors that can provide multiaxial stretching of TEVs.
Mironov et al. designed a perfusion bioreactor that was capable of applying axial stretch of up to 200% to both silicone tubing and to native arteries.11 However, this system was not able to support the in vitro growth of biologically based TEVs. Similarly, Zaucha et al. constructed a computer-controlled bioreactor system that applied short-term biaxial stretching to collagen-based TEVs.12 Nevertheless, the capability of this system to sustain long-term tissue growth and mechanical conditioning was not examined. Tranquillo's group built a pulsatile bioreactor that allowed incremental axial retraction (60%) at the unfixed end of TEVs.13 However, the ability to exert a constant or cyclic axial load on TEVs was not reported using this bioreactor. Bose® ElectroForce® BioDynamic® Test Instrument can simulate cyclic torsion, axial loads, and pulsatile perfusion to characterize mechanical properties of vascular constructs. However, these expensive devices are optimized and customized primarily to run mechanical tests on vascular constructs and not specifically designed for the production of engineered arterial grafts.
The aim of this current work was to develop a bioreactor to (i) allow independent control over the amount of stretch in the axial and circumferential directions, (ii) to investigate the impact of long-term biaxial loading on biological and mechanical properties of TEVs. The results show that biaxial loading enhances mechanical properties of TEVs, which is associated with an increase in mature elastin production as well as undulated collagen development.
Materials and Methods
Design of biaxial bioreactor system
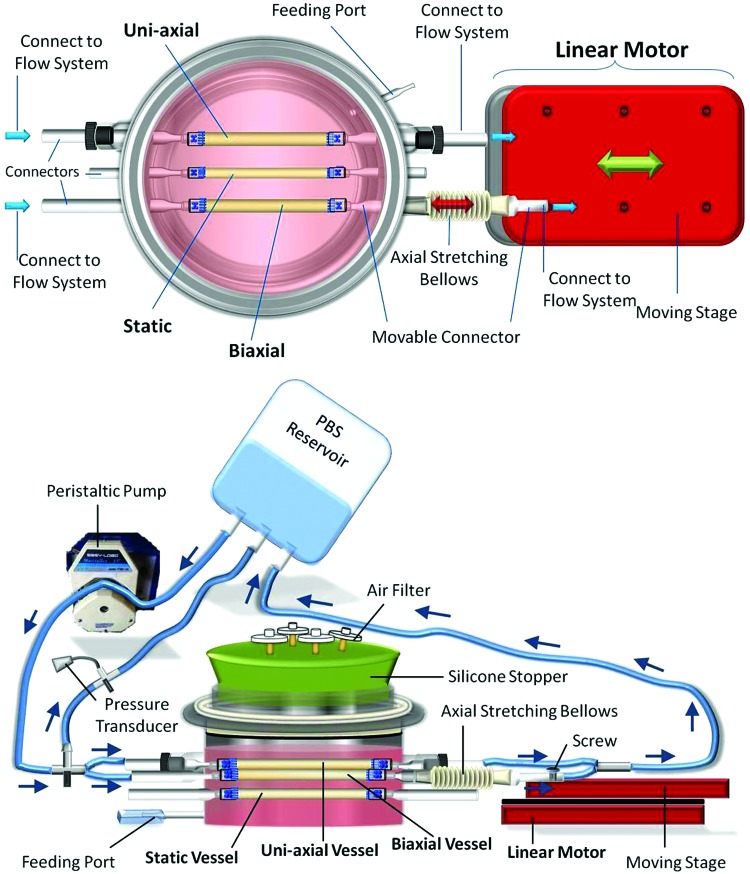
The biaxial bioreactor housing comprised entirely of glass and was made by the Yale University glassblower (makes tailored glassware upon request). All individual parts of the biaxial bioreactor system are listed in Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/tec). This biaxial bioreactor is capable of providing three types of mechanical stretching during in vitro growth of three TEVs: static culture, circumferential stretching only, and biaxial stretching (circumferential and axial stretching combined). A top view of the device is shown in Figure 1. Fluid flow, driven by a peristaltic pump (Masterflex L/S Precision Modular Drive; Cole-Parmer), exerts a pulsatile force (circumferential stretch) on growing engineered vessels. To provide cyclic axial stretch, a linearly moving motor (Nipponpulse, Linear Shaft Motor Stage SCR075) is employed. When both the linear motor and pulsatile flow are initiated, biaxial stretching on TEVs is achieved.
FIG. 1.
Top view and side view of the biaxial bioreactor system. Three tissue-engineered vessels (TEVs) were cultured under different mechanical stimuli within the same biochemical milieu. The biaxial bioreactor is connected to a flow system and linear motor to achieve cyclic biaxial stretching (circumferential and axial stretching). Axial stretch is achieved when the linear motor axially stretches the movable connector of the bioreactor through a bellows. Circumferential stretch is achieved through pulsatile phosphate-buffered saline (PBS) flow that is maintained by a peristaltic pump. Arrows indicate the direction of flow. Color images available online at www.liebertpub.com/tec
Day 1: biaxial bioreactor assembly
Tubular polyglycolic acid scaffold fabrication
A degradable sheet of polyglycolic acid (PGA) mesh (Biomedical Structures) was cut into three 1.3×∼4 cm pieces. Dexon 6.0 suture (Syneture) was used to sew along the edges of PGA scaffolds to form a tubular structure. Prolene 4.0 suture (Syneture) was used to sew small pieces of Dacron cuffs onto each end of the PGA mesh with an overlap of 2–3 mm. The same suture was used to sew five stitches around the ends of Dacron cuffs, leaving at least 15 cm of free suture on the cuffs (Supplementary Fig. S2A). Tubular PGA scaffolds were treated in 1 M NaOH for 1–2 min, followed by three ddH2O rinses. Silicone tubing (≈3 mm diameter×8 cm length; Saint-Gobain) was inserted into the lumen of each tubular PGA scaffold (Supplementary Fig. S2B). Pulsatile phosphate-buffered saline (PBS) flow through the silicone tubing creates cyclic circumferential stretching on both the tubing and the TEVs.
Bioreactor cap assembly
Before assembling, bioreactor parts were autoclaved and submerged in 70% ethanol bath with tubular PGA scaffolds for at least 1 h for further sterilization. Bioreactor components were assembled inside the 70% ethanol bath inside the tissue culture hood. The bioreactor cap consisted of a silicone stopper and Masterflex tubes L/S 16 (Cole-Parmer) (Supplementary Fig. S3). The silicone stopper had four drilled holes where tubes were pulled through to allow for gas exchange. The silicone stopper was inserted into a glass cylinder and the unit was inserted through a Teflon bushing (Supplementary Fig. S3A). A nylon O-ring was pulled over the glass cylinder from the bottom (Supplementary Fig. S3B).
Bioreactor and PGA scaffold assembly
First, Masterflex tubes L/S 16 were attached to the feeding port of the bioreactor for medium exchange (Fig. 2). To set up connectors for the uniaxial TEV, detachable glass connectors (6.0 mm) with tapered and flared ends were inserted into a Teflon bushing and nylon O-ring (4 mm). The units were then inserted into the connector arms at the two sides of the bioreactor. These connector arms were customized to fit those units for the uniaxial TEV. Teflon bushings were screwed tightly to prevent medium leakage and to enclose the inside of the bioreactor from the outside environment.
FIG. 2.
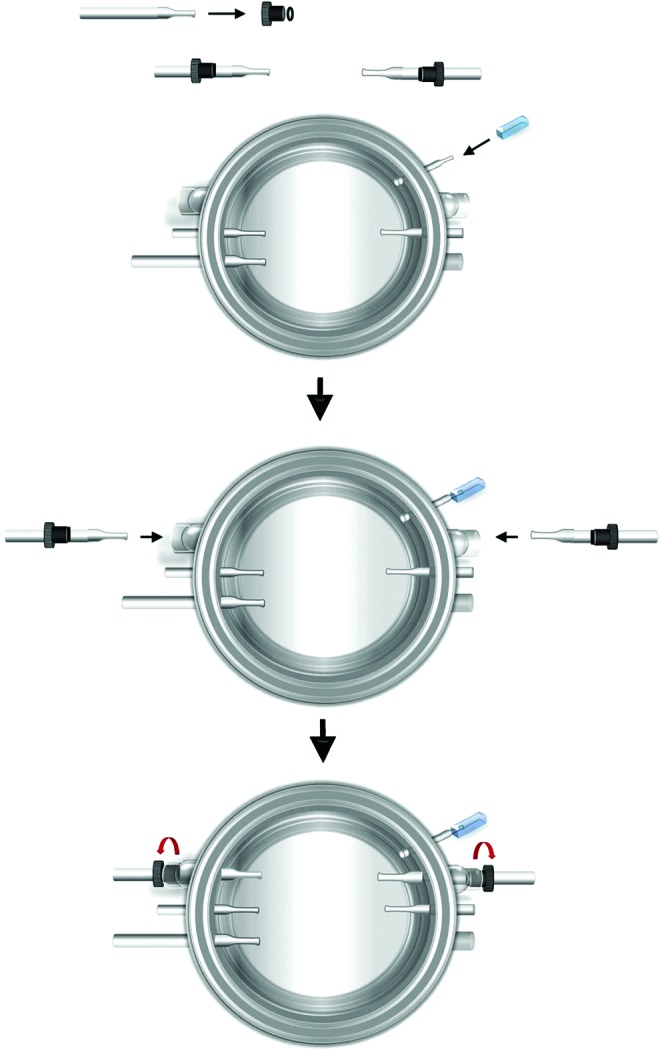
Assembly of uniaxial TEV connectors. A feeding tube is pulled over the feeding port of the bioreactor for medium exchange. Detachable glass connectors (6.0 mm) are fitted into O-rings and Teflon bushings. The units are inserted into corresponding connector arms at two sides of the bioreactor for the uniaxial TEV. The bushings are screwed tightly to enclose the openings. Only a portion of the feeding tube is shown above. Color images available online at www.liebertpub.com/tec
To assemble the connector for the biaxial TEV, the attachable glass connector (6.5 mm) was tightly inserted into a Teflon bellows that was axially extensible (Fig. 3). The unit was tightly fitted into the connector arm at one side of the bioreactor for linear axial stretching. Finally, PGA scaffolds with silicone tubing were mounted onto the bioreactor by pulling two ends of the silicone tubing over flared ends of glass connectors (Supplementary Fig. S4). Dacron cuffs were positioned over the flared ends and were tightened with the 4.0 Prolene suture on the Dacron cuffs. Static, uniaxial, and the biaxial TEVs were assigned to middle, top, and bottom PGA scaffolds, respectively (Fig. 1). Biaxial TEVs would be stretched axially through the extensible Teflon bellows via a linear motor. After the assembly was completed, the bioreactor and cap were submerged in 70% ethanol bath for another hour. Excess ethanol was drained and the bioreactor and cap were dried in the hood overnight.
FIG. 3.
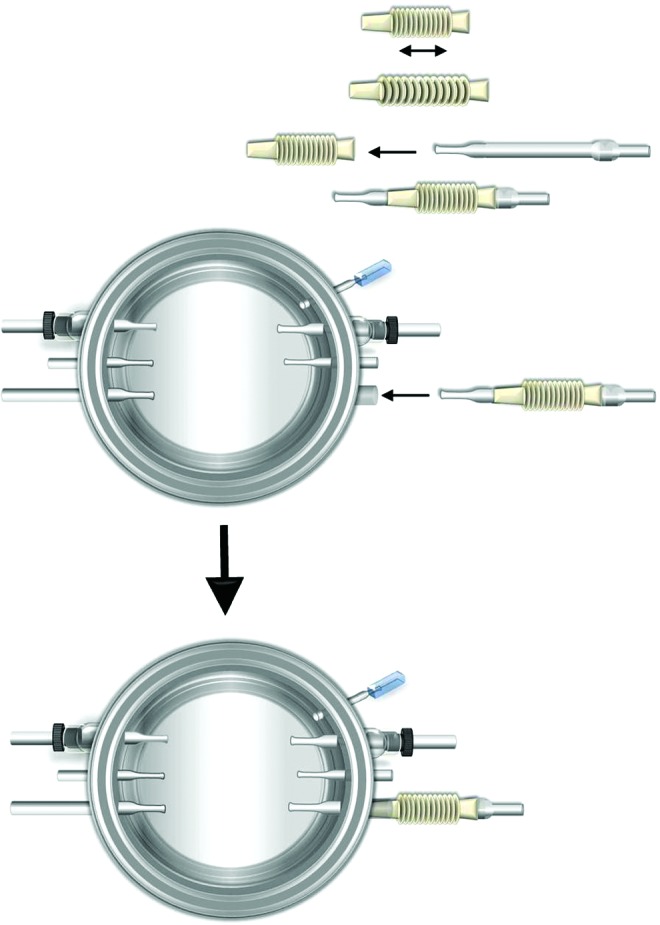
Assembly of biaxial TEV connector. The detachable glass connector (6.5 mm) is tightly fitted into an axially extensible Teflon bellows. The unit is inserted into the corresponding connector arm at one side of the bioreactor for biaxial TEV setup. Color images available online at www.liebertpub.com/tec
Day 2: cell seeding and setting up the flow system and linear motor
Cell seeding
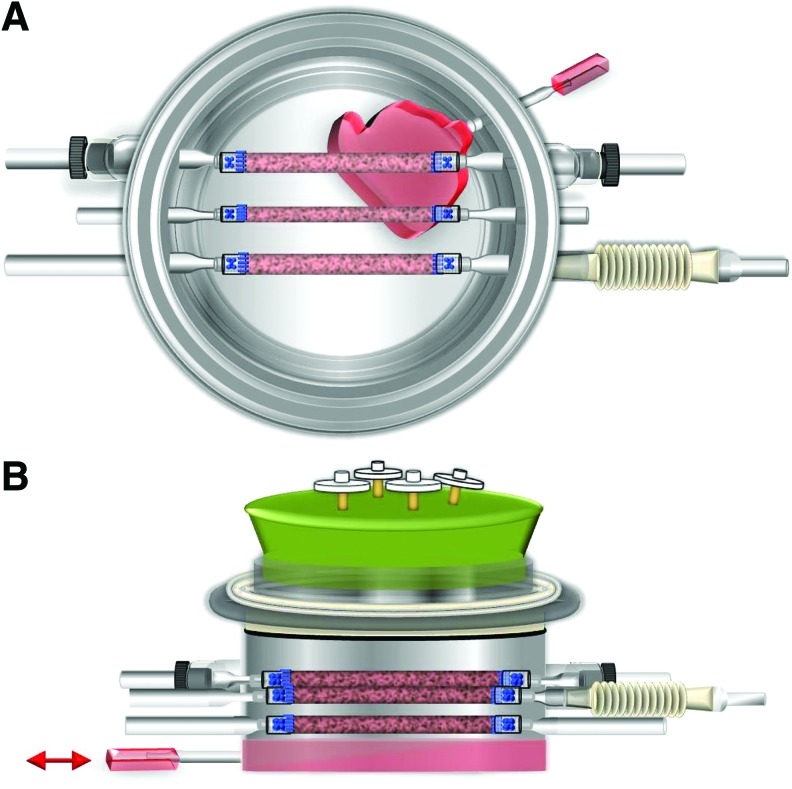
Bovine smooth muscle cells (SMCs) were isolated from the media of bovine aortas that were obtained from the local abattoir. Bovine SMCs were expanded in T-75 flasks till 80% confluence with a standard culture medium (20% fetal bovine serum [FBS], 1% penicillin and streptomycin, and high-glucose Dulbecco's modified Eagle's medium [DMEM]). Passage 2 bovine SMCs were trypsinized, resuspended in 2 mL of fresh medium, and seeded evenly onto three PGA scaffolds. Approximately 7×106 passage 2 bovine SMCs were seeded onto each tubular PGA scaffold (Fig. 4). Cell suspension was carefully dripped onto the entire PGA scaffolds with a pipette until the suspension was absorbed by the entire scaffold.
FIG. 4.
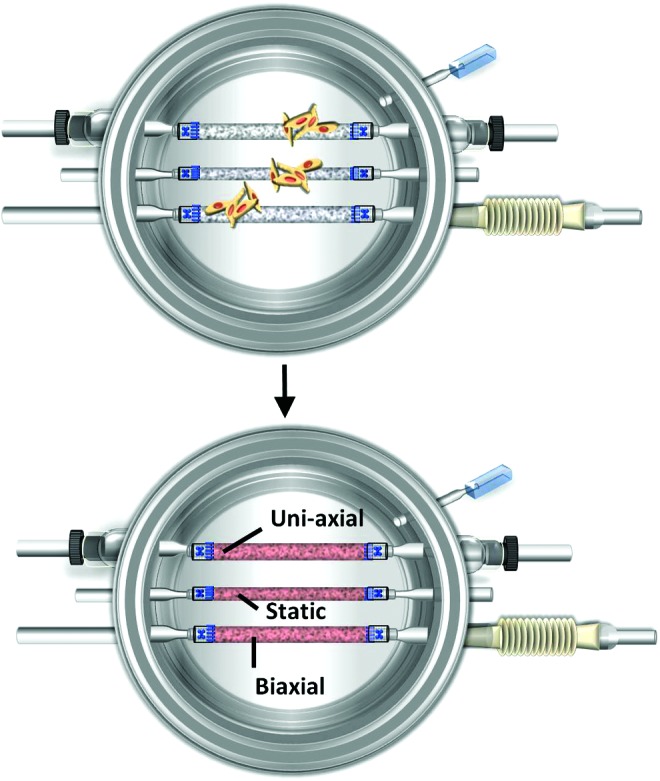
Cell seeding in the biaxial bioreactor. Bovine smooth muscle cells (SMCs) are seeded onto each polyglycolic acid (PGA) scaffold inside the bioreactor. PGA scaffolds absorb seeded cells and appear red. The position for each TEV and corresponding mechanical cues are labeled. Color images available online at www.liebertpub.com/tec
Enclosure of bioreactor
The bioreactor cap was placed on top of the bioreactor, and the Teflon bushing was rotated several times to enclose and seal the bioreactor (Supplementary Fig. 5). Finally, air filters (0.22 μm; Whatman) were inserted into the segments of Masterflex tubes L/S 16 to allow for sterile gas exchange between the incubator and bioreactor environments. The bioreactor was placed into the incubator to allow seeded cells to infiltrate and attach onto PGA scaffolds. After 40 min, 250 mL of fresh medium was added to the bioreactor through the feeding tubing (Fig. 5). TEVs were cultured statically for the first week. Mechanical stretching was applied on the second week of tissue culture.
FIG. 5.
Illustration of medium change through the feeding port. (A) A top view of the bioreactor showing medium change through the feeding port. (B) A side view of the bioreactor during the medium exchange process through the feeding tube. Color images available online at www.liebertpub.com/tec
Connecting the bioreactor to the flow system and linear motor. The biaxial bioreactor system consisted of three completely separate systems: biaxial bioreactor (TEV culture), flow system (cyclic circumferential stretching), and linear motor (circumferential axial stretching). Each of the three TEVs in this biaxial bioreactor was cultured with no loading (static), uniaxial loading (circumferentially stretched), or biaxial loading. The biaxial bioreactor was first connected to the flow system and then to a linear motor (Fig. 6). The static TEV was not connected to either the flow system or linear motor. Both uniaxial and biaxial TEVs were connected to the flow system, while the biaxial TEV was also attached to the linear motor through the axially movable connector. The flow system consisted of a PBS reservoir, Masterflex L/S 16 tubing, and connectors, as well as a pressure transducer. Connecting tubes (bifurcated from two ends of the main flow system through three-way connectors) were connected to corresponding glass connectors that were designed for uniaxial and biaxial TEVs (Fig. 6). Parallel PBS flow through the bioreactor generated cyclic circumferential stretching to both uniaxial and biaxial TEVs. To provide axial stretch to the TEV that was to be stretched biaxially, the axially movable glass connector was fastened to the moving stage of linear motor by using a screw (Fig. 6).
FIG. 6.
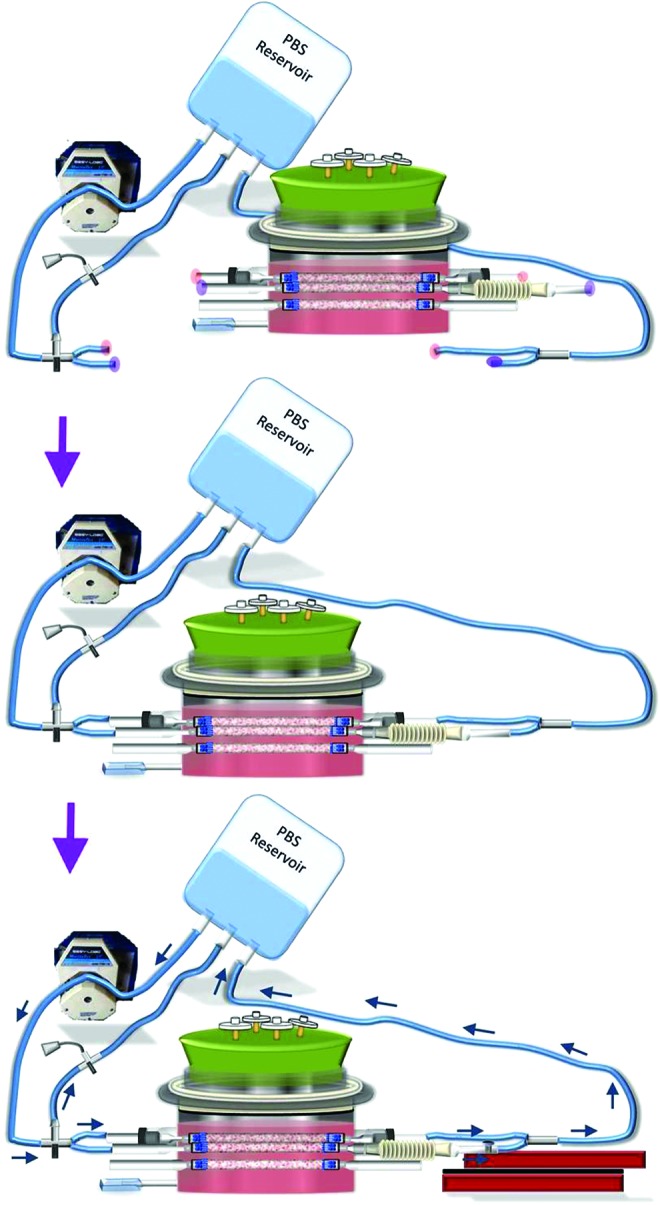
Connecting the biaxial bioreactor to the flow system and linear motor. One week after cell seeding, opaque extracellular matrix (ECM) started to be deposited onto PGA scaffolds of TEVs. The flow system is connected to the bioreactor by attaching connecting tubes to designated connector arms. Pink and purple highlights mark the connection locations for uniaxial and biaxial TEVs, respectively. The bioreactor is then connected to the linear motor by attaching the axially movable connector to the moving stage. The surface of the moving stage is prescrewed with multiple holes. A screw is used to secure the axially movable connector to the moving stage through one of those holes. Arrows indicate the direction of PBS flow. Color images available online at www.liebertpub.com/tec
Bioreactor maintenance
TEVs were maintained in vessel growth medium at 37°C and 5% CO2.2,14 The vessel growth medium is optimized specially to support ECM synthesis and consists of high-glucose DMEM supplemented with 10% FBS, penicillin G (100 U/mL; Sigma), ascorbic acid (50 ng/mL; Sigma), CuSO4 (3 ng/mL; Sigma), HEPES (5 mM; Sigma), proline (50 ng/mL; Sigma), glycine (50 ng/mL; Sigma), and alanine (20 ng/mL; Sigma). The culture medium was refreshed weekly and ascorbic acid was refreshed every 2 days.
Application of mechanical cues: circumferential and axial stretching
Application of axial stretching
Once the bioreactor was set up, the bioreactor was maintained under static culture for the first week. During the first week, no mechanical stimuli were applied to any of the TEVs. The static TEV was maintained under static conditioning for the entire 13-week culture period. Starting from the second week, axial stretching was applied only to the TEV that was to undergo biaxial stretching while keeping the other two TEVs under static conditions. To apply axial stretching, the linear motor was turned on and set to a cyclic displacement of 3.2 mm, which resulted in ∼8% axial stretch on the TEV. To avoid disruption of the PGA matrix, the seeded cells were allowed to form a rudimentary tissue matrix before axial stretching. Since a high axial stretch was applied to the PGA scaffolds, axial stretching was not applied until the second week. The linear motor axially stretched the TEV by cyclically extending the bellows that was connected to the moving stage through the axially movable connector. Axial stretching was maintained for the remaining 11 weeks at a rate of 0.0333 Hz for the biaxial TEV.
Application of circumferential stretching
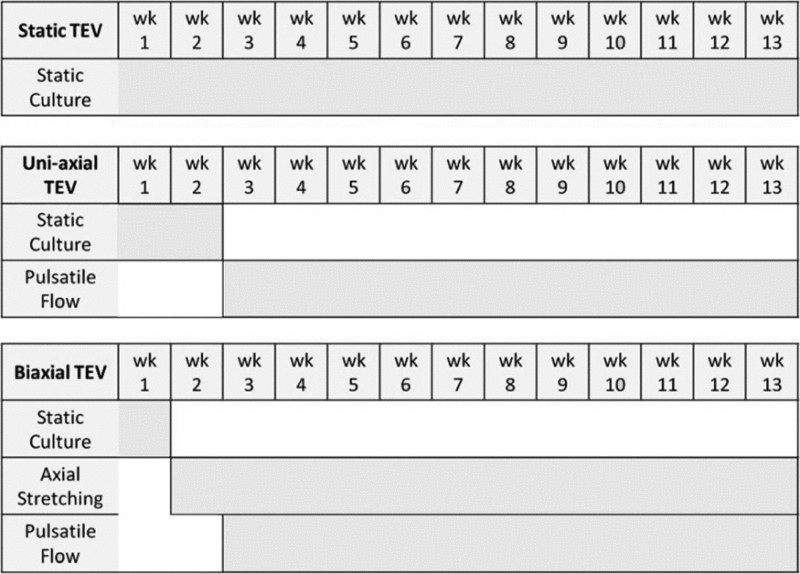
After 1 week of axial stretching (starting from the third week), circumferential stretching was applied to both uniaxial and biaxial TEVs. Since uniaxial and biaxial TEVs were aligned in parallel inside the bioreactor, the pulsatile flow simultaneously exerted cyclic circumferential stretching on both TEVs. For uniaxial and biaxial TEVs, the pulsatile flow was maintained for the remaining 11 weeks at the pressure range of 300 to −30 mmHg at a rate of 245 bpm. Luminal pressure of 270–300 mmHg resulted in 1.5–2.0% distension in silicone tubing. The flow was monitored and measured using a pressure transducer (Edwards Lifesciences) and PowerLab (ADInstruments) with LabChart Pro software (ADInstruments). A summary on the application of mechanical conditioning on TEVs can be found in Table 1.
Table 1.
Chart Describing the Application of Mechanical Conditioning to the Static, Uniaxial, and Biaxial Vessels
 |
Working range of the biaxial bioreactor system
The linear motor (Linear Shaft Motor Stage SCR075; Nipponpulse) was pretreated with a chromium coating (customarily done by Nipponpulse upon request) to enhance anticorrosion and antioxidization properties of the moving stage in a humid environment. The linear motor was capable of operating for 26 weeks in an incubator without any failure. The system can exert an axial displacement between 0 and 4 mm at a rate between 0 and 0.3 Hz. In addition, interchangeable properties of those glass connectors allowed for a versatile selection for TEVs with diameters ranging from 1 to 4 mm. Different combinations of static, circumferential, axial, and biaxial conditions can be applied to study the impact of mechanical cues on ECM remodeling of TEVs using this bioreactor system. Furthermore, second harmonic generation images could be obtained on living TEVs inside the bioreactor by placing a viewing window through the Teflon bushing of the bioreactor cap to assess ECM growth and remodeling in a real-time setting.15 The capacity of this biaxial bioreactor system is summarized in Table 2.
Table 2.
List of Tested Parameters and Capacity of the Bioreactor System
| Parameters | Tested capacity |
|---|---|
| Reliability | >75% success rate |
| System duration | 26 weeks |
| Axial displacement | 0–4 mm (0–10%) |
| Axial stretching rate | 0–0.33 Hz |
| TEV diameter | 1–4 mm (outer diameter) |
| Noninvasive imaging | With a specially designed imaging window |
TEV, tissue-engineered vessel.
Picrosirius red staining and immunofluorescence staining
At the conclusion of the 13-week culture period, engineered vessel segments were fixed in 10% neutral buffered formalin. After embedding the tissue samples in paraffin, the vessels were cut into 5-μm sections for histology and immunofluorescence staining.
The cross sections of TEVs were stained with picrosirius red to visualize fibrillar collagen. Images were acquired with an Olympus BX51TF microscope equipped with an Olympus DP70 camera using Olympus CellSens Dimension 1.4.1 software. We utilized polarizing optics and dark-field imaging as well as bright-field imaging. Magnification was set to 20×and 60×.
Immunofluorescence staining was performed for SMC markers and elastin in the TEVs. Briefly, tissue sections were incubated with primary antibodies overnight at 4°C (1:50 rabbit anti-elastin [ab21610; Abcam] and 1:5 for mouse anti-smoothelin [SC R4A; Santa Cruz]). Secondary antibodies (Invitrogen Alexa Fluor® 555 IgG [H+L], 1:400 dilution) were applied for 1 h at room temperature. Fluorescence images were acquired using Volocity software with 40×magnification.
Mechanical tests
Suture retention strength was measured by hanging weights at 2–3 mm from the edge (axial direction) of TEVs until tearing, at which point the failure load was recorded. Biaxial mechanical tests were performed on TEVs as previously described.16 Before removal from the bioreactors, India ink was used to mark the bioreactor length (in situ) of the TEVs. Subsequently, the unloaded length was measured and used to calculate the stretch ratio between the in situ and unloaded lengths. The TEVs were then secured to custom glass cannulae using a suture and cyanoacrylate glue and placed within Hanks phosphate-buffered solution (1.26 mM CaCl2 at 37°C) in a custom biaxial testing system.17 Luminal pressure, outer diameter in the central region, axial force, and axial extension were measured using protocols established previously.18,19 Briefly, all TEVs were subjected to four cycles of preconditioning, followed by three cycles of pressure–diameter tests (0–140 mmHg).
Desmosine assay
Tissue samples with wet weight of at least 3 mg were first hydrolyzed in 6 N HCl at 110°C for 24 h. The samples were then lyophilized and redissolved in water to measure the total protein mass for each TEV.20 The amount of desmosine was measured using a radioimmunoassay as described previously.21 The desmosine content was expressed as picomoles of desmosine per milligram of protein in tissue samples.
Results
The assembly procedures for our bioreactor system are reliable with a success rate of 75% for culture of 13 weeks. The first of four setups resulted in contamination due to inexperience in the assembly and sterilization processes. After optimization, all three consequent experiments lasted 13 weeks and resulted in successful cultures free of contamination and without failure of the linear motor or peristaltic pump. Once set up properly, both the linear motor and flow system sustained a stable stretching rate and displacement for the entire culture duration. Since the flow system was a separate system from the bioreactor and TEV culture, PBS leakage was readily salvaged by replacing the damaged tubing. The cost for our biaxial bioreactor system is approximately $3200. This cost is significantly lower than the $50,000 for the ElectroForce BioDynamic Test Instrument marketed by Bose.
Engineered arteries upon harvest
After 13 weeks of bioreactor culture, all three engineered vessels developed an opaque appearance as shown in the cross-sectional rings of each TEV (Fig. 7). TEVs cultured with biaxial stretching developed the thickest vascular wall, whereas the static vessel developed the thinnest wall (wall thickness: static [0.40±0.2 mm, n=2], uniaxial [0.56±0.14 mm, n=2], and biaxial [1.0±0.36 mm, n=2]). The average wall thickness of the biaxial TEVs is higher than what is reported for human coronary arteries, which are of comparable diameter (0.75±0.17 mm).22
FIG. 7.
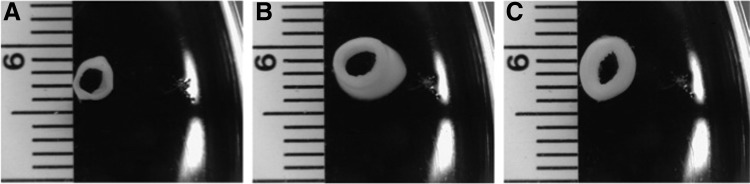
Cross-sectional rings of TEVs cultured in the biaxial bioreactor upon harvest. Differences in the wall thickness were found among static TEVs (A), uniaxial TEVs (B), and biaxial TEVs (C).
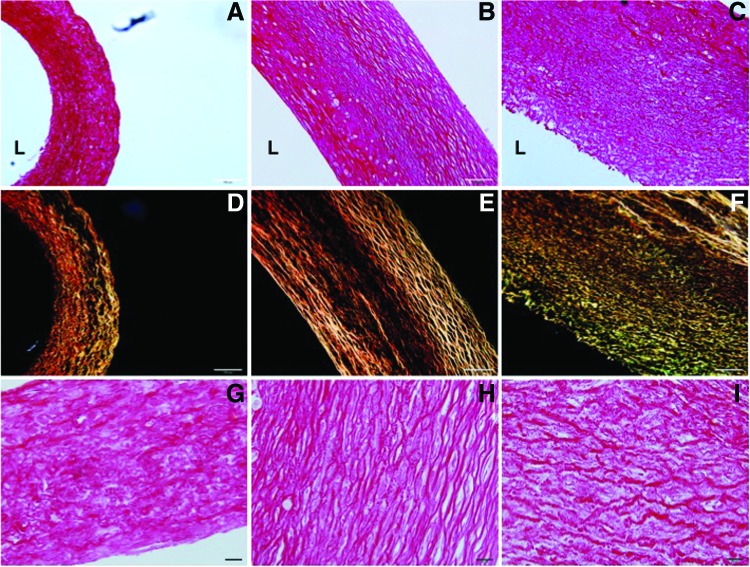
Examination of collagen matrix
Differences in fibrillar collagen structure among the three types of TEVs were visualized through picrosirius red stain under a polarized microscope (Fig. 8). Under dark field, mature or thicker collagen fibers appear bright red/orange, while the thinner fibers appear green/yellow. Shadowed regions found in both static and uniaxial vessels under dark field suggest the presence of thin/immature collagen fibers that were below the threshold for detection (Fig. 8D, E). A large number of green/yellow collagen fibers were detected in biaxially stretched TEVs, indicating active remodeling of the collagen matrix (Fig. 8F). Collagen fibers in the biaxial TEVs appeared more undulated than those in the uniaxial TEVs. In contrast, the undulated collagen structure was essentially absent in the collagen matrix of the uniaxial TEVs (Fig. 8H).
FIG. 8.
Examination of collagen matrix in TEVs. Picrosirius red stain was used to examine fibrillar collagen in TEVs under bright and dark fields of a polarized microscope. (A, D, G) Static vessel; (B, E, H) uniaxial vessel; and (C, F, I) biaxial vessel. (A–F) Scale bar represents 100 μm. (G–I) Scale bar represents 20 μm. The luminal side is indicated by “L.” Color images available online at www.liebertpub.com/tec
SMC contractile marker in TEVs under different loading conditions
Smoothelin, a late-stage SMC contractile marker, was examined in all TEVs (Fig. 9A–C). In comparison with static conditioning, both uniaxial and biaxial loading resulted in robust smoothelin expression in SMCs (Fig. 9A–C). This result implies that mechanical conditioning encourages the expression of late-stage contractile markers compared with no mechanical conditioning.
FIG. 9.
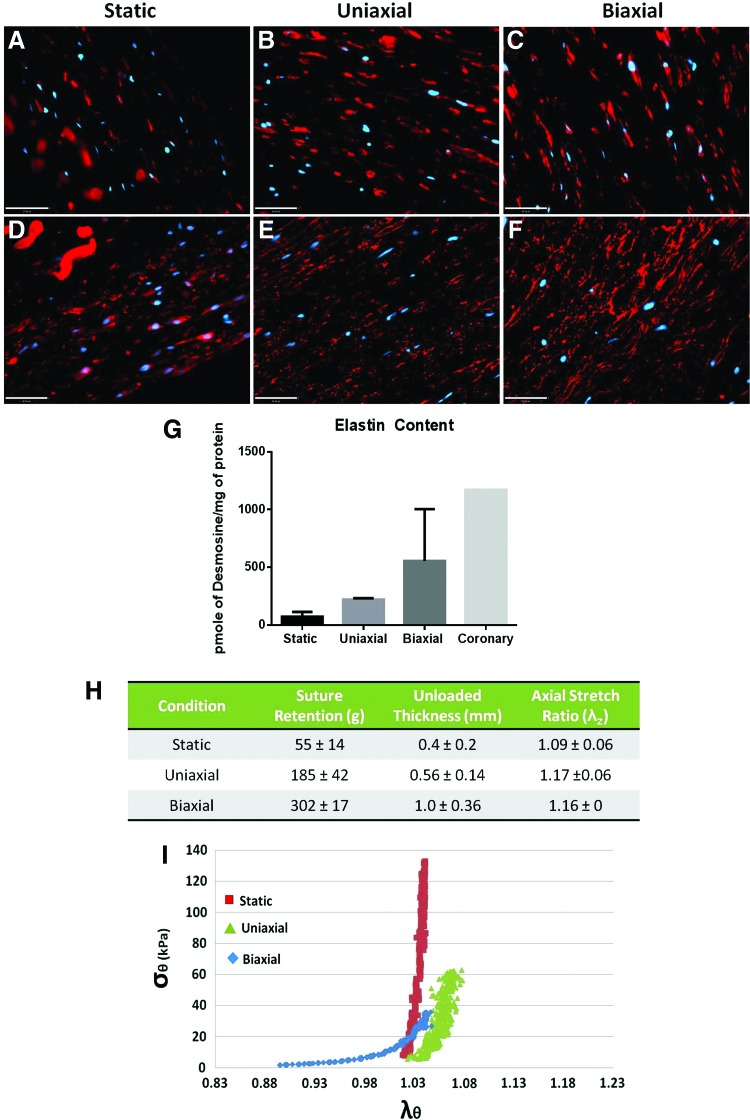
Immunostaining, biochemical, and mechanical analysis on TEVs. (A–C) Smoothelin, a late-stage contractile marker for SMCs. Red indicates smoothelin, blue indicates DAPI. Residual PGA fragments could also be found in TEVs. Scale bar=37 μm. (D–F) Immunofluorescence staining to detect elastin deposition in the ECM in all TEVs. Red indicates elastin, and blue indicates DAPI. Scale bar=50 μm. (G) Desmosine, an elastin cross-link, units of picomoles of desmosine per milligram of total protein. n=2, average±SD. (H) Suture retention, unloaded thickness, and axial retraction ratio, λz, of TEVs. Average±SD, n=2. (I) Circumferential stress (σθ) versus circumferential stretch (λθ) relationship. Color images available online at www.liebertpub.com/tec
Mature elastin analysis
Double staining of cellular marker and elastin was performed to compare intra-and extracellular elastin in the three TEV groups (Fig. 9D–F). The biaxial TEVs exhibited the most extensive elastin deposition in the ECM compared with the static and uniaxial TEVs (Fig. 9D–F). As for quantitative measure of mature elastin, a radioimmunoassay was used to detect desmosine, which is a specific amino acid found in elastin cross-links. The biaxial TEVs showed the highest desmosine content (555±449 pmole/mg protein, n=2) compared with the static (72±41 pmole/mg protein, n=2) and uniaxial TEVs (221±11 pmole/mg protein, n=2) (Fig. 9G). The desmosine content of the biaxial TEVs was ∼50% of that in native coronary artery (1171 pmole/mg protein, n=1). The result suggests that biaxial loading enhances the formation of mature elastin.
Mechanical analysis
The biaxial TEVs showed a suture retention strength of 302 g, which is higher than the reported value of 200 g for native human arteries23 (Fig. 9H). The stretch ratio, λz, indicates TEV retraction after releasing from an axial tension (removal from the bioreactor). Both biaxial (λz=1.16±0, n=2) and uniaxial TEVs (λz=1.17±0.06, n=2) showed a higher degree of retraction than the static TEVs (λz=1.09±0.06, n=2) (Fig. 9H). Stress–stretch relationships of TEVs were also impacted by biaxial conditioning. The biaxial TEVs showed a nonlinear behavior, including a toe region that is reminiscent of that seen in native arteries (Fig. 9I). In contrast, the static and uniaxial TEVs demonstrated a more linear relationship between the circumferential stress (σθ) and the circumferential stretch (λθ), which implies a comparative lack of elasticity (Fig. 9I). This result suggests that biaxial loading appears to increase vascular compliance compared with uniaxial loading or static conditions.
Discussion
Biaxial loading had major quantitative and qualitative impacts on ECM deposition and structure as well as mechanical properties in TEVs. In comparison with the traditional approach, biaxial loading greatly increased elastin deposition in the TEVs. The highest desmosine content in biaxial TEVs suggests that biaxial loading is required for extensive formation of mature elastin. Biaxial loading also had an impact on development of collagen structure by yielding highly undulated collagen fibers, which resemble those in native arteries and are essentially absent in uniaxial TEVs. More importantly, biaxial loading effectively enhanced arterial wall thickness as well as mechanical strength of the grafts. The presence of both mature elastin and undulated collagen fibers could contribute to the improved compliance in this situation. These results suggest that biaxial loading enables regeneration of TEVs that further mimic the physiologic characteristics of native arteries. Although biaxial loading greatly enhances TEV functionalities, either biaxial or uniaxial loading appears to be important for maintaining SMCs in a contractile phenotype.
This study suggests that incorporation of axial stretch into conventional mechanical conditioning plays an important role in matrix development and cellular phenotype. Further study is needed to demonstrate if biaxial stretching is required to achieve a collagen matrix that more completely recapitulates that of native arteries.
Our novel bioreactor is built to sustain long-term multiaxial stretching to engineered vessels during in vitro growth in a sterile environment. This biaxial bioreactor therefore allows us to elucidate the impact of specific mechanical conditions on ECM properties through side-by-side comparison of different mechanical stimuli within the same biochemical environment.
Most commercial bioreactors are not designed to apply multi-axial loading while supporting long-term tissue regeneration. Bose ElectroForce BioDynamic applies multiaxial mechanical stresses primarily for testing and characterizing material properties of tissue samples. Similarly, Instron LumeGen® Chambers (TGT) are designed for vascular tissue culture, but cannot apply multiaxial loads. The LumeGen Chambers share some features with our bioreactor system, but the LumeGen does not apply cyclic axial stretching or biaxial loading. Therefore, our biaxial bioreactor combines the strengths of both the Bose and the Instron systems. This biaxial bioreactor applies long-term multiaxial loading and has also been optimized for neotissue regeneration. In addition, the cost of our biaxial bioreactor is roughly10-fold lower than commercial bioreactors. Low cost, versatility, and reliability also may make this biaxial bioreactor attractive to research laboratories.
Conclusions
In summary, the current study describes the design and construction of a novel bioreactor system that is designed to apply biaxial stretch to TEVs to better simulate physiological conditions of native arteries. We showed that biaxial loading results in mature extracellular elastin as well as undulated collagen fibers. Both elastin and collagen structure contribute to vascular mechanics, such as compliance and mechanical strength.16,24,25 Therefore, additional studies are required to understand the impact of functional elastin and undulated collagen on the mechanics of biaxial TEVs. In conclusion, our study shows that biaxial stretching is a novel and valuable means to regenerate TEVs with improved physiologic properties.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 HL083895-06A1 and R01 1U01HL111016-01 (both to L.E.N.).
Disclosure Statement
L.E.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
References
- 1.Niklason L.E., Gao J., Abbott W.M., Hirschi K.K., Houser S., Marini R., et al. Functional arteries grown in vitro. Science 284, 489, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Quint C., Kondo Y., Manson R.J., Lawson J.H., Dardik A., and Niklason L.E. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A 108, 9214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahl S.L., Vaughn M.E., and Niklason L.E. An ultrastructural analysis of collagen in tissue engineered arteries. Ann Biomed Eng 35, 1749, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahl S.L., Rhim C., Song Y.C., and Niklason L.E. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng 35, 348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson Z.S., Gotlieb A.I., and Langille B.L. Wall tissue remodeling regulates longitudinal tension in arteries. Circ Res 90, 918, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Dobrin P.B. Mechanical properties of arteries. Physiol Rev 58, 397, 1978 [DOI] [PubMed] [Google Scholar]
- 7.Learoyd B.M., and Taylor M.G. Alterations with age in the viscoelastic properties of human arterial walls. Circ Res 18, 278, 1966 [DOI] [PubMed] [Google Scholar]
- 8.Gleason R.L., Wilson E., and Humphrey J.D. Biaxial biomechanical adaptations of mouse carotid arteries cultured at altered axial extension. J Biomec 40, 766, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Lawrence A.R., and Gooch K.J. Transmural pressure and axial loading interactively regulate arterial remodeling ex vivo. Am J Physiol Heart Circ Physiol 297, 22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humphrey J.D., Eberth J.F., Dye W.W., and Gleason R.L. Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech 42, 1, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mironov V., Kasyanov V., McAllister K., Oliver S., Sistino J., and Markwald R. Perfusion bioreactor for vascular tissue engineering with capacities for longitudinal stretch. J Craniofac Surg 14, 340, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Zaucha M.T., Raykin J., Wan W., Gauvin R., Auger F.A., Germain L., et al. A novel cylindrical biaxial computer-controlled bioreactor and biomechanical testing device for vascular tissue engineering. Tissue Eng Part A 15, 3331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syedain Z.H., Meier L.A., Bjork J.W., Lee A., and Tranquillo R.T. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 32, 714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gui L., Muto A., Chan S.A., Breuer C.K., and Niklason L.E. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng Part A 15, 2665, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niklason L.E., Yeh A.T., Calle E.A., Bai Y., Valentin A., and Humphrey J.D. Enabling tools for engineering collagenous tissues integrating bioreactors, intravital imaging, and biomechanical modeling. Proc Natl Acad Sci U S A 107, 3335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferruzzi J., Collins M.J., Yeh A.T., and Humphrey J.D. Mechanical assessment of elastin integrity in fibrillin-1-deficient carotid arteries: implications for Marfan syndrome. Cardiovasc Res 92, 287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gleason R.L., Gray S.P., Wilson E., and Humphrey J.D. A multiaxial computer-controlled organ culture and biomechanical device for mouse carotid arteries. J Biomech Eng 126, 787, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Eberth J.F., Taucer A.I., Wilson E., and Humphrey J.D. Mechanics of carotid arteries in a mouse model of Marfan Syndrome. Ann Biomed Eng 37, 1093, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dye W.W., Gleason R.L., Wilson E., and Humphrey J.D. Altered biomechanical properties of carotid arteries in two mouse models of muscular dystrophy. J Appl Physiol 103, 664, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Starcher B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal Biochem 292, 125, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Starcher B., and Conrad M. A role for neutrophil elastase in the progression of solar elastosis. Connect Tissue Res 31, 133, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Fayad Z.A., Fuster V., Fallon J.T., Jayasundera T., Worthley S.G., Helft G., et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation 102, 506, 2000 [DOI] [PubMed] [Google Scholar]
- 23.L'Heureux N., Dusserre N., Konig G., Victor B., Keire P., Wight T.N., et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med 12, 361, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schriefl A.J., Reinisch A.J., Sankaran S., Pierce D.M., and Holzapfel G.A. Quantitative assessment of collagen fibre orientations from two-dimensional images of soft biological tissues. J R Soc Interface 9, 3081, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schriefl A.J., Zeindlinger G., Pierce D.M., Regitnig P., and Holzapfel G.A. Determination of the layer-specific distributed collagen fibre orientations in human thoracic and abdominal aortas and common iliac arteries. J R Soc Interface 9, 1275, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.