Abstract
The sequences, or primary structures, of existing biopolymers--in particular, proteins--are believed to be a product of evolution. Are the sequences random? If not, what is the character of this nonrandomness? To explore the statistics of protein sequences, we use the idea of mapping the sequence onto the trajectory of a random walk, originally proposed by Peng et al. [Peng, C.-K., Buldyrev, S. V., Goldberger, A. L., Havlin, S., Sciortino, F., Simons, M. & Stanley, H. E. (1992) Nature (London) 356, 168-170] in their analysis of DNA sequences. Using three different mappings, corresponding to three basic physical interactions between amino acids, we found pronounced deviations from pure randomness, and these deviations seem directed toward minimization of the energy of the three-dimensional structure. We consider this result as evidence for a physically driven stage of evolution.
Full text
PDF
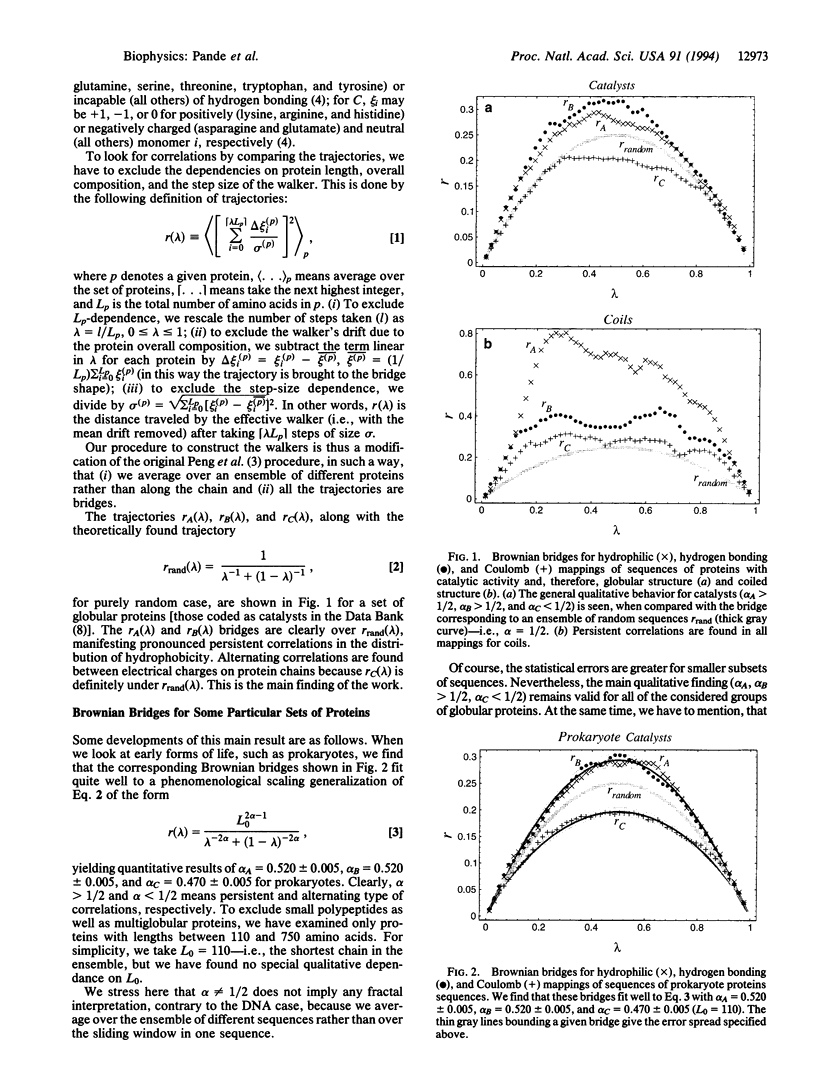
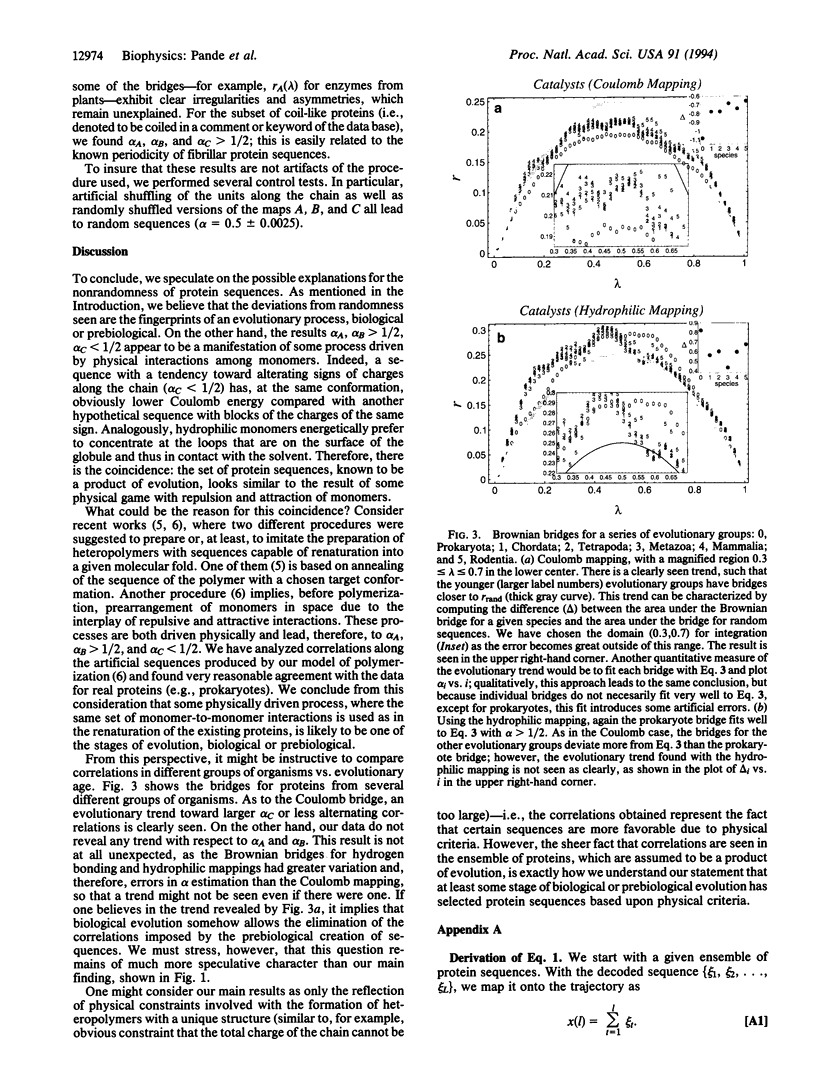

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bairoch A., Boeckmann B. The SWISS-PROT protein sequence data bank. Nucleic Acids Res. 1992 May 11;20 (Suppl):2019–2022. doi: 10.1093/nar/20.suppl.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande V. S., Grosberg A. Y., Tanaka T. Thermodynamic procedure to synthesize heteropolymers that can renature to recognize a given target molecule. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12976–12979. doi: 10.1073/pnas.91.26.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C. K., Buldyrev S. V., Goldberger A. L., Havlin S., Sciortino F., Simons M., Stanley H. E. Long-range correlations in nucleotide sequences. Nature. 1992 Mar 12;356(6365):168–170. doi: 10.1038/356168a0. [DOI] [PubMed] [Google Scholar]
- Ptitsyn O. B., Volkenstein M. V. Protein structure and neutral theory of evolution. J Biomol Struct Dyn. 1986 Aug;4(1):137–156. doi: 10.1080/07391102.1986.10507651. [DOI] [PubMed] [Google Scholar]
- Shakhnovich E. I., Gutin A. M. Engineering of stable and fast-folding sequences of model proteins. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7195–7199. doi: 10.1073/pnas.90.15.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]