ABSTRACT
The increasing emergence of antibiotic-resistant bacterial pathogens represents a serious risk to human health and the entire health care system. Many currently circulating strains of Acinetobacter baumannii exhibit resistance to multiple antibiotics. A key limitation in combating A. baumannii is that our understanding of the molecular mechanisms underlying the pathogenesis of A. baumannii is lacking. To identify potential virulence determinants of a contemporary multidrug-resistant isolate of A. baumannii, we used transposon insertion sequencing (TnSeq) of strain AB5075. A collection of 250,000 A. baumannii transposon mutants was analyzed for growth within Galleria mellonella larvae, an insect-based infection model. The screen identified 300 genes that were specifically required for survival and/or growth of A. baumannii inside G. mellonella larvae. These genes encompass both known, established virulence factors and several novel genes. Among these were more than 30 transcription factors required for growth in G. mellonella. A subset of the transcription factors was also found to be required for resistance to antibiotics and environmental stress. This work thus establishes a novel connection between virulence and resistance to both antibiotics and environmental stress in A. baumannii.
IMPORTANCE
Acinetobacter baumannii is rapidly emerging as a significant human pathogen, largely because of disinfectant and antibiotic resistance, causing lethal infection in fragile hosts. Despite the increasing prevalence of infections with multidrug-resistant A. baumannii strains, little is known regarding not only the molecular mechanisms that allow A. baumannii to resist environmental stresses (i.e., antibiotics and disinfectants) but also how these pathogens survive within an infected host to cause disease. We employed a large-scale genetic screen to identify genes required for A. baumannii to survive and grow in an insect disease model. While we identified many known virulence factors harbored by A. baumannii, we also discovered many novel genes that likely play key roles in A. baumannii survival of exposure to antibiotics and other stress-inducing chemicals. These results suggest that selection for increased resistance to antibiotics and environmental stress may inadvertently select for increased virulence in A. baumannii.
INTRODUCTION
Acinetobacter baumannii is rapidly emerging as a significant human pathogen (1). Current estimates predict that of the approximately 45,000 A. baumannii infections per year in the United States (and roughly one million infections annually worldwide), half (~23,000) are due to strains resistant to carbapenem antibiotics and have a mortality rate approaching 20% (2). Indeed, many of the currently circulating strains of this opportunistic pathogen exhibit a multidrug-resistant (MDR) phenotype, and even panresistant strains have been reported (3). In A. baumannii, the MDR phenotype can be attributed to a combination of intrinsic and acquired traits (4). While work to identify and characterize the repertoire of A. baumannii virulence determinants has begun, additional studies are required to understand the genetic basis of virulence and to translate how these determinants impact human disease (5).
Bacterial genetics represents an extremely powerful tool for the identification of virulence factors (6, 7), and as genetic technology has advanced, so too has the scale of genetic screens (8–11). We examined genes required for A. baumannii virulence by using strain AB5075, a contemporary MDR isolate that represents currently circulating isolates of A. baumannii (12–14). Using transposon insertion sequencing (TnSeq), we identified 300 genes required for survival and/or growth within Galleria mellonella larvae, an established insect model system for a variety of human microbial diseases (15). In addition to some known virulence factors, the screen reported here identified many novel genes, including several transcriptional regulators that control both virulence-related genes and those required for antibiotic resistance.
As A. baumannii clinical isolates are increasingly resistant to antibiotic therapy (16), we hypothesized that environmental virulence factors may be associated with resistance to antimicrobial compounds. Thus, we assessed the antibiotic sensitivity of a subset of the mutants. We found that several mutants defective for growth in G. mellonella larvae also exhibit increased susceptibility to antibiotics and other environmental stress conditions. These results highlight the existence of links between virulence and resistance to antibiotic and environmental stresses in A. baumannii (17–19) and suggest that selection for increased environmental stress resistance may also inadvertently select for strains with enhanced virulence.
RESULTS
TnSeq of A. baumannii in G. mellonella larvae.
In order to screen for virulence determinants in A. baumannii, we employed the G. mellonella infection model (15, 20, 21). Previous work with several microbial pathogens has demonstrated a positive correlation between the Galleria model and mammalian disease models (22–24). Infection of Galleria larvae with various Acinetobacter strains showed that the model easily distinguishes nonpathogenic from pathogenic Acinetobacter spp. For example, Fig. 1 shows that Acinetobacter baylyi, a well-characterized, nonpathogenic Acinetobacter species, does not survive for 4 h within larvae, whereas the human-pathogenic isolates ATCC 17978 and AB5075 survive and grow within the larvae during the same time period. Furthermore, larvae infected with these strains displayed dramatic differences in survival, with strain AB5075 leading to rapid death, A. baylyi ADP1 causing minimal larval killing over the course of the experiment, and ATCC 17978 leading to intermediate levels of killing (Fig. 1). The enhanced growth within larvae and the rapid larval killing kinetics are consistent with previous reports that AB5075 is more virulent than the ATCC 17978 strain and supports a correlation between growth in G. mellonella larvae with the previously reported virulence of specific A. baumannii strains in mammalian models (13) (Fig. 1).
FIG 1 .
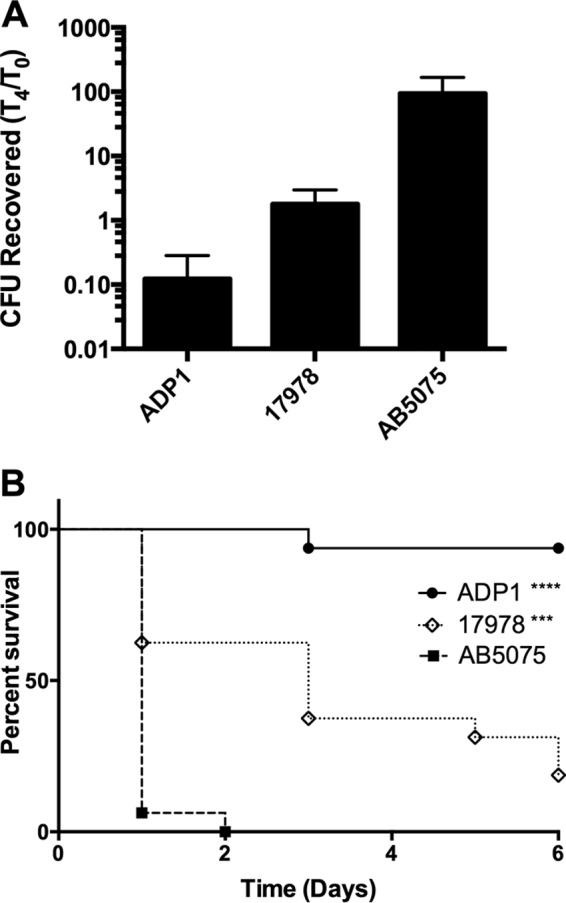
G. mellonella differentiates pathogenic and nonpathogenic Acinetobacter strains. (A) G. mellonella larvae were inoculated with 106 CFU of the strains indicated. Larvae were homogenized and bacteria were quantified immediately following infection (t = 0) and after 4 h at 37°C (t = 4). Data from a representative experiment are presented as the ratio of the number of CFU recovered at t = 4 to the number of CFU recovered at t = 0 (error bars, 1 standard deviation). (B) G. mellonella larvae were inoculated with 106 CFU of the strains indicated. Survival was monitored daily for 6 days. ****, P < 0.0001; ***, P < 0.001. ADP1, A. baylyi ADP1; 17978, A. baumannii ATCC 17978; AB5075, A. baumannii AB5075.
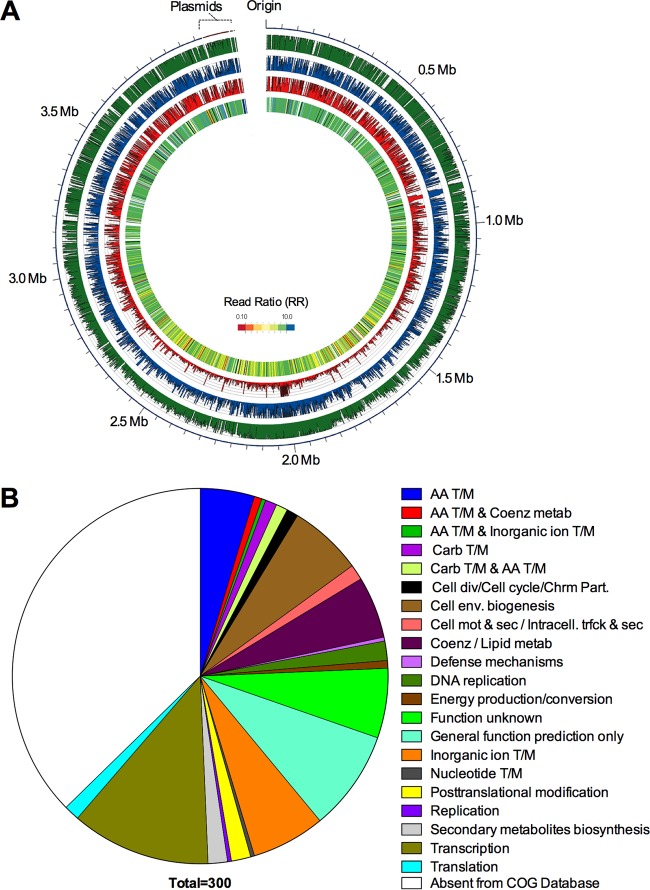
Given that the G. mellonella infection model readily differentiates between pathogenic and nonpathogenic Acinetobacter spp., as well as between more and less virulent A. baumannii strains, we sought to identify the genetic elements of A. baumannii strain AB5075 that are required for growth and/or survival within G. mellonella larvae. We performed a TnSeq experiment by the TnSeq Circle method with a previously described library of transposon mutants (8, 12). Aliquots of the transposon library were inoculated into either LB or G. mellonella larvae, incubated for 4 h at 37°C (see Fig. S1 in the supplemental material), and processed as described in Materials and Methods. To identify mutants that were underrepresented following growth in G. mellonella, a read ratio (RR) was determined for each gene in the G. mellonella and LB samples. Using a 10-fold reduction (RR of ≤0.10) as a significance cutoff, 300 genes were defined as essential for growth of AB5075 within G. mellonella larvae (Fig. 2; see Table S1 in the supplemental material). We anticipated that the G. mellonella TnSeq screen would identify three main categories of genes, (i) genes required for nutrient acquisition within G. mellonella, (ii) genes required to resist the G. mellonella immune responses, and (iii) genes performing an as-yet-unknown role during infection.
FIG 2 .
TnSeq experiment. (A) TnSeq experiment data. The outermost ring depicts the AB5075 chromosome and plasmids. The three middle rings depict the numbers of hits per gene in the pregrowth, LB growth, and Galleria growth samples (green, blue, and red, respectively). The innermost ring is a heat map of the RR of the Gm to the LB samples (Gm-essential genes are red). Essential genes are white, and genes with a general growth defect are black. The image was created with Circos (61). (B) Pie chart depicting Gm-essential hits grouped by COG categories. AA, amino acid; T/M, transport and metabolism; Coenz, coenzyme; metab, metabolism; Carb, carbohydrate; div, division; Chrm Part., chromosome partitioning; env., envelope; mot & sec, motility and secretion; Intracell. trfck & sec, intracellular trafficking and secretion.
Nutrient acquisition/metabolic functions.
A recently described concept of innate immune defense to bacterial infection is “nutritional immunity” or the ability of the host to restrict access of essential nutrients (25). The TnSeq screen identified several genes involved in acquisition systems for two essential micronutrients, zinc and iron (Table 1). Several components of the acinetobactin siderophore system were found to be required for growth in G. mellonella (Table 1) (26, 27), as well as ABUW_2074, a Fur family transcriptional regulator (RRfur of 0.050). Additionally, mutants with insertions in each of the three structural genes of the znuABC zinc uptake system and the transcriptional regulator controlling the expression of these genes (zur) exhibited very low RRs (Table 1). Zinc acquisition has been previously demonstrated as an important virulence determinant for A. baumannii disease in a murine lung infection model (28). Consistent with previous reports, a znuB mutant showed decreased survival after 4 h of infection, confirming the requirement for zinc acquisition in the Galleria model (Fig. 3) (28, 29). Recently, transcriptome data obtained from a zur mutant of A. baumannii was published and comparison of the zur-controlled genes with our TnSeq data set identified eight genes that were both differentially expressed in the zur mutant and required for growth in G. mellonella (29). These genes include znuA, a TetR family regulator (ABUW_1692), otsB (ABUW_3122), an isochorismatase hydrolase (ABUW_2374), and four genes of unknown function (ABUW_2145, ABUW_2439, ABUW_2442, and ABUW_2679). Identification of these well-known metal ion acquisition systems provides proof of the principle that the Galleria model imposes nutrient limitation stresses similar to those in mammalian infection models.
TABLE 1 .
Genes required for growth of A. baumannii in G. mellonellaa
| Function and annotation | Gene | Product | RR |
|---|---|---|---|
| Micronutrient acquisition | |||
| ABUW_1173 | bauD | Acinetobactin permease | 0.0119 |
| ABUW_1174 | bauC | Acinetobactin permease | 0.0976 |
| ABUW_1176 | bauB | Acinetobactin periplasmic binding protein | 0.0472 |
| ABUW_1177 | bauA | Acinetobactin receptor | 0.0111 |
| ABUW_3740 | znuA | High-affinity Zn transport protein | 0.0312 |
| ABUW_3741 | Zur | Fur family transcriptional regulator | 0.0730 |
| ABUW_3742 | znuC | Zinc import ATP-binding protein | 0.0685 |
| ABUW_3743 | znuB | High-affinity Zn transport protein | 0.0449 |
| Cysteine metabolism/sulfur assimilation | |||
| ABUW_0643 | cysI | Sulfite reductase | 0.0217 |
| ABUW_0722 | cysH | PAPSb reductase | 0 |
| ABUW_0853 | cobA | Uroporphyrin-III C-methyltransferase | 0 |
| ABUW_1760 | Sulfate permease | 0.0959 | |
| ABUW_2218 | cysQ | 3′(2′),5′-Bisphosphate nucleotidase | 0.0503 |
| ABUW_2362 | cysE | Serine acetyltransferase | 0.0361 |
| ABUW_2895 | cysN | Sulfate adenylyltransferase subunit 1 | 0 |
| ABUW_2896 | cysD | Sulfate adenylyltransferase subunit 2 | 0 |
| Aromatic hydrocarbon metabolism | |||
| ABUW_1835 | pcaD | 3-Oxoadipate enol-lactonase | 0.0469 |
| ABUW_1837 | pcaC | 4-Carboxymuconolactone decarboxylase | 0.0652 |
| ABUW_1848 | pcaU | pca operon regulatory protein | 0.0852 |
| ABUW_1854 | benP | Benzoate transport porin | 0.0305 |
| ABUW_2090 | 4-Hydroxybenzoate transporter | 0.0909 | |
| ABUW_2349 | 4-Oxalocrotonate tautomerase | 0.0086 | |
| ABUW_2374 | Isochorismatase hydrolase | 0.0631 | |
| ABUW_2523 | paaI1 | Thioesterase domain protein | 0.0069 |
| ABUW_2524 | paaY | Phenylacetic acid degradation protein | 0.0934 |
| Cell envelope/membrane/Wall | |||
| ABUW_3360 | lptE | LPS assembly | 0 |
| ABUW_3447 | lpxL | Lipid A biosynthesis acyltransferase | 0 |
| ABUW_3448 | lptB | Glycosyl transferase, group 1 | 0.0248 |
| ABUW_3638 | pbpG | d-Alanyl-d-alanyl carboxypeptidase | 0 |
| ABUW_3831 | Wza | Polysaccharide export protein | 0.0133 |
| ABUW_3832 | Ptp | Protein tyrosine phosphatase | 0 |
| ABUW_3833 | Ptk | Tyrosine protein kinase | 0 |
| Stress response genes | |||
| ABUW_0655 | typA | GTP-binding protein TypA/BipA | 0.0223 |
| ABUW_1595 | Kef | Ion transport protein | 0.0666 |
| ABUW_1648 | mscS | Mechanosensitive ion channel | 0.0658 |
| ABUW_1740 | rseP | Intramembrane metallopeptidase | 0.0279 |
| ABUW_1763 | uspA | UspA domain protein | 0 |
| ABUW_1804 | trkH | K+ uptake system component | 0.0639 |
| ABUW_2521 | uvrD | UvrD/REP helicase | 0.0460 |
| ABUW_2590 | kefF | NADPH oxidoreductase | 0.0405 |
| ABUW_3122 | otsB | Trehalose phosphatase | 0.0859 |
| Antibiotic resistance | |||
| ABUW_0842 | adeK | Multidrug efflux protein | 0 |
| ABUW_0843 | adeJ | Multidrug efflux protein | 0.0157 |
| ABUW_0844 | adeI | Multidrug efflux protein | 0.0154 |
| ABUW_1156 | Drug/metabolite exporter | 0.0442 | |
| ABUW_1499 | EamA-like transporter | 0.0619 | |
| ABUW_1520 | EamA-like transporter | 0.0635 | |
| ABUW_1673 | Bacterial TMc pair family protein | 0.0458 | |
| ABUW_1851 | Aminoglycoside phosphotransferase | 0.0912 | |
| ABUW_2123 | Metallo-β-lactamase family protein | 0.0828 | |
| ABUW_2550 | EamA-like transporter | 0.0584 | |
| Transcriptional regulation | |||
| ABUW_1645 | TetR family transcriptional regulator | 0.0437 | |
| ABUW_1672 | LysR family transcriptional regulator | 0.0676 | |
| ABUW_1692 | TetR family transcriptional regulator | 0.0493 | |
| ABUW_1755 | gigD | AsnC family transcriptional regulator | 0 |
| ABUW_1768 | MarR family transcriptional regulator | 0.0272 | |
| ABUW_1849 | LysR family transcriptional regulator | 0.0547 | |
| ABUW_1966 | LysR family transcriptional regulator | 0.0556 | |
| ABUW_2074 | Fur family transcriptional regulator | 0.0495 | |
| ABUW_2196 | alkR | AraC family transcriptional regulator | 0.0516 |
| ABUW_2236 | AraC family transcriptional regulator | 0.0550 | |
| ABUW_2370 | arsR | ArsR family transcriptional regulator | 0 |
| ABUW_2520 | TetR family transcriptional regulator | 0.0666 | |
| ABUW_2544 | AraC family transcriptional regulator | 0.0373 | |
| ABUW_2555 | soxR | Redox-sensitive transcriptional activator | 0.0934 |
| ABUW_3161 | gigC | LysR family transcriptional regulator | 0.0007 |
| ABUW_3180 | bfmS | TCS sensor kinase protein | 0 |
| ABUW_3260 | gigA | Putative TCS response regulator | 0.0671 |
Listed is a subset of genes identified in the TnSeq screen as outlined in the text. For the full list of hits, see the supplemental material.
PAPS, phosphoadenosine phosphosulfate.
TM, transmembrane.
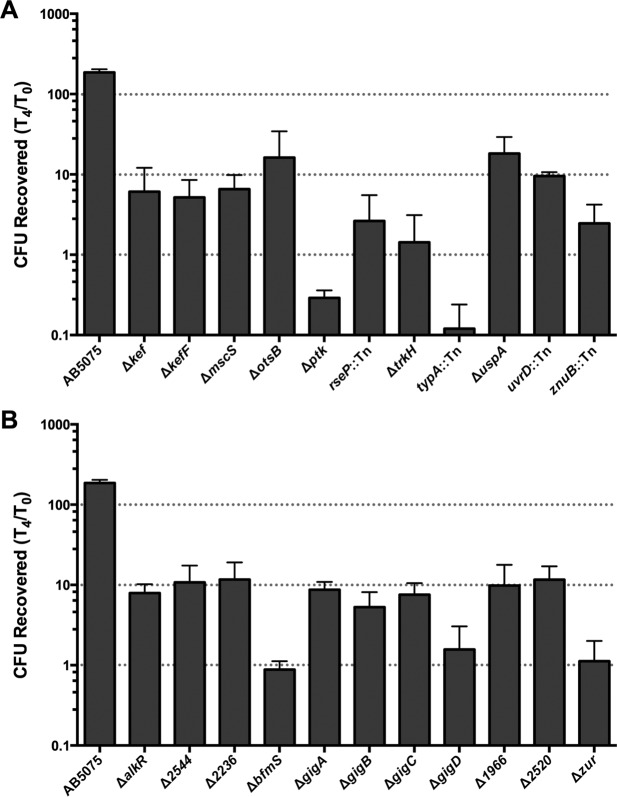
FIG 3 .
Growth of selected mutants in G. mellonella larvae. The mutant strains indicated were inoculated into G. mellonella larvae. Data are presented as described in the legend to Fig. 1. Panels: A, stress response genes; B, transcriptional regulators.
Another pathway required for growth in the larvae is cysteine metabolism/sulfur assimilation (Table 1), which is well conserved and has been characterized in many bacterial species (30, 31). One explanation for the requirement of these genes is that G. mellonella limits free cysteine as a pathogen restriction strategy, as shown previously (32). An alternative hypothesis for the cysteine requirement in the Galleria model is that cysteine serves as a protectant from oxidative and/or nitrosative stresses, which are widely conserved antimicrobial defense strategies. Indeed, cys genes have been linked to increased susceptibility to oxidative stress in several other bacterial species (33–35).
Eight genes predicted to play a role in the catabolism of aromatic compounds were identified as required for growth in G. mellonella (Table 1). This could indicate that the Galleria hemolymph contains aromatic hydrocarbons as carbon sources for Acinetobacter. Mutants predicted to be defective in the metabolism of various aromatic compounds, including benzoic acid and protocatechuate, were reduced following growth in Galleria larvae, indicating a link between aromatic compound catabolism and the ability to grow in the larvae. Additionally, two of the identified genes, paaI and paaY, predicted to be involved in phenylacetic acid catabolism, have previously been implicated in virulence (36).
Capsule, cell envelope, and membrane biogenesis.
Transposon insertions within several genes required for capsule, cell wall, and outer membrane biogenesis reduced the ability of A. baumannii to survive and grow within Galleria larvae (Table 1). This result is not surprising, as many of the immune defense mechanisms employed by the larvae will first encounter the bacterial outer membrane/cell envelope. Indeed, mutants with transposon insertions in several genes required for capsule biosynthesis were completely absent from the output DNA pool after growth in Galleria (see Fig. S2 in the supplemental material). Also, strains harboring insertions in the pbpG gene, encoding a d-alanyl-d-alanine endopeptidase required for modification of peptidoglycan, were unable to grow in the larvae (RRpbpG of 0). This gene was previously shown to be required for the growth of A. baumannii in human serum, a murine pneumonia model, and a rat pneumonia model (11, 37). In addition to the capsule operon and pbpG, transposon insertions in several other genes involved in lipopolysaccharide (LPS) biosynthesis, including lptE, lpxL, and lpsB, were undetected or underrepresented in the larval samples (RRs of 0, 0, and 0.025, respectively). In many Gram-negative bacteria, like Escherichia coli, LptE is an essential protein required for the proper insertion of LPS into the outer membrane (reviewed in reference 38). In some Gram-negative organisms, however, such as Neisseria meningitidis, LptE is not essential for LPS transport (39). Analysis of a previously published list of candidate essential genes in A. baumannii AB5075 indicates that other LPS transport genes (lptA, lptB, lptC, and lptD) are essential, whereas lptE is not required for growth in rich medium (12). The requirement of lptE in the Galleria model suggests that there may be environments where LptE is required for proper LPS assembly.
To validate and establish a protective role for capsule production in the G. mellonella disease model, as has been shown in other disease models (40), we constructed a strain with an in-frame deletion of the ptk gene. Ptk is a structural component of the capsular polysaccharide export machinery, along with Wza and Ptp. Recovery of mutants with changes in all three genes was significantly reduced in the G. mellonella larval output pool (RRwza of 0.013, RRptp of 0, RRptk of 0). When we infected G. mellonella larvae with the ptk mutant, larval killing and bacterial growth were dramatically reduced compared to those of wild-type AB5075 (see Fig. S2 in the supplemental material) and were restored by expression of the wild-type ptk gene in trans, confirming the importance of the capsule as a virulence factor in A. baumannii.
Stress response genes.
Sixteen genes required for growth within G. mellonella are predicted to mediate stress resistance (Table 1). These genes include those involved in the general stress response (cinA, csp, typA, and uspA), DNA repair, uvrD, membrane/envelope stress (ABUW_1447 [HptX homolog] and rseP), oxidation-induced stress (pqiA [paraquat] and arsC [arsenate]), and osmotic stress resistance (ABUW_2237 [cation transport], otsB, kef, kefF, mscS, trkA, and trkH) (Table 1). These results suggest that the ability of A. baumannii to grow and/or survive in G. mellonella may be dependent upon the organism’s ability to cope with environmental stresses imposed by the innate immune response to infection. As the G. mellonella larva contains hemocytes, phagocytic cells that, much like human neutrophils, generate an oxidative burst and produce antimicrobial peptides, which can mimic osmotic shock (15, 21, 23, 41, 42), we infer that these stress response genes may also play a role in human disease. To confirm the phenotypes of the stress response genes identified in the TnSeq screen, we constructed mutants containing in-frame, unmarked deletions of several of the hits and tested these strains for virulence defects in the G. mellonella model (Fig. 3). In the few instances where attempts to generate in-frame deletions were unsuccessful, we used strains harboring a transposon insertion in the gene of interest. Deletion strains showing defective survival and/or growth within G. mellonella include both genes with known/predicted functions, including ptk; bfmS (the gene for a two-component system [TCS] sensor kinase described in further detail below); znuB; stress response genes rseP, typA, and uspA; and genes with uncharacterized functions (Fig. 3).
As several of the stress response genes identified in the TnSeq screen are predicted to mediate osmotic stress resistance, we conducted additional experiments to demonstrate a role for osmotic stress resistance in the G. mellonella model. The osmotic stress gene mutants used in the experiment shown in Fig. 3 were defective for growth in freshly collected larval hemolymph, consistent with the results obtained in the TnSeq experiment (Fig. 4A). Decreased killing of larvae by several strains harboring deletions of osmotic stress genes was also observed (Fig. 4B).
FIG 4 .
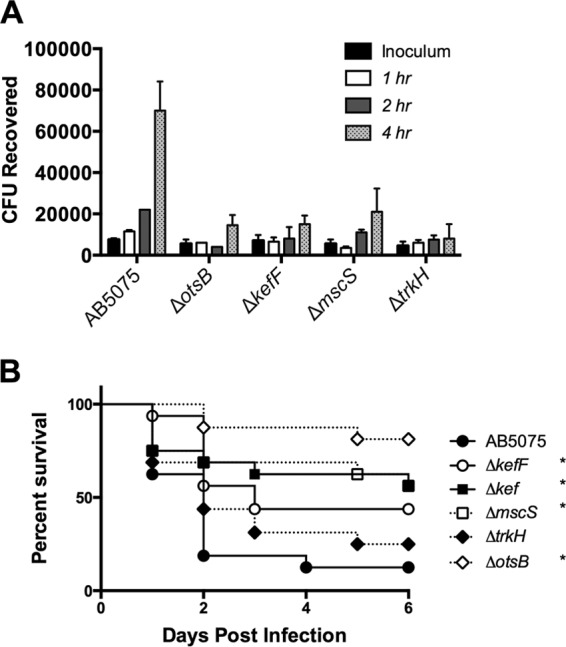
Osmotic stress genes are required for virulence in G. mellonella. (A) Growth of osmotic stress mutants in freshly collected hemolymph monitored over time. (B) G. mellonella larvae (n = 16) were infected with 105 CFU of wild-type AB5075 or isogenic mutants. Larval survival was monitored daily for 6 days. *, P < 0.05.
Furthermore, we performed additional complementation experiments with a subset of genes (rseP, typA, and uspA) predicted to be involved in both stress sensing and survival. In other proteobacteria, the rseP gene encodes an intramembrane protease that transmits outer membrane stress via RpoE/σE (43), while typA and uspA are predicted to be involved in the general stress response and have previously been shown to play a role in virulence (44–46). We confirmed the initial TnSeq results by assessing the ability of these mutants to grow within and kill G. mellonella. Strains defective for these genes displayed a Galleria growth defect, which was restored to nearly wild-type levels by complementation with wild-type copies of the genes expressed in trans (Fig. 5).
FIG 5 .
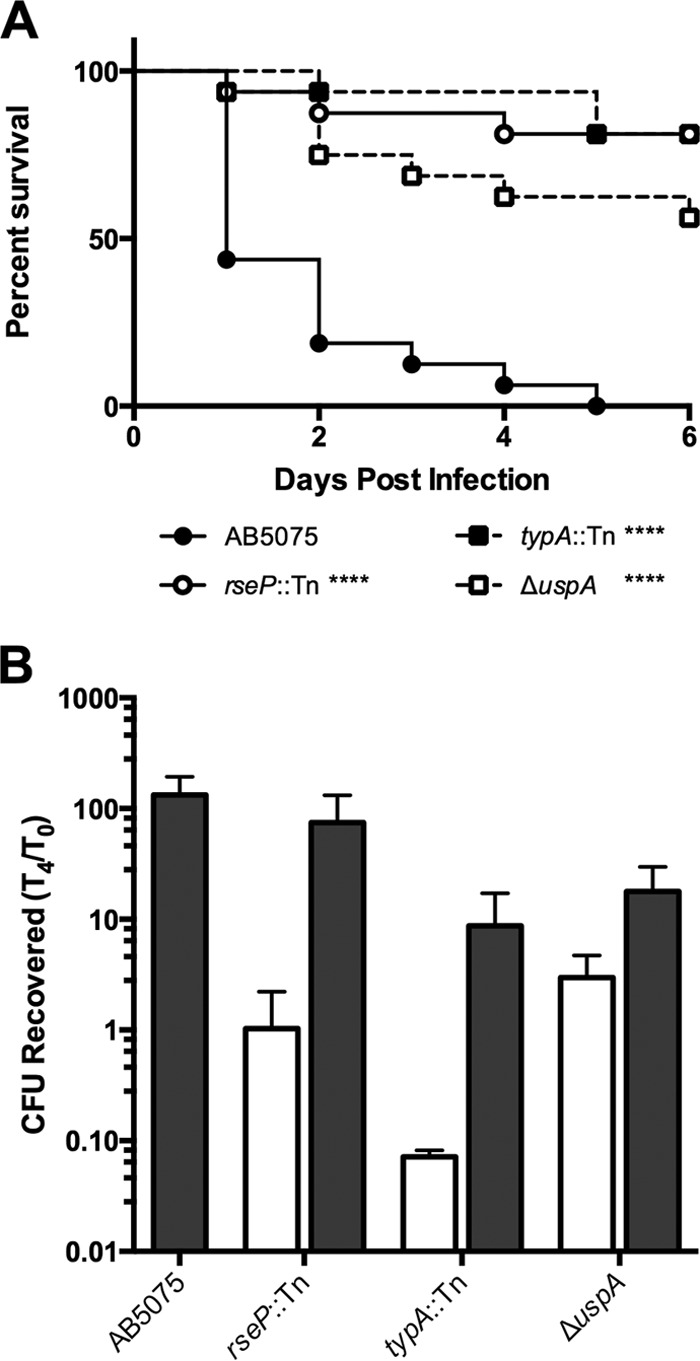
Stress response genes are required for G. mellonella killing and growth in Galleria. (A) G. mellonella larvae were infected with the strains indicated as described in the legend to Fig. 1. (B) Growth of wild-type AB5075 (gray bar) or the strains indicated harboring a hygromycin resistance gene (white bars) or a wild-type copy of the deleted gene at the Tn7 locus (gray bars) following inoculation of G. mellonella larvae as described in the legend to Fig. 1. ****, P < 0.0001.
Antibiotic resistance genes.
All strains of A. baumannii harbor efflux pumps associated with the removal of toxic compounds from bacterial cells (47). The TnSeq screen identified genes belonging to three different types of efflux systems, adeI, adeJ, and adeK (RRs of 0.0247, 0.0233, and 0, respectively), which encode the resistance-nodulation-cell division pump AdeIJK (48); four different genes containing EamA-like transporter domains of the metabolite/drug transporter family (ABUW_1156 [RR of 0.044], ABUW_1499 [RR of 0.062], ABUW_1520 [RR of 0.064], ABUW_2550 [RR of 0.058]) and a member of the proteobacterial antimicrobial compound efflux family (ABUW_1673 [RR of 0]) (49) (Table 1). Decreased recovery of strains harboring interruptions of these genes following growth in Galleria larvae relative to rich medium suggests that Galleria produces toxic compounds during infection that A. baumannii must export for survival. In addition to these drug efflux systems, the recovery of strains harboring insertions within two genes (ABUW_1851 and ABUW_2123) encoding a putative aminoglycoside phosphotransferase and a beta-lactamase family protein was also reduced following G. mellonella growth. Although annotated as encoding potential antibiotic resistance mechanisms, we hypothesize that the proteins produced by these genes may perform other functions, as G. mellonella larvae likely do not produce either aminoglycosides or β-lactams.
Transcriptional regulators.
Other than genes of unknown function, the largest single category of hits from the TnSeq screen includes genes annotated as having signal transduction or transcriptional regulation functions (Table 1; Fig 2). Among the 32 genes in this group, only a few have previously recognized functions, i.e., arsR (arsenic resistance), alkR (alkane metabolism), bfmS (biofilm formation), and soxR (redox homeostasis) (50–53). An insufficient number of transposon insertions mapped to bfmR (the cognate response regulator for bfmS) in the LB sample to be included in the TnSeq analysis. Several other putative transcriptional regulatory genes were found (see Table S1 in the supplemental material), i.e., two TCS response regulators, seven LysR family regulators, five TetR family regulators, four AraC family regulators, two AsnC/Lrp family regulators, a MarR family regulator, and several others. One of the TetR family regulators (ABUW_1692) and one of the AsnC/Lrp regulators (ABUW_1755, described below) were reported to be differentially expressed in the zur mutant (29), as well as during biofilm growth (54). Coupled with the bfmS requirement for growth in G. mellonella, this finding suggests that similar genetic pathways are required for biofilm formation and growth/survival in G. mellonella. Follow-up studies identified a subset of genes that are required for both growth within and killing of G. mellonella larvae. We term these gig genes for growth in Galleria. While many additional genes listed in Table 1 (see also Table S1 in the supplemental material) could be considered to encode the gig phenotype, we formally classify only a small subset as gig genes because loss of these particular genes leads to a robust defect in both growth within and killing of Galleria larvae. The gig genes include ABUW_3260, a predicted TCS response regulator with a PP2C protein phosphatase domain (gigA); ABUW_3261, a putative anti-anti-sigma factor (gigB); ABUW_3161, a LysR family regulator (gigC); and ABUW_1755, an AsnC/Lrp family regulator (gigD) (Fig. 6). Expression of the gigB, gigC, and gigD genes in trans restored the Galleria growth defect of these strains (Fig. 6). Attempts to complement the gigA mutant yielded ambiguous results.
FIG 6 .
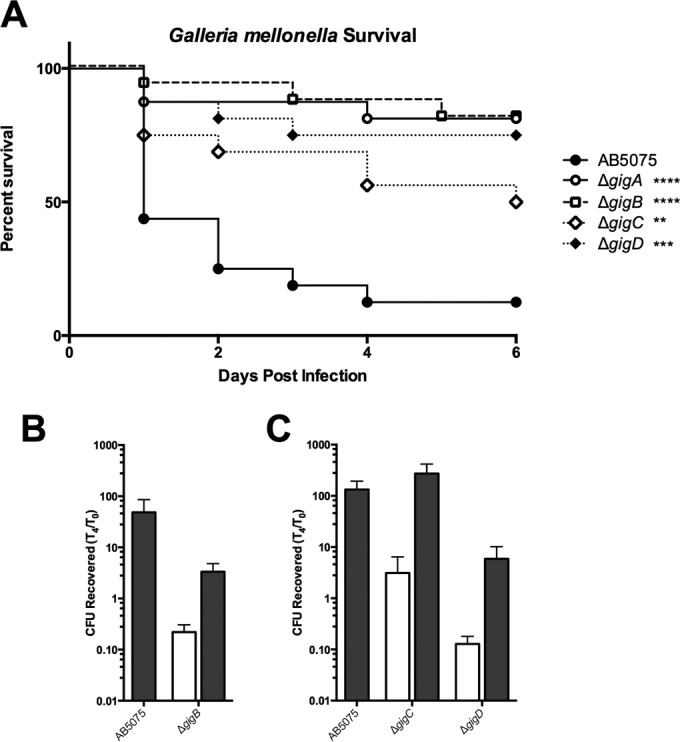
Gig genes are required for virulence in G. mellonella. (A) G. mellonella larvae were infected with the strains indicated and monitored for survival as described in Fig. 1. (B) Growth of AB5075 with the empty vector (left gray bar) or the gigB mutant harboring the empty vector (white bar) or a complementing clone of gigB (right gray bar). (C) Growth of wild-type AB5075 (left gray bar) or the strains indicated harboring a hygromycin resistance gene (white bars) or a wild-type copy of the deleted gene at the Tn7 locus (center and right gray bars). In panels B and C, growth is depicted as described in the legend to Fig. 1. ****, P < 0.0001; ***, P < 0.001; **, P < 0.01.
Mutations in gig genes produce decreased antibiotic resistance.
As discussed above, several genes required for growth in G. mellonella appeared to be involved in resistance to environmental stress and/or antibiotics (Table 1). This finding led to the hypothesis that genes required for virulence may also have an important role in resistance to environmental stress(es), such as exposure to antibiotics. In order to test this hypothesis, we performed antibiotic susceptibility testing according to the CLSI guidelines (55) and discovered that several genes that exhibited an RR of <0.10 in the G. mellonella model also mediate resistance to antibiotics. For example, strains with either gigA or gigB deleted showed increased sensitivity to several antibiotics, including meropenem, aminoglycosides, and tigecycline (Table 2; Fig. 7). The Δptk mutant strain showed enhanced susceptibility to several antibiotic categories, suggesting that capsular polysaccharides also serve to protect A. baumannii from antibiotic killing (Table 2). In addition, deletion of a LysR family regulator (ABUW_1966) or zur led to increased susceptibility to peptidoglycan-targeting antibiotics, including penicillins and meropenem (Table 2). The strain harboring a transposon insertion in typA also exhibited sensitivity to several antibiotics (Table 2). The finding that these genes are also required for growth within and/or killing of G. mellonella larvae supports the hypothesis that there is a link between virulence and the ability to resist environmental and/or antibiotic stresses.
TABLE 2 .
Antibiotic susceptibilities of selected TnSeq hits
| Straina |
Sensitivity to:b,c |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Amp-sulb | Pip-tazo | Merop | Amik | Tobra | Levofl | Col | Minocyc | Gent | Tigecycd | |
| ATCC 17978 | 29 | 23 | 27 | 25 | 24 | 26 | 14 | 28 | 25 | 0.13 |
| AB5075 | 6 | 6 | 8.2 | 8.4 | 10 | 11.2 | 12.7 | 24 | 6 | 1.5 |
| ΔalkR | 6 | 6 | 7 | 9 | 10.5 | 11 | 12 | 23 | 6 | 1 |
| Δ2544 | 6 | 6 | 8 | 8 | 10 | 11 | 13 | 25 | 6 | 1 |
| Δ2236 | 6 | 6 | 7 | 9 | 10 | 11 | 13 | 24 | 6 | 1 |
| ΔarsR | 6 | 6 | 8 | 8 | 10 | 12 | 12 | 21 | 6 | 2 |
| bfmR::Tn | 6 | 6 | 8 | 9 | 10.5 | 12 | 14 | 25 | 6 | 1 |
| ΔbfmS | 8 | 6 | 9 | 6 | 8 | 8 | 12 | 21 | 6 | 2 |
| ΔgigA | 6 | 6 | 9.2 | 14.6 | 17.8 | 12.1 | 13.5 | 24 | 13 | 0.75 |
| ΔgigB | 8.4 | 7.5 | 10.8 | 18.2 | 20.7 | 13 | 14.7 | 27.5 | 16 | 0.125 |
| ΔgigC | 6 | 6 | 8 | 6 | 9 | 12 | 14 | 27 | 6 | 0.5 |
| ΔgigD | 9 | 6 | 9 | 6 | 10 | 11 | 14 | 27 | 6 | 0.75 |
| Δfur | 6 | 6 | 8 | 9 | 10 | 10 | 14 | 25 | 6 | 1.5 |
| Δ1849 | 6 | 6 | 8 | 7 | 10 | 11 | 12 | 22 | 6 | 1 |
| Δ1966 | 9 | 6.5 | 9.8 | 7.1 | 8.7 | 10.7 | 13.1 | 25 | 6 | 1.5 |
| Δ1672 | 6 | 6 | 8 | 7.5 | 10 | 11 | 14 | 27 | 6 | 0.75 |
| Δ1768 | 6 | 6 | 8 | 9 | 9 | 11.5 | 13 | 25 | 6 | 1 |
| Δptk | 10 | 7.8 | 10.5 | 7.4 | 10 | 9.3 | 14.5 | 27.5 | 6 | 0.75 |
| Δ1645 | 6 | 6 | 8 | 9 | 11 | 11 | 13 | 24 | 6 | 0.75 |
| Δ1692 | 6 | 6 | 8 | 6 | 10 | 11 | 12 | 20 | 6 | 2 |
| Δ2520 | 6 | 6 | 8 | 9 | 9 | 11 | 14 | 25 | 6 | 1 |
| typA::Tn | 6 | 6 | 9 | 15 | 11 | 10 | 15 | 28 | 6 | 1.0 |
| ΔuspA | 8 | 6 | 9 | 6 | 9 | 9 | 12 | 22 | 6 | 2 |
| Δzur | 12.5 | 10 | 11 | 9 | 11.5 | 11.4 | 12 | 26 | 6 | 2.0 |
Numbers indicate strains harboring a deletion/interruption of the annotation number (ABUW_0000). Amp-sulb, ampicillin-sulbactam; Pip-tazo, piperacillin-tazobactam; Merop, meropenem; Amik, amikacin; Tobra, tobramycin; Levofl, levofloxacin; Col, colistin; Minocyc, minocycline; Gent, gentamicin; Tigecyc, tigecycline.
Unless indicated otherwise, sensitivity is represented as the zone of growth inhibition (in millimeters) surrounding a filter disc impregnated with the antibiotic indicated.
Bold values are statistically significant different from those of wild-type AB5075.
Tigecycline sensitivity is reported as the MIC (in micrograms per milliliter) determined by E test.
FIG 7 .
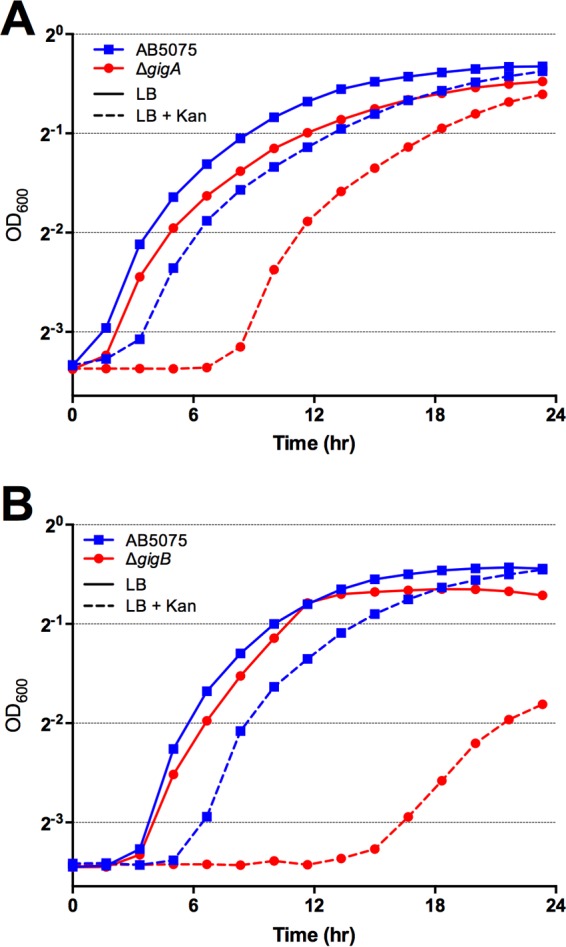
Genes required for growth in Galleria are also required for growth in subinhibitory concentrations of antibacterials. The strains indicated were grown for 24 h at 37°C in LB with or without 625 µg/ml kanamycin (Kan). Growth was measured by determining the OD600 every 10 min in a Tecan 96-well plate reader.
DISCUSSION
A. baumannii is a serious health risk because of its rapid acquisition of antimicrobial resistance and the inherent ability of the species to survive in the clinical environment by resisting decontamination practices employed in health care facilities (56), thus providing the potential for spread to susceptible patients. Despite the increasing prevalence of MDR isolates, however, the molecular mechanisms underlying the pathogenesis of A. baumannii remain poorly defined. In this work, we confirmed many known virulence genes and also identified novel genes that contribute to A. baumannii virulence as assessed in a well-established invertebrate model.
Recently, Geisinger and Isberg published the results of experiments investigating the capsule synthetic genes of A. baumannii (18). Other work indicates that the capsule locus is quite variable across the sequenced genomes of A. baumannii isolates, suggesting that this important surface structure is under positive selection by environmental stresses and perhaps immune recognition (57), and as our data suggest, the presence of capsule is protective during antibiotic stress (Table 2). Indeed, there is a consensus that production of exopolysaccharide capsule is important for A. baumannii virulence in all models of infection, including the Galleria model used for the studies described here (18, 40). In accordance with this, we confirmed the requirement for capsule synthesis in AB5075 by showing that deletion of ptk, a gene required for capsule export, impaired growth within the larval cavity and attenuated larval killing (see Fig. S2 in the supplemental material). Geisinger and colleagues reported difficulty in generating ptk mutants of strain ATCC 17978 and found that strains with a ptk mutation had acquired suppressor mutations in the bfmRS locus (18). We readily obtained ptk null mutants of the AB5075 strain, and sequencing of the bfmRS locus in four independent ptk deletion strains did not reveal any differences from the wild-type bfmRS sequence (data not shown), although we cannot exclude the possibility that these strains have acquired other suppressor mutations. Additional observations from our lab and others demonstrate that A. baumannii strains harboring deletions of the BfmS sensor kinase generate excess polysaccharides (data not shown; 18), suggesting that the BfmRS TCS may modulate capsule production. On the basis of this observation, we speculate that BfmS may act as a phosphatase for BfmR, and loss of BfmS leads to increased BfmR-P, which in turn activates capsule production.
As capsular polysaccharides are commonly associated with protection from host defenses and antibiotics and because G. mellonella produces antimicrobial peptides in response to bacterial infection, we originally hypothesized that the increased capsule production of the bfmS mutant may confer protection from the insect defenses. On the contrary, we observed that bfmS null strains were defective in growth/persistence in G. mellonella (Fig. 3). For the cognate response regulator bfmR, we were unsuccessful in obtaining a deletion strain in the AB5075 background, and strains harboring a transposon insertion in this gene appear to have a growth defect. In light of these observations; we propose that the bfmRS TCS has a broader role beyond the mediation of biofilm formation.
Wang et al. recently published the results of an investigation of the genetic requirements for A. baumannii persistence in a murine pneumonia model (11). Despite the use of different strains (Wang and colleagues used strain ATCC 17978) and model systems, the two studies did identify many of the same genes. For instance, both experiments identified known virulence factors for A. baumannii, including zinc and iron acquisition systems, capsule and LPS biosynthesis genes, amino acid metabolism and acquisition genes, and the bfmRS TCS (11). The correlation between the present study and that of Wang et al., which used a mammalian infection model, demonstrates the utility of the G. mellonella system for studying A. baumannii infection. Beyond these similarities, however, the work described here, as discussed below, identified additional virulence genes that were not previously identified.
When we looked more closely at the genes involved in amino acid biosynthesis, we observed that many genes involved in the cysteine metabolism/sulfur assimilation pathway were required for A. baumannii to grow in Galleria. This pathway is well conserved across bacterial species (58), and aside from generating cysteine for protein synthesis, the sulfur assimilation pathway appears to prevent damage due to oxidative stress, possibly by maintaining a reducing environment inside the cell (34). As oxidative stress is a widely conserved pathogen restriction factor, we hypothesize that this pathway may be involved in the oxidative stress resistance of A. baumannii. Interestingly, when we interrogated the data of Wang and colleagues, we observed that several genes in the cysteine/sulfur assimilation were also required for persistence in the murine pneumonia model, even though the authors did not comment on these genes (11). The correlation between the two studies further emphasizes the importance of the cysteine/sulfur pathway for A. baumannii virulence. As the sulfur assimilation pathway has recently attracted renewed interest as a target for novel antimicrobial therapeutics (reviewed in reference 30), it will be important to investigate whether pharmacological perturbation of this pathway represents a viable strategy to treat A. baumannii infection.
Our experiment also identified many genes involved in transcriptional regulation (n = 32), which is significant because one of the key differences distinguishing A. baumannii from the closely related but nonpathogenic species A. baylyi is the presence of transcription regulatory genes. Indeed, Adams and colleagues recently found that one major difference between A. baylyi and A. baumannii is the presence of substantially more transcriptional regulators in A. baumannii (~10% of the pan-A. baumannii genes) (59). We conclude that these transcriptional regulators may control genes that allow A. baumannii to be a human pathogen. Of the 32 regulator genes identified in this study, half do not have an ortholog in A. baylyi, supporting the conclusions of the aforementioned study. Future studies to define genes controlled by the transcriptional regulators identified in our screen will likely highlight the key regulatory networks involved in A. baumannii sensing and responding to the host environment.
Interestingly, several of the genes required for growth in Galleria identified in our screen also show a growth defect in subinhibitory concentrations of various antibiotics (Fig. 7; Table 2). The functions of genes required for surviving both within Galleria and during environmental and/or antibiotic stress encompass signal transduction, transcriptional regulation, and genes predicted to play a role in stress survival. We thus conclude that the stresses imposed upon A. baumannii during infection overlap those faced by bacteria during exposure to antibiotics and other environmental stressors. Antibiotics mediate cell killing through a variety of mechanisms, and we have shown that strains harboring deletions of specific transcriptional regulators display increased antibiotic sensitivity; therefore, we infer that these regulators may also control genes required for detoxification of stress or for alteration of cell/envelope permeability—a property that could impact sensitivity to both antimicrobial peptides and antibiotics. We further propose that selection for increased survival of environmental stresses, such as clinical disinfectant strategies, antimicrobial compounds, etc., inadvertently selects for strains displaying a hypervirulence phenotype and may, in fact, select for MDR in clinical settings. This hypothesis is supported by work by Geisinger and Isberg in which they observe increased virulence after exposure to sublethal levels of antibiotics (18) and Roux et al., who recently reported loss of virulence in antibiotic-sensitive bacterial mutants (19). Future experiments will be aimed at identifying genes controlled by the transcriptional regulators described here.
MATERIALS AND METHODS
Strains and growth conditions.
For the strains, plasmids, and oligonucleotide primers used in this study, see Tables S2 and S3 in the supplemental material. E. coli and A. baumannii strains were cultured in LB medium at 37°C. When required, the antibiotics added for selection were tetracycline (10 and 5 µg/ml), hygromycin (500 and 250 µg/ml), and apramycin (50 and 25 µg/ml) for growth on solid agar and in liquid medium, respectively.
G. mellonella infection.
G. mellonella larvae were purchased from Knutson’s Live Bait (Brooklyn, MI). Infection of G. mellonella was performed with overnight bacterial cultures as previously described (13). For larval survival experiments, infected larvae were stored in the dark at 37°C and monitored daily for survival for 6 days. Significance of survival differences was assessed with the log rank test. For bacterial recovery experiments, larvae were infected with ~106 CFU of the strains indicated. The CFU recovery experiments were repeated at least three times, and data from a representative experiment are shown. Larvae (n = 2 per time point) were homogenized with a D1000 Benchtop homogenizer in 2 ml of phosphate-buffered saline (PBS) immediately following infection (t = 0) and after 4 h of incubation at 37°C (t = 4). Homogenates were diluted and plated onto LB agar with 5 µg/ml chloramphenicol to eliminate the growth of the normal flora of G. mellonella without impacting the growth of AB5075 and its derivatives. Bacterial growth is represented as the ratio of the number of CFU per larva at t = 4 to the number of CFU at t = 0. To assess bacterial growth in hemolymph, the bacterial strains indicated (~104 CFU) were mixed with freshly collected hemolymph. Larvae were lanced, and hemolymph was collected from several larvae and pooled prior to bacterial inoculation. Bacterium-hemolymph mixtures were incubated at 37°C, and bacteria were enumerated at the times indicated. Hemolymph experiments were repeated three times, and the results of a single experiment are shown.
Transposon mutant library and TnSeq experiment.
The A. baumannii AB5075 transposon mutant library was prepared as previously described (8, 12). For the TnSeq experiment, aliquots of the transposon library (stored at −80°C) were thawed on ice for 30 min and diluted and ~106 CFU were inoculated either into 5 ml of LB or into G. mellonella larvae. The LB and G. mellonella pools were grown at 37°C for 4 h, at which time larvae were homogenized as described above and aliquots of the LB cultures or larval homogenates were plated onto LB agar with tetracycline (10 µg/ml) to eliminate the normal bacterial flora of G. mellonella. The plates were incubated at 37°C for 4 h. Bacteria were then washed from the plates with 1.5 ml of sterile PBS. Genomic DNA was prepared with the Qiagen DNeasy kit according to the manufacturer’s recommendations and processed and sequenced as previously described (8). Following the mapping of reads to the AB5075 genome, genes with fewer than five unique insertions mapped in the LB sample were omitted from further analysis. To identify underrepresented genes following growth in G. mellonella, the number of reads per gene in the G. mellonella sample was divided by the number of reads per gene in the LB sample to generate an RR. An RR of 0 is reported for cases in which no reads mapped to a given gene in the G. mellonella sample. A hit was defined as a gene with an RR of <0.1, i.e., a 1-log reduction in the number of reads following passage through G. mellonella. For the full TnSeq data set, see Data Set S1 in the supplemental material.
Generation of mutant strains and mutant strain complementation.
Gene deletions were performed with an allelic exchange plasmid (pMJG42) harboring the sacB gene for counterselection via growth on medium containing sucrose as previously described (60). Sucrose-resistant colonies were subjected to colony PCR to identify clones that had lost the wild-type gene, and deletions were confirmed by sequencing. For complementation at the Tn7 site, DNA containing the entire open reading frame of the deleted gene, including the native promoter region of the targeted gene, was amplified by PCR, cloned into pMJG111, and introduced into AB5075 as previously described (13, 60). For plasmid-based complementation of the gigB mutant strain, the gigB region was amplified and cloned into pMJG120. For the oligonucleotides used for Tn7- and pMJG120-based complementation, see Table S3 in the supplemental material.
Antibiotic susceptibility testing.
Antibiotic sensitivity testing was performed according to CLSI guidelines (55). Disc diffusion assay (DDA) and E test experiments were performed five times. Data are reported as the mean zone of inhibition (DDA)/MIC (E test). Growth curves were performed with a Tecan M200 microtiter plate reader. Overnight cultures of the strains indicated were diluted 1:100 into fresh LB medium with or without kanamycin, and growth was monitored for 24 h. The growth curves were repeated three times, and results are shown as the mean optical density at 600 nm (OD600) obtained from triplicate wells of a representative experiment.
SUPPLEMENTAL MATERIAL
Full TnSeq data set. Download
Growth of AB5075 Tn mutant pools in LB and G. mellonella. The graph depicts CFU recovery following growth of the Tn mutant pool over time in LB (black) and G. mellonella larvae (gray). Download
Capsule production is required for virulence in G. mellonella. (A) TnSeq data for the capsule biosynthetic operon depicted as the numbers of Tn reads per gene mapped to the capsule operon from the LB growth sample (black) and from the G. mellonella growth samples (gray). The gene RR (Gm/LB) is shown above each bar. (B) G. mellonella larvae were infected with either wild-type or ptk-deficient bacteria as indicated. Survival was monitored for 6 days. (C) Growth of wild-type AB5075 carrying a hygromycin resistance cassette at the Tn7 locus (gray) or a ptk deletion strain harboring either a hygromycin resistance cassette at the Tn7 attachment site (white) or a wild-type copy of the ptk gene (gray) after injection into G. mellonella larvae. Growth is presented as described in the legend to Fig. 1. ***, P < 0.001; ****, P < 0.0001. Download
Genes required for growth in G. mellonella larvae.
Strains and plasmids used in this study.
Oligonucleotide primers used in this study.
ACKNOWLEDGMENTS
M.J.G. was supported by National Institutes of Health (NIH) grant T32 GM007183. H.A.S. was supported by the NIH (award AI115203) and by a NorthShore/UCM collaborative research award. L.A.G. was supported by grant U19AI107775 from the National Institute of Allergy and Infectious Diseases (NIAID). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. D.V.Z. is supported by grants from the MIDRP and DMRDP programs and is grateful for continued support. The findings and opinions expressed herein belong to the authors and do not necessarily reflect the official views of the WRAIR, the U.S. Army, or the Department of Defense.
We acknowledge Colin Manoil for technical advice and critical reading of the manuscript.
Footnotes
Citation Gebhardt MJ, Gallagher LA, Jacobson RK, Usacheva EA, Peterson LR, Zurawski DV, Shuman HA. 2015. Joint transcriptional control of virulence and resistance to antibiotic and environmental stress in Acinetobacter baumannii. mBio 6(6):e01660-15. doi:10.1128/mBio.01660-15.
REFERENCES
- 1.Visca P, Seifert H, Towner KJ. 2011. Acinetobacter infection—an emerging threat to human health. IUBMB Life 63:1048–1054. doi: 10.1002/iub.534. [DOI] [PubMed] [Google Scholar]
- 2.Spellberg B, Rex JH. 2013. The value of single-pathogen antibacterial agents. Nat Rev Drug Discov 12:963. doi: 10.1038/nrd3957-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Göttig S, Gruber TM, Higgins PG, Wachsmuth M, Seifert H, Kempf VAJ. 2014. Detection of pan drug-resistant Acinetobacter baumannii in Germany. J Antimicrob Chemother 69:2578–2579. doi: 10.1093/jac/dku170. [DOI] [PubMed] [Google Scholar]
- 4.Peleg AY, de Breij A, Adams MD, Cerqueira GM, Mocali S, Galardini M, Nibbering PH, Earl AM, Ward DV, Paterson DL, Seifert H, Dijkshoorn L. 2012. The success of Acinetobacter species; genetic, metabolic and virulence attributes. PLoS One 7:e46984. doi: 10.1371/journal.pone.0046984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antunes LCS, Visca P, Towner KJ. 2014. Acinetobacter baumannii: evolution of a global pathogen. Pathog Dis 71:292–301. doi: 10.1111/2049-632X.12125. [DOI] [PubMed] [Google Scholar]
- 6.Cox JS, Chen B, McNeil M, Jacobs WR Jr. 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402:79–83. doi: 10.1038/47042. [DOI] [PubMed] [Google Scholar]
- 7.Slauch JM, Camilli A. 2000. IVET and RIVET: use of gene fusions to identify bacterial virulence factors specifically induced in host tissues. Methods Enzymol 326:73–96. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher LA, Shendure J, Manoil C. 2011. Genome-scale identification of resistance functions in Pseudomonas aeruginosa using Tn-seq. mBio 2:e00315-00310. doi: 10.1128/mBio.00315-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Opijnen T, Bodi KL, Camilli A. 2009. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat Methods 6:767–772. doi: 10.1038/nmeth.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Opijnen T, Camilli A. 2013. Transposon insertion sequencing: a new tool for systems-level analysis of microorganisms. Nat Rev Microbiol 11:435–442. doi: 10.1038/nrmicro3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang N, Ozer EA, Mandel MJ, Hauser AR. 2014. Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. mBio 5:e01163-01114. doi: 10.1128/mBio.01163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher LA, Ramage E, Weiss EJ, Radey M, Hayden HS, Held KG, Huse HK, Zurawski DV, Brittnacher MJ, Manoil C. 2015. Resources for genetic and genomic analysis of emerging pathogen Acinetobacter baumannii. J Bacteriol 197:2027–2035. doi: 10.1128/JB.00131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs AC, Thompson MG, Black CC, Kessler JL, Clark LP, McQueary CN, Gancz HY, Corey BW, Moon JK, Si Y, Owen MT, Hallock JD, Kwak YI, Summers A, Li CZ, Rasko DA, Penwell WF, Honnold CL, Wise MC, Waterman PE, Lesho EP, Stewart RL, Actis LA, Palys TJ, Craft DW, Zurawski DV. 2014. AB5075, a highly virulent isolate of Acinetobacter baumannii, as a model strain for the evaluation of pathogenesis and antimicrobial treatments. mBio 5:e01076-14. doi: 10.1128/mBio.01076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zurawski DV, Thompson MG, McQueary CN, Matalka MN, Sahl JW, Craft DW, Rasko DA. 2012. Genome sequences of four divergent multidrug-resistant Acinetobacter baumannii strains isolated from patients with sepsis or osteomyelitis. J Bacteriol 194:1619–1620. doi: 10.1128/JB.06749-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC Jr., Mylonakis E. 2009. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother 53:2605–2609. doi: 10.1128/AAC.01533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boucher H, Talbot G, Bradley J, Edwards J, Gilbert D, Rice L, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 17.Wright MS, Haft DH, Harkins DM, Perez F, Hujer KM, Bajaksouzian S, Benard MF, Jacobs MR, Bonomo RA, Adams MD. 2014. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. mBio 5:e00963-13. doi: 10.1128/mBio.00963-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geisinger E, Isberg RR. 2015. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog 11:e1004691. doi: 10.1371/journal.ppat.1004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roux D, Danilchanka O, Guillard T, Cattoir V, Aschard H, Fu Y, Angoulvant F, Messika J, Ricard J-, Mekalanos JJ, Lory S, Pier GB, Skurnik D. 2015. Fitness cost of antibiotic susceptibility during bacterial infection. Sci Transl Med 7:297ra114. doi: 10.1126/scitranslmed.aab1621. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs BB, O’Brien E, El Khoury JB, Mylonakis E. 2010. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 1:475–482. doi: 10.4161/viru.1.6.12985. [DOI] [PubMed] [Google Scholar]
- 21.Kavanagh K, Reeves EP. 2004. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev 28:101–112. doi: 10.1016/j.femsre.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Jander G, Rahme LG, Ausubel FM. 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J Bacteriol 182:3843–3845. doi: 10.1128/JB.182.13.3843-3845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lionakis MS. 2011. Drosophila and Galleria insect model hosts: new tools for the study of fungal virulence, pharmacology and immunology. Virulence 2:521–527. doi: 10.4161/viru.2.6.18520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee K, Altincicek B, Hain T, Domann E, Vilcinskas A, Chakraborty T. 2010. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl Environ Microbiol 76:310–317. doi: 10.1128/AEM.01301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaddy JA, Arivett BA, McConnell MJ, Lopez-Rojas R, Pachon J, Actis LA. 2012. Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect Immun 80:1015–1024. doi: 10.1128/IAI.06279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimbler DL, Penwell WF, Gaddy JA, Menke SM, Tomaras AP, Connerly PL, Actis LA. 2009. Iron acquisition functions expressed by the human pathogen Acinetobacter baumannii. Biometals 22:23–32. doi: 10.1007/s10534-008-9202-3. [DOI] [PubMed] [Google Scholar]
- 28.Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, Chazin WJ, Caprioli RM, Skaar EP. 2012. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog 8:e1003068. doi: 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mortensen BL, Rathi S, Chazin WJ, Skaar EP. 2014. Acinetobacter baumannii response to host-mediated zinc limitation requires the transcriptional regulator Zur. J Bacteriol 196:2616–2626. doi: 10.1128/JB.01650-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campanini B, Pieroni M, Raboni S, Bettati S, Benoni R, Pecchini C, Costantino G, Mozzarelli A. 2015. Inhibitors of the sulfur assimilation pathway in bacterial pathogens as enhancers of antibiotic therapy. Curr Med Chem 22:187–213. doi: 10.2174/0929867321666141112122553. [DOI] [PubMed] [Google Scholar]
- 31.van der Ploeg J, Eichhorn E, Leisinger T. 2001. Sulfonate-sulfur metabolism and its regulation in Escherichia coli. Arch Microbiol 176:1–8. doi: 10.1007/s002030100298. [DOI] [PubMed] [Google Scholar]
- 32.Wyatt GR, Loughheed TC, Wyatt SS. 1956. The chemistry of insect hemolymph; organic components of the hemolymph of the silkworm, Bombyx mori, and two other species. J Gen Physiol 39:853–868. doi: 10.1085/jgp.39.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Álvarez R, Neumann G, Frávega J, Díaz F, Tejías C, Collao B, Fuentes JA, Paredes-Sabja D, Calderón IL, Gil F. 2015. CysB-dependent upregulation of the Salmonella typhimurium cysJIH operon in response to antimicrobial compounds that induce oxidative stress. Biochem Biophys Res Commun 458:46–51 doi: 10.1016/j.bbrc.2015.01.058. [DOI] [PubMed] [Google Scholar]
- 34.Turnbull AL, Surette MG. 2010. Cysteine biosynthesis, oxidative stress and antibiotic resistance in Salmonella typhimurium. Res Microbiol 161:643–650. doi: 10.1016/j.resmic.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Shatalin K, Shatalina E, Mironov A, Nudler E. 2011. H2S: a universal defense against antibiotics in bacteria. Science 334:986–990. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 36.Cerqueira GM, Kostoulias X, Khoo C, Aibinu I, Qu Y, Traven A, Peleg AY. 2014. A global virulence regulator in Acinetobacter baumannii and its control of the phenylacetic acid catabolic pathway. J Infect Dis 210:46–55. doi: 10.1093/infdis/jiu024. [DOI] [PubMed] [Google Scholar]
- 37.Russo T, MacDonald U, Beanan J, Olson R, MacDonald I, Sauberan S, Luke N, Schultz L, Umland T. 2009. Penicillin-binding protein 7/8 contributes to the survival of Acinetobacter baumannii in vitro and in vivo. J Infect Dis 199:513–521. doi: 10.1086/596317. [DOI] [PubMed] [Google Scholar]
- 38.May JM, Sherman DJ, Simpson BW, Ruiz N, Kahne D. 2015. Lipopolysaccharide transport to the cell surface: periplasmic transport and assembly into the outer membrane. Philos Trans R Soc Lond B Biol Sci 370:20150027. doi: 10.1098/rstb.2015.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bos MP, Tommassen J. 2011. The LptD chaperone LptE is not directly involved in lipopolysaccharide transport in Neisseria meningitidis. J Biol Chem 286:28688–28696. doi: 10.1074/jbc.M111.239673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russo TA, Luke NR, Beanan JM, Olson R, Sauberan SL, MacDonald U, Schultz LW, Umland TC, Campagnari AA. 2010. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun 78:3993–4000. doi: 10.1128/IAI.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan N, Hyršl P, Šimek V. 2006. Nitric oxide production by hemocytes of larva and pharate prepupa of Galleria mellonella in response to bacterial lipopolysaccharide: cytoprotective or cytotoxic? Comp Biochem Physiol C Toxicol Pharmacol 142:103–110. doi: 10.1016/j.cbpc.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Kim B, Richards SM, Gunn JS, Slauch JM. 2010. Protecting against antimicrobial effectors in the phagosome allows SodCII to contribute to virulence in Salmonella enterica serovar Typhimurium. J Bacteriol 192:2140–2149. doi: 10.1128/JB.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makinoshima H, Glickman MS. 2006. Site-2 proteases in prokaryotes: regulated intramembrane proteolysis expands to microbial pathogenesis. Microbes Infect 8:1882–1888. doi: 10.1016/j.micinf.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Micklinghoff JC, Schmidt M, Geffers R, Tegge W, Bange F. 2010. Analysis of expression and regulatory functions of the ribosome-binding protein TypA in Mycobacterium tuberculosis under stress conditions. Arch Microbiol 192:499–504. doi: 10.1007/s00203-010-0571-y. [DOI] [PubMed] [Google Scholar]
- 45.Neidig A, Yeung AT, Rosay T, Tettmann B, Strempel N, Rueger M, Lesouhaitier O, Overhage J. 2013. TypA is involved in virulence, antimicrobial resistance and biofilm formation in Pseudomonas aeruginosa. BMC Microbiol 13:77. doi: 10.1186/1471-2180-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kvint K, Nachin L, Diez A, Nyström T. 2003. The bacterial universal stress protein: function and regulation. Curr Opin Microbiol 6:140–145. doi: 10.1016/S1369-5274(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 47.Yoon E, Nait Chabane Y, Goussard S, Snesrud E, Courvalin P, Dé E, Grillot-Courvalin C. 2015. Contribution of resistance-nodulation-cell division efflux systems to antibiotic resistance and biofilm formation in Acinetobacter baumannii. mBio 6:e00309-15. doi: 10.1128/mBio.00309-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Damier-Piolle L, Magnet S, Bremont S, Lambert T, Courvalin P, Magnet S. 2008. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob Agents Chemother 52:557–562. doi: 10.1128/AAC.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassan KA, Liu Q, Henderson PJF, Paulsen IT. 2015. Homologs of the Acinetobacter baumannii AceI transporter represent a new family of bacterial multidrug efflux systems. mBio 6:e01982-14. doi: 10.1128/mBio.01982-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liou M, Soo P, Ling S, Kuo H, Tang CY, Chang K. 2014. The sensor kinase BfmS mediates virulence in Acinetobacter baumannii. J Microbiol Immunol Infect 47:275–281. doi: 10.1016/j.jmii.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Tomaras AP, Flagler MJ, Dorsey CW, Gaddy JA, Actis LA. 2008. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology 154:3398–3409. doi: 10.1099/mic.0.2008/019471-0. [DOI] [PubMed] [Google Scholar]
- 52.Ratajczak A, Geissdorfer W, Hillen W. 1998. Expression of alkane hydroxylase from Acinetobacter sp. strain ADP1 is induced by a broad range of n-alkanes and requires the transcriptional activator AlkR. J Bacteriol 180:5822–5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobayashi K, Fujikawa M, Kozawa T. 2014. Oxidative stress sensing by the iron-sulfur cluster in the transcription factor, SoxR. J Inorg Biochem 133:87–91. doi: 10.1016/j.jinorgbio.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Rumbo-Feal S, Gómez MJ, Gayoso C, Álvarez-Fraga L, Cabral MP, Aransay AM, Rodríguez-Ezpeleta N, Fullaondo A, Valle J, Tomás M, Bou G, Poza M. 2013. Whole transcriptome analysis of Acinetobacter baumannii Assessed by RNA-sequencing reveals different mRNA expression profiles in biofilm compared to planktonic cells. PLoS One 8:e72968. doi: 10.1371/journal.pone.0072968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.CLSI 2014. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational Supplement. CLSI document M100-S24 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 56.Kawamura-Sato K, Wachino J-, Kondo T, Ito H, Arakawa Y. 2010. Correlation between reduced susceptibility to disinfectants and multidrug resistance among clinical isolates of Acinetobacter species. J Antimicrob Chemother 65:1975–1983. doi: 10.1093/jac/dkq227. [DOI] [PubMed] [Google Scholar]
- 57.Scott NE, Kinsella RL, Edwards AVG, Larsen MR, Dutta S, Saba J, Foster LJ, Feldman MF. 2014. Diversity within the O-linked protein glycosylation systems of Acinetobacter species. Mol Cell Proteomics 13:2354–2370. doi: 10.1074/mcp.M114.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kessler D. 2006. Enzymatic activation of sulfur for incorporation into biomolecules in prokaryotes. FEMS Microbiol Rev 30:825–840. doi: 10.1111/j.1574-6976.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 59.Adams MD, Goglin K, Molyneaux N, Hujer KM, Lavender H, Jamison JJ, MacDonald IJ, Martin KM, Russo T, Campagnari AA, Hujer AM, Bonomo RA, Gill SR. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J Bacteriol 190:8053–8064. doi: 10.1128/JB.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacobs AC, Thompson MG, Gebhardt M, Corey BW, Yildirim S, Shuman HA, Zurawski DV. 2014. Genetic manipulation of Acinetobacter baumannii. Curr Protoc Microbiol 35:6G.2.1–6G.2.11. doi: 10.1002/9780471729259.mc06g02s35. [DOI] [PubMed] [Google Scholar]
- 61.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. 2009. Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645 doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full TnSeq data set. Download
Growth of AB5075 Tn mutant pools in LB and G. mellonella. The graph depicts CFU recovery following growth of the Tn mutant pool over time in LB (black) and G. mellonella larvae (gray). Download
Capsule production is required for virulence in G. mellonella. (A) TnSeq data for the capsule biosynthetic operon depicted as the numbers of Tn reads per gene mapped to the capsule operon from the LB growth sample (black) and from the G. mellonella growth samples (gray). The gene RR (Gm/LB) is shown above each bar. (B) G. mellonella larvae were infected with either wild-type or ptk-deficient bacteria as indicated. Survival was monitored for 6 days. (C) Growth of wild-type AB5075 carrying a hygromycin resistance cassette at the Tn7 locus (gray) or a ptk deletion strain harboring either a hygromycin resistance cassette at the Tn7 attachment site (white) or a wild-type copy of the ptk gene (gray) after injection into G. mellonella larvae. Growth is presented as described in the legend to Fig. 1. ***, P < 0.001; ****, P < 0.0001. Download
Genes required for growth in G. mellonella larvae.
Strains and plasmids used in this study.
Oligonucleotide primers used in this study.