Abstract
Prolonged human interactions and artificial selection have influenced the genotypic and phenotypic diversity among dog breeds. Because humans and dogs occupy diverse habitats, ecological contexts have likely contributed to breed-specific positive selection. Prior to the advent of modern dog-feeding practices, there was likely substantial variation in dietary landscapes among disparate dog breeds. As such, we investigated one type of genetic variant, copy number variation, in three metabolic genes: glucokinase regulatory protein (GCKR), phytanol-CoA 2-hydroxylase (PHYH), and pancreatic α-amylase 2B (AMY2B). These genes code for proteins that are responsible for metabolizing dietary products that originate from distinctly different food types: sugar, meat, and starch, respectively. After surveying copy number variation among dogs with diverse dietary histories, we found no correlation between diet and positive selection in either GCKR or PHYH. Although it has been previously demonstrated that dogs experienced a copy number increase in AMY2B relative to wolves during or after the dog domestication process, we demonstrate that positive selection continued to act on amylase copy number in dog breeds that consumed starch-rich diets in time periods after domestication. Furthermore, we found that introgression with wolves is not responsible for deterioration of positive selection on AMY2B among diverse dog breeds. Together, this supports the hypothesis that the amylase copy number expansion is found universally in dogs.
Introduction
The remarkable phenotypic diversity amongst dog breeds has been affected in part by intensive and prolonged human-facilitated artificial selection since the initial period of dog domestication [1]. Following domestication, human influence has exerted a selection pressure, which, alongside natural selection and stochastic genetic processes such as genetic drift [2], shaped dog biology. For example, metabolic adaptations to new diets arose in dog breeds spanning geographic and climatic ranges that mirror the cohabitation patterns of humans [3]. In this context, selective pressures directly and indirectly imposed by humans likely impacted the evolution of canine biology by leading to the emergence and persistence of positively-selected traits over the course of human and dog coevolution. On the other hand, stochastic evolutionary forces such as drift and bottlenecks may have changed the trajectory of positive selection in canines during human and dog co-migration.
Though the exact timespan and location of initial dog-human cohabitation and domestication remains unclear, humans and dogs have lived amongst one another for thousands of years [4–6]. Consensus from fossil and genetic evidence places the lower bound date of domestication around 14,000 BP [7–10]. While most genetic research supports a single center of domestication [7,9,11,12], the location of this center is contentiously debated.
Although the driving forces of dog domestication by humans remains unclear, dogs have nonetheless evolved suites of dietary and metabolic adaptations that have been argued to reflect their direct interaction with humans. It has been suggested that humans provided food for local dogs both directly and indirectly throughout dog-human coevolution [13]. For example, historically, it was believed that dogs were fed directly by their caretakers from human provisions [14]. However, more recently it has been argued that initially humans may have indirectly fed dogs by creating food waste from which wild canids scavenged [13,15]. Regardless of intention, this (direct or indirect) provisioning may have acted as a defining selective agent in the dog domestication process [13,15], with important impacts on canine metabolism.
Over time, and via cohabitation, the canine diet has been transformed from the carnivorous diet of its ancestor, the wolf, to a diet more closely matching that of omnivorous humans [13]. This transformation increased variation in the domesticated dog’s diet, potentiating impact on numerous biological pathways. This increase in variation was extreme in some cases: isotopic analysis has shown that by 7000 BC in northern China, 65–90% of domesticated dogs’ diets were comprised of millet alone [5]. However, the onset and sources of dietary variation were not universal—in 3500–2000 BC, dogs living among Korean shell midden cultures received the majority of their calories from marine mammals and other fish [16]. Because humans populated a variety of habitats with different dietary staples, dog breeds from different places also consumed diets composed of unique combinations of food items. For many breeds, dietary changes resulted in increases in novel food constituents that may have required new, better, or more digestive mechanisms, thereby exerting differential selective forces on dogs living among different groups of humans. For example, starch digestion presented a new dietary challenge to which the dog likely adapted through alteration of three key genes in the starch digestion pathway [15]. Other novel dietary changes for certain breeds likely include high intake of marine mammals and fish [17,18].
Selection on variability in both single-base pair and copy number variants may underlie key metabolic changes in the domesticated dog. Interestingly, in instances of prolonged dog-human cohabitation, there is evidence of parallel evolution in metabolic genes. For example, compared to native Chinese dogs, Tibetan mastiffs have 33 SNPs in 11 genes that display signatures of positive selection, the majority of which cluster in 9 genes related to high-altitude adaptations [19]. Likewise, Tibetans have experienced selective sweeps for haplotypes in genes involved in high-altitude adaptations some of which are likely the introduced through interbreeding with archaic humans [20]. More pertinently, amylase genes responsible for digesting starch have undergone copy number duplications in both dogs and humans [15,21]. These duplications translate to a functional increase in the ability to digest starches into sugar [21–23].
The goal of this study was to investigate how potential selection pressures created by exposure to different diets affected copy number variation (CNV) in six dog breeds from diverse dietary backgrounds: Pekingese, Shar Pei, Shiba Inu, Akita, Siberian Husky, and Alaskan Malamute. The Pekingese and Shar Pei likely consumed high-starch diets [5,24–26], while the Akita and Shiba Inu (Japanese dogs), Siberian Husky, and Alaskan Malamute likely consumed low-starch, seafood-rich diets [27–30]. We specifically focused on three metabolic and dietary genes: glucokinase regulator (GCKR), which plays a key role in maintaining blood glucose homeostasis; phytanol CoA 2-hydroxylase (PHYH), which codes for the protein responsible for the first step of phytanic acid digestion [31] often found in ruminant meat and seafood [32]; and pancreatic α-amylase 2B (AMY2B), which, as discussed above, is involved in the starch digestion.
By further investigating copy number variation in the AMY2B gene of dog breeds with both starch-rich and starch-poor diets, we were able to explore the relationship between diet and copy number post-domestication when diet likely depended greatly on location and human interactions. We found evidence that positive selection continued to affect AMY2B CNV in dog breeds that consumed starch-rich diets, but that genetic drift affected AMY2B CNV more in dogs that began consuming starch-poor diets. The data presented in our study support early positive selection on increased AMY2B CNV across all breeds of domesticated dogs.
Results
Diet does not predict GCKR CNV or PHYH CNV
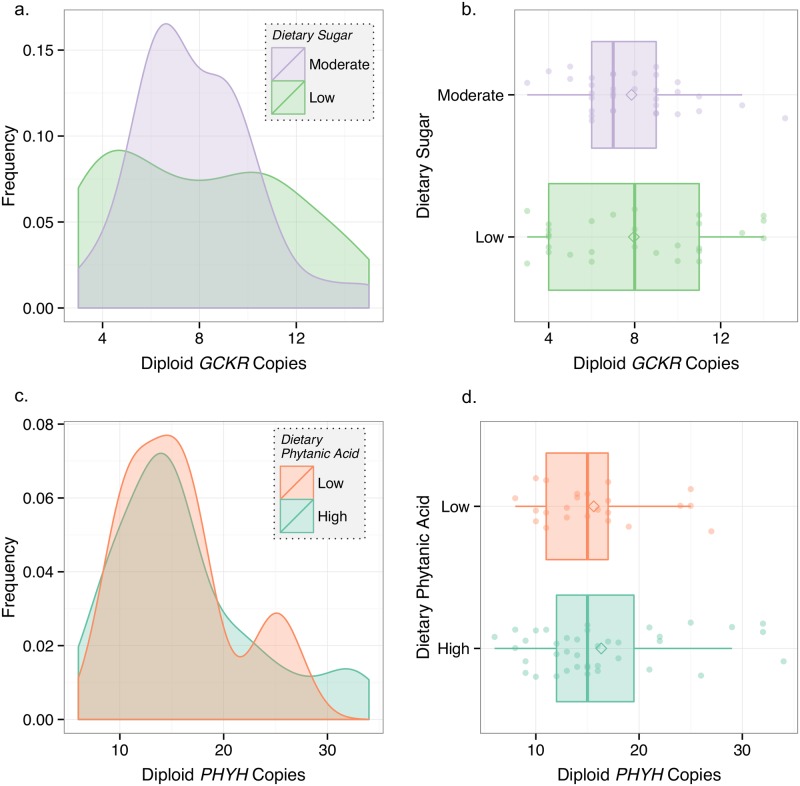
To investigate the impact of diet on copy number variation in GCKR, PHYH, and AMY2B, we determined a crude dietary composition by probing archaeological and ethnographic records (Methods). We also determined the copy number of each individual in each gene using digital droplet PCR (Methods). We found that sugar intake did not predict GCKR copy number across breeds. Specifically, dogs with moderate sugar intake (Shar Pei, Pekingese, and Japanese dogs; n = 34) had a mean CNV of 8.1±3.6, while dogs with low sugar intake (Siberian Husky and Alaskan Malamute; n = 28) had a mean CNV of 7.8±2.5 (Robust rank order test: p = .98; Fligner-Killeen test: p = .01) (Fig 1). Although our proxies indicate that the Alaskan Malamute and Siberian Husky consumed the least sugar of the six dog breeds, the averages of these dog breeds fell at disparate ends of the breed-wide distribution.
Fig 1. Diet and GCKR and PHYH copy number variation.
a) Density plot of ddPCR diploid GCKR copy number for dogs that traditionally consumed high sugar diets and low sugar diets. Density reflects frequency with which a given diploid copy number appears in each population. b) Tukey boxplot of diploid GCKR copy number for dogs that traditionally consumed high sugar diets and low sugar diets. c) Density plot of ddPCR diploid PHYH copy number for dogs that traditionally consumed high phytanic acid diets and low phytanic acid diets. d) Tukey boxplot of diploid PHYH copy number for dogs that traditionally consumed high phytanic acid diets and low phytanic acid diets.
Because of its role in regulating blood glucose homeostasis, it has previously been suggested that GCKR copy number may play a role in diabetes incidence in some dog breeds [33]. To investigate this further, diploid GCKR CNV was compared to diabetes risk for breeds for which this information was available (Pekingese, Siberian Husky, and Alaskan Malamute). Two breeds, the Siberian Husky and the Pekingese, both had duplications relative to the reference sequence CanFam2. However, the diabetes risk for these two breeds was not the same, with Pekingese having lower risk and the Siberian Husky having higher risk. In addition, the Alaskan Malamute, which experienced both gains and losses relative to CanFam2, displayed no evidence of increased risk for diabetes (S1 Table).
We next investigated the relationship between our proxies of dietary intake of phytanic acid and PHYH copy number, and similarly found no relationship; intake did not predict PHYH copy number. Specifically, the mean CNV of dogs that consumed high phytanic acid diets (Alaskan Malamute, Siberian Husky, and Japanese dogs; n = 39), 16.2±7.1 was not significantly different from that of dogs that consumed low phytanic acid diets (Pekingese, and Shar Pei; n = 23) which had a mean CNV of 15.5±5.4 (Robust rank order test: p = .92; Fligner-Killeen test: p = .48) (Fig 1). Though these results preliminarily suggest that dietary intake does not correlate with copy number variation in these genes, it is important to note that ddPCR has limited power to accurately predict copy number at high numbers of duplications (Discussion).
Starch intake predicts diploid AMY2B CNV in some dog breeds
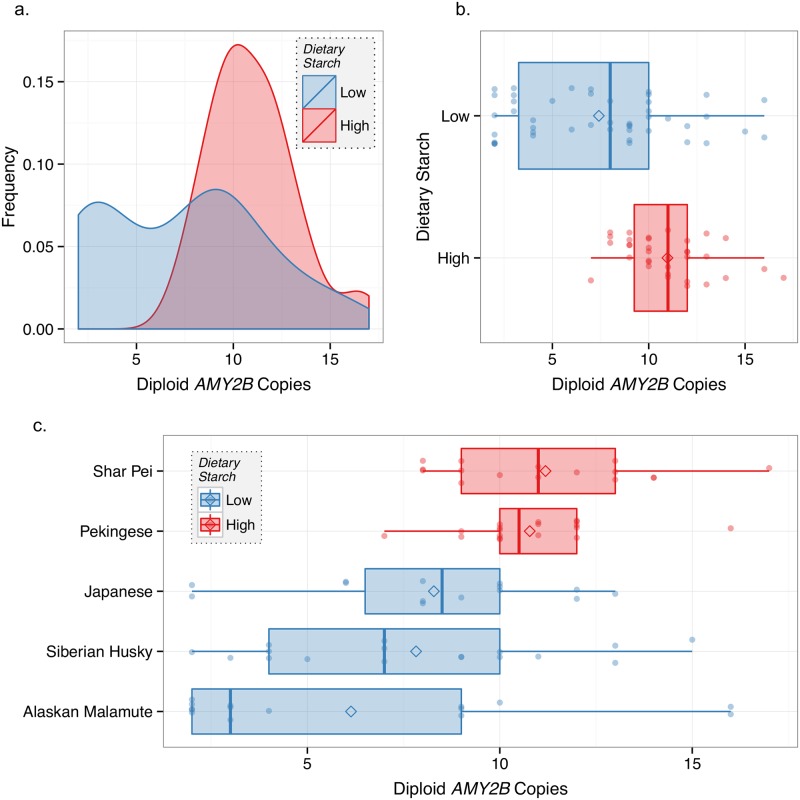
Given the history of studies on the role of amylase CNV and starch digestion in humans [21] and dogs [15,22], we next examined AMY2B copy number in our expanded dataset, which includes dog breeds representing localities with historically low levels of dietary starch intake (Methods). Consistent with studies with more limited datasets, we found that AMY2B CNV did vary with dietary starch intake. Dogs with high-starch diets (Pekingese and Shar Pei; n = 34) had a statistically significant higher mean CNV of 10.9±2.2, compared to dogs with low-starch diets (Siberian Husky, Alaskan Malamute, and Japanese dogs; n = 46) with a mean CNV of 7.4±4.2 (Robust rank order test: p < .0001; Fligner-Killeen test: p = .0004) (Fig 2, S1 Appendix). Importantly, we identified the same significant relationship regardless if we used aggregated data (n = 80), and/or collected samples (n = 62) (Robust rank order test: p = .0002; Fligner-Killeen test: p = .001). The proportion of dogs from combined high starch consuming breeds with at least 10 AMY2B copies (74%) was more than two times greater than that for the low starch consuming dogs (33%).
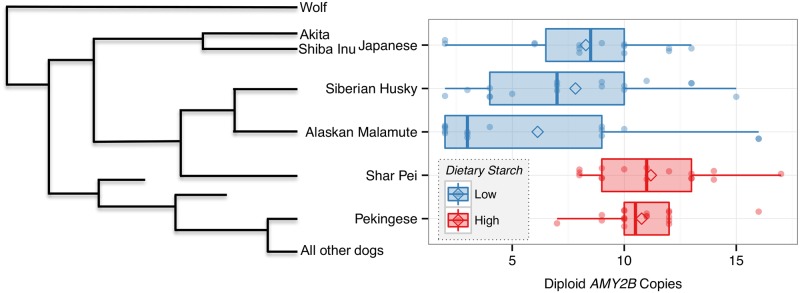
Fig 2. Diet and AMY2B copy number variation.
a) Density plot of ddPCR diploid AMY2B copy number for dogs that traditionally consumed high-starch diets and low-starch diets. Density reflects frequency with which a given diploid copy number appears in each population. b) Tukey boxplot of diploid AMY2B copy number for dogs that traditionally consumed high-starch diets and low-starch diets. c) Tukey boxplot of diploid AMY2B copy number for specific dog breeds that traditionally consumed high-starch diets and low-starch diets.
We next compared AMY2B CNV within breeds and found that AMY2B copy number also differed between high and low starch consuming dogs (Table 1). For example, the Shar Pei (n = 16) had a mean CNV of 11.1±2.7, the Pekingese (n = 18) of 10.7±1.7, the Japanese dogs (n = 14) of 8.3±3.4, the Siberian Husky (n = 17) of 7.8±3.9, and the Alaskan Malamute (n = 15) of 6.1±5.0 (Fig 2). Importantly, dog breeds that consumed high-starch diets were also more similar to each other both in mean copy number and in variation of copy number than to dog breeds that consumed low-starch diets. Breeds that consumed low-starch diets had markedly higher variation in copy number and lower average copy number than breeds that consumed high-starch diets. This could be evidence of positive selection, however we cannot rule out the possibility that stochastic forces led to this pattern from ddPCR data alone.
Table 1. Average diploid AMY2B copy number and number of samples taken for specific breeds.
| Breed | Samples | Starch Intake | Avg Diploid AMY2B Copy Number |
|---|---|---|---|
| Chinese Shar Pei | 16 | High | 11.1±2.7 |
| Pekingese | 18 | High | 10.7±1.7 |
| Japanese Dogs | 14 | Low | 8.3±3.4 |
| Siberian Husky | 17 | Low | 7.8±3.9 |
| Alaskan Malamute | 15 | Low | 6.1±5.0 |
Diploid AMY2B CNV differs from other copy number variable regions
Although our ddPCR data preliminarily suggest and build upon previous suppositions relating to positive selection acting on AMY2B copy number, such a selective signature cannot be detected directly from diploid copy number alone. Because traditional methods of identifying positive selection cannot be employed for analysis of diploid copy number, we acquired array-based comparative genomic hybridization data (aCGH) for the Alaskan Malamute and Shar Pei in order to compare patterns in AMY2B CNV to genome-wide patterns in CNV. This comparison allowed us to investigate if positive selection acted on AMY2B copy number differently in dog breeds with disparate starch intake. Previously, aCGH data from copy number variable sites was compiled for four Alaskan Malamutes and five Shar Pei dogs [34] (GEO http://www.ncbi.nlm.nih.gov/geo/ accession number GSE26170). This distribution was used to compare the extent of breed-level differentiation at the AMY2B locus to other genomic loci for the Alaskan Malamute and the Shar Pei.
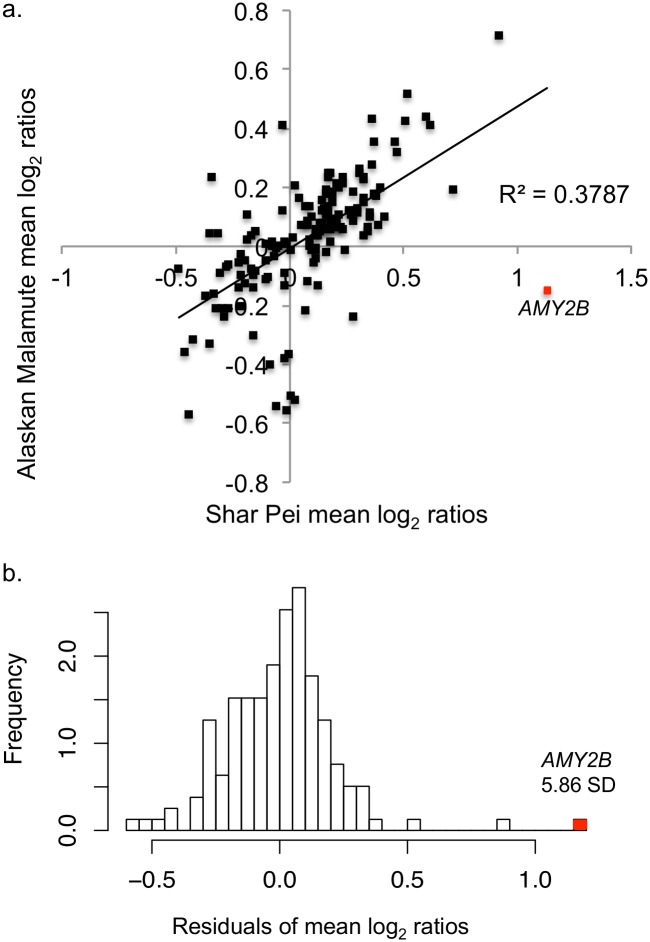
To determine if AMY2B CNV mean log2 ratio (Methods) deviated substantially from the null distribution of other copy number variable sites in the genome, we analyzed residuals of AMY2B to a linear regression line fit to the aCGH data. We found that AMY2B fell 5.8 standard deviations away from the mean, indicating that in one or both breeds, AMY2B has experienced unusual evolutionary pressures dissimilar to those experienced at other copy number variable sites throughout the genome (Fig 3). However, because aCGH data was only available for nine dogs, this analysis was supplemented by comparisons generated by 55 samples from 10 other breeds: Border Collie, Beagle, Brittany Spaniel, Boxer, Dachshund, Greyhound, German Shepard, Jack Russell Terrier, Labrador Retriever, and Standard Poodle (S2 Appendix). When compared to other null distributions of copy number variation, AMY2B fell 15 standard deviations away from the mean.
Fig 3. Results from analysis of aCGH data.
a) Relationship between Shar Pei and Alaskan Malamute mean log2 ratios for 157 copy number variable sites in the dog genome. AMY2B mean log2 ratio is plotted in red, and deviates significantly from the null distribution. b) Histogram of residuals from linear regression performed on mean log2 ratios from aCGH data in the Alaskan Malamute and Shar Pei. Value of residuals appears on the x-axis while density, or proportion of a specific residual occurring, falls on the y-axis. The residual for mean log2 ratio in AMY2B is indicated in red and falls 5.86 standard deviations away from the mean (0±.2).
When these results are supplemented by data obtained by ddPCR, these patterns support that the Shar Pei continued to experience positive selection at AMY2B after the initial duplication event, while AMY2B CNV in the Alaskan Malamute may have been subject to genetic drift or weak negative selection.
Starch intake predicts species-wide differences in diploid AMY2B CNV
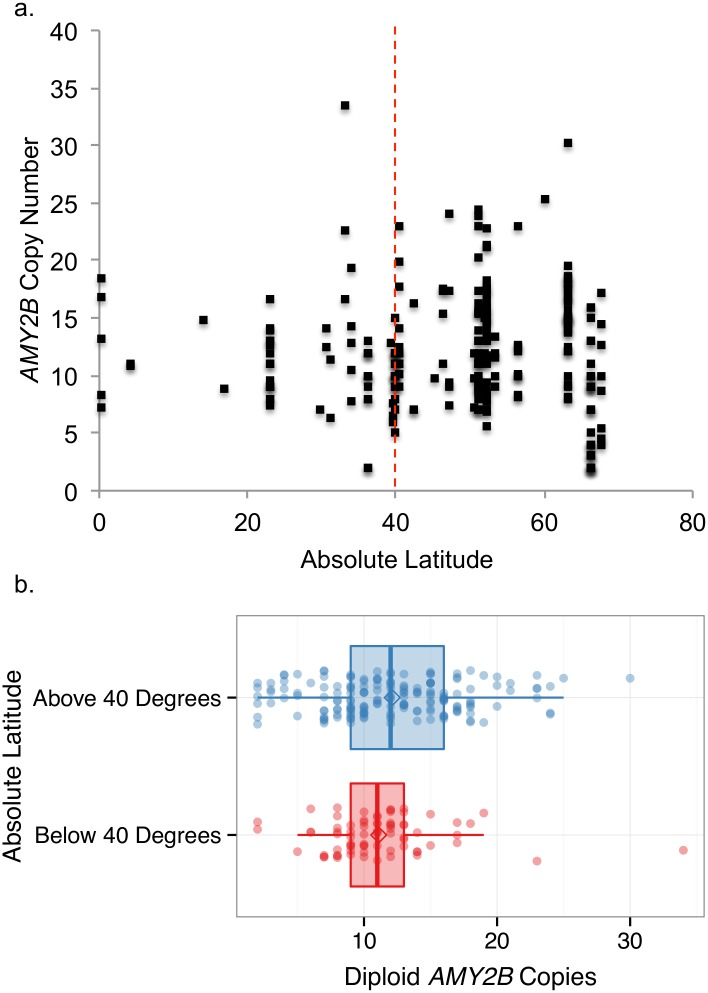
Prior to the adoption and global dominion of agriculture, human starch intake dramatically decreased above 40° latitude [35,36]. With the beginning of agriculture, archaeological accounts indicate that all domestication centers were below 40° latitude and agriculture then spread more slowly on a north-south axis than on an east-west axis [37]. Because starch intake was prehistorically and historically high below 40° latitude and low above 40° latitude, we used latitude as a proxy for starch intake in line with several other studies [35,36].
We found that absolute latitude did not predict copy number (R2 = .003; p = .4). However, variation in AMY2B CNV increased with increasing absolute latitude (Fig 4) such that variation in CNV calls from below 40° latitude (n = 75; mean = 11.1±4.4) was statistically different from the variation in CNV calls from above 40° latitude (n = 177; mean = 12.1±5.3) (Fligner-Killeen: p < .001) (Fig 4). As illustrated with ddPCR data, variation in AMY2B copy number increases as starch consumption declines, but average AMY2B copy number is universally high in all dog breeds compared to wolves [15].
Fig 4. Absolute latitude and starch intake.
a) AMY2B CNV regressed by absolute latitude at breed location of origin for 252 dogs. When absolute latitude is used as a proxy for starch intake, absolute latitude does not predict AMY2B copy number. Variation increases at 40° latitude. b) Tukey boxplot of AMY2B copy number in dog breeds originating from below 40° latitude and above 40° latitude. Variation in AMY2B copy number increases at higher latitudes.
Phylogeny does not predict AMY2B CNV between breeds
In order to determine whether the above patterns in mean CNV or CNV variation reflect some historical (artificial) selection signal or random forces, we sought to determine if phylogeny played a role. Given the limited statistical power to detect phylogenetic signal using a dataset consisting of only six dog breeds, phylogeny could not be controlled for with traditional methods [38]. Instead, to gauge how phylogenetic signals could potentially explain our findings we mapped AMY2B CNV onto two different cladograms [12,39], each of which depicted the phylogenetic relationship between each breed (Fig 5; S1 Fig). In both cases, phylogeny does not appear to significantly impact AMY2B copy number across breeds. Instead, our results indicate that starch intake appears to have influenced AMY2B copy number. For example, although both phylogenies reveal that the Shar Pei is more closely related to the Alaskan Malamute, Siberian Husky, and Japanese dogs [12] (Fig 5), the Shar Pei has a higher average copy number and a lower range in possible CNVs more similar to that of the Pekingese.
Fig 5. Cladogram depicting the relationship between the dog breeds evaluated in this study and diploid AMY2B copy number.
Cladogram relations were determined from SNP data and was adapted from vonHoldt et al. [12].
Wolf introgression does not explain the low AMY2B CNV in low-starch breeds
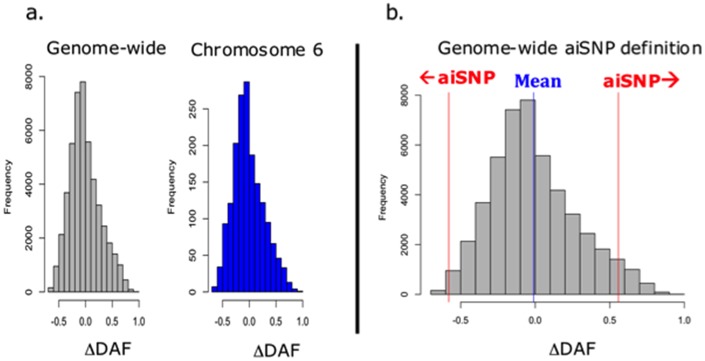
Finally, to investigate whether introgression from wolves explains the AMY2B low copy number in the low-starch breeds, we determined the difference of derived allele frequency (ΔDAF) between our dog samples and our wolf samples for every locus in the genome for which we had genotype data (Methods). As a control, we confirmed that there was no difference between the genome-wide ΔDAF distribution and that on chromosome 6 (Fig 6) (Welch Two Sample t-test, p-value = 0.5867). We then defined “ancestry informative SNPs” (hereafter, “aiSNPs’”) as those with a ΔDAF with an absolute value greater than two standard deviations higher than the genome-wide mean (see Fig 6).
Fig 6. Distribution of dog-wolf difference of derived allele frequency (DAF).
(a) There is no significant difference between the distribution of DAF genome-wide on chromosome 6 (Welch Two Sample t-test, p-value = 0.5867.) (b) Ancestry Informative SNPs (aiSNPs) are defined as those with an absolute DAF greater than two standard deviations from the genome-wide mean. Threshold are greater than 0.557 or less than -0.579.
If the high frequency of low AMY2B copy numbers in the Alaskan Malamute, Siberian Husky, and the Japanese dogs is due to introgression from wolves, we would expect that at aiSNP loci within the AMY2B genomic region, low-starch breeds would have the “wolf allele” (the allele that is at high frequency in wolves) at higher frequency than the high-starch breeds. We identified three aiSNPs in the AMY2B region of the genome. Of the two dog breeds with the highest frequency of the “wolf” allele at these three loci, one was a high-starch, high-CNV breed, and the other was a low-starch, low-CNV breed (S2 Table). Therefore, these data indicate that it is unlikely that wolf introgression explains the low copy number of the low starch-breeds at this locus. For consistency, we repeated this procedure on the GCKR and PHYH loci and similarly found no correlation between ΔDAF at aiSNPs and starch intake (S3 and S4 Tables).
Discussion
In this study, we investigated the relationship between diet and CNVs in three metabolic genes in the context of the evolutionary forces that have potentially operated since initial dog domestication. Of these three loci, only copy number variation for AMY2B, a gene with important roles in starch digestion [15,40], correlated with diet. While it is possible that GCKR or PHYH copy number were influenced by positive selection, we did not find evidence that either dietary intake of sugar or phytanic acid drove positive selection at these loci, respectively.
Specifically, our findings indicate that it is unlikely that sugar intake drove positive selection in GCKR CNV. We find no evidence that positive selection acted on this locus, though this study cannot definitively rule out the possibility of selection. Results from our aCGH analysis indicate that unusual pressures may have acted on GCKR CNV (S2 Fig) and we cannot exclude the possibility that other factors drove positive or negative selection at this locus, or that CNV at this locus was predominately influenced by random forces. Although it is primarily expressed in the liver, GCKR is also expressed in the ovaries and the adrenal glands of humans [41]. In dogs, GCKR expression has been identified in colonic mucosa [42]. It is possible that the expression profile of humans is conserved in dogs, or that GCKR plays a key unique role in the colonic mucosa of dogs, and that selection on GCKR CNV provides functional benefits other than maintenance of blood glucose homeostasis. Additionally, while our sample sizes are limited, it is unlikely that GCKR CNV alone plays a role in diabetes risk in dogs. Further research is needed to determine whether specific GCKR haplotypes are associated with increased or decreased risk of diabetes in dogs.
Our copy number analysis and results obtained for PHYH were also inconclusive. We identified that CNV at PHYH were variable with high CNV values being quite inaccurate and difficult to determine. When the available ddPCR data were analyzed on the aCGH distribution, the PHYH coordinate fell well outside of the cluster of copy number variable loci, indicating that no other surveyed loci had a profile similar to that of the PHYH ddPCR results and reflecting error in copy number calls. This inaccuracy reflects that, with all CNV computation methods used to date, the detection and resolution of high-copy number genotypes assumes high error (Methods). This highlights a broader need for technologies with increased accuracy in copy number resolutions especially for high copy variants. Without reliable copy number reads especially for those at the extreme high end of the distribution (and likely reflecting natural selection), it is difficult to speculate what selective factors may have driven copy number changes within each dog breed. However, given that dietary phytanic acid is toxic at high concentrations [43,44] and that it is an activator of genetic pathways involved in non-shivering thermogenesis [45], further analysis of PHYH function and CNV detection, using better methods when they become available, should examine if copy number balances the relative importance of each pathway.
Importantly, data obtained from ddPCR and aCGH suggested that AMY2B copy number may have been subject to positive selection in multiple breeds that consumed starch-rich diets. Because aCGH data were only available for the Alaskan Malamute and the Shar Pei, the null hypothesis cannot be fully tested in other breeds. However, the pattern of AMY2B CNV observed in all dogs that consumed high starch is similar, as is the pattern observed in all dogs that consumed low starch. Analysis of global trends in AMY2B CNV in a variety of breeds also showed that this pattern is not isolated to the six breeds examined in-depth. Although structural instability in this genomic region could create a high degree of variability in copy number variation, there is no evidence that the AMY2B region shows an unusually high level of instability [46] and any instability would be expected to affect all dog breeds randomly, and therefore does not explain our finding of the correlation between CNV and starch consumption.
These findings also extend species-wide. For example, when absolute latitude is used as a proxy for starch intake, global variation in AMY2B copy number mirrors results obtained from ddPCR. Indeed, AMY2B variation increases with increasing latitude, and although latitude does not predict copy number, as starch consumption decreases, variation in copy number increases.
These findings expand upon the recent study of Axelsson and colleagues [15], which found that AMY2B copy number is substantially increased in domestic dogs relative to wolves. This study presents evidence that in dog breeds that were exposed to starch-rich diets, positive selection continued to influence AMY2B copy number after this initial copy number expansion. Our findings also indicate that in dog breeds that adopted starch poor diets after copy number expansion, copy number likely evolved via genetic drift or via weak negative selection.
Previously, it has been reported that the Siberian Husky has not experienced copy number expansion at the AMY2B locus, suggesting that “the AMY2B copy number expansion was not fixed across all dogs early in the domestication process” [7]. This was inferred because in those huskies sampled AMY2B CNV ranged from three to four. On the contrary, with our expanded sampling strategy and use of ddPCR, which has slightly better ability to accurately call high CNV genotypes, we observed a copy number range from 2–15 in huskies, which, like all other breeds that have been surveyed, suggests that the copy number expansion event may likely have occurred prior to the split of modern major breeds worldwide. Of the 17 huskies we included in this study, four had a CNV of four or lower, which likely reflects genetic drift rather than possible introgression of alleles from wolves, though we cannot rule out introgression by other low CNV breeds. In summary, our observed patterns further validate that dogs experienced copy number expansion and that variation in CNV distribution likely reflects early positive selection at the AMY2B locus after initial expansion [15] and the possible impact of genetic drift or weak negative selection on the locus thereafter.
Importantly, the Japanese dog breeds we sampled may represent breeds in transition between drift and positive selection. It is likely that both breeds lived among the ancient hunter-gatherer cultures that existed prior to the agriculturalists that began dominating Japan roughly 2000 years ago [47]. The timing of this transition for each breed is not known, and may have differed for individual dogs. Although Japanese dogs display decreased mean CNV and increased variation compared to the Pekingese or Shar Pei, they have higher mean CNV and less variation than the Alaskan Malamute or Siberian Husky. This likely does not reflect the effects of island biogeography given that their variation is higher than nearby continental breeds, but could reflect a transition between a low- and high-starch diet that the breeds experienced. Because the transition to consuming a high-starch diet was likely recent in these breeds histories, and because of limited sampling, the Japanese breeds were included in the low-starch diet category.
These findings provide support for the idea that starch-rich diets were consumed after AMY2B CNV expansion, and likely before global migration of the ancestors to modern dogs. Given the genetic patterns identified in this study, we propose a model in which starch-rich diets predominated among most dog breeds coinciding with or shortly after the AMY2B CNV expansion and proliferation.
Although our findings suggest that AMY2B copy number expansion was likely influenced by positive selection pressure due to high-starch intake, we still find several individuals in low-starch breeds with high AMY2B copy numbers. For example, of Alaskan Malamutes sampled, close to 1/3 of individuals have a high CNV. We see three possible scenarios that could explain this finding. Scenario one calls for relatively recent positive selection for the high CNV, such that this pressure leads to the high CNV in already-differentiated high-starch dog breeds. In this case, the presence of some high copy numbers in the low-starch breeds is due to recent admixture between high- and low-starch dog breeds. We consider this scenario to be unlikely as there is no evidence of such introgression [48]. Both scenarios two and three place positive selection for the high CNV earlier in dog domestication, before the high- and low-starch breeds differentiated from each other. In scenario two, the low copy numbers in low-starch dogs is due to admixture between these breeds and wolves, which brought in low CNV haplotypes into the low-starch population. Given that our study found no evidence that wolf introgression is related to AMY2B copy number, we reject this scenario.
We therefore find a third scenario to be the most likely. We propose a model in which starch-rich diets predominated among most dogs after AMY2B expansion and before migration and differentiation of high- and low-starch breeds, coinciding with local starch consumption by humans. In turn, high starch consumption created positive selective pressure that likely acted on standing variation in AMY2B CNV to increase copy number species-wide. As some dog breeds migrated away, the selection pressure for high AMY2B CNV was maintained if dogs continued to consume starch-rich diets, or AMY2B CNV was subject to genetic drift if starch-poor diets were adopted. In the case of persistent positive selection, variation in AMY2B was kept low as selection pushed copy numbers toward an adaptive optimum. In the case of relaxed selection and genetic drift, variation increased throughout the dog breed and in certain locations, variation increased throughout the dog breed, possibly combined with positive selection on the low copy number variant in these populations.
In order to further test our hypotheses, future research should analyze AMY2B haplotype diversity using structural analyses in a variety of dog breeds in order to assess age and global distribution of haplotypes as has been carried out for the AMY2A locus in humans using 1000 Genomes datasets [49]. Future analyses would also be aided by improved copy number detection technologies. Particularly, it will be important to discern haploid copy number in order to detect selection via traditional methods. Finally, at this time little is understood regarding the functional ramifications of increased copy number and so future experiments should be targeted to these goals.
Materials and Methods
Dog samples
To investigate the relationship between diet and genetic polymorphisms located in and around key metabolic genes, DNA samples and dietary information were collected from multiple dog breeds (S5–S8 Tables). Sixty-two buccal swab samples were taken from six different dog breeds. These breeds are more genetically distinct than breeds created in the last few hundred years [12,39]. Dietary information was also collected from the Human Relations Area Files (OCM code 220) and from archaeological and isotopic analyses [5,18,50] and in general, dogs had variable dietary intake during their breed histories. CNV was surveyed in the genes AMY2B, GCKR, and PHYH.
For statistical analysis of AMY2B, CNV calls were also aggregated from other published studies [7,15,22]. Eighteen additional CNV calls from the same six breeds were used, bringing the sample total to eighty. For regression analyses, 172 additional AMY2B CNV calls from sixty-five other dog breeds were also included [7,15,22]. For these tests only, 252 total samples were used from seventy-one distinct breeds.
Finally, array-based comparative genomic hybridization (aCGH) data were obtained for four Alaskan Malamutes and five Shar Peis [34]. No AMY2B CNV calls were made on these samples; however, this data provided a comparative framework for analyses of other AMY2B CNV calls.
DNA sample collection and processing
DNA samples were swabbed from the buccal side of each dog’s cheek using VWR Foam-Tipped Swab (catalog # 89009–338). Age and sex were not regarded, except all dogs sampled were older than six months of age. Following standard procedures, each sample was placed in an open 2.0 mL tube to dry for two hours and then closed and stored at -20°C until DNA extraction. DNA was extracted with the QIAamp mini DNA kit (Qiagen) using the Buccal Swab Protocol (Qiagen). Extracted samples were stored in 150 μL Buffer AE (Qiagen) at -20°C.
All dog samples were collected with owner’s permission. We received exemption from the oversight of the Institutional Animal Care and Use Committee of Harvard University. No dogs were hurt or adversely affected by the collection process.
Copy number variation assays
Three CNV assays were performed on all collected samples. All copy number variants were determined using droplet digital PCR (ddPCR). Previously, real-time PCR (qPCR) has been used to study CNV in select breeds [7,15], however qPCR has limited ability to discern high copy number gene duplications [51,52], ddPCR allows absolute measure of oligonucleotides that are separated into an emulsified oil solution. This method generates a more accurate estimation of DNA copy number by decreasing the potential exponential error in a reaction and by producing technical error estimates for a single replicate. ddPCR was performed using QX200 Droplet Digital PCR system designed by Bio-Rad. ddPCR assay for AMY2B was performed as described in Arendt et al. [22]. ddPCR assays for GCKR and PHYH were adapted from Nicholas et al. [33]. For all assays, thermocyler settings were programmed as described by manufacturer (Bio-Rad) and under the following conditions: Amplification was done under the following conditions: one cycle at 95°C for 10 minutes, 40 cycles at 94°C for 30 seconds and 60°C for 1 minute, and one cycle at 98°C for 10 minutes. DNA was digested by restriction enzyme DraI (New England Bio Labs), allowing for separation of individual gene copies for better segregation and quantification in each droplet. ddPCR settings were sufficient to inactivate the enzyme. Restriction digests were diluted at varying concentrations from 10X to 1X and tested for replication. DNA concentrations varied after extraction, however because ddPCR does not have strict parameters for input DNA concentration, equal volumes of DNA were added to the assays regardless of concentration. Concentration of the added 2 μL of DNA ranged from .055-31.6 ng/μL. After ddPCR assays were completed, copy number was regressed by DNA concentration to see if a relationship was produced between concentration and copy number. No correlation was identified. Because of the nature of ddPCR, technical error reports replaced traditional replicates (S6–S8 Tables). Raw copy number data were rounded to the nearest whole number and with technical error estimates (S6–S8 Tables). Primer and probe sequences for AMY2B, GCKR, PHYH and reference genes are located in S9 Table.
Statistical analyses
Statistical analyses of copy number variants were performed using the StatPlus package for Microsoft Excel, as well as in R. Modeling and graphics were created using Excel and R. The Robust Rank Order Test was employed to test differences in medians because it does not assume that the populations have the same shape or variances [53]. Fligner-Killeen test was employed to test homogeneity of variances of copy number in dogs that consumed different diets [54].
aCGH analysis
To determine if AMY2B CNV mean log2 ratio deviated substantially from the null distribution of other copy number variable sites in the genome, we analyzed residuals of AMY2B to a linear regression line fit to the aCGH data. 156 CNV sites were present in at least one Alaskan Malamute and one Shar Pei in the aCGH data. aCGH data are presented as log2 ratios that represent the amount of genetic material present relative to a common control (CanFam2) at each surveyed SNP. The mean log2 ratio was calculated for each site in both the Alaskan Malamute and the Shar Pei by averaging all log2 ratios within each copy number variable site. Next, mean log2 ratios for the Alaskan Malamute and the Shar Pei were plotted against each other to produce a null distribution for the relationship between standard genomic CNV in the Alaskan Malamute and the Shar Pei.
The mean log2 ratio for AMY2B in Alaskan Malamutes and Shar Peis were added to the null distribution. Mean log2 ratios were calculated relative to CanFam2 and by using the AMY2B CNV data produced by ddPCR.
A linear regression analysis was conducted on the distribution of Alaskan Malamute and Shar Pei mean log2 ratios to determine if AMY2B CNV deviated (Fig 3). If no difference in variation existed between synonymous copy number variable regions, then the breed log2 ratios would be similar, even if dissimilar from zero. Any copy number variant with a mean log2 ratio that deviates substantially from the null distribution may have been subject to unusual evolutionary pressure in one or both of the breeds.
To determine how AMY2B compared to this null distribution, residuals were calculated from the linear regression model. Mean and standard deviation for these residuals were calculated and used to compare deviation in AMY2B CNV in these breeds. Because of limited samples size, this analysis was performed again taking into consideration other breeds from previously published studies employing qPCR datasets in order to generate a more robust null distribution (S2 Appendix).
Regression of absolute latitude by CNV
Latitude at location of dog origin was used to determine breed-level designation of starch intake for breeds with published AMY2B CNV calls. Latitude was next adjusted to reflect absolute distance from the equator. Absolute latitude was next regressed by AMY2B CNV of 252 dogs to determine if starch intake predicted copy number, and calls were separated into two categories: those below 40° latitude (n = 75) and those above 40° latitude (n = 177). A Fligner-Killeen test was run between the two groups to test homogeneity of variances [54].
Difference of derived allele frequency and introgression analyses
The difference of derived allele frequency (ΔDAF) was determined according to the following formula, ΔDAF = DAFdogs—DAFwolves. Genotype information came from analyses conducted by vonHoldt and colleagues [12]. The mean and standard deviation of ΔDAF throughout the genome was determined using the R “mean()” and “sd()” functions. Ancestry informative SNPs (aiSNPs) were those with a ΔDAF with an absolute value greater than two standard deviations above the mean. In determining the region in which to search for aiSNPs for each gene, our goal was to insure that we included any potentially relevant aiSNPs in either the coding or non-coding regions of each gene. We therefore initially considered the region between the transcription start sites of the flanking genes on either side of our genes of interest. Given the relatively poor annotation of the dog genome, we additionally further expanded these regions by 500kb on either side due to a convention established by vonHoldt et al. [12] in which wolf-introgressed haplotypes in dogs were found to be 500kb in length. The coordinates we considered for each gene and the location of the aiSNPs are shown in S10 Table.
Supporting Information
Cladogram depicting the relationship between the dog breeds evaluated in this study and diploid AMY2B copy number.
(PDF)
Relationship between Shar Pei and Alaskan malamute mean log2 ratios for 157 copy number variable sites in the dog genome. GCKR deviates from the null distribution. The location of PHYH likely reflects error in ddPCR measurements due to high copy number.
(PDF)
Analyses of AMY2B CNV using additional datasets.
(PDF)
Analyses of aCGH datasets across a number of dog breeds.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
For funding we thank the Cordeiro Fellowship and the Museum of Comparative Zoology of Harvard University. We would like to thank Joshua Akey for providing primer and probe sequences. We also thank Bridgett vonHoldt for her critical discussions and advice, as well as Linda Reynard and Richard Wrangham for their early input. Finally, we thank the breeders and owners who provided access to all the dogs used to acquire samples.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by Global Health and Health Policy Secondary Field, Harvard University (TR), fellowship name: Cordiero Fellowship, http://ghhp.fas.harvard.edu/cordeiro-summer-research-fellowship; and Museum of Comparative Zoology, Harvard University (TR), grant name: Grants-in-aid of undergraduate research, http://www.mcz.harvard.edu/grants_and_funding/gur.html. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Akey JM, Ruhe AL, Akey DT, Wong AK, Connelly CF, et al. (2010) Tracking footprints of artificial selection in the dog genome. Proc Natl Acad Sci U S A 107: 1160–1165. 10.1073/pnas.0909918107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyko AR, Quignon P, Li L, Schoenebeck JJ, Degenhardt JD, et al. (2010) A simple genetic architecture underlies morphological variation in dogs. PLoS Biol 8: e1000451 10.1371/journal.pbio.1000451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang GD, Zhai W, Yang HC, Fan RX, Cao X, et al. (2013) The genomics of selection in dogs and the parallel evolution between dogs and humans. Nat Commun 4: 1860 10.1038/ncomms2814 [DOI] [PubMed] [Google Scholar]
- 4.Dayan T (1994) Early Domesticated Dogs of the Near East. Journal of Archaeological Science 21: 633–640. [Google Scholar]
- 5.Pechenkina EA, Ambrose SH, Xiaolin M, Benfer RA Jr (2005) Reconstructing northern Chinese Neolithic subsistence practices by isotopic analysis. Journal of Archaeological Science 32: 1176–1189. [Google Scholar]
- 6.Esaka T (1970) The burial skeleton of dog in Jomon period. Archaeological Journal 40: 6–8. [Google Scholar]
- 7.Freedman AH, Gronau I, Schweizer RM, Ortega-Del Vecchyo D, Han E, et al. (2014) Genome sequencing highlights the dynamic early history of dogs. PLoS Genet 10: e1004016 10.1371/journal.pgen.1004016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larson G, Karlsson EK, Perri A, Webster MT, Ho SY, et al. (2012) Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc Natl Acad Sci U S A 109: 8878–8883. 10.1073/pnas.1203005109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pang J-F, Kluetsch C, Zou X-J, Zhang A-b, Luo L-Y, et al. (2009) mtDNA Data Indicate a Single Origin for Dogs South of Yangtze River, Less Than 16,300 Years Ago, from Numerous Wolves. Molecular Biology and Evolution 26: 2849–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skoglund P, Götherström A, Jakobsson M (2011) Estimation of Population Divergence Times from Non-Overlapping Genomic Sequences: Examples from Dogs and Wolves. Molecular Biology and Evolution 28: 1505–1517. 10.1093/molbev/msq342 [DOI] [PubMed] [Google Scholar]
- 11.Ding ZL, Oskarsson M, Ardalan A, Angleby H, Dahlgren LG, et al. (2012) Origins of domestic dog in southern East Asia is supported by analysis of Y-chromosome DNA. Heredity (Edinb) 108: 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vonholdt BM, Pollinger JP, Lohmueller KE, Han E, Parker HG, et al. (2010) Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature 464: 898–902. 10.1038/nature08837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coppinger R, Coppinger L (2001) Dogs: a new understanding of canine origin, behavior and evolution. New York: Scribner. [Google Scholar]
- 14.Clutton-Brock J (1995) Origins of the dog: domestication and early history In: Serpell J, editor. The Domestic Dob, its Evolution, Behaviour and Interactions with People. Cambridge: Cambridge University Press; pp. 7–20. [Google Scholar]
- 15.Axelsson E, Ratnakumar A, Arendt ML, Maqbool K, Webster MT, et al. (2013) The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495: 360–364. 10.1038/nature11837 [DOI] [PubMed] [Google Scholar]
- 16.Choy K, Richards M (2010) Isotopic evidence for diet in the Middle Chulmun period: a case study from the Tongsamdong shell midden, Korea. Archaeological and Anthropological Sciences 2: 1–10. [Google Scholar]
- 17.Losey RJ, Garvie-Lok S, Leonard JA, Katzenberg MA, Germonpre M, et al. (2013) Burying dogs in ancient Cis-Baikal, Siberia: temporal trends and relationships with human diet and subsistence practices. PLoS One 8: e63740 10.1371/journal.pone.0063740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsutaya T, Naito YI, Ishida H, Yoneda M (2014) Carbon and nitrogen isotope analyses of human and dog diet in the Okhotsk culture: perspectives from the Moyoro site, Japan. Anthropological Science 122: 89–99. [Google Scholar]
- 19.Li Y, Wu DD, Boyko AR, Wang GD, Wu SF, et al. (2014) Population variation revealed high-altitude adaptation of Tibetan mastiffs. Mol Biol Evol 31: 1200–1205. [DOI] [PubMed] [Google Scholar]
- 20.Simonson TS, Yang Y, Huff CD, Yun H, Qin G, et al. (2010) Genetic evidence for high-altitude adaptation in Tibet. Science 329: 72–75. 10.1126/science.1189406 [DOI] [PubMed] [Google Scholar]
- 21.Perry GH, Dominy NJ, Claw KG, Lee AS, Fiegler H, et al. (2007) Diet and the evolution of human amylase gene copy number variation. Nat Genet 39: 1256–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arendt M, Fall T, Lindblad-Toh K, Axelsson E (2014) Amylase activity is associated with AMY2B copy numbers in dog: implications for dog domestication, diet and diabetes. Anim Genet 45: 716–722. 10.1111/age.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandel AL, Peyrot des Gachons C, Plank KL, Alarcon S, Breslin PA (2010) Individual differences in AMY1 gene copy number, salivary alpha-amylase levels, and the perception of oral starch. PLoS One 5: e13352 10.1371/journal.pone.0013352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weng Q (2000) Human-environment interactions in agricultural land use in a South China's wetland region: A study on the Zhujiang Delta in the Holocene. GeoJournal 51: 191–202. [Google Scholar]
- 25.Yang X, Barton HJ, Wan Z, Li Q, Ma Z, et al. (2013) Sago-Type Palms Were an Important Plant Food Prior to Rice in Southern Subtropical China. PLoS ONE 8: e63148 10.1371/journal.pone.0063148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Yu J, Lü H, Cui T, Guo J, et al. (2009) Starch grain analysis reveals function of grinding stone tools at Shangzhai site, Beijing. Science in China Series D: Earth Sciences 52: 1164–1171. [Google Scholar]
- 27.Bogoras W (1901) THE CHUKCHI OF NORTHEASTERN ASIA. American Anthropologist 3: 80–108. [Google Scholar]
- 28.Habu J (2004) Ancient Jomon of Japan. Cambridge, UK: Cambridge University Press; 350 p. [Google Scholar]
- 29.Porsild AE (1953) Edible Plants of the Arctic. 1953 6. [Google Scholar]
- 30.Shinichiro T (1960) The Ainu of northern Japan: a study in conquest and acculturation. Harrison JA, translator. Philadelphia: American Philosophical Society. [Google Scholar]
- 31.Croes K, Casteels M, De Hoffmann E, Mannaerts GP, Van Veldhoven PP (1996) alpha-Oxidation of 3-methyl-substituted fatty acids in rat liver. Production of formic acid instead of CO2, cofactor requirements, subcellular localization and formation of a 2-hydroxy-3-methylacyl-CoA intermediate. Eur J Biochem 240: 674–683. [DOI] [PubMed] [Google Scholar]
- 32.van den Brink DM, Wanders RJ (2006) Phytanic acid: production from phytol, its breakdown and role in human disease. Cell Mol Life Sci 63: 1752–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholas TJ, Cheng Z, Ventura M, Mealey K, Eichler EE, et al. (2009) The genomic architecture of segmental duplications and associated copy number variants in dogs. Genome Res 19: 491–499. 10.1101/gr.084715.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholas TJ, Baker C, Eichler EE, Akey JM (2011) A high-resolution integrated map of copy number polymorphisms within and between breeds of the modern domesticated dog. BMC Genomics 12: 414 10.1186/1471-2164-12-414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marlowe FW (2005) Hunter-gatherers and human evolution. Evolutionary Anthropology: Issues, News, and Reviews 14: 54–67. [Google Scholar]
- 36.Strohle A, Hahn A (2011) Diets of modern hunter-gatherers vary substantially in their carbohydrate content depending on ecoenvironments: results from an ethnographic analysis. Nutr Res 31: 429–435. 10.1016/j.nutres.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 37.Diamond J (2002) Evolution, consequences and future of plant and animal domestication. Nature 418: 700–707. [DOI] [PubMed] [Google Scholar]
- 38.Blomberg SP, Garland T Jr., Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57: 717–745. [DOI] [PubMed] [Google Scholar]
- 39.Parker HG, Kim LV, Sutter NB, Carlson S, Lorentzen TD, et al. (2004) Genetic structure of the purebred domestic dog. Science 304: 1160–1164. [DOI] [PubMed] [Google Scholar]
- 40.Horii A, Emi M, Tomita N, Nishide T, Ogawa M, et al. (1987) Primary structure of human pancreatic alpha-amylase gene: its comparison with human salivary alpha-amylase gene. Gene 60: 57–64. [DOI] [PubMed] [Google Scholar]
- 41.Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, et al. (2013) The Genotype-Tissue Expression (GTEx) project. Nat Genet 45: 580–585. 10.1038/ng.2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kil DY, Vester Boler BM, Apanavicius CJ, Schook LB, Swanson KS (2010) Gene expression profiles of colonic mucosa in healthy young adult and senior dogs. PLoS One 5: e12882 10.1371/journal.pone.0012882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jansen GA, Ofman R, Ferdinandusse S, Ijlst L, Muijsers AO, et al. (1997) Refsum disease is caused by mutations in the phytanoyl-CoA hydroxylase gene. Nat Genet 17: 190–193. [DOI] [PubMed] [Google Scholar]
- 44.Mihalik SJ, Morrell JC, Kim D, Sacksteder KA, Watkins PA, et al. (1997) Identification of PAHX, a Refsum disease gene. Nat Genet 17: 185–189. [DOI] [PubMed] [Google Scholar]
- 45.Schluter A, Barbera MJ, Iglesias R, Giralt M, Villarroya F (2002) Phytanic acid, a novel activator of uncoupling protein-1 gene transcription and brown adipocyte differentiation. Biochem J 362: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong AK, Ruhe AL, Dumont BL, Robertson KR, Guerrero G, et al. (2010) A comprehensive linkage map of the dog genome. Genetics 184: 595–605. 10.1534/genetics.109.106831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okumura N, Ishiguro N, Nakano M, Matsui A, Shigehara N, et al. (1999) Variations in Mitochondrial DNA of Dogs Isolated from Archaeological Sites in Japan and Neighbouring Islands. Anthropological Science 107: 213–228. [Google Scholar]
- 48.Vilà C, Savolainen P, Maldonado JE, Amorim IR, Rice JE, et al. (1997) Multiple and Ancient Origins of the Domestic Dog. Science 276: 1687–1689. [DOI] [PubMed] [Google Scholar]
- 49.Usher CL, Handsaker RE, Esko T, Tuke MA, Weedon MN, et al. (2015) Structural forms of the human amylase locus and their relationships to SNPs, haplotypes and obesity. Nat Genet 47: 921–925. 10.1038/ng.3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murdock GP, Ford CS, Hudson AE, Kennedy R, Simmons LW, et al. (2008) Outline of Cultural Materials. New Haven. [Google Scholar]
- 51.Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, et al. (2011) High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem 83: 8604–8610. 10.1021/ac202028g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinheiro LB, Coleman VA, Hindson CM, Herrmann J, Hindson BJ, et al. (2012) Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal Chem 84: 1003–1011. 10.1021/ac202578x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feltovich N (2003) Nonparametric Tests of Differences in Medians: Comparison of the Wilcoxon–Mann–Whitney and Robust Rank-Order Tests. Experimental Economics 6: 273–297. [Google Scholar]
- 54.Fligner MA, Killeen TJ (1976) Distribution-Free Two-Sample Tests for Scale. Journal of the American Statistical Association 71: 210–213. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cladogram depicting the relationship between the dog breeds evaluated in this study and diploid AMY2B copy number.
(PDF)
Relationship between Shar Pei and Alaskan malamute mean log2 ratios for 157 copy number variable sites in the dog genome. GCKR deviates from the null distribution. The location of PHYH likely reflects error in ddPCR measurements due to high copy number.
(PDF)
Analyses of AMY2B CNV using additional datasets.
(PDF)
Analyses of aCGH datasets across a number of dog breeds.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.