Abstract
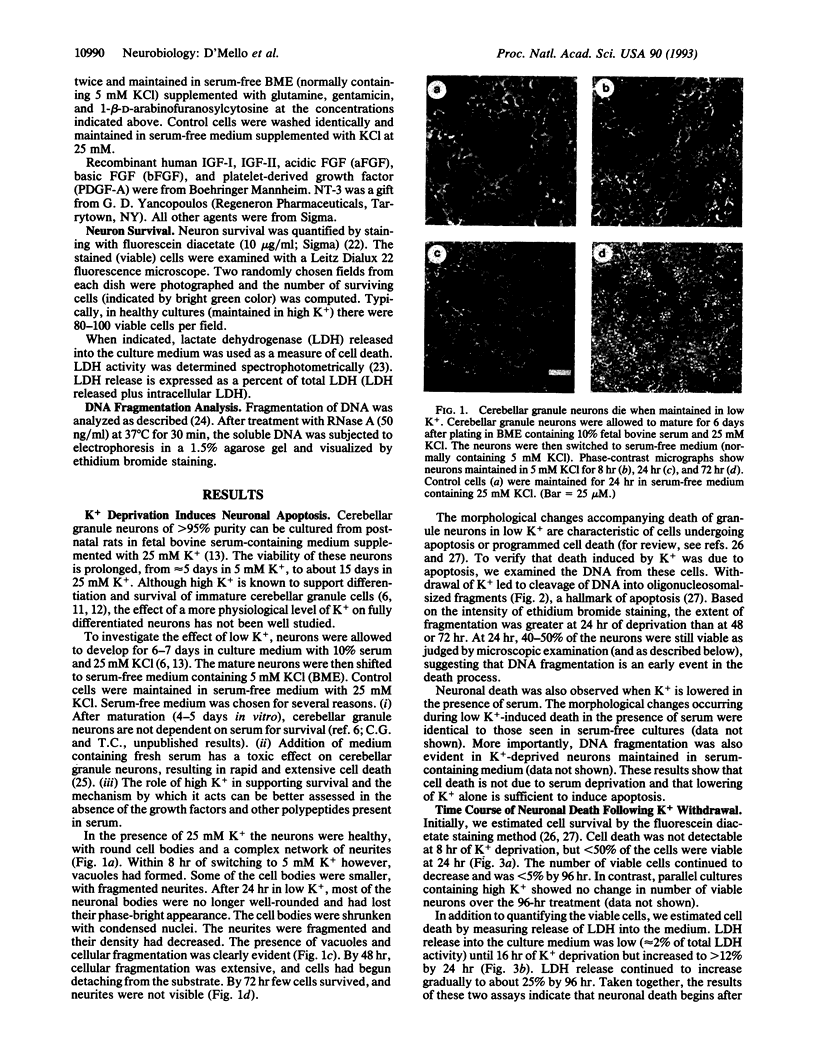
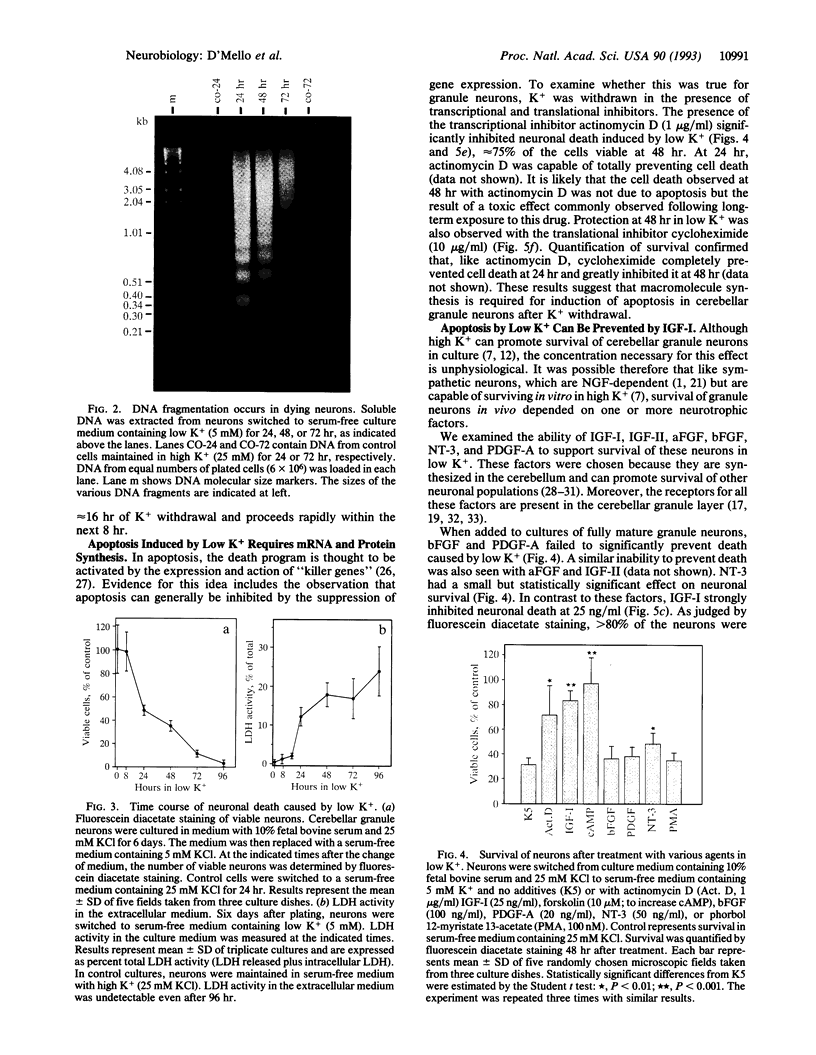
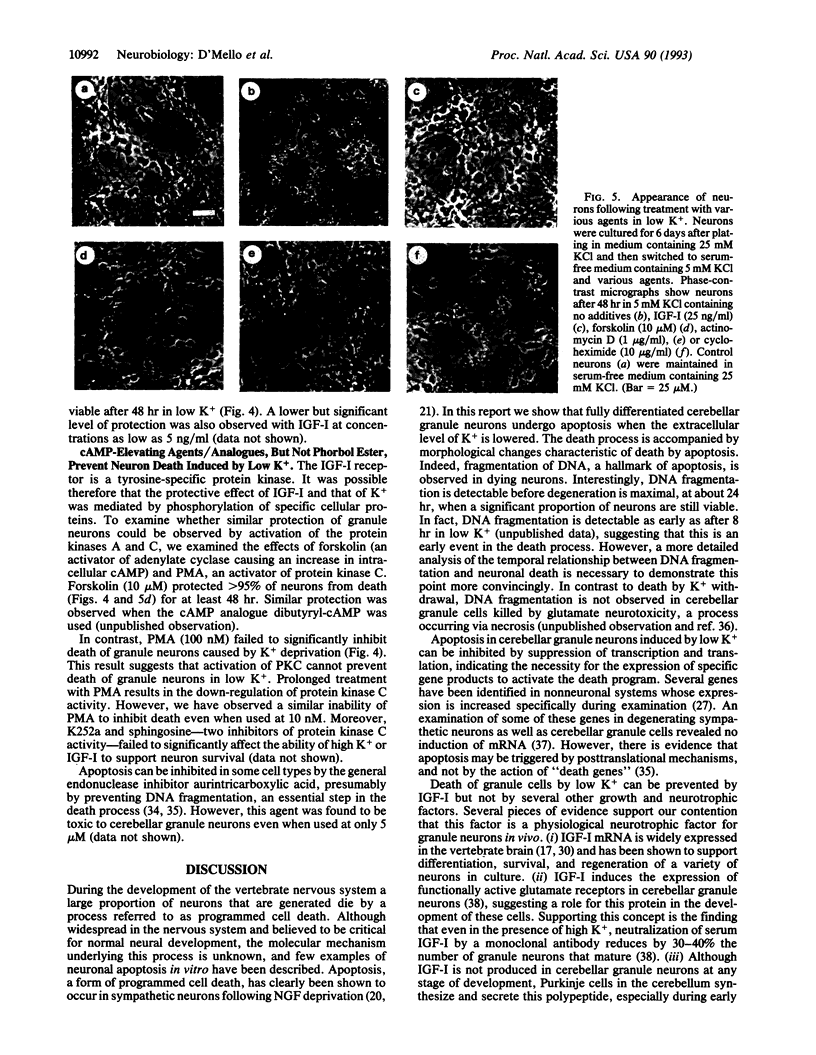
High levels of extracellular K+ ensure proper development and prolong survival of cerebellar granule neurons in culture. We find that when switched from a culture medium containing high K+ (25 mM) to one containing a low but more physiological K+ concentration (5 mM), differentiated granule neurons degenerate and die. Death induced by low K+ is due to apoptosis (programmed cell death), a form of cell death observed extensively in the developing nervous system and believed to be necessary for proper neurogenesis. The death process is accompanied by cleavage of genomic DNA into internucleosome-sized fragments, a hallmark of apoptosis. Inhibitors of transcription and translation suppress apoptosis induced by low K+, suggesting the necessity for newly synthesized gene products for activation of the process. Death can be prevented by insulin-like growth factor I but not by several other growth/neurotrophic factors. cAMP but not the protein kinase C activator phorbol 12-myristate 13-acetate can also support survival in low K+. In view of the large numbers of granule neurons that can be homogeneously cultured, our results offer the prospect of an excellent model system to study the mechanisms underlying apoptosis in the central nervous system and the suppression of this process by survival factors such as insulin-like growth factor I.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson I. K., Edwall D., Norstedt G., Rozell B., Skottner A., Hansson H. A. Differing expression of insulin-like growth factor I in the developing and in the adult rat cerebellum. Acta Physiol Scand. 1988 Feb;132(2):167–173. doi: 10.1111/j.1748-1716.1988.tb08314.x. [DOI] [PubMed] [Google Scholar]
- Barde Y. A. Trophic factors and neuronal survival. Neuron. 1989 Jun;2(6):1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Bartlett W. P., Li X. S., Williams M., Benkovic S. Localization of insulin-like growth factor-1 mRNA in murine central nervous system during postnatal development. Dev Biol. 1991 Sep;147(1):239–250. doi: 10.1016/s0012-1606(05)80021-1. [DOI] [PubMed] [Google Scholar]
- Batistatou A., Greene L. A. Aurintricarboxylic acid rescues PC12 cells and sympathetic neurons from cell death caused by nerve growth factor deprivation: correlation with suppression of endonuclease activity. J Cell Biol. 1991 Oct;115(2):461–471. doi: 10.1083/jcb.115.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy C. A. Transient IGF-I gene expression during the maturation of functionally related central projection neurons. J Neurosci. 1991 Nov;11(11):3442–3455. doi: 10.1523/JNEUROSCI.11-11-03442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calissano P., Ciotti M. T., Battistini L., Zona C., Angelini A., Merlo D., Mercanti D. Recombinant human insulin-like growth factor I exerts a trophic action and confers glutamate sensitivity on glutamate-resistant cerebellar granule cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8752–8756. doi: 10.1073/pnas.90.18.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A., Fischbach G. D. Elevated potassium induces morphological differentiation of dorsal root ganglionic neurons in dissociated cell culture. Dev Biol. 1980 Jul;78(1):173–183. doi: 10.1016/0012-1606(80)90327-9. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Howe J. R., Ogden D. C. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988 Jun;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello S. R., Galli C. SGP2, ubiquitin, 14K lectin and RP8 mRNAs are not induced in neuronal apoptosis. Neuroreport. 1993 Apr;4(4):355–358. doi: 10.1097/00001756-199304000-00003. [DOI] [PubMed] [Google Scholar]
- Dessi F., Charriaut-Marlangue C., Khrestchatisky M., Ben-Ari Y. Glutamate-induced neuronal death is not a programmed cell death in cerebellar culture. J Neurochem. 1993 May;60(5):1953–1955. doi: 10.1111/j.1471-4159.1993.tb13427.x. [DOI] [PubMed] [Google Scholar]
- Edwards S. N., Buckmaster A. E., Tolkovsky A. M. The death programme in cultured sympathetic neurones can be suppressed at the posttranslational level by nerve growth factor, cyclic AMP, and depolarization. J Neurochem. 1991 Dec;57(6):2140–2143. doi: 10.1111/j.1471-4159.1991.tb06434.x. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Lönnerberg P., Ayer-LeLievre C., Persson H. Developmental and regional expression of basic fibroblast growth factor mRNA in the rat central nervous system. J Neurosci Res. 1990 Sep;27(1):10–15. doi: 10.1002/jnr.490270103. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Minozzi M. C., Toffano G., Leon A., Skaper S. D. Basic fibroblast growth factor promotes the survival and development of mesencephalic neurons in culture. Dev Biol. 1989 May;133(1):140–147. doi: 10.1016/0012-1606(89)90305-9. [DOI] [PubMed] [Google Scholar]
- Franklin J. L., Johnson E. M., Jr Suppression of programmed neuronal death by sustained elevation of cytoplasmic calcium. Trends Neurosci. 1992 Dec;15(12):501–508. doi: 10.1016/0166-2236(92)90103-f. [DOI] [PubMed] [Google Scholar]
- Gallo V., Giovannini C., Levi G. Modulation of non-N-methyl-D-aspartate receptors in cultured cerebellar granule cells. J Neurochem. 1990 May;54(5):1619–1625. doi: 10.1111/j.1471-4159.1990.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Hockberger P. E., Tseng H. Y., Connor J. A. Immunocytochemical and electrophysiological differentiation of rat cerebellar granule cells in explant cultures. J Neurosci. 1987 May;7(5):1370–1383. doi: 10.1523/JNEUROSCI.07-05-01370.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Ishida I., Deguchi T. Effect of depolarizing agents on choline acetyltransferase and acetylcholinesterase activities in primary cell cultures of spinal cord. J Neurosci. 1983 Sep;3(9):1818–1823. doi: 10.1523/JNEUROSCI.03-09-01818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. H., Senft J. A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985 Jan;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Knusel B., Michel P. P., Schwaber J. S., Hefti F. Selective and nonselective stimulation of central cholinergic and dopaminergic development in vitro by nerve growth factor, basic fibroblast growth factor, epidermal growth factor, insulin and the insulin-like growth factors I and II. J Neurosci. 1990 Feb;10(2):558–570. doi: 10.1523/JNEUROSCI.10-02-00558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T., Martin D. P., Johnson E. M., Jr Role of Ca2+ channels in the ability of membrane depolarization to prevent neuronal death induced by trophic-factor deprivation: evidence that levels of internal Ca2+ determine nerve growth factor dependence of sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6421–6425. doi: 10.1073/pnas.86.16.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamballe F., Klein R., Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991 Sep 6;66(5):967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Lasher R. S., Zagon I. S. The effect of potassium on neuronal differentiation in cultures of dissociated newborn rat cerebellum. Brain Res. 1972 Jun 22;41(2):482–488. doi: 10.1016/0006-8993(72)90521-5. [DOI] [PubMed] [Google Scholar]
- Lesniak M. A., Hill J. M., Kiess W., Rojeski M., Pert C. B., Roth J. Receptors for insulin-like growth factors I and II: autoradiographic localization in rat brain and comparison to receptors for insulin. Endocrinology. 1988 Oct;123(4):2089–2099. doi: 10.1210/endo-123-4-2089. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: thirty-five years later. EMBO J. 1987 May;6(5):1145–1154. doi: 10.1002/j.1460-2075.1987.tb02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G., Aloisi F., Ciotti M. T., Gallo V. Autoradiographic localization and depolarization-induced release of acidic amino acids in differentiating cerebellar granule cell cultures. Brain Res. 1984 Jan 2;290(1):77–86. doi: 10.1016/0006-8993(84)90737-6. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Friedman B., Alderson R. F., Wiegand S. J., Furth M. E., Lindsay R. M., Yancopoulos G. D. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990 Oct;5(4):501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Squinto S., Ip N. Y., Furth M. E., Lindsay R. M., Yancopoulos G. D. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990 Mar 23;247(4949 Pt 1):1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Orrenius S. Rapid turnover of endogenous endonuclease activity in thymocytes: effects of inhibitors of macromolecular synthesis. Arch Biochem Biophys. 1990 Apr;278(1):284–287. doi: 10.1016/0003-9861(90)90261-v. [DOI] [PubMed] [Google Scholar]
- Murphy T. H., Malouf A. T., Sastre A., Schnaar R. L., Coyle J. T. Calcium-dependent glutamate cytotoxicity in a neuronal cell line. Brain Res. 1988 Mar 22;444(2):325–332. doi: 10.1016/0006-8993(88)90941-9. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Resink A., Boer G. J., Balázs R. Treatment with excitatory amino acids or high K+ and NMDA receptors in cerebellar granule cells. Neuroreport. 1992 Sep;3(9):757–760. doi: 10.1097/00001756-199209000-00009. [DOI] [PubMed] [Google Scholar]
- Rukenstein A., Rydel R. E., Greene L. A. Multiple agents rescue PC12 cells from serum-free cell death by translation- and transcription-independent mechanisms. J Neurosci. 1991 Aug;11(8):2552–2563. doi: 10.1523/JNEUROSCI.11-08-02552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydel R. E., Greene L. A. cAMP analogs promote survival and neurite outgrowth in cultures of rat sympathetic and sensory neurons independently of nerve growth factor. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1257–1261. doi: 10.1073/pnas.85.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm M., Eimerl S., Costa E. Serum and depolarizing agents cause acute neurotoxicity in cultured cerebellar granule cells: role of the glutamate receptor responsive to N-methyl-D-aspartate. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1193–1197. doi: 10.1073/pnas.87.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal R. A., Takahashi H., McKay R. D. Changes in neurotrophin responsiveness during the development of cerebellar granule neurons. Neuron. 1992 Dec;9(6):1041–1052. doi: 10.1016/0896-6273(92)90064-k. [DOI] [PubMed] [Google Scholar]
- Wanaka A., Johnson E. M., Jr, Milbrandt J. Localization of FGF receptor mRNA in the adult rat central nervous system by in situ hybridization. Neuron. 1990 Sep;5(3):267–281. doi: 10.1016/0896-6273(90)90164-b. [DOI] [PubMed] [Google Scholar]
- Yeh H. J., Ruit K. G., Wang Y. X., Parks W. C., Snider W. D., Deuel T. F. PDGF A-chain gene is expressed by mammalian neurons during development and in maturity. Cell. 1991 Jan 11;64(1):209–216. doi: 10.1016/0092-8674(91)90222-k. [DOI] [PubMed] [Google Scholar]





