Abstract
Following the acquisition of chloroplasts and mitochondria by eukaryotic cells during endosymbiotic evolution, most of the genes in these organelles were either lost or transferred to the nucleus. Encoding organelle-destined proteins in the nucleus allows for host control of the organelle. In return, organelles send signals to the nucleus to coordinate nuclear and organellar activities. In photosynthetic eukaryotes, additional interactions exist between mitochondria and chloroplasts. Here we review recent advances in elucidating the intracellular signalling pathways that coordinate gene expression between organelles and the nucleus, with a focus on photosynthetic plants.
It is widely accepted that chloroplasts and mitochondria descended from free-living bacterial ancestors1. Phylogenetic, biochemical, and structural analyses suggest that mitochondria descended from an α-proteobacterium-like ancestor that invaded or was engulfed by an archaeal-like host 1.5 billion years ago or earlier, and that primary chloroplasts descended from a cyanobacterium-like ancestor that invaded or was engulfed by a mitochondrion-possessing eukaryote between 1.5 and 1.2 billion years ago1.
In most cases these organelles have retained a genome, but present-day organelle genomes are severely reduced and encode few proteins (from 3 in the Plasmodium falciparum mitochondrion2 to 209 in the Porphyra purpurea chloroplast3) (BOX 1). On the other hand, proteomics and genome analyses of protein-localization sequences estimate that organelles might contain up to several thousand different proteins4,5. This estimate is within the range of the number of predicted protein-encoding genes in current-day cyanobacteria5 and α-proteobacteria6. As these numbers imply, most proteins (93–99%) that are found in organelles are encoded in the nucleus, synthesized in the cytoplasm and then imported into the organelles.
BOX 1. Why maintain an organellar genome?
The gene composition of organellar genomes varies between species, but they usually consist of genes encoding part of the translation machinery: 70S ribosome subunits, a variable set of tRNA genes (that can be complete, incomplete or even absent) and a small (~2–4) and incomplete set of rRNA genes101. Most sequenced chloroplasts from photosynthetic organisms contain a core constituency of ~44 protein-encoding genes encoding subunits for transcription (bacterial-like plastid-encoded RNA polymerase), photosynthesis (photosystem I, photosystem II, cytochrome b6f complex and the coupling ATPase) and the large subunit for ribulose-1,5-bisphosphate carboxylase/oxygenase102. Mitochondrial genomes encode protein subunits for respiration (cytochrome c biogenesis and complexes I–IV)4.
The transfer of organellar genes to the nucleus is presumed to be advantageous — it allows simpler gene coordination and reduces the risk of mutations from free-radical by-products of electron transfer reactions103. Maintaining a separate genetic system in an organelle creates logistical challenges for the cell, as discussed in the main text, and imposes a high energetic cost; for example, in Saccharomyces cerevisiae ~25% of the mitochondrial proteome is dedicated to maintaining and expressing the 19 mitochondrial proteins104. It is therefore unclear why the genes mentioned above are still found in organelle genomes. It seems reasonable to conclude that the complete transfer of organellar genes to the nucleus is evolutionarily difficult and is occurring slowly, or that there is an advantage to maintaining separate genomes. Either way, there needs to be communication between the nucleus and the organelle (retrograde and anterograde, as discussed in the main text) to coordinate the separate genomes.
Two principal and non-exclusive hypotheses (reviewed in REF. 103) have tried to explain this mystery. In the first hypothesis, proteins that are involved in photosynthesis and respiration cannot be efficiently transported from the cytoplasm to the organelle, because they might be too hydrophobic or they are toxic if allowed to accumulate in the cytoplasm105. Alternatively, the efficient assembly of multi-subunit complexes might require on-site synthesis106. The second hypothesis states that organellar proteins that are involved in redox reactions are encoded in the organelles to enable a rapid regulatory response to changes in the redox state of an organelle103. This allows a single organelle to rapidly change the concentration of a particular protein without having to send a signal to the nucleus, which, in a cell with multiple chloroplasts and/or mitochondria, would have no way of responding only to that individual organelle. This hypothesis is supported, for example, by the transcriptional regulation of chloroplast photosystem protein-encoding genes by the redox state of the chloroplast in mustard plants107. However, neither of these two hypotheses explains the retention of chloroplast genomes in non-photosynthetic organisms; other reasons must therefore exist for the incomplete transfer of organelle genes101.
Having organelle proteins encoded on two or more separate and compartmentalized genomes requires coordinated expression to produce the correct concentrations of organelle proteins and to maintain organelle function. These genome-coordinating mechanisms include both anterograde (nucleus to organelle) and retrograde (organelle to nucleus) signals (FIG. 1). Anterograde mechanisms coordinate gene expression in organelles in response to endogenous and environmental stimuli that are perceived by the nucleus. Retrograde mechanisms transmit signals that originate in the organelles to regulate nuclear gene expression, which can then alter anterograde control. In photosynthetic organisms this regulation is even more complex owing to cross-talk between mitochondria and chloroplasts. Tight coordination between the nucleus and organelles is crucial to the survival of eukaryotic cells: not only are chloroplasts and mitochondria of great bioenergetic importance, but they synthesize many different cellular metabolites, including amino acids, lipids, nucleotides, vitamins and porphyrins.
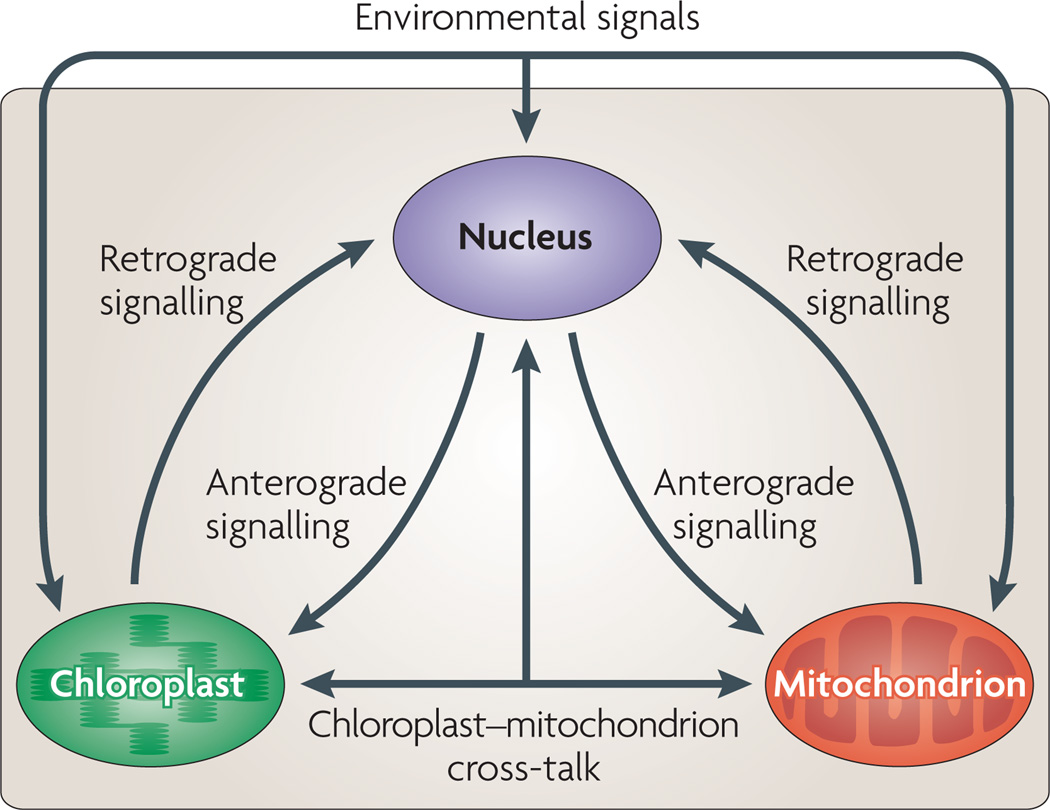
Figure 1. An overview of genome co-ordination between the nucleus and intracellular organelles.
The diagram depicts communication between the nucleus, chloroplast and mitochondrion. Details of anterograde signalling and retrograde signalling between the nucleus and the organelles, and of chloroplast–mitochondrion cross-talk are discussed in the main text. Environmental signals such as stress, oxygen or nutrient availability, light intensity or quality, developmental cues, and hormones affect the expression of nuclear genes that encode organellar proteins. This process will, in turn, affect organelle function and gene expression through anterograde mechanisms. Chloroplasts and mitochondria are also able to sense certain environmental conditions and stimuli that can affect their functional activities, for example, light intensity or quality (chloroplasts) and O2 availability (mitochondria). Using retrograde signals, organelles communicate these received stimuli and their functional status to the nucleus, which leads to nuclear gene regulation.
This Review summarizes recent progress on anterograde and retrograde signalling mechanisms between mitochondria or chloroplasts and the nucleus, with an emphasis on chloroplast-to-nucleus communication. During the past few years, there have been significant advances in uncovering specific signals from chloroplasts and their mechanisms of action. The availability of genome sequences has accelerated forward genetic analysis and allowed for the use of reverse genetics techniques to uncover signalling mechanisms and their protein components in model organisms such as the green alga Chlamydomonas reinhardtii and the flowering plant Arabidopsis thaliana. In addition, both transcriptomics and proteomics have provided insight into the effects that retrograde and anterograde signals have on nuclear and organellar gene expression, respectively. As well as being a fundamental feature of eukaryotic cell physiology, correct coordination of activities between the nucleus and organelles has recently been implicated in fertility in plants7, ageing in yeast8 and tumorigenesis in mammals9. Thus, it is becoming clear that retrograde and anterograde signalling mechanisms have broad roles within the cell and diverse roles between eukaryotic kingdoms.
Challenges associated with separate genomes
The presence of organelles that contain their own genomes presents the cell with several challenges for regulating gene expression and for controlling organelle protein levels. An individual cell can contain multiple organelles, each containing several copies of their genome; as a result there can be up to a 1:5,000 disparity between the copy number of nuclear and organellar genomes in metazoans10 and plants11 that can fluctuate according to cell type, age and growth conditions11. Because many organellar genes encode subunits for protein complexes that are largely encoded in the nucleus (BOX 1), regulation is required to ensure the correct amount of protein is available for assembly. Furthermore, organelles do not encode any known autonomous regulators of gene expression and must rely on the nucleus for such proteins. These types of mechanisms are achieved through anterograde control, as discussed below.
In addition to developmental changes, organelles experience shifting environmental conditions, including rapid redox changes, oxidative damage and changes in nutrient availability. For the most part, they are not genetically equipped to manage these changes because the relevant genes are in the nucleus. Organelles must therefore be able to emit different signals that the nucleus can interpret and respond to by altering gene expression. This type of regulatory mechanism has been achieved through retrograde signalling, which is discussed in a later section.
Anterograde signalling
By and large, the nucleus and nuclear-encoded proteins control organelle function and proteome composition. The concentrations of many nuclear-encoded organelle proteins are efficiently regulated by nuclear transcription12. In other cases, nuclear-encoded proteins regulate post-translational events such as protein transport, the assembly of large multi-subunit complexes (that is, photosynthetic and respiratory complexes) and the regulation of enzyme activities (for example, tetrapyrrole biosynthesis)13,14. Organelle development, division and, in plastids, differentiation into chloroplasts, amyloplasts and chromoplasts15 is also largely, if not completely, dependent on nuclear-encoded proteins. Nuclear-encoded proteins also primarily control organelle gene expression. Because nuclear control of organelle function is such a broad subject, this Review only covers how it pertains to the coordination of nuclear and organelle genomes, and is limited to the anterograde mechanisms that control organelle gene expression (FIG. 2).
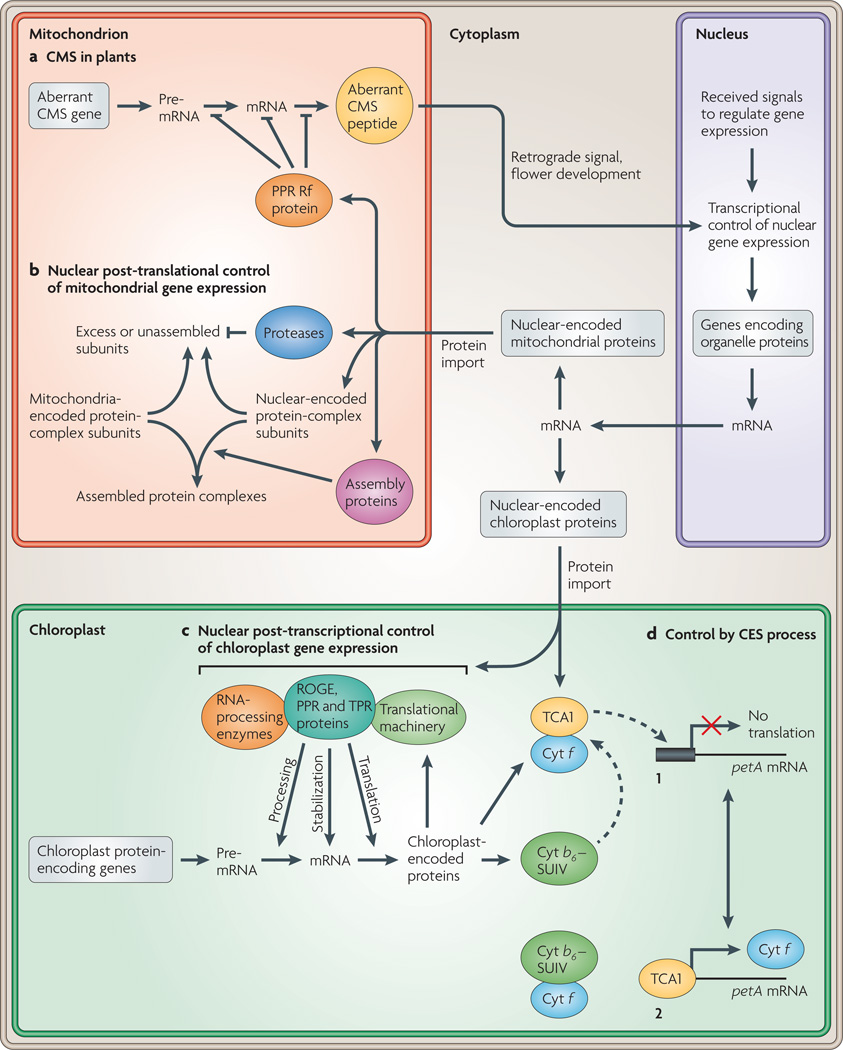
Figure 2. Nuclear anterograde control of organelle gene expression.
A generalized model of the coordination of organelle gene expression through nuclear anterograde control in eukaryotes. Several processes are highlighted. a | In flowering plants, restorers of fertility (Rf) proteins reverse the cytoplasmic male sterility (CMS) phenotype by reducing the expression of the aberrant mitochondrial gene using post-transcriptional mechanisms, b | Post-translational control of mitochondrial gene expression using nuclear-encoded proteases and assembly proteins, c | Post-transcriptional control of chloroplast gene expression using nuclear-encoded regulators of organelle gene expression (ROGE) proteins that target specific RNA transcripts, d | Control by epistasy of synthesis (CES) is an autoregulatory process in which unassembled organelle proteins repress their own translation. The autoregulation of the petA gene encoding the cytochrome f subunit (Cyt f) of the cytochrome b6f complex in Chlamydomonas reinhardtii is used as an example of the CES process. In scenario 1, unassembled Cyt f binds the ROGE protein translation factor TCA1, and translation of the petA mRNA is inhibited. In scenario 2, the presence of cognate protein subunits cytochrome b6 (Cyt b6) and cytochrome subunit IV (SUIV) assemble with Cyt f. TCA1 is now able to bind petA mRNA and activate its translation. A discussion of which proteins are organelle encoded can be found in BOX 1. A more complete model of retrograde signalling than that highlighted in panel a can be viewed in FIG. 3. Proteins and protein complexes are designated as ovals. PPR pentatricopeptide repeat; TPR, tetratricopeptide repeat.
Transcriptional regulation
The regulation of organelle gene expression mostly occurs through post-transcriptional mechanisms and involves nuclear-encoded proteins. However, some degree of transcriptional regulation does occur in both mitochondria and chloroplasts16–19, but all known organelle transcription factors are nuclearencoded proteins in yeast (mtTFB), mammals (TFB1M, TFB2M and the termination factor family20) and plants21, with the exception of the plastid-encoded RNA polymerase (PEP) in plant chloroplasts.
Mitochondria use a nuclear-encoded RNA polymerase (NEP), chloroplasts use PEP, and chloroplasts of higher plants use both. The reason for having two polymerases is unclear as most chloroplast genes have promoters that are recognized by both enzymes22. In general, PEP transcribes photosynthesis-related genes and it has been proposed that chloroplast development in higher plants proceeds by switching from NEP- to PEP-dominated transcription22 (possibly through the inhibition of NEP activity by PEP-transcribed tRNAGlu (REF. 23)), which, along with post-transcriptional mechanisms18, leads to differential transcript accumulation during primary leaf and chloroplast development24. This switch, which is probably used for large developmental switches rather than subtle regulatory changes17, also leads to a general increase of transcription, perhaps as a mechanism to increase photosynthesis-related protein accumulation24.
Furthermore, nuclear-encoded sigma factors (A. thaliana encodes six, SIG1–SIG6) regulate PEP activity to express different sets of genes25. Again, these mechanisms of nuclear control appear to be used more on the developmental scale, as different sigma factors are important during different stages of A. thaliana growth25. However, SIG5 does appear to be involved in the response to blue light26 and stress27, which might allow the nucleus to relay a specific stimulus to the chloroplast through transcriptional control.
Although transcriptional control and activities vary in organelles, they do not correlate much with protein levels in yeast28 or plant mitochondria29 or chloroplasts30,31. Transcript levels might increase in some situations31,32 to ‘prime’ the organelle, but ultimately the post-transcriptional and post-translational mechanisms that are controlled by the nucleus determine the protein levels.
Post-transcriptional regulatory proteins
Most anterograde control of organellar gene expression is post-transcriptional13,17,19. The processes that are involved, which include transcript maturation (for example, splicing, processing and editing) and translation, are controlled by nuclear-encoded proteins13,17,33. Genetic analysis of mutants defective in organellar functions has revealed many nuclear-encoded post-transcriptional regulators of organelle gene expression (ROGEs). Often, ROGEs are dedicated to the proper expression of only one specific organellar gene. ROGEs fall into two principal classes of regulation: those that are required for the proper maturation and/or stabilization of organellar transcripts33,34 and those that affect the translation (translational initiation, elongation) of organellar transcripts13,33 (FIG. 2c). It is likely that ROGE proteins act as adaptors; they bind specific mRNA transcripts and recruit translation machinery or RNA metabolism enzymes. In most, if not all, cases, ROGE proteins are either pioneer proteins that are not conserved among eukaryotes, or they belong to protein families that are identified only by their sequence motifs that are involved in protein–protein interactions (tetratricopeptide repeat motifs)35 or protein–RNA interactions (pentatricopeptide repeat (PPR) motifs)34,36 (BOX 2; Supplementary information S1 (table)).
BOX 2. Pentatricopeptide-repeat proteins.
Genome sequencing has revealed the existence of large families of proteins, the functions of which have not been assigned or validated experimentally. One such large group of proteins, the pentatricopeptide repeat (PPR)-containing proteins, are found in most eukaryotes and are important in organelle communication and development34.
The PPR motif is a degenerate 35-amino-acid sequence similar to the tetratricopeptide repeat (TPR) motif that is involved in protein-protein interactions36. Like TPR motifs, PPR motifs are predicted to form a pair of anti-parallel a helices108. It is suggested that tandem arrays of these motifs form a superhelical structure containing a groove that is lined with positively charged residues that allows for binding to nucleic acids in a sequence-specific manner36. Whereas all known PPR proteins are nuclear-encoded, most are predicted to be targeted to chloroplasts or mitochondria, and most of the ~30 genetically characterized PPR proteins are proposed to affect post-transcriptional gene regulation in organelles by interacting with one specific RNA or a small set of RNA molecules (FIG. 2c; Supplementary information S1 (table)). Studies in fungi, plants, protists, insects and humans have strongly implicated PPR proteins in processes such as RNA editing109,110, transcript processing (splicing)111–113, transcript stabilization33,39,112,113, transcript translation111,114 or some combination of these functions111,114. Although several PPR proteins bind RNA in vitro110,115, they have not been shown to catalyse any RNA processing. Instead, PPR proteins might act as adaptors, recognizing specific RNA molecules and facilitating interactions with processing enzymes.
In plants, PPR proteins have a larger role in organelle communication than in other eukaryotes. Whereas animal genomes encode fewer than ten PPR proteins, plant genomes encode hundreds116. Most plant PPR proteins are predicted to be localized to mitochondria, indicating that the presence of chloroplasts cannot fully explain the reason that plants have so many PPR proteins116. Most of the cytoplasmic male sterility (CMS) restorers of fertility (Rf) genes encode PPR proteins71,13,116 (FIG. 2a; Supplementary information S1 (table)), suggesting that CMS increases any selective pressure for PPR diversity in plants (BOX 4).
Although redundancy is common in large gene families in plants117, no redundancy in PPR proteins has been demonstrated. In fact, many PPR loss-of-function mutants have strong and/or lethal phenotypes, suggesting they have important and non-redundant roles in plant organelle physiology116. The severe phenotypes caused by some PPR loss-of-function mutants might be due to pleiotropic effects that are caused by the loss of a specific organelle gene product. Alternatively, PPR proteins might have direct roles in plant development and signalling, perhaps by regulating developmentally significant RNAs or by being involved in an unidentified developmental process. This interpretation is supported by the PPR protein GENOMES UNCOUPLED 1 (GUN1), which functions in many retrograde signalling pathways in chloroplasts62. Whichever is the case, the study of PPR proteins should see some fascinating developments into the understanding of this large group of proteins and their involvement in genome coordination.
Although most of the research on ROGE proteins has suggested that they might enable the nucleus to control organelle gene expression, our understanding of their physiological roles is limited. Some ROGE proteins might be constantly present in organelles to control the assembly-dependent regulatory process named control by epistasy of synthesis (FIG. 2d). This mechanism, for which several cases have been reported33,37,38, involves negative feedback by the unassembled protein on the translation of its own mRNA. In the best-studied example, in C. reinhardtii chloroplasts, the ROGE protein translation factor TCA1 (a non-conserved pioneer protein required for petA translation)33 binds the exposed C-terminus of unassembled cytochrome f. When petA-encoded cytochrome f is assembled with the cytochrome b6–cytochrome subunit IV complex, its C-terminus is unexposed and TCA1 is able to activate translation of petA mRNA.
The nuclear-controlled fluctuation of ROGE protein levels in response to environmental stimuli can directly regulate protein abundance in organelles. In C. reinhardtii chloroplasts, expression of the petA gene is controlled by the abundance of TCA1 (REF. 33) and another ROGE protein: RNA stability factor MCA1, a PPR protein required for the stable accumulation of the petA transcript39. Nitrogen starvation conditions led to the nuclear downregulation of both MCA1 and TCA1, resulting in a decreased accumulation of petA transcripts and its protein product.
A similar situation exists in Saccharomyces cerevisiae. Here, translation of the mitochondrial-encoded cytochrome c oxidase subunit III gene (coxIII) is regulated by Pet494 (REF. 28), a nuclear-encoded ROGE pioneer protein, the accumulation of which is regulated by the nucleus in response to oxygen availability and type of carbon source40. The availability of ROGE proteins for organelle gene expression can be regulated by other mechanisms that are controlled by the state of the organelle. For example, the translation of photosystem II protein D1 (PsbA) in C. reinhardtii chloroplasts is regulated by an already present ROGE protein that responds to the redox state of the organelle37.
It is unclear why so many organelle-encoded proteins are controlled by separate nuclear-encoded proteins (many, if not most, of the respiratory and photosynthesis complex proteins in S. cerevisiae and C. reinhardtii, respectively, are regulated by ROGE proteins 13,38), but it might indicate that intricate control of multi-subunit complexes is necessary for protein assembly or function. The lack of conservation among the ROGE proteins (most of the proteins that are involved in the regulation of yeast mitochondrial cytochrome c oxidase (COX) genes are absent in higher eukaryotes13) and the extra reliance on PPR proteins in plants (BOX 2) suggests that this type of regulation is evolving to suit the specific needs of a particular organelle in a particular species. This is surprising considering the sequence conservation of organellar genes, and it might reflect the differences in organellar transcriptional and translational mechanisms among species. For instance, most of the translational activators of the mitochondrial COX genes in yeast recognize the 5′ UTR13. Mitochondrial transcripts in humans lack 5′ UTRs and the existence of specific translational activators has not been confirmed13.
Post-translational regulation in mitochondria
Studies in both plant41 and yeast42 mitochondria have suggested that the rapid degradation of the excess number of unassembled mitochondrial-encoded proteins and the regulation of multi-subunit protein assembly are conserved mechanisms of post-translational regulation in these and other organelles. Given that there is little evidence of translational regulation in plant mitochondria17, such an anterograde mechanism could explain how the nucleus coordinates gene expression in these organelles (FIG. 2b).
It was recently shown that sucrose-starved A. thaliana cells had a decreased level of mitochondrial ribosomes and respiration protein complexes: nuclear (not mitochondrial) transcripts for these protein complexes became limiting29. The levels of mitochondrial-encoded proteins and their assembly with nuclear-encoded proteins must be adjusted accordingly by post-translational mechanisms to control the correct concentrations of mitochondria-encoded protein complexes compared with nuclear-encoded protein complexes. It is expected that under sugar-starvation conditions, protein assembly is regulated by specific nuclearencoded chaperones, and that excess unassembled mitochondrial subunits are degraded by specific nuclear-encoded proteases.
In yeast, mitochondrial transcripts and protein levels fluctuate during glucose repression and derepression, but protein levels can be controlled, at least in part, by limiting the amount of ROGE proteins13. Therefore, as is the case in plants, the modulation of yeast respiration according to sugar availability is under the control of the nucleus and uses a mitochondrial system that is limited by nuclear-encoded proteins28,32.
There is no universal mode of anterograde control of organellar gene expression, but certain themes have emerged. Organelle genome copy number fluctuates but it contributes little to gene expression18,43, whereas transcriptional regulation with nuclear-encoded transcription factors leads to global rather than subtle changes in transcript accumulation. In general, however, post-transcriptional mechanisms dominate by using specific ROGE proteins to regulate gene expression, by the turnover of excess unassembled proteins and by the control of protein assembly. Nuclear control of organelle function can therefore be separated from any fluctuation in genome copy number and in mRNA transcript abundance that is caused by variable growth rates or nutrient availability30.
Retrograde signalling
Retrograde mechanisms have evolved to communicate the functional and developmental state of organelles to the nucleus, which can then modulate anterograde control and cellular metabolism accordingly (FIG. 3). These signals are used for genome coordination, instructing adaptive responses to fluctuating or stressful environmental conditions, or to inform the nucleus of cellular stress. Although retrograde pathways appear to be used by virtually all eukaryotic organisms, there is little conservation between these signals as they vary according to organelle type, species and purpose. Like anterograde signalling, this might indicate that the relationship between organelles and the nucleus is continually evolving to suit an organism’s needs.
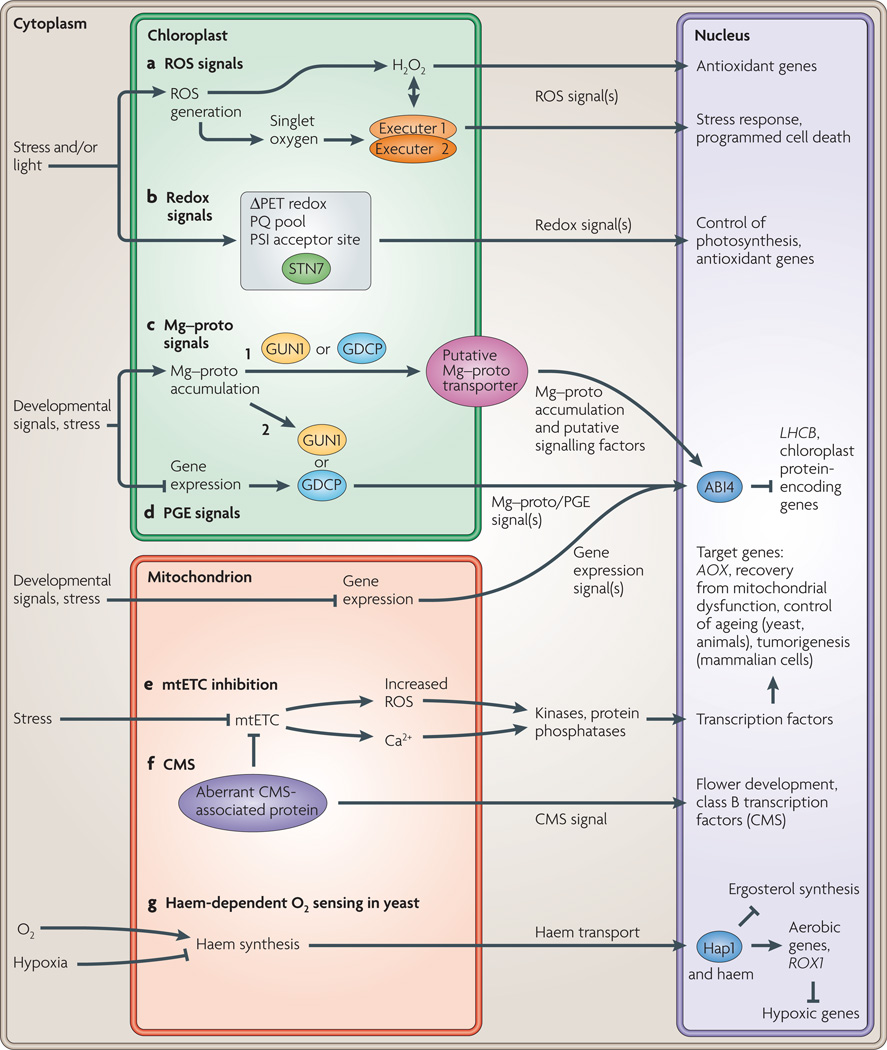
Figure 3. Retrograde signalling pathways and chloroplast-mitochondrion cross-talk in higher plant cells.
This figure depicts chloroplast-to-nucleus and mitochondrion-to-nucleus retrograde signalling pathways in the higher plant cell (and in yeast and animals where noted). Seven different pathways are highlighted, a | The use of chloroplast-generated reactive oxygen species (ROS) to induce nuclear gene transcription, b | Control of nuclear gene regulation by the redox state of the photosynthetic electron transport chain (PET), c | Chloroplast Mg–protoporphyrin IX (Mg–proto) accumulation, d | Inhibition of plastid gene expression (PGE). c and d lead to the repression of nuclear-encoded chloroplast protein genes. Signals from inhibited mitochondrial gene expression act synergistically with the PGE pathway. Two putative Mg–proto signalling pathways are depicted: in pathway 1, GENOMES UNCOUPLED 1 (GUN1) or a putative GUN1-dependent chloroplast protein (GDCP) facilitate the export of Mg–proto from the chloroplast where it interacts with cytoplasmic signalling factors; in pathway 2, GUN1 or GDCP sense Mg–proto accumulation and other retrograde signals within the chloroplast and send an unidentified signal to the nucleus to control transcription of chloroplast protein-encoding genes, e | Mitochondrial electron transport chain (mtETC) dysfunction leads to transcriptional changes in the nucleus in several phyla, f | An aberrant mitochondrial protein leads to cytoplasmic male sterility (CMS) by affecting nuclear gene expression, g | Mitochondrial haem synthesis as a cellular sensor for O2 availability in yeast. Proteins that are known to be involved in these pathways are designated as ovals. ABI4, abscisic acid insensitive 4; AOX, gene encoding the mitochondrial alternative oxidase; HAP1, haem activation protein; LHCB, gene encoding photosystem II chlorophyll a/b-binding protein; PQ, plastoquinone; PSI, photosystem I; ROX1, repressor of hypoxic genes; STN7, a thylakoid protein kinase.
Mitochondrial retrograde signalling: yeast and plants
The physiological state of mitochondria can reflect their degree of energy production or O2 availability, or levels of reactive oxygen species (ROS). These metabolites allow mitochondria to inform the nucleus of these fluctuating conditions, and induce adaptive measures. Not surprisingly, nuclear genes that are related to respiration, peroxisomal biogenesis and oxidative-stress responses are regulated at the transcriptional level in response to the physiological state of mitochondria8,9,44. Mitochondrial retrograde signalling might also be involved in other processes, including ageing and tumorigenesis in yeast and animals, respectively; (BOX 3).
BOX 3. Retrograde signalling: yeast, mammals and tumorigenesis.
The budding yeast, Saccharomyces cerevisiae, is an ideal system for understanding mitochondrial retrograde signalling; as yeast do not require mitochondria to ferment sugars it is possible to propagate cells with severe mitochondrial defects. Most of the work on the retrograde pathway has focused on the induction of the nuclear-encoded peroxisomal citrate synthase (Cit2) as an adaptive response to reduced respiration caused by mitochondrial dysfunction8,9. In addition to increasing Cit2, which allows yeast to use acetate and ethanol as sole carbon and energy sources, the retrograde pathway induces α-ketoglutarate production in mitochondria to ensure glutamate pools meet the demand for nitrogen8,9. It is unclear whether these signals are conserved in other phyla, because the regulators of these pathways have been identified only in yeast8.
These signals have also been implicated with ageing in yeast: the loss of mitochondrial DNA (mtDNA) extends the life span of some strains and is dependent on the retrograde positive regulator Rtg2 (REF. 118). Rtg2 might reduce the formation of extrachromosomal ribosomal DNA circles, which decrease the life span of yeast119. Mutations that affect ubiquinone biosyntheses, and thus mitochondrial function, increase the life spans of both Caenorhabditis elegans120 and mice121, suggesting a conserved role of retrograde signalling and ageing across kingdoms. Although the decrease of reactive oxygen species might be involved, it is unclear how mitochondrial retrograde signals influence ageing in any species.
In mammalian cells, mitochondrial dysfunction also leads to retrograde signals that influence nuclear transcription. The signal involves increased Ca2+ levels in the cytoplasm, possibly owing to a change in the mitochondrial membrane potential or a reduced efflux of Ca2+ into storage organelles that is due to the decrease in ATP production9. The subsequent activation of cytosolic calcineurin and Ca2+-dependent kinases might then start a signalling cascade that induces transcription factors including nuclear factor-kappa B, nuclear factor of activated T-cells and activating transcription factor9,122.
Recently, a link between retrograde signalling and tumorigenesis has been suggested. Inducing the retrograde response by depleting mitochondria of their DNA, or by using inhibitors to block respiration, increases the invasive behaviour of otherwise non-tumorigenic rhabdomyoblasts123, of human lung carcinomas123,124 and of human osteosarcomas125, possibly by increasing Ca2+ levels followed by induction of calcineurin and Ca2+-dependent kinases123,124. Furthermore, retrograde signals that are elicited in response to mitochondrial dysfunction increase the resistance to apoptosis and the levels of anti-apoptotic proteins122,126, and reduce the levels of pro-apoptotic proteins126. Proteomic analyses of mtDNA-depleted rat myocyte127 and human osteosarcoma125 cells have detected altered levels of cell-cycle and apoptosis-related proteins, which is often observed in tumour cells. Because resistance to apoptosis is a hallmark of cancer cells at advanced stages of tumour progression, future work in this field should determine whether mitochondrial dysfunction and the activation of retrograde signalling are contributing factors in cancer.
Organelle retrograde signalling was first studied in S. cerevisiae mitochondria, where work has focused on the retrograde signals resulting from mitochondrial dysfunction (BOX 3) and the haem signalling pathway. Because haem biosynthesis in the mitochondrion is oxygen dependent, cellular haem concentrations can reflect oxygen availability45. By interacting directly with the haem activator protein (Hap1) in the nucleus, haem switches the cell between aerobic and anaerobic growth through transcriptional regulation46 (FIG. 3g). Under aerobic conditions, haem is available to bind Hap1, which then activates the transcription of many aerobic genes, including those required for respiration and controlling oxidative damage, and the repressor of hypoxic genes (Rox1)45,47 by direct DNA binding. Under low-oxygen conditions, when haem is not synthesized, Hap1 acts as a repressor and represses at least three genes involved in ergosterol biosynthesis48.
It is unclear whether other phyla share this mechanism of oxygen sensing, as Hap1 is not conserved outside of fungi. In mammals and humans, the activity of basic leucine zipper transcription factor 1 (BACH1) is regulated by haem49. Because BACH1 regulates genes that are involved in haem metabolism and the oxidativestress response49, this might be an analogous system to yeast in which haem concentrations inform the nucleus of cellular oxygen concentrations or mitochondrial function. Chloroplasts use structurally related tetrapyrrole molecules of the chlorophyll biosynthetic pathway for chloroplast retrograde signalling; however, it is unknown whether a mechanism common to yeast haem signalling will be used.
In plants, mitochondrial retrograde signalling has been implicated in ROS signalling, O2 sensing, heat shock, pathogen sensing and programmed cell death44. Retrograde signals from dysfunctional mitochondria might also be the cause of at least some types of cytoplasmic male sterility (CMS) in flowering plants (BOX 4). No protein component of any plant mitochondrial retrograde signalling pathway has been identified, although Ca2+ signalling, protein kinases and nuclear transcription factors are predicted to be involved. The best-studied retrograde signal(s) in plant mitochondria involve increased expression of the nuclear-encoded alternative oxidase (AOX) as an adaptive response to recover from mitochondrial electron transport chain (mtETC) inhibition44 (FIG. 3e). AOX reduces the production of ROS50, and A. thaliana mitochondrial retrograde regulation deficient (mrrd) mutants (the identities of which are unknown) that are unable to induce AOX after mtETC inhibition exhibit ROS-like cellular damage51. There might be several signals for AOX induction as these mutants do not all respond equally to different mechanisms of mtETC inhibition51, but these separate signals appear to lead to different elements within the same 93 bp promoter region of AOX52.
BOX 4. Cytoplasmic male sterility in plants.
In plants, cytoplasmic male sterility (CMS) is a maternally inherited phenotype that is characterized by the inability to produce fertile pollen7,128. CMS has been characterized in over 150 plant species and is associated with aberrant mitochondrial genes (hence the maternal inheritance). The ~14 characterized mitochondrial genes that determine CMS are chimeric ORFs that often encode part of the ATP synthase gene fused to unique sequences. These fused genes are usually gain-of-function alleles that can lead to mitochondrial dysfunction, without altering the general phenotype of the plant. Instead, CMS lines are usually specifically defective in producing fertile pollen — which can be caused by abnormal pollen development, degeneration of male reproductive organs (stamens) or their components, or homeotic changes (stamens converted to non-reproductive petals or other organs)7,128.
The exact causes of CMS are unknown, but in cases in which there are homeotic changes, retrograde signals from dysfunctional mitochondria might lead to transcriptional changes in nuclear genes that control organ identity and patterning128 (FIG. 3f). Recent studies have associated CMS phenotypes with decreased expression of nuclear genes that encode class-B function MADS-box transcription factors (that is, APETALA3, GLOBOSA, DEFICIENS) that control flower morphology7,128,130. This could be caused by a reduction of mitochondrial ATP that prevents the proteolysis of negative regulators of class-B gene expression, or by an unidentified mechanism129. Other types of CMS might also be caused by mitochondrial retrograde signals. A microarray analysis of a CMS line of rice that produces morphologically normal but infertile pollen revealed ~140 differentially regulated nuclear genes in mature anther cells, but not in leaf, root or stem cells when compared with fertile lines44,131.
CMS phenotypes can be reversed by nuclear restorers of fertility (Rf) genes that reduce the accumulation of the aberrant mitochondrial CMS protein7,34 (FIG. 2A). The one exception is Rf2, from Texas-type CMS maize, that encodes a mitochondrial-localized aldehyde dehydrogenase and restores fertility by an unknown mechanism132. All other cloned Rf genes encode mitochondrial-localized pentatricopeptide repeat (PPR) proteins (BOX 2, Supplementary information S1 (table)), most of which act dominantly. There is no conserved mechanism to reduce production of the CMS protein, but PPR proteins probably act post-transcriptionally on CMS mRNA transcripts by promoting endonucleolytic cleavage113,133, editing113 and destabilization113,134, or by reducing translation113,134 (Supplementary information S1 (table)).
In some circumstances, CMS can be advantageous as it allows female plants to avoid investing resources in pollen production and might also lead to reactive oxygen species resistance owing to retrograde-signalled stress responses. The diversity in Rf alleles, however, suggests that there is strong selective pressure to restore fertility, and this interaction between the two genomes has been dubbed the ‘intragenomic arms race’135. This cross-talk between the mitochondria and nucleus in CMS lines should open opportunities to understand both anterograde and retrograde signalling in plants.
Signals from chloroplasts
The first evidence that signals from chloroplasts regulate nuclear gene expression came from studies on albostrians mutants of barley, which contain undifferentiated plastids that lack ribosomes53. Cells with these albino plastids did not accumulate nuclear-encoded photosynthetic mRNA transcripts54,55. This retrograde signal from chloroplasts, originally termed ‘the plastid factor’56, alters the transcriptional regulation of nuclear genes and is quite complex. Although no chloroplast retrograde pathway is well-understood mechanistically, several signals have been reported to trigger retrograde signalling from chloroplasts, including accumulation of Mg–protoporphyrin IX (Mg–proto, the first unique intermediate of chlorophyll biosynthesis), redox signalling, and signals that are generated by inhibiting plastid gene expression or by accumulating various ROS.
The best-characterized chloroplast retrograde signalling pathway involves tetrapyrrole intermediates of the chlorophyll biosynthetic pathway. In C. reinhardtii, Mg–proto and its methyl ester, Mg–proto–Me, can substitute light to induce the transcription of at least four nuclear genes: HEMA57 (the gene encoding glutamyl-tRNA reductase, which catalyses the first step of tetrapy-rrole biosynthesis in plants) and three heat shock protein 70 genes58. Because these chlorophyll intermediates are chloroplast specific, using them as retrograde signals — instead of haem (as used in yeast), which can be synthesized in both plant chloroplasts and mitochondria59 — informs the nucleus as to their origin.
Higher plants use Mg–proto as a chloroplast signalling molecule, but in a different way. In A. thaliana, undeveloped chloroplasts that have suffered photo-oxidative damage, owing to the lack of protective carotenoids, accumulate Mg–proto, which leads to the repression of nuclear genes that encode photosynthesisrelated proteins60 (FIG. 3c). Mutants that block this retrograde signalling have been isolated (genomes uncoupled or gun mutants61), and all but gun1 (REF. 62) mutants (GUN1 encodes a chloroplast-localized PPR protein62, see BOX 2; Supplementary information S1 (table)) interfere with chlorophyll metabolism and affect Mg–proto accumulation60,63,64. This role for Mg–proto is also corroborated by work with barley Mg–proto biosynthetic mutants65, transgenic tobacco lines with altered expression of the Mg–proto methyl transferase gene (CHLM)66, and A. thaliana CHLM knockout lines67.
Work in tobacco has suggested that Mg–proto can have the opposite effect on nuclear gene expression. Transgenic plants with lowered levels of Mg–proto synthesis have repressed nuclear photosynthetic genes68. Unlike the work with barley and A. thaliana, these studies were performed using adult plants with relatively developed chloroplasts. This might mean that the Mg–proto signal in higher plants as described above is used to coordinate genome expression in young seedlings during organelle development or during severe stress conditions, as opposed to throughout development under normal conditions60. In developed chloroplasts, the accumulation of chlorophyll intermediates might have different effects (for example, to modulate levels of photosynthetic machinery in response to available chlorophyll).
Using in vivo visualization techniques, Mg–proto has been reported to exit the cytoplasm, where presumably it interacts with unidentified signalling factors69 (FIG. 3c). This mechanism would probably require active transport of Mg–proto, although no transporters have been identified. Because the Mg–proto signal is dependent on the expression of GUN1 (REF. 62), GUN1 or a GUN1-dependent chloroplast protein (discussed below) might be necessary for this export. However, these studies did not analyse Mg–proto export in gun1 mutant cells, nor have they demonstrated that Mg–proto export is required for nuclear gene repression. Alternatively, as discussed below, the Mg–proto signal might converge with other retrograde pathways inside the nucleus in a GUN 1-dependent fashion and involve an unidentified signal that exits the chloroplast (FIG. 3c).
Different cis-acting elements in the promoters of nuclear genes have been identified in C. reinhardtii (the plastid response element70) and A. thaliana (the CUF1 element of the G-box60) that respond to the Mg–proto-induced signal(s). Both elements are also used for light signalling in these organisms and, at least in C. reinhardtii, Mg–proto might be mediating the light activation of genes harbouring these elements70. In higher plants, the repressive effect of Mg–proto suggests that it antagonizes light activation, possibly by activating a transcriptional repressor that recognizes proximal or overlapping sequences of the CUF1 element (that is, abscisic acid insensitive 4 (AB14), see below)71. Because not all Mg–protoresponsive nuclear promoters contain the CUF1 element, other cis-acting factors are probably involved in receiving the Mg–proto signal in A. thaliana.
A second retrograde signalling pathway (called the plastid gene expression (PGE) pathway) was discovered using inhibitors of chloroplast translation, which resulted in the repression of nuclear photosynthesis genes72. This pathway seems to be used to coordinate genomes during organelle development, as it is only active in young seedlings. In A. thaliana, the nuclear mutations prpl11 (chloroplast ribosome large subunit 11 (REF. 73)) and prors1 (chloroplast and mitochondrial prolyl-tRNA synthetase 1 (REF. 74)) negatively affect translation in the chloroplast and also lead to similar light-independent repressive effects on nuclear genes. The repression of nuclear genes in prpl11 mutant plants was dependent on an additional mutation, mrpl11 (mitochondrial ribosomal protein L11), impairing mitochondrial translation, suggesting that both organelles send retrograde signals when protein synthesis is impaired, and that their signals are synergistic74 (FIG. 3d).
Of the A. thaliana gun mutants mentioned above, only gun1 affects the PGE pathway. GUN1 functions downstream of Mg–proto accumulation, as concluded by a recent microarray experiment (covering ~75% of A. thaliana genes) that showed that gun1 and gun5 mutants (the latter being defective in Mg–proto synthesis) misexpress similar sets of genes in cells with photobleached plastids62. This evidence supports a model in which Mg–proto and PGE signalling pathways converge within the chloroplast and that GUN1 is involved after the convergence point (FIG. 3c,d). Because Mg–proto accumulation does not affect the PGE signalling pathway62, the converged retrograde signal probably does not involve the export of Mg–proto from the chloroplast. As such, this model could be in conflict with the model of Mg–proto export from the chloroplast69.
The nuclear-localized AP2-type transcription factor ABI4, which was originally characterized for its involvement in hormone (abscisic acid) signalling, also acts downstream of both Mg–proto and PGE signalling, where it can bind a CCAC motif (often found within close proximity or overlapping CUF1 elements) upstream of light-responsive genes and repress transcription62. A converged pathway would allow the chloroplast to integrate multiple retrograde signals to regulate similar genes in the nucleus.
The biochemical function of the GUN1 protein remains elusive, but its implicated role in other signalling events — such as circadian-clock regulation75, de-etiolation and greening76, and, along with AB14, sugar and redox signalling62 — suggests that it is used to integrate multiple retrograde signalling pathways within the chloroplast. The identity of GUN1 as a PPR protein suggests that it might be required for the expression (or repression) of one specific chloroplast gene, the product of which (GUN 1-dependent chloroplast protein) might be a plastid factor that interacts with Mg–proto and that is involved with multiple retrograde signals (FIG. 3c).
Reactive oxygen species as signals
Exposure to both biotic and abiotic stresses can lead to the increased accumulation of ROS, which cause irreversible oxidative damage to cells. ROS that are generated in chloroplasts, or the damage that they cause, act as retrograde signals to inform the nucleus to increase antioxidant enzyme production and to adjust the photosynthetic machinery for more efficient light harvesting77,78.
ROS production often involves multiple reactive species such as singlet oxygen (1O2) and H2O2 in multiple locations, but a breakthrough in understanding ROS signalling in chloroplasts came from using the A. thaliana fluorescent in blue light (flu) mutant79, which over-accumulates the photo-excitable chlorophyll precursor, protochlorophyllide, in the dark. A subsequent shift from dark to light leads to the rapid accumulation of 1O2 in the chloroplast, followed by growth arrest and cell death. The nuclear transcriptional response is distinct from that when chloroplast H2O2 accumulates because of the application of the herbicide paraquat, which suggests that separate retrograde signalling pathways respond to 1O2 and H2O2 accumulation in the chloroplast80 (FIG. 3a). 1O2 has a shorter half-life than H2O2, but is now suggested to be able to travel over short distances (250 µM in vivo) and across membranes81, which introduces the possibility of 1O2 acting outside of the chloroplast. Nonetheless, a mutational suppressor screen identified the chloroplast-localized protein EXECUTER 1 as being involved in the 1O2 signal82 and mutations in EXECUTER 1 allow flu mutants to be resistant to 1O2 stress. Both growth inhibition and cell death were avoided in the double flu/executer 1 mutant, suggesting that these phenotypes are genetically programmed responses to 1O2 rather than the direct consequence of oxidative damage. 1O2 accumulation in the flu mutant affects ~1,400 nuclear genes, but only ~200 of those encode chloroplast-localized proteins77. Unlike the PGE–GUN signalling pathway, the ROS signalling pathway might be primarily used for stress signalling rather than genome coordination. Further work has shown that 1O2 signalling involves at least one other chloroplast protein, EXECUTER 2 (REF. 77), and that there is an interaction with the H2O2 signalling pathway83 (FIG. 3a).
Redox signals
As light intensity fluctuates, the chloroplast modulates the expression of photosynthesis-related genes to maximize photosynthesis and decrease ROS production. The redox state of the photosynthetic electron transport chain (PET), which is the link between photosynthetic light reactions and metabolism, fluctuates owing to varying light intensity and quality, and has been shown by microarray analyses to exert retrograde control of nuclear gene expression of photosynthetic genes in A. thaliana84. Two main sources of retrograde signals have been proposed that involve assembled PET in developed chloroplasts; the redox state of the plastoquinone pool85 and the photosystem I (PSI) acceptor site86 (FIG. 3b).
Unlike ROS signalling, which is used as a stress response, the redox signal appears to be used to modulate metabolism in response to fluctuating light conditions84. Mechanistically, little is known about the redox retrograde signal(s). However, genetic analyses in A. thaliana have identified components in chloroplast redox signalling including STN7 (REF. 87), a dual-function thylakoid protein kinase that is required for state transitions and photosynthetic acclimation, and five redox imbalanced (rimb) mutants, which have uncoupled transcriptional control of the nuclear-encoded chloroplast antioxidant enzyme 2-cys peroxiredoxin from the redox state of the PSI acceptor site88.
The nuclear response to chloroplast retrograde signals
To understand how the nucleus integrates various retrograde signals from the chloroplast, two studies analysed the accumulation of over 3,000 nuclear gene transcripts of A. thaliana, most of them encoding chloroplast-localized proteins, under 101 different genetic and environmental conditions89,90. This work led to the conclusion that, together, these retrograde signalling pathways result in several layers of transcriptional regulation of nuclear genes (that is, up, down or mixed response). Overall, these chloroplast-protein-encoding genes comprise 23 ‘regulons’ of co-expressed, but physically dispersed, sets of genes90. The existence of a ‘master switch’ was proposed because most of these regulons are predominantly repressed or induced in over half of the conditions. The involvement of GUN 1 and AB14 in several retrograde pathways suggests that they could be part of this regulatory system in plants62.
Mitochondrion-chloroplast cross-talk
In plant cells, chloroplasts and mitochondria have complex metabolic interdependencies91. For instance, photosynthesis can use mitochondrial products (that is, CO2 and ATP) and it provides compounds for mitochondrial respiration (that is, O2 and malate). Mitochondria dissipate redox equivalents from the chloroplast, which protects the chloroplast from photoinhibition. The chloroplast provides haem precursors to the mitochondria92 and metabolic compounds that are involved in photorespiration, such as serine and glycine, are also exchanged through the peroxisome91. However, there is no evidence of direct signalling between chloroplasts and mitochondria, although molecules such as active oxygen species and ascorbate, and mitochondrially produced nitric oxide, have been suggested as candidate signalling molecules91. Additionally, the exchange of redox equivalents could potentially alter organelles’ redox environments and affect gene expression.
The existence of cross-talk signalling mechanisms has mostly been surmised from genetic studies. Mutations affecting glycine decarboxylase93,94 and NADH dehydrogenase95,96 activities in mitochondria led to impaired photorespiration and reduced photosynthesis, respectively. In the other direction, mutations in the C. reinhardtii chloroplast have been shown to act as mitochondrial mutant suppressors, suggesting that tRNA exchange occurs between the organelles97.
Other cases suggest that chloroplast–mitochondrion cross-talk involves a retrograde signal from one of the organelles that modulates the anterograde control of the other. In C. reinhardtii, activation of the cytochrome respiratory pathway in mitochondria leads to an increase in expression of photosynthesis-related genes in the nucleus98. In mutant barley cells that lack chloroplast ribosomes, the nuclear-encoded mitochondrial RNA polymerase is upregulated, resulting in increased mitochondrial transcription99. The redox state of the plastoquinone pool in chloroplasts affects transcription of the mitochondrial RNA polymerase in the nucleus84. The maize non-chromosomal stripe6 (ncs6) mutant has a dysfunctional mtETC that leads to a decrease in both chloroplast- and nuclear-encoded subunits of PSI100. It remains to be determined whether mitochondria are signalling directly to the chloroplast or indirectly to the nucleus to affect anterograde mechanisms. Simultaneous retrograde signals from both organelles (such as the gene expression pathway) might also interact to affect nuclear gene transcription (FIG. 3d), although it remains to be revealed how this or any of these signals are perceived74.
Conclusions
A major challenge to understanding the coordination of nuclear and organelle genomes will be not only to identify proteins that are involved in organelle gene expression, but to determine how their functions or levels are controlled by the nucleus and to place anterograde signalling into a larger context. Because organelle gene expression appears to occur mostly at the post-transcriptional level, the measurement of protein levels rather than transcript levels will be necessary to understand the effect of anterograde control. Our understanding of retrograde signal mechanisms remains limited, in part because the identity of many of the proteins involved is not known, particularly on the ‘receiving side’ in the cytoplasm or the nucleus. This might be due to protein redundancy, to the essentiality of these signalling factors or to overlapping retrograde signals masking the effects of mutations. Innovative genetic or biochemical screens in model organisms should help to identify more of the proteins and signals in these pathways. Other than being a central and important feature of eukaryotic cell physiology, the increasing implication of genome coordination in fertility, ageing and tumorigenesis will produce exciting future research in this field.
Supplementary Material
Acknowledgments
Our work on genome coordination is supported by grants from the US Department of Energy (J. C), and the Howard Hughes Medical Institute (J. C). J. W. is supported by the Ruth L. Kirschstein National Research Service Awards postdoctoral fellowship from the National Institutes of Health. The authors wish to thank S. S. Orchard, G. H. Anderson, A. Nott, and J. M. Pérez-Ruiz for their reading and discussion of the manuscript.
Glossary
- Amyloplasts
A non-pigmented heterotrophic type of plastid that is used for starch storage, mostly found in underground tissues such as roots and tubers. When the plant requires energy amyloplasts can convert the stored starch into usable sugars
- Chromoplasts
Plastids that are dedicated to pigment synthesis and storage. Chromoplast colours vary according to the type of pigment they contain, and they are responsible for the distinctive colours of fruits and of flower petals
- Pioneer protein
A protein that has no known homologue in other species. Therefore, pioneer proteins often lack any identifiable sequence motifs other than those that are used for protein localization
- De-etiolation
In plants, de-etiolation is the irreversible process of shifting from a heterotrophic dark-grown (etiolated) lifestyle to a phototrophic light-grown lifestyle. De-etiolation involves both morphological and physiological changes that are needed to acclimatize to light and to harvest its energy
- Greening
The shift of non-green plant seedlings grown in the dark (etiolated) to a green colour as a result of chloroplast biogenesis and chlorophyll accumulation during the process of de-etiolation
Footnotes
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene EXECUTER 1 | flu | GUN1 | petA | Rox1
UniProtKB: http://ca.expasy.org/sprot ABI4 | BACH1 | Hap1 | MCA1 | TCA1
FURTHER INFORMATION
Joanne Chory’s homepage: http://www.salk.edu/faculty/faculty_details.php?id=12
References
- 1.Dyall SD, Brown MT, Johnson PJ. Ancient invasions: from endosymbionts to organelles. Science. 2004;304:253–257. doi: 10.1126/science.1094884. [DOI] [PubMed] [Google Scholar]
- 2.Conway DJ, et al. Origin of Plasmodium falciparum malaria is traced by mitochondrial DNA. Mol. Biochem. Parasitol. 2000;111:163–171. doi: 10.1016/s0166-6851(00)00313-3. [DOI] [PubMed] [Google Scholar]
- 3.Reith M, Munholland J. A high-resolution gene map of the chloroplast genome of the red alga Porphyra purpurea . Plant Cell. 1993;5:465–475. doi: 10.1105/tpc.5.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson SC, Karlberg O, Canback B, Kurland CG. On the origin of mitochondria: a genomics perspective. Philos. Trans. R. Soc. Land., B, Biol. Sci. 2003;358:165–177. doi: 10.1098/rstb.2002.1193. discussion 177–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richly E, Leister D. An improved prediction of chloroplast proteins reveals diversities and commonalities in the chloroplast proteomes of Arabidopsis and rice. Gene. 2004;329:11–16. doi: 10.1016/j.gene.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Richly E, Chinnery PF, Leister D. Evolutionary diversification of mitochondrial proteomes: implications for human disease. Trends Genet. 2003;19:356–362. doi: 10.1016/S0168-9525(03)00137-9. [DOI] [PubMed] [Google Scholar]
- 7.Chase CD. Cytoplasmic male sterility: a window to the world of plant mitochondrial-nuclear interactions. Trends Genet. 2007;23:81–90. doi: 10.1016/j.tig.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Butow RA. Mitochondrial retrograde signaling. Annu. Rev. Genet. 2006;40:159–185. doi: 10.1146/annurev.genet.40.110405.090613. [DOI] [PubMed] [Google Scholar]
- 9.Butow RA, Avadhani NG. Mitochondrial signaling: the retrograde response. Mol. Cell. 2004;14:1–15. doi: 10.1016/s1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- 10.Cavelier L, Johannisson A, Gyllensten U. Analysis of mtDNA copy number and composition of single mitochondrial particles using flow cytometry and PCR. Exp. Cell Res. 2000;259:79–85. doi: 10.1006/excr.2000.4949. [DOI] [PubMed] [Google Scholar]
- 11.Bendich AJ. Why do chloroplasts and mitochondria contain so many copies of their genome? Bioessays. 1987;6:279–282. doi: 10.1002/bies.950060608. [DOI] [PubMed] [Google Scholar]
- 12.Kleffmann T, et al. The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr. Biol. 2004;14:354–362. doi: 10.1016/j.cub.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 13.Fontanesi F, Soto IC, Horn D, Barrientos A. Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process. Am. J. Physiol. Cell Physiol. 2006;291:C1129–C1147. doi: 10.1152/ajpcell.00233.2006. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka R, Tanaka A. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 2007;58:321–346. doi: 10.1146/annurev.arplant.57.032905.105448. [DOI] [PubMed] [Google Scholar]
- 15.Waters M, Pyke P. In: Plastids. Moller SG, editor. Boca Raton: Blackwell Publishing; 2005. pp. 30–59. [Google Scholar]
- 16.Giege P, Hoffmann M, Binder S, Brennicke A. RNA degradation buffers asymmetries of transcription in Arabidopsis mitochondria. EMBO Rep. 2000;1:164–170. doi: 10.1093/embo-reports/kvd024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leon P, Arroyo A, Mackenzie S. Nuclear control of plastid and mitochondrial development in higher plants. Annu. Rev. Plant Biol. 1998;49:453–480. doi: 10.1146/annurev.arplant.49.1.453. [DOI] [PubMed] [Google Scholar]
- 18.Zoschke R, Liere K, Borner T. From seedling to mature plant: Arabidopsis plastidial genome copy number, RNA accumulation and transcription are differentially regulated during leaf development. Plant J. 2007;50:710–722. doi: 10.1111/j.1365-313X.2007.03084.x. [DOI] [PubMed] [Google Scholar]
- 19.Binder S, Brennicke A. Gene expression in plant mitochondria: transcriptional and post-transcriptional control. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2003;358:181–188. doi: 10.1098/rstb.2002.1179. discussion 188–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asin-Cayuela J, Gustafsson CM. Mitochondria] transcription and its regulation in mammalian cells. Trends Biochem. Sci. 2007;32:111–117. doi: 10.1016/j.tibs.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda TM, Gray MW. Characterization of a DNA-binding protein implicated in transcription in wheat mitochondria. Mol. Cell Biol. 1999;19:8113–8122. doi: 10.1128/mcb.19.12.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maliga P. Two plastid polymerases of higher plants: an evolving story. Trends Plant Sci. 1998;3:4–6. [Google Scholar]
- 23.Hanaoka M, Kanamaru K, Fujiwara M, Takahashi H, Tanaka K. Glutamyl-tRNA mediates a switch in RNA polymerase use during chloroplast biogenesis. EMBO Rep. 2005;6:545–550. doi: 10.1038/sj.embor.7400411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DuBell AN, Mullet JE. Differential transcription of pea chloroplast genes during light-induced leaf development (continuous far-red light activates chloroplast transcription) Plant Physiol. 1995;109:105–112. doi: 10.1104/pp.109.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanamaru K, Tanaka K. Roles of chloroplast RNA polymerase sigma factors in chloroplast development and stress response in higher plants. Biosci. Biotechnol. Biochem. 2004;68:2215–2223. doi: 10.1271/bbb.68.2215. [DOI] [PubMed] [Google Scholar]
- 26.Tsunoyama Y, Morikawa K, Shiina T, Toyoshima Y. Blue light specific and differential expression of a plastid sigma factor, SIG5 in Arabidopsis thaliana . FEBS Lett. 2002;516:225–228. doi: 10.1016/s0014-5793(02)02538-3. [DOI] [PubMed] [Google Scholar]
- 27.Nagashima A, et al. The multiple-stress responsive plastid sigma factor, SIG5, directs activation of the psbD blue light-responsive promoter (BLRP) in Arabidopsis thaliana . Plant Cell Physiol. 2004;45:357–368. doi: 10.1093/pcp/pch050. [DOI] [PubMed] [Google Scholar]
- 28.Steele DF, Butler CA, Fox TD. Expression of a recoded nuclear gene inserted into yeast mitochondrial DNA is limited by mRNA-specific translational activation. Proc. Natl Acad. Sci. USA. 1996;93:5253–5257. doi: 10.1073/pnas.93.11.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giege P, Sweetlove LJ, Cognat V, Leaver CJ. Coordination of nuclear and mitochondrial genome expression during mitochondrial biogenesis in Arabidopsis . Plant Cell. 2005;17:1497–1512. doi: 10.1105/tpc.104.030254.. These authors provide a compelling model for how the expression of mitochondrial and nuclear genomes is coordinated during sugar starvation in A. thaliana cells. Coordination occurs by post-translational mechanisms within the mitochondria and transcriptional changes in the nucleus
- 30.Eberhard S, Drapier D&Wollman, F A. Searching limiting steps in the expression of chloroplast-encoded proteins: relations between gene copy number, transcription, transcript abundance and translation rate in the chloroplast of Chlamydomonas reinhardtii . Plant J. 2002;31:149–160. doi: 10.1046/j.1365-313x.2002.01340.x. [DOI] [PubMed] [Google Scholar]
- 31. Matsuo M, Obokata J. Dual roles of photosynthetic electron transport in photosystem 1 biogenesis: light induction of mRNAs and chromatic regulation at post-mRNA level. Plant Cell Physiol. 2002;43:1189–1197. doi: 10.1093/pcp/pcf146.. These authors provide a model for the regulation by light of PSI in C. reinhardtii chloroplasts: the photosynthetic electron transport chain would control both the accumulation of chloroplast-encoded PSI transcripts and their protein products, but by separate mechanisms
- 32.Ulery TL, Jang SH, Jaehning JA. Glucose repression of yeast mitochondrial transcription: kinetics of derepression and role of nuclear genes. Mol. Cell Biol. 1994;14:1160–1170. doi: 10.1128/mcb.14.2.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raynaud C, et al. Evidence for regulatory function of nucleus-encoded factors on mRNA stabilization and translation in the chloroplast. Proc. Natl Acad. Sci. USA. 2007;104:9093–9098. doi: 10.1073/pnas.0703162104.. Using C. reinhardtii, these authors demonstrate that altered expression of nuclear-encoded ROGE proteins can affect organelle gene expression in vivo
- 34.Saha D, Prasad AM, Srinivasan R. Pentatricopeptide repeat proteins and their emerging roles in plants. Plant Physiol. Biochem. 2007;45:521–534. doi: 10.1016/j.plaphy.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 35.Blatch GL, Lassie M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays. 1999;21:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.Small ID, Peeters N. The PPR motif — a TPR-related motif prevalent in plant organellar proteins. Trends Biochem. Sci. 2000;25:46–47. doi: 10.1016/s0968-0004(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 37.Marin-Navarro J, Manuell AL, Wu JSPM. Chloroplast translation regulation. Photosynth. Res. 2007;94:359–374. doi: 10.1007/s11120-007-9183-z. [DOI] [PubMed] [Google Scholar]
- 38.Wostrikoff K, Girard-Bascou J, Wollman FA, Choquet Y. Biogenesis of PSI involves a cascade of translational autoregulation in the chloroplast of Chlamydomonas . EMBO J. 2004;23:2696–2705. doi: 10.1038/sj.emboj.7600266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lown FJ, Watson AT, Purton S. Chlamydomonas nuclear mutants that fail to assemble respiratory or photosynthetic electron transfer complexes. Biochem. Soc. Trans. 2001;29:452–455. doi: 10.1042/bst0290452. [DOI] [PubMed] [Google Scholar]
- 40.Marykwas DL, Fox TD. Control of the Saccharomyces cerevisiae regulatory gene PET494: transcriptional repression by glucose and translational induction by oxygen. Mol. Cell. Biol. 1989;9:484–491. doi: 10.1128/mcb.9.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu B, Wilson RK, Phreaner CG, Mulligan RM, Hanson MR. Protein polymorphism generated by differential RNA editing of a plant mitochondrial rps12 gene. Mol. Cell Biol. 1996;16:1543–1549. doi: 10.1128/mcb.16.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rep M, Grivell LA. The role of protein degradation in mitochondrial function and biogenesis. Curr. Genet. 1996;30:367–380. doi: 10.1007/s002940050145. [DOI] [PubMed] [Google Scholar]
- 43.Li W, Ruf S, Bock R. Constancy of organellar genome copy numbers during leaf development and senescence in higher plants. Mol. Genet. Genomics. 2006;275:185–192. doi: 10.1007/s00438-005-0075-7. [DOI] [PubMed] [Google Scholar]
- 44.Rhoads DM, Subbaiah CC. Mitochondrial retrograde regulation in plants. Mitochondrion. 2007;7:177–194. doi: 10.1016/j.mito.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Kwast KE, Burke PV, Poyton RO. Oxygen sensing and the transcriptional regulation of oxygen-responsive genes in yeast. J. Exp. Biol. 1998;201:1177–1195. doi: 10.1242/jeb.201.8.1177. [DOI] [PubMed] [Google Scholar]
- 46.Lai LC, Kosorukoff AL, Burke PV, Kwast KE. Metabolic-state-dependent remodeling of the transcriptome in response to anoxia and subsequent reoxygenation in Saccharomyces cerevisiae . Eukaryot. Cell. 2006;5:1468–1489. doi: 10.1128/EC.00107-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ter Linde JJ, Steensma HY. A microarray-assisted screen for potential Hap1 and Rox1 target genes in Saccharomyces cerevisiae . Yeast. 2002;19:825–840. doi: 10.1002/yea.879. [DOI] [PubMed] [Google Scholar]
- 48.Hickman MJ, Winston F. Heme levels switch the function of Hap1 of Saccharomyces cerevisiae between transcriptional activator and transcriptional repressor. Mol. Cell Biol. 2007;27:7414–7424. doi: 10.1128/MCB.00887-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mense SM, Zhang L. Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res. 2006;16:681–692. doi: 10.1038/sj.cr.7310086. [DOI] [PubMed] [Google Scholar]
- 50.Rhoads DM, Umbach AL, Subbaiah CC, Siedow JN. Mitochondrial reactive oxygen species Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006;141:357–366. doi: 10.1104/pp.106.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zarkovic J, Anderson SL, Rhoads DM. A reporter gene system used to study developmental expression of alternative oxidase and isolate mitochondrial retrograde regulation mutants in Arabidopsis . Plant Mol. Biol. 2005;57:871–888. doi: 10.1007/s11103-005-3249-0.. This paper describes the first mutant screen in plants to isolate individuals that are defective in the retrograde signals emitted from dysfunctional mitochondria to induce nuclear gene expression
- 52.Dojcinovic D, Krosting J, Harris AJ, Wagner DJ, Rhoads DM. Identification of a region of the Arabidopsis AtAOX1a promoter necessary for mitochondrial retrograde regulation of expression. Plant Mol. Biol. 2005;58:159–175. doi: 10.1007/s11103-005-5390-1. [DOI] [PubMed] [Google Scholar]
- 53.Bradbeer JW, Atkinson YA, Borner T, Hagemann R. Cytoplasmic synthesis of plastid polypeptide may be controlled by plastid-synthesized RNA. Nature. 1979;279:816–817. [Google Scholar]
- 54.Taylor WC. Regulatory interactions between nuclear and plastid genes. Rev. Plant Physiol. Plant Mol. Biol. 1989;40:211–233. [Google Scholar]
- 55.Nott A, Jung HS, Koussevitzky S, Chory J. Plastid-to-nucleus retrograde signaling. Annu. Rev. Plant Biol. 2006;57:739–759. doi: 10.1146/annurev.arplant.57.032905.105310. [DOI] [PubMed] [Google Scholar]
- 56.Oelmuller R, Mohr H. Photooxidative destruction of chloroplast and its consequences for expression of nuclear genes. Planta. 1986;167:106–113. doi: 10.1007/BF00446376. [DOI] [PubMed] [Google Scholar]
- 57.Vasileuskaya Z, Oster U, Beck CF. Involvement of tetrapyrroles in inter-organellar signaling in plants and algae. Photosynth. Res. 2004;82:289–299. doi: 10.1007/s11120-004-2160-x. [DOI] [PubMed] [Google Scholar]
- 58.Kropat J, Oster U, Rudiger W, Beck CF. Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc. Natl Acad. Sci. USA. 1997;94:14168–14172. doi: 10.1073/pnas.94.25.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cornah JE, Roper JM, Pal Singh D, Smith AG. Measurement of ferrochelatase activity using a novel assay suggests that plastids are the major site of haem biosynthesis in both photosynthetic and non-photosynthetic cells of pea (Pisum sativum L.) Biochem. J. 2002;362:423–432. doi: 10.1042/0264-6021:3620423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strand A, Asami T, Alonso J, Ecker JR, Chory J. Chloroplast to nucleus communication triggered by accumulation of Mg–protoporphyrin IX. Nature. 2003;421:79–83. doi: 10.1038/nature01204. [DOI] [PubMed] [Google Scholar]
- 61.Susek RE, Ausubel FM, Chory J. Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell. 1993;74:787–799. doi: 10.1016/0092-8674(93)90459-4. [DOI] [PubMed] [Google Scholar]
- 62. Koussevitzky S, et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;316:715–719.. These authors provide genetic evidence that multiple retrograde signals converge within the chloroplast in A. thaliana. They also reveal the identity of two new proteins that are involved in this converged retrograde signal: a pentatricopeptide-repeat protein and a nuclear transcription factor
- 63.Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl Acad. Sci. USA. 2001;98:2053–2058. doi: 10.1073/pnas.98.4.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larkin RM, Alonso JM, Ecker JR, Chory J. GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science. 2003;299:902–906. doi: 10.1126/science.1079978. [DOI] [PubMed] [Google Scholar]
- 65.Gadjieva R, Axelsson E, Olsson U, Hansson M. Analysis of gun phenotype in barley magnesium chelatase and Mg–protoporphyrin IX monomethyl ester cyclase mutants. Plant Physiol. Biochem. 2005;43:901–908. doi: 10.1016/j.plaphy.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Alawady AE, Grimm B. Tobacco Mg–protoporphyrin IX methyltransferase is involved in inverse activation of Mg porphyrin and protoheme synthesis. Plant J. 2005;41:282–290. doi: 10.1111/j.1365-313X.2004.02291.x. [DOI] [PubMed] [Google Scholar]
- 67.Pontier D, Albrieux C, Joyard J, Lagrange T, Block MA. Knock-out of the magnesium protoporphyrin IX methyltransferase gene in Arabidopsis Effects on chloroplast development and on chloroplast-to-nucleus signaling. J. Biol. Chem. 2007;282:2297–2304. doi: 10.1074/jbc.M610286200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Papenbrock J, Mock HP, Tanaka R, Kruse E, Grimm B. Role of magnesium chelatase activity in the early steps of the tetrapyrrole biosynthetic pathway. Plant Physiol. 2000;122:1161–1169. doi: 10.1104/pp.122.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ankele E, Kindgren P, Pesquet E, Strand A. In vivo visualization of Mg–protoporphyrin IX, a coordinator of photosynthetic gene expression in the nucleus and the chloroplast. Plant Cell. 2007;19:1964–1979. doi: 10.1105/tpc.106.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Gromoff ED, Schroda M, Oster U, Beck CF. Identification of a plastid response element that acts as an enhancer within the Chlamydomonas HSP70A promoter. Nucleic Acids Res. 2006;34:4767–4779. doi: 10.1093/nar/gkl602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beck CF. Signaling pathways from the chloroplast to the nucleus. Planta. 2005;222:743–756. doi: 10.1007/s00425-005-0021-2. [DOI] [PubMed] [Google Scholar]
- 72.Gray JC, Sullivan JA, Wang JH, Jerome CA, MacLean D. Coordination of plastid and nuclear gene expression. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2003;358:135–145. doi: 10.1098/rstb.2002.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pesaresi P, et al. Knock-out of the plastid ribosomal protein L11 in Arabidopsis: effects on mRNA translation and photosynthesis. Plant J. 2001;27:179–189. doi: 10.1046/j.1365-313x.2001.01076.x. [DOI] [PubMed] [Google Scholar]
- 74. Pesaresi P, et al. Nuclear photosynthetic gene expression is synergistically modulated by rates of protein synthesis in chloroplasts and mitochondria. Plant Cell. 2006;18:970–991. doi: 10.1105/tpc.105.039073.. This paper provides strong genetic evidence that retrograde signals from protein-synthesis-deficient chloroplasts and mitochondria act synergistically to repress nuclear photosynthesis genes in A. thaliana
- 75.Hassidim M, et al. Mutations in CHLOROPLAST RNA BINDING provide evidence for the involvement of the chloroplast in the regulation of the circadian clock in Arabidopsis . Plant J. 2007;51:551–562. doi: 10.1111/j.1365-313X.2007.03160.x. [DOI] [PubMed] [Google Scholar]
- 76.Mochizuki N, Susek R, Chory J. An intracellular signal transduction pathway between the chloroplast and nucleus is involved in de-etiolation. Plant Physiol. 1996;112:1465–1469. doi: 10.1104/pp.112.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee KP, Kim C, Landgraf F, Apel K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana . Proc. Natl Acad. Sci. USA. 2007;104:10270–10275. doi: 10.1073/pnas.0702061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vandenabeele S, et al. Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana . Plant J. 2004;39:45–58. doi: 10.1111/j.1365-313X.2004.02105.x. [DOI] [PubMed] [Google Scholar]
- 79.Meskauskiene R, et al. FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana . Proc. Natl Acad. Sci. USA. 2001;98:12826–12831. doi: 10.1073/pnas.221252798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.op den Camp RG, et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis . Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skovsen E, Snyder JW, Lambert JD, Ogilby PR. Lifetime and diffusion of singlet oxygen in a cell. J. Phys. Chem. B. 2005;109:8570–8573. doi: 10.1021/jp051163i. [DOI] [PubMed] [Google Scholar]
- 82. Wagner D, et al. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana . Science. 2004;306:1183–1185. doi: 10.1126/science.1103178.. This paper shows that seedling lethality and growth inhibition of A. thaliana in response to singlet oxygen in the chloroplast is due to a genetically activated response that involves a chloroplast retrograde signal and the EXECUTER 1 protein
- 83.Laloi C, et al. Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana . Proc. Natl Acad. Sci. USA. 2007;104:672–677. doi: 10.1073/pnas.0609063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fey V, et al. Retrograde plastid redox signals in the expression of nuclear genes for chloroplast proteins of Arabidopsis thaliana . J. Biol. Chem. 2005;280:5318–5328. doi: 10.1074/jbc.M406358200. [DOI] [PubMed] [Google Scholar]
- 85.Escoubas JM, Lomas M, LaRoche J, Falkowski PG. Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc. Natl Acad. Sci. USA. 1995;92:10237–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baier M, Dietz KJ. Chloroplasts as source and target of cellular redox regulation: a discussion on chloroplast redox signals in the context of plant physiology. J. Exp. Bot. 2005;56:1449–1462. doi: 10.1093/jxb/eri161. [DOI] [PubMed] [Google Scholar]
- 87.Bonardi V, et al. Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature. 2005;437:1179–1182. doi: 10.1038/nature04016. [DOI] [PubMed] [Google Scholar]
- 88.Heiber I, et al. The redox imbalanced mutants of Arabidopsis differentiate signaling pathways for redox regulation of chloroplast antioxidant enzymes. Plant Physiol. 2007;143:1774–1788. doi: 10.1104/pp.106.093328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Richly E, et al. Covariations in the nuclear chloroplast transcriptome reveal a regulatory master-switch. EMBO Rep. 2003;4:491–498. doi: 10.1038/sj.embor.embor828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Biehl A, Richly E, Noutsos C, Salamini F, Leister D. Analysis of 101 nuclear transcriptomes reveals 23 distinct regulons and their relationship to metabolism, chromosomal gene distribution and co-ordination of nuclear and plastid gene expression. Gene. 2005;344:33–41. doi: 10.1016/j.gene.2004.09.009.. Along with reference 89, these authors provide the most detailed analysis to date of the nuclear transcriptional response to chloroplast retrograde signals
- 91.Raghavendra AS, Padmasree K. Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci. 2003;8:546–553. doi: 10.1016/j.tplants.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 92.Lindemann P, et al. A novel Arabidopsis thaliana protein is a functional peripheral-type benzodiazepine receptor. Plant Cell Physiol. 2004;45:723–733. doi: 10.1093/pcp/pch088. [DOI] [PubMed] [Google Scholar]
- 93.Heineke D, Bykova N, Gardestrom P, Bauwe H. Metabolic response of potato plants to an antisense reduction of the P-protein of glycine decarboxylase. Planta. 2001;212:880–887. doi: 10.1007/s004250000460. [DOI] [PubMed] [Google Scholar]
- 94.Igamberdiev AU, Bykova NV, Lea PJ, Gardestrom P. The role of photorespiration in redox and energy balance of photosynthetic plant cells: A study with a barley mutant deficient in glycine decarboxylase. Physiol. Plant. 2001;111:427–438. doi: 10.1034/j.1399-3054.2001.1110402.x. [DOI] [PubMed] [Google Scholar]
- 95.Sabar M, De Paepe R, de Kouchkovsky Y. Complex I impairment, respiratory compensations, and photosynthetic decrease in nuclear and mitochondrial male sterile mutants of Nicotiana sylvestris . Plant Physiol. 2000;124:1239–1250. doi: 10.1104/pp.124.3.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dutilleul C, et al. Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. Plant Cell. 2003;15:1212–1226. doi: 10.1105/tpc.009464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bennoun P, Delosme M. Chloroplast suppressors that act on a mitochondrial mutation in Chlamydomonas reinhardtii . Mol. Gen. Genet. 1999;262:85–89. doi: 10.1007/s004380051062. [DOI] [PubMed] [Google Scholar]
- 98.Matsuo M, Obokata J. Remote control of photosynthetic genes by the mitochondrial respiratory chain. Plant J. 2006;47:873–882. doi: 10.1111/j.1365-313X.2006.02839.x. [DOI] [PubMed] [Google Scholar]
- 99.Emanuel C, Weihe A, Graner A, Hess WR, Borner T. Chloroplast development affects expression of phage-type RNA polymerases in barley leaves. Plant J. 2004;38:460–472. doi: 10.1111/j.0960-7412.2004.02060.x. [DOI] [PubMed] [Google Scholar]
- 100.Jiao S, Thornsberry JM, Elthon TE, Newton KJ. Biochemical and molecular characterization of photosystem I deficiency in the NCS6 mitochondrial mutant of maize. Plant Mol. Biol. 2005;57:303–313. doi: 10.1007/s11103-004-7792-x. [DOI] [PubMed] [Google Scholar]
- 101.Barbrook AC, Howe CJ, Purton S. Why are plastid genomes retained in non-photosynthetic organisms? Trends Plant Sci. 2006;11:101–108. doi: 10.1016/j.tplants.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 102.Martin W, et al. Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thousands of cyanobacterial genes in the nucleus. Proc. Natl Acad. Sci. USA. 2002;99:12246–12251. doi: 10.1073/pnas.182432999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allen JF. The function of genomes in bioenergetic organelles. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2003;358:19–37. doi: 10.1098/rstb.2002.1191. discussion 37–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sickmann A, et al. The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl Acad. Sci. USA. 2003;100:13207–13212. doi: 10.1073/pnas.2135385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bogorad L. Evolution of organelles and eukaryotic genomes. Science. 1975;188:891–898. doi: 10.1126/science.1138359. [DOI] [PubMed] [Google Scholar]
- 106.Daley DO, Whelan J. Why genes persist in organelle genomes. Genome Biol. 2005;6:110. doi: 10.1186/gb-2005-6-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pfannschmidt T, Nilsson A, Tullberg A, Link G, Allen JF. Direct transcriptional control of the chloroplast genes psbA and psaAB adjusts photosynthesis to light energy distribution in plants. IUBMB Life. 1999;48:271–276. doi: 10.1080/713803507. [DOI] [PubMed] [Google Scholar]
- 108.Das AK, Cohen PW, Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 1998;17:1192–1199. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kotera E, Tasaka M, Shikanai T. A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature. 2005;433:326–330. doi: 10.1038/nature03229. [DOI] [PubMed] [Google Scholar]
- 110.Okuda K, Nakamura T, Sugita M, Shimizu T, Shikanai T. A pentatricopeptide repeat protein is a site recognition factor in chloroplast RNA editing. J. Biol. Chem. 2006;281:37661–37667. doi: 10.1074/jbc.M608184200. [DOI] [PubMed] [Google Scholar]
- 111.Fisk DG, Walker MB, Barkan A. Molecular cloning of the maize gene crp1 reveals similarity between regulators of mitochondrial and chloroplast gene expression. EMBO J. 1999;18:2621–2630. doi: 10.1093/emboj/18.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meierhoff K, Felder S, Nakamura T, Bechtold N, Schuster G. HCF152, an Arabidopsis RNA binding pentatricopeptide repeat protein involved in the processing of chloroplast psbB–psbT–psbH–petB–petD RNAs. Plant Cell. 2003;15:1480–1495. doi: 10.1105/tpc.010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Z, et al. Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell. 2006;18:676–687. doi: 10.1105/tpc.105.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schmitz-Linneweber C, Williams-Carrier R, Barkan A. RNA immunoprecipitation and microarray analysis show a chloroplast pentatricopeptide repeat protein to be associated with the 5′ region of mRNAs whose translation it activates. Plant Cell. 2005;17:2791–2804. doi: 10.1105/tpc.105.034454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lahmy S, et al. A chloroplastic RNA-binding protein is a new member of the PPR family. FEBS Lett. 2000;480:255–260. doi: 10.1016/s0014-5793(00)01935-9. [DOI] [PubMed] [Google Scholar]
- 116. Lurin C, et al. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell. 2004;16:2089–2103. doi: 10.1105/tpc.104.022236.. These authors attempt the broadest analysis yet of PPR proteins in plants. They describe a bioinformatics analysis of A. thaliana PPR proteins and present data on the expression, localization and function of several of these proteins
- 117.Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 118.Borghouts C, Benguria A, Wawryn J, Jazwinski SM. Rtg2 protein links metabolism and genome stability in yeast longevity. Genetics. 2004;166:765–777. doi: 10.1534/genetics.166.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sinclair DA, Guarente L. Extrachromosomal rDNA circles — a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 120.Felkai S, et al. CLK-1 controls respiration, behavior and aging in the nematode Caenorhabditis elegans . EMBO J. 1999;18:1783–1792. doi: 10.1093/emboj/18.7.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu X, et al. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 2005;19:2424–2434. doi: 10.1101/gad.1352905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Biswas G, Guha M, Avadhani NG. Mitochondria-to-nucleus stress signaling in mammalian cells: nature of nuclear gene targets, transcription regulation, and induced resistance to apoptosis. Gene. 2005;354:132–139. doi: 10.1016/j.gene.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Amuthan G, et al. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J. 2001;20:1910–1920. doi: 10.1093/emboj/20.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Amuthan G, et al. Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. OncoGene. 2002;21:7839–7849. doi: 10.1038/sj.onc.1205983. [DOI] [PubMed] [Google Scholar]
- 125.Kulawiec M, et al. Proteomic analysis of mitochondria-to-nucleus retrograde response in human cancer. Cancer Biol. Ther. 2006;5:967–975. doi: 10.4161/cbt.5.8.2880. [DOI] [PubMed] [Google Scholar]
- 126.Biswas G, Anandatheerthavarada HK, Avadhani NG. Mechanism of mitochondrial stress-induced resistance to apoptosis in mitochondrial DNA-depleted C2C12 myocytes. Cell Death Differ. 2005;12:266–278. doi: 10.1038/sj.cdd.4401553. [DOI] [PubMed] [Google Scholar]
- 127.Park SY, Lee S, Park KS, Lee HK, Lee W. Proteomic analysis of cellular change involved in mitochondria-to-nucleus communication in L6 GLUT4myc myocytes. Proteomics. 2006;6:1210–1222. doi: 10.1002/pmic.200500284. [DOI] [PubMed] [Google Scholar]
- 128.Zubko MK. Mitochondrial tuning fork in nuclear homeotic functions. Trends Plant Sci. 2004;9:61–64. doi: 10.1016/j.tplants.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 129.Teixeira RT, Farbos I, Glimelius K. Expression levels of meristem identity and homeotic genes are modified by nuclear-mitochondrial interactions in alloplasmic male-sterile lines of Brassica napus . Plant J. 2005;42:731–742. doi: 10.1111/j.1365-313X.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- 130. Carlsson J, et al. Microarray analysis reveals altered expression of a large number of nuclear genes in developing cytoplasmic male sterile Brassica napus flowers. Plant J. 2007;49:452–462. doi: 10.1111/j.1365-313X.2006.02975.x.. This paper provides a comparison of gene expression profiles between Brassica napus CMS and fertile lines. The large difference in expression of nuclear genes between the two lines adds considerable support to the hypothesis that retrograde signals from dysfunctional mitochondria can be responsible for the CMS phenotype in plants
- 131.Fujii S, Komatsu S, Toriyama K. Retrograde regulation of nuclear gene expression in CW-CMS of rice. Plant Mol. Biol. 2007;63:405–417. doi: 10.1007/s11103-006-9097-8. [DOI] [PubMed] [Google Scholar]
- 132.Liu F, Cui X, Horner HT, Weiner H, Schnable PS. Mitochondrial aldehyde dehydrogenase activity is required for male fertility in maize. Plant Cell. 2001;13:1063–1078. doi: 10.1105/tpc.13.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kazama T, Toriyama K. A pentatricopeptide repeat-containing gene that promotes the processing of aberrant atp6 RNA of cytoplasmic male-sterile rice. FEBS Lett. 2003;544:99–102. doi: 10.1016/s0014-5793(03)00480-0. [DOI] [PubMed] [Google Scholar]
- 134.Akagi H, et al. Positional cloning of the rice Rf-1 gene, a restorer of BT-type cytoplasmic male sterility that encodes a mitochondria-targeting PPR protein. Theor. Appl. Genet. 2004;108:1449–1457. doi: 10.1007/s00122-004-1591-2. [DOI] [PubMed] [Google Scholar]
- 135.Geddy R, Brown GG. Genes encoding pentatricopeptide repeat (PPR) proteins are not conserved in location in plant genomes and may be subject to diversifying selection. BMC Genomics. 2007;8:130. doi: 10.1186/1471-2164-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.