Abstract
Cytosolic DNA sensing is an important process during the innate immune response that activates the Stimulator of Interferon Genes (STING) adaptor and induce interferon type I (IFN-I). STING incites spontaneous immunity during immunogenic tumor growth and accordingly, STING agonists induce regression of therapy-resistant tumors. However DNA, STING agonists and apoptotic cells can also promote tolerogenic responses via STING by activating immunoregulatory mechanisms such as indoleamine 2,3 dioxygenase (IDO). Here, we show that IDO activity induced by STING activity in the tumor microenvironment (TME) promoted the growth of Lewis lung carcinoma (LLC). While STING also induced IDO in tumor-draining lymph nodes (TDLNs) during EL4 thymoma growth, this event was insufficient to promote tumorigenesis. In the LLC model, STING ablation enhanced CD8+ T cell infiltration and tumor cell killing while decreasing myeloid-derived suppressor cell infiltration and IL-10 production in the TME. Depletion of CD8+ T cells also eliminated the growth disadvantage of LLC tumors in STING-deficient mice, indicating that STING signaling attenuated CD8+ T cell effector functions during tumorigenesis. In contrast to native LLC tumors, STING signaling did not promote growth of neoantigen-expressing LLC, nor did it induce IDO in TDLN. Similarly, STING failed to promote growth of B16 melanoma or to induce IDO activity in TDLN in this setting. Thus, our results show how STING-dependent DNA sensing can enhance tolerogenic states in tumors characterized by low antigenicity, and how IDO inhibition can overcome this state by attenuating tumor tolerance. Further, our results reveal a greater complexity in the role of STING signaling in cancer, underscoring how innate immune pathways in the TME modify tumorigenesis in distinct tumor settings, with implications for designing effective immunotherapy trials.
Keywords: DNA sensing, STING, IDO
Introduction
Eukaryotic cells express cytosolic DNA sensors that activate the Stimulator of Interferon Genes (STING) adaptor to induce interferon type I (IFN-I) production (1). STING/IFN-I signaling incites host immunity to some pathogens but sustained DNA sensing in mice with defective DNA catabolizing enzymes incited spontaneous autoimmunity (2). Moreover, STING/IFN-I signaling in the tumor microenvironment (TME) incited therapeutic responses to some murine tumors, including B16 melanoma (3,4).
DNA, STING agonists and apoptotic cells also induce STING-dependent tolerogenic responses in mice (5–7). Immune regulation was the dominant response to these treatments because STING/IFN-I signaling induced the regulatory indoleamine 2,3 dioxygenase (IDO) activity. STING ablation also accelerated onset of autoimmune syndromes in susceptible mice (8). IDO is an immune checkpoint that attenuates tumor immunity and elevated IDO is a common TME feature in mice and cancer patients (9). Moreover, tumor-infiltrating lymphocytes (TILs) elicited counter-regulatory responses to reinforce immune checkpoints in melanomas, including IDO (10). Accordingly, cancer therapies must overcome tolerance established during tumorigenesis and reinforced by TILs.
How tumor-associated inflammation establishes immune checkpoints like IDO is unclear. We hypothesized that DNA from dying cells is sensed to induce IDO via STING during tumorigenesis. We used the Lewis lung carcinoma (LLC) model to test this hypothesis, as IDO promotes LLC tumorigenesis and T cell evasion in this model (11). We show that cytosolic DNA sensing promoted LLC tumorigenesis and induced IDO but only if tumors had low antigenicity.
Materials and Methods
Mice
C57BL/6 (B6) mice were purchased (Taconic) or bred in a barrier facility at GRU. IDO1 knockout (KO), STING-KO, IFNAR-KO, and IFNγR-KO mice were described previously (7). cGAS-KO mice were obtained From Dr. Skip Virgin (Washington University). All KO mice were fully backcrossed to B6 backgrounds. All procedures in mice were approved by the local IACUC at GRU.
Tumor growth
LLC, EL4 (ATCC) or LLC cells transfected to express RFP cells were injected intra-dermally (i/d, 1.5 x 105/mouse) into the right flank of female mice and tumor growth monitored. Tumor sizes were calculated using the formula v=(d1×d2)3/2 × (π/6), where d1 and d2 are perpendicular tumor diameters. Some mice were treated with rat anti-CD8 (Clone: YTS 169.4, BioXcell) or IgG2a isotype-matched (clone 2A3, BioXcell) mAbs 0, 4, 7, 12 days after LLC challenge (150μg/injection). LLC cells were transduced with lentiviral vectors (from Dr. Yukai He) encoding influenza A nucleoprotein (NP) or melanoma-specific gp100 antigen and selected using blasticidine (5μg/ml). For lung tumors, LLC cells were injected intravenously (i/v, 5x105/mouse) and mice were subjected to computer tomography (CT) scans 10 days later using a dedicated small animal imaging system (nanoScan SPECT/CT, Mediso, USA). Lung CT images were acquired using X-ray energy 50kvp, exposure time 270ms/projection, 480 projections/360 degree with medium zoom, acquisition time 2.14 minutes. Multiplanar reconstruction of projection images (spatial resolution 137 micron/slice) was performed and images were converted from DICOM to Analyze format to analyze CT signal intensity.
IDO enzyme activity
IDO activity was measured in TDLNs or tumor lesions as described (6). In brief, tissues were homogenized in PBS, added to IDO enzyme cocktails and kynurenine generated after 2hr. was measured by HPLC.
Flow cytometry
Tumors were disrupted using gentleMACSTM (Miltenyi Biotec) in PBS containing dispase I (2.5U/ml) and collagenase IV (200U/ml) and TDLNs were digested in PBS containing collagenase IV (400U/ml) at 370C, 30min. Digestion was stopped with PBS/10mM EDTA, 2% serum, and debris removed on discontinuous Percoll gradients (40–80%). Cells were stained for CD45 (30F-11,), CD11b (M1/70), Ly6C (HK1.4), Ly6G (1A8, all Biolegend), CD11c (N418, Ebioscience), CCR2 (475301, R&D systems). TILs were analyzed to exclude circulating leukocytes as described with minor modifications (12). Briefly, anti-CD45.2 PECy7 to mark circulating leukocytes was injected (2.5μg, i/v) 15mins before sacrificing mice. Tumors cell suspensions were then stained with anti-CD45-brilliant violet 605. CD45+CD45.2neg cells are tumor-infiltrating cells while CD45+CD45.2+ cells are circulating leukocytes. Data were acquired using a LSRII flow cytometer and analyzed using DIVA or FlowJo software.
Multiplex Analyses
TDLNs were homogenized in PBS, frozen and thawed, centrifuged (10,000g), and protein concentration adjusted to 200μg/ml before multiplexing (23-plex panel) to detect mouse cytokines & chemokines (Bio-Rad, cat. No. M60-009RDPD). Data were acquired using a Luminex 200 reader.
Statistical analysis
Data were analyzed using GraphPad Prism. For tumor growth, two-way ANOVA with Bonferroni post-hoc tests were performed. Unpaired two-tailed Student’s t tests were performed for two group comparisons.
Results and Discussion
Cytosolic DNA sensing to activate STING promotes LLC growth
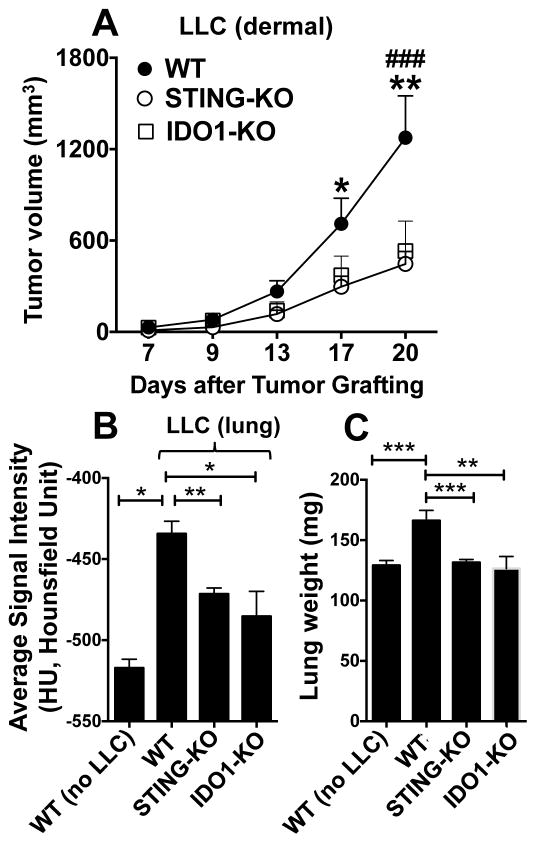
To test if cytosolic DNA sensing influences LLC growth, tumors were grown in B6 (WT) mice and STING-deficient (STING-KO) mice with B6 backgrounds. After 20 days LLC tumors were smaller (~50%) in STING-KO than WT mice (Fig. 1A). Consistent with a previous study using IDO inhibitors (11), LLC growth was attenuated in IDO1-KO mice and was comparable with growth in STING-KO mice (Fig. 1A). To test if DNA promoted LLC growth in other tissues, mice were challenged with LLC cells intravenously (5x105 cells/mouse) and lung CT scans were performed after 10 days. Lung growth (Fig. 1B) and lung weights (Fig. 1C) were lower in STING-KO and IDO1-KO than in WT mice. Thus mice lacking STING or IDO1 genes were more resistant to LLC growth at distinct sites.
Figure 1. Cytosolic DNA sensing to activate STING promotes LLC growth in dermis and lungs.
A. LLC cells were injected intradermally into B6 (WT), STING-KO or IDO1-KO mice and tumor growth was monitored. BC. LLC cells were injected intravenously and CT scans were performed to assess tumor growth in lungs after 10 days (B) and lung weights after 12 days (C). Statistical significance was determined by two-way ANOVA with Bonferroni post hoc test (A) and two tailed unpaired Student’s t test (B,C); *p<0.05, **p<0.01, ***p<0.001, except IDO1-KO vs. WT in panel A, ###p<0.001.
STING stimulates local IDO activity during LLC tumorigenesis
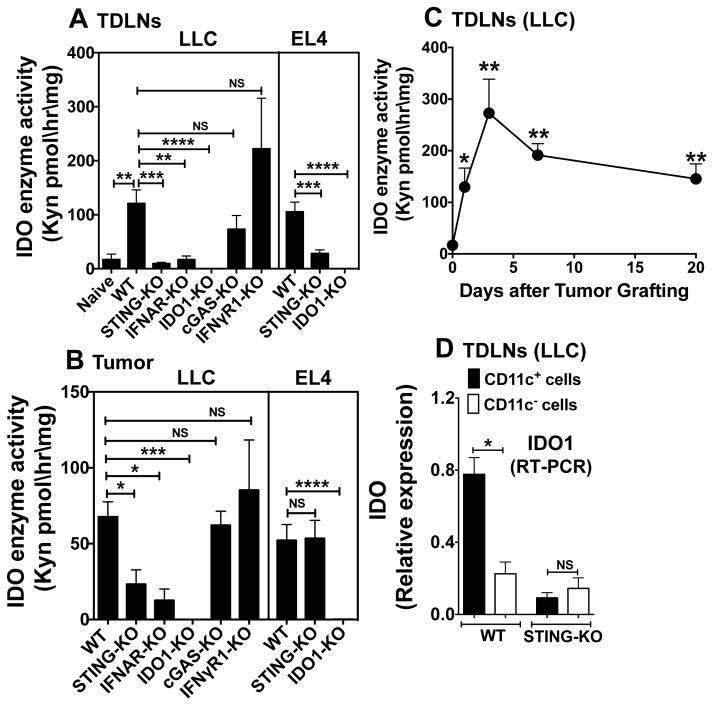
DNA incited tolerogenic responses to STING agonists or apoptotic cells in mice by stimulating IDO activity (5,7). We hypothesized that DNA sensing to activate STING induces IDO and tolerogenic processes that promote LLC growth. To test this hypothesis homogenates of tumor-draining lymph nodes (TDLNs) and tumor lesions (20 days) were incubated in IDO enzyme cocktail to assess production of kynurenine (Kyn), a tryptophan catabolite produced by IDO. IDO activity was elevated significantly in TDLNs from WT mice, relative to LNs from naïve WT mice (Fig. 2A). In contrast, TDLN IDO activity was not induced in mice lacking STING or IFN-I receptor (IFNAR) genes but was induced in mice lacking IFN-II receptors (IFNγR1) or the cytosolic DNA sensor cyclic GAMP synthase (cGAS, Fig. 2A). IDO1 (not IDO2 or TDO genes) encoded IDO activity in TDLNs since IDO activity was not detected in TDLNs from IDO1-KO mice. STING also induced IDO in TDLNs of mice with EL4 thymomas (Fig. 2A). Thus, STING/IFN-I signaling induces IDO in TDLNs, while cGAS DNA sensing and IFNγ signaling are not required for this response.
Figure 2. STING/IFN-I signaling induces IDO during LLC tumor growth.
A–C. LLC or EL4 cells were injected (i/d, day 0) into mice (7–10 mice/group). IDO activity was assessed after 20 days for LLC, 14 days for EL4 (AB) or over time (C) in TDLNs (A, C) or in tumor lesions (B). IDO activity is expressed as Kyn generated ex vivo in tissue homogenates. D. DCs (CD11c+) and non-DCs (CD11c-) were enriched from TDLNs (MACS) of mice with LLC tumors (5 mice/group, day 20). IDO1 transcripts in RNA from enriched DCs and non-DCs were analyzed by RT-PCR. Statistical significance was determined by two tailed unpaired Student’s t test; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; NS, not significant.
Similar requirements to induce IDO manifested in LLC tumor lesions. LLC tumors did not express IDO since no activity was detected in tumors from IDO1-KO mice (Fig. 2B). LLC tumors grew at comparable rates in IFNAR-KO, cGAS-KO and WT mice (Supplemental Fig. S1AB), suggesting that cGAS sensing and IFN-I signaling does not promote tumorigenesis, even though IFN-I induced IDO. EL4 growth induced tumor-associated cells to express IDO via a STING independent pathway (Fig. 2B), providing an explanation for IDO-dependent, STING-independent tumorigenesis in this model (Supplemental Fig. S2C). IDO activity was elevated significantly one day after LLC challenge and remained high thereafter, relative to levels in LNs from naïve mice (Fig. 2C). Thus LLC growth induced rapid and sustained increase in IDO activity, which did not correlate with tumor size.
IDO-expressing TDLN dendritic cells (DCs) inhibited T cells and activated Foxp3+ regulatory CD4 T cells to suppress immunity to B16 melanomas (13). To assess if DCs expressed IDO during LLC growth TDLNs were fractionated to enrich DC (CD11c+) and non-DC (CD11c-) populations and IDO1 transcripts were detected by qRT-PCR. IDO transcription was elevated in TDLN DCs in WT mice (Fig. 2D) and was not elevated in TDLN cells from STING-KO mice. Thus DNA sensing induced selective IDO1 expression by TDLN DCs.
STING attenuates tumor cell killing and promotes tolerogenic responses during LLC growth
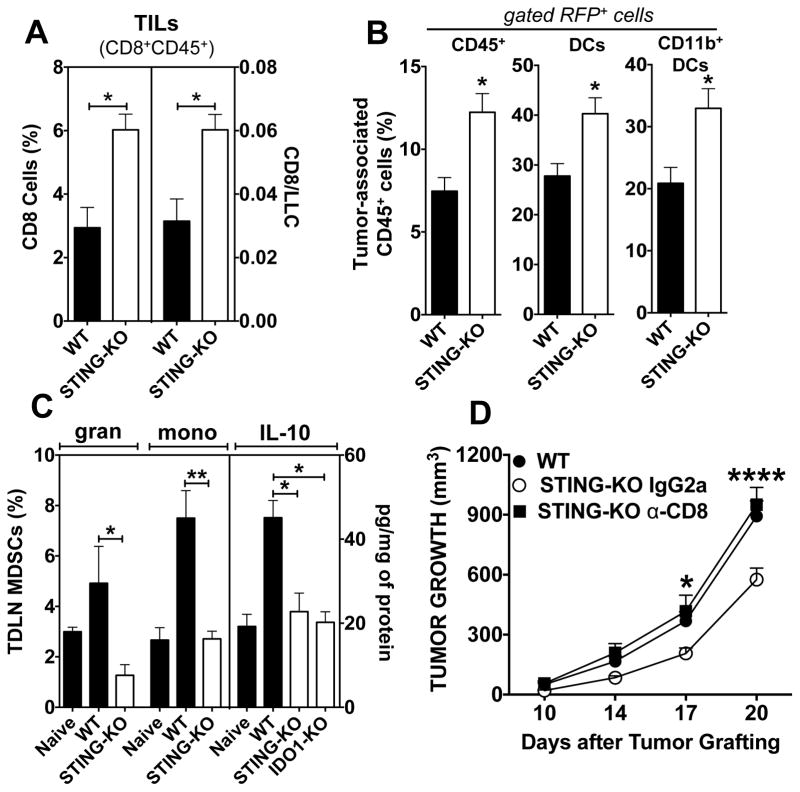
Elevated IDO is a TME hallmark in mice and cancer patients (14). We assessed if STING ablation impacted tumor-induced inflammation using transfected LLC cells expressing red fluorescent protein (LLC-RFP). As for LLC tumors, STING-KO mice were more resistant to LLC-RFP growth (Supplemental Fig. 2A). To detect tumor-infiltrating CD8 T cells (TILs) circulating CD45+ cells were pre-stained in vivo and were distinguished from tumor-associated CD45+ cells (12) by staining excised tumors ex vivo with a different CD45 (Supplemental Fig. S2B) and CD8 mAbs. More TILs and higher TIL:LLC ratios were detected in tumors from STING-KO mice (Fig. 3A). Higher proportions of CD45+ cells that ingested tumor RFP were detected in STING-KO mice (Fig. 3B and Supplemental Fig. S2C). Phenotypic analyses revealed increased proportions of CD45+RFP+ cells expressing CD11c and CD11b in STING-KO mice, suggesting that CD11b+ DCs were a major cell type that ingested LLC-RFP tumor cells. Infiltration of granulocytic (Ly6ChighGint) and monocytic (Ly6CnegGhigh) CD11b+CCR2+ myeloid-derived suppressor cells (MDSCs) into TDLNs decreased when STING was ablated, as did levels of the immunoregulatory cytokine IL-10 in TDLNs (Fig. 3C). STING ablation also attenuated expression of other cytokines, growth factors and chemokines linked to recruitment of regulatory cells such as MDSCs into the TME (Supplemental Fig. S3). Thus STING ablation led to increased TILs in tumor lesions, tumor cell ingestion by tumor-associated cells, and attenuated MDSC infiltration and IL-10 production in TDLNs during tumorigenesis.
Figure 3. STING attenuates tumor cell killing and promotes tolerogenic responses during LLC tumorigenesis.
AB. Flow cytometric analyses of TILs (CD8+CD45+, A) and tumor-associated hematopoietic cells (CD45+RFP+, B) that ingested LLC-RFP cells in tumor lesions 13 and 20 days post LLC challenge, respectively. C. Flow cytometric analyses of MDSCs and multiplex analyses of IL-10 levels in TDLNs 20 days post LLC challenge. For FACS gating strategies see Methods and Supplemental Fig. S2. D. Mice were pre-treated with anti-CD8 depleting mAbs or isotype-matched IgG2a mAbs before LLC challenge. LLC growth in naïve WT mice was monitored in parallel until day 20. Statistical significance was determined by two tailed unpaired Student’s t test (A–C) or two-way ANOVA with Bonferroni post hoc test (D) and; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
We hypothesized that increased resistance to LLC growth in the absence of STING was due to enhanced CD8 T cell (TIL) functions. STING-KO mice were treated with anti-CD8 depleting mAbs or irrelevant IgG2a (isotype-matched) mAbs. CD8 depletion accelerated LLC growth in STING-KO mice, which was comparable with tumor growth in WT mice. Thus CD8 cells mediated increased resistance to LLC growth in STING-KO mice.
Tumor antigenicity influences responses to DNA in the TME
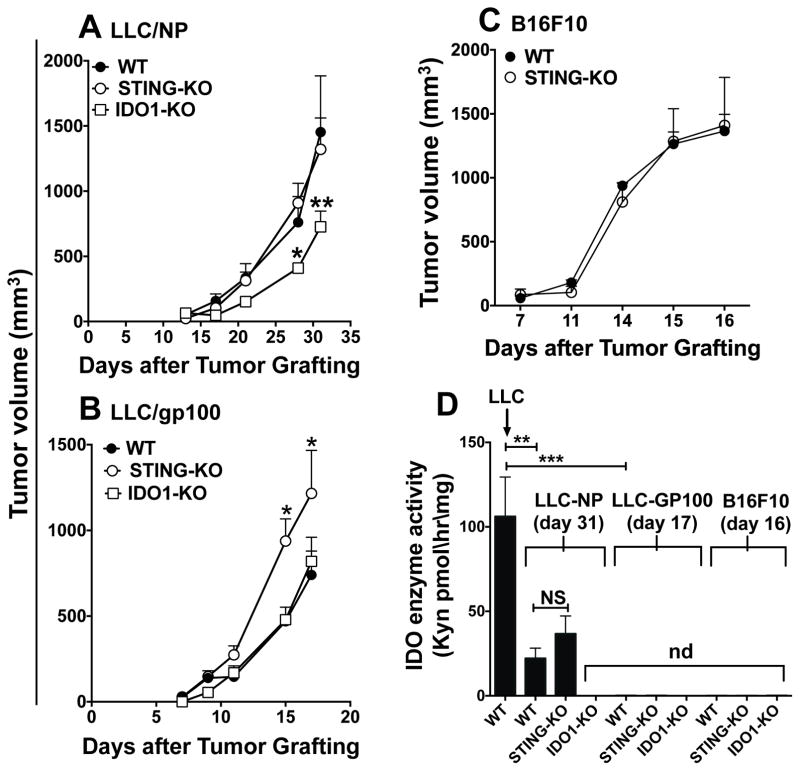
Tumor cells expressing defined neo-antigens are used to monitor tumor-specific immunity. We generated LLC cells expressing influenza nucleoprotein (NP) or the dermal autoantigen gp100. Compared with native LLC tumors, LLC-NP and LLC-gp100 tumors grew at slower or comparable rates, respectively (Fig. 4AB). Unlike native tumors, STING ablation did not attenuate LLC-NP and LLC-gp100 growth and LLP-gp100 tumors grew faster (Fig. 4AB). Consistent with previous reports (15), STING ablation did not slow B16 melanoma growth (Fig. 4C). As for native tumors, IDO1 ablation slowed LLC-NP growth but did not impact LLC-gp100 growth (Fig. 4AB). Moreover, enhanced LLC tumor antigenicity attenuated (LLC-NP) or abolished (LLC-gp100, B16) IDO induction in TDLNs in WT mice (Fig. 4D). Thus DNA was sensed to induce IDO and promote LLC growth only for native LLC tumors, while DNA did not promote growth of LLC tumors with increased antigenicity or B16 melanoma. Collectively, our findings reveal that cytosolic DNA sensing activates STING/IFN-I signaling to induce local IDO activity and other tolerogenic responses that promote LLC growth. Moreover tumor antigenicity was a pivotal factor influencing dependence on STING to induce IDO and promote LLC growth. These findings highlight tumor antigenicity as an underappreciated factor influencing immune responses to DNA in the TME.
Figure 4. LLC tumor antigenicity is a pivotal factor influencing responses to DNA in the TME.
AB. Growth of LLC tumors transfected to express NP (A) or gp100 (B) was monitored in the mice indicated. Due to higher incidence of ulceration, experiments were terminated on day 17 for LLC-gp100 tumors. C. B16F10 melanoma growth in WT and STING-KO mice. D. IDO activity in TDLNs was assessed after LLC-NP (day 31), LLC-gp100 (day 17) or B16F10 melanoma (day 16) challenge, when tumor sizes were comparable in WT mice. Statistical significance was determined by two-way ANOVA with Bonferroni post hoc test (A, B, D) or two tailed unpaired Student’s t test (C); *p<0.05, **p<0.01, ***p<0.001; NS, not significant; nd, not detected.
We used the LLC model because IDO promoted LLC growth and T cell evasion (11), though how IDO was induced was unknown. We hypothesized that DNA sensing to activate STING/IFN-I signaling induced IDO to promote tolerogenic responses and LLC growth. Our findings support this hypothesis since STING ablation attenuated LLC growth and abolished local IDO induction. STING-dependent tolerogenic responses to DNA were described in mice treated with STING agonists or apoptotic cells to suppress immunity and autoimmunity, and in mice prone to autoimmune syndromes (5–8). Our findings show that tolerogenic responses to DNA feature in some tumor settings.
LLC challenge induced rapid and sustained increase in local IDO activity. Moreover STING/IFN-I signaling was essential to induce IDO in tumor-associated cells and TDLNs. Thus innate immune cells incited IDO, while IFNγ from activated lymphoid cells (NK cells, TILs) was not required. The cytosolic DNA sensor cGAS was not essential to induce IDO, suggesting that other DNA sensors induce IDO; alternatively, other DNA sensors may compensate for loss of cGAS.
TME analyses in mice lacking STING revealed profound changes in inflammatory responses to LLC growth, including more TILs, tumor cell ingestion, (especially by myeloid DCs) and attenuated MDSC infiltration and IL-10 production in TDLNs. Previously, we showed that splenic myeloid DCs ingested DNA nanoparticles and sensed cargo DNA to activate STING/IFN-I signaling, which induced tolerogenic responses via IDO (5). Moreover, apoptotic cells induced IDO via a STING-dependent pathway. Myeloid DCs may also mediate tolerogenic responses to DNA from dying cells in the TME. Consistent with these findings, CD8 cell depletion in STING-KO mice accelerated LLC growth, suggesting that STING-dependent tolerogenic responses impeded effector CD8 cell functions in the TME.
Our findings in the LLC model contrast with reports showing that STING/IFN-I signaling is a critical immunostimulatory pathway required for effective responses to chemotherapy and radiotherapy in the TME (3,4). These reports focused on immunogenic tumor models that underwent spontaneous regression or responded to chemotherapy or radiotherapy. However DNA sensing to activate STING had no effect on native B16 melanoma growth and did not induce IDO activity, suggesting that IDO expressed in TDLN DCs is not enzymatically active (16). Unlike native LLC tumors, LLC-NP and LLC-gp100 tumors exhibited similar attributes to B16 melanoma, as STING did not promote tumorigenesis or induce robust TDLN IDO activity. Thus native LLC tumor growth incited inflammation favoring tolerogenic responses to DNA, while enhanced LLC antigenicity overcame tolerogenic responses to promote immunogenic responses. These findings help reconcile observations of diametric responses to DNA in distinct tumor settings and reflect dual roles of the immune system in killing malignant cells and creating inflammatory microenvironments that promote tumorigenesis (17). Further evidence of the complex role of STING in the developing TME emerged from a study showing that STING ablation enhanced resistance to papilloma formation following chronic exposure to the carcinogen DMBA, revealing a critical role for STING in promoting carcinogenesis (18). Like the current study, these findings identify DNA sensing to activate STING as a pivotal factor in inflammatory responses that promote tumorigenesis. Our findings also suggest that innate tumor antigenicity or interventions to enhance tumor antigenicity can override tolerogenic responses to DNA to promote immunogenic responses to DNA. Tumor neoantigens are key factors influencing clinical responses to immune checkpoint blockade (19). Thus tumors with high antigenicity are more responsive and tumor neoantigens may be useful biomarkers to stratify patients (19). Treatments with synthetic STING agonists also promoted robust anti-tumor responses when administered into tumors (15). Our findings suggest that STING agonists may not be effective in all tumor settings, particularly those where robust tolerogenic responses to DNA and low tumor antigenicity prevail. Indeed resistance to ipilimumab (anti-CTLA4 mAb) therapy in some melanoma patients and in mice with B16 melanomas was mediated by IDO (20). Thus targeting multiple pathways may be necessary to elicit effective therapeutic responses in some cancer patients.
Supplementary Material
Acknowledgments
Financial support: This study was supported by NIH grant AI1043347 and the Mason Trust (to ALM). HL was supported by a postdoctoral research fellowship from the JDRF (grant No. 3-2013-219).
We thank Janice Randall for expert technical assistance, and our colleagues in the Georgia Regents University Cancer Immunology, Inflammation and Tolerance program for constructive comments on aspects of this study.
Footnotes
Disclosure of Potential Conflicts of Interest: ALM & DHM serve as scientific consultants for NewLink Genetics Inc. and receive income from this source.
Authors’ Contributions
Conception and design: H. Lemos, E. Mohamed, L. Huang, A. Mellor.
Development of methodology: H. Lemos, E. Mohamed, L. Huang, A. Mellor.
Acquisition of data (provided animals, facilities, etc.): H. Lemos, E. Mohamed, L. Huang, R. Ou, G. Pacholczyk, Ali Arbab.
Analysis and interpretation of data: H. Lemos, E. Mohamed, L. Huang, Ali Arbab, A. Mellor.
Writing, review, and/or revision of the manuscript: H. Lemos, E. Mohamed, L. Huang, D. Munn, Ali Arbab, A. Mellor.
Administrative, technical, or material support (i.e., reporting or organizing data): H. Lemos, E. Mohamed, L. Huang, A. Mellor.
References
- 1.Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends Immunol. 2014;35(2):88–93. doi: 10.1016/j.it.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Gall A, Treuting P, Elkon KB, Loo YM, Gale M, Jr, Barber GN, et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36(1):120–31. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MYK, et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity. 2014;41:830–42. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent Cytosolic DNA Sensing Promotes Radiation-induced Type I interferon-dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang L, Li L, Lemos H, Chandler PR, Pacholczyk G, Baban B, et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. Journal of immunology. 2013;191(7):3509–13. doi: 10.4049/jimmunol.1301419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L, Lemos HP, Li L, Li M, Chandler PR, Baban B, et al. Engineering DNA Nanoparticles as Immunomodulatory Reagents that Activate Regulatory T Cells. Journal of immunology. 2012;188(10):4913–20. doi: 10.4049/jimmunol.1103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemos H, Huang L, Chandler PR, Mohamed E, Souza GS, Li L, et al. Activation of the STING Adaptor Attenuates Experimental Autoimmune Encephalitis. Journal of immunology. 2014;192:5571–8. doi: 10.4049/jimmunol.1303258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma S, Campbell AM, Chan J, Schattgen SA, Orlowski GM, Nayar R, et al. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1420217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2012;34:137–43. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Science translational medicine. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. International Journal of Cancer. 2002;101:151–55. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 12.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. Journal of immunology. 2011;187(11):5510–4. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via IDO. The Journal of clinical investigation. 2007;117(9):2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munn DH. Indoleamine 2,3-dioxygenase, Tregs and Cancer. Curr Med Chem. 2011;18(15):2240–6. doi: 10.2174/092986711795656045. [DOI] [PubMed] [Google Scholar]
- 15.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell reports. 2015;11(7):1018–30. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munn DH, Sharma MD, Hou D, Baban B, Lee J, Antonia SJ, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. The Journal of clinical investigation. 2004;114:280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 18.Ahn J, Xia T, Konno H, Konno K, Ruiz P, Barber GN. Inflammation-driven carcinogenesis is mediated through STING. Nature communications. 2014;5:5166. doi: 10.1038/ncomms6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 20.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. The Journal of experimental medicine. 2013;210(7):1389–402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.