Abstract
Aging is a complex, multifaceted process that induces a myriad of physiological changes over an extended period of time. Aging is accompanied by major biochemical and biomechanical changes at macroscopic and microscopic length scales that affect not only tissues and organs but also cells and subcellular organelles. These changes include transcriptional and epigenetic modifications; changes in energy production within mitochondria; and alterations in the overall mechanics of cells, their nuclei, and their surrounding extracellular matrix. In addition, aging influences the ability of cells to sense changes in extracellular-matrix compliance (mechanosensation) and to transduce these changes into biochemical signals (mechanotransduction). Moreover, following a complex positive-feedback loop, aging is accompanied by changes in the composition and structure of the extracellular matrix, resulting in changes in the mechanics of connective tissues in older individuals. Consequently, these progressive dysfunctions facilitate many human pathologies and deficits that are associated with aging, including cardiovascular, musculoskeletal, and neurodegenerative disorders and diseases. Here, we critically review recent work highlighting some of the primary biophysical changes occurring in cells and tissues that accompany the aging process.
Keywords: cellular mechanics, nuclear mechanics, extracellular matrix, mitochondrial dysfunction
MECHANICS AND THE ECM
The extracellular matrix (ECM) plays an essential role in the architecture and function of composite tissue networks. This noncellular component provides the structural scaffold in which cells grow and assume their niche to ensure the proper functioning of tissues and organs in living organisms. The ECM primarily consists of water, polysaccharides, and proteins; and the composition and topology of the ECM in each tissue is unique and remarkably heterogeneous, which stems from the dynamic biochemical and biomechanical dialogue between various cellular and noncellular components during development (1). In mammals, the ECM is composed of approximately 300 proteins that regulate tissue homeostasis, organ development, inflammation, and disease (2, 3). The major constituents of the ECM are fibrous proteins (i.e., collagen, elastins, fibronectins, and laminins) and proteoglycans (i.e., hyaluronic acid, heparan sulfate, and keratin sulfate). These proteins are primarily secreted and assembled by fibroblasts, and form the organized meshwork that constitutes the mechanostructural framework of tissue networks (1, 2).
Briefly, proteoglycans have a variety of roles in maintaining proper tissue homeostasis and function including environmental buffering, tissue hydration, and providing force-resistance properties through the formation of hydrated gels within the interstitial space (4). However, the main contributors to the content, mechanical structure, and rates of remodeling and degradation of the ECM are the fibrous proteins and their associated proteinases and enzymes. These dynamic interactions between cells and their surrounding microenvironments undergo significant changes as a function of age, both locally and in bulk. As a result, these changes can lead to systemic dysfunctions and the onset of pathogenesis, i.e., impaired wound healing (5), the formation of scar tissue, enhanced metastasis, and cancer progression (2). In healthy individuals, the unique contents and ratios of the ECM’s components (e.g., the ratio of fibrous proteins to proteinase and enzyme levels) dictate the homeostatic relationship between ECM remodeling and degradation. Therefore, for proper mechanical integrity of the ECM there must exist a balance in the coordinated secretions of ECM components and in its subsequent remodeling and degradation by the resident cells (6–9).
Degradation and Remodeling of the ECM
The timely deposition and degradation of the ECM is an important feature in the development, morphogenesis, repair, and remodeling of tissues in living organisms (6). Under physiological conditions, the degradation and remodeling of the ECM is tightly regulated by a large family of enzymatic proteins, primarily matrix metalloproteinases (MMPs) (7, 9), tissue inhibitors of metalloproteinases (TIMPs) (6, 8), crosslinking transglutaminases, and enzymes such as lysyl oxidases (1). MMPs are small-molecule endopeptidases that can break the peptide bonds of nonessential amino acids. These enzymes are collectively able to degrade a wide array of ECM proteins, and also cleave cell-surface receptors, which can lead to downstream activation or inactivation of various pathways and interactions, including cell–matrix interactions, cell–cell interactions, and secretions of growth factors (6). Humans have approximately 24 MMP genes, which code for 23 MMPs (two genes code for MMP-23) that are involved in the degradation of various components of the ECM. For instance, MMP-2 and MMP-9 are able to cleave elastin, type IV collagen, and several other ECM molecules, and MMP-2 can digest type I, II, and III collagen. Studies have shown that the activity of MMPs tends to be low or negligible at normal steady-state tissue conditions; however, the precise expression levels are transcriptionally controlled through feedback mechanisms, which include signaling from inflammatory cytokines, growth factor hormones, cell–cell interactions, and cell–matrix interactions (6, 7). In addition, the functional regulation of MMPs is controlled by (a) the local availability and expression levels of endogenous precursor zymogens, and (b) inhibition by the complimentary inhibitor TIMPs (6, 7). Thus, the intimate homeostatic interactions among MMPs, TIMPs, and other components of the ECM are critical to proper remodeling and the proper functioning of tissues.
The Role of ECM Components in Aging
The ECM has been a central topic of discussion in aging studies for several years. It is associated with numerous age-related diseases and dysfunctions, most of them involving connective tissue, cartilage, bone, blood vessels, and skin (10). The current view is that the ECM’s control of the molecular network is driven by the synthesis of components that are genetically and transcriptionally regulated but also environmentally influenced, thus facilitating a cohort of complex biochemical and biomechanical cues. In the following sections, we discuss the roles and interactions of the various components of the ECM, and the alterations that lead to progressive dysfunction in aging tissues (Figure 1).
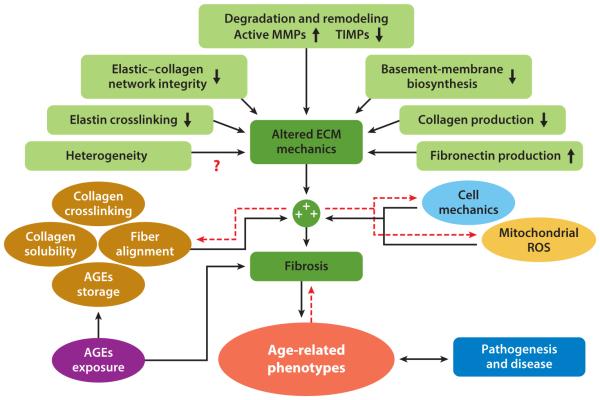
Figure 1.
Age-related dysfunction of the extracellular matrix (ECM). Interactome of the effects of ECM deregulation and mechanical deficiency, cellular and nuclear mechanics, mitochondrial dysfunctions, and cellular damage—e.g., exposure to, and buildup of, advanced glycation end-products (AGEs) in long-lived proteins, such as collagen and elastin (1, 11)—on the aging phenotype. Concomitantly, these effects combine to foster a multidirectional feedback cascade that leads to pathogenesis and disease. Red dashed arrows represent potential bidirectional interactions. Red question marks illustrate potential functional interactions that warrant further study to identify the magnitude of their contribution to age-dependent functional decline. Abbreviations: MMPs, matrix metalloproteinases; ROS, reactive oxygen species; TIMPs, tissue inhibitors of metalloproteinases.
Collagen, being the most abundant and the primary structural element of the ECM—comprising more than 90% of the ECM and 30% of all proteins present in the human body (2, 12)—provides the structural integrity and tensile strength of tissues and organs (such as tendons, ligaments, bone, and cartilage), regulates cell adhesion and migration, and directs tissue development (13). The secretion and assembly of collagen fibers are mediated by fibroblasts that reside within the stromal matrix, or fibroblasts that are recruited from neighboring tissues (1, 2, 14). By exerting tangential stretching forces on the collagen, the fibroblasts under the physiological conditions of the tissues can organize the collagen fibrils into sheets and cables, which in turn drastically changes the alignment of the collagen-fiber network (1). This dynamic change in the alignment of collagen fibers by the fibroblasts results in reciprocal changes through feed-forward signaling that further dictates the orientation, morphology, and alignment of neighboring cells (e.g., keratinocytes and epithelial cells) (15). Collagen molecules and fibers typically bind to other components of the ECM, such as elastins, whose fibers provide the elastic and recoil properties of tissues that undergo repetitive stretching. However, tissue stretching is limited by the intrinsic properties of this heterogeneous, composite mixture of collagens with high tensile strength and the highly elastic elastins (1, 16).
Another major component of the ECM is fibronectin (FN), which is intimately involved in directing the organization of the interstitial ECM, and has a crucial role in cell adhesion and attachment, and cell migration. When FNs are stretched and unfolded via forces exerted by resident fibroblasts, cryptic binding sites are exposed, resulting in changes in cellular behavior and in the mechanical regulation of the ECM (17). Laminins, unlike FNs, which are ubiquitous, primarily constitute the basement membrane, which experiences age-associated changes (18). Although the various fibrous protein components of the ECM differ in structure and function, they collectively play a vital part in the overall function, or dysfunction, of the ECM.
Numerous changes occur during the aging process and these lead to changes at the gross physioanatomical level. Tissues from elderly individuals have a characteristic thinning of the basement membrane, which is in part due to reductions in the biosynthesis of basement membrane protein, and elevated levels of MMP-mediated degradation and remodeling (1, 19). Separate studies have provided evidence that on the transcriptional level there is an increase in the amount of messenger RNA (mRNA) coding for FNs, as well as a subsequent increase in downstream FN biosynthesis with age, as measured by enzyme-linked immunosorbent assays (ELISAs) (10). Although FN is present in abundance during development, and decreases postdevelopment, the marked increase that occurs with age may suggest that FN has alternating roles as a function of age. In addition, resident fibroblasts in tissues from older donors tend to be growth-arrested, have reduced sensitivity to apoptotic signals, and express elevated levels of interleukins, cytokines, and reactive oxygen species (ROS)—a phenotype that is indicative of senescent cells (see Mechanobiology of Senescence and Frailty, below) (1, 11) (Figure 1).
The presence of these senescent cells within the tissue microenvironment, which increases with age (20), induces a state of chronic inflammation. This inflammation, combined with reduced glycosaminoglycans (1), reduced expression levels of TIMPs, reduced crosslinked elastin content (16), elevated levels of activated MMPs, elevated levels of plasminogen activator inhibitors, elevated levels of elastase activity, and elevated levels of mitochondrial-related ROS, compromises the integrity of the elastin and collagen networks, and the basement membrane (1, 21). This deficiency in the structural integrity of the ECM is also associated with a decrease in the biosynthesis of precollagen 1; a subsequent decrease in collagen fibrils (22, 23); and deregulation of the activity of MMPs, which is controlled partly through an increase in cJUN/AP-1 and cell-bound integrins such as α2β1, which has been observed by assessing the levels of both mRNA and protein in tissues, such as skin, bone, and cardiac tissue (22). In addition, studies conducted on samples of sun-protected skin from elderly donors (older than 80 years) exhibited a marked decrease in collagen production relative to controls from a younger cohort (aged 18–29 years), suggesting that these reciprocal effects stem from complex contributions associated with both somatic damage and intrinsic aging (11). Coupled with the fact that there are fewer interstitial fibroblasts in aged skin relative to young skin, this speaks to reductions in growth capacity and reductions in ECM alignment capabilities occurring as a function of age (23).
Although the production and organization of collagen in aged tissue correlate with loose, poorly organized structures, surprisingly, these collagen fibers tend to be inappropriately crosslinked. This increased collagen crosslinking (24)—which has been observed in skin (22, 23), cardiac tissue, and blood vessels (19)—has been partially attributed to a buildup of advanced glycation end-products (AGEs) and byproducts of lipid oxidation occurring through exposure to UV light (1). The elevated levels of AGEs that occur with age result from somatic damage to the regulatory machinery that is tasked with the sensing, degradation, and clearing of these products from the local tissue; the damage to the regulatory machinery fosters the accumulation of AGEs in long-lived structural proteins, such as collagen and elastins (11). Taken together, this loose, disorganized, fragmented collagen network with local regions of enhanced crosslinking is associated with an increase in local tissue stiffness, reduced tissue resilience, and fibrosis, which is characterized by areas of low collagen solubility and degradation (19), poor fiber alignment, and increased Wnt signaling (25). These ECM changes further facilitate mechanically weak tissue and less deformable blood vessels, leading to impairments in the overall function of organs, such as bone, heart, and kidney (1, 11, 19, 22). This uncharacteristic mechanical state of the ECM fuels a vicious feed-forward signaling cascade that severely compromises local and bulk organization, and the integrity of both the fiber network and the residing cells (i.e., fibroblasts and epithelial cells), leading to perpetual tissue dysfunction. These changes further potentiate age-related pathologies, which account for the increases in mortality and comorbidities observed in elderly individuals (11, 26) (Figure 1).
Effects of Age and ECM Mechanics on Functional Wound Healing
Aging entails numerous functional and structural changes, many, but not all, of which adversely affect life span and survival. Although intrinsic aging may begin to explain the convergence in aging phenotypes expressed in various living organisms, the accumulation of damage, along with the propagated stochastic errors in bioinformational processes, and the attenuation of such damages, could explain the differences in longevity seen in various living organisms (11). Exposure to acute injury activates a cascade of intra- and extracellular signaling pathways that induces repair and wound healing (1). In humans, optimal wound healing is a complex, dynamic process that encompasses a number of overlapping and coordinated phases that include hemostasis, inflammation, proliferation, and remodeling (27–29). This continuum of wound healing, and its corresponding biophysical functions, must occur in the proper sequence, at specific times, for specific durations, and at the optimal magnitude of response (27). The first events that characterize wound response are hemostasis and inflammation—that is, the formation of a fibrin clot and the aggregation of platelets that stimulates the infiltration of inflammatory cells—such as monocytes and macrophages—to the sites of injury and the damaged ECM (1, 27). These inflammatory cells serve to (a) release an abundance of chemotactic signaling molecules, such as proinflammatory cytokines, interleukins, growth factors, and MMPs (27); (b) ingest foreign materials and apoptotic cells; (c) increase vascular permeation and promote angiogenesis (28); and (d ) recruit and stimulate fibroblast activity, such as proliferation, migration, and ECM remodeling through chemotactic signal gradients (1). Once fibroblasts and other cell types are recruited, they synthesize and deposit the required ECM proteins, including collagen type I and III, FNs, and the proteoglycan hyaluronic acid. This elevated mechanical state of the wounded environment induces the mechanical and chemical transdifferentiation of cells, for instance of mesenchymal stem cells into myofibroblasts (14, 27, 28, 30). The highly contractile myofibroblasts (27) are able to secrete ECM proteins that degrade and remodel the ECM, and promote mechanically and chemically induced directional migration toward the wound site, a process termed epithelialization (1, 28, 29).
A result of the age-related deterioration of the cellular-response machinery is that the wound-healing process in older adult individuals is impaired, not only in ECM modification but also in the sensing and interpretation of biomechanical and biochemical signals. In vitro studies conducted to measure the motility as a function of age of fibroblast cells plated on flat substrates have indicated that there is a decrease in overall single-cell translocation with age (31). Wound healing in elderly individuals is hampered (32) by a cohort of factors including a decreased capacity to produce ECM components, such as collagens and elastins; increased collagen fragmentation; increased MMPs; reduced collagen solubility and increased fibrosis; a reduced rate of HIF-1α (hypoxia-inducible factor-1α) mRNA and HIF-1α production (33), which influence ECM production (2, 34); a decreased sensitivity to chemotactic and mechanical stimulation (35); reduced motility and translocation by single cells and clusters of cells (31, 34); a reduced proliferation and number of fibroblasts in older tissue; an increased ratio of senescent to normal cells (20); enhanced ROS (1); depleted adenosine triphosphate (ATP) generation (11); and reduced epithelialization (29).
Together with these intracellular mechanical and extracellular changes, there are numerous other factors that affect wound healing, which are both directly and indirectly affected by the aging process. These include nutritional status; activity levels; cigarette and alcohol consumption; chronic diseases, such as diabetes and peripheral vascular conditions; and chronic psychological stress (27). Studies have shown that sex hormones play a major part in age-related deficits in wound healing. Compared with older females, older males show delayed healing of acute wounds, which is partially attributable to the hormones estrogen and androgen and their precursor steroids, all of which appear to have significant effects on wound healing (27). In one study, it was demonstrated that estrogen has significant effects on wound healing. When estrogen was topically applied, there was a marked acceleration in wound closure, an effect observed in both males and females (36). Although we have only briefly discussed some of the mechanobiological effects of age on wound healing, extensive studies have been conducted and reviews have been written; for more elaborate descriptions of wound healing and changes in the process as it applies to different organ types, such as the skin and cardiac tissue, please see reviews by Wu et al. (35) and Guo & Dipietro (27).
AGING AND CELL MECHANICS
Accumulating evidence indicates that aging correlates with progressive changes in the mechanical integrity and impaired response of cells and tissues to mechanical forces (37–40). It has long been hypothesized that the altered mechanical compliance of aging tissues is primarily attributable to changes in the composition, micro- and nanostructure, and organization of the ECM (39, 41, 42). However, the complex interactions of biological, biophysical, and biochemical processes, which are characteristic of living organisms, result from the combined effects of not only physical changes in the ECM but also in the mechanical compliance of cells. These changes in cell compliance in response to stress perturbations affect the intrinsic ability of cells to sense and transduce mechanical signals (35), ultimately mediating physiological degradation and loss of function at the gross physioanatomical level. These changes include the increased incidences of cardiovascular disease and cancer (43), reduced muscle mass, and weakening of the bone through the onset of disorders such as osteoporosis, osteoarthritis, and frailty (44).
Cell Mechanics
Aging correlates with modulations in cell mechanics. This matters because a multitude of cellular and subcellular processes depend critically on the dynamic mechanical deformability of the cytoplasm (45); these processes include the regulation of gene expression (46), the translocation and replication of organelles within the cytoplasm (47, 48), the movement and biogenesis of mitochondrial bodies along cytoskeletal tracks (49), and cell polarization during wound healing (50). These mechanical changes also regulate the ability of cells to protrude, adhere, migrate, and squeeze through 3D matrices and blood vessels (51).
Technological advances during the past 15 years have led to a wide range of sophisticated methods for measuring both the global and local viscoelastic properties of cells. These methods include atomic force microscopy (AFM) (52–54), particle-tracking microrheology (45, 55), optical stretching (56, 57), magnetic twisting cytometry (58, 59), and micropipette aspirations (46, 60). These methods feature different spatiotemporal resolutions, leading to complementary mechanical measurements of cells and tissues.
Many key cellular components and their mutual interactions orchestrate the complex response of cells to changes in their mechanical properties, which undergo dysfunctional changes with age and age-associated pathogenesis. A critical player in this mechanical regulation of cells is the cytoskeleton, the highly entangled network of filamentous proteins (39) that provides cells with their structure and morphology (45). The cytoskeleton consists of three types of filamentous proteins: microfilaments (F-actin), intermediate filaments, and microtubules, all of whose content, organization, and dynamics greatly influence the ability of cells to sense and respond to mechanical stimuli. Other players in this mechanical regulation of cellular compliance include (a) the biomechanical properties of the cell membrane and cytoplasm (e.g., membrane viscosity, which is influenced by lipid and protein contents, ratios of cholesterol to phospholipids, and the local and/or bulk viscosity of the protein and lipid solutions within the cytoplasm, leading to so-called non-Newtonian behavior whereby force and deformation are not proportional) (61); (b) the density of intracellular organelles [e.g., the mitochondria and endoplasmic reticulum (ER) within a local region of the cell, which is indirectly linked to the local cytoskeletal architecture, because these organelles are anchored to cytoskeletal tracks and consequently have coupled dynamics (62)]; (c) the local concentration and activity of cytoskeletal proteins [Rho guanosine triphosphatases (GTPases), crosslinkers, and motor proteins) (63); and (d ) the transport of water throughout the cell, either via external osmotic stimulation or from the internal dynamics of the cytoskeleton and other organelles (64, 65).
Another important contributor to cellular mechanics, which is often overlooked but could potentially play a major part in the functional mechanics of cells, is the memory and homeostasis of cells’ internal machinery in the production and organization of proteins. To obtain a more intuitive understanding of this concept, we consider the following example. Cells that are repeatedly exposed to mechanical stimulation—for instance, in vitro through repeated tapping with an AFM tip (66, 67) or in vivo in patients with dilated cardiomyopathy that has been induced via prolonged tachycardia and atrial fibrillation (68), leading to irregular and asynchronous heart beats—will develop changes in the compliance of cells and tissue in the local area experiencing the mechanical stimulation. Similarly, recent in vitro studies have shown that when serum-starved cells, which have low levels of organized F-actin, are exposed to external mechanical stimulation generated by shear flows or mechanical stretching of the underlying substrate, their cytoplasm rapidly assembles actin into highly organized fibers, and a distinct perinuclear actin structure called the actin cap (69–72) emerges. Through activation of the ROCK (Rho-associated coiled-coil kinase) pathway, this increased F-actin organization adds to the mechanical rigidity of the cytoplasm by strengthening physical connections among the cytoskeleton and the nucleus and the ECM (45). Although F-actin structures are highly dynamic in nature, progressive shifts occur during aging in regards to the homeostatic ratio of cells with organized versus loosely organized structures, and cells with high versus low F-actin content. These shifts in the content and structure of F-actin, in turn, facilitate increased dysfunction in the mechanical phenotypes of cells and their surrounding tissues. However, this impaired homeostasis and the inability of cells to elastically respond to stresses, which are characteristic of the age-related phenotype, are, in part, due to intrinsic aging and to prolonged exposure to mechanical stimulation, coupled with somatic damage to proteins and organelles (11), which fuel mechanical deterioration and loss of function.
Mechanical Changes as a Function of Age
Changes in the mechanical properties of cells are hallmarks of the aging process (62). Indeed, numerous studies have demonstrated that there is a strong correlation between age and cytoplasmic stiffness (i.e., cytoplasmic compliance or deformability is reduced with age) (Figure 2). Studies that have applied AFM to adherent human cells [epithelial cells (37, 42, 73), fibroblasts (38), and cardiac myocytes (40)] seeded on flat substrates have shown that cells consistently respond to mechanical activation with a stiffening response as a function of increasing age. This suggests that age-dependent cytoplasmic stiffening is not cell-type specific. Moreover, this stiffening affects all cell regions (the cell edge, cytoplasm, and perinuclear region) (37). Experiments conducted using a microfluidic optical stretching device that measures the elasticity of detached cells also have shown that there is enhanced stiffening with increasing age (39). Even suspended samples of red blood cells derived from healthy donors experience reduced deformability as a result of stiffening with increasing age (61, 74).
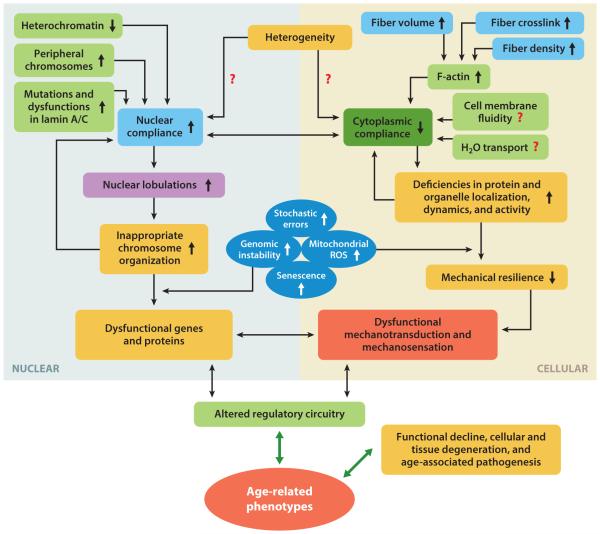
Figure 2.
Interactome illustrating the functional coupling of cellular and nuclear mechanics, and their influences on biochemical mediators and responses. Age-related phenotypes, which foster functional decline and cellular degeneration, are associated with the disruption of multidirectional interactions between biomechanical and biochemical pathways. Red question marks illustrate potential functional interactions that warrant further study to identify the magnitude of their contribution to age-dependent functional decline. Abbreviation: ROS, reactive oxygen species.
Amid these studies, there exist conflicting results that show cytoplasmic softening with age (75). However, this study brings up an interesting premise that warrants further investigation: cellular heterogeneity. Cellular heterogeneity plays an important part in dictating cell function—that is, how cells process information and respond to perturbations (76, 77). Although often neglected in aging studies, heterogeneity can be dictated by many factors, in vitro and in vivo, from both cell-intrinsic and cell-extrinsic factors, such as stochasticity in cellular morphogenesis, the cell-cycle state, cell–cell and cell–matrix interactions, genetic predispositions, lifestyle (factors such as nutrition or diet, and exercise), and environmental factors and exposures. To some degree, cell-to-cell differences are always present in any cell population, and the ensemble-averaged behavior of a population (cellular as well as for an individual patient or donor) may not represent the behavior of any single entity (78). In light of this, further studies are needed that are based on large sets of samples from donors, that address both the single-cell and single-individual levels, to decipher the relationship between heterogeneity and aging. These studies should place special emphasis on the bidirectional effects of aging and on the rate and emergence of dysfunctional age-related phenotypes with respect to both cell-intrinsic and cell-extrinsic factors. Results from such studies may lead to the development of novel approaches for patient stratification and therapeutic interventions to combat age-associated dysfunctions and pathologies.
The age-dependent increase in the rigidity of cells is accompanied by the onset of numerous diseases, including vascular degeneration (79), cardiac dysfunction (80), and cancer (81). Considering the importance of cellular mechanics to the correct physiological functioning of cells (39), an improved understanding of the underlying molecular basis for this mechanical dysfunction is essential. Clearly, the cytoskeleton plays a central role in modulating cell mechanics with age (39). Since the state of actin, intermediate filaments, and microtubule polymerization contribute to the mechanical rigidity of cells, it is important to understand how the content, structure, and organization of these cytoskeletal components changes with age. Fluorescence-activated cell-sorting (FACS) flow cytometry analysis has indicated that the level of globular actin (G-actin) is maintained and does not change significantly with increasing age. However, F-actin content increased significantly with age in dermal fibroblasts from a young donor cohort ranging in age from 20 to 27 years to those from an older donor cohort ranging in age from 61 to 72 years (39). Hence, increased levels of F-actin and constant levels of G-actin, corresponding to an increased ratio of F-actin to G-actin, suggests that actin assembly is enhanced in cells derived from older donors versus those from younger donors (39). Not only is there more F-actin in cells from older donors (Figure 3), but there is also (a) increased cytoskeletal volume in cells from older donors relative to cells from younger donors, (b) increased cytoskeletal crosslinking and bundling [that is, effects from crosslinking proteins, such as fascin and α-actinin (63, 82, 83)], (c) an associated increase in cytoskeletal density (more fibers per unit area), and (d ) reduced variation in fiber density (that is, a narrower distribution equals a more similar fiber density per cell) associated with increased age (37, 73) (Figure 2).
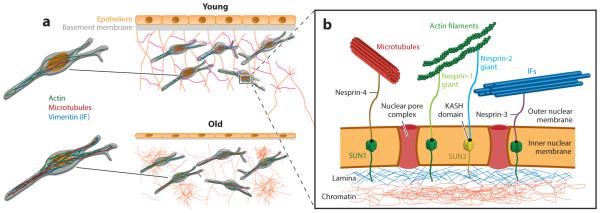
Figure 3.
Overview of age-dependent cellular, nuclear, and extracellular changes associated with age. (a) Young and old epithelial microenvironments where age-dependent changes involve cellular atrophy, thinning of the basement membrane, degradation of the extracellular matrix, local regions of fibrosis, an increased number of and thicker actin filaments, and enhanced nuclear lobulations. (b) Overview of LINC (linker of nucleoskeleton and cytoskeleton) complex proteins that physically connect the cytoskeleton to the nuclear lamina. The nuclear envelope comprises nuclear pore complexes that enable the transport of cargo in and out of the nucleus. The envelope also contains SUN1 and SUN2 proteins, which span the inner nuclear membrane and interact with the nuclear lamina, namely with lamin proteins. SUN proteins also contain a KASH-binding domain, which enables their interactions with KASH-domain proteins. KASH proteins span the outer nuclear membrane and provide a direct link to various cytoskeletal filaments, including microtubules, F-actin, and intermediate filaments. KASH-domain proteins include Nesprin-1, -2, -3, and -4. Directly underneath the nuclear lamina is nuclear DNA in the form of chromatin. The structural connection of KASH and SUN proteins between the cytoskeleton and chromatin facilitate mechanotransduction between the cell exterior and the nuclear interior. Abbreviation: IF, intermediate filament.
The intermediate filament vimentin (84), and to a lesser extent microtubules, can contribute to cell mechanics (50). However, FACS analysis has indicated no statistically significant changes in the content of microtubules and vimentin in suspended dermal fibroblasts from donors of different ages (39). In addition, the location of F-actin just beneath the plasma membrane and the high mechanical strength of the actin cortex suggest that F-actin and its associated proteins collectively dominate the viscoelastic response of cells to small deformations (39). This provides further confirmation of the hypothesis that the mechanically rigid phenotypes observed in older individuals are linked to altered (a) actin polymerization and (b) actomyosin contractility, which is dictated by the content and organization of F-actin fibers and the associated motor protein, myosin (37–39). Although the vast majority of the literature has focused on how the properties of the cytoskeleton (primarily F-actin) drive enhanced stiffness with age, it will be interesting to understand how the other aforementioned factors, such as the properties of cell membranes and the local distribution of intracellular organelles, contribute to the cellular stiffening response that occurs with age.
Reversal of Age-Associated Mechanical Phenotypes
The characteristic changes in the mechanical properties of aging cells induce physiological dysfunctions and present avenues for deficiencies in mechanosensation and mechanotransduction (35, 41). In efforts to better understand how to potentially reduce these age-dependent mechanical dysfunctions in cells and tissues, studies have been geared toward assessing the possibility of reversing the mechanical phenotypes of cells derived from elderly individuals (38, 42). Due to the dominating effects of the actin cytoskeleton and the increased F-actin content (38, 39) observed in cells from older donors (i.e., fibroblasts, cardiac myocytes, and red blood cells), a widely studied target for possible phenotypic reversal is the use of pharmacological agents that affect F-actin. Studies conducted using the F-actin-depolymerizing drug cytochalasin B and the F-actin-stabilizing drug jasplakinolide indicate that fibroblasts treated with cytochalasin B that were aged in vitro on a dish (>50 doublings) regressed to levels of cytoplasmic stiffness comparable to those of earlier passage cells (<25 doublings) (42, 73). In addition, dermal fibroblast cells from a young donor cohort resembled the cytoplasmic mechanics of samples from older donors after treatment with jasplakinolide (39). Similarly, human fibroblasts exposed to an antiwrinkle tripeptide demonstrated decreased cytoplasmic stiffness, with larger effects seen in samples from older donors (aged 60 years) relative to samples from younger donors (aged 40 and 30 years). This reversal was partly due to direct effects on the cytoskeleton, primarily F-actin, as seen from changes in the content, structure, and contractility after treatment (38). However, although these studies show evidence of mechanical reversal in vitro by manipulating actin directly, additional studies should be performed to further elucidate and identify reversal in vivo and to evaluate the role of cytoskeletal control on cellular aging.
Mechanical Properties of Cells in Models of Accelerated Aging
In recent years, much effort has been made to elucidate and understand the mechanisms of various aging pathologies, as well the onset of maladies that resemble the normal aging process, which are termed accelerated aging (85). A well-studied set of such accelerated aging diseases falls under the general category of laminopathies, which arise from content and structural dysfunctions in the intermediate filament lamin A/C, encoded by the LMNA gene. In somatic cells, lamins are separated into two subcategories: A-type lamin (lamins A and C, which result from alternative splicing of the LMNA gene) and B-type lamin (lamins B1 and lamin B2/B3, encoded by the LMNB1 and LMNB2 genes, respectively) (86). These laminopathies include Hutchinson–Gilford progeria syndrome (HGPS), Werner’s syndrome and Emery–Dreifuss muscular dystrophy (85). A study using cells derived from mice models deficient in lamin A/C has shown that there is a significant loss of cytoplasmic stiffness relative to wild-type controls as assessed by cellular microrheology (87). LMNA−/− cells treated with the actin- and microtubule-depolymerizing drugs, latrunculin B and nocodazole, respectively, demonstrated cytoplasmic stiffness similar to that of control cells. As expected, wild-type LMNA+/+ cells treated with these drugs showed a decrease in cytoplasmic stiffness, down to levels comparable with that of LMNA−/− cells (87). Taken together, these results indicate that direct cytoplasmic mechanical properties stemming from deficiencies in lamin A/C (in both content and structure)—which are characteristic of models of accelerated aging—occur contrary to the widely accepted body of knowledge with regards to cytoplasmic stiffness increasing with increasing age. This suggests that although numerous studies have shown that there is a decrease in the fraction of cells expressing lamin A/C as age increases (88, 89), there may be compensatory effects in lamin A/C structure, localization, and organization that dominate these accelerated-aging diseases but have diminished effects on normal aging processes, thus providing avenues for further study.
NUCLEAR MECHANICS
The nucleus, which stores a cell’s genetic information, is directly involved in the functional activity of the entire cell. Although conventionally thought of as a static structure, the nucleus is a dynamic organelle that is constantly subjected to mechanical forces, which in the context of aging and disease can result in nuclear alterations and deformities. Nuclear mechanics in response to mechanical perturbations are highly dependent on the structure and components of the nucleus.
The nuclear envelope consists of an inner and outer membrane, with approximately 30–50 nm of perinuclear space; the nuclear envelope separates the cell’s cytosol from its genetic material. It is composed of two phospholipid bilayers that have approximately 50–100 associated membrane-bound and integral proteins (90). The two membranes are interrupted by nuclear pore complexes, which mediate the transport of macromolecules between the nucleus and the cytoplasm. Directly beneath the inner nuclear membrane is a dense protein network, termed the nuclear lamina (91) (Figure 3). The nuclear lamina has an essential role in determining the mechanical properties of the nucleus (92). The nuclear lamina is composed mostly of V-type intermediate filaments, or lamins. Although B-type lamins are essential for viability, A-type lamins are developmentally regulated. Lamins A and C provide the nucleus with structural support, contribute to the stiffness and stability of the nucleus, and are essential for the direct connection between the cell’s cytoskeleton and the nucleus (86). As a result, lamin A/C is required for enabling the mechanotransduction of forces from the extracellular environment via focal adhesions through the cytoskeleton and into the nuclear interior (93, 94), a connection that is mediated by lamin-associating proteins. These include SUN-domain-containing proteins, which are localized at the inner nuclear membrane, and the KASH-domain proteins, which are localized at the outer nuclear membrane (91) (Figure 3). SUN proteins interact with the nuclear lamina and the nuclear pore complex as well as with other nuclear proteins, such as emerin. KASH-domain-containing proteins bind to major networks of cytoskeletal filaments. These include nesprin 1 and 2 giant (nuclear envelope spectrin repeat) isoforms, which bind to, respectively, actin and microtubule filaments; nesprin 3, which interacts with intermediate filaments via plectin and nesprin 4; and KASH 5, which binds to microtubule filaments (95). Together lamins and SUN and KASH proteins comprise the LINC (linker of nucleoskeleton and cytoskeleton) complexes (Figure 3).
Through these nucleus–cytoskeleton connections, nuclear responses to external or extracellular forces can be transmitted from the plasma membrane to the nucleus (96), resulting in intranuclear rearrangement and deformation. Recent studies have demonstrated that disrupting the LINC complex by using dominant negative constructs of nesprin and SUN proteins prevented nuclear deformations in response to the stretching of the cell’s underlying substrate (97). Nuclear deformations and architectural rearrangements can promote conformational changes in nuclear proteins as well in the nuclear interior, namely in chromatin. These changes include the release of transcription factors as well as the movement of segments of chromatin to and from transcriptionally active or repressed regions (91). This physical tethering is a direct result of the interactions between nuclear lamins and chromatin (91). Ultimately, the mechanical properties exhibited by the nucleus are a synergy of (a) the nuclear lamina content; (b) the interconnections among the nuclear lamina, the cytoskeleton (through the LINC complexes), and chromosomal DNA (through lamin-associated proteins); and (c) the content of the nuclear interior (which is partially mediated by the osmotic flux of water and ions in and out of the nucleus).
Aging and Nuclear Mechanics
Cytoskeletal mechanics are tightly coupled to nuclear mechanics (91) (Figure 2). Hence, aging promotes not only cytoskeletal changes but also nuclear changes (91, 98). During development, when stem cells differentiate into distinct lineages, nuclear mechanics change with differentiation. Live-cell confocal tracking of nuclear lamina and the use of micromanipulation methods have indicated that nuclear stiffness greatly increases when terminal differentiation is induced in human adult stem cells and hematopoietic stem cells (92, 99). In addition, mouse embryonic stem cells display greater mechanical plasticity than their differentiated counterparts (92). In part, this may be due to the lack of expressed Lamin A in embryonic stem cells, which is a key mediator of mechanical and structural support for the nucleus cells (100).
Nuclear alterations and the physiological function of the nuclear structure associated with human aging and disease have been extensively studied with regards to laminopathic models of aging. Cells from HGPS patients show progressive abnormalities in their nuclear shape and architecture, with excessive nuclear lobulations or blebbing, and invaginations (101, 102). The nuclear morphological changes that occur in accelerated-aging models also occur in normal aging (98, 103). In the HGPS model, the observed nuclear abnormalities have been linked to the accumulation of mutated lamin (progerin) in the nuclear lamina, and have more recently been observed in cells from healthy aged donors (98). Progerin accumulation has been observed in the nuclei of cells in the blood vessels and skin of normally aging individuals (104, 105). Lamin A/C, which is required for the structural link between the nucleoskeleton and cytoskeleton, is essential for mechanical support of the nucleus, and enables force transmission across the nuclear envelope (91, 106). Thus, a weakened, dysfunctional nuclear lamina is more susceptible to the various stresses and types of mechanical loading that occur within the human body. Nuclei from cells harboring mutations or deletions in lamins display decreased nuclear stiffness and increased vulnerability to mechanical strain (107–110). Further, vascular smooth muscle cells from normally aging individuals have higher levels of prelamin A, a precursor of lamin A that is present in cells from HGPS, where the cells of younger individuals lack detectible amounts (111). Furthermore, the Caenorhabditis elegans model displays age-dependent changes in nuclear structure, such as aberrant shape changes (103). As a consequence of this age-associated softening of the nucleus, intracellular and extracellular mechanical cues may drive inappropriate reorganization of chromatin (which is intimately tethered to the cytoskeleton), thus facilitating genomic instability, as well as errors in heterochromatin, and epigenetic errors and defects (43) (Figure 2).
Heterochromatin and Epigenetics
Chromatin is a complex of nuclear DNA associating with histone proteins (112). The basic structural unit of chromatin, the nucleosome, is approximately 147 base pairs of DNA wrapped around histone octamers. Histones are positively charged structures that facilitate DNA compaction by acting as spools for negatively charged DNA to wrap around. Dynamic and highly regulated modifications to DNA and histones occur via chromatin-modifying enzymes. These modifications alter DNA accessibility and chromatin structure, and concomitantly regulate gene expression without making direct changes to the genome. Heritable modifications to the cellular phenotype that occur independently of changes to the DNA sequence are termed epigenetics (112). Epigenetic modifications are essential for normal development and enable the differentiation of cells into different lineages (113). For DNA, the primary modifications are the covalent methylation of cytosine residues at the C5 position. This modification is associated with gene silencing in eukaryotes and is essential for controlling the architecture of the nucleus (114, 115). DNA methylation occurs with the DNA methyltransferase 3 family of de novo DNA methyltransferase 1. However, pathways involving the establishment of DNA methylation patterns, including the addition and removal of methyl groups and their maintenance, require further characterization and insight.
Histones are essential structures that regulate gene expression through posttranslational modifications at various residues through the modulation of DNA packing. These modifications include acetylation and methylation at lysine (K), methylation at arginine (R), and phosphorylation and methylation at serine (S) (114). Each modification and its location influence differential gene expression. For example, the acetylation of histone H3 at lysine sites is typically associated with transcriptional activation. Methylation of histone H3 at K9 and K27, and histone H4 at K20, are associated with translational repression and DNA compaction, but methylation of histone H3 at K36 is associated with transcriptional activation (116). Histone modifications occur as a result of enzyme activity, i.e., the activity of histone acetylases, deacetylases, methylases, and demethylases. Further, chromatin is divided into two major categories: first, heterochromatin, which is condensed DNA containing transcriptionally inactive genes (117), and, second, euchromatin, which is a relatively open DNA structure containing mostly transcriptionally active genes (118).
The nuclear interior is also a major contributor to the mechanical properties of the nucleus. Experiments using micropipette aspiration have demonstrated that chromatin provides the majority of the resistance to deformation in unswollen nuclei (119, 120). In addition, alterations to the structure and organization of chromatin—such as the inhibition of histone deacetylase, differentiation, or the increased expression of heterochromatin proteins—promote changes in nuclear mechanical properties (92, 119, 121).
Aging, Chromatin, and Epigenetics
Chromatin and epigenetic modifications are critical, from early development through to older age. For example, the ability of stem cells to differentiate into various lineages requires not only changes to the structure of chromatin but also distinct and directed epigenetic alterations that allow greater access for the binding of transcription factors (121, 122). Because the structure and organization of chromatin influence the mechanics of the nucleus, their changes may explain the changes in nuclear deformability that occur with differentiation.
In HGPS models, the accumulation of mutated lamin within the nuclear envelope is accompanied by the loss of peripheral heterochromatin directly underneath the nuclear lamina (123). A similar reorganization of chromatin throughout aging has been observed both in human cell lines and in C. elegans (103). There are numerous changes in histone modification that are associated with increased age, including an increase in histone acetylation of H4K16, trimethylation of H4K20 and H3K4, and a decrease in methylation of H3K9 and H3K27 (124, 125). In addition, a number of histone modifications have been associated with an increase in life span and survival. For example, the loss of histone demethylases for H3K27 promotes longevity in worms by altering insulin and insulin-like growth factor (IGF) signaling (126), which are key pathways known to regulate life span in yeast, C. elegans, fruit flies, and rodents.
In addition, an age-dependent decrease in total genomic DNA methylation has also been observed (127): Because DNA methylation elicits the formation of constitutive heterochromatin, its decrease promotes deheterochromatinization, leading to reduced nuclear compliance. Although a decrease in heterochromatin occurs with age, its accumulation has also been observed at specific sites. In rat liver and kidney cells, total levels of histone H4 methylation at lysine 20 (H4K20me) increase with age (128). In addition, recent studies have observed that the accumulation of heterochromatic domains was associated with senescence (129) and cellular aging. These domains, termed senescence-associated heterochromatic foci, form repressive chromatin structures that are directly involved in the repression of genes that promote cell growth (129). Recognition of the loss of peripheral chromatin and the changes in cellular epigenetics that occur with age has led to a notion of age-associated heterochromatin reorganization, which would result in genome-wide transcriptional changes (130).
MITOCHONDRIAL DYSFUNCTION
As organisms age, specific changes occur that lead to diminished integrity in the mitochondrial structure–function relationship (43, 131). For many years, it was postulated that there was an intimate relationship between organismal aging and mitochondrial dysfunction, but the question of whether dysfunctions in mitochondria drive the aging phenotype or vice versa, is still under debate, and resolving this issue remains a major challenge in aging research (132–134). Regardless of the order of the chicken or the egg, it is clear that the decline in mitochondrial function has a key role in the aging process, and this decline is associated with the onset and progression of age-related disorders (134). The popular free-radical theory of aging proposes that this cumulative damage to biological macromolecules caused by ROS leads to irreversible cellular damage and overall functional decline (43, 134, 135). This theory has been further extended to include mitochondrial ROS and shows that the accumulation of age-associated alterations in mitochondrial DNA (136) and global cellular damage leads to impaired efficacy of the respiratory–electron transport chain and mitochondrial biogenesis, and induces a perpetual feed-forward production of ROS. This results in a vicious positive-feedback loop that fosters exponential increases in oxidative damage and dysfunctions, which resonate at the cellular, tissue, and gross physioanatomical levels (134, 136–138).
Mitochondria are highly complex and dynamic organelles that can alter their organization, morphology, size, and bioenergetics in response to intra- and extracellular cues (134). Mitochondrial bodies undergo bidirectional cycles of regulated processes—fusion and fission—that, in turn, influence their morphology (134, 139), dynamics (140, 141), and bioenergetics (142, 143), and even play a part in cell-cycle regulation and growth (144, 145). As with any properly functioning, adaptive system, the cell maintains regulative control over mitochondrial fusion and fission processes. At steady-state conditions, homeostasis results in functionally stable, yet heterogeneous, mitochondria (146). However, this balance can be shifted in favor of either fusion or fission, based on the time-dependent, functional, and energetic needs of the cell. Decreased mitochondrial fusion results in mitochondrial fragmentation due to continuous fission, and is associated with increased mitochondrial outer membrane permeabilization and decreased mitochondrial membrane potential. In contrast, decreased fission induces long, highly interconnected mitochondrial networks, which are associated with enhanced mitochondrial membrane potential (134).
Different cell types exhibit different mitochondrial structures as a function of their location and roles [e.g., in terms of energetic needs, consider heart cells versus lung cells (147)], their stress levels (apoptotic versus healthy) as well as their phase within the cell cycle (G0/G1 versus S versus G2M phases) (142–144). Healthy myoblasts and fibroblasts are often highly networked structures, possessing both fragmented and tubular mitochondria. However, during the G1/S transition, mitochondria coalesce into a giant, single tubular network, displaying hyperpolarization and increased generation of ATP (144). This characteristic increase in ATP production, brought about by electrical coupling of the mitochondria, is not surprising because the membrane potential serves as the ionic gradient for ATP generation (145). During this transition, increased ATP production—the energy currency of the cell—is understandable because during the S phase the cell is involved in the synthesis and reorganization of numerous proteins (e.g., cytoskeletal proteins, such as F-actin and microtubules) and organelles (such as, the Golgi apparatus and the ER). As the cells continue into the different phases of the cell cycle and progress toward mitosis, the mitochondria attain a larger fraction of fragmented, topologically distributed mitochondria (145). Moreover, as an organism ages, there are characteristic changes in, and deterioration of, essential components that make up the mitochondrial regulatory and repair machinery (i.e., mitophagy, cell-cycle control and proliferation), consequently giving rise to dysfunctional phenotypes. These dysfunctional phenotypes further induce and facilitate adverse effects on overall mitochondrial health (e.g., morphology and biogenesis) and on cellular bioenergetics (ATP production). In the next section, we discuss some of the primary mitochondrial age-dependent dysfunctions.
Many time-dependent mitochondrial dysfunctions occur with age, including diminished efficacy of the electron-transport chain [due to increased electron leakage, increased oxidative stress (148), and ROS production], reduced ATP production, and a marked decline in mitochondrial function [especially in samples of muscle tissue (149)] and turnover (due to reduced biogenesis, inefficient mitochondrial degradation, or both; and mitophagy) (43, 134, 150) (Figure 4). The findings of many studies have supported an increase in ROS as chronological age increases; however, confounding results regarding the negative, positive, or neutral effects of mitochondrial ROS, or a combination of these, have recently sparked a reevaluation mitochondrial free-radical theory (43). Studies conducted in C. elegans (151) and mice (152) suggest that ROS may prolong life span and survival. However, comprehensive studies on mice that had genetic modifications to increase mitochondrial ROS production and oxidative damage did not find accelerated aging. Similarly, separate studies that increased antioxidant defenses (153) and impaired mitochondrial function in mice (133, 154) did not extend life span or accelerate aging, respectively. However, a novel framework postulated by Lopez-Otin et al. (43) may help explain the confounding evidence regarding the roles of ROS. As organisms age, there is an increase in the associated cellular stresses and damage, which concomitantly facilitate an increase in ROS production that maintains survival. However, there is an upper limit (and this limit may be inter- and intraorganism dependent) beyond which the ROS levels betray their original homeostatic purpose and eventually aggravate, rather than alleviate. This increased level of ROS facilitates the perpetual cell-associated damage (43) and fuels bidirectional ECM dysfunction through enhanced collagen fragmentation and activation of MMPs (22), as well as by altering the architecture and activity of actomyosin cytoskeletal contractility (155) (Figure 4).
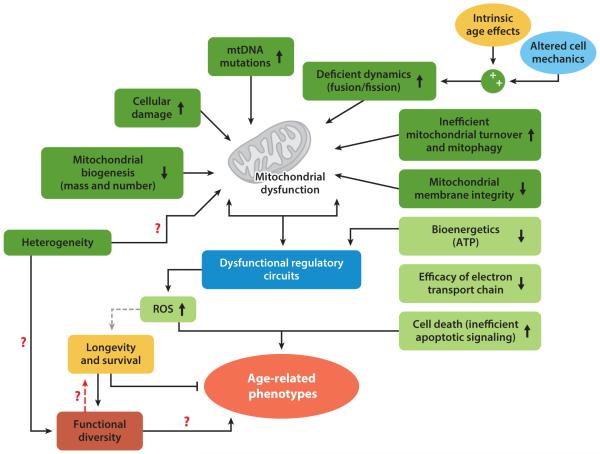
Figure 4.
Age-related mitochondrial dysfunction. Mitochondrial function becomes deficient with age-associated changes, including cellular damage, decreased mitochondrial biogenesis, and compromised membrane integrity. This leads to dysfunctional regulation of cellular processes, and a complex feedback cascade that perpetuates the dysfunction. In healthy, young individuals, levels of reactive oxygen species (ROS) are maintained within the optimal range that promotes longevity and survival. However, during aging, when ROS regulation becomes progressively more inefficient in dictating cellular responses to stress, it leads to impaired bioenergetics and cell death (43). A key question that remains to be answered is how heterogeneity and functional diversity relate to the perpetuation or remediation of these dysfunctions, and whether this occurs through mitochondrial dysfunction, functional diversity, or both. The red dashed arrow represents a potential bidirectional interaction; the gray dashed arrow represents the interaction that has been proposed in the literature but warrants further study. Red question marks illustrate potential functional interactions that warrant further study to identify the magnitude of their contribution to age-dependent functional decline. Abbreviations: ATP, adenosine triphosphate; mtDNA, mitochondrial DNA.
As previously stated, mitochondria are highly dynamic structures that can rapidly adapt their morphology and function in response to a wide range of chemical and mechanical stimuli, whether intracellular or extracellular, or both. Progressively, mitochondria experience age-dependent decreases in morphological plasticity and the capacity for biogenesis (134). In healthy cells, mitochondrial fusion provides a synchronized internal cable for the translocation and mixing of metabolites, whereas mitochondrial fission facilitates the equal distribution of mitochondria into daughter cells during cell division, and allows for the regulated degradation of damaged mitochondria through autophagy (134, 156). The reduction in the efficiency of mitochondrial bioenergetics that occurs as a function of age may result from multiple converging mechanisms, including (a) reduced mitochondrial biogenesis, (b) the accumulation of mutations and deletions in mitochondrial DNA, (c) an increase in ROS and oxidative damage to mitochondrial proteins, (d ) an increased destabilization of the macromolecular organization of the respiratory chain complexes and super complexes, (e) changes in the lipid composition of the mitochondrial membranes, ( f ) deficiencies in mitochondrial dynamics resulting from an imbalance of fusion and fission events, and ( g) defective mitochondrial turnover and quality control by mitophagy (43). As a direct result of these age-dependent dysfunctions—which are functions of intrinsic and extrinsic aging (132)—oxidative damage may surpass a crucial threshold, resulting in ineffective maintenance of functional mitochondria, thus fueling senescent phenotypes and triggering apoptosis, and leading to substantial changes in mitochondrial morphology, irreversible cell death (134), altered cytoskeletal dynamics (155), and deficient ECM (Figure 4).
Other studies have shown that mitochondrial integrity and function can be preserved and ameliorated during the aging process by lifestyle practices (e.g., by ensuring appropriate nutrition, or engaging in alternate-day fasting or calorie restriction) (133); physical stimulation, primarily for muscle cells (e.g., through endurance training or exercise); early diagnosis of age-related phenotypes (e.g., prefrail or frail phenotypes) (157); and therapeutic interventions (43, 134). Here, we have only briefly described some of the age-associated mitochondrial dysfunctions as they relate to mechanobiology; for a more in-depth review of age-associated biochemical aspects please refer to Seo et al. (134), Trifunovic & Larsson (133), and Terman et al. (150).
AGING AND DISEASE
Despite improvements in health-care delivery and life expectancy during the past century, age continues to be the greatest risk factor for most chronic diseases and pathologies, including a range of cardiovascular and neurodegenerative conditions. The mitochondrial, cellular, and extracellular changes described above are likely to contribute to accumulating cellular damage and, ultimately, to these pathophysiological disease processes. Although further study is needed to find clearer connections among these age-related cellular changes and chronic disease states, some components and mechanisms are already understood and are described below.
Mechanobiology of Senescence and Frailty
In the 1950s it was believed that the aging process was solely perpetuated by an increase in damage to proteins, lipids, and nucleic acids resulting from oxidative damage, i.e., oxygen radicals. Indeed, recent studies have shown that increasing or decreasing the activity of cell-defense pathways against such radicals modulates the longevity of an organism (158, 159). Moreover, some have postulated that aging occurs as a result of the accumulation of cellular damage, i.e., the accumulation of nuclear DNA damage, misfolded proteins, and telomere erosion (160, 161). Interestingly, similar cellular damage is associated with senescence. Senescence is a state of irreversibly arrested growth that occurs as a result of genomic stress or oncogenic stimulation. In response to damaging stimuli, cells enter senescence via the initiation of either one or two tumor-suppressive pathways. These include the p53 and pRB pathways. Both proteins are important transcription factors and cell-cycle regulators. In response to cellular damage, these proteins halt the cell cycle in an attempt to rectify the damage via a p53-dependent response to damage (161). When damage is irreparable, cells are permanently halted from cell-cycle progression and fail to undergo cell division. This is a means of preventing the perpetuation of cellular damage from one generation to the next, and thus provides a tumor-protection mechanism for cells in response to oncogenic stimuli (162, 163).
Although senescence is thought to be beneficial in this regard, it has recently been demonstrated to promote an increase in the secretion of cytokines, growth factors, and proteinases (163). This enhanced secretion is termed the senescence-associated secretory phenotype. This phenotype contributes to age-related pathologies by stimulating tissue remodeling and promoting tumor progression. These processes include enhanced invasion, proliferation, loss of cell-to-cell contacts, and an apparent epithelial-to-mesenchymal transition (163, 164). Senescent cells increase as a function of age (161, 165). Studies conducted to clear senescent cells from mice models have resulted in delayed age-related pathologies, including reductions in lordokyphosis and cataracts. In addition, such manipulations have shown that the mice had an increased ability to perform exercise and displayed increased adiposity (165), resulting in a less frail phenotype.
Mechanical changes have also been shown to occur as cells enter into senescence. The most apparent is the dramatic change in cell morphology. Senescent cells acquire significantly enlarged cell morphology due to the continued stimulation of the cell-growth pathways, MAPK (mitogen-activated protein kinase) and mTOR (mammalian target of rapamycin) (166). Senescence is also linked to increased expression of the intermediate filament vimentin, decreased expression of actin and tubulin, and decreases in the focal adhesion protein paxillin and c-Src (167). Further, senescence is associated with spatial alterations to the nuclear lamina, along with the resulting changes in the shapes of the nuclear lamina and nucleus. This includes increased nuclear lobulations and invagination, as well as the local accumulation of lamin A in the nuclear envelope (168). Changes in the expression of mechanosensing and mechanotransducing proteins may alter how senescent cells in vivo properly respond to internal and external stressors. Although more knowledge is required in regard to the mechanical changes that occur with senescence, recent work using multipotent human mesenchymal stem cells has demonstrated that senescent cells can be characterized by decreased cytoskeletal stiffness, contractility, and motility (169).
Frailty is an age-dependent syndrome that is often synonymous with disability and comorbidity, in which frail individuals have a high risk of falls, hospitalization, and morbidity (170). In a study performed by Fried et al. (170), a frail individual was further defined as one who met at least three of the following criteria: an unintentional decrease in weight during a year, fatigue, weakness as measured by grip strength, slow walking speed, and low levels of physical activity. This definition is predictive of deteriorating mobility and falls, and is much more likely to predict mortality when these individuals are compared with age-matched nonfrail individuals. Despite the potential of cell mechanics to act as a label-free marker and the clinical importance of frailty, it remains to be assessed and clinically validated whether cell mechanics can predict frailty.
Cardiovascular Disease
Cardiovascular disease, which includes all pathologies related to the heart and circulatory system, continues to be the leading cause of death in the United States (171). The primary cause of cardiovascular disease is atherosclerosis, which results from the buildup of plaque inside the arteries, which, in turn, causes hardening and narrowing of blood vessels. However, evidence suggests that age-related changes in cardiomyocytes and valvular tissues that result in heart-valve malfunction and congestive heart failure are increasingly important causes of morbidity and mortality in older adults. Although important risk factors for cardiovascular disease include obesity, high blood pressure, and diabetes (172), age is a major contributor, as evidenced by the fact that most cardiovascular diseases are not present until middle or older age. This may be due to the decline of normal heart function with age: for example, the increase in cardiomyocyte apoptosis, the decrease in cell contractility, as well as hypertrophy and fibrosis (173). Apoptosis, which is typically characterized morphologically by cell shrinkage and chromatin compaction, is postulated to occur partly as a result of a decline in mitochondrial function (172). Contractile cells, such as cardiomyocytes, require large amounts of energy to maintain proper cellular function. However, as dysfunctional mitochondria accumulate with age (172), cells lose the ability to meet their energy demands. The accumulation of dysfunctional mitochondria initiates cell death and can lead to a chronic loss of cells in the heart (174). The contractile properties of the heart are especially important in cardiovascular diseases such as dilated cardiomyopathy, which is characterized by thinning of the ventricular wall and enlargement of the ventricular chamber (175). Dilated cardiomyopathy is marked by mutations in various cytoskeletal-associating and -regulating proteins, as well as proteins critical to the mechanotransduction of forces between the cell exterior and interior. Some of these include mutations in β-myosin heavy chain protein, which can cause ventricular wall thinning; α-tropomyosin 1, which may cause ventricular wall stiffening; and the intermediate filament desmin, which is linked to enlargement of the ventricular chamber. Mutations in genes encoding lamin A/C and the focal adhesion protein vinculin, have also been associated with dilated cardiomyopathy (175). The functional loss of these proteins internally affects the contractility of cardiomyocytes and influences their ability to respond to contractile changes, which in turn disrupts cell mechanotransduction.
Heart disease is not solely attributed to the loss of cellular function in cardiomyocytes but also to the decline in function of surrounding cells. Cardiac fibroblasts—which produce, maintain, and remodel the ECM within cardiac tissue—decline as a function of age (176); in addition, aging hearts tend to harbor more fibrotic regions compared with younger hearts. The excessive deposition of collagen and the resulting fibrotic scars promote stiffening of the heart and impede the contractile function of cardiomyocytes (175).
Changes in the ECM have also been linked to age-associated cardiovascular disease. For example, the increased production and deposition of FN, together with the increased inappropriate crosslinking of collagen, is associated with increasing age (177, 178). Changes in the structure of the ECM influence cardiomyocytes by causing changes in their morphology and in tissue architecture, which, in turn, influence the orientation of actin filaments, the organization of sarcomeres, contractility, and myofibrillogenesis (179, 180). Excessive crosslinking of ECM proteins further induces alterations in the mechanics of the cardiomyocyte microenvironment, where the contractile activity and organization of myofibrils increase with extracellular stiffness. The mechanosensation of changes in the extracellular environment and focal adhesions is critically dependent on cytoskeletal tension (181). Moreover, epigenetic modifications have been shown to be associated with cardiovascular disease, hypertension, and diabetes, which could be the missing hereditary link among these diseases (182, 183). For example, methylation status is directly correlated with type II diabetes, a possible contributor to heart disease, but this warrants further study.
The aging of blood vessels appears to be an important contributor to cardiovascular pathology. The aging of vessels results in vascular remodeling and decreases the elasticity of arteries, and together these promote vascular stiffening (184). Age-dependent changes, such as increased inappropriate collagen crosslinking and decreased elastin content, are major contributors to vascular stiffness and hypertension. Increased hypertension further propagates deficiencies in the collagen microstructure and vessel stiffness, thus, acting as a positive-feedback mechanism that perpetuates the pathology (184). Together, the total contributions of mechanobiology to the proper function of the heart are critical. Minute intrinsic and environmental alterations seem to reap large effects and are associated with the development and progression of various cardiac deficiencies.
Neurodegenerative Diseases
Neurodegenerative diseases encompass a group of disorders that are characterized by the progressive loss of the function or structure of neurons and the central nervous system. These include diseases such as Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis. Similar to cardiovascular diseases, aging remains the leading risk factor for neurodegenerative diseases—that is, age makes patients more prone to these diseases and their cells less capable of self-repair. One major cause of age-related neurodegenerative diseases is the accumulation of disease-specific misfolded proteins within regions of the central nervous system. These proteins are insoluble, filamentous aggregates of normally soluble proteins (184). The aggregates contain fibers that display the properties of amyloid fibrils having β-sheet structure (184, 185). The accumulation of these proteins leads to the progressive loss of neuronal function and inflicts damage on synapses (186). These aggregates begin to accumulate early in life, but manifest as various diseases during midor late life (184). Although, most neurodegenerative diseases are characterized by the accumulation of protein, there remains striking diversity among the diseases. These differences arise primarily from the diversity of proteins deposited in each disease. For example, β-amyloid peptides and tau or tau phosphorylated proteins accumulate in Alzheimer’s disease, α-synuclein and ubiquitin accumulate in Parkinson’s disease, and mutant huntingtin proteins accumulate in Huntington’s disease (186, 187). Each disease differs in its spatiotemporal pattern of protein aggregates. Depending on the particular neurodegenerative disease, aggregates have been shown to occur in either the extracellular, intracytoplasmic, or intranuclear regions of neurons, astrocytes, and oligodendroglia.
In addition to protein aggregation, chronic inflammation is largely associated with age-related neurodegenerative diseases. Neuroinflammation results from the chronic activation of immune responses, which include those mediated by active microglia in the degenerating areas (187, 188). Microglia cells are macrophages that colonize the central nervous system during embryonic development and are responsible for controlling inflammation, repair, and regeneration (187, 189). In response to pathology or injury, microglia are activated and rapidly change their morphology to express the required inflammatory proteins, which, in turn, accelerate many pathophysiological processes in the central nervous system. Moreover, these morphologically active microglia are present in the central nervous system of a large number of patients with neurodegenerative diseases and might attest to the chronicity of inflammation found in these pathologies (190).
SUMMARY
Cellular properties change markedly with aging, and likely have a profound impact on age-related phenotypes and a host of age-related chronic disease states. Alterations in intra- and extracellular support structures, mitochondria, chromatin, and histones, and the emergence of senescent cells, all likely contribute to these changes. Future studies that help to facilitate the understanding of the connections between these cellular changes and the evolution of chronic disease states will be important next steps in the development of novel prevention and treatment strategies.
ACKNOWLEDGMENTS
The authors would like to thank Pei-Hsun Wu and Daniele Gilkes for helpful feedback and fruitful discussions. We acknowledge financial support from the United States National Institutes of Health and the National Institute on Aging (grant numbers R01CA174388, U54CA143868, and P30AG021334).
Glossary
- ECM
extracellular matrix
- MMPs
matrix metalloproteinases
- TIMPs
tissue inhibitors of metalloproteinases
- FN
fibronectin
- ROS
reactive oxygen species
- AGEs
advanced glycation end-products
- ATP
adenosine triphosphate
- AFM
atomic force microscopy
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J. Cell Sci. 2010;123:4195–200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat. Rev. Cancer. 2014;14:430–39. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteomics. 2012;11:M111.014647. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarvelainen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol. Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minimas DA. Ageing and its influence on wound healing. Wounds UK. 2007;3:42–50. [Google Scholar]
- 6.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Nagase H, Woessner JF. Matrix metalloproteinases. J. Biol. Chem. 1999;274:21491–94. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Munoz W, Khokha R. The role of tissue inhibitors of metalloproteinases in tumorigenesis and metastasis. Crit. Rev. Clin. Lab. Sci. 2008;45:291–338. doi: 10.1080/10408360801973244. [DOI] [PubMed] [Google Scholar]
- 9.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004;16:558–64. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labat-Robert J. Cell-matrix interactions in aging: role of receptors and matricryptins. Ageing Res. Rev. 2004;3:233–47. doi: 10.1016/j.arr.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–71. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–23. [PubMed] [Google Scholar]
- 13.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 2010;341:126–40. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int. J. Cancer. 2008;123:2229–38. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 15.Guo Q, Phillip JM, Majumdar S, Wu PH, Chen J, et al. Modulation of keratocyte phenotype by collagen fibril nanoarchitecture in membranes for corneal repair. Biomaterials. 2013;34:9365–72. doi: 10.1016/j.biomaterials.2013.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert L. Mechanisms of aging of the extracellular matrix: role of the elastin–laminin receptor. Gerontology. 1998;44:307–17. doi: 10.1159/000022034. [DOI] [PubMed] [Google Scholar]
- 17.Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, et al. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLOS Biol. 2007;5:e268. doi: 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labat-Robert J. Age-dependent remodeling of connective tissue: role of fibronectin and laminin. Pathol. Biol. 2003;51:563–68. doi: 10.1016/j.patbio.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Kwak HB. Aging, exercise, and extracellular matrix in the heart. J. Exercise Rehabil. 2013;9:338–47. doi: 10.12965/jer.130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–36. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callaghan TM, Wilhelm KP. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Int. J. Cosmet. Sci. 2008;30:313–22. doi: 10.1111/j.1468-2494.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- 22.Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009;174:101–14. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, et al. Decreased collagen production in chrono-logically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006;168:1861–68. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 25.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–10. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 26.Sprenger CC, Plymate SR, Reed MJ. Aging-related alterations in the extracellular matrix modulate the microenvironment and influence tumor progression. Int. J. Cancer. 2010;127:2739–48. doi: 10.1002/ijc.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo S, Dipietro LA. Factors affecting wound healing. J. Dent. Res. 2010;89:219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gosain A, DiPietro LA. Aging and wound healing. World J. Surg. 2004;28:321–26. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 29.Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology. 2002;3:337–45. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- 30.Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012;11:642–49. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 31.Pienta KJ, Coffey DS. Characterization of the subtypes of cell motility in ageing human fibroblasts. Mech. Ageing Dev. 1990;56:99–105. doi: 10.1016/0047-6374(90)90001-v. [DOI] [PubMed] [Google Scholar]
- 32.Goodson WH, 3rd, Hunt TK. Wound healing and aging. J. Investig. Dermatol. 1979;73:88–91. doi: 10.1111/1523-1747.ep12532775. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Marti GP, Wei X, Zhang X, Zhang H, et al. Age-dependent impairment of HIF-1α expression in diabetic mice: correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J. Cell. Physiol. 2008;217:319–27. doi: 10.1002/jcp.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williamson KA, Hamilton A, Reynolds JA, Sipos P, Crocker I, et al. Age-related impairment of endothelial progenitor cell migration correlates with structural alterations of heparan sulfate proteoglycans. Aging Cell. 2013;12:139–47. doi: 10.1111/acel.12031. [DOI] [PubMed] [Google Scholar]
- 35.Wu M, Fannin J, Rice KM, Wang B, Blough ER. Effect of aging on cellular mechanotransduction. Ageing Res. Rev. 2011;10:1–15. doi: 10.1016/j.arr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MWJ. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am. J. Pathol. 1999;155:1137–46. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berdyyeva TK, Woodworth CD, Sokolov I. Human epithelial cells increase their rigidity with ageing in vitro: direct measurements. Phys. Med. Biol. 2005;50:81–92. doi: 10.1088/0031-9155/50/1/007. [DOI] [PubMed] [Google Scholar]
- 38.Dulinska-Molak I, Pasikowska M, Pogoda K, Lewandowska M, Eris I, Lekka M. Age-related changes in the mechanical properties of human fibroblasts and its prospective reversal after anti-wrinkle tripeptide treatment. Int. J. Pept. Res. Ther. 2014;20:77–85. doi: 10.1007/s10989-013-9370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulze C, Wetzel F, Kueper T, Malsen A, Muhr G, et al. Stiffening of human skin fibroblasts with age. Clin. Plast. Surg. 2012;39:9–20. doi: 10.1016/j.cps.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Lieber SC, Aubry N, Pain J, Diaz G, Kim SJ, Vatner SF. Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H645–51. doi: 10.1152/ajpheart.00564.2003. [DOI] [PubMed] [Google Scholar]
- 41.Pelissier FA, Garbe JC, Ananthanarayanan B, Miyano M, Lin C, et al. Age-related dysfunction in mechanotransduction impairs differentiation of human mammary epithelial progenitors. Cell Rep. 2014;7:1926–39. doi: 10.1016/j.celrep.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sokolov I, Iyer S, Woodworth CD. Recovery of elasticity of aged human epithelial cells in vitro. Nanomedicine. 2006;2:31–36. doi: 10.1016/j.nano.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J. Am. Geriatr. Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 45.Wirtz D. Particle-tracking microrheology of living cells: principles and applications. Annu. Rev. Biophys. 2009;38:301–26. doi: 10.1146/annurev.biophys.050708.133724. [DOI] [PubMed] [Google Scholar]
- 46.Pravincumar P, Bader DL, Knight MM. Viscoelastic cell mechanics and actin remodelling are dependent on the rate of applied pressure. PLOS ONE. 2012;7:e43938. doi: 10.1371/journal.pone.0043938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minin AA, Kulik AV, Gyoeva FK, Li Y, Goshima G, Gelfand VI. Regulation of mitochondria distribution by RhoA and formins. J. Cell Sci. 2006;119:659–70. doi: 10.1242/jcs.02762. [DOI] [PubMed] [Google Scholar]
- 48.Lee JSH, Chang MI, Tseng Y, Wirtz D. Cdc42 mediates nucleus movement and MTOC polarization in Swiss 3T3 fibroblasts under mechanical shear stress. Mol. Biol. Cell. 2005;16:871–80. doi: 10.1091/mbc.E03-12-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anesti V, Scorrano L. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim. Biophys. Acta. 2006;1757:692–99. doi: 10.1016/j.bbabio.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Kole TP, Tseng Y, Jiang I, Katz JL, Wirtz D. Intracellular mechanics of migrating fibroblasts. Mol. Biol. Cell. 2005;16:328–38. doi: 10.1091/mbc.E04-06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer. 2011;11:512–22. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirmizis DL, Logothetidis S. Atomic force microscopy probing in the measurement of cell mechanics. Int. J. Nanomed. 2010;5:137–45. doi: 10.2147/ijn.s5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nawaz S, Sanchez P, Bodensiek K, Li S, Simons M, Schaap IA. Cell visco-elasticity measured with AFM and optical trapping at sub-micrometer deformations. PLOS ONE. 2012;7:e45297. doi: 10.1371/journal.pone.0045297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sokolov I. Atomic force microscopy in cancer cell research. In: Nalwa HS, Webster T, editors. Cancer Nanotechnology. Am. Sci. Publ.; New York: 2007. pp. 1–17. [Google Scholar]
- 55.Wu PH, Hale CM, Chen WC, Lee JS, Tseng Y, Wirtz D. High-throughput ballistic injection nanorheology to measure cell mechanics. Nat. Protoc. 2012;7:155–70. doi: 10.1038/nprot.2011.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Liu KK. Optical tweezers for single cells. J. R. Soc. Interface. 2008;5:671–90. doi: 10.1098/rsif.2008.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roth KB, Eggleton CD, Neeves KB, Marr DW. Measuring cell mechanics by optical alignment compression cytometry. Lab Chip. 2013;13:1571–77. doi: 10.1039/c3lc41253a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Celedon A, Hale CM, Wirtz D. Magnetic manipulation of nanorods in the nucleus of living cells. Biophys. J. 2011;101:1880–86. doi: 10.1016/j.bpj.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puig-De-Morales M, Grabulosa M, Alcaraz J, Mullol J, Maksym GN, et al. Measurement of cell microrheology by magnetic twisting cytometry with frequency domain demodulation. J. Appl. Physiol. 2001;91:1152–59. doi: 10.1152/jappl.2001.91.3.1152. [DOI] [PubMed] [Google Scholar]
- 60.Lim CT, Zhou EH, Quek ST. Mechanical models for living cells—a review. J. Biomech. 2006;39:195–216. doi: 10.1016/j.jbiomech.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Ajmani RS, Rifkind JM. Hemorheological changes during human aging. Gerontology. 1998;44:111–20. doi: 10.1159/000021993. [DOI] [PubMed] [Google Scholar]
- 62.Starodubtseva MN. Mechanical properties of cells and ageing. Ageing Res. Rev. 2011;10:16–25. doi: 10.1016/j.arr.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Tseng Y, Kole TP, Lee JS, Fedorov E, Almo SC, et al. How actin crosslinking and bundling proteins cooperate to generate an enhanced cell mechanical response. Biochem. Biophys. Res. Commun. 2005;334:183–92. doi: 10.1016/j.bbrc.2005.05.205. [DOI] [PubMed] [Google Scholar]
- 64.Jiang H, Sun SX. Cellular pressure and volume regulation and implications for cell mechanics. Biophys. J. 2013;105:609–19. doi: 10.1016/j.bpj.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stroka KM, Jiang H, Chen SH, Tong Z, Wirtz D, et al. Water permeation drives tumor cell migration in confined microenvironments. Cell. 2014;157:611–23. doi: 10.1016/j.cell.2014.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng L, Fairbank NJ, Cole DJ, Fredberg JJ, Maksym GN. Airway smooth muscle tone modulates mechanically induced cytoskeletal stiffening and remodeling. J. Appl. Physiol. 2005;99:634–41. doi: 10.1152/japplphysiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 67.Icard-Arcizet D, Cardoso O, Richert A, Henon S. Cell stiffening in response to external stress is correlated to actin recruitment. Biophys. J. 2008;94:2906–13. doi: 10.1529/biophysj.107.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Popescu WM. Stoelting’s Anesthesia and Co-existing Disease. Elsevier; Philadelphia: 2012. Heart failure and cardiomyopathies; pp. 120–43. [Google Scholar]
- 69.Chambliss AB, Khatau SB, Erdenberger N, Robinson DK, Hodzic D, et al. The LINC-anchored actin cap connects the extracellular milieu to the nucleus for ultrafast mechanotransduction. Sci. Rep. 2013;3:1087. doi: 10.1038/srep01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khatau SB, Hale CM, Stewart-Hutchinson PJ, Patel MS, Stewart CL, et al. A perinuclear actin cap regulates nuclear shape. PNAS. 2009;106:19017–22. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim DH, Khatau SB, Feng Y, Walcott S, Sun SX, et al. Actin cap associated focal adhesions and their distinct role in cellular mechanosensing. Sci. Rep. 2012;2:555. doi: 10.1038/srep00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gay O, Gilquin B, Nakamura F, Jenkins ZA, McCartney R, et al. RefilinB (FAM101B) targets filamin A to organize perinuclear actin networks and regulates nuclear shape. PNAS. 2011;108:11464–69. doi: 10.1073/pnas.1104211108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sokolov I, Woodworth CD. Technical Proceedings of the 2005 NSTI Nanotechnology Conference and Trade Show. Vol. 1. Nano Sci. Technol. Inst.; Austin, TX: 2005. Loss of elasticity of ageing epithelial cells, and its possible reversal; pp. 63–66. [Google Scholar]
- 74.Ward KA, Baker C, Roebuck L, Wickline K, Schwartz RW. Red blood cell deformability: effect of age and smoking. Age. 1991;14:73–77. [Google Scholar]
- 75.Zahn JT, Louban I, Jungbauer S, Bissinger M, Kaufmann D, et al. Age-dependent changes in microscale stiffness and mechanoresponses of cells. Small. 2011;7:1480–87. doi: 10.1002/smll.201100146. [DOI] [PubMed] [Google Scholar]
- 76.Muller-Sieburg CE, Sieburg BH, Bernitz JM, Cattarossi G. Stem cell heterogeneity-implications for aging and regenerative medicine. Blood. 2012;119:3900–7. doi: 10.1182/blood-2011-12-376749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glauche I, Thielecke L, Roeder I. Cellular aging leads to functional heterogeneity of hematopoietic stem cells: a modeling perspective. Aging Cell. 2011;10:457–65. doi: 10.1111/j.1474-9726.2011.00692.x. [DOI] [PubMed] [Google Scholar]
- 78.Altschuler SJ, Wu LF. Cellular heterogeneity: Do differences make a difference? Cell. 2010;141:559–63. doi: 10.1016/j.cell.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005;25:932–43. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 80.Harvey PA, Leinwand LA. The cell biology of disease: cellular mechanisms of cardiomyopathy. J. Cell Biol. 2011;194:355–65. doi: 10.1083/jcb.201101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3:413–38. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Esue O, Tseng Y, Wirtz D. Alpha-actinin and filamin cooperatively enhance the stiffness of actin filament networks. PLOS ONE. 2009;4:e4411. doi: 10.1371/journal.pone.0004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tseng Y, Fedorov E, McCaffery JM, Almo SC, Wirtz D. Micromechanics and ultrastructure of actin filament networks crosslinked by human fascin: a comparison with α-actinin. J. Mol. Biol. 2001;310:351–66. doi: 10.1006/jmbi.2001.4716. [DOI] [PubMed] [Google Scholar]
- 84.Mendez MG, Restle D, Janmey PA. Vimentin enhances cell elastic behavior and protects against compressive stress. Biophys. J. 2014;107:314–23. doi: 10.1016/j.bpj.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Capell BC, Collins FS, Nabel EG. Mechanisms of cardiovascular disease in accelerated aging syndromes. Circ. Res. 2007;101:13–26. doi: 10.1161/CIRCRESAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- 86.Zwerger M, Ho CY, Lammerding J. Nuclear mechanics in disease. Annu. Rev. Biomed. Eng. 2011;13:397–428. doi: 10.1146/annurev-bioeng-071910-124736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, et al. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys. J. 2007;93:2542–52. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Afilalo J, Sebag IA, Chalifour LE, Rivas D, Akter R, et al. Age-related changes in lamin A/C expression in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1451–56. doi: 10.1152/ajpheart.01194.2006. [DOI] [PubMed] [Google Scholar]
- 89.Duque G, Rivas D. Age-related changes in lamin A/C expression in the osteoarticular system: laminopathies as a potential new aging mechanism. Mech. Ageing Dev. 2006;127:378–83. doi: 10.1016/j.mad.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 90.Schirmer EC, Florens L, Guan TL, Yates JR, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301:1380–82. doi: 10.1126/science.1088176. [DOI] [PubMed] [Google Scholar]
- 91.Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr. Biol. 2013;23:R1113–21. doi: 10.1016/j.cub.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. PNAS. 2007;104:15619–24. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ostlund C, Folker ES, Choi JC, Gomes ER, Gundersen GG, Worman HJ. Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J. Cell Sci. 2009;122:4099–108. doi: 10.1242/jcs.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zwerger M, Jaalouk DE, Lombardi ML, Isermann P, Mauermann M, et al. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum. Mol. Genet. 2013;22:2335–49. doi: 10.1093/hmg/ddt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gundersen GG, Worman HJ. Nuclear positioning. Cell. 2013;152:1376–89. doi: 10.1016/j.cell.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chambliss AB, Khatau SB, Erdenberger N, Robinson DK, Hodzic D, et al. The LINC-anchored actin cap connects the extracellular milieu to the nucleus for ultrafast mechanotransduction. Sci. Rep. 2013;3:1087. doi: 10.1038/srep01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem. 2011;286:26743–53. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–63. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhattacharya D, Talwar S, Mazumder A, Shivashankar GV. Spatio-temporal plasticity in chromatin organization in mouse cell differentiation and during Drosophila embryogenesis. Biophys. J. 2009;96:3832–39. doi: 10.1016/j.bpj.2008.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stewart C, Burke B. Teratocarcinoma stem-cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin-B. Cell. 1987;51:383–92. doi: 10.1016/0092-8674(87)90634-9. [DOI] [PubMed] [Google Scholar]
- 101.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature. 2003;423:293–98. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taimen P, Pfleghaar K, Shimi T, Moller D, Ben-Harush K, et al. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. PNAS. 2009;106:20788–93. doi: 10.1073/pnas.0911895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haithcock E, Dayani Y, Neufeld E, Zahand AJ, Feinstein N, et al. Age-related changes of nuclear architecture in Caenorhabditis elegans. PNAS. 2005;102:16690–95. doi: 10.1073/pnas.0506955102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, et al. The mutant form of lamin a that causes Hutchinson–Gilford progeria is a biomarker of cellular aging in human skin. PLOS ONE. 2007;2:e1269. doi: 10.1371/journal.pone.0001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Olive M, Harten I, Mitchell R, Beers JK, Djabali K, et al. Cardiovascular pathology in Hutchinson–Gilford progeria: correlation with the vascular pathology of aging. Arterioscl. Thromb. Vasc. Biol. 2010;30:2301–9. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dahl KN, Ribeiro AJS, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ. Res. 2008;102:1307–18. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Broers JLV, Bronnenberg NMHJ, Kuijpers HJH, Schutte B, Hutchison CJ, Ramaekers FCS. Partial cleavage of A-type lamins concurs with their total disintegration from the nuclear lamina during apoptosis. Eur. J. Cell Biol. 2002;81:677–91. doi: 10.1078/0171-9335-00282. [DOI] [PubMed] [Google Scholar]
- 108.Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J. Biol. Chem. 2006;281:25768–80. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 109.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Investig. 2004;113:370–78. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 1999;147:913–19. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ragnauth CD, Warren DT, Liu YW, McNair R, Tajsic T, et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200–10. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
- 112.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 113.Handy DE, Castro R, Loscalzo J. Epigenetic modifications basic mechanisms and role in cardiovascular disease. Circulation. 2011;123:2145–56. doi: 10.1161/CIRCULATIONAHA.110.956839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Esteller M. Molecular origins of cancer: epigenetics in cancer. N. Engl. J. Med. 2008;358:1148–59. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 115.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010;11:204–20. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karpf AR, Matsui S. Genetic disruption of cytosine DNA methyltransferase enzymes induces chromosomal instability in human cancer cells. Cancer Res. 2005;65:8635–39. doi: 10.1158/0008-5472.CAN-05-1961. [DOI] [PubMed] [Google Scholar]
- 117.Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. PNAS. 1964;51:786–94. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–38. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 119.Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys. J. 2005;89:2855–64. doi: 10.1529/biophysj.105.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rowat AC, Lammerding J, Herrmann H, Aebi U. Towards an integrated understanding of the structure and mechanics of the cell nucleus. BioEssays. 2008;30:226–36. doi: 10.1002/bies.20720. [DOI] [PubMed] [Google Scholar]
- 121.Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Mistell T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev. Cell. 2006;10:105–16. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bibikova M, Laurent LC, Ren B, Loring JF, Fan JB. Unraveling epigenetic regulation in embryonic stem cells. Cell Stem Cell. 2008;2:123–34. doi: 10.1016/j.stem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 123.Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson–Gilford progeria syndrome. PNAS. 2004;101:8963–68. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fraga MF, Esteller M. Epigenetics and aging: the targets and the marks. Trends Genet. 2007;23:413–18. doi: 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 125.Han S, Brunet A. Histone methylation makes its mark on longevity. Trends Cell Biol. 2012;22:42–49. doi: 10.1016/j.tcb.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jin CY, Li J, Green CD, Yu XM, Tang X, et al. Histone demethylase UTX-1 regulates C. elegans life span by targeting the insulin/IGF-1 signaling pathway. Cell Metab. 2011;14:161–72. doi: 10.1016/j.cmet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 127.Romanov GA, Vanyushin BF. Methylation of reiterated sequences in mammalian DNAs: effects of the tissue-type, age, malignancy and hormonal induction. Biochim. Biophys. Acta. 1981;653:204–18. doi: 10.1016/0005-2787(81)90156-8. [DOI] [PubMed] [Google Scholar]
- 128.Sarg B, Koutzamani E, Helliger W, Rundquist I, Lindner HH. Postsynthetic trimethylation of histone H4 at lysine 20 in mammalian tissues is associated with aging. J. Biol. Chem. 2002;277:39195–201. doi: 10.1074/jbc.M205166200. [DOI] [PubMed] [Google Scholar]
- 129.Narita M, Nunez S, Heard E, Narita M, Lin AW, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–16. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 130.Oberdoerffer P, Sinclair DA. The role of nuclear architecture in genomic instability and ageing. Nat. Rev. Mol. Cell Biol. 2007;8:692–702. doi: 10.1038/nrm2238. [DOI] [PubMed] [Google Scholar]
- 131.Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat. Rev. Mol. Cell Biol. 2012;13:397–404. doi: 10.1038/nrm3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Conley KE, Marcinek DJ, Villarin J. Mitochondrial dysfunction and age. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:688–92. doi: 10.1097/MCO.0b013e3282f0dbfb. [DOI] [PubMed] [Google Scholar]
- 133.Trifunovic A, Larsson NG. Mitochondrial dysfunction as a cause of ageing. J. Intern. Med. 2008;263:167–78. doi: 10.1111/j.1365-2796.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 134.Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J. Cell Sci. 2010;123:2533–42. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Harman D. The free radical theory of aging: effect of age on serum copper levels. J. Gerontol. 1965;20:151–53. doi: 10.1093/geronj/20.2.151. [DOI] [PubMed] [Google Scholar]
- 136.Chomyn A, Attardi G. MtDNA mutations in aging and apoptosis. Biochem. Biophys. Res. Commun. 2003;304:519–29. doi: 10.1016/s0006-291x(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 137.Seo AY, Xu J, Servais S, Hofer T, Marzetti E, et al. Mitochondrial iron accumulation with age and functional consequences. Aging Cell. 2008;7:706–16. doi: 10.1111/j.1474-9726.2008.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal. Transduct. 2012;2012:646354. doi: 10.1155/2012/646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J. Cell Sci. 2000;114:867–74. doi: 10.1242/jcs.114.5.867. Pt. 5. [DOI] [PubMed] [Google Scholar]
- 140.Karbowski M, Arnoult D, Chen H, Chan DC, Smith CL, Youle RJ. Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J. Cell Biol. 2004;164:493–99. doi: 10.1083/jcb.200309082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koopman WJ, Visch HJ, Smeitink JA, Willems PH. Simultaneous quantitative measurement and automated analysis of mitochondrial morphology, mass, potential, and motility in living human skin fibroblasts. Cytometry A. 2006;69:1–12. doi: 10.1002/cyto.a.20198. [DOI] [PubMed] [Google Scholar]
- 142.Benard G, Bellance N, James D, Parrone P, Fernandez H, et al. Mitochondrial bioenergetics and structural network organization. J. Cell Sci. 2007;120:838–48. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 143.Benard G, Rossignol R. Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid. Redox Signal. 2008;10:1313–42. doi: 10.1089/ars.2007.2000. [DOI] [PubMed] [Google Scholar]
- 144.Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. PNAS. 2009;106:11960–65. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Finkel T, Hwang PM. The Krebs cycle meets the cell cycle: mitochondria and the G1-S transition. PNAS. 2009;106:11825–26. doi: 10.1073/pnas.0906430106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Collins TJ, Berridge MJ, Lipp P, Bootman MD. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J. 2002;21:1616–27. doi: 10.1093/emboj/21.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Calvo SE, Mootha VK. The mitochondrial proteome and human disease. Annu. Rev. Genomics Hum. Genet. 2010;11:25–44. doi: 10.1146/annurev-genom-082509-141720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLOS ONE. 2012;7:e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. PNAS. 2005;102:5618–23. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Terman AK, Kurtz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial–lysosomal axis theory of aging. Antioxid. Redox Signal. 2010;12:503–35. doi: 10.1089/ars.2009.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Doonan R, McElwee JJ, Matthijssens F, Walker GA, Houthoofd K, et al. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008;22:3236–41. doi: 10.1101/gad.504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 153.Pérez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–23. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 155.Muliyil S, Narasimha M. Mitochondrial ROS regulates cytoskeletal and mitochondrial remodeling to tune cell and tissue dynamics in a model for wound healing. Dev. Cell. 2014;28:239–52. doi: 10.1016/j.devcel.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 156.Chen H, Chan DC. Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum. Mol. Genet. 2009;18:R169–76. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Scheibye-Knudsen M, Scheibye-Alsing K, Canugovi C, Croteau DL, Bohr VA. A novel diagnostic tool reveals mitochondrial pathology in human diseases and aging. Aging. 2013;5:192–208. doi: 10.18632/aging.100546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech. Ageing Dev. 2005;126:365–79. doi: 10.1016/j.mad.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 159.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–11. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 160.Burtner CR, Kennedy BK. Progeria syndromes and ageing: what is the connection? Nat. Rev. Mol. Cell Biol. 2010;11:567–78. doi: 10.1038/nrm2944. [DOI] [PubMed] [Google Scholar]
- 161.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 162.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 163.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLOS Biol. 2008;6:2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Coppe JP, Patil CK, Rodier F, Krtolica A, Beausejour CM, et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLOS ONE. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, et al. Clearance of p16(Ink4a)-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–36. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging. 2012;4:159–65. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nishio K, Inoue A. Senescence-associated alterations of cytoskeleton: extraordinary production of vimentin that anchors cytoplasmic p53 in senescent human fibroblasts. Histochem. Cell Biol. 2005;123:263–73. doi: 10.1007/s00418-005-0766-5. [DOI] [PubMed] [Google Scholar]
- 168.Righolt CH, van ‘t Hoff MLR, Vermolen BJ, Young IT, Raz V. Robust nuclear lamina-based cell classification of aging and senescent cells. Aging. 2011;3:1192–201. doi: 10.18632/aging.100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McGrail DJ, McAndrews KM, Dawson MR. Biomechanical analysis predicts decreased human mesenchymal stem cell function before molecular differences. Exp. Cell Res. 2013;319:684–96. doi: 10.1016/j.yexcr.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 170.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 171.Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: a review of global methodologies of mortality measurement. Circulation. 2013;127:749–56. doi: 10.1161/CIRCULATIONAHA.112.128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post-infarction left ventricular remodeling. J. Am. Coll. Cardiol. 2010;57:9–17. doi: 10.1016/j.jacc.2010.08.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Russell RR, Li J, Coven DL, Pypaert M, Zechner C, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Investig. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ. Res. 2004;95:957–70. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 175.Harvey PA, Leinwand LA. Cellular mechanisms of cardiomyopathy. J. Cell Biol. 2011;194:355–65. doi: 10.1083/jcb.201101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr. Biol. 2012;22:R741–52. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 177.Burgess ML, McCrea JC, Hedrick HL. Age-associated changes in cardiac matrix and integrins. Mech. Ageing Dev. 2001;122:1739–56. doi: 10.1016/s0047-6374(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 178.Thomas DP, Cotter TA, Li X, McCormick RJ, Gosselin LE. Exercise training attenuates aging-associated increases in collagen and collagen crosslinking of the left but not the right ventricle in the rat. Eur. J. Appl. Physiol. 2001;85:164–69. doi: 10.1007/s004210100447. [DOI] [PubMed] [Google Scholar]
- 179.Alford PW, Nesmith AP, Seywerd JN, Grosberg A, Parker KK. Vascular smooth muscle contractility depends on cell shape. Integr. Biol. Camb. 2011;3:1063–70. doi: 10.1039/c1ib00061f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil. Cytoskelet. 2008;65:641–51. doi: 10.1002/cm.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zajac AL, Discher DE. Cell differentiation through tissue elasticity-coupled, myosin-driven remodeling. Curr. Opin. Cell Biol. 2008;20:609–15. doi: 10.1016/j.ceb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Slatkin M. Epigenetic inheritance and the missing heritability problem. Genetics. 2009;182:845–50. doi: 10.1534/genetics.109.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Skovronsky DM, Lee VM, Trojanowski JQ. Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu. Rev. Pathol. 2006;1:151–70. doi: 10.1146/annurev.pathol.1.110304.100113. [DOI] [PubMed] [Google Scholar]
- 185.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat. Med. 2004;10:S10–17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 186.Hung CW, Chen YC, Hsieh WL, Chiou SH, Kao CL. Ageing and neurodegenerative diseases. Ageing Res. Rev. 2010;9(Suppl. 1):S36–46. doi: 10.1016/j.arr.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 187.Amor S, Peferoen LA, Vogel DY, Breur M, van der Valk P, et al. Inflammation in neurodegenerative diseases—an update. Immunology. 2014;142:151–66. doi: 10.1111/imm.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann. N.Y. Acad. Sci. 2004;1035:104–16. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- 189.Cappellano G, Carecchio M, Fleetwood T, Magistrelli L, Cantello R, et al. Immunity and inflammation in neurodegenerative diseases. Am. J. Neurodegen. Dis. 2013;2:89–107. [PMC free article] [PubMed] [Google Scholar]
- 190.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]