Abstract
Objective:
The aims of the current study were to determine whether children with the 6 different APOE ε genotypes show differences in gray matter maturation, particularly for those with ε4 and ε2 alleles, which are associated with poorer outcomes in many neurologic disorders.
Methods:
A total of 1,187 healthy children (aged 3–20 years, 52.1% boys, 47.9% girls) with acceptable data from the cross-sectional Pediatric Imaging Neurocognition and Genetics Study were evaluated for the effects of 6 APOE ε genotypes on macroscopic and microscopic cortical and subcortical gray matter structures (measured with 3-tesla MRI and FreeSurfer for automated morphometry) and on cognition (NIH Toolbox).
Results:
Among APOE ε4 carriers, age-related changes in brain structures and cognition varied depending on genotype, with the smallest hippocampi in ε2ε4 children, the lowest hippocampal fractional anisotropy in younger ε4ε4 children, the largest medial orbitofrontal cortical areas in ε3ε4 children, and age-dependent thinning of the entorhinal cortex in ε4ε4 children. Younger ε4ε4 children had the lowest scores on executive function and working memory, while younger ε2ε4 children performed worse on attention tasks. Larger parietal gyri in the younger ε2ε4 children, and thinner temporal and cingulate isthmus cortices or smaller hippocampi in the younger ε4ε4 children, predicted poorer performance on attention or working memory.
Conclusions:
Our findings validated and extended prior smaller studies that showed altered brain development in APOE ε4–carrier children. The ε4ε4 and ε2ε4 genotypes may negatively influence brain development and brain aging at the extremes of age. Studying APOE ε polymorphisms in young children may provide the earliest indicators for individuals who might benefit from early interventions or preventive measures for future brain injuries and dementia.
APOE ε4 is a well-known risk allele for Alzheimer disease (AD), especially late-onset AD, and may lead to poorer outcome in neurologic disorders.1–4 In addition, APOE ε4 may influence brain development.5–7 However, the APOE ε4 allele demonstrates antagonistic pleiotropy, with deleterious effects on cognition, brain morphometry, and activation primarily after 55 years of age, but no negative8 or even beneficial effects in adults younger than 50 years9,10 and children aged 6 to 15 years.11–13 Compared to non–ε4 carriers, healthy children carrying ε4 (8–20 years) tended to have thinner entorhinal cortex,5 while healthy infants carrying ε4 showed altered brain measures in regions affected by AD.6,7 Whether these structural differences influence cognitive performance in children with ε4 remains controversial.5,12–14
APOE ε2 also may affect brain variably. The ε2 carriers were less likely to develop clinical dementia15,16 and had greater cognitive reserve,17 since APOE ε2 may be neuroprotective.17 However, ε2 carriers also had more cortical amyloid plaques,18 with elevated risks of cerebral amyloid angiopathy17 and cortical infarctions.2 Furthermore, children carrying ε2 performed worse on visuospatial tasks12 and had thicker temporal cortices5 than non–ε2 carriers, similar to adults carrying ε2 with mild cognitive impairment or AD.19
Because of potentially opposite influences of APOE ε4 and APOE ε2, prior studies typically evaluated homozygous or heterozygous ε4 or ε2 individuals, but frequently excluded ε2ε4 participants.5,20,21 One study assessed all 6 APOE genotypes but only on IQ and academic achievements.13 Therefore, we evaluated group differences across all 6 APOE ε genotypes on gray matter morphometry and cognition in typically developing children. We hypothesized that children with different APOE ε genotypes, especially ε4 and ε2 carriers (including ε2ε4), would show differential gray matter measures and cognitive function across the age span of childhood.
METHODS
Participants.
A total of 1,493 typically developing children aged 3 to 20 years were enrolled in the Pediatric Imaging, Neurocognition, and Genetics (PING) Study (http://ping.chd.ucsd.edu) at 10 US academic institutions from September 2010 to August 2012. The PING Study was designed to cross-sectionally investigate how genes influence brain maturation and cognitive measures across childhood. Children were enrolled from diverse ethnic groups and socioeconomic status (SES); detailed participant criteria were reported previously.22 Specifically, the 1,187 children in the current study were excluded for any confounding neurologic or psychiatric disorders, history of head trauma, mental retardation, preterm birth (<36 weeks), prenatal drug exposure (daily maternal illicit drug use >1 trimester), or any MRI contraindications (including pregnancy).
Standard protocol approvals, registrations, and patient consents.
All participants provided written assents (older than 7 years) or consents (18 years or older), and parental consents (3–17 years), which along with the protocol were approved by each of the local institutional review boards for human subject studies.
Genotyping.
Genomic DNA extracted from saliva was genotyped in 1,187 children for APOE ε (rs429358 and rs7412) using the iPLEX Gold assay at the Sequenom MassARRAY genotyping platform (Sequenom, San Diego, CA). Final genotypes were called using the MassARRAY Type, version 4.0. Replication and quality-control procedures were described previously.22 Genetic ancestry factor (GAF) was determined with the ADMIXTURE software (https://www.genetics.ucla.edu/software/admixture/) and the Illumina Human660W-Quad BeadChip for the 6 major continental populations (African, Central Asian, East Asian, European, Native American, and Oceanic) in each child.
Magnetic resonance imaging.
Image acquisition and processing were detailed previously.22 All scans were performed on 3-tesla scanners (9 Siemens, 2 General Electric, 2 Philips) using closely matched sequences. The protocol included 3-dimensional T1-weighted structural MRI (magnetization-prepared rapid-acquisition gradient echo, 1.0 × 1.0 × 1.2 mm3, 8 minutes) and diffusion-tensor imaging (echo-planar imaging, 2.5-mm isotropic, b = 1,000 s/mm2, 30 directions, 10 minutes) (http://ping.chd.ucsd.edu). Image processing included verification of protocol compliance and quality assurance. Automated morphometry using a modified FreeSurfer software was performed on the whole brain, and 7 subcortical and 20 cortical regions of interest (ROIs) from the standard FreeSurfer atlas were selected based on reported APOE ε effects in children5 and neonates,6,7 or patients with AD.4 Fractional anisotropy (FA) (corrected for B0 inhomogeneities) in the same 7 subcortical ROIs was also assessed, since FA in these regions often increases during neurodevelopment.23 Of 1,187 children, 1,080 met quality criteria for morphometry and 988 for FA.
NIH Toolbox.
The NIH Toolbox Cognition Battery comprised 7 tests that assessed 8 cognitive domains.24 Four domains found abnormal in patients with AD or ε2 or ε4 carriers25–27 were analyzed: (1) executive function–cognitive flexibility (Dimensional Change Card Sort Test); (2) visual attention (Flanker Inhibitory Control and Attention Test); (3) episodic memory (Picture Sequence Memory Test); and (4) working memory (WM) (List Sorting WM Test). For these domains, 1,060 children had acceptable data.
Statistical analyses.
Statistical analyses were performed using the PING data portal (https://ping-dataportal.ucsd.edu).22 Genotype and genotype-by-age effects on morphometry and FA, and their relationships with cognition, were assessed with a general additive model (GAM) in R program (http://www.r-project.or/). GAM is a multiple linear regression model including smooth functions of variables that are data driven. Each model used age as a smooth independent variable and included a linear term for genotype and a smooth age-by-genotype interaction.22 GAMs used thin plate regression splines for the smoothing basis (using the bs = “ts” specification), with basis dimensions k = 4 for main-effect smooth terms (e.g., age) and k = 3 for smooth interaction terms (e.g., age-by-sex).
All models covaried for SES (highest parental education and household income), sex, GAF, and scanner device. Subcortical volumes were adjusted for intracranial volume but not for average cortical thickness and area. For vertex-wise analyses, significance maps were thresholded at 5% using the false-discovery rate to correct for multiple comparisons. ROI-based analyses were corrected for multiple comparisons using the Holm-Bonferroni sequential method, which controls the family-wise error rate, using a step-wise procedure to adjust for significance levels instead of p values, and is uniformly more powerful than the Bonferroni correction.28 Pairwise post hoc analyses were explored for contrasts with group differences on the GAM. Two children with ε2ε2 were not included in group analyses but are described separately.
RESULTS
Participant characteristics.
The 1,187 children were aged 12.1 ± 5.0 years; 569 were girls (table e-1 on the Neurology® Web site at Neurology.org). The 5 APOE ε allele groups were similar in sex proportion. The ε2ε2 children were the youngest and ε2ε3 children were the oldest; ε3ε3 was most common (61.78%), followed by ε3ε4 (21.8%), ε2ε3 (11.9%), ε2ε4 (2.6%), and ε4ε4 (1.75%), with ε2ε2 the rarest (0.17%). All genotype groups showed significant European GAF (60%–70%), except for ε4ε4 (35% European, but higher African ancestry than other groups). Parents/guardians of ε4ε4 children had the lowest household income, education, and occupation levels.
Subcortical volume differences across genotypes.
The hippocampi differed across genotype groups (figure 1, A and B; table e-2). Independent of age, ε3ε4 children had the largest, while ε2ε4 children had the smallest hippocampi across groups (figure 1B). Hippocampal volumes increased linearly with age but differed by APOE ε genotype, with an inverted U shape in ε3ε3 children (peaking at 13.2 years). The 2 children with ε2ε2 had relatively large hippocampi.
Figure 1. APOE ε genotypic variations on age-dependent changes of subcortical volumes and FA.
(A) Right and left hippocampal volumes are shown as averaged values (since they were not significantly different) across the 6 APOE ε genotypes. Note the relatively larger volumes in the 2 individuals homozygous for ε2 (black) and the smallest volumes across the age range and genotype groups in those with ε2ε4 (blue, ε2ε4 < ε3ε3, p = 0.007). (B) Right hippocampal FA is relatively stable across the age range except for the children with ε4ε4 (red), especially those at younger ages (similar age-related curve is seen in the left hippocampus; table e-2). The younger child with ε2ε2 also showed the lowest FA in the hippocampus (black). (C) Segmented hippocampi shown in 3 orientations (arrows). (D) The younger children (<10 years) with ε2 allele (ε2ε2, ε2ε3, and ε2ε4) and ε4ε4 showed relatively lower FA in left amygdala. (E) Younger children with ε2ε4 and ε4ε4 also showed relatively lower FA in left thalamus (post hoc test: ε2ε4 < ε3ε3, p = 0.01). (F) Log p value maps of the brain regions showing age-by-genotype interactions (see also table e-2). *Data for the ε2ε2 children are not included in the group analyses. FA = fractional anisotropy.
Genotype-by-age on subcortical FA.
Age-dependent FA changes were evaluated in all subcortical structures (table e-2; figure 1, B–F). In the right hippocampus, FAs in ε4ε4 children were lower at younger ages (younger than 7 years) but normalized thereafter, with no changes with age (figure 1B). As a group, ε2ε4 children had the highest FA, while the younger ε2ε2 child showed the lowest hippocampal FA. Conversely, in the left amygdala, younger children (<7 years) with ε4ε4, ε2ε4, and ε2ε3 had lower FA than ε3ε3 and ε3ε4 groups, whose FA was constant with age (figure 1D). The left thalamus FA increased with age in all children (figure 1E), but children younger than 7 years with ε2ε4 and ε4ε4 had lower FA, while children older than 12 years with ε4ε4 showed higher FA (figure 1E).
APOE ε genotype on cortical measures.
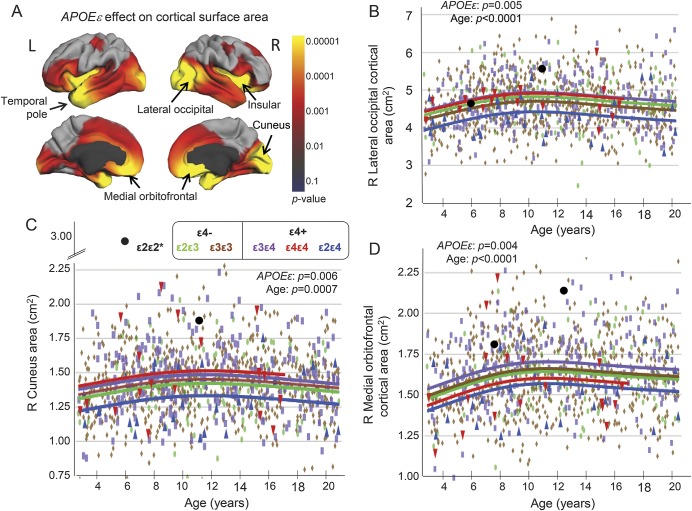
Several cortical areas differed by APOE ε genotype (figure 2A), with parallel age-related trajectories (validated in the ROI model) (figure 2, A–D; table e-3). For these regions, ε2ε4 children showed the smallest areas, while ε4ε4 or ε3ε4 children had the largest areas (figure 2, B–D). The 2 children with ε2ε2 had exceptionally large areas. The selected cortical volumes decreased with age for all genotypes. Compared with other groups, ε2ε4 children had larger left inferior parietal gyrus and right superior parietal gyrus at younger age (<10 years) but smaller volumes during adolescence (figure 3, A and B).
Figure 2. APOE ε genotypic variations on cortical areas.
(A) The p value maps using the vertex model reveal significant APOE ε effects (red and yellow) on cortical surfaces of the insular cortex, the temporal poles, as well as the 3 right hemisphere regions (lateral occipital, medial orbitofrontal, and cuneus) shown to be different across genotypes on the region-of-interest model (see B–D). The ε2ε4 group showed the smallest cortical areas across the age range in the right lateral occipital cortex (B), right cuneus (C), and right medial orbitofrontal cortex (D). Post hoc analysis shows that, relative to the reference ε3ε3 group (brown), the ε2ε4 group (blue) had significantly smaller right medial orbitofrontal cortical areas (p = 0.006) and smaller right cuneus areas (p = 0.002), while the ε3ε4 group (purple) had larger lateral occipital (p = 0.016) and medial orbitofrontal cortical areas (p = 0.006). The ε4ε4 group (red) also had the largest areas among all groups in the right cuneus (ε4ε4 > ε2ε4, p = 0.07) and right lateral occipital area (ε4ε4 > ε2ε4, p = 0.04). The children with ε2ε2 (black) had relatively large cortical areas in the cuneus and right medial orbitofrontal regions, largest in the younger ε2ε2 child. See also table e-3 for additional results. *Data for the ε2ε2 children are not included in the group analyses.
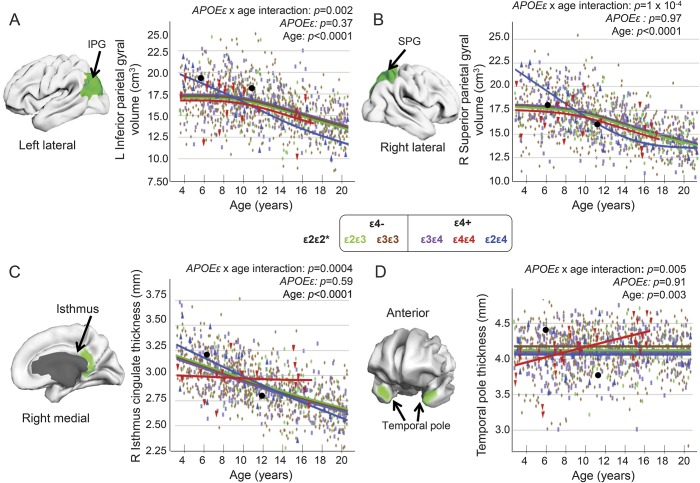
Figure 3. APOE ε-by-age interactions or APOE ε effects on cortical volumes and thickness.
Brain regions with APOE ε-by-age interactions are shown (green). (A and B) The ε2ε4 group showed the largest average volumes in inferior and superior parietal cortices at younger age (<10 years), but the smallest average volumes during adolescence. (C) The isthmus of the cingulate showed age-dependent thinning in all children except for those with ε4ε4. (D) In contrast, all children showed relatively stable temporal pole thickness (averaged left and right) across the age range, except for the children homozygous for ε4. All models for regions of interest were generated from the general additive model with thickness or volume of the region of interest as dependent variable, covarying for sex, scanner device, socioeconomic status, and genetic ancestry factor. *Data for the ε2ε2 children are not included in the group analyses. IPG = inferior parietal gyrus; SPG = superior parietal gyrus.
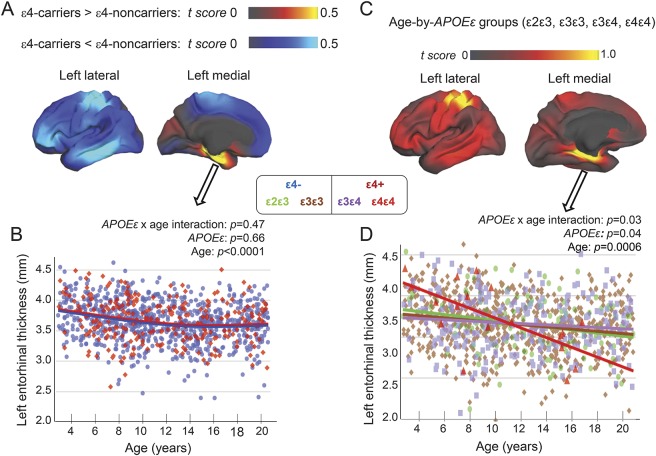
For cortical thickness, the right isthmus cingulate showed age-dependent thinning, except for ε4ε4 children who showed no change with age (figure 3C). Conversely, temporal pole thickness was constant with age, except for ε4ε4 children who showed age-related increases, with thinner cortices in younger and thicker cortices in older individuals (figure 3D). Furthermore, since prior reports compared ε4 carriers (ε4ε4, ε4ε3) with non–ε4 carriers (ε3ε3, ε2ε3),5,29 we performed vertex-based, whole-brain analyses using the same grouping. Compared to non–ε4 carriers, ε4 carriers had nonsignificantly thicker left entorhinal cortex (figure 4A, yellow region), verified on ROI analyses (figure 4B), and nonsignificantly thinner dorsal postcentral and lateral temporal cortices (figure 4A, lighter blue). However, cortical thickness showed a trend for group differences on age-dependent measures in parahippocampal regions and the left postcentral and entorhinal cortices (figure 4C, yellow regions). ROI analyses verified group differences in age-dependent thinning in the left entorhinal cortex. Specifically, ε4ε4 children showed the steepest slope (r = −0.66, p = 0.02), with thicker cortices in younger children but thinner cortices in older children (>11 years; figure 4D), as reported for adolescents.5
Figure 4. Cortical thickness differences between ε4 carriers and non–ε4 carriers.
These analyses were performed using the same grouping as prior studies.5 (A) On the vertex-based analyses (covaried for age, sex, scanner device, socioeconomic status, and genetic ancestry factor), compared to ε4 noncarriers (668 ε3ε3+ and 124 ε2ε3), ε4 carriers (235 ε3ε4+ and 21 ε4ε4) showed nonsignificantly thicker right precuneus (data not shown) and left entorhinal cortices (red-yellow regions), and nonsignificantly thinner left postcentral (parietal) and left lateral temporal cortices (blue areas). (B) Region-of-interest analysis of the left entorhinal region verified the nonsignificantly thicker cortex in ε4 carriers (red dots) than non–ε4 carriers (blue dots). (C) The 4 genotype groups showed a trend for group differences (p = 0.13) on age-dependent changes in cortical thickness in the left entorhinal and parahippocampal regions as well as the left postcentral cortex (yellow regions). (D) Region-of-interest analyses verified the age-dependent group differences; however, the ε4ε4 children (red dots) showed steepest age-dependent thinning in this brain region, with thicker cortices in the younger children but thinner cortices in the older children (>11 years).
APOE ε genotypes and age on cognitive performance.
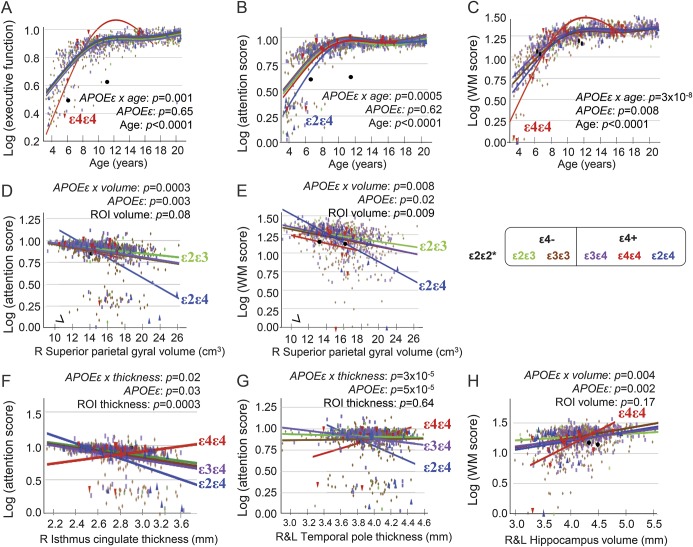
Age-by-genotype interactions were found on executive function, attention, and WM (figure 5, A–C) but not episodic memory. Compared to other genotype groups, younger ε4ε4 children had lower scores on executive function and WM, but similar or better performance after age 8 years (figure 5, A and B). On the attention task, younger ε2ε4 children performed worse than other genotype groups, but their scores normalized after age 10 years (figure 5C).
Figure 5. Cognitive performance in relation to age, brain volumes, or cortical thickness.
APOE ε genotype-by-age interactions on executive function (A), attention (B), and WM (C). Children with larger right superior parietal gyral volumes had poorer attention scores (D) or WM scores (E) across all genotype groups, especially those with APOE ε2ε4. Similarly, those with thicker cortices in the isthmus cingulate cortical thickness (F) and temporal poles (G) had poorer attention and WM scores, especially children with APOE ε2ε4. In contrast, children with APOE ε4ε4 with thicker cortices had better attention and WM scores (F and G). All children with larger hippocampal volumes had higher WM scores (H), especially children homozygous for APOE ε4 (H). *Data for the ε2ε2 children (black dots) are not included in the group analyses. ROI = region of interest; WM = working memory.
Relationships between brain morphometry and cognition.
After adjustments for sex, SES, and GAF, brain regions with age-related genotype variations also showed differential correlations with attention or WM. In the right superior parietal gyrus, ε2ε4 children differed from other groups; those with larger superior parietal gyral volumes had the lowest attention (r = −0.58, p = 0.001; figure 5D) or WM scores (r = −0.59, p = 0.0005; figure 5E). Similarly, across genotypes, ε2ε4 children with thicker cortices had the poorest attention (right isthmus cingulate: r = −0.62, p = 0.0002, figure 5F; temporal pole: r = −0.43, p = 0.01, figure 5G), while ε4ε4 children with thinner temporal poles had poorer attention (r = +0.5, p = 0.02; figure 5G). All children with smaller hippocampal volumes had poorer WM performance, especially ε4ε4 children (r = +0.48, p = 0.03; figure 5H).
DISCUSSION
The APOE ε gene is polymorphic with 3 alleles, with ε3 being the most common (approximately 78%), followed by ε4 (14%) and ε2 (8%).15 Evaluating all 6 APOE ε genotypes in a large cohort of typically developing children clarified the associations of heterozygous vs homozygous ε2 and ε4 on brain development. The major findings are as follows: (1) compared to other genotype groups, ε4ε4, ε2ε2, and ε2ε4 children had altered age-related slopes in brain regions often affected in AD30; (2) smaller hippocampal volumes in younger ε2ε4 children and lower hippocampal FA in younger ε4ε4 children mirror the smaller volumes and steeper age-dependent atrophy of the hippocampi in elderly ε4 and/or ε2 carriers31; and (3) the younger ε2ε4 and ε4ε4 children with altered age-related changes in brain measures also showed poorer performance on attention or WM tasks.
Children with the most common ε3ε3 genotype served as our reference group. These children showed typical age-related increases in hippocampal volumes32 and the medial orbitofrontal and occipital cortical areas until early adolescence.33 They also showed the typical age-dependent increase in thalamic FA,23 reflecting ongoing myelination, but not age-related increases in hippocampal and amygdala FA, as reported in healthy children without genotype groupings.23 Age-dependent decreases in parietal cortical volume and thinning of the isthmus likely reflect pruning of neuronal synapses and cell shrinkage.33 Furthermore, our ε3ε3 children with thinner isthmus had similarly better performance in WM and attention.34
The children with APOE ε2ε3 genotype had comparable brain morphometry and cognitive performance as ε3ε3 children. However, younger (<7 years) ε2ε3 children, like those with ε2ε4 or ε4ε4, had relatively lower FA in left amygdala, suggesting lesser microstructural integrity, such as lower cellular density or lesser myelination. Since the amygdala is involved in emotional processing, these children may have greater vulnerability to emotional problems. Lower amygdala FA was also found in infants whose mothers had greater prenatal depressive symptoms35; hence, future studies should include maternal depressive symptoms as a covariate to determine whether these symptoms might account for the lower amygdala FA in younger ε2ε3 children.
Relative to ε3ε3 children, those with one ε4 allele, specifically APOE ε3ε4, had larger hippocampi, occipital and frontal cortical areas, and thicker temporal poles, but similar cognitive functioning. These larger brain measures are consistent with an antagonistic pleiotropic effect of ε4, similar to findings in middle-aged (51–59 years) heterozygous ε4 carriers (primarily ε3ε4).3,10 Normal or thicker cortices in our ε3ε4 children contrast with prior findings of thinner left entorhinal and orbitofrontal cortices (92% ε3ε4 compared to ε3ε3 children).5 However, larger cortical areas in our ε3ε4 children resemble larger parietal volumes in ε3ε4 infants (ages 8.5–14 months)7 relative to ε3ε3 participants. Moreover, consistent with our results, prior studies found similar IQ or cognitive performance between ε3ε4 and ε3ε3 children.5,13
Despite the relatively normal hippocampal volumes, the younger ε4ε4 participants had the lowest FA in hippocampus, amygdala, and thalamus, suggesting slower development initially with lesser myelination or lower cellularity in these regions. These children also did not show typical age-dependent cortical thinning in posterior cingulate (isthmus) cortex, which suggests aberrant brain maturation, possibly due to reduced synaptic pruning. Such possible aberrant brain maturation with lesser cortical thinning was found in children with prenatal alcohol exposure and fetal alcohol syndrome,36 although such children were excluded in the current study. Furthermore, ε4ε4 children showed age-dependent thickening of the temporal pole, which along with the isthmus, is often affected in AD30,37 and in cognitively healthy ε4 carriers,30 who showed age-dependent increases in amyloid deposition.18
Younger ε4ε4 children showed the poorest executive function and WM among groups, even after SES adjustment, similar to school-age ε4 carriers with AD family history.14 The normal cognition of our older ε4ε4 children is consistent with prior findings in older ε4ε4 children5,13 and in young and middle-aged adults.10 The ε4ε4 children with smaller hippocampal volumes and thinner temporal pole had poorer WM and attention; these findings resemble the thinnest frontal cortices29 in middle-aged ε4ε4 individuals without dementia, who showed the most rapid decline on mental arithmetic tasks requiring WM.26 Hence, ε4 homozygosity might slow maturation of the hippocampus and cortical thickness, which in turn might negatively affect WM and attention. Mirroring these findings, older individuals with ε4 homozygosity had the highest prevalence for AD (50%–91%)4,15 and the greatest hippocampal and temporal lobe atrophy30 among genotypes.
Unlike ε3ε4 children with the largest hippocampi, ε2ε4 children had the smallest hippocampi and orbitofrontal and occipital surface areas among groups and across ages. Therefore, ε4 allele effects differ greatly when combined with ε2 vs ε3. Although the younger ε2ε4 children had larger parietal cortices and thicker posterior cingulate cortices, they had poorer attention and WM. These findings also mirror those in the 3 ε2ε4 oldest old (>90 years) among 89 participants, since all 3 met criteria for dementia but not neuropathology for AD.38 While ε2 carriers showed reduced cognitive decline, ε2ε4 is a risk genotype for AD across ethnicity.15 Unfortunately, ε2ε4 participants are often excluded from studies because of potentially opposite effects of ε4 and ε2.11,12,21,38
In the ε2ε2 children, the relatively large hippocampi are similar to the case reports of ε2ε2 adults,31 and the lower hippocampal FA in the younger child suggests less coherent fibers in the large hippocampi. They also had relatively higher thalamic FA, similar to findings in ε2 heterozygous adults.39 Our ε2ε2 children also had poorer attention and executive function, while ε2ε2 children in a prior study showed above-average IQ.13 Furthermore, a 92-year-old ε2ε2 woman showed no cognitive deficits until her stroke, despite the postmortem finding of prominent AD neuropathology.16 The relative preservation of cognition in aging ε2ε2 individuals may be due to ApoE ε2's antioxidant, anti-inflammatory, and antiproteolytic effects.17 However, these processes may not be relevant in the developing brain.
For comparison with the literature, we also evaluated all ε4 carriers. Hippocampal volumes in ε4 carriers ranged from smaller (ε2ε4) to no difference (ε4ε4) or slightly larger (ε3ε4) relative to ε3ε3 children. Similarly, cortical surface areas were smallest in ε2ε4 but largest in ε3ε4 or ε4ε4. Hence, combining all ε4 carriers might have attenuated or abolished group differences compared to non–ε4 carriers,5–7,12,14,40 and the larger parietal volumes in the youngest ε2ε4 children or the thinnest isthmus gyrus and temporal poles in the youngest ε4ε4 children might have been missed. In fact, our ε4 carriers collectively had nonsignificantly thicker temporal lobes, contrasting with thinner entorhinal cortices in a prior study of healthy ε4 children.5 However, further analysis showed that ε4ε4 children had steeper age-dependent thinning in this region, leading to thinner entorhinal cortices in adolescents, similar to prior findings.5 Moreover, our youngest ε4 carriers performed poorer on WM than the non–ε4 carriers, which resemble WM deficits in older healthy ε4 carriers.25
The age-dependent brain measures of ε4ε4 and ε2ε4 children often deviated from those in the other genotype groups. The youngest children with one of these genotypes had less mature brain structures and poorer cognitive function, but tended to normalize or exceed the other genotype groups during late adolescence. Prior studies of ε4ε4 and ε2ε4 young adults showed larger specific brain structures and better cognitive performance, whereas older adults showed poorer cognitive performance or less efficient neural networks relative to other genotype groups.9,10 Incidentally, ε2ε4 participants have a low odds ratio of AD until age 50 years, but the highest odds at age 70 years,15 while ε4 homozygosity leads to earliest AD onset (approximately 68 years).4
This study has several limitations. Despite this relatively large sample, the age-related brain measures may be biased by the cohort effect, driven by participants with these rare genotypes at the extremes of age range, in this cross-sectional study. Longitudinal follow-ups are needed to confirm the true developmental trajectories in the less prevalent ε4 or ε2 carriers. In addition, some younger children (<5 years) could not perform all NIH Toolbox tasks; future studies with more young children using age-appropriate assessments are needed. Lastly, although we covaried for GAF, ethnicity may influence the effects of ε4 and ε2 on brain and cognitive measures.4,15 However, repeating all the analyses only in children with >50% European ancestry yielded similar results (table e-4, figures e-1 to e-3). Future studies should include a larger sample of children with other ancestry and evaluate them separately.
This large sample of children validated and extended prior smaller studies that showed altered brain development in ε4 carriers. The ε4ε4 and ε2ε4 carriers appear to show the strongest antagonistic pleiotropic effects, with negative influences on brain structures and cognition at younger age, mirroring those in elderly participants and patients with AD. Future studies of APOE ε should evaluate each genotype separately, since brain development, and possibly brain aging and recovery, may vary substantially across specific ε4 or ε2 genotypes. Finally, studying APOE ε polymorphism in young children may provide early indications of risk of future brain injuries and dementia. Given the urgent need to determine how early patients with AD should receive interventions or preventive treatments, a thorough understanding of how AD risk genes, such as APOE ε4, might independently or interactively influence the brain across the ages, is needed.
Supplementary Material
ACKNOWLEDGMENT
The authors thank all of the children and their families who have participated in our study, the physicians who referred some of the participants, and all the clinical and technical staff of the research teams in the PING Consortium. The authors also thank Hauke Bartsch, PhD, for PING Portal support and James Davis, PhD, and Wesley Thompson, PhD, for statistical advice.
GLOSSARY
- AD
Alzheimer disease
- FA
fractional anisotropy
- GAF
genetic ancestry factor
- GAM
general additive model
- PING
Pediatric Imaging, Neurocognition, and Genetics
- ROI
region of interest
- SES
socioeconomic status
- WM
working memory
Footnotes
Supplemental data at Neurology.org
Editorial, page 558
Contributor Information
Collaborators: Pediatric Imaging, Neurocognition, and Genetics (PING) Study Consortium, Terry L. Jernigan, Connor McCabe, Linda Chang, Natacha Akshoomoff, Erik Newman, Anders M. Dale, Thomas Ernst, Anders M. Dale, Peter Van Zijl, Joshua Kuperman, Sarah Murray, Cinnamon Bloss, Mark Appelbaum, Anthony Gamst, Wesley Thompson, Brian Keating, David Amaral, Walter Kaufmann, Peter Van Zijl, Stewart Mostofsky, B.J. Casey, Erika J. Ruberry, Alisa Powers, Bruce Rosen, Tal Kenet, Jean Frazier, David Kennedy, and Jeffrey Gruen
AUTHOR CONTRIBUTIONS
Linda Chang, Vanessa Douet, and Thomas Ernst developed the concept, designed the study, performed the statistical analyses, interpreted the data, drafted and revised the manuscript. Linda Chang, Thomas Ernst, Alexandra Pritchett, Kristin Lee, Terry Jernigan, Natacha Akshoomoff, Sarah Murray, Cinnamon Bloss, David Kennedy, Jean Frazier, David Amaral, Jeffrey Gruen, Walter Kaufmann, B.J. Casey, and Elizabeth Sowell were all involved in the data acquisition. Sarah Murray was responsible for all genotyping and genetic imputations. The USCD team was responsible for all image processing. All authors were involved in the critical revision and approval of the manuscript.
STUDY FUNDING
Funded by the NIH (RC2-DA29475, 2K24-DA016170, U54-NS56883, G12-MD007601).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Teasdale GM, Nicoll JA, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet 1997;350:1069–1071. [DOI] [PubMed] [Google Scholar]
- 2.Kokubo Y, Chowdhury AH, Date C, Yokoyama T, Sobue H, Tanaka H. Age-dependent association of apolipoprotein E genotypes with stroke subtypes in a Japanese rural population. Stroke 2000;31:1299–1306. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Andres M, Sadino J, et al. . Impact of apolipoprotein E epsilon4 and HIV on cognition and brain atrophy: antagonistic pleiotropy and premature brain aging. Neuroimage 2011;58:1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013;9:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw P, Lerch JP, Pruessner JC, et al. . Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol 2007;6:494–500. [DOI] [PubMed] [Google Scholar]
- 6.Dean DC III, Jerskey BA, Chen K, et al. . Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurol 2014;71:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knickmeyer RC, Wang J, Zhu H, et al. . Common variants in psychiatric risk genes predict brain structure at birth. Cereb Cortex 2014;24:1230–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunce D, Bielak AA, Anstey KJ, Cherbuin N, Batterham PJ, Easteal S. APOE genotype and cognitive change in young, middle-aged, and older adults living in the community. J Gerontol A Biol Sci Med Sci 2014;69:379–386. [DOI] [PubMed] [Google Scholar]
- 9.Jochemsen HM, Muller M, van der Graaf Y, Geerlings MI. APOE epsilon4 differentially influences change in memory performance depending on age. The SMART-MR Study. Neurobiol Aging 2012;33:e815–e822. [DOI] [PubMed] [Google Scholar]
- 10.Evans S, Dowell NG, Tabet N, Tofts PS, King SL, Rusted JM. Cognitive and neural signatures of the APOE E4 allele in mid-aged adults. Neurobiol Aging 2014;35:1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander DM, Williams LM, Gatt JM, et al. . The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biol Psychol 2007;75:229–238. [DOI] [PubMed] [Google Scholar]
- 12.Bloss CS, Delis DC, Salmon DP, Bondi MW. APOE genotype is associated with left-handedness and visuospatial skills in children. Neurobiol Aging 2010;31:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor AE, Guthrie PA, Smith GD, et al. . IQ, educational attainment, memory and plasma lipids: associations with apolipoprotein E genotype in 5995 children. Biol Psychiatry 2011;70:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloss CS, Delis DC, Salmon DP, Bondi MW. Decreased cognition in children with risk factors for Alzheimer's disease. Biol Psychiatry 2008;64:904–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrer LA, Cupples LA, Haines JL, et al. . Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997;278:1349–1356. [PubMed] [Google Scholar]
- 16.Berlau DJ, Kahle-Wrobleski K, Head E, Goodus M, Kim R, Kawas C. Dissociation of neuropathologic findings and cognition: case report of an apolipoprotein E epsilon2/epsilon2 genotype. Arch Neurol 2007;64:1193–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE epsilon2. Neurosci Biobehav Rev 2013;37:2878–2886. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC, Roe CM, Xiong C, et al. . APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010;67:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Paajanen T, Westman E, et al. . APOE epsilon2 allele is associated with larger regional cortical thicknesses and volumes. Dement Geriatr Cogn Disord 2010;30:229–237. [DOI] [PubMed] [Google Scholar]
- 20.den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MM. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology 2002;59:746–748. [DOI] [PubMed] [Google Scholar]
- 21.Bunce D, Anstey KJ, Cherbuin N, Gautam P, Sachdev P, Easteal S. APOE genotype and entorhinal cortex volume in non-demented community-dwelling adults in midlife and early old age. J Alzheimers Dis 2012;30:935–942. [DOI] [PubMed] [Google Scholar]
- 22.Jernigan TL, Brown TT, Hagler DJ Jr, et al. . The Pediatric Imaging, Neurocognition, and Genetics (PING) data repository. Neuroimage 2015;124:1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yap QJ, Teh I, Fusar-Poli P, Sum MY, Kuswanto C, Sim K. Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. J Neural Transm 2013;120:1369–1395. [DOI] [PubMed] [Google Scholar]
- 24.Weintraub S, Dikmen SS, Heaton RK, et al. . Cognition assessment using the NIH Toolbox. Neurology 2013;80:S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinvang I, Winjevoll IL, Rootwelt H, Espeseth T. Working memory deficits in healthy APOE epsilon 4 carriers. Neuropsychologia 2010;48:566–573. [DOI] [PubMed] [Google Scholar]
- 26.Caselli RJ, Dueck AC, Locke DE, et al. . Longitudinal modeling of frontal cognition in APOE epsilon4 homozygotes, heterozygotes, and noncarriers. Neurology 2011;76:1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantarci K, Lowe V, Przybelski SA, et al. . APOE modifies the association between Abeta load and cognition in cognitively normal older adults. Neurology 2012;78:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health 1996;86:726–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fennema-Notestine C, Panizzon MS, Thompson WR, et al. . Presence of ApoE epsilon4 allele associated with thinner frontal cortex in middle age. J Alzheimers Dis 2011;26(suppl 3):49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Paajanen T, Westman E, et al. . Effect of APOE epsilon4 allele on cortical thicknesses and volumes: the AddNeuroMed Study. J Alzheimers Dis 2010;21:947–966. [DOI] [PubMed] [Google Scholar]
- 31.Hostage CA, Roy Choudhury K, Doraiswamy PM, Petrella JR; Alzheimer's Disease Neuroimaging Initiative. Dissecting the gene dose-effects of the APOE epsilon4 and epsilon2 alleles on hippocampal volumes in aging and Alzheimer's disease. PLoS One 2013;8:e54483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci 2009;29:11772–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gogtay N, Giedd JN, Lusk L, et al. . Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 2004;101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowell E, Thompson P, Welcome S, Henkenius A, Toga A, Peterson B. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet 2003;362:1699–1707. [DOI] [PubMed] [Google Scholar]
- 35.Rifkin-Graboi A, Bai J, Chen H, et al. . Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol Psychiatry 2013;74:837–844. [DOI] [PubMed] [Google Scholar]
- 36.Treit S, Zhou D, Lebel C, Rasmussen C, Andrew G, Beaulieu C. Longitudinal MRI reveals impaired cortical thinning in children and adolescents prenatally exposed to alcohol. Hum Brain Mapp 2014;35:4892–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hua X, Leow AD, Parikshak N, et al. . Tensor-based morphometry as a neuroimaging biomarker for Alzheimer's disease: an MRI study of 676 AD, MCI, and normal subjects. Neuroimage 2008;43:458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berlau DJ, Corrada MM, Head E, Kawas CH. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology 2009;72:829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiang GC, Zhan W, Schuff N, Weiner MW. White matter alterations in cognitively normal apoE epsilon2 carriers: insight into Alzheimer resistance? AJNR Am J Neuroradiol 2012;33:1392–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan W, Giampietro V, Ginestet C, et al. . No differences in hippocampal volume between carriers and non-carriers of the ApoE epsilon4 and epsilon2 alleles in young healthy adolescents. J Alzheimers Dis 2014;40:37–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.