Abstract
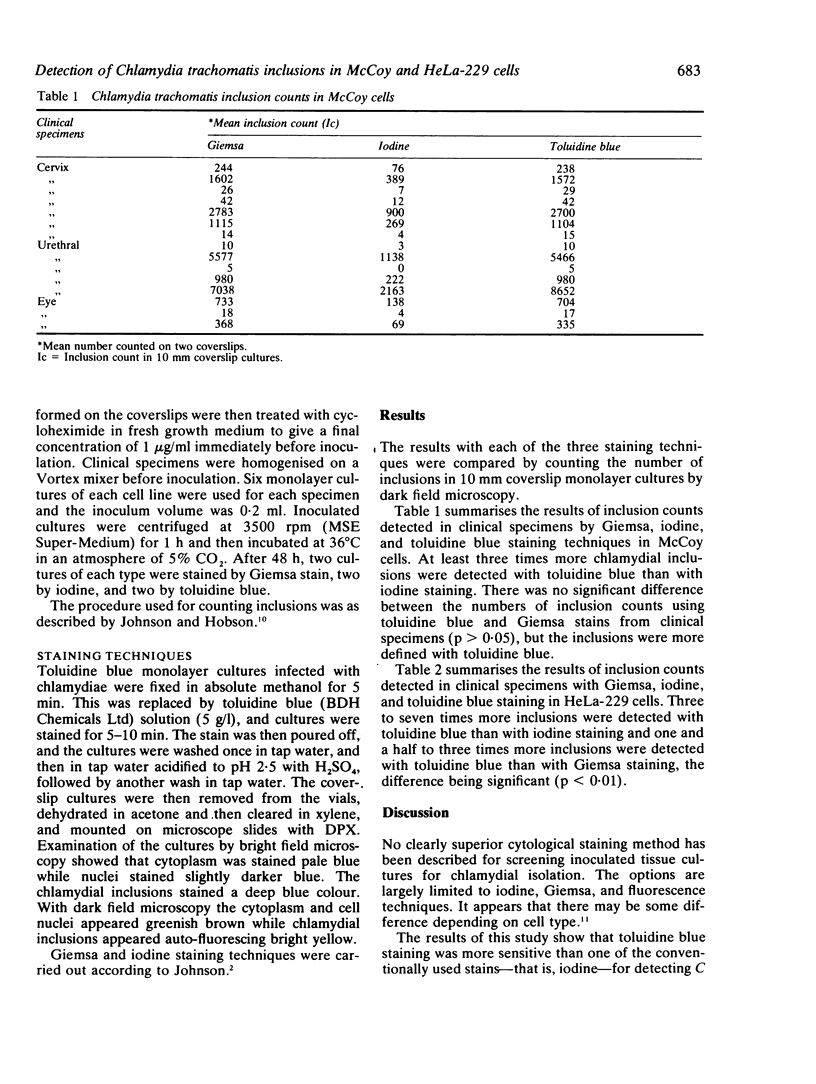
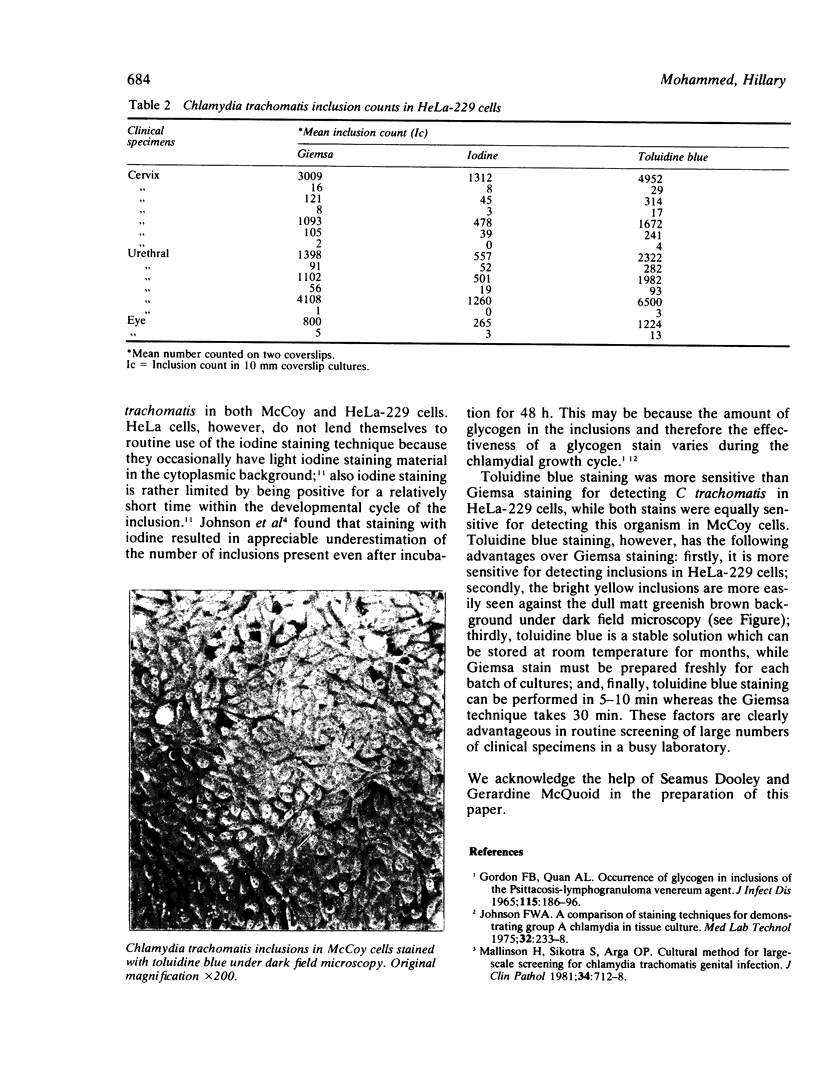
Toluidine blue staining was used to detect Chlamydia trachomatis inclusions in both McCoy and HeLa-229 cells from clinical specimens. This method was more sensitive than iodine staining for detecting C trachomatis inclusions in both McCoy and HeLa-229 cells and also more sensitive than Giemsa staining for detecting chlamydial inclusions in HeLa-229 cells. While its sensitivity for detection of chlamydial inclusions in McCoy cells is equal to that of Giemsa staining, toluidine blue staining is easier and faster to perform.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Croy T. R., Kuo C. C., Wang S. P. Comparative susceptibility of eleven mammalian cell lines to infection with trachoma organisms. J Clin Microbiol. 1975 May;1(5):434–439. doi: 10.1128/jcm.1.5.434-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. A., Rees E., Hobson D., Karayiannis P. Isolation of Chlamydia trachomatis from Bartholin's ducts. Br J Vener Dis. 1978 Dec;54(6):409–413. doi: 10.1136/sti.54.6.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. T., Woodland R. M. Detection of chlamydiae by isolation and direct examination. Br Med Bull. 1983 Apr;39(2):181–186. doi: 10.1093/oxfordjournals.bmb.a071813. [DOI] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. OCCURENCE OF GLYCOGEN IN INCLUSIONS OF THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM-TRACHOMA AGENTS. J Infect Dis. 1965 Apr;115:186–196. doi: 10.1093/infdis/115.2.186. [DOI] [PubMed] [Google Scholar]
- Johnson F. W. A comparison of staining techniques for demonstrating group A chlamydia in tissue culture. Med Lab Technol. 1975 Jul;32(3):233–238. [PubMed] [Google Scholar]
- Johnson F. W., Chancerelle L. Y., Hobson D. An improved method for demonstrating the growth of Chlamydiae in tissue culture. Med Lab Sci. 1978 Jan;35(1):67–74. [PubMed] [Google Scholar]
- Johnson F. W., Hobson D. Factors affecting the sensitivity of replicating McCoy cells in the isolation and growth of chlamydia A (TRIC agents). J Hyg (Lond) 1976 Jun;76(3):441–451. doi: 10.1017/s0022172400055376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallinson H., Sikotra S., Arya O. P. Cultural method for large-scale screening for Chlamydia trachomatis genital infection. J Clin Pathol. 1981 Jul;34(7):712–718. doi: 10.1136/jcp.34.7.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland R. M., El-Sheikh H., Darougar S., Squires S. Sensitivity of immunoperoxidase and immunofluorescence staining for detecting chlamydia in conjunctival scrapings and in cell culture. J Clin Pathol. 1978 Nov;31(11):1073–1077. doi: 10.1136/jcp.31.11.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland R. M., Malam J., Darougar S. A rapid method for staining inclusions of Chlamydia psittaci and Chlamydia trachomatis. J Clin Pathol. 1982 Jun;35(6):642–644. doi: 10.1136/jcp.35.6.642. [DOI] [PMC free article] [PubMed] [Google Scholar]



