Abstract
Drug abuse represents a considerable burden of disease and has enormous economic impacts on societies. Over the years, few medications have been developed for clinical use. Their utilization is endowed with several limitations, including partial efficacy or significant side effects. On the other hand, the successful advancement of these compounds provides an important proof-of-concept for the feasibility of drug development programs in addiction. In recent years, a wealth of information has been generated on the psychological mechanisms, genetic or epigenetic predisposing factors, and neurobiological adaptations induced by drug consumption that interact with each other to contribute to disease progression. It is now clear that addiction develops through phases, from initial recreational use to excessive consumption and compulsive drug seeking, with a shift from positive to negative reinforcement driving motivated behaviors. A greater understanding of these mechanisms has opened new vistas in drug development programs. Researchers’ attention has been shifted from investigation of classical targets associated with reward to biological substrates responsible for negative reinforcement, impulse loss of control and maladaptive mechanisms resulting from protracted drug use. From this research, several new biological targets for the development of innovative therapies have started to emerge. The present article offers an overview of targets currently under scrutiny for the development of new medications for addiction. This work is not exhaustive but rather it provides a few examples of how this research has advanced in recent years by virtue of studies carried out in our laboratory.
Keywords: PPARα, PPARγ, Neurokinins, NK1, CRF, Nociceptin, NOP, CREB, Phosphodiesterase, Neuropetide S, Orexin
Introduction
Drug abuse and addiction represent a considerable burden of disease and cost for society (Uhl and Grow, 2004). Despite this, effective pharmacotreatments are still lacking, and the few currently available medications suffer from significant limitations, including important side effects or restricted efficacy. For instance, drugs that are registered for the treatment of heroin addiction, such as methadone and buprenorphine, have reinforcing properties and thus can be themselves abused. Whereas the opioid antagonist naltrexone, which is used in the clinic for opioid and alcohol addiction appears to have limited efficacy restricted to a specific subpopulation of patients (Heilig et al., 2011). One of the factors that has hampered the development of effective medication in addiction is the extreme complexity of the biological mechanisms responsible for this psychiatric disease in which genetic vulnerability, environmental risk factors and their interaction play a determining role (also see the chapter of Quednow & Herdener “Human pharmacology for addiction medicine” in this volume).
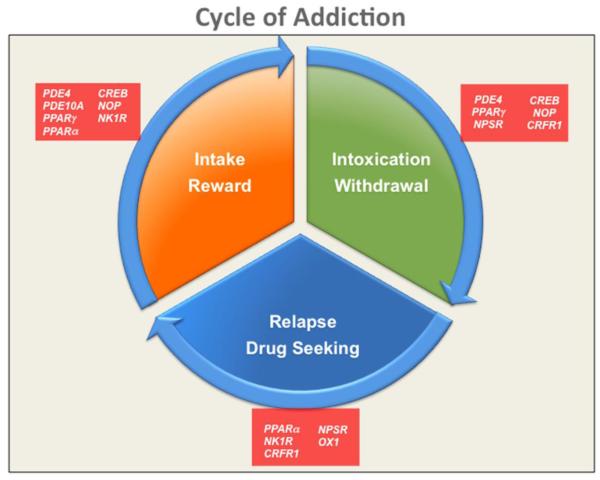
Over the past decades, a wealth of information has been collected that has helped us unravel the neurobiological mechanisms responsible for maladaptive behaviors associated with the use of addictive drugs. The research in this field has advanced enormously, and the functions of several neurotransmitter systems, molecular pathways, and transcriptional and epigenetic mechanisms have started to be revealed (Robinson and Berridge, 1993; Shaham and Hope, 2005; Le Moal and Koob, 2007; Thomas et al., 2008; Robison and Nestler, 2011). This new acquired knowledge has allowed for a more accurate dissection of the major facets of addiction, which includes drug reward and positive reinforcement, abstinence and negative reinforcement, craving, relapse to drug seeking, and impulsivity and compulsivity (Fig. 1).
Figure 1.
Schematic representation of the addiction cycle characterized by initial recreational drug use, followed by drug dose escalation, intoxication and episodic withdrawal and terminating in drug seeking and relapse. Drug targets for these different domains are depicted.
With few exceptions, all addictive substances engage the mesolimbic dopamine reward pathway of the brain. It has been recognized that activation of this catecholaminergic circuit is critical for the rewarding and reinforcing properties of these drugs. The pleasurable effect experienced following exposure to a drug promotes the recreational use that often terminates into abuse and dependence (Gardner, 2000). However the role played by dopamine on human addiction is not fully disclosed yet. For example there are little evidences that opioids and cannabinoids induce reward and cause addiction through mesolimbic DA mechanisms. Additional or alternative processes may be involved in the acquisition of dependence for these substances (Nutt et al., 2015).
For a long time researchers in the drug of abuse field have mostly concentrated their attention on the study of positive reinforcement mechanisms (Koob et al., 2003); however, through the years, research has progressively highlighted other important but initially elusive aspects of substance abuse disorder.
For instance, it has been recognized that with protracted exposure to increasing daily doses of drugs, the reward or hedonic states associated with their consumption significantly decrease and the negative emotional states, such as anhedonia, dysphoria and anxiety, start to emerge. After this shift, substance use is no longer so pleasurable, and the drug is mostly taken to alleviate the aversive conditions (negative reinforcement) associated with its abstinence. At the beginning, drug use is episodic, but it rapidly progresses to a habitual consumption and, finally, to a compulsive, uncontrolled use characterized by chronic intoxication, followed by abstinence episodes that are usually accompanied by an intense and persistent urge for the drug and relapse (Fig. 1). Several factors may contribute to the exacerbation of drug seeking and relapse; among the major ones are stress and environmental cues predictive of drug availability or previously associated with the pleasurable effect of the drug.
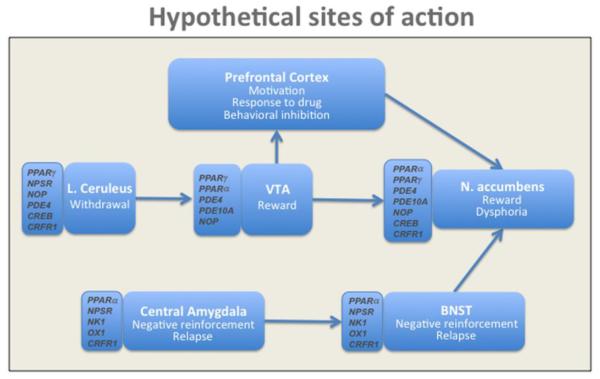
At the neuroanatomical level, the circuits that mediate the rewarding effects of drugs are partially different from those mediating drug-seeking and relapse. For instance, drug reward is largely controlled by the mesolimbic dopamine system that originates from the ventral tegmental area (VTA) and that sends afferents to the nucleus accumbens (Nac) (Koob et al., 1998). On the contrary, drug-seeking and relapse are mediated by a complex network that includes the extended amygdala, hippocampus, dorsal striatum, prefrontal cortical structures and insula (Koob and Volkow, 2010). Moreover, several neurotransmitter systems and cellular mechanisms have been suggested to modulate the function of these circuitries; therefore, their potential involvement in shaping addictive responses has been proposed (Fig. 2).
Figure 2.
Schematic representation of the hypothetical brain sites of action and neuronal substrates for new targets in addiction.
As a consequence of these advancements, new or previously unexplored drug targets have been proposed and are now under scrutiny for the development of innovative pharmacotherapeutic approaches beyond agents acting as drug reward modulators. In the present article, we reviewed some of the recent and most promising advancements in the identification and validation of these new targets. This work is not intended to be exhaustive but will focus on a few specific areas of development over the past decade that have shown major promise.
Peroxisome Proliferating Activator Receptors (PPARs)
PPARs are intracellular receptors that function as transcription factors (Issemann and Green, 1990). Once activated by their ligand, PPARs translocate to the nucleus where they attach the retinoid receptor (RXR). The PPAR–RXR complex binds to PPAR response elements in DNA to modulate the transcription of different genes.
Three isotypes of PPARs have been identified (PPARα, PPARγ and PPARβ/δ). They are differentially distributed in body tissues but all are present in the brain (Woods et al., 2003; Moreno et al., 2004; Gofflot et al., 2007; Sarruf et al., 2009). PPARs are involved in insulin sensitization (Moller and Berger, 2003), fatty acid homeostasis (Aoyama et al., 1998), apoptosis (Roberts et al., 1998), inflammatory response and neuroprotection (Landreth and Heneka, 2001; Berger and Moller, 2002; Kapadia et al., 2008).
Endogenous ligands of PPARs are unsaturated fatty acids, such as palmitic, oleic, linoleic and arachidonic acid and leukotrienes. Unsaturated fatty acids are also ligands of PPARγ, and they also bind to prostaglandins and low density lipoprotein components (Varga et al., 2011). Over the years, a number of synthetic ligands for PPARs have been developed, and some are marketed as drugs for the treatment of metabolic disorders (Schupp and Lazar, 2010). PPARα ligands, such as gemfibrozil, bezafibrate, clofibrate and fenofibrate, are used for the treatments of dyslipidemia, whereas PPARγ agonists, such as pioglitazone and rosiglitazone, are used for the therapy of type 2 diabetes.
Recent evidence supports the role of PPARα and PPARγ in addiction. For instance, PPARα and PPARγ are expressed in addiction-related brain areas, such as the lateral hypothalamus and the ventral tegmental area (VTA), from which dopamine (DA) release into the NAc can be modulated (Moreno et al., 2004; Sarruf et al., 2009; Melis et al., 2010; de Guglielmo et al., 2015b). In fact, electrophysiological experiments have shown that the activation of PPARα by fibrates decreased the ability of nicotine to enhance the firing rate of VTA DA neurons. This effect was accompanied with decreased levels of extracellular DA in the Nac (Melis et al., 2010; Panlilio et al., 2012). At the behavioral level, preclinical findings have shown that the PPARα agonist clofibrate blocked the acquisition of nicotine intake in rats and monkeys. Moreover, this drug reduced nicotine self-administration and prevented relapse to nicotine seeking precipitated by cues predictive of its availability or by nicotine priming (Panlilio et al., 2012). Similar results were observed with two other PPARα agonists, WY14643 and methyl oleoylethanolamide (Mascia et al., 2011). The behavioral and neurochemical effects of PPARα agonists were reversed following pretreatment with MK886, a selective PPARα antagonist. More recently, the efficacy of PPARα agonists in attenuating alcohol consumption and relapse to drug seeking in rodents have also been documented (Bilbao et al., 2015; Blednov et al., 2015).
Moreover, gene expression experiments have provided initial evidence of a link between PPARγ function and nicotine dependence (Amoruso et al., 2007); however, the most convincing data linking PPARγ to addiction came from pharmacological studies on alcohol and heroin. In these studies, it has been shown that the activation of PPARγ by chronic pioglitazone and rosiglitazone selectively decreased voluntary alcohol consumption in rodents (Stopponi et al., 2011). Moreover, pioglitazone markedly attenuated operant ethanol self-administration and reinstatement to alcohol seeking elicited by exposure to stress. Conversely, pioglitazone was not effective in controlling cue-induced relapse. Of note, when pioglitazone was administered in combination with naltrexone, a drug approved for the treatment of alcohol addiction in humans, a more robust inhibition of alcohol intake and a broader effect on relapse for alcohol resulted than when the two agents were given separately (Stopponi et al., 2013). These effects were selective for alcohol because it was shown in similar experiments that pioglitazone did not modify operant responding for saccharin. Interestingly, pioglitazone was also able to decrease the aversive symptoms associated with ethanol withdrawal that have an important clinical significance in alcohol relapse prevention (Stopponi et al., 2011).
Studies were also carried out to evaluate the effect of PPARγ agonists on opioid abuse. The results revealed that pioglitazone reduced heroin self-administration under both a fixed-ratio and progressive-ratio schedule of reinforcement. This effect was accompanied by a significant decrease of Nac extracellular DA levels following acute heroin infusion and a reduced VTA DA firing rate (de Guglielmo et al., 2015b). The activation of PPARγ was also shown to prevent the development of tolerance to the analgesic effects of morphine. Together, these findings suggest the possibility of combining PPARγ agonists with opioid agents to enhance the anti-addictive efficacy of antagonists (i.e., naltrexone) or to attenuate the abuse potential of agonists (i.e., buprenorphine or methadone). The possibility of combining PPARγ activators and opioid agonists for the development of analgesic therapies endowed with low abuse liability is also envisioned. In this regard, it is worth mentioning that both pioglitazone and rosiglitazone have anti-inflammatory properties and appear to be effective in treating neuropathic pain in rodent models (Morgenweck et al., 2013).
CREB and the inhibition of phosphodiesterase (PDE) enzymes
Chronic drug use causes long-term structural and functional modifications in the brain. Underlying this process are alterations in the transcription of specific target genes. The modified expression will reshape the function of neuronal cells causing the remodeling of neurocircuitries formed by those neurons. The cAMP response element binding protein (CREB) is an important transcription factor that mediates the action of cAMP. A large body of evidence links CREB to the acquisition and the maintenance of dependence to drugs of abuse. Few excellent exhaustive reviews have been published that discuss the role of CREB in addiction (Carlezon et al., 2005; Robison and Nestler, 2011; Nestler, 2014). Hence, we will summarize only a few major findings generated over the past two decades.
One of the first findings was that mice with reduced CREB gene function showed decreased symptoms of morphine withdrawal (Maldonado et al., 1996). Consistently, the infusion of CREB antisense oligonucleotides in the locus coeruleus attenuated the appearance of morphine withdrawal symptoms (Lane-Ladd et al., 1997).
CREB is activated following chronic amphetamine administration in the rat striatum (Cole et al., 1995) and cocaine administration in the rat Nac (Carlezon et al., 1998), and it has been demonstrated that the rewarding effects of drugs of abuse are related to CREB activity within the Nac (Koob et al., 1998). The expression of a dominant-negative mutant CREB in this brain region enhanced the rewarding effects of cocaine and morphine, whereas CREB overexpression decreased the rewarding properties of both drugs (Carlezon et al., 1998; Barrot et al., 2002). Based on this latter evidence, the finding that overexpressing CREB in the Nac leads to a higher rate of cocaine self-administration should be interpreted as an attempt of the animal to load more drug to achieve reward (Larson et al., 2011). On the other hand, both exposure to chronic alcohol or nicotine decreased CREB activity within the Nac, which may reflect the classical hypohedonic state associated with protracted exposure to drugs of abuse (Misra et al., 2001; Pluzarev and Pandey, 2004).
To a large extent, evidence linking CREB function to drug abuse has been generated using mice with genetic modifications causing reduction in its expression or by manipulating the levels of this transcription factor in selected brain areas via viral mediated technologies (Carlezon et al., 1998; Barrot et al., 2002). These molecular approaches have provided advanced knowledge of the role of CREB in drug abuse; however, it is much harder to develop compounds that directly target this transcription factor as a clinical remedy for addiction.
An alternative possibility to modulate CREB is via protein kinase A (PKA)-mediated mechanisms. PKA is an enzyme located upstream of CREB that is responsible for its activation in dopamine receptor-containing neurons in mesolimbic circuitries (Greengard, 2001). A fine-tuning of PKA activity in these areas can be achieved by the regulation of phosphodiesterase (PDE) enzymes.
PDEs are enzymes that hydrolyze adenosine and guanosine cyclic nucleotides (cAMP and cGMP), the second messengers involved in a variety of physiological processes, which are responsible for PKA phosphorylation and whose activity has also been linked to addiction. For example, adaptive changes of the cAMP signaling within the mesocorticolimbic system occur following cocaine exposure and appear to play a role in the progression to dependence (Self et al., 1998; Lu et al., 2003).
Eleven members of the PDE family have been identified, and they differ in their specificity toward cAMP and cGMP, their kinetics, their intracellular localization, their expression in different brain nuclei, and their distinct roles in the regulation of CNS functions (Bender and Beavo, 2006).
Experience has demonstrated that PDEs represent suitable targets for pharmacological manipulation, and there are successful stories of PDE inhibitors being developed in the clinic. For instance, PDE5 selective blockers, such as sildenafil, vardenafil, and tadalafil, are used for erectile dysfunction and pulmonary hypertension (Montani et al., 2009; Chen et al., 2015), whereas compounds that preferentially target PDE4, such as rolipram, have been developed for the treatment of asthma and chronic obstructive pulmonary disease (Spina, 2004). In the CNS, the eleven PDE isoforms are all expressed (Bender and Beavo, 2006), but with respect to drugs of abuse, particularly attractive are PDE4, PDE7 and PDE10 because they are widely expressed in brain areas that are responsible for the regulation of motivated behavior, reward, learning and memory. Evidence linking the activity of these isoforms to drug abuse has started to emerge (Fujishige et al., 1999; Loughney et al., 1999; Soderling et al., 1999) (Miro et al., 2001; Reyes-Irisarri et al., 2005).
PDE4
PDE4 consists of four variants, PDE4 A, B, C, D, that are characterized by a “low Km” and cAMP-specific activity (Bender and Beavo, 2006). All four members are widely expressed in the brain with the highest levels in the basal ganglia, where the most expressed is PDE4B, the anterior cortex, the hippocampus and the hypothalamus, where all four variants have been found (Johansson et al., 2012).
Converging evidence reveals that the manipulation of PDE4 could result in a future of promising approaches for the treatment of opioid, alcohol and psychostimulant abuse. Initial findings showed that the inhibition of this PDE attenuated morphine tolerance and prevented the physical symptoms of morphine withdrawal in mice and rats (Itoh et al., 1998; Nunez et al., 2009). The PDE4 inhibitor, rolipram, has also been shown to be able to block relapse to heroin seeking induced by cues predictive of drug availability and by heroin priming in the rat (Lai et al., 2014). Rolipram also prevented the acquisition of morphine-induced Conditioned Place Preference (CPP) in mice (Thompson et al., 2004). This CPP result was later replicated in another study in which it was demonstrated that the VTA is an important brain site of action for the effect of rolipram on opioid reward.
Substantial evidence also links PDE4 activity to cocaine. For instance, it was shown that rolipram was able to prevent the acquisition of cocaine-induced locomotor sensitization (Janes et al., 2009), whereas the activation of the cAMP cascade by both rolipram and Ro 20-1724, another PDE4 inhibitor, was capable to block the acquistion of cocaine self-administration (Knapp et al., 1999).
Finally, rolipram showed efficacy in reducing alcohol self-administration and intake in the rat (Wen et al., 2012). One potential limitation in the development of PDE4 inhibitors are side effects associated with their use in particular, vomiting and nausea, which are caused by inhibition of this enzyme in the nucleus of the solitary tract. These side effects appear to be predominantly associated with the inhibition of PDE4D (Lipworth, 2005). Hence, space for the development of selective PDE4 A, B, C inhibitors exists. New generations of brain penetrating PDE4 are under development, and their potential use for CNS disorders, including drug dependence, is envisioned.
PDE10A
In in vitro studies, PDE10A is responsible for the hydrolysis of both cAMP and cGMP; less is known in vivo. In the CNS, PDE10A shows the highest signal in the striatum, but substantial levels have also been identified in the hippocampus, thalamus and cerebellum (Fujishige et al., 1999; Loughney et al., 1999; Soderling et al., 1999). Due its large expression in striatal medium spiny neurons, a role for PDE10A in the modulation of basal ganglia function has been proposed (Wilson and Brandon, 2015). Several studies have demonstrated the preclinical efficacy of PDE10A inhibitors in models of Parkinson’s and psychosis (Chappie et al., 2009; Garcia et al., 2014). These findings have promoted aggressive drug development programs on this target, and few compounds are now under clinical development for the treatment of these disorders (Chappie et al., 2009; Garcia et al., 2014). However, an alternative medical indication is addiction because their efficacy in animal models of drug abuse have recently been demonstrated.
For instance, it has been found that MP-10, a highly selective PDE10Ai blocked the acquisition of morphine CPP and facilitated extinction. Conversely, PDE10Ai did not prevent the expression of morphine CPP (Mu et al., 2014). A similar effect was observed with cocaine. Papaverine, which acts as a PDE10Ai, had no significant effect on the expression of cocaine-induced CPP (Liddie et al., 2012). Recently, it was also shown that MP-10 was able to reduce alcohol self-administration in rats with or without a history of exposure to stress. Moreover, MP-10 reduced alcohol operant responding in rats genetically selected for high alcohol preference and in alcohol-dependent rats. Brain microinjection experiments with MP-10 revealed that this effect was mediated by the dorsolateral striatum. Of note, the inhibition of PDE10A also decreased saccharin self-administration, suggesting a rather general involvement of this enzyme in the modulation of reward-related behaviors (Logrip et al., 2014). Consistent with this view, it was also shown that MP-10 impaired mouse performance when trained to achieve highly appetitive stimuli probably by affecting incentive salience attribution as suggested by authors (Piccart et al., 2013).
PDE7
Another PDE member of potential interest in addiction is PDE7. Like PDE4, PDE7 is highly selective for cAMP, especially at low levels of substrate. PDE7 consists of two variants, PDE7A and PDE7B, which are differentially distributed in the brain. PDE7A is highly represented in the substantia nigra, VTA, habenula and hippocampus, whereas the highest expression of PDE7B has been identified in the striatum, Nac and olfactory tubercles (Miro et al., 2001; Reyes-Irisarri et al., 2005). Importantly, approximately 70% of PDE7B-positive neurons in the dorsal striatum, Nac and olfactory tubercles are DA receptor-containing GABAergic neurons, which suggests an important role of this enzyme in the regulation of basal ganglion DA function (Reyes-Irisarri et al., 2005). Recently, the first evidence linking PDE7 to addiction was reported (Ciccocioppo et al., 2014b). In this work, it has been documented that the inhibition of PDE7 reduced nicotine self-administration under fixed- and progressive-ratio contingencies, whereas no effects on FR-1 food self-administration were observed. Enzyme inhibition also attenuated the cue- and stress-induced reinstatement of nicotine seeking. Based on the results of brain microinjection experiments, it was suggested that the effect of PDE7 inhibitors may be linked to their ability to modulate the mesolimbic DA system; thus, it is tempting to speculate that in addition to PDE4 and PDE10, PDE7 may also represent an interesting novel target for drug development in addiction.
Stress-related Neuropeptides
The modulation of appetitive behavior via direct or indirect manipulation of the mesolimbic DA system is the largest explored approach for the development of drug abuse treatments. The drug targets described above offer new possibilities to modulate positive reinforcement via the regulation of mesolimbic activity, which may be responsible, at least in part, for their promising efficacy in drug abuse. An alternative way to reduce the detrimental effects of drugs and to facilitate recovery from addiction is by acting on stress mechanisms and negative reinforcement.
In fact, protracted exposure to drugs leads to maladaptive alterations in physiological stress mechanisms and triggers negative reinforcement. These events represent two main landmarks of the transition from recreational drug use to abuse and dependence (Koob et al., 1998; Schank et al., 2012). Once dependence is established, addicts tend to perseverate in the use of drugs to attenuate negative emotional states (i.e., anxiety, depression, anhedonia) and distress caused by drug abstinence (Koob, 2008). A large, and rather heterogeneous, family of neuropeptides has been shown to play a primary role as stress modulators, and a strong link with addiction has been documented for a few of them (Schank et al., 2012).
Corticotropin-Releasing Factor
A prototypical example of one of the neuropeptides involved in the modulation of the stress mechanism, negative reinforcement and addiction is the corticotropin-releasing factor (CRF), also known as corticotropin-releasing hormone. This stress-related neuropeptide drives both the peripheral and the central stress-response by binding to its cognate G-coupled receptors CRF1 and CRF2 (Bale and Vale, 2004). CRF initiates the neuroendocrine stress response by activating the hypothalamic-pituitary-adrenal (HPA) axis. In addition to this neuroendocrine role, CRF modulates the emotional aspects of the stress response by acting on receptors distributed in extrahypothalamic regions and that are responsible for mediating negative mood and distress (Primus et al., 1997; Sanchez et al., 1999; Van Pett et al., 2000).
As briefly mentioned above, addiction is characterized by three major domains, also conceptualized as a three-stage cycle in which the initial recreational use of drugs is followed by dependence and a withdrawal/negative effect, which is then followed by preoccupation/urge and, therefore, relapse to uncontrolled drug use (Koob and Volkow, 2010). CRF is known to play a role in all three stages, but it is the primary actor in the regulation of the withdrawal/negative effect stage (Zorrilla et al., 2014), which is largely mediated by CRF1 receptors located in the extended amygdala, as has been demonstrated for alcohol (Hansson et al., 2007; Merlo Pich et al., 1995; Zorrilla et al., 2001; Olive et al., 2002; Funk et al., 2006; Roberto et al., 2010), nicotine (George et al., 2007), cocaine (Richter and Weiss, 1999), opioids (Weiss et al., 2001) and cannabinoids (Rodriguez de Fonseca et al., 1997).
Preclinical studies have provided strong evidence supporting the potential usefulness of CRF1 receptor antagonists in treating addiction. CRF antagonists reduced elevated alcohol withdrawal-induced anxiety in dependent rats (Knapp et al., 2004; Breese et al., 2005; Gehlert et al., 2007; Sommer et al., 2008) and the anxiogenic-like response induced by cocaine, nicotine, cannabinoids, opiates, and benzodiazepines (Rodriguez de Fonseca et al., 1997; Basso et al., 1999; Tucci et al., 2003; George et al., 2007; Skelton et al., 2007; Park et al., 2013). CRF1 antagonists were also able to decrease the self-administration of alcohol (Sabino et al., 2006; Chu et al., 2007; Funk et al., 2007; Gehlert et al., 2007; Gilpin et al., 2008; Richardson et al., 2008), cocaine (Specio et al., 2008), nicotine (George et al., 2007), and heroin (Greenwell et al., 2009) in rats and mice. Most importantly, CRF1 antagonists have been shown to possess a marked ability to prevent relapse to drug seeking elicited by stress and to prolong drug abstinence in laboratory animals. Moreover, human genetic studies have suggested that CRF1R gene polymorphisms are associated with binge drinking and excessive drinking in humans (Treutlein et al., 2006). Together, these findings support the hypothesis that genetic variation at the CRF1R locus may represent an important element for the evolution of alcohol dependence, and according to the promising perspective highlighted by preclinical studies, there is hope that CRF1R antagonists may be efficacious in treating drug addiction. To date, none of the clinically tested CRF1R antagonists have passed to phase III for lack of efficacy, adverse side effects or because Phase II clinical trials are stil ongoing (Zorrilla et al., 2013; Kwako et al., 2015); however, a CRF1 antagonist, GSK561679, is now being tested in phase II (ClinicalTrials.gov Identifier: NCT01187511). It will be exciting to know the results of this ongoing investigation.
N/OFQ-NOP
A large body of evidence supports the possibility that Nociceptin/orphanin FQ receptor (NOP) agonists represent a promising approach to treat addiction, especially alcoholism.
Nociceptin/orphanin FQ (N/OFQ), the endogenous ligand of NOP receptors, is a 17-amino acid neuropeptide that is structurally related to the opioid peptide dynorphin A (Meunier et al., 1995; Reinscheid et al., 1995; Nothacker et al., 1996). Despite its structural homology with opioid peptides, N/OFQ does not bind to mu, delta and kappa opioid receptors (MOP, DOP, KOP respectively), nor do opioid peptides activate the NOP receptor (Reinscheid et al., 1996). Functional studies have demonstrated that N/OFQ possesses anti-opioid and anti-CRF properties because the activation of NOP attenuates the rewarding effects of morphine and prevents the anorectic, anxiogenic and stress-like effects of CRF (Ciccocioppo et al., 2000; Ciccocioppo et al., 2001). Consistent with the anti-opioid nature of N/OFQ, it has been shown that the activation of NOP receptors blunts the reinforcing and motivational effects of alcohol across a range of behavioral measures, including alcohol intake (Ciccocioppo et al., 1999), CPP (Kuzmin et al., 2003) and relapse to alcohol seeking triggered by alcohol-associated cues (Ciccocioppo et al., 2004). In agreement with its anti-CRF properties it has also been shown that N/OFQ administration prevents the foot-shock stress-induced reinstatement of alcohol seeking in the rat (Martin-Fardon et al., 2000). This property of N/OFQ to attenuate both forms of relapse is particularly noteworthy because it has been previously shown that blockade of the MOP opioid receptor by naltrexone blocks alcohol self-administration and cues but not stress-induced relapse (Le et al., 1999; Le et al., 2000; Liu and Weiss, 2002), whereas CRF1 antagonism prevents relapse associated with stress but is unable to attenuate alcohol seeking elicited by conditioning factors (Le et al., 1999; Le et al., 2000; Liu and Weiss, 2002).
Recent data have shown that the inhibition of drinking by a newly developed NOP agonist, namely MT1677, is highly pronounced following chronic administration, whereas drug effects appeared to be absent or very low following an acute injection. These data have been obtained in genetically selected alcohol-preferring Marchigian Sardiniam (msP) rats, an animal model of pathological drinking in which most of the published studies supporting a role of the NOP system in alcohol abuse have been carried out (Ciccocioppo et al., 1999; Ciccocioppo et al., 2004; Economidou et al., 2006; Economidou et al., 2011; Ciccocioppo et al., 2014a). In contrast with these findings, it was shown that NOP agonists did not attenuate drinking in heterogeneous Wistar rats, from which msP rats originate (Ciccocioppo et al., 2006; Economidou et al., 2008). On the other hand, if heterogeneous Wistar rats are subjected to a history of alcohol intoxication, NOP agonists appear to gain efficacy (de Guglielmo et al., 2015a). Studies using in situ hybridization showed that compared to Wistar rats, msP have an upregulation of the N/OFQ system, with major differences occurring in the CeA, BNST, VTA and some cortical structures (Economidou et al., 2008).
Noteworthy, enhanced NOP and N/OFQ levels have been documented also in the CeA and BNST of heterogeneous Wistar rats following a history of alcohol intoxication (Aujla et al., 2013). These findings prompt an intriguing hypothesis that in apparent contrast with the current view of a role of NOP agonists in addiction, suggests that enhanced NOP function may represent a vulnerable factor for the development of alcohol abuse and that the documented efficacy of NOP agonists may be related to their ability to desensitize the system. This hypothesis is supported by the following evidence: 1) NOP receptors are subjected to a very rapid desensitization following exposure to agonists (Spampinato et al., 2007) and 2) NOP agonists are efficacious after protracted administration and under circumstances in which the receptor system is hyperfunctioning (Ciccocioppo et al., 2014a). If this hypothesis is proven to be true, we should expect that not only NOP agonists but also NOP antagonists to reduce alcohol drinking. In addition, different from what is observed with agonists, the antagonists should also be efficacious following acute administration.
Recently, 11C-NOP-1A, a new radioligand for the nociceptin/orphanin FQ peptide (NOP) receptor with high affinity (Ki, 0.15 nM) and adequate lipophilicity (measured logD, 3.4) for PET brain imaging, has been developed (Pike et al., 2011). Using this ligand, it will be possible to obtain some information on whether genetic predisposition to alcoholism or protracted exposure to alcohol is associated with the upregulation of NOP receptor levels in rodents and humans. This study will help further clarify the potential of NOP as a treatment target for alcoholism and possibly other forms of addiction, opening new vistas for drug development programs on this peptidergic system.
Non-peptide, orally available and brain penetrant NOP receptor agonists and antagonists have been developed, and seem to have acceptable safety and tolerability. Some of these compounds are in relatively advanced stages of development, and a hypothesis concerning their efficacy in alcohol addiction can be tested in clinical trials (Witkin et al., 2014).
Compared to alcohol, less is known about the role of the N/OFQ system in the abuse of other drugs. A few studies have shown that N/OFQ blocked CCP elicited by morphine (Murphy et al., 1999; Ciccocioppo et al., 2000), whereas microdialysis data demonstrated that N/OFQ reduced morphine-induced dopamine release in the Nac (Di Giannuario and Pieretti, 2000). On the other hand, when N/OFQ was tested on operant heroin self-administration, no effects of the peptide were reported (Walker et al., 1998). Of note, CPP is highly influenced by mesolimbic DA transmission DA whereas heroin-self-administration is largely independent from DA. This could explain why NOP agonists, even though able to blunt Nac DA levels and morphine CPP does not affect operant responsing for heroin (Gerrits and Van Ree, 1996; Sanchis-Segura and Spanagel, 2006; Tzschentke, 2007) NOP activation was shown to be effective also in preventing cocaine- and amphetamine-induced CPP (Kotlinska et al., 2002; Zhao et al., 2003); moreover, mice with constitutive deletion of NOP receptors were more sensitive to the effects of cocaine in a CPP paradigm (Marquez et al., 2008; Sakoori and Murphy, 2008).
Hypocretins/Orexins
Hypocretin-1/Orexin A and Hypocretin-2/Orexin B (Hcrt-1/OxA; Hcrt-2/OxB) are neuropeptides produced in the lateral, dorsomedial and perifornical hypothalamus (de Lecea et al., 1998; Sakurai et al., 1998) by neurons projecting throughout the brain (Peyron et al., 1998). Both Hcrt-1/OxA and Hcrt-2/OxB bind to their cognate receptors orexin-1 (OX1) and orexin-2 (OX2), which are extensively expressed in the central nervous system due to the wide projections of Hcrt/Ox neurons (Trivedi et al., 1998). OX1 and OX2 seem to have differential roles with respect to drug addiction. OX1 has been implicated in the modulation of morphine, cocaine, nicotine, and alcohol seeking (Boutrel et al., 2005; Harris et al., 2005; Borgland et al., 2006; Lawrence et al., 2006; Pasumarthi et al., 2006; Harris et al., 2007; Dayas et al., 2008; Hollander et al., 2008; Richards et al., 2008; Cannella et al., 2009a; Moorman and Aston-Jones, 2009; Kallupi et al., 2010; Plaza-Zabala et al., 2010; Jupp et al., 2011; Plaza-Zabala et al., 2012; Ubaldi et al., 2015), whereas OX2 seems to have a less important role. Yet, it was recently documented that OX2 antagonism selectively reduced alcohol self-administration in respect to sucrose while showing no effect on cue-induced reinstatement (Brown et al., 2013).
The modulation of the drug-seeking response mediated by OX1 appeared to be mainly linked to the modulation of VTA-mediated mechanisms (Mahler et al., 2013). However, besides the VTA, other areas reach of Hcrt-1/OxA terminals, such as the prelimbic and orbitofrontal cortices, have been implicated in cue-induced alcohol seeking (Jupp et al., 2011). An OX1 antagonist also blocked stress-induced reinstatement (Winsky-Sommerer et al., 2004; Boutrel et al., 2005; Richards et al., 2008), but this effect was independent of the VTA (Wang et al., 2009). A possible target for Hcrt-1/OxA in stress-induced reinstatement may be the extended amygdala, which is composed of the central amygdala (CeA) and the bed nucleus of the stria terminalis (BNST), where Hcrt-1/OxA neurons send afferent projections (Peyron et al., 1998; Schmitt et al., 2012). In addition, Hcrt-1/Ox-A may also modulate stress-induced reinstatement via the paraventricular hypothalamic nucleus (PVN) as it is highly innervated by orexin fibers. Indeed, the Hcrt-1/Ox-A-induced activation of approximately 96% and 45% of CRF-containing neurons in the PVN and the CeA, respectively (Sakamoto et al., 2004), which increases CRF and vasopressin expression in the PVN and activates the HPA axis (Al-Barazanji et al., 2001).
Several orexin receptor antagonists are under development; they can be divided into three classes: 1) dual orexin receptor antagonist (DORA); 2) OX1 selective receptor antagonist (1-SORA) and 3) OX2 selective receptor antagonist (2-SORA). The most promising approach for addiction treatment is with 1-SORA because a wealth of preclinical data has shown that the inhibition of Ox1 attenuates the motivation for most drugs of abuse while being devoid of side effects, such as sleepiness and alteration of vigilance, two effects typically mediated by Ox2; however, nonselective antagonists may also have some space in addiction medicine. For example, they could have potential in cocaine abuse because the antagonism of Ox1 appears to play a primary role in attenuating the motivation for this addictive agent, whereas blockade of Ox2 may lead to some sedative effects that in the case of psychostimulants abuse, are tolerable if not advantageous. Instead, it is less likely that DORA could be used for the treatment of addictive drugs, such as alcohol or opioids. In fact, in this case, blockade of Ox2 may enhance the risk of excessive inhibition of CNS function by potentiating the depressant properties of the addictive agents. Few orexin antagonists have been tested in the clinic, and recently, the US FDA approved suvorexant, the first dual antagonist registered for the treatment of primary insomnia (Boss and Roch, 2015). Another DORA, almorexant, was also tested in the clinic, but its use was stopped due to tolerability issues (Boss and Roch, 2015). Selective Ox2 antagonists are also entering into clinical investigation for sleep disorders, and for some of them, phase I studies have already been successfully completed (Boss and Roch, 2015). On the other hand, less advanced is the development of selective Ox1 blockers; However, a number of molecules are rapidly making their way through preclinical stages, and soon, some of them could be available for their first clinical trials. Based on preclinical evidence, addiction is one of the major disease areas toward which these compounds could be oriented.
Neuropeptide S
The Neuropeptide S (NPS) is a 20 amino-acid peptide identified as the endogenous ligand for the deorphanized G-protein-coupled receptor 154 (GPCR 154), which is currently named the NPS receptor (NPSR). NPS is produced exclusively in three brainstem regions, the peri-locus coeruleus (LC) area, the principal sensory trigeminal nucleus, and the lateral parabrachial nucleus. Conversely, the NPSR is widely distributed throughout the brain. The most peculiar feature of NPS is its paradoxical physio-pharmacological profile as it is a pro-stress neuropeptide endowed with anxiolytic-like properties (Xu et al., 2004; Xu et al., 2007; Cannella et al., 2013).
The central administration of NPS reduced alcohol drinking and self-administration selectively in alcohol-preferring P rats with respect to non-preferring NP controls (Badia-Elder et al., 2008; Cannella et al., 2009a; Cannella et al., 2009b). Additionally, an effect that was associated with the anxiolytic effects of NPS in excessive alcohol drinking in P rats is associated to alcohol's ability to relieve them from anxiety (Ciccocioppo et al., 2006; Badia-Elder et al., 2008). Recently, it has been shown that the NPSR antagonist can decrease alcohol self-administration in non-preferring rats (Thorsell et al., 2013), which suggest that this system may have differential effects in alcohol-preferring subjects with respect to non-preferring ones. NPSR antagonists have also been shown to blunt cocaine self-administration (Schmoutz et al., 2012) and some consequences of alcohol and morphine intoxication and withdrawal (Ruggeri et al., 2010; Ghazal et al., 2013). For instance, NPSR mRNA was increased in post-dependent rats, in which the anxiolytic-like effects of NPS were more pronounced (Ruggeri et al., 2010; Ghazal et al., 2013). This finding may suggest that increased NPSR expression may be a neuroadaptation aimed to cope with withdrawal syndrome. Several studies have linked the pro-arousal and pro-stress effects of NPS with the reinstatement of drug seeking. It has been demonstrated that NPS, given ICV or into the lateral hypothalamus (LH), potentiated the reinstatement of ethanol seeking induced by environmental stimuli previously paired with ethanol and cocaine availability. The effect of NPS was specific and was not observed following re-exposure to cues predictive of non-rewarding solutions (Cannella et al., 2009a; Kallupi et al., 2010). The permissive role of NPS on ethanol and cocaine seeking was mediated by the Hcrt-1/Ox-A system because peripheral administration of the OX1 receptor antagonist SB334867 completely blocked it (Cannella et al., 2009a; Kallupi et al., 2010). Further investigation demonstrated that NPS activates Hcrt-1/Ox-A neurons that project from the LH to the BNST and PVN, two areas classically involved in the modulation of the stress response; selective inhibition of Ox1 receptors in these two areas completely abolished drug-seeking elicited by NPS (Ubaldi et al., 2015). Together, these findings suggest that the enhancement of the seeking response by NPS is mediated by pro-stress and pro-arousal mechanisms linked to the activation of the Ox1 system (Smith et al., 2006). NPSR antagonists represent a possible new way to develop relapse prevention treatment that may represent an alternative to Hcrt-1/Ox-A antagonists. Research linking the NPS system to addiction in humans is still at its infancy; however, interesting findings are starting to emerge. For instance, it is now known that in humans, NPSR is present in two isoforms deriving from a A>T single nucleotide polymorphism, resulting in an Asn107Ile exchange, which confers ten-fold higher potency to the NPSR107Ile isoform (Reinscheid et al., 2005). This isoform of NPSR is associated with impulsivity, stress-sensitivity and alcohol use disorder (Laas et al., 2014; Laas et al., 2015), which is in line with preclinical data indicating that overfunction of the system may be linked to enhanced relapse propensity (Cannella et al., 2009a; Paneda et al., 2009; Kallupi et al., 2010).
NK1 Receptor
The neurokinin1 receptor (NK1R) is a member of the tachykinin receptor family that preferentially binds the tachykinin Substance P (Pennefather et al., 2004). It is well known that the activation of NK1 by Substance P (SP) regulates the stress response and induces anxiety-like behavior and that NK1R antagonists have anxiolytic-like properties (Santarelli et al., 2001; Ebner and Singewald, 2006; Ebner et al., 2008). The PVN receives substance P innervation, and NK1R stimulation activates the HPA axis, enhancing corticosterone release and the expression of CRF1R (Kawano and Masuko, 1992; Hamke et al., 2006; Mello et al., 2007; Womack and Barrett-Jolley, 2007; Womack et al., 2007; Ebner et al., 2008). On the other hand, it was also reported that NK antagonist administration can increase adrenocorticotropic hormone (ACTH) release and CRF expression (Jessop et al., 2000) and that SP can suppress ACTH release (Jones et al., 1978)r. These effects of NK1R antagonists, however, occur in unstressed animals, and therefore, they suggest a tonic suppression of HPA axis activity by SP/NK1R. Thus, it is envisioned that under resting conditions, SP tonically inhibits HPA axis activity, but in stressful conditions, SP activates HPA. In humans, it seems more likely that the inhibitory effect of an NK1R antagonist on the HPA axis predominates as basal cortisol levels are not influenced but the stress-induced release of both ACTH and cortisol is blocked (George et al., 2008). Other than the stress response, NK1R also mediates catecholamine signaling in the mesolimbic, mesocortical, and nigrostriatal pathways. NK1R is expressed throughout the striatum, in dendrites of cholinergic interneurons (Pickel et al., 2000; Commons and Serock, 2009), whereas D1 medium spiny neurons in the Nac express SP and feed back to the substantia nigra (Le Moine and Bloch, 1995; Whitty et al., 1995; Futami et al., 1998). The stimulation of NK1R in the substantia nigra or VTA induces dopaminergic firing (Barnes et al., 1990; West and Michael, 1991) and conditioned place preference (Boix et al., 1995; Nikolaus et al., 1999). The role of SP/NK1R in addiction has been tested on opioid, cocaine and alcohol. NK1R knockout mice did not develop CPP for morphine and displayed reduced psychomotor sensitization and morphine self-administration (Murtra et al., 2000; Ripley et al., 2002), Moreover, the selective inactivation of NK1R in the amygdala attenuated morphine consumption (Gadd et al., 2003). These findings suggest the potential of NK1R antagonism on opioid addiction, but initial human studies point to the opposite direction as the NK1R antagonist aprepitant potentiated the subjective and physiologic responses in opioid abusers (Walsh et al., 2013). NK1R have also been tested against psychostimulants, but the results are rather inconclusive. In fact, although they suppress cocaine-induced locomotion (Kraft et al., 2001), receptor blockade neither prevents cocaine self-administration nor attenuates cocaine-induced CPP (Murtra et al., 2000; Ripley et al., 2002; Gadd et al., 2003). Controversial effects have also been reported in the case of reinstatement experiments showing that NK1R agonism facilitated the reinstatement of cocaine seeking, but receptor antagonists did not block cocaine-priming induced reinstatement (Placenza et al., 2004; Placenza et al., 2005). The data obtained with alcohol are more promising.
For instance, it was shown that NK1R knockout mice did not develop alcohol CPP, consumed less alcohol in a two-bottle choice paradigm and did not escalate alcohol consumption following repeated cycles of deprivation (George et al., 2008; Thorsell et al., 2010). Moreover, NK1R antagonism reduced the stress-induced reinstatement of alcohol seeking (Schank et al., 2011). Yet, the operant alcohol self-administration and cue-induced reinstatement of alcohol seeking were not affected by treatments (Schank et al., 2011) (Steensland et al., 2010).
Initial human data are in line with preclinical findings as antagonists decreased alcohol craving in alcohol-dependent subjects in unprovoked conditions, under social stressors and upon exposure to alcohol-associated cues. In addition, an NK1R antagonist also decreased cortisol release induced by stress and cues (George et al., 2008).
Conclusion and Future Directions
Drug addiction is a serious disabling condition that has a dramatic impact on our societies and public health systems. The processes involved in the development of addiction are complex and involve interactions of several neurotransmitter systems, cell signaling and transcription mechanisms. Following chronic exposure to drugs of abuse, the brain is subjected to profound functional alterations to which genetic and environmental determinants also contribute. Despite the severity of the disorder and the dramatic impact it has on our lives, only few medications have been approved for addiction treatment. At present, only a minority of patients can benefit from the use of these medications, whereas the majority of addicts still remain poorly treated. To improve the impact of pharmacotherapy in addiction, it is critical to develop more efficacious and safer drugs. Over the past decade, a number of new mechanisms involved in the regulation of drug abuse and in the transition to addiction have been identified (Table 1), thus allowing the possibility to develop new pharmacological strategies. Here, we discussed a few of these potential new approaches that offer hope to obtaining useful medications in the near future. This review is not exhaustive as several other drug targets are under exploration; therefore, there is hope that many more therapeutic options will be available in the clinic in the near future.
Table 1.
List of novel pharmacological targets explored for development of innovative treatments in addiction.
| Target | Pharmacological Tool | Drug of Abuse |
Experimental Procedure |
Key Finding |
Reference |
|---|---|---|---|---|---|
| PPARγ | Pioglitazone, Rosiglitazone |
Alcohol | SA, stress-induced reinstatement |
reduce | (Stopponi et al., 2011) |
| Pioglitazone | Heroin | SA | reduce | (de Guglielmo et al., 2015) | |
| 15-deoxy-delta(12,14)- prostaglandin J(2), Ciglitazone |
Nicotine | PPARγ gene expression | increase | (Amoruso et al., 2007) | |
| Pioglitazone | Morphine | tolerance | reduce | (de Guglielmo et al., 2015) | |
| PPARα | Fenofibrate, Tesaglitazar, Bezafibrate |
Alcohol | SA | reduce | (Blednov et al., 2015) |
| Clofibrate, WY14643, methyl oleoylethanolamide |
Nicotine | SA, cue-, priming- induced reinstatement |
reduce | (Panlilio et al., 2012) (Mascia et al., 2011) |
|
| PDE4 | Rolipram | Alcohol | SA | reduce | (Wen et al., 2012) |
| Rolipram | Morphine | withdrawal, tolerance, CPP |
reduce | (Itoh et al., 1998) (Nunez et al., 2009) (Thompson et al., 2004) |
|
| Rolipram | Cocaine | CPP, locomotor sensitisation, SA |
reduce | (Thompson et al., 2004) (Janes et al., 2009) (Knapp et al., 1999) |
|
| PDE10A | MP-10 | Alcohol | SA | reduce | (Logrip et al., 2014) |
| MP-10 | Morphine | CPP | reduce | (Mu et al., 2014) | |
| Papaverine | Cocaine | CPP | reduce | (Liddie et al., 2012) | |
| CREB | KO mice, antisense oligonucleotide |
Morphine | Withdrawal | reduce | (Maldonado et al., 1996) (Lane-Ladd et al., 1997) |
| Over-expression | Cocaine | SA | increase | (Carlezon et al., 1998) (Barrot et al., 2002) (Larson et al., 2011) (Bilbao et al., 2014) |
|
| Dominant-negative CREB variant |
CPP Priming-induced reinstatement |
||||
| CRF1R | CRA1000 MTIP MPZP LWH-63 Antalarmin KO mice D-Phe-CRF(12-41) |
Alcohol | withdrawal, SA | reduce | (Knapp et al., 2004) (Gehlert et al., 2007) (Sommer et al., 2008) (Sabino et al., 2006) (Chu et al., 2007) (Funk et al., 2007) (Gilpin et al., 2008) (Richardson et al., 2008) |
| D-phe CRF(12-41) Antalarmin MPZP |
Cocaine | Withdrawal, SA | reduce | (Basso et al., 1999) (Specio et al., 2008) |
|
| alpha-helical CRF(9-41) MPZP |
Nicotine | Withdrawal, SA | reduce | (Tucci et al., 2003) (George et al., 2007) |
|
| MPZP MJL-1-109-2 R121919 |
Heroin | Withdrawal, SA | reduce | (Park et al., 2013) (Greenwell et al., 2009) |
|
| NOP | N/OFQ Ro 64-6198 |
Alcohol | CPP, SA, withdrawal |
reduce | (Ciccocioppo et al., 1999) (Kuzmin et al., 2003) (Ciccocioppo et al., 2004) (Martin-Fardon et al., 2000) (Economidou et al., 2011) |
| N/OFQ | Morphine | CPP | reduce | (Ciccocioppo et al., 2000) (Murphy et al., 1999) |
|
| N/OFQ | Cocaine Amphetamine |
CPP | reduce | (Kotlinska et al., 2002) (Zhao et al., 2003) |
|
| OX1 | SB-334867 | Alcohol | cue-, stress-induced reinstatement |
reduce | (Lawrence et al., 2006) (Richards et al., 2008) |
| SB-334867 | Cocaine | cue-, stress-induced reinstatement |
reduce | (Smith et al., 2009) (Boutrel et al., 2005) |
|
| SB-334867 | Morphine | cue-induced reinstatement |
reduce | (Harris et al., 2005) | |
| NPSR | NPS | Alcohol | SA | no effect | (Cannella et al., 2009) (Ruggeri et al., 2010) |
| cue-induced reinstatement |
increase | ||||
| withdrawal | reduce | ||||
| NPS | Morphine | CPP | reduce | (Li et al., 2009) | |
| NPS SHA 68 NPSR-QA1 |
Cocaine | SA, CPP | no effect | (Kallupi et al., 2010) (Kallupi et al., 2013) (Cannella et al., 2013) |
|
| SHA 68 NPSR-QA1 [D-Cys(tBu)5 ]NPS |
cue-induced reinstatement |
reduce | |||
| NK1R | KO mice, L822429 | Alcohol | SA, CPP, stress-induced reinstatement |
reduce | (George et al., 2008) (Thorsell et al., 2010) (Schank et al., 2011) |
| KO mice | Morphine | SA, CPP | reduce | (Murtra et al., 2000) (Ripley et al., 2002) |
|
| KO mice | Cocaine | SA, CPP, locomotor sensitisation |
no effect | (Ripley et al., 2002) |
Abbreviations: Peroxisome proliferating activator receptors gamma (PPARγ), peroxisome proliferating activator receptors alpha (PPARα), cAMP response element-binding protein (CREB), phosphodiesterase 4 (PDE4), phosphodiesterase 10A (PDE10A), corticotropin-releasing factor receptor 1 (CRF1R), nociceptin opioid receptor (NOP), hypocretin-1/orexin A receptor (Ox1), neuropeptide S receptor (NPSR), neurokinin 1 receptor (NK1R), operant self-administration (SA), conditioned place preference (CPP).
Acknowledgements
This work was supported by the National Institutes Alcohol Abuse and Alcoholism (grants: RO1 AA017447 and RO1 AA014351).
References
- Al-Barazanji KA, Wilson S, Baker J, Jessop DS, Harbuz MS. Central orexin-A activates hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats. J. Neuroendocrinol. 2001;13:421–424. doi: 10.1046/j.1365-2826.2001.00655.x. [DOI] [PubMed] [Google Scholar]
- Amoruso A, Bardelli C, Gunella G, Fresu LG, Ferrero V, Brunelleschi S. Quantification of PPAR-gamma protein in monocyte/macrophages from healthy smokers and non-smokers: a possible direct effect of nicotine. Life Sci. 2007;81:906–915. doi: 10.1016/j.lfs.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J. Biol. Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- Aujla H, Cannarsa R, Romualdi P, Ciccocioppo R, Martin-Fardon R, Weiss F. Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict Biol. 2013;18:467–479. doi: 10.1111/j.1369-1600.2012.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia-Elder NE, Henderson AN, Bertholomey ML, Dodge NC, Stewart RB. The effects of neuropeptide S on ethanol drinking and other related behaviors in alcohol-preferring and -nonpreferring rats. Alcohol. Clin. Exp. Res. 2008;32:1380–1387. doi: 10.1111/j.1530-0277.2008.00713.x. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Costall B, Cox AJ, Domeney AM, Kelly ME, Naylor RJ. Neurochemical consequences following injection of the substance P analogue, DiMe-C7, into the rat ventral tegmental area. Pharmacol. Biochem. Behav. 1990;37:839–841. doi: 10.1016/0091-3057(90)90572-y. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the "anxiogenic-like" effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol. Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu. Rev. Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Bilbao A, Rieker C, Cannella N, Parlato R, Golda S, Piechota M, Korostynski M, Engblom D, Przewlocki R, Schutz G, Spanagel R, Parkitna JR. CREB activity in dopamine D1 receptor expressing neurons regulates cocaine-induced behavioral effects. Frontiers in behavioral neuroscience. 2014;8:212. doi: 10.3389/fnbeh.2014.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbao A, Serrano A, Cippitelli A, Pavon FJ, Giuffrida A, Suarez J, Garcia-Marchena N, Baixeras E, Gomez de Heras R, Orio L, Alen F, Ciccocioppo R, Cravatt BF, Parsons LH, Piomelli D, Rodriguez de Fonseca F. Role of the satiety factor oleoylethanolamide in alcoholism. Addict Biol. 2015 doi: 10.1111/adb.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Ferguson LB, Schoenhard GL, Goate AM, Edenberg HJ, Wetherill L, Hesselbrock V, Foroud T, Harris RA. Peroxisome proliferator-activated receptors alpha and gamma are linked with alcohol consumption in mice and withdrawal and dependence in humans. Alcohol. Clin. Exp. Res. 2015;39:136–145. doi: 10.1111/acer.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boix F, Sandor P, Nogueira PJ, Huston JP, Schwarting RK. Relationship between dopamine release in nucleus accumbens and place preference induced by substance P injected into the nucleus basalis magnocellularis region. Neuroscience. 1995;64:1045–1055. doi: 10.1016/0306-4522(94)00425-5. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boss C, Roch C. Recent trends in orexin research-2010 to 2015. Bioorg. Med. Chem. Lett. 2015 doi: 10.1016/j.bmcl.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Khoo SY, Lawrence AJ. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol. 2013;16:2067–2079. doi: 10.1017/S1461145713000333. [DOI] [PubMed] [Google Scholar]
- Cannella N, Kallupi M, Ruggeri B, Ciccocioppo R, Ubaldi M. The role of the neuropeptide S system in addiction: focus on its interaction with the CRF and hypocretin/orexin neurotransmission. Prog. Neurobiol. 2013;100:48–59. doi: 10.1016/j.pneurobio.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Cannella N, Ruggeri B, Ubaldi M, Braconi S, Kallupi M, Massi M, Ciccocioppo R. Neuropeptide S differently modulate ethanol self-administration and cue-induced reinstatement of ethanol seeking in msP and wistar rats. Behavioral Pharmacology Special Issue 1, S31P30; 13th Biennal Meeting of the European Behavioral Pharmacology Society; Rome, Italy. 2009a. [Google Scholar]
- Cannella N, Economidou D, Kallupi M, Stopponi S, Heilig M, Massi M, Ciccocioppo R. Persistent increase of alcohol-seeking evoked by neuropeptide S: an effect mediated by the hypothalamic hypocretin system. Neuropsychopharmacology. 2009b;34:2125–2134. doi: 10.1038/npp.2009.37. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Chappie T, Humphrey J, Menniti F, Schmidt C. PDE10A inhibitors: an assessment of the current CNS drug discovery landscape. Current opinion in drug discovery & development. 2009;12:458–467. [PubMed] [Google Scholar]
- Chen L, Staubli SE, Schneider MP, Kessels AG, Ivic S, Bachmann LM, Kessler TM. Phosphodiesterase 5 Inhibitors for the Treatment of Erectile Dysfunction: A Trade-off Network Meta-analysis. Eur. Urol. 2015 doi: 10.1016/j.eururo.2015.03.031. [DOI] [PubMed] [Google Scholar]
- Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol. Biochem. Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F, Massi M. Nociceptin/orphanin FQ inhibits stress- and CRF-induced anorexia in rats. Neuroreport. 2001;12:1145–1149. doi: 10.1097/00001756-200105080-00019. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 1999;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur. J. Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Li H, de Guglielmo G, Ubaldi M, Soverchia L, Caffino L, Fumagalli F, Demopulos G, Gaitanaris G. SY40-2phosphodiesterase type 7: a novel target for smoking cessation -preclinical evidence. Alcohol Alcohol. 2014a:49. [Google Scholar]
- Ciccocioppo R, Stopponi S, Economidou D, Kuriyama M, Kinoshita H, Heilig M, Roberto M, Weiss F, Teshima K. Chronic treatment with novel brain-penetrating selective NOP receptor agonist MT-7716 reduces alcohol drinking and seeking in the rat. Neuropsychopharmacology. 2014b;39:2601–2610. doi: 10.1038/npp.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Serock MR. Coincidence of neurokinin 1 receptor with the vesicular glutamate transporter 3 (VGLUT3) in the rat forebrain. Neurosci. Lett. 2009;464:188–192. doi: 10.1016/j.neulet.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol. Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Martin-Fardon R, Teshima K, Ciccocioppo R, Weiss F. MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addict Biol. 2015a;20:643–651. doi: 10.1111/adb.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Melis M, De Luca MA, Kallupi M, Li HW, Niswender K, Giordano A, Senzacqua M, Somaini L, Cippitelli A, Gaitanaris G, Demopulos G, Damadzic R, Tapocik J, Heilig M, Ciccocioppo R. PPARgamma activation attenuates opioid consumption and modulates mesolimbic dopamine transmission. Neuropsychopharmacology. 2015b;40:927–937. doi: 10.1038/npp.2014.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S. Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides. 2000;21:1125–1130. doi: 10.1016/s0196-9781(00)00250-3. [DOI] [PubMed] [Google Scholar]
- Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31:251–272. doi: 10.1007/s00726-006-0335-9. [DOI] [PubMed] [Google Scholar]
- Ebner K, Muigg P, Singewald G, Singewald N. Substance P in stress and anxiety: NK-1 receptor antagonism interacts with key brain areas of the stress circuitry. Ann. N. Y. Acad. Sci. 2008;1144:61–73. doi: 10.1196/annals.1418.018. [DOI] [PubMed] [Google Scholar]
- Economidou D, Fedeli A, Fardon RM, Weiss F, Massi M, Ciccocioppo R. Effect of novel nociceptin/orphanin FQ-NOP receptor ligands on ethanol drinking in alcohol-preferring msP rats. Peptides. 2006;27:3299–3306. doi: 10.1016/j.peptides.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, Martin-Fardon R, Weiss F, Massi M, Ciccocioppo R. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol. Clin. Exp. Res. 2011;35:747–755. doi: 10.1111/j.1530-0277.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol. Psychiatry. 2008;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishige K, Kotera J, Michibata H, Yuasa K, Takebayashi S, Okumura K, Omori K. Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A) J. Biol. Chem. 1999;274:18438–18445. doi: 10.1074/jbc.274.26.18438. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J. Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol. Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futami T, Hatanaka Y, Matsushita K, Furuya S. Expression of substance P receptor in the substantia nigra. Brain Res. Mol. Brain Res. 1998;54:183–198. doi: 10.1016/s0169-328x(97)00307-0. [DOI] [PubMed] [Google Scholar]
- Gadd CA, Murtra P, De Felipe C, Hunt SP. Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. J. Neurosci. 2003;23:8271–8280. doi: 10.1523/JNEUROSCI.23-23-08271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AM, Redondo M, Martinez A, Gil C. Phosphodiesterase 10 inhibitors: new disease modifying drugs for Parkinson's disease? Curr. Med. Chem. 2014;21:1171–1187. doi: 10.2174/0929867321666131228221749. [DOI] [PubMed] [Google Scholar]
- Gardner EL. What we have learned about addiction from animal models of drug self-administration. Am. J. Addict. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J. Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits MA, Van Ree JM. Effect of nucleus accumbens dopamine depletion on motivational aspects involved in initiation of cocaine and heroin self-administration in rats. Brain Res. 1996;713:114–124. doi: 10.1016/0006-8993(95)01491-8. [DOI] [PubMed] [Google Scholar]
- Ghazal P, Ciccocioppo R, Ubaldi M. Morphine dependence is associated with changes in neuropeptide S receptor expression and function in rat brain. Peptides. 2013;46:6–12. doi: 10.1016/j.peptides.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol. Clin. Exp. Res. 2008;32:1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofflot F, Chartoire N, Vasseur L, Heikkinen S, Dembele D, Le Merrer J, Auwerx J. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell. 2007;131:405–418. doi: 10.1016/j.cell.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol. 2009;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamke M, Herpfer I, Lieb K, Wandelt C, Fiebich BL. Substance P induces expression of the corticotropin-releasing factor receptor 1 by activation of the neurokinin-1 receptor. Brain Res. 2006;1102:135–144. doi: 10.1016/j.brainres.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav. Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nature reviews. Neuroscience. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Itoh A, Noda Y, Mamiya T, Hasegawa T, Nabeshima T. A therapeutic strategy to prevent morphine dependence and tolerance by coadministration of cAMP-related reagents with morphine. Methods Find. Exp. Clin. Pharmacol. 1998;20:619–625. doi: 10.1358/mf.1998.20.7.485728. [DOI] [PubMed] [Google Scholar]
- Janes AC, Kantak KM, Cherry JA. The involvement of type IV phosphodiesterases in cocaine-induced sensitization and subsequent pERK expression in the mouse nucleus accumbens. Psychopharmacology (Berl) 2009;206:177–185. doi: 10.1007/s00213-009-1594-4. [DOI] [PubMed] [Google Scholar]
- Jessop DS, Renshaw D, Larsen PJ, Chowdrey HS, Harbuz MS. Substance P is involved in terminating the hypothalamo-pituitary-adrenal axis response to acute stress through centrally located neurokinin-1 receptors. Stress. 2000;3:209–220. doi: 10.3109/10253890009001125. [DOI] [PubMed] [Google Scholar]
- Johansson EM, Reyes-Irisarri E, Mengod G. Comparison of cAMP-specific phosphodiesterase mRNAs distribution in mouse and rat brain. Neurosci. Lett. 2012;525:1–6. doi: 10.1016/j.neulet.2012.07.050. [DOI] [PubMed] [Google Scholar]
- Jones MT, Gillham B, Holmes MC, Hodges JR, Buckingham JC. Influence of substance P on hypothalamo-pituitary-adrenocorticol activity in the rat. J. Endocrinol. 1978;76:183–184. doi: 10.1677/joe.0.0760183. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, Lawrence AJ. Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin(1) receptors. Br. J. Pharmacol. 2011;162:880–889. doi: 10.1111/j.1476-5381.2010.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, de Guglielmo G, Cannella N, Li HW, Calo G, Guerrini R, Ubaldi M, Renger JJ, Uebele VN, Ciccocioppo R. Hypothalamic neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2013;226:347–355. doi: 10.1007/s00213-012-2910-y. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Cannella N, Economidou D, Ubaldi M, Ruggeri B, Weiss F, Massi M, Marugan J, Heilig M, Bonnavion P, de Lecea L, Ciccocioppo R. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19567–19572. doi: 10.1073/pnas.1004100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front. Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H, Masuko S. Met-enkephalin-Arg6-Gly7-Leu8- and substance P-containing projections from the nucleus preopticus medianus to the paraventricular hypothalamic nucleus. Neurosci. Lett. 1992;148:211–215. doi: 10.1016/0304-3940(92)90841-t. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Foye MM, Ciraulo DA, Kornetsky C. The type IV phosphodiesterase inhibitors, Ro 20-1724 and rolipram, block the initiation of cocaine self-administration. Pharmacol. Biochem. Behav. 1999;62:151–158. doi: 10.1016/s0091-3057(98)00154-3. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Kieffer BL, Heyser CJ, Katner SN, Ciccocioppo R, Weiss F. Animal models of motivation for drinking in rodents with a focus on opioid receptor neuropharmacology. Recent Dev Alcohol. 2003;16:263–281. doi: 10.1007/0-306-47939-7_19. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Wichmann J, Legowska A, Rolka K, Silberring J. Orphanin FQ/nociceptin but not Ro 65-6570 inhibits the expression of cocaine-induced conditioned place preference. Behav. Pharmacol. 2002;13:229–235. doi: 10.1097/00008877-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Kraft M, Ahluwahlia S, Angulo JA. Neurokinin-1 receptor antagonists block acute cocaine-induced horizontal locomotion. Ann. N. Y. Acad. Sci. 2001;937:132–139. doi: 10.1111/j.1749-6632.2001.tb03562.x. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J. Pharmacol. Exp. Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, Rio DE, Huestis M, Anizan S, Concheiro M, Sinha R, Heilig M. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology. 2015;40:1053–1063. doi: 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laas K, Eensoo D, Paaver M, Lesch KP, Reif A, Harro J. Further evidence for the association of the NPSR1 gene A/T polymorphism (Asn107Ile) with impulsivity and hyperactivity. J Psychopharmacol. 2015 doi: 10.1177/0269881115573803. [DOI] [PubMed] [Google Scholar]
- Laas K, Reif A, Akkermann K, Kiive E, Domschke K, Lesch KP, Veidebaum T, Harro J. Neuropeptide S receptor gene variant and environment: contribution to alcohol use disorders and alcohol consumption. Addict Biol. 2014 doi: 10.1111/adb.12149. [DOI] [PubMed] [Google Scholar]
- Lai M, Zhu H, Sun A, Zhuang D, Fu D, Chen W, Zhang HT, Zhou W. The phosphodiesterase-4 inhibitor rolipram attenuates heroin-seeking behavior induced by cues or heroin priming in rats. Int J Neuropsychopharmacol. 2014;17:1397–1407. doi: 10.1017/S1461145714000595. [DOI] [PubMed] [Google Scholar]
- Landreth GE, Heneka MT. Anti-inflammatory actions of peroxisome proliferator-activated receptor gamma agonists in Alzheimer's disease. Neurobiol. Aging. 2001;22:937–944. doi: 10.1016/s0197-4580(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, Nestler EJ. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J. Neurosci. 1997;17:7890–7901. doi: 10.1523/JNEUROSCI.17-20-07890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Graham DL, Arzaga RR, Buzin N, Webb J, Green TA, Bass CE, Neve RL, Terwilliger EF, Nestler EJ, Self DW. Overexpression of CREB in the nucleus accumbens shell increases cocaine reinforcement in self-administering rats. J. Neurosci. 2011;31:16447–16457. doi: 10.1523/JNEUROSCI.3070-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br. J. Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur. Neuropsychopharmacol. 2007;17:377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J. Comp. Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- Li W, Gao YH, Chang M, Peng YL, Yao J, Han RW, Wang R. Neuropeptide S inhibits the acquisition and the expression of conditioned place preference to morphine in mice. Peptides. 2009;30:234–240. doi: 10.1016/j.peptides.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Liddie S, Anderson KL, Paz A, Itzhak Y. The effect of phosphodiesterase inhibitors on the extinction of cocaine-induced conditioned place preference in mice. J Psychopharmacol. 2012;26:1375–1382. doi: 10.1177/0269881112447991. [DOI] [PubMed] [Google Scholar]
- Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;365:167–175. doi: 10.1016/S0140-6736(05)17708-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Vendruscolo LF, Schlosburg JE, Koob GF, Zorrilla EP. Phosphodiesterase 10A regulates alcohol and saccharin self-administration in rats. Neuropsychopharmacology. 2014;39:1722–1731. doi: 10.1038/npp.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughney K, Snyder PB, Uher L, Rosman GJ, Ferguson K, Florio VA. Isolation and characterization of PDE10A, a novel human 3', 5'-cyclic nucleotide phosphodiesterase. Gene. 1999;234:109–117. doi: 10.1016/s0378-1119(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J. Neurochem. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2013;226:687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Blendy JA, Tzavara E, Gass P, Roques BP, Hanoune J, Schutz G. Reduction of morphine abstinence in mice with a mutation in the gene encoding CREB. Science. 1996;273:657–659. doi: 10.1126/science.273.5275.657. [DOI] [PubMed] [Google Scholar]
- Marquez P, Borse J, Nguyen AT, Hamid A, Lutfy K. The role of the opioid receptor-like (ORL1) receptor in motor stimulatory and rewarding actions of buprenorphine and morphine. Neuroscience. 2008;155:597–602. doi: 10.1016/j.neuroscience.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le Foll B, Tanda G, Piomelli D, Goldberg SR. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol. Psychiatry. 2011;69:633–641. doi: 10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Carta S, Fattore L, Tolu S, Yasar S, Goldberg SR, Fratta W, Maskos U, Pistis M. Peroxisome proliferator-activated receptors-alpha modulate dopamine cell activity through nicotinic receptors. Biol. Psychiatry. 2010;68:256–264. doi: 10.1016/j.biopsych.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello DM, Marcinichen DR, Madruga D, Branco R, Paschoalini MA, De Lima TC. Involvement of NK1 receptors in metabolic stress markers after the central administration of substance P. Behav. Brain Res. 2007;181:232–238. doi: 10.1016/j.bbr.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J. Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Miro X, Perez-Torres S, Palacios JM, Puigdomenech P, Mengod G. Differential distribution of cAMP-specific phosphodiesterase 7A mRNA in rat brain and peripheral organs. Synapse. 2001;40:201–214. doi: 10.1002/syn.1043. [DOI] [PubMed] [Google Scholar]
- Misra K, Roy A, Pandey SC. Effects of voluntary ethanol intake on the expression of Ca(2+) /calmodulin-dependent protein kinase IV and on CREB expression and phosphorylation in the rat nucleus accumbens. Neuroreport. 2001;12:4133–4137. doi: 10.1097/00001756-200112210-00054. [DOI] [PubMed] [Google Scholar]
- Moller DE, Berger JP. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation. Int. J. Obes. Relat. Metab. Disord. 2003;27(Suppl 3):S17–21. doi: 10.1038/sj.ijo.0802494. [DOI] [PubMed] [Google Scholar]
- Montani D, Chaumais MC, Savale L, Natali D, Price LC, Jais X, Humbert M, Simonneau G, Sitbon O. Phosphodiesterase type 5 inhibitors in pulmonary arterial hypertension. Adv. Ther. 2009;26:813–825. doi: 10.1007/s12325-009-0064-z. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Morgenweck J, Griggs RB, Donahue RR, Zadina JE, Taylor BK. PPARgamma activation blocks development and reduces established neuropathic pain in rats. Neuropharmacology. 2013;70:236–246. doi: 10.1016/j.neuropharm.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Ren Z, Jia J, Gao B, Zheng L, Wang G, Friedman E, Zhen X. Inhibition of phosphodiesterase10A attenuates morphine-induced conditioned place preference. Molecular brain. 2014;7:70. doi: 10.1186/s13041-014-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- Murtra P, Sheasby AM, Hunt SP, De Felipe C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature. 2000;405:180–183. doi: 10.1038/35012069. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76:259–268. doi: 10.1016/j.neuropharm.2013.04.004. Pt B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaus S, Huston JP, Hasenohrl RU. Reinforcing effects of neurokinin substance P in the ventral pallidum: mediation by the tachykinin NK1 receptor. Eur. J. Pharmacol. 1999;370:93–99. doi: 10.1016/s0014-2999(99)00105-3. [DOI] [PubMed] [Google Scholar]
- Nothacker HP, Reinscheid RK, Mansour A, Henningsen RA, Ardati A, Monsma FJ, Jr., Watson SJ, Civelli O. Primary structure and tissue distribution of the orphanin FQ precursor. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8677–8682. doi: 10.1073/pnas.93.16.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez C, Gonzalez-Cuello A, Sanchez L, Vargas ML, Milanes MV, Laorden ML. Effects of rolipram and diazepam on the adaptive changes induced by morphine withdrawal in the hypothalamic paraventricular nucleus. Eur. J. Pharmacol. 2009;620:1–8. doi: 10.1016/j.ejphar.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci. 2015;16:305–12. doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol. Biochem. Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Paneda C, Huitron-Resendiz S, Frago LM, Chowen JA, Picetti R, de Lecea L, Roberts AJ. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. J. Neurosci. 2009;29:4155–4161. doi: 10.1523/JNEUROSCI.5256-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, Barnes C, Redhi GH, Adair J, Heishman SJ, Yasar S, Aliczki M, Haller J, Goldberg SR. Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: preclinical findings. Neuropsychopharmacology. 2012;37:1838–1847. doi: 10.1038/npp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PE, Vendruscolo LF, Schlosburg JE, Edwards S, Schulteis G, Koob GF. Corticotropin-releasing factor (CRF) and alpha 2 adrenergic receptors mediate heroin withdrawal-potentiated startle in rats. Int J Neuropsychopharmacol. 2013;16:1867–1875. doi: 10.1017/S1461145713000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi RK, Reznikov LR, Fadel J. Activation of orexin neurons by acute nicotine. Eur. J. Pharmacol. 2006;535:172–176. doi: 10.1016/j.ejphar.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, Maggi CA. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74:1445–1463. doi: 10.1016/j.lfs.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccart E, Langlois X, Vanhoof G, D'Hooge R. Selective inhibition of phosphodiesterase 10A impairs appetitive and aversive conditioning and incentive salience attribution. Neuropharmacology. 2013;75:437–444. doi: 10.1016/j.neuropharm.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Douglas J, Chan J, Gamp PD, Bunnett NW. Neurokinin 1 receptor distribution in cholinergic neurons and targets of substance P terminals in the rat nucleus accumbens. J. Comp. Neurol. 2000;423:500–511. [PubMed] [Google Scholar]
- Pike VW, Rash KS, Chen Z, Pedregal C, Statnick MA, Kimura Y, Hong J, Zoghbi SS, Fujita M, Toledo MA, Diaz N, Gackenheimer SL, Tauscher JT, Barth VN, Innis RB. Synthesis and evaluation of radioligands for imaging brain nociceptin/orphanin FQ peptide (NOP) receptors with positron emission tomography. J. Med. Chem. 2011;54:2687–2700. doi: 10.1021/jm101487v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placenza FM, Fletcher PJ, Rotzinger S, Vaccarino FJ. Infusion of the substance P analogue, DiMe-C7, into the ventral tegmental area induces reinstatement of cocaine-seeking behaviour in rats. Psychopharmacology (Berl) 2004;177:111–120. doi: 10.1007/s00213-004-1912-9. [DOI] [PubMed] [Google Scholar]
- Placenza FM, Vaccarino FJ, Fletcher PJ, Erb S. Activation of central neurokinin-1 receptors induces reinstatement of cocaine-seeking behavior. Neurosci. Lett. 2005;390:42–47. doi: 10.1016/j.neulet.2005.07.050. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Flores A, Maldonado R, Berrendero F. Hypocretin/orexin signaling in the hypothalamic paraventricular nucleus is essential for the expression of nicotine withdrawal. Biol. Psychiatry. 2012;71:214–223. doi: 10.1016/j.biopsych.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J. Neurosci. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluzarev O, Pandey SC. Modulation of CREB expression and phosphorylation in the rat nucleus accumbens during nicotine exposure and withdrawal. J. Neurosci. Res. 2004;77:884–891. doi: 10.1002/jnr.20216. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Yevich E, Baltazar C, Gallager DW. Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology. 1997;17:308–316. doi: 10.1016/S0893-133X(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Ardati A, Monsma FJ, Jr., Civelli O. Structure-activity relationship studies on the novel neuropeptide orphanin FQ. J. Biol. Chem. 1996;271:14163–14168. doi: 10.1074/jbc.271.24.14163. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu YL, Okamura N, Zeng J, Chung S, Pai R, Wang Z, Civelli O. Pharmacological characterization of human and murine neuropeptide s receptor variants. J. Pharmacol. Exp. Ther. 2005;315:1338–1345. doi: 10.1124/jpet.105.093427. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr., Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Reyes-Irisarri E, Perez-Torres S, Mengod G. Neuronal expression of cAMP-specific phosphodiesterase 7B mRNA in the rat brain. Neuroscience. 2005;132:1173–1185. doi: 10.1016/j.neuroscience.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol. Biochem. Behav. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ripley TL, Gadd CA, De Felipe C, Hunt SP, Stephens DN. Lack of self-administration and behavioural sensitisation to morphine, but not cocaine, in mice lacking NK1 receptors. Neuropharmacology. 2002;43:1258–1268. doi: 10.1016/s0028-3908(02)00295-2. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol. Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RA, James NH, Woodyatt NJ, Macdonald N, Tugwood JD. Evidence for the suppression of apoptosis by the peroxisome proliferator activated receptor alpha (PPAR alpha) Carcinogenesis. 1998;19:43–48. doi: 10.1093/carcin/19.1.43. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nature reviews. Neuroscience. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Ruggeri B, Braconi S, Cannella N, Kallupi M, Soverchia L, Ciccocioppo R, Ubaldi M. Neuropeptide S receptor gene expression in alcohol withdrawal and protracted abstinence in postdependent rats. Alcohol. Clin. Exp. Res. 2010;34:90–97. doi: 10.1111/j.1530-0277.2009.01070.x. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul. Pept. 2004;118:183–191. doi: 10.1016/j.regpep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Endogenous nociceptin (orphanin FQ) suppresses basal hedonic state and acute reward responses to methamphetamine and ethanol, but facilitates chronic responses. Neuropsychopharmacology. 2008;33:877–891. doi: 10.1038/sj.npp.1301459. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J. Comp. Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Gobbi G, Debs PC, Sibille ET, Blier P, Hen R, Heath MJ. Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1912–1917. doi: 10.1073/pnas.041596398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD, Schwartz MW. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology. 2009;150:707–712. doi: 10.1210/en.2008-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M. Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron. 2012;76:192–208. doi: 10.1016/j.neuron.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, Shaham Y, Heilig M. Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl) 2011;218:111–119. doi: 10.1007/s00213-011-2201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt O, Usunoff KG, Lazarov NE, Itzev DE, Eipert P, Rolfs A, Wree A. Orexinergic innervation of the extended amygdala and basal ganglia in the rat. Brain structure & function. 2012;217:233–256. doi: 10.1007/s00429-011-0343-8. [DOI] [PubMed] [Google Scholar]
- Schmoutz CD, Zhang Y, Runyon SP, Goeders NE. Antagonism of the neuropeptide S receptor with RTI-118 decreases cocaine self-administration and cocaine-seeking behavior in rats. Pharmacol. Biochem. Behav. 2012;103:332–337. doi: 10.1016/j.pbb.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp M, Lazar MA. Endogenous ligands for nuclear receptors: digging deeper. J. Biol. Chem. 2010;285:40409–40415. doi: 10.1074/jbc.R110.182451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J. Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat. Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- Skelton KH, Gutman DA, Thrivikraman KV, Nemeroff CB, Owens MJ. The CRF1 receptor antagonist R121919 attenuates the neuroendocrine and behavioral effects of precipitated lorazepam withdrawal. Psychopharmacology (Berl) 2007;192:385–396. doi: 10.1007/s00213-007-0713-3. [DOI] [PubMed] [Google Scholar]
- Smith KL, Patterson M, Dhillo WS, Patel SR, Semjonous NM, Gardiner JV, Ghatei MA, Bloom SR. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology. 2006;147:3510–3518. doi: 10.1210/en.2005-1280. [DOI] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur. J. Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Bayuga SJ, Beavo JA. Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7071–7076. doi: 10.1073/pnas.96.12.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol. Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Spampinato S, Baiula M, Calienni M. Agonist-regulated internalization and desensitization of the human nociceptin receptor expressed in CHO cells. Current drug targets. 2007;8:137–146. doi: 10.2174/138945007779315641. [DOI] [PubMed] [Google Scholar]
- Specio SE, Wee S, O'Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina D. The potential of PDE4 inhibitors in respiratory disease. Current drug targets. Inflammation and allergy. 2004;3:231–236. doi: 10.2174/1568010043343822. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Nielsen CK, Holgate J, Bito-Onon JJ, Bartlett SE. The neurokinin 1 receptor antagonist, ezlopitant, reduces appetitive responding for sucrose and ethanol. PloS one. 2010:5. doi: 10.1371/journal.pone.0012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M, Ubaldi M, Heilig M, Demopulos G, Gaitanaris G, Ciccocioppo R. Activation of PPARgamma by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcohol. Clin. Exp. Res. 2013;37:1351–1360. doi: 10.1111/acer.12091. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, Ruggeri B, Heilig M, Demopulos G, Gaitanaris G, Massi M, Ciccocioppo R. Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol. Psychiatry. 2011;69:642–649. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br. J. Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BE, Sachs BD, Kantak KM, Cherry JA. The Type IV phosphodiesterase inhibitor rolipram interferes with drug-induced conditioned place preference but not immediate early gene induction in mice. Eur. J. Neurosci. 2004;19:2561–2568. doi: 10.1111/j.0953-816X.2004.03357.x. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Schank JR, Singley E, Hunt SP, Heilig M. Neurokinin-1 receptors (NK1R:s), alcohol consumption, and alcohol reward in mice. Psychopharmacology (Berl) 2010;209:103–111. doi: 10.1007/s00213-010-1775-1. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Tapocik JD, Liu K, Zook M, Bell L, Flanigan M, Patnaik S, Marugan J, Damadzic R, Dehdashti SJ, Schwandt ML, Southall N, Austin CP, Eskay R, Ciccocioppo R, Zheng W, Heilig M. A novel brain penetrant NPS receptor antagonist, NCGC00185684, blocks alcohol-induced ERK-phosphorylation in the central amygdala and decreases operant alcohol self-administration in rats. J. Neurosci. 2013;33:10132–10142. doi: 10.1523/JNEUROSCI.4742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol. Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Tucci S, Cheeta S, Seth P, File SE. Corticotropin releasing factor antagonist, alpha-helical CRF(9-41), reverses nicotine-induced conditioned, but not unconditioned, anxiety. Psychopharmacology (Berl) 2003;167:251–256. doi: 10.1007/s00213-003-1403-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Ubaldi M, Giordano A, Severi I, Li H, Kallupi M, de Guglielmo G, Ruggeri B, Stopponi S, Ciccocioppo R, Cannella N. Activation of Hypocretin-1/Orexin-A Neurons Projecting to the Bed Nucleus of the Stria Terminalis and Paraventricular Nucleus Is Critical for Reinstatement of Alcohol Seeking by Neuropeptide S. Biol. Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Grow RW. The burden of complex genetics in brain disorders. Arch. Gen. Psychiatry. 2004;61:223–229. doi: 10.1001/archpsyc.61.3.223. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta. 2011;1812:1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JR, Spina M, Terenius L, Koob GF. Nociceptin fails to affect heroin self-administration in the rat. Neuroreport. 1998;9:2243–2247. doi: 10.1097/00001756-199807130-00017. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Heilig M, Nuzzo PA, Henderson P, Lofwall MR. Effects of the NK1 antagonist, aprepitant, on response to oral and intranasal oxycodone in prescription opioid abusers. Addict Biol. 2013;18:332–343. doi: 10.1111/j.1369-1600.2011.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol. Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann. N. Y. Acad. Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Wen RT, Zhang M, Qin WJ, Liu Q, Wang WP, Lawrence AJ, Zhang HT, Liang JH. The phosphodiesterase-4 (PDE4) inhibitor rolipram decreases ethanol seeking and consumption in alcohol-preferring Fawn-Hooded rats. Alcohol. Clin. Exp. Res. 2012;36:2157–2167. doi: 10.1111/j.1530-0277.2012.01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CH, Michael RP. Substance P injections into the ventral tegmentum affect unit activity in mesolimbic terminal regions. Brain Res. Bull. 1991;26:229–233. doi: 10.1016/0361-9230(91)90232-9. [DOI] [PubMed] [Google Scholar]
- Whitty CJ, Walker PD, Goebel DJ, Poosch MS, Bannon MJ. Quantitation, cellular localization and regulation of neurokinin receptor gene expression within the rat substantia nigra. Neuroscience. 1995;64:419–425. doi: 10.1016/0306-4522(94)00373-d. [DOI] [PubMed] [Google Scholar]
- Wilson LS, Brandon NJ. Emerging biology of PDE10A. Curr. Pharm. Des. 2015;21:378–388. doi: 10.2174/1381612820666140826114744. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J. Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, Tucker RC, Ciccocioppo R. The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol. Ther. 2014;141:283–299. doi: 10.1016/j.pharmthera.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Barrett-Jolley R. Activation of paraventricular nucleus neurones by the dorsomedial hypothalamus via a tachykinin pathway in rats. Exp. Physiol. 2007;92:671–676. doi: 10.1113/expphysiol.2007.037457. [DOI] [PubMed] [Google Scholar]
- Womack MD, Morris R, Gent TC, Barrett-Jolley R. Substance P targets sympathetic control neurons in the paraventricular nucleus. Circ. Res. 2007;100:1650–1658. doi: 10.1161/CIRCRESAHA.107.153494. [DOI] [PubMed] [Google Scholar]
- Woods JW, Tanen M, Figueroa DJ, Biswas C, Zycband E, Moller DE, Austin CP, Berger JP. Localization of PPARdelta in murine central nervous system: expression in oligodendrocytes and neurons. Brain Res. 2003;975:10–21. doi: 10.1016/s0006-8993(03)02515-0. [DOI] [PubMed] [Google Scholar]
- Xu Y-L, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J. Comp. Neurol. 2007;500:84–8102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- Xu Y-L, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Zhao RJ, Woo RS, Jeong MS, Shin BS, Kim DG, Kim KW. Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport. 2003;14:2383–2385. doi: 10.1097/00001756-200312190-00019. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front. Neuroendocrinol. 2014;35:234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Heilig M, de Wit H, Shaham Y. Behavioral, biological, and chemical perspectives on targeting CRF(1) receptor antagonists to treat alcoholism. Drug Alcohol Depend. 2013;128:175–186. doi: 10.1016/j.drugalcdep.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]