Abstract
Non-medical use of amphetamine (AMPH) among adolescents is prevalent, which is problematic given the potential consequences of developmental drug exposure on brain function and behavior. Previously we found in adult male rats that AMPH exposure starting before puberty induces a persistent decrease in dopamine D1 receptor (D1R) function in the medial prefrontal cortex (mPFC). Here we investigated if this dysfunction was associated with changes in D1R expression in the mPFC and nucleus accumbens (NAc). We also determined if starting drug exposure well before or near the onset of puberty would influence AMPH-induced changes in D1R expression and behavior. Male and female Sprague-Dawley rats were treated once every other day (10 injections total) with saline or 3 mg/kg AMPH (i.p.) from either postnatal day (P) 27 to 45 (pre-puberty groups; pre-P) or P37 to 55 (peri-puberty groups; peri-P). After 1, 7 and 21 days of withdrawal, sucrose preference tests were performed to assess anhedonia. Exploratory behavior was studied in an open-field arena and on an elevated plus maze (EPM). Rats were then sacrificed for western blot analysis of D1R expression. We found that AMPH withdrawal induced decreases in sucrose preference that persisted in rats with peri-P onset treatment. Pre-P onset AMPH exposure led to increased open arm exploration in the EPM test, as well as a decreased D1R level in the mPFC but not NAc. Our results demonstrated that AMPH exposure starting at different developmental stages resulted in distinct neurobehavioral abnormalities, suggesting an important role of exposure timing in drug-induced plasticity.
Keywords: adolescence, prefrontal cortex, D1 receptor, puberty, elevated plus maze, risk taking
1. Introduction
Empirical evidence suggests that illicit substance use during adolescence may profoundly alter brain development (Gulley and Juraska, 2013; Spear, 2015) and potentially increase the risk of future substance abuse and addiction (Paus et al., 2008; Hammerslag and Gulley, 2016). Amphetamine (AMPH) is among the most widely abused drugs and it has been estimated that about 10.3% of the US population has used AMPH at least once by the 10th grade (UNODC, 2010). Studies of the impact of adolescent AMPH exposure have largely utilized laboratory rodent models as a means to investigate potential neurobiological and cognitive changes. In rats, adolescence is generally accepted to begin near postnatal day (P) 28 and extend until approximately P60 (Spear, 2000; Brenhouse and Andersen, 2011). It is characterized by a rise in gonadal hormones, which leads to pubertal onset as identified by vaginal opening in females and preputial separation in males at approximately P35 and P45, respectively (Korenbrot et al., 1977; Castellano et al., 2011). Rat adolescence also involves numerous neural and behavioral changes that are noted as early as P28 and are similar to those seen in humans (Sisk and Foster, 2004; Spear, 2011).
Adult rats that had been previously exposed to moderate doses of AMPH (2–3 mg/kg, i.p.) during different portions of adolescence have been shown to have deficits in associative learning (Richetto et al., 2012), behavioral flexibility (Hankosky et al., 2013), impulse control (Hankosky and Gulley, 2013; Hammerslag et al., 2014) and working memory (Sherrill et al., 2013). These cognitive changes may be due in part to alterations in dopaminergic function, as the mesocorticolimbic dopamine system undergoes extensive changes throughout adolescent development and thus may be especially vulnerable to drug-induced adaptations (Wahlstrom et al., 2010; McCutcheon et al., 2012; Gulley and Juraska, 2013).
One notable developmental change in dopamine is in the expression and function of its receptors. For example, D1 receptor (D1R) levels in the medial prefrontal cortex (mPFC) increase rapidly in early adolescence, peak around postnatal day (P) 40, and continue to decrease into young adulthood (Andersen et al., 2000). The localization of these receptors on particular cell types within the mPFC also changes during this time (Brenhouse et al., 2008), and this redistribution is associated with an increase in the excitability of mPFC interneurons following stimulation of D1R or D2 receptors (Tseng and O’Donnell, 2007). In the nucleus accumbens (NAc), D1R levels peak relatively earlier around P30 (Naneix et al., 2012); this receptor’s functional maturation is less understood. These developmental changes in dopamine receptor expression and function are thought to be critical for the maturation of the mesocortical circuit and its role in mediating various cognitive and affective behaviors (Tseng and O’Donnell, 2007; Casey et al., 2008; Naneix et al., 2012). We recently used in vitro, whole-cell recordings to demonstrate that adult rats exposed to AMPH during adolescence had reduced function of D1Rs in the mPFC (Kang et al., 2016). A potential mechanism for this effect is an AMPH-induced change in the typical developmental shift in the expression of D1R. A primary goal of the current study was to investigate if adolescent AMPH exposure induces changes in D1R expression in the mPFC and in one of the target structures whose ontogeny it influences, the NAc (Casey et al., 2008).
A secondary aim of the current study was to determine if developmental exposure to AMPH would induce lasting changes in affective behavior that is also known to be sensitive to changes in dopamine function (Rodgers et al., 1994; Dunlop and Nemeroff, 2007; Labonte et al., 2012; Yu et al., 2014). Increases in anxiety and depression are frequently observed in those who abuse AMPHs, especially during early stage of withdrawal (London, 2004; Thompson et al. 2004; Zorick et al., 2010; Leventhal et al., 2010). In adult rodents, withdrawal from chronic exposure to AMPHs is associated with increases in anxiety-like behavior (Barr et al., 2010) and a transient anhedonic state (Barr and Phillips, 1999; Che et al., 2013; Pathak et al., 2015). To date, few studies have examined affective behavior following exposure to AMPH in adolescence and the available results are mixed (Labonte et al., 2012; Kolyaduke and Hughes, 2013). Moreover, increasing evidence suggests that the timing of drug exposure during the peri-adolescent period is likely to be an important determining factor for adaptations in the brain and behavior that result. For example, an earlier study examined the long-term effects of repeated exposure to nicotine starting in pre-, mid- or post-adolescence in mice (Adriani et al., 2004). Exposure with mid-adolescence onset (P36) produced a heightened locomotor response to novel environment in adulthood, whereas pre- (P23) and late-adolescent onset (P49) exposure had no or very modest effects on this behavior. Some of these differences may be related to the onset of puberty, which in female rats is between P31 and P39 and in male rats is between P40 and P48 (Juraska and Willing, 2016).
Using the same adolescent drug exposure paradigm that we have used previously to demonstrate changes in behavior and neurophysiology that last well into adulthood (Hankosky and Gulley, 2013; Hankosky et al., 2013; Sherrill et al., 2013; Hammerslag et al., 2014; Kang et al., 2016; Paul et al., 2016), we injected male and female rats with 3 mg/kg AMPH every other day starting well before puberty onset (P27) or near female puberty onset (P37). We then assessed withdrawal associated anhedonia and anxiety-related behavior in these pre-pubertal (Pre-P) and peri-pubertal (Peri-P) groups of rats using tests of sucrose preference and measures of activity in open-field arena and an elevated plus maze. Lastly, rats were sacrificed and their brains were removed for analysis of D1R expression in the mPFC and NAc.
2. Materials and Methods
2.1 Subjects
Subjects were male (n = 45) and female (n = 76) Sprague-Dawley rats born in our animal facility from dedicated breeders originally obtained from Harlan (Indianapolis, IN, USA). Following weaning on P22, rats were housed 2 per cage with same-sex littermates. All rats were maintained on a 12:12 h light/dark cycle (lights on at 0800 h) with food and water available ad libitum. Experimental procedures, which were performed between 1300 and 1800 h, were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign and were consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
2.2 Apparatus
For testing of sucrose preference, an acrylic divider was inserted into each pair-housed rats’ home cage. This divider, which was transparent and contained multiple small holes (~8 mm diameter) that allowed for olfactory and tactile interactions between cage mates, temporarily separated rats so that accurate measures of individual consumption could be obtained. During the 48-h test, food was available ad libitum to both rats.
Locomotor activity was assessed in open-field arenas (Coulbourn Instruments; Whitehall, PA USA) that each consisted of a clear acrylic box (41 × 41 × 41 cm) fitted with two photobeam frames (16 beams/dimension; 2.5 cm between beams). A lower frame was located 2.5 cm above the arena floor and was used to determine ambulation; an upper frame was located 15 cm above the floor and was used to determine rearing. These chambers were located inside a 76 × 80 × 63cm sound attenuating cubicle that had a 76 mm speaker mounted on the inside of one wall and two ceiling-mounted white lights (4 W each) that provided dim illumination. White noise (70 dB) was played continuously through the speakers when rats were in the testing room. Each open-field apparatus was connected to a nearby computer running activity monitoring software (TruScan, v 2.01; Coulbourn Instruments) that recorded beam breaks with a 500 msec sampling rate.
Anxiety-related behavior was measured on an elevated plus maze (EPM) that was located in a testing room illuminated by a dim red light (60 W). The EPM consisted of two open arms (50 cm × 10 cm, each with a 2 mm raised edge along the sides to discourage falls) and two enclosed arms (50 cm × 10 cm, with 40 cm high walls). Open and enclosed arms were arranged opposite to each other, and all arms had an open top. The maze arms were elevated 100 cm above the floor and a padded surface was placed 50 cm below the arms to provide cushion in the case of a rat falling from open arms.
2.3 AMPH exposure
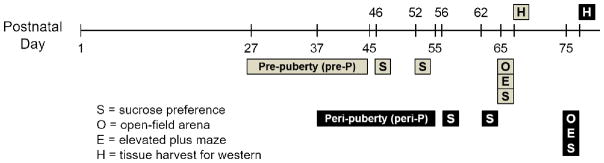
d-Amphetamine sulfate (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.9% saline at a concentration of 1 mg/mL and dose was calculated based on the weight of the salt. Rats were assigned to the following adolescent treatment groups: pre-pubertal onset (pre-P) females (n = 10 given AMPH; n = 9 given saline), pre-P males (n = 10 given AMPH; n = 13 given saline), peri-pubertal onset (peri-P) females (n = 13 given AMPH; n = 12 given saline) and peri-P males (n = 12 given AMPH; n = 12 given saline). Assignment to groups was pseudorandom such that all groups were represented in each of the 15 litters used in this study. Injections (i.p.) of 3 mg/kg AMPH or saline were given every other day over a 3-week period for a total of 10 injections (see Fig. 1). These began on P27 for rats in the pre-P groups and P37 for rats in the peri-P groups. For each treatment, animals were transported to a testing room, given their assigned injection, and placed individually in a clear plastic tub (46 × 25 × 22 cm) lined with beta-chip bedding. After 60 min, rats were returned to their home cages and the colony room.
Figure 1.
Timeline for adolescent treatments and behavioral assessments. Rats were given injections of saline or 3 mg/kg AMPH every other day (10 injections total) starting on either P27 (pre-P groups) or P37 (peri-P groups). Each sucrose preference test (S) lasted 48 h. Rats were sacrificed 48 h after their last S and brain tissue was harvested (H) for subsequent analysis of D1R expression.
2.4 Assessment of puberty onset
We performed once daily checks for evidence of vaginal opening in females or preputial separation in males (Juraska and Willing, 2016) in a subset of rats that underwent the behavioral testing described below (n = 3–6/group). These checks were also performed on a separate cohort of female rats that were experimentally naïve (n = 8) or were treated with saline (n = 6–7) or AMPH (n = 5–7) with pre- or peri-pubertal onset. Rats were examined starting on P27 and continuing until these external signs of puberty onset were noted.
2.5 Behavioral assessments
The timeline for behavioral testing is illustrated in Fig. 1. One and seven days following their last injection with saline or AMPH (Fig. 1), rats were given 48-h free access to a sipper tube (100 mL) filled with tap water and one filled with a 1% sucrose solution. The amount of water and sucrose solution consumed was measured after the first 24 h, at which time the relative position of the water and sucrose tubes was also switched.
Approximately three weeks (20–21 days) after their last injection, rats were transported individually from their colony to a testing room where they remained for a 5 min habituation period. They were then placed in an open-field arena and allowed to behave undisturbed for 10min. Subsequently, they were moved to another testing room, allowed to habituate for 5 min, and were then tested on the EPM. For this test, rats were placed in the center of the maze with their head oriented towards an open arm and they were allowed to behave undisturbed for 5 min. Following the EPM test, rats were returned to the colony. A final 48-h sucrose preference test was performed approximately 30 min after the completion of the EPM test.
The order of testing was determined based on studies showing both anxiogenic (Barr et al., 2010) and anxiolytic (Labonte et al., 2012) effects of repeated AMPH in the EPM. Pre-exposure to environmental novelty, such as in an open-field arena, has been shown to increase open-arm entry and exploration in a subsequent EPM tests (Walf and Frye, 2007). Because the goal here was to perform the EPM test in such a way as to prevent a “floor effect” and thereby allow for the potential observation of a reduction in open-arm activity induced by AMPH exposure, rats were first tested in the open-field prior to the EPM.
2.6 D1R expression
Two days after the last sucrose preference test, rats were deeply anesthetized with Fatal-Plus (Vortech Pharmaceuticals; Dearborn, MI USA) and transcardially perfused with ice-cold saline. Their brains were removed rapidly and placed in a chilled metal matrix that was used to cut 1-mm coronal sections containing the mPFC and NAc. A 2-mm diameter punch was used to extract samples containing both shell and core of the NAc and both infralimbic and prelimbic regions of the mPFC. The subregions within each brain region were combined as our previous study (Kang et al., 2016) showed no differential effect of AMPH exposure on D1 function in the infralimbic compared to prelimbic regions. After storage at −80 °C for up to 8 weeks, brain tissue from individual rats was homogenized in lysis buffer containing (in mM) 50 Tris-HCl (pH 7.4), 150 NaCl, 30 EDTA, 1.5% Triton-X, and 0.1% sodium dodecyl sulfate. The homogenate was centrifuged at 10,000 RPM for 10 min at 4 °C. The supernatant was assessed for protein concentration using Precision Red Advanced Protein Assay (Cytoskeleton, CA) and then combined with 2X laemmli loading buffer (Bio-Rad Laboratories; Hercules, CA USA) and 2-mercaptoethanol (0.25%, Sigma-Aldrich), followed by heat-shock at 98 °C for 10 min. Samples were then stored at −80 °C until processing for gel electrophoresis and Western blotting.
Samples (20 μg protein/well) were then fractionated in pre-cast gels (4–15%, Biorad) using a dual miniature vertical polyacrylamide gel electrophoresis system (Biorad). Gels were run first at 100 V and 40 mA for 15 min, followed by 200 V and 40 mA for 35–45 min. Separated proteins were wet-transferred onto polyvinyl difluoride membranes using a Trans-Blot electrophoresis transfer cell (Biorad) run at 100 V for 1 h. Non-specific binding sites were blocked for 20 min at room temperature on an orbital shaker with a solution of 5% non-fat milk in Tris-buffered saline and 0.1% Tween 20 (TBST). Membranes were then incubated overnight at 4°C on an orbital shaker with 1:1000 primary D1R antibody (ab20066, Abcam; Cambridge, MA USA) in 5% non-fat milk/TBST.
On the second day of processing, membranes were washed three times for 10 min using TBST. Subsequently, membranes were incubated for 2 h at room temperature on an orbital shaker in 1:1000 anti-rabbit secondary antibody (7074A, Cell Signaling) in 5% non-fat milk/TBST, followed by three more TBST washes. Lastly, membranes were incubated with horseradish substrate (SuperSignal™ West Dura Extended Duration Substrate, ThermoFisher; Waltham, MA USA) and imaged using the ChemiDoc Touch imaging system (Biorad). Following the first imaging, membranes were washed in TBST for 20–30 min and then 45 min at 50°C in stripping buffer containing 20% SDS, 0.5 M Tris-HCl (pH 6.8), 0.8% 2-mercaptoethanol. Subsequently, the membrane was washed with nanopure H2O four times for 15 min each, followed by three 10-min washes using TBST. Non-fat milk (5%) with TBST was again added for 20 min to block non-specific binding sites, followed by incubation in 1:1000 anti-GAPDH antibody in 5% non-fat milk overnight at 4°C on an orbital shaker. On the subsequent day, membranes were again subjected to the TBST washes, secondary antibody and horseradish substrate incubation, and imaging processes as described above.
2.7 Data analysis
Puberty onset data were analyzed using two-way ANOVA with group (pre-P, peri-P) and treatment (control, AMPH) as between-subjects factors. Fluid consumption during sucrose preference tests was measured every 24 h by weighing sipper tubes and estimating consumption by subtracting remaining fluid weight from the starting weight. A preference ratio (%) was then calculated as follows: sucrose intake/(sucrose intake + water intake) X 100. Data from the one- and seven-day tests were analyzed using a four-way, mixed factor ANOVA with sex (male, female), group (pre-P, peri-P) and treatment (control, AMPH) as the between-subjects factors and withdrawal day (1 and 7) as the repeated factor. Data from the final preference test (20–21 day withdrawal) was analyzed using a three-way, between-subjects ANOVA (sex × group × treatment). In all cases, significant main effects and interactions (p values ≤ 0.05) were followed up with Tukey post-hoc analyses.
Measures of locomotor activity in the open-field arena were obtained from analysis software (TruScan; Coulbourn Instruments) and included ambulation, rearing, and time spent in the center of the open-field arena. Ambulation was calculated by tabulating consecutive photobeam breaks in the lower photobeam plane and converting this to distance (m). Rearing, which was calculated as photobeam breaks in the upper photobeam frame, occurred when rats raised their forelimbs off the floor and stood on their hindlimbs. For analysis of time spent in the center, the arena was evenly divided into 16 grids (10.25 X 10.25 cm/each) and the center was designated as the centermost 4 grids. Cumulative measures during the 10-min test session were analyzed with three-way ANOVAs (sex × group × treatment). Tukey post-hoc analyses were used to investigate significant main effects and interactions.
Anxiety-related behavior in the EPM was assessed by quantifying the number of open and closed arm entries as well as the time spent in open arms. These measures were recorded during each test session by an experimenter who was blind to the group status of the rat being tested. Rats were considered to have entered an arm when all four of its paws crossed the borderline of the center area. These data were analyzed using three-way ANOVAs (sex × group × treatment) followed by Tukey post-hoc tests. Data from 3 males (2 from the pre-P and 1 from the peri-P control groups) and 2 females from the pre-P AMPH group were excluded from the analysis because they fell off the maze onto the protective cushion before they completed the 5-min test.
D1R expression was determined by first measuring the optical density of each band using the ChemiDoc™ Touch Imaging System (Biorad) and Image Lab software (Biorad). The density of the putative D1R band (~50 KD) was subsequently divided by the density of the corresponding loading control GAPDH (39 KD) to obtain an adjusted density that corrects for differences in loading. To pool the adjusted density for each group across different gels, same-sex samples from the same gel were normalized to the average of the same-sex controls across all gels. These normalized densities were analyzed using two-way, between subjects ANOVA (group × treatment) followed by Tukey post-hoc tests. Data from the mPFC of one female in the pre-P saline group was lost due to problems with sample preparation.
3. Results
3.1 Effects of injections on puberty onset
In the subset of rats (n=3–6/group) that underwent daily checks for pubertal status (Table 1), we noticed that onset in males occurred within a range between P42 and P48. Furthermore, mean puberty onset was similar in the different treatment groups and there was no dependency on age at the start of treatment. Females, in contrast, exhibited a wider range (P32 to P44) and the mean age of puberty onset was earlier in rats who began treatment at P27 compared to P37. Three-way ANOVA revealed a significant main effect of sex (F1,31=144, p<0.001), group (F1,31=16.6, p<0.001) and sex by group interaction (F1,31=10.4, p<0.01). Post hoc analysis suggested a significant difference between female pre-P and peri-P groups. We further investigated this effect in a separate cohort of female rats (n=5–7/group) that also included an experimentally naïve group (n = 8). As shown in Fig. 2, we found that females who received injections beginning on P27 had earlier puberty onset than those who had their first injection on P37. Two-way ANOVA of these data revealed a significant main effect of group (F1,21 = 4.54, p < 0.05). In the experimentally naïve females, puberty onset ranged between P31 and P40 and the mean onset (~P 36) was similar to that observed in the peri-P groups, but later than in the pre-P groups.
Table 1.
Mean postnatal day (± SEM) when markers of pubertal onset were first noted in females and males in the pre-pubertal onset (pre-P) and peri-pubertal onset (peri-P) treatment groups. Saline or 3 mg/kg AMPH treatment began on P27 for rats in the pre-P group and P37 for those in the peri-P group.
| pre-P | peri-P | |||
|---|---|---|---|---|
| saline | AMPH | saline | AMPH | |
| Female | 34.8 ± 0.49 (n = 6) | 34.3 ± 0.61 (n = 5) | 38.7 ± 2.40* (n = 6) | 40.0 ± 1.27* (n = 4) |
| Male | 44.7 ± 0.84 (n = 4) | 45.0 ± 0.45 (n =6 | 45.2 ± 0.91 (n = 3) | 45.3 ± 0.75 (n = 5) |
The number of subject per group is indicated in parentheses.
p < 0.05, vs female pre-P groups (collapsed across treatment).
Figure 2.
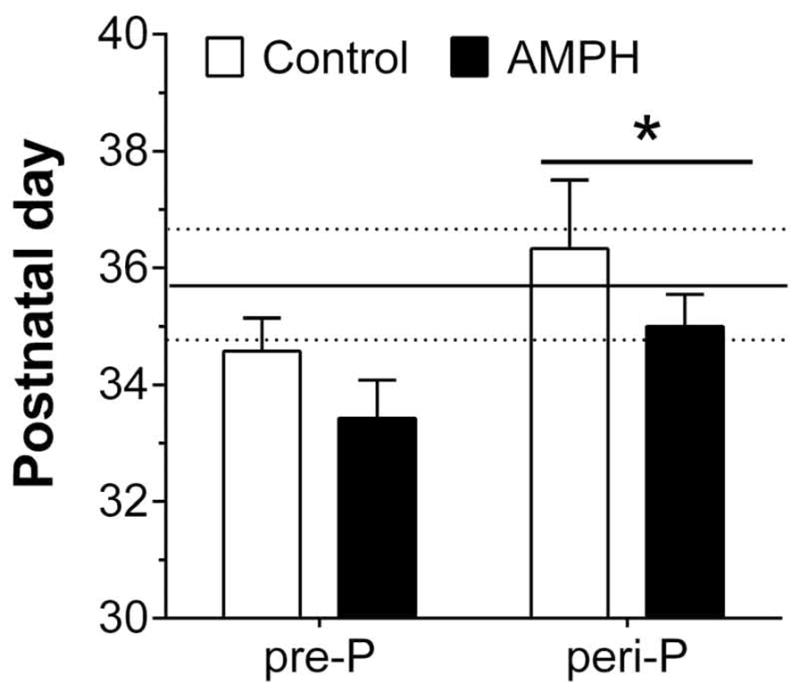
Puberty onset, as defined by age at vaginal opening, in females (n=5–7/group) given injections of saline (Control) or 3 mg/kg AMPH. The horizontal lines in the figure represent the mean (solid line) and SEM (dotted lines) postnatal day of puberty onset for a separate group of female rats (n = 8) that were otherwise experimentally naïve. *p < 0.05, vs. pre-P collapsed across treatment
3.2 Effects of AMPH on hedonic and anxiety-related behavior
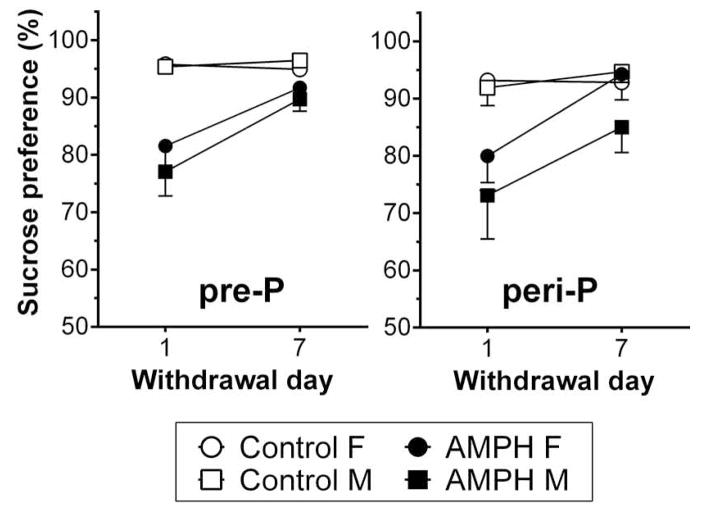
As shown in Fig. 3, repeated exposure to 3 mg/kg AMPH was associated with a decrease in sucrose preference following withdrawal. Four-way ANOVA revealed significant main effects of treatment (F1, 166 = 36.0, p <0.001), withdrawal day (F1,166 = 13.2, p<0.001) and a treatment by withdrawal day interaction (F1,166 = 10.5, p<0.01). It appeared that males had a greater decrease but the main effect of sex (F1,166 = 0.48, p > 0.05) and interactions with sex were not statistically significant. Post-hoc analysis of the treatment by withdrawal day interaction suggested that the difference between AMPH treated rats and control was significant on withdrawal day 1 and nearly significant on day 7 (p = 0.052). Within AMPH groups, sucrose preference on day 1 was significantly different from day 7. We also found a near-significant interaction of group by treatment (F1,166=3.31, p = 0.071) and post-hoc tests suggested a significant difference between pre-P AMPH and peri-P AMPH groups.
Figure 3.
Preference for a 1% sucrose solution in females (F; n = 9–13/group) and males (M; n = 10–13/group) following withdrawal from repeated injections with saline or 3 mg/kg AMPH. Sucrose preference, which is expressed as a percentage of sucrose solution intake relative to total intake of both sucrose and water, was measured for a 48-h period beginning 1 or 7 days after the last injection.
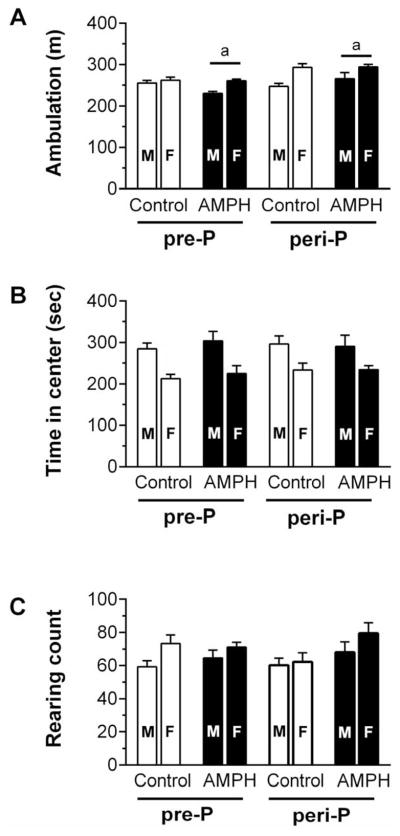
Following a 20–21 day withdrawal from AMPH, spontaneous locomotor activity and anxiety-related behavior in the EPM were assessed. Three-way ANOVA of ambulation (Fig. 4A) revealed a significant main effect of sex (F1,83 = 22.2, p<0.001), group (F1,83 = 15.2, p<0.001) and a near-significant interaction of group by treatment (F1,83=5.33, p = 0.056). Post-hoc test suggested that peri-P AMPH group traveled more distance than pre-P AMPH group. We also found significant main effects of sex for time spent in the arena center (F1,83 = 26.4, p < 0.001) and rearing (F1,83 = 5.38, p < 0.05). As shown in Fig 4 panels B and C, females spent less time in the center of the arena but had more rearing behavior than males. We did not find any other group differences in these measures of open-field activity.
Figure 4.
Spontaneous locomotor activity in an open-field arena measured 20–21 days after the last saline or AMPH injection (n = 10–13 M/group; n = 9–13 F/group). Cumulative measures of ambulation (A), time spent in the center quadrant (B) and number of rearing events (C) areshown for the 10-min test period. Matching letters indicate a significant group difference (p < 0.05) collapsed across sex.
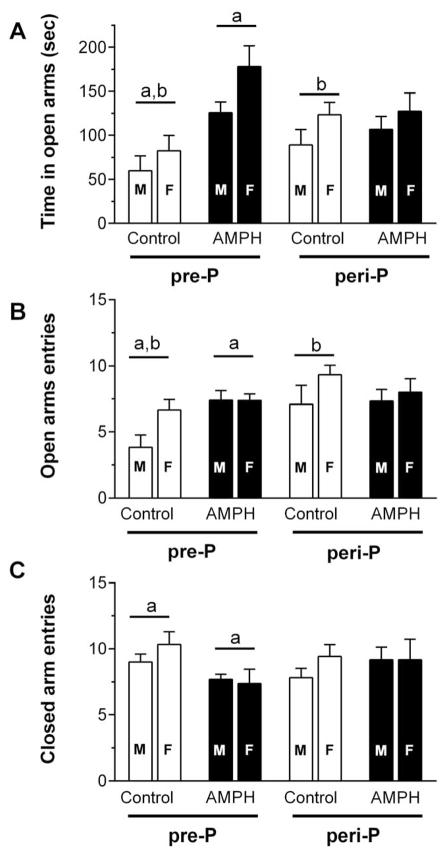
In the EPM test, we found increased open-arm activity in rats exposed to AMPH beginning on P27. Three-way ANOVA of time spent in open arms (Fig. 5A) revealed a significant main effect of sex (F1, 78 = 6.90, p < 0.05), treatment (F1, 78 = 13.6, p < 0.01) and treatment by group interaction (F1, 78 = 7.96, p < 0.01). Post hoc analysis suggested a significant group difference within the pre-P group such that the AMPH treated rats spent more time in open arms than their control. There was also a significant difference between pre-P and peri-P controls, with the latter spending more time in open arms. Three-way ANOVA of open arm entry (Fig. 5B) revealed a significant main effect of sex (F1, 78 = 4.42, p <0.05), group (F1, 78 = 5.67, p < 0.05) and a treatment by group interaction that was near-significant (F1,78 = 3.89, p = 0.052). Post hoc analysis suggested a significant difference within the pre-P group such that AMPH-treated rats had more entries. Post hoc tests also indicated that peri-P controls had significantly more open arm entries than pre-P controls. Three-way ANOVA of close arm entry (Fig. 5C) revealed a significant treatment by group interaction (F1,78=3.95, p = 0.050), with post-hoc analysis suggesting that AMPH-treated rats had significantly fewer closed arm entries than their controls within the pre-P group (Fig. 5C). There were no statistically significant group differences in total arm entries (data not shown).
Figure 5.
Activity in an elevated plus maze measured 20–21 days after the last saline or AMPH injection (n = 8–12 males/group; n = 8–13 females/group). Measures of time spent in the open arms (A), number of open arm entries (B) and number of closed arm entries (C) were obtained during a 5-min test session. Matching letters indicate a significant group difference (p < 0.05) collapsed across sex.
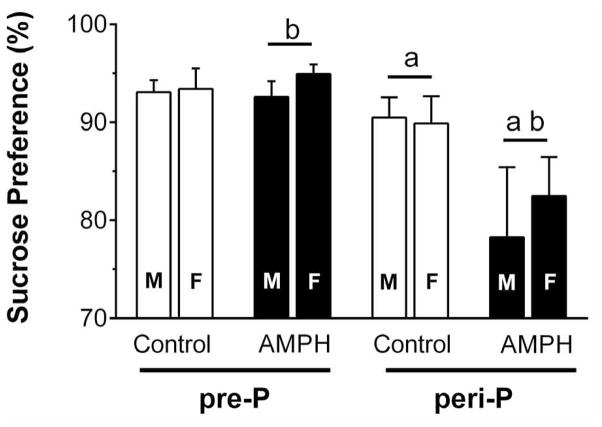
Following the EPM test, all rats were given a third sucrose preference test (Fig. 6). Three-way ANOVA revealed a significant main effect of group (F1,83 = 10.8, p < 0.01) and a treatment by group interaction (F1,83 = 4.27, p < 0.05). Post hoc tests suggested a significant difference between AMPH-treated and control within the peri-P group, as well as between the pre-P and peri-P groups injected with AMPH. In other words, sucrose preference was significantly decreased, and to an approximately equal extent both male and females, in rats exposed to AMPH during the peri-pubertal period.
Figure 6.
Sucrose preference (n = 9–13 F/group and n = 10–13 M/group) following 20–21 days withdrawal from repeated injections with saline or 3 mg/kg AMPH. Matching letters indicate a significant group difference (p < 0.05) collapsed across sex.
3.3 Effects of AMPH on D1R expression
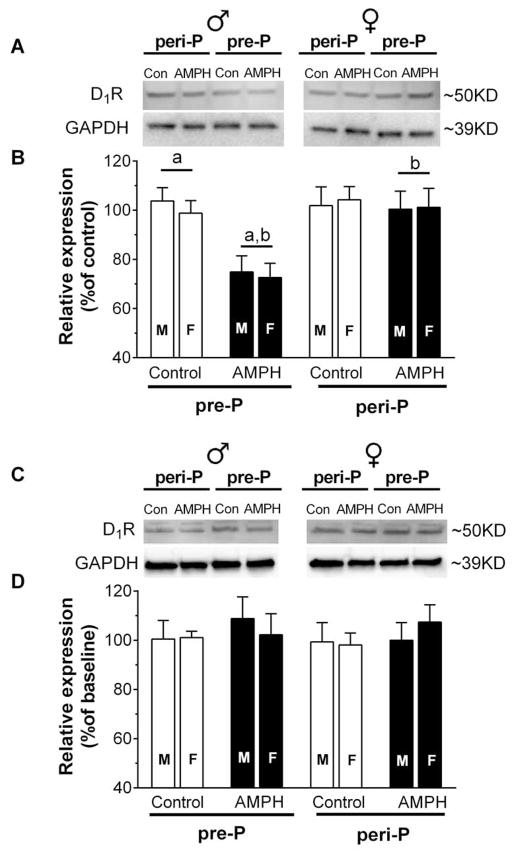
D1R expression levels in the mPFC and NAc were assessed using Western blot analysis of brain tissue taken two days after the last behavioral test. In the mPFC, two-way ANOVA of D1R levels (Fig 7B) revealed a significant main effect of treatment (F1, 87 = 10.9, p < 0.01), group (F1, 87 = 9.58, p < 0.01), and a group by treatment interaction (F1,87 = 7.89, p < 0.01). Post hoc tests confirmed that AMPH exposure beginning at P27 led to a significant reduction in D1 expression in the mPFC compared to pre-P controls and the per-P group exposed to AMPH. We did not find any significant group differences in D1 expression in the NAc (Fig. 7D).
Figure 7.
Analysis of D1R expression in the mPFC (A,B) and NAc (C,D) of rats (n = 10–13 M/group; n = 8–13 F/group) exposed to saline or 3 mg/kg AMPH. Representative blot images are shown in A and C, with group means for relative expression shown in B and D. Brains were analyzed 21–22 days after the last injection during adolescence. Data used for group means within sex were normalized to the mean expression level of same-sex controls. Matching letters indicate a significant group difference (p < 0.05) collapsed across sex.
4. Discussion
We recently found that repeated AMPH exposure during adolescence induced a long lasting reduction in D1R function in the mPFC (Kang et al., 2016). A primary aim of the current study was to determine if this functional change was associated with a reduction in D1R expression in the mPFC and one of its major targets, the NAc. A secondary goal was to investigate if abnormalities in affective behavior were associated with potential dopaminergic changes and the extent to which AMPH’s effects depended on exposure beginning well before, or right near, the onset of puberty.
Our results suggest that repeated exposure to AMPH starting before puberty (pre-P group) led to a significant reduction in mPFC D1R level and increased exploratory behavior in the EPM when measured in young adulthood. These effects of AMPH were not observed in rats who began exposure at or near puberty onset (peri-P group). In the peri-P group, however, we found evidence for a lasting (21 days) impact of withdrawal-induced anhedonia, as measured by a decreases in sucrose preference that was not apparent in the pre-P rats exposed to AMPH. Together, these results demonstrate persistent alterations in D1R expression and in anxiety- and depression-related behaviors following chronic AMPH exposure during adolescence. Moreover, the treatment by group interaction we observed reinforces a key role for the timing of exposure onset in directing drug-induced neurobiological plasticity and its behavioral manifestations.
4.1 Injections hastened puberty onset in females
In two different assessments from separate cohorts of female rats, we found that repeated injection experience before puberty accelerated puberty onset. This effect occurred regardless of injection type (saline or AMPH) and may be due to stress associated with the injection procedure. Previous studies of pre-pubertal exposure to stressors (Mendle et al., 2011) or pre-pubertal activation of stress-signaling pathways (Li et al., 2014) have also shown that female puberty can be induced to occur earlier than normal. We did not observe an effect of injection experience in males, but this should be interpreted cautiously given the relatively low sample size in this group. An earlier study showed that repeated exposure to cocaine during adolescence led to lasting decrease in plasma testosterone level (Alves et al., 2014) that may potentially affect male puberty. In light of recent reports suggesting that specific neuroanatomical refinements occurring in the mPFC may be caused by the hormonal changes associated with puberty onset (Juraska and Willing, 2016; Drzewiecki et al., 2016), our findings in females suggest that a shift in puberty timing may alter the ontogeny of the mPFC and other brain regions to which it is connected and in turn alter behavior. This is further supported by the group differences we observed between the pre-P and peri-P controls in the EPM test. Specifically, the peri-P control group had significantly more open arm activity than pre-P controls. Importantly, these apparent stress-related changes may interact with the effects of AMPH exposure during different stages of adolescence.
4.2 Withdrawal-induced anhedonic state
Human studies suggest one of the most common symptoms associated with repeated AMPH use is an increase in depression-related behaviors, including anhedonia (Thompson et al. 2004; Zorick et al., 2010; Leventhal et al., 2010) that usually becomes undetectable after 4 to7 days in adult human (London, 2004) and animals (Barr and Phillips, 1999; Jang et al, 2013). Anhedonic individuals are suggested to be more prone to relapse and dependence than those who experience less anhedonia during drug withdrawal (Leventhal et al. 2008; Leventhal et al., 2010). In human adolescents, depression-related symptoms are also observed following AMPH use (Degenhardt et al., 2007; Brière et al., 2012) and evidence suggests that the rate of depression comorbid with illicit drug use is higher in adolescents than adults (Rao, 2006). However, there is a large gap in understanding the characteristics and neurobiology of depression-related behaviors following exposure to stimulants in adolescents. A few earlier studies found that repeated exposure to methylphenidate (Bolaños et al., 2003; Carlezon et al., 2003) or nicotine (Iñiguez et al., 2008) during pre- or peri-adolescence led to detectable depression-related behaviors in adulthood. However, the effect of AMPH exposure during adolescence on depression-related behaviors had not previously been reported.
Using sucrose preference test, we found decreased hedonic response in AMPH-exposed rats during the first 48 hours of withdrawal, regardless of sex and age at treatment onset. This anhedonic state became less evident after 7 days of abstinence, showing a transient nature similar to that found in adult animals following chronic AMPH (Barr and Phillips, 1999; Jang et al., 2013; Pathak et al., 2015). Although this anhedonic response appeared similar between pre-P and peri-P group, our statistical analysis indicated that peri-P AMPH exposure was associated with a relatively greater decrease in sucrose preference (Fig. 3). In the third test following the EPM (~3 weeks after the last AMPH injection), a significant reduction in sucrose preference reemerged in the peri-P AMPH group (Fig. 6). This result may correspond to the earlier finding of the long-lasting depressive symptoms associated with stimulants exposure during adolescence (Bolaños et al., 2003; Iñiguez et al., 2008). An alternative explanation is that rats in the peri-P AMPH group were more prone to an influence of the two behavioral tests that preceded the final sucrose preference test. A previous study showed that an EPM testing led to significant increases in plasma corticosterone levels in rats (Alves et al., 2014), which is an effect of stress that may interact with the effect of AMPH. It is unclear if the peri-P AMPH group would have still shown an anhedonia if they had been tested before the open-field and EPM assessments. In addition, the near maximal sucrose preference we observed for most groups during the second and third test (≥ 95% preference) may have made it difficult to reveal subtle groups differences. Recently, preference for 0.25–1% sucrose has been shown to be sensitive to changes in D1 receptor levels in the mPFC (Freund et al., 2016). It is thus possible that our use of repeated testing of a 1% sucrose solution made it difficult to detect an altered hedonic response in the pre-P group that had a decreased mPFC D1 level. Although this possibility would need to be assessed in a future study, the current results nonetheless indicate that repeated AMPH exposure during adolescence induced abnormalities in hedonic homeostasis that can persist into adulthood.
The detailed neuronal mechanisms underlying reduced sucrose preference are not clear. Notably, reduced dopamine activity in the mesocortical circuit, especially in the NAc, is suggested to play a critical role in expressing anhedonia (Salamone et al., 1997; Orsini et al., 2001; Wise, 2008). In adult animals, there is a marked decrease of extracellular dopamine level 1 to 7 days after repeated AMPH or methamphetamine exposure that is likely related to a homeostatic response to drug-induced increases in dopamine (Kitanaka et al., 2008). We speculate that the decrease in sucrose preference at the beginning of AMPH withdrawal in the current study may be a reflection of this mechanism. At this time, the neuronal adaptation underlying the reoccurrence of anhedonia in peri-P AMPH group remains unknown. Notably, we did not find any changes in D1R level in either mPFC or NAC following peri-P AMPH exposure, suggesting there are other mechanisms contributing to this enduring effect of AMPH. For example, the reduction in sucrose intake may be due to attenuated “hedonic liking” that is mediated by opioid signaling in the NAc (Smith et al., 2011). It has been shown that repeated AMPH exposure decreases mu-opioid receptor mRNA in the NAc shell in young adult rats (Vecchiola et al., 1999). Thus, the repeated AMPH injection in the current study may potentially alter mu receptor function and result in related changes in hedonic information processing. It is also important to recognize that the sucrose preference test may not fully capture the characteristics of anhedonia, as the psychopathological expression of anhedonia may include not only changes in the motivation to obtain reward but also in reward anticipation, prediction, evaluation and reward-associated decision-making (Der-Avakian and Markou, 2012). Future work is necessary to more clearly identify the mechanisms of adolescent AMPH exposure-induced changes in anhedonia.
4.3 Increased approach behavior is associated with decreased D1R expression in the mPFC
We found modest effects of AMPH on spontaneous locomotor activity, with peri-P AMPH group ambulating more than pre-P AMPH group but neither group behaving significantly different from their respective controls. The EPM test, in contrast, revealed that pre-P rats exposed to AMPH had a marked increase in open arm behavior. This was evident in terms of open arm entries, as well as time spent in the open arms. The peri-P group exposed to AMPH was not different from their controls. The results from our pre-P group are consistent with an earlier report showing that repeated AMPH exposure starting from P30 led to increased open arm activity when measured in adulthood (Labonte et al., 2012). This “anxiolytic-like” effect of repeated AMPH exposure is in contrast to the anxiogenic effect that results from repeated AMPH exposure during adulthood (Barr et al., 2010; Reinbold et al., 2014) and from the effect of repeated cocaine exposure in adolescent mice (Estelles et al., 2007; Santucci and Maderia, 2008; Santucci and Maderia, 2010). This discrepancy may indicate that there are unique neuroadaptations associated with AMPH exposure during early adolescence, but it also may be the result of differences in experimental conditions used across studies. For example, our rats were put in the EPM approximately 5 min after the open-field test, while other studies had no pre-exposure to an open-field arena or used a longer delay (e.g., 1 h) before the EPM test. The methodology we used for the open-field test may also explain the lack of any group differences in time spent in the center of the arena. When that measure has been used as an index of anxiety-like behavior (Prut and Belzung, 2003), the test is usually done in a brightly illuminated environment so as to promote an anxiogenic state. Here, we assessed open-field activity in low light conditions to increase the probability that rats would engage in spontaneous locomotion.
The EPM has been widely used for assessing anxiety-related behaviors in rodents, as it utilizes the inherent conflict between the rodent’s exploratory nature and their avoidance of potential harm (e.g., elevation and open space; Pawlak et al., 2012). Under this conflict between approach and avoidance, it is suggested that behavioral inhibition mechanisms become active and generate anxiety in normal animals that in turn leads to their limited approach behavior (Gray and McNaughton, 2000; Pawlak et al., 2012). Generally, control rats or mice spend less than 30% of their total test duration exploring open arms. Our results showed that the time in open arms of rats exposed with the pre-P onset was about twice as much as their controls, with pre-exposed females spending over 50% of the total testing period to the open arms. This considerable shift in the approach-avoidance balance suggests an impaired inhibitory control mechanism (Pawlak et al., 2012). Such a shift in this balance has also been interpreted as a result of increased motivation for risk (Labonte et al., 2012; Hodgson et al, 2008), which may result from impaired risk-based decision making. Previous work has shown that repeated drug exposure in adult rats will also increase risk-taking behavior in other tasks (Zhou et al., 2015). Dopamine signaling in the mPFC has previously been shown to mediate decision making, including risk-based decisions (St Onge and Floresco, 2010; Simon et al., 2011), and it is noteworthy that temporary inactivation of the mPFC by local infusion of muscimol increases open arm activity (Shah et al., 2004; Solati et al., 2013).
In association with the increased approach behavior we observed, pre-P rats exposed to AMPH also had a significant reduction in D1R expression in the mPFC. Labonte et al. (2012) showed that the baseline firing rate of dopaminergic neurons in the ventral tegmental area, which project to the mPFC and other target regions, was elevated 3 to 4 weeks following an AMPH exposure (1.5mg/kg) that was similar to the method used in our pre-P group. We speculate that the decrease in D1R expression we observed may be the result of an adaptation to the putatively elevated levels of mPFC dopamine that AMPH would have induced. While this hypothesis requires a more direct test, our finding is also in keeping with the result of an earlier study showing that increased open-arm activity was associated with dopamine hypofunction in the mPFC (Watt et al., 2009). The extent to which a decreased level of D1Rs in the mPFC is associated with altered risk-based decision-making remains unclear. Using an operant behavior task, Simon et al. (2011) demonstrated that D1R mRNA expression in the mPFC was not correlated with choice of a large, risky reward and a D1R agonist had no significant effect on risk preference.
The precise mechanism by which changes in mPFC dopamine tone contribute to an altered balance of approach-avoidance behavior in the EPM requires further investigation. Recently, it was reported that activating a subpopulation of VTA cells that project to the mPFC leads to aversion and this effect could be blocked by antagonism of D1Rs in the mPFC (Lammel et al., 2012). This finding suggests that mPFC D1R signaling is involved in the perception or processing of information associated with aversive stimuli. Hence, the reduced mPFC D1R level in the pre-P AMPH group may result in an attenuated sensitivity or response to the aversive experience associated with open arms and thereby lead to increased exploration. Notably, using the same pre-P AMPH treatment and withdrawal schedule used here, we found an impairment in D1R-mediated inhibition in the mPFC (Kang et al., 2016). Our current study suggests this functional impairment of D1R could be attributed to a decrease in D1R expression level. Such impairment of dopamine-mediated inhibitory function may result in aberrant mPFC output that influences down-stream regions and in turn leads to abnormal behavior output. One limitation of the current study is that we did not differentiate D1R levels in the prelimbic and infralimbic regions of the mPFC, which are known to have distinct afferent innervation and projections (Heidbreder and Groenewegen, 2003). Future studies will be needed to determine if the effect of AMPH on mPFC D1R level is region-specific.
4.5 The role of exposure timing in AMPH’s effect
In the current study, we consistently found group by treatment interactions (Fig. 4A, Fig. 5A,B, Fig. 6 and Fig. 7A), suggesting an important role of age of exposure onset in determining drug-induced plasticity. The importance of exposure age has been highlighted in multiple studies of alcohol, nicotine and cannabinoids (Spear, 2015). Indeed, the developmental trajectory of many neurotransmitter systems usually proceeds in a non-linear fashion (Casey et al, 2008) during adolescence. In this regard, it is not surprising that the neurobiological or behavioral outcomes resulting from drug exposure depend on when during this development that drugs are introduced to the brain. For example, the pre-P onset treatment used in the current study overlapped the developmental window when D1Rs are over-produced in the mPFC [from early adolescence to about P40 (Andersen et al., 2000) or 45 (Naneix et al., 2012)], whereas the later occurring peri-P treatment may have more of an influence on the subsequent pruning process.
It is notable that only the pre-P treatment induced a long-lasting reduction of D1R level in the mPFC. It has been shown that the continuous maturation of dopamine function in the mPFC throughout late-adolescence to adulthood coincides with improvement or emergence of complex cognitive behavior (Naneix et al., 2012). Thus, disruption of such delayed development by drug exposure during adolescence may induce specific neural and behavioral adaptations that contribute to a heightened vulnerability to addiction and comorbid mental disorders (Gulley and Juraska, 2013; Hammerslag and Gulley, 2016). An early study (Brandon et al., 2001) showing that exposure to methylphenidate during adolescence led to cross-sensitization to cocaine and increased cocaine self-administration in adulthood is consistent with this hypothesis. The present results also raise potential concerns for the timing of therapeutic AMPH use, such as for the treatment of attention deficit hyperactivity disorder (ADHD), in pre- and peri-pubertal young people. Whether or not those prescribed the drug before puberty onset would be more susceptible to side effects is not clear, especially since the exposure dose used here (3 mg/kg/day injected i.p.) is likely to be beyond what would be used therapeutically. Other laboratory animal studies that used more clinically relevant dosing of AMPH have reported no adverse effects on cognitive function (Soto et al., 2012) or responses to other drugs of abuse (Jordan et al., 2016), but there has not been a systematic investigation of how sex or pubertal status at the time of treatment onset impacts drug effects.
4.6 Conclusion
In the current study, we found long-lasting behavioral and neurobiological changes following repeated AMPH exposure during adolescence that depended on the timing of exposure relative to puberty onset. Exposure beginning at or near puberty onset was associated with a potentially higher propensity of exhibiting anhedonia, whereas drug exposure beginning several days or more than two weeks before puberty onset in females and males, respectively, was marked by increases in approach or risk taking behavior and reductions in D1R expression in the mPFC. Further investigation is needed to understand the detailed mechanisms underlying these neurobiological changes and behavioral abnormalities, especially since they may confer heightened vulnerability to future drug use or abuse its associated consequences.
Highlights
Anxiety-related behavior was lower in adults first given amphetamine pre-puberty
This effect was associated with a decrease in D1 receptor expression in the cortex
Anhedonia after withdrawal was greater when drug exposure onset was near puberty
Acknowledgments
The authors thank Amogh Belagodu and Wendy M. Soler for technical assistance. This work was supported by NIH grant DA029815. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves CJ, Magalhães A, Melo P, de Sousa L, Tavares MA, Monteiro PR, Summavielle T. Long-term effects of chronic cocaine exposure throughout adolescence on anxiety and stress responsivity in a Wistar rat model. Neurosci. 2014;277:343–55. doi: 10.1016/j.neuroscience.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–9. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Adriani W, Granstrem O, Macri S, Izykenova G, Dambinova S, Laviola G. Behavioral and neurochemical vulnerability during adolescence in mice: Studies with nicotine. Neuropsychopharmacology. 2004;29:869–878. doi: 10.1038/sj.npp.1300366. [DOI] [PubMed] [Google Scholar]
- Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacoloy. 1999;141:99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- Barr JL, Renner KJ, Forster GL. Withdrawal from chronic amphetamine produces persistent anxiety-like behavior but temporally-limited reductions in monoamines and neurogenesis in the adult rat dentate gyrus. Neuropharmacology. 2010;59:395–405. doi: 10.1016/j.neuropharm.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters Behavioral Responses to Emotional Stimuli at Adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharm. 2001;25:651–61. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–82. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brière FN, Fallu J-S, Janosz M, Pagani LS. Prospective associations between meth/amphetamine (speed) and MDMA (ecstasy) use and depressive symptoms in secondary school students. J Epidemiol Community Health. 2012;66:990–994. doi: 10.1136/jech-2011-200706. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54:1330–7. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Bentsen AH, Sanchez-Garrido MA, Ruiz-Pino F, Romero M, Garcia-Galiano D, Aguilar E, Pinilla L, Dieguez C, Mikkelsen JD, Tena-Sempere M. Early metabolic programming of puberty onset: Impact of changes in postnatal feeding and rearing conditions on the timing of puberty and the development of the hypothalamic kisspeptin system. Endocrinology. 2011;152:3396–3408. doi: 10.1210/en.2010-1415. [DOI] [PubMed] [Google Scholar]
- Che Y, Cui YH, Tan H, Andreazza AC, Young LT, Wang JF. Abstinence from repeated amphetamine treatment induces depressive-like behaviors and oxidative damage in rat brain. Psychopharmacology (Berl) 2013;227:605–614. doi: 10.1007/s00213-013-2993-0. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Tennant C, Gilmour S, Schofield D, Nash L, Hall W, McKay D. The temporal dynamics of relationships between cannabis, psychosis and depression among young adults with psychotic disorders: findings from a 10-month prospective study. Psychol Med. 2007;37:927–34. doi: 10.1017/S0033291707009956. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzewiecki CM, Willing J, Juraska JM. Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: A role for pubertal onset. Synapse. 2016 doi: 10.1002/syn.21909. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Espejo EF. Selective dopamine depletion within the medial prefrontal cortex induces anxiogenic-like effects in rats placed on the elevated plus maze. Brain Res. 1997;762:281–284. doi: 10.1016/s0006-8993(97)00593-3. [DOI] [PubMed] [Google Scholar]
- Estelles J, Lluch J, Rodríguez-Arias M, Aguilar MA, Miñarro J. Cocaine exposure during adolescence affects anxiety in adult mice. Brain Res Bulletin. 2007;71:393–403. doi: 10.1016/j.brainresbull.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Freund N, Thompson BS, Sonntag K, Meda S, Andersen SL. When the party is over: depressive-like states in rats following termination of cortical D1 receptor overexpression. Psychopharm. 2016;233:1191–1201. doi: 10.1007/s00213-015-4200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JS, McNaughton N. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. 2. Oxford, UK: Oxford University Press; 2000. [Google Scholar]
- Gulley JM, Juraska JM. The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience. 2013;249:3–20. doi: 10.1016/j.neuroscience.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerslag LR, Waldman AJ, Gulley JM. Effects of amphetamine exposure in adolescence or young adulthood on inhibitory control in adult male and female rats. Behav Brain Res. 2014;263:22–33. doi: 10.1016/j.bbr.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerslag LR, Gulley JM. Sex differences in behavior and neural development and their role in adolescent vulnerability to substance use. Behav Brain Res. 2016;298:15–26. doi: 10.1016/j.bbr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankosky ER, Gulley JM. Performance on an impulse control task is altered in adult rats exposed to amphetamine during adolescence. Dev Psychobiol. 2013;55:733–44. doi: 10.1002/dev.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankosky ER, Kofsky NM, Gulley JM. Age of exposure-dependent effects of amphetamine on behavioral flexibility. Behav Brain Res. 2013;252:117–25. doi: 10.1016/j.bbr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Norris CJ, Eitan S. Increased elevated plus maze open-arm time in mice during naloxone-precipitated morphine withdrawal. Behav Pharma. 2008;19:805–811. doi: 10.1097/FBP.0b013e32831c3b57. [DOI] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Monojlovic Z, Bolanos-Guzmán C. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009;34:1609–1624. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang C-G, Whitfield T, Schulteis G, Koob GF, Wee S. A dysphoric-like state during early withdrawal from extended access to methamphetamine self-administration in rats. Psychopharmacology (Berl) 2013;225:753–763. doi: 10.1007/s00213-012-2864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Taylor DM, Dwoskin LP, Kantak KM. Adolescent d-amphetamine treatment in a rodent model of ADHD: Pro-cognitive effects in adolescence without an impact on cocaine cue reactivity in adulthood. Behav Brain Res. 2016;297:165–79. doi: 10.1016/j.bbr.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, Willing J. Pubertal onset as a critical transition for neural development and cognition. Brain Res. 2016 doi: 10.1016/j.brainres.2016.04.012. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Paul K, Hankosky ER, Cox CL, Gulley JM. D1 receptor-mediated inhibition of medial prefrontal cortex neurons is disrupted in adult rats exposed to amphetamine in adolescence. Neuroscience. 2016;324:40–49. doi: 10.1016/j.neuroscience.2016.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka JN, Takemura M. Neurochemical consequences of dysphoric state during amphetamine withdrawal in animal models: A review. Neurochem Res. 2008;33:204–219. doi: 10.1007/s11064-007-9409-7. [DOI] [PubMed] [Google Scholar]
- Kolyaduke OV, Hughes RN. Increased anxiety-related behavior in male and female adult rats following early and late adolescent exposure to 3,4-methylenedioxymethamphetamine (MDMA) Pharma Biochem Behav. 2013;103:742–749. doi: 10.1016/j.pbb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Labonte B, McLaughlin RJ, Dominguez-Lopez S, Bambico FR, Lucchino I, Ochoa-Sanchez R, Leyton M, Gobbi G. Adolescent amphetamine exposure elicits dose-specific effects on monoaminergic neurotransmission and behaviour in adulthood. Int J Neuropsychopharmacol. 2012;15:1319–30. doi: 10.1017/S1461145711001544. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Stone K, Young D, Chelminski I, Zimmerman M. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. Am J Addict. 2008;17(3):218–23. doi: 10.1080/10550490802019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Brightman M, Ameringer KJ, Greengerg J, Mickens L, Ray LA, Sun P, Sussman S. Anhedonia associated with stimulant use and dependence in a population-based sample of american adults. Exp Clin Psychopharmacol. 2010;18:562–569. doi: 10.1037/a0021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Hu MH, Li SY, Geach C, Hikima A, Rose S, Greenwood MP, Greenwood M, Murphy D, Poston L, Lightman SL, O’Byrne KT. Overexpression of corticotropin releasing factor in the central nucleus of the amygdala advances puberty and disrupts reproductive cycles in female rats. Endocrinology. 2014;155:3934–44. doi: 10.1210/en.2014-1339. [DOI] [PubMed] [Google Scholar]
- London ED. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol. 2012;108:1620–30. doi: 10.1152/jn.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Leve LD, Van Ryzin M, Natsuaki MN, Ge X. Associations between early life stress, child maltreatment, and pubertal development among girls in foster care. J Res Adolesc. 2011;21:871–880. doi: 10.1111/j.1532-7795.2011.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini C, Koob GF, Pulvirenti L. Dopamine Partial Agonist Reverses Amphetamine Withdrawal in Rats. Neuropsychopharmacology. 2001;25:789–792. doi: 10.1016/S0893-133X(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Pathak G, Ibrahim BA, McCarthy SA, Baker K, Kelly MP. Amphetamine sensitization in mice is sufficient to produce both manic- and depressive-related behaviors as well as changes in the functional connectivity of corticolimbic structures. Neuropharmacology. 2015;95:434–447. doi: 10.1016/j.neuropharm.2015.04.026. [DOI] [PubMed] [Google Scholar]
- Paul K, Kang S, Cox CL, Gulley JM. Repeated exposure to amphetamine during adolescence alters inhibitory tone in the medial prefrontal cortex following drug re-exposure in adulthood. Behav Brain Res. 2016;309:9–13. doi: 10.1016/j.bbr.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak CR, Karrenbauer BD, Schneider P, Ho Y-J. The Elevated Plus-Maze Test: Differential Psychopharmacology of Anxiety-Related Behavior. Emotion Rev. 2012;4:98–115. [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Euro J Pharmaco. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rao U. Links between depression and substance abuse in adolescents. Am J Prev Med. 2006;31:S161–S174. doi: 10.1016/j.amepre.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Reinbold ED, Scholl JL, Oliver KM, Watt MJ, Forster GL. Central CRF2 receptor antagonism reduces anxiety states during amphetamine withdrawal. Neurosci Res. 2014;89:37–43. doi: 10.1016/j.neures.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetto J, Feldon J, Riva MA, Meyer U. Comparison of the long-term consequences of withdrawal from repeated amphetamine exposure in adolescence and adulthood on information processing and locomotor sensitization in mice. Eur Neuropsychopharmacol. 2013;23:160–70. doi: 10.1016/j.euroneuro.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Nikulina EM, Cole JC. Dopamine D1 and D2 receptor ligands modulate the behavior of mice in the elevated plus maze. Pharmaco Biochem Behav. 1994;49:985–95. doi: 10.1016/0091-3057(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: Empirical and conceptual problems with the anhedonia hypothesis. Neurosci& Biobehav Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Madeira E. Anxiogenesis in adult rats treated chronically with cocaine during adolescence: Effects of extended abstinence and 8-OH-DPAT treatment. Brain Res Bulletin. 2008;76:402–411. doi: 10.1016/j.brainresbull.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Madeira E. Anxiety-like responses in adolescent rats following a 10–11-day withdrawal period from repeated cocaine administration. Brain Res Bulletin. 2010;81:441–444. doi: 10.1016/j.brainresbull.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Shah AA, Sjovold T, Treit D. Inactivation of the medial prefrontal cortex with the GABAA receptor agonist muscimol increases open-arm activity in the elevated plus-maze and attenuates shock-probe burying in rats. Brain Res. 2004;1028:112–115. doi: 10.1016/j.brainres.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Sherrill LK, Stanis JJ, Gulley JM. Age-dependent effects of repeated amphetamine exposure on working memory in rats. Behav Brain Res. 2013;242:84–94. doi: 10.1016/j.bbr.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, Bañuelos C, Vokes CM, Taylor AB, Haberman RP, Bizon JL, Setlow B. Dopaminergic modulation of risky decision-making. J Neurosci. 2011;31:17460–70. doi: 10.1523/JNEUROSCI.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Nat Acad Sci. 2011;108:E255–64. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solati J, Hajikhani R, Golub Y. Activation of GABAA receptors in the medial prefrontal cortex produces an anxiolytic-like response. Acta Neuropsychiatr. 2013;25:221–6. doi: 10.1111/acn.12016. [DOI] [PubMed] [Google Scholar]
- Soto PL, Wilcox KM, Zhou Y, Ator NA, Riddle MA, Wong DF, Weed MR. Long-term exposure to oral methylphenidate or dl-amphetamine mixture in peri-adolescent rhesus monkeys: Effects on physiology, behavior, and dopamine system development. Neuropsychopharmacology. 2012;37:2566–2579. doi: 10.1038/npp.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24:115–23. [PMC free article] [PubMed] [Google Scholar]
- Spear L. Adolescent neurobehavioral characteristics, alcohol sensitivities, and intake: Setting the stage for alcohol use disorders? Child Dev Perspect. 2011;5:231–238. doi: 10.1111/j.1750-8606.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav. 2015;148:122–130. doi: 10.1016/j.physbeh.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Prefrontal cortical contribution to risk-based decision making. Cereb Cortex. 2010;20:1816–28. doi: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- Thompson MR, Li KM, Clemens KJ, Gurtman CG, Hunt GE, Cornish JL, McGregor IS. Chronic fluoxetine treatment partly attenuates the long-term anxiety and depressive symptoms induced by MDMA (‘Ecstasy’) in rats. Neuropsychopharmacology. 2004;29:694–704. doi: 10.1038/sj.npp.1300347. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–40. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC. World drug report. United Nations Office on Drugs and Crime; 2010. ( http://www.unodc.org/documents/data-and-analysis/WDR2010/Youth-school-amphetamines.pdf) [Google Scholar]
- Vecchiola A, Collyer P, Figueroa R, Labarca R, Bustos G, Magendzo K. Differential regulation of mu-opioid receptor mRNA in the nucleus accumbens shell and core accompanying amphetamine behavioral sensitization. Brain Res Mol Brain Res. 1999;69:1–9. doi: 10.1016/s0169-328x(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn. 2010;72:146–59. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behavioral Neurosci. 2009;123:564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–83. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Teixeira CM, Mahadevia D, Huang Y, Balsam D, Mann JJ, Gingrich JA, Ansorge MS. Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Mol Psychiatry. 2014;19:688–698. doi: 10.1038/mp.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wang Y, Tan H, Bharti V, Che Y, Wang J. Chronic treatment with mood stabilizer lithium inhibits amphetamine-induced risk taking manic-like behaviors. Neurosci letters. 2015;603:84–88. doi: 10.1016/j.neulet.2015.07.027. [DOI] [PubMed] [Google Scholar]
- Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanlon G, Rawson R, London ED. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction. 2010;105:1809–1818. doi: 10.1111/j.1360-0443.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]