Abstract
Objective
The objectives of this study were to determine the impact of in vivo reactive oxygen species (ROS) on microvascular endothelial function in obese human subjects and to determine the efficacy of an aerobic exercise intervention on alleviating obesity-associated dysfunctionality.
Approach and Results
Young, sedentary men and women were divided into lean (BMI 18–25; n=14), intermediate (BMI 28–32.5; n=13), and obese (BMI 33–40; n=15) groups. A novel microdialysis technique was utilized to detect elevated interstitial hydrogen peroxide (H2O2) and superoxide levels in the vastus lateralis of obese compared to both lean and intermediate subjects. Nutritive blood flow was monitored in the vastus lateralis via the microdialysis-ethanol technique. A decrement in acetylcholine-stimulated blood flow revealed impaired microvascular endothelial function in the obese subjects. Perfusion of apocynin, an NADPH oxidase (Nox) inhibitor, lowered (normalized) H2O2 and superoxide levels and reversed microvascular endothelial dysfunction in obese subjects. Following 8-weeks of exercise, H2O2 levels were decreased in the obese subjects and microvascular endothelial function in these subjects was restored to levels similar to lean subjects. Skeletal muscle protein expression of the Nox subunits p22phox, p47phox, and p67phox were increased in obese relative to lean subjects, where p22phox and p67phox expression was attenuated by exercise training in obese subjects.
Conclusions
This study implicates Nox as a source of excessive ROS production in skeletal muscle of obese individuals, and links excessive Nox derived ROS to microvascular endothelial dysfunction in obesity. Furthermore, aerobic exercise training proved to be an effective strategy for alleviating these maladies.
Keywords: H2O2, superoxide, microdialysis, obesity, ROS detection
INTRODUCTION
Worldwide obesity rates have risen to pandemic levels, as the number of overweight and obese individuals has recently been estimated at 2.1 billion.1 Obesity has become a major cause of mortality,2 while obesity greatly increases the relative risk of death from cardiovascular disease (CVD).3 Impaired endothelium-dependent vasodilation is a key early step in atherosclerotic progression and is predictive of future cardiovascular risk.4 Such impairments to microvascular resistance vessels lead to decreased capillary recruitment and have been shown to be exacerbated with increasing adiposity.5,6 Microvascular dysfunction is thought to contribute to the development of hypertension due to increased peripheral vascular resistance7 and progression of insulin resistance by limiting nutrient and/or insulin delivery to skeletal muscle,8 both pathologies which could further compound CVD risk. Thus, obesity may promote microvascular dysfunction and the ensuing elevated cardiovascular risk.
Oxidative stress is a systemic feature of obesity that is well-documented in clinical and experimental studies. Excessive reactive oxygen species (ROS) production can result in apoptosis and increased cellular permeability, which may promote inflammation, endothelial dysfunction, and vascular remodeling.9 NADPH oxidase (Nox), in particular, is considered a prominent source of vascular-derived ROS and is known to promote endothelial dysfunction and play a pathophysiological role in hypertension, atherosclerosis, and diabetic microvascular complications.10 Recently, we developed and validated a novel microdialysis technique to measure in vivo production of the ROS hydrogen peroxide (H2O2) and superoxide.11 We developed this technique because direct measurement of oxidative stress in humans is extremely difficult, with investigators typically relying on indirect byproducts of lipid peroxidation in plasma or urine,12 or from in vitro measurements of ROS production from excised tissue.13
Aerobic exercise training has been found to promote far-reaching health benefits in multiple organ systems that extend well beyond reducing the traditional CVD risk factors.14 Aerobic interval training (AIT), in particular, has been shown to induce improvements in aerobic capacity, endothelial function, and insulin signaling in patients with the metabolic syndrome.15 Although exercise has been shown to induce ROS generation during exercise in an intensity- and duration-dependent manner, the overall net effect of chronic exercise training tends to promote a reduced oxidative burden.14 Previous studies suggest that aerobic exercise training reduces oxidative stress and reverses endothelial dysfunction through an attenuation of Nox activity.16,17 Given the important pathological role of Nox-derived ROS, we sought to investigate the role of Nox in microvascular endothelial dysfunction in skeletal muscle of obese individuals, and to determine whether aerobic exercise training mitigates this dysfunction by modulating Nox or ROS scavenging. To accomplish these goals, we coupled our newly developed ROS measurement technique with our previously established microdialysis methodology of monitoring microvascular blood flow18,19 to simultaneously measure in vivo ROS levels and microvascular endothelial function in skeletal muscle of human subjects.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Subject characteristics
Subject characteristics are listed in Table 1. Body fat percentage was increased in the intermediate relative to the lean group (P<0.001), and the obese relative to the intermediate group (P=0.005). There were no group differences for fasting serum glucose levels; however, higher fasting insulin levels in the obese group (P=0.007) resulted in a higher HOMA-IR (P=0.014) compared to the lean group. Triglyceride levels appear higher in the intermediate than the obese group, though this trend did not reach statistical significance (P=0.265).
Table 1.
Pre-training subject characteristics and metabolic parameters.
| Lean | Intermediate | Obese | |
|---|---|---|---|
| Age (y) | 23.8±1.0 | 29.0±2.0* | 25.0±1.2 |
| Sex (M/F) | 5/9 | 7/6 | 3/12 |
| Race (AA/C) | 2/12 | 4/9 | 7/8 |
| Height (m) | 1.70±0.03 | 1.73±0.03 | 1.68±0.02 |
| Weight (kg) | 63.3±3.5 | 91.3±3.4* | 103.7±3.4*† |
| BMI (kg/m2) | 21.6±0.6 | 30.1±0.4* | 36.6±0.7*† |
| Body Fat % | 25.2±2.3 | 36.2±1.8* | 46.0±1.2*† |
| Glucose (mg/dl) | 86.1±2.4 | 91.7±1.9 | 89.7±1.8 |
| Insulin (μIU/ml) | 7.1±0.8 | 14.1±3.5 | 18.7±2.9* |
| HOMA-IR | 1.54±0.20 | 3.30±0.93 | 4.23±0.71* |
| Triglycerides (mg/dl) | 86.2±5.8 | 116±22.2 | 85.0±11.0 |
| Cholesterol (mg/dl) | 160±8.1 | 161±6.1 | 154±8.6 |
| HDL-C (mg/dl) | 57.3±3.7 | 42.8±3.8* | 46.3±2.7 |
| LDL-C (mg/dl) | 85.4±6.2 | 94.7±7.2 | 91.1±9.0 |
| VO2peak (ml/kg/min) | 35.3±1.9 | 30.3±1.6 | 22.9±1.0*† |
P<0.05 vs. Lean,
P<0.05 vs. Intermediate.
Subject characteristics before and after the exercise intervention are listed in Supplemental Table II. None of the groups lost weight, nor were there any changes in body fat percentage or fasting glucose or insulin levels. AIT did not alter blood lipid profiles in the intermediate or obese groups, although training significantly reduced total (P=0.013), and LDL (P=0.007) cholesterol levels in the lean group. AIT increased VO2peak in the intermediate (P=0.001) and obese (P<0.001) groups, though the increase in the lean group did not reach statistical significance (P=0.092).
Vascular Injury Markers
Pre-training serum markers of vascular injury are presented in Table 2. Serum concentrations of CRP (P=0.003), VCAM-1 (P=0.021), ICAM-1 (P=0.044), E-Selectin (P<0.001), and SAA (P=0.004) were higher in the obese relative to the lean group, with sICAM-3 higher in the obese relative to the intermediate group (P=0.015). There were no significant differences between the intermediate and lean groups for any of the vascular injury markers. There were no AIT-induced changes for any vascular injury marker in any group (Supplemental Table III).
Table 2.
Pre-training markers of vascular injury.
| Lean | Intermediate | Obese | |
|---|---|---|---|
| CRP (ng/ml) | 5.89±2.04 | 19.9±6.71 | 33.4±6.02* |
| VCAM-1 (ng/ml) | 1.07±0.08 | 1.10±0.13 | 1.56±0.15*† |
| ICAM-1 (ng/ml) | 0.62±0.05 | 0.79±0.19 | 1.04±0.11* |
| sICAM-3 (ng/ml) | 0.64±0.05 | 0.61±0.07 | 0.88±0.06† |
| E-Selectin (ng/ml) | 5.69±2.10 | 13.0±2.93 | 20.0±2.06* |
| P-Selectin (ng/ml) | 64.7±9.2 | 62.7±8.5 | 66.4±6.0 |
| Thrombomodulin (ng/ml) | 3.77±0.46 | 3.36±0.41 | 3.47±0.15 |
| SAA (ng/ml) | 12.4±3.8 | 16.0±4.6 | 36.0±5.8*† |
P<0.05 vs. Lean,
P<0.05 vs. Intermediate.
In vivo ROS
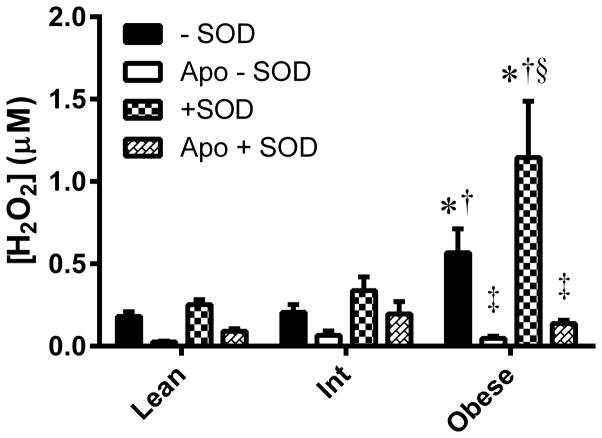
Endogenous H2O2 was elevated in the obese group compared to both the lean and intermediate groups (P<0.001; Figure 1). There were no significant differences across groups for ROS measured in the apocynin perfused probe; however apocynin significantly attenuated H2O2 only in the obese group (P<0.001). The increase in ROS signal upon addition of SOD to the perfusate, indicative of superoxide, was elevated in the obese group compared to both the lean (P=0.004) and intermediate (P=0.038) groups. The increased levels of H2O2 and superoxide in the obese group were independent of sex and race of the participants in each group.
Figure 1. In vivo NADPH oxidase-mediated ROS in skeletal muscle of lean, overweight/mildly obese (Int), and obese subjects.
ROS was measured without superoxide dismutase (−SOD) in the perfusate, indicative of H2O2 produced endogenously. ROS detected with SOD added (+SOD) to the perfusate is indicative of endogenous superoxide in addition to H2O2. ROS were also measured in the absence (−Apo) and presence of apocynin (+Apo), an NADPH oxidase inhibitor. Values are mean±SEM for n = 13–15 subjects in each group. *P<0.05 vs. lean group. †P<0.05 vs. Int group. ‡Significant effect of apocynin (P<0.05). §Effect of SOD in obese significantly (P<0.05) greater than lean and Int groups.
Microvascular Endothelial Function
Resting microvascular nutritive blood flow was not different between any groups, nor was it significantly altered by apocynin perfusion (Figure 2A). Microvascular endothelial function, tested by ACh-stimulated blood flow, was attenuated in the obese relative to the lean (P=0.016) and intermediate (P=0.044) groups (Figure 2B), which was not dependent upon the race or sex distribution of subjects in each group. Apocynin co-perfusion augmented ACh-stimulated blood flow only in the obese group (P=0.041). Endothelium-independent vasodilatory function was tested by SNP-stimulated blood flow, which was not significantly different amongst any groups (Figure 2C).
Figure 2. Association between obesity and NADPH oxidase mediated skeletal muscle microvascular endothelial function.
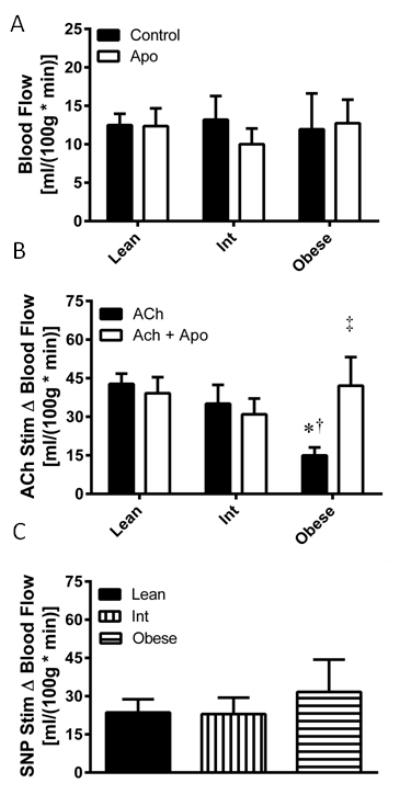
(A) Basal microvascular blood flow was assessed in the absence (control) and presence (Apo) of apocynin in lean, overweight/mildly obese (Int), and obese subjects. (B) Microvascular endothelial function was assessed as the change in blood flow from baseline (Δ Blood Flow) upon addition of acetylcholine (ACh) to the perfusate. (C) Microvascular endothelium-independent blood flow was assessed by change in blood flow from baseline (Δ Blood Flow) upon addition of sodium nitroprusside (SNP) to the perfusate. Values are mean±SEM for n = 13–15 subjects per group. *P<0.05 vs. lean group. †P<0.05 vs. Int group. ‡P<0.05 vs. ACh only condition.
Exercise Training Effects on ROS
Eight weeks of AIT decreased endogenous H2O2 in the obese group (P=0.033), while the lean and intermediate groups were unchanged (Figure 3A). However, the increase in total ROS signal upon SOD perfusion was not significantly changed by AIT in any group. Subtracting ROS concentrations measured in the apocynin perfused probe from that of the control probe revealed decreased Nox-mediated H2O2 following AIT in the obese group (P=0.019; Figure 3B). ROS detected in the apocynin perfused probe revealed no AIT-mediated changes in Nox-independent ROS (Figure 3C).
Figure 3. Effect of exercise training on NADPH oxidase-mediated ROS production.
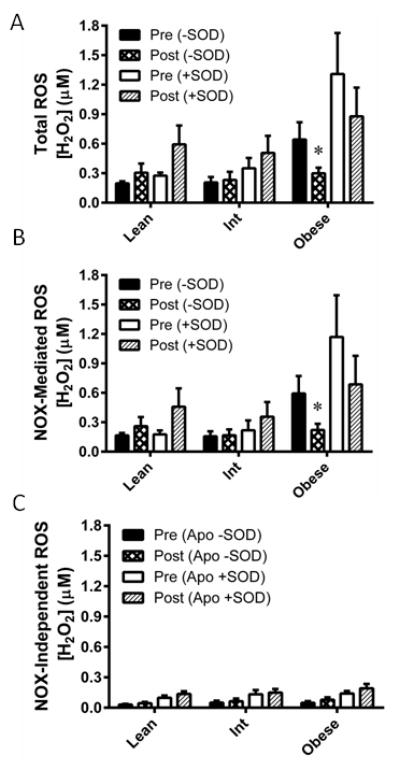
ROS were measured before (Pre) and after (Post) eight weeks of aerobic exercise training in lean, overweight/mildly obese (Int), and obese individuals. (A) ROS was measured without superoxide dismutase (−SOD) in the perfusate, indicative of H2O2 produced endogenously. ROS detected with SOD added (+SOD) to the perfusate is indicative of endogenous superoxide in addition to H2O2. (B) NADPH oxidase (NOX) mediated ROS were determined by calculation of the ROS that was inhibited by the NOX inhibitor apocynin (Apo). NOX-independent ROS were calculated as the fraction of ROS not inhibited by Apo. Values are mean±SEM for n = 9–12 subjects in each group. *P<0.05 vs. pre-training.
Exercise Training Effects on Microvascular Endothelial Function
Eight weeks of AIT did not alter resting nutritive skeletal muscle blood flow in any group (Figure 4A), nor did it affect nutritive blood flow under apocynin perfusion (Figure 4B). AIT augmented microvascular endothelial function in the obese (P=0.033), but had no effect on the lean or intermediate groups (Figure 4C). There were no AIT-induced changes in apocynin co-perfused ACh-stimulated blood flow in any group (Figure 4D). There were no AIT-mediated changes in endothelium-independent vasodilatory function in any group (Figure 4E).
Figure 4. Exercise training effects on skeletal muscle microvascular endothelial function.
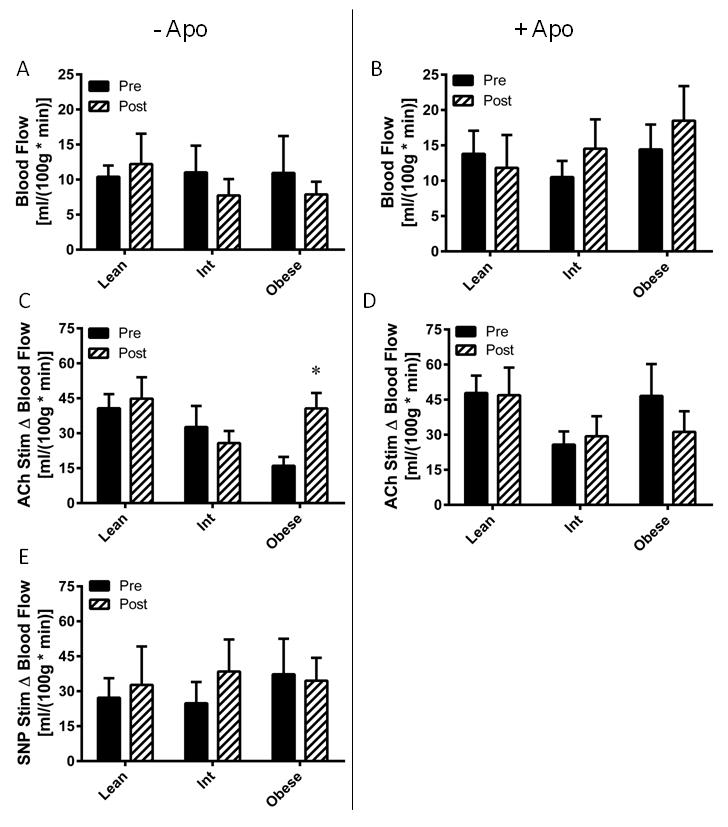
Microvascular blood flow was assessed before (Pre) and after (Post) eight weeks of aerobic exercise training in lean, overweight/mildly obese (Int), and obese subjects. (A) Basal blood flow. (B) Blood flow with apocynin perfusion. (C) Microvascular endothelial function, assessed by change in blood flow from basal (Δ Blood Flow) in response to acetylcholine perfusion. (D) Effect of NADPH oxidase on microvascular endothelial function, assessed by change in blood flow upon acetylcholine addition to the apocynin perfused probe. (E) Microvascular endothelium-independent blood flow was assessed by change in blood flow from baseline upon addition of sodium nitroprusside (SNP) to the perfusate. Values are mean±SEM for n = 9–12 subjects per group. *P<0.05 vs. pre-training.
Nox Subunit and Xanthine Oxidase Expression and Nox activity
Representative images for Western blots ran against the Nox subunits gp91phox, p22phox, p47phox, p67phox and xanthine oxidase (XO) in skeletal muscle samples from a subset of lean and obese subjects are presented in Figure 5A. Densitometry analysis revealed a trend (p=0.06) for increased gp91phox (Figure 5B) expression, and significant (P<0.05) increases in expression for p22phox (Figure 5C), p47phox (Figure 5D), and p67phox (Figure 5E) in obese relative to lean subjects. AIT significantly depressed p22phox and p67phox expression in obese subjects, although no AIT-induced changes were observed for gp91phox or p47phox expression. There were no obesity- or AIT-induced changes observed in XO expression (Figure 5F). Nox activity was significantly elevated in sedentary obese vs. lean skeletal muscle samples, where AIT induced increased Nox activity in the lean tissue, but suppressed Nox activity in obese tissue (Figure 5G).
Figure 5. Protein expression of NADPH oxidase subunits, xanthine oxidase, and NADPH oxidase activity in lean and obese skeletal muscle.
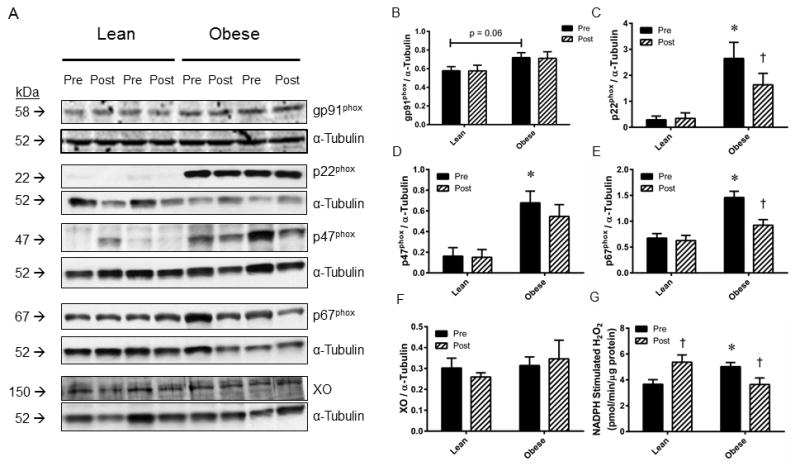
(A) Representative Western blot images for NADPH oxidase subunits gp91phox, p22phox, p47phox, p67phox, and xanthine oxidase from skeletal muscle obtained from lean and obese subjects before (Pre) and after (Post) eight weeks of aerobic exercise training. Images of α-tubulin are provided for a loading control. Densitometry analyses for (B) gp91phox, (C) p22phox, (D) p47phox, (E) p67phox, (F) xanthine oxidase expression normalized to α-tubulin. (G) NADPH-stimulated H2O2 generation was measured as an index of NADPH oxidase activity. Values are mean±SEM for n=5 subjects per group. *P<0.05 vs. lean group. †P<0.05 vs. pre-training.
DISCUSSION
In the present study, we utilized a novel microdialysis technique to demonstrate that in vivo superoxide and H2O2 production are increased in skeletal muscle of obese relative to lean or overweight/mildly obese individuals. In addition, microvascular endothelial dysfunction was evident in these obese individuals relative to both of the other groups. A role for Nox in obesity associated oxidative stress and microvascular endothelial dysfunction was confirmed by three independent experiments, in that ROS production and endothelial dysfunction were attenuated by apocynin perfusion, that immunoblot analysis demonstrated increased expression of Nox subunits gp91phox, p22phox, p47phox, and p67phox in obese skeletal muscle, and that Nox activity was increased in obese skeletal muscle samples. Finally, we demonstrated that eight weeks of AIT attenuated H2O2 levels and reversed microvascular endothelial dysfunction in obese individuals, which coincided with decreased expression of two of the four Nox subunits investigated, and decreased ex vivo Nox activity.
Superoxide is a very short lived, highly reactive molecule with well documented detrimental effects on vascular function driven by oxidative damage to lipids, proteins, and DNA, apoptosis, increased endothelial cell permeability, and quenching of NO bioavailability in the formation of peroxynitrite.9,10 H2O2, in contrast, is less reactive and thus more stable and more readily permeates membranes. H2O2 is critical for redox-based signal transduction with several properties that may influence vascular function. H2O2 activates several signaling cascades which modulate vascular function, including angiogenesis, endothelial barrier dysfunction and apoptosis, and induction of inflammatory proteins.20 Of particular interest, acute H2O2 exposure has been found to impair endothelium-dependent dilation of porcine coronary arterioles via induction of arginase activity.21 Hellsten et al.22 have previously utilized a microdialysis approach based on cytochrome c reduction to demonstrate that superoxide levels are increased by exercise in human skeletal muscle. However, the present study is the first to directly measure both H2O2 and superoxide levels in vivo in human skeletal muscle, and to demonstrate that both ROS are elevated in obese subjects.
A recent study by Walther et al.23 demonstrated impaired brachial artery endothelial function and cutaneous microvascular endothelial function in obese subjects with the metabolic syndrome. The present study extends these findings to the lower limb skeletal muscle microvasculature and adds an important role for the degree of obesity, as subjects with a BMI of 28–32.5 were free of microvascular endothelial dysfunction as opposed to subjects with a BMI of 33–40 in which microvascular endothelial dysfunction was evident. Recent large scale population-based studies have concluded that non-invasive assessment of vascular function in conduit vessels adds little predictive value to CVD risk above the traditional CVD risk factors, whereas infusion of ACh into the resistance vasculature and assessment of vascular function in the resistance vessels improves CVD risk prediction beyond the Framingham risk score.24–26 These studies highlight the applicability of stimulating endothelium-dependent dilation in the resistance vasculature for assessment of cardiovascular health. Additionally, two previous studies6,27 demonstrate that brachial artery endothelial function is augmented upon infusion of the antioxidant ascorbic acid in obese individuals, further demonstrating the detrimental nature of acute ROS production in the obese human vasculature. A stated limitation of one of these studies was that local, physiologically relevant levels of ROS were not detectable with the methods used in the study.27 The present study adds to these findings by detecting elevated in vivo H2O2 and superoxide concentrations in the obese cohort, as well as by demonstrating improved endothelial function in the resistance vasculature in this cohort by drastically attenuating ROS levels by apocynin perfusion, further implicating Nox as both a source of increased ROS and deterrent of vascular function in the resistance vasculature in the obese subjects. These findings are further corroborated by the increased skeletal muscle p22phox, p47phox, and p67phox content and ex vivo Nox activity in the obese subjects.
The finding that SNP-stimulated blood flow was not different between any of the groups indicates that the impairment of ACh-stimulated blood flow in the obese was indeed due to impaired endothelium-dependent vasodilation rather than impairment of soluble guanylate cyclase-mediated smooth muscle relaxation. Furthermore, the finding that impaired ACh-stimulated blood flow in the obese was reversed upon apocynin perfusion implicates a role for Nox-mediated ROS in skeletal muscle microvascular endothelial dysfunction in human obesity. The observation that resting microvascular blood flow was not different between groups or altered by apocynin suggests that regulation of resting microvascular blood flow is also controlled by factors in addition to ROS and NO. Nonetheless, Nox-mediated ROS appear to be significantly impacting NO production in the obese group, which can have important ramifications regarding CVD development. Nox is a likely important source of vascular ROS in human obesity, as expression of Nox subunits are elevated in venous endothelial cells obtained from obese human subjects.28 Furthermore, Nox has previously been implicated in human vascular disease, as Nox activity and subunit expression have been found to be elevated in excised arterial segments from Type II diabetic patients and coronary artery disease patients.29, 30 In addition, apocynin perfusion through microdialysis probes has previously been found to blunt local angiotensin II-mediated vasoconstriction in the human cutaneous microvasculature,31 and to restore local cutaneous vascular conductance in chronic kidney disease patients.32 Thus, the findings in the present study that apocynin delivery via microdialysis augments local microvascular function are not without precedent. Conversely, apocynin incubation has been shown to have no effect on flow-induced dilation in coronary resistance arteries from cardiopulmonary bypass patients.33 The finding that Nox-mediated ROS and microvascular endothelial function were essentially unchanged in the overweight/mildly obese intermediate BMI group relative to the lean group was unexpected and interesting. This intermediate group demonstrated normal levels of VCAM-1, ICAM-1, and sICAM-3, which were all elevated in the obese group. The discrepancy in these inflammatory and atherogenic molecules between the obese and overweight/mildly obese subjects could have long-term consequences of accelerated development of more severe vascular pathologies in the obese. However, that acute apocynin perfusion completely abrogated ROS levels and restored ACh-stimulated blood flow implicates a more profound role for Nox in driving the microvascular endothelial dysfunction observed in this obese group. It is important to note that these obese individuals were young and free of overt disease. As such, it is quite possible that more factors than ROS may be involved in regulating microvascular function in more severe disease states. These data also implicate an important role for the degree of obesity, as no functional abnormalities were observed in the overweight/mildly obese intermediate BMI group. Nonetheless, this study demonstrates a critical, acute role for Nox-mediated ROS in regulating skeletal muscle microvascular endothelial function in obese individuals.
A recent meta-analysis concluded that a large proportion of the protective effects of exercise on the vasculature occur independent of changes in the traditional CVD risk factors and are more likely explained by direct effects of exercise on the arterial wall or cellular environment.34 In the present study, we observed significant decreases in both total and Nox-mediated H2O2 in the obese skeletal muscle extracellular environment post-training. Despite no AIT-induced changes in the traditional cardiovascular risk factors, we observed augmented microvascular endothelial function in the obese group, while apocynin no longer affected endothelial function post-training. These AIT-induced changes were unique to the obese group, as neither the lean nor intermediate groups demonstrated any changes in interstitial ROS levels or microvascular function post-training. Previous studies suggest that exercise-induced improvements in endothelial function are inversely proportional to pre-training functionality.34 Given that the lean and intermediate groups demonstrated large pre-training vasodilatory responses to acetylcholine, failure to improve further was unsurprising. However, exercise-induced reversal of endothelial dysfunction through attenuation of Nox-derived ROS has been implicated in several contexts.16,35–37 Exercise training has been shown to decrease Nox activity or subunit expression in coronary arteries of diet-induced obese rodents36,37 and carotid arteries of aged rodents35, while apocynin improves endothelial function in sedentary, but not exercise trained vessels in these studies. Importantly, CAD patients that performed four weeks of aerobic exercise training prior to bypass surgery demonstrated reduced Nox activity and increased vasodilatory function in internal mammary artery segments relative to patients that remained sedentary prior to surgery.16 These findings demonstrate an ability of Nox activity to be beneficially altered in severely diseased human arteries by relatively short-term exercise training.16 The present study adds to these findings by demonstrating an ability of exercise training to act in a restorative manner and reverse endothelial dysfunction in the lower limb microvasculature of obese individuals, even those without overt vascular or metabolic disease.
The decrement in interstitial apocynin-inhibitable H2O2 induced by AIT in the obese is supported by AIT-induced decreases in p22phox and p67phox protein content and ex vivo Nox activity in skeletal muscle tissue in a subset of these subjects. Aside from Nox, there are several factors that could potentially influence interstitial ROS levels. It is quite possible that exercise training induces an upregulation in the H2O2 detoxification pathways in the obese, such as the glutathione and thioredoxin systems, catalase, and the Nrf2/Keap1 phase II antioxidant system.38 As H2O2 has a longer half-life and is much more membrane permeable than superoxide, it is very plausible that some of the H2O2 detected via microdialysis is mitochondria derived. In this context, it is also likely that exercise training in the obese subjects promotes a reduction in mitochondrial derived ROS emission, which could manifest as reduced interstitial H2O2. We have previously demonstrated that AIT attenuates mitochondrial H2O2 emission in red gastrocnemius of high-fat, high sucrose-fed rats through an upregulation of the thioredoxin system.39 In addition, Gram et al.13 recently demonstrated that exercise training attenuates mitochondrial H2O2 emission in skeletal muscle biopsy samples from subjects that had previously undergone leg immobilization. Importantly, excessive mitochondrial derived ROS has been found to promote endothelial dysfunction.40,41 There also remains a possibility that Nox-derived ROS stimulates mitochondrial ROS production.42 Mitochondrial ROS production from Nox overexpressing transgenic mice fed a high-fat diet is increased threefold over wild type mice fed the same high-fat diet.43 Thus, it remains possible that apocynin perfusion attenuates mitochondrial ROS production through inhibition of ROS-induced ROS release. In addition, synergy between Nox- and XO-derived ROS has been demonstrated, whereby Nox inhibition prevents XO activation.44 Both mitochondrial ROS and XO activity have been shown to be increased in non-diabetic obese subjects relative to lean counterparts.45,46 Regardless of the enzymatic source of ROS, we observed a decrease in skeletal muscle H2O2 and apocynin inhibitable H2O2 following exercise training in the obese group, which coincided with improved microvascular endothelial function in these individuals.
Apocynin was the only Nox inhibitor used in this study, thus all inferences made on the microvascular effects of Nox are derived from the influence of apocynin. Apocynin is reported to inhibit the assembly of p47phox and p67phox within the membrane complex, and inhibit activation of NADPH oxidase isoforms that require subunit translocation.47 However, apocynin has been reported to be both an antioxidant48 and a pro-oxidant.49 Thus, we cannot be certain that the effects of apocynin were mediated specifically through Nox inhibition. All of the ROS measurements from the apocynin perfused probe were converted from fluorescence units to [H2O2] based off of an H2O2 standard curve constructed in the apocynin containing perfusate. Thus, any possible H2O2 scavenging effect of apocynin or any possible interference of apocynin on the amplex ultrared assay would be accounted for by the standard curve. The marked inhibition of ROS by apocynin strongly suggests that apocynin does not have a pro-oxidant effect in this system. Despite the potential limitations of apocynin, it is by far the most commonly used Nox inhibitor available31,32,35,37,40, and at the concentrations employed by use in these studies, it is unrealistic to expect that any potential antioxidant effect of this compound could explain the effects observed here. A few subjects from each group dropped out of the exercise program prior to completion, thus data was not attained from these subjects for post-training measures. In addition, the muscle biopsy was an optional procedure to which only five subjects per group consented. Despite the small sample size, the marked elevation in Nox activity and expression of Nox subunits in the obese subjects conferred clear differences that were statistically significant. Furthermore, the obese subjects failed to lose weight in response to exercise training. There were no improvements in traditional cardiometabolic risk factors or inflammatory markers following exercise training, although there is evidence to suggest that weight loss is necessary for a reduction in fasting glucose, triglyceride, or cholesterol levels.50,51 High-intensity exercise has been shown to induce an acute pro-inflammatory state, which may last for up to 72 hours following intense exercise.52 Thus, any long-term anti-inflammatory effects of the exercise program could be masked by blood sampling 48 hours following the final exercise bout. It is noteworthy that exercise training altered the ROS levels, Nox activity and expression levels, and microvascular endothelial function without improvements in any of these inflammatory or traditional risk factors, which highlights the beneficial effects of exercise in the obese population, which extend well beyond these markers.
In conclusion, we demonstrate for the first time that in vivo ROS are elevated in skeletal muscle of obese human subjects. This study is the first utilization of the newly developed microdialysis approach to measure in vivo H2O2 and superoxide,11 and is the first demonstration of increased in vivo ROS in human skeletal muscle under a pathological condition. Perfusion of apocynin normalized ROS levels and reversed microvascular endothelial dysfunction in obese subjects, providing a mechanistic link between ROS and microvascular function in the in vivo setting. Additionally, AIT was proven effective at attenuating in vivo H2O2 and reversing microvascular endothelial dysfunction in the obese cohort, providing further evidence that AIT is a practical, clinically relevant means to alleviate these obesity associated maladies. Furthermore, these obese subjects were young with normal blood chemistries of the traditional clinically evaluated cardiometabolic risk factors, though were likely in an early state of CVD pathogenesis as evidenced by microvascular endothelial dysfunction. This study implicates Nox-mediated ROS as a potential target to prevent further CVD progression in obesity.
Supplementary Material
HIGHLIGHTS.
A novel technique to directly measure in vivo H2O2 and superoxide revealed increased levels of both ROS in obese human subjects relative to lean and overweight/mildly obese subjects.
Microvascular endothelial function was impaired in obese relative to lean and overweight/mildly obese subjects.
NADPH oxidase inhibition normalized H2O2 and superoxide levels and reversed endothelial dysfunction in the obese subjects.
Eight weeks of aerobic exercise training decreased H2O2 levels and improved microvascular endothelial function in the obese subjects.
Skeletal muscle NADPH oxidase subunit expression and activity were increased in obese subjects, both of which were decreased with exercise training.
Acknowledgments
Sources of Funding: This work was supported by grants from the American College of Sports Medicine Foundation (J.D.L.) and the National Institutes of Health 1R15HL113854-01A1 (R.C.H.) and 5R01HL122863 (E.J.A.). J.D.L. is supported by Ruth L. Kirschstein National Research Service Award 1F32DK100082 from the National Institutes of Health.
ABBREVIATIONS
- ACh
Acetylcholine
- AIT
Aerobic Interval Training
- Apo
Apocynin
- CRP
C-Reactive Protein
- CVD
Cardiovascular Disease
- H2O2
Hydrogen Peroxide
- HRP
Horseradish Peroxidase
- ICAM-1
Intracellular Adhesion Moledule-1
- Nox
NADPH Oxidase
- ROS
Reactive Oxygen Species
- SNP
Sodium Nitroprusside
- SAA
Serum Amyloid A
- sICAM-3
Soluble Intracellular Adhesion Molecule-3
- SOD
Superoxide Dismutase
- VCAM-1
Vascular Cell Adhesion Molecule-1
- XO
Xanthine Oxidase
Footnotes
Disclosures: None.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 4.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III-27–III-32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 5.de Jongh RT, Ijzerman RG, Serné EH, Voordouw JJ, Yudkin JS, de Wall HA, Stehouwer CD, van Weissenbruch MM. Visceral and truncal subcutaneous adipose tissue are associated with impaired capillary recruitment in healthy individuals. J Clin Endocrinol Metab. 2006;91:5100–5106. doi: 10.1210/jc.2006-1103. [DOI] [PubMed] [Google Scholar]
- 6.Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50:159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- 7.Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–740. doi: 10.1161/hc3101.091158. [DOI] [PubMed] [Google Scholar]
- 8.Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Heart Circ Physiol. 2003;284:E241–E258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 9.Weseler AR, Bast A. Oxidative stress and vascular function: implications for pharmacologic treatments. Curr Hypertens Rep. 2010;12:154–161. doi: 10.1007/s11906-010-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Favor JD, Anderson EJ, Hickner RC. Novel method for detection of reactive oxygen species in vivo in human skeletal muscle. Physiol Res. 2014;63:387–392. doi: 10.33549/physiolres.932587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho E, Karimi Galougahi K, Liu CC, Bhindi R, Figtree GA. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1:483–491. doi: 10.1016/j.redox.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gram M, Vigelsø A, Yokota T, Helge JW, Dela F, Hey-Mogensen M. Skeletal muscle mitochondrial H2O2 emission increases with immobilization and decreases after aerobic training in young and older men. J Physiol. 2015;593:4011–4027. doi: 10.1113/JP270211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology. 2013;28:330–358. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 15.Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatement for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrect R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- 17.Laufs U, Wassmann S, Czech T, Münzel T, Eisenhauer M, Böhm M, Nickenig G. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Anterioscler Thromb Vasc Biol. 2005;25:809–814. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 18.La Favor JD, Kraus RM, Carrithers JA, Roseno SL, Gavin TP, Hickner RC. Sex differences with aging in nutritive skeletal muscle blood flow: impact of exercise training, nitric oxide, and α-adrenergic-mediated mechanisms. Am J Physiol Heart Circ Physiol. 2014;307:H524–H532. doi: 10.1152/ajpheart.00247.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallgren F, Amberg G, Hickner RC, Ekelund U, Jorfeldt L, Henriksson J. A mathematical model for measuring blood flow in skeletal muscle with the microdialysis ethanol technique. J Appl Physiol. 1995;79:648–659. doi: 10.1152/jappl.1995.79.2.648. [DOI] [PubMed] [Google Scholar]
- 20.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res. 2005;96:818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 21.Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, Kuo L. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol. 2006;26:2035–2042. doi: 10.1161/01.ATV.0000233334.24805.62. [DOI] [PubMed] [Google Scholar]
- 22.Hellsten Y, Nielsen JJ, Lykkesfeldt J, Bruhn M, Silveira L, Pilegaard H, Bangsbo J. Antioxidant supplementation enhances the exercise-induced increase in mitochondrial uncoupling protein 3 and endothelial nitric oxide synthase mRNA content in human skeletal muscle. Free Radic Biol Med. 2007;43:353–361. doi: 10.1016/j.freeradbiomed.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 23.Walther G, Obert P, Dutheil F, Chapier R, Lesourd B, Naughton G, Courteix D, Vinet A. Metabolic syndrome individuals with and without type 2 diabetes mellitus present generalized vascular dysfunction: cross-sectional study. Arterioscler Thromb Vasc Biol. 2015;35:1022–1029. doi: 10.1161/ATVBAHA.114.304591. [DOI] [PubMed] [Google Scholar]
- 24.Lind L, Berglund L, Larsson A, Sundström J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation. 2011;123:1545–1551. doi: 10.1161/CIRCULATIONAHA.110.984047. [DOI] [PubMed] [Google Scholar]
- 25.Schnabel RB, Schulz A, Wild PS, Sinning CR, Wilde S, Eleftheriadis M, Herkenhoff S, Zeller T, Lubos E, Lackner KJ, Warnholtz A, Gori T, Blankenberg S, Münzel T. Noninvasive vascular function measurement in the community: cross-sectional relations and comparison of methods. Circ Cardiovasc Imaging. 2011;4:371–380. doi: 10.1161/CIRCIMAGING.110.961557. [DOI] [PubMed] [Google Scholar]
- 26.Schnabel RB, Wild PS, Schulz A, Zeller T, Sinning CR, Wilde S, Kunde J, Lubos E, Lackner KJ, Warnholtz A, Gori T, Blankenberg S, Munzel T Gutenberg Health Study Investigators. Multiple endothelial biomarkers and noninvasive vascular function in the general population: the Gutenberg Health Study. Hypertension. 2012;60:288–295. doi: 10.1161/HYPERTENSIONAHA.112.191874. [DOI] [PubMed] [Google Scholar]
- 27.Limberg JK, Harrell JW, Johansson RE, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Microvascular function in younger adults with obesity and metabolic syndrome: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2013;305:H1230–H1237. doi: 10.1152/ajpheart.00291.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47phox expression and evidence of endothelial oxidative stress. Circulation. 2007;115:627–637. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- 29.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 30.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG, Channon KM. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:333–339. doi: 10.1161/01.ATV.0000196651.64776.51. [DOI] [PubMed] [Google Scholar]
- 31.Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J Appl Physiol. 2011;111:20–26. doi: 10.1152/japplphysiol.01448.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DuPont JJ, Ramick MG, Farquhar WB, Townsend RR, Edwards DG. NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. Am J Physiol Renal Physiol. 2014;306:F1499–F1506. doi: 10.1152/ajprenal.00058.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green DJ, Eijsvogels T, Bouts YM, Maiorana AJ, Naylor LH, Scholten RR, Spaanderman MEA, Pugh CJA, Sprung VS, Schreuder T, Jones H, Cable T, Hopman MTE, Thijssen DHJ. Exercise training and artery function in humans: nonresponse and its relationship to cardiovascular risk factors. J Appl Physiol. 2014;117:345–352. doi: 10.1152/japplphysiol.00354.2014. [DOI] [PubMed] [Google Scholar]
- 35.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.La Favor JD, Anderson EJ, Dawkins JT, Hickner RC, Wingard CJ. Exercise prevents Western diet-associated erectile dysfunction and coronary artery endothelial dysfunction: response to acute apocynin and sepiapterin treatment. Am J Physiol Regul Integr Comp Physiol. 2013;305:R423–R434. doi: 10.1152/ajpregu.00049.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park Y, Booth FW, Lee S, Laye MJ, Zhang C. Physical activity opposes coronary vascular dysfunction induced during high fat feeding in mice. J Physiol. 2012;590:4255–4268. doi: 10.1113/jphysiol.2012.234856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alleman RJ, Katunga LA, Nelson MA, Brown DA, Anderson EJ. The “Goldilocks Zone” from a redox perspective-adaptive vs. deleterious responses to oxidative stress in striated muscle. Front Physiol. 2014;5:358. doi: 10.3389/fphys.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher-Wellman KH, Mattox TA, Thayne K, Katunga LA, La Favor JD, Neufer PD, Hickner RC, Wingard CJ, Anderson EJ. Novel role for thioredoxin reductase-2 in mitochondrial redox adaptations to obesogenic diet and exercise in heart and skeletal muscle. J Physiol. 2013;591:3471–3486. doi: 10.1113/jphysiol.2013.254193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 41.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youn JY, Siu KL, Lob HE, Itani H, Harrison DG, Cai H. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes. 2014;63:2344–2355. doi: 10.2337/db13-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isabelle M, Vergeade A, Moritz F, Dautréaux B, Henry JP, Lallemand F, Richard V, Mulder P, Thuillez C, Monteil C. NADPH oxidase inhibition prevents cocaine-induced up-regulation of xanthine oxidoreductase and cardiac dysfunction. J Mol Cell Cardiol. 2007;42:326–332. doi: 10.1016/j.yjmcc.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Armutcu F, Ataymen M, Atmaca H, Gurel A. Oxidative stress markers, C-reactive protein and heat shock protein 70 levels in subjects with metabolic syndrome. Clin Chem Lab Med. 2008;46:785–790. doi: 10.1515/CCLM.2008.166. [DOI] [PubMed] [Google Scholar]
- 46.Chattopadhyay M, Khemka VK, Chatterjee G, Ganguly A, Mukhopadhyay S, Chakrabarti S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Mol Cell Biochem. 2015;399:95–103. doi: 10.1007/s11010-014-2236-7. [DOI] [PubMed] [Google Scholar]
- 47.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophil by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 48.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HHHW, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 49.Castor LR, Locatelli KA, Ximenes VF. Pro-oxidant activity of apocynin radical. Free Radic Biol Med. 2010;48:1636–1643. doi: 10.1016/j.freeradbiomed.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Reid CM, Dart AM, Dewar EM, Jennings GL. Interactions between the effects of exercise and weight loss on risk factors, cariovascular haemodynamics and left ventricular structure in overweight subjects. J Hypertens. 1994;12:291–301. [PubMed] [Google Scholar]
- 51.Watkins LL, Sherwood A, Feinglos M, Hinderliter A, Babyak M, Gullette E, Waugh R, Blumenthal JA. Effects of exercise and weight loss on cardiac risk factors associated with syndrome X. Arch Intern Med. 2003;163:1889–1895. doi: 10.1001/archinte.163.16.1889. [DOI] [PubMed] [Google Scholar]
- 52.Tuan TC, Hsu TG, Fong MC, Hsu CF, Tsai KK, Lee CY, Kong CW. Deleterious effects of short-term, high-intensity exercise on immune function: evidence from leucocyte mitochondrial alterations and apoptosis. Br J Sports Med. 2008;42:11–15. doi: 10.1136/bjsm.2006.029314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.