ABSTRACT
Filter feeding shellfish can concentrate pathogenic bacteria, including Vibrio vulnificus and Vibrio parahaemolyticus, as much as 100-fold from the overlying water. These shellfish, especially clams and oysters, are often consumed raw, providing a route of entry for concentrated doses of pathogenic bacteria into the human body. The numbers of foodborne infections with these microbes are increasing, and a better understanding of the conditions that might trigger elevated concentrations of these bacteria in seafood is needed. In addition, if bacterial concentrations in water are correlated with those in shellfish, then sampling regimens could be simplified, as water samples can be more rapidly and easily obtained. After sampling of oysters and clams, either simultaneously or separately, for over 2 years, it was concluded that while Vibrio concentrations in oysters and water were related, this was not the case for levels in clams and water. When clams and oysters were collected simultaneously from the same site, the clams were found to have lower Vibrio levels than the oysters. Furthermore, the environmental parameters that were correlated with levels of Vibrio spp. in oysters and water were found to be quite different from those that were correlated with levels of Vibrio spp. in clams.
IMPORTANCE This study shows that clams are a potential source of infection in North Carolina, especially for V. parahaemolyticus. These findings also highlight the need for clam-specific environmental research to develop accurate Vibrio abundance models and to broaden the ecological understanding of clam-Vibrio interactions. This is especially relevant as foodborne Vibrio infections from clams are being reported.
KEYWORDS: clams, ecology, food, oysters, shellfish, Vibrio
INTRODUCTION
An estimated 84,000 people contract foodborne Vibrio infections each year in the United States, resulting in 500 hospitalizations and 100 deaths (1, 2). Unlike most other major foodborne bacterial pathogens, the number of cases caused by Vibrio spp. is increasing and currently is the highest since national reporting began (2, 3). While at least 12 Vibrio spp. are potentially pathogenic to humans, the two foodborne Vibrio spp. that cause the most infections and the most deaths in the United States are Vibrio parahaemolyticus and Vibrio vulnificus, respectively (2, 4).
V. vulnificus is the single most fatal foodborne pathogen in the United States, and perhaps the world (4), accounting for 95% of all U.S. seafood-related deaths, with a fatality rate approaching 50% (5). Infections resulting from ingestion typically produce symptoms such as fever, chills, nausea, abdominal pain, hypotension, and the development of secondary lesions on the extremities (5, 6). V. parahaemolyticus infections are far more common but are less severe and generally self-limiting. Infection with this species produces a variety of outcomes, with gastroenteritis representing approximately 60 to 80% of cases. Symptoms include diarrhea with cramping, nausea and vomiting, headache, chills, and low-grade fever (7, 8).
Both of these bacterial pathogens occur naturally in estuarine waters worldwide. Molluscan shellfish, such as oysters and clams, concentrate cells from the surrounding water, and efficient filter feeding ability can lead to levels of 105 CFU/g of tissue or more (9, 10). Because these concentrations are up to 100 times those of the water column, the consumption of raw or undercooked shellfish represents the primary route for contracting foodborne Vibrio infections (9, 11).
Although there are protective regulations in place in the United States to limit the risk of infection from these pathogens, the numbers of cases of vibriosis are still increasing (3, 12). Infections follow a seasonal trend, and the numbers peak in the months of late spring and early summer, corresponding to warm waters and increased abundance of vibrios (10, 13–16). However, temperature differences only partially explain the variations in concentrations seen in shellfish (10, 15, 17, 18). To reduce the number of infections, there needs to be a broader understanding of the environmental conditions that contribute to pathogenic Vibrio abundance in shellfish. Moreover, while a growing oyster-environment-Vibrio data set is being produced by the scientific community, there have been only a few investigations on the occurrence of these pathogens in clams. This is especially relevant because foodborne Vibrio infections from clams are being reported (19–21).
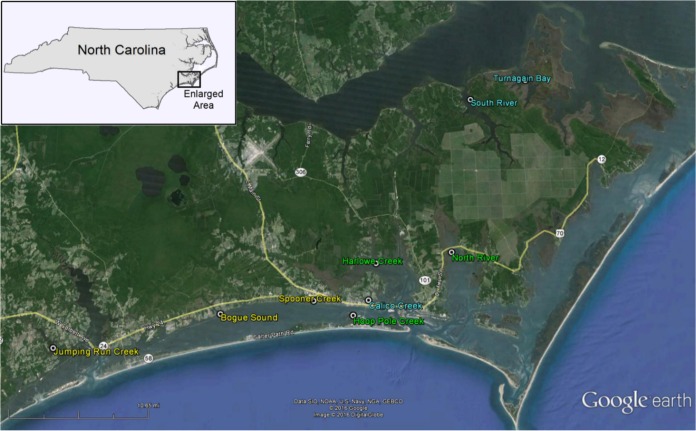
In this work, oysters and clams were collected from various sites in North Carolina (Fig. 1); several locations were habitats for both oysters and clams. It was hypothesized that, because both oysters and clams are filter feeders, the concentrations of Vibrio spp. within the shellfish would correlate with the concentrations in the water from which they were harvested. Additionally, it was hypothesized that, when oysters and clams were harvested from the same site, they would have correlating Vibrio abundances. Finally, it was hypothesized that the environmental factors that influenced Vibrio loads in oysters and clams would be similar. Thus, our investigations were designed to characterize the presence and levels of V. vulnificus and V. parahaemolyticus in water, oysters, and clams and to attempt to correlate these levels with several environmental parameters.
FIG 1.
Locations of shellfish harvest sites. The colors of the site names indicate the type of shellfish harvested. Yellow, clams; blue, oysters; green, both clams and oysters. The main map is reprinted from Google Earth; the inset map is reprinted from reference 33.
RESULTS
Comparing clams and oysters harvested from the same sites.
The concentrations of Vibrio spp., V. parahaemolyticus, and V. vulnificus in clams were significantly lower than those in oysters when the shellfish were harvested simultaneously from the same site (P = 0.0002, P = 0.0336, and P = 0.0093, respectively, by paired t test). The combined results are shown in Fig. 2. Each paired sampling event was also examined individually. For Vibrio spp., all except three samples showed lower concentrations in clams than in oysters (see Fig. S1 in the supplemental material). For V. parahaemolyticus, generally two patterns were observed (Fig. S2). In pattern 1, oysters contained V. parahaemolyticus while levels in clams were nondetectable. In pattern 2, if both types of shellfish contained V. parahaemolyticus at the same sampling event, then the concentrations in the clams were usually slightly higher than those in the oysters. There was only one paired sampling event in which V. parahaemolyticus was present in clams but not oysters. All except three clam samples collected from the same sites as oysters had nondetectable concentrations of V. vulnificus (Fig. S3), and in only one sample set did the clams have more V. vulnificus than the oysters.
FIG 2.
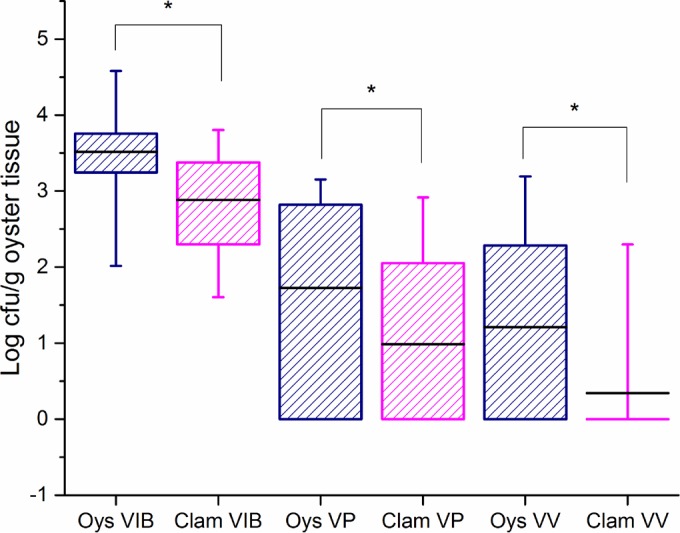
Box plots of levels of total Vibrio spp. (VIB), V. parahaemolyticus (VP), and V. vulnificus (VV) in oysters (Oys) and clams. Horizontal black lines, means; boxes, 25th to 75th percentile values; whiskers, minimum and maximum values. *, significant difference in means.
Harvest season and Vibrio risk.
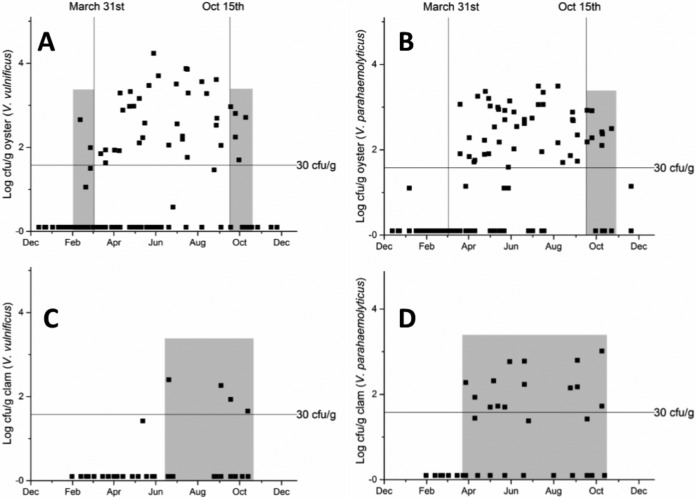
Oyster season in North Carolina is from 15 October through 31 March. Figure 3A and B show the concentrations of V. vulnificus and V. parahaemolyticus, respectively, in oysters harvested both in and out of oyster season (there is no set clam season in North Carolina). Figure 3C and D show the concentrations of V. vulnificus and V. parahaemolyticus, respectively, in clams. For both V. vulnificus and V. parahaemolyticus, the U.S. Food and Drug Administration (FDA) has determined that Vibrio concentrations of less than 30 CFU/g are sufficient for post-harvest-processed shellfish or for shellfish that are claimed to have nondetectable levels (22).
FIG 3.
Concentrations of V. vulnificus in oysters (A) and clams (C) and concentrations of V. parahaemolyticus in oysters (B) and clams (D), according to the time of year of sample collection. Vertical lines in panels A and B, the dates of the opening (15 October) and closing (31 March) of the oyster season in North Carolina. Horizontal lines, concentration of bacteria that the FDA considers nondetectable in oysters. Gray boxes, times of the year when the shellfish are in season and there are Vibrio concentrations greater than the FDA nondetectable level. The actual limit of detection was log 1 CFU/g. Values below the limit of detection were assigned a value of log 0 CFU/g.
Environmental and biological factors correlated with Vibrio abundance.
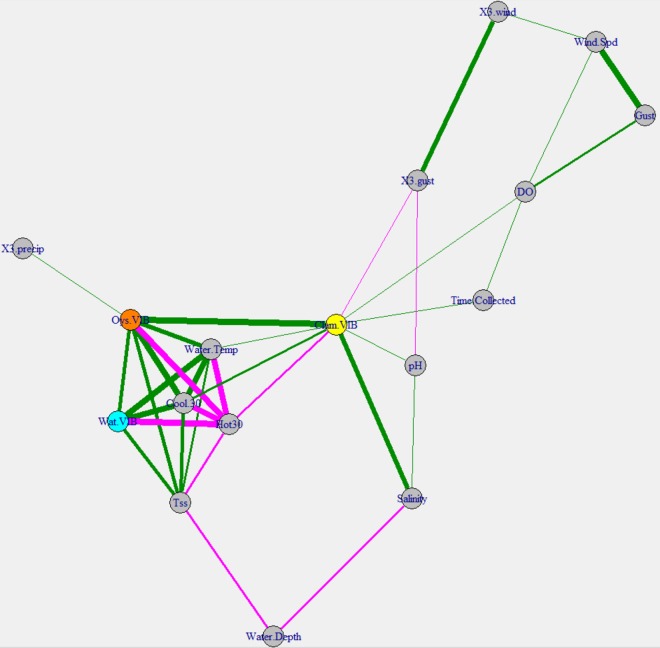
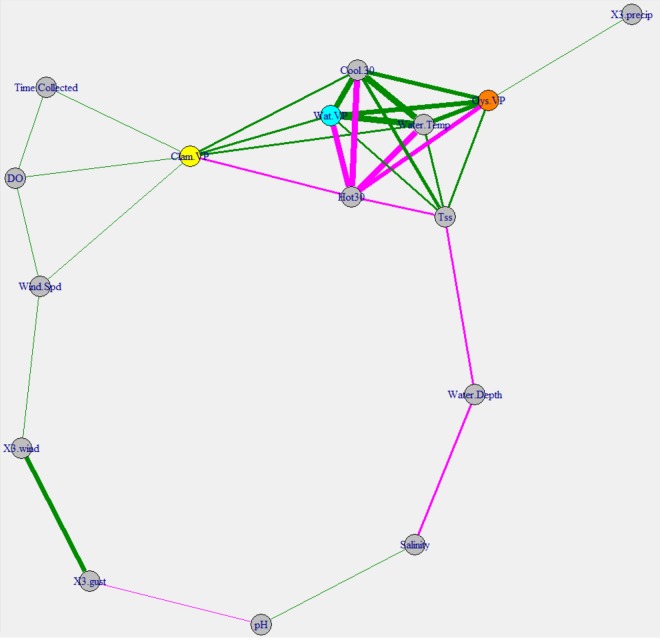
Vibrio levels in oysters were correlated with the Vibrio abundance in water; however, this was not true for Vibrio levels in clams (Fig. 4). Oyster Vibrio levels and water Vibrio levels shared all of the same environmental correlations with the exception of 3-day antecedent rainfall, with water temperature and total suspended solid (TSS) levels having some of the highest coefficients. This similarity in response to environmental conditions is demonstrated in Fig. 4 by the temperature-TSS level-oyster-water vertices clustering together. The concentrations of Vibrio in clams and oysters were significantly correlated. Water temperature was less strong in determining clam Vibrio levels than in determining oyster or water levels. Figure S4 shows that, while water temperature did appear to influence clam Vibrio levels, the levels had more variation than water or oyster levels. Nevertheless, heating degree days, which are a measure of cumulative cold days, had a tighter negative correlation with clam Vibrio levels than did the instantaneous water temperature (Fig. 4). Clam Vibrio levels were apparently affected by salinity, a factor not connected with oyster or water Vibrio levels in thus study. Further evidence of this difference is shown in Fig. S5 in the supplemental material, with increases in clam Vibrio levels being seen above 25% salinity.
FIG 4.
Correlation network map for total Vibrio spp. The network is based on Spearman's rank correlation coefficients. Vertices are connected by edges only if the correlation coefficient is greater than 0.25. Edges are colored based on positive (green) or negative (magenta) correlations. The thickness of the edges represents the correlation strength. Vertices were arranged using the Fruchterman-Reingold method, which clusters similar vertices together. Three-day antecedent precipitation values (X3.precip) were summed, while 3-day antecedent wind speed (X3.wind) and wind gust (X3.gust) values were averaged. The values for heating degree days (Hot 30) and cooling degree days (Cool 30) were obtained by summing the degree days 30 days before (and including) the collection date. Vertices representing the total Vibrio concentrations in clams (Clam.VIB), oysters (Oys.VIB), and water (Wat.VIB) are colored only for ease of viewing.
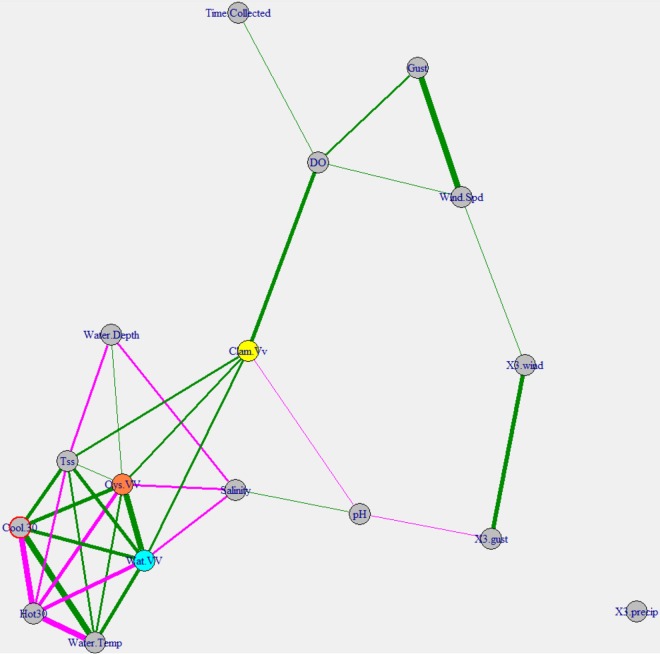
For V. vulnificus, oyster and water levels again shared nearly all correlating factors, with water depth having a small but significant correlation with V. vulnificus levels in oysters (Fig. 5). The factors included temperature and TSS levels, similar to the Vibrio cluster. Salinity was negatively correlated with V. vulnificus concentrations in water and oysters but not clams. In both water and oysters, V. vulnificus levels increased up to ∼17% salinity and then decreased above that, while clam concentrations changed very little along the salinity gradient (Fig. S6). Dissolved oxygen (DO) levels and pH were correlated with clam V. vulnificus abundance but not water or oyster levels (Fig. 5).
FIG 5.
Correlation network map for V. vulnificus. The network is based on Spearman's rank correlation coefficients. Vertices are connected by edges only if the correlation coefficient is greater than 0.25. Edges are colored based on positive (green) or negative (magenta) correlations. The thickness of the edges represents the correlation strength. Vertices were arranged using the Fruchterman-Reingold method, which clusters similar vertices together. Three-day antecedent precipitation values (X3.precip) were summed, while 3-day antecedent wind speed (X3.wind) and wind gust (X3.gust) values were averaged. The values for heating degree days (Hot 30) and cooling degree days (Cool 30) were obtained by summing the degree days 30 days before (and including) the collection date. Vertices representing the V. vulnificus concentrations in clams (Clam.Vv), oysters (Oys.VV), and water (Wat.VV) are colored only for ease of viewing.
Once again, the clam V. parahaemolyticus vertex did not cluster with those for oysters and water (Fig. 6). As with V. vulnificus, temperature and TSS levels were the most critical correlating environmental factors for oyster and water levels. Water temperature and wind velocity were weakly correlated with V. parahaemolyticus levels in clams.
FIG 6.
Correlation network map for V. parahaemolyticus. The network is based on Spearman's rank correlation coefficients. Vertices are connected by edges only if the correlation coefficient is greater than 0.25. Edges are colored based on positive (green) or negative (magenta) correlations. The thickness of the edges represents the correlation strength. Vertices were arranged using the Fruchterman-Reingold method, which clusters similar vertices together. Three-day antecedent precipitation values (X3.precip) were summed, while 3-day antecedent wind speed (X3.wind) and wind gust (X3.gust) values were averaged. The values for heating degree days (Hot 30) and cooling degree days (Cool 30) were obtained by summing the degree days 30 days before (and including) the collection date. Vertices representing the V. parahaemolyticus concentrations in clams (Clam.VP), oysters (Oys.VP), and water (Wat.VP) are colored only for ease of viewing.
DISCUSSION
There are several approaches to reducing the number of human infections caused by the seafood-borne bacteria V. vulnificus and V. parahaemolyticus. One approach is the development of easy-to-use predictive models that can provide shellfish consumers or producers with early warnings when shellfish harvested from particular sites might contain dangerous concentrations of either bacterium. To develop such models, an understanding of the biological and ecological parameters that influence bacterial abundance in shellfish is required. To that end, numerous recent studies have compared environmental conditions and Vibrio concentrations in oysters. A recent review of the topic presents data from these reports and highlights the site-to-site differences observed in these types of studies (12). Infections with these bacteria are most commonly acquired by eating raw oysters, but a significant number of infections are caused by consuming raw or undercooked clams (19–21). There are strikingly few reports on the environmental levels of these pathogens in hard clams (Mercenaria mercenaria), and such data are needed for the development of predictive models. Initially, it might be assumed that, because they are both filter feeding shellfish, clams and oysters would have correlated (if not similar) concentrations of Vibrio spp., especially when the shellfish are harvested from the same sites and growing areas. After collecting samples from three sites in eastern North Carolina where clams and oysters could be harvested simultaneously, we found that clams had significantly lower levels of V. parahaemolyticus, V. vulnificus, and Vibrio spp. than did oysters. A study by Jones et al. (23) that was conducted in Long Island Sound also found that the levels of these human pathogens, as well as Vibrio cholerae, were lower in clams than in oysters.
The finding of lower concentrations of these pathogens in clams is welcome news, suggesting that clams are less likely to result in human infections. The oyster season in North Carolina runs from 15 October to 31 March. The timing of the oyster season was established when, historically, oysters were eaten mostly in the winter months to avoid the spawning period during the summer months. The timing of the oyster season exists today because of the scarcity of oysters (S. Jenkins, North Carolina Division of Marine Fisheries, personal communication). Still, this timing likely has a strong effect in keeping the number of oyster-based Vibrio infections low. For V. vulnificus, the out-of-season period corresponds to the period when the most oysters with levels of >30 CFU/g are found in North Carolina. For V. vulnificus and V. parahaemolyticus, 30 CFU per gram of oyster is a value that the FDA considers to be below the limit of detection and therefore safe (22). It should be noted that concentrations above 30 CFU/g are not labeled unsafe but do carry increased risk of infection. In this study, only seven in-season oyster samples, which were collected in February and October/November, had levels above this limit for V. vulnificus. V. parahaemolyticus concentrations above 30 CFU/g occurred in season only in October and November, again showing the effectiveness of the oyster season regulations for reducing potential infections. It is important to note that private cultured oyster harvests are permitted in the summer off season; these shellfish pose a potential infection risk, and research is under way to understand the risk differences for farmed versus wild shellfish.
While the concentrations of Vibrio spp. were found to be lower in clams, there is no clam season in North Carolina, as clams are more abundant than oysters. While there is no FDA limit for Vibrio in clams, if the same value of 30 CFU/g that is used as a nondetectable threshold in oysters is applied, then the potential for infection appears greater. Interestingly, there were only five clam samples in this study that contained detectable V. vulnificus and the level in one of those samples was below the threshold of 30 CFU/g. Samples with levels above the threshold were found between mid-June and mid-October. The small number of V. vulnificus isolates recovered from clams could be due in part to the relatively higher salinity (>20%) of clam growing sites, considering that V. vulnificus is sensitive to high salinity. When clam and oyster samples that were collected simultaneously from the same sites were examined, however, it was evident that oysters more often contained these pathogens. Thus, the higher salinity is certainly not the sole cause, and clam biology must play a role. Nevertheless, these data suggest that V. vulnificus infections arising from clam consumption will be rare in North Carolina. The V. parahaemolyticus data tell a different story; many of the clam samples collected between mid-March and mid-October had concentrations well above 30 CFU/g, and these represent a potential health risk.
Unsurprisingly, water temperature and temperature-related factors, such as heating and cooling days, had strong significant effects on Vibrio levels in oysters and water but less so for levels in clams. For both oysters and water, Vibrio levels increased as the temperature increased, although the increases slowed at ∼22°C. Vibrio levels in clams did increase with the temperature but the data were highly varied, making the relationship less obvious. Interestingly, salinity was positively correlated with Vibrio levels in clams but not in water or oysters. This is striking, because a previous study conducted in a different body of water in North Carolina also showed a correlation between salinity and Vibrio levels in water (24). Furthermore, salinity is one of the variables most often correlated with Vibrio levels in water in other studies (25–30). The concentrations of Vibrio spp. in oysters remained mostly flat along the salinity gradient, with salt-tolerant Vibrio spp. and other bacteria likely occupying oyster matrices vacated by less-salt-tolerant species such as V. vulnificus (31, 32). In clams, Vibrio levels began increasing at ∼25% salinity; currently, we have no explanation for this observation. The temperature factor of heating degree days had a closer relationship with clam Vibrio levels than did water temperature at the time of collection. Heating degree days is a measure of how long (in days) the air temperature is below a base temperature; cooling degree days is the opposite. These values can be quickly calculated and represent simple cumulative measurements of hot or cold days. The data in this study provide evidence that lengthy series of cold days are more important (negative correlation) in determining clam Vibrio levels than are daily water temperatures.
Like Vibrio findings, clam V. vulnificus findings clustered more distantly than findings for oysters and water in the Fruchterman-Reingold layout of the correlation network map, indicating that they were less related than the others. Water temperatures and TSS levels were positively correlated with oyster and water V. vulnificus levels, while salinity was negatively correlated. For both oysters and water, the levels of V. vulnificus appeared to increase to ∼17% salinity and then began to decrease, as commonly reported (10, 31–34). Clam concentrations remained mostly stable as salinity increased. Again, the water temperature did not appear to have a significant effect on clam V. vulnificus concentrations in this study. Also, surprisingly, salinity was not related to clam bacterial concentrations. These conclusions must be considered carefully, however, as there were few clam samples with detectable V. vulnificus levels.
There were fewer V. parahaemolyticus isolates in clams than in oysters, but the clam samples with confirmed V. parahaemolyticus isolates vastly outnumbered the clam samples with confirmed V. vulnificus isolates. Again, temperatures were not as closely related to bacterial concentrations in clams as they were to those in water and oysters. TSS levels were related to oyster and water but not clam V. parahaemolyticus levels. Finally, wind-related factors were unique in being related to clam levels, perhaps suggesting that wind-driven mixing or resuspension plays a larger role in the uptake of Vibrio by clams than in that by oysters.
This study confirms and expands findings by Jones et al. (23) that clams and oysters harvested simultaneously from the same locations had different, and often uncorrelated, concentrations of Vibrio spp., including human pathogens. The concentrations of Vibrio spp., V. vulnificus, and V. parahaemolyticus in oysters and water were significantly correlated and had most of the same environmental determinates. Clam Vibrio concentrations, on the other hand, were either unrelated or only weakly related to water column Vibrio numbers. Furthermore, clam Vibrio concentrations always had influential environmental parameters that were unique (i.e., not shared by levels in oysters or water). Similarly, oyster and water levels had common correlates that clam levels did not have. These findings appear to indicate that oysters contain greater numbers of transient Vibrio organisms, which is why the levels are correlated with the Vibrio levels in the surrounding water. Clams, however, with weaker correlations with water Vibrio levels, might have a more stable Vibrio population. This stable population is further evidenced by the resistance to changes in daily water temperatures. With so many unique and unshared environmental variables contributing to oyster and clam bacterial concentrations, we hypothesize that clam biology plays a larger factor in internal Vibrio abundance than originally thought. More research is clearly needed, specifically regarding clam Vibrio uptake and depuration, for a better understanding of this interesting relationship.
MATERIALS AND METHODS
Sampling sites.
Oysters (Crassostrea virginica), clams (Mercenaria mercenaria), and water samples were collected from nine sites along the eastern North Carolina coast (Fig. 1). At Calico Creek, Turnagain Bay, and South River, only oysters were harvested. Only clams were collected at Jumping Run Creek, Bogue Sound, and Spooner Creek. Oysters and clams were collected simultaneously from Hoop Pole Creek, Harlowe Creek, and North River. Water samples were collected from all sites at each sampling event. Sites were chosen to represent a range of salinity minima and maxima, salinity fluctuations, and suitability for growth for oysters and/or clams.
Shellfish collection and processing.
Oyster samples were collected between 4 February 2013 and 11 November 2015, and clam samples were collected between 25 September 2013 and 16 October 2015; this resulted in 112 sampling events for oysters, 36 for clams, and 125 for water. At each sampling event, 5 oysters, 5 clams, or 5 oysters and 5 clams were collected, transported on ice to the laboratory, and processed within 5 h. Shellfish were shucked aseptically, and the hemolymph was drained. Meats were pooled, weighed, and diluted with sterile phosphate-buffered saline (PBS) at a 1:1 (wt/vol) ratio. Shellfish meats were blended for 10 min in a paddle blender (Fisher Scientific, Waltham, MA) at 280 rpm. Homogenates were diluted 1:10 in PBS, and 100-μl aliquots of both diluted and undiluted homogenates were plated on medium as described below.
Water collection and processing.
At each site and sampling event, water was collected simultaneously with shellfish. Water was collected in sterile 1-liter bottles, which were rinsed three times with water immediately above the shellfish. As with shellfish, water samples were placed on ice in coolers that were transported to the laboratory. A Hanna HI96822 digital refractometer (Hanna Instruments, Carrollton, TX) was used to measure salinity. Water samples of 1 to 10 ml were passed through a mixed cellulose ester filter (0.45-μm pore size; Pall, Port Washington, NY) and placed on media as described below. To determine TSS levels, water samples were vacuum filtered through predried, preweighed, 25-mm, glass microfiber filters (GE Life Sciences, Pittsburgh, PA), using a minimum of 100 ml of water or continuing until the filter was visibly discolored. Filters were placed in a drying oven until the water was evaporated, and then they were reweighed.
Measurement of environmental parameters.
Water temperature and shellfish depth were measured by hand at the time of each collection. DO levels were measured using an Orion 5 Star handheld probe (Thermo Scientific, Waltham, MA). Measurements of pH were made with a Denver Instrument UB-5 pH meter (Denver Instrument, Bohemia, NY). Data on wind velocity or gusts, precipitation, air temperature, and heating and cooling degree days were collected from individual weather stations near each sampling site. A list of the weather stations is included in Table S1 in the supplemental material. Heating and cooling degree days were determined relative to a base temperature of 18°C. Heating degree days for a particular day were calculated by determining the mean of the maximum and minimum temperatures for that day and subtracting that from the base temperature. Cooling days were calculated by subtracting the base temperature from the mean of the daily temperature. Then, the calculated degree days for individual days were summed for a particular period of time (in this study, 30 days) to determine a cumulative degree day value, representing how far and for how long the daily temperature was above or below a base temperature.
Media and growth conditions.
For total presumed Vibrio enumeration, thiosulfate-citrate-bile salts-sucrose (TCBS) agar, prepared according to the manufacturer's instructions (Becton, Dickinson, Franklin Lakes, NJ), was used. Green and yellow colonies were counted, and values were summed to determine total Vibrio abundance. CHROMagar Vibrio plates (CHROMagar, Paris, France), prepared as instructed, were used to isolate presumptive V. vulnificus (dark blue) and V. parahaemolyticus (dark purple) colonies. To grow pure cultures of each isolate, heart infusion (HI) agar plates were used (Becton, Dickinson). All media were incubated at 37°C for 24 h. After incubation, colonies on plates were counted and the data were converted to CFU per gram of shellfish or CFU per milliliter of water. This number was then multiplied by the fraction of isolates that were confirmed, using the molecular methods detailed below, to be either V. parahaemolyticus or V. vulnificus; This resulted in a presumptive value of confirmed bacterial abundance for each sample.
Molecular confirmation of isolates.
Between 5 and 25 colonies (or all colonies, if fewer than 5 were present) were isolated from each type of sample (water or shellfish) from each sampling event. Isolates were grown overnight in HI broth as pure cultures and then were boiled for 10 min. Centrifugation at 10,000 × g for 10 min separated the aqueous DNA from cellular material. Supernatants, to be used as PCR templates, were stored at −20°C until they were examined. Using the primers described by Tarr et al. (35), V. parahaemolyticus was identified on the basis of the flaE gene. Confirmation of V. vulnificus utilized the vvhA gene, with the primers described by Warner and Oliver (36).
Statistical analyses.
All data were combined, regardless of the harvest site, and Spearman correlations were determined for all factors. To simplify analysis, the correlation tables were converted into correlation matrices using the igraph package for R (37, 38). Only correlations with absolute correlation coefficients of at least 0.25 were linked with edges in the figures. Vertices were arranged using the Fruchterman-Reingold method, to ensure that related vertices were clustered together (39). The correlation coefficients and P values can be found in Tables S2 to S4.
Supplementary Material
ACKNOWLEDGMENTS
We thank everyone at the North Carolina Division of Marine Fisheries, including Shannon Jenkins, Paul Moore, Mike Millard, Timmy Moore, Phil Piner, and Steve Murphy, for assistance with oyster collection and for scientific input. We thank Mezrop Ayrepetyan, Kelsey Jesser, Justin Hart, Tiffany Williams, and Leigh Robertson for sample processing and Denene Blackwood for general assistance. Funding was provided by Agriculture and Food Research Initiative Competitive Grant 11352692 from the USDA National Institute of Food and Agriculture and by a Saltonstall-Kennedy grant from NOAA (grant no. NA14NMF270041).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02265-16.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States: major pathogens. Emerg Infect Dis 17:1–11. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foodborne Diseases Active Surveillance Network. 2016. FoodNet 2015 annual report. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 3.Newton A, Kendall M, Vugia DJ, Henao OL, Mahon BE. 2012. Increasing rates of vibriosis in the United States, 1996–2010: review of surveillance data from 2 systems. Clin Infect Dis 54(Suppl 5):S391–S395. doi: 10.1093/cid/cis243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mead PS, Slusker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RBV. 1999. Food-related illness and death in the United States. Emerg Infect Dis 5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones MK, Oliver JD. 2009. Vibrio vulnificus: disease and pathogenesis. Infect Immun 77:1723–1733. doi: 10.1128/IAI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisharat N, Agmon V, Finkelstein R, Raz R, Ben-Dror G, Lerner L, Soboh S, Colodner R, Cameron DN, Wykstra DL, Swerdlow DL, Farmer JJ III. 1999. Clinical, epidemiological, and microbiological features of Vibrio vulnificus biogroup 3 causing outbreaks of wound infection and bacteraemia in Israel. Lancet 354:1421–1424. doi: 10.1016/S0140-6736(99)02471-X. [DOI] [PubMed] [Google Scholar]
- 7.Yeung PSM, Boor KJ. 2004. Epidemiology, pathogenesis, and prevention of foodborne Vibrio parahaemolyticus infections. Foodborne Pathog Dis 1:74–88. doi: 10.1089/153531404323143594. [DOI] [PubMed] [Google Scholar]
- 8.Letchumanan V, Chan K-G, Lee L-H. 2014. Vibrio parahaemolyticus: a review on the pathogenesis, prevalence, and advance molecular identification techniques. Front Microbiol 5:705. doi: 10.3389/fmicb.2014.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DePaola A, Nordstrom JL, Bowers JC, Wells JG, Cook DW. 2003. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl Environ Microbiol 69:1521–1526. doi: 10.1128/AEM.69.3.1521-1526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motes ML, DePaola A, Cook DW, Veazey JE, Hunsucker JC, Garthright WE, Blodgett RJ, Chirtel SJ. 1998. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl Environ Microbiol 64:1459–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colwell RR. 2006. A global perspective, p 3–11. In Thompson FL, Austin B, Swings J (ed), The biology of vibrios. American Society of Microbiology, Washington, DC. [Google Scholar]
- 12.Froelich BA, Noble RT. 2016. Vibrio bacteria in raw oysters: managing risks to human health. Philos Trans R Soc B 371:20150209. doi: 10.1098/rstb.2015.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniels NA, MacKinnon L, Bishop R, Altekruse S, Ray B, Hammond RM, Thompson S, Wilson S, Bean NH, Griffin PM, Slutsker L. 2000. Vibrio parahaemolyticus infections in the United States, 1973–1998. J Infect Dis 181:1661–1666. doi: 10.1086/315459. [DOI] [PubMed] [Google Scholar]
- 14.Kaspar CW, Tamplin ML. 1993. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl Environ Microbiol 59:2425–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Givens CE, Bowers JC, DePaola A, Hollibaugh JT, Jones JL. 2014. Occurrence and distribution of Vibrio vulnificus and Vibrio parahaemolyticus: potential roles for fish, oyster, sediment and water. Lett Appl Microbiol 58:503–510. doi: 10.1111/lam.12226. [DOI] [PubMed] [Google Scholar]
- 16.Wright AC, Hill RT, Johnson JA, Roghman MC, Colwell RR, Morris JG. 1996. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl Environ Microbiol 62:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Froelich B, Oliver JD. 2013. The interactions of Vibrio vulnificus and the oyster Crassostrea virginica. Microb Ecol 65:807–816. doi: 10.1007/s00248-012-0162-3. [DOI] [PubMed] [Google Scholar]
- 18.Johnson CN, Flowers AR, Noriea NF, Zimmerman AM, Bowers JC, DePaola A, Grimes DJ. 2010. Relationships between environmental factors and pathogenic vibrios in the northern Gulf of Mexico. Appl Environ Microbiol 76:7076–7084. doi: 10.1128/AEM.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. 1999. Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters and clams harvested from Long Island Sound: Connecticut, New Jersey, and New York. MMWR Morb Mortal Wkly Rep 48:48–51. [PubMed] [Google Scholar]
- 20.Slayton RB, Newton AE, Depaola A, Jones JL, Mahon BE. 2014. Clam-associated vibriosis, USA, 1988–2010. Epidemiol Infect 142:1083–1088. doi: 10.1017/S0950268813001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace BJ, Guzewich JJ, Cambridge M, Altekruse S, Morse DL. 1999. Seafood-associated disease outbreaks in New York, 1980–1994. Am J Prev Med 17:48–54. doi: 10.1016/S0749-3797(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 22.Center for Food Safety and Applied Nutrition. 2011. Fish and fishery products hazards and controls guidance, 4th ed Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 23.Jones JL, Lüdeke CHM, Bowers JC, DeRosia-Banick K, Carey DH, Hastback W. 2014. Abundance of Vibrio cholerae, V. vulnificus, and V. parahaemolyticus in oysters (Crassostrea virginica) and clams (Mercenaria mercenaria) from Long Island Sound. Appl Environ Microbiol 80:7667–7672. doi: 10.1128/AEM.02820-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Froelich B, Bowen J, Gonzalez R, Snedeker A, Noble R. 2013. Mechanistic and statistical models of total Vibrio abundance in the Neuse River Estuary. Water Res 47:5783–5793. doi: 10.1016/j.watres.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 25.Takemura AF, Chien DM, Polz MF. 2014. Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front Microbiol 5:38. doi: 10.3389/fmicb.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wetz JJ, Blackwood AD, Fries JS, Williams ZF, Noble RT. 2008. Trends in total Vibrio spp. and Vibrio vulnificus concentrations in the eutrophic Neuse River Estuary, North Carolina, during storm events. Aquat Microb Ecol 53:141–149. doi: 10.3354/ame01223. [DOI] [Google Scholar]
- 27.Vezzulli L, Brettar I, Pezzati E, Reid PC, Colwell RR, Hofle M, Pruzzo C. 2012. Effect of global warming on Vibrio spp. in the temperate marine environment, p 7–11. In Pathogenic Vibrio spp. in Northern European Waters: international symposium, 31 May–1 June 2012 in Koblenz German Federal Institute of Hydrology, Koblenz, Germany. [Google Scholar]
- 28.Oberbeckmann S, Fuchs BM, Meiners M, Wichels A, Wiltshire KH, Gerdts G. 2012. Seasonal dynamics and modeling of a Vibrio community in coastal waters of the North Sea. Microb Ecol 63:543–551. doi: 10.1007/s00248-011-9990-9. [DOI] [PubMed] [Google Scholar]
- 29.Nigro OD, Steward GF. 2015. Differential specificity of selective culture media for enumeration of pathogenic vibrios: advantages and limitations of multi-plating methods. J Microbiol Methods 111:24–30. doi: 10.1016/j.mimet.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Turner JW, Good B, Cole D, Lipp EK. 2009. Plankton composition and environmental factors contribute to Vibrio seasonality. ISME J 3:1082–1092. doi: 10.1038/ismej.2009.50. [DOI] [PubMed] [Google Scholar]
- 31.Froelich BA, Williams TC, Noble RT, Oliver JD. 2012. Apparent loss of Vibrio vulnificus from North Carolina oysters coincides with a drought-induced increase in salinity. Appl Environ Microbiol 78:3885–3889. doi: 10.1128/AEM.07855-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staley C, Chase E, Harwood VJ. 2013. Detection and differentiation of Vibrio vulnificus and V. sinaloensis in water and oysters of a Gulf of Mexico estuary. Environ Microbiol 15:623–633. doi: 10.1111/1462-2920.12045. [DOI] [PubMed] [Google Scholar]
- 33.Froelich BA, Ayrapetyan M, Fowler P, Oliver JD, Noble RT. 2015. Development of a matrix tool for the prediction of Vibrio species in oysters harvested from North Carolina. Appl Environ Microbiol 81:1111–1119. doi: 10.1128/AEM.03206-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warner EB, Oliver JD. 2008. Population structures of two genotypes of Vibrio vulnificus in oysters (Crassostrea virginica) and seawater. Appl Environ Microbiol 74:80–85. doi: 10.1128/AEM.01434-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarr CL, Patel JS, Puhr ND, Sowers EG, Bopp CA, Strockbine NA. 2007. Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J Clin Microbiol 45:134–140. doi: 10.1128/JCM.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warner EB, Oliver JD. 2008. Multiplex PCR assay for detection and simultaneous differentiation of genotypes of Vibrio vulnificus biotype 1. Foodborne Pathog Dis 5:691–693. doi: 10.1089/fpd.2008.0120. [DOI] [PubMed] [Google Scholar]
- 37.Csardi G, Nepusz T. 2006. The igraph software package for complex network research. InterJournal Complex Syst 1695:1–9. [Google Scholar]
- 38.Ihaka R, Gentleman R. 1996. R: a language for data analysis and graphics. J Comput Graph Stat 5:299–314. doi: 10.1080/10618600.1996.10474713. [DOI] [Google Scholar]
- 39.Fruchterman TMJ, Reingold EM. 1991. Graph drawing by force-directed placement. Softw Pract Exp 21:1129–1164. doi: 10.1002/spe.4380211102. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.