Abstract
Successful treatment of aspergillosis caused by Aspergillus fumigatus is threatened by an increasing incidence of drug resistance. This situation is further complicated by the finding that strains resistant to azoles, the major antifungal drugs for aspergillosis, have been widely disseminated across the globe. To elucidate mechanisms underlying azole resistance, we identified a novel transcription factor that is required for normal azole resistance in Aspergillus fungi including A. fumigatus, Aspergillus oryzae, and Aspergillus nidulans. This fungal-specific Zn2-Cys6 type transcription factor AtrR was found to regulate expression of the genes related to ergosterol biosynthesis, including cyp51A that encodes a target protein of azoles. The atrR deletion mutant showed impaired growth under hypoxic conditions and attenuation of virulence in murine infection model for aspergillosis. These results were similar to the phenotypes for a mutant strain lacking SrbA that is also a direct regulator for the cyp51A gene. Notably, AtrR was responsible for the expression of cdr1B that encodes an ABC transporter related to azole resistance, whereas SrbA was not involved in the regulation. Chromatin immunoprecipitation assays indicated that AtrR directly bound both the cyp51A and cdr1B promoters. In the clinically isolated itraconazole resistant strain that harbors a mutant Cyp51A (G54E), deletion of the atrR gene resulted in a hypersensitivity to the azole drugs. Together, our results revealed that AtrR plays a pivotal role in a novel azole resistance mechanism by co-regulating the drug target (Cyp51A) and putative drug efflux pump (Cdr1B).
Author Summary
Better survival of chronically ill patients has produced an increased number of cases involving fungal infectious disease. This is partly due to a larger number of immunocompromised patients. Meanwhile, the catalogue of antifungal drugs remains quite limited and the appearance of resistant isolates is becoming more common. The filamentous fungus Aspergillus fumigatus is the main causative pathogen for deep-seated aspergillosis. For this fungus, resistance to azoles, which are the most commonly utilized drug for treatment of aspergillosis, has exhibited an alarming increase linked with elevated mortality. We know relatively little of the molecular details underpinning azole resistance in A. fumigatus other than alterations in the target enzyme. Here, we identified and characterized a novel transcription factor AtrR that is required for wild-type azole resistance in this fungus as well as other Aspergillus fungi, such as Aspergillus oryzae and Aspergillus nidulans. We discovered that AtrR co-regulates the target and pump, both of which are essential for azole resistance. More importantly, deletion of the atrR gene could sensitize a clinically isolated multi-azole resistant strain and exhibited a virulence defect in a mouse model. This success in overcoming an existing resistance mutation provides a new avenue for the prevention and treatment of aspergillosis.
Introduction
Aspergillosis is one of the most common infectious diseases by mold, with Aspergillus fumigatus being the most frequently causative fungus with high mortality (more than 60%). Despite the importance of this disease, approved antifungals for treatment of aspergillosis are quite limited. Together with difficulties in early diagnosis, today aspergillosis is a major threat especially for immunocompromised patients. Further worsening the outlook for successful antifungal therapy, azole resistant A. fumigatus strains have been found with increasing incidence worldwide [1,2]. Azoles are an essential antifungal drug for therapy for chronic aspergillosis. In fact, azole resistant A. fumigatus strains exhibit a poor clinical outcome [3]. Therefore it is an urgent issue to unravel the molecular mechanisms underlying azole resistance and to develop new antifungal drugs or agents that are able to reverse azole resistance.
Mutations in the cyp51A gene locus have been frequently reported in azole resistant A. fumigatus strains. This locus encodes lanosterol-α-14-demethylase, the target enzyme of azole drugs [1,3]. Inhibition of the Cyp51A enzyme results in a significant change in sterol profile in A. fumigatus cells, where ergosterol deficiency as well as accumulation of toxic intermediates is thought to cause the antifungal effect [4,5]. Several reports have revealed that G54 and M220 are hot spots for azole resistance mutations in the Cyp51A protein [6]. Resistant mutations tend to arise during prolonged treatment of chronic aspergillosis with azole drugs [3,7,8]. In addition to resistance acquired during therapy, environmentally-derived resistance mutations have emerged as a major source of resistant organisms. Azole resistant strains with a combination of a tandem repeat in the promoter region of cyp51A and amino acid mutation(s) (TR34/L98H and TR46/Y121F/T289A) have been isolated from patients regardless of azole therapy history [9–11]. This pre-acquired azole resistant strain limits the alternatives for effective drug therapy of aspergillosis. Recently emerging mutations were described worldwide and range across isolates from Europe [10–13], Asia [14–16], and North America [17].
To date, one surveillance report provided data that azole resistant strains were up to 38% of the isolates from aspergillosis patients in the Netherlands, about 90% of which are related to Cyp51A [18]. Apart from alterations in cyp51A, a mutation in hapE [19] and overexpression of cdr1B [20] or cyp51B [21] were reported to be responsible for azole resistance in a clinical setting. Importantly, cdr1B encodes an ATP-binding cassette (ABC) transporter sharing high sequence similarity with Saccharomyces cerevisiae Pdr15 and Pdr5, Candida albicans Cdr4 and Cdr1, and Candida glabrata Cdr1 and Pdh1. This shared homology is suggestive of a role of Cdr1B in drug efflux. The fact that deletion of the cdr1B resulted in azole sensitive phenotype further supported the important role in azole resistance in A. fumigatus [20,22].
The transcription factors involved in azole resistance in fungi have been intensively investigated in S. cerevisiae. The well-studied Pdr1 and its paralog, Pdr3, are zinc finger transcription factors that regulate the pleiotropic drug response in the yeast [23]. Pdr1 and Pdr3 serve as transcriptional activators and repressors by binding to pleiotropic drug response elements (PDREs) that are found in the promoter regions of target genes [24]. The targets include the ABC transporters encoded by PDR5, PDR10, and PDR15 [25].
In filamentous fungi including plant and animal pathogens, only one regulatory protein involved in azole resistance has been studied. This protein was designated SrbA and encodes a homologue of the Sterol Regulatory Element-Binding Proteins (SREBPs). The SREBP is a transcription factor with a basic Helix-Loop-Helix (bHLH) DNA-binding domain and is well conserved in mammalian cells, where it regulates cholesterol synthesis and uptake [26]. Roles of the A. fumigatus SREBP, SrbA, were recently demonstrated [27,28]. SrbA was shown to regulate expression of multiple genes related to ergosterol biosynthesis pathway, including erg3, erg24A, and erg25A. A mutant strain defective in srbA showed hypersensitivity to azoles. Further experiments revealed that SrbA exerts a direct regulation on these genes, suggesting that this factor is a central regulator of ergosterol biosynthesis in the fungus [29,30]. Importantly, the srbA mutant was unable to grow under hypoxic condition. This is because SREBPs sense sterol level in the cells as an indirect indicator of oxygen. The srbA mutant was unable to adapt to hypoxic conditions that the fungi are thought to encounter at infection sites. This inability of the mutant to grow under hypoxia is also thought to lead to a strong attenuation in virulence in a mouse infection model. Whereas Candida species have no obvious SREBP orthologs, Cryptococcus neoformans has a SREBP, Sre1, which is also involved in azole sensitivity, hypoxia growth, and virulence [31,32]. These findings highlighted that ergosterol biosynthesis is crucial for not only drug resistance but also for adaptation to hypoxia and virulence. Besides SREBPs, however, current knowledge about the transcription factors responsible for azole resistance and sterol biosynthesis is limited particularly in pathogenic fungi.
In the present study, we identified a novel transcription factor involved in azole resistance mechanism in Aspergillus fungi. From a genetic screen in Aspergillus oryzae designed to identify transcriptional regulators of azole drug resistance, we found a novel Gal4-type Zn2-Cys6 zinc finger domain-containing transcription factor designated AtrR. Deletion of the atrR gene in A. oryzae and Aspergillus nidulans resulted in hypersensitivity to azole drugs. Similarly, deletion of the atrR gene in A. fumigatus resulted in hypersensitivity to azole antifungals, inability to grow under hypoxic conditions, attenuated virulence, and decreased expression of cyp51A as well as the ABC transporter-encoding gene cdr1B. Even in a clinical azole resistant strain (cyp51A G54E), deletion of atrR led to an azole-hypersensitive phenotype. All our data indicate that AtrR plays an essential role in azole resistance in A. fumigatus. Importantly, this is the first identification of a regulatory protein coordinating expression of not only an azole drug target (Cyp51A) but also for the putative drug efflux transporter (Cdr1B).
Results
Identification of Aspergillus oryzae AtrR transcription factor responsible for azole resistance
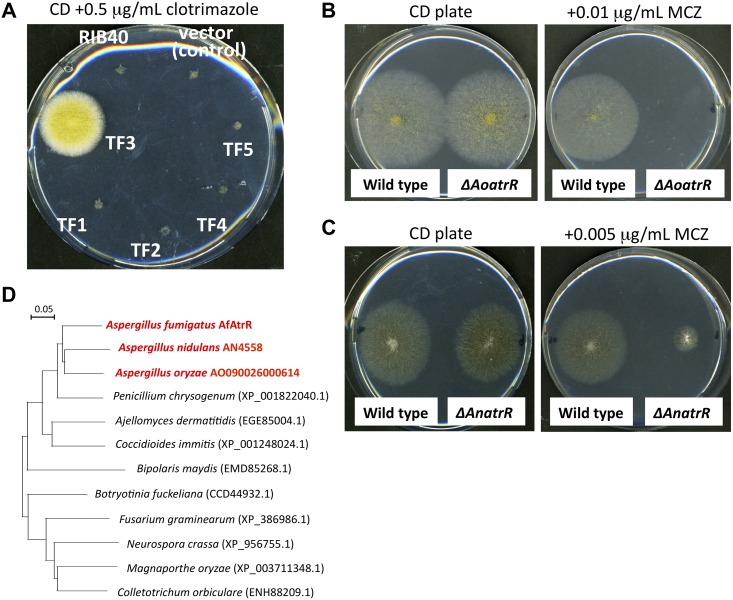
To identify the transcription factors (TFs) responsible for azole resistance in filamentous fungi, we utilized a transcription factor-overexpressing library of Aspergillus oryzae that was previously constructed in the Noda Institute for Scientific Research [33] First, we searched for A. oryzae transcription factors with a DNA-binding domain related to S. cerevisiae Pdr1 and Pdr3, resulting in 5 candidate proteins (designated TF1 to TF5) (S1A Fig). To evaluate a role of these TFs in azole resistance, strains overexpressing each TF were tested for growth in the presence of azoles. Only A. oryzae strain expressing TF3 showed a significant hyper-resistance to clotrimazole (Fig 1A). TF3 corresponds to AO090026000614 which is a protein with 894 amino acids containing two characteristic motifs designated as GAL4-like Zn(II)2/Cys6 binuclear cluster DNA-binding domains (IPR001138) and a fungal specific transcription factor domain (IPR007219) (S1B Fig). Based on the screening design to find transcription factors homologous to ScPdr1 and/or ScPdr3 that regulate ABC transporter gene expression, we named this novel TF AoAtrR (A. oryzae ABC-transporter regulating transcription factor). To further characterize the role of AoAtrR, the corresponding gene was deleted in A. oryzae host strain (RIB40) (S2A and S2B Fig), and azole resistance was examined. The AoatrR mutant showed hyper-sensitivity in the presence of 0.01 μg/mL miconazole, whereas the host strain was able to grow under this condition (Fig 1B). These results indicated that AoAtrR is involved in azole resistance in A. oryzae. The closely related species Aspergillus nidulans has an orthologous protein (designated AnAtrR; amino acids sequence identity: 79.9%) (S1B Fig), and the AnatrR deletion mutant strain (S2C and S2D Fig) also clearly exhibited azole sensitive phenotype (Fig 1C). As a result, the AtrR-family protein appears to be a novel TF responsible for azole resistance mechanisms in Aspergillus species. This AtrR-family TF is widely conserved in filamentous fungi including important plant pathogenic fungi (Fusarium graminearum, Magnaporthe oryzae, and Collectotrichum orbiculare) and human pathogenic fungi (Aspergillus fumigatus, Ajellomyces dermatitidis, and Coccidioides immitis) (Fig 1D).
Fig 1. Identification of AtrR-family genes in A. oryzae and A. nidulans.
(A) Growth of A. oryzae strains overexpressing the candidate genes on medium plate in the presence of clotrimazole. 102 conidia of the strains were inoculated on CD plate (1% maltose) containing 0.5 μg/ml clotrimazole and incubate at 30°C for 7 days. (B) Growth of the AoatrR deletion strain on medium plate in the presence of miconazole. 102 conidia of A. oryzae LigD (wild type) and the AoatrR deletion mutant (ΔAoatrR) were inoculated on CD plate (1% glucose) containing 0.01 μg/ml miconazole (MCZ) and incubate at 30°C for 5 days. (C) Growth of the AnatrR deletion strain on medium plate in the presence of MCZ. 102 conidia of A. nidulans KU70 (wild type) and the AnatrR deletion mutant (ΔAnatrR) were inoculated on CD plate (1% glucose) containing 0.005 μg/ml miconazole and incubate at 37°C for 5 days. (D) Phylogenetic tree of fungal AtrR ortholog proteins. The protein sequences were retrieved from NCBI database, and the sequence IDs were shown in brackets. The sequences were aligned by Clustal W program and the distances were calculated. The phylogenetic tree was drawn with an NJplot program.
AtrR is involved in azole resistance of A. fumigatus
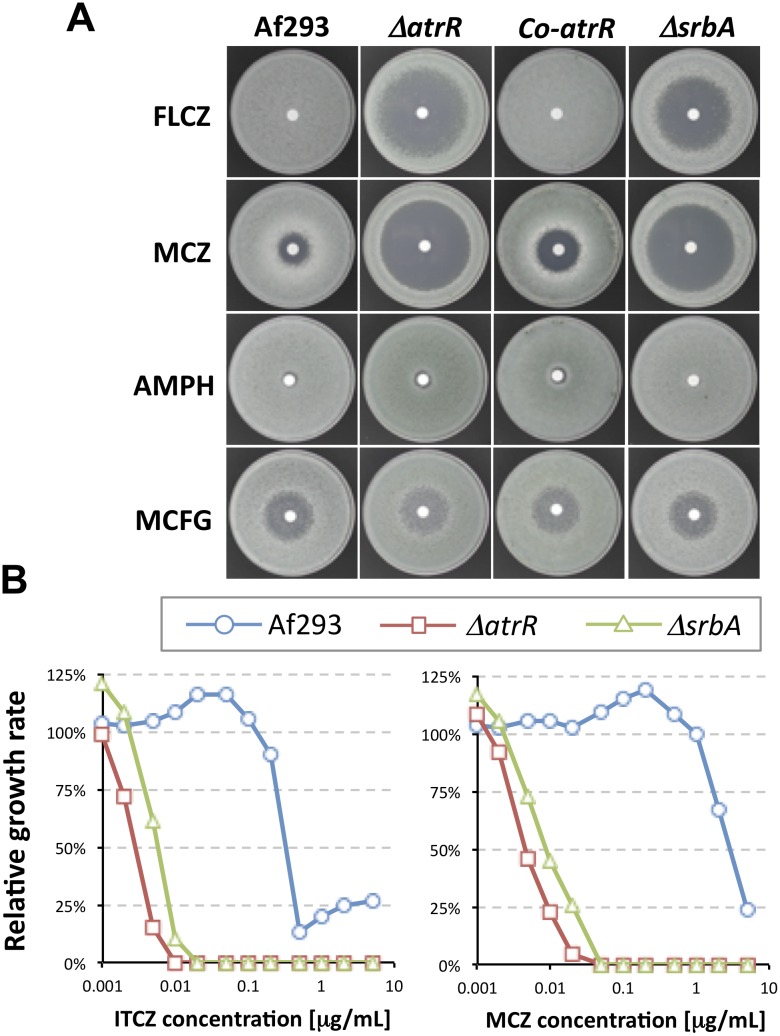
To expand our understanding of molecular mechanism underlying azole resistance with clinically important fungi, we searched for an AtrR homologue in the human pathogenic fungus A. fumigatus. The AfAtrR in A. fumigatus (hereafter, designated AtrR to simplify descriptions) is homologous to AoAtrR and AnAtrR, and the deletion mutant and the complementing strains (designated ΔatrR and Co-atrR) were constructed in the A. fumigatus host strain (Af293) (S3 Fig). A disc-diffusion assay revealed that ΔatrR was hypersensitive to fluconazole and miconazole, whereas the mutant showed no distinguishable sensitivity to amphotericin B and micafungin (Fig 2A). In addition to the medical azoles, ΔatrR showed hypersensitivity to widely used azole fungicides, bromuconazole, tebuconazole, difenoconazole, and propiconazole (S4 Fig). Growth inhibition assay with varied concentrations of drugs in plate media indicated that ΔatrR was highly susceptible to itraconazole and miconazole (Fig 2B). The complemented strain showed comparable drug sensitivity to the WT, indicating that these phenotypes were caused by deletion of the atrR gene.
Fig 2. Drug susceptibility of A. fumigatus strains linked to modifications of the atrR and srbA genes.
(A) Antifungal drug susceptibility test by paper-disc diffusion assay. GMM plates containing conidia of each strain were prepared. A paper-disc was placed on center of the plate, and 10 μl of drug solution indicated was dropped on it (fluconazole (FLCZ): 10 mg/mL; MCZ: 10 mg/mL; amphotericin B (AMPH): 0.25 mg/mL; micafungin (MCFG): 0.02 mg/mL). The plates were incubated for 48 h before photographed. (B) Inhibition test for colony growth by antifungal drugs. The conidia of each strain were center-inoculated onto GMM plates containing indicated concentrations of itraconazole (ITCZ) or MCZ. The plates were incubated for 70 h. The colony diameter was measured in triplicates, and the mean values for plates with drugs were compared with those without drugs. The percentage of growth was plotted in the graphs.
AtrR regulates cyp51A expression
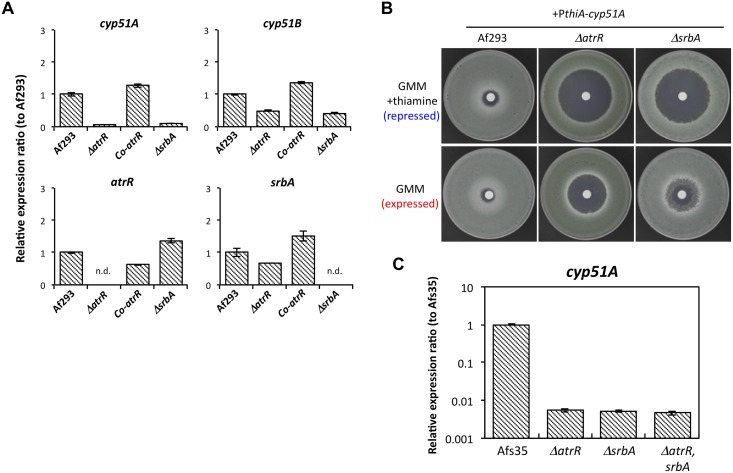
We assumed that hypersensitivity to azoles in ΔatrR was associated with a defect in expression of the target protein Cyp51A. In fact, the expression level of cyp51A gene was quite low in the ΔatrR strain (less than 5% of that in the WT), whereas cyp51B expression was also reduced (approximately 50% of the WT) (Fig 3A). Since SrbA was previously characterized to regulate cyp51A [29], we also constructed a null mutant of srbA and compared the two mutants (ΔatrR and ΔsrbA) for their relative effects on cyp51A and cyp51B expression levels. We found that the expression levels of cyp51A and cyp51B were largely comparable between ΔsrbA and ΔatrR (Fig 3A). Although not large, there was a slight increase in expression level for srbA in ΔatrR mutant and a slight decrease in atrR expression in ΔsrbA, suggesting effects of AtrR and SrbA on each other’s mRNA expression (Fig 3A). To determine if the heterologous expression of cyp51A was sufficient to restore azole resistance in mutants lacking either atrR or srbA, we introduced an ectopic cyp51A gene regulated by the thiA promoter (PthiA-cyp51A) into the ΔatrR and ΔsrbA backgrounds. When the thiA promoter was maximally active (absence of thiamine), the resulting expression level of Cyp51A was able to partially suppress loss of either transcription factor gene in both strains. ΔatrR+PthiA-cyp51A and ΔsrbA+PthiA-cyp51A showed smaller inhibition halos against miconazole compared to those under the repressed condition (presence of thiamine) (Fig 3B). This suggested that azole susceptibility in ΔatrR and ΔsrbA are partly a result of decreased cyp51A expression.
Fig 3. Expression analysis in atrR and srbA deletion mutants.
(A) Expression levels of the cyp51A and cyp51B as well as the atrR and srbA genes were determined by real-time RT-PCR method. The strains were cultivated in GMM for 24 h. The expression levels of gene of interest were normalized with that of actin gene. Relative expression ratios against Af293 (WT) are shown. Error bars represent the standard deviations based on three independent replicates. (B) MCZ susceptibility test for the Af293, ΔatrR, and ΔsrbA strains carrying a cyp51A gene fused with a thiA promoter. MCZ susceptibility was examined as described above. When thiamine was present, expression of the functional cyp51A mRNA driven by thiA promoter was repressed. The plates were incubated for 48 h before photographed. (C) Expression levels of cyp51A were determined by real-time RT-PCR in the mutant strains of ΔatrR, ΔsrbA, ΔatrR ΔsrbA of Afs35-background. The strains were cultivated in YGMM for 18 h.
Hypersensitivities to fluconazole, miconazole, and itraconazole were also seen in the ΔsrbA strain (Fig 2A and 2B). The ΔatrR strain was slightly more sensitive to fluconazole and itraconazole compared to a mutant lacking the srbA gene. However, both of these factors are required for normal azole resistance in A. fumigatus. In order to confirm the relative role of these genes across strains, these mutations were produced in a different genetic background (Afs35). The double ΔatrR ΔsrbA mutation was also produced in this same strain. The expression level of cyp51A was comparable between the ΔatrR, ΔsrbA, as well as ΔatrR ΔsrbA, supporting the required function of both AtrR and SrbA (Fig 3C).
AtrR regulates genes related to ergosterol biosynthesis
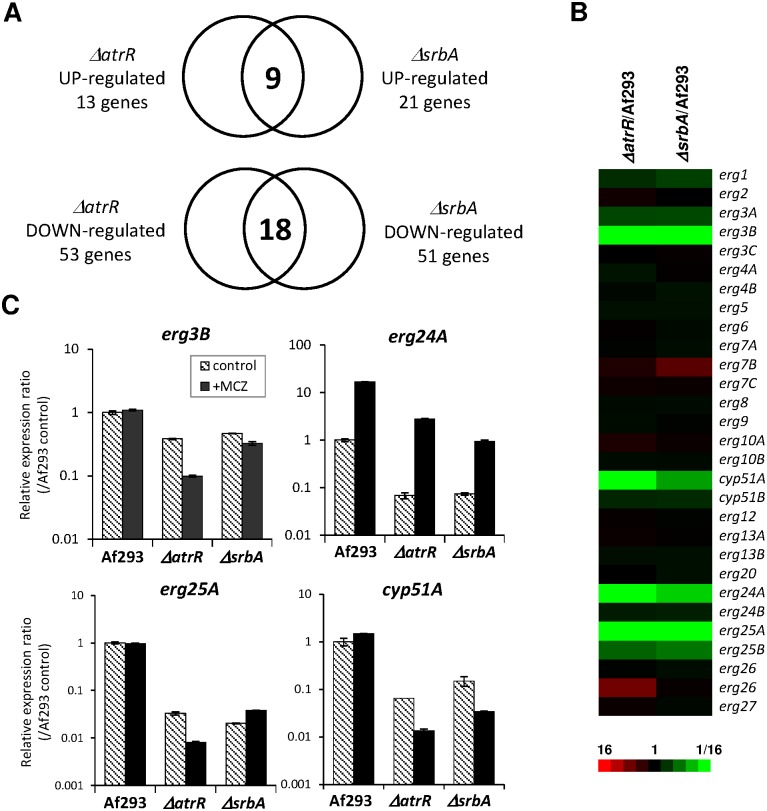
To globally identify the genes responsive to AtrR, we performed transcriptome analysis using RNA-sequencing. Compared to the WT, 13 and 53 genes were up-regulated and down-regulated, respectively, in the ΔatrR strain, showing AtrR-dependent genes. We also determined SrbA-dependent genes in a similar fashion, resulting in 21 up-regulated genes and 51 down-regulated genes. Comparing each group of up- and down-regulated genes between the ΔatrR and ΔsrbA strains, we found that 9 genes were commonly negatively regulated by AtrR and SrbA whereas 18 genes were positively regulated (Fig 4A, Table 1, S1 and S2 Tables). This comparative transcriptome analysis revealed that AtrR and SrbA were required for the expression of erg3B, erg24A, and erg25A as well as cyp51A genes, all of which are related to ergosterol biosynthesis. Interestingly, the other genes related to ergosterol biosynthesis were only modestly affected by deletion of either atrR or srbA genes (Fig 4B). Involvement of AtrR and SrbA in the transcriptional regulation of the erg3B, erg24A, erg25A, and cyp51A genes was further verified by real-time RT PCR analysis (Fig 4C). As a previous report demonstrated that SrbA regulates genes related to the ergosterol biosynthesis pathway [30], our data indicate that AtrR also functions in regulating this pathway.
Fig 4. AtrR and SrbA regulate genes associated with the ergosterol biosynthesis pathway.
(A) The AtrR- and SrbA-dependent genes determined by RNA-sequencing analysis. Numbers of gene with more than 4-fold or less than 1/4-fold change in expression level compared to Af293 are shown. The expression level of nine genes in ΔatrR and ΔsrbA strains was more than 4-fold of Af293, and the expression level of eighteen genes in ΔatrR and ΔsrbA strains was less than 1/4-fold of Af293. (B) Heat map of expression of the erg genes. The levels of expression ratio were shown. (C) Expression levels of erg3B, erg24A, erg25A, and cyp51A genes in response to MCZ addition were determined by real-time RT PCR method. The strains were cultivated in GMM for 24 h followed by 2 h culture with MCZ (final concentration, 2 μg/mL) or without MCZ (control). The expression levels of gene of interest were normalized with that of actin gene. Relative expression ratios against Af293 (WT without MCZ) are shown. Error bars represent the standard deviations based on three independent replicates.
Table 1. RNA-sequencing expression data for the genes regulated by AtrR and SrbA.
| RPKM | Expression ratio | |||||||
|---|---|---|---|---|---|---|---|---|
| ID | Gene name | Af293 | ΔatrR | ΔsrbA | ΔatrR/Af293 | ΔsrbA/Af293 | Annotation | |
| UP in ΔatrR and ΔsrbA | ||||||||
| Afu1g03800 | 13.8 | 83.1 | 205.3 | 6.01 | 14.84 | C6 transcription factor, putative | ||
| Afu2g05060 | aoxA | 474.7 | 4255.6 | 6685.0 | 8.97 | 14.08 | alternative oxidase AlxA, putative | |
| Afu3g03280 | 46.7 | 324.7 | 542.2 | 6.95 | 11.60 | FAD binding monooxygenase, putative | ||
| Afu3g13010 | 52.5 | 390.8 | 567.2 | 7.44 | 10.80 | Zn-dependent hydrolase/oxidoreductase family protein, putative | ||
| Afu4g09450 | 24.0 | 97.0 | 171.1 | 4.04 | 7.13 | hypothetical protein | ||
| Afu4g14380 | 203.2 | 1313.3 | 1894.0 | 6.46 | 9.32 | hypothetical protein | ||
| Afu5g06070 | mdr1 | 11.9 | 65.9 | 108.2 | 5.53 | 9.09 | ABC multidrug transporter Mdr1 | |
| Afu7g00950 | 10.3 | 60.5 | 77.7 | 5.87 | 7.54 | MFS monosaccharide transporter, putative | ||
| Afu8g01860 | 30.6 | 163.0 | 218.8 | 5.32 | 7.15 | hypothetical protein | ||
| DOWN in ΔatrR and ΔsrbA | ||||||||
| Afu1g03150 | erg24A | 25.5 | 1.1 | 2.5 | 0.04 | 0.10 | c-14 sterol reductase | |
| Afu2g00320 | erg3B | 116.8 | 0.4 | 0.8 | 0.00 | 0.01 | sterol delta 5,6-desaturase, putative | |
| Afu2g00330 | 10.5 | 1.5 | 0.6 | 0.15 | 0.06 | beta-alanine synthase, putative | ||
| Afu2g17660 | 11.7 | 1.5 | 0.8 | 0.13 | 0.07 | C4-dicarboxylate transporter/malic acid transport protein, putative | ||
| Afu3g00810 | hyd1 | 39.7 | 1.2 | 0.0 | 0.03 | 0.00 | cholestenol delta-isomerase, putative | |
| Afu3g00820 | 14.5 | 0.3 | 1.0 | 0.02 | 0.07 | conserved hypothetical protein | ||
| Afu4g06890 | cyp51A | 261.4 | 14.9 | 40.2 | 0.06 | 0.15 | 14-alpha sterol demethylase Cyp51A | |
| Afu5g01248 | 24.5 | 0.8 | 0.8 | 0.03 | 0.03 | conserved hypothetical protein | ||
| Afu5g14740 | fleA | 91.6 | 9.3 | 6.7 | 0.10 | 0.07 | fucose-specific lectin FleA | |
| Afu7g01490 | ptr2 | 149.7 | 23.4 | 27.8 | 0.16 | 0.19 | MFS peptide transporter Ptr2, putative | |
| Afu704920 | 138.3 | 20.1 | 16.6 | 0.15 | 0.12 | hypothetical protein | ||
| Afu7g04930 | pr1 | 26.7 | 0.7 | 2.2 | 0.03 | 0.08 | alkaline serine protease (PR1)/allergen F18-like | |
| Afu7g06981 | 13.0 | 1.5 | 2.5 | 0.12 | 0.19 | conserved hypothetical protein | ||
| Afu8g00830 | 135.7 | 13.2 | 23.9 | 0.10 | 0.18 | conserved hypothetical protein | ||
| Afu8g01330 | 10.2 | 0.6 | 1.8 | 0.06 | 0.18 | hypothetical protein | ||
| Afu8g02440 | erg25A | 1345.1 | 84.5 | 40.5 | 0.06 | 0.03 | C-4 methyl sterol oxidase, putative | |
| Afu8g06000 | 18.2 | 4.3 | 4.3 | 0.24 | 0.24 | FMN dependent dehydrogenase, putative | ||
| Afu8g06010 | 36.8 | 6.4 | 8.8 | 0.17 | 0.24 | C6 transcription factor, putative | ||
AtrR is necessary for hypoxia adaptation and virulence
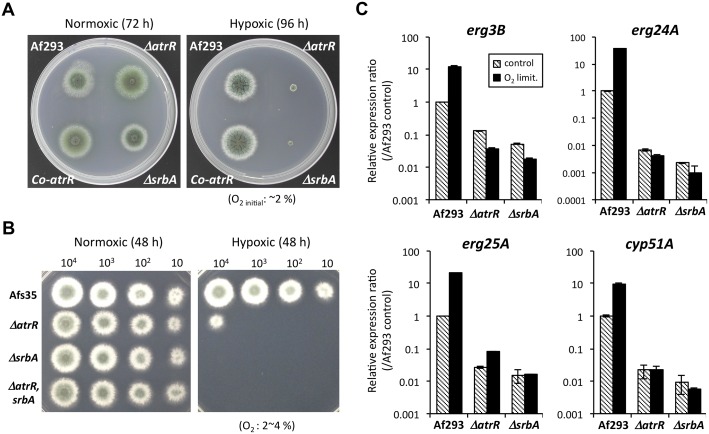
As both AtrR and SrbA have a role in transcriptional regulation of the ergosterol biosynthesis pathway, we wanted to assess if AtrR also contributed to hypoxic growth and virulence as previously shown for SrbA [27,28]. We tested growth under hypoxic conditions and found that growth of both ΔatrR and ΔsrbA mutants, constructed in either the Af293 or Afs35 genetic backgrounds, was strongly inhibited under this condition (Fig 5A and 5B). These results indicate a crucial role for AtrR in adaptation to hypoxia. To confirm the participation of AtrR in the hypoxic response, the transcriptional regulation of erg3B, erg24A, erg25A, and cyp51A upon oxygen limitation (1 h) was compared in ΔatrR and ΔsrbA strains. The induction of these genes upon oxygen limitation was diminished or eliminated in the both mutants (Fig 5C). These results indicated that AtrR also plays an important role in hypoxia adaptation in A. fumigatus physiology as found for SrbA.
Fig 5. AtrR is involved in adaptation to hypoxia.
(A) Growth test under normoxic and hypoxic conditions. The conidia of each strain were inoculated on GMM plate, and the plate was incubated in anaeropack system (a sealed pack) where initial concentration of oxygen was set at 2% (hypoxia condition). After 96 h, oxygen in the pack appeared to be completely consumed as estimated from no more growth of all colonies on the plate. The plate for normoxia was incubated in a regular incubator (21% oxygen) for 72 h. (B) Growth of Afs35-background strains under normoxic and hypoxic conditions. The 10 to 104 conidia of each strain were inoculated on GMM plate, and the plate was incubated in anaeropack system (a sealed pack) for 48 h, where concentration of oxygen was maintained at 2 to 4% (moderate hypoxia condition). (C) Expression levels of erg3B, erg24A, erg25A, and cyp51A genes in response to oxygen limitation (O2 limit.) were determined by real-time RT PCR method. The strains were cultivated in YGMM for 18 h. A half potion of the culture was transferred into a 50 mL tube with a screwed cap. The tube was closed with a cap carefully eliminating air, and was incubated for another 1 h. The expression levels of gene of interest were normalized with that of actin gene. Relative expression ratios against Af293 (WT before oxygen limitation) are shown. Error bars represent the standard deviations based on three independent replicates.
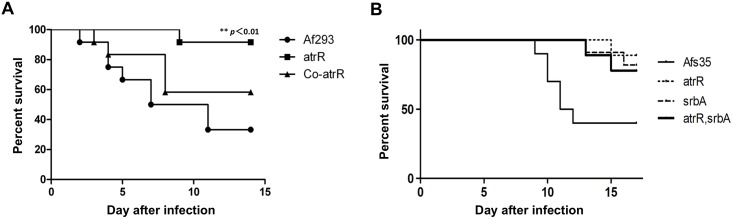
We also examined the role of AtrR in pathogenesis using a murine infection model. In this model for aspergillosis, virulence of the ΔatrR strain was significantly reduced compared to the WT and Co-atrR strains (Fig 6A). The reduction in virulence was also seen in the Afs35-background ΔatrR as well as ΔsrbA and ΔatrR ΔsrbA (Fig 6B). These results indicated that AtrR was required for full pathogenicity of A. fumigatus. This is again reminiscent of the role of SrbA in virulence, where ΔsrbA showed significantly attenuated virulence in immune-compromised mice [27].
Fig 6. AtrR is involved in A. fumigatus virulence.
(A) Mouse infection test with the Af293-background strains. Outbred ICR mice (n = 12) were immune-compromised by intraperitoneally injected cyclophosphamide (200 mg/kg) at Day -4, -2, 1, 3, 5, and 7. Mice were infected intratracheally with 2.5×107 conidia in a volume of 25 μL of each strain (Af293, ΔatrR, and Co-atrR). Statistical significance was examined by log-rank test. P value for comparison between ΔatrR and Af293 was lower than 0.01. (B) Mouse infection test with the Afs35-background strains. Outbred ICR mice (n = 9 to 11) were immune-compromised by intraperitoneally injected cyclophosphamide (150 mg/kg) at Day -4, -1, 3, 6, 9, and 12. Cortisone acetate was also administrated subcutaneously at a concentration of 200 mg per kg of body weight on day -1. Mice were infected intratracheally with 3×105 conidia in a volume of 30 μL of each strain (Afs35, ΔatrR, ΔsrbA, and ΔatrR ΔsrbA) on Day 0.
AtrR is a regulator of ABC transporter gene cdr1B
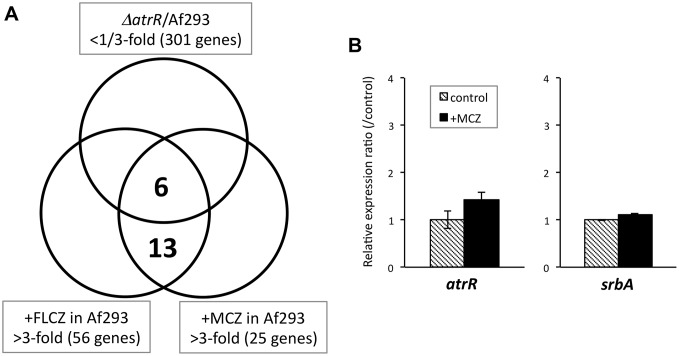
To determine the role of AtrR in drug-induced gene expression in the A. fumigatus azole response, we performed further RNA-sequencing analysis following azole challenges. WT and ΔatrR were cultivated in PDB for 20 h, and then fluconazole (final concentration, 64 μg/mL) or miconazole (2 μg/mL) were added. After 2 h, the mycelia were harvested, and the transcriptomes were determined by RNA-sequencing. Of 7015 genes that showed >10 reads per kilobase of exon per million mapped reads (RPKM) in WT without drug treatment, 301 genes were reduced to <33% in ΔatrR compared to the WT (Fig 7A). Upon fluconazole and miconazole treatment, 56 and 25 genes were upregulated in WT, respectively. Among them, 19 genes were commonly responsive to fluconazole and miconazole, 6 genes of which were dependent on AtrR. The azole-responsive AtrR-dependent genes included cyp51A, erg24A, erg24B, erg25A as well as hyd1 and Afu3g00820. These results again confirmed that AtrR regulated multiple genes related to the ergosterol biosynthesis pathway (Table 2). While atrR and srbA expression was slightly affected in response to fluconazole (1.22- and 1.62-fold, respectively) and miconazole (1.27- and 1.37-fold) treatment, real-time RT-PCR analysis suggested that the effect of azoles on the expressions were only subtle (Fig 7B).
Fig 7. FLCZ- and MCZ-responsive and AtrR-dependent genes determined by RNA-sequencing analysis.
(A) The ΔatrR and Af293 strains were cultivated in PDB for 20 h followed by FLCZ (final concentration, 64 μg/mL), MCZ (2 μg/mL), or DMSO (as a control) treatment for 2 h. Number of gene with less than 1/3-fold change in expression level in ΔatrR (DMSO) compared to Af293 (DMSO) was shown. Numbers of gene with more than 3-fold upregulation upon FLCZ or MCZ treatment (2 h) in Af293 were shown. Expression of six genes was induced upon FLCZ and MCZ treatment and these genes were dependent on AtrR, and expression of thirteen genes was induced upon FLCZ and MCZ treatment and these genes were independent on AtrR. (B) Expression level of atrR and srbA genes in response to MCZ addition was determined by real-time RT PCR method. The Af293 strain was cultivated in GMM for 24 h followed by 2 h culture with MCZ (final concentration, 2 μg/mL) or without MCZ (control). The expression levels of gene of interest were normalized with that of actin gene. Relative expression ratios against control (Af293 without MCZ) are shown. Error bars represent the standard deviations based on three independent replicates.
Table 2. RNA-sequencing analysis for Af293 and ΔatrR in response to azole drugs.
| RPKM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Af293 | ΔatrR | ||||||||||
| ID | Gene name | +DMSO | +FLCZ | +MCZ | +DMSO | +FLCZ | +MCZ | ΔatrR (DMSO) /Af293 (DMSO) | Af293 (FLCZ) /Af293 (DMSO) | Af293 (MCZ) /Af293 (DMSO) | Predicted function |
| AtrR- and azole treatment-dependent genes | |||||||||||
| Afu4g06890 | cyp51A | 249.0 | 901.5 | 952.8 | 3.9 | 15.3 | 18.9 | 0.02 | 3.62 | 3.83 | 14-alpha sterol demethylase |
| Afu1g03150 | erg24A | 45.6 | 605.5 | 736.4 | 2.0 | 16.7 | 35.7 | 0.04 | 13.28 | 16.15 | C-14 sterol reductase |
| Afu1g05720 | erg24B | 95.0 | 441.6 | 409.8 | 26.2 | 23.6 | 32.1 | 0.28 | 4.65 | 4.31 | C-14 sterol reductase |
| Afu8g02440 | erg25A | 597.8 | 1965.1 | 1906.6 | 10.0 | 13.0 | 24.7 | 0.02 | 3.29 | 3.19 | C-4 methyl sterol oxidase |
| Afu3g00810 | hyd1 | 62.94 | 320.39 | 402.68 | 0.00 | 19.36 | 20.35 | 0.00 | 5.09 | 6.40 | cholestenol delta-isomerase, putative |
| Afu3g00820 | 15.96 | 123.97 | 118.89 | 3.63 | 2.10 | 13.82 | 0.23 | 7.77 | 7.45 | putative exported protein | |
| PDR sub-family ABC transporters | |||||||||||
| Afu1g14330 | cdr1B | 61.1 | 125.5 | 111.3 | 5.7 | 9.9 | 6.9 | 0.09 | 2.06 | 1.82 | ABC transporter, putative |
| Afu1g17440 | abcA | 0.3 | 0.0 | 0.0 | 0.3 | 0.0 | 0.4 | 1.06 | 0.00 | 0.00 | ABC drug exporter AbcA |
| Afu2g15130 | 24.0 | 36.1 | 28.4 | 24.8 | 22.2 | 14.9 | 1.03 | 1.50 | 1.18 | ABC multidrug transporter, putative | |
| Afu3g01400 | 10.6 | 26.7 | 10.1 | 27.6 | 112.7 | 59.7 | 2.59 | 2.51 | 0.95 | ABC multidrug transporter, putative | |
| Afu3g07300 | atrI | 9.7 | 11.0 | 11.1 | 18.5 | 20.2 | 18.7 | 1.91 | 1.14 | 1.15 | ABC multidrug transporter, putative |
| Afu4g01050 | 0.4 | 3.1 | 0.0 | 0.4 | 5.2 | 3.8 | 1.06 | 8.79 | 0.00 | ABC multidrug transporter, putative | |
| Afu5g00790 | 2.6 | 11.3 | 10.1 | 6.8 | 17.8 | 60.1 | 2.65 | 4.39 | 3.91 | ABC multidrug transporter, putative | |
| Afu5g02260 | 5.2 | 5.3 | 2.7 | 4.1 | 6.0 | 3.1 | 0.80 | 1.03 | 0.52 | ABC multidrug transporter, putative | |
| Afu5g09460 | 29.4 | 20.7 | 18.6 | 37.6 | 23.6 | 24.8 | 1.28 | 0.70 | 0.63 | ABC transporter, putative | |
| Afu6g04360 | atrF | 3.4 | 3.7 | 1.9 | 8.5 | 6.8 | 3.0 | 2.51 | 1.10 | 0.57 | ABC drug exporter AtrF |
| Afu6g08020 | 8.1 | 9.2 | 8.1 | 7.6 | 13.8 | 7.2 | 0.94 | 1.14 | 1.00 | ABC transporter, putative | |
| Afu8g02650 | 3.3 | 5.4 | 6.8 | 5.2 | 5.1 | 5.9 | 1.59 | 1.65 | 2.09 | ABC multidrug transporter, putative | |
| Transcription factors | |||||||||||
| Afu5g09420 | ccg-8 | 46.4 | 40.8 | 43.6 | 41.4 | 41.1 | 47.2 | 0.89 | 0.88 | 0.94 | clock controled protein (Ccg-8), putative |
| Afu1g16460 | ads-4 | 88.0 | 103.1 | 94.8 | 83.4 | 129.5 | 135.8 | 0.95 | 1.17 | 1.08 | bZIP transcription factor (LziP), putative |
| Members of SREBP pathway | |||||||||||
| Afu2g01260 | srbA | 59.0 | 95.3 | 80.5 | 37.7 | 27.0 | 30.8 | 0.64 | 1.62 | 1.37 | HLH transcription factor, putative |
| Afu1g12080 | dscA | 23.0 | 18.8 | 16.0 | 21.9 | 26.2 | 14.3 | 0.95 | 0.82 | 0.70 | RING finger ubiquitin ligase (Tul1), putative |
| Afu4g11680 | dscB | 70.6 | 60.4 | 65.5 | 28.3 | 43.5 | 53.3 | 0.40 | 0.85 | 0.93 | conserved hypothetical protein |
| Afu4g10700 | dscC | 44.4 | 55.5 | 41.5 | 29.2 | 45.2 | 48.2 | 0.66 | 1.25 | 0.94 | conserved membrane protein, putative |
| Afu3g08340 | dscD | 10.0 | 12.6 | 6.0 | 15.2 | 23.7 | 10.4 | 1.52 | 1.26 | 0.60 | conserved hypothetical protein |
| Afu1g14320 | dscE | 47.9 | 61.7 | 75.9 | 52.8 | 56.9 | 59.2 | 1.10 | 1.29 | 1.58 | UBX domain protein |
| Afu6g12750 | rbdB(rbdA) | 55.6 | 45.8 | 48.9 | 35.0 | 43.3 | 21.1 | 0.63 | 0.82 | 0.88 | rhomboid family protein, putative |
| Afu6g02150 | sppA | 28.6 | 36.4 | 26.7 | 27.2 | 16.7 | 21.9 | 0.95 | 1.27 | 0.93 | signal peptide peptidase, putative |
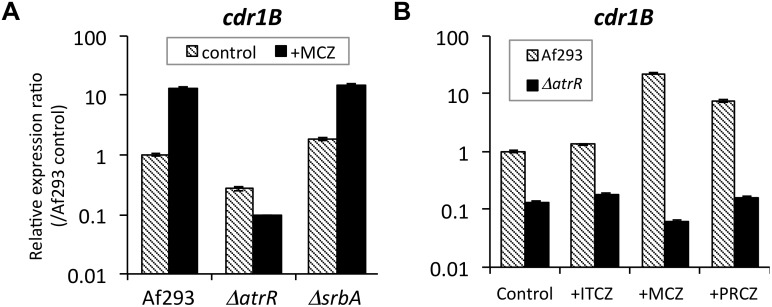
By comparing amino acid sequences, A. fumigatus was found to possess 12 PDR-type ABC transporters. Among them, cdr1B expression was highest in WT according to the transcriptome data, whereas the expression level was reduced to less than 10% in the ΔatrR strain (Table 2). On the contrary, expressions of some transporter genes (i.e. Afu3g01400 and AtrI) were slightly higher in the ΔatrR compared to the WT, which suggests a negative role for AtrR. We then verified the expression profiles for the cdr1B by real-time RT PCR analysis, showing that the basal cdr1B expression was reduced in the ΔatrR strain (approximately 25% relative to the WT), and there was no induced expression in ΔatrR (Fig 8A). In a sharp contrast, the ΔsrbA strain showed WT-level basal expression and upregulation of cdr1B. This result clearly indicated that AtrR is responsible for cdr1B expression while SrbA is not involved in control of this transporter. Furthermore, we found that when treated with propiconazole, a representative of an azole fungicide, cdr1B expression was upregulated. This induction was also dependent on AtrR (Fig 8B) and supported a crucial role of AtrR in cdr1B expression in A. fumigatus.
Fig 8. Expression levels of the cdr1B gene in response to azoles were determined by real-time RT PCR method.
(A) AtrR is involved in regulating cdr1B expression. Af293, ΔatrR, and ΔsrbA strains were cultivated in GMM for 24 h followed by 2 h culture with or without MCZ (final concentration, 2 μg/mL). (B) Af293 and ΔatrR strains were cultivated in GMM for 24 h followed by 2h culture with ITCZ, MCZ, or propiconazole (PRCZ) (final concentrations, 2 μg/mL). The expression level of cdr1B was normalized with that of actin gene. Relative expression ratios against Af293 (WT without drug) are shown. Error bars represent the standard deviations based on three independent replicates.
AtrR exerts direct regulation for cyp51A and cdr1B
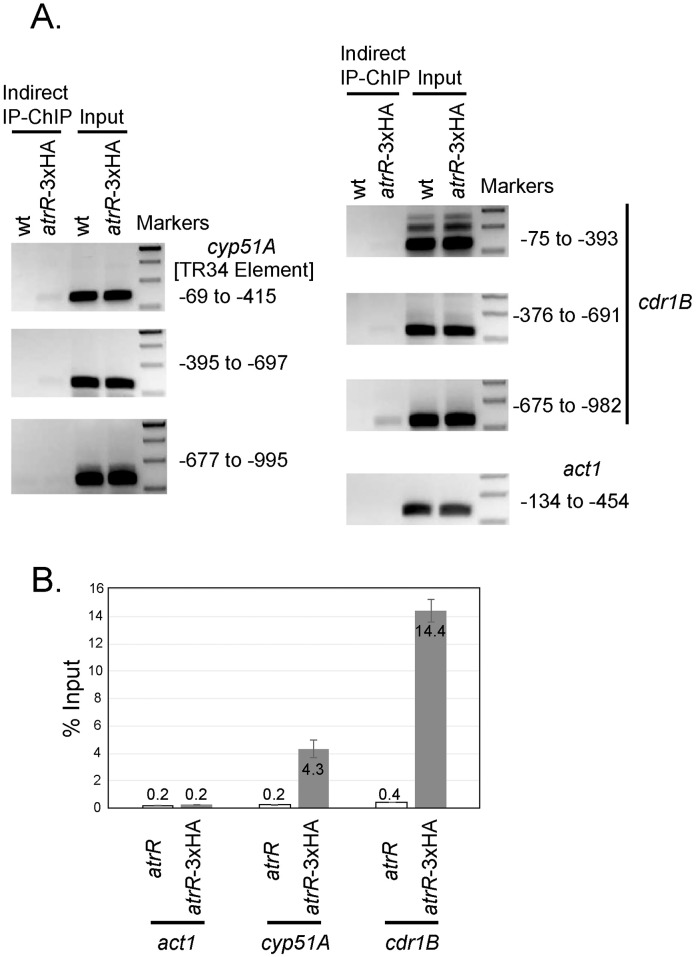
To examine the mode of AtrR interaction with the promoters of cyp51A and cdr1B, we carried out chromatin immunoprecipitation (ChIP) analysis using an epitope-tagged allele of atrR. A 3x hemagglutinin (HA) tag was placed at the carboxy-terminus of the chromosomal atrR gene (S5A Fig). This allele was still able to confer azole resistance indicating that AtrR function was maintained (S5B Fig). ChIP experiments were carried out as described [30] with immunoprecipitated chromatin examined for enrichment of fragments corresponding to the promoter regions of cyp51A, cdr1B and act1. We found the strongest level of enrichment for the cdr1B promoter with easily detectable recovery of the cyp51A promoter (Fig 9A). ChIP experiments using the act1 promoter did not produce any enrichment above background when comparing ChIP reactions carried out on strains lacking the HA tag versus a strain expressing the tagged AtrR protein. This specific enrichment for the cdr1B and cyp51A promoters was further confirmed by quantitative real-time PCR (Fig 9B). These data are consistent with AtrR acting at sites upstream from both cdr1B and cyp51A. Specificity of this interaction is supported by the lack of enrichment of the act1 promoter region.
Fig 9. Chromatin immunoprecipitation assay showing binding of AtrR protein to the promoter regions of cyp51A and cdr1B.
(A) Chromatin immunoprecipitation (ChIP) was done using a mouse monoclonal antibody directed against the HA epitope. Crosslinked chromatin was prepared from a WT strain or an isogenic strain expressing a 3x HA-tagged form of atrR (shown as atrR-3× HA). Immunoprecipitated DNA was examined by semiquantitative RT PCR analysis using primers that amplified the cyp51A and cdr1B promoters. The relative enrichment of the act1 promoter was also assessed as a control for a promoter not responsive to AtrR. (B) Quantitative analysis for enrichment of the cyp51A and cdr1B promoters by real-time RT PCR method. Chromatin immunoprecipitated from the atrR-3× HA strains exhibited a 20-fold enrichment of cyp51A promoter DNA along with a ~33-fold enrichment of cdr1B promoter sequence. No detectable enrichment of act1 was found. Data are presented as the mean and standard error of two separate ChIP experiments. % input represent signals obtained from the ChIP that are divided by signals obtained from the input sample.
The role of AtrR, SrbA, Cdr1B, and Cyp51A in sensitivity to azoles antifungals
To compare the physiological importance of the atrR, srbA, cdr1B, and cyp51A genes, we generated a series of isogenic strains lacking these important drug resistance loci in the Afs35 background. Representative isolates were then assessed for their drug resistance by determining their minimum inhibitory concentration (MIC) for a variety of antifungal drugs. The MICs of fluconazole, itraconazole, voriconazole, and miconazole were lowered in ΔatrR and ΔsrbA compared to the WT (Afs35) (Table 3). The MICs of voriconazole and miconazole were more depressed in the ΔatrR strain than in the corresponding ΔsrbA mutant, which are consistent with the results of growth inhibition assay using the Af293 background strains (Fig 2B). The double deletion mutant (ΔatrR ΔsrbA) exhibited increased susceptibility for azoles compared to either of single mutants. In the Δcdr1B and Δcyp51A, the MICs of itraconazole, voriconazole, and miconazole were lowered (50%-87.5%), compared to the WT. These drug susceptibility tests confirmed that the transcription factors AtrR and SrbA, the ABC transporter Cdr1B, and the target enzyme Cyp51A were required for normal azole resistance. Importantly, simultaneous loss of the transcription factors AtrR and SrbA caused the most profound azole sensitivity of all the genetic alterations tested.
Table 3. Minimal inhibitory concentrations of antifungals in A. fumigatus mutant strains.
| MEC (mg/L)*1 | MIC (mg/L)*1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | MCFG | CPFG | AMPH | 5-FC | FLCZ | ITCZ | VCZ | MCZ | PCZ |
| Afs35 (WT) | <0.015 | n.d. | 1 | >64 | >64 | 0.5 | 0.5 | 2 | n.d. |
| Δcdr1B | <0.015 | n.d. | 1 | >64 | >64 | 0.25 | 0.125 | 1 | n.d. |
| Δcyp51A | <0.015 | n.d. | 1 | >64 | 16 | 0.25 | 0.25 | 0.25 | n.d. |
| ΔatrR | <0.015 | n.d. | 1 | 16 | 4 | 0.125 | <0.015 | <0.03 | n.d. |
| ΔsrbA | <0.015 | n.d. | 1 | >64 | 2 | 0.125 | 0.03 | 0.06 | n.d. |
| ΔatrR,srbA | <0.015 | n.d. | 1 | >64 | 0.5 | 0.06 | <0.015 | <0.03 | n.d. |
| IFM 61567 | <0.015 | 0.25 | 2 | 64 | >64 | >8 | 1 | 0.5 | 8 |
| IFM 61567 ΔatrR No.2 | <0.015 | 0.25 | 2 | >64 | 4 | 0.125 | 0.06 | 0.06 | 0.06 |
| IFM 61567 ΔatrR No.3 | <0.015 | 0.25 | 2 | 64 | 4 | 0.125 | 0.06 | 0.06 | ≦0.06 |
| IFM 61567 ΔatrR No.4 | <0.015 | 0.25 | 2 | 64 | 4 | 0.25 | 0.06 | 0.06 | ≦0.06 |
*1 n.d. indicates 'not determined'.
Micafungin: MCFG; caspofungin: CPFG; amphotericin B: AMPH; flucytosine: 5-FC; fluconazole: FLCZ; itraconazole: ITCZ; voriconazole: VRCZ; miconazole: MCZ; posaconazole: PCZ.
Deleting atrR cures azole resistant phenotype in the clinical strain with Cyp51A G54E mutation
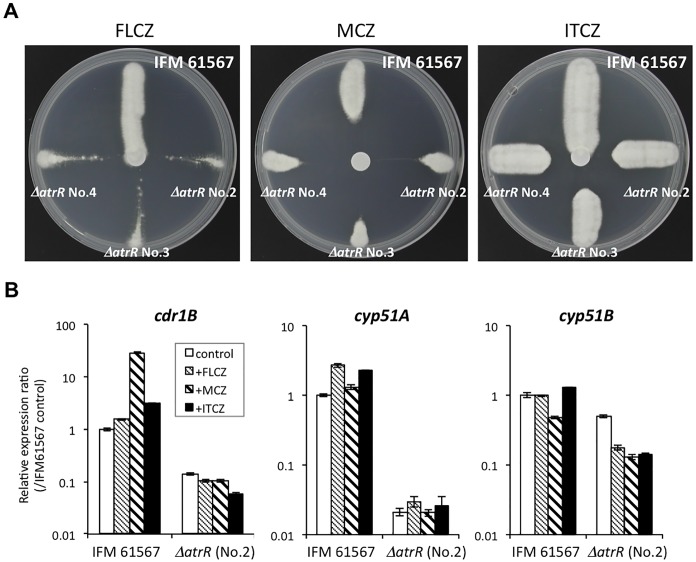
Considering the important role of AtrR in azole resistance, we wanted to evaluate the contribution of the atrR gene to azole resistance in a clinical isolate. We deleted the atrR gene from the genome of the clinical isolate IFM 61567 that contains a known itraconazole resistance-conferring mutation in its cyp51A gene (Cyp51A G54E). We obtained three independent deletion mutant strains (IFM 61567 ΔatrR No.2-4) and compared the MICs of antifungals with the starting strain (Table 3). The parental strain IFM 61567 showed resistance to itraconazole and posaconazole, but retained susceptibility to voriconazole and miconazole. All the three ΔatrR deletant strains were highly susceptible to itraconazole (MIC: 0.125~0.25) and posaconazole (MIC: ≤0.06~0.06), as well as to voriconazole (MIC: 0.06) and miconazole (MIC: 0.06), which revealed these strains are not resistant to azoles, even if judged from a clinical endpoint. We also used a disc-diffusion assay to confirm azole susceptibility for these mutants upon fluconazole, miconazole, and itraconazole treatment (Fig 10A). Together, these different assays demonstrated that the ΔatrR strains were more sensitive to these azoles compared to the parental resistant strain (IFM 61567). We confirmed that the expression levels of cdr1B, cyp51A, and cyp51B were markedly decreased in the ΔatrR derivatives (Fig 10B), as was observed earlier for ΔatrR in Af293 background. Collectively, our results showed that the atrR gene deletion led to azole sensitization even in this clinically isolated multi-azole resistant strain with a G54E mutation in Cyp51A.
Fig 10. The clinical azole resistant strain with an atrR gene deleted shows azole hyper-susceptibility.
(A) Drug susceptibility test by paper-disc diffusion assay. A paper-disc was placed on center of the GMM plate, and 10 μl of drug solution indicated was dropped on it (FLCZ: 10 mg/mL; MCZ: 10 mg/mL; ITCZ: 10 mg/mL). An azole resistant strain IFM 61567 and the independently obtained atrR deleted strains (ΔatrR No. 2–4) were streaked outward from the center. The plates were incubated for 48 h before photographed. The IFM 61567 fully grew along the streaked line regardless of the presence of FLCZ and ITCZ, whereas the ΔatrR mutants showed growth inhibition. (B) Expression levels of cdr1B, cyp51A, and cyp51B genes in IFM 61567 and the atrR-deleted strain (ΔatrR No.2) were determined by real-time RT PCR method. IFM 61567 and the ΔatrR were cultivated in YPG for 18 h followed by 1 h culture with or without FLCZ, MCZ, and ITCZ (final concentrations, 2 μg/mL). The expression levels of genes of interest were normalized with that of actin gene. Relative expression ratios against IFM 61567 without drug (control) are shown. Error bars represent the standard deviations based on three independent replicates.
Discussion
In the present study, we identified a novel transcription factor called AtrR that is essential for azole resistance in the human pathogen A. fumigatus as well as the industrially important fungus A. oryzae and the model fungus A. nidulans. Our results using the ChIP assay support the view that AtrR exerts direct transcriptional regulation on cyp51A and cdr1B expression via promoter association. In filamentous fungal pathogens, AtrR is the second transcriptional regulator, after SrbA, regulating the cyp51A gene. To the best of our knowledge, this is the first report of a direct transcriptional regulator for azole resistance-related ABC transporter genes in filamentous fungi including A. fumigatus. AtrR is widely found in an array of filamentous fungi including plant and human pathogenic fungi. Therefore, this finding sheds important new light on how filamentous fungi react and adapt to azole challenge via the coordinate upregulation of both sterol biosynthesis and ABC transporter gene expression.
Regulation of ABC transporters in A. fumigatus
The ABC transporters in the PDR sub-family are one of the main players implicated in azole resistance in fungi [34]. It has been demonstrated in many fungi, including plant and human pathogens, that certain ABC transporter genes were up-regulated in response to azole treatment, and loss of genes encoding PDR-type ABC transporters resulted in hypersensitivity to azoles [35–37]. According to phylogenetic analysis, the A. fumigatus PDR sub-family (also called ABCG family) contains 12 ABC transporters [38]. Among these include the already characterized Cdr1B, AbcA, AtrF, and AtrI, deletion mutants of which showed decreased susceptibility to azoles [20,22,39,40]. Our RNA-seq analysis showed that only a few ABC transporter genes were upregulated upon miconazole and fluconazole treatment, and that Cdr1B seemed to be the only transporter regulated by AtrR (Table 2). Studies of Cdr1B revealed a clinical relevance as an azole resistant strain overexpressing cdr1B has been isolated from a patient [20]. In that report, genetic evidence was provided that azole resistance was caused by cdr1B overexpression. However, the mechanism of that overexpression was not assessed in the paper. It is possible that elevated activation via AtrR led to the increased cdr1B transcription.
In a model filamentous fungus Neurospora crassa, Cdr4, an ortholog of Cdr1B or S. cerevisiae Pdr5p, plays a major role in azole tolerance [41]. Although direct interaction with the promoter region was not investigated, two transcription factors were characterized to be involved in regulating the cdr4 expression. The putative transcription factor CCG-8, which has been identified as a clock-controlled gene, positively regulates cdr4 as well as the azole target gene erg11, an ortholog of A. fumigatus cyp51A [42]. Ketoconazole challenge induced cdr4 and erg11 expression in the WT, whereas the induction levels were partly decreased in the ccg-8 deletion mutant. The CCG-8 ortholog (Afu5g09420) in A. fumigatus has not been characterized so far. A role of the bZip transcription factor ADS-4 was also investigated with regard to azole resistance in N. crassa [43]. A null mutant lacking ads-4 showed hypersensitivity to itraconazole and ketoconazole. ADS-4 was involved in regulating cdr4 as well as erg5 encoding a sterol C-22 desaturase. The ortholog of ads-4 (Afu1g16460) was also characterized in A. fumigatus with its deletion strain, which showed a slightly higher sensitivity to azoles compared to the parental strain. Our RNA-seq data revealed that expression of the two transcription factors (CCG-8 and ADS-4) was not affected by atrR deletion in A. fumigatus (Table 2), which supports the idea that AtrR is an independent regulator of cdr1B and cyp51A expression. The molecular mechanism of how AtrR functions will be an essential goal of future study.
In the pathogenic yeasts, C. albicans and C. glabrata, the molecular mechanisms underlying transcriptional regulation of ABC transporters (functioning as drug efflux pump) have been more intensively studied. The azole resistance-related ABC transporter Cdr1 (an ortholog of A. fumigatus Cdr1B and S. cerevisiae Pdr5p) is directly regulated by a Zn2-Cys6 transcription factor Pdr1 in C. glabrata [44]. In a clinical setting, azole resistant strains with elevated CDR1 expression were commonly recovered. In most of the strains, CDR1 overexpression was attributed to constitutively activated Pdr1 that possessed a gain-of-function mutation [45,46]. As stated earlier, Candida has no ortholog of AtrR. Indeed, Candida Pdr1 and A. fumigatus AtrR only share sequence homology in a Zn2-Cys6 domain at the N-terminus. The most notable distinctive role between the ABC transporter regulating transcription factors, Pdr1 and AtrR, is that AtrR expression is not substantially upregulated in response to azole treatment (Fig 7B), whereas Pdr1 itself is induced by azole treatment [47]. Collectively our results suggest that the transcriptional regulator of azole resistance-related ABC transporters, particularly for a Cdr1-type transporter, is evolutionarily distinct between yeasts and filamentous fungi.
Regulation of cyp51A gene by AtrR in association with SrbA
Ergosterol biosynthesis is a crucial process that is specific to fungi. Antifungal drugs such as azoles and polyenes target this biosynthetic pathway or the ergosterol molecule directly. The SREBP SrbA is a well-studied transcription factor that regulates ergosterol pathway-related genes including erg3, erg24, erg25, and cyp51A [27,30]. Deletion of srbA resulted in a disturbed sterol profile and hypersusceptibility to azole drugs. Intriguingly, a deletion mutant of atrR revealed strikingly similar phenotypes to those of the srbA deletion mutant. In addition to the regulation of erg genes and azole susceptibility, hypoxia adaptation and virulence attenuation were phenocopied between the srbA and atrR null mutants. This striking overlap argues that AtrR and SrbA closely function to regulate the ergosterol biosynthesis pathway. Recent studies provided information on the contributors in the SREBP pathway including the Golgi Dsc E3 ligase complex members (DscA-E), a rhomboid family protease RbdB (also reported as RbdA), and an aspartyl peptidase SppA [48–51]. Although these contributors are essential for SrbA function, transcription levels of these genes including srbA were not largely affected by deleting AtrR (Table 2). This suggested that AtrR did not act upstream via the SREBP pathway at a transcriptional level but rather may have its own independent regulatory input. The possibility that AtrR interacts with SrbA to function as a transcriptional regulator complex over the genes related to ergosterol biosynthesis, thus far, cannot be ruled out.
Previous studies by others revealed that SrbA binds to the promoter region of the cyp51A gene (also known as erg11A) [27,30]. ChIP-seq data indicated that SrbA-enrichment peaks resided in the cyp51A promoter region at the site 302 bp upstream from the initiation codon [30]. Accordingly, a putative SrbA binding site similar to the mammal SRE binding motif (TCACNCCAC) was found in this same region. Notably, the peak of SrbA-enrichment and the putative binding site are contained within a 34 bp sequence that had been identified as a tandem repeat sequence in azole resistant strains [11,13]. In our ChIP data for cyp51A with AtrR-3HA, a slightly more intense band was exhibited by probing with -69 to -415 region than with -395 to -697 (Fig 9A). This suggested that the binding motif of AtrR in the cyp51A promoter is more likely to be present at the region from -69 to -415 rather than -395 to -697. Gal4-type Zn2-Cys6 transcription factors typically bind to inverted CGG triplets as homodimers [52]. We therefore searched for CGG-Nx-CCG motifs in the cyp51A promoter region (to -1000). Interestingly, we found three regions with the motifs, CGG-N10-CCG, CGG-N18(9)-CCG, CGG-N17(12)-CCG, at -949 to -934, -796(-787) to -773, and -314(-309) to -292, respectively. The last one is located in the 34 bp of tandem repeat-related sequence and thus is adjacent to the putative SrbA-binding site. From these data, we anticipate that AtrR and SrbA could cooperatively function to regulate cyp51A expression at the proximal sites in the promoter region. Additionally, three motifs (CGG-N5-CCG, CGG-N21-CCG, and CGG-N20-CCG) were found in the cdr1B promoter at the sites (-723 to -713, -537 to -511, and -495 to -470). For the other 51 AtrR-dependent genes identified in RNA-sequencing analysis, the potential CGG-Nx-CCG motifs were widely detected in the promoter region (-1000 to 0) of several genes (S1 Table). Determination of the accurate binding sequences for AtrR is crucial to allow detailed understanding of the molecular function of AtrR.
Physiological impact of AtrR on hypoxia adaptation and pathogenicity
As molecular oxygen is a critical component for several biochemical processes, fungi must have the ability to adapt to hypoxic conditions. Previous studies showed that ergosterol biosynthesis is one of the fungal metabolic pathways most responsive to oxygen level [27,32,53,54]. Several enzymes in the fungal ergosterol biosynthesis pathway, such as the cytochrome P450 Erg3 and Erg11/Cyp51, require molecular oxygen as well as heme. Heme biosynthesis is also an oxygen-requiring pathway. The SREBP pathway coordinates oxygen levels with sterol and heme biosynthesis since these pathways are responsive to levels of this gas [28,30,55]. In the present study, AtrR was found to be involved in hypoxia adaptation as seen before for SrbA. In fact, the expression levels of cyp51A, erg3B, erg24A, and erg25A were induced in response to oxygen limitation in an AtrR- and SrbA-dependent manner (Fig 5B). Although the mechanism is still unknown, AtrR is responsible for sterol regulation under oxygen-limited condition, likely in association with SREBP pathway.
Sterol regulation of S. cerevisiae and Candida species involves a Gal4-type Zn2-Cys6 transcription factor Upc2. Interestingly, these yeasts lack an SREBP pathway. A recent finding in the lipid degrading yeast Yarrowia lipolytica that retains an intact SREBP, called YlSre1, revealed that YlSre1 only played a minor role while Upc2 played a major role in sterol regulation, hypoxia adaptation and azole resistance [56]. Therefore it was proposed that Upc2 has replaced the role of SREBPs in sterol regulation in these yeasts. From a comparison of the amino acid sequences, it is evident that AtrR is not an orthologue of Upc2.
Several lines of data have supported an intimate relationship between hypoxic adaptation and pathogenicity of pathogenic fungi. 1) The murine lung infected by fungi is a hypoxic environment at the infection site, which was revealed by a hypoxic indicator [57], and 2) SREBP mutants of C. neoformans (Sre1) and A. fumigatus (SrbA) which are unable to grow under hypoxic condition showed an avirulent phenotype [27,32]. We demonstrated that AtrR is also required for both hypoxic adaptation and A. fumigatus virulence. As AtrR is a fungal specific transcription factor, it would be a potential drug target, inactivation of which could suppress A. fumigatus in vivo growth during the infection process.
Deleting AtrR overcame Cyp51A-related azole resistance
One of the most noteworthy results in the present study is that deleting the atrR gene effectively sensitized the clinically isolated multi azole-resistant strain (Fig 10A and Table 3). Loss of atrR from this clinical strain reduced expression levels of cyp51A and cyp51B to the low level seen in the other laboratory lineage (Af293-background) (Fig 10B). This, in turn, likely reduced the amount of target proteins, Cyp51A and Cyp51B, in the cells. Even though the strain carried a G54E substitution in the Cyp51A protein, the reduction in gene transcription contributed to the resulting strains showing hypersensitivity to azoles in contrast to the resistant parental strain IFM 61567. Alternatively, accumulation of toxic intermediates at multiple AtrR-regulated steps (Erg3B, Cyp51A, Erg24A, and Erg25A) in the ergosterol biosynthesis pathway could also contribute to reacquisition of azole sensitivity. Even when cyp51A expression was heterologously elevated in an atrR background, this was insufficient to suppress the azole-susceptibility of this mutant strain (Fig 3B). The same result was also observed in srbA mutant background (Fig 3B), and is consistent with earlier work [29]. These results suggested that defective cyp51A expression was not the only reason for the azole-sensitivity in atrR or srbA deletion mutants. If judged by MIC values caused by loss of AtrR (itraconazole: 0.125–0.25; voriconazole: 0.06; posaconazole: ≦0.06–0.06), the resultant deletion mutants would be curable in a conventional azole drug therapy. This fact clearly illuminates a path toward development of new azole-sensitizing agent that could overcome Cyp51A-related azole resistance mechanisms.
In this study, we characterized a transcriptional factor designated AtrR that is responsible for a novel A. fumigatus intrinsic azole resistance mechanism. AtrR crucially governs adaptation to azole treatment in the sense that both target (cyp51A) and efflux pump (cdr1B) are transcriptionally co-regulated by AtrR. The experiment using a clinical azole-resistant isolate revealed a potential clinical relevance of AtrR. Our findings provide the first direct evidence that AtrR serves as a critical coordinator of both ergosterol biosynthesis and levels of a plasma membrane ABC transporter that can efflux azole drugs, the major antifungal drug in current use against pathogenic fungi.
Materials and Methods
Ethics statement
The principles that guide our studies are based on the Guidelines for Proper Conduct of Animal Experiments formulated by Science Council of Japan in June 1, 2006. All protocol used in this study were approved by the institutional animal care and use committee of Chiba University (Permit Number: DOU25-207, DOU26-228, and DOU28-345). All efforts were made to minimize suffering in strict accordance with the principles outlined by the Guidelines for Proper Conduct of Animal Experiments. Animals were clinically monitored at least daily and humanely sacrificed if moribund (defined by lethargy, dyspnea and weight loss). All stressed animals were sacrificed by cervical dislocation.
Strains and growth media
A. oryzae ΔligD::sC (niaD−) [58] and A. nidulans Δku70 (pyrG−, pyroA−, biA1−, ku70::argB) strains (derived from the strains, RIB40 and ABPU1, respectively) were used for a generation of deletion mutants of the AtrR-family genes. For identification of the AoatrR, the A. oryzae strains overexpressing a TF gene were used, which were obtained from a genetic library prepared by Noda Institute for Scientific Research, Kikkoman Corporation [33]. Briefly, the transcription factor genes of interest were cloned into an overexpression vector (pAPLTN) [59], which drives the expression under the amyB promoter system in the presence of maltose as a sole carbon source. A. fumigatus strains Af293 and Afs35 (akuA::loxP) were used to generate the following deletion strains and atrR complemented strain: ΔatrR, Co-atrR, ΔsrbA, Δcyp51A, Δcdr1B, ΔatrR,srbA, Af293+PthiA-cyp51A, ΔatrR+PthiA-cyp51A, and ΔsrbA+PthiA-cyp51A. The clinical strain IFM 61567 was isolated in 2011 in Japan from a patient with itraconazole treatment. The mutation in cyp51A gene in the strain was identified as a procedure described previously [60]. The fungal strains used in this study are listed in Table 4. All strains were routinely cultivated in potato dextrose agar (PDA), potato dextrose broth (PDB), 0.1% yeast extract-containing glucose minimal medium (YGMM), or glucose minimal medium (GMM) at 37°C. For A. oryzae or A. nidulans culture, Czapek-Dox (CD) medium was used, which contained 1% maltose or 1% glucose, 68.8 mM (NH4)2SO4, trace elements (1 μg/ml FeSO4·7H2O, 8.8 μg/ml ZnSO4·7H2O, 0.4 μg/ml CuSO4·5H2O, 0.15 μg/ml MnSO4·4H2O, 0.1 μg/ml Na2B4O7·10H2O, 0.05 μg/ml (NH4)6Mo7O24·4H2O) and appropriate supplements such as 1 mg/ml uridine, 0.02 μg/ml biotin, 2.5 μg/ml pyridoxine. To collect conidia of each A. fumigatus strain, PDA was used. The antifungal chemicals were commercially obtained as follows: fluconazole, miconazole, itraconazole, propiconazole, bromuconazole, tebuconazole, difenoconazole, clotrimazole (Wako Pure Chemical Industries, Osaka, Japan), and amphotericin B (Sigma-Aldrich Co., St. Louis, MO, USA). Micafungin was a generous gift from Dr. Keietsu Abe, Tohoku University.
Table 4. Strains used in this study.
| Strain | Background strain | Genotype |
|---|---|---|
| A. oryzae RIB40 niaD300 | WT | niaD- |
| A. oryzae+pAPLTN | RIB40 | niaD+ |
| A. oryzae+TF1 | RIB40 | niaD+, PamyB-TF1 |
| A. oryzae+TF2 | RIB40 | niaD+, PamyB-TF2 |
| A. oryzae+TF3 | RIB40 | niaD+, PamyB-TF3 |
| A. oryzae+TF4 | RIB40 | niaD+, PamyB-TF4 |
| A. oryzae+TF5 | RIB40 | niaD+, PamyB-TF5 |
| A. oryzae LigD | RIB40 | ΔligD::sC, niaD- |
| A. oryzae ΔatrR | RIB40 | ΔligD::sC, niaD-, AoatrR::ptrA |
| A. nidulans KU70 | ABPU1 | pyrG-, pyroA-, biA1-, ku70::argB |
| A. nidulans ΔatrR | KU70 | pyrG-, pyroA-, biA1-, ku70::argB, AnatrR::ptrA |
| Af293 | WT | - |
| ΔatrR | Af293 | atrR::hph |
| Co-atrR | Af293 | atrR::hph, atrR+-ptrA |
| ΔsrbA | Af293 | srbA::ptrA |
| Af293+PthiA-cyp51A | Af293 | PthiA-cyp51A-phle |
| ΔatrR+PthiA-cyp51A | Af293 | atrR::hph, PthiA-cyp51A-phle |
| ΔsrbA+PthiA-cyp51A | Af293 | srbA::ptrA, PthiA-cyp51A-phle |
| AfS35 | WT | ΔakuA-loxP |
| ΔatrR | AfS35 | ΔakuA-loxP, atrR::hph |
| ΔsrbA | AfS35 | ΔakuA-loxP, srbA::ptrA |
| ΔatrR,srbA | AfS35 | ΔakuA-loxP, atrR::hph, srbA::ptrA |
| Δcdr1B | AfS35 | ΔakuA-loxP, cdr1B::ptrA |
| Δcyp51A | AfS35 | ΔakuA-loxP, cyp51A::ptrA |
| SPF89 | AfS35 | ΔakuA-loxP, atrR-3xHA-hph |
| IFM 61567 | - | cyp51A(G54E) |
| IFM 61567 ΔatrR No2 | IFM 61567 | cyp51A(G54E), atrR::hph |
| IFM 61567 ΔatrR No3 | IFM 61567 | cyp51A(G54E), atrR::hph |
| IFM 61567 ΔatrR No4 | IFM 61567 | cyp51A(G54E), atrR::hph |
Construction of the gene deletion and complemented strains
For the deletion of the AoatrR gene in A. oryzae, approximately 1-kb fragments upstream and downstream of the coding region of the gene were amplified by PCR using primers YESAoAtrRFw + AoAtrRPtrARv and PtrAAoAtrRFw + AoAtrRYESRv, respectively. The selectable marker gene, ptrA, was isolated by digesting pPTRI (TaKaRa Bio, Otsu, Japan) with NheI. Then, the PCR-amplified two DNA fragments, the ptrA gene, and a yeast vector, pYES2 (Invitrogen, Tokyo, Japan), digested with EcoRI and BamHI, were assembled in S. cerevisiae BY4741 (MATa, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0) as described previously [61,62]. The resulting plasmid DNA was digested with KpnI and NotI to prepare a deletion construct, which was used for deletion of AoatrR. Similarly, for the deletion of the AnatrR gene in A. nidulans, DNA fragments upstream and downstream of the coding region of the gene were PCR-amplified using primers YESAnAtrRFw + AnAtrRPtrARv and PtrAAnAtrRFw + AnAtrRYESRv, respectively. The PCR-amplified two DNA fragments, the ptrA gene, and pYES2 digested with EcoRI and BamHI were assembled in S. cerevisiae, and used for deletion of AnatrR. Southern blot analyses demonstrated that the transformation cassettes had integrated homologously at the targeted loci and the target ORF was replaced with a selectable marker gene. To construct the A. fumigatus deletion mutants and the complemented strain, plasmids for each purpose were generated. DNA manipulation was performed according to standard laboratory procedures. To amplify DNA fragments from the genome, Prime STAR HS (TaKaRa Bio) was used. To prepare gene replacement cassettes for A. fumigatus, the fragments and plasmids were constructed by one-step fusion PCR and GeneArt Seamless Cloning and Assembly Kit (Invitrogen), respectively. Primers used in the present study were listed in S3 Table.
To generate a replacement cassette for atrR gene, the 5′- and 3′-flanking regions were obtained using the primers AtrR-5.UP and AtrR-5.LP(hph) (for the 5′-region) and AtrR-3.UP(hph) and AtrR-3.LP (for the 3′-region). These flanking regions and an hph fragment, hygromycin B resistant marker that was amplified from pCB1004 plasmid, were fused by one-step fusion PCR, which were then used for transformation.
For complementation of atrR gene, the atrR fragment containing both upstream and downstream sequences were PCR amplified using the primers AtrR-5.UPHind and AtrR-3.LP. The PCR fragment was digested by HindIII and XmaI and ligated into HindIII- and HpaI-digested pKIS518 (derived from a binary vector pPZP-HYG2, in which the hygB gene was removed by BamHI digestion, and a ptrA cassette is ligated at KpnI and EcoRV cites between the right and left borders of T-DNA), resulting in a plasmid pKIS518-atrR, which was used for Agrobacterium tumefaciens-mediated transformation.
To generate replacement cassettes for the srbA, cyp51A, and cdr1B genes, the 5′- and 3′-flanking regions were obtained using the primers srbA-U-F(pUC119E) and srbA-U-R(ptrA) (for the 5′-region of srbA) and srbA-D-F(ptrA) and srbA-D-R(pUC119B) (for the 3′-region of srbA), cyp51A-U-F(pBC-ph-s) and cyp51A-U-R(ptrA) (for the 5′-region of cyp51A) and cyp51A-D-F(ptrA) and cyp51A-D-R(pBC-ph-x) (for the 3′-region of cyp51A), and cdr1B-U-F(pUC119B) and cdr1B-U-R(ptrA) (for the 5′-region of cdr1B) and cdr1B-D-F(ptrA) and cdr1B-D-R(pUC119E) (for the 3′-region of cdr1B), respectively. These flanking regions and a ptrA fragment, pyrithiamine resistant marker that was amplified from pPTRI plasmid (TaKaRa Bio), were fused into pUC119 or pBC-phleo by GeneArt cloning system, resulting in plasmids pUC119-srbA::ptrA, pBC-phleo-cyp51A::ptrA, and pBC119-cdr1B::ptrA. The cassettes used for transformation were amplified from these plasmids.
For generation of a plasmid pBC-phleo-PthiA::cyp51A, promoter fragment (approximately 800bp) of A. fumigatus thiA (Afu6g08360) and the cyp51A ORF and terminator (approximately 450bp) fragment were obtained using primers PthiA-F(pBC-ph-s) and PthiA-R(cyp51A) (for the PthiA) and RT-cyp51A-F and cyp51A+T-R(pBC-ph-x) (for the cyp51A ORF+ter), respectively. These fragments were fused into pBC-phleo by GeneArt cloning system, resulting in a plasmid pBC-phleo-PthiA::cyp51A, which was used for transformation to obtain strains, Af293+PthiA-cyp51A, ΔatrR+PthiA-cyp51A, and ΔsrbA+PthiA-cyp51A.
A. fumigatus transformation was performed according to a protoplast-polyethylene glycol transformation method for Aspergillus [63]. Precise recombination and integration were confirmed by Southern Blot analysis and/or PCR of the genomic DNA, and in case of gene deletion the absence of mRNA of the target gene was confirmatively verified using real-time RT-PCR analysis. To generate a complemented strain for the atrR gene, A. tumefaciens strain EHA105 carrying the pKIS518-atrR was used for transformation of A. fumigatus ΔatrR strain by the method described elsewhere [64].
Antifungal drugs susceptibility test
Paper-disc diffusion assay: The conidia of each strain of interest were mixed with 20 mL of GMM (final concentration, 104 conidia/mL), and they were pored into a petri dish to solidify. A paper-disc was placed on center of the plate, and 10 μl of drug solution was dropped on it (fluconazole: 10 mg/mL; miconazole: 10 mg/mL; amphotericin B: 0.25 mg/mL; micafungin: 0.02 mg/mL). The plates were incubated at 37°C for 48 h before photographed. Instead of mixing conidia with pre-solidified GMM, the strains were inoculated by streaking conidia on a GMM plate when multiple strains were investigated.
Colony growth inhibition test: The 104 conidia of each strain were inoculated on the center of GMM plates containing 0 to 5 μg/mL of itraconazole or miconazole. The plates were incubated at 37°C for 70 h. The colony diameter was measured from three independent plates, and the mean values for plates with drugs were compared with those without drugs.
MIC test: MICs of each strain against antifungal drugs were investigated as described previously [65]. Tests were performed in triplicates using micafungin, amphotericin B, flucytosine, fluconazole, itraconazole, voriconazole, miconazole, and posaconazole in RPMI 1640 medium (pH 7.0) at 35°C. This was performed according to the Clinical and Laboratory Standards Institute reference broth microdilution method, document M38-A2, with partial modifications in term of using the dried plate for antifungal susceptibility testing of yeasts method (Eiken Chemicals, Tokyo, Japan).
RNA and cDNA preparation
The mycelia were cultured in GMM, YGMM, or PDB at 37°C and harvested at time-points (18~24 h) appropriate for the applications. The mycelia were frozen in liquid nitrogen, and total RNA was isolated using the FastRNA Pro Red Kit (MP Biomedicals, Santa Ana, CA, USA). To obtain cDNA pools from the total RNA, reverse transcription was performed using the ReverTra Ace qPCR RT Master Mix with gDNA remover (Toyobo, Osaka, Japan).
Quantitative real-time RT-PCR
Real-time RT-PCR was performed using SYBR Green detection as described previously [63]. The Thunderbird SYBR qPCR Mix was used for reaction mixture preparation (Toyobo). The primer sets are listed in S3 Table. The relative expression ratios were calculated by the comparative cycle threshold (Ct) (ΔΔCt) method. The actin gene was used as a normalization reference (internal control). Each sample was tested in triplicate.
RNA-sequencing analysis
RNA-sequencing analysis was performed as described previously [66]. Briefly, mRNA libraries and cDNA libraries were prepared by Illumina TruSeq RNA Sample Prep Kit v2 according to the standard protocols (Illumina, San Diego, CA, USA). Each total RNA sample (1 μg) was enriched for mRNA using oligo (dT)-tagged beads. The library construction involved cDNA synthesis, end repair, A-tailing, adapter ligation, and amplification. The mean length for each library was approximately 280 to 300 bp. Sequencing was performed in a pair-end 50 base mode on a Miseq system (Illumina). In this study, we performed two independent experimental settings: one containing Af293, ΔatrR, and ΔsrbA (total 3 samples) and the other containing Af293 and ΔatrR with DMSO, fluconazole, or miconazole treatment (total 6 samples). These read data were deposited to the DDBJ Sequence Read Archive under accession No. PRJDB5273.
To compare expression levels for each gene, the sequences were analyzed using CLC genomics workbench (CLC Bio, Aarhus, Denmark). The sequence reads were trimmed on the program software. The only reads with quality values higher than Q30 were used for mapping. The A. fumigatus Af293 genome data retrieved from NCBI (http://www.ncbi.nlm.nih.gov/bioproject/PRJNA14003) was used as the template for mapping. From the mapping data, RPKM values were calculated. Heat map presentation was performed using Cluster 3.0 and Java TreeView ver 1.1.6r2 according to the instructions.
Growth under hypoxia condition
The conidia of each strain were inoculated on GMM plate, and the plate was incubated at 37°C in anaeropack system (Mitsubishi Gas Chemical, Tokyo, Japan) where initial concentration of oxygen was set at 2% (hypoxia condition). After 96 h, oxygen in the pack appeared to be completely consumed as estimated from no more growth of all colonies on the plate. The plate for normoxia was incubated at 37°C in a regular incubator without gas control (21% oxygen) for 72 h.
Murine model of pulmonary aspergillosis
The mouse model of pulmonary aspergillosis was performed according to [67] with slight modifications. A. fumigatus strains Af293, ΔatrR, and Co-atrR were used to infect immunosuppressed mice (12 mice per group). Outbreed male ICR mice (5w, body weight, 20 to 24 g) were housed in sterile cages with sterile bedding and provided with sterile feed and drinking water containing 300 μg/mL tetracycline hydrochloride to prevent bacterial infection. Mice were immunosuppressed with cyclophosphamide at a concentration of 200 mg per kg of body weight, which was administered intraperitoneally on days −4, −2, 1, 3, 5, and 7 prior to and post-infection (day 0). The A. fumigatus conidia used for inoculation were grown on PDA for 5 days prior to infection. Fresh conidia were harvested in PBS+0.01% Tween 20 and filtered through a Falcon Cell Strainer (Corning Inc., NY, USA). Conidial suspensions were spun for 5 min at 3,000 × g, washed twice with PBS+0.01% Tween 20, counted using a hemocytometer, then resuspended at a concentration of 106 conidia/μl. Mice were anesthetized by ketamine and xylazine and infected by intratracheal instillation of 2.5 × 107 conidia in 25 μl of PBS. Mice were weighed and visually inspected every 24 h from the day of infection. The endpoint for survival experimentation was identified when a 30% reduction in body weight was recorded, at which time the mice were sacrificed. The statistical significance of comparative survival values was calculated using log rank analysis using the Prism statistical analysis package.
For A. fumigatus strains Afs35, ΔatrR, ΔsrbA, and ΔatrR ΔsrbA, immunosuppressed ICR mice (5w, body weight, 20 to 24 g) were used as described above (9 to 11 mice per group). Mice were immunosuppressed with cyclophosphamide at a concentration of 150 mg per kg of body weight, which was administered intraperitoneally on days −4, −1, 3, 6, 9, and 12 prior to and post-infection (day 0). Cortisone acetate (Sigma-Aldrich Co) was also administrated subcutaneously at a concentration of 200 mg per kg of body weight on day -1. Mice were anesthetized by ketamine and xylazine and infected by intratracheal instillation of 3 × 105 conidia in 30 μl of PBS. Mice were weighed and visually inspected every 24 h from the day of infection. The endpoint for survival experimentation was identified when a 30% reduction in body weight was recorded, at which time the mice were sacrificed.
Chromatin immunoprecipitation
For ChIP experiments, a strain possessing 3x HA-tagged allele of atrR was constructed. A cassette consisting of 750 bp of the C-terminus of atrR fused to a 3x HA tag, a trpC terminator followed by a hygromycin resistance gene and 750 bp of the 3’ untranslated region of the atrR gene was transformed into Afs35 cells with selection for hygromycin resistance. Resistant clones were confirmed to contain a homologous integration by PCR (Designated SPF89 strain). 1×106 spores/mL of A. fumigatus strains AfS35 (atrR) and SPF89 (atrR-3xHA) were grown in 50 mL of Sabouroud dextrose medium in 250 mL shaking flask cultures for 16 h. Cross-linking was done in (0.4 M Sucrose, 10 mM Tris-HCl, pH 8.0, 1 mM EDTA, adding 1 mM PMSF and 1% formaldehyde just before use) for 15 min under shaking (100 rpm) at 30°C. Crosslinking was stopped by adding 2.5 mL of 2 M glycine, and continued shaking incubation for 10 min. Mycelia were collected and dried using vacuum filtration and rinsed with sterile ddH2O and frozen immediately with liquid nitrogen and stored at −80°C. Approximately 100 mg of frozen mycelia were ground to a fine powder in a chilled mortar and pestle with liquid nitrogen added. Powder was transferred to 0.5 mL of ChIP lysis buffer (CLB: 50 mM HEPES pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% Deoxycholate (Sigma D6750), 0.1% SDS, 1 mM PMSF, 1× fungal proteinase inhibitor cocktail (Sigma)). Each sample was vortexed for 2 min and then split into 3*130 μl volume in AFA Fiber Pre-Slit Snap-Cap [6 x 15mm] microTUBE [Covaris] (130μl sample per tube). Chromatin was sheared with E220 Focused-ultrasonicator [Covaris] under following conditions: Peak Incident Power (W): 175; Duty Factor: 10%; Cycles per burst: 200; Treatment Time: 420 sec; Temperature: 7°C; Sample volume: 130 μl; in the presence of E220 –Intensifier (pn500141). Tubes from each sample were then pooled and centrifuged at 10,000 g for 5 min at 4°C. Supernatant was transferred into new tube. 25 μL was reserved as input control (IC) fraction for reverse crosslinking to verify sonication and control for ChIP and qPCR.
The sheared chromatin was incubated with HA.11(16B12) mouse monoclonal antibody (Covance) at 1:150 dilution overnight (12 h) on a nutator at 4°C. This sample was further incubated with 30 μL of washed Protein G Dynabeads (Life Technologies) for another 12 h. Washing, reverse-crosslinking and purification of ChIP-ed DNA was performed as described in Chung et al. [30].
ChIP qRT-PCR
Semiquantitative PCR was initially performed across the cyp51A and cdr1B promoters to serve as initial verification of enrichment of these promoter regions upon ChIP with HA. This PCR was done using Phusion DNA polymerase (NEB) under following conditions: 95°C for 30 s followed by 25 cycles of 95°C for 15 s, 60°C for 10 s and 72°C for 10 s. The primers used for the reaction were cyp51A-ChIP-F1 and cyp51A-ChIP-R1, cyp51A-ChIP-F2 and cyp51A-ChIP-R2, and cyp51A-ChIP-F3 and cyp51A-ChIP-R3 (for the cyp51A promoter (-69 to -415, -395 to -697, and -677 to -995, respectively)), cdr1B-ChIP-F1 and cdr1B-ChIP-R1, cdr1B-ChIP-F2 and cdr1B-ChIP-R2, and cdr1B-ChIP-F3 and cdr1B-ChIP-R3 (for the cdr1B promoter (-75 to -393, -376 to -691, and -675 to -982, respectively)), and act1-ChIP-F and act1-ChIP-R (for the act1 promoter (-134 to -454)) (S3 Table). Real-time PCR was performed in triplicate for each separate ChIP experiment using primers designed for regions as identified above as enriched in preliminary analysis, under the following conditions: 1 cycle of 95°C for 30 s followed by 40 cycles of 95°C for 15 s and 60°C for 30 s on an MyiQ2 BioRad machine. 1 μl of ChIP-ed or input (diluted 25-fold to bring it to 1%) DNA was used in 20 μl total volume reaction using SYBR green master mix (BioRad) and 0.4 μM of each primer. Percent input method was used to calculate the signal of enrichment of the promoter region for each gene (http://www.thermofisher.com/us/en/home/life-science/epigenetics-noncoding-rna-research/chromatin-remodeling/chromatin-immunoprecipitation-chip/chip-analysis.html).
Supporting Information
(A) Amino acid sequence alignment for Zn-finger motifs. Amino acid sequences of the candidate A. oryzae proteins (TF1 to TF5) were compared with that of S. cerevisiae Zn-finger type transcription factors, Pdr1 and Pdr3. The sequence numbers of amino acid in each protein were shown in brackets. (B) Protein motifs in the AtrR proteins. The Gal4-like Zn2-Cys6 DNA binding motif (IPR001138) is indicated by the diagonal line-containing box. The fungal specific sequence motif associated with transcription factor (IPR007219) is indicated by a dotted box. The number above the boxes show the amino acid sequence numbers counted from an initial methionine.
(PPTX)
(A) Genomic structures of the AoatrR loci in A. oryzae LigD and ΔAoatrR. The AoatrR gene was replaced with a ptrA marker in the deletion strain. SphI restriction sites are indicated. The fragment used as a probe is indicated by the black bar. (B) Southern blot analysis to confirm deletion of the AoatrR gene. The expected sizes of the bands detected by probing were 5.7 kb and 3.4 kb in WT and ΔAoatrR strain, respectively. (C) Genomic structures of AnatrR loci in A. nidulans KU70 and ΔAnatrR. The AnatrR gene was replaced with a ptrA marker in the deletion strain. SalI restriction sites are indicated. The fragment used as a probe is indicated by the black bar. (D) Southern blot analysis to confirm deletion of the AoatrR gene. The expected sizes of the bands detected by probing were 11.3 kb and 2.8 kb in WT and ΔAnatrR strains, respectively.
(PPTX)
(A) Genomic structures of the atrR loci in Af293 and ΔatrR. The atrR gene was replaced with an hph marker (1.35kb) in the mutant strain. NspV restriction sites are indicated by the blue arrowheads. The fragment used as a probe is indicated as a purple line. (B) Southern blot analysis for checking deletion of atrR gene. The expected size of the bands detected by probing were 3.9 kb and 3.6 kb in the WT and ΔatrR strains, respectively.
(PPTX)
GMM plates containing conidia of each strain were prepared. A paper-disc was placed on center of the plate, and 10 μl of drug solution indicated was dropped on it (bromuconazole: 10 mg/mL; tebuconazole: 10 mg/mL; difenoconazole: 10 mg/mL; propiconazole: 10 mg/mL). The plates were incubated for 48 h before photographed.
(PPTX)
(A) Western blot analysis of AtrR containing a 3x HA tag at its C-terminus. Whole cell protein extracts were prepared from wild-type and atrR-3x HA-containing cells. Equal amounts of protein were resolved by SDS-PAGE and then stained with Ponceau S (top panel) to confirm equal loading. This membrane was then subjected to western blotting using an anti-HA mouse monoclonal antibody. The ~100 kDa AtrR-3x HA is only detected in extracts from cells containing the epitope-tagged allele. (B) Azole sensitivity test for the strain containing 3x HA-tagged allele of atrR. Spores from the indicated strains were placed on either minimal medium or the same medium containing 0.15μg/ml voriconazole. Plates were incubated at 37°C for 72 hours and then photographed. Note that the strain containing the epitope-tagged atrR allele grows at least as well as the wild-type cells confirming that this allele retains function.
(PPTX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We acknowledge experimental assistance of Dr. Misako Ohkusu and Ms. Yumiko Nakano.
Data Availability
All sequence read files are available from the DDBJ Sequence Read Archive (http://trace.ddbj.nig.ac.jp/dra/index.html) database (accession number PRJDB5273).
Funding Statement
This work was supported by Joint Usage/Research Program of Medical Mycology Research Center, Chiba University (12-2, 13-1, 14-1, and 15-2). This work was also supported in part by the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) Special Budget for Research Project: The Project on Controlling Aspergillosis and the Related Emerging Mycoses (KK), a Cooperative Research Grant of NEKKEN (2011-15) (TG), the National BioResource project for Pathogenic Microbes funded by the MEXT, Japan (http://www.nbrp.jp/), a Grant-in-Aid for Scientific Research on Priority Areas, Applied Genomics (KAKENHI no. 17019001), from MEXT (KG), and NIH GM49825 and AI113748 (WSMR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vermeulen E, Lagrou K, Verweij PE. Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr Opin Infect Dis [Internet]. 2013;26(6):493–500. [DOI] [PubMed] [Google Scholar]
- 2.van der Linden JWM, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis. Centers for Disease Control and Prevention (CDC); 2015;21(6):1041–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, et al. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis. 2009;15(7):1068–76. 10.3201/eid1507.090043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghannoum MA, Rice LB. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Vol. 12, Clinical Microbiology Reviews. 1999. p. 501–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcazar-Fuoli L, Mellado E, Garcia-Effron G, Lopez JF, Grimalt JO, Cuenca-Estrella JM, et al. Ergosterol biosynthesis pathway in Aspergillus fumigatus. Steroids. 2008;73(3):339–47. 10.1016/j.steroids.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 6.Lelièvre L, Groh M, Angebault C, Maherault AC, Didier E, Bougnoux ME. Azole resistant Aspergillus fumigatus: An emerging problem. Vol. 43, Medecine et Maladies Infectieuses. 2013. p. 139–45. [DOI] [PubMed] [Google Scholar]
- 7.Tashiro M, Izumikawa K, Hirano K, Ide S, Mihara T, Hosogaya N, et al. Correlation between triazole treatment history and susceptibility in clinically isolated Aspergillus fumigatus. Antimicrob Agents Chemother. 2012;56(9):4870–5. 10.1128/AAC.00514-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camps SM, van der Linden JW, Li Y, Kuijper EJ, van Dissel JT, Verweij PE, et al. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature. Antimicrob Agents Chemother [Internet]. 2012;56(1):10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verweij PE, Chowdhary A, Melchers WJG, Meis JF. Azole Resistance in Aspergillus fumigatus : Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Weinstein RA, editor. Clin Infect Dis [Internet]. 2016;62(3):362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermeulen E, Maertens J, Schoemans H, Lagrou K. Azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation emerging in Belgium, July 2012. Eurosurveillance. 2012;17(48). [PubMed] [Google Scholar]
- 11.Snelders E, Van Der Lee HAL, Kuijpers J, Rijs AJMM, Varga J, Samson RA, et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008;5(11):1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelaez T, Monteiro MC, Garcia-Rubio R, Bouza E, Gomez-Lopez A, Mellado E. First detection of Aspergillus fumigatus azole-resistant strain due to Cyp51A TR46/Y121F/T289A in an azole-naive patient in Spain. New microbes new Infect [Internet]. 2015;6:33–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellado E, Garcia-Effron G, Alcázar-Fuoli L, Melchers WJG, Verweij PE, Cuenca-Estrella M, et al. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother. 2007;51(6):1897–904. 10.1128/AAC.01092-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. Azole-resistant aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. Vol. 69, Journal of Antimicrobial Chemotherapy. 2014. p. 555–7. [DOI] [PubMed] [Google Scholar]
- 15.Wu CJ, Wang HC, Lee JC, Lo HJ, Dai CT, Chou PH, et al. Azole-resistant Aspergillus fumigatus isolates carrying TR34/L98H mutations in Taiwan. Mycoses. Blackwell Publishing Ltd; 2015;58(9):544–9. [DOI] [PubMed] [Google Scholar]
- 16.Hagiwara D, Takahashi H, Fujimoto M, Sugahara M, Misawa Y, Gonoi T, et al. Multi-azole resistant Aspergillus fumigatus harboring Cyp51A TR46/Y121F/T289A isolated in Japan. J Infect Chemother. 2015; [DOI] [PubMed] [Google Scholar]
- 17.Wiederhold NP, Gil VG, Gutierrez F, Lindner JR, Albataineh MT, McCarthy DI, et al. First Detection of TR34 L98H and TR46 Y121F T289A Cyp51 Mutations in Aspergillus fumigatus Isolates in the United States. Warnock DW, editor. J Clin Microbiol [Internet]. 2016;54(1):168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Ingen J, van der Lee HA, Rijs TAJ, Zoll J, Leenstra T, Melchers WJG, et al. Azole, polyene and echinocandin MIC distributions for wild-type, TR34/L98H and TR46/Y121F/T289A Aspergillus fumigatus isolates in the Netherlands. J Antimicrob Chemother. 2015;70(1):178–81. 10.1093/jac/dku364 [DOI] [PubMed] [Google Scholar]
- 19.Camps SMT, Dutilh BE, Arendrup MC, Rijs AJMM, Snelders E, Huynen MA, et al. Discovery of a hapE Mutation That Causes Azole Resistance in Aspergillus fumigatus through Whole Genome Sequencing and Sexual Crossing. PLoS One. 2012;7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraczek MG, Bromley M, Buied A, Moore CB, Rajendran R, Rautemaa R, et al. The cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J Antimicrob Chemother. 2013;68(7):1486–96. 10.1093/jac/dkt075 [DOI] [PubMed] [Google Scholar]
- 21.Buied A, Moore CB, Denning DW, Bowyer P. High-level expression of cyp51B in azole-resistant clinical aspergillus fumigatus isolates. J Antimicrob Chemother. 2013;68(3):512–4. 10.1093/jac/dks451 [DOI] [PubMed] [Google Scholar]
- 22.Paul S, Diekema D, Moye-Rowley WS. Contributions of Aspergillus fumigatus ATP-binding cassette transporter proteins to drug resistance and virulence. Eukaryot Cell. 2013;12(12):1619–28. 10.1128/EC.00171-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delaveau T, Delahodde A, Carvajal E, Subik J, Jacq C. PDR3, a new yeast regulatory gene, is homologous to PDR1 and controls the multidrug resistance phenomenon. Mol Gen Genet [Internet]. 1994;244(5):501–11. [DOI] [PubMed] [Google Scholar]
- 24.Moye-Rowley WS. Transcriptional Control of Multidrug Resistance in the Yeast Saccharomyces. Prog Nucleic Acid Res Mol Biol. 2003;73:251–79. [DOI] [PubMed] [Google Scholar]
- 25.Jungwirth H, Kuchler K. Yeast ABC transporters—A tale of sex, stress, drugs and aging. Vol. 580, FEBS Letters. 2006. p. 1131–8. [DOI] [PubMed] [Google Scholar]
- 26.Brown MS, Goldstein JL. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Vol. 89, Cell. 1997. p. 331–40. [DOI] [PubMed] [Google Scholar]
- 27.Willger SD, Puttikamonkul S, Kim K-H, Burritt JB, Grahl N, Metzler LJ, et al. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog [Internet]. 2008;4(11):e1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blatzer M, Barker BM, Willger SD, Beckmann N, Blosser SJ, Cornish EJ, et al. SREBP coordinates iron and ergosterol homeostasis to mediate triazole drug and hypoxia responses in the human fungal pathogen Aspergillus fumigatus. PLoS Genet. 2011;7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blosser SJ, Cramer RA. SREBP-dependent triazole susceptibility in Aspergillus fumigatus is mediated through direct transcriptional regulation of erg11A (cyp51A). Antimicrob Agents Chemother. 2012;56(1):248–57. 10.1128/AAC.05027-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung D, Barker BM, Carey CC, Merriman B, Werner ER, Lechner BE, et al. ChIP-seq and In Vivo Transcriptome Analyses of the Aspergillus fumigatus SREBP SrbA Reveals a New Regulator of the Fungal Hypoxia Response and Virulence. PLoS Pathog. Public Library of Science; 2014;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang YC, Bien CM, Lee H, Espenshade PJ, Kwon-Chung KJ. Sre1p, a regulator of oxygen sensing and sterol homeostasis, is required for virulence in Cryptococcus neoformans. Mol Microbiol. 2007;64(3):614–29. [DOI] [PubMed] [Google Scholar]
- 32.Chun CD, Liu OW, Madhani HD. A link between virulence and homeostatic responses to hypoxia during infection by the human fungal pathogen Cryptococcus neoformans. PLoS Pathog. 2007;3(2):0225–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatamoto O, Umitsuki G, Machida M, Sano M, Tanaka A, Oka C, Maeda H, Tainaka H, Ito T, Uchikawa T, Masuda T, Matsushima K (2010) Recombinant vector capable of increasing secretion of Koji mold protease. US patent, US 7842799
- 34.Paul S, Moye-Rowley WS. Multidrug resistance in fungi: Regulation of transporter-encoding gene expression. Vol. 5 APR, Frontiers in Physiology. Frontiers Media SA; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abou Ammar G, Tryono R, Döll K, Karlovsky P, Deising HB, Wirsel SGR. Identification of ABC transporter genes of Fusarium graminearum with roles in azole tolerance and/or virulence. PLoS One [Internet]. Public Library of Science; 2013. January 11 [cited 2016 Apr 21];8(11):e79042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi K, Schoonbeek H, De Waard MA. Expression of the ABC transporter BcatrD from Botrytis cinerea reduces sensitivity to sterol demethylation inhibitor fungicides. Pestic Biochem Physiol [Internet]. 2002. June [cited 2016 Apr 21];73(2):110–21. [Google Scholar]
- 37.Hulvey J, Popko JT, Sang H, Berg A, Jung G. Overexpression of ShCYP51B and ShatrD in Sclerotinia homoeocarpa isolates exhibiting practical field resistance to a demethylation inhibitor fungicide. Appl Environ Microbiol [Internet]. 2012. September 15 [cited 2016 Apr 21];78(18):6674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagiwara D, Watanabe A, Kamei K, Goldman GH. Epidemiological and Genomic Landscape of Azole Resistance Mechanisms in Aspergillus Fungi. Front Microbiol [Internet]. 2016. [cited 2016 Oct 25];7:1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slaven JW, Anderson MJ, Sanglard D, Dixon GK, Bille J, Roberts IS, et al. Increased expression of a novel Aspergillus fumigatus ABC transporter gene, atrF, in the presence of itraconazole in an itraconazole resistant clinical isolate. Fungal Genet Biol. 2002;36(3):199–206. [DOI] [PubMed] [Google Scholar]
- 40.Meneau I, Coste AT, Sanglard D. Identification of Aspergillus fumigatus multidrug transporter genes and their potential involvement in antifungal resistance. Med Mycol [Internet]. 2016. March 1 [cited 2016 Mar 11]; [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Zhang Z, Zhang X, Zhang H, Sun X, Hu C, et al. CDR4 is the major contributor to azole resistance among four Pdr5p-like ABC transporters in Neurospora crassa. Fungal Biol. 2012;116(7):848–54. 10.1016/j.funbio.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 42.Sun X, Wang K, Yu X, Liu J, Zhang H, Zhou F, et al. Transcription factor CCG-8 as a new regulator in the adaptation to antifungal azole stress. Antimicrob Agents Chemother. 2014;58(3):1434–42. 10.1128/AAC.02244-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K, Zhang Z, Chen X, Sun X, Jin C, Liu H, et al. Transcription factor ADS-4 regulates adaptive responses and resistance to antifungal azole stress. Antimicrob Agents Chemother [Internet]. 2015. September [cited 2016 Apr 21];59(9):5396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paul S, Schmidt JA, Moye-Rowley WS. Regulation of the CgPdr1 transcription factor from the pathogen Candida glabrata. Eukaryot Cell [Internet]. 2011. February [cited 2016 Apr 21];10(2):187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrari S, Ischer F, Calabrese D, Posteraro B, Sanguinetti M, Fadda G, et al. Gain of function mutations in CgPDR1 of Candida glabrata not only mediate antifungal resistance but also enhance virulence. PLoS Pathog [Internet]. 2009. January [cited 2016 Apr 21];5(1):e1000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vermitsky J-P, Edlind TD. Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob Agents Chemother [Internet]. 2004. October [cited 2016 Apr 21];48(10):3773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vermitsky J-P, Earhart KD, Smith WL, Homayouni R, Edlind TD, Rogers PD. Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies. Mol Microbiol. 2006;61(3):704–22. [DOI] [PubMed] [Google Scholar]
- 48.Willger SD, Cornish EJ, Chung D, Fleming BA, Lehmann MM, Puttikamonkul S, et al. Dsc orthologs are required for hypoxia adaptation, triazole drug responses, and fungal virulence in Aspergillus fumigatus. Eukaryot Cell. 2012;11(12):1557–67. 10.1128/EC.00252-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dhingra S, Kowlaski CH, Thammahong A, Beattie SR, Bultman KM, Cramer RA. RbdB, a Rhomboid Protease Critical for SREBP Activation and Virulence in Aspergillus fumigatus. Bahn Y-S, editor. mSphere [Internet]. American Society for Microbiology Journals; 2016. March 2 [cited 2016 Apr 21];1(2):e00035–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaknin Y, Hillmann F, Iannitti R, Ben Baruch N, Sandovsky-Losica H, Shadkchan Y, et al. Identification and characterization of a novel Aspergillus fumigatus rhomboid family putative protease RbdA involved in hypoxia sensing and virulence. Infect Immun [Internet]. 2016. April 11 [cited 2016 Apr 16];IAI.00011–6 –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bat-Ochir C, Kwak J-Y, Koh S-K, Jeon M-H, Chung D, Lee Y-W, et al. The signal peptide peptidase SppA is involved in sterol regulatory element-binding protein cleavage and hypoxia adaptation in Aspergillus nidulans. Mol Microbiol [Internet]. 2016. January 29 [cited 2016 Apr 21]; [DOI] [PubMed] [Google Scholar]
- 52.MacPherson S, Larochelle M, Turcotte B. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol Mol Biol Rev [Internet]. 2006;70(3):583–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Synnott JM, Guida A, Mulhern-Haughey S, Higgins DG, Butler G. Regulation of the hypoxic response in Candida albicans. Eukaryot Cell. 2010;9(11):1734–46. 10.1128/EC.00159-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barker BM, Kroll K, Vödisch M, Mazurie A, Kniemeyer O, Cramer R a. Transcriptomic and proteomic analyses of the Aspergillus fumigatus hypoxia response using an oxygen-controlled fermenter. BMC Genomics [Internet]. 2012;13(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung D, Haas H, Cramer R. Coordination of hypoxia adaptation and iron homeostasis in human pathogenic fungi. Vol. 3, Frontiers in Microbiology. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maguire SL, Wang C, Holland LM, Brunel F, Neuvéglise C, Nicaud JM, et al. Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution. PLoS Genet. 2014;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grahl N, Puttikamonkul S, Macdonald JM, Gamcsik MP, Ngo LY, Hohl TM, et al. In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathog. 2011;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mizutani O, Kudo Y, Saito A, Matsuura T, Inoue H, Abe K, et al. A defect of LigD (human Lig4 homolog) for nonhomologous end joining significantly improves efficiency of gene-targeting in Aspergillus oryzae. Fungal Genet Biol. 2008;45(6):878–89. 10.1016/j.fgb.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 59.Jin FJ, Takahashi T, Matsushima KI, Hara S, Shinohara Y, Maruyama JI, et al. SclR, a basic helix-loop-helix transcription factor, regulates hyphal morphology and promotes sclerotial formation in Aspergillus oryzae. Eukaryot Cell. 2011;10(7):945–55. 10.1128/EC.00013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hagiwara D, Takahashi H, Watanabe A, Takahashi-Nakaguchi A, Kawamoto S, Kamei K, et al. Whole-genome comparison of aspergillus fumigatus strains serially isolated from patients with aspergillosis. J Clin Microbiol. American Society for Microbiology; 2014;52(12):4202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oldenburg KR, Vo KT, Michaelis S, Paddon C. Recombination-mediated PCR-directed plasmid construction in vivo in yeast. Nucleic Acids Res. 1997;25(2):451–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colot H V, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, et al. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci U S A [Internet]. 2006;103(27):10352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagiwara D, Takahashi-Nakaguchi A, Toyotome T, Yoshimi A, Abe K, Kamei K, et al. NikA/TcsC histidine kinase is involved in conidiation, hyphal morphology, and responses to osmotic stress and antifungal chemicals in Aspergillus fumigatus. PLoS One [Internet]. 2013;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimizu K, Imanishi Y, Toh-e A, Uno J, Chibana H, Hull CM, et al. Functional characterization of PMT2, encoding a protein-O-mannosyltransferase, in the human pathogen Cryptococcus neoformans. Fungal Genet Biol. 2014;69:13–22. 10.1016/j.fgb.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 65.Kikuchi K, Watanabe A, Ito J, Oku Y, Wuren T, Taguchi H, et al. Antifungal susceptibility of Aspergillus fumigatus clinical isolates collected from various areas in Japan. J Infect Chemother. 2014;20(5):336–8. 10.1016/j.jiac.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 66.Hagiwara D, Suzuki S, Kamei K, Gonoi T, Kawamoto S. The role of AtfA and HOG MAPK pathway in stress tolerance in conidia of Aspergillus fumigatus. Fungal Genet Biol. 2014;73:138–49. 10.1016/j.fgb.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 67.Wang DN, Toyotome T, Muraosa Y, Watanabe A, Wuren T, Bunsupa S, et al. GliA in Aspergillus fumigatus is required for its tolerance to gliotoxin and affects the amount of extracellular and intracellular gliotoxin. Med Mycol. 2014;52(5):506–18. 10.1093/mmy/myu007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Amino acid sequence alignment for Zn-finger motifs. Amino acid sequences of the candidate A. oryzae proteins (TF1 to TF5) were compared with that of S. cerevisiae Zn-finger type transcription factors, Pdr1 and Pdr3. The sequence numbers of amino acid in each protein were shown in brackets. (B) Protein motifs in the AtrR proteins. The Gal4-like Zn2-Cys6 DNA binding motif (IPR001138) is indicated by the diagonal line-containing box. The fungal specific sequence motif associated with transcription factor (IPR007219) is indicated by a dotted box. The number above the boxes show the amino acid sequence numbers counted from an initial methionine.
(PPTX)
(A) Genomic structures of the AoatrR loci in A. oryzae LigD and ΔAoatrR. The AoatrR gene was replaced with a ptrA marker in the deletion strain. SphI restriction sites are indicated. The fragment used as a probe is indicated by the black bar. (B) Southern blot analysis to confirm deletion of the AoatrR gene. The expected sizes of the bands detected by probing were 5.7 kb and 3.4 kb in WT and ΔAoatrR strain, respectively. (C) Genomic structures of AnatrR loci in A. nidulans KU70 and ΔAnatrR. The AnatrR gene was replaced with a ptrA marker in the deletion strain. SalI restriction sites are indicated. The fragment used as a probe is indicated by the black bar. (D) Southern blot analysis to confirm deletion of the AoatrR gene. The expected sizes of the bands detected by probing were 11.3 kb and 2.8 kb in WT and ΔAnatrR strains, respectively.
(PPTX)
(A) Genomic structures of the atrR loci in Af293 and ΔatrR. The atrR gene was replaced with an hph marker (1.35kb) in the mutant strain. NspV restriction sites are indicated by the blue arrowheads. The fragment used as a probe is indicated as a purple line. (B) Southern blot analysis for checking deletion of atrR gene. The expected size of the bands detected by probing were 3.9 kb and 3.6 kb in the WT and ΔatrR strains, respectively.
(PPTX)
GMM plates containing conidia of each strain were prepared. A paper-disc was placed on center of the plate, and 10 μl of drug solution indicated was dropped on it (bromuconazole: 10 mg/mL; tebuconazole: 10 mg/mL; difenoconazole: 10 mg/mL; propiconazole: 10 mg/mL). The plates were incubated for 48 h before photographed.
(PPTX)
(A) Western blot analysis of AtrR containing a 3x HA tag at its C-terminus. Whole cell protein extracts were prepared from wild-type and atrR-3x HA-containing cells. Equal amounts of protein were resolved by SDS-PAGE and then stained with Ponceau S (top panel) to confirm equal loading. This membrane was then subjected to western blotting using an anti-HA mouse monoclonal antibody. The ~100 kDa AtrR-3x HA is only detected in extracts from cells containing the epitope-tagged allele. (B) Azole sensitivity test for the strain containing 3x HA-tagged allele of atrR. Spores from the indicated strains were placed on either minimal medium or the same medium containing 0.15μg/ml voriconazole. Plates were incubated at 37°C for 72 hours and then photographed. Note that the strain containing the epitope-tagged atrR allele grows at least as well as the wild-type cells confirming that this allele retains function.
(PPTX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All sequence read files are available from the DDBJ Sequence Read Archive (http://trace.ddbj.nig.ac.jp/dra/index.html) database (accession number PRJDB5273).