Abstract
Purpose
Despite the efficacy of abiraterone, a CYP17A1 inhibitor, in metastatic castration-resistant prostate cancer (CRPC), nearly all patients develop resistance. The purpose of this phase II study was to evaluate mechanisms of resistance to more complete androgen synthesis inhibition with abiraterone and dutasteride.
Experimental Design
Eligible metastatic CRPC patients underwent a baseline metastasis biopsy. Patients received abiraterone and prednisone for two 4-week cycles. After this time, high-dose dutasteride (3.5mg daily) was added. Patients continued therapy until study withdrawal or radiographic progression. Repeat metastasis biopsy was obtained at progression. The primary endpoint was to assess mechanisms of resistance. Serum hormone and abiraterone levels were assessed. Tissue was assessed for androgen receptor (AR) and AR splice variant-7 (ARV7) expression.
Results
Forty patients were enrolled. 60% (n=24) achieved a ≥50% reduction in PSA. The median time to radiographic progression was 11 months. Nearly all baseline (n=29/30) and post-treatment (n=16/16) tumors tested for AR nuclear expression were positive. Of those tested, ARV7 expression was present in 48% (n=10/21) of baseline and 42% (n=5/12) of treatment discontinuation specimens. Compared to patients with higher serum abiraterone levels at treatment discontinuation, patients with lower levels had higher circulating androgens.
Conclusions
Despite increased androgen synthesis inhibition, we demonstrate that tumor AR axis remains important in disease progression. We highlight that abiraterone metabolism and pharmacokinetics may play a role in resistance. The non-comparative design limits conclusions on the efficacy of dual therapy with abiraterone and dutasteride, but the results support development of further multifaceted approaches towards AR inhibition.
Keywords: Abiraterone, Dutasteride, Castration-resistance, Prostate cancer, Resistance
Introduction
Persistent androgen receptor (AR) signaling is a critical mechanism of resistance in patients with metastatic castration-resistant prostate cancer (CRPC). Increased intratumoral androgen synthesis contributes to reactivation of the AR signaling pathway in CRPC (1). Increased expression of enzymes mediating testosterone and dihydrotestosterone (DHT) synthesis from weak adrenal androgens including dehydroepiandrostereone (DHEA) and androstenedione has been observed in CRPC tumors (2). Additionally, enzymes required for de novo steroid synthesis including CYP17A1 are upregulated in some tumors rendering them resistant to castration levels of circulating androgens (3).
Abiraterone, administered orally as abiraterone acetate, is a novel CYP17A1 inhibitor demonstrated to prolong survival in metastatic CRPC (4,5). CYP17A1, a rate-limiting enzyme in steroidogenesis, has two important enzymatic activities required for androgen biosynthesis. Although studies of abiraterone have demonstrated responses in a substantial number of men with CRPC, disease progression occurs in nearly all individuals. Thus, a better understanding of the drivers of resistance is needed to develop therapeutic strategies that offer patients durable clinical benefit.
Postulated mechanisms of acquired resistance to abiraterone include the persistence of adrenal androgens including high levels of circulating DHEA-sulfate (DHEAS), which can be transported into tumor cells and desulfated, thereby serving as an androgen reservoir. More robust inhibition of adrenal androgen synthesis is hypothesized as an approach that may overcome this resistance mechanism. Moreover, intratumoral de novo synthesis of androgens is another mechanism by which tumors may escape abiraterone (3,6). Alterations of the AR including mutations, substitute steroid receptors (7,8), and the presence of constitutively active AR splice variants (ARVs) lacking a ligand-binding domain (9) are additional potential resistance mechanisms.
Dutasteride is a type I and II 5-α reductase inhibitor which prevents the conversion of testosterone to DHT, a more potent androgen that has the highest binding affinity for the AR. Studies have demonstrated that type I 5-α reductase expression is increased in both primary prostate cancer (PCa) and CRPC (10). Though the role of 5-α reductase inhibition in the prevention and treatment of PCa has been controversial, combination CYP17A1 and 5-α reductase blockade is hypothesized to result in more complete blockade of androgen biosynthetic pathways (11,12). More complete androgen synthesis blockade through this multipronged approach could provide greater clinical benefit than solitary pathway inhibition. Preclinical studies have demonstrated that the combination of abiraterone and dutasteride decreased intratumoral testosterone and DHT (13). Additionally, we previously conducted a phase II trial of ketoconazole, a less potent CYP17A1 inhibitor, with dutasteride and hydrocortisone in patients with CRPC (14). We demonstrated that 56% of patients had a ≥50% decline in prostate specific antigen (PSA), which compared favorably to published response rates of ketoconazole alone. Additionally, the median duration of response was a favorable 20 months, supporting ongoing investigation of CYP17A1 and 5-α reductase combination therapy.
Based on these observations, we evaluated the effect of more complete androgen synthesis blockade through potent CYP171A inhibition with abiraterone combined with inhibition of type I and II 5-α reductase with dutasteride in patients with metastatic CRPC. We chose to use high dose dutasteride (3.5 versus 0.5 mg) to further reduce tissue DHT levels. Herein, we present the results of a phase II trial evaluating the combination of abiraterone and dutasteride in men with metastatic CRPC. The primary aim of this study was to evaluate tumor AR status in response to androgen synthesis blockade and investigate mechanisms of resistance to androgen synthesis blockade with abiraterone and dutasteride.
Patients and Methods
Patients
This is a phase II, single arm, open label study of abiraterone and dutasteride in patients with metastatic CRPC (NCT01393730). In total, 40 patients were enrolled on the study between 9/2011 and 10/2012. Patients were enrolled at three institutions: Dana-Farber Cancer Institute (n=27, Boston, MA), Beth Israel Deaconess Medical Center (n=5, Boston, MA), and University of Washington (n=8, Seattle, WA). Eligible patients had CRPC defined as disease progression despite a serum total testosterone <50 ng/dL and: 1) PSA progression as defined by the Prostate Cancer Clinical Trials Working Group (PCWG) 2, 2) soft tissue disease progression as defined by Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1, or 3) bone disease progression as defined by PCWG2. Additionally, patients had evidence of metastases with at least one metastatic site amendable to biopsy. Patients may have had any number of prior hormonal therapies (including antiandrogens, steroids, estrogens, finasteride, dutasteride, and ketoconazole), up to two previous cytotoxic therapies, radiotherapy, radiopharmaceuticals, or immunotherapy provided these were discontinued ≥4 weeks prior to study treatment initiation. Other eligibility criteria included: Eastern Cooperative Oncology Group (ECOG) performance status ≤2, predefined hematologic and laboratory criteria including serum potassium ≥3.5 mmol/L, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) <1.5 x institutional upper limit of normal (ULN), total serum bilirubin ≤1.5 x institutional ULN (except for participants with documented Gilbert’s disease), and left ventricular ejection fraction ≥50%. Patients were excluded if they had received prior abiraterone, had known brain metastases, uncontrolled intercurrent illness, uncontrolled hypertension (≥160 mmHg/≥95 mmHg), liver disease, pituitary or adrenal dysfunction, clinically significant cardiovascular events or thromboembolism within six months, surgery or local prostatic intervention within one month of study treatment initiation, gastrointestinal disorders which could interfere with drug absorption, or requirement for chronic steroids greater than the equivalent of prednisone 5 mg daily. All patients provided written informed consent.
Treatment
Following enrollment, patients had a baseline research biopsy of a soft tissue or bone metastasis. Biopsies were performed after informed consent in the interventional radiology department. Subsequently, patients were treated with abiraterone (1,000 mg daily) and prednisone (5 mg daily) for two 4-week cycles. After this time, high-dose dutasteride (3.5 mg daily) was added. Patients continued on the three-drug regimen until study withdrawal or radiographic disease progression. A repeat metastasis research biopsy, while patients were still on therapy, was obtained at progression in patients completing at least four treatment cycles. When possible the second biopsy was at the same site as the baseline biopsy. Though baseline and progression biopsies were mandatory, not all patients underwent biopsy at progression given lack of feasible biopsy site, clinical disease progression, or patient withdrawal. Imaging assessments occurred every 12 weeks. PSA was measured every 4-weeks and pre and post-dutasteride PSA levels were analyzed.
Assessment of flare on imaging
Flare on imaging was determined by two independent radiologists (KZ, LD). A flare was recorded on bone or computed tomography (CT) scan (at 3-months) if there was a stable or decreasing PSA at 3-months and there was an increase in intensity (for bone scan), sclerosis (for CT) or number of lesions between the baseline and 3-month scan and stable disease or reduction in intensity, sclerosis, or number of lesions on the subsequent bone scan or CT (6-months).
Immunohistochemistry
Biopsies were fixed in formalin or frozen in Optimal Cutting Temperature (OCT) compound. Bone biopsy samples were processed without decalcification. Immunohistochemistry (IHC) for AR (#M3562, clone AR441, Dako, dilution 1:200), pan-cytokeratin (CK, #MU357-UC, clone C11, Biogenex, dilution 1:500), and ARV7 (Abcam, ab198394 Anti-AR [EPR15656], dilution 1:200) were performed as previously described (15–17).
All available sections were evaluated by the study pathologist (RL) for hematoxylin and eosin (HE), CK, AR, and ARV7. Presence of tumor was recorded for each of the stains. AR and ARV7 expression in the nucleus and cytoplasm was scored as ‘positive’ (+1: weak; +2: moderate; +3: strong) or ‘negative’ when there was no perceptible staining in the tumor cells, and the percent of cells staining at a given intensity was specified.
Serum hormone and abiraterone levels
Serum hormone and abiraterone levels were obtained at baseline, at initiation of cycles 3, 4, 7, and treatment discontinuation. Methods for determination of hormone and abiraterone levels in the serum by mass spectrometry were as described (17,18). The lower limits of quantification were 0.12 ng/dL for testosterone, 0.49 ng/dL for progesterone, DHEA, and androstenedione, 0.98 ng/dL for pregnenolone and DHT, 120 ng/dL for DHEAS, and 0.01 ng/mL for abiraterone.
Laser-capture microdissection and RNA-seq analysis
In cases with adequate tumor in biopsies prior to starting therapy and at progression, laser-capture microdissection (LCM) from FFPE sections was used to isolate relatively pure populations of tumor cells. Extracted RNA (20 – 100 ng) was used for library construction after ribosomal RNA reduction using standard manufacture protocol for paired-end sequencing. Library quality was checked using the Agilent DNA High Sensitivity Chip and qRT-PCR. High quality libraries were sequenced on an Illumina HiSeq 2500 to generate 10–50 million paired-end reads per sample (HudsonAlpha, Huntsville, Al). The raw sequencing data were processed using FASTQC and Trimomatic software, and reads were aligned against human genome using tophat2 and bowtie2 software packages.
Statistical analysis
The primary endpoint of this study was to evaluate changes in AR activity at progression. This was assessed by tumor nuclear AR expression, tumor transcriptome analysis in a subset of cases, and serum hormone and abiraterone levels. The power and sample size was calculated for the primary endpoint based on serial biopsies in 40 patients. The current analysis had over 90% power to detect 0.75 standard deviation changes in serum hormone and abiraterone levels at progression compared to baseline with 2-sided alpha=0.1 using Wilcoxon’s signed rank test. IHC data were summarized with 95% confidence interval (CI). Secondary objectives were to assess PSA response and duration, radiographic response, flare on imaging, and safety.
Patient and disease characteristics were summarized as numbers and percentages for categorical variables and median with interquartile ranges (IQRs) for continuous variables. PSA nadir and percentage of patients with ≥50% and ≥90% PSA decline were reported. Radiographic response was summarized as numbers and percentages with 95% CI using an exact binomial test. Toxicity was summarized based on Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 by toxicity type and maximum grade for all patients regardless of attributes. Time to PSA progression and radiographic progression was summarized using the Kaplan Meier method. For patients who demonstrated any initial decline in PSA, PSA progression was defined as a PSA ≥2 ng/mL and ≥25% increase above the nadir confirmed ≥3 weeks later. For patients without an initial decline in PSA, PSA progression was defined as ≥25% increase in PSA and PSA ≥2 ng/mL after 12 weeks of treatment. Radiographic progression was defined by RECIST version 1.1 for soft tissue and visceral disease and PCWG2 for bone disease.
Hormone and abiraterone levels at baseline, at initiation of cycles 3, 4, 7, and progression were summarized as median and IQRs. Changes in hormone levels between cycle 7 and treatment completion were summarized using Wilcoxon’s signed rank test. Patients were divided into two groups based on abiraterone levels at progression compared to cycle 7: 1) decreased abiraterone levels, or 2) stable or increased abiraterone levels. Additionally, associations between abiraterone and hormones levels at cycle 3 initiation were summarized using Wilcoxon’s rank sum test. Patients were divided into two groups by dichotomizing abiraterone levels at the median: 1) low abiraterone levels, or 2) high abiraterone. A nominal p-value <0.05 was used to determine significance.
Results
Baseline characteristics
Forty patients were enrolled on the study. Patient and disease characteristics are summarized in Table 1. The median age at enrollment was 69 years. Ten patients (25%) received prior ketoconazole, seven (18%) received prior chemotherapy, and four (10%) received prior enzalutamide. The most common sites of metastasis were bone (80%) followed by lymph nodes (28%).
Table 1.
Baseline patient and disease characteristics.
| Characteristic | N | Median (q1-q3) or % |
|---|---|---|
| Age at diagnosis (years) | 39 | 61 (59, 65) |
| Gleason score at diagnosis | ||
| ≤ 6 | 6 | 15% |
| 7 | 14 | 35% |
| ≥ 8 | 18 | 45% |
| Unknown | 2 | 5% |
| Prior radical prostatectomy | 20 | 50% |
| Prior radiation therapy | 21 | 53% |
| Prior chemotherapy | 7 | 18% |
| Prior ketoconazole | 10 | 25% |
| Median duration of ketoconazole | 10 | 2.5 months (range <1–25 months) |
| Prior enzalutamide | 4 | 10% |
| Median duration of enzalutamide | 4 | 5 months (range 2–18 months) |
| At therapy initiation | ||
| Age (years) | 40 | 69 (65, 74) |
| Sites of metastasis | ||
| Bone metastases | 32 | 80% |
| Lymph nodes | 11 | 28% |
| Liver | 2 | 5% |
| Lung | 1 | 3% |
| ECOG performance status | ||
| 0 | 34 | 85% |
| 1 | 6 | 15% |
| Laboratory data | ||
| PSA (ng/mL) | 40 | 28.8 (9.5, 74.0) |
| Testosterone (ng/dL) | 40 | 10 (7, 12.5) |
| Alkaline phosphatase (U/L) | 40 | 89.5 (67.5, 153.5) |
| Calcium (mg/dL) | 34 | 9.4 (9.1, 9.7) |
| Hemoglobin (g/dL) | 40 | 13.2 (12.5, 14.0) |
| Platelets (K/UL) | 40 | 230 (186.5, 255) |
ECOG=Eastern Oncology Cooperative Group, PSA=prostate specific antigen.
PSA and radiographic response
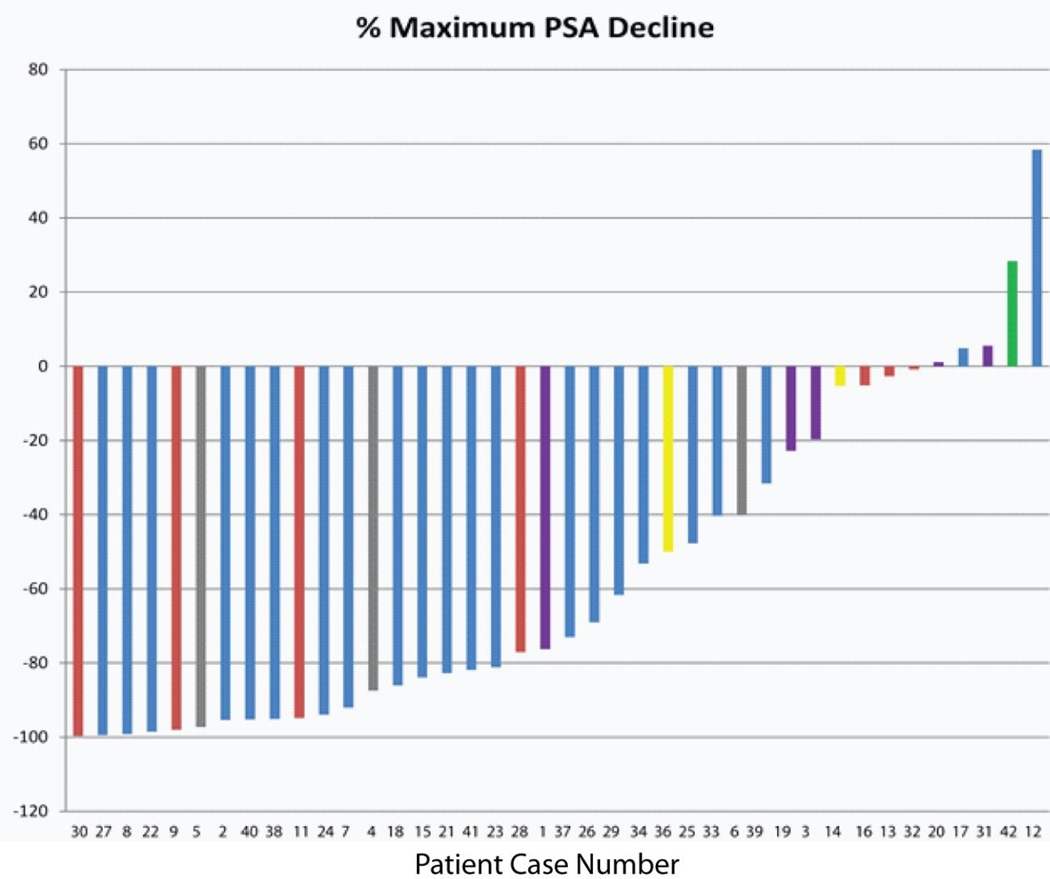
The degree of maximal PSA decline on treatment is displayed in Figure 1. The median PSA at therapy initiation was 28.8 ng/mL. After two cycles of abiraterone, the median PSA declined to 10.9 ng/mL [median 54% decline in PSA with IQR (<1%, 86%)]. Median PSA nadir was 6.3 ng/mL and median time to nadir was 3.2 months. Twenty-four patients (60%) achieved a ≥50% reduction in PSA from baseline at a median of 1.4 months (IQR: 1.3, 1.9 months) and 12 patients (30%) achieved a ≥90% reduction in PSA from baseline at a median of 2.4 months (IQR: 1.6, 2.9 months). Of the 34 patients who experienced a decline in PSA, 24% (n=8) had a PSA nadir at the beginning of cycle 2, 21% (n=7) at the beginning of cycle 3 prior to the addition of dutasteride, and 48% (n=19) after the addition of dutasteride.
Figure 1.
Waterfall plot of best PSA response to therapy with abiraterone and dutasteride. Red bars indicate prior ketoconazole. Purple bars indicate prior chemotherapy. Gray bars indicate prior enzalutamide. Yellow bars indicate prior ketoconazole and chemotherapy. Green bar indicates prior ketoconazole and enzalutamide.
There were five patients (12.5%) who did not experience any decline in PSA on therapy. Two had received prior chemotherapy (abiraterone duration 2.5 and 2.7 months) and one had received prior ketoconazole and enzalutamide (abiraterone duration 2.5 months) (Figure 1). Two patients did not receive prior chemotherapy, ketoconazole, or enzalutamide and remained on treatment without a PSA decline for 8 and 17 months. All five patients discontinued treatment for disease progression.
For the overall study, the objective response rate (ORR) was 15% (n=6, 95% CI 6%, 30%) with one patient having bone-only metastases experiencing a complete response. Fifty-five percent of patients (n=22, 95% CI 38%, 71%) experienced stable disease. Overall, 70% of patients (n=28) demonstrated clinical benefit with therapy, defined as an objective response or stable disease.
PSA and radiographic progression
A total of 32 patients experienced PSA progression at a median time of 5 months from therapy initiation. Thirty-two patients experienced radiographic progression and the median time to radiographic progression was 11 months. Of the 27 patients who experienced both PSA and radiographic progression, one patient experienced radiographic progression prior to PSA progression. The median duration between PSA progression and radiographic progression was five months (IQR: 2, 9 months). Median progression-free survival (PFS) as defined by clinical or radiographic progression was 8 months.
Toxicity
The most common any grade treatment-associated adverse events included fatigue, musculoskeletal pain, hypertension, hypokalemia, hot flashes, ALT elevation, and AST elevation (Supplement Table 1). The most common grade 3 toxicity was hypertension. There were no ≥ grade 4 toxicities. Only one patient required dose modification for grade 3 liver dysfunction.
Imaging flare phenomenon
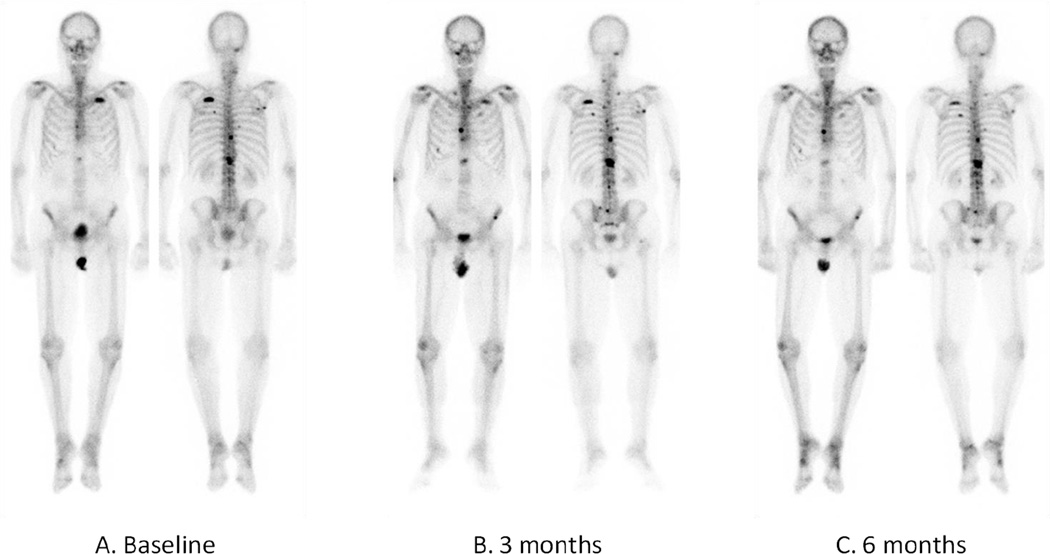
Data for flare on imaging assessment was available on 33 patients. Flare was observed on bone scan in 21% of patients (n=7/33) and CT scan in 42% of patients (n=14/33) (Figure 2). Five patients (15%) demonstrated flare on both CT and bone scan.
Figure 2.
Example of bone scan flare in a patient receiving abiraterone and dutasteride. A) Baseline bone scan PSA=45.96 ng/mL. B) 3-month bone scan PSA=10.19 ng/mL demonstrating increased and new areas of uptake in the ribs and spine. C) 6-month bone scan PSA=6.72 ng/mL demonstrating resolution of increased and new areas of uptake in the ribs and spine on 3-month scan.
Pathologic assessment and immunohistochemistry
Thirty-eight patients had baseline biopsies of which 82% (n=31) were positive for tumor (Figure 3, Supplement Table 2). Twenty-two had biopsies at therapy completion of which 73% (n=16) were positive for tumor. Nearly all baseline [n=29/30, 97%, 95% CI (83%, 100%)] and treatment discontinuation [n=16/16, 100%, 95% CI (79%, 100%)] tumor samples tested for AR nuclear staining were positive. In all positive cases, nuclear staining was more intense than cytoplasmic staining.
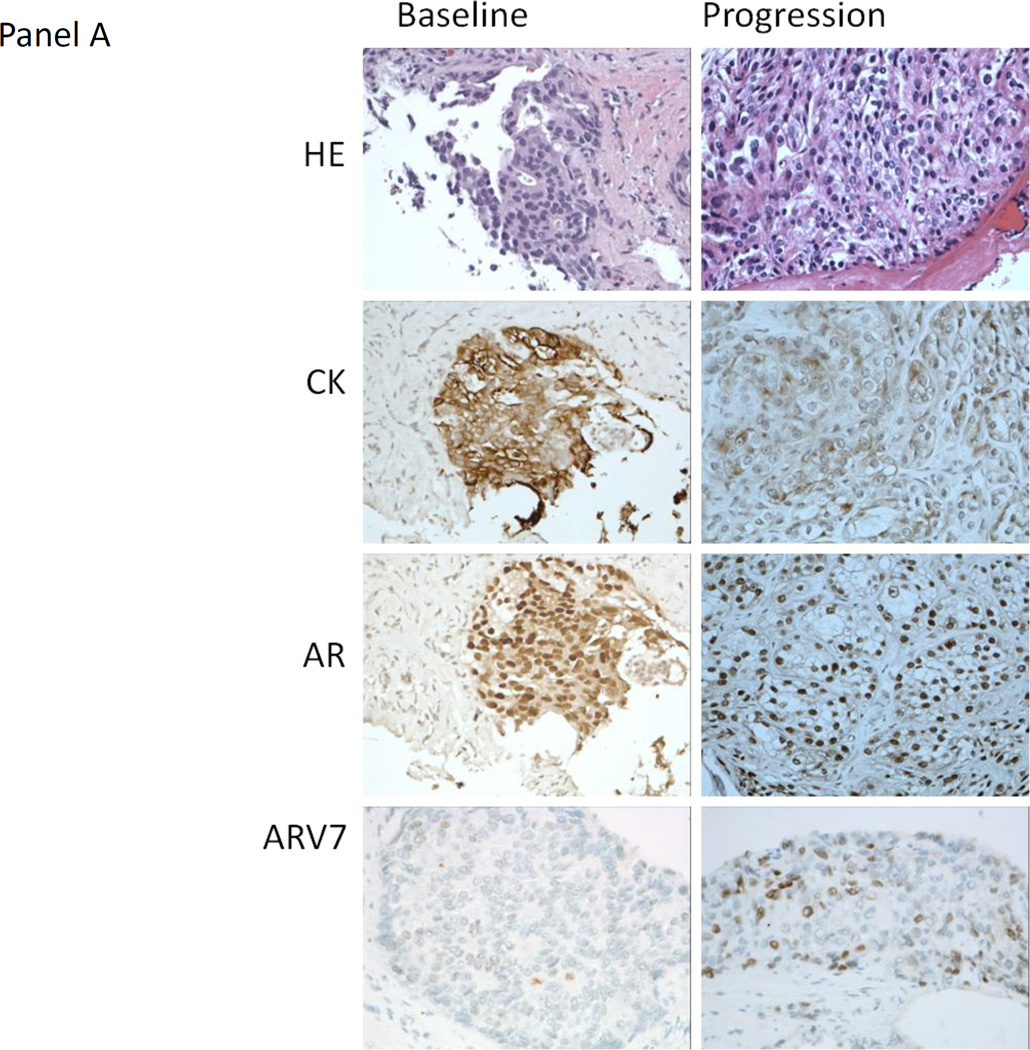
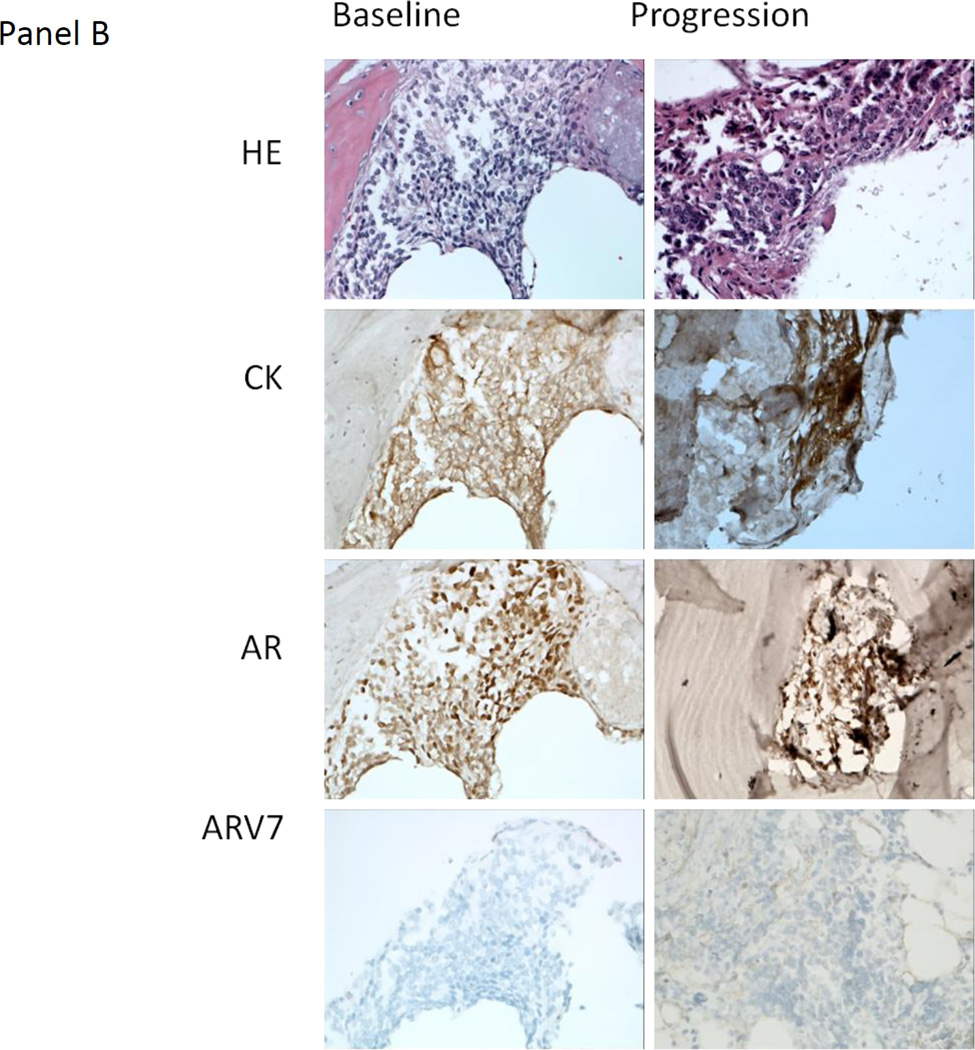
Figure 3.
A) Representative hematoxylin and eosin (HE) staining, and immunohistochemistry staining for cytokeratin (CK), the androgen receptor (AR) and AR variant 7 (ARV7) from the baseline and progression lymph node metastasis biopsy obtained from patient 36 (40X). The HE morphology of the baseline and progression biopsies was similar. ARV7 was weakly staining (+1, <10% of tumor nuclei at given intensity) at baseline and moderate staining (+2, ~50% of tumor nuclei at given intensity) at progression. This patient had lymph node only metastases and remained on treatment for 5.5 months after experiencing disease progression. B) Representative HE staining, and IHC staining for CK, the AR and ARV7 from the baseline and progression bone metastasis biopsy obtained from patient 33 (40X). The HE morphology of the baseline and progression biopsies was similar. There was no ARV7 staining at baseline or progression. The patient had bone only metastases and remained on treatment for 8 months after experiencing disease progression.
A subset of patient samples was stained for ARV7. Forty-eight percent of evaluated baseline tumors (n=10/21) and 42% of evaluated therapy completion tumors (n=5/12) were positive for ARV7 staining. The intensity of the staining was variable between samples (Supplement Table 2). Of patients with tissue available for baseline ARV7 testing (n=21), the median time-to-progression was 10 months for ARV7 positive individuals and 14 months for ARV7 negative patients. Of patients with tissue available for treatment discontinuation ARV7 testing (n=12), the median time-to-progression was 6 months in the ARV7 positive patients and 12 months in the ARV7 negative patients.
Expression of AR regulated genes
We have thus far been able to obtain adequate tumor from baseline and progression biopsies for LCM purification and analysis in six cases, and from progression biopsies only in two additional cases. RNA from these samples was examined by RNA-seq and assessed for expression of AR regulated genes based on a previously reported AR transcription signature.(19) Consistent with previous reports indicating that AR is substantially reactivated in CRPC, the AR signature scores in the baseline biopsies were comparable to those in untreated primary tumors from the TCGA data set (Supplement Figure 1) (20). Significantly, while AR activity was decreased in most of the matched progression biopsies, this decrease was modest and activity in all of the progression samples was markedly higher than in a series of non-prostate tumors or in a series of neuroendocrine prostate cancers. A similar result was obtained by examining expression of a small subset of highly AR regulated genes (Supplement Figure 1). Consistent with this AR activity, there were no increases in the neuroendocrine related genes CHGA or SYP (not shown). AR mRNA was slightly increased in most progression versus matched baseline samples, while there were no consistent changes in other steroid receptors (Supplement Figure 2). Interestingly, the largest changes were observed in the mineralocorticoid receptor, but this was increased in some progression biopsies and decreased in others.
Serum hormone and abiraterone levels
A total of 26 patients had abiraterone measurements prior to the addition of dutasteride at the start of cycle 3 and the median level was 35.0 ng/mL (IQR: 20.0, 65.0). After the addition of dutasteride, median abiraterone levels at cycle 4, 7, and at therapy discontinuation were 67 ng/mL (IQR: 31.0, 152.0), 43.0 ng/mL (IQR: 23.0, 85.0), and 39.3 ng/mL (IQR: 8.8, 63.2), respectively. The addition of dutasteride resulted in a significant 2-fold increase in abiraterone levels at cycle 4 (p=0.02) when compared to cycle 3, however abiraterone levels subsequently declined and there was no difference in abiraterone levels between cycle 3 and cycle 7 (p=0.26) and cycle 3 and treatment discontinuation (p=0.76). There was no difference in median abiraterone levels in patients with a nadir PSA prior to the addition of dutasteride [n=13, 46.0 ng/mL, IQR: (24.0, 70.0)] compared to those with nadir PSA after the addition of dutasteride [n=17, 44.0 ng/mL, IQR (23.0, 76.0)]. Though median DHEAS was reduced from baseline (50,110.0 ng/dL), levels were still detectable at proportionately higher amounts compared to other androgens at therapy discontinuation (500.0 ng/dL) (Supplement Table 3, Supplement Figure 3).
We dichotomized the group at the median into abiraterone low versus high levels and summarized the association of abiraterone and hormone levels at cycle 3 (Table 2). Pregnenolone and progesterone were higher in patients with high abiraterone levels at cycle 3, however this was not statistically significant. Additionally, there was a trend towards lower DHEAS in patients with high abiraterone levels at cycle 3.
Table 2.
Association of abiraterone and hormone levels at cycle 3.
| Abiraterone ≤ 35 ng/mL |
Abiraterone > 35 ng/mL |
All Patients | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | Median (q1, q3) | N | Median (q1, q3) | N | Median (q1, q3) | ||
| Pregnenolone (ng/dL) |
13 | 374 (268, 584) | 13 | 588 (418.0, 929) | 26 | 516 (312, 879) | 0.05 |
| Progesterone (ng/dL) |
13 | 87 (51, 163) | 13 | 141 (112, 298) | 26 | 123.5 (70, 217) | 0.09 |
| DHEAS (ng/dL) | 13 | 1095 (380, 1300) | 13 | 645.0 (410.0, 1172.5) |
26 | 505 (170, 1140) | 0.06 |
| DHEA (ng/dL) | 13 | 1.9 (1.8, 2.7) | 13 | 2.4 (1.74, 2.9) | 26 | 2.1 (1.8, 2.9) | 0.90 |
| Androstenedione (ng/dL) |
13 | 0.5 (0.3, 0.5) | 13 | 0.5 (0.4, 0.6) | 26 | 0.5 (0.63, 0.6) | 0.35 |
| Testosterone (ng/dL) |
13 | 0.4 (0.3, 0.7) | 13 | 0.5, (0.3, 0.6) | 26 | 0.45 (0.3, 0.6) | 0.74 |
| DHT (ng/dL) | 13 | 1.30 (1.0, 1.0) | 13 | 1.0 (1.0, 1.0) | 26 | 1.0 (1.0, 1.0) | - |
DHEAS= dehydroepiandrosterone sulfate, DHEA= dehydroepiandrosterone, DHT=dihydrotestosterone.
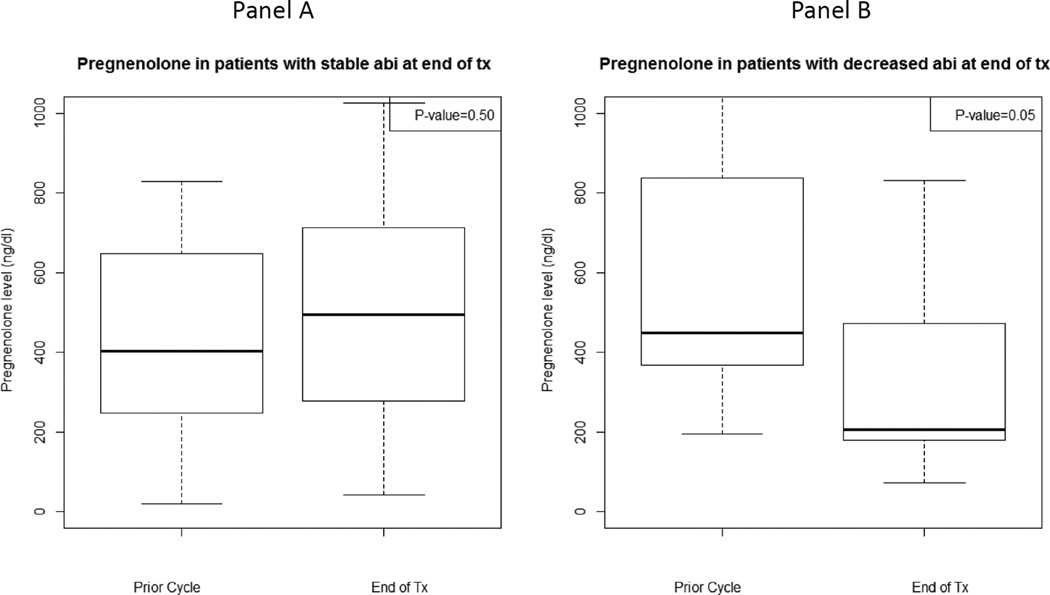
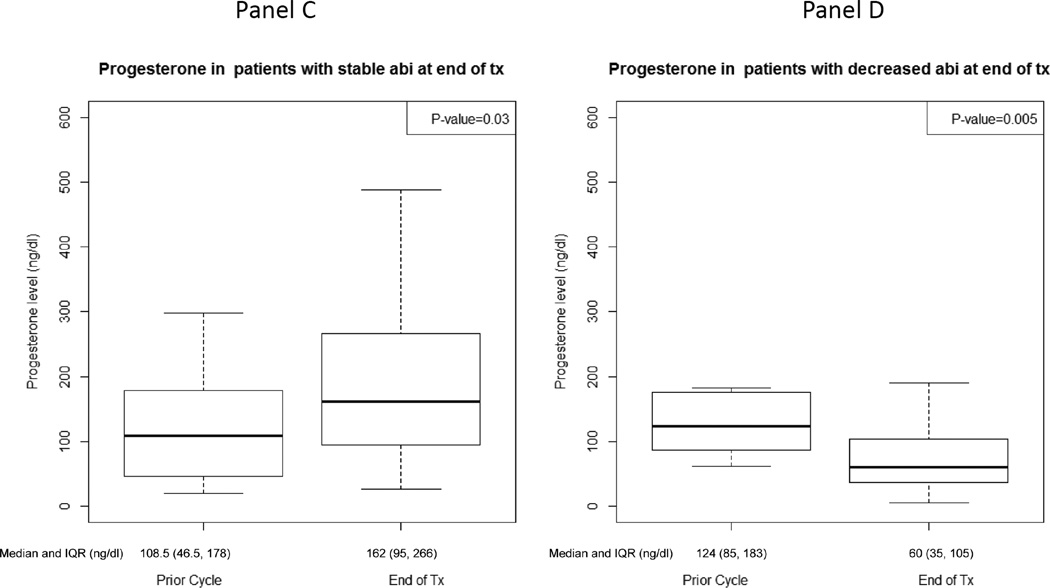
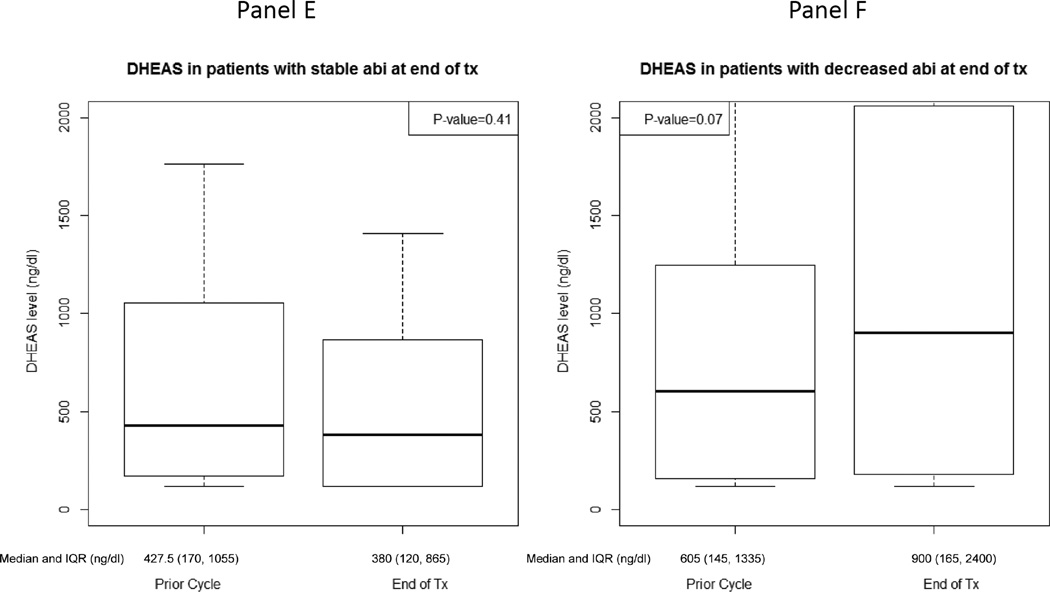
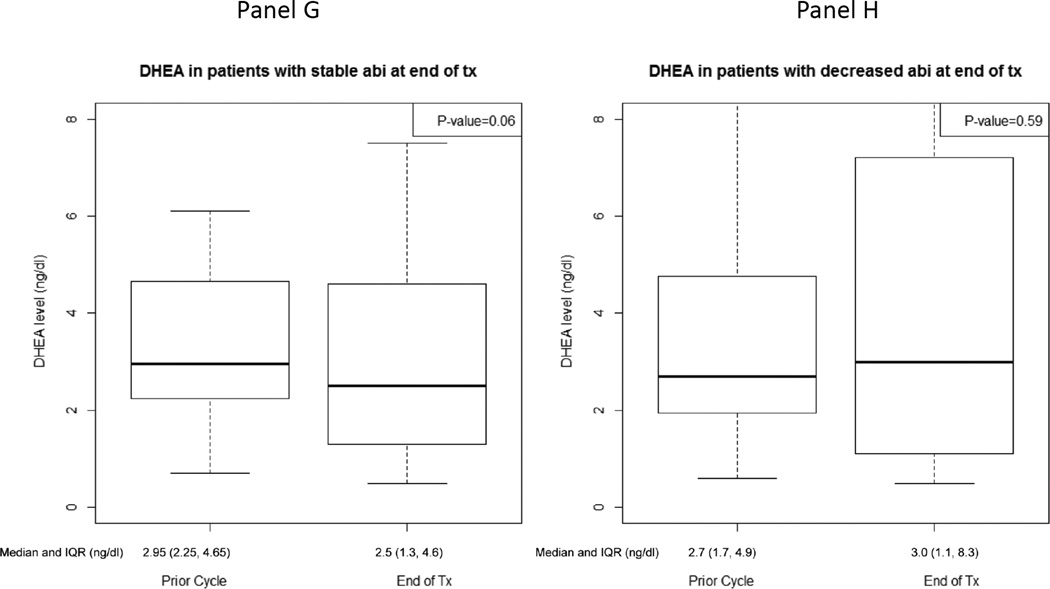
At the time of therapy discontinuation, abiraterone levels were lower than in prior cycles in 19 patients (48%) (prescribed dosing was confirmed in all patients based on drug diary). Patients with decreased abiraterone levels at therapy discontinuation were accompanied by a statistically significant decrease in pregnenolone and progesterone and a trend towards an increase in DHEAS (Figure 4, Supplement Table 3). Additionally, those with increased abiraterone levels at therapy discontinuation had a statistically significant rise in progesterone and trend towards a decline in DHEA (Figure 4, Supplement Table 3). Twelve of 19 patients (63%) with decreased abiraterone levels at therapy discontinuation had elevated DHEAS levels for whom there was a statistically significant decline in progesterone [-51.5 ng/dL, IQR: (-277.0, −41.5), p=0.01].
Figure 4.
Hormone levels at treatment-end and the prior cycle for patients with stable/increased (n=21) or decreased (n=19) abiraterone levels at treatment-end: A) Pregnenolone for patients with stable/increased abiraterone levels, B) Pregnenolone for patients with decreased abiraterone levels, C) Progesterone for patients with stable/increased abiraterone levels, D) Progesterone for patients with decreased abiraterone levels, E) Dehydroepiandrosterone sulfate (DHEAS) for patients with stable/increased abiraterone levels, F) DHEAS for patients with decreased abiraterone levels, G) Dehydroepiandrosterone (DHEA) for patients with stable/increased abiraterone levels, H) DHEA for patients with decreased abiraterone levels.
There was no statistical difference in clinical outcomes based on abiraterone levels at therapy discontinuation. The median duration of time to clinical progression for patients with stable or increased abiraterone levels at therapy discontinuation was 8.4 months (IQR: 4.1, 13.7). The median duration of time to clinical progression for patients with decreased abiraterone levels at therapy discontinuation was 11.1 months (IQR: 5.7, 17.3). Of the 21 patients with stable or increased abiraterone levels at therapy discontinuation, 12 (57%) had a 50% decline in PSA. Of the 19 patients with decreased abiraterone levels at therapy discontinuation, 11 (58%) had a 50% decline in PSA.
In the five patients with no PSA decline to therapy, three had lower abiraterone levels (range 0.1–30.8 ng/mL) at therapy discontinuation compared to median abiraterone levels for the total cohort at that time point (45.0 ng/mL). In case 12, at progression, DHEAS was at 80% (97,365.0 ng/dL) of baseline, DHEA was at 52% (125.5 ng/dL) of baseline, androstenedione was elevated by 13% (58.4 ng/dL) from baseline, and testosterone was at 4.9 ng/dL. This individual had not received prior chemotherapy, enzalutamide or ketoconzole, was on treatment at the time of therapy discontinuation, did not undergo any dosing modifications, and discontinued treatment for disease progression after remaining on therapy for 8 months.
Discussion
In this multicenter phase II study, we evaluated hormone levels and AR status following increased androgen synthesis suppression with abiraterone and dutasteride, and explored mechanisms of resistance. Via assessment of paired tumor biopsies and serum hormone and abiraterone levels, we provide evidence indicating that the AR axis remains active at disease progression despite aggressive suppression of androgen synthesis. All but one (97%) evaluable patients had AR nuclear expression on their baseline biopsy, and all had AR nuclear expression on their progression biopsy. Consistent with this nuclear AR expression, our limited transcriptome analyses on paired baseline and progression biopsies showed that AR transcriptional activity was largely intact in the progression biopsy samples. These findings indicate that persistent activation of the AR signaling program continues to be a major driver of resistance despite aggressive suppression of androgen synthesis.
Dutasteride was added at the beginning of cycle 3 in order to further suppress residual DHT synthesis. Although there were no detectable effects on serum DHT levels, it is possible that intratumoral DHT levels were decreased, but there were no clear further decreases in PSA levels. Interestingly, the addition of dutasteride resulted in a 2-fold increase in serum abiraterone, which may reflect decreased abiraterone metabolism by 5α-reductases. This would be consistent with recent data indicating that an abiraterone metabolite, Δ4-abiraterone (D4A), is metabolized by 5α-reductases (8,21). We also observed a trend towards lower serum androgen levels (and higher progestin levels) in patients with higher serum abiraterone, and found that a subset of patients had lower abiraterone levels at progression with reciprocal changes in upstream and downstream hormone levels. Due to the small sample size we did not correlate these findings with clinical outcomes, but these data highlight that variability in abiraterone exposure could serve as a mechanism contributing to drug sensitivity versus resistance. In addition to abiraterone serum levels, we found previously in a neoadjuvant abiraterone trial that germ-line variations in SLCO genes were significantly associated with differences in mean abiraterone tissue levels at prostatectomy (22). Given its steroidal structure, abiraterone may undergo SLCO-mediated cellular transport and these transport genes may be pharmacogenic determinants of intracellular abiraterone levels and potentially predictors of response to abiraterone. Further studies are warranted to assess the clinical significance of abiraterone levels and dutasteride-mediated increases in abiraterone and D4A.
Our study did not directly compare the efficacy of abiraterone and dutasteride versus abiraterone alone, and comparisons with historical controls are confounded by differences in patient populations and methods. Our study population included chemotherapy (18%) and/or ketoconazole (25%) pretreated patients, and 60% of all patients experienced a ≥50% decline in PSA from baseline (with the majority of declines occurring during the first 12 weeks of therapy). The PSA response is consistent with data from phase II studies of abiraterone in which 51–79% of patients experienced a ≥ 50% decline in PSA from baseline, commonly occurring within the first 12 weeks of treatment (25,26). Additionally, median radiologic PFS in our study was 8 months, which falls between the radiologic PFS from COU-301 (5.6 months, docetaxel-pretreated, ketoconazole naïve patients) and COU-302 (16.5 months, chemotherapy and ketoconazole naive) (4,5). The data presented here do not support investment in a large randomized trial of abiraterone with and without dutasteride, however the strategy of more effective ways to suppress AR signaling and delay tumor resistance remains of great importance.
Numerous mechanisms may have contributed to the persistence of nuclear AR and signaling in the progression biopsies. Data from multiple studies have shown that AR expression is increased in resistant tumors, although further increases in this study may have been modest as the tumors were castration-resistant at baseline (29,30). An adaptive increase in AR expression has also been observed in circulating tumor cells (CTCs) and circulating-free DNA (cfDNA) in patients with CRPC treated with abiraterone or enzalutamide (7,31,32). Furthermore, data from 150 men (the majority pre-abiraterone or enzalutamide) enrolled in a large metastatic biopsy program to characterize the molecular landscape of CRPC revealed that the most frequently detected genetic alteration was AR copy number gain, observed in 45% of case (33).
AR point mutations in the ligand-binding domain or alternative splicing events that lead to constitutively active AR variants have been described as mechanisms of resistance in CRPC. We previously examined the AR by targeted sequencing of 18 patients with CRPC progressing on CYP17A1 inhibition (including 15 patients treated on this clinical trial) (8). We demonstrated that the progesterone-activated T878A-mutant AR was present in high allele frequency in 3/18 cases (including patient 6 from this study who had liver and lymph node metastases and remained on treatment for nine cycles). A subset of patients had ARV7 expression at baseline and/or progression, and ARV7 expression was associated with decreased time to progression, suggesting it may contribute to intrinsic or acquired resistance to abiraterone, respectively. However, given the lack of standardization of ARV7 IHC testing, our results warrant confirmation with more specific antibodies that are becoming available and with molecular testing for ARV7.
We also found that substantial levels of DHEAS persist despite treatment with abiraterone. This is consistent with prior data from our group in two neoadjuvant studies investigating pre-operative intense androgen blockage in which DHEAS levels persisted at 10–30% of baseline levels at prostatectomy (17,34). In the neoadjuvant abiraterone trial, serum DHEAS levels remained in the 20 µg/dL range (17). These levels may provide a depot of androgen precursors that can be transported into PCa cells by solute carrier organic anion transporters (SLCO) transporters, unconjugated by steroid sulfatase, and converted to testosterone and DHT by steroidogenic enzymes to drive canonical AR signaling.
In our study, flare at 3-months was seen on bone scan in 21% of patients and on CT in 42% of patients. Flare on imaging has been previously reported in patients receiving treatment with abiraterone; this phenomenon may be under appreciated by clinicians and thus deserves highlighting here (26,39). Additionally, the presence of “bone flare” phenomena has been associated with long-term favorable outcomes in CRPC (39). These findings highlight that though there may be an initial discordance between PSA and imaging findings, patients need to be followed over time to confirm radiographic findings. The PCWG2 criteria formally address the issue of “bone flare” and recommend a confirmatory bone scan ≥6 weeks from first radiologic assessment if this demonstrates evidence of progression (40).
Lastly, this work describes practical methods for optimizing tumor acquisition in patients with metastatic CRPC with bone metastases. Historically, this process has been a challenge given the paucity of soft tissue metastases and dense sclerotic reaction associated with bone metastases, making biopsies and tissue processing technically difficult resulting in low tumor yield. We previously demonstrated that imaging, procedural, and clinical variables have an impact on image-guided bone biopsy tumor yield and this work demonstrates that bone biopsy in metastatic CRPC is feasible resulting in sufficient tumor yield in 80% of samples for correlative assessment (41).
Supplementary Material
Statement of Translational Relevance.
Abiraterone is a potent CYP17A1 inhibitor with demonstrated efficacy in patients with metastatic castration-resistant prostate cancer (CRPC). Unfortunately, durable responses to abiraterone are limited and most patients develop progressive disease. Therapies that achieve more robust androgen synthesis ablation may result in improved clinical outcomes. Additionally, a better understanding of mechanisms of resistance to therapy is highly relevant to optimizing the current treatment paradigm for patients with metastatic CRPC. In this study, we evaluate the effect of more complete androgen synthesis inhibition with abiraterone and dutasteride on androgen receptor (AR) signaling and explore mechanisms of resistance to combination therapy. We demonstrate persistent nuclear AR expression and signaling at progression despite more complete androgen synthesis inhibition, highlighting that the AR axis remains relevant after progression on abiraterone and dutasteride.
Acknowledgments
We would like to thank the patients and family members who participated in this clinical trial
Funding: This study was funded by Janssen. It was also supported by the Fairweather Family Fund and Uribe Family Fund at the Dana-Farber Cancer Institute (MET), Prostate Cancer Clinical Trials Consortium, Prostate Cancer Foundation Challenge Award (MET, SPB), NCI P01 CA163227 (SPB, EAM), and the DF/HCC Prostate Cancer SPORE (NCI P50 CA090381).
Footnotes
ClinicalTrials.gov Identifier: NCT01393730
Disclosures: MET and PWK serve on the Advisory Board for Janssen and receive clinical research funding from Janssen. The remaining authors have no disclosures.
References
- 1.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 3.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68(15):6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leon CG, Locke JA, Adomat HH, Etinger SL, Twiddy AL, Neumann RD, et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2010;70(4):390–400. doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- 7.Azad AA, Volik SV, Wyatt AW, Haegert A, Le Bihan S, Bell RH, et al. Androgen Receptor Gene Aberrations in Circulating Cell-Free DNA: Biomarkers of Therapeutic Resistance in Castration-Resistant Prostate Cancer. Clin Cancer Res. 2015;21(10):2315–2324. doi: 10.1158/1078-0432.CCR-14-2666. [DOI] [PubMed] [Google Scholar]
- 8.Chen EJ, Sowalsky AG, Gao S, Cai C, Voznesensky O, Schaefer R, et al. Abiraterone treatment in castration-resistant prostate cancer selects for progesterone responsive mutant androgen receptors. Clin Cancer Res. 2015;21(6):1273–1280. doi: 10.1158/1078-0432.CCR-14-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas LN, Douglas RC, Lazier CB, Too CK, Rittmaster RS, Tindall DJ. Type 1 and type 2 5alpha-reductase expression in the development and progression of prostate cancer. Eur Urol. 2008;53(2):244–252. doi: 10.1016/j.eururo.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 11.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362(13):1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 12.Fleshner NE investigators Rt. Dutasteride and active surveillance of low-risk prostate cancer. Lancet. 2012;379(9826):1590. doi: 10.1016/S0140-6736(12)60678-3. [DOI] [PubMed] [Google Scholar]
- 13.Pham S, Deb S, Ming DS, Adomat H, Hosseini-Beheshti E, Zoubeidi A, et al. Next-generation steroidogenesis inhibitors, dutasteride and abiraterone, attenuate but still do not eliminate androgen biosynthesis in 22RV1 cells in vitro. J Steroid Biochem Mol Biol. 2014;144(Pt B):436–444. doi: 10.1016/j.jsbmb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Taplin ME, Regan MM, Ko YJ, Bubley GJ, Duggan SE, Werner L, et al. Phase II study of androgen synthesis inhibition with ketoconazole, hydrocortisone, and dutasteride in asymptomatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15(22):7099–7105. doi: 10.1158/1078-0432.CCR-09-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efstathiou E, Titus M, Wen S, Hoang A, Karlou M, Ashe R, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol. 2015;67(1):53–60. doi: 10.1016/j.eururo.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu Y, Dai B, Ye D, Kong Y, Chang K, Jia Z, et al. Constitutively active AR-V7 plays an essential role in the development and progression of castration-resistant prostate cancer. Sci Rep. 2015;5:7654. doi: 10.1038/srep07654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol. 2014;32(33):3705–3715. doi: 10.1200/JCO.2013.53.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho E, Mostaghel EA, Russell KJ, Liao JJ, Konodi MA, Kurland BF, et al. External beam radiation therapy and abiraterone in men with localized prostate cancer: safety and effect on tissue androgens. Int J Radiat Oncol Biol Phys. 2015;92(2):236–243. doi: 10.1016/j.ijrobp.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendiratta P, Mostaghel E, Guinney J, Tewari AK, Porrello A, Barry WT, et al. Genomic strategy for targeting therapy in castration-resistant prostate cancer. J Clin Oncol. 2009;27(12):2022–2029. doi: 10.1200/JCO.2008.17.2882. [DOI] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas Research N. The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163(4):1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Alyamani M, Li J, Rogacki K, Abazeed M, Upadhyay SK, et al. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature. 2016;533(7604):547–551. doi: 10.1038/nature17954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mostaghel EA, Cho E, Wright JL, Loda M, Marck B, Matsumoto AM, et al. Association of SLCO transport genes with intraprostatic abiraterone (ABI) levels and pathologic outcomes in men with high-risk localized prostate cancer (PCa) ASCO Meeting Abstracts. 2015;33(15_suppl):5013. [Google Scholar]
- 23.Chang KH, Li R, Papari-Zareei M, Watumull L, Zhao YD, Auchus RJ, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2011;108(33):13728–13733. doi: 10.1073/pnas.1107898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah SK, Trump DL, Sartor O, Tan W, Wilding GE, Mohler JL. Phase II study of Dutasteride for recurrent prostate cancer during androgen deprivation therapy. J Urol. 2009;181(2):621–626. doi: 10.1016/j.juro.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28(9):1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan CJ, Shah S, Efstathiou E, Smith MR, Taplin ME, Bubley GJ, et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17(14):4854–4861. doi: 10.1158/1078-0432.CCR-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazier CB, Thomas LN, Douglas RC, Vessey JP, Rittmaster RS. Dutasteride, the dual 5alpha-reductase inhibitor, inhibits androgen action and promotes cell death in the LNCaP prostate cancer cell line. Prostate. 2004;58(2):130–144. doi: 10.1002/pros.10340. [DOI] [PubMed] [Google Scholar]
- 28.Keizman D, Ish-Shalom M, Peer A, Chish A, Sella A, Gottfried M, et al. Metformin (met) use and outcome of sunitinib (Su) treatment (tx) in diabetic patients (pts) with metastatic renal cell carcinoma (mRCC) ASCO Meeting Abstracts. 2015;33(15_suppl):e15618. [Google Scholar]
- 29.Mostaghel EA, Morgan A, Zhang X, Marck BT, Xia J, Hunter-Merrill R, et al. Prostate cancer characteristics associated with response to pre-receptor targeting of the androgen axis. PLoS One. 2014;9(10):e111545. doi: 10.1371/journal.pone.0111545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64(24):9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 31.Crespo M, van Dalum G, Ferraldeschi R, Zafeiriou Z, Sideris S, Lorente D, et al. Androgen receptor expression in circulating tumour cells from castration-resistant prostate cancer patients treated with novel endocrine agents. Br J Cancer. 2015;112(7):1166–1174. doi: 10.1038/bjc.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvi S, Casadio V, Conteduca V, Burgio SL, Menna C, Bianchi E, et al. Circulating cell-free AR and CYP17A1 copy number variations may associate with outcome of metastatic castration-resistant prostate cancer patients treated with abiraterone. Br J Cancer. 2015;112(10):1717–1724. doi: 10.1038/bjc.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamae D, Mostaghel E, Montgomery B, Nelson PS, Balk SP, Kantoff PW, et al. The DHEA-sulfate depot following P450c17 inhibition supports the case for AKR1C3 inhibition in high risk localized and advanced castration resistant prostate cancer. Chem Biol Interact. 2015;234:332–338. doi: 10.1016/j.cbi.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3(9):1020–1029. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 36.Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide) Cancer Discov. 2013;3(9):1030–1043. doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- 37.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M, et al. Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2015;1(5):582–591. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Efstathiou E, Titus M, Tsavachidou D, Tzelepi V, Wen S, Hoang A, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30(6):637–643. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Giorgi U, Caroli P, Burgio SL, Menna C, Conteduca V, Bianchi E, et al. Early outcome prediction on 18F-fluorocholine PET/CT in metastatic castration-resistant prostate cancer patients treated with abiraterone. Oncotarget. 2014;5(23):12448–12458. doi: 10.18632/oncotarget.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKay RR, Zukotynski KA, Werner L, Voznesensky O, Wu JS, Smith SE, et al. Imaging, procedural and clinical variables associated with tumor yield on bone biopsy in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2014;17(4):325–331. doi: 10.1038/pcan.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.