Abstract
In pancreatic ductal adenocarcinoma (PDAC), mutant KRAS stimulates the translation initiation factor eIF5A and upregulates the focal adhesion kinase PEAK1, which transmits integrin and growth factor signals mediated by the tumor microenvironment. Although eIF5A-PEAK1 signaling contributes to multiple aggressive cancer cell phenotypes, the downstream signaling processes that mediate these responses are uncharacterized. Through proteomics and informatic analyses of PEAK1-depleted PDAC cells, we defined protein translation, cytoskeleton organization and cell cycle regulatory pathways as major pathways controlled by PEAK1. Biochemical and functional studies revealed that the transcription factors YAP1 and TAZ are key targets of eIF5A-PEAK1 signaling. YAP1/TAZ co-immunoprecipitated with PEAK1. Interfering with eIF5A-PEAK1 signaling in PDAC cells inhibited YAP/TAZ protein expression, decreasing expression of stem cell-associated transcription factors (STF) including Oct4, Nanog, c-Myc and TEAD, thereby decreasing 3D tumor sphere growth. Conversely, amplified eIF5A-PEAK1 signaling increased YAP1/TAZ expression, increasing expression of STF and enhancing 3D tumor sphere growth. Informatic interrogation of mRNA sequence databases revealed upregulation of the eIF5A-PEAK1-YAP1-TEAD signaling module in PDAC patients. Taken together, our findings indicate that eIF5A-PEAK1-YAP signaling contributes to PDAC development by regulating an STF program associated with increased tumorigenicity.
Keywords: pancreatic ductal adenocarcinoma, PEAK1, eIF5A, YAP1
Introduction
Deregulation of protein synthesis is a hallmark of cancer characterized by hyperactive ribosome biogenesis and reprogramming of mRNA translation in a manner that favors proliferation, survival, and metastasis (1, 2). eIF5A (eukaryotic translation initiation factor 5A) is an 18-kDa protein that is highly conserved from archae to humans. eIF5A is indispensible for normal mammalian development, is involved in translation elongation, mRNA transport, and is important for cell-cycle progression and proliferation (3–5). Vertebrates carry two genes that encode two highly homologous eIF5A isoforms, eIF5A1 and eIF5A2, which in humans are 84% identical. eIF5A1 is ubiquitously expressed in all tissues, whereas eIF5A2 expression is primarily restricted to brain and testis. Although the role that eIF5A proteins play in protein synthesis is not fully understood, emerging evidence indicates that eIF5A does not regulate global protein synthesis, but rather enhances and fine-tunes the production of subsets of proteins crucial for hyper-proliferating cancer cells, which have substantial demands for oncogenic and metabolic proteins (5–7). Increased demands for such proteins may explain why eIF5A expression is increased in several cancers, including glioblastoma, leukemia, liver, colon, lung, cervical, PDAC, and ovarian cancer (4, 8).
Previous work showed that eIF5A1 (eIF5A) is upregulated in human PDAC tissues and in premalignant pancreatic intraepithelial neoplasia tissues isolated from Pdx-1-Cre: LSL-KRASG12D mice (1). Knockdown of eIF5A in PDAC cells inhibited their growth in vitro and orthotopic tumor growth in vivo, whereas amplification of eIF5A protein increased PDAC cell growth and tumor formation in mice. eIF5A regulates PDAC cell growth by modulating the expression of PEAK1, a nonreceptor tyrosine kinase essential for PDAC cell growth and gemcitabine resistance (1). PEAK1 is a non-receptor, cytoskeleton-associated tyrosine kinase that plays an essential role in driving PDAC malignancy downstream of eIF5A (1, 9, 10). Like eIF5A, PEAK1 expression is induced by activated KRasG12D and is amplified in PanINs from Pdx-1-Cre: LSL-KRASG12D mice, and in the majority of PDAC patient tissues samples. While results from these studies demonstrate that eIF5A utilizes PEAK1 as a downstream effector to drive PDAC pathogenesis, the downstream effectors of this pathway are poorly understood. To begin to address this limitation, we depleted PDAC cells of PEAK1 and examined them for changes in protein expression using liquid chromatography-tandem mass spectrometry LC/MS/MS. The signaling pathways altered in these cells were identified using computational methods and functional annotation programs. Here we report that the major pathways controlled by PEAK1 include protein translation, cytoskeleton organization, and cell cycle regulation. We also report that eIF5A-PEAK1 signaling controls YAP1/TAZ expression, which in turn, regulate Oct4, Nanog, c-Myc, and TEAD transcription factors, associated with increased PDAC tumorigencity and poor patient outcome.
Material and methods
PDAC cell lines
The 779E cell line (obtained from A.M. Lowy, Moores Cancer Center, UCSD, La Jolla CA.) was recently established from a moderate-to-poorly differentiated patient-derived tumor that harbored KRasG12F mutation. 1334 cell line (obtained from A.M. Lowy, Moores Cancer Center, UCSD, La Jolla CA.) was recently established from a PDAC patient liver metastasis and harbors mutated KRasG12D. Both of the cell lines were authenticated by validating mutations in the KRas gene and by assessing the histology of tumors derived from xenografted lines. The FG cell line is a well-differentiated PDAC line that harbors mutated KRASG12D, and was authenticated by assessing cell morphology, the KRASG12D mutation and cell doubling time, and was kindly provided by Dr. David Cheresh, UCSD. 4964 and 4313 cells were provided by A.M. Lowy, Moores Cancer Center, UCSD, La Jolla CA. Murine PanIN 4313 cells were isolated from a mouse PanIN (Pdx1-cre; LSL-KrasG12D/+). Murine PDAC 4964 cells were derived from an established primary PDAC tumor (Pdx1-cre; LSL-KrasG12D/+; p53R172H/+) (11). Panc1 and BxPC3 are established PDAC cell lines obtained from the American Type Tissue Culture Collection (ATCC) in 2008 and have been subcultured for less than 16 passages and not further authenticated. All the cells have been tested to be free of mycoplasma contamination.
3D spheroid culture
Cells cultured previously on 2D were re-suspended in serum-free media containing DMEM/F12, B27 Supplement (Gibco), 1% penicillin/streptomycin, 20 ng/mL of human bFGF, EGF (20ng/mL) and heparin and plated on Corning Ultra-Low Attachment polystyrene plates. Fresh medium was added every 3 days and cells were monitored for formation of tumorspheres for 12–17 days after which they were used for western blotting, immunoprecipitation, and biochemical analyses. In some cases, cells were placed in 3D cultures in the continued presence of 20 μm GC7 or vehicle (DMSO). 3D spheroid culture experiments were also performed in the extracellular matrix protein gel. Briefly, single cell suspension was re-suspended in the mixture of ice-cold media and PathClear Cultrex (Trevigen, USA) growth factor reduced basement membrane extract (1:3) and aliquoted into 12-well Ultra-Low Attachment polystyrene plates. Next day, a small amount of media was added to the wells. Fresh media was added every 4 days and plates were monitored for formation of spheroids using a Leica DMi8 or Nikon Eclipse Ti inverted microscope running Nikon Elements software, equipped with a temperature-controlled chamber and Hamamatsu Orca CCD camera. Images were taken at 4x magnification with at least 5 fields per sample. Number of spheroids was quantified (per field) as well as the diameter of each spheroid measured using Photoshop, Metamorph, or ImageJ. Data is represented with standard deviation error bars and a t-test for statistical analysis.
Statistical analyses
The data were plotted and analyzed in GraphPad Prism 6.0 with ANOVA, Student-test. Data are representative of at least three independent experiments (or as described in Figure legends) and are reported as mean ± SD. Corresponding P values are indicated within each graph.
Results
Depletion of PEAK1 in PDAC cells alters protein translation, cytoskeleton, and cell cycle regulation
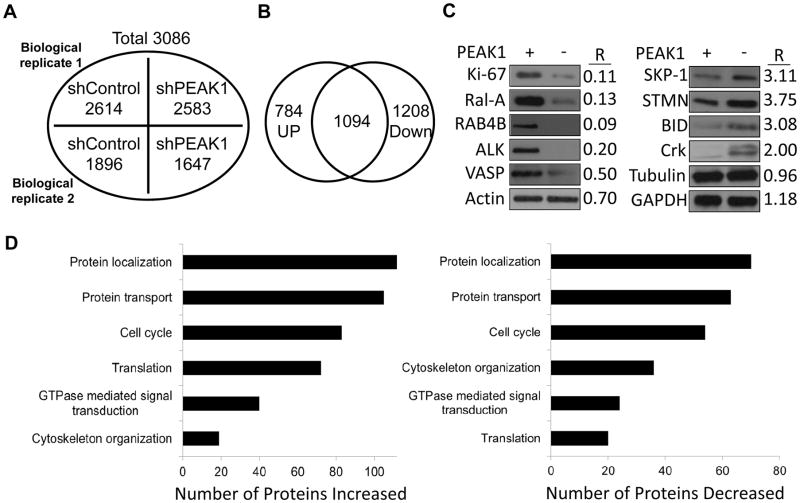
Label-free comparative LC-MS/MS analysis was performed to profile protein expression changes on 779E cells with or without stable PEAK1 knockdown using shRNA. 779E cells were recently established from a moderate-to-poorly differentiated patient-derived PDAC tumor that harbored KRasG12F mutation. PEAK1 was stably depleted in these cells using shRNA and showed approximately a 90% decrease in protein levels (supplementary Fig. S1) (1). Our proteomic strategy is shown in supplementary figure S1. To reliably identify protein changes resulting from PEAK1 knockdown, we analyzed two biological replicates in triplicate and considered only proteins with more than 2-fold differences in spectral counts (supplementary Fig. S1). Out of a total of 3086 proteins identified in both biological replicates, 784 proteins were down-regulated and 1028 proteins were up-regulated (Fig. 1A and B, supplementary Table S1). Western blot analyses of selected up and down regulated proteins recapitulated the mass spectrometry results indicating that our biological samples and protein quantification methods were valid (Fig. 1C).
Figure 1.
Proteomic and informatic analyses of proteins changes in PEAK1 knockdown PDAC cells. (A) Total number of proteins identified in two independent biological replicates from shRNA control (shControl) or shRNA PEAK1 (shPEAK1) treated 779E cells. (B) Venn diagram showing the overlap of proteins significantly down- or up-regulated by eIF5A knockdown from two biological replicates. (C) Cell lysates from 779E cells expressing shControl (PEAK1+) or shPEAK1 (PEAK1-) were western blotted for the indicated proteins. R = ratio of shPEAK/shControl protein levels as quantified by LC/MS/MS. (D) Functional classification of proteins increased or (E) decreased by shPEAK1 knockdown based on Gene Ontology (GO) analysis.
The functional relevance of proteins altered by PEAK1 knockdown was classified using Gene Ontology (GO) analysis. Protein translation, cytoskeleton organization, cell cycle, GTPase mediated signal transduction, protein localization, and protein transport were the major cellular functions altered by PEAK1 depletion (Fig. 1D and E). Protein localization, protein transport, and cell cycle were identfied as major cellular pathways in both the up regulated and downregulated data sets (Fig. 1D and E). These findings suggest that PEAK1’s scaffolding and/or kinase functions may serve to regulate the translation, transport and localization of proteins involved in cell cycle and cytoskeletal dynamics. It is notable that the upregulated protein group strongly annotated with protein translation functions (Fig 1D). A complete list of these proteins is shown in supplementary Table S2 and their protein-protein interaction network and quantitative features analyzed by STRING database and Cytoscape are shown in supplmentary figures S2A and B. In this group of proteins, eukaryotic translation initiation factors and ribosomal proteins (including mitochondrial ribosomal proteins) were significantly increased (supplementary Table S2). These findings suggest that global protein synthesis might be increased due to PEAK1 depletion. However, total protein levels were similar in control and PEAK1 knockdown cells (supplmentary Fig. S1B) and several house keeping proteins were similar under these conditions including actin, GAPDH, and tubulin (Fig. 1C). One possibility is that the observed increase in the protein translation machinery is a compensatory response resulting from cellular stress induced by the loss of PEAK1. PEAK1 knockdown cells did show reduced cell cycle/proliferation associated proteins (Ki-67, CCAR) and increased apoptosis associated proteins Bid, BAX, BIRC6, and several BCL2 family proteins (supplementary Table S1). Also, stress conditions can induce increased ribosomal protein expression, which regulate cell cycle progression and apoptosis through various mechanisms including enhanced translation (2).
Several proteins associated with cytoskeleton organization were down-regulated by PEAK1 knockdown (Fig. 1E). A complete list of these proteins is shown in supplementary Table S3 and their protein-protein interaction network and quantitative features analyzed by STRING database and Cytoscape are shown in supplmentary figures S3A and B. Notable proteins in this group include PTK2 (FAK, focal adhesion kinase 1), PAK2 (p21-activated kinase 2), FSCN1 (fascin actin-bundling proteins 1), YAP1 (yes-associated protein 1, Yorkie homolog) RALA (ras-related proteins Ral-A), ADD1 (α-adducin), ARHGEF11 (Rho guanine nucleotide exchange factor 11), CIT (Citron Rho-interacting kinase) and VASP (vasodilator-stimulated phosphoprotein). All of these proteins have been linked to critical cellular processes including cell migration, cytokinesis, proliferation, oncogenic transformation and membrane trafficking. These findings are consistant with previous work showing that PEAK1 associates with the actin cytoskeleton and localizes to focal adhesions where it plays a central role in cell migration, proliferation, and cancer metastasis (9, 10, 12–15).
PEAK1 controls YAP1 and TAZ protein expression in PDAC cells
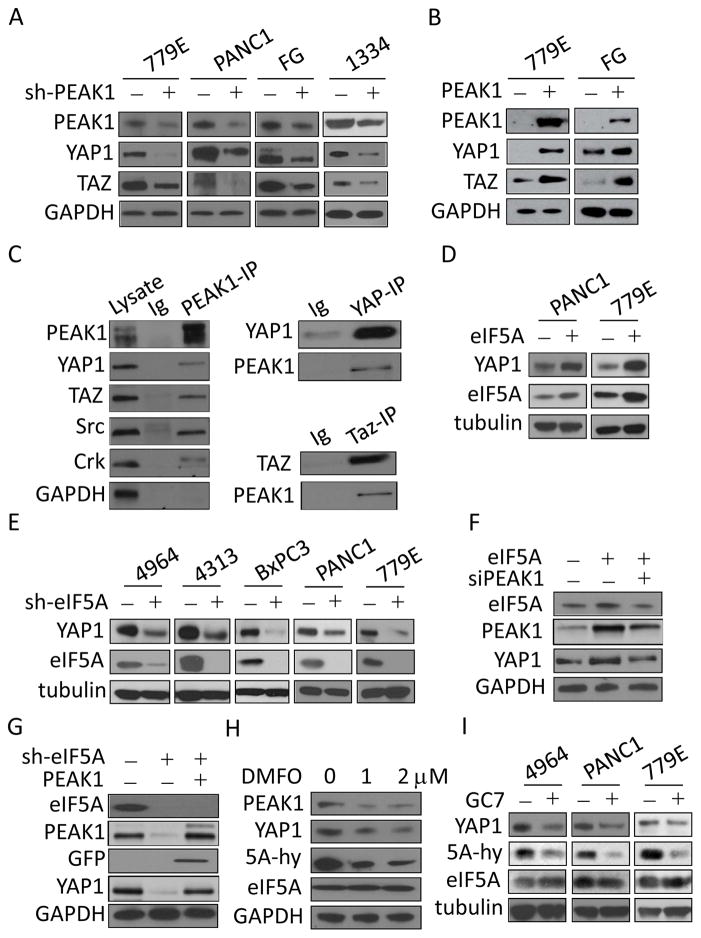
The downregulation of YAP1 in PEAK1 deficient cells was of particular significance as it is a central cytoskeletal sensor and transcriptional co-activator that controls cell proliferation, shape, and size downstream of extracellular cues (16). YAP1 is also essential for neoplastic progression of PDAC and it regulates important transcription factors linked to cancer malignacy including Nanog, Myc, Oct4, and TEAD (17–24). These findings prompted us to investigate whether PEAK1 regulates YAP1 and TAZ (YAP1 ortholog) protein expression and whether this process is important for PDAC progression. Western blotting revealed that YAP1 and TAZ protein levels were strongly decreased in response to PEAK1 depletion in FG, 779E, Panc1, and 1334 PDAC cells (Fig. 2A), which is consistant with our mass spectrometry findings (supplementary Table S1). Conversely, PEAK1 overexpression in PDAC cells increased endogenous YAP1/Taz protein levels (Fig. 2B). Depletion of PEAK1 with a second independent shRNA (supplementary Fig. S4) or with siRNA (Fig. 2F) reduced PEAK1 and YAP1 protein expression eliminating possible off-target effects. In addition, PEAK1/YAP1 and PEAK1/Taz were found to co-precipiated together in PDAC cells (Fig. 2C). These findings indicate that PEAK1 co-associates with YAP1/Taz proteins and regulates their expression levels in PDAC cells.
Figure 2.
PEAK1 associates with and regulates YAP1/TAZ protein levels in PDAC cells. (A) The indicated PDAC cells lines were depleted of PEAK1 using shPEAK1 (+) or control shRNAs (−) and western blotted for the indicated proteins. GAPDH served as a loading control. (B) The indicated cell lines were infected with lentiviruses encoding GFP-PEAK1 (+) or control viruses (−) and western blotted as in A. (C) Protein lysates were prepared from FG cells and western blotted for the indicated proteins. Also, PEAK1, YAP1, or TAZ were immunoprecipitated (IP) and then western blotted for the indicated proteins. Immunoglobulin coupled beads (Ig) served as a control. (D) The indicated cell lines were infected with lentiviruses encoding eIF5A (+) or control lentivirus (−) and western blotted for the indicated proteins. Tubulin served as a loading control. (E) The indicated cells were depleted of eIF5A using shRNAs to eIF5A (+) or control shRNAs (−) and western blotted for the indicated proteins. (F) eIF5A exexpressing cells as in D were depleted of PEAK1 protein using siRNA (+) or control siRNAs (−) and western blotted for the indicated proteins. (G) eIF5A knockdown cells as in E were infected with lentiviruses encoding PEAK1 (+) or control viruses (−) and western blotted for the indicated proteins. (H) FG cells were treated with the 1 or 2 μM α-difluoromethylornithine (DMFO) or vehicle (0) for 48 hours then lysed and western blottted for the indicated proteins. 5A-hy represents hypusine modified eIF5A which was detected with a eIF5A-hypusine specific antibody. (I) The indicate cell lines were treated with 20 μM GC7 (N(1)-guanyl-1,7,-diamineoheptane) or vehicle (0) for 48 hours then lysed and western blottted for the indicated proteins.
eIF5A-PEAK1 signaling controls YAP1 protein expression in PDAC cells
Our previous work showed that eIF5A controls PEAK1 protein expression in PDAC cells, which is involved in tumor formation and metastasis (1). Exogenous overexpression of eIF5A in PDAC cells increased YAP1 expression (Fig. 2D), whereas knockdown of eIF5A signficantly inhibited endogenous PEAK1 and YAP1 expression (Fig. 2E). Importantly, the increased YAP1 expression induced by eIF5A overexpression was inhibited in response to PEAK1 depletion (Fig. 2F). Also, exogenous expression of GFP tagged PEAK1 in eIF5A knockdown PDAC cells restored YAP1 protein levels (Fig. 2G). These findings indicate that eIF5A regulates YAP1 protein levels in PDAC cells in a PEAK1 dependent manner. Similar findings were also observed for TAZ in eIF5A knockdown and overxpressing cells (data not shown). In subsequent experiments, we focus primarily on YAP1 expression since YAP1 and TAZ are co-regulated by eIF5A-PEAK1 signaling in a similar manner.
eIF5A’s ability to mediate mRNA translation is uniquely regulated by hypusination. Hypusine (N-(4-amino-2-hydroxybutyl)lysine) is formed by the transfer of the butylamine portion of spermidine to the ε-amino group of a specific lysine substrate of eIF5A, which is catalyzed by deoxyhypusine synthase (DHPS) (1, 6, 7). Carbon 2 of the transferred 4-aminobutyl moiety is then hydroxylated by deoxyhypusine hydroxylase (DOHH). GC7 (N(1)-guanyl-1,7,-diamineoheptane) is an inhibitor of DHPS and DMFO (α-difluoromethylornithine) is an inhibitor of ornithine decarboxylase which prevents spermidine synthesis (25). Both drugs inhibit eIF5A hypusination leading to decreased PEAK1 expression in PDAC cells (Fig. 2H) and (1). Treatment of PDAC cells with DMFO or GC7 reduced eIF5A hypusination and YAP1 expression (Fig. 2H and I). These findings demonstrate that PEAK1 and YAP1 expression require activated hypusinated eIF5A.
eIF5A-PEAK1-YAP1 signaling regulates TEAD and CTGF protein expression in PDAC cells
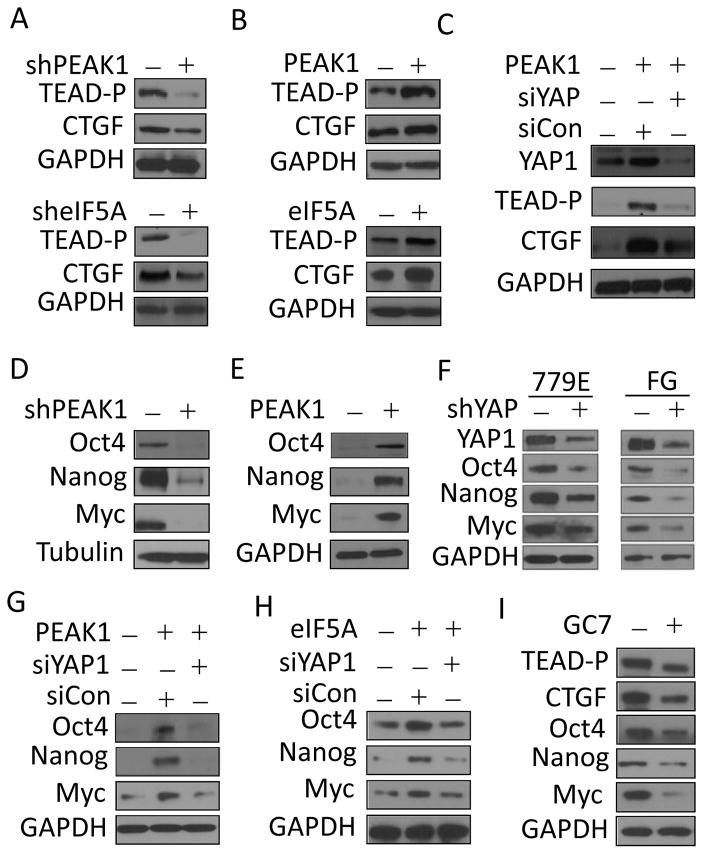
YAP1 binds to the TEAD family of transcription factors, which regulate genes important for cell growth, apoptosis and cancer progression (16). The YAP1/TEAD complex has been previously linked to several cancers including PDAC (16). Our proteomic results indicate that TEAD expression is strongly reduced in PEAK1 knockdown PDAC cells (supplementary Table S1). Western blot analyses using a pan TEAD antibody confirmed the reduced TEAD expression in PEAK1-deficient cells (Fig. 3A). TEAD is well known to regulate connective tissue growth factor (CTGF) expression, which has important roles in cell migration, proliferation and angiogenesis (16). PEAK1 knockdown in PDAC cells also reduced CTGF protein expression, whereas PEAK1 overexpression increased TEAD and CTGF expression (Fig. 3A and B). The increased TEAD/CTGF expression induced by PEAK1 was attenuated in response to YAP1 depletion indicating that YAP1 is downstream of PEAK1 and necessary for this process (Fig. 3C). Similar to PEAK1 depletion, eIF5A knockdown reduced TEAD and CTGF protein expression, whereas eIF5A overexpression increased TEAD and CTGF protein levels (Fig. 3A and B, lower panels). These findings indicate that eIF5A-PEAK1 signaling regulates YAP1 protein expression and its downstream signaling in PDAC cells.
Figure 3.
eIF5A-PEAK1-YAP1 signaling control the expression of stem cell-associated transcription factors. (A) 779E cells were depleted of either PEAK1 or eIF5A using shRNAs to PEAK1/eIF5A (+) or control shRNAs (−) and western blotted for the indicated proteins. (B) 779E cells were infected with lentiviruses encoding GFP-PEAK1 or eIF5A (+) or control lentivirus (−) and western blotted for the indicated proteins. (C) 779E cells overexpressing GFP-PEAK1 as in B were depleted of YAP1 using siRNA (siYAP1) or control siRNA (siCon) and western blotted for the indicated proteins. (D) 779E cells were depleted of PEAK1 using shPEAK1 (+) or control shRNAs (−) and western blotted for the indicated proteins. (E) 779E cells were infected with lentiviruses encoding GFP-PEAK1 (+) or control lentivirus (−) and western blotted for the indicated proteins. (F) FG and 779E cells were depleted of YAP1 using shRNAs to YAP1 (+) or control shRNAs (−) and western blotted for the indicated proteins. (G and H) 779E cells treated as in B were depleted of YAP1 using YAP1 siRNA (siYAP1) or control siRNA (siCon) and western blotted for the indicated proteins. (I) FG cells were treated with 20 μM GC7 or vehicle (0) for 48 hours then lysed and western blottted for the indicated proteins.
eIF5A-PEAK1-YAP1 signaling controls the expression of stem cell-associated transcription factors
In addition to TEAD/CTGF, YAP1 has been shown to regulate Oct4, c-Myc, and Nanog expression (17, 26–28). These stem cell-associated transcription factors (STFs) are highly relevant to PDAC progression, patient outcome, and drug resistance making them possible biomarkers of disease and therapeutic targets (26, 29). Importantly, Oct4, Nanog and c-Myc were reduced in PEAK1 knockdown PDAC cells (Fig. 3D). Conversely, PEAK1 overexpression increased the expression of these STFs (Fig 3E). Similar findings were obtained in YAP1 depleted cells (Fig. 3F). Importantly, the increased Oct4, Nanog and Myc expression induced by PEAK1 overexpression was inhibited upon YAP1 depletion indicating that this response was YAP1 dependent (Fig. 3G). eIF5A overexpression also increased Oct4, Nanog, and Myc levels, which was YAP1 dependent (Fig. 3H). Furthermore, inhibition of eIF5A hypusination with GC7 reduced Oct4, Nanog, and Myc as well as TEAD and CTGF expression in PDAC cells (Fig. 3I). These findings indicate that the eIF5A-PEAK1-YAP1 signaling module controls TEAD, Oct4, Nanog and Myc expression in PDAC cells.
eIF5A-PEAK1-YAP1 signaling mediate 3D tumor sphere formation
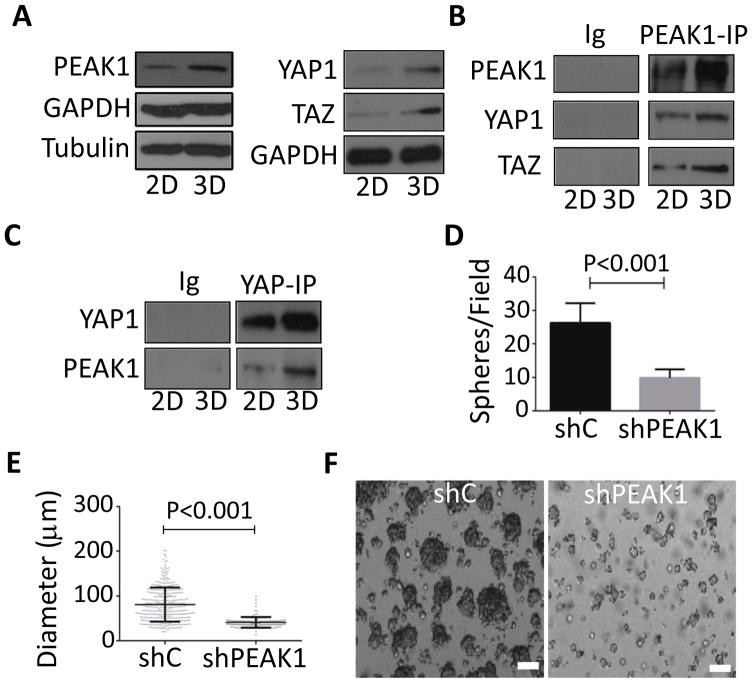
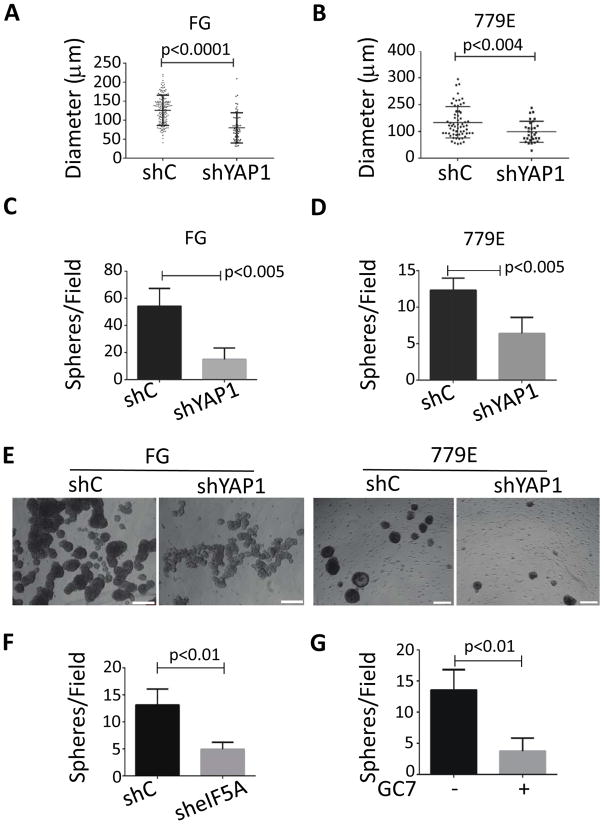
The aberrant expression of STFs by cancer cells increases tumorgenicity, which is commonly reflected in increased 3D sphere forming potential in vitro (30). Therefore, we investigate the role of eIF5A-PEAK1-YAP1 signaling in tumor sphere formation using a common 3D tumor colony forming assay under defined serum-free conditions. PEAK1 and YAP1/TAZ levels were increased in 3D cultures compared to cells cultured on plastic dishes in 2D in the presence of serum (Fig. 4A). Also, the level of PEAK1-YAP1-TAZ complexes were increased in 3D compared to 2D cultures (Fig. 4B and C). We did not detect eIF5A in PEAK1, YAP1 or TAZ immunoprecipitates. Importantly, PEAK1 and YAP1 were necessary for 3D sphere formation as PDAC cells depleted of PEAK1 or YAP1 showed reduced number and size of spheres compared to control cells (Fig. 4D–F and Fig 5A–E). eIF5A knockdown or treatment with GC7 also inhbited 3D sphere formation (Fig. 5F and G). We also attempted double knockdowns of PEAK1 and YAP1, but this strongly inhibited cell growth in 2D cultures and, thus, we were unable to generate viable cell numbers for sphere forming and cell-based assays in vivo. These findings indicate that hypusinated eIF5A, PEAK1, and YAP1 are necessary for PDAC tumor sphere formation in vitro.
Figure 4.
3D sphere formation increases PEAK1-YAP1 complexes in PDAC cells. (A) 779E cells were cultured on plastic dishes in the presence of serum (2D) or placed in non-adherent culture dishes without serum to induce 3D sphere formation (3D) as described in Materials and Methods. Cells were then lysed and western blotted for the indicated proteins. (B and C) 779E cells treated as in A were lysed and analysed for PEAK1, YAP1, and TAZ complexes by co-immunoprecipitation (IP) and western blotting. Immunoglobulin coupled beads (Ig) served as a control. (D) The number of 3D spheres per microscopic field and (E) 3D sphere diameter was determined for 779E cells depleted of PEAK1 by shPEAK1 or treated with control shRNA (shC). (F) Representative phase-contrast photomicrographs of shC and shPEAK1 779E cells in 3D culture. Bar = 50μm.
Figure 5.
eIF5A-PEAK1-YAP1 signaling is required for 3D sphere formation. (A–D) The number of 3D spheres per microscopic field and (E) 3D sphere diameter was determined for FG and 779E cells depleted of YAP1 by shRNA (shYAP1) or treated with control shRNA (shC). (E) Representative phase contrast photomicrographs of shC and shYAP1 FG and 779E cells in 3D culture. Bar = 50μm. (F) The number of spheres per microscopic field was determined for 779E cells depleted of eIF5A by shRNA (sheIF5A) or treated with control shRNA (shC). (G) The number of 3D spheres per microscopic field was determined for 779E cells cultured in 3D in the continued presence of 20 μM GC7 (+) or vehicle (−).
PDAC cells with increased Oct4 expression show increased eIF5A-PEAK1-YAP1-STF expression and increased tumor and 3D sphere formation
The expression of Oct4 is regulated by the Oct4 gene promoter whose structure has been studied extensively (31–33). Currently, GFP-Oct4 expression analysis under the control of its respective promoter is considered a valid biomarker and reporter system to study cell differentiation and the stem cell state in many cell types. Therefore, the GFP-Oct4 promoter reporter system provides an opportunity to isolate and study the endogenous differentiation state and tumor forming potentials of PDAC cells using fluorescence microscopy and/or flow cytometry. A FG-GFP-Oct4 reporter line (FG-pOct4-GFP) was developed as described (supplementary Materials and Methods).
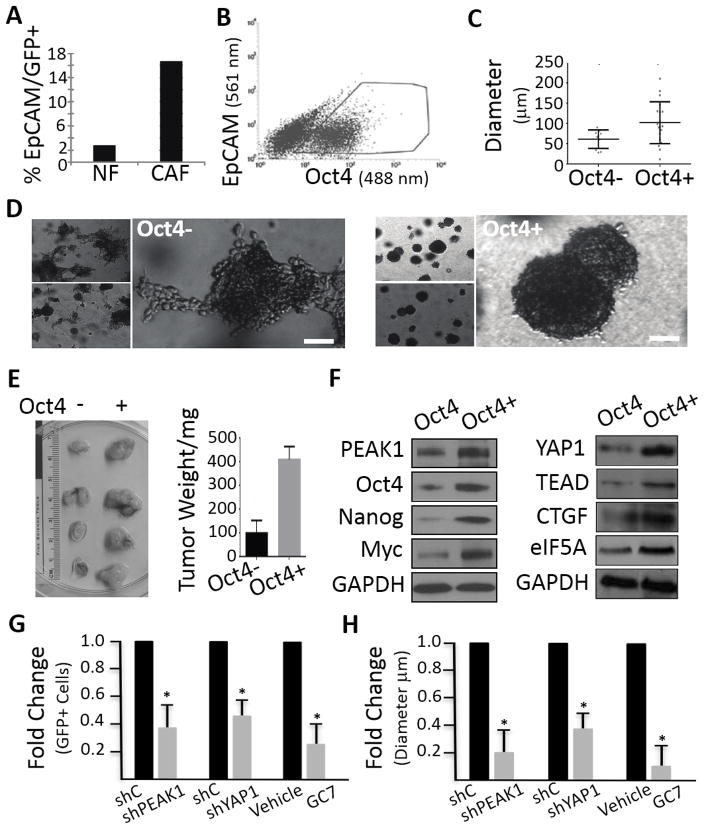
We first measured the number of EpCAM+/Oct4-GFP+ FG cells co-cultured with either freshly isolated cancer-associated fibroblasts (BK14 CAFs) or normal human fibroblasts (NUFF, control) by FACS (Fig. 6A). Unlike normal human fibroblasts, CAFs and their secreted products are believed to contribute to the upregulation of stem-like properties in cancer cells, which increases Oct4 reporter expression (34, 35). Indeed, FG-Oct4-GFP cells co-cultured with CAFs showed an 8 fold increase in reporter positive cells compared to these same cells cocultured with normal human fibroblasts, indicating that the Oct4-GFP promoter reporter is regulated and functioning in these cells (Fig. 6A).
Figure 6.
eIF5A-PEAK1-YAP1 signaling in Oct4 reporter PDAC cells. (A) FG cells expressing GFP fused to the Oct4 promoter were analysed for expression changes in co-cultures with normal human fibroblasts (NF) or freshly isolated cancer-associated fibroblasts (CAFs) derived from PDAC patients as described in supplementary Materials and Methods. The percentage of cells expressing both the Oct4 reporter (GFP+) and the epithelial marker (EpCAM+) were detemined for each of the co-cultures by FACS. (B) Representative FACS profile and gating used for the enrichment of FG cells expressing the GFP-Oct4 reporter in traditional 2D cultures. (C) The size of tumor spheres in 3D cultures was determined for GFP-Oct4+ and GFP-Oct4- cell populations isolated as in B. (D) Representative phase contrast photomicrographs of GFP-Oct4+ and GFP-Oct4- cell populations in 3D cultures. Bar = 20μm. Note that GFP-Oct4+ cells form round compact spheres, whereas GFP-Oct4- cells form smaller and loosely adherent, disorganized cell-cell clusters. (E) Tumor size in mice was determined for GFP-Oct4+ and GFP-Oct4- cell populations isolated as in B. p<0.05 by Student t test. (F) Western blots for the indicated proteins from GFP-Oct4+ and GFP-Oct4- cell populations isolated as in B. (G) FG cells expressing the GFP-Oct4 promoter reporter and depleted of PEAK1 (shPEAK1), YAP1 (shYAP1), or treated with GC7 were examined for changes in the percentage of GFP+ cells by FACS relative to control cells treated with non-specific shRNAs (shC) or drug vehicle. * p< 0.01 Student t test. (H) GFP-Oct4+ and GFP-Oct4- cells treated as in G and examined for changes in sphere diameter. Fold changes are relative to shControl and vehicle treat cells as in G. * p< 0.01 Student t test.
If Oct4 transcriptional expression is associated with increased tumorigenicity, then FGpOct4-GFP+ cells should show increased spheroid formation and tumor growth in vivo. To test this notion, we sorted FG-Oct4-GFP+ and FG-Oct4-GFP- cell populations by FACS (Fig. 6B) and then examined their ability to form 3D spheroids and tumors in mice. FG-Oct4-GFP+ cells formed significantly larger spheroids (Fig. 6C and D), and showed increased tumor size in vivo compared to FG-Oct4-GFP- cells (Fig. 6E). FG-Oct4-GFP+ cells also displayed a unique morphology characterized by a more rounded and compact shape compared to FG-Oct4-GFP- cells, which grew as loosely attached and disorganized cell clusters (Fig. 6D). Importantly, associated with increased tumorigenicity of the FG-Oct4-GFP+ cells was increased Oct4, PEAK1, Nanog, c-Myc, YAP1, TEAD, CTGF and eIF5A (Fig. 4F). Depletion of PEAK1 and YAP1 as well as treatment with GC7 signficantly reduced the number of FG-Oct4-GFP+ cells in 2D cultured cells and reduced their ability to form 3D spheres (Fig. 6G and H). These findings suggest that the eIF5A-PEAK1-YAP1 signaling module regulates an STF program important for PDAC sphere formation and tumorgenicity.
PEAK1, YAP1, TEAD1, and CTGF mRNAs are increased in PDAC patient samples
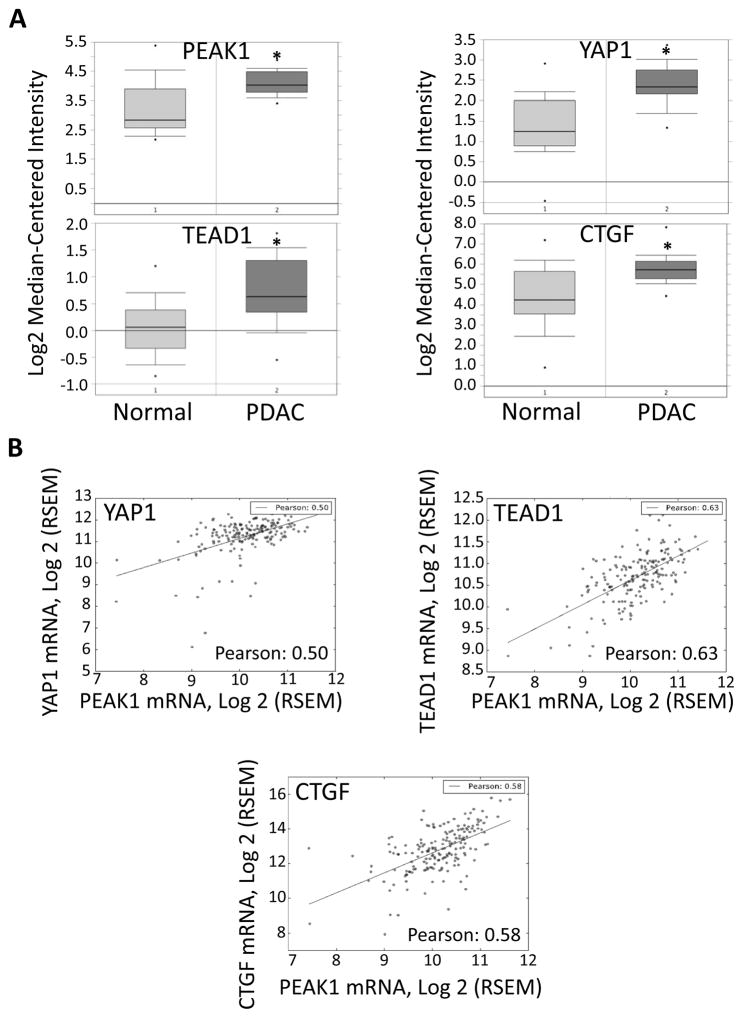
Finally, we integrated Oncomine and The Cancer Genome Atlas (TCGA) RNA databases to determine if the increased RNA expression levels of PEAK1 correlated with YAP1, TEAD, and CTGF RNA expression levels in PDAC. Indeed, PEAK1, YAP1, TEAD, and CTGF RNA levels all showed a strong correlative increase in PDAC patients (Fig. 7A and B). A similar strong correlation was also observed for breast, prostrate, and thymoma (supplementary Table S4). However, Oct4, Nanog and c-Myc mRNA expression levels did not strongly correlated with PEAK1, eIF5A, or YAP1 expression levels in PDAC (data not shown). These findings suggest that the eIF5A-PEAK1-YAP1-TEAD-CTGF signaling module is amplified at the mRNA and protein levels in PDAC patients, and that this signaling pathway may modulate STF protein expression post-transcriptionally.
Figure 7.
Correlation of PEAK1, YAP1, TEAD, and CTGF mRNA levels in PDAC.
(A) Oncomine analyses of PEAK1, YAP1, TEAD1 and CTGF mRNA levels in normal human tissue and PDAC tumor tissue obtained from the Badea pancreas dataset using the Oncomine database consisting of 39 normal and 39 PDAC tissue samples. The log2 median centered intensity value was recorded and graphed as shown. * Student’s t-test: normal vs PDAC samples for PEAK1 (p=1.88E-7), YAP1 (p=2.71E-10), TEAD1 (p=2.08E-7), and CTGF (p=2.37E-7). (B) Pearson’s correlation analysis of mRNA expression in PDAC patients using the RSEM (RNA-Seq by Expectation Maximization) normalized RNA-seq data from the TCGA collection. The log2 transformed RNA-seq data, and the calculated Pearson’s correlation coefficient is shown between the mRNA expression of PEAK1 and the indicated genes. A linear regression fit was plotted and overlaid with scatter plot for visualization. Supplementary Table S4 shows Pearson’s correlation coefficients for all 32 cancer types from the TCGA database. Values of 0.50 and greater indicate high correlation.
Discussion
Despite the critical role of KRas-eIF5A-PEAK1 signaling in regulating cancer cell proliferation, migration, and metastasis, the downstream signalings that control these cellular processes are not understood (1, 9, 10, 12–14). Here we provide evidence that eIF5A and PEAK1 are major upstream regulators controlling YAP1/TAZ protein levels in PDAC cells. These co-transcriptional activators, in turn, control STFs in the nucleus to regulate cell proliferation and differentiation in normal and malignant cells (2, 17, 19, 21, 27–29, 32, 36–39). Our discovery that eIF5A-PEAK1 couples to YAP1/TAZ signaling provides a plausible mechanism for how PEAK1, a focal adhesion and cytoskeleton-associated kinase, can communicate with the nucleus to control cancer cell proliferation and differentiation. Understanding how this signaling pathway is regulated has clinical relevance as eIF5A, PEAK1, YAP1/TAZ and their downstream STFs have all been shown to be amplified in PDAC patient tissues (16, 18–20, 23, 24, 28, 37, 39, 40).
Our previous work showed that eIF5A and PEAK1 proteins are amplified in response to mutational activation of KRas (i.e. KRasG12D), the major oncogenic driver in PDAC patients (Fig. 6) (1). In this work, we demonstrated that eIF5A regulates PEAK1 expression and that eIF5A-PEAK1 signaling is necessary for KRasG12D dependent oncogenesis, cell migration, metastasis, and orthotopic tumor fomation in mice. Our work here indicates that YAP1/TAZ expression is a critical downstream target modulated by eIF5A-PEAK1 signaling. eIF5A, PEAK1, and YAP1/TAZ are known to integrate and relay extracellular information through integrins, growth factors and cell-cell adhesion receptors to the actin cytoskeleton (1, 4, 8, 12, 14, 16). Amplification of this cytoskeleton signaling module may contribute to PDAC in several ways. First, KRas-induced upregulation of this pathway may serve to bypass normal anchorage-dependent cell growth control mechanisms and promote significant morphological and hyperplastic responses (10, 18, 20, 23, 24, 28). Indeed, eIF5A, PEAK1, and YAP1 are all indispensible for KRas-mediated PDAC growth in non-adherent 3D spheroid cultures (Figs. 4 and 5) (1, 10, 24). Second, YAP1 is a potent co-transcription activator that relays information from the cytoskeleton to the nucleus where it can control transcription factors like TEAD and c-Myc, known to regulate cell growth and apoptosis (16, 21, 23, 28). Third, YAP1 has been reported to control normal stem cell functions including self-renewal and differentiation by targeting STFs including Oct4, Nanog, c-Myc, and TEAD (16, 17, 26, 27, 29, 36). These transcription factors have clinical significance their expression are indicative of poor patient prognosis in PDAC and several other cancers (16, 17, 26, 27, 29, 36). It is also notable that in preclinical mouse models of PDAC progression, eIF5A, PEAK1, and YAP1 protein levels are low in normal pancreatic ducts, but become upregulated in early-stage PanINs in response to KRas activation (1, 10, 20, 24). These findings suggest that eIF5A-PEAK1-YAP1/TAZ may work downstream of KRas to drive early events involved in tumor initiation. In support of these findings, we were unable to generate double PEAK1 and YAP1 knockdown PDAC cell lines for analyses in in vitro and in vivo tumor forming assays. These cells failed to grow in standard 2D cultures even in the presence of high concentrations of serum. The inability to generate stable PEAK1/YAP1 knockdown cell lines in vitro precluded tumor initiation studies in mice and will require the use of inducible gene knockdown model or the development of mouse transgenic lines. However, single knockdown of PEAK1, eIF5A or YAP1 have been shown to reduce tumor formation in mice indicating that these genes do play a central role in tumor formation in animals (1, 4, 8–10, 14, 18–20). Also, eIF5A, PEAK1, and YAP1 regulate epithelial to mesenchymal transition, which is associated with cytoskeleton alterations and a de-differentiated stem cell-like phenotype (13, 15, 16, 23). Together these findings are consistant with the idea that eIF5A-PEAK1-YAP1 signaling contributes to tumor initiation by reactivating an stem cell-like transcription program in adult PDAC cancer cells, which is associated with increased tumorigenicity and poor patient outcomes.
A question that needs further study is how eIF5A-PEAK1 signaling regulates the protein levels of YAP1 and TAZ. Protein synthesis demands are increased in PDAC cells due to increased cell proliferation and metabolism (2). In this regard, it is interesting that eIF5A does not regulate global protein synthesis, but rather is specifically recruited to the ribosome to fine tune the production of specific subsets of proteins, including proteins that contain one or more polyproline motifs (6, 7). It has been proposed that eIF5A is upregulated in hyperactive cancer cells due to increased demands for such proteins (1, 5–7). PEAK1 and YAP1 do contain polyproline motifs making them good candidates for regulation by eIF5A using this translational mechanism. However, while this mechanism is likely responsible for regulation of PEAK1/YAP1/Taz protein levels, other possible mechanisms may be involved in regulating this process. For example, the fact that PEAK1 and YAP1/TAZ co-immunoprecipitate from PDAC cell lysates (Fig. 2C and Fig. 4B and C) suggest that they may form a stable molecular complex in the cytosol, protected from protein degradation. eIF5A-PEAK1 amplification in malignant cells could also upregulate YAP1/TAZ gene transcription. This possibility is consistant with our informatic analyses showing that PEAK1 and YAP1 as well as TEAD1 and CTGF mRNA levels are all co-elevated in PDAC patient samples (Fig. 7). Furthermore, eIF5A can directly shuttle certain mRNA messages from the nucleus to the cytosol for translation (4, 6, 41). Therefore, eIF5A may use a similar mRNA transport mechanism to control PEAK1 and/or YAP1/TAZ levels. Finally, eIF5A-PEAK1 signaling may directly regulate Lats1 activity, which is a well described upstream kinase that mediates YAP1 protein phosphorylation and its degradation in the cytosol (16, 42). Furthermore, all of these mechanisms may not be mutually exclusive, but may work cooperatively to modulate YAP1/TAZ protein expression in PDAC cells.
In summary, our findings demonstrate an important new link between eIF5A-PEAK1 and YAP1/TAZ signaling, which controls the transcriptional network of stemness-associated genes involved in PDAC development and growth. The fact that eIF5A hypusination can be therapeutically targeted, provides a possible means to block this pathway with small molecule inhibitors like GC7 and DMFO, which could benefit PDAC patients (1, 2, 4, 6, 8, 25, 43). Such new biologiocal targets and therapeutic approaches are sorely needed to treat this deadly disease.
Supplementary Material
Acknowledgments
We would like to thank Dr. Andrew Lowy (UCSD, Moores Cancer Center) for kindly providing 779E and 1334 cells and Drs. Cristina Metildi, MD and Sharmeela Kaushal, Ph.D. for help with initial cell grafting. This work was supported by funding from NIH to R.L.K (CA182495 and CA097022), from NCI to K.F (CA180374), M.B (CA142669 and CA132971), Hartwell Foundation, NIH NCI CA157885 to J.B., and NIH R35 CA196878 and R01 GM51586 to K.L.G. The Kelber Lab acknowledges the CSUN CSM/ORSP, CSUPERB, Medtronic and the Sidney Stern Memorial Trust for ongoing support of research.
Financial Support: R.L.Klemke, NIH CA182495, CA097022; K. Fujimura, NIH CA180374; M. Bouvet, NIH CA142669, CA132971; J. Bui, Hartwell Foundation, NIH CA157885; K.L.Guan, NIH CA196878, GM51586; J. Kelber, CSM/ORSP, CSUPERB.
Footnotes
Conflict of Interest: The authors have read and understood Cancer Research policy on declaration of interests, and declare that they have no competing interests.
References
- 1.Fujimura K, Wright T, Strnadel J, Kaushal S, Metildi C, Lowy AM, et al. A hypusine-eIF5A-PEAK1 switch regulates the pathogenesis of pancreatic cancer. Cancer Res. 2014;74:6671–81. doi: 10.1158/0008-5472.CAN-14-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I. Targeting the translation machinery in cancer. Nat Rev Drug Discov. 2015;14:261–78. doi: 10.1038/nrd4505. [DOI] [PubMed] [Google Scholar]
- 3.Michael AJ. Polyamines in Eukaryotes, Bacteria, and Archaea. J Biol Chem. 2016;291:14896–903. doi: 10.1074/jbc.R116.734780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caraglia M, Park MH, Wolff EC, Marra M, Abbruzzese A. eIF5A isoforms and cancer: two brothers for two functions? Amino Acids. 2013;44:103–9. doi: 10.1007/s00726-011-1182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujimura K, Choi S, Wyse M, Strnadel J, Wright T, Klemke R. Eukaryotic Translation Initiation Factor 5A (EIF5A) Regulates Pancreatic Cancer Metastasis by Modulating RhoA and Rho-associated Kinase (ROCK) Protein Expression Levels. J Biol Chem. 2015;290:29907–19. doi: 10.1074/jbc.M115.687418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dever TE, Gutierrez E, Shin BS. The hypusine-containing translation factor eIF5A. Crit Rev Biochem Mol Biol. 2014;49:413–25. doi: 10.3109/10409238.2014.939608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez E, Shin BS, Woolstenhulme CJ, Kim JR, Saini P, Buskirk AR, et al. eIF5A promotes translation of polyproline motifs. Mol Cell. 2013;51:35–45. doi: 10.1016/j.molcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathews MB, Hershey JW. The translation factor eIF5A and human cancer. Biochim Biophys Acta. 2015;1849:836–44. doi: 10.1016/j.bbagrm.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelber JA, Klemke RL. PEAK1, a novel kinase target in the fight against cancer. Oncotarget. 2010;1:219–23. doi: 10.18632/oncotarget.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelber JA, Reno T, Kaushal S, Metildi C, Wright T, Stoletov K, et al. KRas induces a Src/PEAK1/ErbB2 kinase amplification loop that drives metastatic growth and therapy resistance in pancreatic cancer. Cancer Res. 2012;72:2554–64. doi: 10.1158/0008-5472.CAN-11-3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Bristow JM, Reno TA, Jo M, Gonias SL, Klemke RL. Dynamic phosphorylation of tyrosine 665 in pseudopodium-enriched atypical kinase 1 (PEAK1) is essential for the regulation of cell migration and focal adhesion turnover. J Biol Chem. 2013;288:123–31. doi: 10.1074/jbc.M112.410910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agajanian M, Campeau A, Hoover M, Hou A, Brambilla D, Kim SL, et al. PEAK1 Acts as a Molecular Switch to Regulate Context-Dependent TGFbeta Responses in Breast Cancer. PLoS One. 2015;10:e0135748. doi: 10.1371/journal.pone.0135748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Kelber JA, Tran Cao HS, Cantin GT, Lin R, Wang W, et al. Pseudopodium-enriched atypical kinase 1 regulates the cytoskeleton and cancer progression [corrected] Proc Natl Acad Sci U S A. 2010;107:10920–5. doi: 10.1073/pnas.0914776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tactacan CM, Phua YW, Liu L, Zhang L, Humphrey ES, Cowley M, et al. The pseudokinase SgK223 promotes invasion of pancreatic ductal epithelial cells through JAK1/Stat3 signaling. Mol Cancer. 2015;14:139. doi: 10.1186/s12943-015-0412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73–9. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bora-Singhal N, Nguyen J, Schaal C, Perumal D, Singh S, Coppola D, et al. YAP1 Regulates OCT4 Activity and SOX2 Expression to Facilitate Self-Renewal and Vascular Mimicry of Stem-Like Cells. Stem Cells. 2015;33:1705–18. doi: 10.1002/stem.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greten FR. YAP1 takes over when oncogenic K-Ras slumbers. Cell. 2014;158:11–2. doi: 10.1016/j.cell.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Gruber R, Panayiotou R, Nye E, Spencer-Dene B, Stamp G, Behrens A. YAP1 and TAZ Control Pancreatic Cancer Initiation in Mice by Direct Up-regulation of JAK-STAT3 Signaling. Gastroenterology. 2016;151:526–39. doi: 10.1053/j.gastro.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–97. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–18. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morvaridi S, Dhall D, Greene MI, Pandol SJ, Wang Q. Role of YAP and TAZ in pancreatic ductal adenocarcinoma and in stellate cells associated with cancer and chronic pancreatitis. Sci Rep. 2015;5:16759. doi: 10.1038/srep16759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–84. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakanishi S, Cleveland JL. Targeting the polyamine-hypusine circuit for the prevention and treatment of cancer. Amino Acids. 2016 doi: 10.1007/s00726-016-2275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herreros-Villanueva M, Bujanda L, Billadeau DD, Zhang JS. Embryonic stem cell factors and pancreatic cancer. World J Gastroenterol. 2014;20:2247–54. doi: 10.3748/wjg.v20.i9.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao W, Wang J, Ou C, Zhang Y, Ma L, Weng W, et al. Mutual interaction between YAP and c-Myc is critical for carcinogenesis in liver cancer. Biochem Biophys Res Commun. 2013;439:167–72. doi: 10.1016/j.bbrc.2013.08.071. [DOI] [PubMed] [Google Scholar]
- 28.Nussinov R, Tsai CJ, Jang H, Korcsmaros T, Csermely P. Oncogenic KRAS signaling and YAP1/beta-catenin: Similar cell cycle control in tumor initiation. Semin Cell Dev Biol. 2016 doi: 10.1016/j.semcdb.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimshaw MJ, Cooper L, Papazisis K, Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, et al. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10:R52. doi: 10.1186/bcr2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiagarajan PS, Hitomi M, Hale JS, Alvarado AG, Otvos B, Sinyuk M, et al. Development of a Fluorescent Reporter System to Delineate Cancer Stem Cells in Triple-Negative Breast Cancer. Stem Cells. 2015;33:2114–25. doi: 10.1002/stem.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu G, Wilson G, Zhou G, Hebbard L, George J, Qiao L. Oct4 is a reliable marker of liver tumor propagating cells in hepatocellular carcinoma. Discov Med. 2015;20:219–29. [PubMed] [Google Scholar]
- 33.Levings PP, McGarry SV, Currie TP, Nickerson DM, McClellan S, Ghivizzani SC, et al. Expression of an exogenous human Oct-4 promoter identifies tumor-initiating cells in osteosarcoma. Cancer Res. 2009;69:5648–55. doi: 10.1158/0008-5472.CAN-08-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–56. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 35.Chen WJ, Ho CC, Chang YL, Chen HY, Lin CA, Ling TY, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun. 2014;5:3472. doi: 10.1038/ncomms4472. [DOI] [PubMed] [Google Scholar]
- 36.Diep CH, Zucker KM, Hostetter G, Watanabe A, Hu C, Munoz RM, et al. Down-regulation of Yes Associated Protein 1 expression reduces cell proliferation and clonogenicity of pancreatic cancer cells. PLoS One. 2012;7:e32783. doi: 10.1371/journal.pone.0032783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin H, Sun LH, Han W, He TY, Xu XJ, Cheng K, et al. Knockdown of OCT4 suppresses the growth and invasion of pancreatic cancer cells through inhibition of the AKT pathway. Mol Med Rep. 2014;10:1335–42. doi: 10.3892/mmr.2014.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162:780–94. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wen J, Park JY, Park KH, Chung HW, Bang S, Park SW, et al. Oct4 and Nanog expression is associated with early stages of pancreatic carcinogenesis. Pancreas. 2010;39:622–6. doi: 10.1097/MPA.0b013e3181c75f5e. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Zhu H, Shan H, Lu J, Chang X, Li X, et al. Knockdown of Oct4 and Nanog expression inhibits the stemness of pancreatic cancer cells. Cancer Lett. 2013;340:113–23. doi: 10.1016/j.canlet.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Kaiser A. Translational control of eIF5A in various diseases. Amino Acids. 2012;42:679–84. doi: 10.1007/s00726-011-1042-8. [DOI] [PubMed] [Google Scholar]
- 42.Moroishi T, Park HW, Qin B, Chen Q, Meng Z, Plouffe SW, et al. A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis. Genes Dev. 2015;29:1271–84. doi: 10.1101/gad.262816.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammed A, Janakiram NB, Madka V, Ritchie RL, Brewer M, Biddick L, et al. Eflornithine (DFMO) prevents progression of pancreatic cancer by modulating ornithine decarboxylase signaling. Cancer Prev Res (Phila) 2014;7:1198–209. doi: 10.1158/1940-6207.CAPR-14-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.