Abstract
Objectives
To evaluate the associations between intakes of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and the intermediate and advanced stages of age-related macular degeneration (AMD).
Design
Prospective cohort study.
Participants
We followed 75,889 women from the Nurses’ Health Study and 38,961 men from the Health Professionals Follow-Up Study who were at least 50 years old from 1984 to 2012 and 1986 to 2010, respectively. Cohort participants are mostly white (≥ 95%).
Methods
We assessed dietary intake by a validated food frequency questionnaire (FFQ) at baseline and every four years. We calculated cumulative average intakes of EPA and DHA from FFQs and also computed predicted erythrocyte and plasma scores directly from food intake using regression models. Cox proportional hazards models were used to compute the associations with AMD outcomes.
Main Outcome Measures
We confirmed 1,589 incident intermediate and 1,356 advanced AMD cases (primarily neovascular AMD) with a visual acuity of 20/30 or worse due primarily to AMD by medical record review.
Results
For intermediate AMD, the pooled hazard ratio (HR) between the two cohorts for DHA comparing the extreme quintiles of intake was 0.78 (95% CI, 0.66 – 0.92; p trend, 0.008) and for EPA + DHA was 0.83 (95% CI, 0.71 – 0.98; p trend, 0.03). The pooled HR for fatty fish comparing ≥ 5 servings/week to almost never was 0.61 (95% CI, 0.46 – 0.81; p trend, < 0.001). For advanced AMD, the pooled HR for DHA was 1.01 (95% CI, 0.84 – 1.21; p trend, 0.75) and for fatty fish was 0.80 (95% CI, 0.59 – 1.08; p trend, 0.11). Secondary analyses using predicted erythrocyte and plasma scores of EPA and DHA yielded slightly stronger inverse associations for intermediate AMD and similar results for advanced AMD.
Conclusions
Higher intakes of EPA and DHA may prevent or delay the occurrence of visually significant intermediate AMD. However, the totality of current evidence for EPA and DHA and advanced AMD is discordant, though there was no association with advanced AMD in the present study.
Introduction
Age-related macular degeneration (AMD) is a chronic, degenerative disease of the macula, which can result in loss of the ability to read, write or drive.1 Although the advanced stages of AMD are often debilitating, some forms of advanced AMD can now be successfully managed with intravitreal injection of anti-VEGF agents, allowing patients to maintain or even restore vision for variable periods of time.2, 3 On the other hand, although usually less visually debilitating, early/intermediate AMD affects a much larger number of persons worldwide, and increases risk of development of advanced AMD. According to the 2005–2008 US National Health and Nutrition Examination Survey (NHANES), the estimated prevalence in persons aged 40 years and older was 5.7% for early/intermediate AMD versus 0.8% for advanced AMD.4 Globally, among people of European ancestry, the prevalence was 11.2% for early/intermediate AMD versus 0.5% for advanced AMD.5 Although decreasing exposure to some risk factors (e.g. smoking and blood pressure) in recent years might eventually help mitigate the incidence of early/intermediate AMD,4 owing to rapidly aging populations and lack of other effective means of primary prevention, the number of early/intermediate AMD cases is expected to double in the next few decades.5–7 Therefore, identification of other means of primary prevention, especially for vision-threatening early/intermediate AMD cases, would carry marked public health significance.
Docosahexaenoic acid (DHA), a long-chain omega3 (n3) fatty acid, is a major lipid component of retinal photoreceptor outer segment membranes that has anti-inflammation and anti-angiogenesis properties that could protect against AMD.8 The retinal concentration of DHA is dependent upon and modifiable by diet. Eicosapentaenoic acid (EPA), although not concentrated in the retina, is a precursor to DHA, and its metabolites could similarly affect the pathogenic processes of AMD.8 Our earlier study9 and investigations by others10–12 suggested that long-chain n3 fatty acids (DHA, EPA, and other 20 and 22-carbon n3 fatty acids) may reduce the risk of early/intermediate AMD. With respect to advanced AMD, intake of DHA was inversely associated with advanced AMD in several prospective cohort studies;10, 13–15 but this finding was not corroborated by the Age-Related Eye Disease Study 2 (AREDS2) trial in which supplementation with DHA and EPA for 5 years did not slow the progression to advanced AMD among patients with intermediate AMD.16
In light of the mixed findings from prior literature, we aimed to evaluate the relations of intakes of EPA and DHA to different forms of AMD in large prospective cohorts over 28 years of follow-up.
Methods
Study Population
The two large ongoing US prospective cohorts, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS), have been described in detail before.17, 18 Briefly, the NHS includes 121,701 US female registered nurses aged 30 to 55 years in 1976. The HPFS includes 51,529 US male health professionals aged 40 to 75 years in 1986. Both cohorts are predominantly white (96.8% in the NHS and 95.7% in the HPFS). The long-term follow-up rates are >95%. Questionnaires were mailed to all participants biennially to inquire updated lifestyle factors and disease outcomes. Food frequency questionnaires (FFQ’s) were mailed every four years to assess diet in the preceding year. Submission of a completed self-administered questionnaire was deemed to imply informed consent. The study protocol was approved by the Institutional Review Boards at the Brigham and Women's Hospital and Harvard T.H. Chan School of Public Health.
At study baseline (1984 in the NHS and 1986 in the HPFS), we excluded participants who did not return the initial FFQ, left more than 70 food items blank, reported implausible dietary intake (<600 or >3500 Kcal/d for the NHS and <800 or > 4200 Kcal/d for the HPFS), had prevalent AMD, or serious chronic diseases including cancer (except nonmelanoma skin cancer), diabetes and cardiovascular disease. To minimize detection bias, we also excluded participants who never reported an eye exam over the entire follow-up and excluded from analysis the person-time during any two-year interval in which a participant did not report an eye exam. Results did not materially change in sensitivity analyses including time intervals lacking an eye exam. Participants were included in the analysis when ≥ 50 years old, and were censored at age 90 to alleviate concerns of low reporting validity (NHS, n=15; HPFS, n=528). By the end of follow-up, a total of 75,889 women and 38,961 men contributed to the analysis. Those excluded participants tended to be slightly older, less physically active and smoked more than those included (eTable 1).
AMD Ascertainment
Our case definition has been previously described19 and also validated by comparison with retinal images and medical records.20 Briefly, when a participant reported a diagnosis of AMD on a biennial questionnaire, we requested informed consent and then contacted the participant’s eye doctor to confirm the diagnosis by reviewing medical records. We defined intermediate AMD as having at least one of the following signs: intermediate drusen (≥ 63 and <125 µm), pigment abnormalities, large drusen (≥ 125 µm) or any non-central geographic atrophy (GA). Cases with only small hard drusen (< 63µm diameter) were excluded. We defined neovascular AMD as having any of the following: RPE detachment, subretinal neovascular membrane, disciform scar, or history of treatment with laser, photodynamic, or anti-VEGF therapies for AMD. Central GA was defined as having a central geographic atrophy lesion involving the center of the macula. Advanced AMD included neovascular AMD and central GA. Additionally, all case definitions, except those recent neovascular AMD cases that had anti-VEGF therapies, included a visual acuity of 20/30 or worse due primarily to AMD. This magnitude of vision loss is not only of clinical significance, but also is severe enough to warrant medical attention so as to minimize potential detection bias arising from differential health consciousness. The person was used as the unit of analysis, and the worst eye was used for classification.
Dietary Assessment
We began follow up in 1984 for the NHS and 1986 for the HPFS, when the first comprehensive FFQ with an expanded section on fish was administered, and assessed dietary intake every four years thereafter. FFQ items on fish or seafood consumption include 1) canned tuna (3–4 oz); 2) dark meat fish (3–5 oz); 3) other fish (mainly white fish, 3–5 oz); 4) shrimp, lobster or scallops as main dish (3–5 oz). On the FFQs, commonly used units or portion sizes (e.g., 1 orange or ½ cup broccoli) are specified for the approximately 130 items. Participants were asked to report how often, on average over the past year, they had consumed each food item (responses ranging from “≤1 time per month” to “≥6 times/day”). Fish oil supplements including marine and cod liver oils were assessed from 1990 in the NHS and 1988 in the HPFS. We calculated nutrient intakes by multiplying the consumption frequency of each food by the nutrient content of the specified food portion summing across all foods. The nutrient composition data were primarily based on the US Department of Agriculture Nutrient Database supplemented with information from manufacturers and published reports. Nutrient values were energy-adjusted using the residual method.21
The validity and reproducibility of FFQs in measuring polyunsaturated fatty acids and fish intake has been assessed in a random sample of 118 HPFS participants who completed two consecutive FFQs (1986 & 1987), two 1-week dietary records ~ 7 months apart and provided subcutaneous fat samples.22 The correlation was 0.61 for fish between two FFQs.23 The correlation between energy-adjusted EPA from FFQs and percentage of EPA in the adipose tissue was 0.47.22 Earlier validation studies in the NHS cohort had similar findings.24, 25
Measurement of Erythrocyte and Plasma EPA and DHA
Measurement error in FFQs and imprecision in the nutrient composition database may introduce error into the calculated intakes of EPA and DHA. Because erythrocyte and plasma EPA and DHA reflect long-term dietary intake,26 we used an empirical prediction model to predict the erythrocyte and plasma levels of EPA and DHA directly from food intake based on previous blood measurements among participants of nested case-control studies of cardiovascular disease in the NHS and HPFS. We included all the cases and controls because all were free of disease at the time of blood collection. The details on blood collection and measurements have been described previously.26 Briefly, we collected whole blood samples from 32,826 women between 1989 and 1990 and from 18,225 men between 1993 and 1995. Risk set sampling was used to select 1–2 controls for each confirmed cases matched on age (within 2 years), time of blood donation, and other factors (e.g. smoking and fasting status) from 1990 to 2006 in the NHS and 1994 to 2004 in the HPFS.
Statistical Analysis
Participants contributed person-time to the analysis from the return of the baseline questionnaire if over age 50 years at baseline or from reaching 50 years old to the confirmed diagnosis of AMD, death, loss to follow-up or the end of follow-up (05/31/2012 for the NHS and 01/31/2010 for the HPFS), whichever occurred first. To best represent long-term intake and minimize measurement error,27 we calculated the cumulative average of intakes of EPA and DHA. All cumulative averages were categorized into quintiles based on the distribution in each cohort.
We used time-varying Cox proportional hazards model to estimate the hazard ratio (HR) and 95% confidence interval (CI) controlling for known and suspected risk factors, including race, body mass index (BMI), pack-years of smoking, physical activity, aspirin use, history of hypertension, history of hypercholesterolemia, menopausal status and postmenopausal hormone use (in the NHS only), and the alternative Healthy Eating Index28 (aHEI, modified by excluding EPA + DHA) to account for confounding by healthy dietary pattern. We further adjusted for α-linoleic acid (ALA), the 18-carbon n3 fatty acid that was positively associated with AMD in our cohorts.9 We also tested whether the associations would vary by intake of linoleic acid (LA), an omega6 fatty acid, and by age. We created binary variables using the median intake of LA in each cohort, and the median age of onset of AMD cases (73 years old). We created the interaction terms and used a likelihood ratio test to test the significance of interactions by comparing models with and without the interaction terms.
The empirical prediction model has been described previously.29 Briefly, among food sources of EPA and DHA, we used stepwise linear regression to select foods that were significantly predictive of EPA and DHA blood measurements (p < 0.05). We used the average of food intake between 1986 and 1990 FFQs in the NHS and between 1990 and 1994 FFQs in the HPFS to correspond with the time of blood draw and to reduce within-person variation. The prediction models were separately created in each cohort. We specifically excluded fish oil supplement users so that the predicted blood scores, when extended to all cohort participants, would more accurately reflect long-term dietary intake because intake of fish oil supplements during the follow-up was intermittent (only 4% participants in each cohort had a consistent intake for ≥ 4 years). We then computed predicted scores based on food intake from the FFQ at each 4-year cycle for the full cohort and used the cumulative average value in the analysis.
We performed the analyses separately in each cohort using SAS 9.3. To derive a pooled HR, we first combined the two cohorts and then used a Cox proportional hazards model in the pooled data stratified by the cohort. Interpretation of the results was mainly based on pooled HRs unless otherwise specified. All hypothesis tests were two-sided and used an α-value of 0.05.
Results
In 1998 (the middle of follow-up), participants at the highest cumulative average intake of EPA + DHA were likely to be more physically active, smoke less and consume a healthier diet. However, they were more likely to have a history of hypertension and hypercholesterolemia (Table 1).
Table 1.
Age-standardized characteristics of participants according to cumulative average intake of EPA + DHA in 1998 (middle of follow-up)
| EPA + DHA (diet + supplements), Quintiles | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| NHS | ||||||
| Participants, No. | 13,329 | 13,198 | 13,268 | 13,208 | 13,267 | |
| Age, y | 63 | 63 | 64 | 64 | 64 | |
| ¶BMI, kg/m2 | 26.5 | 26.6 | 26.8 | 26.8 | 26.8 | |
| Caucasians, % | 98 | 98 | 98 | 97 | 96 | |
| Current smokers, % | 12 | 11 | 10 | 9 | 8 | |
| Pack years of smoking | 27 | 25 | 24 | 23 | 22 | |
| Physical activity, MET-h/wk | 15 | 16 | 17 | 19 | 21 | |
| Hypertension, % | 42 | 43 | 43 | 45 | 46 | |
| Hypercholesterolemia, % | 54 | 56 | 57 | 59 | 60 | |
| Postmenopausal, % | 94 | 94 | 94 | 94 | 94 | |
| §Current menopausal hormone use, % | 42 | 44 | 46 | 46 | 46 | |
| Current aspirin use, % | 44 | 48 | 50 | 50 | 50 | |
| Fish oil supplements, % | 0 | 0 | 1 | 3 | 12 | |
| Dietary intake | ||||||
| ALA, mg/d | 956 | 962 | 961 | 963 | 978 | |
| EPA + DHA, mg/d | 67 | 123 | 174 | 240 | 416 | |
| LA, g/d | 9 | 9 | 9 | 9 | 9 | |
| Fruits and vegetables, servings/d | 5 | 5 | 6 | 6 | 6 | |
| Red meat, servings/d | 0.6 | 0.6 | 0.6 | 0.5 | 0.4 | |
| aHEI (excluding EPA + DHA) | 42 | 43 | 45 | 47 | 50 | |
| Total energy intake, Kcal/d | 1,728 | 1,761 | 1,769 | 1,748 | 1,700 | |
| HPFS | ||||||
| Participants, No. | 6,132 | 6,148 | 6,140 | 6,163 | 6,112 | |
| Age, y | 63 | 64 | 64 | 64 | 65 | |
| ¶BMI, kg/m2 | 26.2 | 26.2 | 26.3 | 26.1 | 26.0 | |
| Caucasians, % | 97 | 96 | 96 | 95 | 93 | |
| Current smokers, % | 6 | 6 | 5 | 5 | 4 | |
| Pack years of smoking | 13 | 12 | 12 | 11 | 10 | |
| Physical activity, MET-h/wk | 32 | 33 | 35 | 35 | 39 | |
| Hypertension, % | 35 | 36 | 37 | 38 | 39 | |
| Hypercholesterolemia, % | 42 | 46 | 49 | 51 | 52 | |
| Current aspirin use, % | 59 | 62 | 62 | 62 | 62 | |
| Fish oil supplements, % | 0 | 1 | 2 | 6 | 16 | |
| Dietary intake | ||||||
| ALA, mg/d | 1,113 | 1,111 | 1,110 | 1,102 | 1,122 | |
| EPA + DHA, mg/d | 93 | 184 | 268 | 373 | 697 | |
| LA, g/d | 11 | 11 | 11 | 11 | 11 | |
| Fruits and vegetables, servings/d | 5 | 5 | 6 | 6 | 7 | |
| Red meat, servings/d | 0.7 | 0.7 | 0.6 | 0.5 | 0.4 | |
| aHEI (excluding EPA + DHA) | 43 | 44 | 46 | 48 | 51 | |
| Total energy intake, Kcal/d | 1,982 | 2,015 | 2,011 | 1,951 | 1,933 | |
All the values (except for age) are medians or percentages and are standardized to the age distribution of the study population
Abbreviations: MET-h, hours of metabolic equivalent tasks; aHEI, alternative healthy eating index; BMI, body mass index; ALA, α-linolenic acid; LA, linoleic acid.
BMI is calculated as weight in kilograms divided by height in meters squared.
Current menopausal hormone use among postmenopausal women.
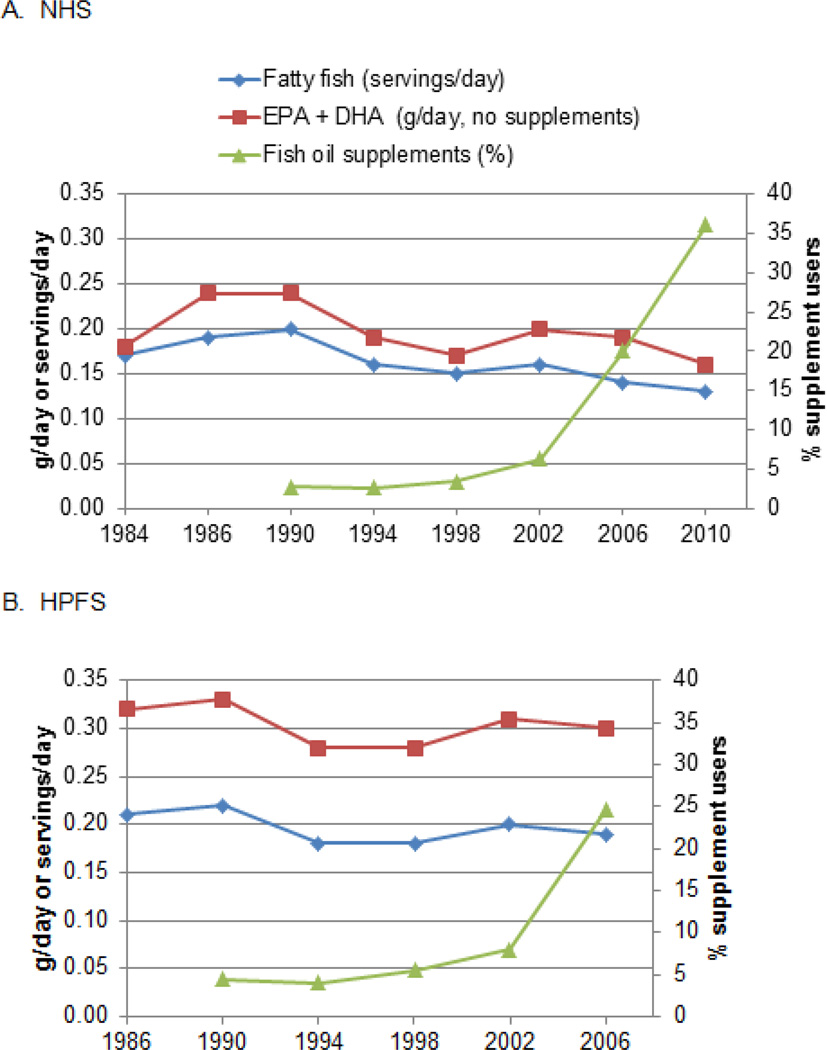
Intakes of EPA and DHA were strongly correlated (Pearson r,0.88). During the follow-up, intake of food-sourced EPA + DHA declined slightly (and the same for fatty fish), whereas the intake of fish oil supplements increased markedly especially after 2002 (Figure1).
Figure 1. Time trend of age-adjusted mean intake of EPA + DHA, fatty fish and fish oil supplements.
None
There were 1,209 intermediate AMD cases in the NHS and 380 in the HPFS, among which 364 in the NHS and 79 in the HPFS were featured by either large drusen or noncentral geographic atrophy. There were 1,010 advanced AMD cases in the NHS and 346 in the HPFS and 96% of them were neovascular types. The overall age- and sex-adjusted incident rate in the pooled cohort was 75 cases/100,000 person-years for intermediate AMD and 64 cases/100,000 person-years for advanced AMD. In pooled primary analysis (Table 2), the HR comparing extreme quintiles of intake of EPA + DHA for risk of intermediate AMD was 0.83 (95% CI, 0.71 – 0.89; p trend, 0.03) but the inverse association was primarily attributable to DHA (HR, 0.78; 95% CI, 0.66 – 0.92; p trend, 0.008). Results excluding fish oil supplement users were similar. With respect to advanced AMD, we did not find any associations in the pooled analysis, with the exception of a significant inverse association in the HPFS for intake of EPA + DHA when excluding fish oil supplement users. The results for advanced AMD were not materially altered in sensitivity analyses excluding African American participants, as they generally have a lower risk of AMD,1, 30 as well as in analyses using deciles of intake (data not shown).
Table2.
Hazard ratios (HR’s) of intermediate and advanced AMD according to cumulative average intake of EPA and DHA
| Quintiles | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p trend | |
| Intermediate AMD | ||||||
| EPA | ||||||
| NHS | ||||||
| Median (mg/d) | 15 | 35 | 54 | 82 | 140 | |
| Cases/person-years | 236 /300,256 | 260 /297,224 | 229 /303,226 | 254 /306,019 | 230 /302,315 | |
| Age-adjusted HR | 1 (ref) | 1.10 | 0.95 | 1.03 | 0.90 | 0.14 |
| Multivariate HR (95% CI) | 1 (ref) | 1.06 (0.89,1.27) | 0.92 (0.76,1.10) | 1.00 (0.83,1.2) | 0.88 (0.73,1.07) | 0.13 |
| HPFS | ||||||
| Median (mg/d) | 25 | 58 | 93 | 135 | 248 | |
| Cases/person-years | 80 /122,739 | 86 /123,084 | 68 /124,924 | 67 /121,765 | 79 /122,436 | |
| Age-adjusted HR | 1 (ref) | 1.02 | 0.81 | 0.8 | 0.86 | 0.21 |
| Multivariate HR (95% CI) | 1 (ref) | 1.01 (0.74,1.37) | 0.80 (0.58,1.11) | 0.84 (0.6,01.17) | 0.90 (0.65,1.25) | 0.47 |
| Pooled | 1 (ref) | 1.06 (0.91,1.23) | 0.90 (0.76,1.05) | 0.96 (0.82,1.13) | 0.89 (0.75,1.05) | 0.09 |
| DHA | ||||||
| NHS | ||||||
| Median (mg/d) | 52 | 89 | 123 | 169 | 279 | |
| Cases/person-years | 259 /302,824 | 238 /297,361 | 249 /305,730 | 253 /300,047 | 210 /303,078 | |
| Age-adjusted HR | 1 (ref) | 0.94 | 0.96 | 0.97 | 0.77 | 0.008 |
| Multivariate HR (95% CI) | 1 (ref) | 0.93 (0.78,1.11) | 0.94 (0.79,1.12) | 0.95 (0.80,1.14) | 0.76 (0.62,0.92) | 0.008 |
| HPFS | ||||||
| Median (mg/d) | 70 | 128 | 180 | 246 | 390 | |
| Cases/person-years | 84 /122,484 | 82 /124,126 | 68 /122,714 | 67 /121,959 | 79 /123,665 | |
| Age-adjusted HR | 1 (ref) | 0.93 | 0.80 | 0.77 | 0.81 | 0.14 |
| Multivariate HR (95% CI) | 1 (ref) | 0.91 (0.67,1.23) | 0.80 (0.58,1.11) | 0.80 (0.58,1.12) | 0.85 (0.61,1.18) | 0.35 |
| Pooled | 1 (ref) | 0.93 (0.80,1.08) | 0.91 (0.78,1.07) | 0.92 (0.79,1.07) | 0.78 (0.66,0.92) | 0.008 |
| EPA + DHA | ||||||
| NHS | ||||||
| Median (mg/d) | 70 | 123 | 179 | 250 | 390 | |
| Cases/person-years | 252 /301,969 | 248 /299,460 | 228 /302,148 | 262 /302,831 | 219 /302,632 | |
| Age-adjusted HR | 1 (ref) | 1 | 0.90 | 1.02 | 0.82 | 0.04 |
| Multivariate HR (95% CI) | 1 (ref) | 0.98 (0.82,1.17) | 0.88 (0.74,1.06) | 1.00 (0.84,1.19) | 0.80 (0.66,0.97) | 0.03 |
| HPFS | ||||||
| Median (mg/d) | 94 | 187 | 275 | 380 | 625 | |
| Cases/person-years | 80 /121,668 | 89 /124,756 | 71 /122,400 | 58 /122,937 | 82 /123,188 | |
| Age-adjusted HR | 1 (ref) | 1.05 | 0.86 | 0.69 | 0.87 | 0.15 |
| Multivariate HR (95% CI) | 1 (ref) | 1.03 (0.76,1.40) | 0.86 (0.62,1.19) | 0.73 (0.52,1.03) | 0.92 (0.66,1.27) | 0.38 |
| Pooled | 1 (ref) | 1.00 (0.86,1.17) | 0.88 (0.75,1.04) | 0.94 (0.80,1.10) | 0.83 (0.71,0.98) | 0.03 |
| EPA + DHA (supplement users excluded) | ||||||
| NHS | ||||||
| Median (mg/d) | 66 | 118 | 170 | 233 | 355 | |
| Cases/person-years | 237 /286,521 | 234 /281,743 | 213 /281,446 | 232 /280,475 | 207 /275,902 | |
| Age-adjusted HR | 1 (ref) | 1.01 | 0.91 | 0.98 | 0.86 | 0.09 |
| Multivariate HR (95% CI) | 1 (ref) | 0.99 (0.82,1.19) | 0.89 (0.74,1.08) | 0.96 (0.80,1.16) | 0.84 (0.69,1.03) | 0.09 |
| HPFS | ||||||
| Median (mg/d) | 90 | 180 | 260 | 358 | 564 | |
| Cases/person-years | 76 /114,609 | 83 /114,692 | 61 /111,874 | 55 /111,261 | 69 /109,760 | |
| Age-adjusted HR | 1 (ref) | 1.04 | 0.81 | 0.71 | 0.81 | 0.07 |
| Multivariate HR (95% CI) | 1 (ref) | 1.03 (0.75,1.41) | 0.80 (0.57,1.13) | 0.76 (0.53,1.08) | 0.88 (0.63,1.25) | 0.29 |
| Pooled | 1 (ref) | 1.01 (0.86,1.18) | 0.88 (0.75,1.04) | 0.92 (0.78,1.08) | 0.86 (0.72,1.02) | 0.04 |
| Advanced AMD | ||||||
| EPA | ||||||
| NHS | ||||||
| Cases/person-years | 182 /300,294 | 201 /297,277 | 198 /303,243 | 225 /306,041 | 204 /302,325 | |
| Age-adjusted HR | 1 (ref) | 1.11 | 1.07 | 1.20 | 1.06 | 0.60 |
| Multivariate HR (95% CI) | 1 (ref) | 1.07 (0.88,1.31) | 1.05 (0.86,1.29) | 1.21 (0.99,1.48) | 1.11 (0.89,1.36) | 0.29 |
| HPFS | ||||||
| Cases/person-years | 80 /122,749 | 66 /123,111 | 64 /124,929 | 62 /121,771 | 74 /122,437 | |
| Age-adjusted HR | 1 (ref) | 0.81 | 0.77 | 0.77 | 0.82 | 0.37 |
| Multivariate HR (95% CI) | 1 (ref) | 0.84 (0.60,1.16) | 0.82 (0.59,1.14) | 0.88 (0.62,1.23) | 0.99 (0.71,1.38) | 0.70 |
| Pooled | 1 (ref) | 1.00 (0.84,1.19) | 0.98 (0.83,1.17) | 1.11 (0.94,1.32) | 1.07 (0.89,1.28) | 0.37 |
| DHA | ||||||
| NHS | ||||||
| Cases/person-years | 185 /302,871 | 216 /297,373 | 205 /305,774 | 210 /300,073 | 194 /303,089 | |
| Age-adjusted HR | 1 (ref) | 1.21 | 1.12 | 1.15 | 1.02 | 0.66 |
| Multivariate HR (95% CI) | 1 (ref) | 1.20 (0.98,1.46) | 1.12 (0.92,1.37) | 1.17 (0.96,1.44) | 1.06 (0.86,1.32) | 0.90 |
| HPFS | ||||||
| Cases/person-years | 84 /122,496 | 65 /124,148 | 71 /122,708 | 54 /121,976 | 72 /123,669 | |
| Age-adjusted HR | 1 (ref) | 0.75 | 0.82 | 0.62 | 0.75 | 0.10 |
| Multivariate HR (95% CI) | 1 (ref) | 0.76 (0.55,1.05) | 0.87 (0.63,1.21) | 0.71 (0.50,1.01) | 0.89 (0.64,1.24) | 0.67 |
| Pooled | 1 (ref) | 1.06 (0.90,1.26) | 1.04 (0.88,1.24) | 1.03 (0.86,1.23) | 1.01 (0.84,1.21) | 0.75 |
| EPA + DHA | ||||||
| NHS | ||||||
| Cases/person-years | 177 /302,026 | 216 /299,479 | 205 /302,179 | 211 /302,856 | 201 /302,639 | |
| Age-adjusted HR | 1 (ref) | 1.25 | 1.17 | 1.20 | 1.09 | 0.93 |
| Multivariate HR (95% CI) | 1 (ref) | 1.23 (1.01,1.50) | 1.17 (0.96,1.44) | 1.22 (0.99,1.5) | 1.15 (0.93,1.42) | 0.49 |
| HPFS | ||||||
| Cases/person-years | 78 /121,683 | 74 /124,775 | 62 /122,405 | 62 /122,938 | 70 /123,196 | |
| Age-adjusted HR | 1 (ref) | 0.91 | 0.76 | 0.77 | 0.78 | 0.13 |
| Multivariate HR (95% CI) | 1 (ref) | 0.93 (0.68,1.28) | 0.82 (0.58,1.15) | 0.88 (0.62,1.24) | 0.93 (0.66,1.31) | 0.77 |
| Pooled | 1 (ref) | 1.14 (0.96,1.35) | 1.06 (0.89,1.27) | 1.11 (0.93,1.33) | 1.08 (0.90,1.29) | 0.86 |
| EPA + DHA (supplement users excluded) | ||||||
| NHS | ||||||
| Cases/person-years | 170 /286,565 | 189 /281,778 | 187 /281,460 | 195 /280,510 | 178 /275,921 | |
| Age-adjusted HR | 1 (ref) | 1.15 | 1.14 | 1.17 | 1.06 | 0.82 |
| Multivariate HR (95% CI) | 1 (ref) | 1.12 (0.91,1.38) | 1.13 (0.91,1.39) | 1.18 (0.95,1.45) | 1.08 (0.87,1.35) | 0.57 |
| HPFS | ||||||
| Cases/person-years | 77 /114,618 | 64 /114,715 | 63 /111,870 | 52 /111,272 | 47 /109,775 | |
| Age-adjusted HR | 1 (ref) | 0.81 | 0.81 | 0.68 | 0.56 | 0.002 |
| Multivariate HR (95% CI) | 1 (ref) | 0.82 (0.59,1.15) | 0.85 (0.61,1.20) | 0.77 (0.53,1.10) | 0.68 (0.46,0.99) | 0.05 |
| Pooled | 1 (ref) | 1.03 (0.86,1.23) | 1.04 (0.87,1.24) | 1.05 (0.88,1.26) | 0.96 (0.79,1.16) | 0.29 |
Multivariate models were adjusted for: age (continuous), race (Caucasians or not), BMI (<18.5, 18.5–23, 23–25, 25–30, 30–35, ≥ 35 kg/m2), pack-years of smoking (never, 1–9, 10–24, 25–44, 45–64, ≥65y), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, ≥27 MET-h/wk), current aspirin use (≥1 tablets/wk or none), history of hypertension and hypercholesterolemia, dietary variables including aHEI (excluding EPA and DHA), ALA, total energy intake (all in quintiles). In NHS, models were additionally adjusted for postmenopausal status and menopausal hormone use (never, current and past).
DHA and EPA included intake from diet and supplements unless otherwise specified.
We further examined associations between more remote dietary intake from the average of the first two FFQs and the risk of AMD in case an association with AMD would be missed if there was a long latency period between the intake and AMD onset. However, remote intakes of EPA and DHA were not associated with either intermediate or advanced AMD (data not shown).
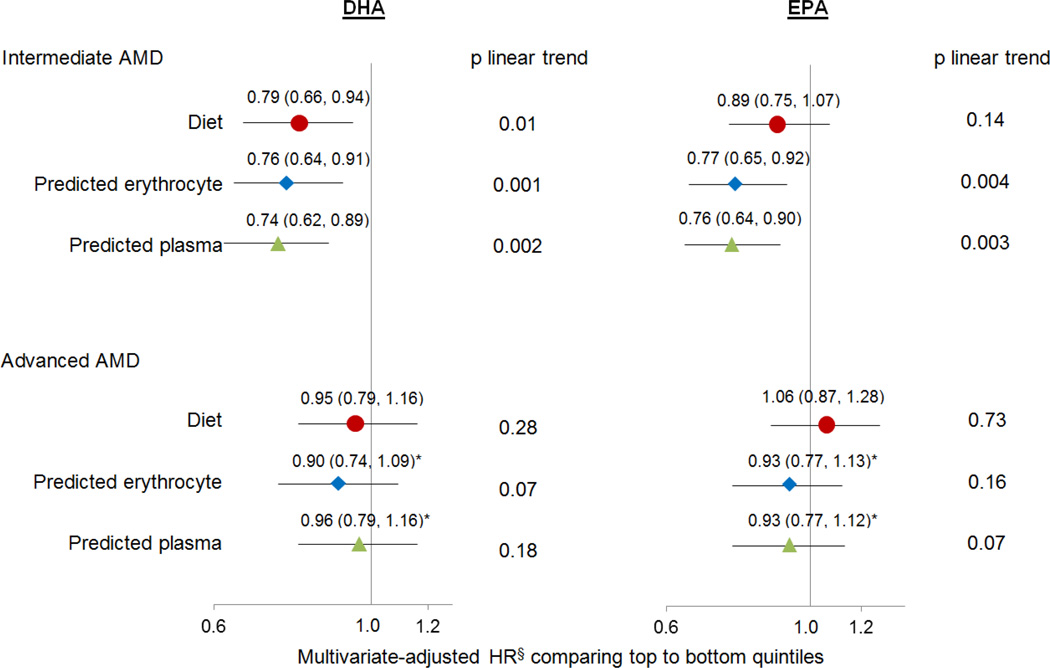
eTable 2 provides the empirical prediction models used for computing predicted erythrocyte and plasma scores of EPA and DHA for all cohort participants. The Spearman correlations between predicted blood scores and measured blood levels ranged from 0.21 to 0.56 with stronger correlations for DHA and for measurements in the HPFS (eTable 3). Among non-users of fish oil supplements, the pooled HRs of intermediate AMD for DHA were similar across analyses based on diet, predicted erythrocyte and plasma scores; the associations for EPA were strengthened when using predicted erythrocyte and plasma scores (Figure 2). With respect to advanced AMD, although the pooled HRs were similar across analyses, we observed significant heterogeneity between the HRs in the NHS and HPFS. In the HPFS, there were significant inverse associations for predicted erythrocyte and plasma scores of EPA and DHA with advanced AMD (eTable 4).
Figure 2. The pooled hazard ratios of AMD according to dietary intake, predicted erythrocyte and plasma scores of EPA and DHA among non-users of fish oil supplements.
§Multivariate models were adjusted for the same variables as in the Table 2
*p for heterogeneity between the HRs from the NHS and HPFS was < 0.05
Intake of total fatty fish was significantly inversely related to intermediate AMD (p for trend across intake categories < 0.001); the association was primarily attributed to canned tuna (Table 3), for which the median intake was 3.5 times that of dark oily fish. In contrast, intake of other fish (mainly white fish) was not associated with the risk of intermediate AMD. We did not find any significant association between any type of fish and advanced AMD.
Table 3.
Pooled hazard ratios of intermediate and advanced AMD according to cumulative average intake of fish
| Fish‡ | Servings/wk Median |
Multivariate HR (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Almost never | 1–3 servings/mo | 1 serving/wk | 2–4 servings/wk | ≥ 5 servings/wk | p trend | ||
| Intermediate AMD | |||||||
| Canned tuna | 0.70 | 1 (ref) | 0.94 (0.83,1.07) | 0.84 (0.73, 0.96) | 0.68 (0.44,1.05) | 0.005 | |
| Dark fish | 0.20 | 1 (ref) | 1.08 (0.91,1.27) | 0.92 (0.74, 1.14) | 0.74 | ||
| Other fisha | 0.58 | 1 (ref) | 0.96 (0.85,1.09) | 0.91 (0.79, 1.05) | 0.91 (0.45,1.83) | 0.25 | |
| Total fatty fishb | 0.98 | 1 (ref) | 0.92 (0.80,1.06) | 0.95 (0.82,1.10) | 0.79 (0.65,0.96) | 0.61 (0.46,0.81) | <.001 |
| Advanced AMD | |||||||
| Canned tuna | 0.70 | 1 (ref) | 1.10 (0.96,1.26) | 1.00 (0.86,1.16) | 0.76 (0.46,1.23) | 0.34 | |
| Dark fish | 0.20 | 1 (ref) | 1.04 (0.87,1.24) | 0.98 (0.78,1.23) | 0.97 | ||
| Other fisha | 0.58 | 1 (ref) | 1.08 (0.94,1.23) | 1.04 (0.89,1.22) | 1.00 (0.44,2.24) | 0.63 | |
| Total fatty fishb | 0.98 | 1 (ref) | 1.10 (0.94,1.29) | 1.05 (0.90,1.24) | 0.99 (0.80,1.22) | 0.80 (0.59,1.08) | 0.11 |
Multivariate models were adjusted for the same variable as in Table 2
Other fish were mainly white fish
Total fatty fish was the sum of canned tuna and dark fish. Finer intake categories were created to show detailed associations
Two percent of the population in each cohort had a consistent intake of fish oil supplements for ≥ 6 years. In terms of intermediate AMD, in the NHS after controlling for food-sourced intake of EPA + DHA and other risk factors, consistent users compared to irregular users and non-users had a 40% (HR, 0.60; 95% CI, 0.36 – 1.01; p, 0.05) lower risk. When further restricting the comparison to consistent versus irregular users, the risk reduction was 45% (HR, 0.55; 95% CI, 0.32 – 0.93; p, 0.02). With respect to advanced AMD, in the NHS consistent users compared to irregular users did not have a reduced risk (HR, 1.07; 95% CI, 0.73 – 1.58). In contrast, there were no significant associations in the HPFS between fish oil supplements and any type of AMD, but the 95% CIs surrounding the HRs were wide (e.g. for intermediate AMD comparing consistent to irregular users, HR, 0.95; 95% CI, 0.45 – 1.98; p, 0.88; other data not shown).
High intake of omega6 fatty acids is hypothesized to negate the inverse associations for long-chain n3 fatty acids due to competition for the same enzymes.13, 31, 32 To explore this possibility, we stratified the analyses by the median intake level of LA, a major omega6 fatty acid. We did not find any statistically significant interactions with the intake of LA in the pooled analysis (eTable 5), nor with age (eTable 6).
Discussion
In this large prospective study with 24–28 years of follow-up, high intakes of DHA + EPA (especially DHA) and fatty fish, were associated with a 17 to 40% lower risk of visually significant intermediate AMD, but no reduction in the risk of advanced AMD. Results based on predicted erythrocyte and plasma DHA and EPA scores supported findings based on dietary intake. Overall, these data support the hypothesis that DHA and EPA may prevent or delay the onset of AMD.
Among existing studies on the relation between intakes of long-chain n3 fatty acids or fish with early/intermediate AMD, two33, 34 out of four cross-sectional studies33–36 reported an inverse association whereas 1 case-control study did not.37 In all 3 prospective cohort studies an inverse association was found.10–12 In particular, the 10-year prospective Women’s Health Study, which has a similar study methodology to ours (e.g. a cohort of female health professionals, the same Willett FFQs and classification of AMD types)11 showed similar results (HR,0.62, 95% CI, 0.44–0.86, for intake of DHA comparing tertile 3 (median, 230mg/d) to tertile 1(median, 60mg/d)). Ours is the only study that has further explored and showed an inverse association for a consistent intake of fish oil supplements, although the HR was only significant in the NHS. A much smaller number of cases in the HPFS may have resulted in an inadequate power to detect an association; however, the wide confidence interval for the HR in the HPFS did include the point estimate in the NHS.
With respect to advanced AMD, an apparent discrepancy exists between observational studies across several populations and randomized trials. Observational studies10, 13–15, 31–35 were almost all suggestive of an inverse association, including four prospective cohort studies,10, 13–15 two case-control studies,31, 32 and three34, 35, 38 out of four cross-sectional studies33–35, 38. In those studies, the median intake of EPA + DHA was 200–350mg/d for participants at the highest intake category, compared to 400–600mg/d in our study. In contrast, in two double-blinded, placebo-controlled, randomized trials, AREDS2 from US16 and NAT2 from France39, supplementation of high dose EPA and DHA (AREDS2, 350mg/d DHA and 650mg/d EPA; NAT2, 840mg/d DHA and 270mg/d EPA) did not reduce the risk of progression to advanced AMD over 3–5 years of follow-up. While confounding may cause the inverse associations in observational studies, several other reasons could explain the null findings in randomized trials including short duration of follow-up, timing of interventions that did not encompass the true latent period, poor compliance (NAT2 trial), high baseline intakes of EPA and DHA, etc. In this study, although the associations with advanced AMD were null in the pooled analysis, in the HPFS we found significant inverse associations especially when using the predicted biomarker scores. Two cross-sectional studies based on erythrocyte or plasma EPA and DHA among a French population also suggested an inverse association.40, 41 The correlations between calculated intakes of EPA and DHA and measured blood levels were stronger in the HPFS than in the NHS perhaps due to a higher intake of fatty fish in the HPFS. However, the possibility of a chance finding cannot be excluded due to a much smaller number of advanced cases in the HPFS. Therefore, totaling all existing evidence, the associations between intakes of EPA and DHA with advanced AMD remain unclear.
Intuitively, a reduction in the incidence of intermediate AMD with EPA and DHA could ultimately decrease that of advanced AMD. However, AMD is a complex disease with different clinical manifestations and underlying genetic profiles.42, 43 Clinically-defined intermediate AMD cases could harbor variable underlying genetic backgrounds with different propensities for progression. Our AMD ascertainment scheme may have accrued mostly those intermediate AMD cases that were slow progressing or not destined to progress further. The existence of this subset of intermediate AMD cases is plausible in view of: 1) the high prevalence of intermediate AMD (>20%) at older ages in white populations;4, 6, 44 2) the relatively small proportion of intermediate AMD that progresses (18% progression rate over 5 years in the AREDS study45 and 16% over 20 years by our crude estimation in the Beaver Dam Eye Study44).
Multiple biological mechanisms by which EPA and DHA could affect the development of AMD have been proposed.8, 46 To summarize, first, DHA is a major structural component of the retinal photoreceptor and neural membranes. Tissue level of DHA affects the physical and biochemical properties of membranes that are important to normal retinal function. Second, EPA and DHA serve as potent ligands for nuclear hormone receptors (e.g., PPAR-α RXR, NFκB) that are transcriptional factors regulating the expression of genes implicated in cell differentiation and survival. Furthermore, EPA and DHA inhibit the conversion of omega6 fatty acids (e.g., arachidonic acid) to angiogenic and proinflammatory eicosanoids whereas the metabolites of EPA and DHA such as resolvins and protectins are anti-inflammatory.
Our study has several strengths and limitations. The prospective design and high follow-up rate minimized the likelihood of recall and selection bias, respectively. To our knowledge, this is the only observational study that has repeated dietary assessment, which not only dampens within-person variation but also reflects dietary change over time. Also to our knowledge, we are the first study to make use of food intake and existing blood data to develop predicted blood scores for AMD research. The empirical prediction model provides an alternative way of estimating exposure that accounts for the variation in accuracy across self-reported foods, imprecision in food composition database and variation in absorption and metabolism. Consistent findings between blood and dietary analyses lend support to the validity of our results. The observational design cannot exclude residual or unmeasured confounding, but this is unlikely to have a major impact on the current results, as the magnitude of associations did not appreciably change after extensive adjustment for known and suspected risk factors. To rule out the possibility that high intakes of EPA and DHA are a marker for healthy dietary pattern, we specifically adjusted for a recognized indicator of diet quality,28 and the results were essentially the same pre and post-adjustment. EPA and DHA could also be a marker for other beneficial components of fatty fish, e.g., vitamin D, and we were not able to adjust for serum 25(OH)D in the model. However, the suggestive inverse association between fish oil supplements and intermediate AMD supports the hypothesis that EPA and DHA are the primary causal factors because the vitamin D-rich cod liver oil47 only accounts for a small proportion of fish oil supplements consumed by our study participants. Another issue of concern is that participants at the high intake of EPA and DHA were more likely to be health-conscious and to have an eye exam, and thus to have existing AMD diagnosed. This could result in detection bias that may particularly affect early/intermediate AMD, which is usually asymptomatic, and may lead to an underestimation of a true benefit. To minimize the effect of such bias, we restricted to those AMD cases that were sufficiently visually significant to warrant medical attention and also excluded those who did not report an eye exam in the past 2 years. Because our advanced AMD cases primarily consisted of neovascular forms, cautions should be taken when extrapolating our results to central GA. A lower than usual proportion of GA cases is likely in large due to our strict case definition (GA without any signs of exudation and must involve the center of macula). It could also be due to death from competing events, such as cardiovascular disease, prior to developing central GA which usually takes longer time to progress than neovascular AMD. Lastly, due to the relatively more homogenous demographic features of our cohorts, our results may not be generalizable to other populations.
In summary, this prospective study over more than two decades of follow-up supports beneficial effects of EPA and DHA on the risk of visually significant intermediate AMD. Specifically, intake of EPA + DHA ≥ 350 mg/d or fatty fish starting from ≥ 2 servings/week may provide a moderate reduction in risk. Given that less than 5% of US adults and fewer than 1 in 4 adults consumed that amount of EPA + DHA and fatty fish, respectively, according to 2009 – 2010 NHANES,48 increasing the average intake on a population basis may have implications for the primary prevention of visually significant intermediate AMD. Consistent long-term intake of fish oil supplements, which on average contain 590 mg of EPA + DHA,49 may be an appealing alternative to achieve the same benefit. Whether intakes of EPA and DHA may protect against the development of advanced AMD is still inconclusive but current evidence does not suggest any harm.
Supplementary Material
Marine-sourced omega3 fatty acids were protective against the development of visually significant intermediate age-related macular degeneration. However, they were unrelated to the advanced, neovascular stage of this disease.
Acknowledgments
None
Financial Support
This study was supported by the grants EY017362, EY013834, EY000365, EY009611, EY021900, UM1 CA186107 and UM1 CA167552 and R01 CA49449 from the National Institute of Health.
The funding organizations have no role in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- AMD
age-related macular degeneration
- NHANES
National Health and Nutrition Examination Survey
- n3
omega3
- AREDS2
Age-Related Eye Disease Study 2
- NHS
Nurses’ Health Study
- HPFS
Health Professionals Follow-up Study
- FFQ
food frequency questionnaire
- GA
geographic atrophy
- HR
hazard ratio
- CI
confidence interval
- BMI
body mass index
- aHEI
alternative Healthy Eating Index
- ALA
α-linoleic acid
- LA
linoleic acid
- WHS
Women’s Health Study
- BMES
Blue Mountain Eye Study
- RES
Reykjavik Eye Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1.Coleman HR, Chan CC, Ferris FL, 3rd, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bressler NM, Doan QV, Varma R, et al. Estimated cases of legal blindness and visual impairment avoided using ranibizumab for choroidal neovascularization: non-Hispanic white population in the United States with age-related macular degeneration. Arch Ophthalmol. 2011;129:709–717. doi: 10.1001/archophthalmol.2011.140. [DOI] [PubMed] [Google Scholar]
- 3.Wong TY, Liew G, Mitchell P. Clinical update: new treatments for age-related macular degeneration. Lancet. 2007;370:204–206. doi: 10.1016/S0140-6736(07)61104-0. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Chou CF, Klein BE, et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129:75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 5.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 7.Rein DB, Wittenborn JS, Zhang X, et al. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127:533–540. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- 8.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Cho E, Hung S, Willett WC, et al. Prospective study of dietary fat and the risk of age-related macular degeneration. Am J Clin Nutr. 2001;73:209–218. doi: 10.1093/ajcn/73.2.209. [DOI] [PubMed] [Google Scholar]
- 10.Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2009;127:656–665. doi: 10.1001/archophthalmol.2009.76. [DOI] [PubMed] [Google Scholar]
- 11.Christen WG, Schaumberg DA, Glynn RJ, Buring JE. Dietary omega-3 fatty acid and fish intake and incident age-related macular degeneration in women. Arch Ophthalmol. 2011;129:921–929. doi: 10.1001/archophthalmol.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnarsson A, Sverrisson T, Stefansson E, et al. Risk factors for five-year incident age-related macular degeneration: the Reykjavik Eye Study. Am J Ophthalmol. 2006;142:419–428. doi: 10.1016/j.ajo.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003;121:1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sangiovanni JP, Agron E, Meleth AD, et al. {omega}-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2009;90:1601–1607. doi: 10.3945/ajcn.2009.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SanGiovanni JP, Chew EY, Agron E, et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126:1274–1279. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 17.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Cho E, Willett WC, et al. Intakes of Lutein, Zeaxanthin, and Other Carotenoids and Age-Related Macular Degeneration During 2 Decades of Prospective Follow-up. JAMA Ophthalmol. 2015;133:1415–1424. doi: 10.1001/jamaophthalmol.2015.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA. 1996;276:1141–1146. [PubMed] [Google Scholar]
- 21.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65 doi: 10.1093/ajcn/65.4.1220S. 1220S-8S; discussion 9S-31S. [DOI] [PubMed] [Google Scholar]
- 22.Hunter DJ, Rimm EB, Sacks FM, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol. 1992;135:418–427. doi: 10.1093/oxfordjournals.aje.a116302. [DOI] [PubMed] [Google Scholar]
- 23.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 24.Garland M, Sacks FM, Colditz GA, et al. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr. 1998;67:25–30. doi: 10.1093/ajcn/67.1.25. [DOI] [PubMed] [Google Scholar]
- 25.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 26.Sun Q, Ma J, Campos H, et al. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86:74–81. doi: 10.1093/ajcn/86.1.74. [DOI] [PubMed] [Google Scholar]
- 27.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 28.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrickson SJ, Willett WC, Rosner BA, Eliassen AH. Food predictors of plasma carotenoids. Nutrients. 2013;5:4051–4066. doi: 10.3390/nu5104051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein R, Klein BE, Jensen SC, et al. Age-related maculopathy in a multiracial United States population: the National Health and Nutrition Examination Survey III. Ophthalmology. 1999;106:1056–1065. doi: 10.1016/S0161-6420(99)90255-5. [DOI] [PubMed] [Google Scholar]
- 31.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124:995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- 32.Seddon JM, Rosner B, Sperduto RD, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119:1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- 33.Chong EW, Robman LD, Simpson JA, et al. Fat consumption and its association with age-related macular degeneration. Arch Ophthalmol. 2009;127:674–680. doi: 10.1001/archophthalmol.2009.60. [DOI] [PubMed] [Google Scholar]
- 34.Merle B, Delyfer MN, Korobelnik JF, et al. Dietary omega-3 fatty acids and the risk for age-related maculopathy: the Alienor Study. Invest Ophthalmol Vis Sci. 2011;52:6004–6011. doi: 10.1167/iovs.11-7254. [DOI] [PubMed] [Google Scholar]
- 35.Heuberger RA, Mares-Perlman JA, Klein R, et al. Relationship of dietary fat to age-related maculopathy in the Third National Health and Nutrition Examination Survey. Arch Ophthalmol. 2001;119:1833–1838. doi: 10.1001/archopht.119.12.1833. [DOI] [PubMed] [Google Scholar]
- 36.Parekh N, Voland RP, Moeller SM, et al. Association between dietary fat intake and age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS): an ancillary study of the Women's Health Initiative. Arch Ophthalmol. 2009;127:1483–1493. doi: 10.1001/archophthalmol.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch Ophthalmol. 2007;125:671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- 38.Augood C, Chakravarthy U, Young I, et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr. 2008;88:398–406. doi: 10.1093/ajcn/88.2.398. [DOI] [PubMed] [Google Scholar]
- 39.Souied EH, Delcourt C, Querques G, et al. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology. 2013;120:1619–1631. doi: 10.1016/j.ophtha.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Merle BM, Delyfer MN, Korobelnik JF, et al. High concentrations of plasma n3 fatty acids are associated with decreased risk for late age-related macular degeneration. J Nutr. 2013;143:505–511. doi: 10.3945/jn.112.171033. [DOI] [PubMed] [Google Scholar]
- 41.Merle BM, Benlian P, Puche N, et al. Circulating omega-3 Fatty acids and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:2010–2019. doi: 10.1167/iovs.14-13916. [DOI] [PubMed] [Google Scholar]
- 42.Chong EW, Amirul Islam FM, Robman LD, et al. Age-related macular degeneration phenotypes associated with mutually exclusive homozygous risk variants in CFH and HTRA1 genes. Retina. 2015;35:989–998. doi: 10.1097/IAE.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 43.Cantsilieris S, White SJ, Richardson AJ, et al. Comprehensive analysis of Copy Number Variation of genes at chromosome 1 and 10 loci associated with late age related macular degeneration. PLoS One. 2012;7:e35255. doi: 10.1371/journal.pone.0035255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myers CE, Klein BE, Gangnon R, et al. Cigarette smoking and the natural history of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2014;121:1949–1955. doi: 10.1016/j.ophtha.2014.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Querques G, Souied EH. The role of omega-3 and micronutrients in age-related macular degeneration. Surv Ophthalmol. 2014;59:532–539. doi: 10.1016/j.survophthal.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Ross CM. Fish oil versus cod liver oil: is vitamin D a reason to go back to the future. J Am Board Fam Pract. 2005;18:445–446. doi: 10.3122/jabfm.18.5.445-b. author reply 6. [DOI] [PubMed] [Google Scholar]
- 48.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 49.Srigley CT, Rader JI. Content and composition of fatty acids in marine oil omega-3 supplements. J Agric Food Chem. 2014;62:7268–7278. doi: 10.1021/jf5016973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.