Abstract
Kynurenic acid (KYNA) is an endogenous antagonist of N-methyl-D-aspartate and α7 nicotinic acetylcholine receptors that is derived from astrocytes as part of the kynurenine pathway of tryptophan degradation. Evidence suggests that abnormal KYNA levels are involved in the pathophysiology of schizophrenia. However, this has never been assessed through a meta-analysis. A literature search was conducted through Ovid using Embase, Medline, and PsycINFO databases (last search: December 2016) with the search terms: (kynuren* or KYNA) and (schizophreni* or psychosis). English language studies measuring KYNA levels using any method in patients with schizophrenia and healthy controls (HCs) were identified. Standardized mean differences (SMDs) were calculated to determine differences in KYNA levels between groups. Subgroup analyses were separately performed for nonoverlapping participant samples, KYNA measurement techniques, and KYNA sample source. The influences of patients’ age, antipsychotic status (%medicated), and sex (%male) on study SMDs were assessed through a meta-regression. Thirteen studies were deemed eligible for inclusion in the meta-analysis. In the main analysis, KYNA levels were elevated in the patient group. Subgroup analyses demonstrated that KYNA levels were increased in nonoverlapping participant samples, and centrally (cerebrospinal fluid and brain tissue) but not peripherally. Patients’ age, %medicated, and %male were each positively associated with study SMDs. Overall, KYNA levels are increased in patients with schizophrenia, specifically within the central nervous system. An improved understanding of KYNA in patients with schizophrenia may contribute to the development of novel diagnostic approaches and therapeutic strategies.
Keywords: kynurenine, tryptophan, psychosis, neuroinflammation
Introduction
Schizophrenia
While schizophrenia is characterized by positive, negative, and cognitive symptoms, neurometabolic abnormalities have also been identified as key features of the illness.1,2 The longstanding dopamine hypothesis of schizophrenia suggests that dysregulated functioning of the dopaminergic system underlies its pathophysiology.3–7 However, the dopamine hypothesis does not readily explain negative and cognitive symptoms.8,9 Moreover, a subset of patients (20%–35%) show partial or no response to standard antipsychotic treatments, which exert their effect primarily through dopamine receptor antagonism.10,11
Another widely purported pathophysiological mechanism is the glutamatergic hypothesis of schizophrenia. Evidence for this hypothesis arises from pharmacological studies in which N-methyl-D-aspartate receptor (NMDAR) antagonist administration leads to the emergence of positive, negative, and cognitive symptoms in human volunteers.12–17 These agents also elicit symptom exacerbation in patients with schizophrenia.16,18,19 Olney and Farber proposed that hypofunctioning NMDARs on gamma-aminobutyric acid (GABA)-ergic inhibitory interneurons result in the disinhibition of downstream pyramidal neurons, increasing presynaptic glutamate release within various brain regions.20 In support, disturbed glutamatergic signaling has been observed in healthy volunteers following acute exposure to an NMDAR antagonist21,22 and in patients with schizophrenia.23–28 The known effects of exogenous NMDAR antagonists on glutamatergic dysregulation and schizophrenia-like symptomatology have resulted in increased attention towards kynurenic acid (KYNA), the only currently known endogenous NMDAR antagonist.
Kynurenine Pathway
KYNA is produced through the kynurenine (KYN) pathway of tryptophan (TRP) degradation, accounting for over 90% of the metabolism of this essential amino acid.29 TRP is oxidized to N-formylkynurenine by 1 of 3 enzymes: indoleamine 2,3-dioxygenase 1 (IDO1), IDO2, or tryptophan 2,3-dioxygenase (TDO2). Next, deformylation of N-formylkynurenine by formamidase produces KYN. KYN is thereafter metabolized through 3 distinct branches of the KYN pathway. KYN can be irreversibly transaminated to KYNA by 4 kynurenine aminotransferases (KATs). KYN can also be oxidized by kynurenine 3-monooxygenase (KMO) to produce 3-hydroxykynurenine (3-HK). Lastly, KYN can undergo oxidative cleavage by kynureninase to form anthranilic acid (for additional details on this pathway, see reviews by Dounay et al,30 Schwarcz et al,31 and Vécsei et al32).
The KYN pathway of TRP degradation is initiated by IDO and TDO2.30 These enzymes are known to exist at higher levels in the periphery compared to the central nervous system (CNS).31 Downstream, KYN readily crosses the blood-brain barrier through the large neutral amino acid transporter33; approximately 60% of brain KYN is believed to be contributed from the periphery.34 In contrast, due to its polar structure, KYNA does not cross the blood-brain barrier.33 Thus, brain KYNA is predominantly derived from brain KYN.31 The conversion of KYN to KYNA takes place primarily within astrocytes, as these cells contain KATs but not KMO and therefore cannot degrade KYN to 3-HK and its metabolites.35 Of the 4 existing KATs, KAT II is thought to be the main enzyme of KYNA production.36
KYNA acts as an antagonist of all 3 ionotropic glutamate receptors, including NMDARs, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, and kainate receptors.37 However, of these, KYNA preferentially and competitively inhibits the glycine site of the NMDAR.38,39 KYNA is also an antagonist of α7 nicotinic acetylcholine receptors (α7nAChR)40; its inhibitory effect on these receptors is achieved noncompetitively through its interaction with an allosteric potentiating site, which is oppositely stimulated by galantamine, an α7nAChR positive allosteric modulator.41 KYNA also activates the G-protein-coupled receptor GPR 35 and the aryl hydrocarbon receptor.42,43 Additionally, KYNA functions as a free radical scavenger and an antioxidant.44 Given its capacity to block neuronal excitation and scavenge free radicals, KYNA is widely considered to have neuroprotective and anticonvulsant properties.45
KYNA Hypothesis of Schizophrenia
The KYNA hypothesis of schizophrenia posits that disrupted KYNA levels are implicated in the pathophysiology of the illness.46 This hypothesis is supported by the notion that KYNA, as an endogenous glutamate receptor antagonist, may mimic schizophrenia-like phenomena induced by exogenous glutamate receptor antagonists, along with evidence from both preclinical and clinical literature.41,47,48 Preclinical studies manipulating levels of KYNA have demonstrated its influence on both behavior (eg, cognitive functioning) and neurotransmission (eg, glutamatergic, dopaminergic) observed to be aberrant in patients with schizophrenia.41,48 Furthermore, KYNA levels have also been measured in schizophrenia patient populations and deviations from healthy controls (HCs) have often been reported.41
Study Aims
Although individual studies have reported KYNA disruptions in patients with schizophrenia, their findings have not been assessed through a meta-analysis. The primary aim of this systematic review and meta-analysis was to evaluate the difference in KYNA levels between patients with schizophrenia and HCs. As secondary aims, subgroup analyses examined nonoverlapping participant samples, KYNA measurement techniques, and KYNA sample source. Also, the influences of patients’ age, antipsychotic status, and sex were explored through a meta-regression.
Methods
Literature Search
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis group.49 Two authors (E.P., J.K.) independently performed the search (last search: December 2016) and assessed eligibility, and 2 authors (E.P., J.K.C.) independently extracted data. English language human published articles were searched for using Embase, Medline, and PsycINFO. The Ovid search was conducted using the following terms: (kynuren* or KYNA) and (schizophreni* or psychosis). The reference sections of major review articles30–32,41,46–48,50–54 were also searched.
Inclusion Criteria
Full-length English language articles were included if: (1) they included patients with schizophrenia or related disorders, (2) they included a HC group, (3) KYNA levels were measured in both groups using any method, and (4) data were sufficient to calculate standardized mean differences (SMDs).
Exclusion Criteria
When studies reported upon a sample completely overlapping with another study, as described within their texts, the study with the largest sample size was used and the other excluded. Where publications reported partially overlapping samples, both were included in the primary analysis. Studies missing baseline KYNA levels or examining KYNA production were excluded.
Outcome Measures
The main outcome measure was KYNA levels. We aimed to investigate group differences in KYNA between patients with schizophrenia and HCs.
Recorded Variables
The variables recorded from each included study were KYNA levels, diagnoses, age, sex, antipsychotic status, method of KYNA measurement, and participant sample overlap with other studies.
Data Analysis
Meta-analysis.
The primary meta-analysis, subgroup analyses, and sensitivity analyses were conducted using Review Manager Version 5.2 (http://tech.cochrane.org/revman). The meta-regression was carried out using Comprehensive Meta Analysis (www.meta-analysis.com). Differences in KYNA levels between patients with schizophrenia and HCs were determined by calculating SMDs.55 If the total number of study participants exceeded the number that underwent KYNA measurement, only subjects in whom KYNA was measured were included. When studies separately reported KYNA levels from multiple brain areas, average SMDs were calculated and utilized. Where mean values were not stated, authors were contacted for additional data or, if reported, median values were utilized. Where SD values were not reported, values were obtained through calculations from available data according to the Cochrane Handbook for Systematic Reviews of Interventions (http://www.handbook.cochrane.org). Effects were interpreted as small (SMD = 0.2), moderate (SMD = 0.5) or large (SMD = 0.8),55 with positive values indicating elevated KYNA levels in the schizophrenia group. To adjust for study heterogeneity, the inverse variance statistical method and random effects model were employed.56 Significance was assessed using 2-sided 95% confidence intervals (CIs).
The I2 statistic was utilized to assess study heterogeneity for the primary analysis; I2 ≥ 50% represented significant heterogeneity. If heterogeneity was found, one-leave-out sensitivity analyses were performed to examine influences of any single study on the pooled SMD and associated P values. The possibility of publication bias was assessed using funnel plots and Egger’s regression test57; if identified, the trim-and-fill procedure58 was utilized.
Moderator Analyses.
Moderator analyses were conducted to investigate the influence of study and patient characteristics on KYNA levels. Subgroup analyses were separately examined for: (1) nonoverlapping participant samples using the study with the largest sample size, (2) KYNA measurement technique (ie, cerebrospinal fluid (CSF), brain tissue, plasma/serum, saliva), and (3) KYNA sample source (ie, central, peripheral). Meta-regression analyses were conducted for patients’ age, the proportion of antipsychotic-medicated patients (%medicated), and the proportion of male patients (%male). When participant information was presented only for the full sample, this data was used for meta-regression analyses.
Risk of Bias.
The Risk of Bias Assessment tool for Nonrandomized Studies59 was employed, using the following factors: participant selection, confounding variables, measurement of exposure, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting.
Significance for all tests was set at P < .05 (2-tailed). Continuous variables are reported as mean ± SD.
Results
Included Individual Studies
Thirteen studies were deemed eligible for inclusion in the meta-analysis (total number of subjects, n = 961).60–72 The PRISMA flow diagram is presented in supplementary figure 1 and characteristics of included studies are summarized in table 1. The average number of subjects was 73.9 ± 47.1 (range: 26 to 174). Average age and %male of the patient group were 37.7 ± 7.0 years and 68.0% ± 17.5%, respectively. Average age and %male of the control group were 34.2 ± 9.7 years and 64.0% ± 18.6%, respectively. Average %medicated was 69.0% ± 35.3%. Four studies measured KYNA in CSF,64,65,68,72 3 in brain tissue,66,70,71 5 in plasma/serum,60,62,63,67,69 and 1 in saliva.61 Of the 13 included studies, 10 had completely nonoverlapping samples.60–63,66–71
Table 1.
Summary of Included Studies (n = 13)
| Authors (Year) | n | Mean Age (SD) | Sex (%Male) | Antipsychotic Status (%Medicated) | KYNA Measurement Technique | SCZ KYNA Mean (SD) [Units], HC KYNA Mean (SD) [Units], P valuea | Key Findingsb |
|---|---|---|---|---|---|---|---|
| Fazio et al (2015) | 90 SCZ; 84 HC | SCZ: 33.4 (11.7); HC: 32.8 (10.4) | SCZ: 68.9%; HC: 44.0% | 66.7%; 30 patients unmedicated | Serum | SCZ: 3.79 (2.03) [ng/ml]; HC: 3.28 (1.84) [ng/ml] | KYNA levels higher in SCZ group |
| Schwieler et al (2015)c | 23 SCZd; 37 HC | Median (IQR), SCZ: 35.0 (32.0– 41.0); HC: 23.0 (22.0–25.5) | SCZ: 65.2%; HC: 62.2% | 100.0% | CSF | Median (IQR), SCZ: 1.87 (1.63–2.29) [nM]; HC: 1.50 (1.14–1.92) [nM]; P = .006 | KYNA levels higher in SCZ group |
| Chiappelli et al (2014) | 64 SCZd; 64 HC | SCZ: 37.7 (12.4); HC: 38.9 (12.9) | SCZ: 65.6%; HC: 54.7% | 82.8%; 11 patients unmedicated for at least 1 mo | Saliva | SCZ: 7.40 (1.05) [nM]; HC: 6.02 (0.74) [nM] | Mean KYNA level higher in SCZ group |
| Fukushima et al (2014) | 25 SCZ; 27 HC | SCZ: 28.2 (4.4); HC: 26.5 (5.6) | SCZ: 44.0%; HC: 44.4% | 100.0% | Serum | SCZ: 26.5 (11.95) [nmol/L]; HC: 28.7 (11.17) [nmol/L] | No difference in KYNA levels between groups |
| Kegel et al (2014)c | 19 SCZd,e; 26 HC | SCZ: 37.5 (7.5); HC: 24.9 (5.8) | SCZ: 57.1%; HC: 69.2% | 100.0% | CSF | SCZ: 2.1 (0.87) [nM]; HC: 1.6 (0.51) [nM] | KYNA levels elevated in SCZ group |
| Linderholm et al (2012)c | 16 SCZ; 29 HC | SCZ: 36.8 (7.9); HC: 25.4 (7.3) | SCZ: 100.0%; HC: 100.0% | 100.0% | CSF | SCZ: 2.03 (0.92) [nM]; HC: 1.36 (0.43) [nM] | KYNA levels elevated in SCZ group |
| Myint et al (2011) | 53 SCZ; 48 HC | SCZ: 33.3 (12.2); HC: 32.6 (10.3) | SCZ: 43.4%; HC: 43.8% | 0.0%; all patients unmedicated for at least 4 mo | Plasma | SCZ: 26.90 (16.38) [nmol/L]; HC: 35.95 (9.49) [nmol/L] | KYNA levels lower in SCZ group |
| Sathyasaikumar et al (2011) | 15 SCZ; 15 HC | SCZ: 50.0 (17.0); HC: 46.7 (16.3) | SCZ: 73.3%; HC: 66.7% | 80.0%; 3 patients unmedicated | Brain tissue | BA 10, SCZ: 4.03 [pmol/mg protein]; HC: 2.2 (0.77) [pmol/mg protein]; P = .011 | KYNA levels elevated within BA 10 in SCZ group |
| BA 9, SCZ: 2.50 [pmol/mg protein]; HC: 1.7 (0.77) [pmol/mg protein]; P = .058 | KYNA level elevation within BA 9 in SCZ group approached significance | ||||||
| Barry et al (2009) | 34 SCZf; 36 HC | SCZ: 37.3 (8.9); HC: 33.7 (6.6) | SCZ: 76.5%; HC: 72.2% | 85.3%; 5 patients unmedicated | Plasma | SCZ: 6.433 (2.93) [ng/mL]; HC: 6.785 (2.80) [ng/mL] | No difference in KYNA levels between groups |
| Miller et al (2006) | 12 SCZ; 14 HC | SCZ: 43.8 (12.8); HC: 48.6 (10.9) | SCZ: 66.7%; HC: 57.1% | 75.0%; 3 patients unmedicated | Brain tissue | SCZ: 1.719 (1.45) [pmol/10mg tissue]; HC: 1.034 (0.32) [pmol/10mg tissue] | Mean KYNA level higher in SCZ group |
| Nilsson et al (2005) | 90 SCZ; 49 HC | SCZ: 29.9 (8.5); HC: 27.0 (5.6) | SCZ: 100.0%; HC: 100.0% | 37.8%; 56 patients unmedicated for at least 21 d | CSF | SCZ: 1.45 (0.95) [nM]; HC: 1.06 (0.42) [nM] | KYNA levels elevated in SCZ group |
| Schwarcz et al (2001) | 30 SCZ; 31 HC | SCZ: 49.8 (15.1); HC: 49.8 (15.9) | SCZ: 70.0%; HC: 64.5% | 70.0%; 9 patients unmedicated for at least 6 mo | Brain tissue | BA 9e: SCZ: 2.9 (2.2) [pmol/mg protein]; HC: 1.9 (1.3) [pmol/mg protein] | KYNA levels increased within BA 9 in SCZ group |
| BA 10: SCZ: 2.7 (2.2) [pmol/mg protein]; HC: 2.0 (1.3) [pmol/mg protein] | Trend towards increased KYNA levels within BA 10 and 19 in SCZ group | ||||||
| BA 19: SCZ: 1.1 (0.6) [pmol/mg protein]; HC: 0.9 (0.4) [pmol/mg protein] | |||||||
| Ravikumar et al (2000) | 15 SCZ; 15 HC | Range, SCZ: 20–35 | SCZ: 53.3%; HC: 53.3% | 0.0% | Plasma | SCZ: 271.21 (22.44) [ng/ml]; HC: 172.60 (16.46) [ng/ml] | KYNA levels elevated in SCZ group |
Note: BA, Brodmann area; HC, healthy controls; KYNA, kynurenic acid; SCZ, schizophrenia.
aWhere P values were utilized to calculate SD, corresponding P values are presented in this table.
bFor ease of presentation, only findings concerning group differences in KYNA levels are included in this table.
cConsist of partially overlapping samples.
dIncluded patients with schizoaffective disorder.
eSample size presented here does not reflect total sample size, from which variables for meta-regression analyses were utilized.
fIncluded patients with schizoaffective disorder and psychosis not otherwise specified.
Risk of Bias
Six (46.2%) of 13 studies showed a “low” risk of bias for all items. The detailed assessment is displayed in supplementary figure 2.
Meta-analyses
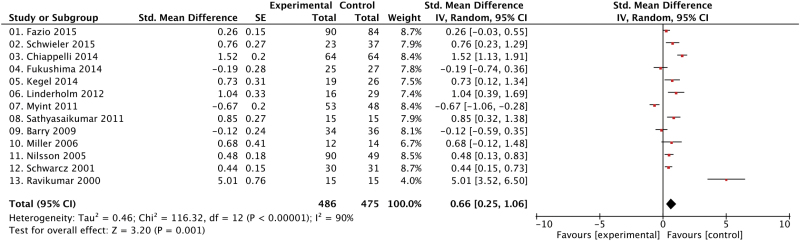
KYNA levels were moderately higher in patients with schizophrenia in comparison to HCs (SMD = 0.66, CI = 0.25 to 1.06, P = .001) (figure 1).
Fig. 1.
Group differences in KYNA levels between patients with schizophrenia and healthy controls. CI, confidence interval; IV, inverse variance; Std, standardized.
Moderator Analyses
Subgroup Analyses.
Nonoverlapping Samples
Excluding 2 studies64,65 with smaller, partially overlapping samples with another study,72 KYNA levels were still moderately elevated in patients with schizophrenia compared to HCs (SMD = 0.62, CI = 0.17 to 1.07, P = .007) (supplementary figure 3).
KYNA Measurement Technique
KYNA levels were moderately increased in patients with schizophrenia compared to HCs in studies using CSF (SMD = 0.66, CI = 0.42 to 0.91, P < .00001) and brain tissue samples (SMD = 0.55, CI = 0.31 to 0.79, P < .0001). KYNA levels did not differ between groups in studies using plasma/serum measurement techniques (SMD = 0.51, CI = −0.32 to 1.33, P = .23) (supplementary figure 4). There were insufficient studies using saliva to permit an analysis in this subgroup.
KYNA Sample Source
In the 7 studies measuring KYNA centrally, KYNA levels were moderately higher in patients with schizophrenia in comparison to HCs (SMD = 0.61, CI = 0.43 to 0.78, P < .00001). In contrast, in the 6 studies measuring KYNA peripherally, KYNA levels did not differ between groups (SMD = 0.74, CI = −0.12 to 1.59, P = .09) (supplementary figure 5).
Meta-regression Analyses.
Meta-regression analyses showed that the higher the patients’ age, the higher (ie, more positive) the study SMD (12 studies, n = 931, slope = 0.022, 95% CI: 0.005 to 0.039, P = .012). Also, the higher the %medicated, the higher the study SMD (13 studies, n = 961, slope = 0.008, 95% CI: 0.004 to 0.013, P < .001). Lastly, the higher the patients’ %male, the higher the study SMD (13 studies, n = 961, slope = 0.012, 95% CI: 0.004 to 0.020, P = .002) (supplementary figure 6). Notably, excluding the study with the lowest SMD67 led to the loss of significance for the meta-regression analyses mentioned above (all P values > .17). In contrast, excluding the study with the highest SMD61,69 did not alter findings (all P values < .012).
Sensitivity Analysis
Significant study heterogeneity existed in the main analysis (I2 = 90%). Sensitivity analyses indicated that no single study significantly contributed to heterogeneity.
Publication Bias
Egger’s test showed no publication bias in the analysis. The funnel plot is displayed in supplementary figure 7.
Discussion
Main Findings
This is the first meta-analysis to compare KYNA levels between patients with schizophrenia and HCs. The main analysis found elevated KYNA in patients with schizophrenia. Subgroup analyses demonstrated that: (1) this group difference remained when studies with partially overlapping samples were removed, (2) KYNA was increased in patients with schizophrenia when measured in CSF and brain tissue samples, and (3) KYNA was increased in patients with schizophrenia when measured in the CNS but not in the periphery. Lastly, meta-regression analyses revealed that the higher patients’ age, %medicated, and %male, the more positive the SMDs comparing KYNA between groups. Upon removing the study with the lowest SMD, significance for these relationships was lost.
Analysis of Included Studies
Four included studies measured KYNA in CSF. Nilsson et al68 found elevated KYNA levels in a mostly unmedicated sample of patients with schizophrenia compared to HCs. The other 3 studies measuring KYNA in CSF used partially overlapping participant samples, each investigating a unique primary objective. In their samples of olanzapine-treated patients with schizophrenia or schizoaffective disorder (SA), each of the 3 studies found increased KYNA levels in the patient group compared to HCs.64,65,72
Three included studies measured KYNA in brain tissue samples. In a seminal study, Schwarcz et al71 found increased KYNA in a sample of mostly medicated patients with schizophrenia within Brodmann area (BA) 9 but not 10 or 19, although a trend towards an increase was seen in the latter 2 areas. Sathyasaikumar et al70 found increased KYNA within BA 10 but not 9 in a mostly medicated sample of patients with schizophrenia; the elevation in BA 9 approached significance. Miller et al66 noted an increase in KYNA in samples of the anterior cingulate gyrus from mostly medicated patients with schizophrenia as compared to HCs, but the study was only powered to assess significance for a greater degree of change than that seen.
Five included studies measured KYNA in the plasma or serum. Fazio et al62 reported increased KYNA levels in a mostly medicated sample of patients with schizophrenia. Ravikumar et al69 found elevated plasma KYNA levels in unmedicated patients with schizophrenia. Contrastingly, Myint et al67 reported decreased plasma KYNA in antipsychotic-naïve or antipsychotic-free patients with schizophrenia. Also, Fukushima et al63 reported no difference in serum KYNA between medicated patients with schizophrenia and HCs, and Barry et al60 found no difference in plasma KYNA between mostly medicated patients with schizophrenia, SA, or psychosis not otherwise specified (NOS) and HCs.
Lastly, 1 included study measured KYNA in saliva. Chiappelli et al61 reported higher mean saliva KYNA in a mostly medicated sample of patients with schizophrenia or SA compared to HCs.
Analysis of Meta-regression Findings
The findings from meta-regression analyses suggest that patients’ age, %medicated, and %male are positively related to study SMDs. First, with respect to age, the current meta-regression results are in line with previous studies that report a positive correlation between age and KYNA in patients with schizophrenia.68,73 This supports the notion that increasing KYNA levels may explain cognitive deterioration with age.50,54 Also, given that α7nAChRs may be the preferred target of endogenous KYNA,40 and have been linked to cognitive impairment, increases in KYNA with age may also explain why cognitive symptoms arise early in the course of schizophrenia.74 However, it should be noted that not all studies find an association between age and KYNA levels.65,71 Second, in terms of antipsychotic status, these findings contrast those of previous studies suggesting that antipsychotic medication reduces brain KYNA levels.71,75 One included study showed a trend towards decreased KYNA within brain tissue samples of treated vs untreated patients,66 although other studies have found no relationship between antipsychotic status and KYNA levels.61,65 Finally, with respect to sex, results from the present meta-analysis contrast those of a previous study that found higher KYNA levels in female HCs than male HCs.76
However, the removal of Myint et al67 from the meta-regression analysis led to the loss of significance in each of the aforementioned relationships. Thus, the meta-regression results may in fact hold greater implications for interpreting the findings from Myint et al67 than those of the entire meta-analysis. It is proposed that the results of Myint et al,67 which was the only study to report decreased KYNA in the patient group, were influenced by their comparatively young, unmedicated, and mostly female patient sample.
Putative Mechanisms of KYNA Elevation in Schizophrenia
One explanation for elevated KYNA in schizophrenia might be a greater availability of KYN to be metabolized by KAT II to KYNA. In keeping with the notion that schizophrenia has an inflammatory component,77–79 evidence suggests that inflammatory processes activate KYN pathway enzymes in the periphery, leading to increases in peripheral KYN.31,47,53,80,81 As KYN readily crosses the blood-brain barrier, elevated peripheral KYN may contribute to elevated brain KYNA. Accordingly, elevated KYN has been detected centrally and peripherally in patients with schizophrenia63–66,71,72 and has been shown to correlate with brain KYNA.65,71
Further, studies examining KYN pathway enzyme expression and activity within brain areas highly implicated in schizophrenia pathophysiology have reported increased TDO2 and decreased KMO, with no change in IDO or KAT II.66,70,82,83 An increase in TDO2 would contribute to elevated brain KYN. This would be increasingly directed towards KYNA production in the presence of KMO disturbances, as supported by genetic studies that report KMO gene alterations to be related to increased KYNA.84,85 Likewise, preclinical studies administering a KMO blocker or genetically disrupting KMO have observed KYNA elevations.86–89
In addition, astrocytic activation may have an important role in explaining elevated KYNA. As previously described, KAT II, the enzyme primarily responsible for converting KYN to KYNA in the brain, has been found to exist preferentially in astrocytes. Thus, astrocytic activation may increase KYNA production. In support, increases in S100B, a marker for astrocyte function, have been found in patients with schizophrenia, reflecting increased astrocytic activity.90 Moreover, administration of interleukin 6 to cultured human astrocytes has been shown to increase KYNA, consistent with the aforementioned inflammation and astrocyte mechanisms.72
Overall, peripheral inflammation, altered brain TDO2 and KMO, and astrocytic activation may provide a framework through which to understand elevated KYNA in schizophrenia.
Implications of KYNA Dysregulation
KYNA and Behavior.
KYNA has a demonstrated capacity to affect behavior and has been posited to be especially influential in cognitive dysfunction.31,52 In the present review, 4 included studies reported upon relationships between KYNA and behavior. Fazio et al62 found negative correlations between KYNA levels and Positive and Negative Syndrome Scale (PANSS) positive symptom scores, and between KYNA levels and speed of processing, in subgroups of patients with multi-episode schizophrenia and first-episode schizophrenia, respectively; the authors identified no other relationships between KYNA levels and measures of symptomatology and functioning. Chiappelli et al61 noted that patients who experienced distress intolerance had higher KYNA levels both at baseline and following a stressor paradigm than patients who tolerated the psychological stressor and HCs. Also, in patients with distress intolerance, the change in KYNA was positively related to Brief Psychiatric Rating Scale (BPRS) total scores; however, baseline KYNA levels were not related to BPRS total scores. In addition, neither baseline KYNA nor change in KYNA levels were correlated with processing speed or working memory in patients or HCs.61 Linderholm et al65 noted no relationship between KYNA levels and BPRS and the Global Assessment of Functioning scores. Finally, Myint et al67 found that initial plasma KYNA levels were associated with a greater reduction in PANSS positive symptom scores as well as Korean Version of the Calgary Depression Scale for Schizophrenia depressive symptom scores after 6 weeks of antipsychotic treatment, though no cross-sectional relationships existed.
Beyond the included studies, other investigations in humans have provided evidence for associations between KYNA and behavior in patients with schizophrenia. Wonodi et al83 found that a single-nucleotide polymorphism in the KMO gene (the rate-limiting enzyme of KYN breakdown) was related to impaired smooth pursuit eye movement and visuospatial working memory in a clinical sample. Similarly, Wonodi et al91 found an association between variations in the KMO gene and deficits in cognitive function, an effect that was more marked in patients with schizophrenia than in HCs.
While human studies provide some evidence for the role of KYNA in modulating schizophrenia-like behavior, stronger support arises from preclinical work. In animal studies, KYNA levels can be raised through focal application of KYNA, administration of KYN, genomic KMO elimination, or KMO blockade.41,52 These manipulations cause cognitive impairments similar to those observed in patients with schizophrenia, including deficits in prepulse inhibition,92,93 auditory sensory-gating,94 stimulus processing and conditioned responding,95 spatial working memory,96 contextual fear conditioning and context discrimination,97 spatial learning and memory,98–100 and cognitive flexibility.101–103 In addition, KYNA increases have been shown to enhance spontaneous and amphetamine-induced locomotor activity.104
Conversely, experimentally induced reductions in KYNA by genetic deletion or acute inhibition of KAT II have led to improved cognitive functioning. Improvements have been noted in contextual memory and spatial discrimination,105 spatial learning and memory,100 sustained attention, amphetamine- and ketamine-induced disruptions in auditory gating, and ketamine-induced deficits in working memory and spatial memory.106
In summary, while human literature on the topic is emergent, preclinical studies provide evidence to suggest that increased KYNA levels may account for certain schizophrenia-like behaviors, specifically those observed within cognitive and social domains.
KYNA and Neurotransmission.
Of the studies included in the current review, only one measured indices of neurotransmission. Among other neurometabolites, Fukushima et al63 found decreased plasma serotonin and increased glutamate in the schizophrenia group, although relationships with KYNA were not reported. Moreover, another human study reported positive correlations between CSF KYNA and CSF homovanillic acid and 5-hydroxy-indoleacetic acid, indicative of dopamine and serotonin turnover, respectively.107
Unlike currently available human studies, preclinical literature has provided ample evidence to suggest that KYNA has inverse bi-directional relationships with several neurotransmitters, including glutamate, dopamine, acetylcholine, and GABA. Studies have demonstrated that increasing KYNA results in decreased glutamate99,100,102,108–112; notably galantamine administration normalizes this effect.102,110,112 Accordingly, decreasing KYNA leads to increased glutamate.100,105,108,110,112
Similarly, studies investigating dopaminergic neurotransmission have found that increasing KYNA results in decreased dopamine levels87,113,114—an effect that can also be attenuated by galantamine113,114—whereas decreasing KYNA increases dopamine levels.114,115 Furthermore, KYNA’s influence on midbrain dopamine neurons has been thoroughly studied; reliably, increased KYNA leads to increased firing rate and burst firing activity,93,116–119 whereas decreased KYNA has an opposite effect.119,120 These effects are believed to result from KYNA’s blockade of glutamate receptors.116,118,119 Moreover, the influence of KYNA on the dopamine system has been explored through the assessment of its effect on amphetamine-induced responses. Akin to NMDAR antagonists, KYNA has an amplifying effect on amphetamine-induced dopamine release through a mechanism involving reduced inhibition by amphetamine on firing rate and burst activity of ventral tegmental area dopamine neurons.121,122 Finally, it deserves mention that KYNA modulates the effects of clozapine—an atypical antipsychotic with particular efficacy in patients with treatment-resistant schizophrenia—and nicotine on midbrain dopamine neurons.46,120,123
Further, an inverse relationship between KYNA and acetylcholine is observed, with a decrease in KYNA leading to increased acetylcholine levels.124 Lastly, a bi-directional relationship between KYNA and GABA has been noted. An increase in KYNA results in decreased GABA—an effect prevented by galantamine—while a decrease in KYNA increases GABA.108,125
In summary, preclinical literature supports KYNA’s inverse effects on glutamate, dopamine, acetylcholine, and GABA, through its antagonism of α7nAChRs. In contrast, KYNA’s influence on midbrain dopamine neurons’ firing rate and burst firing activity, and amphetamine-induced responses, is likely related to its antagonism of glutamate receptors. These inverse associations remain unclear in schizophrenia, as hallmark neurotransmitter disruptions such as elevated striatal dopamine synthesis and release,4,6 and increased subcortical glutamate,2 seem incongruent with observed elevations in KYNA levels; heterogeneous brain KYNA distribution might explain this discrepancy.
KYNA and Drugs.
Several pharmacological agents can be utilized to manipulate KYNA levels. As per the above evidence, treatments that are intended to benefit patients with schizophrenia might aim to reduce KYNA levels. Given that there are no known KYNA degradation enzymes or specific targetable reuptake sites, the optimal method to lower KYNA appears to be via KAT II inhibition.41 KAT II has been shown to be highly substrate-specific, further making it an attractive target.41 Preclinical studies have shown that agents inhibiting KAT II lower KYNA levels by approximately 30% to 40%.52,100,106,112,124,126–128 Additionally, preclinical work suggests that these agents are procognitive100,106,128 and increase neurotransmitter levels described to be influenced by KYNA above.100,108,110,112,115,124,125
Pharmacological treatments might also attempt to counter KYNA’s mechanism of action. Preclinical findings presented above suggest that agonism of α7nAChRs or NMDARs might mitigate schizophrenia-like behavior and/or neurotransmission derangements. However, studies examining such agents in patients with schizophrenia have shown minimal efficacy to-date.55,129–131
Other possible targets to reduce KYNA are peripheral IDO and TDO2. Decreasing their activity might attenuate overproduction of peripheral KYN, thereby preventing increases in brain KYN and ultimately, brain KYNA. While IDO and TDO2 inhibitors have been studied as possible cancer treatments, their use in psychiatric diseases has been limited.31 IDO and TDO2 have important physiological functions, including immunomodulation and NAD production, respectively, and their inhibition can cause significant adverse effects.132–135
Nonselective inhibitors of cyclooxygenase (COX)-1 and COX-2 have also been found to influence KYNA levels: the former elevating KYNA and the latter decreasing it.136 COX-2 inhibitors have also been suggested to rebalance a disrupted immune response74,137 and have demonstrated beneficial effects for patients with schizophrenia80,138; however, the latter notion may be influenced by publication bias.139
Another strategy is activation of central KMO and its downstream enzymes along the quinolinic acid (QUIN)-producing branch, which may be decreased in schizophrenia.70 Doing so may shift brain KYN degradation towards QUIN and away from KYNA production. However, this would increase production of potentially harmful neurotoxins.32,53,54
Finally, nonspecific reduction of KYNA through TRP depletion has been attempted. Thus far, it has produced mixed results with respect to symptoms in patients with schizophrenia.140–142
Limitations of Present Study
The present work should be considered in light of its limitations. First, the primary aim may have been too narrow in that other KYN pathway metabolites were not evaluated. Second, some studies included patients with SA and psychosis NOS, which may have alternate pathophysiologies. Third, since some included studies did not report upon certain variables, such as duration of illness, antipsychotic dose, and symptom severity, and multiple measurement scales were utilized for the latter, the present study was unable to include these variables in meta-regression analyses. Fourth, some included studies did not account for the influence of food, smoking, or drug use. Fifth, compared to other major meta-analyses, our sample size was small. This may be especially relevant for the interpretation of subgroup analyses, as accumulating evidence may also reveal disruptions in peripheral KYNA levels. Finally, the possibility of publication bias should not be discounted.
Conclusion
The present meta-analysis found increased KYNA levels in patients with schizophrenia, a phenomenon that appears to be localized to the CNS. While age, antipsychotic status, and sex may have modulating effects, elevated central KYNA might help to explain disruptions in behavior and neurotransmission in patients with schizophrenia, thereby providing further clarity towards the understanding of schizophrenia pathophysiology and contributing to the development of novel potential treatment targets.
Future Directions
Future clinical studies should aim to replicate preclinical findings by testing the relationships between KYNA levels and measures of behavior and neurotransmission. The former can be achieved by utilizing well-characterized symptom (eg, PANSS143) and neuropsychological (eg, MATRICS144) batteries while the latter can be studied using in vivo brain imaging. Moreover, the regional distribution of KYNA levels should be explored in these relationships. Specifically, the investigation of possible relationships among KYNA levels, elevated striatal presynaptic dopamine synthesis, and increased subcortical glutamatergic neurometabolites early in the illness is warranted. Furthermore, measuring KYNA longitudinally over the course of illness would help define its role in schizophrenia pathophysiology. Additionally, future investigations should clarify the relationship between CSF, brain, plasma, and saliva KYNA levels, while ensuring that methodological issues such as fasting status are accounted for. This may elucidate whether studies in patients with schizophrenia should employ a particular KYNA sampling method. It may also be beneficial for future work to concurrently measure KYNA with other KYN pathway components to further examine pathway dysregulation. Overall, a better understanding of the cause and consequences of elevated KYNA in patients with schizophrenia may lead to the development of improved diagnostic and therapeutic strategies.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
Vanier Canada Graduate Scholarship (E.P.); Canadian Institute of Health Research (CIHR) (MOP-142493 and 14196 to A.G-G).
Supplementary Material
Acknowledgments
Thank you to Dr Christine Miller and Dr Ravikumar Kurup for kindly providing additional study information. Thank you to Dr Paul Fletcher for providing guidance and support. E.P. has received funding from the Vanier Canada Graduate Scholarship, the Ontario Graduate Scholarship, and the Canada Graduate Scholarship—Master’s. Y.I. has received fellowship grants from Keio University Medical Science Foundation, Mitsukoshi Foundation, Japan Foundation for Aging and Health, and manuscript fees from Dainippon Sumitomo Pharma. S.N. has received fellowship grants from CIHR, research support from Japan Society for the Promotion of Science, and manuscript fees or speaker’s honoraria from Dainippon Sumitomo Pharma and Yoshitomi Yakuhin. J.K.C. has received funding from the CIHR Doctoral Award and the Canada Graduate Scholarship—Master’s. P.G. has received fellowship awards from CIHR, the Ontario Mental Health Foundation (OMHF) and the Centre for Addiction and Mental Health (CAMH). H.T. has received fellowship grants from the CAMH Foundation, the Japanese Society of Clinical Neuropsychopharmacology, and Astellas Foundation for Research on Metabolic Disorders, and manuscript fees from Dainippon Sumitomo Pharma. G.R. has received consultant fees from Neurocrine Biosciences and Synchroneuron, as well as research support from Novartis. A.G.-G. has received support from the United States National Institute of Health, CIHR, OMHF, Consejo Nacional de Ciencia y Tecnología, the Instituto de Ciencia y Tecnología del DF, the Brain & Behavior Research Foundation (Formerly NARSAD), the Ontario Ministry of Health and Long-Term Care, the Ontario Ministry of Research and Innovation Early Research Award, and Janssen. All other authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol. 2015;29:97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poels EM, Kegeles LS, Kantrowitz JT, et al. Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Mol Psychiatry. 2014;19:20–29. [DOI] [PubMed] [Google Scholar]
- 3. Hietala J, Syvälahti E, Vuorio K, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346:1130–1131. [DOI] [PubMed] [Google Scholar]
- 4. Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169:1203–1210. [DOI] [PubMed] [Google Scholar]
- 5. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laruelle M, Abi-Dargham A, Van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–1219. [DOI] [PubMed] [Google Scholar]
- 8. Fusar-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry. 2012;17:1206–1227. [DOI] [PubMed] [Google Scholar]
- 10. Lindenmayer JP. Treatment refractory schizophrenia. Psychiatr Q. 2000;71:373–384. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki T, Remington G, Mulsant BH, et al. Treatment resistant schizophrenia and response to antipsychotics: a review. Schizophr Res. 2011;133:54–62. [DOI] [PubMed] [Google Scholar]
- 12. Adler CM, Malhotra AK, Elman I, et al. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156:1646–1649. [DOI] [PubMed] [Google Scholar]
- 13. Krystal JH, Perry EB, Jr, Gueorguieva R, et al. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry. 2005;62:985–994. [DOI] [PubMed] [Google Scholar]
- 14. Krystal JH, Bennett A, Abi-Saab D, et al. Dissociation of ketamine effects on rule acquisition and rule implementation: possible relevance to NMDA receptor contributions to executive cognitive functions. Biol Psychiatry. 2000;47:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. [DOI] [PubMed] [Google Scholar]
- 16. Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. [DOI] [PubMed] [Google Scholar]
- 17. Malhotra AK, Pinals DA, Weingartner H, et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. [DOI] [PubMed] [Google Scholar]
- 18. Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6:869–872. [DOI] [PubMed] [Google Scholar]
- 19. Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. [DOI] [PubMed] [Google Scholar]
- 20. Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. [DOI] [PubMed] [Google Scholar]
- 21. Rowland LM, Bustillo JR, Mullins PG, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162:394–396. [DOI] [PubMed] [Google Scholar]
- 22. Stone JM, Dietrich C, Edden R, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17:664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bartha R, Williamson PC, Drost DJ, et al. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54:959–965. [DOI] [PubMed] [Google Scholar]
- 24. de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology. 2011;36:1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. [DOI] [PubMed] [Google Scholar]
- 26. Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plitman E, de la Fuente-Sandoval C, Reyes-Madrigal F, et al. Elevated myo-inositol, choline, and glutamate levels in the associative striatum of antipsychotic-naive patients with first-episode psychosis: a proton magnetic resonance spectroscopy study with implications for glial dysfunction. Schizophr Bull. 2016;42:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Théberge J, Bartha R, Drost DJ, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. [DOI] [PubMed] [Google Scholar]
- 29. Leklem JE. Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am J Clin Nutr. 1971;24:659–672. [DOI] [PubMed] [Google Scholar]
- 30. Dounay AB, Tuttle JB, Verhoest PR. Challenges and opportunities in the discovery of new therapeutics targeting the kynurenine pathway. J Med Chem. 2015;58:8762–8782. [DOI] [PubMed] [Google Scholar]
- 31. Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vécsei L, Szalárdy L, Fülöp F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. 2013;12:64–82. [DOI] [PubMed] [Google Scholar]
- 33. Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. [DOI] [PubMed] [Google Scholar]
- 34. Gál EM, Sherman AD. L-kynurenine: its synthesis and possible regulatory function in brain. Neurochem Res. 1980;5:223–239. [DOI] [PubMed] [Google Scholar]
- 35. Guillemin GJ, Kerr SJ, Smythe GA, et al. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78:842–853. [DOI] [PubMed] [Google Scholar]
- 36. Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem. 2007;102:103–111. [DOI] [PubMed] [Google Scholar]
- 37. Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. [DOI] [PubMed] [Google Scholar]
- 38. Birch PJ, Grossman CJ, Hayes AG. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988;154:85–87. [DOI] [PubMed] [Google Scholar]
- 39. Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–1328. [DOI] [PubMed] [Google Scholar]
- 40. Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pocivavsek A, Notarangelo FM, Wu H-Q, Bruno JP, Schwarcz R. Chapter 25 - Astrocytes as pharmacological targets in the treatment of schizophrenia: focus on kynurenic acid. In: Mikhail VP, John LW, eds. Handbook of Behavioral Neuroscience. Vol 23 San Diego, CA: Elsevier; 2016:423–443. [Google Scholar]
- 42. Wang J, Simonavicius N, Wu X, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. 2006;281:22021–22028. [DOI] [PubMed] [Google Scholar]
- 43. DiNatale BC, Murray IA, Schroeder JC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lugo-Huitrón R, Blanco-Ayala T, Ugalde-Muñiz P, et al. On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol Teratol. 2011;33:538–547. [DOI] [PubMed] [Google Scholar]
- 45. Foster AC, Vezzani A, French ED, Schwarcz R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci Lett. 1984;48:273–278. [DOI] [PubMed] [Google Scholar]
- 46. Erhardt S, Schwieler L, Nilsson L, Linderholm K, Engberg G. The kynurenic acid hypothesis of schizophrenia. Physiol Behav. 2007;92:203–209. [DOI] [PubMed] [Google Scholar]
- 47. Myint AM. Kynurenines: from the perspective of major psychiatric disorders. FEBS J. 2012;279:1375–1385. [DOI] [PubMed] [Google Scholar]
- 48. Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia. Schizophr Bull. 2010;36:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 50. Erhardt S, Olsson SK, Engberg G. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs. 2009;23:91–101. [DOI] [PubMed] [Google Scholar]
- 51. Müller N, Myint AM, Schwarz MJ. Kynurenine pathway in schizophrenia: pathophysiological and therapeutic aspects. Curr Pharm Des. 2011;17:130–136. [DOI] [PubMed] [Google Scholar]
- 52. Schwarcz R. Kynurenines and glutamate: multiple links and therapeutic implications. Adv Pharmacol. 2016;76:13–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stone TW, Darlington LG. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br J Pharmacol. 2013;169:1211–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci. 2013;34:136–143. [DOI] [PubMed] [Google Scholar]
- 55. Iwata Y, Nakajima S, Suzuki T, et al. Effects of glutamate positive modulators on cognitive deficits in schizophrenia: a systematic review and meta-analysis of double-blind randomized controlled trials. Mol Psychiatry. 2015;20:1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 57. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 59. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66:408–414. [DOI] [PubMed] [Google Scholar]
- 60. Barry S, Clarke G, Scully P, Dinan TG. Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol. 2009;23:287–294. [DOI] [PubMed] [Google Scholar]
- 61. Chiappelli J, Pocivavsek A, Nugent KL, et al. Stress-induced increase in kynurenic acid as a potential biomarker for patients with schizophrenia and distress intolerance. JAMA Psychiatry. 2014;71:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fazio F, Lionetto L, Curto M, et al. Xanthurenic acid activates mGlu2/3 metabotropic glutamate receptors and is a potential trait marker for schizophrenia. Sci Rep. 2015;5:17799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fukushima T, Iizuka H, Yokota A, et al. Quantitative analyses of schizophrenia-associated metabolites in serum: serum D-lactate levels are negatively correlated with gamma-glutamylcysteine in medicated schizophrenia patients. PLoS One. 2014;9:e101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kegel ME, Bhat M, Skogh E, et al. Imbalanced kynurenine pathway in schizophrenia. Int J Tryptophan Res. 2014;7:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Linderholm KR, Skogh E, Olsson SK, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073-1074:25–37. [DOI] [PubMed] [Google Scholar]
- 67. Myint AM, Schwarz MJ, Verkerk R, et al. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naïve and medication-free schizophrenic patients. Brain Behav Immun. 2011;25:1576–1581. [DOI] [PubMed] [Google Scholar]
- 68. Nilsson LK, Linderholm KR, Engberg G, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80:315–322. [DOI] [PubMed] [Google Scholar]
- 69. Ravikumar A, Deepadevi KV, Arun P, Manojkumar V, Kurup PA. Tryptophan and tyrosine catabolic pattern in neuropsychiatric disorders. Neurol India. 2000;48:231–238. [PubMed] [Google Scholar]
- 70. Sathyasaikumar KV, Stachowski EK, Wonodi I, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37:1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–530. [DOI] [PubMed] [Google Scholar]
- 72. Schwieler L, Larsson MK, Skogh E, et al. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia–significance for activation of the kynurenine pathway. J Psychiatry Neurosci. 2015;40:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–98. [DOI] [PubMed] [Google Scholar]
- 74. Muller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006;10:131–148. [DOI] [PubMed] [Google Scholar]
- 75. Ceresoli-Borroni G, Rassoulpour A, Wu HQ, Guidetti P, Schwarcz R. Chronic neuroleptic treatment reduces endogenous kynurenic acid levels in rat brain. J Neural Transm (Vienna). 2006;113:1355–1365. [DOI] [PubMed] [Google Scholar]
- 76. Nilsson LK, Nordin C, Jönsson EG, Engberg G, Linderholm KR, Erhardt S. Cerebrospinal fluid kynurenic acid in male and female controls - correlation with monoamine metabolites and influences of confounding factors. J Psychiatr Res. 2007;41:144–151. [DOI] [PubMed] [Google Scholar]
- 77. Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tourjman V, Kouassi É, Koué MÈ, et al. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res. 2013;151:43–47. [DOI] [PubMed] [Google Scholar]
- 79. Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2014;155:101–108. [DOI] [PubMed] [Google Scholar]
- 80. Müller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci. 2015;9:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Steiner J, Bogerts B, Sarnyai Z, et al. Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World J Biol Psychiatry. 2012;13:482–492. [DOI] [PubMed] [Google Scholar]
- 82. Miller CL, Llenos IC, Dulay JR, Barillo MM, Yolken RH, Weis S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol Dis. 2004;15:618–629. [DOI] [PubMed] [Google Scholar]
- 83. Wonodi I, Stine OC, Sathyasaikumar KV, et al. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch Gen Psychiatry. 2011;68:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Holtze M, Saetre P, Engberg G, et al. Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls. J Psychiatry Neurosci. 2012;37:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lavebratt C, Olsson S, Backlund L, et al. The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol Psychiatry. 2014;19:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Giorgini F, Huang SY, Sathyasaikumar KV, et al. Targeted deletion of kynurenine 3-monooxygenase in mice: a new tool for studying kynurenine pathway metabolism in periphery and brain. J Biol Chem. 2013;288:36554–36566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rassoulpour A, Wu HQ, Ferre S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005;93:762–765. [DOI] [PubMed] [Google Scholar]
- 88. Röver S, Cesura AM, Huguenin P, Kettler R, Szente A. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J Med Chem. 1997;40:4378–4385. [DOI] [PubMed] [Google Scholar]
- 89. Speciale C, Wu HQ, Cini M, Marconi M, Varasi M, Schwarcz R. (R,S)-3,4-dichlorobenzoylalanine (FCE 28833A) causes a large and persistent increase in brain kynurenic acid levels in rats. Eur J Pharmacol. 1996;315:263–267. [DOI] [PubMed] [Google Scholar]
- 90. Rothermundt M, Ahn JN, Jörgens S. S100B in schizophrenia: an update. Gen Physiol Biophys. 2009;28 Spec No Focus:F76–F81. [PubMed] [Google Scholar]
- 91. Wonodi I, McMahon RP, Krishna N, et al. Influence of kynurenine 3-monooxygenase (KMO) gene polymorphism on cognitive function in schizophrenia. Schizophr Res. 2014;160:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry. 2004;56:255–260. [DOI] [PubMed] [Google Scholar]
- 93. Nilsson LK, Linderholm KR, Erhardt S. Subchronic treatment with kynurenine and probenecid: effects on prepulse inhibition and firing of midbrain dopamine neurons. J Neural Transm (Vienna). 2006;113:557–571. [DOI] [PubMed] [Google Scholar]
- 94. Shepard PD, Joy B, Clerkin L, Schwarcz R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology. 2003;28:1454–1462. [DOI] [PubMed] [Google Scholar]
- 95. Chess AC, Bucci DJ. Increased concentration of cerebral kynurenic acid alters stimulus processing and conditioned responding. Behav Brain Res. 2006;170:326–332. [DOI] [PubMed] [Google Scholar]
- 96. Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res. 2009;201:325–331. [DOI] [PubMed] [Google Scholar]
- 98. Pocivavsek A, Thomas MA, Elmer GI, Bruno JP, Schwarcz R. Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology (Berl). 2014;231:2799–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur J Neurosci. 2012;35:1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Alexander KS, Pocivavsek A, Wu HQ, Pershing ML, Schwarcz R, Bruno JP. Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine. Neuroscience. 2013;238:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Alexander KS, Wu HQ, Schwarcz R, Bruno JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl). 2012;220:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pershing ML, Bortz DM, Pocivavsek A, et al. Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology. 2015;90:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Olsson SK, Larsson MK, Erhardt S. Subchronic elevation of brain kynurenic acid augments amphetamine-induced locomotor response in mice. J Neural Transm (Vienna). 2012;119:155–163. [DOI] [PubMed] [Google Scholar]
- 105. Potter MC, Elmer GI, Bergeron R, et al. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35:1734–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kozak R, Campbell BM, Strick CA, et al. Reduction of brain kynurenic acid improves cognitive function. J Neurosci. 2014;34:10592–10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nilsson-Todd LK, Nordin C, Jönsson EG, Skogh E, Erhardt S. Cerebrospinal fluid kynurenic acid in male patients with schizophrenia - correlation with monoamine metabolites. Acta Neuropsychiatr. 2007;19:45–52. [DOI] [PubMed] [Google Scholar]
- 108. Beggiato S, Antonelli T, Tomasini MC, et al. Kynurenic acid, by targeting α7 nicotinic acetylcholine receptors, modulates extracellular GABA levels in the rat striatum in vivo. Eur J Neurosci. 2013;37:1470–1477. [DOI] [PubMed] [Google Scholar]
- 109. Carpenedo R, Pittaluga A, Cozzi A, et al. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur J Neurosci. 2001;13:2141–2147. [DOI] [PubMed] [Google Scholar]
- 110. Konradsson-Geuken A, Wu HQ, Gash CR, et al. Cortical kynurenic acid bi-directionally modulates prefrontal glutamate levels as assessed by microdialysis and rapid electrochemistry. Neuroscience. 2010;169:1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Konradsson-Geuken A, Gash CR, Alexander K, et al. Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse. 2009;63:1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wu HQ, Pereira EF, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci. 2010;40:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lopes C, Pereira EF, Wu HQ, et al. Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at alpha7* nicotinic receptors. J Pharmacol Exp Ther. 2007;322:48–58. [DOI] [PubMed] [Google Scholar]
- 114. Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm (Vienna). 2007;114:33–41. [DOI] [PubMed] [Google Scholar]
- 115. Amori L, Wu HQ, Marinozzi M, Pellicciari R, Guidetti P, Schwarcz R. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience. 2009;159:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Erhardt S, Engberg G. Increased phasic activity of dopaminergic neurones in the rat ventral tegmental area following pharmacologically elevated levels of endogenous kynurenic acid. Acta Physiol Scand. 2002;175:45–53. [DOI] [PubMed] [Google Scholar]
- 117. Erhardt S, Oberg H, Mathé JM, Engberg G. Pharmacological elevation of endogenous kynurenic acid levels activates nigral dopamine neurons. Amino Acids. 2001;20:353–362. [DOI] [PubMed] [Google Scholar]
- 118. Linderholm KR, Andersson A, Olsson S, et al. Activation of rat ventral tegmental area dopamine neurons by endogenous kynurenic acid: a pharmacological analysis. Neuropharmacology. 2007;53:918–924. [DOI] [PubMed] [Google Scholar]
- 119. Schwieler L, Erhardt S, Nilsson L, Linderholm K, Engberg G. Effects of COX-1 and COX-2 inhibitors on the firing of rat midbrain dopaminergic neurons–possible involvement of endogenous kynurenic acid. Synapse. 2006;59:290–298. [DOI] [PubMed] [Google Scholar]
- 120. Schwieler L, Linderholm KR, Nilsson-Todd LK, Erhardt S, Engberg G. Clozapine interacts with the glycine site of the NMDA receptor: electrophysiological studies of dopamine neurons in the rat ventral tegmental area. Life Sci. 2008;83:170–175. [DOI] [PubMed] [Google Scholar]
- 121. Liu XC, Holtze M, Powell SB, et al. Behavioral disturbances in adult mice following neonatal virus infection or kynurenine treatment–role of brain kynurenic acid. Brain Behav Immun. 2014;36:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Olsson SK, Andersson AS, Linderholm KR, et al. Elevated levels of kynurenic acid change the dopaminergic response to amphetamine: implications for schizophrenia. Int J Neuropsychopharmacol. 2009;12:501–512. [DOI] [PubMed] [Google Scholar]
- 123. Schwieler L, Erhardt S. Inhibitory action of clozapine on rat ventral tegmental area dopamine neurons following increased levels of endogenous kynurenic acid. Neuropsychopharmacology. 2003;28:1770–1777. [DOI] [PubMed] [Google Scholar]
- 124. Zmarowski A, Wu HQ, Brooks JM, et al. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur J Neurosci. 2009;29:529–538. [DOI] [PubMed] [Google Scholar]
- 125. Beggiato S, Tanganelli S, Fuxe K, Antonelli T, Schwarcz R, Ferraro L. Endogenous kynurenic acid regulates extracellular GABA levels in the rat prefrontal cortex. Neuropharmacology. 2014;82:11–18. [DOI] [PubMed] [Google Scholar]
- 126. Amori L, Guidetti P, Pellicciari R, Kajii Y, Schwarcz R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J Neurochem. 2009;109:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pellicciari R, Rizzo RC, Costantino G, et al. Modulators of the kynurenine pathway of tryptophan metabolism: synthesis and preliminary biological evaluation of (S)-4-(ethylsulfonyl)benzoylalanine, a potent and selective kynurenine aminotransferase II (KAT II) inhibitor. ChemMedChem. 2006;1:528–531. [DOI] [PubMed] [Google Scholar]
- 128. Wu HQ, Okuyama M, Kajii Y, Pocivavsek A, Bruno JP, Schwarcz R. Targeting kynurenine aminotransferase II in psychiatric diseases: promising effects of an orally active enzyme inhibitor. Schizophr Bull. 2014;40(suppl 2):S152–S158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Choi KH, Wykes T, Kurtz MM. Adjunctive pharmacotherapy for cognitive deficits in schizophrenia: meta-analytical investigation of efficacy. Br J Psychiatry. 2013;203:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Conley RR, Boggs DL, Kelly DL, et al. The effects of galantamine on psychopathology in chronic stable schizophrenia. Clin Neuropharmacol. 2009;32:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lindenmayer JP, Khan A. Galantamine augmentation of long-acting injectable risperidone for cognitive impairments in chronic schizophrenia. Schizophr Res. 2011;125:267–277. [DOI] [PubMed] [Google Scholar]
- 132. Ball HJ, Jusof FF, Bakmiwewa SM, Hunt NH, Yuasa HJ. Tryptophan-catabolizing enzymes - party of three. Front Immunol. 2014;5:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Fatokun AA, Hunt NH, Ball HJ. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids. 2013;45:1319–1329. [DOI] [PubMed] [Google Scholar]
- 134. Miller CL. The evolution of schizophrenia: a model for selection by infection, with a focus on NAD. Curr Pharm Des. 2009;15:100–109. [DOI] [PubMed] [Google Scholar]
- 135. Yu CP, Pan ZZ, Luo DY. TDO as a therapeutic target in brain diseases. Metab Brain Dis. 2016;31:737–747. [DOI] [PubMed] [Google Scholar]
- 136. Schwieler L, Erhardt S, Erhardt C, Engberg G. Prostaglandin-mediated control of rat brain kynurenic acid synthesis--opposite actions by COX-1 and COX-2 isoforms. J Neural Transm (Vienna). 2005;112:863–872. [DOI] [PubMed] [Google Scholar]
- 137. Müller N. Inflammation and the glutamate system in schizophrenia: implications for therapeutic targets and drug development. Expert Opin Ther Targets. 2008;12:1497–1507. [DOI] [PubMed] [Google Scholar]
- 138. Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res. 2007;90:179–185. [DOI] [PubMed] [Google Scholar]
- 139. Rappard F, Müller N. Celecoxib add-on therapy does not have beneficial antipsychotic effects over risperidone alone in schizophrenia. Neuropsychopharmacology. 2004;29:S222. [Google Scholar]
- 140. Golightly KL, Lloyd JA, Hobson JE, Gallagher P, Mercer G, Young AH. Acute tryptophan depletion in schizophrenia. Psychol Med. 2001;31:75–84. [DOI] [PubMed] [Google Scholar]
- 141. Hitsman B, Spring B, Wolf W, Pingitore R, Crayton JW, Hedeker D. Effects of acute tryptophan depletion on negative symptoms and smoking topography in nicotine-dependent schizophrenics and nonpsychiatric controls. Neuropsychopharmacology. 2005;30:640–648. [DOI] [PubMed] [Google Scholar]
- 142. Sharma RP, Shapiro LE, Kamath SK, Soll EA, Watanabe MD, Davis JM. Acute dietary tryptophan depletion: effects on schizophrenic positive and negative symptoms. Neuropsychobiology. 1997;35:5–10. [DOI] [PubMed] [Google Scholar]
- 143. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 144. Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.