Abstract
Molecular biology studies the cause-and-effect relationships among microscopic processes initiated by individual molecules within a cell and observes their macroscopic phenotypic effects on cells and organisms. These studies provide a wealth of information about the underlying networks and pathways responsible for the basic functionality and robustness of biological systems. At the same time, these studies create exciting opportunities for the development of quantitative and predictive models that connect the mechanism to its phenotype then examine various modular structures and the range of their dynamical behavior. The use of such models enables a deeper understanding of the design principles underlying biological organization and makes their reverse engineering and manipulation both possible and tractable The heat shock response presents an interesting mechanism where such an endeavor is possible. Using a model of heat shock, we extract the design motifs in the system and justify their existence in terms of various performance objectives. We also offer a modular decomposition that parallels that of traditional engineering control architectures.
Keywords: control theory, heat shock stress response, mathematical modeling
Asystems approach is a promising venue for studying biological complexity. The hallmark of this approach is an analysis of the dynamics of the biological network and the protocols used for their interactions. Experiences from fields like engineering, where model-based analysis and design have been the central paradigm, provide a glimpse of what is achievable (1). Biology and engineering share many similarities at the system level (2), including the use of complexity to achieve robustness and performance rather than for minimal functionality. We define robustness as the capability of a system to operate reliably when its physical parameters vary within their expected ranges; conversely, fragility is defined as hypersensitivity to such parametric variations. We suspect that the comparative study of genetic networks will confirm that robustness and the ability to achieve prompt adaptation in the presence of environmental challenges impose severe constraints on the underlying architecture of a system and are the source of the general design motifs repeated in many cellular networks.
Here, we study the heat shock response (hsr) in Escherichia coli. The hsr is universally conserved among organisms and serves to remedy protein damage induced by heat and other stresses (reviewed in ref. 3). We build a model for the E. coli hsr system, analyze its layers of regulation, and argue that its complexity is a necessary outcome of design requirements such as robustness, speed of response, noise rejection, and efficiency. This model provides useful insight into the heat shock system design architecture. It also suggests a mathematical and conceptual modular decomposition that defines the functional blocks or submodules of the heat shock system. This decomposition is drawn by analogy to manmade control systems and is found to constitute a canonical blueprint representation for the heat shock network. This analysis further complements and validates conclusions obtained on simpler and sometimes “designer”-built networks.
The hsr
High temperatures cause protein unfolding and malfunction, eventually resulting in cell death. Heat shock proteins (hsps) counter the effects of heat by serving as molecular chaperones that assist in the refolding of denatured proteins or proteases that degrade and remove the denatured proteins. In E. coli, hsps represent <2% of the total protein at 30°C and 20–25% of the total protein at 46°C, close to its growth cutoff (4). The cell must maintain a fine balance between the protective effect of the hsps and the metabolic burden (i.e., material and energy costs) of overexpressing these proteins. In E. coli, this balance is achieved through an intricate architecture of feedback loops centered around the σ32 factor that promotes transcription of the hsps under all conditions. The enzyme RNA polymerase (RNAP) bound to σ32 recognizes the heat shock gene (hsg) promoters and then transcribes specific hsgs. hsgs encode molecular chaperones such as GroEL/S and DnaK/J and proteases such as Lon and FtsH. Transcription of hsgs is tightly controlled by regulating the synthesis, activity, and stability of σ32 by using intricate feedback and feedforward loops that incorporate information about both the temperature and the level of folded proteins in the cell (5).
σ32 synthesis is regulated primarily at the translational level. At low temperatures, the translation start site of rpoH mRNA (encoding σ32) is occluded by base pairing; temperature upshift destabilizes this inhibitory base pairing, thereby increasing translation efficiency (6). By using both a temperature sensor and a feedforward element, this mechanism uses temperature information independently of the folding state of the cellular proteins to affect the production of hsps. Thus, the system can sense and react to a disturbance (e.g., heat) before its effects appear in the output (protein unfolding).
σ32 activity is regulated through its interaction with chaperones such as DnaK and its cochaperone DnaJ. In addition to their role in protein folding, chaperones bind to σ32 itself, thereby limiting its binding to core RNAP. Upon temperature increase, the cellular levels of unfolded proteins increase, thereby titrating chaperones away from σ32. Consequently, more σ32 binds RNAP, and transcription of hsgs increases (5). The accumulation of high levels of hsps leads to the efficient refolding of the denatured proteins, freeing up DnaK/J to sequester σ32 from RNAP. This implements a negative feedback loop referred to as a sequestration feedback loop.
σ32 stability is regulated through its interaction with proteases, primarily FtsH. During steady-state growth, σ32 is rapidly degraded (t1/2 = 1 min) but is stabilized for the first 5 min after temperature upshift. Possibly the direct titration of proteases by unfolded proteins may underlie σ32 stabilization (7). Alternatively, the DnaK/J chaperones, which are required in some capacity for rapid σ32 degradation, may be unavailable for the degradation reaction, because they are sequestered by unfolded proteins (8, 9). This mechanism yields a feedback-regulated degradation of σ32 and is referred to as the FtsH degradation feedback loop. Together, these mechanisms result in a transient 15- to 20-fold increase in the amount of σ32 at the peak of the hsr (≈5 min after temperature increase), followed by a decrease in 20–30 min to a new steady-state concentration dictated by the balance between the temperature-dependent translation of rpoH mRNA and the regulated degradation of σ32 (6).
A thorough study of the hsr has yielded a wealth of information about the different components of the system. Here, we use this qualitative biological information to formulate a quantitative description of the response and then use this description to pose questions about the regulatory architecture of the system. A largely qualitative description of some dynamic properties revealed by our work on the heat-shock system appeared in a review article published in IEEE Control Systems Magazine (10).
Models and Modularity of the hsr
The hsr under σ32 control consists of ≈50–100 genes and includes many chaperones and proteases (4). We developed a tractable mechanistic description of the σ32 hsr. In this model, we choose DnaK as the chaperone representative and FtsH as the primary protease degrading σ32. We assume that FtsH action in degrading σ32 is mediated by the interaction with the [σ32:DnaK] complex (9). We note that the actual mechanistic details of this degradation will not change the conclusions in this article. We also use the HslVU protease in our model to account for the slow degradation of σ32 in FtsH-null mutants (11). The model built to describe these components uses first-order mass-action kinetics and consists of a set of 31 differential-algebraic equations with 27 kinetic parameters of the form
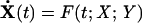 |
[1] |
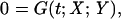 |
[2] |
where X is an 11-dimensional vector whose elements are differential (slow) variables, and Y is a 20-dimensional vector whose elements are algebraic (fast) variables. Some kinetic rate parameters are picked from the relevant literature. The unavailable parameters are tuned to reproduce the steady-state levels of σ32 and chaperones at low temperature. Those parameter sets reproducing steady-state behavior at high temperature and transient response upon upshift are retained. We then use data from heat shock mutants to discriminate among the remaining parameter sets, adopting the set that reproduces all of the data without additional tuning. The model equations, parameters, and data, along with time trajectories, are available in supporting information, which is published on the PNAS web site. For numerical simulations of the deterministic models, we used the differential-algebraic equations solver dassl (12). For sensitivity analysis, we used daspk, a software for the sensitivity analysis of large-scale differential algebraic systems (13). Stochastic simulations were implemented by using the Gillepsie stochastic simulation algorithm (14). The full-order heat shock model was also reduced to a simplified yet reasonably accurate version of the original model. This model reduction was made possible by various simplifications warranted by drastic time- and concentration-scale separations in the original model, in addition to the salient modularity of the heat shock system.
Functional Modules: Building Cellular Block Diagrams
Both biological and engineering systems share the propensity for modular decompositions (1). Control and dynamical systems theory is a discipline that uses modular decompositions extensively to make modeling and model reduction of systems more tractable. Because biological networks are themselves complex regulation systems, it is reasonable to expect that seeking similarities with the functional modules traditionally identified in control engineering schemes can be particularly useful.
The typical starting point of modular decompositions in engineering systems is the isolation of the process to be regulated. This process is commonly labeled as a plant. The remaining components in the system are then classified in terms of the function they accomplish to facilitate this regulation. For example, sensing and detection mechanisms constitute sensing modules, whereas mechanisms responsible for making decisions based on information provided by sensor modules constitute controller modules. The typical modular list of an engineering system also includes actuation modules. Actuation is necessary to transform the information-rich signal computed by the controller into a quantity of sufficient magnitude to drive the plant in the desired direction. A simple technological example where this decomposition is transparent is the heating of a house to a desired temperature (set point). For this purpose, temperature is measured (sensor), and its deviation from the desired temperature is assessed (error signal). The error signal is then fed to the thermostat (controller) that devises that appropriate control action. The output of the controller is used to operate the heat fuel valve (actuator), therefore generating an appropriate actuation signal (fuel to furnace). Errors or deviations from temperature setpoint are therefore corrected.
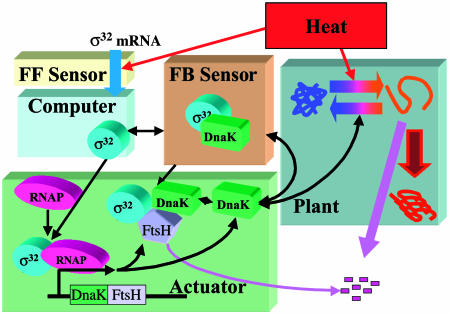
A similar modular decomposition can be carried in the hsr system if the protein-folding task is viewed as the process to be regulated. This plant is actuated by chaperones. The chaperone “signal” is produced by the high-gain transcription/translation machinery, which amplifies a modest σ32 input signal (few copies per cell) into a large chaperone output signal (≈10,000 per cell), much like an actuator. The σ32 control signal is the output of the computational or controller unit, which, based on the sensed temperature and folding state of the cell, modulates the number and activity of the σ molecules. The direct temperature measurement provided by σ32-mRNA heat-induced melting, in addition to the indirect protein-folding information, is assessed by the σ computational unit, producing an adequate control action by adjusting the synthesis, degradation, and activity of σ32. The conceptual modular decomposition of the heat shock system is shown in Fig. 1. At the same time, writing the equations describing the basic interactions of the molecular components that fit into any one of the specified modules generates a distinct mathematical modular decomposition. The usefulness of this mathematical decomposition resides in defining the boundaries of the functional modules through appropriate characteristic signals and generating a reduced-order model that qualitatively describes the core functionality of the heat shock system (see supporting information). Subsequently, the use of this reduced-order model is limited to analytical investigation and guiding numerical simulation. All simulation results are given for the full-order model.
Fig. 1.
Modular decomposition of the hsr. The functional modules, such as the plant, sensors, computational unit, and actuator, consist of various molecular species and their interactions
Structural Organization of the hsr System: A Necessary Complexity
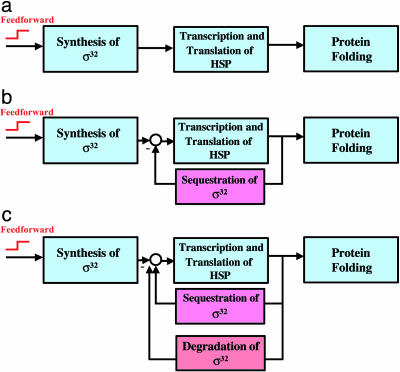
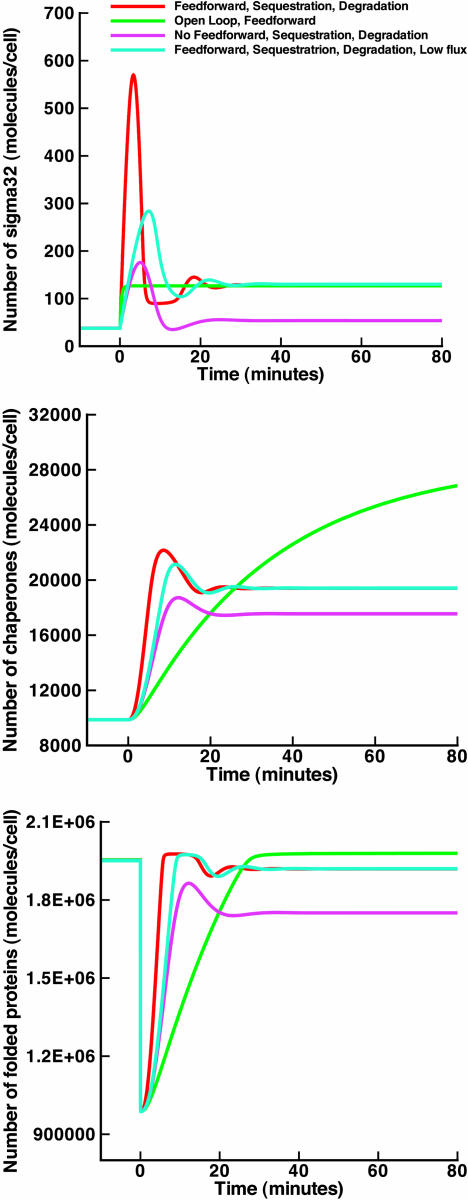
The modular decomposition of the hsr shows a level of complexity not justified by the basic functionality demanded from an operational heat shock system. A simple and operational heat-shock system would consist solely of a temperature sensor (for example, melting of σ32 mRNA) and a transcriptional/translational apparatus that responds appropriately to temperature changes (for example, hsp synthesis dictated by the number of σ32) (see Fig. 2a). Any number of σ32 and hsps is achievable through the careful tuning of the synthesis rates of these proteins. In engineering terms, this is referred to as an open-loop design. It differs from closed-loop designs, which use the measurement of internal variables to make decisions about future courses of action in the system. To verify that an open-loop scheme is sufficient to produce a functional design, we built a model incorporating the basic building blocks shown in Fig. 2a. We adjusted the levels of σ32, chaperones, and unfolded proteins in the open-loop design to make them identical to those of wild-type heat shock at a low temperature and investigated the behavior of the system after temperature upshift. This procedure is necessary to reduce the accidental difference between the two models and ensure that any remaining dissimilarities in their dynamical behavior are the correct indicators of their actual differences. This procedure is related to mathematically controlled comparison (15) and will be used for all models presented in this work. The levels of σ32, DnaK, and unfolded protein obtained from the simple open-loop model are shown in Fig. 3 (green line), where it can be seen that the design provides an acceptable folding profile after heat shock. One cannot but wonder whether the complexity of the actual hsr is merely accidental. To investigate this, we start with the simple open-loop model in Fig. 2a, then add each experimentally verified layer of regulation, one after another, each time demonstrating how that layer is needed to improve the performance indices. Thus, complexity is indeed necessary to achieve robustness, noise rejection, speed of response, and economical use of cellular resources, much like engineering systems.
Fig. 2.
Hypothetical design models for the hsr. (a) Simple open-loop design. The feedforward element achieves temperature sensing (b). Closed-loop design with feedforward and sequestration loop to regulate the activity of σ32. (c) Closed loop with feedforward, sequestration, and degradation of σ32 loop, which corresponds to the wild-type heat shock system.
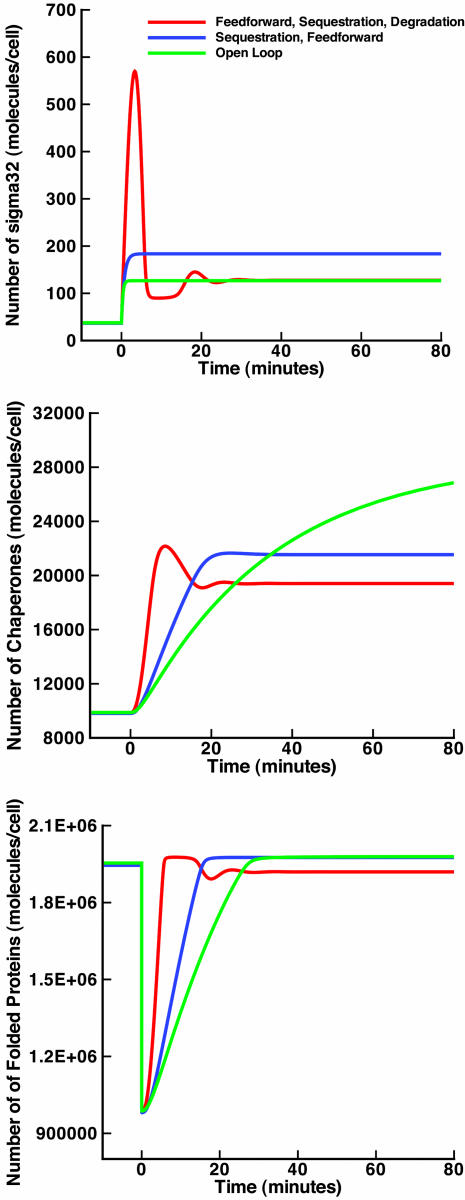
Fig. 3.
Levels of σ32, DnaK, and unfolded proteins for the open-loop design in Fig. 2a, closed-loop design with sequestration loop in Fig. 2b, and closed loop with both sequestration and degradation loops in Fig. 2c. Heat shock occurs at time 0. The rate of production of σ32 is tuned to produce the same levels at low temperature as the wild-type design, and performance is assessed at a high temperature.
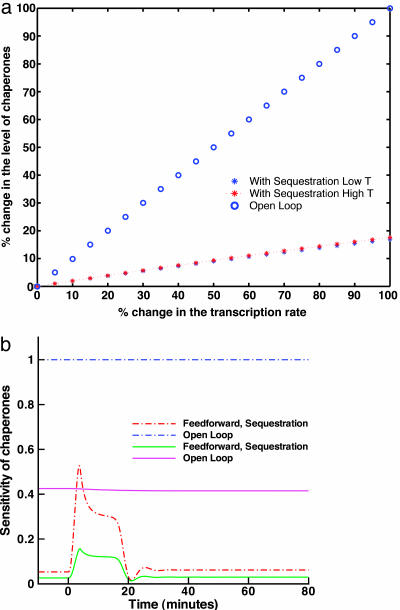
First, we investigate the contribution of the regulation of σ32 activity, replacing the open-loop system in Fig. 2a by the closed-loop system in Fig. 2b, where chaperones sequester free σ32 and modulate its activity. Comparison of the performance of both indicates that the open-loop system is alarmingly sensitive to parameter variations. Indeed, in the open-loop design of Fig. 2a, the slightest change in transcription and translation rates of chaperones results in a corresponding change in the number of hsps produced, even under normal circumstances and at low temperatures (see Fig. 4a). Parametric variations are directly transmitted to the output. This is a salient drawback of open-loop designs that makes them adequate only when the cellular environment is constant and the system components are precise. Thus, feedback control can be used to ensure robustness in the presence of imprecise components and the ever-changing cellular environment. To investigate this effect of feedback on the system in Fig. 2b, we compute the variations in the steady-state chaperone output after fluctuations in their synthesis rate. The result of this computation is plotted along with the open-loop fluctuations in Fig. 4a and shows a dramatic increase in the robustness of the system as measured by the attenuation of the effect of parametric fluctuations. A more systematic sensitivity analysis is also possible and can be carried out by computing the derivative of chaperone time evolution with respect to any parameters of interest (see supporting information for details on this procedure). In addition to steady-state analysis, this gives the temporal behavior of sensitivity. We have performed these computations, and the results confirm increased robustness in the presence of the sequestration loop. For illustration purposes, we show examples of the sensitivities in Fig. 4b. We also show in Fig. 3 (blue line) the dynamics of σ32, DnaK, and unfolded proteins for the design of Fig. 2b and point to an important observation. Although the steady-state number of chaperones at high temperature in the open-loop case is much higher than in the closed-loop case, the number of folded proteins in the two cases is comparable. This effect is mainly due to the fact that in the closed-loop case, the activity of σ32 is tightly regulated, and any excess of unneeded hsps is hindered by feeding back a measure of the quantity of unfolded proteins in the cell (through the σ32–chaperone complex) to regulate the pool of free σ32. Hence, the unnecessary use of materials and energy that the open-loop design suffers from is completely avoided through the utilization of feedback.
Fig. 4.
Robustness as an outcome of feedback in hsr. (a) Plot of the percentage change in the level of chaperones vs. the percentage change in the transcription rate for the open-loop model in Fig. 2a and the closed-loop model with sequestration loop in Fig. 2b. (b) Small signal sensitivity of the level of chaperones to their synthesis rate (dotted lines) and to the binding between σ32 and the core RNAP (solid lines). The plots are shown for the open-loop design in Fig. 2a and the closed-loop design with sequestrion in Fig. 2b. Sensitivity is computed as the derivative of the chaperone level to the corresponding parameters along the trajectories of the system. The plots are in log space. Heat shock occurs at time 0.
In the hypothetical models up to this point, the production and activity of σ32 are regulated, whereas its degradation is assumed to take place at a constant rate. If the speed of the repair response depends on the immediate accumulation of σ32, then one might easily foresee the benefits of stabilizing σ32 for that purpose, i.e., regulating its degradation based on the protein-folding state of the cell. In wild-type heat shock, the stability of σ32 is feedback-regulated by a number of proteases, the most investigated being FtsH. Adding the FtsH-mediated layer of regulation to our sequence of hypothetical designs, we obtain the system in Fig. 2c. The corresponding plots for σ32, DnaK, and unfolded proteins are shown in Fig. 3 (red line) and illustrate the anticipated role of the regulated degradation of σ32 in reducing the transient time required for protein folding.
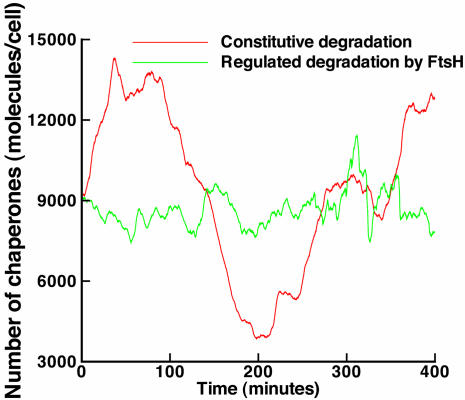
One feature of primary importance in cellular processes is their ability to attenuate undesirable noise. Numerous sources of noise induce fluctuations in the concentration of cellular molecular species. The magnitude and nature of the fluctuations are thought to depend on the structure of the molecular networks, the concentration of molecules that populate this structure, and the reaction rates of the underlying biochemical reactions. At the same time, molecular networks are expected to function reliably and robustly in the presence of this noise. It has been suggested that this robust operation is in part the outcome of feedback regulatory loops (16). To verify this prediction in the context of the hsr, we investigate the noise-rejection merits of the various negative feedback loops. Specifically, we consider here the FtsH degradation loop. We look at a virtual mutant, where the degradation of σ32 is constitutive, i.e., independent of any proteases belonging to the σ32 regulon, and compare it with the wild-type, where the stability of σ32 is dynamically regulated. Starting with the reduced-order model and using an approximation technique commonly known as the linear noise approximation (17), it is possible to establish analytically that the regulated stability of σ32 yields a chaperone steady-state level that is more narrowly distributed than its unregulated counterpart. This narrower distribution of chaperones (and consequently of unfolded proteins) is an indication of a superior ability to attenuate intrinsic biochemical noise and is mostly due to the contribution of the degradation loop in creating a larger feedback gain. The derivation of this result is omitted here for brevity; however, we illustrate its validity by using the Gillespie stochastic simulation algorithm (SSA) (14). The SSA reproduces the exact stochastic behavior of a system. Hence, the rejection/amplification properties of a system can be assessed by observing the excursions of a quantity around its ensemble average. Fig. 5 clearly shows that stochastic fluctuations are much more pronounced in the constitutive degradation case. Probability density functions compiled for the two cases also show a noticeably wider distribution for the constitutive degradation case as compared with regulated degradation.
Fig. 5.
A stochastic realization using the Gillespie stochastic simulation algorithm (10). The heat shock model with constitutive degradation of σ32 (red) shows larger excursions around the mean than the model with regulated degradation of σ32 (green). The mean value for the number of chaperones per cell is comparable for both cases, <DnaK> ≃8,950. The standard deviation for the regulated degradation case was computed to be σr ≃ 1,500, whereas that for the constitutive degradation case was σu ≃ 2,700. [Reproduced with permission from ref. 10 (Copyright 2004, IEEE)].
Feedforward: A Dynamic Sensor
Having established that a closed-loop design implementing feedback through the regulated activity and degradation of σ32 is necessary to implement a fast, efficient, and robust response, we look back at properties of the feedforward loop. The role of this loop as a temperature sensor was essential in the open-loop design. However, in the closed-loop heat shock design, sensing is already implemented through the temperature-induced titration of DnaK by unfolded proteins. Does this second feedforward thermosensor confer any benefits to the response, or is it just a redundancy measure? To answer this question, we investigate a virtual mutant where this translation thermosensor is disabled by locking the translational switch in the OFF position upon temperature upshift, therefore imposing the same translational efficiency at both low and high temperatures. In this case, the accumulation of σ32 in the induction phase of the response is achieved through the stabilization rather than increase in synthesis rate of σ32. This yields a more modest accumulation of σ32 at the peak of the hsr, after which the level of σ32 recovers to a value slightly higher than that at the lower temperature. This, in turn, results in insufficient chaperone production and consequently impaired and slightly delayed protein folding (Fig. 6). The verification of these specific outcomes in the absence of feedforward was partially accomplished experimentally, and the results closely coincide with our observations (Takashi Yura, personal communication). However, one might be tempted to question whether any tuning of the feedback components themselves could compensate for the response deficiency in the absence of feedforward.
Fig. 6.
Levels of σ32, DnaK, and unfolded proteins for the open-loop design in Fig. 2a; closed loop with sequestration and degradations loops in Fig. 2c; closed loop with sequestration and degradation but lacking the feedforward component; and closed loop with feedforward, sequestration, and degradation and a lower σ32 turnover rate. The rate of production of σ32 is tuned to produce the same levels at low temperature as the wild-type design, and performance is assessed at high temperature. Heat shock occurs at time 0.
The lower level of σ32 at steady state in the feedforward mutant, as compared with its counterpart in the wild type, is the result of a new setpoint dictated by the balance between σ32 lower synthesis rate at high temperature and its degradation rate. As a certain level of folding is recovered in the adaptation phase of the response and due to the small number of σ32 as compared with chaperones, the majority of σ32 returns to the sequestered form, therefore limiting the number of new chaperones produced. The number of unfolded proteins is stabilized at this specific level. This process possesses higher thresholds when the synthesis of σ32 is larger in the presence of feedforward. To recover this threshold using feedback alone, the degradation feedback gain should be decreased to balance its reduced synthesis, for example, by making σ32 less susceptible to degradation by FtsH. However, this would imply the presence of a higher concentration of σ32 even at low temperature, hence an unneeded excess of hsps. Additionally, the slower transient response of the feedforward mutant is attributed to the accumulation of σ32 through its feedback-mediated stabilization solely, a process possessing an inherent delay, because it relies on protein unfolding rather than the direct sensing of temperature. A possible mechanism that can compensate for this delay in the absence of the feedforward term is an increase in the turnover rate of σ32. The steady state for σ32 (and subsequently chaperones and unfolded proteins) can be kept constant by simultaneously manipulating its synthesis and degradation rates. This, however, results in modified response dynamics. We show the results of a simulation where we simultaneously decrease both the translation and degradation of σ32 5-fold, yielding a lower turnover rate for σ32 and a slower response (see Fig. 6). Note that in this case, the translational switch is still operational in the sense that at high temperature, σ32 translation is still being increased by 5-fold relative to its low-temperature value. Based on this analysis, one can postulate a scenario where the synthesis and degradation of σ32 are tuned appropriately at low temperature can compensate for the delayed and impaired response in the absence of the feedforward loop at high temperature. However, the byproduct of such tuning is at the least a high metabolic cost at low temperature. Alternatively, feedforward presents a simple and economical means of achieving the same objective.
Discussion
A functional criterion, universally present in manmade and naturally occurring systems, is the need to be competitively robust in uncertain environments. Technologies and biological mechanisms that suffer from unremedied fragilities to frequently occurring disturbances in their environment are bound to be outcompeted (2). Battling such fragilities in engineering systems through the use of feedback has a rich history, starting in antiquity with simple schemes of flow-rate control to regulate water clocks and extending to recent times, where machines, such as planes, possess thousands of computers to regulate various functions. The use of increasingly sophisticated control mechanisms resulted in more reliable systems, all generating spiraling levels of complexity. It is increasingly apparent that robustness, implemented through complex feedback structures rather than through the use of simple but precise and finely tuned components, is also a salient feature of biological organization. In the heat shock system, we have illustrated how the use of feedforward and feedback loops is justified by robust operation in fluctuating environments and fast response to heat disturbance. Considering the limited cellular energies and materials, these performance objectives often form a contradictory set of constraints and require the presence of various tradeoffs. For example, a large turnover rate for σ32 necessarily yields a fast response but comes at the expense of fast production and degradation, mechanisms that require a substantial amount of cellular materials and energy. High feedback gains also contribute to improving the transient response while attenuating harmful cellular noise. Implementing these gains requires, among other things, increased binding specificities that result in highly complex or specialized proteins. This, again, is not without similarities to tradeoffs considered in engineering systems where typical design requirements include the simultaneous minimization of deviation from some desired operation and the control effort needed to accomplish that, obviously competing objective. Drawing a parallel to heat shock, this would correspond to the objective of limiting the deviation from an optimal number of unfolded proteins by using a minimal number of chaperones and proteases. Adding transient performance and noise rejection considerations generates additional complexity and results in a rather delicate optimization problem. The formulation of such a problem aside, the physical implementation of any of its solutions seems to have been evolutionarily solved by using a number of recurring motifs (18). An example is the use of negative feedback as a motif to speed slow dynamics. Strong supporting evidence suggested in ref. 19 and verified in ref. 20 indicates that the rise time in the level of a protein, following a step induction in an open-loop transcription unit, can be dramatically improved by adding a negative feedback loop. Accordingly, in the hsr, we have observed that both sequestration and degradation negative-feedback loops work precisely to this effect. The use of high turnover rates in speeding slow kinetics is another recurring motif whose effect was observed in a variety of biological systems, including the regulation of enzyme levels in mammalian tissues (21), and metabolic networks (22). Here, it is also shown to play an important role. The role of feedback motifs as noise-attenuating mechanisms has also been investigated theoretically and experimentally (16, 23). In ref. 16, for example, it was found that a bacterial gene whose product autoregulates its own synthesis is characterized by smaller deviation of the protein distribution from Poissonian statistics compared with its open-loop counterpart. This study and others mainly focus on the noise rejection and stability properties related to regulation of protein synthesis (23). In contrast, in the hsr system, it is mainly the stability of σ32 rather than its synthesis that is regulated. We have demonstrated how this scheme generates an equally effective or possibly superior method for noise rejection. This finding is of substantial importance, because regulated protein degradation plays a crucial role in a multitude of processes, including cell growth, division, differentiation, and responses to various stressors (24). Regulated proteolysis is characterized by a fast and irreversible response. To this list of desirable properties, we append here noise attenuation as an additional benefit. In addition to the analysis of recurring regulatory feedforward and feedback motifs, our study of the hsr uncovered an intriguing modular decomposition of the system. Traditionally, functional modules have been defined as a collection of proteins that participate in a particular cellular process (25). Common examples of functional modules include the CDK/cyclin module, responsible for cell-cycle progression; the yeast pheromone response pathway; and mitogen-activated protein kinase cascades. Viewed from this perspective, heat shock itself constitutes an integral functional module. Such a characterization of functional modules is extremely useful, because it provides an inventory list of cellular processes. An analogy would be a list of machines and their function in a factory. However, to understand the operational principles of a certain machine, to repair it, or to optimize its performance, it is often necessary to consider a modular decomposition of the machine itself. Such a decomposition does not necessarily require stripping the machine down to the component level but rather identifying its submodules with their predefined functionalities. A particularly successful such modular decomposition has been extensively used in the field of control and dynamical systems, where components of a system are classified in terms of their role with respect to the regulation objective. Similar decompositions exist in computer science, for example, because modularity is a basic principle of good programming. Indeed, in higher-level languages, a complicated programming task is usually divided into a set of modules, subroutines, or objects, with simple well defined interfaces. This results in flexible and robust programs, whose modules can be designed almost separately and, as such, are more easily evolvable.
In an insightful paper, Hartwell et al. (1) argue that ideas borrowed from “synthetic” sciences, such as engineering and computer science, need to be used for a broader understanding of functional biological modules and the dynamical range of their interactions. Examples across biological scales, ranging from physiology (26) to gene regulation (27), demonstrate the validity of their predictions. The analysis of the hsr system along the general guidelines they advocate provides yet more evidence in that direction.
Supplementary Material
Acknowledgments
We thank Irina Grigorova for critical reading of the manuscript. This work was supported by National Science Foundation Grant CCF-0326576 and Institute for Collaborative Biotechnologies Grant DAAD19-03-D-0004 from the U.S. Army Research Office.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: hsr, heat shock response; hsps, heat shock proteins; hsg, heat shock gene; RNAP, RNA polymerase.
References
- 1.Hartwell, L. H., Hopfield, J., Leibler, S. & Murray, A. W. (1999) Nature 81, C47–C52. [DOI] [PubMed] [Google Scholar]
- 2.Csete, M. & Doyle, J. C. (2002) Science 295, 1664–1669. [DOI] [PubMed] [Google Scholar]
- 3.Craig, E. A. (1985) Crit. Rev. Biochem. 18, 239–280. [DOI] [PubMed] [Google Scholar]
- 4.Gross, C. A. (1996) in Escherichia coli and Salmonella: Cellular and Molecular Biology, eds. Neidhart, F. C., Curtis, R. I., Ingraham, J. L., Lin, C. C., Low, K. B., Magasnik, B., Reznikoff, W. S., Riley, M., Schaechter, M. & Umbarger, H. E. (Am. Soc. Microbiol., Washington, DC), pp. 1384–1394.
- 5.Straus, D. B., Walter, W. A. & Gross, C. A. (1990) Genes Dev. 4, 2202–2209. [DOI] [PubMed] [Google Scholar]
- 6.Morita, M., Kanemori, M., Yanagi, H. & Yura, T. (1999) J. Bacteriol. 181, 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman, C., Prakash, S., Lu, C. Z., Matouschek, A. & Gross, C. A. (2003) Mol. Cell 11, 659–669. [DOI] [PubMed] [Google Scholar]
- 8.Morita, M., Kanemori, M., Yanagi, H. & Yura, T. (2000) Proc. Natl. Acad. Sci. USA 97, 5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatsuta, T., Joo, D., Calender, R., Akiyama, Y. & Ogura, T. (2000) FEBS Lett. 478, 271–275. [DOI] [PubMed] [Google Scholar]
- 10.Khammash, M. & El-Samad, H. (2004) IEEE Control Syst. Mag. 24, 62–76. [Google Scholar]
- 11.Kanemori, M., Nishihara, K., Yanagi, H. & Takashi, Y. (1997) J. Bacteriol. 179, 7219–7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petzold, L. R. (1983) in Scientific Computing, ed. Stepleman, R. S. (North–Holland, Amsterdam), pp. 65–68.
- 13.Li, S. & Petzold, L. R. (2000) J. Comput. Appl. Math. 125, 131–146. [Google Scholar]
- 14.Gillespie, D. T. (1976) J. Comput. Phys. 22, 403–434. [Google Scholar]
- 15.Alves, R. & Savageau, M. A. (2000) Bioinformatics 16, 786–798. [DOI] [PubMed] [Google Scholar]
- 16.Thattai, M. & Van Oudenaarden, A. (2001) Proc. Natl. Acad. Sci. USA 98, 8614–8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elf, J. & Ehrenberg, M. (2003) Genes Res. 13, 2475–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen-Orr, S., Milo, R., Mangan, S. & Alon, U. (2002) Nat. Genet. 31, 64–68. [DOI] [PubMed] [Google Scholar]
- 19.Savageau, M. A. (1974) Nature 252, 546–549. [DOI] [PubMed] [Google Scholar]
- 20.Rosenfeld, N., Elowitz, M. B. & Alon, U. (2002) J. Mol. Biol. 323, 785–793. [DOI] [PubMed] [Google Scholar]
- 21.Schimke, R. T. (1969) Curr. Top. Cell. Regul. 1, 77–124. [Google Scholar]
- 22.Covert, M. W., Schilling, C. H. & Palsson, B. (2001) J. Theor. Biol. 213, 73–88. [DOI] [PubMed] [Google Scholar]
- 23.Becskei, A. & Serrano, L. (2000) Nature 405, 590–593. [DOI] [PubMed] [Google Scholar]
- 24.Hilt, W. & Wolf, D. H. (1996) Trends Biochem. Sci. 21, 96–102. [PubMed] [Google Scholar]
- 25.Spirin, V. & Mirny, L. A. (2003) Proc. Natl. Acad. Sci. USA 100, 12123–12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Samad, H., Goff, J. P. & Khammash, M. (2002) J. Theor. Biol. 214, 17–29. [DOI] [PubMed] [Google Scholar]
- 27.Yi, T. M., Huang, Y., Simon, M. I. & Doyle, J. (2000) Proc. Natl. Acad. Sci. USA 97, 4649–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.