Abstract
The RANK/RANKL/OPG pathway is well known for bone destruction in skeletal metastases but has also been implicated in osteoclast‐independent roles in tumorigenesis and de novo metastasis. Experimental data suggest contribution of progesterone to tumorigenesis may be mediated by RANKL. Importantly, modulation of this pathway became possible through the availability of denosumab, an artificial counterpart of OPG, but significant gaps remain in the translation of preclinical findings on the pathway. We analyzed gene expression of RANK, RANKL and OPG from 40 Affymetrix datasets encompassing 4467 primary breast cancers and focused on ER positive disease.
We did not observe a significant prognostic value of RANK and RANKL mRNA expression. In contrast, OPG was associated with a better prognosis among 1941 ER positive cancers (HR 0.64, 95% CI 0.53–0.77; P < 0.0001) using a cutoff from its highly bimodal expression. We detected considerable heterogeneity regarding the prognostic value of OPG between different datasets. This heterogeneity could neither be attributed to technical reasons nor to differences in standard clinical parameters or treatments of the cohorts. Interestingly, the prognostic value of the progesterone receptor and of OPG showed similar cohort specific effects. Still both factors were no surrogates for each other but contributed independent prognostic value in multivariate analyses.
Thus, both OPG and PgR are independently associated with good prognosis in ER positive breast cancer. However both markers share common cohort specific differences in contrast to proliferation markers as Ki67 which may be based on the underlying biology.
Keywords: Osteoprotegerin, RANK, RANKL, Progesteron receptor, Breast cancer, Gene expression profiling, Cohort bias, Dataset pooling
Highlights
The RANK/RANKL/OPG pathway is known for bone destruction in skeletal metastases.
It has also been implicated in tumorigenesis and de novo metastasis in breast cancer.
We performed a large meta‐analysis of gene expression data on RANK/RANKL/OPG.
OPG and PgR are independently associated with good prognosis in ER positive breast cancer.
But both share common cohort specific differences in contrast to proliferation markers as Ki67.
1. Introduction
The cell surface receptor RANK (receptor activator of NFkB), its ligand (RANKL), and the decoy receptor of RANKL, osteoprotegerin (OPG), play an important functional role in bone physiology and in bone metastasis by regulating osteoclasts (González‐Suárez, 2011). RANK/RANKL signaling has also been shown to be involved in mammary gland development (Fata et al., 2000) as well as mammary tumorigenesis (Gonzalez‐Suarez et al., 2010; Schramek et al., 2010) and progression (Palafox et al., 2012) in mice. In normal mammary gland development RANKL expression is strongly induced by PgR signaling and required for progesterone induced side branching (Brisken, 2013). Recent data show that RANKL protein expression in breast tissue correlates with progesterone levels in women (Tanos et al., 2013). Soluble RANKL administered intravenously can elicit proliferation in the mammary epithelium, and systemic administration of its decoy receptor osteoprotegerin (OPG) can inhibit proliferation (Brisken, 2013). Adding progesterone (MPA) to estrogen (CEE) treatment resulted in higher expression of RANK and RANKL, and lower expression of OPG in normal breast tissue of macaque monkeys (Wood et al., 2013). RANKL protein was localized exclusively in PgR expressing luminal epithelial cells in that study, similar to what has been described in mice and humans (Gonzalez‐Suarez et al., 2010; Tanos et al., 2013). The important role of the RANKL decoy receptor OPG is demonstrated by the effects of its artificial counterpart, a human monoclonal antibody to RANKL (Denosumab) (Dougall, 2012). This antibody has been developed to prevent skeletal‐related events in patients with bone metastases (Stopeck et al., 2010; Lipton et al., 2012; Coleman et al., 2012) based on its role in modulating formation, function, and survival of bone‐resorbing osteoclasts (Nakashima and Takayanagi, 2009).
Breast cancer is a heterogeneous disease composed of at least four major subtypes which differ by their expression of estrogen (ER) and progesterone (PgR) receptors, HER2, and the proliferative status of the tumor (Reis‐Filho, 2011). The analysis of the protein expression patterns of RANK, RANKL, and OPG in human breast cancers has been difficult due to the lack of highly sensitive and specific antibodies, the down‐modulation of RANK by RANKL, and the expression of RANK, RANKL, and OPG in several different cell types (González‐Suárez, 2011). For instance, RANKL expression by regulatory T cells (Tregs) within the tumor seems to stimulate metastasis of breast cancers through RANK signaling (Tan et al., 2011). RANKL mRNA expression was strongly associated with young age independent of breast cancer subtype in a comprehensive gene expression profiling study on breast cancer in young women (Azim et al., 2012). The mRNA expression of its receptor RANK has been shown to be increased in the basal‐like subtype of breast cancer (Santini et al., 2011), and consequently it has been associated with a worse survival. However, as we and others have previously shown it is pivotal to perform gene expression analyses separately by breast cancer subtype to avoid rediscovering the well known differences between the subtypes (Rody et al., 2011, 2012, 2011).
Comprehensive data on RANK, RANKL, and OPG gene expression in breast cancer subtypes are yet missing especially for ER positive disease which is in particular associated with bone metastasis. Therefore, in the present study we set out to characterize the prognostic value of the mRNA expression of RANK, RANKL, and OPG in several larger microarray datasets of breast cancer. Surprisingly, we found discrepant results on the prognostic value of OPG in different ER positive datasets. After enlarging the analysis to 4467 patients we observed a clear positive prognostic value of OPG but heterogeneity between datasets still remained which could not be attributed to technical dissimilarities or to differences in standard clinical parameters or treatments between cohorts. Interestingly, the cohort effects for the prognostic value of OPG paralleled those for the progesterone receptor. Still the two factors are no proxies for each other but independent in multivariate analyses including Ki67 and clinical parameters. Our study also highlights that unrecognized biases between cohorts need careful consideration.
2. Materials and methods
All analyses were performed according to the ”REporting recommendations for tumor MARKer prognostic studies” (REMARK) (McShane et al., 2005; Simon et al., 2009) and the respective guidelines to microarray‐based studies for clinical outcomes (Dupuy and Simon, 2007). A diagram of the complete analytical strategy and the flow of patients through the study, including the number of patients included in each stage of the analysis, is given in Figure 1. We compiled Affymetrix gene expression data (U133A or U133Plus2.0 arrays) of 4467 breast cancer patients from 40 publicly available datasets (Supplementary Table S1). Affymetrix CEL files were processed with the MAS5.0 algorithm of the affy package (Gautier et al., 2004) of the Bioconductor software project (Gentleman et al., 2004). Data from each array were log2‐transformed, median‐centered, and the expression values of all the probesets from the U133A array were multiplied by a scale factor S so that the magnitude (sum of the squares of the values) equals one. The Bimodality Index (BI) according to Wang and colleagues was used as a metric to measure the degree of bimodal expression of genes (Wang et al., 2009). The bimodal distributions of ESR1, PgR, HER2, and OPG gene expression were used to derive cutoffs to differentiate high and low expression, or positive and negative status, respectively, as described previously (Karn et al., 2010).
Figure 1.
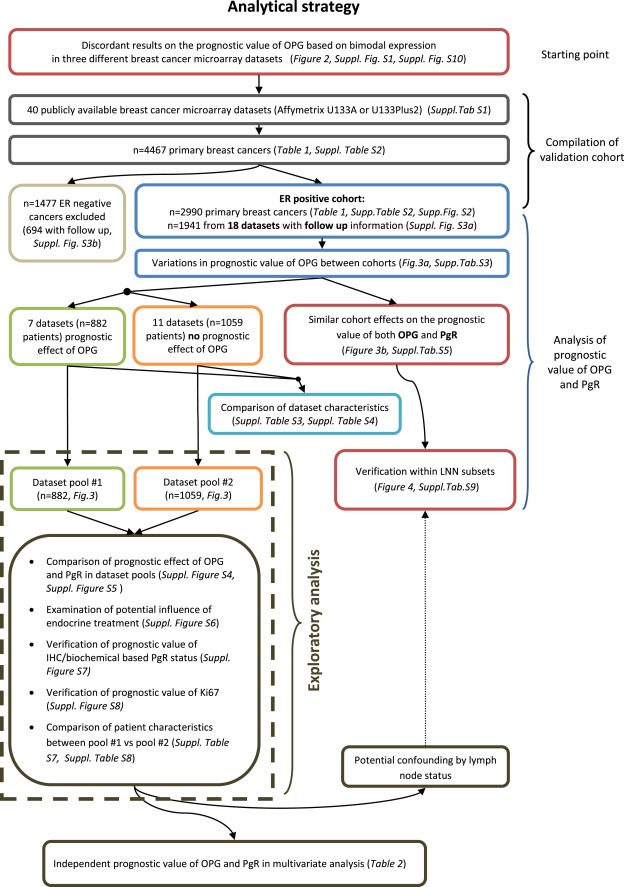
Diagram of the analytical strategy and the flow of patients through the study according to REMARK criteria (”REporting recommendations for tumor MARKer prognostic studies” McShane et al. J Clin Oncol. 2005; 23:9067).
To approximate the intrinsic subtypes of breast cancer we used the simple method according to Hugh et al. (Hugh et al., 2009) which is based on the expression of single marker genes (ESR1, PgR, HER2, Ki67) to define TNBC‐, HER2‐, Luminal A‐, and Luminal B‐subtypes. For a distinction of Luminal A and Luminal B subgroups all 2884 ERpositive/HER2negative samples were selected and a median split according to Ki67 expression was performed. In addition all 106 ERpositive/HER2positive cases were also assigned to the Luminal B subtype according to this method (Hugh et al., 2009). The individual assignments of molecular subtypes are given for each sample in Supplementary Table S2.
Follow up information was available for 2590 of the 4467 samples. In the conduct of the presented analysis event free survival (EFS) was calculated as preferentially corresponding to the RFS endpoint, but measured with respect to the DMFS endpoint if RFS was not available. All results from survival analyses were verified by examining the effect of the different endpoints in stratified analyses. Follow up data for those women in whom the envisaged end point was not reached were censored as of the last follow‐up date or at 120 months. Subjects with missing values were excluded from the analyses. We constructed Kaplan–Meier curves and used the log‐rank test to determine the univariate significance of the variables. A Cox proportional‐hazards model was used to simultaneously examine the effects of multiple covariates on survival. The effect of each individual variable was assessed with the use of the Wald test and described by the hazard ratio, with a 95 percent confidence interval (95% CI). The rmeta package (Thomas, 2012) was used to generate forest plots. All analyses were performed using SPSS Statistics Version 21 (IBM Corp.) and R 3.0.1 (www.r‐project.org). Supplementary Table S2 gives complete information including weblinks to all the samples that were analyzed. It also contains the normalized expression values for all genes analyzed, the stratification for each sample and the different filter used for subcohort analyses. An R workspace and script for the correlation analysis in Supplementary Figure S2 and the generation of the forest plots from univariate cox regression data in Figures 3 and 4 is provided as Supplementary Material.
Figure 3.
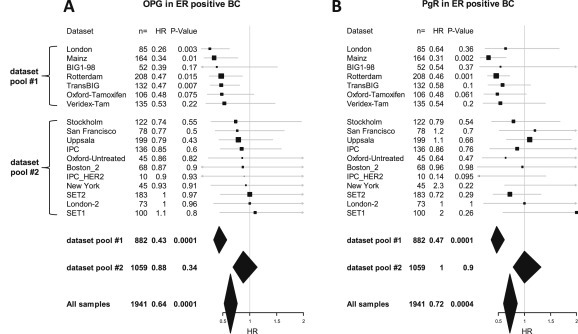
Prognostic value of OPG and PgR expression in different dataset. Forest plot illustration of the results from univariate Cox regression analysis of survival according to OPG (A) and PgR (B) gene expression in ER positive patients from individual datasets. Datasets are sorted according to the hazard ration of OPG in both figures. Cutoffs for OPG and PgR were derived from their bimodal distribution. Size of squares relate to sample size, horizontal lines to 95% CI. Diamonds give summary measures of two dataset pools (#1 and #2) as well as all samples. The detailed results from the Cox regression analyses are given in Supplementary Table S5.
Figure 4.
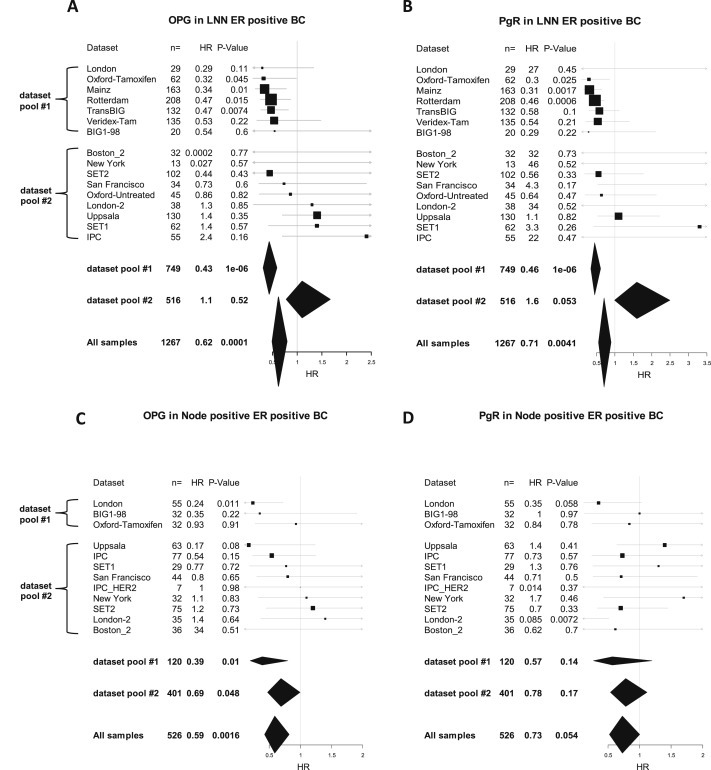
Prognostic value of OPG and PgR expression in different dataset stratified according to lymph node status. Forest plot illustration of the results from univariate Cox regression analysis of survival according to OPG (A) and PgR (B) gene expression in lymph node negative (LNN) ER positive patients from individual datasets. The results for node positive patients are presented in panels (C) and (D) for OPG and PgR, respectively. Datasets are sorted according to the hazard ration of OPG within the two dataset pools in all four figures. Cutoffs for OPG and PgR were derived from their bimodal distribution. Size of squares relate to sample size, horizontal lines to 95% CI. Diamonds give summary measures of two dataset pools (#1 and #2) as well as all samples. The detailed results from the Cox regression analyses are given in Supplementary Table S9.
3. Results
3.1. Discordant results on the prognostic value of OPG in some datasets
We studied the prognostic value of RANK, RANKL, and OPG gene expression in a well known microarray dataset (“Rotterdam”) of 286 samples from breast cancer patients which were not treated with adjuvant therapy (Wang et al., 2005; Minn et al., 2007). We detected no relationship with patients' prognosis for both RANK and RANKL mRNA when analyzing quartiles of expression levels among the overall population or within subtypes. For OPG mRNA expression two probesets are available on the Affymetrix array (probesets 204932_at and 204933_s_at, respectively). Both correlated well with each other and displayed a positive relationship with favorable prognosis of the patients when analyzed in quartiles. Notably, probeset 204933_s_at displayed a strong bimodal distribution suggesting an intrinsic cutoff value to stratify tumors with high and low OPG (Supplementary Figure S1). The extraordinary degree of bimodal expression was revealed when compared to other genes on the Affymetrix array: Within the group of ER positive samples from the Rotterdam dataset we used the Bimodality Index (BI) as a metric for bimodal expression (Wang et al., 2009). OPG probeset 204933_s_at displayed a BI of more than 1.91 which ranks at position 187 within the 1% highest BI values among all 22,283 probesets on the Affymetrix U133 Array. Such bimodality has been observed for many powerful biomarkers as e.g. ER, PgR, and HER2 revealing distinct disease subsets (Ertel, 2010; Karn et al., 2010; Hellwig et al., 2010; Karrila et al., 2011; Karn et al., 2012). We applied the cutoff deduced from the bimodal distribution in Kaplan–Meier survival analysis and found a positive correlation of OPG expression with better outcome both for the overall cohort (P = 0.005; Figure 2A) as well as the ER positive subset (P = 0.017, Figure 2B) of dataset “Rotterdam”, while only a trend was observed in ER negative cancers (P = 0.14). However, when we subsequently tried to validate this observation in two additional independent datasets (“Mainz”(Schmidt et al., 2008) and “Uppsala” (Miller et al., 2005)), we got inconsistent results. Despite that OPG expression values displayed the bimodal distribution in all three datasets allowing the use of the same cutoff value (Supplementary Figure S1), we detected a positive prognostic value only in the “Rotterdam” (Figure 2B) and “Mainz”(Schmidt et al., 2008) datasets (Figure 2C) but not in the “Uppsala” dataset(Miller et al., 2005) (Figure 2D). Interestingly, the patients in the Uppsala dataset were treated with adjuvant chemotherapy and endocrine therapy. In contrast, patients in both the Rotterdam and Mainz datasets did not receive any adjuvant therapy. However, an additional difference was the use of relapse free survival (RFS) as outcome variable in the Uppsala dataset but distant metastasis free survival (DMFS) in both the Rotterdam and Mainz datasets. Thus, we assembled additional datasets to verify whether OPG might have a potential predictive value for adjuvant therapy and/or distant metastasis rather than local relapse.
Figure 2.
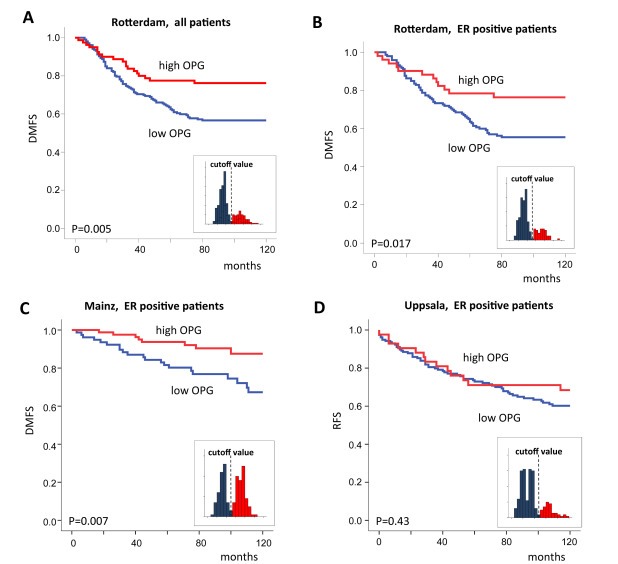
Discordant results on the prognostic value of OPG in three datasets. Kaplan–Meier analysis of all patients (A) and the ER positive subset (B) in the Rotterdam dataset according to OPG mRNA expression. OPG was dichotomized using the cutoff derived from its bimodal expression (Affymetrix probeset 204933_s_at). The same cutoff was used for validation in the ER positive subset of the Mainz dataset (C) and Uppsala dataset (D), respectively. Data in panels B, C, and D are shown for the ER positive subset in order to exclude bias from different proportions of ER positive and ER negative samples in the datasets. Similar results were also obtained when including the complete cohorts (Supplementary Figure S10). Distant metastasis free survival was used in Panels A, B, and C. Relapse free survival was used in Panel D. The small inset figures display the bimodal distribution and the applied cutoff.
3.2. Comprehensive compilation of Affymetrix microarray datasets
We compiled Affymetrix gene expression profiles of a total of 4467 breast cancer patients from 40 publicly available datasets as previously reported (Hanker et al., 2013) (see Methods section and Supplementary Table S1 and Figure 1). The clinical characteristics of all 4467 samples are given in Table 1. We observed the bimodal distribution of OPG mRNA expression (probeset 204933_s_at) among all datasets and used it for stratification of tumors with either high or low OPG. Within the 4467 samples those tumors with high OPG are characterized by a somewhat lower fraction of ER positive cases (62.5% vs 70.4%, P < 0.001) and a higher fraction of TNBC (28.2% vs 21.6%, P < 0.001; Table 1). Contrasting with this observation, we found that the OPG high group is also characterized by a higher fraction of PgR positive cases (62.4% vs 95.5%). A separate analysis of the ER positive subset of the patients shown also in Table 1 demonstrated that this observation is mainly due to the fact that three quarters of the ER‐positive/PgR‐negative cases were in the OPG low group. Among the ER positive tumors high OPG was clearly associated with a positive PgR status (OR 2.8, P < 0.001; Table 1). We also found significant differences in both the type of systemic treatment and the available outcome variable for survival (DMFS vs RFS) of the datasets for the groups of high and low OPG in the ER positive subset (Table 1). In addition we used the mRNA expression of OPG, ER, PgR, HER2, and Ki67 as continuous parameters to analyze their correlation through hierarchical cluster analysis within the subset of ER positive tumors. As shown in the resulting dendrogram in Supplementary Figure S2 the two OPG probesets clustered in the same branch as PgR in Iine with the observed positive association of OPG and PgR in the ER positive subset in Table 1.
Table 1.
Clinical characteristics of all 4467 samples and the 2990 ER positive samples from the 40 datasets.
| Parameter | Total | OPG low | OPG high | OR | P‐Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| All tumors (n = 4467) | |||||||||
| Lymph node status | LNN | 2040 | 62.5% | 1142 | 62.1% | 898 | 63.0% | 1.0 | |
| N+ | 1225 | 37.5% | 697 | 37.9% | 528 | 37.0% | n.s. | ||
| Age | Age >50 | 1908 | 61.1% | 977 | 61.8% | 931 | 60.3% | 0.9 | |
| Age ≤50 | 1217 | 38.9% | 604 | 38.2% | 613 | 39.7% | n.s. | ||
| Tumor size | ≤2 cm | 358 | 20.3% | 197 | 20.5% | 161 | 20.1% | 1.0 | |
| >2 cm | 1403 | 79.7% | 763 | 79.5% | 640 | 79.9% | n.s. | ||
| Grade | G3 | 1524 | 49.2% | 798 | 49.0% | 726 | 49.4% | 1.0 | |
| G1 & G2 | 1575 | 50.8% | 831 | 51.0% | 744 | 50.6% | n.s. | ||
| ER status | Positive | 2990 | 66.9% | 1756 | 70.4% | 1234 | 62.5% | 0.7 | |
| Negative | 1477 | 33.1% | 737 | 29.6% | 740 | 37.5% | <0.001 | ||
| PgR status | Positive | 2466 | 55.2% | 1234 | 49.5% | 1232 | 62.4% | 1.7 | |
| Negative | 2001 | 44.8% | 1259 | 50.5% | 742 | 37.6% | <0.001 | ||
| HER2 status | Positive | 589 | 13.2% | 318 | 12.8% | 271 | 13.7% | 1.1 | |
| Negative | 3878 | 86.8% | 2175 | 87.2% | 1703 | 86.3% | n.s. | ||
| Molecular subtype (Hugh et al.) | HER2 | 381 | 8.5% | 198 | 7.9% | 183 | 9.3% | 1.2 | |
| Luminal A | 1442 | 32.3% | 740 | 29.7% | 702 | 35.6% | 1.3 | ||
| Luminal B | 1548 | 34.7% | 1016 | 40.8% | 532 | 27.0% | 0.5 | ||
| Triple negative | 1096 | 24.5% | 539 | 21.6% | 557 | 28.2% | 1.4 | <0.001 | |
| Systemic treatment | Untreated | 1108 | 38.3% | 654 | 36.8% | 454 | 40.6% | 1.2 | |
| Endocrine only | 1182 | 40.8% | 765 | 43.1% | 417 | 37.3% | |||
| Chemotherapy | 604 | 20.9% | 357 | 20.1% | 247 | 22.1% | 0.008 | ||
| Outcome variable | RFS | 1412 | 50.9% | 740 | 47.6% | 672 | 55.1% | 1.3 | |
| DMFS | 1361 | 49.1% | 813 | 52.4% | 548 | 44.9% | <0.001 | ||
| ER positive tumors (n = 2990), only parameters significant among all 4467 samples above | |||||||||
| PgR status | Positive | 2088 | 69.8% | 1080 | 61.5% | 1008 | 81.7% | 2.8 | |
| Negative | 902 | 30.2% | 676 | 38.5% | 226 | 18.3% | <0.001 | ||
| Molecular subtype (Hugh et al.) | Luminal A | 1442 | 48.2% | 740 | 42.1% | 702 | 59.9% | 1.8 | |
| Luminal B | 1548 | 51.8% | 1016 | 57.9% | 532 | 43.1% | <0.001 | ||
| Systemic treatment | Untreated | 750 | 34.6% | 448 | 32.9% | 302 | 37.4% | 1.2 | |
| Endocrine only | 1102 | 50.9% | 718 | 52.8% | 384 | 47.6% | |||
| Chemotherapy | 315 | 14.5% | 194 | 14.3% | 121 | 15.0% | <0.001 | ||
| Outcome variable | RFS | 1011 | 50.1% | 548 | 47.0% | 463 | 54.5% | 1.4 | |
| DMFS | 1006 | 49.9% | 619 | 53.0% | 387 | 45.5% | 0.001 | ||
3.3. Prognostic value of OPG in different cohorts of ER positive breast cancer
We performed separate Kaplan Meier analyses within the 1941 ER positive and 694 ER negative samples with follow up data from the combined cohorts using the cutoff from the bimodal expression of OPG. As shown in Supplementary Figure S3 we detected a significant prognostic value in the ER positive subset (P < 0.0001) but only a trend among ER negative patients (P = 0.26). We then performed univariate Cox regression analyses for survival separately in each individual dataset with follow up information to examine potential differences between datasets. We considered only the subsets of ER positive patients for all analyses to reduce potential influences of subtype composition of the different cohorts. The samples with follow up were derived from 18 different datasets as given in Supplementary Table S3. Only for seven of the datasets we detected a significant effect or a trend for a better prognosis in the OPG high group. Hazard ratios were between 0.26 and 0.53 as given in Supplementary Table S3. These seven datasets in total encompassed 882 of the patients. The remaining 11 datasets encompassing 1059 patients did not show a difference in survival according to OPG. Even if the sample size of some of these datasets with a negative result may have been too small, at least five of the datasets encompassed more then 100 cases (Supplementary Table S3). We next examined whether we could detect a systematic difference between those datasets displaying a potential positive result and a negative result, respectively. Therefore we inspected the following parameters of the patients as given in Supplementary Table S3: type of adjuvant treatment, age, tumor size, lymph node status, grading, relative proportion of luminal B tumors, and PgR status. We also checked for systematic differences regarding the applied Affymetrix array type (U133A vs. U133Plus2.0), the available outcome variable (DMFS vs. RFS), and the frequency of early events in the different datasets. However, none of the parameters in Supplementary Table S3 did show a significant difference between those dataset which displayed a potential positive and negative prognostic result (Supplementary Table S4). Also quantification of heterogeneity by the I2 metric (Higgins et al., 2003) suggested that all variation that we observed between the datasets could be attributed to chance.
3.4. Similar cohort effects on the prognostic value of both OPG and PgR
Because of the observed correlation of PgR and OPG gene expression we also tested the prognostic value of PgR in the different datasets. Surprisingly, we detected a prognostic value of PgR mainly in those datasets for which we also observed a prognostic value of OPG. To demonstrate this effect a forest plot of Cox regression results for OPG is shown in Figure 3A where the datasets are sorted according to the observed hazard ratio of OPG. Figure 3B shows a corresponding forest plot of the results for PgR with datasets sorted in the same order as in Figure 3A. We also exploratory pooled the upper seven datasets in the figure which displayed a prognostic effect (dataset pool #1 containing 882 ER positive samples) and the remaining 11 datasets (dataset pool #2 containing 1059 ER positive samples), respectively. Naturally, these two arbitrary dataset pools showed a strong difference regarding the prognostic value of OPG as shown in their summary measures presented as diamonds in Figure 3A. However, as shown in Figure 3B a similar result was also obtained for PgR with a HR of 0.47 (95% CI 0.36–0.61, P < 0.001) in dataset pool #1 but a HR of 1.0 (95% CI 0.79–1.32, P = 0.9) in dataset pool #2 (Supplementary Table S5). For illustrative purpose the results of the pooled groups are also presented in Kaplan–Meier analyses in Supplementary Figure S4 for OPG and Supplementary Figure S5 for PgR. The obtained results were not related to differences in adjuvant treatment since they were observed both within the subcohorts which obtained endocrine treatment (Mihály et al., 2013) and those without adjuvant treatment (Supplementary Figure S6).
In the analyses so far we derived both OPG and PgR status from mRNA expression as detected by microarray in order to include a maximum number of samples. Thus, the similarity in prognosis for both markers could be related to technical discrepancies between the microarray data from the different datasets and not to a biological background. However, as we had previously shown (Karn et al., 2010) there was a reasonable concordance with biochemical or immunohistochemical (IHC) determination of PgR status and we found no systematic difference between the dataset pools #1 and #2 (Supplementary Table S6). Importantly, when we used only the PgR status from biochemical/IHC assay for stratification in Kaplan–Meier analysis we obtained a similar result as above with a prognostic value in dataset pool #1 and no prognostic value in dataset pool #2 (Supplementary Figure S7). This result demonstrates that the observed differences between the datasets are not related to technical discrepancies between microarray analyses but to clinical or biological differences of the analyzed patient cohorts.
3.5. Exploratory analyses of differences between cohorts
The observed cohort differences regarding the prognostic value of both OPG and PgR did not extend to other prognostic markers as for instance proliferation associated genes. As shown in Supplementary Figure S8 the prognostic value of Ki67 expression was similar in both dataset pools #1 and #2. We next exploratory compared the clinical parameters of the two dataset pools #1 and #2. As shown in Supplementary Table S7 we observed in dataset pool #1 significant higher proportions of lymph node negative (LNN) patients (85.7% vs 56.3%, P < 0.001), smaller tumors (≤2 cm; 39.8% vs 17.5%, P < 0.001), and patients without adjuvant treatment (57.1% vs 21.5% P < 0.001), among others, collectively suggesting that dataset pool #1 is enriched in patients with lower risk. Thus, to exclude a potential bias that may be caused from different proportions of lymph node positive patients we also restricted our analyses to LNN patients. The analysis from Supplementary Table S7 was then repeated within the subset of LNN patient (Supplementary Table S8). Within this subset of 1267 LNN ER positive patients we detected no difference for tumor size between dataset pool #1 and #2. Nevertheless, as shown in the forest plots in Figures 4A and 4B and in Supplementary Table S9 we still detected a strong concordance of the differences between dataset pools regarding the prognostic value of both OPG and PgR in LNN patients.
3.6. Independent prognostic value of OPG and PgR
The concordance regarding the prognostic value of OPG and PgR in different datasets could suggest that the two markers may just be proxies for each other. Thus, to study this possibility we performed multivariate Cox regression analysis of survival including both OPG, PgR, and all standard parameters in dataset pool #1. We used three different Cox models including different variables (Table 2): (1.) Information on eight parameters (age, lymph node status, tumor size, histological grading, Her2 status, proliferation status as Luminal A vs B, PgR, and OPG) was available for a total of only 303 of the 808 samples from dataset pool #1. In this model only PgR status (HR 0.52, 95% CI 0.35–0.77; P = 0.001) and the Luminal A vs. B distinction (HR 0.51, 95% CI 0.33–0.79; P = 0.003) remained significant while OPG (HR 1.56, 95% CI 0.99–2.45; P = 0.055) only trended to significance. (2.) Leaving out tumor size, all the remaining parameters were available for a total of 704 samples. In this model four variables were significant: PgR status (HR 0.56, 95% CI 0.42–0.75; P < 0.001), the Luminal A vs. B distinction (HR 0.46, 95% CI 0.34–0.62; P < 0.001), OPG (HR 1.80, 95% CI 1.31–2.47; P < 0.001), and age (HR 0.73, 95% CI 0.54–0.98; P = 0.036; Table 2). (3.) Finally, when including only the three most significant variables (PgR, Luminal A vs B, and OPG) in a model, data were available for all 882 samples from the dataset pool #1. In this model all three parameters retained high significance for an independent effect on survival (Luminal A vs B, HR 0.45, 95% CI 0.35–0.59; PgR, HR 0.59, 95% CI 0.45–0.77; OPG, HR 1.74, 95% CI 1.31–2.33; all P < 0.001; Table 2). Similar results were also obtained when Ki67 as measure for proliferation was used as a continuous parameter instead of the binary distinction of Luminal A vs B (Supplementary Table S11).
Table 2.
Multivariate Cox regression models among ER positive cancers from dataset pool#1. Significant P‐values are given in bold.
| Model 1 (n = 303)a | n= | HR | 95% CI | P‐Value | |
| Age | >50 vs. ≤50 | 154 vs. 149 | 0.81 | 0.56–1.19 | 0.28 |
| Lymph node status | LNN vs. N1 | 303 vs. 0 | – | – | – |
| Tumor size | ≤2 cm vs. >2 cm | 121 vs. 182 | 0.82 | 0.55–1.22 | 0.32 |
| Grading | G3 vs. G1/G2 | 155 vs. 148 | 0.94 | 0.64–1.37 | 0.75 |
| HER2 | Positive vs. negative | 33 vs. 270 | 0.82 | 0.46–1.47 | 0.51 |
| Subtype (Hugh et al.) | Luminal A vs B | 144 vs. 159 | 0.51 | 0.33–0.79 | 0.003 |
| PgR | Positive vs. negative | 209 vs. 94 | 0.52 | 0.35–0.77 | 0.001 |
| OPG | High vs. low | 109 vs. 194 | 0.64 | 0.41–1.01 | 0.055 |
| Model 2 (n = 704)a | n = | HR | 95% CI | P‐Value | |
| Age | >50 vs. ≤ 50 | 468 vs. 236 | 0.73 | 0.54–0.98 | 0.036 |
| Lymph node status | LNN vs. N1 | 959 vs. 109 | 0.80 | 0.51–1.24 | 0.31 |
| Grading | G3 vs. G1/G2 | 226 vs 478 | 1.16 | 0.87–1.55 | 0.33 |
| HER2 | Positive vs. negative | 52 vs. 652 | 0.94 | 0.60–1.48 | 0.78 |
| Subtype (Hugh et al.) | Luminal A vs B | 401 vs. 303 | 0.46 | 0.34–0.62 | <0.001 |
| PgR | Positive vs. negative | 528 vs. 176 | 0.56 | 0.42–0.75 | <0.001 |
| OPG | High vs. low | 336 vs. 368 | 0.56 | 0.40–0.76 | <0.001 |
| Model 3 (n = 882)a | n = | HR | 95% CI | P‐Value | |
| Subtype (Hugh et al.) | Luminal A vs B | 504 vs. 378 | 0.45 | 0.35–0.59 | <0.001 |
| PgR | Positive vs. negative | 655 vs. 227 | 0.59 | 0.45–0.77 | <0.001 |
| OPG | High vs. low | 418 vs. 464 | 0.57 | 0.43–0.76 | <0.001 |
In each of the three Cox regression models all patients were included for whom information on all the respective parameters were available (number in parentheses).
These results demonstrate that in those datasets where a prognostic value of OPG and PgR was detected, the two parameters are no surrogate for each other or the proliferative status of the tumor, but provide independent prognostic information.
4. Discussion
In the present study we set out to characterize the prognostic value of the mRNA expression of RANK, RANKL, and OPG in several larger microarray datasets of breast cancer. Surprisingly, we found discrepant results on the prognostic value of OPG in ER positive breast cancer between three different datasets. We therefore enlarged our samples size by including 4467 patients from a total of 40 datasets and thoroughly analyzed potential reasons for these discrepancies. In the overall series of 1941 ER positive breast cancers with follow up OPG was clearly prognostic (HR 0.64, P < 0.0001; Figure 3 and Supplementary Figure S3A). Nevertheless, we detected considerable heterogeneity regarding the prognostic value of OPG between different datasets. This heterogeneity could neither be attributed to technical reasons nor to differences in standard clinical parameters or treatments of the cohorts. Moreover quantification by the I2 metric (Higgins et al., 2003) suggested that the heterogeneity may be just attributed to chance. Interestingly however we detected similar cohort effects for PgR as for OPG (Figure 3B). PgR was prognostic in the overall series (HR 0.72, P = 0.0004; Figure 3B) but showed similar cohort effects either when measured by microarray (Figure 3B, Supplementary Figure S5, Supplementary Figure S6) or IHC (Supplementary Figure S7). Since a prognostic value of PgR in ER positive breast cancer is known (Grann et al., 2005; Dunnwald et al., 2007; Bauer et al., 2010), we speculate that the heterogeneity may rather be attributed to false negative observations in the cohorts not showing an effect than false positive errors in those that do. Among >150.000 patients from the SEER registry ER+/PgR‐ tumors were associated with a HR of 1.46 (95% C 1.39–1.53) as compared to ER+/PgR + tumors in multivariate analysis (Grann et al., 2005). There are few studies on subcohort differences regarding the prognostic potential of PgR in ER positive breast cancer. In a study from the California Cancer Registry a profound effect of PgR status among ER positive tumors was only seen in the lymph node positive subset (Bauer et al., 2010). Thus nodal status could be a strong confounding factor. However, we observed concordance of prognostic values of PgR and OPG also and especially in lymph node negative patients (Figure 4 and Supplementary Table S9). A predictive value of PgR for endocrine treatment in ER positive patients was not observed in a recent patient‐level meta analysis (Davies et al., 2011) and two clinical trials (Viale et al., 2007; Dowsett et al., 2008). Similarly, in our analysis we also observed the concordance of the prognostic values of PgR and OPG independent of endocrine treatment (Supplementary Figure S6). The value of PgR status and its potential to better define the luminal A subtype has been brought up recently (Prat et al., 2013). In our study we used a simple adaption of the luminal A vs luminal B distinction based on the expression of Ki67 or HER2 (Hugh et al., 2009). However, in the dataset pool #1 that showed a strong prognostic effect for OPG and PgR in our study, all three parameters (OPG, PgR, and Luminal A vs B) were independently associated with prognosis in multivariate analysis (Table 2). This effect was also still observed albeit less significant when including those datasets that showed no prognostic effect of OPG and PgR (dataset pool #2) in a combined analysis (Supplementary Table S10). Thus, despite OPG and PgR displayed similar cohort effects (in contrast to Ki67) they are not just simple proxies for each other but provide independent prognostic information in multivariate analysis.
In those cohorts where we detected a prognostic effect, the expression of OPG correlates with a better prognosis in ER positive breast cancer. In line with this result OPG would be suggested mechanistically to inhibit bone metastasis. OPG has been used experimentally to decrease bone resorption in women with postmenopausal osteoporosis (Bekker et al., 2001) as well as the treatment of bone metastasis (Body et al., 2003). Further development of this strategy led to denosumab an artificial counterpart of OPG which reduces the rate of skeletal‐related events in breast cancer (Stopeck et al., 2010; Lipton et al., 2012). Thus it is intuitive to ask whether the improved prognosis we observed might be especially related to bone metastasis. Information on different metastatic sites was available for only 242 ER positive patients. 197 patients were from the Rotterdam dataset (out of dataset pool#1) and 45 patients from the NewYork dataset (out of dataset pool#2). All patients from Rotterdam but only 13 from the NewYork dataset were lymph node negative. In the Rotterdam dataset both OPG and PgR were prognostic when analyzing either bone metastasis or lung metastasis while the number of brain metastasis events was too small to reach significance (Supplementary Figure S9). No prognostic value was detected in the smaller NewYork dataset. Thus we obtained no evidence for a specific prognostic value of OPG for bone relapse.
The use of retrospective datasets has been put forward regarding the evaluation of biomarkers (Simon et al., 2009). Thus, in a broader sense an important aspect of our study is also that caution has to be applied when pooling multiple gene expression datasets (Győrffy et al., 2012; Gyorffy et al., 2012). Resulting “batch effects” are widespread and of critical impact (Leek et al., 2010). Several systematic biases may be controlled (Karn et al., 2011) but others cannot easily be identified as e.g. the quality of the clinical information and potential selection bias. Therefore it is imperative, to carry the original dataset allocations throughout all analyses and to run controls for dataset bias repeatedly. Recent whole genome sequencing studies demonstrate that cancer heterogeneity continues on the mutational level with large numbers of individual alterations per tumor (Koboldt et al., 2012; Banerji et al., 2012). Thus, very large datasets will also be needed to answer the complex questions about driver versus passenger mutations, tumor cell heterogeneity (Shibata, 2012) as well as tumor evolution (Burrell et al., 2013) and cohort bias will probably become even more important here.
Funding
This work was supported by grants from the H.W. & J. Hector‐Stiftung, Mannheim; the Margarete Bonifer‐Stiftung, Bad Soden; and the BANSS‐Stiftung, Biedenkopf.
Disclosure
The authors have declared no conflicts of interest.
Supporting information
The following are the supplementary data related to this article:
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary Figure S1 Bimodal distribution of OPG expression values. Histogram of normalized OPG (TNFRSF11B) mRNA expression values according to Affymetrix probeset 204933_s_at among 286 breast cancer samples of the Rotterdam dataset (Wang et al., 2005; Lancet 365, 671–679) is given in Panel A. Coloring according to ER status (blue: ER positive; red: ER negative) demonstrates that the bimodal shape of the distribution did not originate from subtype composition. A dashed line represents the cutoff for OPG gene expression derived from the distribution. Panels B and C demonstrate the same analyses for the datasets Mainz and Uppsala, respectively. The cutoff derived from the Rotterdam dataset in (A) is shown to also separate the bimodal peaks in those datasets.
Supplementary Figure S2 Correlation analysis of OPG and other genes as continuous variables. The correlation of gene expression values of OPG, estrogen receptor (ESR1), progesterone receptor (PGR), Ki67, and HER2 as continuous factors was analyzed in the dataset of 2990 ER positive tumors. Hierarchical clustering was performed to visualize the relationship of all parameters. The two OPG probesets cluster in the same branch of the dendrogram as PGR.
Supplementary Figure S3 Prognostic value of OPG in the combined cohorts. Kaplan–Meier analysis of all patients from the combined cohorts with ER positive (A) or ER negative (B) tumors according to OPG mRNA expression. OPG was dichotomized using the cutoff derived from its bimodal expression (Affymetrix probeset 204933_s_at). The small inset figures display the bimodal distribution of expression values and the applied cutoff (the same cutoff was used as for the analyses of individual datasets).
Supplementary Figure S4 Illustration of the effect of dataset composition on the prognostic value of OPG expression. Kaplan–Meier analysis was performed in two arbitrarily defined dataset pools: In Panel (A) the 882 ER positive patients from the seven datasets in which a prognostic value of OPG was detected (see Supplementary Table S3) were combined as dataset‐pool #1. In Panel (B) all 1059 ER positive patients from the 11 datasets in which no prognostic value of OPG has been detected were combined as dataset‐pool #2. The Kaplan–Meier graphs display the differences of event free survival according to OPG expression in these two voluntarily combined datasets for an illustrative purpose only.
Supplementary Figure S5 Illustration of the effect of dataset composition on the prognostic value of PgR expression. Kaplan–Meier analysis was performed in two arbitrarily defined dataset pools: In Panel (A) the 882 ER positive patients from the seven datasets in which a prognostic value of OPG was detected (see Supplementary Table S3) were combined as dataset‐pool #1. In Panel (B) all 1059 ER positive patients from the 11 datasets in which no prognostic value of OPG has been detected were combined as dataset‐pool #2. The Kaplan–Meier graphs display the differences of event free survival according to PgR expression in these two voluntarily combined datasets. The two small inset graphs display the bimodal distribution of PgR expression and the applied cutoff value for PgR positivety.
Supplementary Figure S6 Illustration of the effect of dataset composition on the prognostic value of PgR and OPG expression independent of adjuvant treatment. Kaplan–Meier analysis was performed in two arbitrarily defined dataset pools: For Panels (A), (B), (E), and (F) the 882 ER positive patients from the seven datasets in which a prognostic value of OPG was detected (see Supplementary Table S3) were combined as dataset‐pool #1. Kaplan–Meier graphs of event free survival according to PgR expression (A and B) or OPG expression (E and F) are shown for those 863 patients who were either untreated (A, E) or treated with endocrine therapy (B, F). For Panels (C), (D), (G), and (H) the 1059 ER positive patients from the 11 datasets in which no prognostic value of OPG has been detected were combined as dataset‐pool #2. Again, Kaplan–Meier graphs of event free survival according to PgR (C, D) expression or OPG expression (G, H) are shown for those 733 patients who were either untreated (C, G) or treated with endocrine therapy (D, H). Independent of treatment, the prognostic effect of both PgR and OPG was only detected in dataset pool #1 (Panels A, B, E, and F) and not in dataset pool #2 (Panels C, D, G, and H). In Panels I and K we also included results for the prognostic effect of OPG in ER positive breast cancer patients obtained using the KM plotter website (kmplot.com). For this analysis the upper quartiles of the cohorts were applied as optimal cutoff for high expression (Gyorffy et al., 2012; Endocr. Relat. Cancer 19, 197–208). A highly prognostic value of OPG was detected for both ER positive patients which had either received no adjuvant treatment (Panel I) or tamoxifen (Panel K) (Mihály et al., 2013; Breast Cancer Res. Treat. 140, 219–232).
Supplementary Figure S7 Illustration of the effect of dataset composition on the prognostic value of biochemical/IHC based PgR status. Kaplan–Meier analysis was performed in two arbitrarily defined dataset pools: The 882 ER positive patients from the seven datasets in which a prognostic value of OPG was detected (see Supplementary Table S3) were combined as dataset‐pool #1. All 1059 ER positive patients from the 11 datasets in which no prognostic value of OPG has been detected were combined as dataset‐pool #2. For 335 of the 882 patients from dataset‐pool #1 and 606 of the 1059 patients from dataset‐pool #2 information on PgR status from biochemical/IHC assay was available. The Kaplan–Meier graphs display the differences of event free survival according to PgR status as determined by biochemical/IHC assay separately for dataset‐pool #1 in (A) and dataset‐pool #2 in (B) for exploratory purpose.
Supplementary Figure S8 Verification of the prognostic value of Ki67 in both dataset pools. Kaplan–Meier analysis according to Ki67 expression was performed in the same dataset pool #1 (A, n = 882) and dataset pool #2 (B, n = 1059) that were used for the Kaplan–Meier analyses of OPG and PgR in Supplementary Figures S4 and S5, respectively. Samples were stratified according to median Ki67 expression among all 2990 ER positive patients of the full cohort. In panels (C) and (D), the Kaplan–Meier analyses were restricted to the lymph node negative patients of dataset pool #1 (n = 751) and dataset pool #2 (n = 516), respectively.
Supplementary Figure S9 Prognostic value of OPG and PgR for different types of distant relapse. Kaplan–Meier analysis according to OPG (A–C) and PgR (D–F) gene expression was performed among 242 ER positive tumors from the Rotterdam dataset with information on either bone metastasis (A, D), lung metastasis (B, E), or brain metastasis (C, F). All patients are lymph node negative.
Supplementary Figure S10 Prognostic value of OPG in different dataset. Kaplan–Meier analysis of all patients in the Rotterdam dataset (A), the Mainz dataset (B), and the Uppsala dataset (C), according to OPG mRNA expression. OPG was dichotomized using the cutoff derived from its bimodal expression (Affymetrix probeset 204933_s_at). The same cutoff was used for validation the Mainz dataset (B) and Uppsala dataset (C), respectively. The small inset figures display the bimodal distribution of expression values and the applied cutoff. DMFS was used in (A) and (B), while RFS was used in (C). This figure analyzing mixed cohorts of ER positive and ER negative samples is included here only for completeness. Similar results were obtained when only the ER positive subsets of the cohorts were separately analyzed (see Figure 2 in the main manuscript).
Supplementary Files OPG_analysis.zip. This ZIP package contains two files. An R workspace file (OPG_analysis.RData) and and the respective R script file (OPG_analysis.R) to perform the analyses generating Figures 3, 4, and Supplementary Figure S2.
Acknowledgments
We thank Katerina Brinkmann and Samira Adel for expert technical assistance.
Supplementary data 1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2014.04.003.
Sänger Nicole, Ruckhäberle Eugen, Bianchini Giampaolo, Heinrich Tomas, Milde-Langosch Karin, Müller Volkmar, Rody Achim, Solomayer Erich Franz, Fehm Tanja, Holtrich Uwe, Becker Sven, Karn Thomas, (2014), OPG and PgR show similar cohort specific effects as prognostic factors in ER positive breast cancer, Molecular Oncology, 8, doi: 10.1016/j.molonc.2014.04.003.
References
- Azim, H.A. , Michiels, S. , Bedard, P.L. , Singhal, S.K. , Criscitiello, C. , Ignatiadis, M. , Haibe-Kains, B. , Piccart, M.J. , Sotiriou, C. , Loi, S. , 2012. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin. Cancer Res. 18, 1341–1351. [DOI] [PubMed] [Google Scholar]
- Banerji, S. , Cibulskis, K. , Rangel-Escareno, C. , Brown, K.K. , Carter, S.L. , Frederick, A.M. , Lawrence, M.S. , Sivachenko, A.Y. , Sougnez, C. , Zou, L. , Cortes, M.L. , Fernandez-Lopez, J.C. , Peng, S. , Ardlie, K.G. , Auclair, D. , 2012. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 486, 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, K. , Parise, C. , Caggiano, V. , 2010. Use of ER/PR/HER2 subtypes in conjunction with the 2007 St Gallen Consensus Statement for early breast cancer. BMC Cancer. 10, 228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker, P.J. , Holloway, D. , Nakanishi, A. , Arrighi, M. , Leese, P.T. , Dunstan, C.R. , 2001. The effect of a single dose of osteoprotegerin in postmenopausal women. J. Bone Miner. Res. 16, 348–360. [DOI] [PubMed] [Google Scholar]
- Body, J. , Greipp, P. , Coleman, R.E. , Facon, T. , Geurs, F. , Fermand, J. , Harousseau, J. , Lipton, A. , Mariette, X. , Williams, C.D. , Nakanishi, A. , Holloway, D. , Martin, S.W. , Dunstan, C.R. , Bekker, P.J. , 2003. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 97, (3 Suppl) 887–892. [DOI] [PubMed] [Google Scholar]
- Brisken, C. , 2013. Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. Nat. Rev. Cancer. 13, 385–396. [DOI] [PubMed] [Google Scholar]
- Burrell, R.A. , McGranahan, N. , Bartek, J. , Swanton, C. , 2013. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 501, 338–345. [DOI] [PubMed] [Google Scholar]
- Coleman, R. , Gnant, M. , Morgan, G. , Clezardin, P. , 2012. Effects of bone-targeted agents on cancer progression and mortality. J. Natl. Cancer Inst. 104, 1059–1067. [DOI] [PubMed] [Google Scholar]
- Davies, C. , Godwin, J. , Gray, R. , Clarke, M. , Cutter, D. , Darby, S. , McGale, P. , Pan, H.C. , Taylor, C. , Wang, Y.C. , Dowsett, M. , Ingle, J. , Peto, R. , 2011. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 378, 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall, W.C. , 2012. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin. Cancer Res. 18, 326–335. [DOI] [PubMed] [Google Scholar]
- Dowsett, M. , Allred, C. , Knox, J. , Quinn, E. , Salter, J. , Wale, C. , Cuzick, J. , Houghton, J. , Williams, N. , Mallon, E. , Bishop, H. , Ellis, I. , Larsimont, D. , Sasano, H. , Carder, P. , Cussac, A.L. , Knox, F. , Speirs, V. , Forbes, J. , Buzdar, A. , 2008. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J. Clin. Oncol. 26, 1059–1065. [DOI] [PubMed] [Google Scholar]
- Dunnwald, L.K. , Rossing, M.A. , Li, C.I. , 2007. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 9, R6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy, A. , Simon, R.M. , 2007. Critical review of published microarray studies for cancer outcome and guidelines on statistical analysis and reporting. J. Natl. Cancer Inst. 99, 147–157. [DOI] [PubMed] [Google Scholar]
- Ertel, A. , 2010. Bimodal gene expression and biomarker discovery. Cancer Inform. 9, 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata, J.E. , Kong, Y.Y. , Li, J. , Sasaki, T. , Irie-Sasaki, J. , Moorehead, R.A. , Elliott, R. , Scully, S. , Voura, E.B. , Lacey, D.L. , Boyle, W.J. , Khokha, R. , Penninger, J.M. , 2000. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 103, 41–50. [DOI] [PubMed] [Google Scholar]
- Gautier, L. , Cope, L. , Bolstad, B.M. , Irizarry, R.A. , 2004. affy–analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 20, 307–315. [DOI] [PubMed] [Google Scholar]
- Gentleman, R.C. , Carey, V.J. , Bates, D.M. , Bolstad, B. , Dettling, M. , Dudoit, S. , Ellis, B. , Gautier, L. , Ge, Y. , Gentry, J. , Hornik, K. , Hothorn, T. , Huber, W. , Iacus, S. , Irizarry, R. , 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Suarez, E. , Jacob, A.P. , Jones, J. , Miller, R. , Roudier-Meyer, M.P. , Erwert, R. , Pinkas, J. , Branstetter, D. , Dougall, W.C. , 2010. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 468, 103–107. [DOI] [PubMed] [Google Scholar]
- González-Suárez, E. , 2011. RANKL inhibition: a promising novel strategy for breast cancer treatment. Clin. Transl Oncol. 13, 222–228. [DOI] [PubMed] [Google Scholar]
- Grann, V.R. , Troxel, A.B. , Zojwalla, N.J. , Jacobson, J.S. , Hershman, D. , Neugut, A.I. , 2005. Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer. 103, 2241–2251. [DOI] [PubMed] [Google Scholar]
- Gyorffy, B. , Lánczky, A. , Szállási, Z. , 2012. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr. Relat. Cancer. 19, 197–208. [DOI] [PubMed] [Google Scholar]
- Győrffy, B. , Benke, Z. , Lánczky, A. , Balázs, B. , Szállási, Z. , Timár, J. , Schäfer, R. , 2012. RecurrenceOnline: an online analysis tool to determine breast cancer recurrence and hormone receptor status using microarray data. Breast Cancer Res. Treat. 132, 1025–1034. [DOI] [PubMed] [Google Scholar]
- Hanker, L.C. , Rody, A. , Holtrich, U. , Pusztai, L. , Ruckhaeberle, E. , Liedtke, C. , Ahr, A. , Heinrich, T.M. , Sänger, N. , Becker, S. , Karn, T. , 2013. Prognostic evaluation of the B cell/IL-8 metagene in different intrinsic breast cancer subtypes. Breast Cancer Res. Treat. 137, 407–416. [DOI] [PubMed] [Google Scholar]
- Hellwig, B. , Hengstler, J.G. , Schmidt, M. , Gehrmann, M.C. , Schormann, W. , Rahnenführer, J. , 2010. Comparison of scores for bimodality of gene expression distributions and genome-wide evaluation of the prognostic relevance of high-scoring genes. BMC Bioinformatics. 11, 276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J.P. , Thompson, S.G. , Deeks, J.J. , Altman, D.G. , 2003. Measuring inconsistency in meta-analyses. BMJ. 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugh, J. , Hanson, J. , Cheang, M.C.U. , Nielsen, T.O. , Perou, C.M. , Dumontet, C. , Reed, J. , Krajewska, M. , Treilleux, I. , Rupin, M. , Magherini, E. , Mackey, J. , Martin, M. , Vogel, C. , 2009. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J. Clin. Oncol. 27, 1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn, T. , Metzler, D. , Ruckhäberle, E. , Hanker, L. , Gätje, R. , Solbach, C. , Ahr, A. , Schmidt, M. , Holtrich, U. , Kaufmann, M. , Rody, A. , 2010. Data driven derivation of cutoffs from a pool of 3,030 Affymetrix arrays to stratify distinct clinical types of breast cancer. Breast Cancer Res. Treat. 120, 567–579. [DOI] [PubMed] [Google Scholar]
- Karn, T. , Pusztai, L. , Holtrich, U. , Iwamoto, T. , Shiang, C.Y. , Schmidt, M. , Müller, V. , Solbach, C. , Gaetje, R. , Hanker, L. , Ahr, A. , Liedtke, C. , Ruckhäberle, E. , Kaufmann, M. , Rody, A. , 2011. Homogeneous datasets of triple negative breast cancers enable the identification of novel prognostic and predictive signatures. PLoS ONE. 6, e28403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn, T. , Pusztai, L. , Ruckhäberle, E. , Liedtke, C. , Müller, V. , Schmidt, M. , Metzler, D. , Wang, J. , Coombes, K.R. , Gätje, R. , Hanker, L. , Solbach, C. , Ahr, A. , Holtrich, U. , Rody, A. , Kaufmann, M. , 2012. Melanoma antigen family A identified by the bimodality index defines a subset of triple negative breast cancers as candidates for immune response augmentation. Eur. J. Cancer. 48, 12–23. [DOI] [PubMed] [Google Scholar]
- Karrila, S. , Lee, Julian Hock Ean , Tucker-Kellogg, G. , 2011. A comparison of methods for data-driven cancer outlier discovery, and an application scheme to semisupervised predictive biomarker discovery. Cancer Inform. 10, 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koboldt, D.C. , Fulton, R.S. , McLellan, M.D. , Schmidt, H. , Kalicki-Veizer, J. , McMichael, J.F. , Fulton, L.L. , Dooling, D.J. , Ding, L. , Mardis, E.R. , Wilson, R.K. , Ally, A. , Balasundaram, M. , Butterfield, Y.S.N. , Carlsen, R. , 2012. Comprehensive molecular portraits of human breast tumours. Nature. 490, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek, J.T. , Scharpf, R.B. , Bravo, H.C. , Simcha, D. , Langmead, B. , Johnson, W.E. , Geman, D. , Baggerly, K. , Irizarry, R.A. , 2010. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat. Rev. Genet. 11, 733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton, A. , Fizazi, K. , Stopeck, A.T. , Henry, D.H. , Brown, J.E. , Yardley, D.A. , Richardson, G.E. , Siena, S. , Maroto, P. , Clemens, M. , Bilynskyy, B. , Charu, V. , Beuzeboc, P. , Rader, M. , Viniegra, M. , Saad, F. , Ke, C. , Braun, A. , Jun, S. , 2012. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur. J. Cancer. 48, 3082–3092. [DOI] [PubMed] [Google Scholar]
- McShane, L.M. , Altman, D.G. , Sauerbrei, W. , Taube, S.E. , Gion, M. , Clark, G.M. , 2005. Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. J. Clin. Oncol. 23, 9067–9072. Reporting recommendations for tumor marker prognostic studies [DOI] [PubMed] [Google Scholar]
- Mihály, Z. , Kormos, M. , Lánczky, A. , Dank, M. , Budczies, J. , Szász, M.A. , Győrffy, B. , 2013. A meta-analysis of gene expression-based biomarkers predicting outcome after tamoxifen treatment in breast cancer. Breast Cancer Res. Treat. 140, 219–232. [DOI] [PubMed] [Google Scholar]
- Miller, L.D. , Smeds, J. , George, J. , Vega, V.B. , Vergara, L. , Ploner, A. , Pawitan, Y. , Hall, P. , Klaar, S. , Liu, E.T. , Bergh, J. , 2005. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc. Natl. Acad. Sci. U S A. 102, 13550–13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn, A.J. , Gupta, G.P. , Padua, D. , Bos, P. , Nguyen, D.X. , Nuyten, D. , Kreike, B. , Zhang, Y. , Wang, Y. , Ishwaran, H. , Foekens, J.A. , van de Vijver, Marc , Massagué, J. , 2007. Lung metastasis genes couple breast tumor size and metastatic spread. Proc. Natl. Acad. Sci. U. S. A. 104, 6740–6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima, T. , Takayanagi, H. , 2009. Osteoimmunology: crosstalk between the immune and bone systems. J. Clin. Immunol. 29, 555–567. [DOI] [PubMed] [Google Scholar]
- Palafox, M. , Ferrer, I. , Pellegrini, P. , Vila, S. , Hernandez-Ortega, S. , Urruticoechea, A. , Climent, F. , Soler, M.T. , Muñoz, P. , Viñals, F. , Tometsko, M. , Branstetter, D. , Dougall, W.C. , González-Suárez, E. , 2012. RANK induces epithelial-mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res. 72, 2879–2888. [DOI] [PubMed] [Google Scholar]
- Prat, A. , Cheang, M.C.U. , Martín, M. , Parker, J.S. , Carrasco, E. , Caballero, R. , Tyldesley, S. , Gelmon, K. , Bernard, P.S. , Nielsen, T.O. , Perou, C.M. , 2013. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal a breast cancer. J. Clin. Oncol. 31, 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis-Filho, J.S. , Pusztai, L. , 2011. Gene expression profiling in breast cancer: classification, prognostication, and prediction. Lancet. 378, 1812–1823. [DOI] [PubMed] [Google Scholar]
- Rody, A. , Karn, T. , Liedtke, C. , Pusztai, L. , Ruckhaeberle, E. , Hanker, L. , Gaetje, R. , Solbach, C. , Ahr, A. , Metzler, D. , Schmidt, M. , Müller, V. , Holtrich, U. , Kaufmann, M. , 2011. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res. 13, R97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini, D. , Perrone, G. , Roato, I. , Godio, L. , Pantano, F. , Grasso, D. , Russo, A. , Vincenzi, B. , Fratto, M.E. , Sabbatini, R. , Della Pepa, C. , Porta, C. , Del Conte, A. , Schiavon, G. , Berruti, A. , 2011. Expression pattern of receptor activator of NFκB (RANK) in a series of primary solid tumors and related bone metastases. J. Cell. Physiol. 226, 780–784. [DOI] [PubMed] [Google Scholar]
- Schmidt, M. , Böhm, D. , Törne, C. , von Steiner, E. , Puhl, A. , Pilch, H. , Lehr, H.-A. , Hengstler, J.G. , Kölbl, H. , Gehrmann, M. , 2008. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 68, 5405–5413. [DOI] [PubMed] [Google Scholar]
- Schramek, D. , Leibbrandt, A. , Sigl, V. , Kenner, L. , Pospisilik, J.A. , Lee, H.J. , Hanada, R. , Joshi, P.A. , Aliprantis, A. , Glimcher, L. , Pasparakis, M. , Khokha, R. , Ormandy, C.J. , Widschwendter, M. , Schett, G. , Penninger, J.M. , 2010. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 468, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata, D. , 2012. Cancer. Heterogeneity and tumor history. Science. 336, 304–305. [DOI] [PubMed] [Google Scholar]
- Simon, R.M. , Paik, S. , Hayes, D.F. , 2009. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J. Natl. Cancer Inst. 101, 1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopeck, A.T. , Lipton, A. , Body, J.-J. , Steger, G.G. , Tonkin, K. , de Boer, Richard H. , Lichinitser, M. , Fujiwara, Y. , Yardley, D.A. , Viniegra, M. , Fan, M. , Jiang, Q. , Dansey, R. , Jun, S. , Braun, A. , 2010. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 28, 5132–5139. [DOI] [PubMed] [Google Scholar]
- Tan, W. , Zhang, W. , Strasner, A. , Grivennikov, S. , Cheng, J.Q. , Hoffman, R.M. , Karin, M. , 2011. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 470, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanos, T. , Sflomos, G. , Echeverria, P.C. , Ayyanan, A. , Gutierrez, M. , Delaloye, J.-F. , Raffoul, W. , Fiche, M. , Dougall, W. , Schneider, P. , Yalcin-Ozuysal, O. , Brisken, C. , 2013. Progesterone/RANKL is a major regulatory axis in the human breast. Sci. Transl Med. 5, 182ra55 [DOI] [PubMed] [Google Scholar]
- Thomas, Lumley , 2012. rmeta: Meta-Analysis http://CRAN.R-project.org/package=rmeta [Google Scholar]
- Viale, G. , Regan, M.M. , Maiorano, E. , Mastropasqua, M.G. , Dell'Orto, P. , Rasmussen, B.B. , Raffoul, J. , Neven, P. , Orosz, Z. , Braye, S. , Ohlschlegel, C. , Thürlimann, B. , Gelber, R.D. , Castiglione-Gertsch, M. , Price, K.N. , Goldhirsch, A. , Gusterson, B.A. , Coates, A.S. , 2007. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J. Clin. Oncol. 25, 3846–3852. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Wen, S. , Symmans, W.F. , Pusztai, L. , Coombes, K.R. , 2009. The bimodality index: a criterion for discovering and ranking bimodal signatures from cancer gene expression profiling data. Cancer Inform. 7, 199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Klijn, Jan G.M. , Zhang, Y. , Sieuwerts, A.M. , Look, M.P. , Yang, F. , Talantov, D. , Timmermans, M. , Meijer-van, Gelder , Marion, E. , Yu, J. , Jatkoe, T. , Berns, Els M.J. J. , Atkins, D. , Foekens, J.A. , 2005. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 365, 671–679. [DOI] [PubMed] [Google Scholar]
- Wood, C.E. , Branstetter, D. , Jacob, A.P. , Cline, J.M. , Register, T.C. , Rohrbach, K. , Huang, L.-Y. , Borgerink, H. , Dougall, W.C. , 2013. Progestin effects on cell proliferation pathways in the postmenopausal mammary gland. Breast Cancer Res. 15, R62 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary Figure S1 Bimodal distribution of OPG expression values. Histogram of normalized OPG (TNFRSF11B) mRNA expression values according to Affymetrix probeset 204933_s_at among 286 breast cancer samples of the Rotterdam dataset (Wang et al., 2005; Lancet 365, 671–679) is given in Panel A. Coloring according to ER status (blue: ER positive; red: ER negative) demonstrates that the bimodal shape of the distribution did not originate from subtype composition. A dashed line represents the cutoff for OPG gene expression derived from the distribution. Panels B and C demonstrate the same analyses for the datasets Mainz and Uppsala, respectively. The cutoff derived from the Rotterdam dataset in (A) is shown to also separate the bimodal peaks in those datasets.
Supplementary Figure S2 Correlation analysis of OPG and other genes as continuous variables. The correlation of gene expression values of OPG, estrogen receptor (ESR1), progesterone receptor (PGR), Ki67, and HER2 as continuous factors was analyzed in the dataset of 2990 ER positive tumors. Hierarchical clustering was performed to visualize the relationship of all parameters. The two OPG probesets cluster in the same branch of the dendrogram as PGR.
Supplementary Figure S3 Prognostic value of OPG in the combined cohorts. Kaplan–Meier analysis of all patients from the combined cohorts with ER positive (A) or ER negative (B) tumors according to OPG mRNA expression. OPG was dichotomized using the cutoff derived from its bimodal expression (Affymetrix probeset 204933_s_at). The small inset figures display the bimodal distribution of expression values and the applied cutoff (the same cutoff was used as for the analyses of individual datasets).
Supplementary Figure S4 Illustration of the effect of dataset composition on the prognostic value of OPG expression. Kaplan–Meier analysis was performed in two arbitrarily defined dataset pools: In Panel (A) the 882 ER positive patients from the seven datasets in which a prognostic value of OPG was detected (see Supplementary Table S3) were combined as dataset‐pool #1. In Panel (B) all 1059 ER positive patients from the 11 datasets in which no prognostic value of OPG has been detected were combined as dataset‐pool #2. The Kaplan–Meier graphs display the differences of event free survival according to OPG expression in these two voluntarily combined datasets for an illustrative purpose only.
Supplementary Figure S5 Illustration of the effect of dataset composition on the prognostic value of PgR expression. Kaplan–Meier analysis was performed in two arbitrarily defined dataset pools: In Panel (A) the 882 ER positive patients from the seven datasets in which a prognostic value of OPG was detected (see Supplementary Table S3) were combined as dataset‐pool #1. In Panel (B) all 1059 ER positive patients from the 11 datasets in which no prognostic value of OPG has been detected were combined as dataset‐pool #2. The Kaplan–Meier graphs display the differences of event free survival according to PgR expression in these two voluntarily combined datasets. The two small inset graphs display the bimodal distribution of PgR expression and the applied cutoff value for PgR positivety.
Supplementary Figure S6 Illustration of the effect of dataset composition on the prognostic value of PgR and OPG expression independent of adjuvant treatment. Kaplan–Meier analysis was performed in two arbitrarily defined dataset pools: For Panels (A), (B), (E), and (F) the 882 ER positive patients from the seven datasets in which a prognostic value of OPG was detected (see Supplementary Table S3) were combined as dataset‐pool #1. Kaplan–Meier graphs of event free survival according to PgR expression (A and B) or OPG expression (E and F) are shown for those 863 patients who were either untreated (A, E) or treated with endocrine therapy (B, F). For Panels (C), (D), (G), and (H) the 1059 ER positive patients from the 11 datasets in which no prognostic value of OPG has been detected were combined as dataset‐pool #2. Again, Kaplan–Meier graphs of event free survival according to PgR (C, D) expression or OPG expression (G, H) are shown for those 733 patients who were either untreated (C, G) or treated with endocrine therapy (D, H). Independent of treatment, the prognostic effect of both PgR and OPG was only detected in dataset pool #1 (Panels A, B, E, and F) and not in dataset pool #2 (Panels C, D, G, and H). In Panels I and K we also included results for the prognostic effect of OPG in ER positive breast cancer patients obtained using the KM plotter website (kmplot.com). For this analysis the upper quartiles of the cohorts were applied as optimal cutoff for high expression (Gyorffy et al., 2012; Endocr. Relat. Cancer 19, 197–208). A highly prognostic value of OPG was detected for both ER positive patients which had either received no adjuvant treatment (Panel I) or tamoxifen (Panel K) (Mihály et al., 2013; Breast Cancer Res. Treat. 140, 219–232).
Supplementary Figure S7 Illustration of the effect of dataset composition on the prognostic value of biochemical/IHC based PgR status. Kaplan–Meier analysis was performed in two arbitrarily defined dataset pools: The 882 ER positive patients from the seven datasets in which a prognostic value of OPG was detected (see Supplementary Table S3) were combined as dataset‐pool #1. All 1059 ER positive patients from the 11 datasets in which no prognostic value of OPG has been detected were combined as dataset‐pool #2. For 335 of the 882 patients from dataset‐pool #1 and 606 of the 1059 patients from dataset‐pool #2 information on PgR status from biochemical/IHC assay was available. The Kaplan–Meier graphs display the differences of event free survival according to PgR status as determined by biochemical/IHC assay separately for dataset‐pool #1 in (A) and dataset‐pool #2 in (B) for exploratory purpose.
Supplementary Figure S8 Verification of the prognostic value of Ki67 in both dataset pools. Kaplan–Meier analysis according to Ki67 expression was performed in the same dataset pool #1 (A, n = 882) and dataset pool #2 (B, n = 1059) that were used for the Kaplan–Meier analyses of OPG and PgR in Supplementary Figures S4 and S5, respectively. Samples were stratified according to median Ki67 expression among all 2990 ER positive patients of the full cohort. In panels (C) and (D), the Kaplan–Meier analyses were restricted to the lymph node negative patients of dataset pool #1 (n = 751) and dataset pool #2 (n = 516), respectively.
Supplementary Figure S9 Prognostic value of OPG and PgR for different types of distant relapse. Kaplan–Meier analysis according to OPG (A–C) and PgR (D–F) gene expression was performed among 242 ER positive tumors from the Rotterdam dataset with information on either bone metastasis (A, D), lung metastasis (B, E), or brain metastasis (C, F). All patients are lymph node negative.
Supplementary Figure S10 Prognostic value of OPG in different dataset. Kaplan–Meier analysis of all patients in the Rotterdam dataset (A), the Mainz dataset (B), and the Uppsala dataset (C), according to OPG mRNA expression. OPG was dichotomized using the cutoff derived from its bimodal expression (Affymetrix probeset 204933_s_at). The same cutoff was used for validation the Mainz dataset (B) and Uppsala dataset (C), respectively. The small inset figures display the bimodal distribution of expression values and the applied cutoff. DMFS was used in (A) and (B), while RFS was used in (C). This figure analyzing mixed cohorts of ER positive and ER negative samples is included here only for completeness. Similar results were obtained when only the ER positive subsets of the cohorts were separately analyzed (see Figure 2 in the main manuscript).
Supplementary Files OPG_analysis.zip. This ZIP package contains two files. An R workspace file (OPG_analysis.RData) and and the respective R script file (OPG_analysis.R) to perform the analyses generating Figures 3, 4, and Supplementary Figure S2.


