Abstract
Background: Chinese clearhead icefish, Protosalanx hyalocranius, is a representative icefish species with economic importance and special appearance. Due to its great economic value in China, the fish was introduced into Lake Dianchi and several other lakes from the Lake Taihu half a century ago. Similar to the Sinocyclocheilus cavefish, the clearhead icefish has certain cavefish-like traits, such as transparent body and nearly scaleless skin. Here, we provide the whole genome sequence of this surface-dwelling fish and generated a draft genome assembly, aiming at exploring molecular mechanisms for the biological interests. Findings: A total of 252.1 Gb of raw reads were sequenced. Subsequently, a novel draft genome assembly was generated, with the scaffold N50 reaching 1.163 Mb. The genome completeness was estimated to be 98.39 % by using the CEGMA evaluation. Finally, we annotated 19 884 protein-coding genes and observed that repeat sequences account for 24.43 % of the genome assembly. Conclusion: We report the first draft genome of the Chinese clearhead icefish. The genome assembly will provide a solid foundation for further molecular breeding and germplasm resource protection in Chinese clearhead icefish, as well as other icefishes. It is also a valuable genetic resource for revealing the molecular mechanisms for the cavefish-like characters.
Keywords: Clearhead icefish, Protosalanx hyalocranius, Whole genome sequencing, Genome assembly, Gene prediction, Repetitive sequences
Data description
Introduction
Icefishes (Osmeriformes, Salangidae) are widely distributed in freshwater, coastal, and estuarine habitats in East Asian countries [1–3]. Chinese clearhead icefish (Protosalanx hyalocranius; Fig. 1), a diadromous fish, mainly inhabits in coastal areas and adjacent freshwaters [4–6]. As an economically important fish in China, the clearhead icefish was widely introduced into some lakes from the original Lake Taihu half a century ago, and it has developed a resident life history in these water areas [2, 7, 8]. Because of its transparent body and nearly scaleless skin, similar to the Sinocyclocheilus cavefishes [9], we are very interested in this surface-dwelling fish and are performing comparative genomics studies to explore the mechanisms for these biological phenotypes. However, with the rapid development of the Chinese economy in recent decades, the population size of the clearhead icefish has been seriously declining because of overfishing, construction of water conservancy facilities, and water pollution in the ecological systems [10]. To maintain its sustainable development in China, here we performed whole genome sequencing of Chinese clearhead icefish to support its biological and economic importance.
Figure 1:
Picture of a Chinese clearhead icefish. It was captured from the Taihu Lake of Jiangsu Province, China.
Sample and sequencing
In this study, we applied Illumina whole genome sequencing strategy to sequence the genome of Chinese clearhead icefish (NCBI taxonomy ID: 418454; Fishbase ID: 12236). Genomic DNA was isolated from the muscle tissue of an individual collected from the Lake Taihu of Jiangsu Province in China. We constructed seven paired-end libraries with three short-insert libraries (250, 500, and 800 bp) and four long-insert libraries (2, 5, 10, and 20 kb) using the standard protocol provided by Illumina (San Diego, CA, USA). Subsequent paired-end sequencing was performed by the Illumina HiSeq 2000 platform for each library. Finally, we obtained 252.1 Gb of raw reads for further analysis.
Genome size estimation and genome assembly
The SOAPfilter v2.2 software [11] with optimized parameters (-y -p -g 1 -o clean -M 2 -f 0) was utilized to remove low-quality raw reads (including reads with 10 or more Ns and low-quality bases) and PCR replicates as well as adaptor sequences. In total, we obtained 169.0 Gb of clean reads. Subsequently, we estimated the genome size based on the 17-mer depth frequency distribution method [12]. We applied the following formula to calculate the genome size: G = k_num/k_depth = b_num/b_depth (k_num is the total number of K-mers from the sequencing data, k_depth is the expected coverage depth for k-mers, b_num is the total number of bases, b_depth is the expected coverage depth of bases; As one read with length L generates L-K+1 k-mers, k_num/b_num = (L-K+1)/L). In our current study, the K_num was 10 500 000 000 and the K_depth was 20. Hence, we estimated that the genome size of Chinese clearhead icefish is 525 Mb.
The filtered reads were assembled using SOAPdenovo2 v2.04.4 software [13] with optimized parameters (pregraph -K 79 -d 1; contig -M 1; scaff -F -b 1.5 -p 16) to generate contigs and original scaffolds. The gaps were filled using GapCloser v1.12 software [14] with default parameters and –p set to 25. Finally, we generated a draft genome assembly of 536 Mb, with the scaffold N50 reaching 1.163 Mb (Table 1).
Table 1:
The statistics of genome assembly and annotation for P. hyalocranius
| Genome assembly | |
|---|---|
| Contig N50 size (kb) | 17.2 |
| Scaffold N50 size (Mb) | 1.163 |
| Estimated genome size (Mb) | 525 |
| Assembled genome size (Mb) | 536 |
| Genome coverage (X) | 315 |
| The longest scaffold (bp) | 5 398 389 |
| Gap length (Mb) | 122 |
| Genome annotation | |
| Protein-coding gene number | 19 884 |
| Annotated functional gene number | 19 125 (96.2 %) |
| Unannotated functional gene number | 759 (3.8 %) |
| Repeat content | 24.43 % |
The completeness of our assembly was evaluated by using both CEGMA [15] and BUSCO [16]. The CEGMA program (Core Eukaryotic Genes Mapping Approach; version 2.4) assessment with 248 conserved Core Eukaryotic Genes was performed for evaluation of the gene space completeness. Our results revealed that the assembled genome had a CEGMA completeness score at 90.32 % and 98.39 %, which was calculated from the complete gene set and the partial gene set, respectively. Meanwhile, we used the representative metazoa gene set [17], which contains 843 single-copy genes that are widely present in metazoan, as a reference. The assessment demonstrated that the BUSCO value is 89 %, containing [D: 10 %], F: 7.7 %, M: 2.9 %, n: 843 (C: complete [D: duplicated], F: fragmented, M: missed, n: genes). These data from CEGMA and BUSCO indicate that the assembled genome covered majority of the gene space.
Repeat annotation
Firstly, a de novo repeat library was constructed by the RepeatModeller v1.05 [18] and LTR_FINDER.x86_64-1.0.6 [10] with default parameters. Then, the assembled genome sequences were aligned against the RepBase v21.01 [19] and the de novo repeat libraries to recognize the known and novel transposable elements using the RepeatMasker v4.06 [20]. Meantime, the Tandem Repeat Finder v4.07 [21] with parameters “Match = 2, Mismatch = 7, Delta = 7, PM = 80, PI = 10, Minscore = 50, and MaxPeriod = 2000” was utilized for annotation of tandem repeats. Furthermore, the RepeatProteinMask software v4.0.6 [20] was used to predict transposable element relevant proteins in our genome assembly. Finally, we observed that the repeat sequences account for 24.43 % of the assembled genome (Table 1), and the de novo annotation method predicted the most abundant repeat sequence among the four methods (Table 2).
Table 2:
Detailed classification of repeat sequences in the assembled genome
| Type | Repeat size (bp) | % of Genome |
|---|---|---|
| ProteinMask | 9 925 152 | 1.85 |
| RepeatMasker | 5 948 136 | 1.11 |
| Tandem Repeat Finder | 66 595 756 | 12.41 |
| De novo | 93 726 009 | 17.47 |
| Total | 131 090 229 | 24.43 |
Genome Annotation
In brief, we utilized two different methods to predict total gene set of the clearhead icefish.
de novo annotation
The AUGUSTUS v2.5 [22] and GENSCAN v1.0 [23] were executed to ab initio predict genes within the assembled genome, with the repetitive sequences masked as “N” to discard pseudo gene prediction. Those low-quality genes with short length (<150 bp), premature termination, or frame-shifting were removed. Finally, we identified 23 132 and 21 379 pro-coding genes by using the AUGUSTUS and GENSCAN software (Table 3).
Table 3:
Gene annotation statistics of the genome of P. hyalocranius
| Average transcript | Average CDS | Average Exons | Average Exons | Average Intron | |||
|---|---|---|---|---|---|---|---|
| Method | Number | length (bp) | length (bp) | Per Gene | Length (bp) | Length (bp) | |
| De novo | AUGUSTUS | 23 132 | 4897.24 | 1264.61 | 5.78 | 218.81 | 760.04 |
| GeneScan | 21 379 | 17 213.49 | 1973.56 | 10.22 | 193.05 | 1652.41 | |
| Homolog | Danio rerio | 25 390 | 7156.92 | 1312.32 | 6.17 | 212.62 | 1129.99 |
| Oryzias latipes | 25 319 | 6411.36 | 1194.58 | 5.89 | 202.73 | 1066.29 | |
| Takifugu rubripes | 16 563 | 7990.91 | 1759.17 | 11.59 | 151.75 | 588.32 | |
| Tetraodon nigroviridis | 19 128 | 8335.40 | 1351.98 | 7.44 | 181.78 | 1084.78 | |
| Esox lucius | 24 861 | 8019.18 | 1375.58 | 6.92 | 198.85 | 1122.70 | |
| Gasterosteus aculeatus | 25 354 | 6819.62 | 1183.46 | 6.18 | 191.44 | 1087.68 | |
| Final gene set | 19 884 | 12 889.35 | 1821.79 | 9.13 | 199.49 | 1360.92 |
Homology annotation
We aligned the protein sequences from six published genomes, including Danio rerio [24], Oryzias latipes [25], Takifugu rubripes [26], Tetraodon nigroviridis [27], Esox lucius [28], and Gasterosteus aculeatus [29], against our assembly to predict homology-based genes. The potential homology-based genes were searched by TblastN [30] with an e-value of 10−5. The TblastN results were then processed by Sorting Out Local Alignment Result [31] to obtain the best hit of each alignment. Subsequently, GeneWise v2.2.0 [32] was performed to detect the possible gene structure for the best hit of each alignment. The low-quality genes were also removed as described in the above-mentioned de novo annotation.
Integration of annotation results
We employed the GLEAN [33] to generate a nonredundant and comprehensive gene set. Finally, the best hit of each protein was obtained through all protein sequences from the GLEAN results aligned to the databases of the SwissProt and TrEMBL [34] (Uniprot release 2011.06) by BlastP with an e-value of 10−5. Overall, we generated a final gene set with 19,884 genes for the Chinese clearhead icefish (Table 3).
CEGMA was performed again to evaluate the coverage rate between eukaryotic orthologous group genes predicted by CEGMA and the predicted total gene set. It demonstrates that the predicted gene set mapped 96.4 % of the eukaryotic orthologous groups. Simultaneously, the BUSCO was implemented again to assess completeness of the predicted gene set. The BUSCO values were calculated as follows: C: 79 % [D: 16 %], F: 9.8 %, M: 10, n: 843 (C: complete [D: duplicated], F: fragmented, M: missed, n: genes). The assessment values from both CEGMA and BUSCO proved high accuracy of the annotation.
Function annotation
The predicted protein sequences of the clearhead icefish were aligned against several public databases (Pfam [35], PRINTS [36], ProDom [37], and SMART [38]) for detection of functional motifs and domains. Finally, we found that 96.2 % of the predicted total gene set had been annotated with at least one functional assignment from other public databases (Swiss-Prot [39], Interpro [40], TrEMBL [41], and KEGG [42]).
Genome evolution
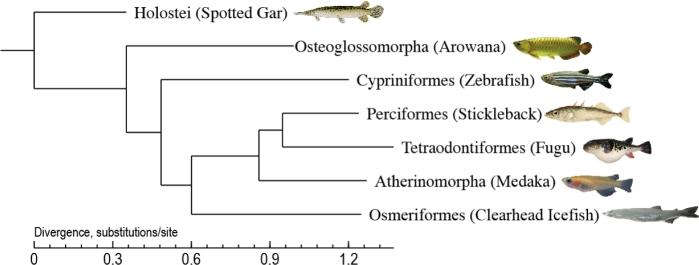
We performed phylogenomic analyses with orthologues from representative species for each clade. We used the Ensembl BioMart (www.ensembl.org; Ensembl version 76) to extract orthologues for zebrafish [24], fugu [26], stickleback [29], medaka [25], and spotted gar [43]. This generated orthologue dataset from six species was filtered out to retain only one-to-one orthologues. Meanwhile, a new Asian arowana gene set stemmed from our recent work [44]. To extrapolate the Biomart orthologues to the arowana and clearhead icefish gene sets, we used zebrafish as the reference. We ran InParanoid [45] for the three species pairs (zebrafish-arowana and zebrafish-clearhead icefish) at default settings (i.e., a minimum BLASTP score of 40 bits, minimum 50 % alignment span, minimum 25 % alignment coverage, and minimum inparalog confidence level of 0.05). By comparing the three InParanoid outputs, we narrowed down the list of one-to-one orthologues, presented in all seven species, to 454 genes. Multiple alignments were subsequently performed on proteins of each selected family using MUSCLE (version 3.8.31) [46], and protein alignments were converted to their corresponding CDS alignments using an in-house perl script (see supporting data). All the translated CDS sequences were linked into one “supergene” for each species. Nondegenerated sites extracted from the supergenes were subsequently joined into the new sequence of each species to construct a phylogenetic tree (Fig. 2) using MrBayes [47] (GTR+gamma model, Version 3.2). Our phylogenetic data demonstrate the phylogenetic position of the clearhead icefish (Fig. 2).
Figure 2:
Phylogeny of seven representative ray-finned fishes. The spotted gar was used as the outgroup species.
Synteny blocks and genome duplication
Genomic homology between the clearhead icefish and Nile tilapia [48] was examined using i-ADHoRe 3.0 [49] using the following settings: alignment method gg2, gap size 30, tandem gap 30, cluster gap 35, q value of 0.85, prob cutoff 0.01, anchor points 5, and using multiple hypothesis correction FDR. The output of this was processed by the pipeline and incorporated in a relational database to which visualization programs can connect and on which additional statistical analysis can then be performed. For synteny detection, the cloud mode was enabled (cluster_type = cloud) and appropriate settings were selected as follows: cloud_gap_size 20, cloud_cluster_gap 20, cloud_filter_method binomial, prob cutoff 0.01, anchor points 5, multiple hypothesis correction FDR, and level_2_only true. Finally, we identified 771 synteny blocks containing 7057 genes between the clearhead icefish and Nile tilapia.
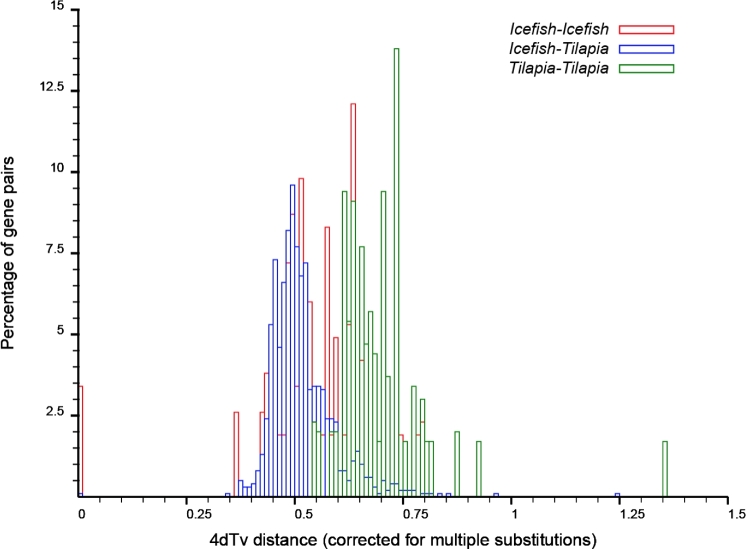
Subsequently, protein sequences of homologous gene pairs in the identified syntenic regions were aligned using MUSCLE [46], and the protein alignments were then converted to the CDS alignments. Finally, 4-fold degenerative third-codon transversion (4DTV) values were calculated on these CDS alignments and corrected using the HKY model in the PAML package [50]. These data indicate that the clearhead icefish also experienced the teleost-specific whole genome duplication (Fig. 3).
Figure 3:
Distribution of 4DTV distances between the clearhead icefish and tilapia. The horizontal axis stands for the 4DTV distance corrected using the HKY model. The vertical axis represents the percentage of collinear gene pairs.
Conclusion
We generated a draft genome assembly of the Chinese clearhead icefish. The novel genome data were deposited in publicly accessible repositories to promote further biological research, molecular breeding, and resource protection of this representative and valuable icefish.
Availability of supporting data
Supporting data and materials are available in the GigaScience GigaDB database [51], with the raw genome sequences deposited in the SRA under the bioproject number PRJNA328051.
Competing interests
The authors declare that they have no competing interests.
Funding
This study was supported by a grant from the Natural Science Foundation of Jiangsu Province (No. BK2012093), fish investigation in Taihu Lake (No. TH2016WT007), National Infrastructure of Fishery Germplasm Resources (No. 2016DKA30470), Basic Research Funds from Freshwater Fisheries Research Center (No. 2013JBFM07), Special Project on the Integration of Industry, Education and Research of Guangdong Province (No. 2013B090800017), Shenzhen Special Program for Future Industrial Development (N o. JSGG20141020113728803), and Zhenjiang Leading Talent Program for Innovation and Entrepreneurship.
Author Contributions
KL, PX, QS, DX, JX, CB, and ZZ conceived the project. MZ, XY, HY, JC, GX, DF, JQ, SJ, and JH collected the samples and extracted the genomic DNA. JL, CB, and HY performed the genome assembly and data analysis. JL, CB, QS, KL, XP, KL, YY, and ZZ wrote the paper.
Supplementary Material
References
- 1. Wang ZS, Cui Zhang FU. Biodiversity of Chinese Icefishes (Salangidae) and their conserving strategies. Chin Biodivers. 2002;10(4):416–24. [Google Scholar]
- 2. Zhang J, Li M, Xu M et al. Molecular phylogeny of icefish Salangidae based on complete mtDNA cytochrome b sequences, with comments on estuarine fish evolution. Biol J Linn Soc. 2007;91(2):325–40. [Google Scholar]
- 3. Wang Z, Lu C, Hu H et al. Dynamics of Icefish (Salangidae) stocks in Nanyi Lake, Eastern China: degradation and overfishing. J Freshw Ecol. 2004;19(2):271–78. [Google Scholar]
- 4. Xia DQ, Cao Y, Ting ting WU et al. Study on lineages of Protosalanx chinensis, Neosalanx taihuensis and N. oligodontis in Taihu Lake with RAPD technique. J Fish Sci China. 2000;7(01):12–15. [Google Scholar]
- 5. Xia DQ, Cao Y, Ting Ting WU et al. Genetic structures of population of Protosalanx Chinensis, Neosalanx Taihuensis and Neosalanx Oligodontis in Lake Taihu. J Fish China. 1999;(03):254–60. [Google Scholar]
- 6. Armani A, Castigliego L, Tinacci L et al. Molecular characterization of icefish, (S alangidae family), using direct sequencing of mitochondrial cytochrome b gene. Food Control. 2011;22(6):888–95. [Google Scholar]
- 7. Wang Z, Lu C, Hu H et al. Freshwater icefishes (Salangidae) in the Yangtze River basin of China: spatial distribution patterns and environmental determinants. Environ Biol Fishes. 2005;73(3):253–62. [Google Scholar]
- 8. Ye S, Yang J, Liu H et al. Use of elemental fingerprint analysis to identify localities of collection for the large icefish protosalanx chinensis in Taihu Lake, China. J Fac Agric Kyushu Univ. 2011;56(1):41–45. [Google Scholar]
- 9. Yang J, Chen X, Jie B et al. The Sinocyclocheilus cavefish genome provides insights into cave adaptation. BMC Biol. 2016;14(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu J, Xie P, Zhang M et al. Icefish (salangidae) as an indicator of anthropogenic pollution in freshwater systems using nitrogen isotope analysis. Bull Environ Contam Toxicol. 2007;79(3):323–26. [DOI] [PubMed] [Google Scholar]
- 11. Kar HK, Narayan R, Gautam RK et al. Mucocutaneous disorders in Hiv positive patients. Indian J Dermatol Venereol. 1996;62(5):283–85. [PubMed] [Google Scholar]
- 12. Liu B, Shi Y, Yuan J et al. Estimation of genomic characteristics by analyzing k-mer frequency in de novo genome projects. Quant Biol. 2013;35(s 1–3):62–67. [Google Scholar]
- 13. Luo R, Liu B, Xie Y et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li R, Yu C, Li Y et al. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics. 2009;25(15):1966–67. [DOI] [PubMed] [Google Scholar]
- 15. Parra G, Bradnam K, Korf I. CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics. 2007;23(9):1061–67. [DOI] [PubMed] [Google Scholar]
- 16. Sim AFO, Waterhouse MR, Ioannidis P et al. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–12. [DOI] [PubMed] [Google Scholar]
- 17. Kriventseva EV, Tegenfeldt F, Petty TJ et al. OrthoDB v8: update of the hierarchical catalog of orthologs and the underlying free software. Nucleic Acids Res. 2015;43(Database issue):D250–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maziade M, Bouchard S, Gingras N et al. Long-term stability of diagnosis and symptom dimensions in a systematic sample of patients with onset of schizophrenia in childhood and early adolescence. II: postnegative distinction and childhood predictors of adult outcome. Br J Psychiatry J Ment Sci. 1996;169(3):371–78. [DOI] [PubMed] [Google Scholar]
- 19. Jurka J, Kapitonov VV, Pavlicek A et al. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005;110(1–4):462–67. [DOI] [PubMed] [Google Scholar]
- 20. Chen N. Using RepeatMasker to identify repetitive elements in genomic sequences. John Wiley & Sons, Inc., New Jersey, USA: 2004;Chapter 4(Unit 4):4.10.1–4.10.14. [DOI] [PubMed] [Google Scholar]
- 21. Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27(2):573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mario S, Oliver K, Irfan G et al. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 2006;34:435–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burge C, Karlin S, Prediction of complete gene structures in human genomic DNA. J Mol Biol. 1997;268(1):78–94. [DOI] [PubMed] [Google Scholar]
- 24. Collins JE, White S, Searle SMJ et al. Incorporating RNA-seq data into the zebrafish Ensembl genebuild. Genome Res. 2012;22(10):2067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kasahara M, Naruse K, Sasaki S et al. The medaka draft genome and insights into vertebrate genome evolution. Nature. 2007;447(7145):714–19. [DOI] [PubMed] [Google Scholar]
- 26. Kesteven GL. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science. 2002;297(5585):130–10. [DOI] [PubMed] [Google Scholar]
- 27. Jaillon O, Aury JM, Brunet F et al. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature. 2004;431(7011):946–57. [DOI] [PubMed] [Google Scholar]
- 28. Rondeau EB, Minkley DR, Leong JS et al. The genome and linkage map of the northern pike (Esox lucius): conserved synteny revealed between the salmonid sister group and the Neoteleostei. PLoS ONE. 2014;9(7):e102089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones FC, Grabherr MG, Chan YF et al. The genomic basis of adaptive evolution in threespine sticklebacks. Nature. 2012;484(7392):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pevsner J. Basic Local Alignment Search Tool (BLAST), John Wiley & Sons, Inc., New Jersey, USA: 2005. [Google Scholar]
- 31. Yu XJ, Zheng HK, Wang J et al. Detecting lineage-specific adaptive evolution of brain-expressed genes in human using rhesus macaque as outgroup. Genomics. 2006;88(6):745–51. [DOI] [PubMed] [Google Scholar]
- 32. Birney E, Clamp M, Durbin R. GeneWise and Genomewise. Genome Res. 2004;14(5):988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elsik CG, Mackey AJ, Reese JT et al. Creating a honey bee consensus gene set. Genome Biol. 2007;8(1):90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bairoch A, Apweiler R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 2000;28(1):45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Finn RD. Pfam: the protein families database. Nucleic Acids Res. 2014;42(Database issue):D222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Attwood TK. The PRINTS database: a resource for identification of protein families. Brief Bioinform. 2002;3(3):252–63. [DOI] [PubMed] [Google Scholar]
- 37. Bru C, Courcelle E, Beausse Y et al. The ProDom database of protein domain families: more emphasis on 3D. Nucleic Acids Res. 2005;33(Database issue):212–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Letunic I, Copley RR, Schmidt S et al. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 2004;32(Databaseissue):D142–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boeckmann B, Bairoch A, Apweiler R et al. The Swiss-Prot knowledgebase and its supplement TREMBL in 2003. Nucleic Acids Res. 2003;31(1):365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hunter S, Apweiler R, Attwood TK et al. InterPro: the integrative protein signature database. Nucleic Acids Res. 2009;37(suppl 1):D211–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hingamp P, Broek AEVD, Stoesser G et al. The EMBL nucleotide sequence database. Mol Biotechnol. 1999;12(3):255–67. [DOI] [PubMed] [Google Scholar]
- 42. Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;27(1):29–34 (26). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Braasch I, Gehrke AR, Smith JJ et al. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat Genet. 2016;48(4):427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bian C, Hu Y, Ravi V et al. The Asian arowana (Scleropages formosus) genome provides new insights into the evolution of an early lineage of teleosts. Sci Rep. 2016;6:24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ostlund G, Schmitt T, Forslund K et al. InParanoid 7: new algorithms and tools for eukaryotic orthology analysis. Nucleic Acids Res. 2010;38(Database issue):D196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ronquist F, Teslenko M, van der Mark P et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brawand D, Wagner CE, Li YI et al. The genomic substrate for adaptive radiation in African cichlid fish. Nature. 2014;513(7518):375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Proost S, Fostier J, De Witte D et al. i-ADHoRe 3.0–fast and sensitive detection of genomic homology in extremely large data sets. Nucleic Acids Res. 2012;40(2):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13(5):555–56. [DOI] [PubMed] [Google Scholar]
- 51. Liu K, Xu D, Li J et al. Supporting data for "Whole genome sequencing of Chinese clearhead icefish, Protosalanx hyalocranius". GigaScience Database. 2016. http://doi.org/10.5524/100262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.