Abstract
Whereas transformation events in hematopoietic malignancies may occur at different developmental stages, the initial mutation originates in hematopoietic stem cells (HSCs), creating a preleukemic stem cell (PLSC). Subsequent mutations at either stem cell or progenitor cell levels transform the PLSC into lymphoma/leukemia initiating cells (LIC). Thymic lymphomas have been thought to develop from developing thymocytes. T cell progenitors are generated from HSCs in the bone marrow (BM), but maturation and proliferation of T cells as well as T-lymphomagenesis depends on both regulatory mechanisms and microenvironment within the thymus. We studied PLSC linked to thymic lymphomas. In this study, we use MSH2-/- mice as a model to investigate the existence of PLSC and the evolution of PLSC to LIC. Following BM transplantation, we found that MSH2-/- BM cells from young mice are able to fully reconstitute multiple hematopoietic lineages of lethally irradiated wild-type recipients. However, all recipients developed thymic lymphomas within three and four months post transplantation. Transplantation of different fractions of BM cells or thymocytes from young health MSH2-/- mice showed that an HSC enriched fraction always reconstituted hematopoiesis followed by lymphoma development. In addition, lymphomas did not occur in thymectomized recipients of MSH2-/- BM. These results suggest that HSCs with DNA repair defects such as MSH2-/- are PLSCs because they retain hematopoietic function, but also carry an obligate lymphomagenic potential within their T-cell progeny that is dependent on the thymic microenvironment.
Introduction
Mismatch repair (MMR) is an essential pathway for maintaining genomic integrity mainly by removing base mismatches and small insertion/deletion loops (IDLs) introduced during replication [1]. In humans, MMR gene defects have been most closely associated with hereditary nonpolyposis colorectal cancer (HNPCC) [2,3,4]. Deficiency in MMR is associated with subsequent mutation of critical downstream genes, resulting in deregulated cell proliferation and tissue-specific tumorigenesis [5,6].
Although human HNPCC patients primarily develop cancers of the gastrointestinal tract, lymphomas and leukemias have been observed in certain kindreds [7,8]. Several human patients with germline mutations in both copies of any one of the mismatch repair genes, MLH1, MSH2, or PMS2, have presented with early-onset childhood T- or B-cell malignancies [9,10,11,12,13]. MMR deficiency has also been identified in primary and secondary hematopoietic malignancies and in leukemia and lymphoma cell lines [14,15,16]. In addition, leukemia cells from a substantial proportion of children with newly diagnosed acute lymphoblastic leukemia have low or undetectable MSH2 protein levels, despite abundant wild-type MSH2 mRNA[17]. These reports suggest that functional MMR suppresses lymphoma/leukemia development.
Mice deficient in Msh2 most commonly develop early-onset thymic lymphomas although other tumors including small intestinal tumors occur with a lower frequency at later stage [18,19], this may be analog to the early-onset childhood T-cell malignancies in humans with biallelic MSH2 mutations [12]. Immunohistochemistry with T-cell markers, and histology, showed that these thymic lymphomas are very homogeneous, predominantly of T-cell origin, characterized by a ‘‘starry sky” appearance, enlarged nuclei, reduced cytoplasm, and numerous mitotic figures [18]. Msh2-deficient thymic lymphomas are thought to represent a single histopathologic entity and the tumor homogeneity suggests specific recurring genetic events may underlie the lymphocyte transformation and expression of a malignant phenotype [20].
Thymic lymphomas have been thought to develop from disregulated differentiation and proliferation of developing thymocytes. T cells develop in the thymus from precursors that are generated in the bone marrow (BM) and continuously seed the thymus through the blood. Maturation and proliferation of T cells depend on regulatory mechanisms in the thymus where the T-progenitors must interact with the microenvironment in the thymus to be able to differentiate [21,22]. Thymus environment is also important for lymphoma development. Earlier in vivo transplantation experiments have shown that whole body X-irradiation exposure or leukemia virus induced thymus-dependent pre-leukemic cells which required the thymus microenvironment for progression to full malignancy [23,24,25,26]. Thymectomy at birth or young age abolished spontaneous development in a lymphoma prone mouse stain AKR [27,28]. Thymectomy also reduced the incidence of radiation induced lymphoma in C57BL mice [29]. These data were interpreted as showing that lymphoma development is the final outcome of a series of events in which bone marrow-derived thymocyte progenitors are transformed in the thymic environment [30].
Hematopoietic stem cells (HSCs) are responsible for generation and maintenance of multiple lineages in the blood supply [31]. Recent evidence suggests that, in hematological malignancies, whereas transformation events may occur at different developmental stages, the initial mutation often originates in the HSCs, and creates a preleukemic stem cell (PLSC) [32]. PLSCs retain the ability to differentiate into the full spectrum of mature myeloid and lymphoid cells. Subsequent mutations at either stem cell or progenitor cell levels would transform the PLSC into leukemia stem cell (LSC) or leukemia initiating cells (LIC)[33,34].
MSH2-deficient cells display a mutator phenotype due to lack of MMR capacity [35], HPCs from MSH2-/- BM after serial transplantation display microsatellite instability (MSI) [36]. Though hematopoiesis in young MSH2-deficient mice appears normal, the high incidence of thymic lymphomas [18,19] in MSH2-deficient mice lead us to hypothesize that MSH2-deficient HSCs are PLSCs, and the transformation of MSH2-deficient PLSC into LSC or LIC requires the thymus microenvironment. In this study, we investigate the function of MSH2-/- HSCs, the cellular source of lymphomas, and the role of the thymic microenvironment in lymphoma development in MSH2-/- mice.
Materials and methods
Mice
The C57BL/6 (CD45.2) and congenic strains B6.SJL-PtprcaPep3b/BoyJ (BoyJ, CD45.1) mice were obtained from Jackson Laboratory. MSH2+/- mice were kindly provided by Dr. T. W. Mak. Thymectomized BoyJ mice were obtained from NCI Frederick. All the mice were housed in specific pathogen-free facility. All mouse studies were approved by the institutional animal care and use committee at Case Western Reserve University (Cleveland, OH).
Flow cytometry and cell sorting
Flow cytometry was performed on a BD LSRI or LSRII (BD Biosciences, San Jose, CA), and data were analyzed using FlowJo software (TreeStar, Ashland, OR). Antibodies include CD45.2, CD45.1, Ly-6G (Gr-1), CD11b (Mac-1), CD45R/B220, CD4 (L3T4), CD8 (Ly2), and Ter119/Ly76, Sca1 (Ly-6A/E), c-Kit (CD117), CD34, CD16/32, IL7Ra (BD Bioscience).
BD Aria was used for cell sorting. BM cells were lineage depleted using a lineage depletion kit (Miltenyi Biotec, Auburn, CA), and labeled with phycoerythrin (PE)-conjugated lineage antibodies, fluorescein isothiocyanate (FITC)-conjugated Sca-1 and allophycocyanin (APC)-conjugated c-Kit, LSK cells were sorted.
Transplantation assay
Single cell suspension was prepared from BM, thymus, and spleen. For BM transplantation, whole BM cells from WT or MSH2-/- mice were injected into lethally irradiated (11Gy) recipient mice through the lateral tail vein. For transplantation with thymocytes or splenocytes, isolated thymocytes or splenocytes were mixed with 2x105 BM cells from BoyJ mice, and transplanted into lethally irradiated BoyJ recipients. For transplantation with lymphoma cells, isolated single cells from lymphomas were transplanted into sublethally irradiated (6Gy) BoyJ mice.
Statistical analysis
Logrank test was used to determine the significance between survival curves. The Student’s t test was used to determine the significance in the chimerisms studies.
Results
Thymic lymphoma initiating cells originate from the BM hematopoietic stem/progenitor pool in MSH2-deficient mice
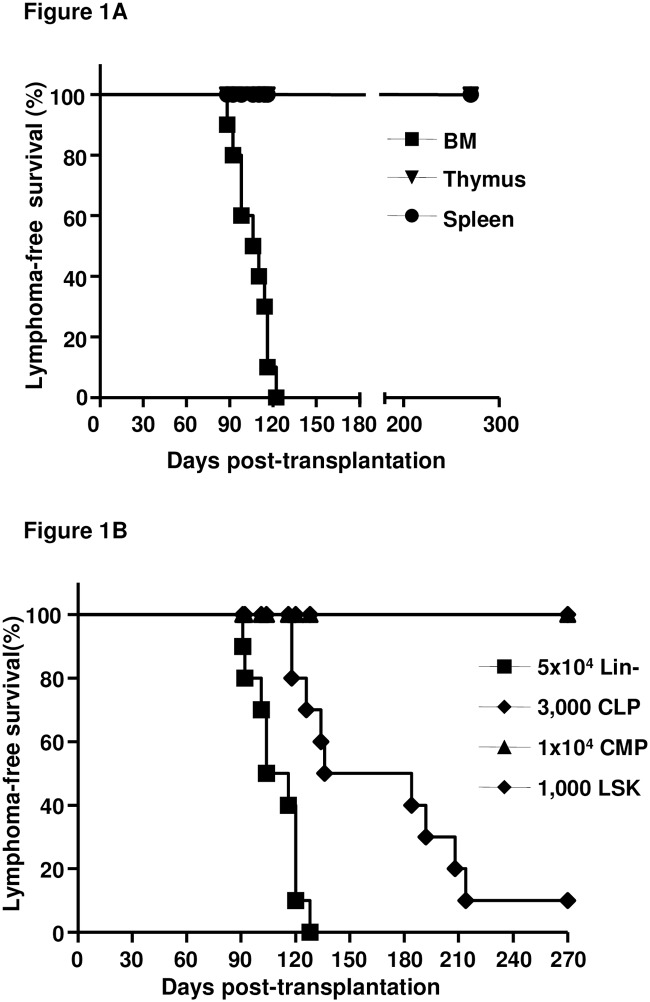
To determine the cellular source of the thymic lymphomas in MSH2-deficient mice, 2x106 cells from several hematopoietic tissues including BM, thymus, and spleen, of young healthy (6–8 weeks old) mice were transplanted into WT mice and thymic lymphoma development was monitored. All recipients receiving MSH2-/- BM cells developed thymic lymphomas within 3 to 4 months, while no lymphomas were observed in recipients of MSH2-/- thymocytes or splenocytes up to 9 months (Fig 1A). However, even when 1x107 thymocytes or splenocytes were transplanted into WT recipients, no lymphomas or other types of hematopoietic malignancies were observed after up to 9 months (data not shown). BM transplantation with different cell doses was also performed, and recipients of all cell doses from 1x106 to 1x105 developed T-lymphomas, and recipients of 1x105 MSH2-/- BM cells developed thymic lymphomas with slightly longer, but not significant, latency compared to the recipients of 1x106 (S1 Fig). These results indicate that the cellular source of thymic lymphoma in MSH2-/- mice is in the BM.
Fig 1. Kaplan-Meier survival curves of the recipients of transplantation with different cellular source of MSH2-/- mice.
(A). 2x106 cells from BM, thymus and spleen of MSH2-/- mice (n = 3) were transplanted into lethally irradiated BoyJ mice (n = 10 per group). The development of thymic lymphomas in the recipients was monitored. (B). Lineage depleted BM cells (Lin-), sorted LSK, CLP or CMP cells were transplanted along with 2x105 BM cells from BoyJ mice into lethally irradiated BoyJ mice (n = 10 per group). The development of thymic lymphomas in the recipients was monitored.
The lymphomas observed all resulted in enlarged thymus (data not shown). The immunophenotypes of these lymphomas are similar in MSH2 -/- mice and in irradiated normal recipients transplanted with MSH2-/- BM cells. The predominant phenotype was CD4+CD8+ double positive (DP) cells (13/20 lymphomas in MSH2-/- mice vs 14/26 lymphomas of the BMT recipients). Some showed a mixed population of DP and single positive populations (5/20 MSH2-/- mice vs 9/26 BMT recipients). A minority showed a large proportion of DN thymic cells (2/20 MSH2-/- lymphomas vs 3/26 lymphomas in BMT recipients of MSH2-/- BM cells (S2 Fig). These results suggest that lymphoma development in BMT recipients is similar to lymphomagenesis in MSH2-/- mice, predominantly evolving from DP cells; and lymphomas develop from different stages of T cells in the thymus.
To further identify the cellular population leading to the development of thymic lymphomas, BM from MSH2-/- mice were fractioned based on surface markers [37], and transplanted into WT BoyJ mice (n = 10 per group). MSH2-/- mice displayed similar frequencies of HSC/progenitors (Lin-, Sca1+,c-Kit+, LSK), common lymphoid progenitor (CLP, Lin-, CD127+, Sca1med, c-Kitmed) and common myeloid progenitor (CMP, Lin-, Sca1-, c-Kit+, CD34+/low,CD16/32int) compared to WT mice (S3 Fig). All the recipients of MSH2-/- Lin- cells and 90% of MSH2-/- LSK cells recipients developed thymic lymphomas, while no lymphomas were observed in recipients of CLP or CMP cells up to 9 months (Fig 1B). Recipients of T cells (CD3+), B cells (B220+) or myeloid cells (Mac1+) did not develop hematopoietic malignancies of any type (data not shown). We also note that recipients of 5x104 Lin- cells developed T-lymphomas with similar latency to that of 2x106 whole BM recipients (median latency, 110 days vs 108 days), while the latency in recipients of LSK cells was significantly longer than BM recipients (median latency, 160 days vs 108 days, P<0.0005) (Fig 1B). These results suggested that thymic lymphomas in MSH2-/- mice derived from HSC/progenitor cells, rather than lymphoid specific progenitors.
MSH2-/- HSCs are able to reconstitute hematopoiesis in lethally irradiated recipients
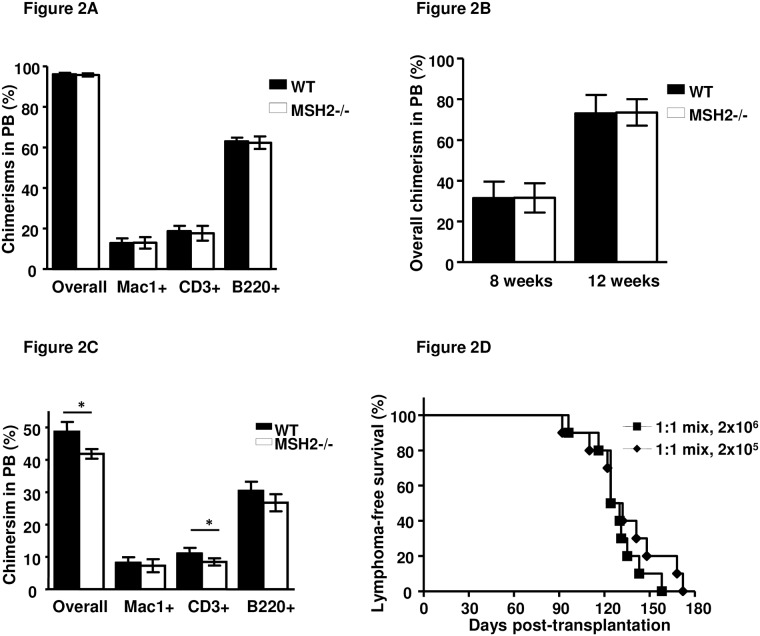
We have previously shown that MSH2-/- HSCs are defective in repopulation in serial transplantation by CFU assays [36], which mainly assess the myelopoiesis potential of progenitors. To assess the hematopoietic reconstitution potential of MSH2-/- HSCs including both myelopoiesis and lymphopoiesis, 2x106 BM cells from WT and MSH2-/- mice were transplanted into WT recipients. Similar to the results from WT BM transplantation, MSH2-/- BM contributed more than 95% overall chimerism in the peripheral blood of the recipients eight weeks post transplantation, and levels of MSH2-/- derived donor chimerism of each lineage including myeloid, T and B cells were similar to those from WT BM donor (Fig 2A). We could not determine the long-term hematopoiesis function of MSH2-/- HSCs in this setting due to the high incidence of fatal lymphoma development between 12 and 18 weeks (Fig 1). Purified LSK cells were also transplanted into WT recipients to examine their hematopoiesis activity. The contribution of MSH2-/- LSK cells to hematopoiesis was comparable to WT LSK cells at 8 and 12 weeks post transplantation (Fig 2B), and the recipients developed T-lymphomas 16–30 weeks post transplantation (Fig 1B), suggesting that the hematopoietic reconstitution function of MSH2-/- HSCs is intact, and lymphoma initiating cells evolved from the HSC/progenitor cells after hematopoietic reconstitution.
Fig 2. Functional assessment of HSCs from young healthy MSH2-/- mice.
(A). 2x106 whole BM pooled from 6–8 weeks old WT (n = 3) or MSH2-/- (n = 3) donor mice (CD45.2) into irradiated recipients (CD45.1, n = 5 per group). 8 weeks after transplantation, donor chimerism in the peripheral blood was analyzed and quantitated. Similar results were obtained in three independent experiments. (B). 1x103 sorted LSK cells from 6–8 weeks old WT or MSH2-/- (n = 3 each) donor mice (CD45.2) along with 2x105 BoyJ BM cells were transplanted into irradiated recipients (CD45.1, n = 10 per group). 8 and 12 weeks after transplantation, donor chimerism in the peripheral blood was analyzed and quantitated. Similar results were obtained in two independent experiments. (C). BM from 6–8 weeks old WT and MSH2-/- mice (CD45.2) was harvested and mixed with WT BM (CD45.1) at 1:1 ratio and transplanted into lethally irradiated WT mice (CD45.1, n = 10 per group). 8 weeks after transplantation, donor chimerism in the peripheral blood was analyzed and quantitated. Similar results were obtained in two independent experiments. Error bars indicate the SD, and significance was determined by a two-tailed t test. Asterisk, P<0.05. (D). Survival curve of the recipients of the experimental group described in (C).
To determine if hematopoiesis and lymphomagenesis initiated by MSH2-/- BMT would be suppressed by WT BM, competitive repopulation assays were performed. When mixed with WT BM at 1:1 ratio, MSH2-/- BM contributed slightly less to the overall chimerism in the peripheral blood of the recipients 8 weeks post transplantation compared to WT BM (41.8+/-1.5% vs 48.6+/-3.0%) (Fig 2C), with similar contributions to myeloid (7.3+/-2.0% vs 8.2+/-1.7%) and B cell (26.7+/-2.6% vs 30.4+/-2.8%), but slightly less contribution to T cell (8.4+/-1.1% vs11.1+/-1.7%) chimerism (Fig 2C). All recipients developed MSH2-/- BM derived lymphomas (Fig 2D). Mice with signs of lymphomas including labored breathing, loss of body weight and mobility, were examined for donor chimerism in the peripheral blood and thymus. While about 50% of the leukocytes in the peripheral blood derived from MSH2-/- cells, more than 95% of the cells in the lymphomas are MSH2-/-, and as noted, most of the lymphomas are double positive for CD4 and CD8 (S4 Fig). Interestingly, lymphoma latencies from mixtures of 2x105 and 2x106 total cells are similar (median latency, 127days vs 128 Days, P = 0.39) (Fig 2D). These results indicate that MSH2-/- HSCs were not defective in full hematopoietic repopulation—and thus of HSC function—compared to WT HSCs. However, whereas MSH2-/- cells were responsible for initiating T-lymphomas.
MSH2-/- thymic lymphomas are transplantable
To determine whether the T-lymphomas are transplantable, MSH2-/- mice bearing lymphomas were used as donors for transplantation. Lymphoma cells were transplanted into sublethally irradiated WT mice at different cell doses, as few as 40 cells was able to initiate T-cell leukemia within 5 weeks (Table 1).
Table 1. Lymphoma/T-leukemia development in secondary recipients.
| Cell doses | T-cell lymphoma/leukemia incidence | Latency (days) |
|---|---|---|
| Experiment #1 | ||
| 4x105 | 5/5 | 14–18 |
| 4x104 | 5/5 | 14–20 |
| 4x103 | 5/5 | 20–24 |
| 400 | 5/5 | 22–25 |
| 40 | 2/5 | 35–38 |
| Experiment #2 | ||
| 4x104 | 5/5 | 15–18 |
| 4x103 | 5/5 | 18–26 |
| 400 | 5/5 | 24–28 |
| 40 | 0/5 | |
| Experiment #3 | ||
| 4x104 | 5/5 | 14–16 |
| 4x103 | 5/5 | 14–20 |
| 400 | 5/5 | 20–24 |
| 40 | 1/5 | 34 |
Indicated numbers of lymphoma cells were transplanted into sublethally irradiated WT recipients (n = 5 per group). Lymphoma development in the recipients was monitored. Similar results were obtained in three independent experiments.
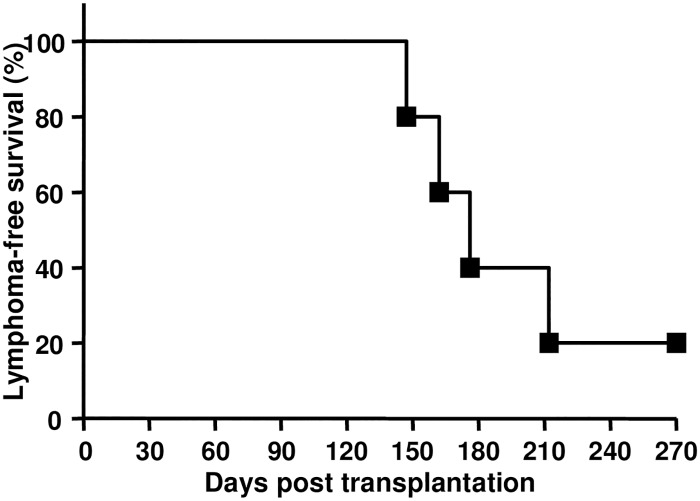
To determine if the HSCs in the mice with lymphomas have increased lymphomagenetic potential, 1x103 sorted LSK cells were transplanted into lethally irradiated WT mice. LSK cells contributed to mutlilineage hematopoiesis (data not shown), and 80% of the recipients developed T-lymphomas with a mean latency of 176 days post-transplantation (Fig 3). A similar T-lymphoma phenotype and latency was observed after transplanting HSC-LSK cells isolated from young healthy and from the lymphoma-bearing MSH2-/- mice (Figs 1B and 3) suggested that HSC/progenitors of MSH2-/- mice retained hematopoietic function and were not altered by the presence of thymic lymphomas.
Fig 3. Functional assessment of MSH2-/- HSCs from lymphoma bearing mice.
1x103 sorted LSK cells from MSH2-/- mouse with lymphoma symptoms along with 2x105 BoyJ BM cells were transplanted into irradiated recipients (n = 10 per group). The development of thymic lymphomas in the recipients was monitored.
Thymus is required for the development of thymic lymphomas in MSH2-/- mice
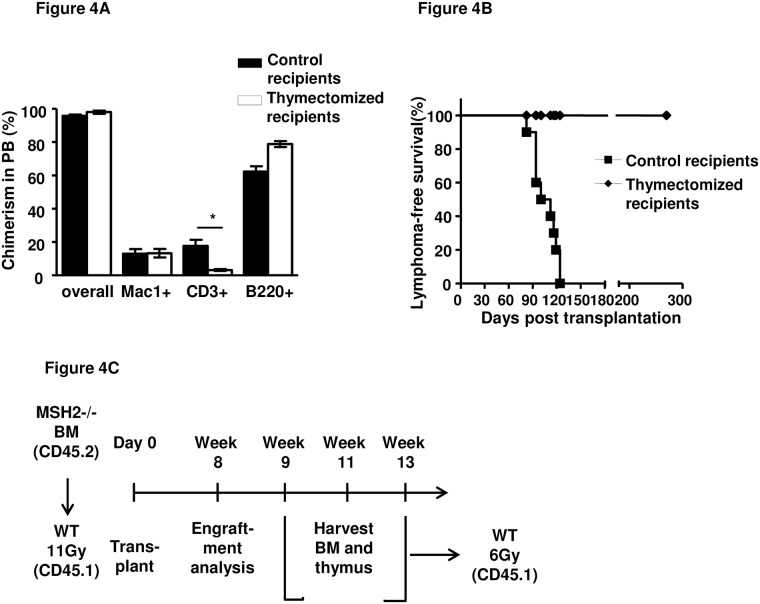
All the tumors were thymus-centric, suggesting a strong dependence on signals received from the thymic environment and that lymphoma initiating cells may evolve in the thymus. To determine whether the thymus was necessary for T-lymphoma development, MSH2-/- BM cells were transplanted into thymectomized irradiated WT recipients. Reduced T-cell reconstitution was observed in the recipients due to the lack of host thymus (Fig 4A). While MSH2-/- lymphomas developed 3–4 months post-transplantation in thymus-intact WT recipients, no T-lymphomas was observed in thymectomized recipients of MSH2-/- BM cells observed for up to 9 months (Fig 4B).
Fig 4. Requirement of thymus for the development of MSH2-/- BM derived thymic lymphomas.
(A). 2x106 BM cells from 6–8 weeks old MSH2-/- mice (n = 3) were transplanted into lethally irradiated WT control or thymectomized mice (n = 10 per group). 8 weeks after transplantation, donor chimerism in the peripheral blood was analyzed and quantitated. Similar results were obtained in two independent experiments. Error bars indicate the SD, and significance was determined by a two-tailed t test. (B). Survival curve of the recipients of the experimental group described in (A). (C). Scheme of the transplantation to determine the initial appearance of lymphoma initiating cells.
We also tested the requirement of the thymus for the expansion of lymphoma cells. T-cells were taken from the lymphomas, and transplanted into control and thymectomized mice. Each of the control and thymectomized recipients rapidly developed lymphoma/leukemia with a median latency of 15 days (S5 Fig). These results indicate that lymphoma cells do not need the thymus to propagate or expand, but that the thymus was required for the evolution of lymphoma initiating cells (LICs).
Next, we tested whether the evolution of LICs from normal HSC occurred in the thymus. 2x106 MSH2-/- BM cells were transplanted into irradiated WT recipients, 8 weeks post transplantation and hematopoietic reconstitution by MSH2-/- BM was confirmed (data not shown). Thymus and BM from the primary recipients were harvested at 9, 11, and 13 weeks post transplantation, and transplanted into sublethally irradiated WT secondary recipients (Fig 4C). While secondary recipients of BM cells at all three time points developed T-lymphomas 4–6 months post transplantation, all the recipients of thymocytes harvested from primary recipients at 9 weeks post transplantation developed T-cell leukemia within 8 weeks, whereas recipients of thymocytes harvested from primary recipients at 11 and 13 weeks post transplantation developed T-cell leukemia within 4 weeks (Table 2). The significantly shorter latency of lymphoma/leukemogenesis observed after thymocyte transplantation compared to the BM recipients reaffirmed that the thymus is the site of, and is required for, the evolution of LICs.
Table 2. Thymocytes and BM from primary recipients of MSH2-/- BM induced lymphoma/T-leukemia in secondary recipients.
| Donor Cell source | T-cell lymphoma/leukemia incidence | Latency (days) | Median Latency (days) |
|---|---|---|---|
| BM from primary recipients at weeks post transplantation | |||
| 9 weeks | 5/5 | 128–186 | 154 |
| 11 weeks | 5/5 | 122–174 | 150 |
| 13 weeks | 5/5 | 104–188 | 152 |
| Thymocytes from primary recipients at weeks post transplantation | |||
| 9 weeks | 5/5 | 33–57 | 48 |
| 11 weeks | 5/5 | 12–28 | 26 |
| 13 weeks | 5/5 | 21–27 | 23 |
2x106 MSH2-/- BM cells were transplanted into WT irradiated recipients. Thymus and BM from the primary recipients were harvested at 9, 11, and 13 weeks post transplantation (pooled from three mice each group), and 2x106 BM cells or thymocytes were transplanted into sublethally irradiated WT secondary recipients (n = 5 per group). Lymphoma/leukemia development in the secondary recipients was monitored. Similar results were obtained in two independent experiments.
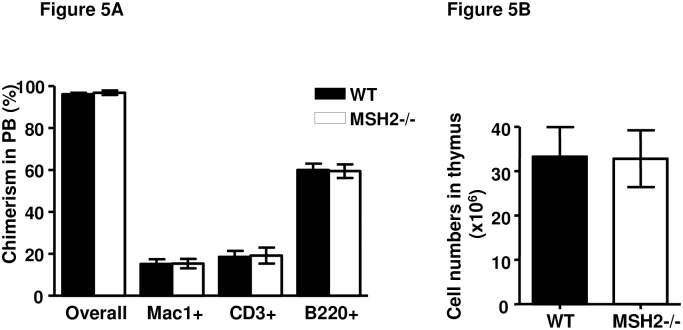
To further determine the impact of the microenvironment in MSH2-/- mice on lymphomagenesis, 2x106 WT BM cells were transplanted into lethally irradiated MSH2-/- mice, resulting in WT BM reconstitution of hematopoiesis with more than 95% overall chimerism in the peripheral blood of recipients, and normal lineage (Fig 5A). No recipients developed T-lymphomas or other hematopoietic malignancies observed for up to 9 months (data not shown). WT BM derived-T cell development within the thymus of MSH2-/- mice was also examined at 8 weeks post transplantation. Within the thymus, the total cell numbers (Fig 5B) and composition of T-cell subsets are comparable to WT controls (S6 Fig). These results indicated that the MSH2-/- BM microenvironment is not impaired in supporting hematopoiesis and that the MSH2-/- thymus microenvironment does not promote lymphomagenesis from WT progenitors.
Fig 5. Normal thymus microenviroment of MSH2-/- mice.
(A).2x106 BM cells from BoyJ mice (n = 3) were transplanted into 6–8 weeks old WT or MSH2-/- mice (n = 10 per group). 8 weeks after transplantation, donor chimerism in the peripheral blood was analyzed and quantitated. (B) Total cell numbers within thymus of the recipients at 8 weeks post transplantation. Error bars indicate the SD, and significance was determined by a two-tailed t test.
Discussion
The primary purpose of this study was to investigate the process of HSC-derived thymic lymphomagenesis in MSH2-/- mice. We set out to determine the cellular source of the LICs and the sites at which LICs develop into thymic lymphomas. Our results provide evidence that MSH2-/- HSCs are PLSCs, and while they retain full hematopoietic potential, their progeny gain lymphomagenetic potential in the thymic microenvironment and become LICs and initiate lymphomagenesis. Thus, the HSC is an obligate precursor carrier of the LIC in MSH2-/- mice.
Though MSH2-/- mice display somatic hyper mutation defects [38], it appears that MSH2 is not involved in T cell development. When MSH2-/- BM cells were transplanted into WT recipients, the hematopoietic reconstitution was similar with those receiving WT BM cells (Fig 2A). These results suggested that MSH2 is not critical for T-cell development or hematopoietic reconstitution. When MSH2-/- BM cells were mixed with WT BM at 1:1 ratio, MSH2-/- showed modest defects in competitive repopulation activity in the primary recipients (Fig 2C). These results indicate that the hematopoiesis function of MSH2-/- HSCs was largely intact.
MSH2-/- mice develop spontaneous thymic lymphomas starting at 3 months of age, most mice die from the disease within 5–7 months old ([18,19] and our unpublished results). Using BM transplantation, we found that BM cells from young healthy MSH2-/- mice were able to initiate thymic lymphomas in the recipients within 3–4 months post transplantation; whereas thymocytes and splenocytes were not able to initiate lymphomas (Fig 1A). These results indicate that the cellular source for lymphomagenesis originates from the BM, rather than the more specific lymphoid organs. Only the fractions enriched with HSC/progenitors (Lin- and LSK cells) were able to initiate lymphomagenesis, whereas the lineage positive populations or committed progenitors were not able to do so (Fig 1B and data not shown). In fact, the lineage positive cells and CLP/CMP were not able to maintain engraftment in the recipients [39,40] (and data not shown). These results suggest that HSC/progenitors in MSH2-/- mice are the cellular sources for lymphomagenesis. It is likely that sustained engraftment and hematopoiesis are required for the progenitor cells to initiate lymphomagenesis. Importantly, the limiting dilution BM transplantation studies showed that all recipients of even 1x105 BM from MSH2-/- mice developed thymic lymphomas (Fig 2C), and the frequency of HSCs in mouse BM is about 0.01% of total nucleated cells[37,41], suggesting that perhaps every HSC in MSH2-/- mice has the lymphomagenetic potential. Of note, LSK cells from lymphoma bearing mice does not cause early-onset lymphoma in the recipients (Fig 3), and are able to contribute to multilineage hematopoietic reconstitution in the recipients (data not shown), suggesting that LSK cells are not the direct source for LIC.
These results allow us to propose that HSCs in the BM of MSH2-/- mice are PLSCs, retaining full hematopoietic potential, but are an obligate carrier of full lymphomagenic potential. In this regard, the PLSCs in MSH2-/- mice have similar characteristics to LSCs in human acute myelogenous leukemia (AML). For instance, the LSCs in human AML share the immunophenotype of normal HSCs, possesses proliferation and differentiation potential[42]. An example is AML derived from AML1/ETO [43]. By surveying AML1/ETO mRNA expression in rigorously purified HSC, progenitors, and mature hematopoietic cells of various lineages, Miyamoto et al. demonstrated that acquisition of AML1/ETO occurs in HSC/progenitors, and that these progenitors contribute to B lymphopoiesis as well as myelopoiesis throughout the clinical course [44]. Interestingly, AML1/ETO is necessary, but not sufficient for transformation or leukemia development, and a fraction of the AML1/ETO-expressing stem cells undergo additional oncogenic event(s) that ultimately leads to transformation into AML[44,45]. Future studies of lymphomagenesis with MSH2-/- mouse model will provide important insight onto the process of human AML LSCs evolution from normal HSCs.
We also show that the thymus microenvironment is required for the MSH2-/- BM derived lymphomagenesis. While all the recipients of MSH2-/- BM cells developed lymphomas, the thymectomized recipients did not develop lymphomas or leukemias (Fig 4B). And when thymocytes and BM from the primary recipients of MSH2-/- BM transplantation were transplanted into secondary recipients, recipients of thymocytes developed lymphomas with a much shorter latency compared to BM recipients (Table 2). These results indicate that the evolution of PLSC to lymphoma requires T cell progenitors to migrate to the thymus, and thymocyte differentiation within the thymic microenvironment. On the other hand, intrinsic function of the thymic microenvironment in MSH2-/- mice appears normal, since no malignancies developed when WT BM were transplanted into MSH2-/- mice, and the development of T cells in the thymus appear normal (Fig 5 and S6 Fig).
Our observations do not appear isolated to MSH2. MLH1 is another key component in the MMR pathway. Mice deficient in MLH1 develop lymphomas at a lower frequency compared to MSH2-/- mice [46], while mice with thymocyte-specific deletion of MLH1 develop lymphomas at a remarkably reduced frequency compared to MLH1-/- mice (6% vs 26%), and most of the lymphomas are double positive [46]. These results indicate that the MMR pathway prevents genetic events that lead to cellular transformation at an early T cell development stage or an even earlier stage of hematopoiesis [47].
Loss of MMR allows cells to accumulate mutations over time. MSH2-/- hematopoietic progenitors displayed increased MSI in the BM from secondary recipients [36],MSI and mutational spectrum of the thymic lymphomas in MSH2-/- mice have been demonstrated [18,48,49,50,51]. However, a single targeted mutational lymphomagenic gene has not been identified and rather appears pleotropic. Insertion/deletion mutations of TGFbeta receptor II have been observed in lymphomas from MSH2-/-mice, the mutations correlate with loss of TGFbeta receptor II expression [52], but TGFbeta receptor II deficient thymocytes develop normally, without lymphoma incidence [53]. Expression levels of three human lymphoma-related genes LMO2, SCL and HOX11, have been examined in MSH2-/- mouse lymphomas. Expression of LMO2, SCL, and HOX11 is detected in 100%, 40%, and 0% of the cases, respectively [54]. However, whether LMO2 and SCL are direct targets of the mutator phenotype is not known. In addition, overexpression of LMO2 or SCL alone in developing thymocytes in the mouse results in the development of T-ALL with long latency, LMO2 and SCL cooperate to accelerate lymphomagenesis [55].
Lymphomagenesis is thought to require disruption of multiple pathways [56], MSH2-/- lymphomas might be a good model to identify lymphomagenic genes. In patients with Lynch syndrome and HNPCC, loss of MMR leads to many second hits, and accumulation of specific genetic alterations in MSI-positive colorectal cancers is markedly heterogeneous [57,58,59]. It is likely that MSH2-/- HSCs in mice like MMR-deficient colorectal cancer stem cells do not contain driver transformational mutations. Rather, during the expansion and differentiation process, their progeny acquire such driver mutations, with clonal expansion result in lympomagenesis. However, it is instructive to appreciate that in MSH2-/- mice, these data indicate that the HSC are, for the most part, obligate PLSC.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank the staff at the Cytometry & Imaging Microscopy Core Facility of the Case Comprehensive Cancer Center for their technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work is supported by the Cytometry & Imaging Microscopy Core Facility of the Case Comprehensive Cancer Center (P30 CA43703) (SLG) and by National Institute of Health (nih.gov) grant R01CA063193 (SLG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jiricny J (2006) The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 7: 335–346. doi: 10.1038/nrm1907 [DOI] [PubMed] [Google Scholar]
- 2.Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, et al. (1993) The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 75: 1027–1038. [DOI] [PubMed] [Google Scholar]
- 3.Bronner CE, Baker SM, Morrison PT, Warren G, Smith LG, et al. (1994) Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 368: 258–261. doi: 10.1038/368258a0 [DOI] [PubMed] [Google Scholar]
- 4.Liu B, Parsons R, Papadopoulos N, Nicolaides NC, Lynch HT, et al. (1996) Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med 2: 169–174. [DOI] [PubMed] [Google Scholar]
- 5.Peltomaki P (2001) Deficient DNA mismatch repair: a common etiologic factor for colon cancer. Hum Mol Genet 10: 735–740. [DOI] [PubMed] [Google Scholar]
- 6.Woerner SM, Kloor M, Mueller A, Rueschoff J, Friedrichs N, et al. (2005) Microsatellite instability of selective target genes in HNPCC-associated colon adenomas. Oncogene 24: 2525–2535. doi: 10.1038/sj.onc.1208456 [DOI] [PubMed] [Google Scholar]
- 7.Hirano K, Yamashita K, Yamashita N, Nakatsumi Y, Esumi H, et al. (2002) Non-Hodgkin's lymphoma in a patient with probable hereditary nonpolyposis colon cancer: report of a case and review of the literature. Dis Colon Rectum 45: 273–279. [DOI] [PubMed] [Google Scholar]
- 8.Rosty C, Briere J, Cellier C, Delabesse E, Carnot F, et al. (2000) Association of a duodenal follicular lymphoma and hereditary nonpolyposis colorectal cancer. Mod Pathol 13: 586–590. doi: 10.1038/modpathol.3880100 [DOI] [PubMed] [Google Scholar]
- 9.Bougeard G, Charbonnier F, Moerman A, Martin C, Ruchoux MM, et al. (2003) Early onset brain tumor and lymphoma in MSH2-deficient children. Am J Hum Genet 72: 213–216. doi: 10.1086/345297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricciardone MD, Ozcelik T, Cevher B, Ozdag H, Tuncer M, et al. (1999) Human MLH1 deficiency predisposes to hematological malignancy and neurofibromatosis type 1. Cancer Res 59: 290–293. [PubMed] [Google Scholar]
- 11.Wang Q, Lasset C, Desseigne F, Frappaz D, Bergeron C, et al. (1999) Neurofibromatosis and early onset of cancers in hMLH1-deficient children. Cancer Res 59: 294–297. [PubMed] [Google Scholar]
- 12.Whiteside D, McLeod R, Graham G, Steckley JL, Booth K, et al. (2002) A homozygous germ-line mutation in the human MSH2 gene predisposes to hematological malignancy and multiple cafe-au-lait spots. Cancer Res 62: 359–362. [PubMed] [Google Scholar]
- 13.De Vos M, Hayward BE, Picton S, Sheridan E, Bonthron DT (2004) Novel PMS2 pseudogenes can conceal recessive mutations causing a distinctive childhood cancer syndrome. Am J Hum Genet 74: 954–964. doi: 10.1086/420796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada C, Shionoya S, Fujino Y, Tokuhiro H, Akahoshi T, et al. (1994) Genomic instability of microsatellite repeats and its association with the evolution of chronic myelogenous leukemia. Blood 83: 3449–3456. [PubMed] [Google Scholar]
- 15.Zhu YM, Das-Gupta EP, Russell NH (1999) Microsatellite instability and p53 mutations are associated with abnormal expression of the MSH2 gene in adult acute leukemia. Blood 94: 733–740. [PubMed] [Google Scholar]
- 16.Robledo M, Martinez B, Arranz E, Trujillo MJ, Gonzalez Ageitos A, et al. (1995) Genetic instability of microsatellites in hematological neoplasms. Leukemia 9: 960–964. [PubMed] [Google Scholar]
- 17.Diouf B, Cheng Q, Krynetskaia NF, Yang W, Cheok M, et al. (2011) Somatic deletions of genes regulating MSH2 protein stability cause DNA mismatch repair deficiency and drug resistance in human leukemia cells. Nat Med 17: 1298–1303. doi: 10.1038/nm.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reitmair AH, Schmits R, Ewel A, Bapat B, Redston M, et al. (1995) MSH2 deficient mice are viable and susceptible to lymphoid tumours. Nat Genet 11: 64–70. doi: 10.1038/ng0995-64 [DOI] [PubMed] [Google Scholar]
- 19.de Wind N, Dekker M, Berns A, Radman M, te Riele H (1995) Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell 82: 321–330. [DOI] [PubMed] [Google Scholar]
- 20.Campbell MR, Nation PN, Andrew SE (2005) A lack of DNA mismatch repair on an athymic murine background predisposes to hematologic malignancy. Cancer Res 65: 2626–2635. doi: 10.1158/0008-5472.CAN-04-3158 [DOI] [PubMed] [Google Scholar]
- 21.Takahama Y (2006) Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol 6: 127–135. doi: 10.1038/nri1781 [DOI] [PubMed] [Google Scholar]
- 22.Ladi E, Yin X, Chtanova T, Robey EA (2006) Thymic microenvironments for T cell differentiation and selection. Nat Immunol 7: 338–343. doi: 10.1038/ni1323 [DOI] [PubMed] [Google Scholar]
- 23.Haran-Ghera N (1978) Spontaneous and induced preleukemia cells in C57BL/6 mice:brief communication. J Natl Cancer Inst 60: 707–710. [DOI] [PubMed] [Google Scholar]
- 24.Boniver J, Decleve A, Lieberman M, Honsik C, Travis M, et al. (1981) Marrow-thymus interactions during radiation leukemogenesis in C57BL/Ka mice. Cancer Res 41: 390–392. [PubMed] [Google Scholar]
- 25.Haran-Ghera N, Peled A, Leef F, Hoffman AD, Levy JA (1987) Enhanced AKR leukemogenesis by the dual tropic viruses. I. The time and site of origin of potential leukemic cells. Leukemia 1: 442–449. [PubMed] [Google Scholar]
- 26.Hays EF, Bristol GC, McDougall S, Klotz JL, Kronenberg M (1989) Development of lymphoma in the thymus of AKR mice treated with the lymphomagenic virus SL 3–3. Cancer Res 49: 4225–4230. [PubMed] [Google Scholar]
- 27.Metcalf D (1966) The Thymus: Rentchnick P, editor. New York: Springer-Verlag. [Google Scholar]
- 28.Furth J (1946) Prolongation of life with prevention of leukemia by thymectomy in mice. J Gerontol 1: 46–54. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan HS (1950) Influence of thymectomy, splenectomy, and gonadectomy on incidence of radiation-induced lymphoid tumors in strain C57 black mice. J Natl Cancer Inst 11: 83–90. [PubMed] [Google Scholar]
- 30.Kato A, Hays EF (1985) Development of virus-accelerated thymic lymphoma in AKR mice. J Natl Cancer Inst 75: 491–497. [PubMed] [Google Scholar]
- 31.He S, Nakada D, Morrison SJ (2009) Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol 25: 377–406. doi: 10.1146/annurev.cellbio.042308.113248 [DOI] [PubMed] [Google Scholar]
- 32.Jan M, Snyder TM, Corces-Zimmerman MR, Vyas P, Weissman IL, et al. (2012) Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med 4: 149ra118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, et al. (2014) Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 506: 328–333. doi: 10.1038/nature13038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corces-Zimmerman MR, Hong WJ, Weissman IL, Medeiros BC, Majeti R (2014) Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci U S A 111: 2548–2553. doi: 10.1073/pnas.1324297111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reitmair AH, Risley R, Bristow RG, Wilson T, Ganesh A, et al. (1997) Mutator phenotype in Msh2-deficient murine embryonic fibroblasts. Cancer Res 57: 3765–3771. [PubMed] [Google Scholar]
- 36.Reese JS, Liu L, Gerson SL (2003) Repopulating defect of mismatch repair-deficient hematopoietic stem cells. Blood 102: 1626–1633. doi: 10.1182/blood-2002-10-3035 [DOI] [PubMed] [Google Scholar]
- 37.Bryder D, Rossi DJ, Weissman IL (2006) Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol 169: 338–346. doi: 10.2353/ajpath.2006.060312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim N, Storb U (1998) The role of DNA repair in somatic hypermutation of immunoglobulin genes. J Exp Med 187: 1729–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kondo M, Weissman IL, Akashi K (1997) Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91: 661–672. [DOI] [PubMed] [Google Scholar]
- 40.Akashi K, Traver D, Miyamoto T, Weissman IL (2000) A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404: 193–197. doi: 10.1038/35004599 [DOI] [PubMed] [Google Scholar]
- 41.Mayle A, Luo M, Jeong M, Goodell MA (2013) Flow cytometry analysis of murine hematopoietic stem cells. Cytometry A 83: 27–37. doi: 10.1002/cyto.a.22093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3: 730–737. [DOI] [PubMed] [Google Scholar]
- 43.Licht JD (2001) AML1 and the AML1-ETO fusion protein in the pathogenesis of t(8;21) AML. Oncogene 20: 5660–5679. doi: 10.1038/sj.onc.1204593 [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto T, Weissman IL, Akashi K (2000) AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci U S A 97: 7521–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan Y, Zhou L, Miyamoto T, Iwasaki H, Harakawa N, et al. (2001) AML1-ETO expression is directly involved in the development of acute myeloid leukemia in the presence of additional mutations. Proc Natl Acad Sci U S A 98: 10398–10403. doi: 10.1073/pnas.171321298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiss C, Haneke T, Volker HU, Spahn M, Rosenwald A, et al. (2010) Conditional inactivation of MLH1 in thymic and naive T-cells in mice leads to a limited incidence of lymphoblastic T-cell lymphomas. Leuk Lymphoma 51: 1875–1886. doi: 10.3109/10428194.2010.510360 [DOI] [PubMed] [Google Scholar]
- 47.Belcheva A, Kolaj B, Martin A (2010) Missing mismatch repair: a key to T cell immortality. Leuk Lymphoma 51: 1777–1778. doi: 10.3109/10428194.2010.516377 [DOI] [PubMed] [Google Scholar]
- 48.Chalastanis A, Penard-Lacronique V, Svrcek M, Defaweux V, Antoine N, et al. (2010) Azathioprine-induced carcinogenesis in mice according to Msh2 genotype. J Natl Cancer Inst 102: 1731–1740. doi: 10.1093/jnci/djq389 [DOI] [PubMed] [Google Scholar]
- 49.Baross-Francis A, Milhausen MK, Andrew SE, Jevon G, Jirik FR (2000) Tumors arising in DNA mismatch repair-deficient mice show a wide variation in mutation frequency as assessed by a transgenic reporter gene. Carcinogenesis 21: 1259–1262. [PubMed] [Google Scholar]
- 50.Baross-Francis A, Andrew SE, Penney JE, Jirik FR (1998) Tumors of DNA mismatch repair-deficient hosts exhibit dramatic increases in genomic instability. Proc Natl Acad Sci U S A 95: 8739–8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang S, Lloyd R, Bowden G, Glickman BW, de Boer JG (2002) Thymic lymphomas arising in Msh2 deficient mice display a large increase in mutation frequency and an altered mutational spectrum. Mutat Res 500: 67–74. [DOI] [PubMed] [Google Scholar]
- 52.Lowsky R, Magliocco A, Ichinohasama R, Reitmair A, Scott S, et al. (2000) MSH2-deficient murine lymphomas harbor insertion/deletion mutations in the transforming growth factor beta receptor type 2 gene and display low not high frequency microsatellite instability. Blood 95: 1767–1772. [PubMed] [Google Scholar]
- 53.Leveen P, Carlsen M, Makowska A, Oddsson S, Larsson J, et al. (2005) TGF-beta type II receptor-deficient thymocytes develop normally but demonstrate increased CD8+ proliferation in vivo. Blood 106: 4234–4240. doi: 10.1182/blood-2005-05-1871 [DOI] [PubMed] [Google Scholar]
- 54.Lowsky R, DeCoteau JF, Reitmair AH, Ichinohasama R, Dong WF, et al. (1997) Defects of the mismatch repair gene MSH2 are implicated in the development of murine and human lymphoblastic lymphomas and are associated with the aberrant expression of rhombotin-2 (Lmo-2) and Tal-1 (SCL). Blood 89: 2276–2282. [PubMed] [Google Scholar]
- 55.Larson RC, Lavenir I, Larson TA, Baer R, Warren AJ, et al. (1996) Protein dimerization between Lmo2 (Rbtn2) and Tal1 alters thymocyte development and potentiates T cell tumorigenesis in transgenic mice. Embo J 15: 1021–1027. [PMC free article] [PubMed] [Google Scholar]
- 56.Aifantis I, Raetz E, Buonamici S (2008) Molecular pathogenesis of T-cell leukaemia and lymphoma. Nat Rev Immunol 8: 380–390. doi: 10.1038/nri2304 [DOI] [PubMed] [Google Scholar]
- 57.Fujiwara T, Stolker JM, Watanabe T, Rashid A, Longo P, et al. (1998) Accumulated clonal genetic alterations in familial and sporadic colorectal carcinomas with widespread instability in microsatellite sequences. Am J Pathol 153: 1063–1078. doi: 10.1016/S0002-9440(10)65651-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duval A, Hamelin R (2002) Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Cancer Res 62: 2447–2454. [PubMed] [Google Scholar]
- 59.Kim TM, Laird PW, Park PJ (2013) The landscape of microsatellite instability in colorectal and endometrial cancer genomes. Cell 155: 858–868. doi: 10.1016/j.cell.2013.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.