Abstract
In response to DNA damage during S phase, cells slow DNA replication. This slowing is orchestrated by the intra-S checkpoint and involves inhibition of origin firing and reduction of replication fork speed. Slowing of replication allows for tolerance of DNA damage and suppresses genomic instability. Although the mechanisms of origin inhibition by the intra-S checkpoint are understood, major questions remain about how the checkpoint regulates replication forks: Does the checkpoint regulate the rate of fork progression? Does the checkpoint affect all forks, or only those encountering damage? Does the checkpoint facilitate the replication of polymerase-blocking lesions? To address these questions, we have analyzed the checkpoint in the fission yeast Schizosaccharomyces pombe using a single-molecule DNA combing assay, which allows us to unambiguously separate the contribution of origin and fork regulation towards replication slowing, and allows us to investigate the behavior of individual forks. Moreover, we have interrogated the role of forks interacting with individual sites of damage by using three damaging agents—MMS, 4NQO and bleomycin—that cause similar levels of replication slowing with very different frequency of DNA lesions. We find that the checkpoint slows replication by inhibiting origin firing, but not by decreasing fork rates. However, the checkpoint appears to facilitate replication of damaged templates, allowing forks to more quickly pass lesions. Finally, using a novel analytic approach, we rigorously identify fork stalling events in our combing data and show that they play a previously unappreciated role in shaping replication kinetics in response to DNA damage.
Author summary
Faithful duplication of the genome is essential for genetic stability of organisms and species. To ensure faithful duplication, cells must be able to replicate damaged DNA. To do so, they employ checkpoints that regulate replication in response to DNA damage. However, the mechanisms by which checkpoints regulate DNA replication forks, the macromolecular machines that contain the helicases and polymerases required to unwind and copy the parental DNA, is unknown. We have used DNA combing, a single-molecule technique that allows us to monitor the progression of individual replication forks, to characterize the response of fission yeast replication forks to DNA damage that blocks the replicative polymerases. We find that forks pass most lesions with only a brief pause and that this lesion bypass is checkpoint independent. However, at a low frequency, forks stall at lesions, and that the checkpoint is required to prevent these stalls from accumulating single-stranded DNA. Our results suggest that the major role of the checkpoint is not to regulate the interaction of replication forks with DNA damage, per se, but to mitigate the consequences of fork stalling when forks are unable to successfully navigate DNA damage on their own.
Introduction
In response to DNA damage during the G1 or G2 phase of the cell cycle, DNA damage checkpoints block cell cycle progression, giving cells time to repair damage before proceeding to the next phase of the cell cycle [1,2]. The response to DNA damage during S phase is more complicated, because repair has to be coordinated with ongoing DNA replication [3]. DNA damage during S phase activates the intra-S DNA damage checkpoint, which does not completely block S-phase progression, but rather slows DNA replication, presumably allowing for replication-coupled repair [4]. Lack of the intra-S DNA damage checkpoint predisposes cells to genomic instability [5]. Nonetheless, the mechanisms by which replication is slowed, and the roles of checkpoint-dependent and -independent regulation in the S-phase DNA damage response, are not well understood.
The slowing of S phase in response to damage involves both inhibition of origin firing and reduction in fork rate [6–12]. The effect of the checkpoint on origin firing has been characterized in budding yeast and mammalian cells. The checkpoint prevents activation of late origins by targeting initiation factors required for origin firing. In mammals, checkpoint kinase 1 (Chk1) inhibits origin firing by targeting the replication kinases, cyclin-dependent kinase (CDK) and Dbf4-dependent kinase (DDK) [13–15]. In budding yeast, Rad53 targets Sld3, an origin initiation factor, and Dbf4, the regulatory subunit of DDK [16,17]. In addition to Sld3 and Dbf4, Mcm4 plays a critical role in suppressing late origin firing in response to replication stress [18].
Although checkpoint inhibition of origin firing is conserved from yeast to mammals, the contribution of origin regulation to damage tolerance is not clear. For instance, budding yeast mutants such as mec1-100, SLD3-m25 and dbf4-m25, which cannot block origin firing in response to damage, are not sensitive to damaging agents such as methyl methanesulfonate (MMS) [16,17,19,20] suggesting that checkpoint regulation of origin firing is not as critical as the checkpoint’s contribution to damage tolerance via fork regulation.
The effect of checkpoint activation on replication forks is less well understood. Because many DNA damage lesions block the replicative polymerases, for forks to pass leading-strand lesions, they must have some way to reestablish leading-strand synthesis downstream of the lesion. Recruitment of trans-lesion polymerases, template switching and leading-strand repriming have all been proposed to be involved in the fork by-pass of lesions, but which is actually used in vivo and how the checkpoint may affect that choice, is unclear [21–26].
A consistent observation is that forks slow in response to DNA damage. Whether slowing of forks in the presence of damage is checkpoint-dependent or simply due to the physical presence of lesions is not clear [27–29]. Initial work in budding yeast showed that replication forks in checkpoint mutant and wild-type cells were slowed to the same extent in the presence of damage, suggesting that slowing is checkpoint-independent [27]. However, subsequent work showed that checkpoint activation inhibited replication of damaged DNA, suggesting an active role in the slowing of replication forks [28]. Furthermore, work in mammalian cells showed a role for checkpoint signaling in DNA-damage-dependent slowing of replication forks [29]. Checkpoint regulation clearly affects fork stability [30–33], but that conclusion is largely based on the response to hydroxyurea (HU)-induced replication stress, which blocks fork progression due to deoxynucleoside triphosphate (dNTP) depletion, and thus does not directly address the role of checkpoint regulation in the replication of damaged templates.
The question of how replication forks are regulated in response to DNA damage is complicated by the difficulty of measuring fork progression rates. To directly address this question requires single-molecule resolution. Bulk assays of replication kinetics, such as the quantitation of radioactive thymidine incorporation or flow cytometry, provide only an average profile of replication kinetics, convolving the effects of origin firing and fork progression and obscuring any heterogeneity in fork slowing. The effects of DNA damage on specific origins and on forks replicating specific loci can be analyzed by gel- or sequence-based methods [9,12,27,28,34], but these techniques still only reveal the average response to DNA damage. Such approaches lack the single-molecule resolution necessary to identify heterogeneity in response to damage and to distinguish, for instance, if all forks pause briefly at all lesions or if only a fraction of forks stall, but for a longer time. Therefore, to investigate the effect of polymerase blocking lesions on individual origins and forks on a global scale, we have used a single-molecule DNA combing assay.
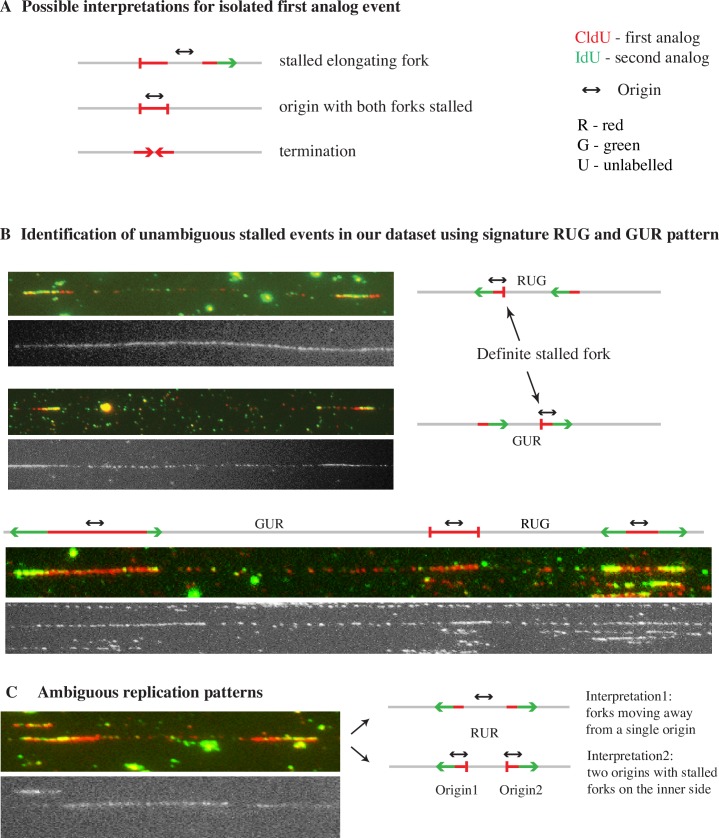
DNA combing is a single-fiber visualization technique that allows mapping of thymidine-analog incorporation patches on uniformly-stretched, megabase-length DNA fibers [35–41]. The analysis of replication kinetics by DNA combing involves isolating DNA from cells pulse-labeled in vivo with thymidine analogs. Labeled DNA is stretched on a coverslip and DNA replicated during the pulse is identified by immunofluorescence. Sequential labeling with two different analogs allows us to determine the direction and speed of replication of labeled tracks on a fiber [37,38] (Fig 1). Because combing reveals the behavior of individual replication forks, it allows us to unambiguously study the effect of checkpoint on origin firing and fork rates. In particular, it allows us to measure the heterogeneity of fork rates and determine if all forks respond the same way to lesions. The importance of measuring the heterogeneity of fork rates can be illustrated in a case where some forks pass lesions without slowing, but others stop at the lesion and do not resume replication for the duration of the experiment, a condition we refer to as fork stalling. The fork stalling is particularly difficult to analyze because stalled forks create ambiguous patterns of nucleotide incorporation which cannot be definitively interpreted in isolation [42]. However, such stalling events can, in principle, be unambiguously identified on fibers that are long enough to contain multiple replicons, allowing potentially ambiguous incorporation patterns to be definitively interpreted from the context of surrounding forks (Fig 2). We have, for the first time, developed an analysis strategy that rigorously incorporates fork stalling and used it to unambiguously quantitate fork stalling rates and the effect of checkpoint activation on fork stalling.
Fig 1.
Visualization of replication fork progression Wild-type cells (yFS940) were G1 synchronized and pulse labeled at 50 minutes after release into S phase with CldU (visualized using red antibody) for 5 minutes, and chased with IdU (visualized using green antibody) for 10 minutes. Shown are a sample fiber from the wild-type untreated dataset and the various replication patterns observed in the dataset with their simplest interpretations. See Fig 2 for other possible interpretations of ambiguous patterns.
Fig 2.
Identification of stalled forks using the context of neighboring forks (A) Ambiguity in interpreting isolated first analog events. Possible interpretations of isolated first analog event based on the neighboring event are listed. (B) The signature we have used to identify definite stalled forks: RUG (red-unlabeled-green) and GUR (green-unlabeled-red). Two forks moving in the same direction indicate that the fork moving in between them in the opposite direction must have stalled. (C) Ambiguous events such as RUR (red-unlabeled-red) and their possible interpretations.
A fundamental question about the regulation of fork progression in response to DNA damage is whether it is a global or local effect [43]. The effect of checkpoint on origins is inherently a global response because origins distant to sites of damage are blocked from firing. However slowing of forks could be a local or a global effect. If all forks are slowed by checkpoint activation, irrespective of whether they encounter damage or not, then slowing is a global effect. On the other hand, if forks are slowed only when they encounter a lesion, then it is a local effect [43]. It should be possible to distinguish between global and local regulation of fork progression by examining the effects of different lesion densities. Previously, we showed that the extent of replication slowing correlated with the density of MMS lesion, suggesting a local effect on fork progression [44]. However, we used a bulk replication assay, which did not allow us to directly observe replication fork rates.
Here, we have assayed checkpoint-dependent slowing of S phase in fission yeast in response to three DNA damaging drugs that activate the checkpoint at significantly different densities of lesions: methyl methanesulfonate (MMS), which mainly methylates purines and creates a relatively small adduct, 4-nitroquinoline 1-oxide (4NQO), which adds a quinoline group to purines resulting in a bulkier adduct [45–47] and bleomycin, which mainly creates single strand and double strand DNA breaks [48]. MMS and 4NQO create polymerase-stalling lesions and have been shown to activate the intra-S checkpoint [17,44,49–52]. The standard dose of 3.5 mM (0.03%) MMS causes about one lesion every 1 kb, whereas a physiologically similar dose of 1 μM 4NQO in CHO cells causes one lesion about every 25 kb and we estimate 16.5 μM of bleomycin causes about one double-strand break per 50 kb (S1 Fig) [53–56]. Although these are only rough approximations of lesion density, they show that forks will encounter many more MMS lesions than 4NQO lesions or bleomycin-induced double-strand breaks. This wide disparity in lesion density allows us to address differences in global and local effects of the checkpoint. In case of 4NQO, since the lesions are rare, we expect few forks to encounter lesions. Therefore, if all forks slow in response to 4NQO then we can conclude that fork regulation by checkpoint is global in nature. On the contrary, if fork regulation is a local effect then very few forks will actually encounter the lesion and slow. Similarly since the double-strand breaks caused by bleomycin are infrequent we expect very few forks to be affected by the breaks unless the checkpoint actively regulates all forks. In case of MMS, since the lesions are frequent we expect all forks to encounter lesions, and thus to slow regardless of whether slowing is a local or global effect. By comparing the effects of these three drugs, we can differentiate between global and local effects on fork regulation by the checkpoint. We find that fork slowing is a local, checkpoint-independent effect, but that persistent fork stalling plays a more significant role in replication kinetics than previously appreciated.
Results
Assaying replication slowing by DNA combing
To investigate the mechanism by which the checkpoint slows replication in response to MMS, 4NQO and bleomycin we used DNA combing. We pulsed cells in S phase with 5-chloro-2’-deoxyuridine (CldU) for 5 minutes and chased it with 5-iodo-2’-deoxyuridine (IdU) for 10 minutes, isolated and combed DNA, and visualized the CldU and IdU analogs with red and green antibodies, respectively. Fig 1 shows a sample fiber from the dataset. The fiber contains a rightward moving fork (red-green [RG]), an origin that fired during IdU labeling (green [G]), and three origins that fired during CldU labeling (green-red-green [GRG]). Further replication patterns observed in our combing dataset are shown with an interpretation of each pattern. The patterns observed were most simply interpreted as a leftward fork (green-red [GR]), a rightward fork (RG), origins that fired during CldU (GRG) or IdU (G), and terminations during IdU (red-green-red [RGR]) or CldU (red [R]). However, a more sophisticated analysis allowing the possibility of fork stalling reveals that many of these patterns—in particular termination during CldU (R)—are ambiguous, as described in the Methods section and Fig 2 and S2 Fig. For each experiment we collected about 25 Mb of DNA, which is about twice the size of the fission yeast genome, ensuring representation of most genomic loci in our analysis. From the combing data we estimated four parameters: origin firing rate, fork density, fork rate and fork stalling frequency (see Methods for details).
Identification of stalled forks in the combing dataset using the context of neighboring events
Difficulty in identifying fork stalling events in combing data arises form the fact that stalls during the first pulse can result in ambiguous analog incorporation patterns [42]. Specifically, an isolated red patch can be interpreted as an elongating fork that stalled during the first pulse or an origin with both its forks stalled or as a termination event during the first pulse (Fig 2A). Therefore, we cannot use first-pulse events alone as rigorous evidence for stalled forks. However, using double-labeled combing data we can unambiguously identify fork stalling. In particular, two neighboring forks moving in the same direction show that the fork in between them moving in the opposite direction must have stalled (Fig 2B). Thus, a red-unlabeled-green (RUG) or green-unlabeled-red (GUR) pattern is diagnostic of a fork stall. Since the fibers in our datasets average over 400 kb, we observed many neighboring forks, allowing us to robustly measure fork stalling.
Although stalled forks can be identified using the RUG and GUR patterns, some stalled forks still produce ambiguous patterns. For instance, an RUR pattern can be produced both by forks moving away from an origin and two converging forks that have stalled (Fig 2C). Thus, in order to approximate the fork stalling rate in our entire dataset, we used a probabilistic approach to estimate how many of the ambiguous patterns arise from fork stalls. We enumerated all possible incorporation patterns and classified them by the possibility that they arose from a fork stall (see Methods for details, S2 Fig). We then used the stall rate from the unambiguous signals (GUR or RUG) to calculate the likelihood of ambiguous events being due to stalled forks and used that frequency for our final estimation of stall rate for each fiber.
MMS, bleomycin and 4NQO-induced DNA damage show a similar effect on the overall replication rate
To determine the effect of MMS, bleomycin, and 4NQO—three compounds that activate the checkpoint at very different densities of lesions—on the replication rate at a population level, we analyzed cells' response to them by flow cytometry. G1 synchronized cells were released into S phase with or without DNA damage, using the commonly used dose of 3.5 mM (0.03%) MMS or a dose of 1 μM 4NQO or 16.5 μM bleomycin chosen to produce a similar slowing of bulk replication (S3 and S4 Figs). These doses of MMS, 4NQO and bleomycin cause lesions about once every 1 kb, 25 kb, and 50 kb respectively (S1 Fig) [53–56]. By flow cytometry, control cells completed replication by 80 minutes, while in the presence of either drug cells slowed replication, reaching only about 60% replicated by the end of the time course (Fig 3A and S5A Fig). Thus, despite the disparity in the number of lesions created by MMS, 4NQO and bleomycin, all three drugs led to a similar extent of replication slowing at the doses used. In all cases, the slowing in response to DNA damage was largely checkpoint-dependent. In the absence of the Cds1 checkpoint kinase, cells completed replication by 100 minutes even in the presence of damage, as reported previously (Fig 3B and S5B Fig) [52,57]. Although the three drugs appeared to have similar extent of slowing in wild-type, their effects in cds1Δ cells differed, with cds1Δ cells showing more checkpoint-independent slowing in MMS than in 4NQO or bleomycin (Fig 3B). We therefore examined if there is a difference in the mechanism by which DNA replication is slowed in response to MMS, 4NQO and bleomycin.
Fig 3.
MMS-, 4NQO and bleomycin-induced DNA damage show a similar effect on the overall replication rate (A) Wild-type cells (yFS940) show similar slowing of replication in response to 3.5 mM (0.03%) MMS, 1 μM 4NQO, and 16.5 μM bleomycin by flow cytometry. (B) Slowing in response to damage is largely checkpoint dependent by bulk assay. Wild-type (yFS940, A) and cds1Δ (yFS941, B) cells were synchronized in G1 and released into S phase in the presence or absence of 3.5 mM MMS, or 1μM 4NQO, or 16.5 μM bleomycin. S-phase progression was monitored by taking samples every 20 minutes for flow cytometry. The error bars represent standard deviation.
Inhibition of origin firing is immediate in response to 4NQO and Bleomycin, but delayed in the case of MMS
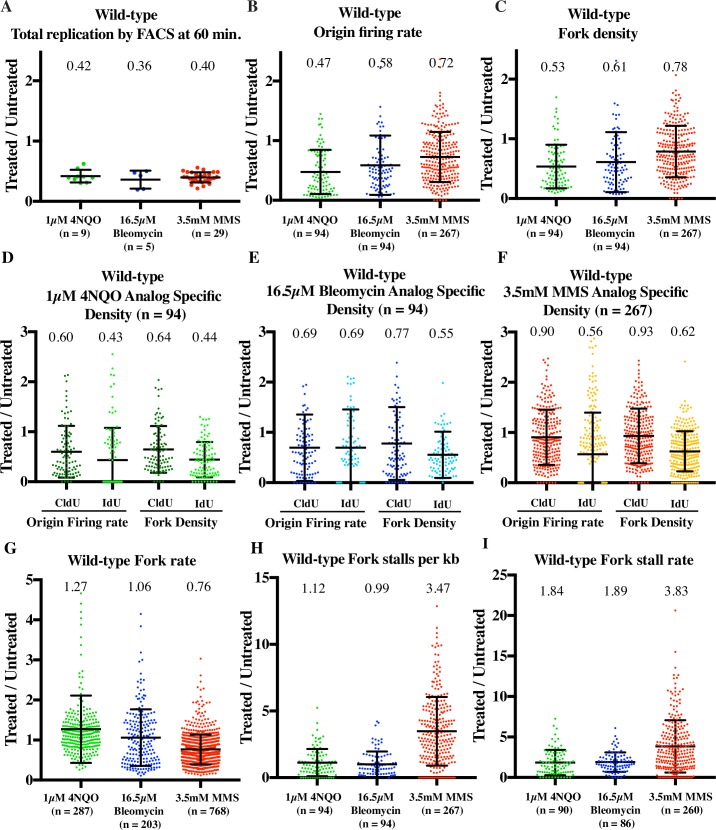
As shown in Fig 3 we see similar levels of bulk slowing for all three damage treatments at 60 minutes in S phase (Figs 3 and 4A). We first analyzed the effect of DNA damage on origin firing. To measure the rate of origin firing, we directly measured the number of new origins fired during the CldU pulse (GRG patches) or IdU pulse (isolated green patches). The overall origin firing rate was calculated as the total number of origin firing events in each fiber normalized to total length of un-replicated DNA of that fiber and to the length of the analog pulse (Fig 4B). Additionally, the origin firing rate during first analog and second analog was determined separately (Fig 4D, 4E and 4F). In untreated controls, the rate of origin firing in the first analog was 2.3±0.7 origins per Mb per minute, similar to previous estimates of origin firing rates (S1 Table) [58].
Fig 4. 4NQO, MMS and bleomycin slow S phase using distinct mechanisms.
Wild-type cells (yFS940) were synchronized in G1 and released into S phase untreated, or treated with 3.5 mM MMS, or 1μM 4NQO, or 16.5 μM bleomycin. Cells were labeled at mid-S phase with CldU followed by IdU for DNA combing. All parameters are represented as a ratio of treated v. untreated sample. (A) 4NQO, bleomycin and MMS slow S phase to similar extent by flow cytometry. Total replication by FACS was calculated at 60 minutes after release, which is close to the mid-point of analog labeling. (B) and (C) 4NQO, bleomycin and MMS show differing levels of reduction in origin firing rate and fork density. (D), (E), (F) Analog specific estimations of origin firing rate and fork density for 4NQO, bleomycin and MMS respectively. 4NQO and bleomycin treatment leads to immediate reduction in origin firing rate in the first analog while in MMS the reduction is apparent only during the second analog. (G) Fork rate decreases in response to MMS, but not in response to 4NQO or bleomycin. (H) Fork stalls per kb increases in response to MMS, but not in response to 4NQO or bleomycin (I) Fork stall rate increases in response to MMS, 4NQO and bleomycin. For calculations of origin firing rate, fork density, fork rate, fork stalls per kb and fork stall rate refer to text and methods. For each sample of each experiment, about 25Mb of DNA was collected. All error bars represent SD.
In the presence of 4NQO, the origin firing rate in wild-type cells decreased to 47% of the untreated control (p = 5.17x10-12, t tests were used for all statistical tests) (Fig 4B and S1 Table). Since, the origin firing rate only measures the origins that fired during the analog pulses, we also measured the density of active forks in the datasets in order to estimate the effect on origin firing prior to the analog pulses (see Methods for details). Fork density in 4NQO-treated cells was 64% of the untreated control (p = 1.28x10-6) during the first analog pulse and 44% (p = 3.08x10-13) in the second, consistent with the trend seen in origin firing rates (Fig 4D). Thus, the response to 4NQO included an immediate reduction in origin firing rate. We see a similar trend for bleomycin treated sample. The origin firing rate decreases to 58% (p = 7.45x10-5) and the fork density decreases to 61% (p = 1.33x10-5) (Fig 4B and 4C). Analog specific estimation shows that bleomycin treatment leads to reduction in the origin firing rate in the first analog as well as second analog to 69% (3.91x10-4) and shows a corresponding decrease in fork density during the first (77%, p = 7.65x10-4) and the second analog (55%, p = 8.54x10-7) (Fig 4E).
In case of MMS, the overall origin firing rate was reduced to 72% (p = 1.18x10-6) (Fig 4B). Analyzing origin firing during the first and second pulse separately, we saw no statistically significant reduction in the first analog (90%, p = 0.0748) followed by a stronger reduction to 56% (p = 7.15x10-11) in the second analog (Fig 4F). Therefore, the effect of MMS on origin firing rate is delayed. This conclusion was supported by two observations. Firstly, the average fork density across both analogs in MMS showed a modest reduction to 78% (p = 2.59x10-5) as compared to 53% (p = 5.7x10-12) in 4NQO (Fig 4C). Second, the analog-specific fork density estimations for MMS followed a similar trend as the origin firing rate showing a greater reduction during the second analog (0.93 v. 0.62, Fig 4F). Hence, the effect of MMS-induced damage on origin firing is only manifest late in S-phase, after significant bulk slowing has already occurred, whereas 4NQO- and bleomycin-induced damage inhibit origin firing immediately in early S phase.
Fork rate declines in response to MMS but not 4NQO or bleomycin
Since 4NQO and MMS both create polymerase-blocking lesions [17,49–51], we next studied how these drugs affect fork speed. We measured fork rate in the combing data as the length of the green track (second analog) continuing from a red track (first analog), divided by the length of the chase time (10 minutes) (Fig 1). The average fork rate in our untreated samples was 0.91±0.02 kb/minute, which is within the range of previous estimates [42,59–62].
In MMS, the fork rate decreases to 76% of the untreated control (p = 6.13x10-38) (Fig 4G and S9 Fig). The reduction in fork rate was expected since the lesions are so frequent that every fork encounters about ten of lesions during the 10 minutes pulse. In case of 4NQO, the lesions are significantly less common, so only about half of the forks should encounter a lesion during the second pulse. Consistent with the low density of lesions, fork rates in 4NQO treated cells were similar to untreated cells (Fig 4G). In fact, the fork rate showed a 27% increase in the presence of 4NQO as compared to untreated cells (p = 5.75x10-4). The dose of bleomycin used appears to create about one break per 50 kb (S1B Fig). However, this estimate of lesion density may be an overestimation, because we have analyzed fibers on average 450 kb in length in bleomycin-treated wild-type samples by combing. Regardless, we do not see any statistically significant reduction in fork rate for bleomycin treated sample as compared to untreated sample (p = 0.3138) (Fig 4G).
Fork stalling increases in response to MMS, 4NQO and bleomycin
In case of MMS treated sample, we only see a modest reduction in fork density as compared to 4NQO and bleomycin (Fig 4C). In particular, there is no statistically significant reduction in fork density in the MMS treated sample during the first analog (93%, p = 0.1951) (Fig 4F). However by bulk assay we see the same kinetics of slowing for all three damage treatments (Figs 3A and 4A). A possible explanation for this discrepancy is that forks stall during the first pulse in response to damage and thus are not observed during the second pulse (Fig 2). We therefore interrogated our combing data for evidence of fork stalling.
We first determined the absolute number of stalls per kb for each dataset (see Methods for details). The absolute number of stalls per kb determines the contribution of stalled forks towards total replication slowing. In MMS treated samples, we see a 3.47-fold increase in the number of stalls as compared to untreated (p = 6.23x10-27), while we see no significant increase in stalling in response to 4NQO (1.12, p = 0.604) and bleomycin (0.99, p = 0.346) (Fig 4H). Thus an increase in the total number of stalls per kb in response to MMS helps explain the slowing of total replication to similar levels as compared to 4NQO and bleomycin despite having a delayed effect on origin firing rate.
Next we estimated the fork stall rate. We define the fork stall rate as the total number of stall events per fiber before and during the first analog pulse divided by the total number of ongoing forks in that fiber during the first analog. Although the absolute number of stalls per kb in response to 4NQO and bleomycin treatment is similar to the wild-type untreated sample, the treated samples have far fewer origins firing as compared to untreated sample (Fig 4B and 4H). Therefore the rate of stalling normalized to the origin firing rate is higher in the treated sample (Fig 4I). The average stall rate per fiber in the untreated sample was 14%. The fork stall rate showed a 1.8-fold increase in response to 4NQO (p = 1.2x10-4) and a 1.9-fold increase in response to bleomycin (p = 2.26x10-7), whereas MMS caused a 3.8-fold increase relative to untreated cells (p = 1.55x10-26) (Fig 4I). Combining our stall-rate data with estimates of lesion density, we estimate that forks stall at 5% of 4NQO lesions and at 0.5% of MMS lesions, consistent with the fact that 4NQO creates a bulkier lesion than MMS. The stall rate per lesion is more complicated to interpret for bleomycin. Since the stalls we detect are not at the ends of our fibers, they cannot be at the sites of bleomycin-induced double-strand breaks. However, bleomycin is reported to create a 6 to 20-fold excess of single-strand nicks to double strand breaks, which, at the dose we used should produce one nick about every 2.5–9 kb (S1B Fig) [48]. Since we expect that every leading-strand nick will lead to fork collapse, resulting in a single-ended DSB on one strand and a terminated nascent strand, which we would score as a fork stall, on the other, we presume that most nicks are rapidly repaired and estimate that forks encounter bleomycin-induced leading-strand nicks about every 300 kb (S1 Table). Although the stall events seem to be infrequent relative to lesion density, approximately 53% of forks in MMS-treated cells and 25% of forks in 4NQO- and bleomycin-treated cells stalled, contributing significantly to slowing, and ensuring that all treated cells had many stalled forks.
Inhibition of origin firing is checkpoint dependent
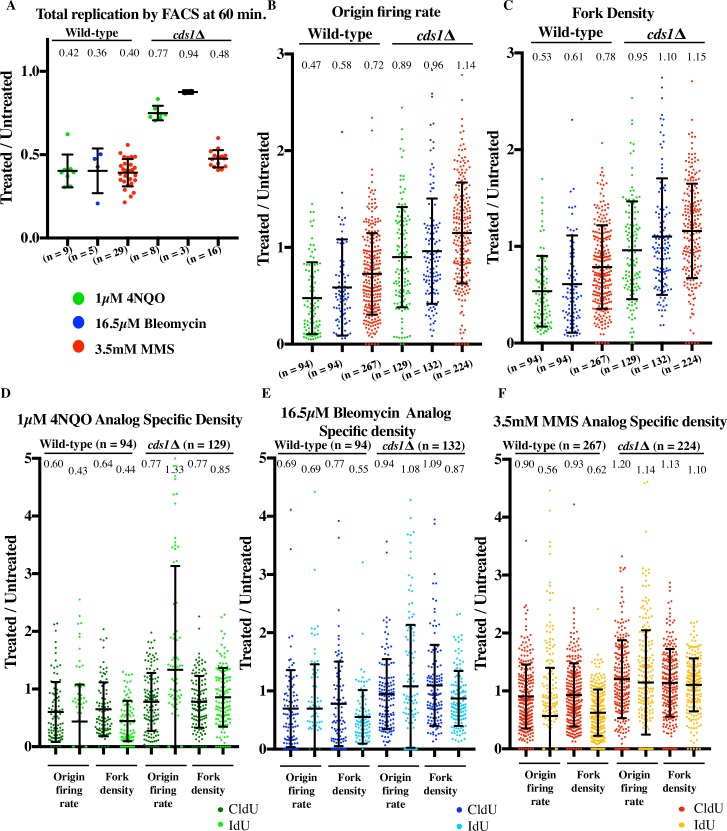
To determine the role of the intra-S checkpoint in the observed DNA-damage dependent changes in replication kinetics, we repeated our combing experiments in checkpoint-deficient cds1Δ cells. In the presence of 4NQO, wild-type cells replicated 42% (p = 5.55x10-16) as much as untreated cells, as assayed by flow cytometry at 60 minutes after release, whereas, in the absence of checkpoint, cds1Δ 4NQO treated cells replicated 77% as compared to untreated cells (p = 5.82x10-8) (Fig 5A). 4NQO did not significantly reduce origin firing in cds1Δ cells (89%, p = 0.206), as opposed to 47% in wild-type cells (p = 5.17x10-12) (Fig 5B). Thus, the inhibition of origin firing in response to 4NQO is checkpoint-dependent (Figs 4B and 5B). Analog specific origin density and fork density followed similar trends as the total origin firing rate data (Fig 5D). During both the analog pulses, cds1Δ cells had a higher origin firing rate and fork density than wild-type cells (Fig 5D).
Fig 5. Reduction in origin firing rate is checkpoint dependent.
cds1Δ cells (yFS941) were synchronized in G1 and released into S phase untreated, or treated with 3.5 mM MMS, or 1μM 4NQO, or 16.5 μM bleomycin. Cells were labeled at mid-S phase with CldU followed by IdU for DNA combing. All parameters from combing data of cds1Δ are represented as a ratio of treated v. untreated. Wild-type dataset is plotted alongside cds1Δ for comparison. (A) Total replication by FACS was calculated at 60 minutes after release, which is close to the mid-point of analog labeling. (B) and (C) Reduction in origin firing and fork density is checkpoint dependent for 4NQO, bleomycin and MMS. (D, E, F) Analog specific estimations of origin firing rate and fork density for 4NQO, bleomycin and MMS respectively shows higher values in cds1Δ than wild-type. For calculations of origin firing rate, fork density refer to text and methods. For each sample of each experiment, about 25Mb of DNA was collected. All error bars represent SD.
Likewise, inhibition of origin firing in response to bleomycin and MMS is checkpoint-dependent (Fig 5E and 5F). In wild-type cells, the overall origin firing rate was reduced to 58% by bleomycin (p = 7.45x10-5) and 72% by MMS (p = 1.18x10-6) treatment (Figs 4B and 5B). In contrast, cds1Δ cells showed no significant decrease in origin firing relative to untreated cells when treated with bleomycin (96%, p = 0.389) or MMS (114%, p = 0.011) (Fig 5B). Analog specific estimation of fork density and origin firing rate in cds1Δ treated with bleomycin or MMS showed a similar trend (Fig 5E and 5F). cds1Δ cells had higher fork density and origin firing rate in response to bleomycin and MMS than wild-type cells for both analog pulses (Fig 5E and 5F).
In the absence of DNA damage, the lack of Cds1 caused a 51% increase in the rate of origin firing (3.5±0.6 v. 2.3±0.6 origins firing/Mb/minute, S1 Table), consistent with previous reports in other systems of checkpoint inhibition of origin firing in unperturbed S phase [62,63]. Nonetheless, loss of Cds1 increases origin firing in damaged cells to the same level as in undamaged cells, showing that there is no checkpoint independent inhibition of origin firing (S6 Fig).
Reduction in fork rate is checkpoint independent
In MMS-treated wild-type cells, fork rate was reduced to 76% of the untreated cells (p = 6.14x10-38) (Figs 4G and 6A). This effect was not checkpoint-dependent. In fact, at 61% (p = 6.13x10-66), cds1Δ cells showed a greater reduction in fork rate than seen in wild-type cells in response to MMS (Fig 6A). Thus, the observed reduction in fork rate in response to MMS seems to be due to the physical presence of the lesions and the checkpoint activation may facilitate efficient by-pass of the lesions.
Fig 6. Reduction in fork rate and increase in fork stalling is checkpoint independent.
(A) Fork rate, (B) fork stalls per kb and (C) fork stall rate data for cds1Δ (yFS941) treated with 4NQO, bleomycin and MMS. All three parameters are represented as a ratio of treated v. untreated. Wild-type dataset is plotted alongside cds1Δ for comparison. See Methods for calculation of fork rate, fork stall per kb and fork stall rate.
Fork rates showed similar increase in cds1Δ cells treated with 4NQO (126%, p = 1.17x10-5) as they did in wild-type cells (127%, p = 5.75x10-4) (Figs 4G and 6A). Although the lack of Cds1 does not seem to have an effect on the relative fork rate between untreated and 4NQO-treated cells, it does have a significant effect on the absolute fork rate in untreated cells. Forks moved significantly slower in untreated cds1Δ cells than in wild-type cells (0.72 v 0.91 kb/min, p < 10−9, S1 Table). This difference may be an indirect effect of the higher origin firing rate and fork density in cds1Δ cells (S1 Table). Several groups working in different systems have made the similar observation that fork rate is inversely correlated to the number of active forks, perhaps due to constrains on a limiting factor required for replication, such as the dNTP pool [64–66].
Fork stalling in response to damage is largely checkpoint independent
Similar to wild-type cells, we see an increase in fork stalls per kb in response to MMS treatment in cds1Δ but not in case of 4NQO and bleomycin (Figs 4H and 6B). The fork stalls per kb shows a 1.9-fold increase in response to MMS as compared to untreated (p = 4.42x10-10) cds1Δ cells. Fork stalls per kb does not change significantly between 4NQO (1.39, p = 0.987) and bleomycin (1.05, p = 0.839) treated cds1Δ cells as compared to untreated cds1Δ (Fig 6B).
In wild-type cells, the fork stalling rate was increased 1.8-fold by 4NQO (p = 1.2x10-4), 1.9-fold by bleomycin (p = 2.26x10-7) and 3.8-fold (p = 1.55x10-26) by MMS treatment, relative to untreated cells (Figs 4H and 6C). In cds1Δ cells, we saw a 1.7-, 2.3- and 2.8-fold increase of the stall rate in 4NQO (p = 2.59x10-4), bleomycin (p = 7.96x10-13) and MMS (p = 2.93x10-15) treated cells respectively as compared to untreated cells (Fig 6C). This increase in stall rate is slightly lower in MMS-treated cds1Δ cells than in wild-type cells (2.8 fold v 3.8 fold, p = 9.86x10-5), showing that there are checkpoint-dependent and -independent contributions to stalling in response to MMS, however the differences in stall rates for cds1Δ cells treated with 4NQO (1.7 fold v 1.8 fold, p = 0.746) and bleomycin (2.3 fold v 1.89 fold, p = 0.01) as compared to wild-type cells are less pronounced. Therefore the bulk of fork stalling events appears to be checkpoint-independent (Fig 6C).
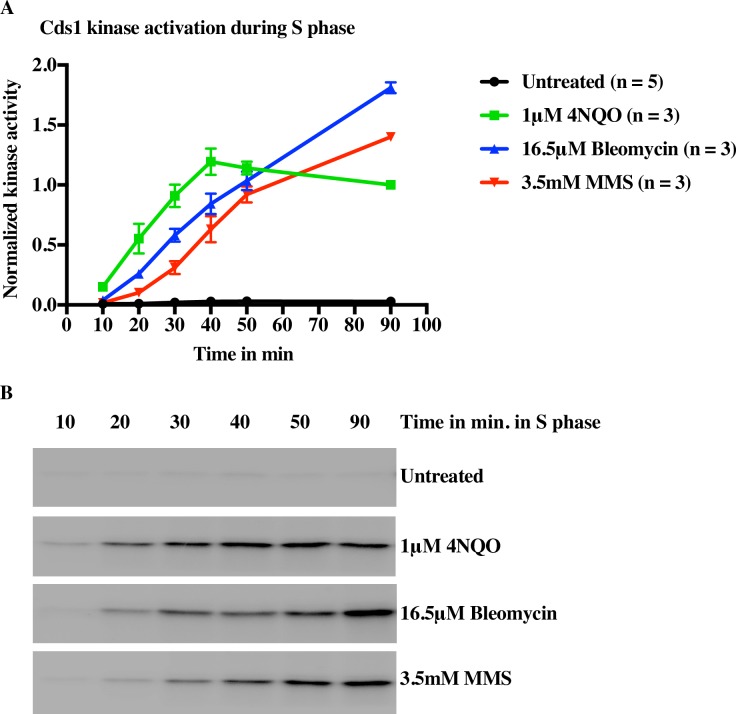
Delayed inhibition of origin firing by MMS correlates with delayed checkpoint kinase activation
To test the possibility that the later inhibition of origin firing seen in response to MMS (Fig 4F) is due to delayed checkpoint activation, and to confirm that the doses of the various damaging agents we use cause comparable checkpoint activation, we assayed activation of the Cds1 S-phase checkpoint kinase in response to MMS, 4NQO and bleomycin. Cells with an HA-tagged Cds1 were synchronized in G1 and released into DNA damage (S7 Fig). Cells were harvested throughout S phase and Cds1 was immunopurified for in vitro kinase assays (Fig 7). We observe a significant and reproducible delay of Cds1 activation in response to MMS, relative to both 4NQO and bleomycin. These results are consistent with MMS taking longer to disrupt a sufficient number of replication forks to trigger a robust checkpoint response.
Fig 7.
Delayed Inhibition of Origin Firing by MMS Correlates with Delayed Checkpoint Kinase Activation A) Wild-type cells with an HA-tagged Cds1 (yFS988) were G1 synchronized, released into S phase is the presence of 1μM 4NQO, 16.5 μM bleomycin or 3.5 mM MMS, harvested at the indicated times and processed for Cds1 IP kinase assays. Average signal normalized to the 4NQO 90-minute timepoint is plotted ± standard error of the mean. B) Representative kinase assays. See S8 Fig for data from each replicate.
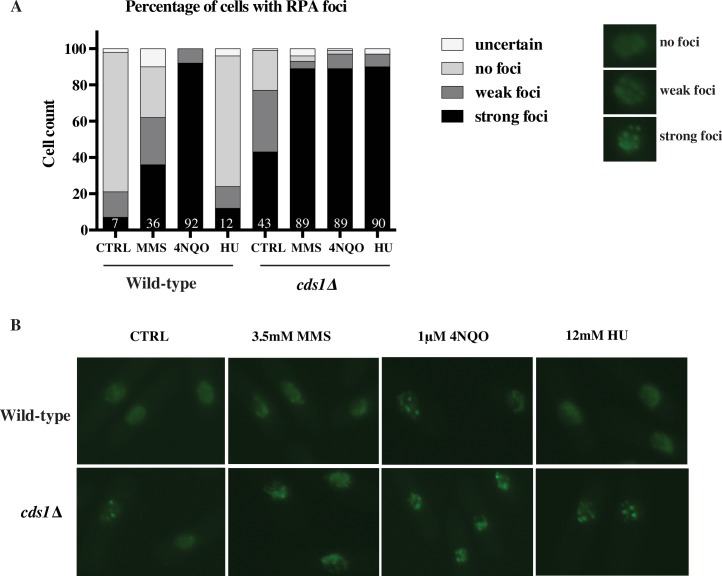
Accumulation of RPA foci in response to 4NQO and MMS
To investigate the relationship between the fork stalling that we measured by DNA combing and the fate of forks in living cells, we visualized replication protein A (RPA)-GFP foci, which mark the accumulation of single-stranded DNA (ssDNA), in cells treated with MMS or 4NQO. Because RPA-coated single-stranded DNA is a trigger for the activation of the intra-S checkpoint [67–71], we hypothesized that stalls that accumulate substantial amounts of RPA are likely to correlate with checkpoint activation, but that stalls that lead to less RPA accumulation may be checkpoint silent.
We analyzed 100 cells for each sample and sorted them into three categories based on the intensity and number of foci: strong foci, weak foci or no foci (see Methods for details). 21% of untreated wild-type cells have some foci, consistent with previous reports [72], but the majority of these are weak foci, consistent with the hypothesis that weak foci do not activate the checkpoint (Fig 8A). In the presence of damage, we saw distinctly different responses to MMS and 4NQO. In the presence of MMS, 36% of cells had strong foci (Fig 8A). Since all MMS-treated cells have stalled forks (S1 Table), we conclude that only a minority of MMS-induced stalls accumulate substantial ssDNA. On the contrary, essentially all 4NQO-treated cells had strong foci, suggesting a qualitatively different nature of 4NQO-induced fork stalls, in which the majority of 4NQO-induced lesions accumulated substantial ssDNA (Fig 8A). As previously reported [73], RPA did not accumulate at HU-stalled forks in checkpoint-proficient cells.
Fig 8. Accumulation of RPA foci in response to 4NQO and MMS.
(A) Percentage of cells with RPA foci in each sample with the numbers on the bars indicating the percentage of cells with strong foci. Wild-type (yFS956) and cds1Δ (yFS957) cells were synchronized and released into S phase in the presence of 3.5 mM MMS, 1 μM 4NQO or 12 mM HU or left untreated. Samples were collected for microscopy at 100 minutes after release. Cells were fixed and stained with DAPI and imaged in the DIC, GFP and DAPI channels. For each sample 100 cells were blinded and scored independently by two individuals. (B) Representative images for all samples quantified in (A).
In the absence of checkpoint all treated samples displayed strong foci in about 90% of cells. In particular, in MMS and HU treated cells we saw a large increase in the number of cells with strong foci in cds1Δ as compared to wild-type, suggesting that in response to MMS treatment the checkpoint plays a role in preventing accumulation of excess ssDNA at stalled forks, as it does in HU [73,74] (Fig 8A).
Discussion
The regulation of DNA replication in response to DNA damage involves both inhibition of origin firing and reduction of fork speed, but relative contributions of the two effects have been unclear. Furthermore, although genetic evidence has suggested that the checkpoint regulation of fork progression is critical for genomic stability, it has been unclear if the checkpoint regulates fork speed, per se, and, if so, whether the regulation is at a global or local level [11,28,29,43,75]. One of the reasons that these questions have persisted is that bulk methods, such as gel- or sequence-based approaches, provide an average profile of checkpoint regulation of origins and forks in response to damage and often convolve origin and fork dynamics. We have used DNA combing in fission yeast to systematically look at the effect of the checkpoint on individual origins and forks at a global scale and hence to capture the heterogeneity in response to damage. To understand the behavior of the checkpoint at a global and local scale we have used three different types of lesion inducing agents at vastly differing concentrations—3.5mM (0.03%) for MMS, 1μM for 4NQO and 16.5 μM for bleomycin—which thus produce very different densities of lesions—1000 per Mb for MMS, 40 per Mb for 4NQO and 20 per Mb for bleomycin (S1 Fig) [53–56]—but nonetheless have very similar effects on bulk replication kinetics (Fig 3, S3 and S4 Figs). We find that the inhibition of origin firing is a checkpoint-dependent response, but one that develops more slowly in response to the many fork encounters with MMS than the less frequent but more severe fork interactions with 4NQO lesions or in response to repair of bleomycin-induced double-strand breaks. The slowing of fork progression, in contrast, is checkpoint-independent and therefore a local effect in which forks only slow when they encounter DNA damage. Finally, we discover that fork stalling, an event in which a fork stops and does not resume for the duration of the experiment, plays a significant role, with as many as 53% of forks stalling in response to DNA damage caused by MMS.
Inhibition of origin firing is a global, checkpoint-dependent response to interactions of forks with DNA damage
Consistent with previous studies from S. cerevisiae [9] and human cells [7,8,15] reduction in origin firing in response to 4NQO, bleomycin and MMS is checkpoint-dependent. By flow cytometry, 4NQO, bleomycin and MMS lead to similar extent of slowing, however the combing data reveals important differences in the regulation of origin firing in response to the two drugs. In case of 4NQO and bleomycin reduction in origin firing is robust and immediate. The fork density during the first analog, which is a proxy for origin firing occurring prior to analog labeling, decreases to 64% (p = 1.28x10-6) in case of 4NQO and 77% (p = 7.65x10-4) in case of bleomycin, as compared to untreated cells (Fig 4D and 4E). Therefore, origin firing inhibition starts early in S phase in response to 4NQO and bleomycin. On the contrary, in the case of MMS, fork density in the first analog is barely reduced (93%, p = 0.195) suggesting no significant reduction in origin firing during early S phase (Fig 4F). Furthermore, even during the second analog, the fork density is 40% higher in MMS than in 4NQO (62% v. 44%, Fig 4D and 4F). Therefore reduction of origin firing in MMS is delayed and modest as compared to 4NQO.
The disparity in origin firing inhibition response can be explained by the observation that a threshold of damage has to be met for the activation of the intra-S checkpoint. A certain number of arrested forks are necessary for checkpoint activation during S phase [76]. Therefore robust reduction in origin firing in response to 4NQO in early S phase suggests that 4NQO lesions, although less frequent than MMS lesion at the concentrations used in our studies, have more severe effects on forks and hence are more efficient at activating the checkpoint than MMS. Consistent with this interpretation, 4NQO lesions lead to a 10- fold higher rate of fork stalls per lesion than MMS (5% v. 0.5%) as detected by combing. The low density of stalls per lesion in response to MMS suggests that MMS takes longer to induce sufficient fork stalls to activate a checkpoint signal, and explains why the checkpoint mainly inhibits origin firing later in S phase. Our analysis of RPA accumulation is also consistent with this interpretation, showing that 4NQO-induced lesions accumulate RPA to a much greater extent than MMS-induced lesions (Fig 8).
Reduction in fork rate is a local checkpoint-independent response to interactions of forks with DNA damage
The fork rate in wild-type cells is reduced in response to MMS to 76% (p = 6.14x10-38) (Fig 4G). The fork rate is also reduced to 61% in cds1Δ cells treated with MMS (p = 6.13x10-66) (Fig 6A) consistent with previous reports [27]. Thus, reduction in fork rate is checkpoint-independent and seems to be simply due to the physical presence of the lesions. In fact, by combing we see a slower fork rate in response to MMS in cds1Δ cells than in wild-type cells as compared to untreated (61% vs 76%, Fig 6A). The previously discussed correlation between fork rate and fork density in undamaged cells notwithstanding [66], we do not believe this effect in MMS-treated cds1Δ cells is an indirect consequence of the increased origin firing, because we do not see a similar decrease in fork rate in the 4NQO-treated cells, which show much greater increase in origin firing and fork density in cds1Δ cells relative to wild-type cells (Figs 6A, 5D and 5F). Instead, we prefer the interpretation that the checkpoint facilitates fork progression across a damaged template. Recent work shows that even in the absence of the checkpoint, the replisome is intact at stalled forks [77]. Therefore, it has been speculated that the checkpoint does not affect the stability of the replisome per se, but instead helps maintain the replisome in a replication competent state at sites of DNA damage [78], a possibility supported by our data. In contrast, previous studies have reported similar extent of slowing in both wild-type and the checkpoint mutants in response to MMS [27,28]. One explanation for the discrepancy could be that the previous methods—density transfer approach and BrdU-IP-seq—offer an average profile at lower resolution and mask the difference between wild-type and checkpoint-deficient cells.
Reduction of fork rate in response to MMS but not to 4NQO supports the model that slowing of forks is simply due to a transient physical slowing of forks at each lesion encountered and is not due to a global checkpoint-dependent effect on fork progression rates. Consistent with this model, we do not see a reduction in fork rate in response to bleomycin treatment (1.06, p = 0.31), which activates the checkpoint robustly (Figs 4G and 7). This result is consistent with our previous observations that slowing is dependent on MMS dose [44] and occurs in response to frequent UV-induced lesions, but not rare IR-induced double-strand breaks [57]. Since the lesions induced by 4NQO and bleomycin are 25 and 50 times rarer than those caused by MMS, the forks are less likely to encounter damage and slow down in 4NQO-treated cells. The corollary to this conclusion is that activation of the checkpoint does not slow replication forks, as demonstrated by the fact that forks in 4NQO- and bleomycin-treated cells fail to slow despite the strong Cds1 activation and checkpoint-dependent inhibition of origin function.
Fork stalling is a qualitatively different response to damage than fork slowing
The interaction of a fork with a DNA lesion is a first step towards recognition of damaged template during S phase and is a critical mechanism for checkpoint activation [67]. Activation of the intra-S checkpoint by stalled forks allows the cell to activate repair pathways, tolerate damage and prevent genomic instability [68]. Although it is believed that forks can pass polymerase-blocking lesions on the leading strand using translesion polymerases, leading-strand repriming or recombinational lesion bypass, the role of the checkpoint in such responses is unclear [21–26].
Here we show that forks can bypass both MMS- and 4NQO-induced lesions and that the checkpoint is not required for that bypass. However, in the case of MMS-induced lesion, the checkpoint seems to facilitate bypass, since forks move past lesions more slowly in cds1Δ cells as compared to wild-type (0.61% v. 0.76%, Fig 6A). We also show that, whereas forks can bypass most lesions efficiently, at a small fraction of lesions—0.5% of MMS-induced lesions and 5% of 4NQO-induces lesions—forks stall for the duration of the experiment. Given the large number of lesions throughout the genome, even these small numbers lead to a large number of stalled forks: 53% in MMS-treated cells and 25% in 4NQO- and bleomycin-treated cells. However, since any stall that occurs prior to the beginning of the second label will be identified by our analysis (see Methods for details), only a fraction of the stalls will occur during either of the labeling pulses.
Detection of fork stalling events is complicated due to lack of consensus on how to identify them in the DNA fiber datasets [42]. Generally, signal from the first (red) analog alone on a fiber (a unlabeled-red-unlabeled [URU] event) is presumed to be either an elongating fork that stalled or an origin that fired in the first pulse followed by stalling of both its forks (Fig 2A) [7,42,60,79,80]. Alternatively the events from the first analog alone can be interpreted as terminations (Fig 2A) [42,81–83]. However, both interpretations rely on heuristic arguments and are unable to quantitate ambiguous signals, such as URU. Therefore we developed a new, rigorous way of quantitating stalled forks in double-labeled data. We have used the context in which the first analog event occurs to define it as a stalled fork or not. As discussed in greater detail in the Results and Methods sections we have used RUG and GUR patterns as a diagnostic for fork stall event occurring during the first analog (Fig 2B). We then used a probabilistic approach to quantitate the frequency of stall events in other ambiguous patterns (Fig 2C and S2 Fig). It should be noted that we can detect a stall only if it occurs before or during the first (red) analog pulse and persists throughout the second (green) analog pulse.
Fork stalling does not appear to be simply an extreme example of the transient fork pausing that leads to observed fork slowing. If it were, we would expect to see a continuum of fork pause lengths form very short pauses to full stalls. Such heterogeneity would lead to a greater variation in apparent fork speeds and, in particular an increase in the asymmetry of rates in fork pairs, neither of which we see (S9 and S10 Figs and S1 Table). Therefore, we conclude that there are two distinct possible fate for a fork that encounters damage. It can pause briefly as it bypasses the lesion or it can stall permanently. A permanent stall does not appear to be a catastrophic event, as a majority of MMS-treated cells, which all have many stalls (S1 Table), do not have strong RPA foci (Fig 8A). However, such stalls do appear to require the checkpoint to restrain ssDNA accumulation. 4NQO-induced lesions appear to cause more severe stalls, since 4NQO-treated cells have many more strong RPA foci, even though they have fewer stalls (S1 Table and Fig 8A).
The replication fork dynamics that we observe in response to MMS- and 4NQO-induced DNA damage demonstrate that forks interact with DNA damage largely in a checkpoint-independent manner. Forks are able to bypass lesions that stall the replicative polymerases with only a modest reduction in speed (Fig 6A) and are no more prone to stall at lesions in the absence of checkpoint (Fig 6B and 6C). However, stalled forks do appear to accumulate more ssDNA in the absence of the checkpoint (Fig 8). These results suggest that the major role of the checkpoint is not to regulate the interaction of replication forks with DNA damage, per se, but to mitigate the consequences of fork stalling when forks are unable to successfully navigate DNA damage on their own.
Materials and methods
General methods
The following strains used in this study were created by standard methods and grown in YES at 25°C [84]: yFS105 (h- leu1-32 ura4-D18), yFS940 (h+ ura4-D18 his7-366 cdc10-M17 leu1::pFS181 (leu1 adh1:hENT1) pJL218 (his7 adh1:tk)), yFS941 (h- ura4-D18 his7-366 cdc10-M17 cds1::kanMX leu1-32::pFS181(leu1 adh1:hENT1) pJL218 (his7 adh1:tk)), yFS956 (h+ leu1-32 ura4-D18 cdc10-M17 rpa1-GFP::hph-MX6), yFS957 (h+ leu1-32 ura4-D18 cdc10-M17 cds1::ura4 rpa1-GFP::hph-MX6), yFS988 (h- ura4-D18 ade-? cdc10-M17 leu1-32::pYJ294(leu1 cds1-6his2HA).
S phase progression assay by flow cytometry
Cells were synchronized in G1 phase using cdc10-M17 temperature sensitive allele combined with centrifugal elutriation, which selects cells that have been arrested in G1 for as little time as possible [85]. Cells were grown to mid log phase at 25°C and arrested at 35°C for 2 hours followed by centrifugal-elutriation-based size selection at 35°C to collect cells that had most recently arrested in G1. The cells were then immediately released into S phase by shifting them to 25°C, untreated or treated with 3.5mM MMS or 1 μM 4NQO or 16.5 μM bleomycin. S-phase progression was followed by flow cytometry using a nuclei isolation protocol, as previously described [85] with some minor modifications. Briefly, 0.6 O.D. of cells were pelleted every 20 minutes for 2 hours after release into S phase. Pelleted cells were fixed by resuspension in 70% ethanol and stored overnight at 4°C. Fixed cells were spheroplasted at 37°C for 1 hour in 0.6 M KCl with 1 mg/ml Lysing enzyme (Sigma # L1412) and 0.5 mg/ml Zymolyase 20T (Sunrise Science Products # N0766391). Cells were then washed with 0.1 M KCl containing 0.1% Triton X-100 followed by 20 mM Tris-HCl, 5 mM EDTA, pH 8.0. The cells were then resuspended in 20 mM Tris-HCl, 5 mM EDTA, pH 8.0 containing 250 μg/ml RNaseA and incubated at 37°C overnight. Cells were pelleted, chilled and sonicated for 7 seconds with a Sonifier (Branson Sonifier 450) equipped with a micro tip at power 5 and constant duty cycle to release nuclei. Nuclei were mixed with equal amount of 1x PBS containing 2 μM Sytox Green and analyzed by flow cytometry. S-phase progression values were obtained from the histograms as previously described [85].
We have used three different drugs (3.5mM MMS, 1 μM 4NQO and 16.5 μM bleomycin) to create significantly differing lesion densities (of 1 every 1 kb, 25 kb or 50 kb, respectively) in order to differentiate between global v. local regulation of forks. Conceivably, variable lesion density could be achieved by just titrating one of the drugs. However, we cannot achieve a 25-fold difference in lesion density by just titrating either drug alone. In case of 4NQO, increasing dose concentration to even 2 μM 4NQO almost inhibits replication (S3 Fig), while decreasing MMS dose below 3.5mM to 0.875mM greatly reduces the effect of damage on replication kinetics [44].
Estimation of lesion density
Cells were harvested from asynchronous log-phase cultures of yFS105 untreated or treated with 3.5mM MMS for 1 hour. Genomic DNA was isolated by bead beating, followed by phenol-chloroform extraction and ethanol precipitation of DNA [84]. The genomic DNA was digested with EcoRV and ScaI at 37°C for 6–8 hours. The DNA was then heated at 65°C for 2 hours to create nicks at sites of MMS-induced lesions [53]. Restriction fragments were analyzed by alkaline agarose gel electrophoresis (0.8%). The lanes were quantified using Image J software. The ratio of DNA molecules at peak1 and 2 remaining in the MMS-treated sample relative to the untreated samples was used to infer the lesion density, assuming a Poisson distribution of MMS-induced lesions and calculating the frequency of nicks predicted to result in the observed fraction of molecules with no nicks (S1A Fig). We have used the value estimated from peak1 (1 lesion per kb) for calculation. We maybe under-estimating the lesion density because we are not certain about the efficiency with which the 65°C treatment converts MMS-induced lesions to ssDNA breaks.
Cells were harvested from asynchronous log-phase cultures of yFS105 untreated or treated with 16.5 μM bleomycin for 1 hour. 10 O.D. of cells were pelleted, frozen in liquid N2 and stored at -80°C. Plugs were prepared as described before [38]. After the cell wall digestion, Proteinase K treatment and TE washes the plugs were processed and digested with EcoRV and ScaI as described [86]. The digested plugs were analyzed by neutral agarose gel electrophoresis (0.8%). The lanes were quantified and analyzed as for MMS (S1B Fig). For S1B Fig, the length of DNA of peak1, which represents the exclusion volume of the gel, containing molecules from 10 to ~40 kb, was assumed to average 20kb. The value obtained for break per kb from both the peaks were averaged to get the estimate 1 break per 50 kb. However we suspect that we are over-estimating the number of breaks. perhaps due to shearing at single-strand nicks, since we are able to analyze fibers on average 450 kb long from bleomycin-treated wild-type samples by combing.
DNA combing
Cell labeling and plug preparation
Cells were pulse labeled with 2 μM CldU for 5 minutes and chased with 20 μM IdU for 10 minutes. For the second replicate of 4NQO dataset in wild-type and cds1Δ cells and all the bleomycin datasets, cells were labeled with 5 μM CldU for 5 minutes and chased with 20 μM IdU for 10 minutes. For MMS, experiments were done 5 times in wild-type cells and 3 times in cds1Δ cells; 4NQO and bleomycin experiments were done twice in each wild-type and cds1Δ cells. Cells were pulse labeled at different time points across S phase for both wild-type and cds1Δ, however they gave similar results and hence have been combined and represented together in the figures for simplicity. Refer to S1 Table for the exact timing of analog labeling during S phase for the various replicates. Analog labeling was stopped by adding sodium azide to a final concentration of 0.1% and cooling the cells on ice. 10 O.D. of cells were pelleted for combing, frozen in liquid N2 and stored at -80°C. Cells were processed as previously described [38] with minor modifications. For MMS experiments the plugs were digested with proteinase K at 37°C instead of 50°C to facilitate isolation of longer fibers based on the observation that MMS creates heat-labile DNA damage [53]. Higher pH of MES (6.25 or 6.35) was used for combing instead of pH 5.4 to isolate longer fibers [87]. Combing and immuno-staining of samples were done as previously described [38]. For the second replicate of 4NQO dataset in wild-type and cds1Δ cells, and for all the bleomycin datasets, data collection was done in collaboration with Genomic Vision, France. For experiments done in collaboration with Genomic Vision the cells were labeled and processed for combing as before, except for immuno-detection of the fibers a different set of secondary antibodies were used. The dilutions at which they were used are goat anti-rat (Abcam ab6565) 1:50, goat anti-mouse Cy3.5 (Abcam ab6946) 1:50, and goat anti-rabbit BV480 (BD Horizon 564879) 1:10.
Data collection
Fibers were measured in pixels using Image J [88]. Pixels were converted to kb using λ DNA as a standard. Only fibers longer than 120 kb were analyzed. For experiments done in collaboration with Genomic Vision, the images were acquired using the FiberVision scanner and the fibers were manually measured using FiberStudio software. About 25 Mb of DNA was collected for each dataset. Refer to S11 Fig and S1 Table for details of fiber lengths and dataset sizes. For each fiber, the length of each green, red and unlabeled track was manually measured. These measurements were compiled into a master data table (S2 Table). Analysis of this data was automated using MATLAB scripts, which are available upon request.
Fork rate measurement
Green tracks (containing IdU, the second analog added) continuing from red tracks (containing CldU, the first analog added) were used to determine fork rate. The length of the green track was measured from green-red (GR), red-green (RG) and green-red-green (GRG) events and divided by the length of the chase time (10 minutes). For each dataset the fork rate distribution was plotted as a histogram and was fit to a Gaussian curve. The mean fork rate was obtained from the fit (S9 Fig).
We also considered that there could be a lag between the addition of analog and its incorporation into the DNA, which might lead to an underestimation of the actual fork rate. To estimate the lag we labeled the cells as previously described with 2μM CldU for 5 minutes and chased it with 20μM IdU but collected samples after different lengths of IdU incubation: 3, 4, 5, 6, 7, 8, 10, 14 and 16 minutes. However we did not find any lag in analog incorporation by plotting the lengths of IdU labeled forks across different lengths of time after IdU addition (S12 Fig).
Fork rate was also analyzed by excluding forks occurring at the ends of the fiber, as broken forks may lead to an under-estimation of the actual fork rate. However, excluding the forks occurring at the ends of the fiber does not significantly affect fork rate estimations, consistent with the observation that only 6% of the forks in our dataset occur at the end of a fiber.
We note that the fork rate for wild-type untreated samples varies across different replicates (0.65±0.4 kb/min for the first three replicates v. 0.91±0.2 kb/min for the last three replicates, S1 Table). We ascribe this variation to the pH of MES (6.35) used for combing the first three replicates, which we used to facilitate isolation of longer fibers. We have concluded that the stretching factor estimated from lambda DNA at 6.35 is not reliable. Hence we switched to a lower pH of 6.25 for further combing experiments. We believe that the values obtained at pH 6.25 are more reliable, since we have obtained 0.9 kb/min fork rate value from wild-type untreated samples stretched at pH 5.4, which is close to the standard pH used in most combing experiments (S14 Fig) [36,41,89,90]. Despite the variation in the absolute fork rate values the relative trend observed between treated and untreated sample holds true across both pH. For example consider experiments WT3-M and WT4-M, which are similar except for the pH of the combing solution used (S1 Table). The absolute value of wild-type untreated fork rate differs between the experiments (0.60 kb/min vs 0.92 kb/min, S1 Table). However the change in fork rate in treated v. untreated sample is 0.72 for both the experiments.
Origin firing rate measurement
To estimate the rate of origin firing, the total number of origin firing events in each fiber was divided by the length of the unreplicated DNA of that fiber and by the total length of the analog pulses (15 minutes). The total number of origins firing in the fiber is the sum of origins that fire during the first and the second analog. Origins that fire during the first analog are identified as GRG events. Origins that fire during the second analog are identified as isolated green events. However, origins that fire in the first analog will appear as GRG only if both its forks progress into the second analog labeling. In the event of a unidirectional fork stall during the first analog pulse, origins that fire will appear as GR or RG. An origin may also appear as an isolated red event if both its forks stall during the first analog pulse. Therefore, the origin firing rates were corrected by accounting for the probability of forks stalling during the first analog pulse which would have disrupted GRG events, based on the measured fork stall rate (see below for stall rate calculation).
Fork density measurement
Origin firing rate captures the origins that fire during the course of the analog pulses. However, the rate of origin firing prior to labeling influences the density of forks during the pulses and provides a parallel measure of origin activity. Therefore, we measured fork density to assess origin firing rate during the period prior to our S-phase labeling pulses. Fork density, the total number of forks in each fiber was divided by the length of the unreplicated DNA of that fiber, and was calculated independently for the two labeling pulses. The basic strategy for measuring the fork density during either analog pulse is straight-forward. For the first pulse, it is done by counting the following events in each fiber and dividing by the length of the unreplicated DNA of that fiber: the number of origins firing (GRG) and terminations (R and RGR) during the first pulse multiplied by two, since each origin or termination comprises two forks, and the unidirectional forks (GR and RG) counted once. Likewise, for the second analog pulse it is done by counting the following events in each fiber and dividing by the length of the unreplicated DNA of that fiber: the number of origin firing during the second pulse (G), forks from origins firing in the first pulse (GRG) and terminations during the second pulse (RGR) multiplied by two, and the unidirectional forks (GR and RG) counted once. We also calculated the total fork density for each fiber, which is done by counting the following events in each fiber and dividing by the length of the unreplicated DNA of that fiber: number of origins firing during each analog (GRG and G) and terminations (R and RGR) multiplied by two, and the unidirectional forks (GR and RG) counted once. However, these calculations are confounded by forks that stall during the first pulse, leading to the misclassification of events (Fig 2). To account for such stalling events, we took a probabilistic approach, in which the measured stall rate is used to more accurately estimate fork density, as described below.
Fork stall rate measurement
We can estimate the rate at which forks stall before and during the first analog pulse. Stalls occurring during the second analog pulse simply reduce the length of the second analog incorporation track and thus get interpreted as a reduction in fork rate. Fork stall events occurring before the second analog pulse can lead to ambiguous incorporation track patterns (Fig 2A). For instance, an isolated first analog event is open to any of the following three interpretations: an unidirectional elongating fork that stalled, or an origin firing event for which both forks stalled, or a termination event. However, two apparently unidirectional forks moving in the same direction on a fiber must have had the fork in between them stall (Fig 2B and S2A Fig). Such unambiguous stall events leave signature red-unlabeled-green (RUG) or green-unlabeled-red (GUR) patterns. On the other hand the signature for two unstalled forks is green-unlabeled-green (GUG) (S2A Fig). To estimate the apparent stall rate in the dataset we counted every RUG and GUR event and divided it by the sum of GUR, RUG and GUG events (S2A Fig). The GUR and RUG events are included in the denominator because every stall event represents a loss of one fork. For example, consider an origin (GRG) on a fiber. If one of its fork is stalled then it will appear as GR or RG and we would calculate the stall rate as 50% since one fork is stalled but ideally there should be two (one fork which we can visualize and one which is stalled).
Although the unambiguous events allow us to determine the stall rate with certainty, not every isolated first analog event is flanked by second analog events to help us determine whether it is stalled or not. For example consider Fig 2C, which shows two forks moving away from one another. It can be interpreted as forks moving away from a single origin or as two origins with stalled forks on their inner side. To estimate the stall rate across the ambiguous events we used a probabilistic approach. We considered all combinations in which ambiguous events could occur (e.g. UR, RU, RUR, GRU, URG, GRUR, RURG, URUR, RURU, RURUR etc.) (S2B Fig). The ambiguous events were assigned a probability of being either stalled or not, using the apparent stall rate as the probability of a fork stalling (S2B Fig). For example, consider red-unlabeled-red (RUR) event represented in Fig 2C, if the leftward moving fork is interpreted as an origin with stalled rightward fork then it automatically implies that the rightward moving fork on the fiber is also an origin with stalled leftward moving fork. Therefore the probability of both the events being considered as origins with a stalled fork, is the product of two stall events occurring at the same time i.e. square of the apparent stall rate. If the apparent stall rate is 10%, then RUR events are assigned a 1% probability of as having two stalled forks. The probabilistic interpretation of the ambiguous events also changes the absolute number of forks in the dataset. For example according to interpretation 1 in Fig 2C there is only one origin with two elongating forks on the fiber. However according to interpretation 2 there are two origins with a total of 4 expected and 2 apparent forks. To estimate the stalls per kb for each fiber the unambiguous stall events (RUG and GUR) in each fiber were combined with the fraction of the ambiguous events that were predicted to be stalled and this total was divided by the length of the un-replicated DNA. To estimate the stall rate per fiber the unambiguous stall events (RUG and GUR) in each fiber were combined with the fraction of the ambiguous events that were predicted to be stalled and this total was divided by the total number of ongoing forks in the first analog, which was calculated as the sum of GR, RG, and R events (counted once or twice based on whether they were interpreted as elongating forks or as an origin with a stalled fork) plus two forks for each GRG.
The fork stall rate in untreated wild-type and cds1Δ cells is about 15% (S1 Table). This result reveals an unexpectedly high rate of spontaneous fork stalling. To exclude the possibility that the high stall rate is a result of previously identified combing artifacts [86], we re-analyzed our dataset, excluding the labeled events occurring at the ends of the fibers, where such artifacts can occur. However the stall rate estimations remain unchanged after re-analysis (S13 Fig). We cannot rule out the possibility that the observed fork stall rate is increased by the thymidine analogs used to label the DNA. Nonetheless, since we normalize our quantitation of fork-stalling to an untreated sample, our results are internally controlled. Furthermore, double-labeled combing studies done in mammalian cells, Plasmodium falciparum, and Trypansoma brucei show similar rate of fork stalling in the untreated sample (Merav Socolovsky, personal communication) [91,92].
The effect of analog addition on replication kinetics
Addition of analog early in S phase causes slowing of bulk replication, probably due to checkpoint-dependent origin inhibition (S15A Fig). Therefore, we have labeled cells in a manner so as to incur minimal effect on S phase progression due to analog addition (S15B Fig). By adding analogs at different time points across S phase and examining the effect on S phase progression by FACS, we chose to add analog at 50 minutes after release and harvest cells for combing by 65 minutes after release for wild-type (S15B Fig). Furthermore, all of our interpretations are based on comparison to analog-treated cells, so any analog-specific effects are controlled for.
Cds1 kinase assay
To estimate the Cds1 kinase activity S phase progression assay was done in triplicate as described earlier in response to 3.5 mM MMS, 1 μM 4NQO, and 16.5 μM bleomycin in yFS988 strain (S7 Fig). Approximately 10 OD of cells were pelleted at 10, 20, 30, 40, 50, and 90 minutes in S phase for measuring Cds1 activity. Cells were lysed by bead beating in 400μl ice-cold lysis buffer (150 mM NaCl, 50 mM Tris pH 8.0, 5 mM EDTA pH 8.0, 10% Glycerol, 1%IGEPAL CA630, 50 mM NaF, freshly added 1 mM Na3VO4 and protease inhibitor cocktail (Sigma 11836170001)) for 15 minutes at 4°C. Lysate was cleared by centrifuging at 1000g, 5 minutes and combined with anti-HA Ab conjugated agarose beads (Pierce 26181) and incubated with constant mixing at 4°C for 4 hours. Beads were washed twice with lysis buffer and twice with kinase buffer (5 mM HEPES pH 7.5, 37.5 mM KCl, 2.5 mM MgCl2, 1 mM DTT). The sample was then split into two portions, one was used to estimate the amount of Cds1 pulled down by western blot and the other was processed to estimate the kinase activity. For western, the Cds1 was eluted off the beads by boiling in 2x SDS PAGE gel loading dye. Cds1 was detected by using rabbit anti-Cds1 Ab at 1:1000 and anti-rabbit HRP conjugated secondary Ab at 1:10000. For kinase assay, the beads were re-suspended in 10 μl 2x kinase buffer, 0.5 μl 10 μCi/μl γP32-ATP, 2 μl 1 mM ATP, 5 μl 1 mg/ml myelin basic protein and incubated at 30°C for 15 minutes. The reaction was quenched by adding SDS PAGE gel loading buffer and boiling at 95°C for 5 minutes. Kinase reactions were run on a 15% gel normalized to the amount of Cds1 pulled down in each lane. The gel was dried under vacuum and exposed to phosphoimager screen for 48 hours. The screen was scanned on Typhoon FLA-9000 and quantitated using ImageJ [88]. Asynchronous culture of yFS988 treated with 3.5 mM MMS for 4 hours was used as a control to normalize across gels and blots.
RPA foci estimation
In order to estimate RPA foci formation due to damage, S-phase progression assays were performed as described above with strains expressing GFP-tagged Rpa1 (yFS956 and yFS957). Cells were collected at 100 minutes after release from untreated, 3.5 mM MMS, 1 μM 4NQO and 12 mM HU treated cultures and fixed in ice-cold 100% methanol and stored at -20°C for at least 20 minutes. Fixed cells were washed thrice with 1x PBS and re-suspended in 10 μl Vectashield mounting medium with DAPI at 2 μg/ml. Cells were visualized using a Zeiss Axioskop 2 Plus epifluorescence microscope with 100X Plan-NEOFLUAR oil objective and imaged using SPOT monochrome cooled-CCD camera. Images were analyzed using ImageJ and Microsoft Excel. Image of each nuclei in the GFP channel was cut and pasted into cells in an excel file. The nuclei were then blinded, randomly sorted and scored independently by two individuals. 100 nuclei were analyzed for each sample. The nuclei were scored into three categories: uncertain, foci negative, and foci positive. Foci positive cells were further characterized into nuclei with few weak foci (1 or 2) or containing many weak foci, strong foci or a combination of strong and weak foci.
Supporting information
yFS105 asynchronous, log-phase cultures were untreated or treated with 3.5mM MMS or 16.5μM bleomycin for 1 hour. (A) Estimation of lesion density caused by MMS. Genomic DNA was isolated from untreated and MMS treated samples and digested with EcoRV and ScaI and heated at 65°C for 2 hours. The resulting satellite bands were analyzed by alkaline agarose gel electrophoresis. (B) Estimation of lesion density caused by bleomycin. Agarose plugs were prepared from untreated and bleomycin treated samples. The plugs were digested with EcoRV and ScaI and the resulting satellite bands were analyzed by neutral agarose gel electrophoresis. The DNA in Peak1, which represents the exclusion volume of the gel, is assumed to average ~20kb. The lanes were quantified using ImageJ software. Lesion density was inferred by assuming a Poisson distribution of lesions and calculating the frequency of lesions predicted to result in the observed fraction of molecules with no breaks.
(PDF)
(A) Estimation of apparent stall rate from unambiguous events. (B) Estimation of stall rate from all events–ambiguous as well as unambiguous events.
(PDF)
Wild-type (yFS940) and cds1Δ (yFS941) cells were synchronized and released into S phase with 3.5 mM MMS, 0.5 μM, 1 μM, 2 μM 4NQO or left untreated. S-phase progression was monitored by taking samples every 20 minutes for flow cytometry.
(PDF)
Wild-type (yFS940) and cds1Δ (yFS941) cells were synchronized and released into S phase with different concentrations of bleomycin 16.5 μM, 23.79 μM, 47.9 μM or left untreated. S phase progression was monitored by taking samples every 20 minutes for flow cytometry.
(PDF)
WT (yFS940) (A) and cds1Δ (yFS941) (B) cells were synchronized in G1 phase using cdc10-M17 temperature sensitive allele followed by elutriation. Elutriated G1 cells were released into permissive temperature untreated or treated with 3.5 mM MMS or 1 μM 4NQO or 16.5 μM Bleomycin.
(PDF)
(PDF)
(PDF)
For each time course, Cds1 western was done to estimate the amount of Cds1 pulled down in each condition. Kinase assay reactions were run normalized to the amount of Cds1 pull down. Signal from each lane of the kinase assay gel was normalized to the asynchronous MMS sample to get the normalized kinase activity.
(PDF)
Fork rate distribution in wild-type and cds1Δ cells untreated or treated with MMS from a single experiment at time points 1 and 3, respectively (A and C) and from two experiments at time points 2 and 4, respectively (B and D). For the exact timings refer to S1 Table. Each distribution of fork rate was fit to a Gaussian curve. Mean fork rate was obtained from the fit.
(PDF)
(PDF)
Length of fibers from wild-type 4NQO (A) and MMS (B) samples from a single experiment. Approximately 25 Mb of total DNA was collected for each sample (S1 Table).
(PDF)
Cells were pulse labeled with 2 μM CldU for 5 minutes and chased with 20 μM IdU for varying lengths of time: 3, 4, 5, 6, 7, 8, 10, 14, 16 minutes. At least 100 forks were collected for each time point except for 3 and 4 minutes of IdU labeling for which only 11 and 44 forks were measured, respectively, due to lack IdU labeled forks at such short lengths of labeling period.
(PDF)
We re-analyzed the fibers considering stretching artifacts, which may lead to incorrect interpretation of analog incorporation patterns [86]. Ends of the fiber are most susceptible to such stretching artifacts and hence we ignored the labeled events occurring at the ends of the fiber and re-calculated the stall rate. However we do not see a significant change between the stall rate calculated from the whole fiber or after ignoring the labeled events occurring at the ends of the fiber. 4NQO and bleomycin experiments were done twice in each wild-type and cds1Δ. MMS experiments were done 5 and 3 times in wild-type and cds1Δ respectively.
(PDF)
Fork rate value was estimated from the second analog track continuing from the first analog as explained in the Methods section.
(PDF)
A) Wild-type cells were synchronized and released into S phase with analog added at different concentrations—0.1 μM, 0.5 μM, 1 μM, 2 μM CldU—and chased with 20 μM IdU at 35 minutes after release or without any analog. B) The S-phase progression of cultures with and without analog addition used for combing experiments. 2 μM CldU analog was added at 50 minutes after release for 5 minutes and chased with 20 μM IdU for 10 minutes and the cells were harvested for combing at 65 minutes after release so as to have minimal effects of analog addition on replication kinetics.
(PDF)
(XLS)
(XLS)
Acknowledgments
We thank Atanas Kaykov for suggestion regarding the preparation of silanized coverslips and the pH of the combing buffer, which allowed us isolate longer fibers, Merav Socolovsky and Shankar Das for their helpful suggestions regarding the combing protocol and Julien Cottineau, Nicolas Lambert and their colleagues at Genomic Vision for their assistance with their FiberVision and FiberStudio technology. We also thank members of the Rhind lab for constructive comments of the project and this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported National Institutes of Health <https://www.nih.gov> grant GM098815 to NR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hartwell LH, Weinert TA (1989) Checkpoints: controls that ensure the order of cell cycle events. Science 246: 629–634. [DOI] [PubMed] [Google Scholar]
- 2.Rhind N, Russell P (2012) Signaling pathways that regulate cell division. Cold Spring Harb Perspect Biol 4: a005942 doi: 10.1101/cshperspect.a005942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartek J, Lukas C, Lukas J (2004) Checking on DNA damage in S phase. Nat Rev Mol Cell Biol 5: 792–804. doi: 10.1038/nrm1493 [DOI] [PubMed] [Google Scholar]
- 4.Rhind N, Russell P (2000) Checkpoints: it takes more than time to heal some wounds. Curr Biol 10: R908–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou BB, Elledge SJ (2000) The DNA damage response: putting checkpoints in perspective. Nature 408: 433–439. doi: 10.1038/35044005 [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann WK, Cleaver JE, Painter RB (1980) Ultraviolet radiation inhibits replicon initiation in S phase human cells. Biochim Biophys Acta 608: 191–195. [DOI] [PubMed] [Google Scholar]
- 7.Merrick CJ, Jackson D, Diffley JF (2004) Visualization of altered replication dynamics after DNA damage in human cells. J Biol Chem 279: 20067–20075. doi: 10.1074/jbc.M400022200 [DOI] [PubMed] [Google Scholar]
- 8.Falck J, Petrini JH, Williams BR, Lukas J, Bartek J (2002) The DNA damage-dependent intra-S phase checkpoint is regulated by parallel pathways. Nat Genet 30: 290–294. doi: 10.1038/ng845 [DOI] [PubMed] [Google Scholar]
- 9.Santocanale C, Diffley JF (1998) A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395: 615–618. doi: 10.1038/27001 [DOI] [PubMed] [Google Scholar]
- 10.Chastain PD, Heffernan TP, Nevis KR, Lin L, Kaufmann WK, Kaufman DG et al. (2006) Checkpoint regulation of replication dynamics in UV-irradiated human cells. Cell Cycle 5: 2160–2167. doi: 10.4161/cc.5.18.3236 [DOI] [PubMed] [Google Scholar]
- 11.Seiler JA, Conti C, Syed A, Aladjem MI, Pommier Y (2007) The intra-S-phase checkpoint affects both DNA replication initiation and elongation: single-cell and -DNA fiber analyses. Mol Cell Biol 27: 5806–5818. doi: 10.1128/MCB.02278-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Huberman JA (2009) Checkpoint-dependent regulation of origin firing and replication fork movement in response to DNA damage in fission yeast. Mol Cell Biol 29: 602–611. doi: 10.1128/MCB.01319-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao H, Piwnica-Worms H (2001) ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol 21: 4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falck J, Mailand N, Syljuåsen RG, Bartek J, Lukas J (2001) The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 410: 842–847. doi: 10.1038/35071124 [DOI] [PubMed] [Google Scholar]
- 15.Sørensen CS, Syljuåsen RG, Falck J, Schroeder T, Rönnstrand L, Khanna KK et al. (2003) Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell 3: 247–258. [DOI] [PubMed] [Google Scholar]
- 16.Zegerman P, Diffley JF (2010) Checkpoint-dependent inhibition of DNA replication initiation by Sld3 and Dbf4 phosphorylation. Nature 467: 474–478. doi: 10.1038/nature09373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Mosqueda J, Maas NL, Jonsson ZO, Defazio-Eli LG, Wohlschlegel J, Toczyski DP (2010) Damage-induced phosphorylation of Sld3 is important to block late origin firing. Nature 467: 479–483. doi: 10.1038/nature09377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheu YJ, Kinney JB, Stillman B (2016) Concerted activities of Mcm4, Sld3, and Dbf4 in control of origin activation and DNA replication fork progression. Genome Res 26: 315–330. doi: 10.1101/gr.195248.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tercero JA, Longhese MP, Diffley JF (2003) A central role for DNA replication forks in checkpoint activation and response. Mol Cell 11: 1323–1336. [DOI] [PubMed] [Google Scholar]
- 20.Paciotti V, Clerici M, Scotti M, Lucchini G, Longhese MP (2001) Characterization of mec1 kinase-deficient mutants and of new hypomorphic mec1 alleles impairing subsets of the DNA damage response pathway. Mol Cell Biol 21: 3913–3925. doi: 10.1128/MCB.21.12.3913-3925.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branzei D, Foiani M (2009) The checkpoint response to replication stress. DNA Repair (Amst) 8: 1038–1046. [DOI] [PubMed] [Google Scholar]
- 22.Branzei D, Foiani M (2005) The DNA damage response during DNA replication. Curr Opin Cell Biol 17: 568–575. doi: 10.1016/j.ceb.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 23.Ulrich HD (2012) Ubiquitin and SUMO in DNA repair at a glance. J Cell Sci 125: 249–254. doi: 10.1242/jcs.091801 [DOI] [PubMed] [Google Scholar]
- 24.Daigaku Y, Davies AA, Ulrich HD (2010) Ubiquitin-dependent DNA damage bypass is separable from genome replication. Nature 465: 951–955. doi: 10.1038/nature09097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sale JE (2012) Competition, collaboration and coordination—determining how cells bypass DNA damage. J Cell Sci 125: 1633–1643. doi: 10.1242/jcs.094748 [DOI] [PubMed] [Google Scholar]
- 26.Lee KY, Myung K (2008) PCNA modifications for regulation of post-replication repair pathways. Mol Cells 26: 5–11. [PMC free article] [PubMed] [Google Scholar]
- 27.Tercero JA, Diffley JF (2001) Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412: 553–557. doi: 10.1038/35087607 [DOI] [PubMed] [Google Scholar]
- 28.Szyjka SJ, Aparicio JG, Viggiani CJ, Knott S, Xu W, Tavaré S et al. (2008) Rad53 regulates replication fork restart after DNA damage in Saccharomyces cerevisiae. Genes Dev 22: 1906–1920. doi: 10.1101/gad.1660408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unsal-Kaçmaz K, Chastain PD, Qu PP, Minoo P, Cordeiro-Stone M, Sancar A et al. (2007) The human Tim/Tipin complex coordinates an Intra-S checkpoint response to UV that slows replication fork displacement. Mol Cell Biol 27: 3131–3142. doi: 10.1128/MCB.02190-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes M, Cotta-Ramusino C, Pellicioli A, Liberi G, Plevani P, Muzi-Falconi M et al. (2001) The DNA replication checkpoint response stabilizes stalled replication forks. Nature 412: 557–561. doi: 10.1038/35087613 [DOI] [PubMed] [Google Scholar]
- 31.Cobb JA, Bjergbaek L, Shimada K, Frei C, Gasser SM (2003) DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J 22: 4325–4336. doi: 10.1093/emboj/cdg391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cobb JA, Schleker T, Rojas V, Bjergbaek L, Tercero JA, Gasser SM (2005) Replisome instability, fork collapse, and gross chromosomal rearrangements arise synergistically from Mec1 kinase and RecQ helicase mutations. Genes Dev 19: 3055–3069. doi: 10.1101/gad.361805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noguchi E, Noguchi C, McDonald WH, Yates JR, Russell P (2004) Swi1 and Swi3 are components of a replication fork protection complex in fission yeast. Mol Cell Biol 24: 8342–8355. doi: 10.1128/MCB.24.19.8342-8355.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirahige K, Hori Y, Shiraishi K, Yamashita M, Takahashi K, Obuse C et al. (1998) Regulation of DNA-replication origins during cell-cycle progression. Nature 395: 618–621. doi: 10.1038/27007 [DOI] [PubMed] [Google Scholar]
- 35.Bensimon A, Simon A, Chiffaudel A, Croquette V, Heslot F, Bensimon D (1994) Alignment and sensitive detection of DNA by a moving interface. Science 265: 2096–2098. [DOI] [PubMed] [Google Scholar]
- 36.Michalet X, Ekong R, Fougerousse F, Rousseaux S, Schurra C, Hornigold N et al. (1997) Dynamic molecular combing: stretching the whole human genome for high-resolution studies. Science 277: 1518–1523. [DOI] [PubMed] [Google Scholar]
- 37.Jackson DA, Pombo A (1998) Replicon clusters are stable units of chromosome structure: evidence that nuclear organization contributes to the efficient activation and propagation of S phase in human cells. J Cell Biol 140: 1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iyer DR, Das S, Rhind N (in press) Analysis of DNA Replication in Fission Yeast by Combing In: Hagan I, Carr AM, Nurse P, editors. Fission Yeast: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; pp. 490. [DOI] [PubMed] [Google Scholar]
- 39.Gallo D, Wang G, Yip CM, Brown GW (2016) Analysis of Replicating Yeast Chromosomes by DNA Combing. Cold Spring Harb Protoc 2016: pdb.prot085118. [DOI] [PubMed]
- 40.Gallo D, Wang G, Yip CM, Brown GW (2016) Single-Molecule Analysis of Replicating Yeast Chromosomes. Cold Spring Harb Protoc 2016: pdb.top077784. [DOI] [PubMed]
- 41.Bianco JN, Poli J, Saksouk J, Bacal J, Silva MJ, Yoshida K et al. (2012) Analysis of DNA replication profiles in budding yeast and mammalian cells using DNA combing. Methods 57: 149–157. doi: 10.1016/j.ymeth.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 42.Técher H, Koundrioukoff S, Azar D, Wilhelm T, Carignon S, Brison O et al. (2013) Replication dynamics: biases and robustness of DNA fiber analysis. J Mol Biol 425: 4845–4855. doi: 10.1016/j.jmb.2013.03.040 [DOI] [PubMed] [Google Scholar]
- 43.Iyer DR, Rhind N (2013) Checkpoint regulation of replication forks: global or local. Biochem Soc Trans 41: 1701–1705. doi: 10.1042/BST20130197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willis N, Rhind N (2009) Mus81, Rhp51(Rad51), and Rqh1 form an epistatic pathway required for the S-phase DNA damage checkpoint. Mol Biol Cell 20: 819–833. doi: 10.1091/mbc.E08-08-0798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sikora A, Mielecki D, Chojnacka A, Nieminuszczy J, Wrzesinski M, Grzesiuk E (2010) Lethal and mutagenic properties of MMS-generated DNA lesions in Escherichia coli cells deficient in BER and AlkB-directed DNA repair. Mutagenesis 25: 139–147. doi: 10.1093/mutage/gep052 [DOI] [PubMed] [Google Scholar]
- 46.Galiègue-Zouitina S, Bailleul B, Loucheux-Lefebvre MH (1985) Adducts from in vivo action of the carcinogen 4-hydroxyaminoquinoline 1-oxide in rats and from in vitro reaction of 4-acetoxyaminoquinoline 1-oxide with DNA and polynucleotides. Cancer Res 45: 520–525. [PubMed] [Google Scholar]
- 47.Galiègue-Zouitina S, Bailleul B, Ginot YM, Perly B, Vigny P, Loucheux-Lefebvre MH (1986) N2-guanyl and N6-adenyl arylation of chicken erythrocyte DNA by the ultimate carcinogen of 4-nitroquinoline 1-oxide. Cancer Res 46: 1858–1863. [PubMed] [Google Scholar]
- 48.Chen J, Stubbe J (2005) Bleomycins: towards better therapeutics. Nat Rev Cancer 5: 102–112. doi: 10.1038/nrc1547 [DOI] [PubMed] [Google Scholar]
- 49.Friedberg EC, Walker GC, Siede W (1995) DNA Repair and Mutagenesis. Washington, D.C., USA: ASM Press. 698 p. [Google Scholar]
- 50.Larson K, Sahm J, Shenkar R, Strauss B (1985) Methylation-induced blocks to in vitro DNA replication. Mutat Res 150: 77–84. [DOI] [PubMed] [Google Scholar]
- 51.Minca EC, Kowalski D (2011) Replication fork stalling by bulky DNA damage: localization at active origins and checkpoint modulation. Nucleic Acids Res 39: 2610–2623. doi: 10.1093/nar/gkq1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindsay HD, Griffiths DJ, Edwards RJ, Christensen PU, Murray JM, Osman F et al. (1998) S-phase-specific activation of Cds1 kinase defines a subpathway of the checkpoint response in Schizosaccharomyces pombe. Genes Dev 12: 382–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundin C, North M, Erixon K, Walters K, Jenssen D, Goldman AS et al. (2005) Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic Acids Res 33: 3799–3811. doi: 10.1093/nar/gki681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snyderwine EG, Bohr VA (1992) Gene- and strand-specific damage and repair in Chinese hamster ovary cells treated with 4-nitroquinoline 1-oxide. Cancer Res 52: 4183–4189. [PubMed] [Google Scholar]
- 55.Ma W, Resnick MA, Gordenin DA (2008) Apn1 and Apn2 endonucleases prevent accumulation of repair-associated DNA breaks in budding yeast as revealed by direct chromosomal analysis. Nucleic Acids Res 36: 1836–1846. doi: 10.1093/nar/gkm1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kearsey SE, Stevenson AL, Toda T, Wang SW (2007) Fission yeast Cut8 is required for the repair of DNA double-strand breaks, ribosomal DNA maintenance, and cell survival in the absence of Rqh1 helicase. Mol Cell Biol 27: 1558–1567. doi: 10.1128/MCB.01495-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rhind N, Russell P (1998) The Schizosaccharomyces pombe S-phase checkpoint differentiates between different types of DNA damage. Genetics 149: 1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldar A, Marsolier-Kergoat MC, Hyrien O (2009) Universal temporal profile of replication origin activation in eukaryotes. PLoS One 4: e5899 doi: 10.1371/journal.pone.0005899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conti C, Seiler JA, Pommier Y (2007) The mammalian DNA replication elongation checkpoint: implication of Chk1 and relationship with origin firing as determined by single DNA molecule and single cell analyses. Cell Cycle 6: 2760–2767. doi: 10.4161/cc.6.22.4932 [DOI] [PubMed] [Google Scholar]
- 60.Conti C, Leo E, Eichler GS, Sordet O, Martin MM, Fan A et al. (2010) Inhibition of histone deacetylase in cancer cells slows down replication forks, activates dormant origins, and induces DNA damage. Cancer Res 70: 4470–4480. doi: 10.1158/0008-5472.CAN-09-3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petermann E, Helleday T, Caldecott KW (2008) Claspin promotes normal replication fork rates in human cells. Mol Biol Cell 19: 2373–2378. doi: 10.1091/mbc.E07-10-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petermann E, Woodcock M, Helleday T (2010) Chk1 promotes replication fork progression by controlling replication initiation. Proc Natl Acad Sci U S A 107: 16090–16095. doi: 10.1073/pnas.1005031107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shechter D, Costanzo V, Gautier J (2004) ATR and ATM regulate the timing of DNA replication origin firing. Nat Cell Biol 6: 648–655. doi: 10.1038/ncb1145 [DOI] [PubMed] [Google Scholar]
- 64.Herrick J, Sclavi B (2007) Ribonucleotide reductase and the regulation of DNA replication: an old story and an ancient heritage. Mol Microbiol 63: 22–34. doi: 10.1111/j.1365-2958.2006.05493.x [DOI] [PubMed] [Google Scholar]
- 65.Poli J, Tsaponina O, Crabbé L, Keszthelyi A, Pantesco V, Chabes A et al. (2012) dNTP pools determine fork progression and origin usage under replication stress. EMBO J 31: 883–894. doi: 10.1038/emboj.2011.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herrick J, Bensimon A (2008) Global regulation of genome duplication in eukaryotes: an overview from the epifluorescence microscope. Chromosoma 117: 243–260. doi: 10.1007/s00412-007-0145-1 [DOI] [PubMed] [Google Scholar]
- 67.Zou L, Elledge SJ (2003) Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300: 1542–1548. doi: 10.1126/science.1083430 [DOI] [PubMed] [Google Scholar]
- 68.Cimprich KA, Cortez D (2008) ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol 9: 616–627. doi: 10.1038/nrm2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Byun TS, Pacek M, Yee MC, Walter JC, Cimprich KA (2005) Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev 19: 1040–1052. doi: 10.1101/gad.1301205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu X, Vaithiyalingam S, Glick GG, Mordes DA, Chazin WJ, Cortez D (2008) The basic cleft of RPA70N binds multiple checkpoint proteins, including RAD9, to regulate ATR signaling. Mol Cell Biol 28: 7345–7353. doi: 10.1128/MCB.01079-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu R, Terry AV, Singh PB, Gilbert DM (2005) Differential subnuclear localization and replication timing of histone H3 lysine 9 methylation states. Mol Biol Cell 16: 2872–2881. doi: 10.1091/mbc.E04-11-0997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sabatinos SA, Forsburg SL (2015) Managing Single-Stranded DNA during Replication Stress in Fission Yeast. Biomolecules 5: 2123–2139. doi: 10.3390/biom5032123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabatinos SA, Green MD, Forsburg SL (2012) Continued DNA synthesis in replication checkpoint mutants leads to fork collapse. Mol Cell Biol 32: 4986–4997. doi: 10.1128/MCB.01060-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sogo JM, Lopes M, Foiani M (2002) Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science 297: 599–602. doi: 10.1126/science.1074023 [DOI] [PubMed] [Google Scholar]
- 75.Wang X, Guan J, Hu B, Weiss RS, Iliakis G, Wang Y (2004) Involvement of Hus1 in the chain elongation step of DNA replication after exposure to camptothecin or ionizing radiation. Nucleic Acids Res 32: 767–775. doi: 10.1093/nar/gkh243 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Shimada K, Pasero P, Gasser SM (2002) ORC and the intra-S-phase checkpoint: a threshold regulates Rad53p activation in S phase. Genes Dev 16: 3236–3252. doi: 10.1101/gad.239802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Piccoli G, Katou Y, Itoh T, Nakato R, Shirahige K, Labib K (2012) Replisome stability at defective DNA replication forks is independent of S phase checkpoint kinases. Mol Cell 45: 696–704. doi: 10.1016/j.molcel.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 78.Segurado M, Diffley JF (2008) Separate roles for the DNA damage checkpoint protein kinases in stabilizing DNA replication forks. Genes Dev 22: 1816–1827. doi: 10.1101/gad.477208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scorah J, McGowan CH (2009) Claspin and Chk1 regulate replication fork stability by different mechanisms. Cell Cycle 8: 1036–1043. doi: 10.4161/cc.8.7.8040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilsker D, Petermann E, Helleday T, Bunz F (2008) Essential function of Chk1 can be uncoupled from DNA damage checkpoint and replication control. Proc Natl Acad Sci U S A 105: 20752–20757. doi: 10.1073/pnas.0806917106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anglana M, Apiou F, Bensimon A, Debatisse M (2003) Dynamics of DNA replication in mammalian somatic cells: nucleotide pool modulates origin choice and interorigin spacing. Cell 114: 385–394. [DOI] [PubMed] [Google Scholar]
- 82.Courbet S, Gay S, Arnoult N, Wronka G, Anglana M, Brison O et al. (2008) Replication fork movement sets chromatin loop size and origin choice in mammalian cells. Nature 455: 557–560. doi: 10.1038/nature07233 [DOI] [PubMed] [Google Scholar]
- 83.Letessier A, Millot GA, Koundrioukoff S, Lachagès AM, Vogt N, Hansen RS et al. (2011) Cell-type-specific replication initiation programs set fragility of the FRA3B fragile site. Nature 470: 120–123. doi: 10.1038/nature09745 [DOI] [PubMed] [Google Scholar]
- 84.Forsburg SL, Rhind N (2006) Basic methods for fission yeast. Yeast 23: 173–183. doi: 10.1002/yea.1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Willis N, Rhind N (2011) Studying S-phase DNA damage checkpoints using the fission yeast Schizosaccharomyces pombe. Methods Mol Biol 782: 13–21. doi: 10.1007/978-1-61779-273-1_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Demczuk A, Norio P (2009) Determining the replication dynamics of specific gene loci by single-molecule analysis of replicated DNA. Methods Mol Biol 521: 633–671. doi: 10.1007/978-1-60327-815-7_35 [DOI] [PubMed] [Google Scholar]
- 87.Kaykov A, Nurse P (2015) The spatial and temporal organization of origin firing during the S-phase of fission yeast. Genome Res 25: 391–401. doi: 10.1101/gr.180372.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allemand JF, Bensimon D, Jullien L, Bensimon A, Croquette V (1997) pH-dependent specific binding and combing of DNA. Biophys J 73: 2064–2070. doi: 10.1016/S0006-3495(97)78236-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herrick J, Bensimon A (1999) Single molecule analysis of DNA replication. Biochimie 81: 859–871. [DOI] [PubMed] [Google Scholar]
- 91.Stanojcic S, Sollelis L, Kuk N, Crobu L, Balard Y, Schwob E et al. (2016) Single-molecule analysis of DNA replication reveals novel features in the divergent eukaryotes Leishmania and Trypanosoma brucei versus mammalian cells. Sci Rep 6: 23142 doi: 10.1038/srep23142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stanojcic S, Kuk N, Ullah I, Sterkers Y, Merrick CJ (2017) Single-molecule analysis reveals that DNA replication dynamics vary across the course of schizogony in the malaria parasite Plasmodium falciparum. Sci Rep 7: 4003 doi: 10.1038/s41598-017-04407-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
yFS105 asynchronous, log-phase cultures were untreated or treated with 3.5mM MMS or 16.5μM bleomycin for 1 hour. (A) Estimation of lesion density caused by MMS. Genomic DNA was isolated from untreated and MMS treated samples and digested with EcoRV and ScaI and heated at 65°C for 2 hours. The resulting satellite bands were analyzed by alkaline agarose gel electrophoresis. (B) Estimation of lesion density caused by bleomycin. Agarose plugs were prepared from untreated and bleomycin treated samples. The plugs were digested with EcoRV and ScaI and the resulting satellite bands were analyzed by neutral agarose gel electrophoresis. The DNA in Peak1, which represents the exclusion volume of the gel, is assumed to average ~20kb. The lanes were quantified using ImageJ software. Lesion density was inferred by assuming a Poisson distribution of lesions and calculating the frequency of lesions predicted to result in the observed fraction of molecules with no breaks.
(PDF)
(A) Estimation of apparent stall rate from unambiguous events. (B) Estimation of stall rate from all events–ambiguous as well as unambiguous events.
(PDF)
Wild-type (yFS940) and cds1Δ (yFS941) cells were synchronized and released into S phase with 3.5 mM MMS, 0.5 μM, 1 μM, 2 μM 4NQO or left untreated. S-phase progression was monitored by taking samples every 20 minutes for flow cytometry.
(PDF)
Wild-type (yFS940) and cds1Δ (yFS941) cells were synchronized and released into S phase with different concentrations of bleomycin 16.5 μM, 23.79 μM, 47.9 μM or left untreated. S phase progression was monitored by taking samples every 20 minutes for flow cytometry.
(PDF)
WT (yFS940) (A) and cds1Δ (yFS941) (B) cells were synchronized in G1 phase using cdc10-M17 temperature sensitive allele followed by elutriation. Elutriated G1 cells were released into permissive temperature untreated or treated with 3.5 mM MMS or 1 μM 4NQO or 16.5 μM Bleomycin.
(PDF)
(PDF)
(PDF)
For each time course, Cds1 western was done to estimate the amount of Cds1 pulled down in each condition. Kinase assay reactions were run normalized to the amount of Cds1 pull down. Signal from each lane of the kinase assay gel was normalized to the asynchronous MMS sample to get the normalized kinase activity.
(PDF)
Fork rate distribution in wild-type and cds1Δ cells untreated or treated with MMS from a single experiment at time points 1 and 3, respectively (A and C) and from two experiments at time points 2 and 4, respectively (B and D). For the exact timings refer to S1 Table. Each distribution of fork rate was fit to a Gaussian curve. Mean fork rate was obtained from the fit.
(PDF)
(PDF)
Length of fibers from wild-type 4NQO (A) and MMS (B) samples from a single experiment. Approximately 25 Mb of total DNA was collected for each sample (S1 Table).
(PDF)
Cells were pulse labeled with 2 μM CldU for 5 minutes and chased with 20 μM IdU for varying lengths of time: 3, 4, 5, 6, 7, 8, 10, 14, 16 minutes. At least 100 forks were collected for each time point except for 3 and 4 minutes of IdU labeling for which only 11 and 44 forks were measured, respectively, due to lack IdU labeled forks at such short lengths of labeling period.
(PDF)
We re-analyzed the fibers considering stretching artifacts, which may lead to incorrect interpretation of analog incorporation patterns [86]. Ends of the fiber are most susceptible to such stretching artifacts and hence we ignored the labeled events occurring at the ends of the fiber and re-calculated the stall rate. However we do not see a significant change between the stall rate calculated from the whole fiber or after ignoring the labeled events occurring at the ends of the fiber. 4NQO and bleomycin experiments were done twice in each wild-type and cds1Δ. MMS experiments were done 5 and 3 times in wild-type and cds1Δ respectively.
(PDF)
Fork rate value was estimated from the second analog track continuing from the first analog as explained in the Methods section.
(PDF)
A) Wild-type cells were synchronized and released into S phase with analog added at different concentrations—0.1 μM, 0.5 μM, 1 μM, 2 μM CldU—and chased with 20 μM IdU at 35 minutes after release or without any analog. B) The S-phase progression of cultures with and without analog addition used for combing experiments. 2 μM CldU analog was added at 50 minutes after release for 5 minutes and chased with 20 μM IdU for 10 minutes and the cells were harvested for combing at 65 minutes after release so as to have minimal effects of analog addition on replication kinetics.
(PDF)
(XLS)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.