Abstract
Vascular tone of resistance arteries and arterioles determines peripheral vascular resistance, contributing to the regulation of blood pressure and blood flow to, and within the body’s tissues and organs. Ion channels in the plasma membrane and endoplasmic reticulum of vascular smooth muscle cells (SMCs) in these blood vessels importantly contribute to the regulation of intracellular Ca2+ concentration, the primary determinant of SMC contractile activity and vascular tone. Ion channels provide the main source of activator Ca2+ that determines vascular tone, and strongly contribute to setting and regulating membrane potential, which, in turn, regulates the open-state-probability of voltage gated Ca2+ channels (VGCCs), the primary source of Ca2+ in resistance artery and arteriolar SMCs. Ion channel function is also modulated by vasoconstrictors and vasodilators, contributing to all aspects of the regulation of vascular tone. This review will focus on the physiology of VGCCs, voltage-gated K+ (KV) channels, large-conductance Ca2+-activated K+ (BKCa) channels, strong-inward-rectifier K+ (KIR) channels, ATP-sensitive K+ (KATP) channels, ryanodine receptors (RyRs), inositol 1,4,5-trisphosphate receptors (IP3Rs), and a variety of transient receptor potential (TRP) channels that contribute to pressure-induced myogenic tone in resistance arteries and arterioles, the modulation of the function of these ion channels by vasoconstrictors and vasodilators, their role in the functional regulation of tissue blood flow and their dysfunction in diseases such as hypertension, obesity, and diabetes.
Introduction
Resistance arteries and arterioles importantly contribute to cardiovascular and whole-body homeostasis by serving as the primary location of vascular resistance that contributes to blood pressure regulation and the distribution of cardiac output among and within the body’s organs and tissues to meet their metabolic and physiological demands (316). Vascular smooth muscle cells (SMCs) that make up the wall of resistance arteries and arterioles serve as the primary effectors in the minute-to-minute, active regulation of vascular resistance via modulation of the steady-state contraction of these cells, or vascular tone (316). Vascular tone of resistance arteries and arterioles depends on the blood pressure within these vessels, as well as the balance between vasoconstrictor and vasodilator signals that impinge upon them (316). Blood pressure, by stretching the SMCs, activates signaling pathways to produce myogenic tone, a hallmark of resistance arteries and arterioles (299, 314, 315, 488, 595, 1036). Myogenic tone serves as the baseline SMC contraction upon which vasoconstrictor and vasodilator signals from neurotransmitters, hormones, endothelium-derived substances, local metabolites and ions act (316). Intracellular Ca2+ in SMCs serves as a key determinant of vascular tone by controlling the activity of myosin-light-chain kinase and the degree of phosphorylation of the 20-kD myosin light chains (748). Ion channels in both the plasma membrane and endoplasmic reticulum (ER) serve as the primary sources of activator Ca2+ (152, 555, 748, 835). Plasma membrane ion channels also importantly contribute to setting and modulating membrane potential, which not only determines the magnitude of Ca2+ influx through voltage-gated Ca2+ channels (VGCCs), but also, in part, sets the electrochemical gradient for the movement of ions through all plasmalemmal ion channels (666). Membrane potential also impacts the release of Ca2+ from intracellular stores and the Ca2+ sensitivity of the contractile machinery in vascular SMCs (327, 419, 420, 459, 798, 895, 930, 1105, 1448, 1571, 1574, 1580). Therefore, ion channels importantly contribute to all aspects of the determination and modulation of vascular tone in resistance arteries and arterioles.
Resistance artery and arteriolar SMCs express a plethora of ion channels including, but not limited to: two or more classes of VGCCs (Table 1, Fig. 1), four or more classes of K+ channels (Table 2, Fig. 1), two or more intracellular Ca2+ release channels (Table 3, Fig. 1), multiple members of five classes from the transient receptor potential (TRP) family of ion channels (Table 4, Fig. 1), two or more classes of Cl− channels (184, 303, 304, 584, 632, 829, 956, 957) and members of the epithelial Na+/acid-sensing channel (ENaC) (340–342, 460, 502, 510, 698, 697, 1465). This review will focus on VGCCs, K+ channels, ER Ca2+ release channels and TRP channels that contribute to myogenic and agonist-modulated tone in vascular SMCs of resistance arteries and arterioles. Chloride channels (184, 303, 304, 584, 632, 829, 956, 957) and ENaC (340–342, 460, 502, 510, 698, 697, 1465) that may contribute to myogenic- and agonist-induced tone of some resistance arteries and arterioles will not be discussed. Ion channel function in the pulmonary circulation also will not be reviewed in detail (937). We will focus on ion channel function in establishing myogenic tone in resistance arteries and arterioles, the role played by these channels in the mechanism of action of vasodilators and vasoconstrictors that modulate vascular tone and the effects of disease states such as hypertension, obesity, and diabetes on ion channel expression and function. The relationships between these diseases and SMC ion channel expression and function are complex and multifaceted: changes may directly contribute to disease progression, changes may result from disease progression, or changes may be a means of compensation to account for the pathophysiological progression of a disease. Where possible, this review presents the relationship between SMC ion channels and disease from these different perspectives.
Table 1.
Vascular Voltage-Gated Ca2+ Channels and Their Pharmacology
| Channel | Gene | Alternative names |
Accessory subunits |
Inhibitors/antagonists (IC50) | Activators/agonists (EC50) |
|---|---|---|---|---|---|
| CaV 1.2 | CACNA1C | L-type | β2 and β3, α2δ1 | Nifedipine (10–100 nmol/L) (872) | BayK 8644 (6 nmol/L) (1638) |
| Nimodipine (139 nmol/L) (1563) | FPL64176 (211 nmol/L) (1638) | ||||
| Diltiazem (500 nmol/L) (615) | |||||
| Verapamil (60 nmol/L) (615) | |||||
| Mibefradil (1.4–13 µmol/L) (576, 948) | |||||
| Cd2+ (7 µmol/L) (1054) | |||||
| Ni2+ (280 µmol/L) (1054) | |||||
| Kurtoxin (> 10 µmol/L) (251) | |||||
| ML218 (> 10 µmol/L) (1560) | |||||
| CaV 3.1 | CACNA1G | T-type, α1G | Mibefradil (0.4–1.2 µmol/L) (576) | ||
| Cd2+ (160 µmol/L) (833) | |||||
| Ni2+ (167–250 µmol/L) (833) | |||||
| Kurtoxin (15 nmol/L) (251) | |||||
| ML218 (~300 nmol/L) (1560) | |||||
| CaV 3.2 | CACNA1H | T-type, α1H | Mibefradil (1.1–1.2 µmol/L) (576) | ||
| Cd2+ (160 µmol/L) (833) | |||||
| Ni2+ (5.7–12 µmol/L) (833) | |||||
| Kurtoxin (61 nmol/L) (251) | |||||
| ML218 (310 nmol/L) (1560) |
Figure 1.
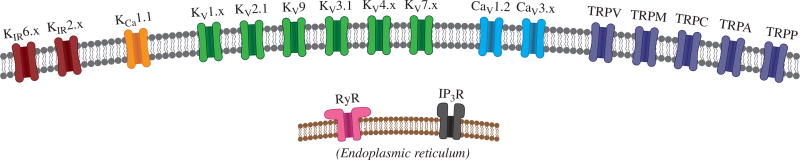
Principal ion channels expressed in vascular SMCs. In the plasma membrane (gray), the following channels are expressed: at least two members of the inward-rectifier K+ channel (KIR) family; large-conductance, Ca2+-activated K+ channels (KCa 1.1); at least six members of the voltage-dependent K+ channel (Kv) family; at least two voltage-dependent Ca2+ channels (Cav); and a number of TRP channels. In the endoplasmic reticular membrane (brown), RyRs and IP3R are expressed.
Table 2.
Vascular K+ Channels and Their Pharmacology
| Channel | Gene | Alternative names |
Accessory subunits |
Inhibitors/antagonists (IC50) | Activators/agonists (EC50) |
|---|---|---|---|---|---|
| KV 1.1 | KCNA1 | KV β1, KV β2 | TEA (0.3 mmol/L) (504) | ||
| 4-AP (0.29 mmol/L) (504) | |||||
| correolide (430 nmol/L) (414) | |||||
| α-dendrotoxin (20 nmol/L) (504) | |||||
| dendrotoxin-k (2.5 nmol/L) (1208) | |||||
| hongotoxin (31 pmol/L) (783) | |||||
| margatoxin (144 pmol/L) (783) | |||||
| kaliotoxin (41 nmol/L) (504) | |||||
| ShK toxin (16 pmol/L) (723) | |||||
| Psora-4 (62 nmol/L) (1472) | |||||
| PAP-1 (65 nmol/L) (1267) | |||||
| KV 1.2 | KCNA2 | KV β1, KV β2 | TEA (0.56 µmol/L) (504) | ||
| 4-AP (0.59 mmol/L) (504) | |||||
| correolide (700 nmol/L) (414) | |||||
| charybdotoxin (14 nmol/L) (504) | |||||
| α-dendrotoxin (17 nmol/L) (504) | |||||
| hongotoxin (170 pmol/L) (783) | |||||
| margatoxin (675 pmol/L) (783) | |||||
| ShK toxin (9 nmol/L) (723) | |||||
| Psora-4 (49 nmol/L) (1472) | |||||
| PAP-1 (250 nmol/L) (1267) | |||||
| KV 1.3 | KCNA3 | KV β2 | TEA (10 mmol/L) (504) | ||
| 4-AP (0.195 mmol/L) (504) | |||||
| correolide (86 nmol/L) (414) | |||||
| α-dendrotoxin (250 nmol/L) (504) | |||||
| dendrotoxin-k (2.5 nmol/L) (1208) | |||||
| hongotoxin (86 pmol/L) (783) | |||||
| margatoxin (230 pmol/L) (783) | |||||
| kaliotoxin (0.65 nmol/L) (504) | |||||
| ShK toxin (11 pmol/L) (723) | |||||
| Psora-4 (3 nmol/L) (1472) | |||||
| PAP-1 (2 nmol/L) (1267) | |||||
| KV 1.5 | KCNA5 | KV β1.2, KV | TEA (330 mmol/L) (504) | ||
| β2, KV β3 | 4-AP (0.27 mmol/L) (504) | ||||
| correolide (1.1 µmol/L) (414) | |||||
| Psora-4 (7.7 nmol/L) (1472) | |||||
| PAP-1 (45 nmol/L) (1267) | |||||
| KV 1.6 | KCNA6 | KV β1, KV β2 | TEA (1.7–7 mmol/L) (485, 509, 754) | ||
| 4-AP (0.3–1.5 mmol/L) (509, 754) | |||||
| correolide (450 nmol/L) (414) | |||||
| charybdotoxin (1 nmol/L) (509) | |||||
| α-dendrotoxin (25 nmol/L) (754) | |||||
| hongotoxin (6 nmol/L) (783) | |||||
| margatoxin (144 pmol/L) (783) | |||||
| ShK toxin (165 pmol/L) (723) | |||||
| PAP-1 (62 nmol/L) (1267) | |||||
| KV 2.1 | KCNB1 | KV 9.3 | TEA (4.9 mmol/L) (575) | ||
| 4-AP (18 mmol/L) (755) | |||||
| Ba2+ (30 mmol/L) (1369) | |||||
| SsmTx-1 (41.7 nmol/L) (230) | |||||
| stromotoxin-1 (12.7 nmol/L) (387) | |||||
| PAP-1 (3 µmol/L) (1267) | |||||
| KV 3.1 | KCNC1 | TEA (0.2 mmol/L) (504) | |||
| 4-AP (29 µmol/L) (504) | |||||
| PAP-1 (5 µmol/L) (1267) | |||||
| KV 4.1 | KCND1 | TEA (11 mmol/L) (1324) | |||
| 4-AP (1 mmol/L) (1324) | |||||
| phrixotoxin 1 (>250 nmol/L) (335) | |||||
| phrixotoxin 2 (>300 nmol/L) (335) | |||||
| KV 4.2 | KCND2 | TEA (11 mmol/L) (1324) | |||
| 4-AP (1 mmol/L) (1324) | |||||
| phrixotoxin 1 (5 nmol/L) (335) | |||||
| phrixotoxin 2 (34 nmol/L) (335) | |||||
| PAP-1 (1.2 µmol/L) (1267) | |||||
| KV 4.3 | KCND3 | TEA (~11 mmol/L) (1324) | |||
| 4-AP (1.2 mmol/L) (1380) | |||||
| phrixotoxin 1 (28 nmol/L) (335) | |||||
| phrixotoxin 2 (71 nmol/L) (335) | |||||
| KV 7.1 | KCND4 | KCNE1–5 | TEA (5 mmol/L) (1205) | ML277 (0.26 µmol/L) (964) | |
| Linopirdine (40 µmol/L) (1102) | ML213 (>10 µmol/L) (1609) | ||||
| XE991 (0.8 µmol/L) (1205) | R-L3 (L-364, 373; <1 µmol/L) (1236) | ||||
| Chromanol 293B (0.5–63 µmol/L) (130) | |||||
| HMR-1556 (120 nmol/L) (481) | |||||
| L735821 (173 nmol/L) (1275) | |||||
| Retigabine (~100 µmol/L) (517) | |||||
| KV 7.4 | KCNQ4 | KCNE1–5 | TEA (3 mmol/L) (1205) | ML277 (>30 µmol/L) (964) | |
| Linopirdine (14 µmol/L) (1321) | ML213 (0.5–0.8 µmol/L) (179, 1609) | ||||
| XE991 (5.5 µmol/L) (1321) | Retigabine (5.3 µmol/L) (517) | ||||
| KV 7.5 | KCNE1–5 | XE991 (65 µmol/L) (1205) | Retigabine (6.4 µmol/L) (517) | ||
| ML213 (700 nmol/L) (179) | |||||
| KV 9.3 | KCNS3 | KV 2.1 | See KV 2.1 earlier | ||
| KCa 1.1 | KCNMA1 | BKCa, Slo1 | β1–4 | Iberiotoxin (1.7 nmol/L) (1509) | NS1619 (1107) |
| (KCNMB1–4) | Charybdotoxin (2.9 nmol/L) (1509) | BMS204352 (352 nmol/L) (500) | |||
| LRRC26 | Paxilline (1.9 nmol/L) (1509) | Dehydrosoyasaponin-I (DHS-I) (60 nmol/L) (476) | |||
| TEA (0.14 mmol/L) (1509) | |||||
| Psora-4 (5 µmol/L) (1472) | 17β-Estradiol (2.6 µmol/L) (1454) | ||||
| PAP-1 (2.5 µmol/L) (1267) | |||||
| KCa 2.3 | KCNN3 | SKCa 3, SK3 | Calmodulin | Apamin (10 nmol/L) (1509) | EBIO (87–600 µmol/L) (1554) |
| UCL1684 (9.5 nmol/L) (1509) | NS309 (120–900 nmol/L) (1554) | ||||
| TRAM-34 (20 µmol/L) (1554) | SKA-31 (3 µmol/L) (1554) | ||||
| Psora-4 (5 µmol/L) (1472) | |||||
| PAP-1 (5 µmol/L) (1267) | |||||
| KCa 3.1 | KCNN4 | IKCa 1, IK1 | Calmodulin | Charybdotoxin (5 nmol/L) (1554) | EBIO (24–80 µmol/L) (1554) |
| Chlotrimazole (70 nmol/L) (1509) | NS309 (10–27 nmol/L) (1554) | ||||
| TRAM-34 (10–25 nmol/L) (1554) | SKA-31 (260 nmol/L) (1554) | ||||
| NS6180 (11 nmol/L) (1554) | |||||
| Psora-4 (5 µmol/L) (1472) | |||||
| PAP-1 (10 µmol/L) (1267) | |||||
| KIR 2.1 | KCNJ2 | Ba2+ (2 µmol/L at −100 mV; 19–30 µmol/L at −40 mV) (26, 881) | Extracellular K+ (3–20 mmol/L) (903) | ||
| Intracellular Mg2+ and polyamines (594) | |||||
| ML133 (1.9 µmol/L) (1496) | |||||
| PAP-1 (15 µmol/L) (1267) | |||||
| KIR 2.2 | KCNJ12 | Ba2+ (0.5 µmol/L at −100 mV; 9 µmol/L at −40 mV) (881) | Extracellular K+ (3–20 mmol/L) (903) | ||
| ML133 (2.9 µmol/L) (1496) | |||||
| Intracellular Mg2+ and polyamines (594) | |||||
| KIR 2.3 | KCNJ4 | Ba2+ (10.3 µmol/L at −100 mV; 70 µmol/L at −40 mV) (881) | Extracellular K+ (3–20 mmol/L) (903) | ||
| ML133 (4 µmol/L) (1496) | |||||
| Intracellular Mg2+ and polyamines (594) | |||||
| KIR 6.1 | KCNJ8 | SUR2b | Ba2+ (100 µmol/L) (154) | Diazoxide (32 µmol/L) (941) | |
| Glibenclamide (20–100 nmol/L) (1074, 1187) | Pinacidil (0.6 µmol/L) (941) | ||||
| Tolbutamide (350 µmol/L) (1187) | Levcromakalim (79 nmol/L) (941) | ||||
| KIR 6.2 | KCNJ11 | SUR2b | Ba2+ (100 µmol/L) (154) | Diazoxide (32 µmol/L) (941) | |
| Glibenclamide (20–100 nmol/L) (1074, 1187) | Pinacidil (0.6 µmol/L) (941) | ||||
| Tolbutamide (350 µmol/L) (1187) | Levcromakalim (79 nmol/L) (941) | ||||
| ML133 (7.7 µmol/L) (1496) |
Table 3.
Vascular RyRs and IP3Rs and Their Pharmacology
| Channel | Gene | Alternative names |
Accessory subunits | Inhibitors/antagonists (IC50) | Activators/agonists (EC50) |
|---|---|---|---|---|---|
| RyR1 | RYR1 | See (425, 921) for list of interacting proteins | Ryanodine (> 10 µmol/L)b (1657) | Ryanodine (100 nmol/L-1 µmol/L) (1657) | |
| Tetracaine (100 µmol/L) (1657) | Caffeine (0.2–0.5 mmol/L) (1657) | ||||
| RyR2 | RYR2 | See RyR1 | See RyR1 | See RyR1 | |
| RyR3 | RYR3 | See RyR1 | See RyR1 | See RyR1 | |
| IP3R1 | ITPR1 | See (434) for list of interacting proteins | Ca2+ (1.3–52 µmol/L) (434) | Ca2+ (57–348 nmol/L) (434, 1485) | |
| Heparin (4.1 µg/mL) (1237) | IP3 (34 nmol/L) (1237) | ||||
| Xestospongin C/D (358–844 nmol/L) (456) | Adenophostine A (4.5 nmol/L) (1237) | ||||
| 2-Aminoethoxydiphenyl borate (2-APB) (42 µmol/L) (950) | |||||
| IP3R2 | ITPR2 | See (434) for list of interacting proteins | Ca2+ (1.3–52 µmol/L)a (434) | Ca2+ (58 nmol/L) (1485) | |
| Heparin (22 µg/mL) (1237) | IP3 (151 nmol/L) (1237) | ||||
| 2-Aminoethoxydiphenyl borate (2-APB) (~100 µmol/L) (1237) | |||||
| IP3R3 | ITPR3 | See (434) for list of interacting proteins | Ca2+ (0.3–39 µmol/L) (434) | Ca2+ (77 nmol/L) (434) | |
| Heparin (2.8 µg/mL) (1237) | IP3 (219 nmol/L) (1237) | ||||
| 2-Aminoethoxydiphenyl borate (2-APB) (> 100 µmol/L) (1237) | Adenophostine A (19.5 nmol/L) (1237) |
Table 4.
Vascular Transient Receptor Potential Channels and Their Pharmacology (259)
| Channel | Physiological activation | Selectivity [ratio] | Inhibitors/antagonists [IC50] | Activators/agonists [EC50] |
|---|---|---|---|---|
| TRPC1 | Gq signaling; SOCE with STIM1 (1078, 1238) | Ca2+:Na+ [~1:1] (1347) | La3+ [n.d.] (1347) | n.d. |
| Gd3+ [n.d.] (1655) | ||||
| TRPC3 | Diacylglycerols (612) | Ca2+:Na+ [1.6:1] (1654) | Gd3+ [0.1 µmol/L] (539) | OAG [n.d.] (612) |
| La3+ [4 µmol/L] (539) | ||||
| 2-APB [10 µmol/L] (874) | ||||
| BTP2 [0.3 µmol/L] (574) | ||||
| TRPC4 | Gi signaling; protons (693, 1279) | Ca2+:Na+ [1.1:1] (1258) | Niflumic acid [n.d.] (773) | (−)-englerin A [11.2 nmol/L] (25) |
| ML204 [3.2 µmol/L] (1002) | ||||
| TRPC5 | Gq/Gi/Go signaling; calpain cleavage (693, 721, 1258) | Ca2+:Na+ [1.8:1] (1258) | 2-APB [20 µmol/L] (1568) | rosiglitazone [32 µmol/L] (932) |
| KB-R7943 [1.3 µmol/L] (789) | Ca2+ [1 µmol/L] (507) | |||
| Mg2+ [500 µmol/L] (1101) | genistein [n.a.] (1543) | |||
| TRPC6 | Diacylglycerols; stretch (indirectly) (612, 1331) | Ca2+:Na+ [4.5:1] (647) | Gd3+ [1.9 µmol/L] (647) | OAG [n.d.] (612) |
| La3+ [3.9 µmol/L] (647) | SLG [n.d.] (612) | |||
| SKF96365 [3.9 µmol/L] (647) | SAG [n.d.] (612) | |||
| TRPV1 | Depolarization; heat; protons (206, 823) | Ca2+:Na+ [9.6:1] (206) | Capsazepine [40 nmol/L] (979) | capsaicin [30 nmol/L] (1481) |
| JNJ17203212 [16 nmol/L] (1365) | resiniferatoxin [4 nmol/L] (1310) | |||
| AMG517 [1 nmol/L] (143) | anandamide [1.3 µmol/L] (1309) | |||
| TRPV2 | Osmolarity/stretch; heat (rodent); IGF-1 (rodent) (205, 731, 1067) | Ca2+:Na+ [2.9:1] (205) | Tranilast [10 µmol/L] (1081) | probenecid [3.9 µmol/L] (83) |
| ruthenium red [0.6 µmol/L] (205) | Δ9-THC [16 µmol/L] (1181) | |||
| SKF96365 [n.d.] (720) | cannabidiol [79 µmol/L] (1181) | |||
| TRPV3 | Depolarization; heat (1566) | Ca2+:Na+ [12.1:1] (1566) | 17(R)-resolvin D1 [0.4 µmol/L] (84) | eugenol [2 mmol/L] (1562) |
| 2,2-di(phenyl)oxolane [8 µmol/L] (255) | carvacrol [4 µmol/L] (363) | |||
| 2-APB [25 µmol/L] (256) | ||||
| TRPV4 | Heat; mechanical stimuli; Gq signalling (994, 1345, 1501) | Ca2+:Na+ [6.9:1] (1482) | GSK2193874 [5 nmol/L] (1407) | 4α-PDD [0.2 µmol/L] (1499) |
| HC067047 [50 nmol/L] (392) | GSK1016790A [20 nmol/L] (1408) | |||
| RN1734 [2.5 µmol/L] (1480) | 5,6-EET [0.1 µmol/L] (1500) | |||
| TRPM4 | Intracellular Ca2+ (824) | Ca2+:Na+ [< 0.001:1] (1088) | 9-phenanthrol [20 µmol/L] (497) | BTP2 [8 nmol/L] (1378) |
| flufenamic acid [2.5 µmol/L] (1445) | decavanadate [2 µmol/L] (1089) | |||
| ATP [1 µmol/L] (1092) | PIP2 [5 µmol/L] (1085) | |||
| TRPM8 | Depolarization; cooling (1481) | Ca2+:Na+ [3.2:1] (980) | BCTC [0.8 µmol/L] (107) | Icilin [0.2 µmol/L] (48) |
| M8-B [0.8 µmol/L] (38) | menthol [16 µmol/L] (107) | |||
| PBMC [0.5 nmol/L] (767) | WS-12 [13 µmol/L] (1289) | |||
| TRPA1 | Chemosensation (129, 1490) | Ca2+:Na+ [0.84:1] (1046) | Resolvin D2 [2 nmol/L] (1137) | AITC [10 µmol/L] (714) |
| A967079 [63 nmol/L] (227) | acrolein [5 µmol/L] (97) | |||
| HC030031 [6.3 µmol/L] (983) | PF-4840154 [25 nmol/L] (1229) | |||
| TRPP1* | Cilial mechanosensation; Development (1278) | Ca2+:Na+ [6:1] (323) | SKF96365 [n.d.] (1148) | Calmidazolium [n.d.] (323) |
In this instance, “TRPP1” is used to describe the Pkd2 gene product. Prior to 2014, this same gene product is often referred to as “TRPP2” or “PKD2” in the literature. For further clarification, see the Section titled, “Polycystin (TRPP) Channels.”
n.d. = not determined.
Approaches Used to Study Ion Channels in Resistance Arteries and Arterioles
The study of ion channel expression and function in SMCs of resistance arteries and arterioles requires the application of multiple methods applied to preparations that range from patch-clamp of freshly isolated SMCs, to pressure myography of isolated vessels, to in vivo imaging of arterioles in the living microcirculation of anesthetized animals, to the measurement of blood flow and blood pressure in conscious, freely moving animals. While cultured cells can be utilized for patch-clamp study of the biophysical properties of ion channels and the potential for their modulation by interactions with other proteins and post-transcriptional modification, this approach is not viable for the study of the physiological function of a given channel due to the ion channel remodeling that occurs as cells proliferate in culture (103, 625, 1037, 1425, 1443). Patch-clamp (102, 105, 106, 110, 154, 175, 308, 458, 646, 672, 673, 1072, 1075, 1544) and imaging (41, 266, 994, 1061, 1071) approaches applied to SMCs freshly isolated from resistance arteries or arterioles (114, 673, 1072, 1075, 1186) are required to define the functional expression and activity of an ion channel in its native context. However, it should be understood that this approach also is limited because isolated cells are usually studied at room temperature and are not exposed to the signaling environment (pressure-induced cell strain, exposure to hormones and neurotransmitters, signaling generated from interactions with the extracellular matrix, homotypic and heterotypic interactions with other cells, etc.) that occurs in the native environment of the vessels.
Isolated resistance arteries and arterioles studied by pressure myography (349, 542) provide additional information about the physiological function of an ion channel in a more complete system where the SMCs are exposed to physiological temperature, pressure-induced stress and strain, interactions with the extracellular matrix and interactions with other cells. In this setting, the use of microelectrodes to monitor membrane potential (185, 380, 763), imaging approaches to visualize global and local Ca2+ signals (393, 683, 764, 1527, 1528), the measurement of diameter to assess SMC contractile function, the judicious use of channel blockers and the use of genetically modified animal models provides an integrated view of ion channel function in these important vessels. However, it is not yet possible to apply patch-clamp methods to SMCs in this native environment in pressurized vessels because these cells are embedded in extracellular matrix proteins. Hence, the ion channel “signature” information (single channel conductance, channel kinetics, current-voltage relationship, etc.) that can be resolved by patch-clamp is not yet available with the study of intact vessels. Therefore, the ability to resolve the function of a single class of ion channels in pressure myography experiments is limited to the selectivity of available blockers (which is often lacking) and/or the availability of genetically modified animal models. In addition, isolated vessels are not tethered to the extracellular matrix as they are, in vivo, and neural and hormonal input are absent. Isolated vessels also lack input from upstream and downstream segments of the microvascular network from which they were removed. Furthermore, pressure myography is limited by our ability to dissect vessels with sufficient unbranched length to be cannulated, and have an anatomical location that is amenable to surgical isolation of the vessels; hence not all vessels can be studied.
The study of resistance arteries and arterioles by intravital microscopy (79, 147, 244, 348, 587, 847, 1276, 1524, 1603) allows interrogation of vessels in their native environment, removing some of the shortcomings of the study of isolated vessels while adding additional limitations that include lack of control of the environment (blood pressure, blood flow, hormonal and neural input, etc.) and difficulty identifying the cellular site of action of ion channel blockers, which are necessary to define the role of a specific ion channel in a physiological process. The use of cell-specific, conditional knockout animal models coupled with careful pharmacology can help resolve some of these issues. However, the necessary use of anesthetics adds additional constraints on interpretation of data from intravital experiments. Approaches to visualize resistance arteries and arterioles in conscious animals, in the absence of anesthetics have been developed, but are limited in their application (393, 586, 1093).
The measurement of blood pressure (1253) and blood flow (463, 516, 812, 1159, 1368) in intact animals provides a 30, 000 ft view of the integrated function of resistance artery and arteriolar networks. However, these approaches do not provide the resolution to define the site of action of drugs or interventions on ion channel function that can be achieved by the study of single cells or isolated vessels, in vitro, or imaging of single vessels or microvascular networks, in vivo.
Thus, all of the approaches available to study ion channel function in resistance arteries and arterioles have unique limitations that should be acknowledged. Only when combined do they provide a complete view of the function of a given SMC ion channel in the physiology and pathophysiology of these vessels.
Voltage-Gated Ca2+ Channels
VGCCs transduce membrane depolarization into augmented Ca2+ influx into vascular SMCs and play an important role in the regulation of both contraction and gene expression in vascular SMCs (316, 599, 600, 1026, 1073). They are members of a gene superfamily of plasma membrane ion channels that includes voltage-gated Na+ channels and voltage-gated K+ channels (210).
Discovery of VGCCs
Calcium-based action potentials, the first evidence for VGCCs, were recorded from crustacean muscle by Fatt and Katz (412). Later, currents through Ca2+-selective channels were recorded in cardiac Purkinje fibers (1200) and it subsequently became apparent that there were multiple classes of VGCCs (101, 208). The original characterization of VGCCs was based on the pharmacology and electrophysiological characteristics of currents through these channels (101, 208). L-type Ca2+ currents activated at relatively positive potentials (high voltage of activation: threshold at −30 to −40 mV), had high single channel conductances (20–27 pS with 110 mmol/L Ba2+ as the charge carrier), showed slow voltage-dependent inactivation, displayed long lasting currents with Ba2+ as the charge carrier, and were inhibited by dihydropyridine, phenylalkylamine, and benzothiazepine organic Ca2+ channel blockers (101, 208, 1433). T-type Ca2+ currents activated at relatively negative membrane potentials (−60 to −70 mV), showed rapid voltage-dependent inactivation, had small single channel conductances (8 pS in 110 mmol/L Ba2+), and were relatively insensitive to organic Ca2+ channel blockers (101, 208, 1433). Currents through some neuronal Ca2+ channels appeared to be intermediate between L- and T-type and could be distinguished by their sensitivity to ω-conotoxin and were categorized as N-type currents (101, 208, 1433). P-type Ca2+ currents were later identified in Purkinje neurons and were recognized by their sensitivity to blockade by ω-agitoxin, but not organic Ca2+ channel blockers or ω-conotoxin (101, 208). Q-type Ca2+ currents have similar characteristics to P-type, but show lower sensitivity to block by ω-agitoxin (208). Finally, R-type Ca2+ currents were identified that were high-voltage-activated, but resistant to organic Ca2+ channel blockers and the toxins that block N-, P-, and Q-type currents (208).
Ten distinct genes are now recognized, divided into three main subfamilies that encode the pore-forming α1 subunits of VGCCs (210): CaV 1, CaV 2, and CaV 3 (210). The CaV 1 subfamily contains four members (CaV 1.1, 1.2, 1.3, and 1.4) that represent channels that carry high-voltage-activated, long lasting (L)-type Ca2+ currents. The CaV 2 subfamily (CaV 2.1, 2.2, and 2.3) includes channels that carry P/Q-, N-, and R-type currents, respectively. The CaV 3 family members (CaV 3.1, 3.2, and 3.3) constitute low-voltage-activated, transient (T)-type VGCCs.
Alternative splicing amplifies the number of gene products from the ten genes encoding these channels. For example, splice variants of CaV 1.2 in domain I, segment 6 distinguish L-type Ca2+ channels expressed in the heart (CaV 1.2a) from those in vascular SMC (CaV 1.2b), and account for differences in the pharmacology and biophysics of L-type Ca2+ channels expressed in these tissues (1515).
Structure of VGCCs
VGCCs consist of a large, ~190 kD α1 subunit, that forms the ion-conducting pore and contains the voltage sensors, gating apparatus and sites of channel regulation by drugs, second messengers and toxins (209, 210). There is a common theme regarding the structure of the pore-forming portion of all of the ion channels covered in this review, as depicted in Figure 2. Two membrane spanning domains (either alone as for KIR channels, or as part of 6-transmembrane domain structures) and a connecting pore-forming loop (P-Loop) contribute to the channels’ pores, with four such units coalescing to form the ion conducting portion of the channels. Modulatory β, α2, and δ subunits are present in most types of VGCCs, with γ subunits also found in channels expressed in skeletal muscle and parts of the brain (209, 210). The β and α2δ subunits are important for expression of the channels in the plasma membrane (209). These accessory subunits also modulate the voltage dependence and the gating kinetics of VGCCs (209). There are four genes that encode the β-subunits (β1–4) and four that encode the α2δ subunits (α2δ1–4) (195, 209). Both are also subject to alternative splicing (195, 209). Vascular SMCs express β2 (283), β3 (745, 1038, 1039), α2δ1 (87, 88, 283) and α2δ3 (283) isoforms of these accessory subunits.
Figure 2.
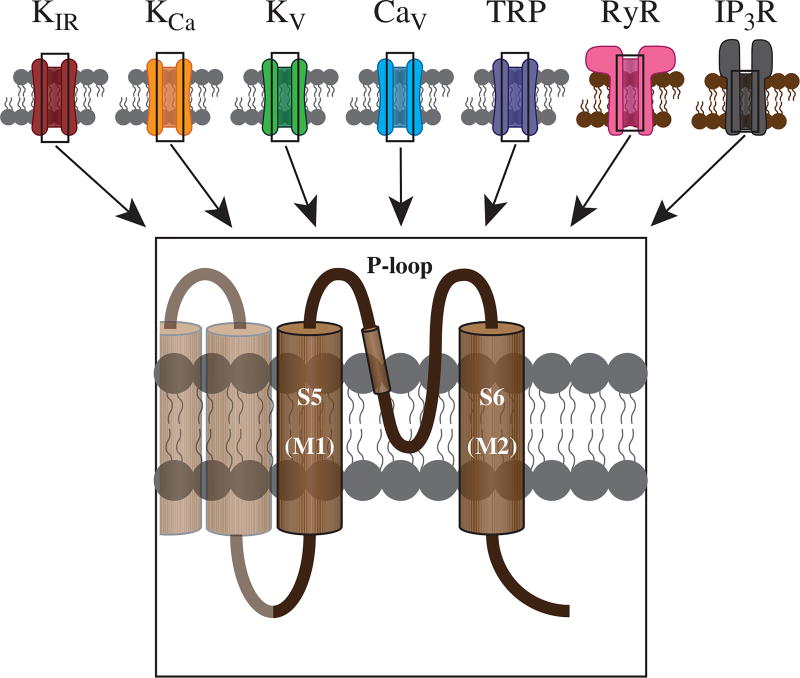
Pore-forming subunits of ion channels. All ion channels share a similar topology, wherein the S5 and S6 transmembrane domains (M1 and M2 for KIR channels) form the ion-permeable pore. These two domains are linked by a pore-loop (P-loop), which contains multiple residues responsible for regulating pore function and ion selectivity. See text for details.
The α1 subunit of VGCCs consists of four sets (I–IV) of six transmembrane spanning domains (S1–S6) linked by intracellular peptide loops (208, 209), similar to the structure of voltage-gated sodium channels (207, 209). The pore of the channel is formed by segment 6 and the peptide loop (P-loops) between segments 5 and 6 of the four domains (Fig. 2), and the voltage sensor is located in the S4 segments (208, 209). Glutamate residues in the P-loops form the selectivity filter and residues in segment 6 form the binding site for organic Ca2+ channel blockers (209). The N- and C-terminal domains are both intracellular (209). Accessory β subunits are located intracellularly and bind to the α-interaction domain in the intracellular P-loop between domains I and II (209). The α2 subunit is located extracellularly and is linked to the δ subunit by a disulfide bridge (209). The δ subunits are postranscriptionally modified with a glycophosphatidylinositol membrane anchor, tethering these proteins to the membrane in association with the α1-pore-forming subunit (209). Cerebrovascular SMCs express α2δ1 subunits that are essential for trafficking and targeting CaV 1.2 VGCCs to the plasma membrane (87, 88). Vascular SMCs also express β2 (283) and/or β3 subunits (745) that increase the stability of the channel proteins by inhibiting their degradation, and which mediate upregulation of the channels by angiotensin II.
Calcium channels exist in signaling complexes with a large number of binding partners that contribute to the localization and regulation of these VGCCs (195, 302, 565, 1064, 1442). For example, in addition to the β3, and α2δ1 subunits, CaV 1.2 in vascular SMCs also associates with the scaffolding protein, A-kinase anchoring protein (AKAP) 150, which targets protein kinase C (PKC) (1063), protein kinase A (PKA) (1065), and the protein phosphatase, calcineurin (PP2b) (1064) to the channel and is required for kinase-dependent regulation of the CaV 1.2 (see following text for more on this topic). The C-terminus of CaV 1.2 also binds calmodulin, which contributes to both Ca2+-dependent inactivation and facilitation of CaV 1.2 (1458, 1658). Based on studies in other systems, such as the heart (565) and neurons (195) additional binding partners are likely.
Evidence for VGCCs in vascular SMCs
Early evidence for the presence of VGCCs in vascular SMCs came from studies demonstrating inhibition of vascular SMC contraction (429, 482, 1147) and block of vascular SMC Ca2+-dependent action potentials (108, 554) by organic VGCC blockers (Table 1). Subsequently, application of patch clamp approaches identified voltage-gated, Ca2+-selective single channel currents in membrane patches from vascular SMCs isolated from rabbit mesenteric arteries that were potently inhibited by the VGCC blocker, nisoldipine and activated by the dihydropyridine Ca2+ channel agonist, BayK 8644 (1544). Two populations of single channel events were detected in these experiments with conductances of 8 and 15 pS (in 80 mmol/L Ba2+) suggesting the expression of two classes of VGCCs. Whole-cell recordings of macroscopic Ca2+ currents from vascular SMCs from rat mesenteric arteries also identified two separable Ca2+ currents (102): a fast-inactivating current with activation threshold at −40 mV and a slowly inactivating current with threshold activation at −10 mV with 115 mmol/L Ba2+ as the charge carrier. The slowly inactivating current displayed voltage-dependent block by nitrendipine and activation by Bay K 8644, whereas the fast-inactivating current was resistant to block by nitrendipine. The voltage dependence, kinetics and pharmacology of the currents reported by Bean et al. (102) were very similar to those reported in cardiac myocytes (100), and were the first to provide evidence for the presence of both L-type and T-type VGCCs in vascular SMCs. Similar results were reported in SMCs isolated from rat Azygos vein (1349). Currents resembling those through L-type and T-type channels were also reported in vascular SMCs isolated from rabbit ear artery at both the whole-cell and single channel levels (113).
Pharmacology of vascular SMC VGCCs
VGCCs display distinct pharmacology that can help in the identification of the specific channels that function in systems where multiple VGCCs are expressed (Table 1). Dihydropiridines such as nifedipine and nimodipine are relatively selective blockers of CaV 1.2 channels expressed in vascular SMCs, (Table 1). The phenylalkylamine, verapamil, and the benzothiazepine, diltiazem are likewise relatively selective blockers for this class of VGCCs (Table 1) and can allow separation of processes mediated by CaV 1.2 and CaV 3 channels, for example. However, as with all drugs, care must be taken with the concentrations used. For example, nifedipine at nanomolar concentrations (IC50 = 10–100 nmol/L) (872) and diltiazem at low micromolar concentrations (IC50 = 500 nmol/L) (615) are quite selective for CaV 1.2 channels. However, both of these drugs also block some voltage-gated K+ channels at higher concentrations: for example, the IC50 for KV 1.2 is 20 µmol/L for nifedipine and 200 µmol/L for diltiazem (504). This is not a problem in patch clamp studies where the voltage and ionic characteristics of currents can be isolated. However, in studies of isolated vessels and especially in vivo, where a number of channels are functioning simultaneously, the typical use of high concentrations of these drugs to ensure channel blockade also insures the likelihood of off-target effects.
Dihydropyridine blockers and activators of CaV 1.2 channels display significant voltage dependence: they are more effective at depolarized membrane potentials, and bind preferentially to the inactivated state of the channels (1252). Thus, translation of IC50 or EC50 data for these compounds from typical patch clamp experiments, where SMCs are held at very negative membrane potentials (e.g., −80 mV), to experiments in pressurized vessels that develop myogenic tone in which SMCs are relatively depolarized (e.g., −30 to −40 mV), is difficult. This likely explains the potent activation of L-type VGCCs by Bay K 8644 (EC50 = 6 nmol/L) reported by Zheng et al. (1638) for contraction of vascular smooth muscle, relative to patch clamp reports for cardiac myocytes (EC50 = 30 nmol/L) (546).
The divalent metal ions, Cd2+ and Ni2+ also display some selectivity for classes of VGCCs expressed in vascular SMCs (Table 1). For Cd2+, CaV 1.2 channels (IC50 = 7 µmol/L) (1054) are blocked at lower concentrations than are CaV 3.1 and 3.2 channels (IC50 = 160 µmol/L) (833). Nickel ions can be used to separate currents through CaV 3.2 channels (IC50 = 5.7–12 µmol/L) (833) from currents through CaV 1.2 (IC50 = 280 µmol/L) (1054) and CaV 3.1 (IC50 = 167–250 µmol/L) (833) channels. However, Cd2+ also blocks the Na+/Ca2+ exchanger with an IC50 = 321 µmol/L such that off target effects are likely at concentrations of Cd2+ of 100 µmol/L or greater (605).
Selective blockade of CaV 3, T-type channels can be achieved with kurtoxin, which is quite selective for these channels, relative to CaV 1.2 channels (Table 1). Mibefradil, on the other hand, is not as selective (Table 1), making its use in intact tissue and in vivo, problematic. Newer, small molecule blockers of CaV 3 channels, such as ML 218 (1560) may prove useful. However, the use of this compound in vascular systems has not been reported.
L-type VGCCs and myogenic tone
The early studies outlined earlier provided the first evidence that vascular SMCs express multiple classes of VGCCs. Importantly, they demonstrated that the inhibitory effects of dihydropyridine Ca2+ channel blockers on SMC tone (429) were consistent with effects of this class of VGCC blocker on L-type channels which appear to provide a major source of activator Ca2+ in vascular SMCs in the wall of resistance arteries (1073). This has been confirmed in rat middle cerebral arteries where the voltage dependence of intracellular Ca2+ and myogenic tone matches that for currents through L-type VGCCs, and both depolarization-induced increases in intracellular Ca2+ and myogenic tone are prevented or reversed by the L-type VGCC blockers nisoldipine and diltiazem (764). These SMCs express a splice variant of CaV 1.2 in exon 1 that alters the effects of α2δ1 and β3 VGCC subunits on the membrane insertion and protein stability of these channels and produces a leftward (negative) shift in the voltage-dependent activation of the channels (236). Additional splice variants have also been identified in these cells (237).
As noted earlier, data from rat cerebral arteries indicate that Ca2+ influx through CaV 1.2-containing L-type VGCCs is the major source of activator Ca2+ involved in pressure-dependent, myogenic tone. This conclusion is supported by studies of skeletal muscle resistance arteries from conditional, SMC-specific knockout of CaV 1.2, in which pressure-induced myogenic tone was absent from vessels isolated from the knockouts at pressures above 40 mmHg, and where reduced vascular resistance of perfused hind limb preparations was observed (1026). However, interpretation of these data is complicated by findings that L-type VGCCs are essential for initiation of myogenic tone, but perhaps not all of the steady-state tone of some vessels (785).
Studies of other vessels support a significant, but not exclusive role for Ca2+ influx through L-type channels in myogenic tone. In first-order Sprague-Dawley rat cremaster muscle arterioles, the relationship between membrane potential and tone is steeper than observed in cerebral arteries (764), and in these rat cremaster arterioles, only 33% of Ca2+-dependent tone was eliminated by nifedipine (1 µmol/L) (785), suggesting that Ca2+ influx through L-type VGCCs is not the sole source of activator Ca2+ in this tissue. In contrast, cumulative nifedipine concentration-response studies by this same group indicated that Ca2+ influx through L-type VGCCs accounted for >70% of Ca2+-dependent myogenic tone in a subsequent study in the same arterioles (1171). The reason for this difference in the role played by L-type VGCCs between these studies is not apparent. Nonetheless, the former study showed that preincubation of the arterioles with nifedipine (1 µmol/L) prevented the development of pressure-induced myogenic tone indicating an essential role for L-type VGCCs in the initiation phase of the response to increased transmural pressure in cremaster arterioles, consistent with studies in most other vessels (315, 316, 596, 600).
Several in vivo studies also support a major role for L-type VGCCs in myogenic tone, at rest. Nifedipine, at concentrations where it selectively blocks L-type VGCCs, produces nonmaximal dilation of pial arterioles in anesthetized cats (169). This dihydropyridine also eliminates norepinephrine-induced myogenic reactivity in first-order rat cremaster arterioles, in vivo (885). Diltiazem or verapamil dilated arterioles in rat cremaster muscles, in vivo (717). In anesthetized pigs, diltiazem increases blood flow, nonmaximally, at a normal perfusion pressure of 100 mmHg and severely impairs blood flow autoregulation (127). Similarly, the L-type VGCC blocker, nicardipine, increases coronary blood flow in the hearts of anesthetized dogs (768). In some instances, nifedipine has been shown to dilate arterioles in the cremaster muscle of anesthetized mice (627, 634, 967) [although contrary results have been reported (1079, 1149)—see later]. Nifedipine also dilates rat epi-neural arterioles in vivo, supporting a role for L-type VGCCs in resting myogenic tone in these arterioles (1234).
In distinct contrast, there are a number of studies showing little or no effect of L-type VGCC blockers on resting myogenic tone or blood flow. Hill and Meininger (599) studied rat cremaster arterioles by intravital microscopy. These vessels had substantial myogenic tone (resting diameter was ~50% of maximal diameter), and the hyperpolarizing vasodilator, pinacidil, produced 94% dilation, suggesting that tone in these vessels arose from a voltage-dependent mechanism. However, neither nifedipine nor methoxyverapamil significantly dilated the arterioles at concentrations where they should maximally block L-type VGCCs. The authors did find that the VGCC blockers abolished vasomotion, establishing the efficacy of the drugs in this system. Similar results have been obtained for arterioles in hamster cremaster muscles (670), where nifedipine did not produce steady-state dilation of arterioles with substantial myogenic tone, but abolished vasomotion of these vessels. A lack of effect of nifedipine on resting diameter of cheek pouch arterioles, in vivo, was reported by Boric and colleagues (159). Similarly, myogenic tone resistant to L-type VGCC blockade was also reported by Welsh et al. (1522) in the hamster cheek pouch where resting diameters of arterioles were not significantly influenced, in the steady state, by either nifedipine or diltiazem at concentrations that blocked constrictions induced by elevated extracellular K+ or elevated solution PO2. As in rat cremaster muscle, arterioles in hamster cremaster and cheek pouch dilate when exposed to K+ channel agonists such as cromakalim or pinacidil (664) indicating the voltage-dependence of resting tone in these preparations. In the cremaster muscle of anesthetized mice, nifedipine (1079) or diltiazem (1149) had no effect on resting arteriolar tone, in contrast to studies noted earlier (627, 634, 967). Nifedipine also is without effect on resting coronary blood flow in conscious dogs (77) and pigs (126) instrumented for coronary blood flow measurements. The lack of effect of L-type VGCC blockers on resting myogenic tone in these systems suggests that voltage-dependent Ca2+ influx pathways other than L-type VGCCs are involved in resting myogenic tone, in vivo, whereas the same arterioles studied by pressure myography, in vitro, invariably depend heavily on L-type VGCCs. The presence of T-type VGCCs in addition to L-type VGCCs, as reported in rat cremaster arterioles (1460), might provide an explanation, particularly if membrane potential was slightly more hyperpolarized, in vivo. While not statistically significant, SMC membrane potential in hamster cheek pouch arterioles has been reported to be slightly more hyperpolarized in vivo (−41 ± 4 mV) than what was measured in similar vessels, in vitro (−33 ± 1 mV) (670).
L-type VGCCs and vasomotion
Vasomotion, rhythmic oscillations in vessel diameter, is a hallmark characteristic of arterioles in the microcirculation. A number of studies, in vivo and in vitro, have shown that blockers of L-type VGCCs inhibit vasomotion (1, 93, 522, 523, 529, 599, 670, 998, 1010). While there are exceptions to this rule (530), the majority of published studies indicate that vasomotion depends on Ca2+ influx through L-type VGCCs.
Vasoconstrictors and L-type VGCCs
With few exceptions, vasoconstrictor agonists that act through Gq/11-coupled receptors cause contraction of SMCs in blood vessels that can be inhibited, at least in part, by organic L-type VGCC blockers (53, 55, 133, 138, 146, 211, 212, 369, 482, 532, 611, 635, 657, 808, 809, 901, 959, 1007, 1073, 1075, 1138, 1242, 1337, 1367, 1447, 1449, 1545, 1649). This is due to not only agonist-induced depolarization of the SMC membrane, but also to a direct augmentation of the function of L-type VGCCs by the agonists (611). Supporting these pharmacological findings, SMC-specific knockout of CaV 1.2 decreases contractions induced by the α1-adrenoreceptor agonist, phenylephrine, in mouse resistance arteries (1026). Similarly, siRNA knockdown of CaV 1.2 in rat mesenteric arteries severely compromises norepinephrine-induced contraction (795).
In SMCs from rabbit ear artery, Benham and Tsien (114) found that norepinephrine increased whole-cell currents through L-type VGCCs. These results were difficult to interpret, however, because the effects of norepinephrine could not be inhibited by phentolamine, prazosin, or propranolol. Nelson et al. (1075) demonstrated that the voltage dependence of contraction induced by norepinephrine in rabbit mesenteric arteries was similar to that for L-type VGCCs and that this adrenergic agonist increased the open-state probability of single L-Type VGCCs independent from changes in membrane potential. As the agonist was applied outside the membrane patch (i.e., in the bath solution), the authors concluded that a second-messenger system must be involved in the adrenoreceptor augmentation of L-type VGCC function. Subsequent studies of SMCs from a number of vascular beds have confirmed these initial reports and extended them to different G-protein coupled receptor agonists including serotonin (5-HT) (1545), histamine (657), angiotensin II (64, 199, 204, 927, 928, 1470, 1477), and endothelin (494, 496, 648, 1304).
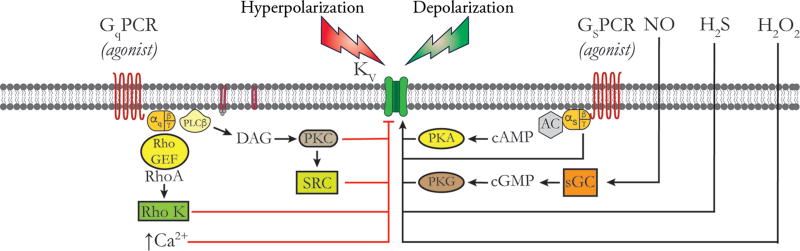
The mechanisms by which Gq/11-coupled receptor agonists increase the open-state probability of VGCCs appears to depend on activation of PKC (740) (Fig. 3). Based on the effects of antagonists of different PKC isoforms, it appears that both conventional (α, β1, β2, and γ) and novel (δ, ε, η, and θ) isoforms of PKC can modulate CaV 1.2 VGCCs. PKC-α is targeted to CaV 1.2 via AKAP150 (1063) and produces persistent opening of CaV 1.2 clusters, resulting in high activity CaV 1.2-Ca2+ sparklets that appear to significantly contribute to global intracellular Ca2+ levels in SMCs from murine cerebral and mesenteric arteries (41, 1061, 1063) and myogenic tone (1063). This mechanism may not be functional in all vessels or species as there are several instances in the literature where effective inhibition of PKC does not substantially inhibit nifedipine-sensitive myogenic tone (597, 662, 690). In SMCs from portal vein, activation of α1-adrenoreceptors coupled via Gq/11 or α2-adrenoreceptors coupled to Gi each activate L-type VGCCs via a PKC-dependent pathway (845, 1009). A novel PKC has been proposed to contribute to Gq/11-coupled receptor-mediated enhancement of current through L-type VGCCs in these same cells (192). In this pathway, the βγ subunit of the G-protein activates phosphatidylinositol 3,4,5-trisphosphate (PiP3) Kinase γ (PiP3Kγ), which, in turn, activates a novel PKC, phosphorylating and activating the proto-oncogene tyrosine-protein kinase Src (c-SRC), which then acts on the L-type-VGCCs to increase channel activity (192) (Fig. 3). The role of the PKC-c-SRC pathway in agonist-induced enhancement of L-type VGCC currents and vasoconstriction to other G-protein coupled receptors in other vessels has not been established. However, a role for the Gβγ, PiP3Kγ, and PKC also has been proposed for angiotensin II-induced stimulation of currents through L-type VGCCs (927, 928, 1189, 1478). In contrast, it has been shown that phosphatidylinositol 3,4,5-trisphosphate (PiP3) produced by PiP3Kγ increases the activity of L-type VGCCs directly and accounts for angiotensin II-induced augmentation of Ca2+ channel activity independent from PKC (828) (Fig. 3). This may account for angiotensin II signaling via receptors coupled to G12/13 and PiP3Kγ (828, 927, 1189, 1478). Chronic stimulation of vascular SMCs with angiotensin II leads to increased expression of L-type VGCCs in the plasma membrane that is mediated by PiP3Kγ induced activation of PKB/Akt, phosphorylation of β2a subunits and inhibition of degradation (increased stability) of these channels in other systems (204, 1477).
Figure 3.
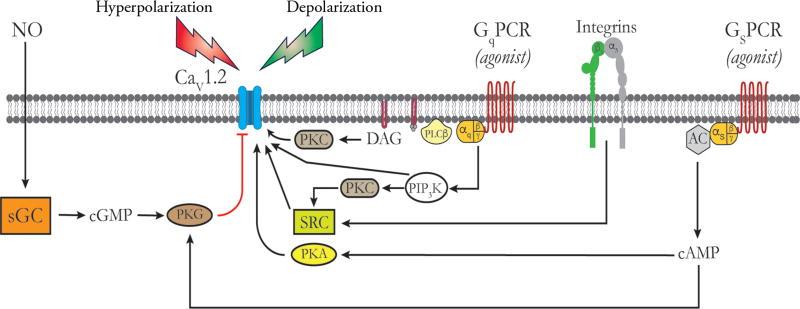
Regulation of CaV 1.2 channels by vasoconstrictors and vasodilators. Schematic of the plasma membrane of a vascular SMC showing, from left to right, a CaV 1.2 channel, a Gq-protein coupled receptor (GqPCR), an α5β1 Integrin and a Gs-protein coupled receptor (GSPCR). Black lines and arrows indicate stimulation, activativation or increases; red lines indicate inhibition. Pathways to the right of the CaV 1.2 activate these channels, while those to the left are inhibitory. Membrane depolarization due to opening of membrane channels that conduct Na+, Ca2+, or Cl− or due to closue of K+ channels represents the major stimulus for opening CaV 1.2 channels. Vasoconstictor agonists that act through GqPCRs norepinephrine, endothelin, angiotensin II, 5-HT, etc.) are coupled to phospholipase Cβ (PLCβ), which acts on q membrane phophoinositol bisphosphate to form diacylglycerol (DAG), which, in the presence of Ca2+, activates PKC. PKC phosphorylates CaV 1.2 to increase its open-state probability. GqPCR activation can also stimulate phosphatidyl inostitol trisphosphate kinase (PIP3K), which acts on novel PKCs to activate the tyrosine kinase SRC, as shown. SRC phosphorylates CaV 1.2 channels, increasing their activity. Activation of CaV 1.2 by PIP3K independent of PKC and SRC has also been reported. SRC can also be activated by activated inetgrins as shown, also increasing CaV 1.2 activity. Agonists for GsPCR (isoproterenol, adenosine, prostacyclin, CGRP, etc.) activate adenylate cyclase (AC) to increase the formation of cAMP which activates PKA. PKA phosphorylates CaV 1.2 to increase the activity of this channel. Membrane hyperpolarization due to opening of K+ channels or closure of channels conducting Na+, Ca2+, or Cl− represents the main stimulus for deactivation of CaV 1.2 channels. In addition, nitric oxide (NO) acting through soluble guanylate cyclase (sGC), and other agents that increase cGMP, activate protein kinase G (PKG) which can phosphorylate CaV 1.2 channels to decrease their activity. In addition, high levels of cAMP can transactivate PKG accounting for the inhibitory effects of high levels of activation of GsPCR or direct activators of AC such as forskolin on CaV 1.2 channel activity. See text for details and references.
As noted earlier, c-SRC also appears to modulate the activity of CaV 1.2 VGCCs through PKC-dependent (192) and independent mechanisms (515). Like PKC α, c-SRC has been shown to promote persistent activity of CaV 1.2 and resulting VGCC-Ca2+ sparklets via phosphorylation of a tyrosine residue (Y2122) in the C-terminus of the channel’s α subunit (515). c-SRC resides in macromolecular complexes with CaV 1.2 and associates with the C terminus (515). Davis and colleagues (221, 513, 515, 1550) demonstrated that fibronectin engagement of α5β1 integrin leads to association of c-SRC and the integrin complex with CaV 1.2 and enhancement of currents through the channel (Fig. 3). This has been proposed to be involved in the myogenic response and mechanotransduction by SMCs (315–317, 600). In contrast, engagement of αvβ3 integrins reduces currents through CaV 1.2 (1552). This latter response may be involved in the vascular response to local injury (1552).
L-type VGCCs and voltage-dependent release of intracellular Ca2+
In some vascular SMCs, membrane depolarization induces release of Ca2+ from internal stores (326, 327, 419, 1448). In this pathway it has been proposed that membrane depolarization is sensed by the VGCCs, stimulating a G-protein coupled phospholipase C (PLC) to produce inositol 1,4,5-trisphosphate (IP3) and the resultant release of Ca2+ from internal stores through inositol 1,4,5-trisphosphate receptors (IP3R), which is amplified by release of Ca2+ through ryanodine receptors (RyRs) (326, 327, 1448, 1574). Subsequent studies of aorta from SMC-specific CaV 1.2 knockout mice demonstrated loss of depolarization-induced Ca2+ release (419), supporting this hypothesis. In contrast, IP3R-dependent Ca2+ waves in SMCs of rat cerebral arteries appear to be independent of membrane potential (1035). These data suggest that depolarization-induced Ca2+ release may not be a general phenomenon.
Vasodilators and L-type VGCCs
Effects of cAMP-PKA signaling on L-type VGCC function
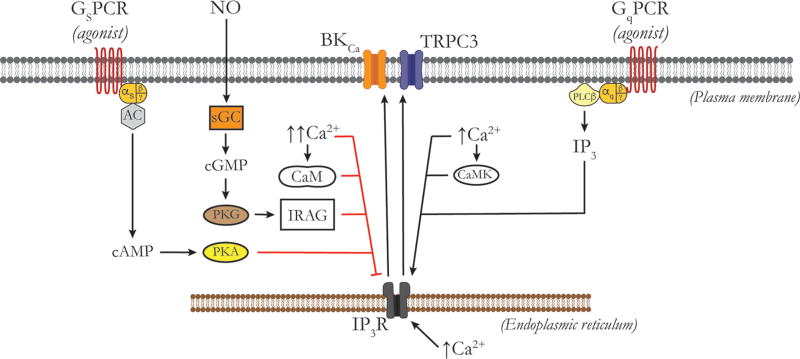
Vasoactive agents such as isoproterenol, adenosine, calcitonin-gene-related peptide (CGRP), and prostacyclin that act through the cAMP-PKA signaling cascade invariably cause relaxation of vascular SMCs and vasodilation. As will be discussed in the sections on K+ channels, a significant portion of their mechanism of action involves the activation of K+ channels, membrane hyperpolarization and deactivation of VGCCs. In addition, activation of cAMP-PKA signaling pathway in SMCs has been shown to both stimulate and inhibit currents through SMC CaV 1.2 channels [see (740) and references therein]. As in cardiac myocytes, the direct effect of cAMP-mediated activation of PKA appears to be stimulatory to SMC CaV 1.2 channels (740) (Fig. 3). However, high concentrations of agonists or direct activators of adenylate cyclase, such as forskolin, which result in high levels of cAMP, lead to transactivation of cGMP-activated protein kinase G (PKG) and subsequent inhibition of currents through CaV 1.2 channels (740) (Fig. 3). In the heart, CaV 1.2 channels are proteolytically processed with cleavage of the distal portion of the C-terminus of the protein, termed the DCT (451). The DCT remains associated with the channel and serves as an autoinhibitor of CaV 1.2 (633), requiring association with AKAPs (451). CaV 1.2 is similarly processed in cerebrovascular SMCs, and the DCT may play a similar role (89). Phosphorylation of the proximal C-terminal serine 1700 of the DCT relieves this inhibition and results in augmentation of channel activity (444). In neurons and the heart, β-adrenoreceptors, associated trimeric G-proteins, adenyl cyclase, PKA, and protein phosphatases exist in macromolecular complexes with CaV 1.2 targeted by AKAPs (302, 312). Studies of SMCs isolated from first-order rat cremaster arterioles and cerebral artery SMCs (1065) also indicate that PKA associates with CaV 1.2 (221) suggesting that similar protein complexes are present in the vasculature.
The excitatory action of PKA on L-type VGCCs in vascular SMCs appears counter to the established vasodilator activity of agonists that activate this kinase. However, it is likely that through Ca2+-dependent activation of nearby large-conductance Ca2+-activated K+ (BKCa) channels (514, 891) and/or stimulation of Ca2+ sparks (241, 683), PKA-dependent activation of currents through CaV 1.2 channels could promote membrane hyperpolarization, be self-limiting and produce vasodilation in arteries and arterioles. In contrast, recent evidence suggests that PKA inhibits currents through CaV 3.2 channels in SMCs from rat cerebral arteries (560). The authors suggest that this might reduce intracellular Ca2+ and promote vasodilation. However, this same group has shown that Ca2+ influx through CaV 3.2 channels activates RyRs to produce Ca2+ sparks and subsequent activation of nearby BKCa channels, contributing to the negative feedback regulation of myogenic tone (557) (see BKCa Channels Section for more on this topic). Inhibition of CaV 3.2 activity by PKA would dampen this negative feedback and actually promote vasoconstriction, all other factors constant. Thus, additional research is necessary to clarify the precise mechanism by which PKA modulates the activity of vascular SMC CaV 1.2 and CaV 3.X VGCCs and how these effects translate into modulation of myogenic tone.
Effects of cGMP-PKG signaling on L-type VGCC function
Activation of PKG inhibits currents through SMC CaV 1.2 channels (740) contributing to the vasodilator actions of NO and other cGMP-related vasodilators, although the precise mechanism has not been established (Fig. 3). In cardiac muscle CaV 1.2, PKG phosphorylates a number of residues on the α1 subunit as well as a serine residue on the associated β-subunit (1588). However, site-directed mutagenesis revealed that it is phosphorylation of the channel’s β subunit (1588) that mediates inhibition of currents through these VGCCs. It is not known whether the inhibitory effects of PKG on SMC CaV 1.2 VGCCs are also mediated by phosphorylation of the β subunits found in vascular tissue. Currents through T-type channels also are inhibited by PKG in SMCs from rat cerebral arteries and appear to contribute to the mechanism of dilation of NO and other cGMP-related dilators in this system (558). The molecular mechanism of this inhibition has not been established.
Calcium influx through L-type VGCCs also stimulates Ca2+ sparks (241, 389, 678, 683). However, this appears to result from Ca2+ loading of the ER rather than from a direct effect of Ca2+ entry through L-type channels on underlying RyRs (241, 389).
T-type VGCCs and myogenic tone
The expression and functional role of T-type VGCCs in resistance vessels has been demonstrated in the literature. Whole vessel lysates of first-order Wistar rat cremaster arterioles revealed expression of message for CaV 1.2, CaV 3.1 and CaV 3.2, although the cell type from which the RNA originated was not established (1460). VanBavel et al. (1460) found that myogenic tone in these vessels was potently and efficaciously inhibited by not only the L-type VGCC blocker, verapamil, but also the T-type VGCC blockers Ni2+ and mibefradil, supporting a potential role for T-type VGCCs in SMCs from these arterioles. Patch clamp recording of T-type VGCC currents have not been reported in cremaster arteriolar SMCs, only high-voltage-activated currents through VGCCs in SMCs from Sprague-Dawley rats (1552) and golden-Syrian hamsters (266). Furthermore, earlier studies showed that mibefradil dilated cremaster arterioles without reducing intracellular Ca2+ suggesting significant off-target effects of this putative T-type VGCC blocker (1171). The effects of Ni2+ cannot be so easily dismissed (1460) such that Ca2+ entry through T-type VGCCs also may contribute to myogenic tone in rat first-order arterioles. Because verapamil can also block T-type VGCCs, and L-Type VGCCs can be blocked by mibefradil (321), the precise contribution of L- and T-type VGCCs to myogenic tone in these vessels remains to be established. That nifedipine (785, 1171) and diltiazem (185), which are both selective for L-type VGCCs, substantially inhibit myogenic tone in cremaster arterioles, in vitro, suggests that L-type channels provide a major contribution (70%–90%) in vessels studied in vitro, but the potential for significant regional-, strain-, and species-dependent differences in the contribution of different classes of VGCCs are acknowledged. In mouse cremaster muscle, in vivo, in contrast to studies of rat (599) and hamster (670), 60% of resting tone is due to L-type VGCCs, with <20% from T-type VGCCs (628). Inhibition of NO synthesis increased the contribution of T-type VGCCs to ~38% in this model.
In addition to cremaster arterioles (1460), expression and function of T-type VGCCs have also been implicated in mesenteric (173, 521, 692), renal (550) and cerebral (3, 557, 802, 1060) resistance arteries and arterioles. However, many of these studies were based on the use of drugs like mibefradil (1025), which are notoriously non-specific, and patch clamp electrophysiological characterization of the SMCs from the vessels studied is often lacking. In the rat renal afferent arterioles, for example, where expression of CaV 3, 1 and 3.2 have been detected in whole vessel lysates (550), and where putative T-type VGCC blockers have been reported to affect myogenic tone and vasoconstrictor reactivity (417, 550), no rapidly inactivating, kurtoxin-sensitive Ca2+ or Ba2+ current is detected (1312). These data cast doubt on a role for T-type channels in rat afferent arterioles. However, alterations in renal function are also observed in CaV 3.X-deficient mice supporting a role for T-type VGCCs in renal vascular function in the mouse (1409). It should be noted that CaV 3.2 has been detected in endothelial cells in the renal microcirculation (1409) and CaV 3.1 in endothelial cells in both mesenteric arterioles (173) and the pulmonary circulation (1646). Thus, indirect effects mediated by changes in endothelial cell function cannot be excluded.
Rapidly-inactivating, nifedipine-insensitive, high voltage-activated Ba2+ currents that are inhibited by putative blockers of T-type VGCCs have been demonstrated in SMCs isolated from guinea pig and rat mesenteric arterioles (1032). Similar currents have also been reported in SMCs isolated from branches of rat basilar arteries (802). The molecular identity of these channels has not been established, but it has been speculated that splice variants of CaV 3.1 and/or 3.2 may alter the biophysical properties of these VGCCs such that they activate and inactivate at more positive potentials than the full length CaV 3.X VGCCs (803). Nifedipine-insensitive currents with the characteristics of T-type VGCCs have also been reported in SMCs isolated from rat middle cerebral arteries (3). Pressure-induced tone of mesenteric arteries from CaV 3.1 knock-out mice is inhibited between 40 and 80 mm Hg, supporting a role for T-type VGCCs in myogenic tone at low pressures in these vessels (140). Tone at higher pressures appears dependent on the activity of L-type VGCCs. Studies in rat middle cerebral arteries which express CaV 1.2, 3.1, and 3.2 support this hypothesis: at 20 mmHg T-type and L-type VGCCs contribute equally to what little myogenic tone exists at this low pressure, while at 80 mmHg, where there is substantial tone, L-type channels play the dominant role (3). Examination of the pressure-diameter relationship of tibialis anterior feed arteries from mice with SMC-specific knockout of CaV 1.2 reveals that pressure-induced tone between 20 and 40 mmHg appears unaffected by the loss of L-type VGCCs (1026). This has been argued as support for a role for T-type channels in myogenic tone at low intravascular pressures (803). Thus, where expressed in combination with L-type VGCCs, T-type VGCCs may contribute to myogenic tone, particularly at low intravascular pressure where SMCs are relatively hyperpolarized.
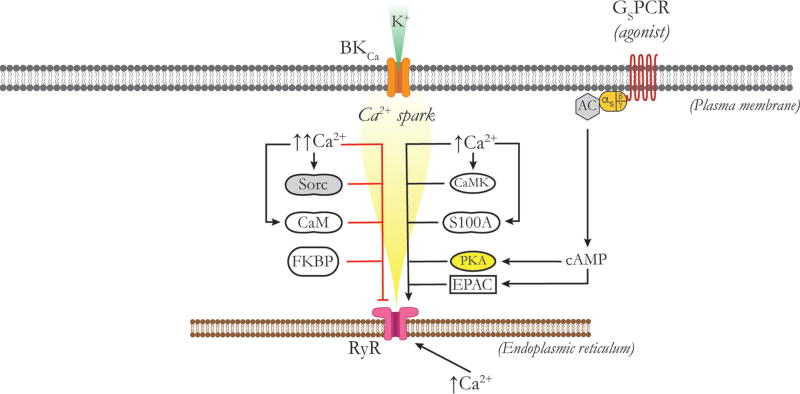
T-type VGCCs and the negative-feedback regulation of myogenic tone
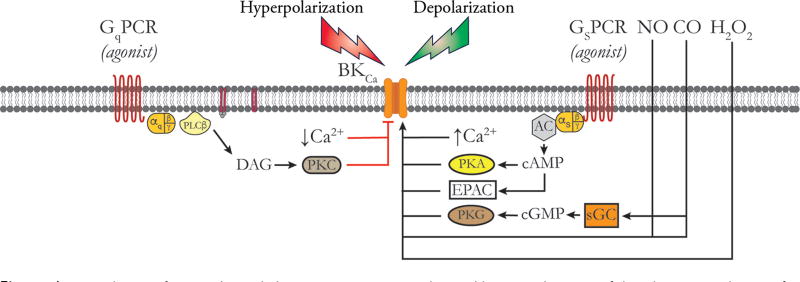
In rat middle cerebral arteries, CaV 3.2 VGCCs participate in the negative feedback regulation of membrane potential and myogenic tone (557, 559). Harraz et al. (557, 559) propose that Ca2+ influx through CaV 3.2 activates subsarcolemmal RyRs to induce Ca2+ sparks, which then activate overlying BKCa channels to produce membrane hyperpolarization, reducing the activity of CaV 1.2 and CaV 3.1 VGCCs and limiting myogenic tone (Fig. 4). They also propose that this mechanism may explain the paradoxical loss of vasodilator reactivity that has been observed in vessels isolated from CaV 3.2 knockouts (228) or in vessels exposed to low concentrations of Ni2+ that selectively block CaV 3.2 (1172). However, this mechanism has not been established in other blood vessels.
Figure 4.
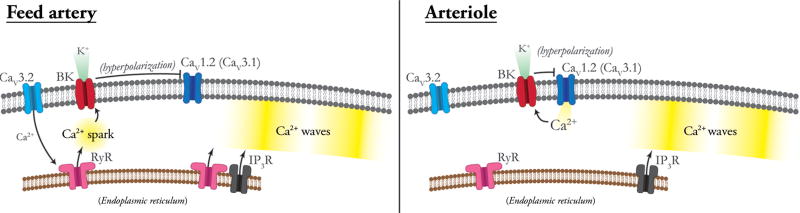
Calcium signaling in feed arteries versus downstream arterioles. Feed arteries display both Ca2+ sparks and Ca2+ waves, as shown. Ca2+ sparks in feed arteries arise from RyRs that may be activated by Ca2+ influx through CaV 3.2 channels via Ca2+-induced Ca2+ release. In feed arteries, Ca2+ sparks activate BKCa channels, hyperpolarizing the membrane and deactivating CaV 1.2 channels, which contributes to the negative feedback regulation of myogenic tone. Ca2+ waves in feed arteries depend on the activity of both RyRs and IP3Rs. In arterioles, Ca2+ influx through CaV 1.2 and other VGCCs provides the Ca2+ signal for activation of BKCa channels and the negative feedback regulation of membrane potential and VGCC activity. Ca2+ waves in arterioles depend solely on the activity of IP3R. RyRs are expressed in arteriolar SMCs but are silent under resting conditions. See text for details.
Other VGCCs in vascular tissue
Rat (549), mouse (552) and human (552) renal afferent arterioles appear to express the α1 subunits for CaV 2.1 P/Q-type VGCCs. Despite expression of CaV 2.1 across species, functional differences in channel function are evident: ω-agatoxin dose-dependently inhibits K+-induced constriction in human but not murine intrarenal arteries (552). In cerebral arteries expression and function of R-type VGCCs (CaV 2.3) appears after exposure to oxyhemoglobin as occurs during hemorrhagic stroke, contributing to the vasospasm that often occurs after this type of cerebrovascular accident (879).
VGCCs and pathophysiology
Hypertension
There is increased expression and function of CaV 1.2 channels in hypertension that contributes to increased myogenic tone and vasoconstrictor reactivity, and decreased vasodilator reactivity, all of which likely contribute to the increase in peripheral vascular resistance that is a hallmark of hypertension (see (716) for numerous references). The mechanisms responsible for the increased number of functional channels expressed in hypertension is not well understood, but may relate to increased trafficking of CaV 1.2 α-subunits to the plasma membrane via the increased expression of α2δ1 subunits (88) or β3 subunit (745), and/or altered posttranscriptional processing of the CaV 1.2 α1 subunits by micro-RNAs (miRs) such as miR-328 (518). Altered regulation of CaV 1.2 channel function via increased clustering of these channels into macromolecular signaling complexes may also contribute to the channels increased activity in hypertension (1062). In mesenteric artery SMCs from the spontaneously hypertensive rat (SHR), L-type VGCCs display higher current densities, activate at more negative potentials, display a slower inactivation and faster rate of recovery from inactivation than observed in SMCs from Wistar-Kyoto rats (287). Subsequent studies showed that there were significant differences in the expression of α2δ and β subunits that likely accounted for the different properties of the VGCC currents recorded in SMCs from the SHR (283). Also, consistent with studies in cerebral arteries (88), the increased L-type current densities recorded in mesenteric SMCs from the SHR were associated with increased expression of the α2δ1 subunits (283).
In angiotensin-induced hypertension, there is increased expression of CaV 1.2 due to activation of PiP3K-γ (1470). PiP3K-γ is activated by the βγ subunits of G12/13-protein coupled receptors such as the angiotensin receptor 1 (1335). Activation of PiP3K-γ promotes trafficking of L-type Ca2+ channels to the plasma membrane (1477) that appears to be mediated by PKB/Akt (199, 1470). In addition, phosphorylation of the β subunit of the channel (1477) protects the α1 subunit from proteolysis, increasing protein stability (1189), which contributes to the increased expression of the channels in the plasma membrane. Inhibitors of PIP3K-γ blunt angiotensin II-induced hypertension, cause peripheral vasodilation in angiotensin-induced hypertension and reduce the augmentation in Ca2+ currents in vascular SMCs from angiotensin-treated animals (199).
Obesity and the metabolic syndrome
Obesity and the metabolic syndrome also appear to cause ion channel remodeling. However, how this impacts VGCC expression and function is not clear. Increased L-type Ca2+ channel function was observed in SMCs from obese Zucker rats (1115). Similarly, a high fat diet produced increased Ca2+ current densities in cerebral artery SMCs from the Osborne-Mendel rat (1531). Increased resting Ca2+ levels and increased constriction to L-type VGCC agonist was observed in coronary vessels from obese swine (157). The expression and activity of L-type VGCCs is upregulated in obese pigs displaying the metabolic syndrome, and blockade of these channels with nifedipine increases resting coronary blood flow and exercise-induced increases in flow, whereas this dihydropyridine had no effect on resting flow or functional hyperemia in the hearts of lean pigs (126). In vascular SMCs from diabetic, obese and dyslipidemic db/db mice or in cells acutely exposed to elevated glucose, increased CaV 1.2-based Ca2+ sparklets were observed that resulted from elevated PKA-signaling that required PKA coupling to CaV 1.2 via AKAP150 (1065).
In contrast to the studies outlined earlier indicating increased expression and function of VGCCs, it was shown that in miniature pigs fed a high-fat diet to induce obesity and diet-induced hypercholesterolemia, L-type VGCC currents were reduced in SMCs isolated from large coronary arteries, with no effect on currents through these channels in SMCs from coronary arterioles (165). The reasons for these different outcomes is not known, but may be related to regional or species-dependent differences, the time course of the induced metabolic state, and the precise metabolic status of the model that was studied (1348).
Aging
Aging has been shown to impair the development of myogenic tone in murine mesenteric arteries (505). As a test of the hypothesis that a diminished functional expression of L-type Ca2+ channels are responsible for this loss of myogenic tone, Ba2+ currents through L-type VGCCs were measured (325). Not supporting this hypothesis, aging was found to increase SMC cell size, but did not affect L-type VGCC current density (325).
Diabetes
With the exception of a study of mesenteric artery SMCs from streptozotocin-treated rats that showed no change in L-type VGCC current density (1607), other studies have shown increased expression and function of VGCCs in type 1 diabetes or exposure of vascular SMCs to high glucose levels (421). Increased functional coupling of angiotensin II receptors with L-type Ca2+ channels was observed in SMCs from rat thoracic aorta after streptozotocin-induced diabetes (64). Similarly, CaV 1.2 function is upregulated by a mechanism involving PiP3K-δ in streptozotocin-induced diabetes in the mouse and accounts for increased reactivity to phenylephrine that is observed in this model (1160). As noted above, exposure of cerebrovascular SMCs to elevated glucose results in increased L-type VGCC current density, increased clustering of VGCCs and increased Ca2+ sparklet activity (1065). Species or regional differences in the adaptation to the diabetic state, time course of development of diabetes and the severity of diabetes may account for the different outcomes that have been observed (1348).
Potassium Channels
Vascular SMCs express a diverse array of K+ channels that contribute to the regulation and modulation of myogenic tone in resistance arteries and arterioles (40, 270, 332, 403, 520, 630, 665–668, 696, 769, 832, 920, 1000, 1069, 1073, 1074, 1128, 1142, 1187, 1277, 1315, 1397). This includes multiple types of voltage-gated K+ (KV) channels, members of the Ca2+-activated K+ (KCa) channel family, members of the inward-rectifier K+ (KIR) channel family, and several types of two-pore K+ (K2P) channels. The structure, expression and function of K2P channels will not be addressed in the present review (489, 1199, 1283, 1529).
KV Channels
Discovery of KV channels
Currents through KV channels were first reported by Hodgkin and Huxley in voltage-clamp experiments on the squid giant axon (606, 607). In general, these channels activate in response to membrane depolarization and then inactivate in a voltage-dependent manner with maintained depolarization (489, 688, 1074). There is considerable heterogeneity in the properties of KV channels found within and among tissues, indicating that there is considerable diversity among the different KV channels that are expressed (489, 524, 688, 1074). Molecular studies performed over the last 20 years have identified 40 genes encoding mammalian KV channels representing 12 families (KV 1–12) that contribute to this heterogeneity (489, 524). Members of the KV 1–4, 7, and 10–12 form functional channels as homomers, whereas KV 5, 6, 8, and 9 must coassemble with KV 2 or 3 subunits to form functional channels (489).
Structure of KV channels
Each KV channel is composed of a tetramer of pore forming α subunits (489, 688). Each α subunit has six transmembrane domains, S1–S6. The fourth membrane spanning region, S4, contains the voltage sensor of these channels, and the P-loop between S5 and S6, along with S6 forms the channel pore (82, 489, 687, 797, 1074, 1127) (Fig. 2). The N-terminal portion of the α subunit may be involved in fast (N-type) inactivation that occurs in some forms of these channels (623, 624, 687, 797, 1616). Slow (C-type) inactivation has been linked to the C-terminal domain and resides within or close to the pore of the channel (624, 797). Most KV channel α subunits are accompanied by modulatory accessory subunits and also interact with numerous proteins in macromolecular signaling complexes [see Gutman et al. (524) for details and references]. Heterogeneity in the function of expressed KV channels arises not only from the expression of different KV channel gene products, but also from heteromultimerization of channel subunits, the presence (or absence) of modifier subunits, association of the channels with accessory subunits, alternative splicing, and posttranslational modifications (489, 524).
KV channels expressed in vascular SMCs
Beech and Bolton (104, 105) and Okabe et al. (1104) were the first to identify currents through KV channels in vascular SMCs. Subsequent studies have shown their presence in virtually every vascular muscle studied (281, 499, 666, 668, 696, 920, 1074). Like KV channels expressed in other tissues (688), those in vascular SMCs appear to represent a diverse group of channels with a range of single channel conductances, voltage dependencies, kinetics and pharmacology (1074). Channels in the KV 1.X family appear to be widely expressed in vascular SMCs. Early studies showed expression of KV 1.5 in rat vascular SMCs (1120). Subsequent studies by numerous investigators confirmed and extended these findings by showing expression at the mRNA and protein levels of KV 1.1, 1.2, 1.3, 1.5, and 1.6 in a variety of blood vessels [see Table 5 in Ref. (281) for a summary and original references]. In addition, KV 2.1 expression has been observed in a number of vessels (281). Expression of members of the KV 3.X, 4.X, and 9.X also have been reported (281). More recent studies have also demonstrated expression of message (mRNA) and protein for members of the KV 7.X (KCNQ) family of channels (particularly KV 7.1, 7.4, and 7.5) in SMCs from a number of blood vessels (499, 696, 920).
Pharmacology of vascular SMC KV channels
As shown in Table 2, KV channels display a diverse pharmacology, and given the large number of KV channels expressed in a typical vascular SMC, pharmacological dissection of the function of individual channels, particularly in isolated vessel experiments or in vivo is challenging. However, some selectivity exists allowing pharmacological dissection of the function of the complex array of KV channels that are expressed in vascular SMCs (284, 285). Correolide is selective for KV 1 family members at micromolar concentrations (414). Derivatives of psoralen (Psora-4 (1472) and PAP-1 (1267), Table 2) are potent, selective inhibitors of members of the KV 1 family at nanomolar concentrations. However, at micromolar concentrations, these inhibitors also block a number of other K+ channels (Table 2), such that care must be taken in selection of blocker concentrations. Toxins, such as stromotoxin-1 (KV 2.1) (387) or phrixotoxins (KV 4) (335) are particularly useful in vitro, but are difficult to implement for in vivo experiments due to cost and protein binding issues. Nonetheless, through use of a combination of inhibitors as well as the kinetic analysis of currents in patch clamp experiments, a “fingerprint” can be developed to identify the functional expression of KV channels that contribute to the regulation of vascular tone in resistance arteries and arterioles (284, 285).
KV channels and myogenic tone
Early studies demonstrated that KV channel blockers such as 3,4-diaminopyridine or 4-aminopyridine (4-AP) caused SMCs to contract in a variety of blood vessels (271, 553, 1444), supporting a role for KV channels in the regulation of vascular tone. It was then shown that millimolar concentrations of 4-AP, a blocker of KV 1–4 channels (281), inhibited currents around the resting membrane potential of rabbit portal vein myocytes and depolarized SMCs isolated from renal (471) or coronary (830) arteries, indicating that KV channels contribute to the resting membrane potential of vascular SMCs. Knot and Nelson (763) then showed, in intact, pressurized rabbit cerebral arteries, that 4-AP depolarized SMCs and augmented myogenic tone at intraluminal pressures greater than 40 mmHg, consistent with the hypothesis that 4-AP-sensitive KV channels contribute to the resting membrane potential and to the negative feedback regulation of myogenic tone. Subsequent studies confirmed these findings in a number of arteries and arterioles (281, 665–668, 1074).
KV 1 channels in vascular SMCs
The specific KV channel α-subunits that contribute to the 4-AP-sensitive currents, membrane potential and tone responses have only been examined in a few instances. Cheong et al. (240) found that SMCs in mouse pial arterioles express KV 1.3, and 1.6, with KV 1.6 being prominent. Expression of KV 1.5 was present in perivascular nerves, but not in the SMCs. Consistent with this protein expression pattern they found that agitoxin-2 and margatoxin, peptide blockers of KV 1.3 and 1.6 channels, constricted arterioles pretreated with endothelin to depolarize the SMCs to about −40 mV. They concluded that KV 1.3 and 1.6 contribute significantly to 4-AP-sensitive current and regulation of agonist-induced tone. In contrast, in rabbit cerebral arterioles, Cheong et al. (239), found that SMCs express predominantly KV 1.5 and 1.6. In these cells, currents sensitive to the KV 1 family blocker, correolide, were active at a physiologically relevant membrane potential (−45 mV), and correolide further constricted arterioles preconstricted with endothelin-1. However, agitoxin-2, which blocks channels formed from homomers of KV 1.6, had no effect on currents or vascular tone. These data led the authors to suggest that channels formed from heteromers of KV 1.5 and 1.6 composed the 4-AP and correolide-sensitive currents in these vessels. In rabbit portal vein myocytes, based on the biophysical properties and pharmacology of native current compared with heterologously expressed KV 1.2 and 1.5, Kerr et al. (741) suggested that the 4-AP-sensitive currents in these vascular myocytes arose from heteromers of KV 1.2 and 1.5. Subsequent expression and immunoprecipitation studies supported this hypothesis (1406). Alberwani et al. (28) came to a similar conclusion and reported expression of message and protein for KV 1.2 and 1.5 in rat cerebral arteries. Correolide inhibited currents at physiological membrane potentials, depolarized SMCs in pressurized arteries and constricted these vessels consistent with a major role for KV 1 channels in the negative feedback regulation of myogenic tone. Based on the biophysical and pharmacological properties of the native channels and their finding that KV 1.2 and KV 1.5 coimmunoprecipitated, they concluded that the correolide-sensitive currents originated from heteromers of KV 1.2 and 1.5. Using dialysis of cells with anti-KV channel subunit antibodies, it was proposed that KV currents in rat mesenteric arteries were composed of currents through KV 1.2, KV 1.5, and KV 2.1 (909). Dialysis with an anti-KV 1.3 antibody was without effect despite a prior report of expression of these channels in rat mesenteric artery SMCs (1564). In another study of rat mesenteric resistance arteries, based on the channel expression profile, the biophysical properties and the pharmacology, it was proposed that heteromers of KV 1.2, KV 1.6, and KV 1.5 composed the 4-AP and correolide-sensitive currents that were responsible for negative feedback regulation of myogenic tone (1163). Chen et al. (231) then showed that in rat middle cerebral arteries, overexpression of KV 1.5 dampened myogenic tone, whereas expression of loss-of-function mutants of this channel enhanced myogenic tone consistent with a major role for KV 1.5 in whatever native channels are expressed in these cells. Rat retinal arterioles display rapidly inactivating (A-type) KV currents (976, 977), in contrast to the slowly inactivating delayed rectifier-type currents observed in most vascular SMCs (281, 665–668, 1074). However, as with the other vessels types presented thus far, KV 1.5-based channels appear to play a major role, because the currents were inhibited by correolide or intracellular application of an anti-KV 1.5 antibody (976). McGahon et al. found coexpression of KVβ1 accessory subunits in these vessels, which may account for the kinetics of the currents observed in SMCs from retinal arterioles (976). Rapidly inactivating A-type currents also have been observed in SMCs from rabbit portal veins (106), human mesenteric arteries (1311), rat renal microvessels (494), and rabbit aorta (541). However, the molecular constituents of these channels have not been ascertained. In mouse mesenteric arteries, mRNAs for KV 1.1, 1.2, 1.3, 1.5, 1.6, 2.1, 3.3, 3.4, 4.1–4.3, and KV 9.3 along with a number of accessory subunits were detected by PCR using TaqMan low density arrays, with message for KV 1.2, 1.5, 1.6, and 2.1 being most prominent (1028). Pharmacological dissection of KV channel currents in patch clamp studies demonstrated major contributions from KV 1 (56% at +80 mV) and KV 2 (27% at +80 mV) channels similar to what has been reported in mesenteric arteries from other species (see earlier).
KV 2 channels in vascular SMCs
As noted earlier, currents through KV 2.1 channels also appear to be functional in resistance arteries and arterioles. Rat cerebral artery SMCs express KV 2.1, and stromatoxin, which blocks this class of KV channels, inhibits whole-cell currents and enhances myogenic tone suggesting that these channels contribute to the regulation of membrane potential and the negative feedback of myogenic tone in these vessels (44). In rat middle cerebral artery, KV 2.1 may form heteromeric channels with KV 9.3 and be particularly important in regulation of resting membrane potential at negative membrane potentials associated with low intravascular pressure (1642). Rat and mouse mesenteric arteries also express KV 2.1 that contributes to whole-cell KV currents in these vessels (909, 1028).
KV 7 channels in vascular SMCs
Members of the KV 7 family also appear to contribute to resting membrane potential and the negative feedback regulation of myogenic and vasoconstrictor-induced tone in several vessels (499, 696, 920). Ohya et al. (1102) reported that only KV 7.1 was expressed in SMCs from portal vein and showed that the KV 7 channel blocker, linopirdine (23), significantly inhibited whole-cell currents in these cells and increased the duration of evoked action potentials. Subsequent studies utilizing additional KV 7 antagonists confirmed and extended these studies to show that KV 7 channels control electrical excitability of murine portal vein myocytes (1598) and that KV 7.4 and 7.5 are also expressed in SMCs of this vessel (1600). In SMCs from murine aorta, carotid artery, femoral artery and mesenteric arteries, KV 7.1, 7.4, and 7.5 mRNA was detected, and expression of protein for these isoforms was confirmed in SMCs from aorta (1599). Yeung et al. (1599) also found that KV 7 channel blockers constricted the vessels, whereas retigabine, which activates KV 7 channels, relaxed preconstricted vessels. These data support a significant role for KV 7 channels in the regulation of membrane potential in vascular SMCs. In cerebral arteries, KV 7 channels appear to contribute to the negative feedback control of myogenic tone, because inhibitors of these channels enhance myogenic tone at pressures greater than 20 mmHg (1643). These SMCs also were shown to express KV 7.1, 7.4, and 7.5 (1643). SMCs in rat coronary arteries also express KV 7.1, 7.4, and 7.5 (744). Blockade of KV 7.1 with HMR 1556 had no effect on resting tone of these vessels, whereas application of pan-KV 7 blockers contracted the vessels supporting a role for KV 7.4 and 7.5 in the regulation of resting membrane potential. Consistent with this hypothesis, selective activators of KV 7.2–7.5 relaxed preconstricted vessels, whereas an activator of KV 7.1 was without effect. Supporting the findings from isolated coronary arteries, application of KV 7 blockers to Langendorff-perfused hearts increased vascular resistance indicating a significant role for KV 7 channels in the regulation of coronary vascular tone. In coronary arteries KV 7.1 does not appear to play a significant functional role (744). A selective activator of KV 7.1, R-L3 (1236), relaxes preconstricted rat mesenteric arteries, although blockade of these channels has no effect on resting or agonist induced tone (215). As pan-KV 7 channel blockers contract rat mesenteric arteries (215, 695), these data suggest that there may be distinct roles for KV 7 channel isoforms in different regions of the vasculature. In rat cerebral arteries, both KV 7.4 and 7.5 appear to contribute to the negative feedback regulation of myogenic tone particularly at low intravascular pressures (214). Expression and function of KV 7.4 SMCs in rat mesenteric arteries require expression of and colocalization with the auxiliary subunit KNCE4 (694). These channels also interact with G-protein βγ subunits, that appear to be required for channel activity and participate the in the regulation of myogenic tone in rat renal arteries (1342).
Vasoconstrictors and KV channels
Vasoconstrictors modulate the activity of KV channels. Given the voltage dependence of these channels, one would expect that vasoconstrictors that depolarize vascular SMCs should activate KV channels (Fig. 5). Thus, depolarization-induced activation of KV channels should limit the degree of SMC depolarization, and hence vasoconstriction, in a negative feedback manner. Consistent with this hypothesis it has been shown that block of KV channels potentiates constriction induced by Gq/11-coupled receptor agonists (214, 239, 240, 271, 540, 949, 1124, 1300).
Figure 5.
Regulation of KV channels by vasoconstrictors and vasodilators. Schematic of the plasma membrane of a vascular SMC showing, from left to right, a Gq-protein-coupled receptor (GqPCR), associated G-proteins and phospholipase C-β (PLCβ); a generic KV channel; and a Gs-protein-coupled receptor, associated G-proteins and adenylate cyclase (AC). Black lines and arrows indicate stimulation, activation or increases; red lines indicate inhibition. Pathways to the right of the KV channel activate these channels, while those to the left are inhibitory. Membrane depolarization due to opening of membrane channels that conduct Na+, Ca2+, or Cl− or due to closure of other K+ channels represents the major stimulus for opening KV channels. Vasodilator agonists that act at GsPCR (isoproterenol, adenosine, prostacyclin, CGRP, etc.), stimulate the formation of cAMP, activation of PKA and phosphorylation of KV channels leading to their activation. In addition, the Gβγ-subunits can directly interact with some KV channels also leading to their activation. NO, acting through sGC, and other vasodilators that stimulate the production of cGMP, activeate PKG, phosphorylating KV channels and increasing their activity. Other vasodilators, such as H2S and H2O2 also can activate KV channels as shown. Hyperpolarization, induced by opening of other K+ channels or closure of channels conducting Na+, Ca2+, or Cl−, represents the major stimulus for closure of KV channels. In addition, vasoconstrictors that act through Gq-coupled receptors can inhibit KV channels through several mecahisms including: (A) the activation of PLCβ, the formation of DAG and activation of PKC; (B) PKC-dependent activation of the tyrosine kinase SRC; (C) Rho-guanine-nuclotide exchange factor (Rho-GEF)-dependent activation of RhoA and Rho kinase (Rho K); and (D) agonist-induced increases in intracellular Ca2+. See text for more information.
Kinase-mediated inhibition of KV channels
However, evidence also suggests that KV channel closure may contribute to the mechanism of action of vasoconstrictors. Agents such as phenylephrine (1012), 5-HT (78, 770, 1360), and angiotensin II (263) inhibit vascular SMC KV channels (Fig. 5). This closure may involve protein kinase-mediated or Ca2+-dependent inhibition of KV channels (Fig. 5). Activation of PKC, either by phorbol esters (18) or by Gq/11-coupled receptor agonists (263, 571, 770) inhibits currents through 4-AP-sensitive KV channels, which should contribute to vasoconstrictor-induced SMC depolarization (Fig. 5). In pulmonary vascular SMCs, 5-HT inhibits 4-AP-sensitive KV currents through a mechanism involving PKC and a tyrosine kinase via a mechanism that involves channel internalization (265). A similar mechanism may be involved in peripheral arteries, because 5-HT inhibits 4-AP-sensitive currents to mediate contraction of mesenteric arteries through a mechanism involving the tyrosine kinase, c-SRC (1360) in addition to PKC (770) (Fig. 5). Active PKC also inhibits currents through KV 7 channels, and this PKC-dependent inhibition of current has been shown to depolarize SMCs and lead to contraction of rat mesenteric arteries to low concentrations of arginine vasopressin (919). In rat cerebral arteries, uridine triphosphate (UTP), and U46619 inhibit 4-AP-sensitive KV currents through a mechanism involving Rho-kinase and modulation of the actin cytoskeleton that is independent from PKC (912, 913) (Fig. 5). Vasodilators that act through the cAMP and cGMP signaling cascades may act, in part, by antagonizing this Rho-kinase-mediated KV channel downregulation (914).
Calcium-mediated inhibition of KV channels
In addition to kinase-mediated inhibition of currents through KV channels, agonist-induced increases in intracellular Ca2+ concentration also have been shown to inhibit 4-AP-sensitive KV channel activity (289, 471, 657) (Fig. 5). Both the kinase-dependent and Ca2+-dependent processes would provide a positive feedback signal and could support depolarization and constriction for vasoconstrictors that activate PKC or Rho-kinase and/or raise intracellular Ca2+.
Vasodilators and KV channels
cAMP-PKA-mediated activation of KV 1 channels
A number of vasodilators have been proposed to act, in part, by activation of KV channels in the membrane of vascular SMCs. Dilators that act through the cAMP-PKA pathway were shown to activate 4-AP-sensitive KV channels in rabbit vascular SMCs (19–21) (Fig. 5). Subsequent studies by other investigators indicated a significant role for 4-AP-sensitive KV channels in the mechanism of action of vasodilators that act on Gs-coupled receptors to activate adenylate cyclase, increase the production of cAMP, and activate PKA (128, 331, 336, 577, 578, 849, 1254). Phosphorylation of KV 1.2 by PKA at serine 449 in the C-terminus of this channel mediates the increase in whole-cell currents (710), which may underlie the 4-AP-sensitive effects of cAMP-PKA-related vasodilators in vessels where heteromers of KV 1.2 and 1.5 or 1.6 predominate (28, 231, 741, 1163, 1406). In rat cerebral artery SMCs, PKA-dependent phosphorylation of KV 1.2 depends on interaction of the channels with the scaffolding protein PSD95, which may be involved in targeting PKA to KV 1.2 (1024).
cAMP-PKA-mediated activation of KV 7 channels
The cAMP-PKA signaling pathway also modulates the activity of KV 7 channels. In rat renal arteries the β-adrenoreceptor agonist, isoproterenol, or the adenylate cyclase activator, forskolin, activate currents through KV 7 channels to dilate these vessels, and siRNA knockdown of KV 7.4 attenuated these responses (216). The C-terminus of KV 7 channels has binding domains for AKAPs (536), consistent with the idea that vascular KV 7 channels may exist in signaling domains with PKA (216). Activation of KV 7.4 channels by isoproterenol involves G-protein βγ subunits in rat renal arteries (1342).
Adenosine-induced dilation of coronary arteries also appears to be mediated in part by KV 7.4 (744). Vasodilation of rat cerebral arteries induced by CGRP is inhibited by the pan-KV 7 blocker linopirdine, but not 4-AP (214). Furthermore, siRNA knockdown of KV 7.4, but not KV 7.5 also inhibited CGRP-induced vasodilation indicating a major role for KV 7.4 in the mechanism of action of CGRP in rat cerebral arteries. Thus, agonists that act at Gs-coupled receptors also modulate the activity of KV 7 channels in the vasculature.
NO-cGMP-PKG-mediated activation of KV channels
Early studies suggested roles for 4-AP-sensitive KV channels in various aspects of endothelium-dependent vasodilation in several vascular beds (370, 589, 1299, 1612). However, subsequent studies showed that inhibitory effects of 4-AP likely resulted from depolarization-induced effects on myoendothelial electrical coupling, rather than blockade of effects of endothelium-derived autacoids on 4-AP-sensitive KV channels in SMCs (36, 519, 588, 1299). Nonetheless, endothelium-dependent dilation or dilation induced by NO or cGMP analogs are inhibited by KV channel blockers in rat basilar arteries (1317) (Fig. 5). Studies in rat aortic SMCs indicated that NO and atrial natriuretic peptide-induced relaxation can be inhibited by high concentrations of 4-AP and tetraethylammonium (TEA), and that these dilators activate KV channel currents in aortic A7R5 cells that appear to be carried by KV 2.1 containing channels (1384). Sodium nitroprusside (SNP)-induced dilation of canine coronary circulation is inhibited by 4-AP or correolide suggesting a role for KV 1 channels in NO-mediated vasodilation (331). More recently, cGMP has been shown to increase currents through KV 7 channels. Blockade of these channels inhibits relaxation of rat renal arteries induced by atrial natriuretic peptide, and of rat aorta induced by atrial natriuretic peptide or SNP, both of which act through the cGMP-signaling cascade (1341). In contrast, SNP-induced relaxation of porcine coronary arteries is unaffected by KV 7 channel blockade (579). Thus, there may be species and regional differences.
Other dilators that activate KV channels
In addition to NO, endothelium-derived hydrogen sulfide (H2S) (223, 946, 1262) and hydrogen peroxide (H2O2) (1210) also have been proposed to act, in part, via activation of SMC KV channels (Fig. 5). Hydrogen sulfide-induced relaxation and SMC hyperpolarization of rat aorta are blunted by 4-AP suggesting a role for KV channels in the mechanism of action of this dilator (223). Other studies suggest that H2S activates vascular SMC KV 7 channels to hyperpolarize and relax the SMCs (946, 1262). Hydrogen peroxide activates 4-AP-sensitive KV channels in SMCs from canine coronary (1210) and rat mesenteric (1135) arteries. Importantly, 4-AP also inhibits H2O2-induced relaxation of canine coronary (1210, 1211) and rat mesenteric (1135) arteries, dilation of isolated canine coronary arterioles, and the H2O2-induced increase in coronary blood flow, in vivo (1211). The effects of H2O2 on K+ currents and SMC tone can be inhibited by dithiothreitol (DTT), suggesting that the effects of H2O2 on KV channels involve thiol oxidation (1135, 1210, 1211). In mesenteric arteries, the effects of H2O2 may involve S-glutathionylation of KV channels, with KV 2.1 being a likely target (1135).
Voltage-gated K+ channels also may serve as sensors mediating hypoxia- and acidosis-induced dilation in coronary arteries. Hypoxia-induced relaxation of porcine coronary arteries is inhibited by KV 7 channel blockers, and hypoxia activates currents through KV 7 channels in SMCs from these vessels (579). It has also been shown that acidosis activates 4-AP-sensitive KV channels in coronary vascular muscle cells (119), although the functional significance of this effect was not studied.
Perivascular adipose tissue release one or more anticontractile substances, often referred to as adipocyte-derived relaxing factors that appear to activate KV 7 channels (1389). In resistance arteries upstream from the microcirculation of the gracilis muscle in the rat, this seems to involve KV 7.4 (1624).
Functional vasodilation and KV channels
The role played by vascular KV channels in the local regulation of blood flow is not clear. Functional hyperemia is impaired and resting blood flow reduced by 4-AP in canine hearts, in vivo, supporting a role for 4-AP-sensitive KV channels in coupling metabolism to blood flow (125, 1233). Also, the duration of reactive hyperemia is impaired by 4-AP in this model (331). Similarly, the KV 1 channel blocker correolide inhibited dobutamine-induced hyperemia at every level of myocardial oxygen consumption and also impaired reactive hyperemia in anesthetized pigs (492). In pigs, 4-AP also impairs reactive hyperemia (126), but does not affect blood flow autoregulation (127). Also, in contrast to the findings in dogs (1233) and the correolide studies in pigs (492), 4-AP had no effect on the relationship between myocardial oxygen consumption and coronary blood flow suggesting that there may be model-dependent differences in the role played by KV channels (127). In Langendorff-perfused rat hearts, KV 7 channel blockers increase resting vascular resistance and inhibit reactive hyperemia (744). However, pacing-induced functional hyperemia and blood flow autoregulation are unaffected (744). In the coronary circulation of pigs, block of KV 7 channels with linopirdine had no effect on resting blood flow, or the increase in blood flow after ischemia, H2O2 or exercise despite evidence for expression and function of these channels in coronary SMCs from this model (491). Thus, there appears to be species or model-dependent differences in the participation of KV channels in the local regulation of blood flow in the heart. In skeletal muscle, exercise training is accompanied by an increase in the functional expression of KV channels in resistance arteries (709), but their function in the local regulation of blood flow was not studied.
KV channels and pathophysiology
Hypertension
The effects of hypertension on KV channel expression and function are not clear (280, 290, 716, 771). Electrophysiological studies have reported increased (282, 286), decreased (170, 171, 279, 288, 886, 947, 1414), or no change (887, 890) in KV current density. One possible explanation for these differences relates to the experimental conditions used to study KV currents; examination of studies on SMCs from the same vessel, recorded in the same lab are particularly illuminating (282, 288). In SMCs from mesenteric arteries, KV channel currents recorded using conventional whole-cell methods with intracellular Ca2+ buffered to low levels with 10 mmol/L 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (BAPTA), 4-AP-sensitive KV currents were observed to be higher in cells from SHR than from Wistar-Kyoto (WKY) rats (282). In contrast, in the same cells studied using the perforated-patch method in which intracellular Ca2+ is not buffered, KV channel current density was reported to be lower in cells from SHR versus WKY (288). As increased intracellular Ca2+ inhibits KV channels (289, 471, 657), this could explain why opposing results were obtained in the same SMCs and rat model of hypertension. Regional differences in the impact of hypertension on KV channel expression and function as well and differences in the model of hypertension used, also may contribute to the varied reports in the literature.
As with electrophysiological studies, examination of mRNA and protein expression of KV channels in models of hypertension also has led to conflicting results. Cox (282, 286) has reported an increase in mRNA for KV 1.2, KV 1.5, and KV 2.1 that is supported by increased protein expression for these KV channel subunits by Western blot and increased KV channel current densities in patch clamp experiments of mesenteric artery from SHR versus WKY (282, 286). It is worthy to note that examination of mRNA and protein for KV channel subunits in aortic SMCs revealed no difference in expression between SHR and WKY (286). These data support the idea that there are regional differences in the effects of hypertension on ion channel expression.
In support of the hypothesis that KV channel function is upregulated in hypertension, it has been reported that 4-AP or 3,4 diaminopyridine causes an immediate increase in peripheral vascular resistance that is greater in SHR than in WKY, and was attributed to closure of KV channels on vascular SMCs (117). However, these data are difficult to interpret, because of the concomitant upregulation of VGCC expression and function, as outlined in the section on VGCCs in hypertension.
In contrast to the studies reporting upregulation of KV channel expression and function in hypertension, several investigators have found decreased expression and function of KV channels in vessels from hypertensive animals. Both whole-cell KV currents measured using the perforated patch technique, and KV 1.2 and KV 1.5 protein expression were lower in mesenteric arteries from 6 to 8 month old SHR versus WKY (1628). In superior mesenteric arteries, membrane potential was more depolarized in vessels from rats with L-NAME-induced hypertension (170). This was accompanied by decreased protein expression for KV 1.5 (170), and decreased KV channel current densities (171). In two forms of hypertension, there was a reduced protein expression of KV 1.2 and 1.5, decreased whole-cell KV currents in SMCs and reduced functional role of KV 1.X channels in the regulation of myogenic tone in rat cerebral arteries (1414). The protein expression for KV 1.2 and 1.5 were reduced in aortas of SHR versus WKY (860). These changes were correlated with reduced contraction induced by 4-AP, maurotoxin, and mephetyl tetrazole, inhibitors of KV 1.2 and 1.5 channels, respectively. In a mouse model of hypertension, decreased KV channel current densities were observed in patch clamp studies of SMCs isolated from mesenteric arteries from BPH (Blood Pressure–hypertensive) versus BPN (Blood Pressure–normotensive) mice (1028). Currents in cells from BPN were predominantly due to KV 1, KV 2, KV 4, and KV 3 channels in order of decreasing importance. In cells from BPH, the proportional contribution from KV 1 channels was similar to those in cells from BPN, whereas there was a reduced contribution of currents through KV 2 channels. KV 2 currents were also slower to activate and deactivate. Intracellular application of an anti-KV 6.3 antibody, which is expressed only in cells from BPH, decreased KV 2 currents that could be reproduced by expression of both KV 2.1 and 6.3 in HEK293 cells suggesting heteromerization of these channels. BPH cells were more depolarized and guangxitoxin (selective KV 2 blocker) produced less depolarization in BPH cells consistent with the expression of KV channel heteromers in the cells from BPH. The decreased expression of KV 1 channels that was observed could be due to decreased expression of the K+ channel-associated protein (KChAP), a chaperone for these channels.
KV 7.4 protein expression and function are reduced in thoracic aorta and mesenteric arteries from SHR and angiotensin-induced hypertensive mice compared with vessels from normotensive counterparts (695). Decreased protein expression and function of KV 7.4 in mesenteric arteries from SHR was also observed by Zavaritskaya et al. (1624). Loss of KV 7.4 also accounts for reduced β-adrenoreceptor-mediated dilation of renal arteries in SHR (216). However, in the gracilis artery, KV 7.4 expression and function does not seem to be altered in the SHR (1624).
In cerebral arteries of rats, angiotensin II-induced hypertension, independent from the increase in blood pressure, resulted in decreased expression of KV 2.1 protein and a reduction in whole-cell KV channel currents (42). The reduced expression was mediated by activation of the transcription factor, Nuclear Factor of Activated T-cells C3 (NFATC3) through a pathway that involves Ca2+ influx through L-type VGCCs and activation of calcineurin (42). It was also shown that KV 2.1 function is reduced, with no change in KV 1 function in angiotensin II-induced hypertension (44). Thus, the bulk of the literature seems to support the hypothesis that there is reduced KV channel expression and function in hypertension. However, the specifics may depend on the vascular bed examined, the model of hypertension studied and the duration and extent of hypertension.
Obesity and the metabolic syndrome
As in models of hypertension, there is evidence for both increased and decreased expression and function of KV channels in models of obesity and the metabolic syndrome. Hypercholesterolemia also impairs KV channel function in coronary SMCs (332, 1581). In pigs with diet-induced metabolic syndrome, coronary artery KV channel expression and function are impaired and correlate with the impairment in metabolic vasodilation in this model (125). There is selective downregulation of KV 2.1 expression and function in both cerebral and mesenteric arteries that contributes to enhanced arterial tone in mice fed a high fat diet (1083). The decreased expression and function of KV 2.1 is mediated by calcineurin-dependent activation of the transcription factor NFATC3 that is associated with AKAP150 (1083).
In contrast, in Otsuka Long-Evans Tokushima fatty (OLETF) rats, the reactivity of mesenteric arteries to 5-HT is enhanced compared the reactivity of vessels from Long-Evans Tokushima Otsuka (LETO) rats (770). It was found that 4-AP-sensitive KV currents were higher in SMCs isolated from OLETF rats compared to SMCs from LETO rats, and importantly, that 5-HT inhibited KV channel currents in SMCs from OLETF rats to a greater extent via a mechanism involving PKC (770). There is increased reactivity to 4-AP in aortas from atherosclerosis-prone mice suggesting KV channel function is augmented in aortic SMCs (704). Thus, as with hypertension there appears to be heterogeneity in the effects of obesity and the metabolic syndrome on KV channel expression and function.
Aging
In Fisher 344 rats KV 1.5 protein expression is unchanged with advanced age, but in soleus muscle feed arteries 4-AP induced constriction is increased in vessels from aged rats suggesting increased function of KV channels (730). In soleus muscle feed arteries, the KV 1 selective inhibitor, correolide had similar effects (475). These data suggest that there may be regional differences in the impact of aging on expression and function of KV channels. Subsequent studies showed that exercise training could reverse the effects of aging on KV 1 channel function in soleus feed arteries (475).
Diabetes
In experimental models of type 1 diabetes, there appears to be a decreased expression and function of KV channels that may contribute to the increased vascular reactivity observed in this disease (771). High glucose impairs KV channel function in coronary arteries (852, 892) by nitration of KV channels (852). Exposure of coronary arteries to high glucose impairs cAMP-mediated vasodilation by impairing the function of KV channels (849). Similarly, dilation induced by cAMP-related vasodilators is impaired in coronary arteries isolated from streptozotocin-treated rats due to reactive oxygen species (ROS)-related impairment of KV channel function (181). Exposure of SMCs isolated from mesenteric arteries from Wistar rats to elevated glucose reduces KV channel currents, depolarizes the SMCs and inhibits endothelin-induced inhibition of the KV current that remains through a mechanism that involves PKC (1192). Streptozotocin-induced diabetes is associated with decreased KV channel current densities and impaired cAMP-mediated dilation in rat coronary arteries (217). Protein expression of KV 1.2 but not KV 1.5 is reduced in coronary arteries from these streptozotocin-treated rats (218).
Other disease states
Vasospasm associated with subarachnoid hemorrhage results, in part, from oxyhemoglobin-induced activation of tyrosine kinases that stimulate endocytosis of KV 1.5 channels, a reduction in KV currents, the resultant depolarization of cerebrovascular SMCs and activation of VGCCs in the rabbit (655). Reduced KV channel function is also observed in dog models of subarachnoid hemorrhage (685) that may be related to decreased expression of KV 2 channel subunits (22, 685).
Ca2+-activated K+ Channels
Discovery of Ca2+-activated K+ channels
Calcium-activated K+ (KCa) channels are a group of ubiquitous, abundant K+ channels that are activated by increases in intracellular Ca2+ concentration (602, 822). They are the most abundant K+ channels found in the membrane of most cells, including vascular SMCs (1074). The first evidence for KCa channels came from the observation that chelation of Ca2+ in red blood cells inhibited the release of K+ ions during metabolic inhibition (464). This was followed by microelectrode studies in which the K+ conductance of molluscan neurons was shown to increase after they were injected with solutions containing Ca2+ (986, 987). Subsequent patch clamp studies in the early 1980s by numerous investigators showed that KCa channels were found in essentially all cells (602, 822). Two general classes of KCa channels were originally identified and named based on their single channel conductances, voltage sensitivities and pharmacology: large conductance Ca2+- and voltage-activated BKCa channels and small conductance Ca2+-activated KCa (SKCa) channels (602, 822). Subsequent molecular studies identified the KCNMA1 gene that encodes the α-pore-forming subunit of BKCa channels (KCa 1.1) (187, 1126), and KCNN1–3 (774) and KCNN4 (656, 713) genes that encode the α-subunits for KCa 2.1–2.3, small conductance, apamin-sensitive SKCa channels and KCa 3.1, the intermediate conductance, IKCa channel, respectively (1509). The focus in this section will be on BKCa channels as only two studies have identified SKCa channels in vascular SMCs from systemic blood vessels (468, 646), and IKCa channels appear only to be expressed in proliferating SMCs (135, 1077, 1402, 1421).
BKCa Channels
Structure of BKCa channels
The pore-forming, α subunit of BKCa channels has seven transmembrane spanning domains (S0–S6), with the pore formed by the P-loop between S5 and S6, and the S6 domains (989). What differentiates BKCa channels from other members of the KV family is the presence of an additional transmembrane spanning region (S0) at the NH2-terminus of the molecule and a long C-terminus cytoplasmic tail region (989). Positively charged residues in S2, S3 and S4 serve as the voltage sensors in BKCa channels (916), less centralized than in the S4-segment of KV channels. Residues in two, tandem regulator of conductance of K+ (RCK) domains (RCK1 and RCK2) in the large cytosolic C-terminus of the α subunit serve as the Ca2+ sensors of the channel (see (621) for refs). The α subunit of BKCa channels is subject to considerable alternative splicing, with greater than 20 spliced variants identified (13, 810, 1431, 1561, 1578). This allows considerable functional diversity among BKCa channels expressed in different tissues and also allows for dynamic regulation of BKCa channel function (1561, 1578).
Association with β1 subunits
The α subunit of BKCa channels often associates with modulatory β subunits; of the four genes (KCNMB1–4, encoding KCaβ1–4 subunits), KCaβ1 appears to be the main isoform expressed in vascular SMCs, altering channel gating kinetics and increasing the Ca2+ sensitivity of the α-subunit (974, 982, 988, 1432). Association of the α subunit with β1 is required for activation of BKCa channels by agonists such as dehydrosoyasaponin I (982) and 17β-estradiol (1454). Variation in the degree of coupling of α and β subunits may explain, in part, the heterogeneity of Ca2+ sensitivity of BKCa channels between and within vascular SMCs from various sources (1382) (see later for more on this topic). Studies in rat cerebral artery SMCs show that while most of the α subunits of BKCa channels reside in the plasma membrane, the β1 subunits are located in Rab11A-positive recycling endosomes and can rapidly traffic to the plasma membrane to associate with the α subunits, providing a dynamic means to regulate the function of BKCa channels (842).
LRRCs as BKCa channel subunits
In addition to β subunits, the α subunit of BKCa channels also associates with leucine-rich-repeat-containing proteins (LRRCs) that have been suggested to be γ subunits of BKCa channels (37, 391, 490, 1576). These LRRCs increase the voltage sensitivity of the channels allowing activation of BKCa channels at negative membrane potentials in the absence of Ca2+ (490, 1576). The presence of LRRCs also increases the sensitivity of the channels to activators such as docosahexaenoic acid (622). In vascular SMCs from rat cerebral arteries, LRRC26 appears to be the isoform expressed (391). Small interfering RNA knock down of LRRC26 increased myogenic tone, reduced constriction induced by the BKCa channel blocker, iberiotoxin, and reduced dilation induced by the BKCa channel activator, NS-1619 (391) supporting a functional role for this auxiliary subunit. The expression and function of LRRCs in other vascular SMCs has not been established.
KCa channels expressed in vascular SMCs
Benham et al. (1514) were among the first to identify BKCa channels in patch clamp studies of vascular SMCs isolated from guinea pig mesenteric arteries. They found a high density of Ca2+- and voltage-sensitive K+ channels in inside-out patches of membrane with a single channel conductance of ~200 pS in symmetrical 126:126 mmol/L K+ solution. Subsequently, numerous investigators have reported similar findings (1074). These studies indicate that vascular muscle cells from a variety of large systemic blood vessels express BKCa channels with single channel conductances of ~250 pS in inside-out patches of membrane exposed to symmetrical 140 to 150 mmol/LK+ solutions (198, 981, 1074). Similar findings were reported for arteriolar SMCs from rat and hamster cremaster muscle (672) and rat kidneys (468). Thus, BKCa channels appear to be ubiquitously expressed in systemic vascular SMCs.
Calcium-activated K+ channels of smaller conductance (SKCa) have also been reported (468, 646). In cells from rabbit portal vein, a KCa channel with a single channel conductance of 92 pS in symmetrical 142 mmol/L K+ that is insensitive to extracellular TEA has been reported (646). The sensitivity of these channels to other pharmacological agents was not evaluated. Gebremedhin et al. (468) reported an apamin-sensitive KCa channel present in the membranes of rat renal arteriolar muscle cells with a unitary conductance of 68 pS in symmetrical 145 mmol/L K+. In both of these instances, BKCa channels were also identified. These data suggest that SKCa channels may be present in the membrane of some vascular SMCs.
Pharmacology of BKCa channels
As shown in Table 2, BKCa channels display a distinct pharmacological profile that has allowed pharmacological dissection of their function, even in in vivo experiments. Both iberiotoxin and paxilline, for example are very selective for BKCa channels (1509). However, caution must be used with charybdotoxin and TEA, because these blockers affect several other channels that may be expressed in vascular smooth muscle (Table 2). Charybdotoxin blocks IKCa channels, KV 1.2 and KV 1.6 in addition to BKCa channels (Table 2). In some systems, TEA, at concentrations of 1 mmol/L or less, is quite selective for BKCa channels. However, TEA also potently blocks KV 1.1, KV 1.2, and KV 3.1, channels also expressed in some vascular SMCs (Table 2).
BKCa channels and myogenic tone
Evidence for BKCa channels in the negative-feedback regulation of myogenic tone
Early studies showed that inhibitors of BKCa channels (charybdotoxin, iberiotoxin, or millimolar concentrations of TEA) depolarized vascular SMCs and constricted small, myogenically active rabbit cerebral arteries when they were pressurized, in vitro (175, 1071). Similar results have been reported in a large number of different preparations studied by pressure myography including rat tail arteries (1270), rat saphenous arteries (116), rabbit renal arcuate arteries (1176), canine subepicardial arteries (191), canine coronary arterioles (1210), first-order hamster (1596) or rat cremaster arterioles (629, 785), rat small mesenteric arteries (677, 911), hamster (1528), or murine (1527) cremaster muscle feed arteries and second-order arterioles, and murine superior epigastric arteries (573). Charybdotoxin contracts rings of porcine coronary arteries (467) and strips of rat femoral and mesenteric arteries (65) and enhances stretch-induced myogenic tone of dog basilar (66), middle cerebral (65), posterior cerebral (65), and coronary (65) artery strips. However, strips of canine mesenteric artery (65) and rat carotid artery (65) did not respond to this BKCa channel blocker, likely because they did not display stretch-induced myogenic tone. Resting membrane potential of porcine coronary arteriolar SMCs is not affected by iberiotoxin, but stretch-induced depolarization of these cells is potentiated in the presence of this BKCa channel blocker supporting their role in the negative feedback regulation of myogenic tone (1549). Both TEA (5 mmol/L) and charybdotoxin depolarize SMCs in rat superior mesenteric arteries (225).
The in vitro data cited earlier suggest that BKCa channels are active under resting conditions, contribute to resting membrane potential and participate in the negative feedback regulation of myogenic tone. In vivo studies of canine diaphragm have demonstrated that the BKCa blocker, iberiotoxin, decreases resting blood flow when infused into the blood perfusing this muscle (1462, 1463). These data suggest that BKCa channels may play a role in the regulation of resting blood flow in the canine diaphragm. However, these and all in vivo data must be interpreted cautiously because the site of action of the K+ channel blockers was not established; vascular SMCs, endothelial, neural or parenchymal BKCa channels could have been affected. In rat spinotrapezeus muscle, in vivo, both TEA and iberiotoxin produce arteriolar constriction (951), consistent with a negative feedback role in the regulation of myogenic tone. Similarly, TEA has been shown to increase human forearm vascular resistance supporting a role for BKCa channels in resting vascular tone in this vascular bed (1123). However, in another study using the same protocol, TEA did not significantly increase forearm vascular resistance in healthy individuals (1122). Rat basilar arteries, in vivo, constrict when exposed to 10 mmol/L TEA suggesting that BKCa channels contribute to resting membrane potential and tone in these arteries, although a role for TEA-sensitive KV channels cannot be excluded due to the high concentration of TEA used in this study (445). Other investigators have shown that a lower concentration of TEA (1 mmol/L) reduced rat basilar artery diameter by only 5% (1140), 10% (1316), or 13% (404). In this same model, iberiotoxin either had a small effect [5% constriction (1140)], or no significant effect (404). Taken together, these data suggest a small role for BKCa channels in determining the resting myogenic tone of basilar arteries in the rat, in vivo. Iberiotoxin has also been shown to depolarize SMC membrane potential in rat mesenteric resistance arteries, in vivo (778), consistent with a role for BKCa channels in the negative feedback regulation of myogenic tone in this vessel.
Evidence against BKCa channels in the negative-feedback regulation of myogenic tone
In contrast to the reports cited earlier, a number of studies have failed to implicate BKCa channels in the regulation of resting membrane potential and tone, particularly in arterioles. Perforated patch (673) or conventional whole-cell (665) recording of K+ currents in second-order rat and hamster cremasteric arteriolar muscle cells have failed to find any effect of iberiotoxin on currents at physiological membrane potentials (−90 to 0 mV), even when cells are dialyzed with solutions containing 300 nmol/L free Ca2+ (665). Currents inhibited by iberiotoxin could be detected at positive membrane potentials in these studies (665). Consistent with these findings, iberiotoxin has no significant effect on resting membrane potential of single cremasteric arteriolar muscle cells (673). These data suggest that BKCa channels do not contribute to resting membrane potential in relaxed SMCs from these arterioles or even when cytosolic Ca2+ is raised to 300 nmol/L. This hypothesis is supported by in vivo studies demonstrating that neither iberiotoxin nor TEA affect resting diameter of hamster (672, 1532), rat (898), or mouse (1303) cremasteric arterioles in vivo, despite these vessels having substantial resting, myogenic tone. We have observed similar results in the hamster cheek pouch preparation: TEA (1 mmol/L, selective for BKCa channels) has no effect on resting diameter of second-order arterioles in this preparation (Jackson, unpublished observations). Similarly, iberiotoxin, TEA or charybdotoxin do not affect resting diameter of rat pial arterioles, in vivo, despite significant resting myogenic tone (617, 816, 969, 1139, 1141, 1319, 1320, 1489). Identical results were reported in cats (1511) and newborn pigs (58, 59). In rabbits, 50 nmol/L iberiotoxin had no effect on resting pial arteriolar diameter, whereas 100 nmol/L produced only a 3% constriction (1371). In fawn hooded rats, administration of penetrem A or iberiotoxin into eyes results in no significant change in retinal arteriolar diameter, although both produced a 10% decrease in arteriolar blood flow, which was computed from the vessel diameter and red blood cell velocity suggesting upstream effects (1066). Other investigators have previously reported no effect of iberiotoxin on resting retinal arteriolar diameter in Wistar rats (1030). Iberiotoxin has little effect on renal microvessels, in vivo (929). A lack of effect of TEA on resting blood flow to feline hind limb has also been reported (220). In addition, there are a few in vitro studies where BKCa channel blockers have little or no effect on pressure-induced arteriolar tone. Neither iberiotoxin nor TEA significantly affects resting diameter of isolated-cannulated porcine retinal arterioles (1044, 1385), Iberiotoxin has little effect on the diameter of pressurized rat cerebral parenchymal arterioles, (257, 301). First-order hamster cremaster arterioles studied by pressure myography constrict when superfused with charybdotoxin (10% constriction) or TEA (1 mmol/L; 19% constriction), but not iberiotoxin (3% constriction) (1596). Iberiotoxin was reported to have no effect on resting diameter of rat gracilis feed arteries, studied by pressure myography, in vitro, whereas charybdotoxin, which also inhibits endothelial IKCa channels, and a high concentration (10 mmol/L TEA) caused constriction (1446). In contrast, in studies by Samora et al. (1248) iberiotoxin was found to significantly constrict isolated rat gracilis feed arteries. TEA (1 mmol/L) reportedly has no significant effect on the diameter of cannulated coronary arterioles isolated from Yucatan minipigs (577). Iberiotoxin enhanced myogenic tone at low pressures in first-order arterioles from soleus muscle isolated from aged rats, but had no effect on myogenic tone of these same vessels isolated from young rats (730).
BKCa channel Ca2+ setpoint and the negative-feedback regulation of myogenic tone
Thus, the role played by vascular SMC BKCa channels on resting myogenic tone in systemic microvascular beds remains unclear. The lack of apparent activity of BKCa channels in some systems could be due to a lack or low expression of the channels; low voltage sensitivity (reduced slope of the voltage activation relationship); low Ca2+ sensitivity (reduced slope of the Ca2+-activation relationship); a high Ca2+ setpoint (Ca2+ threshold for voltage-dependent activation) of the channels expressed; differences in the source and magnitude of Ca2+ signals that activate these channels; or differences in membrane potential (672). In SMCs isolated from hamster second-order cremaster arterioles, a high Ca2+ setpoint appears to contribute to the lack of activity of BKCa channels in these cells (672). In this model, as noted earlier, no activity of BKCa channels was observed in relaxed resting cells studied by the perforated patch technique (673), in cells dialyzed with up to 300 nmol/L Ca2+ (665), or inferred from functional studies of arterioles under resting conditions, in vivo (672). This lack of activity of BKCa channels was not due to the absence of these channels as a normal, relatively high density (1–8 channels in essentially every patch) of high conductance (242 pS in symmetrical 140 mmol/L K+), iberiotoxin-sensitive channels were observed in inside-out patches of membrane from these cells (672). Furthermore, the voltage (16 mV per e-fold change in activity) and Ca2+ (85 mV/ten-fold change in Ca2+ concentration) sensitivities of the channels were similar to what had been reported in other SMCs (672). What appeared to be responsible for the low BKCa channel activity was a relatively high (9 µmol/L) Ca2+ setpoint (672). This value represents the concentration of Ca2+ required for activation of the channels to 50% of maximum at 0 mV (198, 672) and is an index of the threshold of Ca2+ required for physiological activation of the channels. In vascular SMCs isolated from larger arteries and other SMCs, the Ca2+ setpoint is on the order of 1 µmol/L (29, 111, 198, 646, 981); values that are 6- to 18-fold lower than the setpoint measured in hamster cremaster arteriolar muscle cells (672). Put in more physiological terms, the high Ca2+ setpoint in cremasteric arteriolar muscle cells means that the internal face of the membrane of these cells must be exposed to Ca2+ concentrations on the order of 3 µM for any activity of the channels to be observed at negative membrane potentials (665, 672). A high Ca2+ setpoint (~12 µmol/L) has also been measured in SMCs isolated from rat first-order cremaster arterioles (1593), and appears to arise from reduced expression of β1 subunits in these arteriolar SMCs relative to cerebral arteries (1589, 1593) and possible differences in expression of spliced variants (1098). It was also shown that siRNA knockdown of β1 subunit expression in SMCs isolated from cerebral arteries produced a phenotype (increased Ca2+ setpoint) similar to what was observed in cremaster arteriolar SMCs (1593). These data are consistent with data from heterologous expression systems where expression of BKCa channel α subunits alone yields channels with a Ca2+ setpoint on the order of 30 µmol/L, whereas expression of both α and β1 subunits produces channels with a Ca2+ setpoint on the order of 5 µmol/L [estimated from data in (974)]. Differences in coupling of α and β1 subunits, or differences in β1 expression were proposed to explain the heterogeneity of activity of BKCa channels in vascular muscle cells isolated from human coronary arteries (1382). Consistent with the hypothesis that there are regional differences in β1 subunit expression, SMCs from second-order mouse cremaster arterioles express only 55% of the β1 subunit expressed in SMCs isolated from upstream feed arteries (Jackson, unpublished observations). Thus, a high Ca2+ setpoint due to reduced β1 subunit expression in the SMCs in arterioles may account for some of the apparent lack of activity of these channels under resting conditions. However, this high Ca2+ setpoint cannot explain why BKCa channels are silent in arterioles studied in vivo [see, e.g., (672)], yet are active in the same vessels when studied, in vitro [see, e.g., (1528)]. Instead, it is proposed that the source of activator Ca2+ for BKCa channels accounts for the differences observed in vivo versus study of the same vessels, in vitro.
Sources of activator Ca2+ for BKCa channels
The source of Ca2+ that controls the activity of BKCa channels in resistance arteries and arterioles appears to display regional differences. Studies in cerebral arteries have shown that BKCa channels in isolated cells and in vessels studied, in vitro, using pressure myography, are controlled largely through Ca2+ released from RyRs in the form of Ca2+ sparks (Fig. 4) (176, 483, 682–684, 765, 1072, 1075, 1154, 1155, 1170, 1518, 1519), and similar results have been obtained in SMCs from coronary arteries (189, 453).
Benham and Bolton (110) were the first to describe spontaneous transient outward currents (STOCs), bursts of activity of BKCa channels in SMCs isolated from rabbit ear arteries and jejunum that arose from cyclical release of Ca2+ from internal stores. However, they did not establish a physiological function for these events. Subsequently, STOCs were identified in many vascular SMC preparations including, but not limited to: rabbit portal vein (104), hog carotid artery (329), guinea pig coronary artery (458), porcine coronary arteries (1333), rat basilar arteries (1338), human mesenteric arteries (1311), bovine coronary arteries (1334), rat cerebral arteries (1071), rat aorta (888), human coronary arteries (484), guinea pig mesenteric and submucosal arterioles (598), rat hepatic arteries (1660), canine renal arteries (663), rat renal arteries (131), mouse cerebral arteries (1168), rat mesenteric arteries (850), rat tail artery (243), porcine coronary arterioles (1020), guinea pig mesenteric arteries (1178), rat coronary arteries (264), rabbit basilar artery (1132), rat saphenous arteries (428), rat cremaster first-order arterioles (1589), and mouse superior epigastric arteries (573).
Early studies showed that STOCs were inhibited by ryanodine in SMCs from rabbit portal vein (782), but this was interpreted as a result of depletion of internal stores, not a specific role for RyRs in these events. However, subsequent studies by Nelson and colleagues (1071) identified Ca2+ sparks through RyRs as the source of Ca2+ the controls BKCa channel activity underlying STOCs. More importantly, they found that inhibition of RyR function with ryanodine, or inhibition of BKCa channel activity with TEA or iberiotoxin caused equivalent constriction of pressurized rat cerebral arteries, and that prior application of either ryanodine or one of the BKCa channel blockers eliminated subsequent effects of application of the other blocker. These data implied that BKCa channel activity in an intact, myogenically active artery was controlled by the activity of RyRs in the form of Ca2+ sparks and that this mechanism operates to provide an important negative feedback signal to limit myogenic tone.
While many studies have confirmed a role for RyR-based Ca2+ sparks in the control of BKCa channel activity, not all vessels use this mechanism. In vitro studies of second-order hamster (1528) and mouse (1527) cremaster arterioles have failed to detect Ca2+ sparks, and ryanodine is without effect on myogenic tone in these vessels. This did not appear to be methodological, because Ca2+ sparks were detected in upstream cremaster feed arteries, and ryanodine abolished these events and produced the expected vasoconstriction using identical methods (1527, 1528). In the arterioles, BKCa channels were active and contributed to the negative feedback control of myogenic tone (1527, 1528). However, it appeared that the source of Ca2+ controlling these channels originated from Ca2+ influx through L-type VGCCs, rather than from RyR-based Ca2+ sparks (1527, 1528) (Fig. 4). Studies in neurons (120, 508, 1355), in SMCs from rabbit coronary arteries (514) and in SMCs from mouse mesenteric arteries (1364) have shown that Ca2+ influx through L-type VGCCs can directly activate BKCa channels, supporting this idea. BKCa channels, L-type VGCCs and caveolin-1 colocalize in rat mesenteric vascular SMCs where tight coupling between BKCa channels and L-type Ca2+ channels has been demonstrated (1364). It should be noted that in contrast to the findings in rat cerebral arteries where BKCa channels appear to be solely controlled by RyR-based Ca2+ sparks (1071), in hamster (1528) and mouse (1527) cremaster feed arteries, Ca2+ influx, in addition to RyR-based Ca2+ sparks, contributes to the control of BKCa channel activity. Thus, there may be a spectrum of phenotypes for control of BKCa channel activity from solely RyR-based Ca2+ sparks to solely Ca2+-influx through L-type VGCCs.
We think that control of BKCa channels by VGCCs in cremaster arterioles explains why BKCa channels appear silent at rest, in vivo, but contribute to the negative-feedback regulation of myogenic tone when these same vessels are studied, in vitro, by pressure myography. In vivo, myogenic tone of second-order cremaster arterioles appears resistant to L-type Ca2+ channel blockers (599, 670). In contrast, blockade of L-type VGCCs produces substantial dilation of these vessels when they are studied, in vitro (185). Thus, in vivo, at rest when L-type VGCCs are silent, BKCa channels are silent (672). In contrast, in vitro, where pressure-induced myogenic tone depends on Ca2+-influx through L-type-VGCCs, BKCa channels are active and contribute to the negative-feedback control of myogenic tone (1527, 1528). Note that in vivo, induction of agonist or oxygen-induced tone, which depends on Ca2+-influx through L-type VGCCs, activates BKCa channels that limit the vasoconstriction (672).
Coupling of Ca2+-influx through VGCCs to the activity of BKCa channels does not appear to explain the lack or low activity of BKCa channels in cerebral penetrating arterioles. Instead, RyR-based Ca2+ sparks appear to be absent under resting conditions, limiting the activity of the BKCa channels (301). However, exposure of SMCs to acidic solutions activates the RyRs, generating Ca2+ sparks which then activate BKCa channels to contribute to vasodilation during acidosis. These data further support the idea that there are regional differences in the mechanisms controlling BKCa channel function.
Vasoconstrictors and BKCa channels
Vasoconstrictor-mediated inhibition of BKCa channels
Calcium-activated K+ channels have been reported to play both a positive (817, 1272, 1417, 1526) and negative (116, 175, 458, 566, 672, 1071, 1074, 1228, 1486) feedback role in the mechanism of action of vasoconstrictors. As with KV channels (earlier) and KATP channels (later), activation of PKC, a common step in the mechanism of action of many vasoconstrictors (620, 836), appears to inhibit BKCa channels in some vascular SMCs (817, 1004) and in other systems (1201, 1631) (Fig. 6). The functional significance of this inhibition has not been well studied. However, in small mesenteric arteries, blockade of BKCa channels with charybdotoxin, TEA or iberiotoxin inhibits pressure-induced depolarization and myogenic reactivity (1526) suggesting that closure of BKCa channels plays a role in pressure-induced vasoconstriction in some systemic arteries. In addition, the vasoconstrictor angiotensin II has been shown to cause internalization and degradation of BKCa channels in a PKC-dependent fashion, providing an additional mechanism to contribute to vasoconstriction induced by this vasoactive agent (843).
Figure 6.
Regulation of BKCa channels by vasoconstrictors and vasodilators. Schematic of the plasma membrane of a vascular SMC showing, from left to right, a Gq-protein-coupled receptor (GqPCR), associated G-proteins and PLCβ; a BKCa channel; and a Gs-protein-coupled receptor, associated G-proteins and AC. Black lines and arrows indicate stimulation, activation or increases; red lines indicate inhibition. Pathways to the right of the BKCa channel activate these channels, while those to the left are inhibitory. Membrane depolarization due to opening of membrane channels that conduct Na+, Ca2+, or Cl− or due to closure of other K+ channels as well as increases in subsarcolemmal Ca2+ are the major stimulae for opening BKCa channels. Vasodilator agonists that act at GsPCR (isoproterenol, adenosine, prostacyclin, CGRP, etc.), stimulate the formation of cAMP, activation of PKA and phosphorylation of BKCa channels leading to their activation. Vasodilators that lead to increased production of cAMP als may active BKCa channels through exchange EPACs. NO, acting through sGC, and other vasodilators that stimulate the production of cGMP, activate PKG, phosphorylating BKCa channels and increasing their activity. Other vasodilators, such as H2S and H2O2 also can activate KV channels as shown. NO or carbon monoxide (CO) also may directly interact with BKCa channels or associated heme-proteins to increase channel activity. BKCa channels also are activated by H2O2. Conversely, hyperpolarization, induced by opening of other K+ channels or closure of channels conducting Na+, Ca2+, or Cl−, and/or a fall in subsarcolemmal Ca2+ represent the major stimulae for closure of KV channels. In addition, vasoconstrictors that act through Gq-coupled receptors can inhibit BKCa channels through activation of PLCβ, the formation of diacylglycerol (DAG) and activation of PKC. See text for more information.
Vasoconstrictor-mediated activation of BKCa channels
Despite the evidence presented earlier, other studies suggest that BKCa channels play a negative feedback role in the mechanism of action of vasoconstrictors including elevated intravascular pressure. In a number of systems, inhibition of BKCa channels augments the effects of vasoconstrictors (220, 672, 1487, 1526) in the absence of effects on basal tone (Fig. 6). For example, in hamster cremasteric arteriolar SMCs, iberiotoxin increases the response of single cells to norepinephrine (672). Contraction of these SMCs by norepinephrine is correlated with a large, reversible increase in the opening of single BKCa channels in cell attached patches that can be inhibited by inclusion of 1 mmol/L TEA in the pipette solution (667). Vasopressin (1486), endothelin-1 (1244) and histamine (566) have also been reported to increase BKCa channel activity in vascular SMCs. Arteriolar vasoconstriction induced by elevated PO2 in hamster cremaster muscle is enhanced by TEA, suggesting that BKCa channels are activated during this process (672). In porcine coronary arteries, IP3 has been proposed to directly activate BKCa channels, contributing to the negative-feedback regulation of vasoconstrictor-induced SMC tone (1582). Thus, vasoconstriction appears to be associated with an increase in BKCa channel activity, rather than a decrease as predicted from the studies mentioned above. In addition, pressure-induced activation of BKCa channels has been reported in several systems (116, 175, 1071). It may be that while activation of PKC might cause some channel inhibition, the increase in intracellular Ca2+ as well as the membrane depolarization induced by vasoconstrictors, remain sufficient to activate enough BKCa channels to limit the degree of membrane depolarization that occurs. Future studies in which membrane potential, subsarcolemmal calcium, BKCa channel activity and vascular SMC tone are measured simultaneously during application of vasoconstrictors, or elevated pressure will be required to test this hypothesis.
Vasodilators and BKCa channels
cAMP signaling and BKCa channels
The role played by BKCa channels in the mechanism of action of vasodilators remains controversial. Sadoshima et al. (1230) first demonstrated that cAMP-mediated activation of PKA activates BKCa channels in patch clamp studies of cultured rat aortic SMCs. Subsequent studies in other systems have confirmed and extended these results (799, 801) (Fig. 6). Phosphorylation of the BKCa α-subunit at serine 869 appears to be involved in effects of PKA on BKCa channel activity (1053, 1410, 1411). These K+ channels may also be activated by the guanine nucleotide binding protein, Gαs, independent from cAMP and PKA (799, 800, 1271). Vasodilators that act via cAMP, such as isoproterenol, may also activate BKCa channels by increasing subsarcolemmal Ca2+ concentrations (1572) and the frequency of Ca2+ sparks (1169). This latter effect may involve cAMP-mediated, PKA-dependent phosphorylation of phospholamban and the resultant increase in SERCA activity, which elevates store Ca2+ load (1521). Finally, dilators that act via cyclic nucleotides can also increase the trafficking of β1 subunits to the plasma membrane, dynamically increasing the association of these accessory proteins with the α-subunits, increasing the Ca2+ sensitivity of the BKCa channels (961). In aortic SMCs, β2-adrenergic receptors associate with BKCa channels in complexes with AKAP150 and CaV 1.2 VGCCs suggesting an intimate relationship between a Gαs-coupled receptor and BKCa channels (891). All of the aforementioned studies suggest that vasodilators, such as isoproterenol, prostacyclin, adenosine, CGRP, etc., which bind to receptors coupled to adenylate cyclase via Gαs-proteins, may relax vascular SMCs, at least in part, by activation of BKCa channels and membrane hyperpolarization.
Agonists that activate adenylate cyclase to increase the production of cAMP also may activate BKCa channels via exchange proteins activated by cAMP [EPACs (480)] as has been demonstrated in rat mesenteric artery (1206) (Fig. 6). In this model, a selective agonist of EPACs, 8-pCPT-2’-O-Me-cAMP, increased the frequency of Ca2+ sparks and BKCa-associated STOCs, resulting in SMC cell membrane hyperpolarization and relaxation (1206). In addition to the effects of the EPAC agonist on Ca2+ spark and STOC frequency, Roberts et al. (1206) found that the amplitude of STOCs was increased, despite no change in the amplitude of Ca2+ sparks. These data suggest that EPACs may mediate effects on RYRs (resulting in the increase in spark and STOC frequency) and directly on BKCa channels (resulting in the increase in STOC amplitude).
The hypothesis that BKCa channels mediate at least a portion of the dilator response of blood vessels to cAMP-related vasodilators is supported by many, but not all, functional studies of isolated blood vessels. Dilation or relaxation induced by the adenylate cyclase activator, forskolin (90, 961, 1110, 1169, 1371); the cell-permeant cAMP analog, dibutyryl cAMP (1139, 1383); or receptor-mediated agonists such as adenosine (191, 201, 1139); di-adenosine poly phosphate (1354); CGRP (617); isoproterenol (961, 1254); iloprost (1270); prostacyclin (60); PGE2 (60); vasoactive intestinal polypeptide (VIP) (1383); adenylate-cyclase-activating peptides (178); and 11,12-EET (642) are all inhibited by iberiotoxin, charybdotoxin and/or TEA (1 mmol/L).
In contrast, dilation of the vasculature in cat hind limb induced by albuterol and prostaglandin E1, which should be mediated by cAMP, are not affected by blockade of BKCa channels with TEA (220). Systemic dilation induced by adenosine is only slightly attenuated by infusion of iberiotoxin in anesthetized pigs (1619). Relaxation of rat aorta by dibutyryl cAMP is not inhibited by iberiotoxin (1254). Adenosine-induced dilation of pig coronary arterioles is not inhibited by iberiotoxin (580). Forskolin-induced dilation of the renal vascular bed is not inhibited by iberiotoxin (929). In contrast to previous studies utilizing iberiotoxin (1139), adenosine-induced dilation of rat pial arterioles was not inhibited by an effective concentration of paxilline (1125). Dilation of porcine coronary arteries to PGE2 is not inhibited by iberiotoxin (1403). These data suggest that there may be regional or species differences in the role played by BKCa channels in cAMP-related vasodilation or that subtle methodological differences can alter the role played by these channels.
NO-cGMP-PKG-signaling and BKCa channels
Vasodilators that signal through the cGMP-PKG pathway, such as the nitrovasodilators and endogenous NO, also activate BKCa channels (Fig. 6). Early studies showed that SMC relaxation induced by the NO-donor, SNP or atrial natriuretic peptide (which activates particulate guanylate cyclase to produce cGMP) were impaired in rabbit aorta contracted with 80 mmol/L K+ (1539), consistent with a role for K+ channels in the mechanism of action of these cGMP-related vasodilators, and 10 mmol/L TEA inhibited relaxation of rabbit aorta to natriuretic peptide (1538). Subsequent patch clamp studies demonstrated that SNP, atrial natriuretic peptide, or dibutyryl cGMP increased the open-state probability of BKCa channels in cell-attached patches of cultured bovine aortic SMCs (1533). However, the authors concluded that it was cGMP that activated the channels directly, rather than an effect mediated via cGMP-dependent activation of PKG. Nitroglycerin and 8-Br-cGMP were then shown to increase BKCa channel activity in cell-attached patches of primary cultured SMCs from porcine coronary arteries (446). Subsequently, the NO-donor, SIN-1, and the membrane permeant analog of cGMP, 8(4-chlorophenylthio)-cGMP, were shown to activate BKCa channels in cell-attached patches of rabbit basilar artery SMCs (1209). Importantly, Robertson et al. (1209) were the first to show that PKG specifically activated BKCa channels in inside-out patches of rabbit basilar artery SMCs. This was confirmed in membrane patches from canine coronary SMCs (1388). Taniguchi et al. (1388) also reported that charybdotoxin inhibited relaxation induced by atrial natriuretic peptide, consistent with a role for cGMP/PKG activation of BKCa channels. The NO-donor, NONOate increased the activity of BKCa channels in cell-attached patches of bovine coronary artery SMCs (867). In addition, NO modulates the frequency of Ca2+ sparks affecting the activity of BKCa channels (699, 938, 1613). In contrast, endothelium-derived NO inhibits the relaxation of the porcine coronary artery to natriuretic peptides by desensitizing BKCa channels (870).
Consistent with the electrophysiological results outlined earlier, studies in rabbit superior mesenteric arteries (743), horse penile arteries (1307), rat coronary arteries (1174, 1517), rat mesenteric arteries (1162), rat pial arterioles, in vivo, (1139), canine middle cerebral arteries (1108, 1109), human coronary arteries (190), rat middle cerebral arteries (1356) indicate that blockade of BKCa channels inhibits relaxation or dilation of these vessels. In addition, insulin-induced increases in human skin blood flow are mediated by NO and BKCa channels (472). These data all support a role for BKCa channels in the mechanism of action of NO on vascular SMCs.
Supporting a role for phosphorylation in NO-mediated activation of BKCa channels, phosphatase inhibition potentiated cGMP-dependent activation of pulmonary artery SMC BKCa channels (54). Phosphorylation of the α subunit of BKCa channels by PKG, in vitro, was reported in channels isolated from tracheal SMCs (34), human BKCa channels expressed in Xenopus oocytes (35), and murine BKCa channels (1366). Site-directed mutagenesis identified serine 1072 as an essential PKG phosphorylation site in studies of human embryonic kidney (HEK) cells expressing BKCa channels cloned from canine colonic SMCs (cSlo) (450). In contrast, site-directed mutagenesis indicated that PKG-dependent phosphorylation at serines 855 and 869 are required for full activation of BKCa channels in Xenopus oocytes stimulated with NO or atrial natriuretic peptide and expressing hSlo and hβ1 (1052). Expression of a kinase-dead mutant of PKG eliminates effects of NO donors and cGMP analogs on murine BKCa channels expressed in HEK cells supporting a role for PKG-dependent phosphorylation (1366). Phosphopeptide and phophoamino acid mapping and site directed mutagenesis of murine BKCa channels have identified serine 873 and serines 1111–1113 [serine 1112 is equivalent to serine 1072 reported by (450)] as essential sites for PKG-mediated channel phosphorylation and cGMP/PKG-dependent modulation of BKCa channel function (807). However, in mesangial cells and an HEK cell expression system, dibutyryl cGMP-induced activation of BKCa channels depended on the presence of the β1 subunit (794). Furthermore, in rat cerebral vascular myocytes, NO, via activation of PKG, stimulates anterograde trafficking of β1 subunit to the plasma membrane, association with BKCa α subunits, and subsequent activation of BKCa channels which contributes to NO-mediated vasodilation in these vessels (842).
Nitric oxide also appears to have the potential to activate BKCa channels independent from cGMP. Bolotina et al. (151) showed that NO could activate these channels in inside-out patches of rabbit aortic SMC cell membranes and that charybdotoxin inhibited NO-induced, cGMP-independent relaxation of the same tissue. Similarly, the NO-donor, SNP, increased the open-state probability of BKCa channels in cell-free patches of rat tail artery (1546) and rat middle cerebral arteries (1356), supporting this hypothesis. Activation of BKCa channels by NO, independent from cGMP/PKG also has been reported in other systems (16, 188, 814, 815). Charybdotoxin-sensitive dilation induced by the NO-donor, 3-morpholinosydnonimine (SIN-1) of rat mesenteric arteries (1162, 1164) and activation of BKCa channels in SMCs from these vessels (1011) also appears to occur independent of activation of guanylate cyclase. Studies in rat small mesenteric arteries also suggest that NO may activate BKCa channels in a cGMP-independent manner, although functional studies were not performed to demonstrate a role for this process in NO-induced vasodilation (1512). Inhibition of soluble guanylate cyclase inhibits, but does not abolish NO-induced activation of BKCa channels (854). Inhibition of guanylate cyclase does not inhibit charybdotoxin-sensitive relaxation of canine middle cerebral arteries (1109). Similarly, activation of BKCa channel currents in SMCs isolated from human radial arteries by an NO donor is resistant to inhibition of guanylate cyclase suggesting cGMP/PKG-independent effects (1630), consistent with a direct effect of NO on BKCa channels. These data also suggest that cGMP-dependent and independent mechanisms for NO-induced activation of BKCa channels may exist in the same cells. Nitric oxide-induced relaxation of human umbilical arteries is inhibited by iberiotoxin, but appears independent from cGMP (907). Dilation of rat middle cerebral arteries induced by DEA-NONOate is inhibited by iberiotoxin, whereas this BKCa channel blocker has little effect on dilation induced by 8-Br-cGMP (1608), suggesting cGMP-independent activation of BKCa channels in this model.
Despite the evidence presented earlier, other studies suggest no direct effect of NO on BKCa channels. Nitric oxide donors increase BKCa channel activity in cell-attached patches, but have no effect on BKCa channels in cell-free patches of membrane from cultured bovine aortic SMCs (1533). Similar results have been reported for heterologously expressed BKCa channels (450). NO-donor-induced activation of BKCa channels is absent in aortic SMCs isolated from PKG knockout mice (1255). Similarly, in an expression system, NO-donor induced activation of BKCa channels could be prevented by coexpression of a kinase-dead mutant of PKG (1366). Thus, the bulk of evidence suggests that NO and NO-donors activate BKCa channels through cGMP/PKG-dependent mechanisms.
In contrast to the studies supporting NO-induced activation of BKCa channels, there is also a body of evidence suggesting that the mechanism of action of NO does not involve the activation of these channels. Studies in feline hind limb have failed to demonstrate inhibition of nitrovasodilator-induced vasodilation with TEA, despite other effects consistent with effective blockade of BKCa channels (220). Other studies have also failed to demonstrate effects of BKCa channel blockade on NO-induced vasodilation (59, 136, 473, 1647) or relaxation of isolated vessels (136, 274, 337, 449, 551, 593, 747, 1165, 1371, 1516, 1647). In addition, NO does not hyperpolarize SMCs in rabbit middle cerebral arteries (174) or rabbit basilar arteries (1161). Nitric oxide hyperpolarizes SMCs in rat mesenteric arteries, but this hyperpolarization is inhibited by the KATP channel blocker, glibenclamide (465). Relaxation and hyperpolarization of rat superior mesenteric arteries to SNAP is not inhibited by charybdotoxin (473). Thus, as with cAMP-related vasodilators, there may be species or regional differences in the role played by BKCa channels in NO-induced vasodilation.
Other vasodilators and BKCa channels
In addition to endothelium-derived NO, other endothelium-derived vasodilators have also been proposed to act, in part, by activation of BKCa channels. Carbon monoxide produced endogenously from metabolism of heme groups by the heme oxygenases activates BKCa channels (7, 1494, 1495) (Fig. 6). In rat tail artery, vasodilation induced by CO is inhibited by charybdotoxin, but not by apamin (1494). These data are supported by studies showing a direct effect of CO on BKCa channel activity in inside-out patches of rat tail artery membranes (1494, 1495), independent from changes in cGMP, as CO also activates guanylate cyclase (746). BKCa channels bind heme proteins, which serve as the receptor for CO-dependent activation of these channels (680). Carbon monoxide also increases the frequency of Ca2+ sparks and increases the coupling of Ca2+ sparks to BKCa channels in cerebral SMCs (679), likely by increasing the Ca2+ sensitivity of the channels (1556). Glutamate-induced dilation of cerebral arterioles in brain slices of neonatal pigs involves astrocyte-derived CO, activation of guanylate cyclase, and increase in Ca2+ spark frequency and a reduction in global Ca2+ concentration in cerebral arteriolar SMCs (865, 1557).
Epoxyeicosatrienoic acids (EETs), which serve as endothelium-derived relaxing factors in some blood vessels, also have been shown to activate BKCa channels. In bovine coronary arteries EET-induced relaxation is inhibited by TEA (1 mmol/L) or charybdotoxin (194). These arachidonic acid (AA) metabolites also hyperpolarized SMCs in these vessels and increased the activity of BKCa channels in patch clamp experiments (194), providing evidence that EETs act, in part, by activation of BKCa channels. Similarly, relaxation and hyperpolarization of guinea pig coronary arteries induced by 11,12 EET is inhibited by iberiotoxin (370). Iberiotoxin also inhibits dilation and SMC hyperpolarization induced by endothelium-derived EETs in rat gracilis arteries (631). It appears that the effects of EETs on BKCa channels may be indirect as it has been proposed that EETs activate TRPV4 channels inducing Ca2+ influx, that stimulates RyR-mediated Ca2+ sparks, which, in turn, activate overlying BKCa channels (364).
There is also evidence that H2O2 and H2S act through BKCa channels in some blood vessels. In porcine coronary artery SMCs, H2O2 activates BKCa channels (92), and H2O2-induced dilation of porcine coronary arteries is inhibited by iberiotoxin (1403) (Fig. 6). Tumor necrosis factor (TNF) α dilates cerebral resistance arteries, in part, by stimulation of Ca2+ sparks and activation of BKCa channels by a mechanism involving activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and production of H2O2 (242).
In cerebral arterioles isolated from newborn pigs, H2S stimulates Ca2+ sparks which activate BKCa channels to hyperpolarize the SMCs and produce vasodilation (871). An indirect mechanism involving H2S-induced Ca2+ spark stimulation of BKCa channels also has been proposed in rat mesenteric arteries (676).
Functional vasodilation and BKCa channels
Relatively few functional studies have been performed to determine the role played by BKCa channels in the regulation of blood flow in systemic vascular beds. Hyperosmolarity (319) and low extracellular pH (301, 877), two conditions that are associated with functional hyperemia in several organ systems, are mediated, in part, by activation of BKCa channels. Active hyperemia in canine diaphragm is partially inhibited by iberiotoxin, particularly at low levels of activity (1462). Similarly, BKCa channels modestly contribute to functional hyperemia in human forearm (1122). These data suggest that BKCa channels play at least a small role in the functional regulation of blood flow in skeletal muscle. However, the role played by BKCa channels in reactive hyperemia is unclear. In canine diaphragm, iberiotoxin inhibits a portion of the hyperemia induced by vascular occlusion (1463), supporting a role for BKCa channels in this response. Exercise training also has been shown to enhance BKCa channel function in mesenteric arteries through increases in the expression of the β1 subunit (1295). In contrast, studies in the cat hind limb found no effect of TEA on reactive hyperemia (220), suggesting no role for BKCa channels. Thus, there may be species-dependent differences in the contribution of BKCa channels to the hyperemia after vascular occlusion.
Reactive-hyperemia to 5 min vascular occlusion is inhibited by both TEA and charybdotoxin in isolated rat hearts (1302). Block of BKCa channels with TEA in the pig coronary circulation resulted in a decrease in resting vascular conductance and a decrease in exercise-induced hyperemia (996). These data suggest that in the BKCa channels may participate in reactive- and functional-hyperemia in the heart. Opposing this view are studies in the coronary circulation of Ossabaw pigs, using penitrem A to block BKCa channels that show no role for BKCa channels in resting coronary vascular resistance or in exercise-induced increases in blood flow in lean or obese pigs (158). Borbouse et al. (158) suggested that ganglionic blocking effects of TEA, rather than inhibition of BKCa channels might reconcile their study with that of Merkus et al. (996). Thus, the role played by BKCa channels in the local regulation of blood flow remains unclear.
Pathophysiology and BKCa channels
Hypertension
As with KV channels discussed earlier, hypertension has been reported to both increase and decrease the expression and function of BKCa channels in resistance arteries and arterioles. A number of functional studies have shown that inhibition of BKCa channels causes contraction of vascular SMCs from hypertensive animals that is substantially larger than that observed in normotensive counterparts (65, 67, 890, 1140, 1227, 1228). This apparent increase in BKCa channel activity is associated with an increased BKCa channel current density in patch clamp experiments (890, 1227, 1228, 1628) and increased expression of BKCa channel proteins (890, 1228, 1628). This increased expression and function of BKCa channels may occur as a negative feedback response to the increased vascular reactivity observed in hypertension (1228).
In contrast, in Sprague-Dawley rats, angiotensin II-induced hypertension is associated with a decrease in BKCa channel function due to reduced Ca2+ sensitivity of BKCa channels resulting from decreased expression of the β1 subunit in cerebral arteries (39). Reduced expression of β1 subunits was also observed in vessels from SHR, but also in WKY rats with blood pressures that were intermediate between the Sprague-Dawley and SHR (43). Reduced function of BKCa channels correlated with reduced expression of β1 subunits also has been reported in aortic SMCs from SHR (860) and in human hypertension (1583). It was proposed that activation of NFATC3 resulted in the down regulation of the β1 subunits in angiotensin-induced hypertension (1082). BKCa channel function is also impaired in aortic SMCs from two-kidney: one-clip hypertensive rats (193).
In addition, BKCa channel currents are reduced with decreased expression of BKCa channel α subunits in mesenteric arteries from L-nitroarginine methyl ester-induced hypertensive rats (170, 171). In a mouse model of hypertension associated with increases in aldosterone, coronary artery dysfunction involves downregulation of expression and function of SMC BKCa channels (45). There also is decreased expression of BKCa channel α and β1 subunits in mesenteric artery SMCs from BPH versus BPN mice and a corresponding decrease in functional activity of BKCa channels in patch clamp studies (1028). The reason for these differences in BKCa channel expression and function is not known, but may relate to methodological differences, heterogeneity in the expression, function and regulation of BKCa channels in different vascular beds as well as strain- or species-dependent differences.
Obesity and the metabolic syndrome
The effects of obesity, hypercholesteremia, and the metabolic syndrome on BKCa channel expression and function is complex (1226). In obese pigs displaying the metabolic syndrome, the expression of BKCa channels is increased; however, their function is severely depressed relative to their lean counterparts (157, 158). Similarly, there is decreased BKCa channel function in SMCs from obese Zucker rats (440). Hypercholesterolemia also impairs BKCa channel function in porcine coronary artery SMCs (1581) and it has been shown that hypercholesterolemia impairs the function of BKCa channels in human forearm circulation (1123). In a mouse model of type 2 diabetes, BKCa channel function is depressed in both cerebral and mesenteric arteries due to decreased expression of β1 subunits (1099). Hyperglycemia was associated with activation of the transcription factor NFATC3 via calcineurin that is targeted to the appropriate cellular compartment by AKAP150 (1099). That hyperglycemia, alone, can induce this phenotype suggests that this mechanism may also be active in type 1 diabetes.
Aging
Aging impairs BKCa channel expression and function in coronary arteries of rats that can be partially restored by low intensity exercise training (27). In rat skeletal muscle feed arteries, advanced age reduces expression of BKCa channel subunits. However, their function in the regulation of myogenic tone appears to be increased in soleus arteries, but not affected in gracilis muscle arteries (730). There is decreased function and expression of BKCa channels in coronary arteries from Fisher 344 rats (942). Decreased expression was also reported in coronary arteries from aged humans (942). Aging also is associated with decreased expression of the β1 subunit that contributes to the age-related decrease in channel function (1094). In contrast, advanced age increases the amplitude of STOCs in SMCs from murine superior epigastric arteries (573) with no change in the expression of BKCa channels. However, despite the increase in STOC amplitude, the integrated function of these channels in the regulation of myogenic tone was similar in vessels from young (3–6 mo) and old (>22 mo) mice (573). Thus, the effects of aging on BKCa channel expression and function appear to be complex, and likely display regional variation.
Diabetes
The expression and function of BKCa channels appears to be reduced in type 1 diabetes (421, 884). It has been proposed that this may involve hyperglycemia-induced oxidative stress and the formation of peroxynitrite (884). In high fat/high cholesterol-fed pigs treated with alloxan to induce diabetes, there is a reduction in baseline coronary blood flow, enhanced constriction induced by PGF2α and impaired adenosine-mediated dilation (1020). These functional changes were associated with a reduction in Ca2+ sparks and BKCa channel-mediated STOCs in SMCs isolated from coronary arteries from these animals, suggesting impairment of Ca2+ signaling and BKCa channel function (1020). Moderate exercise training prevented the changes that were observed except for the impaired vasodilator reactivity. Arachidonic acid–induced dilation of rat coronary arteries, which involves BKCa channels, is impaired in streptozotocin-induced diabetes (1645). Chronic (but not acute) treatment with a PKCβ inhibitor or with superoxide-dismutase restores AA-induced dilation implicating a role for ROS in the downregulation of BKCa channel function in this model system (1645). Streptozotocin-induced diabetes impairs BKCa channel function in SMCs from rat retinal arterioles due to decreased expression of β1 subunits (975). Similarly, in cerebrovascular SMCs, BKCa channel function is depressed in diabetes (338, 1497) due to increased ROS (338) and decreased expression of β1 subunit (1497). These data indicate that BKCa channel expression and function are depressed in diabetes and that this may contribute to the increased vascular tone and vasoconstrictor reactivity observed in this disease state (421, 884).
In contrast, diabetic dyslipidemia in alloxan-treated Yucatan mini-pigs resulted in increased vasoconstrictor reactivity of coronary arteries studied in vivo and in vitro (1021). However, whole-cell K+ current densities, and caffeine-induced BKCa channel current densities were elevated, changes that were reduced by endurance exercise (1021). It was observed that STOC frequency was either decreased or remained the same relative to events in SMCs from untreated pigs, with no change in BKCa α subunit expression as assessed by Western blot. The increased K+ current density may be compensatory to increased Ca2+ signaling. Disparate results between this study and others in the literature (see earlier) could be due to species differences, vessel heterogeneity and/or the duration or magnitude of the diabetic dyslipidemia.
Other disease states
Alcohol-induced cerebral vasoconstriction is mediated by closure of BKCa channels (893). Depletion of membrane cholesterol with methyl-β-cyclodextrin abolished this effect, which appeared to be mediated by interactions of cholesterol with the β1 subunit (183).
In mice, heart failure induced by myocardial infarction results in increased myogenic tone in mesenteric resistance arteries due to decreased expression of both the α and β1 subunits of BKCa channels, reduced BKCa channel function, and membrane depolarization (1488). However, the mechanism for this BKCa channel remodeling was not established.
BKCa channels may also be involved in the decreased vascular reactivity observed in sepsis. In LPS or TNF-alpha-induced shock in mice, block of BKCa channels reduces mortality if SKCa channels also are blocked (213). This observation suggests an interplay between K+ channel signaling in the endothelium with that in vascular SMCs.
KIR Channels
Discovery of KIR channels
Inward-rectifier K+ channels derive their name from the fact that at membrane potentials negative to the K+ equilibrium potential, these channels conduct K+ ions into cells, whereas at more positive potentials, outward K+ current is limited (594, 791, 1080, 1187). Inward rectification of membrane K+ currents was first described by Katz in skeletal muscle (737). Subsequently seven families of 15 genes in the potassium channel family J (KCNJ) have been identified that encode K+ channels which display inward rectification (791). This section will focus on the KCNJ family members that encode strong inward rectifiers, particularly KNCJ2 (KIR 2.1) and KCNJ12 (KIR 2.2) as these channels are highly expressed in vascular SMCs of resistance arteries and arterioles and determine the inward rectification of K+ currents through SMC membranes (168, 689, 1313, 1621) as discussed below.
Structure of KIR channels
Inward-rectifier K+ (KIR) channels are composed of a tetramer of pore-forming α subunits (594, 1080). Each α subunit has two membrane spanning domains (M1 and M2, Fig. 2) with intracellular carboxy and amino termini (594, 1080). The membrane spanning domains are linked by a P-loop that, along with M2, forms the ion-conducting pore and contains the K+ ion selectivity filter of a KIR channel (594, 1080). The characteristic inward rectification of K+ current through KIR channels occurs due to block of outward K+ currents by intracellular polyamines (396, 424, 904) and Mg2+ (960, 1461) [see (908) for a review of this topic]. These positively charge moieties interact with negatively charged amino acids in M2 (D171 in KIR 2.1) and in the carboxy terminal domains (E224 and E299 in KIR 2.1) to block efflux of K+ ions at membrane potentials positive to the K+ equilibrium potential (908). In addition, and important for the physiological function of these channels, the conductance of KIR channels increases in proportion to the square-root of the extracellular K+ concentration (534, 792, 905, 936, 1235). This results from the presence of two K+-binding sites in the P-Loop K+ selectivity filter (594). Also important is the outward “hump” in the current-voltage relationship [i.e., a region of negative slope conductance (372, 1235)] that is observed at potentials positive to the K+ equilibrium potential but negative to the resting membrane potential of most vascular SMCs of resistance arteries and arterioles (~−30 mV) (185, 380). Hyperpolarization of the membrane from the resting potential will activate KIR channels and amplify the initial hyperpolarization (185, 668, 689, 903, 1313). Also, small increases in extracellular K+ concentration will shift this region of negative slope conductance to more depolarized potentials (as the K+ equilibrium potential shifts to more positive potentials), which, if this region encompasses the prevailing membrane potential, will lead to activation of outward current through the KIR channels accounting for at least a part of the membrane hyperpolarization that is observed in vascular SMCs exposed to increases in extracellular K+ up to about 20 mmol/L.
Modulation of KIR channels by membrane lipids
Currents through KIR 2 channels are strongly regulated by their lipid environment. Phosphatidylinositol 4,5-bisphosphate (PIP2) activates KIR 2 channels through interactions with positively charged residues in M2 and the cytoplasmic tails of the channels (594) opening the potential for modulation of KIR channel function by PIP2 hydrolysis and synthesis (594). Thus, agonists that act on Gq/11-coupled receptors could, potentially, inhibit KIR channel function by stimulation of phospholipase Cβ and subsequent hydrolysis of PIP2 to IP3 and diacylglycerol. However, the affinity of KIR 2.1 for PIP2 binding is sufficiently high that local depletion of PIP2 (by the action of phospholipases) does not strip these channels of activator PIP2 (344). In contrast, KIR 2.2 and 2.3 have lower PIP2 affinity allowing stronger regulation by local hydrolysis of PIP2 (344). Thus, it is possible that Gq/11-coupled receptor activity could modulate the function of KIR 2.2 containing vascular KIR channels, although this has not been adequately explored in SMCs from resistance arteries and arterioles.
Membrane cholesterol also substantially impacts the function of KIR channels, although the physiological significance of this has not been explored in vascular SMC KIR channels. Increases in membrane cholesterol induced by exposure of cells to cholesterol-saturated methyl-β-cyclodextrin inhibits, while decreases in membrane cholesterol produced by methyl-β-cyclodextrin stimulates currents through KIR 2 channels in endothelial cells (382, 400, 1215, 1217, 1218) and in heterologously expressed KIR 2 channels (1214), with KIR 2.1 and 2.2 being particularly sensitive to cholesterol manipulation (1214). Two cholesterol-binding domains have been identified: one in the hinge region of M1 and a second at the interface between M1 and the cytosolic domains (1217). This cholesterol sensitivity involves several amino acids (L222, N216, and K219) in the CD loop of the carboxy terminus of KIR 2 channels that are also important for sensitivity to PIP2 (382), and are part of a group of cytosolic residues that form a “cholesterol-sensitivity belt” around the putative pore of KIR 2 channels affecting gating of the channels (1216). Studies of the bacterial K+ channel KIR Bac1.1 and KIR 2.1 proteins incorporated into liposomes indicate that it is likely that cholesterol binds directly to the channel to modulate function (1308), through novel cholesterol binding motifs located near the hinge region of M1 and at the interface between M1 and the cytosolic domains (1217).
KIR 2 channels preferentially partition into cholesterol-rich lipid rafts (1214). Removal of membrane cholesterol results in channel translocation out of these microdomains, whereas adding cholesterol has the opposite effect (1413). In addition to modulation of channel function by cholesterol, these channels also interact with caveolin-1, which also has a negative impact on KIR channel function, stabilizing the channel in a closed state (548). The location of KIR channels in caveolae positions them to potentially interact with and be modulated by a large number of other receptors, ion channels, protein kinases, etc. that assemble in these membrane signaling microdomains (139), similar to what has been described for KIR 2 channels in cardiac myocytes (844, 1534). In heterologous expression systems, KIR 2.1 has been shown to interact with AKAP79 that may help target PKA, calcineurin and other signaling proteins to these channels (307).
Evidence for KIR channels in vascular SMCs
Edwards and Hirst were the first to describe vascular Ba2+-sensitive inwardly rectified currents in guinea pig sub-mucosal (372) and rat cerebral (373) arterioles. However, because these currents were measured in intact arterioles, the cell-type conducting these currents could not be identified. Because SMCs are electrically coupled to endothelial cells (380, 1251) and endothelial cells also express KIR channels (668, 903, 1084, 1326), the currents measured by Edwards and colleagues (372, 373) could have originated from either cell type. Specific currents through Ba2+-sensitive KIR channels in vascular SMCs were first demonstrated in SMCs from rat posterior cerebral arteries by Quayle et al. (1186) and subsequently identified in SMCs from rat (1207) and pig coronary arteries (1185); hamster cremaster arterioles (185); rat renal afferent arterioles (245, 840); hamster retractor feed arteries (1313); and rat renal interlobular arteries (247). The expression and function of KIR channels appears to be inversely related to vessel diameter with microvascular SMCs displaying higher functional expression than upstream arteries (903, 1128, 1185, 1187).
Expression of KIR channel transcripts and robust Ba2+-sensitive KIR channel currents were observed in SMCs isolated from small mesenteric arteries from inbred normotensive and hypertensive mouse strains (BPN and BPH mice) (1373). In contrast, while mRNA for KIR 2.1 has been reported in SMCs from rat mesenteric arteries (168, 177, 1313), patch-clamp studies of SMCs isolated from small mesenteric arteries in rats (291, 1313) and some mice strains (1326) have failed to identify Ba2+-sensitive KIR channel currents. Lack of functional evidence for KIR channels in rat mesenteric arteries also has been presented (177). These data conflict with a report of robust expression of KIR 2.1 transcripts from primary cultures of SMCs isolated from rat mesenteric arteries, with highest expression observed in third-order branches off of the superior mesenteric artery. Substantial Ba2+-sensitive currents recorded from these cells and Ba2+-sensitive, K+-induced vasodilation was observed in endothelium-denuded rat mesenteric arteries (749). There is no current explanation for these conflicting results, but it may be related to differences in animal strains, species or methods.
Vascular SMCs have been reported to express only KIR 2.1 (168, 1130), or KIR 2.1 and 2.2 (670, 689, 1313, 1373, 1551). However, in the mouse, knockout of KIR 2.1 eliminates KIR channel currents in cerebral SMCs from neonates (1621). These data suggest that, at the least, KIR 2.1 is essential for KIR channel currents in this model. KIR 2.1 also has been proposed to compose the KIR channels that are observed in rat afferent arteriole SMCs (245, 840). In the myocardium where KIR 2.1, 2.2 and 2.3 are expressed, it has been proposed that channels composed of predominately KIR 2.1/2.2 heteromers underlie the native KIR channel currents (1652). Here too, knockout of KIR 2.1 abolishes native KIR currents indicating a critical role for KIR 2.1 in the native channels (1622).
Pharmacology of KIR channels
The primary pharmacological tool for the study of KIR channels in cells, tissues, and in vivo has been Ba2+ ions (Table 2). Early studies identified Ba2+ ions as potent and effective inhibitors of KIR channels (533). Extracellular Ba2+ produces voltage-dependent block of strongly rectifying KIR channels: at physiological membrane potentials (−30 to −40 mV), the Kd for Ba2+ block of whole-cell KIR channels currents in rat cerebral artery SMCs is on the order of 8 to 10 µmol/L (1186), with concentrations up to 300 µmol/L required for complete block. These data are consistent with the block of KIR channels in other systems. Note that Ba2+ also blocks KATP channels with an IC50 = 100 µmol/L (154), so appropriate controls must be implemented when using higher concentrations of this ion. Cesium ions also block the channel in a voltage-dependent manner (594). More recently, a small molecule inhibitor of KIR 2 channels, ML133, has been reported (1496). However, there are as yet no complete studies of the action of this drug on vascular SMC KIR channels, and it should be noted that ML133 also blocks KATP channels composed of KIR 6.2 subunits (1496).
KIR channels and myogenic tone
Currents through KIR channels contribute to resting membrane potential and tone of isolated SMCs, and SMC’s in isolated resistance arteries and arterioles from several vascular beds. Extracellular Ba2+ was reported to depolarize unpressurized male guinea pig submucosal arterioles (372) and male rat cerebral arterioles (373) suggesting that KIR channels contribute to the resting membrane potential of SMCs in these vessels. Consistent with this hypothesis, Ba2+ has also been shown to constrict male rat posterior cerebral arteries (973), constrict cerebral and brainstem penetrating arterioles (619), depolarize and constrict hamster cremaster arterioles (185), constrict male hamster retractor feed arteries (689), constrict male (711) and female (1313, 1551) rat middle cerebral and coronary septal arteries (1313), depolarize (245, 246), and constrict (245, 246, 1429) male rat renal afferent arterioles and interlobular arteries (247) in pressure myography studies, in vitro. Micromolar concentrations of Ba2+ also have been reported to depolarize guinea pig spiral modiolar artery SMCs (703).
In contrast, in female rat posterior cerebral arteries and coronary septal arteries, Ba2+ was reported to have little effect on resting membrane potential or diameter (766). No effect of Ba2+ on resting tone of isolated porcine coronary arterioles also has been reported (1204). Similarly, Ba2+ had no effect on resting tone of rat parenchymal arterioles in brain slices (426). These data suggest that experimental conditions may modulate the function of KIR channels.
Consistent with the in vitro studies of isolated SMCs and cannulated, pressurized vessels presented above, there also are in vivo data supporting a role for KIR channels in the control of resting vascular tone and blood flow. It should be noted, however, that the in vivo experiments are difficult to interpret because the site of action of Ba2+ (SMCs, endothelial cells, parenchymal cells, nerves, etc.) cannot be established, and because of often complex compensatory mechanisms. Nonetheless, micromolar concentrations of Ba2+ constrict cremaster arterioles in both anesthetized rats (898) and hamsters (63, 1596). Rat (970, 1359) and cat (371) pial arterioles also constrict in response to Ba2+. With the exception of one study (292), infusion of Ba2+ into human forearm [plasma concentrations of Ba2+ of ~50 µmol/L (318)] causes vasoconstriction (294, 295, 318). Given that the arteriolar endothelium poses a significant barrier to the passage of charged molecules (848), the precise concentration of Ba2+ to which the vascular SMCs were exposed in these in vivo experiments is unknown. In contrast, topically applied Ba2+ reportedly has no effect on resting cerebral blood flow in anesthetized mice (479). Thus, the bulk of evidence indicates that KIR channels are active in resistance arteries and arterioles at rest and contribute to resting membrane potential, tone and tissue blood flow.
K+-induced vasodilation: Functional evidence for vascular KIR channels
Extracellular K+ has long been proposed as an important signaling molecule that may be involved in the mechanisms responsible for matching tissue blood flow with the level of activity in electrically excitable tissues (531). In addition, K+ released from adjacent endothelial cells may accumulate in the intercellular space next to SMCs and also provide a signal coupling endothelial cell K+ channel activation to SMC function (186, 531). Thus, a large number of studies have examined the effects of elevated extracellular K+ on vascular tone and blood flow either as tests of the hypothesis that K+ is a mediator of functional vasodilation or, as will be seen, as a sensitive assay for the presence and function of vascular KIR channels (Fig. 7).
Figure 7.
Regulation of KIR channels by vasoconstrictors and vasodilators. Schematic of the plasma membrane of a vascular SMC showing, from left to right, a Gq-protein-coupled receptor (GqPCR), associated G-proteins and PLCβ; a KIR channel; and a Gs-protein-coupled receptor, associated G-proteins and AC. Black lines and arrows indicate stimulation, activation or increases; red lines indicate inhibition. Hyperpolarization induced by the activation of other K+ channels, or the closure of channels conducting Na+, Ca2+, or Cl− and/or increases in extracellular K+ concentration are the major stimuli for activation of vascular SMC KIR channels. In addition, vasodilators that act at GsPCRs (isoproterenol, adenosine, prostacyclin, CGRP, etc.), stimulate AC, increase the production of cAMP and activate PKA lead to activation of KIR channels. Similarly, NO, acting through sGC to increase production of cGMP, activated protein kinase G which can activate KIR channels. Conversely, membrane depolarization due to closure of other K+ channels or opening of channels that conduct Na+, Ca2+, or Cl− will close KIR channels. Vasoconstrictors that act through GqPCRs (norepinephrine, endothelin, angiotensin II, 5-HT, etc.) to activate PLCβ, the production of DAG and PKC activation lead to closure of KIR channels. See text for more information.
Early studies showed that isotonic elevation of extracellular K+ causes vasodilation and a decrease in vascular resistance in canine forelimb (441, 1119), coronary (1273), renal (441), gastrointestinal (1401), and cerebral (805) circulations. However, the site of action of the elevated K+ concentration (SMCs, endothelial cells, and parenchymal cells) and its precise mechanism of action could not be established. Subsequently, Edwards et al. (373) showed that small cerebral arterioles hyperpolarized when exposed to small elevations in extracellular K+ concentration and that this effect could be blocked by micromolar concentrations of Ba2+, suggesting that KIR channels were part of the mechanism of action. However, as these experiments were performed on intact arterioles, the site of action of the K+ and Ba2+ could not be established. Later, in isolated cerebral (711, 766, 972, 973) and coronary (766) resistance arteries it was shown that these vessels dilate when exposed to elevated extracellular K+ from a resting level of 3 to 5 mmol/L up to approximately 20 mmol/L, that these dilations were preceded by SMC hyperpolarization (766) and that these K+-induced effects could be blocked by micromolar concentrations of Ba2+. Furthermore, removal of the endothelium did not eliminate the responses to elevated K+, targeting the effects to the SMCs. Barium also blocks K+-induced dilation of rat afferent arterioles (245, 246), rat interlobular arterioles (247), and parenchymal arterioles studied by pressure myography and in brain slices (426, 902) supporting a role for KIR channels in a variety of vascular SMCs.
It should be noted that not all K+-induced vasodilation can be explained by SMC KIR channels. For example, in rat cerebral arteries, dilation of isolated vessels induced by exposure to 5 mmol/L K+ after exposure to 0 mmol/L K+ is mediated by the Na+/K+ ATPase and can be inhibited by ouabain (973). In these vessels elevation of K+ from 5 mmol/L to higher levels up to 20 mmol/L results in dilation that is fully blocked by Ba2+. These data suggest that there are two independent mechanisms of action dependent on the K+ range and the initial conditions. In human forearm, vasodilation induced by infusion of KCl is attenuated by infusion of Ba2+ (~50 µmol/L in plasma) (318), but is abolished by ouabain + Ba2+ (292, 318). In isolated hamster cremaster arterioles (185), K+-induced SMC hyperpolarization and dilation are only attenuated by Ba2+ at a concentration that was shown to fully block vascular KIR channels, whereas the Na+/K+ ATPase inhibitor, ouabain, alone, abolished K+-induced vasodilation. Burns et al. (185) proposed that the KIR channels in this system acted mostly to amplify hyperpolarization produced by K+-induced activation of the Na+/K+ ATPase in these hamster arterioles. It should be noted, that in mouse cremaster arterioles, Ba2+ alone abolishes K+-induced dilation, suggesting that only KIR channels mediate K+-induced dilation in these arterioles (Jackson, unpublished observations). In rat renal arcuate arteries, K+-induced dilation appears to be mediated solely by the Na+/K+ ATPase, with no role for KIR channels (1175). These data suggest that there may be regional and/or species differences in the function of KIR channels in resistance arteries and arterioles.
Vasoconstrictors and KIR channels
KIR channels are open under resting conditions in SMCs of resistance arteries and arterioles (see above). Thus, closure of these channels by signaling pathways activated by vasoconstrictors could contribute to the membrane depolarization that is observed when these vessels constrict. Studies in the literature have shown that signaling pathways that are commonly activated by vasoconstrictors such as those involving PKC (591, 1653) or tyrosine kinases (1540, 1652) can inhibit KIR 2-containing KIR channels (Fig. 7).
Currents through KIR channels in rabbit coronary SMCs are inhibited by α1-adrenergic receptor activation, although the signaling pathway that was involved was not investigated (1567). Both endothelin-1 (1130) and angiotensin II (1133) inhibit currents through KIR channels in rabbit coronary SMCs through PKC α-dependent mechanisms that were mimicked by phorbol 12,13-dibutyrate or 1-oleoyl-2-acetylsn-glycerol, direct activators of PKC (1130). Endothelin also has been shown to inhibit KIR channel currents in endothelial cells (1629). In addition, superfusion with phorbol 12,13-dibutyrate inhibits K+-induced dilation of rat pial arterioles, in vivo (1476). These data suggest that vasoconstrictors that act on Gq/11-coupled receptors to activate the PLCβ-DAG-PKC signaling cascade have the potential to inhibit vascular SMC KIR channels, which would contribute to vasoconstrictor-induced SMC depolarization (Fig. 7). However, it has also been shown that phorbol ester-induced activation of PKC inhibits K+-induced dilation of rat cerebral arteries without affecting K+-induced hyperpolarization, data that argue against PKC-dependent inhibition of KIR channel function (249). These data also indicate that inhibition of K+-induced dilation does not always mean a reduction of KIR channel function.
Vasoconstrictor-induced inhibition of KIR channel function may be vessel and agonist specific. For example, UTP, the thromboxane analog, U46619 and 5-HT had no effect on KIR currents in SMCs from female rat cerebral arteries (1551). All 3 of these agonists act on receptors coupled to the Gq/11 signaling cascade, as do endothelin-1 and angiotensin II, noted above (1133). In these same cerebral SMCs it was shown that direct activation of PKC using phorbol esters, or hypoosmotic challenge rapidly inhibited KIR channel currents in a PKC-dependent manner (1551). These data suggest that there may be regional or species-dependent differences in the signaling pathways coupled to KIR channels. The studies by Wu et al. (1551) also suggest that, in the cerebral circulation, inhibition of KIR channels may contribute to pressure- or stretch-induced depolarization of these cells. However, these findings are difficult to reconcile with previous studies showing lack of effect of PKC activation on K+-induced SMC hyperpolarization (249).
The specific KIR channel isoform involved in vasoconstrictor-induced inhibition of vascular KIR channels has not been established. Studies in heterologous expression systems have shown that activation of PKC via phorbol 12,13-dibutyrate or phorbol mysterate acetate inhibits currents through KIR 2.3 channels [and by inference, KIR 2.2 channels (344, 1653), but not KIR 2.1 channels (344, 591)]. These data suggest that PKC-dependent effects may involve KIR 2.2 alone or heteromeric channels containing KIR 2.2.
However, activation of α1A-adrenergic receptors, which also is coupled to Gq/11, inhibits currents through homomeric KIR 2.2 channels and heteromeric K2.1/2.2 channels, but not KIR 2.1 channels in a heterologous expression system by a mechanism involving Src tyrosine kinases, independent from PKC (1652), which also has inhibitory effects on KIR 2.2-containing channels (1653). A Src tyrosine kinase was also shown to mediate α1A-adrenergic receptor mediated inhibition of native KIR channel currents in rat ventricular myocytes which are likely heteromers of KIR 2.1 and 2.2 (1652). In contrast, α1A-adrenergic receptor-mediated modulation of KIR 2.3 channels is mediated by PKC (1652). Also, KIR 2.3 is inhibited by Gβγ-subunits when expressed as homomers or heteromers with KIR 2.1 (267). Thus, the specific KIR channel isoforms and the signaling pathways involved in vasoconstrictor-induced inhibition of KIR channel currents in vascular SMCs remain in question.
Vasodilators and KIR channels
Increased activity of KIR channels will produce membrane hyperpolarization and vasodilation, and, as will be outlined below, studies support a role for KIR channels in the mechanism of action of several vasodilators. However, it should also be noted that currents through KIR channels can be recruited simply by membrane hyperpolarization due to activation of other K+ channels, for example, with KIR channels acting to amplify the initial hyperpolarization (668, 689, 903, 1313, 1326) (Fig. 7). SMC KIR channels also have the potential to be activated by K+ ions released during activation of endothelial cell K+ channels (i.e., endothelial-derived hyperpolarization at myoendothelial junctions) (186, 531, 903).
Barium inhibits bradykinin-induced dilation of coronary arterioles implicating KIR channels in the mechanism of action of this dilator (1204). These channels also participate in propagation of hyperpolarizing signals along arterioles (689, 1204, 1313). Inward rectifier K+ channels also have been proposed to contribute to bradykinin-induced dilation in human forearm (356). C-type natriuretic peptide, a putative endothelium-derived hyperpolarizing factor (EDHF) may act, in part, by activation of KIR channels (222).
cAMP signaling and KIR channels
In other systems, KIR channels can be modulated by protein kinases (1540) or G-proteins (726) suggesting that their vascular counterparts may also be regulated in a similar fashion. Adenosine activates currents through Ba2+-sensitive KIR channels in rabbit small coronary artery SMCs and increases coronary blood flow via a mechanism involving A3-adenosine receptors, adenylate cyclase, cAMP and PKA (1323). Adenosine-induced dilation of pial arterioles is inhibited by Ba2+, inhibitors of adenylate cyclase and PKA (1125) also supporting a role for cAMP-PKA signaling pathway in modulating the function of vascular KIR channels. Hypoxia activates KIR channels in rabbit small coronary artery SMCs through a mechanism involving Gs, adenylate cyclase, cAMP and PKA (1129). Thus, there is support for cAMP-PKA mediated activation of vascular KIR channels (Fig. 7).
NO-cGMP-PKG signaling and KIR channels
Additional studies in porcine pial arterioles demonstrated a second, potentially species-specific signaling pathway in which adenosine acts on endothelial cells to stimulate NO production that then relaxes pial SMCs via a Ba2+-sensitive mechanism (582). These data suggest that NO may activate SMC KIR channels in this preparation, although the precise signaling pathway was not established (582). SNP activates KIR channels composed of KIR 2.1 channels in rat tail artery SMCs and contributes to vasodilation induced by this NO donor in that tissue (1269) (Fig. 7). In addition, Ba2+ inhibits relaxation of rat tail artery to the PGI2 receptor agonist, cisaprost, through a mechanism that does not appear to be mediated by PKA (1112).
Heterologous expression systems have provided further insight into the regulation of KIR channels. Currents through KIR 2.2 channels are increased by activated PKA through phosphorylation of S430 (1653). Additional studies have shown that activation of β3-adrenoreceptors increases currents through KIR 2.1 and KIR 2.2 channels (1259). For KIR 2.1, this appears to involve PKC, for KIR 2.2, cAMP and PKA-mediated activation (1259). Heteromeric channels containing KIR 2.1 and 2.2 were regulated similar to homomeric KIR 2.2 channels (1259).
Functional vasodilation and KIR channels
There is considerable evidence that KIR channels contribute to functional vasodilation in skeletal muscle and in the brain. In hamster cremaster muscles, Ba2+ attenuates vasodilation induced by muscle contraction, particularly the rapid onset of dilation, suggesting a role for KIR channels in coupling muscle contraction to vascular function in this tissue (63). Studies in human forearm also have shown that Ba2+ inhibits the rapid-onset vasodilation induced by muscle contraction (293), as well as the steady-state functional vasodilation in that tissue (294). Barium also attenuates reactive hyperemia induced by blood flow occlusion to the forearm, supporting a role for KIR channels in this response (295).
Barium ions reduce hypoxia-induced coronary vasodilation in Langendorff-perfused rabbit hearts that involves adenylate cyclase, cAMP and PKA suggesting a role for the KIR channels in hypoxia-induced vasodilation in this preparation (1129, 1136).
Arteriolar KIR channels also play a role in neurovascular coupling in the brain. Barium attenuates electrical field-stimulation-induced dilation of parenchymal arterioles in brain slices (426). Barium also has been shown to reduce functional vasodilation induced by whisker stimulation in the mouse cerebral cortex (479), and vasodilation of pial arterioles induced by peripheral nerve stimulation (1125, 1476).
KIR channels and pathophysiology
Hypertension
Early studies demonstrated that K+-induced vasodilation in dog hindlimb (1116), rat hindlimb (1117) and human forearm (1118) was impaired with hypertension. These data are consistent with a reduced function of KIR channels in hypertension.
However, several subsequent studies in isolated vascular preparations found that K+-induced relaxation of strips of rat tail artery (1504), pig tail artery (1503) or rat basilar artery (592) was increased in vessels from hypertensive animals. However, in all of these in vitro studies, vessels were exposed to solutions with 0 mmol/L K+ to induce K+ reactivity. It was later shown that in isolated cerebral vessels, the SMC response to elevated K+ after exposure to 0 mmol/L K+ was largely mediated by the Na+/K+ ATPase, whereas K+-induced dilation initiated from physiological K+ levels (3–5 mmol/L K+) were Ba2+-sensitive and likely mediated by KIR channels. Further, the KIR channel-mediated responses were reduced in vessels from hypertensive rats (972).
Dilation of basilar arteries to increased K+ was elevated in SHR (250). However, these K+-induced dilations in the SHR were relatively insensitive to block by Ba2+, in contrast to what is observed in basilar arteries in normotensive WKY rats (250). Instead, the augmented response in the SHR resulted from nNOS-derived NO released from perivascular nerves (250). These data suggest that there is a loss of KIR channel function in hypertension with compensatory upregulation of the nNOS pathway to maintain reactivity to elevated K+.
Expression of KIR 2.1 was reduced in mesenteric SMCs from hypertensive BPH mice relative to normotensive controls (BPN) (1373). The decreased expression correlated with a decrease in KIR channel current density in SMCs, SMC depolarization, and increased myogenic reactivity of resistance arteries from BPH that may contribute to the hypertensive phenotype in this model of essential hypertension (1373). However, despite the reduction in KIR currents, Ba2+-induced constriction of mesenteric arteries was not reduced in vessels from the hypertensive BPH mice (1373). These data suggest that despite reduced expression and currents, that the function of KIR channels was maintained, likely by remodeling of other ion channels in the SMCs of the BPH mice to maintain homeostasis (1373).
In rats, angiotensin II-induced hypertension reduces SMC KIR channel current density and K+-induced dilation of skeletal muscle and cerebral arteries (1281). In the skeletal muscle artery SMCs, 2 weeks of exercise training reversed the effects of hypertension on KIR channel currents and K+-induced dilation (1281). While KIR channel currents were restored by exercise in cerebrovascular myocytes, K+-induced dilation remained depressed (1281) suggesting regional heterogeneity in the response to exercise training. Previous studies showed that exercise training augments expression and function of vascular SMC KIR channels (709).
In contrast to the findings outlined earlier, Ba2+-sensitive, K+-induced dilation of parenchymal arterioles in rat cerebral cortex was augmented in brain slices from SHR compared to arterioles in brain slices from normotensive WKY (1047). This may indicate that there are regional differences in the impact of hypertension on KIR channel expression and function in the vasculature.
Obesity and the metabolic syndrome
The effects of obesity and the metabolic syndrome on vascular SMC KIR channels have not been well studied. Diet-induced obesity is associated with reduced KIR channel function in rat mesenteric arteries (528), although this likely represents effects on endothelial cell KIR channels and not channels in SMCs. K+-induced vasodilation in human forearm is reduced in obese subjects (1479), suggesting reduced KIR channel function. Consistent with this hypothesis, Ba2+-induced vasoconstriction was also depressed in the forearms of obese subjects (1479) and in subjects infused with elevated levels of non-esterified fatty acids (320). These data suggest that KIR channel function may be decreased in the obese, which could significantly impact peripheral vascular function. Additional research will be required to precisely define the effects of obesity on KIR channel function in the SMCs of resistance arteries and arterioles around the body.
Diabetes
The effects of type 1 diabetes on KIR channel function is not clear, because there is evidence for both increased and decreased function in animal models of this disease. Ba2+-induced constriction of afferent arterioles is increased in streptozotocin-induced diabetic rats (1429, 1430), with no change in expression of mRNA or protein for KIR 2.1 (1430). The increased Ba2+-induced constriction could be normalized by treatment with the superoxide dismutase mimetic, Tempol, suggesting a role for ROS in mediating the increased KIR channel function (1430). No change in mRNA expression of KIR 2.1 in aortic SMCs from streptozotocin-induced diabetic rats has also been reported (1197). These data suggest that KIR channel function is upregulated in diabetes, with no change in expression.
In contrast to the studies of renal arterioles cited above, studies of pial arterioles suggest that KIR channel function is impaired in diabetes. Barium-induced constriction and K+-induced dilation were impaired in pial arterioles of streptozotocin-induced diabetic rats (970), suggesting reduced KIR channel function. Similarly, KIR channel function in pial vessels was impaired in diabetes through a mechanism involving PKC (1476). Diabetes was associated with an increase in the rectification of KIR currents in pericytes located at the arteriolar end of capillaries in the retinas of streptozotocin-treated rats (962). This results in a decrease in the outward “hump” in the current positive to the K+ equilibrium potential, and a slight depolarization of these cells (962). The increased rectification appears to be caused by increased spermine production in this model of diabetes. Thus, there may be regional differences in the effects of diabetes on KIR channel function.
Hypercholesterolemia
As noted above, KIR 2.1 and 2.2 are strongly regulated by membrane cholesterol, and hypercholesterolemia results in silencing of endothelial cell KIR channels (400). However, comparable studies have yet to be performed on SMC KIR channels. In a rat model, KIR 2.1 mRNA expression was unchanged by hypercholesterolemia, but KIR channel function was not examined (1198). In humans, infusion with elevated levels of non-esterified fatty acids reduces the magnitude of Ba2+-induced vasoconstriction, suggesting reduced KIR channel function (320). In mouse models of hypercholesterolemia, conducted vasodilation, which may involve SMC KIR channels (689, 1313), is not impaired (109, 1542). Although not directly tested, these data suggest no impairment of KIR channel function in this model of hypercholesterolemia. Reactive hyperemia of second-order arterioles is impaired in hypercholesterolemic mice (1466), although a specific role for KIR channels was not assessed. Thus, the effects of hypercholesterolemia on SMC KIR channel expression and function remain unclear.
Other disease states
Subarachnoid hemorrhage converts K+-induced dilation into K+-induced constriction due to elevated release of K+ from glial BKCa channels, with no change in the function of parenchymal arteriolar KIR channels (775). Stress impairs KIR channel function in rat cerebral parenchymal arterioles, which, in turn, reduces neurovascular coupling potentially contributing to the reduced cognitive function associated with stress (902). There is increased angiotensin II-induced inhibition of KIR channels in small coronary arteries after isoproterenol-induced cardiac hypertrophy (1134). Potassium ion-induced vasodilation is impaired after ischemia/reperfusion injury in middle cerebral arteries (944), although the mechanism was not explored. Similarly, brain ischemia reduces KIR currents in cerebrovascular SMCs in the rat (94, 95). Pretreatment with LPS eliminates the decrease in functional KIR channels and K+-induced vasodilation after ischemia (95).
KATP Channels
Discovery of KATP channels
Noma discovered ATP-sensitive K+ channels in cardiac myocytes (1097), and currents through similar channels were subsequently identified in many other cell types including vascular SMCs [see (9, 15, 68–72, 76, 166, 180, 219, 272, 273, 311, 322, 374, 375, 436, 506, 544, 661, 786, 826, 827, 984, 1070, 1111, 1182, 1183, 1187, 1188, 1332, 1464, 1595, 1604) for reviews]. These K+ channels derive their name from their property that increases in intracellular ATP decrease their activity. However, and probably of more physiological relevance, their activity is modulated by a number of signaling pathways independent from their ATP sensitivity.
Structure and Expression of KATP channels
KATP channels are hetero-octamers composed of four inward rectifier K+ channel subunits in the KIR 6.X family, coupled with four regulatory subunits, the so-called sulfonylurea receptors (SURs) (15, 76, 1187). The hallmark ATP sensitivity of these channels resides in the KIR 6.X channel subunits as indicated by site directed mutagenesis of KIR 6.2 (1436, 1437), which is the pore-forming subunit in cardiac myocytes (1363). Vascular SMCs express KIR 6.1 subunits (75, 855, 1001, 1013, 1363), as suggested by Yamada et al. (1570). However, KATP channels that are heteromultimers of KIR 6.1 and 6.2 have been described in guinea pig urethral SMCs (1398), suggesting that there could be regional variability in the structure of SMC KATP channels.
The regulatory SUR subunits confer sensitivity to blockade by sulphonylureas, such as tolbutamide and glibenclamide, and also are necessary for activation of the channels by KATP channel openers such as diaxozide, cromakalim and pinacidil, as well as nucleotide diphosphates (15, 76, 1187). There are two different classes of SUR subunits, with expression varying by cell type. The type of SUR expressed determines the sensitivity to blockade of the channels by sulphonylureas, and possibly the sensitivity to activation by KATP channel openers (15, 76, 1187). Pancreatic β-cells express a 140 kDa receptor that has been termed SUR1, which has a high affinity for glibenclamide (Kd ~ 10 nM) (15, 76, 1187). Cardiac myocytes and vascular SMCs express sulphonylurea receptors that are in the SUR2 class. These receptors show 68% homology to SUR1, and have an affinity for glibenclamide that is more than an order of magnitude lower (100–600 nmol/L) than SUR1 (15, 76, 1187). There are two spliced variants of SUR2 (SUR2A and SUR2B) (644, 660, 1570); vascular muscle cells express the SUR2B isoform of these receptors (10, 1013, 1187).
Pharmacology of KATP channels
Sulfonylureas, such as glibenclamide (Table 2), block KATP channels by binding to their SUR subunits, and are the primary tools used to study KATP channels in vitro and in vivo. At concentrations of 1 µmol/L or less, glibenclamide is very selective. However, because this drug has to diffuse through the plasma membrane to bind to the intracellular SUR, it has a slow rate of onset of effect [>15 min in organ bath type experiments to achieve maximal blockade (990)]. Therefore, particularly in patch clamp experiments, many investigators have resorted to using higher concentrations, such that the time to maximal block is reduced. However, in whole tissue experiments, and in vivo, the use of concentrations higher than 1 µmol/L is fraught with difficulties because glibenclamide can have significant off-target effects including nonspecific vasodilation (705) and block of Cl− channels (1627). Caution is also urged with the use of the KATP channel agonist, pinacidil, because at concentrations higher than 10 µmol/L, this drug relaxes vascular SMCs by mechanisms other than activation of K+ channels (991).
KATP channels and myogenic tone
KATP channels contribute to resting vascular tone and tissue blood flow in the coronary (128, 305, 354, 407–409, 641, 674, 995, 996, 1029, 1193, 1202, 1203, 1246, 1286, 1336, 1644), skin (2, 196, 613), splanchnic, and renal circulations (351, 614). In the heart it has been shown that the beating myocardium releases a KATP channel agonist that activates these channels and dilates coronary arterioles (1376).
In the resistance vasculature of skeletal muscle there is evidence both for (664, 784, 1231, 1462, 1463) and against (85, 86, 137, 351, 405, 406, 547, 614, 1041) a contribution of KATP channels to resting vascular tone and blood flow. The reason for these different findings has not been established, but could relate to methodological differences. For example, it has been shown that the rate of inhibition of KATP channels by glibenclamide is quite slow, requiring up to 15 min for maximal block to occur (990). In addition, glibenclamide has been shown to nonspecifically relax SMCs with an EC50 = 40 µmol/L (705) (the IC50 for block of KATP channels is on the order of 100 nmol/L for vascular KATP channels). Thus, constriction due to block of KATP channels by glibenclamide may have been offset by nonspecific vasodilation dependent on the concentration of glibenclamide used and the duration of exposure to the KATP channel blocker. In addition, particularly in human studies with infused glibenclamide, there is substantial binding of this drug to plasma proteins such that the free concentration of the drug available to block SMC KATP channels will be low. Also, lower concentrations of the blockers are usually used in human studies (137).
KATP channels do not appear to be active in the cerebral circulation under resting conditions in a variety of model systems [see (402) for numerous references and (618, 838, 877, 1096, 1422, 1510)].
Vasoconstrictors and KATP channels
Vasoconstrictors decrease KATP channel activity through several mechanisms. Considerable evidence indicates that PKC inhibits KATP channels (155, 249, 268, 570, 1190, 1249) both by altered channel gating (268) and by promoting internalization of the channels (708) (Fig. 8). Vasoconstrictor agonist-induced elevation of intracellular Ca2+, acting through PP2B (calcineurin), also has been shown to inhibit KATP channel function (1537) (Fig. 8). Finally, vasoconstrictors that signal through Gi/o can inhibit constitutively active adenylate cyclase to reduce KATP channel activity (570). Vasoconstrictors that have been shown to inhibit KATP channels include angiotensin II (570, 1016), endothelin (1018), histamine (155, 762), norepinephrine (666), neuropeptide Y (155), phenylephrine (155), 5-HT (155, 762), and vasopressin (1291, 1487) (Fig. 8). Closure of KATP channels also has been implicated in vasoconstriction of cremaster arterioles induced by the α2-adrenergic agonist, UK-14,304 (1393), although the mechanism responsible for this effect was not established. Inhibition of KATP channels by angiotensin II has been shown to involve PKCε (570, 1249) and its recruitment to caveolae (1249). This isoform of PKC also is responsible for signaling internalization of KATP channels from caveolae (708). Studies of KIR 6.2-containing-KATP channels have shown that the channels undergo constitutive, rapid endocytosis and recycling, and that PKC reduces recycling and targets the channels for lysosomal destruction (940). It is assumed that a similar process is involved in PKC-dependent internalization of KATP channels in the vascular KIR 6.1-containing channels.
Figure 8.
Regulation of KATP channels by vasoconstrictors and vasodilators. Schematic of the plasma membrane of a vascular SMC showing, from left to right, a Gq-protein-coupled receptor (GqPCR), associated G-proteins and PLCβ; a KATP channel; and a Gs-protein-coupled receptor, associated G-proteins and AC. Black lines and arrows indicate stimulation, activation or increases; red lines indicate inhibition. These channels can be activated by a fall in intracellular ATP in the environment of these channels. In addition, vasodilators that act at GSPCRs (isoproterenol, adenosine, prostacyclin, CGRP, etc.), stimulate AC, increase the production of cAMP and activate PKA lead to activation of KATP channels. Similarly, NO, acting through sGC to increase production of cGMP, activating PKG which can activate KATP channels. These channels also can be activated by H2S, as shown. Conversely, increases in ATP close KATP channels. Vasoconstrictors that act through GqPCRs (norepinephrine, endothelin, angiotensin II, serotonin, etc.) to activate PLCβ, the production of DAG and PKC activation will lead to closure of KIR channels. Increases in intracellular Ca2+ that accompany SMC stimulation by vasoconstrictors activates protein phosphatase 2B (calcineurin), which also closes KATP channels by dephosphorylation. See text for more information.
Vasodilators and KATP channels
cAMP-PKA signaling and KATP channels
KATP channels have been implicated in the mechanism of action of vasodilators that are thought to act through the cAMP-signaling cascade such as adenosine (24, 308, 664, 761, 1193), PGI2 (161, 664), PGE1 (378), PGE2 (161), CGRP (759, 1072, 1184, 1520), vasoactive intestinal peptide (1257, 1592), and agonists of β-adrenergic receptors (664, 1005, 1048, 1068) (Fig. 8). This involves receptor-mediated activation of adenylate cyclase type 6 [AC6 (1068)], formation of cAMP and activation of PKA, which is targeted to the channels by AKAPs (569), with the channels localized in caveolae (1250). Exogenous caveolin-1 inhibits KATP channel activity suggesting that localization in caveolae is also important in regulation of the channel’s basal activity (313). Phosphorylation of both the KIR 6.1 (1191) and SUR2B (1290, 1296) subunits by PKA underlies the effects of cAMP-related vasodilators on KATP channel activity in vascular SMCs.
NO-cGMP-PKG signaling and KATP channels
There is evidence both for and against NO and cGMP signaling in modulating the activity of KATP channels in vascular SMCs (Fig. 8). Patch clamp studies of vascular SMCs from porcine coronary arteries found that NO, via activation of guanylate cyclase, increases the activity of KATP channels (1017). Similarly, atrial natriuretic peptide, an NO-donor and 8-Br-cGMP activated KATP channels in cultured rat aortic vascular SMCs (793). Nitric oxide was also shown to hyperpolarize SMCs in rabbit mesenteric arteries via a mechanism that involves cGMP and activation of KATP channels (1040). Hyperpolarization induced by NO was also shown to involve KATP channels in mesenteric SMCs (1547). KATP channels have also been implicated in vasodilation induced by NO in several other systems (57, 581).
However, studies in rabbit coronary circulation (674), rabbit mesenteric arteries (743, 1184), porcine retinal arterioles (478) and mouse mesenteric arteries (1043) have failed to demonstrate a role for KATP channels in the mechanism of action of exogenous NO, NO-donors or endothelium-derived NO. The reasons for this heterogeneity are not clear, but may indicate that subtle differences in methodology affect coupling of the NO-cGMP-PKG signaling pathway to KATP channels, and that there are regional or species differences in mechanisms that modulate these channels.
The mechanism by which NO activates KATP channels in vascular SMCs has not been firmly established. In cardiac myocytes, NO-induced activation of KIR 6.2-based KATP channels involves cGMP, PKG, ERK1/2, and CAMK II (1625). It has also been speculated that NO-induced cGMP formation may cross-activate PKA to modulate KATP channel activity (1187).
Other dilators and KATP channels
Hydrogen sulfide also appears to act, at least in part, by activation of KATP channels. This gasotransmitter was shown to activate KATP channel currents in rat aortic SMCs, and H2S-induced relaxation of rat aortas could be inhibited by very high concentrations of glibenclamide (1636). Glibenclamide also was shown to inhibit H2S-induced vasodilation of perfused rat mesenteries (238) and canine myocardium (202), consistent with a role for KATP channels in its mechanism of action. H2S also has been shown to activate KATP channels in cerebral arteriolar SMCs to cause vasodilation (838, 869). H2S appears to sulfhydrate KATP channels leading to their activation and subsequent vasodilation (1043).
Acidosis-induced dilation of coronary arterioles is mediated by KATP channels (659). Hypercapnic acidosis-induced increases in blood flow are inhibited by glibenclamide in mouse hearts (583) and in the cerebral circulation (401, 877).
Early studies showed that hypoxic dilation of the coronary circulation could be reduced by glibenclamide (310, 1049, 1050, 1483). Similar results have been obtained in the cerebral (1370, 1416), systemic (945), and renal (906) circulations. Although hypoxia has been demonstrated to activate KATP channels in SMCs from rabbit coronary arteries (309), other studies have suggested that hypoxia stimulates endothelial cells to release PGI2 (437) [or other substances (882)], or parenchymal cells to release adenosine (883, 945, 1050, 1483) or opioids (56) which then activate SMC KATP channels to hyperpolarize SMCs and produce vasodilation. However, in fetal pigs, hypoxia-induced pial arteriolar vasodilation does not involve KATP channels (839) and in rat coronary resistance arteries endothelium-derived NO appears to mediate hypoxic vasodilation (915). Hypoxia-induced cerebral vasodilation in the rat also involves EETs (883).
Volatile anesthetics such as halothane (819), isoflurane (203, 296, 447), and enflurane (296) cause coronary vasodilation that can be inhibited by glibenclamide. Similar results have been reported for volatile anesthetic-induced dilation of pial arterioles (637). In isolated porcine coronary arterioles, isoflurane-induced dilation is endothelium dependent and can be inhibited by glibenclamide (457). However, isoflurane has been shown to activate KATP channel currents in isolated vascular SMCs and that activation of KATP channels by isoflurane requires SUR2B (447) and likely results from PKA-mediated phosphorylation of both KIR 6.1 and SUR2B subunits (1381).
Functional hyperemia and KATP channels
There is evidence both for and against a role for KATP channels in the local regulation of blood flow to tissues and organs. Muscle contraction-induced dilation of arterioles in hamster cremaster muscles is inhibited by glibenclamide (547, 1041, 1231). Glibenclamide also inhibited functional hyperemia in rat (952) and mouse (1559) spinotrapezeus muscles. Consistent with these data, normal functional hyperemia in rat hindlimb muscles requires functional KATP channels (614). In contrast, studies in human forearm have failed to identify a role for KATP channels in exercise-induced increases in blood flow (405, 406, 1268). These data suggest that there may be species differences in the role played by KATP channels in the regulation of skeletal muscle blood flow. Methodological differences also cannot be excluded as the dose or concentration of glibenclamide used in human studies is often much lower than what is usually used in animal studies.
There is evidence both for and against a role for KATP channels contributing to functional hyperemia in the coronary circulation. Exercise-induced hyperemia in the canine coronary circulation was reduced by glibenclamide supporting a role for KATP channels in this system (353, 654). KATP channels also have been implicated in pacing-induced hyperemia in dog (736), human (408), and mouse (1644) coronary circulations. The increased coronary blood flow induced by β1-adrenergic agonists in canine hearts also is inhibited by glibenclamide (1057). KATP channels also appear to contribute to exercise-induced vasodilation in the porcine coronary circulation (995).
In contrast, a number of studies suggest that KATP channels do not contribute to functional hyperemia in the coronary circulation. Glibencamide reduced coronary blood flow at rest, but had no effect on the increase in coronary flow produced by phenylephrine-induced hypertension and rapid atrial pacing in dogs (74). Block of KATP channels had no effect on dobutamine-induced increases in coronary flow in anesthetized female rats (1412). Glibenclamide also had little effect on increases in coronary blood flow produced by infusion of dobutamine and rapid atrial pacing in the dog (379). Similarly, pacing-induced increases in coronary blood flow were not inhibited by glibenclamide in anesthetized dogs, although this KATP antagonist did reduce resting blood flow (1202). Block of KATP channels did not alter exercise-induced vasodilation in conscious dogs (1203, 1439). Thus, there is evidence both for and against a major role for KATP channels in coupling metabolism and blood flow in the coronary circulation, with no clear explanation for the disparate findings other than the usual suspects of species and methodological differences.
Reactive hyperemia and KATP channels
KATP channels may be involved in the vasodilation and increase in blood flow that is observed after occlusion of blood flow (reactive hyperemia) in skeletal muscle. Ingestion of glybenclamide (7.5 mg) reduced resting human calf muscle blood flow and reduced both the peak and duration of the blood flow increase induced by 10 min occlusion of blood flow (784). In human forearm, block of KATP channels with tolbutamide (0.4 µmol/min) had no effect on resting blood flow or the peak increase in flow observed after 5 min occlusion (85). However, the total blood flow response (area under the curve) was reduced by KATP channel blockade (85) supporting a role for these channels in forearm reactive hyperemia. Similarly, infusion of glibenclamide to yield a plasma concentration of 60 ng/ml reduced peak flow after 2 min and flow repayment after both 2 and 5 min occlusion in human forearm (137). In contrast, infusion of glibenclamide at 0.03 µmol/min had no effect on forearm reactive hyperemia in another study (406). However, in this latter study, the infused glibenclamide barely inhibited dilation induced by the KATP channel agonist, diazoxide. Thus, a lack of efficacy may explain the lack of inhibition that was observed.
In the heart, KATP channels also are involved in reactive hyperemia. Daut et al. (310) first showed that glibenclamide inhibited reactive hyperemia in Langendorff-perfused guinea pig hearts, in vitro. Glibenclamide also inhibited reactive hyperemia induced by 15 to 120 s occlusion in canine coronary circulation, in vivo (73, 128, 262, 352, 654, 728, 780, 1569). Similarly, glibenclamide inhibits reactive hyperemia induced by 30 s occlusion in isolated rat hearts (1302). Reactive hyperemia also was reduced by 30% after 5 min occlusion in pig hearts, in vivo (1605). In murine hearts, in vitro, glibenclamide inhibits reactive hyperemia induced by 15 s ischemia (1286), confirming earlier studies (1623).
Autoregulation of blood flow and KATP channels
Blood flow to organs like the brain and heart displays autoregulation: blood flow tends to remain relatively constant in the face of changes in blood pressure or organ perfusion pressure. KATP channels have been implicated in autoregulation in both the heart and brain. Dilation of small coronary arterioles in response to reductions in perfusion pressure in anesthetized dogs (termed microvascular autoregulation) was abolished by glibenclamide (780, 781). Similarly, coronary autoregulation of blood flow was abolished by glibenclamide in anesthetized dogs (1058). Dilation of rat pial arterioles induced by a reduction in perfusion pressure was also impaired by sulfonylureas such as glibenclamide and glipizide (616, 834). Similar results were reported for blood flow to the brainstem as well as autoregulatory diameter increases to reduced blood pressure in small branches off of the rat basilar artery (1422).
In contrast, it has been reported that glibenclamide does not inhibit coronary autoregulation in anesthetized dogs (1336). No clear explanation for the lack of effect in this study versus those mentioned earlier, in the same tissue are apparent.
Pathophysiology and KATP channels
Hypertension
A number of studies suggest that KATP channel function is reduced in models of hypertension. SMC relaxation induced by KATP channel agonists has been shown to be reduced in: aortas in two-kidney-one-clip hypertensive rats (1455); basilar arteries from SHRSP (758); mesenteric arteries from SHR (724); aortas from DOCA-salt hypertensive rats (474); mesenteric arteries from L-NAME-induced hypertensive rats (722); and mesenteric resistance arteries from hypertensive BPH mice (1373). Similarly, KATP channel-opener-induced vasodilation is reduced in the brains of SHRSP (1374) and in the kidney of SHR (739). In mesenteric arteries of hypertensive BPH mice, expression of KIR 6.1 and SUR2B are reduced, as are currents through KATP channels in this model of hypertension (1373). Reduced currents through KATP channels have also been reported in mesenteric arteries from SHR (1103). Reversal of hypertension by administration of antihypertensives has been reported to restore KATP channel function (1103, 1374). These data suggest that hypertension is the cause of the downregulated KATP channel function observed in those models.
However, there are several studies that indicate that KATP channel function is either not changed (141, 635, 779), or actually enhanced (452, 1014) in models of hypertension. The study by Blanco-Rivero et al. (141) is particularly interesting because these investigators found a significant reduction in the expression of mRNA and protein for KIR 6.1 and SUR2B in vessels from SHR, and yet patch clamp studies revealed no reduction in KATP channel currents and no effect on pinacidil-induced relaxation (141). Thus, our understanding of the effects of hypertension on KATP channel expression and function is incomplete.
Obesity and the metabolic syndrome
KATP channel expression and function appear to be downregulated in animal models of obesity and the metabolic syndrome. Insulin-induced vasodilation of mesenteric arteries, which appears to be mediated by KATP channels, was abolished in vessels from fructose-fed rats, a model of the metabolic syndrome (1003). Dilation of middle cerebral arteries mediated by KATP channels also is impaired in this model of insulin resistance (384, 385). Similarly, KATP channel-mediated arteriolar vasodilation is impaired in obese Zucker rats (608, 910). In this model, exercise capacity is reduced, an effect that can be reproduced by inhibition of KATP channels in lean control animals. Reactivity to cromakalim in human internal mammary arteries from patients with type 2 diabetes is also depressed suggesting a similar down regulation of KATP channel function in humans (652). Consistent with down regulation of KATP channels in obesity/metabolic syndrome, chronic treatment with sulfonylureas does not significantly affect reactive hyperemia in forearms of patients with type 2 diabetes (1330), a response that is mediated, in part, by activation of KATP channels (85, 137).
The mechanisms responsible for the down regulation of KATP channel function in obesity and the metabolic syndrome are not clear. The decreased insulin-reactivity, mediated by KATP channels, in vessels from fructose-fed rats can be restored by block of endothelin-A receptors (1003), suggesting increased endothelial cell production of endothelin may contribute to the reduced KATP channel function in this model. In diet-induced obesity in rats, relaxation of aortas and mesenteric arteries to a KATP channel agonist, KATP channel currents in SMCs and mRNA and protein expression from these two vessels were reduced (397, 399). Expression of SUR2B also is down regulated (398). However, the mechanisms responsible for the reduced KATP channel function and expression was not addressed. Pinacidil-induced dilation is reduced in mesenteric arteries of obese SHR rats relative to non-obese SHR and WKY rats, and reactivity can be restored with rosiglitazone treatment (643). The reduced function of KATP channels in fructose-fed rats appears to be mediated by ROS (385). Also, obesity-induced decreases in KATP channel function in Obese Zucker rats are associated with increased vascular levels of ROS (910). Inhibition of NADPH oxidase with apocynin reduces ROS and improves KATP channel function in this model (910). Thus the down-regulation of KATP channel expression and function may be induced by an increase in ROS production in obesity and the metabolic syndrome.
In contrast to studies showing decreased function and expression of KATP channels in models of obesity, adipose tissue in Ob−/Ob− mice releases substances that activate vascular SMC KATP channels, reducing basal vascular tone and vasodilator capacity (i.e., the vessels are already dilated, therefore the magnitude of any additional dilation is blunted) (1559). Also, it has been shown that hypercholesterolemia does not impair KATP channel-mediated vasodilation in the mesenteric bed of rabbits (1221). These data suggest that there are experimental model-dependent differences in the impact of obesity on vascular KATP channel function.
Aging
There is limited information concerning the impact of aging on the expression and function of vascular KATP channels. Diazoxide-induced increases in muscle blood flow are reduced in senescent Fischer 344 rats (653). Similarly, levcromakalim-induced dilation of small branches off of the basilar artery was impaired in aged Sprague-Dawley rats (1423).
Diabetes
Type 1 diabetes is also associated with a decrease in vascular KATP channel function. Streptozotocin-induced diabetes in rats impairs KATP channel agonist-induced relaxation or dilation in: aortas (725), pial arterioles (969), basilar arteries (968), and the coronary circulation (162). In addition, there are decreased basal and pinacidil-induced KIR channel currents in aortic SMCs from streptozotocin-induced diabetic mice (851). Pinacidil-induced dilation was also impaired in mesenteric arteries and in the coronary circulation of the diabetic mice (851). Relaxation of human mesenteric arteries to cromakalim is impaired when exposed to elevated D-glucose (20 mM) (752). Also, hypoxia-induced dilation of human coronary arterioles is reduced due to decreased function of KATP channels in vessels from diabetic patients (1013). Exposure of vascular SMCs to elevated extracellular glucose impairs isoflurane-induced activation of KATP channels (738). The reduced vascular KATP channel function in streptozotocin-induced diabetic mice appears to result from increased S-glutathionylation of KIR 6.1 in this model of type 1 diabetes (851). Previous studies have shown that S-glutathionylation of KIR 6.1 at cysteine 176 induced by oxidative stress inhibits KATP channel function (1590, 1591). Treatment with the antioxidant MnTBAP restores KATP channel function in coronary arteries from human diabetic patients (1013), consistent with a role of ROS. High glucose-induced inhibition of KATP channel function in human mesenteric artery SMCs can be reversed by peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists, which have antioxidant effects (752). In this same system, high glucose has been demonstrated to inhibit KATP channel function through increased NADPH oxidase activity and production of superoxide anions by a mechanism involving phosphatidylinositol 3-kinase (753). It has also been shown that long-term exposure to methyl glyoxal, an oxidant species produced during hyperglycemia, leads to down regulation of the expression (protein and mRNA) and function of KATP channels (1584) due to microRNA-mediated decreases in the expression of SUR2B (864). High glucose-induced activation of PKC, independent from ROS, has been proposed as another mechanism of downregulation of KATP channel function in vascular SMCs (738).
In contrast to the studies showing depressed KATP channel function in diabetes, it was shown that cromakalim-induced relaxation was enhanced in mesenteric resistance arteries from streptozotocin-induced diabetic SHR and WKY rats (724). Also, Lemakalim-induced relaxation of aortas and vasodilation of the mesenteric vascular bed were unaffected in streptozotocin-treated rats, despite depression of responses in the coronary circulation (162). Alloxan-induced diabetes in dogs or hyperglycemia increases aprikalim-induced dilation of coronary arterioles, although ischemia-induced dilation, which is mediated by KATP channels, is depressed (742). Also, in contrast to normal dogs, KATP channels contributed significantly to the local regulation of coronary blood flow during exercise (1440), suggesting increased, rather than decreased KATP channel function in this model of diabetes. An explanation for these results is not apparent, but could relate to the degree or duration of diabetes.
Other disease states
Perfusion of hearts with cardioplegic solutions results in a postcardioplegia-induced coronary hyperemia (1493). In pigs, this hyperemia can be inhibited by glibenclamide, suggesting a role for KATP channels (1493).
Global cerebral ischemia (91) and traumatic brain injury (61, 62, 735) impair vasodilation of the cerebral circulation by KATP channel agonists. In traumatic brain injury, the reduced KATP channel function has been proposed to result from a process involving elevated endothelin (735) production or release of vasopressin (62), activation of PKC and the production of superoxide anions (61, 62, 735) through a mechanism involving tyrosine kinase and ERK (1219).
In contrast to the down-regulation of KATP channel function that is observed in hypertension, obesity, the metabolic syndrome and diabetes, KATP channel function appears to be enhanced in models of heart failure (686, 1424, 1573) and subarachnoid hemorrhage (1318). However, the mechanisms responsible for the upregulated function have not been explored.
Sepsis represents another pathology in which vascular KATP channel function is upregulated. It was shown that KATP channels mediate, to a large extent, the hypotension that occurs in sepsis (813), that glibenclamide can reverse this hypotension, that cardiovascular function can be improved by block of KATP channels (1245) and that lipopolysaccharide stimulates vascular SMC expression of KIR 6.1 and SUR2B augmenting KATP channel function via Nfkappa B signaling (1292). KATP channels are also likely activated by the lactic acidosis that is a hallmark of septic shock (813). However, this knowledge has not translated into clinical practice. Oral glibenclamide (10–30 mg enteral), at a therapeutic dose used to treat hyperglycemia, did not restore blood pressure or reduce norepinephrine requirement in septic patients (1027, 1498). However, these glibenclamide doses are much lower than required to block vascular KATP channels in sepsis (182). Also, early treatment with TEA (to block BKCa channels), but not glibenclamide improves cardiovascular function and organ damage in sepsis (1327). This is likely because KATP channels in vascular SMCs are protective: induction of sepsis is lethal in animals with knockout of KIR 6.1 with a major effect mediated by KATP channels in the coronary circulation (729).
Ryanodine Receptors
Discovery of RyRs
RyRs are the largest known ion channel proteins (1457, 1577, 1617). They derive their name from the alkaloid, ryanodine, produced by the Columbian flowering shrub, Ryania speciosa (1212). Ryanodine was originally isolated and characterized for its insecticidal properties (1212), in which it produced paralysis, a transient increase in O2 consumption and ultimately death (568). Skeletal muscle paralysis induced by ryanodine was subsequently demonstrated in vertebrates, including mammals (376), with the contraction process in the muscle identified as the site of action (376), but no effect on the muscle membrane or the activity of the contractile proteins (144). Blum et al. (144) speculated that ryanodine acted by combining with a substance that couples excitation of the muscle membrane with activation of the contractile proteins; the first hint of the existence of RyRs.
Ryanodine was shown to increase both the uptake and release of Ca2+ from skeletal muscle (17), and in particular, to increase the efflux of Ca2+ from the sarcoplasmic reticulum (394, 395). In parallel, Ca2+ release from the terminal cisternae in skeletal muscle was established as the trigger for muscle contraction [see (381) for a review]. Subsequently, specific binding of ryanodine to terminal cisternae membrane vesicles of skeletal and cardiac muscle was reported (432, 1157), with the binding sites named Ca2+-RyRs (1157). Proteins from these membranes could be inserted into lipid bilayers to reconstitute a functional Ca2+ channel (1314). This knowledge then was used to isolate and purify RyRs from both skeletal and cardiac muscle (636, 649, 650, 811, 1232), to show that RyRs formed functional Ca2+ release channels when incorporated into planer lipid bi-layers (636, 811, 1045) and to demonstrate that these channels were ryanodine-sensitive (1045). The lipid bilayer studies also showed that low concentrations of ryanodine locked the channels into a subconductance state, allowing leak of Ca2+ from the sarcoplasmic reticulum, consistent with the vesicle studies (394, 395), while high concentrations of ryanodine blocked the channel (1045).
Structure of RyRs
RyRs are composed of a homo-tetramer of ~560 kDa subunits that form ryanodine-sensitive, Ca2+-selective channels in the membrane of the ER (1577, 1617). The ion conductive portion of the RyR is located in the C-terminal portion of the peptide and arises from association of 6 transmembrane spanning domains (S1–S6) from each of the four subunits, much like the structure of KV channels, with a loop between S5 and S6 (P-loop), and S6 contributing to the ion conducting pore of the channel (1577, 1617) (Fig. 2). The P-loop and S6 contain a large number of negatively charged residues contributing to the high Ca2+ conductance of RyRs [~120 pS (346, 1617)]. Large N-terminal and smaller C-terminal domains are located in the cell cytoplasm and constitute the bulk of the mass of the protein (~80%) and contain the Ca2+ sensor (responsible for Ca2+-induced-Ca2+ release), binding sites for a large number of interacting protein binding partners, and phosphorylation sites for regulation by protein kinases (1100, 1457). The N-terminal domain of each subunit consists of two β-trefoil domains (domains A and B) and a group of α-helices (domain C (1441) or N-Sol (1617)) that interact with each other through a number of hydrophilic interfaces (1441). These three domains form a large vestibule of the channel pore, and disease-related mutations in a number of residues in these domains have been identified (1441). The bulk (56%) of the remainder of the cytosolic domain consists of a scaffold of α-solenoid repeats with three large SPRY domains [domains found in SplA kinase and RyRs (1577)], two pairs of RyR repeats, the calstabin (FK506 binding protein, FKBP)-binding domain and an EF-hand pair, which is the presumed Ca2+-binding domain (1577, 1617). The most proximal portion of the α-solenoid repeats (termed the core solenoid by Zalk et al.) contains the EF-hand pair and interacts with the C-terminus of the channel (1617). The RyR domains contain presumed regulatory phosphorylation sites (1577). This large flexible domain structure serves both as a scaffold for recruitment of regulatory proteins to the channel and to allow coupling of conformational changes induced by ligand binding and protein phosphorylation and other post-translational modifications (1617). A cytosolic loop connecting segments 2 and 3 of the pore domain appears to interact with the Ca2+-sensing EF-hand domain providing a means to couple Ca2+ binding with gating of the RyR Ca2+ channel (377, 1617).
RyR isoforms
There are three isoforms of RyRs (RyR1, RyR2, and RyR3) that are the products of three genes (818) with high sequence homology (66% between RyR1 and RyR2, 67% between RyR1 and RyR3, and 70% between RyR2 and RyR3) (538, 1377, 1656). Sequence differences among the three isoforms are located in three regions, termed D1, D2, and D3 that are located in the large N-terminal cytoplasmic domain and which likely account for differences in the function of these isoforms (818, 1456).
RyR expression
It was initially shown that skeletal muscle cells express predominantly RyR1, the heart predominantly RyR2, and the brain and other cells in the body, RyR3 (477, 831, 1196). However, subsequent studies have demonstrated expression and function of all three isoforms around the body (477, 921, 1618).
Vascular SMCs display regional heterogeneity in the expression of RyR isoforms (1241, 1451, 1453, 1527, 1528, 1587). Expression of predominantly RyR2 was found in rat aorta and pulmonary arteries (1587). Similarly, in SMCs from posterior, middle or anterior cerebral arteries, RyR2 message and protein were more highly expressed than RyR1 or RyR3 (1451). Studies of SMCs isolated from murine skeletal muscle resistance arteries and arterioles also demonstrated predominant expression of RyR2 (1527). However, in these SMCs, RyR3 was expressed at a much lower level, and RyR1 was not detected (1527). In contrast, Salomone et al. (1241), found expression of only RyR3, but not RyR1 or RyR2 in basilar artery SMCs. These data suggest that there are significant regional differences in RyR isoform expression. Consistent with this hypothesis, it was shown that rat pulmonary conduit and mesenteric arteries express RyR1 and RyR2 with little expression of RyR3 (1639). However, all three isoforms were expressed at similar levels in pulmonary resistance arteries. In a separate study of mesenteric resistance arteries, RyR1 and RyR3 expression were greater than RyR2 (857).
The expression of RyR isoforms also may change during proliferation as it has been shown that cultured rat aortic SMCs, express predominantly RyR3 followed by RyR2 and RyR1 (1453), whereas RyR2 predominated in freshly isolated tissue (1587). Consistent with the idea of isoform expression heterogeneity, studies of primary cultures of portal vein SMCs by Coussin et al. (278) showed similar expression of RyR1 and RyR2, with lower RyR3 expression. Thus, in addition to regional heterogeneity in isoform expression, proliferating SMCs likely express different isoforms of RyRs suggesting varied roles for the three RyR isoforms.
RyR alternative splicing
The complexity of the pattern of expression of RyR isoforms is also increased by alternative splicing that may contribute to their functional heterogeneity in the vasculature. RyR1 has two splice variants, ASI and ASII (Alternative Splice sites I and II) (455). Expression of these variants appears to increase RyR1 activity (750, 751). Alternative splicing of RyR3 has been detected in vascular SMCs (837, 955, 1015). One of these RyR variants (AS-8a, with a deletion in exon 8) is unique to SMCs and leads to expression of an RyR with decreased function (300, 706). Heterodimerization of the AS8a with normal RyR3 may decrease channel activity (706).
Regulation of RyR function
Calcium
Cytosolic Ca2+ displays a bell-shaped concentration-response relationship with low concentrations (0.1–10 µmol/L) activating the channel and higher concentrations inhibiting channel activity (232, 275, 543, 545, 700, 707, 825, 1152, 1428, 1457). RyR1 is inhibited by cytosolic Ca2+ concentrations of 1 mmol/L or higher (545, 707). RyR2 and RyR3 also can be inhibited by Ca2+, but at even higher concentrations (229, 1260). The binding site for Ca2+-dependent gating of RyRs is presumably mediated via the EF-hand domain located in the proximal α-solenoid domain of the N-terminus, as noted above (377, 1617). Ca2+-dependent inhibition of the channel likely involves several lower affinity Ca2+-binding sites on the protein (818).
The Ca2+ concentration in the lumen of the ER also impacts the function of RyRs (233, 425, 525, 825). Increased Ca2+ load in ER stores leads to increased opening of RyRs (233, 425, 525). Released Ca2+ may interact with cytosolic Ca2+ activation sites to increase RyR activity (1428). In addition, the helix bundle-crossing region, part of the proposed Ca2+ gate in RyR2, has been shown to be essential for luminal Ca2+ sensing (233). Finally, luminal Ca2+ may interact with Ca2+-binding proteins within the ER such as calsequestrin to modulate RyR activity (526).
Phosphorylation
Examination of the amino acid sequences of RyRs reveal a large number of potential phosphorylation sites (425, 545, 992, 1100, 1375, 1618). Consensus sequences for PKA, CamKII, and PKG are present (347, 992, 1618). However, PKA and CamKII appear to be the most important (347, 992, 1618) (Fig. 9). Both kinases have specific anchoring regions on RyR (98, 954). In addition, Protein Phosphatase 1 (PP1) associates both with RyR1 and RyR2, Protein Phosphatase 2A (PP2A) associates with RyR2 and PP2B (calcineurin) associates with RyR1 (992). The kinases and phosphatases are targeted to RyRs by anchoring proteins: PKA and phosphodiesterase 4D3 via mAKAP, PP1 via sinophilin, PP2A via PR130 (aPP2A targeting protein) (1618), and PP2B (calcineurin) via mAKAP (96).
Figure 9.
Regulation of RyRs. Schematic of the plasma membrane and the ER membrane of a vascular SMC showing, from left to right in the plasma membrane, a BKCa channel and a Gs-protein-coupled receptor, associated G-proteins and AC, and a RyR in the membrane of the ER. Moderate increases in cytoplasmic Ca2+ in the environment of a RyR, or increases in the concentration of Ca2+ in the lumen of the ER are the primary stimulae for activation of RyRs. Activation of RyR by cytosolic Ca2+ is mediated by direct actions of Ca2+ on the channels, through activation of calcium-calmodulin-dependent protein kinase (CaMK) and phosphorylation of the channels, or interactions of Ca2+ with the Ca2+-binding protein S100A which competes with calmodulin for binding to the RyR. Vasodilators that act at GsPCRs (isoproterenol, adenosine, prostacyclin, CGRP, etc.), activate AC to increase production of cAMP which then can activate PKA to phosphorylate RyRs and increase their activity increasing the frequency of production of Ca2+ sparks. Elevated cAMP can also increase RyR activity through activation of EPACs. The increase in RyR-depedent Ca2+ spark activity is transduced into membrane hyperpolarization and vasodilation through activation of overlying BKCa channels, as shown. Conversely, high levels of intracellular Ca2+ inhibit RyR activity through mechanisms involving the Ca2+-binding proteins sorcin (SORC) or calmodulin (CaM). The activity of RyRs also may be decreased by interactions with FK-506 binding proteins 12 and 12A FKBPs. See text for more information.
The functional effects of RyR phosphorylation remain a matter of debate (992, 1100). Most studies suggest that phosphorylation by CamKII and PKA leads to increased RyR activity (425, 1508, 1541, 1618) (Fig. 9). However, contrary results have been reported (115, 355, 921). Hyperphosphorylation (phosphorylation of all four RyR subunits) of RyR2 has been reported to cause dissociation of FKBP12.6, a RyR-associated protein, resulting in leaky channel function (1506, 1507). However, acute phosphorylation of RyR2 has been reported to increase activity (535), decrease channel opening (1452), or produce no change in RyR Ca2+ handling (856). How RyR phosphorylation affects vascular SMC function has not been established.
Calmodulin, Sorcin, and S100A1
The Ca2+ binding protein, calmodulin (248), associates with RyRs in the proximal portion of the N-terminal domain near the transmembrane spanning domains (1484). High-micromolar, concentrations of Ca2+ increase calmodulin binding to RyRs, and RyR inhibition (thus limiting CICR at high Ca2+), whereas nanomolar Ca2+ concentrations decrease binding and increase RyR activity (425, 545). RyR1 and RyR3 are more sensitive to activation by calmodulin in the presence of low Ca2+ concentrations, while Ca2+-calmodulin-dependent inhibition of RyR2 occurs at lower Ca2+ concentrations relative to RyR1 or RyR3 (442, 443) (Fig. 9).
RyRs also can be modulated by another Ca2+-binding protein, Sorcin, which inhibits RyRs at high-micromolar concentration of Ca2+ (347) (Fig. 9). Sorcin immunoprecipitates with RyRs in cardiac muscle (999). In rat aortic and cerebral artery SMCs, Sorcin expression is higher and RyR2 expression is lower than what is observed in cardiac myocytes (1223). They proposed an increased role for Sorcin-RyR interaction in vascular SMCs, confirmed by a lower frequency of RyR-mediated Ca2+ sparks (1223). Sorcin has also been proposed to prevent the propagation of Ca2+ sparks to adjacent groups of RyRs by detecting Ca2+ released from a spark site, binding to nearby RyRs, and inhibiting Ca2+ spark propagation (410, 411).
The protein S100A1, another EF-hand-containing protein, also interacts with RyRs (1177, 1427), with a functional outcome distinct from calmodulin or Sorcin. In the presence of Ca2+, S100A1 increases RyR activity, promoting Ca2+ release (1427) (Fig. 9). This process may involve competition of S100A1 with calmodulin (1177). Vascular SMCs express S100A1 (939), however, the function of this protein in the regulation of vascular SMC RyRs has not been explored.
FKBP12/FKBP12.6
FKBPs are a family of 20 immunophillins named because they serve as binding sites for the immunosuppressant, FK506 (922). Two of this family, FKBP12 and FKBP12.6 (numbered according to molecular mass and also referred to as calstabin 1 and 2, respectively), regulate the function of RyRs (14, 224, 922). FKBPs bind near the interface among the SPRY1, SPRY2 and linking α-solenoid region 2 domains of the N-terminus (1617). FKBPs bind to each RyR monomer with such high affinity that the two proteins co-purify (943). It has been proposed that binding of FKBPs to RyRs clamps SPRY2 and α-solenoid 1 domains together restricting their motion, and hence, decreasing channel activity (377) (Fig. 9). All three RyR isoforms can interact with both FKBP12 and FKBP12.6 (943). However, RyR2 preferentially binds FKBP12.6, and RyR1 and RyR3 preferentially bind FKBP12 (943). For RyR1, removal of FKBP12 causes increased channel activity (425, 922). However, less is known about the regulation of RyR2 and RyR3 by FKBPs (425, 922). The physiological significance of the interaction between FKBPs and RyRs has not fully emerged. Mutations in RyR1 related to malignant hyperthermia are near the FKBP-binding site (224), suggesting that the RyR1 dysfunction observed in this disease could be related to altered RyR-FKBP interactions. In heart failure, it has been proposed that displacement of FKBP12.6 from the RyR2 due to hyperphosphorylation of the channel increasing RyR activity resulting in leak of Ca2+ from the ER (115, 355). Overexpression of FKBP12 in cardiac myocytes decreases the amplitude and duration of Ca2+ sparks (702). Conversely, pharmacological dissociation of FKBPs from RyRs in bladder SMCs leads to increased Ca2+ spark amplitude and frequency (971). Increased expression of FKBP12.6 in cerebral arteries has been proposed to be the cause of decreased expression and activity of RyR2 (775).
However, there appears to be regional and perhaps species differences in the role played by FKBPs in the regulation of SMC function. In pulmonary artery SMCs, FKBP12.6 associates with RyR2, but not RyR1 or RyR3 (1637). FKBP12 associates with and modulates the activity of RyR2 in guinea pig colonic SMCs (924). However, although FKBP12 is expressed in aortic SMCs, it does not associate with RyR3 (the major isoform expressed in this tissue) and does not modulate RyR3 function (923, 924). Similarly, RyR function in porcine coronary artery SMCs (1597) and bovine renal, coronary or mesenteric arteries (383) appears not to be modulated by FKBPs. In contrast, FKBPs appear to attenuate RyR function in guinea pig bladder (1513) and portal vein (925) SMCs independent from calcineurin (see later). An inhibitory role for FKBPs have also been proposed in the regulation of RyR function in rat aortic SMCs (234). Thus, there appears to be regional and likely species dependent differences in role played by FKBPs in the regulation of RyR function.
It should be noted that FKBPs also serve other actions that have the potential to modulate the function of RyRs independent from effects mediated by direct binding of FKBPs to RyRs (922). The drug FK506 (Tacrolimus) binds to FKBPs resulting in dissociation of the FK506-FKBP complex from RyRs. The FK506-FKBP complex then inhibits the Ca2+-calmodulin-dependent phosphatase, calcineurin, which is responsible for the immunosuppressive effects of FK506 (922). Calcineurin may be tethered to RyRs by FKBPs targeting this phosphatase to RyRs, and providing a mechanism for modulation of RyR function via dephosphorylation (922). However, in SMCs, some studies suggest that calcineurin does not modulate RyR function (922, 924, 925). In contrast, inhibition of calcineurin or its genetic knockout inhibits RyR1 function in murine airway smooth SMCs (1256). Thus, there may be regional or species differences in the role played by calcineurin in the modulation of SMC RyR function.
The immunosuppressant drug, rapamycin, also results in dissociation of FKBPs from RyRs, but inhibits the function of the protein kinase, mammalian target of rapamycin (mTOR) (922). This pathway also does not appear to contribute to acute modulation of RyR function in SMCs (922). However, the mTOR pathway may participate in more long-term modulation of RyR expression and function (1031).
Cyclic adenosine diphosphate ribose and RyRs
Cyclic adenosine diphosphate ribose (cADPR) has been proposed to modulate RyR function in vascular SMCs (390). This second messenger is synthesized by SMC microsomes from coronary, renal and pulmonary arteries (390). However, there seems to be substantial regional heterogeneity: in the rabbit, pulmonary artery SMCs synthesize more than tenfold higher levels of cADPR compared to aortic or mesenteric resistance artery SMCs (1535). Similarly, in rat kidneys, preglomerular vessels synthesize much more cADPR than do postglomerular vessels (861). Calcium release induced from intracellular stores by cADPR has been demonstrated in rabbit small intestine longitudinal (but not circular) SMCs (796), bovine coronary SMCs (1610), rabbit (1535) and rat (148) pulmonary artery SMCs, and renal microvessels (861). Dialysis of rat tail artery SMCs with cADPR increases the frequency of RyR-dependent STOCs (243). This second messenger also has been shown to activate bovine coronary artery RyRs reconstituted into lipid bilayers (1386). The effects of cADPR on Ca2+ release does not appear to result from direct binding of this messenger to RyRs (390). Because cADPR activates RyRs in bovine coronary SMCs only when FKBP12.6 is associated with the RyR (1386), FKBP12.6 has been proposed as the receptor for cADPR, or at least to be required for its action on RyRs. This hypothesis is supported by studies in renal microvessels (1396) and murine bladder SMCs (1640). In contrast to the studies supporting a role for cADPR in modulating RyR function, studies in guinea pig colonic SMC found no effect of cADPR on RyR function even in the presence of FKBP12.6 (167). Instead, effects on removal of Ca2+ from the cytosol were reported (167). However, these studies have not been confirmed.
It has been proposed that cADPR-RyR signaling may be involved in the mechanism of action of vasodilators that act through both the cAMP and cGMP signaling cascades, via effects on Ca2+ sparks and RyR-dependent STOCs (390). However, block of cADPR signaling has also been shown to cause vasodilation, rather than the predicted vasoconstriction if this hypothesis were correct, and to not affect vasodilation induced by dilators that act through the cAMP-signaling cascade, data that also are not consistent with this hypothesis (469).
Scaffolding and anchoring proteins
There are several scaffolding and anchoring proteins that may contribute to the regulation of the RyR function (224). The scaffolding protein Homer binds to proline-rich sequences of proteins, and couples membrane receptors to intracellular Ca2+ stores (347). Homer interacts with RyR1, but not RyR2 or RyR3, which lack the consensus sequence for this interaction (347). In skeletal muscle, interaction of Homer with RyR1 increases channel activity (418). However, it is not known if a similar interaction occurs in SMCs.
Anchoring proteins, such as AKAPs tether PKA to RyRs (992). In cardiac muscle, AKAP immunoprecipitates with RyR2, suggesting a close association (953). In skeletal muscle, mAKAP targets PKA to RyR1, with phosphorylation increasing RyR1 activity (1225). The expression and function of anchoring proteins in the regulation of RyR function in vascular SMCs has not been explored.
Oxidation and nitrosylation
RyRs are sensitive to the redox status of cells because they have more than 100 cysteine residues in each RyR monomer (347, 992). Oxidation of exposed cysteines has been shown to increase RyR activity in RyR1 and RyR2 (345, 347, 357, 358, 527), likely by altering interactions with RyR-associated proteins (1632). For example, in diseases characterized by contractile dysfunction and muscle weakness such as heart failure, muscular dystrophy and age-related sarcopenia, RyR oxidation results in dissociation of FKBP12 and/or FKBP12.6 from the RyR, Ca2+ leak and muscle dysfunction (1195).
Nitric oxide also can interact directly with RyRs resulting in S-nitrosylation (992). Low levels of S-nitrosylation appear to activate RyR1, whereas high levels of S-nitrosylation appear to be inhibitory (992). In contrast, RyR2 requires S-nitrosoglutathionylation via peroxynitrite (992).
Intracellular ion channels and RyR function
Release of Ca2+ from the SR results in loss of positive charge from ER stores and development of a negative potential across the ER membrane, with the opposite occurring when Ca2+ is pumped back into the SR (1475). To counter this charge imbalance, there is a flux of cations (principally K+) through Trimeric Intracellular Cation-selective (TRIC) channels (1475). There are two isoforms of TRIC channels, TRIC-A and TRIC-B (1475). Knockout of TRIC-A compromises RyR1 function in skeletal muscle (1475) and in vascular SMC (1575).
Another ER-membrane ion channel, TRPP1 interacts with and inhibits the function of RyR2 (5, 52). The N-terminus of TRPP1 binds to RyR2, and the C-terminus binds only when the RyR2 are in an open state (52). However, the mechanism of inhibition of RyR2 function has not been established.
Pharmacology of RyRs
The pharmacology of RyRs is outlined in Table 3. As noted above, ryanodine acts as both an agonist, and a blocker of RyRs, dependent on the concentration (1657). At concentrations less than 1 µmol/L, ryanodine locks the RyR into a subconductance state, releasing Ca2+ from the ER that can lead to store depletion (1657). At concentrations above 10 µmol/L, ryanodine blocks RyRs (1657). Tetracaine also blocks RyRs, but does not lead to store depletion (1657). However, this local anesthetic also has a number of off-target effects including block of vascular SMC K+ channels that limits its usefulness in whole tissue studies (573). The RyR agonist, caffeine also has significant off-target effects such as phosphodiesterase inhibition that also make its use in whole tissue or in vivo studies problematic (1528).
RyR function in vascular SMCs
In skeletal and cardiac muscle, RyRs amplify signals from other ion channels and importantly contribute to the increase in intracellular Ca2+ that triggers muscle contraction. The function of RyRs in SMCs is more variable and less well understood (269). In some vessels, RyRs amplify Ca2+ signals generated by other ion channels contributing to increases in intracellular Ca2+ and promoting vasoconstriction. For example, Ca2+-induced-Ca2+ release (CICR) stimulated by Ca2+ influx through L-type VGCCs (269, 493, 590, 638, 639) or due to Ca2+ release through IP3Rs (142, 149) has been reported in some SMCs. Furthermore, in some blood vessels RyRs contribute to IP3R-dependent Ca2+ waves that are excitatory and promote vasoconstriction (678, 1035, 1036, 1527, 1528) (Fig. 4, see IP3R Section for more on this topic). However, unlike cardiac or skeletal muscle, RyR-dependent CICR may not be essential for vasoconstriction, because many SMCs contract at cytosolic Ca2+ concentrations lower than required to activate RyR-mediated CICR (512). The variability in RyR function likely results from differences in expression and localization of RyRs in SMCs in various blood vessels (1241, 1451, 1453, 1528, 1587).
Role of Ca2+ sparks in SMCs
RyRs contribute to at least two types of Ca2+ signaling events in vascular SMCs: Ca2+ waves, that will be discussed further in the IP3R section, and Ca2+ sparks, which have already been mentioned in the section on BKCa channels, above. Calcium sparks result from the rapid opening of a small group of RyRs in the ER, leading to transient, focal increases in subplasmalemmal Ca2+ concentrations, without a significant effect on global Ca2+ levels (388). In skeletal (760) and cardiac muscle (1328), and in some SMCs (81, 297, 298, 415, 416, 804, 1438), RyR-dependent Ca2+ sparks function in a positive-feedback manner that is critical for excitation-contraction coupling (197, 235). In some vascular SMCs, Ca2+ sparks also may activate Ca2+-activated Cl− (ClCa) channels to produce spontaneous transient inward currents (STICs) (235). These currents depolarize cells, activating L-type VGCC and promoting vasoconstriction (1650).
In contrast, in some vascular SMCs, RyR-mediated Ca2+ sparks importantly participate in a negative-feedback loop that contributes to the regulation of myogenic tone (235, 683, 1071) (Fig. 4). As covered in the BKCa channel Section, this negative-feedback mechanism involves Ca2+ spark-dependent activation of overlying BKCa channels to produce STOCs, membrane hyperpolarization, deactivation of L-type VGCCs and reduced myogenic tone (235, 683, 1071).
In some arterioles in the microcirculation, RyRs appear to be silent at rest and do not contribute to the regulation of myogenic tone. In first-order rat cremaster arterioles (1589), second-order hamster (1528) and mouse (1527) cremaster arterioles, rat cerebral penetrating arterioles at rest (301), and ureteral arterioles (160), RyRs do not seem to participate in the negative-feedback regulation of myogenic tone. In cerebral penetrating arterioles, low pH stimulates generation of Ca2+ sparks, STOCs and BKCa channel-mediated vasodilation (301).
RyR isoforms and Ca2+ sparks
Differences in the pattern of expression of RyR isoforms may explain variation in the occurrence and properties of Ca2+ sparks (388). For example, RyR1 and RyR2 are required for Ca2+ sparks (278, 866, 1008), while the function of RyR3 is unclear (388). In rat portal vein, antisense knockdown showed Ca2+ sparks depend on expression of RyR1 and RyR2, whereas knockdown of RyR3 has no impact (278, 1008). However, silencing of RyR3 altered global Ca2+ levels in this model (278, 1008). In contrast, vascular SMCs from RyR3(−/−) mice display increased Ca2+ spark frequency, suggesting an inhibitory role for RyR3 (900). Consistent with this hypothesis, Ca2+ sparks were absent from murine cremaster arteriole SMCs which showed an elevated ratio of expression of RyR3/RyR2 relative to SMCs from upstream arteries that displayed Ca2+ sparks (1527).
Vasoconstrictors, RyRs and Ca2+ sparks
PKC activation contributes to myogenic tone in some blood vessels (597, 1113), and activation of PKC signaling can decrease the occurrence of Ca2+ sparks in some SMCs (153, 681, 896). In rat cerebral SMCs, UTP-induced decreases in Ca2+ spark frequency occur independent from the ER Ca2+ load suggesting a direct effect on RyR function (681). However, it has been shown that norepinephrine, which should activate PKC, stimulates Ca2+ sparks in guinea pig mesenteric SMCs (1179). Thus, there may be regional or species differences in the role played by PKC in modulating Ca2+ spark activity.
Vasodilators, RyRs and Ca2+ sparks
A number of vasodilators have been suggested to act, in part by activation of SMC RyRs leading to an increase in the frequency of Ca2+ sparks, increased activity of BKCa channels, membrane hyperpolarization, deactivation of VGCCs, reduced intracellular Ca2+ and vasodilation. Early studies showed that the adenylate cyclase activator, forskolin, increased Ca2+ spark frequency in SMCs isolated from rat basilar or coronary arteries and that vasodilation induced by forskolin could be substantially inhibited by ryanodine (1169) (Fig. 9). Similarly, adenosine in rat cerebral and coronary artery SMCs (1169), arginine vasopressin in rat retinal arteriolar SMCs (691) and pituitary adenylate cyclase activating polypeptide in rat cerebral artery SMCs (776) act via the cAMP-PKA signaling cascade to stimulate Ca2+ spark frequency (Fig. 9). The increase in Ca2+ spark frequency in these studies could be due to cAMP-PKA targeting either RyRs (992, 1100) or phospholamban (1306, 1521). Phosphorylation of phospholamban by PKA disinhibits the ER Ca2+ ATPase, SERCA, increasing Ca2+ in the lumen of the ER. Studies using phospholamban knockout mice revealed that the increase in Ca2+ spark frequency induced by forskolin appeared to be mainly mediated by cAMP-PKA-dependent phosphorylation of phospholamban (1521). This would increase the load of Ca2+ in the SR, which is known to increase the activity of RyRs and the frequency of Ca2+ sparks (241, 1651). It has also been proposed that the effects of cAMP-related vasodilators on Ca2+ sparks may be mediated through EPACs in SMCs from rat mesenteric arteries (1206) (Fig. 9).
Like the cAMP pathway, NO-dependent signaling via cGMP-dependent PKG also may increase RyR activity by increasing ER Ca2+ content and by sensitizing RyRs to Ca2+-dependent activation (1169). NO also can directly S-nitrosylate RyR1 to increase RyR activity (1357), and other mechanisms of action have been proposed (992).
However, there are data that conflict with these early findings. Pucovský et al. (1179) found that in SMCs from guinea pig mesenteric arteries, high concentrations of the NO donors SNP (100 µmol/L) or SNAP (50 µmol/L) had no significant effect on Ca2+ spark frequency unless these agents were applied in the presence of norepinephrine, in which case a decrease in Ca2+ spark frequency was observed. The inhibition of Ca2+ spark activity appeared to be due to a direct effect of NO, because inhibition of guanylate cyclase did not block the effect (1179). Nitric oxide also has been shown to inhibit caffeine-induced Ca2+ transients in bovine coronary artery SMCs (868). It was suggested that NO inhibits ADP-ribosyl cyclase and cADPR formation to inhibit RyR function in these cells (1610). This effect also appeared to be a direct effect of NO. On the other hand, a lack of effect of NO on caffeine-induced Ca2+ transients also has been reported in rat aortic SMCs (701) suggesting no effect of NO on RyR function. Thus the effects of the NO and cGMP pathways on RyR function in vascular SMCs is far from clear.
Hydrogen sulfide has been shown to increase Ca2+ spark frequency in SMCs from piglet cerebral arteries by increasing the Ca2+ load in the SR, a mechanism that contributes to the vasodilation induced by H2S in this vessel (871). In rat mesenteric SMCs, H2S increases Ca2+ spark frequency, but only in arteries with an intact endothelium suggesting an indirect effect in this system (675, 676).
RyRs and pathophysiology
Hypertension
Early studies found that caffeine-induced contraction of mesenteric resistance arteries (1033) and aortas (727) is increased in vessels from SHRSP versus WKY rats and that this is likely due to an increased Ca2+ load in the SR (727). RyR function also appears to be enhanced in SMCs from SHR versus WKY rats (276). However, no difference in the amplitude or frequency of Ca2+ sparks in cerebral artery SMCs from SHR versus WKY or Sprague-Dawley rats was reported (43). In angiotensin II-induced hypertension, Ca2+ spark frequency was unchanged, but Ca2+ spark amplitude was increased in SMCs from rat cerebral arteries (39). These data suggest that different causes of hypertension may have different effects on RyR function. There also may be regional differences, as increased expression of RyR2, increased Ca2+ spark frequency and increased function of RyRs were found that contribute to purinergic P2X receptor-mediated Ca2+ signaling in rat renal preglomerular arterial SMCs in SHR versus WKY (495).
Knockout of TRIC-A channels results in reduced Ca2+ spark frequency with no change in amplitude, leading to membrane depolarization due to less spark-dependent activation of BKCa channels (1575). This may contribute to the increased myogenic tone and hypertension in this mouse model and in humans with TRIC-A mutations (1575). Overexpression of TRIC-A results in hypotension and increased Ca2+ spark frequency (1390).
Maternal consumption of caffeine in rats results in decreased expression of RyR1 and RyR3 in mesenteric arteries of offspring, decreased frequency of STOCs (as a surrogate marker of Ca2+ sparks), membrane depolarization and increased vascular reactivity that correlates with increased pressor responses in the offspring (857). These changes may contribute to development of hypertension in these offspring as they age (857).
Aging
Expression of TRPP1 in the ER has a stimulatory effect on RyR function (5). Aging is associated with a decrease in the function of RyRs and reduced modulation of RyRs by TRPP1, which is associated with no change in the apparent expression of TRPP1 (5).
Diabetes
The effects of diabetes on RyR expression and function are not clear (421). For example, no change in RyR function was reported in mesenteric arteries from streptozotocin-induced diabetic rats (1505). In tail arteries from this same model, an increase in RyR function was found (1379). Aortic SMCs from two models of diabetes in rats, and high glucose exposure of A7R5 cells resulted in an increase in RyR protein expression and redistribution of RyRs from SR to nuclear membranes (1274).
However, decreased expression of RyR protein in aortic SMCs from streptozotocin-induced diabetic rats was reported (918). A reduced release of Ca2+ induced by ryanodine also was observed in these cells in contrast to what was reported by (1505). In cerebrovascular SMCs from male diabetic db/db mice, RyR expression also is reduced and is accompanied by a modest reduction in the amplitude, duration and mean rise time of the sparks with no change in frequency (1222). While Ca2+ spark frequency was unchanged, STOC frequency and amplitude were reduced due to reduced coupling of sparks to STOCs (1222). In cerebrovascular SMCs from female db/db mice, while the frequency of Ca2+ sparks was reduced, there was no difference in other spark properties and no difference in properties of STOCs indicating that there are likely gender differences in the effects of diabetes on RyR function in this model of type 2 diabetes (1222). Thus, there is not a clear view of how diabetes influences the expression and function of RyRs and how this contributes to the vascular pathologies associated with this disease. Little is also known of the mechanisms responsible for the changes in RyRs that have been observed. In cardiac myocytes from rats on a high-fructose diet, ROS-activated CamKII leads to increased phosphorylation of RyR2 and an increase in RyR2 activity that may contribute to arrhythmias in the model (1322). It is not known if a similar mechanism is operational in vascular SMCs.
Subarachnoid hemorrhage
Subarachnoid hemorrhage (SAH) results in substantial increases in vascular tone that can progress into vasospasm in large arteries, resistance arteries and arterioles (775). In a rabbit model of SAH, there was a reduction in the frequency of Ca2+ sparks in SMCs isolated from posterior cerebral and cerebellar arteries in this model that resulted in a decrease in the frequency of BKCa channel STOCs (775). Associated with this change in RyR function, there was a reduction in the expression (message and protein) of RyR2 and an increase in the expression of FKBP12.6 (775). It was proposed that the substantially increased ratio of FKBP12.6 to RyR2 was responsible for the reduced frequency of Ca2+ sparks, and that via reduced activation of BKCa channels, the reduction in RyR channel function contributes to the increased vascular tone observed after SAH (775). However, the mechanism responsible for the altered expression of RyR2 and FKBP12.6 were not established, and the contribution of the altered RyR2 function to the changes in cerebral resistance artery tone after SAH is not known.
Inositol-1,4,5-triphosphate Receptors
Discovery of IP3Rs
Release of Ca2+ from nonmitochondrial stores by IP3 was first described in pancreatic acinar cells (1344). Subsequent studies identified a similar process in a variety of cells (124, 1395) including vascular SMCs (1350), and it was proposed that IP3 bound to a receptor in the membrane of the ER to stimulate Ca2+ release (124). Subsequently, a 260 kD IP3R was isolated and purified from rat cerebellum (1361), which, when reconstituted into lipid bi-layer vesicles, allowed IP3-dependent Ca2+ flux (423). The full length IP3R1 was then cloned from mouse cerebellum (454) and subsequent studies showed that there are three IP3R family members (IP3R1, IP3R2, and IP3R3), as outlined later (434).
Structure of IP3Rs
IP3Rs are homotetramers of large subunits (~310 kD) that form Ca2+ release channels in the membrane of the ER (434). Like RyRs (1280, 1282), IP3Rs consist of a long, cytoplasmic N-terminus, 6 transmembrane domains (S1–S6) that form the ion conductive portion of the channel, followed by a short cytoplasmic C-terminal domain (145). The ion conductive pore is likely formed by transmembrane S5 and S6, and the P-loop between these segments similar to RyRs (145) (Fig. 2). Each IP3R monomer contains one binding site for IP3 that is located toward the N-terminus of each monomer and is referred to as the IP3-binding core (IBC) (434, 1280, 1282, 1395). Each IBC consists of α- and β-domains; these interact with the 5- and 4-phosphate groups of IP3, respectively, and lead to a conformational change that gates the channels open (1280, 1282, 1395). At the N-terminus is a suppressor domain (SD) that interacts with, and stabilizes the IBC in the absence of ligand, and is essential for channel gating (1280, 1282, 1395). Peptide loops within the IBC β-domain and the SD domain of each monomer interact with adjacent monomers, and stabilize the IP3R in the closed state in the absence of ligands (1280, 1282, 1395). This configuration of the SD, α- and β-domains of IP3R1s is similar to the A, B, and C domains of RyR1 (1280, 1282). Between the IBC and the transmembrane spanning domains is a long regulatory domain that contains sites for interaction with a number of proteins including TRPC coupling domains, putative binding sites for Ca2+ and ATP and consensus sequences for phosphorylation by several protein kinases (434).
IP3R isoforms and expression
Mammalian IP3Rs exist in three isoforms (IP3R1-IP3R3) that are the products of three genes (434). The isoforms display approximately 60% to 80% sequence homology, with the pore and ligand-binding regions being highly conserved (933). The majority of tissues, including vascular SMCs, express multiple IP3R isoforms, whereas invertebrates and some neural tissue express only IP3R1 (434, 715, 933).
There is regional heterogeneity in the expression of IP3R isoforms in SMCs [see (1055) for review]. Early studies identified IP3R1 as the major isoform expressed in the vas deferens and in aortic SMCs (1095). Tasker et al. (1391), demonstrated that the other isoforms are expressed in SMCs during development, but their expression wanes as the expression of IP3R1 increases. Proliferating SMCs express predominantly IP3R2 and IP3R3, suggesting specialized functions of IP3R isoforms (1392). Rat basilar, mesenteric and thoracic arteries express predominantly IP3R1, followed by IP3R3 and IP3R2 with aortas displaying lower levels of expression of all isoforms than the other vessels (498). While feed arteries and arterioles in mouse skeletal muscle express predominantly IP3R1, in these vessels IP3R2 was then next most highly expressed with negligible IP3R3 (1527). Thus, IP3R1 appears to be the most highly expressed isoform in vascular SMCs, with significant heterogeneity in the expression of IP3R2 and IP3R3. The functional significance of these differences is not known.
Alternative splicing of IP3Rs
The diversity of expression of IP3Rs is increased by alternative splicing. Three main regions of the IP3R1 gene, all located in the long N-terminal domain, termed SI, SII, and SIII have been detected (306, 434); most studies have focused on the SII variants. The functional consequences of these splice variants in SMCs is not clear. In regions of the brain where only IP3R1 is expressed, expression of the SII variant, which occurs only in IP3R1, alters allosteric modulation of the IP3R by phosphorylation (1343). The impact of alternative splicing on IP3R function in SMCs where multiple IP3Rs exist is not known (434).
Regulation of IP3Rs by ligands
Inositol 1,4,5 trisphosphate
Inositol 1,4,5 trisphosphate is formed from hydrolysis of membrane phosphatidylinositol 1,4 bisphosphate (PIP2) by membrane associated phospholipases whose activities are controlled by Gαq/11-coupled heptihelical receptors (phospholipase Cβ; PLCβ) (Fig. 10) or tyrosine kinase receptors (PLCΥ) (122). Although IP3Rs derive their name from IP3, both IP3 and cytosolic Ca2+ are required for normal activation to occur (434). IP3 alters the sensitivity of the IP3R for Ca2+-induced inhibition: at low concentrations of IP3, IP3Rs are more sensitive to inhibition by Ca2+, while higher concentrations of IP3 decrease the sensitivity for inhibition by Ca2+ (651, 935). As noted earlier, IP3 binds to the IBC to modulate channel gating in a Ca2+-dependent fashion (330, 1280, 1282, 1395).
Figure 10.
Regulation of IP3 receptors by vasoconstrictors and vasodilators. Schematic of the plasma membrane and the ER membrane of a vascular SMC showing, from left to right in the plasma membrane, a Gs-protein-coupled receptor, associated G-proteins and AC, a BKCa channel, a TRPC3 channel and a Gq-protein-coupled receptor (GqPCR), associated G-proteins and phospholipase C-β (PLCβ), and an IP3 receptor (IP3R) in the membrane of the ER. Increases in IP3 and moderate increases in cytoplasmic Ca2+ in the environment of an IP3R are the primary stimulae for activation. Activation of IP3Rs by cytosolic Ca2+ is mediated by direct actions of Ca2+ on the channels, or through activation of calcium-calmodulin-dependent protein kinase (CaMK) and phosphorylation of the channels. Vasoconstrictors acting through GqPCRs and activation of PLCβ increase the production of IP3, stimulating Ca2+ release through IP3R. Activated IP3Rs have been shown to physically interact with, and activate plasma membrane BKCa and TRPC3 channels, as shown. Conversely, high levels of intracellular Ca2+ inhibit IP3R activity through mechanisms involving the Ca2+ binding protein, CaM. NO, through activation of soluble guanylate cyclase, increased production of cGMP and activation of PKG phosphorylates IRAG which inhibits IP3R activity. Vasodilators that act at GsPCRs (isoproterenol, adenosine, prostacyclin, CGRP, etc.), activate AC to increase producting of cAMP which then can activate PKA to phosphorylate IP3Rs to decrease their activity. See text for more information.
Calcium
Calcium ions also are important regulators of IP3R function (Fig. 10). In the absence of IP3, Ca2+ is not capable of gating the channel open (434). In the presence of IP3, Ca2+ displays a bell-shaped concentration-response relationship (1434) (Fig. 10). At least eight putative Ca2+ binding sites have been identified, with sites located both in the SD/IBC domains, the regulatory domain, a luminal loop between transmembrane spanning domains, and within the C-terminal domain (1284). However, because the complete structure of IP3Rs has not been resolved, the exact number and precise location of Ca2+ binding sites involved in gating the channels are not clear (435). It also has been shown that at nanomolar concentrations of Ca2+, IP3 binding is increased via stabilization of the IBC (1394). The inhibitory Ca2+ binding site may be located either on the receptor itself, or on an accessory protein (330). Low-affinity Ca2+-binding sites may account for Ca2+-dependent inhibition, but the location of such sites remains unclear (330).
Calmodulin
Calmodulin has been reported to bind to and modulate the function of IP3Rs, although the significance of this interaction to the function of IP3Rs, in vivo remains to be established (435). Putative calmodulin binding sites are located both in the SD and the regulatory domains of IP3Rs (1284). As with RyRs, it has been proposed that Ca2+ binding to calmodulin causes a conformational change that alters its interaction with IP3Rs and decreases channel activity (1220) (Fig. 10). However, calmodulin does not bind to IP3R3, which still displays Ca2+-dependent inhibition (435). This observation suggests that calmodulin is not required for Ca2+-dependent inhibition. The function of calmodulin on IP3Rs, in vivo, is still unknown. Binding studies have been conducted in vitro, and concrete evidence of complex formation such as coimmunoprecipitation of calmodulin and IP3Rs have not been reported (434, 435).
In addition to calmodulin, the Ca2+-binding protein 1 (CaBP1) or related proteins such as Calmylin, in the presence of Ca2+ interacts with the SD and β-domains of the IBC and may stabilize interactions with adjacent monomers inhibiting channel opening (1395).
Adenosine triphosphate
Adenosine triphosphate also modulates the activity of IP3Rs (434, 435, 933, 1485). Putative binding sites for ATP are located in the regulatory domain of IP3Rs (434, 1284). For IP3R1 and IP3R3, ATP increases the sensitivity of the channels to activation by Ca2+ without affecting the maximal channel open probability (434, 1485). In contrast, ATP modulates the maximal open probability induced by Ca2+ of IP3R2, without affecting the channel’s Ca2+ sensitivity (434, 1485). This difference in ATP reactivity of the isoforms may allow local ATP concentrations, due to alterations in consumption or production of ATP, to differentially modulate the function of the different isoforms. However, the impact of local ATP concentrations on the function of IP3Rs in vascular SMCs has not been established.
Protein-binding partners
Many proteins interact with IP3Rs and potentially alter their activity (434). IRBIT (IP3-binding protein released with IP3) is a protein that, when phosphorylated, interacts with residues in the SD domain and IBC at the N-terminus of IP3Rs (50, 434). Binding of IRBIT to the receptor reduces IP3R activity, whereas IRBIT knockdown increases IP3R activity (49). It has been proposed that this peptide functions in the negative feedback regulation of IP3Rs.
In addition to its effects on RyRs, FKBP12 may modulate the activity of IP3Rs (434). In colonic SMCs, FKBP12 associates with IP3Rs and modulates IP3-dependent Ca2+ release through its effects on the kinase, mTOR, and the phosphatase, calcineurin (923). IP3Rs are also modulated by FKBP12 in portal vein SMCs (925). However, in aortic SMCs, although FKBP12 is expressed, it is not bound to IP3Rs and does not modulate IP3-dependent Ca2+ release (923). These data suggest that there are regional differences in the role played by FKBP12 in the modulation of IP3Rs in vascular SMCs. It has also been proposed that FKBP12 alters the interaction between PKC and IP3Rs to augment Ca2+ release (1143). This mechanism, however, has not been demonstrated in vascular SMCs.
Scaffolding proteins also interact with IP3Rs. RACK1, a scaffold protein linking PKC to its substrates, also interacts with IP3Rs within the SD and the IBC domains at the N-terminus (434, 1144). Binding of RACK1 to IP3R increases IP3 sensitivity and activity (1144). Ankyrin, an adaptor protein linked to the cytoskeleton, binds IP3Rs near the pore sequence and inhibits IP3R activity (572). Ankyrin also appears to be important for localization of IP3Rs (434). Subcellular localization may also be mediated by interaction of IP3Rs with the Homer family of scaffolding proteins (434). Homer binds in the SD domain at the N-terminus (434, 1435), and it has been shown that disruption of Homer cross-links to TRPC1 channels alters the activity of both channels (1611).
IP3Rs interact with and affect the function of a number of TRPC channels including TRPC1 (163), TRPC3 (163, 756), TRPC4 (997), TRPC6 (163) and TRPC7 (1468), either directly (163) or as part of larger protein complexes (1611). Coupling sequences for interactions between IP3Rs and TRPCs are located in the regulatory domain of IP3Rs (434). In addition to TRPC channels, TRPP1 channels, when expressed in the ER, also interact with and enhance the function of IP3Rs (5, 788, 863, 1247). This involves an interaction of negatively charged amino acids in the C-terminus of TRPP1 and positively charged residues in the SD of IP3R (1247).
IP3Rs also have been shown to interact with proteins involved in protecting cells from apoptosis. The Bcl family members Bcl-xL, Mcl, and Bcl-2 bind to the C-terminus of IP3Rs and enhance channel activity contributing to their antiapoptotic actions (368, 862).
Pharmacology of IP3Rs
The pharmacology of IP3Rs is outlined in Table 3. As with RyRs, the toolkit is limited, particularly because the standard method for assessing IP3R function (Ca2+ imaging on whole cells) does not have the precision offered by patch clamp approaches applied to channels in the plasma membrane. There are also significant limitations with two of the agents listed; heparin and adenophostine A are not cell permeant, they must be injected into cells or dialyzed through a patch pipette. The selectivity of 2-aminoethoxydiphenyl borate (2-APB) has been questioned because it also blocks some TRP channels (156). However, in vascular SMCs this compound appears to effectively block IP3Rs and it is the block of IP3Rs that modulates TRPC3 channel function (1555). Nonetheless, prudent use of these compounds requires comparison of the effects of multiple inhibitors with different structures and mechanisms of action.
IP3R function in vascular SMCs
Early studies demonstrated that inhibition of PLC attenuated myogenic tone of cerebral arteries (1114), suggesting a role for IP3 and IP3Rs in this process. More recent studies in the same vessels suggest a central role for IP3Rs in generation and maintenance of myogenic tone (461, 486, 488), with Ca2+ release through IP3R-activating TRPM4 channels, contributing to pressure-induced depolarization of the SMCs and subsequent activation of VGCCs. Integrin-mediated activation of PLCγ1 and subsequent formation of IP3 have been proposed to be the link between distending pressure and activation of IP3Rs in this system (1036). An alternative mechanism involving pressure-induced activation of angiotensin II receptors and subsequent activation of PLCβ also has been proposed to account for IP3 formation in renal and mesenteric arteries and could account for IP3R activation in the myogenic response (985, 1261). Membrane depolarization, per se, also has been suggested to activate some G-protein-coupled receptors leading to activation of PLC, IP3 formation, and IP3R-dependent Ca2+ release (327, 419, 459, 895, 930, 1448, 1574). Thus, there may be multiple mechanisms by which intravascular pressure can lead to IP3R signaling in vascular SMCs.
In addition to cerebral vessels, myogenic tone in skeletal muscle feed arteries and arterioles in hamsters (1528) and mice (967, 1527) also appears dependent on IP3R signaling. In contrast, studies in fourth-order murine mesenteric arteries found no role for IP3 and IP3Rs in myogenic tone (966). Instead, they propose that PLC hydrolyzes phosphatidylcholine to produce DAG that is essential for myogenic tone in this murine resistance artery (966).
Role of IP3Rs in Ca2+ waves and Ca2+ oscillations
Regenerative release of Ca2+ through IP3Rs can produce Ca2+ waves that propagate along cells and which can result in oscillations in intracellular Ca2+ (123, 434). It is thought that IP3 primes IP3Rs for activation by Ca2+, which then, via CICR, recruits Ca2+ release from adjacent IP3Rs allowing the signal to propagate along a cell (123, 434). The elevated Ca2+ then terminates release by Ca2+-induced inhibition of the IP3Rs, with released Ca2+ being transported back into the ER via SERCA (123, 434). If IP3 levels remain elevated, this cycle can repeat resulting in oscillations in intracellular Ca2+ (123, 434). Calcium-dependent inhibition of PLC may lead to oscillations in IP3, contributing to Ca2+ oscillations (556). The DAG produced along with IP3 may activate PKC which, in turn, can inhibit PLC and IP3 formation and also contribute to Ca2+ oscillations (537).
Role of Ca2+ waves in myogenic tone
Ca2+ waves have been reported in many types of vascular SMCs, but their role in the modulation of myogenic tone is uncertain (316). Pressurization of rat cerebral arteries leads to development of myogenic tone and an increase in the frequency of SMC Ca2+ waves (678, 1035, 1036). In this system Ca2+ waves involve both IP3Rs (1036) and RyRs (678, 1035, 1036), and these Ca2+ signals appear to contribute to development of myogenic tone independent from VGCCs (1035, 1036). Pressure-induced Ca2+ waves that contribute to myogenic tone and which are dependent on both IP3Rs and RyRs also have been observed in hamster and mouse cremaster muscle feed arteries (1527, 1528) (Fig. 4). However, in second-order arterioles, downstream from these feed arteries, Ca2+ waves also are observed, but are dependent only on the activity of IP3Rs. In both cremaster feed arteries and arterioles Ca2+ waves appeared to contribute to myogenic tone, in that global intracellular Ca2+ fell and the vessels dilated when PLC or IP3Rs were inhibited (1527, 1528). In cremaster arterioles, IP3R-mediated Ca2+ waves appeared to be dependent on Ca2+ influx through VGCCs, and it was proposed that IP3Rs amplified Ca2+ signals produced by Ca2+ influx through VGCCs (1527, 1528) (Fig. 4).
In contrast to the findings outlined in the preceding paragraph, studies in both rat (1007) and mouse (1615) mesenteric resistance arteries revealed a decrease in asynchronous Ca2+ waves as pressure-induced myogenic tone increased, presumably because Ca2+ influx through VGCCs led to inactivation of IP3Rs. In murine mesenteric resistance arteries it was also shown that block of IP3Rs with xestospongin C had no effect on myogenic tone (966). Thus, in these vessels IP3Rs do appear to contribute to myogenic tone.
Studies of mouse cremaster arterioles, in vivo, also failed to observe Ca2+ waves (967), however, the sampling rate used by these authors (2 Hz) may have limited their ability to detect higher frequency events. Despite the lack of detected Ca2+ waves, inhibition of PLC or block of IP3Rs dilated mouse cremaster arterioles, in vivo (967), consistent with in vitro studies of cremaster arterioles from hamsters (1528) and mice (1527). Thus, there may be regional heterogeneity in the role played by IP3Rs in the development and maintenance of myogenic tone.
Vasoconstrictors and IP3Rs
Many vasoconstrictors act on vascular SMCs through heptihelical receptors coupled to heterotrimeric Gq/11 and downstream PLC resulting in hydrolysis of membrane phospholipids, formation of DAG and IP3, activation of IP3Rs and subsequent release of Ca2+ that contributes to SMC contraction (1055, 1502) (Fig. 10). Early studies in cultured SMCs found that agonists such as thrombin (1076), vasopressin (142), ATP (931) or norepinephrine (149) stimulated oscillatory Ca2+ waves. Subsequent studies imaging intracellular Ca2+ in SMCs in the wall of resistance arteries or arterioles showed that agonists such as norepinephrine (339, 640, 734, 1150, 1602), phenylephrine (835, 965, 1007, 1059, 1224, 1288, 1530), UTP (681, 1634), U46619 (1288) or endothelin (1288) induced Ca2+ waves in the SMCs that were either asynchronous, inducing stable vasoconstriction, or synchronous, resulting in vasomotion (1288, 1530). Studies in SMCs isolated from rat portal vein (149), isolated rat inferior vena cava (835), rat cerebral arteries (1634) and human mesenteric arteries (1059) then provided evidence that IP3Rs contributed to these oscillatory changes in intracellular Ca2+. In several instances, RyRs also were involved in agonist-induced Ca2+ waves (149, 681, 1634). In rat tail arteries, downregulation of RyRs by organ culture in the presence of ryanodine eliminated RyR function, but had no effect on norepinephrine-induced Ca2+ waves (339). These data suggest that IP3Rs alone are capable of supporting Ca2+ waves as has been shown for Ca2+ waves observed during myogenic tone in cremaster arterioles (1527, 1528). In rat cerebral arteries, it has been shown that IP3R1 is the isoform responsible for UTP-generated Ca2+ waves (1634).
The DAG produced concomitantly with IP3 after receptor activation, along with elevated Ca2+ activates PKC, which can also phosphorylate IP3Rs and potentially modulate their function (132, 434). However, the consequence of such phosphorylation on IP3R function is not clear (132, 434). Phorbol ester-induced activation of PKC was shown to phosphorylate IP3Rs and increase IP3-stimulated Ca2+ release from isolated hepatocyte nuclei (963). In contrast, activation of PKC decreased the activity of IP3R2 (200) and IP3R3 (200) in cell-based systems. Detailed studies of the effects of PKC activation on IP3R properties have not been performed (132, 434). Thus, the role played by PKC in modulation of IP3R function in vascular SMCs is not known.
IP3Rs can also be phosphorylated by CamKII, although there is limited evidence that these modifications affect IP3R activity, and the functional consequences are not known (132) (Fig. 10). There is also evidence that other serine/threonine kinases such as MAP kinases and Akt (protein kinase B), and tyrosine kinases such as Src, Fyn, and Lyn can phosphorylate IP3Rs, but nothing is known of the functional consequences of such phosphorylation, particularly in SMCs (132).
In cerebral SMCs, IP3R1 interacts with TRPC3 to regulate the activity of TRPC3 through a process that does not involve release of Ca2+ through the IP3R1 channels (11, 1555, 1634) (Fig. 10). Jaggar and colleagues (11, 1555, 1634) propose that agonists, such as UTP, that bind to Gq/11-coupled receptors and activate PLCβ to produce IP3 activate IP3R1, which then interact with and activate TRPC3. The inward Na+ and Ca2+ currents through TRPC3 channels depolarize the cells to activate VGCCs, increase intracellular Ca2+ and significantly contribute to the vasoconstriction produced by these agonists.
Vasodilators and IP3Rs
The effects of vasodilators on IP3R function, per se, in vascular SMCs have not been well studied. Both PKA (422) and PKG (1329) can phosphorylate IP3Rs at identical sites in the regulatory domain and potentially modulate IP3R activity (132, 330, 434) (Fig. 10). PKA is present in signaling complexes at IP3R1 with AKAP9 and PP1 (132, 330, 434). Studies of IP3Rs in heterologous expression systems have shown that PKA-dependent phosphorylation of IP3R1 increases the channel’s activity (132) (Fig. 10). In contrast, IP3R3 seems resistant to modulation by PKA and PKG (132). However, the effects of PKA-dependent phosphorylation on IP3R function under physiological conditions remain unclear (132, 330, 434). It has been shown in several systems, including airway SMCs (80), that elevated cAMP, via PKA, inhibits IP3-dependent release of Ca2+ through IP3Rs (4, 1400, 1614). Thus, it is possible that vasodilators that act through the cAMP-PKA signaling cascade, could act, in part, by inhibition of IP3R function in vascular SMCs. Increased cAMP-PKA activity also can inhibit the production of IP3 via inhibition of PLCs (4, 1051). This would indirectly inhibit Ca2+ release through IP3Rs.
The cGMP-PKG signaling pathway has also been shown to inhibit IP3-dependent Ca2+ release through IP3Rs (4, 1042, 1399, 1400) (Fig. 10). However, this appears to result from PKG-mediated phosphorylation of the protein-binding partner, IP3R-associated cGMP-kinase substrate (IRAG) (132). IRAG is a membrane bound protein in the ER that couples PKG1β to IP3R1 (1264). Phosphorylation of IRAG at S696 inhibits agonist-induced release of Ca2+ through IP3R1 (1264) and mediates cGMP-mediated relaxation of vascular SMCs (470) (Fig. 10). The cGMP-PKG signaling pathway also can inhibit formation of IP3 via PLCs to inhibit Ca2+ release through IP3Rs (4, 1051).
In rat cerebral SMCs, IP3R1 has been shown to interact with plasmalemmal BKCa channels, and that activation of the IP3Rs with IP3 or adenophostin A activates the BKCa channels, providing another negative feedback mechanism to regulate myogenic tone in resistance arteries and arterioles (1635) (Fig. 10). IP3R-related modulation of BKCa channels also has been proposed to contribute to the negative feedback regulation of tone in porcine coronary arteries (1582).
IP3Rs and pathophysiology
Hypertension
Studies of the effects of hypertension on vascular SMC IP3R function are limited. There is increased basal and phenylephrine-stimulated IP3 formation in vascular SMCs from SHR (1548), and IP3 binding capacity is increased in microsomes from vascular SMCs in the SHR (121). These data suggest that there may be increased IP3R signaling in hypertension. However, this hypothesis has not been directly tested.
Activation of ionotropic P2X purinergic receptors has been shown to stimulate Ca2+ release through IP3Rs by a mechanism that involves activation of Ca2+ entry through VGCCs and stimulation of PLC (1173, 1351, 1352). In renal preglomerular vascular SMCs, P2X receptor-induced IP3R-mediated Ca2+ release is decreased in cells from SHR due to a decrease in the Ca2+ load in the SR (495). This may account, in part, for the loss of preglomerular P2X receptor-mediated reactivity that is observed in hypertension.
In contrast, in mesenteric artery SMCs, hypertension is associated with an increase in IP3R1 protein expression and increased IP3R function that contributes to enhanced reactivity to norepinephrine observed in hypertension (6). The increased expression of IP3Rs appears to result from Ca2+-dependent activation of calcineurin-NFAT signaling (6).
Aging
As noted earlier in the Protein-Binding Partners section, IP3Rs interact with, and their function is modulated by, TRPP1 channels expressed in the ER (5, 788, 863, 1247). In the cerebral circulation advanced age is not associated with a change in TRPP1 protein expression, but a decrease in the function of IP3Rs and an apparent decrease in the modulation of IP3Rs by TRPP1 (5). The cause of the differences observed was not established.
Diabetes
The effects of diabetes on vascular SMC IP3R function are not clear. In streptozotocin-induced diabetes, renal glomerular, and preglomerular vascular expression of IP3R mRNA and protein are reduced (978, 1287). Consistent with the reduced IP3R expression, agonist stimulated Ca2+ signals in whole cells and IP3-induced Ca2+ release in permeabilized cells also were reduced (978). These effects appeared to be mediated through transforming growth factor-β. Decreased expression and function of IP3Rs have also been reported in aortic (918, 1274) and femoral (1274) SMCs from diabetic rats, and in A7R5 cells exposed to elevated glucose (1274).
In contrast, expression of antiapoptotic protein, Bcl-xL, is increased in db/db diabetic mice resulting in augmented mesenteric vascular SMC IP3R Ca2+ signaling (1471). However, the increased IP3R-mediated Ca2+ signals do not appear to be the cause of the increased vasoconstrictor reactivity observed in this model of type 2 diabetes, because pharmacological inhibition of Bcl-xL effects reduced Ca2+ signals, but had no effect on contractility (1471).
Transient Receptor Potential Channels
Discovery of TRP channels
Transient receptor potential (TRP) channels were first discovered and characterized in Drosophila melanogaster, after a mutation to Chromosome 3 resulted in offspring that were visually impaired in normal light but showed normal phototaxis under low light (277). Further experiments uncovered that the normal, prolonged increase in photoreceptor membrane potential associated with light exposure was absent; instead, exposure to light resulted in a transient increase in voltage that rapidly decayed back to baseline. This characteristic phenotype lead to these mutants being named “transient receptor potential,” or trp, mutants (277, 1006). It was postulated later that the protein product of the trp locus was probably a cation-permeable ion channel, since hydrophobicity plots predicted a 6 to 8 transmembrane domain structure similar to that of known voltage-dependent ion channels (1023). However, the “trp channel” lacked the positively-charged amino acid sequence in the S4 region that normally imparts voltage sensitivity, suggesting the channel did not respond to depolarization (1158). Instead, the trp channel was found to be a Ca2+-permeable nonselective cation channel, which was activated by the SERCA inhibitor thapsigargin and opened subsequent to IP3-mediated Ca2+ release from intracellular ER Ca2+ stores (1023, 1450). Expression of “TRP” and “TRP-like” proteins was subsequently discovered in most eukaryotic species, where the channels are key mediators of cation flux in both excitable and nonexcitable cells (260, 1086).
Structure and general function of TRP channels
In mammals, 28 TRP channels have been identified and grouped by sequence homology into 6 distinct families: canonical (TRPC1–7), melanostatin (TRPM1–8), vanilloid (TRPV1–6), ankyrin (TRPA1), polycystin (TRPP1–3), and mucolipin (TRPML1–3) (1121). Individual TRP channels are tetramers, with each subunit containing six transmembrane domains and intracellular C- and N-termini of varying amino acid length. These termini contain multiple protein binding sites, enzymatic domains, and regulatory elements that also differ between families and channel subtypes (258). TRP channels can exist as both homo- and heteromultimers, which further increases the diversity of their function and activity (258, 259, 333, 361, 658, 1347). With regard to the function of TRP channels in vascular SMCs, members of each family (with the exception of TRPML) have been implicated in regulating SMC contractility in different vascular beds (361).
The general properties of each TRP channel family, common familial characteristics, and relevant differences in channel structure are described in this section. Their individual contributions to vascular tone are discussed in subsequent sections.
Canonical (TRPC) channels
The seven members of the TRPC family were named “canonical” [or “classical” (260)] due to their close structural resemblance and sequence homology to the original trp channel discovered in D. Melanogaster (1525, 1648, 1655). With the exception of TRPC2 (a pseudogene in humans and rats), the remaining TRPC channels can be subdivided into two groups by sequence homology: TRPC1/C4/C5 and TRPC3/C6/C7 (875, 1553, 1601). The general structure of TRPC channels is relatively similar. Each subunit contains the requisite six transmembrane domains, 3–4 ankyrin repeats and a coiled-coil domain on the N-terminus, and a C-terminus containing a calmodulin/IP3R binding (CIRB) domain and the “TRP box” sequence (EWKFAR) common to all TRP channels (258, 1101, 1469, 1553). Additionally, TRPC4 and TRPC5 contain a common structural domain originally found in Postsynaptic density protein 95, Drosophila disk large tumor suppressor protein and Zona occludens 1 (PDZ-binding motif) on their C-termini that is crucial for protein/protein interactions between these channels and several signaling molecules (46, 757, 1387). The PDZ motif, and an additional coiled-coil domain, may also lead to the extensive heteromultimerization that exists between TRPC1, TRPC4 and TRPC5 (880, 1346, 1347). TRPC3/C6/C7, however, largely exist as homomultimers (1553). TRPC channels show little ion selectivity, favoring Ca2+ conductance over Na+ with a ratio of 1:1 to 5:1 (1258, 1347, 1469, 1654).
Members of the TRPC family are part of receptor-operated Ca2+ entry (ROCE) pathways, since they are activated downstream of Gq/11-coupled receptors and receptor tyrosine kinases that activate phospholipase C (1166, 1167, 1387, 1426, 1553). A detailed description of G protein-coupled receptors that activate TRPC channels is presented in (8). Several canonical family members are also activated by DAG, through a mechanism that is independent of traditional PKC-mediated channel phosphorylation (562, 612, 836, 880, 1536). This finding further reinforces the direct relationship between TRPC channels and the activation of membrane-bound receptors. Given their downstream association with PLC activation and the presence of a CIRB-binding site, it has been long-proposed that TRPC channels open in response depletion of intracellular Ca2+ stores, and thus would be the dominant source of store-operated Ca2+ entry (SOCE) as well (47, 669, 756, 1240, 1263, 1525). However, TRPC channels lack the typical store-operated gating mechanisms present in other SOCE channel complexes (e.g., STIM1/Orai) (51, 328, 858). Simultaneous knockout of TRPC1/4/5 channels resulted in no change to neuronal SOCE, indicating that activation of TRPC channels is indeed not required (564). Furthermore, the lack of Ca2+ selectivity inherent to the TRPC channels (601) and the absence of SOCE in many types of contractile SMCs (1170, 1425, 1555) have largely eliminated TRPC channels as SOCE channels in vascular smooth muscle. Thus, TRPC channels are most appropriately described as receptor-operated cation channels.
Melastatin (TRPM) channels
With its 8 members, the TRPM channels are the largest subfamily of TRP channels (431). TRPM channels received their name because the first identified member, TRPM1, was discovered while screening for genes downregulated in mouse melanoma tumor-cell lines (350, 1565). Structurally, all TRPM channels contain the typical C-terminal TRP-box sequence and an N-terminal coiled-coil region that is also common to most TRP channels (386, 448, 503, 1022, 1091, 1156). However, TRPM channels lack the N-terminal ankyrin repeats present in TRPC, TRPA, and TRPV channels (561). Instead, an additional ~700 amino acid TRPM homology domain on the N-terminus is conserved in all 8 TRPM family members (431). As with the TRPC channel family, TRPM channels can be further subdivided into four pairs, based on mechanisms of activation, structural homology, and biophysical properties: TRPM1/M3, TRPM2/M8, TRPM4/M5, and TRPM6/M7 (1553).
TRPM1 and TRPM3 channels are both constitutively active and nonselective for Ca2+ versus Na+ (431, 503). The activity of TRPM3 can also be augmented by hypotonic solutions, suggesting a role in osmolarity sensation and Ca2+ homeostasis in the kidney (503, 563). While TRPM2/M8 are the closest-related family members (42% sequence homology and relatively little cationic selectivity (980, 1146, 1155)), their activation and regulation are entirely different from one another. TRPM2 channels are activated by oxidative and nitrositive stress, due to in part to a C-terminal Nudix-like domain that hydrolyzes ADP ribose and leads to channel opening (790, 1155, 1156). This domain is absent in TRPM8. Instead, the C-terminus of the TRPM8 channel contains a PIP2 binding domain and structural elements involved in temperature-dependent gating, which imparts TRPM8’s well-characterized cold/menthol-sensitivity and PIP2-dependent activation (172, 1213).
TRPM4/5 are the only two members of the TRPM family that show marked selectivity for Na+ over Ca2+ (Ca2+/Na+ = <0.05), and are virtually impermeable to all other divalent cations (610, 824). TRPM4/M5 are activated by increases in intracellular Ca2+ concentration, and are thus purported to function primarily as a Ca2+-activated, monovalent cation-conducting channel (610, 1085, 1087, 1091). Structurally, TRPM4 and TRPM5 both contain voltage-sensing domains (originally thought to be absent from TRP channels), PIP2/calmodulin binding sites, and phosphorylation sites that regulate the Ca2+ sensitivity and voltage dependence of channel opening (431, 1088, 1091).
The ubiquitously-expressed TRPM6/M7 channels are fusion proteins, comprised of the traditional ion channel transmembrane domains coupled to an enzymatically active α-kinase domain (252, 430). While selective for divalent cations over monovalent cations, TRPM6/M7 have a fivefold greater selectivity for Mg2+ over Ca2+ and thus are key to Mg2+ homeostasis (1633). Both channels are also activated by acidic pH (853). The endogenous substrate(s) for TRPM6/M7 α-kinase activity are unclear, although in vitro studies suggest that both TRPM6 and TRPM7’s α-kinases can phosphorylate myosin IA, IIB, and IIC on identical residues (261). Furthermore, the TRPM6 and TRPM7 channels coassemble into heteromultimers, leading to regulation by both auto- and cross-phosphorylation by their respective kinase domains (253, 261, 1266, 1633).
Vanilloid (TRPV) channels
The six members of the TRPV family are so named because of their activation by vanilloid-like compounds (e.g., capsaicin) (206, 1415). While vanilloid-dependent activation is a common trait of TRPV channels, they are critical for the regulation of nociception, thermosensation, mechanosensation, and Ca2+ absorption/reabsorption (112, 1415, 1473). TRPV channels can be broadly subdivided into two groups, based on their Ca2+ selectivity: TRPV1–V4 (PCa/PNa = ~1–10) and TRPV5/V6 (PCa/PNa>100) (258, 259, 1473, 1474). Similar to the TRPC family, heteromultimerization between channels within each group has been reported (585, 787, 873). All TRPV channels contain 3–5 N-terminal ankyrin repeats, as well as the conserved TRP-box sequence (1474). Specific residues in the transmembrane domain linkers of TRPV1–V4 impart thermal and proton sensitivity, and TRPV1, V4, V5, and V6 also contain C-terminal CaM-binding sites (134, 413, 462, 777, 1594). TRPV5/V6 are weakly voltage-sensitive, and are the only TRP channels that are almost completely selective for Ca2+ (1473).
Ankyrin (TRPA) channels
A sole member of the TRPA family, TRPA1, is expressed in mammalian cells (1659). This family is named “ankyrin” due to the 14 to 18 ankyrin repeats on the N-terminus of the channel, which makes up over half the total size of the TRPA1 monomeric protein (466, 1090). The TRPA1 channel is nonselective for monovalent or divalent cations (PCa/PNa = 0.84) (1046), but is bi-modally regulated by intracellular [Ca2+]—much like the IP3 receptor (discussed earlier). Low concentrations of intracellular Ca2+ potentiate TRPA1 activation, whereas high concentrations inhibit channel opening (1491). The physiological roles of TRPA1 channels include nociception, cold temperature sensation, and chemosensation (324, 732, 1090, 1340, 1492). Pungent chemicals, such as mustard oil [allyl isothiocyanate (AITC)] and cinnamaldehyde, reversibly activate TRPA1 through covalent modifications of N-terminal cysteine residues (603). The massive relative size of the N-terminal tail begat the hypothesis that TRPA1 may also be a mechanosensitive channel (626), but this has yet to be confirmed in vivo (324, 1340).
Polycystin (TRPP) channels
The TRPP family originally included two subsets of proteins, both of which are related to the development of polycystic kidney disease (PKD): PKD1 and PKD2 (1278). Members of the PKD1 group turned out to have 11 transmembrane domains that did not form functional ion channels (609). Members of the PKD2 group, however, are 6-TM domain proteins that assemble to form an ion-permeable pore, and are generally now regarded as sole members of the “TRPP” family of TRP channels (259, 609, 1278). This early confusion has led to relatively confusing nomenclature surrounding the TRPP channel family, from a historical perspective. To alleviate such confusion, and based on the nomenclature given in the 2016 International Union of Basic and Clinical Pharmacology (IUPHAR) Database (259), “TRPP1,” “TRPP2,” and “TRPP3” in this review will refer to “PKD2,” “PKDL1,” and “PKDL2,” respectively, as found in earlier literature. While evidence suggests PKD1 proteins do associate with TRPP channels in macromolecular signaling complexes (1278), their role and function will not be discussed in this review. See several outstanding reviews on this subject for more information (609, 1278, 1553).
The TRPP channels share a similar topology to the other TRP channels, with the exception of a large extracellular loop between the S1 and S2 transmembrane helices (609, 1019, 1278). TRPP1–P3 channels are relatively nonselective for Ca2+ (PCa/PNa = 6) (258, 1086). Little is known about the physiological significance for TRPP channels other than their roles in left/right symmetry development and in the pathogenesis of autosomal dominant polycystic kidney disease (ADPKD) (609). Activation of TRPP1 is vaguely understood, and is further complicated by the fact that TRPP1 channels locate in multiple subcellular compartments, including the endoplasmic reticular membrane, the plasma membrane and primary cilia (433, 788, 1145). TRPP2 channels are implicated in sour taste sensation as they are activated by intracellular Ca2+ and inhibited by low extracellular pH (658). Recent evidence also suggests that TRPP2 channels are weakly voltage-dependent and sensitive to cell swelling (1297, 1298). No spontaneous activity has been observed when the TRPP3 channel is overexpressed, and thus no relevant function is yet clear (1362).
Expression and function of TRP channels in vascular SMCs
Of the TRP channel subfamilies described earlier, at least 12 have been detected in SMCs across most vascular beds. In this section, the important functions of the vascular TRP channels will be covered for each family, in terms of the physiological stimuli to which they respond: endogenous messengers (e.g., DAG, intracellular Ca2+, and GPCR activation); activation by exogenous stimuli (e.g., temperature, noxious chemicals, and osmotic stress); and mechanical stresses (e.g., pressure-induced tone). In some cases, the functions of TRP channels in the vasculature have relied heavily on the use of nonselective pharmacological agents (see Section “Pharmacology”). As such, prudence should be used when interpreting these data with regard to the role of TRP channels in SMC contractility. Also, for more information regarding TRP channel distribution in different vascular beds, see Earley and Brayden (361).
TRPC channels: Receptor-operated Ca2+ entry and membrane depolarization
As stated above, TRPC channels have long been implicated in ROCE and SOCE, especially in vascular SMCs (30, 846, 1166). The complex heteromultimerization of the TRPC channels, and differences in subtype expression and association throughout the vasculature, led to significant difficulty in isolating the exact contributions of each TRPC channel subtype to these two Ca2+ entry processes. To date, evidence supports an important role for 5 members of the TRPC family in smooth muscle: TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6. Their individual and collective contributions to vascular tone are described below.
TRPC1 was originally linked to ROCE and SOCE in and of itself (118, 645). Endothelin-1 caused ROCE in cerebral arteries and cultured aortic SMCs in a TRPC1-dependent manner (1372), and in pulmonary artery SMCs, activation of SOCE (by blocking SERCA) was mediated by STIM1 and subsequent Ca2+ entry through TRPC1 channels (1078). However, this was refuted in later studies using TRPC1 knockout mice, which showed no differences in SOCE in aortas and cerebral arteries (334). Phenylephrine-induced constriction was also augmented in TRPC1 knockout mice and was unchanged in TRPC3 knockout animals, suggesting that TRPC1 channels are involved in ROCE instead (772). The role of TRPC1 in vascular contractility is further complicated by the finding that TRPC1 forms heteromultimers with TRPC5 (1293, 1294). It has also been proposed that TRPC1 channels are part of a larger signaling complex with non-TRP ion channels in vascular SMCs. In aorta and mesenteric arteries, TRPC1 immunoprecipitated with BKCa channels, and that Ca2+ influx through TRPC1 activated BKCa channels to hyperpolarize the SMC membrane and oppose vasoconstriction (806). Another model proposed an association between TRPC1, STIM1, and IP3 receptors that accounted for SMC contraction (150, 361, 1620). TRPC1 may be involved in ROCE only, regulating GPCR-mediated vascular contractility through interactions with BKCa channels or TRPC5 channels.
As with TRPC1, TRPC3 is implicated in vasoconstriction in response to several endogenous ligands for GPCRs. Responses to UTP in cerebral arteries, ET-1 in coronary arteries, and angiotensin-II in aorta, all involve activation of TRPC3 (894, 1151, 1194). TRPC3 is also capable of forming heteromultimers with TRPC1 and TRPC6, and this complex participates in norepinephrine-induced constriction of afferent arterioles (1243). Downregulation of TRPC3 channels also had no effect on pressure-induced (“myogenic”) tone development in pial arteries (1194), suggesting the role for TRPC3 was limited to GPCR-mediated responses—perhaps through direct activation of the channel by DAG, as had been previously suggested (32, 821, 1459). However, several reports associated opening of TRPC3 channels with activation of IP3 receptors on the sarcoplasmic reticulum. IP3 constricts cerebral arteries via IP3R-mediated activation of TRPC3 (1555). Additionally, ET-1 causes vasoconstriction via direct interaction of IP3R1 and TRPC3 (12). TRPC3 and IP3R1 associate with caveolin-1 in a macromolecular complex which, when disrupted, interferes with IP3-induced activation of TRPC3 channels (11). Lastly, TRPC3 function can be mediated by direct action of several different kinases, although this regulation may be tissue specific (361). WNK4 and PKG inhibit TRPC3 function in rat aorta and carotid artery, respectively (226, 1131). However, studies with TRPC3 knockout mice failed to confirm this relationship in mouse aorta or hind limb vasculature (899). Taken together, these data suggest TRPC3 function is controlled by multiple mechanisms initiated by phospholipase activation, but these pathways may involve both DAG- and IP3R-mediated regulation of the TRPC3 channel and direct interaction between TRPC3 and IP3Rs (see Fig. 11).
Figure 11.
TRP channel regulation of myogenic and agonist-induced smooth muscle contractility. Increased intravascular pressure activates a stretch-sensitive Gq-protein-coupled-receptor (GqPCR; e.g., angiotensin type 1 receptor, AT1R), which then causes the hydrolysis of PIP2 by phospholipase C- γ1 (PLCγ1) to form DAG and IP3. DAG activates TRPC6 channels to increase cytosolic Ca2+ concentration, combined with IP3-mediated Ca2+ release through IP3Rs in the ER. This local increase in cytosolic Ca2+ concentration activates TRPM4-dependent Na+ influx, membrane depolarization, and opening of Cav 1.2 channels. This results in SMC contraction (myogenic tone). A similar mechanism is activated in response to a GqPCR agonist, through activation of PLCβ and TRPC3 channels. Figure adapted, with permission, from Earley and Brayden (361). See text for more information.
Even though both channels are expressed throughout the vascular tree (439, 1641), the individual roles of TRPC4 and TRPC5 in smooth muscle contractility are the least clear of the canonical TRP family members. TRPC4 channels (along with TRPC1 and TRPC5) have been implicated in SOCE in pulmonary arterial SMCs (859). Prolonged, cyclic stretch reduced TRPC4 expression and SOCE in rat mesenteric arteries (878). However, no direct measures of TRPC4-mediated changes in vascular SMC contractility have been reported. Changes in vascular tone have been reported after removal of TRPC4 from endothelial cells (438), but even these findings are not without controversy (361, 1301). Little information exists as to the role of TRPC5 alone; rather, TRPC5 is best described as a heteromultimer with TRPC1, TRPC6, or TRPC7 (1238).
TRPC6 channels are well described in both venous and arterial smooth muscle, where they play an important role in regulating contractile function downstream of PLC activation (see Fig. 11). Cationic currents seen subsequent to α1-adrenoreceptor activation in portal vein myocytes were discovered to be through TRPC6 channels (647). Other GPCRs also activate TRPC6 currents: vasopressin activates TRPC6 in A7r5 cells (719), and angiotensin II also does so in mesenteric artery SMCs (33). This activation is due to direct interaction of DAG with the TRPC6 channel, and not through indirect phosphorylation by protein kinases (820, 1239). Interestingly, the precursor of DAG—PIP2—has an inhibitory effect on TRPC6 channel function in arterial SMCs, which is not seen in other expression systems (33, 841). This suggests a coregulatory mechanism, by which PLC activation both relieves TRPC6 channel inhibition by PIP2 and causes channel activation by DAG. Additional reports describe a synergistic relationship between activation of TRPC6 channels by both DAG and IP3, with IP3 alone having no effect on channel opening (31, 718).
In addition to receptor-mediated Ca2+ signaling, TRPC6 is also involved in the development of pressure-induced (myogenic) tone (see Fig. 11). Myogenic tone is largely regulated by pressure-dependent depolarization of SMCs, which results in the opening of VGCCs, Ca2+ influx, and vasoconstriction (315, 600, 993, 1073). TRPC6 channels appear to be integral in the development of myogenic tone of cerebral arteries (1523), but not mesenteric arteries (1261). However, it is not clear how mechanical forces activate the TRPC6 channel, nor is it clear the functional role that cationic influx through TRPC6 channels has in regulating myogenic tone. It is possible that, as with receptor-mediated constriction, TRPC6 channels are activated downstream of PLC activation. Activation of PLC by mechanosensitive GPCRs plays a role in myogenic tone development in small cerebral arteries (985, 1114), which would support the idea that TRPC6 serves as a parallel Ca2+ influx pathway to VGCCs. Others have described direct mechanical activation of TRPC6 in SMCs, independent of PLC activation (1331, 1523), but not without controversy. Calcium influx through TRPC6 channels during myogenic tone development appears to supplement or trigger IP3R-mediated Ca2+ release, which subsequently activates TRPM4 channels, contributing to pressure-dependent membrane depolarization (488). Given the level of mechanistic complexity and variability across the vasculature, the importance of TRPC6 channels in regulating vascular tone remains unclear and their function in the development and regulation of myogenic tone should be further explored.
TRPM channels: SMC depolarization and myogenic tone
While the TRPM channels represent the largest family of TRP channels in quantity, only two members are expressed in vascular SMCs: TRPM4 and TRPM8 (360, 712, 1358). TRPM4 channels have been found throughout the vascular tree, and are present in myogenically active resistance arteries and large conduit arteries alike (360). As mentioned earlier, TRPM4 is impermeable to divalent cations; TRPM4 is instead responsible for Na+ influx that causes membrane depolarization of SMCs (367). To that end, TRM4 channels show some degree of voltage dependence in their activation. These channels inactivate rapidly at negative membrane potentials, but have large, slow-activating currents at more positive potentials (1087). This voltage sensitivity appears to be due to an intrinsic voltage sensor and not blockade of the ion pore by divalent cations (1087). Also, Na+ currents through TRPM4 channels exhibit time-dependent inactivation and are regulated both by PKC and intracellular Ca2+ concentration (360, 366, 367). The concentrations of Ca2+ required to activate TRPM4 channels are extremely high (10–100 µmol/L), which far exceed even the highest global intracellular Ca2+ concentrations in normal SMCs and suggests a local Ca2+ event (similar to a Ca2+ spark) is required. As shown in Figure 11, the local bursts of Ca2+ needed to activate TRPM4 channels were found to originate from IP3 receptors in close proximity to TRPM4 channels in the plasma membrane (486, 487).
In cerebral arteries, TRPM4 channels appear to mediate myogenic tone development through a mechanism that also involves PLCγ1 activation, TRPC6, and IP3Rs (488). Inhibition of TRPM4 with either 9-phenanthrol or siRNA, inhibited pressure-induced tone development, but not UTP-mediated constriction, suggesting that TRPM4 channels are specifically involved in regulating membrane depolarization in response to changes in intravascular pressure and not contractile agonists (367, 1194). However, knockout of TRPM4 resulted in a hypertensive phenotype and no appreciable change to either myogenic or agonist-induced vascular tone of the hind limb (958). While the hypertensive phenotype was attributed to increases in circulating catecholamines, no explanation for the lack of change in myogenic tone in the hind limb resistance vasculature was readily apparent. Thus, it remains unknown if the regulation of myogenic tone by TRPM4 is specific to the cerebral vasculature, or if parallel mechanisms exist by which myogenic tone can be controlled in other vascular beds.
To date, few studies show the presence of TRPM8 channels in vascular SMCs. In aorta, mesenteric artery, and femoral artery, TRPM8 activation results in a modest relaxation that is endothelium independent (712). Another study concluded that TRPM8 played a part in mesenteric artery vasodilation in rats, but some of the pharmacological tools used bring the specificity of this conclusion into question (1305). Thus, the role of TRPM8 channels is still relatively unknown.
TRPV channels: Responses to temperature, endocannabinoids and EETs
Of the vanilloid family, TRPV1, TRPV2, and TRPV4 channels have all been found to play a functional role in vascular SMC tone in different vascular beds (99, 427, 1419). TRPV1 channels are expressed in aortic and skeletal muscle arteriolar SMCs as well as arterioles from thermoregulatory tissues (e.g., skin and ear) (733, 897, 1034, 1419, 1467). In these tissues, activation of TRPV1 by capsaicin resulted in endothelium-independent vasoconstriction, decreased hind limb perfusion, and increased gracilis muscle vascular resistance (733, 1034, 1467). TRPV1 activation can also cause vasodilation, but this is in an endothelium-dependent manner and not attributed to SMC TRPV1 (1626).
In isolated mesenteric artery SMCs, TRPV2 channels are activated by endocannabinoids and may result in vasodilation (99, 1181). Although not related to contraction, TRPV2 channels appear to be activated by several vascular growth factors to contribute to SMC migration (1339). However, TRPV2 channels in pulmonary artery SMCs are responsible for nonselective cation currents activated by thromboxane A2 during hypoxia (1606). Nonetheless, whole-vessel studies are still lacking, and as such the role of TRPV2 channels in vascular tone is not yet clearly defined.
A broad body of evidence links endothelial TRPV4 channels to vasodilation in mesenteric arteries and peripheral arterioles (365, 604, 1325), but TRPV4 channels are also expressed in SMCs and have a role in regulating vascular tone. In cerebral artery myocytes, TRPV4 channels temper the vasoconstrictor responses to angiotensin-II through a mechanism dependent on PKC and AKAP150 (994). There is also an important connection between SMC TRPV4 and BKCa channels, which drive SMC hyperpolarization in response to EETs (365, 427, 917, 994, 1482). These findings suggest that smooth muscle TRPV4 channels, similar to endothelial TRPV4 channels, are anchored by AKAP150 into a subcellular signaling complex. In SMCs, however, this complex includes PKC, BKCa channels and GPCRs, and regulates vasodilation.
TRPA and TRPP channels: Mechanosensitive vasodilation
The least well-characterized TRP channels in vascular smooth muscle are the TRPA1 channel and the TRPP1 channel. Unlike most of the TRP channel family members described above, both TRPA1 and TRPP1 are linked to vasodilation, either directly or indirectly. TRPA1 channels are predominantly involved in endothelium-dependent vasodilation (362, 1265, 1353), but were also shown to be involved in endothelium-independent relaxation of aortic rings by cinnamaldehyde (1579). However, this study did not confirm SMC-specific expression of TRPA1. Cerebellar arteries did express mRNA and positive immunofluorescence for TRPA1, but no endothelium-independent response to the TRPA1 agonist AITC was seen (359, 363). This suggests that TRPA1 may only play a role in large conduit arteries, or the response was due to non-specific effects of cinnamaldehyde (926). In either case, the function of TRPA1 channels in vascular SMCs remains unclear.
Originally, TRPP1 channels were assumed to aid in cytoskeletal arrangement and organization during SMC differentiation through associations with PKD1 (501, 1285). In the presence of PKD1, TRPP1 seemed to have little effect on contractility; it was only after knockout of PKD1 that myogenic tone was decreased in mesenteric arteries (1285). Interestingly, knockdown of TRPP1 in the same PKD1 knockout animals recovered normal myogenic constriction of mesenteric arteries (1285). TRPP1 knockdown also resulted in increased constriction of aortae and mesenteric arteries in response to the adrenergic agonist, phenylephrine (343, 1180). These data suggested that, when activated, TRPP1 currents opposed the development of myogenic constriction in peripheral and conduit arteries. In the cerebral arteries, however, TRPP1 knockdown had the opposite effect: myogenic tone development was decreased, and cell swelling-induced cation currents were reduced (1056). The exact role of TRPP1 in vascular SMCs will require more investigation to determine the reasons for these diametric roles in different segments of the vascular tree.
TRP channel pharmacology
The vast overlap and interplay among TRP channels has resulted in an extremely complex and confusing pharmacopoeia. With the exception of capsaicin (TRPV1), the selectivity and specificity of the pharmacological tools used to investigate TRP channels is questionable, at best (926, 1553). Table 4 summarizes TRP channel pharmacology, in terms of activation of the channel, ionic selectivity, agonists, and antagonists. The agonists and antagonists in the table were selected by their relative specificity for each TRP channel subtype. Due to the aforementioned lack of specificity of many of these compounds at higher concentrations, drugs with defined IC50 and EC50 values were used wherever possible. Individual concentrations are not reported to avoid confusion; however, the provided references will contain such information. A more in-depth description of TRP channel pharmacology can be found in the IUPHAR/BPS Database of Receptors and Ion Channels (259).
Most agents were originally believed to exhibit subfamily specificity, but were later found to have broad actions across multiple TRP channels and channel families. This includes drugs such as SKF96365, ruthenium red, and flufenamic acid (647, 720, 979, 1046, 1148). Other agents, such as 2-APB, were found to both activate and inhibit TRP channels in a concentration- and subtype-specific manner (853, 1568). Nonetheless, the broad-spectrum TRP channel agonists and antagonists have been used with much success as part of a “process of elimination” of TRP channel currents measured from vascular SMCs. Large di- and trivalent metal cations (e.g., La3+, Gd3+, Ni2+, and Zn2+) have also been used to differentiate between different TRP channel family members (164). However, these have proven difficult to use in whole vascular tissues due to their propensity to interfere with other ion channels and the often high concentrations required for TRP channel block or activation (259). This “dirty” pharmacology highlights the need for parallel studies, using tissue-specific knockout animals or alternative inhibition techniques (e.g., siRNA and viral vectors), to properly understand and elucidate individual TRP channel functions.
TRP channels and pathophysiology
Due to the broad involvement of TRP channels in regulating mechanical- and agonist-induced responses in the vasculature, defining specific roles for individual TRP channels in disease has proven extremely difficult. Nonetheless, several TRP channels are identified as having important roles in the pathophysiology of cardiovascular diseases.
Hypertension
Considering that SMC TRP channels largely mediate cationic fluxes that are necessary for contraction, augmentation of TRP channel function should lead to increased SMC tone and reactivity. Thus, it is not surprising that many of the TRP channels expressed in vascular SMCs have been implicated in the pathogenesis of hypertension. TRPC3 channels are implicated in the pathogenesis of hypertension through differential phosphorylation by the serine threonine kinase, WNK4, which suppresses channel activation and leads to SMC hypertrophy, increased myogenic tone and hypertension (1131). Regulation of TRPC3 is also linked to vascular pathology in hypertension through mechanisms affecting endothelial cell and immune cell signaling (1404, 1405). While TRPM4 knockout animals are also hypertensive, this appears to stem from an increase in circulating catecholamines and not a change in SMC reactivity alone (958). TRPM7 channels may also play a role in essential hypertension: SHRs show decreased TRPM7 expression and decreased intracellular Mg2+, both of which are linked to the actions of angiotensin II (1420).
Unlike essential hypertension, TRP channels may be a promising target for the treatment of pulmonary arterial hypertension (PAH). Both TRPC1 and TRPC6 expression is increased in pulmonary arteries during hypoxic conditions similar to those caused by PAH (876). Additionally, TRPV4 channels are upregulated in chronic hypoxia-induced PAH in mice and rats (1558, 1585) with no change in TRPM channel expression under the same conditions (1585). However, this is in conflict with other researchers, who showed a dramatic decrease in TRPM8 expression in pulmonary arterial myocytes in animal models of PAH (889, 1586).
Aging
Generally, the roles of TRP channels in age-dependent changes to vascular tone are vastly undefined beyond a few specific examples. Knockout mice lacking TRPP channels develop age-dependent hypercontractility in large conduit vessels (567). Aged hypertensive rats also showed maladaptive changes to middle cerebral artery myogenic tone and Ca2+ signaling, which was associated with decreased TRP channel-mediated Ca2+ responses (1418). Further research is needed to determine the roles of other TRP channels in aging.
Diabetes
Vessels from diabetic patients are more reactive than nondiabetic controls (1106), a finding which may be linked to changes in SMC TRP channel function. In human saphenous vein, diabetic vessels were more reactive to cyclopiazonic acid; this response was also inhibited by the TRP channel blocker SKF-96365 (254). This change in response was associated with increased TRPC4 expression, and decreased TRPC1 and TRPC6 expression in the diabetic vessels (254). Additionally, TRPV1 channel expression and capsaicin-mediated vasodilation are decreased in coronary arteries from diabetic mice (511).
Conclusions and Remaining Questions
Decades of studies have widely advanced our knowledge of the expression of ion channels in vascular smooth muscle and their roles in regulating tone and tissue perfusion. However, a broad analysis of the current literature still leaves fundamental questions unanswered while providing new insight into the complex interplay of these channels in health and disease. We suggest several such questions that warrant further investigation.
While it is clear that L-type VGCCs composed of CaV 1.2 channels importantly contribute to myogenic tone and its modulation by vasoconstrictors and vasodilators, a number of questions remain concerning these channels and the expression and function of other VGCCs in resistance arteries and arterioles around the body. Why do L-type VGCCs appear silent in some in vivo preparations? Do CaV 3.2-based T-type channels contribute to the negative-feedback regulation of myogenic tone in all vascular beds? What is the role of other VGCCs?
Studies have shown a remarkable number of KV channel isoforms expressed in vascular SMCs around the body. However, our understanding of the integrated function of the different classes of KV channels is limited. For example, studies in rat middle cerebral arteries indicate that at least three classes of KV channels (KV 1, KV 2, and KV 7) are expressed and contribute to the regulation of SMC membrane potential and the negative-feedback regulation of myogenic tone [see (1643) and references therein]. In these vessels, it has been proposed that the unique voltage dependence of activation and inactivation of each of these KV channels provides precise negative-feedback control of membrane potential across of broad range of voltages, allowing myogenic tone to be precisely regulated across a wide spectrum of blood pressures (1643). However, this remains speculation and has not been critically tested in other blood vessels, and particularly, in vivo.
Our understanding of the expression and function of RyR and IP3R isoforms and their regulation in the context of vascular SMCs in resistance arteries and arterioles is very limited. Why do RyRs appear to be silent in arterioles? Why do IP3R-dependent Ca2+ waves not activate BKCa channels? Do Ca2+ waves contribute to functions other than contributing to global Ca2+ signals in the regulation of resistance artery and arteriolar SMC function?
While evidence continues to mount as to the importance of TRP channels in the development of vascular tone, our understanding of the mechanisms by which TRP channels are regulated in vascular SMCs has only just begun. TRP channels represent the most logical means to transduce changes in the physical environment into changes into vascular function, but the paucity of selective pharmacological agents has limited our ability to explore these possibilities. Future questions include: how does the proximity of TRP channels to one another affect vascular tone? What endogenous ligands activate and/or inhibit TRP channel function? How can we differentiate the functions of homomeric from heteromeric TRP channels, and do these arrangements change in disease? How can we design better drugs to target these channels with some degree of specificity? Even with these questions unanswered, the evidence that TRP channels are integral in regulation of myogenic and agonist-induced tone is without contestation.
Disease states often lead to altered SMC ion channel expression and/or function. However, it is not often clear whether the changes that occur are part of the disease progression, or a compensatory change in an attempt to maintain homeostasis. It is suspected that the use of different disease models with different time courses of progression, severity and root cause, while important to try and understand the spectrum of human and animal disease, has left a confusing picture of the contribution of altered SMC ion channel expression and function to a given disease. Additional research identifying the signaling pathways responsible for altered SMC ion channel function and expression, the time course and the dose-response relationships between disease severity and ion channel dysfunction are needed to resolve these issues.
We have focused on SMC ion channels in this review. However, we fully acknowledge that in intact resistance arteries and arterioles, SMCs are electrically coupled to underlying endothelial cells by myoendothelial gap junctions, and that endothelial cells also express their own cadre of ion channels (671). While it is known that hyperpolarization of endothelial cells (induced by opening of KCa channels in these cells) can be transmitted to overlying SMCs (i.e., via EDHF), the impact of other endothelial cell ion channels on the regulation of membrane potential and function in the overlying SMC remains largely speculative, because the tools currently available to electrically uncouple these cell layers (mechanical denudation, available gap junction inhibitors, etc.) are simply too blunt.
Ion channels in the plasma membrane and in the ER of SMCs importantly contribute to the generation and maintenance of myogenic tone, as well as the mechanism of action of vasodilators and vasoconstrictors in resistance arteries and arterioles. Diseases such as hypertension, obesity, the metabolic syndrome, and diabetes alter the expression and function of SMC ion channels, contributing to vascular dysfunction and pathogenesis. While much has been learned about the structure, biophysics and function of the myriad of channels expressed in vascular SMCs, significant gaps remain in our understanding. Regional and species heterogeneity have made the study of ion channel function in vascular SMCs difficult because investigators cannot extrapolate between regions and systems, except in the broadest terms. Also, because we now know that ion channels exist in large macromolecular complexes, our understanding of the entire repertoire of regulatory mechanisms is hindered by lack of knowledge of all proteins with which a given channel interacts within the context of the vascular bed and physiological (or pathophysiological) status of the blood vessel in question. Trying to understand the function of an ion channel in a vascular SMCs in a resistance artery or arteriole, in vivo, is particularly challenging. Pharmacological approaches that are so useful in a patch clamp experiment, where specific currents can be readily identified, are problematic in a complex tissue because of expression of similar channels in multiple cell types as well as the complex pattern of expression of ion channels within SMCs. The use of blockers in the absence of electrophysiological characterization of the repertoire of channel currents expressed within SMCs is particularly problematic because of the lack of selectivity of most ion channel blockers (Table 2). This situation is compounded, in vivo, where multiple cell types interact and where the site of action of a drug cannot be established. The use of cell specific, conditional knockout and knockin models can help resolve some of these issues, but given the complex interactions among proteins in signaling complexes, even this approach can be a relatively blunt tool to use in vivo without detailed electrophysiological characterization of the cell in question. The bottom line is, that if you want to understand the function of a given ion channel, multiple approaches should be applied and should always include patch clamp studies characterizing the currents through the channels in question.
As pointed out repeatedly, there is considerable heterogeneity in the spectrum of ion channels expressed in vascular SMCs in different vascular beds and at different levels of the circulation within a given vascular bed. With the exception of rat mesenteric arteries, rat cerebral arteries, and mouse mesenteric arteries where there is extensive knowledge of the spectrum of ion channels expressed as well as their function, there are only bits and pieces of information available for other vessels, particularly for SMCs in arterioles in the microcirculation around the body. The microvasculature deserves more study in the future.
Acknowledgments
Supported by National Institutes of Health Grants: K01 DK103840 (N. R. Tykocki); F32 HL118836 and 1K99 HL129196 (E. M. Boerman); and PO1 HL070687 (W. F. Jackson)
References
- 1.Aalkjaer C, Nilsson H. Vasomotion: Cellular background for the oscillator and for the synchronization of smooth muscle cells. Br J Pharmacol. 2005;144:605–616. doi: 10.1038/sj.bjp.0706084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbink EJ, Wollersheim H, Netten PM, Russel FG, Lutterman JA, Smits P. Microcirculatory effects of KATP channel blockade by sulphonylurea derivatives in humans. Eur J Clin Invest. 2002;32:163–171. doi: 10.1046/j.1365-2362.2002.00964.x. [DOI] [PubMed] [Google Scholar]
- 3.Abd El-Rahman RR, Harraz OF, Brett SE, Anfinogenova Y, Mufti RE, Goldman D, Welsh DG. Identification of L- and T-type Ca2+ channels in rat cerebral arteries: Role in myogenic tone development. Am J Physiol Heart Circ Physiol. 2013;304:H58–H71. doi: 10.1152/ajpheart.00476.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Latif AA. Cross talk between cyclic nucleotides and polyphos-phoinositide hydrolysis, protein kinases, and contraction in smooth muscle. Exp Biol Med (Maywood) 2001;226:153–163. doi: 10.1177/153537020122600302. [DOI] [PubMed] [Google Scholar]
- 5.Abdi A, Mazzocco C, Legeron FP, Yvert B, Macrez N, Morel JL. TRPP2 modulates ryanodine- and inositol-1,4,5-trisphosphate receptors-dependent Ca2+ signals in opposite ways in cerebral arteries. Cell Calcium. 2015;58:467–475. doi: 10.1016/j.ceca.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Abou-Saleh H, Pathan AR, Daalis A, Hubrack S, Abou-Jassoum H, Al-Naeimi H, Rusch NJ, Machaca K. Inositol 1,4,5-trisphosphate (IP3) receptor up-regulation in hypertension is associated with sensitization of Ca2+ release and vascular smooth muscle contractility. J Biol Chem. 2013;288:32941–32951. doi: 10.1074/jbc.M113.496802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraham NG, Drummond GS, Lutton JD, Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Biochem. 1996;6:129–168. [Google Scholar]
- 8.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams DJ. Ionic channels in vascular endothelial cells. Trends Cardiovasc Med. 1994;4:18–26. doi: 10.1016/1050-1738(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 10.Adebiyi A, McNally EM, Jaggar JH. Vasodilation induced by oxygen/glucose deprivation is attenuated in cerebral arteries of SUR2 null mice. Am J Physiol Heart Circ Physiol. 2011;301:H1360–H1368. doi: 10.1152/ajpheart.00406.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adebiyi A, Narayanan D, Jaggar JH. Caveolin-1 assembles type 1 inositol 1,4,5-trisphosphate receptors and canonical transient receptor potential 3 channels into a functional signaling complex in arterial smooth muscle cells. J Biol Chem. 2011;286:4341–4348. doi: 10.1074/jbc.M110.179747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adebiyi A, Zhao G, Narayanan D, Thomas-Gatewood CM, Bannister JP, Jaggar JH. Isoform-selective physical coupling of TRPC3 channels to IP3 receptors in smooth muscle cells regulates arterial contractility. Circ Res. 2010;106:1603–1612. doi: 10.1161/CIRCRESAHA.110.216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- 14.Aghdasi B, Ye K, Resnick A, Huang A, Ha HC, Guo X, Dawson TM, Dawson VL, Snyder SH. FKBP12, the 12-kDa FK506-binding protein, is a physiologic regulator of the cell cycle. Proc Natl Acad Sci U S A. 2001;98:2425–2430. doi: 10.1073/pnas.041614198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguilar-Bryan L, Clement JPt, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- 16.Ahern GP, Hsu SF, Jackson MB. Direct actions of nitric oxide on rat neurohypophysial K+ channels. J Physiol. 1999;520(Pt 1):165–176. doi: 10.1111/j.1469-7793.1999.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad K, Lewis JJ. The effects of gallamine, carbachol, nicotine, ryanodine and protoveratrine A and B upon flux of calcium-47 in frog skeletal muscle. J Pharm Pharmacol. 1961;13:383–384. doi: 10.1111/j.2042-7158.1961.tb11841.x. [DOI] [PubMed] [Google Scholar]
- 18.Aiello EA, Clement-Chomienne O, Sontag DP, Walsh MP, Cole WC. Protein kinase C inhibits delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1996;271:H109–H119. doi: 10.1152/ajpheart.1996.271.1.H109. [DOI] [PubMed] [Google Scholar]
- 19.Aiello EA, Malcolm AT, Walsh MP, Cole WC. Beta-adrenoceptor activation and PKA regulate delayed rectifier K+ channels of vascular smooth muscle cells. Am J Physiol. 1998;275:H448–H459. doi: 10.1152/ajpheart.1998.275.2.H448. [DOI] [PubMed] [Google Scholar]
- 20.Aiello EA, Walsh MP, Cole WC. Isoproterenol and forskolin increase and PKI inhibits delayed rectifier K+ current in vascular myocytes isolated from rabbit coronary artery and portal vein. Can J Physiol Pharmacol. 1994;72:47. [Google Scholar]
- 21.Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1995;268:H926–H934. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- 22.Aihara Y, Jahromi BS, Yassari R, Nikitina E, Agbaje-Williams M, Macdonald RL. Molecular profile of vascular ion channels after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:75–83. doi: 10.1097/01.WCB.0000095803.98378.D8. [DOI] [PubMed] [Google Scholar]
- 23.Aiken SP, Zaczek R, Brown BS. Pharmacology of the neurotransmitter release enhancer linopirdine (DuP 996), and insights into its mechanism of action. Adv Pharmacol. 1996;35:349–384. doi: 10.1016/s1054-3589(08)60281-1. [DOI] [PubMed] [Google Scholar]
- 24.Akatsuka Y, Egashira K, Katsuda Y, Narishige T, Ueno H, Shimokawa H, Takeshita A. ATP sensitive potassium channels are involved in adenosine A2 receptor mediated coronary vasodilatation in the dog. Cardiovasc Res. 1994;28:906–911. doi: 10.1093/cvr/28.6.906. [DOI] [PubMed] [Google Scholar]
- 25.Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, Vasudev NS, Radtke L, Willot M, Hahn S, Seitz T, Ziegler S, Christmann M, Beech DJ, Waldmann H. (−)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl. 2015;54:3787–3791. doi: 10.1002/anie.201411511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alagem N, Dvir M, Reuveny E. Mechanism of Ba(2+) block of a mouse inwardly rectifying K+ channel: Differential contribution by two discrete residues. J Physiol. 2001;534:381–393. doi: 10.1111/j.1469-7793.2001.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albarwani S, Al-Siyabi S, Baomar H, Hassan MO. Exercise training attenuates ageing-induced BKCa channel downregulation in rat coronary arteries. Exp Physiol. 2010;95:746–755. doi: 10.1113/expphysiol.2009.051250. [DOI] [PubMed] [Google Scholar]
- 28.Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, Rusch NJ. Voltage-gated K+ channels in rat small cerebral arteries: Molecular identity of the functional channels. J Physiol. 2003;551:751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albarwani S, Robertson BE, Nye PC, Kozlowski RZ. Biophysical properties of Ca(2+)- and Mg-ATP-activated K+ channels in pulmonary arterial smooth muscle cells isolated from the rat. Pflugers Arch. 1994;428:446–454. doi: 10.1007/BF00374564. [DOI] [PubMed] [Google Scholar]
- 30.Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium. 2003;33:345–356. doi: 10.1016/s0143-4160(03)00048-4. [DOI] [PubMed] [Google Scholar]
- 31.Albert AP, Large WA. Synergism between inositol phosphates and diacylglycerol on native TRPC6-like channels in rabbit portal vein myocytes. J Physiol. 2003;552:789–795. doi: 10.1113/jphysiol.2003.052977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albert AP, Piper AS, Large WA. Role of phospholipase D and diacylglycerol in activating constitutive TRPC-like cation channels in rabbit ear artery myocytes. J Physiol. 2005;566:769–780. doi: 10.1113/jphysiol.2005.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albert AP, Saleh SN, Large WA. Inhibition of native TRPC6 channel activity by phosphatidylinositol 4,5-bisphosphate in mesenteric artery myocytes. J Physiol. 2008;586:3087–3095. doi: 10.1113/jphysiol.2008.153676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alioua A, Huggins JP, Rousseau E. PKG-I alpha phosphorylates the alpha-subunit and upregulates reconstituted GKCa channels from tracheal smooth muscle. Am J Physiol. 1995;268:L1057–L1063. doi: 10.1152/ajplung.1995.268.6.L1057. [DOI] [PubMed] [Google Scholar]
- 35.Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P, Toro L. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J Biol Chem. 1998;273:32950–32956. doi: 10.1074/jbc.273.49.32950. [DOI] [PubMed] [Google Scholar]
- 36.Allen T, Iftinca M, Cole WC, Plane F. Smooth muscle membrane potential modulates endothelium-dependent relaxation of rat basilar artery via myo-endothelial gap junctions. J Physiol. 2002;545:975–986. doi: 10.1113/jphysiol.2002.031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almassy J, Begenisich T. The LRRC26 protein selectively alters the efficacy of BK channel activators. Mol Pharmacol. 2012;81:21–30. doi: 10.1124/mol.111.075234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, Garami A, Bautista D, Gavva NR, Romanovsky AA. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32:2086–2099. doi: 10.1523/JNEUROSCI.5606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca(2+) activated K(+) channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amberg GC, Koh SD, Imaizumi Y, Ohya S, Sanders KM. A-type potassium currents in smooth muscle. Am J Physiol Cell Physiol. 2003;284:C583–595. doi: 10.1152/ajpcell.00301.2002. [DOI] [PubMed] [Google Scholar]
- 41.Amberg GC, Navedo MF, Nieves-Cintron M, Molkentin JD, Santana LF. Calcium sparklets regulate local and global calcium in murine arterial smooth muscle. J Physiol. 2007;579:187–201. doi: 10.1113/jphysiol.2006.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 regulates Kv2.1 expression in arterial smooth muscle. J Biol Chem. 2004;279:47326–47334. doi: 10.1074/jbc.M408789200. [DOI] [PubMed] [Google Scholar]
- 43.Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- 44.Amberg GC, Santana LF. Kv2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Cell Physiol. 2006;291:C348–356. doi: 10.1152/ajpcell.00086.2006. [DOI] [PubMed] [Google Scholar]
- 45.Ambroisine ML, Favre J, Oliviero P, Rodriguez C, Gao J, Thuillez C, Samuel JL, Richard V, Delcayre C. Aldosterone-induced coronary dysfunction in transgenic mice involves the calcium-activated potassium (BKCa) channels of vascular smooth muscle cells. Circulation. 2007;116:2435–2443. doi: 10.1161/CIRCULATIONAHA.107.722009. [DOI] [PubMed] [Google Scholar]
- 46.Ambudkar IS, Ong HL. Organization and function of TRPC channelosomes. Pflugers Arch. 2007;455:187–200. doi: 10.1007/s00424-007-0252-0. [DOI] [PubMed] [Google Scholar]
- 47.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay BC, Cheng KT. TRPC1: The link between functionally distinct store-operated calcium channels. Cell Calcium. 2007;42:213–223. doi: 10.1016/j.ceca.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Andersson DA, Chase HW, Bevan S. TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH. J Neurosci. 2004;24:5364–5369. doi: 10.1523/JNEUROSCI.0890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell. 2006;22:795–806. doi: 10.1016/j.molcel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 50.Ando H, Mizutani A, Matsu-ura T, Mikoshiba K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem. 2003;278:10602–10612. doi: 10.1074/jbc.M210119200. [DOI] [PubMed] [Google Scholar]
- 51.Antigny F, Jousset H, Konig S, Frieden M. Thapsigargin activates Ca(2)+ entry both by store-dependent, STIM1/Orai1-mediated, and store-independent, TRPC3/PLC/PKC-mediated pathways in human endothelial cells. Cell Calcium. 2011;49:115–127. doi: 10.1016/j.ceca.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Anyatonwu GI, Estrada M, Tian X, Somlo S, Ehrlich BE. Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc Natl Acad Sci USA. 2007;104:6454–6459. doi: 10.1073/pnas.0610324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aoki K, Asano M. Effects of Bay K 8644 and nifedipine on femoral arteries of spontaneously hypertensive rats. Br J Pharmacol. 1986;88:221–230. doi: 10.1111/j.1476-5381.1986.tb09490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arii T, Ohyanagi M, Shibuya J, Iwasaki T. Increased function of the voltage-dependent calcium channels, without increase of Ca2+ release from the sarcoplasmic reticulum in the arterioles of spontaneous hypertensive rats. Am J Hypertens. 1999;12:1236–1242. doi: 10.1016/s0895-7061(99)00159-4. [DOI] [PubMed] [Google Scholar]
- 56.Armstead WM. Opioids and nitric oxide contribute to hypoxia-induced pial arterial vasodilation in newborn pigs. Am J Physiol. 1995;268:H226–232. doi: 10.1152/ajpheart.1995.268.1.H226. [DOI] [PubMed] [Google Scholar]
- 57.Armstead WM. Role of ATP-sensitive K+ channels in cGMP-mediated pial artery vasodilation. Am J Physiol. 1996;270:H423–426. doi: 10.1152/ajpheart.1996.270.2.H423. [DOI] [PubMed] [Google Scholar]
- 58.Armstead WM. Role of activation of calcium-sensitive K+ channels and cAMP in opioid-induced pial artery dilation. Brain Res. 1997;747:252–258. doi: 10.1016/s0006-8993(96)01284-x. [DOI] [PubMed] [Google Scholar]
- 59.Armstead WM. Role of activation of calcium-sensitive K+ channels in NO- and hypoxia-induced pial artery vasodilation. Am J Physiol. 1997;272:H1785–1790. doi: 10.1152/ajpheart.1997.272.4.H1785. [DOI] [PubMed] [Google Scholar]
- 60.Armstead WM. Brain injury impairs prostaglandin cerebrovasodilation. J Neurotrauma. 1998;15:721–729. doi: 10.1089/neu.1998.15.721. [DOI] [PubMed] [Google Scholar]
- 61.Armstead WM. Superoxide generation links protein kinase C activation to impaired ATP-sensitive K+ channel function after brain injury. Stroke. 1999;30:153–159. doi: 10.1161/01.str.30.1.153. [DOI] [PubMed] [Google Scholar]
- 62.Armstead WM. Vasopressin-induced protein kinase C-dependent superoxide generation contributes to atp-sensitive potassium channel but not calcium-sensitive potassium channel function impairment after brain injury. Stroke. 2001;32:1408–1414. doi: 10.1161/01.str.32.6.1408. [DOI] [PubMed] [Google Scholar]
- 63.Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol. 2007;581:841–852. doi: 10.1113/jphysiol.2007.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arun KH, Kaul CL, Ramarao P. AT1 receptors and L-type calcium channels: Functional coupling in supersensitivity to angiotensin II in diabetic rats. Cardiovasc Res. 2005;65:374–386. doi: 10.1016/j.cardiores.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 65.Asano M, Masuzawa-Ito K, Matsuda T. Charybdotoxin-sensitive K+ channels regulate the myogenic tone in the resting state of arteries from spontaneously hypertensive rats. Br J Pharmacol. 1993;108:214–222. doi: 10.1111/j.1476-5381.1993.tb13465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asano M, Masuzawa-Ito K, Matsuda T, Suzuki Y, Oyama H, Shibuya M, Sugita K. Increased Ca2+ influx in the resting state maintains the myogenic tone and activates charybdotoxin-sensitive K+ channels in dog basilar artery. J Cereb Blood Flow Metab. 1993;13:969–977. doi: 10.1038/jcbfm.1993.121. [DOI] [PubMed] [Google Scholar]
- 67.Asano M, Matsuda T, Hayakawa M, Ito KM, Ito K. Increased resting Ca2+ maintains the myogenic tone and activates K+ channels in arteries from young spontaneously hypertensive rats. Eur J Pharmacol. 1993;247:295–304. doi: 10.1016/0922-4106(93)90198-i. [DOI] [PubMed] [Google Scholar]
- 68.Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- 69.Ashcroft SJ, Ashcroft FM. The sulfonylurea receptor. Biochim Biophys Acta. 1992;1175:45–59. doi: 10.1016/0167-4889(92)90008-y. [DOI] [PubMed] [Google Scholar]
- 70.Ashcroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21:288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- 71.Atwal KS. Advances in the structure-activity-relationships, mechanisms of action, and therapeutic utilities of Atp-sensitive potassium channel openers. Drug Dev Res. 1994;33:250–262. [Google Scholar]
- 72.Atwal KS. Pharmacology and structure-activity relationships for KATP modulators: Tissue-selective KATP openers. J Cardiovasc Pharmacol. 1994;24(Suppl 4):S12–S17. [PubMed] [Google Scholar]
- 73.Aversano T, Ouyang P, Silverman H. Blockade of the ATP-sensitive potassium channel modulates reactive hyperemia in the canine coronary circulation. Circ Res. 1991;69:618–622. doi: 10.1161/01.res.69.3.618. [DOI] [PubMed] [Google Scholar]
- 74.Aversano T, Ouyang P, Silverman H, Ziegelstein RC, Gips S. Effect of blockade of the ATP-sensitive potassium channel on metabolic coronary vasodilation in the dog. Pharmacology. 1993;47:360–368. doi: 10.1159/000139119. [DOI] [PubMed] [Google Scholar]
- 75.Aziz Q, Thomas AM, Gomes J, Ang R, Sones WR, Li Y, Ng KE, Gee L, Tinker A. The ATP-sensitive potassium channel subunit, Kir6.1, in vascular smooth muscle plays a major role in blood pressure control. Hypertension. 2014;64:523–529. doi: 10.1161/HYPERTENSIONAHA.114.03116. [DOI] [PubMed] [Google Scholar]
- 76.Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- 77.Bache RJ, Quanbeck D, Homans DC, Dai XZ. Effects of nifedipine on coronary reactive and exercise induced hyperaemia. Cardiovasc Res. 1987;21:766–771. doi: 10.1093/cvr/21.10.766. [DOI] [PubMed] [Google Scholar]
- 78.Bae YM, Kim A, Kim J, Park SW, Kim TK, Lee YR, Kim B, Cho SI. Serotonin depolarizes the membrane potential in rat mesenteric artery myocytes by decreasing voltage-gated K+ currents. Biochem Biophys Res Commun. 2006;347:468–476. doi: 10.1016/j.bbrc.2006.06.116. [DOI] [PubMed] [Google Scholar]
- 79.Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc Res. 1973;5:384–394. doi: 10.1016/0026-2862(73)90054-x. [DOI] [PubMed] [Google Scholar]
- 80.Bai Y, Sanderson MJ. Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a cAMP-mediated inhibition of the IP3 receptor. Respir Res. 2006;7:34. doi: 10.1186/1465-9921-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balasubramanian L, Ahmed A, Lo CM, Sham JS, Yip KP. Integrin-mediated mechanotransduction in renal vascular smooth muscle cells: Activation of calcium sparks. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1586–R1594. doi: 10.1152/ajpregu.00025.2007. [DOI] [PubMed] [Google Scholar]
- 82.Baldwin TJ, Isacoff E, Li M, Lopez GA, Sheng M, Tsaur ML, Yan YN, Jan LY. Elucidation of biophysical and biological properties of voltage-gated potassium channels. Cold Spring Harb Symp Quant Biol. 1992;57:491–499. doi: 10.1101/sqb.1992.057.01.054. [DOI] [PubMed] [Google Scholar]
- 83.Bang S, Kim KY, Yoo S, Lee SH, Hwang SW. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett. 2007;425:120–125. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 84.Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br J Pharmacol. 2012;165:683–692. doi: 10.1111/j.1476-5381.2011.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banitt PF, Smits P, Williams SB, Ganz P, Creager MA. Activation of ATP-sensitive potassium channels contributes to reactive hyperemia in humans. Am J Physiol. 1996;271:H1594–H1598. doi: 10.1152/ajpheart.1996.271.4.H1594. [DOI] [PubMed] [Google Scholar]
- 86.Bank AJ, Sih R, Mullen K, Osayamwen M, Lee PC. Vascular ATP-dependent potassium channels, nitric oxide, and human forearm reactive hyperemia. Cardiovasc Drugs Ther. 2000;14:23–29. doi: 10.1023/a:1007835003493. [DOI] [PubMed] [Google Scholar]
- 87.Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. Smooth muscle cell alpha2delta-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res. 2009;105:948–955. doi: 10.1161/CIRCRESAHA.109.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bannister JP, Bulley S, Narayanan D, Thomas-Gatewood C, Luzny P, Pachuau J, Jaggar JH. Transcriptional upregulation of alpha2delta-1 elevates arterial smooth muscle cell voltage-dependent Ca2+ channel surface expression and cerebrovascular constriction in genetic hypertension. Hypertension. 2012;60:1006–1015. doi: 10.1161/HYPERTENSIONAHA.112.199661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bannister JP, Leo MD, Narayanan D, Jangsangthong W, Nair A, Evanson KW, Pachuau J, Gabrick KS, Boop FA, Jaggar JH. The voltage-dependent L-type Ca2+ (CaV1.2) channel C-terminus fragment is a bi-modal vasodilator. J Physiol. 2013;591:2987–2998. doi: 10.1113/jphysiol.2013.251926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bari F, Louis TM, Busija DW. Calcium-activated K+ channels in cerebral arterioles in piglets are resistant to ischemia. J Cereb Blood Flow Metab. 1997;17:1152–1156. doi: 10.1097/00004647-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 91.Bari F, Louis TM, Meng W, Busija DW. Global ischemia impairs ATP-sensitive K+ channel function in cerebral arterioles in piglets. Stroke. 1996;27:1874–1880. doi: 10.1161/01.str.27.10.1874. discussion 1880-1871. [DOI] [PubMed] [Google Scholar]
- 92.Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol. 1998;275:H1283–H1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- 93.Bartlett IS, Crane GJ, Neild TO, Segal SS. Electrophysiological basis of arteriolar vasomotion in vivo. J Vasc Res. 2000;37:568–575. doi: 10.1159/000054090. [DOI] [PubMed] [Google Scholar]
- 94.Bastide M, Bordet R, Pu Q, Robin E, Puisieux F, Dupuis B. Relationship between inward rectifier potassium current impairment and brain injury after cerebral ischemia/reperfusion. J Cereb Blood Flow Metab. 1999;19:1309–1315. doi: 10.1097/00004647-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 95.Bastide M, Gele P, Petrault O, Pu Q, Caliez A, Robin E, Deplanque D, Duriez P, Bordet R. Delayed cerebrovascular protective effect of lipopolysaccharide in parallel to brain ischemic tolerance. J Cereb Blood Flow Metab. 2003;23:399–405. doi: 10.1097/01.WCB.0000050064.57184.F2. [DOI] [PubMed] [Google Scholar]
- 96.Bauman AL, Michel JJ, Henson E, Dodge-Kafka KL, Kapiloff MS. The mAKAP signalosome and cardiac myocyte hypertrophy. IUBMB Life. 2007;59:163–169. doi: 10.1080/15216540701358593. [DOI] [PubMed] [Google Scholar]
- 97.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 98.Bayer KU, Harbers K, Schulman H. alphaKAP is an anchoring protein for a novel CaM kinase II isoform in skeletal muscle. Embo J. 1998;17:5598–5605. doi: 10.1093/emboj/17.19.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baylie RL, Brayden JE. TRPV channels and vascular function. Acta Physiol (Oxf) 2011;203:99–116. doi: 10.1111/j.1748-1716.2010.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bean BP. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985;86:1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bean BP. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- 102.Bean BP, Sturek M, Puga A, Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: Modulation by dihydropyridine drugs. Circ Res. 1986;59:229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- 103.Beech DJ. Ion channel switching and activation in smooth-muscle cells of occlusive vascular diseases. Biochem Soc Trans. 2007;35:890–894. doi: 10.1042/BST0350890. [DOI] [PubMed] [Google Scholar]
- 104.Beech DJ, Bolton TB. The effects of tetraethylammonium ions, 4-aminopyridine or quinidine on K+-currents in single smooth muscle cells of the rabbit portal vein. Biomed Biochim Acta. 1987;46:S673–S676. [PubMed] [Google Scholar]
- 105.Beech DJ, Bolton TB. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beech DJ, Bolton TB. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J Physiol. 1989;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Belardinelli L, Harder D, Sperelakis N, Rubio R, Berne RM. Cardiac glycoside stimulation of inward Ca++ current in vascular smooth muscle of canine coronary artery. J Pharmacol Exp Ther. 1979;209:62–66. [PubMed] [Google Scholar]
- 109.Beleznai T, Takano H, Hamill C, Yarova P, Douglas G, Channon K, Dora K. Enhanced K(+)-channel-mediated endothelium-dependent local and conducted dilation of small mesenteric arteries from ApoE(−/−) mice. Cardiovasc Res. 2011;92:199–208. doi: 10.1093/cvr181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Benham CD, Bolton TB. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Benham CD, Bolton TB, Lang RJ, Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell Calcium. 2003;33:479–487. doi: 10.1016/s0143-4160(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 113.Benham CD, Hess P, Tsien RW. Two types of calcium channels in single smooth muscle cells from rabbit ear artery studied with whole-cell and single-channel recordings. Circ Res. 1987;61:I10–16. [PubMed] [Google Scholar]
- 114.Benham CD, Tsien RW. Noradrenaline modulation of calcium channels in single smooth muscle cells from rabbit ear artery. J Physiol. 1988;404:767–784. doi: 10.1113/jphysiol.1988.sp017318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Benkusky NA, Farrell EF, Valdivia HH. Ryanodine receptor channelopathies. Biochem Biophys Res Commun. 2004;322:1280–1285. doi: 10.1016/j.bbrc.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 116.Berczi V, Stekiel WJ, Contney SJ, Rusch NJ. Pressure-induced activation of membrane K+ current in rat saphenous artery. Hypertension. 1992;19:725–729. doi: 10.1161/01.hyp.19.6.725. [DOI] [PubMed] [Google Scholar]
- 117.Berg T. The vascular response to the K+ channel inhibitor 4-aminopyridine in hypertensive rats. Eur J Pharmacol. 2003;466:301–310. doi: 10.1016/s0014-2999(03)01555-3. [DOI] [PubMed] [Google Scholar]
- 118.Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res. 2003;93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- 119.Berger MG, Vandier C, Bonnet P, Jackson WF, Rusch NJ. Intracellular acidosis differentially regulates KV channels in coronary and pulmonary vascular muscle. Am J Physiol. 1998;275:H1351–H1359. doi: 10.1152/ajpheart.1998.275.4.H1351. [DOI] [PubMed] [Google Scholar]
- 120.Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- 121.Bernier S, Guillemette G. Increased inositol 1,4,5-trisphosphate binding capacity in vascular smooth muscle of spontaneously hypertensive rats. Am J Hypertens. 1993;6:217–225. [PubMed] [Google Scholar]
- 122.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 123.Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499(Pt 2):291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 125.Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K(+) channels to metabolic control of coronary blood flow. J Mol Cell Cardiol. 2012;52:912–919. doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berwick ZC, Dick GM, O’Leary HA, Bender SB, Goodwill AG, Moberly SP, Owen MK, Miller SJ, Obukhov AG, Tune JD. Contribution of electromechanical coupling between Kv and Ca v1.2 channels to coronary dysfunction in obesity. Basic Res Cardiol. 2013;108:370. doi: 10.1007/s00395-013-0370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berwick ZC, Moberly SP, Kohr MC, Morrical EB, Kurian MM, Dick GM, Tune JD. Contribution of voltage-dependent K+ and Ca2+ channels to coronary pressure-flow autoregulation. Basic Res Cardiol. 2012;107:264. doi: 10.1007/s00395-012-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Berwick ZC, Payne GA, Lynch B, Dick GM, Sturek M, Tune JD. Contribution of adenosine A(2A) and A(2B) receptors to ischemic coronary dilation: Role of K(V) and K(ATP) channels. Microcirculation. 2010;17:600–607. doi: 10.1111/j.1549-8719.2010.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bett GC, Morales MJ, Beahm DL, Duffey ME, Rasmusson RL. Ancillary subunits and stimulation frequency determine the potency of chromanol 293B block of the KCNQ1 potassium channel. J Physiol. 2006;576:755–767. doi: 10.1113/jphysiol.2006.116012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Betts LC, Kozlowski RZ. Electrophysiological effects of endothelin-1 and their relationship to contraction in rat renal arterial smooth muscle. Br J Pharmacol. 2000;130:787–796. doi: 10.1038/sj.bjp.0703377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Betzenhauser MJ, Yule DI. Chapter 12: Regulation of inositol 1,4,5-trisphosphate receptors by phosphorylation and adenine nucleotides. In: Serysheva I, editor. Curr Top Membr. Burlington, MA: Academic Press; 2010. pp. 273–298. [DOI] [PubMed] [Google Scholar]
- 133.Bevan JA. Selective action of diltiazem on cerebral vascular smooth muscle in the rabbit: Antagonism of extrinsic but not intrinsic maintained tone. Am J Cardiol. 1982;49:519–524. doi: 10.1016/s0002-9149(82)80005-2. [DOI] [PubMed] [Google Scholar]
- 134.Bevan S, Quallo T, Andersson DA. Trpv1. Handb Exp Pharmacol. 2014;222:207–245. doi: 10.1007/978-3-642-54215-2_9. [DOI] [PubMed] [Google Scholar]
- 135.Bi D, Toyama K, Lemaitre V, Takai J, Fan F, Jenkins DP, Wulff H, Gutterman DD, Park F, Miura H. The intermediate conductance calcium-activated potassium channel KCa3.1 regulates vascular smooth muscle cell proliferation via controlling calcium-dependent signaling. J Biol Chem. 2013;288:15843–15853. doi: 10.1074/jbc.M112.427187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bialecki RA, Stinson-Fisher C. KCa channel antagonists reduce NO donor-mediated relaxation of vascular and tracheal smooth muscle. Am J Physiol. 1995;268:L152–L159. doi: 10.1152/ajplung.1995.268.1.L152. [DOI] [PubMed] [Google Scholar]
- 137.Bijlstra PJ, den Arend JA, Lutterman JA, Russel FG, Thien T, Smits P. Blockade of vascular ATP-sensitive potassium channels reduces the vasodilator response to ischaemia in humans. Diabetologia. 1996;39:1562–1568. doi: 10.1007/s001250050615. [DOI] [PubMed] [Google Scholar]
- 138.Bilek I, Laven R, Peiper U, Regnat K. The effect of verapamil on the response to noradrenaline or to potassium-depolarization in isolated vascular strips. Microvasc Res. 1974;7:181–189. doi: 10.1016/0026-2862(74)90004-1. [DOI] [PubMed] [Google Scholar]
- 139.Billaud M, Lohman AW, Johnstone SR, Biwer LA, Mutchler S, Isakson BE. Regulation of cellular communication by signaling microdomains in the blood vessel wall. Pharmacol Rev. 2014;66:513–569. doi: 10.1124/pr.112.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bjorling K, Morita H, Olsen MF, Prodan A, Hansen PB, Lory P, Holstein-Rathlou NH, Jensen LJ. Myogenic tone is impaired at low arterial pressure in mice deficient in the low-voltage-activated CaV 3.1 T-type Ca(2+) channel. Acta Physiol (Oxf) 2013;207:709–720. doi: 10.1111/apha.12066. [DOI] [PubMed] [Google Scholar]
- 141.Blanco-Rivero J, Gamallo C, Aras-Lopez R, Cobeno L, Cogolludo A, Perez-Vizcaino F, Ferrer M, Balfagon G. Decreased expression of aortic KIR6.1 and SUR2B in hypertension does not correlate with changes in the functional role of K(ATP) channels. Eur J Pharmacol. 2008;587:204–208. doi: 10.1016/j.ejphar.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 142.Blatter LA, Wier WG. Agonist-induced [Ca2+]i waves and Ca(2+)-induced Ca2+ release in mammalian vascular smooth muscle cells. Am J Physiol. 1992;263:H576–H586. doi: 10.1152/ajpheart.1992.263.2.H576. [DOI] [PubMed] [Google Scholar]
- 143.Blum CA, Caldwell T, Zheng X, Bakthavatchalam R, Capitosti S, Brielmann H, De Lombaert S, Kershaw MT, Matson D, Krause JE, Cortright D, Crandall M, Martin WJ, Murphy BA, Boyce S, Jones AB, Mason G, Rycroft W, Perrett H, Conley R, Burnaby-Davies N, Chenard BL, Hodgetts KJ. Discovery of novel 6,6-heterocycles as transient receptor potential vanilloid (TRPV1) antagonists. J Med Chem. 2010;53:3330–3348. doi: 10.1021/jm100051g. [DOI] [PubMed] [Google Scholar]
- 144.Blum JJ, Creese R, Jenden DJ, Scholes NW. The mechanism of action of ryanodine on skeletal muscle. J Pharmacol Exp Ther. 1957;121:477–486. [PubMed] [Google Scholar]
- 145.Boehning DF. Chapter 9: Molecular architecture of the inositol 1,4,5-trisphosphate receptor pore. In: Serysheva I, editor. Curr Top Membr. Burlington, MA: Academic Press; 2010. pp. 191–207. [DOI] [PubMed] [Google Scholar]
- 146.Bognar IT, Enero MA. Influence of a receptor reserve on the inhibition by calcium channel blockers of alpha adrenoceptor-mediated responses in rat isolated vascular tissues. J Pharmacol Exp Ther. 1988;245:673–681. [PubMed] [Google Scholar]
- 147.Bohlen HG, Gore RW. Preparation of rat intestinal muscle and mucosa for quantitative microcirculatory studies. Microvasc Res. 1976;11:103–110. doi: 10.1016/0026-2862(76)90081-9. [DOI] [PubMed] [Google Scholar]
- 148.Boittin FX, Dipp M, Kinnear NP, Galione A, Evans AM. Vasodilation by the calcium-mobilizing messenger cyclic ADP-ribose. J Biol Chem. 2003;278:9602–9608. doi: 10.1074/jbc.M204891200. [DOI] [PubMed] [Google Scholar]
- 149.Boittin FX, Macrez N, Halet G, Mironneau J. Norepinephrine-induced Ca(2+) waves depend on InsP(3) and ryanodine receptor activation in vascular myocytes. Am J Physiol. 1999;277:C139–C151. doi: 10.1152/ajpcell.1999.277.1.C139. [DOI] [PubMed] [Google Scholar]
- 150.Bolotina VM. Orai, STIM1 and iPLA2beta: A view from a different perspective. J Physiol. 2008;586:3035–3042. doi: 10.1113/jphysiol.2008.154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 152.Bolton TB, Prestwich SA, Zholos AV, Gordienko DV. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu Rev Physiol. 1999;61:85–115. doi: 10.1146/annurev.physiol.61.1.85. [DOI] [PubMed] [Google Scholar]
- 153.Bonev AD, Jaggar JH, Rubart M, Nelson MT. Activators of protein kinase C decrease Ca2+ spark frequency in smooth muscle cells from cerebral arteries. Am J Physiol. 1997;273:C2090–C2095. doi: 10.1152/ajpcell.1997.273.6.C2090. [DOI] [PubMed] [Google Scholar]
- 154.Bonev AD, Nelson MT. ATP-sensitive potassium channels in smooth muscle cells from guinea pig urinary bladder. Am J Physiol. 1993;264:C1190–C1200. doi: 10.1152/ajpcell.1993.264.5.C1190. [DOI] [PubMed] [Google Scholar]
- 155.Bonev AD, Nelson MT. Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. J Gen Physiol. 1996;108:315–323. doi: 10.1085/jgp.108.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 157.Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, Neeb ZP, Bratz IN, Sturek M, Tune JD. Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H1629–H1637. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Borbouse L, Dick GM, Payne GA, Payne BD, Svendsen MC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. Contribution of BK(Ca) channels to local metabolic coronary vasodilation: Effects of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2010;298:H966–H973. doi: 10.1152/ajpheart.00876.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boric MP, Donoso V, Fournier A, St Pierre S, Huidobro-Toro JP. Endothelin reduces microvascular blood flow by acting on arterioles and venules of the hamster cheek pouch. Eur J Pharmacol. 1990;190:123–133. doi: 10.1016/0014-2999(90)94119-i. [DOI] [PubMed] [Google Scholar]
- 160.Borisova L, Wray S, Eisner DA, Burdyga T. How structure, Ca signals, and cellular communications underlie function in precapillary arterioles. Circ Res. 2009;105:803–810. doi: 10.1161/CIRCRESAHA.109.202960. [DOI] [PubMed] [Google Scholar]
- 161.Bouchard JF, Dumont E, Lamontagne D. Evidence that prostaglandins I2 E2 and D2 may activate ATP sensitive potassium channels in the isolated rat heart. Cardiovasc Res. 1994;28:901–905. doi: 10.1093/cvr/28.6.901. [DOI] [PubMed] [Google Scholar]
- 162.Bouchard JF, Dumont EC, Lamontagne D. Modification of vasodilator response in streptozotocin-induced diabetic rat. Can J Physiol Pharmacol. 1999;77:980–985. [PubMed] [Google Scholar]
- 163.Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Modulation of Ca(2+) entry by polypeptides of the inositol 1,4, 5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): Evidence for roles of TRP and IP3R in store depletion-activated Ca(2+) entry. Proc Natl Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bouron A, Kiselyov K, Oberwinkler J. Permeation, regulation and control of expression of TRP channels by trace metal ions. Pflugers Arch. 2015;467:1143–1164. doi: 10.1007/s00424-014-1590-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bowles DK, Heaps CL, Turk JR, Maddali KK, Price EM. Hypercholesterolemia inhibits L-type calcium current in coronary macro-, not microcirculation. J Appl Physiol (1985) 2004;96:2240–2248. doi: 10.1152/japplphysiol.01229.2003. [DOI] [PubMed] [Google Scholar]
- 166.Boyd AE, III, Aguilar-Bryan L, Nelson DA. Molecular mechanisms of action of glyburide on the beta cell. Am J Med. 1990;89:3S–10S. doi: 10.1016/0002-9343(90)90330-g. discussion 51S–53S. [DOI] [PubMed] [Google Scholar]
- 167.Bradley KN, Currie S, MacMillan D, Muir TC, McCarron JG. Cyclic ADP-ribose increases Ca2+ removal in smooth muscle. J Cell Sci. 2003;116:4291–4306. doi: 10.1242/jcs.00713. [DOI] [PubMed] [Google Scholar]
- 168.Bradley KK, Jaggar JH, Bonev AD, Heppner TJ, Flynn ER, Nelson MT, Horowitz B. Kir2.1 encodes the inward rectifier potassium channel in rat arterial smooth muscle cells. J Physiol. 1999;515(Pt 3):639–651. doi: 10.1111/j.1469-7793.1999.639ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brandt L, Ljunggren B, Andersson KE, Edvinsson L, MacKenzie E, Tamura A, Teasdale G. Effects of topical application of a calcium antagonist (nifedipine) on feline cortical pial microvasculature under normal conditions and in focal ischemia. J Cereb Blood Flow Metab. 1983;3:44–50. doi: 10.1038/jcbfm.1983.5. [DOI] [PubMed] [Google Scholar]
- 170.Bratz IN, Dick GM, Partridge LD, Kanagy NL. Reduced molecular expression of K(+) channel proteins in vascular smooth muscle from rats made hypertensive with N{omega}-nitro-L-arginine. Am J Physiol Heart Circ Physiol. 2005;289:H1277–H1283. doi: 10.1152/ajpheart.01052.2004. [DOI] [PubMed] [Google Scholar]
- 171.Bratz IN, Swafford AN, Jr, Kanagy NL, Dick GM. Reduced functional expression of K(+) channels in vascular smooth muscle cells from rats made hypertensive with N{omega}-nitro-L-arginine. Am J Physiol Heart Circ Physiol. 2005;289:H1284–H1290. doi: 10.1152/ajpheart.01053.2004. [DOI] [PubMed] [Google Scholar]
- 172.Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci. 2006;26:4835–4840. doi: 10.1523/JNEUROSCI.5080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Braunstein TH, Inoue R, Cribbs L, Oike M, Ito Y, Holstein-Rathlou NH, Jensen LJ. The role of L- and T-type calcium channels in local and remote calcium responses in rat mesenteric terminal arterioles. J Vasc Res. 2009;46:138–151. doi: 10.1159/000151767. [DOI] [PubMed] [Google Scholar]
- 174.Brayden JE. Membrane hyperpolarization is a mechanism of endothelium-dependent cerebral vasodilation. Am J Physiol. 1990;259:H668–H673. doi: 10.1152/ajpheart.1990.259.3.H668. [DOI] [PubMed] [Google Scholar]
- 175.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 176.Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 177.Brochet DX, Langton PD. Dual effect of initial [K] on vascular tone in rat mesenteric arteries. Pflugers Arch. 2006;453:33–41. doi: 10.1007/s00424-006-0106-1. [DOI] [PubMed] [Google Scholar]
- 178.Bruch L, Bychkov R, Kastner A, Bulow T, Ried C, Gollasch M, Baumann G, Luft FC, Haller H. Pituitary adenylate-cyclase-activating peptides relax human coronary arteries by activating K(ATP) and K(Ca) channels in smooth muscle cells. J Vasc Res. 1997;34:11–18. doi: 10.1159/000159197. [DOI] [PubMed] [Google Scholar]
- 179.Brueggemann LI, Haick JM, Cribbs LL, Byron KL. Differential activation of vascular smooth muscle Kv7.4, Kv7.5, and Kv7.4/7.5 channels by ML213 and ICA-069673. Mol Pharmacol. 2014;86:330–341. doi: 10.1124/mol.114.093799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bryan J, Aguilar-Bryan L. The ABCs of ATP-sensitive potassium channels: More pieces of the puzzle. Curr Opin Cell Biol. 1997;9:553–559. doi: 10.1016/s0955-0674(97)80033-6. [DOI] [PubMed] [Google Scholar]
- 181.Bubolz AH, Li H, Wu Q, Liu Y. Enhanced oxidative stress impairs cAMP-mediated dilation by reducing Kv channel function in small coronary arteries of diabetic rats. Am J Physiol Heart Circ Physiol. 2005;289:H1873–H1880. doi: 10.1152/ajpheart.00357.2005. [DOI] [PubMed] [Google Scholar]
- 182.Buckley JF, Singer M, Clapp LH. Role of KATP channels in sepsis. Cardiovasc Res. 2006;72:220–230. doi: 10.1016/j.cardiores.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 183.Bukiya AN, Vaithianathan T, Kuntamallappanavar G, Asuncion-Chin M, Dopico AM. Smooth muscle cholesterol enables BK beta1 subunit-mediated channel inhibition and subsequent vasoconstriction evoked by alcohol. Arterioscler Thromb Vasc Biol. 2011;31:2410–2423. doi: 10.1161/ATVBAHA.111.233965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bulley S, Neeb ZP, Burris SK, Bannister JP, Thomas-Gatewood CM, Jangsangthong W, Jaggar JH. TMEM16A/ANO1 channels contribute to the myogenic response in cerebral arteries. Circ Res. 2012;111:1027–1036. doi: 10.1161/CIRCRESAHA.112.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation. 2004;11:279–293. doi: 10.1080/10739680490425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: Bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 187.Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- 188.Buxton IL, Kaiser RA, Malmquist NA, Tichenor S. NO-induced relaxation of labouring and non-labouring human myometrium is not mediated by cyclic GMP. Br J Pharmacol. 2001;134:206–214. doi: 10.1038/sj.bjp.0704226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bychkov R, Gollasch M, Ried C, Luft FC, Haller H. Regulation of spontaneous transient outward potassium currents in human coronary arteries. Circulation. 1997;95:503–510. doi: 10.1161/01.cir.95.2.503. [DOI] [PubMed] [Google Scholar]
- 190.Bychkov R, Gollasch M, Steinke T, Ried C, Luft FC, Haller H. Calcium-activated potassium channels and nitrate-induced vasodilation in human coronary arteries. J Pharmacol Exp Ther. 1998;285:293–298. [PubMed] [Google Scholar]
- 191.Cabell F, Weiss DS, Price JM. Inhibition of adenosine-induced coronary vasodilation by block of large-conductance Ca(2+)-activated K+ channels. Am J Physiol. 1994;267:H1455–H1460. doi: 10.1152/ajpheart.1994.267.4.H1455. [DOI] [PubMed] [Google Scholar]
- 192.Callaghan B, Koh SD, Keef KD. Muscarinic M2 receptor stimulation of Cav1.2b requires phosphatidylinositol 3-kinase, protein kinase C, c-Src. Circ Res. 2004;94:626–633. doi: 10.1161/01.RES.0000118248.17466.B7. [DOI] [PubMed] [Google Scholar]
- 193.Callera GE, Yogi A, Tostes RC, Rossoni LV, Bendhack LM. Ca2+-activated K+ channels underlying the impaired acetylcholine-induced vasodilation in 2K-1C hypertensive rats. J Pharmacol Exp Ther. 2004;309:1036–1042. doi: 10.1124/jpet.103.062810. [DOI] [PubMed] [Google Scholar]
- 194.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 195.Campiglio M, Flucher BE. The role of auxiliary subunits for the functional diversity of voltage-gated calcium channels. J Cell Physiol. 2015;230:2019–2031. doi: 10.1002/jcp.24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cankar K, Strucl M. The effect of glibenclamide on cutaneous laser-Doppler flux. Microvasc Res. 2008;75:97–103. doi: 10.1016/j.mvr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 197.Cannell MB, Cheng H, Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268:1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- 198.Carl A, Lee HK, Sanders KM. Regulation of ion channels in smooth muscles by calcium. Am J Physiol. 1996;271:C9–C34. doi: 10.1152/ajpcell.1996.271.1.C9. [DOI] [PubMed] [Google Scholar]
- 199.Carnevale D, Vecchione C, Mascio G, Esposito G, Cifelli G, Martinello K, Landolfi A, Selvetella G, Grieco P, Damato A, Franco E, Haase H, Maffei A, Ciraolo E, Fucile S, Frati G, Mazzoni O, Hirsch E, Lembo G. PI3Kgamma inhibition reduces blood pressure by a vasorelaxant Akt/L-type calcium channel mechanism. Cardiovasc Res. 2012;93:200–209. doi: 10.1093/cvr/cvr288. [DOI] [PubMed] [Google Scholar]
- 200.Caron AZ, Chaloux B, Arguin G, Guillemette G. Protein kinase C decreases the apparent affinity of the inositol 1,4,5-trisphosphate receptor type 3 in RINm5F cells. Cell Calcium. 2007;42:323–331. doi: 10.1016/j.ceca.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 201.Carroll MA, Doumad AB, Li J, Cheng MK, Falck JR, McGiff JC. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol. 2006;291:F155–F161. doi: 10.1152/ajprenal.00231.2005. [DOI] [PubMed] [Google Scholar]
- 202.Casalini ED, Goodwill AG, Owen MK, Moberly SP, Berwick ZC, Tune JD. Contribution of hydrogen sulfide to the control of coronary blood flow. Microcirculation. 2014;21:104–111. doi: 10.1111/micc.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cason BA, Shubayev I, Hickey RF. Blockade of adenosine triphosphate-sensitive potassium channels eliminates isoflurane-induced coronary artery vasodilation. Anesthesiology. 1994;81:1245–1255. doi: 10.1097/00000542-199411000-00019. discussion 1227A–1228A. [DOI] [PubMed] [Google Scholar]
- 204.Catalucci D, Zhang DH, DeSantiago J, Aimond F, Barbara G, Chemin J, Bonci D, Picht E, Rusconi F, Dalton ND, Peterson KL, Richard S, Bers DM, Brown JH, Condorelli G. Akt regulates L-type Ca2+ channel activity by modulating Cavalpha1 protein stability. J Cell Biol. 2009;184:923–933. doi: 10.1083/jcb.200805063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 206.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 207.Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 208.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 209.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Catterall WA, Striessnig J, Snutch TP, Perez-Reyes E International Union of P. International Union of Pharmacology. XL. Compendium of voltage-gated ion channels: Calcium channels. Pharmacol Rev. 2003;55:579–581. doi: 10.1124/pr.55.4.8. [DOI] [PubMed] [Google Scholar]
- 211.Cauvin C, Tejerina M, Hwang O, Kai-Yamamoto M, van Breemen C. The effects of Ca2+ antagonists on isolated rat and rabbit mesenteric resistance vessels. What determines the sensitivity of agonist-activated vessels to Ca2+ antagonists? Ann NY Acad Sci. 1988;522:338–350. doi: 10.1111/j.1749-6632.1988.tb33375.x. [DOI] [PubMed] [Google Scholar]
- 212.Cauvin C, Weir SW, Wallnofer A, Ruegg U. Agonist-induced activation of rat mesenteric resistance vessels: Comparison between noradrenaline and vasopressin. J Cardiovasc Pharmacol. 1988;12(Suppl 5):S128–S133. [PubMed] [Google Scholar]
- 213.Cauwels A, Brouckaert P. Critical role for small and large conductance calcium-dependent potassium channels in endotoxemia and TNF toxicity. Shock. 2008;29:577–582. doi: 10.1097/shk.0b013e31815071e9. [DOI] [PubMed] [Google Scholar]
- 214.Chadha PS, Jepps TA, Carr G, Stott JB, Zhu HL, Cole WC, Greenwood IA. Contribution of kv7.4/kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler Thromb Vasc Biol. 2014;34:887–893. doi: 10.1161/ATVBAHA.114.303405. [DOI] [PubMed] [Google Scholar]
- 215.Chadha PS, Zunke F, Davis AJ, Jepps TA, Linders JT, Schwake M, Towart R, Greenwood IA. Pharmacological dissection of K(v)7.1 channels in systemic and pulmonary arteries. Br J Pharmacol. 2012;166:1377–1387. doi: 10.1111/j.1476-5381.2012.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chadha PS, Zunke F, Zhu HL, Davis AJ, Jepps TA, Olesen SP, Cole WC, Moffatt JD, Greenwood IA. Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired beta-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertension. 2012;59:877–884. doi: 10.1161/HYPERTENSIONAHA.111.187427. [DOI] [PubMed] [Google Scholar]
- 217.Chai Q, Liu Z, Chen L. Effects of streptozotocin-induced diabetes on Kv channels in rat small coronary smooth muscle cells. Chin J Physiol. 2005;48:57–63. [PubMed] [Google Scholar]
- 218.Chai Q, Xu X, Jia Q, Dong Q, Liu Z, Zhang W, Chen L. Molecular basis of dysfunctional Kv channels in small coronary artery smooth muscle cells of streptozotocin-induced diabetic rats. Chin J Physiol. 2007;50:171–177. [PubMed] [Google Scholar]
- 219.Challinor-Rogers JL, McPherson GA. Potassium channel openers and other regulators of KATP channels. Clin Exp Pharmacol Physiol. 1994;21:583–597. doi: 10.1111/j.1440-1681.1994.tb02559.x. [DOI] [PubMed] [Google Scholar]
- 220.Champion HC, Kadowitz PJ. Vasodilator responses to acetylcholine, bradykinin, and substance P are mediated by a TEA-sensitive mechanism. Am J Physiol. 1997;273:R414–R422. doi: 10.1152/ajpregu.1997.273.1.R414. [DOI] [PubMed] [Google Scholar]
- 221.Chao JT, Gui P, Zamponi GW, Davis GE, Davis MJ. Spatial association of the Cav1.2 calcium channel with alpha5beta1-integrin. Am J Physiol Cell Physiol. 2011;300:C477–C489. doi: 10.1152/ajpcell.00171.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cheang WS, Wong WT, Shen B, Lau CW, Tian XY, Tsang SY, Yao X, Chen ZY, Huang Y. 4-aminopyridine-sensitive K+ channels contributes to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery. Vascul Pharmacol. 2010;53:94–98. doi: 10.1016/j.vph.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 224.Chelu MG, Danila CI, Gilman CP, Hamilton SL. Regulation of ryanodine receptors by FK506 binding proteins. Trends Cardiovasc Med. 2004;14:227–234. doi: 10.1016/j.tcm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 225.Chen G, Cheung DW. Effect of K(+)-channel blockers on ACh-induced hyperpolarization and relaxation in mesenteric arteries. Am J Physiol. 1997;272:H2306–H2312. doi: 10.1152/ajpheart.1997.272.5.H2306. [DOI] [PubMed] [Google Scholar]
- 226.Chen J, Crossland RF, Noorani MM, Marrelli SP. Inhibition of TRPC1/TRPC3 by PKG contributes to NO-mediated vasorelaxation. Am J Physiol Heart Circ Physiol. 2009;297:H417–H424. doi: 10.1152/ajpheart.01130.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, Segreti JA, Han P, Zhang XF, Niforatos W, Bianchi BR, Baker SJ, Zhong C, Simler GH, McDonald HA, Schmidt RG, McGaraughty SP, Chu KL, Faltynek CR, Kort ME, Reilly RM, Kym PR. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain. 2011;152:1165–1172. doi: 10.1016/j.pain.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 228.Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science. 2003;302:1416–1418. doi: 10.1126/science.1089268. [DOI] [PubMed] [Google Scholar]
- 229.Chen SR, Li X, Ebisawa K, Zhang L. Functional characterization of the recombinant type 3 Ca2+ release channel (ryanodine receptor) expressed in HEK293 cells. J Biol Chem. 1997;272:24234–24246. doi: 10.1074/jbc.272.39.24234. [DOI] [PubMed] [Google Scholar]
- 230.Chen M, Li J, Zhang F, Liu Z. Isolation and characterization of SsmTx-I, a Specific Kv2.1 blocker from the venom of the centipede Scolopendra Subspinipes Mutilans L Koch. J Pept Sci. 2014;20:159–164. doi: 10.1002/psc.2588. [DOI] [PubMed] [Google Scholar]
- 231.Chen TT, Luykenaar KD, Walsh EJ, Walsh MP, Cole WC. Key role of Kv1 channels in vasoregulation. Circ Res. 2006;99:53–60. doi: 10.1161/01.RES.0000229654.45090.57. [DOI] [PubMed] [Google Scholar]
- 232.Chen SR, MacLennan DH. Identification of calmodulin-, Ca(2+)-, and ruthenium red-binding domains in the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1994;269:22698–22704. [PubMed] [Google Scholar]
- 233.Chen W, Wang R, Chen B, Zhong X, Kong H, Bai Y, Zhou Q, Xie C, Zhang J, Guo A, Tian X, Jones PP, O’Mara ML, Liu Y, Mi T, Zhang L, Bolstad J, Semeniuk L, Cheng H, Zhang J, Chen J, Tieleman DP, Gillis AM, Duff HJ, Fill M, Song LS, Chen SR. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat Med. 2014;20:184–192. doi: 10.1038/nm.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chen YF, Wang C, Zhang R, Wang H, Ma R, Jin S, Xiang JZ, Tang Q. Tacrolimus inhibits vasoconstriction by increasing Ca(2+) sparks in rat aorta. J Huazhong Univ Sci Technolog Med Sci. 2016;36:8–13. doi: 10.1007/s11596-016-1534-6. [DOI] [PubMed] [Google Scholar]
- 235.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 236.Cheng X, Liu J, Asuncion-Chin M, Blaskova E, Bannister JP, Dopico AM, Jaggar JH. A novel Ca(V)1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J Biol Chem. 2007;282:29211–29221. doi: 10.1074/jbc.M610623200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cheng X, Pachuau J, Blaskova E, Asuncion-Chin M, Liu J, Dopico AM, Jaggar JH. Alternative splicing of Cav1.2 channel exons in smooth muscle cells of resistance-size arteries generates currents with unique electrophysiological properties. Am J Physiol Heart Circ Physiol. 2009;297:H680–H688. doi: 10.1152/ajpheart.00109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 239.Cheong A, Dedman AM, Beech DJ. Expression and function of native potassium channel [K(V)alpha1] subunits in terminal arterioles of rabbit. J Physiol. 2001;534:691–700. doi: 10.1111/j.1469-7793.2001.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cheong A, Dedman AM, Xu SZ, Beech DJ. K(V)alpha1 channels in murine arterioles: Differential cellular expression and regulation of diameter. Am J Physiol Heart Circ Physiol. 2001;281:H1057–H1065. doi: 10.1152/ajpheart.2001.281.3.H1057. [DOI] [PubMed] [Google Scholar]
- 241.Cheranov SY, Jaggar JH. Sarcoplasmic reticulum calcium load regulates rat arterial smooth muscle calcium sparks and transient K(Ca) currents. J Physiol. 2002;544:71–84. doi: 10.1113/jphysiol.2002.025197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cheranov SY, Jaggar JH. TNF-alpha dilates cerebral arteries via NAD(P)H oxidase-dependent Ca2+ spark activation. Am J Physiol Cell Physiol. 2006;290:C964–C971. doi: 10.1152/ajpcell.00499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Cheung DW. Modulation of spontaneous transient Ca2+-activated K+ channel currents by cADP-ribose in vascular smooth muscle cells. Eur J Pharmacol. 2003;458:57–59. doi: 10.1016/s0014-2999(02)02816-9. [DOI] [PubMed] [Google Scholar]
- 244.Chilian WM, Layne SM, Eastham CL, Marcus ML. Heterogeneous microvascular coronary alpha-adrenergic vasoconstriction. Circ Res. 1989;64:376–388. doi: 10.1161/01.res.64.2.376. [DOI] [PubMed] [Google Scholar]
- 245.Chilton L, Loutzenhiser K, Morales E, Breaks J, Kargacin GJ, Loutzenhiser R. Inward rectifier K(+) currents and Kir2.1 expression in renal afferent and efferent arterioles. J Am Soc Nephrol. 2008;19:69–76. doi: 10.1681/ASN.2007010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chilton L, Loutzenhiser R. Functional evidence for an inward rectifier potassium current in rat renal afferent arterioles. Circ Res. 2001;88:152–158. doi: 10.1161/01.res.88.2.152. [DOI] [PubMed] [Google Scholar]
- 247.Chilton L, Smirnov SV, Loutzenhiser K, Wang X, Loutzenhiser R. Segment-specific differences in the inward rectifier K(+) current along the renal interlobular artery. Cardiovasc Res. 2011;92:169–177. doi: 10.1093/cvr/cvr179. [DOI] [PubMed] [Google Scholar]
- 248.Chin D, Means AR. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 249.Chrissobolis S, Sobey CG. Inhibitory effects of protein kinase C on inwardly rectifying K+- and ATP-sensitive K+ channel-mediated responses of the basilar artery. Stroke. 2002;33:1692–1697. doi: 10.1161/01.str.0000016966.89226.67. [DOI] [PubMed] [Google Scholar]
- 250.Chrissobolis S, Ziogas J, Anderson CR, Chu Y, Faraci FM, Sobey CG. Neuronal NO mediates cerebral vasodilator responses to K+ in hypertensive rats. Hypertension. 2002;39:880–885. doi: 10.1161/01.hyp.0000013056.74554.ce. [DOI] [PubMed] [Google Scholar]
- 251.Chuang RS, Jaffe H, Cribbs L, Perez-Reyes E, Swartz KJ. Inhibition of T-type voltage-gated calcium channels by a new scorpion toxin. Nat Neurosci. 1998;1:668–674. doi: 10.1038/3669. [DOI] [PubMed] [Google Scholar]
- 252.Chubanov V, Gudermann T. Trpm6. Handb Exp Pharmacol. 2014;222:503–520. doi: 10.1007/978-3-642-54215-2_20. [DOI] [PubMed] [Google Scholar]
- 253.Chubanov V, Waldegger S, Mederosy Schnitzler M, Vitzthum H, Sassen MC, Seyberth HW, Konrad M, Gudermann T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci USA. 2004;101:2894–2899. doi: 10.1073/pnas.0305252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chung AW, Au Yeung K, Chum E, Okon EB, van Breemen C. Diabetes modulates capacitative calcium entry and expression of transient receptor potential canonical channels in human saphenous vein. Eur J Pharmacol. 2009;613:114–118. doi: 10.1016/j.ejphar.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 255.Chung MK, Guler AD, Caterina MJ. Biphasic currents evoked by chemical or thermal activation of the heat-gated ion channel, TRPV3. J Biol Chem. 2005;280:15928–15941. doi: 10.1074/jbc.M500596200. [DOI] [PubMed] [Google Scholar]
- 256.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. 2-Aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci. 2004;24:5177–5182. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cipolla MJ, Sweet J, Chan SL, Tavares MJ, Gokina N, Brayden JE. Increased pressure-induced tone in rat parenchymal arterioles vs. middle cerebral arteries: Role of ion channels and calcium sensitivity. J Appl Physiol (1985) 2014;117:53–59. doi: 10.1152/japplphysiol.00253.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 259.Clapham DE, DeCaen P, Carvacho I, Chaudhuri D, Doerner JF, Julius D, Kahle KT, McKemy D, Oancea E, Sah R, Stotz SC, Tong D, Wu L-J, Xu H, Nilius B, Owsianik G. Transient Receptor Potential channels. 2016 Feb 9; http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=78 2016.
- 260.Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 261.Clark K, Middelbeek J, Morrice NA, Figdor CG, Lasonder E, van Leeuwen FN. Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS One. 2008;3:e1876. doi: 10.1371/journal.pone.0001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Clayton FC, Hess TA, Smith MA, Grover GJ. Coronary reactive hyperemia and adenosine-induced vasodilation are mediated partially by a glyburide-sensitive mechanism. Pharmacology. 1992;44:92–100. doi: 10.1159/000138877. [DOI] [PubMed] [Google Scholar]
- 263.Clement-Chomienne O, Walsh MP, Cole WC. Angiotensin II activation of protein kinase C decreases delayed rectifier K+ current in rabbit vascular myocytes. J Physiol. 1996;495(Pt 3):689–700. doi: 10.1113/jphysiol.1996.sp021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cogolludo A, Frazziano G, Briones AM, Cobeno L, Moreno L, Lodi F, Salaices M, Tamargo J, Perez-Vizcaino F. The dietary flavonoid quercetin activates BKCa currents in coronary arteries via production of H2O2. Role in vasodilatation. Cardiovasc Res. 2007;73:424–431. doi: 10.1016/j.cardiores.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 265.Cogolludo A, Moreno L, Lodi F, Frazziano G, Cobeno L, Tamargo J, Perez-Vizcaino F. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: Role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ Res. 2006;98:931–938. doi: 10.1161/01.RES.0000216858.04599.e1. [DOI] [PubMed] [Google Scholar]
- 266.Cohen KD, Jackson WF. Hypoxia inhibits contraction but not calcium channel currents or changes in intracellular calcium in arteriolar muscle cells. Microcirculation. 2003;10:133–141. doi: 10.1038/sj/mn.7800178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cohen NA, Sha Q, Makhina EN, Lopatin AN, Linder ME, Snyder SH, Nichols CG. Inhibition of an inward rectifier potassium channel (Kir2.3) by G-protein betagamma subunits. J Biol Chem. 1996;271:32301–32305. doi: 10.1074/jbc.271.50.32301. [DOI] [PubMed] [Google Scholar]
- 268.Cole WC, Malcolm T, Walsh MP, Light PE. Inhibition by protein kinase C of the K(NDP) subtype of vascular smooth muscle ATP-sensitive potassium channel. Circ Res. 2000;87:112–117. doi: 10.1161/01.res.87.2.112. [DOI] [PubMed] [Google Scholar]
- 269.Collier ML, Ji G, Wang Y, Kotlikoff MI. Calcium-induced calcium release in smooth muscle: Loose coupling between the action potential and calcium release. J Gen Physiol. 2000;115:653–662. doi: 10.1085/jgp.115.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Contreras GF, Castillo K, Enrique N, Carrasquel-Ursulaez W, Castillo JP, Milesi V, Neely A, Alvarez O, Ferreira G, Gonzalez C, Latorre R. A BK (Slo1) channel journey from molecule to physiology. Channels (Austin) 2013;7:442–458. doi: 10.4161/chan.26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Cook NS. Effect of some potassium channel blockers on contractile responses of the rabbit aorta. J Cardiovasc Pharmacol. 1989;13:299–306. doi: 10.1097/00005344-198902000-00019. [DOI] [PubMed] [Google Scholar]
- 272.Cook NS, Chapman ID. Therapeutic potential of potassium channel openers in peripheral vascular disease and asthma. Cardiovasc Drugs Ther. 1993;7(Suppl 3):555–563. doi: 10.1007/BF00877621. [DOI] [PubMed] [Google Scholar]
- 273.Cook NS, Quast U. Potassium channel pharmacology. In: Cook NS, editor. Potassium channels: Structure, Classification, Function and Thereapeutic Potential. Chichester: Ellis Horwood; 1989. pp. 181–255. [Google Scholar]
- 274.Cooke JP, Rossitch E, Jr, Andon NA, Loscalzo J, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest. 1991;88:1663–1671. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Copello JA, Barg S, Onoue H, Fleischer S. Heterogeneity of Ca2+ gating of skeletal muscle and cardiac ryanodine receptors. Biophys J. 1997;73:141–156. doi: 10.1016/S0006-3495(97)78055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Cortes SF, Lemos VS, Stoclet JC. Alterations in calcium stores in aortic myocytes from spontaneously hypertensive rats. Hypertension. 1997;29:1322–1328. doi: 10.1161/01.hyp.29.6.1322. [DOI] [PubMed] [Google Scholar]
- 277.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 278.Coussin F, Macrez N, Morel JL, Mironneau J. Requirement of ryanodine receptor subtypes 1 and 2 for Ca(2+)-induced Ca(2+) release in vascular myocytes. J Biol Chem. 2000;275:9596–9603. doi: 10.1074/jbc.275.13.9596. [DOI] [PubMed] [Google Scholar]
- 279.Cox RH. Comparison of K+ channel properties in freshly isolated myocytes from thoracic aorta of WKY and SHR. Am J Hypertens. 1996;9:884–894. doi: 10.1016/s0895-7061(96)00179-3. [DOI] [PubMed] [Google Scholar]
- 280.Cox RH. Changes in the expression and function of arterial potassium channels during hypertension. Vascul Pharmacol. 2002;38:13–23. doi: 10.1016/s1537-1891(02)00122-2. [DOI] [PubMed] [Google Scholar]
- 281.Cox RH. Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophys. 2005;42:167–195. doi: 10.1385/CBB:42:2:167. [DOI] [PubMed] [Google Scholar]
- 282.Cox RH, Folander K, Swanson R. Differential expression of voltage-gated K(+) channel genes in arteries from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 2001;37:1315–1322. doi: 10.1161/01.hyp.37.5.1315. [DOI] [PubMed] [Google Scholar]
- 283.Cox RH, Fromme S. Expression of calcium channel subunit variants in small mesenteric arteries of WKY and SHR. Am J Hypertens. 2015;28:1229–1239. doi: 10.1093/ajh/hpv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Cox RH, Fromme S. Comparison of voltage gated K+ currents in arterial myocytes with heterologously expressed Kv subunits. Cell Biochem Biophys. 2016;74:499–511. doi: 10.1007/s12013-016-0763-4. [DOI] [PubMed] [Google Scholar]
- 285.Cox RH, Fromme S. Functional expression profile of voltage-gated K(+) channel subunits in rat small mesenteric arteries. Cell Biochem Biophys. 2016;74:263–276. doi: 10.1007/s12013-015-0715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Cox RH, Fromme SJ, Folander KL, Swanson RJ. Voltage gated K+ channel expression in arteries of Wistar-Kyoto and spontaneously hypertensive rats. Am J Hypertens. 2008;21:213–218. doi: 10.1038/ajh.2007.44. [DOI] [PubMed] [Google Scholar]
- 287.Cox RH, Lozinskaya IM. Ca2+ channel inactivation in small mesenteric arteries of WKY and SHR. Am J Hypertens. 2008;21:406–412. doi: 10.1038/ajh.2007.73. [DOI] [PubMed] [Google Scholar]
- 288.Cox RH, Lozinskaya I, Dietz NJ. Differences in K+ current components in mesenteric artery myocytes from WKY and SHR. Am J Hypertens. 2001;14:897–907. doi: 10.1016/s0895-7061(01)02145-8. [DOI] [PubMed] [Google Scholar]
- 289.Cox RH, Petrou S. Ca(2+) influx inhibits voltage-dependent and augments Ca(2+)-dependent K(+) currents in arterial myocytes. Am J Physiol. 1999;277:C51–C63. doi: 10.1152/ajpcell.1999.277.1.C51. [DOI] [PubMed] [Google Scholar]
- 290.Cox RH, Rusch NJ. New expression profiles of voltage-gated ion channels in arteries exposed to high blood pressure. Microcirculation. 2002;9:243–257. doi: 10.1038/sj.mn.7800140. [DOI] [PubMed] [Google Scholar]
- 291.Crane GJ, Walker SD, Dora KA, Garland CJ. Evidence for a differential cellular distribution of inward rectifier K channels in the rat isolated mesenteric artery. J Vasc Res. 2003;40:159–168. doi: 10.1159/000070713. [DOI] [PubMed] [Google Scholar]
- 292.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. ATP-mediated vasodilatation occurs via activation of inwardly rectifying potassium channels in humans. J Physiol. 2012;590:5349–5359. doi: 10.1113/jphysiol.2012.234245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2013;305:H29–H40. doi: 10.1152/ajpheart.00298.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Crecelius AR, Luckasen GJ, Larson DG, Dinenno FA. KIR channel activation contributes to onset and steady-state exercise hyperemia in humans. Am J Physiol Heart Circ Physiol. 2014;307:H782–H791. doi: 10.1152/ajpheart.00212.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Crecelius AR, Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+-ATPase in humans. Circ Res. 2013;113:1023–1032. doi: 10.1161/CIRCRESAHA.113.301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Crystal GJ, Gurevicius J, Salem MR, Zhou X. Role of adenosine triphosphate-sensitive potassium channels in coronary vasodilation by halothane, isoflurane, and enflurane. Anesthesiology. 1997;86:448–458. doi: 10.1097/00000542-199702000-00020. [DOI] [PubMed] [Google Scholar]
- 297.Curtis TM, Tumelty J, Dawicki J, Scholfield CN, McGeown JG. Identification and spatiotemporal characterization of spontaneous Ca2+ sparks and global Ca2+ oscillations in retinal arteriolar smooth muscle cells. Invest Ophthalmol Vis Sci. 2004;45:4409–4414. doi: 10.1167/iovs.04-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Curtis TM, Tumelty J, Stewart MT, Arora AR, Lai FA, McGahon MK, Scholfield CN, McGeown JG. Modification of smooth muscle Ca2+-sparks by tetracaine: Evidence for sequential RyR activation. Cell Calcium. 2008;43:142–154. doi: 10.1016/j.ceca.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 299.D’Angelo G, Davis MJ, Meininger GA. Calcium and mechanotransduction of the myogenic response. Am J Physiol. 1997;273:H175–H182. doi: 10.1152/ajpheart.1997.273.1.H175. [DOI] [PubMed] [Google Scholar]
- 300.Dabertrand F, Morel JL, Sorrentino V, Mironneau J, Mironneau C, Macrez N. Modulation of calcium signalling by dominant negative splice variant of ryanodine receptor subtype 3 in native smooth muscle cells. Cell Calcium. 2006;40:11–21. doi: 10.1016/j.ceca.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 301.Dabertrand F, Nelson MT, Brayden JE. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ Res. 2012;110:285–294. doi: 10.1161/CIRCRESAHA.111.258145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Dam VS, Boedtkjer DM, Aalkjaer C, Matchkov V. The bestrophin- and TMEM16A-associated Ca(2+)-activated Cl(−) channels in vascular smooth muscles. Channels (Austin) 2014;8:361–369. doi: 10.4161/chan.29531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Dam VS, Boedtkjer DM, Nyvad J, Aalkjaer C, Matchkov V. TMEM16A knockdown abrogates two different Ca(2+)-activated Cl(−) currents and contractility of smooth muscle in rat mesenteric small arteries. Pflugers Arch. 2014;466:1391–1409. doi: 10.1007/s00424-013-1382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Dankelman J, Van der Ploeg CP, Spaan JA. Glibenclamide decelerates the responses of coronary regulation in the goat. Am J Physiol. 1994;266:H1715–H1721. doi: 10.1152/ajpheart.1994.266.5.H1715. [DOI] [PubMed] [Google Scholar]
- 306.Danoff SK, Ferris CD, Donath C, Fischer GA, Munemitsu S, Ullrich A, Snyder SH, Ross CA. Inositol 1,4,5-trisphosphate receptors: distinct neuronal and nonneuronal forms derived by alternative splicing differ in phosphorylation. Proc Natl Acad Sci USA. 1991;88:2951–2955. doi: 10.1073/pnas.88.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Dart C, Leyland ML. Targeting of an A kinase-anchoring protein, AKAP79, to an inwardly rectifying potassium channel, Kir2.1. J Biol Chem. 2001;276:20499–20505. doi: 10.1074/jbc.M101425200. [DOI] [PubMed] [Google Scholar]
- 308.Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Dart C, Standen NB. Activation of ATP-dependent K+ channels by hypoxia in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1995;483(Pt 1):29–39. doi: 10.1113/jphysiol.1995.sp020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Gunther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990;247:1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- 311.Daut J, Standen NB, Nelson MT. The role of the membrane potential of endothelial and smooth muscle cells in the regulation of coronary blood flow. J Cardiovasc Electrophysiol. 1994;5:154–181. doi: 10.1111/j.1540-8167.1994.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 312.Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 313.Davies LM, Purves GI, Barrett-Jolley R, Dart C. Interaction with caveolin-1 modulates vascular ATP-sensitive potassium (KATP) channel activity. J Physiol. 2010;588:3255–3266. doi: 10.1113/jphysiol.2010.194779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Davis MJ. Perspective: Physiological role(s) of the vascular myogenic response. Microcirculation. 2012;19:99–114. doi: 10.1111/j.1549-8719.2011.00131.x. [DOI] [PubMed] [Google Scholar]
- 315.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 316.Davis MJ, Hill MA, Kuo L. Compr Physiol. Hoboken, NJ: John Wiley & Sons; 2011. Local regulation of microvascular perfusion; pp. 161–284. [Google Scholar]
- 317.Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Davis GE, Hill MA, Meininger GA. Integrins and mechanotransduction of the vascular myogenic response. Am J Physiol Heart Circ Physiol. 2001;280:H1427–H1433. doi: 10.1152/ajpheart.2001.280.4.H1427. [DOI] [PubMed] [Google Scholar]
- 318.Dawes M, Sieniawska C, Delves T, Dwivedi R, Chowienczyk PJ, Ritter JM. Barium reduces resting blood flow and inhibits potassium-induced vasodilation in the human forearm. Circulation. 2002;105:1323–1328. doi: 10.1161/hc1102.105651. [DOI] [PubMed] [Google Scholar]
- 319.de Clerck I, Guyssens B, Pannier JL, Van de Voorde J. Hyperosmolarity causes BK Ca-dependent vasodilatations in rat skeletal muscle arteries. Med Sci Sports Exerc. 2005;37:1697–1703. doi: 10.1249/01.mss.0000176446.13607.b0. [DOI] [PubMed] [Google Scholar]
- 320.de Kreutzenberg SV, Puato M, Kiwanuka E, Del Prato S, Pauletto P, Pasini L, Tiengo A, Avogaro A. Elevated non-esterified fatty acids impair nitric oxide independent vasodilation, in humans: Evidence for a role of inwardly rectifying potassium channels. Atherosclerosis. 2003;169:147–153. doi: 10.1016/s0021-9150(03)00153-9. [DOI] [PubMed] [Google Scholar]
- 321.De Paoli P, Cerbai E, Koidl B, Kirchengast M, Sartiani L, Mugelli A. Selectivity of different calcium antagonists on T- and L-type calcium currents in guinea-pig ventricular myocytes. Pharmacol Res. 2002;46:491–497. doi: 10.1016/s1043661802002360. [DOI] [PubMed] [Google Scholar]
- 322.de Weille JR. Modulation of ATP sensitive potassium channels. Cardiovasc Res. 1992;26:1017–1020. doi: 10.1093/cvr/26.11.1017. [DOI] [PubMed] [Google Scholar]
- 323.DeCaen PG, Delling M, Vien TN, Clapham DE. Direct recording and molecular identification of the calcium channel of primary cilia. Nature. 2013;504:315–318. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D’Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong JA, Fanger CM, Woolf CJ, Patapoutian A, Moran MM. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.del Corsso C, Ostrovskaya O, McAllister CE, Murray K, Hatton WJ, Gurney AM, Spencer NJ, Wilson SM. Effects of aging on Ca2+ signaling in murine mesenteric arterial smooth muscle cells. Mech Ageing Dev. 2006;127:315–323. doi: 10.1016/j.mad.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 326.Del Valle-Rodriguez A, Calderon E, Ruiz M, Ordonez A, Lopez-Barneo J, Urena J. Metabotropic Ca(2+) channel-induced Ca(2+) release and ATP-dependent facilitation of arterial myocyte contraction. Proc Natl Acad Sci USA. 2006;103:4316–4321. doi: 10.1073/pnas.0508781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.del Valle-Rodriguez A, Lopez-Barneo J, Urena J. Ca2+ channel-sarcoplasmic reticulum coupling: A mechanism of arterial myocyte contraction without Ca2+ influx. Embo J. 2003;22:4337–4345. doi: 10.1093/emboj/cdg432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Desai PN, Zhang X, Wu S, Janoshazi A, Bolimuntha S, Putney JW, Trebak M. Multiple types of calcium channels arising from alternative translation initiation of the Orai1 message. Sci Signal. 2015;8:ra74. doi: 10.1126/scisignal.aaa8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Desilets M, Driska SP, Baumgarten CM. Current fluctuations and oscillations in smooth muscle cells from hog carotid artery. Role of the sarcoplasmic reticulum. Circ Res. 1989;65:708–722. doi: 10.1161/01.res.65.3.708. [DOI] [PubMed] [Google Scholar]
- 330.Devogelaere B, Verbert L, Parys JB, Missiaen L, De Smedt H. The complex regulatory function of the ligand-binding domain of the inositol 1,4,5-trisphosphate receptor. Cell Calcium. 2008;43:17–27. doi: 10.1016/j.ceca.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 331.Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol. 2008;294:H2371–H2381. doi: 10.1152/ajpheart.01279.2007. [DOI] [PubMed] [Google Scholar]
- 332.Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Exp Biol Med (Maywood) 2010;235:10–22. doi: 10.1258/ebm.2009.009201. [DOI] [PubMed] [Google Scholar]
- 333.Dietrich A, Kalwa H, Rost BR, Gudermann T. The diacylgylcerol-sensitive TRPC3/6/7 subfamily of cation channels: Functional characterization and physiological relevance. Pflugers Arch. 2005;451:72–80. doi: 10.1007/s00424-005-1460-0. [DOI] [PubMed] [Google Scholar]
- 334.Dietrich A, Kalwa H, Storch U, Mederos y Schnitzler M, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch. 2007;455:465–477. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- 335.Diochot S, Drici MD, Moinier D, Fink M, Lazdunski M. Effects of phrixotoxins on the Kv4 family of potassium channels and implications for the role of Ito1 in cardiac electrogenesis. Br J Pharmacol. 1999;126:251–263. doi: 10.1038/sj.bjp.0702283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Dong H, Waldron GJ, Cole WC, Triggle CR. Roles of calcium-activated and voltage-gated delayed rectifier potassium channels in endothelium-dependent vasorelaxation of the rabbit middle cerebral artery. Br J Pharmacol. 1998;123:821–832. doi: 10.1038/sj.bjp.0701680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Dong H, Waldron GJ, Galipeau D, Cole WC, Triggle CR. NO/PGI2-independent vasorelaxation and the cytochrome P450 pathway in rabbit carotid artery. Br J Pharmacol. 1997;120:695–701. doi: 10.1038/sj.bjp.0700945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Dong L, Xie MJ, Zhang P, Ji LL, Liu WC, Dong MQ, Gao F. Rotenone partially reverses decreased BK Ca currents in cerebral artery smooth muscle cells from streptozotocin-induced diabetic mice. Clin Exp Pharmacol Physiol. 2009;36:e57–e64. doi: 10.1111/j.1440-1681.2009.05222.x. [DOI] [PubMed] [Google Scholar]
- 339.Dreja K, Nordstrom I, Hellstrand P. Rat arterial smooth muscle devoid of ryanodine receptor function: Effects on cellular Ca(2+) handling. Br J Pharmacol. 2001;132:1957–1966. doi: 10.1038/sj.bjp.0703986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Drummond HA. Yes, no, maybe so: ENaC proteins as mediators of renal myogenic constriction. Hypertension. 2009;54:962–963. doi: 10.1161/HYPERTENSIONAHA.109.139014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: Components of a vascular mechanosensor. Hypertension. 2004;44:643–648. doi: 10.1161/01.HYP.0000144465.56360.ad. [DOI] [PubMed] [Google Scholar]
- 342.Drummond HA, Grifoni SC, Jernigan NL. A new trick for an old dogma: ENaC proteins as mechanotransducers in vascular smooth muscle. Physiology (Bethesda) 2008;23:23–31. doi: 10.1152/physiol.00034.2007. [DOI] [PubMed] [Google Scholar]
- 343.Du H, Wang X, Wu J, Qian Q. Phenylephrine induces elevated RhoA activation and smooth muscle alpha-actin expression in Pkd2+/− vascular smooth muscle cells. Hypertens Res. 2010;33:37–42. doi: 10.1038/hr.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of kir channels by diverse modulators. J Biol Chem. 2004;279:37271–37281. doi: 10.1074/jbc.M403413200. [DOI] [PubMed] [Google Scholar]
- 345.Dulhunty A, Haarmann C, Green D, Hart J. How many cysteine residues regulate ryanodine receptor channel activity? Antioxid Redox Signal. 2000;2:27–34. doi: 10.1089/ars.2000.2.1-27. [DOI] [PubMed] [Google Scholar]
- 346.Dulhunty AF, Junankar PR, Eager KR, Ahern GP, Laver DR. Ion channels in the sarcoplasmic reticulum of striated muscle. Acta Physiol Scand. 1996;156:375–385. doi: 10.1046/j.1365-201X.1996.193000.x. [DOI] [PubMed] [Google Scholar]
- 347.Dulhunty AF, Pouliquin P. What we don’t know about the structure of ryanodine receptor calcium release channels. Clin Exp Pharmacol Physiol. 2003;30:713–723. doi: 10.1046/j.1440-1681.2003.03904.x. [DOI] [PubMed] [Google Scholar]
- 348.Duling BR. The preparation and use of the hamster cheek pouch for studies of the microcirculation. Microvasc Res. 1973;5:423–429. doi: 10.1016/0026-2862(73)90059-9. [DOI] [PubMed] [Google Scholar]
- 349.Duling BR, Gore RW, Dacey RG, Jr, Damon DN. Methods for isolation, cannulation, and in vitro study of single microvessels. Am J Physiol. 1981;241:H108–H116. doi: 10.1152/ajpheart.1981.241.1.H108. [DOI] [PubMed] [Google Scholar]
- 350.Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998;58:1515–1520. [PubMed] [Google Scholar]
- 351.Duncker DJ, Oei HH, Hu F, Stubenitsky R, Verdouw PD. Role of K(ATP)(+) channels in regulation of systemic, pulmonary, and coronary vasomotor tone in exercising swine. Am J Physiol Heart Circ Physiol. 2001;280:H22–H33. doi: 10.1152/ajpheart.2001.280.1.H22. [DOI] [PubMed] [Google Scholar]
- 352.Duncker DJ, Van Zon NS, Altman JD, Pavek TJ, Bache RJ. Role of K+ATP channels in coronary vasodilation during exercise. Circulation. 1993;88:1245–1253. doi: 10.1161/01.cir.88.3.1245. [DOI] [PubMed] [Google Scholar]
- 353.Duncker DJ, van Zon NS, Ishibashi Y, Bache RJ. Role of K+ ATP channels and adenosine in the regulation of coronary blood flow during exercise with normal and restricted coronary blood flow. J Clin Invest. 1996;97:996–1009. doi: 10.1172/JCI118524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Duncker DJ, van Zon NS, Pavek TJ, Herrlinger SK, Bache RJ. Endogenous adenosine mediates coronary vasodilation during exercise after K(ATP)+ channel blockade. J Clin Invest. 1995;95:285–295. doi: 10.1172/JCI117653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Durham WJ, Wehrens XH, Sood S, Hamilton SL. Diseases associated with altered ryanodine receptor activity. Subcell Biochem. 2007;45:273–321. doi: 10.1007/978-1-4020-6191-2_10. [DOI] [PubMed] [Google Scholar]
- 356.Dwivedi R, Saha S, Chowienczyk PJ, Ritter JM. Block of inward rectifying K+ channels (KIR) inhibits bradykinin-induced vasodilatation in human forearm resistance vasculature. Arterioscler Thromb Vasc Biol. 2005;25:e7–e9. doi: 10.1161/01.ATV.0000152610.40086.31. [DOI] [PubMed] [Google Scholar]
- 357.Eager KR, Dulhunty AF. Cardiac ryanodine receptor activity is altered by oxidizing reagents in either the luminal or cytoplasmic solution. J Membr Biol. 1999;167:205–214. doi: 10.1007/s002329900484. [DOI] [PubMed] [Google Scholar]
- 358.Eager KR, Roden LD, Dulhunty AF. Actions of sulfhydryl reagents on single ryanodine receptor Ca(2+)-release channels from sheep myocardium. Am J Physiol. 1997;272:C1908–C1918. doi: 10.1152/ajpcell.1997.272.6.C1908. [DOI] [PubMed] [Google Scholar]
- 359.Earley S. TRPA1 channels in the vasculature. Br J Pharmacol. 2012;167:13–22. doi: 10.1111/j.1476-5381.2012.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Earley S. TRPM4 channels in smooth muscle function. Pflugers Arch. 2013;465:1223–1231. doi: 10.1007/s00424-013-1250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev. 2015;95:645–690. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ Res. 2009;104:987–994. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Earley S, Gonzales AL, Garcia ZI. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol Pharmacol. 2010;77:612–620. doi: 10.1124/mol.109.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 365.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol. 2009;297:H1096–H1102. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol. 2007;292:H2613–H2622. doi: 10.1152/ajpheart.01286.2006. [DOI] [PubMed] [Google Scholar]
- 367.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004;95:922–929. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 368.Eckenrode EF, Yang J, Velmurugan GV, Foskett JK, White C. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem. 2010;285:13678–13684. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Eckert RE, Karsten AJ, Utz J, Ziegler M. Regulation of renal artery smooth muscle tone by alpha1-adrenoceptors: Role of voltage-gated calcium channels and intracellular calcium stores. Urol Res. 2000;28:122–127. doi: 10.1007/s002400050149. [DOI] [PubMed] [Google Scholar]
- 370.Eckman DM, Hopkins N, McBride C, Keef KD. Endothelium-dependent relaxation and hyperpolarization in guinea-pig coronary artery: Role of epoxyeicosatrienoic acid. Br J Pharmacol. 1998;124:181–189. doi: 10.1038/sj.bjp.0701778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Edvinsson L, Jansen Olesen I, Kingman TA, McCulloch J, Uddman R. Modification of vasoconstrictor responses in cerebral blood vessels by lesioning of the trigeminal nerve: Possible involvement of CGRP. Cephalalgia. 1995;15:373–383. doi: 10.1046/j.1468-2982.1995.1505373.x. [DOI] [PubMed] [Google Scholar]
- 372.Edwards FR, Hirst GD. Inward rectification in submucosal arterioles of guinea-pig ileum. J Physiol. 1988;404:437–454. doi: 10.1113/jphysiol.1988.sp017298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Edwards FR, Hirst GD, Silverberg GD. Inward rectification in rat cerebral arterioles; involvement of potassium ions in autoregulation. J Physiol. 1988;404:455–466. doi: 10.1113/jphysiol.1988.sp017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Edwards G, Weston AH. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- 375.Edwards G, Weston AH. Pharmacology of the potassium channel openers. Cardiovasc Drugs Ther. 1995;9(Suppl 2):185–193. doi: 10.1007/BF00878465. [DOI] [PubMed] [Google Scholar]
- 376.Edwards GA, Weiant EA, Slocombe AG, Roeder KD. The action of ryanodine on the contractile process in striated muscle. Science. 1948;108:330–332. doi: 10.1126/science.108.2804.330. [DOI] [PubMed] [Google Scholar]
- 377.Efremov RG, Leitner A, Aebersold R, Raunser S. Architecture and conformational switch mechanism of the ryanodine receptor. Nature. 2015;517:39–43. doi: 10.1038/nature13916. [DOI] [PubMed] [Google Scholar]
- 378.Eguchi S, Kawano T, Yinhua, Tanaka K, Yasui S, Mawatari K, Takahashi A, Nakaya Y, Oshita S, Nakajo N. Effects of prostaglandin E1 on vascular ATP-sensitive potassium channels. J Cardiovasc Pharmacol. 2007;50:686–691. doi: 10.1097/FJC.0b013e3181583d9b. [DOI] [PubMed] [Google Scholar]
- 379.Embrey RP, Brooks LA, Dellsperger KC. Mechanism of coronary microvascular responses to metabolic stimulation. Cardiovasc Res. 1997;35:148–157. doi: 10.1016/s0008-6363(97)00096-5. [DOI] [PubMed] [Google Scholar]
- 380.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: Role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 381.Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977;57:71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- 382.Epshtein Y, Chopra AP, Rosenhouse-Dantsker A, Kowalsky GB, Logothetis DE, Levitan I. Identification of a C-terminus domain critical for the sensitivity of Kir2.1 to cholesterol. Proc Natl Acad Sci U S A. 2009;106:8055–8060. doi: 10.1073/pnas.0809847106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Epstein A, Beall A, Wynn J, Mulloy L, Brophy CM. Cyclosporine, but not FK506, selectively induces renal and coronary artery smooth muscle contraction. Surgery. 1998;123:456–460. [PubMed] [Google Scholar]
- 384.Erdos B, Miller AW, Busija DW. Alterations in KATP and KCa channel function in cerebral arteries of insulin-resistant rats. Am J Physiol Heart Circ Physiol. 2002;283:H2472–H2477. doi: 10.1152/ajpheart.00516.2002. [DOI] [PubMed] [Google Scholar]
- 385.Erdos B, Simandle SA, Snipes JA, Miller AW, Busija DW. Potassium channel dysfunction in cerebral arteries of insulin-resistant rats is mediated by reactive oxygen species. Stroke. 2004;35:964–969. doi: 10.1161/01.STR.0000119753.05670.F1. [DOI] [PubMed] [Google Scholar]
- 386.Erler I, Al-Ansary DM, Wissenbach U, Wagner TF, Flockerzi V, Niemeyer BA. Trafficking and assembly of the cold-sensitive TRPM8 channel. J Biol Chem. 2006;281:38396–38404. doi: 10.1074/jbc.M607756200. [DOI] [PubMed] [Google Scholar]
- 387.Escoubas P, Diochot S, Celerier ML, Nakajima T, Lazdunski M. Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Mol Pharmacol. 2002;62:48–57. doi: 10.1124/mol.62.1.48. [DOI] [PubMed] [Google Scholar]
- 388.Essin K, Gollasch M. Role of ryanodine receptor subtypes in initiation and formation of calcium sparks in arterial smooth muscle: comparison with striated muscle. J Biomed Biotechnol. 2009;2009:135249. doi: 10.1155/2009/135249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Essin K, Welling A, Hofmann F, Luft FC, Gollasch M, Moosmang S. Indirect coupling between Cav1.2 channels and ryanodine receptors to generate Ca2+ sparks in murine arterial smooth muscle cells. J Physiol. 2007;584:205–219. doi: 10.1113/jphysiol.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Evans AM, Wyatt CN, Kinnear NP, Clark JH, Blanco EA. Pyridine nucleotides and calcium signalling in arterial smooth muscle: From cell physiology to pharmacology. Pharmacol Ther. 2005;107:286–313. doi: 10.1016/j.pharmthera.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 391.Evanson KW, Bannister JP, Leo MD, Jaggar JH. LRRC26 is a functional BK channel auxiliary gamma subunit in arterial smooth muscle cells. Circ Res. 2014;115:423–431. doi: 10.1161/CIRCRESAHA.115.303407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A. 2010;107:19084–19089. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Fairfax ST, Mauban JR, Hao S, Rizzo MA, Zhang J, Wier WG. Ca(2+) signaling in arterioles and small arteries of conscious, restrained, optical biosensor mice. Front Physiol. 2014;5:387. doi: 10.3389/fphys.2014.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Fairhurst AS. A ryanodine-caffeine-sensitive membrane fraction of skeletal muscle. Am J Physiol. 1974;227:1124–1131. doi: 10.1152/ajplegacy.1974.227.5.1124. [DOI] [PubMed] [Google Scholar]
- 395.Fairhurst AS, Hasselbach W. Calcium efflux from a heavy sarcotubular fraction. Effects of ryanodine, caffeine and magnesium. Eur J Biochem. 1970;13:504–509. doi: 10.1111/j.1432-1033.1970.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 396.Fakler B, Brandle U, Glowatzki E, Weidemann S, Zenner HP, Ruppersberg JP. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995;80:149–154. doi: 10.1016/0092-8674(95)90459-x. [DOI] [PubMed] [Google Scholar]
- 397.Fan LH, Tian HY, Ma AQ, Hu Z, Huo JH, Cao YX. Altered ATP-sensitive potassium channels may underscore obesity-triggered increase in blood pressure. Acta Pharmacol Sin. 2008;29:1167–1174. doi: 10.1111/j.1745-7254.2008.00810.x. [DOI] [PubMed] [Google Scholar]
- 398.Fan LH, Tian HY, Wang J, Huo JH, Hu Z, Ma AQ, Cao YX. Down-regulation of Kir6.1/SUR2B channels in the obese rat aorta. Nutrition. 2009;25:359–363. doi: 10.1016/j.nut.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 399.Fan LH, Tian HY, Yang ML, Ma AQ, Hu Z, Bai XJ, Cao YX. High-fat diet may impair K(ATP) channels in vascular smooth muscle cells. Biomed Pharmacother. 2009;63:165–170. doi: 10.1016/j.biopha.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 400.Fang Y, Mohler ER, III, Hsieh E, Osman H, Hashemi SM, Davies PF, Rothblat GH, Wilensky RL, Levitan I. Hypercholesterolemia suppresses inwardly rectifying K+ channels in aortic endothelium in vitro and in vivo. Circ Res. 2006;98:1064–1071. doi: 10.1161/01.RES.0000218776.87842.43. [DOI] [PubMed] [Google Scholar]
- 401.Faraci FM, Breese KR, Heistad DD. Cerebral vasodilation during hypercapnia. Role of glibenclamide-sensitive potassium channels and nitric oxide. Stroke. 1994;25:1679–1683. doi: 10.1161/01.str.25.8.1679. [DOI] [PubMed] [Google Scholar]
- 402.Faraci FM, Heistad DD. Regulation of the cerebral circulation: Role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 403.Faraci FM, Sobey CG. Role of potassium channels in regulation of cerebral vascular tone. J Cereb Blood Flow Metab. 1998;18:1047–1063. doi: 10.1097/00004647-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 404.Faraci FM, Sobey CG, Chrissobolis S, Lund DD, Heistad DD, Weintraub NL. Arachidonate dilates basilar artery by lipoxygenase-dependent mechanism and activation of K(+) channels. Am J Physiol Regul Integr Comp Physiol. 2001;281:R246–R253. doi: 10.1152/ajpregu.2001.281.1.R246. [DOI] [PubMed] [Google Scholar]
- 405.Farouque HM, Meredith IT. Effects of inhibition of ATP-sensitive potassium channels on metabolic vasodilation in the human forearm. Clin Sci (Lond) 2003;104:39–46. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 406.Farouque HM, Meredith IT. Inhibition of vascular ATP-sensitive K+ channels does not affect reactive hyperemia in human forearm. Am J Physiol Heart Circ Physiol. 2003;284:H711–H718. doi: 10.1152/ajpheart.00315.2002. [DOI] [PubMed] [Google Scholar]
- 407.Farouque HM, Meredith IT. Effect of adenosine triphosphate-sensitive potassium channel inhibitors on coronary metabolic vasodilation. Trends Cardiovasc Med. 2007;17:63–68. doi: 10.1016/j.tcm.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 408.Farouque HM, Worthley SG, Meredith IT. Effect of ATP-sensitive potassium channel inhibition on coronary metabolic vasodilation in humans. Arterioscler Thromb Vasc Biol. 2004;24:905–910. doi: 10.1161/01.ATV.0000125701.18648.48. [DOI] [PubMed] [Google Scholar]
- 409.Farouque HM, Worthley SG, Meredith IT, Skyrme-Jones RA, Zhang MJ. Effect of ATP-sensitive potassium channel inhibition on resting coronary vascular responses in humans. Circ Res. 2002;90:231–236. doi: 10.1161/hh0202.103713. [DOI] [PubMed] [Google Scholar]
- 410.Farrell EF, Antaramian A, Benkusky N, Zhu X, Rueda A, Gomez AM, Valdivia HH. Regulation of cardiac excitation-contraction coupling by sorcin, a novel modulator of ryanodine receptors. Biol Res. 2004;37:609–612. doi: 10.4067/s0716-97602004000400015. [DOI] [PubMed] [Google Scholar]
- 411.Farrell EF, Antaramian A, Rueda A, Gomez AM, Valdivia HH. Sorcin inhibits calcium release and modulates excitation-contraction coupling in the heart. J Biol Chem. 2003;278:34660–34666. doi: 10.1074/jbc.M305931200. [DOI] [PubMed] [Google Scholar]
- 412.Fatt P, Katz B. The electrical properties of crustacean muscle fibres. J Physiol. 1953;120:171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Fecher-Trost C, Weissgerber P, Wissenbach U. TRPV6 channels. Handb Exp Pharmacol. 2014;222:359–384. doi: 10.1007/978-3-642-54215-2_14. [DOI] [PubMed] [Google Scholar]
- 414.Felix JP, Bugianesi RM, Schmalhofer WA, Borris R, Goetz MA, Hensens OD, Bao JM, Kayser F, Parsons WH, Rupprecht K, Garcia ML, Kaczorowski GJ, Slaughter RS. Identification and biochemical characterization of a novel nortriterpene inhibitor of the human lymphocyte voltage-gated potassium channel, Kv1.3. Biochemistry. 1999;38:4922–4930. doi: 10.1021/bi982954w. [DOI] [PubMed] [Google Scholar]
- 415.Fellner SK, Arendshorst WJ. Angiotensin II Ca2+ signaling in rat afferent arterioles: Stimulation of cyclic ADP ribose and IP3 pathways. Am J Physiol Renal Physiol. 2005;288:F785–F791. doi: 10.1152/ajprenal.00372.2004. [DOI] [PubMed] [Google Scholar]
- 416.Fellner SK, Arendshorst WJ. Voltage-gated Ca2+ entry and ryanodine receptor Ca2+-induced Ca2+ release in preglomerular arterioles. Am J Physiol Renal Physiol. 2007;292:F1568–F1572. doi: 10.1152/ajprenal.00459.2006. [DOI] [PubMed] [Google Scholar]
- 417.Feng MG, Li M, Navar LG. T-type calcium channels in the regulation of afferent and efferent arterioles in rats. Am J Physiol Renal Physiol. 2004;286:F331–F337. doi: 10.1152/ajprenal.00251.2003. [DOI] [PubMed] [Google Scholar]
- 418.Feng W, Tu J, Pouliquin P, Cabrales E, Shen X, Dulhunty A, Worley PF, Allen PD, Pessah IN. Dynamic regulation of ryanodine receptor type 1 (RyR1) channel activity by Homer 1. Cell Calcium. 2008;43:307–314. doi: 10.1016/j.ceca.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Fernandez-Tenorio M, Gonzalez-Rodriguez P, Porras C, Castellano A, Moosmang S, Hofmann F, Urena J, Lopez-Barneo J. Short communication: Genetic ablation of L-type Ca2+ channels abolishes depolarization-induced Ca2+ release in arterial smooth muscle. Circ Res. 2010;106:1285–1289. doi: 10.1161/CIRCRESAHA.109.213967. [DOI] [PubMed] [Google Scholar]
- 420.Fernandez-Tenorio M, Porras-Gonzalez C, Castellano A, Del Valle-Rodriguez A, Lopez-Barneo J, Urena J. Metabotropic regulation of RhoA/Rho-associated kinase by L-type Ca2+ channels: New mechanism for depolarization-evoked mammalian arterial contraction. Circ Res. 2011;108:1348–1357. doi: 10.1161/CIRCRESAHA.111.240127. [DOI] [PubMed] [Google Scholar]
- 421.Fernandez-Velasco M, Ruiz-Hurtado G, Gomez AM, Rueda A. Ca(2+) handling alterations and vascular dysfunction in diabetes. Cell Calcium. 2014;56:397–407. doi: 10.1016/j.ceca.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 422.Ferris CD, Cameron AM, Bredt DS, Huganir RL, Snyder SH. Inositol 1,4,5-trisphosphate receptor is phosphorylated by cyclic AMP-dependent protein kinase at serines 1755 and 1589. Biochem Biophys Res Commun. 1991;175:192–198. doi: 10.1016/s0006-291x(05)81219-7. [DOI] [PubMed] [Google Scholar]
- 423.Ferris CD, Huganir RL, Supattapone S, Snyder SH. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989;342:87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- 424.Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- 425.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 426.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 427.Filosa JA, Yao X, Rath G. TRPV4 and the regulation of vascular tone. J Cardiovasc Pharmacol. 2013;61:113–119. doi: 10.1097/FJC.0b013e318279ba42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Fioretti B, Trequattrini C, Sforna L, Harper A, Catacuzzeno L, Franciolini F. Cromakalim activates the K(ATP) and enhances spontaneous transient outward potassium currents in rat saphenous arterial myocytes. Pharmacol Res. 2008;57:398–402. doi: 10.1016/j.phrs.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 429.Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- 430.Fleig A, Chubanov V. Trpm7. Handb Exp Pharmacol. 2014;222:521–546. doi: 10.1007/978-3-642-54215-2_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Fleig A, Penner R. The TRPM ion channel subfamily: Molecular, biophysical and functional features. Trends Pharmacol Sci. 2004;25:633–639. doi: 10.1016/j.tips.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 432.Fleischer S, Ogunbunmi EM, Dixon MC, Fleer EA. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci USA. 1985;82:7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Foggensteiner L, Bevan AP, Thomas R, Coleman N, Boulter C, Bradley J, Ibraghimov-Beskrovnaya O, Klinger K, Sandford R. Cellular and subcellular distribution of polycystin-2, the protein product of the PKD2 gene. J Am Soc Nephrol. 2000;11:814–827. doi: 10.1681/ASN.V115814. [DOI] [PubMed] [Google Scholar]
- 434.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Foskett KJ, Mak DD. Chapter 11: Regulation of IP3R channel gating by Ca2+ and Ca2+ binding proteins. In: Serysheva I, editor. Curr Top Membr. Burlington, MA: Academic Press; 2010. pp. 235–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Foster MN, Coetzee WA. KATP channels in the cardiovascular system. Physiol Rev. 2016;96:177–252. doi: 10.1152/physrev.00003.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Fredricks KT, Liu Y, Rusch NJ, Lombard JH. Role of endothelium and arterial K+ channels in mediating hypoxic dilation of middle cerebral arteries. Am J Physiol. 1994;267:H580–H586. doi: 10.1152/ajpheart.1994.267.2.H580. [DOI] [PubMed] [Google Scholar]
- 438.Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- 439.Freichel M, Tsvilovskyy V, Camacho-Londono JE. TRPC4- and TRPC4-containing channels. Handb Exp Pharmacol. 2014;222:85–128. doi: 10.1007/978-3-642-54215-2_5. [DOI] [PubMed] [Google Scholar]
- 440.Frisbee JC, Maier KG, Stepp DW. Oxidant stress-induced increase in myogenic activation of skeletal muscle resistance arteries in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2002;283:H2160–H2168. doi: 10.1152/ajpheart.00379.2002. [DOI] [PubMed] [Google Scholar]
- 441.Frohlich ED, Scott JB, Haddy FJ. Effect of cations on resistance and responsiveness of renal and forelimb vascular beds. Am J Physiol. 1962;203:583–587. doi: 10.1152/ajplegacy.1962.203.3.583. [DOI] [PubMed] [Google Scholar]
- 442.Fruen BR, Bardy JM, Byrem TM, Strasburg GM, Louis CF. Differential Ca(2+) sensitivity of skeletal and cardiac muscle ryanodine receptors in the presence of calmodulin. Am J Physiol Cell Physiol. 2000;279:C724–C733. doi: 10.1152/ajpcell.2000.279.3.C724. [DOI] [PubMed] [Google Scholar]
- 443.Fruen BR, Black DJ, Bloomquist RA, Bardy JM, Johnson JD, Louis CF, Balog EM. Regulation of the RYR1 and RYR2 Ca2+ release channel isoforms by Ca2+-insensitive mutants of calmodulin. Biochemistry. 2003;42:2740–2747. doi: 10.1021/bi0267689. [DOI] [PubMed] [Google Scholar]
- 444.Fu Y, Westenbroek RE, Scheuer T, Catterall WA. Basal and beta-adrenergic regulation of the cardiac calcium channel CaV1.2 requires phosphorylation of serine 1700. Proc Natl Acad Sci USA. 2014;111:16598–16603. doi: 10.1073/pnas.1419129111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Fujii K, Heistad DD, Faraci FM. Flow-mediated dilatation of the basilar artery in vivo. Circ Res. 1991;69:697–705. doi: 10.1161/01.res.69.3.697. [DOI] [PubMed] [Google Scholar]
- 446.Fujino K, Nakaya S, Wakatsuki T, Miyoshi Y, Nakaya Y, Mori H, Inoue I. Effects of nitroglycerin on ATP-induced Ca(++)-mobilization, Ca(++)-activated K channels and contraction of cultured smooth muscle cells of porcine coronary artery. J Pharmacol Exp Ther. 1991;256:371–377. [PubMed] [Google Scholar]
- 447.Fujita H, Ogura T, Tamagawa M, Uemura H, Sato T, Ishida A, Imamaki M, Kimura F, Miyazaki M, Nakaya H. A key role for the subunit SUR2B in the preferential activation of vascular KATP channels by isoflurane. Br J Pharmacol. 2006;149:573–580. doi: 10.1038/sj.bjp.0706891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Fujiwara Y, Minor DL., Jr X-ray crystal structure of a TRPM assembly domain reveals an antiparallel four-stranded coiled-coil. J Mol Biol. 2008;383:854–870. doi: 10.1016/j.jmb.2008.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Fukami Y, Toki Y, Numaguchi Y, Nakashima Y, Mukawa H, Matsui H, Okumura K, Ito T. Nitroglycerin-induced aortic relaxation mediated by calcium-activated potassium channel is markedly diminished in hypertensive rats. Life Sci. 1998;63:1047–1055. doi: 10.1016/s0024-3205(98)00366-x. [DOI] [PubMed] [Google Scholar]
- 450.Fukao M, Mason HS, Britton FC, Kenyon JL, Horowitz B, Keef KD. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J Biol Chem. 1999;274:10927–10935. doi: 10.1074/jbc.274.16.10927. [DOI] [PubMed] [Google Scholar]
- 451.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010;3:ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Furspan PB, Webb RC. Decreased ATP sensitivity of a K+ channel and enhanced vascular smooth muscle relaxation in genetically hypertensive rats. J Hypertens. 1993;11:1067–1072. doi: 10.1097/00004872-199310000-00010. [DOI] [PubMed] [Google Scholar]
- 453.Furstenau M, Lohn M, Ried C, Luft FC, Haller H, Gollasch M. Calcium sparks in human coronary artery smooth muscle cells resolved by confocal imaging. J Hypertens. 2000;18:1215–1222. doi: 10.1097/00004872-200018090-00007. [DOI] [PubMed] [Google Scholar]
- 454.Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 455.Futatsugi A, Kuwajima G, Mikoshiba K. Tissue-specific and developmentally regulated alternative splicing in mouse skeletal muscle ryanodine receptor mRNA. Biochem J. 1995;305(Pt 2):373–378. doi: 10.1042/bj3050373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 457.Gamperl AK, Hein TW, Kuo L, Cason BA. Isoflurane-induced dilation of porcine coronary microvessels is endothelium dependent and inhibited by glibenclamide. Anesthesiology. 2002;96:1465–1471. doi: 10.1097/00000542-200206000-00028. [DOI] [PubMed] [Google Scholar]
- 458.Ganitkevich V, Isenberg G. Isolated guinea pig coronary smooth muscle cells. Acetylcholine induces hyperpolarization due to sarcoplasmic reticulum calcium release activating potassium channels. Circ Res. 1990;67:525–528. doi: 10.1161/01.res.67.2.525. [DOI] [PubMed] [Google Scholar]
- 459.Ganitkevich V, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. J Physiol. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Gannon KP, Vanlandingham LG, Jernigan NL, Grifoni SC, Hamilton G, Drummond HA. Impaired pressure-induced constriction in mouse middle cerebral arteries of ASIC2 knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H1793–H1803. doi: 10.1152/ajpheart.01380.2007. [DOI] [PubMed] [Google Scholar]
- 461.Garcia Z, Earley S. PLC gamma-1 is required for IP3-mediated activation of TRPM4 and pressure-induced depolarization and vasoconstriction in cerebral arteries. Faseb Journal. 2011;25:1024.1016. [Google Scholar]
- 462.Garcia-Elias A, Mrkonjic S, Jung C, Pardo-Pastor C, Vicente R, Valverde MA. The TRPV4 channel. Handb Exp Pharmacol. 2014;222:293–319. doi: 10.1007/978-3-642-54215-2_12. [DOI] [PubMed] [Google Scholar]
- 463.Gardiner SM, March JE, Kemp PA, Maguire JJ, Kuc RE, Davenport AP, Bennett T. Regional heterogeneity in the haemodynamic responses to urotensin II infusion in relation to UT receptor localisation. Br J Pharmacol. 2006;147:612–621. doi: 10.1038/sj.bjp.0706503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Gardos G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958;30:653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- 465.Garland CJ, McPherson GA. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Gaudet R. A primer on ankyrin repeat function in TRP channels and beyond. Mol Biosyst. 2008;4:372–379. doi: 10.1039/b801481g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Ge ZD, Zhang XH, Fung PC, He GW. Endothelium-dependent hyperpolarization and relaxation resistance to N(G)-nitro-L-arginine and indomethacin in coronary circulation. Cardiovasc Res. 2000;46:547–556. doi: 10.1016/s0008-6363(00)00040-7. [DOI] [PubMed] [Google Scholar]
- 468.Gebremedhin D, Kaldunski M, Jacobs ER, Harder DR, Roman RJ. Coexistence of two types of Ca(2+)-activated K+ channels in rat renal arterioles. Am J Physiol. 1996;270:F69–F81. doi: 10.1152/ajprenal.1996.270.1.F69. [DOI] [PubMed] [Google Scholar]
- 469.Geiger J, Zou AP, Campbell WB, Li PL. Inhibition of cADP-ribose formation produces vasodilation in bovine coronary arteries. Hypertension. 2000;35:397–402. doi: 10.1161/01.hyp.35.1.397. [DOI] [PubMed] [Google Scholar]
- 470.Geiselhoringer A, Werner M, Sigl K, Smital P, Worner R, Acheo L, Stieber J, Weinmeister P, Feil R, Feil S, Wegener J, Hofmann F, Schlossmann J. IRAG is essential for relaxation of receptor-triggered smooth muscle contraction by cGMP kinase. Embo J. 2004;23:4222–4231. doi: 10.1038/sj.emboj.7600440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Gelband CH, Ishikawa T, Post JM, Keef KD, Hume JR. Intracellular divalent cations block smooth muscle K+ channels. Circ Res. 1993;73:24–34. doi: 10.1161/01.res.73.1.24. [DOI] [PubMed] [Google Scholar]
- 472.Ghafouri S, Hajizadeh S, Mani AR. Enhancement of insulin-induced cutaneous vasorelaxation by exercise in rats: A role for nitric oxide and K(Ca2+) channels. Eur J Pharmacol. 2011;652:89–95. doi: 10.1016/j.ejphar.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 473.Ghisdal P, Gomez JP, Morel N. Action of a NO donor on the excitation-contraction pathway activated by noradrenaline in rat superior mesenteric artery. J Physiol. 2000;522(Pt 1):83–96. doi: 10.1111/j.1469-7793.2000.t01-3-00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Ghosh M, Hanna ST, Wang R, McNeill JR. Altered vascular reactivity and KATP channel currents in vascular smooth muscle cells from deoxycorticosterone acetate (DOCA)-salt hypertensive rats. J Cardiovasc Pharmacol. 2004;44:525–531. doi: 10.1097/00005344-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 475.Ghosh P, Mora Solis FR, Dominguez JM, II, Spier SA, Donato AJ, Delp MD, Muller-Delp JM. Exercise training reverses aging-induced impairment of myogenic constriction in skeletal muscle arterioles. J Appl Physiol (1985) 2015;118:904–911. doi: 10.1152/japplphysiol.00277.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Giangiacomo KM, Kamassah A, Harris G, McManus OB. Mechanism of maxi-K channel activation by dehydrosoyasaponin-I. J Gen Physiol. 1998;112:485–501. doi: 10.1085/jgp.112.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Gidday JM, Maceren RG, Shah AR, Meier JA, Zhu Y. KATP channels mediate adenosine-induced hyperemia in retina. Invest Ophthalmol Vis Sci. 1996;37:2624–2633. [PubMed] [Google Scholar]
- 479.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Gloerich M, Bos JL. Epac: Defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–375. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- 481.Gogelein H, Bruggemann A, Gerlach U, Brendel J, Busch AE. Inhibition of IKs channels by HMR 1556. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:480–488. doi: 10.1007/s002100000284. [DOI] [PubMed] [Google Scholar]
- 482.Golenhofen K, Hermstein N, Lammel E. Membrane potential and contraction of vascular smooth muscle (portal vein) during application of noradrenaline and high potassium, and selective inhibitory effects of iproveratril (verapamil) Microvasc Res. 1973;5:73–80. doi: 10.1016/s0026-2862(73)80007-x. [DOI] [PubMed] [Google Scholar]
- 483.Gollasch M, Lohn M, Furstenau M, Nelson MT, Luft FC, Haller H. Ca2+ channels, Ca2+ sparks, and regulation of arterial smooth muscle function. Z Kardiol. 2000;89(Suppl 2):15–19. doi: 10.1007/s003920070095. [DOI] [PubMed] [Google Scholar]
- 484.Gollasch M, Ried C, Bychkov R, Luft FC, Haller H. K+ currents in human coronary artery vascular smooth muscle cells. Circ Res. 1996;78:676–688. doi: 10.1161/01.res.78.4.676. [DOI] [PubMed] [Google Scholar]
- 485.Gomez-Hernandez JM, Lorra C, Pardo LA, Stuhmer W, Pongs O, Heinemann SH, Elliott AA. Molecular basis for different pore properties of potassium channels from the rat brain Kv1 gene family. Pflugers Arch. 1997;434:661–668. doi: 10.1007/s004240050449. [DOI] [PubMed] [Google Scholar]
- 486.Gonzales AL, Amberg GC, Earley S. Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2010;299:C279–C288. doi: 10.1152/ajpcell.00550.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Gonzales AL, Earley S. Endogenous cytosolic Ca(2+) buffering is necessary for TRPM4 activity in cerebral artery smooth muscle cells. Cell Calcium. 2012;51:82–93. doi: 10.1016/j.ceca.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Gonzales AL, Yang Y, Sullivan MN, Sanders L, Dabertrand F, Hill-Eubanks DC, Nelson MT, Earley S. A PLCgamma1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci Signal. 2014;7:ra49. doi: 10.1126/scisignal.2004732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Gonzalez C, Baez-Nieto D, Valencia I, Oyarzun I, Rojas P, Naranjo D, Latorre R. K(+) channels: Function-structural overview. Compr Physiol. 2012;2:2087–2149. doi: 10.1002/cphy.c110047. [DOI] [PubMed] [Google Scholar]
- 490.Gonzalez-Perez V, Xia XM, Lingle CJ. Functional regulation of BK potassium channels by gamma1 auxiliary subunits. Proc Natl Acad Sci U S A. 2014;111:4868–4873. doi: 10.1073/pnas.1322123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Goodwill AG, Fu L, Noblet JN, Casalini ED, Sassoon D, Berwick ZC, Kassab GS, Tune JD, Dick GM. KV7 channels contribute to paracrine, but not metabolic or ischemic, regulation of coronary vascular reactivity in swine. Am J Physiol Heart Circ Physiol. 2016;310:H693–H704. doi: 10.1152/ajpheart.00688.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Goodwill AG, Noblet JN, Sassoon D, Fu L, Kassab GS, Schepers L, Herring BP, Rottgen TS, Tune JD, Dick GM. Critical contribution of KV1 channels to the regulation of coronary blood flow. Basic Res Cardiol. 2016;111:56. doi: 10.1007/s00395-016-0575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Gordienko DV, Bolton TB. Crosstalk between ryanodine receptors and IP(3) receptors as a factor shaping spontaneous Ca(2+)-release events in rabbit portal vein myocytes. J Physiol. 2002;542:743–762. doi: 10.1113/jphysiol.2001.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Gordienko DV, Clausen C, Goligorsky MS. Ionic currents and endothelin signaling in smooth muscle cells from rat renal resistance arteries. Am J Physiol. 1994;266:F325–F341. doi: 10.1152/ajprenal.1994.266.2.F325. [DOI] [PubMed] [Google Scholar]
- 495.Gordienko D, Povstyan O, Sukhanova K, Raphael M, Harhun M, Dyskina Y, Lehen’kyi V, Jama A, Lu ZL, Skryma R, Prevarskaya N. Impaired P2X signalling pathways in renal microvascular myocytes in genetic hypertension. Cardiovasc Res. 2015;105:131–142. doi: 10.1093/cvr/cvu249. [DOI] [PubMed] [Google Scholar]
- 496.Goto K, Kasuya Y, Matsuki N, Takuwa Y, Kurihara H, Ishikawa T, Kimura S, Yanagisawa M, Masaki T. Endothelin activates the dihydropyridine-sensitive, voltage-dependent Ca2+ channel in vascular smooth muscle. Proc Natl Acad Sci USA. 1989;86:3915–3918. doi: 10.1073/pnas.86.10.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Grand T, Demion M, Norez C, Mettey Y, Launay P, Becq F, Bois P, Guinamard R. 9-Phenanthrol inhibits human TRPM4 but not TRPM5 cationic channels. Br J Pharmacol. 2008;153:1697–1705. doi: 10.1038/bjp.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Grayson TH, Haddock RE, Murray TP, Wojcikiewicz RJ, Hill CE. Inositol 1,4,5-trisphosphate receptor subtypes are differentially distributed between smooth muscle and endothelial layers of rat arteries. Cell Calcium. 2004;36:447–458. doi: 10.1016/j.ceca.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 499.Greenwood IA, Ohya S. New tricks for old dogs: KCNQ expression and role in smooth muscle. Br J Pharmacol. 2009;156:1196–1203. doi: 10.1111/j.1476-5381.2009.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Gribkoff VK, Starrett JE, Jr, Dworetzky SI, Hewawasam P, Boissard CG, Cook DA, Frantz SW, Heman K, Hibbard JR, Huston K, Johnson G, Krishnan BS, Kinney GG, Lombardo LA, Meanwell NA, Molinoff PB, Myers RA, Moon SL, Ortiz A, Pajor L, Pieschl RL, Post-Munson DJ, Signor LJ, Srinivas N, Taber MT, Thalody G, Trojnacki JT, Wiener H, Yeleswaram K, Yeola SW. Targeting acute ischemic stroke with a calcium-sensitive opener of maxi-K potassium channels. Nat Med. 2001;7:471–477. doi: 10.1038/86546. [DOI] [PubMed] [Google Scholar]
- 501.Griffin MD, Torres VE, Grande JP, Kumar R. Vascular expression of polycystin. J Am Soc Nephrol. 1997;8:616–626. doi: 10.1681/ASN.V84616. [DOI] [PubMed] [Google Scholar]
- 502.Grifoni SC, Chiposi R, McKey SE, Ryan MJ, Drummond HA. Altered whole kidney blood flow autoregulation in a mouse model of reduced beta-ENaC. Am J Physiol Renal Physiol. 2010;298:F285–F292. doi: 10.1152/ajprenal.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem. 2003;278:21493–21501. doi: 10.1074/jbc.M300945200. [DOI] [PubMed] [Google Scholar]
- 504.Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol. 1994;45:1227–1234. [PubMed] [Google Scholar]
- 505.Gros R, Van Wert R, You X, Thorin E, Husain M. Effects of age, gender, and blood pressure on myogenic responses of mesenteric arteries from C57BL/6 mice. Am J Physiol Heart Circ Physiol. 2002;282:H380–H388. doi: 10.1152/ajpheart.2002.282.1.H380. [DOI] [PubMed] [Google Scholar]
- 506.Gross GJ, Auchampach JA. Role of ATP dependent potassium channels in myocardial ischaemia. Cardiovasc Res. 1992;26:1011–1016. doi: 10.1093/cvr/26.11.1011. [DOI] [PubMed] [Google Scholar]
- 507.Gross SA, Guzman GA, Wissenbach U, Philipp SE, Zhu MX, Bruns D, Cavalie A. TRPC5 is a Ca2+-activated channel functionally coupled to Ca2+-selective ion channels. J Biol Chem. 2009;284:34423–34432. doi: 10.1074/jbc.M109.018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Grunnet M, Kaufmann WA. Coassembly of big conductance Ca2+-activated K+ channels and L-type voltage-gated Ca2+ channels in rat brain. J Biol Chem. 2004;279:36445–36453. doi: 10.1074/jbc.M402254200. [DOI] [PubMed] [Google Scholar]
- 509.Grupe A, Schroter KH, Ruppersberg JP, Stocker M, Drewes T, Beckh S, Pongs O. Cloning and expression of a human voltage-gated potassium channel. A novel member of the RCK potassium channel family. Embo J. 1990;9:1749–1756. doi: 10.1002/j.1460-2075.1990.tb08299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Guan Z, Pollock JS, Cook AK, Hobbs JL, Inscho EW. Effect of epithelial sodium channel blockade on the myogenic response of rat juxtamedullary afferent arterioles. Hypertension. 2009;54:1062–1069. doi: 10.1161/HYPERTENSIONAHA.109.137992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Guarini G, Ohanyan VA, Kmetz JG, DelloStritto DJ, Thoppil RJ, Thodeti CK, Meszaros JG, Damron DS, Bratz IN. Disruption of TRPV1-mediated coupling of coronary blood flow to cardiac metabolism in diabetic mice: Role of nitric oxide and BK channels. Am J Physiol Heart Circ Physiol. 2012;303:H216–H223. doi: 10.1152/ajpheart.00011.2012. [DOI] [PubMed] [Google Scholar]
- 512.Guerrero-Hernandez A, Gomez-Viquez L, Guerrero-Serna G, Rueda A. Ryanodine receptors in smooth muscle. Front Biosci. 2002;7:d1676–d1688. doi: 10.2741/a871. [DOI] [PubMed] [Google Scholar]
- 513.Gui P, Wu X, Ling S, Stotz SC, Winkfein RJ, Wilson E, Davis GE, Braun AP, Zamponi GW, Davis MJ. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J Biol Chem. 2006;281:14015–14025. doi: 10.1074/jbc.M600433200. [DOI] [PubMed] [Google Scholar]
- 514.Guia A, Wan X, Courtemanche M, Leblanc N. Local Ca2+ entry through L-type Ca2+ channels activates Ca2+-dependent K+ channels in rabbit coronary myocytes. Circ Res. 1999;84:1032–1042. doi: 10.1161/01.res.84.9.1032. [DOI] [PubMed] [Google Scholar]
- 515.Gulia J, Navedo MF, Gui P, Chao JT, Mercado JL, Santana LF, Davis MJ. Regulation of L-type calcium channel sparklet activity by c-Src and PKC-alpha. Am J Physiol Cell Physiol. 2013;305:C568–C577. doi: 10.1152/ajpcell.00381.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Gunther M. Perfusion imaging. J Magn Reson Imaging. 2014;40:269–279. doi: 10.1002/jmri.24382. [DOI] [PubMed] [Google Scholar]
- 517.Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia. 2012;53:412–424. doi: 10.1111/j.1528-1167.2011.03365.x. [DOI] [PubMed] [Google Scholar]
- 518.Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, Tian H, Jiang C, Zhu D. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-alpha1C. Hypertension. 2012;59:1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 519.Gupta PK, Subramani J, Leo MD, Sikarwar AS, Parida S, Prakash VR, Mishra SK. Role of voltage-dependent potassium channels and myo-endothelial gap junctions in 4-aminopyridine-induced inhibition of acetylcholine relaxation in rat carotid artery. Eur J Pharmacol. 2008;591:171–176. doi: 10.1016/j.ejphar.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 520.Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J. 2009;38:305–318. doi: 10.1007/s00249-008-0326-8. [DOI] [PubMed] [Google Scholar]
- 521.Gustafsson F, Andreasen D, Salomonsson M, Jensen BL, Holstein-Rathlou N. Conducted vasoconstriction in rat mesenteric arterioles: Role for dihydropyridine-insensitive Ca(2+) channels. Am J Physiol Heart Circ Physiol. 2001;280:H582–H590. doi: 10.1152/ajpheart.2001.280.2.H582. [DOI] [PubMed] [Google Scholar]
- 522.Gustafsson H, Bulow A, Nilsson H. Rhythmic contractions of isolated, pressurized small arteries from rat. Acta Physiol Scand. 1994;152:145–152. doi: 10.1111/j.1748-1716.1994.tb09794.x. [DOI] [PubMed] [Google Scholar]
- 523.Gustafsson H, Nilsson H. Rhythmic contractions of isolated small arteries from rat: Role of calcium. Acta Physiol Scand. 1993;149:283–291. doi: 10.1111/j.1748-1716.1993.tb09623.x. [DOI] [PubMed] [Google Scholar]
- 524.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 525.Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Gyorke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 527.Haarmann CS, Fink RH, Dulhunty AF. Oxidation and reduction of pig skeletal muscle ryanodine receptors. Biophys J. 1999;77:3010–3022. doi: 10.1016/S0006-3495(99)77132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Haddock RE, Grayson TH, Morris MJ, Howitt L, Chadha PS, Sandow SL. Diet-induced obesity impairs endothelium-derived hyperpolarization via altered potassium channel signaling mechanisms. PLoS One. 2011;6:e16423. doi: 10.1371/journal.pone.0016423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Haddock RE, Hill CE. Differential activation of ion channels by inositol 1,4,5-trisphosphate (IP3)- and ryanodine-sensitive calcium stores in rat basilar artery vasomotion. J Physiol. 2002;545:615–627. doi: 10.1113/jphysiol.2002.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Haddock RE, Hirst GD, Hill CE. Voltage independence of vasomotion in isolated irideal arterioles of the rat. J Physiol. 2002;540:219–229. doi: 10.1113/jphysiol.2001.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R546–R552. doi: 10.1152/ajpregu.00491.2005. [DOI] [PubMed] [Google Scholar]
- 532.Hagiwara S, Mitsui M, Karaki H. Effects of felodipine, nifedipine and verapamil on cytosolic Ca2+ and contraction in vascular smooth muscle. Eur J Pharmacol. 1993;234:1–7. doi: 10.1016/0014-2999(93)90698-h. [DOI] [PubMed] [Google Scholar]
- 533.Hagiwara S, Miyazaki S, Moody W, Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Hagiwara S, Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18:61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- 535.Hain J, Onoue H, Mayrleitner M, Fleischer S, Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. J Biol Chem. 1995;270:2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]
- 536.Haitin Y, Attali B. The C-terminus of Kv7 channels: A multifunctional module. J Physiol. 2008;586:1803–1810. doi: 10.1113/jphysiol.2007.149187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Hajnoczky G, Thomas AP. Minimal requirements for calcium oscillations driven by the IP3 receptor. Embo J. 1997;16:3533–3543. doi: 10.1093/emboj/16.12.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Hakamata Y, Nakai J, Takeshima H, Imoto K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett. 1992;312:229–235. doi: 10.1016/0014-5793(92)80941-9. [DOI] [PubMed] [Google Scholar]
- 539.Halaszovich CR, Zitt C, Jungling E, Luckhoff A. Inhibition of TRP3 channels by lanthanides. Block from the cytosolic side of the plasma membrane. J Biol Chem. 2000;275:37423–37428. doi: 10.1074/jbc.M007010200. [DOI] [PubMed] [Google Scholar]
- 540.Hald BO, Jacobsen JC, Braunstein TH, Inoue R, Ito Y, Sorensen PG, Holstein-Rathlou NH, Jensen LJ. BKCa and KV channels limit conducted vasomotor responses in rat mesenteric terminal arterioles. Pflugers Arch. 2012;463:279–295. doi: 10.1007/s00424-011-1049-8. [DOI] [PubMed] [Google Scholar]
- 541.Halliday FC, Aaronson PI, Evans AM, Gurney AM. The pharmacological properties of K +currents from rabbit isolated aortic smooth muscle cells. Br J Pharmacol. 1995;116:3139–3148. doi: 10.1111/j.1476-5381.1995.tb15116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Halpern W, Osol G, Coy GS. Mechanical behavior of pressurized in vitro prearteriolar vessels determined with a video system. Ann Biomed Eng. 1984;12:463–479. doi: 10.1007/BF02363917. [DOI] [PubMed] [Google Scholar]
- 543.Hamilton SL. Ryanodine receptors. Cell Calcium. 2005;38:253–260. doi: 10.1016/j.ceca.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 544.Hamilton TC, Beerahee A, Moen JS, Price RK, Ramji JV, Clapham JC. Levcromakalim. Cardiovascular Drug Reviews. 1993;11:199–222. [Google Scholar]
- 545.Hamilton SL, Serysheva II. Ryanodine receptor structure: Progress and challenges. J Biol Chem. 2009;284:4047–4051. doi: 10.1074/jbc.R800054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Hamilton SL, Yatani A, Brush K, Schwartz A, Brown AM. A comparison between the binding and electrophysiological effects of dihydropyridines on cardiac membranes. Mol Pharmacol. 1987;31:221–231. [PubMed] [Google Scholar]
- 547.Hammer LW, Ligon AL, Hester RL. Differential inhibition of functional dilation of small arterioles by indomethacin and glibenclamide. Hypertension. 2001;37:599–603. doi: 10.1161/01.hyp.37.2.599. [DOI] [PubMed] [Google Scholar]
- 548.Han H, Rosenhouse-Dantsker A, Gnanasambandam R, Epshtein Y, Chen Z, Sachs F, Minshall RD, Levitan I. Silencing of Kir2 channels by caveolin-1: Cross-talk with cholesterol. J Physiol. 2014;592:4025–4038. doi: 10.1113/jphysiol.2014.273177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Hansen PB, Jensen BL, Andreasen D, Friis UG, Skott O. Vascular smooth muscle cells express the alpha(1A) subunit of a P-/Q-type voltage-dependent Ca(2+)channel, and it is functionally important in renal afferent arterioles. Circ Res. 2000;87:896–902. doi: 10.1161/01.res.87.10.896. [DOI] [PubMed] [Google Scholar]
- 550.Hansen PB, Jensen BL, Andreasen D, Skott O. Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ Res. 2001;89:630–638. doi: 10.1161/hh1901.097126. [DOI] [PubMed] [Google Scholar]
- 551.Hansen PR, Olesen SP. Relaxation of rat resistance arteries by acetylcholine involves a dual mechanism: Activation of K+ channels and formation of nitric oxide. Pharmacol Toxicol. 1997;80:280–285. doi: 10.1111/j.1600-0773.1997.tb01974.x. [DOI] [PubMed] [Google Scholar]
- 552.Hansen PB, Poulsen CB, Walter S, Marcussen N, Cribbs LL, Skott O, Jensen BL. Functional importance of L- and P/Q-type voltage-gated calcium channels in human renal vasculature. Hypertension. 2011;58:464–470. doi: 10.1161/HYPERTENSIONAHA.111.170845. [DOI] [PubMed] [Google Scholar]
- 553.Hara Y, Kitamura K, Kuriyama H. Actions of 4-aminopyridine on vascular smooth muscle tissues of the guinea-pig. Br J Pharmacol. 1980;68:99–106. doi: 10.1111/j.1476-5381.1980.tb10704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Harder DR, Sperelakis N. Action potentials induced in guinea pig arterial smooth muscle by tetraethylammonium. Am J Physiol. 1979;237:C75–C80. doi: 10.1152/ajpcell.1979.237.1.C75. [DOI] [PubMed] [Google Scholar]
- 555.Harnett KM, Biancani P. Calcium-dependent and calcium-independent contractions in smooth muscles. Am J Med. 2003;115(Suppl 3A):24S–30S. doi: 10.1016/s0002-9343(03)00232-8. [DOI] [PubMed] [Google Scholar]
- 556.Harootunian AT, Kao JP, Paranjape S, Tsien RY. Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP3. Science. 1991;251:75–78. doi: 10.1126/science.1986413. [DOI] [PubMed] [Google Scholar]
- 557.Harraz OF, Abd El-Rahman RR, Bigdely-Shamloo K, Wilson SM, Brett SE, Romero M, Gonzales AL, Earley S, Vigmond EJ, Nygren A, Menon BK, Mufti RE, Watson T, Starreveld Y, Furstenhaupt T, Muellerleile PR, Kurjiaka DT, Kyle BD, Braun AP, Welsh DG. Ca(V)3.2 channels and the induction of negative feedback in cerebral arteries. Circ Res. 2014;115:650–661. doi: 10.1161/CIRCRESAHA.114.304056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Harraz OF, Brett SE, Welsh DG. Nitric oxide suppresses vascular voltage-gated T-type Ca2+ channels through cGMP/PKG signaling. Am J Physiol Heart Circ Physiol. 2014;306:H279–H285. doi: 10.1152/ajpheart.00743.2013. [DOI] [PubMed] [Google Scholar]
- 559.Harraz OF, Brett SE, Zechariah A, Romero M, Puglisi JL, Wilson SM, Welsh DG. Genetic ablation of CaV3.2 channels enhances the arterial myogenic response by modulating the RyR-BKCa axis. Arterioscler Thromb Vasc Biol. 2015;35:1843–1851. doi: 10.1161/ATVBAHA.115.305736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Harraz OF, Welsh DG. Protein kinase A regulation of T-type Ca2+ channels in rat cerebral arterial smooth muscle. J Cell Sci. 2013;126:2944–2954. doi: 10.1242/jcs.128363. [DOI] [PubMed] [Google Scholar]
- 561.Harteneck C. Function and pharmacology of TRPM cation channels. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:307–314. doi: 10.1007/s00210-005-1034-x. [DOI] [PubMed] [Google Scholar]
- 562.Harteneck C, Gollasch M. Pharmacological modulation of diacylglycerol-sensitive TRPC3/6/7 channels. Curr Pharm Biotechnol. 2011;12:35–41. doi: 10.2174/138920111793937943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Harteneck C, Reiter B. TRP channels activated by extracellular hypoosmoticity in epithelia. Biochem Soc Trans. 2007;35:91–95. doi: 10.1042/BST0350091. [DOI] [PubMed] [Google Scholar]
- 564.Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Harvey RD, Hell JW. CaV1.2 signaling complexes in the heart. J Mol Cell Cardiol. 2013;58:143–152. doi: 10.1016/j.yjmcc.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Hashemzadeh-Gargari H, Rembold CM. Histamine activates whole cell K+ currents in swine carotid arterial smooth muscle cell. Comp Biochem Physiol C. 1992;102:33–37. doi: 10.1016/0742-8413(92)90039-a. [DOI] [PubMed] [Google Scholar]
- 567.Hassane S, Claij N, Jodar M, Dedman A, Lauritzen I, Duprat F, Koenderman JS, van der Wal A, Breuning MH, de Heer E, Honore E, DeRuiter MC, Peters DJ. Pkd1-inactivation in vascular smooth muscle cells and adaptation to hypertension. Lab Invest. 2011;91:24–32. doi: 10.1038/labinvest.2010.159. [DOI] [PubMed] [Google Scholar]
- 568.Hassett CC. Effect of ryanodine on the oxygen consumption of periplaneta americana. Science. 1948;108:138–139. doi: 10.1126/science.108.2797.138. [DOI] [PubMed] [Google Scholar]
- 569.Hayabuchi Y, Dart C, Standen NB. Evidence for involvement of A-kinase anchoring protein in activation of rat arterial K(ATP) channels by protein kinase A. J Physiol. 2001;536:421–427. doi: 10.1111/j.1469-7793.2001.0421c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Hayabuchi Y, Davies NW, Standen NB. Angiotensin II inhibits rat arterial KATP channels by inhibiting steady-state protein kinase A activity and activating protein kinase Ce. J Physiol. 2001;530:193–205. doi: 10.1111/j.1469-7793.2001.0193l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Hayabuchi Y, Standen NB, Davies NW. Angiotensin II inhibits and alters kinetics of voltage-gated K(+) channels of rat arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2001;281:H2480–H2489. doi: 10.1152/ajpheart.2001.281.6.H2480. [DOI] [PubMed] [Google Scholar]
- 572.Hayashi T, Su TP. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc Natl Acad Sci U S A. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Hayoz S, Bradley V, Boerman EM, Nourian Z, Segal SS, Jackson WF. Aging increases capacitance and spontaneous transient outward current amplitude of smooth muscle cells from murine superior epigastric arteries. Am J Physiol Heart Circ Physiol. 2014;306:H1512–H1524. doi: 10.1152/ajpheart.00492.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.He LP, Hewavitharana T, Soboloff J, Spassova MA, Gill DL. A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J Biol Chem. 2005;280:10997–11006. doi: 10.1074/jbc.M411797200. [DOI] [PubMed] [Google Scholar]
- 575.He Y, Kang Y, Leung YM, Xia F, Gao X, Xie H, Gaisano HY, Tsushima RG. Modulation of Kv2.1 channel gating and TEA sensitivity by distinct domains of SNAP-25. Biochem J. 2006;396:363–369. doi: 10.1042/BJ20051478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Heady TN, Gomora JC, Macdonald TL, Perez-Reyes E. Molecular pharmacology of T-type Ca2+ channels. Jpn J Pharmacol. 2001;85:339–350. doi: 10.1254/jjp.85.339. [DOI] [PubMed] [Google Scholar]
- 577.Heaps CL, Bowles DK. Gender-specific K(+)-channel contribution to adenosine-induced relaxation in coronary arterioles. J Appl Physiol (1985) 2002;92:550–558. doi: 10.1152/japplphysiol.00566.2001. [DOI] [PubMed] [Google Scholar]
- 578.Heaps CL, Tharp DL, Bowles DK. Hypercholesterolemia abolishes voltage-dependent K+ channel contribution to adenosine-mediated relaxation in porcine coronary arterioles. Am J Physiol Heart Circ Physiol. 2005;288:H568–H576. doi: 10.1152/ajpheart.00157.2004. [DOI] [PubMed] [Google Scholar]
- 579.Hedegaard ER, Nielsen BD, Kun A, Hughes AD, Kroigaard C, Mogensen S, Matchkov VV, Frobert O, Simonsen U. KV 7 channels are involved in hypoxia-induced vasodilatation of porcine coronary arteries. Br J Pharmacol. 2014;171:69–82. doi: 10.1111/bph.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Hein TW, Kuo L. cAMP-independent dilation of coronary arterioles to adenosine: Role of nitric oxide, G proteins, and K(ATP) channels. Circ Res. 1999;85:634–642. doi: 10.1161/01.res.85.7.634. [DOI] [PubMed] [Google Scholar]
- 581.Hein TW, Xu W, Kuo L. Dilation of retinal arterioles in response to lactate: Role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Invest Ophthalmol Vis Sci. 2006;47:693–699. doi: 10.1167/iovs.05-1224. [DOI] [PubMed] [Google Scholar]
- 582.Hein TW, Xu W, Ren Y, Kuo L. Cellular signalling pathways mediating dilation of porcine pial arterioles to adenosine A(2)A receptor activation. Cardiovasc Res. 2013;99:156–163. doi: 10.1093/cvr/cvt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Heintz A, Damm M, Brand M, Koch T, Deussen A. Coronary flow regulation in mouse heart during hypercapnic acidosis: role of NO and its compensation during eNOS impairment. Cardiovasc Res. 2008;77:188–196. doi: 10.1093/cvr/cvm014. [DOI] [PubMed] [Google Scholar]
- 584.Heinze C, Seniuk A, Sokolov MV, Huebner AK, Klementowicz AE, Szijarto IA, Schleifenbaum J, Vitzthum H, Gollasch M, Ehmke H, Schroeder BC, Hubner CA. Disruption of vascular Ca2+-activated chloride currents lowers blood pressure. J Clin Invest. 2014;124:675–686. doi: 10.1172/JCI70025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci. 2005;118:917–928. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- 586.Helmchen F, Denk W, Kerr JN. Miniaturization of two-photon microscopy for imaging in freely moving animals. Cold Spring Harb Protoc. 2013;2013:904–913. doi: 10.1101/pdb.top078147. [DOI] [PubMed] [Google Scholar]
- 587.Helmchen F, Kleinfeld D. Chapter 10. In vivo measurements of blood flow and glial cell function with two-photon laser-scanning microscopy. Methods Enzymol. 2008;444:231–254. doi: 10.1016/S0076-6879(08)02810-3. [DOI] [PubMed] [Google Scholar]
- 588.Hempelmann RG, Seebeck J, Kruse ML, Ziegler A, Mehdorn HM. Role of potassium channels in the relaxation induced by the nitric oxide (NO) donor DEA/NO in the isolated rat basilar artery. Neurosci Lett. 2001;313:21–24. doi: 10.1016/s0304-3940(01)02225-x. [DOI] [PubMed] [Google Scholar]
- 589.Hendriks MG, Pfaffendorf M, van Zwieten PA. The role of nitric oxide and potassium channels in endothelium-dependent vasodilation in SHR. Blood Press. 1993;2:233–243. doi: 10.3109/08037059309077557. [DOI] [PubMed] [Google Scholar]
- 590.Hennig GW, Smith CB, O’Shea DM, Smith TK. Patterns of intracellular and intercellular Ca2+ waves in the longitudinal muscle layer of the murine large intestine in vitro. J Physiol. 2002;543:233–253. doi: 10.1113/jphysiol.2002.018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Henry P, Pearson WL, Nichols CG. Protein kinase C inhibition of cloned inward rectifier (HRK1/KIR2.3) K+ channels expressed in Xenopus oocytes. J Physiol. 1996;495(Pt 3):681–688. doi: 10.1113/jphysiol.1996.sp021625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Hermsmeyer K, Harder D. Membrane ATPase mechanism of K+-return relaxation in arterial muscles of stroke-prone SHR and WKY. Am J Physiol. 1986;250:C557–C562. doi: 10.1152/ajpcell.1986.250.4.C557. [DOI] [PubMed] [Google Scholar]
- 593.Hernanz R, Alonso MJ, Baena AB, Salaices M, Alvarez L, Castillo-Olivares JL, Marin J. Mechanisms involved in relaxation induced by exogenous nitric oxide in pig coronary arteries. Methods Find Exp Clin Pharmacol. 1999;21:155–160. doi: 10.1358/mf.1999.21.3.534823. [DOI] [PubMed] [Google Scholar]
- 594.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 595.Hill MA, Davis MJ. Coupling a change in intraluminal pressure to vascular smooth muscle depolarization: Still stretching for an explanation. Am J Physiol Heart Circ Physiol. 2007;292:H2570–H2572. doi: 10.1152/ajpheart.00331.2007. [DOI] [PubMed] [Google Scholar]
- 596.Hill MA, Davis MJ, Meininger GA, Potocnik SJ, Murphy TV. Arteriolar myogenic signalling mechanisms: Implications for local vascular function. Clin Hemorheol Microcirc. 2006;34:67–79. [PubMed] [Google Scholar]
- 597.Hill MA, Falcone JC, Meininger GA. Evidence for protein kinase C involvement in arteriolar myogenic reactivity. Am J Physiol. 1990;259:H1586–H1594. doi: 10.1152/ajpheart.1990.259.5.H1586. [DOI] [PubMed] [Google Scholar]
- 598.Hill CE, Kirton A, Wu DD, Vanner SJ. Role of maxi-K+ channels in endothelin-induced vasoconstriction of mesenteric and submucosal arterioles. Am J Physiol. 1997;273:G1087–G1093. doi: 10.1152/ajpgi.1997.273.5.G1087. [DOI] [PubMed] [Google Scholar]
- 599.Hill MA, Meininger GA. Calcium entry and myogenic phenomena in skeletal muscle arterioles. Am J Physiol. 1994;267:H1085–H1092. doi: 10.1152/ajpheart.1994.267.3.H1085. [DOI] [PubMed] [Google Scholar]
- 600.Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Invited review: Arteriolar smooth muscle mechanotransduction: Ca(2+) signaling pathways underlying myogenic reactivity. J Appl Physiol (1985) 2001;91:973–983. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- 601.Hill-Eubanks DC, Gonzales AL, Sonkusare SK, Nelson MT. Vascular TRP channels: Performing under pressure and going with the flow. Physiology (Bethesda) 2014;29:343–360. doi: 10.1152/physiol.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Hille B. Ionic Channels of Excitable Membranes. Sunderland: Sinauer Associates; 2001. p. 814. [Google Scholar]
- 603.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Ho WS, Zheng X, Zhang DX. Role of endothelial TRPV4 channels in vascular actions of the endocannabinoid, 2-arachidonoylglycerol. Br J Pharmacol. 2015;172:5251–5264. doi: 10.1111/bph.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Hobai IA, Bates JA, Howarth FC, Levi AJ. Inhibition by external Cd2+ of Na/Ca exchange and L-type Ca channel in rabbit ventricular myocytes. Am J Physiol. 1997;272:H2164–H2172. doi: 10.1152/ajpheart.1997.272.5.H2164. [DOI] [PubMed] [Google Scholar]
- 606.Hodgkin AL, Huxley AF. Current carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952;116:449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Hodgkin AL, Huxley AF, Katz B. Ionic currents underlying activity in the giant axon of the squid. Arch Sci Physiol. 1949;3:129–150. [Google Scholar]
- 608.Hodnett BL, Xiang L, Dearman JA, Carter CB, Hester RL. K(ATP)-mediated vasodilation is impaired in obese Zucker rats. Microcirculation. 2008;15:485–494. doi: 10.1080/10739680801942240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Hofherr A, Kottgen M. TRPP channels and polycystins. Adv Exp Med Biol. 2011;704:287–313. doi: 10.1007/978-94-007-0265-3_16. [DOI] [PubMed] [Google Scholar]
- 610.Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- 611.Hofmann F, Flockerzi V, Kahl S, Wegener JW. L-type CaV1.2 calcium channels: From in vitro findings to in vivo function. Physiol Rev. 2014;94:303–326. doi: 10.1152/physrev.00016.2013. [DOI] [PubMed] [Google Scholar]
- 612.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 613.Hojs N, Strucl M, Cankar K. The effect of glibenclamide on acetylcholine and sodium nitroprusside induced vasodilatation in human cutaneous microcirculation. Clin Physiol Funct Imaging. 2009;29:38–44. doi: 10.1111/j.1475-097X.2008.00833.x. [DOI] [PubMed] [Google Scholar]
- 614.Holdsworth CT, Copp SW, Ferguson SK, Sims GE, Poole DC, Musch TI. Acute inhibition of ATP-sensitive K+ channels impairs skeletal muscle vascular control in rats during treadmill exercise. Am J Physiol Heart Circ Physiol. 2015;308:H1434–H1442. doi: 10.1152/ajpheart.00772.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Hondeghem LM, Ayad MJ, Robertson RM. Verapamil, diltiazem and nifedipine block the depolarization-induced potentiation of norepinephrine contractions in rabbit aorta and porcine coronary arteries. J Pharmacol Exp Ther. 1986;239:808–813. [PubMed] [Google Scholar]
- 616.Hong KW, Pyo KM, Lee WS, Yu SS, Rhim BY. Pharmacological evidence that calcitonin gene-related peptide is implicated in cerebral autoregulation. Am J Physiol. 1994;266:H11–H16. doi: 10.1152/ajpheart.1994.266.1.H11. [DOI] [PubMed] [Google Scholar]
- 617.Hong KW, Yoo SE, Yu SS, Lee JY, Rhim BY. Pharmacological coupling and functional role for CGRP receptors in the vasodilation of rat pial arterioles. Am J Physiol. 1996;270:H317–H323. doi: 10.1152/ajpheart.1996.270.1.H317. [DOI] [PubMed] [Google Scholar]
- 618.Horinaka N, Kuang TY, Pak H, Wang R, Jehle J, Kennedy C, Sokoloff L. Blockade of cerebral blood flow response to insulin-induced hypoglycemia by caffeine and glibenclamide in conscious rats. J Cereb Blood Flow Metab. 1997;17:1309–1318. doi: 10.1097/00004647-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 619.Horiuchi T, Dietrich HH, Tsugane S, Dacey RG., Jr Role of potassium channels in regulation of brain arteriolar tone: Comparison of cerebrum versus brain stem. Stroke. 2001;32:218–224. doi: 10.1161/01.str.32.1.218. [DOI] [PubMed] [Google Scholar]
- 620.Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev. 1996;76:967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- 621.Hoshi T, Pantazis A, Olcese R. Transduction of voltage and Ca2+ signals by Slo1 BK channels. Physiology (Bethesda) 2013;28:172–189. doi: 10.1152/physiol.00055.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Hoshi T, Tian Y, Xu R, Heinemann SH, Hou S. Mechanism of the modulation of BK potassium channel complexes with different auxiliary subunit compositions by the omega-3 fatty acid DHA. Proc Natl Acad Sci U S A. 2013;110:4822–4827. doi: 10.1073/pnas.1222003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- 624.Hoshi T, Zagotta WN, Aldrich RW. Two types of inactivation in Shaker K+ channels: Effects of alterations in the carboxy-terminal region. Neuron. 1991;7:547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- 625.House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: Calcium signaling and phenotypic switching during vascular disease. Pflugers Arch. 2008;456:769–785. doi: 10.1007/s00424-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Howard J, Bechstedt S. Hypothesis: A helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol. 2004;14:R224–R226. doi: 10.1016/j.cub.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 627.Howitt L, Chaston DJ, Sandow SL, Matthaei KI, Edwards FR, Hill CE. Spreading vasodilatation in the murine microcirculation: Attenuation by oxidative stress-induced change in electromechanical coupling. J Physiol. 2013;591:2157–2173. doi: 10.1113/jphysiol.2013.250928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Howitt L, Kuo IY, Ellis A, Chaston DJ, Shin HS, Hansen PB, Hill CE. Chronic deficit in nitric oxide elicits oxidative stress and augments T-type calcium-channel contribution to vascular tone of rodent arteries and arterioles. Cardiovasc Res. 2013;98:449–457. doi: 10.1093/cvr/cvt043. [DOI] [PubMed] [Google Scholar]
- 629.Howitt L, Sandow SL, Grayson TH, Ellis ZE, Morris MJ, Murphy TV. Differential effects of diet-induced obesity on BKCa {beta} 1-subunit expression and function in rat skeletal muscle arterioles and small cerebral arteries. Am J Physiol Heart Circ Physiol. 2011;301:H29–H40. doi: 10.1152/ajpheart.00134.2011. [DOI] [PubMed] [Google Scholar]
- 630.Hu XQ, Zhang L. Function and regulation of large conductance Ca(2+)-activated K+ channel in vascular smooth muscle cells. Drug Discov Today. 2012;17:974–987. doi: 10.1016/j.drudis.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res. 2005;96:376–383. doi: 10.1161/01.RES.0000155332.17783.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Hubner CA, Schroeder BC, Ehmke H. Regulation of vascular tone and arterial blood pressure: Role of chloride transport in vascular smooth muscle. Pflugers Arch. 2015;467:605–614. doi: 10.1007/s00424-014-1684-y. [DOI] [PubMed] [Google Scholar]
- 633.Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Autoinhibitory control of the CaV1.2 channel by its proteolytically processed distal C-terminal domain. J Physiol. 2006;576:87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Hungerford JE, Sessa WC, Segal SS. Vasomotor control in arterioles of the mouse cremaster muscle. FASEB J. 2000;14:197–207. doi: 10.1096/fasebj.14.1.197. [DOI] [PubMed] [Google Scholar]
- 635.Hutri-Kahonen N, Kahonen M, Wu X, Sand J, Nordback I, Taurio J, Porsti I. Control of vascular tone in isolated mesenteric arterial segments from hypertensive patients. Br J Pharmacol. 1999;127:1735–1743. doi: 10.1038/sj.bjp.0702716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Hymel L, Inui M, Fleischer S, Schindler H. Purified ryanodine receptor of skeletal muscle sarcoplasmic reticulum forms Ca2+-activated oligomeric Ca2+ channels in planar bilayers. Proc Natl Acad Sci U S A. 1988;85:441–445. doi: 10.1073/pnas.85.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Iida H, Ohata H, Iida M, Watanabe Y, Dohi S. Isoflurane and sevoflurane induce vasodilation of cerebral vessels via ATP-sensitive K+ channel activation. Anesthesiology. 1998;89:954–960. doi: 10.1097/00000542-199810000-00020. [DOI] [PubMed] [Google Scholar]
- 638.Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989;94:363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Iino M. Calcium release mechanisms in smooth muscle. Jpn J Pharmacol. 1990;54:345–354. doi: 10.1254/jjp.54.345. [DOI] [PubMed] [Google Scholar]
- 640.Iino M, Kasai H, Yamazawa T. Visualization of neural control of intracellular Ca2+ concentration in single vascular smooth muscle cells in situ. Embo J. 1994;13:5026–5031. doi: 10.1002/j.1460-2075.1994.tb06831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Imamura Y, Tomoike H, Narishige T, Takahashi T, Kasuya H, Takeshita A. Glibenclamide decreases basal coronary blood flow in anesthetized dogs. Am J Physiol. 1992;263:H399–H404. doi: 10.1152/ajpheart.1992.263.2.H399. [DOI] [PubMed] [Google Scholar]
- 642.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11, 12-EET analogs involves PP2A activity and Ca2+-activated K+ channels. Microcirculation. 2008;15:137–150. doi: 10.1080/10739680701456960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Imig JD, Walsh KA, Hye Khan MA, Nagasawa T, Cherian-Shaw M, Shaw SM, Hammock BD. Soluble epoxide hydrolase inhibition and peroxisome proliferator activated receptor gamma agonist improve vascular function and decrease renal injury in hypertensive obese rats. Exp Biol Med (Maywood) 2012;237:1402–1412. doi: 10.1258/ebm.2012.012225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 645.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 646.Inoue R, Kitamura K, Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985;405:173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- 647.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- 648.Inoue Y, Oike M, Nakao K, Kitamura K, Kuriyama H. Endothelin augments unitary calcium channel currents on the smooth muscle cell membrane of guinea-pig portal vein. J Physiol. 1990;423:171–191. doi: 10.1113/jphysiol.1990.sp018017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Inui M, Saito A, Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem. 1987;262:15637–15642. [PubMed] [Google Scholar]
- 650.Inui M, Saito A, Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987;262:1740–1747. [PubMed] [Google Scholar]
- 651.Ionescu L, Cheung KH, Vais H, Mak DO, White C, Foskett JK. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: Implications for quantal [corrected] Ca2+ release. J Physiol. 2006;573:645–662. doi: 10.1113/jphysiol.2006.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Irat AM, Aslamaci S, Karasu C, Ari N. Alteration of vascular reactivity in diabetic human mammary artery and the effects of thiazolidinediones. J Pharm Pharmacol. 2006;58:1647–1653. doi: 10.1211/jpp.58.12.0012. [DOI] [PubMed] [Google Scholar]
- 653.Irion GL, Vasthare US, Tuma RF. Age-related change in skeletal muscle blood flow in the rat. J Gerontol. 1987;42:660–665. doi: 10.1093/geronj/42.6.660. [DOI] [PubMed] [Google Scholar]
- 654.Ishibashi Y, Duncker DJ, Zhang J, Bache RJ. ATP-sensitive K+ channels, adenosine, and nitric oxide-mediated mechanisms account for coronary vasodilation during exercise. Circ Res. 1998;82:346–359. doi: 10.1161/01.res.82.3.346. [DOI] [PubMed] [Google Scholar]
- 655.Ishiguro M, Morielli AD, Zvarova K, Tranmer BI, Penar PL, Wellman GC. Oxyhemoglobin-induced suppression of voltage-dependent K+ channels in cerebral arteries by enhanced tyrosine kinase activity. Circ Res. 2006;99:1252–1260. doi: 10.1161/01.RES.0000250821.32324.e1. [DOI] [PubMed] [Google Scholar]
- 656.Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci U S A. 1997;94:11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Ishikawa T, Hume JR, Keef KD. Modulation of K+ and Ca2+ channels by histamine H1-receptor stimulation in rabbit coronary artery cells. J Physiol. 1993;468:379–400. doi: 10.1113/jphysiol.1993.sp019777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci U S A. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Ishizaka H, Kuo L. Acidosis-induced coronary arteriolar dilation is mediated by ATP-sensitive potassium channels in vascular smooth muscle. Circ Res. 1996;78:50–57. doi: 10.1161/01.res.78.1.50. [DOI] [PubMed] [Google Scholar]
- 660.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 661.Isomoto S, Yamada M, Horio Y, Kurachi Y. Molecular aspects of ATP-sensitive K+ channels in the cardiovascular system. Jpn J Physiol. 1997;47(Suppl 1):S5–S6. [PubMed] [Google Scholar]
- 662.Ito I, Jarajapu YP, Grant MB, Knot HJ. Characteristics of myogenic tone in the rat ophthalmic artery. Am J Physiol Heart Circ Physiol. 2007;292:H360–H368. doi: 10.1152/ajpheart.00630.2006. [DOI] [PubMed] [Google Scholar]
- 663.Jabr RI, Yamazaki J, Hume JR. Lysophosphatidylcholine triggers intracellular calcium release and activation of non-selective cation channels in renal arterial smooth muscle cells. Pflugers Arch. 2000;439:495–500. doi: 10.1007/s004249900206. [DOI] [PubMed] [Google Scholar]
- 664.Jackson WF. Arteriolar tone is determined by activity of ATP-sensitive potassium channels. Am J Physiol. 1993;265:H1797–H1803. doi: 10.1152/ajpheart.1993.265.5.H1797. [DOI] [PubMed] [Google Scholar]
- 665.Jackson WF. Potassium channels and regulation of the microcirculation. Microcirculation. 1998;5:85–90. [PubMed] [Google Scholar]
- 666.Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35:173–178. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Jackson WF. Potassium channels in the circulation of skeletal muscle. In: Archer SL, Rusch NJ, editors. Potassium Channels in the Cardiovascular Biology. New York: Kluwer Academic/Plenum Publishers; 2001. pp. 505–522. [Google Scholar]
- 668.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Jackson WF. Vascular smooth muscle store-operated Ca2+ channels: What a TRP! Am J Physiol Heart Circ Physiol. 2006;291:H2592–H2594. doi: 10.1152/ajpheart.00869.2006. [DOI] [PubMed] [Google Scholar]
- 670.Jackson WF. Chapter 89: Microcirculation. In: Olson JA, Hill EN, editors. Muscle. Boston/Waltham: Academic Press; 2012. pp. 1197–1206. [Google Scholar]
- 671.Jackson WF. Endothelial cell ion channel expression and function in arterioles and resistance arteries. In: Levitan I, Dopico AM, editors. Vascular Ion Channels in Physiology and Disease. Switzerland: Springer International Publishing; 2016. p. 431. [Google Scholar]
- 672.Jackson WF, Blair KL. Characterization and function of Ca(2+)-activated K+ channels in arteriolar muscle cells. Am J Physiol. 1998;274:H27–H34. doi: 10.1152/ajpheart.1998.274.1.H27. [DOI] [PubMed] [Google Scholar]
- 673.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation. 1997;4:35–50. doi: 10.3109/10739689709148316. [DOI] [PubMed] [Google Scholar]
- 674.Jackson WF, Konig A, Dambacher T, Busse R. Prostacyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am J Physiol. 1993;264:H238–H243. doi: 10.1152/ajpheart.1993.264.1.H238. [DOI] [PubMed] [Google Scholar]
- 675.Jackson-Weaver O, Osmond JM, Naik JS, Gonzalez Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats reduces activation of Ca2+ sparks in mesenteric arteries. Am J Physiol Heart Circ Physiol. 2015;309:H1915–H1922. doi: 10.1152/ajpheart.00179.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Jackson-Weaver O, Osmond JM, Riddle MA, Naik JS, Gonzalez Bosc LV, Walker BR, Kanagy NL. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca(2)(+)-activated K(+) channels and smooth muscle Ca(2)(+) sparks. Am J Physiol Heart Circ Physiol. 2013;304:H1446–H1454. doi: 10.1152/ajpheart.00506.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circ Res. 2011;108:1439–1447. doi: 10.1161/CIRCRESAHA.110.228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Jaggar JH. Intravascular pressure regulates local and global Ca(2+) signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- 679.Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, Shuyu E, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res. 2002;91:610–617. doi: 10.1161/01.res.0000036900.76780.95. [DOI] [PubMed] [Google Scholar]
- 680.Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97:805–812. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Jaggar JH, Nelson MT. Differential regulation of Ca(2+) sparks and Ca(2+) waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C1528–C1539. doi: 10.1152/ajpcell.2000.279.5.C1528. [DOI] [PubMed] [Google Scholar]
- 682.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 683.Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol. 1998;274:C1755–C1761. doi: 10.1152/ajpcell.1998.274.6.C1755. [DOI] [PubMed] [Google Scholar]
- 684.Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Kleppisch T, Rubart M, Stevenson AS, Lederer WJ, Knot HJ, Bonev AD, Nelson MT. Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: A functional unit for regulating arterial tone. Acta Physiol Scand. 1998;164:577–587. doi: 10.1046/j.1365-201X.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- 685.Jahromi BS, Aihara Y, Ai J, Zhang ZD, Weyer G, Nikitina E, Yassari R, Houamed KM, Macdonald RL. Temporal profile of potassium channel dysfunction in cerebrovascular smooth muscle after experimental subarachnoid haemorrhage. Neurosci Lett. 2008;440:81–86. doi: 10.1016/j.neulet.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Jameel MN, Xiong Q, Mansoor A, Bache RJ, Zhang J. ATP sensitive K(+) channels are critical for maintaining myocardial perfusion and high energy phosphates in the failing heart. J Mol Cell Cardiol. 2016;92:116–121. doi: 10.1016/j.yjmcc.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Jan LY, Jan YN. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- 688.Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. J Physiol. 1997;505(Pt 2):267–282. doi: 10.1111/j.1469-7793.1997.267bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Jantzi MC, Brett SE, Jackson WF, Corteling R, Vigmond EJ, Welsh DG. Inward rectifying potassium channels facilitate cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol. 2006;291:H1319–H1328. doi: 10.1152/ajpheart.00217.2006. [DOI] [PubMed] [Google Scholar]
- 690.Jarajapu YP, Knot HJ. Relative contribution of Rho kinase and protein kinase C to myogenic tone in rat cerebral arteries in hypertension. Am J Physiol Heart Circ Physiol. 2005;289:H1917–H1922. doi: 10.1152/ajpheart.01012.2004. [DOI] [PubMed] [Google Scholar]
- 691.Jeffries O, McGahon MK, Bankhead P, Lozano MM, Scholfield CN, Curtis TM, McGeown JG. cAMP/PKA-dependent increases in Ca Sparks, oscillations and SR Ca stores in retinal arteriolar myocytes after exposure to vasopressin. Invest Ophthalmol Vis Sci. 2010;51:1591–1598. doi: 10.1167/iovs.09-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Jensen LJ, Salomonsson M, Jensen BL, Holstein-Rathlou NH. Depolarization-induced calcium influx in rat mesenteric small arterioles is mediated exclusively via mibefradil-sensitive calcium channels. Br J Pharmacol. 2004;142:709–718. doi: 10.1038/sj.bjp.0705841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Jeon JP, Hong C, Park EJ, Jeon JH, Cho NH, Kim IG, Choe H, Muallem S, Kim HJ, So I. Selective Galphai subunits as novel direct activators of transient receptor potential canonical (TRPC)4 and TRPC5 channels. J Biol Chem. 2012;287:17029–17039. doi: 10.1074/jbc.M111.326553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Jepps TA, Carr G, Lundegaard PR, Olesen SP, Greenwood IA. Fundamental role for the KCNE4 ancillary subunit in Kv7.4 regulation of arterial tone. J Physiol. 2015;593:5325–5340. doi: 10.1113/JP271286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Jepps TA, Chadha PS, Davis AJ, Harhun MI, Cockerill GW, Olesen SP, Hansen RS, Greenwood IA. Downregulation of Kv7.4 channel activity in primary and secondary hypertension. Circulation. 2011;124:602–611. doi: 10.1161/CIRCULATIONAHA.111.032136. [DOI] [PubMed] [Google Scholar]
- 696.Jepps TA, Olesen SP, Greenwood IA. One man’s side effect is another man’s therapeutic opportunity: Targeting Kv7 channels in smooth muscle disorders. Br J Pharmacol. 2013;168:19–27. doi: 10.1111/j.1476-5381.2012.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol. 2005;289:F891–F901. doi: 10.1152/ajprenal.00019.2005. [DOI] [PubMed] [Google Scholar]
- 698.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: Role of endogenous beta and gammaENaC. Am J Physiol Renal Physiol. 2006;291:F1184–F1191. doi: 10.1152/ajprenal.00177.2006. [DOI] [PubMed] [Google Scholar]
- 699.Jewell RP, Saundry CM, Bonev AD, Tranmer BI, Wellman GC. Inhibition of Ca++ sparks by oxyhemoglobin in rabbit cerebral arteries. J Neurosurg. 2004;100:295–302. doi: 10.3171/jns.2004.100.2.0295. [DOI] [PubMed] [Google Scholar]
- 700.Jeyakumar LH, Copello JA, O’Malley AM, Wu GM, Grassucci R, Wagenknecht T, Fleischer S. Purification and characterization of ryanodine receptor 3 from mammalian tissue. J Biol Chem. 1998;273:16011–16020. doi: 10.1074/jbc.273.26.16011. [DOI] [PubMed] [Google Scholar]
- 701.Ji J, Benishin CG, Pang PK. Nitric oxide selectively inhibits intracellular Ca++ release elicited by inositol trisphosphate but not caffeine in rat vascular smooth muscle. J Pharmacol Exp Ther. 1998;285:16–21. [PubMed] [Google Scholar]
- 702.Ji G, Feldman ME, Greene KS, Sorrentino V, Xin HB, Kotlikoff MI. RYR2 proteins contribute to the formation of Ca(2+) sparks in smooth muscle. J Gen Physiol. 2004;123:377–386. doi: 10.1085/jgp.200308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Jiang ZG, Si JQ, Lasarev MR, Nuttall AL. Two resting potential levels regulated by the inward-rectifier potassium channel in the guinea-pig spiral modiolar artery. J Physiol. 2001;537:829–842. doi: 10.1111/j.1469-7793.2001.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Jiang J, Thoren P, Caligiuri G, Hansson GK, Pernow J. Enhanced phenylephrine-induced rhythmic activity in the atherosclerotic mouse aorta via an increase in opening of KCa channels: Relation to Kv channels and nitric oxide. Br J Pharmacol. 1999;128:637–646. doi: 10.1038/sj.bjp.0702855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Jiang B, Wu L, Wang R. Sulphonylureas induced vasorelaxation of mouse arteries. Eur J Pharmacol. 2007;577:124–128. doi: 10.1016/j.ejphar.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 706.Jiang D, Xiao B, Li X, Chen SR. Smooth muscle tissues express a major dominant negative splice variant of the type 3 Ca2+ release channel (ryanodine receptor) J Biol Chem. 2003;278:4763–4769. doi: 10.1074/jbc.M210410200. [DOI] [PubMed] [Google Scholar]
- 707.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SR. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci U S A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Jiao J, Garg V, Yang B, Elton TS, Hu K. Protein kinase C-epsilon induces caveolin-dependent internalization of vascular adenosine 5′-triphosphate-sensitive K+ channels. Hypertension. 2008;52:499–506. doi: 10.1161/HYPERTENSIONAHA.108.110817. [DOI] [PubMed] [Google Scholar]
- 709.Jin CZ, Kim HS, Seo EY, Shin DH, Park KS, Chun YS, Zhang YH, Kim SJ. Exercise training increases inwardly rectifying K(+) current and augments K(+)-mediated vasodilatation in deep femoral artery of rats. Cardiovasc Res. 2011;91:142–150. doi: 10.1093/cvr/cvr050. [DOI] [PubMed] [Google Scholar]
- 710.Johnson RP, El-Yazbi AF, Hughes MF, Schriemer DC, Walsh EJ, Walsh MP, Cole WC. Identification and functional characterization of protein kinase A-catalyzed phosphorylation of potassium channel Kv1.2 at serine 449. J Biol Chem. 2009;284:16562–16574. doi: 10.1074/jbc.M109.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Johnson TD, Marrelli SP, Steenberg ML, Childres WF, Bryan RM., Jr Inward rectifier potassium channels in the rat middle cerebral artery. Am J Physiol. 1998;274:R541–R547. doi: 10.1152/ajpregu.1998.274.2.R541. [DOI] [PubMed] [Google Scholar]
- 712.Johnson CD, Melanaphy D, Purse A, Stokesberry SA, Dickson P, Zholos AV. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2009;296:H1868–H1877. doi: 10.1152/ajpheart.01112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Joiner WJ, Wang LY, Tang MD, Kaczmarek LK. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci U S A. 1997;94:11013–11018. doi: 10.1073/pnas.94.20.11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 715.Joseph SK. The inositol triphosphate receptor family. Cell Signal. 1996;8:1–7. doi: 10.1016/0898-6568(95)02012-8. [DOI] [PubMed] [Google Scholar]
- 716.Joseph BK, Thakali KM, Moore CL, Rhee SW. Ion channel remodeling in vascular smooth muscle during hypertension: Implications for novel therapeutic approaches. Pharmacol Res. 2013;70:126–138. doi: 10.1016/j.phrs.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Joshua IG, Miller FN, Dowe JP. In vivo arteriolar reactivity to norepinephrine and calcium in one-kidney, one-clip Goldblatt hypertensive rats. Clin Exp Hypertens A. 1987;9:1691–1711. doi: 10.3109/10641968709158967. [DOI] [PubMed] [Google Scholar]
- 718.Ju M, Shi J, Saleh SN, Albert AP, Large WA. Ins(1,4,5)P3 interacts with PIP2 to regulate activation of TRPC6/C7 channels by diacylglycerol in native vascular myocytes. J Physiol. 2010;588:1419–1433. doi: 10.1113/jphysiol.2009.185256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C347–C359. doi: 10.1152/ajpcell.00283.2001. [DOI] [PubMed] [Google Scholar]
- 720.Juvin V, Penna A, Chemin J, Lin YL, Rassendren FA. Pharmacological characterization and molecular determinants of the activation of transient receptor potential V2 channel orthologs by 2-aminoethoxydiphenyl borate. Mol Pharmacol. 2007;72:1258–1268. doi: 10.1124/mol.107.037044. [DOI] [PubMed] [Google Scholar]
- 721.Kaczmarek JS, Riccio A, Clapham DE. Calpain cleaves and activates the TRPC5 channel to participate in semaphorin 3A-induced neuronal growth cone collapse. Proc Natl Acad Sci U S A. 2012;109:7888–7892. doi: 10.1073/pnas.1205869109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Kalliovalkama J, Jolma P, Tolvanen JP, Kahonen M, Hutri-Kahonen N, Wu X, Holm P, Porsti I. Arterial function in nitric oxide-deficient hypertension: Influence of long-term angiotensin II receptor antagonism. Cardiovasc Res. 1999;42:773–782. doi: 10.1016/s0008-6363(98)00346-0. [DOI] [PubMed] [Google Scholar]
- 723.Kalman K, Pennington MW, Lanigan MD, Nguyen A, Rauer H, Mahnir V, Paschetto K, Kem WR, Grissmer S, Gutman GA, Christian EP, Cahalan MD, Norton RS, Chandy KG. ShK-Dap22, a potent Kv1.3-specific immunosuppressive polypeptide. J Biol Chem. 1998;273:32697–32707. doi: 10.1074/jbc.273.49.32697. [DOI] [PubMed] [Google Scholar]
- 724.Kam KL, Pfaffendorf M, van Zwieten PA. Drug-induced endothelium-dependent and -independent relaxations in isolated resistance vessels taken from simultaneously hypertensive and streptozotocin-diabetic rats. Blood Press. 1994;3:418–427. doi: 10.3109/08037059409102296. [DOI] [PubMed] [Google Scholar]
- 725.Kamata K, Miyata N, Kasuya Y. Functional changes in potassium channels in aortas from rats with streptozotocin-induced diabetes. Eur J Pharmacol. 1989;166:319–323. doi: 10.1016/0014-2999(89)90076-9. [DOI] [PubMed] [Google Scholar]
- 726.Kamouchi M, Van Den Bremt K, Eggermont J, Droogmans G, Nilius B. Modulation of inwardly rectifying potassium channels in cultured bovine pulmonary artery endothelial cells. J Physiol. 1997;504(Pt 3):545–556. doi: 10.1111/j.1469-7793.1997.545bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Kanagy NL, Ansari MN, Ghosh S, Webb RC. Recycling and buffering of intracellular calcium in vascular smooth muscle from genetically hypertensive rats. J Hypertens. 1994;12:1365–1372. [PubMed] [Google Scholar]
- 728.Kanatsuka H, Sekiguchi N, Sato K, Akai K, Wang Y, Komaru T, Ashikawa K, Takishima T. Microvascular sites and mechanisms responsible for reactive hyperemia in the coronary circulation of the beating canine heart. Circ Res. 1992;71:912–922. doi: 10.1161/01.res.71.4.912. [DOI] [PubMed] [Google Scholar]
- 729.Kane GC, Lam CF, O’Cochlain F, Hodgson DM, Reyes S, Liu XK, Miki T, Seino S, Katusic ZS, Terzic A. Gene knockout of the KCNJ8-encoded Kir6.1 K(ATP) channel imparts fatal susceptibility to endotoxemia. FASEB J. 2006;20:2271–2280. doi: 10.1096/fj.06-6349com. [DOI] [PubMed] [Google Scholar]
- 730.Kang LS, Kim S, Dominguez JM, II, Sindler AL, Dick GM, Muller-Delp JM. Aging and muscle fiber type alter K+ channel contributions to the myogenic response in skeletal muscle arterioles. J Appl Physiol (1985) 2009;107:389–398. doi: 10.1152/japplphysiol.91245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nature Cell Biology. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- 732.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Kark T, Bagi Z, Lizanecz E, Pasztor ET, Erdei N, Czikora A, Papp Z, Edes I, Porszasz R, Toth A. Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol. 2008;73:1405–1412. doi: 10.1124/mol.107.043323. [DOI] [PubMed] [Google Scholar]
- 734.Kasai Y, Yamazawa T, Sakurai T, Taketani Y, Iino M. Endothelium-dependent frequency modulation of Ca2+ signalling in individual vascular smooth muscle cells of the rat. J Physiol. 1997;504(Pt 2):349–357. doi: 10.1111/j.1469-7793.1997.349be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Kasemsri T, Armstead WM. Endothelin impairs ATP-sensitive K+ channel function after brain injury. Am J Physiol. 1997;273:H2639–H2647. doi: 10.1152/ajpheart.1997.273.6.H2639. [DOI] [PubMed] [Google Scholar]
- 736.Katsuda Y, Egashira K, Ueno H, Akatsuka Y, Narishige T, Arai Y, Takayanagi T, Shimokawa H, Takeshita A. Glibenclamide, a selective inhibitor of ATP-sensitive K+ channels, attenuates metabolic coronary vasodilatation induced by pacing tachycardia in dogs. Circulation. 1995;92:511–517. doi: 10.1161/01.cir.92.3.511. [DOI] [PubMed] [Google Scholar]
- 737.Katz B. Les Constantes electriques de la membrane du muscle. Arch Sci Physiol. 1949;3:285–299. [Google Scholar]
- 738.Kawano T, Tanaka K, Mawatari K, Oshita S, Takahashi A, Nakaya Y. Hyperglycemia impairs isoflurane-induced adenosine triphosphate-sensitive potassium channel activation in vascular smooth muscle cells. Anesth Analg. 2008;106:858–864. doi: 10.1213/ane.0b013e318163fd5b. [DOI] [PubMed] [Google Scholar]
- 739.Kawata T, Mimuro T, Onuki T, Tsuchiya K, Nihei H, Koike T. The K(ATP) channel opener nicorandil: Effect on renal hemodynamics in spontaneously hypertensive and Wistar Kyoto rats. Kidney Int Suppl. 1998;67:S231–S233. doi: 10.1046/j.1523-1755.1998.06758.x. [DOI] [PubMed] [Google Scholar]
- 740.Keef KD, Hume JR, Zhong J. Regulation of cardiac and smooth muscle Ca(2+) channels (Ca(V)1.2a,b) by protein kinases. Am J Physiol Cell Physiol. 2001;281:C1743–C1756. doi: 10.1152/ajpcell.2001.281.6.C1743. [DOI] [PubMed] [Google Scholar]
- 741.Kerr PM, Clement-Chomienne O, Thorneloe KS, Chen TT, Ishii K, Sontag DP, Walsh MP, Cole WC. Heteromultimeric Kv1.2–Kv1.5 channels underlie 4-aminopyridine-sensitive delayed rectifier K(+) current of rabbit vascular myocytes. Circ Res. 2001;89:1038–1044. doi: 10.1161/hh2301.100803. [DOI] [PubMed] [Google Scholar]
- 742.Kersten JR, Brooks LA, Dellsperger KC. Impaired microvascular response to graded coronary occlusion in diabetic and hyperglycemic dogs. Am J Physiol. 1995;268:H1667–H1674. doi: 10.1152/ajpheart.1995.268.4.H1667. [DOI] [PubMed] [Google Scholar]
- 743.Khan SA, Mathews WR, Meisheri KD. Role of calcium-activated K+ channels in vasodilation induced by nitroglycerine, acetylcholine and nitric oxide. J Pharmacol Exp Ther. 1993;267:1327–1335. [PubMed] [Google Scholar]
- 744.Khanamiri S, Soltysinska E, Jepps TA, Bentzen BH, Chadha PS, Schmitt N, Greenwood IA, Olesen SP. Contribution of Kv7 channels to basal coronary flow and active response to ischemia. Hypertension. 2013;62:1090–1097. doi: 10.1161/HYPERTENSIONAHA.113.01244. [DOI] [PubMed] [Google Scholar]
- 745.Kharade SV, Sonkusare SK, Srivastava AK, Thakali KM, Fletcher TW, Rhee SW, Rusch NJ. The beta3 subunit contributes to vascular calcium channel upregulation and hypertension in angiotensin II-infused C57BL/6 mice. Hypertension. 2013;61:137–142. doi: 10.1161/HYPERTENSIONAHA.112.197863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 746.Kharitonov VG, Sharma VS, Pilz RB, Magde D, Koesling D. Basis of guanylate cyclase activation by carbon monoxide. Proc Natl Acad Sci U S A. 1995;92:2568–2571. doi: 10.1073/pnas.92.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Kilpatrick EV, Cocks TM. Evidence for differential roles of nitric oxide (NO) and hyperpolarization in endothelium-dependent relaxation of pig isolated coronary artery. Br J Pharmacol. 1994;112:557–565. doi: 10.1111/j.1476-5381.1994.tb13110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signalling pathways in health and disease. J Cell Mol Med. 2008;12:2165–2180. doi: 10.1111/j.1582-4934.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749.Kim MY, Liang GH, Kim JA, Park SH, Hah JS, Suh SH. Contribution of Na+-K+ pump and KIR currents to extracellular pH-dependent changes of contractility in rat superior mesenteric artery. Am J Physiol Heart Circ Physiol. 2005;289:H792–H800. doi: 10.1152/ajpheart.00050.2005. [DOI] [PubMed] [Google Scholar]
- 750.Kimura T, Lueck JD, Harvey PJ, Pace SM, Ikemoto N, Casarotto MG, Dirksen RT, Dulhunty AF. Alternative splicing of RyR1 alters the efficacy of skeletal EC coupling. Cell Calcium. 2009;45:264–274. doi: 10.1016/j.ceca.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Kimura T, Pace SM, Wei L, Beard NA, Dirksen RT, Dulhunty AF. A variably spliced region in the type 1 ryanodine receptor may participate in an inter-domain interaction. Biochem J. 2007;401:317–324. doi: 10.1042/BJ20060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Kinoshita H, Azma T, Iranami H, Nakahata K, Kimoto Y, Dojo M, Yuge O, Hatano Y. Synthetic peroxisome proliferator-activated receptor-gamma agonists restore impaired vasorelaxation via ATP-sensitive K+ channels by high glucose. J Pharmacol Exp Ther. 2006;318:312–318. doi: 10.1124/jpet.106.100958. [DOI] [PubMed] [Google Scholar]
- 753.Kinoshita H, Matsuda N, Kaba H, Hatakeyama N, Azma T, Nakahata K, Kuroda Y, Tange K, Iranami H, Hatano Y. Roles of phosphatidylinositol 3-kinase-Akt and NADPH oxidase in adenosine 5′-triphosphate-sensitive K+ channel function impaired by high glucose in the human artery. Hypertension. 2008;52:507–513. doi: 10.1161/HYPERTENSIONAHA.108.118216. [DOI] [PubMed] [Google Scholar]
- 754.Kirsch GE, Drewe JA, Verma S, Brown AM, Joho RH. Electrophysiological characterization of a new member of the RCK family of rat brain K+ channels. FEBS Lett. 1991;278:55–60. doi: 10.1016/0014-5793(91)80082-e. [DOI] [PubMed] [Google Scholar]
- 755.Kirsch GE, Shieh CC, Drewe JA, Vener DF, Brown AM. Segmental exchanges define 4-aminopyridine binding and the inner mouth of K+ pores. Neuron. 1993;11:503–512. doi: 10.1016/0896-6273(93)90154-j. [DOI] [PubMed] [Google Scholar]
- 756.Kiselyov K, Mignery GA, Zhu MX, Muallem S. The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol Cell. 1999;4:423–429. doi: 10.1016/s1097-2765(00)80344-5. [DOI] [PubMed] [Google Scholar]
- 757.Kiselyov K, Shin DM, Kim JY, Yuan JP, Muallem S. TRPC channels: Interacting proteins. Handb Exp Pharmacol. 2007:559–574. doi: 10.1007/978-3-540-34891-7_33. [DOI] [PubMed] [Google Scholar]
- 758.Kitazono T, Heistad DD, Faraci FM. ATP-sensitive potassium channels in the basilar artery during chronic hypertension. Hypertension. 1993;22:677–681. doi: 10.1161/01.hyp.22.5.677. [DOI] [PubMed] [Google Scholar]
- 759.Kitazono T, Heistad DD, Faraci FM. Role of ATP-sensitive K+ channels in CGRP-induced dilatation of basilar artery in vivo. Am J Physiol. 1993;265:H581–H585. doi: 10.1152/ajpheart.1993.265.2.H581. [DOI] [PubMed] [Google Scholar]
- 760.Klein MG, Cheng H, Santana LF, Jiang YH, Lederer WJ, Schneider MF. Two mechanisms of quantized calcium release in skeletal muscle. Nature. 1996;379:455–458. doi: 10.1038/379455a0. [DOI] [PubMed] [Google Scholar]
- 761.Kleppisch T, Nelson MT. Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1995;92:12441–12445. doi: 10.1073/pnas.92.26.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Kleppisch T, Nelson MT. ATP-sensitive K+ currents in cerebral arterial smooth muscle: Pharmacological and hormonal modulation. Am J Physiol. 1995;269:H1634–H1640. doi: 10.1152/ajpheart.1995.269.5.H1634. [DOI] [PubMed] [Google Scholar]
- 763.Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol. 1995;269:H348–H355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- 764.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508(Pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol. 1998;508(Pt 1):211–221. doi: 10.1111/j.1469-7793.1998.211br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Knot HJ, Zimmermann PA, Nelson MT. Extracellular K(+)-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J Physiol. 1996;492(Pt 2):419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One. 2011;6:e25894. doi: 10.1371/journal.pone.0025894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.Knudson JD, Dincer UD, Bratz IN, Sturek M, Dick GM, Tune JD. Mechanisms of coronary dysfunction in obesity and insulin resistance. Microcirculation. 2007;14:317–338. doi: 10.1080/10739680701282887. [DOI] [PubMed] [Google Scholar]
- 769.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 770.Ko EA, Park WS, Firth AL, Hong DH, Choi SW, Heo HJ, Kim MH, Noh JH, Ko JH, Kim N, Earm YE, Song DK, Han J. Increased sensitivity of serotonin on the voltage-dependent K+ channels in mesenteric arterial smooth muscle cells of OLETF rats. Prog Biophys Mol Biol. 2010;103:88–94. doi: 10.1016/j.pbiomolbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 771.Ko EA, Park WS, Firth AL, Kim N, Yuan JX, Han J. Pathophysiology of voltage-gated K+ channels in vascular smooth muscle cells: Modulation by protein kinases. Prog Biophys Mol Biol. 2010;103:95–101. doi: 10.1016/j.pbiomolbio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 772.Kochukov MY, Balasubramanian A, Noel RC, Marrelli SP. Role of TRPC1 and TRPC3 channels in contraction and relaxation of mouse thoracic aorta. J Vasc Res. 2013;50:11–20. doi: 10.1159/000342461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Koh SD, Jun JY, Kim TW, Sanders KM. A Ca(2+)-inhibited nonselective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002;540:803–814. doi: 10.1113/jphysiol.2001.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774.Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 775.Koide M, Nystoriak MA, Krishnamoorthy G, O’Connor KP, Bonev AD, Nelson MT, Wellman GC. Reduced Ca2+ spark activity after subarachnoid hemorrhage disables BK channel control of cerebral artery tone. J Cereb Blood Flow Metab. 2011;31:3–16. doi: 10.1038/jcbfm.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Koide M, Syed AU, Braas KM, May V, Wellman GC. Pituitary adenylate cyclase activating polypeptide (PACAP) dilates cerebellar arteries through activation of large-conductance Ca(2+)-activated (BK) and ATP-sensitive (K ATP) K (+) channels. J Mol Neurosci. 2014;54:443–450. doi: 10.1007/s12031-014-0301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777.Kojima I, Nagasawa M. Trpv2. Handb Exp Pharmacol. 2014;222:247–272. doi: 10.1007/978-3-642-54215-2_10. [DOI] [PubMed] [Google Scholar]
- 778.Kokita N, Stekiel TA, Yamazaki M, Bosnjak ZJ, Kampine JP, Stekiel WJ. Potassium channel-mediated hyperpolarization of mesenteric vascular smooth muscle by isoflurane. Anesthesiology. 1999;90:779–788. doi: 10.1097/00000542-199903000-00021. [DOI] [PubMed] [Google Scholar]
- 779.Kolias TJ, Chai S, Webb RC. Potassium channel antagonists and vascular reactivity in stroke-prone spontaneously hypertensive rats. Am J Hypertens. 1993;6:528–533. doi: 10.1093/ajh/6.6.528. [DOI] [PubMed] [Google Scholar]
- 780.Komaru T, Kanatsuka H, Dellsperger K, Takishima T. The role of ATP-sensitive potassium channels in regulating coronary microcirculation. Biorheology. 1993;30:371–380. doi: 10.3233/bir-1993-305-608. [DOI] [PubMed] [Google Scholar]
- 781.Komaru T, Lamping KG, Eastham CL, Dellsperger KC. Role of ATP-sensitive potassium channels in coronary microvascular autoregulatory responses. Circ Res. 1991;69:1146–1151. doi: 10.1161/01.res.69.4.1146. [DOI] [PubMed] [Google Scholar]
- 782.Komori S, Bolton TB. Actions of guanine nucleotides and cyclic nucleotides on calcium stores in single patch-clamped smooth muscle cells from rabbit portal vein. Br J Pharmacol. 1989;97:973–982. doi: 10.1111/j.1476-5381.1989.tb12039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 783.Koschak A, Bugianesi RM, Mitterdorfer J, Kaczorowski GJ, Garcia ML, Knaus HG. Subunit composition of brain voltage-gated potassium channels determined by hongotoxin-1, a novel peptide derived from Centruroides limbatus venom. J Biol Chem. 1998;273:2639–2644. doi: 10.1074/jbc.273.5.2639. [DOI] [PubMed] [Google Scholar]
- 784.Kosmas EN, Levy RD, Hussain SN. Acute effects of glyburide on the regulation of peripheral blood flow in normal humans. Eur J Pharmacol. 1995;274:193–199. doi: 10.1016/0014-2999(94)00732-m. [DOI] [PubMed] [Google Scholar]
- 785.Kotecha N, Hill MA. Myogenic contraction in rat skeletal muscle arterioles: Smooth muscle membrane potential and Ca(2+) signaling. Am J Physiol Heart Circ Physiol. 2005;289:H1326–H1334. doi: 10.1152/ajpheart.00323.2005. [DOI] [PubMed] [Google Scholar]
- 786.Kotlikoff MI. Potassium channels in airway smooth muscle: a tale of two channels. Pharmacol Ther. 1993;58:1–12. doi: 10.1016/0163-7258(93)90064-k. [DOI] [PubMed] [Google Scholar]
- 787.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 789.Kraft R. The Na+/Ca2+ exchange inhibitor KB-R7943 potently blocks TRPC channels. Biochem Biophys Res Commun. 2007;361:230–236. doi: 10.1016/j.bbrc.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 790.Kraft R, Harteneck C. The mammalian melastatin-related transient receptor potential cation channels: An overview. Pflugers Arch. 2005;451:204–211. doi: 10.1007/s00424-005-1428-0. [DOI] [PubMed] [Google Scholar]
- 791.Kubo Y, Adelman JP, Clapham DE, Jan LY, Karschin A, Kurachi Y, Lazdunski M, Nichols CG, Seino S, Vandenberg CA. International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol Rev. 2005;57:509–526. doi: 10.1124/pr.57.4.11. [DOI] [PubMed] [Google Scholar]
- 792.Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- 793.Kubo M, Nakaya Y, Matsuoka S, Saito K, Kuroda Y. Atrial natriuretic factor and isosorbide dinitrate modulate the gating of ATP-sensitive K+ channels in cultured vascular smooth muscle cells. Circ Res. 1994;74:471–476. doi: 10.1161/01.res.74.3.471. [DOI] [PubMed] [Google Scholar]
- 794.Kudlacek PE, Pluznick JL, Ma R, Padanilam B, Sansom SC. Role of hbeta1 in activation of human mesangial BK channels by cGMP kinase. Am J Physiol Renal Physiol. 2003;285:F289–F294. doi: 10.1152/ajprenal.00046.2003. [DOI] [PubMed] [Google Scholar]
- 795.Kudryavtseva O, Herum KM, Dam VS, Straarup MS, Kamaev D, Briggs Boedtkjer DM, Matchkov VV, Aalkjaer C. Downregulation of L-type Ca2+ channel in rat mesenteric arteries leads to loss of smooth muscle contractile phenotype and inward hypertrophic remodeling. Am J Physiol Heart Circ Physiol. 2014;306:H1287–H1301. doi: 10.1152/ajpheart.00503.2013. [DOI] [PubMed] [Google Scholar]
- 796.Kuemmerle JF, Makhlouf GM. Agonist-stimulated cyclic ADP ribose. Endogenous modulator of Ca(2+)-induced Ca2+ release in intestinal longitudinal muscle. J Biol Chem. 1995;270:25488–25494. doi: 10.1074/jbc.270.43.25488. [DOI] [PubMed] [Google Scholar]
- 797.Kukuljan M, Labarca P, Latorre R. Molecular determinants of ion conduction and inactivation in K+ channels. Am J Physiol. 1995;268:C535–C556. doi: 10.1152/ajpcell.1995.268.3.C535. [DOI] [PubMed] [Google Scholar]
- 798.Kukuljan M, Rojas E, Catt KJ, Stojilkovic SS. Membrane potential regulates inositol 1,4,5-trisphosphate-controlled cytoplasmic Ca2+ oscillations in pituitary gonadotrophs. J Biol Chem. 1994;269:4860–4865. [PubMed] [Google Scholar]
- 799.Kume H, Graziano MP, Kotlikoff MI. Stimulatory and inhibitory regulation of calcium-activated potassium channels by guanine nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1992;89:11051–11055. doi: 10.1073/pnas.89.22.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Kume H, Hall IP, Washabau RJ, Takagi K, Kotlikoff MI. Beta-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J Clin Invest. 1994;93:371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Kume H, Takai A, Tokuno H, Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989;341:152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- 802.Kuo IY, Ellis A, Seymour VA, Sandow SL, Hill CE. Dihydropyridine-insensitive calcium currents contribute to function of small cerebral arteries. J Cereb Blood Flow Metab. 2010;30:1226–1239. doi: 10.1038/jcbfm.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803.Kuo IY, Howitt L, Sandow SL, McFarlane A, Hansen PB, Hill CE. Role of T-type channels in vasomotor function: Team player or chameleon? Pflugers Arch. 2014;466:767–779. doi: 10.1007/s00424-013-1430-x. [DOI] [PubMed] [Google Scholar]
- 804.Kur J, Bankhead P, Scholfield CN, Curtis TM, McGeown JG. Ca(2+) sparks promote myogenic tone in retinal arterioles. Br J Pharmacol. 2013;168:1675–1686. doi: 10.1111/bph.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Kuschinsky W, Wahl M, Bosse O, Thurau K. Perivascular potassium and pH as determinants of local pial arterial diameter in cats. A microapplication study. Circ Res. 1972;31:240–247. doi: 10.1161/01.res.31.2.240. [DOI] [PubMed] [Google Scholar]
- 806.Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, Yu S, Yao X. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009;104:670–678. doi: 10.1161/CIRCRESAHA.108.188748. [DOI] [PubMed] [Google Scholar]
- 807.Kyle BD, Hurst S, Swayze RD, Sheng J, Braun AP. Specific phosphorylation sites underlie the stimulation of a large conductance, Ca(2+)-activated K(+) channel by cGMP-dependent protein kinase. FASEB J. 2013;27:2027–2038. doi: 10.1096/fj.12-223669. [DOI] [PubMed] [Google Scholar]
- 808.Labadia A, Costa G, Jimenez E, Triguero D, Garcia-Pascual A. Endothelin receptor-mediated Ca2+ mobilization and contraction in bovine oviductal arteries: Comparison with noradrenaline and potassium. Gen Pharmacol. 1997;29:611–619. doi: 10.1016/s0306-3623(96)00565-4. [DOI] [PubMed] [Google Scholar]
- 809.Lagaud GJ, Randriamboavonjy V, Roul G, Stoclet JC, Andriantsitohaina R. Mechanism of Ca2+ release and entry during contraction elicited by norepinephrine in rat resistance arteries. Am J Physiol. 1999;276:H300–H308. doi: 10.1152/ajpheart.1999.276.1.H300. [DOI] [PubMed] [Google Scholar]
- 810.Lagrutta A, Shen KZ, North RA, Adelman JP. Functional differences among alternatively spliced variants of Slowpoke, a Drosophila calcium-activated potassium channel. J Biol Chem. 1994;269:20347–20351. [PubMed] [Google Scholar]
- 811.Lai FA, Erickson HP, Rousseau E, Liu QY, Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988;331:315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- 812.Lal C, Leahy MJ. An updated review of methods and advancements in microvascular blood flow imaging. Microcirculation. 2016;23:345–363. doi: 10.1111/micc.12284. [DOI] [PubMed] [Google Scholar]
- 813.Landry DW, Oliver JA. The ATP-sensitive K+ channel mediates hypotension in endotoxemia and hypoxic lactic acidosis in dog. J Clin Invest. 1992;89:2071–2074. doi: 10.1172/JCI115820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 814.Lang RJ, Harvey JR, McPhee GJ, Klemm MF. Nitric oxide and thiol reagent modulation of Ca2+-activated K+ (BKCa) channels in myocytes of the guinea-pig taenia caeci. J Physiol. 2000;525(Pt 2):363–376. doi: 10.1111/j.1469-7793.2000.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Lang RJ, Harvey JR, Mulholland EL. Sodium (2-sulfonatoethyl) methanethiosulfonate prevents S-nitroso-l-cysteine activation of Ca2+-activated K+ (BKCa) channels in myocytes of the guinea-pig taenia caeca. Br J Pharmacol. 2003;139:1153–1163. doi: 10.1038/sj.bjp.0705349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 816.Lang MG, Paterno R, Faraci FM, Heistad DD. Mechanisms of adrenomedullin-induced dilatation of cerebral arterioles. Stroke. 1997;28:181–185. doi: 10.1161/01.str.28.1.181. [DOI] [PubMed] [Google Scholar]
- 817.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272:27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- 818.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819.Larach DR, Schuler HG. Potassium channel blockade and halothane vasodilation in conducting and resistance coronary arteries. J Pharmacol Exp Ther. 1993;267:72–81. [PubMed] [Google Scholar]
- 820.Large WA. Receptor-operated Ca2(+)-permeable nonselective cation channels in vascular smooth muscle: A physiologic perspective. J Cardiovasc Electrophysiol. 2002;13:493–501. doi: 10.1046/j.1540-8167.2002.00493.x. [DOI] [PubMed] [Google Scholar]
- 821.Large WA, Saleh SN, Albert AP. Role of phosphoinositol 4,5-bisphosphate and diacylglycerol in regulating native TRPC channel proteins in vascular smooth muscle. Cell Calcium. 2009;45:574–582. doi: 10.1016/j.ceca.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 822.Latorre R, Oberhauser A, Labarca P, Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- 823.Latorre R, Vargas G, Orta G, Brauchi S. Voltage and temperature gating of thermoTRP channels. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton, FL: CRC Press; 2007. [PubMed] [Google Scholar]
- 824.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 825.Laver DR, Roden LD, Ahern GP, Eager KR, Junankar PR, Dulhunty AF. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J Membr Biol. 1995;147:7–22. doi: 10.1007/BF00235394. [DOI] [PubMed] [Google Scholar]
- 826.Lazdunski M. ATP-sensitive potassium channels: An overview. J Cardiovasc Pharmacol. 1994;24(Suppl 4):S1–S5. [PubMed] [Google Scholar]
- 827.Lazdunski M, Allard B, Bernardi H, De Weille J, Fosset M, Heurteaux C, Honore E. ATP-sensitive K+ channels. Ren Physiol Biochem. 1994;17:118–120. [PubMed] [Google Scholar]
- 828.Le Blanc C, Mironneau C, Barbot C, Henaff M, Bondeva T, Wetzker R, Macrez N. Regulation of vascular L-type Ca2+ channels by phosphatidylinositol 3,4,5-trisphosphate. Circ Res. 2004;95:300–307. doi: 10.1161/01.RES.0000138017.76125.8b. [DOI] [PubMed] [Google Scholar]
- 829.Leblanc N, Forrest AS, Ayon RJ, Wiwchar M, Angermann JE, Pritchard HA, Singer CA, Valencik ML, Britton F, Greenwood IA. Molecular and functional significance of Ca(2+)-activated Cl(−) channels in pulmonary arterial smooth muscle. Pulm Circ. 2015;5:244–268. doi: 10.1086/680189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.Leblanc N, Wan X, Leung PM. Physiological role of Ca(2+)-activated and voltage-dependent K+ currents in rabbit coronary myocytes. Am J Physiol. 1994;266:C1523–C1537. doi: 10.1152/ajpcell.1994.266.6.C1523. [DOI] [PubMed] [Google Scholar]
- 831.Ledbetter MW, Preiner JK, Louis CF, Mickelson JR. Tissue distribution of ryanodine receptor isoforms and alleles determined by reverse transcription polymerase chain reaction. J Biol Chem. 1994;269:31544–31551. [PubMed] [Google Scholar]
- 832.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 833.Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: Low concentrations selectively block alpha1H. Biophys J. 1999;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.Lee WS, Kwon YJ, Yu SS, Rhim BY, Hong KW. Disturbances in autoregulatory responses of rat pial arteries by sulfonylureas. Life Sci. 1993;52:1527–1534. doi: 10.1016/0024-3205(93)90053-6. [DOI] [PubMed] [Google Scholar]
- 835.Lee CH, Poburko D, Kuo KH, Seow CY, van Breemen C. Ca(2+) oscillations, gradients, and homeostasis in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;282:H1571–H1583. doi: 10.1152/ajpheart.01035.2001. [DOI] [PubMed] [Google Scholar]
- 836.Lee MW, Severson DL. Signal transduction in vascular smooth muscle: Diacylglycerol second messengers and PKC action. Am J Physiol. 1994;267:C659–C678. doi: 10.1152/ajpcell.1994.267.3.C659. [DOI] [PubMed] [Google Scholar]
- 837.Leeb T, Brenig B. cDNA cloning and sequencing of the human ryanodine receptor type 3 (RYR3) reveals a novel alternative splice site in the RYR3 gene. FEBS Lett. 1998;423:367–370. doi: 10.1016/s0014-5793(98)00124-0. [DOI] [PubMed] [Google Scholar]
- 838.Leffler CW, Parfenova H, Basuroy S, Jaggar JH, Umstot ES, Fedinec AL. Hydrogen sulfide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol. 2011;300:H440–H447. doi: 10.1152/ajpheart.00722.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839.Leffler CW, Smith JS, Edrington JL, Zuckerman SL, Parfenova H. Mechanisms of hypoxia-induced cerebrovascular dilation in the newborn pig. Am J Physiol. 1997;272:H1323–H1332. doi: 10.1152/ajpheart.1997.272.3.H1323. [DOI] [PubMed] [Google Scholar]
- 840.Leichtle A, Rauch U, Albinus M, Benohr P, Kalbacher H, Mack AF, Veh RW, Quast U, Russ U. Electrophysiological and molecular characterization of the inward rectifier in juxtaglomerular cells from rat kidney. J Physiol. 2004;560:365–376. doi: 10.1113/jphysiol.2004.070359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841.Lemonnier L, Trebak M, Putney JW., Jr Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium. 2008;43:506–514. doi: 10.1016/j.ceca.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 842.Leo MD, Bannister JP, Narayanan D, Nair A, Grubbs JE, Gabrick KS, Boop FA, Jaggar JH. Dynamic regulation of beta1 subunit trafficking controls vascular contractility. Proc Natl Acad Sci U S A. 2014;111:2361–2366. doi: 10.1073/pnas.1317527111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 843.Leo MD, Bulley S, Bannister JP, Kuruvilla KP, Narayanan D, Jaggar JH. Angiotensin II stimulates internalization and degradation of arterial myocyte plasma membrane BK channels to induce vasoconstriction. Am J Physiol Cell Physiol. 2015;309:C392–C402. doi: 10.1152/ajpcell.00127.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844.Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, Froehner SC, Yates JR, III, Vandenberg CA. Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2.x)-associated proteins. J Biol Chem. 2004;279:22331–22346. doi: 10.1074/jbc.M400285200. [DOI] [PubMed] [Google Scholar]
- 845.Lepretre N, Mironneau J, Morel JL. Both alpha 1A- and alpha 2A-adrenoreceptor subtypes stimulate voltage-operated L-type calcium channels in rat portal vein myocytes. Evidence for two distinct transduction pathways. J Biol Chem. 1994;269:29546–29552. [PubMed] [Google Scholar]
- 846.Leung FP, Yung LM, Yao X, Laher I, Huang Y. Store-operated calcium entry in vascular smooth muscle. Br J Pharmacol. 2008;153:846–857. doi: 10.1038/sj.bjp.0707455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.Levasseur JE, Wei EP, Raper AJ, Kontos AA, Patterson JL. Detailed description of a cranial window technique for acute and chronic experiments. Stroke. 1975;6:308–317. doi: 10.1161/01.str.6.3.308. [DOI] [PubMed] [Google Scholar]
- 848.Lew MJ, Rivers RJ, Duling BR. Arteriolar smooth muscle responses are modulated by an intramural diffusion barrier. Am J Physiol. 1989;257:H10–H16. doi: 10.1152/ajpheart.1989.257.1.H10. [DOI] [PubMed] [Google Scholar]
- 849.Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs cAMP-mediated dilation by reducing Kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H1213–H1219. doi: 10.1152/ajpheart.00226.2003. [DOI] [PubMed] [Google Scholar]
- 850.Li G, Cheung DW. Modulation of Ca(2+)-dependent K(+) currents in mesenteric arterial smooth muscle cells by adenosine. Eur J Pharmacol. 2000;394:35–40. doi: 10.1016/s0014-2999(00)00142-4. [DOI] [PubMed] [Google Scholar]
- 851.Li SS, Cui N, Yang Y, Trower TC, Wei YM, Wu Y, Zhang S, Jin X, Jiang C. Impairment of the vascular KATP channel imposes fatal susceptibility to experimental diabetes due to multi-organ injuries. J Cell Physiol. 2015;230:2915–2926. doi: 10.1002/jcp.25003. [DOI] [PubMed] [Google Scholar]
- 852.Li H, Gutterman DD, Rusch NJ, Bubolz A, Liu Y. Nitration and functional loss of voltage-gated K+ channels in rat coronary microvessels exposed to high glucose. Diabetes. 2004;53:2436–2442. doi: 10.2337/diabetes.53.9.2436. [DOI] [PubMed] [Google Scholar]
- 853.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 854.Li PL, Jin MW, Campbell WB. Effect of selective inhibition of soluble guanylyl cyclase on the K(Ca) channel activity in coronary artery smooth muscle. Hypertension. 1998;31:303–308. doi: 10.1161/01.hyp.31.1.303. [DOI] [PubMed] [Google Scholar]
- 855.Li A, Knutsen RH, Zhang H, Osei-Owusu P, Moreno-Dominguez A, Harter TM, Uchida K, Remedi MS, Dietrich HH, Bernal-Mizrachi C, Blumer KJ, Mecham RP, Koster JC, Nichols CG. Hypotension due to Kir6.1 gain-of-function in vascular smooth muscle. J Am Heart Assoc. 2013;2:e000365. doi: 10.1161/JAHA.113.000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856.Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- 857.Li N, Li Y, Gao Q, Li D, Tang J, Sun M, Zhang P, Liu B, Mao C, Xu Z. Chronic fetal exposure to caffeine altered resistance vessel functions via RyRs-BKCa down-regulation in rat offspring. Sci Rep. 2015;5:13225. doi: 10.1038/srep13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858.Li Z, Liu L, Deng Y, Ji W, Du W, Xu P, Chen L, Xu T. Graded activation of CRAC channel by binding of different numbers of STIM1 to Orai1 subunits. Cell Res. 2011;21:305–315. doi: 10.1038/cr.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 859.Li X, Lu W, Fu X, Zhang Y, Yang K, Zhong N, Ran P, Wang J. BMP4 increases canonical transient receptor potential protein expression by activating p38 MAPK and ERK1/2 signaling pathways in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol. 2013;49:212–220. doi: 10.1165/rcmb.2012-0051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 860.Li Z, Lu N, Shi L. Exercise training reverses alterations in Kv and BKCa channel molecular expression in thoracic aorta smooth muscle cells from spontaneously hypertensive rats. J Vasc Res. 2014;51:447–457. doi: 10.1159/000369928. [DOI] [PubMed] [Google Scholar]
- 861.Li N, Teggatz EG, Li PL, Allaire R, Zou AP. Formation and actions of cyclic ADP-ribose in renal microvessels. Microvasc Res. 2000;60:149–159. doi: 10.1006/mvre.2000.2255. [DOI] [PubMed] [Google Scholar]
- 862.Li C, Wang X, Vais H, Thompson CB, Foskett JK, White C. Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci U S A. 2007;104:12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863.Li Y, Wright JM, Qian F, Germino GG, Guggino WB. Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J Biol Chem. 2005;280:41298–41306. doi: 10.1074/jbc.M510082200. [DOI] [PubMed] [Google Scholar]
- 864.Li SS, Wu Y, Jin X, Jiang C. The SUR2B subunit of rat vascular KATP channel is targeted by miR-9a-3p induced by prolonged exposure to methylglyoxal. Am J Physiol Cell Physiol. 2015;308:C139–C145. doi: 10.1152/ajpcell.00311.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865.Li A, Xi Q, Umstot ES, Bellner L, Schwartzman ML, Jaggar JH, Leffler CW. Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced cerebral arteriolar dilation by activating smooth muscle Cell KCa channels. Circ Res. 2008;102:234–241. doi: 10.1161/CIRCRESAHA.107.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866.Li XQ, Zheng YM, Rathore R, Ma J, Takeshima H, Wang YX. Genetic evidence for functional role of ryanodine receptor 1 in pulmonary artery smooth muscle cells. Pflugers Arch. 2009;457:771–783. doi: 10.1007/s00424-008-0556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 867.Li PL, Zou AP, Campbell WB. Regulation of potassium channels in coronary arterial smooth muscle by endothelium-derived vasodilators. Hypertension. 1997;29:262–267. doi: 10.1161/01.hyp.29.1.262. [DOI] [PubMed] [Google Scholar]
- 868.Li N, Zou AP, Ge ZD, Campbell WB, Li PL. Effect of nitric oxide on calcium-induced calcium release in coronary arterial smooth muscle. Gen Pharmacol. 2000;35:37–45. doi: 10.1016/s0306-3623(01)00089-1. [DOI] [PubMed] [Google Scholar]
- 869.Liang GH, Adebiyi A, Leo MD, McNally EM, Leffler CW, Jaggar JH. Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels. Am J Physiol Heart Circ Physiol. 2011;300:H2088–H2095. doi: 10.1152/ajpheart.01290.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 870.Liang CF, Au AL, Leung SW, Ng KF, Feletou M, Kwan YW, Man RY, Vanhoutte PM. Endothelium-derived nitric oxide inhibits the relaxation of the porcine coronary artery to natriuretic peptides by desensitizing big conductance calcium-activated potassium channels of vascular smooth muscle. J Pharmacol Exp Ther. 2010;334:223–231. doi: 10.1124/jpet.110.166652. [DOI] [PubMed] [Google Scholar]
- 871.Liang GH, Xi Q, Leffler CW, Jaggar JH. Hydrogen sulfide activates Ca(2)(+) sparks to induce cerebral arteriole dilatation. J Physiol. 2012;590:2709–2720. doi: 10.1113/jphysiol.2011.225128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 872.Liao P, Yu D, Li G, Yong TF, Soon JL, Chua YL, Soong TW. A smooth muscle Cav1.2 calcium channel splice variant underlies hyperpolarized window current and enhanced state-dependent inhibition by nifedipine. J Biol Chem. 2007;282:35133–35142. doi: 10.1074/jbc.M705478200. [DOI] [PubMed] [Google Scholar]
- 873.Liapi A, Wood JN. Extensive co-localization and heteromultimer formation of the vanilloid receptor-like protein TRPV2 and the capsaicin receptor TRPV1 in the adult rat cerebral cortex. Eur J Neurosci. 2005;22:825–834. doi: 10.1111/j.1460-9568.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- 874.Lievremont JP, Bird GS, Putney JW., Jr Mechanism of inhibition of TRPC cation channels by 2-aminoethoxydiphenylborane. Mol Pharmacol. 2005;68:758–762. doi: 10.1124/mol.105.012856. [DOI] [PubMed] [Google Scholar]
- 875.Liman ER, Corey DP, Dulac C. TRP2: A candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci U S A. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 876.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- 877.Lindauer U, Vogt J, Schuh-Hofer S, Dreier JP, Dirnagl U. Cerebrovascular vasodilation to extraluminal acidosis occurs via combined activation of ATP-sensitive and Ca2+-activated potassium channels. J Cereb Blood Flow Metab. 2003;23:1227–1238. doi: 10.1097/01.WCB.0000088764.02615.B7. [DOI] [PubMed] [Google Scholar]
- 878.Lindsey SH, Songu-Mize E. Stretch-induced TRPC4 downregulation is accompanied by reduced capacitative Ca2+ entry in WKY but not SHR mesenteric smooth muscle cells. Clin Exp Hypertens. 2010;32:288–292. doi: 10.3109/10641960903443525. [DOI] [PubMed] [Google Scholar]
- 879.Link TE, Murakami K, Beem-Miller M, Tranmer BI, Wellman GC. Oxyhemoglobin-induced expression of R-type Ca2+ channels in cerebral arteries. Stroke. 2008;39:2122–2128. doi: 10.1161/STROKEAHA.107.508754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 880.Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, Groschner K. Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J Biol Chem. 2000;275:27799–27805. doi: 10.1074/jbc.M002705200. [DOI] [PubMed] [Google Scholar]
- 881.Liu GX, Derst C, Schlichthorl G, Heinen S, Seebohm G, Bruggemann A, Kummer W, Veh RW, Daut J, Preisig-Muller R. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol. 2001;532:115–126. doi: 10.1111/j.1469-7793.2001.0115g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 882.Liu Q, Flavahan NA. Hypoxic dilatation of porcine small coronary arteries: role of endothelium and KATP-channels. Br J Pharmacol. 1997;120:728–734. doi: 10.1038/sj.bjp.0700939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 883.Liu X, Gebremedhin D, Harder DR, Koehler RC. Contribution of epoxyeicosatrienoic acids to the cerebral blood flow response to hypoxemia. J Appl Physiol (1985) 2015;119:1202–1209. doi: 10.1152/japplphysiol.01043.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 884.Liu Y, Gutterman DD. The coronary circulation in diabetes: Influence of reactive oxygen species on K+ channel-mediated vasodilation. Vascul Pharmacol. 2002;38:43–49. doi: 10.1016/s1537-1891(02)00125-8. [DOI] [PubMed] [Google Scholar]
- 885.Liu J, Hill MA, Meininger GA. Mechanisms of myogenic enhancement by norepinephrine. Am J Physiol. 1994;266:H440–H446. doi: 10.1152/ajpheart.1994.266.2.H440. [DOI] [PubMed] [Google Scholar]
- 886.Liu Y, Hudetz AG, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in the cerebral microcirculation of genetically hypertensive rats: Evidence for their protection against cerebral vasospasm. Circ Res. 1998;82:729–737. doi: 10.1161/01.res.82.6.729. [DOI] [PubMed] [Google Scholar]
- 887.Liu Y, Jones AW, Sturek M. Increased barium influx and potassium current in stroke-prone spontaneously hypertensive rats. Hypertension. 1994;23:1091–1095. doi: 10.1161/01.hyp.23.6.1091. [DOI] [PubMed] [Google Scholar]
- 888.Liu Y, Jones AW, Sturek M. Ca(2+)-dependent K+ current in arterial smooth muscle cells from aldosterone-salt hypertensive rats. Am J Physiol. 1995;269:H1246–H1257. doi: 10.1152/ajpheart.1995.269.4.H1246. [DOI] [PubMed] [Google Scholar]
- 889.Liu XR, Liu Q, Chen GY, Hu Y, Sham JS, Lin MJ. Down-regulation of TRPM8 in pulmonary arteries of pulmonary hypertensive rats. Cell Physiol Biochem. 2013;31:892–904. doi: 10.1159/000350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890.Liu Y, Pleyte K, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension. 1997;30:1403–1409. doi: 10.1161/01.hyp.30.6.1403. [DOI] [PubMed] [Google Scholar]
- 891.Liu G, Shi J, Yang L, Cao L, Park SM, Cui J, Marx SO. Assembly of a Ca2+-dependent BK channel signaling complex by binding to beta2 adrenergic receptor. Embo J. 2004;23:2196–2205. doi: 10.1038/sj.emboj.7600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892.Liu Y, Terata K, Rusch NJ, Gutterman DD. High glucose impairs voltage-gated K(+) channel current in rat small coronary arteries. Circ Res. 2001;89:146–152. doi: 10.1161/hh1401.093294. [DOI] [PubMed] [Google Scholar]
- 893.Liu P, Xi Q, Ahmed A, Jaggar JH, Dopico AM. Essential role for smooth muscle BK channels in alcohol-induced cerebrovascular constriction. Proc Natl Acad Sci U S A. 2004;101:18217–18222. doi: 10.1073/pnas.0406096102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 894.Liu D, Yang D, He H, Chen X, Cao T, Feng X, Ma L, Luo Z, Wang L, Yan Z, Zhu Z, Tepel M. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension. 2009;53:70–76. doi: 10.1161/HYPERTENSIONAHA.108.116947. [DOI] [PubMed] [Google Scholar]
- 895.Liu QH, Zheng YM, Korde AS, Yadav VR, Rathore R, Wess J, Wang YX. Membrane depolarization causes a direct activation of G protein-coupled receptors leading to local Ca2+ release in smooth muscle. Proc Natl Acad Sci U S A. 2009;106:11418–11423. doi: 10.1073/pnas.0813307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 896.Liu QH, Zheng YM, Wang YX. Two distinct signaling pathways for regulation of spontaneous local Ca2+ release by phospholipase C in airway smooth muscle cells. Pflugers Arch. 2007;453:531–541. doi: 10.1007/s00424-006-0130-1. [DOI] [PubMed] [Google Scholar]
- 897.Lizanecz E, Bagi Z, Pasztor ET, Papp Z, Edes I, Kedei N, Blumberg PM, Toth A. Phosphorylation-dependent desensitization by anandamide of vanilloid receptor-1 (TRPV1) function in rat skeletal muscle arterioles and in Chinese hamster ovary cells expressing TRPV1. Mol Pharmacol. 2006;69:1015–1023. doi: 10.1124/mol.105.015644. [DOI] [PubMed] [Google Scholar]
- 898.Loeb AL, Godeny I, Longnecker DE. Functional evidence for inward-rectifier potassium channels in rat cremaster muscle arterioles. Microvasc Res. 2000;59:1–6. doi: 10.1006/mvre.1999.2187. [DOI] [PubMed] [Google Scholar]
- 899.Loga F, Domes K, Freichel M, Flockerzi V, Dietrich A, Birnbaumer L, Hofmann F, Wegener JW. The role of cGMP/cGKI signalling and Trpc channels in regulation of vascular tone. Cardiovasc Res. 2013;100:280–287. doi: 10.1093/cvr/cvt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 900.Lohn M, Jessner W, Furstenau M, Wellner M, Sorrentino V, Haller H, Luft FC, Gollasch M. Regulation of calcium sparks and spontaneous transient outward currents by RyR3 in arterial vascular smooth muscle cells. Circ Res. 2001;89:1051–1057. doi: 10.1161/hh2301.100250. [DOI] [PubMed] [Google Scholar]
- 901.Long W, Zhao Y, Zhang L, Longo LD. Role of Ca(2+) channels in NE-induced increase in [Ca(2+)](i) and tension in fetal and adult cerebral arteries. Am J Physiol. 1999;277:R286–R294. doi: 10.1152/ajpregu.1999.277.1.R286. [DOI] [PubMed] [Google Scholar]
- 902.Longden TA, Dabertrand F, Hill-Eubanks DC, Hammack SE, Nelson MT. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci U S A. 2014;111:7462–7467. doi: 10.1073/pnas.1401811111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 903.Longden TA, Nelson MT. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation. 2015;22:183–196. doi: 10.1111/micc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 904.Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- 905.Lopatin AN, Nichols CG. [K+] dependence of open-channel conductance in cloned inward rectifier potassium channels (IRK1, Kir2.1) Biophys J. 1996;71:682–694. doi: 10.1016/S0006-3495(96)79268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 906.Loutzenhiser RD, Parker MJ. Hypoxia inhibits myogenic reactivity of renal afferent arterioles by activating ATP-sensitive K+ channels. Circ Res. 1994;74:861–869. doi: 10.1161/01.res.74.5.861. [DOI] [PubMed] [Google Scholar]
- 907.Lovren F, Triggle C. Nitric oxide and sodium nitroprusside-induced relaxation of the human umbilical artery. Br J Pharmacol. 2000;131:521–529. doi: 10.1038/sj.bjp.0703588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 908.Lu Z. Mechanism of rectification in inward-rectifier K+ channels. Annu Rev Physiol. 2004;66:103–129. doi: 10.1146/annurev.physiol.66.032102.150822. [DOI] [PubMed] [Google Scholar]
- 909.Lu Y, Hanna ST, Tang G, Wang R. Contributions of Kv1.2, Kv1.5 and Kv2.1 subunits to the native delayed rectifier K(+) current in rat mesenteric artery smooth muscle cells. Life Sci. 2002;71:1465–1473. doi: 10.1016/s0024-3205(02)01922-7. [DOI] [PubMed] [Google Scholar]
- 910.Lu S, Xiang L, Clemmer JS, Gowdey AR, Mittwede PN, Hester RL. Impaired vascular KATP function attenuates exercise capacity in obese zucker rats. Microcirculation. 2013;20:662–669. doi: 10.1111/micc.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 911.Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens. 2005;23:571–579. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- 912.Luykenaar KD, Brett SE, Wu BN, Wiehler WB, Welsh DG. Pyrimidine nucleotides suppress KDR currents and depolarize rat cerebral arteries by activating Rho kinase. Am J Physiol Heart Circ Physiol. 2004;286:H1088–H1100. doi: 10.1152/ajpheart.00903.2003. [DOI] [PubMed] [Google Scholar]
- 913.Luykenaar KD, El-Rahman RA, Walsh MP, Welsh DG. Rho-kinase-mediated suppression of KDR current in cerebral arteries requires an intact actin cytoskeleton. Am J Physiol Heart Circ Physiol. 2009;296:H917–H926. doi: 10.1152/ajpheart.01206.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 914.Luykenaar KD, Welsh DG. Activators of the PKA and PKG pathways attenuate RhoA-mediated suppression of the KDR current in cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;292:H2654–H2663. doi: 10.1152/ajpheart.01255.2006. [DOI] [PubMed] [Google Scholar]
- 915.Lynch FM, Austin C, Heagerty AM, Izzard AS. Adenosine and hypoxic dilation of rat coronary small arteries: Roles of the ATP-sensitive potassium channel, endothelium, and nitric oxide. Am J Physiol Heart Circ Physiol. 2006;290:H1145–H1150. doi: 10.1152/ajpheart.00314.2005. [DOI] [PubMed] [Google Scholar]
- 916.Ma Z, Lou XJ, Horrigan FT. Role of charged residues in the S1–S4 voltage sensor of BK channels. J Gen Physiol. 2006;127:309–328. doi: 10.1085/jgp.200509421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 917.Ma Y, Zhang P, Li J, Lu J, Ge J, Zhao Z, Ma X, Wan S, Yao X, Shen B. Epoxyeicosatrienoic acids act through TRPV4-TRPC1-KCa1.1 complex to induce smooth muscle membrane hyperpolarization and relaxation in human internal mammary arteries. Biochim Biophys Acta. 2015;1852:552–559. doi: 10.1016/j.bbadis.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 918.Ma L, Zhu B, Chen X, Liu J, Guan Y, Ren J. Abnormalities of sarcoplasmic reticulum Ca2+ mobilization in aortic smooth muscle cells from streptozotocin-induced diabetic rats. Clin Exp Pharmacol Physiol. 2008;35:568–573. doi: 10.1111/j.1440-1681.2007.04832.x. [DOI] [PubMed] [Google Scholar]
- 919.Mackie AR, Brueggemann LI, Henderson KK, Shiels AJ, Cribbs LL, Scrogin KE, Byron KL. Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther. 2008;325:475–483. doi: 10.1124/jpet.107.135764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 920.Mackie AR, Byron KL. Cardiovascular KCNQ (Kv7) potassium channels: Physiological regulators and new targets for therapeutic intervention. Mol Pharmacol. 2008;74:1171–1179. doi: 10.1124/mol.108.049825. [DOI] [PubMed] [Google Scholar]
- 921.Mackrill JJ. Ryanodine receptor calcium channels and their partners as drug targets. Biochem Pharmacol. 2010;79:1535–1543. doi: 10.1016/j.bcp.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 922.MacMillan D. FK506 binding proteins: Cellular regulators of intracellular Ca2+ signalling. Eur J Pharmacol. 2013;700:181–193. doi: 10.1016/j.ejphar.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 923.MacMillan D, Currie S, Bradley KN, Muir TC, McCarron JG. In smooth muscle, FK506-binding protein modulates IP3 receptor-evoked Ca2+ release by mTOR and calcineurin. J Cell Sci. 2005;118:5443–5451. doi: 10.1242/jcs.02657. [DOI] [PubMed] [Google Scholar]
- 924.MacMillan D, Currie S, McCarron JG. FK506-binding protein (FKBP12) regulates ryanodine receptor-evoked Ca2+ release in colonic but not aortic smooth muscle. Cell Calcium. 2008;43:539–549. doi: 10.1016/j.ceca.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 925.MacMillan D, McCarron JG. Regulation by FK506 and rapamycin of Ca2+ release from the sarcoplasmic reticulum in vascular smooth muscle: The role of FK506 binding proteins and mTOR. Br J Pharmacol. 2009;158:1112–1120. doi: 10.1111/j.1476-5381.2009.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 926.Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: Promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 927.Macrez N, Morel JL, Kalkbrenner F, Viard P, Schultz G, Mironneau J. A betagamma dimer derived from G13 transduces the angiotensin AT1 receptor signal to stimulation of Ca2+ channels in rat portal vein myocytes. J Biol Chem. 1997;272:23180–23185. doi: 10.1074/jbc.272.37.23180. [DOI] [PubMed] [Google Scholar]
- 928.Macrez-Lepretre N, Kalkbrenner F, Morel JL, Schultz G, Mironneau J. G protein heterotrimer Galpha13beta1gamma3 couples the angiotensin AT1A receptor to increases in cytoplasmic Ca2+ in rat portal vein myocytes. J Biol Chem. 1997;272:10095–10102. doi: 10.1074/jbc.272.15.10095. [DOI] [PubMed] [Google Scholar]
- 929.Magnusson L, Sorensen CM, Braunstein TH, Holstein-Rathlou NH, Salomonsson M. Renovascular BK(Ca) channels are not activated in vivo under resting conditions and during agonist stimulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R345–R353. doi: 10.1152/ajpregu.00337.2006. [DOI] [PubMed] [Google Scholar]
- 930.Mahaut-Smith MP, Martinez-Pinna J, Gurung IS. A role for membrane potential in regulating GPCRs? Trends Pharmacol Sci. 2008;29:421–429. doi: 10.1016/j.tips.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 931.Mahoney MG, Slakey LL, Hepler PK, Gross DJ. Independent modes of propagation of calcium waves in smooth muscle cells. J Cell Sci. 1993;104(Pt 4):1101–1107. doi: 10.1242/jcs.104.4.1101. [DOI] [PubMed] [Google Scholar]
- 932.Majeed Y, Bahnasi Y, Seymour VA, Wilson LA, Milligan CJ, Agarwal AK, Sukumar P, Naylor J, Beech DJ. Rapid and contrasting effects of rosiglitazone on transient receptor potential TRPM3 and TRPC5 channels. Mol Pharmacol. 2011;79:1023–1030. doi: 10.1124/mol.110.069922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 933.Mak DO, Foskett JK. Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: A single-channel point of view. Cell Calcium. 2015;58:67–78. doi: 10.1016/j.ceca.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 934.Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate [correction of tris-phosphate] activation of inositol trisphosphate [correction of tris-phosphate] receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci U S A. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935.Mak DO, McBride S, Foskett JK. Regulation by Ca2+ and inositol 1,4,5-trisphosphate (InsP3) of single recombinant type 3 InsP3 receptor channels. Ca2+ activation uniquely distinguishes types 1 and 3 insp3 receptors. J Gen Physiol. 2001;117:435–446. doi: 10.1085/jgp.117.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 936.Makhina EN, Kelly AJ, Lopatin AN, Mercer RW, Nichols CG. Cloning and expression of a novel human brain inward rectifier potassium channel. J Biol Chem. 1994;269:20468–20474. [PubMed] [Google Scholar]
- 937.Makino A, Firth AL, Yuan JX. Endothelial and smooth muscle cell ion channels in pulmonary vasoconstriction and vascular remodeling. Compr Physiol. 2011;1:1555–1602. doi: 10.1002/cphy.c100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 938.Mandala M, Heppner TJ, Bonev AD, Nelson MT. Effect of endogenous and exogenous nitric oxide on calcium sparks as targets for vasodilation in rat cerebral artery. Nitric Oxide. 2007;16:104–109. doi: 10.1016/j.niox.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 939.Mandinova A, Atar D, Schafer BW, Spiess M, Aebi U, Heizmann CW. Distinct subcellular localization of calcium binding S100 proteins in human smooth muscle cells and their relocation in response to rises in intracellular calcium. J Cell Sci. 1998;111(Pt 14):2043–2054. doi: 10.1242/jcs.111.14.2043. [DOI] [PubMed] [Google Scholar]
- 940.Manna PT, Smith AJ, Taneja TK, Howell GJ, Lippiat JD, Sivaprasadarao A. Constitutive endocytic recycling and protein kinase C-mediated lysosomal degradation control K(ATP) channel surface density. J Biol Chem. 2010;285:5963–5973. doi: 10.1074/jbc.M109.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 941.Mannhold R. KATP channel openers: Structure-activity relationships and therapeutic potential. Med Res Rev. 2004;24:213–266. doi: 10.1002/med.10060. [DOI] [PubMed] [Google Scholar]
- 942.Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L. Decreased expression of voltage- and Ca(2+)-activated K(+) channels in coronary smooth muscle during aging. Circ Res. 2001;88:210–216. doi: 10.1161/01.res.88.2.210. [DOI] [PubMed] [Google Scholar]
- 943.Marks AR. Cellular functions of immunophilins. Physiol Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- 944.Marrelli SP, Johnson TD, Khorovets A, Childres WF, Bryan RM., Jr Altered function of inward rectifier potassium channels in cerebrovascular smooth muscle after ischemia/reperfusion. Stroke. 1998;29:1469–1474. doi: 10.1161/01.str.29.7.1469. [DOI] [PubMed] [Google Scholar]
- 945.Marshall JM, Thomas T, Turner L. A link between adenosine, ATP-sensitive K+ channels, potassium and muscle vasodilatation in the rat in systemic hypoxia. J Physiol. 1993;472:1–9. doi: 10.1113/jphysiol.1993.sp019931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 946.Martelli A, Testai L, Breschi MC, Lawson K, McKay NG, Miceli F, Taglialatela M, Calderone V. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol Res. 2013;70:27–34. doi: 10.1016/j.phrs.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 947.Martens JR, Gelband CH. Alterations in rat interlobar artery membrane potential and K +channels in genetic and nongenetic hypertension. Circ Res. 1996;79:295–301. doi: 10.1161/01.res.79.2.295. [DOI] [PubMed] [Google Scholar]
- 948.Martin RL, Lee JH, Cribbs LL, Perez-Reyes E, Hanck DA. Mibefradil block of cloned T-type calcium channels. J Pharmacol Exp Ther. 2000;295:302–308. [PubMed] [Google Scholar]
- 949.Martinez AC, Pagan RM, Prieto D, Recio P, Garcia-Sacristan A, Hernandez M, Benedito S. Modulation of noradrenergic neurotransmission in isolated rat radial artery. J Pharmacol Sci. 2009;111:299–311. doi: 10.1254/jphs.09135fp. [DOI] [PubMed] [Google Scholar]
- 950.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 951.Marvar PJ, Falck JR, Boegehold MA. High dietary salt reduces the contribution of 20-HETE to arteriolar oxygen responsiveness in skeletal muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1507–H1515. doi: 10.1152/ajpheart.00754.2006. [DOI] [PubMed] [Google Scholar]
- 952.Marvar PJ, Hammer LW, Boegehold MA. Hydrogen peroxide-dependent arteriolar dilation in contracting muscle of rats fed normal and high salt diets. Microcirculation. 2007;14:779–791. doi: 10.1080/10739680701444057. [DOI] [PubMed] [Google Scholar]
- 953.Marx SO, Ondrias K, Marks AR. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors) Science. 1998;281:818–821. doi: 10.1126/science.281.5378.818. [DOI] [PubMed] [Google Scholar]
- 954.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 955.Marziali G, Rossi D, Giannini G, Charlesworth A, Sorrentino V. cDNA cloning reveals a tissue specific expression of alternatively spliced transcripts of the ryanodine receptor type 3 (RyR3) calcium release channel. FEBS Lett. 1996;394:76–82. doi: 10.1016/0014-5793(96)00944-1. [DOI] [PubMed] [Google Scholar]
- 956.Matchkov VV, Boedtkjer DM, Aalkjaer C. The role of Ca(2+) activated Cl(−) channels in blood pressure control. Curr Opin Pharmacol. 2015;21:127–137. doi: 10.1016/j.coph.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 957.Matchkov VV, Secher Dam V, Bodtkjer DM, Aalkjaer C. Transport and function of chloride in vascular smooth muscles. J Vasc Res. 2013;50:69–87. doi: 10.1159/000345242. [DOI] [PubMed] [Google Scholar]
- 958.Mathar I, Vennekens R, Meissner M, Kees F, Van der Mieren G, Camacho Londono JE, Uhl S, Voets T, Hummel B, van den Bergh A, Herijgers P, Nilius B, Flockerzi V, Schweda F, Freichel M. Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J Clin Invest. 2010;120:3267–3279. doi: 10.1172/JCI41348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 959.Matsuda K, Lozinskaya I, Cox RH. Augmented contributions of voltage-gated Ca2+ channels to contractile responses in spontaneously hypertensive rat mesenteric arteries. Am J Hypertens. 1997;10:1231–1239. doi: 10.1016/s0895-7061(97)00225-2. [DOI] [PubMed] [Google Scholar]
- 960.Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+ Nature. 1987;325:156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- 961.Matsumoto T, Szasz T, Tostes RC, Webb RC. Impaired beta-adrenoceptor-induced relaxation in small mesenteric arteries from DOCA-salt hypertensive rats is due to reduced K(Ca) channel activity. Pharmacol Res. 2012;65:537–545. doi: 10.1016/j.phrs.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 962.Matsushita K, Puro DG. Topographical heterogeneity of K(IR) currents in pericyte-containing microvessels of the rat retina: Effect of diabetes. J Physiol. 2006;573:483–495. doi: 10.1113/jphysiol.2006.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 963.Matter N, Ritz MF, Freyermuth S, Rogue P, Malviya AN. Stimulation of nuclear protein kinase C leads to phosphorylation of nuclear inositol 1,4,5-trisphosphate receptor and accelerated calcium release by inositol 1,4,5-trisphosphate from isolated rat liver nuclei. J Biol Chem. 1993;268:732–736. [PubMed] [Google Scholar]
- 964.Mattmann ME, Yu H, Lin Z, Xu K, Huang X, Long S, Wu M, McManus OB, Engers DW, Le UM, Li M, Lindsley CW, Hopkins CR. Identification of (R)-N-(4-(4-methoxyphenyl)thiazol-2-yl)-1-tosylpiperidine-2-carboxamide, ML277, as a novel, potent and selective K(v)7.1 (KCNQ1) potassium channel activator. Bioorg Med Chem Lett. 2012;22:5936–5941. doi: 10.1016/j.bmcl.2012.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 965.Mauban JR, Lamont C, Balke CW, Wier WG. Adrenergic stimulation of rat resistance arteries affects Ca(2+) sparks, Ca(2+) waves, and Ca(2+) oscillations. Am J Physiol Heart Circ Physiol. 2001;280:H2399–H2405. doi: 10.1152/ajpheart.2001.280.5.H2399. [DOI] [PubMed] [Google Scholar]
- 966.Mauban JR, Zacharia J, Fairfax S, Wier WG. PC-PLC/sphingomyelin synthase activity plays a central role in the development of myogenic tone in murine resistance arteries. Am J Physiol Heart Circ Physiol. 2015;308:H1517–H1524. doi: 10.1152/ajpheart.00594.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 967.Mauban JR, Zacharia J, Zhang J, Wier WG. Vascular tone and Ca(2+) signaling in murine cremaster muscle arterioles in vivo. Microcirculation. 2013;20:269–277. doi: 10.1111/micc.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 968.Mayhan WG. Effect of diabetes mellitus on response of the basilar artery to activation of ATP-sensitive potassium channels. Brain Res. 1994;636:35–39. doi: 10.1016/0006-8993(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 969.Mayhan WG, Faraci FM. Responses of cerebral arterioles in diabetic rats to activation of ATP-sensitive potassium channels. Am J Physiol. 1993;265:H152–H157. doi: 10.1152/ajpheart.1993.265.1.H152. [DOI] [PubMed] [Google Scholar]
- 970.Mayhan WG, Mayhan JF, Sun H, Patel KP. In vivo properties of potassium channels in cerebral blood vessels during diabetes mellitus. Microcirculation. 2004;11:605–613. doi: 10.1080/10739680490503410. [DOI] [PubMed] [Google Scholar]
- 971.McCarron JG, Chalmers S, Bradley KN, MacMillan D, Muir TC. Ca2+ microdomains in smooth muscle. Cell Calcium. 2006;40:461–493. doi: 10.1016/j.ceca.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 972.McCarron JG, Halpern W. Impaired potassium-induced dilation in hypertensive rat cerebral arteries does not reflect altered Na+,K(+)-ATPase dilation. Circ Res. 1990;67:1035–1039. doi: 10.1161/01.res.67.4.1035. [DOI] [PubMed] [Google Scholar]
- 973.McCarron JG, Halpern W. Potassium dilates rat cerebral arteries by two independent mechanisms. Am J Physiol. 1990;259:H902–908. doi: 10.1152/ajpheart.1990.259.3.H902. [DOI] [PubMed] [Google Scholar]
- 974.McCobb DP, Fowler NL, Featherstone T, Lingle CJ, Saito M, Krause JE, Salkoff L. A human calcium-activated potassium channel gene expressed in vascular smooth muscle. Am J Physiol. 1995;269:H767–H777. doi: 10.1152/ajpheart.1995.269.3.H767. [DOI] [PubMed] [Google Scholar]
- 975.McGahon MK, Dash DP, Arora A, Wall N, Dawicki J, Simpson DA, Scholfield CN, McGeown JG, Curtis TM. Diabetes downregulates large-conductance Ca2+-activated potassium beta 1 channel subunit in retinal arteriolar smooth muscle. Circ Res. 2007;100:703–711. doi: 10.1161/01.RES.0000260182.36481.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 976.McGahon MK, Dawicki JM, Arora A, Simpson DA, Gardiner TA, Stitt AW, Scholfield CN, McGeown JG, Curtis TM. Kv1.5 is a major component underlying the A-type potassium current in retinal arteriolar smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1001–H1008. doi: 10.1152/ajpheart.01003.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 977.McGahon MK, Dawicki JM, Scholfield CN, McGeown JG, Curtis TM. A-type potassium current in retinal arteriolar smooth muscle cells. Invest Ophthalmol Vis Sci. 2005;46:3281–3287. doi: 10.1167/iovs.04-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 978.McGowan TA, Sharma K. Regulation of inositol 1,4,5-trisphosphate receptors by transforming growth factor-beta: Implications for vascular dysfunction in diabetes. Kidney Int Suppl. 2000;77:S99–S103. doi: 10.1046/j.1523-1755.2000.07716.x. [DOI] [PubMed] [Google Scholar]
- 979.McIntyre P, McLatchie LM, Chambers A, Phillips E, Clarke M, Savidge J, Toms C, Peacock M, Shah K, Winter J, Weerasakera N, Webb M, Rang HP, Bevan S, James IF. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1) Br J Pharmacol. 2001;132:1084–1094. doi: 10.1038/sj.bjp.0703918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 980.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 981.McManus OB. Calcium-activated potassium channels: Regulation by calcium. J Bioenerg Biomembr. 1991;23:537–560. doi: 10.1007/BF00785810. [DOI] [PubMed] [Google Scholar]
- 982.McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 983.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984.McPherson GA. Current trends in the study of potassium channel openers. Gen Pharmacol. 1993;24:275–281. doi: 10.1016/0306-3623(93)90303-f. [DOI] [PubMed] [Google Scholar]
- 985.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. Embo J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 986.Meech RW. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972;42:493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- 987.Meech RW. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974;237:259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 988.Meera P, Wallner M, Jiang Z, Toro L. A calcium switch for the functional coupling between alpha (hslo) and beta subunits (KV, Ca beta) of maxi K channels. FEBS Lett. 1996;382:84–88. doi: 10.1016/0014-5793(96)00151-2. [DOI] [PubMed] [Google Scholar]
- 989.Meera P, Wallner M, Song M, Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0–S6), an extracellular N terminus, and an intracellular (S9–S10) C terminus. Proc Natl Acad Sci U S A. 1997;94:14066–14071. doi: 10.1073/pnas.94.25.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 990.Meisheri KD, Khan SA, Martin JL. Vascular pharmacology of ATP-sensitive K+ channels: Interactions between glyburide and K+ channel openers. J Vasc Res. 1993;30:2–12. doi: 10.1159/000158969. [DOI] [PubMed] [Google Scholar]
- 991.Meisheri KD, Swirtz MA, Purohit SS, Cipkus-Dubray LA, Khan SA, Oleynek JJ. Characterization of K+ channel-dependent as well as -independent components of pinacidil-induced vasodilation. J Pharmacol Exp Ther. 1991;256:492–499. [PubMed] [Google Scholar]
- 992.Meissner G. Regulation of ryanodine receptor ion channels through posttranslational modifications. Curr Top Membr. 2010;66:91–113. doi: 10.1016/S1063-5823(10)66005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 993.Mellander S. Functional aspects of myogenic vascular control. J Hypertens Suppl. 1989;7:S21–S30. discussion S31. [PubMed] [Google Scholar]
- 994.Mercado J, Baylie R, Navedo MF, Yuan C, Scott JD, Nelson MT, Brayden JE, Santana LF. Local control of TRPV4 channels by AKAP150-targeted PKC in arterial smooth muscle. J Gen Physiol. 2014;143:559–575. doi: 10.1085/jgp.201311050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 995.Merkus D, Haitsma DB, Fung TY, Assen YJ, Verdouw PD, Duncker DJ. Coronary blood flow regulation in exercising swine involves parallel rather than redundant vasodilator pathways. Am J Physiol Heart Circ Physiol. 2003;285:H424–H433. doi: 10.1152/ajpheart.00916.2002. [DOI] [PubMed] [Google Scholar]
- 996.Merkus D, Sorop O, Houweling B, Hoogteijling BA, Duncker DJ. KCa+ channels contribute to exercise-induced coronary vasodilation in swine. Am J Physiol Heart Circ Physiol. 2006;291:H2090–H2097. doi: 10.1152/ajpheart.00315.2006. [DOI] [PubMed] [Google Scholar]
- 997.Mery L, Magnino F, Schmidt K, Krause KH, Dufour JF. Alternative splice variants of hTrp4 differentially interact with the C-terminal portion of the inositol 1,4,5-trisphosphate receptors. FEBS Lett. 2001;487:377–383. doi: 10.1016/s0014-5793(00)02362-0. [DOI] [PubMed] [Google Scholar]
- 998.Messing M, Van Essen H, Smith TL, Smits JF, Struyker-Boudier HA. Microvascular actions of calcium channel antagonists. Eur J Pharmacol. 1991;198:189–195. doi: 10.1016/0014-2999(91)90620-6. [DOI] [PubMed] [Google Scholar]
- 999.Meyers MB, Pickel VM, Sheu SS, Sharma VK, Scotto KW, Fishman GI. Association of sorcin with the cardiac ryanodine receptor. J Biol Chem. 1995;270:26411–26418. doi: 10.1074/jbc.270.44.26411. [DOI] [PubMed] [Google Scholar]
- 1000.Michelakis ED, Reeve HL, Huang JM, Tolarova S, Nelson DP, Weir EK, Archer SL. Potassium channel diversity in vascular smooth muscle cells. Can J Physiol Pharmacol. 1997;75:889–897. [PubMed] [Google Scholar]
- 1001.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 1002.Miller M, Shi J, Zhu Y, Kustov M, Tian JB, Stevens A, Wu M, Xu J, Long S, Yang P, Zholos AV, Salovich JM, Weaver CD, Hopkins CR, Lindsley CW, McManus O, Li M, Zhu MX. Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J Biol Chem. 2011;286:33436–33446. doi: 10.1074/jbc.M111.274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1003.Miller AW, Tulbert C, Puskar M, Busija DW. Enhanced endothelin activity prevents vasodilation to insulin in insulin resistance. Hypertension. 2002;40:78–82. doi: 10.1161/01.hyp.0000022806.87281.62. [DOI] [PubMed] [Google Scholar]
- 1004.Minami K, Fukuzawa K, Nakaya Y. Protein kinase C inhibits the Ca(2+)-activated K+ channel of cultured porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1993;190:263–269. doi: 10.1006/bbrc.1993.1040. [DOI] [PubMed] [Google Scholar]
- 1005.Ming Z, Parent R, Lavallee M. Beta 2-adrenergic dilation of resistance coronary vessels involves KATP channels and nitric oxide in conscious dogs. Circulation. 1997;95:1568–1576. doi: 10.1161/01.cir.95.6.1568. [DOI] [PubMed] [Google Scholar]
- 1006.Minke B, Wu C, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258:84–87. doi: 10.1038/258084a0. [DOI] [PubMed] [Google Scholar]
- 1007.Miriel VA, Mauban JR, Blaustein MP, Wier WG. Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J Physiol. 1999;518(Pt 3):815–824. doi: 10.1111/j.1469-7793.1999.0815p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1008.Mironneau J, Coussin F, Jeyakumar LH, Fleischer S, Mironneau C, Macrez N. Contribution of ryanodine receptor subtype 3 to Ca2+ responses in Ca2+-overloaded cultured rat portal vein myocytes. J Biol Chem. 2001;276:11257–11264. doi: 10.1074/jbc.M005994200. [DOI] [PubMed] [Google Scholar]
- 1009.Mironneau J, Macrez-Lepretre N. Modulation of Ca2+ channels by alpha 1A- and alpha 2A-adrenoceptors in vascular myocytes: Involvement of different transduction pathways. Cell Signal. 1995;7:471–479. doi: 10.1016/0898-6568(95)00014-g. [DOI] [PubMed] [Google Scholar]
- 1010.Misfeldt MW, Aalkjaer C, Simonsen U, Bek T. Voltage-gated calcium channels are involved in the regulation of calcium oscillations in vascular smooth muscle cells from isolated porcine retinal arterioles. Exp Eye Res. 2010;91:69–75. doi: 10.1016/j.exer.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 1011.Mistry DK, Garland CJ. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BK(Ca)) in smooth muscle cells isolated from the rat mesenteric artery. Br J Pharmacol. 1998;124:1131–1140. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1012.Mistry DK, Garland CJ. The influence of phenylephrine outward potassium currents in single smooth muscle cells from the rabbit mesenteric artery. Gen Pharmacol. 1999;33:389–399. doi: 10.1016/s0306-3623(99)00031-2. [DOI] [PubMed] [Google Scholar]
- 1013.Miura H, Wachtel RE, Loberiza FR, Jr, Saito T, Miura M, Nicolosi AC, Gutterman DD. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: Reduced activity of ATP-sensitive potassium channels. Circ Res. 2003;92:151–158. doi: 10.1161/01.res.0000052671.53256.49. [DOI] [PubMed] [Google Scholar]
- 1014.Miyata N, Tsuchida K, Otomo S. Functional changes in potassium channels in carotid arteries from stroke-prone spontaneously hypertensive rats. Eur J Pharmacol. 1990;182:209–210. doi: 10.1016/0014-2999(90)90517-a. [DOI] [PubMed] [Google Scholar]
- 1015.Miyatake R, Furukawa A, Matsushita M, Iwahashi K, Nakamura K, Ichikawa Y, Suwaki H. Tissue-specific alternative splicing of mouse brain type ryanodine receptor/calcium release channel mRNA. FEBS Lett. 1996;395:123–126. doi: 10.1016/0014-5793(96)01022-8. [DOI] [PubMed] [Google Scholar]
- 1016.Miyoshi Y, Nakaya Y. Angiotensin II blocks ATP-sensitive K+ channels in porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1991;181:700–706. doi: 10.1016/0006-291x(91)91247-a. [DOI] [PubMed] [Google Scholar]
- 1017.Miyoshi H, Nakaya Y, Moritoki H. Nonendothelial-derived nitric oxide activates the ATP-sensitive K+ channel of vascular smooth muscle cells. FEBS Lett. 1994;345:47–49. doi: 10.1016/0014-5793(94)00417-x. [DOI] [PubMed] [Google Scholar]
- 1018.Miyoshi Y, Nakaya Y, Wakatsuki T, Nakaya S, Fujino K, Saito K, Inoue I. Endothelin blocks ATP-sensitive K+ channels and depolarizes smooth muscle cells of porcine coronary artery. Circ Res. 1992;70:612–616. doi: 10.1161/01.res.70.3.612. [DOI] [PubMed] [Google Scholar]
- 1019.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 1020.Mokelke EA, Dietz NJ, Eckman DM, Nelson MT, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol. 2005;288:H1233–H1241. doi: 10.1152/ajpheart.00732.2004. [DOI] [PubMed] [Google Scholar]
- 1021.Mokelke EA, Hu Q, Song M, Toro L, Reddy HK, Sturek M. Altered functional coupling of coronary K+ channels in diabetic dyslipidemic pigs is prevented by exercise. J Appl Physiol (1985) 2003;95:1179–1193. doi: 10.1152/japplphysiol.00972.2002. [DOI] [PubMed] [Google Scholar]
- 1022.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 1023.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 1024.Moore CL, Nelson PL, Parelkar NK, Rusch NJ, Rhee SW. Protein kinase A-phosphorylated KV1 channels in PSD95 signaling complex contribute to the resting membrane potential and diameter of cerebral arteries. Circ Res. 2014;114:1258–1267. doi: 10.1161/CIRCRESAHA.114.303167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1025.Moosmang S, Haider N, Bruderl B, Welling A, Hofmann F. Antihypertensive effects of the putative T-type calcium channel antagonist mibefradil are mediated by the L-type calcium channel Cav1.2. Circ Res. 2006;98:105–110. doi: 10.1161/01.RES.0000197851.11031.9c. [DOI] [PubMed] [Google Scholar]
- 1026.Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. Embo J. 2003;22:6027–6034. doi: 10.1093/emboj/cdg583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1027.Morelli A, Lange M, Ertmer C, Broeking K, Van Aken H, Orecchioni A, Rocco M, Bachetoni A, Traber DL, Landoni G, Pietropaoli P, Westphal M. Glibenclamide dose response in patients with septic shock: Effects on norepinephrine requirements, cardiopulmonary performance, and global oxygen transport. Shock. 2007;28:530–535. doi: 10.1097/shk.0b013e3180556a3c. [DOI] [PubMed] [Google Scholar]
- 1028.Moreno-Dominguez A, Cidad P, Miguel-Velado E, Lopez-Lopez JR, Perez-Garcia MT. De novo expression of Kv6.3 contributes to changes in vascular smooth muscle cell excitability in a hypertensive mice strain. J Physiol. 2009;587:625–640. doi: 10.1113/jphysiol.2008.165217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1029.Mori H, Chujo M, Tanaka E, Yamakawa A, Shinozaki Y, Mohamed MU, Nakazawa H. Modulation of adrenergic coronary vasoconstriction via ATP-sensitive potassium channel. Am J Physiol. 1995;268:H1077–H1085. doi: 10.1152/ajpheart.1995.268.3.H1077. [DOI] [PubMed] [Google Scholar]
- 1030.Mori A, Suzuki S, Sakamoto K, Nakahara T, Ishii K. BMS-191011, an opener of large-conductance Ca2+-activated potassium channels, dilates rat retinal arterioles in vivo. Biol Pharm Bull. 2011;34:150–152. doi: 10.1248/bpb.34.150. [DOI] [PubMed] [Google Scholar]
- 1031.Morita T, Okada M, Yamawaki H. Mechanisms underlying a decrease in KCl-induced contraction after long-term serum-free organ culture of rat isolated mesenteric artery. J Vet Med Sci. 2014;76:963–969. doi: 10.1292/jvms.14-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1032.Morita H, Shi J, Ito Y, Inoue R. T-channel-like pharmacological properties of high voltage-activated, nifedipine-insensitive Ca2+ currents in the rat terminal mesenteric artery. Br J Pharmacol. 2002;137:467–476. doi: 10.1038/sj.bjp.0704892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1033.Moriyama K, Osugi S, Shimamura K, Sunano S. Caffeine-induced contraction in arteries from stroke-prone spontaneously hypertensive rats. Blood Vessels. 1989;26:280–289. doi: 10.1159/000158777. [DOI] [PubMed] [Google Scholar]
- 1034.Movahed P, Evilevitch V, Andersson TL, Jonsson BA, Wollmer P, Zygmunt PM, Hogestatt ED. Vascular effects of anandamide and N-acylvanillylamines in the human forearm and skin microcirculation. Br J Pharmacol. 2005;146:171–179. doi: 10.1038/sj.bjp.0706313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1035.Mufti RE, Brett SE, Tran CH, Abd El-Rahman R, Anfinogenova Y, El-Yazbi A, Cole WC, Jones PP, Chen SR, Welsh DG. Intravascular pressure augments cerebral arterial constriction by inducing voltage-insensitive Ca2+ waves. J Physiol. 2010;588:3983–4005. doi: 10.1113/jphysiol.2010.193300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1036.Mufti RE, Zechariah A, Sancho M, Mazumdar N, Brett SE, Welsh DG. Implications of alphavbeta3 integrin signaling in the regulation of Ca2+ waves and myogenic tone in cerebral arteries. Arterioscler Thromb Vasc Biol. 2015;35:2571–2578. doi: 10.1161/ATVBAHA.115.305619. [DOI] [PubMed] [Google Scholar]
- 1037.Munoz E, Hernandez-Morales M, Sobradillo D, Rocher A, Nunez L, Villalobos C. Intracellular Ca(2+) remodeling during the phenotypic journey of human coronary smooth muscle cells. Cell Calcium. 2013;54:375–385. doi: 10.1016/j.ceca.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 1038.Murakami M, Yamamura H, Murakami A, Okamura T, Nunoki K, Mitui-Saito M, Muraki K, Hano T, Imaizumi Y, Flockerzi T, Yanagisawa T. Conserved smooth muscle contractility and blood pressure increase in response to high-salt diet in mice lacking the beta3 subunit of the voltage-dependent calcium channel. J Cardiovasc Pharmacol. 2000;36(Suppl 2):S69–S73. doi: 10.1097/00005344-200000006-00015. [DOI] [PubMed] [Google Scholar]
- 1039.Murakami M, Yamamura H, Suzuki T, Kang MG, Ohya S, Murakami A, Miyoshi I, Sasano H, Muraki K, Hano T, Kasai N, Nakayama S, Campbell KP, Flockerzi V, Imaizumi Y, Yanagisawa T, Iijima T. Modified cardiovascular L-type channels in mice lacking the voltage-dependent Ca2+ channel beta3 subunit. J Biol Chem. 2003;278:43261–43267. doi: 10.1074/jbc.M211380200. [DOI] [PubMed] [Google Scholar]
- 1040.Murphy ME, Brayden JE. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J Physiol. 1995;486(Pt 1):47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1041.Murrant CL, Sarelius IH. Multiple dilator pathways in skeletal muscle contraction-induced arteriolar dilations. Am J Physiol Regul Integr Comp Physiol. 2002;282:R969–R978. doi: 10.1152/ajpregu.00405.2001. [DOI] [PubMed] [Google Scholar]
- 1042.Murthy KS, Zhou H. Selective phosphorylation of the IP3R-I in vivo by cGMP-dependent protein kinase in smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;284:G221–G230. doi: 10.1152/ajpgi.00401.2002. [DOI] [PubMed] [Google Scholar]
- 1043.Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Barodka VM, Gazi FK, Barrow RK, Wang R, Amzel LM, Berkowitz DE, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1044.Nagaoka T, Hein TW, Yoshida A, Kuo L. Resveratrol, a component of red wine, elicits dilation of isolated porcine retinal arterioles: Role of nitric oxide and potassium channels. Invest Ophthalmol Vis Sci. 2007;48:4232–4239. doi: 10.1167/iovs.07-0094. [DOI] [PubMed] [Google Scholar]
- 1045.Nagasaki K, Fleischer S. Ryanodine sensitivity of the calcium release channel of sarcoplasmic reticulum. Cell Calcium. 1988;9:1–7. doi: 10.1016/0143-4160(88)90032-2. [DOI] [PubMed] [Google Scholar]
- 1046.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1047.Nakahata K, Kinoshita H, Tokinaga Y, Ishida Y, Kimoto Y, Dojo M, Mizumoto K, Ogawa K, Hatano Y. Vasodilation mediated by inward rectifier K+ channels in cerebral microvessels of hypertensive and normotensive rats. Anesth Analg. 2006;102:571–576. doi: 10.1213/01.ane.0000194303.00844.5e. [DOI] [PubMed] [Google Scholar]
- 1048.Nakashima M, Vanhoutte PM. Isoproterenol causes hyperpolarization through opening of ATP-sensitive potassium channels in vascular smooth muscle of the canine saphenous vein. J Pharmacol Exp Ther. 1995;272:379–384. [PubMed] [Google Scholar]
- 1049.Nakhostine N, Lamontagne D. Adenosine contributes to hypoxia-induced vasodilation through ATP-sensitive K+ channel activation. Am J Physiol. 1993;265:H1289–H1293. doi: 10.1152/ajpheart.1993.265.4.H1289. [DOI] [PubMed] [Google Scholar]
- 1050.Nakhostine N, Lamontagne D. Contribution of prostaglandins in hypoxia-induced vasodilation in isolated rabbit hearts. Relation to adenosine and KATP channels. Pflugers Arch. 1994;428:526–532. doi: 10.1007/BF00374574. [DOI] [PubMed] [Google Scholar]
- 1051.Nalli AD, Kumar DP, Al-Shboul O, Mahavadi S, Kuemmerle JF, Grider JR, Murthy KS. Regulation of Gbetagammai-dependent PLC-beta3 activity in smooth muscle: Inhibitory phosphorylation of PLC-beta3 by PKA and PKG and stimulatory phosphorylation of Galphai-GTPase-activating protein RGS2 by PKG. Cell Biochem Biophys. 2014;70:867–880. doi: 10.1007/s12013-014-9992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1052.Nara M, Dhulipala PD, Ji GJ, Kamasani UR, Wang YX, Matalon S, Kotlikoff MI. Guanylyl cyclase stimulatory coupling to K(Ca) channels. Am J Physiol Cell Physiol. 2000;279:C1938–C1945. doi: 10.1152/ajpcell.2000.279.6.C1938. [DOI] [PubMed] [Google Scholar]
- 1053.Nara M, Dhulipala PD, Wang YX, Kotlikoff MI. Reconstitution of beta-adrenergic modulation of large conductance, calcium-activated potassium (maxi-K) channels in Xenopus oocytes. Identification of the camp-dependent protein kinase phosphorylation site. J Biol Chem. 1998;273:14920–14924. doi: 10.1074/jbc.273.24.14920. [DOI] [PubMed] [Google Scholar]
- 1054.Narahashi T, Tsunoo A, Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1055.Narayanan D, Adebiyi A, Jaggar JH. Inositol trisphosphate receptors in smooth muscsle cells. Am J Physiol Heart Circ Physiol. 2012;302:H2190–H2210. doi: 10.1152/ajpheart.01146.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1056.Narayanan D, Bulley S, Leo MD, Burris SK, Gabrick KS, Boop FA, Jaggar JH. Smooth muscle cell transient receptor potential polycystin-2 (TRPP2) channels contribute to the myogenic response in cerebral arteries. J Physiol. 2013;591:5031–5046. doi: 10.1113/jphysiol.2013.258319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1057.Narishige T, Egashira K, Akatsuka Y, Imamura Y, Takahashi T, Kasuya H, Takeshita A. Glibenclamide prevents coronary vasodilation induced by beta 1-adrenoceptor stimulation in dogs. Am J Physiol. 1994;266:H84–H92. doi: 10.1152/ajpheart.1994.266.1.H84. [DOI] [PubMed] [Google Scholar]
- 1058.Narishige T, Egashira K, Akatsuka Y, Katsuda Y, Numaguchi K, Sakata M, Takeshita A. Glibenclamide, a putative ATP-sensitive K+ channel blocker, inhibits coronary autoregulation in anesthetized dogs. Circ Res. 1993;73:771–776. doi: 10.1161/01.res.73.4.771. [DOI] [PubMed] [Google Scholar]
- 1059.Navarro-Dorado J, Garcia-Alonso M, van Breemen C, Tejerina T, Fameli N. Calcium oscillations in human mesenteric vascular smooth muscle. Biochem Biophys Res Commun. 2014;445:84–88. doi: 10.1016/j.bbrc.2014.01.150. [DOI] [PubMed] [Google Scholar]
- 1060.Navarro-Gonzalez MF, Grayson TH, Meaney KR, Cribbs LL, Hill CE. Non-L-type voltage-dependent calcium channels control vascular tone of the rat basilar artery. Clin Exp Pharmacol Physiol. 2009;36:55–66. doi: 10.1111/j.1440-1681.2008.05035.x. [DOI] [PubMed] [Google Scholar]
- 1061.Navedo MF, Amberg GC, Votaw VS, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102:11112–11117. doi: 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1062.Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, Santana LF. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ Res. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1063.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 1064.Navedo MF, Santana LF. CaV1.2 sparklets in heart and vascular smooth muscle. J Mol Cell Cardiol. 2013;58:67–76. doi: 10.1016/j.yjmcc.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1065.Navedo MF, Takeda Y, Nieves-Cintron M, Molkentin JD, Santana LF. Elevated Ca2+ sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells. Am J Physiol Cell Physiol. 2010;298:C211–C220. doi: 10.1152/ajpcell.00267.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1066.Needham M, McGahon MK, Bankhead P, Gardiner TA, Scholfield CN, Curtis TM, McGeown JG. The role of K+ and Cl− channels in the regulation of retinal arteriolar tone and blood flow. Invest Ophthalmol Vis Sci. 2014;55:2157–2165. doi: 10.1167/iovs.13-12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1067.Neeper MP, Liu Y, Hutchinson TL, Wang Y, Flores CM, Qin N. Activation properties of heterologously expressed mammalian TRPV2: Evidence for species dependence. J Biol Chem. 2007;282:15894–15902. doi: 10.1074/jbc.M608287200. [DOI] [PubMed] [Google Scholar]
- 1068.Nelson CP, Rainbow RD, Brignell JL, Perry MD, Willets JM, Davies NW, Standen NB, Challiss RA. Principal role of adenylyl cyclase 6 in K(+) channel regulation and vasodilator signalling in vascular smooth muscle cells. Cardiovasc Res. 2011;91:694–702. doi: 10.1093/cvr/cvr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1069.Nelson MT. Regulation of arterial tone by potassium channels. Jpn J Pharmacol. 1992;58(Suppl 2):238P–242P. [PubMed] [Google Scholar]
- 1070.Nelson MT. Ca(2+)-activated potassium channels and ATP-sensitive potassium channels as modulators of vascular tone. Trends Cardiovasc Med. 1993;3:54–60. doi: 10.1016/1050-1738(93)90037-7. [DOI] [PubMed] [Google Scholar]
- 1071.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 1072.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344:770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- 1073.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 1074.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 1075.Nelson MT, Standen NB, Brayden JE, Worley JF., III Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988;336:382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- 1076.Neylon CB, Hoyland J, Mason WT, Irvine RF. Spatial dynamics of intracellular calcium in agonist-stimulated vascular smooth muscle cells. Am J Physiol. 1990;259:C675–C686. doi: 10.1152/ajpcell.1990.259.4.C675. [DOI] [PubMed] [Google Scholar]
- 1077.Neylon CB, Lang RJ, Fu Y, Bobik A, Reinhart PH. Molecular cloning and characterization of the intermediate-conductance Ca(2+)-activated K(+) channel in vascular smooth muscle: Relationship between K(Ca) channel diversity and smooth muscle cell function. Circ Res. 1999;85:e33–e43. doi: 10.1161/01.res.85.9.e33. [DOI] [PubMed] [Google Scholar]
- 1078.Ng LC, McCormack MD, Airey JA, Singer CA, Keller PS, Shen XM, Hume JR. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol. 2009;587:2429–2442. doi: 10.1113/jphysiol.2009.172254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1079.Ngo AT, Riemann M, Holstein-Rathlou NH, Torp-Pedersen C, Jensen LJ. Significance of K(ATP) channels, L-type Ca(2)(+) channels and CYP450-4A enzymes in oxygen sensing in mouse cremaster muscle arterioles in vivo. BMC Physiol. 2013;13:8. doi: 10.1186/1472-6793-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1080.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 1081.Nie L, Oishi Y, Doi I, Shibata H, Kojima I. Inhibition of proliferation of MCF-7 breast cancer cells by a blocker of Ca(2+)-permeable channel. Cell Calcium. 1997;22:75–82. doi: 10.1016/s0143-4160(97)90107-x. [DOI] [PubMed] [Google Scholar]
- 1082.Nieves-Cintron M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 down-regulates the beta1 subunit of large conductance, calcium-activated K +channels in arterial smooth muscle and contributes to hypertension. J Biol Chem. 2007;282:3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- 1083.Nieves-Cintron M, Nystoriak MA, Prada MP, Johnson K, Fayer W, Dell’Acqua ML, Scott JD, Navedo MF. Selective down-regulation of KV2.1 function contributes to enhanced arterial tone during diabetes. J Biol Chem. 2015;290:7918–7929. doi: 10.1074/jbc.M114.622811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1084.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 1085.Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. Embo J. 2006;25:467–478. doi: 10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1086.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1087.Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V. Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem. 2003;278:30813–30820. doi: 10.1074/jbc.M305127200. [DOI] [PubMed] [Google Scholar]
- 1088.Nilius B, Prenen J, Janssens A, Owsianik G, Wang C, Zhu MX, Voets T. The selectivity filter of the cation channel TRPM4. J Biol Chem. 2005;280:22899–22906. doi: 10.1074/jbc.M501686200. [DOI] [PubMed] [Google Scholar]
- 1089.Nilius B, Prenen J, Janssens A, Voets T, Droogmans G. Decavanadate modulates gating of TRPM4 cation channels. J Physiol. 2004;560:753–765. doi: 10.1113/jphysiol.2004.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1090.Nilius B, Prenen J, Owsianik G. Irritating channels: The case of TRPA1. J Physiol. 2011;589:1543–1549. doi: 10.1113/jphysiol.2010.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1091.Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem. 2005;280:6423–6433. doi: 10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- 1092.Nilius B, Prenen J, Voets T, Droogmans G. Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Pflugers Arch. 2004;448:70–75. doi: 10.1007/s00424-003-1221-x. [DOI] [PubMed] [Google Scholar]
- 1093.Nims JC, Irwin JW. Chamber techniques to study the microvasculature. Microvasc Res. 1973;5:105–118. doi: 10.1016/s0026-2862(73)80013-5. [DOI] [PubMed] [Google Scholar]
- 1094.Nishimaru K, Eghbali M, Lu R, Marijic J, Stefani E, Toro L. Functional and molecular evidence of MaxiK channel beta1 subunit decrease with coronary artery ageing in the rat. J Physiol. 2004;559:849–862. doi: 10.1113/jphysiol.2004.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1095.Nixon GF, Mignery GA, Somlyo AV. Immunogold localization of inositol 1,4,5-trisphosphate receptors and characterization of ultra-structural features of the sarcoplasmic reticulum in phasic and tonic smooth muscle. J Muscle Res Cell Motil. 1994;15:682–700. doi: 10.1007/BF00121075. [DOI] [PubMed] [Google Scholar]
- 1096.Nnorom CC, Davis C, Fedinec AL, Howell K, Jaggar JH, Parfenova H, Pourcyrous M, Leffler CW. Contributions of KATP and KCa channels to cerebral arteriolar dilation to hypercapnia in neonatal brain. Physiol Rep. 2014;2:e12127. doi: 10.14814/phy2.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1097.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 1098.Nourian Z, Li M, Leo MD, Jaggar JH, Braun AP, Hill MA. Large conductance Ca2+-activated K +channel (BKCa) alpha-subunit splice variants in resistance arteries from rat cerebral and skeletal muscle vasculature. PLoS One. 2014;9:e98863. doi: 10.1371/journal.pone.0098863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1099.Nystoriak MA, Nieves-Cintron M, Nygren PJ, Hinke SA, Nichols CB, Chen CY, Puglisi JL, Izu LT, Bers DM, Dell’acqua ML, Scott JD, Santana LF, Navedo MF. AKAP150 contributes to enhanced vascular tone by facilitating large-conductance Ca2+-activated K+ channel remodeling in hyperglycemia and diabetes mellitus. Circ Res. 2014;114:607–615. doi: 10.1161/CIRCRESAHA.114.302168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1100.O’Brien F, Venturi E, Sitsapesan R. The ryanodine receptor provides high throughput Ca2+-release but is precisely regulated by networks of associated proteins: A focus on proteins relevant to phosphorylation. Biochem Soc Trans. 2015;43:426–433. doi: 10.1042/BST20140297. [DOI] [PubMed] [Google Scholar]
- 1101.Obukhov AG, Nowycky MC. A cytosolic residue mediates Mg2+ block and regulates inward current amplitude of a transient receptor potential channel. J Neurosci. 2005;25:1234–1239. doi: 10.1523/JNEUROSCI.4451-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1102.Ohya S, Sergeant GP, Greenwood IA, Horowitz B. Molecular variants of KCNQ channels expressed in murine portal vein myocytes: A role in delayed rectifier current. Circ Res. 2003;92:1016–1023. doi: 10.1161/01.RES.0000070880.20955.F4. [DOI] [PubMed] [Google Scholar]
- 1103.Ohya Y, Setoguchi M, Fujii K, Nagao T, Abe I, Fujishima M. Impaired action of levcromakalim on ATP-sensitive K+ channels in mesenteric artery cells from spontaneously hypertensive rats. Hypertension. 1996;27:1234–1239. doi: 10.1161/01.hyp.27.6.1234. [DOI] [PubMed] [Google Scholar]
- 1104.Okabe K, Kitamura K, Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987;409:561–568. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- 1105.Okada Y, Yanagisawa T, Taira N. BRL 38227 (levcromakalim)-induced hyperpolarization reduces the sensitivity to Ca2+ of contractile elements in canine coronary artery. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:438–444. doi: 10.1007/BF00165396. [DOI] [PubMed] [Google Scholar]
- 1106.Okon EB, Chung AW, Rauniyar P, Padilla E, Tejerina T, McManus BM, Luo H, van Breemen C. Compromised arterial function in human type 2 diabetic patients. Diabetes. 2005;54:2415–2423. doi: 10.2337/diabetes.54.8.2415. [DOI] [PubMed] [Google Scholar]
- 1107.Olesen SP, Munch E, Moldt P, Drejer J. Selective activation of Ca(2+)-dependent K+ channels by novel benzimidazolone. Eur J Pharmacol. 1994;251:53–59. doi: 10.1016/0014-2999(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 1108.Onoue H, Katusic ZS. Role of potassium channels in relaxations of canine middle cerebral arteries induced by nitric oxide donors. Stroke. 1997;28:1264–1270. doi: 10.1161/01.str.28.6.1264. discussion 1270-1261. [DOI] [PubMed] [Google Scholar]
- 1109.Onoue H, Katusic ZS. The effect of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and charybdotoxin (CTX) on relaxations of isolated cerebral arteries to nitric oxide. Brain Res. 1998;785:107–113. doi: 10.1016/s0006-8993(97)01393-0. [DOI] [PubMed] [Google Scholar]
- 1110.Onoue H, Katusic ZS. The effect of subarachnoid hemorrhage on mechanisms of vasodilation mediated by cyclic adenosine monophosphate. J Neurosurg. 1998;89:111–117. doi: 10.3171/jns.1998.89.1.0111. [DOI] [PubMed] [Google Scholar]
- 1111.Opie LH. Modulation of ischemia by regulation of the ATP-sensitive potassium channel. Cardiovasc Drugs Ther. 1993;7(Suppl 3):507–513. doi: 10.1007/BF00877615. [DOI] [PubMed] [Google Scholar]
- 1112.Orie NN, Fry CH, Clapp LH. Evidence that inward rectifier K+ channels mediate relaxation by the PGI2 receptor agonist cicaprost via a cyclic AMP-independent mechanism. Cardiovasc Res. 2006;69:107–115. doi: 10.1016/j.cardiores.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 1113.Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res. 1991;68:359–367. doi: 10.1161/01.res.68.2.359. [DOI] [PubMed] [Google Scholar]
- 1114.Osol G, Laher I, Kelley M. Myogenic tone is coupled to phospholipase C and G protein activation in small cerebral arteries. Am J Physiol. 1993;265:H415–H420. doi: 10.1152/ajpheart.1993.265.1.H415. [DOI] [PubMed] [Google Scholar]
- 1115.Ouchi Y, Han SZ, Kim S, Akishita M, Kozaki K, Toba K, Orimo H. Augmented contractile function and abnormal Ca2+ handling in the aorta of Zucker obese rats with insulin resistance. Diabetes. 1996;45(Suppl 3):S55–S58. doi: 10.2337/diab.45.3.s55. [DOI] [PubMed] [Google Scholar]
- 1116.Overbeck HW. Vascular responses to cations, osmolality, and angiotensin in renal hypertensive dogs. Am J Physiol. 1972;223:1358–1364. doi: 10.1152/ajplegacy.1972.223.6.1358. [DOI] [PubMed] [Google Scholar]
- 1117.Overbeck HW, Clark DW. Vasodilator responses to K+ in genetic hypertensive and in renal hypertensive rats. J Lab Clin Med. 1975;86:973–983. [PubMed] [Google Scholar]
- 1118.Overbeck HW, Derifield RS, Pamnani MB, Sozen T. Attenuated vasodilator responses to K +in essential hypertensive men. J Clin Invest. 1974;53:678–686. doi: 10.1172/JCI107605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1119.Overbeck HW, Molnar JI, Haddy FJ. Resistance to blood flow through the vascular bed of the dog forelimb. Local effects of sodium, potassium, calcium, magnesium, acetate, hypertonicity and hypotonicity. Am J Cardiol. 1961;8:533–541. doi: 10.1016/0002-9149(61)90131-x. [DOI] [PubMed] [Google Scholar]
- 1120.Overturf KE, Russell SN, Carl A, Vogalis F, Hart PJ, Hume JR, Sanders KM, Horowitz B. Cloning and characterization of a Kv 1.5 delayed rectifier K+ channel from vascular and visceral smooth muscles. Am J Physiol. 1994;267:C1231–C1238. doi: 10.1152/ajpcell.1994.267.5.C1231. [DOI] [PubMed] [Google Scholar]
- 1121.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 1122.Ozkor MA, Hayek SS, Rahman AM, Murrow JR, Kavtaradze N, Lin J, Manatunga A, Quyyumi AA. Contribution of endothelium-derived hyperpolarizing factor to exercise-induced vasodilation in health and hypercholesterolemia. Vasc Med. 2015;20:14–22. doi: 10.1177/1358863X14565374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1123.Ozkor MA, Murrow JR, Rahman AM, Kavtaradze N, Lin J, Manatunga A, Quyyumi AA. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation. 2011;123:2244–2253. doi: 10.1161/CIRCULATIONAHA.110.990317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1124.Pagan RM, Martinez AC, Martinez MP, Hernandez M, Garcia-Sacristan A, Correa C, Prieto D, Benedito S. Endothelial and potassium channel dependent modulation of noradrenergic vasoconstriction in the pig radial artery. Eur J Pharmacol. 2009;616:166–174. doi: 10.1016/j.ejphar.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 1125.Paisansathan C, Xu H, Vetri F, Hernandez M, Pelligrino DA. Interactions between adenosine and K+ channel-related pathways in the coupling of somatosensory activation and pial arteriolar dilation. Am J Physiol Heart Circ Physiol. 2010;299:H2009–H2017. doi: 10.1152/ajpheart.00702.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1126.Pallanck L, Ganetzky B. Cloning and characterization of human and mouse homologs of the Drosophila calcium-activated potassium channel gene, slowpoke. Hum Mol Genet. 1994;3:1239–1243. doi: 10.1093/hmg/3.8.1239. [DOI] [PubMed] [Google Scholar]
- 1127.Pallotta BS, Wagoner PK. Voltage-dependent potassium channels since Hodgkin and Huxley. Physiol Rev. 1992;72:S49–S67. doi: 10.1152/physrev.1992.72.suppl_4.S49. [DOI] [PubMed] [Google Scholar]
- 1128.Park WS, Han J, Earm YE. Physiological role of inward rectifier K(+) channels in vascular smooth muscle cells. Pflugers Arch. 2008;457:137–147. doi: 10.1007/s00424-008-0512-7. [DOI] [PubMed] [Google Scholar]
- 1129.Park WS, Han J, Kim N, Ko JH, Kim SJ, Earm YE. Activation of inward rectifier K+ channels by hypoxia in rabbit coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;289:H2461–H2467. doi: 10.1152/ajpheart.00331.2005. [DOI] [PubMed] [Google Scholar]
- 1130.Park WS, Han J, Kim N, Youm JB, Joo H, Kim HK, Ko JH, Earm YE. Endothelin-1 inhibits inward rectifier K+ channels in rabbit coronary arterial smooth muscle cells through protein kinase C. J Cardiovasc Pharmacol. 2005;46:681–689. doi: 10.1097/01.fjc.0000182846.08357.ed. [DOI] [PubMed] [Google Scholar]
- 1131.Park HW, Kim JY, Choi SK, Lee YH, Zeng W, Kim KH, Muallem S, Lee MG. Serine-threonine kinase with-no-lysine 4 (WNK4) controls blood pressure via transient receptor potential canonical 3 (TRPC3) in the vasculature. Proc Natl Acad Sci U S A. 2011;108:10750–10755. doi: 10.1073/pnas.1104271108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1132.Park JK, Kim YC, Sim JH, Choi MY, Choi W, Hwang KK, Cho MC, Kim KW, Lim SW, Lee SJ. Regulation of membrane excitability by intracellular pH (pHi) changers through Ca2+-activated K+ current (BK channel) in single smooth muscle cells from rabbit basilar artery. Pflugers Arch. 2007;454:307–319. doi: 10.1007/s00424-007-0204-8. [DOI] [PubMed] [Google Scholar]
- 1133.Park WS, Kim N, Youm JB, Warda M, Ko JH, Kim SJ, Earm YE, Han J. Angiotensin II inhibits inward rectifier K+ channels in rabbit coronary arterial smooth muscle cells through protein kinase Calpha. Biochem Biophys Res Commun. 2006;341:728–735. doi: 10.1016/j.bbrc.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 1134.Park WS, Ko JH, Kim N, Son YK, Kang SH, Warda M, Jung ID, Park YM, Han J. Increased inhibition of inward rectifier K+ channels by angiotensin II in small-diameter coronary artery of isoproterenol-induced hypertrophied model. Arterioscler Thromb Vasc Biol. 2007;27:1768–1775. doi: 10.1161/ATVBAHA.107.143339. [DOI] [PubMed] [Google Scholar]
- 1135.Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu SY, Kang K, Kim B, Bae YM, Cho H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflugers Arch. 2015;467:285–297. doi: 10.1007/s00424-014-1513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1136.Park WS, Son YK, Kim N, Ko JH, Kang SH, Warda M, Earm YE, Jung ID, Park YM, Han J. Acute hypoxia induces vasodilation and increases coronary blood flow by activating inward rectifier K(+) channels. Pflugers Arch. 2007;454:1023–1030. doi: 10.1007/s00424-007-0269-4. [DOI] [PubMed] [Google Scholar]
- 1137.Park CK, Xu ZZ, Liu T, Lu N, Serhan CN, Ji RR. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: Distinct roles of resolvin D1, D2, and E1. J Neurosci. 2011;31:18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1138.Parkinson NA, Hughes AD. The mechanism of action of alpha 2-adrenoceptors in human isolated subcutaneous resistance arteries. Br J Pharmacol. 1995;115:1463–1468. doi: 10.1111/j.1476-5381.1995.tb16638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1139.Paterno R, Faraci FM, Heistad DD. Role of Ca(2+)-dependent K+ channels in cerebral vasodilatation induced by increases in cyclic GMP and cyclic AMP in the rat. Stroke. 1996;27:1603–1607. doi: 10.1161/01.str.27.9.1603. discussion 1607–1608. [DOI] [PubMed] [Google Scholar]
- 1140.Paterno R, Heistad DD, Faraci FM. Functional activity of Ca2+-dependent K+ channels is increased in basilar artery during chronic hypertension. Am J Physiol. 1997;272:H1287–H1291. doi: 10.1152/ajpheart.1997.272.3.H1287. [DOI] [PubMed] [Google Scholar]
- 1141.Paterno R, Heistad DD, Faraci FM. Potassium channels modulate cerebral autoregulation during acute hypertension. Am J Physiol Heart Circ Physiol. 2000;278:H2003–H2007. doi: 10.1152/ajpheart.2000.278.6.H2003. [DOI] [PubMed] [Google Scholar]
- 1142.Pathan AR, Rusch NJ. Two-pore domain K(+) channels: Evidence for TWIK-2 in blood pressure regulation. Hypertension. 2011;58:539–541. doi: 10.1161/HYPERTENSIONAHA.111.179390. [DOI] [PubMed] [Google Scholar]
- 1143.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 1144.Patterson RL, van Rossum DB, Barrow RK, Snyder SH. RACK1 binds to inositol 1,4,5-trisphosphate receptors and mediates Ca2+ release. Proc Natl Acad Sci U S A. 2004;101:2328–2332. doi: 10.1073/pnas.0308567100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1145.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 1146.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 1147.Peiper U, Griebel L, Wende W. Activation of vascular smooth muscle of rat aorta by noradrenaline and depolarization: Two different mechanisms. Pflugers Arch. 1971;330:74–89. doi: 10.1007/BF00588736. [DOI] [PubMed] [Google Scholar]
- 1148.Pelucchi B, Aguiari G, Pignatelli A, Manzati E, Witzgall R, Del Senno L, Belluzzi O. Nonspecific cation current associated with native polycystin-2 in HEK-293 cells. J Am Soc Nephrol. 2006;17:388–397. doi: 10.1681/ASN.2004121146. [DOI] [PubMed] [Google Scholar]
- 1149.Pemberton M, Anderson GL, Barker JH. Characterization of microvascular vasoconstriction following ischemia/reperfusion in skeletal muscle using videomicroscopy. Microsurgery. 1996;17:9–16. doi: 10.1002/(SICI)1098-2752(1996)17:1<9::AID-MICR2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 1150.Peng H, Matchkov V, Ivarsen A, Aalkjaer C, Nilsson H. Hypothesis for the initiation of vasomotion. Circ Res. 2001;88:810–815. doi: 10.1161/hh0801.089603. [DOI] [PubMed] [Google Scholar]
- 1151.Peppiatt-Wildman CM, Albert AP, Saleh SN, Large WA. Endothelin-1 activates a Ca2+-permeable cation channel with TRPC3 and TRPC7 properties in rabbit coronary artery myocytes. J Physiol. 2007;580:755–764. doi: 10.1113/jphysiol.2006.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1152.Percival AL, Williams AJ, Kenyon JL, Grinsell MM, Airey JA, Sutko JL. Chicken skeletal muscle ryanodine receptor isoforms: Ion channel properties. Biophys J. 1994;67:1834–1850. doi: 10.1016/S0006-3495(94)80665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1153.Perez GJ, Bonev AD, Nelson MT. Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol. 2001;281:C1769–C1775. doi: 10.1152/ajpcell.2001.281.6.C1769. [DOI] [PubMed] [Google Scholar]
- 1154.Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1155.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 1156.Perraud AL, Schmitz C, Scharenberg AM. TRPM2 Ca2+ permeable cation channels: From gene to biological function. Cell Calcium. 2003;33:519–531. doi: 10.1016/s0143-4160(03)00057-5. [DOI] [PubMed] [Google Scholar]
- 1157.Pessah IN, Waterhouse AL, Casida JE. The calcium-ryanodine receptor complex of skeletal and cardiac muscle. Biochem Biophys Res Commun. 1985;128:449–456. doi: 10.1016/0006-291x(85)91699-7. [DOI] [PubMed] [Google Scholar]
- 1158.Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- 1159.Phillips AA, Chan FH, Zheng MM, Krassioukov AV, Ainslie PN. Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab. 2016;36:647–664. doi: 10.1177/0271678X15617954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1160.Pinho JF, Medeiros MA, Capettini LS, Rezende BA, Campos PP, Andrade SP, Cortes SF, Cruz JS, Lemos VS. Phosphatidylinositol 3-kinase-delta up-regulates L-type Ca2+ currents and increases vascular contractility in a mouse model of type 1 diabetes. Br J Pharmacol. 2010;161:1458–1471. doi: 10.1111/j.1476-5381.2010.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1161.Plane F, Garland CJ. Differential effects of acetylcholine, nitric oxide and levcromakalim on smooth muscle membrane potential and tone in the rabbit basilar artery. Br J Pharmacol. 1993;110:651–656. doi: 10.1111/j.1476-5381.1993.tb13861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1162.Plane F, Hurrell A, Jeremy JY, Garland CJ. Evidence that potassium channels make a major contribution to SIN-1-evoked relaxation of rat isolated mesenteric artery. Br J Pharmacol. 1996;119:1557–1562. doi: 10.1111/j.1476-5381.1996.tb16072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1163.Plane F, Johnson R, Kerr P, Wiehler W, Thorneloe K, Ishii K, Chen T, Cole W. Heteromultimeric Kv1 channels contribute to myogenic control of arterial diameter. Circ Res. 2005;96:216–224. doi: 10.1161/01.RES.0000154070.06421.25. [DOI] [PubMed] [Google Scholar]
- 1164.Plane F, Sampson LJ, Smith JJ, Garland CJ. Relaxation to authentic nitric oxide and SIN-1 in rat isolated mesenteric arteries: Variable role for smooth muscle hyperpolarization. Br J Pharmacol. 2001;133:665–672. doi: 10.1038/sj.bjp.0704127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1165.Plane F, Wiley KE, Jeremy JY, Cohen RA, Garland CJ. Evidence that different mechanisms underlie smooth muscle relaxation to nitric oxide and nitric oxide donors in the rabbit isolated carotid artery. Br J Pharmacol. 1998;123:1351–1358. doi: 10.1038/sj.bjp.0701746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1166.Plant TD, Schaefer M. TRPC4 and TRPC5: Receptor-operated Ca2+-permeable nonselective cation channels. Cell Calcium. 2003;33:441–450. doi: 10.1016/s0143-4160(03)00055-1. [DOI] [PubMed] [Google Scholar]
- 1167.Plant TD, Schaefer M. Receptor-operated cation channels formed by TRPC4 and TRPC5. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:266–276. doi: 10.1007/s00210-005-1055-5. [DOI] [PubMed] [Google Scholar]
- 1168.Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res. 2000;87:E53–E60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 1169.Porter VA, Bonev AD, Knot HJ, Heppner TJ, Stevenson AS, Kleppisch T, Lederer WJ, Nelson MT. Frequency modulation of Ca2+ sparks is involved in regulation of arterial diameter by cyclic nucleotides. Am J Physiol. 1998;274:C1346–C1355. doi: 10.1152/ajpcell.1998.274.5.C1346. [DOI] [PubMed] [Google Scholar]
- 1170.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: Role in proliferation and migration. FASEB J. 2009;23:2425–2437. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1171.Potocnik SJ, Murphy TV, Kotecha N, Hill MA. Effects of mibefradil and nifedipine on arteriolar myogenic responsiveness and intracellular Ca(2+) Br J Pharmacol. 2000;131:1065–1072. doi: 10.1038/sj.bjp.0703650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1172.Poulsen CB, Al-Mashhadi RH, Cribbs LL, Skott O, Hansen PB. T-type voltage-gated calcium channels regulate the tone of mouse efferent arterioles. Kidney Int. 2011;79:443–451. doi: 10.1038/ki.2010.429. [DOI] [PubMed] [Google Scholar]
- 1173.Povstyan OV, Harhun MI, Gordienko DV. Ca2+ entry following P2X receptor activation induces IP3 receptor-mediated Ca2+ release in myocytes from small renal arteries. Br J Pharmacol. 2011;162:1618–1638. doi: 10.1111/j.1476-5381.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1174.Price JM, Hellermann A. Inhibition of cGMP mediated relaxation in small rat coronary arteries by block of CA2+ activated K+ channels. Life Sci. 1997;61:1185–1192. doi: 10.1016/s0024-3205(97)00660-7. [DOI] [PubMed] [Google Scholar]
- 1175.Prior HM, Webster N, Quinn K, Beech DJ, Yates MS. K(+)-induced dilation of a small renal artery: no role for inward rectifier K+ channels. Cardiovasc Res. 1998;37:780–790. doi: 10.1016/s0008-6363(97)00237-x. [DOI] [PubMed] [Google Scholar]
- 1176.Prior HM, Yates MS, Beech DJ. Functions of large conductance Ca2+-activated (BKCa), delayed rectifier (KV) and background K+ channels in the control of membrane potential in rabbit renal arcuate artery. J Physiol. 1998;511(Pt 1):159–169. doi: 10.1111/j.1469-7793.1998.159bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1177.Prosser BL, Wright NT, Hernandez-Ochoa EO, Varney KM, Liu Y, Olojo RO, Zimmer DB, Weber DJ, Schneider MF. S100A1 binds to the calmodulin-binding site of ryanodine receptor and modulates skeletal muscle excitation-contraction coupling. J Biol Chem. 2008;283:5046–5057. doi: 10.1074/jbc.M709231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1178.Pucovsky V, Bolton TB. Localisation, function and composition of primary Ca(2+) spark discharge region in isolated smooth muscle cells from guinea-pig mesenteric arteries. Cell Calcium. 2006;39:113–129. doi: 10.1016/j.ceca.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 1179.Pucovsky V, Gordienko DV, Bolton TB. Effect of nitric oxide donors and noradrenaline on Ca2+ release sites and global intracellular Ca2+ in myocytes from guinea-pig small mesenteric arteries. J Physiol. 2002;539:25–39. doi: 10.1113/jphysiol.2001.012978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1180.Qian Q, Hunter LW, Du H, Ren Q, Han Y, Sieck GC. Pkd2+/− vascular smooth muscles develop exaggerated vasocontraction in response to phenylephrine stimulation. J Am Soc Nephrol. 2007;18:485–493. doi: 10.1681/ASN.2006050501. [DOI] [PubMed] [Google Scholar]
- 1181.Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1182.Quast U. Potassium channel openers: Pharmacological and clinical aspects. Fundam Clin Pharmacol. 1992;6:279–293. doi: 10.1111/j.1472-8206.1992.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 1183.Quast U, Guillon JM, Cavero I. Cellular pharmacology of potassium channel openers in vascular smooth muscle. Cardiovasc Res. 1994;28:805–810. doi: 10.1093/cvr/28.6.805. [DOI] [PubMed] [Google Scholar]
- 1184.Quayle JM, Bonev AD, Brayden JE, Nelson MT. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994;475:9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1185.Quayle JM, Dart C, Standen NB. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth muscle. J Physiol. 1996;494(Pt 3):715–726. doi: 10.1113/jphysiol.1996.sp021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1186.Quayle JM, McCarron JG, Brayden JE, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am J Physiol. 1993;265:C1363–C1370. doi: 10.1152/ajpcell.1993.265.5.C1363. [DOI] [PubMed] [Google Scholar]
- 1187.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 1188.Quayle JM, Standen NB. KATP channels in vascular smooth muscle. Cardiovasc Res. 1994;28:797–804. doi: 10.1093/cvr/28.6.797. [DOI] [PubMed] [Google Scholar]
- 1189.Quignard JF, Mironneau J, Carricaburu V, Fournier B, Babich A, Nurnberg B, Mironneau C, Macrez N. Phosphoinositide 3-kinase gamma mediates angiotensin II-induced stimulation of L-type calcium channels in vascular myocytes. J Biol Chem. 2001;276:32545–32551. doi: 10.1074/jbc.M102582200. [DOI] [PubMed] [Google Scholar]
- 1190.Quinn KV, Cui Y, Giblin JP, Clapp LH, Tinker A. Do anionic phospholipids serve as cofactors or second messengers for the regulation of activity of cloned ATP-sensitive K +channels? Circ Res. 2003;93:646–655. doi: 10.1161/01.RES.0000095247.81449.8E. [DOI] [PubMed] [Google Scholar]
- 1191.Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res. 2004;94:1359–1366. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- 1192.Rainbow RD, Hardy ME, Standen NB, Davies NW. Glucose reduces endothelin inhibition of voltage-gated potassium channels in rat arterial smooth muscle cells. J Physiol. 2006;575:833–844. doi: 10.1113/jphysiol.2006.114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1193.Randall MD. The involvement of ATP-sensitive potassium channels and adenosine in the regulation of coronary flow in the isolated perfused rat heart. Br J Pharmacol. 1995;116:3068–3074. doi: 10.1111/j.1476-5381.1995.tb15965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1194.Reading SA, Earley S, Waldron BJ, Welsh DG, Brayden JE. TRPC3 mediates pyrimidine receptor-induced depolarization of cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;288:H2055–H2061. doi: 10.1152/ajpheart.00861.2004. [DOI] [PubMed] [Google Scholar]
- 1195.Regan JN, Waning DL, Guise TA. Skeletal muscle Ca(2+) mishandling: Another effect of bone-to-muscle signaling. Semin Cell Dev Biol. 2016;49:24–29. doi: 10.1016/j.semcdb.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1196.Reggiani C, te Kronnie T. RyR isoforms and fibre type-specific expression of proteins controlling intracellular calcium concentration in skeletal muscles. J Muscle Res Cell Motil. 2006;27:327–335. doi: 10.1007/s10974-006-9076-3. [DOI] [PubMed] [Google Scholar]
- 1197.Ren Y, Xu X, Wang X. Altered mRNA expression of ATP-sensitive and inward rectifier potassium channel subunits in streptozotocin-induced diabetic rat heart and aorta. J Pharmacol Sci. 2003;93:478–483. doi: 10.1254/jphs.93.478. [DOI] [PubMed] [Google Scholar]
- 1198.Ren YJ, Xu XH, Zhong CB, Feng N, Wang XL. Hypercholesterolemia alters vascular functions and gene expression of potassium channels in rat aortic smooth muscle cells. Acta Pharmacol Sin. 2001;22:274–278. [PubMed] [Google Scholar]
- 1199.Renigunta V, Schlichthorl G, Daut J. Much more than a leak: Structure and function of K(2)p-channels. Pflugers Arch. 2015;467:867–894. doi: 10.1007/s00424-015-1703-7. [DOI] [PubMed] [Google Scholar]
- 1200.Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calcium-concentration. J Physiol. 1967;192:479–492. doi: 10.1113/jphysiol.1967.sp008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1201.Ribalet B, Eddlestone GT. Characterization of the G protein coupling of SRIF and beta-adrenergic receptors to the maxi KCa channel in insulin-secreting cells. J Membr Biol. 1995;148:111–125. doi: 10.1007/BF00207268. [DOI] [PubMed] [Google Scholar]
- 1202.Richmond KN, Tune JD, Gorman MW, Feigl EO. Role of K+ ATP channels in local metabolic coronary vasodilation. Am J Physiol. 1999;277:H2115–H2123. doi: 10.1152/ajpheart.1999.277.6.H2115. [DOI] [PubMed] [Google Scholar]
- 1203.Richmond KN, Tune JD, Gorman MW, Feigl EO. Role of K(ATP)(+) channels and adenosine in the control of coronary blood flow during exercise. J Appl Physiol (1985) 2000;89:529–536. doi: 10.1152/jappl.2000.89.2.529. [DOI] [PubMed] [Google Scholar]
- 1204.Rivers RJ, Hein TW, Zhang C, Kuo L. Activation of barium-sensitive inward rectifier potassium channels mediates remote dilation of coronary arterioles. Circulation. 2001;104:1749–1753. doi: 10.1161/hc4001.098053. [DOI] [PubMed] [Google Scholar]
- 1205.Robbins J. KCNQ potassium channels: Physiology, pathophysiology, and pharmacology. Pharmacol Ther. 2001;90:1–19. doi: 10.1016/s0163-7258(01)00116-4. [DOI] [PubMed] [Google Scholar]
- 1206.Roberts OL, Kamishima T, Barrett-Jolley R, Quayle JM, Dart C. Exchange protein activated by cAMP (Epac) induces vascular relaxation by activating Ca2+-sensitive K+ channels in rat mesenteric artery. J Physiol. 2013;591:5107–5123. doi: 10.1113/jphysiol.2013.262006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1207.Robertson BE, Bonev AD, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat coronary arteries: Block by Mg2+, Ca2+, and Ba2+ Am J Physiol. 1996;271:H696–H705. doi: 10.1152/ajpheart.1996.271.2.H696. [DOI] [PubMed] [Google Scholar]
- 1208.Robertson B, Owen D, Stow J, Butler C, Newland C. Novel effects of dendrotoxin homologues on subtypes of mammalian Kv1 potassium channels expressed in Xenopus oocytes. FEBS Lett. 1996;383:26–30. doi: 10.1016/0014-5793(96)00211-6. [DOI] [PubMed] [Google Scholar]
- 1209.Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993;265:C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- 1210.Rogers PA, Chilian WM, Bratz IN, Bryan RM, Jr, Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1404–H1411. doi: 10.1152/ajpheart.00696.2006. [DOI] [PubMed] [Google Scholar]
- 1211.Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr, Saitoh S, Tune JD, Chilian WM. H2O2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2006;291:H2473–H2482. doi: 10.1152/ajpheart.00172.2006. [DOI] [PubMed] [Google Scholar]
- 1212.Rogers EF, Koniuszy FR, Shavel J, Folkers K. Plant insecticides; ryanodine, a new alkaloid from Ryania speciosa Vahl. J Am Chem Soc. 1948;70:3086–3088. doi: 10.1021/ja01189a074. [DOI] [PubMed] [Google Scholar]
- 1213.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 1214.Romanenko VG, Fang Y, Byfield F, Travis AJ, Vandenberg CA, Rothblat GH, Levitan I. Cholesterol sensitivity and lipid raft targeting of Kir2.1 channels. Biophys J. 2004;87:3850–3861. doi: 10.1529/biophysj.104.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1215.Romanenko VG, Rothblat GH, Levitan I. Modulation of endothelial inward-rectifier K+ current by optical isomers of cholesterol. Biophys J. 2002;83:3211–3222. doi: 10.1016/S0006-3495(02)75323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1216.Rosenhouse-Dantsker A, Logothetis DE, Levitan I. Cholesterol sensitivity of KIR2.1 is controlled by a belt of residues around the cytosolic pore. Biophys J. 2011;100:381–389. doi: 10.1016/j.bpj.2010.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1217.Rosenhouse-Dantsker A, Noskov S, Durdagi S, Logothetis DE, Levitan I. Identification of novel cholesterol-binding regions in Kir2 channels. J Biol Chem. 2013;288:31154–31164. doi: 10.1074/jbc.M113.496117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1218.Rosenhouse-Dantsker A, Noskov S, Logothetis DE, Levitan I. Cholesterol sensitivity of KIR2.1 depends on functional inter-links between the N and C termini. Channels (Austin) 2013;7:303–312. doi: 10.4161/chan.25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1219.Ross J, Armstead WM. Differential role of PTK and ERK MAPK in superoxide impairment of K(ATP) and K(Ca) channel cerebrovasodilation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R149–R154. doi: 10.1152/ajpregu.00003.2003. [DOI] [PubMed] [Google Scholar]
- 1220.Rossi AM, Taylor CW. Ca2+ regulation of inositol 1,4,5-trisphosphate receptors: Can Ca2+ function without calmodulin? Mol Pharmacol. 2004;66:199–203. doi: 10.1124/mol.104.002592. [DOI] [PubMed] [Google Scholar]
- 1221.Rozsa Z, Pataricza J, Nemeth J, Papp JG. Differential efficacy of vasodilators in hypercholesterolaemic rabbits. J Pharm Pharmacol. 1998;50:1035–1044. doi: 10.1111/j.2042-7158.1998.tb06919.x. [DOI] [PubMed] [Google Scholar]
- 1222.Rueda A, Fernandez-Velasco M, Benitah JP, Gomez AM. Abnormal Ca2+ spark/STOC coupling in cerebral artery smooth muscle cells of obese type 2 diabetic mice. PLoS One. 2013;8:e53321. doi: 10.1371/journal.pone.0053321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1223.Rueda A, Song M, Toro L, Stefani E, Valdivia HH. Sorcin modulation of Ca2+ sparks in rat vascular smooth muscle cells. J Physiol. 2006;576:887–901. doi: 10.1113/jphysiol.2006.113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1224.Ruehlmann DO, Lee CH, Poburko D, van Breemen C. Asynchronous Ca(2+) waves in intact venous smooth muscle. Circ Res. 2000;86:E72–E79. doi: 10.1161/01.res.86.4.e72. [DOI] [PubMed] [Google Scholar]
- 1225.Ruehr ML, Russell MA, Ferguson DG, Bhat M, Ma J, Damron DS, Scott JD, Bond M. Targeting of protein kinase A by muscle A kinase-anchoring protein (mAKAP) regulates phosphorylation and function of the skeletal muscle ryanodine receptor. J Biol Chem. 2003;278:24831–24836. doi: 10.1074/jbc.M213279200. [DOI] [PubMed] [Google Scholar]
- 1226.Rusch NJ. BK channels in cardiovascular disease: A complex story of channel dysregulation. Am J Physiol Heart Circ Physiol. 2009;297:H1580–H1582. doi: 10.1152/ajpheart.00852.2009. [DOI] [PubMed] [Google Scholar]
- 1227.Rusch NJ, De Lucena RG, Wooldridge TA, England SK, Cowley AW., Jr A Ca(2+)-dependent K+ current is enhanced in arterial membranes of hypertensive rats. Hypertension. 1992;19:301–307. doi: 10.1161/01.hyp.19.4.301. [DOI] [PubMed] [Google Scholar]
- 1228.Rusch NJ, Liu Y. Potassium channels in hypertension: Homeostatic pathways to buffer arterial contraction. J Lab Clin Med. 1997;130:245–251. doi: 10.1016/s0022-2143(97)90018-4. [DOI] [PubMed] [Google Scholar]
- 1229.Ryckmans T, Aubdool AA, Bodkin JV, Cox P, Brain SD, Dupont T, Fairman E, Hashizume Y, Ishii N, Kato T, Kitching L, Newman J, Omoto K, Rawson D, Strover J. Design and pharmacological evaluation of PF-4840154, a non-electrophilic reference agonist of the TrpA1 channel. Bioorg Med Chem Lett. 2011;21:4857–4859. doi: 10.1016/j.bmcl.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 1230.Sadoshima J, Akaike N, Kanaide H, Nakamura M. Cyclic AMP modulates Ca-activated K channel in cultured smooth muscle cells of rat aortas. Am J Physiol. 1988;255:H754–H759. doi: 10.1152/ajpheart.1988.255.4.H754. [DOI] [PubMed] [Google Scholar]
- 1231.Saito Y, McKay M, Eraslan A, Hester RL. Functional hyperemia in striated muscle is reduced following blockade of ATP-sensitive potassium channels. Am J Physiol. 1996;270:H1649–1654. doi: 10.1152/ajpheart.1996.270.5.H1649. [DOI] [PubMed] [Google Scholar]
- 1232.Saito A, Seiler S, Chu A, Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984;99:875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1233.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: A feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–2621. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 1234.Sakai N, Mizuno R, Ono N, Kato H, Ohhashi T. High oxygen tension constricts epineurial arterioles of the rat sciatic nerve via reactive oxygen species. Am J Physiol Heart Circ Physiol. 2007;293:H1498–H1507. doi: 10.1152/ajpheart.01190.2006. [DOI] [PubMed] [Google Scholar]
- 1235.Sakmann B, Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1236.Salata JJ, Jurkiewicz NK, Wang J, Evans BE, Orme HT, Sanguinetti MC. A novel benzodiazepine that activates cardiac slow delayed rectifier K+ currents. Mol Pharmacol. 1998;54:220–230. doi: 10.1124/mol.54.1.220. [DOI] [PubMed] [Google Scholar]
- 1237.Saleem H, Tovey SC, Molinski TF, Taylor CW. Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br J Pharmacol. 2014;171:3298–3312. doi: 10.1111/bph.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1238.Saleh SN, Albert AP, Large WA. Activation of native TRPC1/C5/C6 channels by endothelin-1 is mediated by both PIP3 and PIP2 in rabbit coronary artery myocytes. J Physiol. 2009;587:5361–5375. doi: 10.1113/jphysiol.2009.180331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1239.Saleh SN, Albert AP, Peppiatt CM, Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol. 2006;577:479–495. doi: 10.1113/jphysiol.2006.119305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1240.Salido GM, Sage SO, Rosado JA. TRPC channels and store-operated Ca(2+) entry. Biochim Biophys Acta. 2009;1793:223–230. doi: 10.1016/j.bbamcr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 1241.Salomone S, Soydan G, Moskowitz MA, Sims JR. Inhibition of cerebral vasoconstriction by dantrolene and nimodipine. Neurocrit Care. 2009;10:93–102. doi: 10.1007/s12028-008-9153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1242.Salomonsson M, Arendshorst WJ. Calcium recruitment in renal vasculature: NE effects on blood flow and cytosolic calcium concentration. Am J Physiol. 1999;276:F700–F710. doi: 10.1152/ajprenal.1999.276.5.F700. [DOI] [PubMed] [Google Scholar]
- 1243.Salomonsson M, Braunstein TH, Holstein-Rathlou NH, Jensen LJ. Na+-independent, nifedipine-resistant rat afferent arteriolar Ca2+ responses to noradrenaline: Possible role of TRPC channels. Acta Physiol (Oxf) 2010;200:265–278. doi: 10.1111/j.1748-1716.2010.02141.x. [DOI] [PubMed] [Google Scholar]
- 1244.Salter KJ, Kozlowski RZ. Differential electrophysiological actions of endothelin-1 on Cl− and K+ currents in myocytes isolated from aorta, basilar and pulmonary artery. J Pharmacol Exp Ther. 1998;284:1122–1131. [PubMed] [Google Scholar]
- 1245.Salzman AL, Vromen A, Denenberg A, Szabo C. K(ATP)-channel inhibition improves hemodynamics and cellular energetics in hemorrhagic shock. Am J Physiol. 1997;272:H688–H694. doi: 10.1152/ajpheart.1997.272.2.H688. [DOI] [PubMed] [Google Scholar]
- 1246.Samaha FF, Heineman FW, Ince C, Fleming J, Balaban RS. ATP-sensitive potassium channel is essential to maintain basal coronary vascular tone in vivo. Am J Physiol. 1992;262:C1220–C1227. doi: 10.1152/ajpcell.1992.262.5.C1220. [DOI] [PubMed] [Google Scholar]
- 1247.Sammels E, Devogelaere B, Mekahli D, Bultynck G, Missiaen L, Parys JB, Cai Y, Somlo S, De Smedt H. Polycystin-2 activation by inositol 1,4,5-trisphosphate-induced Ca2+ release requires its direct association with the inositol 1,4,5-trisphosphate receptor in a signaling microdomain. J Biol Chem. 2010;285:18794–18805. doi: 10.1074/jbc.M109.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1248.Samora JB, Frisbee JC, Boegehold MA. Growth-dependent changes in endothelial factors regulating arteriolar tone. Am J Physiol Heart Circ Physiol. 2007;292:H207–H214. doi: 10.1152/ajpheart.00677.2006. [DOI] [PubMed] [Google Scholar]
- 1249.Sampson LJ, Davies LM, Barrett-Jolley R, Standen NB, Dart C. Angiotensin II-activated protein kinase C targets caveolae to inhibit aortic ATP-sensitive potassium channels. Cardiovasc Res. 2007;76:61–70. doi: 10.1016/j.cardiores.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 1250.Sampson LJ, Hayabuchi Y, Standen NB, Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ Res. 2004;95:1012–1018. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- 1251.Sandow SL, Senadheera S, Bertrand PP, Murphy TV, Tare M. Myoendothelial contacts, gap junctions, and microdomains: Anatomical links to function? Microcirculation. 2012;19:403–415. doi: 10.1111/j.1549-8719.2011.00146.x. [DOI] [PubMed] [Google Scholar]
- 1252.Sanguinetti MC, Kass RS. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984;55:336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- 1253.Sarazan RD. Cardiovascular pressure measurement in safety assessment studies: Technology requirements and potential errors. J Pharmacol Toxicol Methods. 2014;70:210–223. doi: 10.1016/j.vascn.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 1254.Satake N, Shibata M, Shibata S. The inhibitory effects of iberiotoxin and 4-aminopyridine on the relaxation induced by beta 1- and beta 2-adrenoceptor activation in rat aortic rings. Br J Pharmacol. 1996;119:505–510. doi: 10.1111/j.1476-5381.1996.tb15700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1255.Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, Kleppisch T, Ruth P, Hofmann F. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res. 2000;87:825–830. doi: 10.1161/01.res.87.9.825. [DOI] [PubMed] [Google Scholar]
- 1256.Savoia CP, Liu QH, Zheng YM, Yadav V, Zhang Z, Wu LG, Wang YX. Calcineurin upregulates local Ca(2+) signaling through ryanodine receptor-1 in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2014;307:L781–L790. doi: 10.1152/ajplung.00149.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1257.Sawmiller DR, Ashtari M, Urueta H, Leschinsky M, Henning RJ. Mechanisms of vasoactive intestinal peptide-elicited coronary vasodilation in the isolated perfused rat heart. Neuropeptides. 2006;40:349–355. doi: 10.1016/j.npep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 1258.Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- 1259.Scherer D, Kiesecker C, Kulzer M, Gunth M, Scholz EP, Kathofer S, Thomas D, Maurer M, Kreuzer J, Bauer A, Katus HA, Karle CA, Zitron E. Activation of inwardly rectifying Kir2.x potassium channels by beta 3-adrenoceptors is mediated via different signaling pathways with a predominant role of PKC for Kir2.1 and of PKA for Kir2.2. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:311–322. doi: 10.1007/s00210-007-0167-5. [DOI] [PubMed] [Google Scholar]
- 1260.Schiefer A, Meissner G, Isenberg G. Ca2+ activation and Ca2+ inactivation of canine reconstituted cardiac sarcoplasmic reticulum Ca(2+)-release channels. J Physiol. 1995;489(Pt 2):337–348. doi: 10.1113/jphysiol.1995.sp021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1261.Schleifenbaum J, Kassmann M, Szijarto IA, Hercule HC, Tano JY, Weinert S, Heidenreich M, Pathan AR, Anistan YM, Alenina N, Rusch NJ, Bader M, Jentsch TJ, Gollasch M. Stretch-activation of angiotensin II type 1a receptors contributes to the myogenic response of mouse mesenteric and renal arteries. Circ Res. 2014;115:263–272. doi: 10.1161/CIRCRESAHA.115.302882. [DOI] [PubMed] [Google Scholar]
- 1262.Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, Crean CS, Luft FC, Huang Y, Schubert R, Gollasch M. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens. 2010;28:1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]
- 1263.Schleifer H, Doleschal B, Lichtenegger M, Oppenrieder R, Derler I, Frischauf I, Glasnov TN, Kappe CO, Romanin C, Groschner K. Novel pyrazole compounds for pharmacological discrimination between receptor-operated and store-operated Ca(2+) entry pathways. Br J Pharmacol. 2012;167:1712–1722. doi: 10.1111/j.1476-5381.2012.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1264.Schlossmann J, Desch M. IRAG and novel PKG targeting in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2011;301:H672–H682. doi: 10.1152/ajpheart.00198.2011. [DOI] [PubMed] [Google Scholar]
- 1265.Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64:498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1266.Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG, Perraud AL. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem. 2005;280:37763–37771. doi: 10.1074/jbc.M509175200. [DOI] [PubMed] [Google Scholar]
- 1267.Schmitz A, Sankaranarayanan A, Azam P, Schmidt-Lassen K, Homerick D, Hansel W, Wulff H. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol Pharmacol. 2005;68:1254–1270. doi: 10.1124/mol.105.015669. [DOI] [PubMed] [Google Scholar]
- 1268.Schrage WG, Dietz NM, Joyner MJ. Effects of combined inhibition of ATP-sensitive potassium channels, nitric oxide, and prostaglandins on hyperemia during moderate exercise. J Appl Physiol (1985) 2006;100:1506–1512. doi: 10.1152/japplphysiol.01639.2005. [DOI] [PubMed] [Google Scholar]
- 1269.Schubert R, Krien U, Wulfsen I, Schiemann D, Lehmann G, Ulfig N, Veh RW, Schwarz JR, Gago H. Nitric oxide donor sodium nitroprusside dilates rat small arteries by activation of inward rectifier potassium channels. Hypertension. 2004;43:891–896. doi: 10.1161/01.HYP.0000121882.42731.6b. [DOI] [PubMed] [Google Scholar]
- 1270.Schubert R, Serebryakov VN, Mewes H, Hopp HH. Iloprost dilates rat small arteries: Role of K(ATP)- and K(Ca)-channel activation by cAMP-dependent protein kinase. Am J Physiol. 1997;272:H1147–H1156. doi: 10.1152/ajpheart.1997.272.3.H1147. [DOI] [PubMed] [Google Scholar]
- 1271.Scornik FS, Codina J, Birnbaumer L, Toro L. Modulation of coronary smooth muscle KCa channels by Gs alpha independent of phosphorylation by protein kinase A. Am J Physiol. 1993;265:H1460–H1465. doi: 10.1152/ajpheart.1993.265.4.H1460. [DOI] [PubMed] [Google Scholar]
- 1272.Scornik FS, Toro L. U46619, a thromboxane A2 agonist, inhibits KCa channel activity from pig coronary artery. Am J Physiol. 1992;262:C708–C713. doi: 10.1152/ajpcell.1992.262.3.C708. [DOI] [PubMed] [Google Scholar]
- 1273.Scott JB, Frohlich ED, Hardin RA, Haddy FJ. Na, K, Ca, and Mg ion action on coronary vascular resistance in the dog heart. Am J Physiol. 1961;201:1095–1100. doi: 10.1152/ajplegacy.1961.201.6.1095. [DOI] [PubMed] [Google Scholar]
- 1274.Searls YM, Loganathan R, Smirnova IV, Stehno-Bittel L. Intracellular Ca2+ regulating proteins in vascular smooth muscle cells are altered with type 1 diabetes due to the direct effects of hyperglycemia. Cardiovasc Diabetol. 2010;9:8. doi: 10.1186/1475-2840-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1275.Seebohm G, Chen J, Strutz N, Culberson C, Lerche C, Sanguinetti MC. Molecular determinants of KCNQ1 channel block by a benzodiazepine. Mol Pharmacol. 2003;64:70–77. doi: 10.1124/mol.64.1.70. [DOI] [PubMed] [Google Scholar]
- 1276.Segal SS, Duling BR. Communication between feed arteries and microvessels in hamster striated-muscle—segmental vascular-responses are functionally coordinated. Circulation Research. 1986;59:283–290. doi: 10.1161/01.res.59.3.283. [DOI] [PubMed] [Google Scholar]
- 1277.Seino S, Miki T. Gene targeting approach to clarification of ion channel function: Studies of Kir6.x null mice. J Physiol. 2004;554:295–300. doi: 10.1113/jphysiol.2003.047175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1278.Semmo M, Kottgen M, Hofherr A. The TRPP subfamily and polycystin-1 proteins. Handb Exp Pharmacol. 2014;222:675–711. doi: 10.1007/978-3-642-54215-2_27. [DOI] [PubMed] [Google Scholar]
- 1279.Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J Biol Chem. 2007;282:33868–33878. doi: 10.1074/jbc.M702577200. [DOI] [PubMed] [Google Scholar]
- 1280.Seo MD, Enomoto M, Ishiyama N, Stathopulos PB, Ikura M. Structural insights into endoplasmic reticulum stored calcium regulation by inositol 1,4,5-trisphosphate and ryanodine receptors. Biochim Biophys Acta. 2015;1853:1980–1991. doi: 10.1016/j.bbamcr.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 1281.Seo EY, Kim HJ, Zhao ZH, Jang JH, Jin CZ, Yoo HY, Zhang YH, Kim SJ. Low K(+) current in arterial myocytes with impaired K(+)-vasodilation and its recovery by exercise in hypertensive rats. Pflugers Arch. 2014;466:2101–2111. doi: 10.1007/s00424-014-1473-7. [DOI] [PubMed] [Google Scholar]
- 1282.Seo MD, Velamakanni S, Ishiyama N, Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M, Taylor CW. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature. 2012;483:108–112. doi: 10.1038/nature10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1283.Sepulveda FV, Pablo Cid L, Teulon J, Niemeyer MI. Molecular aspects of structure, gating, and physiology of pH-sensitive background K2P and Kir K+-transport channels. Physiol Rev. 2015;95:179–217. doi: 10.1152/physrev.00016.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1284.Serysheva II. Toward a high-resolution structure of IP(3)R channel. Cell Calcium. 2014;56:125–132. doi: 10.1016/j.ceca.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1285.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, Retailleau K, Loufrani L, Patel A, Sachs F, Delmas P, Peters DJ, Honore E. Polycystin-1 and-2 dosage regulates pressure sensing. Cell. 2009;139:587–596. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 1286.Sharifi-Sanjani M, Zhou X, Asano S, Tilley S, Ledent C, Teng B, Dick GM, Mustafa SJ. Interactions between A(2A) adenosine receptors, hydrogen peroxide, and KATP channels in coronary reactive hyperemia. Am J Physiol Heart Circ Physiol. 2013;304:H1294–H1301. doi: 10.1152/ajpheart.00637.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1287.Sharma K, Wang L, Zhu Y, DeGuzman A, Cao GY, Lynn RB, Joseph SK. Renal type I inositol 1,4,5-trisphosphate receptor is reduced in streptozotocin-induced diabetic rats and mice. Am J Physiol. 1999;276:F54–F61. doi: 10.1152/ajprenal.1999.276.1.F54. [DOI] [PubMed] [Google Scholar]
- 1288.Shaw L, O’Neill S, Jones CJ, Austin C, Taggart MJ. Comparison of U46619-, endothelin-1- or phenylephrine-induced changes in cellular Ca2+ profiles and Ca2+ sensitisation of constriction of pressurised rat resistance arteries. Br J Pharmacol. 2004;141:678–688. doi: 10.1038/sj.bjp.0705647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1289.Sherkheli MA, Gisselman G, Vogt-Eisele AK, Doerner JF, Hatt HHH. Menthol derivative WS-12 selectively activates transient receptor potential melastatin-8 (TRPM8) ion channels. Pak J Pharm Sci. 2008;21:370–378. [PubMed] [Google Scholar]
- 1290.Shi Y, Chen X, Wu Z, Shi W, Yang Y, Cui N, Jiang C, Harrison RW. cAMP-dependent protein kinase phosphorylation produces interdomain movement in SUR2B leading to activation of the vascular KATP channel. J Biol Chem. 2008;283:7523–7530. doi: 10.1074/jbc.M709941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1291.Shi W, Cui N, Shi Y, Zhang X, Yang Y, Jiang C. Arginine vasopressin inhibits Kir6.1/SUR2B channel and constricts the mesenteric artery via V1a receptor and protein kinase C. Am J Physiol Regul Integr Comp Physiol. 2007;293:R191–R199. doi: 10.1152/ajpregu.00047.2007. [DOI] [PubMed] [Google Scholar]
- 1292.Shi W, Cui N, Wu Z, Yang Y, Zhang S, Gai H, Zhu D, Jiang C. Lipopolysaccharides up-regulate Kir6.1/SUR2B channel expression and enhance vascular KATP channel activity via NF-kappaB-dependent signaling. J Biol Chem. 2010;285:3021–3029. doi: 10.1074/jbc.M109.058313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1293.Shi J, Ju M, Abramowitz J, Large WA, Birnbaumer L, Albert AP. TRPC1 proteins confer PKC and phosphoinositol activation on native heteromeric TRPC1/C5 channels in vascular smooth muscle: Comparative study of wild-type and TRPC1−/− mice. FASEB J. 2012;26:409–419. doi: 10.1096/fj.11-185611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1294.Shi J, Ju M, Saleh SN, Albert AP, Large WA. TRPC6 channels stimulated by angiotensin II are inhibited by TRPC1/C5 channel activity through a Ca2+- and PKC-dependent mechanism in native vascular myocytes. J Physiol. 2010;588:3671–3682. doi: 10.1113/jphysiol.2010.194621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1295.Shi L, Liu B, Li N, Xue Z, Liu X. Aerobic exercise increases BK(Ca) channel contribution to regulation of mesenteric arterial tone by upregulating beta1-subunit. Exp Physiol. 2013;98:326–336. doi: 10.1113/expphysiol.2012.066225. [DOI] [PubMed] [Google Scholar]
- 1296.Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Rojas A, Ha BT, Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1205–R1214. doi: 10.1152/ajpregu.00337.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1297.Shimizu T, Higuchi T, Fujii T, Nilius B, Sakai H. Bimodal effect of alkalization on the polycystin transient receptor potential channel, PKD2L1. Pflugers Arch. 2011;461:507–513. doi: 10.1007/s00424-011-0934-5. [DOI] [PubMed] [Google Scholar]
- 1298.Shimizu T, Janssens A, Voets T, Nilius B. Regulation of the murine TRPP3 channel by voltage, pH, and changes in cell volume. Pflugers Arch. 2009;457:795–807. doi: 10.1007/s00424-008-0558-6. [DOI] [PubMed] [Google Scholar]
- 1299.Shimizu S, Paul RJ. The endothelium-dependent, substance P relaxation of porcine coronary arteries resistant to nitric oxide synthesis inhibition is partially mediated by 4-aminopyridine-sensitive voltage-dependent K+ channels. Endothelium. 1997;5:287–295. doi: 10.3109/10623329709052593. [DOI] [PubMed] [Google Scholar]
- 1300.Shimizu S, Yokoshiki H, Sperelakis N, Paul RJ. Role of voltage-dependent and Ca(2+)-activated K(+) channels on the regulation of isometric force in porcine coronary artery. J Vasc Res. 2000;37:16–25. doi: 10.1159/000025709. [DOI] [PubMed] [Google Scholar]
- 1301.Shinde AV, Motiani RK, Zhang X, Abdullaev IF, Adam AP, Gonzalez-Cobos JC, Zhang W, Matrougui K, Vincent PA, Trebak M. STIM1 controls endothelial barrier function independently of Orai1 and Ca2+ entry. Sci Signal. 2013;6:ra18. doi: 10.1126/scisignal.2003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1302.Shinoda M, Toki Y, Murase K, Mokuno S, Okumura K, Ito T. Types of potassium channels involved in coronary reactive hyperemia depend on duration of preceding ischemia in rat hearts. Life Sci. 1997;61:997–1007. doi: 10.1016/s0024-3205(97)00604-8. [DOI] [PubMed] [Google Scholar]
- 1303.Siegl D, Koeppen M, Wolfle SE, Pohl U, de Wit C. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res. 2005;97:781–788. doi: 10.1161/01.RES.0000186193.22438.6c. [DOI] [PubMed] [Google Scholar]
- 1304.Silberberg SD, Poder TC, Lacerda AE. Endothelin increases single-channel calcium currents in coronary arterial smooth muscle cells. FEBS Lett. 1989;247:68–72. doi: 10.1016/0014-5793(89)81242-6. [DOI] [PubMed] [Google Scholar]
- 1305.Silva DF, de Almeida MM, Chaves CG, Braz AL, Gomes MA, Pinho-da-Silva L, Pesquero JL, Andrade VA, Leite Mde F, de Albuquerque JG, Araujo IG, Nunes XP, Barbosa-Filho JM, Cruz Jdos S, Correia Nde A, de Medeiros IA. TRPM8 channel activation induced by monoterpenoid rotundifolone underlies mesenteric artery relaxation. PLoS One. 2015;10:e0143171. doi: 10.1371/journal.pone.0143171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1306.Simmerman HK, Jones LR. Phospholamban: Protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- 1307.Simonsen U, Prieto D, Sanez de Tejada I, Garcia-Sacristan A. Involvement of nitric oxide in the non-adrenergic non-cholinergic neurotransmission of horse deep penile arteries: Role of charybdotoxin-sensitive K(+)-channels. Br J Pharmacol. 1995;116:2582–2590. doi: 10.1111/j.1476-5381.1995.tb17211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1308.Singh DK, Shentu TP, Enkvetchakul D, Levitan I. Cholesterol regulates prokaryotic Kir channel by direct binding to channel protein. Biochim Biophys Acta. 2011;1808:2527–2533. doi: 10.1016/j.bbamem.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1309.Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1310.Smart D, Jerman JC, Gunthorpe MJ, Brough SJ, Ranson J, Cairns W, Hayes PD, Randall AD, Davis JB. Characterisation using FLIPR of human vanilloid VR1 receptor pharmacology. Eur J Pharmacol. 2001;417:51–58. doi: 10.1016/s0014-2999(01)00901-3. [DOI] [PubMed] [Google Scholar]
- 1311.Smirnov SV, Aaronson PI. Ca(2+)-activated and voltage-gated K+ currents in smooth muscle cells isolated from human mesenteric arteries. J Physiol. 1992;457:431–454. doi: 10.1113/jphysiol.1992.sp019386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1312.Smirnov SV, Loutzenhiser K, Loutzenhiser R. Voltage-activated Ca(2+) channels in rat renal afferent and efferent myocytes: No evidence for the T-type Ca(2+) current. Cardiovasc Res. 2013;97:293–301. doi: 10.1093/cvr/cvs310. [DOI] [PubMed] [Google Scholar]
- 1313.Smith PD, Brett SE, Luykenaar KD, Sandow SL, Marrelli SP, Vigmond EJ, Welsh DG. KIR channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol. 2008;586:1147–1160. doi: 10.1113/jphysiol.2007.145474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1314.Smith JS, Coronado R, Meissner G. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature. 1985;316:446–449. doi: 10.1038/316446a0. [DOI] [PubMed] [Google Scholar]
- 1315.Sobey CG. Potassium channel function in vascular disease. Arterioscler Thromb Vasc Biol. 2001;21:28–38. doi: 10.1161/01.atv.21.1.28. [DOI] [PubMed] [Google Scholar]
- 1316.Sobey CG, Faraci FM. Effect of nitric oxide and potassium channel agonists and inhibitors on basilar artery diameter. Am J Physiol. 1997;272:H256–H262. doi: 10.1152/ajpheart.1997.272.1.H256. [DOI] [PubMed] [Google Scholar]
- 1317.Sobey CG, Faraci FM. Inhibitory effect of 4-aminopyridine on responses of the basilar artery to nitric oxide. Br J Pharmacol. 1999;126:1437–1443. doi: 10.1038/sj.bjp.0702439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1318.Sobey CG, Heistad DD, Faraci FM. Effect of subarachnoid hemorrhage on cerebral vasodilatation in response to activation of ATP-sensitive K+ channels in chronically hypertensive rats. Stroke. 1997;28:392–396. doi: 10.1161/01.str.28.2.392. discussion 396–397. [DOI] [PubMed] [Google Scholar]
- 1319.Sobey CG, Heistad DD, Faraci FM. Mechanisms of bradykinin-induced cerebral vasodilatation in rats. Evidence that reactive oxygen species activate K+ channels. Stroke. 1997;28:2290–2294. doi: 10.1161/01.str.28.11.2290. discussion 2295. [DOI] [PubMed] [Google Scholar]
- 1320.Sobey CG, Heistad DD, Faraci FM. Potassium channels mediate dilatation of cerebral arterioles in response to arachidonate. Am J Physiol. 1998;275:H1606–H1612. doi: 10.1152/ajpheart.1998.275.5.H1606. [DOI] [PubMed] [Google Scholar]
- 1321.Sogaard R, Ljungstrom T, Pedersen KA, Olesen SP, Jensen BS. KCNQ4 channels expressed in mammalian cells: Functional characteristics and pharmacology. Am J Physiol Cell Physiol. 2001;280:C859–C866. doi: 10.1152/ajpcell.2001.280.4.C859. [DOI] [PubMed] [Google Scholar]
- 1322.Sommese L, Valverde CA, Blanco P, Castro MC, Rueda OV, Kaetzel M, Dedman J, Anderson ME, Mattiazzi A, Palomeque J. Ryanodine receptor phosphorylation by CaMKII promotes spontaneous Ca(2+) release events in a rodent model of early stage diabetes: The arrhythmogenic substrate. Int J Cardiol. 2016;202:394–406. doi: 10.1016/j.ijcard.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1323.Son YK, Park WS, Ko JH, Han J, Kim N, Earm YE. Protein kinase A-dependent activation of inward rectifier potassium channels by adenosine in rabbit coronary smooth muscle cells. Biochem Biophys Res Commun. 2005;337:1145–1152. doi: 10.1016/j.bbrc.2005.09.176. [DOI] [PubMed] [Google Scholar]
- 1324.Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. J Neurosci. 1998;18:3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1325.Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, Santana LF, Nelson MT. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal. 2014;7:ra66. doi: 10.1126/scisignal.2005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1326.Sonkusare SK, Dalsgaard T, Bonev AD, Nelson MT. Inward rectifier potassium (Kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators. J Physiol. 2016;594:3271–3285. doi: 10.1113/JP271652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1327.Sordi R, Fernandes D, Heckert BT, Assreuy J. Early potassium channel blockade improves sepsis-induced organ damage and cardiovascular dysfunction. Br J Pharmacol. 2011;163:1289–1301. doi: 10.1111/j.1476-5381.2011.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1328.Sorrentino V, Volpe P. Ryanodine receptors: How many, where and why? Trends Pharmacol Sci. 1993;14:98–103. doi: 10.1016/0165-6147(93)90072-r. [DOI] [PubMed] [Google Scholar]
- 1329.Soulsby MD, Alzayady K, Xu Q, Wojcikiewicz RJ. The contribution of serine residues 1588 and 1755 to phosphorylation of the type I inositol 1,4,5-trisphosphate receptor by PKA and PKG. FEBS Lett. 2004;557:181–184. doi: 10.1016/s0014-5793(03)01487-x. [DOI] [PubMed] [Google Scholar]
- 1330.Spallarossa P, Schiavo M, Rossettin P, Cordone S, Olivotti L, Cordera R, Brunelli C. Sulfonylurea treatment of type 2 diabetic patients does not reduce the vasodilator response to ischemia. Diabetes Care. 2001;24:738–742. doi: 10.2337/diacare.24.4.738. [DOI] [PubMed] [Google Scholar]
- 1331.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1332.Standen NB. The G. L. Brown Lecture. Potassium channels, metabolism and muscle. Exp Physiol. 1992;77:1–25. doi: 10.1113/expphysiol.1992.sp003564. [DOI] [PubMed] [Google Scholar]
- 1333.Stehno-Bittel L, Laughlin MH, Sturek M. Exercise training depletes sarcoplasmic reticulum calcium in coronary smooth muscle. J Appl Physiol (1985) 1991;71:1764–1773. doi: 10.1152/jappl.1991.71.5.1764. [DOI] [PubMed] [Google Scholar]
- 1334.Stehno-Bittel L, Sturek M. Spontaneous sarcoplasmic reticulum calcium release and extrusion from bovine, not porcine, coronary artery smooth muscle. J Physiol. 1992;451:49–78. doi: 10.1113/jphysiol.1992.sp019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1335.Steinberg SF. PI3King the L-type calcium channel activation mechanism. Circ Res. 2001;89:641–644. [PubMed] [Google Scholar]
- 1336.Stepp DW, Kroll K, Feigl EO. K+ ATP channels and adenosine are not necessary for coronary autoregulation. Am J Physiol. 1997;273:H1299–H1308. doi: 10.1152/ajpheart.1997.273.3.H1299. [DOI] [PubMed] [Google Scholar]
- 1337.Stevens MJ, Moulds RF. Antagonism by nifedipine of alpha-1 and alpha-2 adrenoceptor-mediated responses of human digital arteries. J Pharmacol Exp Ther. 1986;236:764–769. [PubMed] [Google Scholar]
- 1338.Stockbridge N, Zhang H, Weir B. Potassium currents of rat basilar artery smooth muscle cells. Pflugers Arch. 1992;421:37–42. doi: 10.1007/BF00374731. [DOI] [PubMed] [Google Scholar]
- 1339.Stokes AJ, Wakano C, Del Carmen KA, Koblan-Huberson M, Turner H. Formation of a physiological complex between TRPV2 and RGA protein promotes cell surface expression of TRPV2. J Cell Biochem. 2005;94:669–683. doi: 10.1002/jcb.20331. [DOI] [PubMed] [Google Scholar]
- 1340.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 1341.Stott JB, Barrese V, Jepps TA, Leighton EV, Greenwood IA. Contribution of Kv7 channels to natriuretic peptide mediated vasodilation in normal and hypertensive rats. Hypertension. 2015;65:676–682. doi: 10.1161/HYPERTENSIONAHA.114.04373. [DOI] [PubMed] [Google Scholar]
- 1342.Stott JB, Povstyan OV, Carr G, Barrese V, Greenwood IA. G-protein betagamma subunits are positive regulators of Kv7.4 and native vascular Kv7 channel activity. Proc Natl Acad Sci U S A. 2015;112:6497–6502. doi: 10.1073/pnas.1418605112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1343.Straub SV, Wagner LE, II, Bruce JI, Yule DI. Modulation of cytosolic calcium signaling by protein kinase A-mediated phosphorylation of inositol 1,4,5-trisphosphate receptors. Biol Res. 2004;37:593–602. doi: 10.4067/s0716-97602004000400013. [DOI] [PubMed] [Google Scholar]
- 1344.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 1345.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 1346.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- 1347.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 1348.Sturek M. Tuning in to the ‘right’ calcium channel regulation in experimental models of diabetes. Br J Pharmacol. 2010;161:1455–1457. doi: 10.1111/j.1476-5381.2010.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1349.Sturek M, Hermsmeyer K. Calcium and sodium channels in spontaneously contracting vascular muscle cells. Science. 1986;233:475–478. doi: 10.1126/science.2425434. [DOI] [PubMed] [Google Scholar]
- 1350.Suematsu E, Hirata M, Hashimoto T, Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984;120:481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- 1351.Sukhanova KY, Harhun MI, Bouryi VA, Gordienko DV. Mechanisms of [Ca2+]i elevation following P2X receptor activation in the guinea-pig small mesenteric artery myocytes. Pharmacol Rep. 2013;65:152–163. doi: 10.1016/s1734-1140(13)70973-3. [DOI] [PubMed] [Google Scholar]
- 1352.Sukhanova KY, Thugorka OM, Bouryi VA, Harhun MI, Gordienko DV. Mechanisms of the sarcoplasmic reticulum Ca2+ release induced by P2X receptor activation in mesenteric artery myocytes. Pharmacol Rep. 2014;66:363–372. doi: 10.1016/j.pharep.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 1353.Sullivan MN, Gonzales AL, Pires PW, Bruhl A, Leo MD, Li W, Oulidi A, Boop FA, Feng Y, Jaggar JH, Welsh DG, Earley S. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci Signal. 2015;8:ra2. doi: 10.1126/scisignal.2005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1354.Sumiyoshi R, Nishimura J, Kawasaki J, Kobayashi S, Takahashi S, Kanaide H. Diadenosine polyphosphates directly relax porcine coronary arterial smooth muscle. J Pharmacol Exp Ther. 1997;283:548–556. [PubMed] [Google Scholar]
- 1355.Sun X, Gu XQ, Haddad GG. Calcium influx via L- and N-type calcium channels activates a transient large-conductance Ca2+-activated K+ current in mouse neocortical pyramidal neurons. J Neurosci. 2003;23:3639–3648. doi: 10.1523/JNEUROSCI.23-09-03639.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1356.Sun CW, Falck JR, Okamoto H, Harder DR, Roman RJ. Role of cGMP versus 20-HETE in the vasodilator response to nitric oxide in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2000;279:H339–H350. doi: 10.1152/ajpheart.2000.279.1.H339. [DOI] [PubMed] [Google Scholar]
- 1357.Sun J, Xin C, Eu JP, Stamler JS, Meissner G. Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc Natl Acad Sci U S A. 2001;98:11158–11162. doi: 10.1073/pnas.201289098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1358.Sun J, Yang T, Wang P, Ma S, Zhu Z, Pu Y, Li L, Zhao Y, Xiong S, Liu D, Zhu Z. Activation of cold-sensing transient receptor potential melastatin subtype 8 antagonizes vasoconstriction and hypertension through attenuating RhoA/Rho kinase pathway. Hypertension. 2014;63:1354–1363. doi: 10.1161/HYPERTENSIONAHA.113.02573. [DOI] [PubMed] [Google Scholar]
- 1359.Sun H, Zhao H, Sharpe GM, Arrick DM, Mayhan WG. Influence of chronic alcohol consumption on inward rectifier potassium channels in cerebral arterioles. Microvasc Res. 2008;75:367–372. doi: 10.1016/j.mvr.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1360.Sung DJ, Noh HJ, Kim JG, Park SW, Kim B, Cho H, Bae YM. Serotonin contracts the rat mesenteric artery by inhibiting 4-aminopyridine-sensitive Kv channels via the 5-HT2A receptor and Src tyrosine kinase. Exp Mol Med. 2013;45:e67. doi: 10.1038/emm.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1361.Supattapone S, Worley PF, Baraban JM, Snyder SH. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988;263:1530–1534. [PubMed] [Google Scholar]
- 1362.Sutton KA, Jungnickel MK, Ward CJ, Harris PC, Florman HM. Functional characterization of PKDREJ, a male germ cell-restricted polycystin. J Cell Physiol. 2006;209:493–500. doi: 10.1002/jcp.20755. [DOI] [PubMed] [Google Scholar]
- 1363.Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marban E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- 1364.Suzuki Y, Yamamura H, Ohya S, Imaizumi Y. Caveolin-1 facilitates the direct coupling between large conductance Ca2+-activated K+ (BKCa) and Cav1.2 Ca2+ channels and their clustering to regulate membrane excitability in vascular myocytes. J Biol Chem. 2013;288:36750–36761. doi: 10.1074/jbc.M113.511485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1365.Swanson DM, Dubin AE, Shah C, Nasser N, Chang L, Dax SL, Jetter M, Breitenbucher JG, Liu C, Mazur C, Lord B, Gonzales L, Hoey K, Rizzolio M, Bogenstaetter M, Codd EE, Lee DH, Zhang SP, Chaplan SR, Carruthers NI. Identification and biological evaluation of 4-(3-trifluoromethylpyridin-2-yl)piperazine-1-carboxylic acid (5-trifluoromethylpyridin-2-yl)amide, a high affinity TRPV1 (VR1) vanilloid receptor antagonist. J Med Chem. 2005;48:1857–1872. doi: 10.1021/jm0495071. [DOI] [PubMed] [Google Scholar]
- 1366.Swayze RD, Braun AP. A catalytically inactive mutant of type I cGMP-dependent protein kinase prevents enhancement of large conductance, calcium-sensitive K+ channels by sodium nitroprusside and cGMP. J Biol Chem. 2001;276:19729–19737. doi: 10.1074/jbc.M005711200. [DOI] [PubMed] [Google Scholar]
- 1367.Sybertz EJ, Vander Vliet G, Baum T. Analysis of the vasoconstrictor responses to potassium depolarization and norepinephrine and their antagonism by differing classes of vasodilators in the perfused rat hindquarters. J Pharmacol Exp Ther. 1983;227:621–626. [PubMed] [Google Scholar]
- 1368.Tabrizchi R, Pugsley MK. Methods of blood flow measurement in the arterial circulatory system. J Pharmacol Toxicol Methods. 2000;44:375–384. doi: 10.1016/s1056-8719(00)00123-4. [DOI] [PubMed] [Google Scholar]
- 1369.Taglialatela M, Drewe JA, Brown AM. Barium blockade of a clonal potassium channel and its regulation by a critical pore residue. Mol Pharmacol. 1993;44:180–190. [PubMed] [Google Scholar]
- 1370.Taguchi H, Heistad DD, Kitazono T, Faraci FM. ATP-sensitive K+ channels mediate dilatation of cerebral arterioles during hypoxia. Circ Res. 1994;74:1005–1008. doi: 10.1161/01.res.74.5.1005. [DOI] [PubMed] [Google Scholar]
- 1371.Taguchi H, Heistad DD, Kitazono T, Faraci FM. Dilatation of cerebral arterioles in response to activation of adenylate cyclase is dependent on activation of Ca(2+)-dependent K+ channels. Circ Res. 1995;76:1057–1062. doi: 10.1161/01.res.76.6.1057. [DOI] [PubMed] [Google Scholar]
- 1372.Tai K, Hamaide MC, Debaix H, Gailly P, Wibo M, Morel N. Agonist-evoked calcium entry in vascular smooth muscle cells requires IP3 receptor-mediated activation of TRPC1. Eur J Pharmacol. 2008;583:135–147. doi: 10.1016/j.ejphar.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 1373.Tajada S, Cidad P, Moreno-Dominguez A, Perez-Garcia MT, Lopez-Lopez JR. High blood pressure associates with the remodelling of inward rectifier K+ channels in mice mesenteric vascular smooth muscle cells. J Physiol. 2012;590:6075–6091. doi: 10.1113/jphysiol.2012.236190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1374.Takaba H, Nagao T, Ibayashi S, Kitazono T, Fujii K, Fujishima M. Altered cerebrovascular response to a potassium channel opener in hypertensive rats. Hypertension. 1996;28:143–146. doi: 10.1161/01.hyp.28.1.143. [DOI] [PubMed] [Google Scholar]
- 1375.Takasago T, Imagawa T, Furukawa K, Ogurusu T, Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem. 1991;109:163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- 1376.Takeda S, Komaru T, Takahashi K, Sato K, Kanatsuka H, Kokusho Y, Shirato K, Shimokawa H. Beating myocardium counteracts myogenic tone of coronary microvessels: Involvement of ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 2006;291:H3050–H3057. doi: 10.1152/ajpheart.00039.2006. [DOI] [PubMed] [Google Scholar]
- 1377.Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, Numa S. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989;339:439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- 1378.Takezawa R, Cheng H, Beck A, Ishikawa J, Launay P, Kubota H, Kinet JP, Fleig A, Yamada T, Penner R. A pyrazole derivative potently inhibits lymphocyte Ca2+ influx and cytokine production by facilitating transient receptor potential melastatin 4 channel activity. Mol Pharmacol. 2006;69:1413–1420. doi: 10.1124/mol.105.021154. [DOI] [PubMed] [Google Scholar]
- 1379.Tam ES, Ferguson DG, Bielefeld DR, Lorenz JN, Cohen RM, Pun RY. Norepinephrine-mediated calcium signaling is altered in vascular smooth muscle of diabetic rat. Cell Calcium. 1997;21:143–150. doi: 10.1016/s0143-4160(97)90038-5. [DOI] [PubMed] [Google Scholar]
- 1380.Tamargo J, Caballero R, Gomez R, Valenzuela C, Delpon E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 1381.Tanaka K, Kawano T, Nakamura A, Nazari H, Kawahito S, Oshita S, Takahashi A, Nakaya Y. Isoflurane activates sarcolemmal adenosine triphosphate-sensitive potassium channels in vascular smooth muscle cells: A role for protein kinase A. Anesthesiology. 2007;106:984–991. doi: 10.1097/01.anes.0000265158.47556.73. [DOI] [PubMed] [Google Scholar]
- 1382.Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J Physiol. 1997;502(Pt 3):545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1383.Tanaka Y, Mochizuki Y, Hirano H, Aida M, Tanaka H, Toro L, Shigenobu K. Role of Maxi K channels in vasoactive intestinal peptide-induced relaxation of rat mesenteric artery. Eur J Pharmacol. 1999;383:291–296. doi: 10.1016/s0014-2999(99)00647-0. [DOI] [PubMed] [Google Scholar]
- 1384.Tanaka Y, Tang G, Takizawa K, Otsuka K, Eghbali M, Song M, Nishimaru K, Shigenobu K, Koike K, Stefani E, Toro L. Kv channels contribute to nitric oxide- and a trial natriuretic peptide-induced relaxation of a rat conduit artery. J Pharmacol Exp Ther. 2006;317:341–354. doi: 10.1124/jpet.105.096115. [DOI] [PubMed] [Google Scholar]
- 1385.Tanano I, Nagaoka T, Omae T, Ishibazawa A, Kamiya T, Ono S, Yoshida A. Dilation of porcine retinal arterioles to cilostazol: Roles of eNOS phosphorylation via cAMP/protein kinase A and AMP-activated protein kinase and potassium channels. Invest Ophthalmol Vis Sci. 2013;54:1443–1449. doi: 10.1167/iovs.12-10115. [DOI] [PubMed] [Google Scholar]
- 1386.Tang WX, Chen YF, Zou AP, Campbell WB, Li PL. Role of FKBP12.6 in cADPR-induced activation of reconstituted ryanodine receptors from arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2002;282:H1304–H1310. doi: 10.1152/ajpheart.00843.2001. [DOI] [PubMed] [Google Scholar]
- 1387.Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, Ramesh V, Zhu MX. Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J Biol Chem. 2000;275:37559–37564. doi: 10.1074/jbc.M006635200. [DOI] [PubMed] [Google Scholar]
- 1388.Taniguchi J, Furukawa KI, Shigekawa M. Maxi K+ channels are stimulated by cyclic guanosine monophosphate-dependent protein kinase in canine coronary artery smooth muscle cells. Pflugers Arch. 1993;423:167–172. doi: 10.1007/BF00374390. [DOI] [PubMed] [Google Scholar]
- 1389.Tano JY, Schleifenbaum J, Gollasch M. Perivascular adipose tissue, potassium channels, and vascular dysfunction. Arterioscler Thromb Vasc Biol. 2014;34:1827–1830. doi: 10.1161/ATVBAHA.114.303032. [DOI] [PubMed] [Google Scholar]
- 1390.Tao S, Yamazaki D, Komazaki S, Zhao C, Iida T, Kakizawa S, Imaizumi Y, Takeshima H. Facilitated hyperpolarization signaling in vascular smooth muscle-overexpressing TRIC-A channels. J Biol Chem. 2013;288:15581–15589. doi: 10.1074/jbc.M112.435396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1391.Tasker PN, Michelangeli F, Nixon GF. Expression and distribution of the type 1 and type 3 inositol 1,4, 5-trisphosphate receptor in developing vascular smooth muscle. Circ Res. 1999;84:536–542. doi: 10.1161/01.res.84.5.536. [DOI] [PubMed] [Google Scholar]
- 1392.Tasker PN, Taylor CW, Nixon GF. Expression and distribution of InsP(3) receptor subtypes in proliferating vascular smooth muscle cells. Biochem Biophys Res Commun. 2000;273:907–912. doi: 10.1006/bbrc.2000.3036. [DOI] [PubMed] [Google Scholar]
- 1393.Tateishi J, Faber JE. ATP-sensitive K+ channels mediate alpha 2D-adrenergic receptor contraction of arteriolar smooth muscle and reversal of contraction by hypoxia. Circ Res. 1995;76:53–63. doi: 10.1161/01.res.76.1.53. [DOI] [PubMed] [Google Scholar]
- 1394.Taylor CW, da Fonseca PC, Morris EP. IP(3) receptors: The search for structure. Trends Biochem Sci. 2004;29:210–219. doi: 10.1016/j.tibs.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 1395.Taylor CW, Tovey SC, Rossi AM, Lopez Sanjurjo CI, Prole DL, Rahman T. Structural organization of signalling to and from IP3 receptors. Biochem Soc Trans. 2014;42:63–70. doi: 10.1042/BST20130205. [DOI] [PubMed] [Google Scholar]
- 1396.Teggatz EG, Zhang G, Zhang AY, Yi F, Li N, Zou AP, Li PL. Role of cyclic ADP-ribose in Ca2+-induced Ca2+ release and vasoconstriction in small renal arteries. Microvasc Res. 2005;70:65–75. doi: 10.1016/j.mvr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 1397.Teramoto N. Physiological roles of ATP-sensitive K+ channels in smooth muscle. J Physiol. 2006;572:617–624. doi: 10.1113/jphysiol.2006.105973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1398.Teramoto N, Zhu HL, Shibata A, Aishima M, Walsh EJ, Nagao M, Cole WC. ATP-sensitive K+ channels in pig urethral smooth muscle cells are heteromultimers of Kir6.1 and Kir6.2. Am J Physiol Renal Physiol. 2009;296:F107–F117. doi: 10.1152/ajprenal.90440.2008. [DOI] [PubMed] [Google Scholar]
- 1399.Tertyshnikova S, Fein A. [Ca2+]i oscillations and [Ca2+]i waves in rat megakaryocytes. Cell Calcium. 1997;21:331–344. doi: 10.1016/s0143-4160(97)90026-9. [DOI] [PubMed] [Google Scholar]
- 1400.Tertyshnikova S, Fein A. Inhibition of inositol 1,4,5-trisphosphate-induced Ca2+ release by cAMP-dependent protein kinase in a living cell. Proc Natl Acad Sci U S A. 1998;95:1613–1617. doi: 10.1073/pnas.95.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1401.Texter EC, Jr, Laureta HC, Frohlich ED, Chou CC. Effects of major cations on gastric and mesenteric vascular resistances. Am J Physiol. 1967;212:569–573. doi: 10.1152/ajplegacy.1967.212.3.569. [DOI] [PubMed] [Google Scholar]
- 1402.Tharp DL, Wamhoff BR, Turk JR, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channel (IKCa1) mediates phenotypic modulation of coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2006;291:H2493–H2503. doi: 10.1152/ajpheart.01254.2005. [DOI] [PubMed] [Google Scholar]
- 1403.Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol. 2003;285:H2255–H2263. doi: 10.1152/ajpheart.00487.2003. [DOI] [PubMed] [Google Scholar]
- 1404.Thilo F, Loddenkemper C, Berg E, Zidek W, Tepel M. Increased TRPC3 expression in vascular endothelium of patients with malignant hypertension. Mod Pathol. 2009;22:426–430. doi: 10.1038/modpathol.2008.200. [DOI] [PubMed] [Google Scholar]
- 1405.Thilo F, Scholze A, Liu DY, Zidek W, Tepel M. Association of transient receptor potential canonical type 3 (TRPC3) channel transcripts with proinflammatory cytokines. Arch Biochem Biophys. 2008;471:57–62. doi: 10.1016/j.abb.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 1406.Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. Molecular composition of 4-aminopyridine-sensitive voltage-gated K(+) channels of vascular smooth muscle. Circ Res. 2001;89:1030–1037. doi: 10.1161/hh2301.100817. [DOI] [PubMed] [Google Scholar]
- 1407.Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, Costell M, Maniscalco-Hauk K, Krawiec JA, Olzinski A, Gordon E, Lozinskaya I, Elefante L, Qin P, Matasic DS, James C, Tunstead J, Donovan B, Kallal L, Waszkiewicz A, Vaidya K, Davenport EA, Larkin J, Burgert M, Casillas LN, Marquis RW, Ye G, Eidam HS, Goodman KB, Toomey JR, Roethke TJ, Jucker BM, Schnackenberg CG, Townsley MI, Lepore JJ, Willette RN. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med. 2012;4:159ra148. doi: 10.1126/scitranslmed.3004276. [DOI] [PubMed] [Google Scholar]
- 1408.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 1409.Thuesen AD, Andersen H, Cardel M, Toft A, Walter S, Marcussen N, Jensen BL, Bie P, Hansen PB. Differential effect of T-type voltage-gated Ca2+ channel disruption on renal plasma flow and glomerular filtration rate in vivo. Am J Physiol Renal Physiol. 2014;307:F445–F452. doi: 10.1152/ajprenal.00016.2014. [DOI] [PubMed] [Google Scholar]
- 1410.Tian L, Coghill LS, McClafferty H, MacDonald SH, Antoni FA, Ruth P, Knaus HG, Shipston MJ. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 2004;101:11897–11902. doi: 10.1073/pnas.0402590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1411.Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem. 2001;276:7717–7720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- 1412.Tiefenbacher CP, Tillmanns H, Niroomand F, Zimmermann R, Kubler W. Adaptation of myocardial blood flow to increased metabolic demand is not dependent on endothelial vasodilators in the rat heart. Heart. 1997;77:147–153. doi: 10.1136/hrt.77.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1413.Tikku S, Epshtein Y, Collins H, Travis AJ, Rothblat GH, Levitan I. Relationship between Kir2.1/Kir2.3 activity and their distributions between cholesterol-rich and cholesterol-poor membrane domains. Am J Physiol Cell Physiol. 2007;293:C440–C450. doi: 10.1152/ajpcell.00492.2006. [DOI] [PubMed] [Google Scholar]
- 1414.Tobin AA, Joseph BK, Al-Kindi HN, Albarwani S, Madden JA, Nemetz LT, Rusch NJ, Rhee SW. Loss of cerebrovascular Shaker-type K(+) channels: A shared vasodilator defect of genetic and renal hypertensive rats. Am J Physiol Heart Circ Physiol. 2009;297:H293–H303. doi: 10.1152/ajpheart.00991.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1415.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 1416.Tomiyama Y, Brian JE, Jr, Todd MM. Cerebral blood flow during hemodilution and hypoxia in rats: Role of ATP-sensitive potassium channels. Stroke. 1999;30:1942–1947. doi: 10.1161/01.str.30.9.1942. discussion 1947–1948. [DOI] [PubMed] [Google Scholar]
- 1417.Toro L, Amador M, Stefani E. ANG II inhibits calcium-activated potassium channels from coronary smooth muscle in lipid bilayers. Am J Physiol. 1990;258:H912–H915. doi: 10.1152/ajpheart.1990.258.3.H912. [DOI] [PubMed] [Google Scholar]
- 1418.Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol. 2013;305:H1698–H1708. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1419.Toth A, Czikora A, Pasztor ET, Dienes B, Bai P, Csernoch L, Rutkai I, Csato V, Manyine IS, Porszasz R, Edes I, Papp Z, Boczan J. Vanilloid receptor-1 (TRPV1) expression and function in the vasculature of the rat. J Histochem Cytochem. 2014;62:129–144. doi: 10.1369/0022155413513589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1420.Touyz RM, He Y, Montezano AC, Yao G, Chubanov V, Gudermann T, Callera GE. Differential regulation of transient receptor potential melastatin 6 and 7 cation channels by ANG II in vascular smooth muscle cells from spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R73–R78. doi: 10.1152/ajpregu.00515.2005. [DOI] [PubMed] [Google Scholar]
- 1421.Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin JE, Pratt PF, Hatoum OA, Gutterman DD, Harder DR, Miura H. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118:3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1422.Toyoda K, Fujii K, Ibayashi S, Kitazono T, Nagao T, Fujishima M. Role of ATP-sensitive potassium channels in brain stem circulation during hypotension. Am J Physiol. 1997;273:H1342–H1346. doi: 10.1152/ajpheart.1997.273.3.H1342. [DOI] [PubMed] [Google Scholar]
- 1423.Toyoda K, Fujii K, Takata Y, Ibayashi S, Kitazono T, Nagao T, Fujikawa M, Fujishima M. Age-related changes in response of brain stem vessels to opening of ATP-sensitive potassium channels. Stroke. 1997;28:171–175. doi: 10.1161/01.str.28.1.171. [DOI] [PubMed] [Google Scholar]
- 1424.Traverse JH, Chen Y, Hou M, Li Y, Bache RJ. Effect of K+ ATP channel and adenosine receptor blockade during rest and exercise in congestive heart failure. Circ Res. 2007;100:1643–1649. doi: 10.1161/CIRCRESAHA.107.150219. [DOI] [PubMed] [Google Scholar]
- 1425.Trebak M. STIM/Orai signalling complexes in vascular smooth muscle. J Physiol. 2012;590:4201–4208. doi: 10.1113/jphysiol.2012.233353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1426.Trebak M, Lemonnier L, Smyth JT, Vazquez G, Putney JW., Jr Phospholipase C-coupled receptors and activation of TRPC channels. Handb Exp Pharmacol. 2007:593–614. doi: 10.1007/978-3-540-34891-7_35. [DOI] [PubMed] [Google Scholar]
- 1427.Treves S, Scutari E, Robert M, Groh S, Ottolia M, Prestipino G, Ronjat M, Zorzato F. Interaction of S100A1 with the Ca2+ release channel (ryanodine receptor) of skeletal muscle. Biochemistry. 1997;36:11496–11503. doi: 10.1021/bi970160w. [DOI] [PubMed] [Google Scholar]
- 1428.Tripathy A, Meissner G. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys J. 1996;70:2600–2615. doi: 10.1016/S0006-3495(96)79831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1429.Troncoso Brindeiro CM, Fallet RW, Lane PH, Carmines PK. Potassium channel contributions to afferent arteriolar tone in normal and diabetic rat kidney. Am J Physiol Renal Physiol. 2008;295:F171–F178. doi: 10.1152/ajprenal.00563.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1430.Troncoso Brindeiro CM, Lane PH, Carmines PK. Tempol prevents altered K(+) channel regulation of afferent arteriolar tone in diabetic rat kidney. Hypertension. 2012;59:657–664. doi: 10.1161/HYPERTENSIONAHA.111.184218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1431.Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca(2+)-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 1432.Tseng-Crank J, Godinot N, Johansen TE, Ahring PK, Strobaek D, Mertz R, Foster CD, Olesen SP, Reinhart PH. Cloning, expression, and distribution of a Ca(2+)-activated K+ channel beta-subunit from human brain. Proc Natl Acad Sci U S A. 1996;93:9200–9205. doi: 10.1073/pnas.93.17.9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1433.Tsien RW, Tsien RY. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- 1434.Tu H, Wang Z, Bezprozvanny I. Modulation of mammalian inositol 1,4,5-trisphosphate receptor isoforms by calcium: A role of calcium sensor region. Biophys J. 2005;88:1056–1069. doi: 10.1529/biophysj.104.049601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1435.Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 1436.Tucker SJ, Gribble FM, Proks P, Trapp S, Ryder TJ, Haug T, Reimann F, Ashcroft FM. Molecular determinants of KATP channel inhibition by ATP. Embo J. 1998;17:3290–3296. doi: 10.1093/emboj/17.12.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1437.Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- 1438.Tumelty J, Scholfield N, Stewart M, Curtis T, McGeown G. Ca2+-sparks constitute elementary building blocks for global Ca2+-signals in myocytes of retinal arterioles. Cell Calcium. 2007;41:451–466. doi: 10.1016/j.ceca.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1439.Tune JD, Richmond KN, Gorman MW, Feigl EO. K(ATP)(+) channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2001;280:H868–H875. doi: 10.1152/ajpheart.2001.280.2.H868. [DOI] [PubMed] [Google Scholar]
- 1440.Tune JD, Yeh C, Setty S, Downey HF. ATP-dependent K(+) channels contribute to local metabolic coronary vasodilation in experimental diabetes. Diabetes. 2002;51:1201–1207. doi: 10.2337/diabetes.51.4.1201. [DOI] [PubMed] [Google Scholar]
- 1441.Tung CC, Lobo PA, Kimlicka L, Van Petegem F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature. 2010;468:585–588. doi: 10.1038/nature09471. [DOI] [PubMed] [Google Scholar]
- 1442.Turner RW, Anderson D, Zamponi GW. Signaling complexes of voltage-gated calcium channels. Channels (Austin) 2011;5:440–448. doi: 10.4161/chan.5.5.16473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1443.Tzeng BH, Chen YH, Huang CH, Lin SS, Lee KR, Chen CC. The Ca(v)3.1 T-type calcium channel is required for neointimal formation in response to vascular injury in mice. Cardiovasc Res. 2012;96:533–542. doi: 10.1093/cvr/cvs257. [DOI] [PubMed] [Google Scholar]
- 1444.Uchida Y, Nakamura F, Tomaru T, Sumino S, Kato A, Sugimoto T. Phasic contractions of canine and human coronary arteries induced by potassium channel blockers. Jpn Heart J. 1986;27:727–740. doi: 10.1536/ihj.27.727. [DOI] [PubMed] [Google Scholar]
- 1445.Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B. Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium. 2005;37:267–278. doi: 10.1016/j.ceca.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 1446.Ungvari Z, Koller A. Mediation of EDHF-induced reduction of smooth muscle [Ca(2+)](i) and arteriolar dilation by K(+) channels, 5,6-EET, and gap junctions. Microcirculation. 2001;8:265–274. doi: 10.1038/sj/mn/7800080. [DOI] [PubMed] [Google Scholar]
- 1447.Ungvari Z, Pacher P, Kecskemeti V, Papp G, Szollar L, Koller A. Increased myogenic tone in skeletal muscle arterioles of diabetic rats. Possible role of increased activity of smooth muscle Ca2+ channels and protein kinase C. Cardiovasc Res. 1999;43:1018–1028. doi: 10.1016/s0008-6363(99)00106-6. [DOI] [PubMed] [Google Scholar]
- 1448.Urena J, del Valle-Rodriguez A, Lopez-Barneo J. Metabotropic Ca2+ channel-induced calcium release in vascular smooth muscle. Cell Calcium. 2007;42:513–520. doi: 10.1016/j.ceca.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 1449.Utz J, Eckert R, Trautwein W. Changes of intracellular calcium concentrations by phenylephrine in renal arterial smooth muscle cells. Pflugers Arch. 1999;438:725–731. doi: 10.1007/s004249900091. [DOI] [PubMed] [Google Scholar]
- 1450.Vaca L, Sinkins WG, Hu Y, Kunze DL, Schilling WP. Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am J Physiol. 1994;267:C1501–C1505. doi: 10.1152/ajpcell.1994.267.5.C1501. [DOI] [PubMed] [Google Scholar]
- 1451.Vaithianathan T, Narayanan D, Asuncion-Chin MT, Jeyakumar LH, Liu J, Fleischer S, Jaggar JH, Dopico AM. Subtype identification and functional characterization of ryanodine receptors in rat cerebral artery myocytes. Am J Physiol Cell Physiol. 2010;299:C264–C278. doi: 10.1152/ajpcell.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1452.Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1453.Vallot O, Combettes L, Jourdon P, Inamo J, Marty I, Claret M, Lompre AM. Intracellular Ca(2+) handling in vascular smooth muscle cells is affected by proliferation. Arterioscler Thromb Vasc Biol. 2000;20:1225–1235. doi: 10.1161/01.atv.20.5.1225. [DOI] [PubMed] [Google Scholar]
- 1454.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 1455.Van de Voorde J, Vanheel B, Leusen I. Endothelium-dependent relaxation and hyperpolarization in aorta from control and renal hypertensive rats. Circ Res. 1992;70:1–8. doi: 10.1161/01.res.70.1.1. [DOI] [PubMed] [Google Scholar]
- 1456.Van Petegem F. Ryanodine receptors: Structure and function. J Biol Chem. 2012;287:31624–31632. doi: 10.1074/jbc.R112.349068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1457.Van Petegem F. Ryanodine receptors: Allosteric ion channel giants. J Mol Biol. 2015;427:31–53. doi: 10.1016/j.jmb.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 1458.Van Petegem F, Chatelain FC, Minor DL., Jr Insights into voltage-gated calcium channel regulation from the structure of the CaV1.2 IQ domain-Ca2+/calmodulin complex. Nat Struct Mol Biol. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1459.van Rossum DB, Patterson RL, Ma HT, Gill DL. Ca2+ entry mediated by store depletion, S-nitrosylation, and TRP3 channels. Comparison of coupling and function. J Biol Chem. 2000;275:28562–28568. doi: 10.1074/jbc.M003147200. [DOI] [PubMed] [Google Scholar]
- 1460.VanBavel E, Sorop O, Andreasen D, Pfaffendorf M, Jensen BL. Role of T-type calcium channels in myogenic tone of skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2002;283:H2239–H2243. doi: 10.1152/ajpheart.00531.2002. [DOI] [PubMed] [Google Scholar]
- 1461.Vandenberg CA. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987;84:2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1462.Vanelli G, Chang HY, Gatensby AG, Hussain SN. Contribution of potassium channels to active hyperemia of the canine diaphragm. J Appl Physiol (1985) 1994;76:1098–1105. doi: 10.1152/jappl.1994.76.3.1098. [DOI] [PubMed] [Google Scholar]
- 1463.Vanelli G, Hussain SN. Effects of potassium channel blockers on basal vascular tone and reactive hyperemia of canine diaphragm. Am J Physiol. 1994;266:H43–H51. doi: 10.1152/ajpheart.1994.266.1.H43. [DOI] [PubMed] [Google Scholar]
- 1464.Vanheel B, de Hemptinne A. Influence of KATP channel modulation on net potassium efflux from ischaemic mammalian cardiac tissue. Cardiovasc Res. 1992;26:1030–1039. doi: 10.1093/cvr/26.11.1030. [DOI] [PubMed] [Google Scholar]
- 1465.VanLandingham LG, Gannon KP, Drummond HA. Pressure-induced constriction is inhibited in a mouse model of reduced betaENaC. Am J Physiol Regul Integr Comp Physiol. 2009;297:R723–R728. doi: 10.1152/ajpregu.00212.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1466.VanTeeffelen JW, Constantinescu AA, Vink H, Spaan JA. Hypercholesterolemia impairs reactive hyperemic vasodilation of 2A but not 3A arterioles in mouse cremaster muscle. Am J Physiol Heart Circ Physiol. 2005;289:H447–H454. doi: 10.1152/ajpheart.01298.2004. [DOI] [PubMed] [Google Scholar]
- 1467.Vass Z, Dai CF, Steyger PS, Jancso G, Trune DR, Nuttall AL. Colocalization of the vanilloid capsaicin receptor and substance P in sensory nerve fibers innervating cochlear and vertebro-basilar arteries. Neuroscience. 2004;124:919–927. doi: 10.1016/j.neuroscience.2003.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1468.Vazquez G, Bird GS, Mori Y, Putney JW., Jr Native TRPC7 channel activation by an inositol trisphosphate receptor-dependent mechanism. J Biol Chem. 2006;281:25250–25258. doi: 10.1074/jbc.M604994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1469.Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney JW., Jr The mammalian TRPC cation channels. Biochim Biophys Acta. 2004;1742:21–36. doi: 10.1016/j.bbamcr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 1470.Vecchione C, Patrucco E, Marino G, Barberis L, Poulet R, Aretini A, Maffei A, Gentile MT, Storto M, Azzolino O, Brancaccio M, Colussi GL, Bettarini U, Altruda F, Silengo L, Tarone G, Wymann MP, Hirsch E, Lembo G. Protection from angiotensin II-mediated vasculotoxic and hypertensive response in mice lacking PI3Kgamma. J Exp Med. 2005;201:1217–1228. doi: 10.1084/jem.20040995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1471.Velmurugan GV, White C. Calcium homeostasis in vascular smooth muscle cells is altered in type 2 diabetes by Bcl-2 protein modulation of InsP3R calcium release channels. Am J Physiol Heart Circ Physiol. 2012;302:H124–H134. doi: 10.1152/ajpheart.00218.2011. [DOI] [PubMed] [Google Scholar]
- 1472.Vennekamp J, Wulff H, Beeton C, Calabresi PA, Grissmer S, Hansel W, Chandy KG. Kv1.3-blocking 5-phenylalkoxypsoralens: A new class of immunomodulators. Mol Pharmacol. 2004;65:1364–1374. doi: 10.1124/mol.65.6.1364. [DOI] [PubMed] [Google Scholar]
- 1473.Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels RJ. Permeation and gating properties of the novel epithelial Ca(2+) channel. J Biol Chem. 2000;275:3963–3969. doi: 10.1074/jbc.275.6.3963. [DOI] [PubMed] [Google Scholar]
- 1474.Vennekens R, Owsianik G, Nilius B. Vanilloid transient receptor potential cation channels: An overview. Curr Pharm Des. 2008;14:18–31. doi: 10.2174/138161208783330763. [DOI] [PubMed] [Google Scholar]
- 1475.Venturi E, Sitsapesan R, Yamazaki D, Takeshima H. TRIC channels supporting efficient Ca(2+) release from intracellular stores. Pflugers Arch. 2013;465:187–195. doi: 10.1007/s00424-012-1197-5. [DOI] [PubMed] [Google Scholar]
- 1476.Vetri F, Xu H, Paisansathan C, Pelligrino DA. Impairment of neurovascular coupling in type 1 diabetes mellitus in rats is linked to PKC modulation of BK(Ca) and Kir channels. Am J Physiol Heart Circ Physiol. 2012;302:H1274–H1284. doi: 10.1152/ajpheart.01067.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1477.Viard P, Butcher AJ, Halet G, Davies A, Nurnberg B, Heblich F, Dolphin AC. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- 1478.Viard P, Exner T, Maier U, Mironneau J, Nurnberg B, Macrez N. Gbetagamma dimers stimulate vascular L-type Ca2+ channels via phosphoinositide 3-kinase. FASEB J. 1999;13:685–694. doi: 10.1096/fasebj.13.6.685. [DOI] [PubMed] [Google Scholar]
- 1479.Vigili de Kreutzenberg S, Kiwanuka E, Tiengo A, Avogaro A. Visceral obesity is characterized by impaired nitric oxide-independent vasodilation. Eur Heart J. 2003;24:1210–1215. doi: 10.1016/s0195-668x(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 1480.Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MA. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 1481.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 1482.Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bodding M, Droogmans G, Nilius B. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem. 2002;277:33704–33710. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- 1483.von Beckerath N, Cyrys S, Dischner A, Daut J. Hypoxic vasodilatation in isolated, perfused guinea-pig heart: An analysis of the underlying mechanisms. J Physiol. 1991;442:297–319. doi: 10.1113/jphysiol.1991.sp018794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1484.Wagenknecht T, Radermacher M, Grassucci R, Berkowitz J, Xin HB, Fleischer S. Locations of calmodulin and FK506-binding protein on the three-dimensional architecture of the skeletal muscle ryanodine receptor. J Biol Chem. 1997;272:32463–32471. doi: 10.1074/jbc.272.51.32463. [DOI] [PubMed] [Google Scholar]
- 1485.Wagner LE, II, Yule DI. Differential regulation of the InsP(3) receptor type-1 and-2 single channel properties by InsP(3), Ca(2)(+) and ATP. J Physiol. 2012;590:3245–3259. doi: 10.1113/jphysiol.2012.228320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1486.Wakatsuki T, Nakaya Y, Inoue I. Vasopressin modulates K(+)-channel activities of cultured smooth muscle cells from porcine coronary artery. Am J Physiol. 1992;263:H491–H496. doi: 10.1152/ajpheart.1992.263.2.H491. [DOI] [PubMed] [Google Scholar]
- 1487.Wakatsuki T, Nakaya Y, Miyoshi Y, Zeng XR, Nomura M, Saito K, Inoue I. Effects of vasopressin on ATP-sensitive and Ca(2+)-activated K+ channels of coronary arterial smooth muscle cells. Jpn J Pharmacol. 1992;58(Suppl 2):339P. [PubMed] [Google Scholar]
- 1488.Wan E, Kushner JS, Zakharov S, Nui XW, Chudasama N, Kelly C, Waase M, Doshi D, Liu G, Iwata S, Shiomi T, Katchman A, D’Armiento J, Homma S, Marx SO. Reduced vascular smooth muscle BK channel current underlies heart failure-induced vasoconstriction in mice. FASEB J. 2013;27:1859–1867. doi: 10.1096/fj.12-223511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1489.Wang Q, Bryan RM, Jr, Pelligrino DA. Calcium-dependent and ATP-sensitive potassium channels and the ‘permissive’ function of cyclic GMP in hypercapnia-induced pial arteriolar relaxation. Brain Res. 1998;793:187–196. doi: 10.1016/s0006-8993(98)00173-5. [DOI] [PubMed] [Google Scholar]
- 1490.Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER. A TRPA1-dependent mechanism for the pungent sensation of weak acids. J Gen Physiol. 2011;137:493–505. doi: 10.1085/jgp.201110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1491.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283:32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1492.Wang L, Cvetkov TL, Chance MR, Moiseenkova-Bell VY. Identification of in vivo disulfide conformation of TRPA1 ion channel. J Biol Chem. 2012;287:6169–6176. doi: 10.1074/jbc.M111.329748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1493.Wang SY, Friedman M, Johnson RG, Zeind AJ, Sellke FW. Adenosine triphosphate-sensitive K+ channels mediate postcardioplegia coronary hyperemia. J Thorac Cardiovasc Surg. 1995;110:1073–1082. doi: 10.1016/s0022-5223(05)80177-1. [DOI] [PubMed] [Google Scholar]
- 1494.Wang R, Wu L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J Biol Chem. 1997;272:8222–8226. doi: 10.1074/jbc.272.13.8222. [DOI] [PubMed] [Google Scholar]
- 1495.Wang R, Wu L, Wang Z. The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Arch. 1997;434:285–291. doi: 10.1007/s004240050398. [DOI] [PubMed] [Google Scholar]
- 1496.Wang HR, Wu M, Yu H, Long S, Stevens A, Engers DW, Sackin H, Daniels JS, Dawson ES, Hopkins CR, Lindsley CW, Li M, McManus OB. Selective inhibition of the K(ir)2 family of inward rectifier potassium channels by a small molecule probe: The discovery, SAR, and pharmacological characterization of ML133. ACS Chem Biol. 2011;6:845–856. doi: 10.1021/cb200146a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1497.Wang Y, Zhang HT, Su XL, Deng XL, Yuan BX, Zhang W, Wang XF, Yang YB. Experimental diabetes mellitus down-regulates large-conductance Ca2+-activated K+ channels in cerebral artery smooth muscle and alters functional conductance. Curr Neurovasc Res. 2010;7:75–84. doi: 10.2174/156720210791184925. [DOI] [PubMed] [Google Scholar]
- 1498.Warrillow S, Egi M, Bellomo R. Randomized, double-blind, placebo-controlled crossover pilot study of a potassium channel blocker in patients with septic shock. Crit Care Med. 2006;34:980–985. doi: 10.1097/01.CCM.0000206114.19707.7C. [DOI] [PubMed] [Google Scholar]
- 1499.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 1500.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 1501.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 1502.Watts SW, Kanagy NL, Lombard JH. Compr Physiol. Hoboken, NJ: John Wiley & Sons; 2010. Receptor-mediated events in the microcirculation. [Google Scholar]
- 1503.Webb RC. Potassium relaxation of vascular smooth muscle from DOCA hypertensive pigs. Hypertension. 1982;4:609–619. doi: 10.1161/01.hyp.4.5.609. [DOI] [PubMed] [Google Scholar]
- 1504.Webb RC, Bohr DF. Potassium relaxation of vascular smooth muscle from spontaneously hypertensive rats. Blood Vessels. 1979;16:71–79. [PubMed] [Google Scholar]
- 1505.Weber LP, Chow WL, Abebe W, MacLeod KM. Enhanced contractile responses of arteries from streptozotocin diabetic rats to sodium fluoride. Br J Pharmacol. 1996;118:115–122. doi: 10.1111/j.1476-5381.1996.tb15373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1506.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D’Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 1507.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin 2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 1508.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 1509.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;57:463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 1510.Wei EP, Kontos HA. Blockade of ATP-sensitive potassium channels in cerebral arterioles inhibits vasoconstriction from hypocapnic alkalosis in cats. Stroke. 1999;30:851–853. doi: 10.1161/01.str.30.4.851. discussion 854. [DOI] [PubMed] [Google Scholar]
- 1511.Wei EP, Kontos HA, Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol. 1996;271:H1262–H1266. doi: 10.1152/ajpheart.1996.271.3.H1262. [DOI] [PubMed] [Google Scholar]
- 1512.Weidelt T, Boldt W, Markwardt F. Acetylcholine-induced K+ currents in smooth muscle cells of intact rat small arteries. J Physiol. 1997;500(Pt 3):617–630. doi: 10.1113/jphysiol.1997.sp022047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1513.Weidelt T, Isenberg G. Augmentation of SR Ca(2+) release by rapamycin and FK506 causes K(+)-channel activation and membrane hyperpolarization in bladder smooth muscle. Br J Pharmacol. 2000;129:1293–1300. doi: 10.1038/sj.bjp.0703223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1514.Weiss T, Gheber L, Shoshan-Barmatz V, Priel Z. Possible mechanism of ciliary stimulation by extracellular ATP: involvement of calcium-dependent potassium channels and exogenous Ca2+ J Membr Biol. 1992;127:185–193. doi: 10.1007/BF00231506. [DOI] [PubMed] [Google Scholar]
- 1515.Welling A, Ludwig A, Zimmer S, Klugbauer N, Flockerzi V, Hofmann F. Alternatively spliced IS6 segments of the alpha 1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ Res. 1997;81:526–532. doi: 10.1161/01.res.81.4.526. [DOI] [PubMed] [Google Scholar]
- 1516.Wellman GC, Bevan JA. Barium inhibits the endothelium-dependent component of flowbut not acetylcholine-induced relaxation in isolated rabbit cerebral arteries. J Pharmacol Exp Ther. 1995;274:47–53. [PubMed] [Google Scholar]
- 1517.Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca(2+)-dependent K+ channels. Circ Res. 1996;79:1024–1030. doi: 10.1161/01.res.79.5.1024. [DOI] [PubMed] [Google Scholar]
- 1518.Wellman GC, Nathan DJ, Saundry CM, Perez G, Bonev AD, Penar PL, Tranmer BI, Nelson MT. Ca2+ sparks and their function in human cerebral arteries. Stroke. 2002;33:802–808. doi: 10.1161/hs0302.104089. [DOI] [PubMed] [Google Scholar]
- 1519.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: Sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34:211–229. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 1520.Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol. 1998;507(Pt 1):117–129. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1521.Wellman GC, Santana LF, Bonev AD, Nelson MT. Role of phospholamban in the modulation of arterial Ca(2+) sparks and Ca(2+)-activated K(+) channels by cAMP. Am J Physiol Cell Physiol. 2001;281:C1029–C1037. doi: 10.1152/ajpcell.2001.281.3.C1029. [DOI] [PubMed] [Google Scholar]
- 1522.Welsh DG, Jackson WF, Segal SS. Oxygen induces electromechanical coupling in arteriolar smooth muscle cells: A role for L-type Ca2+ channels. Am J Physiol. 1998;274:H2018–H2024. doi: 10.1152/ajpheart.1998.274.6.H2018. [DOI] [PubMed] [Google Scholar]
- 1523.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 1524.Welsh DG, Segal SS. Muscle length directs sympathetic nerve activity and vasomotor tone in resistance vessels of hamster retractor. Circ Res. 1996;79:551–559. doi: 10.1161/01.res.79.3.551. [DOI] [PubMed] [Google Scholar]
- 1525.Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1526.Wesselman JP, Schubert R, VanBavel ED, Nilsson H, Mulvany MJ. KCa-channel blockade prevents sustained pressure-induced depolarization in rat mesenteric small arteries. Am J Physiol. 1997;272:H2241–H2249. doi: 10.1152/ajpheart.1997.272.5.H2241. [DOI] [PubMed] [Google Scholar]
- 1527.Westcott EB, Goodwin EL, Segal SS, Jackson WF. Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J Physiol. 2012;590:1849–1869. doi: 10.1113/jphysiol.2011.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1528.Westcott EB, Jackson WF. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. Am J Physiol Heart Circ Physiol. 2011;300:H1616–H1630. doi: 10.1152/ajpheart.00728.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1529.Wiedmann F, Schmidt C, Lugenbiel P, Staudacher I, Rahm AK, Seyler C, Schweizer PA, Katus HA, Thomas D. Therapeutic targeting of two-pore-domain potassium (K(2P)) channels in the cardiovascular system. Clin Sci (Lond) 2016;130:643–650. doi: 10.1042/CS20150533. [DOI] [PubMed] [Google Scholar]
- 1530.Wier WG, Morgan KG. Alpha1-adrenergic signaling mechanisms in contraction of resistance arteries. Rev Physiol Biochem Pharmacol. 2003;150:91–139. doi: 10.1007/s10254-003-0019-8. [DOI] [PubMed] [Google Scholar]
- 1531.Wilde DW, Massey KD, Walker GK, Vollmer A, Grekin RJ. High-fat diet elevates blood pressure and cerebrovascular muscle Ca(2+) current. Hypertension. 2000;35:832–837. doi: 10.1161/01.hyp.35.3.832. [DOI] [PubMed] [Google Scholar]
- 1532.Willcox JM, Summerlee AJ, Murrant CL. Relaxin induces rapid, transient vasodilation in the microcirculation of hamster skeletal muscle. J Endocrinol. 2013;218:179–191. doi: 10.1530/JOE-13-0115. [DOI] [PubMed] [Google Scholar]
- 1533.Williams DL, Jr, Katz GM, Roy-Contancin L, Reuben JP. Guanosine 5′-monophosphate modulates gating of high-conductance Ca2+-activated K+ channels in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1988;85:9360–9364. doi: 10.1073/pnas.85.23.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1534.Willis BC, Ponce-Balbuena D, Jalife J. Protein assemblies of sodium and inward rectifier potassium channels control cardiac excitability and arrhythmogenesis. Am J Physiol Heart Circ Physiol. 2015;308:H1463–H1473. doi: 10.1152/ajpheart.00176.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1535.Wilson HL, Dipp M, Thomas JM, Lad C, Galione A, Evans AM. Adp-ribosyl cyclase and cyclic ADP-ribose hydrolase act as a redox sensor. A primary role for cyclic ADP-ribose in hypoxic pulmonary vasoconstriction. J Biol Chem. 2001;276:11180–11188. doi: 10.1074/jbc.M004849200. [DOI] [PubMed] [Google Scholar]
- 1536.Wilson C, Dryer SE. A mutation in TRPC6 channels abolishes their activation by hypoosmotic stretch but does not affect activation by diacylglycerol or G protein signaling cascades. Am J Physiol Renal Physiol. 2014;306:F1018–F1025. doi: 10.1152/ajprenal.00662.2013. [DOI] [PubMed] [Google Scholar]
- 1537.Wilson AJ, Jabr RI, Clapp LH. Calcium modulation of vascular smooth muscle ATP-sensitive K(+) channels: Role of protein phosphatase-2B. Circ Res. 2000;87:1019–1025. doi: 10.1161/01.res.87.11.1019. [DOI] [PubMed] [Google Scholar]
- 1538.Winquist RJ, Faison EP, Napier M, Vandlen R, Waldman SA, Murad F. The effects of atrial natriuretic factor on vascular smooth muscle. In: Bevan JA, Godfraind T, Maxwell RA, Stoclet JC, Worcel M, editors. Vascular Neuroeffector Mechanisms. Amsetrdam: Elsevier; 1985. pp. 349–353. [Google Scholar]
- 1539.Winquist RJ, Faison EP, Nutt RF. Vasodilator profile of synthetic atrial natriuretic factor. Eur J Pharmacol. 1984;102:169–173. doi: 10.1016/0014-2999(84)90353-4. [DOI] [PubMed] [Google Scholar]
- 1540.Wischmeyer E, Doring F, Karschin A. Acute suppression of inwardly rectifying Kir2.1 channels by direct tyrosine kinase phosphorylation. J Biol Chem. 1998;273:34063–34068. doi: 10.1074/jbc.273.51.34063. [DOI] [PubMed] [Google Scholar]
- 1541.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 1542.Wolfle SE, de Wit C. Intact endothelium-dependent dilation and conducted responses in resistance vessels of hypercholesterolemic mice in vivo. J Vasc Res. 2005;42:475–482. doi: 10.1159/000088101. [DOI] [PubMed] [Google Scholar]
- 1543.Wong CO, Huang Y, Yao X. Genistein potentiates activity of the cation channel TRPC5 independently of tyrosine kinases. Br J Pharmacol. 2010;159:1486–1496. doi: 10.1111/j.1476-5381.2010.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1544.Worley JF, III, Deitmer JW, Nelson MT. Single nisoldipine-sensitive calcium channels in smooth muscle cells isolated from rabbit mesenteric artery. Proc Natl Acad Sci U S A. 1986;83:5746–5750. doi: 10.1073/pnas.83.15.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1545.Worley JF, Quayle JM, Standen NB, Nelson MT. Regulation of single calcium channels in cerebral arteries by voltage, serotonin, and dihydropyridines. Am J Physiol. 1991;261:H1951–H1960. doi: 10.1152/ajpheart.1991.261.6.H1951. [DOI] [PubMed] [Google Scholar]
- 1546.Wu L, Cao K, Lu Y, Wang R. Different mechanisms underlying the stimulation of K(Ca) channels by nitric oxide and carbon monoxide. J Clin Invest. 2002;110:691–700. doi: 10.1172/JCI15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1547.Wu CC, Chen SJ, Garland CJ. NO and KATP channels underlie endotoxin-induced smooth muscle hyperpolarization in rat mesenteric resistance arteries. Br J Pharmacol. 2004;142:479–484. doi: 10.1038/sj.bjp.0705794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1548.Wu L, de Champlain J. Inhibition by cyclic AMP of basal and induced inositol phosphate production in cultured aortic smooth muscle cells from Wistar-Kyoto and spontaneously hypertensive rats. J Hypertens. 1996;14:593–599. doi: 10.1097/00004872-199605000-00008. [DOI] [PubMed] [Google Scholar]
- 1549.Wu X, Davis MJ. Characterization of stretch-activated cation current in coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H1751–H1761. doi: 10.1152/ajpheart.2001.280.4.H1751. [DOI] [PubMed] [Google Scholar]
- 1550.Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by alpha 5beta 1 integrin requires signaling between focal adhesion proteins. J Biol Chem. 2001;276:30285–30292. doi: 10.1074/jbc.M102436200. [DOI] [PubMed] [Google Scholar]
- 1551.Wu BN, Luykenaar KD, Brayden JE, Giles WR, Corteling RL, Wiehler WB, Welsh DG. Hyposmotic challenge inhibits inward rectifying K+ channels in cerebral arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H1085–H1094. doi: 10.1152/ajpheart.00926.2006. [DOI] [PubMed] [Google Scholar]
- 1552.Wu X, Mogford JE, Platts SH, Davis GE, Meininger GA, Davis MJ. Modulation of calcium current in arteriolar smooth muscle by alphav beta3 and alpha5 beta1 integrin ligands. J Cell Biol. 1998;143:241–252. doi: 10.1083/jcb.143.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1553.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1554.Wulff H, Kohler R. Endothelial small-conductance and intermediate-conductance KCa channels: An update on their pharmacology and usefulness as cardiovascular targets. J Cardiovasc Pharmacol. 2013;61:102–112. doi: 10.1097/FJC.0b013e318279ba20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1555.Xi Q, Adebiyi A, Zhao G, Chapman KE, Waters CM, Hassid A, Jaggar JH. IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ Res. 2008;102:1118–1126. doi: 10.1161/CIRCRESAHA.108.173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1556.Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of alpha-subunits. Am J Physiol Heart Circ Physiol. 2004;286:H610–H618. doi: 10.1152/ajpheart.00782.2003. [DOI] [PubMed] [Google Scholar]
- 1557.Xi Q, Umstot E, Zhao G, Narayanan D, Leffler CW, Jaggar JH. Glutamate regulates Ca2+ signals in smooth muscle cells of newborn piglet brain slice arterioles through astrocyte- and heme oxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol. 2010;298:H562–H569. doi: 10.1152/ajpheart.00823.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1558.Xia Y, Fu Z, Hu J, Huang C, Paudel O, Cai S, Liedtke W, Sham JS. TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am J Physiol Cell Physiol. 2013;305:C704–C715. doi: 10.1152/ajpcell.00099.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1559.Xiang L, Hester RL. Adipocyte-derived factor reduces vasodilatory capability in ob-/ob- mice. Am J Physiol Heart Circ Physiol. 2009;297:H689–H695. doi: 10.1152/ajpheart.01327.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1560.Xiang Z, Thompson AD, Brogan JT, Schulte ML, Melancon BJ, Mi D, Lewis LM, Zou B, Yang L, Morrison R, Santomango T, Byers F, Brewer K, Aldrich JS, Yu H, Dawson ES, Li M, McManus O, Jones CK, Daniels JS, Hopkins CR, Xie XS, Conn PJ, Weaver CD, Lindsley CW. The discovery and characterization of ML218: A novel, centrally active T-type calcium channel inhibitor with robust effects in STN neurons and in a rodent model of Parkinson’s disease. ACS Chem Neurosci. 2011;2:730–742. doi: 10.1021/cn200090z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1561.Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280:443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- 1562.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 1563.Xu W, Lipscombe D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1564.Xu C, Lu Y, Tang G, Wang R. Expression of voltage-dependent K(+) channel genes in mesenteric artery smooth muscle cells. Am J Physiol. 1999;277:G1055–G1063. doi: 10.1152/ajpgi.1999.277.5.G1055. [DOI] [PubMed] [Google Scholar]
- 1565.Xu XZ, Moebius F, Gill DL, Montell C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci U S A. 2001;98:10692–10697. doi: 10.1073/pnas.191360198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1566.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 1567.Xu X, Rials SJ, Wu Y, Marinchak RA, Kowey PR. The properties of the inward rectifier potassium currents in rabbit coronary arterial smooth muscle cells. Pflugers Arch. 1999;438:187–194. doi: 10.1007/s004240050897. [DOI] [PubMed] [Google Scholar]
- 1568.Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: A differential, extracellular and voltage-dependent effect. Br J Pharmacol. 2005;145:405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1569.Yada T, Hiramatsu O, Kimura A, Tachibana H, Chiba Y, Lu S, Goto M, Ogasawara Y, Tsujioka K, Kajiya F. Direct in vivo observation of subendocardial arteriolar response during reactive hyperemia. Circ Res. 1995;77:622–631. doi: 10.1161/01.res.77.3.622. [DOI] [PubMed] [Google Scholar]
- 1570.Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol. 1997;499(Pt 3):715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1571.Yamagishi T, Yanagisawa T, Taira N. K+ channel openers, cromakalim and Ki4032, inhibit agonist-induced Ca2+ release in canine coronary artery. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:691–700. doi: 10.1007/BF00168744. [DOI] [PubMed] [Google Scholar]
- 1572.Yamaguchi H, Kajita J, Madison JM. Isoproterenol increases peripheral [Ca2+]i and decreases inner [Ca2+]i in single airway smooth muscle cells. Am J Physiol. 1995;268:C771–C779. doi: 10.1152/ajpcell.1995.268.3.C771. [DOI] [PubMed] [Google Scholar]
- 1573.Yamamoto M, Egashira K, Arimura K, Tada H, Shimokawa H, Takeshita A. Coronary vascular K+ ATP channels contribute to the maintenance of myocardial perfusion in dogs with pacing-induced heart failure. Jpn Circ J. 2000;64:701–707. doi: 10.1253/jcj.64.701. [DOI] [PubMed] [Google Scholar]
- 1574.Yamamura H, Ohya S, Muraki K, Imaizumi Y. Involvement of inositol 1,4,5-trisphosphate formation in the voltage-dependent regulation of the Ca(2+) concentration in porcine coronary arterial smooth muscle cells. J Pharmacol Exp Ther. 2012;342:486–496. doi: 10.1124/jpet.112.194233. [DOI] [PubMed] [Google Scholar]
- 1575.Yamazaki D, Tabara Y, Kita S, Hanada H, Komazaki S, Naitou D, Mishima A, Nishi M, Yamamura H, Yamamoto S, Kakizawa S, Miyachi H, Yamamoto S, Miyata T, Kawano Y, Kamide K, Ogihara T, Hata A, Umemura S, Soma M, Takahashi N, Imaizumi Y, Miki T, Iwamoto T, Takeshima H. TRIC-A channels in vascular smooth muscle contribute to blood pressure maintenance. Cell Metab. 2011;14:231–241. doi: 10.1016/j.cmet.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 1576.Yan J, Aldrich RW. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature. 2010;466:513–516. doi: 10.1038/nature09162. [DOI] [PubMed] [Google Scholar]
- 1577.Yan Z, Bai XC, Yan C, Wu J, Li Z, Xie T, Peng W, Yin CC, Li X, Scheres SH, Shi Y, Yan N. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature. 2015;517:50–55. doi: 10.1038/nature14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1578.Yan J, Olsen JV, Park KS, Li W, Bildl W, Schulte U, Aldrich RW, Fakler B, Trimmer JS. Profiling the phospho-status of the BKCa channel alpha subunit in rat brain reveals unexpected patterns and complexity. Mol Cell Proteomics. 2008;7:2188–2198. doi: 10.1074/mcp.M800063-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1579.Yanaga A, Goto H, Nakagawa T, Hikiami H, Shibahara N, Shimada Y. Cinnamaldehyde induces endothelium-dependent and -independent vasorelaxant action on isolated rat aorta. Biol Pharm Bull. 2006;29:2415–2418. doi: 10.1248/bpb.29.2415. [DOI] [PubMed] [Google Scholar]
- 1580.Yanagisawa T, Yamagishi T, Okada Y. Hyperpolarization induced by K+ channel openers inhibits Ca2+ influx and Ca2+ release in coronary artery. Cardiovasc Drugs Ther. 1993;7(Suppl 3):565–574. doi: 10.1007/BF00877622. [DOI] [PubMed] [Google Scholar]
- 1581.Yang Y, Jones AW, Thomas TR, Rubin LJ. Influence of sex, high-fat diet, and exercise training on potassium currents of swine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2007;293:H1553–H1563. doi: 10.1152/ajpheart.00151.2007. [DOI] [PubMed] [Google Scholar]
- 1582.Yang Y, Li PY, Cheng J, Cai F, Lei M, Tan XQ, Li ML, Liu ZF, Zeng XR. IP3 decreases coronary artery tone via activating the BKCa channel of coronary artery smooth muscle cells in pigs. Biochem Biophys Res Commun. 2013;439:363–368. doi: 10.1016/j.bbrc.2013.08.079. [DOI] [PubMed] [Google Scholar]
- 1583.Yang Y, Li PY, Cheng J, Mao L, Wen J, Tan XQ, Liu ZF, Zeng XR. Function of BKCa channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension. Hypertension. 2013;61:519–525. doi: 10.1161/HYPERTENSIONAHA.111.00211. [DOI] [PubMed] [Google Scholar]
- 1584.Yang Y, Li S, Konduru AS, Zhang S, Trower TC, Shi W, Cui N, Yu L, Wang Y, Zhu D, Jiang C. Prolonged exposure to methylglyoxal causes disruption of vascular KATP channel by mRNA instability. Am J Physiol Cell Physiol. 2012;303:C1045–C1054. doi: 10.1152/ajpcell.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1585.Yang XR, Lin AH, Hughes JM, Flavahan NA, Cao YN, Liedtke W, Sham JS. Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302:L555–L568. doi: 10.1152/ajplung.00005.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1586.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1267–L1276. doi: 10.1152/ajplung.00515.2005. [DOI] [PubMed] [Google Scholar]
- 1587.Yang XR, Lin MJ, Yip KP, Jeyakumar LH, Fleischer S, Leung GP, Sham JS. Multiple ryanodine receptor subtypes and heterogeneous ryanodine receptor-gated Ca2+ stores in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L338–L348. doi: 10.1152/ajplung.00328.2004. [DOI] [PubMed] [Google Scholar]
- 1588.Yang L, Liu G, Zakharov SI, Bellinger AM, Mongillo M, Marx SO. Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ Res. 2007;101:465–474. doi: 10.1161/CIRCRESAHA.107.156976. [DOI] [PubMed] [Google Scholar]
- 1589.Yang Y, Murphy TV, Ella SR, Grayson TH, Haddock R, Hwang YT, Braun AP, Peichun G, Korthuis RJ, Davis MJ, Hill MA. Heterogeneity in function of small artery smooth muscle BKCa: involvement of the beta1-subunit. J Physiol. 2009;587:3025–3044. doi: 10.1113/jphysiol.2009.169920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1590.Yang Y, Shi W, Chen X, Cui N, Konduru AS, Shi Y, Trower TC, Zhang S, Jiang C. Molecular basis and structural insight of vascular K(ATP) channel gating by S-glutathionylation. J Biol Chem. 2011;286:9298–9307. doi: 10.1074/jbc.M110.195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1591.Yang Y, Shi W, Cui N, Wu Z, Jiang C. Oxidative stress inhibits vascular K(ATP) channels by S-glutathionylation. J Biol Chem. 2010;285:38641–38648. doi: 10.1074/jbc.M110.162578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1592.Yang Y, Shi Y, Guo S, Zhang S, Cui N, Shi W, Zhu D, Jiang C. PKA-dependent activation of the vascular smooth muscle isoform of KATP channels by vasoactive intestinal polypeptide and its effect on relaxation of the mesenteric resistance artery. Biochim Biophys Acta. 2008;1778:88–96. doi: 10.1016/j.bbamem.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1593.Yang Y, Sohma Y, Nourian Z, Ella SR, Li M, Stupica A, Korthuis RJ, Davis MJ, Braun AP, Hill MA. Mechanisms underlying regional differences in the Ca2+ sensitivity of BK(Ca) current in arteriolar smooth muscle. J Physiol. 2013;591:1277–1293. doi: 10.1113/jphysiol.2012.241562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1594.Yang P, Zhu MX. Trpv3. Handb Exp Pharmacol. 2014;222:273–291. doi: 10.1007/978-3-642-54215-2_11. [DOI] [PubMed] [Google Scholar]
- 1595.Yao Z, Gross GJ. The ATP-dependent potassium channel: An endogenous cardioprotective mechanism. J Cardiovasc Pharmacol. 1994;24(Suppl 4):S28–S34. [PubMed] [Google Scholar]
- 1596.Yashiro Y, Duling BR. Participation of intracellular Ca2+ stores in arteriolar conducted responses. Am J Physiol Heart Circ Physiol. 2003;285:H65–H73. doi: 10.1152/ajpheart.00662.2002. [DOI] [PubMed] [Google Scholar]
- 1597.Yasutsune T, Kawakami N, Hirano K, Nishimura J, Yasui H, Kitamura K, Kanaide H. Vasorelaxation and inhibition of the voltage-operated Ca2+ channels by FK506 in the porcine coronary artery. Br J Pharmacol. 1999;126:717–729. doi: 10.1038/sj.bjp.0702339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1598.Yeung SY, Greenwood IA. Electrophysiological and functional effects of the KCNQ channel blocker XE991 on murine portal vein smooth muscle cells. Br J Pharmacol. 2005;146:585–595. doi: 10.1038/sj.bjp.0706342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1599.Yeung SY, Pucovsky V, Moffatt JD, Saldanha L, Schwake M, Ohya S, Greenwood IA. Molecular expression and pharmacological identification of a role for K(v)7 channels in murine vascular reactivity. Br J Pharmacol. 2007;151:758–770. doi: 10.1038/sj.bjp.0707284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1600.Yeung S, Schwake M, Pucovsky V, Greenwood I. Bimodal effects of the Kv7 channel activator retigabine on vascular K+ currents. Br J Pharmacol. 2008;155:62–72. doi: 10.1038/bjp.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1601.Yildirim E, Dietrich A, Birnbaumer L. The mouse C-type transient receptor potential 2 (TRPC2) channel: Alternative splicing and calmodulin binding to its N terminus. Proc Natl Acad Sci U S A. 2003;100:2220–2225. doi: 10.1073/pnas.0438036100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1602.Yip KP, Marsh DJ. [Ca2+]i in rat afferent arteriole during constriction measured with confocal fluorescence microscopy. Am J Physiol. 1996;271:F1004–F1011. doi: 10.1152/ajprenal.1996.271.5.F1004. [DOI] [PubMed] [Google Scholar]
- 1603.Yoder EJ, Kleinfeld D. Cortical imaging through the intact mouse skull using two-photon excitation laser scanning microscopy. Microsc Res Tech. 2002;56:304–305. doi: 10.1002/jemt.10002. [DOI] [PubMed] [Google Scholar]
- 1604.Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Physiol. 1998;274:C25–C37. doi: 10.1152/ajpcell.1998.274.1.C25. [DOI] [PubMed] [Google Scholar]
- 1605.Yokota R, Tanaka M, Yamasaki K, Araki M, Miyamae M, Maeda T, Koga K, Yabuuchi Y, Sasayama S. Blockade of ATP-sensitive K+ channels attenuates preconditioning effect on myocardial metabolism in swine: Myocardial metabolism and ATP-sensitive K+ channels. Int J Cardiol. 1998;67:225–236. doi: 10.1016/s0167-5273(98)00257-5. [DOI] [PubMed] [Google Scholar]
- 1606.Yoo HY, Park SJ, Seo EY, Park KS, Han JA, Kim KS, Shin DH, Earm YE, Zhang YH, Kim SJ. Role of thromboxane A(2)-activated nonselective cation channels in hypoxic pulmonary vasoconstriction of rat. Am J Physiol Cell Physiol. 2012;302:C307–C317. doi: 10.1152/ajpcell.00153.2011. [DOI] [PubMed] [Google Scholar]
- 1607.Youm JB, Park KS, Jang YJ, Leem CH. Effects of streptozotocin and unilateral nephrectomy on L-type Ca(2)(+) channels and membrane capacitance in arteriolar smooth muscle cells. Pflugers Arch. 2015;467:1689–1697. doi: 10.1007/s00424-014-1604-1. [DOI] [PubMed] [Google Scholar]
- 1608.Yu M, Sun CW, Maier KG, Harder DR, Roman RJ. Mechanism of cGMP contribution to the vasodilator response to NO in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;282:H1724–H1731. doi: 10.1152/ajpheart.00699.2001. [DOI] [PubMed] [Google Scholar]
- 1609.Yu H, Wu M, Townsend SD, Zou B, Long S, Daniels JS, McManus OB, Li M, Lindsley CW, Hopkins CR. Discovery, synthesis, and structure activity relationship of a series of N-aryl-bicyclo[2.2.1]heptane-2-carboxamides: Characterization of ML213 as a novel KCNQ2 and KCNQ4 potassium channel opener. ACS Chem Neurosci. 2011;2:572–577. doi: 10.1021/cn200065b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1610.Yu JZ, Zhang DX, Zou AP, Campbell WB, Li PL. Nitric oxide inhibits Ca(2+) mobilization through cADP-ribose signaling in coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;279:H873–H881. doi: 10.1152/ajpheart.2000.279.3.H873. [DOI] [PubMed] [Google Scholar]
- 1611.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 1612.Yuan XJ, Tod ML, Rubin LJ, Blaustein MP. NO hyperpolarizes pulmonary artery smooth muscle cells and decreases the intracellular Ca2+ concentration by activating voltage-gated K+ channels. Proc Natl Acad Sci U S A. 1996;93:10489–10494. doi: 10.1073/pnas.93.19.10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1613.Yuill KH, McNeish AJ, Kansui Y, Garland CJ, Dora KA. Nitric oxide suppresses cerebral vasomotion by sGC-independent effects on ryanodine receptors and voltage-gated calcium channels. J Vasc Res. 2010;47:93–107. doi: 10.1159/000235964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1614.Yule DI, Straub SV, Bruce JI. Modulation of Ca2+ oscillations by phosphorylation of Ins(1,4,5)P3 receptors. Biochem Soc Trans. 2003;31:954–957. doi: 10.1042/bst0310954. [DOI] [PubMed] [Google Scholar]
- 1615.Zacharia J, Zhang J, Wier WG. Ca2+ signaling in mouse mesenteric small arteries: Myogenic tone and adrenergic vasoconstriction. Am J Physiol Heart Circ Physiol. 2007;292:H1523–H1532. doi: 10.1152/ajpheart.00670.2006. [DOI] [PubMed] [Google Scholar]
- 1616.Zagotta WN, Hoshi T, Aldrich RW. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990;250:568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]
- 1617.Zalk R, Clarke OB, des Georges A, Grassucci RA, Reiken S, Mancia F, Hendrickson WA, Frank J, Marks AR. Structure of a mammalian ryanodine receptor. Nature. 2015;517:44–49. doi: 10.1038/nature13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1618.Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- 1619.Zanzinger J, Czachurski J, Seller H. Role of calcium-dependent K+ channels in the regulation of arterial and venous tone by nitric oxide in pigs. Pflugers Arch. 1996;432:671–677. doi: 10.1007/s004240050184. [DOI] [PubMed] [Google Scholar]
- 1620.Zarayskiy V, Monje F, Peter K, Csutora P, Khodorov BI, Bolotina VM. Store-operated Orai1 and IP3 receptor-operated TRPC1 channel. Channels (Austin) 2007;1:246–252. doi: 10.4161/chan.4835. [DOI] [PubMed] [Google Scholar]
- 1621.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K(+) current in K(+)-mediated vasodilation. Circ Res. 2000;87:160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- 1622.Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL. The consequences of disrupting cardiac inwardly rectifying K(+) current (I(K1)) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol. 2001;533:697–710. doi: 10.1111/j.1469-7793.2001.t01-1-00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1623.Zatta AJ, Headrick JP. Mediators of coronary reactive hyperaemia in isolated mouse heart. Br J Pharmacol. 2005;144:576–587. doi: 10.1038/sj.bjp.0706099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1624.Zavaritskaya O, Zhuravleva N, Schleifenbaum J, Gloe T, Devermann L, Kluge R, Mladenov M, Frey M, Gagov H, Fesus G, Gollasch M, Schubert R. Role of KCNQ channels in skeletal muscle arteries and periadventitial vascular dysfunction. Hypertension. 2013;61:151–159. doi: 10.1161/HYPERTENSIONAHA.112.197566. [DOI] [PubMed] [Google Scholar]
- 1625.Zhang DM, Chai Y, Erickson JR, Brown JH, Bers DM, Lin YF. Intracellular signalling mechanism responsible for modulation of sarcolemmal ATP-sensitive potassium channels by nitric oxide in ventricular cardiomyocytes. J Physiol. 2014;592:971–990. doi: 10.1113/jphysiol.2013.264697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1626.Zhang Y, Chen Q, Sun Z, Han J, Wang L, Zheng L. Impaired capsaicin-induced relaxation in diabetic mesenteric arteries. J Diabetes Complications. 2015;29:747–754. doi: 10.1016/j.jdiacomp.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 1627.Zhang ZR, Cui G, Zeltwanger S, McCarty NA. Time-dependent interactions of glibenclamide with CFTR: Kinetically complex block of macroscopic currents. J Membr Biol. 2004;201:139–155. doi: 10.1007/s00232-004-0712-9. [DOI] [PubMed] [Google Scholar]
- 1628.Zhang Y, Gao YJ, Zuo J, Lee RM, Janssen LJ. Alteration of arterial smooth muscle potassium channel composition and BKCa current modulation in hypertension. Eur J Pharmacol. 2005;514:111–119. doi: 10.1016/j.ejphar.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 1629.Zhang H, Inazu M, Weir B, Daniel E. Endothelin-1 inhibits inward rectifier potassium channels and activates nonspecific cation channels in cultured endothelial cells. Pharmacology. 1994;49:11–22. doi: 10.1159/000139212. [DOI] [PubMed] [Google Scholar]
- 1630.Zhang Y, Tazzeo T, Chu V, Janssen LJ. Membrane potassium currents in human radial artery and their regulation by nitric oxide donor. Cardiovasc Res. 2006;71:383–392. doi: 10.1016/j.cardiores.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 1631.Zhang H, Weir B, Daniel EE. Activation of protein kinase C inhibits potassium currents in cultured endothelial cells. Pharmacology. 1995;50:247–256. doi: 10.1159/000139289. [DOI] [PubMed] [Google Scholar]
- 1632.Zhang JZ, Wu Y, Williams BY, Rodney G, Mandel F, Strasburg GM, Hamilton SL. Oxidation of the skeletal muscle Ca2+ release channel alters calmodulin binding. Am J Physiol. 1999;276:C46–C53. doi: 10.1152/ajpcell.1999.276.1.c46. [DOI] [PubMed] [Google Scholar]
- 1633.Zhang Z, Yu H, Huang J, Faouzi M, Schmitz C, Penner R, Fleig A. The TRPM6 kinase domain determines the Mg. ATP sensitivity of TRPM7/M6 heteromeric ion channels. J Biol Chem. 2014;289:5217–5227. doi: 10.1074/jbc.M113.512285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1634.Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol Cell Physiol. 2008;295:C1376–C1384. doi: 10.1152/ajpcell.00362.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1635.Zhao G, Neeb ZP, Leo MD, Pachuau J, Adebiyi A, Ouyang K, Chen J, Jaggar JH. Type 1 IP3 receptors activate BKCa channels via local molecular coupling in arterial smooth muscle cells. J Gen Physiol. 2010;136:283–291. doi: 10.1085/jgp.201010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1636.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. Embo J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1637.Zheng YM, Mei QB, Wang QS, Abdullaev I, Lai FA, Xin HB, Kotlikoff MI, Wang YX. Role of FKBP12.6 in hypoxia- and norepinephrine-induced Ca2+ release and contraction in pulmonary artery myocytes. Cell Calcium. 2004;35:345–355. doi: 10.1016/j.ceca.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 1638.Zheng W, Rampe D, Triggle DJ. Pharmacological, radioligand binding, and electrophysiological characteristics of FPL 64176, a novel nondihydropyridine Ca2+ channel activator, in cardiac and vascular preparations. Mol Pharmacol. 1991;40:734–741. [PubMed] [Google Scholar]
- 1639.Zheng YM, Wang QS, Liu QH, Rathore R, Yadav V, Wang YX. Heterogeneous gene expression and functional activity of ryanodine receptors in resistance and conduit pulmonary as well as mesenteric artery smooth muscle cells. J Vasc Res. 2008;45:469–479. doi: 10.1159/000127438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1640.Zheng J, Wenzhi B, Miao L, Hao Y, Zhang X, Yin W, Pan J, Yuan Z, Song B, Ji G. Ca(2+) release induced by cADP-ribose is mediated by FKBP12.6 proteins in mouse bladder smooth muscle. Cell Calcium. 2010;47:449–457. doi: 10.1016/j.ceca.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 1641.Zholos AV. Trpc5. Handb Exp Pharmacol. 2014;222:129–156. doi: 10.1007/978-3-642-54215-2_6. [DOI] [PubMed] [Google Scholar]
- 1642.Zhong XZ, Abd-Elrahman KS, Liao CH, El-Yazbi AF, Walsh EJ, Walsh MP, Cole WC. Stromatoxin-sensitive, heteromultimeric Kv2.1/Kv9.3 channels contribute to myogenic control of cerebral arterial diameter. J Physiol. 2010;588:4519–4537. doi: 10.1113/jphysiol.2010.196618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1643.Zhong XZ, Harhun MI, Olesen SP, Ohya S, Moffatt JD, Cole WC, Greenwood IA. Participation of KCNQ (Kv7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol. 2010;588:3277–3293. doi: 10.1113/jphysiol.2010.192823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1644.Zhou X, Teng B, Tilley S, Ledent C, Mustafa SJ. Metabolic hyperemia requires ATP-sensitive K+ channels and H2O2 but not adenosine in isolated mouse hearts. Am J Physiol Heart Circ Physiol. 2014;307:H1046–H1055. doi: 10.1152/ajpheart.00421.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1645.Zhou W, Wang XL, Lamping KG, Lee HC. Inhibition of protein kinase Cbeta protects against diabetes-induced impairment in arachidonic acid dilation of small coronary arteries. J Pharmacol Exp Ther. 2006;319:199–207. doi: 10.1124/jpet.106.106666. [DOI] [PubMed] [Google Scholar]
- 1646.Zhou C, Wu S. T-type calcium channels in pulmonary vascular endothelium. Microcirculation. 2006;13:645–656. doi: 10.1080/10739680600930289. [DOI] [PubMed] [Google Scholar]
- 1647.Zhu P, Beny JL, Flammer J, Luscher TF, Haefliger IO. Relaxation by bradykinin in porcine ciliary artery. Role of nitric oxide and K(+)-channels. Invest Ophthalmol Vis Sci. 1997;38:1761–1767. [PubMed] [Google Scholar]
- 1648.Zhu X, Chu PB, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–198. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- 1649.Zhu WZ, Kwan CY, Han C. Ca2+-dependence of vasoconstriction mediated by alpha1A-adrenoceptors in perfused rat hindlimb: A pharmacological approach. Life Sci. 1998;63:PL89–PL94. doi: 10.1016/s0024-3205(98)00290-2. [DOI] [PubMed] [Google Scholar]
- 1650.ZhuGe R, Sims SM, Tuft RA, Fogarty KE, Walsh JV., Jr Ca2+ sparks activate K+ and Cl− channels, resulting in spontaneous transient currents in guinea-pig tracheal myocytes. J Physiol. 1998;513(Pt 3):711–718. doi: 10.1111/j.1469-7793.1998.711ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1651.ZhuGe R, Tuft RA, Fogarty KE, Bellve K, Fay FS, Walsh JV., Jr The influence of sarcoplasmic reticulum Ca2+ concentration on Ca2+ sparks and spontaneous transient outward currents in single smooth muscle cells. J Gen Physiol. 1999;113:215–228. doi: 10.1085/jgp.113.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1652.Zitron E, Gunth M, Scherer D, Kiesecker C, Kulzer M, Bloehs R, Scholz EP, Thomas D, Weidenhammer C, Kathofer S, Bauer A, Katus HA, Karle CA. Kir2.x inward rectifier potassium channels are differentially regulated by adrenergic alpha1A receptors. J Mol Cell Cardiol. 2008;44:84–94. doi: 10.1016/j.yjmcc.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 1653.Zitron E, Kiesecker C, Luck S, Kathofer S, Thomas D, Kreye VA, Kiehn J, Katus HA, Schoels W, Karle CA. Human cardiac inwardly rectifying current IKir2.2 is upregulated by activation of protein kinase A. Cardiovasc Res. 2004;63:520–527. doi: 10.1016/j.cardiores.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 1654.Zitt C, Obukhov AG, Strubing C, Zobel A, Kalkbrenner F, Luckhoff A, Schultz G. Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J Cell Biol. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1655.Zitt C, Zobel A, Obukhov AG, Harteneck C, Kalkbrenner F, Luckhoff A, Schultz G. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron. 1996;16:1189–1196. doi: 10.1016/s0896-6273(00)80145-2. [DOI] [PubMed] [Google Scholar]
- 1656.Zorzato F, Fujii J, Otsu K, Phillips M, Green NM, Lai FA, Meissner G, MacLennan DH. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990;265:2244–2256. [PubMed] [Google Scholar]
- 1657.Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: Modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997;49:1–51. [PubMed] [Google Scholar]
- 1658.Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- 1659.Zygmunt PM, Hogestatt ED. Trpa1. Handb Exp Pharmacol. 2014;222:583–630. doi: 10.1007/978-3-642-54215-2_23. [DOI] [PubMed] [Google Scholar]
- 1660.Zygmunt PM, Hogestatt ED, Waldeck K, Edwards G, Kirkup AJ, Weston AH. Studies on the effects of anandamide in rat hepatic artery. Br J Pharmacol. 1997;122:1679–1686. doi: 10.1038/sj.bjp.0701601. [DOI] [PMC free article] [PubMed] [Google Scholar]