Introduction
Food allergy is defined as an immune response that occurs reproducibly to a given food, typically an immunoglobulin E (IgE)-mediated clinical reaction to specific protein epitopes.1–3 Over the last 20–30 years, food allergy has grown into a major public health problem especially in developed countries, including the United States.4–6 Some recent studies estimate the prevalence at 2–8% of the general population.4 Peanut allergy is a common type of food allergy that accounts for a disproportionate number of fatal and near-fatal anaphylactic events amongst all the common food allergens.7–9 Some studies now estimate that this condition affects between 1.5%–3% of all children, making it one of the most common chronic conditions of childhood.4,10–12 Therefore, identifying additional prevention and treatment strategies for this disease is of major clinical importance.
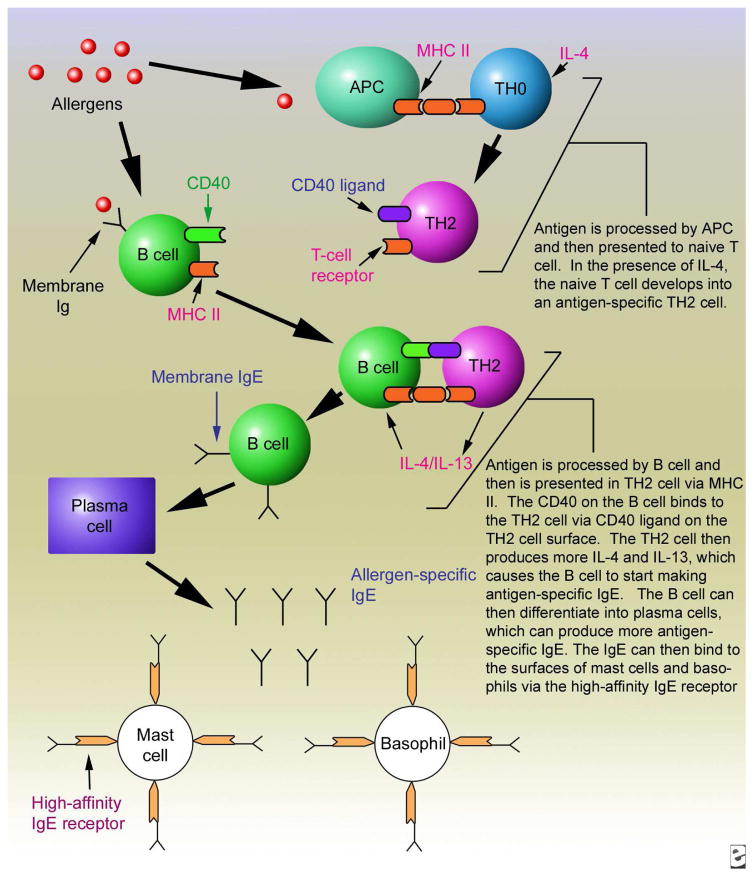
One hindrance to developing such strategies is lack of understanding of the mechanisms governing peanut allergy’s biological development in vivo. There are data to suggest that genetic factors play a significant role in this disease based on familial aggregation13,14 and twin studies.15 Therefore studying the interplay of certain genes in the development of peanut allergy is warranted. One particular area of the genome that has garnered interest in regard to atopic disease, including food allergy, is the human leukocyte antigen (HLA) locus. A number of recent studies suggest that HLA plays a role in these diseases,16,17 including a recent large scale genome-wide association study (GWAS) that identified the HLA-DQB1 locus to be associated with asthma.18 There is also a significant body of literature implicating the MHC in the immunopathogenesis of drug hypersensitivity reactions, suggesting that HLA class I and/or class II alleles may be necessary but not sufficient for the development of severe immunologically-mediated drug reactions.19–21 In humans, genes of the HLA family are part of the Major Histocompatibility Complex locus located on chromosome 6p21.22,23 These genes are crucial to the functioning of the immune system and to the development of allergy (Fig 1). Protein antigens, including those found in foods, are internalized by antigen presenting cells (APC) such as dendritic cells, macrophages and B cells. These antigens are processed into peptides (12–25 amino acids long) that bind to a limited number of MHC class II molecules (Fig 2).24 Antigen-specific T cell receptors (TCRs) on CD4+ T cells recognize their peptide in complex with MHC II. This, in combination with the presence of various cytokines and a variety of cell-cell interactions, leads to activation of the T cells and their development into TH2 cells. B cells are also antigen-specific, recognizing proteins via membrane bound antibodies on their surfaces that bind and internalize proteins, allowing concentration and processing of specific antigens for loading into the MHC class II. Thus, TCRs on allergen-specific CD4+ TH2 cells interact with MHC-peptide on B cells that recognize the same antigen (Fig 1). This cognate T-B interaction, in concert with production of TH2 cytokines, induces IgE class switch, and further differentiation of the B cells into IgE-secreting plasma cells.25,26 Therefore MHC class II is critical both for initial antigen-presentation to T cells, and for cognate T-B interactions that drive IgE production. Since each MHC class II allele is able to bind only a limited number of peptides, it is reasonable to infer that specific HLA alleles are responsible for the ability of the immune system to recognize allergenic proteins, including peanut proteins, which then lead to specific IgE responses.27
Figure 1. Diagram illustrating the role of MHC class II in antigen-presentation of allergens to T cells and antigen-specific T-B interactions that give rise to allergen-specific IgE.
Image reprinted with permission from Medscape Drugs & Diseases (http://emedicine.medscape.com/), 2015, available at: http://emedicine.medscape.com/article/136217-overview
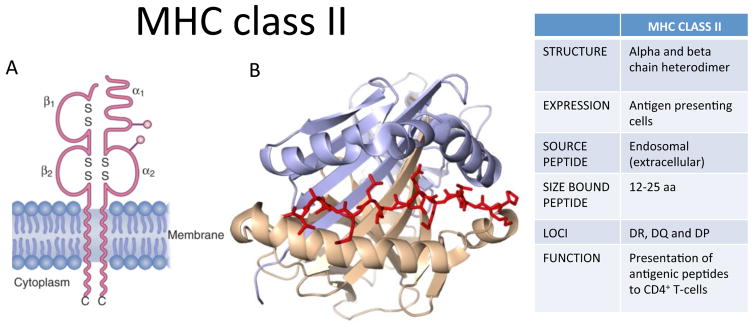
Figure 2. MHC class II structure and function.
A) Schematic of MHC class II consisting of α and β chains, with extracellular domains, transmembrane region and cytoplasmic domains. B) Ribbon diagram of a typical MHC class II protein, with beta-pleated sheet flanked by alpha helices, forming a groove for a peptide antigen (red). Figure adapted from White K, Gaudieri S, Phillips E. HLA and the Pharmacogenomics of Drug Hypersensitivity. In Handbook of Pharmacogenomics and Stratified Medicine. Vol 1, ed. S. Padmanabhan 2014;437–59. Elsevier press.
The purpose of this review was to systematically search the published literature for studies that have examined the associations between HLA and peanut allergy and to provide an objective review of the studies. There have been few candidate gene studies performed over the past two decades and sample size and clinical phenotype have likely limited the ability to associate a specific HLA risk allele with peanut allergy. A recent GWAS examining HLA in food allergy has provided the first convincing evidence of an association between peanut allergy and the class II HLA region. Overall, this literature review indicates that this area of investigation requires sophisticated techniques and large, well-defined study populations, including attention to sub-phenotypes, in order to build a biological model to help define the specific immunopathogenesis of this potentially fatal disease.
Methods
The literature search was conducted using PubMed, with no time limitation and with results limited to English-language, human data. The MeSH keyword human leukocyte antigen was searched and combined with the following terms: peanut hypersensitivity and peanut allergy. Additional searches using the terms HLA, MHC, and peanut were performed to ensure capture of all relevant literature. Bibliographies of identified citations were also searched for relevant references. The search criteria identified seven articles of interest that specifically dealt with studies evaluating genetic associations of HLA alleles with peanut allergy.
Studies showing association between HLA and peanut allergy
The first study of an association with HLA alleles and peanut allergy was performed in 1996 by Donovan and colleagues (Table I and 28). They studied a single family in which four of the five siblings had strong allergy to peanut and several other allergens. The study’s aim was to determine whether either peanut-specific allergy or more general allergy involved shared TCR-α, TCR-β, or HLA haplotypes. They found that peanut allergy segregated with the paternal HLA-DR4 haplotype and not with the TCR-α or TCR-β alleles. This study is obviously limited by its small sample size; however, it did offer the first evidence that HLA genes might play a role in peanut allergy.
Table 1.
Studies included in the Literature Review
| Study | Year | Study design | No. of patients | Findings |
|---|---|---|---|---|
| Donovan et. al28 | 1996 | Case study, Candidate gene | Single family; 7 total patients | Peanut allergy (PA) segregated with paternal HLA-DR4 haplotype |
| Howell et. al29 | 1998 | Case-control, discordant sibling pairs, candidate gene | 50 PA subjects, 34 non–PA siblings, non-PA parents, and 293 unrelated controls | HLA DRB1*08, DQB1*04, and DPB1*0301 significantly increased in the overall study group vs. controls. In PA individuals vs controls, genotypes DRB1*08 and DQB1*04 were increased. DRB1*03 trended toward significance in PA vs non-PA siblings. |
| Boehncke et. al30 | 1998 | Case-control, candidate gene | 80 patients with pollen-associated food allergy, 9 of whom had peanut allergy, in association with grass pollen allergy. 4251 unrelated controls | Peanut allergic patients had HLA-DRB1*08 association vs. controls. No association when compared with patients who were grass pollen allergic. |
| Shreffler et. al34 | 2006 | Case-control, discordant sibling pairs, candidate gene | Discordant sibling pairs: 73 children with PA, 75 peanut tolerant siblings | No association could be established between 25 class II alleles studied |
| Dreskin et. al35 | 2010 | Case-control, discordant sibling pairs, candidate gene | Discordant sibling pairs: 53 children with PA, 63 peanut tolerant siblings | No significant differences in HLA class II between these discordant sibling pairs was found. DRB1*0803 may be a marker of families with a propensity toward peanut allergy |
| Madore et. al32 | 2013 | Case-control, candidate gene, specifically HLA-DQB1 locus | 590 PA children, 332 controls | Higher frequency of DQB1*06:03P allele and decreased frequency of DQB1*02 allele in PA vs. controls. High number of ambiguous genotypes. |
| Hong et. al38 | 2015 | GWAS, three stages | 2759 subjects (853 families) from the Chicago Food Allergy Study (1315 children and 1444 parents) | Identified two peanut allergy-associated SNPs (rs9275596 and rs7192) mapping to HLA-DR and HLA-DQ genes. Differential DNA methylation of the HLA-DQB1 and HLA-DRB1 genes partially mediate the identified SNP-peanut allergy association |
Howell et al29 performed the first larger study evaluating HLA class II loci and peanut allergy. They examined 50 peanut allergic subjects, 34 non–peanut allergic siblings, non-peanut allergic parents, and 293 unrelated controls. Genotype frequencies were compared between the overall study group (peanut allergic patients plus non-peanut allergic family members) and the control group, between the peanut allergic patients only and the control group, and between peanut allergic patients and non-peanut allergic siblings (both atopic and non-atopic). HLA-DRB1*08 and DQB1*04 were increased in frequency in the overall study group and in peanut allergic patients compared with controls, but no significant HLA class II associations were found when comparing peanut allergic vs. non-peanut allergic siblings. The fact that there were no significant differences between peanut and non-peanut allergic siblings, and that the HLA alleles identified in peanut allergic patients compared with controls also were identified in the overall study group compared with controls, suggests that this group identified HLA alleles that are important for atopy overall rather than just for peanut allergy.
Another interesting study by Boehncke et al30 examined cohorts of patients with pollen allergy and pollen-associated food allergy and analyzed the HLA class II genotypes of these patients. In terms of peanut allergy, patients who were allergic to both grass pollen and peanut were analyzed, given the association between those allergens that the authors had previously reported.31 Patients with peanut allergy showed strong association with HLA-DRB1*08 when compared with the control population, the same allele found in the previous study by Howell. However, this association lost significance when compared with patients who were grass pollen allergic, again suggesting that this allele may be a marker for atopy rather than being specific for peanut allergy. Major limitations of this study included small sample size (9 peanut allergic patients) and the fact that it was not designed specifically to identify HLA and peanut allergy associations.
The final study using a candidate gene approach that identified an HLA association with peanut allergy was performed in 2013 by Madore et al.32 These authors examined the HLA-DQB1 locus due to its association with asthma found in a large GWAS.18 The study included 590 children with peanut allergy recruited from a Canadian peanut allergy case group.33 The control group consisted of 332 subjects selected from the Canadian Asthma Primary Prevention Study (CAPPS) and the Study of Asthma Genes and the Environment (SAGE), who had no history of atopy or asthma and negative skin prick testing to peanut. Genomic analysis and exon sequencing resulted in good quality and unambiguous genotyping for 311 patients in the peanut allergy group and 226 patients in the control group, which is more than triple the number analyzed in all the prior studies outlined above. This study found a significant difference between the two groups, when adjusted for asthma status and sex, for two HLA-DQB1 alleles: higher frequency of the DQB1*06:03P allele (possibly indicating increased risk) and decreased frequency of the DQB1*02 allele (possibly indicating a protective role). This study differed from the Howell study, in which DQB1*04, rather than DQB1*06, was associated with peanut allergy. Strengths of this study include its well-defined cohorts of relatively large size. A weakness is the high number of ambiguous genotypes at the allele type level indicating a high failure rate in genotyping calls. Again, the significant differences found here were between patients with peanut allergy compared to normal, unrelated controls, and the possibility that these genes are associated primarily with an overarching atopic phenotype cannot be ruled out.
Studies showing no association between HLA and peanut allergy
In 2006, Shreffler et al34 attempted to replicate findings from the Howell study, focusing on a weak association found between sibling pairs (DRB1*03) that failed to reach significance.29 The Shreffler group planned to perform hi-resolution DNA typing of MHC class II alleles if an association were found. They recruited sibling pairs discordant for peanut allergy and genotyped them (low resolution typing) by PCR to 7 DQ and 18 DR allele groups. Seventy-three children with confirmed peanut allergy and 75 of their siblings who ate peanut were analyzed. After statistical adjustments, no association could be established between the 25 alleles studied in these sibling pairs. An editorial by Dreskin that accompanied the article27 noted that this study was powered at 80% to detect the level of association seen in the study by Howell et al. Explanations offered for this included population differences, insufficient numbers of study participants, and the possibility that there truly is no association between HLA and peanut allergy, or if there is, that the complexity of the immune response makes it difficult to detect and identify.
A study by Dreskin et al35 in 2010 studied peanut-specific IgG levels in peanut allergic vs. non-peanut allergic subjects related to HLA class II alleles. The authors hypothesized that differences in peanut-specific IgG between peanut-allergic subjects and those who are tolerant to peanuts may be related to differences in the ability to present these allergens via HLA class II molecules. In this study, they determined anti-peanut IgG (total IgG and IgG4), peanut specific IgE levels, and HLA class II alleles via high resolution typing in a population of peanut-allergic subjects and their peanut-tolerant siblings. No significant differences in HLA class II between these discordant sibling pairs were found. Furthermore, there were highly significant differences in both peanut-specific IgG and IgE between the peanut-allergic and peanut-tolerant siblings with identical HLA class II alleles. Notably, there was an increased frequency of a rare allele, HLA-DRB1*08:03, in both the peanut-allergic and peanut-tolerant subjects compared to a large control group of bone marrow donors of European descent, similar to Howell’s lower resolution finding for HLA-DRB1*08. Dreskin concludes that DRB1*08 and particularly DRB1*08:03 appear to be a marker of families with a propensity toward peanut allergy and not a marker of peanut allergy itself or it could be a statistical aberration of no biological consequence.
Genome-wide association study
The above reports were candidate gene studies, which limits analysis to one small area of the genome. Large scale, adequately powered GWAS have now become the method of choice for identifying genes that influence complex disease.18,36 GWAS investigate associations between genetic variables and a specified condition, and test hundreds of thousands of genes simultaneously with the benefit of microarray technology. The variable most commonly studied is called a single nucleotide polymorphism (SNP) which is a change in a base pair of DNA that exists in at least 1% of the population.37 Studies using this method are typically case-control studies with the cases being patients with the disease of interest and controls selected from the general population. The advantages are evident in that the entire genome can be examined for genetic associations with a particular disease.37
The first GWAS of a well-defined food allergy cohort of US children and their biological parents was published in February 2015.38 This impressive study was performed in three stages. The first stage (“gene discovery”) used the modified quasi-likelihood score (MQLS) test to detect genetic associations with any food allergy (including peanut, egg white, cow’s milk, soy, wheat, walnut, fish, shellfish, and sesame seed) and the three most common types of food allergy independently (peanut, egg white, and cow’s milk). The MQLS test is a statistical tool used in genetic studies designed to maximally use available information in complex family data sets.39 2759 subjects (853 families) from the Chicago Food Allergy Study (1315 children and 1444 parents) were included to begin the analysis. These families included one or both parents with at least one biological child, with or without food allergy, verified by clinical history questionnaires, allergy skin prick testing, and food-specific IgE levels. Normal controls were defined as children with neither clinical allergic reaction nor evidence of sensitization to any of the nine foods. Other children were categorized as having an “uncertain phenotype” if the above criteria could not be met. All parents were defined as having uncertain food allergy phenotypes. 2197 individuals of European ancestry (671 food allergic children) and 497 individuals of non-European ancestry (155 food allergic children) were included in the final sample size for gene discovery analysis using the MQLS test after quality control exclusions. In the MQLS analysis for any food allergy, no SNP reached genome-wide significance. Subtype analyses specifically examining peanut allergy, egg allergy, or milk allergy identified genome-wide significant associations for peanut, but no significant associations for milk or egg. In 319 peanut allergic patients, 144 nonallergic controls, and 1737 controls of uncertain type from the families of European descent, 40 SNPs spanning the HLA class II DQ genes at the 6p21.32 region were identified as significant. The SNP rs9275596, which is intergenic between HLA-DQB1 and HLA-DQA2 genes, showed the most significant association with peanut allergy. The other 39 were found to be in linkage disequilibrium with rs9275596, and were not significant when conditioned on that SNP. A second SNP, rs7192, which leads to a Leu242Val change in the HLA-DRA gene product, was also found to be significant, and the association between rs9275596 and peanut allergy was reduced when conditioned on rs7192, suggesting that these two SNPs may represent a single risk factor. Of note, these associations were not borne out in studies of the smaller cohorts of non-European ancestry.
In the second stage, the authors narrowed the scope of the study to focus only on peanut allergy, as it was the only significant association found. In this stage, a replication study was performed for the two peanut allergy SNPs using an independent sample of 86 peanut allergy cases and 127 controls from the same Chicago Food Allergy cohort. These samples were all independent of those used in stage 1. They found that both rs9275596 (the most significant SNP) and rs7192 (potential functional SNP) were significantly associated with peanut allergy in children of European ancestry, and that both SNPs had a similar effect size as seen in the Stage 1 GWAS results.
The third stage examined relationships between the two peanut allergy associated SNPs and a regulator of gene expression, DNA methylation (DNAm). They found that both SNPs were significantly associated with differential DNAm at multiple sites and that differential DNAm of the HLA-DQB1 and HLA-DRB1 genes partially mediated the identified SNP-peanut allergy association.
This study presented the first GWAS of food allergy, identifying two SNPs associated with HLA class II genes, specifically, HLA-DR and HLA-DQ. It then replicated these findings with an independent sample, and discovered that both SNPs significantly affected DNA methylation in several nearby genes. This study is the first to provide powerful evidence that the HLA-DR and –DQ gene region harbors significant genetic risk for peanut allergy in subjects of European ancestry.
Conclusion
The HLA region of the genome presents a complex area of study for identifying genes that may contribute to peanut allergy. This is the most highly polymorphic component of the genome, and all genes are co-dominantly expressed. This emerging field tracks with technical advances in cellular and molecular biology. Interestingly, the very first study, using only a single family at a time when PCR was laborious, was able to show an association with HLA, but not TCR genes. Of note, these early findings linked peanut allergy and HLA with atopy (dust mite allergy), and thus did not suggest any independent relationship between peanut allergy and HLA. Subsequent studies, using candidate gene approaches and increasingly larger cohorts, produced data with similar overall outcomes: significant associations emerged when patients were compared with normal controls, but not when compared within families, again suggesting that HLA may govern atopy, without confirming any specific link to peanut allergy. These studies were intriguing, especially as some found associations with the same alleles. However, they had inherent limitations that may have masked true associations between HLA and peanut allergy, including small sample sizes, inadequately or imprecisely defined phenotypes, inadequate racial stratification and the use of low-resolution DNA typing.
Technology has now moved another step forward, and the recent GWAS took advantage of higher-powered approaches and larger sample sizes to clearly identify a relationship between HLA and peanut allergy for the first time, even among family members, that is independent of atopy. This study found strong association between specific SNPs that tag the HLA class II DR and DQ region and peanut allergy that appears to explain about 20% of peanut allergic children. Both these SNPs are also associated with differential DNA methylation of the HLA-DRB1 and HLA-DQB1 genes, suggesting that alteration of the expression of HLA genes through epigenetic mechanisms may be a potential explanation for why not all children who carry specific HLA class II risk alleles develop peanut allergy.
These studies highlight the complex mechanisms underlying development of tolerance to foods. The roadmap of immunogenetic factors necessary but not sufficient for the development of peanut allergy is likely to be equally complex. As with any immunologically mediated response, antigen presentation via HLA-encoded MHC molecules should be a primary factor governing the peptides available to generate food-specific responses. Yet only with the advent of large databases and refined molecular techniques do patterns implicating HLA in this disease begin to emerge. This indicates that the relationship between HLA and food allergy is not simple. Interestingly, HLA may also confer risk or protection in a less direct manner, by helping to shape the microbiome, for example.40 Future study of HLA in oral tolerance studies might therefore help explain why early feeding of peanut antigen induces tolerance in some, but not all, patients at risk.41 In addition, since studies to-date have focused mainly on European populations, studies across diverse populations, including African and African American populations where the burden of peanut allergy is increasing, are warranted.
Acknowledgments
This study was supported by grant R01 DK084246 (to PK) from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampson HA, Aceves S, Bock SA, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134(5):1016–1025. e1043. doi: 10.1016/j.jaci.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133(2):291–307. doi: 10.1016/j.jaci.2013.11.020. quiz 308. [DOI] [PubMed] [Google Scholar]
- 4.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 5.Longo G, Berti I, Burks AW, Krauss B, Barbi E. IgE-mediated food allergy in children. Lancet. 2013;382(9905):1656–1664. doi: 10.1016/S0140-6736(13)60309-8. [DOI] [PubMed] [Google Scholar]
- 6.Gupta RS, Springston EE, Smith B, Warrier MR, Pongracic J, Holl JL. Geographic variability of childhood food allergy in the United States. Clin Pediatr (Phila) 2012;51(9):856–861. doi: 10.1177/0009922812448526. [DOI] [PubMed] [Google Scholar]
- 7.Ross MP, Ferguson M, Street D, Klontz K, Schroeder T, Luccioli S. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. J Allergy Clin Immunol. 2008;121(1):166–171. doi: 10.1016/j.jaci.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Bock SA, Muñoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119(4):1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 9.Umasunthar T, Leonardi-Bee J, Hodes M, et al. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2013;43(12):1333–1341. doi: 10.1111/cea.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hourihane JO. Peanut allergy. Pediatr Clin North Am. 2011;58(2):445–458. xi. doi: 10.1016/j.pcl.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125(6):1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Osborne NJ, Koplin JJ, Martin PE, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127(3):668–676. e661–662. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 13.Hong X, Tsai HJ, Wang X. Genetics of food allergy. Curr Opin Pediatr. 2009;21(6):770–776. doi: 10.1097/MOP.0b013e32833252dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai HJ, Kumar R, Pongracic J, et al. Familial aggregation of food allergy and sensitization to food allergens: a family-based study. Clin Exp Allergy. 2009;39(1):101–109. doi: 10.1111/j.1365-2222.2008.03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sicherer SH, Furlong TJ, Maes HH, Desnick RJ, Sampson HA, Gelb BD. Genetics of peanut allergy: a twin study. J Allergy Clin Immunol. 2000;106(1 Pt 1):53–56. doi: 10.1067/mai.2000.108105. [DOI] [PubMed] [Google Scholar]
- 16.Pino-Yanes M, Corrales A, Acosta-Herrera M, et al. HLA-DRB1*15:01 allele protects from asthma susceptibility. J Allergy Clin Immunol. 2014;134(5):1201–1203. doi: 10.1016/j.jaci.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Mishra MN, Dudeja P, Gupta RK. Association of HLA-Class II and IgE serum levels in pediatric asthma. Iran J Immunol. 2014;11(1):21–28. [PubMed] [Google Scholar]
- 18.Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallal S, Phillips E, Carosi G, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358(6):568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 20.White K, Gaudieri S, Phillips E. HLA and the Pharmacogenomics of Drug Hypersensitivity. Handbook of Pharmacogenomics and Stratified Medicine. 12014 [Google Scholar]
- 21.White K, Chung W, Hung S, Mallal S, Phillips E. Evolving models of the immunopathogenesis of T-cell mediated drug allergy: the role of host, pathogens and drug response. Journal of Allergy and Clinical Immunology. 2015;136(2):219–34. doi: 10.1016/j.jaci.2015.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chinen J, Shearer W, Fleischer T. Middleton’s Allergy: Principles & Practice. 8. Vol. 1. Philadelphia, PA: Elsevier Saunders; 2014. Adaptive Immunity. [Google Scholar]
- 23.Winchester RJ. The major histocompatibility complex In: Clinical Immunology: Principles and Practice. 4. Vol. 1. London, England: Elsevier; 2013. [Google Scholar]
- 24.Abbas A, Lichtman A, Pillai S. Cellular and Molecular Immunology. 8. Philadelphia, PA: Elsevier Saunders; 2015. The Major Histocompatibility Complex. [Google Scholar]
- 25.Robinson JH, Delvig AA. Diversity in MHC class II antigen presentation. Immunology. 2002;105(3):252–262. doi: 10.1046/j.0019-2805.2001.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinke J, Rosenwasser L, Borish L. Middleton’s Allergy: Principles and Practice. 8. Vol. 1. Philadelphia, PA: Elsevier; 2014. Cytokines in Allergic Inflammation. [Google Scholar]
- 27.Dreskin SC. Do HLA genes play a role in the genetics of peanut allergy? Ann Allergy Asthma Immunol. 2006;96(6):766–768. doi: 10.1016/S1081-1206(10)61337-3. [DOI] [PubMed] [Google Scholar]
- 28.Donovan GR, Manolios N, Weiner JM, et al. A family study of allergy: segregation with HLA but not with T-cell receptor genes. J Allergy Clin Immunol. 1996;97(2):712–713. doi: 10.1016/s0091-6749(96)70320-2. [DOI] [PubMed] [Google Scholar]
- 29.Howell WM, Turner SJ, Hourihane JO, Dean TP, Warner JO. HLA class II DRB1, DQB1 and DPB1 genotypic associations with peanut allergy: evidence from a family-based and case-control study. Clin Exp Allergy. 1998;28(2):156–162. doi: 10.1046/j.1365-2222.1998.00224.x. [DOI] [PubMed] [Google Scholar]
- 30.Boehncke WH, Loeliger C, Kuehnl P, Kalbacher H, Böhm BO, Gall H. Identification of HLA-DR and -DQ alleles conferring susceptibility to pollen allergy and pollen associated food allergy. Clin Exp Allergy. 1998;28(4):434–441. doi: 10.1046/j.1365-2222.1998.00246.x. [DOI] [PubMed] [Google Scholar]
- 31.Ortolani C, Ispano M, Pastorello E, Bigi A, Ansaloni R. The oral allergy syndrome. Ann Allergy. 1988;61(6 Pt 2):47–52. [PubMed] [Google Scholar]
- 32.Madore AM, Vaillancourt VT, Asai Y, et al. HLA-DQB1*02 and DQB1*06:03P are associated with peanut allergy. Eur J Hum Genet. 2013;21(10):1181–1184. doi: 10.1038/ejhg.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen-Luu NU, Ben-Shoshan M, Alizadehfar R, et al. Inadvertent exposures in children with peanut allergy. Pediatr Allergy Immunol. 2012;23(2):133–139. doi: 10.1111/j.1399-3038.2011.01235.x. [DOI] [PubMed] [Google Scholar]
- 34.Shreffler WG, Charlop-Powers Z, Sicherer SH. Lack of association of HLA class II alleles with peanut allergy. Ann Allergy Asthma Immunol. 2006;96(6):865–869. doi: 10.1016/S1081-1206(10)61351-8. [DOI] [PubMed] [Google Scholar]
- 35.Dreskin SC, Tripputi MT, Aubrey MT, et al. Peanut-allergic subjects and their peanut-tolerant siblings have large differences in peanut-specific IgG that are independent of HLA class II. Clin Immunol. 2010;137(3):366–373. doi: 10.1016/j.clim.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Consortium WTCC. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visscher PM, Montgomery GW. Genome-wide association studies and human disease: from trickle to flood. JAMA. 2009;302(18):2028–2029. doi: 10.1001/jama.2009.1643. [DOI] [PubMed] [Google Scholar]
- 38.Hong X, Hao K, Ladd-Acosta C, et al. Genome-wide association study identifies peanut allergy-specific loci and evidence of epigenetic mediation in US children. Nat Commun. 2015;6:6304. doi: 10.1038/ncomms7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thornton T, McPeek MS. Case-control association testing with related individuals: a more powerful quasi-likelihood score test. Am J Hum Genet. 2007;81(2):321–337. doi: 10.1086/519497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marietta E, Rishi A, Taneja V. Immunogenetic control of the intestinal microbiota. Immunology. 2015;145(3):313–322. doi: 10.1111/imm.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]