Abstract
Background
Failure of procognitive drug trials in schizophrenia may reflect the clinical heterogeneity of schizophrenia, underscoring the need to identify biomarkers of treatment sensitivity. We used an experimental medicine design to test the procognitive effects of a putative procognitive agent, tolcapone, using an electroencephalogram-based cognitive control task in healthy subjects.
Methods
Healthy men and women (n=27; ages 18–35 years), homozygous for either the Met/Met or Val/Val rs4680 genotype, received placebo and tolcapone 200 mg orally across 2 test days separated by 1 week in a double-blind, randomized, counterbalanced, within-subject design. On each test day, neurocognitive performance was assessed using the MATRICS Consensus Cognitive Battery and an electroencephalogram-based 5 Choice-Continuous Performance Test.
Results
Tolcapone enhanced visual learning in low-baseline MATRICS Consensus Cognitive Battery performers (d=0.35) and had an opposite effect in high performers (d=0.5), and enhanced verbal fluency across all subjects (P=.03) but had no effect on overall MATRICS Consensus Cognitive Battery performance. Tolcapone reduced false alarm rate (d=0.8) and enhanced frontal P200 amplitude during correctly identified nontarget trials (d=0.6) in low-baseline 5 Choice-Continuous Performance Test performers and had opposite effects in high performers (d=0.5 and d=0.25, respectively). Tolcapone’s effect on frontal P200 amplitude and false alarm rate was correlated (rs=-0.4, P=.05). All neurocognitive effects of tolcapone were independent of rs4680 genotype.
Conclusion
Tolcapone enhanced neurocognition and engaged electroencephalogram measures relevant to cognitive processes in specific subgroups of healthy individuals. These findings support an experimental medicine model for identifying procognitive treatments and provide a strong basis for future biomarker-informed procognitive studies in schizophrenia patients.
Keywords: tolcapone, COMT inhibitor, cognition, biomarker, event related potential, cognitive control
Significance Statement
The present study uses an experimental medicine approach to examine the effect of the cognitive-enhancing drug tolcapone on cognitive performance and changes in the distribution of electrical activity measured across the surface of the brain during task performance in healthy subjects. Findings from this study inform us about the way brain electrical activity reflects our ability to inhibit unwanted behavioral responses and how this ability is strengthened by tolcapone. These findings provide a strong basis for testing tolcapone’s cognitive enhancing effects in schizophrenia patients who have marked deficits in their ability to inhibit unwanted behavioral responses.
Introduction
Neurocognitive deficits in patients with chronic psychotic disorders are observed across a range of cognitive constructs, including working memory, attention, and cognitive control (Heinrichs and Zakzanis, 1998; Lesh et al., 2011; Barch and Ceaser, 2012), and are strong predictors of functional impairment (Green, 2016). Antipsychotic medications produce only marginal gains in neurocognition (Lieberman et al., 2005). Substantial efforts to develop procognitive agents that mitigate these neurocognitive deficits in schizophrenia (SZ) have thus far been unsuccessful (Harvey and Bowie, 2012). This failure could be due in part to the clinical heterogeneity of SZ and underscores the need to both understand the neural basis for procognitive drug effects and identify predictive biomarkers of treatment sensitivity.
An “experimental medicine” approach to studying putative procognitive drug effectiveness and sensitivity in clinical populations or healthy subjects can include the use of neurophysiological or imaging techniques to identify symptom-relevant neural targets engaged by the procognitive drug (Insel and Gogtay, 2014). Notably, concurrent event-related potential (ERP) recording during cognitive task performance provides a real-time measure of information processing and characterizes the spatial distribution and time-course of neural events associated with specific cognitive processes (Gehring et al., 1993; van der Stelt and Belger, 2007). For example, simultaneous ERP recording during a 5 choice-continuous performance test (5C-CPT) in schizophrenia (SZ) patients vs healthy subjects (HS) showed reduced amplitudes reflecting poorer response selection for both target and nontarget trials, and reduced nontarget amplitudes during cognitive response phase, the latter accounting for 37% of variance in negative symptoms (Young et al., 2017). Engagement of these ERP measures by a procognitive drug could help delineate the neural basis of procognitive drug effect and serve as potential biomarkers of treatment sensitivity.
Tolcapone was selected as the “test” procognitive drug for this study because of its known specific biochemical action and well-established neurocognitive and neurophysiological effects (Apud and Weinberger, 2007). At both a single acute dose of 200 mg and a dose of 200 mg 3 times daily for a week, this centrally acting reversible catechol-O-methyl transferase (COMT) inhibitor (Robertson, 1999) was found to improve neurocognitive task performance (Apud et al., 2007; Giakoumaki et al., 2008; Farrell et al., 2012; Magalona et al., 2013), increase prepulse inhibition of startle in healthy males with a specific COMT genotype (Giakoumaki et al., 2008), and reduce prefrontal cortical blood oxygen-dependent signaling during working memory and attention tasks (Apud et al., 2007; Magalona et al., 2013) in HS. However, the neural basis and biomarker predictive potential of these effects remain unclear.
In advance of future studies in SZ patients, and the potential use of COMT inhibitors as “procognitive therapy” agents (Swerdlow, 2012), we used an experimental medicine model to test tolcapone’s effects in HS on: (1) the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB; viewed as a gold standard for testing procognitive therapeutics in SZ) (Green et al., 2004); and (2) an ERP-based reverse-translated 5C-CPT (Young et al., 2013). Our primary hypotheses were that a “challenge” dose of tolcapone (200 mg p.o.) would improve MCCB and 5C-CPT performance and enhance frontal ERP signaling during nontarget trials in HS and that these behavioral and electrophysiological effects of tolcapone would be moderated by COMT SNP rs4680. Our exploratory hypothesis was that tolcapone-enhanced frontal ERP signaling would correlate with tolcapone-enhanced 5C-CPT performance.
Materials and Methods
This study was conducted at the UCSD Medical Center, with approval from the UCSD Human Subject Institutional Review Board.
Subjects
Psychiatrically and medically healthy men and women between the ages of 18 and 35 years were recruited from the community via public advertisements and compensated monetarily for study participation. First, subjects underwent phone screening to assess current and past medical and psychiatric history, medication and recreational drug use, and family history of psychosis, followed by a prescreening visit that included a consent procedure for genotyping and structured clinical interview (SCID-NP; First et al., 2002). HS homozygous for the COMT SNP rs4680 genotype with no Axis I DSM IV TR diagnosis were invited for a full screening session (see Supplemental Methods). During the screening visit, subjects completed a baseline assessment of the MCCB in addition to a physical examination, urine toxicology screen, urine pregnancy test for females as per our established screening protocol (Chou et al., 2013), and liver function tests. Study inclusion criteria are described in Supplemental Table 1.
Study Design
The study used a double blind, randomized, placebo-controlled, counterbalanced, within-subject design. HS received either placebo or tolcapone 200 mg orally on each of the 2 test days separated by 1 week. The test day schedule is shown in Supplemental Table 2. Briefly, subjects arrived at the testing center at 8:30 am after overnight fasting with exception of water, completed a urine toxicology screen and a urine pregnancy test in females, and ate a standardized breakfast. Vital signs and subjective symptom rating scale scores (Swerdlow et al., 2003) were obtained at specific intervals pre- and post-pill. Starting 115 minutes post-pill, based on the published pharmacokinetics, neurocognitive, and behavioral effects of tolcapone in HS (Dingemanse et al., 1995; Jorga, 1998; Giakoumaki et al., 2008), testing included the MCCB followed by the 5C-CPT with simultaneous ERP recording. One week after the second test day, liver function tests were reassessed.
Neurocognitive Measures
MCCB
The MCCB was designed by an NIMH initiative and accepted by the FDA as a primary outcome measure for clinical trials of procognitive therapies for SZ (Green et al., 2004; Kern et al., 2008; Nuechterlein et al., 2008). It measures 7 key cognitive domains relevant to cognitive deficits in SZ using 10 tests that assess speed of processing (SP), attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition, and provides T-scores for each domain and a composite score of all domains. For analyses of MCCB data, we used MCCB composite and cognitive domain T-scores, not normed for age and gender, and separately analyzed the moderating effects of age and gender on drug effects.
5C-CPT with Simultaneous ERP Recording
The 5C-CPT paradigm was selected because of its direct translational potential: it is a reverse-translated task from mice with predictive validity (Young et al., 2009, 2011; van Enkhuizen et al., 2014; Tomlinson et al., 2015). It has sensitivity to detect attentional and cognitive control deficits in SZ patients (Young et al., 2013, 2017) and is adapted for both EEG and fMRI studies (McKenna et al., 2013; Young et al., 2017). In this task, subjects were instructed to execute a response by moving the joystick towards the circle that turns white one-at-a-time during “target” or “go” trials and withhold or inhibit a response when all circles turn white simultaneously (“non-target” or “No-go” trials) (supplementary Figure 1). Stimuli were presented for 100 milliseconds with a variable inter-trial interval (ITI) of 0.5 to 1.5 seconds between stimuli to minimize temporal mediating strategies. Task details are found in supplemental Methods. Composite metrics of task performance were used in the analysis of performance, including the hit rate (proportion of correct hits, target detection), false alarm rate (proportion of inappropriate responses to nontarget stimuli, response inhibition), and D-prime (d’; vigilance-parametric measure for the difference between p[Hit Rate] and p[False Alarm Rate] to determine differentiation of stimuli). Similar to other vigilance tasks, decrements in 5C-CPT task performance (d’ scores) over time have been noted in both HS and SZ patients (Young et al., 2013); therefore, a within-session analysis was conducted across 3 trial blocks—initial, middle, and late—each block containing approximately 216 trials with a target:nontarget trial ratio of 10:2.
Continuous EEG data were recorded in DC mode from 64 scalp channels using a BioSemi ActiveTwo system (www.biosemi.com). EEG data were continuously digitized at 1048 Hz with an average reference applied offline. Vertical and horizontal electrooculograms, recorded from electrodes above and below the left eye and at the outer canthi of both eyes, respectively, were used to correct EEG for eye movement and blink. EEG setup and data acquisition required approximately 45 minutes.
EEG Data Preprocessing Procedures
EEG data preprocessing occurred offline. First, EEG data were visually inspected to remove segments containing gross artifacts prior to referencing the data. Bad channels were interpolated using a spherical spline interpolation approach and re-referenced to the average reference before applying a bandpass filter of 0.1 to 70 Hz (24 db/oct) using a Butterworth zero phase-shift filter with 48 db/octave rolloff. Eye movement artifacts were corrected using independent component analyses. Only correct target and nontarget trials were used for ERP analysis due to the low number of task-related errors. Epochs were generated from -100 to 700 milliseconds poststimulus onset for correct trials, followed by rejection of segments with residual artifact (amplitudes>±75 μV), and baseline correction (−100 to 0 milliseconds prestimulus baseline) of all remaining epochs. Separate ERP waveforms were generated for target and nontarget trials for each subject for each test day.
ERP Analysis
Analyses primarily focused on correct responses to target and nontarget stimuli at midline scalp sensors, or centroids (supplemental Methods; supplemental Figure 2). Group-level (tolcapone vs placebo) grand average waveforms for target and nontarget stimuli were generated. Three distinct time windows during which subject-level ERP peaks (point with greatest absolute maxima within a time window) were selected for statistical analysis: (1) 100 to 140 milliseconds thought to represent early sensory components, (2) a middle latency transitional peak (150–250 milliseconds) poststimulus corresponding to response selection, and (3) a later temporal window (300–575 milliseconds) poststimulus considered to represent response action and visual feedback processes (Young et al., 2017). Difference waves were calculated by subtracting peak target waveforms from nontarget waveforms at the midline scalp centroids (Fallgater et al., 1997). Greater difference wave amplitude at the frontal centroids is believed to reflect increased frontal activation recruited during correct inhibition of response to nontarget stimuli. Several neuropsychiatric conditions, including attention deficit hyperactivity disorder, SZ, and 22q11.2 deletion syndrome, are characterized by deficits in both response inhibition and P300 amplitude (Fallgater and Muller, 2001; Fallgater et al., 2005; Ehlis et al., 2007; Romanos et al., 2010).
Statistical Analyses
The main effects of tolcapone on MCCB and 5C-CPT performance and ERP measures were tested using a repeated-measures ANOVA with tolcapone (placebo vs active) as a within-subject factor. Secondary analysis with COMT SNP rs4680 as a between-subject factor was conducted to analyze the moderating role of rs4680 on tolcapone effects. A significant 2- or 3-way interaction was followed by appropriate posthoc contrasts. MCCB T-scores and autonomic measures were treated as continuous variables. Symptom rating scales scores were not normally distributed and were analyzed via nonparametric statistics. Based on known effects of age, gender, and baseline cognitive performance on drug-modified neurocognitive task performance, these variables were used as categorical grouping factors in ANOVA (based on a median split: high [upper 50%] vs low [lower 50%] groups). For MCCB, screen day MCCB Composite T-score was considered baseline MCCB performance and the median T-score of 53 was used to split the subjects into low (T-scores between 36 and 53) vs high (T-scores 54–70) baseline performers. For 5C-CPT, placebo day median d’ score of 4.1 was used to split the subjects into low (d’ scores 2.44–4.1) vs high (d’ scores 4.2–5.71) performers. The median age of 22 years was used to split the age into young (18–22 years old) vs older (23–35 years old) groups. Spearman’s correlation was used to assess the relationship between behavioral performance metrics and ERP amplitudes. Alpha for primary hypotheses was 0.05 and for exploratory hypotheses was 0.01.
Results
Subjects
Of the 31 HS enrolled in the study, 27 subjects (Table 1) completed both test days and the follow-up visit. Three subjects were disqualified based on an Axis I diagnosis (n=2) or positive urine toxicology (n=1), and one subject dropped out of the study after the first test day. Subjects in the rs4680 genotype groups were similar in terms of age, college education, gender ratio, baseline MCCB composite T-score, IQ level, snd baseline liver function test, but differed in terms of race distribution (chi-square=6.21, P<.05), with a greater proportion of Caucasians in the Met/Met group, consistent with previous studies (González-Castro et al., 2013).
Table 1.
Subject Characteristics (n=27)
| Subject characteristics | Genotype | |
|---|---|---|
| Val/Val (N=17) | Met/Met (N=10) | |
| Age in years [mean (SD)] | 22 (4) | 24 (6) |
| WRAT IQ score [mean (SD)] | 104 (10) | 106 (12) |
| Baseline ALT [mean (SD)] | 22.65 (12) | 13 (5.3) |
| Baseline T.Bilirubin [mean (SD)] | 0.4 (0.2) | 0.53 (0.33) |
| Gender (M:F) | 14:3 | 5:5 |
| Education in years [mean (SD)] | 14 (2) | 15 (1) |
| Baseline MCCB Composite Tscore [mean (SD)] | 54.5 (6.6) | 52.2 (9.6) |
| Daily Caffeine intake mg/dl [mean (SD)] | 78.7 (109) | 97.5 (120) |
| Race (% Caucasians) | 41 | 90 |
Bioactivity of Tolcapone
Tolcapone was well tolerated. ANOVA of liver function test revealed a small but statistically significant increase in total bilirubin (P<.05) and alanine transaminase (P<.01) levels during follow-up visit (1 week after test day 2) compared with baseline levels; even with this increase, the post study total bilirubin (mean±SD=0.6±0.36) (mg/dL) and alanine transaminase (22.35±11.6) (U/L) were within normal limits (Olanow, 2000) and did not require additional follow-up (supplemental Table 3). Change in autonomic measures from baseline across the full time-course showed trend level main effects of tolcapone on systolic blood pressure but no effects on diastolic blood pressure or heart rate. Analyses focusing on the peak pharmacokinetic time point (210 minutes post pill) (Jorga, 1998) showed a significant elevation in systolic blood pressure associated with tolcapone (F(1,26)=5.610; P=.025). There were no significant effects of tolcapone on subjective symptom ratings (see supplemental Results).
MCCB Performance
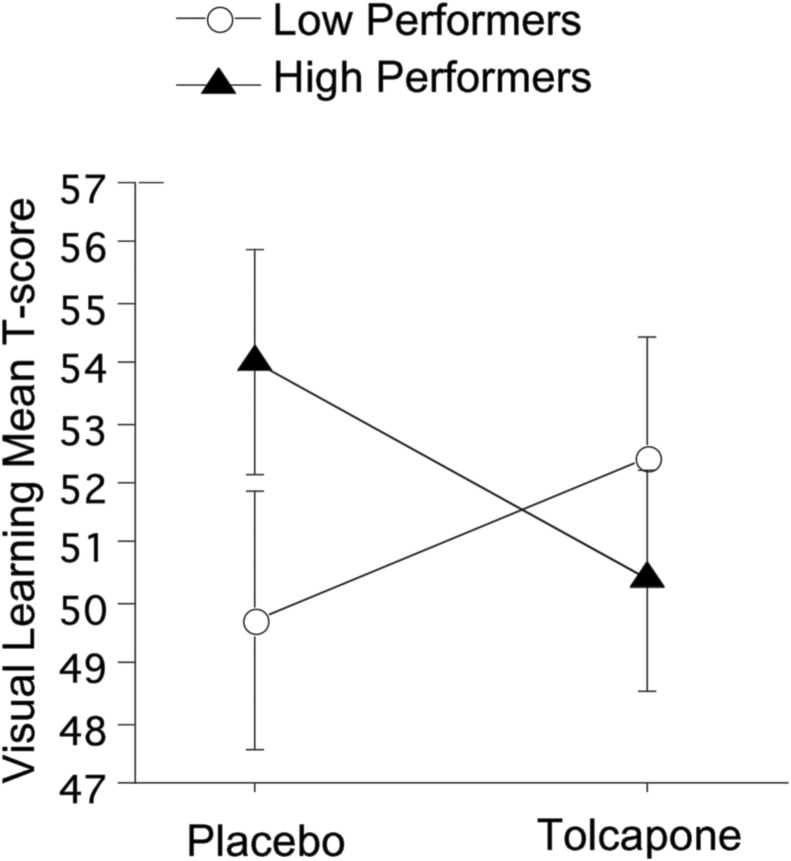
An initial assessment of screen day (baseline) MCCB performance revealed no significant main or interaction effects of either gender (F(1,23)=2.78, NS) or age (median split: F(1,23)=2.05, NS). These variables were omitted from subsequent analyses. ANOVA failed to detect significant main effects of tolcapone or COMT genotype on MCCB global or cognitive domain level T-scores (F<1). There were significant main effects of baseline MCCB performance (F (1,23)=11.34; P<.001) and cognitive domain (F(6,138)=11.25; P<.0001), and a significant 3-way interaction of tolcapone x domain x baseline performance (F(6,138)=2.48; P<.03). Posthoc analyses of each cognitive domain showed only a significant tolcapone x baseline performance 2-way interaction for the visual learning domain (F(1,25)= 5.0; P=.03), with tolcapone-enhancing performance in low-baseline performers (d=0.35) and tolcapone-impairing performance in high-baseline performers (d=0.50) (Figure 1).
Figure 1.
Effect of tolcapone 200 mg on visual learning mean T-score in low- vs high-baseline MATRICS Consensus Cognitive Battery (MCCB) performers. Significant 2-way drug x baseline MCCB performance interaction (P<.05); tolcapone enhances performance in low performers and impairs performance in high performers.
An ANOVA of test order (active pill day) and practice (Day 2 vs Day 1) on MCCB performance revealed a significant main effect of practice for the composite T-score (F(1,26)=4.250; P=.05) and SP cognitive domain T-score (F(1,26)=16.51; P<.001), but no significant main effect of test order (F<1) or 2-way interactions. Posthoc analyses of practice effects for individual tests within SP domain was significant for trail-making test (P=0.02) and BACS-Digit symbol coding (P<.001) but not for verbal fluency (P=ns).
Based on previously published findings that tolcapone significantly enhances verbal fluency (Apud et al., 2007), a posthoc analysis was conducted for this measure. Findings confirmed a significant main effect of tolcapone on verbal fluency (F(1,25)=5.20; P=.03) but no significant main effect of genotype (F<1) and no significant 2-way interactions (F<1) (supplementary Figure 3).
5C-CPT and Task-Related ERP Measures
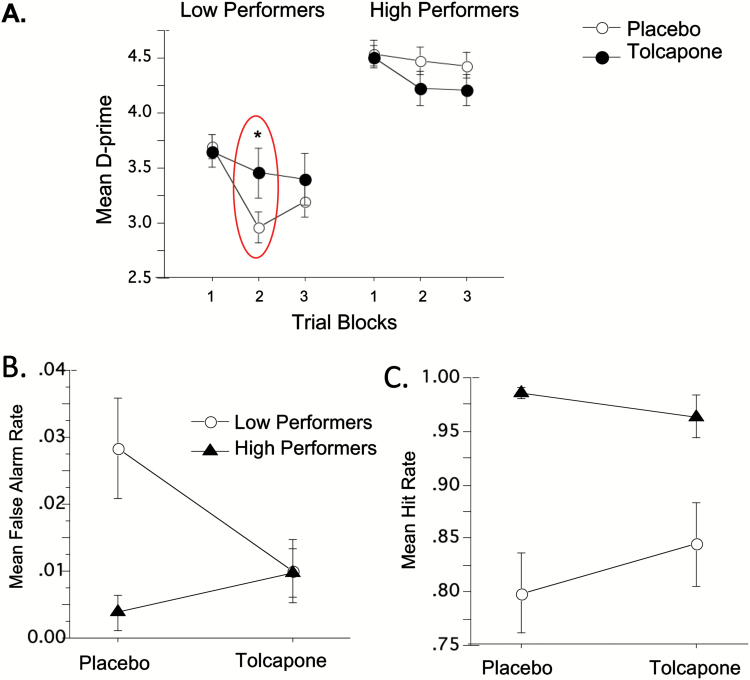
Analyses failed to detect any significant main effect of tolcapone (F<1) or interactions with COMT genotype or median split (low vs high) placebo-day d’ scores (baseline 5C-CPT). ANOVA revealed a significant interaction of tolcapone x trial block x baseline 5C-CPT performance for d’ score (F(2,50)=4.24; P=.02). Posthoc comparisons revealed a strong effect of tolcapone on trial block 2 d’ scores that mainly reflected a robust reduction in trial block 2 false alarm rate (FAR) in low-baseline performers (d=0.8) and an opposite effect in high-baseline performers (d=0.5) (Figure 2A–C).
Figure 2.
(A) Effect of acute administration of tolcapone 200 mg on within session. Mean d’ score in low- vs high-baseline 5 choice-continuous performance test (5C-CPT) performers. *Significant drug x trial block x baseline 5C-CPT performance. {d’score=p[Hit rate] – p[False alarm rate]} (B) Effect of tolcapone on trial block 2 mean false alarm rate (FAR). Significant 2-way drug x baseline 5C-CPT performance interaction (P<.05); tolcapone enhances performance in low performers by decreasing FAR and impairs performance in high performers by increasing FAR. (C) Effect of tolcapone on trial block 2 mean hit rate (P>.05).
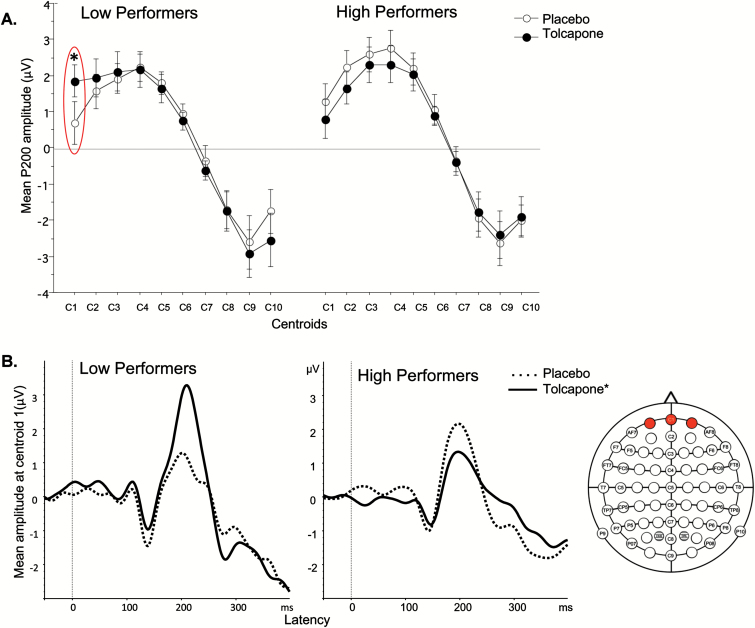
We next analyzed tolcapone effects on stimulus-locked ERP measures during 5C-CPT performance. ANOVA revealed no significant effect of tolcapone or COMT genotype on early, middle, and late peak amplitudes for midline scalp centroids and all channels during correct target trials. In contrast, ANOVA detected a significant drug x centroid x baseline 5C-CPT performance interaction (F(9,225)=2.2; P=.02) for middle latency on nontarget trials requiring inhibition of a prepotent response. Posthoc comparisons showed a strong effect of tolcapone-enhanced P200 amplitude at centroid 1 in low-baseline performers (d=0.6) and a modest opposite effect in high performers (d=0.25) (Figure 3A–B).
Figure 3.
(A) Effect of tolcapone on middle latency peak amplitude across 10 midline centroids in low- vs high-baseline 5 choice-continuous performance test (5C-CPT) performers. *Significant drug x centroid x baseline performance interaction. (B) Waveform of tolcapone’s effect on P200 amplitude at centroid 1 in low vs high performers during nontarget trials. *Significant main effect of drug.
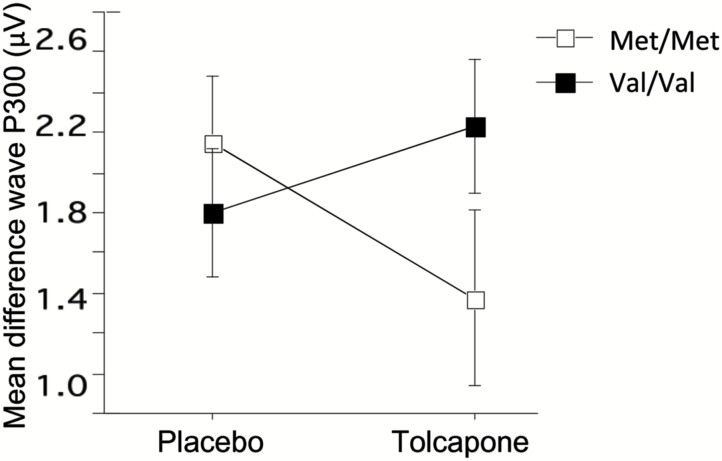
Analyses of difference wave (nontarget waveform minus target waveform) amplitudes at the frontal centroids (centroids 1–4) detected no significant main effects of tolcapone or genotype, but revealed a significant genotype x tolcapone interaction (F(1,25)=6.14; P=0.02) at centroid 2 for difference wave P300 amplitude. Posthoc comparison showed tolcapone-enhanced difference wave amplitude in Val/Val subjects (d=0.4) and tolcapone-reduced difference wave amplitude in Met/Met subjects (d=0.62) (Figure 4).
Figure 4.
Effect of tolcapone on mean difference wave P300 amplitude at centroid 2 in Met/Met vs Val/Val COMT genotype group. Significant tolcapone x genotype interaction (P<.05); tolcapone enhanced P300 amplitude in Val homozygotes and reduced P300 amplitude in Met homozygotes.
Exploratory analysis using Spearman correlation revealed an inverse relationship between tolcapone effects on P200 amplitude at centroid 1 during nontarget trial and tolcapone effects on the false alarm rate (rs=-0.4, P=.05): greater tolcapone-enhancement of P200 amplitude was associated with greater tolcapone-reduction in false-alarm rates.
Discussion
This study investigated the effects of a single dose of tolcapone on MCCB performance and an EEG-based reverse-translated cognitive control task (5C-CPT) in healthy adults homozygous for the rs4680 genotype. Tolcapone significantly enhanced verbal fluency in the inclusive sample. Other effects of tolcapone—on visual learning, 5C-CPT performance, and frontal P200 amplitude during response inhibition—were dependent on the level of baseline performance. Interestingly, tolcapone-enhanced P200 amplitude over midline frontopolar electrodes was associated with tolcapone-reduced false alarm rate. Thus, we demonstrate a successful and feasible experimental medicine model for investigating procognitive drug effects, in which tolcapone engaged a neural target (enhanced frontal P200 amplitude) relevant to a behavior (response inhibition) in a sensitive subgroup (low-baseline performers).
While an acute dose of tolcapone failed to enhance MCCB global cognition, performance on a specific cognitive domain and cognitive tests was enhanced. The limited effects of tolcapone may reflect a performance ceiling, given the fact that most participants were young, healthy, and college educated with a median baseline MCCB composite T-score of 53.
Tolcapone’s Effects on 5C-CPT Performance Were Baseline Dependent
Tolcapone enhanced within-session 5C-CPT performance as reflected by decreased false-alarm rate and increased d’ scores in low-baseline performers and had the opposite effects in high-baseline performers. Interpreting these baseline-dependent tolcapone effects on 5C-CPT performance is complicated; while we cannot rule out the possibility that these effects reflect a “regression to the mean” (Barnett et al., 2005), our findings are consistent with reports of baseline-dependent drug effects on neurocognition with other pro-dopamine agents, including bromocriptine (Kimberg et al., 1997) and amphetamine (Mattay et al., 2003; Chou et al., 2013). More generally, this pattern of tolcapone effects is consistent with an “inverted-U” relationship between baseline cognitive control performance and forebrain dopamine (DA) activity (Lyon and Robbins, 1975; Cools and Esposito, 2011). According to the inverted-U hypothesis, in low-baseline performers (with hypothetically low DA activity in cognitive control-related forebrain regions, such as the prefrontal cortex [PFC]), tolcapone improved performance by increasing DA activity in the PFC, while in high-baseline performers (with hypothetically optimal levels of PFC DA activity), a further increase in PFC DA with tolcapone impaired performance.
Consistent with the behavioral 5C-CPT results, ERP findings showed that tolcapone increased frontal P200 amplitudes during correctly recognized nontarget trials in low-baseline performers and had the opposite effect in high-baseline performers. Further, tolcapone-enhanced frontal P200 amplitude was associated with a reduced false alarm rate. Evidence suggests that this visual P200 is mostly generated from frontal and occipital areas, with frontal scalp areas being the primary source. Functionally, P200 is connected with the automatic identification and classification of stimuli (Lindholm and Koriath, 1985; Kenemans et al., 1993; Heslenfeld et al., 1997); thus, alterations in P200 amplitude are believed to reflect issues with an early orienting or preparatory mechanism that could affect later processing stages (Brandeis et al., 1998). Collectively, these findings suggest that midline frontal P200 amplitude or amplitude malleability might serve as predictive biomarkers of sensitivity to tolcapone-enhanced response inhibition. Future studies using source analysis will be better able to localize the cortical source dynamics associated with tolcapone-enhanced cognitive control.
In this study, tolcapone’s procognitive effects were independent of COMT genotype. This observation might reflect limitations with the study sample, for example, small sample size, or unequal gender and racial distributions across COMT genotypes. Posthoc comparisons limited to Caucasians, or to men, revealed no significant interactions of drug x genotype for MCCB or 5C-CPT performance. Alternatively, the lack of a simple role of COMT genotype in tolcapone sensitivity might reflect more complex underlying mechanisms, such as gene x gene epistatic interactions that can differentially impact synaptic dopamine transmission and procognitive drug effects (Alfimova et al., 2007; Heinzel et al., 2013; Papaleo et al., 2014a, 2014b). It is also possible that COMT genotype may regulate specific aspects of cognition and hence tolcapone sensitivity (e.g., flexibility vs maintenance), such that Met/Met individuals might benefit from tolcapone more or less than Val/Val individuals, depending on the extent to which the task demands cognitive flexibility vs maintenance (Nolan et al., 2004; Frank et al., 2007; Krugel et al., 2009). Lastly, it is possible that the procognitive effects of tolcapone are mediated via mechanisms that are unrelated to COMT enzymatic activity, at least as it is regulated by rs4680. However, in contrast to its procognitive effects, tolcapone’s effect on difference wave P300 amplitude was found to be genotype specific: tolcapone increased frontal P300 amplitude in rs4680 Val homozygotes, but had an opposite effect in Met homozygotes. This finding suggests that EEG can detect regionally specific differences in brain activity associated with the rs4680 SNP that do not reflect SNP-related effects on neurocognitive performance as assessed in this study.
In summary, our findings support the feasibility of an “experimental medicine model” for testing tolcapone’s procognitive effects using an EEG-based reverse-translated 5C-CPT. A single acute dose of 200 mg of tolcapone engaged ERP measures relevant to cognitive processes in specific subgroups of healthy individuals. These findings provide a strong rationale for future biomarker-informed, EEG-guided, procognitive studies of tolcapone in clinical populations, including SZ patients, who show marked deficits in response inhibition.
Statement of Interest
In the past 3 years, N.R.S. has had support from Neurocrine; G.A.L. has served as a consultant for Astellas, Forum Pharmaceuticals, Boehringer Ingelheim, and Neuroverse; and J.W.Y. has received funding from Lundbeck and honoraria from Sunovion and Arena Pharmaceuticals. Neither S.G.B. nor N.R.S. receives or ever received support from companies that develop or market tolcapone. The remaining authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
The authors thank Sarah Lamb for her technical assistance in data collection, Michelle Breier for her assistance in data collation, and Maria Bongiovanni in manuscript preparation.
This work was supported by the Brain and Behavior Research Foundation (S.G.B.: NARSAD 2013 Young Investigator Award; N.R.S.: 2017 NARSAD Distinguished Investigator Award), the NIH (1KL2TR001444 to S.G.B.; MH59803 to N.R.S., G.A.L., J.W.Y.; MH94320 to N.R.S. and G.A.L.; and MH104344 to J.W.Y.), the VISN 22 MIRECC (S.G.B., G.A.L., J.W.Y.), and the Sidney R. Baer Jr Foundation (G.A.L.).
References
- Alfimova MV, Golimbet VE, Gritsenko IK, Lezheiko TV, Abramova LI, Strel’tsova MA, Khlopina IV, Ebstein R (2007) Interaction of dopamine system genes and cognitive functions in patients with schizophrenia and their relatives and in healthy subjects from the general population. Neurosci Behav Physiol 37:643–650. [DOI] [PubMed] [Google Scholar]
- Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, Alce G, Iudicello JE, Akbar N, Egan MF, Goldberg TE, Weinberger DR (2007) Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology 32:1011–1020. [DOI] [PubMed] [Google Scholar]
- Apud JA, Weinberger DR (2007) Treatment of cognitive deficits associated with schizophrenia: potential role of catechol-O-methyltransferase inhibitors. CNS Drugs 21:535–557. [DOI] [PubMed] [Google Scholar]
- Barch DM, Ceaser A (2012) Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci 1:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett AG, van der Pols JC, Dobson AJ (2005) Regression to the mean: what it is and how to deal with it. Int J Epidemiol 34:215–220. [DOI] [PubMed] [Google Scholar]
- Brandeis D, van Leeuwen TH, Rubia K, Vitacco D, Steger J, Pascual-Marqui RD, Steinhausen HC (1998) Neuroelectric mapping reveals precursor of stop failures in children with attention deficits. Behav Brain Res 94:111–125. [DOI] [PubMed] [Google Scholar]
- Chou HH, Talledo JA, Lamb SN, Thompson WK, Swerdlow NR (2013) Amphetamine effects on MATRICS Consensus cognitive battery performance in healthy adults. Psychopharmacology (Berl) 227:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D’Esposito M (2011) Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69:e113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse J, Jorga KM, Schmitt M, Gieschke R, Fotteler B, Zürcher G, DA Prada M, van Brummelen P (1995) Integrated pharmacokinetics and pharmacodynamics of the novel catechol-O-methyltransferase inhibitor tolcapone during first administration to humans. Clin Pharmacol Ther 57:508–517. [DOI] [PubMed] [Google Scholar]
- Ehlis AC, Reif A, Herrmann MJ, Lesch KP, Fallgatter AJ (2007) Impact of catechol-O-methyltransferase on prefrontal brain functioning in schizophrenia spectrum disorders. Neuropsychopharmacology 32:162–170. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Brandeis D, Strik WK (1997) A robust assessment of the NoGo-anteriorisation of P300 microstates in a cued continuous performance test. Brain Topogr 9:295–302. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Müller TJ (2001) Electrophysiological signs of reduced prefrontal response control in schizophrenic patients. Psychiatry Res 107:19–28. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Ehlis AC, Rösler M, Strik WK, Blocher D, Herrmann MJ (2005) Diminished prefrontal brain function in adults with psychopathology in childhood related to attention deficit hyperactivity disorder. Psychiatry Res 138:157–169. [DOI] [PubMed] [Google Scholar]
- Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ (2012) COMTVal158Met genotype determines the direction of cognitive effects produced by Catechol-O-Methyltransferase inhibition. Biol Psychiatry 71:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition. (SCID-I/NP): Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Frank MJ, Moustafa AA, Haughey HM, Curran T, Hutchison KE (2007) Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proc Natl Acad Sci USA 104:16311–16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E (1993) A neural system for error detection and compensation. Psychol Sci 4:385–390. [Google Scholar]
- Giakoumaki SG, Roussos P, Bitsios P (2008) Improvement of prepulse inhibition and executive function by the COMT inhibitor tolcapone depends on COMT Val158Met polymorphism. Neuropsychopharmacology 33:3058–3068. [DOI] [PubMed] [Google Scholar]
- González-Castro TB, Tovilla-Zárate C, Juárez-Rojop I, Pool García S, Genis A, Nicolini H, López Narváez L (2013) Distribution of the Val108/158Met polymorphism ofthe COMT gene in healthy Mexican population. Gene 526:454–458. [DOI] [PubMed] [Google Scholar]
- Green MF. (2016) Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J Clin Psychiatry 77:8–11. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RS, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR (2004) Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry 56:301–307. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Bowie CR (2012) Cognitive enhancement in schizophrenia: pharmacological and cognitive remediation approaches. Psychiatr Clin North Am 35:683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK (1998) Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12:426–445. [DOI] [PubMed] [Google Scholar]
- Heinzel S, Dresler T, Baehne CG, Heine M, Boreatti-Hümmer A, Jacob CP, Renner TJ, Reif A, Lesch KP, Fallgatter AJ, Ehlis AC (2013) COMT x DRD4 epistasis impacts prefrontal cortex function underlying response control. Cereb Cortex 23:1453–1462. [DOI] [PubMed] [Google Scholar]
- Heslenfeld DJ, Kenemans JL, Kok A, Molenaar PC (1997) Feature processing and attention in the human visual system: an overview. Biol Psychol 45:183–215. [DOI] [PubMed] [Google Scholar]
- Insel TR, Gogtay N (2014) National Institute of Mental Health clinical trials: new opportunities, new expectations. JAMA Psychiatry 71:745–756. [DOI] [PubMed] [Google Scholar]
- Jorga KM. (1998) Pharmacokinetics, pharmacodynamics, and tolerability of tolcapone: a review of early studies in volunteers. Neurology 50:31–38. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Kok A, Smulders FT (1993) Event-related potentials to conjunctions of spatial frequency and orientation as a function of stimulus parameters and response requirements. Electroencephalogr Clin Neurophysiol 88:51–63. [DOI] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR (2008) The MATRICS consensus cognitive battery, part 2: co-norming and standardization. Am J Psychiatry 165:214–220. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D’Esposito M, Farah MJ (1997) Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport 8:3581–3585. [DOI] [PubMed] [Google Scholar]
- Krugel LK, Biele G, Mohr PN, Li SC, Heekeren HR (2009) Genetic variation in dopaminergic neuromodulation influences the ability to rapidly and flexibly adapt decisions. Proc Natl Acad Sci USA 106:17951–17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS (2011) Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology 36:316–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223. [DOI] [PubMed] [Google Scholar]
- Lindholm E, Koriath JJ (1985) Analysis of multiple event related potential components in a tone discrimination task. Int J Psychophysiol 3:121–129. [DOI] [PubMed] [Google Scholar]
- Lyon M, Robbins TW (1975) The action of central nervous system stimulant drugs: a general theory concerning amphetamine effects. In Current developments in psychopharmacology (Essman WB, Valzelli L, eds), pp79–163. New York: Spectrum Publications. [Google Scholar]
- Magalona SC, Rasetti R, Chen J, Chen Q, Gold I, Decot H, Calicott JH, Berman KF, Apud JA, Weinberger DR, Mattay VS (2013) Effect of tolcapone on brain activity during a variable attentional control task: a double-blind, placebo-controlled, counter-balanced trial in healthy volunteers. CNS Drugs 27:663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR (2003) Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA 100:6186–6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna BS, Young JW, Dawes SE, Asgaard GL, Eyler LT (2013) Bridging the bench to bedside gap: validation of a reverse-translated rodent continuous performance test using functional magnetic resonance imaging. Psychiatry Res 212:183–191. [DOI] [PubMed] [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, Volavka J (2004). Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry 161:359–361. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, et al. (2008) The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry 165:203–213. [DOI] [PubMed] [Google Scholar]
- Olanow CW. (2000) Tolcapone and hepatotoxic effects. Tasmar Advisory Panel. Arch Neurol 57:263–267. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Burdick MC, Callicott JH, Weinberger DR (2014a) COMT-Dysbindin epistatic interaction. Mol Psychiatry 19:273. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Burdick MC, Callicott JH, Weinberger DR (2014b) Epistatic interaction between COMT and DTNBP1 modulates prefrontal function in mice and in humans. Mol Psychiatry 19:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. (1999) Tolcapone (Tasmar). CMAJ 160:1052–1056. [PMC free article] [PubMed] [Google Scholar]
- Romanos M, Ehlis AC, Baehne CG, Jacob C, Renner TJ, Storch A, Briegel W, Walitza S, Lesch KP, Fallgatter AJ (2010) Reduced NoGo-anteriorisation during continuous performance test in deletion syndrome 22q11.2. J Psychiatr Res 44:768–774. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR. (2012) Beyond antipsychotics: pharmacologically-augmented cognitive therapies (PACTs) for schizophrenia. Neuropsychopharmacology 37:310–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Stephany N, Wasserman LC, Talledo J, Shoemaker J, Auerbach PP (2003) Amphetamine effects on prepulse inhibition across-species: replication and parametric extension. Neuropsychopharmacology 28:640–650. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Grayson B, Marsh S, Hayward A, Marshall KM, Neill JC (2015) Putative therapeutic targets for symptom subtypes of adult ADHD: D4 receptor agonism and COMT inhibition improve attention and response inhibition in a novel translational animal model. Eur Neuropsychopharmacol 25:454–467. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, Belger A (2007) Application of electroencephalography to the study of cognitive and brain functions in schizophrenia. Schizophr Bull 33:955–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Enkhuizen J, Acheson D, Risbrough V, Drummond S, Geyer MA, Young JW (2014) Sleep deprivation impairs performance in the 5-choice continuous performance test: similarities between humans and mice. Behav Brain Res 261:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ (2006) Wide range achievement test – Fourth edition: Professional manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, Geyer MA (2009) The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One 4:e4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Scott CN, Zhou X, Geyer MA (2011) The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: separating response inhibition from premature responding. Behav Brain Res 222:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA, Rissling AJ, Sharp RF, Eyler LT, Asgaard GL, Light GA (2013) Reverse translation of the rodent 5C-CPT reveals that the impaired attention of people with schizophrenia is similar to scopolamine-induced deficits in mice. Transl Psychiatry 3:e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Bismark AW, Sin Y, McIlwain M, Grootendorst I, Light GA (2017) Neurophysiological characterization of attentional performance dysfunction in schizophrenia patients in a reverse-translated task. Neuropsychopharmacology [Epub ahead of print, Feb. 8]. doi: 10.1038/npp.2016.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.