Abstract
A number of investigators have reported that event-related augmentation of high-gamma activity70–110 Hz on electrocorticography (ECoG) can localize functionally-important brain regions in children and adults who undergo epilepsy surgery. The advantages of ECoG-based language mapping over the gold-standard stimulation include: (i) lack of stimulation-induced seizures, (ii) better sensitivity of localization of language areas in young children, and (iii) shorter patient participant time. Despite its potential utility, ECoG-based language mapping is far less commonly practiced than stimulation mapping. Here, we have provided video presentations to explain, point-by-point, our own hardware setting and time-frequency analysis procedures. We also have provided standardized auditory stimuli, in multiple languages, ready to be used for ECoG-based language mapping. Finally, we discussed the technical aspects of ECoG-based mapping, including its pitfalls, to facilitate appropriate interpretation of the data.
Keywords: High-frequency oscillations (HFOs), Ripples, Subdural electroencephalography (EEG), Intracranial electrocorticography (ECoG) recording, Pediatric epilepsy surgery, Language, Speech
1. Need of an alternative mapping tool in presurgical localization of the language areas
The ultimate goal of epilepsy surgery is to completely remove the epileptogenic zone while maximally preserving functionally-important brain areas including the primary language areas (Asano et al., 2013). In case noninvasive evaluation fails to satisfactorily localize these areas of interest, invasive presurgical evaluation is often employed with intracranial electrodes placed on the affected hemisphere for days to weeks (Lesser et al., 2010). The seizure onset zone responsible for habitual seizures and the spatial extent of neuroimaging abnormalities are determined for localization of the epileptogenic zone (Asano et al., 2009). Subsequently, most investigators localize the primary language areas using electrical stimulation, which is the current gold standard mapping method (Wang et al., 2016; Wellmer et al., 2009). In our institution, for example, we ask each patient to answer a question such as ‘What flies in the sky?’ or to name a picture of a common object, while a pair of neighboring subdural electrodes are stimulated with a frequency of 50 Hz for up to five seconds (Kojima et al., 2012; Nakai et al., 2017). Thereby, the stimulated site would be treated as a part of language or speech-related eloquent areas, if a given patient repeatedly fails to answer the question or develops a transient sensorimotor symptom such as tingling or twitching of the mouth. Further specification of the underlying cortical function may require additional tasks, such as syllable repetition or counting, during stimulation (Nakai et al., 2017). Electrical stimulation mapping is a powerful tool to localize the cortical areas essential in performing a given behavioral task, but carries several limitations as summarized in Table 1. Furthermore, the sensitivity of stimulation mapping for localization of the primary language areas might be suboptimal in young children, particularly in those younger than 10 years (Nakai et al., 2017; Schevon et al., 2007; Zea Vera et al., 2017). Unfortunately, non-invasive language mapping using functional MRI (fMRI) may be difficult to employ on young children who cannot minimize head motions during language tasks. Thus, an alternative brain mapping method feasible even in young children is highly desirable, especially one capable in providing accurate results without posing a risk of stimulation-induced seizures.
Table 1.
Technical aspects of two mapping methods.
| Electrical stimulation mapping via intracranial electrodes | ECoG-based brain mapping | |
|---|---|---|
| Patient participation time | Up to several hours. It varies based on the number of intracranial electrodes. | 5–15 minutes per task. |
| Investigator’s analysis time | Short. The results are available in a real-time manner. | Our offline time-frequency analysis, with bootstrap statistics applied, takes up to several hours. Real-time analysis has been reported by others (Miller et al., 2011; Roland et al., 2010; Wang et al., 2016). |
| Risk of non-habitual seizures induced by stimulation | Present (Aungaroon et al., 2017; Blume et al., 2004). | Absent. |
| Factors contributing to difficulty in data interpretation | Remote discharges during stimulation may result in a false positive localization of eloquent areas (Ishitobi et al., 2000; Karakis et al., 2015). Variance of patient’s attention across trials may affect the diagnostic validity. Unlike fMRI, one cannot assess the function of cortical areas not sampled with intracranial electrodes. | If not visually screened and excluded, electromyography (EMG) artifacts and interictal spike discharges might contribute to augmentation of high-gamma activity in an unwanted manner(Nagasawa et al., 2012). One cannot assess the function of cortical areas not sampled with intracranial electrodes. |
| Significance of the findings | It localizes the cortical sites essential to execute a given behavioral task. | It localizes the cortical sites engaged and disengaged in a behavioral task at a given moment(Nakai et al., 2017). |
2. Potential utility of ECoG-based language mapping as a complementary tool
Measurement of event-related high-gamma activity on ECoG can complement electrical stimulation mapping in localization of eloquent areas, including those supporting language function. Historically speaking, ECoG-based presurgical brain mapping was first applied to localize the primary motor area; self-paced movement elicited augmentation of the amplitude of broadband activity including 75–100 Hz (Crone et al., 1998). Such a novel mapping approach drew attention from investigators practicing epilepsy surgery, partly because it does not involve direct electrical stimulation of the cerebral cortex. Event-related high-gamma augmentation has been treated as an excellent summary measure of cortical activation at a given moment, since the amplitude of high-gamma activity is tightly correlated to the firing rate on single neuron recording (Manning et al., 2009; Ray et al., 2008; Whittingstall and Logothetis, 2009), hemodynamic activity on fMRI (Niessing et al., 2005; Shmuel et al., 2006; Scheeringa et al., 2011), and glucose metabolism on positron emission tomography (PET) (Nishida et al., 2008). A number of ECoG studies have demonstrated that cortical sites showing language-related high-gamma augmentation are more likely to be classified as language-related eloquent areas, defined by electrical stimulation mapping (Arya et al., 2017; Babajani-Feremi et al., 2016; Bauer et al., 2013; Genetti et al., 2015; Kojima et al., 2012; Miller et al., 2011; Mooji et al., 2016; Ruescher et al., 2013; Towle et al., 2008; Wang et al., 2016; Wu et al., 2010). Furthermore, surgical resection of cortical sites on the left hemisphere showing naming-related high-gamma augmentation frequently resulted in acute postoperative language impairment (Cervenka et al., 2013; Kojima et al., 2013a; 2013b; Figure 1). It remains to be determined how well measurement of high-gamma activity during naming tasks in either or both auditory and visual domains would improve prediction of long-term language outcome following surgery.
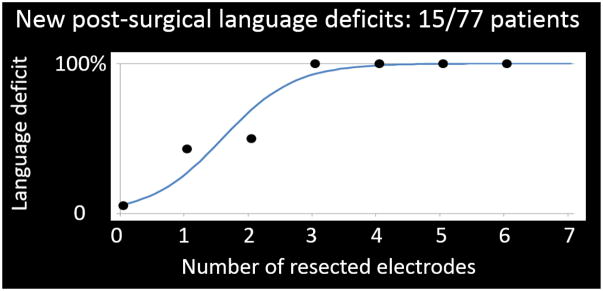
Figure 1. A logistic regression model predicted acute language impairment following epilepsy surgery.
Our ECoG study demonstrated the relationship between the extent of resected high-gamma active sites and the incidence of a post-operative language deficit requiring speech therapy (Kojima et al., 2013b). A total of 77 patients (age range: 4–56 years) were assigned an auditory naming task. The x-axis denotes the number of resected high-gamma active sites in the superior-temporal, inferior-frontal, dorsolateral-premotor, and inferior-Rolandic regions of the left hemisphere assumed to contain essential language function. Resection of each high-gamma active site increased the odds of acute language deficit by six times (odds ratio: 6.04; 95% confidence interval: 2.26–16.15). It should be noted that the left hemisphere was assumed to have essential language function, unless a given patient was left-handed and MRI showed a developmental lesion in the neocortex of the left hemisphere (Akanuma et al., 2003; Möddel et al., 2009; Rasmussen and Milner, 1977). We are fully aware that Wada test and fMRI are useful to lateralize language function, but these tests are not feasible in substantial proportions of young children. It still remains uncertain how well naming-related high-gamma activity can predict the long-term language outcome following epilepsy surgery.
The advantages of ECoG-based language mapping over stimulation mapping are summarized in Table 1. Since it is free from the risk of stimulation-induced non-habitual seizures, one can initiate the mapping procedure while a given patient is prone to develop seizures due to the reduction in interference from antiepileptic drugs. In our institution, many patients undergo extraoperative ECoG recording for 3–5 days. Regardless of antiepileptic medication status, we employ auditory and picture naming tasks typically within 48 hours of implantation of intracranial electrodes. The results of ECoG-based mapping are available for review prior to subsequent resection of the presumed epileptogenic zone. Each naming task is easy to complete and requires 5–15 minutes of patient participation. We have been successful in measuring naming-related high-gamma activity in children as young as 4 years old (Kojima et al., 2013a; 2013b; Nakai et al., 2017).
3. Limitations of language mapping using event-related high-gamma activity
The limitations of ECoG-based language mapping should be understood when this technique is implemented as epilepsy presurgical evaluation (Table 1). First of all, ECoG-based mapping is accompanied by the hardware settings for data acquisition and subsequent data analysis, which are more complicated compared to the gold-standard stimulation mapping. The technical pitfalls include potential misinterpretation of high-gamma augmentation derived from electromyography (EMG) artifacts. Although ECoG is >100 times more resilient to artifacts compared to scalp EEG recording (Ball et al., 2009), it may not be completely free from such artifacts (Nagasawa et al., 2012; Uematsu et al., 2013). Since EMG artifacts could be fairly time-locked to patient behaviors such as overt responses and saccadic eye movements following the onset of picture presentation (Cho-Hisamoto et al., 2015; Crouzet et al., 2010; Fletcher-Watson et al., 2008), cautious data interpretation is recommended (see the details in Chapter 4.6. below).
Naming-related high-gamma augmentation indicates that a given electrode site is activated, but such activation per se does not clarify if resection of the activated site will result in a clinically significant deficit. For example, the right superior-temporal gyrus frequently shows prominent high-gamma augmentation when listening to a sentence question (Nakai et al., 2017), but resection of such a gyrus in the non-dominant hemisphere very rarely causes disabling aphasia in patients with right temporal lobe epilepsy. Thus, additional information determining the language dominant hemisphere would be needed to develop a model to accurately predict the language outcome following epilepsy surgery, as suggested in Figure 1 (Kojima et al., 2013a; 2013b). The aforementioned limitations of ECoG-based language mapping have motivated us to write this technical article, in which we have provided video presentation of the hardware setting and time-frequency analysis for acquisition of high-quality data and proper interpretation of given high-gamma measures (Videos S1–S3).
4. Protocol of our ECoG-based language mapping
The rationale and procedures of ECoG recording, electrical stimulation mapping, and ECoG-based brain mapping are explained to the guardian and patient, and informed consent is obtained accordingly. Below, we present how we measure naming-related high-gamma augmentation to localize language-related eloquent areas for patients undergoing resective epilepsy surgery following extraoperative ECoG recording (Videos S1–S3). The following statements and video presentation include subtle or complex techniques involved in our brain mapping that are visible for other investigators to review or replicate.
4.1. Operational definition of high-gamma activity
High-gamma activity has been defined differently across studies, but the reported frequency band commonly included the range of 70–110 Hz (Arya et al., 2017; Babajani-Feremi et al., 2016; Bauer et al., 2013; Genetti et al., 2015; Kojima et al., 2012; Miller et al., 2011; Mooji et al., 2016; Ruescher et al., 2013; Towle et al., 2008; Wang et al., 2016; Wu et al., 2010). Since event-related amplitude augmentation involves a broadband frequency above 50 Hz (Miller et al., 2014), a slight difference in definition of high-gamma activity is estimated to have only a modest impact, if any, on the spatial-temporal characteristics of event-related amplitude augmentation. Below, we have defined high-gamma activity as that ranging from 70 to 110 Hz unless specified otherwise.
4.2. Subdural electrode placement and subsequent ECoG recording
Our general principle of invasive evaluation is to determine the boundary between the presumed epileptogenic zone and eloquent cortex, and to minimize the risk of under-sampling the true seizure onset zone (Nonoda et al., 2016). Subdural grid and strip electrodes are placed generously over the affected hemisphere based on the results of non-invasive presurgical evaluation using clinical profiles, seizure semiology, scalp EEG, MRI, and glucose-metabolism PET (Asano et al., 2009). Intraoperative photographs of the brain surface are taken to confirm the spatial relationship between electrodes and anatomical landmarks (Wellmer et al., 2002). To maintain the level of consciousness during chronic ECoG monitoring, we make the effort to prevent the intracranial pressure from rising, as presented in Figure 2.
Figure 2. Subdural electrode placement in a 10-year-old boy with drug-resistant focal epilepsy.
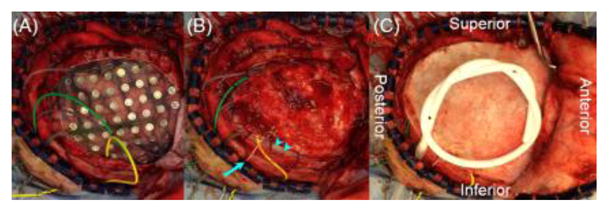
(A) Subdural grid and strip electrodes are placed on the right hemisphere. Electrode plates are stitched to both the edge of dura mater and adjacent electrodes to minimize the risk of unwanted shifting of subdural electrodes after dural closure. Electrode leads are tunneled about an inch away from the main wound to minimize the risk of infection associated with cerebrospinal fluid leakage. (B) The dura is closed in a semi-watertight manner to reduce the pressure to the cortex. Strips of dural repair patches (arrow-heads) are placed in the space between electrodes and dura to minimize the risk of blood draining under electrodes. An intracranial pressure monitor (arrow) is placed in the subdural space to readily detect intracranial hematoma or excessive brain swelling and to treat it accordingly. (C) The bone flap is replaced but not secured, to minimize an increase in the intracranial pressure. A sub-galeal drain is placed to reduce postoperative scalp swelling throughout the ECoG recording period. Intravenous antibiotics are given to reduce the risk of infection; none of our patients in our institution had an incidence of infection of subdural electrodes requiring premature termination of extraoperative ECoG recording.
Once subdural electrode placement is completed, each patient is transferred to the inpatient ward for extraoperative ECoG recording for localization of the seizure onset zone (Asano et al., 2009). Antiepileptic drugs may be reduced or discontinued until habitual seizures are captured and the seizure onset zone is satisfactorily delineated. Pain medications are given to each patient upon request. As long as a given patient is awake and comfortable, we initiate ECoG-based language mapping, either before or after habitual seizures are captured, but not during the postictal period. Conversely, we do not initiate stimulation-based language mapping until antiepileptic drugs are resumed with the epileptogenic zone satisfactorily estimated.
4.3. Recommended EEG recording system for measurement of naming-related high-gamma augmentation
The ECoG acquisition system should have a sampling rate of ≥400 Hz and a low-pass filter of ≥150 Hz to measure event-related high-gamma activity (Gliske et al., 2016; Kunii et al., 2013). In our institute, Nihon Kohden Neurofax EEG system (Nihon Kohden America Inc., Foothill Ranch, CA, USA) has been used to record ECoG signals with a sampling rate of 1,000 Hz and amplifier band-pass filter of 0.016–300 Hz. Though this sampling rate allows recording up to 500 Hz activity based on the Nyquist theory, the low-pass filter of 300 Hz (18dB/oct) attenuates cortical activity at 300 Hz by about one-third and makes the gain of activity above 300 Hz steeply fall to near zero. Thus, with this filter setting, it would be better to restrict the time-frequency analysis to below 300 Hz (Nariai et al., 2011; Nonoda et al., 2016).
4.4. Hardware setting for the auditory naming task
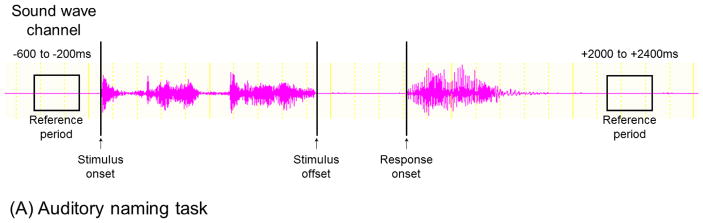
Video S1 shows, in detail, how to integrate the timing of the patient’s behaviors and ECoG signals. While comfortably seated with intracranial electrodes in place, each patient is assigned an auditory naming task at the bedside with extraneous noises minimized. Patients are instructed to overtly name a relevant answer for each question trial. For example, the patient may state ‘Bird’ when presented with ‘What flies in the sky?’. They are instructed to say ‘I don’t know’, in case they fail to generate an answer in mind at any trial. Sound-wave signals from both the speaker and patient are delivered to the DC input (Figure 3I) of the EEG acquisition system via a digital voice recorder (Figure 3G). This procedure effectively synchronizes ECoG and sound-wave signals recorded during the task, and allows us to denote the exact times of question onset, question offset, and response onset. Such ECoG/sound integration, compared to simultaneous video data, is more useful in time-frequency analysis. Since the sampling rate of video recording may vary around 60 Hz, each video frame may have a temporal uncertainty of 17 ms. Conversely, the sampling rate of the voice recorder is 44,100 Hz.
Figure 3. Our hardware setting for measurement of auditory and picture naming-related high-gamma augmentation.
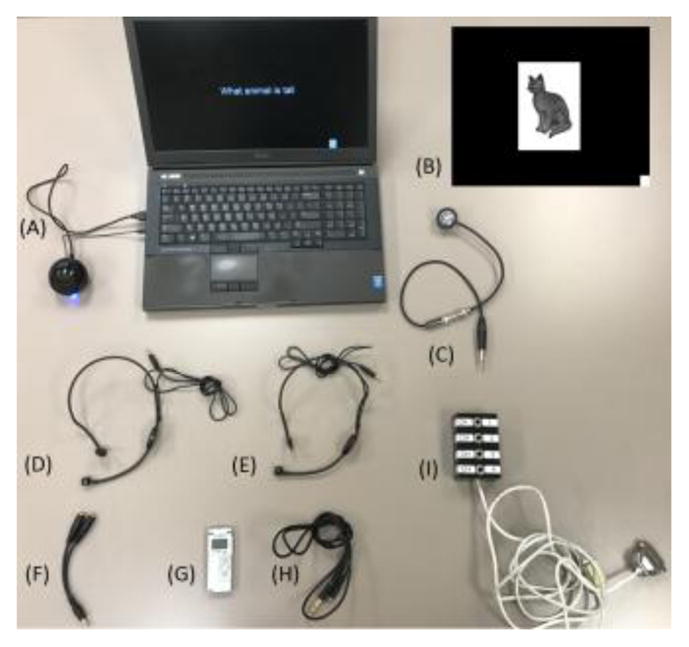
(A) A single regular laptop computer can deliver standardized auditory stimulus sets which can work through Microsoft PowerPoint software (Supplementary documents S1–S7). (B) An example of picture stimulus is presented. A small white square on the right lower corner appears at stimulus onset and disappears at stimulus offset. (C) The photosensor (Newport Research, Fountain Valley, CA, USA) accurately delivers the timing information of stimulus presentation to the DC input, by detecting a white square appearing together with each picture stimulus. (D and E) Hands-free microphones are secured in front of the patient’s mouth as well as in front of a computer speaker. (F) The bifurcated cable connects between microphones and the voice recorder. (G) Olympus Digital Voice Recorder (Olympus America Inc, Hauppauge, NY, USA) constantly delivers amplified sound signals to the DC input. (H) The stereo cable connects between the voice recorder and the DC input. (I) The DC input is a part of the Nihon Kohden ECoG acquisition system.
4.5. Hardware setting for the picture naming task
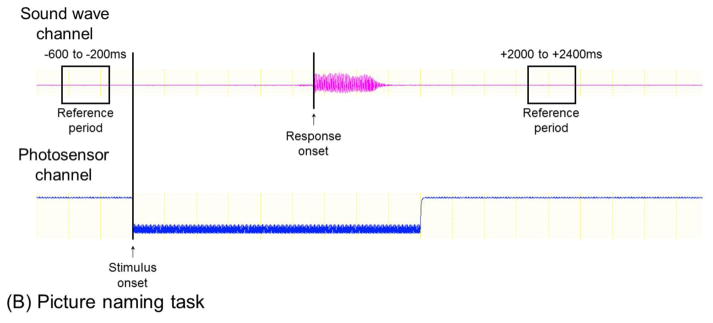
For measurement of picture naming-related high-gamma augmentation, we have utilized a set of stimuli consisting of 60 common line-drawn objects such as ‘cat’ and ‘desk’ (adopted from Rossion and Pourtois, 2004). Each picture stimulus has a small white square in the bottom right corner of the computer screen (Figure 3B). The onset and offset of picture presentation are detected as an increase and decrease of light intensity, respectively, by a photosensor (Figure 3C) attached to the corner of the LCD monitor. The onset of patient response is denoted using sound-wave signals synchronized with ECoG signals.
4.6. Time-frequency analysis and its potential pitfalls
Time-frequency analysis determines ‘when’, ‘at what channel’, ‘at what spectral frequency band’, and ‘how much’ the amplitude of ECoG signals are augmented or attenuated compared to that during the baseline/reference period between trials (Video S2). For this purpose, we utilize BESA EEG Analysis Software (BESA, Gräfelfing, Germany), which has been commercially available for the past two decades and widely used by clinicians and researchers. The detailed method to transform ECoG signals into time-frequency domain has been described elsewhere (Hoechstetter et al., 2004; Papp and Ktonas, 1977). Since our analytic method described here is not designed to generate the results in a real-time manner, it is not suitable for intraoperative language mapping (see Chapter 6. below).
Re-referencing original ECoG signals is a critical process prior to time-frequency software analysis, whereas there is no ideal method securing a true zero reference. In our institution, we record ECoG signals primarily using subdural disk electrodes; Nihon Kohden acquisition system treats the averaged voltage of ECoG signals derived from the fifth and sixth subdural electrodes connected to the amplifier as the default reference (Fukuda et al., 2010). Investigators using other acquisition systems often treat a subdural strip electrode facing the dura or skull as the default reference (Arya et al., 2015). ECoG signals are then re-montaged to a common average reference (Arya et al., 2015; Fukuda et al., 2010); thereby, channels affected by artifacts or interictal spike discharges should be excluded from averaging for calculating the reference voltage. This exclusion procedure is needed to prevent all channels from equally suffering from random noises not related to language function.
When depth electrodes are used for ECoG recording, time-frequency analysis is performed most frequently on bipolar montage with neighboring electrode pairs (Bagshaw et al., 2009; Burke et al., 2014; Mainy et al., 2007). Such a practice might be partly attributed to the notion that depth electrodes, compared to disk electrodes, typically have larger impedance because of the smaller contact areas (Pail et al., 2013). While bipolar montage may not directly discriminate which electrode within a pair elicits high-gamma augmentation, it can reduce contamination of EMG signals from a distant source (Jerbi et al., 2009; Kovach et al., 2011; Nagasawa et al., 2011; Worrell et al., 2012). A recent ECoG study using depth electrodes suggested that electrodes within the white matter also receive signals generated by both nearby and distant gray matters (Mercier et al., 2017); thus, one cannot assume that depth electrodes within the white matter can provide a zero reference.
The strengths of our time-frequency analysis include assessment of cortical activation time-locked to stimulus presentation and patient response (Figure 4). The temporal relationship between amplitude augmentation and patient behavior can better clarify the underlying function of a given site showing event-related high-gamma augmentation (Nakai et al., 2017). For example, high-gamma augmentation immediately following the onset of auditory stimulus presentation is likely to reflect perceptual processing more than motor processing, whereas high-gamma augmentation around response onset is likely to reflect motor processing. High-gamma augmentation in the association cortex immediately following stimulus offset likely reflects cognitive processing to understand the question or generate the relevant answer for it. Such temporal dynamics of cortical activation may be difficult to obtain with fMRI, which has a temporal resolution of seconds.
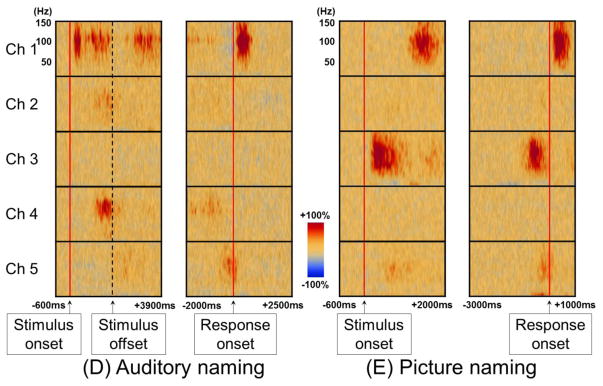
Figure 4. Time-frequency analysis to measure auditory and picture naming-related high-gamma augmentation.
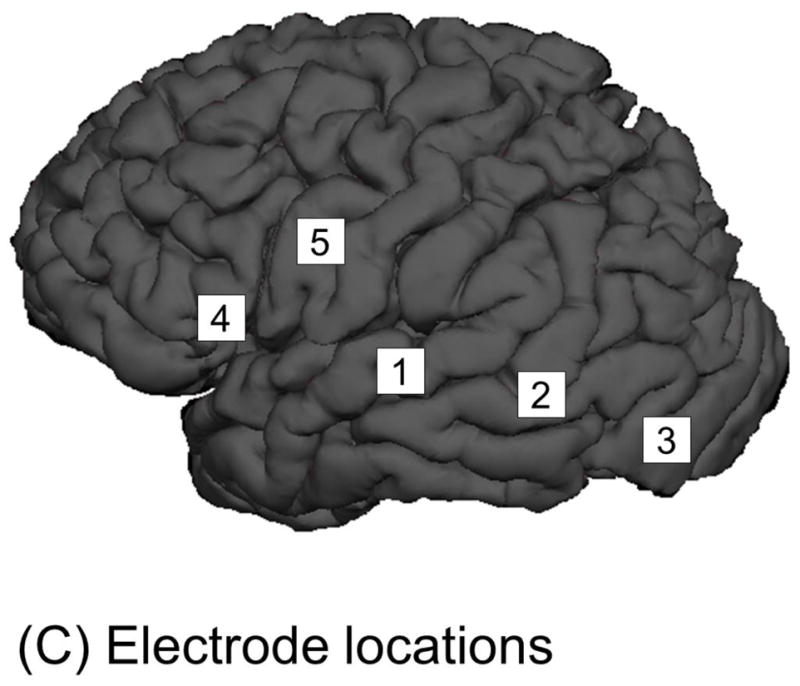
A 12-year-old boy was assigned auditory and picture naming tasks. (A) For analysis of auditory naming-related high-gamma activity, stimulus onset and response onset are visually marked using sound-wave signals integrated into ECoG recording. Users of our standardized auditory stimuli (Supplementary documents S1–S7) do not have to manually mark stimulus offset, since all stimuli have 1.8-s duration. For analysis time-locked to stimulus onset, one can define a period 200–600 ms prior to stimulus onset as the reference (Kojima et al., 2013b). For analysis time-locked to response onset, one can define a silent period of 400 ms several seconds after response onset. We make sure that the defined reference period between trials are not affected by unwanted noises. (B) For analysis of picture naming-related high-gamma activity, response onset is determined using sound-wave signals. Stimulus onset is determined using a deflection of photosensor signal (Figure 3C; Video S1). (C) The locations of subdural electrodes are denoted. (D) The spatial-temporal dynamics of auditory naming-related high-gamma activity is presented. High-gamma augmentation at Channel 2 in the left middle-temporal gyrus is better appreciated in the analysis time-locked to stimulus onset/offset, whereas that at Channel 5 in the precentral gyrus is best seen in that time-locked to response onset. (E) The spatial-temporal dynamics of picture naming-related high-gamma activity suggests that the picture naming task barely elicited high-gamma modulation at Channel 4 in the inferior-frontal gyrus.
Specifically, for measurement of auditory naming-related high-gamma activity, the next analytic procedure is marking of stimulus onset, stimulus offset, and response onset on ECoG signals, based on the sound-wave signals (Figure 4A; Video S2). Likewise, for picture naming, stimulus and response onsets need to be marked (Figure 4B). On the software platform, we then define the reference period between trials. At each channel, in steps of 5 Hz and 10 ms, we determine how much the amplitude of a given ECoG activity is increased or decreased compared to that during the reference period (Figures 4D–4E). The aforementioned procedures take up to 1 hour of investigator’s time based on user experience. This can be followed by the studentized bootstrap statistics to determine if amplitude modulation at each 5-Hz and 10-ms time-frequency bin reaches significance; this statistical analysis may require >1 hour of unmanned computing time, based on the number of channels and trials, the sampling rate of ECoG acquisition, and the central processing unit/random access memory (CPU/RAM) of the analysis computer. On our ordinary PC computer, each channel requires approximately 30–60 seconds of calculation time for the bootstrap statistics.
One should make the best effort to minimize the risk of misinterpretation of high-gamma augmentation spuriously induced by EMG artifacts, which preferentially take place at subdural electrodes placed on the anterior temporal and peri-orbital regions (Figure 5; Jerbi et al., 2009; Kovach et al., 2011; Nagasawa et al., 2011; Uematsu et al., 2013). The temporal muscles are one of the major sources of augmentation of broadband activity (including 70–110 Hz) particularly during overt response. Saccade movements may induce discernible ocular muscle-induced augmentation of broadband activity preferentially at 100–300 ms following the onset of picture presentation (Cho-Hisamoto et al., 2015; Crouzet et al., 2010; Fletcher-Watson et al., 2008). It should be noted that such involuntary saccades cannot be eliminated, by asking patients to fixate their gaze during the entire naming session. Unfortunately, EMG artifacts could be fairly time-locked to stimulus onset or response onset, and might increase the risk of data misinterpretation. Thus, we take the following approach to facilitate proper interpretation of event-related high-gamma augmentation. First, we determine the locations of electrodes prone to artifacts in advance, by observing EMG contamination on ECoG signals during jaw clenching and saccades (Modur et al., 2011; Nagasawa et al., 2011). High-gamma augmentation is treated as artifacts, in case visual assessment of ECoG traces confirms similar EMG contamination during the task or time-frequency analysis on bipolar montage with neighboring electrodes fails to replicate the similar finding (Cho-Hisamoto et al., 2015; Kovach et al., 2011). We routinely record electrooculography (EOG) during chronic ECoG recording to determine the seizure semiology and sleep stage (Bagshaw et al., 2009; Nonoda et al., 2016). We have found that EOG is useful in determining the temporal coupling between high-gamma augmentation on ECoG traces and EMG artifacts recorded on the face (Figure 5; Cho-Hisamoto et al., 2015; Uematsu et al., 2013). Some ECoG investigators have employed independent component analysis (ICA) filtering to reduce saccade-related EMG artifacts, whereas it has been suggested that such filtering, to some extent, alters the time and phase measures present in the original traces (Wallstrom et al., 2004). Further studies are warranted to find an optimal solution to prevent or remove EMG artifacts.
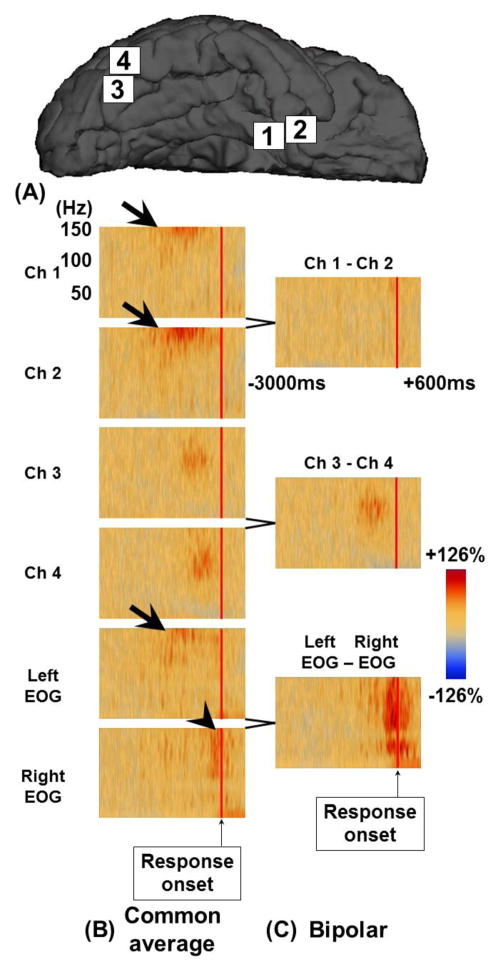
Figure 5. The effects of EMG artifacts on ECoG signals during picture naming task.
A 15-year-old girl was assigned a picture naming task. (A) The locations of subdural electrodes are denoted. (B and C) The results of time-frequency analysis time-locked to response onset are presented using two different montages. On common average montage, Channels 1 and 2 at the right anterior-medial temporal lobe showed amplitude augmentation of activity at ≥100 Hz prior to response onset (thick arrows). Such amplitude augmentation was not replicated on bipolar montage; thus, this amplitude augmentation is likely to reflect artifactual signals from a distant source. Indeed, left electrooculography (EOG) showed similar amplitude augmentation at the same time. In addition, right EOG showed broadband amplitude augmentation related to EMG artifacts during overt response (arrowhead). Channels 3 and 4 at the ventral occipital-temporal region showed augmentation of high-gamma activity at 70–110 Hz, prior to response onset, on both common average and bipolar montage. Thus, high-gamma augmentation at these channels is likely to be of cortical origin.
One should also take into account the effect of interictal spikes on naming-related high-gamma activity. To reduce such unwanted effects, we exclude trials affected by interictal spikes from time-frequency analysis. Failure to do so may result in spurious augmentation of high-gamma activity, since interictal spikes are accompanied by brief augmentation of broadband activity including 70–110 Hz (Jacobs et al., 2011; Zijlmans et al., 2011). Since interictal spikes are very rarely time-locked to stimulus presentation or patient response, the effect of spike-related broadband activity on naming-related high-gamma augmentation most frequently appears negligible on statistical analysis (Figure 6).
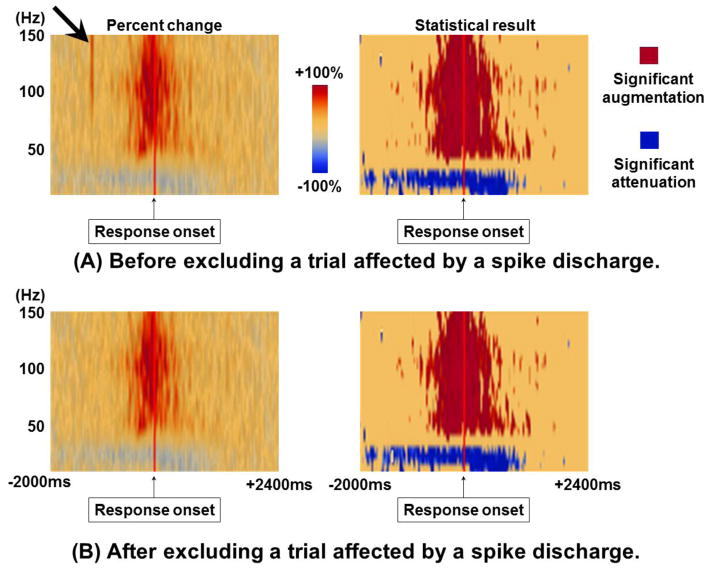
Figure 6. Effect of interictal spikes on time-frequency ECoG analysis.
A 17-year-old boy was assigned an auditory naming task (Nakai et al., 2017), and the temporal dynamics of ECoG amplitude modulation at a left precentral site is shown. (A) Presented are the results of time-frequency analysis including a trial affected by an interictal spike discharge. The plot on the left side shows the percent change in amplitude compared to the baseline period prior to stimulus onset. Immediately following stimulus onset, brief amplitude augmentation of broadband activity was noted (thick arrow); this effect was eliminated by excluding the trial affected by a spike discharge. The plot on the right side shows the statistical plot following the studentized bootstrapping statistics incorporated in BESA software (p<0.05 following Simes correction). The interictal spike discharge did not contribute to statistically-significant augmentation of high-gamma activity. (B) Presented are the results of time-frequency analysis with the trial affected by the spike discharge excluded.
5. Standardized auditory stimuli as open-source materials
To our best knowledge, unlike picture stimuli (e.g.: Rossion and Pourtois, 2004), only few standardized auditory language stimuli have been available for presurgical evaluation. Here, we provide standardized stimuli, in an open-access manner, to investigators who are considering implementation of ECoG-based language mapping (Supplementary documents S1–S7). We have created 100 auditory stimuli for each of English-, Arabic-, Hindi-, and Japanese-speaking patients. Stimuli in other languages can be provided later as a part of post-publication Letters to the Editor. Each stimulus consists of a 1.8-second sentence question articulated by female speakers; for example, ‘What flies in the sky?’ in each of the four languages. The patient is expected to answer each of the questions with an appropriate noun such as ‘Bird’ or ‘Plane’. Questions in English begin with either ‘What’, ‘When’, ‘Where’ or ‘Who’, and are also translated to Arabic, Hindi, and Japanese (Supplementary Table S1). We made our best effort to make the questions as universal and simple as possible so that patients can understand and answer them regardless of gender, age, culture, or religion. We designed all stimulus sets to work through Microsoft PowerPoint software; thus, most investigators can deliver auditory stimuli using a regular laptop computer. Patients are instructed to say: ‘I don’t know’, in case they fail to generate an answer in mind at any trial. For young children with limited vocabulary, we generally ask the family member to customize a set of personalized questions likely to be answered by a given child; for example, ‘What is the name of your dog?’. An investigator or family member can directly deliver questions with a hands-free microphone (Figure 3E), instead of delivering auditory stimuli through a speaker.
6. Future directions
While ECoG-based language mapping has been suggested to predict acute language impairment (Figure 1), it remains uncertain if this mapping is useful in predicting long-term outcome following surgical resection. It also remains to be determined if the extent of resection of high-gamma active sites is indeed correlated to postoperative neuropsychological measures. Further studies on large patient populations are needed to address these questions.
Real-time ECoG-based language mapping has been introduced by other investigators (e.g.: Miller et al., 2011; Roland et al., 2010; Wang et al., 2016), and these studies reported that cortical sites showing event-related high-gamma augmentation were frequently classified as language-related areas by electrical stimulation mapping. Accurate and automatic detection of patient behaviors and accurate detection of artifactual signals would further improve the utility of such real-time functional mapping. Even with hardware and software further improved in the near future, we would still recommend quality assurance by visual assessment of raw ECoG signals, to reduce the risk of misinterpreting artifactual high-gamma augmentation as significant.
Supplementary Material
Supplementary PowerPoint Document S1: Question stimuli in English made by C.K. (a native English speaker)
Supplementary Table S1: List of auditory stimuli.
This video does not present any patient. All persons presented in this video give full permission for the publication.
This video does not present any patient. All persons presented in this video give full permission for the publication.
This video does not present any patient. All persons presented in this video give full permission for the publication.
Supplementary PowerPoint Document S2: Question stimuli in Hindi made by D.R., (a bilingual speaker)
Supplementary PowerPoint Document S3: Question stimuli in English made by D.R. (a bilingual speaker)
Supplementary PowerPoint Document S4: Question stimuli in Arabic made by Z.A. (a bilingual speaker)
Supplementary PowerPoint Document S5: Question stimuli in English made by Z.A. (a bilingual speaker)
Supplementary PowerPoint Document S6: Question stimuli in Japanese made by A.H. (a bilingual speaker)
Supplementary PowerPoint Document S7: Question stimuli in English made by A.H. (a bilingual speaker)
HIGHLIGHTS.
We provide video presentations for ECoG-based language mapping.
Brain mapping with event-related high-gamma activity has pitfalls to be avoided.
We provide standardized auditory stimuli as open-source materials.
Acknowledgments
This work was supported by NIH grant NS64033 (to E. Asano). We are grateful to Jeong-won Jeong, PhD, Hirotaka Motoi, MD, Ayaka Sugiura, PhD, and Karin Halsey, REEG/EPT at Children’s Hospital of Michigan, Wayne State University for the collaboration and assistance in performing the studies described above.
Footnotes
CONFLICT OF INTEREST STATEMENT
None of the authors have potential conflicts of interest to be disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akanuma N, Alarcón G, Lum F, Kissani N, Koutroumanidis M, Adachi N, Binnie CD, Polkey CE, Morris RG. Lateralising value of neuropsychological protocols for presurgical assessment of temporal lobe epilepsy. Epilepsia. 2003;44:408–18. doi: 10.1046/j.1528-1157.2003.24502.x. [DOI] [PubMed] [Google Scholar]
- Arya R, Wilson JA, Vannest J, Byars AW, Greiner HM, Buroker J, Fujiwara H, Mangano FT, Holland KD, Horn PS, Crone NE, Rose DF. Electrocorticographic language mapping in children by high-gamma synchronization during spontaneous conversation: comparison with conventional electrical cortical stimulation. Epilepsy Res. 2015;110:78–87. doi: 10.1016/j.eplepsyres.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Asano E, Brown EC, Juhász C. How to establish causality in epilepsy surgery. Brain Dev. 2013;35:706–20. doi: 10.1016/j.braindev.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Juhász C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009;132:1038–47. doi: 10.1093/brain/awp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aungaroon G, Zea Vera A, Horn PS, Byars AW, Greiner HM, Tenney JR, Arthur TM, Crone NE, Holland KD, Mangano FT, Arya R. After-discharges and seizures during pediatric extra-operative electrical cortical stimulation functional brain mapping: Incidence, thresholds, and determinants. Clin Neurophysiol. 2017;128:2078–2086. doi: 10.1016/j.clinph.2017.06.259. [DOI] [PubMed] [Google Scholar]
- Babajani-Feremi A, Narayana S, Rezaie R, Choudhri AF, Fulton SP, Boop FA, Wheless JW, Papanicolaou AC. Language mapping using high gamma electrocorticography, fMRI, and TMS versus electrocortical stimulation. Clin Neurophysiol. 2016;127:1822–36. doi: 10.1016/j.clinph.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2009;50:617–28. doi: 10.1111/j.1528-1167.2008.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T, Kern M, Mutschler I, Aertsen A, Schulze-Bonhage A. Signal quality of simultaneously recorded invasive and non-invasive EEG. Neuroimage. 2009;46:708–16. doi: 10.1016/j.neuroimage.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Bauer PR, Vansteensel MJ, Bleichner MG, Hermes D, Ferrier CH, Aarnoutse EJ, Ramsey NF. Mismatch between electrocortical stimulation and electrocorticography frequency mapping of language. Brain Stimul. 2013;6:524–31. doi: 10.1016/j.brs.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Blume WT, Jones DC, Pathak P. Properties of after-discharges from cortical electrical stimulation in focal epilepsies. Clin Neurophysiol. 2004;115:982–9. doi: 10.1016/j.clinph.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Burke JF, Long NM, Zaghloul KA, Sharan AD, Sperling MR, Kahana MJ. Human intracranial high-frequency activity maps episodic memory formation in space and time. Neuroimage. 2014;85:834–43. doi: 10.1016/j.neuroimage.2013.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka MC, Corines J, Boatman-Reich DF, Eloyan A, Sheng X, Franaszczuk PJ, Crone NE. Electrocorticographic functional mapping identifies human cortex critical for auditory and visual naming. Neuroimage. 2013;69:267–76. doi: 10.1016/j.neuroimage.2012.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho-Hisamoto Y, Kojima K, Brown EC, Matsuzaki N, Asano E. Gamma activity modulated by naming of ambiguous and unambiguous images: intracranial recording. Clin Neurophysiol. 2015;126:17–26. doi: 10.1016/j.clinph.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet SM, Kirchner H, Thorpe SJ. Fast saccades toward faces: face detection in just 100 ms. J Vis. 2010;10:16.1–17. doi: 10.1167/10.4.16. [DOI] [PubMed] [Google Scholar]
- Fletcher-Watson S, Findlay JM, Leekam SR, Benson V. Rapid detection of person information in a naturalistic scene. Perception. 2008;37:571–83. doi: 10.1068/p5705. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Rothermel R, Juhász C, Nishida M, Sood S, Asano E. Cortical gamma-oscillations modulated by listening and overt repetition of phonemes. Neuroimage. 2010;49:2735–45. doi: 10.1016/j.neuroimage.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetti M, Tyrand R, Grouiller F, Lascano AM, Vulliemoz S, Spinelli L, Seeck M, Schaller K, Michel CM. Comparison of high gamma electrocorticography and fMRI with electrocortical stimulation for localization of somatosensory and language cortex. Clin Neurophysiol. 2015;126:121–30. doi: 10.1016/j.clinph.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Gliske SV, Irwin ZT, Chestek C, Stacey WC. Effect of sampling rate and filter settings on High Frequency Oscillation detections. Clin Neurophysiol. 2016;127:3042–50. doi: 10.1016/j.clinph.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004;16:233–8. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Ishitobi M, Nakasato N, Suzuki K, Nagamatsu K, Shamoto H, Yoshimoto T. Remote discharges in the posterior language area during basal temporal stimulation. Neuroreport. 2000;11:2997–3000. doi: 10.1097/00001756-200009110-00034. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kobayashi K, Gotman J. High-frequency changes during interictal spikes detected by time-frequency analysis. Clin Neurophysiol. 2011;122:32–42. doi: 10.1016/j.clinph.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K, Freyermuth S, Dalal S, Kahane P, Bertrand O, Berthoz A, Lachaux JP. Saccade related gamma-band activity in intracerebral EEG: dissociating neural from ocular muscle activity. Brain Topogr. 2009;22:18–23. doi: 10.1007/s10548-009-0078-5. [DOI] [PubMed] [Google Scholar]
- Karakis I, Leeman-Markowski BA, Leveroni CL, Kilbride RD, Cash SS, Eskandar EN, Simon MV. Intra-stimulation discharges: an overlooked cortical electrographic entity triggered by direct electrical stimulation. Clin Neurophysiol. 2015;126:882–8. doi: 10.1016/j.clinph.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Matsuzaki N, Rothermel R, Fuerst D, Shah A, Mittal S, Sood S, Asano E. Gamma activity modulated by picture and auditory naming tasks: intracranial recording in patients with focal epilepsy. Clin Neurophysiol. 2013a;124:1737–44. doi: 10.1016/j.clinph.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Rothermel R, Carlson A, Fuerst D, Matsuzaki N, Shah A, Atkinson M, Basha M, Mittal S, Sood S, Asano E. Clinical significance and developmental changes of auditory-language-related gamma activity. Clin Neurophysiol. 2013b;124:857–69. doi: 10.1016/j.clinph.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Rothermel R, Carlson A, Matsuzaki N, Shah A, Atkinson M, Mittal S, Fuerst D, Sood S, Asano E. Multimodality language mapping in patients with left-hemispheric language dominance on Wada test. Clin Neurophysiol. 2012;123:1917–24. doi: 10.1016/j.clinph.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach CK, Tsuchiya N, Kawasaki H, Oya H, Howard MA, 3rd, Adolphs R. Manifestation of ocular-muscle EMG contamination in human intracranial recordings. Neuroimage. 2011;54:213–33. doi: 10.1016/j.neuroimage.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunii N, Kamada K, Ota T, Kawai K, Saito N. Characteristic profiles of high gamma activity and blood oxygenation level-dependent responses in various language areas. Neuroimage. 2013;65:242–9. doi: 10.1016/j.neuroimage.2012.09.059. [DOI] [PubMed] [Google Scholar]
- Lesser RP, Crone NE, Webber WR. Subdural electrodes. Clin Neurophysiol. 2010;121:1376–92. doi: 10.1016/j.clinph.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainy N, Kahane P, Minotti L, Hoffmann D, Bertrand O, Lachaux JP. Neural correlates of consolidation in working memory. Hum Brain Mapp. 2007;28:183–93. doi: 10.1002/hbm.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29:13613–20. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier MR, Bickel S, Megevand P, Groppe DM, Schroeder CE, Mehta AD, Lado FA. Evaluation of cortical local field potential diffusion in stereotactic electro-encephalography recordings: A glimpse on white matter signal. Neuroimage. 2017;147:219–232. doi: 10.1016/j.neuroimage.2016.08.037. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Abel TJ, Hebb AO, Ojemann JG. Rapid online language mapping with electrocorticography. J Neurosurg Pediatr. 2011;7:482–90. doi: 10.3171/2011.2.PEDS1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Honey CJ, Hermes D, Rao RP, denNijs M, Ojemann JG. Broadband changes in the cortical surface potential track activation of functionally diverse neuronal populations. Neuroimage. 2014;85:711–20. doi: 10.1016/j.neuroimage.2013.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möddel G, Lineweaver T, Schuele SU, Reinholz J, Loddenkemper T. Atypical language lateralization in epilepsy patients. Epilepsia. 2009;50:1505–16. doi: 10.1111/j.1528-1167.2008.02000.x. [DOI] [PubMed] [Google Scholar]
- Modur PN, Zhang S, Vitaz TW. Ictal high-frequency oscillations in neocortical epilepsy: implications for seizure localization and surgical resection. Epilepsia. 2011;52:1792–801. doi: 10.1111/j.1528-1167.2011.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooij AH, Huiskamp GJ, Gosselaar PH, Ferrier CH. Electrocorticographic language mapping with a listening task consisting of alternating speech and music phrases. Clin Neurophysiol. 2016;127:1113–9. doi: 10.1016/j.clinph.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Matsuzaki N, Juhász C, Hanazawa A, Shah A, Mittal S, Sood S, Asano E. Occipital gamma-oscillations modulated during eye movement tasks: simultaneous eye tracking and electrocorticography recording in epileptic patients. Neuroimage. 2011;58:1101–9. doi: 10.1016/j.neuroimage.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y, Jeong JW, Brown EC, Rothermel R, Kojima K, Kambara T, Shah A, Mittal S, Sood S, Asano E. Three- and four-dimensional mapping of speech and language in patients with epilepsy. Brain. 2017;140:1351–70. doi: 10.1093/brain/awx051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nariai H, Nagasawa T, Juhász C, Sood S, Chugani HT, Asano E. Statistical mapping of ictal high-frequency oscillations in epileptic spasms. Epilepsia. 2011;52:63–74. doi: 10.1111/j.1528-1167.2010.02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–51. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Nishida M, Juhász C, Sood S, Chugani HT, Asano E. Cortical glucose metabolism positively correlates with gamma-oscillations in nonlesional focal epilepsy. Neuroimage. 2008;42:1275–84. doi: 10.1016/j.neuroimage.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoda Y, Miyakoshi M, Ojeda A, Makeig S, Juhász C, Sood S, Asano E. Interictal high-frequency oscillations generated by seizure onset and eloquent areas may be differentially coupled with different slow waves. Clin Neurophysiol. 2016;127:2489–99. doi: 10.1016/j.clinph.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pail M, Halámek J, Daniel P, Kuba R, Tyrlíková I, Chrastina J, Jurák P, Rektor I, Brázdil M. Intracerebrally recorded high frequency oscillations: simple visual assessment versus automated detection. Clin Neurophysiol. 2013;124:1935–42. doi: 10.1016/j.clinph.2013.03.032. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–45. [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–69. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28:11526–36. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland J, Brunner P, Johnston J, Schalk G, Leuthardt EC. Passive real-time identification of speech and motor cortex during an awake craniotomy. Epilepsy Behav. 2010;18:123–8. doi: 10.1016/j.yebeh.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart’s object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33:217–36. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Ruescher J, Iljina O, Altenmüller DM, Aertsen A, Schulze-Bonhage A, Ball T. Somatotopic mapping of natural upper- and lower-extremity movements and speech production with high gamma electrocorticography. Neuroimage. 2013;81:164–77. doi: 10.1016/j.neuroimage.2013.04.102. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Fries P, Petersson KM, Oostenveld R, Grothe I, Norris DG, Hagoort P, Bastiaansen MC. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69:572–83. doi: 10.1016/j.neuron.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Schevon CA, Carlson C, Zaroff CM, Weiner HJ, Doyle WK, Miles D, Lajoie J, Kuzniecky R, Pacia S, Vazquez B, Luciano D, Najjar S, Devinsky O. Pediatric language mapping: sensitivity of neurostimulation and Wada testing in epilepsy surgery. Epilepsia. 2007;48:539–45. doi: 10.1111/j.1528-1167.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9:569–77. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Towle VL, Yoon HA, Castelle M, Edgar JC, Biassou NM, Frim DM, Spire JP, Kohrman MH. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008;131:2013–27. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu M, Matsuzaki N, Brown EC, Kojima K, Asano E. Human occipital cortices differentially exert saccadic suppression: Intracranial recording in children. Neuroimage. 2013;83:224–36. doi: 10.1016/j.neuroimage.2013.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallstrom GL, Kass RE, Miller A, Cohn JF, Fox NA. Automatic correction of ocular artifacts in the EEG: a comparison of regression-based and component-based methods. Int J Psychophysiol. 2004;53:105–19. doi: 10.1016/j.ijpsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fifer MS, Flinker A, Korzeniewska A, Cervenka MC, Anderson WS, Boatman-Reich DF, Crone NE. Spatial-temporal functional mapping of language at the bedside with electrocorticography. Neurology. 2016;86:1181–9. doi: 10.1212/WNL.0000000000002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer J, von Oertzen J, Schaller C, Urbach H, König R, Widman G, Van Roost D, Elger CE. Digital photography and 3D MRI-based multimodal imaging for individualized planning of resective neocortical epilepsy surgery. Epilepsia. 2002;43:1543–50. doi: 10.1046/j.1528-1157.2002.30002.x. [DOI] [PubMed] [Google Scholar]
- Wellmer J, Weber C, Mende M, von der Groeben F, Urbach H, Clusmann H, Elger CE, Helmstaedter C. Multitask electrical stimulation for cortical language mapping: hints for necessity and economic mode of application. Epilepsia. 2009;50:2267–75. doi: 10.1111/j.1528-1167.2009.02192.x. [DOI] [PubMed] [Google Scholar]
- Whittingstall K, Logothetis NK. Frequency-band coupling in surface EEG reflects spiking activity in monkey visual cortex. Neuron. 2009;64:281–9. doi: 10.1016/j.neuron.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Jerbi K, Kobayashi K, Lina JM, Zelmann R, Le Van Quyen M. Recording and analysis techniques for high-frequency oscillations. Prog Neurobiol. 2012;98:265–78. doi: 10.1016/j.pneurobio.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Wisneski K, Schalk G, Sharma M, Roland J, Breshears J, Gaona C, Leuthardt EC. Electrocorticographic frequency alteration mapping for extraoperative localization of speech cortex. Neurosurgery. 2010;66:E407–9. doi: 10.1227/01.NEU.0000345352.13696.6F. [DOI] [PubMed] [Google Scholar]
- Zea Vera A, Aungaroon G, Horn PS, Byars AW, Greiner HM, Tenney JR, Arthur TM, Crone NE, Holland KD, Mangano FT, Arya R. Language and motor function thresholds during pediatric extra-operative electrical cortical stimulation brain mapping. Clin Neurophysiol. 2017;128:2087–2093. doi: 10.1016/j.clinph.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Kahn YU, Zelmann R, Dubeau F, Gotman J. Ictal and interictal high frequency oscillations in patients with focal epilepsy. Clin Neurophysiol. 2011;122:664–71. doi: 10.1016/j.clinph.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary PowerPoint Document S1: Question stimuli in English made by C.K. (a native English speaker)
Supplementary Table S1: List of auditory stimuli.
This video does not present any patient. All persons presented in this video give full permission for the publication.
This video does not present any patient. All persons presented in this video give full permission for the publication.
This video does not present any patient. All persons presented in this video give full permission for the publication.
Supplementary PowerPoint Document S2: Question stimuli in Hindi made by D.R., (a bilingual speaker)
Supplementary PowerPoint Document S3: Question stimuli in English made by D.R. (a bilingual speaker)
Supplementary PowerPoint Document S4: Question stimuli in Arabic made by Z.A. (a bilingual speaker)
Supplementary PowerPoint Document S5: Question stimuli in English made by Z.A. (a bilingual speaker)
Supplementary PowerPoint Document S6: Question stimuli in Japanese made by A.H. (a bilingual speaker)
Supplementary PowerPoint Document S7: Question stimuli in English made by A.H. (a bilingual speaker)