Abstract
Objective
We prospectively examined whether women with physician-diagnosed restless legs syndrome (RLS) had a higher risk of total and cardiovascular disease (CVD) mortality relative to those without RLS.
Methods
The current study included 57,417 women (mean age 67 years) from the Nurses' Health Study without cancer, renal failure, and CVD at baseline (2002). Main outcomes were total and CVD mortality. We used the Cox proportional hazards model to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause and CVD-specific mortality based on RLS status, adjusting for age, presence of major chronic diseases, and other potential confounders.
Results
We documented 6,448 deaths during 10 years of follow-up. We did not observe a significant association between presence of physician-diagnosed RLS and high risk of total mortality (adjusted HR 1.15, 95% CI 0.98–1.34). When cause-specific mortality was studied, participants with RLS had a significantly higher risk of CVD mortality (adjusted HR 1.43, 95% CI 1.02–2.00) relative to those without RLS after adjustment for potential confounders. Longer duration of RLS diagnosis was significantly associated with a higher risk of CVD mortality (p for trend = 0.04). Excluding participants with common RLS comorbidities strengthened the association between RLS and total (adjusted HR 1.43, 95% CI 1.03–1.97) and CVD mortality (adjusted HR 2.27, 95% CI 1.21–4.28). However, we did not find a significant association between RLS and mortality due to cancer and other causes.
Conclusions
Women with RLS had a higher CVD mortality rate, which may not be fully explained by common co-occurring disorders of RLS.
Restless legs syndrome (RLS) is a common sleep disorder with an ≈5% to 10% prevalence in the adult population.1–3 RLS is defined as an urge to move the legs when resting or lying down, especially at night; the urge is relieved by activity.1 RLS can also lead to sleep disturbance and short or prolonged sleep duration.1,2,4 Many previous studies showed that people living with RLS had a higher risk of cardiovascular disease (CVD) and other chronic conditions (e.g., obesity, depression, and disability).5–12
Five previous studies generated inconsistent results regarding whether RLS was associated with altered risk of total mortality.13–18 All 5 studies had some limitations such as small sample size14,15 and missing information on RLS duration.14–18 Studying potential deleterious effects of RLS in women is of particular importance because women have a higher prevalence of RLS relative to men.6 In our previous study, we found that women with RLS had higher risks of developing coronary heart disease (CHD).8 However, the potential influence of RLS on CVD mortality was examined only in a men-only study.17
We thus prospectively examined whether the presence of RLS was associated with a higher risk of total and CVD mortality among ≈57,000 older women independently of other sleep disorders, lifestyle factors and comorbidities. We also explored the association between RLS and deaths due to cancer and other conditions.
Methods
Participants
We used the Nurses' Health Study (NHS) cohort, established in 1976 (cohort baseline) and consisting of 121,700 nurses 30 to 55 years of age from 11 US states. All participants' information was collected and updated with a series of mailed questionnaires (every 2 years). The follow-up rate, which is defined as the number of person-years in the cohort when participants are censored after their last questionnaire response or at death, was 95.6%. In 2002 (study baseline), from 82,160 participants (mean age 67 years) who were still alive and had actively responded to the follow-up questionnaires including several sleep questions (e.g., RLS and sleep duration), we excluded participants with cancer (n = 12,253), renal failure (n = 160), and CVD at baseline (n = 12,074) and those without information on RLS and sleep duration (n = 265), leaving 57,417 participants in the current analyses (figure e-1, http://links.lww.com/WNL/A27).
Standard protocol approvals, registrations, and patient consents
The institutional review boards of the Brigham and Women's Hospital and Harvard School of Public Health approved the study design.
Assessment of physician-diagnosed RLS, sleep duration, and potential confounders
In the 2002 questionnaire, we included the following question “Have you ever had physician-diagnosed restless legs syndrome?” Possible diagnosis dates were 1996 or before, 1997 to 1999, 2000, 2001, or 2002.
From the 2002 questionnaire, baseline characteristics for participants were collected, including age, race, smoking status, body mass index (BMI), physical activity (metabolic equivalent tasks per week), alcohol consumption (grams per day), sleep duration (hours per 24 hours), and snoring frequency; history of major chronic diseases (arthritis, diabetes mellitus, hypertension, hypocholesterolemia, and Parkinson disease) in or before 2002; and use of vitamin supplements, aspirin, antidepressant drugs, and antihypertensives (thiazide diuretic, calcium blocker, β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blocker, and others).
Ascertainment of mortality cases
Deaths were identified according to state vital statistics records, the National Death Index, family reports, and the postal system. Using these methods, we were able to ascertain >98% of the deaths in the cohort.19 Death certificates were obtained, and permission was requested from the next of kin to review medical records when appropriate. Physician reviewers assigned the cause of death according to ICD-8. For this analysis, we assessed all-cause mortality and deaths due to CVD (ICD-8 codes 390-458), cancer (ICD-8 codes 140–207), and other causes.
Statistical analyses
We used SAS 9.4 (SAS Institute, Cary, NC) to perform the statistical analysis. For each participant, person-years were calculated from the return of the 2002 questionnaire, to the date of participants' death or June 1, 2012, or to the date of their last questionnaire returned, depending on which came first. The presence of physician-diagnosed RLS status (yes vs no) was treated as a primary exposure in the current study. Because our previous study suggested that a longer duration of RLS diagnosis was associated with higher risk of CHD,8 we further classified participants into 3 groups based on the existence of physician-diagnosed RLS and duration of RLS diagnosis—no RLS, RLS diagnosis <3 years (the midpoint of the RLS duration), and RLS diagnosis for ≥3 years—to examine the association between RLS duration and mortality. To examine the potential dose-response relationship between the duration of RLS diagnosis and risk of mortality, we treated years of RLS diagnosis as a continuous variable for trend test, adjusting for aforementioned covariates.
The Cox proportional hazards model was used to calculate the hazard ratio (HR) and 95% confidence interval (CI) in each category of RLS. In the multivariable models, we adjusted for potential confounders that could be associated with RLS and mortality, including age (months), race (white, yes/no), smoking status (never smoker, former smoker, or current smoker), alcohol consumption (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, and ≥15 g/d), BMI (kilograms per meter squared), physical activity (quintiles), Adjusted Healthy Eating Index (quintiles), use of estrogen hormone therapy (premenopausal, postmenopausal with no use, past user, or current user), regular use of aspirin (yes/no), use of antidepressant drugs (yes/no), use of antihypertensive drugs (yes/no), and history of chronic diseases (arthritis, diabetes mellitus, hypertension, hypercholesterolemia, and Parkinson disease). We further adjusted for other sleep parameters, including snoring frequency (every night, most night, a few nights a week, occasionally or almost never) and sleep duration (≤5, 6, 7, 8, or ≥9 h/24 h), and use of iron-specific supplements (yes/no).
We examined potential interactions of the presence of RLS (yes vs no) with age (<65 or ≥65 years, approximate mean age of the studied population), overweight (yes/no, based on BMI ≥25 kg/m2), and smoking status (never vs ever) by including multiplicative terms in the Cox models with adjustment for other potential confounders.
Because individuals with RLS were more likely to have other comorbidities, we conducted a sensitivity analysis by excluding women with snoring, short (≤6 h/d) or prolonged (≥9 h/d) sleep duration, Parkinson disease, diabetes mellitus, arthritis, use of antidepressants and iron-specific supplement (surrogates of depression and iron-deficiency anemia, respectively), and obesity (BMI ≥30 kg/m2). To reduce the possibility of reverse causality bias, we further excluded deaths in the first 2 years of follow-up (i.e., 2-year lag analysis).
Results
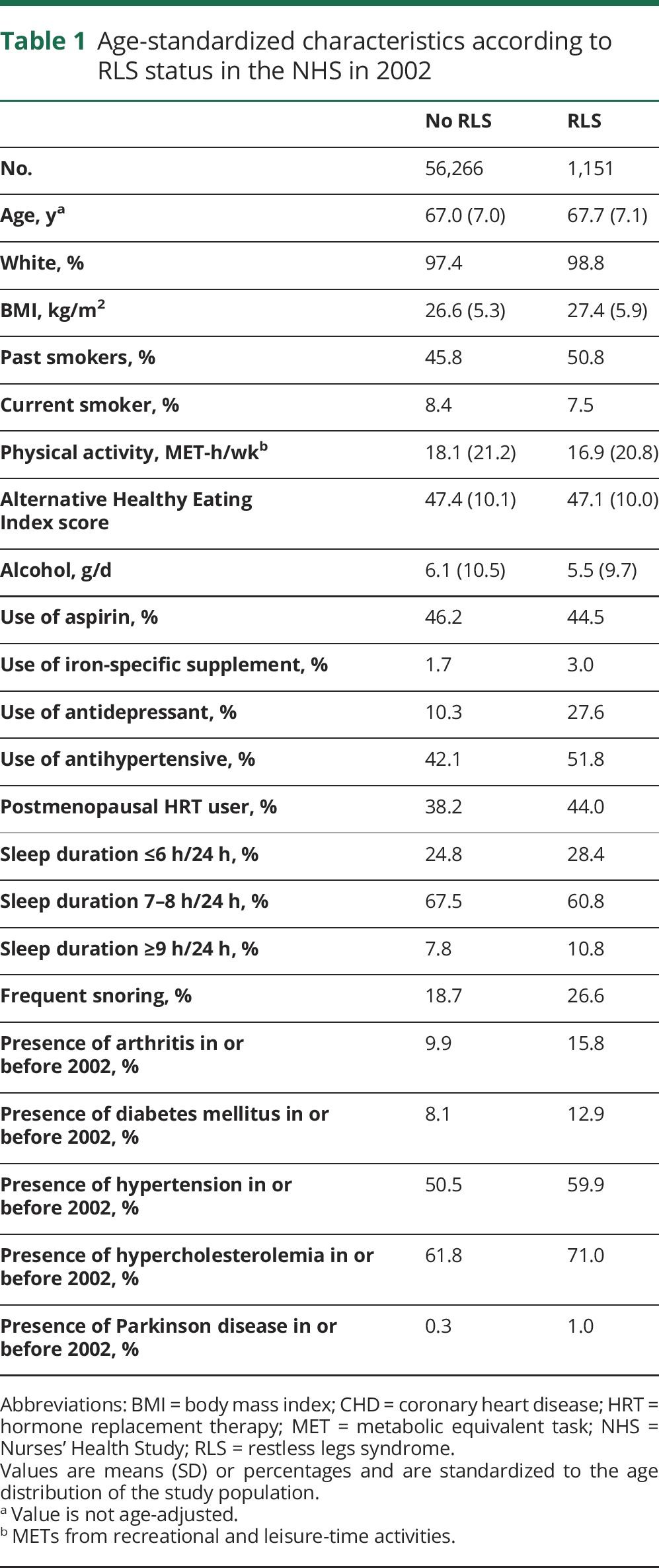
Among 57,417 participants in 2002 (the study baseline), 1,151 (2.0%) women reported physician-diagnosed RLS. Compared with women without RLS, those with RLS tended to be older and white and to have a higher BMI and had a higher prevalence of chronic disease (e.g., hypertension, diabetes mellitus, arthritis, and hypercholesterolemia), sleep disorders (e.g., snoring and short/prolonged sleep duration), and use of iron-specific supplements, antidepressants, and antihypertensives (table 1).
Table 1.
Age-standardized characteristics according to RLS status in the NHS in 2002
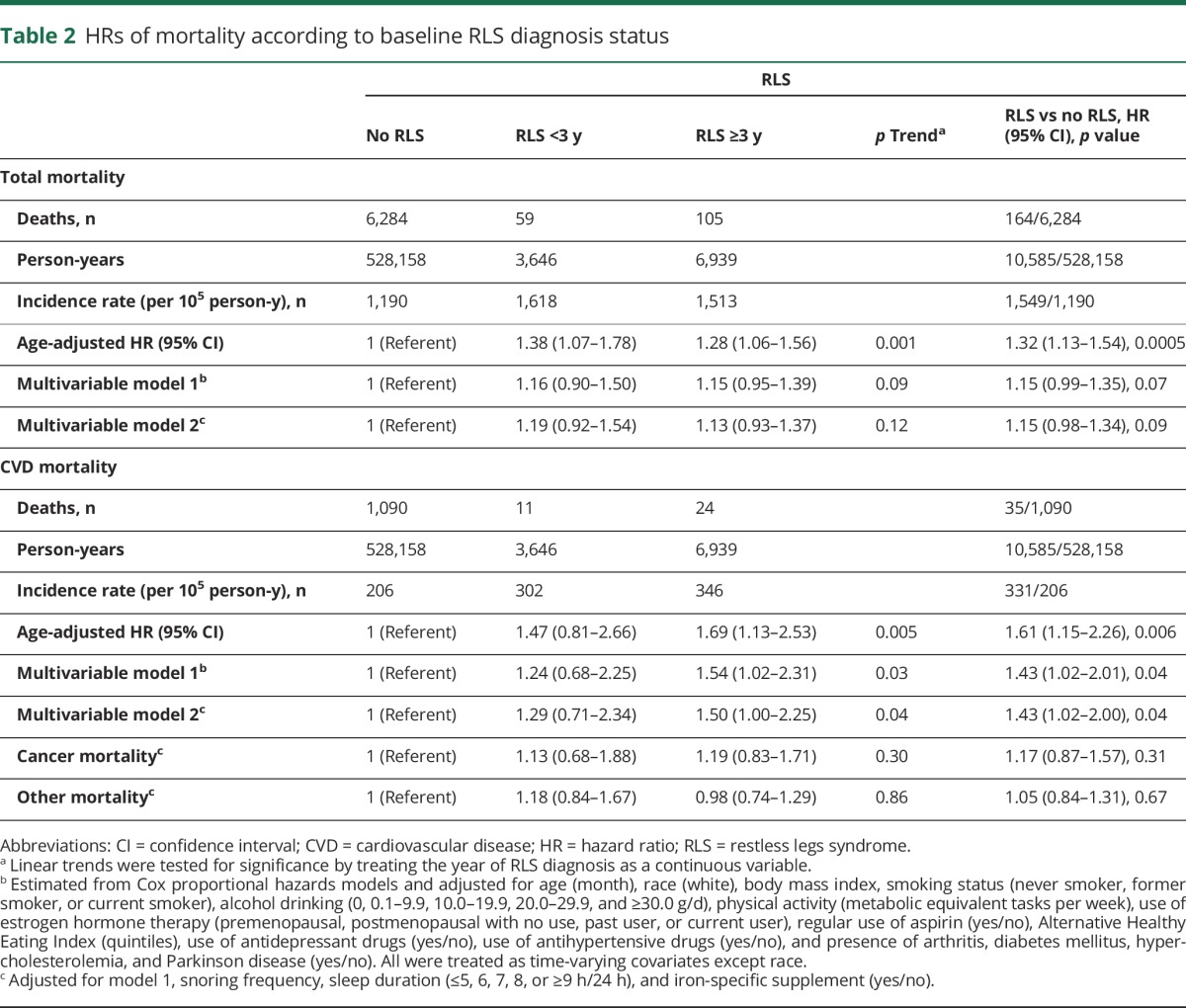
From 2002 to 2012, 6,448 deaths were documented, including 1,125 CVD deaths, 1,943 cancer deaths, and 3,380 other deaths. In the age-adjusted model, women with RLS had a higher risk of total mortality compared with those without RLS (HR 1.32, 95% CI 1.13–1.54) (table 2). After further adjustment for other potential confounders, including use of aspirin, iron and vitamin supplements, antidepressants, and antihypertensive drugs, history of chronic disease, and sleep parameters (sleep duration and snoring), the relationship between RLS and total mortality was not significant (adjusted HR 1.15, 95% CI 0.98–1.34). However, participants with RLS had a 43% higher risk of CVD mortality (adjusted HR 1.43, 95% CI 1.02–2.00) relative to those without RLS with the same model adjustment (table 2). There was a significant dose-response relationship between longer duration of RLS diagnosis and higher CVD mortality (p for trend = 0.04). We did not observe significant interactions between RLS and age, smoking, or overweight in relation to total and CVD mortality (p for interaction >0.1 for all). In contrast, RLS was not associated with deaths due to cancer or other causes (table 2).
Table 2.
HRs of mortality according to baseline RLS diagnosis status
After the exclusion of 36,353 participants with common comorbidities of RLS, including snoring, short or prolonged sleep duration, Parkinson disease, diabetes mellitus, arthritis, use of antidepressants or iron-specific supplements, and obesity, the association between RLS and total and CVD mortality became stronger and statistically significant among women with RLS compared to those without RLS: the adjusted HR was 1.43 (95% CI 1.03–1.97) for total mortality and 2.27 (95% CI 1.21–4.28) for CVD mortality. Duration of RLS diagnosis was significantly associated with total and CVD mortality (p for trend <0.04 for both) (figure 1A). When we further excluded deaths in the first 2 years of follow-up after excluding the comorbidities, the presence of RLS (adjusted HR 1.50, 95% CI 1.07–2.08 for total mortality; adjusted HR 2.77, 95% CI 1.47–5.23 for CVD mortality, p < 0.02 for both) and longer duration of RLS diagnosis (p for trend <0.03 for both) (figure 1B) were significantly associated with a higher total and CVD mortality.
Figure 1. HRs and 95% CIs for total and CVD mortality, sensitivity analyses.
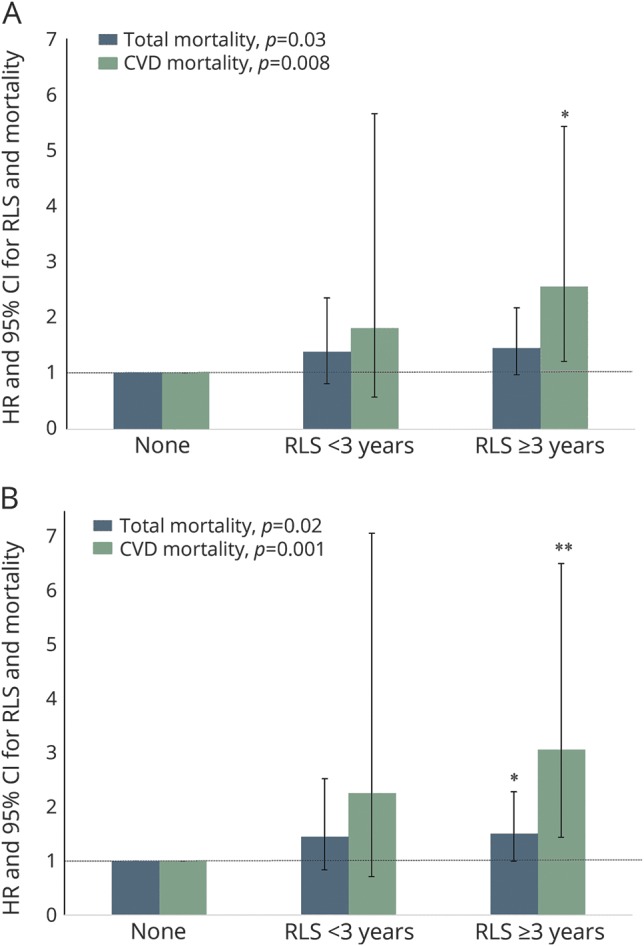
HRs and CIs are according to RLS duration among 21,063 participants (A) without snoring, short (≤6 h/d) or prolonged (≥9 h/d) sleep duration, arthritis, diabetes mellitus, Parkinson disease, obesity, and use of antidepressants or iron-specific supplements or (B) further excluding death in the first 2 years of follow-up. The models were adjusted for age (months), race (white, yes/no), smoking status (never smoker, former smoker, or current smoker), alcohol consumption (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, and ≥15 g/d), physical activity (quintiles), Adjusted Healthy Eating Index (quintiles), history of hypertension and hypercholesterolemia, use of estrogen hormone therapy (premenopausal, postmenopausal with no use, past user, or current user), and regular use of aspirin (yes/no). Linear trends were tested for significance by treating the year of RLS diagnosis as a continuous variable. CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio; RLS = restless legs syndrome. *p < 0.05 relative to women without RLS; **p < 0.01 relative to women without RLS.
Discussion
In this well-established large prospective cohort of women, the presence of RLS was significantly associated with a higher risk of CVD mortality during 10 years of follow-up relative to those without the disorder. The association was dependent on the duration of RLS diagnosis: the longer the presumed RLS disease duration, the higher the risk of CVD mortality. After we excluded participants with common RLS comorbidities, the association between RLS status and duration and CVD mortality became stronger and remained significant even though the sample size decreased greatly. This result suggested that the association might not be explained by the presence of these RLS comorbidities. In contrast, we did not observe any statistically significant association between RLS and deaths due to cancer or other causes. The strengths of our study included a large sample size, long follow-up duration, a large number of outcome events (6,448 deaths vs <55–2,765 deaths in previous studies), and availability of cause-specific mortality data and detailed information on potential confounders.
The findings that RLS, particularly RLS with a longer duration of diagnosis, was significantly associated with a higher risk of CVD mortality are consistent with our previous study based on the same NHS cohort.8 In that NHS study, the multivariable-adjusted HRs were 1.80 (95% CI 1.07–3.01) for nonfatal myocardial infarction and 1.49 (95% CI 0.55–4.04) for fatal CHD compared to women without RLS.8 Consistent with our study, physician-diagnosed RLS was significantly associated with incident myocardial infarction in the Outcomes of Sleep Disorders in Older Men Study after adjustment for the presence of Periodic Limb Movement of Sleep Index and other potential confounders.20 Similar findings were observed in another California cohort study in which only secondary (but not primary) RLS tended to increase the risk of CVD and hypertension.21,22 In distinction, in the current study, when we excluded women with common RLS comorbidities (reducing the number of those with secondary RLS), the association between RLS and CVD became stronger. This is consistent with the notion that the presence of the common comorbidities might weaken the association between RLS and the risk of developing CVD and mortality.13 In other studies of the association of RLS and incident CVD, a significant association was observed in a large cohort of US veterans18 but not in 2 other cohorts.23 In a recent study including 665 adult Amerindians ≥40 years of age, the presence of RLS was not associated with CVD risk factors.24 The lack of significant association could be due to small sample size. Alternatively, it suggests that age may modify the association between RLS and CVD risk/mortality. However, we did not find a significant interaction between age and RLS in relation to CVD mortality.
The mechanisms by which RLS may predispose to CVD and cardiovascular-specific mortality remain unknown. RLS-related poor sleep quality and duration25 may increase the activation of inflammatory cytokines such as serum C-reactive proteins, which contribute to CVD development26 and have been observed to be related to higher blood pressure,27 CHD,28 and CVD mortality.29 Furthermore, periodic limb movements, which are observed in three-fourths of patients with RLS, are associated with an increased CVD risk.20,30 In addition, endothelial dysfunction, related to lower coronary flow,5 and dopamine deficiency are the other 2 proposed mechanisms.26,31 Dopamine deficiency, correlated with hypofunction of the A11 diencephalospinal pathway found in RLS, may result in the disinhibition of somatosensory and sympathetic pathways in the spinal cord and lead to increased sympathetic activation, which may cause hypertension, CVD, and stroke.26,31 Alternatively, impairment in the arterial baroreflex and increased peripheral vascular resistance, potentially as a result of increased sympathetic activity, may also play a role.32
In the age-adjusted model, RLS showed a significant relationship with total mortality, especially in women who had a longer duration of RLS diagnosis. However, after adjustment for other covariates, the relationship between RLS and total mortality was not significant. In contrast, in our previous study based on the Health Professionals Follow-up Study,17 a men-only cohort, RLS (assessed by diagnostic questionnaire with an observed prevalence of 3.7%) had a significant association with a higher risk of total mortality after adjustment for potential confounders (adjusted HR 1.30, 95% CI 1.11–1.52). One possible explanation for this discrepancy is that only physician-diagnosed RLS was considered in our current study. Previous studies reported that RLS was underdiagnosed, with more than half of the patients with RLS not identified.33,34 In our study, in which we relied only on a history of physician-diagnosed RLS, only 2.0% of participants were defined as having had RLS, which is lower than the RLS prevalence observed in the general population (≈5%–6%).1 In our previous study based on the NHS II, which included 65,554 middle-aged nurses, we found that 6.4% of women had RLS on the basis of a set of standardized questions.6 These >2-fold differences in prevalence may have resulted because some patients who had RLS at the time of the current study might not have received a diagnosis of RLS from a physician at the time of the study, thus introducing the misclassification for RLS assessment. Therefore, the true effect size of RLS on total mortality could have been underestimated. However, the use of physician-diagnosed RLS could introduce a selective identification of individuals with severe RLS, which might lead to overestimation of the true effect of RLS on mortality. In a recent US veteran study in which RLS was identified by the ICD code, a significant association between incident RLS and future risk of total mortality was observed (adjusted HR 1.88, 95% CI 1.70–2.08).18 In a 4-cohort study16 in which RLS was assessed via an RLS symptom questionnaire, the investigators did not find a significant association between RLS and total mortality. In this context, more studies are warranted to understand the pros and cons of the use of patient-reported physician diagnosis of RLS vs an RLS symptom questionnaire for RLS ascertainment in large-scale epidemiology studies. Sex difference could be another potential explanation for the discrepancy between studies. Similar sex differences for the RLS-mortality relationship were reported in a previous Swedish-based study15 and a 4-cohort study16 in which the nonsignificant association between RLS and total mortality generally was found to be more pronounced in men than in women. However, the biological mechanism underlying this potential sex difference remains unknown.
Some limitations of our study warrant consideration. As addressed above, using only physician-diagnosed RLS could lead to misclassification of the non-RLS group. Determination of RLS duration was also based on participants' recall, which is vulnerable to misclassification. We did not collect information on RLS severity and use of RLS-related medications, which could confound the observed associations. Our study is also limited by the lack of objective data such as the periodic leg movement burden, which could potentially identify those participants at high risk for CVD outcome. Snoring based on self-report and questionnaires might misclassify many individuals’ sleep-related breathing disorder status. Sleep studies would be a more accurate measure; however, it is not feasible to perform sleep studies on all participants in such a large cohort. It is noteworthy that in a sensitivity analysis excluding participants with snoring and obesity (a major determinant of sleep apnea status), we observed a similar significant association between RLS and CVD mortality. Residual confounding could occur, although we carefully controlled for a wide range of potential confounders. For example, we did not collect information on peripheral neuropathy. However, we adjusted for the presence of diabetes mellitus, which is strongly associated with neuropathy, in the primary analysis and excluded those with diabetes mellitus in the sensitivity analysis. It is also worth noting that we did not have information on RLS severity. Finally, because the majority of the participants in these cohorts are white, the results of this study may not be generalizable to other race groups.
In this large-scale study of women, we found that women with physician-diagnosed RLS tended to have a higher risk of total and CVD mortality. The association between RLS and mortality increased when women had a longer duration of RLS diagnosis.
Glossary
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- HR
hazard ratio
- ICD-8
International Classification of Diseases, Eighth Revision
- NHS
Nurses' Health Study
- RLS
restless legs syndrome
Author contributions
Yinge Li: interpretation of data and manuscript drafting/revising. Yanping Li: collection, analysis, and interpretation of data, statistical analysis, and manuscript drafting/revising. John W. Winkelman, Arthur S. Walters, Jiali Han, and Frank B. Hu: critical revision of manuscript for intellectual content. Xiang Gao: study concept and design, data collection, analysis and interpretation of data, manuscript drafting/revising, and study supervision.
Study funding
This study was supported by grants (UM1 CA186107, R01 HL034594, and 5R01 NS062879) from the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
Yinge Li and Yanping Li report no disclosures relevant to the manuscript. J. Winkelman is a consultant for Merck, Flex Pharma, and Otsuka. He has provided expert testimony for Cantor Colburn. He receives royalties from UpToDate. He has received research grants from Xenoport, UCB Pharma, NeuroMetrix, National Institute of Mental Health, and Luitpold Pharmaceuticals. A. Walter receives an RLS research grant from and was a consultant to Xenoport/Arbor Pharmaceuticals, MundiPharma, and UCB Pharma. J. Han, F. Hu, and X. Gao report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Ekbom K, Ulfberg J. Restless legs syndrome. J Intern Med 2009;266:419–431. [DOI] [PubMed] [Google Scholar]
- 2.Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med 2011;12:623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath 2012;16:987–1007. [DOI] [PubMed] [Google Scholar]
- 4.Lasch KE, Abraham L, Patrick J, Piault EC, Tully SE, Treglia M. Development of a next day functioning measure to assess the impact of sleep disturbance due to restless legs syndrome: the Restless Legs Syndrome-Next Day Impact Questionnaire. Sleep Med 2011;12:754–761. [DOI] [PubMed] [Google Scholar]
- 5.Trenkwalder C, Allen R, Hogl B, Paulus W, Winkelmann J. Restless legs syndrome associated with major diseases: a systematic review and new concept. Neurology 2016;86:1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao X, Schwarzschild MA, Wang H, Ascherio A. Obesity and restless legs syndrome in men and women. Neurology 2009;72:1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Mirzaei F, O'Reilly EJ, et al. Prospective study of restless legs syndrome and risk of depression in women. Am J Epidemiol 2012;176:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation 2012;126:1689–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology 2008;70:35–42. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Li Y, Malhotra A, Ning Y, Gao X. Restless legs syndrome status as a predictor for lower physical function. Neurology 2014;82:1212–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong JC, Li YP, Schwarzschild MA, Ascherio A, Gao X. Restless legs syndrome: an early clinical feature of Parkinson disease in men. Sleep 2014;37:369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Lyall K, Palacios N, Walters AS, Ascherio A. RLS in middle aged women and attention deficit/hyperactivity disorder in their offspring. Sleep Med 2011;12:89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendzerska T, Kamra M, Murray BJ, Boulos MI. Incident cardiovascular events and death in individuals with restless legs syndrome or periodic limb movements in sleep: a systematic review. Sleep 2017;40:zsx013. [DOI] [PubMed] [Google Scholar]
- 14.Pollak CP, Perlick D, Linsner JP, Wenston J, Hsieh F. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health 1990;15:123–135. [DOI] [PubMed] [Google Scholar]
- 15.Mallon L, Broman JE, Hetta J. Restless legs symptoms with sleepiness in relation to mortality: 20-year follow-up study of a middle-aged Swedish population. Psychiatry Clin Neurosciences 2008;62:457–463. [DOI] [PubMed] [Google Scholar]
- 16.Szentkiralyi A, Winter AC, Schurks M, et al. Restless legs syndrome and all-cause mortality in four prospective cohort studies. BMJ Open 2012;2:e001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Wang W, Winkelman JW, Malhotra A, Ma J, Gao X. Prospective study of restless legs syndrome and mortality among men. Neurology 2013;81:52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molnar MZ, Lu JL, Kalantar-Zadeh K, Kovesdy CP. Association of incident restless legs syndrome with outcomes in a large cohort of US veterans. J Sleep Res 2016;25:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax nationwide death search. Am J Epidemiol 1994;140:1016–1019. [DOI] [PubMed] [Google Scholar]
- 20.Winkelman JW, Blackwell T, Stone K, Ancoli-Israel S, Redline S. Associations of incident cardiovascular events with restless legs syndrome and periodic leg movements of sleep in older men, for the outcomes of sleep disorders in older men study (MrOS Sleep Study). Sleep 2017;40:zsx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Den Eeden SK, Albers KB, Davidson JE, et al. Risk of cardiovascular disease associated with a restless legs syndrome diagnosis in a retrospective cohort study from Kaiser Permanente Northern California. Sleep 2015;38:1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong JC, Li W, Gao X. Restless legs syndrome as a prognostic tool for cardiovascular disease. Sleep 2015;38:995–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter AC, Schurks M, Glynn RJ, et al. Restless legs syndrome and risk of incident cardiovascular disease in women and men: prospective cohort study. BMJ Open 2012;2:e000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dredla BK, Del Brutto OH, Lee AS, Castillo PR. Willis-Ekbom disease is not associated with poor cardiovascular health in adults. J Negat Results Biomed 2015;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med 2005;165:1286–1292. [DOI] [PubMed] [Google Scholar]
- 26.Ferini-Strambi L, Walters AS, Sica D. The relationship among restless legs syndrome (Willis-Ekbom Disease), hypertension, cardiovascular disease, and cerebrovascular disease. J Neurol 2014;261:1051–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 2009;32:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sands-Lincoln M, Loucks EB, Lu B, et al. Sleep duration, insomnia, and coronary heart disease among postmenopausal women in the Women's Health Initiative. J Women's Health 2013;22:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li YP, Zhang XH, Winkelman JW, et al. Association between insomnia symptoms and mortality a prospective study of US men. Circulation 2014;129:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koo BB, Blackwell T, Ancoli-Israel S, et al. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: Outcomes of Sleep Disorders in Older Men (MrOS) Study. Circulation 2011;124:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep 2009;32:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertisch SM, Muresan C, Schoerning L, Winkelman JW, Taylor JA. Impact of restless legs syndrome on cardiovascular autonomic control. Sleep 2016;39:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen RP, Stillman P, Myers AJ. Physician-diagnosed restless legs syndrome in a large sample of primary medical care patients in western Europe: prevalence and characteristics. Sleep Med 2010;11:31–37. [DOI] [PubMed] [Google Scholar]
- 34.Gupta R, Lahan V, Goel D. Restless legs syndrome: a common disorder, but rarely diagnosed and barely treated: an Indian experience. Sleep Med 2012;13:838–841. [DOI] [PubMed] [Google Scholar]


