Abstract
Objective
To estimate the incidence densities and cumulative incidence of diabetes-related complications in patients with type 1 diabetes for a maximum of 15-year follow-up. The estimations were further stratified by gender and age at diagnosis (ie, early onset: 0–12 years, late onset:≥13 years).
Design
A population-based retrospective longitudinal cohort study.
Setting
Taiwan’s National Health Insurance medical claims.
Participants
4007 patients newly diagnosed with type 1 diabetes were identified during 1999–2012.
Outcome measures
Acute complications included diabetic ketoacidosis (DKA) and hypoglycaemia. Chronic complications were cardiovascular diseases (CVD), retinopathy, neuropathy and nephropathy.
Results
The incidence density of retinopathy was greatest (97.74 per 1000 person-years), followed by those of nephropathy (31.36), neuropathy (23.93) and CVD (4.39). Among acute complications, the incidence density of DKA was greatest (121.11 per 1000 person-years). The cumulative incidences of acute complications after 12 years following diagnosis were estimated to be 52.1%, 36.1% and 4.1% for DKA, outpatient hypoglycaemia and hospitalised hypoglycaemia, respectively. For chronic complications, the cumulative incidence of retinopathy after 12 years following diagnosis was greatest (65.2%), followed by those of nephropathy (30.2%), neuropathy (23.7%) and CVD (4.1%). Females with late-onset diabetes were greatly affected by advanced retinopathy (ie, sight-threatening diabetic retinopathy) and hospitalised hypoglycaemia, whereas those with early-onset diabetes were more vulnerable to DKA. Chronic complications were more commonly seen in late-onset diabetes, whereas early-onset diabetes were most affected by acute complications.
Conclusions
Ethnic Chinese patients with type 1 diabetes were greatly affected by DKA and retinopathy. The incidence of diabetes-related complications differed by age at diagnosis and sex.
Keywords: DIABETES & ENDOCRINOLOGY, EPIDEMIOLOGY, Diabetic neuropathy
Strengths and limitations of this study.
This is the largest longitudinal cohort study of ethnic Chinese patients with type 1 diabetes followed for a maximum of 15 years to provide up-to-date incidence estimates of acute and chronic complications.
The analyses stratified by gender and age at diabetes onset indicated significant age–gender disparities in the epidemiological data of diabetes-related complications in type 1 diabetes, which highlight importance for clinical attention and developing preventive strategies.
The study limitations resulting from the use of medical reimbursement claims data, including potential misclassifications of diabetes-related complications and lack of clinical biomarkers such as blood glucose, may underestimate rather than overestimate the incidence rates of diabetes-related complications.
The incidence estimates of diabetes-related complications may only be generalisable to ethnic Chinese population with type 1 diabetes.
Introduction
It has been estimated that the incidence of type 1 diabetes increases by about 3%–5% per year worldwide.1–3 The annual incidence rate of childhood (<15 years) type 1 diabetes in Taiwan was 5.3 per 1 00 000 children in the period 2003–2008.4 Type 1 diabetes accounts for only 5%–10% of the diabetic population, but it remains a devastating chronic disorder with acute complications, including diabetic ketoacidosis (DKA) and hypoglycaemia, chronic complications, which can be divided into microvascular (ie, retinopathy, neuropathy, nephropathy) and macrovascular complications (ie, cardiovascular diseases (CVD)). Although treatment and care for type 1 diabetes have improved,5–7 diabetes-related complications are major obstacles to glycaemic control for many patients and contribute to most of the increased morbidity and premature mortality in such individuals.8 The toxicity effect of prolonged chronic hyperglycaemia is a leading cause of microvascular and macrovascular diseases among patients with type 1 diabetes, with hypertension and dyslipidaemia being exacerbating factors.9
Assessing the epidemiology of diabetes-related complications is essential for developing preventive strategies and planning treatment protocols to minimise the impact of the complications. However, there is very little longitudinal data (eg, Pittsburgh Epidemiology of Childhood-Onset Diabetes Complications (EDC) Study,10 EURODIAB IDDM Complications Study11) on the incidence of complications for type 1 diabetes, and previous estimates widely varied with countries (eg, European countries,12 Finland,13 Denmark,14 USA10) and entailed different follow-up periods (eg, 7 years,12 12 years,13 18 years14 and 30 years10). In addition, a limited number of diabetes-related complications have been investigated (eg, microalbuminuria12 14 and CVD13), with no previous study targeting an ethnic Chinese population with type 1 diabetes. Ethnic variations in diabetes-related complications have been recognised; Caucasian patients are greatly affected by CVD,15 16 while the prevalence of end-stage renal failure (ESRD)17 and the odds of microalbuminuria and macroaluminuria18 in Asian populations are much higher compared with those for Caucasian patients. Given the significance of rising life expectancy in recent years among ethnic Chinese patients with type 1 diabetes,19 it is important to provide precise up-to-date estimates of incidence of its complications and compare them to those for other countries. We therefore used a longitudinal population-based cohort of patients newly diagnosed with type 1 diabetes who were followed during the period 1999–2013 to evaluate the incidence densities and cumulative incidences of acute and chronic complications to provide contemporary estimates for an ethnic Chinese population. Efforts were also made to examine whether there were age and sex differences in the incidences of type-1-diabetes-related complications.
Materials and methods
The Institutional Review Board of National Cheng Kung University Hospital approved the study before commencement (A-ER-103–298).
Data source
The present study used the Longitudinal Cohort of Diabetes Patients (LHDB) 1996–2013 data from Taiwan's National Health Insurance Research Database (NHIRD). The LHDB is representative of Taiwan’s population with diabetes sampled from the beneficiaries in Taiwan's National Health Insurance (NHI) programe, which is a mandatory-enrolment and single-payment system that covers over 99% of Taiwan’s population.20 The LHDB consists of a random sample of 120 000 deidentified diabetes incident cases enrolled in Taiwan's NHI programe from each calendar year, who were tracked back to 1996 and followed up to 2013 to establish a longitudinal cohort. The LHDB is a valid national dataset which has been used in many research that evaluated long-term health outcomes of patients.21–26
Cohort
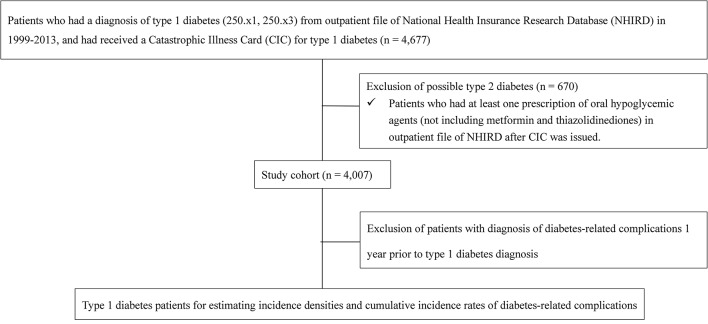
From the LHDB, we selected 4677 patients with a diagnosis of type 1 diabetes (International Classification of Diseases, Ninth Revision, Clinical Modification, ICD-9-CM=250.x1 or 250.x3) from outpatient files of the LHDB and having received a Catastrophic Illness Card (CIC) for type 1 diabetes (figure 1) in the period 1999–2012. Because patients with a CIC are eligible for exemption from copayments, the approval of such a status is subject to evaluation and review by the Bureau of NHI of Taiwan. The CIC patient data are accurate and reliable with a positive predictive value of 98.3% for type 1 diabetes.19 We further excluded 670 potential type 2 diabetes cases who consumed any oral antihypoglycaemic agents (OHAs) after CIC was issued, including sulfonylureas, meglitinides, acarbose, dipeptidyl peptidase-4 inhibitors and glucagon-like peptide-1 receptor agonists; however, those who used metformin alone, thiazolidinediones alone or both were retained. Patients who were prescribed metformin, thiazolidinediones or both were retained because these OHAs are insulin-sensitisers that can be combined with insulin treatments for cases with insulin resistance,27 28 which is also seen in patients with type 1 diabetes in Taiwan based on our expert opinions. To estimate the incidence rates of diabetes-related complications, we further selected cases without a history of the complication before type 1 diabetes diagnosis (table 1). Study patients were stratified by gender and age at first type 1 diabetes diagnosis (ie, early onset: 0–12 years, late onset: ≥13 years). The 25th, 50th (median) and 75th percentiles of age in early-onset group were 5, 8 and 10 years, respectively, with the mean age of 7.69 years (standard deviation; SD: 3.22). And, for late-onset group, the 25th, 50th and 75th percentiles of age were 17, 24 and 33 years, respectively, with the mean age of 26.47 years (SD: 11.60).
Figure 1.
Flowchart of study cohort selection.
Table 1.
Overall incidence density of diabetes-related complications among patients with type 1 diabetes between 1999 and 2013
| Retinopathy | Proliferative retinopathy | STDR | Neuropathy | Nephropathy | Renal failure | CVD | DKA | Outpatient hypoglycaemia | Hospitalised hypoglycaemia | |
| No of cases* | 3359 | 3970 | 3983 | 3742 | 3634 | 4003 | 3916 | 2205 | 3934 | 3987 |
| 1532 | 157 | 90 | 558 | 688 | 36 | 117 | 996 | 913 | 105 | |
| Follow-up time (person-years)† | 15 675 | 26 733 | 27 139 | 23 320 | 21 936 | 27 491 | 26 664 | 8224 | 22 865 | 26 968 |
| Incidence density (1000 person-years) (95% CI) |
97.74 (92.9 to 102.8) |
5.87 (5.0 to 6.9) |
3.32 (2.7 to 4.1) |
23.93 (22.0 to 26.0) |
31.36 (29.1 to 33.8) |
1.31 (0.9 to 1.8) |
4.39 (3.6 to 5.3) |
121.11 (113.7 to 128.9) |
39.93 (37.4 to 42.6) |
3.89 (3.2 to 4.7) |
Patients with type 1 diabetes were retrieved from incident cases from 2000 to 2012. Follow-up time started from the first diagnosis date to the time the event occurred, death, discontinued enrolment from Taiwan’s National Health Insurance Programme, or the end of 2013, whichever came first.
*No of cases refers to the number of patients who had no complication of interest in the baseline year (1 year before diagnosis date).
†No of cases with event refers to the number of patients who had incident events after type 1 diabetes was confirmed.
‡Cumulative follow-up time (person-years) was calculated as the sum of follow-up years during observation period.
CVD, cardiovascular disease, DKA, diabetic ketoacidosis; STDR, sight-threatening diabetic retinopathy.
Diabetes-related complications
The complications of interest included acute complications, namely DKA (confirmed by hospital admission or emergency room visit for DKA), hypoglycaemia (confirmed by defined hypoglycaemic events required for outpatient visits or hospitalisation for medical assistance or interventions) and chronic complications, namely CVD, nephropathy, retinopathy and neuropathy. A list of diabetes-related complications and the corresponding ICD-9-CM codes are provided in online supplementary table 1; this list was confirmed by the expert panel before being applied.
bmjopen-2016-015117supp001.pdf (193.8KB, pdf)
Statistics
The incidence density of diabetes-related complications was calculated by dividing the number of incident cases with individual complication events by the total person-years observed over 15 years of follow-up (1999–2013). The 95% CIs were calculated assuming a Poisson distribution of cases.29 Significant differences in incidence density between age and sex subgroups were indicated by a 95% CI for the difference in incidence density between subgroups.30 Moreover, because a cohort of patients newly diagnosed with type 1 diabetes was used, we were able to provide visual illustrations about the cumulative incidences of diabetes-related complications by diabetes duration since diabetes onset. The cumulative incidence of diabetes-related complications was estimated by using the life table method (using the SAS LIFETEST procedure), and significant difference in cumulative incidence between subgroups was examined according to K-sample tests.31 SAS version 9.4 was used for the aforementioned analyses.
Results
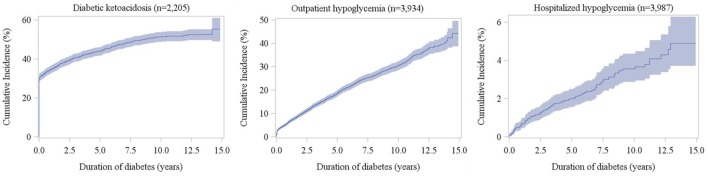
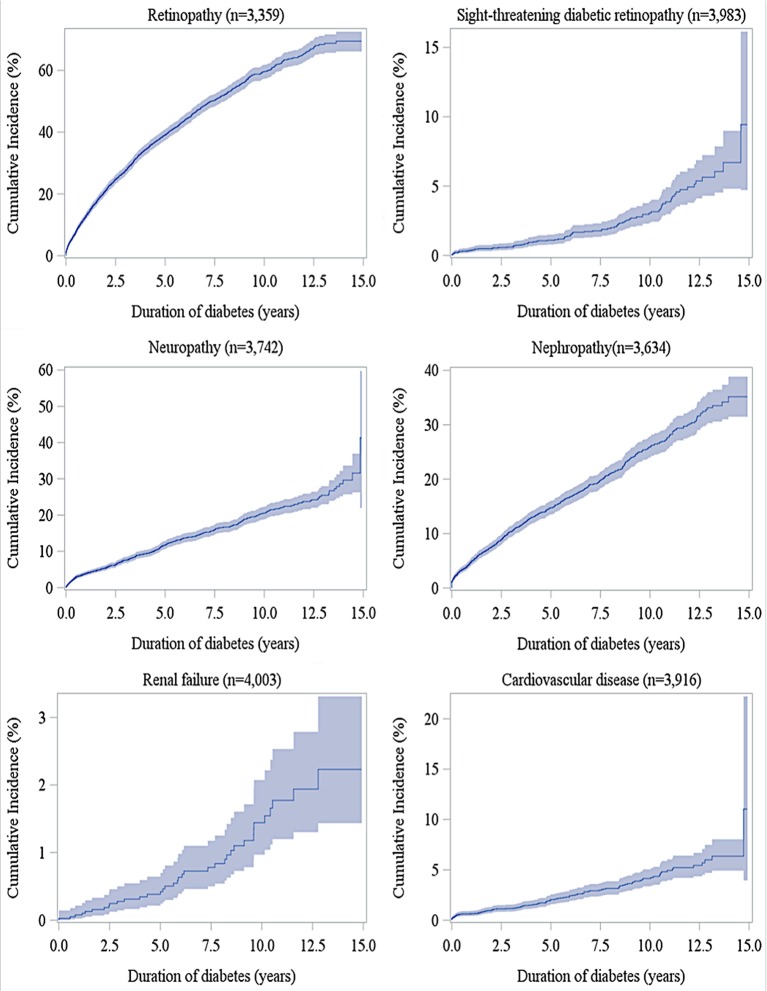
The median (25th and 75th percentiles) of the overall follow-up times (defined as the time from diabetes diagnosis to death, loss-to-follow-up, or the end of study period, whichever came first) is 6.74 years (3.43 and 10.02 years). The overall and age–sex-specific incidence densities of diabetes-related complications are presented in tables 1 and 2, respectively. The incidence rate of retinopathy (97.74 per 1000 person-years) was greatest, followed by those of nephropathy (31.36), neuropathy (23.93) and CVD (4.39). Among acute complications, the incidence density of DKA was greatest (121.11 per 1000 person-years). As shown in table 2, the incidence densities of retinopathy, DKA and hospitalised hypoglycaemia in females were significantly higher than those in males. The incidence densities of DKA and outpatient hypoglycaemia in the early-onset group (0–12 years) were significantly higher than those noted in the late-onset group (≥13 years), while those of advanced retinopathy (ie, sight-threatening diabetic retinopathy; STDR), neuropathy, nephropathy, CVD and hospitalised hypoglycaemia in the late-onset group were significantly higher. Figures 2 and 3 show cumulative incidences for acute and chronic complications, respectively, along with diabetes duration. The cumulative incidences at 12years after diagnosis were 52.1%, 36.1% and 4.1% for DKA, outpatient hypoglycaemia and hospitalised hypoglycaemia, respectively. For chronic complications, the 12-year cumulative incidence of retinopathy was greatest (65.2%), followed by those of nephropathy (30.2%), neuropathy (23.7%) and CVD (5.2%). Age–sex-specific cumulative incidences of diabetes-related complications are illustrated in online supplementary figure 1.
Table 2.
Incidence density of diabetes-related complications by age at diabetes diganosis and sex among patients with type 1 diabetes between 1999 and 2013
| Retinopathy | Proliferative retinopathy | STDR | Neuropathy | Nephropathy | Renal failure | CVD | DKA | Outpatient hypoglycaemia | Hospitalised hypoglycaemia | |
| Male | ||||||||||
| No of cases† | ||||||||||
| All male | 1618 | 1886 | 1892 | 1778 | 1695 | 1901 | 1859 | 1073 | 1872 | 1902 |
| Early onset (0–12 years) | 654 | 719 | 719 | 714 | 700 | 719 | 712 | 396 | 718 | 719 |
| Late onset (≥13 years) | 964 | 1167 | 1173 | 1064 | 995 | 1182 | 1147 | 677 | 1154 | 1183 |
| No of cases with event‡ | ||||||||||
| All male | 693 | 64 | 34 | 261 | 332 | 15 | 60 | 447 | 452 | 28 |
| Early onset | 305 | 14 | 1 | 37 | 86 | 1 | 7 | 239 | 202 | 11 |
| Late onset | 388 | 50 | 33 | 224 | 246 | 14 | 53 | 208 | 250 | 17 |
| Follow-up time (person-years)§ | ||||||||||
| All male | 7813 | 12 908 | 13 102 | 11 345 | 10 403 | 13 248 | 12 798 | 4322 | 11 025 | 13 115 |
| Early onset | 3333 | 5290 | 5368 | 5180 | 4817 | 5358 | 5280 | 1317 | 4277 | 5297 |
| Late onset | 4480 | 7618 | 7734 | 6165 | 5586 | 7891 | 7518 | 3005 | 6749 | 7818 |
| Incidence density (1000 person-years) (95% CI) |
||||||||||
| All male | 88.70 (82.2 to 95.6) |
4.96 (3.8 to 6.3) |
2.60 (1.8 to 3.6) |
23.01 (20.3 to 26.0) |
31.91 (28.6 to 35.5) |
1.13 (0.6 to 1.9) |
4.69 (3.6 to 6.0) |
103.43 (94.1 to 113.5) |
41.00 (37.3 to 45.0) |
2.13 (1.4 to 3.1) |
| Early onset | 91.52 (81.5 to 102.4) |
2.65 (1.4 to 4.4) |
0.19 (0.0 to 1.0) |
7.14 (5.0 to 9.8) |
17.85 (14.3 to 22.0) |
0.19 (0.0 to 1.0) |
1.33 (0.5 to 2.7) |
181.53 (159.2 to 206.1) |
47.23 (40.9 to 54.2) |
2.08 (1.0 to 3.7) |
| Late onset | 86.60 (78.2 to 95.7) |
6.56 (4.9 to 8.7) |
4.27 (2.9 to 6.0) |
36.34 (31.7 to 41.4) |
44.04 (38.7 to 49.9) |
1.77 (1.0 to 3.0) |
7.05 (5.3 to 9.2) |
69.22 (60.1 to 79.3) |
37.05 (32.6 to 41.9) |
2.17 (1.3 to 3.5) |
| 95% CI of incidence density difference for male, early vs late onset | −8.5 to 18.3 | −6.4 to −1.4* | −5.9 to −2.3* | −34.8 to −23.6* | −33.1 to −19.3* | −2.8 to −0.4* | −8.1 to −3.3* | 91.5 to 133.1* | 2.4 to 17.9* | −1.7 to 1.5 |
| Female | ||||||||||
| No of cases† | ||||||||||
| All female | 1741 | 2084 | 2091 | 1964 | 1939 | 2102 | 2057 | 1132 | 2062 | 2085 |
| Early onset | 721 | 777 | 777 | 772 | 764 | 777 | 773 | 413 | 774 | 774 |
| Late onset | 1020 | 1307 | 1314 | 1192 | 1175 | 1325 | 1284 | 719 | 1288 | 1311 |
| No of cases with event‡ | ||||||||||
| All female | 839 | 93 | 56 | 297 | 356 | 21 | 57 | 549 | 461 | 77 |
| Early onset | 358 | 18 | 6 | 50 | 109 | 1 | 11 | 277 | 229 | 21 |
| Late onset | 481 | 75 | 50 | 247 | 247 | 20 | 46 | 272 | 232 | 56 |
| Follow-up time (person-years)§ | ||||||||||
| All female | 7862 | 13 825 | 14 037 | 11 976 | 11 533 | 14 243 | 13 866 | 3902 | 11 840 | 13 853 |
| Early onset | 3616 | 5848 | 5910 | 5610 | 5283 | 5927 | 5871 | 1183 | 4642 | 5777 |
| Late onset | 4246 | 7977 | 8127 | 6365 | 6250 | 8317 | 7995 | 2719 | 7199 | 8076 |
| Incidence density (1000 person-years) (95% CI) | ||||||||||
| All female | 106.72 (99.6 to 114.2) |
6.73 (5.4 to 8.2) |
3.99 (3.0 to 5.2) |
24.80 (22.1 to 27.8) |
30.87 (27.7 to 34.2) |
1.47 (0.9 to 2.3) |
4.11 (3.1 to 5.3) |
140.69 (129.2 to 153.0) |
38.94 (35.5 to 42.7) |
5.56 (4.4 to 6.9) |
| Early onset | 99.01 (89.0 to 109.8) |
3.08 (1.8 to 4.9) |
1.02 (0.4 to 2.2) |
8.91 (6.6 to 11.8) |
20.63 (16.9 to 24.9) |
0.17 (0.0 to 0.9) |
1.87 (0.9 to 3.4) |
234.05 (207.3 to 263.3) |
49.34 (43.1 to 56.2) |
3.63 (2.3 to 5.6) |
| Late onset | 113.29 (103.4to 123.9) |
9.40 (7.4 to 11.8) |
6.15 (4.6 to 8.1) |
38.80 (34.1 to 44.0) |
39.52 (34.7 to 44.8) |
2.40 (1.5 to 3.7) |
5.75 (4.2 to 7.7) |
100.05 (88.5 to 112.7) |
32.23 (28.2 to 36.7) |
6.93 (5.2 to 9.0) |
| 95% CI of incidence density difference for female, early onset vs late onset | −28.8 to 0.2 | −9.1 to −3.6* | −7.3 to −3.0* | −35.5 to −24.2* | −25.3 to −12.5* | −3.5 to −1.0* | −6.0 to −1.7* | 108.4 to 159.6* | 9.8 to 24.4* | −5.8 to -0.8* |
| 95% CI of incidence density difference for male vs female | −27.8 to −8.2* | −3.6 to 0.1 | −2.8 to −0.02* | −5.8 to 2.2 | −3.6 to 5.7 | −1.2 to 0.5 | −1.0 to 2.2 | −52.3 to −22.2* | −3.1 to 7.2 | −4.9 to −1.9* |
Patients with type 1 diabetes were retrieved from incident cases from 2000 to 2012. Follow-up time started from the first diagnosis date to the time the event occurred, death, discontinued enrolment from Taiwan’s National Health Insurance Programme, or the end of 2013, whichever came first.
*p<0.05.
†No of cases refers to the number of patients who had no complication of interest in the baseline year (1 year before diagnosis date).
‡No of cases with event refers to the number of patients who had incident events after type 1 diabetes was confirmed.
§Cumulative follow-up time (person-years) was calculated as the sum of follow-up years during observation period.
CVD, cardiovascular disease, DKA, diabetic ketoacidosis; STDR, sight-threatening diabetic retinopathy.
Figure 2.
Cumulative incidences of diabetic ketoacidosis and hypoglycaemia according to the duration of diabetes in patients with type 1 diabetes (shadow area indicates 95% CI).
Figure 3.
Cumulative incidences of retinopathy, sight-threatening diabetic retinopathy, neuropathy, nephropathy, renal failure and cardiovascular diseases according to the duration of diabetes in patients with type 1 diabetes (shadow area indicates 95% CI).
bmjopen-2016-015117supp002.pdf (934KB, pdf)
Discussion
To the best of our knowledge, this is the largest cohort study of ethnic Chinese patients newly diagnosed with type 1 diabetes. We provided up-to-date estimates of the incidence of acute and chronic complications in type 1 diabetes patients followed for a maximum of 15 years. We observed age–gender disparities in the incidence of diabetes-related complications in type 1 diabetes. Although comparisons of the epidemiology of diabetes-related complications between studies are difficult, as potential determinants of the complications (eg, age, gender, diabetes duration) differ, the estimates from different studies may reveal some racial or ethnic differences. In the following, we compare our results for ethnic Chinese patients with those reported for other countries or ethnicities.
Acute diabetes-related complications in patients with type 1 diabetes
Diabetic ketoacidosis
Among acute complications, hyperglycaemic events, including DKA and hyperglycaemic hyperosmolar syndrome, are leading causes of morbidity and mortality among individuals with diabetes,32 and use significant healthcare resources.33 DKA was the most common acute complication among the Taiwanese population with type 1 diabetes; the incidence density followed for 15 years was 121.11 per 1000 person-years, and half of the study population (~52%) experienced DKA at 12 years after diabetes diagnosis. Consistent with previous studies from USA,34 Australia35 and Canada,36 we found that the incidence of DKA in female patients, especially those with early-onset diabetes (ie, 0–12 years), was higher than that in male patients. A cohort of 1234 children with type 1 diabetes in USA showed that female patients were greatly affected by DKA. A female preponderance of DKA was observed in a longitudinal study of childhood type 1 diabetes in Australia.35 Similarly, a Canadian study of childhood type 1 diabetes showed that female sex was a significant predictor of DKA.36 In fact, insulin omission or intentional insulin undertreatment due to fear of weight gain37 and high prevalence of eating disorders38 and psychiatric disorders34 among female patients with type 1 diabetes have been recognised as precipitating causes of DKA. Hence, effective interventions such as health education and communication for females with type 1 diabetes are needed to reduce the incidence of DKA.
Hypoglycaemia
Increased hypoglycaemic events have been recognised as a result of the undesired effects of intensive insulin therapy with strict glycaemic control.39 The present study showed that the incidence rates of hospitalised and outpatient hypoglycaemia in the Taiwanese population with type 1 diabetes were 3.89 and 39.93 per 1000 person-years, respectively, which are much lower than that reported in type 1 diabetes children (0–19 years) in the USA (incidence of severe hypoglycaemia: 190 per 1000 person-years).34 Such discrepancies in international data may be explained by different definitions and assessment approaches for hypoglycaemic events. We targeted hospital admissions for hypoglycaemia based on ICD-9 CM codes, whereas the US study used patients’ reported survey data and classified severe hypoglycaemia as acute episodes requiring the assistance of another person for treatment reported in the preceding 3 months.40
Moreover, we observed that early-onset patients were greatly affected by acute complications (ie, DKA, hypoglycaemia). It has been documented that, among young children with type 1 diabetes, inconsistent eating patterns and lesser ability to recognise and report acute symptoms make it difficult to achieve glycaemic control, leading to glycaemic fluctuations that cause multiple episodes of hyperglycaemia (ie, DKA) and hypoglycaemia.41 Frequent exposures to hyperglycaemia and hypoglycaemia in early-onset patients with type 1 diabetes could lead to a range of neurocognitive dysfunctions and brain changes.42 Also, structural brain changes in type 1 diabetes children may occur due to recurrent hypoglycaemia.43 Hence, given the high rates of acute complications and associated serious consequences, effective management protocols and identification and treatment of precipitating causes are needed.44 In particular, regular glycaemic monitoring and identification of risk factors in young patients with type 1 diabetes are needed to reduce the frequency and severity of DKA and hypoglycaemia.
Chronic diabetes-related complications in type 1 diabetes
Diabetic retinopathy
Diabetic retinopathy is the main cause of blindness in the adult population.45 Almost all patients with type 1 diabetes develop evident retinopathy in the first 20 years of diagnosis.46 The present study showed that more than half (~69%) of the patients with type 1 diabetes experienced some form of diabetic retinopathy at 12 years after diagnosis. We observed that the incidence density of diabetes retinopathy is greatest among chronic complications in Taiwanese patients with type 1 diabetes (4.53 per 100 person-years over a period of 15 years of follow-up). As compared with the incident density of proliferative retinopathy (19.5 per 1000 person-years) in the Pittsburgh EDC Study of patients with type 1 diabetes with a mean age of 28 years and diabetes duration of 19 years at baseline examination,10 our estimate (5.87 per 1000 person-years) based on a cohort of patients newly diagnosed with type 1 diabetes is lower. Such a difference between studies may be explained by diabetes duration and age at baseline of study examination. Moreover, comparing the prevalence of STDR in patients with type 1 diabetes in this study (2.00% for women and 1.66% for men) with that previously observed in Taiwanese patients with type 2 diabetes (2.75% for women and 2.87% for men)47 reveals a slightly lower advanced diabetic retinopathy (ie, STDR) in the patients with type 1 diabetes versus type 2 diabetes. However, the lower rate of STDR in our study may be due to the other study’s inclusion of prevalent type 2 diabetes cases with longer diabetes duration47 as compared with incident type 1 diabetes targeted in this study.
Consistent with previous studies,48 49 the present study demonstrated a female preponderance in diabetic retinopathy. A large cohort of 8114 patients with type 1 diabetes and their families assembled over 25 years from the USA showed that females had 1.7-fold higher retinopathy risk (p<0.001) when compared with that of males.48 Also, a cross-sectional study of 247 Italian patients with type 1 diabetes showed a significant relationship between diabetic retinopathy and female gender (p=0.01).49 Although exact hormone, genetic, lifestyle or environmental factors are unclear, a differential effect of sex steroid hormones has been proposed to explain this gender discrepancy.50 Also, age at diabetes onset has been shown to be associated with the development of diabetic retinopathy.49 51 An early age at onset (5–14 years) appears to modify the long-term risk of proliferative retinopathy.51 Consistent with other studies, we observed lower incidence of diabetic retinopathy in early-onset patients as compared with that in late-onset patients. Nevertheless, given a high rate of diabetes retinopathy observed among Taiwanese patients with type 1 diabetes, early detection using routine eye examination, control for risk factors of diabetic retinopathy (eg, hypertension, hyperglycaemia, hyperlipidaemia)9 as well as development of tailored intervention strategies for age–sex subgroups are important.
Diabetic nephropathy
Our results show that diabetic nephropathy is the second most common microvascular complication among the Taiwanese population with type 1 diabetes. Without interventions, patients with diabetes with microalbuminuria typically progress to proteinuria and overt diabetic nephropathy.52 Diabetic nephropathy is a leading cause of ESRD among patients with diabetes.52 As estimated, individuals with type 1 diabetes face a 20%–50% chance of developing ESDR that requires dialysis or renal transplantation.53 The Pittsburgh EDC study reported that the incidence density of renal failure (based on self-reported renal transplantation and dialysis) was 6.3 per 1000 person-years over 12 years of follow-up,10 while the present study based on ICD-9 codes of renal failure found that the incidence of renal failure was 1.31 per 1000 person-years over 15 years of follow-up. Of note, the EDC study enrolled more cases with advanced type 1 diabetes (ie, mean age of 28 years and diabetes duration of 19 years at baseline examination10) than those in our study (ie, cases newly diagnosed with type 1 diabetes in 2000–2012), which may explain the higher rate of renal failure in the EDC study. A large inception cohort study of Danish patients newly diagnosed with type 1 diabetes followed for a median of 18 years reported that the cumulative incidences of persistent microalbuminuria and macroalbuminuria were 33.6% and 14.6%, respectively, while the present study found that overall cumulative incidence of any form of diabetic nephropathy was 30.2% at 12 years after diabetes diagnosis. Moreover, early-onset diabetes appears to be protective for developing diabetic nephropathy12 54–56 and may delay the time until microalbuminuria.56 Consistently, we found that late-onset diabetes patients were more affected by diabetic nephropathy than were early-onset diabetes patients. Nevertheless, given the fact that Taiwan has the highest number of patients undergoing renal dialysis in the world, where diabetes contributes to about 40% of ESRD cases,57 it is critical for routine annual screening of clinical signs of diabetic nephropathy (ie, proteinuria, microalbuminuria), optimal control of blood glucose and relevant risk factors (eg, retinopathy, smoking, dyslipidaemia, hypertension14 58 59), and early intervening medications for prevention (eg, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker for those with comorbid hypertension).9
Diabetic neuropathy
Diabetic neuropathy refers to the presence of symptoms, signs or both of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes.60 Peripheral neuropathy in diabetes may manifest in several different forms, including sensory, focal/multifocal and autonomic neuropathies.61 The epidemiological data of diabetic neuropathy are very limited. A study of 467 Italian patients with type 1 diabetes showed that the prevalence rates of asymptomatic and symptomatic neuropathy were 7.2% and 21.3%, respectively.62 The present study is the first study to provide epidemiology data on diabetic neuropathy among ethnic Chinese patients with type 1 diabetes from Asia. We found that the incidence rate was 23.93 per 1000 person-years over 15 years of follow-up, and that the cumulative incidence was 23.7% at 12 years after diabetes diagnosis. We also observed that the incidence of diabetic neuropathy in late-onset patients were much higher than that in early-onset patients. Similarly, the Italian study of type 1 diabetes showed that the prevalence of diabetic neuropathy was higher in patients at older ages.62 Since diabetic neuropathy contributes to considerable disabilities and mortality, it is critical for clinicians to understand its manifestations, prevention and treatment.9 Early prevention strategies that control hypertension and hyperglycaemia and identify patients with peripheral neuropathy or peripheral vascular disease and annual screening for these conditions are strongly recommended.9
Cardiovascular diseases
CVD is a leading cause of mortality in patients with type 1 diabetes63 64 and accounts for the greatest proportion of healthcare spending for patients with diabetes.64 65 As compared with patients without diabetes, type 1 diabetes increases the risk of CVD by 10-fold,63 66 which contributes to two-thirds of mortality in patients with type 1 diabetes.67 68 The Pittsburgh EDC study showed an incidence density of 3.6 per 1000 person-years for coronary heart diseases (defined as coronary-artery-disease-related death, a history of myocardial infarction, angiographic stenosis ≥50% including revascularisation) over a period of 12 years,10 while the present study found that the incidence density for a broader category of CVD (including myocardial infarction, ischaemic heart diseases, heart failure, stroke and arrhythmia, as shown in online supplementary table 1) in the Taiwanese population with type 1 diabetes within 15 years of follow-up was 4.39 per 1000 person-years. The cumulative incidence of CVD (including only stroke and coronary heart disease) at 12years following diabetes diagnosis was 1%–2% among Finnish patients with type 1 diabetes,13 which is lower than that for the Taiwanese patients with type 1 diabetes observed in the present study (~5.2%). Moreover, we found that late-onset patients were greatly affected by CVD. In fact, old age is recognised as a predictor of vascular diseases,63 which may be explained by the calcification of extremity arteries and hypertension in older age patients, which are risk factors of macrovascular diseases.69
Methodological concerns
Some limitations of this study should be acknowledged. The classification of diabetes-related complications based on the ICD-9 CM codes in claims data may underestimate the occurrence of the complications. For example, patients experiencing clinical symptoms/signs of diabetes-related complications (eg, hypoglycaemia) may not see doctors if they can tolerate them. Also, the claims data do not capture clinical/minor symptoms or signs of diabetes-related complications such as minor microalbuminuria. The glycaemic biomarkers such as blood glucose were not available from the claims data, so the identification of hyperglycaemia or hypoglycaemia was only based on the ICD-9 CM diagnosis codes. So, we might underestimate the incidence of hypoglycaemic events and may not be able to disentangle the severity of hypoglycaemia. However, the claims records capture defined diabetes-related complications that are required for medical assistance or treatments, which lead to more conservative estimates and reveal important manifestations of diabetes-related complications for clinical attention. Moreover, based on our operational definition for hospitalised hypoglycaemia (ie, any one of diagnosis codes with hypoglycaemia from the five diagnosis codes in the inpatient files of the NHIRD), two types of hypoglycaemic events could be included: (1) hospital admission for hypoglycaemia and (2) other reasons for hospital admission (eg, DKA), and then hypoglycaemia happened during hospitalisation. It is difficult to differentiate these two types of hypoglycaemic events based on the retrospective claims data we used. However, in the clinical practice in Taiwan, the first code from the five diagnosis codes in hospitalisation is typically to be the main/primary reason for hospital admission. With this regard, we re-run the analyses for hospitalised hypoglycaemia which was identified from the first diagnosis code in hospitalisation. The results are provided in the online supplementary table 2 and supplementary figures 2 and 3. These re-analytical results may also ease the concern that patients who came to hospital primarily for reasons that may induce hypoglycaemia during hospitalisation. Lastly, the generalisability of our study results may be limited to ethnic Chinese populations. In addition, our results may represent only ethnic Chinese patients with type 1 diabetes in Taiwan.
Conclusion
Using an incident cohort of patients with type 1 diabetes diagnosed during the period 1999–2012 with a maximum of 15 years of follow-up, we found that most patients with type 1 diabetes were affected by DKA and retinopathy, which highlight the critical need to identify precipitating causes and modifiable factors for developing preventive strategies and intervening treatment protocols to minimise the impact of these complications. Age and sex discrepancies appear in epidemiological data of diabetes-related complications; late-onset diabetes females were greatly affected by advanced retinopathy (ie, STDR) and hospitalised hypoglycaemia, while early-onset females had a high incidence of DKA. Chronic diabetes-related complications were more common in patients with late-onset of type 1 diabetes, while early-onset individuals were most affected by acute complications. More attention should be given to identify potential risk factors and contributors to such age–sex differences in diabetes-related complications. Population-based data on the incidence of diabetes-related complications from this study are important for clinicians to recognise the need for diagnostic awareness and for policy-makers to develop effective treatments for patients with type 1 diabetes.
bmjopen-2016-015117supp003.pdf (53.3KB, pdf)
bmjopen-2016-015117supp004.pdf (143.2KB, pdf)
Supplementary Material
Acknowledgments
We gratefully thank National Cheng Kung University and its affiliated hospital for all their support.
Footnotes
Contributors: H-TO contributed substantially to the study concept and design, acquisition of data, analysis and interpretation of data. T-YL contributed to data collection and the analysis. C-YL, J-SW and Z-JS provided statistical and clinical interpretation of the results. H-TO wrote the first draft of the manuscript, and T-YL, C-YL, J-SW and Z-JS very critically revised the manuscript. All authors gave approval for the publication of the final version.
Funding: This research was supported by the following two grants: the National Cheng Kung University Hospital, Tainan, Taiwan (grant no. NCKUH 10602008), and the Ministry of Science and Technology, Taiwan (grant no. MOST 104-2320-B-006-008-MY3).
Competing interests: None declared.
Patient consent: This study used deidentified personal medical reimbursement claims data. The patient informed consent was waived by the the Institutional Review Board of National Cheng Kung University Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional data available in relation to this manuscript.
Correction notice: This paper has been amended since it was published Online First. Owing to a scripting error, some of the publisher names in the references were replaced with 'BMJ Publishing Group'. This only affected the full text version, not the PDF. We have since corrected theseerrors and the correct publishers have been inserted into the references.
References
- 1. Onkamo P, Väänänen S, Karvonen M, et al. . Worldwide increase in incidence of type I diabetes--the analysis of the data on published incidence trends. Diabetologia 1999;42:1395–403. 10.1007/s001250051309 [DOI] [PubMed] [Google Scholar]
- 2. Karvonen M, Viik-Kajander M, Moltchanova E, et al. . Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000;23:1516–26. 10.2337/diacare.23.10.1516 [DOI] [PubMed] [Google Scholar]
- 3. Soltesz G, Patterson CC, Dahlquist G. EURODIAB Study Group. Worldwide childhood type 1 diabetes incidence--what can we learn from epidemiology? Pediatr Diabetes 2007;8 Suppl 6:6–14. 10.1111/j.1399-5448.2007.00280.x [DOI] [PubMed] [Google Scholar]
- 4. Lu CL, Shen HN, Chen HF, et al. . Epidemiology of childhood Type 1 diabetes in Taiwan, 2003 to 2008. Diabet Med 2014;31:666–73. 10.1111/dme.12407 [DOI] [PubMed] [Google Scholar]
- 5. Goldstein DE, Walker B, Rawlings SS, et al. . Hemoglobin A1c levels in children and adolescents with diabetes mellitus. Diabetes Care 1980;3:503–7. 10.2337/diacare.3.4.503 [DOI] [PubMed] [Google Scholar]
- 6. Ziegler R, Heidtmann B, Hilgard D, et al. . Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes 2011;12:11–17. 10.1111/j.1399-5448.2010.00650.x [DOI] [PubMed] [Google Scholar]
- 7. Lewis EJ, Hunsicker LG, Bain RP, et al. . The effect of Angiotensin-Converting-Enzyme inhibition on Diabetic Nephropathy. N Engl J Med Overseas Ed 1993;329:1456–62. 10.1056/NEJM199311113292004 [DOI] [PubMed] [Google Scholar]
- 8. Secrest AM, Becker DJ, Kelsey SF, et al. . All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny County type 1 diabetes registry. Diabetes Care 2010;33:2573–9. 10.2337/dc10-1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bate KL, Jerums G. 3: preventing complications of diabetes. Med J Aust 2003;179:498–505. [DOI] [PubMed] [Google Scholar]
- 10. Pambianco G, Costacou T, Ellis D, et al. . The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes complications Study experience. Diabetes 2006;55:1463–9. 10.2337/db05-1423 [DOI] [PubMed] [Google Scholar]
- 11. Stephenson J, Fuller JH. Group EICS. Microvascular and acute complications in IDDM patients: the EURODIAB IDDM complications study. Diabetologia 1994;37:278–85. 10.1007/BF00398055 [DOI] [PubMed] [Google Scholar]
- 12. Chaturvedi N, Bandinelli S, Mangili R, et al. . Microalbuminuria in type 1 diabetes: rates, risk factors and glycemic threshold. Kidney Int 2001;60:219–27. 10.1046/j.1523-1755.2001.00789.x [DOI] [PubMed] [Google Scholar]
- 13. Tuomilehto J, Borch-Johnsen K, Molarius A, et al. . Incidence of cardiovascular disease in type 1 (insulin-dependent) diabetic subjects with and without diabetic nephropathy in Finland. Diabetologia 1998;41:784–90. 10.1007/s001250050988 [DOI] [PubMed] [Google Scholar]
- 14. Hovind P, Tarnow L, Rossing P, et al. . Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ 2004;328:1105 10.1136/bmj.38070.450891.FE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malik MO, Govan L, Petrie JR, et al. . Ethnicity and risk of cardiovascular disease (CVD): 4.8 year follow-up of patients with type 2 diabetes living in Scotland. Diabetologia 2015;58:716–25. 10.1007/s00125-015-3492-0 [DOI] [PubMed] [Google Scholar]
- 16. Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep 2013;13:814–23. 10.1007/s11892-013-0421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. U.S. Renal Data System, USRDS 2003 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD, 2003. [Google Scholar]
- 18. Young BA, Katon WJ, Von Korff M, et al. . Racial and ethnic differences in microalbuminuria prevalence in a diabetes population: the pathways study. J Am Soc Nephrol 2005;16:219–28. 10.1681/ASN.2004030162 [DOI] [PubMed] [Google Scholar]
- 19. Lin WH, Wang MC, Wang WM, et al. . Incidence of and mortality from Type I diabetes in Taiwan from 1999 through 2010: a nationwide cohort study. PLoS One 2014;9:e86172 10.1371/journal.pone.0086172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng TM. Taiwan's new national health insurance program: genesis and experience so far. Health Aff 2003;22:61–76. 10.1377/hlthaff.22.3.61 [DOI] [PubMed] [Google Scholar]
- 21. Ou HT, Chang KC, Li CY, et al. . Risks of cardiovascular diseases associated with dipeptidyl peptidase-4 inhibitors and other antidiabetic drugs in patients with type 2 diabetes: a nation-wide longitudinal study. Cardiovasc Diabetol 2016;15:41 10.1186/s12933-016-0350-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ht O, Chang KC, Liu YM, et al. . Recent trends in the use of antidiabetic medications from 2008 to 2013: a nation‐wide population‐based study from Taiwan. Journal of diabetes 2016. [DOI] [PubMed] [Google Scholar]
- 23. Hou WH, Chang KC, Li CY, et al. . Dipeptidyl peptidase-4 inhibitor use is not associated with elevated risk of severe joint pain in patients with type 2 diabetes: a population-based cohort study. Pain 2016;157:1954–9. 10.1097/j.pain.0000000000000596 [DOI] [PubMed] [Google Scholar]
- 24. Ou HT, Yang CY, Wang JD, et al. . Life Expectancy and Lifetime Health Care expenditures for type 1 Diabetes: a Nationwide Longitudinal Cohort of Incident cases followed for 14 years. Value Health 2016;19:976–84. 10.1016/j.jval.2016.05.017 [DOI] [PubMed] [Google Scholar]
- 25. Ht O, Chang KC, Cy L, et al. . Comparative cardiovascular risks of dipeptidyl peptidase‐4 inhibitors with other 2nd and 3rd line antidiabetic drugs in patients with type 2 diabetes. British Journal of Clinical Pharmacology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ou HT, Chen YT, Liu YM, et al. . Comparative cost-effectiveness of metformin-based dual therapies associated with risk of cardiovascular diseases among Chinese patients with type 2 diabetes: Evidence from a population-based national cohort in Taiwan. Diabetes Res Clin Pract 2016;116:14–25. 10.1016/j.diabres.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 27. Vella S, Buetow L, Royle P, et al. . The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia 2010;53:809–20. 10.1007/s00125-009-1636-9 [DOI] [PubMed] [Google Scholar]
- 28. Strowig SM, Raskin P. The effect of rosiglitazone on overweight subjects with type 1 diabetes. Diabetes Care 2005;28:1562–7. 10.2337/diacare.28.7.1562 [DOI] [PubMed] [Google Scholar]
- 29. Gail MH, Benichou J. Encyclopedia of epidemiologic methods: John Wiley & Sons, 2000. [Google Scholar]
- 30. Sahai H, Khurshid A. Statistics in Epidemiology: Methods, Techniques and Applications: CRC press, 1995. [Google Scholar]
- 31. Gray RJ. A class of K-Sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics 1988;16:1141–54. 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 32. Graves EJ, Gillum BS. Detailed diagnoses and procedures, National Hospital Discharge Survey, 1995. Vital Health Stat 13 1997;13:1–146. [PubMed] [Google Scholar]
- 33. Javor KA, Kotsanos JG, McDonald RC, et al. . Diabetic ketoacidosis charges relative to medical charges of adult patients with type I diabetes. Diabetes Care 1997;20:349–54. 10.2337/diacare.20.3.349 [DOI] [PubMed] [Google Scholar]
- 34. Rewers A, Chase HP, Mackenzie T, et al. . Predictors of acute complications in children with type 1 diabetes. JAMA 2002;287:2511–8. 10.1001/jama.287.19.2511 [DOI] [PubMed] [Google Scholar]
- 35. Neu A, Willasch A, Ehehalt S, et al. . DIARY Group Baden-Wuerttemberg. Ketoacidosis at onset of type 1 diabetes mellitus in children--frequency and clinical presentation. Pediatr Diabetes 2003;4:77–81. 10.1034/j.1399-5448.2003.00007.x [DOI] [PubMed] [Google Scholar]
- 36. Alaghehbandan R, Collins KD, Newhook LA, et al. . Childhood type 1 diabetes mellitus in Newfoundland and Labrador, Canada. Diabetes Res Clin Pract 2006;74:82–9. 10.1016/j.diabres.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 37. Meltzer LJ, Johnson SB, Prine JM, et al. . Disordered eating, body mass, and glycemic control in adolescents with type 1 diabetes. Diabetes Care 2001;24:678–82. 10.2337/diacare.24.4.678 [DOI] [PubMed] [Google Scholar]
- 38. Rodin GM, Daneman D. Eating disorders and IDDM. A problematic association. Diabetes Care 1992;15:1402–12. 10.2337/diacare.15.10.1402 [DOI] [PubMed] [Google Scholar]
- 39. Pramming S, Thorsteinsson B, Bendtson I, et al. . Symptomatic hypoglycaemia in 411 type 1 diabetic patients. Diabet Med 1991;8:217–22. 10.1111/j.1464-5491.1991.tb01575.x [DOI] [PubMed] [Google Scholar]
- 40. Craig ME, Jones TW, Silink M, et al. . Diabetes care, glycemic control, and complications in children with type 1 diabetes from Asia and the Western Pacific Region. J Diabetes Complications 2007;21:280–7. 10.1016/j.jdiacomp.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 41. Aye T, Reiss AL, Kesler S, et al. . The feasibility of detecting neuropsychologic and neuroanatomic effects of type 1 diabetes in young children. Diabetes Care 2011;34:1458–62. 10.2337/dc10-2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Northam EA, Anderson PJ, Jacobs R, et al. . Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care 2001;24:1541–6. 10.2337/diacare.24.9.1541 [DOI] [PubMed] [Google Scholar]
- 43. Ho MS, Weller NJ, Ives FJ, et al. . Prevalence of structural central nervous system abnormalities in early-onset type 1 diabetes mellitus. J Pediatr 2008;153:385–90. 10.1016/j.jpeds.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 44. Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic Ketoacidosis and hyperglycemic hyperosmolar syndrome. Diabetes Spectrum 2002;15:28–36. 10.2337/diaspect.15.1.28 [DOI] [Google Scholar]
- 45. VanNewkirk MR, Weih L, McCarty CA, et al. . Cause-specific prevalence of bilateral visual impairment in Victoria, Australia: the Visual Impairment Project. Ophthalmology 2001;108:960–7. [DOI] [PubMed] [Google Scholar]
- 46. Fong DS, Aiello L, Gardner TW, et al. . American Diabetes Association. Diabetic retinopathy. Diabetes Care 2003;26 Suppl 1:S99–S102. [DOI] [PubMed] [Google Scholar]
- 47. Lin JC, Shau WY, Lai MS. Sex- and age-specific prevalence and incidence rates of sight-threatening diabetic retinopathy in Taiwan. JAMA Ophthalmol 2014;132:922–8. 10.1001/jamaophthalmol.2014.859 [DOI] [PubMed] [Google Scholar]
- 48. Monti MC, Lonsdale JT, Montomoli C, et al. . Familial risk factors for microvascular complications and differential male-female risk in a large cohort of American families with type 1 diabetes. J Clin Endocrinol Metab 2007;92:4650–5. 10.1210/jc.2007-1185 [DOI] [PubMed] [Google Scholar]
- 49. Minuto N, Emmanuele V, Vannati M, et al. . Retinopathy screening in patients with type 1 diabetes diagnosed in young age using a non-mydriatic digital stereoscopic retinal imaging. J Endocrinol Invest 2012;35:389–94. 10.3275/8016 [DOI] [PubMed] [Google Scholar]
- 50. Holl RW, Lang GE, Grabert M, et al. . Diabetic retinopathy in pediatric patients with type-1 diabetes: effect of diabetes duration, prepubertal and pubertal onset of diabetes, and metabolic control. J Pediatr 1998;132:790–4. 10.1016/S0022-3476(98)70305-1 [DOI] [PubMed] [Google Scholar]
- 51. Hietala K, Harjutsalo V, Forsblom C, et al. . FinnDiane Study Group. Age at onset and the risk of proliferative retinopathy in type 1 diabetes. Diabetes Care 2010;33:1315–9. 10.2337/dc09-2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marshall SM. Clinical features and management of diabetic nephropathy. Textbook of diabetes Boston: Blackwell Publishing 2003;2003. [Google Scholar]
- 53. Nordwall M, Bojestig M, Arnqvist HJ, et al. . Declining incidence of severe retinopathy and persisting decrease of nephropathy in an unselected population of type 1 diabetes-the Linköping Diabetes complications Study. Diabetologia 2004;47:1266–72. 10.1007/s00125-004-1431-6 [DOI] [PubMed] [Google Scholar]
- 54. Donaghue KC, Fairchild JM, Craig ME, et al. . Do all prepubertal years of diabetes duration contribute equally to diabetes complications? Diabetes Care 2003;26:1224–9. 10.2337/diacare.26.4.1224 [DOI] [PubMed] [Google Scholar]
- 55. Svensson M, Nyström L, Schön S, et al. . Age at onset of childhood-onset type 1 diabetes and the development of end-stage renal disease: a nationwide population-based study. Diabetes Care 2006;29:538–42. 10.2337/diacare.29.03.06.dc05-1531 [DOI] [PubMed] [Google Scholar]
- 56. Raile K, Galler A, Hofer S, et al. . Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care 2007;30:2523–8. 10.2337/dc07-0282 [DOI] [PubMed] [Google Scholar]
- 57. InternationalComparisons American Journal of Kidney Diseases. 57:e383–e396. [Google Scholar]
- 58. Jenkins AJ, Lyons TJ, Zheng D, et al. . DCCT/EDIC Research Group. Lipoproteins in the DCCT/EDIC cohort: associations with diabetic nephropathy. Kidney Int 2003;64:817–28. 10.1046/j.1523-1755.2003.00164.x [DOI] [PubMed] [Google Scholar]
- 59. Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care 2002;25:859–64. 10.2337/diacare.25.5.859 [DOI] [PubMed] [Google Scholar]
- 60. American Diabetes A. Standards of medical care for patients with diabetes mellitus. Puerto Rico Health Sciences Journal 2013;20. [Google Scholar]
- 61. Boulton AJ, Vinik AI, Arezzo JC, et al. . American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–62. 10.2337/diacare.28.4.956 [DOI] [PubMed] [Google Scholar]
- 62. Veglio M, Sivieri R. The Neuropathy Study Group of the Italian Society for the Study of Diabetes, Piemonte Affiliate. Prevalence of Neuropathy in IDDM Patients in Piemonte, Italy. Diabetes Care 1993;16:456–61. 10.2337/diacare.16.2.456 [DOI] [PubMed] [Google Scholar]
- 63. Laing SP, Swerdlow AJ, Slater SD, et al. . Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760–5. 10.1007/s00125-003-1116-6 [DOI] [PubMed] [Google Scholar]
- 64. Paterson AD, Rutledge BN, Cleary PA, et al. . Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. The effect of intensive diabetes treatment on resting heart rate in type 1 diabetes: the Diabetes Control and complications Trial/Epidemiology of Diabetes Interventions and complications study. Diabetes Care 2007;30:2107–12. 10.2337/dc06-1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hogan P, Dall T, Nikolov P. American Diabetes Association. Economic costs of diabetes in the US in 2002. Diabetes Care 2003;26:917. [DOI] [PubMed] [Google Scholar]
- 66. Dorman JS, Laporte RE, Kuller LH, et al. . The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. mortality results. Diabetes 1984;33:271–6. 10.2337/diab.33.3.271 [DOI] [PubMed] [Google Scholar]
- 67. Borch-Johnsen K, Nissen H, Henriksen E, et al. . The natural history of insulin-dependent diabetes mellitus in Denmark: 1. Long-term survival with and without late diabetic complications. Diabet Med 1987;4:201–10. 10.1111/j.1464-5491.1987.tb00863.x [DOI] [PubMed] [Google Scholar]
- 68. Krolewski AS, Warram JH, Christlieb AR, et al. . The changing natural history of nephropathy in type I diabetes. Am J Med 1985;78:785–94. 10.1016/0002-9343(85)90284-0 [DOI] [PubMed] [Google Scholar]
- 69. Maser RE, Wolfson SK, Ellis D, et al. . Cardiovascular disease and arterial calcification in insulin-dependent diabetes mellitus: interrelations and risk factor profiles. Pittsburgh Epidemiology of Diabetes complications Study-V. Arterioscler Thromb 1991;11:958–65. 10.1161/01.ATV.11.4.958 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-015117supp001.pdf (193.8KB, pdf)
bmjopen-2016-015117supp002.pdf (934KB, pdf)
bmjopen-2016-015117supp003.pdf (53.3KB, pdf)
bmjopen-2016-015117supp004.pdf (143.2KB, pdf)