Abstract
Although the genetic basis of Duchenne muscular dystrophy has been known for almost thirty years, the cellular and molecular mechanisms characterizing the disease are not completely understood and an efficacious treatment remains to be developed. In this study we analyzed proteomics data obtained with the SomaLogic technology from blood serum of a cohort of patients and matched healthy subjects. We developed a workflow based on biomarker identification and network-based pathway analysis that allowed us to describe different deregulated pathways. In addition to muscle-related functions, we identified other biological processes such as apoptosis, signaling in the immune system and neurotrophin signaling as significantly modulated in patients compared with controls. Moreover, our network-based analysis identified the involvement of FoxO transcription factors as putative regulators of different pathways. On the whole, this study provided a global view of the molecular processes involved in Duchenne muscular dystrophy that are decipherable from serum proteome.
Introduction
Proteomics, the large scale analysis of proteins, is feasible thanks to the availability of different technologies, such as mass spectrometry, gel-based techniques, antibody-based arrays and recently developed aptamer-based technologies [1–3]. Despite these technological improvements, the extraction of knowledge from the data produced is still challenging and the development of data analysis workflows capable of providing additional insight compared to traditional methods would be highly advantageous. In particular, a first desirable outcome is the identification of accurate diagnostic and prognostic markers suitable for subject stratification, which would shorten the path to the application of precision medicine concepts to the clinical practice [4–6]. Another highly desirable outcome is a better understanding of the disease on the basis of the newly available proteomic profiles. For this second aim a systems biology approach based on network analysis would enable integration of highly descriptive disease biomarkers with existing knowledge and potentially provide additional insight on the affected biological processes [7–9]. In this work we introduce an approach to achieve both these aims using recently published proteomics data obtained from a Duchenne Muscular Dystrophy (DMD) cohort. DMD is a rare disease caused by mutations in the gene that encodes dystrophin. The main clinical feature observed in DMD patients is the presence of muscular damage coupled with chronic inflammation that leads to a progressive muscular degeneration and fibrosis. The average age of diagnosis is usually at four-five years, when the symptoms appear and the disability starts to arise [10]. The availability of disease biomarkers that can be assessed using simple, non-invasive techniques would be useful to better understand the biological pathways altered in the disease and could be used for an early diagnosis. To this second end, serum creatine kinase (CK) level is usually evaluated. However, blood CK concentration shows high variability because it is influenced by age of the child, physical activity extent and pharmacological treatments [11]. In our study we employed an optimized version of the rank-based classification algorithm introduced in [12,13] to identify two biomarker panels based on SomaLogic profiles of DMD patients and controls and characterize their classification performance. We then used the panels as the starting point of an analysis aimed at identifying the biological pathways altered in the disease. To circumvent the limitation represented by the relatively short list of proteins measurable in blood with the SomaLogic array, we resorted to a network-based systems biology approach to identify a list of biological processes that are affected in DMD. Therefore a first contribution of our study is the identification of accurate diagnostic and prognostic markers suitable for subject stratification. Another contribution is a better understanding of the disease in terms of pathways identified as affected on the basis of the altered serum proteomic profiles.
Results
Workflow overview
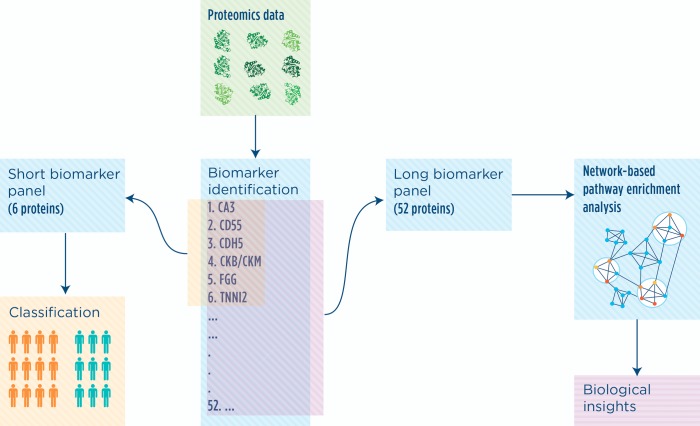
We built a workflow based on a biomarker discovery algorithm and a tool for network analysis that we recently developed [12–14] (Fig 1). The biomarker discovery algorithm identifies subject-specific lists of proteins, herein called signatures, which are then compared in order to classify each subject as belonging to one of the two classes (affected or controls). The aggregation of the individual signatures produces a list of proteins useful for subject classification and we refer to it as a ‘biomarker panel’. Since the method can be tuned to obtain panels of different lengths, we applied it twice with different settings to obtain two biomarker panels, as shown in Fig 1. The shorter one was obtained specifically for classification purposes, while the longer one was derived to identify the biological processes affected by the disease. Specifically, we used the proteins in the long biomarker panel as input to the network-based tool NASFinder that identified functional modules from the sub-networks connecting biomarker proteins to known transcription factors.
Fig 1. Study workflow.
Proteomics data produced with SOMAscan technology were analyzed using a rank-based classification algorithm. We obtained two set of proteins (biomarker panels) useful for subject classification and disease characterization. The longer biomarker panel was used as input for network-based analysis.
Identification of a short biomarker panel that discriminate DMD patients from controls
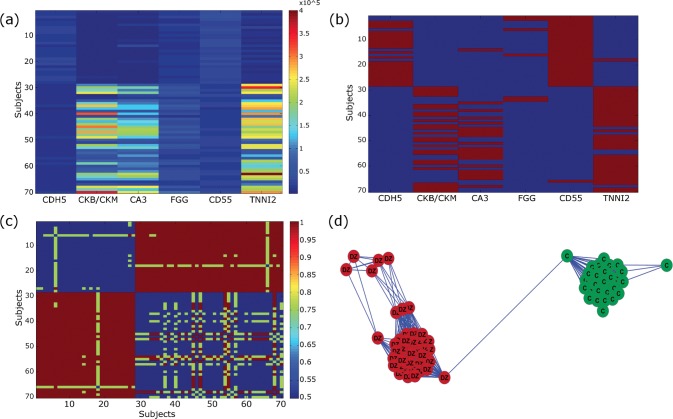
To identify biomarker panels associated with DMD, we analyzed concentration levels of 1128 blood proteins obtained using the SOMAscan technology on a cohort of 42 cases and 28 controls from The Parent Project Muscular Dystrophy-Cincinnati Children’s Hospital Medical Centre study (see details in Material and Methods). Overall, we determined that individual signatures formed of just two proteins out of a panel of six were satisfactory to discriminate DMD patients from controls with perfect accuracy (averaged over all the cross-validation rounds) and permutation test p-value < 0.001. These six protein epitopes correspond to the following genes: CA3, CD55, CDH5, CKB/CKM, FGG and TNNI2 (Table 1). Principal component analysis performed on the dataset containing only data about these six proteins confirmed the separation between DMD patients and controls according to the main axis of variation (S1 Fig). The contribution of each protein in achieving a control/affected classification is illustrated in Fig 2. The heatmap of the measured protein levels shows that no single protein level highly correlates with a partition of the subjects in two groups (Fig 2A). Signatures of length two, as those selected by our method for inclusion in the short panel (Fig 2B, red boxes), are sufficient to correctly identify the control/affected status of each individual. As an intermediate step, our algorithm computes a complete distance matrix based on the similarity between each pair of subject signatures (shown in the form of a heatmap in Fig 2C). As an aid in interpreting the distance matrix, a map of the subjects is drawn by using the shortest N = 20% distances, with colors added afterwards according to control/affected status. In this case, the map clearly shows the emergence of two well-defined groups, with subject perfectly segregated by disease status (Fig 2D).
Table 1. Short biomarker panel.
| SomaLogic ID | UniProt ID | Protein name* | Gene Symbol | p-value |
|---|---|---|---|---|
| 3799–11_2 | P07451 | Carbonic anhydrase 3 | CA3 | 1.31E-10 |
| 5069–9_3 | P08174 | Complement decay-accelerating factor | CD55 | 3.89E-09 |
| 2819–23_2 | P33151 | Cadherin-5 | CDH5 | 2.30E-09 |
| 3714–49_2 | P12277 / P06732 | Creatine kinase B-type / Creatine kinase M-type | CKB CKM | 1.32E-10 |
| 4989–7_1 | P02679 | Fibrinogen gamma chain | FGG | 2.77E-09 |
| 5440–26_3 | P48788 | Troponin I, fast skeletal muscle | TNNI2 | 1.28E-10 |
* From Uniprot database
Fig 2. Signature-based classification of affected vs. control subjects for each individual.
(A) Heatmap of the six proteins included in the biomarker panel (columns) across the 70 subjects (rows). Control subjects: top 28 rows; affected subjects: bottom 52 rows. (B) Signatures composed of at least two proteins (red boxes) out of six are needed to accurately classify each subject as being a member of either the control or the affected group. (C) The heatmap of the distance matrix shows that signatures of length two are actually sufficient to correctly divide subjects into two groups. (D) A map of the subjects based on the distance matrix confirms that the two emerging groups are of homogeneous composition and points to a possible subgroup of affected individuals (green: control subjects, red: affected subjects; colors were added after the map was drawn).
The six proteins are related to different fundamental aspects of the disease. In particular, serum creatine kinase is a marker of muscular damage [15] and it has been known for a long time for being increased in DMD patients, even if it is not DMD-specific [16]. Similarly, carbonic anhydrase 3 is another indicator of muscular damage and it is highly expressed in skeletal muscle. Also troponin is a muscle–specific protein involved in regulating muscle contraction and it was found in blood after prolonged exercise [15]. In addition to these proteins with a muscle origin, the biomarker panel included CD55, which encodes a regulator of complement cascade and, together with fibrinogen and cadherin, is likely involved in fibrosis, a DMD hallmark [17,18].
Identification of a longer biomarker panel to investigate the disease biology
Despite the usefulness of the six proteins in the biomarker panel for classification purposes, they showed a limited applicability in understanding the pathways altered in the disease since only key aspects of the disease could be identified. We speculated that a longer list would be more likely to highlight the set of impaired pathways in a statistically significant way. For this reason, we trained the classification algorithm using different settings to obtain the longest protein list allowing classification accuracy close to 100% and we identified 52 proteins that are able to discriminate between disease and healthy controls with accuracy of prediction of 98.75% and permutation test p-value < 0.001 (Table 2).
Table 2. Long biomarker panel.
| SomaLogic ID | UniProt ID | Gene Symbol | p-value |
|---|---|---|---|
| 5451–1_3 | Q13740 | ALCAM | 5.89E-07 |
| 4194–26_3 | Q92688 | ANP32B | 3.27E-09 |
| 3799–11_2 | P07451 | CA3 | 1.68E-10 |
| 3326–58_2 | Q9BY67 | CADM1 | 7.29E-07 |
| 3350–53_2 | Q9UQM7 | CAMK2A | 9.83E-10 |
| 3351–1_1 | Q13554 | CAMK2B | 5.35E-08 |
| 3419–49_2 | Q13557 | CAMK2D | 5.51E-09 |
| 3290–50_2 | Q6YHK3 | CD109 | 7.00E-07 |
| 5103–30_3 | Q8TD46 | CD200R1 | 3.00E-07 |
| 5069–9_3 | P08174 | CD55 | 6.39E-09 |
| 5337–64_3 | P42081 | CD86 | 2.78E-07 |
| 2819–23_2 | P33151 | CDH5 | 2.81E-09 |
| 3714–49_2 | P12277 P06732 | CKB CKM | 6.67E-10 |
| 2670–67_4 | P06732 | CKM | 1.50E-09 |
| 2827–23_2 | P78423 | CX3CL1 | 1.32E-07 |
| 4545–53_3 | Q96DA6 | DNAJC19 | 1.45E-07 |
| 2677–1_1 | P00533 | EGFR | 1.55E-06 |
| 4908–6_1 | P17813 | ENG | 4.88E-08 |
| 4696–2_2 | P05413 | FABP3 | 1.52E-09 |
| 5029–3_1 | Q12884 | FAP | 1.46E-06 |
| 3052–8_2 | P48023 | FASLG | 1.75E-06 |
| 4907–56_1 | P02671 P02675 P02679 | FGA FGB FGG | 5.03E-09 |
| 2796–62_2 | P02671 P02675 P02679 | FGA FGB FGG | 9.31E-09 |
| 4989–7_1 | P02679 | FGG | 4.31E-09 |
| 2765–4_3 | O95390 | GDF11 | 3.84E-07 |
| 4272–46_2 | P06744 | GPI | 8.07E-10 |
| 3709–4_2 | P24298 | GPT | 3.57E-09 |
| 4775–34_3 | P06396 | GSN | 6.53E-08 |
| 4553–65_3 | Q7Z4V5 | HDGFRP2 | 5.49E-07 |
| 4232–19_2 | P08069 | IGF1R | 7.63E-07 |
| 3073–51_2 | O95998 | IL18BP | 1.58E-06 |
| 5092–51_3 | P78504 | JAG1 | 1.51E-07 |
| 2475–1_3 | P10721 | KIT | 2.48E-07 |
| 3890–8_2 | P07195 | LDHB | 3.05E-08 |
| 5005–4_1 | P53778 | MAPK12 | 2.12E-10 |
| 3042–7_2 | P02144 | MB | 9.01E-10 |
| 3853–56_1 | P40925 | MDH1 | 9.10E-07 |
| 5107–7_2 | P46531 | NOTCH1 | 7.60E-07 |
| 4179–57_3 | None | None | 1.65E-08 |
| 3390–72_2 | P42336 P27986 | PIK3CA PIK3R1 | 2.63E-08 |
| 2692–74_2 | P14555 | PLA2G2A | 1.39E-07 |
| 2212–69_1 | P00750 | PLAT | 1.60E-07 |
| 2961–1_2 | P04070 | PROC | 5.45E-07 |
| 2696–87_2 | O60542 | PSPN | 1.07E-06 |
| 5115–31_3 | Q969Z4 | RELT | 4.87E-08 |
| 3220–40_2 | P07949 | RET | 7.67E-10 |
| 3864–5_2 | P62081 | RPS7 | 7.06E-09 |
| 5122–92_2 | Q9H2E6 | SEMA6A | 9.31E-07 |
| 2665–26_2 | Q02223 | TNFRSF17 | 4.19E-07 |
| 4472–5_2 | P07951 | TPM2 | 1.26E-06 |
| 5440–26_3 | P48788 | TNNI2 | 3.88E-10 |
| 5441–67_3 | P19429 | TNNI3 | 2.31E-09 |
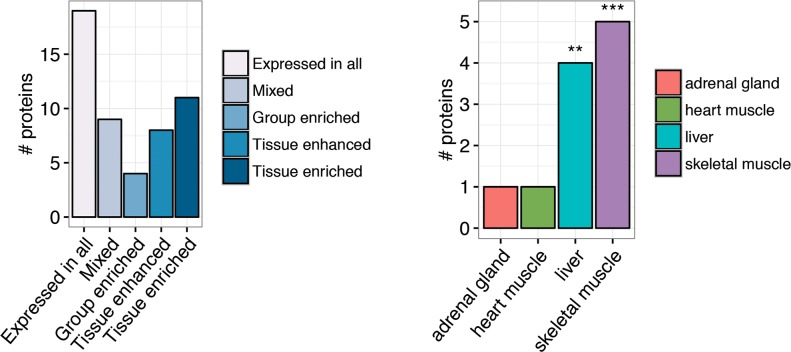
To evaluate the biological role of these proteins we first analyzed their tissue-specific expression using the transcript expression levels in Human Protein Atlas (HPA) dataset [19]. This analysis identified eleven proteins of the biomarker (21%) as tissue specific in HPA and, among them, highlighted an over-representation of muscle-specific proteins (Fig 3 and S1 Table). In fact, skeletal muscle resulted a significantly enriched tissue (p-value = 7.831e-05; Fisher’s exact test) with five proteins showing specific expression: Carbonic anhydrase 3 (CA3), Creatine kinase M-type (CKM), Mitogen-activated protein kinase 12 (MAPK12), Tropomyosin beta chain (TPM2) and Troponin I, fast-twitch isoform (TNNI2). In addition, the biomarker included one protein specific of heart muscle, the Cardiac troponin I (TNNI3). Four proteins, despite not being tissue specific, showed elevated expression in a group of tissues—HPA group enriched proteins—that included skeletal or heart muscle (CaMK-II subunit alpha (CAMK2A), CaMK-II subunit beta (CAMK2B), Heart-type fatty acid-binding protein (FABP3) and myoglobin (MB)). Notably, the biomarker also showed an over-representation of liver-specific proteins (p-value = 0.006; Fisher’s exact test).
Fig 3. Tissue specificity from Human Protein Atlas.
(a) Bar chart showing the number of proteins in each of the categories defined by Human Protein Atlas to classify the proteins according to their level of tissue-specificity. (b) Bar chart showing the tissues in which the tissue-enriched proteins are expressed. The stars above the columns indicate the significance of enrichment analysis (***Fisher’s exact test p-value < 0.0001; ** Fisher’s exact test p-value < 0.001).
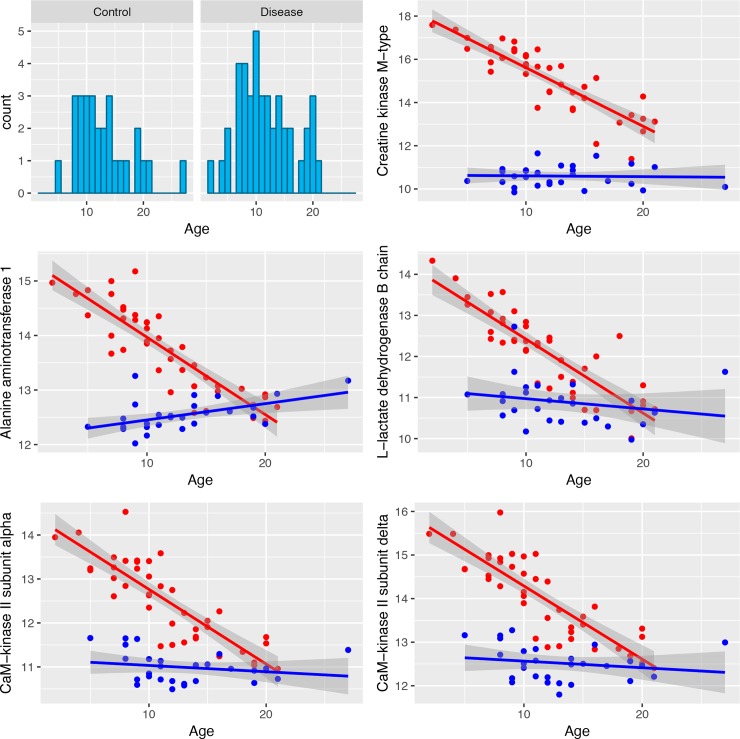
Since the serum protein levels in DMD patients are known to be affected by age, we investigated how the proteins in the biomarker panel change according to the age of patients. After having verified that the age distribution in patients is not significantly different from controls (p-value of Kolmogorov-Smirnov test: 0.82; Fig 4), we tested the presence of a relationship between age and protein levels. We identified 31 proteins whose serum level changes significantly with age. The top 5 proteins are shown in Fig 4 and the full list of biomarker proteins is shown in S2 Fig and S2 Table. With two exceptions, in all cases the protein levels decreased with age in DMD patients while they remain stable in controls. Nine of them showed a significant age-related change also in controls but the variation observed in the two groups was different and did not affect their separation (S3 Fig). These results are not unexpected since the disease is known to affect protein levels along its progression and this change has been related to the progressive loss of muscle mass [20]. Given the presence in the cohort of 28 DMD subjects treated with steroids, we checked if the treatment influenced the protein levels and 32 proteins of the biomarker panel resulted significantly associated with the treatment, indicating proteins potentially affected by the drug (S4 Fig). Despite the presence in the dataset of patients treated with steroids and patients not treated, this did not affect the classification (S5 Fig).
Fig 4. Age-related changes of biomarker levels in DMD patients and control.
The first chart shows the age distribution in cases and controls while the other charts show the variation in serum protein level for the five proteins showing the most significant age related change. The red dots correspond to the data for DMD patients while the blue ones to the controls. The lines in the corresponding colors show the regression line, while the gray area corresponds to the confidence interval.
Gene-set enrichment analysis
To identify the biological processes altered in the disease we first computed the overlap between the 52 biomarker proteins and gene sets retrieved by MSigDB [21]. Specifically, gene set enrichment analysis was performed using hallmark gene sets and the canonical pathways from KEGG, Reactome and Biocarta (Table 3). The analysis with hallmark gene sets confirmed the over-representation of skeletal muscle proteins with a significant enrichment for “myogenesis” gene set (BH corrected p-value 0.000304). The genes coding the biomarker proteins present in the “myogenesis” gene set are: TNNI2, CKM, TPM2, FABP3, MAPK12, GSN, MB, CKB, NOTCH1 and CAMK2B. In addition to the muscle-related biological functions, Biocarta pathway analysis revealed the presence of proteins involved in the coagulation cascade and pointed out the presence of different signaling pathways differentially regulated in DMD individuals compared with controls (Table 3). In particular, significantly enriched Biocarta pathways included different gene sets related to insulin receptor—PI3K/Akt pathway (igf1-mtor pathway, akt pathway and igf1r pathway), consistent with the already described role of PI3K/Akt in muscle atrophy and hypertrophy [22,23].
Table 3. Gene-set enrichment analysis.
| pathway | pathway description | # genes | BH adjusted p-value |
|---|---|---|---|
| HALLMARK_MYOGENESIS | Genes involved in development of skeletal muscle (myogenesis) | 10 | 0.000304117 |
| BIOCARTA_AMI_PATHWAY | Acute Myocardial Infarction | 5 | 0.012630235 |
| BIOCARTA_CREB_PATHWAY | Transcription factor CREB and its extracellular signals | 5 | 0.012630235 |
| BIOCARTA_SARS_PATHWAY | The SARS-coronavirus Life Cycle | 3 | 0.023907602 |
| BIOCARTA FIBRINOLYSIS PATHWAY | Fibrinolysis Pathway | 4 | 0.023907602 |
| BIOCARTA CACAM PATHWAY | Ca++/ Calmodulin-dependent Protein Kinase Activation | 3 | 0.028852412 |
| BIOCARTA STATHMIN PATHWAY | Stathmin and breast cancer resistance to antimicrotubule agents | 3 | 0.028852412 |
| BIOCARTA PGC1A PATHWAY | Regulation of PGC-1a | 3 | 0.028852412 |
| BIOCARTA EXTRINSIC PATHWAY | Extrinsic Prothrombin Activation Pathway | 4 | 0.037936344 |
| BIOCARTA BAD PATHWAY | Regulation of BAD phosphorylation | 4 | 0.037936344 |
| BIOCARTA ACH PATHWAY | Role of nicotinic acetylcholine receptors in the regulation of apoptosis | 3 | 0.037936344 |
| BIOCARTA IGF1MTOR PATHWAY | Skeletal muscle hypertrophy is regulated via AKT/mTOR pathway | 3 | 0.046573009 |
| BIOCARTA AKT PATHWAY | AKT Signaling Pathway | 3 | 0.046573009 |
| BIOCARTA INTRINSIC PATHWAY | Intrinsic Prothrombin Activation Pathway | 4 | 0.046573009 |
| BIOCARTA IGF1R PATHWAY | Multiple antiapoptotic pathways from IGF-1R signaling lead to BAD phosphorylation | 3 | 0.046573009 |
| KEGG GLIOMA | Glioma | 7 | 0.01806724 |
| REACTOME UNBLOCKING OF NMDA RECEPTOR GLUTAMATE BINDING AND ACTIVATION | Genes involved in Unblocking of NMDA receptor, glutamate binding and activation | 3 | 0.02196037 |
| REACTOME CREB PHOSPHORYLATION THROUGH THE ACTIVATION OF CAMKII | Genes involved in CREB phosphorylation through the activation of CaMKII | 3 | 0.02196037 |
| REACTOME RAS ACTIVATION UOPN CA2 INFUX THROUGH NMDA RECEPTOR | Genes involved in Ras activation uopn Ca2+ infux through NMDA receptor | 3 | 0.02196037 |
Identification of disease sub-networks and molecular regulators characterizing DMD
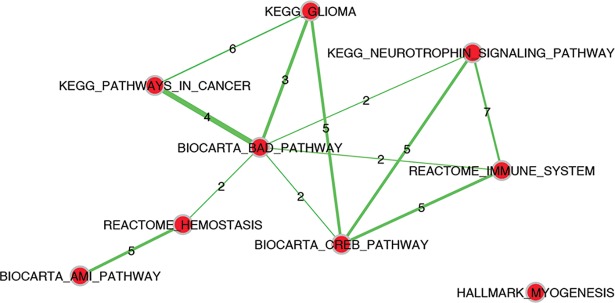
In the next step of the analysis we further investigated the presence of deregulated regulatory circuits in DMD using a network-based approach. In our workflow we applied NASFinder, a network analysis tool we recently developed [14]. Specifically, we mapped on a global signaling interaction network the 52 proteins in the biomarker panel and we identified the sub-networks connecting these proteins with transcription factors (TF). We opted for the use of TF as source nodes because they are at the top of signaling and regulatory cascades. The connections between TF and proteins of interest were established by NASFinder preferentially through differentially expressed proteins (see details in Materials and Methods). To identify the biological processes associated with the disease regulatory circuits we performed a network-based pathway enrichment analysis. The significant pathways are reported in Table 4 together with the transcription factors that NASFinder identified as the most probable regulators. On the whole, the resulting gene sets are partially overlapping, as shown in Fig 5 and S3 Table, suggesting the presence of a shared regulated biological process that involves multiple pathways. Indeed, only myogenesis did not show genes in common with other pathways and, as a confirmation of its peculiarity, the transcription factor selected as putative regulator was MEF2C, a transcription activator specific of muscle genes. In the enrichment map it is interesting to observe the centrality of BioCarta BAD pathway, a gene set related to the regulation of the pro-apoptotic molecule BAD. Among the regulators of the sub-networks the Forkhead family of transcription factors was the most represented with 3 members: FOXO1, FOXO3 and FOXO4. They were respectively identified as regulators of the sub-networks enriched in genes involved in acute myocardial infarction pathway, cancer pathways, neurotrophin signaling and immune system pathway. In particular, eleven proteins in the NASFinder FOXO3 sub-network are present in the neurotrophin pathway (p-value = 1.34 x 10−7). Seven of them (corresponding to the genes CAMK2A, CAMK2B, CAMK2D, FASLG, MAPK12, PIK3CA and PIK3R1) derived from the biomarker while the others (NFKB1, FOXO3, MAPK3 and PIK3CG) were added by NASFinder on the basis of the differentially expressed proteins. It is interesting to observe that 7 proteins (coded by the genes MAPK12, CAMK2A, CAMK2B, CAMK2D, MAPK3, PIK3CA and PIK3R1) were also identified in the Reactome immune system pathway (p-value = 0.003).
Table 4. Network-based pathway enrichment analysis.
| regulator | # genes in the sub-network | Pathway | # pathway overlapping genes | p-value | Empirical p-value of DMD proximity analysis |
|---|---|---|---|---|---|
| TP53 | 83 | KEGG GLIOMA | 11 | 4.82E-10 | 0.513 |
| FOXO1 | 89 | KEGG PATHWAYS IN CANCER | 21 | 2.57E-09 | 0.11 |
| FOXO1 | 89 | BIOCARTA AMI PATHWAY | 7 | 8.23E-09 | 0.086 |
| EGR2 | 76 | BIOCARTA CREB PATHWAY | 7 | 2.55E-08 | 0.411 |
| FOXO3 | 74 | KEGG NEUROTROPHIN SIGNALING PATHWAY | 11 | 1.73E-07 | 0.035 |
| MEF2C | 98 | HALLMARK MYOGENESIS | 15 | 3.38E-07 | 0.312 |
| MYB | 87 | REACTOME HEMOSTASIS | 20 | 3.12E-06 | 0.549 |
| RBPJ | 63 | BIOCARTA BAD PATHWAY | 4 | 0.0001 | 0.276 |
| FOXO4 | 77 | REACTOME IMMUNE SYSTEM | 21 | 0.0028 | 0.481 |
Fig 5. Pathway enrichment map showing the overlap among the pathways identified by NASFinder.
The nodes correspond to the pathways and the thickness of the edges connecting them is proportional to the number of shared genes (indicated on the edges).
Considering all the sub-networks and the related pathways pinpointed by NASFinder, PI3-kinase subunit alpha (PIK3CA) and PI3-kinase regulatory subunit alpha (PIK3R1) were identified as the proteins shared among all pathways, with the exception of myogenesis and myocardial infarction. The two proteins are subunits of phosphatidylinositol 3-kinase, a key element in PI3K/AKT pathway, a regulator of several signaling cascades and in particular of FoxO signaling [24].
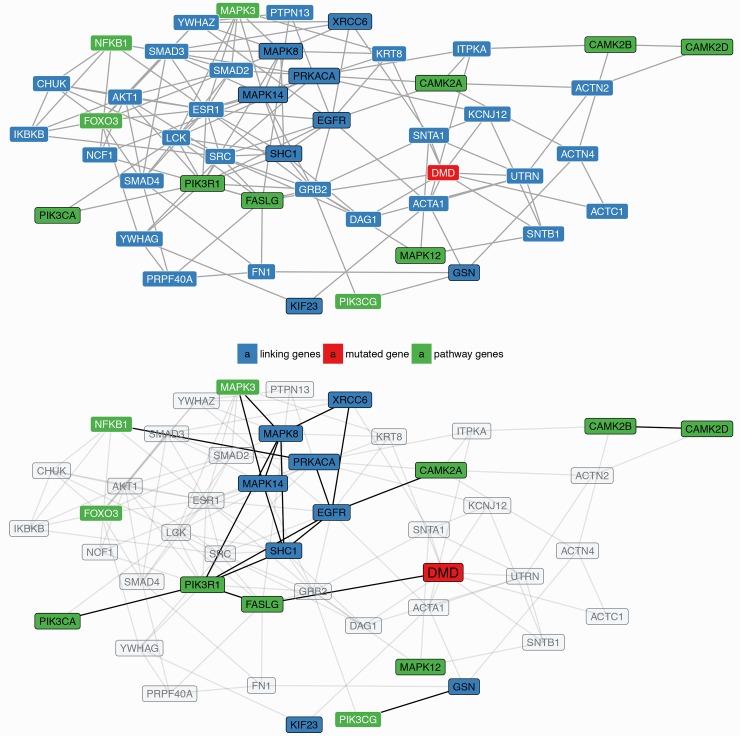
To further investigate the relevance of the identified pathways in the disease biology, we evaluated the closeness in the interactome between the proteins belonging to the pathways described above and dystrophin, the protein mutated in the disease. Expecting that not all the pathway proteins are necessarily involved in the disease, we calculated the shortest path distances between dystrophin and any of the pathway proteins. Among the nine pathways identified using the network-based pathway enrichment analysis (Table 4), the neurotrophin signaling pathway resulted significantly closer to DMD than expected by chance (empirical p-value = 0.035; Fig 6). These results offer a network-based support for the involvement of signaling cascades, and specifically PI3K signaling, in the disease.
Fig 6. Network-based DMD-pathway proximity analysis.
(A) Graphical representation of the subnetwork connecting dystrophin and the proteins in the neurotrophin pathway. The gene symbols corresponding to differentially expressed proteins are written in black, those not modulated or not measured with the SomaLogic platform are in white. When more than one path with the same shortest distance was identified, only those including differentially expressed genes are shown. (B) DMD, the genes in the neurotrophin pathway, the differentially expressed genes and their direct connections are highlighted.
Comparison with published studies about proteomics in DMD
We selected five published proteomics studies performed on blood serum of subjects affected by DMD or DMD mouse models [20,25–28] and checked if the proteins in our panel had been already described as DMD biomarkers (S4 Table). Twenty-seven of the 52 proteins included in our long biomarker panel were already identified as differentially present between serum of DMD cases compared with controls in a previous analysis performed by Hathout and collaborators [20]. To further assess the similarity of the results between the two studies, we investigated their protein signature with our analysis workflow. Using Gene Set Enrichment Analysis, although none was significant after multiple test correction, some of the most enriched pathways are shared between the two studies (see S5 Table). Using NASFinder, the analysis identified 12 statistically significant pathways (S6 Table). Interestingly, three of them (immune system, hemostasis and myogenesis) are shared with our analysis, and for myogenesis the transcription factor identified as a close regulator is the same. Comparing our results with the study by Ayoglu and collaborators [25], which was performed with an antibody bead array platform, creatine kinase and carbonic anidrase were the proteins in common with those of our long panel. GSN was already pinpointed in mass spectrometry analysis of human serum by Cynthia Martin et al. [26]. In the comparison we also included two studies performed using a DMD mouse model. In this case, nine proteins were in common with a study that used the SOMAscan technology for proteomic profiling [27] and five proteins with a study based on mass spectrometry [28].
Evaluation of gene expression patterns in DMD skeletal muscle
The serum proteomics detects the proteins present in the blood, however they can derive from muscle degenerating fibers released into the systemic circulation rather than reflecting expression regulation. Based on this consideration we decided to analyze a type of evidence complementary to our proteomics data; specifically we selected a public dataset of genome-wide expression data obtained from the skeletal muscle of DMD and controls (GSE1004). This dataset contains expression data from quadriceps of patients with clinical symptoms consistent with a DMD diagnosis, whose biopsies were shown to be dystrophin deficient [29]. After having calculated the differentially expressed genes, to investigate the biological processes affected, pathway enrichment analysis was performed using the differentially expressed genes as input and we evaluated the overlap with the pathways identified by NASFinder. With the exception of two Biocarta gene sets, all the pathways identified by NASFinder from the proteome datasets resulted significantly enriched, as reported in Table 5. We further evaluated the dysregulation of these pathways in a dataset of DMD patients in early phases of the disease and controls (GDS3027). These data allowed us to test the dysregulation of the pathways in a pre-symptomatic phase of the disease. With the exception of Biocarta gene sets, the other pathways resulted among the top 20 results (S7 Table).
Table 5. Comparison of NASFinder results with pathway enrichment analysis of dataset GSE1004.
| Pathway | # Genes in Overlap | FDR qvalue |
|---|---|---|
| HALLMARK MYOGENESIS | 31 | 7.03E-24 |
| REACTOME IMMUNE SYSTEM | 50 | 6.71E-16 |
| REACTOME HEMOSTASIS | 31 | 2.67E-12 |
| KEGG PATHWAYS IN CANCER | 22 | 2.83E-09 |
| KEGG GLIOMA | 7 | 0.000083 |
| KEGG NEUROTROPHIN SIGNALING PATHWAY | 8 | 0.000647 |
| BIOCARTA AMI PATHWAY | 3 | 0.0195 |
| BIOCARTA CREB PATHWAY | NA | NA |
| BIOCARTA BAD PATHWAY | NA | NA |
Discussion
In this study we adopted a system biology approach to study Duchenne muscular dystrophy. Our workflow is based on the combination of two complementary methodologies: biomarker identification and network analysis. We applied the algorithm for biomarker identification to point out the essential features of the biological system, i.e. the minimum set of proteins able to fully separate healthy from affected subjects, and then we applied the network analysis to unravel the complex interactions among the proteins. Taking advantage of the flexibility of our algorithm, we calculated two different protein lists, a short one and a long one. All the proteins present in the short biomarker panel have been already described as DMD biomarkers in Hathout et al. [20] and they are related to the main pathological features observed in DMD [6]. However, the short biomarker panel described here, compared with the signature in Hathout et al. [20], has the advantage of being able to classify the subjects with only six proteins achieving perfect accuracy and high statistical significance (permutation test p-value < 0.001). Our long biomarker panel has size comparable to the disease signature reported in Hathout et al. (52 vs 46 proteins, respectively) but only approximately half of the proteins (26) are in common between the two lists. Such low overlap is not surprising in view of the fact that the different approaches used in the two studies (classification-oriented vs statistical significance) have been shown not to be equivalent [30]. From an investigational point of view, our classification-oriented list provides some additional insights on less known aspects of the disease pathophysiology and in particular to identify deregulated pathways and potential regulators. Indeed, in addition to proteins with a muscle origin, likely found in the serum proteome as a consequence of sarcolemma leakage [31], our analysis pointed out several proteins involved in cell signaling that, given their biological role, it is not surprising to detect in blood serum. Our results are in line with growing evidences suggesting that the cellular structural instability caused by the lack of dystrophin affects different signaling pathways [32–34]. In particular, the network-based pathway enrichment analysis implemented in NASFinder identified nine pathways as significantly enriched and, among the influential nodes, different transcription factors belonging to the FoxO family were identified as possible regulators. In humans FoxO proteins are all expressed in skeletal muscle and they have been implicated in muscle homeostasis [35]. Specifically, regarding muscle mass, FoxO proteins were shown to enhance proteolysis through the ubiquitin-proteasome and the autophagy-lysosome system [36]. Notably, a study on sarcopenia, the physiological age-related loss of muscle mass, identified FOXO3 as an up-regulated gene in muscles from old subjects [37] and another study found increased FOXO1 mRNA in aged muscle [38]. However, findings from a more recent study suggest that sarcopenia is not due to FoxO activation [39], highlighting the need for further studies to better understand the role of FoxO proteins in physiological and pathological muscle conditions. Among the sub-networks regulated by FoxO proteins, the neurotrophin signaling pathway, a cascade of biological processes regulating cell survival and apoptosis [40], was identified as enriched in our analysis. Although neurotrophins have been mainly linked to the nervous system [41], recent studies indicated their involvement in skeletal muscle adaptation and regeneration [42]. Specifically, experimental evidences showed that neurotrophins are involved in muscle regeneration in dystrophic mice [43] and the nerve growth factor, the paradigm of neurotrophin family, was identified in muscles from patients affected by DMD [44]. Interestingly, a recent study performed using the mdx mouse, a model of DMD, linked apoptosis of myofibers to the presence of connexin, a protein channel involved in NF-κB activation, iNOS expression and apoptotic cell death [45]. In our analysis NFKB1, a DNA binding subunit of the NFKB protein complex, is present in the FOXO3 sub-network defined by NASFinder and the protein is part of the neurotrophin-related signaling. The network proximity analysis (Fig 6) suggested that the dysregulation of the neurotrophin signaling pathway in patients affected by DMD might be mediated by Fas ligand (FASLG), a mediator of the cellular apoptotic signal that is a direct interactor of the dystrophin protein [46]. A link between Duchenne muscular dystrophy and FASLG has already been reported by Abdel-Salam et al. [47], which observed significantly increased levels of FasLG mRNA expression in blood of DMD patients compared to controls. In addition to neurotrophin signaling, our analysis pointed out, among the FoxO-related sub-networks, an enrichment for the immune system signaling with FOXO4 identified as a regulator. The role of FoxO proteins in immune system has been already described [48] and interestingly, this process, together with hemostasis, another significant pathway in NASFinder analysis, is a key player in fibrosis, a prominent feature of dystrophic muscle [49]. It is thus conceivable that the dysregulation of blood proteins belonging to these pathways is a consequence of the fibrotic process ongoing in muscles of DMD patients. Notably, modulation of the immune response was suggested as a potential means to alleviate the disease intensity [50]. The other FoxO transcription factor with a pathway enriched sub-network was FOXO1. In this case two pathways resulted enriched: BioCarta acute myocardial infarction and KEGG pathways in cancer. While the presence of cardiac dysfunctions in subjects affected by DMD is common [51], we consider the presence of enrichment in cancer pathways (also KEGG glioma in TP53 network resulted enriched) a consequence of the bias introduced by the high number of genes annotated in cancer pathways. Indeed, we noticed that most of the genes in cancer pathways also belong to other enriched gene sets (S3 Table).
In conclusion, in this study we presented a new bioinformatics workflow based on biomarker identification and network analysis that we used to extract biological insights from proteomics data obtained from a cohort of DMD subjects and controls. This methodology allowed us to identify different pathways deregulated in the disease and to pinpoint a putative role of FoxO signaling in DMD. One limitation of this study is the lack of validation of the biomarker panel classification performance using a second independent cohort of subjects. To partially address this issue and avoid overfitting, we trained the algorithm that computed the biomarkers according to a 5-fold cross-validation scheme. We also used a second dataset of a very different nature (transcriptional profile of muscle from an unrelated cohort) to confirm the end results of our analysis and we obtained a substantial confirmation of the results. Another limitation of this study, as any form of in silico analysis, is that it relies entirely on observational data. Therefore, our results will have to be validated by means of functional studies. Despite these limitations, our workflow derives its strength from the convergence of multiple types of evidences from independent experiments, crossed with existing knowledge about the biology of the disease.
Materials and methods
Dataset
The dataset analyzed in this study was obtained from a collaboration with SomaLogic and includes subjects previously analyzed by Hathout and coworkers [20]. The present dataset consists of 42 subjects affected by DMD and 28 healthy, age-matched volunteers (PPMD-C cohort). The group of patients included 28 individuals treated with steroids. For each subject a proteomic profile was obtained using the SOMAscan technology by SomaLogic. Briefly, this high throughput method is based on the use of aptamers with high affinity for the proteins. The presence in the aptamers of a DNA sequence allows the quantification of the protein levels in a simple way using microarray-based technology. Further details about the cohort and the proteomic assay can be obtained from [20]. The original study was approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board and informed consent was obtained from patients or their parents or legal guardians. All methods were performed in accordance with relevant guidelines and regulations.
Biomarkers identification
Protein biomarkers have been identified by means of an enhanced version of the rank-based classification method previously introduced in [12,13]. Briefly, after a preliminary protein selection phase based on the Wilcoxon test, the classification method ranks the filtered proteins by expressions level separately for each sample and then it produces a set of subject-specific signatures, where each signature is the list of the first n1 and the last n2 proteins in the ranking (n1 and n2 have the same value for all subjects and they are parameters estimated by the method). An all-to-all signature comparison is then carried out using a distance metric based on a weighted enrichment score [52], resulting in a distance matrix that systematically quantifies the degree of similarity between the subjects. Subjects are then classified by the algorithm into the two groups of controls and diseased, by assigning each sample to the group of subjects whose elements have the lowest averaged distance from the sample. Finally, a protein biomarker is extracted, which collects all the proteins included in at least one subject-specific signature. Therefore, the proteins included in a biomarker are those required to compute the corresponding subject classification.
In the enhanced version used here, the original classification method has been extended as in our previous studies [53,54] with a genetic optimizer that automatically selects the method parameters (signature length and feature selection stringency) to find the best compromise between biomarker length and classification accuracy. In particular, the two biomarkers herein presented provide the shortest and the longest protein list allowing classification accuracy close to 100%. In the case of the longest biomarker, protein levels have also been preprocessed to emphasize expression fold-changes by dividing protein levels by their averaged value in the dataset.
To avoid overfitting, in all the considered cases the algorithm has been trained according to a 5-fold cross-validation scheme, where 20% of the subjects were set aside for validation at each round, and the remaining ones are used as training set for determining the biomarker. Moreover, we also evaluated the statistical significance of the analysis by means of a permutation test in which we compared the observed classification accuracy of the method with an empirical distribution of accuracy values obtained by 1000 random permutations of the protein labels. As a result of the permutation test we obtained in all cases a p-value < 0.001.
Linear regression was run to identify proteins in the biomarker panel associated with age, after having log2 scaled the data. Furthermore, the presence of a treatment effect on protein levels was tested using Wilcoxon test. For both analyses, p-values were adjusted for multiple test correction using the Benjamini-Hochberg procedure.
Tissue specificity and gene set enrichment analysis
Tissue specificity was evaluated using the transcriptomic data in the Human Protein Atlas (http://www.proteinatlas.org/) [19]. In this database proteins are classified as tissue enriched (mRNA levels in one tissue at least five times higher than all other tissues), group enriched (mRNA levels in a group of 2 to 7 tissues at least five times higher than all other tissues), tissue enhanced (mRNA levels in a particular tissue at least five times the average level in all tissues), expressed in all (mRNA detected in all tissues), mixed (detected in a subset of tissues and expression not markedly elevated in any) and not detected. To evaluate the enrichment in a particular tissue we performed a Fisher’s exact test comparing the tissue enriched proteins in the biomarker panel with the entire SomaLogic panel.
To perform gene set enrichment analysis we downloaded the gene sets from the Molecular Signatures Database website [21], v5.1, updated in January 2016. In particular, the following gene set collections were investigated: hallmark, BioCarta (http://www.biocarta.com/), KEGG[55] and Reactome [56]. Hallmark is a refined collection defined by MSigDB derived from multiple founders to point out relevant information about biological conditions avoiding redundancy [57]. The enrichment was evaluated using the SomaLogic panel as a background and the statistical significance was tested using the hypergeometric distribution (phyper function implemented in the R software).
Network analysis
To explore the interactions among the proteins in the biomarker panel and identifying putative regulators, we applied NASFinder, a tool we recently developed to analyze the connections among genes of interest and their upstream regulators [14]. Briefly, the proteins in the biomarker panel are mapped to a reference protein-protein interaction network, that is, the human signaling network from the Wang Lab - http://www.cancer-systemsbiology.org/dataandsoftware.htm. Then, the subnetwork connecting the proteins of interest, in our case the proteins in the biomarker panel, and the closest transcriptional factor (molecules selected as regulators) is defined. If a protein of interest is not present in the reference network it is added, if possible, from BioGRID [58] as detailed in [14]. The paths that connect transcription factors and biomarker proteins are established by NASFinder preferentially through differentially expressed proteins and they were identified in our study using the Bioconductor package limma. Specifically, proteins were selected as significant when they showed a Benjamini-Hochberg adjusted p-value less than 0.05 in the comparison between DMD subjects and controls. Once the subnetworks were identified, we evaluated their overlap with reference pathways from KEGG, Reactome, Biocarta and the hallmark collection in MSigDB, as in the gene-set enrichment analysis. The significance of the overlap was calculated using one-sided Fisher's exact test. The degree of overlap among the pathways identified by NASFinder was visualized using the Cytoscape App Enrichment Map v2.1.0 [59], run using Cytoscape 3.4.0. Enrichment Map was run using default parameters and Overlap coefficient as a measure of similarity.
The network-based proximity analysis between DMD and the pathway genes was performed with the R package igraph, using the protein-protein interaction network from the Human Protein Reference Database (HPRD) [60]. This background network was preferred to the signaling network used for the identification of pathways and regulators because it includes only physical interactions among proteins instead of regulatory interactions. For all the pathways, all the shortest paths from DMD to the genes were calculated and the shortest path distance was defined as the minimal distance found. To assess the significance, we selected 1000 random genes and, for each of them, we calculated the minimal shortest distance to the pathway. We thus obtained an empirical distribution that was used to calculate the proportion of samples with a minimal shortest distance less or equal to the distance obtained using DMD.
Gene expression analysis
The datasets GSE1004 and GDS3027 were downloaded from NCBI GEO using the R Bioconductor package GEOquery. Dataset GSE1004 was obtained measuring RNA expression in quadriceps biopsies and it includes 12 DMD patients and 12 controls. The DMD biopsies were from young (5- to 7-year-old) males showing clinical symptoms consistent with a DMD diagnosis, and the biopsies were shown to be dystrophin deficient by immunofluorescence and/or Western blotting [29]. For the purpose of this study we analyzed the data from the platform HG_U95Av2 (GPL8300) which includes 11 controls and all the 12 cases. Dataset GDS3027 includes the expression profiles of skeletal muscles from 23 children in a pre-symptomatic phase of Duchenne muscular dystrophy and 14 controls [61]. The analysis of differentially expressed genes was performed with the R Bioconductor package limma. The gene-set enrichment analysis was performed using the MSigDB tool of the GSEA package (http://www.broadinstitute.org/gsea/msigdb/index.jsp) querying the same gene sets collections used in the network analysis (obtained from hallmark, KEGG, Biocarta and Reactome).
Supporting information
The data were retrieved from Human Protein Atlas.
(PDF)
For all the proteins in the long biomarker panel the results of the association analysis with age are shown.
(PDF)
For each pathway that resulted overrepresented the genes in the corresponding sub-network are reported.
(PDF)
For all the proteins in the long biomarker panel the result of a literature search about DMD proteomics studies identifying the same protein is reported.
(PDF)
(PDF)
(PDF)
The top 20 pathways for each database are shown.
(PDF)
(PDF)
The plots show all the proteins in the long biomarker panel showing a significant correlation with age.
(PDF)
(PDF)
Only the proteins in the long biomarker panel with a significant difference are shown.
(PDF)
The nodes of the network represent the samples, while colors indicate their status (controls, disease without treatment, diseases with treatment). The length of the edges is proportional to level of similarity between the sample signatures. In both cases we can observe that controls and disease samples cluster together forming two separate groups, while this does not happen if we consider the treatment (red nodes and orange ones are not clearly separated). (A) Short biomarker panel. (B) Long biomarker panel.
(PDF)
Acknowledgments
The authors are grateful to SomaLogic for having provided the proteomics data produced with SOMAscan technology.
Data Availability
Transcriptomic data were retrieved from NCBI GEO with accession number GDS3027 and GSE1004. Proteomic data used in this study are third party data. They will be made available by contacting the corresponding author of the article in which they were originally described: Hathout et al, PNAS 2015, doi: 10.1073/pnas.1507719112. The authors of this article did not have any special access privileges that others would not have.
Funding Statement
This project was partially funded by Provincia Autonoma di Trento - Zoomer project. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mayne J, Ning Z, Zhang X, Starr AE, Chen R, Deeke S, et al. Bottom-Up Proteomics (2013–2015): Keeping up in the Era of Systems Biology. Anal. Chem. American Chemical Society; 2016;88:95–121. doi: 10.1021/acs.analchem.5b04230 [DOI] [PubMed] [Google Scholar]
- 2.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One. 2010;5:e15004 doi: 10.1371/journal.pone.0015004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold L, Walker JJ, Wilcox SK, Williams S. Advances in human proteomics at high scale with the SOMAscan proteomics platform. N. Biotechnol. 2012;29:543–9. doi: 10.1016/j.nbt.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 4.Biankin A V., Piantadosi S, Hollingsworth SJ. Patient-centric trials for therapeutic development in precision oncology. Nature. Nature Publishing Group; 2015;526:361–70. doi: 10.1038/nature15819 [DOI] [PubMed] [Google Scholar]
- 5.Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat. Biotechnol. Nature Publishing Group; 2006;24:971–83. doi: 10.1038/nbt1235 [DOI] [PubMed] [Google Scholar]
- 6.Hathout Y, Seol H, Han MHJ, Zhang A, Brown KJ, Hoffman EP, et al. Clinical utility of serum biomarkers in Duchenne muscular dystrophy. Clin. Proteomics. BioMed Central; 2016;13:9 doi: 10.1186/s12014-016-9109-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barabási A-L, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat. Rev. Genet. Nature Publishing Group; 2011;12:56–68. doi: 10.1038/nrg2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghiassian SD, Menche J, Chasman DI, Giulianini F, Wang R, Ricchiuto P, et al. Endophenotype Network Models: Common Core of Complex Diseases. Sci. Rep. Nature Publishing Group; 2016;6:27414 doi: 10.1038/srep27414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim J, Hao T, Shaw C, Patel AJ, Szabó G, Rual JF, et al. A Protein-Protein Interaction Network for Human Inherited Ataxias and Disorders of Purkinje Cell Degeneration. Cell. 2006;125:801–14. doi: 10.1016/j.cell.2006.03.032 [DOI] [PubMed] [Google Scholar]
- 10.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6 [DOI] [PubMed] [Google Scholar]
- 11.Malm C, Nyberg P, Engström M, Sjödin B, Lenkei R, Ekblom B, et al. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J. Physiol. Blackwell Science Ltd; 2000;529:243–62. doi: 10.1111/j.1469-7793.2000.00243.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauria M. Rank-based transcriptional signatures: A novel approach to diagnostic biomarker definition and analysis. Syst. Biomed. 2013;1:35–46. [Google Scholar]
- 13.Lauria M, Moyseos P, Priami C. SCUDO: a tool for signature-based clustering of expression profiles. Nucleic Acids Res. 2015;43:W188–92. doi: 10.1093/nar/gkv449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nassiri I, Lombardo R, Lauria M, Morine MJ, Moyseos P, Varma V, et al. Systems view of adipogenesis via novel omics-driven and tissue-specific activity scoring of network functional modules. Sci. Rep. Nature Publishing Group; 2016;6:28851 doi: 10.1038/srep28851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010;48:757–67. doi: 10.1515/CCLM.2010.179 [DOI] [PubMed] [Google Scholar]
- 16.Ozawa E, Hagiwara Y, Yoshida M. Creatine kinase, cell membrane and Duchenne muscular dystrophy. Mol. Cell. Biochem. 1999;190:143–51. [PubMed] [Google Scholar]
- 17.Mastellos DC, DeAngelis RA, Lambris JD. Complement-triggered pathways orchestrate regenerative responses throughout phylogenesis. Semin. Immunol. 2013;25:29–38. doi: 10.1016/j.smim.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharraz Y, Guerra J, Pessina P, Serrano AL, Muñoz-Cánoves P. Understanding the process of fibrosis in Duchenne muscular dystrophy. Biomed Res. Int. Hindawi Publishing Corporation; 2014;2014:965631 doi: 10.1155/2014/965631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science (80-.) 2015;347:1260419 doi: 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- 20.Hathout Y, Brody E, Clemens PR, Cripe L, DeLisle RK, Furlong P, et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. National Academy of Sciences; 2015;112:7153–8. doi: 10.1073/pnas.1507719112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. National Academy of Sciences; 2005;102:15545–50. doi: 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen S, Lee D, Zhai B, Gygi SP, Goldberg AL. Trim32 reduces PI3K–Akt–FoxO signaling in muscle atrophy by promoting plakoglobin–PI3K dissociation. J. Cell Biol. Rockefeller University Press; 2014;204:747–58. doi: 10.1083/jcb.201304167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt Pathway Prevents Expression of Muscle Atrophy-Induced Ubiquitin Ligases by Inhibiting FOXO Transcription Factors. Mol. Cell. 2004;14:395–403. [DOI] [PubMed] [Google Scholar]
- 24.Carter ME, Brunet A. FOXO transcription factors. Curr. Biol. 2007;17:R113–4. doi: 10.1016/j.cub.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 25.Ayoglu B, Chaouch A, Lochmüller H, Politano L, Bertini E, Spitali P, et al. Affinity proteomics within rare diseases: a BIO-NMD study for blood biomarkers of muscular dystrophies. EMBO Mol. Med. EMBO Press; 2014;6:918–36. doi: 10.15252/emmm.201303724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cynthia Martin F, Hiller M, Spitali P, Oonk S, Dalebout H, Palmblad M, et al. Fibronectin is a serum biomarker for Duchenne muscular dystrophy. PROTEOMICS—Clin. Appl. 2014;8:269–78. doi: 10.1002/prca.201300072 [DOI] [PubMed] [Google Scholar]
- 27.Coenen-Stass AML, McClorey G, Manzano R, Betts CA, Blain A, Saleh AF, et al. Identification of novel, therapy-responsive protein biomarkers in a mouse model of Duchenne muscular dystrophy by aptamer-based serum proteomics. Sci. Rep. Nature Publishing Group; 2015;5:17014 doi: 10.1038/srep17014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hathout Y, Marathi RL, Rayavarapu S, Zhang A, Brown KJ, Seol H, et al. Discovery of serum protein biomarkers in the mdx mouse model and cross-species comparison to Duchenne muscular dystrophy patients. Hum. Mol. Genet. Oxford University Press; 2014;23:6458–69. doi: 10.1093/hmg/ddu366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, Kohane IS, et al. Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15000–5. doi: 10.1073/pnas.192571199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo A, Chernoff H, Zheng T, Lo S-H. Why significant variables aren’t automatically good predictors. Proc. Natl. Acad. Sci. 2015;112:13892–7. doi: 10.1073/pnas.1518285112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahimov F, Kunkel LM. Cellular and molecular mechanisms underlying muscular dystrophy. J. Cell Biol. Rockefeller University Press; 2013;201:499–510. doi: 10.1083/jcb.201212142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramachandran J, Schneider JS, Crassous P-A, Zheng R, Gonzalez JP, Xie L-H, et al. Nitric oxide signalling pathway in Duchenne muscular dystrophy mice: up-regulation of L-arginine transporters. Biochem. J. 2013;449:133–42. doi: 10.1042/BJ20120787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messina S, Vita GL, Aguennouz M, Sframeli M, Romeo S, Rodolico C, et al. Activation of NF-kappaB pathway in Duchenne muscular dystrophy: relation to age. Acta Myol. 2011;30:16–23. [PMC free article] [PubMed] [Google Scholar]
- 34.Fuenzalida M, Espinoza C, Pérez MÁ, Tapia-Rojas C, Cuitino L, Brandan E, et al. Wnt signaling pathway improves central inhibitory synaptic transmission in a mouse model of Duchenne muscular dystrophy. Neurobiol. Dis. 2016;86:109–20. doi: 10.1016/j.nbd.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 35.Sanchez AMJ, Candau RB, Bernardi H. FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis. Cell. Mol. Life Sci. 2014;71:1657–71. doi: 10.1007/s00018-013-1513-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda). American Physiological Society; 2008;23:160–70. [DOI] [PubMed] [Google Scholar]
- 37.Giresi PG. Identification of a molecular signature of sarcopenia. Physiol. Genomics. 2005;21:253–63. doi: 10.1152/physiolgenomics.00249.2004 [DOI] [PubMed] [Google Scholar]
- 38.Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiol. Genomics. 2003;14:149–59. doi: 10.1152/physiolgenomics.00049.2003 [DOI] [PubMed] [Google Scholar]
- 39.Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology. 2013;14:303–23. doi: 10.1007/s10522-013-9432-9 [DOI] [PubMed] [Google Scholar]
- 40.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361:1545–64. doi: 10.1098/rstb.2006.1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao M V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. Nature Publishing Group; 2003;4:299–309. doi: 10.1038/nrn1078 [DOI] [PubMed] [Google Scholar]
- 42.Sakuma K, Aoi W, Yamaguchi A. The intriguing regulators of muscle mass in sarcopenia and muscular dystrophy. Front. Aging Neurosci. 2014;6:230 doi: 10.3389/fnagi.2014.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavasani M, Lu A, Peng H, Cummins J, Huard J. Nerve growth factor improves the muscle regeneration capacity of muscle stem cells in dystrophic muscle. Hum. Gene Ther. 2006;17:180–92. doi: 10.1089/hum.2006.17.180 [DOI] [PubMed] [Google Scholar]
- 44.Toti P, Villanova M, Vatti R, Schuerfeld K, Stumpo M, Barbagli L, et al. Nerve growth factor expression in human dystrophic muscles. Muscle Nerve. 2003;27:370–3. doi: 10.1002/mus.10332 [DOI] [PubMed] [Google Scholar]
- 45.Cea LA, Puebla C, Cisterna BA, Escamilla R, Vargas AA, Frank M, et al. Fast skeletal myofibers of mdx mouse, model of Duchenne muscular dystrophy, express connexin hemichannels that lead to apoptosis. Cell. Mol. Life Sci. Springer International Publishing; 2016;73:2583–99. doi: 10.1007/s00018-016-2132-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wenzel J, Sanzenbacher R, Ghadimi M, Lewitzky M, Zhou Q, Kaplan DR, et al. Multiple interactions of the cytosolic polyproline region of the CD95 ligand: hints for the reverse signal transduction capacity of a death factor. FEBS Lett. 2001;509:255–62. [DOI] [PubMed] [Google Scholar]
- 47.Abdel-Salam E, Abdel-Meguid I, Korraa SS. Markers of degeneration and regeneration in Duchenne muscular dystrophy. Acta Myol. myopathies cardiomyopathies Off. J. Mediterr. Soc. Myol. 2009;28:94–100. [PMC free article] [PubMed] [Google Scholar]
- 48.Peng SL. Foxo in the immune system. Oncogene. Nature Publishing Group; 2008;27:2337–44. doi: 10.1038/onc.2008.26 [DOI] [PubMed] [Google Scholar]
- 49.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. Nature Publishing Group; 2012;18:1028–40. doi: 10.1038/nm.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villalta SA, Rosenberg AS, Bluestone JA. The immune system in Duchenne muscular dystrophy: Friend or foe. Rare Dis. 2015;3:e1010966 doi: 10.1080/21675511.2015.1010966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamdar F, Garry DJ. Dystrophin-Deficient Cardiomyopathy. J. Am. Coll. Cardiol. 2016;67:2533–46. doi: 10.1016/j.jacc.2016.02.081 [DOI] [PubMed] [Google Scholar]
- 52.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A. National Academy of Sciences; 2005;102:15545–50. doi: 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caberlotto L, Marchetti L, Lauria M, Scotti M, Parolo S. Integration of transcriptomic and genomic data suggests candidate mechanisms for APOE4-mediated pathogenic action in Alzheimer’s disease. Sci. Rep. 2016;6:32583 doi: 10.1038/srep32583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lacroix S, Lauria M, Scott-Boyer M-P, Marchetti L, Priami C, Caberlotto L. Systems biology approaches to study the molecular effects of caloric restriction and polyphenols on aging processes. Genes Nutr. Springer Berlin Heidelberg; 2015;10:58 doi: 10.1007/s12263-015-0508-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. Oxford University Press; 2016;44:D457–62. doi: 10.1093/nar/gkv1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Croft D, Mundo AF, Haw R, Milacic M, Weiser J, Wu G, et al. The Reactome pathway knowledgebase. Nucleic Acids Res. Oxford University Press; 2014;42:D472–7. doi: 10.1093/nar/gkt1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst. 2015;1:417–25. doi: 10.1016/j.cels.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stark C, Breitkreutz B-J, Reguly T, Boucher L, Breitkreutz A, Tyers M. BioGRID: a general repository for interaction datasets. Nucleic Acids Res. Oxford University Press; 2006;34:D535–9. doi: 10.1093/nar/gkj109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment Map: A Network-Based Method for Gene-Set Enrichment Visualization and Interpretation. Ravasi T, editor. PLoS One. Public Library of Science; 2010;5:e13984 doi: 10.1371/journal.pone.0013984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, et al. Human Protein Reference Database—2009 update. Nucleic Acids Res. 2009;37:D767–72. doi: 10.1093/nar/gkn892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pescatori M, Broccolini A, Minetti C, Bertini E, Bruno C, D’amico A, et al. Gene expression profiling in the early phases of DMD: a constant molecular signature characterizes DMD muscle from early postnatal life throughout disease progression. FASEB J. 2007;21:1210–26. doi: 10.1096/fj.06-7285com [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The data were retrieved from Human Protein Atlas.
(PDF)
For all the proteins in the long biomarker panel the results of the association analysis with age are shown.
(PDF)
For each pathway that resulted overrepresented the genes in the corresponding sub-network are reported.
(PDF)
For all the proteins in the long biomarker panel the result of a literature search about DMD proteomics studies identifying the same protein is reported.
(PDF)
(PDF)
(PDF)
The top 20 pathways for each database are shown.
(PDF)
(PDF)
The plots show all the proteins in the long biomarker panel showing a significant correlation with age.
(PDF)
(PDF)
Only the proteins in the long biomarker panel with a significant difference are shown.
(PDF)
The nodes of the network represent the samples, while colors indicate their status (controls, disease without treatment, diseases with treatment). The length of the edges is proportional to level of similarity between the sample signatures. In both cases we can observe that controls and disease samples cluster together forming two separate groups, while this does not happen if we consider the treatment (red nodes and orange ones are not clearly separated). (A) Short biomarker panel. (B) Long biomarker panel.
(PDF)
Data Availability Statement
Transcriptomic data were retrieved from NCBI GEO with accession number GDS3027 and GSE1004. Proteomic data used in this study are third party data. They will be made available by contacting the corresponding author of the article in which they were originally described: Hathout et al, PNAS 2015, doi: 10.1073/pnas.1507719112. The authors of this article did not have any special access privileges that others would not have.