Short abstract
Preeclampsia (PE) is a pregnancy complex disease, distinguished by high blood pressure and proteinuria, diagnosed after the 20th gestation week. Depending on the values of blood pressure, urine protein concentrations, symptomatology, and onset of disease there is a wide range of phenotypes, from mild forms developing predominantly at the end of pregnancy to severe forms developing in the early stage of pregnancy. In the worst cases severe forms of PE could lead to systemic endothelial dysfunction, eclampsia, and maternal and/or fetal death. Worldwide the fetal morbidity and mortality related to PE is calculated to be around 8% of the total pregnancies. PE still being an enigma regarding its etiology and pathophysiology, in general a deficient trophoblast invasion during placentation at first stage of pregnancy, in combination with maternal conditions are accepted as a cause of endothelial dysfunction, inflammatory alterations and appearance of symptoms. Depending on the PE multifactorial origin, several in vitro, in vivo, and in silico models have been used to evaluate the PE pathophysiology as well as to identify or test biomarkers predicting, diagnosing or prognosing the syndrome. This review focuses on the most common models used for the study of PE, including those related to placental development, abnormal trophoblast invasion, uteroplacental ischemia, angiogenesis, oxygen deregulation, and immune response to maternal–fetal interactions. The advances in mathematical and computational modeling of metabolic network behavior, gene prioritization, the protein–protein interaction network, the genetics of PE, and the PE prediction/classification are discussed. Finally, the potential of these models to enable understanding of PE pathogenesis and to evaluate new preventative and therapeutic approaches in the management of PE are also highlighted.
Impact statement
This review is important to the field of preeclampsia (PE), because it provides a description of the principal in vitro, in vivo, and in silico models developed for the study of its principal aspects, and to test emerging therapies or biomarkers predicting the syndrome before their evaluation in clinical trials. Despite the current advance, the field still lacking of new methods and original modeling approaches that leads to new knowledge about pathophysiology. The part of in silico models described in this review has not been considered in the previous reports.
Keywords: Model systems, study, preeclampsia
Introduction
Preeclampsia (PE), a pregnancy-specific disorder distinguished by high blood pressure and proteinuria as a consequence of abnormal placentation, is diagnosed after the 20th week of gestation. Increased systemic vascular resistance, endothelial cell dysfunction, platelet aggregation, and altered activation of the coagulation system have been associated with PE, representing one of the principal responsible of perinatal and maternal morbimortality worldwide, especially in developing countries, affecting 5–8% of all pregnancies.1,2 Clinical manifestations of PE include a maternal syndrome (proteinuria [≥300 mg in 24-h urine] and high blood pressure [≥140/90 mmHg]) with or without other multisystem abnormalities such as edema, headache, renal failure, epigastric pain, low platelet count and abnormal liver enzyme values, and fetal syndrome (hypoxemia, diminished amniotic fluid and small-for gestational age).2
Exist several theories about the ultimate cause of PE, and nowadays it is thought that the etiology is most likely multifactorial. The appearance of the disease can be influenced by several predisposing factors. The oftenness and severity of PE are considerably elevated in women with multifetal pregnancies, chronic high blood pressure, a personal and family history of PE, pre-existing diabetes mellitus, and thrombophilia.3 The presence of high concentrations of circulating syncytiotrophoblast debris, genetic susceptibility, maternal immunological alterations, nutritional factors, and an increased sensitivity to angiotensin (AT) II could play a fundamental part in the etiology of PE.2,4–6
As referred before, it is widely recognized that a deficient placentation process is the primary cause of PE. In a normal pregnancy, endovascular trophoblast invasion transform the spiral arteries of the decidual part at 8–10 weeks of gestation (WG) (first invasion) and of the myometrial part at 16–18 WG (second invasion), invading the arterial wall and expanding its diameter from narrow to large by replacing normal musculoelastic to amorphous fibrinoid tissue of the arteries, allowing with this physiological transformation an adequate blood flow of the placenta.7,8 Failure of the second trophoblast invasion with an abnormal physiological transformation of the spiral arteries are observed in PE, directing to placental ischemia-reperfusion, oxidative stress, endothelial dysfunction (a disequilibrium among pro and anti-angiogenic proteins with a predominance of the last one), and the origin of the clinical manifestations of disease.9
Because PE-related cellular and/or molecular abnormalities occur between 8 and 18 WG, the impossibility of obtaining placental tissues from early stages of gestation and the delayed clinical manifestations of PE (until after 26 to 28 WG in its earliest form, or after 34 to 36 WG in late PE10) represent the two biggest problems in the study of this disease. Accordingly, the approaches to study PE mostly depend on specimens (placentas) obtained after delivery, limiting the findings and information to relatively late PE manifestations. Intrinsic complications in the in vitro and in vivo research of placental implication to this human disease require the development of model systems.
Since no model by itself has thoroughly reproduced this affection, a diversity of in vitro, in vivo, and in silico techniques have been employed, as well as several “PE-like” models.4 This review focuses on the most common models used for the study of PE, including those related to placental development, abnormal trophoblast invasion, uteroplacental ischemia, angiogenesis, oxygen deregulation, and immune response to maternal–fetal interactions. The mathematical and computational perspective commonly employed to model gene prioritization, protein–protein interaction network analysis, metabolic network behavior, the genetics of PE, and the PE prediction and classification are discussed. Finally, the potential of these models to understand the pathophysiology of PE, as well as to evaluate prevention actions and new treatment strategies in the control of this pregnancy-specific disorder, is also highlighted.
In vitro models of PE
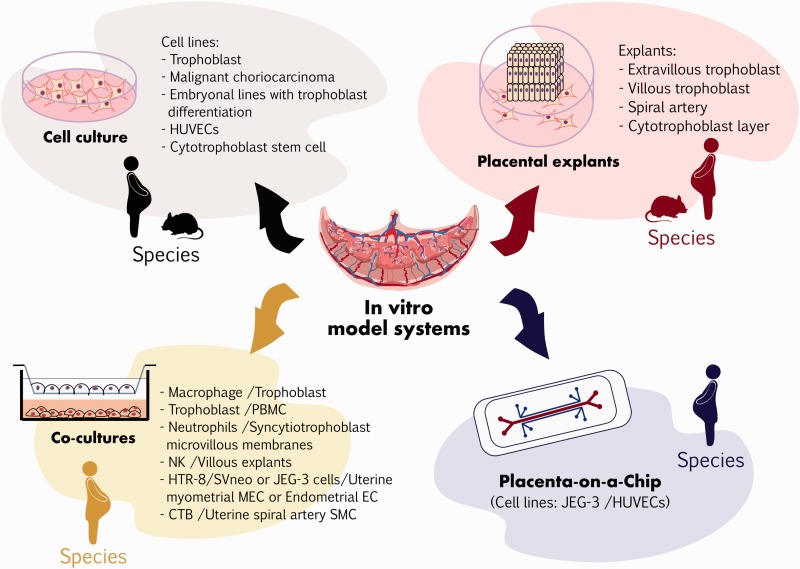
Four types of in vitro models for PE have been described in the literature: (1) cell cultures, (2) placental explants, (3) co-cultures, and (4) placental organ cultures (see Figure 1 and Table 1 in Supplementary material File 1).
Figure 1.
Preeclampsia in vitro research model systems. For detailed information, see the supplementary material File 1.
In PE these models are used to elucidate the mechanism controlling trophoblast cell lineage development, as well as for analysis of trophoblast invasion, endocrine function, immune response, oxygen dysregulation, metabolism, transport, syncytium formation, morphogenesis and adaptation to disease, placental development, and to assess the effect of PE biomarkers or molecules with therapeutic potentials.11–14
The experimental approaches include isolating, culturing, establishing and manipulating primary cell line cultures from trophoblast, malignant choriocarcinoma, embryonal lines with trophoblast differentiation, and stem cells from mammalians (humans, rodents such as mice, golden hamsters, rats and rabbits, bovines, pigs, sheep, and non-human primates such as rhesus monkeys and marmosets).15
In particular, trophoblast cell lines can be obtained through the culture of primary villous, extravillous and placental cells, or from already established trophoblastic cell lines (obtained from normal or malignant cells). Several of these cell lines have been virus transfected, such as cell lines from HTR-8/SVneo and SGHPL cells, acquiring the advantage of longer proliferation in culture than primary cells.16 To extend the utilization of these cell lines, the International Federation of Placenta Associations (IFPA) covenanted the biological criteria for the characterization of established trophoblast cell lines and primary cell cytotrophoblast cultures, in a trophoblast cell line workshop at the European Placenta Group (EPG) meeting in 1999.17 The current villous trophoblast cell model most widely used is the choriocarcinoma cell line BeWo due to it displays most of the attributes of villous trophoblast, such as the formation of syncytiotrophoblast fusion by regulation of syncytin-1 gene expression as well as secretion of human chorionic gonadotropin, prolactin, progesterone, and estradiol.16
Some of the drawbacks of the primary cell cultures are that they cease to proliferate and dedifferentiate when they lose cell–cell interaction, contact with secreted signaling molecules and/or 3D extracellular matrix structure.18 To overcome these limitations, 3D co-culture models and placental explants have been developed, allowing these important cellular interactions to function as an ordered multicellular arrangement where the cells can take advantage of resources and execute functions that would be not possible as individual cells.18 There are two different types of placental explant culture: (A) Early placental cultures for the study of invasion and differentiation of the extravillous trophoblast. This could be first-trimester placental villi explanted on gels of a permissive extracellular matrix or a standard trans-filter trophoblast migration started from a homogeneous preparation of primary cells released from the tissue.19 (B) Term placental cultures for the evaluation of villous trophoblast proliferation and function, and for assessing preventive and therapeutic agents for PE.20
With regard to 3D co-culture models, co-cultures of trophoblasts with peripheral blood mononuclear cells, macrophage, neutrophils or NK cells have been developed to evaluate the immune response and trophoblast–immune cell interactions.13,21–24 In the same sense, in the evaluation of cell proliferation, migration, invasion, and endothelial cell interactions, co-cultures of human umbilical vein endothelial cells (HUVECs) and trophoblasts grown in low oxygenation conditions have been performed.25–27 Co-culture of trophoblast and human uterine myometrial microvascular endothelial cells, or with human endometrial endothelial cells, has also been used to evaluate the oxygen dysregulation effects.28 In all the mentioned models of placental cells, different co-culture matrix compounds in a 3D conformation have been tested, including collagen and matrigel, among others.21,26
Investigate the human placenta constitutes a major experimental feat. In spite of the cell culture, co-cultures and explants have been used as essential tools for the study of different types of placenta-derived cells; they are unable to resemble the placenta-specific structure and functions. To overcome these problems, Lee et al., created, in 2016, a placental organ culture system through the development of a “placenta on a chip” microdevice, which included two polydimethylsiloxane (PDMS) microfluidic channels divided by a membrane. To resemble this model the placental barrier, HUVECs and JEG-3 trophoblast cells were cultured onto the opposite sides of the extracellular matrix membrane (ECM) to form endothelial and epithelial side-by-side layers. This microdevice demonstrated that it is possible to develop a microengineered biomimetic model that replicates the architecture and function of the placenta, and provides new opportunities to simulate and analyze critical physiological responses of the placental barrier.14
Despite the great utility of the in vitro models, the nature of the disease limits their use: specifically, PE implicate modifications in the behavior and communication of fetal trophoblast cells with maternal endothelium4 (see Figure 1 and Table 1 in Supplementary material File 1).
In vivo models of PE
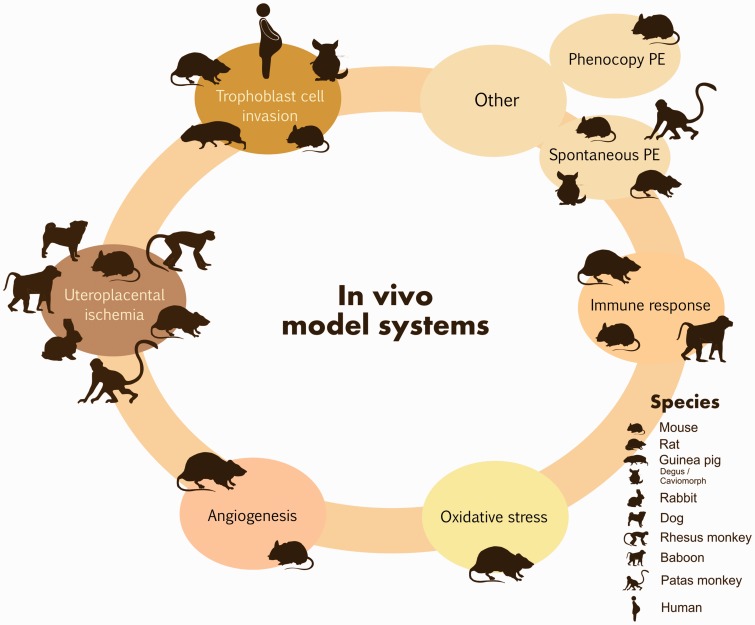
An ideal animal model for PE should reproduce as best as possible the complex pathogenesis and symptoms that underlie the disease, involving the appearance of maternal pregnancy high blood pressure and proteinuria that should be resolved after delivery of the placenta.29 As mammals differ in the placentation process, it is difficult to find a model that satisfies completely these criteria. Table 2 summarizes the in vivo models for the study of PE and their main advantages and limitations (see Table 2 in Supplementary material File 2). In the next paragraphs the most common in vivo models for the study of PE have been described.
Trophoblast invasion models
The placenta plays a fundamental role in the development of PE; it is a specialized pregnancy-organ that is formed with the development of the embryo and fetus, and is comprised of several cell types, but a large part correspond to the trophoblast cells, which participate in the pregnancy-dependent uterine vascular remodeling to provide nutrients to the embryo and gas and waste exchange.30 In some species, trophoblast cells penetrate into the uterine compartment, establishing intimate relationships with the maternal vasculature (hemochorial placentation), a process commonly observed in some Insectivora, Rodentia, Chiroptera, Hyracoidea, Dasypodidae, Lagomorpha, Crocuta, and Primates. It is believed that these groups are intimately linked to the primitive ancestral mammalian stock, conserving numerous of its anatomical characteristics.31 Other species exhibit minimal trophoblast invasion resulting in a segregation of maternal and trophoblast tissues. This type of placentation (epitheliochorial) is seen in domesticated animals, including pigs, and ruminants.31 Intrauterine trophoblast cell invasion is an essential part of hemochorial placentation, and abnormalities in this process are a prominent feature of PE. Nowadays rodents have been reported as in vivo models for the research of trophoblast invasion. Ain et al. generated mice and rats with IFN-gamma genetic deficiency to examine the regulation, the endocrine phenotype and the invasiveness of trophoblast cells.32 In the same sense, Arroyo et al. and Geusens et al. described different in vivo methods using transgenic rat models, to assess endovascular trophoblast cell invasion, spiral artery remodeling, and uteroplacental hemodynamics.33,34 Mess et al. studied caviomorph placentation through the analysis of patterns of trophoblast invasion in guinea pigs and degus, proposing that caviomorphs are appropriate animal models for the study of trophoblast invasion since it should be analogous to humans.35 Finally, Verlohren et al. treated pregnant rats with doxycycline to reduce the trophoblast-vascular remodeling, decreasing following perfusion of the placenta. The model enables the assessment of abnormal vascular remodeling and trophoblast invasion in vivo to obtain significant understanding into PE-related mechanisms.36
Uteroplacental ischemia models
As a consequence of an inadequate trophoblast invasion, uteroplacental ischemia is an important initiating event in PE, is considered a key factor in its pathogenesis and leads to extend vasoconstriction, high blood pressure, and the maternal vascular endothelium dysfunction.37 The first models primarily addressed the mechanism of abruptio placentae in dogs (1953), rabbits (1963), rhesus monkeys (1968), and baboons (Papio anubis, 1974) by ligating permanently or temporarily the inferior vena cava, intercotyledonary vessels and uterine arteries in pregnancy, causing gradual high blood pressure and proteinuria, which concluded after delivery.38–41 Later Abitbol et al. perfected the method of abruptio placentae in rabbit, dogs, and monkeys (Macaca mulatta) by ligating to a specific degree of stricture the terminal aorta, causing high blood pressure, proteinuria, placental lesions, fetal growth restriction, and damage in liver and kidney, similar to those found in human PE.42–44 The murine models for uteroplacental ischemia were developed until 1987, when Eder and MacDonald used laboratory rats (Sprague-Dawley) as an experimental model for pregnancy-induced systemic high blood pressure that was generated through a surgical reduction of blood pressure by 30–35% of aorta capacity.45 Later, Granger et al. developed a reduced uterine perfusion pressure (RUPP) model from modifying and characterizing the rat model of Eder and MacDonald, for the evaluation of cardiovascular–renal dysfunction as a consequence of placental ischemia.46 The oxygen imbalance could result from uteroplacental ischemia due to the hypoxia generated. The transcription factor hypoxia-inducible factor-1 (HIF-1) is a proficient regulator of systemic and cellular homeostatic response to low levels of oxygen and plays a key role in placental development. Several murine models such as C57BL/6J pregnant mice have been developed to evaluate the effects of systemic administration of adenovirus expressing stabilized HIF-1α on pregnancy,47 CITED2-knockout C57BL/6:129 mice, a gene that prevents transactivation of HIF-1α-induced genes through competing with binding of HIF-1α,48 catechol-O-methyltransferase (COMT)-knockout C57BL/6J mice, a gene that generates the HIF-1α inhibitor 2-methoxyestradiol (2-ME),49 and pregnant C57BL/6JArc mice TNF-α infused.50 These models showed elevated blood pressure, proteinuria, reduced embryo and placental weights, fetal intrauterine growth restriction (IUGR), histopathological placental aberrations, and glomerular endotheliosis, hallmark lesions of PE.
Models for angiogenesis study
Increasing evidence suggests that disturbances in angiogenic factor (such as placental growth factor [PGF] and vascular endothelial growth factor [VEGF]) signaling and endothelial health can play a fundamental role in the pathogenesis of PE.51 Exogenous administration of the anti-angiogenic factors soluble endoglin (sENG) and/or soluble fms-like tyrosine kinase-1 (sFlt-1), results in a PE-like phenotype in pregnant rats and mice.51–54 The first model was developed by Maynard et al. They established the sFlt-1-induced rat model of PE through the injection of adenovirus encoding the murine sFlt1 gene product (at 1 × 109 PFU) into the tail vein of Sprague-Dawley rats on early second trimester (day 8–9 of pregnancy), resulting in proteinuria, high blood pressure, and glomerular endotheliosis, the typical injuries of PE.51 Venkatesha et al. intravenously administered Sprague-Dawley pregnant rats with 2 × 109 PFU of adenovirus encoding sFlt1 (Ad.sFlt1), sEng (Ad.sEng) or Ad.sEng + Ad.sFlt1 at day 8 or 9 of pregnancy, resulting in PE-like syndrome, while the coadministration of sEng and sFlt1 amplified the symptoms, leading to severe PE including HELLP syndrome and restriction of fetal growth.53 And finally Kumasawa et al. incubated zona pellucida-free blastocysts collected from B6D2F1 pregnant females in medium containing lentiviral vectors coding for sFlt1 (pLVhsFLT1), sEng (pLV-EGFP) and/or PGF (pLV-mPGF). The transduced blastocysts were implanted into pseudopregnant imprinting control region (ICR) females, resulting in proteinuria and high blood pressure along pregnancy, and the symptoms disappeared after delivery. The model recognizes candidates for PE treatment such as low-dose statins and PGF.52 High levels of sEng and sFlt-1 are commonly an indication of imminent PE and IUGR.55 Two simple animal models for PE were developed by Molnár et al., who caused a chronic inhibition of nitric oxide synthesis (NOS), a mediator of angiogenesis, in pregnant rats through the continuous administration of L-nitro-arginine (a strong inhibitor of NOS), producing a PE-like syndrome,56 and by Carlström et al., who treated pregnant rats with Suramin (an angiogenesis inhibitor) during early placentation, causing high blood pressure and placental dysfunction.57 These models are useful not only for the study of angiogenesis deregulation but also the downstream pathophysiology and treatment of PE.
Oxidative stress models
Placental oxidative stress plays a fundamental role in the pathogenesis of PE. It is a result of increased free radicals and superoxide produced in the course of pregnancy that could attack nucleic acids, proteins, and lipids, generating the injury of both placental cell and tissue and in some cases the complete organ. Oxidative stress promotes maternal vascular endothelial dysfunction and induces leukocyte activation that could compromise fetal growth.58 With the aim of determining whether maternal disturbances during pregnancy induce placental oxidative stress, Beauséjour et al. developed an animal model that shows a PE-like syndrome by making sodium intake in drinking water higher (0.9% or 1.8% NaCl) along the last WG in rats. Markers of oxidative stress were measured in the placenta, identifying alterations in TNF-α expression, vasoactive substances (eNOS and prostanoids) levels, total glutathione content, and apoptotic index, resulting in increased placental cell dysfunction and death. According to their results, maternal perturbations during pregnancy may induce placental oxidative stress.58
Immune models
A rigorous balance between suppression and immune tolerance is necessary for a normal pregnancy, any disparity between pro-inflammatory and anti-inflammatory chemokines and cytokines may lead to abnormal inflammation, which is usually observed in PE.59 Several animal models have been established to investigate the participation of the immune response in the pathogenesis of PE. One of the first models was the one developed by Faas et al., who administered an ultra-low-dose endotoxin (a bacterially derived hydrophobic molecule that induces the immune response) infusion in conscious pregnant rats, resulting in a PE-like syndrome.60
The major findings of several immune models have been achieved by administering inflammatory cytokines to murine and non-human primate models. For example, in pregnant Sprague-Dawley rats, treatment with IL-6 directly enhanced vascular contraction and impaired endothelium-dependent relaxation in systemic vessels.61 In baboons (Papio hamadryas), the intravenous administration of IL-10 and TNF-α in early pregnancy led to vasoconstriction and endothelial dysfunction.62,63 These results demonstrated the usefulness of the administration of cytokines for inducing PE-like symptoms in animal models for the study of PE pathogenesis.
As regard knockout murine models, IL-10-knockout pregnant C57BL/6 mice exposed to low levels of oxygen or Toll-like receptor 3 (TLR-3) agonist polyinosinic-polycytidylic acid (poly I:C) resulted in rated placental injury and proteinuria, high blood pressure, and systemic manifestations of renal pathology.64,65 Pregnant TNF-α infused C57BL/6JArc mice showed placental upregulation of TLR-3 and TLR-4 (molecules responding to inflammation) and PE-like syndrome.50 Chatterjee et al. observed in women with PE a significantly increased level of the TLR3/7/8 family, which has a main participation in the activation of innate immunity, hypothesizing that its activation will be enough to produce PE-like symptoms in mice. They treated pregnant C57BL/6J mice with the TLR7/8 agonist CLO97, the TLR7-specific agonist imiquimod (R-837) and the TLR3 agonist polyinosinic-polycytidylic acid (poly I:C), causing PE-like syndrome.66
A PE mouse model was established by Zenclussen by transferring to allogeneically pregnant BALB/c female mice, activated BALB/c Th1-like splenocytes, during late gestation. This cell transfer only caused PE-like syndrome in pregnant animals, further affecting the pregnancy outcome by increasing fetal rejection through an inflammatory profile of uterine immune cells.67
PE could be a pregnancy-induced autoimmune disorder, result of autoantibody-induced AT receptor activation, a mediator of AT II (a potent vasopressor hormone) observed in women with PE. This hypothesis has led to developed models in both rats and mice, through the administration of anti-AT1-receptor antibodies along pregnancy inducing PE-like syndrome.68,69
A new rat model by administering intraperitoneal low-dose cadmium chloride (CdCl2), an inductor of immune abnormalities, in pregnant Wistar rats at gestational days 9–14 was established by Zhang et al.; in their model, PE-like syndrome was observed.70
Others
Arginine vasopressin (AVP) is a hormone that acts on the kidney as antidiuretic controlling the fluid balance, and also increases the arterial blood pressure through vasoconstriction of the peripheral vessels. Santillan et al. developed a novel and clinically relevant model of PE through the administration of a chronic infusion of AVP along pregnancy of C57BL/6J mice, being enough to phenocopy PE.71
Spontaneous animal models of PE have been discovered. Guinea pigs and patas monkeys were the first inbred animal model reported that spontaneously generates a syndrome that present a close similarity to PE.72,73 Recently, spontaneous murine models have been described: the BPH/5 has borderline high blood pressure before pregnancy, being used to study early feto-placental aberrations prior to the appearance of maternal disease,74,75 and the Dahl salt-sensitive rat, a genetic and spontaneous model of PE, exhibiting a phenotype consistent with several of the features observed in human PE, such as high blood pressure and kidney disease, although it does not exhibit decreasing in uterine artery resistance along late pregnancy.76
Thus, although in vivo studies continue to be necessary, the animal models described above improve our understanding of the pathogenesis of PE and lead to the identification and testing of new therapeutic targets for its treatment (see Figure 2 and Table 2 in Supplementary material File 2).
Figure 2.
Preeclampsia in vivo research model systems. For detailed information, see the supplementary material File 2.
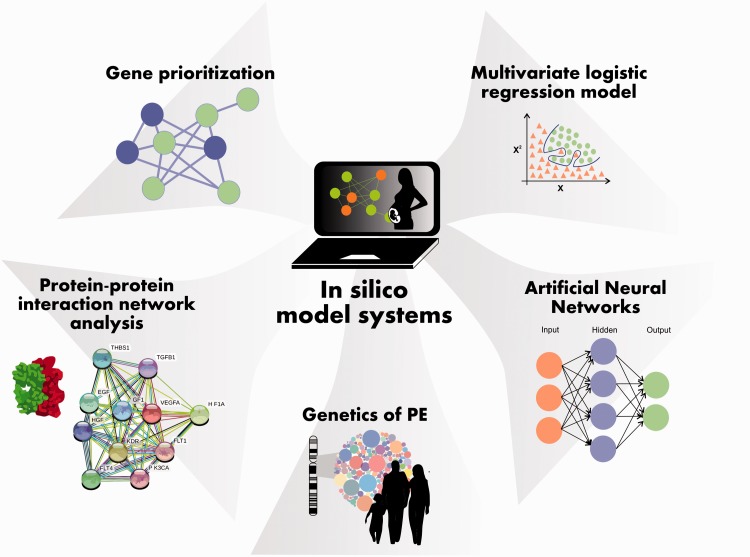
In silico models of PE
PE displays system behaviors that are not easily anticipated, involving numerous and tightly controlled molecular interactions that cannot be comprehended if the biological molecules are treated individually, rather than considering them as an integrated system. The dependence on only employing in vitro and in vivo models to study PE is consequently not enough and the investigation of molecular interactions in detail is required in order to dissect the mechanisms of the etiology, pathophysiology, and progression of the disease in humans.
Several mathematical and computational strategies have been commonly used to model gene prioritization, protein–protein interaction network analysis, metabolic network behavior, the genetics of PE and the rating of women with normal, hypertensive and preeclamptic pregnancy in distinct WG, and to predict the development of PE. These include co-expression network construction, metabolic pathway analysis, genetic algorithms, discriminant analysis classification methods, linear regression, multivariate logistic regression, modular and node analysis, and artificial neural networks (ANNs) (see Figure 3 and Table 3 in Supplementary material File 3).
Figure 3.
Preeclampsia in silico research model systems. For detailed information, see the supplementary material File 3.
Logistic regression is a popular and widely used analysis to build multifactorial models to predict the development of a disease. In PE, several researchers have investigated the utility of clinical variables, maternal characteristics, and biophysical and biochemical markers obtained early during gestation, to develop models for predicting PE (occurrence and severity).77–84
The variables that have been modeled include maternal age, body mass index (BMI), height, parity, blood pressure, hematocrit count, smoking, previous miscarriage, family history of high blood pressure, ultrasound variables such as mean arterial pressure (MAP) and uterine artery pulsatility index (PI),85–88 first-trimester urine and serum metabolomic profiles,89 and biochemical markers such as maternal plasma or serum levels of placental protein-13, PGF, pregnancy-associated plasma protein-A, P-selectin, pentraxin-3, activin-A, inhibin-A, and sENG.90 The prediction efficacy of each model may be compared considering the specificity and sensitivity values and/or with the area under the curve.
Several significant advances have been promoted in cognitive science by the use of economical computer simulations, specifically with the development of ANNs, created by the logician Walter Pitts and the neurophysiologist Warren McCulloch in 194391 as a potential alternative to linear regression, multivariate logistic regression and other conventional statistical analysis. ANNs are a group of statistical learning models inspired by the network of neurons in a brain, integrated by interconnected neurons (processing elements) functioning simultaneously to resolve specific problems.92 The networks can be adjusted based on experience, making them adaptive to inputs and able of learning, with the notable ability to infer meaning from imprecise or complex data that can be used to identify trends and discover profiles that are too difficult to be recognized by either humans or other computer techniques.92
In PE, ANNs have been used as a prediction and classification model. Mello et al. applied an ANN to a group of laboratory and clinical data (ferritin, iron, hematocrit, total proteins, uric acid, creatinine and urea) obtained at 16 and 20 WG to evaluate the efficacy in predicting the development of PE in high-risk pregnant women. Compared with a previous multivariate logistic regression approach, the ANN showed high sensitivity (86.2% vs. 79.3%), specificity (95.4% vs. 97.7%), and positive (86.2% vs. 92%) and negative predictive values (95.5% vs. 93.4%), representing an alternative predictive model that can be performed in early pregnancy (16–20 WG), and considered a tool for primary prevention, breaking off the disease process prior the clinical onset of an overt disease.93 Tejera et al. constructed a model for classifying women with normal, hypertensive and preeclamptic pregnancy in distinct WG employing blood pressure measurements, maternal history, and maternal heart rate variability (HRV) indexes. The obtained results revealed sensitivity for PE of around 80% and an even higher percentage for hypertensive and normal pregnancy groups. Otherwise, specificity in identifying PE women was approximately 85–90%. These results show that the combination of an ANN with HRV indexes could be useful in the characterization and study of pregnancy.94
Conclusion
Despite the great technological developments in health sciences, current in vitro, in vivo, and in silico models still have limitations in recapitulating all the molecular and clinical aspects of PE. The fact that the etiology of PE remains controversial, and the clinical manifestation of the disease presents great heterogeneity, differing from early to late onset of PE and from mild to severe forms of the disease, makes the study of PE a big experimental challenge. Although PE has been complex to study in humans due to difficulties in the delayed appearance of the clinical manifestation, the impossibility of obtaining placental tissues from early stages of gestation and the ethical and technical considerations that preclude the performance of human experiments, human studies are still the gold standard in interpreting the epidemiology and clinical implications of the disease. Therefore, the current model systems offer many advantages in overcoming the research limitations imposed by human studies, and have contributed significantly to understanding the clinical correlations between genetic, molecular biologic, biochemical, physiologic and pathologic analysis, giving great insights into the disease, and in some cases, leading to randomized controlled trials being conducted in humans.
Despite the potential of these models to enable comprehension of the pathogenesis of PE and to test preventative and therapeutic strategies in its management, additional research is needed to develop new model systems that lead to the provision of helpful information on how to prevent placental dysfunction in PE.
Supplemental Material
Supplemental material, supplementary_table1, for Current model systems for the study of preeclampsia by ML Martinez-Fierro, GP Hernández-Delgadillo, V Flores-Morales, E Cardenas-Vargas, M Mercado-Reyes, IP Rodriguez-Sanchez, I Delgado-Enciso, CE Galván-Tejada, JI Galván-Tejada, JM Celaya-Padilla and I Garza-Veloz in Experimental Biology and Medicine
Supplemental Material
Supplemental material, supplementary_table2, for Current model systems for the study of preeclampsia by ML Martinez-Fierro, GP Hernández-Delgadillo, V Flores-Morales, E Cardenas-Vargas, M Mercado-Reyes, IP Rodriguez-Sanchez, I Delgado-Enciso, CE Galván-Tejada, JI Galván-Tejada, JM Celaya-Padilla and I Garza-Veloz in Experimental Biology and Medicine
Supplemental Material
Supplemental material, supplementary_table3, for Current model systems for the study of preeclampsia by ML Martinez-Fierro, GP Hernández-Delgadillo, V Flores-Morales, E Cardenas-Vargas, M Mercado-Reyes, IP Rodriguez-Sanchez, I Delgado-Enciso, CE Galván-Tejada, JI Galván-Tejada, JM Celaya-Padilla and I Garza-Veloz in Experimental Biology and Medicine
Authors’ contributions
All authors participated in the design, review, and editing of this manuscript. MLMF and IGV wrote the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: in part by CONACyT: INFR-2014-01-225520, INFR-2015-01-254106, PDCPN-2015-01-63, SEP-CONACYT-CB-2015-258316 and SS/IMSS/ISSSTE-CONACYT-2016-01-273144.
References
- 1.Kanasaki K, Kalluri R. The biology of preeclampsia. Kidney Int 2009; 76:831–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 2005; 365:785–99 [DOI] [PubMed] [Google Scholar]
- 3.David K, James Philip J, Steer Carl P, Weiner Gonik B. High risk pregnancy: management options. 4 ed. London: Elsevier Health Sciences, 2010 [Google Scholar]
- 4.Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Dise Models Mech 2012; 5:9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balderas Pena LM, Vizcaino Magana CV, Hernandez Higareda S, Vargas Guillen C, Alvarez Romo F, Garcia Iglesias T, del Toro Arreola S, Daneri Navarro A. Lymphocyte subsets and preeclampsia. Ginecol Obstet Mex 2008; 76:327–35 [PubMed] [Google Scholar]
- 6.Gonzalez C, Parra A, Ramirez-Peredo J, Garcia C, Rivera JC, Macotela Y, Aranda J, Lemini M, Arias J, Ibarguengoitia F, de la Escalera GM, Clapp C. Elevated vasoinhibins may contribute to endothelial cell dysfunction and low birth weight in preeclampsia. Lab Invest 2007; 87:1009–17 [DOI] [PubMed] [Google Scholar]
- 7.Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 1983; 4:397–413 [DOI] [PubMed] [Google Scholar]
- 8.Lyall F, Bulmer JN, Duffie E, Cousins F, Theriault A, Robson SC. Human trophoblast invasion and spiral artery transformation: the role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol 2001; 158:1713–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts JM. Pathophysiology of ischemic placental disease. Semin Perinatol 2014; 38:139–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet 2010; 376:631–44 [DOI] [PubMed] [Google Scholar]
- 11.Shiverick KT, King A, Frank H, Whitley GS, Cartwright JE, Schneider H. Cell culture models of human trophoblast II: trophoblast cell lines–a workshop report. Placenta 2001; 22:S104–6 [DOI] [PubMed] [Google Scholar]
- 12.Miller RK, Genbacev O, Turner MA, Aplin JD, Caniggia I, Huppertz B. Human placental explants in culture: approaches and assessments. Placenta 2005; 26:439–48 [DOI] [PubMed] [Google Scholar]
- 13.Mor G, Straszewski-Chavez SL, Abrahams VM. Macrophage-trophoblast interactions. Methods Mol Med 2006; 122:149–63 [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong JS, Huh D. Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med 2016; 29:1046–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amit M, Itskovitz-Eldor J. Derivation spontaneous differentiation of human embryonic stem cells. J Anat 2002; 200:225–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orendi K, Kivity V, Sammar M, Grimpel Y, Gonen R, Meiri H, Lubzens E, Huppertz B. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta 2011; 32:S49–54 [DOI] [PubMed] [Google Scholar]
- 17.King A, Thomas L, Bischof P. Cell culture models of trophoblast II: trophoblast cell lines – a workshop report. Placenta 2000; 21:S113–9 [DOI] [PubMed] [Google Scholar]
- 18.Freytes DO, Wan LQ, Vunjak-Novakovic G. Geometry and force control of cell function. J Cell Biochem 2009; 108:1047–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aplin JD. In vitro analysis of trophoblast invasion. Methods Mol Med 2006; 122:45–57 [DOI] [PubMed] [Google Scholar]
- 20.Tannetta DS, Sargent IL, Linton EA, Redman CW. Vitamins C and E inhibit apoptosis of cultured human term placenta trophoblast. Placenta 2008; 29:680–90 [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, Dutz JP, MacCalman CD, Yong P, Tan R, von Dadelszen P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol 2006; 177:8522–30 [DOI] [PubMed] [Google Scholar]
- 22.Lee SM, Romero R, Lee YJ, Park IS, Park CW, Yoon BH. Systemic inflammatory stimulation by microparticles derived from hypoxic trophoblast as a model for inflammatory response in preeclampsia. Am J Obstet Gynecol 2012; 207:337 e1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aly AS, Khandelwal M, Zhao J, Mehmet AH, Sammel MD, Parry S. Neutrophils are stimulated by syncytiotrophoblast microvillous membranes to generate superoxide radicals in women with preeclampsia. Am J Obstet Gynecol 2004; 190:252–8 [DOI] [PubMed] [Google Scholar]
- 24.Walsh SW. Plasma from preeclamptic women stimulates transendothelial migration of neutrophils. Reprod Sci 2009; 16:320–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez AM, Mulla MJ, Chamley LW, Cadavid AP, Abrahams VM. Aspirin-triggered lipoxin prevents antiphospholipid antibody effects on human trophoblast migration and endothelial cell interactions. Arthritis Rheumatol 2015; 67:488–97 [DOI] [PubMed] [Google Scholar]
- 26.Xu B, Charlton F, Makris A, Hennessy A. Antihypertensive drugs methyldopa, labetalol, hydralazine, and clonidine improve trophoblast interaction with endothelial cellular networks in vitro. J Hypertens 2014; 32:1075–83; discussion 83 [DOI] [PubMed] [Google Scholar]
- 27.Xu B, Nakhla S, Makris A, Hennessy A. TNF-alpha inhibits trophoblast integration into endothelial cellular networks. Placenta 2011; 32:241–6 [DOI] [PubMed] [Google Scholar]
- 28.Graham CH, Fitzpatrick TE, McCrae KR. Hypoxia stimulates urokinase receptor expression through a heme protein-dependent pathway. Blood 1998; 91:3300–7 [PubMed] [Google Scholar]
- 29.McCarthy FP, Kingdom JC, Kenny LC, Walsh SK. Animal models of preeclampsia; uses and limitations. Placenta 2011; 32:413–9 [DOI] [PubMed] [Google Scholar]
- 30.Fonseca BM, Correia-da-Silva G, Almada M, Costa MA, Teixeira NA. The endocannabinoid system in the postimplantation period: a role during decidualization and placentation. Int J Endocrinol 2013; 2013:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wooding FBP, Placentation FFAP. In: Lamming GE. (ed) Marshall’s physiology of reproduction. 4 ed. London: Chapman & Hall, 1994, pp.233–460 [Google Scholar]
- 32.Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol 2003; 260:176–90 [DOI] [PubMed] [Google Scholar]
- 33.Arroyo JA, Konno T, Khalili DC, Soares MJ. A simple in vivo approach to investigate invasive trophoblast cells. Int J Dev Biol 2005; 49:977–80 [DOI] [PubMed] [Google Scholar]
- 34.Geusens N, Verlohren S, Luyten C, Taube M, Hering L, Vercruysse L, Hanssens M, Dudenhausen JW, Dechend R, Pijnenborg R. Endovascular trophoblast invasion, spiral artery remodelling and uteroplacental haemodynamics in a transgenic rat model of pre-eclampsia. Placenta 2008; 29:614–23 [DOI] [PubMed] [Google Scholar]
- 35.Mess A, Zaki N, Kadyrov M, Korr H, Kaufmann P. Caviomorph placentation as a model for trophoblast invasion. Placenta 2007; 28:1234–8 [DOI] [PubMed] [Google Scholar]
- 36.Verlohren S, Geusens N, Morton J, Verhaegen I, Hering L, Herse F, Dudenhausen JW, Muller DN, Luft FC, Cartwright JE, Davidge ST, Pijnenborg R, Dechend R. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension 2010; 56:304–10 [DOI] [PubMed] [Google Scholar]
- 37.Aardema MW, Oosterhof H, Timmer A, van Rooy I, Aarnoudse JG. Uterine artery Doppler flow and uteroplacental vascular pathology in normal pregnancies and pregnancies complicated by pre-eclampsia and small for gestational age fetuses. Placenta 2001; 22:405–11 [DOI] [PubMed] [Google Scholar]
- 38.Howard BK, Goodson JH. Experimental placental abruption. Obstet Gynecol 1953; 2:442–6 [PubMed] [Google Scholar]
- 39.Haynes DM. Experimental abruptio placentae in the rabbit. Am J Obstet Gynecol 1963; 85:626–45 [DOI] [PubMed] [Google Scholar]
- 40.Myers RE, Fujikura T. Placental changes after experimental abruptio placentae and fetal vessel ligation of rhesus monkey placenta. Am J Obstet Gynecol 1968; 100:946–51 [PubMed] [Google Scholar]
- 41.Cavanagh D, Rao PS, Tung KS, Gaston L. Eclamptogenic toxemia: the development of an experimental model in the subhuman primate. Am J Obstet Gynecol 1974; 120:183–96 [DOI] [PubMed] [Google Scholar]
- 42.Abitbol MM, Driscoll SG, Ober WB. Placental lesions in experimental toxemia in the rabbit. Am J Obstet Gynecol 1976; 125:942–8 [DOI] [PubMed] [Google Scholar]
- 43.Abitbol MM, Pirani CL, Ober WB, Driscoll SG, Cohen MW. Production of experimental toxemia in the pregnant dog. Obstet Gynecol 1976; 48:537–48 [PubMed] [Google Scholar]
- 44.Abitbol MM, Ober MB, Gallo GR, Driscoll SG, Pirani CL. Experimental toxemia of pregnancy in the monkey, with a preliminary report on renin and aldosterone. Am J Pathol 1977; 86:573–90 [PMC free article] [PubMed] [Google Scholar]
- 45.Eder DJ, McDonald MT. A role for brain angiotensin II in experimental pregnancy-induced hypertension in laboratory rats. Clin Exp Hypertens B 1987; 6:431–51 [Google Scholar]
- 46.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med 2006; 122:383–92 [DOI] [PubMed] [Google Scholar]
- 47.Tal R, Shaish A, Barshack I, Polak-Charcon S, Afek A, Volkov A, Feldman B, Avivi C, Harats D. Effects of hypoxia-inducible factor-1alpha overexpression in pregnant mice: possible implications for preeclampsia and intrauterine growth restriction. Am J Pathol 2010; 177:2950–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Withington SL, Scott AN, Saunders DN, Lopes Floro K, Preis JI, Michalicek J, Maclean K, Sparrow DB, Barbera JP, Dunwoodie SL. Loss of Cited2 affects trophoblast formation and vascularization of the mouse placenta. Dev Biol 2006; 294:67–82 [DOI] [PubMed] [Google Scholar]
- 49.Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF, Kalluri R. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 2008; 453:1117–21 [DOI] [PubMed] [Google Scholar]
- 50.Bobek G, Surmon L, Mirabito KM, Makris A, Hennessy A. Placental regulation of inflammation and hypoxia after TNF-alpha infusion in mice. Am J Reprod Immunol 2015; 74:407–18 [DOI] [PubMed] [Google Scholar]
- 51.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003; 111:649–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, Okabe M. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci U S A 2011; 108:1451–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006; 12:642–9 [DOI] [PubMed] [Google Scholar]
- 54.Karumanchi SA, Stillman IE. In vivo rat model of preeclampsia. Methods Mol Med 2006; 122:393–9 [DOI] [PubMed] [Google Scholar]
- 55.Zafer E, Demircan Sezer S, Nergiz Avcioglu S, Atakul T, Kurt Omurlu I, Yuksel H. Correlation between maternal serum-amniotic fluid anti-angiogenic factors and uterine artery Doppler indices. J Matern Fetal Neonatal Med 2017;30:2653–7 [DOI] [PubMed]
- 56.Molnar M, Suto T, Toth T, Hertelendy F. Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. Am J Obstet Gynecol 1994; 170:1458–66 [DOI] [PubMed] [Google Scholar]
- 57.Carlstrom M, Wentzel P, Skott O, Persson AE, Eriksson UJ. Angiogenesis inhibition causes hypertension and placental dysfunction in a rat model of preeclampsia. J Hypertens 2009; 27:829–37 [DOI] [PubMed] [Google Scholar]
- 58.Beausejour A, Bibeau K, Lavoie JC, St-Louis J, Brochu M. Placental oxidative stress in a rat model of preeclampsia. Placenta 2007; 28:52–8 [DOI] [PubMed] [Google Scholar]
- 59.Kalagiri RR, Carder T, Choudhury S, Vora N, Ballard AR, Govande V, Drever N, Beeram MR, Uddin MN. Inflammation in complicated pregnancy and its outcome. Am J Perinatol 2016; 33:1337–56 [DOI] [PubMed] [Google Scholar]
- 60.Faas MM, Schuiling GA, Baller JF, Visscher CA, Bakker WW. A new animal model for human preeclampsia: ultra-low-dose endotoxin infusion in pregnant rats. Am J Obstet Gynecol 1994; 171:158–64 [DOI] [PubMed] [Google Scholar]
- 61.Orshal JM, Khalil RA. Interleukin-6 impairs endothelium-dependent NO-cGMP-mediated relaxation and enhances contraction in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol 2004; 286:R1013–23 [DOI] [PubMed] [Google Scholar]
- 62.Orange S, Rasko JE, Thompson JF, Vaughan J, Olive E, Pedler M, Horvath JS, Hennessy A. Interleukin-10 regulates arterial pressure in early primate pregnancy. Cytokine 2005; 29:176–85 [DOI] [PubMed] [Google Scholar]
- 63.Sunderland NS, Thomson SE, Heffernan SJ, Lim S, Thompson J, Ogle R, McKenzie P, Kirwan PJ, Makris A, Hennessy A. Tumor necrosis factor alpha induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 2011; 56:192–9 [DOI] [PubMed] [Google Scholar]
- 64.Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension 2011; 57:505–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chatterjee P, Chiasson VL, Kopriva SE, Young KJ, Chatterjee V, Jones KA, Mitchell BM. Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension 2011; 58:489–96 [DOI] [PubMed] [Google Scholar]
- 66.Chatterjee P, Weaver LE, Doersch KM, Kopriva SE, Chiasson VL, Allen SJ, Narayanan AM, Young KJ, Jones KA, Kuehl TJ, Mitchell BM. Placental Toll-like receptor 3 and Toll-like receptor 7/8 activation contributes to preeclampsia in humans and mice. PloS One 2012; 7:e41884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zenclussen AC. A novel mouse model for preeclampsia by transferring activated th1 cells into normal pregnant mice. Methods Mol Med 2006; 122:401–12 [DOI] [PubMed] [Google Scholar]
- 68.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 2008; 14:855–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wenzel K, Rajakumar A, Haase H, Geusens N, Hubner N, Schulz H, Brewer J, Roberts L, Hubel CA, Herse F, Hering L, Qadri F, Lindschau C, Wallukat G, Pijnenborg R, Heidecke H, Riemekasten G, Luft FC, Muller DN, Lamarca B, Dechend R. Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension 2011; 58:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q, Huang Y, Zhang K, Yan Y, Wang F, Wu J, Wang X, Xu Z, Chen Y, Cheng X, Li Y, Jiao J, Ye D. Cadmium-induced immune abnormality is a key pathogenic event in human and rat models of preeclampsia. Environ Pollut 2016; 218:770–82 [DOI] [PubMed] [Google Scholar]
- 71.Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, Leslie KK, Hunter SK, Zamba GK, Gibson-Corley KN, Grobe JL. Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension 2014; 64:852–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ganaway JR, Allen AM. Obesity predisposes to pregnancy toxemia (ketosis) of guinea pigs. Lab Anim Sci 1971; 21:40–4 [PubMed] [Google Scholar]
- 73.Palmer AE, London WT, Sly DL, Rice JM. Spontaneous preeclamptic toxemia of pregnancy in the patas monkey (Erythrocebus patas). Lab Anim Sci 1979; 29:102–6 [PubMed] [Google Scholar]
- 74.Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, Sethi S, Weiss RM, Bates JN. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension 2002; 39:337–42 [DOI] [PubMed] [Google Scholar]
- 75.Dokras A, Hoffmann DS, Eastvold JS, Kienzle MF, Gruman LM, Kirby PA, Weiss RM, Davisson RL. Severe feto-placental abnormalities precede the onset of hypertension and proteinuria in a mouse model of preeclampsia. Biol Reprod 2006; 75:899–907 [DOI] [PubMed] [Google Scholar]
- 76.Gillis EE, Williams JM, Garrett MR, Mooney JN, Sasser JM. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol 2015; 309:R62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Delic R, Stefanovic M, Krivec S, Weber V. Statistical regression model of standard and new laboratory markers and its usefulness in prediction of preeclampsia. J Matern Fetal Neonatal Med 2014; 27:388–92 [DOI] [PubMed] [Google Scholar]
- 78.Direkvand-Moghadam A, Khosravi A, Sayehmiri K. Predictive factors for preeclampsia in pregnant women: a unvariate and multivariate logistic regression analysis. Acta Biochim Pol 2012; 59:673–7 [PubMed] [Google Scholar]
- 79.Delic R, Stefanovic M. Optimal laboratory panel for predicting preeclampsia. J Matern Fetal Neonatal Med 2010; 23:96–102 [DOI] [PubMed] [Google Scholar]
- 80.Forest JC, Masse J, Bujold E, Rousseau F, Charland M, Theriault S, Lafond J, Giguere Y. OS090. Performance of candidate clinical and biochemical markers in screening early in pregnancy to detect women at high risk to develop preeclampsia. Pregnancy Hypertens 2012; 2:227. [DOI] [PubMed] [Google Scholar]
- 81.Masse J, Forest JC, Moutquin JM, Marcoux S, Brideau NA, Belanger M. A prospective study of several potential biologic markers for early prediction of the development of preeclampsia. Am J Obstet Gynecol 1993; 169:501–8 [DOI] [PubMed] [Google Scholar]
- 82.Harrington K, Carpenter RG, Goldfrad C, Campbell S. Transvaginal Doppler ultrasound of the uteroplacental circulation in the early prediction of pre-eclampsia and intrauterine growth retardation. Br J Obstet Gynaecol 1997; 104:674–81 [DOI] [PubMed] [Google Scholar]
- 83.Romero-Gutierrez G, Malacara JM, Amador N, Fierro MC, Munoz-Guevara LM, Molina RR. Homeostatic model assessment and risk for hypertension during pregnancy: a longitudinal prospective study. Am J Perinatol 2004; 21:455–62 [DOI] [PubMed] [Google Scholar]
- 84.Martinez-Fierro ML, Garza-Veloz I, Castruita-Dela Rosa C, Ortiz-Castro Y, Aceves-Medina MC, Vazquez-Castro R, Delgado-Enciso I, Castaneda-Lopez ME. Plasma cancer biomarker multiplex screening and the risk of subsequent preeclampsia. Int J Cardiol 2015; 179:58–60 [DOI] [PubMed] [Google Scholar]
- 85.Lee LC, Sheu BC, Shau WY, Liu DM, Lai TJ, Lee YH, Huang SC. Mid-trimester beta-hCG levels incorporated in a multifactorial model for the prediction of severe pre-eclampsia. Prenat Diagn 2000; 20:738–43 [DOI] [PubMed] [Google Scholar]
- 86.Nijdam ME, Janssen KJ, Moons KG, Grobbee DE, van der Post JA, Bots ML, Franx A. Prediction model for hypertension in pregnancy in nulliparous women using information obtained at the first antenatal visit. J Hypertens 2010; 28:119–26 [DOI] [PubMed] [Google Scholar]
- 87.Tomoda S, Tamura T, Kitanaka T, Ogita S. First trimester biological markers for the prediction of pregnancy-induced hypertension. Am J Perinatol 1996; 13:89–93 [DOI] [PubMed] [Google Scholar]
- 88.Gabbay-Benziv R, Oliveira N, Baschat AA. Optimal first trimester preeclampsia prediction: a comparison of multi marker algorithm, risk profiles and their sequential application. Prenat Diagn 2015; 36:34–9 [DOI] [PubMed] [Google Scholar]
- 89.Austdal M, Tangeras LH, Skrastad RB, Salvesen K, Austgulen R, Iversen AC, Bathen TF. First trimester urine and serum metabolomics for prediction of preeclampsia and gestational hypertension: a prospective screening study. Int J Mol Sci 2015; 16:21520–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11-13 weeks. Prenat Diagn 2011; 31:66–74 [DOI] [PubMed] [Google Scholar]
- 91.Shanmuganathan S. Artificial neural network modelling: an introduction In: Shanmuganathan S, Samarasinghe S. (eds) Artificial neural network modelling. 1st ed. Switzerland: Springer, 2016, pp.1–14 [Google Scholar]
- 92.del Rosario Martinez-Blanco M, Castañeda-Miranda VH, Ornelas-Vargas G, Guerrero-Osuna HA, Solis-Sanchez LO, Castañeda-Miranda R, Celaya-Padilla JM, Galvan-Tejada CE, Galvan-Tejada JI, Vega-Carrillo HR, Martínez-Fierro M, Garza-Veloz I, Ortiz-Rodriguez JM. Generalized regression neural networks with application in neutron spectrometry In: Rosa DJLG. (ed) Artificial neural networks – models and applications. 1st ed Croatia: InTech, 2016, p.412 [Google Scholar]
- 93.Mello G, Parretti E, Ognibene A, Mecacci F, Cioni R, Scarselli G, Messeri G. Prediction of the development of pregnancy-induced hypertensive disorders in high-risk pregnant women by artificial neural networks. Clin Chem Lab Med CCLM/FESCC 2001; 39:8015. [DOI] [PubMed] [Google Scholar]
- 94.Tejera E, Jose Areias M, Rodrigues A, Ramoa A, Manuel Nieto-Villar J, Rebelo I. Artificial neural network for normal, hypertensive, and preeclamptic pregnancy classification using maternal heart rate variability indexes. J Matern Fetal Neonatal Med 2011; 24:1147–51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supplementary_table1, for Current model systems for the study of preeclampsia by ML Martinez-Fierro, GP Hernández-Delgadillo, V Flores-Morales, E Cardenas-Vargas, M Mercado-Reyes, IP Rodriguez-Sanchez, I Delgado-Enciso, CE Galván-Tejada, JI Galván-Tejada, JM Celaya-Padilla and I Garza-Veloz in Experimental Biology and Medicine
Supplemental material, supplementary_table2, for Current model systems for the study of preeclampsia by ML Martinez-Fierro, GP Hernández-Delgadillo, V Flores-Morales, E Cardenas-Vargas, M Mercado-Reyes, IP Rodriguez-Sanchez, I Delgado-Enciso, CE Galván-Tejada, JI Galván-Tejada, JM Celaya-Padilla and I Garza-Veloz in Experimental Biology and Medicine
Supplemental material, supplementary_table3, for Current model systems for the study of preeclampsia by ML Martinez-Fierro, GP Hernández-Delgadillo, V Flores-Morales, E Cardenas-Vargas, M Mercado-Reyes, IP Rodriguez-Sanchez, I Delgado-Enciso, CE Galván-Tejada, JI Galván-Tejada, JM Celaya-Padilla and I Garza-Veloz in Experimental Biology and Medicine