Abstract
Traumatic brain injury (TBI) is the leading cause of death in young adults. After the initial injury, a poorly understood secondary phase, including a strong inflammatory response determines the final outcome of TBI. The inhibitor of NF-κB kinase (IKK)/NF-κB signaling system is the key regulator of inflammation and also critically involved in regulation of neuronal survival and synaptic plasticity. We addressed the neuron-specific function of IKK2/NF-κB signaling pathway in TBI using an experimental model of closed-head injury (CHI) in combination with mouse models allowing conditional regulation of IKK/NF-κB signaling in excitatory forebrain neurons. We found that repression of IKK2/NF-κB signaling in neurons increases the acute posttraumatic mortality rate, worsens the neurological outcome, and promotes neuronal cell death by apoptosis, thus resulting in enhanced proinflammatory gene expression. As a potential mechanism, we identified elevated levels of the proapoptotic mediators Bax and Bad and enhanced expression of stress response genes. This phenotype is also observed when neuronal IKK/NF-κB activity is inhibited just before CHI. In contrast, neuron-specific activation of IKK/NF-κB signaling does not alter the TBI outcome. Thus, this study demonstrates that physiological neuronal IKK/NF-κB signaling is necessary and sufficient to protect neurons from trauma consequences.—Mettang, M., Reichel, S. N., Lattke, M., Palmer, A., Abaei, A., Rasche, V., Huber-Lang, M., Baumann, B., Wirth, T. IKK2/NF-κB signaling protects neurons after traumatic brain injury.
Keywords: neuroinflammation, apoptosis neurological outcome, neurodegeneration, conditional mouse model
Traumatic brain injury (TBI) represents a leading cause of mortality and morbidity, especially among young individuals below the age of 45 yr (1, 2). It is estimated that 10 million deaths and hospitalizations annually worldwide are direct consequences of TBI. Approximately 57 million people, currently alive, have experienced a brain injury (1). Thus, TBI represents a major global public health problem (3). This statistic translates within the United States alone to ∼1.7 million annual TBI cases, and 5.3 million people are living with a TBI-related disability (4). TBI is typically triggered by a blunt- or sharp-force trauma, with most patients having a closed-head injury (CHI) (5, 6). TBI initiates a complex disease, and the pathophysiology is characterized by great heterogeneity and widely varying outcomes (7). Overall, TBI pathophysiology can be subdivided into two stages: Whereas the primary injury causes direct mechanical damage of blood vessels and parenchymal cells at the lesion site, subsequent secondary injury develops over a period from minutes to weeks and, potentially, months or years (8, 9). Secondary pathology is characterized by an early increase in glutamate and reactive oxygen species, as well as the occurrence of mitochondrial dysfunction and increased expression of cytokines and chemokines (10–12). The secondary pathology is accompanied by neuroinflammation initiated by release of damage-associated molecular patterns, which is supposed to promote delayed cell death, cerebral edema formation and blood–brain barrier damage (4, 11–14). Nevertheless, inflammation may also have beneficial effects by contributing to functional repair, tissue remodeling and neuroplasticity (4, 14–16).
The NF-κB family of transcription factors plays a critical role in the regulation of stress-associated and inflammatory gene expression, as well as in cell survival and neuronal differentiation in the CNS (17–20). The family of NF-κB transcription factors comprises 5 members—RelA, RelB, c-Rel, p105/p50, and p100/p52—that form homo- or heterodimers. In its inactive state, NF-κB is retained in the cytoplasm by IκB inhibitory proteins. The crucial step in NF-κB activation is the phosphorylation of IκB proteins by the inhibitor of NF-κB kinase (IKK) complex, resulting in their proteasomal degradation. This process induces nuclear translocation and transcriptional regulation of target genes. The IKK complex contains 2 protein kinases, IKK1 (IKKα) and IKK2 (IKKβ), as well as the regulatory protein NF-κB essential modulator (IKKγ). IKK2 is the critical kinase subunit inducing the canonical signaling pathway, essentially involved in the regulation of inflammation and cell survival (17, 21). In the CNS, a variety of stimuli, such as damage- and pathogen-associated molecular patterns, synaptic activity, oxidative stress, cytokines, chemokines, neurotransmitters, neurotrophic factors, and neurotoxins induce NF-κB (17, 22, 23). NF-κB is activated in neurons and glial cells upon experimental injury and in the context of neuropathological disorders and has been linked to both neurodegenerative and neuroprotective activities (20, 24–27). NF-κB activation in glial cells has been mainly related to inflammation (28–31), NF-κB activation in neurons is involved in differentiation, synaptic plasticity and neuronal development and survival (26, 32–35). Cytokines such as TNF and IL-1β are typically up-regulated by TBI induction. They are both NF-κB target genes, but can also activate NF-κB, resulting in a self-propagating vicious circle (17, 36). Consistently, NF-κB was elevated in rats after controlled cortical impact and fluid percussion brain injury (37, 38) as well as in biopsies of human contused brain tissue (39). Furthermore, after cortical aspiration, NF-κB is mainly activated in neuronal cells of the degenerating cortex and in astrocytes of the corpus callosum (40). The NF-κB subunit p50 has been was found to be highly expressed in neurons that survive hippocampal injury, arguing for a critical role in the regulation of repair and regeneration (41). In contrast, controlled cortical impact trauma leads to increased brain injury volumes and blood–brain barrier dysfunction in transgenic mice with elevated NF-κB activity in the brain (42). These studies indicate that NF-κB signaling may have a beneficial or detrimental role, depending on the cell type where it is expressed and the nature of the trauma (43–45).
Although activation of NF-κB is a common feature of different pathologies of the CNS, including trauma, excitotoxicity, and ischemia (37, 46–49), the precise role of this transcription factor in damaged neurons is still unknown. To characterize the specific role of neuronal NF-κB in the pathogenesis of TBI, we analyzed the consequences of NF-κB inhibition and activation in neurons, using corresponding neuron-specific loss-of-function and gain-of-function mouse models, respectively. We found that neuronal NF-κB repression increases the acute posttraumatic mortality rate and worsens the neurological outcome of survivors at various time points after CHI, including enhanced reactive astrogliosis and apoptosis. Ectopic neuronal NF-κB activation does not reduce the harmful effects of secondary TBI pathogenesis. Thus, our findings provide the first direct link of CHI and neuronal IKK2/NF-κB signaling, depicting a key pathway in the development of secondary TBI pathology.
MATERIAL AND METHODS
Transgenic mice
Mice were housed in a specific pathogen-free animal facility at Ulm University under standardized conditions with food and water provided ad libitum. Camk2a.tTA×luciferase-(tetO)7-DN-IKK2 (short IKK2-DNCamk2a) and Camk2a.tTA×luciferase-(tetO)7-CA-IKK2 (short IKK2-CACamk2a) were generated by crossing Camk2a.tTA mice with single transgenic mice carrying a luciferase-(tetO)7-IKK2-DN or a luciferase-(tetO)7-IKK2-CA transgene, respectively (49). All mouse lines were bred on an NMRI background. Inactivation of the transgenes was achieved by administration of Dox (0.1 g/L in the drinking water containing 1% sucrose; MP Biomedicals, Eschwege, Germany) to the mothers during pregnancy until weaning (at age 4 wk) and as indicated in Figs. 6 and 7 and Supplemental Fig. S8. Transgene expression was induced by Dox withdrawal. Wild-type and single transgenic mice were used as control groups. Luciferase activity was measured in the living animals with the IVIS200 in vivo imaging system (Caliper Life Sciences, Hopkinton, MA, USA) (49, 50). All animal experiments were performed in compliance with the guidelines of the National Institutes of Health (Bethesda, MD, USA) and the German Animal Protection Act, and were approved by the Regierungspräsidium Tübingen (Tübingen, Germany).
Figure 6.
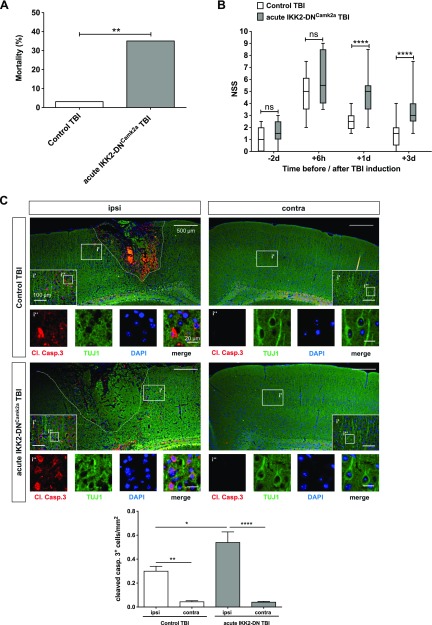
Acute neuronal IKK2-DN transgene expression also results in detrimental TBI outcome. A) Mortality of mice with acute neuronal NF-κB inhibition after CHI. The mortality rate is depicted as the percentage of animals that died. Acute IKK2-DNCamk2a mice showed a significantly enhanced posttraumatic mortality rate compared to control littermates, similar to the chronic IKK2-DNCamk2a mouse model. (Control TBI, n = 30; Acute IKK2-DNCamk2a TBI, n = 17). **P < 0.01, vs. control, by Fisher’s exact test. B) NSS score of mice with acute neuronal NF-κB inhibition. Head-injured acute IKK2-DNCamk2a mice also showed a delayed recovery from TBI, as reflected by a significantly increased NSS, compared to head-injured control animals. Data are presented as box plots with median ± interquartile range; whiskers show minimum and maximum range (n = 11–22). ****P < 0.0001 [not significant (ns), by nonparametric Mann-Whitney U test]. C) Expression of apoptotic cells in mice with acute neuronal NF-κB repression. Increased posttraumatic neuronal cell death 3 d after TBI in the injured (ipsi) hemisphere of acute IKK2-DNCamk2a mice vs. control animals and the uninjured (contra) hemisphere. Immunofluorescent staining of cleaved caspase 3+ neurons (TUJ1+ cells). Quantification of cleaved caspase 3+ cells indicated significantly enhanced apoptosis in mice with acute neuronal NF-κB inhibition vs. control littermates. Means ± sem (n = 3–4). *P < 0.05, **P < 0.01, ****P < 0.0001 (by 1-way ANOVA with Bonferroni’s correction). Scale bars, 500 µm; i′, inset′: 100 µm; i″, inset″: 20 µm.
Figure 7.
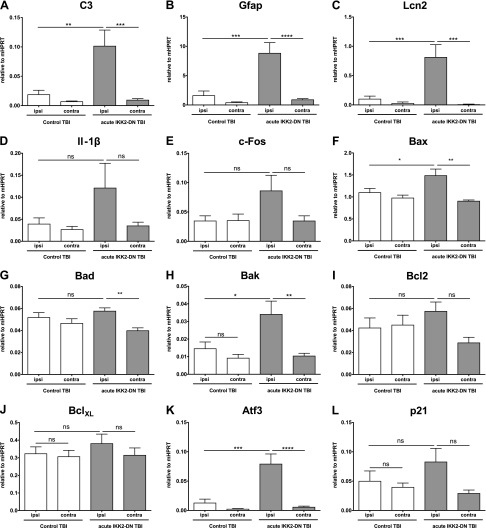
Inflammation and apoptosis are increased in mice with acute neuronal NF-κB inhibition. Expression of proinflammatory and apoptotic genes in the acute IKK2-DNCamk2a mouse model. Acute IKK2-DNCamk2a mice also exhibited prominent neuroinflammation and apoptosis induction 3 d after TBI, similar to chronic IKK2-DNCamk2a mice. This finding is indicated by qPCR analysis of the complement factor C3 (A); the astroglial marker Gfap (B); the acute phase protein Lcn2 (C); the inflammatory cytokine Il1b (D); the neuronal activity marker c-Fos (E); the proapoptotic genes Bax (F), Bad (G), and Bak (H); the antiapoptotic genes Bcl2 (I) and BclXL (J); and the stress response genes in the p53 pathway Atf3 (K) and p21 (L). Measured expression levels are presented relative to Hprt. Means ± sem (n = 3–6). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 [not significant (ns), at 3 d after TBI, between the indicated groups, by 1-way ANOVA, followed by Bonferroni’s post hoc test].
CHI model
Twelve-week-old mice were subjected to experimental CHI with a standardized weight-drop device (51, 52). In brief, the animals were anesthetized with ketamine (Pfizer Pharma, Karlsruhe, Germany), with an intraperitoneal dose of 100 mg/kg body weight, and 2% xylazine (Bayer Health Care, Monheim, Germany), with an i.p. dose of 16 mg/kg body weight Afterward, the skull was exposed by a longitudinal incision of the skin, and a focal blunt injury was induced in the left hemisphere by dropping a 330 g metal rod on the skull from a height of 2.7 cm. Only animals with a visible imprint of the falling rod on the skull were included for further analyses. After trauma, the mice received supporting oxygenation with 100% O2, the wound was sutured, and the animals were placed into a warmed recovery cage with ad libitum access to food and water. Buprenorphine analgesia (Temgesic; Essex Pharma, Munich, Germany) was administered subcutaneously (0.03 mg/kg body weight) immediately after trauma and every 8 h thereafter until 24 h. Sham-procedure mice underwent anesthesia, scalp incision, suturing of the wound, and analgesia, but no experimental head trauma. Animals that died directly after the mechanical insult and during anesthesia (up to 2 h after TBI) were included in the acute posttraumatic mortality rate.
Neurological severity score
A 10-point neurological severity score (NSS) was used to assess the posttraumatic neurological impairments (52–54). This scoring system consists of 10 tests, including tasks to measure cognitive and motor functions (e.g., beam walk, round-stick balance, exit circle, gait pattern, and exploratory interest in new environment), whereby 1 point is given for failure of the task and 0 points for succeeding. Thus, a maximum NSS of 10 points indicates severe neurological dysfunction, with failure of all tasks. In the present study, the NSS was assessed 2 d before and 6 h, 1 d, and 3 d after CHI.
Rotarod, open field, and elevated-plus maze
Motor behavior after CHI was analyzed with the ENV-575M rotarod (Med Associates, St. Albans, VT, USA). After 1 min at 4 rpm for adjustment, the cylinder accelerated within 5 min to 40 rpm. The latency until falling off the accelerating rotarod was recorded. Pretraining was assessed 2 d before TBI (consisting of 4 trials) and analysis was performed at 2, 7, and 30 d post trauma.
To assess general locomotor activity and anxiety/exploratory behavior, the animals were put individually in an open field (OF) arena (50 × 50 cm) (55) and were recorded for 15 min with a video camera with the Tracking System Viewer 2 software (Biobserve, Bonn, Germany). OF analysis was performed at 2 d before injury and 1, 3, 7, and 30 d post trauma.
To further study anxiety and exploratory behavior, mice were subjected to an additional elevated-plus maze test. This maze consists of an elevated plus-shaped platform with 4 arms of equal size (30 × 5 cm), of which 2 opposing arms are surrounded by 16 cm–high walls (55). Animals were placed in the center platform of the maze facing an open arm and were video-tracked for 5 min with the Tracking System Viewer 2 software.
Protein isolation and immunoblot analysis
Tissue samples (cortical impact area) were snap frozen in liquid nitrogen, homogenized with a mortar and pestle and lysed in KA-lysis buffer [25 mM Tris-HCl, 150 mM NaCl, 25 mM sodium pyrophosphate, 50 mM β-glycerophosphate, 50 mM NaF, 2 mM EGTA, 2 mM EDTA, 1 mM DTT, 10% glycerol, 1% Triton X-100 (pH 8.0)] supplemented with protease inhibitors (1 mM PMSF and Complete Mini Tablet; Roche Diagnostics, Mannheim, Germany). After centrifugation (30 min, 13,000 rpm), the supernatant was used as the total protein extract. Equal amounts of protein (30 µg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5% nonfat dry milk in TBS buffer for 1 h at room temperature, primary antibodies (see below) were incubated in blocking solution overnight at 4°C or for 2 h at room temperature. After 3 washing steps, membranes were incubated with horseradish peroxidase–coupled secondary antibody for 1 h at room temperature. Membranes were exposed to ECL detection reagent (Thermo Fisher Scientific) and developed by ECL.
Histology and immunofluorescence staining
Brains were fixed by immersion with 4% paraformaldehyde (overnight at 4°C), dehydrated, embedded in paraffin, and cut to 7 µm–thick coronal sections on a microtome (Microm HM355S; Thermo Fisher Scientific). After rehydration, heat-mediated antigen retrieval was performed with sodium citrate (10 mM, pH 6.0, 0.05% Tween 20) and the tissue sections were additionally incubated with 0.5% Triton X-100 for 30 min. Sections were washed with PBS and blocked with 5% bovine serum albumin (BSA) for 1 h at room temperature. Incubation with primary antibodies (in 5% BSA) was performed overnight at 4°C and incubated afterward with secondary antibodies (in 5% BSA) for 1 h at room temperature with 100 ng/ml DAPI for nuclear counterstaining. Fluorescence images were acquired with the Keyence BZ-9000 BioRevo microscope (Keyence, Neu-Isenburg, Germany) with filter for DAPI, FITC/Alexa Fluor 488, and TexasRed/Alexa Fluor 568/594 and the BZ-II Viewer software (Keyence).
Antibodies for immunoblot analysis and immunostaining
The following antibodies were used for immunoblot analysis: rabbit anti-IKKβ [Y466] (ab32135, 1:2000; Abcam, Cambridge, United Kingdom), goat anti-Lcn2 (AF1857, 1:1000; R&D Systems, Minneapolis, MN, USA), rabbit anti-glial fibrillary acidic protein (GFAP; ab7779, 1:1000; Abcam), rabbit anti-Bax (2772, 1:1000; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-Bad (9292, 1:1000; Cell Signaling Technology), rabbit anti-NF-κB p65 (C20, sc-372, 1:1000; Santa Cruz Biotechnology, Dallas, TX, USA), rabbit anti-phospho NF-κB p65 (Ser536, 3033, 1:1000; Cell Signaling Technology), rabbit anti-GAPDH (FL-335, (sc-25778, 1:2000; Santa Cruz Biotechnology), rabbit anti-β-tubulin (ab6046 1:10000; Abcam) and HRP-conjugated goat anti-rabbit or donkey anti-goat were obtained from Santa Cruz Biotechnology.
For immunofluorescence, the following primary antibodies were used: mouse anti-NeuN (MAB377, 1:300; Millipore-Sigma), rabbit anti-cleaved caspase 3 (ab13847, 1:400; Abcam), and mouse anti-tubulin-β3 (TUJ1) (MMS-435P, 1:500; BioLegend, San Diego, CA, USA). Corresponding Alexa Fluor-conjugated secondary antibodies were obtained from Thermo Fisher Scientific, and DAPI was purchased from Merck (Darmstadt, Germany).
TUNEL staining
For in situ detection of cell death, TUNEL staining was performed with 7 µm–thick coronal paraffin-embedded sections of head-injured mice (3 d after TBI) with the In Situ Cell Death Detection Kit, Fluorescein, according to the manufacturer’s protocol (Roche). Sections were evaluated with the BZ-9000 BioRevo microscope with filters for DAPI and FITC/AlexaFluor 488.
RNA extraction, cDNA synthesis, and quantitative RT-PCR
RNA from the cortex (impact area) was isolated using the PeqGold Trifast Kit (Peqlab, Erlangen, Germany) as described in the manufacturer’s protocol. One microgram of total RNA was used to synthesize cDNA with the Transcriptor High Fidelity cDNA Synthesis kit (Roche) with oligo-dT-primers according to the manufacturer’s instructions. Quantitative PCR (qPCR) assays were run on the Lightcycler 480 Instrument (Roche) with primers and hydrolysis probes designed by the Roche Universal Probe Library (UPL) system. Sequences and UPLs were as follows: C3: forward (F) 5′-ACCTTACCTCGGCAAGTTTCT-3′, reverse (R) 5′-TTGTAGAGCTGCTGGTCAGG-3′, UPL 76; Gfap: F 5′-CCAACTGCAGGCCTTGAC-3′, R 5′-GCTCTAGGGACTCGTTCGTG-3′, UPL 109; Lcn2: F 5′-CCATCTATGAGCTACAAGAGAACAAT-3′, R 5′-TCTGATCCAGTAGCGACAGC-3′, UPL 58; Il1b: F 5′-TGTAATGAAAGACGGCACACC-3′, R 5′-TGTAATGAAAGACGGCACACC-3′, UPL 78; F c-Fos: F 5′-CAGCCTTTCCTACTACCATTCC-3′, R, 5′-ACAGATCTGCGCAAAAGTCC-3′, UPL 67; Bax: F 5′-AGTGTCTCCGGCGAATTG-3′, R 5′-CCACGTCAGCAATCATCCT-3′, UPL 56; Bad: F 5′-CAGCCACCAACAGTCATCAT-3′, R 5′-GCTAAGCTCCTCCTCCATCC-3′, UPL 88; Bak: F 5′-CCACATCTGGAGCAGAGTCA-3′, R 5′-TGTCCAGATGCCATTTTTCA-3′, UPL 22; Bcl2: F 5′-AGTACCTGAACCGGCATCTG-3′, R 5′-GGGGCCATATAGTTCCACAAA-3′, UPL 75; BclXL: F 5′-TGACCACCTAGAGCCTTGGA-3′, R 5′-GCTGCATTGTTCCCGTAGA-3′, UPL 2; Atf3: F 5′-GCTGGAGTCAGTTACCGTCAA-3′, R 5′-CGCCTCCTTTTCCTCTCAT-3′, UPL 80; P21: F 5′-CAGATCCACAGCGATATCCA-3′, R 5′-GGCACACTTTGCTCCTGTG-3′, UPL 21 and Hprt1: F 5′-GGAGCGGTAGCACCTCCT-3′, R 5′-CTGGTTCATCATCGCTAATCA C-3′, UPL 69, which was used as the housekeeping gene.
Thromboelastometry
Rotational thromboelastometry (Rotem Delta; TEM International, Munich, Germany) analysis was performed to determine alterations in coagulation before TBI induction. Whole blood was collected via cardiac puncture from control, IKK2-DNCamk2a, and IKK2-CACamk2a mice and anticoagulated with 3.2% citrate. Immediately after blood drawing, the extrinsic coagulation pathway was determined using the EXTEM test. Clotting time, clot formation time, maximum clot firmness, and α-angle were determined.
GFAP ELISA
Whole blood was taken via decapitation and transferred to sterile plasma EDTA microtubes (Kabe Labortechnik, Nümbrecht-Elsenroth, Germany). EDTA-free protease inhibitor cocktail (10%; Roche) was added, incubated for 10 min on ice, and centrifuged at 4000 rpm for 10 min at 4°C, before storage at −80°C. Plasma samples were analyzed by commercially available ELISA specific for mouse GFAP, according to the manufacturer’s instructions (LSBio, Seattle, WA, USA). Optical density was read at a wavelength of 450 nm on a plate reader (Sunrise Plate Reader; Tecan, Crailsheim, Germany). As a standard curve, a fitted line of the 7-fold standard was used according to the manufacturer’s protocol.
High-resolution MRI
Imaging was performed before and 6 h and 1, 3, and 7 d after trauma. Measurements were performed on a 11.7 T small-animal MRI (BioSpec 117/16; Bruker Biospin, Billerica, MA, USA). All data were acquired with a cryogenically cooled 1H 2-element surface (MRI CryoProbe; Bruker BioSpin) transmit–receive coil. After initiation of anesthesia with 5% isoflurane in air, the mice were placed prone in the cradle. The anesthesia gas was administered via a facial mask, and, during scanning, the isoflurane concentration was adjusted between 1.25 and 1.5% to maintain the respiratory frequency at ∼90 cycles/min. Coronal images were acquired with a T1-weighted multislice Fast Length Adjustment of Short Reads (FLASH; Johns Hopkins University, Baltimore, MD, USA; https://ccb.jhu.edu/software/FLASH/) sequence.
Acquisition parameters were as follows: echo/repetition time, 5/190 ms; flip angle, 17.5°; resolution, 65 × 65 × 500 µm3; field-of-view, 20.8 × 20.8 mm2; and bandwidth, 96 KHz, using 8-signal averages. Total acquisition time for 18 slices was 5 min 20 s.
Statistical analysis
Immunoblot densitometry was analyzed with Image J. Quantifications from microscopy images were performed with the BZ-II Analyzer software with the Hybrid cell-counting tool (Keyence). Statistical analyses were performed with Prism software (GraphPad, San Diego, CA, USA) and are indicated in the specific figure legend. One- or 2-way ANOVA with Bonferroni’s correction was used to compare independent measurements at one or different time points, respectively. For the NSS analysis, the nonparametric Mann-Whitney U test was used. Differences in postinjury mortality and hematoma formation were evaluated in a contingency table with Fisher’s exact test. Statistical significance was set at P < 0.05.
RESULTS
Neuronal IKK/NF-κB inhibition induces a detrimental phenotype after CHI
To study the role of neuronal IKK/NF-κB signaling for the outcome of traumatic brain injury, we used 2 previously generated mouse models called IKK2-DNCamk2a and IKK2-CACamk2a (35, 49, 56). These conditional loss-of-function and gain-of-function models express a dominant negative (IKK2-DN) or a constitutively active (IKK2-CA) allele of human IKK2, respectively. Furthermore, a luciferase reporter gene is coexpressed under the control of the neuron-specific Camk2a promoter in a tetracycline-regulated manner (Fig. 1A). To avoid any impact of the IKK2-DN and -CA transgenes on brain development, animals were bred and housed in the presence of Dox up to the age of 4 wk to block transgene expression. Thereafter transgene expression was induced by Dox withdrawal.
Figure 1.
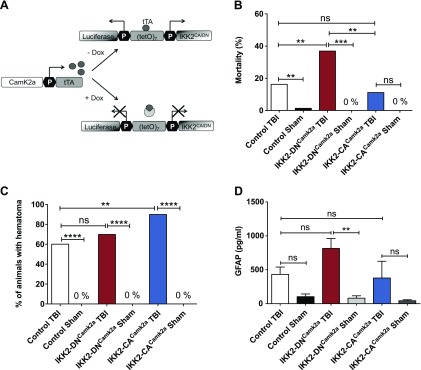
Immediate consequences of CHI on mice with modulated neuronal NF-κB activity. A) Generation of conditional transgenic IKK2-DNCamk2a and IKK2-CACamk2a mouse models using the tetracycline-regulated gene-expression system. Expression of the IKK2-DN (loss-of-function) or IKK2-CA (gain-of-function) transgene and a luciferase reporter gene was controlled by the bidirectional tetracycline-controlled transcriptional activation (tTA)–dependent promoter (tetO7). The Camk2a.tTA module specified transgene expression to neurons with Camk2a-activity. Dox blocked transgene expression. B) Mortality of mice during CHI procedure. Immediate posttraumatic mortality rate is depicted as the percentage of animals that died. IKK2-DNCamk2a mice showed a significantly enhanced posttraumatic mortality compared to control and sham-treated animals, whereas mice with neuronal NF-κB activation (IKK2-CACamk2a) had a mortality similar to that of as control mice. (Control TBI, n = 115; Control Sham-Treated, n = 62; IKK2-DNCamk2a TBI, n = 78; IKK2-DNCamk2a Sham, n = 21; IKK2-CACamk2a TBI, n = 35; IKK2-CACamk2a Sham, n = 20). **P < 0.01, ***P < 0.001 [not significant (ns), by Fisher’s exact test]. C) Quantification of hematoma formation in IKK2-DNCamk2a, IKK2-CACamk2a, and control mice after TBI. CHI induced similar distributed hematoma formation in control and IKK2-DNCamk2a animals. IKK2-CACamk2a mice were slightly more prone to hematoma development than controls. (Control TBI, n = 76; Control Sham, n = 51; IKK2-DNCamk2a TBI, n = 37; IKK2-DNCamk2a Sham, n = 18; IKK2-CACamk2a TBI, n = 31; IKK2-CACamk2a Sham, n = 20). **P < 0.01, ****P < 0.0001 (ns, by Fisher’s exact test). D) Plasma levels of GFAP after CHI. IKK2-DNCamk2a mice showed elevated levels of GFAP in the plasma 6 h after TBI. Means ± sem (n = 6–12). **P < 0.01 (ns, by 1-way ANOVA, followed by Bonferroni’s post hoc test).
TBI was induced in 12-wk-old animals by using a standardized weight-drop device (51, 52). Transgene expression analyzed 6 h after TBI in the cortex of IKK2-DNCamk2a and IKK2-CACamk2a mice by immunoblot analysis revealed robust IKK2 expression on both the ipsilateral and contralateral side (Supplemental Fig. S1A, B respectively). After CHI, the acute postinjury mortality rate was significantly enhanced in IKK2-DNCamk2a mice (37%) compared to controls (16%) suggesting a detrimental effect of impaired IKK2/NF-κB signaling on posttraumatic survival rate (Fig. 1B). Neuronal activation of IKK2/NF-κB in the IKK2-CACamk2a animals did not significantly affect the posttraumatic mortality rate (11% for IKK2-CACamk2a vs. 16% for control mice). Since transgene expression is activated 2 mo before TBI, we asked whether neuronal IKK2/NF-κB modulation affects basic physiologic parameters critical for posttraumatic survival. However, we could not detect obvious differences in body temperature (Supplemental Fig. S2A), blood glucose levels (Supplemental Fig. S2B), and thromboelastometric function (Supplemental Fig. S2C) in IKK2-DNCamk2a, IKK2-CACamk2a, and control mice.
CHI initiates heterogeneous secondary processes including development of cerebral edema and hematoma (6). Therefore, we analyzed the frequency of hematoma formation in the transgenic mouse models. Control and IKK2-DNCamk2a mice show a similar pattern of epidural hematoma formation, whereas IKK2-CACamk2a mice are slightly more prone to induction of hematoma (Fig. 1C). Hematoma and edema formation were confirmed by MRI coronal T1*-weighted imaging (Supplemental Fig. S3).
GFAP is a serum biomarker for TBI that correlates with injury severity (57, 58). The quantification of GFAP in plasma samples of head-injured mice by ELISA revealed slightly enhanced GFAP levels in IKK2-DNCamk2a mice, compared to control and IKK2-CACamk2a animals (Fig. 1D). These data suggest elevated damage in mice with neuronal NF-κB repression.
Neuronal NF-κB inhibition promotes increased neurological deficits after TBI
Functional consequences of CHI were assessed with the 10-point NSS (52–54). This analysis revealed significantly elevated median NSS levels 6 h, 1 d, and 3 d after TBI in IKK2-DNCamk2a mice compared with control mice (Fig. 2A) implying an increased severity and possibly a delayed recovery from experimental CHI. In contrast, no statistically significant difference was noted in the median NSS at any time point between IKK2-CACamk2a and control littermates (Fig. 2B). To further characterize the neurologic deficits of IKK2-DNCamk2a mice after TBI, we performed additional motor and anxiety behavior tests over a time course up to 30 d. In the rotarod test, IKK2-DNCamk2a mice showed deficits 2 and 30 d after CHI in comparison to control littermates (Fig. 2C), indicating a mild permanent motor impairment. In the open-field test, IKK2-DNCamk2a animals showed a hyperactive phenotype 30 d after TBI in comparison to control mice, determined by the length of elevated track covered (Supplemental Fig. S4A). In contrast, IKK2-DNCamk2a mice did not show alterations in anxiety in the elevated plus-maze testing after TBI (Supplemental Fig. S4B).
Figure 2.
IKK2-DNCamk2a mice show enhanced neurologic deficits post TBI. A, B) Assessment of injury severity, neurologic impairment and recovery of control, IKK2-DNCamk2a, IKK2-CACamk2a, and sham-treated mice after CHI, with a standardized 10-point NSS. A) Head-injured IKK2-DNCamk2a mice showed a delayed recovery from TBI, as reflected by a significantly increased NSS, compared to head-injured littermates (Control TBI, n = 18; Control Sham, n = 14; IKK2-DNCamk2a TBI, n = 12; IKK2-DNCamk2a Sham, n = 9). B) No significant difference in NSS of head-injured IKK2-CACamk2a and control mice was seen at any time point assessed (Control TBI, n = 17; Control Sham, n = 10; IKK2-CACamk2a TBI, n = 19; IKK2-CACamk2a Sham, n = 10). All data are presented as box plots with median ± interquartile range; whiskers, minimum–maximum range. *P < 0.05, ***P < 0.001, ****P < 0.0001 [not significant (ns), by nonparametric Mann-Whitney U test]. C) Rotarod performance after TBI. Impaired motor coordination of IKK2-DNCamk2a mice after trauma, demonstrated in a 30 d rotarod experiment. Latency until falling off of an accelerating rotarod was reduced 2 and 30 d after TBI compared to controls. Means ± sem (n = 7–13). *P < 0.05 (2-way-ANOVA followed by Bonferroni’s post hoc test).
In conclusion, neuronal NF-κB repression results in enhanced neurologic and motoric deficits.
Neuronal NF-κB inhibition increases apoptosis of neurons after CHI
Since neurologic deficits correlate with neurodegeneration we investigated the extent of neuronal cell damage after TBI in IKK2-DNCamk2a and control mice. For this purpose, we performed immunofluorescence analyses using an anti-NeuN antibody as a neuron-specific cell marker. Neuronal cell loss was evident in control animals, but significantly enhanced in IKK2-DNCamk2a mice (Fig. 3A). To test whether neuronal cell loss depends on neuronal apoptosis, tissue sections were analyzed by cleaved caspase 3 costained with TUJ1, a marker for neurons. Indeed, prominent costaining was detected, and quantification revealed increased cleaved caspase 3+ cells 3 d after TBI in IKK2-DNCamk2a mice, in comparison to control animals (Fig. 3B), suggesting that neuronal IKK/NF-κB inhibition sensitizes these cells for apoptosis.
Figure 3.
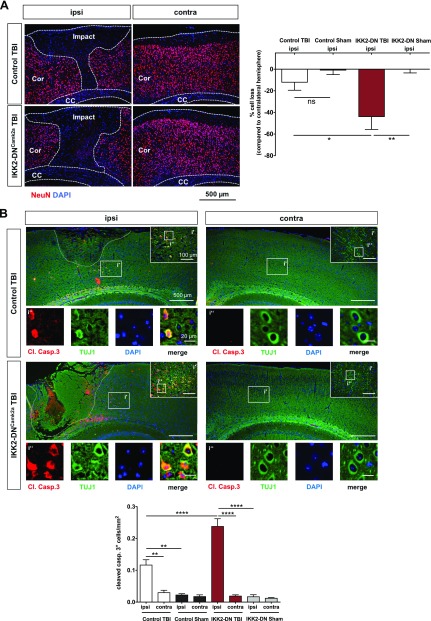
IKK2-DNCamk2a mice exhibit prominent neuronal apoptosis after CHI. A) Neuronal cell loss 3 d after CHI. Significantly enhanced neuronal cell loss in the ipsilateral cortex of IKK2-DNCamk2a mice compared with control TBI and sham-treated mice. Immunofluorescent staining and quantification of NeuN+ cells. The percentage of neuronal cell loss was calculated as the ratio of neuronal cells from the injured (ipsilateral) hemisphere to the number of neurons in the uninjured (contralateral) hemisphere. Impact, TBI impact area; Cor, cortex; CC, corpus callosum. Means ± sem (n = 4–5). *P < 0.05, **P < 0.01 (by 1-way ANOVA with Bonferroni’s correction). Scale bar, 500 µm. B) Apoptotic neurons 3 d after CHI. Increased posttraumatic neuronal cell death 3 d after TBI in the injured (ipsi) hemisphere of IKK2-DNCamk2a mice vs. control TBI and sham-treated animals and vs. the uninjured (contra) hemisphere. Coimmunostaining of the apoptotic marker cleaved caspase 3 (Cl. Casp.3) and the neuronal marker TUJ1. Quantification of cleaved caspase 3+ cells revealed significantly enhanced apoptosis in mice with neuronal NF-κB inhibition vs. control animals. Means ± sem (Control TBI, n = 4; Control Sham, n = 2; IKK2-DNCamk2a TBI, n = 4, IKK2-DNCamk2a Sham, n = 2). **P < 0.01, ****P < 0.0001 (according to 1-way ANOVA with Bonferroni’s correction). Scale bar, 500 µm; i′, inset′: 100 µm; i″, inset″: 20 µm.
Neuronal NF-κB inhibition leads to enhanced up-regulation of neuroinflammatory mediators after trauma
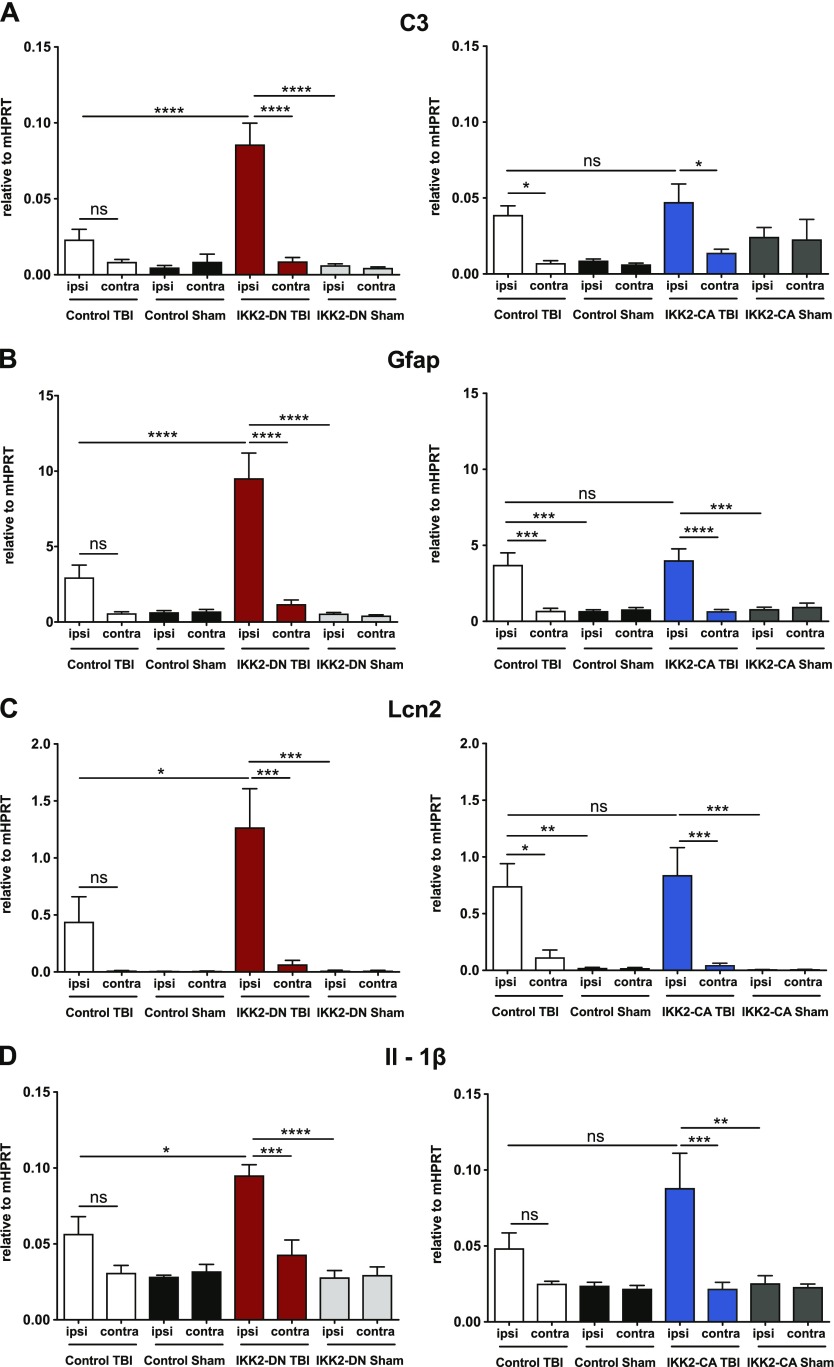
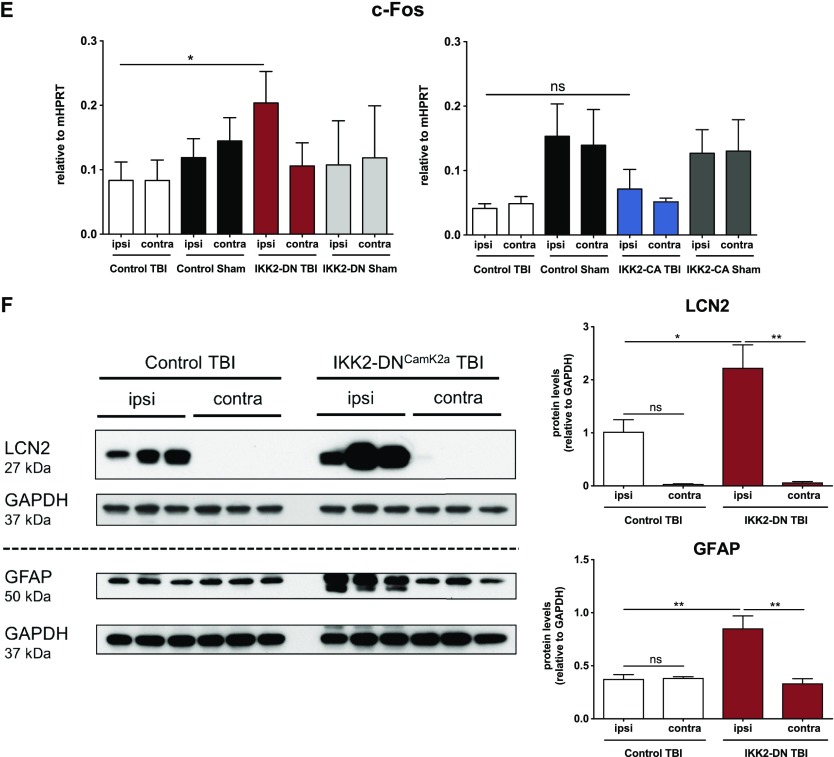
To address the role of inflammatory and apoptotic pathways in the regulation of neuronal survival after TBI in the IKK2-DNCamk2a model, we analyzed marker gene expression in the cortical impact area at various time points after CHI. Whereas 6 h after TBI, we did not see prominent changes in gene expression (Supplemental Fig. S5A–E), 3 d after TBI, significant up-regulation of several proinflammatory genes was seen in the ipsilateral cortex of IKK2-DNCamk2a animals compared to sham-treated animals and control littermates (Fig. 4A–E, left panels). These include the central complement factor C3, the astrogliosis marker gene Gfap, the acute phase gene Lcn2, and the inflammatory cytokine Il1b (Fig. 4A–D, left panels). In control animals, up-regulation of these genes does not reach significance after TBI, compared with sham-treated animals. Protein analysis confirmed enhanced levels of LCN2 and GFAP in IKK2-DNCamk2a animals, compared to control littermates 3 d after trauma (Fig. 4F). We also found the neuronal activity marker c-Fos elevated in IKK2-DNCamk2a animals compared to controls (Fig. 4E, left panel), suggesting enhanced neurogenesis related to elevated neurologic impairments (59). No significant differences in the expression pattern of the different genes were observed between IKK2-CACamk2a and control littermates (Fig. 4A–E, right panels). Analysis of these proinflammatory genes 7 d after CHI did not reveal continuing significant differences between IKK2-DNCamk2a and control mice (Supplemental Fig. S6A–E).
Figure 4.
IKK2-DNCamk2a mice exhibit prominent up-regulation of neuroinflammatory factors after trauma. A–E) Expression of proinflammatory genes 3 d after TBI. Most proinflammatory factors were significantly up-regulated in the cortical impact area of IKK2-DNCamk2a mice, but not in IKK2-CACamk2a mice vs. control littermates. These factors include the complement factor C3 (A), the astroglial marker Gfap (B), the acute phase protein Lcn2 (C), the inflammatory cytokine Il1b (D), and the neuronal activity marker c-Fos (E). Expression levels measured by qPCR are presented relative to Hprt. Means ± sem (n = 4–6). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 [not significant (ns), 3 d after TBI between indicated groups, by 1-way-ANOVA followed by Bonferroni’s post hoc test]. F) Expression of astroglial and acute phase proteins after trauma. Representative immunoblot analysis and quantification of LCN2 and GFAP protein levels in brain lysates of 12-wk-old control and IKK2-DNCamk2a mice 3 d after CHI. GAPDH was the loading control. Means ± sem (n = 3). *P < 0.05, **P < 0.01 (by 1-way ANOVA followed by Bonferroni’s post hoc test).
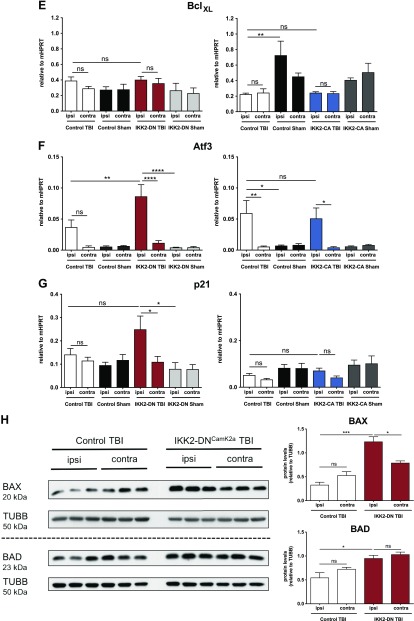
To investigate the underlying mechanism responsible for enhanced apoptosis in the IKK2-DNCamk2a model, we performed a longitudinal gene expression profiling for pro- and anti-apoptotic genes. Three days after CHI, we detected an enhanced expression of the proapoptotic genes Bax and Bad, whereas the expression of Bak and the antiapoptotic genes Bcl2 and BclXL was not affected (Fig. 5A–E, left panels). These results were confirmed on a protein level, as IKK2-DNCamk2a animals showed increased BAX and BAD expression compared to control littermates (Fig. 5H). As a consequence of proapoptotic protein expression, we found an up-regulation of TUNEL+ cells in the ipsilateral cortex of IKK2-DNCamk2a animals compared with control littermates (Supplemental Fig. S7). In addition, stress response genes and p53 target genes, including Atf3 and p21 were up-regulated in these animals (Fig. 5F, G, left panels). Again, expression of these genes was unaltered in IKK2-CACamk2a mice (Fig. 5A–G, right panels). Similar to the proinflammatory genes, we did not identify significant differences in the expression of these pro- and antiapoptotic genes 6 h (Supplemental Fig. S5F–L) and 7 d (Supplemental Fig. S6F–L) after CHI in the IKK2-DNCamk2a mouse model. These results indicate that basal NF-κB activity in neurons is necessary to protect these cells from transiently increased cellular stress resulting from traumatic injury. However, this protective effect is not further enhanced by increased NF-κB activity.
Figure 5.
Neuronal NF-κB inhibition leads to up-regulation of proapoptotic factors after CHI. A–G) Expression of genes regulating apoptosis 3 d after TBI. Proapoptotic, but not antiapoptotic, factors were significantly elevated in the cortical impact area of IKK2-DNCamk2a mice, but not in IKK2-CACamk2a mice vs. control littermates. qPCR analysis of the proapoptotic genes Bax (A), Bad (B), and Bak (C); the antiapoptotic genes Bcl2 (D) and BclXL (E); and the stress response genes in the p53 pathway Atf3 (F) and p21 (G). Expression levels measured by qPCR are presented relative to Hprt. Means ± sem (n = 4–6). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 [not significant (ns), at 3 d TBI between the indicated groups, by 1-way-ANOVA followed by Bonferroni’s post hoc test]. H) Expression of proapoptotic proteins after trauma. Representative immunoblot analysis and quantification of BAX and BAD protein levels in brain lysates of 12-wk-old control and IKK2-DNCamk2a mice. TUBB is used as loading control. Means ± sem (n = 3). *P < 0.05, ***P < 0.001 (ns, 3 d after CHI, between the indicated groups, by 1-way-ANOVA followed by Bonferroni’s post hoc test).
Acute inhibition of IKK/NF-κB signaling in neurons also worsens TBI outcome
IKK2-DN transgene expression and therefore inhibition of IKK/NF-κB signaling was induced at the age of 4 wk for a total period of ∼8 wk before TBI. Therefore, we cannot exclude consequences of chronic NF-κB inhibition that predispose IKK2-DNCamk2a animals to the observed detrimental TBI outcome. To investigate, we activated transgene expression in a more acute paradigm shortly before TBI induction. As the transgene is only slowly activated after Dox withdrawal, we removed Dox 12 d before TBI, which resulted in transgene activation after 8 d (i.e. 4 d before TBI induction), as indicated by the detection of luciferase reporter gene activity by in vivo bioluminescence imaging (Supplemental Fig. S8A). Transgene expression was confirmed by protein immunoblot analysis 6 h after TBI (Supplemental Fig. S8B). Similar to the chronic IKK2-DNCamk2a model, acute NF-κB repression resulted in elevated postinjury mortality (Fig. 6A), and no differences in the hematoma formation were found (data not shown). Again, animals with acute IKK2-DNCamk2a expression showed significantly elevated NSS levels 1 and 3 d after injury compared to controls (Fig. 6B), a tendency for enhanced neuronal cell loss in the ipsilateral cortex after TBI (Supplemental Fig. S8C) and significantly increased neuronal apoptosis measured by cleaved caspase 3 expression costained with TUJ1 (Fig. 6C).
Next, we asked whether acute IKK2-DNCamk2a animals would also show an up-regulation of neuroinflammatory and proapoptotic factors. Gene expression was analyzed 3 d after TBI and revealed elevated levels of C3, Gfap, Lcn2, Il1b, and c-Fos in the injured hemisphere of mice with acute neuronal IKK2-DN expression (Fig. 7A–E). We also detected higher levels of the proapoptotic genes Bax and Bak, whereas the expression of Bad and of the antiapoptotic genes Bcl2 and BclXL were not affected indicating a similar expression pattern in both paradigms (Fig. 7F–J). Furthermore, Atf3 and p21 were also induced in the injured cortex of IKK2-DNCamk2a animals compared to littermates (Fig. 7K, L).
Taken together, our findings showed that appropriate neuronal IKK/NF-κB signaling is critical for neuroprotection after traumatic brain injury.
DISCUSSION
IKK/NF-κB signaling plays an important role in brain development, neuronal survival, and differentiation and has been proposed to be involved in the pathogenesis of various neurologic diseases (22, 26, 32, 60). However, the cell-type–specific contribution of IKK/NF-κB signaling to specific pathophysiology is less clear, and studies have suggested ambivalent roles in the pathogenesis of brain injuries (26, 61). Whereas neuronal NF-κB activation is essential for maintaining homeostasis and integrity of neurons (26, 62–64), NF-κB also controls inflammation and apoptotic cell death after nerve injury (65).
To characterize the specific function of neuronal NF-κB in the pathophysiology of TBI, we used 2 conditional mouse models—loss-of-function (IKK2-DNCamk2a) and gain-of-function (IKK2-CACamk2a)—and subjected the animals to experimental CHI, the most common form of head injuries observed in trauma patients (6). We show that neuronal NF-κB inhibition is detrimental for the outcome of TBI. IKK2-DNCamk2a mice that have a higher immediate posttraumatic mortality and survivors revealed significantly enhanced neurologic deficits. Even though we focused in this study on the analysis of the cerebral cortex, secondary injury of other brain regions, like hippocampus, corpus callosum and cerebellum, can also critically influence the outcome of TBI (52, 66). Despite the well-established proinflammatory role of the NF-κB system, inhibition of neuronal NF-κB signaling in IKK2-DNCamk2a mice unexpectedly resulted in increased expression of inflammatory mediators at the site of injury. This effect could be a consequence of elevated tissue damage, which promotes the activation of microglia and astrocytes. Both cell types are critically involved in the induction of neuroinflammatory responses via the NF-κB pathway (67) and are still able to activate NF-κB signaling in our model. A similar phenotype of enhanced secondary inflammation due to prominent tissue damage upon NF-κB inactivation was observed in models of intestinal NF-κB deficiency (68). Enhanced neuronal NF-κB activation did not alleviate the consequences of TBI, as cell death and neurologic deficits in IKK2-CACamk2a mice did not differ from control littermates. This finding indicates that physiologic NF-κB activation in neurons is sufficient to mediate full neuroprotection. However, it remains open whether a specific timing of activation in neurons (e.g., induction only at later phases of secondary TBI pathogenesis) is able to induce beneficial effects. In line with the hypothesis of a secondary enhancement of inflammation by increased tissue damage, IKK2-CACamk2a animals did not show alterations in the expression of inflammation-associated gene expression compared to control animals. These data indeed suggest that the observed proinflammatory gene expression profile is induced by glial cells. The up-regulation of the proapoptotic genes Bax and Bad and other genes linked to p53-mediated stress responses in IKK2-DNCamk2a, but not IKK2-CACamk2a mice suggests that endogenous physiologic neuronal NF-κB signaling protects neurons in conditions of injury-related cellular stress, possibly by suppressing p53-mediated apoptosis via the intrinsic mitochondrial pathway (69).
In a model of controlled cortical impact, it has been shown that brain-specific NF-κB activation in Nestin-Cre IκBαfl/− mice leads to impaired inflammatory responses after TBI and worsened brain damage (42). In this mouse model, NF-κB is activated because of deletion of the Ikb gene, not only in neurons, but also in astrocytes and oligodendrocytes (70). Thus, the observation that the trauma outcome was not worsened in our neuron-specific, gain-of-function mouse model suggests that NF-κB activation in astrocytes and oligodendrocytes may determine the detrimental processes in the Nestin-Cre IκBαfl/− model. In the setting of cerebral ischemia, a detrimental role of neuronal NF-κB was proposed, as constitutive activation of IKK2 enlarged the infarct size, whereas IKK2 inactivation resulted in neuroprotection (49). These findings indicate a complex role of neuronal NF-κB upon distinct experimental injuries, resulting in different outcomes dependent on the specific injury models. This result may be explained by different mechanisms of the primary injury (e.g., blunt vs. sharp mechanical injury in TBI or injury caused primarily by hypoxia and impaired supply of metabolites in case of ischemic injury). These distinct modes of injury might elicit different protective or maladaptive responses, resulting in different vulnerabilities. We have previously shown that NF-κB regulates synapse formation and spine maturation in adult hippocampal neurons via Igf2 signaling (35). Thus, defective synaptic contacts may contribute to impaired posttraumatic neuronal survival. However, we also found that constitutive IKK2 activation in neurons can generate a selective neuroinflammatory response in the dentate gyrus leading to local neurodegeneration with aging (56). Therefore, neuronal IKK/NF-κB does not exclusively induce survival-promoting genes, in line with our observation that constitutive neuronal NF-κB activation did not further improve the TBI outcome.
Other candidate mechanisms that may underlie the protective role of NF-κB in neurons include the antiapoptotic effects mediated by inducing endogenous caspase inhibitors or by triggering the expression of antioxidant genes, such as manganese superoxide dismutase or calcium-binding protein calbindin D28k (40, 60), as NF-κB transcription factors regulate a wide range of survival-promoting target genes (65).
Prolonged inhibition of neuronal NF-κB may induce indirect structural and functional alterations in the brain, which may sensitize animals for elevated posttraumatic damage. To assess such a detrimental predisposition of IKK2-DNCamk2a animals because of chronic NF-κB inhibition, we also analyzed acute IKK2-DNCamk2a mice in which the NF-κB/IKK2 transgene was activated shortly before CHI induction. Upon TBI, these mice showed a similar detrimental phenotype to mice with chronic IKK2-DNCamk2a expression, excluding that long-term structural changes are responsible for the observed sensitization of IKK2-DNCamk2a mice for TBI.
Current clinical treatment strategies imply the administration of neuroprotective and anti-inflammatory agents; however, until today, most clinical trials have failed (11). Promising therapeutic agents for treatment of head injuries are statins, inhibitors of cholesterol biosynthesis with additional pleiotropic properties (71), as well as progesterone (72). These agents limit the production of inflammatory mediators by attenuating NF-κB, p65 and TNF expression in TBI models (11). However, they most likely target the inflammatory phenotype of M1-like microglia and not neurons (4). Indeed, NF-κB inhibition in microglia and astrocytes has been shown to induce a beneficial outcome from CNS injuries (29, 73–77).
In summary, neuronal IKK2/NF-κB inhibition aggravated neurodetrimental effects on CHI, whereas additional chronic IKK2/NF-κB activation in neurons cannot convert these harmful effects into an improved recovery process. Thus, our findings indicate that basal neuronal NF-κB activity is neuroprotective for the outcome of TBI.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Petra Weihrich, Melanie Gerstenlauer, Annika Gnann, Ute Leschik (all from the Institute of Physiological Chemistry, Ulm University), and Sonja Braumüller (Institute of Clinical and Experimental Trauma-Immunology, University Hospital Ulm) for excellent technical assistance. This work was supported by the German research foundation (CRC1149/Project A03 to T.W. and CRC1149/Project Z03). Melanie Mettang and Stephanie N. Reichel are members of the International Graduate School in Molecular Medicine Ulm (IGradU). The authors declare no conflicts of interest.
Glossary
- BSA
bovine serum albumin
- CA
constitutively active
- CHI
closed head injury
- DN
dominant negative
- Dox
doxycycline
- GFAP
glial fibrillary acidic protein
- IKK
inhibitor of NF-κB kinase
- NSS
neurological severity score
- qPCR
quantitative PCR
- TBI
traumatic brain injury
- TUJ1
anti-tubulin-β3
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. Mettang was responsible for the experimental design, performed most of the experiments, analyzed the data, and wrote and edited the manuscript; S. N. Reichel performed the TBIs and critically reviewed the manuscript; M. Lattke was responsible for experimental design and critically reviewed the manuscript; A. Palmer performed thromboelastometry and GFAP ELISA, helped in establishing the TBI model, and critically reviewed the manuscript; A. Abaei and V. Rasche performed high-resolution MRI and critically reviewed the manuscript; M. Huber-Lang helped in establishing the TBI model, made helpful comments throughout the project, and critically reviewed the manuscript; B. Baumann generated and established the transgenic mouse models, was responsible for experimental design and data interpretation, and wrote and edited the manuscript; and T. Wirth performed experimental design and data interpretation, wrote the manuscript, and edited the document.
REFERENCES
- 1.Langlois J. A., Rutland-Brown W., Wald M. M. (2006) The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 10.1097/00001199-200609000-00001 [DOI] [PubMed] [Google Scholar]
- 2.Thompson H. J., McCormick W. C., Kagan S. H. (2006) Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J. Am. Geriatr. Soc. 54, 1590–1595 10.1111/j.1532-5415.2006.00894.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roozenbeek B., Maas A. I., Menon D. K. (2013) Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 9, 231–236 10.1038/nrneurol.2013.22 [DOI] [PubMed] [Google Scholar]
- 4.Simon D. W., McGeachy M. J., Bayır H., Clark R. S., Loane D. J., Kochanek P. M. (2017) The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 13, 171–191 10.1038/nrneurol.2017.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masson F., Thicoipe M., Aye P., Mokni T., Senjean P., Schmitt V., Dessalles P. H., Cazaugade M., Labadens P.; Aquitaine Group for Severe Brain Injuries Study (2001) Epidemiology of severe brain injuries: a prospective population-based study. J. Trauma 51, 481–489 [DOI] [PubMed] [Google Scholar]
- 6.Namjoshi D. R., Good C., Cheng W. H., Panenka W., Richards D., Cripton P. A., Wellington C. L. (2013) Towards clinical management of traumatic brain injury: a review of models and mechanisms from a biomechanical perspective. Dis. Model. Mech. 6, 1325–1338 10.1242/dmm.011320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menon D. K., Ercole A. (2017) Critical care management of traumatic brain injury. Handb. Clin. Neurol. 140, 239–274 10.1016/B978-0-444-63600-3.00014-3 [DOI] [PubMed] [Google Scholar]
- 8.Bramlett H. M., Dietrich W. D. (2007) Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog. Brain Res. 161, 125–141 10.1016/S0079-6123(06)61009-1 [DOI] [PubMed] [Google Scholar]
- 9.Lozano D., Gonzales-Portillo G. S., Acosta S., de la Pena I., Tajiri N., Kaneko Y., Borlongan C. V. (2015) Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat. 11, 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabadi S. V., Faden A. I. (2014) Neuroprotective strategies for traumatic brain injury: improving clinical translation. Int. J. Mol. Sci. 15, 1216–1236 10.3390/ijms15011216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loane D. J., Faden A. I. (2010) Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 31, 596–604 10.1016/j.tips.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt O. I., Heyde C. E., Ertel W., Stahel P. F. (2005) Closed head injury: an inflammatory disease? Brain Res. Brain Res. Rev. 48, 388–399 10.1016/j.brainresrev.2004.12.028 [DOI] [PubMed] [Google Scholar]
- 13.Gyoneva S., Ransohoff R. M. (2015) Inflammatory reaction after traumatic brain injury: therapeutic potential of targeting cell-cell communication by chemokines. Trends Pharmacol. Sci. 36, 471–480 10.1016/j.tips.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodcock T., Morganti-Kossmann M. C. (2013) The role of markers of inflammation in traumatic brain injury. Front. Neurol. 4, 18. 10.3389/fneur.2013.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morganti-Kossmann M. C., Rancan M., Stahel P. F., Kossmann T. (2002) Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr. Opin. Crit. Care 8, 101–105 10.1097/00075198-200204000-00002 [DOI] [PubMed] [Google Scholar]
- 16.Quillinan N., Herson P. S., Traystman R. J. (2016) Neuropathophysiology of brain injury. Anesthesiol. Clin. 34, 453–464 10.1016/j.anclin.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayden M. S., Ghosh S. (2012) NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 26, 203–234 10.1101/gad.183434.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaltschmidt B., Widera D., Kaltschmidt C. (2005) Signaling via NF-kappaB in the nervous system. Biochim. Biophys. Acta 1745, 287–299 10.1016/j.bbamcr.2005.05.009 [DOI] [PubMed] [Google Scholar]
- 19.Ledoux A. C., Perkins N. D. (2014) NF-κB and the cell cycle. Biochem. Soc. Trans. 42, 76–81 10.1042/BST20130156 [DOI] [PubMed] [Google Scholar]
- 20.Mattson M. P. (2005) NF-kappaB in the survival and plasticity of neurons. Neurochem. Res. 30, 883–893 10.1007/s11064-005-6961-x [DOI] [PubMed] [Google Scholar]
- 21.Oeckinghaus A., Hayden M. S., Ghosh S. (2011) Crosstalk in NF-κB signaling pathways. Nat. Immunol. 12, 695–708 10.1038/ni.2065 [DOI] [PubMed] [Google Scholar]
- 22.Mémet S. (2006) NF-kappaB functions in the nervous system: from development to disease. Biochem. Pharmacol. 72, 1180–1195 10.1016/j.bcp.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 23.Perkins N. D. (2007) Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 8, 49–62 10.1038/nrm2083 [DOI] [PubMed] [Google Scholar]
- 24.Engelmann C., Weih F., Haenold R. (2014) Role of nuclear factor kappa B in central nervous system regeneration. Neural Regen. Res. 9, 707–711 10.4103/1673-5374.131572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmy A., De Simoni M. G., Guilfoyle M. R., Carpenter K. L., Hutchinson P. J. (2011) Cytokines and innate inflammation in the pathogenesis of human traumatic brain injury. Prog. Neurobiol. 95, 352–372 10.1016/j.pneurobio.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 26.Kaltschmidt B., Kaltschmidt C. (2015) NF-KappaB in long-term memory and structural plasticity in the adult mammalian brain. Front. Mol. Neurosci. 8, 69. 10.3389/fnmol.2015.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pennypacker K. R., Kassed C. A., Eidizadeh S., O’Callaghan J. P. (2000) Brain injury: prolonged induction of transcription factors. Acta Neurobiol. Exp. (Warsz.) 60, 515–530 [DOI] [PubMed] [Google Scholar]
- 28.Bales K. R., Du Y., Dodel R. C., Yan G. M., Hamilton-Byrd E., Paul S. M. (1998) The NF-kappaB/Rel family of proteins mediates Abeta-induced neurotoxicity and glial activation. Brain Res. Mol. Brain Res. 57, 63–72 10.1016/S0169-328X(98)00066-7 [DOI] [PubMed] [Google Scholar]
- 29.Brambilla R., Bracchi-Ricard V., Hu W. H., Frydel B., Bramwell A., Karmally S., Green E. J., Bethea J. R. (2005) Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 202, 145–156 10.1084/jem.20041918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lattke M., Magnutzki A., Walther P., Wirth T., Baumann B. (2012) Nuclear factor κB activation impairs ependymal ciliogenesis and links neuroinflammation to hydrocephalus formation. J. Neurosci. 32, 11511–11523 10.1523/JNEUROSCI.0182-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lattke M., Reichel S. N., Magnutzki A., Abaei A., Rasche V., Walther P., Calado D. P., Ferger B., Wirth T., Baumann B. (2017) Transient IKK2 activation in astrocytes initiates selective non-cell-autonomous neurodegeneration. Mol. Neurodegener. 12, 16. 10.1186/s13024-017-0157-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaltschmidt B., Kaltschmidt C. (2009) NF-kappaB in the nervous system. Cold Spring Harb. Perspect. Biol. 1, a001271. 10.1101/cshperspect.a001271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattson M. P., Culmsee C., Yu Z., Camandola S. (2000) Roles of nuclear factor kappaB in neuronal survival and plasticity. J. Neurochem. 74, 443–456 10.1046/j.1471-4159.2000.740443.x [DOI] [PubMed] [Google Scholar]
- 34.O’Neill L. A., Kaltschmidt C. (1997) NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 20, 252–258 10.1016/S0166-2236(96)01035-1 [DOI] [PubMed] [Google Scholar]
- 35.Schmeisser M. J., Baumann B., Johannsen S., Vindedal G. F., Jensen V., Hvalby O. C., Sprengel R., Seither J., Maqbool A., Magnutzki A., Lattke M., Oswald F., Boeckers T. M., Wirth T. (2012) IκB kinase/nuclear factor κB-dependent insulin-like growth factor 2 (Igf2) expression regulates synapse formation and spine maturation via Igf2 receptor signaling. J. Neurosci. 32, 5688–5703 10.1523/JNEUROSCI.0111-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayden M. S., Ghosh S. (2008) Shared principles in NF-kappaB signaling. Cell 132, 344–362 10.1016/j.cell.2008.01.020 [DOI] [PubMed] [Google Scholar]
- 37.Nonaka M., Chen X. H., Pierce J. E., Leoni M. J., McIntosh T. K., Wolf J. A., Smith D. H. (1999) Prolonged activation of NF-kappaB following traumatic brain injury in rats. J. Neurotrauma 16, 1023–1034 10.1089/neu.1999.16.1023 [DOI] [PubMed] [Google Scholar]
- 38.Yang K., Mu X. S., Hayes R. L. (1995) Increased cortical nuclear factor-kappa B (NF-kappa B) DNA binding activity after traumatic brain injury in rats. Neurosci. Lett. 197, 101–104 10.1016/0304-3940(95)11919-N [DOI] [PubMed] [Google Scholar]
- 39.Hang C. H., Chen G., Shi J. X., Zhang X., Li J. S. (2006) Cortical expression of nuclear factor kappaB after human brain contusion. Brain Res. 1109, 14–21 10.1016/j.brainres.2006.06.045 [DOI] [PubMed] [Google Scholar]
- 40.Sanz O., Acarin L., González B., Castellano B. (2002) NF-kappaB and IkappaBalpha expression following traumatic brain injury to the immature rat brain. J. Neurosci. Res. 67, 772–780 10.1002/jnr.10140 [DOI] [PubMed] [Google Scholar]
- 41.Pennypacker K. R., Kassed C. A., Eidizadeh S., Saporta S., Sanberg P. R., Willing A. E. (2001) NF-kappaB p50 is increased in neurons surviving hippocampal injury. Exp. Neurol. 172, 307–319 10.1006/exnr.2001.7817 [DOI] [PubMed] [Google Scholar]
- 42.Lian H., Shim D. J., Gaddam S. S., Rodriguez-Rivera J., Bitner B. R., Pautler R. G., Robertson C. S., Zheng H. (2012) IκBα deficiency in brain leads to elevated basal neuroinflammation and attenuated response following traumatic brain injury: implications for functional recovery. Mol. Neurodegener. 7, 47. 10.1186/1750-1326-7-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattson M. P., Camandola S. (2001) NF-kappaB in neuronal plasticity and neurodegenerative disorders. J. Clin. Invest. 107, 247–254 10.1172/JCI11916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mincheva-Tasheva S., Soler R. M. (2013) NF-κB signaling pathways: role in nervous system physiology and pathology. Neuroscientist 19, 175–194 10.1177/1073858412444007 [DOI] [PubMed] [Google Scholar]
- 45.Turner M. D., Nedjai B., Hurst T., Pennington D. J. (2014) Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 1843, 2563–2582 10.1016/j.bbamcr.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 46.Acarin L., González B., Castellano B. (1998) Stat3 and NFkappaB glial expression after excitotoxic damage to the postnatal brain. Neuroreport 9, 2869–2873 10.1097/00001756-199808240-00035 [DOI] [PubMed] [Google Scholar]
- 47.Acarin L., González B., Castellano B. (2000) STAT3 and NFkappaB activation precedes glial reactivity in the excitotoxically injured young cortex but not in the corresponding distal thalamic nuclei. J. Neuropathol. Exp. Neurol. 59, 151–163 10.1093/jnen/59.2.151 [DOI] [PubMed] [Google Scholar]
- 48.Gabriel C., Justicia C., Camins A., Planas A. M. (1999) Activation of nuclear factor-kappaB in the rat brain after transient focal ischemia. Brain Res. Mol. Brain Res. 65, 61–69 10.1016/S0169-328X(98)00330-1 [DOI] [PubMed] [Google Scholar]
- 49.Herrmann O., Baumann B., de Lorenzi R., Muhammad S., Zhang W., Kleesiek J., Malfertheiner M., Köhrmann M., Potrovita I., Maegele I., Beyer C., Burke J. R., Hasan M. T., Bujard H., Wirth T., Pasparakis M., Schwaninger M. (2005) IKK mediates ischemia-induced neuronal death. Nat. Med. 11, 1322–1329 10.1038/nm1323 [DOI] [PubMed] [Google Scholar]
- 50.Schmidt-Strassburger U., Schips T. G., Maier H. J., Kloiber K., Mannella F., Braunstein K. E., Holzmann K., Ushmorov A., Liebau S., Boeckers T. M., Wirth T. (2012) Expression of constitutively active FoxO3 in murine forebrain leads to a loss of neural progenitors. FASEB J. 26, 4990–5001 10.1096/fj.12-208587 [DOI] [PubMed] [Google Scholar]
- 51.Chen Y., Constantini S., Trembovler V., Weinstock M., Shohami E. (1996) An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma 13, 557–568 [DOI] [PubMed] [Google Scholar]
- 52.Flierl M. A., Stahel P. F., Beauchamp K. M., Morgan S. J., Smith W. R., Shohami E. (2009) Mouse closed head injury model induced by a weight-drop device. Nat. Protoc. 4, 1328–1337 10.1038/nprot.2009.148 [DOI] [PubMed] [Google Scholar]
- 53.Beni-Adani L., Gozes I., Cohen Y., Assaf Y., Steingart R. A., Brenneman D. E., Eizenberg O., Trembolver V., Shohami E. (2001) A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J. Pharmacol. Exp. Ther. 296, 57–63 [PubMed] [Google Scholar]
- 54.Stahel P. F., Shohami E., Younis F. M., Kariya K., Otto V. I., Lenzlinger P. M., Grosjean M. B., Eugster H. P., Trentz O., Kossmann T., Morganti-Kossmann M. C. (2000) Experimental closed head injury: analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb. Blood Flow Metab. 20, 369–380 10.1097/00004647-200002000-00019 [DOI] [PubMed] [Google Scholar]
- 55.Braunstein K. E., Eschbach J., Ròna-Vörös K., Soylu R., Mikrouli E., Larmet Y., René F., Gonzalez De Aguilar J. L., Loeffler J. P., Müller H. P., Bucher S., Kaulisch T., Niessen H. G., Tillmanns J., Fischer K., Schwalenstöcker B., Kassubek J., Pichler B., Stiller D., Petersen A., Ludolph A. C., Dupuis L. (2010) A point mutation in the dynein heavy chain gene leads to striatal atrophy and compromises neurite outgrowth of striatal neurons. Hum. Mol. Genet. 19, 4385–4398 10.1093/hmg/ddq361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maqbool A., Lattke M., Wirth T., Baumann B. (2013) Sustained, neuron-specific IKK/NF-κB activation generates a selective neuroinflammatory response promoting local neurodegeneration with aging. Mol. Neurodegener. 8, 40. 10.1186/1750-1326-8-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nylén K., Ost M., Csajbok L. Z., Nilsson I., Blennow K., Nellgård B., Rosengren L. (2006) Increased serum-GFAP in patients with severe traumatic brain injury is related to outcome. J. Neurol. Sci. 240, 85–91 10.1016/j.jns.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 58.Vos P. E., Lamers K. J., Hendriks J. C., van Haaren M., Beems T., Zimmerman C., van Geel W., de Reus H., Biert J., Verbeek M. M. (2004) Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology 62, 1303–1310 10.1212/01.WNL.0000120550.00643.DC [DOI] [PubMed] [Google Scholar]
- 59.Villasana L. E., Westbrook G. L., Schnell E. (2014) Neurologic impairment following closed head injury predicts post-traumatic neurogenesis. Exp. Neurol. 261, 156–162 10.1016/j.expneurol.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin Z. H., Tao L. Y., Chen X. (2007) Dual roles of NF-kappaB in cell survival and implications of NF-kappaB inhibitors in neuroprotective therapy. Acta Pharmacol. Sin. 28, 1859–1872 10.1111/j.1745-7254.2007.00741.x [DOI] [PubMed] [Google Scholar]
- 61.Pizzi M., Spano P. (2006) Distinct roles of diverse nuclear factor-kappaB complexes in neuropathological mechanisms. Eur. J. Pharmacol. 545, 22–28 10.1016/j.ejphar.2006.06.027 [DOI] [PubMed] [Google Scholar]
- 62.Duckworth E. A., Butler T., Collier L., Collier S., Pennypacker K. R. (2006) NF-kappaB protects neurons from ischemic injury after middle cerebral artery occlusion in mice. Brain Res. 1088, 167–175 10.1016/j.brainres.2006.02.103 [DOI] [PubMed] [Google Scholar]
- 63.Kaltschmidt C., Kaltschmidt B., Neumann H., Wekerle H., Baeuerle P. A. (1994) Constitutive NF-kappa B activity in neurons. Mol. Cell. Biol. 14, 3981–3992 10.1128/MCB.14.6.3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Widera D., Klenke C., Nair D., Heidbreder M., Malkusch S., Sibarita J. B., Choquet D., Kaltschmidt B., Heilemann M., Kaltschmidt C. (2016) Single-particle tracking uncovers dynamics of glutamate-induced retrograde transport of NF-κB p65 in living neurons. Neurophotonics 3, 041804. 10.1117/1.NPh.3.4.041804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang L., Tao L. Y., Chen X. P. (2007) Roles of NF-kappaB in central nervous system damage and repair. Neurosci. Bull. 23, 307–313 10.1007/s12264-007-0046-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mouzon B., Chaytow H., Crynen G., Bachmeier C., Stewart J., Mullan M., Stewart W., Crawford F. (2012) Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J. Neurotrauma 29, 2761–2773 10.1089/neu.2012.2498 [DOI] [PubMed] [Google Scholar]
- 67.Kirkley K. S., Popichak K. A., Afzali M. F., Legare M. E., Tjalkens R. B. (2017) Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. J. Neuroinflammation 14, 99. 10.1186/s12974-017-0871-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nenci A., Becker C., Wullaert A., Gareus R., van Loo G., Danese S., Huth M., Nikolaev A., Neufert C., Madison B., Gumucio D., Neurath M. F., Pasparakis M. (2007) Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 446, 557–561 10.1038/nature05698 [DOI] [PubMed] [Google Scholar]
- 69.Wang D. B., Kinoshita C., Kinoshita Y., Morrison R. S. (2014) p53 and mitochondrial function in neurons. Biochim. Biophys. Acta 1842, 1186–1197 10.1016/j.bbadis.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lothian C., Lendahl U. (1997) An evolutionarily conserved region in the second intron of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. Eur. J. Neurosci. 9, 452–462 10.1111/j.1460-9568.1997.tb01622.x [DOI] [PubMed] [Google Scholar]
- 71.Cucchiara B., Kasner S. E. (2001) Use of statins in CNS disorders. J. Neurol. Sci. 187, 81–89 10.1016/S0022-510X(01)00529-9 [DOI] [PubMed] [Google Scholar]
- 72.Roof R. L., Hall E. D. (2000) Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma 17, 367–388 10.1089/neu.2000.17.367 [DOI] [PubMed] [Google Scholar]
- 73.Chen J., Zhou Y., Mueller-Steiner S., Chen L. F., Kwon H., Yi S., Mucke L., Gan L. (2005) SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J. Biol. Chem. 280, 40364–40374 10.1074/jbc.M509329200 [DOI] [PubMed] [Google Scholar]
- 74.Jayakumar A. R., Tong X. Y., Ruiz-Cordero R., Bregy A., Bethea J. R., Bramlett H. M., Norenberg M. D. (2014) Activation of NF-κB mediates astrocyte swelling and brain edema in traumatic brain injury. J. Neurotrauma 31, 1249–1257 10.1089/neu.2013.3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khorooshi R., Babcock A. A., Owens T. (2008) NF-kappaB-driven STAT2 and CCL2 expression in astrocytes in response to brain injury. J. Immunol. 181, 7284–7291 10.4049/jimmunol.181.10.7284 [DOI] [PubMed] [Google Scholar]
- 76.Lagraoui M., Sukumar G., Latoche J. R., Maynard S. K., Dalgard C. L., Schaefer B. C. (2017) Salsalate treatment following traumatic brain injury reduces inflammation and promotes a neuroprotective and neurogenic transcriptional response with concomitant functional recovery. Brain Behav. Immun. 61, 96–109 10.1016/j.bbi.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan Y., Zhu F., Pu Y., Wang D., Huang A., Hu X., Qin S., Sun X., Su Z., He C. (2015) Neuroprotective effects of nitidine against traumatic CNS injury via inhibiting microglia activation. Brain Behav. Immun. 48, 287–300 10.1016/j.bbi.2015.04.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.