Summary
Food allergy is a major public health problem. Studies have shown that long‐term interactions between activated leucocyte cell adhesion molecule (ALCAM/CD166) on the surface of antigen‐presenting cells, and CD6, a co‐stimulatory molecule, influence immune responses. However, there are currently no studies on the functions of ALCAM in food allergy. Therefore, we aimed to identify the functions of ALCAM in ovalbumin (OVA)‐induced food allergy using ALCAM‐deficient mice. Wild‐type (WT) and ALCAM‐deficient (ALCAM–/–) mice were sensitized intraperitoneally and with orally fed OVA. The mice were killed, and parameters related to food allergy and T helper type 2 (Th2) immune responses were analysed. ALCAM serum levels increased and mRNA expression decreased in OVA‐challenged WT mice. Serum immunoglobulin (Ig)E levels, Th2 cytokine mRNA and histological injuries were higher in OVA‐challenged WT mice than in control mice, and these were attenuated in ALCAM–/– mice. T cell proliferation of total cells, CD3+CD4+ T cells and activated T cells in immune tissues were diminished in OVA‐challenged ALCAM–/– mice. Proliferation of co‐cultured T cells and dendritic cells (DCs) was decreased by the anti‐CD6 antibody. In addition, WT mice sensitized by adoptive transfer of OVA‐pulsed ALCAM–/– BM‐derived DCs showed reduced immune responses. Lastly, serum ALCAM levels were higher in children with food allergy than in control subjects. In this study, serum levels of ALCAM were elevated in food allergy‐induced WT mice and children with food allergy. Moreover, immune responses and T cell activation were attenuated in OVA‐challenged ALCAM–/– mice. These results indicate that ALCAM regulates food allergy by affecting T cell activation.
Keywords: activated leucocyte cell adhesion molecule, ALCAM, CD166, food allergy
Introduction
Food allergy is a serious public health problem, especially as the prevalence has increased significantly in recent years 1, 2. Despite the increasing incidence, the immune responses to food allergens in the gastrointestinal tract are not yet well understood. The gastrointestinal tract, which is the main organ involved in food allergy, is the largest immunological barrier in the body, and constantly encounters microbial antigens and dietary proteins 3. When the balance between tolerance and allergy is disrupted, orally ingested food allergens are processed as small fragments and presented to T cells by antigen‐presenting cells (APCs) 4. This interaction leads to T cell proliferation and secretion of T helper type 2 (Th2) cytokines, such as interleukin (IL)‐4, IL‐5 and IL‐13 5, 6.
Allergen presentation to T cells by APCs is a key component of the immune response. Activation of T cells is dependent upon the formation of synapses, specialized structures that form when the plasma membranes of two cells come into close apposition to transmit signals, between T cells and APCs, called immunological synapses. Numerous co‐stimulatory molecules on immune cells are involved in the maintenance of these immunological synapses 7.
Activated leucocyte cell adhesion molecule (ALCAM/CD166) is a member of the immunoglobulin superfamily (IgSF) 8, and numerous studies have shown that ALCAM is present on APCs, especially dendritic cells (DCs) 8, 9. ALCAM is involved in the maintenance of tissue architecture, immune responses and tumour progression. Previous studies have demonstrated that ALCAM expression is correlated with aggressiveness in a variety of cancers, including melanoma, prostate, breast, ovarian, oesophageal, bladder and intestinal cancers, and has been used as a prognostic marker 10, 11, 12. ALCAM engages in homophilic interactions with ALCAM or heterophilic interactions with CD6 on T cells at immunological synapses 13. These heterophilic interactions are stronger than the homophilic interactions. CD6, ligand for ALCAM, functions in T cell activation as an accessory molecule at the immunological synapse 14, and several studies have shown that long‐term interaction between ALCAM and CD6 activates T cells as co‐stimulatory molecules 8, 13, 15.
ALCAM functions as a co‐stimulatory molecule for T cells and as a biomarker for cancer. In recent years, the function of ALCAM in allergic disease has been studied, and the results suggest that ALCAM plays a role as pattern recognition receptor in delayed‐type hypersensitivity 16. However, this finding is insufficient to explain the role of ALCAM in allergic disease.
The objective of this study was to determine the role of ALCAM in allergy by analysing the systemic immune response and T cell activation in food allergy using a mouse model. We showed that the levels of ALCAM were enhanced in food allergy‐induced model mice and ALCAM‐deficient mice showed diminished IgE and Th2 inflammatory responses. Ex‐vivo experiments demonstrated that ALCAM is related to T cell activation in food allergy. We also showed that serum levels of ALCAM were increased significantly in children with food allergies.
In this study, we have demonstrated that serum ALCAM levels were elevated in food allergy‐induced mice and children with food allergy. ALCAM‐deficient mice showed diminished IgE and Th2 inflammatory responses. Furthermore, ex‐vivo experiments demonstrated that ALCAM is involved in T cell activation in food allergy. Therefore, the objective of this study was to identify the role of ALCAM by analysing the systemic immune response and T cell activation in food allergy using a mouse model.
Materials and methods
Animals
Four‐ to 6‐week‐old female BALB/c mice were purchased from OrientBio, Inc. (Kyeonggi, Korea). ALCAM‐deficient (ALCAM–/–) mice (C57BL/6 background) were purchased from JAX Laboratories (Bar Harbor, ME, USA) and back‐crossed with BALB/c mice for more than seven generations. All animal experiments were performed in accordance with the guidelines of the Korea Research Institute of Bioscience and Biotechnology, and the protocol was approved by the institutional review board of Yonsei University College of Medicine Council of Science and Technology.
Antibodies and reagents
For flow cytometric analysis, cells were stained with allophycocyanin‐conjugated anti‐CD3, fluorescein isothiocyanate (FITC)‐conjugated anti‐CD4, phycoerythrin (PE)‐conjugated anti‐CD44 and allophycocyanin‐conjugated anti‐CD62 ligand (CD62L) antibodies, which were purchased from eBioscience (San Diego, CA, USA). For Western blot anlysis, anti‐β‐actin and anti‐ALCAM rabbit polyclonal antibodies were purchased from Santa Cruz Biotechnology (Dallas, Texas, USA) and an anti‐transferrin rabbit polyclonal antibody from Thermo Fisher Scientific (Waltham, MA, USA). Horseradish peroxidase (HRP)‐conjugated secondary antibodies, streptavidin, and anti‐CD6 and anti‐IgG antibodies were purchased from Santa Cruz Biotechnology, whereas IL‐4 and granulocyte–macrophage colony‐stimulating factor (GM‐CSF) protein from R&D Systems (Minneapolis, MN, USA). Ovalbumin (grade V) was purchased from Sigma‐Aldrich (St Louis, MO, USA). Cholera toxin was purchased from List Biologicals (Campbell, CA, USA).
Experimental food allergy model
Wild‐type (WT) and ALCAM‐deficient mice were sensitized twice with a 2‐week interval by intraperitoneal injection with 50 μg of ovalbumin (OVA) and 10 μg of cholera toxin (CT) as an adjuvant. Then, all mice were challenged with OVA (50 mg in 200 μl of saline) six times at 1‐day intervals. Control mice were sensitized orally and challenged with phosphate‐buffered saline (PBS) alone. The mice were killed 1 day after the final challenge, and allergic responses in the small intestine were analysed (Fig. 1a).
Figure 1.
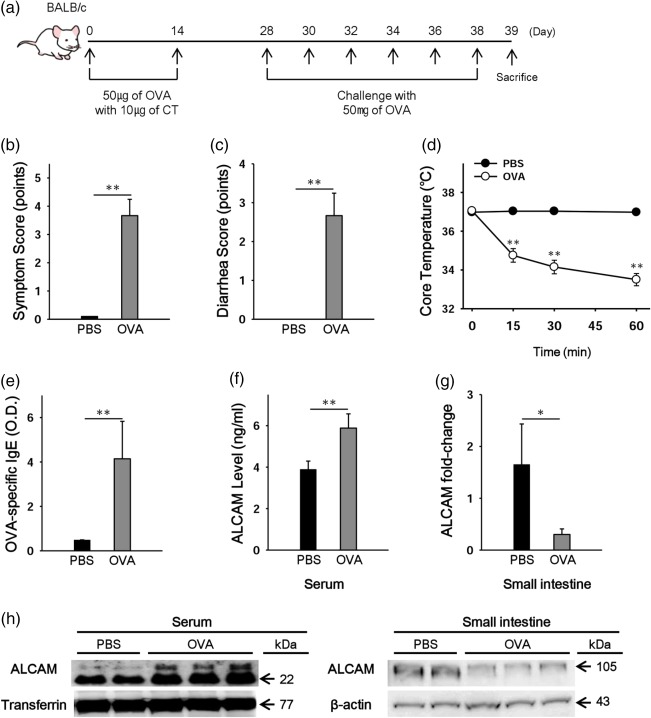
Levels of activated leucocyte cell adhesion molecule (ALCAM) were altered in ovalbumin (OVA)‐challenged wild‐type (WT) mice. (a) Experimental protocol for induction of food allergy in mice. Mice were sensitized with 50 µg of OVA and 10 µg of cholera toxin (CT) and challenged with 50 mg of OVA. Clinical scores (b), diarrhoea scores (c) and core temperature (d) were measured after the last challenge. (e) OVA‐specific immunoglobulin (Ig)E levels in serum of the mice. (f) Serum levels of ALCAM measured by enzyme‐linked immunosorbent assay (ELISA). (g) ALCAM mRNA was measured in the small intestine by real‐time polymerase chain reaction. (h) ALCAM protein levels were measured in serum and small intestine by Western blotting. Data are representative of three independent experiments (n = 3–4 for each group). *P < 0·05, **P < 0·01 versus phosphate‐buffered saline (PBS)‐challenged control mice. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Adoptive transfer of bone marrow‐derived dendritic cells (BMDCs)
For this purpose, we cultured DCs from bone marrow as follows. Both femurs were excised from one mouse and marrow was collected. Marrow cells were purified and plated at a density of 5 × 106/ml in a 100π dish with RPMI medium including 20 ng/ml IL‐4 and GM‐CSF. After 10 days, mature BMDCs were used for adoptive transfer. BMDCs were pulsed with 1·5 ml of OVA (1 mg/ml) and 10 μl of lipopolysaccharide (1 mg/ml) 1 day before the transfer. Mice were injected intraperitoneally (106 cells/mouse) twice with a 2‐week interval and challenged with OVA or PBS six times at 1‐day intervals.
Measurement of parameters of food allergy
At the last challenge, the core temperature of the mice was measured rectally before and 15, 30 and 60 min after challenge with a digital thermometer (Testo, Lenzkirch, Germany). Mice were also observed for 2 h to record diarrhoea and clinical scores, as described previously 5, 17.
Isolation and culture of immune cells
Spleen cells, mesenteric lymph node (mLN) cells and lamina propria mononuclear cells were isolated from the mice. Briefly, the spleen and mLNs were homogenized with a syringe plunger in a cell strainer (BD Falcon, 40 μm; BD Biosciences, San Diego, CA, USA). The cells were centrifuged and washed with RPMI medium supplemented with 5% fetal bovine serum (FBS), and red blood cells (RBCs) were lysed with ammonium–chloride–potassium (ACK) lysis buffer (0·15 M NH4Cl, 0·1 mM KHCO3, 0·1 mM Na2‐ethylemnediamine tetraacetic acid (EDTA) in distilled water; pH 7·2). Then, the cells were washed with RPMI containing 5% FBS, and 1 × 106 cells were cultured in each well of a 96‐well flat‐bottomed plate in the presence or absence of 50 μl of ovalbumin (10 mg/ml). After 72 h of culture, the cells were collected and centrifuged. The supernatant and centrifuged cells were frozen separately. Small intestine (jejunum) lamina propria mononuclear cells were isolated as described previously 18.
Co‐culture
Splenocytes were isolated from WT and ALCAM‐deficient mice. CD3+CD4+ T cells and CD11b+CD11c+ DCs were sorted on a FACS Aria II (BD Biosciences). Next, 2 × 105/well WT T cells and 105/well WT or ALCAM‐deficient DCs were cultured in 96‐well U‐type plates in the presence or absence of 50 μl of OVA (10 mg/ml), 3·5 μg/ml anti‐CD6 antibody and IgG antibody for 5 days. The cells were subjected to a proliferation assay and enzyme‐linked immunosorbent assay (ELISA).
Proliferation assay
Cells isolated from the spleen and mLNs were cultured in 96‐well flat‐bottomed plates (1 × 106/well) in the presence or absence of 50 μl of ovalbumin (10 mg/ml) for 5 days. On the fifth day, 10 μl of solution from the cell counting kit‐8 (CCK‐8; Dojindo Molecular Technologies, Rockville, MD, USA) was added to each well and incubated for 2–4 h. Then, the plates were read at 450 nm with an ELISA reader.
ELISA
The concentrations of IL‐4, IL‐5 and IL‐13 in the cultured supernatants were determined by ELISA (R&D Systems), according to the manufacturer's instructions. Serum total IgE was measured using the mouse IgE ELISA assay kit (BD Biosciences), according to the manufacturer's instructions. To measure ovalbumin‐specific IgE, microplates were coated with an anti‐IgE antibody and ovalbumin in coating buffer.
Flow cytometric analysis
Single‐cell suspensions (containing 1 × 106 to 2 × 106 cells) were resuspended in 100 μl of fluorescence activated cell sorter (FACS) buffer (0·5% FBS in PBS) and dead cells were excluded with Viability dye eFluor780 (eBioscience). Cells were stained simultaneously with the optimal dilutions of monoclonal antibodies (mAbs) specific for CD3, CD4, CD44 and CD62 ligand (CD62L) for 30 min at 4°C. Stained cells were washed twice with cold staining buffer (2% FCS and 0·02% sodium azide in PBS). Samples were run on a FACSVerse flow cytometer (BD Biosciences), and data were analysed using FlowJo software (Tree Star, Ashland, OR, USA).
Measurement of cytokines by quantitative real‐time polymerase chain reaction (RT–PCR)
Quantitative RT–PCR to measure cytokine expression levels was performed as described previously 19. The expressions of the cytokines and ALCAM in the small intestine (jejunum) were calculated relative to β‐actin.
Western blot
Small intestinal and serum protein levels of ALCAM were analysed by Western blotting. Small intestine tissues were homogenized, and proteins were extracted in radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific). The protein concentration was determined using the Bio‐Rad Protein assay kit (Bio‐Rad, Hercules, CA, USA). Next, 5× sodium dodecyl sulphate (SDS) sample loading buffer was added to the protein extracts and boiled for 5 min. After that, 10–20‐μg protein samples were subjected to electrophoresis in 10% SDS polyacrylamide gels and electrotransferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). After blocking with 5% skimmed milk in 1× Tris‐buffered saline containing 0·1% Tween‐20 for 1 h, the membranes were incubated overnight at 4°C with a primary antibody against ALCAM and β‐actin followed by incubation with an HRP‐conjugated anti‐rabbit secondary antibody. Protein bands were visualized using an enhanced chemiluminescence kit (GE Healthcare, Buckinghamshire, UK), with exposure of a film on the ImageQuantTM LAS 4000 Mini Biomolecular Imager (GE Healthcare). For protein analysis in serum, we depleted albumin and IgG with Pierce albumin/IgG removal kit (Thermo Fisher Scientific), and 5× SDS sample loading buffer was added. An anti‐transferrin polyclonal antibody was used for a housekeeping protein control.
Histological evaluation
Small intestine (jejunum) sections were stained with haematoxylin and eosin (H&E). Histological damage scores were measured as follows 20: 0, no damage; 1, a low amount of damage, with distinct structural components (epithelial layer and lamina propria); 2, structural components can still be differentiated, but the epithelial layer is noticeably separating from the lamina propria; 3, some disorganization of the villi and structural components are difficult to differentiate; 4, the structure of the villi is chaotic; and 5, the structure of villi is chaotic and many villi are completely destroyed down to the basal layers of tissue.
Electron microscopy
Small intestine (jejunum) specimens were fixed, dehydrated and embedded. Thin sections were observed by JEM‐1011 transmission electron microscopy (JEOL, Tokyo, Japan) at an acceleration voltage of 80 kV. Images were obtained with a MegaView III camera (Olympus, Tokyo, Japan).
Human subjects
A total of 143 children who visited Severance Children's Hospital for work‐up or treatment for food allergy or a routine health check‐up between April 2012 and September 2014 were enrolled into this study. Food allergy was defined according to the guidelines of the National Institute of Allergy and Infectious Diseases' Expert panel report in 2010 21. A thorough medical history was obtained and a physical examination was performed at the first visit. Patients who were diagnosed with other allergic diseases, such as atopic dermatitis, chronic urticaria, allergic rhinitis or asthma, were excluded considering their potential effects on allergic sensitization. After obtaining consent, a blood sample was obtained and stored at −20°C. We measured the levels of total and specific IgE by the Pharmacia CAP assay (Uppsala, Sweden). A specific IgE test was performed for individuals with suspicion of food allergens and, if not specified, for the five most common food allergens in Korea: cow's milk, egg white, wheat, soybean and peanut. Serum ALCAM levels were assessed with an ELISA kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's instructions. This study was approved by the Institutional Review Board of Severance Hospital (protocol no. 4‐2004‐0036). Written informed consent was obtained from the participants and their parents.
Statistical analysis
Categorical data were presented as counts and percentages. Continuous data were tested for normality using the Kolmogorov–Smirnov test and reported accordingly as the mean [± standard deviation (s.d.)]. The χ2 test was used to analyse categorical variables, and Student's t‐test was used to analyse continuous variables. All statistical analyses were conducted using spss statistics (version 22.0; IBM, Armonk, NY, USA) and r (version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at P ≤ 0·05.
Results
Altered ALCAM levels in OVA‐challenged mice
To address the role of ALCAM in food allergy, we investigated serum ALCAM levels in OVA‐challenged WT mice. WT mice were sensitized intraperitoneally and challenged orally with OVA (Fig. 1a) and parameters of food allergy were evaluated. Clinical and diarrhoea scores were increased and core temperature was decreased in OVA‐challenged WT mice (Fig. 1b–d). OVA‐specific IgE levels were also enhanced in the serum of OVA‐challenged WT mice compared to the levels in control mice (Fig. 1e). In contrast to the elevated serum levels of ALCAM (Fig. 1f), ALCAM mRNA was decreased in the small intestine (jejunum) of OVA‐challenged WT mice (Fig. 1g).
To understand the discrepancy between serum and tissue levels of ALCAM, we performed Western blotting on the mouse serum and small intestine. In the small intestinal lysate, the molecular size of ALCAM was 100–105 kDa, and protein levels decreased in OVA‐challenged WT mice. In contrast, ALCAM fragments appeared at 25 and 22 kDa in serum, and concentrations of these fragments increased in OVA‐challenged WT mice compared to control mice (Fig. 1h). Moreover, to identify the changes in serum ALCAM levels induced by oral food challenges, we compared serum ALCAM protein levels of OVA‐sensitized PBS‐challenged mice against non‐sensitized mice and OVA‐challenged mice (Supporting information, Fig. S1). As a result, although OVA‐sensitized PBS‐challenged mice showed slightly increased shed ALCAM (22kDa), there is no significant difference of serum ALCAM levels between non‐sensitized mice and OVA‐sensitized PBS‐challenged mice. Based on the results we investigated, we think that serum ALCAM levels are not merely reflecting the sensitization to food allergen, but indicate the development of food allergy.
Attenuated immune responses in OVA‐challenged ALCAM–/– mice
To identify the contributions of ALCAM to OVA‐induced food allergy, we induced food allergy to WT and ALCAM–/– mice with OVA. Parameters of food allergy, including the levels of Th2 cytokines, total IgE and OVA‐specific IgE and histological injury in the small intestine, were higher in OVA‐challenged WT mice than in control mice (Fig. 2). In OVA‐challenged ALCAM–/– mice, clinical and diarrhoea scores were lower than those in OVA‐challenged WT mice (Fig. 2a,b). The change of core temperature was smaller in OVA‐challenged ALCAM–/– mice than in OVA‐challenged WT mice (Fig. 2c). In addition, the mRNA expressions of Th2 cytokines, such as IL‐4, IL‐5 and IL‐13, were diminished in the small intestine (jejunum) of OVA‐challenged ALCAM–/– mice compared to those in OVA‐challenged WT mice (Fig. 2d). Levels of serum total IgE and OVA‐specific IgE were also attenuated in OVA‐challenged ALCAM–/– mice (Fig. 2e). In OVA‐challenged ALCAM–/– mice the characteristics of injured small intestines, such as damaged villi and infiltrated cells, were milder than those in OVA‐challenged WT mice (Fig. 2f,g). We observed intestinal tight junctions as a marker of food allergy by scanning electron microscopy (SEM). In control mice, the tight junctions and desmosomes in the small intestine (jejunum) appeared to be clearer, whereas the intestinal tight junctions and desmosomes of OVA‐challenged WT mice were smeared and spread out. However, these findings were less severe in OVA‐challenged ALCAM–/– mice (Fig. 2h).
Figure 2.
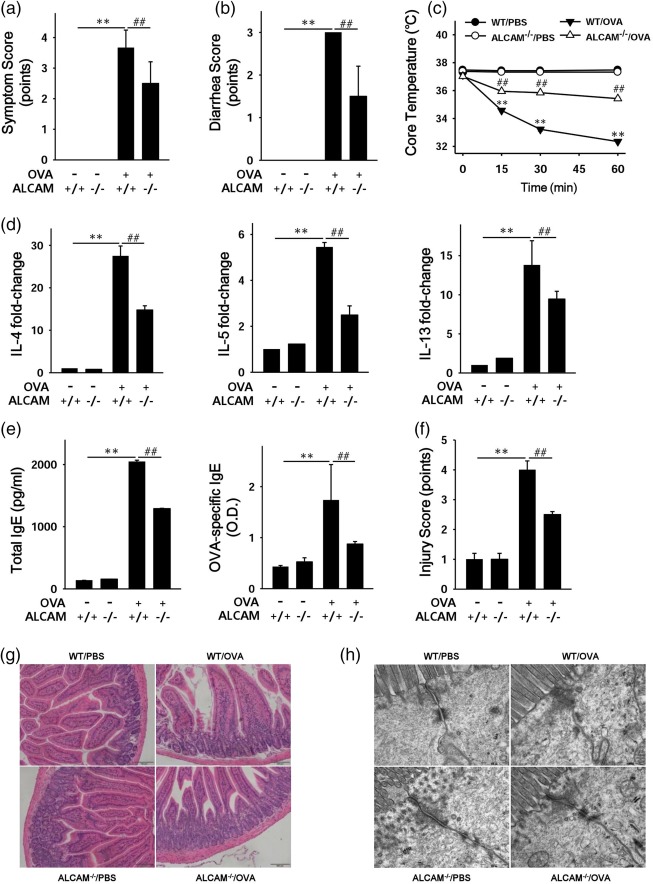
Activated leucocyte cell adhesion molecule (ALCAM) regulates immune responses in a murine model of food allergy. Mice were sensitized with 50 µg of ovalbumin (OVA) and 10 µg of cholera toxin (CT) and challenged with 50 mg of OVA. After the last challenge, clinical score (a), diarrhoea score (b) and core temperature (c) were measured in OVA‐challenged wild‐type (WT) and ALCAM–/– mice (data are representative of three independent experiments, n = 4 for each group). (d) The mRNA expression of T helper type 2 (Th2) cytokine [interleukin (IL)‐4, IL‐5 and IL‐13] in the small intestine of the mice. (e) Levels of total immunoglobulin (Ig)E and optical density (OD) values of OVA‐specific IgE in mouse serum were measured by enzyme‐linked immunosorbent assay (ELISA) for each group of mice (n = 5 for each group) in triplicate for each sample. Histological observations of the small intestine (f) and injury score (data representative of three independent experiments, n = 4 for each group) (g). (h) Morphological observations of intestinal tight junctions by electron microscopy (EM). **P < 0·01 [phosphate‐buffered saline (PBS) versus WT/OVA)]; ## P < 0·01 (WT/OVA versus ALCAM–/–/OVA). Magnification: f, ×200; i, ×50 000. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Diminished T cell responses in ALCAM–/– mice
It is well known that ALCAM on APCs and CD6 on T cells interact at immunological synapses and function as co‐stimulatory molecules 22. Therefore, we attempted to determine the role of ALCAM in T cell responses in an ex‐vivo experiment. Splenocytes and mLN cells were isolated from WT and ALCAM–/– mice and cultured in the presence or absence of OVA. Total T cell proliferation was then measured with the CCK‐8 assay. The rate of total T cell proliferation was diminished in cells obtained from OVA‐challenged ALCAM–/– mice compared with those from OVA‐challenged WT mice (Fig. 3a). The levels of Th2 cytokines, including IL‐4, IL‐5 and IL‐13, were decreased in supernatants of cultured splenocytes from OVA‐challenged ALCAM–/– mice against those of OVA‐challenged WT mice (Fig. 3b).
Figure 3.
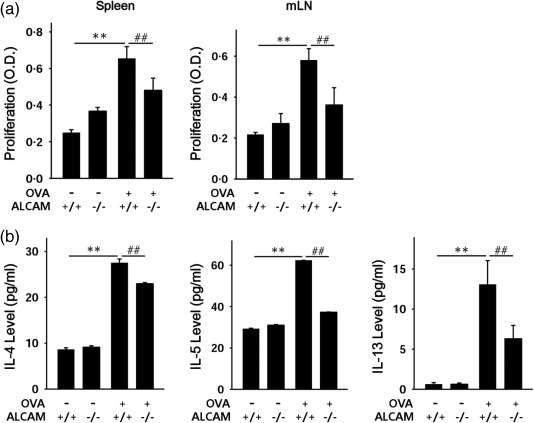
Activated leucocyte cell adhesion molecule (ALCAM) affects T cell proliferation. Spleens and mesenteric lymph nodes (mLNs) were removed from the mice, and cells were isolated from these tissues. Total cells from spleen and mLNs were cultured in the absence (control; CON) or presence of ovalbumin (OVA) for 5 days. T cell proliferation was assessed with the cell counting kit‐8 (CCK‐8) assay. Released T helper type 2 (Th2) cytokines [interleukin (IL)‐4, IL‐5 and IL‐13] were measured in the supernatant from splenocyte cultures. (a) Total cell proliferation in the spleen (left) and mLN (right). (b) Th2 cytokine levels in supernatant from splenocytes cultured medium assessed by enzyme‐linked immunosorbent assay (ELISA). Mean ± standard deviation of triplicate wells; n = 4 for each group, data representative of three independent experiments. **P < 0·01 (CON versus WT/OVA); ## P < 0·01 (WT/OVA versus ALCAM–/–/OVA).
To assess specifically the changes in T cell responses in immune tissues, we isolated and cultured cells from the spleen, mLN, and small intestine. To identify the T cells, CD3 and CD4 were used as markers. The number of CD3+CD4+ T cells was enhanced throughout the immune tissues, especially in the small intestine, of OVA‐challenged WT mice when compared to the numbers of T cells in control WT mice. However, the number of CD3+CD4+ T cells was lower in OVA‐challenged ALCAM–/– mice than in OVA‐challenged WT mice. (Fig. 4a) We also found that the number of activated cells (CD4+CD44highCD62Llow) were more decreased in OVA‐challenged ALCAM–/– mice than in OVA‐challenged WT mice (Fig. 4b). The numbers of CD4+ T cells and effector T cells in the lymphatic tissues of mice are shown as graphs in Fig 4c,d, respectively. These results suggest that ALCAM has an effect on the total population and activation of T cells in the immune system through its interaction with CD6 on T cells.
Figure 4.
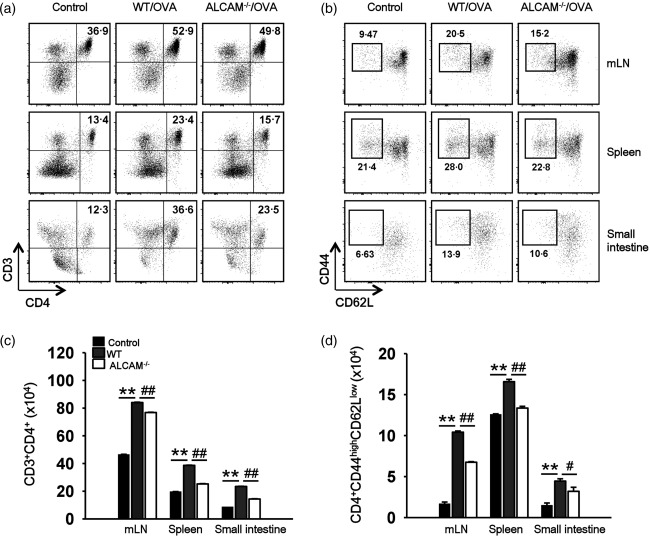
Activated leucocyte cell adhesion molecule (ALCAM) affects the activated T cell populations. Single cells from the spleen, mesenteric lymph nodes (mLNs) and small intestine (jejunum) were gated with CD3 and CD4 for CD4+ T cell populations or CD4, CD44 and CD62 ligand (CD62L) for activated T cells by flow cytometry. (a) The CD3+CD4+ T cell populations in the splenocytes, mLN cells and the small intestinal lamina propria mononuclear cells of ovalbumin (OVA)‐challenged wild‐type (WT) and ALCAM–/– mice. (b) Activated T cells in the lymphatic tissues of OVA‐challenged WT and ALCAM–/– mice were measured by gating with CD4+CD44highCD62Llow. (c) The number of CD4+ T cells in the lymphatic tissues of the mice. (d) The number of effector T cells in the lymphatic tissues of the mice. Data of flow cytometry have reproducibility in three independent experiments, and mean ± standard deviation of triplicate vials, n = 4 for each group, data representative of three independent experiments for (c) and (d). **P < 0·01 (control versus WT/OVA); # P < 0·05, ## P < 0·01 (WT/OVA versus ALCAM–/–/OVA).
Reduced proliferation of CD6 antibody‐treated WT T cells and DCs
To confirm that ALCAM affects a T cell response, we blocked CD6 on T cells with the anti‐CD6 antibody ex vivo. Mice were sensitized twice (2‐week interval) with PBS or OVA. The spleen was removed from the mice, and splenocytes were isolated. T cells and DCs were then sorted from splenocytes of WT and ALCAM–/– mice. We co‐cultured these cells and used mixed T cells (WT T cells and ALCAM–/– T cells) to verify that ALCAM–/– T cells have no problems with proliferation. Mixed T cells and WT or ALCAM–/– DCs were co‐cultured in 96‐well round‐bottomed plates and treated with the anti‐CD6 antibody or an iso‐IgG antibody. Cell proliferation was determined with a CCK‐8 solution, and Th2 cytokine levels in cultured media were measured by ELISA. Proliferation of mixed T cells and WT DCs was higher than that of mixed T cells and ALCAM–/– DCs in the absence of antibodies. Co‐cultured cells treated with the anti‐CD6 antibody showed slower proliferation regardless of the type of DCs. Nevertheless, the reduction in proliferation was greater in mixed T cells and WT DCs than in mixed T cells and ALCAM–/– DCs (Fig. 5a). Levels of IL‐4, IL‐5 and IL‐13 in the culture medium showed a comparable reduction (Fig. 5b).
Figure 5.
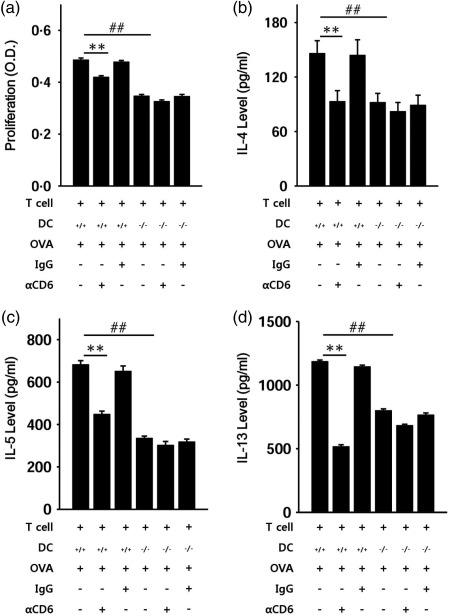
A CD6 deficiency diminishes the proliferation of co‐cultured cells. Wild‐type (WT) (n = 4) and activated leucocyte cell adhesion molecule (ALCAM)–/– (n = 4) mice were sensitized with ovalbumin (OVA) or phosphate‐buffered saline (PBS) twice with a 2‐week interval. Single cells from the spleen were sorted to obtain T cells and dendritic cells (DCs). Next, the mixed T cells and WT DCs or ALCAM–/– DCs were co‐cultured in a 96‐well round‐bottomed plate for 5 days with the anti‐CD6 antibody (αCD6) or immunoglobulin (Ig)G antibody as a control. (a) Proliferation of co‐cultured cells was measured by the cell counting kit‐8 (CCK‐8) assay. (b)–(d) T helper type 2 (Th2) cytokine levels in the culture supernatant were measured by enzyme‐linked immunosorbent assay (ELISA). The data are mean ± standard deviation of triplicate wells; **P < 0·01 (WT T cells with WT DCs versus WT T cells with WT DCs plus αCD6); ## P < 0·01 (WT T cells with WT DCs versus WT T cells with ALCAM–/– DCs).
Alleviated immune responses in WT mice reconstituted with ALCAM–/– BMDCs
OVA‐challenged ALCAM–/– mice and DCs from ALCAM–/– mice showed attenuated immune responses and cell proliferation, respectively, in this study. To clarify the effects of these ALCAM–/– cells from ALCAM–/– mice, we transferred BMDCs adoptively to WT mice. WT mice received naive or OVA‐pulsed BMDCs from WT or ALCAM–/– mice intraperitoneally twice with a 2‐week interval with CT. Two weeks after the last transfer, the mice were challenged orally with OVA six times at 1‐day intervals. There were no signs of food allergy in WT mice that were injected with CT only, but mice that received naive WT BMDCs showed slightly enhanced signs of allergy (Fig. 6a,b,f). Parameters of food allergy, including expression of Th2 cytokine mRNA, levels of OVA‐specific IgE and damage to the small intestine, were significantly greater in the mice that received OVA‐pulsed WT BMDCs compared to naive WT BMDCs (Fig. 6c,d). Moreover, proliferation of splenocytes and cells from mLNs increased in mice that were reconstituted with OVA‐pulsed WT BMDCs (Fig. 6e). Th2 cytokine levels in culture media also decreased in mice that received OVA‐pulsed WT BMDCs (data not shown). Mice that received OVA‐pulsed ALCAM–/– BMDCs showed weaker immune responses compared with mice that received OVA‐pulsed WT BMDCs (Fig. 6). In addition, we verified that ALCAM–/– mice that received OVA‐sensitized WT T cells yielded weaker immune responses compared with WT mice that received OVA‐sensitized WT T cells (Supporting information, Fig. S2).
Figure 6.
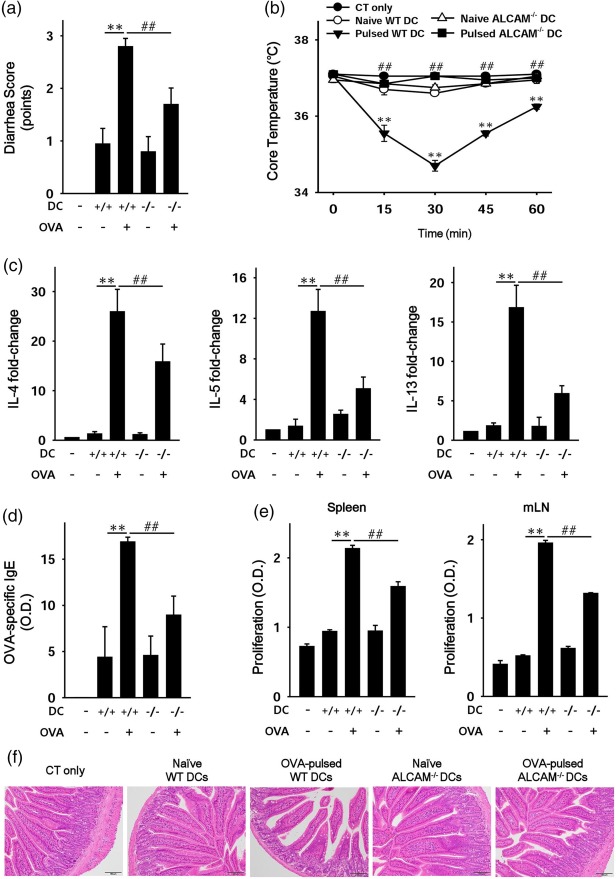
Activated leucocyte cell adhesion molecule (ALCAM)–/– bone marrow‐derived dendritic cells (BMDCs) alleviate immune responses in wild‐type (WT) mice. Bone marrow cells were isolated, and DCs were obtained by differentiation of those cells from WT and ALCAM–/– mice (n = 4). WT mice received naive or ovalbumin (OVA)‐pulsed WT or ALCAM–/– DCs twice with a 2‐week interval with cholera toxin (CT) intraperitoneally. Two weeks later, the mice were challenged orally with OVA or phosphate‐buffered saline (PBS) six times at 1‐day intervals. (a) The diarrhoea score of the mice. (b) Rectally estimated core temperature at the last challenge. (c) T helper type 2 (Th2) cytokine mRNA expression levels in the mouse small intestine according to real‐time polymerase chain reaction (PCR). (d) Serum OVA‐specific immunoglobulin (IgE) was quantified by an enzyme‐linked immunosorbent assay (ELISA). (e) Proliferation of cells from the spleen and mesenteric lymph nodes (mLNs) was measured using a cell counting kit‐8 (CCK‐8) solution. (f) Histological examination of a murine small intestine (haematoxylin and eosin staining); n = 3 for each group and total N = 36. **P < 0·01 (naive WT BMDCs versus OVA‐pulsed WT BMDCs); ## P < 0·01 (OVA‐pulsed WT BMDCs versus OVA‐pulsed ALCAM–/– BMDCs). Magnification ×200. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Increased serum levels of ALCAM in children with food allergy
To determine whether the serum levels of ALCAM were altered in children with food allergy we evaluated a group of 143 children (aged 0·5–10·8 years), including 53 with food allergy and 90 without allergy (control). Among food allergens, egg (n = 35, 66%) was the most common, followed by milk (n = 17, 32%), wheat (n = 5, 9%) and peanut (n = 4, 8%). The total number of children with multiple food allergy was 25 (47%). The clinical characteristics of the subjects are summarized in Table 1. There was no significant difference in gender. The mean age in the control group was significantly higher than that in the food allergy group (7·4 ± 1·44 versus 2·24 ± 1·9, P < 0·0001). Serum total IgE levels were significantly higher in children with food allergy than in the healthy controls (260·8 ± 294·6 versus 40·4 ± 30·5, P < 0·0001), and serum ALCAM levels were significantly higher in those with food allergy than in the healthy controls (35·4 ± 4·7 versus 28·1 ± 4·2, P < 0·0001; Fig. 7). A multiple regression analysis was performed to evaluate whether ALCAM levels were related to food allergy after controlling for age, and the results demonstrated that ALCAM levels were significantly higher in the food allergy group (βo = 5·75, P < 0·0001). In subgroup analysis, children with egg allergy revealed higher serum ALCAM levels compared to those without the egg allergy (36·05 ± 4·66 versus 29·16 ± 4·78, P < 0·0001). The same trend was observed for milk allergy (35·58 ± 4·29 versus 30·21 ± 5·45, P < 0·0001). Among the subjects with food allergy, ALCAM levels were elevated in children with multiple food allergy in comparison with those with only one food allergy; however, the difference was not statistically significant (35·96 ± 4·23 versus 34·95 ± 5·06, P = 0·435).
Table 1.
Characteristics of subjects.
| Characteristics | Control (n = 90) | Food allergy (n = 53) | P‐value |
|---|---|---|---|
| Age, years | 7·4 ± 1·44 | 2·24 ± 1·9 | P < 0·0001 |
| Sex, male (%) | 42 (47) | 32 (60) | 0·158 |
| Serum total immunoglobulin E, kU/l | 40·4 ± 30·5 | 260·8 ± 294·6 | P < 0·0001 |
Figure 7.
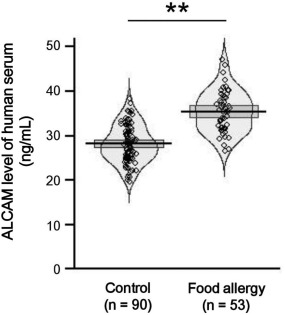
Serum levels of activated leucocyte cell adhesion molecule (ALCAM) were increased in children with food allergy. Serum ALCAM levels were measured in children with food allergy (n = 53) and healthy control subjects (n = 90) by enzyme‐linked immunosorbent assay (ELISA). Pirate plots represent the distribution of ALCAM levels, with a horizontal mean line and boxes representing 95% confidence intervals. **P < 0·01.
Discussion
Allergenic food proteins may not only induce IgE production, but also activate special subsets of T cells to establish food allergy 23. Thus, while the most common food allergy is mediated by IgE antibodies, they may also be induced by T cells 24. Within the small intestine, the main tissue involved in food allergy, lymphocytes are found at various locations in the gut mucosa, and T cells are located in the epithelium 25. When APCs, such as DCs, present food allergens to T cells, an immunological synapse is formed, and there are many co‐stimulatory molecules on immune cells that maintain and prolong these immunological synapses 7. ALCAM, one of these co‐stimulatory molecules, is expressed by DCs and interacts with CD6 on T cells. Many studies have demonstrated that ALCAM mediates immune responses by interacting with CD6 and plays a role in the pathogenesis of some cancers 15. In one study, they characterized ALCAM as intestinal cancer stem cell marker in the human and mouse gastrointestinal tract 26.
We showed that ALCAM levels were enhanced in the serum of OVA‐challenged WT mice and children with food allergy when compared to control mice and children without allergy, respectively. These findings indicate that ALCAM has an effect on OVA‐induced food allergy, which correlates with studies that addressed the role of ALCAM in murine models or human disease. A previous study showed that ALCAM is over‐expressed in a murine model of metastatic prostate cancer and in patients with prostate cancer 27. Moreover, ALCAM levels have been shown to be increased in patients with a variety of cancers, including melanoma, prostate, breast, ovarian, oesophageal, bladder and intestinal cancers 10, 11, 12, 28, 29. We found that increased serum levels of ALCAM were not correlated with decreased ALCAM mRNA and protein expression in the small intestine of OVA‐challenged WT mice. Although seemingly contradictory, this finding paralleled the results of another study examining the role of ALCAM in breast cancer 30. They also found no correlation between soluble ALCAM levels in serum and ALCAM protein levels in the tumour tissues of patients with breast cancer. This discrepancy between serum and tissue of ALCAM levels may result from shedding of the protein into serum by a disintegrin and metalloprotease domain 17 (ADAM17), also called tumour necrosis factor‐α‐converting enzyme (TACE) 31. We confirmed that the molecular size of ALCAM fragments in serum is smaller than that in the small intestine. Therefore, OVA‐challenged WT mice showed different forms of ALCAM in the small intestine and serum. Here, we demonstrated that OVA‐challenged ALCAM–/– mice showed attenuated symptoms of food allergy when compared with WT mice, such as decreased levels of Th2 cytokines, injury of the small intestine and other parameters. Furthermore, WT mice that received OVA‐pulsed ALCAM–/– BMDCs yielded weaker immune responses and slower total cell proliferation. These findings indicate that ALCAM promotes immune responses in food allergy, and these data correlate well with other studies that demonstrated the role of ALCAM in malignant mesothelioma and delayed‐type hypersensitivity 16, 32. In malignant mesothelioma, knock‐down of ALCAM led to 30–50% inhibition of cell migration and invasion of mesothelioma cells 32. In a murine model of delayed‐type hypersensitivity, the clinical score of allergic disease was attenuated in ALCAM–/– mice 16.
One study characterized the T cell response to peanut allergen in a murine model of peanut allergy 33. They showed that the levels of allergen‐specific IgE and systemic anaphylaxis score were significantly higher in peanut‐induced mice compared to the levels in control mice. Moreover, T cell proliferation of splenocytes stimulated by peanut was increased in peanut‐induced mice. In another study, OVA‐challenged BALB/c mice exhibited symptoms of food allergy, including diarrhoea, decreased body temperature, increased levels of OVA‐specific IgE and expanded numbers of mast cells 34. In addition, the levels of Th2 cytokines and CD4+ T cell proliferation in mLN and splenocytes were enhanced in OVA‐challenged mice. It is well known that CD6, ligand for ALCAM, is a co‐stimulatory molecule for T cell activation 14. Both ALCAM and CD6 are recruited actively and contribute to stabilization of the immunological synapse. Moreover, it is also known that ALCAM‐CD6‐mediated adhesion is involved in both the early and later stages of DC‐induced T cell activation and proliferation 15, 22. These findings demonstrate that CD6 and ALCAM form a key adhesive receptor–ligand pair that is involved not only in early DC–T cell binding but also in sustaining DC‐induced T cell proliferation long after the initial contact has been established 15. Following these studies, we investigated the role of ALCAM as a co‐stimulatory molecule of T cells. We measured total T cell proliferation and the CD4+ T cell and activated T cell populations in systemic immune tissues to identify the reasons for the attenuated symptoms in OVA‐challenged ALCAM–/– mice. We observed that the total T cell proliferation of cultured mLN cells and splenocytes was diminished in OVA‐challenged ALCAM–/– mice. More specifically, the CD3+CD4+ T cell and activated T cell populations were lower in the mLN, spleen and small intestine of OVA‐challenged ALCAM–/– mice than in OVA‐challenged WT mice. In the experiments on co‐culture, cell proliferation and levels of Th2 cytokines decreased in WT T cells and DCs when they were treated with the anti‐CD6 antibody. Many studies have determined the role of ALCAM by treatment with an anti‐CD6 antibody in vivo. In one study, authors verified that CD6 is a key molecule for sustaining the activation and differentiation of T cells and is an important target for modulation of autoimmune diseases 35. They found that Itolizumab, a CD6 domain 1‐specific humanized monoclonal antibody, inhibits T cell signalling, activation and proliferation. Moreover, mice that were treated with the anti‐mouse CD6 domain 1 antibody showed a decreased clinical score of experimental autoimmune encephalomyelitis in comparison with the mice that were treated with the mouse isotype control antibody. According to that study and our findings, a lack of ALCAM leads to attenuated T cell activation because it is no longer available to interact with CD6 on T cells.
In this study, although ALCAM expression in the small intestine was lower in OVA‐challenged WT mice than in control mice, serum levels of ALCAM were increased. In accordance with these findings, the serum levels of ALCAM in children with food allergy were also higher than those in healthy control subjects. Immune responses in OVA‐challenged ALCAM–/– mice were attenuated compared to those in OVA‐challenged WT mice. Total T cell proliferation in the spleen and mLN, the CD3+CD4+ T cell population and the activated (CD4+CD44highCD62Llow) T cell in the spleen, mLN and small intestine were also diminished in OVA‐challenged ALCAM–/– mice compared to OVA‐challenged WT mice. Cell proliferation of co‐cultured T cells and DCs was decreased by the anti‐CD6 antibody. In addition, WT mice that were reconstituted with ALCAM–/– BMDCs showed weaker immune responses. Further analysis is necessary to explore the possible usage of soluble ALCAM as a biomarker or therapy for food allergy.
In conclusion, our results showed that the levels of ALCAM are increased in a murine model of food allergy and in children with food allergy. Moreover, food allergy‐induced ALCAM–/– mice showed attenuated systemic immune responses, T cell proliferation and T cell activation. These results suggest that ALCAM regulates allergic disease and affects immune responses by altering T cell activation and the Th2 response in food allergy.
Disclosure
The authors declare no conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Activated leucocyte cell adhesion molecule (ALCAM) protein levels in serum of ovalbumin (OVA)‐sensitized phosphate‐buffered saline (PBS)‐challenged mice. Wild‐type (WT) mice (n = 3) were sensitized and challenged with PBS or OVA. Group 1: non‐sensitized mice (CON); group 2: OVA‐sensitized PBS‐challenged mice (PBS); group 3: OVA‐challenged mice (OVA). Serum ALCAM protein levels of mice were quantified by enzyme‐linked immunosorbent assay (ELISA) (a) and Western blotting (b).
Fig. S2. Immune responses decreased in activated leucocyte cell adhesion molecule (ALCAM)–/– mice that received wild‐type (WT) T cells. WT mice (n = 4) were sensitized with ovalbumin (OVA) or phosphate‐buffered saline (PBS) twice with a 2‐week interval. The spleen was excised from these mice and single cells isolated. CD3+CD4+ T cells were sorted, and we injected these T cells into WT and ALCAM–/– mice once. Two weeks later, the mice were challenged orally with OVA or PBS six times at 1‐day intervals. (a) We estimated core temperature rectally at the last challenge. (b) Serum OVA‐specific immunoglobulin (Ig)E was quantified by enzyme‐linked immunosorbent assay (ELISA). (c) T helper type 2 (Th2) cytokine mRNA expression levels in the murine small intestine were measured by real‐time polymerase chain reaction (PCR). (d) and (e) Proliferation rates of cells from the spleen and mesenteric lymph nodes (mLNs) were measured using a cell counting kit‐8 (CCK‐8) solution. (e) Histological examination of the murine small intestine (haematoxylin and eosin staining); n = 3 for each group and total N = 36. **P < 0·01 (control WT mice versus WT mice that received OVA‐sensitized T cells); ## P < 0·01 (WT mice that received OVA‐sensitized T cells versus ALCAM–/– mice that received OVA‐sensitized T cells). Magnification ×200.
The number of CD3+CD4+ T cells was enhanced throughout the immune tissues, especially in the small intestine, of OVA‐challenged WT mice when compared to the numbers of T cells in control WT mice. However, the number of CD3+CD4+ T cells was lower in OVA‐challenged ALCAM–/– mice than in OVA‐challenged WT mice. We also found that the number of activated cells (CD4+CD44highCD62Llow) decreased in OVA‐challenged ALCAM–/– mice rather than in OVA‐challenged WT mice.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C0234) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A1A01057053) and the Ministry of Science, ICT & Future Planning (NRF‐2017R1A2B2004043).
References
- 1. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 2014; 133:291–307; quiz: 8. [DOI] [PubMed] [Google Scholar]
- 2. Lee SE, Kim H. Update on early nutrition and food allergy in children. Yonsei Med J 2016; 57:542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faria AM, Weiner HL. Oral tolerance. Immunol Rev 2005; 206:232–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eigenmann PA. Mechanisms of food allergy. Pediatr Allergy Immunol 2009; 20:5–11. [DOI] [PubMed] [Google Scholar]
- 5. Shin HS, Bae MJ, Jung SY, Shon DH. Preventive effects of skullcap (Scutellaria baicalensis) extract in a mouse model of food allergy. J Ethnopharmacol 2014; 153:667–73. [DOI] [PubMed] [Google Scholar]
- 6. Otsu K, Dreskin SC. Peanut allergy: an evolving clinical challenge. Discov Med 2011; 12:319–28. [PubMed] [Google Scholar]
- 7. Alarcon B, Mestre D, Martinez‐Martin N. The immunological synapse: a cause or consequence of T‐cell receptor triggering? Immunology 2011; 133:420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowen MA, Patel DD, Li X et al Cloning, mapping, and characterization of activated leukocyte‐cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med 1995; 181:2213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swart GW. Activated leukocyte cell adhesion molecule (CD166/ALCAM): developmental and mechanistic aspects of cell clustering and cell migration. Eur J Cell Biol 2002; 81:313–21. [DOI] [PubMed] [Google Scholar]
- 10. Klein WM, Wu BP, Zhao S, Wu H, Klein‐Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol 2007; 20:102–7. [DOI] [PubMed] [Google Scholar]
- 11. Burkhardt M, Mayordomo E, Winzer KJ et al Cytoplasmic overexpression of ALCAM is prognostic of disease progression in breast cancer. J Clin Pathol 2006; 59:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomita K, van Bokhoven A, Jansen CFJ et al Activated leukocyte cell adhesion molecule (ALCAM) expression is associated with a poor prognosis for bladder cancer patients. Urooncology 2003; 3:121–9. [Google Scholar]
- 13. Hassan NJ, Barclay AN, Brown MH. Frontline: optimal T cell activation requires the engagement of CD6 and CD166. Eur J Immunol 2004; 34:930–40. [DOI] [PubMed] [Google Scholar]
- 14. Resnick D, Pearson A, Krieger M. The SRCR superfamily: a family reminiscent of the Ig superfamily. Trends Biochem Sci 1994; 19:5–8. [DOI] [PubMed] [Google Scholar]
- 15. Zimmerman AW, Joosten B, Torensma R, Parnes JR, van Leeuwen FN, Figdor CG. Long‐term engagement of CD6 and ALCAM is essential for T‐cell proliferation induced by dendritic cells. Blood 2006; 107:3212–20. [DOI] [PubMed] [Google Scholar]
- 16. von Bauer R, Oikonomou D, Sulaj A et al CD166/ALCAM mediates proinflammatory effects of S100B in delayed type hypersensitivity. J Immunol 2013; 191:369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee SY, Oh S, Lee K et al Murine model of buckwheat allergy by intragastric sensitization with fresh buckwheat flour extract. J Korean Med Sci 2005; 20:566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Couter CJ, Surana NK. Isolation and flow cytometric characterization of murine small intestinal lymphocytes. J Vis Exp 2016; 111. doi: 10.3791/54114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polukort SH, Rovatti J, Carlson L et al IL‐10 enhances IgE‐mediated mast cell responses and is essential for the development of experimental food allergy in IL‐10‐deficient mice. J Immunol 2016; 196:4865–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliver SR, Phillips NA, Novosad VL, Bakos MP, Talbert EE, Clanton TL. Hyperthermia induces injury to the intestinal mucosa in the mouse: evidence for an oxidative stress mechanism. Am J Physiol Regul Integr Comp Physiol 2012; 302:R845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boyce JA, AssA'ad A, Burks AW et al Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID‐sponsored expert panel. J Allergy Clin Immunol 2010; 126:S1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weidle UH, Eggle D, Klostermann S, Swart GW. ALCAM/CD166: cancer‐related issues. Cancer Genomics Proteomics 2010; 7:231–43. [PubMed] [Google Scholar]
- 23. Bohle B. T lymphocytes and food allergy. Mol Nutr Food Res 2004; 48:424–33. [DOI] [PubMed] [Google Scholar]
- 24. Abernathy‐Carver KJ, Sampson HA, Picker LJ, Leung DY. Milk‐induced eczema is associated with the expansion of T cells expressing cutaneous lymphocyte antigen. J Clin Invest 1995; 95:913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frossard CP, Asigbetse KE, Burger D, Eigenmann PA. Gut T cell receptor‐gammadelta(+) intraepithelial lymphocytes are activated selectively by cholera toxin to break oral tolerance in mice. Clin Exp Immunol 2015; 180:118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levin TG, Powell AE, Davies PS et al Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology 2010; 139:2072–82.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hansen AG, Arnold SA, Jiang M et al ALCAM/CD166 is a TGF‐beta‐responsive marker and functional regulator of prostate cancer metastasis to bone. Cancer Res 2014; 74:1404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verma A, Shukla NK, Deo SV, Gupta SD, Ralhan R. MEMD/ALCAM: a potential marker for tumor invasion and nodal metastasis in esophageal squamous cell carcinoma. Oncology 2005; 68:462–70. [DOI] [PubMed] [Google Scholar]
- 29. Weichert W, Knosel T, Bellach J, Dietel M, Kristiansen G. ALCAM/CD166 is overexpressed in colorectal carcinoma and correlates with shortened patient survival. J Clin Pathol 2004; 57:1160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Witzel I, Schroder C, Muller V et al Detection of activated leukocyte cell adhesion molecule in the serum of breast cancer patients and implications for prognosis. Oncology 2012; 82:305–12. [DOI] [PubMed] [Google Scholar]
- 31. Rosso O, Piazza T, Bongarzone I et al The ALCAM shedding by the metalloprotease ADAM17/TACE is involved in motility of ovarian carcinoma cells. Mol Cancer Res 2007; 5:1246–53. [DOI] [PubMed] [Google Scholar]
- 32. Ishiguro F, Murakami H, Mizuno T et al Activated leukocyte cell‐adhesion molecule (ALCAM) promotes malignant phenotypes of malignant mesothelioma. J Thorac Oncol 2012; 7:890–9. [DOI] [PubMed] [Google Scholar]
- 33. Li XM, Serebrisky D, Lee SY et al A murine model of peanut anaphylaxis: T‐ and B‐cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol 2000; 106:150–8. [DOI] [PubMed] [Google Scholar]
- 34. Knight AK, Blazquez AB, Zhang S, Mayer L, Sampson HA, Berin MC. CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. Am J Physiol Gastrointest Liver Physiol 2007; 293:G1234–43. [DOI] [PubMed] [Google Scholar]
- 35. Bughani U, Saha A, Kuriakose A et al T cell activation and differentiation is modulated by a CD6 domain 1 antibody Itolizumab. PLOS ONE 2017; 12:e0180088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Activated leucocyte cell adhesion molecule (ALCAM) protein levels in serum of ovalbumin (OVA)‐sensitized phosphate‐buffered saline (PBS)‐challenged mice. Wild‐type (WT) mice (n = 3) were sensitized and challenged with PBS or OVA. Group 1: non‐sensitized mice (CON); group 2: OVA‐sensitized PBS‐challenged mice (PBS); group 3: OVA‐challenged mice (OVA). Serum ALCAM protein levels of mice were quantified by enzyme‐linked immunosorbent assay (ELISA) (a) and Western blotting (b).
Fig. S2. Immune responses decreased in activated leucocyte cell adhesion molecule (ALCAM)–/– mice that received wild‐type (WT) T cells. WT mice (n = 4) were sensitized with ovalbumin (OVA) or phosphate‐buffered saline (PBS) twice with a 2‐week interval. The spleen was excised from these mice and single cells isolated. CD3+CD4+ T cells were sorted, and we injected these T cells into WT and ALCAM–/– mice once. Two weeks later, the mice were challenged orally with OVA or PBS six times at 1‐day intervals. (a) We estimated core temperature rectally at the last challenge. (b) Serum OVA‐specific immunoglobulin (Ig)E was quantified by enzyme‐linked immunosorbent assay (ELISA). (c) T helper type 2 (Th2) cytokine mRNA expression levels in the murine small intestine were measured by real‐time polymerase chain reaction (PCR). (d) and (e) Proliferation rates of cells from the spleen and mesenteric lymph nodes (mLNs) were measured using a cell counting kit‐8 (CCK‐8) solution. (e) Histological examination of the murine small intestine (haematoxylin and eosin staining); n = 3 for each group and total N = 36. **P < 0·01 (control WT mice versus WT mice that received OVA‐sensitized T cells); ## P < 0·01 (WT mice that received OVA‐sensitized T cells versus ALCAM–/– mice that received OVA‐sensitized T cells). Magnification ×200.
The number of CD3+CD4+ T cells was enhanced throughout the immune tissues, especially in the small intestine, of OVA‐challenged WT mice when compared to the numbers of T cells in control WT mice. However, the number of CD3+CD4+ T cells was lower in OVA‐challenged ALCAM–/– mice than in OVA‐challenged WT mice. We also found that the number of activated cells (CD4+CD44highCD62Llow) decreased in OVA‐challenged ALCAM–/– mice rather than in OVA‐challenged WT mice.


