Abstract
The theoretical ability of epigenetic variation to influence the heritable variation of complex traits is gaining traction in the study of adaptation. This theory posits that epigenetic marks can control adaptive phenotypes but the relative potential of epigenetic variation in comparison to genetic variation in these traits is not presently understood. To compare the potential of epigenetic and genetic variation in adaptive traits, we analyzed the influence of DNA methylation variation on the accumulation of chemical defense compounds glucosinolates from the order Brassicales. Several decades of work on glucosinolates has generated extensive knowledge about their synthesis, regulation, genetic variation and contribution to fitness establishing this pathway as a model pathway for complex adaptive traits. Using high-throughput phenotyping with a randomized block design of ddm1 derived Arabidopsis thaliana epigenetic Recombinant Inbred Lines, we measured the correlation between DNA methylation variation and mean glucosinolate variation and within line stochastic variation. Using this information, we identified epigenetic Quantitative Trait Loci that contained specific Differentially Methylated Regions associated with glucosinolate traits. This showed that variation in DNA methylation correlates both with levels and variance of glucosinolates and flowering time with trait-specific loci. By conducting a meta-analysis comparing the results to different genetically variable populations, we conclude that the influence of DNA methylation variation on these adaptive traits is much lower than the corresponding impact of standing genetic variation. As such, selective pressure on these traits should mainly affect standing genetic variation to lead to adaptation.
Key words: Local adaptation, trait stability, DNA methylation, glucosinolates, epiQTL mapping
Genetic polymorphisms in the form of de novo mutations and standing variation are key sources of variation in complex traits that can enable adaptation during selection. This is the foundation of the modern synthesis that linked Darwin’s theories of evolution to Mendelian Genetics (Huxley 1942). In plants, this modern synthesis is well supported by numerous studies showing the adaptive role of genetic variation controlling a range of traits including flowering time, defense metabolism and disease resistance (Wilson et al., 2001; Kliebenstein et al., 2001b; Bäurle and Dean 2006; Salomé et al., 2011). More recently, epigenetic marks have been found to influence a number of developmental traits like flowering time and morphological traits such as root length, leaf area and internode length (Roux et al., 2011; Cortijo et al., 2014; Jia et al., 2015; Kooke et al., 2015; Rosa et al., 2016). Further, some of these marks are potentially inherited across generations although the fraction of these marks that are inherited as pure epi-alleles (i.e., with no associated causal genetic variation) is under intense debate (Richards 2006). These observations have led to the proposal for an “extended evolutionary synthesis” wherein heritable variation in these epigenetic marks could potentiate adaptation (Jablonka and Raz 2009; Weigel and Colot 2012; Laland et al., 2014; Burggren 2016). There is a large number of studies that investigated the potential influence of epigenomic variation on a variety of traits in numerous organisms (Feinberg and Irizarry 2010; Roux et al., 2011; Schmitz and Ecker 2012; Klironomos et al., 2013; Olsson et al., 2014; Kawakatsu et al., 2016). This includes some studies that directly compared genetic and epigenetic variation, but this is a relatively small set of typically genomic studies not inherently focused on specific traits known to be under selection in a wild species (Schmitz and Ecker 2012; Olsson et al., 2014; Kawakatsu et al., 2016). Thus, there is a need for studies that empirically compare the relative heritability and amount of variance created by standing genetic variation vs. epigenetic variation for traits known to be under field selection.
One tool that has been developed to measure how epigenetic variation may influence complex traits of potential adaptive benefit is epigenetic Recombinant Inbred Lines (epiRILs) (Johannes et al., 2009). In Arabidopsis thaliana, an epiRIL population was generated by a cross between Col-0 WT and a Col-0 ddm1 mutant. DDM1 encodes an ATP chromatin remodeler and its mutant shows a 30 to 70% decrease in different types of DNA methylation, CG, CHG and CHH (Johannes et al., 2009; Zemach et al., 2013). The progeny of this cross were backcrossed to Col-0 WT and subsequently went through several generations of selfing to develop a population of 122 lines. This generated a population where the lines contain specific and stably inherited Differentially Methylated Regions (DMRs). Thus, the epiRIL population is isogenic and the lines have distinct epigenetic marks. This allows associating phenotypic differences to DMRs within this population (Lippman et al., 2004). Moreover, since DMRs are stably inherited through generations, they can be used as markers to search for epigenetic quantitative trait loci (epiQTLs). Thereby, one can both quantify and identify specific epigenomic regions correlating with trait variation.
To compare the contribution of epigenetic and genetic variation to phenotypic variation in an adaptive trait, we measured the accumulation of glucosinolate defense compounds in the epiRIL population. Glucosinolates are predominantly produced within the order Brassicales that includes many important crops such as oilseed rape and cabbage. Glucosinolates are well studied and thus, the biosynthetic pathway is often used as a model adaptive pathway based on the elaborate knowledge of enzymes and regulatory elements involved (Prasad et al., 2012; Züst et al., 2012; Brachi et al., 2015; Kerwin et al., 2015). At least 40 glucosinolates exist in A. thaliana (Sønderby et al. 2010b) with the two major glucosinolate groups being indolic glucosinolates and aliphatic glucosinolates, the latter can be further divided into short chain (SC) and long chain (LC). Glucosinolate metabolism is regulated in response to many environmental factors including herbivores and pathogens and also abiotic factors such as light and water availability (Kliebenstein et al., 2005; Mewis et al., 2005; Gigolashvili et al., 2009; Huseby et al., 2013; Mewis et al., 2012; Züst et al., 2012). Glucosinolate responses to attack involve complex interactions between different signaling pathways (Kliebenstein et al., 2002a; Mewis et al., 2005; Dam et al., 2008; Frerigmann and Gigolashvili 2014; Burow et al., 2015). Glucosinolates provide an adaptive benefit in the field as they aid the plant’s response and adaptation to environmental changes and thus genetics and epigenetics may have been selected for in different environments (Prasad et al., 2012; Züst et al., 2012; Brachi et al., 2015; Kerwin et al., 2015; Kerwin et al., 2017).
While there is extensive information about the molecular, quantitative and evolutionary genetics of glucosinolates, there is little known about the potential for epigenetic variation to influence this pathway. We measured whether variation in DNA methylation within the epiRIL population correlates with the accumulation of different glucosinolates while simultaneously measuring adaptive flowering time trait to enable a comparison. Using a replicated randomized block design in multiple independent experiments, we quantified how variation in DNA methylation associates with the mean and the trait stability, within line variation, of these traits. This showed that epigenetic variation significantly correlates with glucosinolate accumulation and this variation differed based on the biosynthetic origins of the glucosinolates. Interestingly, there was no overlap in the epigenetic loci found to correlate with glucosinolates and the major known genes controlling natural variation in glucosinolates. Using a meta-analysis to compare the epigenetic variation in this population to literature measuring genetic variation in this trait, we showed that the influence of the epigenetic variation was dramatically lower than standing genetic variation. This was true across numerous Arabidopsis populations of different origins using both heritability and variance comparisons. Thus, selection on standing genetic variation will provide a stronger and faster response to selection than the detected epigenetic variation in the epiRILs.
Materials and Methods
Germplasm
122 A. thaliana epigenetic Recombinant Inbred Lines (epiRILs) generated in the Col-0 background and 4 Col-0 WT lines were purchased from the Versailles Arabidopsis Stock Center, Institut Jean-Pierre Bourgin. Website: http://publiclines.versailles.inra.fr/epirils/index (Johannes et al., 2009).
Experimental design
Growing of epiRILs was carried out in two independent experimental rounds. In each round, plants were grown in a randomized block design. This yielded a total of 768 plants per experiment and 1536 plants in total, leading to 12 randomized replicates of each epiRIL and 18 randomized replicates of each Col-0 WT lineage. Plants were cold-stratified for 4-6 days and subsequently grown in a light chamber for 21-22 days set to 80-120 μE/ (m2*s), 16 h light, 21°, 70% relative humidity. Flowering time was scored as day upon emergence of an inflorescence stem of at least 1 cm height.
Glucosinolate extraction
Sigma-Aldrich Millipore 96 well filter plates, cat.no. MSHVN45 were charged with 45 mg DEAE Sephadex A25 and 300 µl of water per well and equilibrated at room temperature for minimum 2 hr. The water was removed using a vacuum manifold (Millipore). At day 21-23 day of growing, rosette tissue was harvested, weighted and freeze-dried before the tissue was homogenized with two stainless steel balls by shaking for 2 min at a frequency 1/30 Hz on a Mixer Mill 303 (Retsch, Haan, Germany). Glucosinolates were extracted in 300 µl 85% MeOH (v/v) containing 5 nmol p-OH-benzyl glucosinolate (extracted from seeds of Sinapis alba, SeedCom A/S, Vissenbjerg, Denmark as previously described (Thies 1979; Zrybko et al., 1997) as an internal standard. Samples were centrifuged, the supernatants were applied to the filter plates and absorbed on the ion exchanger by vacuum filtration for 2-4 s. Sephadex material was washed with 2x 100 ml 70% methanol (v/v) and 2x 100 µl water and briefly centrifuged before addition of 20 µl of sulfatase solution (1.25 mg/ml, sulfatase type 1H, Sigma-Aldrich) per sample. After incubation at room temperature over-night, desulfo-glucosinolates were eluted with 100 µl water (Kliebenstein et al., 2001b).
Glucosinolate analysis by UHPLC/TQ-MS
1 µL of a 1:10 dilution of glucosinolates were analyzed as desulfo-glucosinolates by UHPLC/TQ-MS on an Advance-UHPLC/EVOQElite-TQ-MS instrument (Bruker) equipped with a C-18 reversed phase column (Kinetex 1.7 u XB-C18, 10 cm × 2.1 mm, 1.7 µm particle size, Phenomenex) by using a 0.05% formic acid in water (v/v) (solvent A)-0.05% formic acid in acetonitrile (v/v) (solvent B) gradient at a flow rate of 0.4 ml/min at 40°. The gradient applied was as follows: 2% B (0.5 min), 2–30% (0.7 min), 30–100% (0.8 min), 100% B (0.5 min), 100–2% B (0.1 min), and 2% B (1.4 min). Compounds were ionized by ESI with a spray voltage of +3500 V, heated probe temperature 400°, cone temperature 250°. Desulfo-glucosinolates were monitored based on the following MRM transitions: 3-methylthiopropyl (3mtp), (+)328 > 166 [5V]; 3-methylsulfinyl (3msp), (+)344 > 182 [10V]; 4-methylthiobutyl (4mtb), (+)342 > 132 [15V]; 4-methylsulfinylbutyl (4msb), (+)358 > 196 [5V]; 5-methylsulfinylpentyl (5msp), (+)372 > 210 [5V]; 7-methylthioheptyl (7mth), (+)384 > 222 [5V]; 7-methylsulfinylheptyl (7msh), (+)400 > 238 [7V]; 8-methylthiooctyl (8mto), (+)398 > 236 [5V]; 8-methylsulfinyloctyl (8mso), (+)414 > 252 [5V]; p-hydroxybenzyl (pOHB), (+)346 > 184 [10V] (internal standard). N- and 4-methoxy-indol-3-ylmethyl glucosinolate were distinguished based on retention times in comparison to those of known standards. Absolute quantification of the individual glucosinolates was based on response factors relative to pOHB calculated using standard curves in control extracts.
Genetically varying populations
To compare the epiRIL variation in glucosinolates with that found in other genetic populations, we obtained foliar glucosinolate measurements from previous Arabidopsis populations that were measured at the same time under similar growth conditions using the same protocols. This minimizes the potential for technical or biological variation to dramatically influence the comparison. This included data from four previously published biparental RIL populations, Bay-0 x Sha-0 (Wentzell et al., 2007), Kas x Tsu (Joseph et al. 2013), Ler x Cvi (Kliebenstein et al., 2001a) and Ler x Col-0 (Kliebenstein et al., 2002a; Kliebenstein et al., 2002b). We also utilized two previously published datasets from collection of Arabidopsis accessions that was used for genome wide association mapping (Chan et al., 2010; Chan et al., 2011). Other populations measured for glucosinolates were not utilized because they either measured different tissues, did not measure absolute or relative glucosinolates preventing an ability to compare or did not provide the population mean data (Pfalz et al., 2007).
Statistics data visualization
All statistics were done using R version 3.3.2 (R Core Team 2016) and R studio version 0.99.491 (RStudio Team 2015). The package “car” (Fox and Weisberg 2011) and doBy was to conduct ANOVA type II to test for heritability and obtain means for the different phenotypes.
To test for heritability and obtain means for the different phenotypes, we utilized ANOVA and type II sums-of-squares based F tests using the package “car” (Fox and Weisberg 2011) and “doBy” (Højsgaard and Halekoh 2016). The model used was Phenotype = EpiRIL+Experimental_round+Tray:Experimental_round +EpiRIL:Experimental_round:Tray.
The within genotype coefficient of variation for the phenotypes was calculated separately within each of the two planting rounds, and was calculated by dividing the standard deviation for the phenotype across the individuals for each lines individuals by the mean of these same individuals. We then tested for heritability using the following model; Phenotype CV = epiRIL+Experimental_round.
Data visualization
Figures 1 to 4 and Figure S6 were generated in R version 3.3.2 (R Core Team 2016) and R studio version 0.99.491 (RStudio Team 2015) using the package ggplot2 (Wickham 2009). EpiQTL plots were generated from Windows QTL Cartographer Version 2.5 (Wang et al., 2012).
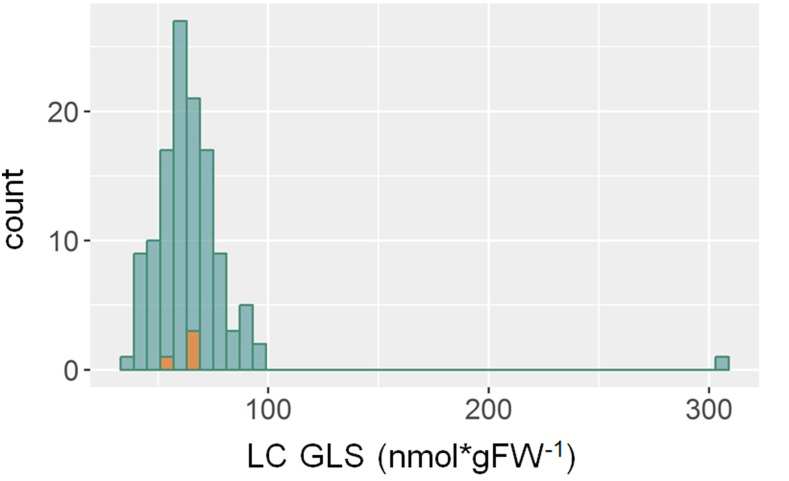
Figure 1.
Distribution of mean LC glucosinolates among the epiRILs showing the outlier epiRIL 573. A histogram of the mean LC glucosinolate content among the 122 epiRILs is shown in green. The orange bars represent the corresponding histogram for the four WT lines.
Composite Interval epi-QTL mapping and statistical analysis of main-effect epi-markers
EpiMarkers were obtained for the 122 epiRILs from http://publiclines.versailles.inra.fr/epirils/index (Colome-Tatche et al., 2012). Windows QTL Cartographer Version 2.5 (Wang et al., 2012) was used to conduct Composite Interval Mapping (CIM) (Jansen and Stam 1994; Zeng 1994). To assess significance, we conducted 1000 permutations and called significant peaks above the permutation estimated significance threshold for each trait (Churchill and Doerge 1994; Doerge and Churchill 1996). To test main effect epiMarkers and potential interactions with environment, we chose the epiMarker nearest each epiQTL peak and used this in a model tested by ANOVA in R version 3.3.2 (R Core Team 2016) using the package “car” (Fox& Weisberg, 2011 and “doBy” (Højsgaard and Halekoh 2016). Individual models were run for means and CVs on groups of SC glucosinolates, LC glucosinolates, indolic glucosinolates and flowering time. The models tested all main effect epiMarkers linked to epiQTLs for the class of phenotypes and the interaction of each marker with the experimental round.
Data availability
File S1 contains phenotyping data for each epiRIL, i.e., mean and CV for glucosinolate traits and flowering time.
Results
Experimental design
To test for potential epigenetic regulation of glucosinolate accumulation, we measured glucosinolate content in the ddm1 derived isogenic epiRIL population (Johannes et al., 2009). We measured glucosinolate accumulation in each line using multiple independent randomized replicates in two independent experiments. This increased the precision of the measurement on each line’s mean and produced a direct measurement of glucosinolate variation across replicates within each line as a distinct trait using the coefficient of variation (CV). We also measured flowering time in all individuals to enable a comparison with another adaptive trait previously measured in the same set of epiRILs and a collection of different genetic populations (Johannes et al., 2009; Atwell et al., 2010; Chiang et al., 2009; Cortijo et al., 2014; Kooke et al., 2015).
Glucosinolate content and flowering time were measured on all 12 individual replicates of each of the 122 epiRILs and 18 individual replicates of four independent WT lines (Johannes et al., 2009). All of the lines were grown in two experiments with 6 biological replicates per epiRIL per experiment and 9 biological replicates per WT line per experiment. This generated a total of 1536 plants to be analyzed for all of the phenotypes. For statistical analysis, glucosinolates were divided into specific biosynthetic groups of SC and LC aliphatic and indolic glucosinolates because these trait groups have previously been shown to have independent genetic, biosynthetic and regulatory control (Sønderby et al., 2010a) (a list of all glucosinolates measured in each group is shown in Table S1).
“Search for Outliers” Analysis of Glucosinolate and Flowering Time in EpiRILs
We first surveyed the phenotypic distributions for extreme outliers (>5 SD outliers) (Haughn et al., 1991; Kliebenstein et al., 2007) to test for the possibility of large effect Mendelian mutations as these may have arisen from an increased rate of transposable element transposition in the epiRILs caused by lower levels of DNA-methylation (Miura et al., 2001; Kato et al., 2004; Johannes et al., 2009). The only phenotype that showed this level of outlier was the accumulation of LC glucosinolates where there was one epiRIL (573) with an extreme phenotype and thus potentially carrying a Mendelian mutation (Figure 1).
To test if epiRIL 573 had a potential Mendelian mutation, we back-crossed it to Col-0 WT and the F1 progeny was selfed to generate an F2 population. The F1 population displayed the WT phenotype and the F2 population showed a 3:1 segregation suggesting that the outlier LC glucosinolate phenotype in epiRIL 573 was caused by a single recessive Mendelian locus (217 plants with WT phenotype and 53 plants with epiRIL573 phenotype in F2). Because this phenotypic outlier is a unique rare outlier and potentially genetic in nature, epiRIL 573 was removed from further analysis of both glucosinolates and flowering time as a rare outlier would conflate genetic and epigenetic variance estimates in the quantitative analysis. No similar rare outliers were observed in any other phenotypes across the epiRIL population, and thus the final epiRIL population consisted of 121 epiRILs compared to 4 WT lines (Figure 2).
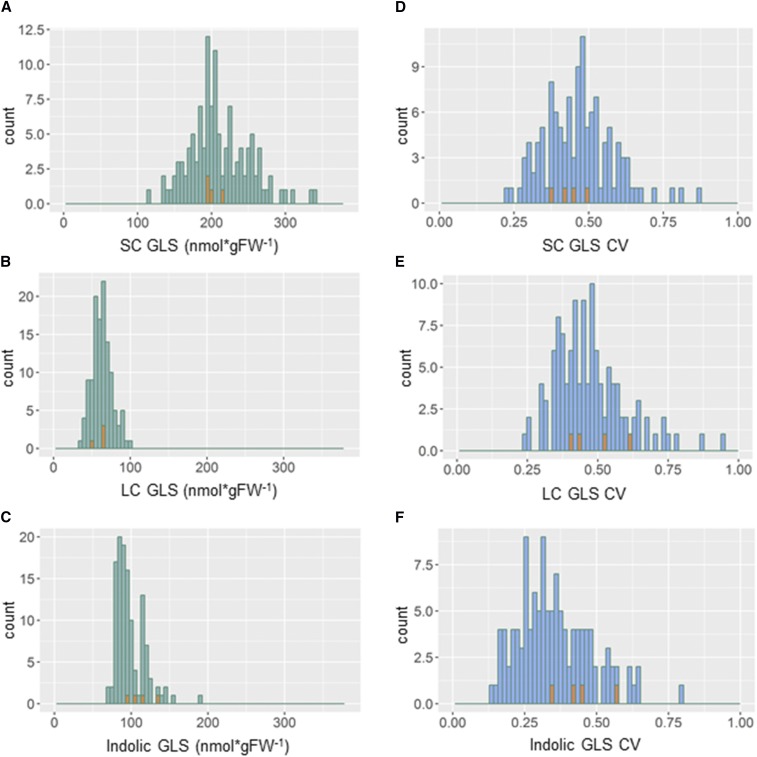
Figure 2.
Distribution of mean and within line (CV) glucosinolate (GLS) traits among the epiRILs. Green bars show the distribution of the mean traits among the epiRILs, blue bars show the histogram of the within line variation among the epiRILs as measured by CV. Orange bars represent the distribution of the corresponding four WT lines for the specific trait shown. A) LSmean SC glucosinolate content, B) LSmean LC glucosinolate content, C) LSmean Indolic glucosinolate content, D) Within line CV for SC glucosinolates, E) Within line CV for LC glucosinolates, F) Within line CV for Indolic glucosinolates.
Variation in DNA Methylation Significantly Correlates With Mean Glucosinolate Accumulation
Phenotypic variation in epiRILs is assumed to mainly associate with heritable variation in DMRs (Johannes et al., 2009). As such, any heritability of phenotypic variation within this population is likely due to epigenetic variation. For all tested groups of glucosinolates, variation in DMRs significantly correlates with glucosinolate variation (Table 1). Linear modeling was utilized to estimate the potential epigenetic heritability (epiheritability) within this population for all measured groups of glucosinolates (Table 1). Glucosinolate variation attributable to epiheritability ranged between 10% and 13% and was thus significantly lower than that found in populations looking at natural genetic heritability of glucosinolates. In these populations, both RILs and GWAS collections, the genetic heritability ranged from ∼30–70% for indolic glucosinolates and ∼40–80% for aliphatic glucosinolates (Kliebenstein et al., 2001a; Wentzell et al., 2007; Chan et al., 2010; Chan et al., 2011; Joseph et al. 2013). Glucosinolate epiheritability was also lower than epiheritability for flowering time in our experiments, 32% (Table 1). This estimate of flowering time epiheritability is similar to other experiments with the exact same population suggesting that there are no major environmental or technical issues affecting our epiheritability estimates (Johannes et al., 2009; Cortijo et al., 2014; Kooke et al., 2015). Thus, epiheritability of glucosinolate accumulation is lower than that found for flowering time and the genetic heritability found in different genetic populations.
Table 1. Epiheritability of mean glucosinolate content and flowering time in the EpiRILs. The Table shows the significant impact of variation between the epiRILs and experimental terms (experiment and tray) as well as interactions on measured traits using a linear model. The top of the Table shows the significance as determined from ANOVA for each term and trait epiheritability (epiRIL variance divided by total variance) is shown on the bottom row.
| SC | LC | Indolic | FT | |
|---|---|---|---|---|
| EpiRILs | 0,003 | 0,016 | <0,001 | <0,001 |
| Experiment | 0,002 | NS | <0,001 | <0,001 |
| Tray | <0,001 | <0,001 | <0,001 | <0,001 |
| ID:Experiment | NS | 0,001 | <0,001 | <0,001 |
| ID:Tray | NS | NS | NS | <0,001 |
| H2 | 0,105 | 0,095 | 0,131 | 0,319 |
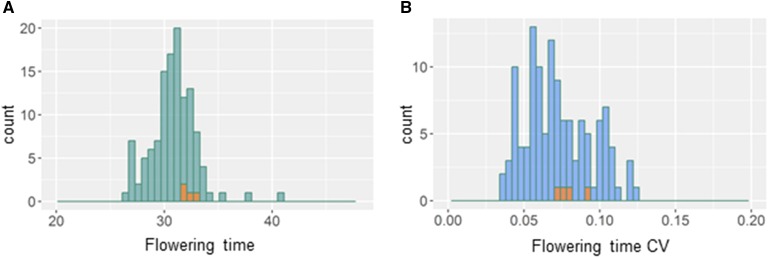
Using the linear model, we obtained the means for each trait in each line for further analysis. The phenotypic distribution of glucosinolate content varied between aliphatic (SC and LC) and indolic glucosinolates. SC and LC glucosinolates showed Gaussian distributions in the epiRIL population (Figure 2A, B) whereas indolic glucosinolates had a bimodal distribution (Figure 2C). SC glucosinolate phenotypes were centered around the WT lines and ranged from 113 to 341 nmol/g. LC glucosinolate content also centered around the WT lines and varied from 37 to 98 nmol/g. Indolic glucosinolate content bi-modally varied from 69 nmol/g to 187 nmol/g with two peaks at 85 nmol/g and at 115 nmol/g (Figure 2C). The four independent WT lines were dispersed across this distribution and did not correlate with the two epiRIL peaks. Flowering time varied across the epiRILs from 26 days to 41 days and had a Gaussian distribution (Figure 3A). In contrast to aliphatic glucosinolates, the distribution of flowering times among epiRILs did not center around the WT sample lines, but instead showed a high skew toward earlier flowering time with only a few epiRILs flowering later than WT. This suggests that variation in DMRs within this background can equally decrease and increase glucosinolate content but shows a bias toward earlier flowering time. As such, there is a bias in the directionality of how ddm1-mediated methylation changes associates with the glucosinolate and flowering time phenotypes.
Figure 3.
Within line distribution of flowering time mean and CV. Green bars show the distribution of the mean flowering time among the epiRILs, blue bars show the histogram of the within line variation in flowering time among the epiRILs as measured by CV. Orange bars represent the distribution of the corresponding four WT lines for the specific trait shown. A) Mean Flowering time, B) Within line CV for Flowering time.
Correlation Between DNA Methylation variation and Within Line Variation in Glucosinolate Accumulation
We also tested how DMR variation correlates with phenotypic stability within a line using the within line coefficient of variation (CV). When looking at line mean only, we are blind to the dispersion of the replicates of a line, which could potentially play an important role in adaption (Hall et al., 2007; Ordas et al. 2008). The experimental design allowed us to obtain independent measures of the within line CV per epiRIL and directly test if DMR variation correlates with this dispersion. While epiheritability of within line CV was not statistically significant for SC and indolic glucosinolates or flowering time, there was a suggestive difference for LC glucosinolates (Table 2). Interestingly, the estimated epiheritability of within line CV was higher than that found for the mean. For glucosinolates it ranged between 36% and 51% and flowering time showed 41% heritability for trait CV. This estimate of within line CV epiheritability is very similar to that found for genetic heritability of within line CV in Arabidopsis for the phenotype (Joseph et al. 2014). The lower statistical significance for the within line CV epiheritability in comparison to the mean is likely because there were only two independent measurements, one per experiment, for this trait in comparison to 12 independent measurements for the mean (Table 1).
Table 2. Epiheritability of within line variation as measured by CV for glucosinolate content and flowering time. The Table shows the significant impact of variation between the epiRILs and experiments on measured traits using a linear model. The top of the Table shows the significance as determined from ANOVA for each term and trait epiheritability (epiRIL variance divided by total variance) is shown on the bottom row.
| SC | LC | Indolic | FT | |
|---|---|---|---|---|
| EpiRILs | NS | 0,064 | NS | NS |
| Experiment | <0,001 | <0,001 | <0,001 | <0,001 |
| H2 | 0,474 | 0,507 | 0,360 | 0,406 |
Using the mean for the within line CV for all the epiRILs showed that the trait distributions for all glucosinolate groups appeared similar (Figure 2D, E, F). All were Gaussian distributed, ranged between 0,2 and 1 and had a peak around 0,5. WT lines were in the peak of epiRILs for SC- and LC glucosinolates and appeared slightly skewed toward higher CV for indolic glucosinolates. Thus, while the trait distributions for the mean accumulation of the different glucosinolates differed in epiRILs, the within line CV was very similar across all of the glucosinolates (Figure 2A-E). Within line CV for flowering time was overall lower than for glucosinolates (Figure 3B). CV values ranged between 0,03 and 0,4 and showed a more dispersed distribution than aliphatic glucosinolates (SC and LC glucosinolates). Thus, within line CV for different traits appears to be associated with DMRs within this population. Moreover, also for CV, flowering time and glucosinolates are distinct traits from each other and the ddm1 epi-QTL are not pleiotropically altering the entire plant.
Comparing Genetic and Epigenetic Influence on Glucosinolate Population Variation
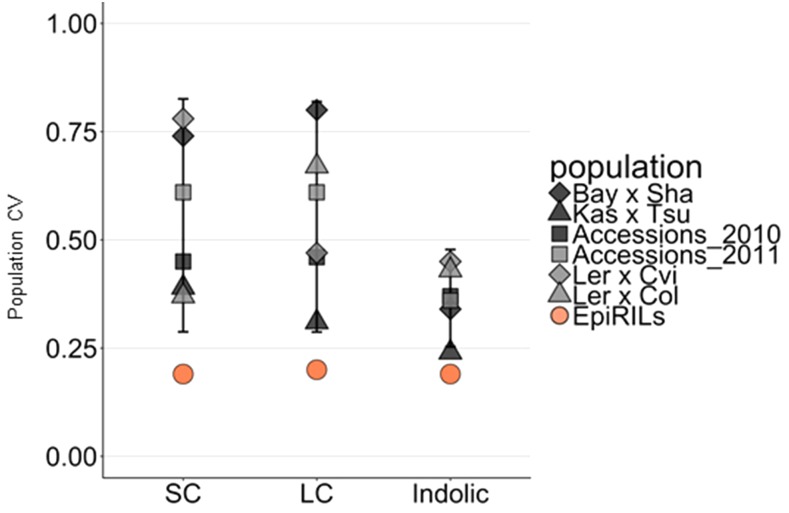
Previous publications using the same experimental design to measure genetic variation in glucosinolates allowed for a direct comparison with the DMR variation (Kliebenstein et al., 2001a; Kliebenstein et al., 2002b; Wentzell et al., 2007; Chan et al., 2010; Chan et al., 2011; Joseph et al. 2013). In combination with the previous comparison of heritability to epiheritability, this can give insight to whether the epigenetic influence on phenotypic variation was similar or different from genetic influence. To facilitate this meta-comparison, we calculated the population CV (an estimate of the range of phenotypic variance) for each trait across the epiRIL and genetic populations using the mean trait values. Using the population CV, we compared the epiRIL population trait variation to the trait variation in four RIL populations based on crosses of Bay x Sha, Kas X Tsu, Ler x Cvi and Ler x Col (Kliebenstein et al., 2001a; Kliebenstein et al., 2002a; Kliebenstein et al., 2002b; Wentzell et al., 2007; Joseph et al. 2013). In addition to the RIL populations, we compared the epiRIL population to two studies on A. thaliana accessions (Chan et al., 2010; Chan et al., 2011) (for details on populations, see materials and methods). For all groups of glucosinolates, the epiRILs had the lowest population CV (around 20%), which was below the 99 percentile confidence interval calculated for genetic variation using the different RIL and accession populations (Figure 4). This shows that the DMR variation caused by the ddm1 mutation within the epiRIL variation associates only with a small fraction of the variation possible from natural genetic variation.
Figure 4.
Meta-analysis of population level variation between epiRILs and genetic populations. The variation within the population (population CV) for mean glucosinolate traits is shown for epiRILs or genetic RILs or genetic association mapping populations are shown. The SC, LC and indolic traits are shown on the x-axis. Error bars for each glucosinolate mark the 99th percentile confidence interval for the genetically variable populations (RILs and accessions).
Besides being lower than the other populations, the epiRIL population was also the only population showing the same level of population CV for all glucosinolate groups. The genetically differing populations had varying population CVs for the different glucosinolate biosynthetic groups with overall lower indolic glucosinolate population CV. Similarly, the flowering time population CV in the epiRIL population was dramatically lower than that found in a collection of accessions, 7% epigenetic population CV and 62% for accessions (Atwell et al., 2010). This points to the potential epigenetic contribution to phenotypic variation caused by the DMR variation being much lower than that found for genetic variation for both glucosinolates and flowering time.
Mapping EpiQTLs for Glucosinolate Levels
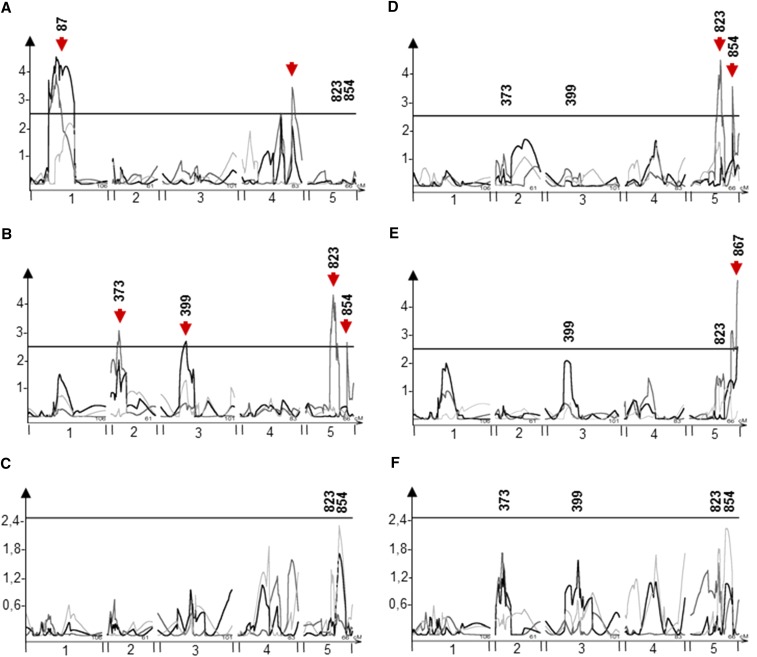
To identify DMRs linked to the observed variation of glucosinolate accumulation, we mapped epigenetic quantitative trait loci (epiQTLs) in the epiRIL population. We used the available map of 126 DMRs for the 121 epiRILs to locate epiQTLs (Colome-Tatche et al., 2012) and performed composite interval mapping (CIM) per experiment and across experiments with 1000 permutations to test for significance. All individual glucosinolates and the pooled data for each biosynthetic group were used to map epiQTLs. As shown for SC glucosinolates, epiQTLs were found for 3MTP, 3MSP, 4MSB and 5MSP glucosinolate accumulation (Figure 5A, B, D, E). Some epiQTLs had broad affects across a set of metabolites such as one epiQTL on chromosome 5 that showed up for 3MSP, 4MSB and 5MSP glucosinolates (Figure 5B, D, E). In contrast, other epiQTLs were specific for a single glucosinolate as in the case of epiQTLs on chromosome 2 and 3 that only showed up for 3MSP (Figure 5B). After locating epiQTLs, we used a linear modeling approach involving all identified epiQTLs to identify the marker with the lowest P-value for each epiQTL. We included the total list of candidate epiQTLs identified for all individual SC glucosinolates and pooled SC glucosinolates within the linear model. This enabled the identification of significant loci not identified using the CIM approach, such as identified 3MSP epiQTL on chromosome 3 centered on marker 399 (Figure 5B); with the modeling approach, this locus was also found to alter the accumulation of 4MSB, 5MSP and pooled SC glucosinolates suggesting that it is a locus altering the accumulation of all sulfinyl SC glucosinolates (Figure 5B, D, E, F). In contrast, an epiQTL on chromosome 5 centered on marker 823 influenced variation of all SC glucosinolates, both methylsulfinyl and methylthiol. In summary, markers were found that linked to all SC glucosinolates, while others were specific for either one SC glucosinolate or subgroups of SC glucosinolates as in the case of methylsulfinyls. LC glucosinolates showed similar patterns, as marker 1 was specific to methylthiols, whereas marker 373 influenced all individual glucosinolates (Figure S1). Indolic glucosinolates showed a slight different pattern when looking at the individual glucosinolates in the biosynthetic group (Figure S2). Most markers were specific to one indolic glucosinolate, whereas marker 859 was significant for all except NMOI3M. NMOI3M did show an epiQTL in this region, but this epiQTL was rejected in the subsequent modeling. Thus, we identified epiQTLs that associated either with an array of glucosinolate accumulation or specific subsets. This blend of specific and general epiQTLs is similar to observations found for QTL analysis of natural genetic variation (Kliebenstein et al., 2001a; Wentzell et al., 2007; Joseph et al. 2014).
Figure 5.
EpiQTL mapping of SC glucosinolate means. Plots show composite interval mapping results and significance estimated from 1000 permutations. The x-axis shows the genome by chromosome and the y-axis shows the LOD score. The significance thresholds are plotted for each trait and significant QTL are re marked with red arrows. The light gray line shows the QTL map using the means from experiment 1, dark gray shows experiment 2, and black lines represent the pooled data from experiment 1 and 2. Marker names show the position of significant makers after using a linear model to assess loci. EpiQTLs not assigned a marker were rejected in the subsequent ANOVA. Glucosinolate abbreviations are shown in Table S1. Analyzed SC glucosinolates are A) 3MTP, B) 3MSP, C) 4MTB, D) 4MSB, E) 5MSP, F) Pooled SC glucosinolates.
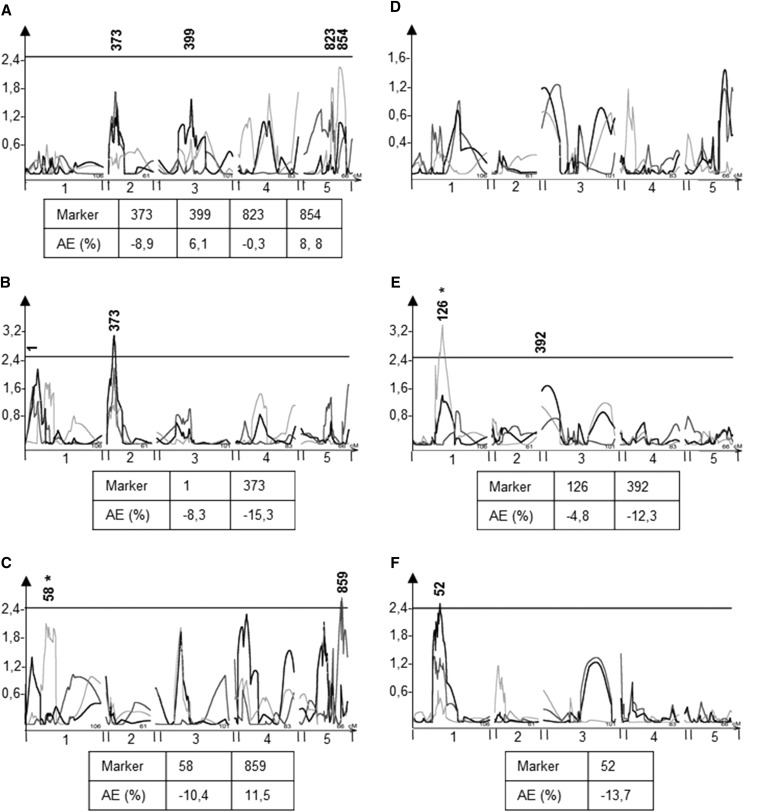
To calculate the effect size of these found epiQTLs, we estimated the additive effect as the percentage difference in phenotypic means of plants when the methylation status of the epiQTL was as the ddm1 parent compared to same marker originating from WT (Figure 6). For SC glucosinolates, marker 399 and 854 correlated with increased glucosinolate accumulation when originating from ddm1 compared to WT, while marker 373 and 823 correlated with decreased glucosinolate accumulation when originating from ddm1 (Figure 6A). Marker 373 also correlated with decreased LC glucosinolate accumulation albeit with a larger effect for LC glucosinolates (Figure 6A, B). Markers found for indolic glucosinolate means also correlated with both increases and decreases in additive effect (Figure 6C), as the two markers, 58 and 859, had a respective a 10,4% decrease and 11,5% increase of the ddm1 derived allele compared to WT (Figure 6C). Thus, the ddm1 derived epialleles associated with a mixture of positive and negative effects on aliphatic glucosinolate accumulation further showing that DDM1-controlled methylation variation can correlate with both increase and decrease in glucosinolate accumulation.
Figure 6.
EpiQTL mapping of glucosinolate mean and CV. Plots show composite interval mapping results and significance estimated from 1000 permutations. The x-axis shows the genome by chromosome and y-axis shows the LOD score. The significance thresholds are plotted for each trait and significant QTL are re marked with red arrows. The light gray line shows the QTL map using the means from experiment 1, dark gray shows experiment 2, and black lines represent the pooled data from experiment 1 and 2. Marker names show the position of significant makers after using a linear model to assess loci. EpiQTLs not assigned a marker were rejected in the subsequent ANOVA. An asterisk after a marker shows that the marker was not significant in both experiments. Beneath plots are shown the additive effect of markers, i.e., the percentage phenotypic change when the marker is ddm1 within the epiRIL lines compared to WT. A) Mean SC glucosinolate content, B) Mean LC glucosinolate content, C) Mean Indolic glucosinolate content, D) Within line CV for SC glucosinolates, E) Within line CV for LC glucosinolates, F) Within line CV for Indolic glucosinolates.
Flowering time epiQTLs
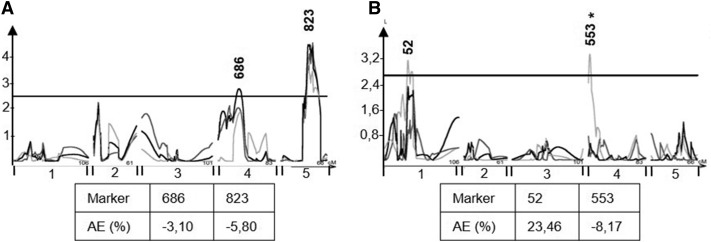
As a control to compare the epiRIL population with previous publications, we mapped epiQTLs associated with flowering time as described above (Figure 7). This analysis identified two epiQTLs for flowering time that centered on marker 686 on chromosome 4 and marker 823 on chromosome 5 which are the same makers as previously identified to correlate with flowering time (Figure 7A) (Cortijo et al., 2014, Kooke et al., 2015). The additive effect on flowering time was a 3,1% decrease for the ddm1 derived allele at marker 686 and 5,8% decrease for the ddm1 derived allele at marker 823. Compared to previous studies (Cortijo et al., 2014; Kooke et al., 2015), both markers showed less correlation with the phenotype in our experiment. The fact that we identified the same flowering time loci as previously found suggests that this population is behaving as in previous studies allowing us to infer that any differences between glucosinolates and flowering time effects in this population are not the result of differences between laboratories and experimental designs. Further, this shows again that DDM1-mediated methylation variation correlates with earlier flowering in this population.
Figure 7.
EpiQTL mapping of flowering time mean and CV. Plots show composite interval mapping results and significance estimated from 1000 permutations. The x-axis shows the genome by chromosome and y-axis shows the LOD score. The significance thresholds are plotted for each trait and significant QTL are re marked with red arrows. The light gray line shows the QTL map using the means from experiment 1, dark gray shows experiment 2, and black lines represent the pooled data from experiment 1 and 2. Marker names show the position of significant makers after using a linear model to assess loci. An asterisk after a marker shows that the marker was not significant in both experiments. Beneath plots are shown the additive effect of markers, i.e., the percentage phenotypic change when the marker is ddm1 within the epiRIL lines compared to WT. EpiQTLs not assigned a marker were rejected in the subsequent ANOVA. A) Mean Flowering time, B) Within line CV for Flowering time.
EpiQTLs Identified for Within Line Trait Variance
The replicated experimental design allowed for the direct measurement of variation between replicates of the same epigenotype as a phenotype that can be mapped to assess if variation in epigenetic marks associates with the stochastic variation or robustness of specific lines. Using phenotypic CV within each line as the measure of variation between replicates, we found epiQTLs that link to within line dispersion for all of the measured traits except SC glucosinolates (Figure 6D, F, Figure S3, Figure S4, Figure S5). For LC glucosinolates, we identified marker 126 on chromosome 1 and marker 392 on chromosome 3 (Figure 6E). Both of these loci have additive effects whereby the ddm1 derived allele links to lower within line variance. Critically, neither of these LC glucosinolate CV markers correlated with the average accumulation of LC glucosinolates within the epiRIL population suggesting that as with genetic QTL, epiQTLs can be specific for either mean glucosinolate accumulation or within line variation showing that these are as affected as separate traits by DDM1-mediated epigenetic marks (Figure 6B, E). In contrast to LC glucosinolate CV, the only identified CV epiQTL for indolic glucosinolates, marker 52 on chromosome 1 was in a region also correlating with indolic glucosinolate mean accumulation (Figure 6C, F). For indolic glucosinolate means however, it was the neighboring marker 58 explaining most of the epiQTL. The two markers are situated ∼200 kbp away from each other indicating that they may be different loci.
Similar to LC glucosinolate CV, two epiQTLs were identified for flowering time CV and these did not link to mean flowering time (Figure 7A, B). These epiQTLs for flowering time CV have not been previously identified (Kooke et al., 2015). Taken together, it is possible to find epiQTLs that associate both within line (CV) and between line variation of glucosinolate accumulation and flowering time. Further, this indicates that DDM1-mediated DNA methylation can influence phenotypic stability within individual lines.
EpiQTLs are Largely in Genomic Regions Unknown for Glucosinolate Phenotypes
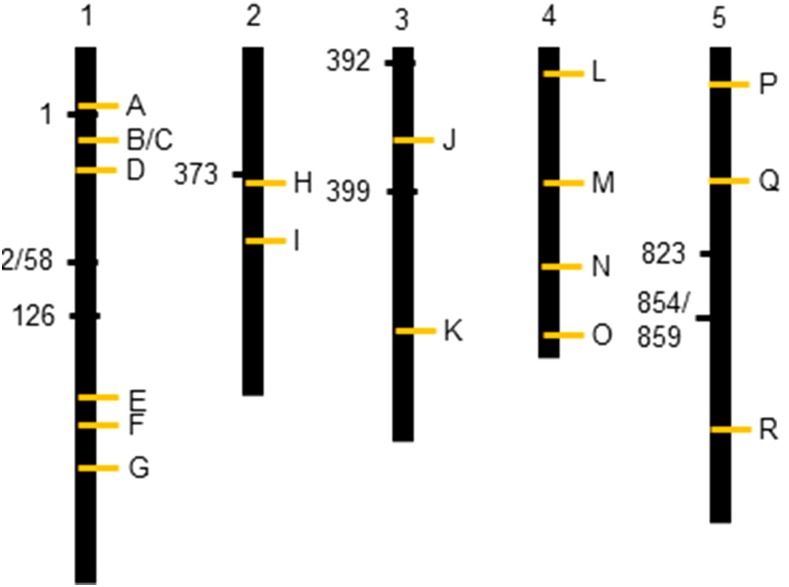
To test if any of the identified epiQTLs may co-locate with known glucosinolate genes, we compared the position of significant epiQTLs with a large collection of known glucosinolate biosynthetic and major regulatory genes (Figure 8). The vast majority of epiQTLs had no overlap with known glucosinolate genes suggesting that they are linked previously unknown causal loci (Figure 8, see marker 52, 58, 126, 392, 823 and 854). Three markers, 373, 399 and 859, were in proximity to glucosinolate loci but the marker closest to the glucosinolate gene was not having the highest LOD score, suggesting DNA methylation of other genetic regions correlating with the phenotype. Marker 1 was the only marker that appeared to link to a known glucosinolate gene as it is located between the CYP79F1/2 and FMO GS-OX5 enzymatic genes. Marker 1 is located >1.2 Mbp away from the CYP79 locus, which is too far to indicate a link between the two. However, it is approximately 200 kbp away from FMO GS-OX5. FMO GS-OX5 is involved in LC glucosinolate synthesis and marker 1 was identified for LC glucosinolates. Together, this might indicate that the methylation status of FMO GS-OX5 is linked to the LC glucosinolate variation observed in epiRILs. To test whether marker 1 associates with this gene, we plotted the additive effect of marker 1 on the ratio between methylthio glucosinolates (7MTH and 8MTO) and total LC glucosinolates which represents a direct approximation of this enzymes function (Figure S6) (Li et al., 2008). This did not point to a correlation between GS-OX5 and marker 1 as the difference in tested ratio levels did not show a significant difference when marker 1 originated from ddm1 compared to WT. Thus, the epiQTLs found are large epigenomic blocks and thus can span many genes. This makes it impossible to precisely define what is correlating to the traits without doing a fine-scale mapping. We can however, conclude that the identified epiQTLs do not appear to link to known glucosinolate genes suggesting that the ddm1 derived epiQTLs are linked to a different suite of genes. This is in contrast to QTL for natural genetic variation of glucosinolates wherein a large number of the loci are due to causal polymorphisms in the biosynthetic enzymes and key regulatory genes (Kliebenstein et al., 2001a; Wentzell et al., 2007).
Figure 8.
Genomic position of significant epiQTL markers and glucosinolate genes. The markers associated with significant epiQTL are shown to the left of each chromsome. Letters denote glucosinolate genes: A, AT1G12140 FMO GS-OX5. B, AT1G16410 CYP79F1 and AT1G16400 CYP79F2. C, AT1G18590 SOT17. D, AT1G24100 UGT74B1. E, AT1G62540 FMO GS-OX2 and AT1G62560 FMO GS-OS3 and AT1G62570 FMO GS-OX4. F, AT1G65860 FMO GS-OX1. G, AT1G74100 SOT16 and AT1G74090 SOT18. H, AT2G20610 SUR1. I, AT2G31790 UGT74C1. J, AT3G19710 BCAT4. K, AT3G49680 BCAT3. L, AT4G03060 AOP2 and AT4G03050 AOP3. M, AT4G13770 CYP83A1. N: AT4G30530 GGP1. O, AT4G39950 CYP79B2 and AT2G22330 CYP79B3. P, AT5G07690 MYB29 and AT5G07700 MYB76. Q, AT5G23010 MAM1 and AT5G23020 MAM3. R, AT5G61420 MYB28.
Discussion
Recent work has implied that epigenetic marks may play a role in the inheritance and evolution of adaptive traits (Roux et al., 2011; Cortijo et al., 2014; Jia et al., 2015; Kooke et al., 2015; Rosa et al., 2016). In this study, we utilized an epiRIL population that varies in DDM1 ediated DNA methylation to test for the potential to influence the heritability of a set of adaptive traits in Arabidopsis thaliana; glucosinolate defense compounds and flowering time (Johannes et al., 2009). We show that both glucosinolates and flowering time display significant epiheritability, meaning that that DMRs associate with the heritable variation of these adaptive traits (Table 1, Table 2). Moreover, there were specific epiQTLs that were unique for each adaptive trait showing that the epiheritability can alter specific subsets of adaptive traits and that they are not globally pleiotropic. Similar to genetic variation, it was possible to find epigenomic regions correlating with the variation of both mean variation between lines and stochastic variation within lines (CV) and some of these regions were specific for controlling the within line variance (Figure 6). As such, epigenetic variation has the potential to influence the inheritance of these adaptive traits.
Existing literature that measured the standing genetic variation affecting these same adaptive traits in Arabidopsis thaliana using the same experimental design and conditions provided the ability to directly compare genetic variation and DDM1-mediated epigenetic variation (Kliebenstein et al., 2001a; Kliebenstein et al., 2002b; Wentzell et al., 2007; Chan et al., 2010; Chan et al., 2011; Joseph et al. 2013). Genetic heritability in both RIL populations and accession collections was dramatically higher than that found in the epiRIL population. Further, the epiRIL population had a significantly lower range of phenotypic variation than the genetic populations (Figure 4); the epigenetic variation in the epiRIL population was ranging between 1,2 to 4 fold lower than the standing genetic variation for the same trait. Thus, if the epiRIL population provides an accurate representation of the potential for epigenetics to influence adaptive trait variation, the standing genetic variation provides a vastly larger pool of phenotypic diversity that is also of higher heritability. As such, selection on this standing genetic variation will provide both a stronger and faster response to selection than the epigenetic variation in the epiRILs. Proving this hypothesis however requires future studies in other epiRIL populations that vary either for different individual epigenetic marks or for a blend of epigenetic marks simultaneously. The large level of standing genetic variation for these traits argues that parsing out the more subtle epigenetic influences in existing genetic populations will be complicated.
Phenotypic Variation in EpiRILs and Potential Mechanistic Insight
The distribution of aliphatic glucosinolates in the epiRILs (Figure 2A, B) showed a Gaussian distribution centered around the WT values. This shows that DMRs link to both positive and negative changes in the accumulation of aliphatic glucosinolates. In contrast, flowering time distributions of epiRILs showed a skew toward earlier flowering compared to WTs (Figure 3A). This might be the overall reduction in DNA methylation levels in the epiRIL that correlates with this earlier onset of flowering time. This suggests that there is a fundamental difference in how DDM1 methylation is linking to these adaptive traits. The observation that DMR variation in epiRILs only associates with earlier flowering times suggests that this methylation may contribute to the irreversible switch like behavior of flowering, which cannot reverse when having started the process of flowering. Previous studies also showed that epiRILs flowered earlier than the parental Col-0 controls grown along when making the epiRILs (Johannes et al., 2009, Table S3). However, the ddm1 mutant parent has been shown to flower either earlier or later than WT parent making the direction of the flowering phenotype based on ddm1-caused hypo-methylation unclear (Soppe et al., 2000; Johannes et al., 2009; Roux et al., 2011). In contrast, glucosinolates are not regulated as an irreversible switch and can be repressed after being induced or vice versa. Thus, DDM1-mediated methylation links to both the activation and repression of glucosinolates to aid in the proper adjustment of glucosinolate levels.
Lack of Overlap in EpiQTLs With Biosynthetic Genes
An interesting observation in this study is that we did not identify instances where the glucosinolate biosynthetic enzyme genes were within an epiQTL region suggesting that the epiQTLs largely do not influence the biosynthetic genes. This is somewhat in contrast to QTL mapping studies in Arabidopsis RILs, which showed that the large-effect variants linked to glucosinolate accumulation are almost entirely in biosynthetic loci (Kliebenstein et al., 2001a; Lambrix et al., 2001; Textor et al., 2004; Kroymann and Mitchell-Olds 2005; Hansen et al., 2007; Sønderby et al., 2007; Hansen et al., 2008; Sønderby et al., 2010a). In the natural accessions, this is similarly true that the major effect polymorphism are in biosynthetic loci (Chan et al., 2010; Chan et al., 2011; Brachi et al., 2015). However, there is also a vast universe of loci that appear to have causal polymorphisms each with small effects (Chan et al., 2010; Chan et al., 2011). As such, any enrichment of genetic causation is only identified within the large-effect polymorphisms. Interestingly, these large effect loci are associated with different epigenetic marks but this is a side-effect of the genetic inversions and duplications present in these genes (Kroymann et al., 2003; Chan et al., 2010; Schmitz and Ecker 2012; Schmitz et al., 2013; Kawakatsu et al., 2016). Thus, it is possible that DDM1 methylation only has the ability to causally influence the peripheral small effect loci within this epiRIL population. Future work studying other epigenetic marks will be required to come up with a reason for this difference.
EpiQTL Mapping of Within Vs. Between Line Variation
Dispersion (or stochastic variation) between replicates of homozygous lines is a trait that is under genetic control and is potentially adaptive (Queitsch et al. 2002; Ansel et al., 2008; Raj and van Oudenaarden 2008; Sangster et al. 2008; Veening et al., 2008; Jimenez-Gomez et al., 2011; Joseph et al., 2015; Lachowiec et al., 2016). These loci can be specific to stochastic variation within lines or can also associate with the mean variation between independent genotypes (Lachowiec et al., 2016). Given the potential for epigenetics to create this stochastic within line variation, we tested the possibility for the epiRILs to have different within line dispersion/stochastic variation within otherwise homozygous epigenotypes. Using within line CV to directly map variation in dispersion, we identified epiQTLs that associated with variation in within line CV for all of the traits from glucosinolate accumulation to flowering time. Previous efforts to identify epiQTLs that linked to within line CV for flowering times did not find these loci (Kooke et al. 2015). The most likely explanation for this difference is that in our experiments, we had independent replication across experiments on within line CV allowing for more power to identify these loci. Thus, the methylation status of the specific epiQTLs specifically associate with within line dispersion and not only the means of glucosinolates and flowering time. Interestingly, despite the mean distributions differing between aliphatic and indolic glucosinolates, their CV distributions were very similar (Figure 2 D, E, F). This is in contrast to flowering time that showed very low variation of within line CV (Figure 3B). Like genetic QTL for within line CV, these epiQTLs were a blend of loci that specifically influence within line CV and loci that shift both within line and between line variation. Thus DDM1-mediated methylation associates with phenotypic stability within a line similar to genetic polymorphisms.
In summary, this shows that while epigenetic mark variation correlates with glucosinolate variation, it is at a point that is much lower than the standing genetic variation. Thus, variation in DDM1-mediated epigenetic marks is unlikely to have a predominant if any influence on adaptation in a polymorphic population via flowering time or glucosinolates. This suggests that any role of epigenetic variation in influencing adaptation is most likely to be identified in isolated homozygous populations that have little to no migration with neighboring populations. Future work is required to assess if variation in other epigenetic marks may have more potential influence on adaptive traits.
Supplementary Material
Supplemental Material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.118.200127/-/DC1.
ACKNOWLEDGMENTS
Financial support for this work was provided by the National Research Foundation DNRF grant 99 (LMJ, MB, DK), The Danish Council for Independent Research, DFF – 4181-00077 (ESTA), US NSF grants IOS 1339125 and MCB 1330337 (DK), and the USDA National Institute of Food and Agriculture, Hatch project number CA-D-PLS-7033-H (DK).
Footnotes
Communicating editor: A. Paterson
Literature Cited
- Ansel J., Bottin H., Rodriguez-Beltran C., Damon C., Nagarajan M., et al. , 2008. Cell-to-cell stochastic variation in gene expression is a complex genetic trait. PLoS Genet. 4(4): e1000049 10.1371/journal.pgen.1000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwell S., Huang Y. S., Vilhjálmsson B. J., Willems G., Li Y., et al. , 2010. Genome-wide association study of 107 phenotypes in a common set of Arabidopsis thaliana inbred lines. Nature 465(7298): 627–631. 10.1038/nature08800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachi B., Meyer C. G., Villoutreix R., Platt A., Morton T. C., et al. , 2015. Coselected genes determine adaptive variation in herbivore resistance throughout the native range of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 112(13): 4032–4037. 10.1073/pnas.1421416112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren W, 2016. Epigenetic inheritance and its role in evolutionary biology: re-evaluation and new perspectives. Biology (Basel) 4: 24 10.3390/biology5020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burow M., Atwell S., Francisco M., Kerwin R. E., Halkier B. A., et al. , 2015. The Glucosinolate Biosynthetic Gene AOP2 Mediates Feed-back Regulation of Jasmonic Acid Signaling in Arabidopsis. Mol. Plant 8(8): 1201–1212. 10.1016/j.molp.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Bäurle I., Dean C., 2006. The Timing of Developmental Transitions in Plants. Cell 125(4): 655–664. 10.1016/j.cell.2006.05.005 [DOI] [PubMed] [Google Scholar]
- Chan E. K. F., Rowe H. C., Kliebenstein D. J., 2010. Understanding the evolution of defense metabolites in Arabidopsis thaliana using genome-wide association mapping. Genetics 185(3): 991–1007. 10.1534/genetics.109.108522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E. K. F., Rowe H. C., Corwin J. A., Joseph B., Kliebenstein D. J., 2011. Combining genome-wide association mapping and transcriptional networks to identify novel genes controlling glucosinolates in Arabidopsis thaliana. PLoS Biol. 9(8): e1001125 10.1371/journal.pbio.1001125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang G. C., Barua D., Kramer E. M., Amasino R. M., Donohue K., et al. , 2009. Major flowering time gene, flowering locus C, regulates seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 106(28): 11661–11666. 10.1073/pnas.0901367106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill G. A., Doerge R. W., 1994. Empirical threshold value for quantitative trait mapping. Genetics 138: 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colome-Tatche M., Cortijo S., Wardenaar R., Morgado L., Lahouze B., et al. , 2012. Features of the Arabidopsis recombination landscape resulting from the combined loss of sequence variation and DNA methylation. Proc. Natl. Acad. Sci. USA 109(40): 16240–16245. 10.1073/pnas.1212955109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortijo S., Wardenaar R., Colomé-Tatché M., Gilly A., Etcheverry M., et al. , 2014. Mapping the epigenetic basis of complex traits. Science 343(6175): 1145–1148. 10.1126/science.1248127 [DOI] [PubMed] [Google Scholar]
- Dam NM Van, Witjes L, Svatoš A (2008) Interactions between aboveground and belowground induction of glucosinolates in two wild Brassica species. 161: 801–810 [DOI] [PubMed]
- Doerge R. W., Churchill G. A., 1996. Permutation tests for multiple loci affecting a quantitative character. Genetics 142: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Irizarry R. A., 2010. Stochastic epigenetic variation as a driving force of development, evolutionary adaptation, and disease. Proc. Natl. Acad. Sci. USA 107(suppl_1): 1757–1764. 10.1073/pnas.0906183107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Weisberg S., 2011. An {R} Companion to Applied Regression, Ed. 2nd Sage, Thousand Oaks, CA. [Google Scholar]
- Frerigmann H., Gigolashvili T., 2014. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in arabidopsis Thaliana. Mol. Plant 7(5): 814–828. 10.1093/mp/ssu004 [DOI] [PubMed] [Google Scholar]
- Gigolashvili T., Berger B., Flügge U. I., 2009. Specific and coordinated control of indolic and aliphatic glucosinolate biosynthesis by R2R3-MYB transcription factors in Arabidopsis thaliana. Phytochem. Rev. 8(1): 3–13. 10.1007/s11101-008-9112-6 [DOI] [Google Scholar]
- Hall M. C., Dworkin I., Ungerer M. C., Purugganan M., 2007. Genetics of microenvironmental canalization in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 104(34): 13717–13722. 10.1073/pnas.0701936104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen B. G., Kliebenstein D. J., Halkier B. A., 2007. Identification of a flavin-monooxygenase as the S-oxygenating enzyme in aliphatic glucosinolate biosynthesis in Arabidopsis. Plant J. 50(5): 902–910. 10.1111/j.1365-313X.2007.03101.x [DOI] [PubMed] [Google Scholar]
- Hansen B. G., Kerwin R. E., Ober J. A., Lambrix V. M., Mitchell-Olds T., et al. , 2008. A novel 2-oxoacid-dependent dioxygenase involved in the formation of the goiterogenic 2-hydroxybut-3-enyl glucosinolate and generalist insect resistance in Arabidopsis. Plant Physiol. 148(4): 2096–2108. 10.1104/pp.108.129981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughn GW, Davin L, Giblin M, Underhill EW (1991) Biochemical Genetics of Plant Secondary Metabolites in Arabidopsis thaliana ’. 217–226. [DOI] [PMC free article] [PubMed]
- Huseby S., Koprivova A., Lee B. R., Saha S., Mithen R., et al. , 2013. Diurnal and light regulation of sulphur assimilation and glucosinolate biosynthesis in Arabidopsis. J. Exp. Bot. 64(4): 1039–1048. 10.1093/jxb/ers378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley J., 1942. Evolution. The Modern Synthesis, George Alien & Unwin Ltd., London. [Google Scholar]
- Højsgaard S, Halekoh U (2016) doBy: Groupwise Statistics, LSmeans, Linear Contrasts, Utilities. R package version 4.5–15.
- Jablonka E., Raz G., 2009. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 84(2): 131–176. 10.1086/598822 [DOI] [PubMed] [Google Scholar]
- Jansen R. C., Stam P., 1994. High resolution of quantitative traits into multiple loci via interval mapping. Genetics 136: 1447–1455. 10.1093/jxb/ers378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Tian H., Li H., Yu Q., Wang L., et al. , 2015. The Arabidopsis thaliana elongator complex subunit 2 epigenetically affects root development. J. Exp. Bot. 66(15): 4631–4642. 10.1093/jxb/erv230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Gomez J. M., Corwin J. A., Joseph B., Maloof J. N., Kliebenstein D. J., 2011. Genomic analysis of QTLs and genes altering natural variation in stochastic noise. PLoS Genet. 7(9): e1002295 10.1371/journal.pgen.1002295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes F., Porcher E., Teixeira F. K., Saliba-Colombani V., Simon M., et al. , 2009. Assessing the impact of transgenerational epigenetic variation on complex traits. PLoS Genet. 5(6): e1000530 10.1371/journal.pgen.1000530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B., Corwin J. A., Züst T., Li B., Iravani M., et al. , 2013. Hierarchical nuclear and cytoplasmic genetic architectures for plant growth and defense within Arabidopsis. Plant Cell Online 25(6): 1929–1945. 10.1105/tpc.113.112615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B., Atwell S., Corwin J. A., Li B., Kliebenstein D. J., 2014. Meta-analysis of metabolome QTLs in Arabidopsis: trying to estimate the network size controlling genetic variation of the metabolome. Front. Plant Sci. 5: 461 10.3389/fpls.2014.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B., Corwin J. A., Kliebenstein D. J., 2015. Genetic Variation in the Nuclear and Organellar Genomes Modulates Stochastic Variation in the Metabolome, Growth, and Defense. PLoS Genet. 11(1): e1004779 10.1371/journal.pgen.1004779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Takashima K., Kakutani T., 2004. Epigenetic control of CACTA transposon mobility in Arabidopsis thaliana. Genetics 168(2): 961–969. 10.1534/genetics.104.029637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakatsu T., Huang S. S. C., Jupe F., Sasaki E., Schmitz R. J., et al. , 2016. Epigenomic Diversity in a Global Collection of Arabidopsis thaliana Accessions. Cell 166(2): 492–505. 10.1016/j.cell.2016.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin R., Feusier J., Corwin J., Rubin M., Lin C., et al. , 2015. Natural genetic variation in Arabidopsis thaliana defense metabolism genes modulates field fitness. eLife 4: 4 10.7554/eLife.05604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin R. E., Feusier J., Muok A., Lin C., Larson B., et al. , 2017. Epistasis x environment interactions among Arabidopsis thaliana glucosinolate genes impact complex traits and fitness in the field. New Phytol. 215(3): 1249–1263. 10.1111/nph.14646 [DOI] [PubMed] [Google Scholar]
- Kliebenstein D. J., Gershenzon J., Mitchell-Olds T., 2001a Comparative quantitative trait loci mapping of aliphatic, indolic and benzylic glucosinolate production in Arabidopsis thaliana leaves and seeds. Genetics 159: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein D. J., Kroymann J., Brown P., Figuth A., Pedersen D., et al. , 2001b Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 126(2): 811–825. 10.1104/pp.126.2.811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein D. J., Figuth A., Mitchell-Olds T., 2002a Genetic architecture of plastic methyl jasmonate responses in Arabidopsis thaliana. Genetics 161: 1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein D. J., Pedersen D., Barker B., Mitchell-Olds T., 2002b Comparative analysis of quantitative trait loci controlling glucosinolates, myrosinase and insect resistance in Arabidopsis thaliana. Genetics 161: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein D. J., Rowe H. C., Denby K. J., 2005. Secondary metabolites influence Arabidopsis/Botrytis interactions: Variation in host production and pathogen sensitivity. Plant J. 44(1): 25–36. 10.1111/j.1365-313X.2005.02508.x [DOI] [PubMed] [Google Scholar]
- Klironomos F. D., Berg J., Collins S., 2013. How epigenetic mutations can affect genetic evolution: Model and mechanism. BioEssays 35(6): 571–578. 10.1002/bies.201200169 [DOI] [PubMed] [Google Scholar]
- Kliebenstein D. J., D’Auria J. C., Behere A. S., Kim J. H., Gunderson K. L., et al. , 2007. Characterization of seed-specific benzoyloxyglucosinolate mutations in Arabidopsis thaliana. Plant J. 51(6): 1062–1076. 10.1111/j.1365-313X.2007.03205.x [DOI] [PubMed] [Google Scholar]
- Kroymann J., Mitchell-Olds T., 2005. Epistasis and balanced polymorphism influencing complex trait variation. Nature 435(7038): 95–98. 10.1038/nature03480 [DOI] [PubMed] [Google Scholar]
- Kroymann J., Donnerhacke S., Schnabelrauch D., Mitchell-Olds T., 2003. Evolutionary dynamics of an Arabidopsis insect resistance quantitative trait locus. Proc. Natl. Acad. Sci. USA 100(Supplement 2): 14587–14592. 10.1073/pnas.1734046100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooke R., Johannes F., Wardenaar R., Becker F., Etcheverry M., et al. , 2015. Epigenetic Basis of Morphological Variation and Phenotypic Plasticity in Arabidopsis thaliana. Plant Cell 27(2): 337–348. 10.1105/tpc.114.133025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowiec J., Queitsch C., Kliebenstein D. J., 2016. Molecular mechanisms governing differential robustness of development and environmental responses in plants. Ann. Bot. 117(5): 795–809. 10.1093/aob/mcv151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland K., Uller T., Feldman M., Sterelny K., Müller G. B., et al. , 2014. Does evolutionary theory need a rethink. Nature 514: 161–164. 10.1038/514161a [DOI] [PubMed] [Google Scholar]
- Lambrix V., Reichelt M., Mitchell-Olds T., Kliebenstein D. J., Gershenzon J., 2001. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell 13(12): 2793–2807. 10.1105/tpc.13.12.2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Hansen B. G., Ober J. A., Kliebenstein D. J., Halkier B. A., 2008. Subclade of Flavin-Monooxygenases Involved in Aliphatic Glucosinolate Biosynthesis. Plant Physiol. 148(3): 1721–1733. 10.1104/pp.108.125757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman Z., Gendrel A.-V., Black M., Vaughn M. W., Dedhia N., et al. , 2004. Role of transposable elements in heterochromatin and epigenetic control. Nature 430(6998): 471–476. 10.1038/nature02651 [DOI] [PubMed] [Google Scholar]
- Mewis I., Appel H. M., Hom A., Raina R., Schultz J. C., 2005. Major Signaling Pathways Modulate Arabidopsis Glucosinolate Accumulation and Response to Both Phloem-Feeding and Chewing Insects 1. Plant Physiol. 138(2): 1149–1162. 10.1104/pp.104.053389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewis I., Khan M. A. M., Glawischnig E., Schreiner M., Ulrichs C., 2012. Water Stress and Aphid Feeding Differentially Influence Metabolite Composition in Arabidopsis thaliana (L.). PLoS One 7(11): e48661 10.1371/journal.pone.0048661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura A., Yonebayashi S., Watanabe K., Toyama T., Shimada H., et al. , 2001. Mobilization of transposons by a mutation abolishing full DNA methylation in Arabidopsis. Nature 411(6834): 212–214. 10.1038/35075612 [DOI] [PubMed] [Google Scholar]
- Olsson A. H., Volkov P., Bacos K., Dayeh T., Hall E., et al. , 2014. Genome-wide associations between genetic and epigenetic variation influence mRNA expression and insulin secretion in human pancreatic islets. PLoS Genet. 10: e1004735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordas B., Malvar R. A., Hill W. G., 2008. Genetic variation and quantitative trait loci associated with developmental stability and the environmental correlation between traits in maize. Genet. Res. 90(05): 385–395. 10.1017/S0016672308009762 [DOI] [PubMed] [Google Scholar]
- Pfalz M., Vogel H., Mitchell-Olds T., Kroymann J., 2007. Mapping of QTL for resistance against the crucifer specialist herbivore Pieris brassicae in a new arabidopsis inbred line population, Da(1)-12 x Ei-2. PLoS One 2(6): e578 10.1371/journal.pone.0000578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KVSK, Song B-H, Olson-Manning C, Anderson JT, Lee C-R, Schranz ME, Windsor a. J, Clauss MJ, Manzaneda a. J, Naqvi I, et al (2012) A Gain-of-Function Polymorphism Controlling Complex Traits and Fitness in Nature. Science (80-) 337: 1081–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queitsch C., Sangster T. A., Lindquist S., 2002. Hsp90 as a capacitor of phenotypic variation. Nature 417(6889): 618–624. 10.1038/nature749 [DOI] [PubMed] [Google Scholar]
- R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria. version 3.3.2.
- Raj A., van Oudenaarden A., 2008. Nature, Nurture, or Chance: Stochastic Gene Expression and Its Consequences. Cell 135(2): 216–226. 10.1016/j.cell.2008.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards E. J., 2006. Inherited epigenetic variation–revisiting soft inheritance. Mycorrhiza 7: 395–401. [DOI] [PubMed] [Google Scholar]
- Rosa S., Duncan S., Dean C., 2016. Mutually exclusive sense–antisense transcription at FLC facilitates environmentally induced gene repression. Nat. Commun. 7: 13031 10.1038/ncomms13031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F., Colomé-Tatché M., Edelist C., Wardenaar R., Guerche P., et al. , 2011. Genome-wide epigenetic perturbation jump-starts patterns of heritable variation found in nature. Genetics 188(4): 1015–1017. 10.1534/genetics.111.128744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team , 2015. RStudio: Integrated Development for R. RStudio, Inc., Boston, MA. [Google Scholar]
- Salomé P. A., Bomblies K., Laitinen R. A. E., Yant L., Mott R., et al. , 2011. Genetic architecture of flowering-time variation in Arabidopsis thaliana. Genetics 188(2): 421–433. 10.1534/genetics.111.126607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster T. A., Salathia N., Lee H. N., Watanabe E., Schellenberg K., et al. , 2008. HSP90-buffered genetic variation is common in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 105(8): 2969–2974. 10.1073/pnas.0712210105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz R. J., Ecker J. R., 2012. Epigenetic and epigenomic variation in Arabidopsis thaliana. Trends Plant Sci. 17(3): 149–154. 10.1016/j.tplants.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz R. J., Schultz M. D., Urich M. A., Nery J. R., Pelizzola M., et al. , 2013. Patterns of population epigenomic diversity. Nature 495(7440): 193–198. 10.1038/nature11968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppe W. J., Jacobsen S. E., Alonso-Blanco C., Jackson J. P., Kakutani T., et al. , 2000. The late flowering phenotype of fwa mutants is caused by gain-of-function epigenetic alleles of a homeodomain gene. Mol. Cell 6(4): 791–802. 10.1016/S1097-2765(05)00090-0 [DOI] [PubMed] [Google Scholar]
- Sønderby I. E., Hansen B. G., Bjarnholt N., Ticconi C., Halkier B. A., et al. , 2007. A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS One 2(12): e1322 10.1371/journal.pone.0001322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderby I. E., Burow M., Rowe H. C., Kliebenstein D. J., Halkier B. A., 2010a A complex interplay of three R2R3 MYB transcription factors determines the profile of aliphatic glucosinolates in Arabidopsis. Plant Physiol. 153(1): 348–363. 10.1104/pp.109.149286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderby I. E., Geu-Flores F., Halkier B. A., 2010b Biosynthesis of glucosinolates - gene discovery and beyond. Trends Plant Sci. 15(5): 283–290. 10.1016/j.tplants.2010.02.005 [DOI] [PubMed] [Google Scholar]
- Textor S., Bartram S., Kroymann J., Falk K. L., Hick A., et al. , 2004. Biosynthesis of methionine-derived glucosinolates in Arabidopsis thaliana: recombinant expression and characterization of methylthioalkylmalate synthase, the condensing enzyme of the chain-elongation cycle. Planta 218(6): 1026–1035. 10.1007/s00425-003-1184-3 [DOI] [PubMed] [Google Scholar]
- Thies W., 1979. Detection and utilization of a glucosinolate sulfohydrolase in the edible snail, Helix pomatia. Naturwissenschaften 66(7): 364–365. 10.1007/BF00368477 [DOI] [Google Scholar]
- Veening J.-W., Smits W. K., Kuipers O. P., 2008. Bistability, Epigenetics, and Bet-Hedging in Bacteria. Annu. Rev. Microbiol. 62(1): 193–210. 10.1146/annurev.micro.62.081307.163002 [DOI] [PubMed] [Google Scholar]
- Wang S., Basten C. J., Zeng Z. B., 2012. Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC. [Google Scholar]
- Weigel D., Colot V., 2012. Epialleles in plant evolution. Genome Biol. 13(10): 249 10.1186/gb-2012-13-10-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzell A. M., Rowe H. C., Hansen B. G., Ticconi C., Halkier B. A., et al. , 2007. Linking metabolic QTLs with network and cis-eQTLs controlling biosynthetic pathways. PLoS Genet. 3(9): e162 10.1371/journal.pgen.0030162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2009) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York.
- Wilson I. W., Schiff C. L., Hughes D. E., Somerville S. C., 2001. Quantitative trait loci analysis of powdery mildew disease resistance in the Arbidopsis thaliana accession Kashmir-1. Genetics 158: 1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemach A., Kim M. Y., Hsieh P. H., Coleman-Derr D., Eshed-Williams L., et al. , 2013. The arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell 153(1): 193–205. 10.1016/j.cell.2013.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z.-B., 1994. Precision Mapping of Quantitative Trait Loci. Genetics 136: 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrybko C. L., Elaine K. F., Robert T. R., 1997. Determination of glucosinolates in domestic and wild mustard by high-performance liquid chromatography with confirmation by electrospray mass spectrometry and photodiode-array detection. J. Chromatogr. A 767(1-2): 43–52. 10.1016/S0021-9673(96)01068-0 [DOI] [Google Scholar]
- Züst T, Heichinger C, Grossniklaus U, Harrington R, Kliebenstein DJ, Turnbull LA. (2012) Natural Enemies Drive Geographic Variation in Plant Defenses. Science (80) 10.1126/science.1226397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
File S1 contains phenotyping data for each epiRIL, i.e., mean and CV for glucosinolate traits and flowering time.