Abstract
Objectives
Delirium occurs in approximately 30% of critically-ill patients, and the risk of dying during admission doubles in those patients. Molecular mechanisms causing delirium are largely unknown. However, critical illness and the intensive care unit environment consistently disrupt circadian rhythms, and circadian disruptions are strongly associated with delirium. Exposure to benzodiazepines and constant light are suspected risk factors for the development of delirium. Thus, we tested the functional role of the circadian rhythm protein Period 2 (PER2) in different mouse models resembling delirium.
Design
Animal study.
Setting
University experimental laboratory.
Subjects
Wildtype, Per2−/− mice.
Interventions
Midazolam, lipopolysaccharide (LPS), constant light, nobiletin or sham treated animals.
Measurements and Main Results
Midazolam significantly reduced the expression of PER2 in the suprachiasmatic nucleus (SCN) and the hippocampus of wildtype mice. Behavioral tests following midazolam exposure revealed a robust phenotype including executive dysfunction and memory impairment suggestive of delirium. These findings indicated a critical role of hippocampal expressed PER2. Similar results were obtained in mice exposed to LPS or constant light. Subsequent studies in Per2−/− mice confirmed a functional role of PER2 in a midazolam-induced delirium-like phenotype. Using the small molecule nobiletin to enhance PER2 function, the cognitive deficits induced by midazolam or constant light were attenuated in wildtype mice.
Conclusions
These experiments identify a novel role for PER2 during a midazolam or constant light induced delirium-like state, highlight the importance of hippocampal PER2 expression for cognitive function, and suggest the PER2 enhancer nobiletin as potential therapy in delirium-like conditions associated with circadian disruption.
Keywords: Delirium, Per2, hippocampus, midazolam, circadian rhythms, nobiletin
Introduction
Nearly 30% of patients admitted to an intensive care unit develop delirium, and these patients are at an increased risk of dying (1). The identification, prevention, and treatment of delirium are increasingly regarded as major public health priorities (1–9) but specific treatments for delirium are unknown. Circadian rhythms orchestrate the mechanism by which environmental factors can regulate biological functions (4). This system couples environmental stimuli with a variety of physiological functions including the integrity of the sleep-wake cycle, motor activity, arousal and cognition. This coupling makes circadian rhythm proteins a logical target for studies exploring delirium pathophysiology. In fact, previous work supports a three-domain model for delirium that includes generalized cognitive impairment, disturbed executive cognition, and behavioral disruption, which are all are under circadian control (4). Recent evidence suggests that the circadian rhythm protein Period2 (PER2) is highly expressed in the hippocampus and therefore might play a role in learning and memory (10). Furthermore, a mutation of the human PER2 gene leads to a significant disruption of normal sleep (11). Circadian disruption and sleep fragmentation often precede the development of delirium. Drugs such as benzodiazepines and constant exposure to light appear to be among the worst offenders in causing sleep disruption and increasing the risk of delirium (12, 13). Given the clinical significance of delirium and the role of PER2 in the control of sleep, we hypothesized that PER2 might play a role in the pathogenesis of delirium and that functional inhibition of PER2 would lead to a delirium-like phenotype. Therefore, we tested our hypothesis in a murine model of midazolam-induced behavioral impairment consistent with components of delirium in humans. We found that midazolam-induced changes in behavior are associated with a significant downregulation of PER2 in the hippocampus. Furthermore, genetic deletion of Per2 resulted in a phenotype that shares several features with acute delirium, but which was not worsened by midazolam treatment. Finally, we successfully attenuated these cognitive deficits using a novel PER2 enhancer.
Methods
Detailed information about the methods is presented in Appendix 1 (Supplemental Digital Content 1)
Mouse Experiments
Experimental protocols were approved by the Institutional Animal Care and Use Committee [IACUC] at the University of Colorado Denver, USA and were in accordance with the NIH guidelines for use of live animals.
Animal
C57BL/6J and Per2−/− mice were obtained from the Jackson Laboratories (14–16).
Transcriptional analysis
mRNA levels were determined by quantitative RT-PCR as described (17).
Immunohistochemistry
30-μm coronal brain sections from untreated or midazolam treated mice were stained using the PER2 antibody R39 made in rabbit as described previously (18).
Murine Model for delirium
Mice were injected with midazolam (PFIZER, 10 mg/kg i.p.), LPS (O111:B4 from E. coli; InvivoGen, 100 μg/kg), or midazolam plus LPS. 24 or 72 hours later mice underwent behavioral testing as described (19–22). In a subset of experiments mice were housed for 7 days under constant light conditions prior to behavioral testing (23, 24). A second, blinded investigator analyzed the behavioral tests.
Data analysis
For multiple comparisons, one-way analysis of variance with Bonferroni adjustment was performed, and for single comparison, the unpaired or paired Student t-test was applied. Values are expressed as mean (SD). P<0.05 was considered statistically significant.
Results
Midazolam downregulates PER2 in the SCN and the hippocampus
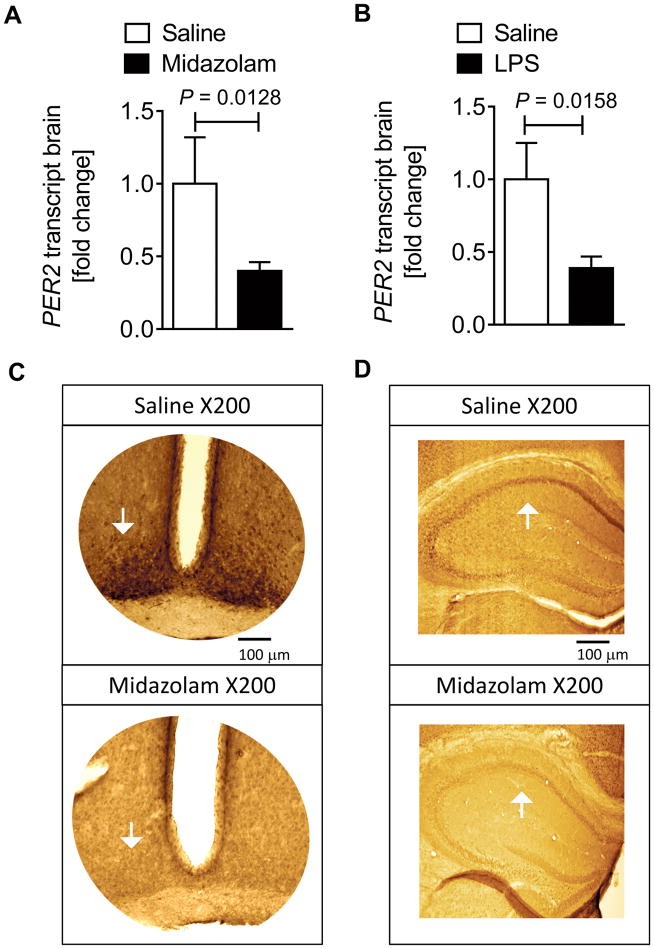
We hypothesized that the circadian rhythm protein PER2 plays a role in midazolam-induced behavioral changes and analyzed murine brain PER2 transcript levels after midazolam administration. Two hours after midazolam administration, brain PER2 mRNA was significantly downregulated (Fig. 1A). Next, to understand if midazolam administration also directly affects other core components of the circadian system, we analyzed circadian Brain and Muscle ARNT-Like 1 (BMAL1) mRNA tissue levels. However, midazolam had no direct effect on BMAL1 transcript, which normally oscillates in antiphase to PER2 (25, 26) (Supplemental Digital Content 2). Because inflammation is an integral component of delirium pathophysiology we next treated mice with LPS, which has been shown to have long term effects on cognitive function (27–29). After 2 hours of LPS administration, PER2 transcript levels were significantly downregulated in murine brain tissue (Fig. 1B), supporting our hypothesis that PER2 mechanisms are involved in delirium pathogenesis. Next, we analyzed various brain regions to localize PER2 downregulation following exposure to midazolam. Immunohistochemistry analysis 24 hours after midazolam administration revealed PER2 protein dominantly downregulated in the SCN or the hippocampus of mice (Fig. 1C and D and Supplemental Digital Content 3). Key findings: these data demonstrate that midazolam significantly downregulates PER2 in the SCN and hippocampus with no effect on circadian antiphase expressed BMAL1.
Figure 1. Midazolam downregulates PER2 in the SCN and the hippocampus.
Wildtype mice (C57BL6/J) were injected with either midazolam (10 mg/kg i.p.) or LPS (100 μg/kg i.p.) and compared to saline treated controls. Brain tissue was harvested, mRNA was isolated using RNeasy Mini Kit (Qiagen), cDNA was generated using miScript RT II kits (Qiagen), and transcript levels were determined by quantitative real-time RT-PCR (iCycler or iCycler IQ; Bio-Rad Laboratories Inc.). In a subset of experiments immunohistochemistry staining for PER2 in the SCN or hippocampus was performed. (A) Brain PER2 transcript levels (n=4 animals) 2 hours following midazolam administration. (B) Brain PER2 transcript levels (n=3 animals) 2 hours following LPS administration. (C, D) Immunohistochemistry of PER2 in the SCN or hippocampus 24 hours following midazolam administration (original magnification, X200, scale bars indicate 100 μm, n=8 animals). All data are presented as mean ± SD; SCN=suprachiasmatic nucleus, LPS=Lipopolysaccharide.
Midazolam induces cognitive deficits in mice
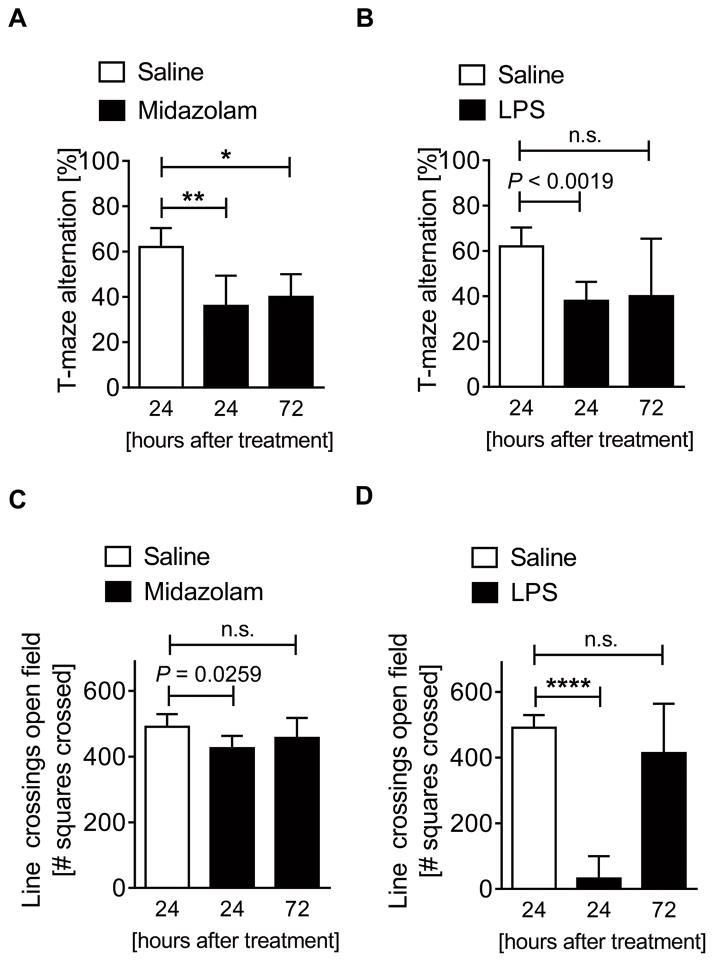
In humans, the hippocampus appears to play a critical role in the pathogenesis of delirium (30). Based on this observation, we hypothesized that Midazolam-induced reduction in hippocampal PER2 protein would lead to delirium-like murine behavioral changes, including cognitive deficits. We therefore studied spontaneous alternation using a T-maze (Supplemental Digital Content 4), a very sensitive test used to determine hippocampal functioning (19) and to detect cognitive impairment (22). After 24 or 72 hours of midazolam administration, mice showed significantly reduced spontaneous alternation in the T-maze (Fig. 2A). Similarly, 24 hours after the administration of inflammation-inducing LPS, spontaneous T-maze alternation was significantly compromised (Fig. 2B). However, 72 hours after LPS treatment, no effects on spontaneous alternation were observed (Fig. 2B). To test if midazolam and LPS would have synergistic effects, we treated mice with both midazolam and LPS. No additional effects on spontaneous alternation in the T-maze were observed (Supplemental Digital Content 5). Key findings: these data demonstrate that midazolam or inflammation, which downregulate brain PER2 levels, lead to significant hippocampus dependent cognitive deficits, as observed in some types of delirium (31).
Figure 2. Midazolam induces cognitive deficits and reduces locomotion in wildtype mice.
Wildtype mice (C57BL6/J) were injected with either midazolam (10 mg/kg i.p.) or LPS (100 μg/kg i.p.) and compared to saline treated controls. 24 or 27 hours later mice underwent behavioral studies using a T-maze alternation test or open-field line-crossing determination. (A) T-maze alternation in %, 24 or 72 hours after a single dose of midazolam. (B) T-maze alternation in %, 24 or 72 hours after a single dose of LPS. (C) Numbers of squares crossed for 10 minutes (line crossing) 24 or 72 hours after a single dose of midazolam. (D) Numbers of squares crossed for 10 minutes (line-crossing) 24 or 72 hours after a single dose of LPS. All data have n=5 individual animals and are presented as mean ± SD, LPS=Lipopolysaccharide, P value denotes t-test and * denotes one-way ANOVA (see also Supplemental Digital Content 1).
Midazolam affects locomotor activity in mice
Psychomotor disturbances are another hallmark of human delirium (32). Therefore, we next assessed locomotor activity after midazolam or LPS exposure using open-field studies (20) (Supplemental Digital Content 6). Mice had significantly compromised locomotor activity 24 hours after midazolam administration compared to saline controls, but this difference was abolished 72 hours after midazolam administration (Fig. 2C). Similarly, inflammation-inducing LPS administration showed significantly reduced locomotor activity at 24 hours but not at 72 hours following treatment (Fig. 2D). Testing for synergistic effects of midazolam and LPS revealed significant additional changes in locomotor activity at 72 hours, but not at 24 hours following midazolam and LPS administration (Supplemental Digital Content 7). Key findings: these data demonstrate that midazolam or inflammation lead to a significant reduction in locomotor activity, indicating that functional brain PER2 inhibition could cause delirium-associated behavioral disruption, such as lethargy or anxiety.
Midazolam leads to memory deficits in mice
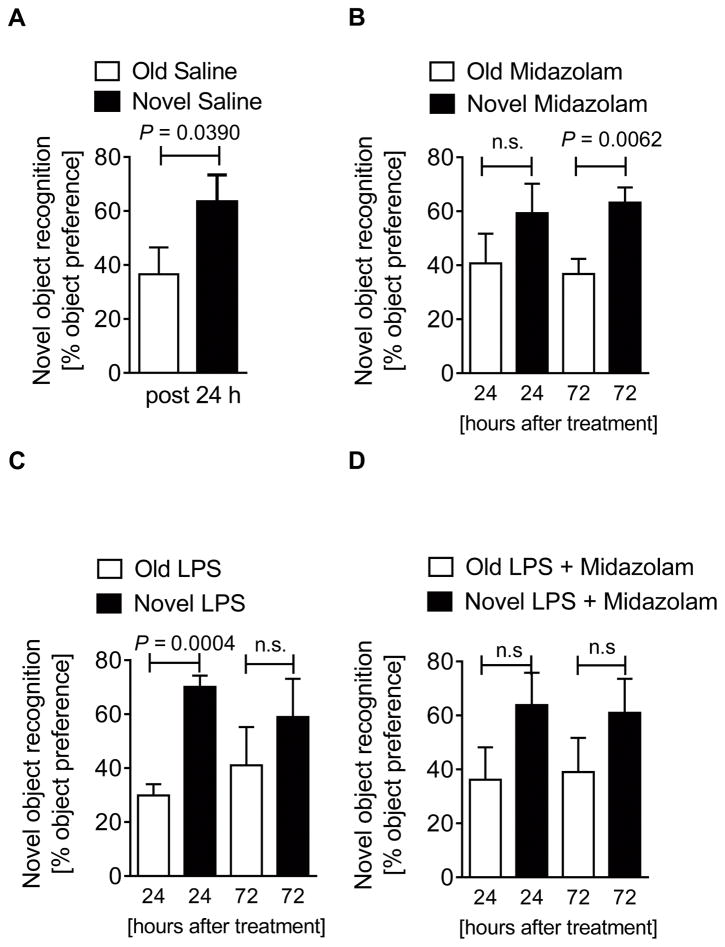
Another aspect of the delirium phenotype is working memory dysfunction, a component of executive cognitive function (4). Thus, we next investigated memory function by using a novel-object-recognition test where mice are habituated to two objects for two days (21). Thereafter, one object is replaced by a new object and the preference to explore the novel object is given as a percentage (Supplemental Digital Content 8). Mice with a normal memory function will have a significantly higher preference for the novel object (Fig. 3A). As observed with our test for locomotion, midazolam affected memory function only at 24 hours, where no significant preference for the novel object was found (Fig. 3B). In contrast, memory function was only compromised 72 hours following LPS treatment (Fig. 3C) and no obvious synergistic effects of midazolam and LPS were observed at 24 or 72 hours (Fig. 3D). Key findings: midazolam or inflammation lead to significant memory deficits with no synergistic effect of the two agents, further supporting our hypothesis that functional brain PER2 inhibition shows characteristics of a delirium-like phenotype (4).
Figure 3. Midazolam induces loss of memory in wildtype mice.
Wildtype (C57BL6/J) were injected with either midazolam (10 mg/kg i.p.), LPS (100 μg/kg i.p.) or midazolam and LPS and compared to saline treated controls. 24 or 27 hours later mice underwent behavioral studies using novel-object-recognition tests. (A) Preference in % for a novel object after 2 days habituation to two old objects 24 hours after a single dose of saline in wildtype mice. (B) Preference in % for a novel object after 2 days habituation to two old objects 24 or 72 hours after a single dose of midazolam in wildtype mice. (C) Preference in % for a novel object after 2 days habituation to two old objects 24 or 72 hours after a single dose of LPS in wildtype mice. (D) Preference in % for a novel object after 2 days habituation to two old objects 24 or 72 hours after a single dose of LPS + midazolam in wildtype mice. All data have n=5 individual animals and are presented as mean ± SD, LPS=Lipopolysaccharide, P value denotes t-test (see also Supplemental Digital Content 1).
Midazolam induced delirium-like phenotype is PER2 dependent
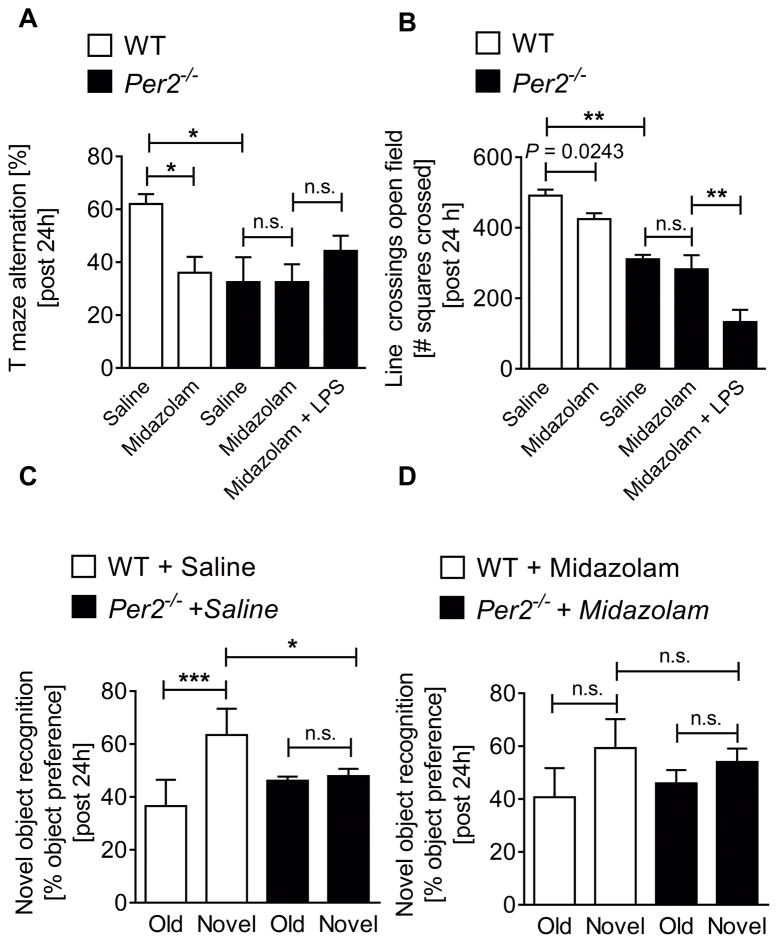
Having demonstrated that midazolam or LPS (28, 29) associated brain PER2 inhibition induced a delirium-like phenotype, similar to a three-domain model for delirium that includes generalized cognitive impairment, disturbed executive cognition, and behavioral disruption (4), we next tested these concepts in mice with a genetic deletion of Per2. T-maze alternation, open-field or novel-object-recognition tests showed significantly reduced cognition, locomotion and memory function in Per2−/− mice at baseline, comparable to midazolam-treated wildtype mice (Fig. 4A–D and Supplemental Digital Content 9). Following treatment of Per2−/− mice with midazolam revealed no further effects in our behavioral tests. However, while midazolam had no further effects, midazolam and LPS administration together revealed significantly less locomotion in Per2−/− mice when compared to saline treated Per2−/− mice (Fig. 4B), suggesting that LPS effects on locomotor activity could also be mediated by systemic inflammation and generalized acute stress or sickness (33). Nonetheless, our data suggest PER2 to be a key mediator of midazolam-induced cognitive, locomotor, and memory deficits (Fig. 4A–D and Supplemental Digital Content 9). Key findings: Per2 deficient mice have baseline deficits in cognition, locomotion and memory that do not change after midazolam treatment, indicating that a midazolam-induced delirium-like phenotype is PER2 dependent.
Figure 4. Midazolam induced delirium is PER2 dependent.
Wildtype (C57BL6/J) or Per2−/− mice were injected with either midazolam (10 mg/kg i.p.), LPS (100 μg/kg i.p.) or midazolam and LPS and compared to saline treated controls. 24 hours later mice underwent behavioral studies using T-maze alternation test, open-field line-crossing determination or novel-object-recognition tests. (A) T-maze alternation in % 24 hours after a single dose of midazolam or LPS + midazolam. (B) Numbers of squares crossed for 10 minutes (line-crossing) 24 hours after a single dose of midazolam or LPS + midazolam. (C, D) Preference in % for the novel object after 2 days habituation to the old object 24 hours after a single dose of midazolam. All data have n=5 individual for wildtype mice and an n=8 individual animals for Per2−/− mice and are presented as mean ± SD, LPS=Lipopolysaccharide P value denotes t-test and * denotes one-way ANOVA (see also Supplemental Digital Content 1).
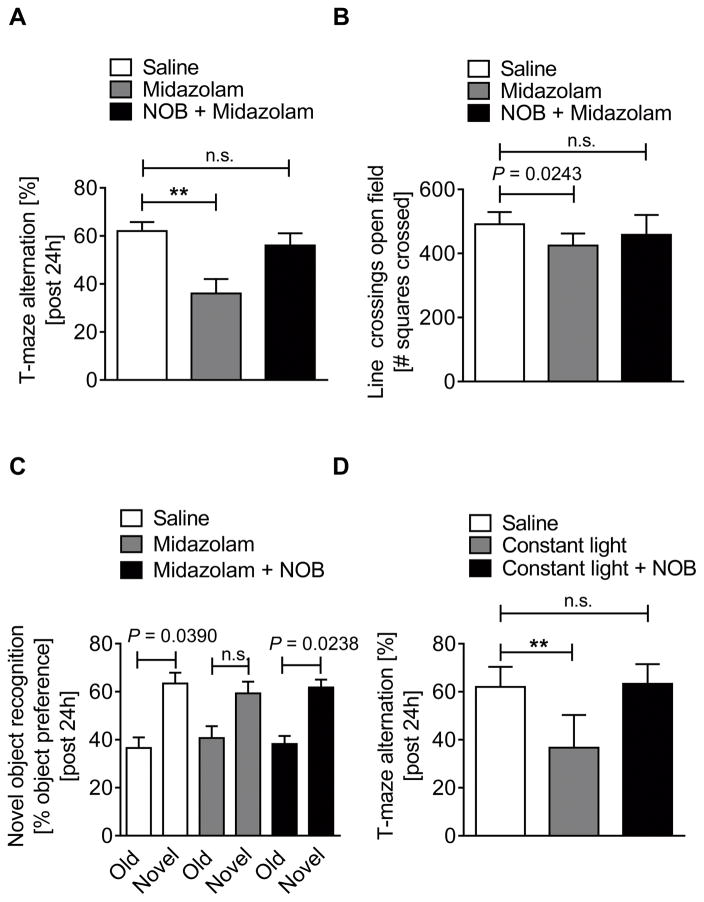
The novel PER2 enhancer nobiletin reverses midazolam or constant-light induced delirium-like behaviors
After we determined midazolam-induced changes in behavior to be PER2 dependent, we next pursued a treatment strategy to enhance brain PER2 levels and function. Previous studies identified nobiletin, a flavonoid from citrus peels, as a highly potent and specific PER2 enhancer (34). In fact, treatment of mice with nobiletin and midazolam significantly increased brain PER2 transcript levels and reversed midazolam mediated downregulation of PER2 (Supplemental Digital Content 10). Using nobiletin together with midazolam in a T-maze alternation, open-field or novel-object-recognition test completely abolished midazolam induced behavioral changes (Fig. 5A–C). To evaluate whether nobiletin could also improve cognitive deficits in other delirium-like models such as prolonged light exposure to mimic artificial lighting in critical care units (35), we used a mouse model where mice were housed under constant light for 7 days to reduce brain PER2 levels (23) and impair hippocampal function (24). Constant light significantly impaired hippocampal mediated cognitive and memory performance, which was fully reversed in nobiletin treated mice (Fig. 5D and Supplemental Digital Content 11). In contrast, no differences in open-field behaviors were found under constant light conditions (Supplemental Digital Content 11), as reported by other investigators (24). Interestingly, nobiletin treatment significantly reduced locomotor activity under constant light conditions, which might be the result of a potential sedative effects of flavonoids (36), despite being a cognitive enhancer (37). Key findings: these data suggest that nobiletin could be used to reverse the effects of midazolam on brain PER2 expression and cognitive function and might be therapeutic in delirium-like models caused by circadian rhythm disruption, such as constant artificial lightning commonly seen in critical care units.
Figure 5. Nobiletin abolished midazolam or constant light induced cognitive deficits.
Wildtype (C57BL6/J) mice were injected with either midazolam (10 mg/kg i.p.) or midazolam + nobiletin (1 mg/kg i.p.) and compared to saline treated controls. 24 hours later mice underwent behavioral studies. In a subset of experiments mice were exposed to 7 days of constant light and tested for behavioral changes 24h later. (A) T-maze alternation in % 24 hours after a single dose of midazolam or midazolam + nobiletin. (B) Numbers of squares crossed for 10 minutes (line crossing) 24 hours after a single dose of midazolam or midazolam + nobiletin. (C) Preference in % for a novel object after 2 days habituation to two old objects 24 hours after a single dose of midazolam or midazolam + nobiletin. (D) T-maze alternation in % 24 hours after 7 days at constant light conditions with and without nobiletin. All data on behavioral tests have n=5 individual wildtype mice and are presented as mean ± SD, NOB=nobiletin, P value denotes t-test and * denotes one-way ANOVA (see also Supplemental Digital Content 1).
Discussion
In the present study, we investigated the putative role of the circadian rhythm protein PER2 in the pathogenesis of delirium using mouse models with induced behavioral impairments consistent with several important components of delirium observed in humans. We identified a critical role for hippocampal expressed PER2 and could reverse hippocampal-mediated cognitive deficits in a delirium-like mouse model using a novel PER2 enhancer. Taken together, these studies identify a novel PER2 mechanism in delirium-like cognitive alterations in mice.
PER2 is expressed in a circadian pattern in the SCN, the primary pacemaker in the mammalian brain (38). The SCN controls the circadian rhythms of locomotion, metabolism, and behavior (4). Per2−/− mice show disrupted circadian oscillations and loss of behavioral rhythms. Recent evidence from mice suggests that PER2 might also play a role in learning and memory due to a robust PER2 expression in the hippocampus (10). However, Per2−/− mice do not show deficits in learning (39). Interestingly, studies in humans suggest a critical role of the hippocampus in the pathogenesis of delirium (30). Moreover, a specific PER2 point mutation in humans leads to a significant disruptive sleep disorder, which can be recapitulated in mice (40). Interrupted sleep can cause circadian disruption resulting in cognitive deficits, and has been linked to delirium in humans (41). Mouse models with disrupted circadian rhythms show similar delirium-like phenotypes (24). The importance of PER2 for circadian rhythmicity has been illustrated in mouse studies, showing that the precise rhythmicity of PER2 is essential for driving cellular circadian oscillations (42). In humans, melatonin levels, a surrogate for a functional circadian rhythm, are consistently low in patients with delirium (13). Furthermore, melatonin tests are used to detect sleep disruption caused by the human PER2 mutation (43). In fact, melatonin treatment may improve delirium in humans (13). However, while disrupted circadian rhythms seem to play an important role in the pathogenesis of delirium, a molecular mechanism has not been identified yet.
In the current study, we show that midazolam or inflammation, each reported to induce delirium in humans (44, 45), lead to a significant dysregulation of PER2 in the SCN and the hippocampus of mice. These findings are consistent with other reports of PER2 dysregulation in the mouse brain following single exposure to anesthetics (46) or LPS (47). Moreover, patients with severe inflammation exhibit lower PER2 and altered melatonin plasma levels (48). While there are no reports to date of midazolam affecting PER2 expression in humans, benzodiazepines do alter sleep patterns and increase the risk of circadian disruption and delirium in humans (13, 49). Using behavioral studies in mice exposed to LPS or midazolam, we found significantly reduced cognitive function, locomotor activity, and memory function with no other pronounced behavioral changes. These findings are in line with current phenomenological models of delirium in humans (31, 32). Therefore, we conclude that functional inhibition of PER2 in mice using midazolam or LPS induces a delirium-like phenotype similar to that seen in humans, and that PER2 upregulation might represent a potential target to reduce the deliriogenic effects of midazolam or inflammation.
To gain more insight into the role of PER2 in the context of delirium, we studied Per2−/− mice, which revealed a phenotype that shares similarities with acute delirium, but with no further effects following midazolam treatment. Per2−/− mice have a significantly shorter night period, like humans with a PER2 mutation. In addition, Per2−/− is associated with changes in daily locomotor activity and disturbance of the resting period (50), further supporting our findings. While our findings in mice may not be generalizable across species, the presented evidence from mice and humans suggests that disrupted PER2 expression could also play a role in the development of some types of delirium in humans.
To test the possibility that PER2 represents a therapeutic target, we tested the novel PER2 enhancer, nobiletin. Nobiletin, a natural polymethoxylated flavone, was recently identified as a PER2 amplitude-enhancing small molecule (34). We found that nobiletin fully abolished midazolam-induced cognitive deficits in mice. Furthermore, we also found nobiletin to be therapeutic in a mouse model of constant light exposure, which impairs hippocampal-mediated cognitive functions (24). These results suggest that nobiletin could improve cognitive dysfunction in different settings of circadian disruption.
This study has some obvious limitations. First, delirium is a very complex disease state and therefore it is difficult to model delirium in mice (9). In addition, we found characteristic differences in our behavioral tests using different interventions, indicating that multiple mechanisms may be involved. In fact, LPS can cause general sickness which may independently affect locomotion (33). As such, many mechanisms might be involved in the pathogenesis of delirium in mice or humans that cannot simply be explained by a PER2 deficiency. In addition, human data on PER2 expression in patients with delirium are unavailable and only indirect measures of circadian disruption exist. Therefore, before a clear connection between human PER2 and delirium can be made, further research on sleep and circadian PER2 expression in patients are warranted.
Conclusion
In conclusion, our studies provide the first mechanistic evidence that murine PER2 plays a critical role in midazolam-induced behavioral impairment that resembles key characteristics of delirium in humans. Using nobiletin as a PER2 enhancer, we abolished the cognitive dysfunction observed in mice following midazolam or constant light exposure. Future work should explore the role of PER2 expression in human sleep and delirium and the effects of midazolam administration, light exposure, and inflammation on PER2 levels in human patients. In addition, further validation of animal models of delirium would help strengthen the intriguing but yet unproven link to human delirium.
Supplementary Material
Acknowledgments
Source of financial support for the work: National Heart, Lung, and Blood Institute (NIH-NHLBI) 5R01HL122472 Grant to T.E.
We thank Wade Shaffer, BS for building the arenas for the open-field task, novel-object recognition, and T-maze alternation and we thank Dr. Yoshimasa Oyama and Colleen Marie Bartman, BS for their extensive help with the revision.
Footnotes
Copyright form disclosure: Drs. Eckle and Scott received support for article research from the National Institutes of Health (NIH), and they disclosed off-label product use of nobiletin, used to treat delirium. Dr. Eckle’s institution received funding from the NIH/National Heart, Lung, and Blood Institute. Ms. Gile disclosed that she does not have any potential conflicts of interest.
Conflict of Interest
The authors declare there are no conflicts of interest
Disclosure
Nobiletin used to treat delirium is not labeled for the use of delirium and is still investigational.
Contributor Information
Jennifer Gile, Anesthesiology, University of Colorado School of Medicine, Aurora, CO/USA.
Benjamin Scott, Anesthesiology, University of Colorado School of Medicine, Aurora, CO/USA.
Tobias Eckle, Anesthesiology, University of Colorado School of Medicine, Aurora, CO/USA.
References
- 1.Salluh JI, Wang H, Schneider EB, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015;350:h2538. doi: 10.1136/bmj.h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott B, Eckle T. The impact of sedation protocols on outcomes in critical illness. Ann Transl Med. 2016;4(2):33. doi: 10.3978/j.issn.2305-5839.2015.12.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellapart J, Boots R. Potential use of melatonin in sleep and delirium in the critically ill. British journal of anaesthesia. 2012;108(4):572–580. doi: 10.1093/bja/aes035. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald JM, Adamis D, Trzepacz PT, et al. Delirium: a disturbance of circadian integrity? Med Hypotheses. 2013;81(4):568–576. doi: 10.1016/j.mehy.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 5.Hatta K, Kishi Y, Wada K, et al. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA psychiatry. 2014;71(4):397–403. doi: 10.1001/jamapsychiatry.2013.3320. [DOI] [PubMed] [Google Scholar]
- 6.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madrid-Navarro CJ, Sanchez-Galvez R, Martinez-Nicolas A, et al. Disruption of Circadian Rhythms and Delirium, Sleep Impairment and Sepsis in Critically ill Patients. Potential Therapeutic Implications for Increased Light-Dark Contrast and Melatonin Therapy in an ICU Environment. Curr Pharm Des. 2015;21(24):3453–3468. doi: 10.2174/1381612821666150706105602. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Choi W, Ko YH, et al. Bright light therapy as an adjunctive treatment with risperidone in patients with delirium: a randomized, open, parallel group study. Gen Hosp Psychiatry. 2012;34(5):546–551. doi: 10.1016/j.genhosppsych.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Scott BK. Disruption of Circadian Rhythms and Sleep in Critical Illness and its Impact on the Development of Delirium. Current pharmaceutical design. 2015;21(24):3443–3452. doi: 10.2174/1381612821666150706110656. [DOI] [PubMed] [Google Scholar]
- 10.Wang LM, Dragich JM, Kudo T, et al. Expression of the circadian clock gene Period2 in the hippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro. 2009;1(3) doi: 10.1042/AN20090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291(5506):1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 12.Brainard J, Gobel M, Bartels K, et al. Circadian rhythms in anesthesia and critical care medicine: potential importance of circadian disruptions. Semin Cardiothorac Vasc Anesth. 2015;19(1):49–60. doi: 10.1177/1089253214553066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brainard J, Gobel M, Scott B, et al. Health implications of disrupted circadian rhythms and the potential for daylight as therapy. Anesthesiology. 2015;122(5):1170–1175. doi: 10.1097/ALN.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng B, Larkin DW, Albrecht U, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400(6740):169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 15.Bonney S, Kominsky D, Brodsky K, et al. Cardiac Per2 functions as novel link between fatty acid metabolism and myocardial inflammation during ischemia and reperfusion injury of the heart. PLoS One. 2013;8(8):e71493. doi: 10.1371/journal.pone.0071493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckle T, Hartmann K, Bonney S, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18(5):774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo SW, Koeppen M, Bonney S, et al. Differential Tissue-Specific Function of Adora2b in Cardioprotection. J Immunol. 2015;195(4):1732–1743. doi: 10.4049/jimmunol.1402288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeSauter J, Lambert CM, Robotham MR, et al. Antibodies for assessing circadian clock proteins in the rodent suprachiasmatic nucleus. PLoS One. 2012;7(4):e35938. doi: 10.1371/journal.pone.0035938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006;1(1):7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- 20.Podhorna J, Brown RE. Strain differences in activity and emotionality do not account for differences in learning and memory performance between C57BL/6 and DBA/2 mice. Genes, brain, and behavior. 2002;1(2):96–110. doi: 10.1034/j.1601-183x.2002.10205.x. [DOI] [PubMed] [Google Scholar]
- 21.Wolf A, Bauer B, Abner EL, et al. A Comprehensive Behavioral Test Battery to Assess Learning and Memory in 129S6/Tg2576 Mice. PLoS One. 2016;11(1):e0147733. doi: 10.1371/journal.pone.0147733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin EW, Skelly DT, Murray CL, et al. Cyclooxygenase-1-dependent prostaglandins mediate susceptibility to systemic inflammation-induced acute cognitive dysfunction. J Neurosci. 2013;33(38):15248–15258. doi: 10.1523/JNEUROSCI.6361-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sudo M, Sasahara K, Moriya T, et al. Constant light housing attenuates circadian rhythms of mPer2 mRNA and mPER2 protein expression in the suprachiasmatic nucleus of mice. Neuroscience. 2003;121(2):493–499. doi: 10.1016/s0306-4522(03)00457-3. [DOI] [PubMed] [Google Scholar]
- 24.Fujioka A, Fujioka T, Tsuruta R, et al. Effects of a constant light environment on hippocampal neurogenesis and memory in mice. Neurosci Lett. 2011;488(1):41–44. doi: 10.1016/j.neulet.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Oishi K, Sakamoto K, Okada T, et al. Antiphase circadian expression between BMAL1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochem Biophys Res Commun. 1998;253(2):199–203. doi: 10.1006/bbrc.1998.9779. [DOI] [PubMed] [Google Scholar]
- 26.Sato TK, Yamada RG, Ukai H, et al. Feedback repression is required for mammalian circadian clock function. Nat Genet. 2006;38(3):312–319. doi: 10.1038/ng1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valero J, Mastrella G, Neiva I, et al. Long-term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Front Neurosci. 2014;8:83. doi: 10.3389/fnins.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoogland IC, Houbolt C, van Westerloo DJ, et al. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ming Z, Sawicki G, Bekar LK. Acute systemic LPS-mediated inflammation induces lasting changes in mouse cortical neuromodulation and behavior. Neurosci Lett. 2015;590:96–100. doi: 10.1016/j.neulet.2015.01.081. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789–856. ix. doi: 10.1016/j.ccc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Meagher DJ, Moran M, Raju B, et al. Phenomenology of delirium. Assessment of 100 adult cases using standardised measures. Br J Psychiatry. 2007;190:135–141. doi: 10.1192/bjp.bp.106.023911. [DOI] [PubMed] [Google Scholar]
- 32.Meagher DJ, Moran M, Raju B, et al. Motor symptoms in 100 patients with delirium versus control subjects: comparison of subtyping methods. Psychosomatics. 2008;49(4):300–308. doi: 10.1176/appi.psy.49.4.300. [DOI] [PubMed] [Google Scholar]
- 33.Skelly DT, Hennessy E, Dansereau MA, et al. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1beta, [corrected] TNF-alpha and IL-6 challenges in C57BL/6 mice. PLoS One. 2013;8(7):e69123. doi: 10.1371/journal.pone.0069123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He B, Nohara K, Park N, et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016;23(4):610–621. doi: 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fonken LK, Finy MS, Walton JC, et al. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res. 2009;205(2):349–354. doi: 10.1016/j.bbr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Martinez MC, Fernandez SP, Loscalzo LM, et al. Hesperidin, a flavonoid glycoside with sedative effect, decreases brain pERK1/2 levels in mice. Pharmacol Biochem Behav. 2009;92(2):291–296. doi: 10.1016/j.pbb.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Banji OJ, Banji D, Ch K. Curcumin and hesperidin improve cognition by suppressing mitochondrial dysfunction and apoptosis induced by D-galactose in rat brain. Food Chem Toxicol. 2014;74:51–59. doi: 10.1016/j.fct.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Ripperger JA, Albrecht U. The circadian clock component PERIOD2: from molecular to cerebral functions. Prog Brain Res. 2012;199:233–245. doi: 10.1016/B978-0-444-59427-3.00014-9. [DOI] [PubMed] [Google Scholar]
- 39.Zueger M, Urani A, Chourbaji S, et al. mPer1 and mPer2 mutant mice show regular spatial and contextual learning in standardized tests for hippocampus-dependent learning. J Neural Transm (Vienna) 2006;113(3):347–356. doi: 10.1007/s00702-005-0322-4. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, Toh KL, Jones CR, et al. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128(1):59–70. doi: 10.1016/j.cell.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamdar BB, Needham DM, Collop NA. Sleep deprivation in critical illness: its role in physical and psychological recovery. J Intensive Care Med. 2012;27(2):97–111. doi: 10.1177/0885066610394322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hallows WC, Ptacek LJ, Fu YH. Solving the mystery of human sleep schedules one mutation at a time. Crit Rev Biochem Mol Biol. 2013;48(5):465–475. doi: 10.3109/10409238.2013.831395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahman SA, Kayumov L, Tchmoutina EA, et al. Clinical efficacy of dim light melatonin onset testing in diagnosing delayed sleep phase syndrome. Sleep Med. 2009;10(5):549–555. doi: 10.1016/j.sleep.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Fraser GL, Devlin JW, Worby CP, et al. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: a systematic review and meta-analysis of randomized trials. Crit Care Med. 2013;41(9 Suppl 1):S30–38. doi: 10.1097/CCM.0b013e3182a16898. [DOI] [PubMed] [Google Scholar]
- 45.Iacobone E, Bailly-Salin J, Polito A, et al. Sepsis-associated encephalopathy and its differential diagnosis. Crit Care Med. 2009;37(10 Suppl):S331–336. doi: 10.1097/CCM.0b013e3181b6ed58. [DOI] [PubMed] [Google Scholar]
- 46.Matsuo I, Iijima N, Takumi K, et al. Characterization of sevoflurane effects on Per2 expression using ex vivo bioluminescence imaging of the suprachiasmatic nucleus in transgenic rats. Neurosci Res. 2016;107:30–37. doi: 10.1016/j.neures.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Okada K, Yano M, Doki Y, et al. Injection of LPS causes transient suppression of biological clock genes in rats. J Surg Res. 2008;145(1):5–12. doi: 10.1016/j.jss.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Li CX, Liang DD, Xie GH, et al. Altered melatonin secretion and circadian gene expression with increased proinflammatory cytokine expression in early-stage sepsis patients. Mol Med Rep. 2013;7(4):1117–1122. doi: 10.3892/mmr.2013.1331. [DOI] [PubMed] [Google Scholar]
- 49.Smith HAB, Gangopadhyay M, Goben CM, et al. Delirium and Benzodiazepines Associated With Prolonged ICU Stay in Critically Ill Infants and Young Children. Crit Care Med. 2017;45(9):1427–1435. doi: 10.1097/CCM.0000000000002515. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura T, Takumi T, Takano A, et al. Characterization and modeling of intermittent locomotor dynamics in clock gene-deficient mice. PLoS One. 2013;8(3):e58884. doi: 10.1371/journal.pone.0058884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.