Dear Editor,
Small cell carcinoma of the esophagus (SCCE) is the most common extrapulmonary small cell carcinoma, accounting for 1%–2.8% of all esophageal carcinomas.1 Most patients with SCCE die within 2 years of diagnosis and experience a median survival of only 8–13 months.1 Due to lack of prospective data and its similarities in histological appearance and clinical behavior to small cell lung cancer (SCLC), treatments for SCCE are adopted from well-established therapeutic strategies for SCLC.2 Chemotherapy is initially effective for SCCE, but most patients suffer a rapid recurrence and die within a few months.2 More effective and precise treatment strategies for SCCE are urgently required, but have been hampered by lack of information on the molecular drivers of this deadly cancer.3 Genome sequencing studies have revealed several potential driver events in two other major subtypes of esophageal carcinoma, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), and showed that they have distinct molecular characteristics, indicating the heterogeneity of esophageal carcinomas.4, 5 To understand the genetic basis of this deadly disease and enable the development of new diagnostic and therapeutic tools for its treatment, we performed genomic profiling of 55 patients with SCCE using whole-exome sequencing (WES) validated by ultra-deep targeted sequencing and copy number microarray assays.
The paired samples were obtained under institutional review board approval (Supplementary information, Table S1). More than half (76.4%) of the patients were men, the primary location of the tumors was the middle thoracic region of the esophagus (72.7%), 52.7% of the tumors were Stages III and IV, and 76.4% of the patients were deceased at the last follow-up (Supplementary information, Table S2). The median overall survival was 18 months, and chemotherapy significantly improved patients’ overall survival (P = 0.012; Supplementary information, Figure S1a). By WES, a total of 4,990 somatic mutations were identified including 3,446 nonsilent, 1,335 silent, 1 stop-loss and 208 short insertions and deletions (indels) (Supplementary information, Figure S1b, Tables S3 and 4, mean depth: 129×). The mutation rate (average: 2.85/Mb) was relatively high among all adult-derived tumors (Supplementary information, Figure S2). Women showed a higher mutation rate than men (P = 0.0017; Supplementary information, Table S5). To validate the findings, 113/115 single nucleotide variations (SNVs) and 9/10 indels were verified by ultra-deep targeted sequencing with consistencies of 98.3% and 90%, respectively (Supplementary information, Figure S3, Tables S6 and 7). Of the 33 candidate somatic mutations, 31 were confirmed with Sanger sequencing (true-positive rate = 94%, Supplementary information, Table S8).
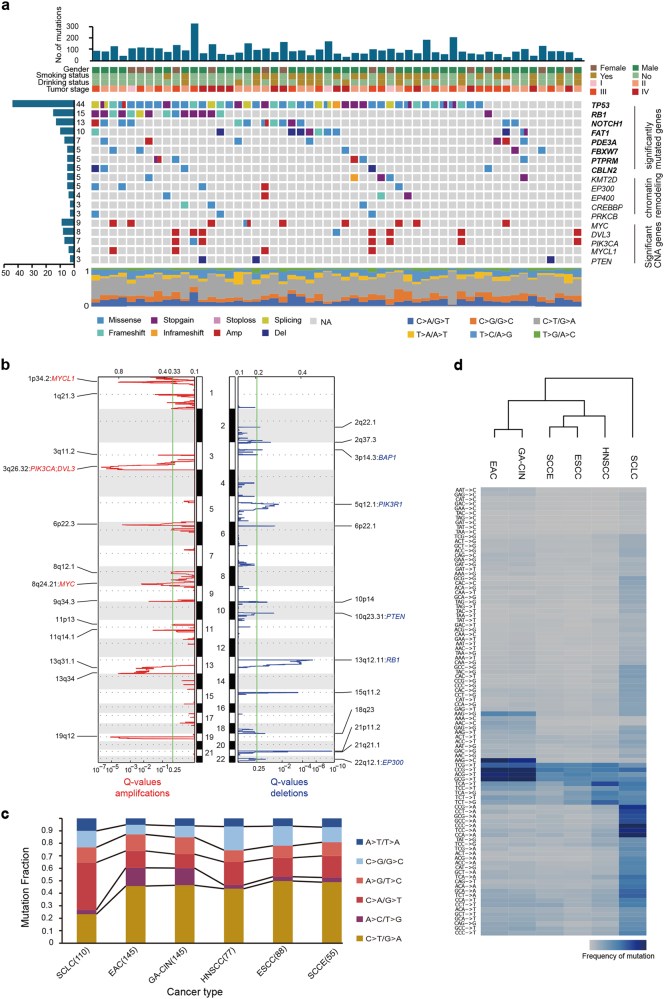
The mutational spectrum of SCCE was dominated by C>T/G>A transitions (Fig. 1a). To discover the mutational processes in SCCE, a mutational signature analysis using the BayesNMF algorithm4 was performed, which identified three mutational signatures in SCCE that matched three of the Sanger signatures in the Catalogue of Somatic Mutations in Cancer (COSMIC) database (Supplementary information, Figure S4). The signature S1 closely resembled COSMIC signature 13 (cosine similarity: 0.79) and was characterized by C>T transitions and C>G transversions at TC[A/T] motifs. This signature has been observed in multiple tumor types and is attributed to APOBEC-mediated mutagenesis.6 The signature S2 was characterized by a broad spectrum of base changes and matched COSMIC signature 5 (cosine similarity: 0.87), the etiology of which remains unknown.6 The last signature, S3, characterized by C>T transitions at NpCpG trinucleotides (analogous to COSMIC signature 1; cosine similarity: 0.89), has been observed in multiple cancer types.6 It is considered age-related and a consequence of spontaneous deamination of 5-methylcytosine.6
Fig. 1.
Genetic landscape of small cell carcinoma of the esophagus. a Genome-wide mutational landscape of small cell carcinoma of the esophagus, characterized by whole-exome sequencing. Top panel: The number of somatic mutations in each tumor. Four key clinicopathological characteristics are presented below. Stage was determined based on the seventh edition of the American Joint Committee on Cancer Staging Manual: Esophagus and Esophagogastric Junction. Left panel: The total number of patients harboring mutations in each gene. Middle panel: The matrix of mutations in a selection of frequently mutated genes, arranged vertically by statistical or functional group and colored by the type of alterations. Amp and Del correspond to amplification and deletion events, respectively, as indicated by a GISTIC value ± 2. Significantly mutated genes are displayed in bold. Columns represent samples. Bottom panel: Mutation fraction of the six-base substitution for each patient. b Significant copy number gains (red) and losses (blue) detected by GISTIC 2.0 (shown as peaks). Cancer-related genes with recurrent copy number alterations in peaks are labeled. c Mutation fraction of the six-base substitution labeled in different colors for small cell carcinoma of the esophagus (SCCE), esophageal squamous cell carcinoma (ESCC), head and neck squamous cell carcinoma (HNSCC), esophageal adenocarcinoma (EAC), chromosomal instability variant of gastric cancer (GA-CIN) and small cell lung cancer (SCLC). d Hierarchical clustering of SCCE, ESCC, HNSCC, EAC, GA-CIN and SCLC according to the mutation frequency of 96 mutation types (six-base substitutions each with 16 possible combinations of neighboring bases)
To explore mutations and genes conferring selective growth advantages, we employed a previously described method that takes background mutation burden, functional impact and gene sequence length into consideration.6 Eight genes were identified as significantly mutated in patients with SCCE (q-value < 0.01), comprising five well-known genes (TP53, RB1, NOTCH1, FAT1 and FBXW7) and three genes (PDE3A, PTPRM and CBLN2) not previously implicated in esophageal cancers (Fig. 1a, Supplementary information, Table S9). Notably, we observed a relatively high frequency (7.3%, 4/55) of the p.C141R/Y mutation in TP53 (Supplementary information, Figure S5a). Of 15 RB1 mutations, 14 were detected, comprising nine nonsense mutations, four frame-shift indels, and one splice site mutation, which caused truncation (Supplementary information, Table S4). Bi-allelic inactivation of TP53 and RB1 was also observed in 62.5% (15) and 29.2% (7), respectively, of the 24 patients profiled by the OncoScan CNV Assay (Supplementary information, Figure S5b), consistent with the two-hit hypothesis of tumor suppressors.
We determined somatic copy number alterations (SCNAs) based on WES data, and 24 WES specimens were analyzed using the OncoScan CNV FFPE Assay (Supplementary information, Figures S1b, 6a, b). SCCE showed a high SCNA burden, with a median of 27.9% per genome (Supplementary information, Figure S6c). To identify recurrent focal SCNAs, the GISTIC algorithm was applied and 13 regions of amplification were detected (Fig. 1b, Supplementary information, Table S10). The significantly amplified regions included 1p34.2, 3q26.32, 8q24.21 and 19q12, encompassing well-known oncogenes such as MYCL1, PIK3CA, MYC and CCNE1 (Fig. 1b, Supplementary information, Table S10). Two members of the MYC family, MYC and MYCL1, were gained or amplified in 80% (44/55) and 56.4% (31/55) of patients, respectively, indicating the activation of MYC signaling in SCCE (Supplementary information, Figure S7). We also manually searched for possible therapeutic inhibitors of amplified cancer-related genes (overlapping with genes included in Cancer Gene Census v77) against DGIdb2.0, a curated and combined gene-drug database from twenty-seven sources. By selecting drug categories with “FDA approved” and “Antineoplastic”, we found 13 genes that are potential clinically actionable therapeutic targets, including genes such as PIK3CA, CCNE1, MYC and ABL2, and 100% of patients with SCCE have amplification of these genes (Supplementary information, Table S11).
GISTIC also identified 13 focal deletions (Fig. 1b, Supplementary information, Table S10), including at 3p14.3, 5q12.1, 10q23.31 and 13q12.11, which encode tumor suppressors such as BAP1, PIK3R1, PTEN and RB1 (Fig. 1b). FAT1 was also frequently altered in SCCE, and we identified 65.5% (36/55) of the tumors as having a loss/deletion and a high frequency (10.9%, 6/55) of somatic mutations (Supplementary information, Figure S8). In addition, SCNAs were detected in a wide variety of histone methyltransferases, including gains/amplifications of EHMT1 (41.8%), EHMT2 (52.7%), and SMYD2 (58.2%) and loss/deletion of SETD2 (69.1%) (Supplementary information, Figure S9).
Pathway analysis showed enrichment of the cell cycle (adjusted P = 0.0027), and the p53 (adjusted P = 0.0053), Notch (adjusted P = 2.38e-06) and Wnt (adjusted P = 0.0022) signaling pathways (Supplementary information, Figure S7, Table S12), which is also the case in ESCC.7 Somatic mutations in NOTCH1 (21.8%), NOTCH2 (5.5%) and NOTCH3 (3.6%) were detected in 29.1% of all 55 patients (Supplementary information, Figures S7b, 10a). Intriguingly, they tended to be mutually exclusive. Furthermore, patients with a NOTCH family mutation experienced a poorer median overall survival (14.5 vs 23 months, P = 0.0045; Supplementary information, Figure S10b), indicating that NOTCH mutations may serve as markers of poor prognosis in SCCE.
Somatic alterations in Wnt pathway components were detected in 96.4% of all patients. These alterations include mutations in APC (3.6%), APC2 (3.6%), AXIN1 (1.8%), FZD5 (1.8%), FZD6 (1.8%) and DVL3 (1.8%), and frequent gain/amplification of FZD6 (78.2%) and DVL3 (72.7%) (Supplementary information, Figure S7c). The expression of DVL3 and β-catenin was upregulated in tumors compared with controls (Supplementary information, Figure S11a), which is consistent with previous findings in multiple cancer types.8, 9 Higher mRNA expression of downstream targets of the Wnt/β-catenin pathway, including LGR5, SNAIL, TWIST, SOX2, OCT4 and AXIN2, was also observed in SCCE (Supplementary information, Figure S11b). These results indicate that the Wnt/β-catenin pathway is highly active in SCCE.
Comparing the molecular portraits of SCCE with those of ESCC,7 HNSCC, EAC, chromosomal instability variant of gastric cancer (GA-CIN) and SCLC (Supplementary information, Data S1), SCCE showed a mutation rate comparable to those of ESCC and HNSCC, but lower than that of SCLC (Supplementary information, Figure S2). The mutation spectrum of six-base substitution types revealed that SCCE more closely resembles ESCC and HNSCC (Fig. 1c). Moreover, clustering of mutation signatures based on 96 possible mutation types suggested that SCCE is more closely related to ESCC and HNSCC (Fig. 1d). The dominant C>A mutation, which was reported to be due to tobacco smoking, discriminates SCLC from other cancers.6 Furthermore, four well-known significantly mutated genes (TP53, NOTCH1, FAT1 and FBXW7) identified in our study show similar high mutation frequencies in ESCC, HNSCC and SCLC (Supplementary information, Figure S12). In addition, the chromosome-wide distribution of G-scores revealed the similarities between SCCE and ESCC or HNSCC (Supplementary information, Figure S13), particularly in the amplifications of 3q and 8q and the deletion of 3p. These features indicate that SCCE genetically resembles ESCC and HNSCC rather than the other cancer types tested.
For the first time, we have provided a comprehensive genomic profile of SCCE. Three significantly mutated genes, PDE3A, PTPRM and CBLN2, have not been previously implicated in esophageal cancers. The functions of these three genes in cancer have not been clarified. PDE3A is a member of a gene family named 3′,5′-cyclic nucleotide phosphodiesterases (PDEs), which negatively regulates the second messengers cAMP and cGMP. The best-studied downstream target of cAMP is protein kinase A (PKA), which can induce a mesenchymal-to-epithelial transition and loss of tumor-initiating ability in human mammary epithelial cells. Moreover, a subset of PDE3A inhibitors such as DNMDP were found to kill cancer cells. Another gene, PTPRM, negatively regulates cell growth and colony formation, and loss of PTPRM promotes oncogenic cell growth in colon cancer. Cerebellin precursor protein 2 (CBLN2) is expressed in the adult nervous system, but its function in cancer has not been reported. Somatic alterations were accumulated in genes involved in the Wnt/β-catenin pathway, suggesting that hyperactivation of this pathway might be an important molecular event leading to SCCE. The mechanisms underlying this phenomenon need to be clarified in the future. We also identified mutations in other well-known genes, such as TP53, RB1, NOTCH1, FAT1 and those related to the chromatin remodeling process. We observed somatic genomic alterations in SCCE similar to those in SCLC such as loss of P53 and RB1 and mutations in the NOTCH family. However, in terms of its mutational spectrum and somatic CNV profile, SCCE more closely resembles ESCC and HNSCC than SCLC, suggesting that small cell carcinoma originating from esophageal cells has a different biological background than small cell carcinomas originating from lung cells. This is consistent with recent evidence that small cell carcinomas originating from different sites may be heterogeneous.10 Our findings reveal key genomic alterations in SCCE and provide insights to better understand the pathogenesis of SCCE and to develop better treatment strategies for patients with this condition.
Electronic supplementary material
Acknowledgements
We are grateful to all the patients who contributed their tumor specimens. We thank Professor Xinyuan Guan (University of Hong Kong, China) and Professor Musheng Zeng (University of Sun Yat-sen University Cancer Center, China) for suggestions. This work was supported by the National Natural Science Foundation of China (81572392), the Major Special Project from Guangzhou Health and Medical Collaborative Innovation (15570006), the Natural Science Foundation of Guangdong Province (2014A030312015), the Science and Technology Program of Guangdong Province (2015B020232008), the Science and Technology Program of Guangzhou (15570006), the Pearl River Nova Program of Guangzhou (201610010068), the Guangdong Esophageal Cancer Institute Science and Technology Program (Q201517), the Fundamental Research Funds for the Central Universities (14ykpy40), the Guangzhou Health-Medical Collaborative Innovation Project (201508020247), the National Key Research and Development Program of China (2017YFC1308900), the China Postdoctoral Science Foundation (2017M610573) and the Guangzhou Health-Medical Collaborative Innovation Project (201400000004-5). F Wang is a recipient of the Outstanding Young Talents Program of Sun Yat-sen University and the Young Physician Scientist Program of Sun Yat-sen University Cancer Center.
Competing interests
The authors declare no competing interests.
Contributor Information
Feng Wang, Email: wangfeng@sysucc.org.cn.
Rui-Hua Xu, Email: xurh@sysucc.org.cn.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41422-018-0039-1.
References
- 1.Hudson E, et al. Br. J. Cancer. 2007;96:708–711. doi: 10.1038/sj.bjc.6603611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner B, Tang LH, Klimstra DS, Kelsen DP. J. Clin. Oncol. 2004;22:2730–2739. doi: 10.1200/JCO.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 3.Lv J, et al. J. Thorac. Oncol. 2008;3:1460–1465. doi: 10.1097/JTO.0b013e31818e1247. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network et al. Nature. 2017;541:169–175. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao YB, et al. Nat. Genet. 2014;46:1097–1102. doi: 10.1038/ng.3076. [DOI] [PubMed] [Google Scholar]
- 6.Alexandrov LB, et al. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song Y, et al. Nature. 2014;509:91–95. doi: 10.1038/nature13176. [DOI] [PubMed] [Google Scholar]
- 8.Uematsu K, et al. Oncogene. 2003;22:7218–7221. doi: 10.1038/sj.onc.1206817. [DOI] [PubMed] [Google Scholar]
- 9.Chan DW, Chan CY, Yam JW, Ching YP, Ng IO. Gastroenterology. 2006;131:1218–1227. doi: 10.1053/j.gastro.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Ramos P, et al. Nat. Genet. 2014;46:427–429. doi: 10.1038/ng.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.