Abstract
Human cytomegalovirus (HCMV) donor positive (D+) serostatus with acute rejection is associated with renal allograft loss, but the impact of recipient positive (R+) serostatus is unclear. In an allogeneic renal transplant model, antiviral natural killer (NK) and CD8+ T cell memory responses in murine CMV (MCMV) D+/R+ transplants were compared to D−/R− and D+/R− transplants, with recipient infection varied by MCMV dose and strain. D+/R− transplants had high primary antiviral cytolytic (interferon-γ+) and cytotoxic (granzyme B+) NK responses, whereas NK memory responses were lower in D+/R+ recipients receiving a high primary MCMV dose. Despite MCMV immunity, D+/R+ recipients receiving a low MCMV dose showed primary-like high cytolytic and cytotoxic NK responses. D+/R+ transplants infected with different D/R strains had low cytolytic NK responses, but high cytotoxic NK responses. NK memory also induced a novel TNF-α+ NK response among high-dose virus recipients. MCMV+ transplants had greater Th17 responses than MCMV uninfected transplants, and Th17 inhibition ameliorated graft injury. All MCMV+ recipients had similar CD8+ T cell responses. In sum, NK and Th17 responses, but not CD8+ T cells, varied according to conditions of primary recipient infection. This variability could contribute to variable graft outcomes in HCMV D+/R+ renal transplantation.
INTRODUCTION
Human cytomegalovirus (HCMV) infection is a risk factor for renal allograft loss in patients with acute rejection (1–3). The risk is greater for HCMV serostatus donor positive (D+) patients compared to donor negative (D−) patients, but the impact of recipient serostatus is unclear, with some studies showing poorest graft survival in the D+/R+ group, but others showing worse outcomes in the D+/R− group (4, 5). Antiviral prophylaxis against HCMV is associated with improved late graft function and survival (5–8). The mechanisms underlying these associations are unknown, and could include direct viral cytolysis or antiviral immune-mediated allograft injury in association with acute rejection.
The immune response to HCMV and murine CMV (MCMV) has been well characterized (reviewed in (9)). Initial control of primary CMV infection is mediated by natural killer (NK) cells (10–12). Among transplant patients, NK cells increase in number and activation status during episodes of HCMV viremia, and NK cells with activating receptors are enriched in peripheral blood during CMV infection (13–17). Patients with NK activating killer cell immunoglobulin-like receptor (KIR) genotypes have lower rates of post-transplant HCMV infection (15, 17, 18). Memory NK cells against MCMV infection preferentially re-expand upon viral rechallenge, and NK memory is also established after HCMV infection (19–22). NK cells assist in shaping the antiviral CD8+ T cell response (9, 23–25).
The CD8+ T cells control acute and persistent CMV infection (reviewed in (9)). In D+/R− transplantation, primary CMV infection induces the development of virus-specific CD8+ T cells with a differentiated phenotype, which are associated with protection from CMV disease (26, 27). Among D+/R+ patients, CMV-specific T cells may expand post-transplant even in the absence of detectable CMV viremia (28). Both HCMV and MCMV infection induce memory inflation, characterized by expansion of a CMV-specific population with an effector memory phenotype (TEM) that differs from the contraction of non-inflated memory T cells that maintain a central memory (TCM) phenotype (29–33). In human populations, HCMV-specific T cells constitute 5–10% of the circulating memory repertoire (34).
Although NK and CD8+ T cells control CMV disease, their impact upon renal allograft injury in CMV immune (R+) patients is not defined. In a murine allogeneic renal transplant model, MCMV D+/R− transplants had increased intragraft CD45+ infiltrates compared to D−/R− transplants, including NK cells, CD4+ and CD8+ T cells (35). MCMV-infected allografts with acute rejection demonstrated more severe late fibrosis compared to MCMV-uninfected grafts, suggesting that MCMV-associated early graft injury combined with acute rejection might contribute to late graft fibrosis (36). NK depletion ameliorated MCMV-associated allograft damage, suggesting that virus-directed NK cells mediate allograft injury (36). In this study, we investigated the impact of antiviral memory NK and CD8+ T cells upon MCMV-associated allograft injury by varying the recipient’s infecting virus dose to generate differential memory CD8+ T cell responses (TCM vs. TEM), or by infecting the recipient with a different MCMV strain from the donor organ.
MATERIALS AND METHODS
Virus and animals
MCMV Smith strains, either wild-type (MCMV-WT) or with a deletion mutation of the m157 open reading frame (MCMVΔm157, kind gift of S. Jonjic, University of Rijeka, Rijeka Croatia) were propagated and stored as previously described (35). BALB/cJ or C57BL/6J mice (Jackson Laboratory, Bar Harbor ME) were housed as described in Supplemental Methods. Murine experimental protocols were approved by the Institutional Animal Care and Use Committees.
Renal transplantation surgery
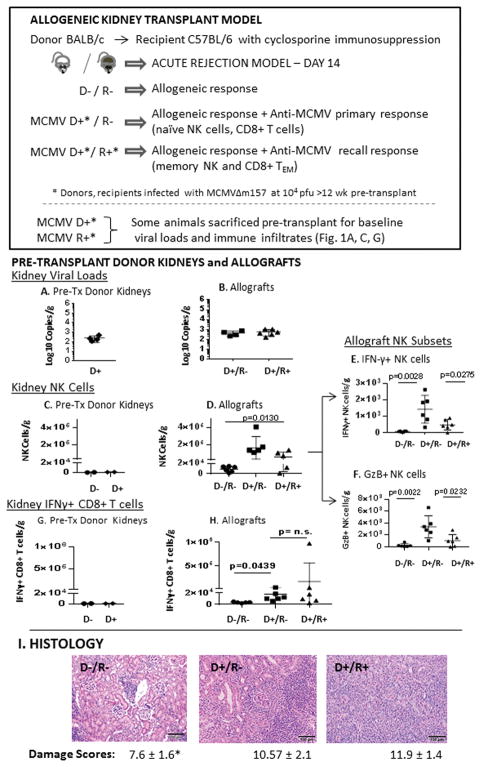
Donor BALB/cJ (“BALB,” H-2d) mice were infected by intraperitoneal injection with MCMVΔm157 at 104 plaque forming units (pfu) for all D+ transplants. Recipient C57BL/6J (“B6,” H-2b) mice were infected (R+) with MCMVΔm157 at either 102 pfu (low-dose) or 104 pfu (high-dose), or with MCMV-WT at 104 pfu. Thus, all D+ donor kidneys had the same virus strain and dose, whereas recipient infection conditions differed for each experimental group. All mice were infected >12 weeks prior to transplant to establish antiviral NK and CD8+ T cell memory (31, 37, 38). Uninfected mice were used for D−/R− transplants. Kidney transplantation was performed as described, retaining the contralateral native kidney for this non-life-sustaining transplant model (35, 39). Recipients were treated with cyclosporine (Novartis Pharmaceuticals Corp., East Hanover NJ) at 10 mg/kg/day, subcutaneously once daily starting immediately postoperatively until terminal sacrifice at day 14 post-transplant (40). Results from 6–8 animals were analyzed per experimental group. In addition, some infected BALB and B6 mice were sacrificed >12 weeks after infection (“pre-transplant” groups, n=3–4/group) to establish baseline viral and immune parameters for comparison with transplant animals.
Flow cytometry
Organs were processed for flow cytometry as described in Supplemental Methods (35). Cells were incubated for 6 hours with brefeldin A (BD Biosciences, San Jose CA), under the following conditions: (1) no stimulation; (2) PMA-ionomycin stimulation (eBiosciences, San Diego CA); (3) MCMV peptide pool consisting of MCMV M45985-993, M38316-323, m139419-426, and IE3416-423 at 10 μmol/ml (Genemed Synthesis, Inc., San Antonio TX); (4) M45985-993 alone; (5) M38316-323 alone (31).
After incubation, cells were washed, stained for flow cytometry, and analyzed as described in Supplemental Methods (41). NK cells, CD4+ and CD8+ T cells were compared using the no-stimulation condition for endogenous cytokine-secreting cells, or peptide-stimulation conditions for MCMV-specific responses. PMA-ionomycin stimulations were used as positive controls but were not analyzed for group comparisons.
Histology and scoring
Allograft tissues were fixed for >24 hours in 10% neutral buffered formalin (Sigma-Aldrich), processed for paraffin embedding and sectioning, and stained with hematoxylin and eosin. Sections were evaluated by a veterinary pathologist (T.R.S.) blinded to sample identity using a previously published 8-criteria scale (Table 1) with a maximum damage score of 24 (36).
TABLE 1.
Histologic Damage Scores for D+/R+ Allografts
| D/R status | D+/R+ | D+/R+ | D+/R+ | |
|---|---|---|---|---|
|
| ||||
| Virus strains | Δ157/Δ157 Same virus | Δ157/Δ157 Same virus | Δ157/WT Different virus | |
| Recipient virus dose | 102 pfu | 104 pfu | 104 pfu | |
|
| ||||
| HISTOLOGY SCORES | p-value | |||
| Glomerulosclerosis | 2.4 ± 0.6 | 2.6 ± 0.5 | 2.9 ± 0.2 | n.s. |
| Tubular degeneration | 2.4 ± 0.9 | 2.6 ± 0.7 | 2.9 ± 0.2 | n.s. |
| Interstitial inflammation | 2.4 ± 0.4 | 2.4 ± 0.4 | 2.9 ± 0.2 | 0.0105 |
| Interstitial fibrosis | 0.0 ± 0.0 | 0.1 ± 0.2 | 0.3 ± 0.4 | n.s. |
| Edema | 0.1 ± 0.4 | 0.2 ± 0.3 | 0.4 ± 0.4 | n.s. |
| Perivascular inflammation | 2.8 ± 0.4 | 2.3 ± 1.0 | 2.4 ± 0.6 | n.s. |
| Arteritis | 0.7 ± 0.8 | 0.8 ± 1.0 | 1.4 ± 0.6 | n.s. |
| Necrosis | 0.7 ± 0.7 | 0.9 ± 0.7 | 1.0 ± 0.7 | n.s. |
|
| ||||
| TOTAL SCORE | 11.5 ± 2.2 | 11.9 ± 1.4 | 14.3 ± 1.8 | 0.0318 |
Cytokine bead immunoassay
Allografts, livers, and spleens were processed for cytokine detection using the LEGENDplex Mouse Inflammation Panel (Biolegend) as described in Supplemental Methods. Cytokine results were analyzed using LEGENDplex Data Analysis Software, Version 7.0 (VigeneTech Inc., Carlisle MA) and depicted as picograms/gram tissue.
IL-6 in vivo depletion
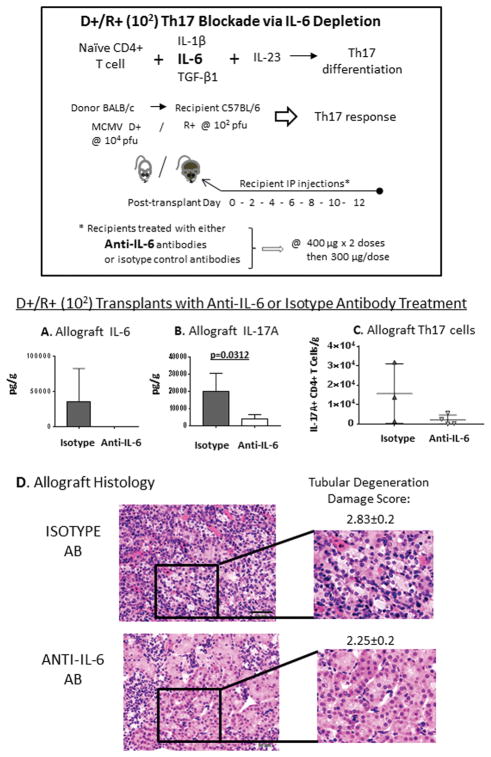
To deplete IL-6 in vivo, kidney transplant recipients were treated with neutralizing rat anti-mouse IL-6 antibodies (clone MP5-20F3) or isotype matched control rat anti-mouse IgG1 antibodies (clone HRPN) (BioXCell, West Lebanon NH) by intraperitoneal injection, starting on day 0 immediately post-transplant and subsequently every second day until terminal sacrifice (Figure 6). Antibodies were dosed at 400 μg/dose x 2 doses, followed by 300 μg/dose (42, 43). Allografts were procured at day 14 post-transplant for histology and quantitation of IL-6, IL-17A, and IL-17A-producing CD4+ T cells.
FIGURE 6. Th17 inhibition via IL-6 blockade reduces intragraft Th17 cell infiltrates and allograft injury.
To test the impact of Th17 cells upon the infected allograft, Th17 cell differentiation was inhibited in D+/R+ low dose (102) recipients by administration of neutralizing anti-IL-6 antibodies post-transplant. Another group of D+/R+ low dose (102) recipients was treated with isotype-matched control antibodies at the same dosing and intervals. At day 14 post-transplant, allograft cytokines, IL-17A+ CD4+ T cells, and histology were examined.
(A–C) Allograft (A) IL-6 and (B) IL-17A cytokines and (C) IL-17A+ CD4+ T cells were quantitated in D+/R+ low dose (102) transplants treated with anti-IL-6 or isotype control antibodies.
(D) Hematoxylin and eosin stained allograft tissues were graded for allograft damage. Tubular degeneration is shown (40x).
Quantitative DNA PCR for MCMV viral load
Quantitative DNA PCR for MCMV viral loads in organs was performed as previously described, using primers and probes for MCMV immediate-early 1 (IE1) exon 4 (44). A PCR assay was developed to distinguish MCMV-WT from MCMVΔm157 viruses, PCR reactions performed, and results expressed as copies per gram tissue as described in Supplemental Methods (45).
Statistical analysis
All experiments were analyzed using 6–8 animals for each experimental group, except for the IL-6 depletion experiment (n=3/group). Comparisons between two groups were analyzed using the Student’s t-test, and comparisons between 3 or more groups were analyzed by ANOVA using Prism 7.0 software, accepting statistically significant differences at a p value of < 0.05 (GraphPad, San Diego CA). Results were depicted as means ± standard deviations (SD).
RESULTS
Recipient immunity influences allograft NK cell infiltrates but not viral loads
MCMV D+/R− allografts have more severe allograft injury compared to D−/R− grafts (35), but recipient immunity could either exacerbate immune-mediated allograft injury or prevent allograft injury by limiting viral replication. To determine whether prior recipient immunity is beneficial or detrimental, D+/R+ transplants (D/R MCMVΔm157, 104 pfu) were compared to D−/R− and D+/R− transplants at day 14 post-transplant (Figure 1). Pre-transplant BALB donor kidneys had similar viral loads compared to both D+/R− and D+/R+ post-transplant allografts (Figure 1A–B), indicating that viral loads were not reduced by recipient immunity. BALB pre-transplant D− and D+ kidneys had low NK cell infiltrates (Figure 1C). Compared to D−/R− transplants, D+/R− and D+/R+ transplants had significantly more abundant intragraft NK infiltrates (p=0.013), comprised of both cytolytic interferon-γ (IFN-γ) producing NK cells (Fig. 1E), and cytotoxic granzyme B (GzB) producing NK cells (Fig.1F). Antiviral memory NK responses in D+/R+ grafts were significantly lower than primary D+/R− responses for both IFN-γ+ NK cells (p=0.0275)and GzB+ NK cells (p=0.0232). Intragraft NK infiltrates did not correlate with viral loads (d.n.s.).
FIGURE 1. NK and CD8+ T cell primary and memory responses in MCMV infected allografts.
Donor BALB/c and recipient C57BL/6 (B6) mice were infected with MCMVΔm157 strain at 104 pfu by intraperitoneal injection at least 12 weeks prior to transplantation to establish NK and CD8+ T cell antiviral memory. MCMV D−/R−, D+/R−, and D+/R+ allogeneic kidney transplants were performed with cyclosporine immunosuppression, and allograft-infiltrating leukocytes were analyzed by flow cytometry on post-transplant day 14. MCMV infected BALB/c and B6 mice were sacrificed > 12 weeks post-infection to establish baseline pre-transplant organ viral loads and leukocyte infiltrates.
(A, B) DNA was extracted from (A) pre-transplant MCMV+ BALB/c kidneys or (B) post-transplant D+/R− and D+/R+ allografts, and MCMV viral loads were determined by quantitative DNA PCR.
(C–F) Kidneys from (C) pre-transplant uninfected (D−) or MCMV infected (D+) BALB/c kidneys, or (D) allografts from D−/R−, D+/R− and D+/R+ transplants were processed for total live cells gated on CD45+/MHCII-/CD3-/NKp46+ NK cells, and the number of NK cells expressing (E) interferon-γ (IFN-γ+ NK cells) or (F) granzyme B (GzB+ NK cells) were compared between groups.
(G, H) Total live cells from (G) pre-transplant uninfected (D−) or MCMV infected (D+) BALB/c kidneys, and (H) D−/R−, D+/R− and D+/R+ allografts were gated on CD45+/MHCII-/CD3+/CD8+ T cells expressing IFN-γ and compared between groups.
(I) Allograft tissues from D−/R−, D+/R−, and D+/R+ transplants were fixed in formalin, paraffin embedded, and stained with hematoxylin and eosin. Allograft damage was graded using an 8-criteria scale (maximum score 24), and scores were compared between groups (n=6–8/group; * p<0.05). Images were collected under identical conditions at 20x magnification. Scale bar=100 μm.
MCMV infection induces intragraft CD8+ T cell infiltrates which are not exacerbated by recipient immunity
BALB pre-transplant D− and D+ kidneys (Figure 1G) had very few IFN-γ+ CD8+ T cell infiltrates. D−/R− allografts had significantly greater IFN-γ+ CD8+ T cell infiltrates than pre-transplant donor kidneys (p=0.0178), consistent with an alloimmune response (Figure 1H). D+/R− allografts had significantly greater IFN-γ+ CD8+ T cell infiltrates compared to D−/R− grafts (p=0.0439), and were similar to D+/R+ grafts (Figure 1H). This result indicates that MCMV D+ infection induces greater CD8+ T cell infiltrates compared to D− grafts, but are not influenced by prior recipient immunity (R+).
MCMV donor and recipient infections are associated with allograft damage
Using an 8-criteria scale to quantitate allograft damage, at day 14 post-transplant D−/R− allografts had lower damage scores (7.6±1.6) compared to D+/R− grafts (10.57±2.1, p=0.027), consistent with prior studies (36). D+/R+ transplant had higher damage scores (11.9±1.4) than D−/R− grafts (p=0.0022), but were similar to D+/R− grafts. This result indicates that MCMV infected (D+) allografts had greater histologic injury compared to uninfected grafts, regardless of recipient (R+ or R-) immune status.
Systemic NK and CD8+ T cell responses in MCMV recipient immune transplants
Pre-transplant viral loads and immune infiltrates in livers and spleens of MCMV+ B6 mice were compared to D+/R− and D+/R+ post-transplant organs. Pre-transplant liver and spleen viral loads, NK cells, and CD8+ T cells (Figure 2A, C, E, G, I, K), were lower than all post-transplant organs, except for liver viral loads which were similar pre- and post-transplant (Fig. 1A–B). Post-transplant D+/R− liver and spleen NK infiltrates were significantly higher than for D−/R− organs, whereas D+/R+ transplants had high NK cells only in the liver (Figure 2D, H). NK infiltrates did not correlate with organ viral loads (d.n.s.). Taken together, MCMV+ transplants had greater systemic NK cell induction compared to MCMV negative transplants, with higher antiviral primary NK responses compared to memory responses.
FIGURE 2. Systemic NK and CD8+ T cell primary and memory responses in MCMV infected transplants.
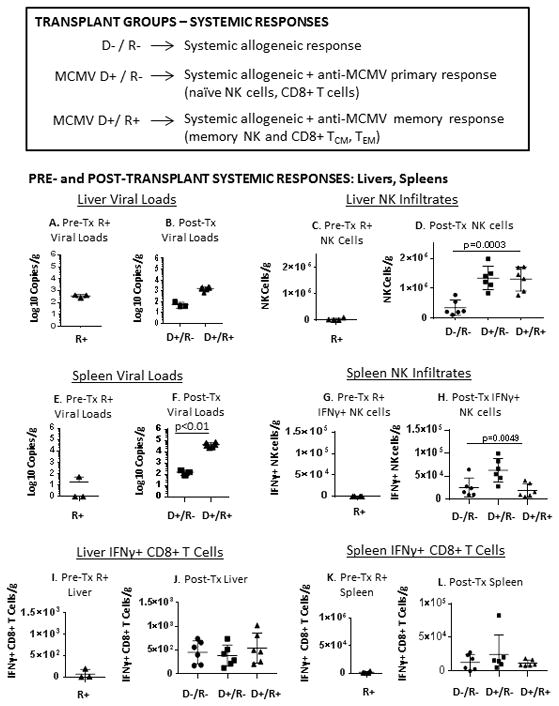
D−/R−, D+/R−, and D+/R+ transplants were performed and livers and spleens were analyzed for viral loads, NK cell and CD8+ T cell infiltrates at day 14 post-transplant, as described for Figure 1. MCMV+ BALB and B6 mice were analyzed for baseline pre-transplant viral loads and immune infiltrates in livers and spleens (as in Figure 1).
(A, B) Liver viral loads were quantitated from (A) pre-transplant MCMV+ B6 mice, and (B) D+/R− and D+/R+ transplant recipients.
(C, D) Liver NK cells were quantitated from (C) pre-transplant MCMV+ B6 mice, and (D) D−/R−, D+/R−, D+/R+ transplant recipients.
(E, F). Spleen viral loads were quantitated from (E) pre-transplant MCMV+ B6 mice, and (F) D+/R− and D+/R+ transplant recipients.
(G, H) Splenic IFN-γ+ NK cells were quantitated from (G) pre-transplant MCMV+ B6 mice, and (H) D−/R−, D+/R−, D+/R+ transplant recipients.
(I–L) IFN-γ+ CD8+ T cells were quantitated for MCMV+ B6 pre-transplant livers (I), spleens (K), and post-transplant D−/R−, D+/R−, and D+/R+ livers (J) and spleens (L).
Liver and spleen IFN-γ+ CD8+ T cells (Figure 2J, L) were similar among D−/R−, D+/R−, and D+/R+ transplants. These results indicate that systemic CD8+ T cell responses were not significantly altered by MCMV infection. However, this experiment could not distinguish allogeneic and antiviral T cell responses.
MCMV-specific CD8+ T cell memory responses in recipient-immune transplants
To better examine MCMV-specific CD8+ T cell responses, we next queried whether the quality of CD8 memory in MCMV R+ recipients might influence CD8+ T cell responses (Figure 3). Recipients were infected >12 weeks prior to transplant with MCMVΔm157 at low dose (102 pfu) or high dose (104 pfu) to establish differences in the memory T cell compartment: low dose (102 pfu) infection generates a central memory (TCM) bias and lower effector memory (TEM) populations, whereas high dose (104 pfu) infection establishes robust TEM with memory inflation (31, 37, 38) . To identify virus-specific CD8+ T cells at day 14 post-transplant, allograft T cells were stimulated ex vivo with either an MCMV peptide pool (M45, M38, m139, IE3) or individual M45 (noninflationary) and M38 (inflationary) peptides (31). Pre-transplant viral loads and virus-specific CD8+ T cell responses were also quantitated in MCMV+ B6 mice.
FIGURE 3. Allograft and systemic CD8+ T cell responses in D+/R+ transplants with central memory or effector memory bias.
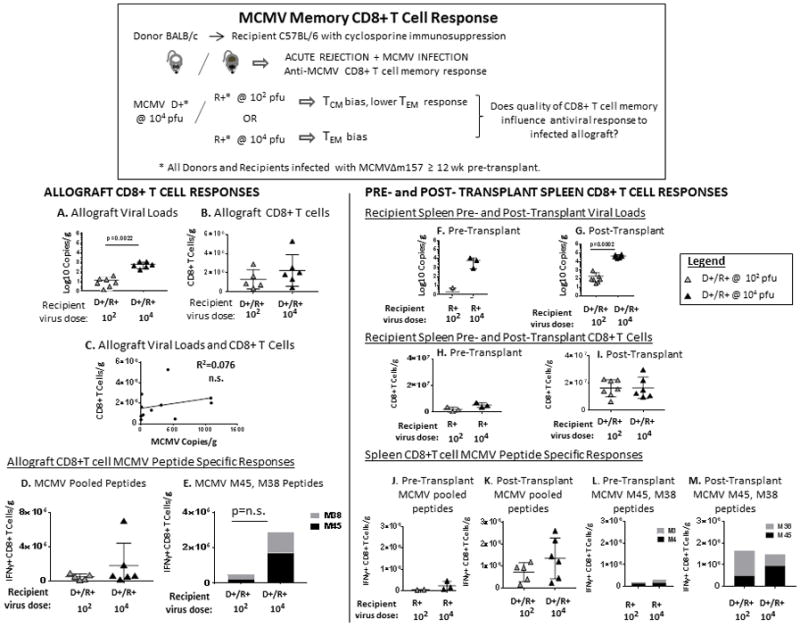
B6 mice were infected with MCMVΔm157 at either low dose (102 pfu) or high dose (104 pfu) to induce central memory (TCM) or effector memory (TEM) bias, respectively. D+/R+ transplants were performed >12 weeks post-infection. Viral loads and CD8+ T cell infiltrates were quantitated in allografts and spleens as described for Figure 1. In addition, CD8+ T cells were isolated from allografts and spleens, stimulated in vitro with either MCMV pooled peptides (M45, M38, m139, IE3), or with individual M45 (noninflationary epitope) or M38 (inflationary epitope) peptides with brefeldin A, and IFN-γ production was measured by intracellular cytokine staining and flow cytometry. Spleens from B6 mice infected with MCMVΔm157 at either low dose (102 pfu) or high dose (104 pfu) for > 12 weeks were analyzed for baseline pre-transplant viral loads, CD8+ T cells, and virus-specific CD8+ T cell responses.
(A) Allograft viral loads and (B) C8+ T cell infiltrates were quantitated from D+/R+ low dose (102) and high dose (104) transplants. (C) Allograft viral loads and CD8+ T cells showed no correlation.
(D, E) MCMV-specific IFN-γ+ CD8+ T cells from D+/R+ low dose (102) or high dose (104) recipient allografts were quantitated after in vitro stimulation with (D) MCMV pooled peptides or (E) M45 or M38 peptides.
(F–I) Spleens from pre-transplant low dose (102) or high dose (104) MCMV+ B6 mice, and from D+/R+ recipients with low dose (102) or high dose (104) infection were analyzed for viral loads (F–G) and CD8+ T cells (H, I).
(J–M) MCMV-specific IFN-γ+ CD8+ T cells were analyzed in spleens from pre-transplant low dose (102) or high dose (104) MCMV+ B6 mice (J, L), or from D+/R+ recipients with low dose (102) or high dose (104) infection (K, M) after stimulation with MCMV pooled peptides (J, K) or individual M45/M38 peptides (L–M).
Pre-transplant BALB donor kidney viral loads and CD8+ T cells were shown in Figure 1(A, G). Post-transplant allograft viral loads from the D+/R+ low dose (102) recipients were lower than those of D+/R+ high dose (104) recipients (p=0.0022), but intragraft CD8+ T cell infiltrates were similar among low dose and high dose recipients (Figure 3B) and did not correlate with viral loads (Figure 3C). MCMV-specific CD8+ T cell responses were similar among low dose (102) and high dose (104) recipients for both pooled and M45/M38 peptides (Figure 3D, E). Similarly, spleen viral loads were lower in the low-dose (102) recipients compared to high dose (104) recipients (Figure 3F, G), but the groups had comparable splenic CD8+ T cells (Figure 3H, I), MCMV pooled peptide-reactive CD8+ T cells (Figure 3J, K), and MCMV M45/M38 specific CD8+ T cells (Figure 3L, M), which did not correlate with viral loads (d.n.s.). Taken together, these results indicate that neither the viral loads nor the characteristics of recipient antiviral CD8+ T cell memory influenced the graft-infiltrating or systemic CD8+ T cell responses after transplantation.
Memory NK responses to MCMV infected allografts vary by primary recipient virus dose
Next, memory NK responses were examined in allografts and spleens from D+/R+ recipients with either low dose (102) or high dose (104) primary infection (Figure 4). Allografts from D+/R+ low dose (102) recipients had high IFN-γ+ NK cells and GzB+ NK cells resembling those found in D+/R− transplants (Figure 4A, B), suggesting that low-dose recipients, although MCMV immune, manifested a primary-like NK response to the infected allograft. In contrast, D+/R+ high dose (104) recipients had significantly lower intragraft IFN-γ+ NK cells and GzB+ NK infiltrates compared to both D+/R− and D+/R+ low dose (102) groups, indicating that the high-dose recipient memory NK responses differed considerably from the responses among D+/R+ low dose recipients.
FIGURE 4. Memory NK responses to MCMV infected allografts differ according to recipient virus dose during primary infection.

B6 mice were infected with MCMVΔm157 at either low dose (102 pfu) or high dose (104 pfu) to induce NK memory, and D+/R+ transplants were performed as in Figure 3. Memory NK responses from low dose and high dose infected recipients were compared to primary NK responses in D+/R− transplants.
(A, B) Allograft IFN-γ+ NK cells (A) or GzB+ NK cells (B) were compared between D+/R−, D+/R+ low dose (102) and D+/R+ high dose (104) infected recipients.
(C) Spleens from pre-transplant low dose (102) or high dose (104) MCMV+ B6 mice were analyzed for pre-transplant baseline IFN-γ+ and GzB+ NK cells.
(D, E) Splenic IFN-γ+ NK cells (D) or GzB+ NK cells (E) were compared between D+/R−, D+/R+ low dose (102) and D+/R+ high dose (104) infected recipients.
Pre-transplant IFN-γ+ and GzB+ NK cells were very low in pre-transplant B6 MCMV+ spleens (Figure 4C). Similar to allografts, splenic IFN-γ+ NK cells were induced among D+/R− and D+/R+ low dose (102) transplants and were lower among D+/R+ high dose (104) transplants (Figure 4D). However, no differences were observed for splenic GzB+ NK cells (Figure 4E), indicating that allograft-infiltrating cytotoxic NK responses (Fig. 4B) were not reflected systemically.
MCMV infection induces intragraft Th17 responses
Next, CD4+ T cell responses were evaluated in allografts and spleens from D−/R−, D+/R−, D+/R+ low dose (102) and D+/R+ high dose (104) transplants (Figure 5). The groups had no differences for allograft-infiltrating IFN-γ+ CD4+ (Th1) cells or IL-4+ CD4+ (Th2) cells (Figure 5D, E), or in spleens (d.n.s.). However, all MCMV+ transplants had greater IL-17A+ CD4+ (Th17) cells compared to D−/R− transplants (Figure 5A). D+/R+ low dose (102) recipients had the highest Th17 infiltrates, compared to both D+/R− (p=0.0191) and D+/R+ high dose allografts (p=0.0056).
FIGURE 5. MCMV infection induces IL-17A+ CD4+ T cell infiltrates and Th17-associated cytokine responses in allografts.
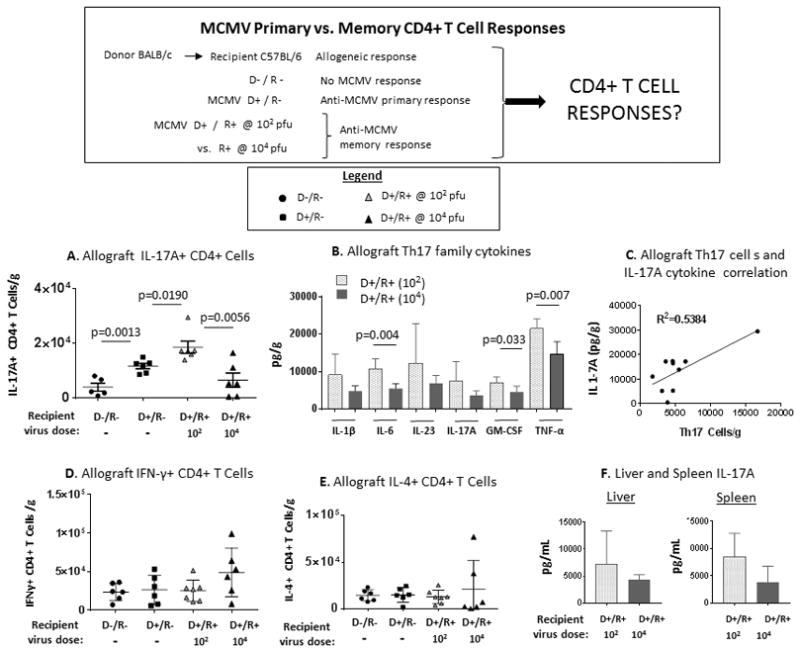
B6 mice were infected with MCMVΔm157 at either low dose (102 pfu) or high dose (104 pfu), and D+/R+ transplants were performed as in Figure 3. Allograft-infiltrating CD4+ T cells were analyzed for IFN-γ, IL-4, or IL-17A production and compared to results from uninfected D−/R− transplants and D+/R− transplants with primary MCMV infection. Cytokines in allografts, livers, and spleens from D+/R+ low dose (102) and high dose (104) infected recipients were quantitated using a bead immunoassay.
(A) Allograft IL-17A+ CD4+ T cell infiltrates were compared between D−/R−, D+/R−, D+/R+ low dose (102) and high dose (104) groups.
(B) Allograft Th17-associated cytokines were compared between D+/R+ low dose and D+/R+ high dose recipients.
(C) Intragraft IL-17A+ CD4+ T cells and IL-17A cytokine levels were correlated for D+/R+ low dose (102) and high dose (104) recipients.
(D, E) Allograft IFN-γ+ (D) and IL-4+ (E) CD4+ T cell infiltrates were compared between D−/R−, D+/R−, D+/R+ low dose (102) and high dose (104) groups.
(F) Liver and spleen IL-17A cytokine levels were compared between D+/R+ low dose (102) and high dose (104) groups.
The cytokine profile was compared between allografts from D+/R+ low dose (102) recipients and D+/R+ high dose (104) recipients using a bead immunoassay (Figure 5B). Both Th17-inducing cytokines (IL-1β, IL-6, IL-23), and Th17-secreted cytokines (IL-17A, GM-CSF, TNF-α), were more abundant in grafts from low-dose recipients (light bars) compared to high-dose recipients (dark bars). Th17 cell infiltrates correlated with IL-17A cytokine quantity (R2=0.5384, p=0.0157) (Figure 5C). Liver and spleen IL-17A cytokine levels (Figure 5F) trended higher among D+/R+ low-dose recipients compared to high-dose recipients but were not statistically significant, indicating that intragraft Th17 differences were not manifested systemically.
IL-6 depletion ameliorates tubular degeneration
As intragraft Th17 cells were most abundant among D+/R+ low dose (102) recipients, the effect of Th17 cell depletion was tested using this group. Th17 cells produce a number of cytokines in addition to IL-17A, such as GM-CSF, TNF-α, and IL-22, so a strategy to prevent Th17 differentiation was utilized, rather than depleting IL-17A alone (46, 47). D+/R+ low dose (102) recipients were treated with either neutralizing anti-IL-6 antibodies or isotype matched control antibodies (Figure 6); animals remained healthy-appearing throughout the antibody treatments. At day 14 post-transplant, intragraft IL-6 cytokine levels (Figure 6A) were lower among anti-IL-6-treated recipients compared to isotype-treated recipients. Among IL-6 depleted recipients, IL-17A cytokine levels (Figure 6B) and IL-17A+ CD4+ cells (Figure 6C) were lower than isotype-treated recipients. Allograft histology showed decreased tubular degeneration in IL-6 depleted recipients compared to non-depleted recipients (Figure 6D). These results indicate that reduction of Th17 cells, IL-6, and IL-17A are associated with amelioration of some aspects of allograft injury.
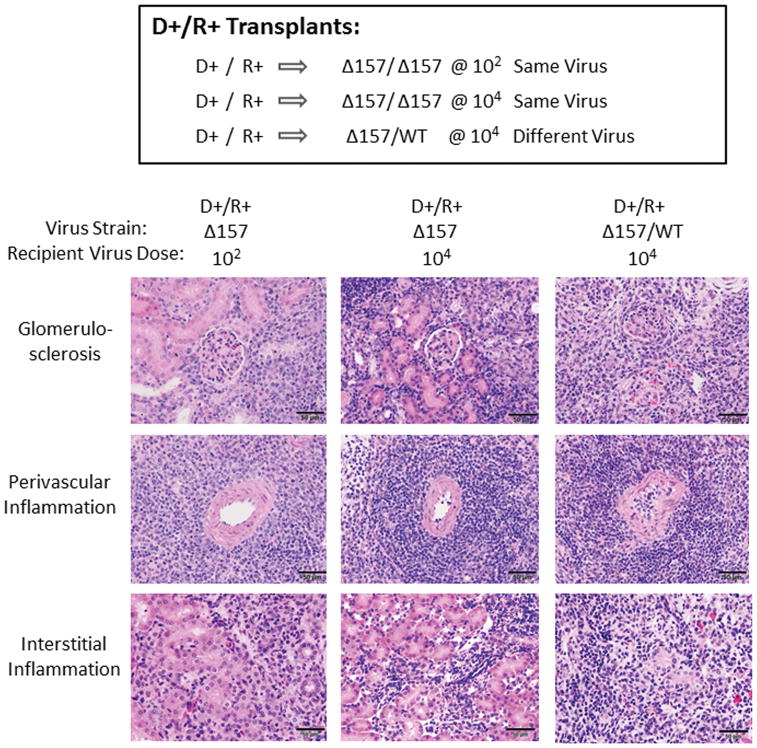
MCMV Donor/Recipient infection with different strains exacerbates NK and T cell responses
Next, to determine R+ recipient responses to an allograft infected with a different virus strain, recipients were infected with MCMV Smith wild-type (MCMV-WT) at 104 pfu and transplanted with donor kidneys infected with MCMVΔm157 at 104 pfu. In immunocompetent mice, the MCMV-WT virus establishes lower end-organ viral loads compared to the MCMVΔm157 virus (45, 48). However, with cyclosporine immunosuppression, at day 14 post-transplant the allograft viral loads (Figure 7A) were comparable for D+/R+ MCMVΔ157/Δ157 (104) same-strain transplants and D+/R+ MCMVΔ157/WT (104) different-strain transplants. To determine whether the virus strain infecting the allografts of D+/R+ different-strain (Δ157/WT) recipients derived from the donor, the recipient, or both, quantitative PCR was performed to amplify both viruses (“pan-MCMV PCR”) or only WT virus (“WT-only PCR”) (Figure 7B). The majority of the allograft-infecting virus was MCMV-WT, indicating that the recipient strain was predominant in allografts.
FIGURE 7. MCMV D+/R+ different-strain infection influences NK and T cell responses to MCMV infected allografts.
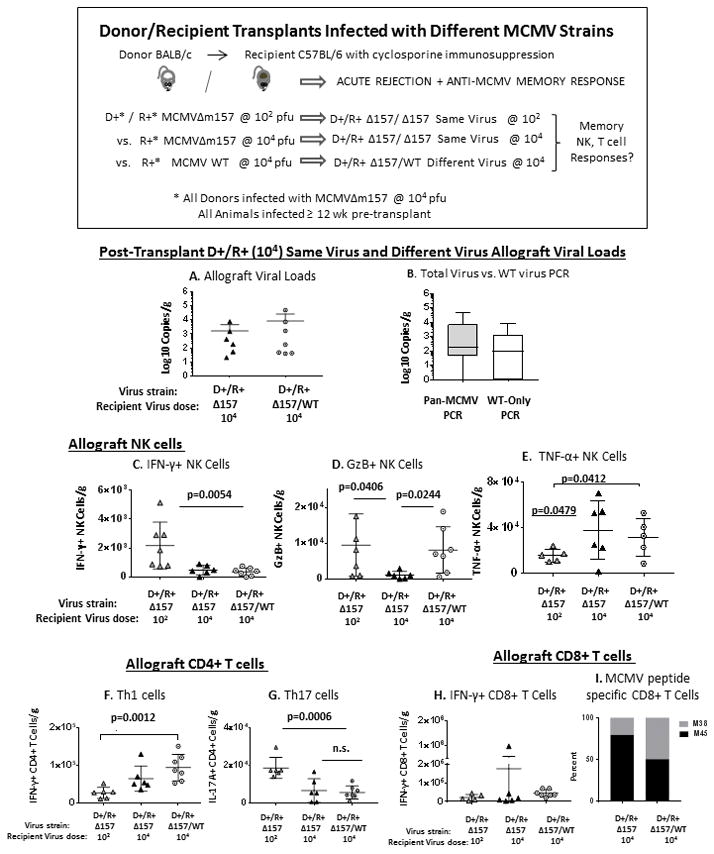
B6 recipients were infected with 104 pfu of MCMV Smith wild-type (MCMV WT) strain > 12 weeks prior to transplant, and received BALB kidneys infected with MCMVΔ157 strain (104 pfu). At day 14 post-transplant, allograft viral loads, NK and T cell infiltrates were analyzed in comparison to same-strain D+/R+ transplants (104 pfu), as well as D+/R+ same-strain transplants with low dose (102 pfu) recipient infection.
(A) Allograft viral loads were compared between different-strain and same-strain transplants for recipients with high dose (104 pfu) primary infection.
(B) DNA was extracted from allografts of D+/R+ different-strain transplants and analyzed by quantitative PCR for MCMV using either a primer/probe set amplifying both MCMVΔ157 and MCMV WT strains (“Pan-MCMV PCR,” grey bar) or a primer/probe set amplifying only MCMV WT virus (“WT-Only PCR,” white bar).
(C–E) D+/R+ transplants with recipient same-strain virus at low dose (102) or high dose (104), or different-strain virus at high dose (104), were compared for allograft-infiltrating (C) IFN-γ+ NK cells, (D) GzB+ NK cells, or (E) TNF-α+ NK cells.
(F–H) Allograft-infiltrating (F) IFN-γ+ CD4+ T cells (Th1); (G) IL-17A+ CD4+ T cells (Th17), or (H) IFN-γ+ CD8+ T cells were compared between the three D+/R+ groups.
(I) Allograft-infiltrating MCMV M45/M38 peptide-specific IFN-γ+ CD8+ T cells were compared between D+/R+ (104 pfu) same-strain and different-strain virus recipients.
Intragraft NK cell, CD4+ T cell, and CD8+ T cell infiltrates in D+/R+ different virus (Δ157/WT, 104 pfu) transplants were compared to D+/R+ same virus (Δ157/ Δ157) transplants with recipient low dose (102) or high dose (104) infection. D+/R+ Δ157/ Δ157 same-strain (104) and D+/R+ Δ157/WT different-strain (104) grafts contained similar quantities of IFN-γ+ NK cells, TNF-α+ NK cells, CD4+ Th1 and Th17 cells, IFN-γ+ CD8+ T cells, and MCMV M45/M38 specific CD8+ T cells (Figures 7C, E–I), indicating that most intragraft responses were similar for recipients with 104 primary virus dose, regardless of virus strain. In contrast, D+/R+ same virus (Δ157/ Δ157) low dose (102) recipient allografts differed significantly from all high dose recipients with greater IFN-γ+ NK cells and Th17 cells, but lower TNF-α+ NK cells and Th1 cells (Figure 7C–G), indicating that the primary MCMV infection dose (low dose vs. high dose) significantly influenced allograft infiltrates.
The only cell type which did not follow this pattern was the GzB+ NK cell (Figure 7D). The D+/R+ different virus transplants mounted a high GzB+ NK cell response which resembled the primary response observed in D+/R− transplants, as well as the response among D+/R+ low dose transplants (Figure 1F). This result indicates that an allograft-directed primary-like cytotoxic NK response can occur in an MCMV R+ recipient with donor/recipient virus strain mismatch.
Systemically, liver and spleen viral loads did not differ pre- or post-transplant for D+/R+ recipients infected with MCMVΔ157 or MCMV WT at 104 pfu (Figure 8A–D). However, D+/R+ transplants infected with different virus strains (Δ157/WT) had greater liver GzB+ NK cells and CD4+ Th1 cells, and greater liver and spleen IFN-γ+ CD8+ T cells (Figure 8E–H). This result shows that systemic responses are exacerbated in transplants with donor/recipient mismatched MCMV strains.
FIGURE 8. Systemic responses in MCMV D+/R+ different-strain infection.
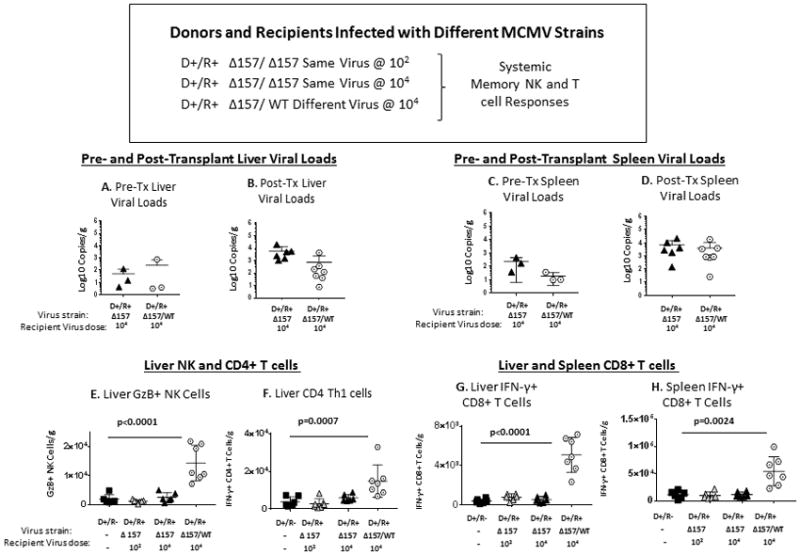
D+/R+ transplants were performed as for Figure 7, using MCMVΔ157 infected donor kidneys and MCMV WT infected recipients. Liver and spleen viral loads, NK cells, Th1, and CD8 cells were compared to D+/R+ same strain transplants and D+/R− transplants with primary viral infection. B6 mice infected with MCMV WT at 104 pfu for >12 weeks were analyzed for baseline pre-transplant organ viral loads and immune cells and compared to B6 mice infected with MCMVΔ157 virus at 104 pfu.
(A–D) Liver and spleen viral loads were compared for pre-transplant organs (A, C) and D+/R+ same-virus and different-virus transplant recipients (B, D).
(E–G). Livers from D+/R+ different-strain transplants were compared to D+/R− and D+/R+ same-strain transplants (low and high dose infection) for (E) GzB+ NK cells, (F) IFN-γ+ CD4+ (Th1) cells, and (G) IFN-γ+ CD8+ T cells. (H) Spleens from the transplant groups were also compared for IFN-γ+ CD8+ T cells.
Allograft histopathology from MCMV immune recipients
Allograft histopathology was evaluated from the D+/R+ same virus (Δ157/ Δ157) transplants at low dose (102) and high dose (104), and D+/R+ different virus (Δ157/WT, 104 pfu) transplants (Figure 9). All allografts had histologic injury, with glomerulosclerosis and arteritis depicted. There was no difference in overall damage score (Table 1) between the D+/R+ same virus (Δ157/ Δ157) low dose (102) and high dose (104) transplants (11.5 ± 2.2 versus 11.9 ± 1.4, p=n.s.). However, D+/R+ different virus (Δ157/WT, 104 pfu) transplants had the most severe tissue injury with obliteration of the renal architecture (damage score 14.3 ± 1.8), with statistically significant differences in the scores for interstitial inflammation (shown, p=0.0105) and total damage (p=0.0318). This result indicates that the most severe allograft damage was associated with donor/recipient infection with different virus strains.
FIGURE 9. Allograft histopathology from MCMV immune recipients.
D+/R+ transplants were performed using MCMVΔ157 same-strain infections for recipients with low dose (102) or high dose (104) infections, and compared to D+/R+ MCMVΔ157/MCMV WT different strain infections (104), as described in Figures 3 and 7. At day 14 post-transplant, allografts were stained with hematoxylin and eosin and allograft damage scored according to 8 histologic criteria (Table 1). Representative images for glomerulosclerosis, arteritis, and interstitial inflammation were collected under identical conditions at 40x. Scale bar=50 μm.
DISCUSSION
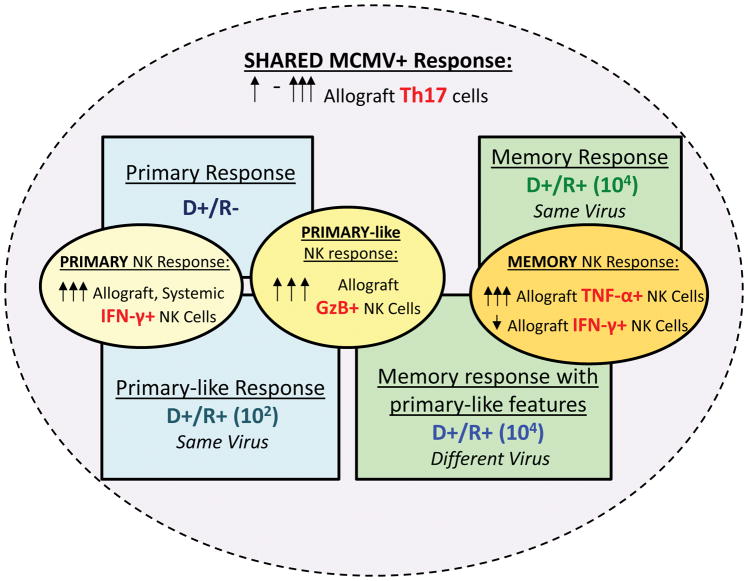
In this study, MCMV recipient immune responses to infected allografts varied according to conditions of recipient primary infection. A model synthesizing these findings is shown in Figure 10. The most striking differences were observed among NK cells. The D+/R− primary NK response consisted of high cytolytic (IFN-γ+) and cytotoxic (GzB+) NK cells, which were lower among recipients with NK memory after high dose primary MCMV infection (104 pfu). Recipients infected with a low MCMV primary inoculum (102 pfu) manifested primary-like high cytolytic and cytotoxic NK responses despite prior MCMV immunity, whereas the NK memory response to a different virus (D+/R+ different virus transplants) had both memory-like (low cytolytic NK) and primary-like (high cytotoxic NK) features. These results suggest that the conditions of primary CMV infection differentially influence the host NK response to an infected allograft.
FIGURE 10. Model for primary and memory NK cell responses according to conditions of recipient infection (virus dose and strain).
Primary NK cell responses (D+/R−, blue box, upper left) were characterized by high cytolytic (IFN-γ+) and cytotoxic (GzB+) NK cell infiltrates (yellow ovals). Memory NK cell responses (D+/R+ at 104 pfu, green box, upper right) were characterized by high TNF-α+ NK cells but low IFN-γ+ NK cells (orange oval).
D+/R+ mice infected with 102 pfu (blue box, lower left) had primary-like NK responses with high cytolytic and cytotoxic NK infiltrates (yellow ovals), despite having prior recipient immunity. Recipients infected with a different virus from the donor (D+/R+ different virus, green box, lower right), had both primary-like GzB+ NK cells (yellow oval) and memory NK responses (orange oval) against the different donor virus strain.
All MCMV+ transplants had allograft-infiltrating Th17 cells (purple oval), which varied in intensity among the different transplant groups (see Figure 5).
Memory NK cells in D+/R+ high-dose (104 pfu) infection demonstrated a novel phenotype characterized by TNF-α production. This antiviral NK response has not been previously described, although an association is suggested by the finding of elevated blood TNF-α levels in renal transplant patients with CMV infection and disease (49, 50). Among patients infected with hepatitis C virus, intrahepatic TNF-α producing NK cells correlated with stage of fibrosis and were postulated to contribute to immune-mediated liver injury during chronic infection (51). It could be hypothesized that TNF-α producing NK cells are induced after HCMV D+/R+ transplantation and might contribute to renal fibrosis, similar to the liver fibrosis observed in viral hepatitis.
The impact of antiviral memory T cell inflation upon allograft injury is of interest, as up to 10% of the human memory T cell repertoire is directed against CMV epitopes (34). In this model, memory-inflated T cells did not preferentially infiltrate the infected allografts compared to non-inflated T cells. This result is reassuring in context of HCMV vaccine development, where antiviral immunity is necessary to protect against CMV disease but might also potentially contribute to allograft damage.
These studies also identify a potential role of Th17 cells in the pathogenesis of viral renal allograft injury. Th17 cells responding to MCMV peptides occur in immunocompetent mice after acute MCMV infection (52). IL-17 is expressed during clinical renal allograft rejection and in animal models for renal, islet xenograft, and cardiac transplant rejection, but CMV was not evaluated in these models (53–59). Liver transplant patients with CMV antigenemia/DNAemia had greater IL-17 mRNA expression in peripheral blood both at baseline and over time compared to patients without CMV infection (60). Among kidney transplants, a correlation between shorter graft survival and the presence of Th17 cells producing IL-17 and IL-21 has been reported (55). In another study, kidney transplant patients with post-transplant CMV infection had higher pre-transplant plasma IL-23 levels compared to patients without post-transplant CMV infection (61). Together, these studies support the potential significance of Th17 cells in CMV associated allograft dysfunction and might be of particular interest given the recent availability of biological response modifiers directed against IL-23 for autoimmune diseases, which could potentially be used to dampen Th17 responses during acute rejection.
In this model, tissue injury and characteristics of allograft-infiltrating immune cells were independent of the tissue viral loads, suggesting that the host response rather than the virus itself might induce allograft injury. This is consistent with our prior work, where MCMV-associated allograft damage was reduced by NK depletion. In addition, results from this study indicate that D/R infections with different strains increase immune-mediated graft injury, a scenario that likely has clinical relevance for HCMV D+/R+ transplants. NK KIR activating polymorphisms can confer protection from CMV infection (DNAemia), but it is unknown whether donor-derived HCMV strains can activate or inhibit NK cells according to recipient KIR polymorphisms (16, 18, 62).
There are limitations to the interpretation of this study. All experiments were conducted using a murine strain combination that acutely rejects allografts, so the impact of host strain MHC differences and less stringent rejection phenotypes were not examined. Due to the technical difficulty of murine kidney transplantation, a limited number of transplants were performed and only one time point was analyzed. Therefore, it is likely that the kinetics of the cellular infiltrates and cytokine profiles among the experimental groups were not well characterized over time. Finally, findings in this animal model were not directly corroborated in patient studies, so the applicability of these findings to clinical transplantation remains inconclusive.
In sum, findings from this MCMV murine renal transplant model suggest that MCMV may influence allograft injury by several previously uncharacterized mechanisms involving the NK and Th17 cells, and that allograft inflammation is influenced by conditions of primary recipient infection. Recipient memory CD8+ T cell characteristics and organ viral loads did not correlate with immune infiltrates. The observations in this model require further evaluation in clinical populations, and a better understanding of these pathways might identify potential interventions to prevent or treat early CMV-associated allograft injury that may contribute to late allograft loss.
Supplementary Material
Acknowledgments
We are grateful for the technical assistance of Irina Kaptsan and Sonya Maher in conducting experiments. This work was supported by NIH R01AI101138 (M.S.), The Research Institute at Nationwide Children’s Hospital, the Children’s Center for Research and Innovation of the Alabama Children’s Hospital Foundation (M.S.), the Kaul Pediatric Research Initiative of The Children’s Hospital of Alabama (M.S.); the UAB-UCSD O’Brien Core Center for Acute Kidney Injury Research and the NIH NIDDK 1P30 DK079337 (B.C.); and the UAB Mucosal HIV and Immunobiology Center, NIH NIDDK DK64400. This work was presented in part at the American Transplant Congress in 2015 and 2016.
ABBREVIATIONS
- bp
base pair
- CMV
cytomegalovirus
- d.n.s
data not shown
- FMO
fluorescence minus one
- GzB
granzyme B
- IFN-γ
interferon-γ
- IL
interleukin
- KIR
killer immunoglobulin-like receptor
- NK
natural killer
- n.s
not significant
- pg
picogram
- pfu
plaque-forming unit
- PMA
phorbol myristate acetate
- TCM
central memory T cells
- TEM
effector memory T cells
- Th
T helper
- TNF-α
tumor necrosis factor-α
- WT
wild-type
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Rubin RH, Tolkoff-Rubin NE, Oliver D, Rota TR, Hamilton J, Betts RF, et al. Multicenter seroepidemiologic study of the impact of cytomegalovirus infection on renal transplantation. Transplantation. 1985;40(3):243–249. doi: 10.1097/00007890-198509000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Humar A, Gillingham KJ, Payne WD, Dunn DL, Sutherland DE, Matas AJ. Association between cytomegalovirus disease and chronic rejection in kidney transplant recipients. Transplantation. 1999;68(12):1879–1883. doi: 10.1097/00007890-199912270-00011. [DOI] [PubMed] [Google Scholar]
- 3.van Ree RM, de Vries AP, Zelle DM, de Vries LV, Oterdoom LH, Gans RO, et al. Latent cytomegalovirus infection is an independent risk factor for late graft failure in renal transplant recipients. Medical science monitor : international medical journal of experimental and clinical research. 2011;17(11):Cr609–617. doi: 10.12659/MSM.882045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerstenkorn C, Balupuri S, Mohamed MA, Manas DM, Ali S, Kirby J, et al. The impact of cytomegalovirus serology for 7-year graft survival in cadaveric kidney transplantation--the Newcastle experience. Transplant International. 2000a;13(S1):S372–374. doi: 10.1007/s001470050364. [DOI] [PubMed] [Google Scholar]
- 5.Opelz G, Dohler B, Ruhenstroth A. Cytomegalovirus prophylaxis and graft outcome in solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4(6):928–936. doi: 10.1111/j.1600-6143.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 6.Bock GH, Sullivan EK, Miller D, Gimon D, Alexander S, Ellis E, et al. Cytomegalovirus infections following renal transplantation--effects on antiviral prophylaxis: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatric nephrology (Berlin, Germany) 1997;11(6):665–671. doi: 10.1007/s004670050361. [DOI] [PubMed] [Google Scholar]
- 7.Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F. Improvement in Long-Term Renal Graft Survival due to CMV Prophylaxis with Oral Ganciclovir: Results of a Randomized Clinical Trial. American Journal of Transplantation. 2008;8(5):975–983. doi: 10.1111/j.1600-6143.2007.02133.x. [DOI] [PubMed] [Google Scholar]
- 8.Kliem V, Fricke L. Additional evidence to support routine cytomegalovirus prophylaxis for all D+/R+ renal graft recipients. Transplantation. 2012;93(6):e21–22. doi: 10.1097/TP.0b013e318247a7d4. [DOI] [PubMed] [Google Scholar]
- 9.Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22(1):76–98. doi: 10.1128/CMR.00034-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983;131(3):1531–1538. [PubMed] [Google Scholar]
- 11.Bukowski JF, Warner JF, Dennert G, Welsh RM. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. The Journal of Experimental Medicine. 1985;161(1):40–52. doi: 10.1084/jem.161.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisnić B, Lisnić VJ, Jonjić S. NK cell interplay with cytomegaloviruses. Current opinion in virology. 2015;15:9–18. doi: 10.1016/j.coviro.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 13.van den Berg AP, van Son WJ, Janssen RA, Brons NH, Heyn AA, Scholten-Sampson A, et al. Recovery from cytomegalovirus infection is associated with activation of peripheral blood lymphocytes. J Infect Dis. 1992;166(6):1228–1235. doi: 10.1093/infdis/166.6.1228. [DOI] [PubMed] [Google Scholar]
- 14.Venema H, Van Den Berg AP, Van Zanten C, Van Son WJ, Van Der Giessen M, Hauw T. Natural killer cell responses in renal transplant patients with cytomegalovirus infection. Journal of Medical Virology. 1994;42(2):188–192. doi: 10.1002/jmv.1890420216. [DOI] [PubMed] [Google Scholar]
- 15.Hadaya K, de Rham C, Bandelier C, Ferrari-Lacraz S, Jendly S, Berney T, et al. Natural Killer Cell Receptor Repertoire and Their Ligands, and the Risk of CMV Infection After Kidney Transplantation. American Journal of Transplantation. 2008;8(12):2674–2683. doi: 10.1111/j.1600-6143.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- 16.de Rham C, Hadaya K, Bandelier C, Ferrari-Lacraz S, Villard J. Expression of killer cell immunoglobulin-like receptors (KIRs) by natural killer cells during acute CMV infection after kidney transplantation. Transpl Immunol. 2014;31(3):157–164. doi: 10.1016/j.trim.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Redondo-Pachon D, Crespo M, Yelamos J, Muntasell A. Adaptive NKG2C+ NK Cell Response and the Risk of Cytomegalovirus Infection in Kidney Transplant Recipients. 2017;198(1):94–101. doi: 10.4049/jimmunol.1601236. [DOI] [PubMed] [Google Scholar]
- 18.Stern M, Elsasser H, Honger G, Steiger J, Schaub S, Hess C. The Number of Activating KIR Genes Inversely Correlates with the Rate of CMV Infection/Reactivation in Kidney Transplant Recipients. American Journal of Transplantation. 2008;8(6):1312–1317. doi: 10.1111/j.1600-6143.2008.02242.x. [DOI] [PubMed] [Google Scholar]
- 19.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120(24):4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang T, Scott JM, Hwang I, Kim S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol. 2013;190(4):1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42(3):431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitrovic M, Arapovic J, Jordan S, Fodil-Cornu N, Ebert S, Vidal SM, et al. The NK Cell Response to Mouse Cytomegalovirus Infection Affects the Level and Kinetics of the Early CD8+ T-Cell Response. Journal of Virology. 2012;86(4):2165–2175. doi: 10.1128/JVI.06042-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitrovic M, Arapovic J, Traven L, Krmpotic A, Jonjic S. Innate immunity regulates adaptive immune response: lessons learned from studying the interplay between NK and CD8+ T cells during MCMV infection. Med Microbiol Immunol. 2012;201(4):487–495. doi: 10.1007/s00430-012-0263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teoh JJ, Gamache AE, Gillespie AL, Stadnisky MD, Yagita H, Bullock TN. Acute Virus Control Mediated by Licensed NK Cells Sets Primary CD8+ T Cell Dependence on CD27 Costimulation. 2016;197(11):4360–4370. doi: 10.4049/jimmunol.1601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meijers RW, Litjens NH, Hesselink DA, Langerak AW, Baan CC, Betjes MG. Primary Cytomegalovirus Infection Significantly Impacts Circulating T Cells in Kidney Transplant Recipients. Am J Transplant. 2015;15(12):3143–3156. doi: 10.1111/ajt.13396. [DOI] [PubMed] [Google Scholar]
- 27.San-Juan R, Navarro D, Garcia-Reyne A, Montejo M, Munoz P, Carratala J, et al. Effect of long-term prophylaxis in the development of cytomegalovirus-specific T-cell immunity in D+/R− solid organ transplant recipients. Transpl Infect Dis. 2015;17(5):637–646. doi: 10.1111/tid.12417. [DOI] [PubMed] [Google Scholar]
- 28.Higdon LE, Trofe-Clark J, Liu S, Margulies KB, Sahoo MK, Blumberg E, et al. Cytomegalovirus-Responsive CD8+ T Cells Expand After Solid Organ Transplantation in the Absence of CMV Disease. Am J Transplant. 2017;17(8):2045–2054. doi: 10.1111/ajt.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170(4):2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 30.Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol. 2005;35(4):1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- 31.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four Distinct Patterns of Memory CD8 T Cell Responses to Chronic Murine Cytomegalovirus Infection. The Journal of Immunology. 2006;177(1):450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- 32.Sylwester A, Nambiar KZ, Caserta S, Klenerman P, Picker LJ, Kern F. A new perspective of the structural complexity of HCMV-specific T-cell responses. Mechanisms of Ageing and Development. 2016;158:14–22. doi: 10.1016/j.mad.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol. 2016;16(6):367–377. doi: 10.1038/nri.2016.38. [DOI] [PubMed] [Google Scholar]
- 34.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. The Journal of Experimental Medicine. 2005;202(5):673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimamura M, Saunders U, Rha B, Guo L, Cassady KA, George JF, et al. Ganciclovir transiently attenuates murine cytomegalovirus-associated renal allograft inflammation. Transplantation. 2011;92(7):759–766. doi: 10.1097/TP.0b013e31822c6e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimamura M, Seleme MC, Guo L, Saunders U, Schoeb TR, George JF, et al. Ganciclovir prophylaxis improves late murine cytomegalovirus-induced renal allograft damage. Transplantation. 2013;95(1):48–53. doi: 10.1097/TP.0b013e3182782efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redeker A, Welten SP, Arens R. Viral inoculum dose impacts memory T-cell inflation. Eur J Immunol. 2014;44(4):1046–1057. doi: 10.1002/eji.201343946. [DOI] [PubMed] [Google Scholar]
- 38.Trgovcich J, Kincaid M, Thomas A, Griessl M, Zimmerman P, Dwivedi V, et al. Cytomegalovirus Reinfections Stimulate CD8 T-Memory Inflation. PLoS ONE. 2016;11(11):e0167097. doi: 10.1371/journal.pone.0167097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, Schlachta C, Duff J, Stiller C, Grant D, Zhong R. Improved techniques for kidney transplantation in mice. Microsurgery. 1995;16(2):103–109. doi: 10.1002/micr.1920160212. [DOI] [PubMed] [Google Scholar]
- 40.Andoh TF, Bennett WM. The synergistic effects of cyclosporine and sirolimus (reply) Transplantation. 1997;63(11):1703–1704. doi: 10.1097/00007890-199706150-00031. [DOI] [PubMed] [Google Scholar]
- 41.Roederer M. Compensation in flow cytometry. Current protocols in cytometry. 2002;Chapter 1(Unit 1.14) doi: 10.1002/0471142956.cy0114s22. [DOI] [PubMed] [Google Scholar]
- 42.Barber DL, Andrade BB, McBerry C, Sereti I, Sher A. Role of IL-6 in Mycobacterium avium--associated immune reconstitution inflammatory syndrome. J Immunol. 2014;192(2):676–682. doi: 10.4049/jimmunol.1301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang Y, Yang K, Guo J, Wroblewska J, Fu YX, Peng H. Innate lymphotoxin receptor mediated signaling promotes HSV-1 associated neuroinflammation and viral replication. Scientific reports. 2015;5:10406. doi: 10.1038/srep10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bantug GR, Cekinovic D, Bradford R, Koontz T, Jonjic S, Britt WJ. CD8+ T lymphocytes control murine cytomegalovirus replication in the central nervous system of newborn animals. J Immunol. 2008;181(3):2111–2123. doi: 10.4049/jimmunol.181.3.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bubic I, Wagner M, Krmpotic A, Saulig T, Kim S, Yokoyama WM, et al. Gain of virulence caused by loss of a gene in murine cytomegalovirus. Journal of Virology. 2004;78(14):7536–7544. doi: 10.1128/JVI.78.14.7536-7544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252(1):116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 47.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redeker A, Welten SPM, Arens R. Viral inoculum dose impacts memory T-cell inflation. European Journal of Immunology. 2014;44(4):1046–1057. doi: 10.1002/eji.201343946. [DOI] [PubMed] [Google Scholar]
- 49.Nordoy I, Muller F, Nordal Knut P, Rollag H, Lien E, Aukrust P, et al. The Role of the Tumor Necrosis Factor System and Interleukin10 during Cytomegalovirus Infection in Renal Transplant Recipients. The Journal of Infectious Diseases. 2000;181(1):51–57. doi: 10.1086/315184. [DOI] [PubMed] [Google Scholar]
- 50.Tong C, Bakran A, Williams H, Cuevas L, Peiris J, Hart C. Association of tumour necrosis factor alpha and interleukin 6 levels with cytomegalovirus DNA detection and disease after renal transplantation. Journal of Medical Virology. 2001;64(1):29–34. doi: 10.1002/jmv.1013. [DOI] [PubMed] [Google Scholar]
- 51.Nel I, Lucar O, Petitdemange C, Beziat V, Lapalus M, Bedossa P, et al. Accumulation of Intrahepatic TNF-alpha-Producing NKp44+ NK Cells Correlates With Liver Fibrosis and Viral Load in Chronic HCV Infection. Medicine. 2016;95(19):e3678. doi: 10.1097/MD.0000000000003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arens R, Wang P, Sidney J, Loewendorf A, Sette A, Schoenberger SP, et al. Cutting Edge: Murine Cytomegalovirus Induces a Polyfunctional CD4 T Cell Response. J Immunol. 2008;180(10):6472–6476. doi: 10.4049/jimmunol.180.10.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Kooten C, Boonstra JG, Paape ME, Fossiez F, Banchereau J, Lebecque S, et al. Interleukin-17 activates human renal epithelial cells in vitro and is expressed during renal allograft rejection. J Am Soc Nephrol. 1998;9(8):1526–1534. doi: 10.1681/ASN.V981526. [DOI] [PubMed] [Google Scholar]
- 54.Hsieh HG, Loong CC, Lui WY, Chen A, Lin CY. IL-17 expression as a possible predictive parameter for subclinical renal allograft rejection. Transpl Int. 2001;14(5):287–298. doi: 10.1007/s001470100344. [DOI] [PubMed] [Google Scholar]
- 55.Deteix C, Attuil-Audenis V, Duthey A, Patey N, McGregor B, Dubois V, et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 2010;184(9):5344–5351. doi: 10.4049/jimmunol.0902999. [DOI] [PubMed] [Google Scholar]
- 56.Gorbacheva V, Fan R, Li X, Valujskikh A. Interleukin-17 promotes early allograft inflammation. Am J Pathol. 2010;177(3):1265–1273. doi: 10.2353/ajpath.2010.091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itoh S, Kimura N, Axtell RC, Velotta JB, Gong Y, Wang X, et al. Interleukin-17 accelerates allograft rejection by suppressing regulatory T cell expansion. Circulation. 2011;124(11 Suppl):S187–196. doi: 10.1161/CIRCULATIONAHA.110.014852. [DOI] [PubMed] [Google Scholar]
- 58.Matignon M, Aissat A, Canoui-Poitrine F, Grondin C, Pilon C, Desvaux D, et al. Th-17 Alloimmune Responses in Renal Allograft Biopsies From Recipients of Kidney Transplants Using Extended Criteria Donors During Acute T Cell-Mediated Rejection. Am J Transplant. 2015;15(10):2718–2725. doi: 10.1111/ajt.13304. [DOI] [PubMed] [Google Scholar]
- 59.Kang HK, Wang S, Dangi A, Zhang X, Singh A, Zhang L, et al. Differential Role of B Cells and IL-17 Versus IFN-gamma During Early and Late Rejection of Pig Islet Xenografts in Mice. Transplantation. 2017;101(8):1801–1810. doi: 10.1097/TP.0000000000001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Afshari A, Yaghobi R, Karimi MH, Darbouy M, Azarpira N, Geramizadeh B, et al. IL-17 mRNA expression and cytomegalovirus infection in liver transplant patients. Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation. 2015;13(Suppl 1):83–89. [PubMed] [Google Scholar]
- 61.Sadeghi M, Lahdou I, Opelz G, Mehrabi A, Zeier M, Schnitzler P, et al. IL-23 plasma level is strongly associated with CMV status and reactivation of CMV in renal transplant recipients. BMC immunology. 2016;17(1):35. doi: 10.1186/s12865-016-0175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzalez A, Schmitter K, Hirsch HH, Garzoni C, van Delden C, Boggian K, et al. KIR-associated protection from CMV replication requires pre-existing immunity: a prospective study in solid organ transplant recipients. Genes and immunity. 2014;15(7):495–499. doi: 10.1038/gene.2014.39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.