Abstract
While vitamin D has been associated with improved overall cancer survival in some investigations, few have prospectively evaluated organ-specific survival. We examined the accepted biomarker of vitamin D status, serum 25-hydroxyvitamin D [25(OH)D], and cancer survival in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Of 4,616 cancer cases with measured serum 25(OH)D, 2,884 died of their cancer during 28 years of follow-up and 1,732 survived or died of other causes. Proportional hazards regression estimated hazard ratios (HR) and 95% confidence intervals (CI) for the association between pre-diagnostic 25(OH)D and overall and site-specific survival. Serum 25(OH)D was significantly lower among cases who subsequently died from their malignancy compared with those who did not (medians 34.7 vs. 36.5 nmol/L, respectively; p=0.01). Higher 25(OH)D was associated with lower overall cancer mortality (HR=0.76, 95% CI 0.67–0.85 for highest vs. lowest quintile, p-trend <0.0001). Higher 25(OH)D was related to lower mortality from the following site-specific malignancies: prostate (HR=0.74, 95% CI 0.55–1.01, p-trend=0.005), kidney (HR=0.59, 95% CI 0.35–0.98, p-trend=0.28), and melanoma (HR=0.39, 95% CI 0.20–0.78, p-trend=0.01), but increased mortality from lung cancer (HR=1.28, 95% CI 1.02–1.61, p-trend=0.19). Improved survival was also suggested for head and neck, gastric, pancreatic, and liver cancers, though not statistically significantly, and case numbers for the latter two organ sites were small. Higher 25(OH)D status years prior to diagnosis were related to improved survival for overall and some site-specific cancers, associations that should be examined in other prospective populations that include women and other racial-ethnic groups.
Keywords: Vitamin D, Cancer, Mortality, Survival Analysis, Prospective Cohort
Introduction
Vitamin D has been associated with improved overall cancer survival in several prospective studies [1], but not all [2, 3], and although low vitamin D status has been observed in cancer patients after diagnosis, fewer studies have examined prospectively measured vitamin D and subsequent site-specific cancer mortality outcomes [1]. We have previously shown that 25-hydroxyvitamin D [25(OH)D], the accepted biomarker of vitamin D status, was associated with improved prostate cancer, but not lung cancer, survival [4, 5]. The present study was undertaken to examine whether prospectively measured 25(OH)D, years in advance of cancer diagnoses, is related to overall and site-specific cancer survival in a cohort of male smokers.
Methods
Participants
The Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study has been previously described [6]. Briefly, 29,133 Finnish male smokers, aged 50–69 years, were recruited from 1985–1988 to participate in a controlled primary prevention trial. Participants were supplemented daily for 5–8 years (median 6.1 years), with alpha-tocopherol (50 mg dl-alpha-tocopheryl acetate), beta-carotene (20 mg), both, or placebo, until death or trial closure in April, 1993. Participants have been followed through linkage with the Finnish Cancer Registry, which provides nearly 100% ascertainment of cancer cases within Finland [7, 8], and the Register of Causes of Death, with survival data available through December 31, 2014. Risk factor questionnaires were completed at baseline, including information about smoking habits, medical history, and dietary intake [6, 9]. Height and weight were measured. Family history of cancer was ascertained in a questionnaire during follow-up. The study was approved by the Institutional Review Boards of the U.S. National Cancer Institute (NCI) and the Finnish National Institute for Health and Welfare, and written informed consent was obtained from all individual participants included in the study.
Cancer cases were drawn from previous nested case-control studies of circulating 25(OH)D and cancer risk within the ATBC Study [10–20]. The majority of cases (82.8%) were assayed together as part of a large multi-organ site study including malignant melanoma (International classification of diseases, ICD9=172), hematological cancers (ICD9 200–208), and cancers of the bladder (ICD9=188), colorectum (ICD9=153 & 154, excluding cancers of appendix and anus), head and neck [including oral cavity (ICD9=140–145), pharynx (ICD9=146–149), and larynx (ICD9=161) and excluding non-squamous cancers], kidney (ICD9=189.0 & 189.1), liver (ICD9=155), lung (ICD9=162), pancreas (ICD9=157, excluding 157.4), prostate (ICD9=185), and upper gastrointestinal tract, including esophageal squamous cell carcinoma (ICD9=150), esophagogastric junctional adenocarcinoma (ICD9=150 and 151.0) and gastric noncardia adenocarcinoma (ICD9=151.1–151.9). Additional cases (7.8%) originated from a prior lymphoid cancer study [19] and a subsequent liver cancer study (2.3%) [20]. To increase the sample size for specific organ sites of interest, we measured serum 25(OH)D in all additional ATBC cases of oropharynx, larynx, upper gastrointestinal, kidney, and melanoma (referred to as the “addendum set”), representing 7.1% of the final dataset. In total, 4,616 cancer cases were included.
Serum 25(OH)D measurements
Fasting baseline serum samples were stored at −70°C until analysis. 25(OH)D from baseline/pre-randomization samples was measured by Heartland Assays, LLC (Ames, Iowa). The majority (multi-site and liver sets) were measured with the direct, competitive chemiluminescence immunoassay, DiaSorin Liaison 25(OH)D TOTAL [21]. The lymphoid set used a radioimmunoassay [19] and the most recent addendum set used liquid chromatography/mass spectrometry. Masked quality control duplicates represented 5–6% of the total sample size, and consisted of pooled serum samples and, for the multi-site set, standard reference material for vitamin D from the US National Institute of Standards and Technology (SRM 972) [22]. Coefficients of variation calculated using nested components of variance analyses [23] were 7.1% (interbatch) and 10.1% (intrabatch) for the multi-site set [24], 1.4% (interbatch) and 3.9% (intrabatch) for the liver set [20], 14% (overall) for the lymphoid set [19], and 8.1% (interbatch) and 8.9% (intrabatch) for the addendum set.
Statistical analyses
Follow-up time was calculated from the date of cancer diagnosis to date of death or the cohort censor date (December 31, 2014). For the overall cancer analyses, men with any cancer as the cause of death were considered cases. For each individual cancer site analysis, cases were defined as men who died of that specific cancer. Men with any other cause of death were censored at their death date and considered cancer survivors, as were men who were alive at the end of follow up.
Wilcoxon rank sum tests (for continuous variables) and chi-square tests (for categorical variables) were used to examine demographic factors for men who had died of cancer compared with cancer survivors. Hazard ratios (HR) and 95% confidence intervals (CI) for 25(OH)D and cancer mortality were calculated using Cox proportional hazards regression. The hazard proportionality assumption was tested and no evidence of a violation was found. Because the assay methods and dates of analysis differed in the multisite, liver, lymphoid, and addendum sets, we created separate 25(OH)D categories based on the 25(OH)D distribution within each of these sets. In addition, because of the known seasonal variation in 25(OH)D concentrations, the 25(OH)D categories were also created separately for two seasons (“darker” and “sunnier”). Therefore, 25(OH)D was modeled using season- and set-specific quantiles (tertiles or quintiles) based on the 25(OH)D distribution of all subjects in each season and set, and entered into the Cox models as indicator variables. To test for linear trends, the quantiles were coded 1–3 or 1–5 (for tertiles and quintiles, respectively) and treated as a continuous variable. Season was defined as “darker” (November-April) and “sunnier” (May-October) based on monthly median 25(OH)D concentrations among a large set of ATBC non-cancer controls [13].
Analyses for overall cancer mortality and most organ-specific models are presented with 25(OH)D quintile categories. We used a smaller number of categories if there were <10 subjects/cell when crossing the 25(OH)D quantile variable with the outcome survival variable. Esophageal, liver, melanoma, and pancreatic cancer are therefore presented as tertiles; however, due to the small number of survivors, models for esophageal and pancreatic cancer still had <10 subjects/cell. Because most of the cancers were prostate (n=1,294) and we had previously observed that higher pre-diagnostic 25(OH)D was associated with lower prostate cancer mortality [4], we conducted a sensitivity analysis excluding those cases from the overall cancer mortality models. We did the same for lung cancer, which had the second largest number of cancers (n=989) and was also previously published [5].
All models were adjusted for age at diagnosis (continuous). No other variables were considered confounders as none produced a >10% change in the 25(OH)D coefficients when added to the models adjusted for age at diagnosis. However, we selected the following for our multivariable models, as they are potentially associated with mortality: body mass index (BMI), number of cigarettes smoked per day, number of years smoked, physical activity in leisure time, serum cholesterol, history of diabetes, family history of cancer, systolic blood pressure, trial intervention group, and calendar year of diagnosis (which could be related to treatment protocol). We further adjusted site-specific models for prior cancer diagnoses by including a yes/no flag variable in the models. The following variables were tested but not included in final models: age at blood collection; height; occupational physical activity; vitamin D, calcium, alcohol, and energy intakes; and supplemental vitamin D and calcium.
Secondary analyses were stratified by median splits of age at diagnosis, BMI, number of cigarettes/day, and serum α-tocopherol, β-carotene, and retinol, as well as trial intervention group, season of blood collection, and season of diagnosis. Effect modification was statistically evaluated by comparing models with and without a cross-product term of 25(OH)D (categorical) and the effect modifier (categorical), using the log-likelihood test. We also examined effect modification for lung and esophageal cancer by smoking intensity. Additional analyses were conducted stratified on year of diagnosis (1985–1994, 1995–2004, 2005–2014) and on median time between blood collection and cancer diagnosis, and excluding the first two years of follow-up to reduce potential reverse causality. Statistical analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, North Carolina) and all P-values were 2-sided.
Data on cancer stage were only available for 49% of cases, and primarily for prostate cancer (84% of prostate cases), lung (56%), colorectal (42%), gastric (51%), liver (47%) and pancreas (76%). The proportion of men with no stage data was similar by quintile of vitamin D. For the overall analysis and for these six cancer sites specifically, we ran additional models adjusting for cancer stage (stages 1–4 and missing). The all-site, prostate, and lung analyses were also stratified by cancer stage.
Results
Men who died from their cancer (n=2,884) were older at study entry/blood collection, diagnosed with cancer at a younger age, heavier smokers, more likely to have a family history of cancer, and had lower serum 25(OH)D at baseline (Table 1). A greater percentage of these men were diagnosed at a later stage (i.e., stage 3 or 4) compared with the cancer survivors (Table 1). Vitamin D status was not, however, associated with cancer stage: medians of 34.3, 34.3, 32.5, 34.1 nmol/L for stages 1–4, respectively (p=0.43).
Table 1:
Selected baseline characteristics by cancer survivor status in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Studya
| Characteristic | Died of cancer (n=2,884) |
Cancer survivorb (n=l,732) |
p-valuec |
|---|---|---|---|
| Age at blood collection (years) | 58 (52–65) | 56 (51–64) | <0.0001 |
| Age at diagnosis (years) | 69 (60–78) | 70 (61–79) | 0.003 |
| Height (cm) | 173 (166–182) | 174 (166–182) | 0.17 |
| Body mass index (kg/m2) | 25.8(21.6–31.1) | 26.0(22.1–31.1) | 0.08 |
| Systolic blood pressure (mmHg) | 140 (120–168) | 140 (120–168) | 0.92 |
| Cigarettes per dayd | 20 (5–80) | 20 (5–60) | 0.01 |
| Years smoked | 38 (28–46) | 36 (26–45) | <0.0001 |
| Physically active, N (% moderate & heavy) | 1,673 (58) | 1,031 (60) | 0.31 |
| History of diabetes, N (% yes) | 103 (3.6) | 58 (3.4) | 0.69 |
| Family history of cancere, N (% yes) | 901 (45) | 561 (41) | 0.05 |
| Stage 3 or4f, N (%) | 996 (67) | 194 (24) | <0.0001 |
| Trial intervention - alpha-tocopherol | 1,431 (50) | 843 (49) | 0.53 |
| Trial intervention - beta-carotene | 1,451 (50) | 864 (50) | 0.78 |
| Energy intake (kcal/day) | 2,554 (1,787–3,620) | 2,595 (1,817–3,646) | 0.11 |
| Dietary vitamin D (ug/day) | 4.7 (2.2–9.3) | 4.6 (2.2–9.1) | 0.37 |
| Dietary calcium (mg/day) | 1,312 (723–2,076) | 1,333 (734–2,101) | 0.35 |
| Alcohol intake (g/day) | 11.4(0–45.7) | 10.7 (0–46.0) | 0.24 |
| Supplemental vitamin D use, N (% yes) | 226 (7.8) | 137 (7.9) | 0.93 |
| Supplemental calcium use, N (% yes) | 367 (12.7) | 191 (11.0) | 0.09 |
| Serum cholesterol (mmol/L) | 6.1 (4.8–7.7) | 6.2 (4.9–7.8) | 0.05 |
| Serum 25(OH)D (nmol/L) | 34.7(14.4–66.1) | 36.5 (15.6–68.0) | 0.01 |
25(OH)D, 25-hydroxyvitamin D
Values are medians (10th-90th) or number (%) unless otherwise noted
Cancer survivors are men who were alive at the end of follow-up or, if deceased, whose cause of death was not cancer
p-values based on Wilcoxon rank sum tests (for continuous variables) and chi-square tests (for categorical variables)
The median values are identical because most men report smoking one pack (n=20 cigarettes) per day. Here we show the full range of the distribution
Family history of lung, bladder, prostate, colon, rectum, breast, stomach, pancreas or “other” cancer; available for 72% of men
Stage data available for 49% of overall cases, primarily for prostate (84% of prostate cases), lung (56% of lung cases), colorectal (42% of colorectal cases), gastric (51% of gastric cases), liver (47% of liver cases), and pancreatic cancer (76% of pancreatic cases).
Higher circulating 25(OH)D years prior to cancer diagnosis was associated with lower cancer mortality (HR=0.76, 95% CI 0.67–0.85 for highest vs. lowest quintile, p-trend <0.0001, Table 2; Kaplan-Meier survival plot of vitamin D quintiles shown in Figure 1). In a sensitivity analysis excluding prostate cancers, the association was attenuated, but cancer mortality remained significantly lower for men in the highest vitamin D category (HR=0.83, 95% CI 0.72–0.94, p-trend=0.02). Cancer mortality also remained significantly lower when lung cancers were excluded, either alone (HR=0.69, 95% CI 0.60–0.80 for highest vs. lowest quintile, p-trend <0.0001) or along with the prostate cancers (HR=0.73, 95% CI 0.62–0.86, p-trend=0.002). When restricted to the 83% of subjects whose serum vitamin D assays were conducted in the same laboratory at the same time using the same assay (DiaSorin Liaison 25(OH)D TOTAL), findings were identical (HR=0.76, 95% CI 0.67–0.87, p-trend <0.0001). Higher vitamin D status was associated with significantly improved survival from prostate cancer, kidney cancer, and malignant melanoma, and non-significantly improved survival from liver, pancreatic, gastric, and head and neck cancers (Table 2). By contrast, higher 25(OH)D was associated with poorer lung and esophageal cancer survival (albeit, significant only for lung cancer, Table 2). Note that the number of cases for several cancer sites was low (see Table 2).
Table 2:
Association between prospectively-measured, circulating 25(OH)D and overall and site-specific cancer mortality
| Season-Specific Quintile of Serum 25(OH)Da | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p-trend | |
| All Cases (n=4,616) | ||||||
| Number of deaths/survivors | 618/309 | 579/346 | 579/344 | 569/353 | 539/380 | |
| Survival time (years, median) | 1.68 | 2.27 | 2.29 | 2.68 | 3.22 | |
| Age-adjusted HR (95% CI)b | 1.00 | 0.84 (0.75–0.94) | 0.83 (0.74–0.93) | 0.79 (0.70–0.89) | 0.71 (0.63–0.80) | <0.0001 |
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.84 (0.75–0.94) | 0.88 (0.78–0.99) | 0.82 (0.73–0.92) | 0.76 (0.67–0.85) | <0.0001 |
| Multivariate and stage-adjusted HR (95% CI) d |
1.00 | 0.88 (0.78–0.98) | 0.89 (0.80–1.00) | 0.87 (0.77–0.97) | 0.76 (0.68–0.86) | <0.0001 |
| Prostate (n=1,294) | ||||||
| Number of deaths/survivors | 83/146 | 83/170 | 82/158 | 67/209 | 91/205 | |
| Survival time (years, median) | 3.99 | 5.02 | 6.08 | 6.12 | 5.90 | |
| Age-adjusted HR (95% CI)b | 1.00 | 0.76 (0.56–1.04) | 0.75 (0.55–1.02) | 0.51 (0.37–0.71) | 0.71 (0.52–0.95) | 0.003 |
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.83 (0.61–1.13) | 0.86 (0.63–1.18) | 0.53 (0.38–0.74) | 0.74 (0.55–1.01) | 0.005 |
| Multivariate and stage-adjusted HR (95% CI) d |
1.00 | 0.88 (0.65–1.21) | 0.87 (0.64–1.19) | 0.59 (0.43–0.82) | 0.77 (0.56–1.05) | 0.02 |
| Lung (n=989) | ||||||
| Number of deaths/survivors | 163/46 | 171/25 | 153/44 | 165/40 | 149/33 | |
| Survival time (years, median) | 0.57 | 0.61 | 0.55 | 0.59 | 0.48 | |
| Age-adjusted HR (95% CI)b | 1.00 | 1.20 (0.96–1.48) | 1.06 (0.85–1.32) | 1.05 (0.84–1.30) | 1.21 (0.97–1.51) | 0.35 |
| Multivariate-adjusted HR (95% CI)c | 1.00 | 1.22 (0.98–1.53) | 1.10 (0.88–1.38) | 1.08 (0.87–1.34) | 1.28 (1.02–1.61) | 0.19 |
| Multivariate and stage-adjusted HR (95% CI) d |
1.00 | 1.26 (1.01–1.58) | 1.11 (0.88–1.39) | 1.14 (0.91–1.42) | 1.34 (1.06–1.69) | 0.08 |
| Bladder (n=833) | ||||||
| Number of deaths/survivors | 18/57 | 15/67 | 11/67 | 21/59 | 16/52 | |
| Survival time (years, median) | 6.09 | 5.93 | 7.33 | 5.33 | 3.83 | |
| Age-adjusted HR (95% CI)b | 1.00 | 0.87 (0.44–1.73) | 0.64 (0.30–1.35) | 1.15 (0.61–2.16) | 1.08 (0.55–2.11) | 0.59 |
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.81 (0.40–1.63) | 0.64 (0.30–1.38) | 1.09 (0.57–2.09) | 0.98 (0.49–1.97) | 0.73 |
| Colorectal (n=497) | ||||||
| Number of deaths/survivors | 40/60 | 35/57 | 38/51 | 40/64 | 49/63 | |
| Survival time (years, median) | 2.14 | 4.40 | 2.35 | 2.88 | 3.60 | |
| Age-adjusted HR (95% CI)b | 1.00 | 0.80 (0.51–1.26) | 1.02 (0.66–1.59) | 0.86 (0.56–1.33) | 0.92 (0.61–1.40) | 0.86 |
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.79 (0.50–1.24) | 1.08 (0.68–1.70) | 0.87 (0.56–1.37) | 0.97 (0.63–1.50) | 0.95 |
| Multivariate and stage-adjusted HR (95% CI) d |
1.00 | 0.89 (0.56–1.42) | 0.86 (0.54–1.37) | 0.81 (0.52–1.28) | 0.96 (0.61–1.49) | 0.77 |
| Head & Neck (n=398) | ||||||
| Number of deaths/survivors | 35/47 | 31/65 | 21/62 | 17/45 | 22/53 | |
| Survival time (years, median) | 3.12 | 2.48 | 3.81 | 4.40 | 4.48 | |
| Age-adjusted HR (95% CI)b | 1.00 | 0.74 (0.45–1.20) | 0.52 (0.30–0.90) | 0.61 (0.34–1.08) | 0.56 (0.33–0.95) | 0.02 |
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.87 (0.53–1.45) | 0.59 (0.33–1.05) | 0.76 (0.41–1.39) | 0.74 (0.42–1.30) | 0.21 |
| Kidney (n=378) | ||||||
| Number of deaths/survivors | 36/34 | 29/41 | 44/40 | 38/34 | 30/52 | |
| Survival time (years, median) | 1.43 | 3.11 | 2.25 | 2.97 | 2.35 | |
| Age-adjusted HR (95% CI)b | 1.00 | 0.64 (0.39–1.05) | 0.86 (0.56–1.34) | 0.84 (0.53–1.33) | 0.64 (0.39–1.04) | 0.27 |
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.62 (0.37–1.05) | 0.89 (0.57–1.41) | 0.94 (0.58–1.52) | 0.59 (0.35–0.98) | 0.28 |
| Hematological (n=349) | ||||||
| Number of deaths/survivors | 28/30 | 40/32 | 48/26 | 40/27 | 45/33 | |
| Survival time (years, median) | 2.26 | 2.19 | 1.62 | 2.87 | 3.85 | |
| Age-adjusted HR (95% CI)b | 1.00 | 1.12 (0.69–1.81) | 1.46 (0.91–2.33) | 1.04 (0.64–1.69) | 0.95 (0.59–1.53) | 0.58 |
| Multivariate-adjusted HR (95% CI)c | 1.00 | 1.08 (0.66–1.76) | 1.48 (0.92–2.39) | 1.10 (0.66–1.81) | 0.97 (0.60–1.58) | 0.77 |
| Gastric (n=339) | ||||||
| Number of deaths/survivors | 50/28 | 45/20 | 38/37 | 43/29 | 30/19 | |
| Survival time (years, median) | 0.80 | 0.94 | 1.15 | 0.79 | 1.74 | |
| Age-adjusted HR (95% CI)b | 1.00 | 0.95 (0.63–1.42) | 0.65 (0.43–1.00) | 0.81 (0.54–1.22) | 0.77 (0.49–1.21) | 0.14 |
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.95 (0.62–1.46) | 0.65 (0.42–1.01) | 0.80 (0.52–1.22) | 0.76 (0.47–1.23) | 0.14 |
| Multivariate and stage-adjusted HR (95% CI) d |
1.00 | 0.72 (0.46–1.12) | 0.53 (0.34–0.83) | 0.73 (0.48–1.12) | 0.66 (0.41–1.07) | 0.11 |
| Liver (n=206) | ||||||
| Number of deaths/survivors | 59/20 | 60/15 | 42/10 | |||
| Survival time (years, median) | 0.14 | 0.19 | 0.29 | |||
| Age-adjusted HR (95% CI)b | 1.00 | 0.91 (0.63–1.32) | 0.75 (0.50–1.13) | 0.18 | ||
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.91 (0.62–1.33) | 0.75 (0.49–1.17) | 0.21 | ||
| Multivariate and stage-adjusted HR (95% CI) d |
1.00 | 0.94 (0.64–1.38) | 0.70 (0.45–1.09) | 0.13 | ||
| Pancreas (n=143) | ||||||
| Number of deaths/survivors | 45/3 | 45/6 | 40/4 | |||
| Survival time (years, median) | 0.24 | 0.26 | 0.21 | |||
| Age-adjusted HR (95% CI)b | 1.00 | 0.91 (0.60–1.37) | 0.81 (0.52–1.25) | 0.34 | ||
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.91 (0.57–1.46) | 0.79 (0.48–1.30) | 0.36 | ||
| Multivariate and stage-adjusted HR (95% CI) d |
1.00 | 0.72 (0.44–1.16) | 0.66 (0.40–1.10) | 0.12 | ||
| Esophagogastric iunctional (n=136) | ||||||
| Number of deaths/survivors | 31/11 | 38/8 | 32/16 | |||
| Survival time (years, median) | 0.68 | 0.60 | 1.03 | |||
| Age-adjusted HR (95% CI)b | 1.00 | 1.05 (0.65–1.71) | 0.74 (0.45–1.22) | 0.21 | ||
| Multivariate-adjusted HR (95% CI)c | 1.00 | 1.09 (0.41–1.21) | 0.70 (0.41–1.21) | 0.18 | ||
| Melanoma (n=135) | ||||||
| Number of deaths/survivors | 19/16 | 13/32 | 17/38 | |||
| Survival time (years, median) | 2.63 | 3.71 | 4.54 | |||
| Age-adjusted HR (95% CI)b | 1.00 | 0.44 (0.22–0.90) | 0.45 (0.23–0.86) | 0.02 | ||
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.47 (0.22–0.78) | 0.39 (0.20–0.78) | 0.01 | ||
| Esophageal (n=97) | ||||||
| Number of deaths/survivors | 40/4 | 29/2 | 19/3 | |||
| Survival time (years, median) | 0.66 | 0.65 | 0.37 | |||
| Age-adjusted HR (95% CI)b | 1.00 | 1.08 (0.67–1.75) | 1.63 (0.91–2.93) | 0.14 | ||
| Multivariate-adjusted HR (95% CI)c | 1.00 | 1.15 (0.65–2.05) | 1.94 (0.91–4.11) | 0.11 | ||
25(OH)D, 25-hydroxyvitamin D; CI, confidence intervals; HR, hazard ratio
Cutpoints for the 25(OH)D concentrations (nmol/L) were based on the distribution in each analysis set and season as follows:
Multi-site darker season (November-April): Q1: < 17.0; Q2 >17.0- < 24.2; Q3; >24.2 - < 33.1; Q4 >33.1 - < 45.4; Q5 >45.4.
Multi-site sunnier season (May-October): Q1: < 25.8; Q2 > 25.8- < 36.5; Q3; >36.5 - < 47.1; >Q4 47.1 - < 61.8; Q5 >61.8.
Liver set darker season: Q1: < 21.9; Q2 >21.9- < 29.8; Q3; >29.8 - < 36.7; Q4 >36.7 - < 46.9; Q5 >46.9.
Liver set sunnier season: Q1: < 26.9; Q2 >26.9- < 46.3; Q3; >46.3 - < 56.7; Q4 >56.7 - < 71.0; Q5 >71.0.
Lymphoma set darker season: Q1: < 31.6; Q2 > 31.6- < 39.5; Q3; />39.5 - < 50.6; >Q4 50.6 - < 65.8; Q5 > 65.8.
Lymphoma set sunnier season: Q1: < 40.0; Q2 >40.0- < 51.8; Q3; >51.8 - < 62.8; Q4 >62.8 - < 77.3; Q5 >77.3.
Addendum set darker season: Q1: < 25.1; Q2 >25.1- < 33.5; Q3; >33.5 - < 45.1; Q4 >45.1 - < 55.9; Q5 >55.9.
Addendum set sunnier season: Q1: < 37.2; Q2 >37.2- < 44.3; Q3; >44.3 - < 55.2; Q4 >55.2 - < 67.8; Q5 >67.8.
Adjusted for age at diagnosis.
Additionally adjusted for body mass index, number of cigarettes smoked per day, years of smoking, physical activity, serum cholesterol, history of diabetes, family history of cancer, systolic blood pressure, trial intervention group, and calendar year of diagnosis. Site-specific models are also adjusted for prior (yes/no) cancer diagnoses
Stage data available for 49% of overall cases, primarily for prostate (84% of prostate cases), lung (56% of lung cases), colorectal (42% of colorectal cases), gastric (51% of gastric cases), liver (47% of liver cases), and pancreatic cancer (76% of pancreatic cases). Stage-adjusted models are shown only for overall and these six cancer sites
Figure 1:
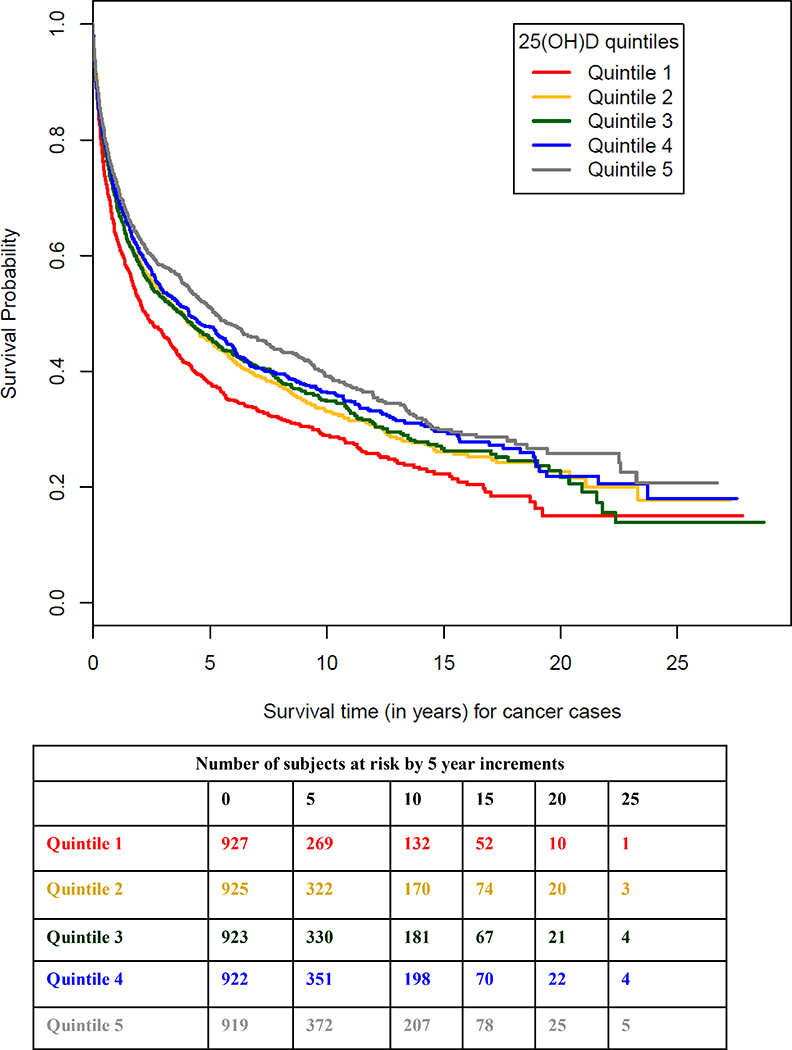
Overall cancer survival by quintile of serum 25(OHD) measured years prior to cancer diagnosis. Cutpoints for the 25(OH)D concentrations (nmol/L) were based on the distribution in each analysis set and season and are given in Table 2.
Findings were similar after adjusting for stage at diagnosis in the 2,278 cases with stage data (HR=0.76, 95% CI 0.68–0.86 for highest vs. lowest quintile, p-trend=<0.0001 for all cases combined, Table 2). This was also true for lung, prostate, colorectal, liver, and pancreatic cancers, while risks for gastric cancer were slightly strengthened with stage adjustment (Table 2). Stratification of overall cancer by disease stage at diagnosis showed a somewhat stronger vitamin D-survival association for stages 1–2 compared with stages 3–4 cancers, but the interaction test was not statistically significant (Table 3, p-interaction=0.77). The reduced prostate cancer mortality for high vitamin D status was limited to stages 1–2 (HR=0.49, 95% CI 0.25–0.96, p-trend 0.03) vs. stages 3–4 disease (HR=0.95, 95% CI 0.66–1.39, p-trend=0.60), while the elevated lung cancer mortality was more apparent for stage 3–4 (HR=1.74, 95% CI 1.18–2.56, p-trend 0.03) vs. stage 1–2 disease (HR=1.19, 95% CI 0.66–2.13, p-trend 0.59), though neither interaction test was significant (p>0.63).
Table 3:
Association between prospectively-measured, circulating 25(OH)D and cancer mortality, stratified by stagea
| Season-Specific Quintile of Serum 25(OH)Db | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p-trend | |
| All Cases - Stage 1–2 10=1,0881 | ||||||
| Number of deaths/survivors | 97/103 | 99/121 | 91/109 | 106/129 | 95/138 | |
| Survival time (years, median) | 4.60 | 7.03 | 7.84 | 6.71 | 7.73 | |
| Age-adjusted HR (95% CI)c | 1.00 | 0.78 (0.59–1.03) | 0.74 (0.56–0.99) | 0.73 (0.55–0.96) | 0.63 (0.47–0.83) | 0.003 |
| Multivariate-adjusted HR (95% CI)d | 1.00 | 0.75 (0.57–1.00) | 0.80 (0.60–1.08) | 0.71 (0.53–0.94) | 0.68 (0.50–0.91) | 0.02 |
| All Cases - Stage 3–4 (n= 1,190) | ||||||
| Number of deaths/survivors | 223/35 | 195/37 | 199/32 | 188/42 | 191/48 | |
| Survival time (years, median) | 0.80 | 1.12 | 0.85 | 0.84 | 1.25 | |
| Age-adjusted HR (95% CI)c | 1.00 | 0.88 (0.72–1.06) | 0.99 (0.82–1.20) | 0.88 (0.72–1.06) | 0.76 (0.62–0.92) | 0.01 |
| Multivariate-adjusted HR (95% CI)d | 1.00 | 0.87 (0.72–1.06) | 1.05 (0.86–1.27) | 0.95 (0.78–1.16) | 0.83 (0.68–1.01) | 0.20 |
25(OH)D, 25-hydroxyvitamin D; CI, confidence intervals; HR, hazard ratio
Stage data available for 49% of overall cases; p-interaction=0.77
Cutpoints for the 25(OH)D concentrations were based on the distribution in each analysis set and season and are reported in Table 2
Adjusted for age at diagnosis
Additionally adjusted for body mass index, number of cigarettes smoked per day, years of smoking, physical activity, serum cholesterol, history of diabetes, family history of cancer, systolic blood pressure, trial intervention group, and calendar year of diagnosis
The 25(OH)D-overall cancer survival association did not differ by calendar year of diagnosis (Table 4), by other factors examined, including time between blood collection and diagnosis, or when excluding the first two years of observation (data not shown).
Table 4:
Association between prospectively-measured, circulating 25(OH)D and cancer mortality, stratified by year of diagnosis
| Season-Specific Quintile of Serum 25(OH)Da | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p-trend | |
| 1985–1994 (n=1,397) | ||||||
| Number of deaths/survivors | 225/80 | 211/91 | 193/73 | 168/95 | 176/85 | |
| Survival time (years, median) | 1.77 | 2.25 | 1.76 | 3.13 | 3.13 | |
| Age-adjusted HR (95% CI)b | 1.00 | 0.80 (0.67–0.97) | 0.89 (0.73–1.08) | 0.67 (0.55–0.82) | 0.70 (0.58–0.86) | 0.0001 |
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.76 (0.63–0.92) | 0.93 (0.77–1.13) | 0.70 (0.57–0.86) | 0.77 (0.63–0.94) | 0.009 |
| 1995–2004 (n=2,581) | ||||||
| Number of deaths/survivors | 320/186 | 302/205 | 316/205 | 310/211 | 299/227 | |
| Survival time (years, median) | 1.75 | 2.77 | 2.99 | 3.13 | 3.70 | |
| Age-adjusted HR (95% CI)b | 1.00 | 0.84 (0.72–0.98) | 0.83 (0.71–0.97) | 0.81 (0.69–0.95) | 0.73 (0.62–0.85) | 0.0002 |
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.87 (0.74–1.02) | 0.87 (0.74–1.02) | 0.84 (0.72–0.99) | 0.77 (0.66–0.91) | 0.003 |
| 2005–2014 (n=638) | ||||||
| Number of deaths/survivors | 73/43 | 66/50 | 70/66 | 91/47 | 64/68 | |
| Survival time (years, median) | 1.22 | 0.94 | 1.13 | 1.18 | 1.54 | |
| Age-adjusted HR (95% CI)b | 1.00 | 0.95 (0.68–1.32) | 0.79 (0.57–1.10) | 1.05 (0.77–1.44) | 0.67 (0.48–0.94) | 0.09 |
| Multivariate-adjusted HR (95% CI)c | 1.00 | 0.95 (0.68–1.34) | 0.81 (0.58–1.13) | 1.10 (0.80–1.52) | 0.68 (0.48–0.96) | 0.13 |
25(OH)D, 25-hydroxyvitamin D; CI, confidence intervals; HR, hazard ratio
Cutpoints for the 25(OH)D concentrations were based on the distribution in each analysis set and season and are reported in Table 2
Adjusted for age at diagnosis
Additionally adjusted for body mass index, number of cigarettes smoked per day, years of smoking, physical activity, serum cholesterol, history of diabetes, family history of cancer, systolic blood pressure, trial intervention group, and calendar year of diagnosis
Discussion
Men with higher serum 25(OH)D years prior to cancer diagnosis experienced significantly longer survival after a cancer diagnosis, compared to those with lower vitamin D status. This was particularly evident for cancers of the prostate and kidney, as well as malignant melanoma. Lung cancer survival was shorter among men with higher pre-diagnostic vitamin D concentrations.
Prior studies examining cancer survival in relation to post-diagnostic (and sometimes post-treatment) vitamin D status have indicated that patients with higher 25(OH)D blood concentrations have improved survival, including for breast, colorectal, head and neck, hematological, kidney, liver, lung, ovarian, prostate, pancreatic, skin (melanoma) and stomach cancers [25–38]. These studies likely suffer from some degree of reverse causality, however; i.e., the cancer or its treatments could have influenced vitamin D status. Prospective studies greatly reduce this bias by measuring 25(OH)D in blood samples obtained years prior to cancer diagnosis, and most show higher circulating vitamin D associated with improved cancer survival [39–42], particularly for women in one study [39] and only in persons with a history of cancer in another [42]. By contrast, two other cohorts [2, 3] and one meta-analysis of eight prospective cohorts [43] showed no association between pre-diagnostic 25(OH)D and cancer survival. Vitamin D supplementation has been associated with increased cancer survival in a meta-analysis of a few trials [44], although risk estimates in the individual studies were not significant [45–47]. Three vitamin D trials of men with advanced prostate cancer were also null [48], and other trials examining effects on cancer progression and survival are on-going [49].
We observed that higher prospectively-measured 25(OH)D was associated with improved survival after a diagnosis of prostate cancer, kidney cancer, and melanoma. Previously we showed this increased prostate cancer survival based on shorter follow-up and fewer cases [4], with similar findings in the Swedish Malmo cohort (HR=0.6 for prostate cancer mortality) [50] and the Health Professionals Follow-up Study (OR=0.4 for fatal or metastatic disease) [51]. By contrast, data from five cohorts within the Breast and Prostate Cancer Cohort Consortium showed no 25(OH)D-survival association [52], and the NHANES III study found non-significant decreased prostate cancer survival for men with higher 25(OH)D status [53]. The EPIC cohort found a U-shaped association between vitamin D and mortality after a kidney cancer diagnosis [54]. We are not aware of prior prospective studies of vitamin D and malignant melanoma survival.
Higher vitamin D status was not associated with survival from bladder, colorectal, or hematological cancers in our study, but better survival was suggested for head and neck, liver, pancreatic, and gastric cancers, and poorer survival for esophageal cancer. The EPIC cohort also reported improved head and neck cancer survival, but no association for esophageal cancer survival [55]. NHANES III found no vitamin D association with non-Hodgkin lymphoma or leukemia survival, but indicated non-significantly increased mortality for digestive cancers (including pancreas, esophagus, stomach and liver cancers combined) in men with higher pre-diagnostic 25(OH)D (RR= 1.6) [53]. Several studies have observed improved colorectal cancer survival with higher vitamin D status [53, 56, 57], although a pooled analysis showed no association [41]. Vitamin D was directly associated with lung cancer mortality in the present analysis, strengthening our earlier finding based on shorter follow-up, of a non-significant 20% elevated mortality [5]. This is in line with a nearly 90% elevated mortality for >100 vs. <50 nmol/L 25(OH)D in NHANES III [53], but contrasts with two Danish cohorts that showed significantly increased survival [41].
Cancer stage data were abstracted from medical record reviews and available for half of our cases. Prediagnostic serum 25(OH)D did not vary by stage, the proportion of men missing stage data was similar across vitamin D categories, and adjusting for stage did not materially alter our findings. Cancer treatment data were not available, but 25(OH)D concentration at study entry is unlikely to have been associated with medical intervention years later, and our models included calendar year of diagnosis to adjust for any secular trends in therapeutics. In addition, improved survival was similar when we stratified on calendar year of diagnosis.
Observational studies of vitamin D status and cancer incidence suggest that increased circulating 25(OH)D is associated with lower colorectal cancer risk and higher prostate cancer risk, with inconsistent data for other organ sites [1, 10, 58–60]. A recent Mendelian randomization analysis found no association between a vitamin D genetic score and risk of seven cancers (colorectal, breast, prostate, ovarian, lung, and pancreatic cancers and neuroblastoma) [61]. Hypothesized mechanisms for protective risk associations for vitamin D include inhibition of angiogenesis, cellular proliferation, and inflammation, and promotion of apoptosis and cellular differentiation [62, 63]. Vitamin D has also been shown to inhibit prostate cancer metastasis in rats [64], and it has been suggested that many vitamin D anti-cancer properties act during cancer progression rather than initiation [65], supporting a role for vitamin D in cancer survival.
How higher vitamin D status years prior to a cancer diagnosis might selectively improve survival for some malignancies but not others can only be speculated. In some cases, lead time bias is possible; e.g., we and others have previously shown elevated serum 25(OH)D to be associated with higher prostate cancer risk [10, 60], and earlier diagnoses could indicate detection of less lethal tumors or effectiveness of earlier treatment, for example. Higher vitamin D status has been related to improved immunity [62], and vitamin D is known to have immunomodulatory functions [66]. For example, a recent study found that the circulating 25(OH)D-colorectal cancer risk association differed by the degree of tumor lymphocyte infiltration [67]. Whether such differences in the tumor microenvironment can explain organ site differences in the vitamin D-survival relation will require examination in other studies.
Our investigation has strengths and limitations. We evaluated mortality outcomes for nearly 5,000 men with cancer, and detailed information regarding potential confounding factors was available. Nonetheless, there were relatively few cases for several rarer organ sites which reduced study power for detecting a survival association. Serum 25(OH)D was measured in fasting blood samples collected up to 28 years prior to cancer diagnosis, thus reducing the potential for cancer-related or treatment-related effects on vitamin D status, which has been a concern regarding many previously conducted studies [1]. Similar to most cohorts, we examined 25(OH)D in samples collected at only one point in time and assume both that the estimated vitamin D status represents the person’s usual condition and that the 25(OH)D concentration remains stable in long-term freezer storage, both of which appear to be the case [68–71]. All of our samples had 25(OH)D measured in one laboratory, but 17% of them were measured at different times using different assay methods (albeit, each considered valid). We analyzed season-specific and study set-specific quantiles to control for both this and the known seasonal variation in 25(OH)D concentrations, and our findings were unchanged in sensitivity analyses of the approximately 3,800 cases whose serum samples were measured at the same time with the same assay. The ATBC Study includes only male smokers, which limits the generalizability of our findings to women and non-smokers. Several studies have reported sex differences in vitamin D and cancer associations [39, 53, 58, 59, 72], but this has not been adequately studied. Smoking intensity and duration were not strongly associated with 25(OH)D and did not confound our findings. In addition, while the vitamin D-cancer survival association could differ in non-smoking populations, our findings would be directly relevant to the approximately 37 million adult current smokers in the US [73] (and possibly to former smokers).
In conclusion, higher serum vitamin D concentrations up to 28 years prior to diagnosis were associated with significantly improved cancer survival, including particularly for men with prostate, kidney, and malignant skin cancers (and possibly for head and neck, pancreas, gastric, and liver cancers) but shorter lung cancer survival. Our findings indicate possible site-specific survival benefits for higher prospective vitamin D status in the face of limited evidence for protective vitamin D-cancer risk associations. Pre-diagnostic vitamin D status in relation to cancer survival outcomes should be examined in other populations that include women and other racial-ethnic groups. Whether vitamin D supplementation may have a role in clinical management of some cancer patients will require further research.
Acknowledgments
Financial Support: The ATBC Study is supported by the Intramural Research Program of the U.S. National Cancer Institute, National Institutes of Health, and by U.S. Public Health Service contract HHSN261201500005C from the National Cancer Institute, Department of Health and Human Services.
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- ATBC
Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study
- BMI
body mass index
- CI
confidence intervals
- HR
hazard ratio
- ICD
International Classification of Diseases
Footnotes
Conflicts of Interest: The authors have no conflicts to disclose.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Mondul AM, Weinstein SJ, Layne TM, Albanes D. Vitamin D and cancer risk and mortality: state of the science, gaps, and challenges. Epidemiol Rev. 2017;39(1):28–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khaw KT, Luben R, Wareham N. Serum 25-hydroxyvitamin D, mortality, and incident cardiovascular disease, respiratory disease, cancers, and fractures: a 13-y prospective population study. Am J Clin Nutr. 2014;100(5):1361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Hilali J, de Koning EJ, van Ballegooijen AJ, Lips P, Sohl E, van Marwijk HWJ et al. Vitamin D, PTH and the risk of overall and disease-specific mortality: Results of the Longitudinal Aging Study Amsterdam. J Steroid Biochem Mol Biol. 2016;164:386–94. [DOI] [PubMed] [Google Scholar]
- 4.Mondul AM, Weinstein SJ, Moy KA, Mannisto S, Albanes D. Circulating 25-hydroxyvitamin D and prostate cancer survival. Cancer Epidemiol Biomarkers Prev. 2016;25(4):665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anic GM, Weinstein SJ, Mondul AM, Mannisto S, Albanes D. Serum vitamin D, vitamin D binding protein, and lung cancer survival. Lung Cancer. 2014;86(3):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- 7.Korhonen P, Malila N, Pukkala E, Teppo L, Albanes D, Virtamo J. The Finnish Cancer Registry as follow-up source of a large trial cohort--accuracy and delay. Acta Oncol. 2002;41(4):381–8. [DOI] [PubMed] [Google Scholar]
- 8.Leinonen MK, Miettinen J, Heikkinen S, Pitkaniemi J, Malila N. Quality measures of the population-based Finnish Cancer Registry indicate sound data quality for solid malignant tumours. Eur J Cancer. 2017;77:31–9. [DOI] [PubMed] [Google Scholar]
- 9.Pietinen P, Hartman AM, Haapa E, Rasanen L, Haapakoski J, Palmgren J et al. Reproducibility and validity of dietary assessment instruments. I. A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol. 1988;128(3):655–66. [DOI] [PubMed] [Google Scholar]
- 10.Albanes D, Mondul AM, Yu K, Parisi D, Horst RL, Virtamo J et al. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1850–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Major JM, Kiruthu C, Weinstein SJ, Horst RL, Snyder K, Virtamo J et al. Pre-diagnostic circulating vitamin D and risk of melanoma in men. PLoS One. 2012;7(4):e35112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purdue MP, Freedman DM, Gapstur SM, Helzlsouer KJ, Laden F, Lim U et al. Circulating 25-hydroxyvitamin D and risk of non-hodgkin lymphoma: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinstein SJ, Yu K, Horst RL, Ashby J, Virtamo J, Albanes D. Serum 25-hydroxyvitamin D and risks of colon and rectal cancer in Finnish men. Am J Epidemiol. 2011;173(5):499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein SJ, Yu K, Horst RL, Parisi D, Virtamo J, Albanes D. Serum 25-hydroxyvitamin D and risk of lung cancer in male smokers: a nested case-control study. PLoS One. 2011;6(6):e20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallicchio L, Moore LE, Stevens VL, Ahn J, Albanes D, Hartmuller V et al. Circulating 25-hydroxyvitamin D and risk of kidney cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abnet CC, Chen Y, Chow WH, Gao YT, Helzlsouer KJ, Le Marchand L et al. Circulating 25-hydroxyvitamin D and risk of esophageal and gastric cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. American Journal of Epidemiology. 2010;172(1):94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arem H, Weinstein SJ, Horst RL, Virtamo J, Yu K, Albanes D et al. Serum 25-hydroxyvitamin D and risk of oropharynx and larynx cancers in Finnish men. Cancer Epidemiol Biomarkers Prev. 2011;20(6): 1178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mondul AM, Weinstein SJ, Mannisto S, Snyder K, Horst RL, Virtamo J et al. Serum vitamin D and risk of bladder cancer. Cancer Res. 2010;70(22):9218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim U, Freedman DM, Hollis BW, Horst RL, Purdue MP, Chatterjee N et al. A prospective investigation of serum 25-hydroxyvitamin D and risk of lymphoid cancers. Int J Cancer. 2009;124(4):979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai GY, Wang JB, Weinstein SJ, Parisi D, Horst RL, McGlynn KA et al. Association of 25-hydroxyvitamin D with liver cancer incidence and chronic liver disease mortality in Finnish male smokers of the ATBC study. Cancer Epidemiol Biomarkers Prev. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem. 2009;42(15):1549–56. [DOI] [PubMed] [Google Scholar]
- 22.Certificate of analysis, standard reference material 972, vitamin D in human serum. National Institute of Standards and Technology, Gaithersburg, MD: 2009. [Google Scholar]
- 23.Fears TR, Ziegler RG, Donaldson JL, Falk RT, Hoover RN, Stanczyk FZ et al. Reproducibility studies and interlaboratory concordance for androgen assays in female plasma. Cancer Epidemiol Biomarkers Prev. 2000;9(4):403–12. [PubMed] [Google Scholar]
- 24.Weinstein SJ, Mondul AM, Kopp W, Rager H, Virtamo J, Albanes D. Circulating 25-hydroxyvitamin D, vitamin D-binding protein and risk of prostate cancer. Int J Cancer. 2013;132(12):2940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robsahm TE, Schwartz GG, Tretli S. The inverse relationship between 25-Hydroxyvitamin D and cancer survival: discussion of causation. Cancers (Basel). 2013;5(4):1439–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76–89. [DOI] [PubMed] [Google Scholar]
- 27.Muller DC, Scelo G, Zaridze D, Janout V, Holcatova I, Navratilova M et al. Circulating 25-hydroxyvitamin D3 and survival after diagnosis with kidney cancer. Cancer Epidemiol Biomarkers Prev. 2015;24(8):1277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren C, Qiu MZ, Wang DS, Luo HY, Zhang DS, Wang ZQ et al. Prognostic effects of 25-hydroxyvitamin D levels in gastric cancer. J Transl Med. 2012; 10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newton-Bishop JA, Beswick S, Randerson-Moor J, Chang YM, Affleck P, Elliott F et al. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. J Clin Oncol. 2009;27(32):5439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton-Bishop JA, Davies JR, Latheef F, Randerson-Moor J, Chan M, Gascoyne J et al. 25-Hydroxyvitamin D2 /D3 levels and factors associated with systemic inflammation and melanoma survival in the Leeds Melanoma Cohort. Int J Cancer. 2015;136(12):2890–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang S, Sui D, Wang Y, Liu H, Chiang YJ, Ross MI et al. Association of vitamin D levels with outcome in patients with melanoma after adjustment for C-reactive protein. J Clin Oncol. 2016;34(15): 1741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timerman D, McEnery-Stonelake M, Joyce CJ, Nambudiri VE, Hodi FS, Claus EB et al. Vitamin D deficiency is associated with a worse prognosis in metastatic melanoma. Oncotarget. 2017;8(4):6873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bade B, Zdebik A, Wagenpfeil S, Graber S, Geisel J, Vogt T et al. Low serum 25-hydroxyvitamin D concentrations are associated with increased risk for melanoma and unfavourable prognosis. PLoS One. 2014;9(12):e112863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkelmeier F, Kronenberger B, Köberle V, Bojunga J, Zeuzem S, Trojan J et al. Severe 25-hydroxyvitamin D deficiency identifies a poor prognosis in patients with hepatocellular carcinoma - a prospective cohort study. Alimentary Pharmacology & Therapeutics. 2014;39(10): 1204–12. [DOI] [PubMed] [Google Scholar]
- 35.Cho M, Peddi PF, Ding K, Chen L, Thomas D, Wang J et al. Vitamin D deficiency and prognostics among patients with pancreatic adenocarcinoma. J Transl Med. 2013;11:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Loon K, Owzar K, Jiang C, Kindler HL, Mulcahy MF, Niedzwiecki D et al. 25-Hydroxyvitamin D levels and survival in advanced pancreatic cancer: findings from CALGB 80303 (Alliance). J Natl Cancer Inst. 2014;106(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Li G, He X, Gao J, Wang R, Wang Y et al. Serum 25-hydroxyvitamin D levels and prognosis in hematological malignancies: a systematic review and meta-analysis. Cell Physiol Biochem. 2015;35(5): 1999–2005. [DOI] [PubMed] [Google Scholar]
- 38.Maalmi H, Walter V, Jansen L, Chang-Claude J, Owen RW, Ulrich A et al. Relationship of very low serum 25-hydroxyvitamin D3 levels with long-term survival in a large cohort of colorectal cancer patients from Germany. Eur J Epidemiol. 2017;32(11):961–71. [DOI] [PubMed] [Google Scholar]
- 39.Yin L, Ordonez-Mena JM, Chen T, Schottker B, Arndt V, Brenner H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: a systematic review and meta-analysis. Prev Med. 2013;57(6):753–64. [DOI] [PubMed] [Google Scholar]
- 40.Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. 2014;349:g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schottker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot L et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaksch M, Jorde R, Grimnes G, Joakimsen R, Schirmer H, Wilsgaard T et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One. 2017;12(2):e0170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Krstic G, Wetterslev J et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014(6):CD007469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J Clin Endocrinol Metab. 2012;97(2):614–22. [DOI] [PubMed] [Google Scholar]
- 46.Brunner RL, Wactawski-Wende J, Caan BJ, Cochrane BB, Chlebowski RT, Gass ML et al. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women’s Health Initiative (WHI) calcium plus vitamin D randomized clinical trial. Nutr Cancer. 2011;63(6):827–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trivedi D, Doll R, Khaw K. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buttigliero C, Monagheddu C, Petroni P, Saini A, Dogliotti L, Ciccone G et al. Prognostic role of vitamin D status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16(9):1215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tagliabue E, Raimondi S, Gandini S. Vitamin D, cancer risk, and mortality. Adv Food Nutr Res. 2015;75:1–52. [DOI] [PubMed] [Google Scholar]
- 50.Brandstedt J, Almquist M, Manjer J, Malm J. Vitamin D, PTH, and calcium in relation to survival following prostate cancer. Cancer Causes Control. 2016;27(5):669–77. [DOI] [PubMed] [Google Scholar]
- 51.Shui IM, Mucci LA, Kraft P, Tamimi RM, Lindstrom S, Penney KL et al. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: a prospective nested case-control study. J Natl Cancer Inst. 2012;104(9):690–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shui IM, Mondul AM, Lindstrom S, Tsilidis KK, Travis RC, Gerke T et al. Circulating vitamin D, vitamin D-related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer. 2015;121(12):1949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freedman DM, Looker AC, Abnet CC, Linet MS, Graubard BI. Serum 25-hydroxyvitamin D and cancer mortality in the NHANES III study (1988–2006). Cancer Res. 2010;70(21):8587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muller DC, Fanidi A, Midttun O, Steffen A, Dossus L, Boutron-Ruault MC et al. Circulating 25-hydroxyvitamin D3 in relation to renal cell carcinoma incidence and survival in the EPIC cohort. Am J Epidemiol. 2014;180(8):810–20. [DOI] [PubMed] [Google Scholar]
- 55.Fanidi A, Muller DC, Midttun O, Ueland PM, Vollset SE, Relton C et al. Circulating vitamin D in relation to cancer incidence and survival of the head and neck and oesophagus in the EPIC cohort. Sci Rep. 2016;6:36017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, Giovannucci EL et al. Circulating 25-hydroxyvitamin D levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26(18):2984–91. [DOI] [PubMed] [Google Scholar]
- 57.Fedirko V, Riboli E, Tjonneland A, Ferrari P, Olsen A, Bueno-de-Mesquita HB et al. Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in western European ppulations. Cancer Epidemiol Biomarkers Prev. 2012;21(4):582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinstein SJ, Purdue MP, Smith-Warner SA, Mondul AM, Black A, Ahn J et al. Serum 25-hydroxyvitamin D, vitamin D binding protein and risk of colorectal cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int J Cancer. 2015;136(6):E654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCullough ML, Zoltick ES, Weinstein SJ, Fedirko V, Wang M, Cook NR et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. JNCI: Journal of the National Cancer Institute. 2018:djy087–djy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu Y, Shao X, Yao Y, Xu L, Chang L, Jiang Z et al. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: new findings from an updated meta-analysis. Journal of Cancer Research and Clinical Oncology. 2014;140(9):1465–77. [DOI] [PubMed] [Google Scholar]
- 61.Dimitrakopoulou VI, Tsilidis KK, Haycock PC, Dimou NL, Al-Dabhani K, Martin RM et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ. 2017;359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. [DOI] [PubMed] [Google Scholar]
- 63.IARC. Vitamin D and cancer. Lyon, France: 2009. 2009. [Google Scholar]
- 64.Lokeshwar BL, Schwartz GG, Selzer MG, Burnstein KL, Zhuang SH, Block NL et al. Inhibition of prostate cancer metastasis in vivo: a comparison of 1,23-dihydroxyvitamin D (calcitriol) and EB1089. Cancer Epidemiol Biomarkers Prev. 1999;8(3):241–8. [PubMed] [Google Scholar]
- 65.Schwartz GG. Vitamin D, sunlight, and the epidemiology of prostate cancer. Anticancer Agents Med Chem. 2013;13(1):45–57. [PubMed] [Google Scholar]
- 66.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4(2):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song M, Nishihara R, Wang M, Chan AT, Qian ZR, Inamura K et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut. 2016;65(2):296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15(3):255–65. [DOI] [PubMed] [Google Scholar]
- 69.Hofmann JN, Yu K, Horst RL, Hayes RB, Purdue MP. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2010;19(4):927–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171(8):903–8. [DOI] [PubMed] [Google Scholar]
- 71.Afzal S, Bojesen SE, Nordestgaard BG. Low plasma 25-hydroxyvitamin D and risk of tobacco-related cancer. Clin Chem. 2013;59(5):771–80. [DOI] [PubMed] [Google Scholar]
- 72.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S. Plasma vitamin D and risk of colorectal cancer: the Japan Public Health Center-Based Prospective Study. Br J Cancer. 2007;97(3):446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults - United States, 2005–2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1205–11. [DOI] [PubMed] [Google Scholar]


