Abstract
Adult plant resistance (APR) is an enigmatic phenomenon in which resistance genes are ineffective in protecting seedlings from disease but confer robust resistance at maturity. Maize has multiple cases in which genes confer APR to northern leaf spot, a lethal disease caused by Cochliobolus carbonum race 1 (CCR1). The first identified case of APR in maize is encoded by a hypomorphic allele, Hm1A, at the hm1 locus. In contrast, wild-type alleles of hm1 provide complete protection at all developmental stages and in every part of the maize plant. Hm1 encodes an NADPH-dependent reductase, which inactivates HC-toxin, a key virulence effector of CCR1. Cloning and characterization of Hm1A ruled out differential transcription or translation for its APR phenotype and identified an amino acid substitution that reduced HC-toxin reductase (HCTR) activity. The possibility of a causal relationship between the weak nature of Hm1A and its APR phenotype was confirmed by the generation of two new APR alleles of Hm1 by mutagenesis. The HCTRs encoded by these new APR alleles had undergone relatively conservative missense changes that partially reduced their enzymatic activity similar to HM1A. No difference in accumulation of HCTR was observed between adult and juvenile plants, suggesting that the susceptibility of seedlings derives from a greater need for HCTR activity, not reduced accumulation of the gene product. Conditions and treatments that altered the photosynthetic output of the host had a dramatic effect on resistance imparted by the APR alleles, demonstrating a link between the energetic or metabolic status of the host and disease resistance affected by HC-toxin catabolism by the APR alleles of HCTR.
Author summary
Adult plant resistance (APR) is a phenomenon in which disease resistance genes are able to confer resistance at the adult stages of the plant but somehow fail to do so at the seedling stages. Despite the widespread occurrence of APR in various plant diseases, the mechanism underlying this trait remains obscure. It is not due to the differential transcription of these genes, and here we show that it is also not due to the differential translation or activity of the APR alleles of the maize hm1 gene at different stages of development. Using a combination of molecular genetics, biochemistry and physiology, we present multiple lines of evidence that demonstrate that APR is a feature or symptom of weak forms of resistance. While the mature parts of the plant are metabolically robust enough to manifest resistance, seedling tissues are not, leaving them vulnerable to disease. Growth conditions that compromise the photosynthetic output of the plant further deteriorate the ability of the seedlings to protect themselves from pathogens.
Introduction
Plant responses to pathogens are dynamic, and they involve a number of inducible mechanisms that are tightly regulated both in space and time [1]. They are called into action only at the time and site of infection. The tight regulation of innate immunity is due to disease resistance genes that plants inherit from their parents and which often segregate with the trait of resistance [1–3]. A vast majority of these disease resistance genes function in every part of the plant and at every stage of development. However, many exceptions exist where resistance is manifested in a tissue- or developmental stage-specific manner. In most instances of developmentally regulated resistance, plants are susceptible at the seedling stage but become increasingly resistant toward maturity. The term commonly used to define such developmentally regulated resistance is adult plant resistance (APR), although other terms such as age-associated resistance, ontogenic resistance, mature plant resistance, or flowering-induced resistance have also been used in the literature to describe the same phenomenon [4–8].
Adult plant resistance (APR) often manifests gradually with the advancement of plant age, but a few cases have been reported where the onset is abrupt, happening sharply at a certain stage of development [9–12]. An example of the latter kind is the wheat Lr34 gene-mediated resistance, in which the onset against the leaf rust pathogen, Puccinia triticina, is largely confined to the uppermost leaf (flag leaf) [13]. In contrast, in the rice-Xanthomonas oryzae pv. oryzae pathosystem, resistance conferred by the Xa21 gene is almost negligible during the first three weeks of age but then increases steadily each week, reaching full efficacy at maturity [14,15]. Similarly, the Yr36-conferred resistance in wheat to Puccinia striiformis [16] and the Hm2-conferred resistance in maize to Cochliobolus carbonum race 1 (CCR1) increase gradually with plant age [12].
In efforts to understand the mechanistic basis of APR, several genes conferring this form of resistance were isolated in different pathosystems. Some of these genes include Cf-9B from tomato conferring resistance to leaf mold [17], Mi-1 from tomato conferring resistance to aphids [18], Xa21 from rice conferring resistance to leaf blight [14], Lr67 and Lr34 from wheat conferring resistance to leaf rust [13,19], Yr36 from wheat conferring resistance to stripe rust [16], and Hm2 from maize conferring resistance to leaf blight [12]. Two of these genes, Cf-9 and Mi-1, clearly follow the gene-for-gene (GFG) paradigm in conferring resistance, while four others, Lr67, Lr34, Yr36 and Hm2, do not, suggesting that any disease resistance gene has the potential to confer an APR phenotype.
What makes a gene behave in an APR manner? This question still eludes us, even though a number of APR genes, including those described in the preceding paragraph, have been cloned and characterized. One logical expectation was that the phenotype of APR genes may derive from their differential expression at different stages of plant development and that the level of gene expression would match their phenotypic efficacy closely. However, this has been ruled out with the majority of the APR genes, as their transcript levels do not reflect changes in their resistance phenotype [12,13,15,17,20]. Other possibilities that may affect the APR behavior of these genes are differential translation, differential post-translational modifications, and developmental changes in plant physiology and metabolism.
To gain insight into the mechanistic basis of APR in maize, we have been studying the northern leaf spot (NLS) disease of maize (Zea mays) caused by C. carbonum. This system is ideally suited because pathogen virulence on maize is determined by a single metabolite, a toxin produced by the pathogen and inactivated by the host. This toxin, HC-toxin, is the disease-causing effector of C. carbonum race 1, the race of this pathogen that causes NLS. Defeat of this effector is accomplished by the host via two loci encoding NADPH-dependent HC-toxin reductases (HCTR), which utilize NADPH as a cofactor to reduce an essential ketone function in HC-toxin [21–23]. A classic APR syndrome is described in this pathosystem [9]. The duplicate HCTR encoding genes, differ in structure and strength of conferred resistance. The HCTR encoded by wild-type (WT) Hm1 contains 356 amino acids and dominantly confers resistance at every stage of plant development. The HCTR encoded by the only functional Hm2 allele, by contrast, is truncated and lacks the last 52 amino acids compared to HM1 [12] and it confers APR against CCR1 when hm1 is homozygous null. Hm2 is expressed throughout the age of the plant [12], ruling out developmentally regulated transcript accumulation as the mechanism of APR. Like Hm2, an allele of hm1 conferring APR has also been described [9]. Designated Hm1A, this APR allele is recessive to the WT Hm1 allele and dominant to the hm1 null allele [9].
To explore why and how the Hm1A allele leads to an APR phenotype, we have cloned and characterized this allele in detail. HCTR activity in Hm1A plants was intermediate between WT (Hm1Hm1) and null mutant (hm1hm1hm2hm2) plants. This, along with the truncated nature of the APR allele at hm2, prompted us to consider if the hypomorphic Hm1 alleles resulted in APR. This hypothesis was addressed by mutagenizing in planta the wild-type allele of hm1. Two new APR alleles also had reduced HCTR activity. Thus, APR was a symptom of partial loss-of-function mutations in Hm1 that result in seedling susceptibility.
Results
Detailed genetics of APR-conferring Hm1A as an allele of hm1
The APR trait attributed to Hm1A was first noticed in the inbred P8, developed at Purdue University in the early 1960s [9]. The genetic evidence linking the APR of P8 with an allele of hm1 (Hm1A) made use of two segregating populations, a testcross and an F2 population, generated by crossing P8 (Hm1AHm1Ahm2hm2) with the resistant inbred WF9 (Hm1Hm1Hm2Hm2). The susceptible inbred for the testcross was Pr, which is homozygous for null mutations at both the hm1 and hm2 loci. There were at least two concerns with this study. First, it used a relatively small number of progenies, comprising about 90 plants each for both the F2 and testcross populations. Second, the resistant inbred WF9 also contained an APR allele at the hm2 locus, leaving room for error in extrapolation from these data.
These concerns necessitated that we revisit these findings, to clone and characterize Hm1A. We acquired P8 from the Germplasm Resources Information Network (GRIN). To confirm that this source of P8 harbored the Hm1A allele reported by Nelson and Ullstrup in 1964 [9], we conducted a thorough analysis of the genetics of P8 resistance to CCR1. We first crossed P8 twice with Pr (hm1hm1hm2hm2) to produce a BC1F1 testcross population. Of 384 BC1F1 plants inoculated with CCR1, 186 plants were susceptible at both the seedling and adult stage while 198 plants were susceptible as seedlings, but later emerging leaves were fully resistant, consistent with the APR phenotype of P8. The recessive null hm1 allele of Pr (designated as hm1Pr) contains a 256-bp Drone transposon insertion in exon 4 [24]. All 186 plants susceptible at maturity were homozygous for hm1Pr, whereas all 198 plants that were initially susceptible and then displayed APR were heterozygous for hm1Pr. This 1:1 ratio of susceptible vs. APR plants (X2–0.375, P > 0.05, 1 d.f.) indicated that a single gene at or near the hm1 locus controlled the APR behavior of P8.
Next we crossed P8 to Pr1, a near isogenic line (NIL) of Pr in which the mutant hm1 allele was replaced by a WT Hm1 [25]. The resulting Hm1AHm1 F1 hybrid was testcrossed to Pr, the hm1hm2 null stock. The inheritance of Hm1Pr1 vs. Hm1A in this population was tracked with a PCR-based marker that differentiated between those two alleles. Of the 540 F1 test cross progeny, 276 were susceptible as seedlings and later exhibited APR, while the remaining 264 were completely resistant to CCR1 regardless of age. All 264 completely resistant plants had inherited the WT Hm1 allele from Pr1, while the 274 plants that exhibited APR had inherited the Hm1A allele from P8. Chi-squared tests supported the 1:1 expected inheritance of monogenic inheritance (X2–0.266667, P > 0.05, 1 d.f.). No recombinants between the genotypes at the hm1 locus and the expression of CCR1 susceptibility were found in either population (924 opportunities for crossover). This confirmed that the source of P8 we obtained recapitulated the phenomenon described in 1964 [9] and that the APR of P8 is likely conferred by the Hm1A allele.
To incorporate Hm1A into a uniform background for detailed phenotypic comparisons, we introgressed this APR allele into the B73 inbred by crossing P8 (Hm1AHm1A hm2hm2) to B73 (Hm1Hm1hm2hm2). As Hm1A is recessive to WT Hm1, we utilized sequence polymorphism between Hm1A and Hm1B73 to construct a PCR-based marker. After seven crosses to B73 with selection for the Hm1A genotype, BC7F2 progeny were generated by self-pollinating a heterozygous plant. This BC7F2 population segregated in a 3:1 ratio for complete resistance and APR, again consistent with Hm1A being responsible for APR of P8. Homozygous Hm1A plants from this population were selected and maintained as an Hm1A near-isogenic line in B73.
Phenotypic manifestation of adult plant resistance in maize to CCR1
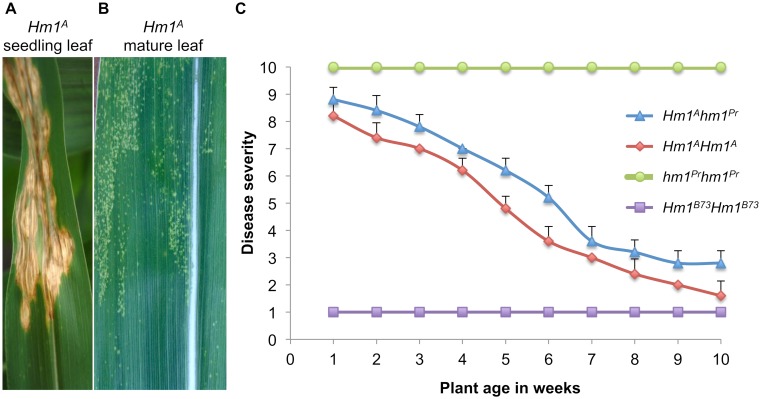
To develop a comprehensive account of the onset of APR by Hm1A, we also introgressed the null hm1Pr allele into the B73 background over seven generations, and crossed with Hm1A B73 NIL to generate plants heterozygous for Hm1A. Both homozygous (Hm1AHm1A) and heterozygous (Hm1Ahm1Pr) Hm1A plants were inoculated with CCR1 at weekly intervals, starting at 1 week-after-planting (wap) and culminating at 10 wap. Their infection phenotypes were measured using a 1–10 disease rating scale [12] and compared with those of B73 and a B73 NIL containing the null hm1 allele (hm1Pr B73 NIL). A rating of 10 on this scale indicated highly susceptible plants, while a rating of 1 indicated complete resistance.
The susceptible hm1Pr B73 NILs scored 10 on the disease rating scale regardless of age, and the resistant controls (B73 inbred), which produced small chlorotic flecks in response to CCR1 infection, scored 1 throughout development. Plants containing Hm1A exhibited very little resistance at the seedling stage, but severity scores decreased with age (Fig 1A and 1C). At the age of week-1, Hm1A seedlings were consistently rated 8 or higher. This disease rating dropped to 5 or less by week-5. At week-10, Hm1A plants resembled the resistant controls, receiving a rating of 1 (Fig 1B and 1C). The level of resistance conferred by Hm1A correlated with the age of the whole plant at the time of inoculation and not the age of the inoculated leaf. Inoculating each leaf of Hm1AHm1A and hm1hm1 plants at week-5 of plant growth confirmed this observation. All the leaves of Hm1A plants were equally resistant regardless of their age, and all the leaves of hm1hm1 plants were equally susceptible.
Fig 1. Developmental onset of the adult plant resistance phenotype of Hm1A.
(A) A seedling Hm1A leaf exhibiting susceptibility to Cochliobolus carbonum race 1 (CCR1) at the 2-week age. (B) A 9-week old Hm1A leaf completely resistant to CCR1. (C) The disease/resistance phenotype of Hm1A plants homozygous and heterozygous (Hm1Ahm1Pr) for the APR allele to CCR1 at weekly intervals from week-1 through week-10. Ratings were established by controls Hm1B73Hm1B73 (rated 1 and resistant throughout) and hm1Prhm1Pr (rated 10 and susceptible throughout). All hm1 alleles were in the B73 genetic background. Error bars represent standard error calculated using R statistical package.
Similar to the APR conferred by the Hm2 gene [12], the resistance conferred by Hm1A was dosage dependent. Plants homozygous for Hm1A were slightly more resistant to CCR1 at almost all stages of development compared to plants heterozygous for Hm1A and the null allele (Hm1Ahm1Pr) indicating that Hm1A is haploinsufficient (Fig 1C). The dosage effect was more pronounced at week-5 and declined after week-7 as the plants matured and became completely resistant.
Molecular characterization of the Hm1A allele
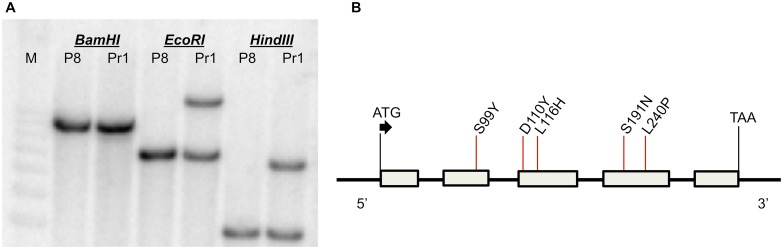
Atypical behavior of a disease resistance gene can sometimes result from complex structural changes at the locus, such as an increase in the copy number of the gene or a part of the gene [26,27]. To address if such a genetic mechanism also led to the Hm1A APR, we conducted a Southern blot analysis with P8 DNA digested with a variety of restriction enzymes. Consistent with the genetic data, a single BamHI restriction fragment hybridized to Hm1-specific probes on these blots, indicating that Hm1A was a single copy gene in the P8 inbred and that the entire gene was present on a 13 kb restriction fragment (Fig 2A). To clone the Hm1A gene, a lambda library was constructed from the BamH1-digested P8 DNA restriction fragments migrating on a gel as 12 to 15 kb fragments. We identified and sequenced a clone containing the 13 kb hm1-encoding fragment. Sequence analysis indicated that our clone contained the entire coding region of the Hm1 gene, as well as 3.8 kb of the promoter region.
Fig 2. Molecular characteristics of Hm1A.
(A) Southern blot analysis of DNA of inbreds P8 (Hm1AHm1A) and Pr1 (Hm1Pr1Hm1Pr1) demonstrating that Hm1A is a single copy gene. Sample genotypes (inbreds P8 or Pr1) are indicated below the restriction endonuclease used for DNA digestions (BamHI, EcoRI, or HindIII) and M corresponds to the DNA marker lane. (B) Schematic representation of the gene structure of Hm1A comprised by five exons (gray boxes) and four introns, identical to Hm1B73. The locations and the nature of five amino acids that differ between HM1A and HM1B73 are indicated by red lines. The locations of the start and termination codons are also indicated.
To determine the structural changes in Hm1A, its sequence was compared with that of the B73 reference sequence. Significant changes were encountered in the promoter regions of Hm1A and Hm1B73. Except for a few indels and SNPs, the first -200 bp from the translation start site of the promoter region are similar in Hm1A and B73 (S1 Fig). The next -1.5 kb region upstream, however, is completely different between the two alleles, though this does not seem to be due to the insertion of a transposable element. Interestingly, the promoter region of Hm1A is identical to that of hm1Pr, the null hm1 allele from the susceptible inbred Pr. To examine if any other resistant lines containing a wild-type Hm1 allele also had a promoter region identical to that of Hm1A, we used a primer pair designed from the Hm1A promoter region to PCR amplify DNA from a number of resistant inbreds. Two inbreds, Pr1 and Va35, were found whose Hm1 WT alleles have the promoter regions identical to that of Hm1A (S1 Fig). Taken together, these results indicate that the promoter polymorphism between Hm1A and Hm1B73 predicted neither resistance nor susceptibility and thus may be inconsequential to the APR phenotype of Hm1A.
The coding region of Hm1A also differed from that of Hm1B73, containing nine SNPs. Although four of these SNPs were silent or synonymous, five led to amino acid substitutions in the predicted HM1A peptide (Fig 2B). Relative to the B73 HM1 reference, these substitutions were: a Serine to Tyrosine change at residue 99 (S99Y), an Aspartic acid to Tyrosine change at residue 110 (D110Y), a Leucine to Histidine change at residue 116 (L116H), a Serine to Asparagine change at residue 191 (S191N), and a Leucine to Proline change at residue 240 (L240P) (Fig 2B).
The L116H substitution is the likely causative polymorphism in the Hm1A allele
As Hm1 is one of the most polymorphic genes in maize [28], we decided to examine the peptide sequence of various resistance alleles to potentially pinpoint the amino acid change(s) responsible for the APR behavior of Hm1A. We first amplified and evaluated the HM1 sequences of Pr1 and Va35, the two resistant inbreds that share their promoters with Hm1A, and compared them with the sequences of both HM1A and HM1B73. HM1Pr1 was found to differ by five amino acids from HM1B73, with two of these polymorphisms, S99Y and L240P, also being present in HM1A (S2 Fig). These same two changes were also found in HM1Va35, which differed from HM1B73 by six amino acids. Another resistant Hm1 allele that differed from B73 by six amino acids was in the inbred W22, but none of those changes matched those of HM1A. However, the predicted HM1 of the landrace Enano from Bolivia [28] shared with HM1A the two polymorphisms D110Y and S191N. And most importantly, the HM1 of the landrace Pira from Colombia [28] shared four of the five amino acid changes between HM1A and HM1B73. These are S99Y, D110Y, S191N, and L240P, thereby leaving only the L116H polymorphism unique to HM1A.
To examine the functional status of the Hm1 allele of Pira, we acquired this landrace from GRIN and inoculated it with CCR1. It was found to be completely resistant to CCR1, even at the seedling stage. This demonstrated that despite having four of the five amino acid changes of HM1A, the Hm1Pira allele is fully functional and not APR. These results highlight the importance of the L116H substitution in defining the phenotype of Hm1A. Consistent with this hypothesis, the Leucine at 116 is highly conserved not only in all the homoeologs and orthologs of the Hm1 gene across the grass lineage, but also in the maize dihydroflavonol 4-reductase (DFR), an NADPH-dependent enzyme of the anthocyanin pathway predicted to be a progenitor of HM1 (S2 Fig). All these findings suggest that the HM1A L116H substitution is unique to Hm1A and may underlie its APR behavior to CCR1 in maize by somehow negatively impacting HCTR activity.
HM1 transcript accumulation is not developmentally regulated in Hm1A
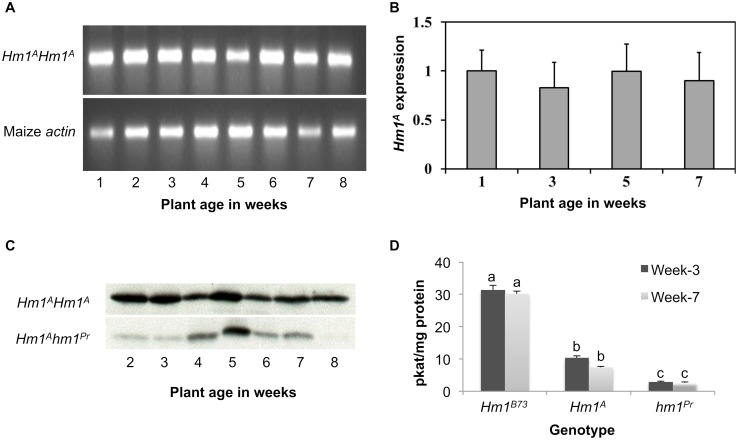
To examine if the transcriptional activity of Hm1A undergoes any change during plant development, reverse transcription (RT)-PCR was conducted on RNA extracted from CCR1-inoculated Hm1A plants of diverse ages. Using a semi-quantitative form of this assay, no dramatic changes could be observed in the level of the Hm1A transcript between the seedling and mature-plant stages (Fig 3A). Likewise, quantitative real time PCR (qRT-PCR) measurements of transcript abundance of Hm1A plants inoculated with CCR1 at different ages did not detect any rise in HM1 expression as the susceptible plants became resistant over time (Fig 3B). These results ruled out the differential transcription of the Hm1A allele as the basis for its APR phenotype.
Fig 3. Transcriptional and translational activities of Hm1A during the seedling and mature stages.
(A) Reverse transcription (RT)-PCR assay showing no change in Hm1A accumulation in leaves from week-1 through week-8 after planting. The actin gene was used as a control. (B) Quantitative real time PCR (qRT-PCR) measurements of the expression of Hm1A also demonstrates no change in Hm1A accumulation across the time period when APR is established. (C) Western blots showing that the level or stability of the HM1A protein do not change over time during plant development. Equal amounts of protein were loaded following quantification with the Bradford method. (D) In vitro HC-toxin reductase (HCTR) assays showing that the relative enzymatic activity encoded by Hm1A is less than Hm1B73 but higher than hm1Pr, the null allele. The specific activity of HCTR varies between alleles but not over time between weeks-3 and -7 in any genotype. The HCTR assay was based on the determination via LC-MS/MS of the amount of HC-toxin reduced by leaf protein extracts from the leaves of all genotypes. Different letters indicate significant differences between genotypes (padj < 0.05).
The level and activity of Hm1A-encoded HCTR stays the same during plant development
To address if the differential translational activity of Hm1A had any impact on its APR behavior, we first conducted western analysis to examine the level and stability of the HM1A protein. While an antibody raised against the entire HM1A peptide lacked specificity, a multiple antigenic peptide (MAP) [29] antibody generated against a 13 aa peptide corresponding to residues 312 to 324 of the HM1 peptide worked well and reacted to a single product on Western blots generated from Hm1A homozygous or heterozygous plants (Fig 3C). No change in the level of the HM1A protein could be detected over time in the Hm1A homozygotes, indicating that the APR phenotype of Hm1A is not due to differential translation either. In the heterozygous Hm1Ahm1Pr plants, with one APR allele and one null allele, the levels of HM1A were lower than in the Hm1A homozygotes, consistent with a dosage effect of the Hm1A allele. In addition, we do detect some variation in HM1A accumulation over time, peaking at week-5 but exhibiting similar levels of accumulation at weeks-2 and -7 (Fig 3C). That young and old plants accumulate similar levels of HM1A demonstrates, again, that preferential accumulation of HM1A protein in older plants cannot explain their resistance.
We next addressed if the activity of HCTR encoded by Hm1A had any role in its APR behavior. To do this, an LC-MS/MS-based in vitro HCTR activity assay that quantified the reduction of HC-toxin by crude protein extracts was developed. The in vitro measurements were normalized to total protein content, allowing us to estimate the level of the functional HCTR in plant tissues. To examine the level of HCTR over time, proteins were extracted from CCR1-inoculated leaves of 3- and 7-week-old plants of Hm1A and control stocks, and their HCTR activity was measured in replicated samples. Two trends were noted as shown in Fig 3D. First, the HCTR activity encoded by HM1A was lower than that of the WT allele but not null as that of hm1Pr. At both stages of development, the HCTR activity of HM1A was about 3-fold lower than that of HM1. Second, the level of active HCTR differed little if any in 3- or 7-week Hm1A plants (Fig 3D). Likewise, the HCTR activity of the WT allele also did not differ between week-3 and week-7-old plants (Fig 3D). Two conclusions can be drawn from these results. First, Hm1A encodes an HCTR that is relatively weaker than the enzyme encoded by the WT allele. Second, the level of the active HCTR stays constant over development and does not account for the APR phenotype of Hm1A.
Partial loss-of-function mutations confer adult plant resistance in the maize-CCR1 pathosystem
What aspect of the Hm1A gene structure or function restricts it to be an APR gene, i.e., conferring resistance only at the mature-plant stage but not the seedling stage? Having ruled out differential transcription or translation as possible mechanisms, we paid attention to an attribute of Hm1A that differentiates it from both the WT and null mutant alleles of hm1—the relatively weak nature of the HCTR activity encoded by Hm1A. This partial enzymatic activity of HM1A mirrored exactly the phenotypic strength of resistance conferred by this APR allele, which is recessive to that of WT Hm1 but dominant to that of null hm1. Given that the APR allele at the hm2 locus also confers partial resistance to CCR1 [12], we pondered if this could be a requirement for a resistance gene to have an APR phenotype.
If this hypothesis that a Hm1 APR allele owes its phenotype to being a weak or partial loss-of-function allele is correct, we should be able to confirm it by generating new APR alleles from the WT Hm1 allele by mutagenesis. To address this possibility, we first tried a random mutagenesis screen to generate new alleles of Hm1, in large part because of the lethal nature of CCR1 infection on field-grown plants lacking functional Hm1. About 1,000 M2 families of B73 were generated by treating pollen with the mutagen ethyl methanesulfonate (EMS). Twenty-four plants per M2 family were planted in a field and inoculated with CCR1 at the seedling stage. One M2 family was identified in which CCR1-susceptible plants segregated in a recessive fashion. These plants remained susceptible throughout their growth, suggesting they were the result of a null mutation. Sequence analysis of the hm1 allele from this mutant (named hm1-2) confirmed its null status and revealed a single G to A transition at the junction of exon3/intron3 as the cause of mutation (S3 Fig). Since this change is expected to abolish the splicing of intron 3, it would result in a truncated protein lacking all the amino acids encoded by exons 4 and 5 (S3 Fig). It is unlikely that such a grossly truncated protein would have any HCTR activity.
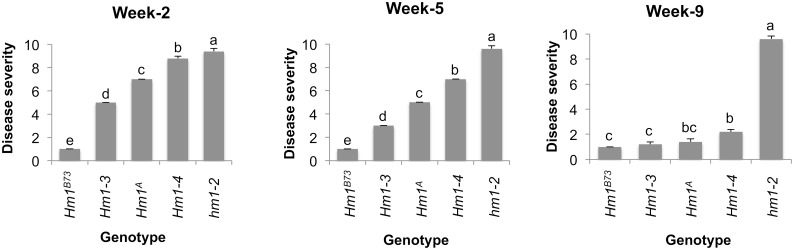
We next conducted a targeted mutagenesis screen to generate a series of mutant alleles of Hm1. To accomplish this, EMS-mutagenized Hm1B73 pollen was applied to ears of completely susceptible Pr plants in a greenhouse (Fig 4). Approximately 4,500 M1 seeds obtained from this cross were planted in the field and inoculated with CCR1 at week-2. Seven plants were identified as CCR1 susceptible at this seedling stage. When inoculated again at week-5, five of them were still fully susceptible, suggesting they were null mutants. The other two plants however exhibited APR as they developed different levels of resistance (S4 Fig). Sequencing the Hm1 gene (S5 Fig) from all seven mutants revealed that they all carried GC to AT transitions in the coding region of Hm1. The two APR-exhibiting alleles (designated Hm1-3 and Hm1-4) had missense mutations resulting in single amino acid substitutions, T90M in Hm1-3 and V210M in Hm1-4, in the HM1 peptide (Table 1). Of the five null mutants, three (named hm1-6 to hm1-8) had nonsense mutations, one a C82Y substitution (hm1-5), and one a splice-site mutation (hm1-9) at the junction of intron 4/exon 5 that also produced a pre-mature stop codon (Table 1). The new APR alleles were introgressed back into B73 for seven generations using CAPs markers. Comparison of the resistance phenotype of the two new APR alleles with Hm1A revealed that all three APR alleles differ markedly from each other in this trait. Hm1-3 confers the highest level of resistance at all stages of development, followed by Hm1A and Hm1-4 (Fig 5). This screen thus provided us with a series of APR alleles at the hm1 locus.
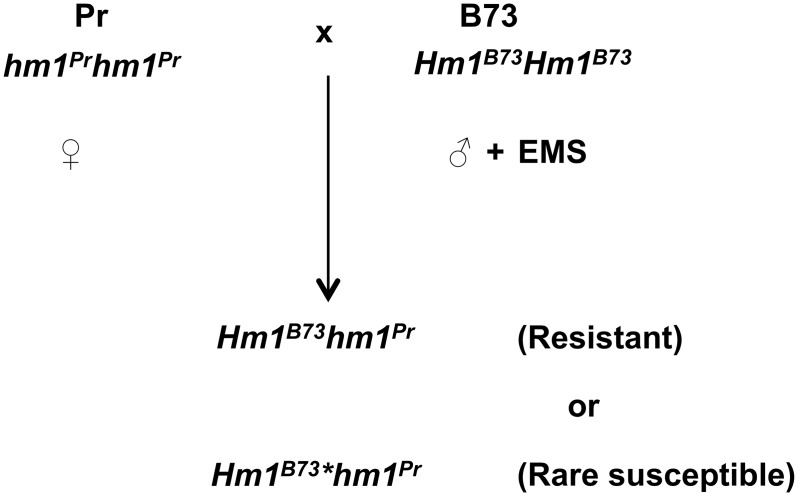
Fig 4. Design of the targeted EMS mutagenesis screen to generate new mutant alleles of Hm1.
Pollen collected from the fully resistant inbred B73 (Hm1B73Hm1B73) was treated with ethyl methanesulfonate (EMS) and used to pollinate ears of the fully susceptible inbred Pr (hm1Prhm1Pr) in a greenhouse. The resultant M1 seeds (Hm1B73hm1Pr) were planted in the field, inoculated with CCR1, and screened for disease resistance at both the seedling stage and at maturity to identify rare susceptible mutants, designated as Hm1B73*hm1Pr. M1 mutants that were susceptible at the seedling stage but became resistant with the progression of age were considered APR. Out of about 4,500 M1 plants screened, 7 susceptible mutants were found and two became resistant at maturity.
Table 1. The nature of molecular changes in the mutant alleles of Hm1 generated by mutagenesis and their respective disease/resistance phenotypes to infection by CCR1 at maturity.
| Allele No. | Disease Response | Mutation |
|---|---|---|
| Hm1-3 | APR | T90M |
| Hm1-4 | APR | V210M |
| hm1-5 | Null | C82Y |
| hm1-6 | Null | Nonsense |
| hm1-7 | Null | Nonsense |
| hm1-8 | Null | Nonsense |
| hm1-9 | Null | Nonsense |
Fig 5. Relative strength of the three APR alleles of hm1 in conferring protection against CCR1.
Like Hm1A, both new APR alleles (Hm1-3 and Hm1-4) were introgressed into B73 for seven generations for comparison of their resistance phenotypes. Plants homozygous for the Hm1B73 and hm1-2 alleles were fully resistant and susceptible, respectively. Disease resistance was evaluated three times, at week-2, week-5 and week-9 after planting, and a scale of 1 (completely resistant) to 10 (completely susceptible) was used to rate the interaction phenotypes. Letters represent whether differences among each age group were significant (padj < 0.05). The relative order of strength observed was Hm1B73 > Hm1-3 > Hm1A > Hm1-4 > hm1-2.
Like Hm1A, the new APR alleles encode HCTRs with intermediate activity
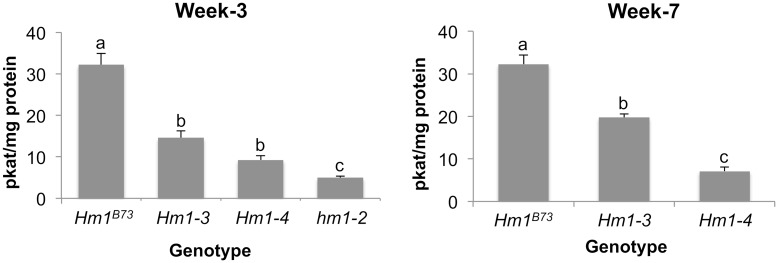
To evaluate if the HCTR activity encoded by Hm1-3 and Hm1-4 was also partially compromised like that of Hm1A, we used the aforementioned LC-MS/MS based activity assay on samples derived from these two mutants as well as their positive and negative controls. During weeks-3 and -7 (when APR plants are susceptible and resistant, respectively), crude protein was extracted from the leaf tissue following inoculation with CCR1. The HCTR activity of extracts from APR plants was found to be significantly reduced when compared with B73 at both week-3 and week-7, indicating that HM1-3 and HM1-4 proteins exhibit partially compromised HCTR activity during both susceptible and resistant plant ages, like HM1A (Fig 6). Furthermore, and consistent with HM1A (Fig 3C), the levels of their HCTR did not change significantly with age (Fig 6) despite the age-dependent manifestation of disease (S4 Fig). Taken together, this demonstrates that the APR encoded by these new alleles was also expressed without a concomitant increase in HCTR levels in mature plants.
Fig 6. In vitro enzymatic activities of HCTRs encoded by the new APR alleles of hm1.
Protein extracts from the leaf tissue of near-isogenic lines of the APR alleles Hm1-3 and Hm1-4 in the B73 background were used to conduct in vitro HCTR assays. The fully resistant (Hm1B73) and susceptible (hm1-2) alleles of hm1 were used as controls. HCTR activities, measured at age week-3 and week-7, relied on determining the amount of HC-toxin reduced via LC-MS/MS. Letters represent whether differences among each age group were significant (padj < 0.05).
Differences in the disease/resistance ratings of the new APR alleles predicted corresponding differences in their HCTR activities. This indeed was found to be true. The disease severity of APR plants at three weeks of age was found to be linearly correlated with HCTR activity (Figs 5 and 6). The APR allele with the highest degree of HCTR activity was HM1-3, followed by HM1A, and HM1-4 being the weakest (Fig 6). This variation in enzymatic activity is consistent with the gradient of CCR1 resistance displayed by Hm1-3, Hm1A, and Hm1-4 plants from strongest to weakest (Fig 5). At maturity, however, plants carrying any of these weak alleles of Hm1 were all indistinguishable from WT B73. This was not the case with plants carrying only the null allele; they remained uniformly susceptible to CCR1 infection even at maturity.
Modulation of photosynthesis output alters susceptibility to CCR1 in Hm1A seedlings
If the HCTR levels of the APR alleles remain largely uniform throughout plant development, why then are weak alleles unable to confer protection at the seedling stage? Some anecdotal observations that we have made about plants with APR alleles suggested that the availability of fixed carbon for energy production played a role in determining the ability of these weak alleles to suppress disease. The APR mutants always exhibited greater disease susceptibility and prolonged sensitivity in winter greenhouses as compared to the field. In the winter greenhouse, those plants closest to supplemental lights were more resistant than plants growing distant from light fixtures. Third, the resistance phenotype of APR alleles was compromised in double-mutant combination with the dominant oil-yellow1-N1989 allele that has a chlorophyll deficiency [30].
We grew the Hm1A plants at extended and reduced photoperiods to test the hypothesis that energy availability from fixed carbon could determine disease susceptibility in APR mutants. Fully-resistant WT B73 and fully-susceptible homozygotes for the null hm1-2 allele were grown side-by-side with Hm1A B73 NIL homozygotes in a growth chamber with a light regimen of 12h light (L) and 12h dark (D) for 2 weeks. Following inoculation with CCR1 and overnight incubation, half of the seedlings were shifted to a growth chamber adjusted at 18h L and 6h D. Hm1A mutant seedlings were strongly influenced by light regime. Hm1A seedlings grown in 12:12 L:D photoperiod were susceptible to CCR1 when examined at 72 hours post-inoculation (hpi) (Fig 7A) and showed no ability to suppress expanding lesions at 96 hpi (Fig 7B). However, the Hm1A plants that were shifted to 18:6 L:D developed a resistant reaction instead (Fig 7C and 7D). Daylight duration of 12h was not sufficient to disrupt the resistance provided by the WT B73 genotype. Similarly, increased daylength was insufficient to overcome the susceptibility of the hm1-2 null homozygotes. Thus, the seedling susceptibility of Hm1A conferred by low HCTR activity could be overcome by providing a longer period of photosynthetically active radiation.
Fig 7. Resistance of Hm1A seedlings to CCR1 is increased by extended photoperiod.
Two-week-old homozygous Hm1A seedlings were inoculated with CCR1 and incubated under two different photoperiods of 12 h daylight (12 h L:12 h D) and 18 h daylight (18 h L:6 h D). Hm1A seedlings grown under 12 h daylight were susceptible to CCR1 at 72 hpi (A) and 96 hpi (B). Hm1A seedlings incubated under the extended photoperiod of 18 h light exhibited notably enhanced resistance at both 72 hpi (C) and 96 hpi (D).
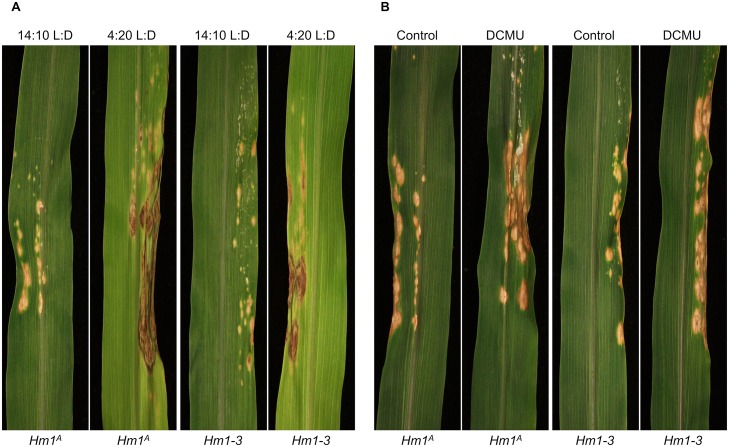
We reasoned that if photosynthate availability enhanced resistance and permitted the weak Hm1 alleles to confer seedling resistance, disruption of energy balance should negate their ability to confer any resistance. To test this, seedlings were either incubated in extended darkness or treated with the herbicide (3-(3,4-dichlorophenyl)-1,1-dimethylurea) (DCMU), which disrupts electron transfer during the light reactions of photosynthesis. For this experiment we used Hm1A and Hm1-3 APR homozygotes as well as WT B73 and the null hm1-2 homozygotes as controls to determine the impact of these treatments on resistance. We inoculated two-week-old plants with CCR1 and grew them in 14:10 L:D or 4:20 L:D. Extending the dark period of the diurnal cycle resulted in an increase in disease severity after seven days of growth for both Hm1A and Hm1-3 plants (Fig 8A) but did not render WT B73 susceptible to CCR1. If the lack of photosynthesis is responsible for susceptibility following extended darkness, then CCR1 susceptibility should also result from disruption of photosynthesis by herbicide treatment. To test this, the same genotypes were grown at 14:10 L:D and inoculated with CCR1. At 24 hpi, plants were divided into two groups with one receiving a solution of DCMU applied to the leaf whorl. At 7 dpi (6 days after DCMU treatment), the DCMU treated Hm1A and Hm1-3 homozygotes were completely susceptible to CCR1 (Fig 8B). As was observed for the extended darkness treatment, DCMU application neither rendered the WT B73 susceptible to CCR1 nor dramatically enhanced the susceptibility of hm1-2 controls.
Fig 8. Decreased photoperiod and photosynthesis inhibition by DCMU enhanced the susceptibility of APR genotypes to CCR1.
(A) Two-week-old homozygous Hm1A and Hm1-3 B73 NIL plants were inoculated with CCR1 and incubated with a shortened photoperiod of 4:20 L:D or longer 14:10 L:D photoperiod. Plants grown under a decreased photoperiod were completely susceptible to CCR1 while control plants were relatively less susceptible. (B) Hm1A and Hm1-3 B73 NIL plants were grown for two-weeks in the longer photoperiod conditions (14:10 L:D) and half of the plants were sprayed with DCMU, a photosynthesis inhibiting herbicide. Application of DCMU rendered both Hm1A and Hm1-3 plants highly susceptible to CCR1 compared to control plants. Pictures were taken 6 days after inoculation.
Together, these two experiments demonstrate that light, and perhaps the energy status of the plant, were key determinants of resistance to CCR1, and provide a direct link between plant primary metabolism and physiology and disease resistance.
Discussion
This study reveals one fundamental aspect of adult plant resistance (APR) in maize to CCR1. APR alleles at the hm1 locus are weak determinants of resistance that fail to protect plants at the seedling stage but are sufficient to confer complete protection to CCR1 at maturity. This conclusion is supported by multiple lines of evidence derived from a combination of genetic, molecular, and biochemical experimentation. Genetic analysis demonstrated that all APR alleles of hm1 confer partial resistance that exhibits haploinsufficiency (gene-dosage sensitivity) during most stages of plant development. This contrasts with resistance conferred by the wild-type (WT) alleles of hm1 that are completely dominant and protect every part of the plant regardless of age or maturity. Plants with null alleles of hm1, on the other hand, are susceptible to CCR1 at all stages of development. CCR1 infection typically results in plant lethality for these alleles, and the ubiquitous nature of this pathogen makes them difficult to propagate in the field. The APR alleles of hm1 are recessive to the WT alleles (e.g., Hm1B73) but dominant to null alleles of hm1 (e.g., hm1Pr).
Consistent with the idea that APR is a symptom of weak or partial loss-of-function alleles, we were able to generate two new APR alleles from the WT Hm1B73 allele by mutagenesis with EMS. Five completely susceptible mutants were also recovered in this mutant screen, which presumably encoded null mutations. In keeping with these predictions, molecular analysis of these null alleles showed that four of the five null mutants were the result of nonsense mutations that truncated their predicted peptides by introducing premature stop codons. The fifth null mutant, which was caused by a missense mutation, changed a highly conserved cysteine residue (C82Y) that is perhaps critical for protein function. In sharp contrast, both novel APR alleles underwent relatively conservative mutational changes: T90M in Hm1-3 and V210M in Hm1-4. Even Hm1A, which differs from the WT Hm1B73 allele by five amino acids, seems to owe its APR phenotype to a single L116H change. HCTR activity was encoded by all of the APR alleles, indicating that none of these mutations completely eliminates the function of the enzyme. Their HCTR activities were compromised, however, being intermediate to that of the fully functional WT allele (which confers completely dominant protection) and the recessive null hm1 alleles, which impart no resistance to CCR1. These results indicate that at some level HCTR activity is unable to deter the pathogen from colonizing maize plants at the seedling stage but that level of activity is sufficient to prevent CCR1 from colonizing at maturity.
A cause-and-effect relationship between APR and partial-loss-of-function alleles of hm1 is further substantiated by the correlation between the strength of the resistance reaction conferred by an APR allele and its HCTR activity. The level of HCTR activity matched perfectly with the strength of CCR1 resistance conditioned by the three APR alleles. These results demonstrate that alleles of hm1 with partial loss-of-function mutations encode HCTR with a compromised activity and that the weaker activity results in later onset of disease resistance. The resistance of seedlings encoding WT Hm1 demonstrates that efficient toxin deactivation is sufficient for maize seedlings to resist CCR1 infection and, therefore, they express all of the required machinery for defense. Likewise, mature plants lacking hm1 function are completely susceptible, demonstrating that HCTR is absolutely required for CCR1 infection, and mature maize plants are not protected from toxin-mediated disease spread. These interpretations depend on the in vitro assay correctly reflecting in vivo activity. Our in vitro HCTR activity assay did not detect the in vivo activity of the enzyme but instead the level of the functional protein present at a given time point. It is possible that in vivo activity did not correspond to the in vitro activity identified by this method.
A seemingly mechanistic relationship between partial resistance and APR is also evident in many other pathosystems where such genes have been cloned and studied in detail. One example is that of Cf-9B, which mediates incomplete resistance to C. fulvum in a developmentally specified fashion [17]. Its paralog Cf9, which encodes a receptor like protein, confers complete protection in all plant tissues at every stage of development [31]. Another example is that of Xa21, a receptor-like kinase that confers weak resistance to Xanthomonas leaf blight in rice [14,15]. The maize Hm2 APR allele provides another example. The weak CCR1 resistance provided by this allele is conferred by a truncated HCTR [12].
In wheat, APR genes are rather common and have been used widely to protect this crop from all forms of the disease caused by three different species of rust pathogens (reviewed in [32]). Even though APR genes confer little or no protection in wheat seedlings, the broad-spectrum and durable nature of resistance provided by such genes in adult plants have many breeders proclaim that breeding for rust resistance should deploy only APR genes [32]. Three of these wheat APR genes have been cloned recently and, interestingly, they all appear to confer resistance by different mechanisms. One of them, Yr36, a mediator of resistance to yellow rust, encodes a kinase with an unusual domain [16], while Lr34 and Lr67, both of which mediate APR to both rust and powdery mildew pathogens, encode an ABC transporter and a hexose transporter, respectively [13,19]. Exactly how these genes confer APR remains unresolved, but one thread that unifies them is their ability to confer only weak or partial resistance [32]. Overexpression of Lr34, one of the best studied APR genes, however, did enable it to confer seedling resistance in durum wheat [33]. Furthermore, the efficacy of this transgene in conferring seedling resistance improved even further under extended daylight conditions [34]. These results echo what we have discovered with the APR alleles in maize and suggest that the connection between weak resistance and APR is not unique to the maize-CCR1 pathosystem but perhaps is a general feature of most disease resistance genes that are weak and provide only partial protection.
A second major finding is that APR is not the result of the enhanced level or activity of proteins encoded by APR alleles at the mature-plant stage. Rather, it must be the result of a change in seedlings vs. mature plants that affects differential resistance. It was previously shown in a number of cases that the differential transcriptional activity of an APR gene did not account for its APR phenotype [12,13,15,17,20]. Here we extend this to the level and HCTR activity of the accumulated HM1 proteins, which remained stable across development. At the onset of APR, resistance manifests uniformly in all parts of the plant, including the youngest leaves that are still unfurled, indicating that the APR-inducing factor is not accumulated over a long period of time in aging tissues, but rather is available in every part of the plant regardless of the age of the organ and determined solely by the plant maturity.
Considering that the HCTR activity is present at equivalent levels in APR mutant extracts regardless of plant stage, why then are seedlings susceptible? Though the studies presented here do not resolve this question, the biochemical mechanism by which hm1 confers resistance to CCR1 suggests a plausible scenario. Although this resistance is conferred by hm1-encoded HCTR, the HC-toxin (HCT) inactivation reaction requires the reducing power of NADPH as a co-substrate. The direct involvement of NADPH in HC-toxin reduction suggests this molecule could be very critical in regulating resistance in the maize-CCR1 pathosystem. Supporting this hypothesis are our results showing that light and photosynthetic activity have a great impact on resistance mediated by APR alleles, either boosting them to confer seedling resistance or limiting them to prevent APR.
Based on these results, it could well be the availability of NADPH that determines the difference in resistance between seedling and mature stages in the hm1 APR mutants. NADPH is produced during the light reactions of photosynthesis, the C4 malate shuttle, and sugar oxidation, along with other energy carriers such as ATP. Maize seedlings not only have a limited photosynthetic capacity to assimilate carbon (C), but also strong sinks to consume these assimilates [35]. As a result, seedling leaves become C-deficient at night and that may negatively impact the availability of NADPH and ATP. Since NADPH is required for HCTR activity, its depletion at night may negatively impact the activity of hypomorphic mutants of HCTR, thereby leaving HC-toxin active to induce susceptibility to CCR1. Bolstering this hypothesis is the observation that the Hm1-3 and Hm1-4 mutations occur at residues predicted to be critical for the binding of NADPH to HCTR [36]. The mutant HCTR may require higher NADPH levels for sufficient activity for CCR1 suppression, while WT HCTR affinity for NADPH retains sufficient activity during the expected dip in NADPH cofactor concentration during the night. Surprisingly, we were unable to find a study of diurnal NADPH concentrations in maize, and such measurements would permit a test of this hypothesis. Such a scenario may also explain why plants with the APR alleles become more resistant as they mature; increased output of photosynthates may outstrip the sink requirements, allowing excess photosynthates to be stored as starch during the day and then used at night to fuel NADPH production.
Several aspects of plant bioenergetics are expected to support the resistance of plants to pathogens. In the case of our APR alleles, NADPH may play an additional critical role in energizing APR in the maize-CCR1 pathosystem. This, of course, is due to the direct involvement of this molecule as a cofactor in the enzyme reaction that is the resistance mechanism mediated by HCTR. This hypothesis is supported by the fact that maize plants carrying the WT Hm1 gene are completely resistant to CCR1 at all stages of development, including as seedlings and in the shorter day-lengths that resulted in loss of resistance in plants carrying APR alleles (Figs 7 and 8). A number of further tests of this hypothesis are available including measurements of HC toxin reduction in vivo in the same conditions employed for our CCR1 inoculations. We have demonstrated that HCTR accumulation is not different (Fig 3) and diurnal modulation in HC toxin reduction could be studied using purified toxin. This hypothesis, if validated, would provide a direct link between primary metabolism in the host and disease resistance.
An intriguing implication of this study concerns the metabolic basis of resistance in plants. This topic is not only of fundamental interest to plant pathologists and entomologists but also has huge agricultural relevance [37–39]. Our study demonstrates that, compared to strong resistance, the weak form of resistance has a much higher metabolic demand from the host. As shown in the case of APR, this can be so high that the seedlings are not able to express such resistance effectively. This argument may also extend to quantitative forms of resistance that are often weak and easily affected by the environment [40,41]. We have shown for the CCR1-maize pathosystem that the vulnerability of seedlings to diseases can increase in conditions that compromise photosynthesis. This phenomenon is analogous to what has been well established in the animal world that malnutrition compromises the immunity of infants much more than that of adults [42,43].
Methods
Plant materials
The inbred P8 and landraces Pira and Enano were obtained from Germplasm Resources Information Network (GRIN) of the U.S. National Plant Germplasm System. The CCR1-susceptible maize inbred Pr, and the CCR1-resistant inbreds B73, Va35, W22, and Pr1 (a near-isogenic line of Pr) were previously available in our research program. To determine whether Hm1A is an allele of Hm1, P8 was crossed with Pr and the F1 hybrid was backcrossed to Pr to generate a BC1F1 population. Additionally, P8 was crossed with Pr1 and the resulting F1 hybrid was testcrossed to the hm1 null stock Pr. Near-isogenic lines of B73 displaying APR to CCR1 infection were generated by backcrossing hm1 APR alleles with the B73 inbred, to determine the behavior of the APR alleles in a uniform genetic background.
Pathogen growth and inoculation
The protocol for culturing CCR1 pathogen on carrot juice agar medium was the same as previously described [23]. One-hundred μl of 105 spores/ml of CCR1 conidial suspension was used for leaf whorl inoculations. To study the phenotypic manifestation of APR by the Hm1A allele, both homozygous (Hm1AHm1A introgressed into B73) and heterozygous (Hm1Ahm1Pr also in B73) plants were planted in isolation at the Purdue ACRE farm and inoculated with 100 μl of 105 spores/mL of CCR1 spore suspension. Wild-type B73 encoding Hm1B73 and the susceptible hm1Pr B73 NIL plants were used as resistant and susceptible controls, respectively. A fresh set of five rows of ~40 plants per row was inoculated every week, and disease severity rating was determined 5 days post-inoculation (dpi) as described previously [12]. To determine if Hm1A is an allele of Hm1, genetic crosses were made at the ACRE farm and the resulting segregating progeny was evaluated under field conditions again at the ACRE farm.
Amplification of Hm1A genomic DNA
Four primer pairs were designed to amplify Hm1A based on its sequence homology with Hm1B73. The promoter region was amplified using a primer pair based on the promoter of hm1 from Pr. Touchdown PCR [44] was carried out with 10 consecutive cycles of denaturation at 94°C for 30 sec, annealing at 63°C for 30 sec with a decrease in 0.5°C per cycle to a “touchdown” of 58°C, and extension at 72°C for 30 sec; followed by 35 cycles of 94°C for 30 sec, 58°C for 30 sec, and 72°C for 45 sec. Three separate PCR reactions were carried out for every primer so that any errors initiated by either the GoTaq DNA Polymerase (Promega, Madison, WI, USA) or by sequencing could be ruled out. The PCR products were cleaned by running them through an agarose column, BigDye sequencing reactions were conducted, and the products precipitated with sodium acetate and ethanol before final resuspension in 20 μl of double-distilled water (ddH2O). These samples were submitted to the Purdue Genomics Facility for low throughput sequencing. Forward and reverse complementary sequences for each primer were compared using the ClustalW2 multiple alignment program. In order to assemble the Hm1A sequence without sequencing errors, only sequences with at least three perfect reads for each primer sequence were considered.
Cloning of Hm1A cDNA
P8 (Hm1AHm1A) seeds were planted in 500M MetroMix and grown in Conviron growth chambers for two weeks. One-hundred μl of 105 spores/mL CCR1 spore suspension was used for whorl inoculation, and plants were covered with a hood overnight to maintain humidity required for spore germination and penetration into the leaf tissue. At 24 h post-inoculation (hpi), affected leaf tissue was collected from the plants and snap-frozen in liquid nitrogen. RNA was extracted with a Qiagen RNeasy extraction kit (Qiagen, Germantown, MD), and cDNA was synthesized by RT-PCR using random hexamer mix (New England BioLabs, Ipswich, MA).
Generating near-isogenic lines of B73 manifesting APR and susceptibility to CCR1
The P8 maize inbred line was crossed with the maize reference B73 inbred, and the resulting F1 hybrid was backcrossed to B73. To introgress Hm1A into the B73 inbred, the resulting BC1F1 progeny was backcrossed to B73 for six generations. Since the promotor region of Hm1B73 differed from that of Hm1A, PCR-based markers designed from the promotor region were used for introgressing Hm1A into B73 (primer sequences are available in S1 Table). After the BC7 generation, Hm1A containing plants (Hm1Hm1A) were self-pollinated to generate homozygous Hm1A B73 NIL plants. Homozygous Hm1A B73 NIL plants were identified with PCR-based markers and were self-pollinated to generate seed. Similar to Hm1A, the two novel APR alleles Hm1-3 and -4 generated through EMS mutagenesis were introgressed into the B73 inbred for seven generations using a Cleaved Amplified Polymorphic sequences (CAPs) assay (primer sequences in S1 Table). The restriction enzyme NlaIII (New England BioLabs, Ipswich, MA) was used to differentiate the Hm1B73 allele from the two novel APR alleles. Similar to the novel APR alleles, the novel null allele hm1-2 identified in the EMS-mutagenized B73 M2 family screen was backcrossed for five generations into B73 using PCR-based markers and self-pollinated to obtain a homozygous hm1-2 NIL in B73. Marker-assisted backcrossing using PCR-based genotyping was conducted on plants grown at the Purdue Agronomy Center for Research and Education (ACRE) farm during the summer and in the Purdue University Botany and Plant Pathology greenhouses during the winter season.
Transcriptional activity of Hm1A
Hm1A plants were inoculated with CCR1 spore suspension as described above at weekly intervals from the seedling stage to maturity (week-1 through week-8). Total RNA was isolated from CCR1-infected leaf tissue as described earlier [45] and treated with RNase-free DNase I to eliminate genomic DNA using the TURBO DNA-free Kit (Ambion, Austin, TX). One μg of treated RNA was reverse-transcribed to cDNA in a total volume of 25 μl using the iScript cDNA Synthesis kit from Bio-Rad (Hercules, CA). RT-PCR was conducted using gene specific primers with the maize actin transcript as a control (see S1 Table for primer information). RT-PCR was conducted under the following conditions: denaturation at 94°C for 1 min, annealing at 60°C for 1 min, extension at 72°C for 1 min, and a terminal extension steps for 10 min. 30 and 28 cycles of PCR were conducted to amplify Hm1A and the control actin gene, respectively. Amplified PCR products were separated on a 0.8% agarose gel to visualize the expression of the Hm1A transcript. Three replicates for each time point were used for this experiment.
Additionally, qRT-PCR was conducted on cDNA from Hm1A plants inoculated with CCR1 at week-1, -3, -5, and -7 using gene specific primers. For relative quantification, Molybdenum co-factor biosynthesis protein (MOL, GRMZM2G067176) was used as a reference gene [46]. All primer combinations had an efficiency of 90–100%. Individual qRT-PCR reactions contained 5 μl of SYBR Select Master Mix (Applied Biosystems, Foster City, CA), 2 μl of cDNA template (20x dilution), and the appropriate amount of forward and reverse primers plus water. A three-step qRT-PCR amplification (40 cycles of 95°C for 5 s followed by 61°C for 20 s and 72°C for 30 s) was performed using the Mx3000P qPCR system (Stratagene–Agilent Technologies, Santa Clara, CA). Three replicates for each time point were used for this experiment.
Translational activity of Hm1A
For Western analysis, samples of homozygous and heterozygous Hm1A (Hm1Ahm1Pr) plants were collected at weekly intervals from week-2 through week-8 after planting. Since Hm1A is infection inducible, plants were inoculated with CCR1 for 24 h before collecting leaf samples. Protein was extracted using an alkaline lysis protocol and quantified using the Bradford method. Equal amounts of the protein (10 μg) was loaded onto SDS-PAGE gels and western blots were developed using a MAP antibody raised against a synthesized 13 amino acid peptide (RPARDRLGELGFK) corresponding to amino acids 312 to 324 of the HM1 peptide.
For the HCTR activity assay, Hm1B73, hm1Pr, and Hm1A plants grown in the field were inoculated with 200 μl of 105 spores/mL CCR1 spore suspension into the leaf whorl at weeks-3 and -7. Four biological replicates of three inoculated plants were sampled 24 hpi and stored at -80°C until used. Total plant protein was extracted using an adapted protocol [47] and desalted using a Sephadex G-50 Fine column (GE Healthcare, Chicago, IL). After determining protein concentration with a Bradford assay, 13.55 μg of protein was used to start reactions containing 25 mM Tris-HCl (pH 7.0), 160 mM NADPH, and 55 μM HC-toxin. The assays were run at 30°C in the dark for 45 min and then stopped by the addition of 1.25 ml cold acetone. After centrifugation at 15,000 x g for 15 min at 4°C, 10 μL of the supernatant was injected onto an Atlantis T3 column (2.1 x 150 mm, 3 μm, 100 Å, Waters) maintained at room temperature and analyzed using an Agilent 1200 series LC instrument coupled to an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA) at the Bindley Bioscience Center in Purdue Discovery Park.
The solvent system contained solvents A (0.1% formic acid in ddH2O) and B (0.1% acetonitrile). The column was eluted with 85% A and 15% B (0 to 1 min), followed by a linear gradient from 1 to 16 min to 40% A and 60% B, and a hold from 16 to 16.5 min at 40% A and 60% B. The column solvent was then reduced from 60% B to 15% B (16.5 to 17 min) and kept isocratic at 15% B from 17 to 22 min with a flow rate of 0.3 ml/min. HC-toxin (Sigma-Aldrich, St. Louis, MO) and its reduced form eluted from the column at 8.5–11.5 min under these conditions. During the analysis, the column effluent was directed to the MS/MS, with the Jetstream ESI set to positive mode with nozzle and capillary voltages at 1000–4000 V. The nebulizer pressure was set at 35 psi, the nitrogen drying gas was set at 325°C with a flow rate of 8 L/min, and the sheath gas was held at 250°C at a flow rate of 7 L/min. Fragmentation was achieved with 70 V for both analytes. Multiple reaction monitoring (MRM) was used to selectively detect HC-toxin and its reduced form. The first quadrupole was set to transition between the [M-H]+ of the analytes, whereas the last quadrupole monitored m/z 411 and 409 for reduced and normal HC-toxin respectively. Each transition was monitored with a dwell time of 150 ms and collision energy of 15 V, with ultrapure nitrogen used as the collision gas. Mass selection was achieved using the following ions: 439.3 for reduced HC-toxin and 437.3 for HC-toxin. Data were collected and analyzed via the MassHunter Workstation (version B.06.00, Agilent Technologies, Santa Clara, CA), and peak areas were determined by integration. Similar to Hm1A, the HCTR activity of the new APR alleles generated by targeted EMS mutagenesis (Hm1-3 and Hm1-4) along with resistant (Hm1) and susceptible (hm1-2) controls were also evaluated by LC-MS/MS.
Generating novel APR manifesting alleles of Hm1 by EMS mutagenesis
The B73 (Hm1Hm1hm2hm2) maize inbred, which exhibits complete resistance to CCR1 at all stages of plant development [23], was the pollen parent for the targeted EMS mutagenesis screen. The CCR1-susceptible maize inbred Pr (hm1hm1hm2hm2) [9,24], which exhibited complete susceptibility to CCR1 at all stages of plant development, was used as the female parent. This experiment was conducted in a greenhouse facility, as the Pr plants do not survive in the field due to high levels of disease pressure.
To conduct pollen EMS mutagenesis, EMS stock solution was prepared by adding 1 ml of EMS (Sigma-Aldrich, St. Louis, MO) to 99 ml of paraffin oil (Sigma-Aldrich, St. Louis, MO). Tassels of the Pr plants were removed before starting the experiment. On the day of conducting pollen mutagenesis, EMS working solution was prepared by mixing 1 ml of EMS stock solution with 14 ml of paraffin oil. This working solution of EMS was mixed gently for one hour to uniformly disperse the EMS in paraffin oil. B73 pollen was collected in tassel bags, measured and transferred to a 50-ml Nalgene bottle. For every 1 ml of pollen collected, 10 ml of EMS working solution was added. The EMS-treated pollen was placed on ice and mixed gently every 5 min for 45 min. About two to three drops of EMS-treated B73 pollen was then applied to the silks of Pr ears. Ears from these Pr plants were harvested 45 days after pollination. The M1 seeds (~4500) obtained from this genetic cross were planted at the Purdue ACRE farm. At both week-2 and week-5, plants were whorl-inoculated with 100 μl of 105 spores/mL of CCR1 conidial suspension and screened for their disease response one week post-inoculation.
Amplification of Hm1B73 allele from heterozygous CCR1-susceptible mutants
Based on sequence polymorphisms between the wild-type Hm1 from B73, Hm1B73 and the null hm1 allele from Pr, hm1Pr, four primer pairs amplifying -560-bp of the promoter region from the translation start site and the entire coding region of Hm1 were designed to preferentially amplify the WT Hm1B73 from heterozygous M1 plants (S5 Fig), which were obtained by crossing Pr plants with EMS-treated B73 pollen. Four overlapping primer combinations (primer sequences in S1 Table) were used to preferentially amplify Hm1B73 over the hm1Pr allele. Amplified PCR fragments were processed as described above for Hm1A amplification and submitted to the Purdue Genomics Facility for low-throughput sequencing.
Differential photoperiod treatments of Hm1A plants
Hm1A B73 NIL plants were grown in Conviron growth chambers providing a 12:12 L:D photoperiod. The WT B73 and hm1-2 were used as resistant and susceptible controls, respectively. Two-week-old plants were inoculated with 100 μl of 105 spores/mL of CCR1 spore suspension into the leaf whorl. CCR1-inoculated plants were incubated overnight in a humidity chamber at 80% relative humidity. These plants were then subjected to 12:12 L:D or 18:6 L:D photoperiods. The response reaction to CCR1 infection was evaluated every 24 h for a 96 h period. Digital photographs of lesion progression were taken using a Canon EOS Digital Rebel XSi camera.
Additional extended darkness and DCMU treatment experiments were performed in growth chambers on plants homozygous Hm1A and Hm1-3 in the B73 genetic background. Again, WT B73 and hm1-2 served as resistant and susceptible controls. Plants were grown in a growth chamber under 14:10 L:D for two weeks. We inoculated these plants with CCR1 and subjected them to two different light regimes, 14:10 L:D or 4:20 L:D. On a subset of CCR1 inoculated plants transferred to 14:10 L:D, the herbicide (3-(3,4-dichlorophenyl)-1,1-dimethylurea) (DCMU) at a concentration of 100 μM was applied to the leaf whorl 24 hpi. Disease severity of these plants was determined at 7 dpi.
Supporting information
Sequence comparison of the Hm1 promotor from different Hm1 alleles that exhibit complete resistance (in B73, Va35, and Pr1 inbreds) against Cochliobolus carbonum race 1 with adult plant resistance (APR) manifesting Hm1A (from P8 inbred) and completely susceptible hm1 (from Pr inbred). Promotor of Hm1 was almost similar for the first -200 bp from the transcription start site (TSS) for all Hm1 alleles. From -200 bp onwards, the promotor sequence of Hm1A differed from CCR1-resistant Hm1B73 but similar to Hm1 alleles from CCR1-resistant inbred Va35 and Pr1, and CCR1-susceptible Pr.
(PDF)
Sequence comparison of HM1A with HM1 from the resistant maize inbreds (B73, Va35, W22, and Pr1), maize cultivars (Enano and Pira), HM1 homologs [sorghum (Sorghum bicolor), rice (Oryza sativa), and barley (Hordeum vulgare)], and maize dihydroflavonol 4-reductase (DFR). Among the five amino acid substitutions present in HM1A (highlighted in red and bold), the L116 residue was a conserved in all Hm1 alleles and orthologs.
(PDF)
(A) Ethyl methanesulfonate (EMS) mutagenesis caused a G to A substitution at the junction of exon3/intron3. (B) Predicted protein sequence of hm1-2 is 176 amino acids similar to HM1-B73 and the mutation in the intron is predicted to generate 26 different amino acids before resulting in a truncated protein.
(PDF)
(A) Hm1B73 plants were resistant at all stages of plant development. Two novel APR alleles, Hm1-3 (B) and Hm1-4 (C), generated by targeted EMS mutagenesis were susceptible as seedlings (week-3) and became resistant to CCR1 at week-7. A novel null allele, hm1-5 (D) remained susceptible throughout the age of the plant.
(PDF)
Four primer pairs that preferentially amplified overlapping sequences of the Hm1B73 allele over the hm1Pr allele from heterozygous M1 plants generated using the targeted EMS mutagenesis screen. Grey boxes represent the exons while the black lines between the exons represent the introns. Different primer pairs used to amplify Hm1B73 are marked using different colors with the amplicon size listed above each fragment.
(PDF)
Using PCR-based markers, APR alleles were introgressed into B73 genetic background.
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was partially supported by GSJ’s Hatch project (IND011280), by the IOS-NSF grant 0547132 (https://www.nsf.gov/awardsearch/showAward?AWD_ID=0547132) to GSJ, and by the National Science Foundation Plant Genome Research Program grant 1444503 (https://www.nsf.gov/awardsearch/showAward?AWD_ID=1444503) to BPD and GSJ. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Johal GS, Gray J, Gruis D, Briggs SP. Convergent insights into mechanisms determining disease and resistance response in plant–fungal interactions. Can J Bot. 1995. December 31;73(S1):468–74. [Google Scholar]
- 2.Jones JDG, Dangl JL. The plant immune system. Nature. 2006. November 16;444(7117):323–9. 10.1038/nature05286 [DOI] [PubMed] [Google Scholar]
- 3.Jones JDG, Vance RE, Dangl JL. Intracellular innate immune surveillance devices in plants and animals. Science. 2016. December 2;354(6316):aaf6395-1–8. [DOI] [PubMed] [Google Scholar]
- 4.Dyck PL, Samborski D. J., Anderson R. G. Inheritance of adult-plant leaf rust resistance derived from the common wheat varieties Exchange and Frontana. Can J Genet Cytol. 1966. December 1;8(4):665–71. [Google Scholar]
- 5.Kus JV, Zaton K, Sarkar R, Cameron RK. Age-related resistance in Arabidopsis is a developmentally regulated defense response to Pseudomonas syringae. Plant Cell. 2002. February;14(2):479–90. 10.1105/tpc.010481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panter SN, Jones DA. Age-related resistance to plant pathogens In: Advances in Botanical Research [Internet]. Academic Press; 2002. [cited 2018 Mar 13]. p. 251–80. http://www.sciencedirect.com/science/article/pii/S0065229602380327 [Google Scholar]
- 7.Whalen MC. Host defence in a developmental context. Mol Plant Pathol. 2005. May 1;6(3):347–60. 10.1111/j.1364-3703.2005.00286.x [DOI] [PubMed] [Google Scholar]
- 8.Develey-Rivière M-P, Galiana E. Resistance to pathogens and host developmental stage: a multifaceted relationship within the plant kingdom. New Phytol. 2007. August 1;175(3):405–16. 10.1111/j.1469-8137.2007.02130.x [DOI] [PubMed] [Google Scholar]
- 9.Nelson OE, Ullstrup AJ. Resistance to leaf spot in maize: genetic control of resistance to Race I of Helminthosporium carbonum Ull. J Hered. 1964. September 1;55(5):195–9. [Google Scholar]
- 10.Jones IT, Hayes JD. The effect of sowing date on adult plant resistance to Erysiphe graminis f.sp. avenae in oats. Ann Appl Biol. 1971. May 1;68(1):31–9. [Google Scholar]
- 11.Abedon BG, Tracy WF. Corngrass 1 of maize (Zea mays L.) delays development of adult plant resistance to common rust (Puccinia sorghi Schw.) and European corn borer (Ostrinia nubilalis Hubner). J Hered. 1996. May 1;87(3):219–23. [Google Scholar]
- 12.Chintamanani S, Multani DS, Ruess H, Johal GS. Distinct mechanisms govern the dosage-dependent and developmentally regulated resistance conferred by the maize Hm2 gene. Mol Plant Microbe Interact. 2008;21(1):79–86. 10.1094/MPMI-21-1-0079 [DOI] [PubMed] [Google Scholar]
- 13.Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, McFadden H, et al. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science. 2009. February 19;323(5919):1360–1363. 10.1126/science.1166453 [DOI] [PubMed] [Google Scholar]
- 14.Song W-Y, Wang G-L, Chen L-L, Kim H-S, Pi L-Y, Holsten T, et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995. December 15;270(5243):1804–6. [DOI] [PubMed] [Google Scholar]
- 15.Century KS, Lagman RA, Adkisson M, Morlan J, Tobias R, Schwartz K, et al. Developmental control of Xa21-mediated disease resistance in rice. Plant J. 1999. October 1;20(2):231–6. [DOI] [PubMed] [Google Scholar]
- 16.Fu D, Uauy C, Distelfeld A, Blechl A, Epstein L, Chen X, et al. A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science. 2009. March 6;323(5919):1357–60. 10.1126/science.1166289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panter SN, Hammond-Kosack KE, Harrison K, Jones JDG, Jones DA. Developmental control of promoter activity Is not responsible for mature onset of Cf-9B-mediated resistance to leaf mold in tomato. Mol Plant Microbe Interact. 2002. November 1;15(11):1099–107. 10.1094/MPMI.2002.15.11.1099 [DOI] [PubMed] [Google Scholar]
- 18.Milligan SB, Bodeau J, Yaghoobi J, Kaloshian I, Zabel P, Williamson VM. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell. 1998. August 1;10(8):1307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore JW, Herrera-Foessel S, Lan C, Schnippenkoetter W, Ayliffe M, Huerta-Espino J, et al. A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat Genet. 2015. December;47(12):1494–8. 10.1038/ng.3439 [DOI] [PubMed] [Google Scholar]
- 20.McDowell JM, Williams SG, Funderburg NT, Eulgem T, Dangl JL. Genetic analysis of developmentally regulated resistance to downy mildew (Hyaloperonospora parasitica) in Arabidopsis thaliana. Mol Plant Microbe Interact. 2005. November 1;18(11):1226–34. 10.1094/MPMI-18-1226 [DOI] [PubMed] [Google Scholar]
- 21.Kim S-D, Knoche HW, Dunkle LD. Essentiality of the ketone function for toxicity of the host-selective toxin produced by Helminthosporium carbonum. Physiol Mol Plant Pathol. 1987. May 1;30(3):433–40. [Google Scholar]
- 22.Meeley RB, Johal GS, Briggs SP, Walton JD. A biochemical phenotype for a disease resistance gene of maize. Plant Cell. 1992. January 1;4(1):71–7. 10.1105/tpc.4.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johal GS, Briggs SP. Reductase activity encoded by the HM1 disease resistance gene in maize. Science. 1992. November 6;258(5084):985–7. [DOI] [PubMed] [Google Scholar]
- 24.Multani DS, Meeley RB, Paterson AH, Gray J, Briggs SP, Johal GS. Plant–pathogen microevolution: Molecular basis for the origin of a fungal disease inmaize. Proc Natl Acad Sci U S A. 1998. February 17;95(4):1686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ullstrup AJ. Further studies on a species of Helminthosporium parasitizing Corn. Phytopathology. 1944;34(2):214–22. [Google Scholar]
- 26.Piffanelli P, Ramsay L, Benabdelmouna A, D’Hont A, Jørgensen JH, Hollricher K, et al. A barley cultivation-associated polymorphism conveys resistance to powdery mildew. Nature. 2004. August 19;430(7002):887 10.1038/nature02781 [DOI] [PubMed] [Google Scholar]
- 27.Cook DE, Lee TG, Guo X, Melito S, Wang K, Bayless AM, et al. Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science. 2012. November 30;338(6111):1206–9. 10.1126/science.1228746 [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Peek AS, Dunams D, Gaut BS. Population genetics of duplicated disease-defense genes, hm1 and hm2, in maize (Zea mays ssp. mays L.) and its wild ancestor (Zea mays ssp. parviglumis). Genetics. 2002. October 1;162(2):851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam JP, Zavala F. Multiple antigen peptide. A novel approach to increase detection sensitivity of synthetic peptides in solid-phase immunoassays. J Immunol Methods. 1989. November 13;124(1):53–61. [DOI] [PubMed] [Google Scholar]
- 30.Sawers RJH, Viney J, Farmer PR, Bussey RR, Olsefski G, Anufrikova K, et al. The maize Oil Yellow1 gene encodes the I subunit of magnesium chelatase. Plant Mol Biol. 2006. January 1;60(1):95–106. 10.1007/s11103-005-2880-0 [DOI] [PubMed] [Google Scholar]
- 31.Parniske M, Hammond-Kosack KE, Golstein C, Thomas CM, Jones DA, Harrison K, et al. Novel disease resistance specificities result from sequence exchange between tandemly repeated genes at the Cf-4/9 locus of tomato. Cell. 1997. December 12;91(6):821–32. [DOI] [PubMed] [Google Scholar]
- 32.Ellis JG, Lagudah ES, Spielmeyer W, Dodds PN. The past, present and future of breeding rust resistant wheat. Front Plant Sci. 2014. November 24;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Risk JM, Selter LL, Krattinger SG, Viccars LA, Richardson TM, Buesing G, et al. Functional variability of the Lr34 durable resistance gene in transgenic wheat. Plant Biotechnol J. 2012. May 1;10(4):477–87. 10.1111/j.1467-7652.2012.00683.x [DOI] [PubMed] [Google Scholar]
- 34.Rinaldo A, Gilbert B, Boni R, Krattinger SG, Singh D, Park RF, et al. The Lr34 adult plant rust resistance gene provides seedling resistance in durum wheat without senescence. Plant Biotechnol J. 2017. July;15(7):894–905. 10.1111/pbi.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalt-Torres W, Kerr PS, Usuda H, Huber SC. Diurnal changes in maize leaf photosynthesis 1. Plant Physiol. 1987. February;83(2):283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dehury B, Patra MC, Maharana J, Sahu J, Sen P, Modi MK, et al. Structure-based computational study of two disease resistance gene homologues (Hm1 and Hm2) in maize (Zea mays L.) with implications in plant-pathogen interactions. PLOS ONE. 2014. May 21;9(5):e97852 10.1371/journal.pone.0097852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huot B, Yao J, Montgomery BL, He SY. Growth–defense tradeoffs in plants: A balancing act to optimize fitness. Mol Plant. 2014. August 1;7(8):1267–87. 10.1093/mp/ssu049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karasov TL, Chae E, Herman JJ, Bergelson J. Mechanisms to mitigate the trade-off between growth and defense. Plant Cell. 2017. April 1;29(4):666–80. 10.1105/tpc.16.00931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Q, Major IT, Howe GA. Resolution of growth–defense conflict: mechanistic insights from jasmonate signaling. Curr Opin Plant Biol. 2018. August 1;44:72–81. 10.1016/j.pbi.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 40.Poland JA, Balint-Kurti PJ, Wisser RJ, Pratt RC, Nelson RJ. Shades of gray: the world of quantitative disease resistance. Trends Plant Sci. 2009. January 1;14(1):21–9. 10.1016/j.tplants.2008.10.006 [DOI] [PubMed] [Google Scholar]
- 41.French E, Kim B-S, Iyer-Pascuzzi AS. Mechanisms of quantitative disease resistance in plants. Semin Cell Dev Biol. 2016. August 1;56:201–8. 10.1016/j.semcdb.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 42.Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008. May 15;46(10):1582–8. 10.1086/587658 [DOI] [PubMed] [Google Scholar]
- 43.Walson JL, Berkley JA. The impact of malnutrition on childhood infections. Curr Opin Infect Dis [Internet]. 2018. March 22 [cited 2018 Apr 3];Publish Ahead of Print. Available from: https://insights.ovid.com/pubmed?pmid=29570495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS. “Touchdown” PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991. July 25;19(14):4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eggermont K, Goderis IJ, Broekaert WF. High-throughput RNA extraction from plant samples based on homogenisation by reciprocal shaking in the presence of a mixture of sand and glass beads. Plant Mol Biol Report. 1996. September 1;14(3):273–9. [Google Scholar]
- 46.Hartwig T, Chuck GS, Fujioka S, Klempien A, Weizbauer R, Potluri DPV, et al. Brassinosteroid control of sex determination in maize. Proc Natl Acad Sci. 2011. December 6;108(49):19814–9. 10.1073/pnas.1108359108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi K, Yoshida K, Matsui Y. A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature. 2005. November 17;438(7066):374–8. 10.1038/nature04112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence comparison of the Hm1 promotor from different Hm1 alleles that exhibit complete resistance (in B73, Va35, and Pr1 inbreds) against Cochliobolus carbonum race 1 with adult plant resistance (APR) manifesting Hm1A (from P8 inbred) and completely susceptible hm1 (from Pr inbred). Promotor of Hm1 was almost similar for the first -200 bp from the transcription start site (TSS) for all Hm1 alleles. From -200 bp onwards, the promotor sequence of Hm1A differed from CCR1-resistant Hm1B73 but similar to Hm1 alleles from CCR1-resistant inbred Va35 and Pr1, and CCR1-susceptible Pr.
(PDF)
Sequence comparison of HM1A with HM1 from the resistant maize inbreds (B73, Va35, W22, and Pr1), maize cultivars (Enano and Pira), HM1 homologs [sorghum (Sorghum bicolor), rice (Oryza sativa), and barley (Hordeum vulgare)], and maize dihydroflavonol 4-reductase (DFR). Among the five amino acid substitutions present in HM1A (highlighted in red and bold), the L116 residue was a conserved in all Hm1 alleles and orthologs.
(PDF)
(A) Ethyl methanesulfonate (EMS) mutagenesis caused a G to A substitution at the junction of exon3/intron3. (B) Predicted protein sequence of hm1-2 is 176 amino acids similar to HM1-B73 and the mutation in the intron is predicted to generate 26 different amino acids before resulting in a truncated protein.
(PDF)
(A) Hm1B73 plants were resistant at all stages of plant development. Two novel APR alleles, Hm1-3 (B) and Hm1-4 (C), generated by targeted EMS mutagenesis were susceptible as seedlings (week-3) and became resistant to CCR1 at week-7. A novel null allele, hm1-5 (D) remained susceptible throughout the age of the plant.
(PDF)
Four primer pairs that preferentially amplified overlapping sequences of the Hm1B73 allele over the hm1Pr allele from heterozygous M1 plants generated using the targeted EMS mutagenesis screen. Grey boxes represent the exons while the black lines between the exons represent the introns. Different primer pairs used to amplify Hm1B73 are marked using different colors with the amplicon size listed above each fragment.
(PDF)
Using PCR-based markers, APR alleles were introgressed into B73 genetic background.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.