Key Points
Question
Is tranexamic acid effective and safe in reducing intraoperative bleeding, postoperative eyelid edema, and periorbital ecchymosis in rhinoplasty?
Findings
In this systematic review and meta-analysis of 5 randomized clinical trials (276 patients), tranexamic acid was shown to reduce bleeding during rhinoplasty and decrease eyelid edema and periorbital ecchymosis within the first postoperative week. There were no reports of thromboembolic events.
Meaning
Preoperative administration of tranexamic acid is safe and may reduce intraoperative bleeding, postoperative eyelid edema, and ecchymosis in patients who undergo rhinoplasty.
Abstract
Importance
Evidence has emerged on the efficacy of tranexamic acid to control blood loss and postoperative complications after rhinoplasty.
Objective
To investigate the results of tranexamic acid use to reduce intraoperative bleeding, postoperative eyelid edema, and periorbital ecchymosis in rhinoplasty.
Data Sources and Study Selection
For this systematic review of randomized clinical trials, searches were performed in PubMed, Cochrane Central Register of Controlled Trials, Web of Science, SCOPUS, Science Direct, Google Scholar, OpenThesis, and ClinicalTrials.gov from inception to December 23, 2017. Key words included tranexamic acid, rhinoplasty, and nasal surgical procedures. The following elements were used to define eligibility criteria: (1) population: patients undergoing rhinoplasty surgery; (2) intervention and controls: tranexamic acid vs placebo solution or no-treatment control group; (3) outcomes: intraoperative bleeding, postoperative eyelid edema and periorbital ecchymosis, and thromboembolic events; and (4) study type: randomized clinical trials.
Data Extraction and Synthesis
Two reviewers extracted data and assessed study quality according to the Cochrane guidelines for randomized clinical trials. Treatment effects were defined as weighted mean difference (WMD) and 95% CIs. The strength of evidence was analyzed using the Grading of Recommendations Assessment, Development, and Evaluation rating system.
Main Outcomes and Measures
Intraoperative bleeding, postoperative eyelid edema and periorbital ecchymosis. To calculate the effect sizes, means and SDs were obtained for each study group and outcome of interest.
Results
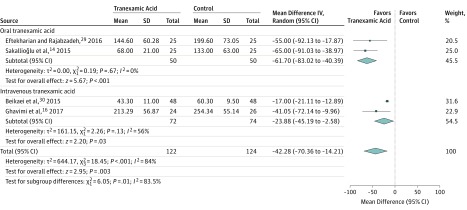
Five studies comprising 276 patients were included in the systematic review: 177 patients (64.1%) were women, and mean age was 26.8 (range, 16-42) years. Four studies comprising 246 patients estimated the amount in intraoperative bleeding as a primary outcome and were included in the meta-analysis. Eyelid edema and ecchymosis were evaluated as outcomes in 2 studies. Tranexamic acid was associated with reduced bleeding during rhinoplasty was found (WMD, −42.28 mL; 95% CI, −70.36 to −14.21 mL), with differences (P = .01) between oral (WMD, −61.70 mL; 95% CI, −83.02 to −40.39 mL; I2 = 0%) and intravenous (WMD, −23.88 mL; 95% CI, −45.19 to −2.58 mL; I2 = 56%) administration. Eyelid edema and ecchymosis scores in patients receiving tranexamic acid were significantly lower compared with the control group within the first postoperative week: lower eyelid edema, WMD, −0.76; 95% CI, −1.04 to −0.49 and lower eyelid ecchymosis, WMD, −0.94; 95% CI, −1.80 to −0.08. No cases of thromboembolic events were reported.
Conclusions and Relevance
Current available evidence suggests that preoperative administration of tranexamic acid is safe and may reduce intraoperative bleeding as well as postoperative eyelid edema and ecchymosis in patients undergoing rhinoplasty.
This systematic review and meta-analysis of randomized clinical trials evaluates the preoperative use of tranexamic acid for reduction of bleeding, eyelid edema, and periorbital ecchymosis in patients undergoing rhinoplasty.
Introduction
Rhinoplasty is a facial plastic surgery indicated to improve nasal aesthetics and airway function. During rhinoplasty, osteotomies are responsible for a significant amount of intraoperative and postoperative complications, including bleeding, eyelid edema, and periorbital ecchymosis.1 Trauma to angular vessels during osteotomy and inadequate local hemostasis may lead to excessive intraoperative bleeding increasing surgical time, risk of morbidities, and postoperative recovery.2,3,4 Eyelid edema and periorbital ecchymosis have been associated with temporary visual impairment and increasing permanent pigmentation, leading to patient dissatisfaction and poor cosmetic results.5,6,7
Several interventions have been found to successfully reduce eyelid edema and ecchymosis after rhinoplasty and include corticosteroid medication, intraoperative hypotension, head elevation, and intraoperative cooling.8 In addition, studies have demonstrated a decrease in intraoperative bleeding using metoprolol as premedication,9 lidocaine injection with epinephrine,10 intravenous infusion of remifentanil with controlled hypotension,11,12 and intravenous desmopressin.13 Evidence has emerged on the use of tranexamic acid to control intraoperative bleeding as well as postoperative eyelid edema and ecchymosis after rhinoplasty.14,15,16
Tranexamic acid is an antifibrinolytic agent that blocks lysine-binding sites in plasminogen by reducing local degradation of fibrin by plasmin. A series of systematic reviews and meta-analyses have been published showing strong evidence that tranexamic acid is associated with reduced bleeding in cardiac surgery,17 orthopedic trauma surgery,18 total knee and hip arthroplasty,19 spinal surgery,20 and orthognathic21 and minor oral procedures22 with no increase in thromboembolic events, such as myocardial infarction, deep venous thrombosis, and pulmonary embolism.
This systematic review and meta-analysis of randomized clinical trials (RCTs) investigated the results of tranexamic acid use for intraoperative bleeding as well as postoperative eyelid edema and periorbital ecchymosis in rhinoplasty.
Methods
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement23 and supplemented by guidance from the Cochrane Collaboration Handbook for Systematic Reviews of Interventions.24
Search Strategy
Searches for RCTs were performed in PubMed, Web of Science, SCOPUS, Science Direct, Cochrane Central Register of Controlled Trials, and the website ClinicalTrials.gov from inception to December 23, 2017. A gray literature search included Google Scholar and OpenThesis. The first 100 results of the Google Scholar search were analyzed. The search was limited to studies published in full-text versions, without language restriction. The reference lists of all eligible studies and reviews were scanned to identify additional studies for inclusion. The structured search strategy used the following terms: tranexamic acid and rhinoplasty or nasal surgical procedures. To expand the number of eligible articles, there was no use of filters in the search.
Study Selection and Eligibility Criteria
Two reviewers (S.J.A.V. and E.M.N.-J.) independently screened the search results and identified studies that were potentially relevant based on their title and abstract. Relevant studies were read in full text and selected according to eligibility criteria. Disagreements between the 2 reviewers were resolved by consensus or by a third reviewer (P.R.S.M.-F.).
The following elements were used to define eligibility criteria: (1) population (patients submitted to rhinoplasty), (2) intervention and controls (preoperative administration of tranexamic acid vs placebo solution or no treatment control group), (3) outcomes (primary outcome was intraoperative bleeding and secondary outcomes were postoperative eyelid edema and periorbital ecchymosis, as well as thromboembolic events, including myocardial infarction, stroke, systemic embolism, thrombosis of a mechanical heart valve, thrombosis of the cardiac chamber, deep vein thrombosis, or pulmonary embolism), and (4) study type (RCTs). Eligible studies must report at least 1 of the outcomes of interest. Patients undergoing functional endoscopic sinus surgery and those receiving tranexamic acid in combination with other medications were excluded.
Data Extraction and Risk of Bias Assessment
Using a standardized data extraction sheet, the following information from the studies was extracted: demographic characteristics of study participants, surgical approach, preoperative medication, postoperative prescription, duration of follow-up, and outcome data.
Risk of bias was assessed according to the Cochrane guidelines for RCTs. Seven domains were assessed for evaluation: sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other potential sources of bias. Risk of bias was rated as low, unclear, or high according to established criteria.24
Data Synthesis
Treatment effects were defined as weighted mean difference (WMD) and 95% CIs. To calculate the effect sizes, means and SDs were obtained for each study group and outcome of interest. A negative effect size indicated that tranexamic acid was beneficial in reducing intraoperative bleeding as well as postoperative eyelid edema and ecchymosis. A forest plot was used to present the effect sizes and the 95% CI. Each study was proportional to the study’s weight in the meta-analysis. A 2-tailed P <.05 was used to determine significance. Statistical heterogeneity was assessed using the Cochran Q test25 and quantified by the I2 index.26 A subgroup analysis was performed according to the tranexamic acid administration and follow-up time. Analyses were conducted using Review Manager, version 5.3 (Cochrane IMS).
Grading the Strength of Evidence
We graded the strength of evidence for the association between topical application of tranexamic acid and the primary outcome as high, moderate, low, or very low using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) rating system. In the GRADE system, RCTs begin as high-quality evidence but may be lowered by 1 or more of 5 categories of limitations: risk of bias, inconsistency (heterogeneity), indirectness of evidence, imprecision, and publication bias.27,28
Results
Data Sources
Search strategy yielded 498 potentially relevant studies. After screening of titles and abstracts, 6 full-text articles were assessed for eligibility and 5 RCTs14,15,16,29,30 were included in the meta-analysis. A flow diagram of the study selection process and specific reasons for exclusion are detailed in eFigure 1 in the Supplement.
Study Characteristics
The total number of patients included in the RCTs was 276. Most surgical procedures were performed in women (177 [64.1%]); mean age of the patients was 26.8 (range, 16-42) years. Studies excluded patients with hematologic, endocrine, metabolic, renal, and gastrointestinal diseases, as well as those using anticoagulant therapy. Details of the anesthetic method were reported in 3 studies16,29,30 and included infusion of propofol and fentanyl and use of atracurium or rocuronium to provide muscle relaxation. In 1 study,16 midazolam was administered intravenously before anesthesia. All studies included patients undergoing cosmetic rhinoplasty or septorhinoplasty, but details about the surgical approach were reported in only 3 studies.14,15,16 In 2 studies,14,15 dorsal hump removal and medial and lateral osteotomies were performed in open rhinoplasties. In 1 study,16 closed rhinoplasty was performed and the authors reported that a Killian incision was chosen for septoplasty and cartilaginous graft harvesting, with subsequent dorsal hump removal. In this study, all patients received the same osteotomy under the internal lateral osteotomy. In 3 studies,15,16,30 patients received intravenous administration of tranexamic acid,10 mg/kg, and in 2 studies14,29 tranexamic acid, 1 g, was administered orally.
Four studies comprising 246 patients14,16,29,30 estimated the amount of intraoperative bleeding as a primary outcome by measuring the level of blood in the suction bottles. The weights of dried and bloody gauzes were also calculated in 2 studies,16,29 but in 1 study30 the authors assumed that a saturated gauze held 10 mL without weighing it. Eyelid edema and ecchymosis were evaluated as outcomes of interest in 2 studies.14,15 Degree of eyelid edema and ecchymosis was recorded on postoperative day 1 (POD1), POD3, and POD7 based on a 0- to 4-point scoring system. The scale for eyelid edema was as follows: 0 (none), 1 (minimal), 2 (extending to the iris), 3 (extending to the pupil), and 4 (massive edema). Periorbital ecchymosis was graded as 0 (none), 1 (present in the medial canthus), 2 (extending to the pupil), 3 (past the pupil), or 4 (extending to the lateral canthus). In 1 study,16 mean and SD of edema and ecchymosis ratings were not provided. The main characteristics (number of patients, age, sex, intervention, follow-up, and key findings) of RCTs are presented in the Table.
Table. Characteristics of Studies Included in the Meta-analysis.
| Source | No. | Age, y | Intervention | Rhinoplasty Surgeon's Approach | Follow-up Visits | Key Findings | |||
|---|---|---|---|---|---|---|---|---|---|
| Intraoperative Bleeding | Eyelid Edema | Periorbital Ecchymosis | Thromboembolic Events | ||||||
| Beikaei et al,30 2015 | 96 | 16-42 | Tranexamic acid: 10 mg/kg IV after anesthesia induction control: placebo solution |
Open rhinoplasty | Not reported | Tranexamic acid was associated with a decrease in intraoperative bleeding | Not evaluated | Not evaluated | Not reported |
| Sakallioğlu et al,14 2015 | 50 | 20-36 | Tranexamic acid: 1 g orally 2 h before surgery and 3 doses every 8 h after surgery for 5 d control: placebo solution |
Open rhinoplasty | Days 1, 3, and 7 | Tranexamic acid was associated with a decrease in intraoperative bleeding | Tranexamic acid reduced eyelid edema during the first postoperative week | Tranexamic acid reduced ecchymosis during the first postoperative week | Not reported |
| Eftekharian and Rajabzadeh,292016 | 50 | 16-40 | Tranexamic acid: 1 g orally 2 h before surgery control: placebo solution |
Not described | Tranexamic acid was associated with a decrease in intraoperative bleeding | Not evaluated | Not evaluated | Not reported | |
| Ghavimi et al,16 2017 | 50 | 19-40 | Tranexamic acid: 10 mg/kg IV immediately before surgery control: placebo solution |
Closed rhinoplasty | Day 1 | Tranexamic acid was associated with a decrease in intraoperative bleeding | Tranexamic acid reduced the eyelid edema | Tranexamic acid reduced ecchymosis | Not reported |
| Mehdizadeh et al,15 2018 | 30 | 18-39 | Tranexamic acid: 10 mg/kg IV 1 h before surgery and 3 doses every 8 h after surgery control: placebo solution |
Open rhinoplasty | Days 1, 3, and 7 | Tranexamic acid was associated with a decrease in intraoperative bleeding | Tranexamic acid reduced eyelid edema during the first postoperative week | Tranexamic acid reduced ecchymosis during the first postoperative week | Not reported |
Abbreviation: IV, intravenous.
Risk of Bias
Most RCTs had a low risk for random sequence generation, blinding, and incomplete outcome data. However, most of the trials had unclear allocation concealment and reporting bias (eFigure 2 in the Supplement).
Data Synthesis and Subgroup Analysis
Primary Outcome
The 4 RCTs included in this meta-analysis randomized 122 patients to receive tranexamic acid and 124 to receive placebo solution assigned as controls to determine the effect of tranexamic acid on intraoperative bleeding. Mean difference between groups was −42.28 mL (95% CI, −70.36 to −14.21 mL), indicating an association between tranexamic acid use and reduced bleeding during rhinoplasty. A significant heterogeneity was found among studies (I2 = 84%) and a random-effects model was used to calculate the effect sizes. Differences (P = .01) between oral (WMD, −61.70 mL; 95% CI, −83.02 mL to −40.39 mL; I2 = 0%) and intravenous (WMD, −23.88 mL; CI 95% − 45.19 mL to −2.58 mL; I2 = 56%) administration of tranexamic acid for intraoperative bleeding were found in the subgroup analysis (Figure).
Figure. Outcomes of Tranexamic Acid Therapy on Intraoperative Bleeding in Rhinoplasty.
The size of the data markers indicates the weight assigned to each study in the meta-analysis. The solid vertical line represents the null effect. The effect estimates in the subgroup analysis are shown.
Secondary Outcomes
Eyelid Edema
Eyelid edema scores in patients receiving tranexamic acid were significantly lower compared with those in the control group within the first postoperative week. Differences in the scores were found on POD1 (upper eyelid: WMD, −1.06; 95% CI, −1.59 to −0.54; lower eyelid: WMD, −0.86, 95% CI, −1.15 to −0.57), POD3 (upper eyelid: WMD, −0.99, 95% CI, −1.63 to −0.36; lower eyelid: WMD, −0.69; 95% CI, −1.03 to −0.34), and POD7 (upper eyelid: WMD, −0.80; 95% CI, −1.15 to −0.45; lower eyelid: WMD, −0.76; 95% CI, −1.04 to −0.49). No significant variability was found across subgroups (I2 = 0%) (eFigure 3 and eFigure 4 in the Supplement).
Periorbital Ecchymosis
Postrhinoplasty ecchymosis scores in patients receiving tranexamic acid were also significantly lower compared with the control group. Differences in the scores were found on POD1 (upper eyelid: WMD, −1.24; 95% CI, −2.34 to −0.14; lower eyelid: WMD, −0.72; 95% CI, −1.32 to −0.11); POD3 (upper eyelid: WMD, −1.53; 95% CI, −2.29 to −0.78; lower eyelid: WMD, −0.95; 95% CI, −1.27 to −0.63), and POD7 (upper eyelid: WMD, −1.33; 95% CI, −2.62 to −0.04; lower eyelid: WMD, −0.94; 95% CI, −1.80 to −0.08). No significant variability was found across subgroups (I2 = 0%) (eFigure 5 and eFigure 6 in the Supplement).
Thromboembolic Events
No cases of thromboembolic events were reported in the included studies in either the tranexamic acid or control groups during the 1- to 7-day follow-up period after rhinoplasty.
Strength of Evidence
We graded the protective ability of tranexamic acid in intraoperative bleeding in patients undergoing rhinoplasty as moderate quality of evidence as per the GRADE criteria with the findings considered to be important. In the 4 RCTs, indirectness and the risk of bias were not serious; however, there was serious heterogeneity (I2 = 84%) and imprecision (large 95% CIs). The mean difference in blood loss between the groups was −42.28 (95% CI, −70.36 to −14.21). Other considerations included the lack of a test for publication bias, a large effect size, and use of different formulations (oral vs intravenous tranexamic acid; P = .01).
Discussion
Although rhinoplasty is a safe surgical procedure, direct trauma to the vessels during the osteotomy can lead to excessive bleeding, prolonged eyelid edema, ecchymosis, and asymmetry resulting in aesthetic deformity and excessive narrowing. Various strategies have been used to reduce morbidity associated with the surgery. In this meta-analysis, we evaluated the results of tranexamic acid use in intraoperative bleeding, postoperative eyelid edema, and periorbital ecchymosis in rhinoplasty. We showed that tranexamic acid was associated with reduced bleeding by 42.28 mL compared with placebo. This finding suggests a protective outcome of tranexamic acid use in reducing intraoperative bleeding during rhinoplasty that may be important to reduce operation time and risk of complications. In addition, we found a decrease in eyelid edema and periorbital ecchymosis within the first postoperative week and no reports of thromboembolic events.
The main purpose of tranexamic acid is the reduction of intraoperative bleeding and transfusion requirements in both cardiac and noncardiac surgery. Tranexamic acid is an antifibrinolytic agent derivative of the amino acid lysine that competitively inhibits activation of plasminogen to plasmin, an enzyme that degrades fibrin clots, fibrinogen, and other plasma proteins, including the procoagulant factors V and VIII.31 Studies have suggested an anti-inflammatory response to tranexamic acid by reducing the levels of interleukin-6, fibrin degradation products (d-dimer), creatine kinase, plasminogen activator inhibitor, and C-reactive protein.32,33,34,35 Interleukin-6 plays a major role in the inflammatory response to surgeries, reaching significant plasma levels after 2 to 4 hours and maximum concentration on POD1.36 In the acute-phase response, interleukin-6 stimulates the production in the liver of C-reactive protein, fibrinogen, and other antiproteinases, which is influenced by operation length and volume of blood loss during surgery.37 Therefore, the use of tranexamic acid in patients undergoing rhinoplasty may lead to reduction of interleukin-6 and acute-phase proteins decreasing the eyelid edema within the first postoperative week. Furthermore, the suppression of d-dimer, a marker of ongoing fibrin formation and degradation, suggests that patients receiving tranexamic acid experienced less secondary fibrinolysis,32 which may lead to reduced intraoperative bleeding and postrhinoplasty ecchymosis.
There are few studies to date comparing outcomes after the use of oral and intravenous tranexamic acid, and most of them were conducted in orthopedic surgeries.38 In the present meta-analysis, oral tranexamic acid, 1 g, 2 hours before rhinoplasty was associated with a greater reduction in intraoperative bleeding compared with intravenous tranexamic acid, 10 mg/kg, and is as safe as intravenous administration regarding short-term surgical outcomes and thromboembolic complications. These findings may be related to differences in plasma concentration of oral and intravenous tranexamic acid during the first few hours after administration. Studies have shown that peak concentrations of oral tranexamic acid are noted within 2 to 4 hours after administration in contrast with monoexponential decays observed for intravenous tranexamic acid. Within 6 hours after administration, the levels of intravenous tranexamic acid seem to be subtherapeutic, but the levels of oral tranexamic acid are still above the minimal required concentration to maintain a hemostatic reaction (10 mg/L).39,40 Moreover, oral tranexamic acid provides greater cost savings to patients41,42,43 and can be recommended to reduce bleeding in rhinoplasty.
In standard rhinoplasty procedures, the trauma of fracturing nasal bones and the injuries to angular vessels that cross the osteotomy sites lead to prolonged postoperative periorbital edema and ecchymosis and can affect the cosmetic results and patient’s social life.44 Recent studies have demonstrated that clinical outcomes in patients who undergo otolaryngologic–head and neck surgery may be improved using different surgical approaches, such as the piezoelectric surgery.45,46 In addition, nonpharmacologic and pharmacologic interventions have been widely used to reduce periorbital edema and ecchymosis after facial plastic surgeries, but evidence for efficacy is conflicting. A recent systematic review evaluated the use of postoperative interventions in rhinoplasty and found that cold compression and arnica administration could reduce eyelid edema and ecchymosis in the short-term.47
Although the use of corticosteroids has become a common practice in facial surgery to reduce postoperative inflammation, nausea, and vomiting, there is no evidence that a single dose of corticosteroids administered prior to rhinoplasty decreases periorbital edema and ecchymosis after POD3.48,49 In addition, a randomized, double-blind, placebo-controlled trial showed no benefit of a single-dose of long-acting dexamethasone in patients who underwent closed rhinoplasty with osteotomies.50 To our knowledge, only 2 studies compared the results of tranexamic acid and corticosteroid use for possible reduction of postrhinoplasty periorbital edema and ecchymosis.14,15 The individual results of these studies showed a reduction of edema and ecchymosis favoring tranexamic acid administration, but differences between groups did not reach statistical significance. Despite the evidence that tranexamic acid reduces intraoperative bleeding and morbidity during the first postoperative week, trials should be performed to confirm the potential superiority of tranexamic acid compared with corticosteroids for rhinoplasty.
Studies have been published showing a possible association between tranexamic acid and increased risk for venous thromboembolism in patients with menorrhagia51 and combat trauma,52 as well as an increased incidence rate of seizures.53 However, a comprehensive systematic review found a low risk of deep vein thrombosis and pulmonary embolism in patients with spontaneous bleeding treated with venous thromboembolism, except for cerebral infarction after a subarachnoid hemorrhage (9.7%; 95% CI, 5.5-14.8). Although concern regarding the harm-benefit balance of tranexamic acid administration owing to the theoretical risk of increasing thromboembolic and neurologic events, there is cumulative evidence that tranexamic acid is a well-tolerated drug when delivered orally, intravenously, or topically with no increased risk for myocardial infarction, stroke, deep vein thrombosis, or pulmonary embolism.22,54,55,56,57,58,59,60 In the present systematic review, studies did not report the occurrence of thromboembolic events during the first postoperative week in patients receiving either oral or intravenous administration, but this finding should be interpreted with caution because most cases of venous thromboembolism are asymptomatic61 and the major adverse cardiovascular events were recorded as secondary outcomes or were not prespecified. Moreover, the low incidence of venous thromboembolism in otolaryngologic–head and neck surgery62 and rhinoplasty63 requires larger sample sizes to reach a conclusion on whether the rate of this complication is increased with tranexamic acid.
Because direct comparisons on safety and efficacy between oral and intravenous tranexamic acid in RCTs are lacking, head-to-head trials are needed to determine the real comparative risk-benefit profiles with different routes of drug administration. Although tranexamic acid appears to have a reasonable safety profile in rhinoplasty, appropriate patient counseling is imperative.
Limitations
This systematic review and meta-analysis presents some limitations, including (1) a relatively small number of studies and the lack of publication bias analysis, (2) the unclear allocation concealment and reporting bias for most studies, (3) potential performance bias owing to differences in surgical techniques, and (4) the lack of meta-regression analysis to evaluate the effect of confounders on outcomes of interest and its influence on between-study heterogeneity. Funnel plots may be useful tools in investigating small-study effects in meta-analyses but are of limited power to detect such effects when there are few studies.64 Therefore, owing to the limited number of included studies, a funnel plot analysis was not performed and the potential for publication bias was not analyzed. Considering the limitations of the present study and moderate quality of evidence, further, larger RCTs are needed to confirm the findings.
Conclusions
Current available evidence suggests that preoperative administration of tranexamic acid is safe and may reduce intraoperative bleeding as well as postoperative eyelid edema and ecchymosis in patients undergoing rhinoplasty. Oral tranexamic acid was associated with a greater reduction in intraoperative bleeding compared with intravenous tranexamic acid.
eFigure 1. PRISMA Flow Diagram
eFigure 2. Risk of Bias Assessment
eFigure 3. Efficacy of Tranexamic Acid on Upper Eyelid Edema Within the First Postoperative week
eFigure 4. Efficacy of Tranexamic Acid on Lower Eyelid Edema Within the First Postoperative Week
eFigure 5. Efficacy of Tranexamic Acid on Upper Eyelid Ecchymosis Within the First Postoperative Week
eFigure 6. Efficacy of Tranexamic Acid on Lower Eyelid Ecchymosis Within the First Postoperative Week
References
- 1.Erişir F, Tahamiler R. Lateral osteotomies in rhinoplasty: a safer and less traumatic method. Aesthet Surg J. 2008;28(5):518-520. doi: 10.1016/j.asj.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 2.Cochran CS, Landecker A. Prevention and management of rhinoplasty complications. Plast Reconstr Surg. 2008;122(2):60e-67e. doi: 10.1097/PRS.0b013e31817d53de [DOI] [PubMed] [Google Scholar]
- 3.Ozkose M, Baykan H, Coşkuner İ. The effect of patient positioning on amount of intraoperative bleeding in rhinoplasty: a randomized controlled trial. Aesthetic Plast Surg. 2016;40(4):453-457. doi: 10.1007/s00266-016-0653-6 [DOI] [PubMed] [Google Scholar]
- 4.Hwang SH, Lee JH, Kim BG, Kim SW, Kang JM. The efficacy of steroids for edema and ecchymosis after rhinoplasty: a meta-analysis. Laryngoscope. 2015;125(1):92-98. doi: 10.1002/lary.24883 [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann DF, Cook TA, Quatela VC, Wang TD, Brownrigg PJ, Brummett RE. Steroids and rhinoplasty: a double-blind study. Arch Otolaryngol Head Neck Surg. 1991;117(9):990-993. doi: 10.1001/archotol.1991.01870210062009 [DOI] [PubMed] [Google Scholar]
- 6.Gürlek A, Fariz A, Aydoğan H, Ersöz-Oztürk A, Evans GRD. Effects of high dose corticosteroids in open rhinoplasty. J Plast Reconstr Aesthet Surg. 2009;62(5):650-655. doi: 10.1016/j.bjps.2007.08.030 [DOI] [PubMed] [Google Scholar]
- 7.Holt GR, Garner ET, McLarey D. Postoperative sequelae and complications of rhinoplasty. Otolaryngol Clin North Am. 1987;20(4):853-876. [PubMed] [Google Scholar]
- 8.Ong AA, Farhood Z, Kyle AR, Patel KG. Interventions to decrease postoperative edema and ecchymosis after rhinoplasty: a systematic review of the literature. Plast Reconstr Surg. 2016;137(5):1448-1462. doi: 10.1097/PRS.0000000000002101 [DOI] [PubMed] [Google Scholar]
- 9.Rahimzadeh P, Faiz SH-R, Alebouyeh MR. Effects of premedication with metoprolol on bleeding and induced hypotension in nasal surgery. Anesth Pain Med. 2012;1(3):157-161. doi: 10.5812/aapm.3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gun R, Yorgancılar E, Yıldırım M, Bakır S, Topcu I, Akkus Z. Effects of lidocaine and adrenaline combination on postoperative edema and ecchymosis in rhinoplasty. Int J Oral Maxillofac Surg. 2011;40(7):722-729. doi: 10.1016/j.ijom.2011.02.022 [DOI] [PubMed] [Google Scholar]
- 11.Koşucu M, Omür S, Beşir A, Uraloğlu M, Topbaş M, Livaoğlu M. Effects of perioperative remifentanil with controlled hypotension on intraoperative bleeding and postoperative edema and ecchymosis in open rhinoplasty. J Craniofac Surg. 2014;25(2):471-475. doi: 10.1097/SCS.0000000000000603 [DOI] [PubMed] [Google Scholar]
- 12.Tuncel U, Turan A, Bayraktar MA, Erkorkmaz U, Kostakoglu N. Efficacy of dexamethasone with controlled hypotension on intraoperative bleeding, postoperative oedema and ecchymosis in rhinoplasty. J Craniomaxillofac Surg. 2013;41(2):124-128. doi: 10.1016/j.jcms.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 13.Gruber RP, Zeidler KR, Berkowitz RL. Desmopressin as a hemostatic agent to provide a dry intraoperative field in rhinoplasty. Plast Reconstr Surg. 2015;135(5):1337-1340. doi: 10.1097/PRS.0000000000001158 [DOI] [PubMed] [Google Scholar]
- 14.Sakallioğlu Ö, Polat C, Soylu E, Düzer S, Orhan İ, Akyiğit A. The efficacy of tranexamic acid and corticosteroid on edema and ecchymosis in septorhinoplasty. Ann Plast Surg. 2015;74(4):392-396. doi: 10.1097/SAP.0b013e3182a1e527 [DOI] [PubMed] [Google Scholar]
- 15.Mehdizadeh M, Ghassemi A, Khakzad M, et al. Comparison of the effect of dexamethasone and tranexamic acid, separately or in combination on post-rhinoplasty edema and ecchymosis. Aesthetic Plast Surg. 2018;42(1):246-252. doi: 10.1007/s00266-017-0969-x [DOI] [PubMed] [Google Scholar]
- 16.Ghavimi MA, Taheri Talesh K, Ghoreishizadeh A, Chavoshzadeh MA, Zarandi A. Efficacy of tranexamic acid on side effects of rhinoplasty: a randomized double-blind study. J Craniomaxillofac Surg. 2017;45(6):897-902. doi: 10.1016/j.jcms.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 17.Dai Z, Chu H, Wang S, Liang Y. The effect of tranexamic acid to reduce blood loss and transfusion on off-pump coronary artery bypass surgery: A systematic review and cumulative meta-analysis. J Clin Anesth. 2018;44:23-31. doi: 10.1016/j.jclinane.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 18.Gausden EB, Qudsi R, Boone MD, OʼGara B, Ruzbarsky JJ, Lorich DG. Tranexamic acid in orthopaedic trauma surgery: a meta-analysis. J Orthop Trauma. 2017;31(10):513-519. doi: 10.1097/BOT.0000000000000913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Zhang MM, Jifeng Li MM, Xiao Wang MM. Combined versus single application of tranexamic acid in total knee and hip arthroplasty: a meta-analysis of randomized controlled trials. Int J Surg. 2017;43:171-180. doi: 10.1016/j.ijsu.2017.05.065 [DOI] [PubMed] [Google Scholar]
- 20.Zhang F, Wang K, Li F-N, et al. Effectiveness of tranexamic acid in reducing blood loss in spinal surgery: a meta-analysis. BMC Musculoskelet Disord. 2014;15(1):448. doi: 10.1186/1471-2474-15-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen JJ, Skov J, Ingerslev J, Thorn JJ, Pinholt EM. Prevention of bleeding in orthognathic surgery—a systematic review and meta-analysis of randomized controlled trials. J Oral Maxillofac Surg. 2016;74(1):139-150. doi: 10.1016/j.joms.2015.05.031 [DOI] [PubMed] [Google Scholar]
- 22.de Vasconcellos SJ de A, de Santana Santos T, Reinheimer DM, Faria-E-Silva AL, de Melo MF, Martins-Filho PR. Topical application of tranexamic acid in anticoagulated patients undergoing minor oral surgery: a systematic review and meta-analysis of randomized clinical trials. J Craniomaxillofac Surg. 2017;45(1):20-26. doi: 10.1016/j.jcms.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101. doi: 10.2307/3001666 [DOI] [Google Scholar]
- 26.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 27.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ; GRADE Working Group . What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336(7651):995-998. doi: 10.1136/bmj.39490.551019.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eftekharian HR, Rajabzadeh Z. The efficacy of preoperative oral tranexamic acid on intraoperative bleeding during rhinoplasty. J Craniofac Surg. 2016;27(1):97-100. doi: 10.1097/SCS.0000000000002273 [DOI] [PubMed] [Google Scholar]
- 30.Beikaei M, Ghazipour A, Derakhshande V, Saki N, Nikakhlagh S. Evaluating the effect of intravenous tranexamic acid on intraoperative bleeding during elective rhinoplasty surgery. Biomed Pharmacol J. 2015;8SE:753-759. doi: 10.13005/bpj/779 [DOI] [Google Scholar]
- 31.Hunt BJ. The current place of tranexamic acid in the management of bleeding. Anaesthesia. 2015;70(suppl 1):50-53, e18. doi: 10.1111/anae.12910 [DOI] [PubMed] [Google Scholar]
- 32.Jimenez JJ, Iribarren JL, Lorente L, et al. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double-blind controlled trial. Crit Care. 2007;11(6):R117. doi: 10.1186/cc6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie J, Hu Q, Ma J, Huang Q, Pei F. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss and the inflammatory response following enhanced-recovery primary total hip arthroplasty: a randomised clinical trial. Bone Joint J. 2017;99-B(11):1442-1449. doi: 10.1302/0301-620X.99B11.BJJ-2017-0488.R1 [DOI] [PubMed] [Google Scholar]
- 34.Jiménez JJ, Iribarren JL, Brouard M, et al. Safety and effectiveness of two treatment regimes with tranexamic acid to minimize inflammatory response in elective cardiopulmonary bypass patients: a randomized double-blind, dose-dependent, phase IV clinical trial. J Cardiothorac Surg. 2011;6:138. doi: 10.1186/1749-8090-6-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Later AFL, Bruggemans EF, Romijn FPHTM, van Pelt J, Klautz RJM. A comparative study of the immune modulating properties of antifibrinolytics in cardiac surgery. Cytokine. 2013;61(2):438-444. doi: 10.1016/j.cyto.2012.10.033 [DOI] [PubMed] [Google Scholar]
- 36.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85(1):109-117. doi: 10.1093/bja/85.1.109 [DOI] [PubMed] [Google Scholar]
- 37.Sakamoto K, Arakawa H, Mita S, et al. Elevation of circulating interleukin 6 after surgery: factors influencing the serum level. Cytokine. 1994;6(2):181-186. doi: 10.1016/1043-4666(94)90040-X [DOI] [PubMed] [Google Scholar]
- 38.Zhang L-K, Ma J-X, Kuang M-J, et al. Comparison of oral versus intravenous application of tranexamic acid in total knee and hip arthroplasty: a systematic review and meta-analysis. Int J Surg. 2017;45:77-84. doi: 10.1016/j.ijsu.2017.07.097 [DOI] [PubMed] [Google Scholar]
- 39.Pilbrant A, Schannong M, Vessman J. Pharmacokinetics and bioavailability of tranexamic acid. Eur J Clin Pharmacol. 1981;20(1):65-72. doi: 10.1007/BF00554669 [DOI] [PubMed] [Google Scholar]
- 40.Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41-47. doi: 10.1136/jcp.33.Suppl_14.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Irwin A, Khan SK, Jameson SS, Tate RC, Copeland C, Reed MR. Oral versus intravenous tranexamic acid in enhanced-recovery primary total hip and knee replacement: results of 3000 procedures. Bone Joint J. 2013;95-B(11):1556-1561. doi: 10.1302/0301-620X.95B11.31055 [DOI] [PubMed] [Google Scholar]
- 42.Kayupov E, Fillingham YA, Okroj K, et al. Oral and intravenous tranexamic acid are equivalent at reducing blood loss following total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2017;99(5):373-378. doi: 10.2106/JBJS.16.00188 [DOI] [PubMed] [Google Scholar]
- 43.Yuan X, Li B, Wang Q, Zhang X. Comparison of 3 routes of administration of tranexamic acid on primary unilateral total knee arthroplasty: a prospective, randomized, controlled study. J Arthroplasty. 2017;32(9):2738-2743. doi: 10.1016/j.arth.2017.03.059 [DOI] [PubMed] [Google Scholar]
- 44.Valente DS, Steffen N, Carvalho LA, Borille GB, Zanella RK, Padoin AV. Preoperative use of dexamethasone in rhinoplasty: a randomized, double-blind, placebo-controlled clinical trial. JAMA Facial Plast Surg. 2015;17(3):169-173. doi: 10.1001/jamafacial.2014.1574 [DOI] [PubMed] [Google Scholar]
- 45.Koc B, Koc EA, Erbek S. Comparison of clinical outcomes using a Piezosurgery device vs. a conventional osteotome for lateral osteotomy in rhinoplasty. Ear Nose Throat J. 2017;96(8):318-326. [DOI] [PubMed] [Google Scholar]
- 46.Pagotto LEC, de Santana Santos T, de Vasconcellos SJA, Santos JS, Martins-Filho PRS. Piezoelectric versus conventional techniques for orthognathic surgery: systematic review and meta-analysis. J Craniomaxillofac Surg. 2017;45(10):1607-1613. doi: 10.1016/j.jcms.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 47.Lee HS, Yoon HY, Kim IH, Hwang SH. The effectiveness of postoperative intervention in patients after rhinoplasty: a meta-analysis. Eur Arch Otorhinolaryngol. 2017;274(7):2685-2694. doi: 10.1007/s00405-017-4535-6 [DOI] [PubMed] [Google Scholar]
- 48.da Silva EMK, Hochman B, Ferreira LM. Perioperative corticosteroids for preventing complications following facial plastic surgery. Cochrane Database Syst Rev. 2014;(6):CD009697. doi: 10.1002/14651858.CD009697.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pulikkottil BJ, Dauwe P, Daniali L, Rohrich RJ. Corticosteroid use in cosmetic plastic surgery. Plast Reconstr Surg. 2013;132(3):352e-360e. doi: 10.1097/PRS.0b013e31829acc60 [DOI] [PubMed] [Google Scholar]
- 50.Gutierrez S, Wuesthoff C. Testing the effects of long-acting steroids in edema and ecchymosis after closed rhinoplasty. Plast Surg (Oakv). 2014;22(2):83-87. doi: 10.1177/229255031402200213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sundström A, Seaman H, Kieler H, Alfredsson L. The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: a case-control study using the General Practice Research Database. BJOG. 2009;116(1):91-97. doi: 10.1111/j.1471-0528.2008.01926.x [DOI] [PubMed] [Google Scholar]
- 52.Johnston LR, Rodriguez CJ, Elster EA, Bradley MJ. Evaluation of military use of tranexamic acid and associated thromboembolic events. JAMA Surg. 2018;153(2):169-175. doi: 10.1001/jamasurg.2017.3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin Z, Xiaoyi Z. Tranexamic acid-associated seizures: a meta-analysis. Seizure. 2016;36:70-73. doi: 10.1016/j.seizure.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 54.Ross J, Al-Shahi Salman R. The frequency of thrombotic events among adults given antifibrinolytic drugs for spontaneous bleeding: systematic review and meta-analysis of observational studies and randomized trials. Curr Drug Saf. 2012;7(1):44-54. doi: 10.2174/157488612800492744 [DOI] [PubMed] [Google Scholar]
- 55.Hutton B, Joseph L, Fergusson D, Mazer CD, Shapiro S, Tinmouth A. Risks of harms using antifibrinolytics in cardiac surgery: systematic review and network meta-analysis of randomised and observational studies. BMJ. 2012;345:e5798. doi: 10.1136/bmj.e5798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z-J, Fu X, Xing D, Zhang H-F, Zang J-C, Ma X-L. Is tranexamic acid effective and safe in spinal surgery? A meta-analysis of randomized controlled trials. Eur Spine J. 2013;22(9):1950-1957. doi: 10.1007/s00586-013-2774-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li C, Gong Y, Dong L, Xie B, Dai Z. Is prophylactic tranexamic acid administration effective and safe for postpartum hemorrhage prevention? a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(1):e5653. doi: 10.1097/MD.0000000000005653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang D, Wang L, Wang Y, Lin X. The efficiency and safety of tranexamic acid for reducing blood loss in open myomectomy: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96(23):e7072. doi: 10.1097/MD.0000000000007072 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Kim HJ, Moon SH, Cho SH, Lee JD, Kim HS. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97(7):776-781. doi: 10.2340/00015555-2668 [DOI] [PubMed] [Google Scholar]
- 60.Franchini M, Mengoli C, Marietta M, et al. Safety of intravenous tranexamic acid in patients undergoing major orthopaedic surgery: a meta-analysis of randomised controlled trials. Blood Transfus. 2018;16(1):36-43. doi: 10.2450/2017.0219-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pannucci CJ, Shanks A, Moote MJ, et al. Identifying patients at high risk for venous thromboembolism requiring treatment after outpatient surgery. Ann Surg. 2012;255(6):1093-1099. doi: 10.1097/SLA.0b013e3182519ccf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cramer JD, Shuman AG, Brenner MJ. Antithrombotic therapy for venous thromboembolism and prevention of thrombosis in otolaryngology–head and neck surgery: state of the art review. Otolaryngol Head Neck Surg. 2018;158(4):627-636. doi: 10.1177/0194599818756599 [DOI] [PubMed] [Google Scholar]
- 63.Moubayed SP, Akdagli S, Most SP. Incidence of venous thromboembolism in rhinoplasty. Aesthet Surg J. 2017;37(3):NP34-NP35. doi: 10.1093/asj/sjw252 [DOI] [PubMed] [Google Scholar]
- 64.Simmonds M. Quantifying the risk of error when interpreting funnel plots. Syst Rev. 2015;4(1):24. doi: 10.1186/s13643-015-0004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA Flow Diagram
eFigure 2. Risk of Bias Assessment
eFigure 3. Efficacy of Tranexamic Acid on Upper Eyelid Edema Within the First Postoperative week
eFigure 4. Efficacy of Tranexamic Acid on Lower Eyelid Edema Within the First Postoperative Week
eFigure 5. Efficacy of Tranexamic Acid on Upper Eyelid Ecchymosis Within the First Postoperative Week
eFigure 6. Efficacy of Tranexamic Acid on Lower Eyelid Ecchymosis Within the First Postoperative Week